User login
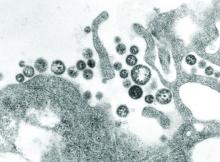
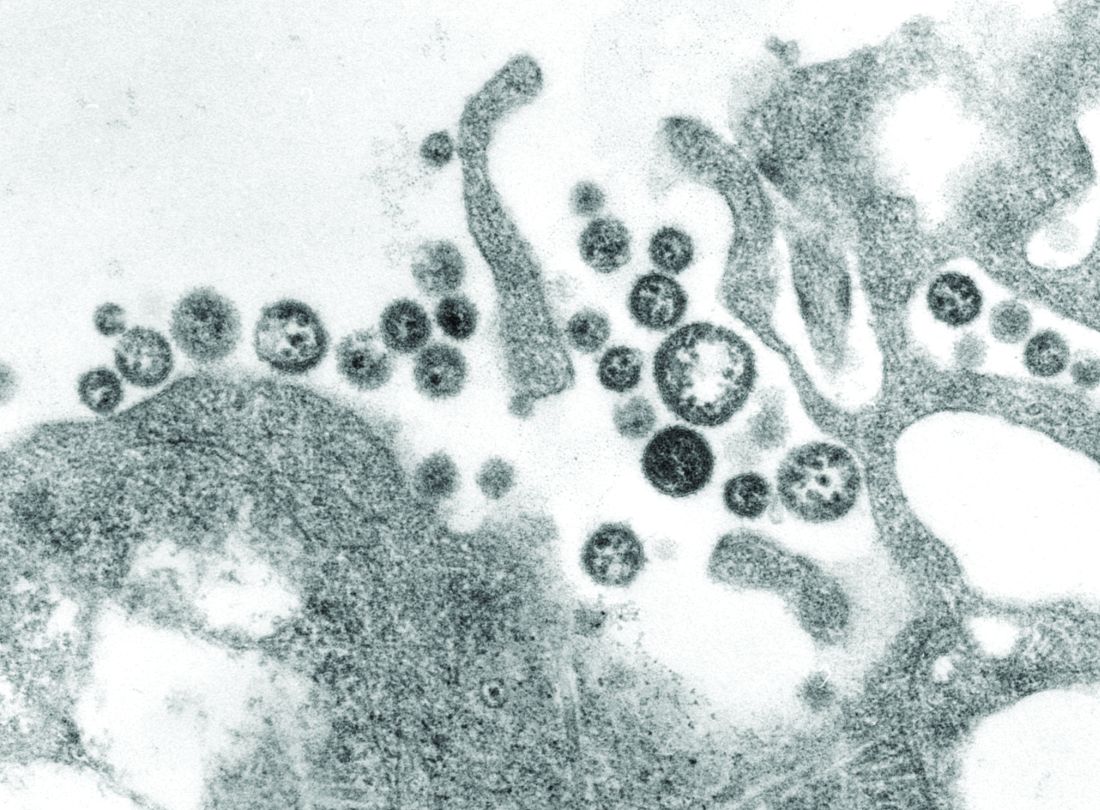
Need blood STAT? Call for a drone
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
AT AABB 2018
Novel recombinant vaccine protects animals from Lassa fever
An inactivated recombinant Lassa virus (LASV) and rabies vaccine candidate was developed that protects both mice and guinea pigs from Lassa fever. The vaccine also elicits a lasting humoral response against both LASV and rabies virus in both animal models. The novel vaccine, which uses a rabies virus–derived vector, expresses a codon-optimized LASV glycoprotein, according to Tiago Abreu Mota, a doctoral student at Jefferson University, Philadelphia, and his colleagues.
Lassa fever, a World Health Organization priority disease with a biosafety level of 4, is a hemorrhagic fever caused by the Lassa virus, which has no approved vaccine or potent antiviral treatment, according to the report published online in Nature Communications. So the development of a vaccine would be an important step in protecting the West African population from this deadly disease, which infects an estimated 100,000-300,000 people annually. As many as 80% of Lassa fever exposures are mildly symptomatic and thus go unreported; however, the case fatality rate of full-blown Lassa fever has been reported to reach as high as 50%, according to Mr. Mota and his colleagues.
An advantage to the new vaccine is that it is inactivated and thus could potentially be used in pregnant women and immunosuppressed patients, both of which are major risk groups for Lassa fever, according to the researchers. The vaccine could also protect against rabies, which is another major health concern in the regions affected by the Lassa virus.
In terms of mechanism of action, the vaccine did not induce virus-neutralizing antibodies, but rather appeared to trigger cell-mediated protection through activating natural killer cells to promote significantly more killing of virus-infected cells than nontriggered NK cells (P less than .01), as seen through in vitro testing.
The ability to assay for this form of vaccine effectiveness was a key development, according to the researchers. “The neutralizing antibody has been something of a gold standard in vaccine development. High levels are usually a good indication that the immune reaction is strong enough to deflect viral disease. In the case of Lassa virus, however, neutralizing antibodies have not been very good surrogates, since they are produced in much lower quantities. ... The new surrogate of protection will aid in the development of a more potent vaccine against Lassa virus,” according to a press release by Jefferson University.
This work was supported by various grants from the National Institutes of Health. Mr. Mota and two of his colleagues are inventors on a U.S. provisional patent application for a recombinant Lassa-rabies vaccine.
SOURCE: Mota TA et al. Nat Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w.
An inactivated recombinant Lassa virus (LASV) and rabies vaccine candidate was developed that protects both mice and guinea pigs from Lassa fever. The vaccine also elicits a lasting humoral response against both LASV and rabies virus in both animal models. The novel vaccine, which uses a rabies virus–derived vector, expresses a codon-optimized LASV glycoprotein, according to Tiago Abreu Mota, a doctoral student at Jefferson University, Philadelphia, and his colleagues.
Lassa fever, a World Health Organization priority disease with a biosafety level of 4, is a hemorrhagic fever caused by the Lassa virus, which has no approved vaccine or potent antiviral treatment, according to the report published online in Nature Communications. So the development of a vaccine would be an important step in protecting the West African population from this deadly disease, which infects an estimated 100,000-300,000 people annually. As many as 80% of Lassa fever exposures are mildly symptomatic and thus go unreported; however, the case fatality rate of full-blown Lassa fever has been reported to reach as high as 50%, according to Mr. Mota and his colleagues.
An advantage to the new vaccine is that it is inactivated and thus could potentially be used in pregnant women and immunosuppressed patients, both of which are major risk groups for Lassa fever, according to the researchers. The vaccine could also protect against rabies, which is another major health concern in the regions affected by the Lassa virus.
In terms of mechanism of action, the vaccine did not induce virus-neutralizing antibodies, but rather appeared to trigger cell-mediated protection through activating natural killer cells to promote significantly more killing of virus-infected cells than nontriggered NK cells (P less than .01), as seen through in vitro testing.
The ability to assay for this form of vaccine effectiveness was a key development, according to the researchers. “The neutralizing antibody has been something of a gold standard in vaccine development. High levels are usually a good indication that the immune reaction is strong enough to deflect viral disease. In the case of Lassa virus, however, neutralizing antibodies have not been very good surrogates, since they are produced in much lower quantities. ... The new surrogate of protection will aid in the development of a more potent vaccine against Lassa virus,” according to a press release by Jefferson University.
