User login
Synthetic HCV glycoproteins elicit narrow, but not widely effective antibodies
Immunization with two synthetic consensus constructs of hepatitis C virus (HCV) glycoproteins was found to elicit narrow, but not widely effective, virus-neutralizing antibodies in guinea pigs, according to the results of a model study published in the journal Antiviral Research.
Researchers created two novel synthetic E2 glycoprotein immunogens (NotC1 and NotC2) using consensus nucleotide sequences deduced from samples of circulating genotype 1 HCV strains, according to Alexander W. Tarr, PhD, of the Nottingham (England) University Hospitals NHS Trust, and his colleagues.
Expression of these constructs in Drosophila melanogaster cells resulted in high yields of correctly folded, monomeric E2 proteins, which were used to immunize guinea pigs. Both proteins generated antibodies capable of neutralizing the H77 strain of HCV, although NotC1 elicited antibodies that were more potent at neutralizing virus entry. The two sets of glycoprotein-induced antibodies neutralized some HCV strains representing genotype 1, but not those strains representing genotype 2 or genotype 3.
“The broader objective of eliciting antibodies that neutralize patient strains representing multiple genotypes will require further refinement of immunization protocols. A vaccine construct comprising the consensus of a minimal CD81 binding domain might be able to focus the antibody response to conserved epitopes on E2. Additionally, boosting immunized animals with glycoproteins representing different strains might be required to focus the antibody response on to conserved conformational epitopes,” the researchers concluded.
The research was funded by the Medical Research Council, the NIHR Nottingham Biomedical Research Centre, and the European Union. Disclosures were not reported.
SOURCE: Tarr AW et al. Antiviral Research 2018;160:25-37.
Immunization with two synthetic consensus constructs of hepatitis C virus (HCV) glycoproteins was found to elicit narrow, but not widely effective, virus-neutralizing antibodies in guinea pigs, according to the results of a model study published in the journal Antiviral Research.
Researchers created two novel synthetic E2 glycoprotein immunogens (NotC1 and NotC2) using consensus nucleotide sequences deduced from samples of circulating genotype 1 HCV strains, according to Alexander W. Tarr, PhD, of the Nottingham (England) University Hospitals NHS Trust, and his colleagues.
Expression of these constructs in Drosophila melanogaster cells resulted in high yields of correctly folded, monomeric E2 proteins, which were used to immunize guinea pigs. Both proteins generated antibodies capable of neutralizing the H77 strain of HCV, although NotC1 elicited antibodies that were more potent at neutralizing virus entry. The two sets of glycoprotein-induced antibodies neutralized some HCV strains representing genotype 1, but not those strains representing genotype 2 or genotype 3.
“The broader objective of eliciting antibodies that neutralize patient strains representing multiple genotypes will require further refinement of immunization protocols. A vaccine construct comprising the consensus of a minimal CD81 binding domain might be able to focus the antibody response to conserved epitopes on E2. Additionally, boosting immunized animals with glycoproteins representing different strains might be required to focus the antibody response on to conserved conformational epitopes,” the researchers concluded.
The research was funded by the Medical Research Council, the NIHR Nottingham Biomedical Research Centre, and the European Union. Disclosures were not reported.
SOURCE: Tarr AW et al. Antiviral Research 2018;160:25-37.
Immunization with two synthetic consensus constructs of hepatitis C virus (HCV) glycoproteins was found to elicit narrow, but not widely effective, virus-neutralizing antibodies in guinea pigs, according to the results of a model study published in the journal Antiviral Research.
Researchers created two novel synthetic E2 glycoprotein immunogens (NotC1 and NotC2) using consensus nucleotide sequences deduced from samples of circulating genotype 1 HCV strains, according to Alexander W. Tarr, PhD, of the Nottingham (England) University Hospitals NHS Trust, and his colleagues.
Expression of these constructs in Drosophila melanogaster cells resulted in high yields of correctly folded, monomeric E2 proteins, which were used to immunize guinea pigs. Both proteins generated antibodies capable of neutralizing the H77 strain of HCV, although NotC1 elicited antibodies that were more potent at neutralizing virus entry. The two sets of glycoprotein-induced antibodies neutralized some HCV strains representing genotype 1, but not those strains representing genotype 2 or genotype 3.
“The broader objective of eliciting antibodies that neutralize patient strains representing multiple genotypes will require further refinement of immunization protocols. A vaccine construct comprising the consensus of a minimal CD81 binding domain might be able to focus the antibody response to conserved epitopes on E2. Additionally, boosting immunized animals with glycoproteins representing different strains might be required to focus the antibody response on to conserved conformational epitopes,” the researchers concluded.
The research was funded by the Medical Research Council, the NIHR Nottingham Biomedical Research Centre, and the European Union. Disclosures were not reported.
SOURCE: Tarr AW et al. Antiviral Research 2018;160:25-37.
FROM ANTIVIRAL RESEARCH
HCV adapts to HIV coinfection
HCV strains evolve differently in HIV-coinfected individuals, according to the results of a database analysis of HCV genetic sequences from patients monoinfected with HCV and those who were coinfected with HIV. The study compared results from 112 coinfected persons (CIPs) and 176 monoinfected persons (MIPs), according to the report published in the journal Infection, Genetics, and Evolution.
Genetic differences between intrahost variants of the HCV hypervariable region 1 (HVR1) were sampled from CIPs and MIPs, and the nucleotide sequences of intrahost HCV HVR1 variants (n = 28,622) obtained were represented using 148 physical-chemical (PhyChem) indexes of DNA nucleotide dimers. Changes of the intrahost HCV population were detected by measuring coevolution among the HVR1 site using new PhyChem properties extracted from the next-generation sequencing of HVR1 data. Small but statistically significant variances in seven of the PhyChem indexes, measured using these intrahost HVR1 variants, was shown to be strongly associated with CIPs and MIPs (P less than .0001).
“The computational models built using these new markers provide novel opportunities for development of cybermolecular diagnostics,” wrote James Lara, PhD, and his colleagues at the Centers for Disease Control and Prevention.
All HVR1 sequences used (n = 28,622) shared only 6,782 profiles of the selected calculated dinucleotide-based auto covariances of the seven PhyChem indexes. The vast majority (98%-99%) of these profiles were found to be specific to CIPs or MIPs, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals, according to the authors.
