User login
Huge underuse of germline testing for cancer patients
Information from germline genetic testing could affect a patient’s cancer care. For example, such testing could indicate that targeted therapies would be beneficial, and it would have implications for close relatives who may carry the same genes.
The finding that so few patients with newly diagnosed cancer were tested comes from an analysis of data on more than 1.3 million individuals across two U.S. states. The data were taken from the Surveillance, Epidemiology, and End Results (SEER) registry.
The rate is “well below guideline recommendations,” said study presenter Allison W. Kurian, MD, department of medicine, Stanford (Calif.) University.
“Innovative care delivery” is needed to tackle the problem, including the streamlining of pretest counseling, making posttest counseling more widely available, and employing long-term follow-up to track patient outcomes, she suggested.
“I do think this is a time for creative solutions of a number of different kinds,” she said. She suggested that lessons could be learned from the use of telemedicine during the COVID-19 pandemic. She also noted that “there have been some interesting studies on embedding genetic counselors in oncology clinics.”
Dr. Kurian presented the study at the annual meeting of the American Society of Clinical Oncology (ASCO). The study was simultaneously published in the Journal of the American Medical Association.
The current results represent a “missed opportunity for decrease the population-level burden of cancer,” experts noted in an accompanying editorial.
“Clinicians should recommend testing to their patients and provide them with the information necessary to make informed decisions about whether to undergo testing,” Zsofia K. Stadler, MD, and Deborah Schrag, MD, MPH, of Memorial Sloan Kettering Cancer Center, New York, wrote in their editorial.
They suggested novel approaches to widen access, such as use of point-of-care testing, telecounseling, and, in the future, chatbots to respond to patient questions.
“With greater emphasis on overcoming both health system and patient-level barriers to genetic cancer susceptibility testing for patients with cancer, treatment outcomes will improve and cancer diagnoses and related deaths in family members will be prevented,” they concluded.
At the meeting, invited discussant Erin Frances Cobain, MD, assistant professor of medical oncology, University of Michigan Health, Ann Arbor, referring to breast cancer as an example, said that progress has “stagnated” in recent years.
The study found a higher rate of gene testing among patients with newly diagnosed breast cancer, at just over 20%.
Dr. Cobain argued that this was still too low. She pointed out that “a recent study suggested that over 60% of individuals with an incident cancer diagnosis would meet criteria for genetic testing by National Comprehensive Cancer Network guidelines.
“This may be because testing is not offered, there may be poor access to genetic counseling resources, or patients may be offered testing but decline it,” she suggested.
One compelling reason to conduct genetic testing for patients newly diagnosed with breast cancer is that it may show that they are candidates for treatment with PARP (poly[ADP]-ribose polymerase) inhibitors, which “may have a direct impact on cancer-related mortality,” she pointed out.
“We need increased awareness and access to genetic testing resources for patients with breast cancer, particularly for racial and ethnic minorities,” she said.
Dr. Cobain also noted that finding variants of uncertain significance (VUS) was more likely among patients from racial and ethnic minorities than among White patients. She said such a finding “increases patient and physician anxiety,” and there may be “unclear optimal management recommendations for these patients.”
Details of the study
Germline genetic testing is “increasingly essential for cancer care,” Dr. Kurian said.
It is central to risk-adapted screening and secondary prevention, the use of targeted therapies, including PARP and checkpoint inhibitors, and cascade testing to identify at-risk relatives.
She pointed out that in clinical practice, testing has “evolved rapidly.” Panels include more and more genes. In addition, the cost of these tests is falling, and guidelines have become “more expansive.”
However, “little is known about genetic testing use and results,” Dr. Kurian noted.
The team therefore undertook the SEER-GeneLINK initiative, which involved patients aged ≥ 20 years who were diagnosed with cancer between Jan. 1, 2013, and March 31, 2019, and who were reported to statewide SEER registries in California and Georgia.
The team looked for patients for whom germline genetic test results had been reported by the four laboratories that performed the majority of patient testing in the two states. Results were categorized as pathogenic, benign, or VUS.
The results were classified on the basis of current guidelines for testing and/or management as related to breast/ovarian cancer, gastrointestinal cancer, other hereditary cancers, or those with no guidelines for testing or management.
Dr. Kurian reported that from an overall population of 1,412,388 patients diagnosed with cancer, 1,369,660 were eligible for inclusion. Of those, about half (51.9%) were women, and the majority (86.3%) were aged 50 years or older.
Many of these patients (61.4%) were non-Hispanic White persons, and slightly fewer than half (49.8%) were deemed to be in medium or high poverty, as determined using U.S. Census tract levels.
Overall, germline genetic testing was performed in 93,052 (6.8%) of patients over the study period.
Women were more likely to have undergone germline mutation testing than men, at 13.9% vs. 2.2%, as were patients aged 20-49 years, at 22.1% vs. 8.2% for those aged 50-69 years, and 3.3% for those aged 70 years and older.
The number of genes for which testing was conducted increased from a median of 2 in 2013 to 34 in 2019. Rates of VUS increased more than that for pathologic variants and substantially more so in non-White patients.
By 2019, the ratio of VUS to pathologic variants stood at 1.7 among White patients, vs. 3.9 among Asian patients, 3.6 among Black patients, and 2.2 among Hispanic patients.
The majority of identified pathologic variants that were related to the diagnosed cancer and genes with testing and/or management guidelines accounted for 67.5% to 94.9% of such variants.
Regarding specific cancer diagnoses, Dr. Kurian said that over the course of the study period, testing rates consistently exceeded 50% only among male breast cancer patients.
There were rapid increases in testing for ovarian cancer, from 28.0% of cases in 2013 to 54.0% in 2019. For pancreatic cancer, rates increased from 1.0% to 19.0% over the same period, and for prostate cancer, rates increased from 0.1% to 4.0%. She suggested that these increases in rates may be related to the approval of PARP inhibitors for use in these indications.
However, there was little change in the rates of germline mutation testing for lung cancer patients, from 01% in 2013 to 0.8% in 2019, and for other cancers, from 0.3% to 2.0%.
The results also revealed racial and ethnic differences in testing after controlling for age, cancer type, and year. Over the course of the study period, 8.0% of White patients underwent genetic testing, compared with 6.0% each for Asian, Black, and Hispanic patients and 5.0% for other patients (P < .001).
With regard specifically to male and female breast cancer and ovarian cancer, testing rates were 31% among White patients, 22% for Asian patients, 25% for Black patients, and 23% for Hispanic patients (P < .001).
Dr. Kurian acknowledged that the study is limited by a lack of testing from other laboratories and direct-to-consumer test data, although a recent survey suggested that this represents fewer than 5% of all germline genetic tests.
She also noted that the SEER registries do not collect data on family history or tumor sequencing.
The study was funded by the National Institutes of Health, and the Centers for Disease Control and Prevention. Dr. Kurian has relationships with Adela, Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, InVitae, and Myriad Genetics. Other authors report numerous relationships with industry. Dr. Cobain has ties with AstraZeneca, Daiichi Sankyo, Athenex, Ayala Pharmaceuticals, bioTheranostics, and Immunomedics. Dr. Schrag has relationships with Merck, JAMA, AACR, and Grail. Dr. Stadler has ties with Adverum Biotechnologies, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Information from germline genetic testing could affect a patient’s cancer care. For example, such testing could indicate that targeted therapies would be beneficial, and it would have implications for close relatives who may carry the same genes.
The finding that so few patients with newly diagnosed cancer were tested comes from an analysis of data on more than 1.3 million individuals across two U.S. states. The data were taken from the Surveillance, Epidemiology, and End Results (SEER) registry.
The rate is “well below guideline recommendations,” said study presenter Allison W. Kurian, MD, department of medicine, Stanford (Calif.) University.
“Innovative care delivery” is needed to tackle the problem, including the streamlining of pretest counseling, making posttest counseling more widely available, and employing long-term follow-up to track patient outcomes, she suggested.
“I do think this is a time for creative solutions of a number of different kinds,” she said. She suggested that lessons could be learned from the use of telemedicine during the COVID-19 pandemic. She also noted that “there have been some interesting studies on embedding genetic counselors in oncology clinics.”
Dr. Kurian presented the study at the annual meeting of the American Society of Clinical Oncology (ASCO). The study was simultaneously published in the Journal of the American Medical Association.
The current results represent a “missed opportunity for decrease the population-level burden of cancer,” experts noted in an accompanying editorial.
“Clinicians should recommend testing to their patients and provide them with the information necessary to make informed decisions about whether to undergo testing,” Zsofia K. Stadler, MD, and Deborah Schrag, MD, MPH, of Memorial Sloan Kettering Cancer Center, New York, wrote in their editorial.
They suggested novel approaches to widen access, such as use of point-of-care testing, telecounseling, and, in the future, chatbots to respond to patient questions.
“With greater emphasis on overcoming both health system and patient-level barriers to genetic cancer susceptibility testing for patients with cancer, treatment outcomes will improve and cancer diagnoses and related deaths in family members will be prevented,” they concluded.
At the meeting, invited discussant Erin Frances Cobain, MD, assistant professor of medical oncology, University of Michigan Health, Ann Arbor, referring to breast cancer as an example, said that progress has “stagnated” in recent years.
The study found a higher rate of gene testing among patients with newly diagnosed breast cancer, at just over 20%.
Dr. Cobain argued that this was still too low. She pointed out that “a recent study suggested that over 60% of individuals with an incident cancer diagnosis would meet criteria for genetic testing by National Comprehensive Cancer Network guidelines.
“This may be because testing is not offered, there may be poor access to genetic counseling resources, or patients may be offered testing but decline it,” she suggested.
One compelling reason to conduct genetic testing for patients newly diagnosed with breast cancer is that it may show that they are candidates for treatment with PARP (poly[ADP]-ribose polymerase) inhibitors, which “may have a direct impact on cancer-related mortality,” she pointed out.
“We need increased awareness and access to genetic testing resources for patients with breast cancer, particularly for racial and ethnic minorities,” she said.
Dr. Cobain also noted that finding variants of uncertain significance (VUS) was more likely among patients from racial and ethnic minorities than among White patients. She said such a finding “increases patient and physician anxiety,” and there may be “unclear optimal management recommendations for these patients.”
Details of the study
Germline genetic testing is “increasingly essential for cancer care,” Dr. Kurian said.
It is central to risk-adapted screening and secondary prevention, the use of targeted therapies, including PARP and checkpoint inhibitors, and cascade testing to identify at-risk relatives.
She pointed out that in clinical practice, testing has “evolved rapidly.” Panels include more and more genes. In addition, the cost of these tests is falling, and guidelines have become “more expansive.”
However, “little is known about genetic testing use and results,” Dr. Kurian noted.
The team therefore undertook the SEER-GeneLINK initiative, which involved patients aged ≥ 20 years who were diagnosed with cancer between Jan. 1, 2013, and March 31, 2019, and who were reported to statewide SEER registries in California and Georgia.
The team looked for patients for whom germline genetic test results had been reported by the four laboratories that performed the majority of patient testing in the two states. Results were categorized as pathogenic, benign, or VUS.
The results were classified on the basis of current guidelines for testing and/or management as related to breast/ovarian cancer, gastrointestinal cancer, other hereditary cancers, or those with no guidelines for testing or management.
Dr. Kurian reported that from an overall population of 1,412,388 patients diagnosed with cancer, 1,369,660 were eligible for inclusion. Of those, about half (51.9%) were women, and the majority (86.3%) were aged 50 years or older.
Many of these patients (61.4%) were non-Hispanic White persons, and slightly fewer than half (49.8%) were deemed to be in medium or high poverty, as determined using U.S. Census tract levels.
Overall, germline genetic testing was performed in 93,052 (6.8%) of patients over the study period.
Women were more likely to have undergone germline mutation testing than men, at 13.9% vs. 2.2%, as were patients aged 20-49 years, at 22.1% vs. 8.2% for those aged 50-69 years, and 3.3% for those aged 70 years and older.
The number of genes for which testing was conducted increased from a median of 2 in 2013 to 34 in 2019. Rates of VUS increased more than that for pathologic variants and substantially more so in non-White patients.
By 2019, the ratio of VUS to pathologic variants stood at 1.7 among White patients, vs. 3.9 among Asian patients, 3.6 among Black patients, and 2.2 among Hispanic patients.
The majority of identified pathologic variants that were related to the diagnosed cancer and genes with testing and/or management guidelines accounted for 67.5% to 94.9% of such variants.
Regarding specific cancer diagnoses, Dr. Kurian said that over the course of the study period, testing rates consistently exceeded 50% only among male breast cancer patients.
There were rapid increases in testing for ovarian cancer, from 28.0% of cases in 2013 to 54.0% in 2019. For pancreatic cancer, rates increased from 1.0% to 19.0% over the same period, and for prostate cancer, rates increased from 0.1% to 4.0%. She suggested that these increases in rates may be related to the approval of PARP inhibitors for use in these indications.
However, there was little change in the rates of germline mutation testing for lung cancer patients, from 01% in 2013 to 0.8% in 2019, and for other cancers, from 0.3% to 2.0%.
