User login
The family physician’s role in long COVID management
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
PRACTICE RECOMMENDATIONS
› Acknowledge and address the persistence of COVID-19 symptoms when meeting with patients. C
› Continue to monitor persistent, fluctuating symptoms of COVID-19 well after hospital discharge or apparent resolution of initial symptoms. C
› Provide psychological support and resources for mental health care to patients regarding their ongoing fears and frustrations with persistent COVID-19 symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Multidrug-resistant gram-negative infections treatable with newer antibiotics, but guidance is needed
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CURRENT OPINION IN INFECTIOUS DISEASES
Rise of the fungi: Pandemic tied to increasing fungal infections
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.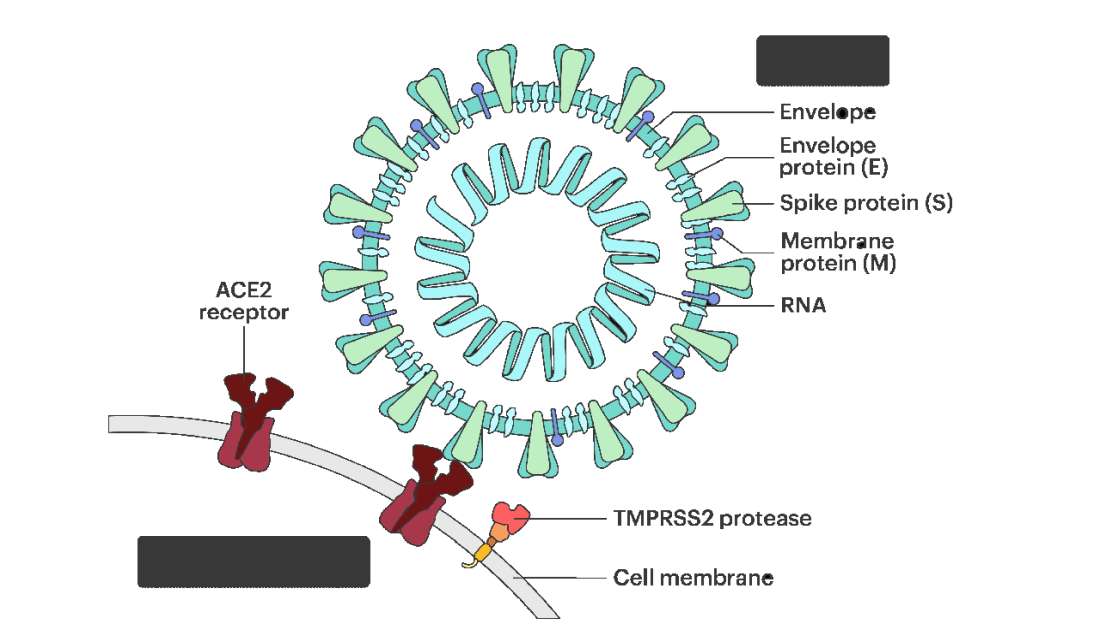
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.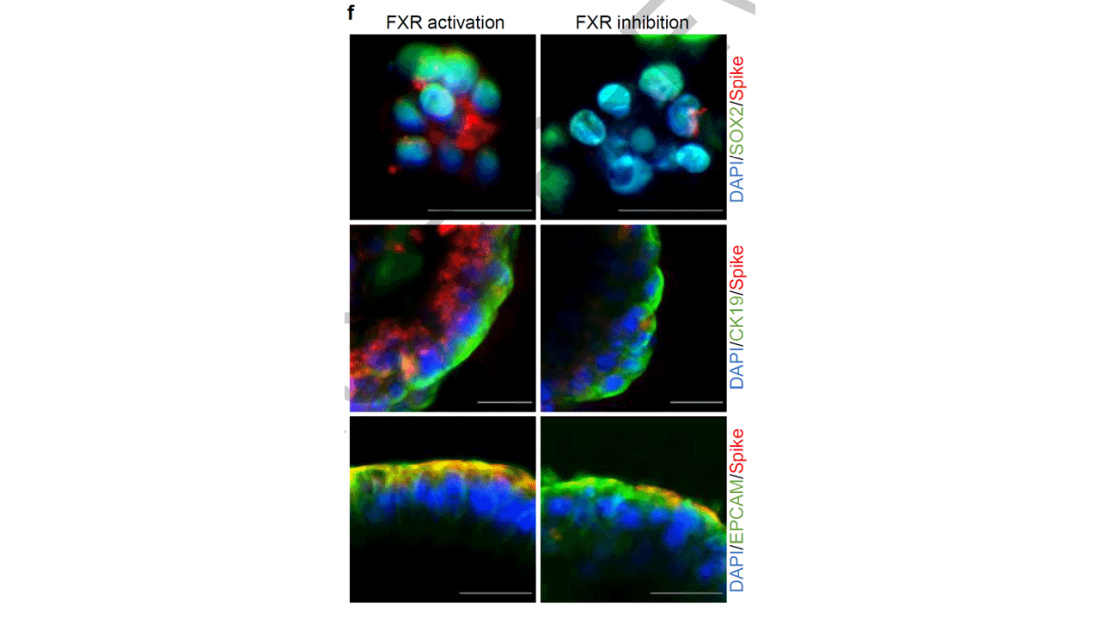
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Applications for laser-assisted drug delivery on the horizon, expert says
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Fungi that cause lung infections now found in most states: Study
Soil-dwelling fungi that can cause lung infections are more widespread than most doctors thought, sometimes leading to missed diagnoses, according to a new study.