Because of the common occurrence of HIV-coinfection in high-risk groups, “HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” Dr. Lara and his colleagues concluded.
The authors reported having no conflicts of interest.
SOURCE: Lara J et al. Infection, Genetics and Evolution. 2018. 65:216-25.
HCV strains evolve differently in HIV-coinfected individuals, according to the results of a database analysis of HCV genetic sequences from patients monoinfected with HCV and those who were coinfected with HIV. The study compared results from 112 coinfected persons (CIPs) and 176 monoinfected persons (MIPs), according to the report published in the journal Infection, Genetics, and Evolution.
Genetic differences between intrahost variants of the HCV hypervariable region 1 (HVR1) were sampled from CIPs and MIPs, and the nucleotide sequences of intrahost HCV HVR1 variants (n = 28,622) obtained were represented using 148 physical-chemical (PhyChem) indexes of DNA nucleotide dimers. Changes of the intrahost HCV population were detected by measuring coevolution among the HVR1 site using new PhyChem properties extracted from the next-generation sequencing of HVR1 data. Small but statistically significant variances in seven of the PhyChem indexes, measured using these intrahost HVR1 variants, was shown to be strongly associated with CIPs and MIPs (P less than .0001).
“The computational models built using these new markers provide novel opportunities for development of cybermolecular diagnostics,” wrote James Lara, PhD, and his colleagues at the Centers for Disease Control and Prevention.
All HVR1 sequences used (n = 28,622) shared only 6,782 profiles of the selected calculated dinucleotide-based auto covariances of the seven PhyChem indexes. The vast majority (98%-99%) of these profiles were found to be specific to CIPs or MIPs, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals, according to the authors.
Because of the common occurrence of HIV-coinfection in high-risk groups, “HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” Dr. Lara and his colleagues concluded.
The authors reported having no conflicts of interest.
SOURCE: Lara J et al. Infection, Genetics and Evolution. 2018. 65:216-25.
HCV strains evolve differently in HIV-coinfected individuals, according to the results of a database analysis of HCV genetic sequences from patients monoinfected with HCV and those who were coinfected with HIV. The study compared results from 112 coinfected persons (CIPs) and 176 monoinfected persons (MIPs), according to the report published in the journal Infection, Genetics, and Evolution.
Genetic differences between intrahost variants of the HCV hypervariable region 1 (HVR1) were sampled from CIPs and MIPs, and the nucleotide sequences of intrahost HCV HVR1 variants (n = 28,622) obtained were represented using 148 physical-chemical (PhyChem) indexes of DNA nucleotide dimers. Changes of the intrahost HCV population were detected by measuring coevolution among the HVR1 site using new PhyChem properties extracted from the next-generation sequencing of HVR1 data. Small but statistically significant variances in seven of the PhyChem indexes, measured using these intrahost HVR1 variants, was shown to be strongly associated with CIPs and MIPs (P less than .0001).
“The computational models built using these new markers provide novel opportunities for development of cybermolecular diagnostics,” wrote James Lara, PhD, and his colleagues at the Centers for Disease Control and Prevention.
All HVR1 sequences used (n = 28,622) shared only 6,782 profiles of the selected calculated dinucleotide-based auto covariances of the seven PhyChem indexes. The vast majority (98%-99%) of these profiles were found to be specific to CIPs or MIPs, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals, according to the authors.
Because of the common occurrence of HIV-coinfection in high-risk groups, “HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” Dr. Lara and his colleagues concluded.
The authors reported having no conflicts of interest.
SOURCE: Lara J et al. Infection, Genetics and Evolution. 2018. 65:216-25.
FROM INFECTION, GENETICS, AND EVOLUTION
Pay attention to kidney disease risk in people living with HIV
The prevalence of chronic kidney disease (CKD) in people living with HIV varied widely, depending on population and criteria, according to a systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
The review included all studies that involved adults older than 21 years of age, investigated people living with HIV with CKD, reported prevalence of CKD, and were published in a peer-reviewed journal, according to Jungmin Park, PhD, RN, of CHA University, Pocheon-Si, South Korea, and her colleague.
Out of an initial search yielding 1,960 citations in PubMed and 5,356 citations in PsycInfo, the results were pared down to 21 articles, which met all of the inclusion/exclusion criteria and were used for the final analysis.
The risk factors for CKD in people living with HIV cited most often in the studies consisted of medications, hypertension, older age, diabetes mellitus, hepatitis coinfection (with hepatitis C virus more prominent than hepatitis B virus), low CD4+ T-cell count, and race, Dr. Park and her colleague reported.
Of the various risk factors, the only ones unique to HIV were viral load and CD4+ T-cell count. One study reporting on 5,538 treatment-naive patients in mainland China suggested that HIV viral replication in renal cells may be the cause of renal damage in patients with high viral loads, meaning that viral suppression would improve renal function. However, all of these risk factors are intrinsically linked, according to Dr. Park and her colleague. They added that managing viral load alone would be ineffective in preventing CKD: “Therefore [people living with HIV] will need to effectively manage every aspect of their health, including metabolic and cardiovascular systems.”
Of the 43,114 people living with HIV across the 21 studies, 3,218 (7.3%) had CKD. The reported prevalence of CKD ranged from 2.3% to 53.3%, with the African population having the highest prevalence. Some of the wide variation was possibly attributable to differences in the definitions of CKD used across the various studies.
“The risk of under-diagnosis of CKD can lead to long-term health complications. Health care providers must monitor kidney function and treatment for renal damage carefully, especially for people living with HIV with additional diagnoses of diabetes and/or hypertension, and for those who are aging,” Dr. Park and her colleague concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Park, J et al. J Assoc Nurses AIDS Care. 2018;29:655-66.
The prevalence of chronic kidney disease (CKD) in people living with HIV varied widely, depending on population and criteria, according to a systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
The review included all studies that involved adults older than 21 years of age, investigated people living with HIV with CKD, reported prevalence of CKD, and were published in a peer-reviewed journal, according to Jungmin Park, PhD, RN, of CHA University, Pocheon-Si, South Korea, and her colleague.
Out of an initial search yielding 1,960 citations in PubMed and 5,356 citations in PsycInfo, the results were pared down to 21 articles, which met all of the inclusion/exclusion criteria and were used for the final analysis.
