User login
Oral flu vaccine protects, evokes mucosal immunity
SAN FRANCISCO – In a phase II study, Vaxart’s oral flu vaccine was compared with a commercial injectable quadrivalent flu vaccine or placebo. The study found rates of illness were comparable between the oral vaccine and quadrivalent vaccinated groups.*

The recombinant adenovirus-based vaccine expresses hemagglutinin. It elicited a mucosal immune response, hinting that the mechanism of protection in flu vaccines may be dependent on the route of administration. It is also believed that a strong mucosal response is key to preventing future infections.
In an interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Nikita Kolhatkar, PhD, a salaried employee of Vaxart, which makes the drug, describes the results of the study and explains the potential advantages of an oral flu vaccine versus a traditional injectable one. The oral formulation is cell-based and so is not vulnerable to the mutation and genetic drift that can occur in egg-based vaccines.
It is also more stable and, of course, less invasive than injectable vaccines, according to Dr. Kolhatkar.
*Correction, 10/9/2018: An earlier vs. of this article did not stress the comparability.
SOURCE: Kolhatkar N. IDWeek 2018. Poster abstract 1947.
SAN FRANCISCO – In a phase II study, Vaxart’s oral flu vaccine was compared with a commercial injectable quadrivalent flu vaccine or placebo. The study found rates of illness were comparable between the oral vaccine and quadrivalent vaccinated groups.*

The recombinant adenovirus-based vaccine expresses hemagglutinin. It elicited a mucosal immune response, hinting that the mechanism of protection in flu vaccines may be dependent on the route of administration. It is also believed that a strong mucosal response is key to preventing future infections.
In an interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Nikita Kolhatkar, PhD, a salaried employee of Vaxart, which makes the drug, describes the results of the study and explains the potential advantages of an oral flu vaccine versus a traditional injectable one. The oral formulation is cell-based and so is not vulnerable to the mutation and genetic drift that can occur in egg-based vaccines.
It is also more stable and, of course, less invasive than injectable vaccines, according to Dr. Kolhatkar.
*Correction, 10/9/2018: An earlier vs. of this article did not stress the comparability.
SOURCE: Kolhatkar N. IDWeek 2018. Poster abstract 1947.
SAN FRANCISCO – In a phase II study, Vaxart’s oral flu vaccine was compared with a commercial injectable quadrivalent flu vaccine or placebo. The study found rates of illness were comparable between the oral vaccine and quadrivalent vaccinated groups.*

The recombinant adenovirus-based vaccine expresses hemagglutinin. It elicited a mucosal immune response, hinting that the mechanism of protection in flu vaccines may be dependent on the route of administration. It is also believed that a strong mucosal response is key to preventing future infections.
In an interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Nikita Kolhatkar, PhD, a salaried employee of Vaxart, which makes the drug, describes the results of the study and explains the potential advantages of an oral flu vaccine versus a traditional injectable one. The oral formulation is cell-based and so is not vulnerable to the mutation and genetic drift that can occur in egg-based vaccines.
It is also more stable and, of course, less invasive than injectable vaccines, according to Dr. Kolhatkar.
*Correction, 10/9/2018: An earlier vs. of this article did not stress the comparability.
SOURCE: Kolhatkar N. IDWeek 2018. Poster abstract 1947.
REPORTING FROM IDWEEK 2018
How to vaccinate patients on biologics
SAN FRANCISCO – The new herpes zoster subunit vaccine (Shingrix) is on the short list of essential vaccines for immunocompromised adults, including those on biologics.
Ongoing research is demonstrating efficacy and safety in renal transplants patients, as well as those with hematologic cancer and stem cell transplants, according to Lorry Rubin, MD, director of pediatric infectious diseases at Cohen Children’s Medical Center, Queens, and professor of pediatrics at Hofstra University, Hempstead, N.Y.
Immunocompromised people, including those on biologics, should be immunized against a variety of diseases just like everyone else, but it’s tricky. There’s considerable variability in how biologics affect the immune system and subsequent vaccine potency. Timing is important, and although live vaccines are generally a no-go, there’s one class of biologics with which they’re safe, he said.
In a wide-ranging interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Dr. Rubin shared his advice on immunizing the immunocompromised, including the other vaccines on the short list. He also tackled the common concern that vaccinations might trigger rejection in transplant patients.
He’s well qualified to address the issues: Dr. Rubin was lead author on the 2013 Infectious Diseases Society of America guidelines on vaccinating immunocompromised patients.
SAN FRANCISCO – The new herpes zoster subunit vaccine (Shingrix) is on the short list of essential vaccines for immunocompromised adults, including those on biologics.
Ongoing research is demonstrating efficacy and safety in renal transplants patients, as well as those with hematologic cancer and stem cell transplants, according to Lorry Rubin, MD, director of pediatric infectious diseases at Cohen Children’s Medical Center, Queens, and professor of pediatrics at Hofstra University, Hempstead, N.Y.
Immunocompromised people, including those on biologics, should be immunized against a variety of diseases just like everyone else, but it’s tricky. There’s considerable variability in how biologics affect the immune system and subsequent vaccine potency. Timing is important, and although live vaccines are generally a no-go, there’s one class of biologics with which they’re safe, he said.
In a wide-ranging interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Dr. Rubin shared his advice on immunizing the immunocompromised, including the other vaccines on the short list. He also tackled the common concern that vaccinations might trigger rejection in transplant patients.