Researchers studying fungi-linked lung infections realized that many infections were occurring in places the fungi weren’t thought to exist. They found that maps doctors use to know if the fungi are a threat in their area hadn’t been updated in half a century.
University of California, Davis infectious disease professor George Thompson, MD, said in a commentary published along with the study.
Published in the journal Clinical Infectious Diseases, the study sought to identify illnesses linked to three types of soil fungi in the United States that are known to cause lung infections. They are called histoplasma, blastomyces, and coccidioides, the latter of which causes an illness known as Valley fever, which has been on the rise in California.
Researchers used data for more than 45 million people who use Medicare and found that at least 1 of these 3 fungi are present in 48 of 50 U.S. states and Washington, D.C.
Symptoms after breathing in the fungi spores include fever and cough and can be similar to symptoms of other illnesses, according to the Centers for Disease Control.
The researchers said health care providers need to increase their suspicion for these fungi, which “would likely result in fewer missed diagnoses, fewer diagnostic delays, and improved patient outcomes.”
A version of this article first appeared on WebMD.com.
Soil-dwelling fungi that can cause lung infections are more widespread than most doctors thought, sometimes leading to missed diagnoses, according to a new study.
Researchers studying fungi-linked lung infections realized that many infections were occurring in places the fungi weren’t thought to exist. They found that maps doctors use to know if the fungi are a threat in their area hadn’t been updated in half a century.
University of California, Davis infectious disease professor George Thompson, MD, said in a commentary published along with the study.
Published in the journal Clinical Infectious Diseases, the study sought to identify illnesses linked to three types of soil fungi in the United States that are known to cause lung infections. They are called histoplasma, blastomyces, and coccidioides, the latter of which causes an illness known as Valley fever, which has been on the rise in California.
Researchers used data for more than 45 million people who use Medicare and found that at least 1 of these 3 fungi are present in 48 of 50 U.S. states and Washington, D.C.
Symptoms after breathing in the fungi spores include fever and cough and can be similar to symptoms of other illnesses, according to the Centers for Disease Control.
The researchers said health care providers need to increase their suspicion for these fungi, which “would likely result in fewer missed diagnoses, fewer diagnostic delays, and improved patient outcomes.”
A version of this article first appeared on WebMD.com.
Soil-dwelling fungi that can cause lung infections are more widespread than most doctors thought, sometimes leading to missed diagnoses, according to a new study.
Researchers studying fungi-linked lung infections realized that many infections were occurring in places the fungi weren’t thought to exist. They found that maps doctors use to know if the fungi are a threat in their area hadn’t been updated in half a century.
University of California, Davis infectious disease professor George Thompson, MD, said in a commentary published along with the study.
Published in the journal Clinical Infectious Diseases, the study sought to identify illnesses linked to three types of soil fungi in the United States that are known to cause lung infections. They are called histoplasma, blastomyces, and coccidioides, the latter of which causes an illness known as Valley fever, which has been on the rise in California.
Researchers used data for more than 45 million people who use Medicare and found that at least 1 of these 3 fungi are present in 48 of 50 U.S. states and Washington, D.C.
Symptoms after breathing in the fungi spores include fever and cough and can be similar to symptoms of other illnesses, according to the Centers for Disease Control.
The researchers said health care providers need to increase their suspicion for these fungi, which “would likely result in fewer missed diagnoses, fewer diagnostic delays, and improved patient outcomes.”
A version of this article first appeared on WebMD.com.
FROM CLINICAL INFECTIOUS DISEASE
Ohio measles outbreak sickens nearly 60 children
None of the children had been fully vaccinated against measles, and 23 of them have been hospitalized, local officials report.
“Measles can be very serious, especially for children under age 5,” Columbus Public Health spokesperson Kelli Newman told CNN.
Nearly all of the infected children are under age 5, with 12 of them being under 1 year old.
“Many children are hospitalized for dehydration,” Ms. Newman told CNN in an email. “Other serious complications also can include pneumonia and neurological conditions such as encephalitis. There’s no way of knowing which children will become so sick they have to be hospitalized. The safest way to protect children from measles is to make sure they are vaccinated with MMR.”
Of the 59 infected children, 56 were unvaccinated and three had been partially vaccinated. The MMR (measles, mumps, and rubella) vaccine is recommended for children beginning at 12 months old, according to the Centers for Disease Control and American Academy of Pediatrics. Two doses are needed to be considered fully vaccinated, and the second dose is usually given between 4 and 6 years old.