The risk factors for CKD in people living with HIV cited most often in the studies consisted of medications, hypertension, older age, diabetes mellitus, hepatitis coinfection (with hepatitis C virus more prominent than hepatitis B virus), low CD4+ T-cell count, and race, Dr. Park and her colleague reported.
Of the various risk factors, the only ones unique to HIV were viral load and CD4+ T-cell count. One study reporting on 5,538 treatment-naive patients in mainland China suggested that HIV viral replication in renal cells may be the cause of renal damage in patients with high viral loads, meaning that viral suppression would improve renal function. However, all of these risk factors are intrinsically linked, according to Dr. Park and her colleague. They added that managing viral load alone would be ineffective in preventing CKD: “Therefore [people living with HIV] will need to effectively manage every aspect of their health, including metabolic and cardiovascular systems.”
Of the 43,114 people living with HIV across the 21 studies, 3,218 (7.3%) had CKD. The reported prevalence of CKD ranged from 2.3% to 53.3%, with the African population having the highest prevalence. Some of the wide variation was possibly attributable to differences in the definitions of CKD used across the various studies.
“The risk of under-diagnosis of CKD can lead to long-term health complications. Health care providers must monitor kidney function and treatment for renal damage carefully, especially for people living with HIV with additional diagnoses of diabetes and/or hypertension, and for those who are aging,” Dr. Park and her colleague concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Park, J et al. J Assoc Nurses AIDS Care. 2018;29:655-66.
The prevalence of chronic kidney disease (CKD) in people living with HIV varied widely, depending on population and criteria, according to a systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
The review included all studies that involved adults older than 21 years of age, investigated people living with HIV with CKD, reported prevalence of CKD, and were published in a peer-reviewed journal, according to Jungmin Park, PhD, RN, of CHA University, Pocheon-Si, South Korea, and her colleague.
Out of an initial search yielding 1,960 citations in PubMed and 5,356 citations in PsycInfo, the results were pared down to 21 articles, which met all of the inclusion/exclusion criteria and were used for the final analysis.
The risk factors for CKD in people living with HIV cited most often in the studies consisted of medications, hypertension, older age, diabetes mellitus, hepatitis coinfection (with hepatitis C virus more prominent than hepatitis B virus), low CD4+ T-cell count, and race, Dr. Park and her colleague reported.
Of the various risk factors, the only ones unique to HIV were viral load and CD4+ T-cell count. One study reporting on 5,538 treatment-naive patients in mainland China suggested that HIV viral replication in renal cells may be the cause of renal damage in patients with high viral loads, meaning that viral suppression would improve renal function. However, all of these risk factors are intrinsically linked, according to Dr. Park and her colleague. They added that managing viral load alone would be ineffective in preventing CKD: “Therefore [people living with HIV] will need to effectively manage every aspect of their health, including metabolic and cardiovascular systems.”
Of the 43,114 people living with HIV across the 21 studies, 3,218 (7.3%) had CKD. The reported prevalence of CKD ranged from 2.3% to 53.3%, with the African population having the highest prevalence. Some of the wide variation was possibly attributable to differences in the definitions of CKD used across the various studies.
“The risk of under-diagnosis of CKD can lead to long-term health complications. Health care providers must monitor kidney function and treatment for renal damage carefully, especially for people living with HIV with additional diagnoses of diabetes and/or hypertension, and for those who are aging,” Dr. Park and her colleague concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Park, J et al. J Assoc Nurses AIDS Care. 2018;29:655-66.
FROM THE JOURNAL OF THE ASSOCIATION OF NURSES IN AIDS CARE
Key clinical point: Chronic kidney disease in people living with HIV varies widely across geographic regions.
Major finding: The reported prevalence of CKD in PLWH ranged from 2.3% to 53.3%, with the African population having the highest prevalence.
Study details: Systematic literature review of the PubMed and PsycInfo databases for articles published from January 2000 through August 2016.
Disclosures: The authors reported that they had no conflicts of interest.
Source: J Assoc Nurses AIDS Care. 2018;29:655-66).
HIV testing low in U.S. women engaged in risky behavior
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point: Health care providers don’t ask sexually active women about risky behavior that would raise their risk of HIV infection.
Major finding: Of women who reported having anal sex, 19% reported that their providers asked about their types of intercourse.
Study details: Data from the 2011-2015 National Survey of Family Growth.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
Glucocorticoids plus tofacitinib may boost herpes zoster risk
Concomitant use of glucocorticoids with tofacitinib in rheumatoid arthritis may double the risk of herpes zoster, but methotrexate does not appear to increase the risk, outcomes from a cohort study of 8,030 rheumatoid arthritis patients – including 222 cases of herpes zoster – suggest.
Using information from Medicare and MarketScan, researchers found that dual therapy with tofacitinib and glucocorticoids was associated with a 96% increase in the risk of herpes zoster, compared with monotherapy with the Janus kinase inhibitor tofacitinib (95% confidence interval, 33%-188%). The crude incidence rate in those taking concomitant tofacitinib and glucocorticoids was 6 cases per 100 patient-years, compared with an incidence rate of 3.4 cases per 100 patient-years with tofacitinib monotherapy.
However, the addition of methotrexate therapy to tofacitinib was not associated with an increased risk of herpes zoster, and the incidence rate in patients on this dual therapy was 3.7 cases per 100 patient-years, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham and his coauthors reported in Arthritis Care & Research.
Women – who constituted 80% of the study population – showed a significantly increased risk of herpes zoster, as did older patients.
The study also found that individuals who had received the live herpes zoster vaccine showed a trend toward a decrease in risk.
“We saw a strong trend for decreased risk related to vaccination with the live agent (Zostavax); the concern with this form of vaccination is that any live vaccination is potentially dangerous in patients receiving potent immunosuppression,” Dr. Curtis and his associates wrote.
“The risks for disease flare, and potentially problematic tolerability related to a relatively high incidence of grade 3 (severe) systemic reactogenicity, may limit enthusiasm until specific data in an RA population is available,” they wrote. However, they noted that a randomized, controlled trial of the live virus vaccine was underway in patients with rheumatoid arthritis who were being treated with tumor necrosis factor inhibitors and suggested that vaccination should be considered in at-risk patients who didn’t have contraindications.
