User login
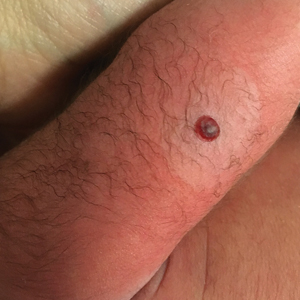
Hemorrhagic Crusted Papule on the Arm
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
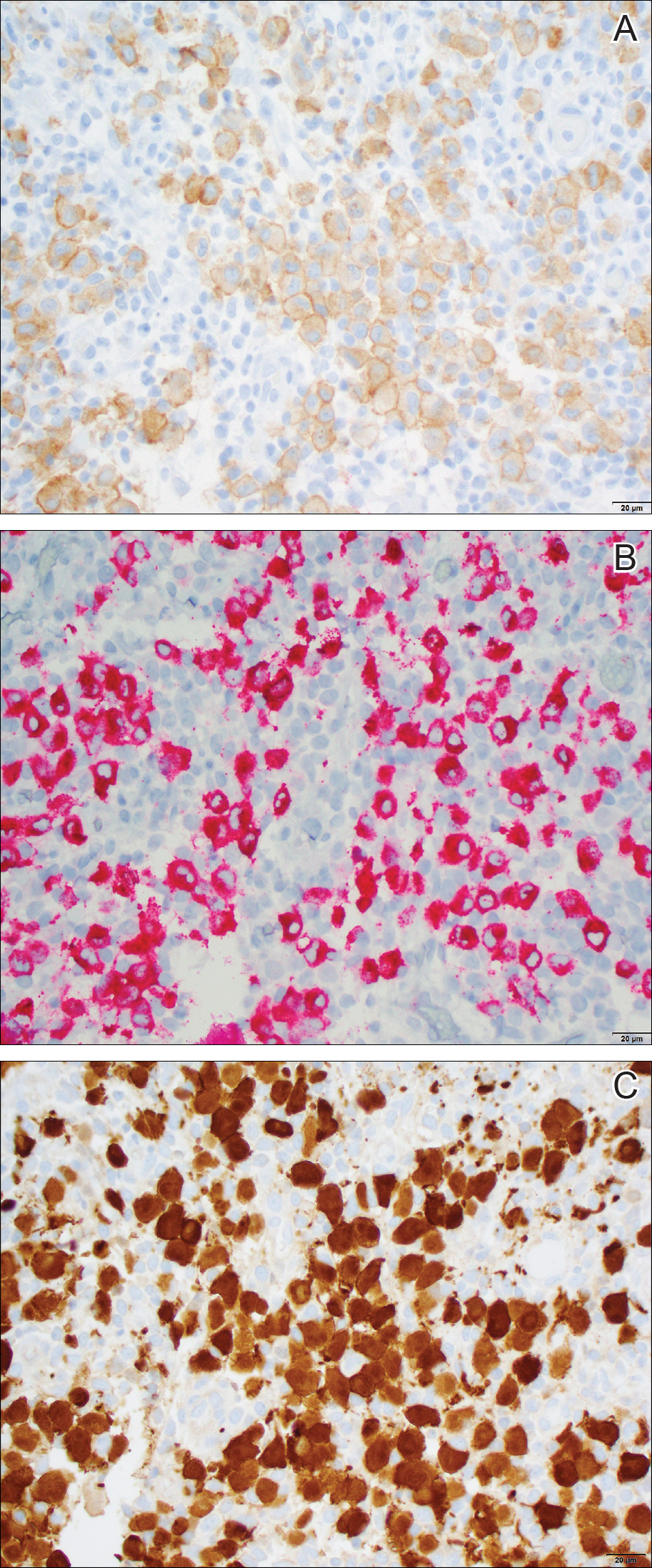
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).
Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).

Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
The Diagnosis: Self-healing Langerhans Cell Histiocytosis
Histopathologic examination showed an infiltrate of mononuclear cells with indented nuclei admixed with a variable dermal inflammatory infiltrate. Immunohistochemistry demonstrated cells that were strongly positive for CD1a (Figure, A) and langerin (Figure, B) antigens as well as S-100 protein (Figure, C), which was consistent with Langerhans cell histiocytosis (LCH).

Histiocytoses are a heterogeneous group of disorders in which the infiltrating cells belong to the mononuclear phagocyte system.1,2 Langerhans cell histiocytosis is the most common dendritic cell-related histiocytosis, occurring in approximately 5 per 1 million children annually, giving it an incidence comparable to pediatric Hodgkin lymphoma and acute myeloid leukemia.1,2
Historically, there has been much debate about the pathogenesis of the disease.2 Until recently it was unknown whether LCH was primarily a neoplastic or an inflammatory disorder. Although the condition initially was thought to have a reactive etiology,1 more recent evidence suggests a clonal neoplastic process. Langerhans cell histiocytosis lesions are clonal and display malignancy-associated mechanisms such as immune evasion. Genome sequencing has revealed several mutations in precursor myeloid cells that result in the common downstream hyperactivation of the mitogen-activated protein kinase signaling pathway that regulates cell proliferation and differentiation.1
Langerhans cell histiocytosis displays a wide spectrum of clinical phenotypes, which historically were subclassified as eosinophilic granulomas (localized lesions in bone), Hand-Schüller-Christian disease (multiple organ involvement with the classic triad of skull defects, diabetes insipidus, and exophthalmos), and Letterer-Siwe disease (visceral lesions involving multiple organs).3 However, in 1997 the Reclassification Working Group of the Histiocyte Society redefined LCH as single-system single site (SS-s) LCH, single-system multisite LCH, and multisystem LCH.4
In SS-s LCH, the most common site is bone (82%), followed by the skin (12%).5 Skin SS-s LCH classically presents as multiple skin lesions at birth without systemic manifestations; the lesions spontaneously involute within a few months.6 Less commonly, skin SS-s LCH can present as a single lesion. Berger et al7 described 4 neonates with unilesional skin SS-s LCH. Since then, more than 30 cases have been reported in the literature,8 and we report herein another unilesional self-healing LCH.
The morphology of skin lesions in self-healing LCH is highly variable, with the most common being multiple erythematous crusted papules (50%), followed by eczematous scaly lesions resembling seborrheic dermatitis in intertriginous areas (37.5%).3,6 Unilesional self-healing LCH typically presents as an ulcerated or crusted nodule or papule on the trunk. This variability results in a large differential diagnosis. Self-healing LCH is easily mistaken for infectious processes including neonatal herpes simplex and varicella-zoster virus infection.9 Often, the dermatology department is consulted to rule out LCH when the asymptomatic neonate does not respond to parenteral acyclovir.
Less commonly, the magenta-colored papulonodules of self-healing LCH can mimic blueberry muffin rash and mandate a workup for intrauterine infections, especially cytomegalovirus, rubella, and blood dyscrasia.10 Other noninfectious processes in the differential of self-healing LCH include congenital infantile hemangioma, neonatal lupus erythematosus, seborrheic dermatitis (cradle cap), pyogenic granuloma, and psoriasis.3,10 Definitive diagnosis requires histopathology.
Because unilesional self-healing LCH has an excellent prognosis and usually resolves on its own, therapy is unnecessary.3,8 One large retrospective study (N=146) found that of all patients with skin lesions, 56% were managed with biopsy only.5 Other options include watchful waiting and topical corticosteroids. If the skin lesions are large, ulcerated, and/or painful, alkylating antitumor agents have been used. For extensive cutaneous disease, systemic corticosteroids combined with chemotherapy and psoralen plus UVA can be effective.6
The primary concern in the management of self-healing LCH is that the solitary skin lesion may be the harbinger of an aggressive disorder that can progress to systemic disease.5 Moreover, recurrent visceral or disseminated disease may occur months to years after resolution of solitary skin lesions.9 Studies have shown that localized and disseminated disease cannot be differentiated on the basis of clinical findings, histology, immunohistochemistry, or biomarkers.3,11 As a result, an evaluation for systemic disease should be performed at the time of diagnosis for cutaneous LCH.3,9 Minimum baseline studies recommended by the Writing Group of the Histiocyte Society include a complete blood cell count, liver function tests, coagulation studies, chest radiography, skeletal surveys, and urine osmolality testing.12 Periodic clinical follow-up is recommended for all variants of LCH.9
Our case was diagnosed as self-healing LCH based on histologic findings. No treatment was required, and at 3-month follow-up the infant was asymptomatic without recurrence and was meeting all developmental milestones.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
- Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to histiocytosis X? Br J Haematol. 2015;169:3-13.
- Jordan MB, Filipovich AH. Histiocytic disorders. In: Hoffman R, Benz EJ Jr, Silberstein LE, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:686-700.
- Stein SL, Paller AS, Haut PR, et al. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778-783.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. Pediatr Blood Cancer. 1997;29:157-166.
- Morimoto A, Ishida Y, Suzuki N, et al. Nationwide survey of single-system single site Langerhans cell histiocytosis in Japan. Pediatr Blood Cancer. 2010;54:98-102.
- Morren MA, Broecke KV, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492.
- Berger TG, Lane AT, Headington JT, et al. A solitary variant of congenital self-healing reticulohistiocytosis: solitary Hashimoto-Pritzker disease. Pediatr Dermatol. 1986;3:230.
- Wheller L, Carman N, Butler G. Unilesional self-limited Langerhans cell histiocytosis: a case report and review of the literature. J Cutan Pathol. 2013;40:595-599.
- Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156.
- Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. 2008;14:8.
- Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children's Medical Center. J Am Acad Dermatol. 2007;56:290-294.
- Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;24:208-209.
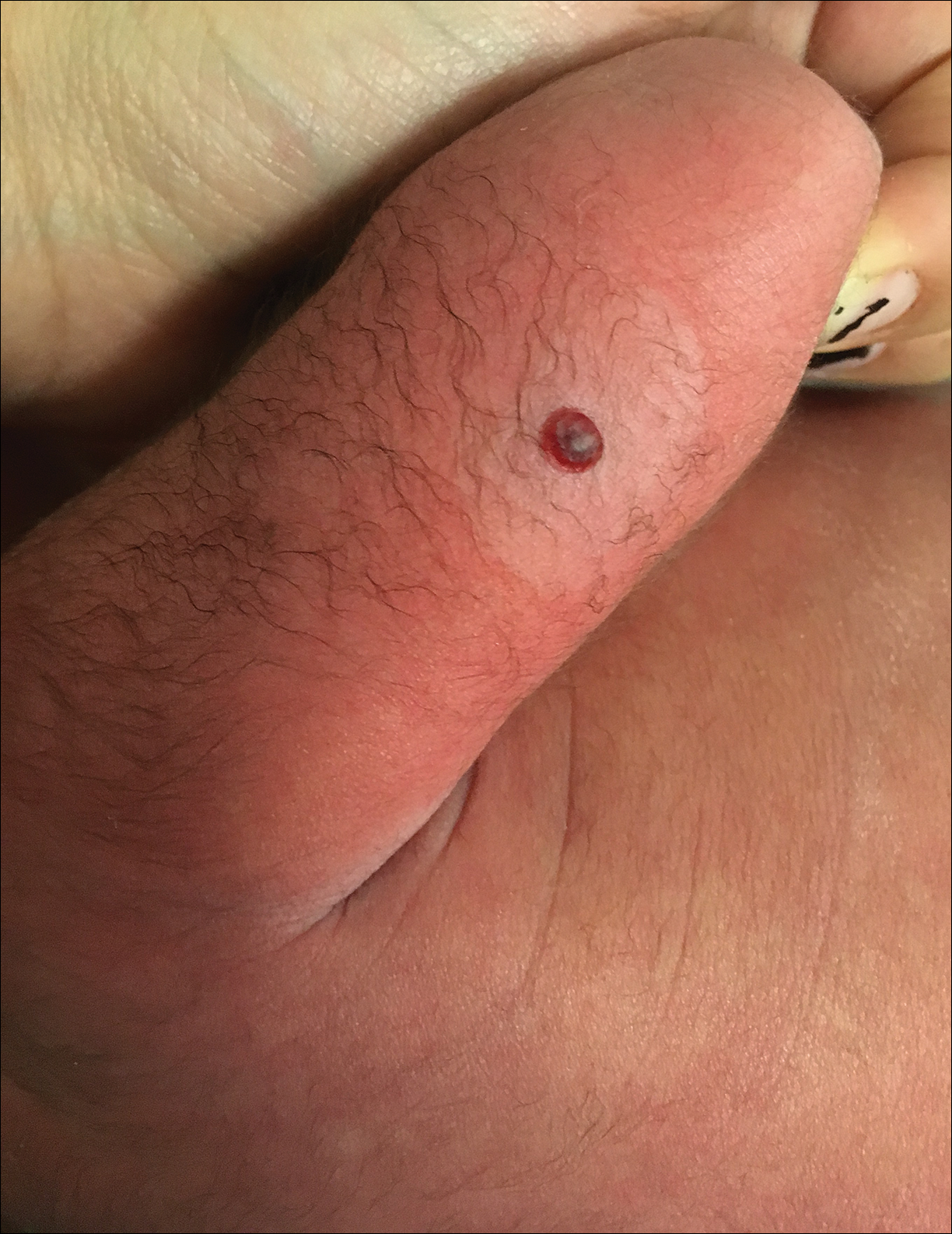
Dermatology consultation was called to the delivery room to evaluate a red, hemorrhagic, crusted, 5-mm papule on the right lateral upper arm of a preterm newborn. He appeared vigorous with an Apgar score of 7 at 1 minute and 8 at 5 minutes. Physical examination was otherwise normal. Of note, the mother presented late to prenatal care. Her herpes simplex and varicella-zoster virus status was unknown. A shave biopsy of the papule was performed at 3 days of age.
Medical exemptions spike after vaccine policy change
according to data from interviews with health officials and immunization staff after implementation of the policy.
In a study published in Pediatrics, Salini Mohanty, DrPH, of the University of Pennsylvania School of Nursing, Philadelphia, and her colleagues conducted semistructured phone interviews with 40 health officers and immunization staff who represented 35 of 61 California heath jurisdictions. The interviews occurred between August 2017 and September 2017, and participants discussed their experiences with medical exemption requests after the policy change.
Although the percentage of fully vaccinated kindergarten students in California increased from 93% in 2015-2016 to 95% in 2017-2018, and the rate of personal belief exemptions declined, overall medical exemption requests rose 250% from 0.2% in 2015-2016 to 0.7% 2017-2018, the researchers noted.
They identified four main issues based on participant responses: the role of stakeholders, the review of medical exemptions received by schools, the medical exemptions perceived as problematic, and the general frustration and concern over medical exemptions.
Based on the interviews, one concerning subtheme involved reports that some physicians wrote medical exemptions for vaccine-hesitant parents based on conditions such as allergies and autoimmune diseases.
“The Internet provides access to physicians who are willing to sign off on exemptions and to websites used to instruct parents on how to get physicians to approve medical exemptions,” the researchers said.
“Understanding how physicians interpret the law is important because they are writing the medical exemptions,” Dr. Mohanty and her associates noted, and they proposed increased outreach and education of physicians about the law to reduce problematic medical exemptions.
Many health officials expressed frustration with their inability to review medical exemptions submitted directly to schools. In fact, interviewees cited one California jurisdiction that was named in a lawsuit for attempting to track medical exemptions, “which had an impact on other jurisdictions decision to track,” they said.
Officials also expressed concern that parents’ use of medical exemptions to replace personal belief exemptions would reduce herd immunity. Overall, regions with high levels of personal belief exemptions showed the largest increases in medical exemptions after SB277, which could put these regions at increased risk for vaccine-preventable outbreaks, the researchers noted.
There also were reports of physicians “who advertised medical exemptions online for a fee.” Officials also reported “receiving medical exemptions signed by physicians who do not typically treat children (cardiologists, dermatologists, surgeons, and physicians at medical marijuana dispensaries) and by unauthorized nonphysician providers, including nurse practitioners,” Dr. Mohanty and her associates said.
The study findings were limited by several factors including small sample size and potential recall bias, the researchers noted. However, the study is the first to include perspectives of local health officials after a change in vaccine exemption policy.
The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
SOURCE: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
Passage of SB277 has had a positive impact on the proportion of California kindergarteners who are fully vaccinated, Richard J. Pan, MD, MPH, and Dorit Rubinstein Reiss, LLB, PhD, wrote in an editorial.
“Vaccines are one of the greatest public health successes in history. Mandating vaccination for school is an effective strategy to prevent outbreaks,” they said. However, “this protection is undermined when unscrupulous physicians monetize their license and abuse the authority delegated to them from the state by granting unwarranted [medical exemptions (MEs)],” they said.
The editorialists emphasized that states have the authority to mandate vaccinations in the interest of public health, and that allowing physicians to grant medical exemptions is appropriate because doctors know their patients and know whether exemptions are needed.
“However, the lack of cooperation by patients’ families who desire unwarranted MEs makes disciplining physicians who are engaged in this unprofessional behavior difficult and costly because licensing boards need to subpoena patient records over families’ objections to obtain evidence. Similarly, professional standard-setting organizations, including professional associations and certification boards, have been reluctant to withdraw credentials or expel members who promote vaccine misinformation and grant unwarranted MEs,” they said. They proposed strategies including establishing a searchable database for MEs, allowing public health officials the option to review and invalidate MEs, and requiring parents to submit MEs to public health departments as well as to schools.
“Pediatricians can partner with public health advocates and proscience parents to pass laws that empower public health officers to protect our children and community. Every child needs community immunity,” they said.
Dr. Pan is a California State Senator, Sacramento, and Dr. Reiss is at the Hastings College of the Law, University of California, San Francisco. Their comments on the article by Mohanty et al. were published in Pediatrics (2018;142[5]:e20182009). Dr. Pan authored legislation (Senate Bill 277) to abolish nonmedical exemption. Dr. Reiss’s family owns regular stock in GlaxoSmithKline.
Passage of SB277 has had a positive impact on the proportion of California kindergarteners who are fully vaccinated, Richard J. Pan, MD, MPH, and Dorit Rubinstein Reiss, LLB, PhD, wrote in an editorial.
“Vaccines are one of the greatest public health successes in history. Mandating vaccination for school is an effective strategy to prevent outbreaks,” they said. However, “this protection is undermined when unscrupulous physicians monetize their license and abuse the authority delegated to them from the state by granting unwarranted [medical exemptions (MEs)],” they said.
The editorialists emphasized that states have the authority to mandate vaccinations in the interest of public health, and that allowing physicians to grant medical exemptions is appropriate because doctors know their patients and know whether exemptions are needed.
