User login
Encourage influenza vaccination in pregnant women
They are at greater risk for more severe illness, and influenza can lead to adverse outcomes in infants. The good news is that recent studies have shown that flu vaccines are safe and effective in pregnant women.
The bad news is that many women are hesitant to be vaccinated out of concerns over safety, in a trend that reflects broader societal worries over vaccination, said Dr. Chu, of the University of Washington, Seattle. In a video interview at an annual scientific meeting on infectious diseases, Dr. Chu advised steps to ensure that pregnant women are aware of the safety and efficacy of flu vaccines, and the benefits to the infant who acquires immunity through the mother. It’s also a good idea to have vaccine on hand to be able to offer it immediately during an office visit.
They are at greater risk for more severe illness, and influenza can lead to adverse outcomes in infants. The good news is that recent studies have shown that flu vaccines are safe and effective in pregnant women.
The bad news is that many women are hesitant to be vaccinated out of concerns over safety, in a trend that reflects broader societal worries over vaccination, said Dr. Chu, of the University of Washington, Seattle. In a video interview at an annual scientific meeting on infectious diseases, Dr. Chu advised steps to ensure that pregnant women are aware of the safety and efficacy of flu vaccines, and the benefits to the infant who acquires immunity through the mother. It’s also a good idea to have vaccine on hand to be able to offer it immediately during an office visit.
They are at greater risk for more severe illness, and influenza can lead to adverse outcomes in infants. The good news is that recent studies have shown that flu vaccines are safe and effective in pregnant women.
The bad news is that many women are hesitant to be vaccinated out of concerns over safety, in a trend that reflects broader societal worries over vaccination, said Dr. Chu, of the University of Washington, Seattle. In a video interview at an annual scientific meeting on infectious diseases, Dr. Chu advised steps to ensure that pregnant women are aware of the safety and efficacy of flu vaccines, and the benefits to the infant who acquires immunity through the mother. It’s also a good idea to have vaccine on hand to be able to offer it immediately during an office visit.
REPORTING FROM ID WEEK 2018
Flu outbreaks may be more intense in small cities
Influenza outbreaks in the United States tend to be concentrated and intense in small cities and more evenly spread throughout the season in large cities, results of a recent study show.
Swings in humidity further intensified the influenza spikes in small cities, but didn’t seem to have as much of an effect in large cities, the results suggest.
These findings help explain differences in influenza transmission patterns between cities that have similar climates and virus epidemiology, according to researcher Benjamin D. Dalziel, PhD, of the departments of integrative biology and mathematics at Oregon State University in Corvallis.
“City size and structure can play a role in determining how other factors such as climate affect and influence transmission,” Dr. Dalziel said in a press conference.
“Our results show how metropolises play a disproportionately important role in this process, as epidemic foci, and as potential sentinel hubs, where epidemiological observatories could integrate local strain dynamics to predict larger-scale patterns. As the growth and form of cities affect their function as climate-driven incubators of infectious disease, it may be possible to design smarter cities that better control epidemics in the face of accelerating global change,” the researchers wrote in their study.
Dr. Dalziel and his coauthors analyzed the weekly incidence of influenza-like illness across 603 U.S. ZIP codes using data obtained from medical claims from 2002 to 2008. They used epidemic intensity as a summary statistic to compare cities. By this variable, low epidemic intensity indicated a diffuse spread evenly across weeks of the flu season, whereas high epidemic intensity indicated intensively focused outbreaks on particular weeks.
In small cities, epidemics were more intensely focused on shorter periods at the peak of flu season, they found. In large cities, incidence was more diffuse, according to results published in Science.
Patterns of where people live and work in a city may account for the more diffuse and prolonged outbreaks seen in large cities, the authors wrote. Large cities have organized population movement patterns and crowding. In more highly established work locations, for example, the population density is pronounced during the day.
“We found the structure makes a difference for how the flu spreads at different times of year,” Dr. Dalziel said of the study, which used U.S. Census data to evaluate spatial population distributions. “In large cities with more highly organized patterns, conditions play a relatively smaller role in flu transmission.”
Humidity’s lower impact on outbreaks in large cities might also be explained by population effects: “If an infected person is sitting beside you, it matters less what the specific humidity is,” Dr. Dalziel said, adding that the proximity helps the virus find hosts even when climatic conditions are not at their most favorable.
The study findings may have implications for health care resources in small cities, which could be strained by intense outbreaks, said coinvestigator Cecile Viboud, PhD, of the Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, Bethesda, Md.
Intense outbreaks could overload the health care system, making it challenging to respond, especially around the peak of the epidemic. Pressure on the health care system may be less intense in cities such as Miami or New York, where flu epidemics are more diffuse and spread out during the year, she said.
Variations in vaccination coverage were not associated with variations in epidemic intensity at the state level. However, the data period that was analyzed ended in 2008, a time when flu vaccination rates were much lower than they are today, according to Dr. Viboud.
“It would be important to revisit the effect of city structure and humidity on flu transmission in a high vaccination regime in more recent years, especially if there is a lot of interest in developing broadly cross-protective flu vaccines, which might become available in the market in the future,” she said.
The researchers declared no competing interests related to their research, which was supported by a grant from the Bill & Melinda Gates Foundation, the RAPIDD program of the Science and Technology Directorate Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
SOURCE: Dalziel BD et al. Science. 2018 Oct 5;362(6410):75-9.
Public health policy makers may need to switch up their thinking about infection control during influenza outbreaks. Instead of targeting the population at large, it may make sense to focus on specific small towns or metropolitan areas for control.
Summary statistics, such as epidemic intensity, help to identify which places require more surge capacity to deal with peak health care demand. They also help to guide locations for active influenza surveillance where long transmission chains of influenza occur, and where new genetic variants of the influenza virus can be detected.
The findings of this study could foster the development of more accurate short-term, small-scale forecasts of the expected health care demand in a season. Most important, they could guide long-term projections that reveal how the shifting demography, growth of cities, and the changing climate alter infection dynamics and required control efforts.
Prof. Jacco Wallinga is with the Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, the Netherlands, and the Department of Biomedical Data Sciences, Leiden (the Netherlands) University Medical Center. These comments appeared in his editorial in Science (2018 Oct 5;362[6410]:29-30).
Public health policy makers may need to switch up their thinking about infection control during influenza outbreaks. Instead of targeting the population at large, it may make sense to focus on specific small towns or metropolitan areas for control.
Summary statistics, such as epidemic intensity, help to identify which places require more surge capacity to deal with peak health care demand. They also help to guide locations for active influenza surveillance where long transmission chains of influenza occur, and where new genetic variants of the influenza virus can be detected.
The findings of this study could foster the development of more accurate short-term, small-scale forecasts of the expected health care demand in a season. Most important, they could guide long-term projections that reveal how the shifting demography, growth of cities, and the changing climate alter infection dynamics and required control efforts.
Prof. Jacco Wallinga is with the Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, the Netherlands, and the Department of Biomedical Data Sciences, Leiden (the Netherlands) University Medical Center. These comments appeared in his editorial in Science (2018 Oct 5;362[6410]:29-30).
Public health policy makers may need to switch up their thinking about infection control during influenza outbreaks. Instead of targeting the population at large, it may make sense to focus on specific small towns or metropolitan areas for control.
Summary statistics, such as epidemic intensity, help to identify which places require more surge capacity to deal with peak health care demand. They also help to guide locations for active influenza surveillance where long transmission chains of influenza occur, and where new genetic variants of the influenza virus can be detected.