The results also revealed racial and ethnic differences in testing after controlling for age, cancer type, and year. Over the course of the study period, 8.0% of White patients underwent genetic testing, compared with 6.0% each for Asian, Black, and Hispanic patients and 5.0% for other patients (P < .001).
With regard specifically to male and female breast cancer and ovarian cancer, testing rates were 31% among White patients, 22% for Asian patients, 25% for Black patients, and 23% for Hispanic patients (P < .001).
Dr. Kurian acknowledged that the study is limited by a lack of testing from other laboratories and direct-to-consumer test data, although a recent survey suggested that this represents fewer than 5% of all germline genetic tests.
She also noted that the SEER registries do not collect data on family history or tumor sequencing.
The study was funded by the National Institutes of Health, and the Centers for Disease Control and Prevention. Dr. Kurian has relationships with Adela, Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, InVitae, and Myriad Genetics. Other authors report numerous relationships with industry. Dr. Cobain has ties with AstraZeneca, Daiichi Sankyo, Athenex, Ayala Pharmaceuticals, bioTheranostics, and Immunomedics. Dr. Schrag has relationships with Merck, JAMA, AACR, and Grail. Dr. Stadler has ties with Adverum Biotechnologies, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Information from germline genetic testing could affect a patient’s cancer care. For example, such testing could indicate that targeted therapies would be beneficial, and it would have implications for close relatives who may carry the same genes.
The finding that so few patients with newly diagnosed cancer were tested comes from an analysis of data on more than 1.3 million individuals across two U.S. states. The data were taken from the Surveillance, Epidemiology, and End Results (SEER) registry.
The rate is “well below guideline recommendations,” said study presenter Allison W. Kurian, MD, department of medicine, Stanford (Calif.) University.
“Innovative care delivery” is needed to tackle the problem, including the streamlining of pretest counseling, making posttest counseling more widely available, and employing long-term follow-up to track patient outcomes, she suggested.
“I do think this is a time for creative solutions of a number of different kinds,” she said. She suggested that lessons could be learned from the use of telemedicine during the COVID-19 pandemic. She also noted that “there have been some interesting studies on embedding genetic counselors in oncology clinics.”
Dr. Kurian presented the study at the annual meeting of the American Society of Clinical Oncology (ASCO). The study was simultaneously published in the Journal of the American Medical Association.
The current results represent a “missed opportunity for decrease the population-level burden of cancer,” experts noted in an accompanying editorial.
“Clinicians should recommend testing to their patients and provide them with the information necessary to make informed decisions about whether to undergo testing,” Zsofia K. Stadler, MD, and Deborah Schrag, MD, MPH, of Memorial Sloan Kettering Cancer Center, New York, wrote in their editorial.
They suggested novel approaches to widen access, such as use of point-of-care testing, telecounseling, and, in the future, chatbots to respond to patient questions.
“With greater emphasis on overcoming both health system and patient-level barriers to genetic cancer susceptibility testing for patients with cancer, treatment outcomes will improve and cancer diagnoses and related deaths in family members will be prevented,” they concluded.
At the meeting, invited discussant Erin Frances Cobain, MD, assistant professor of medical oncology, University of Michigan Health, Ann Arbor, referring to breast cancer as an example, said that progress has “stagnated” in recent years.
The study found a higher rate of gene testing among patients with newly diagnosed breast cancer, at just over 20%.
Dr. Cobain argued that this was still too low. She pointed out that “a recent study suggested that over 60% of individuals with an incident cancer diagnosis would meet criteria for genetic testing by National Comprehensive Cancer Network guidelines.
“This may be because testing is not offered, there may be poor access to genetic counseling resources, or patients may be offered testing but decline it,” she suggested.
One compelling reason to conduct genetic testing for patients newly diagnosed with breast cancer is that it may show that they are candidates for treatment with PARP (poly[ADP]-ribose polymerase) inhibitors, which “may have a direct impact on cancer-related mortality,” she pointed out.
“We need increased awareness and access to genetic testing resources for patients with breast cancer, particularly for racial and ethnic minorities,” she said.
Dr. Cobain also noted that finding variants of uncertain significance (VUS) was more likely among patients from racial and ethnic minorities than among White patients. She said such a finding “increases patient and physician anxiety,” and there may be “unclear optimal management recommendations for these patients.”
Details of the study
Germline genetic testing is “increasingly essential for cancer care,” Dr. Kurian said.
It is central to risk-adapted screening and secondary prevention, the use of targeted therapies, including PARP and checkpoint inhibitors, and cascade testing to identify at-risk relatives.
She pointed out that in clinical practice, testing has “evolved rapidly.” Panels include more and more genes. In addition, the cost of these tests is falling, and guidelines have become “more expansive.”
However, “little is known about genetic testing use and results,” Dr. Kurian noted.
The team therefore undertook the SEER-GeneLINK initiative, which involved patients aged ≥ 20 years who were diagnosed with cancer between Jan. 1, 2013, and March 31, 2019, and who were reported to statewide SEER registries in California and Georgia.
The team looked for patients for whom germline genetic test results had been reported by the four laboratories that performed the majority of patient testing in the two states. Results were categorized as pathogenic, benign, or VUS.
The results were classified on the basis of current guidelines for testing and/or management as related to breast/ovarian cancer, gastrointestinal cancer, other hereditary cancers, or those with no guidelines for testing or management.
Dr. Kurian reported that from an overall population of 1,412,388 patients diagnosed with cancer, 1,369,660 were eligible for inclusion. Of those, about half (51.9%) were women, and the majority (86.3%) were aged 50 years or older.
Many of these patients (61.4%) were non-Hispanic White persons, and slightly fewer than half (49.8%) were deemed to be in medium or high poverty, as determined using U.S. Census tract levels.
Overall, germline genetic testing was performed in 93,052 (6.8%) of patients over the study period.
Women were more likely to have undergone germline mutation testing than men, at 13.9% vs. 2.2%, as were patients aged 20-49 years, at 22.1% vs. 8.2% for those aged 50-69 years, and 3.3% for those aged 70 years and older.
The number of genes for which testing was conducted increased from a median of 2 in 2013 to 34 in 2019. Rates of VUS increased more than that for pathologic variants and substantially more so in non-White patients.
By 2019, the ratio of VUS to pathologic variants stood at 1.7 among White patients, vs. 3.9 among Asian patients, 3.6 among Black patients, and 2.2 among Hispanic patients.
The majority of identified pathologic variants that were related to the diagnosed cancer and genes with testing and/or management guidelines accounted for 67.5% to 94.9% of such variants.
Regarding specific cancer diagnoses, Dr. Kurian said that over the course of the study period, testing rates consistently exceeded 50% only among male breast cancer patients.
There were rapid increases in testing for ovarian cancer, from 28.0% of cases in 2013 to 54.0% in 2019. For pancreatic cancer, rates increased from 1.0% to 19.0% over the same period, and for prostate cancer, rates increased from 0.1% to 4.0%. She suggested that these increases in rates may be related to the approval of PARP inhibitors for use in these indications.
However, there was little change in the rates of germline mutation testing for lung cancer patients, from 01% in 2013 to 0.8% in 2019, and for other cancers, from 0.3% to 2.0%.
The results also revealed racial and ethnic differences in testing after controlling for age, cancer type, and year. Over the course of the study period, 8.0% of White patients underwent genetic testing, compared with 6.0% each for Asian, Black, and Hispanic patients and 5.0% for other patients (P < .001).
With regard specifically to male and female breast cancer and ovarian cancer, testing rates were 31% among White patients, 22% for Asian patients, 25% for Black patients, and 23% for Hispanic patients (P < .001).
Dr. Kurian acknowledged that the study is limited by a lack of testing from other laboratories and direct-to-consumer test data, although a recent survey suggested that this represents fewer than 5% of all germline genetic tests.
She also noted that the SEER registries do not collect data on family history or tumor sequencing.
The study was funded by the National Institutes of Health, and the Centers for Disease Control and Prevention. Dr. Kurian has relationships with Adela, Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, InVitae, and Myriad Genetics. Other authors report numerous relationships with industry. Dr. Cobain has ties with AstraZeneca, Daiichi Sankyo, Athenex, Ayala Pharmaceuticals, bioTheranostics, and Immunomedics. Dr. Schrag has relationships with Merck, JAMA, AACR, and Grail. Dr. Stadler has ties with Adverum Biotechnologies, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
AT ASCO 2023
DEI training gives oncology fellows more confidence
The finding comes from a survey conducted after the introduction of DEI training within the Yale Medical Oncology-Hematology Fellowship Program. The study was reported by Norin Ansari, MD, MPH, of Yale Cancer Center, New Haven, Conn., at the annual meeting of the American Society of Clinical Oncology (ASCO).
Dr. Ansari emphasized the DEI curriculum in fellowship programs by highlighting the racial and gender disparities that exist among physicians.
“There is a significant representation problem – only 2%-3% of practicing oncologists are Black or Hispanic/Latino,” she said. “And that representation decreases with each stage in the pipeline of the workforce.”
Dr. Ansari also noted gender disparities in the oncologist workforce, reporting that about one-third of faculty positions are held by women.
The anonymous survey was sent to 29 fellows; 23 responded, including 8 first-year fellows and 13 senior fellows. Over 57% of respondents rated the importance of DEI education as 10 on a 10-point scale (mean, 8.6).
At the start of this year, the responses of senior fellows who had already received some DEI training during the previous year’s lecture series were compared with first-year fellows who had not had any fellowship DEI education.
First-year fellows reported a mean confidence score of 2.5/5 at navigating bias and microaggressions when experienced personally and a mean score of 2.9/5 when they were directed at others. Senior fellows reported mean confidence scores of 3 and 3.2, respectively.
Yale then compared longitudinal data on fellows’ comfort levels in navigating discrimination in 2021, 2022, and 2023 a month before the ASCO meeting.
Fellows were asked to rate their comfort level from 1 to 10 in navigating different types of discrimination, including racial inequality, sexual harassment, and gender discrimination. In these three categories, fellows rated comfortability as a 5 in 2021 and as 7 in 2023 after the DEI training.
“Our first goal is to normalize talking about DEI and to recognize that different people in our workforce have different experiences and how we can be allies for them and for our patients,” Dr. Ansari said. “And I think for long-term goals we want to take stock of who’s at the table, who’s making decisions, and how does that affect our field, our science, and our patients.”
Yale designed the 3-year longitudinal curriculum with two annual core topics: upstander training and journal club for discussion and reflection. An additional two to three training sessions per year will focus on either race, gender, LGBTQ+, disability, religion, or implicit bias training.
The most popular topics among fellows were upstander training, cancer treatment and outcomes disparities, recruitment and retention, and career promotion and pay disparities.
The preferred platforms of content delivery were lectures from experts in the field, affinity groups or mentorship links, small group discussions, and advocacy education.
Gerald Hsu, MD, PhD, with the San Francisco VA Medical Center, discussed the results of Yale’s DEI curriculum assessment, saying it represented “best practices” in the industry. However, he acknowledged that realistically, not everyone will be receptive to DEI training.
Dr. Hsu said that holding medical staff accountable is the only way to truly incorporate DEI into everyday practice.
“Collectively, we need to be holding ourselves to different standards or holding ourselves to some standard,” Dr. Hsu said. “Maybe we need to be setting goals to the degree to which we diversify our training programs and our faculty, and there needs to be consequences to not doing so.”
No funding for the study was reported.
A version of this article first appeared on Medscape.com.
The finding comes from a survey conducted after the introduction of DEI training within the Yale Medical Oncology-Hematology Fellowship Program. The study was reported by Norin Ansari, MD, MPH, of Yale Cancer Center, New Haven, Conn., at the annual meeting of the American Society of Clinical Oncology (ASCO).
Dr. Ansari emphasized the DEI curriculum in fellowship programs by highlighting the racial and gender disparities that exist among physicians.
“There is a significant representation problem – only 2%-3% of practicing oncologists are Black or Hispanic/Latino,” she said. “And that representation decreases with each stage in the pipeline of the workforce.”
Dr. Ansari also noted gender disparities in the oncologist workforce, reporting that about one-third of faculty positions are held by women.
The anonymous survey was sent to 29 fellows; 23 responded, including 8 first-year fellows and 13 senior fellows. Over 57% of respondents rated the importance of DEI education as 10 on a 10-point scale (mean, 8.6).
At the start of this year, the responses of senior fellows who had already received some DEI training during the previous year’s lecture series were compared with first-year fellows who had not had any fellowship DEI education.
First-year fellows reported a mean confidence score of 2.5/5 at navigating bias and microaggressions when experienced personally and a mean score of 2.9/5 when they were directed at others. Senior fellows reported mean confidence scores of 3 and 3.2, respectively.
Yale then compared longitudinal data on fellows’ comfort levels in navigating discrimination in 2021, 2022, and 2023 a month before the ASCO meeting.
Fellows were asked to rate their comfort level from 1 to 10 in navigating different types of discrimination, including racial inequality, sexual harassment, and gender discrimination. In these three categories, fellows rated comfortability as a 5 in 2021 and as 7 in 2023 after the DEI training.
