User login
FDA approves congenital CMV diagnostic test
, a new test to be used as an aid in the diagnosis of congenital cytomegalovirus (CMV) in newborns less than 21 days of age.
The Alethia CMV Assay Test System detects CMV DNA from a saliva swab. Results from the test should be used only in conjunction with the results of other diagnostic tests and clinical information, according to an FDA statement.
“This test for detecting the virus, when used in conjunction with the results of other diagnostic tests, may help health care providers more quickly identify the virus in newborns,” said Timothy Stenzel, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
In a prospective clinical study, 1,472 saliva samples out of 1,475 samples collected from newborns were correctly identified by the device as negative for the presence of CMV DNA. Three samples were incorrectly identified as positive when they were negative. Five collected saliva specimens were correctly identified as positive for the presence of CMV DNA.
In a testing of 34 samples of archived specimens from babies known to be infected with CMV, all of the archived specimens were correctly identified by the device as positive for the presence of CMV DNA.
The FDA reviewed the Alethia CMV Assay Test System through a regulatory pathway established for novel, low- to moderate-risk devices. Along with this authorization, the FDA is establishing criteria, called special controls, which determine the requirements for demonstrating accuracy, reliability, and effectiveness of tests intended to be used as an aid in the diagnosis of congenital CMV infection.
With this new regulatory classification, subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
The FDA granted marketing authorization of the Alethia CMV Assay Test System to Meridian Bioscience.
, a new test to be used as an aid in the diagnosis of congenital cytomegalovirus (CMV) in newborns less than 21 days of age.
The Alethia CMV Assay Test System detects CMV DNA from a saliva swab. Results from the test should be used only in conjunction with the results of other diagnostic tests and clinical information, according to an FDA statement.
“This test for detecting the virus, when used in conjunction with the results of other diagnostic tests, may help health care providers more quickly identify the virus in newborns,” said Timothy Stenzel, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
In a prospective clinical study, 1,472 saliva samples out of 1,475 samples collected from newborns were correctly identified by the device as negative for the presence of CMV DNA. Three samples were incorrectly identified as positive when they were negative. Five collected saliva specimens were correctly identified as positive for the presence of CMV DNA.
In a testing of 34 samples of archived specimens from babies known to be infected with CMV, all of the archived specimens were correctly identified by the device as positive for the presence of CMV DNA.
The FDA reviewed the Alethia CMV Assay Test System through a regulatory pathway established for novel, low- to moderate-risk devices. Along with this authorization, the FDA is establishing criteria, called special controls, which determine the requirements for demonstrating accuracy, reliability, and effectiveness of tests intended to be used as an aid in the diagnosis of congenital CMV infection.
With this new regulatory classification, subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
The FDA granted marketing authorization of the Alethia CMV Assay Test System to Meridian Bioscience.
, a new test to be used as an aid in the diagnosis of congenital cytomegalovirus (CMV) in newborns less than 21 days of age.
The Alethia CMV Assay Test System detects CMV DNA from a saliva swab. Results from the test should be used only in conjunction with the results of other diagnostic tests and clinical information, according to an FDA statement.
“This test for detecting the virus, when used in conjunction with the results of other diagnostic tests, may help health care providers more quickly identify the virus in newborns,” said Timothy Stenzel, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
In a prospective clinical study, 1,472 saliva samples out of 1,475 samples collected from newborns were correctly identified by the device as negative for the presence of CMV DNA. Three samples were incorrectly identified as positive when they were negative. Five collected saliva specimens were correctly identified as positive for the presence of CMV DNA.
In a testing of 34 samples of archived specimens from babies known to be infected with CMV, all of the archived specimens were correctly identified by the device as positive for the presence of CMV DNA.
The FDA reviewed the Alethia CMV Assay Test System through a regulatory pathway established for novel, low- to moderate-risk devices. Along with this authorization, the FDA is establishing criteria, called special controls, which determine the requirements for demonstrating accuracy, reliability, and effectiveness of tests intended to be used as an aid in the diagnosis of congenital CMV infection.