He’s well qualified to address the issues: Dr. Rubin was lead author on the 2013 Infectious Diseases Society of America guidelines on vaccinating immunocompromised patients.
SAN FRANCISCO – The new herpes zoster subunit vaccine (Shingrix) is on the short list of essential vaccines for immunocompromised adults, including those on biologics.
Ongoing research is demonstrating efficacy and safety in renal transplants patients, as well as those with hematologic cancer and stem cell transplants, according to Lorry Rubin, MD, director of pediatric infectious diseases at Cohen Children’s Medical Center, Queens, and professor of pediatrics at Hofstra University, Hempstead, N.Y.
Immunocompromised people, including those on biologics, should be immunized against a variety of diseases just like everyone else, but it’s tricky. There’s considerable variability in how biologics affect the immune system and subsequent vaccine potency. Timing is important, and although live vaccines are generally a no-go, there’s one class of biologics with which they’re safe, he said.
In a wide-ranging interview at IDWeek 2018, an annual scientific meeting on infectious diseases, Dr. Rubin shared his advice on immunizing the immunocompromised, including the other vaccines on the short list. He also tackled the common concern that vaccinations might trigger rejection in transplant patients.
He’s well qualified to address the issues: Dr. Rubin was lead author on the 2013 Infectious Diseases Society of America guidelines on vaccinating immunocompromised patients.
REPORTING FROM IDWEEK 2018
DAAs top PEG/RBV for reducing HCV cardiovascular risk
SAN FRANCISCO – , according to a review of over 30,000 patients in the ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) database of the Veterans Health Administration system.
In the study, 12,667 patients were treated with direct-acting antiretrovirals (DAAs) and 4,436 with pegylated interferon/ribavirin (PEG/RBV). Each subject was matched to an untreated control based on alcohol use, diabetes, and other confounders. Patients with HIV, hepatitis B, or previously diagnosed cardiovascular disease were excluded.
Over a follow-up of about 10 years, there were 2,361 strokes, heart attacks, or other cardiovascular (CV) events among untreated patients, which translated to an incidence of 30.9 events per 1,000 patient years. In the PEG/RBV group, there were 804 events, yielding an incidence of 23.5 per 1,000 patient years. The DAA group fared better, with 435 events and an incident rate of 16.3.
Sustained virologic response also was associated with lower CV risk, and the odds of attaining it were about 25% greater with DAAs vs. PEG/RBV. That might have played a role in the findings, since hepatitis C is known to be associated with CV disease and the virus has been found in atherosclerotic plaques.
Past investigations have been mixed on whether or not hepatitis C virus treatment reduces CV risk, but lead investigator Adeel Ajwad Butt, MD, professor of medicine at Cornell University, New York, said that his study was stronger than what has come before. He explained why, and also why the findings matter, in an interview at ID Week 2018, an annual scientific meeting on infectious diseases.
Most of the subjects were men, about a quarter were black, and the median age at baseline was 58 years.
Dr. Butt disclosed institutional research grants from Merck and Gilead.
SOURCE: Butt AA et al. ID Week 2018 abstract 930.
SAN FRANCISCO – , according to a review of over 30,000 patients in the ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) database of the Veterans Health Administration system.
In the study, 12,667 patients were treated with direct-acting antiretrovirals (DAAs) and 4,436 with pegylated interferon/ribavirin (PEG/RBV). Each subject was matched to an untreated control based on alcohol use, diabetes, and other confounders. Patients with HIV, hepatitis B, or previously diagnosed cardiovascular disease were excluded.
Over a follow-up of about 10 years, there were 2,361 strokes, heart attacks, or other cardiovascular (CV) events among untreated patients, which translated to an incidence of 30.9 events per 1,000 patient years. In the PEG/RBV group, there were 804 events, yielding an incidence of 23.5 per 1,000 patient years. The DAA group fared better, with 435 events and an incident rate of 16.3.
Sustained virologic response also was associated with lower CV risk, and the odds of attaining it were about 25% greater with DAAs vs. PEG/RBV. That might have played a role in the findings, since hepatitis C is known to be associated with CV disease and the virus has been found in atherosclerotic plaques.
Past investigations have been mixed on whether or not hepatitis C virus treatment reduces CV risk, but lead investigator Adeel Ajwad Butt, MD, professor of medicine at Cornell University, New York, said that his study was stronger than what has come before. He explained why, and also why the findings matter, in an interview at ID Week 2018, an annual scientific meeting on infectious diseases.
Most of the subjects were men, about a quarter were black, and the median age at baseline was 58 years.
Dr. Butt disclosed institutional research grants from Merck and Gilead.
SOURCE: Butt AA et al. ID Week 2018 abstract 930.
SAN FRANCISCO – , according to a review of over 30,000 patients in the ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) database of the Veterans Health Administration system.
In the study, 12,667 patients were treated with direct-acting antiretrovirals (DAAs) and 4,436 with pegylated interferon/ribavirin (PEG/RBV). Each subject was matched to an untreated control based on alcohol use, diabetes, and other confounders. Patients with HIV, hepatitis B, or previously diagnosed cardiovascular disease were excluded.
Over a follow-up of about 10 years, there were 2,361 strokes, heart attacks, or other cardiovascular (CV) events among untreated patients, which translated to an incidence of 30.9 events per 1,000 patient years. In the PEG/RBV group, there were 804 events, yielding an incidence of 23.5 per 1,000 patient years. The DAA group fared better, with 435 events and an incident rate of 16.3.