“Our first goal is to normalize talking about DEI and to recognize that different people in our workforce have different experiences and how we can be allies for them and for our patients,” Dr. Ansari said. “And I think for long-term goals we want to take stock of who’s at the table, who’s making decisions, and how does that affect our field, our science, and our patients.”
Yale designed the 3-year longitudinal curriculum with two annual core topics: upstander training and journal club for discussion and reflection. An additional two to three training sessions per year will focus on either race, gender, LGBTQ+, disability, religion, or implicit bias training.
The most popular topics among fellows were upstander training, cancer treatment and outcomes disparities, recruitment and retention, and career promotion and pay disparities.
The preferred platforms of content delivery were lectures from experts in the field, affinity groups or mentorship links, small group discussions, and advocacy education.
Gerald Hsu, MD, PhD, with the San Francisco VA Medical Center, discussed the results of Yale’s DEI curriculum assessment, saying it represented “best practices” in the industry. However, he acknowledged that realistically, not everyone will be receptive to DEI training.
Dr. Hsu said that holding medical staff accountable is the only way to truly incorporate DEI into everyday practice.
“Collectively, we need to be holding ourselves to different standards or holding ourselves to some standard,” Dr. Hsu said. “Maybe we need to be setting goals to the degree to which we diversify our training programs and our faculty, and there needs to be consequences to not doing so.”
No funding for the study was reported.
A version of this article first appeared on Medscape.com.
The finding comes from a survey conducted after the introduction of DEI training within the Yale Medical Oncology-Hematology Fellowship Program. The study was reported by Norin Ansari, MD, MPH, of Yale Cancer Center, New Haven, Conn., at the annual meeting of the American Society of Clinical Oncology (ASCO).
Dr. Ansari emphasized the DEI curriculum in fellowship programs by highlighting the racial and gender disparities that exist among physicians.
“There is a significant representation problem – only 2%-3% of practicing oncologists are Black or Hispanic/Latino,” she said. “And that representation decreases with each stage in the pipeline of the workforce.”
Dr. Ansari also noted gender disparities in the oncologist workforce, reporting that about one-third of faculty positions are held by women.
The anonymous survey was sent to 29 fellows; 23 responded, including 8 first-year fellows and 13 senior fellows. Over 57% of respondents rated the importance of DEI education as 10 on a 10-point scale (mean, 8.6).
At the start of this year, the responses of senior fellows who had already received some DEI training during the previous year’s lecture series were compared with first-year fellows who had not had any fellowship DEI education.
First-year fellows reported a mean confidence score of 2.5/5 at navigating bias and microaggressions when experienced personally and a mean score of 2.9/5 when they were directed at others. Senior fellows reported mean confidence scores of 3 and 3.2, respectively.
Yale then compared longitudinal data on fellows’ comfort levels in navigating discrimination in 2021, 2022, and 2023 a month before the ASCO meeting.
Fellows were asked to rate their comfort level from 1 to 10 in navigating different types of discrimination, including racial inequality, sexual harassment, and gender discrimination. In these three categories, fellows rated comfortability as a 5 in 2021 and as 7 in 2023 after the DEI training.
“Our first goal is to normalize talking about DEI and to recognize that different people in our workforce have different experiences and how we can be allies for them and for our patients,” Dr. Ansari said. “And I think for long-term goals we want to take stock of who’s at the table, who’s making decisions, and how does that affect our field, our science, and our patients.”
Yale designed the 3-year longitudinal curriculum with two annual core topics: upstander training and journal club for discussion and reflection. An additional two to three training sessions per year will focus on either race, gender, LGBTQ+, disability, religion, or implicit bias training.
The most popular topics among fellows were upstander training, cancer treatment and outcomes disparities, recruitment and retention, and career promotion and pay disparities.
The preferred platforms of content delivery were lectures from experts in the field, affinity groups or mentorship links, small group discussions, and advocacy education.
Gerald Hsu, MD, PhD, with the San Francisco VA Medical Center, discussed the results of Yale’s DEI curriculum assessment, saying it represented “best practices” in the industry. However, he acknowledged that realistically, not everyone will be receptive to DEI training.
Dr. Hsu said that holding medical staff accountable is the only way to truly incorporate DEI into everyday practice.
“Collectively, we need to be holding ourselves to different standards or holding ourselves to some standard,” Dr. Hsu said. “Maybe we need to be setting goals to the degree to which we diversify our training programs and our faculty, and there needs to be consequences to not doing so.”
No funding for the study was reported.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023
Drugmakers are abandoning cheap generics, and now U.S. cancer patients can’t get meds
On Nov. 22, three Food and Drug Administration inspectors arrived at the sprawling Intas Pharmaceuticals plant south of Ahmedabad, India, and found hundreds of trash bags full of shredded documents tossed into a garbage truck. Over the next 10 days, the inspectors assessed what looked like a systematic effort to conceal quality problems at the plant, which provided more than half of the U.S. supply of generic cisplatin and carboplatin, two cheap drugs used to treat as many as 500,000 new cancer cases every year.
Their decisions are likely to result in preventable deaths.
Cisplatin and carboplatin are among scores of drugs in shortage, including 12 other cancer drugs, ADHD pills, blood thinners, and antibiotics. COVID-hangover supply chain issues and limited FDA oversight are part of the problem, but the main cause, experts agree, is the underlying weakness of the generic drug industry. Made mostly overseas, these old but crucial drugs are often sold at a loss or for little profit. Domestic manufacturers have little interest in making them, setting their sights instead on high-priced drugs with plump profit margins.
The problem isn’t new, and that’s particularly infuriating to many clinicians. President Joe Biden, whose son Beau died of an aggressive brain cancer, has focused his Cancer Moonshot on discovering cures – undoubtedly expensive ones. Indeed, existing brand-name cancer drugs often cost tens of thousands of dollars a year.
But what about the thousands of patients today who can’t get a drug like cisplatin, approved by the FDA in 1978 and costing as little as $6 a dose?
“It’s just insane,” said Mark Ratain, MD, a cancer doctor and pharmacologist at the University of Chicago. “Your roof is caving in, but you want to build a basketball court in the backyard because your wife is pregnant with twin boys and you want them to be NBA stars when they grow up?”
“It’s just a travesty that this is the level of health care in the United States of America right now,” said Stephen Divers, MD, an oncologist in Hot Springs, Ark., who in recent weeks has had to delay or change treatment for numerous bladder, breast, and ovarian cancer patients because his clinic cannot find enough cisplatin and carboplatin. Results from a survey of academic cancer centers released June 7 found 93% couldn’t find enough carboplatin and 70% had cisplatin shortages.
“All day, in between patients, we hold staff meetings trying to figure this out,” said Bonny Moore, MD, an oncologist in Fredericksburg, Virginia. “It’s the most nauseous I’ve ever felt. Our office stayed open during COVID; we never had to stop treating patients. We got them vaccinated, kept them safe, and now I can’t get them a $10 drug.”
The cancer clinicians KFF Health News interviewed for this story said that, given current shortages, they prioritize patients who can be cured over later-stage patients, in whom the drugs generally can only slow the disease, and for whom alternatives – though sometimes less effective and often with more side effects – are available. But some doctors are even rationing doses intended to cure.
Isabella McDonald, then a junior at Utah Valley University, was diagnosed in April with a rare, often fatal bone cancer, whose sole treatment for young adults includes the drug methotrexate. When Isabella’s second cycle of treatment began June 5, clinicians advised that she would be getting less than the full dose because of a methotrexate shortage, said her father, Brent.
“They don’t think it will have a negative impact on her treatment, but as far as I am aware, there isn’t any scientific basis to make that conclusion,” he said. “As you can imagine, when they gave us such low odds of her beating this cancer, it feels like we want to give it everything we can and not something short of the standard.”
Mr. McDonald stressed that he didn’t blame the staffers at Intermountain Health who take care of Isabella. The family – his other daughter, Cate, made a TikTok video about her sister’s plight – were simply stunned at such a basic flaw in the health care system.
At Dr. Moore’s practice, in Virginia, clinicians gave 60% of the optimal dose of carboplatin to some uterine cancer patients during the week of May 16, then shifted to 80% after a small shipment came in the following week. The doctors had to omit carboplatin from normal combination treatments for patients with recurrent disease, she said.
On June 2, Dr. Moore and colleagues were glued to their drug distributor’s website, anxious as teenagers waiting for Taylor Swift tickets to go on sale – only with mortal consequences at stake.
She later emailed KFF Health News: “Carboplatin did NOT come back in stock today. Neither did cisplatin.”
Doses remained at 80%, she said. Things hadn’t changed 10 days later.
Generics manufacturers are pulling out
The causes of shortages are well established. Everyone wants to pay less, and the middlemen who procure and distribute generics keep driving down wholesale prices. The average net price of generic drugs fell by more than half between 2016 and 2022, according to research by Anthony Sardella, a business professor at Washington University in St. Louis.
As generics manufacturers compete to win sales contracts with the big negotiators of such purchases, such as Vizient and Premier, their profits sink. Some are going out of business. Akorn, which made 75 common generics, went bankrupt and closed in February. Israeli generics giant Teva, which has a portfolio of 3,600 medicines, announced May 18 it was shifting to brand-name drugs and “high-value generics.” Lannett, with about 120 generics, announced a Chapter 11 reorganization amid declining revenue. Other companies are in trouble too, said David Gaugh, interim CEO of the Association for Accessible Medicines, the leading generics trade group.
The generics industry used to lose money on about a third of the drugs it produced, but now it’s more like half, Mr. Gaugh said. So when a company stops making a drug, others do not necessarily step up, he said. Officials at Fresenius Kabi and Pfizer said they have increased their carboplatin production since March, but not enough to end the shortage. On June 2, FDA Commissioner Robert Califf announced the agency had given emergency authorization for Chinese-made cisplatin to enter the U.S. market, but the impact of the move wasn’t immediately clear.
Cisplatin and carboplatin are made in special production lines under sterile conditions, and expanding or changing the lines requires FDA approval. Bargain-basement prices have pushed production overseas, where it’s harder for the FDA to track quality standards. The Intas plant inspection was a relative rarity in India, where the FDA in 2022 reportedly inspected only 3% of sites that make drugs for the U.S. market. Mr. Sardella testified in May that a quarter of all U.S. drug prescriptions are filled by companies that received FDA warning letters in the past 26 months. And pharmaceutical industry product recalls are at their highest level in 18 years, reflecting fragile supply conditions.
The FDA listed 137 drugs in shortage as of June 13, including many essential medicines made by few companies.
Intas voluntarily shut down its Ahmedabad plant after the FDA inspection, and the agency posted its shocking inspection report in January. Accord Healthcare, the U.S. subsidiary of Intas, said in mid-June it had no date for restarting production.
Asked why it waited 2 months after its inspection to announce the cisplatin shortage, given that Intas supplied more than half the U.S. market for the drug, the FDA said via email that it doesn’t list a drug in shortage until it has “confirmed that overall market demand is not being met.”
Prices for carboplatin, cisplatin, and other drugs have skyrocketed on the so-called gray market, where speculators sell medicines they snapped up in anticipation of shortages. A 600-mg bottle of carboplatin, normally available for $30, was going for $185 in early May and $345 a week later, said Richard Scanlon, the pharmacist at dr. Moore’s clinic.
“It’s hard to have these conversations with patients – ‘I have your dose for this cycle, but not sure about next cycle,’” said Mark Einstein, MD, chair of the department of obstetrics, gynecology and reproductive health at New Jersey Medical School, Newark.
Should government step in?
Despite a drug shortage task force and numerous congressional hearings, progress has been slow at best. The 2020 CARES Act gave the FDA the power to require companies to have contingency plans enabling them to respond to shortages, but the agency has not yet implemented guidance to enforce the provisions.
As a result, neither Accord nor other cisplatin makers had a response plan in place when Intas’ plant was shut down, said Soumi Saha, senior vice president of government affairs for Premier, which arranges wholesale drug purchases for more than 4,400 hospitals and health systems.
Premier understood in December that the shutdown endangered the U.S. supply of cisplatin and carboplatin, but it also didn’t issue an immediate alarm. “It’s a fine balance,” she said. “You don’t want to create panic-buying or hoarding.”
More lasting solutions are under discussion. Mr. Sardella and others have proposed government subsidies to get U.S. generics plants running full time. Their capacity is now half-idle. If federal agencies like the Centers for Medicare & Medicaid Services paid more for more safely and efficiently produced drugs, it would promote a more stable supply chain, he said.
“At a certain point the system needs to recognize there’s a high cost to low-cost drugs,” said Allan Coukell, senior vice president for public policy at Civica Rx, a nonprofit funded by health systems, foundations, and the federal government that provides about 80 drugs to hospitals in its network. Civica is building a $140 million factory near Petersburg, Va., that will produce dozens more, Mr. Coukell said.
Dr. Ratain and his University of Chicago colleague Satyajit Kosuri, MD, recently called for the creation of a strategic inventory buffer for generic medications, something like the Strategic Petroleum Reserve, set up in 1975 in response to the OPEC oil crisis.