This work was supported by various grants from the National Institutes of Health. Mr. Mota and two of his colleagues are inventors on a U.S. provisional patent application for a recombinant Lassa-rabies vaccine.
SOURCE: Mota TA et al. Nat Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w.
An inactivated recombinant Lassa virus (LASV) and rabies vaccine candidate was developed that protects both mice and guinea pigs from Lassa fever. The vaccine also elicits a lasting humoral response against both LASV and rabies virus in both animal models. The novel vaccine, which uses a rabies virus–derived vector, expresses a codon-optimized LASV glycoprotein, according to Tiago Abreu Mota, a doctoral student at Jefferson University, Philadelphia, and his colleagues.
Lassa fever, a World Health Organization priority disease with a biosafety level of 4, is a hemorrhagic fever caused by the Lassa virus, which has no approved vaccine or potent antiviral treatment, according to the report published online in Nature Communications. So the development of a vaccine would be an important step in protecting the West African population from this deadly disease, which infects an estimated 100,000-300,000 people annually. As many as 80% of Lassa fever exposures are mildly symptomatic and thus go unreported; however, the case fatality rate of full-blown Lassa fever has been reported to reach as high as 50%, according to Mr. Mota and his colleagues.
An advantage to the new vaccine is that it is inactivated and thus could potentially be used in pregnant women and immunosuppressed patients, both of which are major risk groups for Lassa fever, according to the researchers. The vaccine could also protect against rabies, which is another major health concern in the regions affected by the Lassa virus.
In terms of mechanism of action, the vaccine did not induce virus-neutralizing antibodies, but rather appeared to trigger cell-mediated protection through activating natural killer cells to promote significantly more killing of virus-infected cells than nontriggered NK cells (P less than .01), as seen through in vitro testing.
The ability to assay for this form of vaccine effectiveness was a key development, according to the researchers. “The neutralizing antibody has been something of a gold standard in vaccine development. High levels are usually a good indication that the immune reaction is strong enough to deflect viral disease. In the case of Lassa virus, however, neutralizing antibodies have not been very good surrogates, since they are produced in much lower quantities. ... The new surrogate of protection will aid in the development of a more potent vaccine against Lassa virus,” according to a press release by Jefferson University.
This work was supported by various grants from the National Institutes of Health. Mr. Mota and two of his colleagues are inventors on a U.S. provisional patent application for a recombinant Lassa-rabies vaccine.
SOURCE: Mota TA et al. Nat Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w.
FROM NATURE COMMUNICATIONS
Key clinical point: A novel recombinant Lassa virus vaccine protected guinea pigs and mice against Lassa fever.
Major finding: A novel recombinant Lassa virus vaccine elicited strong humeral antibody response in guinea pigs and mice against Lassa and rabies viruses.
Study details: Animal models and an in vitro cellular system were used to assay for the effectiveness of a novel Lassa fever vaccine.
Disclosures: This work was supported by grants from the National Institutes of Health. Mr. Mota and two of his colleagues are inventors on a U.S. provisional patent application for a recombinant Lassa-rabies vaccine.
Source: Mota TA et al. Nat Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w.
Group B strep: Short-course IV controls infant infection
Infants with bacteremia caused by group B streptococcus (GBS) who were treated with intravenous antimicrobial therapy for 8 days or less had similarly successful outcomes, compared with those treated longer, based on data from 775 infants.
Current guidelines recommend intravenous antibiotics for a prolonged period of 10 days for infants (defined as 7-90 days old) with uncomplicated, late-onset GBS bacteremia, “however, no studies have compared outcomes among infants who receive prolonged versus shortened durations of IV antibiotic therapy,” wrote Eric R. Coon, MD, of the University of Utah, Salt Lake City, and colleagues. In a retrospective study published in Pediatrics, the researchers compared recurrence rates in infants who received prolonged vs. shortened therapy.
The study population included 612 infants with uncomplicated late-onset GBS bacteremia aged 4 months and younger who received prolonged IV therapy and 163 who received shortened therapy (defined as 8 days or less). Demographics were not significantly different between the groups, although infants who received a shortened treatment were more likely to be older, were more often admitted in later years of the study, and more likely to have a concomitant urinary tract infection.
Overall, 17 infants experienced recurrence; 3 in the shortened therapy group (1.8%) and 14 in the prolonged therapy group (2.3%). The average time to recurrence was 25 days.
Of note, 27 infants in the shortened treatment group received oral antibiotics on the day of their hospital discharge, and none of them experienced GBS recurrence, which suggests that “Early transition to oral antibiotic therapy may be appropriate for carefully selected infants with GBS bacteremia,” the researchers wrote.
In the prolonged treatment group, recurrence rates were 4.0% and 1.5%, respectively, for infants discharged with and without a peripherally inserted central catheter (PICC), but complications from the catheter may have been misclassified as disease recurrence to cause the difference in rate, as the noncatheter patients had similar recurrence rates to the shortened treatment group, the researchers noted.
“We found striking variation in IV antibiotic treatment duration by hospital and whether patients received prolonged or shortened IV courses; rates of GBS disease recurrence and treatment failure were low,” the researchers said. The top three antibiotics prescribed were ampicillin plus a third-generation cephalosporin (37%), third-generation cephalosporin monotherapy (28%), and third-generation cephalosporin monotherapy plus vancomycin (7%).
The findings were limited by the observational design, potential for misclassification of outcomes, and a lack of data to address the total duration of antibiotic therapy, the researchers noted. However, the results suggest that shorter treatment can be an informed decision considered in appropriate patients.
“Beyond decreased health care costs, shortened IV antibiotic courses provide the advantage of a diminished burden for families, allowing for patients to leave the hospital sooner, making it easier to administer the antibiotic at home, and decreasing the likelihood that they would develop a treatment-related complication,” the researchers said. The researchers had no financial conflicts to disclose.
SOURCE: Coon E et al. Pediatrics. 2018 Oct 11. doi: 10.1542/peds.2018-0131.
The 2007 French study investigating oral amoxicillin for early-onset group B streptococcus is one of the few times in the past 3 decades where I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis in the way American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by neonates, even mildly sick ones. It crosses the blood-brain barrier adequately. The French researchers measured serum levels and proved all this using scientific principles as well as clinical trials.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician. I waited for the Red Book to update its recommendations. That didn’t happen.
Meanwhile, I saw other babies kept for 10 days in the hospital for IV therapy, with resultant wasted costs and income loss for the parents. I’ve treated complications and readmissions due to PICC line issues. One baby at home got a syringe of gentamicin given IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Since late-onset GBS can be acquired environmentally, there will always be recurrences. The issue isn’t the rate of recurrence. It is whether the more invasive intervention reduces that rate. Then balance any measured reduction against the adverse effects of the invasive intervention, like PICC line infections. This Bayesian decision making is hard for some risk-adverse humans to assimilate.
Dr. Coon and associates have confirmed, using Big Data, that prolonged IV therapy of late-onset GBS bacteremia does not generate a clinically significant benefit. It is certainly possible to sow doubt by asking for proof in a variety of subpopulations. But this new article is in the context of multiple articles over the past decade that have disproved the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Dr. Coon and associates show that, by 2015, 5 of 49 children’s hospitals were early adopters and had made the switch to mostly using short treatment courses. Fourteen of 49 hadn’t changed at all. Given this new analysis, what are you laggards waiting for?
Dr. Kevin Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He reported no relevant financial disclosures.