Sustained virologic response also was associated with lower CV risk, and the odds of attaining it were about 25% greater with DAAs vs. PEG/RBV. That might have played a role in the findings, since hepatitis C is known to be associated with CV disease and the virus has been found in atherosclerotic plaques.
Past investigations have been mixed on whether or not hepatitis C virus treatment reduces CV risk, but lead investigator Adeel Ajwad Butt, MD, professor of medicine at Cornell University, New York, said that his study was stronger than what has come before. He explained why, and also why the findings matter, in an interview at ID Week 2018, an annual scientific meeting on infectious diseases.
Most of the subjects were men, about a quarter were black, and the median age at baseline was 58 years.
Dr. Butt disclosed institutional research grants from Merck and Gilead.
SOURCE: Butt AA et al. ID Week 2018 abstract 930.
REPORTING FROM ID WEEK 2018
Omadacycline equivalent to linezolid for skin infections
SAN FRANCISCO – In early October, the Food and Drug Administration approved omadacycline (Nuzyra) for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections in adults.
The drug is a synthetic tetracycline designed to overcome some of the resistance mechanisms that can undermine traditional tetracycline drugs. It gained approval on the strength of the Oasis-1 (NCT03482011) and Oasis-2 (NCT03535194) trials, which demonstrated the drug’s noninferiority to linezolid.
At IDWeek 2018, researchers combined the data from the two pivotal trials to gain more power in some of the secondary endpoints, such as adverse events.
Paul McGovern, MD, vice president of clinical and medical affairs at Paratek, which markets omadacycline, discussed the results of the analysis at an annual scientific meeting on infectious diseases.
Combined, the two studies included 691 patients who received omadacycline and 689 who received linezolid. The two drugs achieved similar results for early clinical response, defined as at least a 20% reduction in lesion size 48-72 hours after the first dose. The mean reduction in baseline lesion area at day 3 was 53.4% in the omadacycline group and 53.0% in the linezolid group. At the end of the treatment period, those values were 93.9% and 93.7%, respectively, Dr. McGovern reported.
A total of 28.5% of patients receiving omadacycline reported drug-related treatment-emergent adverse events, compared with 16.1% of the linezolid group. The omadacycline group experienced higher frequency of nausea (21.9% vs. 8.7%) and vomiting (11.4% vs. 3.9%), he said.
The Oasis 1 and Oasis 2 studies were funded by Paratek. Dr. McGovern is an employee of Paratek.
SOURCE: McGovern P et al. IDWeek 2018, poster abstract 1347.
SAN FRANCISCO – In early October, the Food and Drug Administration approved omadacycline (Nuzyra) for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections in adults.
The drug is a synthetic tetracycline designed to overcome some of the resistance mechanisms that can undermine traditional tetracycline drugs. It gained approval on the strength of the Oasis-1 (NCT03482011) and Oasis-2 (NCT03535194) trials, which demonstrated the drug’s noninferiority to linezolid.
At IDWeek 2018, researchers combined the data from the two pivotal trials to gain more power in some of the secondary endpoints, such as adverse events.
Paul McGovern, MD, vice president of clinical and medical affairs at Paratek, which markets omadacycline, discussed the results of the analysis at an annual scientific meeting on infectious diseases.
Combined, the two studies included 691 patients who received omadacycline and 689 who received linezolid. The two drugs achieved similar results for early clinical response, defined as at least a 20% reduction in lesion size 48-72 hours after the first dose. The mean reduction in baseline lesion area at day 3 was 53.4% in the omadacycline group and 53.0% in the linezolid group. At the end of the treatment period, those values were 93.9% and 93.7%, respectively, Dr. McGovern reported.
A total of 28.5% of patients receiving omadacycline reported drug-related treatment-emergent adverse events, compared with 16.1% of the linezolid group. The omadacycline group experienced higher frequency of nausea (21.9% vs. 8.7%) and vomiting (11.4% vs. 3.9%), he said.
The Oasis 1 and Oasis 2 studies were funded by Paratek. Dr. McGovern is an employee of Paratek.
SOURCE: McGovern P et al. IDWeek 2018, poster abstract 1347.
SAN FRANCISCO – In early October, the Food and Drug Administration approved omadacycline (Nuzyra) for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections in adults.
The drug is a synthetic tetracycline designed to overcome some of the resistance mechanisms that can undermine traditional tetracycline drugs. It gained approval on the strength of the Oasis-1 (NCT03482011) and Oasis-2 (NCT03535194) trials, which demonstrated the drug’s noninferiority to linezolid.
At IDWeek 2018, researchers combined the data from the two pivotal trials to gain more power in some of the secondary endpoints, such as adverse events.
Paul McGovern, MD, vice president of clinical and medical affairs at Paratek, which markets omadacycline, discussed the results of the analysis at an annual scientific meeting on infectious diseases.
Combined, the two studies included 691 patients who received omadacycline and 689 who received linezolid. The two drugs achieved similar results for early clinical response, defined as at least a 20% reduction in lesion size 48-72 hours after the first dose. The mean reduction in baseline lesion area at day 3 was 53.4% in the omadacycline group and 53.0% in the linezolid group. At the end of the treatment period, those values were 93.9% and 93.7%, respectively, Dr. McGovern reported.
A total of 28.5% of patients receiving omadacycline reported drug-related treatment-emergent adverse events, compared with 16.1% of the linezolid group. The omadacycline group experienced higher frequency of nausea (21.9% vs. 8.7%) and vomiting (11.4% vs. 3.9%), he said.