In fact, Dr. Ratain reckons, selling a quarter-million barrels of oil would probably generate enough cash to make and store 2 years’ worth of carboplatin and cisplatin.
“It would almost literally be a drop in the bucket.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
On Nov. 22, three Food and Drug Administration inspectors arrived at the sprawling Intas Pharmaceuticals plant south of Ahmedabad, India, and found hundreds of trash bags full of shredded documents tossed into a garbage truck. Over the next 10 days, the inspectors assessed what looked like a systematic effort to conceal quality problems at the plant, which provided more than half of the U.S. supply of generic cisplatin and carboplatin, two cheap drugs used to treat as many as 500,000 new cancer cases every year.
Their decisions are likely to result in preventable deaths.
Cisplatin and carboplatin are among scores of drugs in shortage, including 12 other cancer drugs, ADHD pills, blood thinners, and antibiotics. COVID-hangover supply chain issues and limited FDA oversight are part of the problem, but the main cause, experts agree, is the underlying weakness of the generic drug industry. Made mostly overseas, these old but crucial drugs are often sold at a loss or for little profit. Domestic manufacturers have little interest in making them, setting their sights instead on high-priced drugs with plump profit margins.
The problem isn’t new, and that’s particularly infuriating to many clinicians. President Joe Biden, whose son Beau died of an aggressive brain cancer, has focused his Cancer Moonshot on discovering cures – undoubtedly expensive ones. Indeed, existing brand-name cancer drugs often cost tens of thousands of dollars a year.
But what about the thousands of patients today who can’t get a drug like cisplatin, approved by the FDA in 1978 and costing as little as $6 a dose?
“It’s just insane,” said Mark Ratain, MD, a cancer doctor and pharmacologist at the University of Chicago. “Your roof is caving in, but you want to build a basketball court in the backyard because your wife is pregnant with twin boys and you want them to be NBA stars when they grow up?”
“It’s just a travesty that this is the level of health care in the United States of America right now,” said Stephen Divers, MD, an oncologist in Hot Springs, Ark., who in recent weeks has had to delay or change treatment for numerous bladder, breast, and ovarian cancer patients because his clinic cannot find enough cisplatin and carboplatin. Results from a survey of academic cancer centers released June 7 found 93% couldn’t find enough carboplatin and 70% had cisplatin shortages.
“All day, in between patients, we hold staff meetings trying to figure this out,” said Bonny Moore, MD, an oncologist in Fredericksburg, Virginia. “It’s the most nauseous I’ve ever felt. Our office stayed open during COVID; we never had to stop treating patients. We got them vaccinated, kept them safe, and now I can’t get them a $10 drug.”
The cancer clinicians KFF Health News interviewed for this story said that, given current shortages, they prioritize patients who can be cured over later-stage patients, in whom the drugs generally can only slow the disease, and for whom alternatives – though sometimes less effective and often with more side effects – are available. But some doctors are even rationing doses intended to cure.
Isabella McDonald, then a junior at Utah Valley University, was diagnosed in April with a rare, often fatal bone cancer, whose sole treatment for young adults includes the drug methotrexate. When Isabella’s second cycle of treatment began June 5, clinicians advised that she would be getting less than the full dose because of a methotrexate shortage, said her father, Brent.
“They don’t think it will have a negative impact on her treatment, but as far as I am aware, there isn’t any scientific basis to make that conclusion,” he said. “As you can imagine, when they gave us such low odds of her beating this cancer, it feels like we want to give it everything we can and not something short of the standard.”
Mr. McDonald stressed that he didn’t blame the staffers at Intermountain Health who take care of Isabella. The family – his other daughter, Cate, made a TikTok video about her sister’s plight – were simply stunned at such a basic flaw in the health care system.
At Dr. Moore’s practice, in Virginia, clinicians gave 60% of the optimal dose of carboplatin to some uterine cancer patients during the week of May 16, then shifted to 80% after a small shipment came in the following week. The doctors had to omit carboplatin from normal combination treatments for patients with recurrent disease, she said.
On June 2, Dr. Moore and colleagues were glued to their drug distributor’s website, anxious as teenagers waiting for Taylor Swift tickets to go on sale – only with mortal consequences at stake.
She later emailed KFF Health News: “Carboplatin did NOT come back in stock today. Neither did cisplatin.”
Doses remained at 80%, she said. Things hadn’t changed 10 days later.
Generics manufacturers are pulling out
The causes of shortages are well established. Everyone wants to pay less, and the middlemen who procure and distribute generics keep driving down wholesale prices. The average net price of generic drugs fell by more than half between 2016 and 2022, according to research by Anthony Sardella, a business professor at Washington University in St. Louis.
As generics manufacturers compete to win sales contracts with the big negotiators of such purchases, such as Vizient and Premier, their profits sink. Some are going out of business. Akorn, which made 75 common generics, went bankrupt and closed in February. Israeli generics giant Teva, which has a portfolio of 3,600 medicines, announced May 18 it was shifting to brand-name drugs and “high-value generics.” Lannett, with about 120 generics, announced a Chapter 11 reorganization amid declining revenue. Other companies are in trouble too, said David Gaugh, interim CEO of the Association for Accessible Medicines, the leading generics trade group.
The generics industry used to lose money on about a third of the drugs it produced, but now it’s more like half, Mr. Gaugh said. So when a company stops making a drug, others do not necessarily step up, he said. Officials at Fresenius Kabi and Pfizer said they have increased their carboplatin production since March, but not enough to end the shortage. On June 2, FDA Commissioner Robert Califf announced the agency had given emergency authorization for Chinese-made cisplatin to enter the U.S. market, but the impact of the move wasn’t immediately clear.
Cisplatin and carboplatin are made in special production lines under sterile conditions, and expanding or changing the lines requires FDA approval. Bargain-basement prices have pushed production overseas, where it’s harder for the FDA to track quality standards. The Intas plant inspection was a relative rarity in India, where the FDA in 2022 reportedly inspected only 3% of sites that make drugs for the U.S. market. Mr. Sardella testified in May that a quarter of all U.S. drug prescriptions are filled by companies that received FDA warning letters in the past 26 months. And pharmaceutical industry product recalls are at their highest level in 18 years, reflecting fragile supply conditions.
The FDA listed 137 drugs in shortage as of June 13, including many essential medicines made by few companies.
Intas voluntarily shut down its Ahmedabad plant after the FDA inspection, and the agency posted its shocking inspection report in January. Accord Healthcare, the U.S. subsidiary of Intas, said in mid-June it had no date for restarting production.
Asked why it waited 2 months after its inspection to announce the cisplatin shortage, given that Intas supplied more than half the U.S. market for the drug, the FDA said via email that it doesn’t list a drug in shortage until it has “confirmed that overall market demand is not being met.”
Prices for carboplatin, cisplatin, and other drugs have skyrocketed on the so-called gray market, where speculators sell medicines they snapped up in anticipation of shortages. A 600-mg bottle of carboplatin, normally available for $30, was going for $185 in early May and $345 a week later, said Richard Scanlon, the pharmacist at dr. Moore’s clinic.
“It’s hard to have these conversations with patients – ‘I have your dose for this cycle, but not sure about next cycle,’” said Mark Einstein, MD, chair of the department of obstetrics, gynecology and reproductive health at New Jersey Medical School, Newark.
Should government step in?
Despite a drug shortage task force and numerous congressional hearings, progress has been slow at best. The 2020 CARES Act gave the FDA the power to require companies to have contingency plans enabling them to respond to shortages, but the agency has not yet implemented guidance to enforce the provisions.
As a result, neither Accord nor other cisplatin makers had a response plan in place when Intas’ plant was shut down, said Soumi Saha, senior vice president of government affairs for Premier, which arranges wholesale drug purchases for more than 4,400 hospitals and health systems.
Premier understood in December that the shutdown endangered the U.S. supply of cisplatin and carboplatin, but it also didn’t issue an immediate alarm. “It’s a fine balance,” she said. “You don’t want to create panic-buying or hoarding.”
More lasting solutions are under discussion. Mr. Sardella and others have proposed government subsidies to get U.S. generics plants running full time. Their capacity is now half-idle. If federal agencies like the Centers for Medicare & Medicaid Services paid more for more safely and efficiently produced drugs, it would promote a more stable supply chain, he said.
“At a certain point the system needs to recognize there’s a high cost to low-cost drugs,” said Allan Coukell, senior vice president for public policy at Civica Rx, a nonprofit funded by health systems, foundations, and the federal government that provides about 80 drugs to hospitals in its network. Civica is building a $140 million factory near Petersburg, Va., that will produce dozens more, Mr. Coukell said.
Dr. Ratain and his University of Chicago colleague Satyajit Kosuri, MD, recently called for the creation of a strategic inventory buffer for generic medications, something like the Strategic Petroleum Reserve, set up in 1975 in response to the OPEC oil crisis.
In fact, Dr. Ratain reckons, selling a quarter-million barrels of oil would probably generate enough cash to make and store 2 years’ worth of carboplatin and cisplatin.
“It would almost literally be a drop in the bucket.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
On Nov. 22, three Food and Drug Administration inspectors arrived at the sprawling Intas Pharmaceuticals plant south of Ahmedabad, India, and found hundreds of trash bags full of shredded documents tossed into a garbage truck. Over the next 10 days, the inspectors assessed what looked like a systematic effort to conceal quality problems at the plant, which provided more than half of the U.S. supply of generic cisplatin and carboplatin, two cheap drugs used to treat as many as 500,000 new cancer cases every year.
Their decisions are likely to result in preventable deaths.
Cisplatin and carboplatin are among scores of drugs in shortage, including 12 other cancer drugs, ADHD pills, blood thinners, and antibiotics. COVID-hangover supply chain issues and limited FDA oversight are part of the problem, but the main cause, experts agree, is the underlying weakness of the generic drug industry. Made mostly overseas, these old but crucial drugs are often sold at a loss or for little profit. Domestic manufacturers have little interest in making them, setting their sights instead on high-priced drugs with plump profit margins.
The problem isn’t new, and that’s particularly infuriating to many clinicians. President Joe Biden, whose son Beau died of an aggressive brain cancer, has focused his Cancer Moonshot on discovering cures – undoubtedly expensive ones. Indeed, existing brand-name cancer drugs often cost tens of thousands of dollars a year.
But what about the thousands of patients today who can’t get a drug like cisplatin, approved by the FDA in 1978 and costing as little as $6 a dose?
“It’s just insane,” said Mark Ratain, MD, a cancer doctor and pharmacologist at the University of Chicago. “Your roof is caving in, but you want to build a basketball court in the backyard because your wife is pregnant with twin boys and you want them to be NBA stars when they grow up?”
“It’s just a travesty that this is the level of health care in the United States of America right now,” said Stephen Divers, MD, an oncologist in Hot Springs, Ark., who in recent weeks has had to delay or change treatment for numerous bladder, breast, and ovarian cancer patients because his clinic cannot find enough cisplatin and carboplatin. Results from a survey of academic cancer centers released June 7 found 93% couldn’t find enough carboplatin and 70% had cisplatin shortages.
“All day, in between patients, we hold staff meetings trying to figure this out,” said Bonny Moore, MD, an oncologist in Fredericksburg, Virginia. “It’s the most nauseous I’ve ever felt. Our office stayed open during COVID; we never had to stop treating patients. We got them vaccinated, kept them safe, and now I can’t get them a $10 drug.”
The cancer clinicians KFF Health News interviewed for this story said that, given current shortages, they prioritize patients who can be cured over later-stage patients, in whom the drugs generally can only slow the disease, and for whom alternatives – though sometimes less effective and often with more side effects – are available. But some doctors are even rationing doses intended to cure.
Isabella McDonald, then a junior at Utah Valley University, was diagnosed in April with a rare, often fatal bone cancer, whose sole treatment for young adults includes the drug methotrexate. When Isabella’s second cycle of treatment began June 5, clinicians advised that she would be getting less than the full dose because of a methotrexate shortage, said her father, Brent.
“They don’t think it will have a negative impact on her treatment, but as far as I am aware, there isn’t any scientific basis to make that conclusion,” he said. “As you can imagine, when they gave us such low odds of her beating this cancer, it feels like we want to give it everything we can and not something short of the standard.”
Mr. McDonald stressed that he didn’t blame the staffers at Intermountain Health who take care of Isabella. The family – his other daughter, Cate, made a TikTok video about her sister’s plight – were simply stunned at such a basic flaw in the health care system.
At Dr. Moore’s practice, in Virginia, clinicians gave 60% of the optimal dose of carboplatin to some uterine cancer patients during the week of May 16, then shifted to 80% after a small shipment came in the following week. The doctors had to omit carboplatin from normal combination treatments for patients with recurrent disease, she said.
On June 2, Dr. Moore and colleagues were glued to their drug distributor’s website, anxious as teenagers waiting for Taylor Swift tickets to go on sale – only with mortal consequences at stake.
She later emailed KFF Health News: “Carboplatin did NOT come back in stock today. Neither did cisplatin.”
Doses remained at 80%, she said. Things hadn’t changed 10 days later.