“However, the lack of cooperation by patients’ families who desire unwarranted MEs makes disciplining physicians who are engaged in this unprofessional behavior difficult and costly because licensing boards need to subpoena patient records over families’ objections to obtain evidence. Similarly, professional standard-setting organizations, including professional associations and certification boards, have been reluctant to withdraw credentials or expel members who promote vaccine misinformation and grant unwarranted MEs,” they said. They proposed strategies including establishing a searchable database for MEs, allowing public health officials the option to review and invalidate MEs, and requiring parents to submit MEs to public health departments as well as to schools.
“Pediatricians can partner with public health advocates and proscience parents to pass laws that empower public health officers to protect our children and community. Every child needs community immunity,” they said.
Dr. Pan is a California State Senator, Sacramento, and Dr. Reiss is at the Hastings College of the Law, University of California, San Francisco. Their comments on the article by Mohanty et al. were published in Pediatrics (2018;142[5]:e20182009). Dr. Pan authored legislation (Senate Bill 277) to abolish nonmedical exemption. Dr. Reiss’s family owns regular stock in GlaxoSmithKline.
Passage of SB277 has had a positive impact on the proportion of California kindergarteners who are fully vaccinated, Richard J. Pan, MD, MPH, and Dorit Rubinstein Reiss, LLB, PhD, wrote in an editorial.
“Vaccines are one of the greatest public health successes in history. Mandating vaccination for school is an effective strategy to prevent outbreaks,” they said. However, “this protection is undermined when unscrupulous physicians monetize their license and abuse the authority delegated to them from the state by granting unwarranted [medical exemptions (MEs)],” they said.
The editorialists emphasized that states have the authority to mandate vaccinations in the interest of public health, and that allowing physicians to grant medical exemptions is appropriate because doctors know their patients and know whether exemptions are needed.
“However, the lack of cooperation by patients’ families who desire unwarranted MEs makes disciplining physicians who are engaged in this unprofessional behavior difficult and costly because licensing boards need to subpoena patient records over families’ objections to obtain evidence. Similarly, professional standard-setting organizations, including professional associations and certification boards, have been reluctant to withdraw credentials or expel members who promote vaccine misinformation and grant unwarranted MEs,” they said. They proposed strategies including establishing a searchable database for MEs, allowing public health officials the option to review and invalidate MEs, and requiring parents to submit MEs to public health departments as well as to schools.
“Pediatricians can partner with public health advocates and proscience parents to pass laws that empower public health officers to protect our children and community. Every child needs community immunity,” they said.
Dr. Pan is a California State Senator, Sacramento, and Dr. Reiss is at the Hastings College of the Law, University of California, San Francisco. Their comments on the article by Mohanty et al. were published in Pediatrics (2018;142[5]:e20182009). Dr. Pan authored legislation (Senate Bill 277) to abolish nonmedical exemption. Dr. Reiss’s family owns regular stock in GlaxoSmithKline.
according to data from interviews with health officials and immunization staff after implementation of the policy.
In a study published in Pediatrics, Salini Mohanty, DrPH, of the University of Pennsylvania School of Nursing, Philadelphia, and her colleagues conducted semistructured phone interviews with 40 health officers and immunization staff who represented 35 of 61 California heath jurisdictions. The interviews occurred between August 2017 and September 2017, and participants discussed their experiences with medical exemption requests after the policy change.
Although the percentage of fully vaccinated kindergarten students in California increased from 93% in 2015-2016 to 95% in 2017-2018, and the rate of personal belief exemptions declined, overall medical exemption requests rose 250% from 0.2% in 2015-2016 to 0.7% 2017-2018, the researchers noted.
They identified four main issues based on participant responses: the role of stakeholders, the review of medical exemptions received by schools, the medical exemptions perceived as problematic, and the general frustration and concern over medical exemptions.
Based on the interviews, one concerning subtheme involved reports that some physicians wrote medical exemptions for vaccine-hesitant parents based on conditions such as allergies and autoimmune diseases.
“The Internet provides access to physicians who are willing to sign off on exemptions and to websites used to instruct parents on how to get physicians to approve medical exemptions,” the researchers said.
“Understanding how physicians interpret the law is important because they are writing the medical exemptions,” Dr. Mohanty and her associates noted, and they proposed increased outreach and education of physicians about the law to reduce problematic medical exemptions.
Many health officials expressed frustration with their inability to review medical exemptions submitted directly to schools. In fact, interviewees cited one California jurisdiction that was named in a lawsuit for attempting to track medical exemptions, “which had an impact on other jurisdictions decision to track,” they said.
Officials also expressed concern that parents’ use of medical exemptions to replace personal belief exemptions would reduce herd immunity. Overall, regions with high levels of personal belief exemptions showed the largest increases in medical exemptions after SB277, which could put these regions at increased risk for vaccine-preventable outbreaks, the researchers noted.
There also were reports of physicians “who advertised medical exemptions online for a fee.” Officials also reported “receiving medical exemptions signed by physicians who do not typically treat children (cardiologists, dermatologists, surgeons, and physicians at medical marijuana dispensaries) and by unauthorized nonphysician providers, including nurse practitioners,” Dr. Mohanty and her associates said.
The study findings were limited by several factors including small sample size and potential recall bias, the researchers noted. However, the study is the first to include perspectives of local health officials after a change in vaccine exemption policy.
The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
SOURCE: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
according to data from interviews with health officials and immunization staff after implementation of the policy.
In a study published in Pediatrics, Salini Mohanty, DrPH, of the University of Pennsylvania School of Nursing, Philadelphia, and her colleagues conducted semistructured phone interviews with 40 health officers and immunization staff who represented 35 of 61 California heath jurisdictions. The interviews occurred between August 2017 and September 2017, and participants discussed their experiences with medical exemption requests after the policy change.
Although the percentage of fully vaccinated kindergarten students in California increased from 93% in 2015-2016 to 95% in 2017-2018, and the rate of personal belief exemptions declined, overall medical exemption requests rose 250% from 0.2% in 2015-2016 to 0.7% 2017-2018, the researchers noted.
They identified four main issues based on participant responses: the role of stakeholders, the review of medical exemptions received by schools, the medical exemptions perceived as problematic, and the general frustration and concern over medical exemptions.
Based on the interviews, one concerning subtheme involved reports that some physicians wrote medical exemptions for vaccine-hesitant parents based on conditions such as allergies and autoimmune diseases.
“The Internet provides access to physicians who are willing to sign off on exemptions and to websites used to instruct parents on how to get physicians to approve medical exemptions,” the researchers said.
“Understanding how physicians interpret the law is important because they are writing the medical exemptions,” Dr. Mohanty and her associates noted, and they proposed increased outreach and education of physicians about the law to reduce problematic medical exemptions.
Many health officials expressed frustration with their inability to review medical exemptions submitted directly to schools. In fact, interviewees cited one California jurisdiction that was named in a lawsuit for attempting to track medical exemptions, “which had an impact on other jurisdictions decision to track,” they said.
Officials also expressed concern that parents’ use of medical exemptions to replace personal belief exemptions would reduce herd immunity. Overall, regions with high levels of personal belief exemptions showed the largest increases in medical exemptions after SB277, which could put these regions at increased risk for vaccine-preventable outbreaks, the researchers noted.
There also were reports of physicians “who advertised medical exemptions online for a fee.” Officials also reported “receiving medical exemptions signed by physicians who do not typically treat children (cardiologists, dermatologists, surgeons, and physicians at medical marijuana dispensaries) and by unauthorized nonphysician providers, including nurse practitioners,” Dr. Mohanty and her associates said.
The study findings were limited by several factors including small sample size and potential recall bias, the researchers noted. However, the study is the first to include perspectives of local health officials after a change in vaccine exemption policy.
The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
SOURCE: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
FROM PEDIATRICS
Key clinical point: Medical exemptions for childhood vaccinations in California increased after the implementation of Senate Bill 277 (SB277) eliminating nonmedical exemptions.
Major finding: Medical exemptions in California increased by 250% after the SB277 took effect.
Study details: The data come from 34 interviews with 40 health officers and immunization staff about their experiences with medical exemptions before and after the passage of SB277.
Disclosures: The National Institutes of Health supported the study. Dr. Mohanty had no financial conflicts to disclose; one coauthor disclosed relationships with Merck, Pfizer, and Walgreens.
Source: Mohanty S et al. Pediatrics. 2018. doi: 10.1542/peds.2018-1051.
Is respiratory compromise the new “sepsis”?
Hospitalists can play a key role in prevention
Clinicians and even the general public are aware of the dangers of sepsis, the life-threatening illness caused by a body’s response to an infection. Irrespective of one’s perception of pharmaceutical marketing materials or the evidence-based medicine used, awareness about sepsis has led to earlier diagnosis and interventions that have likely saved countless patients’ lives.
Moreover, hospitalists have played a key role in sepsis prevention. In their research, “Improving Survival from Sepsis in Noncritical Units: Role of Hospitalists and Sepsis Team in Early Detection and Initial Treatment of Septic Patients,” Adriana Ducci, MD, and her colleagues showed that a hospitalist-managed sepsis protocol improved sepsis case notifications and patient outcomes.
Although sepsis and respiratory compromise are clearly very different conditions, I believe that greater awareness about respiratory compromise will lead to earlier diagnosis and interventions, which will theoretically improve patient outcomes. Moreover, as with the sepsis awareness campaign, hospitalists can play a key role in recognizing respiratory compromise and in the implementation of appropriate interventions.