The 2007 French study investigating oral amoxicillin for early-onset group B streptococcus is one of the few times in the past 3 decades where I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis in the way American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by neonates, even mildly sick ones. It crosses the blood-brain barrier adequately. The French researchers measured serum levels and proved all this using scientific principles as well as clinical trials.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician. I waited for the Red Book to update its recommendations. That didn’t happen.
Meanwhile, I saw other babies kept for 10 days in the hospital for IV therapy, with resultant wasted costs and income loss for the parents. I’ve treated complications and readmissions due to PICC line issues. One baby at home got a syringe of gentamicin given IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Since late-onset GBS can be acquired environmentally, there will always be recurrences. The issue isn’t the rate of recurrence. It is whether the more invasive intervention reduces that rate. Then balance any measured reduction against the adverse effects of the invasive intervention, like PICC line infections. This Bayesian decision making is hard for some risk-adverse humans to assimilate.
Dr. Coon and associates have confirmed, using Big Data, that prolonged IV therapy of late-onset GBS bacteremia does not generate a clinically significant benefit. It is certainly possible to sow doubt by asking for proof in a variety of subpopulations. But this new article is in the context of multiple articles over the past decade that have disproved the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Dr. Coon and associates show that, by 2015, 5 of 49 children’s hospitals were early adopters and had made the switch to mostly using short treatment courses. Fourteen of 49 hadn’t changed at all. Given this new analysis, what are you laggards waiting for?
Dr. Kevin Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He reported no relevant financial disclosures.
The 2007 French study investigating oral amoxicillin for early-onset group B streptococcus is one of the few times in the past 3 decades where I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis in the way American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by neonates, even mildly sick ones. It crosses the blood-brain barrier adequately. The French researchers measured serum levels and proved all this using scientific principles as well as clinical trials.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician. I waited for the Red Book to update its recommendations. That didn’t happen.
Meanwhile, I saw other babies kept for 10 days in the hospital for IV therapy, with resultant wasted costs and income loss for the parents. I’ve treated complications and readmissions due to PICC line issues. One baby at home got a syringe of gentamicin given IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Since late-onset GBS can be acquired environmentally, there will always be recurrences. The issue isn’t the rate of recurrence. It is whether the more invasive intervention reduces that rate. Then balance any measured reduction against the adverse effects of the invasive intervention, like PICC line infections. This Bayesian decision making is hard for some risk-adverse humans to assimilate.
Dr. Coon and associates have confirmed, using Big Data, that prolonged IV therapy of late-onset GBS bacteremia does not generate a clinically significant benefit. It is certainly possible to sow doubt by asking for proof in a variety of subpopulations. But this new article is in the context of multiple articles over the past decade that have disproved the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Dr. Coon and associates show that, by 2015, 5 of 49 children’s hospitals were early adopters and had made the switch to mostly using short treatment courses. Fourteen of 49 hadn’t changed at all. Given this new analysis, what are you laggards waiting for?
Dr. Kevin Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He reported no relevant financial disclosures.
Infants with bacteremia caused by group B streptococcus (GBS) who were treated with intravenous antimicrobial therapy for 8 days or less had similarly successful outcomes, compared with those treated longer, based on data from 775 infants.
Current guidelines recommend intravenous antibiotics for a prolonged period of 10 days for infants (defined as 7-90 days old) with uncomplicated, late-onset GBS bacteremia, “however, no studies have compared outcomes among infants who receive prolonged versus shortened durations of IV antibiotic therapy,” wrote Eric R. Coon, MD, of the University of Utah, Salt Lake City, and colleagues. In a retrospective study published in Pediatrics, the researchers compared recurrence rates in infants who received prolonged vs. shortened therapy.
The study population included 612 infants with uncomplicated late-onset GBS bacteremia aged 4 months and younger who received prolonged IV therapy and 163 who received shortened therapy (defined as 8 days or less). Demographics were not significantly different between the groups, although infants who received a shortened treatment were more likely to be older, were more often admitted in later years of the study, and more likely to have a concomitant urinary tract infection.
Overall, 17 infants experienced recurrence; 3 in the shortened therapy group (1.8%) and 14 in the prolonged therapy group (2.3%). The average time to recurrence was 25 days.
Of note, 27 infants in the shortened treatment group received oral antibiotics on the day of their hospital discharge, and none of them experienced GBS recurrence, which suggests that “Early transition to oral antibiotic therapy may be appropriate for carefully selected infants with GBS bacteremia,” the researchers wrote.
In the prolonged treatment group, recurrence rates were 4.0% and 1.5%, respectively, for infants discharged with and without a peripherally inserted central catheter (PICC), but complications from the catheter may have been misclassified as disease recurrence to cause the difference in rate, as the noncatheter patients had similar recurrence rates to the shortened treatment group, the researchers noted.
“We found striking variation in IV antibiotic treatment duration by hospital and whether patients received prolonged or shortened IV courses; rates of GBS disease recurrence and treatment failure were low,” the researchers said. The top three antibiotics prescribed were ampicillin plus a third-generation cephalosporin (37%), third-generation cephalosporin monotherapy (28%), and third-generation cephalosporin monotherapy plus vancomycin (7%).
The findings were limited by the observational design, potential for misclassification of outcomes, and a lack of data to address the total duration of antibiotic therapy, the researchers noted. However, the results suggest that shorter treatment can be an informed decision considered in appropriate patients.
“Beyond decreased health care costs, shortened IV antibiotic courses provide the advantage of a diminished burden for families, allowing for patients to leave the hospital sooner, making it easier to administer the antibiotic at home, and decreasing the likelihood that they would develop a treatment-related complication,” the researchers said. The researchers had no financial conflicts to disclose.
SOURCE: Coon E et al. Pediatrics. 2018 Oct 11. doi: 10.1542/peds.2018-0131.
Infants with bacteremia caused by group B streptococcus (GBS) who were treated with intravenous antimicrobial therapy for 8 days or less had similarly successful outcomes, compared with those treated longer, based on data from 775 infants.
Current guidelines recommend intravenous antibiotics for a prolonged period of 10 days for infants (defined as 7-90 days old) with uncomplicated, late-onset GBS bacteremia, “however, no studies have compared outcomes among infants who receive prolonged versus shortened durations of IV antibiotic therapy,” wrote Eric R. Coon, MD, of the University of Utah, Salt Lake City, and colleagues. In a retrospective study published in Pediatrics, the researchers compared recurrence rates in infants who received prolonged vs. shortened therapy.
The study population included 612 infants with uncomplicated late-onset GBS bacteremia aged 4 months and younger who received prolonged IV therapy and 163 who received shortened therapy (defined as 8 days or less). Demographics were not significantly different between the groups, although infants who received a shortened treatment were more likely to be older, were more often admitted in later years of the study, and more likely to have a concomitant urinary tract infection.
Overall, 17 infants experienced recurrence; 3 in the shortened therapy group (1.8%) and 14 in the prolonged therapy group (2.3%). The average time to recurrence was 25 days.
Of note, 27 infants in the shortened treatment group received oral antibiotics on the day of their hospital discharge, and none of them experienced GBS recurrence, which suggests that “Early transition to oral antibiotic therapy may be appropriate for carefully selected infants with GBS bacteremia,” the researchers wrote.
In the prolonged treatment group, recurrence rates were 4.0% and 1.5%, respectively, for infants discharged with and without a peripherally inserted central catheter (PICC), but complications from the catheter may have been misclassified as disease recurrence to cause the difference in rate, as the noncatheter patients had similar recurrence rates to the shortened treatment group, the researchers noted.