With this new regulatory classification, subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
The FDA granted marketing authorization of the Alethia CMV Assay Test System to Meridian Bioscience.
Acute flaccid myelitis has unique MRI features
Acute flaccid myelitis appears to present most commonly as asymmetric weakness after respiratory viral infection and has distinctive MRI features that could help with early diagnosis.
In a paper published in JAMA Pediatrics, researchers presented the results of a retrospective case series of 45 children who were diagnosed between 2012 and 2016 with acute flaccid myelitis, or “pseudo polio,” using the Centers for Disease Control’s case definition.
Matthew J. Elrick, MD, PhD, of Johns Hopkins University, Baltimore, and his coauthors came up with a set of reproducible and distinctive features of acute flaccid myelitis. These were the presence of a prodromal fever or viral syndrome; weakness in a lower motor neuron pattern involving one or more limbs, neck, face, and/or bulbar muscles; supportive evidence either from MRI, nerve conduction studies, or cerebrospinal fluid; and the absence of objective sensory deficits, supratentorial white matter, cortical lesions greater than 1 cm in size, encephalopathy, elevated cerebrospinal fluid without pleocytosis, or any other alternative diagnosis.
The researchers commented that, while the CDC case definition has helped with epidemiologic surveillance of acute flaccid myelitis, it may also pick up children with acute weakness caused by other conditions such as transverse myelitis, Guillain-Barré syndrome, ischemic myelopathy, and other myelopathies.
To identify clinical features that might help differentiate patients with acute flaccid myelitis, the researchers attempted to see how many alternative diagnoses were captured in the CDC case definition.
The patients in their study all presented with acute flaccid paralysis in at least one limb and with either an MRI showing a spinal cord lesion spanning one or more spinal segments but largely restricted to gray matter or pleocytosis of the cerebrospinal fluid. The researchers divided the cases into those who also met a well-defined alternative diagnosis – who they categorized as “acute flaccid myelitis with possible alternative diagnosis” (AFM-ad) – and those who were categorized as “restrictively defined AFM” (rAFM). Overall, 34 patients were classified as rAFM and 11 as AFM-ad.
Those in the rAFD group nearly all had asymmetric onset of symptoms, while those in the AFM-ad group were more likely to experience bilateral onset in their lower extremities, “reflecting the pattern of symptoms often seen in other causes of myelopathy such as transverse myelitis and ischemic injury,” the authors noted.
While both groups often presented with decreased muscle tone and reflexes, this was more likely to evolve to increased tone or hyperreflexia in the AFM-ad group. Patients with AFM-ad were also more likely to experience impaired bowel or bladder function.
On MRI, lesions were mostly or completely restricted to the spinal cord gray matter in patients with rAFM or to involve the dorsal pons. These patients did not have any supratentorial brain lesions.
Patients in the rAFM category also had lower cerebrospinal fluid protein values than those in the AFM-ad category, but this was the only cerebrospinal fluid difference between the two groups.
All patients categorized as having rAFM had an infectious prodrome – such as viral syndrome, fever, congestion, and cough – compared with 63.6% of the patients categorized as AFM-ad. The pathogen was identified in only 13 of the rAFM patients, and included 5 patients with enterovirus D68, 2 with unspecified enterovirus, 2 with rhinovirus, 2 with adenovirus, and 2 with mycoplasma. Of the three patients in the AFM-ad group whose pathogen was identified, one had an untyped rhinovirus/enterovirus and mycoplasma, one had a rhinovirus B, and one had enterovirus D68.
“These results highlight that the CDC case definition, while appropriately sensitive for epidemiologic ascertainment of possible AFM cases, also encompasses other neurologic diseases that can cause acute weakness,” the authors wrote. However, they acknowledged that acute flaccid myelitis was still poorly understood and their own definition of the disease may change as more children are diagnosed.
“We propose that the definition of rAFM presented here be used as a starting point for developing inclusion and exclusion criteria for future research studies of AFM,” they wrote.