The authors noted that the effect of glucocorticoid exposure on herpes zoster risk in patients taking tofacitinib was similar to that seen in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs or biologic therapies.
The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
SOURCE: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
Concomitant use of glucocorticoids with tofacitinib in rheumatoid arthritis may double the risk of herpes zoster, but methotrexate does not appear to increase the risk, outcomes from a cohort study of 8,030 rheumatoid arthritis patients – including 222 cases of herpes zoster – suggest.
Using information from Medicare and MarketScan, researchers found that dual therapy with tofacitinib and glucocorticoids was associated with a 96% increase in the risk of herpes zoster, compared with monotherapy with the Janus kinase inhibitor tofacitinib (95% confidence interval, 33%-188%). The crude incidence rate in those taking concomitant tofacitinib and glucocorticoids was 6 cases per 100 patient-years, compared with an incidence rate of 3.4 cases per 100 patient-years with tofacitinib monotherapy.
However, the addition of methotrexate therapy to tofacitinib was not associated with an increased risk of herpes zoster, and the incidence rate in patients on this dual therapy was 3.7 cases per 100 patient-years, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham and his coauthors reported in Arthritis Care & Research.
Women – who constituted 80% of the study population – showed a significantly increased risk of herpes zoster, as did older patients.
The study also found that individuals who had received the live herpes zoster vaccine showed a trend toward a decrease in risk.
“We saw a strong trend for decreased risk related to vaccination with the live agent (Zostavax); the concern with this form of vaccination is that any live vaccination is potentially dangerous in patients receiving potent immunosuppression,” Dr. Curtis and his associates wrote.
“The risks for disease flare, and potentially problematic tolerability related to a relatively high incidence of grade 3 (severe) systemic reactogenicity, may limit enthusiasm until specific data in an RA population is available,” they wrote. However, they noted that a randomized, controlled trial of the live virus vaccine was underway in patients with rheumatoid arthritis who were being treated with tumor necrosis factor inhibitors and suggested that vaccination should be considered in at-risk patients who didn’t have contraindications.
The authors noted that the effect of glucocorticoid exposure on herpes zoster risk in patients taking tofacitinib was similar to that seen in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs or biologic therapies.
The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
SOURCE: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
Concomitant use of glucocorticoids with tofacitinib in rheumatoid arthritis may double the risk of herpes zoster, but methotrexate does not appear to increase the risk, outcomes from a cohort study of 8,030 rheumatoid arthritis patients – including 222 cases of herpes zoster – suggest.
Using information from Medicare and MarketScan, researchers found that dual therapy with tofacitinib and glucocorticoids was associated with a 96% increase in the risk of herpes zoster, compared with monotherapy with the Janus kinase inhibitor tofacitinib (95% confidence interval, 33%-188%). The crude incidence rate in those taking concomitant tofacitinib and glucocorticoids was 6 cases per 100 patient-years, compared with an incidence rate of 3.4 cases per 100 patient-years with tofacitinib monotherapy.
However, the addition of methotrexate therapy to tofacitinib was not associated with an increased risk of herpes zoster, and the incidence rate in patients on this dual therapy was 3.7 cases per 100 patient-years, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham and his coauthors reported in Arthritis Care & Research.
Women – who constituted 80% of the study population – showed a significantly increased risk of herpes zoster, as did older patients.
The study also found that individuals who had received the live herpes zoster vaccine showed a trend toward a decrease in risk.
“We saw a strong trend for decreased risk related to vaccination with the live agent (Zostavax); the concern with this form of vaccination is that any live vaccination is potentially dangerous in patients receiving potent immunosuppression,” Dr. Curtis and his associates wrote.
“The risks for disease flare, and potentially problematic tolerability related to a relatively high incidence of grade 3 (severe) systemic reactogenicity, may limit enthusiasm until specific data in an RA population is available,” they wrote. However, they noted that a randomized, controlled trial of the live virus vaccine was underway in patients with rheumatoid arthritis who were being treated with tumor necrosis factor inhibitors and suggested that vaccination should be considered in at-risk patients who didn’t have contraindications.
The authors noted that the effect of glucocorticoid exposure on herpes zoster risk in patients taking tofacitinib was similar to that seen in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs or biologic therapies.
The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
SOURCE: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Patients treated with concomitant glucocorticoids and tofacitinib showed increased risk of herpes zoster.
Major finding: Concomitant use of glucocorticoids and tofacitinib is associated with nearly twofold increase in the risk of herpes zoster.
Study details: Cohort study using data from 8,030 rheumatoid arthritis patients.
Disclosures: The study was partly funded by the Patient-Centered Outcomes Research Institute. Two authors declared research grants and other funding from the pharmaceutical industry, but no other conflicts of interest were declared.
Source: Curtis J et al. Arthritis Care Res. 2018 Oct 8. doi: 10.1002/acr.23769.