Generics manufacturers are pulling out
The causes of shortages are well established. Everyone wants to pay less, and the middlemen who procure and distribute generics keep driving down wholesale prices. The average net price of generic drugs fell by more than half between 2016 and 2022, according to research by Anthony Sardella, a business professor at Washington University in St. Louis.
As generics manufacturers compete to win sales contracts with the big negotiators of such purchases, such as Vizient and Premier, their profits sink. Some are going out of business. Akorn, which made 75 common generics, went bankrupt and closed in February. Israeli generics giant Teva, which has a portfolio of 3,600 medicines, announced May 18 it was shifting to brand-name drugs and “high-value generics.” Lannett, with about 120 generics, announced a Chapter 11 reorganization amid declining revenue. Other companies are in trouble too, said David Gaugh, interim CEO of the Association for Accessible Medicines, the leading generics trade group.
The generics industry used to lose money on about a third of the drugs it produced, but now it’s more like half, Mr. Gaugh said. So when a company stops making a drug, others do not necessarily step up, he said. Officials at Fresenius Kabi and Pfizer said they have increased their carboplatin production since March, but not enough to end the shortage. On June 2, FDA Commissioner Robert Califf announced the agency had given emergency authorization for Chinese-made cisplatin to enter the U.S. market, but the impact of the move wasn’t immediately clear.
Cisplatin and carboplatin are made in special production lines under sterile conditions, and expanding or changing the lines requires FDA approval. Bargain-basement prices have pushed production overseas, where it’s harder for the FDA to track quality standards. The Intas plant inspection was a relative rarity in India, where the FDA in 2022 reportedly inspected only 3% of sites that make drugs for the U.S. market. Mr. Sardella testified in May that a quarter of all U.S. drug prescriptions are filled by companies that received FDA warning letters in the past 26 months. And pharmaceutical industry product recalls are at their highest level in 18 years, reflecting fragile supply conditions.
The FDA listed 137 drugs in shortage as of June 13, including many essential medicines made by few companies.
Intas voluntarily shut down its Ahmedabad plant after the FDA inspection, and the agency posted its shocking inspection report in January. Accord Healthcare, the U.S. subsidiary of Intas, said in mid-June it had no date for restarting production.
Asked why it waited 2 months after its inspection to announce the cisplatin shortage, given that Intas supplied more than half the U.S. market for the drug, the FDA said via email that it doesn’t list a drug in shortage until it has “confirmed that overall market demand is not being met.”
Prices for carboplatin, cisplatin, and other drugs have skyrocketed on the so-called gray market, where speculators sell medicines they snapped up in anticipation of shortages. A 600-mg bottle of carboplatin, normally available for $30, was going for $185 in early May and $345 a week later, said Richard Scanlon, the pharmacist at dr. Moore’s clinic.
“It’s hard to have these conversations with patients – ‘I have your dose for this cycle, but not sure about next cycle,’” said Mark Einstein, MD, chair of the department of obstetrics, gynecology and reproductive health at New Jersey Medical School, Newark.
Should government step in?
Despite a drug shortage task force and numerous congressional hearings, progress has been slow at best. The 2020 CARES Act gave the FDA the power to require companies to have contingency plans enabling them to respond to shortages, but the agency has not yet implemented guidance to enforce the provisions.
As a result, neither Accord nor other cisplatin makers had a response plan in place when Intas’ plant was shut down, said Soumi Saha, senior vice president of government affairs for Premier, which arranges wholesale drug purchases for more than 4,400 hospitals and health systems.
Premier understood in December that the shutdown endangered the U.S. supply of cisplatin and carboplatin, but it also didn’t issue an immediate alarm. “It’s a fine balance,” she said. “You don’t want to create panic-buying or hoarding.”
More lasting solutions are under discussion. Mr. Sardella and others have proposed government subsidies to get U.S. generics plants running full time. Their capacity is now half-idle. If federal agencies like the Centers for Medicare & Medicaid Services paid more for more safely and efficiently produced drugs, it would promote a more stable supply chain, he said.
“At a certain point the system needs to recognize there’s a high cost to low-cost drugs,” said Allan Coukell, senior vice president for public policy at Civica Rx, a nonprofit funded by health systems, foundations, and the federal government that provides about 80 drugs to hospitals in its network. Civica is building a $140 million factory near Petersburg, Va., that will produce dozens more, Mr. Coukell said.
Dr. Ratain and his University of Chicago colleague Satyajit Kosuri, MD, recently called for the creation of a strategic inventory buffer for generic medications, something like the Strategic Petroleum Reserve, set up in 1975 in response to the OPEC oil crisis.
In fact, Dr. Ratain reckons, selling a quarter-million barrels of oil would probably generate enough cash to make and store 2 years’ worth of carboplatin and cisplatin.
“It would almost literally be a drop in the bucket.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
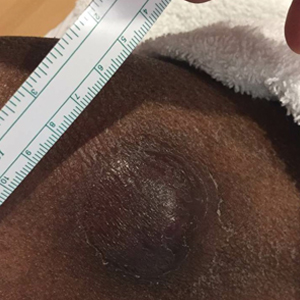
Prognostic factors of SCCs in organ transplant recipients worse compared with general population
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
FROM JAMA DERMATOLOGY
Experts share their sun protection tips for children
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
“I basically say, ‘sun protection means clothing, shade, [considering the] time of day of exposure, and sunscreen if you are going to be otherwise exposed,’ ” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady’s Children’s Hospital, San Diego, said during a panel discussion about sunscreen use at the Hawaii Dermatology Seminar provided by MedscapeLIVE! He recommends photoprotective gear such as rash guards for surfers and other water sport enthusiasts. When patients ask him if they should use sunscreen, he often replies with a question of his own.
“Do you brush your teeth?” he’ll ask.
“Yes, I do.”
“Well, you should put sunscreen on every day.”
Another panelist, Adelaide A. Hebert, MD, professor of dermatology and pediatrics and chief of pediatric dermatology at the University of Texas, Houston, said that she advises new parents to start sun protection efforts early. “Most sunscreens are not approved for use in children under the age of 6 months because testing has not been done in this age group, but I do recommend protective clothing. I also recommend wrap-around sunglasses, which offer 5% more protection from the sun than regular sunglasses.”
In her opinion, stick sunscreens are “a good add-on,” especially for under the eyes and the backs of the hands, but she is not a fan of spray sunscreens, which can leave large areas of skin unprotected if not applied properly.
Fellow panelist Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital, who has a special interest in taking care of dermatologic conditions of children with cancer, generally recommends mineral-based sunscreens. “There is data to suggest that nonmineral sunscreens are less safe than mineral sunscreens for humans, and mineral sunscreens are considered to be better for the environment,” Dr. Huang said. “Plus, there are more elegant versions of mineral sunscreens that don’t make your skin pasty white.” However, for patients with darker skin tones, “it can be hard to apply a pasty white sunscreen, so I lean on some recommendations for tinted sunscreens, too, so there are options. I specifically recommend sunscreens that have iron oxides in them so that it can block physical rays and help with the cosmetic appearance.”
Moise Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, said that his approach to imparting sunscreen advice to children and their parents involves a mix of spoken information, printed information, and sunscreen samples for children to try in the office, in the presence of a parent. To help patients choose among different samples, be they ointments, gels, or lotions, he will often ask the child: “‘What do you like the feel of better?’ If the child says, ‘I like this one,’ I make sure the parent hears that,” Dr. Levy said.
Next, Dr. Eichenfield, who moderated the discussion, asked his fellow panelists how they would counsel someone who comes to their practice for evaluation of moles and has a family history of nonmelanoma skin cancer. “I think this is one of the easier counseling sessions, because there are enough kids who are asked about the moles on their skin when they’re at school,” Dr. Hebert said. “I think they’re very ready to wear sun protective clothing and I certainly don’t want any sun exposure that would pose an increased risk for their child.”
In addition to routine sun protection, Dr. Huang recommends annual mole checks for children who have a first-degree relative with a history of malignant melanoma. Other high-risk groups that should undergo annual skin exams include anyone who has received high doses of radiation, bone marrow transplants, prolonged use of voriconazole, or prolonged systemic immunosuppression. Without a known genetic predisposition syndrome, a family history of nonmelanoma skin cancer would not raise concern for melanoma in an otherwise healthy child.
Dr. Eichenfield added that freckling used to be the secondary risk factor for melanoma, “but it’s flipped over to a primary risk factor. A history of immunosuppression or prior cancer is a major risk factor in childhood and teenage years.”
Dr. Eichenfield disclosed that he is a consultant or adviser for numerous pharmaceutical companies. He has also received research funding from AbbVie, Bausch & Lomb, Galderma Laboratories, and Pfizer. Dr. Hebert disclosed that she is a consultant or adviser for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica. Dr. Levy disclosed that he is consultant or adviser for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. Dr. Huang disclosed that she is an adviser for EllaOla.
MedscapeLive! and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
ACS officer provides ASCO highlights: Targeting hidden cancer, AI in oncology
And it didn’t just sparkle because of the sequined Taylor Swift fans clogging the nearby streets during the meeting.
Arif Kamal, MD, MBA, MHS, who is also an oncologist at Duke University, Durham, N.C., said he was impressed by a pair of landmark studies released at the meeting that show hidden cancer can be targeted with “really remarkable outcomes.” He also highlighted sessions that examined the role of artificial intelligence (AI) in oncology, during an interview.
Below are lightly edited excerpts from a conversation with Dr. Kamal:
Question: What are some of most groundbreaking studies released at ASCO?
Answer: One is an interim analysis of the NATALEE trial, which involved patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2–) breast tumors. This phase 3 randomized trial compared maintenance therapy with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) plus endocrine therapy with an aromatase inhibitor to endocrine therapy alone in patients with node-positive or node-negative and stage II or III HR+/HER– breast cancer.
For a long time, the standard care in these patients has been to use endocrine therapy alone. This is the first big trial to show that upstream usage of additional therapy in early stages is also beneficial for disease-free survival. The 3-year invasive disease-free survival rate was 90.4% in the rebociclib-endocrine therapy group vs. 87.1% for patients who received only endocrine therapy (P = .0014).
Q: How do these findings add to current knowledge?
A: Typically, we let people get metastatic disease before we use CDK4/6 inhibitors. These findings show that systemic treatment beyond endocrine therapy will be helpful in cases where you’ve got smaller disease that has not spread yet.
Even in patients with node-negative breast cancer, micrometastatic disease is clearly there, because the medication killed the negative lymph nodes.
Q: What else struck you as especially important research?
A: The NATALEE findings match what we saw in another study – the ADAURA trial, which looked at adjuvant osimertinib in non–small-cell lung cancer patients with EGFR-mutated, stage IB to IIIA disease – cancer that has not spread to the lymph nodes.
This is another example where you have a treatment being used in earlier-stage disease that’s showing really remarkable outcomes. The study found that 5-year overall survival was 88% in an osimertinib group vs. 78% in a placebo group (P < .001). This is a disease where, in stage IB, we wouldn’t even necessarily give these patients treatment at all, other than surgical resection of the tumor and maybe give them a little bit of chemotherapy.
Even in these smaller, early tumors, osimertinib makes a difference.
Q: As a whole, what are these studies telling us about cancer cells that can’t be easily detected?
A: To find a disease-free survival benefit with adding ribociclib in a stage II, stage III setting, particularly in node-negative disease, is remarkable because it says that the cells in hiding are bad actors, and they are going to cause trouble. The study shows that medications can find these cells and reverse that risk of bad outcomes.
If you think about the paradigm of cancer, that’s pretty remarkable because the ADAURA trial does the same thing: You do surgery for [early-stage] lung cancers that have not spread to the lymph nodes and you figure, “Well, I’ve got it all, right? The margins are real big, healthy, clean.” And yet, people still have recurrences, and you ask the same question: “Can any medicine find those few cells, the hundreds of cells that are still left somewhere in hiding?” And the answer is again, yes. It’s changing the paradigm of our understanding of minimal residual disease.
That’s why there’s so much interest in liquid biopsies. Let’s say that after treatment we don’t see any cancer radiologically, but there’s a signal from a liquid biopsy [detecting residual cancer]. These two trials demonstrate that there’s something we can do about it.
Q: There were quite a few studies about artificial intelligence released at ASCO. Where do we stand on that front?
A: We’re just at the beginning of people thinking about the use of generative AI for clinical decision support, clinical trial matching, and pathology review. But AI, at least for now, still has the issue of making up things that aren’t true. That’s not something patients are going to be okay with.
Q: How can AI be helpful to medical providers considering its limitations?
A: AI is going to be very good at the data-to-information transition. You’ll start seeing people use AI to start clinical notes for them and to match patients to the best clinical trials for them. But fundamentally, the clinician’s role will continue to be to check facts and offer wisdom.
Q: Will AI threaten the careers of oncologists?
A: The body of knowledge about oncology is growing exponentially, and no one can actually keep up. There’s so much data that’s out there that needs to be turned into usable information amid a shortage of oncologists. At the same time, the prevalence of cancer is going up, even though mortality is going down.