“We found striking variation in IV antibiotic treatment duration by hospital and whether patients received prolonged or shortened IV courses; rates of GBS disease recurrence and treatment failure were low,” the researchers said. The top three antibiotics prescribed were ampicillin plus a third-generation cephalosporin (37%), third-generation cephalosporin monotherapy (28%), and third-generation cephalosporin monotherapy plus vancomycin (7%).
The findings were limited by the observational design, potential for misclassification of outcomes, and a lack of data to address the total duration of antibiotic therapy, the researchers noted. However, the results suggest that shorter treatment can be an informed decision considered in appropriate patients.
“Beyond decreased health care costs, shortened IV antibiotic courses provide the advantage of a diminished burden for families, allowing for patients to leave the hospital sooner, making it easier to administer the antibiotic at home, and decreasing the likelihood that they would develop a treatment-related complication,” the researchers said. The researchers had no financial conflicts to disclose.
SOURCE: Coon E et al. Pediatrics. 2018 Oct 11. doi: 10.1542/peds.2018-0131.
FROM PEDIATRICS
Key clinical point: Courses of IV antibiotics shorter than recommended for group B streptococcus bacteremia yield low rates of recurrence and treatment failure.
Major finding: Three children treated with a shorter IV duration had recurrence of group B strep, compared with 14 children in a longer treatment group (1.8% vs. 2.3%).
Study details: The data come from a multicenter retrospective cohort study of 775 infants aged 7 days to 4 months.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Coon E et al. Pediatrics. 2018 Oct 11. doi: 10.1542/peds.2018-0345.
Treating cryptococcal meningitis in patients with HIV
One-week treatment with amphotericin B deoxycholate (AmBd)– and flucytosine (5FC)–based therapy, followed by fluconazole (FLU) on days 8 through 14, is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis, according to the authors of a review of the available literature in the Cochrane Database of Systemic Reviews.
The review is an update of one previous previously published in 2011. The authors found 13 eligible studies that enrolled 2,426 participants and compared 21 interventions. They performed a network meta-analysis using multivariate meta-regression, modeled treatment differences (RR and 95% confidence interval), and determined treatment rankings for 2-week and 10-week mortality outcomes using surface under the cumulative ranking curve, which represents the probability that a treatment will present the best outcome with no uncertainty and was used to develop a hierarchy of treatments for HIV-associated cryptococcal meningitis.
In addition, certainty of the evidence was assessed using the GRADE approach, according to Mark W. Tenforde, MD, of the University of Washington School of Public Health, Seattle, and his colleagues.
They found “reduced 10-week mortality with shortened [AmBd and 5FC] induction therapy, compared to the current gold standard of 2 weeks of AmBd and 5FC, based on moderate-certainty evidence.” They also found no mortality benefit of combination 2 weeks AmBd and FLU, compared with AmBd alone.
“In resource-limited settings, 1-week AmBd- and 5FC-based therapy is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis,” they wrote. “An all-oral regimen of 2 weeks 5FC and FLU may be an alternative in settings where AmBd is unavailable or intravenous therapy cannot be safely administered.” These results indicated the need to expand access to 5FC in resource-limited settings in which HIV-associated cryptococcal meningitis is most common.
They also reported finding no mortality benefit of 2 weeks of combination AmBd and FLU, compared with AmBd alone.
“Given the absence of data from studies in children, and limited data from high-income countries, our findings provide limited guidance for treatment in these patients and settings,” Dr. Tenforde and his colleagues stated.
The authors reported that they had no relevant conflicts of interest.
SOURCE: Tenforde MW et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev. 2018 Jul 25;7:CD005647. doi: 10.1002/14651858.CD005647.
One-week treatment with amphotericin B deoxycholate (AmBd)– and flucytosine (5FC)–based therapy, followed by fluconazole (FLU) on days 8 through 14, is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis, according to the authors of a review of the available literature in the Cochrane Database of Systemic Reviews.
The review is an update of one previous previously published in 2011. The authors found 13 eligible studies that enrolled 2,426 participants and compared 21 interventions. They performed a network meta-analysis using multivariate meta-regression, modeled treatment differences (RR and 95% confidence interval), and determined treatment rankings for 2-week and 10-week mortality outcomes using surface under the cumulative ranking curve, which represents the probability that a treatment will present the best outcome with no uncertainty and was used to develop a hierarchy of treatments for HIV-associated cryptococcal meningitis.
In addition, certainty of the evidence was assessed using the GRADE approach, according to Mark W. Tenforde, MD, of the University of Washington School of Public Health, Seattle, and his colleagues.
They found “reduced 10-week mortality with shortened [AmBd and 5FC] induction therapy, compared to the current gold standard of 2 weeks of AmBd and 5FC, based on moderate-certainty evidence.” They also found no mortality benefit of combination 2 weeks AmBd and FLU, compared with AmBd alone.
“In resource-limited settings, 1-week AmBd- and 5FC-based therapy is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis,” they wrote. “An all-oral regimen of 2 weeks 5FC and FLU may be an alternative in settings where AmBd is unavailable or intravenous therapy cannot be safely administered.” These results indicated the need to expand access to 5FC in resource-limited settings in which HIV-associated cryptococcal meningitis is most common.
They also reported finding no mortality benefit of 2 weeks of combination AmBd and FLU, compared with AmBd alone.
“Given the absence of data from studies in children, and limited data from high-income countries, our findings provide limited guidance for treatment in these patients and settings,” Dr. Tenforde and his colleagues stated.
The authors reported that they had no relevant conflicts of interest.
SOURCE: Tenforde MW et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev. 2018 Jul 25;7:CD005647. doi: 10.1002/14651858.CD005647.
One-week treatment with amphotericin B deoxycholate (AmBd)– and flucytosine (5FC)–based therapy, followed by fluconazole (FLU) on days 8 through 14, is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis, according to the authors of a review of the available literature in the Cochrane Database of Systemic Reviews.
The review is an update of one previous previously published in 2011. The authors found 13 eligible studies that enrolled 2,426 participants and compared 21 interventions. They performed a network meta-analysis using multivariate meta-regression, modeled treatment differences (RR and 95% confidence interval), and determined treatment rankings for 2-week and 10-week mortality outcomes using surface under the cumulative ranking curve, which represents the probability that a treatment will present the best outcome with no uncertainty and was used to develop a hierarchy of treatments for HIV-associated cryptococcal meningitis.
In addition, certainty of the evidence was assessed using the GRADE approach, according to Mark W. Tenforde, MD, of the University of Washington School of Public Health, Seattle, and his colleagues.
They found “reduced 10-week mortality with shortened [AmBd and 5FC] induction therapy, compared to the current gold standard of 2 weeks of AmBd and 5FC, based on moderate-certainty evidence.” They also found no mortality benefit of combination 2 weeks AmBd and FLU, compared with AmBd alone.
“In resource-limited settings, 1-week AmBd- and 5FC-based therapy is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis,” they wrote. “An all-oral regimen of 2 weeks 5FC and FLU may be an alternative in settings where AmBd is unavailable or intravenous therapy cannot be safely administered.” These results indicated the need to expand access to 5FC in resource-limited settings in which HIV-associated cryptococcal meningitis is most common.
They also reported finding no mortality benefit of 2 weeks of combination AmBd and FLU, compared with AmBd alone.