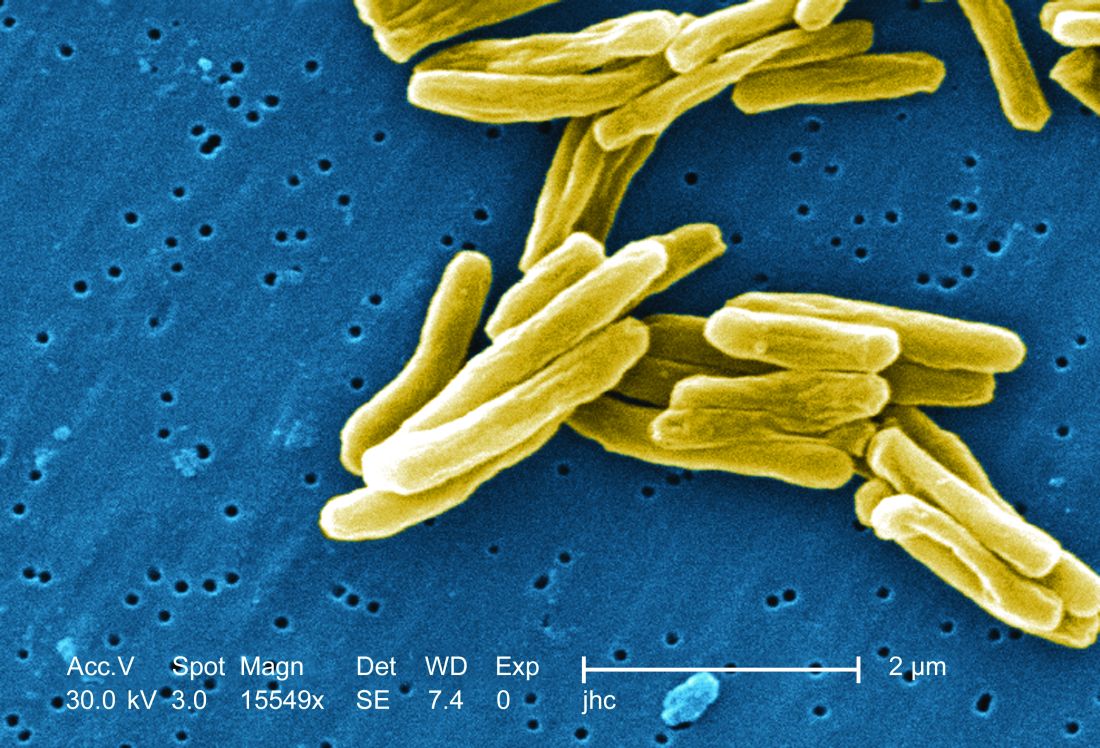
TB vaccine shows promise in previously infected
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
REPORTING FROM IDWEEK 2018
Key clinical point: The vaccine is the first to show efficacy in patients previously exposed to the TB bacterium.
Major finding: The vaccine had a protective efficacy of 54%.
Study details: Randomized, controlled trial with 3,330 participants.
Disclosures: The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
Source: Ginsberg A et al. IDWeek 2018, Abstract 120.
Mysterious polio-like illness baffles medical experts while frightening parents
A spike in the number of children with a rare neurological disease that causes polio-like symptoms has health officials across the country scrambling to understand the illness. Yet, more than 4 years after health officials first recorded the most recent uptick in cases, much about the national outbreak remains a mystery.
Acute flaccid myelitis (AFM) affects the gray matter in the spinal cord, causing sudden muscle weakness and a loss of reflexes. The illness can lead to serious complications – including paralysis or respiratory failure – and requires immediate medical attention.
The Centers for Disease Control and Prevention is investigating 127 cases of possible AFM, including 62 that have been confirmed in 22 states this year. At least 90% of the cases are among patients 18 years old and younger. The average age of a patient is 4 years old.
AFM remains extremely rare, even with the recent increase. The CDC estimates fewer than 1 in a million Americans will get the disease. Officials advised parents not to panic but remain vigilant for any sudden onset of symptoms. They also suggested that children stay up to date with their vaccines and practice good hand washing habits.
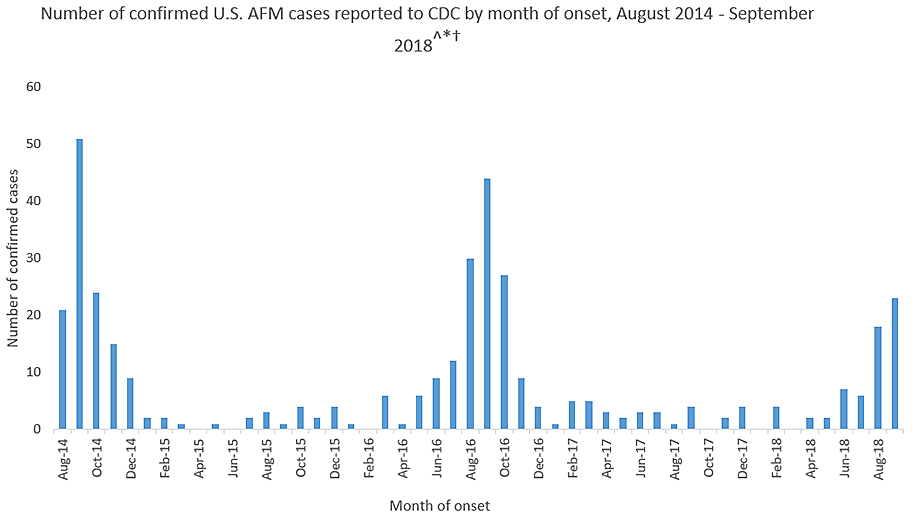
This year’s outbreak marks the third spike of AFM in 4 years. From August 2014 to September 2018, 386 cases have been confirmed. Yet, experts still do not understand crucial aspects of the disease, including its origins and who is most at risk.
“There is a lot we don’t know about AFM,” said Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases. Here’s what puzzles health officials about AFM:
The cause is still unknown
Acute flaccid myelitis can be caused by viruses, such as polio or West Nile. But federal officials said that those viruses have not been linked to the U.S. outbreak over the past 4 years. They have not isolated the cause of these cases.
Despite symptoms reminiscent of polio, no AFM cases have tested positive for that virus, according to the CDC. Investigators have also ruled out a variety of germs. Environmental agents, viruses, and other pathogens are still being considered.
The 2014 outbreak of AFM coincided with a surge of another virus that caused severe respiratory problems, called EV-D68. However, the CDC could not establish a causal link between AFM and the virus. Since then, no large outbreaks of the virus have occurred, according to the CDC.
Carlos Pardo-Villamizar, MD, a neurologist and director of the Johns Hopkins Transverse Myelitis Center, said that the mystery lies in whether the damage seen in AFM is caused by an external agent or the body’s own defenses.
“At this moment, we don’t know if it’s a virus that is coming and producing direct damage of the gray matter in the spinal cord,” he said, “or if a virus is triggering immunological responses that produce a secondary damage in the spinal cord.”
It’s not clear who is at risk
Although the disease appears to target a certain age group, federal disease experts do not know who is likely to get acute flaccid myelitis.
Dr. Pardo-Villamizar said identifying vulnerable populations is “a work in progress.”
Mary Anne Jackson, MD, a pediatric infectious disease specialist and interim dean of the school of medicine at the University of Missouri–Kansas City, said many of the patients she saw were healthy children before falling ill with the disease. She suspects that a host of factors play a role in the likelihood of getting AFM, but more cases must be reviewed in order to find an answer.