The Oasis 1 and Oasis 2 studies were funded by Paratek. Dr. McGovern is an employee of Paratek.
SOURCE: McGovern P et al. IDWeek 2018, poster abstract 1347.
REPORTING FROM IDWEEK 2018
Key clinical point:
Major finding: The mean reduction in lesion area at the end of treatment was 93.9% for omadacycline and 93.7% for linezolid.
Study details: Meta-analysis of two trials with 691 patients receiving omadacycline and 689 patients receiving linezolid.
Disclosures: The Oasis-1 and Oasis-2 studies were funded by Paratek. Dr. McGovern is an employee of Paratek.
Source: McGovern P et al. IDWeek 2018, poster abstract 1347.
FDA expands approval of 9-valent HPV vaccine
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
The , men and women aged 27-45 years, the Food and Drug Administration announced on Oct. 5.
The vaccine (Gardasil 9) was previously approved for those aged 9-26 years.
The approval “represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement announcing the approval.
“The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing,” he added.
Gardasil 9, approved in 2014, covers the four HPV types included in the original Gardasil vaccine approved in 2006, plus five additional HPV types.
The approval is based on the results of a study and follow-up of about 3,200 women aged 27-45 years, followed for an average of 3.5 years, which found that the vaccine was 88% percent effective “in the prevention of a combined endpoint of persistent infection, genital warts, vulvar and vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer related to HPV types covered by the vaccine,” according to the FDA. The vaccine’s effectiveness in men in this age group is “inferred” from these results and from data on Gardasil in men aged 16-26 years, as well as “immunogenicity data from a clinical trial in which 150 men, 27 through 45 years of age, received a 3-dose regimen of Gardasil over 6 months,” the FDA statement noted.
Based on safety data in about 13,000 men and women, injection-site pain, swelling, redness, and headaches are the most common adverse reactions associated with Gardasil 9, the statement said. Gardasil 9 is manufactured by Merck.
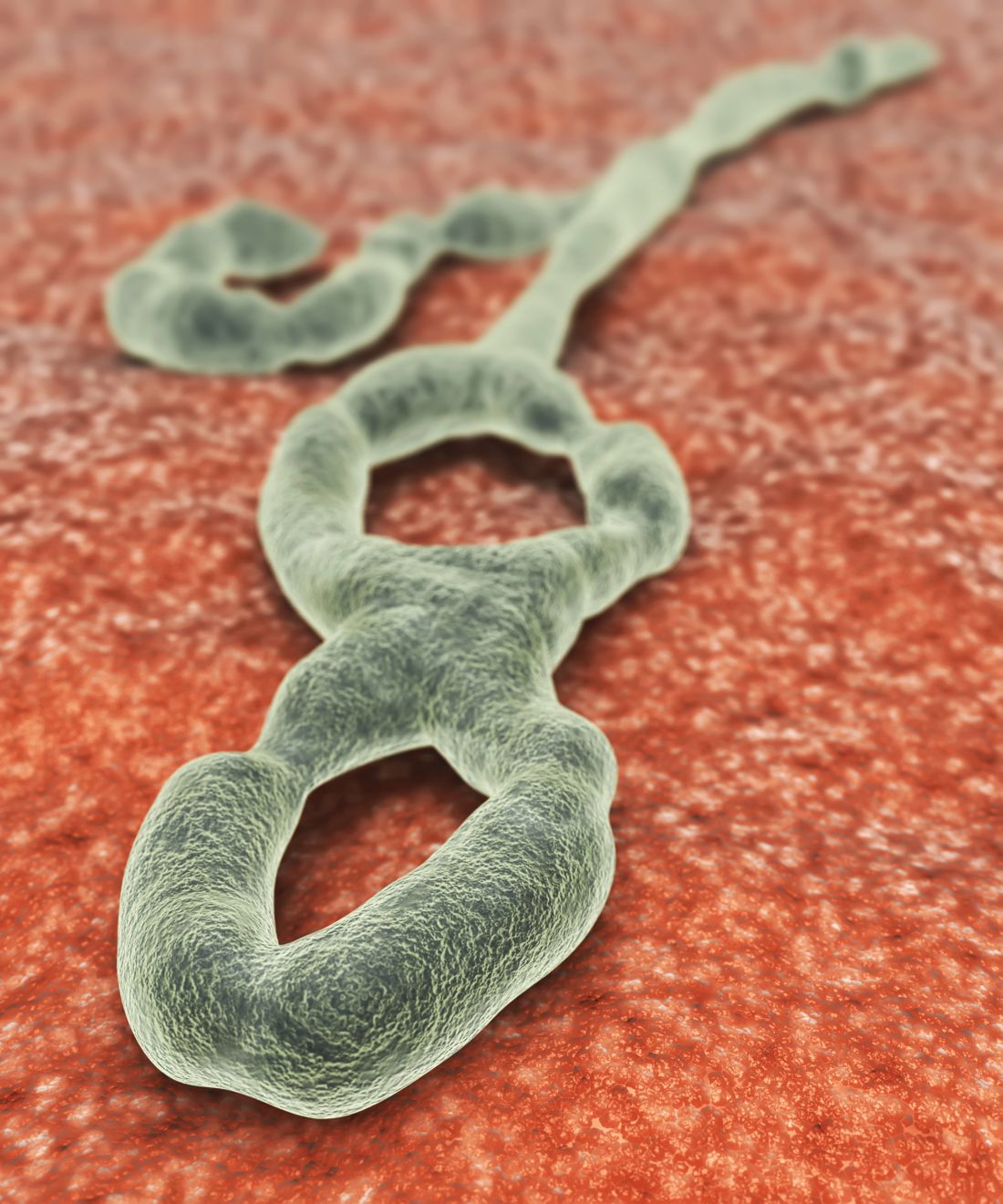
Experimental drugs deployed in Ebola response
SAN FRANCISCO – For the first time, the World Health Organization’s Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI) protocol is being field tested in the Democratic Republic of Congo (DRC), where four different therapeutics are being delivered to Ebola patients. MEURI was created after controversy surrounding the 2014-2016 Ebola outbreak in West Africa, which ultimately led to the decision to integrate research into outbreak responses.