As defined by the Respiratory Compromise Institute, “respiratory compromise” is defined as a state in which there is a high likelihood of decompensation into respiratory failure and/or death, but, in which specific interventions – be it therapeutic and/or monitoring – might prevent or mitigate this decompensation.
A significant segment of patients who may be at risk for respiratory compromise are those receiving opioids. The cost of opioid-related adverse events, in terms of both human life and hospital expenses, remains at the forefront of the public eye. It has been estimated that yearly costs in the United States associated with opioid-related postoperative respiratory failure were estimated at $2 billion.
Thomas W. Frederickson MD, FACP, SFHM, MBA, the lead author of the Society of Hospital Medicine guide for Reducing Adverse Drug Events Related to Opioids (RADEO), emphasized in a podcast with the Physician-Patient Alliance for Health & Safety the need to identify patient conditions that pose a greater risk of respiratory compromise.
In particular, Dr. Frederickson pointed out the need to screen for obstructive sleep apnea (OSA): “Patients with obstructive sleep apnea are dependent upon their arousal mechanism in order to avoid respiratory depression and eventual respiratory failure. When these patients receive opioid medication, it decreases this ability for arousal. That puts them at risk for a sudden spiral that includes respiratory insufficiency and respiratory arrest. This can happen very quickly and part of the risk is that the traditional monitoring for sedation that we use in the hospital – that is on a periodic basis and depends upon nursing interventions and questioning – really becomes much less effective in this patient population that can have a respiratory arrest, because of failure to arouse, very quickly. So, a monitoring regimen that takes place every 60 minutes is likely to be ineffective.”
Patient conditions such as OSA should be considered, along with other comorbidities. As the RADEO Guide states: “Before starting opioid therapy, either in surgical or non-surgical settings, it is important to identify any real or potential risks of respiratory depression or other opioid-related adverse effects. Patient comorbidities such as OSA, neurologic disorders, organ impairment, substance abuse history, and other medication use are important aspects to consider.”
Although we have clearly recognized a significant increase in respiratory complications associated with opioid administration, there are other areas, which are non–opioid related, that can create respiratory compromise. We view many patients with stable or underlying respiratory conditions, whether it be COPD, sleep apnea, or preexisting pathophysiology, where either due to sedative agents, or an acute illness – like pneumonia – they can go from a stable condition to respiratory compromise and become at risk for respiratory failure.
A classic example of that in my world of anesthesia has been the well-recognized area of non–operating room anesthesia – in particular, in endoscopy suites where numerous endoscopy procedures are performed under the administration of propofol or other anxiolytic-like drugs. There has been a well-recognized incidence of sentinel events related to oxygenation and ventilation, including death.
Many clinicians see sedation as a benign introduction of relatively limited-effect drugs, which isn’t always true. So, therefore, it is essential that clinicians understand three things:
1. The drugs we employ as sedative agents can have variable effects on individuals depending on their tolerance and their underlying medical condition.
2. The dosages and particular combination of drugs employed may cause an adverse event – for example, the combination of opioids and benzodiazepines.
3. There are factors that can distract from the clinical assessment of routine vital signs, such as respiratory rate, heart rate, and blood pressure. For example, when pulse oximetry is administered with oxygen therapy, there can often be a delay in the recognition of hypoventilation. Consequently, that’s why more and more clinicians are beginning to utilize capnography, or CO2 monitoring, in the expired gas to earlier detect depressed respiratory rate and/or apnea, as well as signs of hypoventilation or inadequate ventilation.
There clearly are obstacles to continuous patient monitoring, such as the associated cost, familiarity with the utilization, the benefit, as well as the limitations of specific monitors in different clinical situations, which mandates an educational process to employ these. However, currently, patient monitoring provides the best early indicator of a patient’s deterioration and the possibility of respiratory compromise.
In my field, we have become very comfortable with capnography and patient monitoring, because for decades it’s been a standard of care for monitoring in the operating room. The role for utilization of capnography for patients who are receiving an opioid or sedative agent outside of the operating room needs to be further assessed. However, technology is not a silver bullet and should be used as an adjunct to clinical judgment in at-risk populations.
Simple recognition and greater awareness of respiratory compromise, just as with sepsis awareness campaigns, will mean more patients are diagnosed earlier, more appropriate interventions are made, and hopefully more adverse events and patient deaths are averted.
Dr. Vender is the emeritus Harris Family Foundation chairman of the department of anesthesiology at NorthShore University Health System in Evanston, Ill. He is clinical professor at the University of Chicago Pritzker School of Medicine and chairman, Clinical Advisory Committee, Respiratory Compromise Institute. Dr. Vender has consulted with Medtronic.
Hospitalists can play a key role in prevention
Hospitalists can play a key role in prevention
Clinicians and even the general public are aware of the dangers of sepsis, the life-threatening illness caused by a body’s response to an infection. Irrespective of one’s perception of pharmaceutical marketing materials or the evidence-based medicine used, awareness about sepsis has led to earlier diagnosis and interventions that have likely saved countless patients’ lives.
Moreover, hospitalists have played a key role in sepsis prevention. In their research, “Improving Survival from Sepsis in Noncritical Units: Role of Hospitalists and Sepsis Team in Early Detection and Initial Treatment of Septic Patients,” Adriana Ducci, MD, and her colleagues showed that a hospitalist-managed sepsis protocol improved sepsis case notifications and patient outcomes.
Although sepsis and respiratory compromise are clearly very different conditions, I believe that greater awareness about respiratory compromise will lead to earlier diagnosis and interventions, which will theoretically improve patient outcomes. Moreover, as with the sepsis awareness campaign, hospitalists can play a key role in recognizing respiratory compromise and in the implementation of appropriate interventions.
As defined by the Respiratory Compromise Institute, “respiratory compromise” is defined as a state in which there is a high likelihood of decompensation into respiratory failure and/or death, but, in which specific interventions – be it therapeutic and/or monitoring – might prevent or mitigate this decompensation.
A significant segment of patients who may be at risk for respiratory compromise are those receiving opioids. The cost of opioid-related adverse events, in terms of both human life and hospital expenses, remains at the forefront of the public eye. It has been estimated that yearly costs in the United States associated with opioid-related postoperative respiratory failure were estimated at $2 billion.
Thomas W. Frederickson MD, FACP, SFHM, MBA, the lead author of the Society of Hospital Medicine guide for Reducing Adverse Drug Events Related to Opioids (RADEO), emphasized in a podcast with the Physician-Patient Alliance for Health & Safety the need to identify patient conditions that pose a greater risk of respiratory compromise.
In particular, Dr. Frederickson pointed out the need to screen for obstructive sleep apnea (OSA): “Patients with obstructive sleep apnea are dependent upon their arousal mechanism in order to avoid respiratory depression and eventual respiratory failure. When these patients receive opioid medication, it decreases this ability for arousal. That puts them at risk for a sudden spiral that includes respiratory insufficiency and respiratory arrest. This can happen very quickly and part of the risk is that the traditional monitoring for sedation that we use in the hospital – that is on a periodic basis and depends upon nursing interventions and questioning – really becomes much less effective in this patient population that can have a respiratory arrest, because of failure to arouse, very quickly. So, a monitoring regimen that takes place every 60 minutes is likely to be ineffective.”
Patient conditions such as OSA should be considered, along with other comorbidities. As the RADEO Guide states: “Before starting opioid therapy, either in surgical or non-surgical settings, it is important to identify any real or potential risks of respiratory depression or other opioid-related adverse effects. Patient comorbidities such as OSA, neurologic disorders, organ impairment, substance abuse history, and other medication use are important aspects to consider.”
Although we have clearly recognized a significant increase in respiratory complications associated with opioid administration, there are other areas, which are non–opioid related, that can create respiratory compromise. We view many patients with stable or underlying respiratory conditions, whether it be COPD, sleep apnea, or preexisting pathophysiology, where either due to sedative agents, or an acute illness – like pneumonia – they can go from a stable condition to respiratory compromise and become at risk for respiratory failure.
A classic example of that in my world of anesthesia has been the well-recognized area of non–operating room anesthesia – in particular, in endoscopy suites where numerous endoscopy procedures are performed under the administration of propofol or other anxiolytic-like drugs. There has been a well-recognized incidence of sentinel events related to oxygenation and ventilation, including death.
Many clinicians see sedation as a benign introduction of relatively limited-effect drugs, which isn’t always true. So, therefore, it is essential that clinicians understand three things:
1. The drugs we employ as sedative agents can have variable effects on individuals depending on their tolerance and their underlying medical condition.
2. The dosages and particular combination of drugs employed may cause an adverse event – for example, the combination of opioids and benzodiazepines.
3. There are factors that can distract from the clinical assessment of routine vital signs, such as respiratory rate, heart rate, and blood pressure. For example, when pulse oximetry is administered with oxygen therapy, there can often be a delay in the recognition of hypoventilation. Consequently, that’s why more and more clinicians are beginning to utilize capnography, or CO2 monitoring, in the expired gas to earlier detect depressed respiratory rate and/or apnea, as well as signs of hypoventilation or inadequate ventilation.
There clearly are obstacles to continuous patient monitoring, such as the associated cost, familiarity with the utilization, the benefit, as well as the limitations of specific monitors in different clinical situations, which mandates an educational process to employ these. However, currently, patient monitoring provides the best early indicator of a patient’s deterioration and the possibility of respiratory compromise.