The long-term effects are unknown
The CDC said it doesn’t know how long symptoms of the disease will last for patients. However, experts say that initial indications from a small number of cases suggest a grim outlook.
A study published last year found six of eight children in Colorado with acute flaccid myelitis still struggled with motor skills 1 year after their diagnosis. Nonetheless, the researchers found that the patients and families “demonstrated a high degree of resilience and recovery.”
“The majority of these patients are left with extensive problems,” said Dr. Pardo-Villamizar, who was not involved in the study.
Dr. Jackson, who also saw persistent muscle weakness in her patients, said she believes the CDC may be hesitant to specify the long-term effects of the disease because existing studies have included only small numbers of patients. More studies that include a larger proportion of confirmed cases are needed to better understand long-term outcomes, she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
A spike in the number of children with a rare neurological disease that causes polio-like symptoms has health officials across the country scrambling to understand the illness. Yet, more than 4 years after health officials first recorded the most recent uptick in cases, much about the national outbreak remains a mystery.

Acute flaccid myelitis (AFM) affects the gray matter in the spinal cord, causing sudden muscle weakness and a loss of reflexes. The illness can lead to serious complications – including paralysis or respiratory failure – and requires immediate medical attention.
The Centers for Disease Control and Prevention is investigating 127 cases of possible AFM, including 62 that have been confirmed in 22 states this year. At least 90% of the cases are among patients 18 years old and younger. The average age of a patient is 4 years old.
AFM remains extremely rare, even with the recent increase. The CDC estimates fewer than 1 in a million Americans will get the disease. Officials advised parents not to panic but remain vigilant for any sudden onset of symptoms. They also suggested that children stay up to date with their vaccines and practice good hand washing habits.
This year’s outbreak marks the third spike of AFM in 4 years. From August 2014 to September 2018, 386 cases have been confirmed. Yet, experts still do not understand crucial aspects of the disease, including its origins and who is most at risk.
“There is a lot we don’t know about AFM,” said Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases. Here’s what puzzles health officials about AFM:
The cause is still unknown
Acute flaccid myelitis can be caused by viruses, such as polio or West Nile. But federal officials said that those viruses have not been linked to the U.S. outbreak over the past 4 years. They have not isolated the cause of these cases.
Despite symptoms reminiscent of polio, no AFM cases have tested positive for that virus, according to the CDC. Investigators have also ruled out a variety of germs. Environmental agents, viruses, and other pathogens are still being considered.
The 2014 outbreak of AFM coincided with a surge of another virus that caused severe respiratory problems, called EV-D68. However, the CDC could not establish a causal link between AFM and the virus. Since then, no large outbreaks of the virus have occurred, according to the CDC.
Carlos Pardo-Villamizar, MD, a neurologist and director of the Johns Hopkins Transverse Myelitis Center, said that the mystery lies in whether the damage seen in AFM is caused by an external agent or the body’s own defenses.
“At this moment, we don’t know if it’s a virus that is coming and producing direct damage of the gray matter in the spinal cord,” he said, “or if a virus is triggering immunological responses that produce a secondary damage in the spinal cord.”
It’s not clear who is at risk
Although the disease appears to target a certain age group, federal disease experts do not know who is likely to get acute flaccid myelitis.
Dr. Pardo-Villamizar said identifying vulnerable populations is “a work in progress.”
Mary Anne Jackson, MD, a pediatric infectious disease specialist and interim dean of the school of medicine at the University of Missouri–Kansas City, said many of the patients she saw were healthy children before falling ill with the disease. She suspects that a host of factors play a role in the likelihood of getting AFM, but more cases must be reviewed in order to find an answer.
The long-term effects are unknown
The CDC said it doesn’t know how long symptoms of the disease will last for patients. However, experts say that initial indications from a small number of cases suggest a grim outlook.
A study published last year found six of eight children in Colorado with acute flaccid myelitis still struggled with motor skills 1 year after their diagnosis. Nonetheless, the researchers found that the patients and families “demonstrated a high degree of resilience and recovery.”
“The majority of these patients are left with extensive problems,” said Dr. Pardo-Villamizar, who was not involved in the study.
Dr. Jackson, who also saw persistent muscle weakness in her patients, said she believes the CDC may be hesitant to specify the long-term effects of the disease because existing studies have included only small numbers of patients. More studies that include a larger proportion of confirmed cases are needed to better understand long-term outcomes, she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
A spike in the number of children with a rare neurological disease that causes polio-like symptoms has health officials across the country scrambling to understand the illness. Yet, more than 4 years after health officials first recorded the most recent uptick in cases, much about the national outbreak remains a mystery.

Acute flaccid myelitis (AFM) affects the gray matter in the spinal cord, causing sudden muscle weakness and a loss of reflexes. The illness can lead to serious complications – including paralysis or respiratory failure – and requires immediate medical attention.
The Centers for Disease Control and Prevention is investigating 127 cases of possible AFM, including 62 that have been confirmed in 22 states this year. At least 90% of the cases are among patients 18 years old and younger. The average age of a patient is 4 years old.
AFM remains extremely rare, even with the recent increase. The CDC estimates fewer than 1 in a million Americans will get the disease. Officials advised parents not to panic but remain vigilant for any sudden onset of symptoms. They also suggested that children stay up to date with their vaccines and practice good hand washing habits.
This year’s outbreak marks the third spike of AFM in 4 years. From August 2014 to September 2018, 386 cases have been confirmed. Yet, experts still do not understand crucial aspects of the disease, including its origins and who is most at risk.
“There is a lot we don’t know about AFM,” said Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases. Here’s what puzzles health officials about AFM:
The cause is still unknown
Acute flaccid myelitis can be caused by viruses, such as polio or West Nile. But federal officials said that those viruses have not been linked to the U.S. outbreak over the past 4 years. They have not isolated the cause of these cases.
Despite symptoms reminiscent of polio, no AFM cases have tested positive for that virus, according to the CDC. Investigators have also ruled out a variety of germs. Environmental agents, viruses, and other pathogens are still being considered.
The 2014 outbreak of AFM coincided with a surge of another virus that caused severe respiratory problems, called EV-D68. However, the CDC could not establish a causal link between AFM and the virus. Since then, no large outbreaks of the virus have occurred, according to the CDC.