The findings of this study could foster the development of more accurate short-term, small-scale forecasts of the expected health care demand in a season. Most important, they could guide long-term projections that reveal how the shifting demography, growth of cities, and the changing climate alter infection dynamics and required control efforts.
Prof. Jacco Wallinga is with the Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, the Netherlands, and the Department of Biomedical Data Sciences, Leiden (the Netherlands) University Medical Center. These comments appeared in his editorial in Science (2018 Oct 5;362[6410]:29-30).
Influenza outbreaks in the United States tend to be concentrated and intense in small cities and more evenly spread throughout the season in large cities, results of a recent study show.
Swings in humidity further intensified the influenza spikes in small cities, but didn’t seem to have as much of an effect in large cities, the results suggest.
These findings help explain differences in influenza transmission patterns between cities that have similar climates and virus epidemiology, according to researcher Benjamin D. Dalziel, PhD, of the departments of integrative biology and mathematics at Oregon State University in Corvallis.
“City size and structure can play a role in determining how other factors such as climate affect and influence transmission,” Dr. Dalziel said in a press conference.
“Our results show how metropolises play a disproportionately important role in this process, as epidemic foci, and as potential sentinel hubs, where epidemiological observatories could integrate local strain dynamics to predict larger-scale patterns. As the growth and form of cities affect their function as climate-driven incubators of infectious disease, it may be possible to design smarter cities that better control epidemics in the face of accelerating global change,” the researchers wrote in their study.
Dr. Dalziel and his coauthors analyzed the weekly incidence of influenza-like illness across 603 U.S. ZIP codes using data obtained from medical claims from 2002 to 2008. They used epidemic intensity as a summary statistic to compare cities. By this variable, low epidemic intensity indicated a diffuse spread evenly across weeks of the flu season, whereas high epidemic intensity indicated intensively focused outbreaks on particular weeks.
In small cities, epidemics were more intensely focused on shorter periods at the peak of flu season, they found. In large cities, incidence was more diffuse, according to results published in Science.
Patterns of where people live and work in a city may account for the more diffuse and prolonged outbreaks seen in large cities, the authors wrote. Large cities have organized population movement patterns and crowding. In more highly established work locations, for example, the population density is pronounced during the day.
“We found the structure makes a difference for how the flu spreads at different times of year,” Dr. Dalziel said of the study, which used U.S. Census data to evaluate spatial population distributions. “In large cities with more highly organized patterns, conditions play a relatively smaller role in flu transmission.”
Humidity’s lower impact on outbreaks in large cities might also be explained by population effects: “If an infected person is sitting beside you, it matters less what the specific humidity is,” Dr. Dalziel said, adding that the proximity helps the virus find hosts even when climatic conditions are not at their most favorable.
The study findings may have implications for health care resources in small cities, which could be strained by intense outbreaks, said coinvestigator Cecile Viboud, PhD, of the Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, Bethesda, Md.
Intense outbreaks could overload the health care system, making it challenging to respond, especially around the peak of the epidemic. Pressure on the health care system may be less intense in cities such as Miami or New York, where flu epidemics are more diffuse and spread out during the year, she said.
Variations in vaccination coverage were not associated with variations in epidemic intensity at the state level. However, the data period that was analyzed ended in 2008, a time when flu vaccination rates were much lower than they are today, according to Dr. Viboud.
“It would be important to revisit the effect of city structure and humidity on flu transmission in a high vaccination regime in more recent years, especially if there is a lot of interest in developing broadly cross-protective flu vaccines, which might become available in the market in the future,” she said.
The researchers declared no competing interests related to their research, which was supported by a grant from the Bill & Melinda Gates Foundation, the RAPIDD program of the Science and Technology Directorate Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
SOURCE: Dalziel BD et al. Science. 2018 Oct 5;362(6410):75-9.
Influenza outbreaks in the United States tend to be concentrated and intense in small cities and more evenly spread throughout the season in large cities, results of a recent study show.
Swings in humidity further intensified the influenza spikes in small cities, but didn’t seem to have as much of an effect in large cities, the results suggest.
These findings help explain differences in influenza transmission patterns between cities that have similar climates and virus epidemiology, according to researcher Benjamin D. Dalziel, PhD, of the departments of integrative biology and mathematics at Oregon State University in Corvallis.
“City size and structure can play a role in determining how other factors such as climate affect and influence transmission,” Dr. Dalziel said in a press conference.
“Our results show how metropolises play a disproportionately important role in this process, as epidemic foci, and as potential sentinel hubs, where epidemiological observatories could integrate local strain dynamics to predict larger-scale patterns. As the growth and form of cities affect their function as climate-driven incubators of infectious disease, it may be possible to design smarter cities that better control epidemics in the face of accelerating global change,” the researchers wrote in their study.
Dr. Dalziel and his coauthors analyzed the weekly incidence of influenza-like illness across 603 U.S. ZIP codes using data obtained from medical claims from 2002 to 2008. They used epidemic intensity as a summary statistic to compare cities. By this variable, low epidemic intensity indicated a diffuse spread evenly across weeks of the flu season, whereas high epidemic intensity indicated intensively focused outbreaks on particular weeks.
In small cities, epidemics were more intensely focused on shorter periods at the peak of flu season, they found. In large cities, incidence was more diffuse, according to results published in Science.
Patterns of where people live and work in a city may account for the more diffuse and prolonged outbreaks seen in large cities, the authors wrote. Large cities have organized population movement patterns and crowding. In more highly established work locations, for example, the population density is pronounced during the day.
“We found the structure makes a difference for how the flu spreads at different times of year,” Dr. Dalziel said of the study, which used U.S. Census data to evaluate spatial population distributions. “In large cities with more highly organized patterns, conditions play a relatively smaller role in flu transmission.”
Humidity’s lower impact on outbreaks in large cities might also be explained by population effects: “If an infected person is sitting beside you, it matters less what the specific humidity is,” Dr. Dalziel said, adding that the proximity helps the virus find hosts even when climatic conditions are not at their most favorable.
The study findings may have implications for health care resources in small cities, which could be strained by intense outbreaks, said coinvestigator Cecile Viboud, PhD, of the Division of International Epidemiology and Population Studies, Fogarty International Center, National Institutes of Health, Bethesda, Md.
Intense outbreaks could overload the health care system, making it challenging to respond, especially around the peak of the epidemic. Pressure on the health care system may be less intense in cities such as Miami or New York, where flu epidemics are more diffuse and spread out during the year, she said.
Variations in vaccination coverage were not associated with variations in epidemic intensity at the state level. However, the data period that was analyzed ended in 2008, a time when flu vaccination rates were much lower than they are today, according to Dr. Viboud.
“It would be important to revisit the effect of city structure and humidity on flu transmission in a high vaccination regime in more recent years, especially if there is a lot of interest in developing broadly cross-protective flu vaccines, which might become available in the market in the future,” she said.
The researchers declared no competing interests related to their research, which was supported by a grant from the Bill & Melinda Gates Foundation, the RAPIDD program of the Science and Technology Directorate Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
SOURCE: Dalziel BD et al. Science. 2018 Oct 5;362(6410):75-9.
FROM SCIENCE
Key clinical point: The intensity of influenza epidemics in U.S. cities varies according to population.
Major finding: Smaller cities had more intense outbreaks concentrated around the peak of flu season, while larger cities had cases spread throughout the season.
Study details: Analysis of weekly influenza-like illness incidence for 603 U.S. ZIP codes in medical claims data from 2002 to 2008.