Synthesis of data is what oncologists are waiting for from AI. They’ll welcome it as opposed to being worried. That’s the sentiment I heard from my colleagues.
Dr. Kamal has no disclosures.
And it didn’t just sparkle because of the sequined Taylor Swift fans clogging the nearby streets during the meeting.
Arif Kamal, MD, MBA, MHS, who is also an oncologist at Duke University, Durham, N.C., said he was impressed by a pair of landmark studies released at the meeting that show hidden cancer can be targeted with “really remarkable outcomes.” He also highlighted sessions that examined the role of artificial intelligence (AI) in oncology, during an interview.
Below are lightly edited excerpts from a conversation with Dr. Kamal:
Question: What are some of most groundbreaking studies released at ASCO?
Answer: One is an interim analysis of the NATALEE trial, which involved patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2–) breast tumors. This phase 3 randomized trial compared maintenance therapy with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) plus endocrine therapy with an aromatase inhibitor to endocrine therapy alone in patients with node-positive or node-negative and stage II or III HR+/HER– breast cancer.
For a long time, the standard care in these patients has been to use endocrine therapy alone. This is the first big trial to show that upstream usage of additional therapy in early stages is also beneficial for disease-free survival. The 3-year invasive disease-free survival rate was 90.4% in the rebociclib-endocrine therapy group vs. 87.1% for patients who received only endocrine therapy (P = .0014).
Q: How do these findings add to current knowledge?
A: Typically, we let people get metastatic disease before we use CDK4/6 inhibitors. These findings show that systemic treatment beyond endocrine therapy will be helpful in cases where you’ve got smaller disease that has not spread yet.
Even in patients with node-negative breast cancer, micrometastatic disease is clearly there, because the medication killed the negative lymph nodes.
Q: What else struck you as especially important research?
A: The NATALEE findings match what we saw in another study – the ADAURA trial, which looked at adjuvant osimertinib in non–small-cell lung cancer patients with EGFR-mutated, stage IB to IIIA disease – cancer that has not spread to the lymph nodes.
This is another example where you have a treatment being used in earlier-stage disease that’s showing really remarkable outcomes. The study found that 5-year overall survival was 88% in an osimertinib group vs. 78% in a placebo group (P < .001). This is a disease where, in stage IB, we wouldn’t even necessarily give these patients treatment at all, other than surgical resection of the tumor and maybe give them a little bit of chemotherapy.
Even in these smaller, early tumors, osimertinib makes a difference.
Q: As a whole, what are these studies telling us about cancer cells that can’t be easily detected?
A: To find a disease-free survival benefit with adding ribociclib in a stage II, stage III setting, particularly in node-negative disease, is remarkable because it says that the cells in hiding are bad actors, and they are going to cause trouble. The study shows that medications can find these cells and reverse that risk of bad outcomes.
If you think about the paradigm of cancer, that’s pretty remarkable because the ADAURA trial does the same thing: You do surgery for [early-stage] lung cancers that have not spread to the lymph nodes and you figure, “Well, I’ve got it all, right? The margins are real big, healthy, clean.” And yet, people still have recurrences, and you ask the same question: “Can any medicine find those few cells, the hundreds of cells that are still left somewhere in hiding?” And the answer is again, yes. It’s changing the paradigm of our understanding of minimal residual disease.
That’s why there’s so much interest in liquid biopsies. Let’s say that after treatment we don’t see any cancer radiologically, but there’s a signal from a liquid biopsy [detecting residual cancer]. These two trials demonstrate that there’s something we can do about it.
Q: There were quite a few studies about artificial intelligence released at ASCO. Where do we stand on that front?
A: We’re just at the beginning of people thinking about the use of generative AI for clinical decision support, clinical trial matching, and pathology review. But AI, at least for now, still has the issue of making up things that aren’t true. That’s not something patients are going to be okay with.
Q: How can AI be helpful to medical providers considering its limitations?
A: AI is going to be very good at the data-to-information transition. You’ll start seeing people use AI to start clinical notes for them and to match patients to the best clinical trials for them. But fundamentally, the clinician’s role will continue to be to check facts and offer wisdom.
Q: Will AI threaten the careers of oncologists?
A: The body of knowledge about oncology is growing exponentially, and no one can actually keep up. There’s so much data that’s out there that needs to be turned into usable information amid a shortage of oncologists. At the same time, the prevalence of cancer is going up, even though mortality is going down.
Synthesis of data is what oncologists are waiting for from AI. They’ll welcome it as opposed to being worried. That’s the sentiment I heard from my colleagues.
Dr. Kamal has no disclosures.
And it didn’t just sparkle because of the sequined Taylor Swift fans clogging the nearby streets during the meeting.
Arif Kamal, MD, MBA, MHS, who is also an oncologist at Duke University, Durham, N.C., said he was impressed by a pair of landmark studies released at the meeting that show hidden cancer can be targeted with “really remarkable outcomes.” He also highlighted sessions that examined the role of artificial intelligence (AI) in oncology, during an interview.
Below are lightly edited excerpts from a conversation with Dr. Kamal:
Question: What are some of most groundbreaking studies released at ASCO?
Answer: One is an interim analysis of the NATALEE trial, which involved patients with early-stage hormone receptor-positive, HER2-negative (HR+/HER2–) breast tumors. This phase 3 randomized trial compared maintenance therapy with the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor ribociclib (Kisqali) plus endocrine therapy with an aromatase inhibitor to endocrine therapy alone in patients with node-positive or node-negative and stage II or III HR+/HER– breast cancer.
For a long time, the standard care in these patients has been to use endocrine therapy alone. This is the first big trial to show that upstream usage of additional therapy in early stages is also beneficial for disease-free survival. The 3-year invasive disease-free survival rate was 90.4% in the rebociclib-endocrine therapy group vs. 87.1% for patients who received only endocrine therapy (P = .0014).
Q: How do these findings add to current knowledge?
A: Typically, we let people get metastatic disease before we use CDK4/6 inhibitors. These findings show that systemic treatment beyond endocrine therapy will be helpful in cases where you’ve got smaller disease that has not spread yet.
Even in patients with node-negative breast cancer, micrometastatic disease is clearly there, because the medication killed the negative lymph nodes.
Q: What else struck you as especially important research?
A: The NATALEE findings match what we saw in another study – the ADAURA trial, which looked at adjuvant osimertinib in non–small-cell lung cancer patients with EGFR-mutated, stage IB to IIIA disease – cancer that has not spread to the lymph nodes.
This is another example where you have a treatment being used in earlier-stage disease that’s showing really remarkable outcomes. The study found that 5-year overall survival was 88% in an osimertinib group vs. 78% in a placebo group (P < .001). This is a disease where, in stage IB, we wouldn’t even necessarily give these patients treatment at all, other than surgical resection of the tumor and maybe give them a little bit of chemotherapy.
Even in these smaller, early tumors, osimertinib makes a difference.
Q: As a whole, what are these studies telling us about cancer cells that can’t be easily detected?
A: To find a disease-free survival benefit with adding ribociclib in a stage II, stage III setting, particularly in node-negative disease, is remarkable because it says that the cells in hiding are bad actors, and they are going to cause trouble. The study shows that medications can find these cells and reverse that risk of bad outcomes.
If you think about the paradigm of cancer, that’s pretty remarkable because the ADAURA trial does the same thing: You do surgery for [early-stage] lung cancers that have not spread to the lymph nodes and you figure, “Well, I’ve got it all, right? The margins are real big, healthy, clean.” And yet, people still have recurrences, and you ask the same question: “Can any medicine find those few cells, the hundreds of cells that are still left somewhere in hiding?” And the answer is again, yes. It’s changing the paradigm of our understanding of minimal residual disease.
That’s why there’s so much interest in liquid biopsies. Let’s say that after treatment we don’t see any cancer radiologically, but there’s a signal from a liquid biopsy [detecting residual cancer]. These two trials demonstrate that there’s something we can do about it.
Q: There were quite a few studies about artificial intelligence released at ASCO. Where do we stand on that front?
A: We’re just at the beginning of people thinking about the use of generative AI for clinical decision support, clinical trial matching, and pathology review. But AI, at least for now, still has the issue of making up things that aren’t true. That’s not something patients are going to be okay with.
Q: How can AI be helpful to medical providers considering its limitations?
A: AI is going to be very good at the data-to-information transition. You’ll start seeing people use AI to start clinical notes for them and to match patients to the best clinical trials for them. But fundamentally, the clinician’s role will continue to be to check facts and offer wisdom.
Q: Will AI threaten the careers of oncologists?
A: The body of knowledge about oncology is growing exponentially, and no one can actually keep up. There’s so much data that’s out there that needs to be turned into usable information amid a shortage of oncologists. At the same time, the prevalence of cancer is going up, even though mortality is going down.
Synthesis of data is what oncologists are waiting for from AI. They’ll welcome it as opposed to being worried. That’s the sentiment I heard from my colleagues.
Dr. Kamal has no disclosures.
AT ASCO 2023
Antibiotic prophylaxis may lower SSIs in skin cancer surgery
Delivering s, compared with not using incision site antibiotics.
The rate of postoperative SSIs was 2.1% in the clindamycin arm, vs. 5.7% in the control arm and 5.3% in the flucloxacillin arm.
“Based on these results, we recommend the routine adoption of incisional microdosed clindamycin for patients undergoing skin cancer surgery,” Maple Goh, MBChB, of the Auckland Regional Plastic and Reconstructive Surgery Unit, Auckland, New Zealand, and the coauthors conclude. “This strategy appears suitable for widespread implementation because of the magnitude of the effect observed and the absence of adverse events.”
The study was published online in JAMA Surgery.
Skin cancer surgery carries a high risk of SSIs, which represent costly yet largely preventable complications of surgery. Despite the risk, there’s a lack of evidence from randomized clinical trials of the role of antibiotic prophylaxis in reducing SSI rates among patients undergoing skin cancer surgery. Previous studies have investigated incisional antibiotic prophylaxis to reduce SSIs with Mohs micrographic surgery, but these surgeries represent a relatively small proportion of overall skin cancer surgeries.
To understand whether this benefit extends to more general skin cancer surgeries, investigators recruited patients from a high-volume skin cancer center in New Zealand who were treated from February to July 2019. In the double-blind, prospective PICASSo trial, patients were randomly assigned to receive an incision site injection of buffered local anesthetic alone (control group), buffered local anesthetic with microdoses of flucloxacillin (500 mcg/mL), or buffered local anesthetic with microdoses of clindamycin (500 mcg/mL). The most common surgery type was excision and direct closure (approximately 80% in all arms), and the mean volume injected per length of direct closure was 1.5 mL/cm.
The primary endpoint was the rate of postoperative SSIs, defined as a postoperative wound infection score of 5 or more. The SSI rate was calculated as the number of lesions with SSIs per total number of lesions in the group.
Overall, 681 patients with 1,133 total lesions were included in the study. Compared with the control arm, the rate of postoperative SSIs was nearly threefold lower among patients who received clindamycin, –2.1% (9 of 422) vs. 5.7% (22 of 388) in the control arm (P = .01 for clindamycin vs. control).
However, flucloxacillin did not demonstrate the same effectiveness. The flucloxacillin arm and the control arm demonstrated similar postoperative SSI rates – 5.3% (17 of 323) vs. 5.7%.
The results were similar after adjusting for baseline differences and lesion ulceration.
The researchers also found that the proportion of lesions that required postoperative systemic antibiotics was four times higher among the control arm, in comparison with the clindamycin arm (8% vs. 2.1%; P < .001). It was two times higher than in the flucloxacillin arm (8% vs. 4%; P = .03).
Treatment with microdoses of incisional flucloxacillin and clindamycin was safe and well tolerated.
The researchers speculated that clindamycin’s greater effectiveness may come down to its slightly broader coverage of commonly cultured bacteria in skin and soft tissue infections, including community-associated methicillin-resistant Staphylococcus aureus. Clindamycin is known to have more efficacy against anaerobic bacteria that may be lurking in chronically ulcerated skin lesions and is associated with less local tissue inflammation, compared with flucloxacillin.
Overall, “clindamycin was significantly more effective at preventing SSI than flucloxacillin in our study,” the authors conclude. They note that the use of clindamycin as a first-line prophylaxis agent against SSIs for patients undergoing skin cancer surgery is a practical option.
“These results establish evidence-based guidelines for antibiotic prophylaxis in one of the most common surgical interventions performed worldwide, where they have been previously absent,” the researchers say.
The authors of an editorial published with the study underscore other advantages of incisional microdosing with antibiotics.
“One advantage of cutaneous antibiotic administration is improved drug delivery to poorly perfused tissue, which would have limited reach by the systemic circulation,” wrote Amanda R. Sergesketter, MD, of Duke University, Durham, N.C., and Scott T. Hollenbeck, MD, of the University of Virginia, Charlottesville.
“While not evaluated in this study, local antibiotic delivery may be especially relevant to larger and more complex wounds,” the editorialists say. They note that the next step for future studies should be to evaluate prophylaxis in more complex situations.