“Given the absence of data from studies in children, and limited data from high-income countries, our findings provide limited guidance for treatment in these patients and settings,” Dr. Tenforde and his colleagues stated.
The authors reported that they had no relevant conflicts of interest.
SOURCE: Tenforde MW et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev. 2018 Jul 25;7:CD005647. doi: 10.1002/14651858.CD005647.
FROM COCHRANE DATABASE OF SYSTEMIC REVIEWS
Key clinical point: Shorter drug treatment beat the gold standard for HIV-associated cryptococcal meningitis according to a literature review.
Major finding:
Study details: Updated review of articles, registries, and clinical trials during Jan. 1, 1980–July 9, 2018.
Disclosures: The authors reported that they had no relevant conflicts of interest.
Source: Tenforde MW et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev. 2018 Jul 25;7:CD005647. doi: 10.1002/14651858.CD005647.
Sex workers: High rates of HIV, low rates of treatment
The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in comprehensive measures of HIV prevalence and incidence, and in prevention and treatment, according to the results of an updated literature review published in the Lancet.
Kate Shannon, PhD, director of the gender and sexual health initiative at the University of British Columbia, Vancouver, and her colleagues updated a 2013 literature search for the Lancet series on HIV and sex workers to include reports and manuscripts published from Jan. 1, 2006, to Sept. 6, 2017.
They found that In particular, 4 years after their previous Lancet series on HIV and sex workers, this updated analysis showed that the global HIV burden among female sex workers was still similar to the previously determined 11.8% and “unacceptably high” at 10.4%, (95% confidence interval, 9.5-11.5).
Although there has been some improvement in the assessment of HIV in transgender women since the previous analysis, according to Dr. Shannon and her colleagues, small sample sizes and conflation of transgender women and men who have sex with men (MSM) continue to limit the volume of transgender-specific HIV data, particularly in Africa.
Access to HIV prevention and treatment also remains a considerable problem for sex workers, according to the authors. In particular, “qualitative data in sub-Saharan Africa suggest that profound structural barriers of stigma and discrimination impede progress in the HIV care continuum,” with studies confirming that “successful HIV treatment trajectories are impeded by violence and displacement” because of policing, they wrote.
They pointed out that things may well become worse, with evidence-based progress on full decriminalization grounded in health and human rights – which was a key recommendation in their earlier Lancet Series – having stalled in all but South Africa. In fact, they reported that several countries had even rolled back rights further for sex workers.
“HIV prevention and treatment tools are available but, without comprehensive HIV epidemiology, a lack of denominators and failure to address structural determinants (including decriminalisation of sex work) means that progress in achieving health and rights for all sex workers will fall short,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in comprehensive measures of HIV prevalence and incidence, and in prevention and treatment, according to the results of an updated literature review published in the Lancet.
Kate Shannon, PhD, director of the gender and sexual health initiative at the University of British Columbia, Vancouver, and her colleagues updated a 2013 literature search for the Lancet series on HIV and sex workers to include reports and manuscripts published from Jan. 1, 2006, to Sept. 6, 2017.
They found that In particular, 4 years after their previous Lancet series on HIV and sex workers, this updated analysis showed that the global HIV burden among female sex workers was still similar to the previously determined 11.8% and “unacceptably high” at 10.4%, (95% confidence interval, 9.5-11.5).
Although there has been some improvement in the assessment of HIV in transgender women since the previous analysis, according to Dr. Shannon and her colleagues, small sample sizes and conflation of transgender women and men who have sex with men (MSM) continue to limit the volume of transgender-specific HIV data, particularly in Africa.
Access to HIV prevention and treatment also remains a considerable problem for sex workers, according to the authors. In particular, “qualitative data in sub-Saharan Africa suggest that profound structural barriers of stigma and discrimination impede progress in the HIV care continuum,” with studies confirming that “successful HIV treatment trajectories are impeded by violence and displacement” because of policing, they wrote.
They pointed out that things may well become worse, with evidence-based progress on full decriminalization grounded in health and human rights – which was a key recommendation in their earlier Lancet Series – having stalled in all but South Africa. In fact, they reported that several countries had even rolled back rights further for sex workers.
“HIV prevention and treatment tools are available but, without comprehensive HIV epidemiology, a lack of denominators and failure to address structural determinants (including decriminalisation of sex work) means that progress in achieving health and rights for all sex workers will fall short,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in comprehensive measures of HIV prevalence and incidence, and in prevention and treatment, according to the results of an updated literature review published in the Lancet.
Kate Shannon, PhD, director of the gender and sexual health initiative at the University of British Columbia, Vancouver, and her colleagues updated a 2013 literature search for the Lancet series on HIV and sex workers to include reports and manuscripts published from Jan. 1, 2006, to Sept. 6, 2017.
They found that In particular, 4 years after their previous Lancet series on HIV and sex workers, this updated analysis showed that the global HIV burden among female sex workers was still similar to the previously determined 11.8% and “unacceptably high” at 10.4%, (95% confidence interval, 9.5-11.5).
Although there has been some improvement in the assessment of HIV in transgender women since the previous analysis, according to Dr. Shannon and her colleagues, small sample sizes and conflation of transgender women and men who have sex with men (MSM) continue to limit the volume of transgender-specific HIV data, particularly in Africa.
Access to HIV prevention and treatment also remains a considerable problem for sex workers, according to the authors. In particular, “qualitative data in sub-Saharan Africa suggest that profound structural barriers of stigma and discrimination impede progress in the HIV care continuum,” with studies confirming that “successful HIV treatment trajectories are impeded by violence and displacement” because of policing, they wrote.
They pointed out that things may well become worse, with evidence-based progress on full decriminalization grounded in health and human rights – which was a key recommendation in their earlier Lancet Series – having stalled in all but South Africa. In fact, they reported that several countries had even rolled back rights further for sex workers.
“HIV prevention and treatment tools are available but, without comprehensive HIV epidemiology, a lack of denominators and failure to address structural determinants (including decriminalisation of sex work) means that progress in achieving health and rights for all sex workers will fall short,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
FROM THE LANCET
Key clinical point: The HIV pandemic among sex workers remains underaddressed and underresourced, with “glaring gaps” in assessment, treatment.
Major finding: The global HIV burden among female sex workers shows that HIV prevalence was “unacceptably high” at 10.4%.
Study details: Researchers updated a 2013 literature review with reports published from Jan. 1, 2006, to Sept. 6, 2017.
Disclosures: The authors reported that they had no competing interests.
Source: Shannon K et al. Lancet. 2018 Aug 25;392:698-710.