Measles “is one of the most infectious agents known to man,” the academy says.
It is so contagious that if one person has it, up to 9 out of 10 people around that person will also become infected if they are not protected, the CDC explains. Measles infection causes a rash and a fever that can spike beyond 104° F. Sometimes, the illness can lead to brain swelling, brain damage, or death.
Last month, the World Health Organization and CDC warned that 40 million children worldwide missed their measles vaccinations in 2021, partly due to pandemic disruptions. The American Academy of Pediatrics also notes that many parents choose not to vaccinate their children due to misinformation.
Infants are at heightened risk because they are too young to be vaccinated.
The academy offered several tips for protecting unvaccinated infants during a measles outbreak:
- Limit your baby’s exposure to crowds, other children, and people with cold symptoms.
- Disinfect objects and surfaces at home regularly, because the measles virus can live on surfaces or suspended in the air for 2 hours.
- If possible, feed your baby breast milk, because it has antibodies to prevent and fight infections.
A version of this article first appeared on WebMD.com.
None of the children had been fully vaccinated against measles, and 23 of them have been hospitalized, local officials report.
“Measles can be very serious, especially for children under age 5,” Columbus Public Health spokesperson Kelli Newman told CNN.
Nearly all of the infected children are under age 5, with 12 of them being under 1 year old.
“Many children are hospitalized for dehydration,” Ms. Newman told CNN in an email. “Other serious complications also can include pneumonia and neurological conditions such as encephalitis. There’s no way of knowing which children will become so sick they have to be hospitalized. The safest way to protect children from measles is to make sure they are vaccinated with MMR.”
Of the 59 infected children, 56 were unvaccinated and three had been partially vaccinated. The MMR (measles, mumps, and rubella) vaccine is recommended for children beginning at 12 months old, according to the Centers for Disease Control and American Academy of Pediatrics. Two doses are needed to be considered fully vaccinated, and the second dose is usually given between 4 and 6 years old.
Measles “is one of the most infectious agents known to man,” the academy says.
It is so contagious that if one person has it, up to 9 out of 10 people around that person will also become infected if they are not protected, the CDC explains. Measles infection causes a rash and a fever that can spike beyond 104° F. Sometimes, the illness can lead to brain swelling, brain damage, or death.
Last month, the World Health Organization and CDC warned that 40 million children worldwide missed their measles vaccinations in 2021, partly due to pandemic disruptions. The American Academy of Pediatrics also notes that many parents choose not to vaccinate their children due to misinformation.
Infants are at heightened risk because they are too young to be vaccinated.
The academy offered several tips for protecting unvaccinated infants during a measles outbreak:
- Limit your baby’s exposure to crowds, other children, and people with cold symptoms.
- Disinfect objects and surfaces at home regularly, because the measles virus can live on surfaces or suspended in the air for 2 hours.
- If possible, feed your baby breast milk, because it has antibodies to prevent and fight infections.
A version of this article first appeared on WebMD.com.
None of the children had been fully vaccinated against measles, and 23 of them have been hospitalized, local officials report.
“Measles can be very serious, especially for children under age 5,” Columbus Public Health spokesperson Kelli Newman told CNN.
Nearly all of the infected children are under age 5, with 12 of them being under 1 year old.
“Many children are hospitalized for dehydration,” Ms. Newman told CNN in an email. “Other serious complications also can include pneumonia and neurological conditions such as encephalitis. There’s no way of knowing which children will become so sick they have to be hospitalized. The safest way to protect children from measles is to make sure they are vaccinated with MMR.”
Of the 59 infected children, 56 were unvaccinated and three had been partially vaccinated. The MMR (measles, mumps, and rubella) vaccine is recommended for children beginning at 12 months old, according to the Centers for Disease Control and American Academy of Pediatrics. Two doses are needed to be considered fully vaccinated, and the second dose is usually given between 4 and 6 years old.
Measles “is one of the most infectious agents known to man,” the academy says.
It is so contagious that if one person has it, up to 9 out of 10 people around that person will also become infected if they are not protected, the CDC explains. Measles infection causes a rash and a fever that can spike beyond 104° F. Sometimes, the illness can lead to brain swelling, brain damage, or death.
Last month, the World Health Organization and CDC warned that 40 million children worldwide missed their measles vaccinations in 2021, partly due to pandemic disruptions. The American Academy of Pediatrics also notes that many parents choose not to vaccinate their children due to misinformation.
Infants are at heightened risk because they are too young to be vaccinated.
The academy offered several tips for protecting unvaccinated infants during a measles outbreak:
- Limit your baby’s exposure to crowds, other children, and people with cold symptoms.
- Disinfect objects and surfaces at home regularly, because the measles virus can live on surfaces or suspended in the air for 2 hours.
- If possible, feed your baby breast milk, because it has antibodies to prevent and fight infections.
A version of this article first appeared on WebMD.com.
A 9-year old female presented with 1 day of fever, fatigue, and sore throat
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
Erythrasma
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057