MEURI is broadly designed for pathogens that have no proven intervention, on the premise that in a disease with high mortality, it can be ethically appropriate to provide experimental therapies during a response, as long as a set of criteria are met that ensure patient autonomy and give patients a reasonable opportunity to improve their condition.
The original plan was to employ experimental drugs under the MEURI umbrella during an Ebola outbreak in the DRC that began in May, but an effective response contained it so quickly that the program was canceled. However, when another Ebola outbreak occurred on Aug. 27, the material was still in the country and ready to be deployed. “That made it very easy to begin using them,” said Elizabeth Higgs, MD, global health science advisor for the division of clinical research at the National Institute for Allergy and Infectious Disease.
Dr. Higgs spoke about the developments during a late-breakers session at IDWeek 2018, an annual scientific meeting on infectious disease.
The therapies under consideration are Zmapp (Leaf Biopharmaceutical), which includes three chimeric monoclonal antibodies that target the main surface protein of the Ebola virus; mAb114 (NIAID), which was isolated in 1995 from a human survivor of Ebola and binds to the core of the Ebola surface protein; Remdesivir (Gilead), a small molecule that is believed to interfere with viral replication; and Regeneron’s Ebola triple monoclonal antibody cocktail, which also targets the surface protein.
Through Oct. 2, 43 Ebola patients have received one of the four drugs: 19 are cured and have been discharged, 12 have died, and 12 are still in recovery. Dr. Higgs cautioned against reading anything into those numbers, however, since no randomization was involved.
Although compassionate use is the primary aim of MEURI, it may be lead to some scientific benefit. The most that can be hoped for is to glean some insight into the safety of the interventions, although even that information is limited. “A colleague and I were talking about this the other day. After the first X number of patients, they were all still alive. What can you say from that? I think what you can say is that it didn’t kill them,” Dr. Higgs said.
Still, researchers are collecting laboratory data from patients to monitor their responses. “With Remdesivir we’re closely examining their transaminase, for example, so I think it will be helpful,” said Dr. Higgs.
SOURCE: Higgs E et al. IDWeek 2018.
SAN FRANCISCO – For the first time, the World Health Organization’s Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI) protocol is being field tested in the Democratic Republic of Congo (DRC), where four different therapeutics are being delivered to Ebola patients. MEURI was created after controversy surrounding the 2014-2016 Ebola outbreak in West Africa, which ultimately led to the decision to integrate research into outbreak responses.
MEURI is broadly designed for pathogens that have no proven intervention, on the premise that in a disease with high mortality, it can be ethically appropriate to provide experimental therapies during a response, as long as a set of criteria are met that ensure patient autonomy and give patients a reasonable opportunity to improve their condition.
The original plan was to employ experimental drugs under the MEURI umbrella during an Ebola outbreak in the DRC that began in May, but an effective response contained it so quickly that the program was canceled. However, when another Ebola outbreak occurred on Aug. 27, the material was still in the country and ready to be deployed. “That made it very easy to begin using them,” said Elizabeth Higgs, MD, global health science advisor for the division of clinical research at the National Institute for Allergy and Infectious Disease.
Dr. Higgs spoke about the developments during a late-breakers session at IDWeek 2018, an annual scientific meeting on infectious disease.
The therapies under consideration are Zmapp (Leaf Biopharmaceutical), which includes three chimeric monoclonal antibodies that target the main surface protein of the Ebola virus; mAb114 (NIAID), which was isolated in 1995 from a human survivor of Ebola and binds to the core of the Ebola surface protein; Remdesivir (Gilead), a small molecule that is believed to interfere with viral replication; and Regeneron’s Ebola triple monoclonal antibody cocktail, which also targets the surface protein.
Through Oct. 2, 43 Ebola patients have received one of the four drugs: 19 are cured and have been discharged, 12 have died, and 12 are still in recovery. Dr. Higgs cautioned against reading anything into those numbers, however, since no randomization was involved.
Although compassionate use is the primary aim of MEURI, it may be lead to some scientific benefit. The most that can be hoped for is to glean some insight into the safety of the interventions, although even that information is limited. “A colleague and I were talking about this the other day. After the first X number of patients, they were all still alive. What can you say from that? I think what you can say is that it didn’t kill them,” Dr. Higgs said.
Still, researchers are collecting laboratory data from patients to monitor their responses. “With Remdesivir we’re closely examining their transaminase, for example, so I think it will be helpful,” said Dr. Higgs.
SOURCE: Higgs E et al. IDWeek 2018.
SAN FRANCISCO – For the first time, the World Health Organization’s Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI) protocol is being field tested in the Democratic Republic of Congo (DRC), where four different therapeutics are being delivered to Ebola patients. MEURI was created after controversy surrounding the 2014-2016 Ebola outbreak in West Africa, which ultimately led to the decision to integrate research into outbreak responses.