Disclosures: The authors declared no competing interests. Funding came from the Bill & Melinda Gates Foundation, the Science and Technology Directorate Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Source: Dalziel BD et al. Science. 2018 Oct 5;362(6410):75-9.
FDA approves omadacycline for pneumonia and skin infections
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
FDA approves Arikayce for MAC lung diseases
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
that has been caused by members of the Mycobacterium avium complex and is refractory to other treatments.
In a randomized, controlled trial, patients with refractory M. avium complex infections were assigned to receive either Arikayce plus a multidrug antibacterial regimen or just the antibacterial regimen. By 6 months, sputum cultures for 29% of those treated with the combination had shown no mycobacterial growth for 3 consecutive months, whereas this was only true for the cultures for 9% of patients on the multidrug antibacterial regimen alone.
The Arikayce prescribing information includes a boxed warning regarding the increased risk of respiratory conditions, including hypersensitivity pneumonitis, bronchospasm, exacerbation of underlying lung disease, and hemoptysis, some of which have proven serious enough to lead to hospitalization. Other side effects include dysphonia, cough, musculoskeletal pain, nausea, and fatigue.
According to the press announcement from the FDA, this is the first approval under the Limited Population Pathway for Antibacterial and Antifungal Drugs, which was set up by Congress “to advance development and approval of antibacterial and antifungal drugs to treat serious or life-threatening infections in a limited population of patients with unmet need.” It does so by allowing a more streamlined clinical development program that may involve smaller, shorter, or fewer clinical trials.
More information can be found in the full press announcement.
Antibiotics trigger proteolytic activity that leads to chronic colitis
Antibiotics are associated with increased large intestinal proteolytic activity and gut barrier disruption, thereby raising the risk of chronic colitis in susceptible individuals, a recent study found.
Although the association between antibiotics and chronic colitis has been previously described, this is the first study to demonstrate the causative role of high proteolytic activity, reported lead author Hongsup Yoon, PhD, chair of nutrition and immunology at Technische Universität München in Freising-Weihenstephan, Germany, and colleagues. The team’s experiments support development of antiproteolytic strategies in susceptible humans.
“In the context of IBD, several clinical studies have already revealed that early and frequent antibiotic therapies, especially metronidazole or fluoroquinolone treatments, are associated with increased risk for Crohn’s disease,” the authors wrote in Cellular and Molecular Gastroenterology and Hepatology. “However, the causal role of antibiotic therapies in the disease development and the mechanisms underlying this [potentially] serious long-term adverse effect of antibiotics on the intestinal immune homeostasis remain unknown.”
Previous studies have shown that antibiotic therapy often causes high luminal proteolytic activity in the large intestine, likely because of the elimination of antiproteolytic bacteria that normally control pancreatic protease levels. Other studies have shown that exposing murine colonic mucosa to fecal supernatants with high proteolytic activity increases gut barrier permeability, which triggers chronic inflammation via translocation of luminal antigens.
“In view of these data,” the authors wrote, “we hypothesized that the antibiotic-increased proteolytic activity in the large intestine is a relevant risk factor for the development of colitis in susceptible organisms.”
The first component of the study used transwell experiments to evaluate the impact of high proteolytic activity on gut barrier integrity. High proteolytic activity was induced by several antibiotics, including fluoroquinolones with or without an imidazole (ciprofloxacin and levofloxacin plus or minus metronidazole), a beta-lactam (amoxicillin + clavulanate), cephalosporins with or without a macrolide (azithromycin and ceftriaxone plus or minus azithromycin), and a rifamycin (rifaximin).
“All tested antibiotic classes mediated a major proteolytic activity increase in some patients but not in others,” the authors wrote, “demonstrating individual-specific vulnerability of the intestinal microbiota toward antibiotic therapies, which is likely caused by the high interindividual variability of human microbial ecosystems.”
One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy, and several had an increase greater than 900%. Analysis indicated that proteolytic activity was caused by pancreatic proteases such as chymotrypsin and trypsin.
Subsequent cell line testing showed that stool supernatants with high proteolytic activity damaged the epithelial barrier, but samples with low proteolytic activity did not. Of note, the negative impact of high proteolytic activity on epithelial cells could be mitigated by incubating stool supernatants with a serine protease inhibitor.
In analogous experiments, mice were given a combination of vancomycin and metronidazole (V/M). In contrast with the various proteolytic activity levels observed in humans, all mice had high proteolytic activity levels following treatment, suggesting that V/M eliminated almost all antiproteolytic bacteria.
The loss of antiproteolytic bacteria was clarified by cecal microbiota transplantation tests. Transplants from untreated mice were capable of normalizing proteolytic activity levels in germ-free mice (which have high proteolytic activity levels), but transplants from V/M-treated mice were ineffective, suggesting a near-total loss of antiproteolytic bacteria. The identity of these antiproteolytic bacteria remains a mystery.
“Although our data are in line with published literature suggesting specific strains of the order Bacteroidales to play a role in the physiological inactivation of pancreatic proteases,” the authors wrote, “the identity of relevant antiproteolytic species/strains remains to be elucidated.”
The next part of the study involved wild-type and interleukin (IL)-10–/– mice, the latter of which serves as a model of human colitis. Both types of mice were given V/M with or without an oral serine protease inhibitor, a potential therapy intended to limit proteolytic activity and associated intestinal barrier damage.
Although both wild-type and IL-10–/– mice had increased intestinal permeability after V/M treatment, only IL-10–/– mice showed lasting inflammation. Of note, coadministration of an oral serine protease inhibitor with V/M protected against colitis in IL-10–/– mice.
The protective benefit of an oral serine protease inhibitor in IL-10–/– mice prompts the development of antiproteolytic strategies in humans. These would target “large intestinal proteolytic activity [e.g., oral administration of encapsulated serine protease inhibitors, commensal antiproteolytic bacteria, or genetically modified bacteria expressing protease inhibitors] to protect the large intestinal mucosa from adverse effects of antibiotic-induced or diarrhea-induced high proteolytic activity,” the authors wrote.
The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
SOURCE: Yoon H-S et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
Antibiotics are associated with increased large intestinal proteolytic activity and gut barrier disruption, thereby raising the risk of chronic colitis in susceptible individuals, a recent study found.
Although the association between antibiotics and chronic colitis has been previously described, this is the first study to demonstrate the causative role of high proteolytic activity, reported lead author Hongsup Yoon, PhD, chair of nutrition and immunology at Technische Universität München in Freising-Weihenstephan, Germany, and colleagues. The team’s experiments support development of antiproteolytic strategies in susceptible humans.
“In the context of IBD, several clinical studies have already revealed that early and frequent antibiotic therapies, especially metronidazole or fluoroquinolone treatments, are associated with increased risk for Crohn’s disease,” the authors wrote in Cellular and Molecular Gastroenterology and Hepatology. “However, the causal role of antibiotic therapies in the disease development and the mechanisms underlying this [potentially] serious long-term adverse effect of antibiotics on the intestinal immune homeostasis remain unknown.”
Previous studies have shown that antibiotic therapy often causes high luminal proteolytic activity in the large intestine, likely because of the elimination of antiproteolytic bacteria that normally control pancreatic protease levels. Other studies have shown that exposing murine colonic mucosa to fecal supernatants with high proteolytic activity increases gut barrier permeability, which triggers chronic inflammation via translocation of luminal antigens.
“In view of these data,” the authors wrote, “we hypothesized that the antibiotic-increased proteolytic activity in the large intestine is a relevant risk factor for the development of colitis in susceptible organisms.”