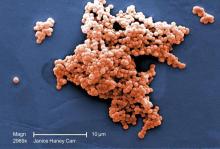
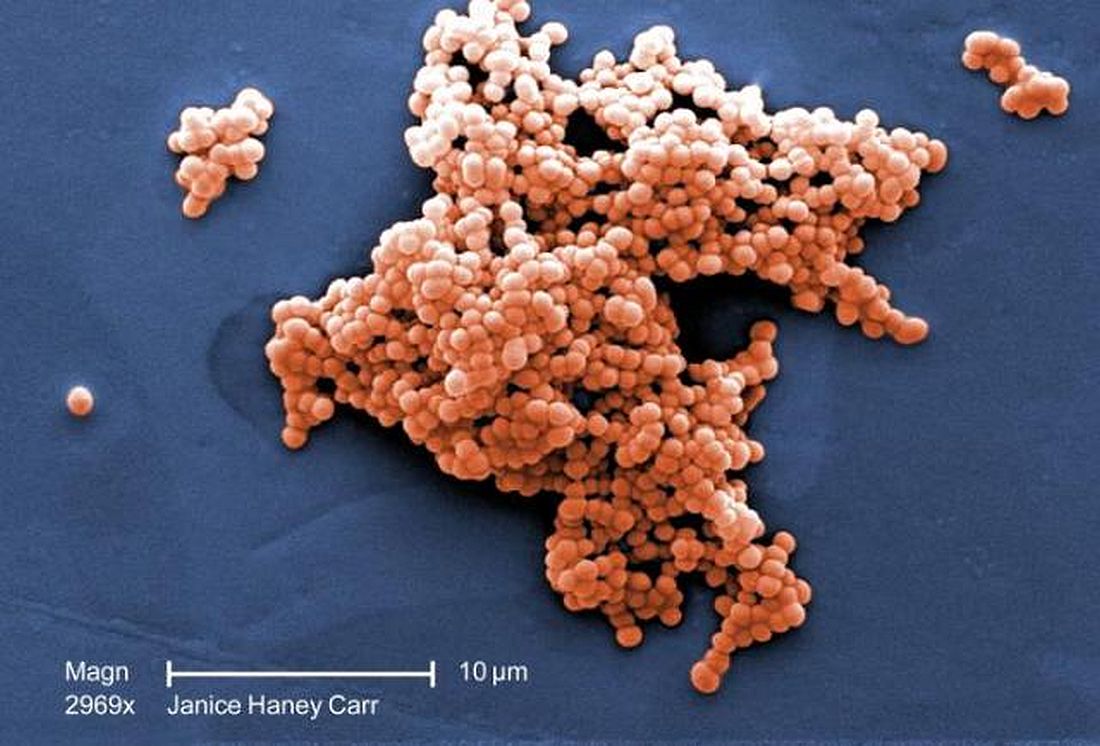
ICU infections: Chlorhexidine wipes tame MRSA, CRE
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
according to a report presented at ID Week 2018.
The move prevented an estimated eight methicillin-resistant Staphylococcus aureus (MRSA) and three carbapenem-resistant Enterobacteriaceae (CRE) infections and saved the medical center more than $150,000 in the year following the November 2016 switch.
The goal was to address the rate of MRSA bacteremia, which was higher than national ICU averages. Contact precautions began to make less sense as MRSA became more common in the surrounding community, and “we just wanted to get rid of contact precautions,” said study lead Jason Moss, DO, an infectious disease fellow at the university.
Contact precautions are expensive, make patients feel isolated, and according to some studies, lead to worse outcomes, he said at the annual scientific meeting on infectious diseases.
Decolonization is not routine in most ICUs, but it’s gaining traction. Guidelines recommend chlorhexidine bathing with wipes to stop CRE transmission, and chlorhexidine is used to prevent central line–associated bloodstream infections (CLABSI).
A recent analysis of 17 trials found marked decreases in MRSA and CLABSI with decolonization and concluded that chlorhexidine bathing “appears to be of the most clinical benefit when infection rates are high for a given ICU population,” as was the case in Kentucky (Crit Care. 2016 Nov 23;20[1]:379).
When researchers compared the year before the change to the year after, “we were pretty surprised at how much the rates of infection and colonization decreased. There have been some people that have been doing this in the ICU, but probably not to our extent. If you want to get rid of contact precautions, this is a great process to do it with,” Dr. Moss said.
Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6 (P = .026). Infection rates fell from 3.9 isolates per 10,000 patient-days to 2 (P = .083). Combined rates of infections and colonizations fell from almost 18 isolates per 10,000 patient-days to fewer than 8 (P = .010).
Decolonization is now standard practice at the university. Every ICU patient gets a one-time povidone iodine nasal swab at admission, then daily baths with 2% chlorhexidine gluconate applied by impregnated wipe. It usually takes four or five wipes to do the entire body.
Spending on gowns fell from about $153,000 per year to just under $60,000, but spending on wipes went up from about $2,700 to $275,000, and spending on povidone iodine nasal swabs went up to more than $100,000.
When balanced against the money not spent on those 11 prevented infections, however, the program saved the medical center about $152,000 in its first year, according to Dr. Moss and his team.
There was no funding for the work, and the investigators had no disclosures.
SOURCE: Moss J et al. ID Week 2018, Abstract 32.
REPORTING FROM ID WEEK 2018
Key clinical point: For high rates of MRSA and CRE in the ICU, consider decolonization instead of contact precautions.
Major finding: Rates of colonization with MRSA or CRE fell from about 14 isolates per 10,000 patient-days to fewer than 6; infection rates fell from 3.9 isolates to 2 per 10,000 patient-days.
Study details: Review of ICU quality improvement initiative
Disclosures: There was no funding for the work, and the investigators had no disclosures.
Source: Moss J et al. ID Week 2018, Abstract 32.
Automated algorithm improves HIV/HCV screening in the ED
More patients had newly diagnosed HIV and hepatitis C virus (HCV) infection during an automated-laboratory-order HIV/HCV screening algorithm than with a nurse-order HIV/HCV screening algorithm, according to the results of a retrospective before/after comparison study of the two electronic health record (EHR)–based protocols.
The results of nurse-order HIV/HCV screening in the 5-month period of March 1, 2016, through July 31, 2016, were compared to the subsequently adopted automated-laboratory-order system results from March 1, 2017, through July 31, 2017, according to Douglas A.E. White, MD, and his colleagues at Highland Hospital Emergency Department, Oakland, Calif.
Via the EHR, nurses were instructed to offer screening to all adults aged 18-75 years unless they were known to be HIV- or HCV-positive, unable to verbally consent (e.g., language barriers, intoxication), or medically unstable. Exclusion was at the discretion of the triage nurse. Using a drop-down menu, nurses could choose “accepts” or “declines” for HIV and HCV testing, according to patient response. Choosing “accepts” automatically ordered the test, according to the report (Ann Emerg Med. 2018 Oct;72[4]:438-48).
Automated-laboratory-order HIV/HCV screening was integrated into clinical care. With this protocol, the EHR-automated annual HIV/hepatitis C virus screening was performed on adult patients aged 18-75 years who had laboratory tests ordered. The EHR was configured to automatically order an HIV or HCV test for age-eligible patients who had any test ordered that required laboratory processing of whole blood (excluding point-of-care tests such as for lactate or glucose level) or a urine or urethral swab for chlamydia or gonorrhea testing, according to the researchers.
There were 20,975 and 19,887 unique, age-eligible patients during the nurse-order HIV/HCV virus screening algorithm and automated-laboratory-order HIV/HCV screening algorithm study periods, respectively. A total of 4,121 patients (19.6%) were screened for HIV and 2,968 (14.2%) patients were screened for HCV during the nurse-order period vs. 6,736 (33.9%) patients screened for HIV and 6,972 (35.1%) screened for HCV during the automated-laboratory-order period.
Overall, HIV screening increased from 19.6% to 33.9% and HCV screening, from 14.2% to 35.1% using the automated vs. the nurse-ordered EHR-based algorithm.