Carlos Pardo-Villamizar, MD, a neurologist and director of the Johns Hopkins Transverse Myelitis Center, said that the mystery lies in whether the damage seen in AFM is caused by an external agent or the body’s own defenses.
“At this moment, we don’t know if it’s a virus that is coming and producing direct damage of the gray matter in the spinal cord,” he said, “or if a virus is triggering immunological responses that produce a secondary damage in the spinal cord.”
It’s not clear who is at risk
Although the disease appears to target a certain age group, federal disease experts do not know who is likely to get acute flaccid myelitis.
Dr. Pardo-Villamizar said identifying vulnerable populations is “a work in progress.”
Mary Anne Jackson, MD, a pediatric infectious disease specialist and interim dean of the school of medicine at the University of Missouri–Kansas City, said many of the patients she saw were healthy children before falling ill with the disease. She suspects that a host of factors play a role in the likelihood of getting AFM, but more cases must be reviewed in order to find an answer.
The long-term effects are unknown
The CDC said it doesn’t know how long symptoms of the disease will last for patients. However, experts say that initial indications from a small number of cases suggest a grim outlook.
A study published last year found six of eight children in Colorado with acute flaccid myelitis still struggled with motor skills 1 year after their diagnosis. Nonetheless, the researchers found that the patients and families “demonstrated a high degree of resilience and recovery.”
“The majority of these patients are left with extensive problems,” said Dr. Pardo-Villamizar, who was not involved in the study.
Dr. Jackson, who also saw persistent muscle weakness in her patients, said she believes the CDC may be hesitant to specify the long-term effects of the disease because existing studies have included only small numbers of patients. More studies that include a larger proportion of confirmed cases are needed to better understand long-term outcomes, she said.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
How much more proof do you need?
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
One piece of wisdom I was given in medical school was to never be the first nor the last to adopt a new treatment. The history of medicine is full of new discoveries that don’t work out as well as the first report. It also is full of long standing dogmas that later were proven false. This balancing act is part of being a professional and being an advocate for your patient. There is science behind this art. Everett Rogers identified innovators, early adopters, and laggards as new ideas are diffused into practice.1
A 2007 French study2 that investigated oral amoxicillin for early-onset group B streptococcal (GBS) disease is one of the few times in the past 3 decades in which I changed my practice based on a single article. It was a large, conclusive study with 222 patients, so it doesn’t need a meta-analysis like American research often requires. The research showed that most of what I had been taught about oral amoxicillin was false. Amoxicillin is absorbed well even at doses above 50 mg/kg per day. It is absorbed reliably by full term neonates, even mildly sick ones. It does adequately cross the blood-brain barrier. The French researchers measured serum levels and proved all this using both scientific principles and through a clinical trial.
I have used this oral protocol (10 days total after 2-3 days IV therapy) on two occasions to treat GBS sepsis when I had informed consent of the parents and buy-in from the primary care pediatrician to be early adopters. I expected the Red Book would update its recommendations. That didn’t happen.
Meanwhile, I have seen other babies kept for 10 days in the hospital for IV therapy with resultant wasted costs (about $20 million/year in the United States) and income loss for the parents. I’ve treated complications and readmissions caused by peripherally inserted central catheter (PICC) line issues. One baby at home got a syringe of gentamicin given as an IV push instead of a normal saline flush. Mistakes happen at home and in the hospital.
Because late-onset GBS can be acquired environmentally, there always will be recurrences. Unless you are practicing defensive medicine, the issue isn’t the rate of recurrence; it is whether the more invasive intervention of prolonged IV therapy reduces that rate. Then balance any measured reduction (which apparently is zero) against the adverse effects of the invasive intervention, such as PICC line infections. This Bayesian decision making is hard for some risk-averse humans to assimilate. (I’m part Borg.)
Coon et al.3 have confirmed, using big data, that prolonged IV therapy of uncomplicated, late-onset GBS bacteremia does not generate a clinically significant benefit. It certainly is possible to sow doubt by asking for proof in a variety of subpopulations. Even in the era of intrapartum antibiotic prophylaxis, which has halved the incidence of GBS disease, GBS disease occurs in about 2,000 babies per year in the United States. However, most are treated in community hospitals and are not included in the database used in this new report. With fewer than 2-3 cases of GBS bacteremia per year per hospital, a multicenter, randomized controlled trial would be an unprecedented undertaking, is ethically problematic, and is not realistically happening soon. So these observational data, skillfully acquired and analyzed, are and will remain the best available data.
This new article is in the context of multiple articles over the past decade that have disproven the myth of the superiority of IV therapy. Given the known risks and costs of PICC lines and prolonged IV therapy, the default should be, absent a credible rationale to the contrary, that oral therapy at home is better.
Coon et al. show that, by 2015, 5 of 49 children’s hospitals (10%) were early adopters and had already made the switch to mostly using short treatment courses for uncomplicated GBS bacteremia; 14 of 49 (29%) hadn’t changed at all from the obsolete Red Book recommendation. Given this new analysis, what are you laggards4 waiting for?
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Diffusion of Innovations,” 5th ed. (New York: Free Press, 2003).
2. Eur J Clin Pharmacol. 2007 Jul;63(7):657-62.
3. Pediatrics. 2018;142(5):e20180345.
4. https://en.wikipedia.org/wiki/Diffusion_of_innovations.
Stepdown to oral ciprofloxacin looks safe in gram-negative bloodstream infections
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
SAN FRANCISCO – In gram-negative bloodstream infections, in patients who are stable at 48 hours, are no longer feverish, and whose infections aren’t invasive, it may be safe to step down from IV antibiotics to oral ciprofloxacin (PO). That is the tentative conclusion from a new single-center, retrospective chart review.
The study adds to growing suspicion among practitioners that stepping down may be safe in gram-negative patients, as well as mounting evidence that shorter treatment durations may also be safe, according to Gregory Cook, PharmD, who presented the study at a poster session at an annual scientific meeting on infectious diseases. “We’re getting more aggressive” in backing off IV treatment, he said in an interview.
Oral medications are associated with shorter hospital stays and decreased costs.