In my field, we have become very comfortable with capnography and patient monitoring, because for decades it’s been a standard of care for monitoring in the operating room. The role for utilization of capnography for patients who are receiving an opioid or sedative agent outside of the operating room needs to be further assessed. However, technology is not a silver bullet and should be used as an adjunct to clinical judgment in at-risk populations.
Simple recognition and greater awareness of respiratory compromise, just as with sepsis awareness campaigns, will mean more patients are diagnosed earlier, more appropriate interventions are made, and hopefully more adverse events and patient deaths are averted.
Dr. Vender is the emeritus Harris Family Foundation chairman of the department of anesthesiology at NorthShore University Health System in Evanston, Ill. He is clinical professor at the University of Chicago Pritzker School of Medicine and chairman, Clinical Advisory Committee, Respiratory Compromise Institute. Dr. Vender has consulted with Medtronic.
Clinicians and even the general public are aware of the dangers of sepsis, the life-threatening illness caused by a body’s response to an infection. Irrespective of one’s perception of pharmaceutical marketing materials or the evidence-based medicine used, awareness about sepsis has led to earlier diagnosis and interventions that have likely saved countless patients’ lives.
Moreover, hospitalists have played a key role in sepsis prevention. In their research, “Improving Survival from Sepsis in Noncritical Units: Role of Hospitalists and Sepsis Team in Early Detection and Initial Treatment of Septic Patients,” Adriana Ducci, MD, and her colleagues showed that a hospitalist-managed sepsis protocol improved sepsis case notifications and patient outcomes.
Although sepsis and respiratory compromise are clearly very different conditions, I believe that greater awareness about respiratory compromise will lead to earlier diagnosis and interventions, which will theoretically improve patient outcomes. Moreover, as with the sepsis awareness campaign, hospitalists can play a key role in recognizing respiratory compromise and in the implementation of appropriate interventions.
As defined by the Respiratory Compromise Institute, “respiratory compromise” is defined as a state in which there is a high likelihood of decompensation into respiratory failure and/or death, but, in which specific interventions – be it therapeutic and/or monitoring – might prevent or mitigate this decompensation.
A significant segment of patients who may be at risk for respiratory compromise are those receiving opioids. The cost of opioid-related adverse events, in terms of both human life and hospital expenses, remains at the forefront of the public eye. It has been estimated that yearly costs in the United States associated with opioid-related postoperative respiratory failure were estimated at $2 billion.
Thomas W. Frederickson MD, FACP, SFHM, MBA, the lead author of the Society of Hospital Medicine guide for Reducing Adverse Drug Events Related to Opioids (RADEO), emphasized in a podcast with the Physician-Patient Alliance for Health & Safety the need to identify patient conditions that pose a greater risk of respiratory compromise.
In particular, Dr. Frederickson pointed out the need to screen for obstructive sleep apnea (OSA): “Patients with obstructive sleep apnea are dependent upon their arousal mechanism in order to avoid respiratory depression and eventual respiratory failure. When these patients receive opioid medication, it decreases this ability for arousal. That puts them at risk for a sudden spiral that includes respiratory insufficiency and respiratory arrest. This can happen very quickly and part of the risk is that the traditional monitoring for sedation that we use in the hospital – that is on a periodic basis and depends upon nursing interventions and questioning – really becomes much less effective in this patient population that can have a respiratory arrest, because of failure to arouse, very quickly. So, a monitoring regimen that takes place every 60 minutes is likely to be ineffective.”
Patient conditions such as OSA should be considered, along with other comorbidities. As the RADEO Guide states: “Before starting opioid therapy, either in surgical or non-surgical settings, it is important to identify any real or potential risks of respiratory depression or other opioid-related adverse effects. Patient comorbidities such as OSA, neurologic disorders, organ impairment, substance abuse history, and other medication use are important aspects to consider.”
Although we have clearly recognized a significant increase in respiratory complications associated with opioid administration, there are other areas, which are non–opioid related, that can create respiratory compromise. We view many patients with stable or underlying respiratory conditions, whether it be COPD, sleep apnea, or preexisting pathophysiology, where either due to sedative agents, or an acute illness – like pneumonia – they can go from a stable condition to respiratory compromise and become at risk for respiratory failure.
A classic example of that in my world of anesthesia has been the well-recognized area of non–operating room anesthesia – in particular, in endoscopy suites where numerous endoscopy procedures are performed under the administration of propofol or other anxiolytic-like drugs. There has been a well-recognized incidence of sentinel events related to oxygenation and ventilation, including death.
Many clinicians see sedation as a benign introduction of relatively limited-effect drugs, which isn’t always true. So, therefore, it is essential that clinicians understand three things:
1. The drugs we employ as sedative agents can have variable effects on individuals depending on their tolerance and their underlying medical condition.
2. The dosages and particular combination of drugs employed may cause an adverse event – for example, the combination of opioids and benzodiazepines.
3. There are factors that can distract from the clinical assessment of routine vital signs, such as respiratory rate, heart rate, and blood pressure. For example, when pulse oximetry is administered with oxygen therapy, there can often be a delay in the recognition of hypoventilation. Consequently, that’s why more and more clinicians are beginning to utilize capnography, or CO2 monitoring, in the expired gas to earlier detect depressed respiratory rate and/or apnea, as well as signs of hypoventilation or inadequate ventilation.
There clearly are obstacles to continuous patient monitoring, such as the associated cost, familiarity with the utilization, the benefit, as well as the limitations of specific monitors in different clinical situations, which mandates an educational process to employ these. However, currently, patient monitoring provides the best early indicator of a patient’s deterioration and the possibility of respiratory compromise.
In my field, we have become very comfortable with capnography and patient monitoring, because for decades it’s been a standard of care for monitoring in the operating room. The role for utilization of capnography for patients who are receiving an opioid or sedative agent outside of the operating room needs to be further assessed. However, technology is not a silver bullet and should be used as an adjunct to clinical judgment in at-risk populations.
Simple recognition and greater awareness of respiratory compromise, just as with sepsis awareness campaigns, will mean more patients are diagnosed earlier, more appropriate interventions are made, and hopefully more adverse events and patient deaths are averted.
Dr. Vender is the emeritus Harris Family Foundation chairman of the department of anesthesiology at NorthShore University Health System in Evanston, Ill. He is clinical professor at the University of Chicago Pritzker School of Medicine and chairman, Clinical Advisory Committee, Respiratory Compromise Institute. Dr. Vender has consulted with Medtronic.
Full-dose quadrivalent flu vaccine shows increased efficacy in children
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
REPORTING FROM AN ACIP MEETING
Vaccine protects against flu-related hospitalizations in pregnancy
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
A review of more than 1,000 hospitalizations revealed a 40% influenza vaccine effectiveness against laboratory-confirmed influenza-associated hospitalizations during pregnancy, Mark Thompson, MD, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
To date, no study has examined influenza vaccine effectiveness (IVE) against hospitalizations among pregnant women, said Dr. Thompson, of the CDC’s influenza division.
He presented results of a study based on data from the Pregnancy Influenza Vaccine Effectiveness Network (PREVENT), which included public health or health care systems with integrated laboratory, medical, and vaccination records in Australia, Canada (Alberta and Ontario), Israel, and three states (California, Oregon, and Washington). The study included women aged 18-50 years who were pregnant during local influenza seasons from 2010 to 2016. Most of the women were older than 35 years (79%), and in the third trimester (65%), and had no high risk medical conditions (66%). The study was published in Clinical Infectious Diseases (2018 Oct 11. doi: 10.1093/cid/ciy737).
The researchers identified 19,450 hospitalizations with an acute respiratory or febrile illness discharge diagnosis and clinician-ordered real-time reverse transcription polymerase chain reaction (rRT-PCR) testing for flu viruses. Of these, 1,030 (6%) of the women underwent rRT-PCR testing, 54% were diagnosed with either influenza or pneumonia, and 58% had detectable influenza A or B virus infections.
Overall, the adjusted IVE was 40%; 13% of rRT-PCR-confirmed influenza-positive pregnant women and 22% of influenza-negative pregnant women were vaccinated; IVE was adjusted for site, season, season timing, and high-risk medical conditions.
“The takeaway is this is the average performance of the vaccine across multiple countries and different seasons,” and the vaccine effectiveness appeared stable across high-risk medical conditions and trimesters of pregnancy, Dr. Thompson said.
The generalizability of the study findings was limited by the lack of data from low- to middle-income countries, he said during the meeting discussion. However, the ICU admission rate is “what we would expect” and similar to results from previous studies. The consistent results showed the need to increase flu vaccination for pregnant women worldwide and to include study populations from lower-income countries in future research.
Dr. Thompson had no financial conflicts to disclose.