The first component of the study used transwell experiments to evaluate the impact of high proteolytic activity on gut barrier integrity. High proteolytic activity was induced by several antibiotics, including fluoroquinolones with or without an imidazole (ciprofloxacin and levofloxacin plus or minus metronidazole), a beta-lactam (amoxicillin + clavulanate), cephalosporins with or without a macrolide (azithromycin and ceftriaxone plus or minus azithromycin), and a rifamycin (rifaximin).
“All tested antibiotic classes mediated a major proteolytic activity increase in some patients but not in others,” the authors wrote, “demonstrating individual-specific vulnerability of the intestinal microbiota toward antibiotic therapies, which is likely caused by the high interindividual variability of human microbial ecosystems.”
One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy, and several had an increase greater than 900%. Analysis indicated that proteolytic activity was caused by pancreatic proteases such as chymotrypsin and trypsin.
Subsequent cell line testing showed that stool supernatants with high proteolytic activity damaged the epithelial barrier, but samples with low proteolytic activity did not. Of note, the negative impact of high proteolytic activity on epithelial cells could be mitigated by incubating stool supernatants with a serine protease inhibitor.
In analogous experiments, mice were given a combination of vancomycin and metronidazole (V/M). In contrast with the various proteolytic activity levels observed in humans, all mice had high proteolytic activity levels following treatment, suggesting that V/M eliminated almost all antiproteolytic bacteria.
The loss of antiproteolytic bacteria was clarified by cecal microbiota transplantation tests. Transplants from untreated mice were capable of normalizing proteolytic activity levels in germ-free mice (which have high proteolytic activity levels), but transplants from V/M-treated mice were ineffective, suggesting a near-total loss of antiproteolytic bacteria. The identity of these antiproteolytic bacteria remains a mystery.
“Although our data are in line with published literature suggesting specific strains of the order Bacteroidales to play a role in the physiological inactivation of pancreatic proteases,” the authors wrote, “the identity of relevant antiproteolytic species/strains remains to be elucidated.”
The next part of the study involved wild-type and interleukin (IL)-10–/– mice, the latter of which serves as a model of human colitis. Both types of mice were given V/M with or without an oral serine protease inhibitor, a potential therapy intended to limit proteolytic activity and associated intestinal barrier damage.
Although both wild-type and IL-10–/– mice had increased intestinal permeability after V/M treatment, only IL-10–/– mice showed lasting inflammation. Of note, coadministration of an oral serine protease inhibitor with V/M protected against colitis in IL-10–/– mice.
The protective benefit of an oral serine protease inhibitor in IL-10–/– mice prompts the development of antiproteolytic strategies in humans. These would target “large intestinal proteolytic activity [e.g., oral administration of encapsulated serine protease inhibitors, commensal antiproteolytic bacteria, or genetically modified bacteria expressing protease inhibitors] to protect the large intestinal mucosa from adverse effects of antibiotic-induced or diarrhea-induced high proteolytic activity,” the authors wrote.
The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
SOURCE: Yoon H-S et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
Antibiotics are associated with increased large intestinal proteolytic activity and gut barrier disruption, thereby raising the risk of chronic colitis in susceptible individuals, a recent study found.
Although the association between antibiotics and chronic colitis has been previously described, this is the first study to demonstrate the causative role of high proteolytic activity, reported lead author Hongsup Yoon, PhD, chair of nutrition and immunology at Technische Universität München in Freising-Weihenstephan, Germany, and colleagues. The team’s experiments support development of antiproteolytic strategies in susceptible humans.
“In the context of IBD, several clinical studies have already revealed that early and frequent antibiotic therapies, especially metronidazole or fluoroquinolone treatments, are associated with increased risk for Crohn’s disease,” the authors wrote in Cellular and Molecular Gastroenterology and Hepatology. “However, the causal role of antibiotic therapies in the disease development and the mechanisms underlying this [potentially] serious long-term adverse effect of antibiotics on the intestinal immune homeostasis remain unknown.”
Previous studies have shown that antibiotic therapy often causes high luminal proteolytic activity in the large intestine, likely because of the elimination of antiproteolytic bacteria that normally control pancreatic protease levels. Other studies have shown that exposing murine colonic mucosa to fecal supernatants with high proteolytic activity increases gut barrier permeability, which triggers chronic inflammation via translocation of luminal antigens.
“In view of these data,” the authors wrote, “we hypothesized that the antibiotic-increased proteolytic activity in the large intestine is a relevant risk factor for the development of colitis in susceptible organisms.”
The first component of the study used transwell experiments to evaluate the impact of high proteolytic activity on gut barrier integrity. High proteolytic activity was induced by several antibiotics, including fluoroquinolones with or without an imidazole (ciprofloxacin and levofloxacin plus or minus metronidazole), a beta-lactam (amoxicillin + clavulanate), cephalosporins with or without a macrolide (azithromycin and ceftriaxone plus or minus azithromycin), and a rifamycin (rifaximin).
“All tested antibiotic classes mediated a major proteolytic activity increase in some patients but not in others,” the authors wrote, “demonstrating individual-specific vulnerability of the intestinal microbiota toward antibiotic therapies, which is likely caused by the high interindividual variability of human microbial ecosystems.”
One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy, and several had an increase greater than 900%. Analysis indicated that proteolytic activity was caused by pancreatic proteases such as chymotrypsin and trypsin.
Subsequent cell line testing showed that stool supernatants with high proteolytic activity damaged the epithelial barrier, but samples with low proteolytic activity did not. Of note, the negative impact of high proteolytic activity on epithelial cells could be mitigated by incubating stool supernatants with a serine protease inhibitor.
In analogous experiments, mice were given a combination of vancomycin and metronidazole (V/M). In contrast with the various proteolytic activity levels observed in humans, all mice had high proteolytic activity levels following treatment, suggesting that V/M eliminated almost all antiproteolytic bacteria.
The loss of antiproteolytic bacteria was clarified by cecal microbiota transplantation tests. Transplants from untreated mice were capable of normalizing proteolytic activity levels in germ-free mice (which have high proteolytic activity levels), but transplants from V/M-treated mice were ineffective, suggesting a near-total loss of antiproteolytic bacteria. The identity of these antiproteolytic bacteria remains a mystery.
“Although our data are in line with published literature suggesting specific strains of the order Bacteroidales to play a role in the physiological inactivation of pancreatic proteases,” the authors wrote, “the identity of relevant antiproteolytic species/strains remains to be elucidated.”
The next part of the study involved wild-type and interleukin (IL)-10–/– mice, the latter of which serves as a model of human colitis. Both types of mice were given V/M with or without an oral serine protease inhibitor, a potential therapy intended to limit proteolytic activity and associated intestinal barrier damage.
Although both wild-type and IL-10–/– mice had increased intestinal permeability after V/M treatment, only IL-10–/– mice showed lasting inflammation. Of note, coadministration of an oral serine protease inhibitor with V/M protected against colitis in IL-10–/– mice.
The protective benefit of an oral serine protease inhibitor in IL-10–/– mice prompts the development of antiproteolytic strategies in humans. These would target “large intestinal proteolytic activity [e.g., oral administration of encapsulated serine protease inhibitors, commensal antiproteolytic bacteria, or genetically modified bacteria expressing protease inhibitors] to protect the large intestinal mucosa from adverse effects of antibiotic-induced or diarrhea-induced high proteolytic activity,” the authors wrote.
The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
SOURCE: Yoon H-S et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: In patients susceptible to inflammatory bowel disease, antibiotics cause increased proteolytic activity in the large intestine that disrupts the gut barrier, thereby increasing risk of chronic colitis.
Major finding: One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy.