“An automated electronic health record algorithm that links nontargeted opt-out HIV and hepatitis C virus screening to physician laboratory ordering more effectively screens ED patients, provides results before discharge, minimizes repeated screening, and diagnoses more new infections than an algorithm that relies on nursing staff to offer screening. Because most EDs in the United States now use EHR systems, this model can be easily replicated and should be considered the standard for future programs,” the researchers concluded.
This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
SOURCE: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
More patients had newly diagnosed HIV and hepatitis C virus (HCV) infection during an automated-laboratory-order HIV/HCV screening algorithm than with a nurse-order HIV/HCV screening algorithm, according to the results of a retrospective before/after comparison study of the two electronic health record (EHR)–based protocols.
The results of nurse-order HIV/HCV screening in the 5-month period of March 1, 2016, through July 31, 2016, were compared to the subsequently adopted automated-laboratory-order system results from March 1, 2017, through July 31, 2017, according to Douglas A.E. White, MD, and his colleagues at Highland Hospital Emergency Department, Oakland, Calif.
Via the EHR, nurses were instructed to offer screening to all adults aged 18-75 years unless they were known to be HIV- or HCV-positive, unable to verbally consent (e.g., language barriers, intoxication), or medically unstable. Exclusion was at the discretion of the triage nurse. Using a drop-down menu, nurses could choose “accepts” or “declines” for HIV and HCV testing, according to patient response. Choosing “accepts” automatically ordered the test, according to the report (Ann Emerg Med. 2018 Oct;72[4]:438-48).
Automated-laboratory-order HIV/HCV screening was integrated into clinical care. With this protocol, the EHR-automated annual HIV/hepatitis C virus screening was performed on adult patients aged 18-75 years who had laboratory tests ordered. The EHR was configured to automatically order an HIV or HCV test for age-eligible patients who had any test ordered that required laboratory processing of whole blood (excluding point-of-care tests such as for lactate or glucose level) or a urine or urethral swab for chlamydia or gonorrhea testing, according to the researchers.
There were 20,975 and 19,887 unique, age-eligible patients during the nurse-order HIV/HCV virus screening algorithm and automated-laboratory-order HIV/HCV screening algorithm study periods, respectively. A total of 4,121 patients (19.6%) were screened for HIV and 2,968 (14.2%) patients were screened for HCV during the nurse-order period vs. 6,736 (33.9%) patients screened for HIV and 6,972 (35.1%) screened for HCV during the automated-laboratory-order period.
Overall, HIV screening increased from 19.6% to 33.9% and HCV screening, from 14.2% to 35.1% using the automated vs. the nurse-ordered EHR-based algorithm.
“An automated electronic health record algorithm that links nontargeted opt-out HIV and hepatitis C virus screening to physician laboratory ordering more effectively screens ED patients, provides results before discharge, minimizes repeated screening, and diagnoses more new infections than an algorithm that relies on nursing staff to offer screening. Because most EDs in the United States now use EHR systems, this model can be easily replicated and should be considered the standard for future programs,” the researchers concluded.
This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
SOURCE: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
More patients had newly diagnosed HIV and hepatitis C virus (HCV) infection during an automated-laboratory-order HIV/HCV screening algorithm than with a nurse-order HIV/HCV screening algorithm, according to the results of a retrospective before/after comparison study of the two electronic health record (EHR)–based protocols.
The results of nurse-order HIV/HCV screening in the 5-month period of March 1, 2016, through July 31, 2016, were compared to the subsequently adopted automated-laboratory-order system results from March 1, 2017, through July 31, 2017, according to Douglas A.E. White, MD, and his colleagues at Highland Hospital Emergency Department, Oakland, Calif.
Via the EHR, nurses were instructed to offer screening to all adults aged 18-75 years unless they were known to be HIV- or HCV-positive, unable to verbally consent (e.g., language barriers, intoxication), or medically unstable. Exclusion was at the discretion of the triage nurse. Using a drop-down menu, nurses could choose “accepts” or “declines” for HIV and HCV testing, according to patient response. Choosing “accepts” automatically ordered the test, according to the report (Ann Emerg Med. 2018 Oct;72[4]:438-48).
Automated-laboratory-order HIV/HCV screening was integrated into clinical care. With this protocol, the EHR-automated annual HIV/hepatitis C virus screening was performed on adult patients aged 18-75 years who had laboratory tests ordered. The EHR was configured to automatically order an HIV or HCV test for age-eligible patients who had any test ordered that required laboratory processing of whole blood (excluding point-of-care tests such as for lactate or glucose level) or a urine or urethral swab for chlamydia or gonorrhea testing, according to the researchers.
There were 20,975 and 19,887 unique, age-eligible patients during the nurse-order HIV/HCV virus screening algorithm and automated-laboratory-order HIV/HCV screening algorithm study periods, respectively. A total of 4,121 patients (19.6%) were screened for HIV and 2,968 (14.2%) patients were screened for HCV during the nurse-order period vs. 6,736 (33.9%) patients screened for HIV and 6,972 (35.1%) screened for HCV during the automated-laboratory-order period.
Overall, HIV screening increased from 19.6% to 33.9% and HCV screening, from 14.2% to 35.1% using the automated vs. the nurse-ordered EHR-based algorithm.
“An automated electronic health record algorithm that links nontargeted opt-out HIV and hepatitis C virus screening to physician laboratory ordering more effectively screens ED patients, provides results before discharge, minimizes repeated screening, and diagnoses more new infections than an algorithm that relies on nursing staff to offer screening. Because most EDs in the United States now use EHR systems, this model can be easily replicated and should be considered the standard for future programs,” the researchers concluded.
This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
SOURCE: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
FROM ANNALS OF EMERGENCY MEDICINE
Key clinical point:
Major finding: HIV screening increased from 19.6% to 33.9%; HCV screening, from 14.2% to 35.1%, with use of an automated vs. nurse-ordered EHR-based algorithm.
Study details: There were 20,975 and 19,887eligible patients assessed during the nurse-order HIV/HCV screening and the automated–laboratory-order screening periods, respectively.
Disclosures: This work was supported, in part, by grant funding through the FOCUS program, Gilead Sciences, which also has provided funding to various of the authors of the study.
Source: White DAE et al. Ann Emerg Med. 2018 Oct;72[4]:438-48.
Adjuvanted flu vaccine reduces hospitalizations in oldest old
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
REPORTING FROM ID WEEK 2018
Vancomycin loading boost yields better C. diff outcomes
SAN FRANCISCO – A heightened loading dose of vancomycin may lead to faster recovery and greater efficacy in Clostridium difficile infections, according to the results of a quasi-experimental study presented at an annual scientific meeting on infectious diseases.
The study looked at a loading dose of 500 mg of vancomycin delivered four times per day for the first 48 hours, followed by a step down to 125 mg every 6 hours. It came on the heels of an attempted randomized, clinical trial that was inconclusive because of insufficient recruitment. Still, the results were promising enough to convince the Yale New Haven Hospital to make it standard practice in C. difficile patients.
Samad Tirmizi, PharmD, an infectious disease pharmacist at Stony Brook University (N.Y.), shares the results of a comparison of outcomes before and after the initiation of this treatment protocol in a video interview.
The approach grew out of concerns that vancomycin may not achieve sufficient concentrations in the colon early in treatment. A pharmacokinetics study published in 2010 suggested that a high initial loading led to higher fecal vancomycin levels, even in patients with increased stool frequency (BMC Infect Dis. 2010 Dec 30;10:363).
SOURCE: Tirmizi S et al. IDWeek 2018, Abstract 1980.