FROM AN ACIP MEETING
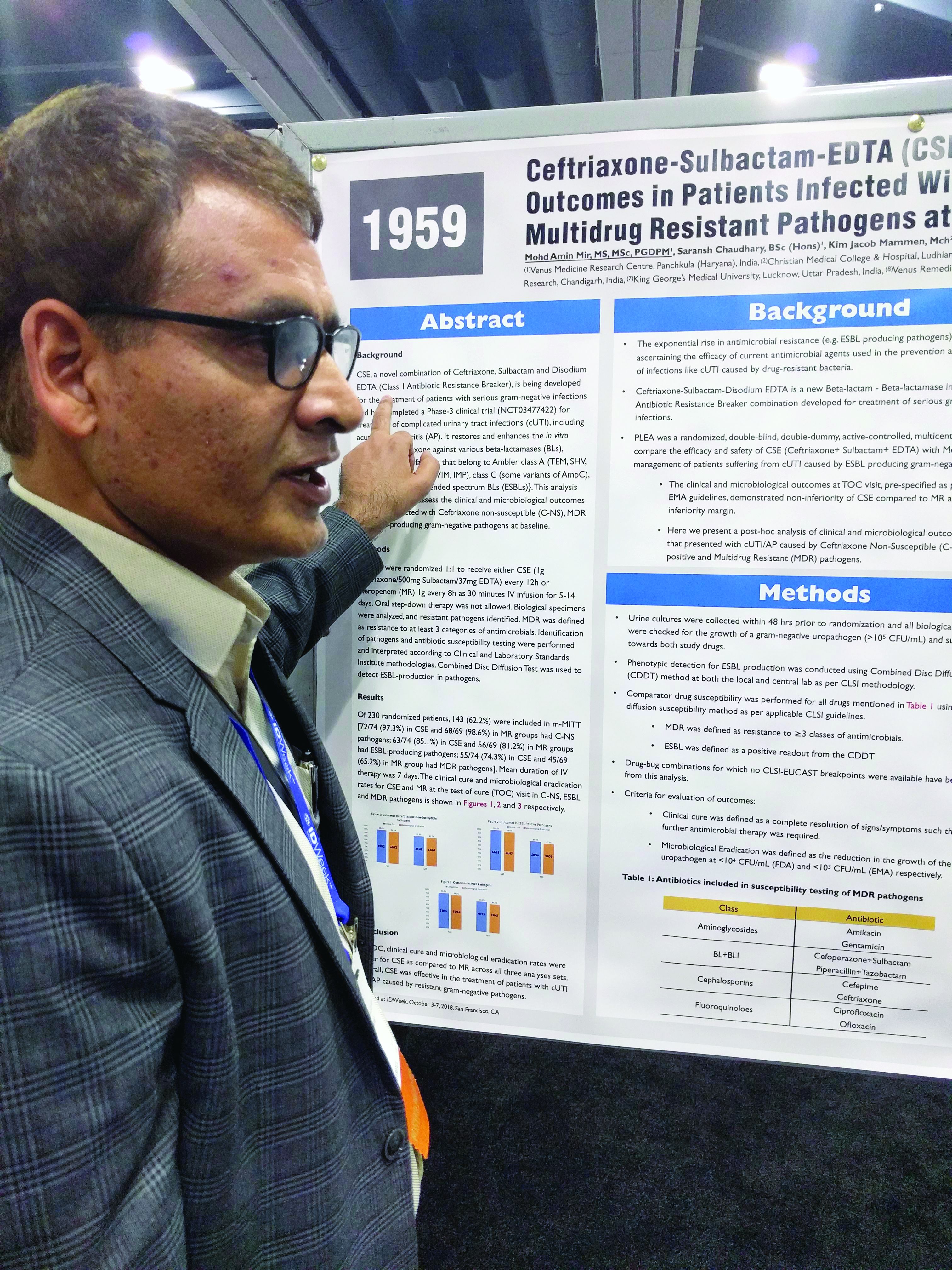
Three-drug combo proves effective against multidrug-resistant UTIs
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
REPORTING FROM IDWEEK 2018
Key clinical point:
Major finding: The combination was noninferior in the context of different resistant subtypes.
Study details: Posthoc analysis of a randomized, controlled trial (n = 143).
Disclosures: The study was funded by Venus Medicine Research Center, which employs Dr. Mir.
Source: Mir MA et al. IDWeek 2018, Abstract 1959.
ACIP resuscitates pertussis working group
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
AT AN ACIP MEETING
Septic shock: Innovative treatment options in the wings
BOSTON– treatment that require more investigation, but nevertheless appear promising, Rishi Rattan, MD, said at the annual clinical congress of the American College of Surgeons.
Trials evaluating vitamin C in this setting have demonstrated a large mortality impact with an absence of side effects, according to Dr. Rattan, a trauma and critical care surgeon with the Ryder Trauma Center at the University of Miami.
“It’s something that I have decided to start early adopting, and many of my colleagues at University of Miami do as well,” Dr. Rattan said in a panel session on updates in septic shock. “We’re anecdotally so far at least seeing good results and are going to be excited to see what these ongoing trials show.”
As an antioxidant, vitamin C has anti-inflammatory properties that may possibly attenuate the overly exuberant inflammatory response seen in septic shock, Dr. Rattan said in his presentation.
The limited clinical data for vitamin C in refractory shock include three studies, of which two are randomized controlled trials, comprising a total of 146 patients, he added.
“I will admit an N of 146 is hardly practice-changing for most people,” Dr. Rattan said. “There’s still a significant and sustained large mortality effect for the use of vitamin C, with nearly no adverse effects.”
Pooled analysis of all three studies revealed a marked reduction in mortality with the use of vitamin C (odds ratio, 0.17, 95% confidence interval 0.07–0.40; P less than .001), according to a meta-analysis recently just published in Critical Care that Dr. Rattan referenced in his presentation (Critical Care 2018;22:258, DOI:10.1186/s13054-018-2191-x).
When taken in recommended dosages, vitamin C given with corticosteroids and thiamine is without known side effects, researcher Paul E. Marik wrote earlier this year in an editorial in Pharmacology & Therapeutics (2018;189[9]:63-70, DOI:10.1016/j.pharmthera.2018.04.007) noted Dr. Rattan, who said he uses the intravenous vitamin C, thiamine, and hydrocortisone protocol previously reported by Dr. Marik and colleagues.
There are 13 ongoing trials, including some prospective blinded, randomized trials, looking at the role of vitamin C in refractory shock, he added.
Angiotensin-II is another intervention that may be promising in refractory septic shock, Dr. Rattan told attendees, pointing to the 2017 publication of the ATHOS-3 trial in the New England Journal of Medicine (2017; 377:419-430,DOI: 10.1056/NEJMoa1704154) showing that treatment increased blood pressure in patients with vasodilatory shock not responding to conventional vasopressors at high doses.
Likewise, methylene blue has shown promise in septic shock, at least in some limited clinical investigations and anecdotally in patients not improving despite standard interventions. “I’ve been able to have a couple patients walk out of the hospital with the use of methylene blue,” Dr. Rattan said. “Again, the plural of ‘anecdote’ is not ‘data,’ but it’s something to consider for the early adopters.”
Dr. Rattan had no disclosures related to his presentation.
BOSTON– treatment that require more investigation, but nevertheless appear promising, Rishi Rattan, MD, said at the annual clinical congress of the American College of Surgeons.
Trials evaluating vitamin C in this setting have demonstrated a large mortality impact with an absence of side effects, according to Dr. Rattan, a trauma and critical care surgeon with the Ryder Trauma Center at the University of Miami.
“It’s something that I have decided to start early adopting, and many of my colleagues at University of Miami do as well,” Dr. Rattan said in a panel session on updates in septic shock. “We’re anecdotally so far at least seeing good results and are going to be excited to see what these ongoing trials show.”
As an antioxidant, vitamin C has anti-inflammatory properties that may possibly attenuate the overly exuberant inflammatory response seen in septic shock, Dr. Rattan said in his presentation.
The limited clinical data for vitamin C in refractory shock include three studies, of which two are randomized controlled trials, comprising a total of 146 patients, he added.
“I will admit an N of 146 is hardly practice-changing for most people,” Dr. Rattan said. “There’s still a significant and sustained large mortality effect for the use of vitamin C, with nearly no adverse effects.”
Pooled analysis of all three studies revealed a marked reduction in mortality with the use of vitamin C (odds ratio, 0.17, 95% confidence interval 0.07–0.40; P less than .001), according to a meta-analysis recently just published in Critical Care that Dr. Rattan referenced in his presentation (Critical Care 2018;22:258, DOI:10.1186/s13054-018-2191-x).
When taken in recommended dosages, vitamin C given with corticosteroids and thiamine is without known side effects, researcher Paul E. Marik wrote earlier this year in an editorial in Pharmacology & Therapeutics (2018;189[9]:63-70, DOI:10.1016/j.pharmthera.2018.04.007) noted Dr. Rattan, who said he uses the intravenous vitamin C, thiamine, and hydrocortisone protocol previously reported by Dr. Marik and colleagues.
There are 13 ongoing trials, including some prospective blinded, randomized trials, looking at the role of vitamin C in refractory shock, he added.
Angiotensin-II is another intervention that may be promising in refractory septic shock, Dr. Rattan told attendees, pointing to the 2017 publication of the ATHOS-3 trial in the New England Journal of Medicine (2017; 377:419-430,DOI: 10.1056/NEJMoa1704154) showing that treatment increased blood pressure in patients with vasodilatory shock not responding to conventional vasopressors at high doses.
Likewise, methylene blue has shown promise in septic shock, at least in some limited clinical investigations and anecdotally in patients not improving despite standard interventions. “I’ve been able to have a couple patients walk out of the hospital with the use of methylene blue,” Dr. Rattan said. “Again, the plural of ‘anecdote’ is not ‘data,’ but it’s something to consider for the early adopters.”
Dr. Rattan had no disclosures related to his presentation.