Froedtert & the Medical College of Wisconsin, where the study was performed, switched some years ago from levofloxacin to ciprofloxacin for cost reasons. But ciprofloxacin has a lower bioavailability, and a recent study showed levofloxacin had less treatment failure at 90 days than ciprofloxacin. Levofloxacin is restricted at the institution and requires antibiotic stewardship approval for use, whereas ciprofloxacin can be used without approval.
But the researchers were concerned about bioavailability. “We like to think of ciprofloxacin as having excellent bioavailability, and it does, it has 80% bioavailability, but it’s still not exactly the same as levofloxacin. We wanted to look into this and see if we were doing our patients a disservice or not (by stepping down to ciprofloxacin),” said Dr. Cook, who is now the antimicrobial stewardship pharmacist at Children’s Hospital New Orleans. The results were reassuring. “Ultimately we were trying to see how our patients were doing on oral ciprofloxacin, and after 2-3 days of IV therapy, most of them did extremely well,” he said.
The researchers analyzed the records of 198 patients who presented with a monomicrobial, gram-negative bloodstream infection between January 2015 and January 2018, and who survived at least 5 days past blood culture collection. One hundred and three switched to PO within 5 days, while 95 remained on intravenous antibiotics for longer than 5 days. On average, patients in the PO group received IV antibiotics for 2 days, while the IV group averaged 15 days. Oral ciprofloxacin treatment length averaged 12 days.
The primary endpoint of treatment failure at 90 days, defined as recurrent infection or all-cause mortality, favored the PO group (1.9% versus 16.8%, P less than .01). This was likely because of patient selection, as those in the IV group tended to be more ill, according to Dr. Cook. More were immunosuppressed (41% IV versus 22% in PO group, P less than .01). There were more nonurinary sources of infection (41% in IV group, P less than .01; 65% urinary source in PO group). Thirty-four percent of the PO group had an infectious disease consult, compared with 60% of the IV group.
SOURCE: Gregory Cook et al. ID Week 2018. Abstract 39.
REPORTING FROM IDWEEK 2018
Key clinical point: Stepping down to oral ciprofloxacin at 48 hours is likely safe in stable patients.
Major finding: The 90-day treatment failure rate was 1.9% in patients switched to oral ciprofloxacin.
Study details: Retrospective analysis of 193 cases.
Disclosures: The study was not funded. Dr. Cook declared no financial conflicts of interest.
Source: ID Week 2018. Abstract 39.
In C. difficile, metronidazole may not benefit ICU patients on vancomycin
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
SAN FRANCISCO – , according to a review of 101 cases at the University of Maryland.
Adding metronidazole is a common move in ICUs when patients start circling the drain with C. difficile, in part because delivery to the gut doesn’t depend on gut motility. “At that point, you are throwing the kitchen sink at them, but it’s” based, like much in C. difficile management, on expert opinion, not evidence, said study lead Ana Vega, PharmD, a former resident at the university’s school of pharmacy in Baltimore, and now an infectious disease pharmacist at Jackson Memorial Hospital, Miami. The investigators wanted to plug the evidence gap. Forty-seven of the 101 patients in their review – all with signs of C. difficile sepsis – had IV metronidazole added to their vancomycin regimens. Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm, and not significantly different (P = .338). There were also no significant differences in resolution rates or normalization of white blood cell counts and temperature.
“Our data question the utility of” of adding IV metronidazole to oral vancomycin in patients with severe disease. “It’s definitely something to think twice about because metronidazole isn’t benign. It makes people feel crummy; you can induce resistance; and it increases the risk of vancomycin-resistant Enterococci colonization,” already a risk with vancomycin, Dr. Vega said at an annual scientific meeting on infectious diseases.
“When you get to the point that you are trying combination therapy based on expert opinion, I think fecal transplants are something to consider” because the success rates are so high. “That would be my suggestion,” she said, even though “it’s much easier to write an order for a drug than to get a fecal transplant.”
The issue is far from resolved, and debate will continue. A similar review of ICU patients at Wake Forest University in Winston-Salem, N.C., did find a significant mortality benefit with combination therapy, regardless of C. difficile severity (Clin Infect Dis. 2015 Sep 15. doi: 10.1093/cid/civ409).
The Maryland investigators excluded patients with toxic megacolon and other life-threatening intra-abdominal complications requiring surgery, because combination therapy is more strongly recommended in fulminant disease. They were interested in people who were not quite ready for the operating room, when what to do is more in doubt.
Subjects were admitted to the ICU from April 2016 to April 2018 with positive C. difficile nucleic acid testing and an order for oral vancomycin. The only statistically significant baseline differences were that patients who got IV metronidazole had higher median white blood cell counts (18,400 versus 13,900 cells/mL; P = .035) and were more likely to receive higher than 500-mg doses of vancomycin (36.2% versus 7.4%; P less than .0001).
The Mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score in the combination group was 23 versus 19 in the monotherapy arm (P = .247). There was no difference in the probability of receiving metronidazole based on the score.
The study again found no significant 30-day mortality differences among 76 patients matched by their APACHE II scores (15.8% in the combination arm versus 9.7%; P = .480).
Severe C. difficile infection was defined as either a white cell count above 15,000 or below 4,000 cells/mL, or a serum creatinine at least 1.5 times above baseline, plus at least one other sign of severe sepsis, such as a mean arterial pressure at or below 60 mm Hg. Metronidazole was started within 72 hours of the first vancomycin dose, and subjects on combination therapy were on both for at least 72 hours.
The mean age in the study was about 60 years old, and just over half of the subjects were men.
Dr. Vega said the investigators hope to expand their sample size and see if patients with more virulent strains of C. difficile do better on combination therapy.
There was no industry funding for the work, and the investigators didn’t have any relevant disclosures.
SOURCE: Vega AD et al. ID Week 2018, Abstract 488.
REPORTING FROM ID WEEK 2018
Key clinical point: The jury is still out on whether adding IV metronidazole helps C. difficile patients already on oral vancomycin in the ICU. Consider fecal transplant.
Major finding: Thirty-day mortality was 14.9% in the combination group versus 7.4% in the monotherapy arm (P = .338).
Study details: Review of 101 ICU patients with severe C. difficile infections
Disclosures: There was no industry funding for the work, and the investigators didn’t have any disclosures.
Source: Vega AD. ID Week 2018, Abstract 488.