SAN FRANCISCO – A heightened loading dose of vancomycin may lead to faster recovery and greater efficacy in Clostridium difficile infections, according to the results of a quasi-experimental study presented at an annual scientific meeting on infectious diseases.
The study looked at a loading dose of 500 mg of vancomycin delivered four times per day for the first 48 hours, followed by a step down to 125 mg every 6 hours. It came on the heels of an attempted randomized, clinical trial that was inconclusive because of insufficient recruitment. Still, the results were promising enough to convince the Yale New Haven Hospital to make it standard practice in C. difficile patients.
Samad Tirmizi, PharmD, an infectious disease pharmacist at Stony Brook University (N.Y.), shares the results of a comparison of outcomes before and after the initiation of this treatment protocol in a video interview.
The approach grew out of concerns that vancomycin may not achieve sufficient concentrations in the colon early in treatment. A pharmacokinetics study published in 2010 suggested that a high initial loading led to higher fecal vancomycin levels, even in patients with increased stool frequency (BMC Infect Dis. 2010 Dec 30;10:363).
SOURCE: Tirmizi S et al. IDWeek 2018, Abstract 1980.
SAN FRANCISCO – A heightened loading dose of vancomycin may lead to faster recovery and greater efficacy in Clostridium difficile infections, according to the results of a quasi-experimental study presented at an annual scientific meeting on infectious diseases.
The study looked at a loading dose of 500 mg of vancomycin delivered four times per day for the first 48 hours, followed by a step down to 125 mg every 6 hours. It came on the heels of an attempted randomized, clinical trial that was inconclusive because of insufficient recruitment. Still, the results were promising enough to convince the Yale New Haven Hospital to make it standard practice in C. difficile patients.
Samad Tirmizi, PharmD, an infectious disease pharmacist at Stony Brook University (N.Y.), shares the results of a comparison of outcomes before and after the initiation of this treatment protocol in a video interview.
The approach grew out of concerns that vancomycin may not achieve sufficient concentrations in the colon early in treatment. A pharmacokinetics study published in 2010 suggested that a high initial loading led to higher fecal vancomycin levels, even in patients with increased stool frequency (BMC Infect Dis. 2010 Dec 30;10:363).
SOURCE: Tirmizi S et al. IDWeek 2018, Abstract 1980.
REPORTING FROM IDWEEK 2018
Myeloperoxidase elevated in HCV-related liver cancer
Myeloperoxidase (MPO) expression was significantly higher in hepatitis C virus (HCV)–related hepatocellular carcinoma (HCC) cases when compared with cirrhotic patients, according to a study of 59 patients with HCV-related liver disease.
HCV is the main cause of liver disease, wrote Mohamed Abdel-Hamed, MD, of Minia University in Egypt, and his colleagues. In addition, HCV is associated with significant oxidative stress, which has been identified as a significant metabolic pathway culminating in hepatic cirrhosis, liver failure, and liver cancer. Thus the researchers studied the role of MPO, an oxidative enzyme released at sites of inflammation, in the possible etiology of HCV-related liver cancer.
The patients were divided into two groups, 25 with HCC and 34 with chronic liver diseases with cirrhosis. All patients were examined immunohistochemically to demonstrate the expression of myeloperoxidase, according to the report published in Meta Gene.
Subjects with HCC showed markedly increased MPO expression when compared with MPO expression in hepatocytes of subjects with liver cirrhosis, who mostly showed a mild degree of expression. In addition, no mild expression of MPO was detected in the subjects with HCC. These findings were highly statistically significant (P less than .0001), according to Dr. Abdel-Hamed and his colleagues.
“The present study showed that marked expression of MPO plays an important role in the pathogenesis of HCV-associated HCC,” the authors stated. “This study could provide valuable, predictive parameters that can be used clinically in the prognosis of HCC patients.”
The authors did not report any disclosures.
SOURCE: Abdel-Hamid M et al. Meta Gene. 2018 Dec;18:1-8.
Myeloperoxidase (MPO) expression was significantly higher in hepatitis C virus (HCV)–related hepatocellular carcinoma (HCC) cases when compared with cirrhotic patients, according to a study of 59 patients with HCV-related liver disease.
HCV is the main cause of liver disease, wrote Mohamed Abdel-Hamed, MD, of Minia University in Egypt, and his colleagues. In addition, HCV is associated with significant oxidative stress, which has been identified as a significant metabolic pathway culminating in hepatic cirrhosis, liver failure, and liver cancer. Thus the researchers studied the role of MPO, an oxidative enzyme released at sites of inflammation, in the possible etiology of HCV-related liver cancer.
The patients were divided into two groups, 25 with HCC and 34 with chronic liver diseases with cirrhosis. All patients were examined immunohistochemically to demonstrate the expression of myeloperoxidase, according to the report published in Meta Gene.
Subjects with HCC showed markedly increased MPO expression when compared with MPO expression in hepatocytes of subjects with liver cirrhosis, who mostly showed a mild degree of expression. In addition, no mild expression of MPO was detected in the subjects with HCC. These findings were highly statistically significant (P less than .0001), according to Dr. Abdel-Hamed and his colleagues.
“The present study showed that marked expression of MPO plays an important role in the pathogenesis of HCV-associated HCC,” the authors stated. “This study could provide valuable, predictive parameters that can be used clinically in the prognosis of HCC patients.”
The authors did not report any disclosures.
SOURCE: Abdel-Hamid M et al. Meta Gene. 2018 Dec;18:1-8.
Myeloperoxidase (MPO) expression was significantly higher in hepatitis C virus (HCV)–related hepatocellular carcinoma (HCC) cases when compared with cirrhotic patients, according to a study of 59 patients with HCV-related liver disease.
HCV is the main cause of liver disease, wrote Mohamed Abdel-Hamed, MD, of Minia University in Egypt, and his colleagues. In addition, HCV is associated with significant oxidative stress, which has been identified as a significant metabolic pathway culminating in hepatic cirrhosis, liver failure, and liver cancer. Thus the researchers studied the role of MPO, an oxidative enzyme released at sites of inflammation, in the possible etiology of HCV-related liver cancer.
The patients were divided into two groups, 25 with HCC and 34 with chronic liver diseases with cirrhosis. All patients were examined immunohistochemically to demonstrate the expression of myeloperoxidase, according to the report published in Meta Gene.
Subjects with HCC showed markedly increased MPO expression when compared with MPO expression in hepatocytes of subjects with liver cirrhosis, who mostly showed a mild degree of expression. In addition, no mild expression of MPO was detected in the subjects with HCC. These findings were highly statistically significant (P less than .0001), according to Dr. Abdel-Hamed and his colleagues.
“The present study showed that marked expression of MPO plays an important role in the pathogenesis of HCV-associated HCC,” the authors stated. “This study could provide valuable, predictive parameters that can be used clinically in the prognosis of HCC patients.”
The authors did not report any disclosures.
SOURCE: Abdel-Hamid M et al. Meta Gene. 2018 Dec;18:1-8.
FROM META GENE
Key clinical point:
Major finding: Hepatocellular carcinoma subjects showed a marked increase of myeloperoxidase expression when compared with subjects with liver cirrhosis (P less than .0001).
Study details: An immunohistochemical analysis of 59 patients infected with hepatitis C virus.
Disclosures: The authors did not report any disclosures.
Source: Abdel-Hamid M et al. Meta Gene. 2018 Dec;18:1-8.