BOSTON– treatment that require more investigation, but nevertheless appear promising, Rishi Rattan, MD, said at the annual clinical congress of the American College of Surgeons.
Trials evaluating vitamin C in this setting have demonstrated a large mortality impact with an absence of side effects, according to Dr. Rattan, a trauma and critical care surgeon with the Ryder Trauma Center at the University of Miami.
“It’s something that I have decided to start early adopting, and many of my colleagues at University of Miami do as well,” Dr. Rattan said in a panel session on updates in septic shock. “We’re anecdotally so far at least seeing good results and are going to be excited to see what these ongoing trials show.”
As an antioxidant, vitamin C has anti-inflammatory properties that may possibly attenuate the overly exuberant inflammatory response seen in septic shock, Dr. Rattan said in his presentation.
The limited clinical data for vitamin C in refractory shock include three studies, of which two are randomized controlled trials, comprising a total of 146 patients, he added.
“I will admit an N of 146 is hardly practice-changing for most people,” Dr. Rattan said. “There’s still a significant and sustained large mortality effect for the use of vitamin C, with nearly no adverse effects.”
Pooled analysis of all three studies revealed a marked reduction in mortality with the use of vitamin C (odds ratio, 0.17, 95% confidence interval 0.07–0.40; P less than .001), according to a meta-analysis recently just published in Critical Care that Dr. Rattan referenced in his presentation (Critical Care 2018;22:258, DOI:10.1186/s13054-018-2191-x).
When taken in recommended dosages, vitamin C given with corticosteroids and thiamine is without known side effects, researcher Paul E. Marik wrote earlier this year in an editorial in Pharmacology & Therapeutics (2018;189[9]:63-70, DOI:10.1016/j.pharmthera.2018.04.007) noted Dr. Rattan, who said he uses the intravenous vitamin C, thiamine, and hydrocortisone protocol previously reported by Dr. Marik and colleagues.
There are 13 ongoing trials, including some prospective blinded, randomized trials, looking at the role of vitamin C in refractory shock, he added.
Angiotensin-II is another intervention that may be promising in refractory septic shock, Dr. Rattan told attendees, pointing to the 2017 publication of the ATHOS-3 trial in the New England Journal of Medicine (2017; 377:419-430,DOI: 10.1056/NEJMoa1704154) showing that treatment increased blood pressure in patients with vasodilatory shock not responding to conventional vasopressors at high doses.
Likewise, methylene blue has shown promise in septic shock, at least in some limited clinical investigations and anecdotally in patients not improving despite standard interventions. “I’ve been able to have a couple patients walk out of the hospital with the use of methylene blue,” Dr. Rattan said. “Again, the plural of ‘anecdote’ is not ‘data,’ but it’s something to consider for the early adopters.”
Dr. Rattan had no disclosures related to his presentation.
AT THE ACS CLINICAL CONGRESS
Endoscopy-related infections found higher than expected, prophylaxis overused
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
SOURCE: Ofstead C., ICEID 2018 Presentation.
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
SOURCE: Ofstead C., ICEID 2018 Presentation.
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
SOURCE: Ofstead C., ICEID 2018 Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Infection risk is about 1 per 1,000 with colonoscopy; 3 per 1,000 with upper gastrointestinal endoscopy; and 4 per 1,000 with cystoscopy.
Study details: Endoscopic procedures performed at ASCs in 2014 all-payer claims data from 6 U.S. states.
Disclosures: Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
Source: Ofstead C et al. ICEID 2018 Presentation.
Pigmented Pruritic Macules in the Genital Area
The Diagnosis: Pediculosis Pubis
Dermoscopy of the pubic hair demonstrated a louse clutching multiple shafts of hairs (Figure) as well as scattered nits, confirming the presence of Phthirus pubis and diagnosis of pediculosis pubis. The key clinical diagnostic feature in this case was severe itching of the pubic area, with visible nits present on pubic hairs. Itching and infestation also can involve other hair-bearing areas such as the chest, legs, and axillae. The patient was treated with permethrin cream 5% applied to the pubic area and chest. Symptoms and infestation were resolved at 1-week follow-up.
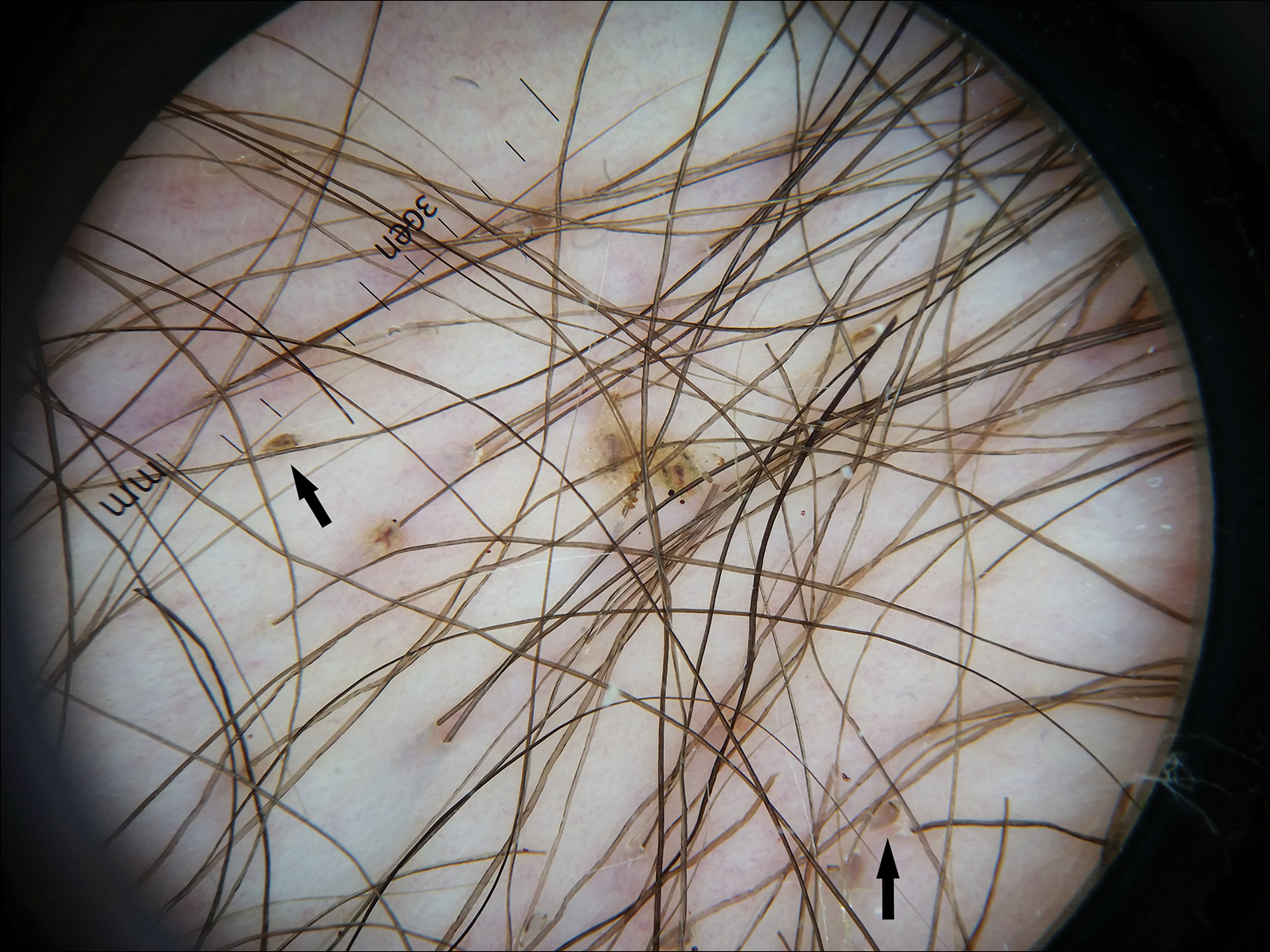
Pediculosis pubis is an infestation of pubic hairs by the pubic (crab) louse P pubis, which feeds on host blood. Other body areas covered with dense hair also may be involved; 60% of patients are infested in at least 2 different sites.1 Pediculosis pubis is most commonly sexually transmitted through direct contact.2 Worldwide prevalence has been estimated at approximately 2% of the adult population, and a survey of 817 US college students in 2009 indicated a lifetime prevalence of 1.3%.3 The prevalence is slightly higher in men, highest in men who have sex with men, and rare in individuals with shaved pubic hair.1 The most common symptom is pruritus of the genital area. Infested patients also may develop asymptomatic bluish gray macules (maculae ceruleae) secondary to hemosiderin deposition from louse bites.4
The diagnosis of pediculosis pubis is made by identification of P pubis, either by examination with the naked eye or confirmation with dermoscopy or microscopy. Although the 0.8- to 1.2-mm lice are visible to the naked eye, they can be difficult to see if not filled with blood, and nits on the hairs can be mistaken for white piedra (fungal infection of the hair shafts) or trichomycosis pubis (bacterial infection of the hair shafts).4 Scabies and tinea cruris do not present with attachments to the hairs. Scabies may present with papules and burrows and tinea cruris with scaly erythematous plaques. Small numbers of lice and nits may be missed by the naked eye or a traditional magnifying glass.5 The use of dermoscopy allows for fast and accurate identification of the characteristic lice and nits, even in these more challenging cases.5 Accurate diagnosis is important, as approximately 31% of infested patients have other concurrent sexually transmitted infections that warrant screening.6
The Centers for Disease Control and Prevention recommends first-line treatment with permethrin cream (1% or 5%) or pyrethrin with piperonyl butoxide applied to all affected areas and washed off after 10 minutes.7 Patients should be reevaluated after 1 week if symptoms persist and re-treated if lice are found on examination.7,8 Malathion lotion 0.5% (applied and washed off after 8-12 hours) or oral ivermectin (250 µg/kg, repeated after 2 weeks) may be used for alternative therapies or cases of permethrin or pyrethrin resistance.7 Ivermectin also is effective for involvement of eyelashes where topical insecticides should not be used.9 Sexual partners should be treated to prevent repeat transmission.7,8 Bedding and clothing can be decontaminated by machine wash on hot cycle or isolating from body contact for 72 hours.7 Patients also should be screened for other sexually transmitted infections, including human immunodeficiency virus.6
- Burkhart CN, Burkhart CG, Morrell DS. Infestations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier/Saunders; 2012:1429-1430.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Anderson AL, Chaney E. Pubic lice (Pthirus pubis): history, biology and treatment vs. knowledge and beliefs of US college students. Int J Environ Res Public Health. 2009;6:592-600.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12; quiz 13-14.
- Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis: a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007;21:837-838.
- Chapel TA, Katta T, Kuszmar T, et al. Pediculosis pubis in a clinic for treatment of sexually transmitted diseases. Sex Transm Dis. 1979;6:257-260.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis. 2007;44(suppl 3):S153-S159.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol. 2000;118:134-135.
The Diagnosis: Pediculosis Pubis
Dermoscopy of the pubic hair demonstrated a louse clutching multiple shafts of hairs (Figure) as well as scattered nits, confirming the presence of Phthirus pubis and diagnosis of pediculosis pubis. The key clinical diagnostic feature in this case was severe itching of the pubic area, with visible nits present on pubic hairs. Itching and infestation also can involve other hair-bearing areas such as the chest, legs, and axillae. The patient was treated with permethrin cream 5% applied to the pubic area and chest. Symptoms and infestation were resolved at 1-week follow-up.

Pediculosis pubis is an infestation of pubic hairs by the pubic (crab) louse P pubis, which feeds on host blood. Other body areas covered with dense hair also may be involved; 60% of patients are infested in at least 2 different sites.1 Pediculosis pubis is most commonly sexually transmitted through direct contact.2 Worldwide prevalence has been estimated at approximately 2% of the adult population, and a survey of 817 US college students in 2009 indicated a lifetime prevalence of 1.3%.3 The prevalence is slightly higher in men, highest in men who have sex with men, and rare in individuals with shaved pubic hair.1 The most common symptom is pruritus of the genital area. Infested patients also may develop asymptomatic bluish gray macules (maculae ceruleae) secondary to hemosiderin deposition from louse bites.4
The diagnosis of pediculosis pubis is made by identification of P pubis, either by examination with the naked eye or confirmation with dermoscopy or microscopy. Although the 0.8- to 1.2-mm lice are visible to the naked eye, they can be difficult to see if not filled with blood, and nits on the hairs can be mistaken for white piedra (fungal infection of the hair shafts) or trichomycosis pubis (bacterial infection of the hair shafts).4 Scabies and tinea cruris do not present with attachments to the hairs. Scabies may present with papules and burrows and tinea cruris with scaly erythematous plaques. Small numbers of lice and nits may be missed by the naked eye or a traditional magnifying glass.5 The use of dermoscopy allows for fast and accurate identification of the characteristic lice and nits, even in these more challenging cases.5 Accurate diagnosis is important, as approximately 31% of infested patients have other concurrent sexually transmitted infections that warrant screening.6
The Centers for Disease Control and Prevention recommends first-line treatment with permethrin cream (1% or 5%) or pyrethrin with piperonyl butoxide applied to all affected areas and washed off after 10 minutes.7 Patients should be reevaluated after 1 week if symptoms persist and re-treated if lice are found on examination.7,8 Malathion lotion 0.5% (applied and washed off after 8-12 hours) or oral ivermectin (250 µg/kg, repeated after 2 weeks) may be used for alternative therapies or cases of permethrin or pyrethrin resistance.7 Ivermectin also is effective for involvement of eyelashes where topical insecticides should not be used.9 Sexual partners should be treated to prevent repeat transmission.7,8 Bedding and clothing can be decontaminated by machine wash on hot cycle or isolating from body contact for 72 hours.7 Patients also should be screened for other sexually transmitted infections, including human immunodeficiency virus.6
The Diagnosis: Pediculosis Pubis
Dermoscopy of the pubic hair demonstrated a louse clutching multiple shafts of hairs (Figure) as well as scattered nits, confirming the presence of Phthirus pubis and diagnosis of pediculosis pubis. The key clinical diagnostic feature in this case was severe itching of the pubic area, with visible nits present on pubic hairs. Itching and infestation also can involve other hair-bearing areas such as the chest, legs, and axillae. The patient was treated with permethrin cream 5% applied to the pubic area and chest. Symptoms and infestation were resolved at 1-week follow-up.

Pediculosis pubis is an infestation of pubic hairs by the pubic (crab) louse P pubis, which feeds on host blood. Other body areas covered with dense hair also may be involved; 60% of patients are infested in at least 2 different sites.1 Pediculosis pubis is most commonly sexually transmitted through direct contact.2 Worldwide prevalence has been estimated at approximately 2% of the adult population, and a survey of 817 US college students in 2009 indicated a lifetime prevalence of 1.3%.3 The prevalence is slightly higher in men, highest in men who have sex with men, and rare in individuals with shaved pubic hair.1 The most common symptom is pruritus of the genital area. Infested patients also may develop asymptomatic bluish gray macules (maculae ceruleae) secondary to hemosiderin deposition from louse bites.4
The diagnosis of pediculosis pubis is made by identification of P pubis, either by examination with the naked eye or confirmation with dermoscopy or microscopy. Although the 0.8- to 1.2-mm lice are visible to the naked eye, they can be difficult to see if not filled with blood, and nits on the hairs can be mistaken for white piedra (fungal infection of the hair shafts) or trichomycosis pubis (bacterial infection of the hair shafts).4 Scabies and tinea cruris do not present with attachments to the hairs. Scabies may present with papules and burrows and tinea cruris with scaly erythematous plaques. Small numbers of lice and nits may be missed by the naked eye or a traditional magnifying glass.5 The use of dermoscopy allows for fast and accurate identification of the characteristic lice and nits, even in these more challenging cases.5 Accurate diagnosis is important, as approximately 31% of infested patients have other concurrent sexually transmitted infections that warrant screening.6
The Centers for Disease Control and Prevention recommends first-line treatment with permethrin cream (1% or 5%) or pyrethrin with piperonyl butoxide applied to all affected areas and washed off after 10 minutes.7 Patients should be reevaluated after 1 week if symptoms persist and re-treated if lice are found on examination.7,8 Malathion lotion 0.5% (applied and washed off after 8-12 hours) or oral ivermectin (250 µg/kg, repeated after 2 weeks) may be used for alternative therapies or cases of permethrin or pyrethrin resistance.7 Ivermectin also is effective for involvement of eyelashes where topical insecticides should not be used.9 Sexual partners should be treated to prevent repeat transmission.7,8 Bedding and clothing can be decontaminated by machine wash on hot cycle or isolating from body contact for 72 hours.7 Patients also should be screened for other sexually transmitted infections, including human immunodeficiency virus.6
- Burkhart CN, Burkhart CG, Morrell DS. Infestations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier/Saunders; 2012:1429-1430.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Anderson AL, Chaney E. Pubic lice (Pthirus pubis): history, biology and treatment vs. knowledge and beliefs of US college students. Int J Environ Res Public Health. 2009;6:592-600.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12; quiz 13-14.
- Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis: a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007;21:837-838.
- Chapel TA, Katta T, Kuszmar T, et al. Pediculosis pubis in a clinic for treatment of sexually transmitted diseases. Sex Transm Dis. 1979;6:257-260.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis. 2007;44(suppl 3):S153-S159.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol. 2000;118:134-135.
- Burkhart CN, Burkhart CG, Morrell DS. Infestations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier/Saunders; 2012:1429-1430.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Anderson AL, Chaney E. Pubic lice (Pthirus pubis): history, biology and treatment vs. knowledge and beliefs of US college students. Int J Environ Res Public Health. 2009;6:592-600.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12; quiz 13-14.
- Chuh A, Lee A, Wong W, et al. Diagnosis of pediculosis pubis: a novel application of digital epiluminescence dermatoscopy. J Eur Acad Dermatol Venereol. 2007;21:837-838.
- Chapel TA, Katta T, Kuszmar T, et al. Pediculosis pubis in a clinic for treatment of sexually transmitted diseases. Sex Transm Dis. 1979;6:257-260.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis. 2007;44(suppl 3):S153-S159.
- Burkhart CN, Burkhart CG. Oral ivermectin therapy for phthiriasis palpebrum. Arch Ophthalmol. 2000;118:134-135.
A 50-year-old man with a history of cerebrovascular accident presented with severe itching along the inguinal folds and over the chest of 2 months' duration. His last sexual encounter was 5 months prior. He had previously seen a primary care physician who told him he needed to clean the hair better. Examination of the genital area revealed pigmented macules and overlying particles among the pubic hair.