“Such studies should be considered enthusiastically, given the clearly favorable impact on surgical site infections demonstrated in the PICASSo trial,” Dr. Sergesketter and Dr. Hollenbeck said.
The study was supported by a grant from the New Zealand Health Research Council. Dr. Hollenbeck reported educational grants to Duke University from Allergan, Acelity, Synovis, Integra, Smith & Nephew, Stryker, Cook, KLs Martin, Bard, VOptix, Scanlan, True Digital Surgery, Nautilus, Mitaka, Checkpoint Surgical, and Omniguide, and he is a founder and equity holder for InSoma Bio, a premarket company focused on tissue regeneration.
A version of this article first appeared on Medscape.com.
Delivering s, compared with not using incision site antibiotics.
The rate of postoperative SSIs was 2.1% in the clindamycin arm, vs. 5.7% in the control arm and 5.3% in the flucloxacillin arm.
“Based on these results, we recommend the routine adoption of incisional microdosed clindamycin for patients undergoing skin cancer surgery,” Maple Goh, MBChB, of the Auckland Regional Plastic and Reconstructive Surgery Unit, Auckland, New Zealand, and the coauthors conclude. “This strategy appears suitable for widespread implementation because of the magnitude of the effect observed and the absence of adverse events.”
The study was published online in JAMA Surgery.
Skin cancer surgery carries a high risk of SSIs, which represent costly yet largely preventable complications of surgery. Despite the risk, there’s a lack of evidence from randomized clinical trials of the role of antibiotic prophylaxis in reducing SSI rates among patients undergoing skin cancer surgery. Previous studies have investigated incisional antibiotic prophylaxis to reduce SSIs with Mohs micrographic surgery, but these surgeries represent a relatively small proportion of overall skin cancer surgeries.
To understand whether this benefit extends to more general skin cancer surgeries, investigators recruited patients from a high-volume skin cancer center in New Zealand who were treated from February to July 2019. In the double-blind, prospective PICASSo trial, patients were randomly assigned to receive an incision site injection of buffered local anesthetic alone (control group), buffered local anesthetic with microdoses of flucloxacillin (500 mcg/mL), or buffered local anesthetic with microdoses of clindamycin (500 mcg/mL). The most common surgery type was excision and direct closure (approximately 80% in all arms), and the mean volume injected per length of direct closure was 1.5 mL/cm.
The primary endpoint was the rate of postoperative SSIs, defined as a postoperative wound infection score of 5 or more. The SSI rate was calculated as the number of lesions with SSIs per total number of lesions in the group.
Overall, 681 patients with 1,133 total lesions were included in the study. Compared with the control arm, the rate of postoperative SSIs was nearly threefold lower among patients who received clindamycin, –2.1% (9 of 422) vs. 5.7% (22 of 388) in the control arm (P = .01 for clindamycin vs. control).
However, flucloxacillin did not demonstrate the same effectiveness. The flucloxacillin arm and the control arm demonstrated similar postoperative SSI rates – 5.3% (17 of 323) vs. 5.7%.
The results were similar after adjusting for baseline differences and lesion ulceration.
The researchers also found that the proportion of lesions that required postoperative systemic antibiotics was four times higher among the control arm, in comparison with the clindamycin arm (8% vs. 2.1%; P < .001). It was two times higher than in the flucloxacillin arm (8% vs. 4%; P = .03).
Treatment with microdoses of incisional flucloxacillin and clindamycin was safe and well tolerated.
The researchers speculated that clindamycin’s greater effectiveness may come down to its slightly broader coverage of commonly cultured bacteria in skin and soft tissue infections, including community-associated methicillin-resistant Staphylococcus aureus. Clindamycin is known to have more efficacy against anaerobic bacteria that may be lurking in chronically ulcerated skin lesions and is associated with less local tissue inflammation, compared with flucloxacillin.
Overall, “clindamycin was significantly more effective at preventing SSI than flucloxacillin in our study,” the authors conclude. They note that the use of clindamycin as a first-line prophylaxis agent against SSIs for patients undergoing skin cancer surgery is a practical option.
“These results establish evidence-based guidelines for antibiotic prophylaxis in one of the most common surgical interventions performed worldwide, where they have been previously absent,” the researchers say.
The authors of an editorial published with the study underscore other advantages of incisional microdosing with antibiotics.
“One advantage of cutaneous antibiotic administration is improved drug delivery to poorly perfused tissue, which would have limited reach by the systemic circulation,” wrote Amanda R. Sergesketter, MD, of Duke University, Durham, N.C., and Scott T. Hollenbeck, MD, of the University of Virginia, Charlottesville.
“While not evaluated in this study, local antibiotic delivery may be especially relevant to larger and more complex wounds,” the editorialists say. They note that the next step for future studies should be to evaluate prophylaxis in more complex situations.
“Such studies should be considered enthusiastically, given the clearly favorable impact on surgical site infections demonstrated in the PICASSo trial,” Dr. Sergesketter and Dr. Hollenbeck said.
The study was supported by a grant from the New Zealand Health Research Council. Dr. Hollenbeck reported educational grants to Duke University from Allergan, Acelity, Synovis, Integra, Smith & Nephew, Stryker, Cook, KLs Martin, Bard, VOptix, Scanlan, True Digital Surgery, Nautilus, Mitaka, Checkpoint Surgical, and Omniguide, and he is a founder and equity holder for InSoma Bio, a premarket company focused on tissue regeneration.
A version of this article first appeared on Medscape.com.
Delivering s, compared with not using incision site antibiotics.
The rate of postoperative SSIs was 2.1% in the clindamycin arm, vs. 5.7% in the control arm and 5.3% in the flucloxacillin arm.
“Based on these results, we recommend the routine adoption of incisional microdosed clindamycin for patients undergoing skin cancer surgery,” Maple Goh, MBChB, of the Auckland Regional Plastic and Reconstructive Surgery Unit, Auckland, New Zealand, and the coauthors conclude. “This strategy appears suitable for widespread implementation because of the magnitude of the effect observed and the absence of adverse events.”
The study was published online in JAMA Surgery.
Skin cancer surgery carries a high risk of SSIs, which represent costly yet largely preventable complications of surgery. Despite the risk, there’s a lack of evidence from randomized clinical trials of the role of antibiotic prophylaxis in reducing SSI rates among patients undergoing skin cancer surgery. Previous studies have investigated incisional antibiotic prophylaxis to reduce SSIs with Mohs micrographic surgery, but these surgeries represent a relatively small proportion of overall skin cancer surgeries.
To understand whether this benefit extends to more general skin cancer surgeries, investigators recruited patients from a high-volume skin cancer center in New Zealand who were treated from February to July 2019. In the double-blind, prospective PICASSo trial, patients were randomly assigned to receive an incision site injection of buffered local anesthetic alone (control group), buffered local anesthetic with microdoses of flucloxacillin (500 mcg/mL), or buffered local anesthetic with microdoses of clindamycin (500 mcg/mL). The most common surgery type was excision and direct closure (approximately 80% in all arms), and the mean volume injected per length of direct closure was 1.5 mL/cm.
The primary endpoint was the rate of postoperative SSIs, defined as a postoperative wound infection score of 5 or more. The SSI rate was calculated as the number of lesions with SSIs per total number of lesions in the group.
Overall, 681 patients with 1,133 total lesions were included in the study. Compared with the control arm, the rate of postoperative SSIs was nearly threefold lower among patients who received clindamycin, –2.1% (9 of 422) vs. 5.7% (22 of 388) in the control arm (P = .01 for clindamycin vs. control).
However, flucloxacillin did not demonstrate the same effectiveness. The flucloxacillin arm and the control arm demonstrated similar postoperative SSI rates – 5.3% (17 of 323) vs. 5.7%.
The results were similar after adjusting for baseline differences and lesion ulceration.
The researchers also found that the proportion of lesions that required postoperative systemic antibiotics was four times higher among the control arm, in comparison with the clindamycin arm (8% vs. 2.1%; P < .001). It was two times higher than in the flucloxacillin arm (8% vs. 4%; P = .03).
Treatment with microdoses of incisional flucloxacillin and clindamycin was safe and well tolerated.
The researchers speculated that clindamycin’s greater effectiveness may come down to its slightly broader coverage of commonly cultured bacteria in skin and soft tissue infections, including community-associated methicillin-resistant Staphylococcus aureus. Clindamycin is known to have more efficacy against anaerobic bacteria that may be lurking in chronically ulcerated skin lesions and is associated with less local tissue inflammation, compared with flucloxacillin.
Overall, “clindamycin was significantly more effective at preventing SSI than flucloxacillin in our study,” the authors conclude. They note that the use of clindamycin as a first-line prophylaxis agent against SSIs for patients undergoing skin cancer surgery is a practical option.
“These results establish evidence-based guidelines for antibiotic prophylaxis in one of the most common surgical interventions performed worldwide, where they have been previously absent,” the researchers say.
The authors of an editorial published with the study underscore other advantages of incisional microdosing with antibiotics.
“One advantage of cutaneous antibiotic administration is improved drug delivery to poorly perfused tissue, which would have limited reach by the systemic circulation,” wrote Amanda R. Sergesketter, MD, of Duke University, Durham, N.C., and Scott T. Hollenbeck, MD, of the University of Virginia, Charlottesville.
“While not evaluated in this study, local antibiotic delivery may be especially relevant to larger and more complex wounds,” the editorialists say. They note that the next step for future studies should be to evaluate prophylaxis in more complex situations.
“Such studies should be considered enthusiastically, given the clearly favorable impact on surgical site infections demonstrated in the PICASSo trial,” Dr. Sergesketter and Dr. Hollenbeck said.
The study was supported by a grant from the New Zealand Health Research Council. Dr. Hollenbeck reported educational grants to Duke University from Allergan, Acelity, Synovis, Integra, Smith & Nephew, Stryker, Cook, KLs Martin, Bard, VOptix, Scanlan, True Digital Surgery, Nautilus, Mitaka, Checkpoint Surgical, and Omniguide, and he is a founder and equity holder for InSoma Bio, a premarket company focused on tissue regeneration.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Widespread carboplatin, cisplatin shortages: NCCN survey
The survey, which included responses from 27 NCCN member institutions, revealed that 93% are experiencing a shortage of carboplatin and that 70% have reported a shortage of cisplatin.
“This is an unacceptable situation,” Robert W. Carlson, MD, NCCN’s chief executive offer, said in the statement released by the network.
“We are hearing from oncologists and pharmacists across the country who have to scramble to find appropriate alternatives for treating their patients with cancer right now,” Dr. Carlson said. And while the survey results show patients are still able to get lifesaving care, “it comes at a burden to our overtaxed medical facilities.”
The NCCN called on the federal government, the pharmaceutical industry, providers, and payers to take steps to “help mitigate any impacts” from this cancer drug shortage.
“We need to work together to improve the current situation and prevent it from happening again in the future,” Dr. Carlson stressed.
Carboplatin and cisplatin, which are frequently used together for systemic treatment, are highly effective therapies prescribed to treat many cancer types, including lung, breast, and prostate cancers, as well as leukemias and lymphomas. An estimated 500,000 new patients with cancer receive these agents each year.
The current survey, conducted over the last week of May, found that 100% of responding centers are able to continue to treat patients who need cisplatin without delays.
The same cannot be said for carboplatin: only 64% of centers said they are still able to continue treating all current patients receiving the platinum-based therapy. Among 19 responding centers, 20% reported that they were continuing carboplatin regimens for some but not all patients. And 16% reported treatment delays from having to obtain prior authorization for modified treatment plans, though none reported denials.
“Carboplatin has been in short supply for months but in the last 4 weeks has reached a critical stage,” according to one survey comment. “Without additional inventory many of our sites will be out of drug by early next week.”
In response to the survey question, “Is your center experiencing a shortage of carboplatin,” others made similar comments:
- “Current shipments from established manufacturers have been paused.”
- “The supply of carboplatin available is not meeting our demands.”
- “Without additional supply in early June, we will have to implement several shortage mitigation strategies.”
Survey respondents also addressed whether manufacturers or suppliers have provided any indication of when these drugs will become readily available again. For both drugs, about 60% of respondents said no. And for those who do receive updates, many noted that the “information is tentative and variable.”
Respondents indicated that other cancer agents, including methotrexate (67%) and 5FU (26%), are also in short supply at their centers.
The shortage and the uncertainty as to when it will end are forcing some centers to develop conservation and mitigation strategies.
The NCCN has broadly outlined how the federal government, the pharmaceutical industry, providers, and payers can help with prevention and mitigation. The NCCN has called on the federal government and the pharmaceutical industry to work to secure a steady supply of core anticancer drugs and has asked payers to “put patients first and provide flexible and efficient systems of providing coverage for alternative therapies replacing anti-cancer drugs that are unavailable or in shortage.”
Overall, the survey results “demonstrate the widespread impact of the chemotherapy shortage,” said Alyssa Schatz, MSW, senior director of policy and advocacy for NCCN. “We hope that by sharing this survey and calling for united action across the oncology community, we can come together to prevent future drug shortages and ensure quality, effective, equitable, and accessible cancer care for all.”
A version of this article first appeared on Medscape.com.