Study details: A prospective study involving mice and humans treated with antibiotics.
Disclosures: The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
Source: Yoon H et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
Trichodysplasia Spinulosa in the Setting of Colon Cancer
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).
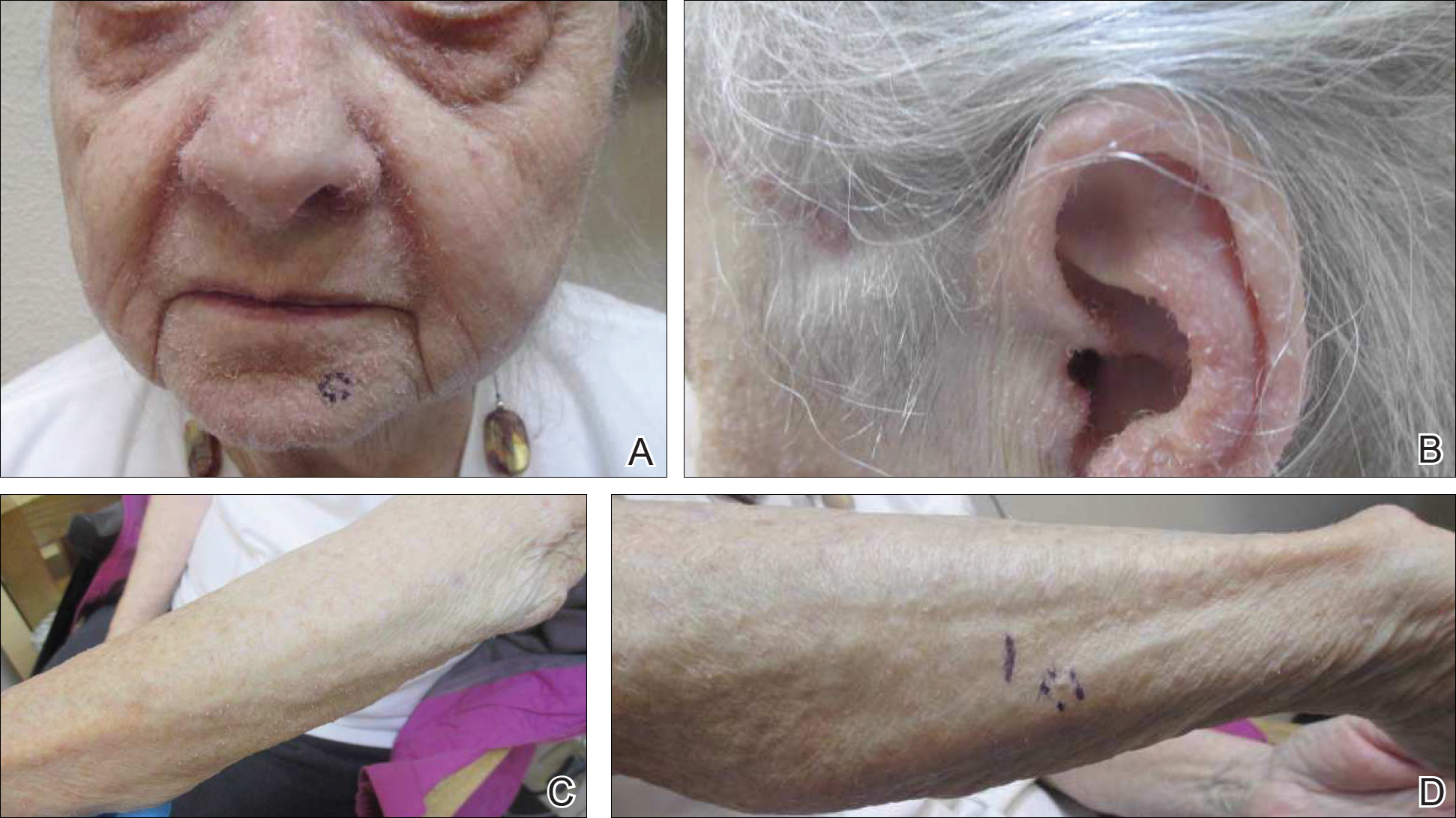
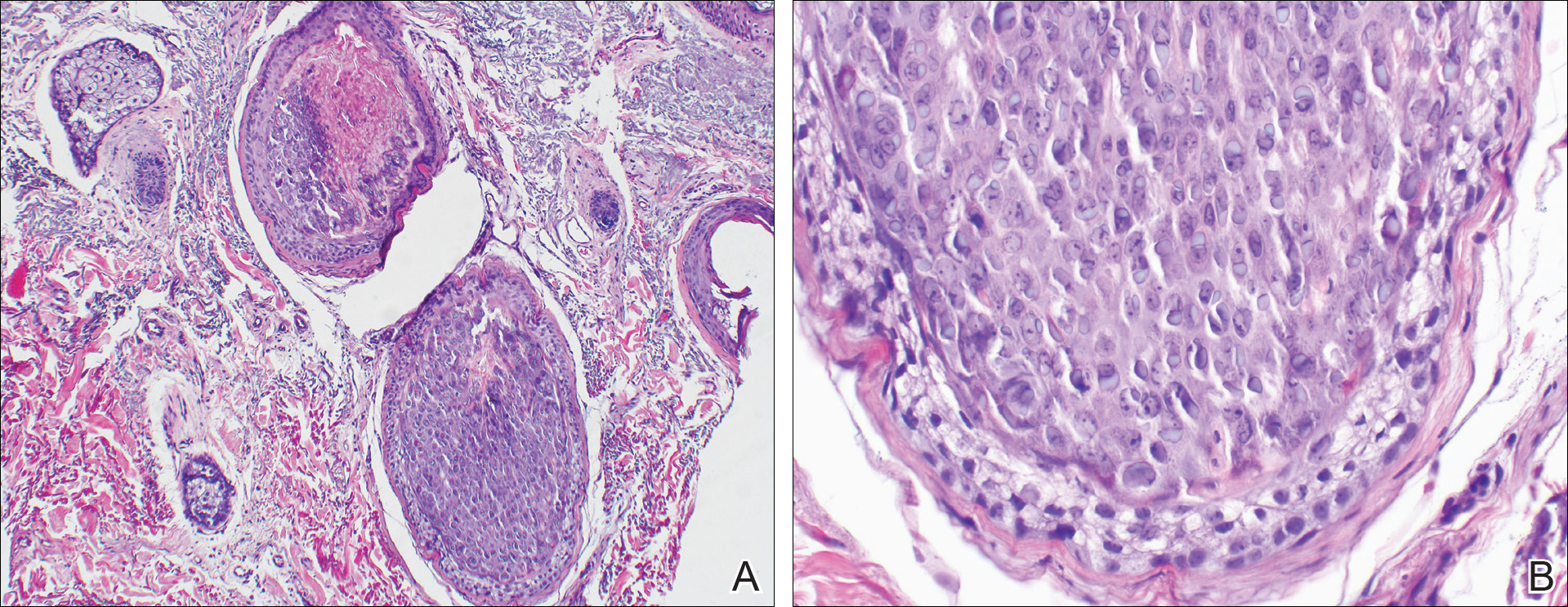
A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.
The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Practice Points
- Rashes have a life span and can evolve with time.
- If apparent straightforward conditions do not appear to respond to standard therapy, start to think outside the box for underlying potential causes.
CDC: Trivalent adjuvanted influenza vaccine aIIV3 safe in elderly adults
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
SOURCE: Haber P et al. ICEID 2018, Board 320.