MEURI is broadly designed for pathogens that have no proven intervention, on the premise that in a disease with high mortality, it can be ethically appropriate to provide experimental therapies during a response, as long as a set of criteria are met that ensure patient autonomy and give patients a reasonable opportunity to improve their condition.
The original plan was to employ experimental drugs under the MEURI umbrella during an Ebola outbreak in the DRC that began in May, but an effective response contained it so quickly that the program was canceled. However, when another Ebola outbreak occurred on Aug. 27, the material was still in the country and ready to be deployed. “That made it very easy to begin using them,” said Elizabeth Higgs, MD, global health science advisor for the division of clinical research at the National Institute for Allergy and Infectious Disease.
Dr. Higgs spoke about the developments during a late-breakers session at IDWeek 2018, an annual scientific meeting on infectious disease.
The therapies under consideration are Zmapp (Leaf Biopharmaceutical), which includes three chimeric monoclonal antibodies that target the main surface protein of the Ebola virus; mAb114 (NIAID), which was isolated in 1995 from a human survivor of Ebola and binds to the core of the Ebola surface protein; Remdesivir (Gilead), a small molecule that is believed to interfere with viral replication; and Regeneron’s Ebola triple monoclonal antibody cocktail, which also targets the surface protein.
Through Oct. 2, 43 Ebola patients have received one of the four drugs: 19 are cured and have been discharged, 12 have died, and 12 are still in recovery. Dr. Higgs cautioned against reading anything into those numbers, however, since no randomization was involved.
Although compassionate use is the primary aim of MEURI, it may be lead to some scientific benefit. The most that can be hoped for is to glean some insight into the safety of the interventions, although even that information is limited. “A colleague and I were talking about this the other day. After the first X number of patients, they were all still alive. What can you say from that? I think what you can say is that it didn’t kill them,” Dr. Higgs said.
Still, researchers are collecting laboratory data from patients to monitor their responses. “With Remdesivir we’re closely examining their transaminase, for example, so I think it will be helpful,” said Dr. Higgs.
SOURCE: Higgs E et al. IDWeek 2018.
REPORTING FROM ID WEEK 2018
Key clinical point:
Major finding: Since Oct. 2, 43 Ebola patients have received one of the four drugs: 19 were cured; 12 died, and 12 are in recovery.
Study details: Therapies are Zmapp (Leaf Biopharmaceutical), mAb114 (NIAID), Remdesivir (Gilead), and Regeneron’s Ebola triple monoclonal antibody cocktail.
Disclosures: Dr. Higgs reported no conflicts of interest.
Source: Higgs E et al. ID Week 2018.
Half of outpatient antibiotics prescribed with no infectious disease code
based on a review of more than half a million outpatient prescriptions to more than a quarter million patients at 514 clinics around Chicago.
The researchers looked to see if prescriptions had an ICD-10 code that indicated an antibiotic; they were liberal in their approach, considering over 21,000 codes to at least possibly signal the need for an antibiotic.
Almost half the time, there was nothing in the codes related to bacterial infection: 29% of scripts were written in connection with codes for high blood pressure, annual visits, and other noninfectious disorders; 17% of prescriptions were written with no diagnosis code at all.
The study is likely the largest to date to look at outpatient antibiotic prescribing patterns in the United States, and the findings are worrisome. “Nearly half the time, clinicians have either a bad reason for prescribing antibiotics, or don’t provide a reason at all. When you consider about 80% of antibiotics are prescribed on an outpatient basis, that’s a concern,” lead investigator Jeffrey A. Linder, MD, MPH, chief of the division of general internal medicine and geriatrics at Northwestern University, Chicago, said in a written statement.
“At busy clinics, sadly, the most efficient thing to do is just call in an antibiotic prescription. We need to dig into the data more, but we believe there is a lot of antibiotic prescribing for colds, the flu, and non-specific symptoms such as just not feeling well,” he said.
With all the concern in recent years about overuse, it’s hard to imagine that prescribers are still being free and easy with antibiotics, and Dr. Linder’s study will certainly have its skeptics.
Sloppy record keeping could be one explanation for the findings. A patient could really have needed an antibiotic, but it just wasn’t captured in coding. There are also valid reasons for prescribing antibiotics over the phone, such as acne and recurrent UTIs.
Dr. Linder, however, thinks it’s more than that. He explained his study, its implications, and the next steps in an interview at ID Week, an annual scientific meeting on infectious diseases.
The 2,413 prescribers in the study included physicians, surgeons, residents, fellows, nurse practitioners, and physician assistants in general and specialty practices. Patients were a mean of 43 years old: 60% were women and 75% were white. The most common antibiotic classes were penicillins, macrolides, and cephalosporins. Prescriptions were written from November 2015 through October 2017.
The work was funded by the Agency for Healthcare Research and Quality. Dr. Linder did not have any disclosures.
SOURCE: Linder JA et al. ID Week 2018 abstract 1632.
based on a review of more than half a million outpatient prescriptions to more than a quarter million patients at 514 clinics around Chicago.
The researchers looked to see if prescriptions had an ICD-10 code that indicated an antibiotic; they were liberal in their approach, considering over 21,000 codes to at least possibly signal the need for an antibiotic.
Almost half the time, there was nothing in the codes related to bacterial infection: 29% of scripts were written in connection with codes for high blood pressure, annual visits, and other noninfectious disorders; 17% of prescriptions were written with no diagnosis code at all.