The survey, which included responses from 27 NCCN member institutions, revealed that 93% are experiencing a shortage of carboplatin and that 70% have reported a shortage of cisplatin.
“This is an unacceptable situation,” Robert W. Carlson, MD, NCCN’s chief executive offer, said in the statement released by the network.
“We are hearing from oncologists and pharmacists across the country who have to scramble to find appropriate alternatives for treating their patients with cancer right now,” Dr. Carlson said. And while the survey results show patients are still able to get lifesaving care, “it comes at a burden to our overtaxed medical facilities.”
The NCCN called on the federal government, the pharmaceutical industry, providers, and payers to take steps to “help mitigate any impacts” from this cancer drug shortage.
“We need to work together to improve the current situation and prevent it from happening again in the future,” Dr. Carlson stressed.
Carboplatin and cisplatin, which are frequently used together for systemic treatment, are highly effective therapies prescribed to treat many cancer types, including lung, breast, and prostate cancers, as well as leukemias and lymphomas. An estimated 500,000 new patients with cancer receive these agents each year.
The current survey, conducted over the last week of May, found that 100% of responding centers are able to continue to treat patients who need cisplatin without delays.
The same cannot be said for carboplatin: only 64% of centers said they are still able to continue treating all current patients receiving the platinum-based therapy. Among 19 responding centers, 20% reported that they were continuing carboplatin regimens for some but not all patients. And 16% reported treatment delays from having to obtain prior authorization for modified treatment plans, though none reported denials.
“Carboplatin has been in short supply for months but in the last 4 weeks has reached a critical stage,” according to one survey comment. “Without additional inventory many of our sites will be out of drug by early next week.”
In response to the survey question, “Is your center experiencing a shortage of carboplatin,” others made similar comments:
- “Current shipments from established manufacturers have been paused.”
- “The supply of carboplatin available is not meeting our demands.”
- “Without additional supply in early June, we will have to implement several shortage mitigation strategies.”
Survey respondents also addressed whether manufacturers or suppliers have provided any indication of when these drugs will become readily available again. For both drugs, about 60% of respondents said no. And for those who do receive updates, many noted that the “information is tentative and variable.”
Respondents indicated that other cancer agents, including methotrexate (67%) and 5FU (26%), are also in short supply at their centers.
The shortage and the uncertainty as to when it will end are forcing some centers to develop conservation and mitigation strategies.
The NCCN has broadly outlined how the federal government, the pharmaceutical industry, providers, and payers can help with prevention and mitigation. The NCCN has called on the federal government and the pharmaceutical industry to work to secure a steady supply of core anticancer drugs and has asked payers to “put patients first and provide flexible and efficient systems of providing coverage for alternative therapies replacing anti-cancer drugs that are unavailable or in shortage.”
Overall, the survey results “demonstrate the widespread impact of the chemotherapy shortage,” said Alyssa Schatz, MSW, senior director of policy and advocacy for NCCN. “We hope that by sharing this survey and calling for united action across the oncology community, we can come together to prevent future drug shortages and ensure quality, effective, equitable, and accessible cancer care for all.”
A version of this article first appeared on Medscape.com.
The survey, which included responses from 27 NCCN member institutions, revealed that 93% are experiencing a shortage of carboplatin and that 70% have reported a shortage of cisplatin.
“This is an unacceptable situation,” Robert W. Carlson, MD, NCCN’s chief executive offer, said in the statement released by the network.
“We are hearing from oncologists and pharmacists across the country who have to scramble to find appropriate alternatives for treating their patients with cancer right now,” Dr. Carlson said. And while the survey results show patients are still able to get lifesaving care, “it comes at a burden to our overtaxed medical facilities.”
The NCCN called on the federal government, the pharmaceutical industry, providers, and payers to take steps to “help mitigate any impacts” from this cancer drug shortage.
“We need to work together to improve the current situation and prevent it from happening again in the future,” Dr. Carlson stressed.
Carboplatin and cisplatin, which are frequently used together for systemic treatment, are highly effective therapies prescribed to treat many cancer types, including lung, breast, and prostate cancers, as well as leukemias and lymphomas. An estimated 500,000 new patients with cancer receive these agents each year.
The current survey, conducted over the last week of May, found that 100% of responding centers are able to continue to treat patients who need cisplatin without delays.
The same cannot be said for carboplatin: only 64% of centers said they are still able to continue treating all current patients receiving the platinum-based therapy. Among 19 responding centers, 20% reported that they were continuing carboplatin regimens for some but not all patients. And 16% reported treatment delays from having to obtain prior authorization for modified treatment plans, though none reported denials.
“Carboplatin has been in short supply for months but in the last 4 weeks has reached a critical stage,” according to one survey comment. “Without additional inventory many of our sites will be out of drug by early next week.”
In response to the survey question, “Is your center experiencing a shortage of carboplatin,” others made similar comments:
- “Current shipments from established manufacturers have been paused.”
- “The supply of carboplatin available is not meeting our demands.”
- “Without additional supply in early June, we will have to implement several shortage mitigation strategies.”
Survey respondents also addressed whether manufacturers or suppliers have provided any indication of when these drugs will become readily available again. For both drugs, about 60% of respondents said no. And for those who do receive updates, many noted that the “information is tentative and variable.”
Respondents indicated that other cancer agents, including methotrexate (67%) and 5FU (26%), are also in short supply at their centers.
The shortage and the uncertainty as to when it will end are forcing some centers to develop conservation and mitigation strategies.
The NCCN has broadly outlined how the federal government, the pharmaceutical industry, providers, and payers can help with prevention and mitigation. The NCCN has called on the federal government and the pharmaceutical industry to work to secure a steady supply of core anticancer drugs and has asked payers to “put patients first and provide flexible and efficient systems of providing coverage for alternative therapies replacing anti-cancer drugs that are unavailable or in shortage.”
Overall, the survey results “demonstrate the widespread impact of the chemotherapy shortage,” said Alyssa Schatz, MSW, senior director of policy and advocacy for NCCN. “We hope that by sharing this survey and calling for united action across the oncology community, we can come together to prevent future drug shortages and ensure quality, effective, equitable, and accessible cancer care for all.”
A version of this article first appeared on Medscape.com.
SPF is only the start when recommending sunscreens
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
CHICAGO – at the inaugural Pigmentary Disorders Exchange Symposium.
Among the first factors physicians should consider before recommending sunscreen are a patient’s Fitzpatrick skin type, risks for burning or tanning, underlying skin disorders, and medications the patient is taking, Dr. Taylor, professor of dermatology at the University of Pennsylvania, Philadelphia, said at the meeting, provided by MedscapeLIVE! If patients are on hypertensives, for example, medications can make them more photosensitive.
Consider skin type
Dr. Taylor said she was dismayed by the results of a recent study, which found that 43% of dermatologists who responded to a survey reported that they never, rarely, or only sometimes took a patient’s skin type into account when making sunscreen recommendations. The article is referenced in a 2022 expert panel consensus paper she coauthored on photoprotection “for skin of all color.” But she pointed out that considering skin type alone is inadequate.
Questions for patients in joint decision-making should include lifestyle and work choices such as whether they work inside or outside, and how much sun exposure they get in a typical day. Heat and humidity levels should also be considered as should a patient’s susceptibility to dyspigmentation. “That could be overall darkening of the skin, mottled hyperpigmentation, actinic dyspigmentation, and, of course, propensity for skin cancer,” she said.
Use differs by race
Dr. Taylor, who is also vice chair for diversity, equity and inclusion in the department of dermatology at the University of Pennsylvania, pointed out that sunscreen use differs considerably by race.
In study of 8,952 adults in the United States who reported that they were sun sensitive found that a subset of adults with skin of color were significantly less likely to use sunscreen when compared with non-Hispanic White adults: Non-Hispanic Black (adjusted odds ratio, 0.43); non-Hispanic Asian (aOR. 0.54); and Hispanic (aOR, 0.70) adults.
In the study, non-Hispanic Black and Hispanic adults were significantly less likely to use sunscreens with an SPF greater than 15. In addition, non-Hispanic Black, non-Hispanic Asian, and Hispanic adults were significantly more likely than non-Hispanic Whites to wear long sleeves when outside. Such differences are important to keep in mind when advising patients about sunscreens, she said.
Protection for lighter-colored skin
Dr. Taylor said that, for patients with lighter skin tones, “we really want to protect against ultraviolet B as well as ultraviolet A, particularly ultraviolet A2. Ultraviolet radiation is going to cause DNA damage.” Patients with Fitzpatrick skin types I, II, or III are most susceptible to the effects of UVB with sunburn inflammation, which will cause erythema and tanning, and immunosuppression.
“For those who are I, II, and III, we do want to recommend a broad-spectrum, photostable sunscreen with a critical wavelength of 370 nanometers, which is going to protect from both UVB and UVA2,” she said.
Sunscreen recommendations are meant to be paired with advice to avoid midday sun from 10 a.m. to 2 p.m., wearing protective clothing and accessories, and seeking shade, she noted.
Dr. Taylor said, for those patients with lighter skin who are more susceptible to photodamage and premature aging, physicians should recommend sunscreens that contain DNA repair enzymes such as photolyases and sunscreens that contain antioxidants that can prevent or reverse DNA damage. “The exogenous form of these lyases have been manufactured and added to sunscreens,” Dr. Taylor said. “They’re readily available in the United States. That is something to consider for patients with significant photodamage.”
Retinoids can also help alleviate or reverse photodamage, she added.
Protection for darker-colored skin
“Many people of color do not believe they need sunscreen,” Dr. Taylor said. But studies show that, although there may be more intrinsic protection, sunscreen is still needed.
Over 30 years ago, Halder and colleagues reported that melanin in skin of color can filter two to five times more UV radiation, and in a paper on the photoprotective role of melanin, Kaidbey and colleagues found that skin types V and VI had an intrinsic SPF of 13 when compared with those who have lighter complexions, which had an SPF of 3.
Sunburns seem to occur less frequently in people with skin of color, but that may be because erythema is less apparent in people with darker skin tones or because of differences in personal definitions of sunburn, Dr. Taylor said.
“Skin of color can and does sustain sunburns and sunscreen will help prevent that,” she said, adding that a recommendation of an SPF 30 is likely sufficient for these patients. Dr. Taylor noted that sunscreens for patients with darker skin often cost substantially more than those for lighter skin, and that should be considered in recommendations.
Tinted sunscreens
Dr. Taylor said that, while broad-spectrum photostable sunscreens protect against UVB and UVA 2, they don’t protect from visible light and UVA1. Two methods to add that protection are using inorganic tinted sunscreens that contain iron oxide or pigmentary titanium dioxide. Dr. Taylor was a coauthor of a practical guide to tinted sunscreens published in 2022.
“For iron oxide, we want a concentration of 3% or greater,” she said, adding that the percentage often is not known because if it is contained in a sunscreen, it is listed as an inactive ingredient.
Another method to address visible light and UVA1 is the use of antioxidant-containing sunscreens with vitamin E, vitamin C, or licochalcone A, Dr. Taylor said.
During the question-and-answer period following her presentation, Amit Pandya, MD, adjunct professor of dermatology at University of Texas Southwestern Medical Center, Dallas, asked why “every makeup, every sunscreen, just says iron oxide,” since it is known that visible light will cause pigmentation, especially in those with darker skin tones.
He urged pushing for a law that would require listing the percentage of iron oxide on products to assure it is sufficient, according to what the literature recommends.
Conference Chair Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute of Southern California, Los Angeles, said that she recommends tinted sunscreens almost exclusively for her patients, but those with darker skin colors struggle to match color.
Dr. Taylor referred to an analysis published in 2022 of 58 over-the counter sunscreens, which found that only 38% of tinted sunscreens was available in more than one shade, “which is a problem for many of our patients.” She said that providing samples with different hues and tactile sensations may help patients find the right product.
Dr. Taylor disclosed being on the advisory boards for AbbVie, Avita Medical, Beiersdorf, Biorez, Eli Lily, EPI Health, Evolus, Galderma, Hugel America, Johnson and Johnson, L’Oreal USA, MedScape, Pfizer, Scientis US, UCB, Vichy Laboratories. She is a consultant for Arcutis Biothermapeutics, Beiersdorf, Bristol-Myers Squibb, Cara Therapeutics, Dior, and Sanofi. She has done contracted research for Allergan Aesthetics, Concert Pharmaceuticals, Croma-Pharma, Eli Lilly, and Pfizer, and has an ownership interest in Armis Scientific, GloGetter, and Piction Health.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! PIGMENTARY DISORDERS SYMPOSIUM
Primary Effusion Lymphoma: An Infiltrative Plaque in a Patient With HIV
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
Practice Points
- Extracavitary primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that occurs solely in the presence of human herpesvirus 8 infection and typically is associated with HIV/AIDS.
- Diagnosis necessitates a thorough workup and correlation of histologic, molecular, and immunophenotypic analysis.
- Antiretroviral therapy in HIV-positive patients and intensive chemotherapy regimens are the current recommended treatments. Despite newer targeted agents, the prognosis remains poor.