REPORTING FROM ICEID 2018
Key clinical point: No new or unexpected adverse events were reported among the 630 reports related to the vaccine during the study period, of which 521 involved adults aged 65 years and older.
Major finding: Of 521 reports, 18 were serious, and there were two deaths.
Study details: A review of 521 reports to the Vaccine Adverse Event Reporting System in 2017-2018.
Disclosures: Ms. Haber reported having no disclosures.
Source: Haber P et al. ICEID 2018, Board 320.
PCV13 moderately effective in older adults
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
REPORTING FROM ICEID 2018
Take these steps to improve your flu season preparedness
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).
Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.
Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).
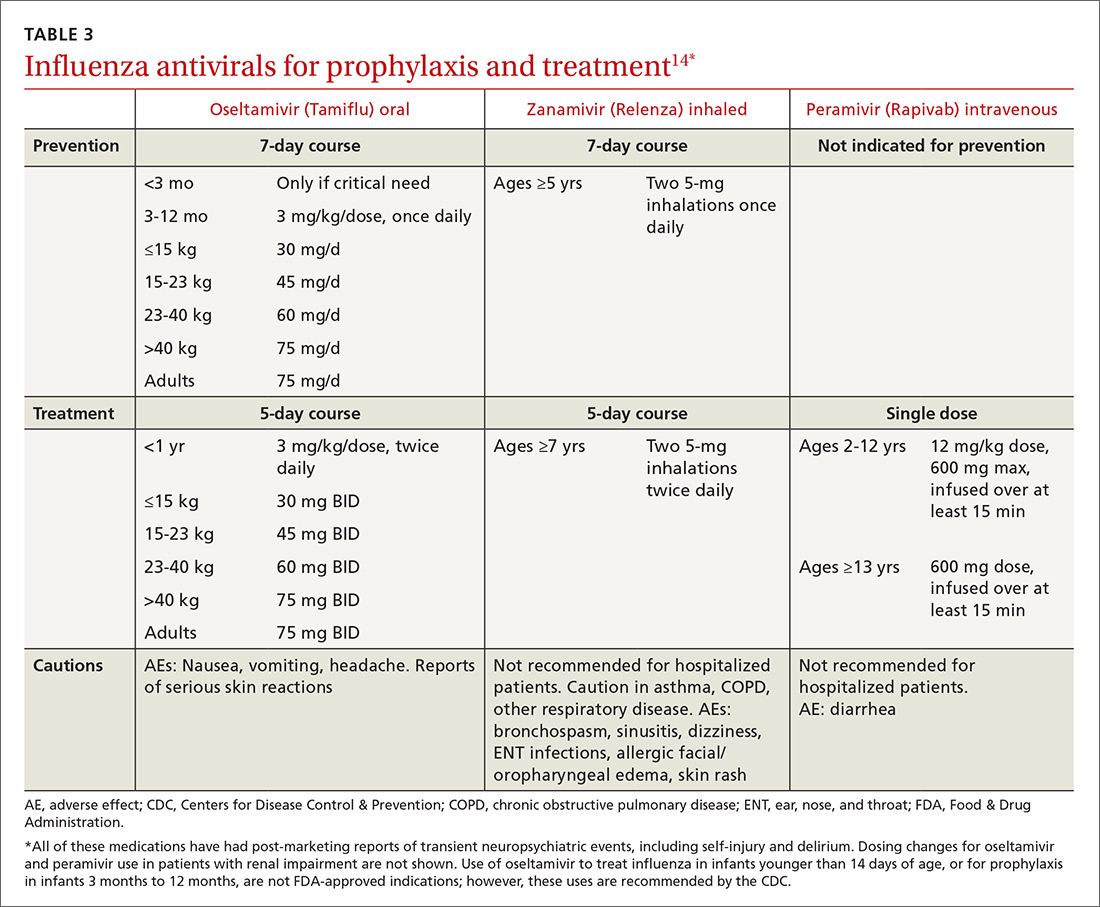
Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14
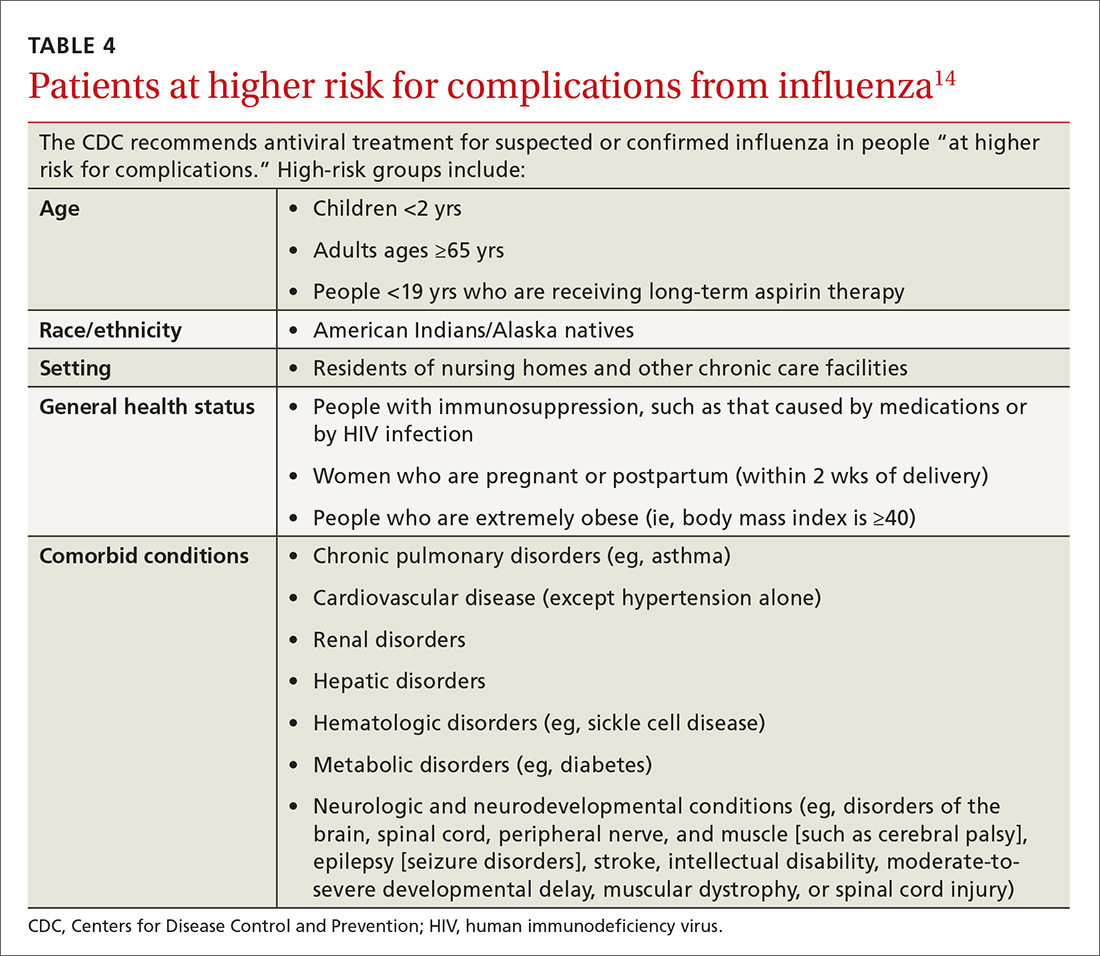
Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
Last year’s influenza season was severe enough that hospitals around the United States set up special evaluation areas beyond their emergency departments, at times spilling over to tents or other temporary structures in what otherwise would be parking lots. The scale and potential severity of the annual epidemic can be difficult to convey to our patients, who sometimes say “just the flu” to refer to an illness responsible for more than 170 pediatric deaths in the United States this past year.1 The Centers for Disease Control and Prevention (CDC) recently updated its 5-year estimates of influenza-related deaths in the United States; influenza mortality ranges from about 12,000 deaths in a mild season (such as 2011-2012) to 56,000 in a more severe season (eg, 2012-2013).2
Although influenza cannot be completely prevented, the following strategies can help reduce the risk for the illness and limit its severity if contracted.