The study is likely the largest to date to look at outpatient antibiotic prescribing patterns in the United States, and the findings are worrisome. “Nearly half the time, clinicians have either a bad reason for prescribing antibiotics, or don’t provide a reason at all. When you consider about 80% of antibiotics are prescribed on an outpatient basis, that’s a concern,” lead investigator Jeffrey A. Linder, MD, MPH, chief of the division of general internal medicine and geriatrics at Northwestern University, Chicago, said in a written statement.
“At busy clinics, sadly, the most efficient thing to do is just call in an antibiotic prescription. We need to dig into the data more, but we believe there is a lot of antibiotic prescribing for colds, the flu, and non-specific symptoms such as just not feeling well,” he said.
With all the concern in recent years about overuse, it’s hard to imagine that prescribers are still being free and easy with antibiotics, and Dr. Linder’s study will certainly have its skeptics.
Sloppy record keeping could be one explanation for the findings. A patient could really have needed an antibiotic, but it just wasn’t captured in coding. There are also valid reasons for prescribing antibiotics over the phone, such as acne and recurrent UTIs.
Dr. Linder, however, thinks it’s more than that. He explained his study, its implications, and the next steps in an interview at ID Week, an annual scientific meeting on infectious diseases.
The 2,413 prescribers in the study included physicians, surgeons, residents, fellows, nurse practitioners, and physician assistants in general and specialty practices. Patients were a mean of 43 years old: 60% were women and 75% were white. The most common antibiotic classes were penicillins, macrolides, and cephalosporins. Prescriptions were written from November 2015 through October 2017.
The work was funded by the Agency for Healthcare Research and Quality. Dr. Linder did not have any disclosures.
SOURCE: Linder JA et al. ID Week 2018 abstract 1632.
based on a review of more than half a million outpatient prescriptions to more than a quarter million patients at 514 clinics around Chicago.
The researchers looked to see if prescriptions had an ICD-10 code that indicated an antibiotic; they were liberal in their approach, considering over 21,000 codes to at least possibly signal the need for an antibiotic.
Almost half the time, there was nothing in the codes related to bacterial infection: 29% of scripts were written in connection with codes for high blood pressure, annual visits, and other noninfectious disorders; 17% of prescriptions were written with no diagnosis code at all.
The study is likely the largest to date to look at outpatient antibiotic prescribing patterns in the United States, and the findings are worrisome. “Nearly half the time, clinicians have either a bad reason for prescribing antibiotics, or don’t provide a reason at all. When you consider about 80% of antibiotics are prescribed on an outpatient basis, that’s a concern,” lead investigator Jeffrey A. Linder, MD, MPH, chief of the division of general internal medicine and geriatrics at Northwestern University, Chicago, said in a written statement.
“At busy clinics, sadly, the most efficient thing to do is just call in an antibiotic prescription. We need to dig into the data more, but we believe there is a lot of antibiotic prescribing for colds, the flu, and non-specific symptoms such as just not feeling well,” he said.
With all the concern in recent years about overuse, it’s hard to imagine that prescribers are still being free and easy with antibiotics, and Dr. Linder’s study will certainly have its skeptics.
Sloppy record keeping could be one explanation for the findings. A patient could really have needed an antibiotic, but it just wasn’t captured in coding. There are also valid reasons for prescribing antibiotics over the phone, such as acne and recurrent UTIs.
Dr. Linder, however, thinks it’s more than that. He explained his study, its implications, and the next steps in an interview at ID Week, an annual scientific meeting on infectious diseases.
The 2,413 prescribers in the study included physicians, surgeons, residents, fellows, nurse practitioners, and physician assistants in general and specialty practices. Patients were a mean of 43 years old: 60% were women and 75% were white. The most common antibiotic classes were penicillins, macrolides, and cephalosporins. Prescriptions were written from November 2015 through October 2017.
The work was funded by the Agency for Healthcare Research and Quality. Dr. Linder did not have any disclosures.
SOURCE: Linder JA et al. ID Week 2018 abstract 1632.
REPORTING FROM ID WEEK 2018
In utero efavirenz, dolutegravir exposure linked to childhood neurologic problems
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
REPORTING FROM ID WEEK 2018
It’s time for universal CMV screening at birth
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
REPORTING FROM IDWEEK 2018
Opiate use tied to hepatitis C risk in youth
SAN FRANCISCO – A new study indicates young adults with opioid use disorder are seldom screened for hepatitis C virus infections; yet 11% of the subjects with opioid use disorder who were tested had been exposed to hepatitis C, and 6.8% had evidence of chronic hepatitis C infection.
Overall, 2.5% (6,812 subjects) of all subjects received hepatitis C testing and 122 (1.8%) tested positive. Based on health records, 23,345 had an ICD-9 code for any illicit drug use and 8.9% of those (2,090) were tested for HCV infection. Of the 933 subjects with an ICD-9 code for opioid use disorder, 35% were tested for HCV.
The results suggest that a group at significant risk of hepatitis C – those with opioid use disorder – is being overlooked in public health efforts to control the disease.
Clinicians may presume, “Oh, you just take opioids orally, you don’t inject drugs,” but oral opiate users can progress to intravenous drug use, Donna Futterman, MD, director of clinical pediatrics, Montefiore Medical Center, and professor of clinical pediatrics at Albert Einstein College of Medicine, both in New York, said during the press conference at the annual scientific meeting on infectious diseases.
Guidelines call for testing for hepatitis C only in individuals with known injected drug use, among other risk factors, but the research suggests that this significantly underestimates the population of teenagers and young adults who are at risk. Many who take opiates go on to use injectable drugs.
Another surprise finding in the study was that only 10.6% of those tested for hepatitis C had also been screened for human immunodeficiency virus (HIV).
The reasons for the low frequency of screening are likely complex, including lack of time, discomfort between the physician and patient, and concerns over privacy and stigma, according to Dr. Epstein, who emphasized the importance of communication to overcome such barriers.
“As a pediatrician, I try to be as open as possible with patients and let them know that anything they tell me is confidential. I start out discussing less private issues, things that are easier to talk about,” Dr. Epstein said.
But the results of the study also suggest that preconceived notions may be holding clinicians back from testing. “How can you test for hepatitis C and not think HIV?” Dr. Futterman said. “What is that differentiator in providers’ heads that makes them focus on one thing and not the other?”
SAN FRANCISCO – A new study indicates young adults with opioid use disorder are seldom screened for hepatitis C virus infections; yet 11% of the subjects with opioid use disorder who were tested had been exposed to hepatitis C, and 6.8% had evidence of chronic hepatitis C infection.
Overall, 2.5% (6,812 subjects) of all subjects received hepatitis C testing and 122 (1.8%) tested positive. Based on health records, 23,345 had an ICD-9 code for any illicit drug use and 8.9% of those (2,090) were tested for HCV infection. Of the 933 subjects with an ICD-9 code for opioid use disorder, 35% were tested for HCV.
The results suggest that a group at significant risk of hepatitis C – those with opioid use disorder – is being overlooked in public health efforts to control the disease.
Clinicians may presume, “Oh, you just take opioids orally, you don’t inject drugs,” but oral opiate users can progress to intravenous drug use, Donna Futterman, MD, director of clinical pediatrics, Montefiore Medical Center, and professor of clinical pediatrics at Albert Einstein College of Medicine, both in New York, said during the press conference at the annual scientific meeting on infectious diseases.
Guidelines call for testing for hepatitis C only in individuals with known injected drug use, among other risk factors, but the research suggests that this significantly underestimates the population of teenagers and young adults who are at risk. Many who take opiates go on to use injectable drugs.
Another surprise finding in the study was that only 10.6% of those tested for hepatitis C had also been screened for human immunodeficiency virus (HIV).
The reasons for the low frequency of screening are likely complex, including lack of time, discomfort between the physician and patient, and concerns over privacy and stigma, according to Dr. Epstein, who emphasized the importance of communication to overcome such barriers.
“As a pediatrician, I try to be as open as possible with patients and let them know that anything they tell me is confidential. I start out discussing less private issues, things that are easier to talk about,” Dr. Epstein said.
But the results of the study also suggest that preconceived notions may be holding clinicians back from testing. “How can you test for hepatitis C and not think HIV?” Dr. Futterman said. “What is that differentiator in providers’ heads that makes them focus on one thing and not the other?”
SAN FRANCISCO – A new study indicates young adults with opioid use disorder are seldom screened for hepatitis C virus infections; yet 11% of the subjects with opioid use disorder who were tested had been exposed to hepatitis C, and 6.8% had evidence of chronic hepatitis C infection.
Overall, 2.5% (6,812 subjects) of all subjects received hepatitis C testing and 122 (1.8%) tested positive. Based on health records, 23,345 had an ICD-9 code for any illicit drug use and 8.9% of those (2,090) were tested for HCV infection. Of the 933 subjects with an ICD-9 code for opioid use disorder, 35% were tested for HCV.
The results suggest that a group at significant risk of hepatitis C – those with opioid use disorder – is being overlooked in public health efforts to control the disease.
Clinicians may presume, “Oh, you just take opioids orally, you don’t inject drugs,” but oral opiate users can progress to intravenous drug use, Donna Futterman, MD, director of clinical pediatrics, Montefiore Medical Center, and professor of clinical pediatrics at Albert Einstein College of Medicine, both in New York, said during the press conference at the annual scientific meeting on infectious diseases.
Guidelines call for testing for hepatitis C only in individuals with known injected drug use, among other risk factors, but the research suggests that this significantly underestimates the population of teenagers and young adults who are at risk. Many who take opiates go on to use injectable drugs.
Another surprise finding in the study was that only 10.6% of those tested for hepatitis C had also been screened for human immunodeficiency virus (HIV).
The reasons for the low frequency of screening are likely complex, including lack of time, discomfort between the physician and patient, and concerns over privacy and stigma, according to Dr. Epstein, who emphasized the importance of communication to overcome such barriers.
“As a pediatrician, I try to be as open as possible with patients and let them know that anything they tell me is confidential. I start out discussing less private issues, things that are easier to talk about,” Dr. Epstein said.
But the results of the study also suggest that preconceived notions may be holding clinicians back from testing. “How can you test for hepatitis C and not think HIV?” Dr. Futterman said. “What is that differentiator in providers’ heads that makes them focus on one thing and not the other?”
REPORTING FROM ID WEEK 2018
Key clinical point: By focusing solely on injectable drug users, clinicians may miss many others who are at risk for hepatitis C infection.
Major finding: Among those with opiate use disorder, 11% tested positive for hepatitis C.
Study details: Survey of 269,124 teenagers and young adults visiting U.S. Federally Qualified Health Centers.
Disclosures: Dr. Epstein and Dr. Futterman have reported no conflicts of interest.