Prevention
Strategy 1: Vaccinate against influenza
While the efficacy of vaccines varies from year to year, vaccination remains the core of influenza prevention efforts. In this decade, vaccine effectiveness has ranged from 19% to 60%.3 However, models suggest that even when the vaccine is only 20% effective, vaccinating 140 million people (the average number of doses delivered annually in the United States over the past 5 seasons) prevents 21 million infections, 130,000 hospitalizations, and more than 61,000 deaths.4 In a case-control study, Flannery et al found that vaccination was 65% effective in preventing laboratory-confirmed influenza-associated death in children over 4 seasons (July 2010 through June 2014).5
Deciding who should be vaccinated is simpler than in prior years: Rather than targeting people who are at higher risk (those ages 65 and older, or those with comorbidities), the current CDC recommendation is to vaccinate nearly everyone ages 6 months or older, with limited exceptions.6,7 (See Table 18).

Formulations. Many types of influenza vaccine are approved for use in the United States; these differ in the number of strains included (3 or 4), the amount of antigen present for each strain, the presence of an adjuvant, the growth medium used for the virus, and the route of administration (see Table 29). The relative merits of each type are a matter of some debate. There is ongoing research into the comparative efficacy of vaccines comprised of egg- vs cell-based cultures, as well as studies comparing high-dose or adjuvant vaccines to standard-dose inactivated vaccines.

Previously, the CDC has recommended preferential use (or avoidance) of some vaccine types, based on their efficacy. For the 2018-2019 flu season, however, the CDC has rescinded its recommendation against vaccine containing live attenuated virus (LAIV; FluMist brand) and expresses no preference for any vaccine formulation for patients of appropriate age and health status.10 The American Academy of Pediatrics (AAP), however, is recommends that LAIV be used only if patients and their families decline injectable vaccines.11
Timing. Influenza vaccines are now distributed as early as July to some locations, raising concerns about waning immunity from early vaccination (July/August) if the influenza season does not peak until February or March.8,12,13 Currently, the CDC recommends balancing the possible benefit of delayed vaccination against the risks of missed opportunities to vaccinate, a possible early season, and logistical problems related to vaccinating the same number of people in a smaller time interval. Offering vaccination by the end of October, if possible, is recommended in order for immunity to develop by mid-November.8 Note: Children ages 6 months to 8 years will need to receive their initial vaccination in 2 half-doses administered at least 28 days apart; completing their vaccination by the end of October would require starting the process weeks earlier.
[polldaddy:10124269]
Continue to: Strategy 2
Strategy 2: Make use of chemoprophylaxis
Preventive use of antiviral medication (chemoprophylaxis) may be a useful adjunct or alternative to vaccination in certain circumstances: if the patient is at high risk for complications, has been exposed to someone with influenza, has contraindications to vaccination, or received the vaccine within the past 2 weeks. The CDC also suggests that chemoprophylaxis be considered for those with immune deficiencies or who are otherwise immunosuppressed after exposure.14 Antivirals can also be used to control outbreaks in long-term care facilities; in these cases, the recommendedregimen is daily administration for at least 2 weeks, continuing until at least 7 days after the identification of the last case.14 Oseltamivir (Tamiflu) and zanamivir (Relenza) are the recommended prophylactic agents; a related intravenous medication, peramivir (Rapivab), is recommend for treatment only (see Table 314).

Strategy 3: Prevent comorbidities and opportunistic infections
Morbidity associated with influenza often comes from secondary infection. Pneumonia is among the most common complications, so influenza season is a good time to ensure that patients are appropriately vaccinated against pneumococcus, as well. Pneumococcal conjugate vaccine (Prevnar or PCV13) is recommended for children younger than 2 years of age, to be administered in a series of 4 doses: at 2, 4, 6, and 12-15 months. Vaccination with PCV13 is also recommended for those ages 65 or older, to be followed at least one year later with pneumococcal polysaccharide vaccine (Pneumovax or PPSV23).15 Additional doses of PCV13, PPSV23, or both may be indicated, depending on health status.
Strategy 4: Encourage good hygiene
The availability of immunizations and antivirals does not replace good hygiene. Frequent handwashing reduces the transmission of respiratory viruses, including influenza.16 Few studies have evaluated the use of alcohol-based hand sanitizers, but available evidence suggests they are effective in lowering viral transmission.16
Barriers, such as masks, gloves, and gowns, are helpful for health care workers.16 Surgical masks are often considered more comfortable to wear than N95 respirators. It may therefore be welcome news that when a 2009 randomized study assessed their use by hospital-based nurses, masks were non-inferior in protecting these health care workers against influenza.17
Presenteeism, the practice of going to work while sick, should be discouraged. People at risk for influenza may wish to avoid crowds during flu season; those with symptoms should be encouraged to stay home and limit contact with others.
Continue to: Treatment
Treatment
Strategy 1: Make prompt use of antivirals
Despite available preventive measures, tens of millions of people in the United States develop influenza every year. Use of antiviral medication, begun early in the course of illness, can reduce the duration of symptoms and may reduce the risk for complications.
The neuraminidase inhibitor (NI) group of antivirals—oseltamivir, zanamivir, and peramivir—is effective against influenza types A and B and current resistance rates are low.
The adamantine family of antivirals, amantadine and rimantadine, treat type A only. Since the circulating influenza strains in the past several seasons have demonstrated resistance >99%, these medications are not currently recommended.14
NIs reduce the duration of influenza symptoms by 10% to 20%, shortening the illness by 6 to 24 hours.18,19 In otherwise healthy patients, this benefit must be balanced against the increased risk for nausea and vomiting (oseltamivir), bronchospasm and sinusitis (zanamivir), and diarrhea (peramivir). In adults, NIs reduce the risk for lower respiratory tract complications and hospitalization. A 2015 meta-analysis by Dobson et al found a relative risk for hospitalization among those prescribed oseltamivir vs placebo of 37%.18
In the past, antivirals were used only in high-risk patients, such as children younger than 2 years, adults older than 65 years, and those with chronic health conditions.14 Now, antivirals are recommended for those who are at higher risk for complications (see Table 4), those with “severe, complicated, or progressive illness,” and hospitalized patients.14

Continue to: Antiviral treatment may have some value...
Antiviral treatment may have some value for hospitalized patients when started even 5 days after symptom onset. Treatment may be extended beyond the usual recommendations (5 days for oseltamivir or zanamivir) in immunosuppressed patients or the critically ill. Additionally, recent guidelines include consideration of antiviral treatment in outpatients who are at normal risk if treatment can be started within 48 hours of symptom onset.14
The CDC currently recommends use of oseltamivir rather than other antivirals for most hospitalized patients, based on the availability of data on its use in this setting.14 Intravenous peramivir is recommended for patients who cannot tolerate or absorb oral medication; inhaled zanamivir or IV peramivir are preferred for patients with end-stage renal disease who are not undergoing dialysis (see Table 3).14
Strategy 2: Exercise caution when it comes to supportive care
There are other medications that may offer symptom relief or prevent complications, especially when antivirals are contraindicated or unavailable.
Corticosteroids are recommended as part of the treatment of community-acquired pneumonia,20 but their role in influenza is controversial. A 2016 Cochrane review21 found no randomized controlled trials on the topic. Although the balance of available data from observational studies indicated that use of corticosteroids was associated with increased mortality, the authors also noted that all the studies included in their meta-analysis were of “very low quality.” They concluded that “the use of steroids in influenza remains a clinical judgement call.”
Statins may be associated with improved outcomes in influenza and pneumonia. Studies thus far have given contradictory results,22,23 and a planned Cochrane review of the question has been withdrawn.24
Continue to: Over-the-counter medications...
Over-the-counter medications, such as aspirin, acetaminophen, and ibuprofen are often used to manage the fever and myalgia associated with influenza. Patients should be cautioned against using the same ingredient in multiple different branded medications. Acetaminophen, for example, is not limited to Tylenol-branded products. To avoid Reye’s syndrome, children and teens with febrile illness, such as influenza, should not use aspirin.
CORRESPONDENCE
Jennifer L. Hamilton, MD, PhD, Drexel Family Medicine, 10 Shurs Lane, Suite 301, Philadelphia, PA 19127; [email protected].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
1. CDC. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Published June 8, 2018. Accessed August 22, 2018.
2. CDC. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. Published April 19, 2017. https://www.cdc.gov/flu/about/disease/2015-16.htm. Accessed Setptember 18, 2018.
3. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Published February 15, 2018. Accessed August 22, 2018.
4. Sah P, Medlock J, Fitzpatrick MC, et al. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci. 2018:201802479.
5. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139: e20164244.
6. Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:158–160.
7. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:156–157.
8. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66:1-20.
9. CDC. Influenza vaccines—United States, 2017–18 influenza season. https://www.cdc.gov/flu/protect/vaccine/vaccines.htm. Published May 16, 2018. Accessed August 22, 2018.
10. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643–645.
11. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. AAP News. May 21, 2018. http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 22, 2018.
12. Glinka ER, Smith DM, Johns ST. Timing matters—influenza vaccination to HIV-infected patients. HIV Med. 2016;17:601-604.
13. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18:20388.
14. CDC. Influenza antiviral medications: summary for clinicians. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Published May 11, 2018. Accessed August 22, 2018.
15. CDC. Pneumococcal vaccination summary: who and when to vaccinate. https://www.cdc.gov/vaccines/vpd/pneumo/hcp/who-when-to-vaccinate.html. Published February 28, 2018. Accessed August 22, 2018.
16. Jefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;(7):CD006207.
17. Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865-1871.
18. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-1737.
19. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ. 2016;355:i6258.
20. Kaysin A, Viera AJ. Community-acquired pneumonia in adults: diagnosis and management. Am Fam Physician. 2016;94:698-706.
21. Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2016;3:CD010406.
22. Brassard P, Wu JW, Ernst P, et al. The effect of statins on influenza-like illness morbidity and mortality. Pharmacoepidemiol Drug Saf. 2017;26:63-70.
23. Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417-435.
24. Khandaker G, Rashid H, Chow MY, et al. Statins for influenza and pneumonia. Cochrane Database Syst Rev. January 9, 2017 [withdrawn].
PRACTICE RECOMMENDATIONS
› Recommend influenza vaccination for all patients at least 6 months old unless a specific contraindication exists. A
› Recommend pneumococcal vaccination to appropriate patients to reduce the risk for a common complication of influenza. A
› Encourage hygiene-based measures to limit infection, including frequent handwashing or use of a hand sanitizer. B
› Prescribe oseltamivir to hospitalized influenza patients to limit the duration and severity of infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Trials need standardized reporting of pediatric fever after flu vaccine
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
Researchers found a lower rate of pediatric fever after applying a standard definition of fever across three different clinical trials of pediatric patients receiving influenza vaccinations, according to research published in the Pediatric Infectious Disease Journal.
Investigators in future studies must adopt a standardized definition of pediatric fever after an influenza vaccination. “Our study demonstrates the variability in results which occur due to minor differences in the definition of fever, methods of analysis and reporting of results,” Jean Li-Kim-Moy, MBBS, of the University of Sydney, and colleagues wrote.
Dr. Li-Kim-Moy and colleagues analyzed pediatric datasets from three different clinical trials using trivalent influenza vaccine (TIV); the primary trial included 3,317 children aged 6-35 months who were randomized to receive Fluarix at 0.25 mL or 0.5 mL, or receive 0.25 mL of Fluzone. The other two trials studied children receiving TIV between 6 months–17 years and 3-17 years. The researchers also performed a multivariable regression analysis to determine the relationship between immunogenicity, antipyretic use, and postvaccination fever.
The primary study initially reported the fever rate 0 days–3 days after vaccination was between 6% and 7%. After reporting the rate of fever separately for each dose and changing the criteria to “defining fever as greater than or equal to 38.0°C by any route of measurement” for the primary study, the researchers found a rate of any-cause fever was 3%-4% for the first dose and 4%-5% for the second dose. The rate of vaccine-related fever in the primary study was 3% for the first dose and 3%-4% for the second dose, with researchers noting vaccine-related fever occurred significantly earlier compared with any-cause fever (mean 1 days vs. 2 days after vaccination; P equals .04).
Impact of fever, antipyretics
The researchers also performed a pooled immunogenicity analysis of 5,902 children from all three trials and found a strong association between fever after vaccination and increased geometric mean titer (GMT) ratios (1.21-1.39; P less than or equal to .01) and an association between antipyretic use and reduced GMT ratios (0.80-0.87; P less than .0006).
“Our pooled analysis of the three trials demonstrated highly significant associations, for all strains, between postvaccination fever and up to 39% higher GMT; Similarly, strong evidence of associations in the opposite direction was found between postvaccination antipyretic use (days 0-3), adjusting for all other factors including fever, and decreased immunogenicity against all vaccine strains in children,” Dr. Li-Kim-Moy and colleagues said.
Antipyretic use was common in the primary study, occurring in one in six of the children, they said. These findings of “significant associations between fever and increased vaccine immunogenicity, and between antipyretic use and reduced immunogenicity in children after influenza vaccination” suggest the need for further study, especially because parents often give antipyretics if their children are febrile after vaccinations.
“There is uncertainty whether our findings, and those of others, on immunogenicity translate into clinically significant effects,” they wrote. “However, the fact that influenza vaccine, unlike many routine childhood vaccines, is only moderately protective may mean that modest reductions in antibody response are more likely to correlate to less protection.”
Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council (NHMRC) and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
SOURCE: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Key clinical point: There is variability in reporting and analysis of pediatric fever rates after administration of the influenza vaccine.
Major finding: Applying the Brighton Collaboration standardized definition for vaccine-related fever to three clinical trials yielded significantly lower rates of fever (3%-4%), compared with the rates reported in the trials (6%-7%).
Study details: An analysis of pediatric fever data from three different clinical trials using Brighton Collaboration criteria.
Disclosures: Dr. Wood reported receiving a fellowship from the National Health and Medical Research Council and being an investigator for GlaxoSmithKline trials. Dr. Booy reported being an advisor for influenza vaccine manufacturing, an advisory board member, on the speaker’s bureau, and a researcher of vaccines for several manufacturers. The other authors reported no relevant conflicts of interest.
Source: Li-Kim-Moy J et al. Pediatr Infect Dis J. 2018 Oct;37(10):971-5.