The study was supported by Johns Hopkins University, the Bart McLean Fund for Neuroimmunology Research, and Project Restore. Two authors reported funding from private industry outside the submitted work and five reported support from or involvement with research and funding bodies.
SOURCE: Elrick MJ et al. JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4890.
Acute flaccid myelitis (AFM) initially presents subtly, complicating its diagnosis. Children present with a rapid onset of weakness that is associated with a febrile illness, which can be respiratory, gastrointestinal, or with symptoms of hand-foot-and-mouth disease. Given the lack of effective treatments, early diagnosis and monitoring are essential for mitigating the risk of respiratory decline and long-term complications.
While patient history and physical examination can provide clues to the presence of AFM, confirming the diagnosis requires lumbar puncture and MRI of the spinal cord. On MRI, diagnostic confirmation will come from findings of longitudinal, butterfly-shaped, anterior horn–predominant T2 and fluid-attenuated inversion recovery hyperintensities of the central gray matter.
Patients with suspected AFM should be hospitalized because they can rapidly deteriorate to the point of respiratory compromise, particularly those with upper extremity and bulbar weakness.
Sarah E. Hopkins, MD, is from the division of neurology at the Children’s Hospital of Philadelphia; Matthew J. Elrick, MD, PhD, is from the department of neurology at Johns Hopkins University, Baltimore; and Kevin Messacar, MD, is from the department of pediatrics at the Children’s Hospital Colorado. These comments are taken from an accompanying viewpoint (JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4896). Dr. Messacar reported support from the National Institutes of Health/National Institute of Allergy and Infectious and Dr. Hopkins reported support from the Centers for Disease Control and Prevention.
Acute flaccid myelitis (AFM) initially presents subtly, complicating its diagnosis. Children present with a rapid onset of weakness that is associated with a febrile illness, which can be respiratory, gastrointestinal, or with symptoms of hand-foot-and-mouth disease. Given the lack of effective treatments, early diagnosis and monitoring are essential for mitigating the risk of respiratory decline and long-term complications.
While patient history and physical examination can provide clues to the presence of AFM, confirming the diagnosis requires lumbar puncture and MRI of the spinal cord. On MRI, diagnostic confirmation will come from findings of longitudinal, butterfly-shaped, anterior horn–predominant T2 and fluid-attenuated inversion recovery hyperintensities of the central gray matter.
Patients with suspected AFM should be hospitalized because they can rapidly deteriorate to the point of respiratory compromise, particularly those with upper extremity and bulbar weakness.
Sarah E. Hopkins, MD, is from the division of neurology at the Children’s Hospital of Philadelphia; Matthew J. Elrick, MD, PhD, is from the department of neurology at Johns Hopkins University, Baltimore; and Kevin Messacar, MD, is from the department of pediatrics at the Children’s Hospital Colorado. These comments are taken from an accompanying viewpoint (JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4896). Dr. Messacar reported support from the National Institutes of Health/National Institute of Allergy and Infectious and Dr. Hopkins reported support from the Centers for Disease Control and Prevention.
Acute flaccid myelitis (AFM) initially presents subtly, complicating its diagnosis. Children present with a rapid onset of weakness that is associated with a febrile illness, which can be respiratory, gastrointestinal, or with symptoms of hand-foot-and-mouth disease. Given the lack of effective treatments, early diagnosis and monitoring are essential for mitigating the risk of respiratory decline and long-term complications.
While patient history and physical examination can provide clues to the presence of AFM, confirming the diagnosis requires lumbar puncture and MRI of the spinal cord. On MRI, diagnostic confirmation will come from findings of longitudinal, butterfly-shaped, anterior horn–predominant T2 and fluid-attenuated inversion recovery hyperintensities of the central gray matter.
Patients with suspected AFM should be hospitalized because they can rapidly deteriorate to the point of respiratory compromise, particularly those with upper extremity and bulbar weakness.
Sarah E. Hopkins, MD, is from the division of neurology at the Children’s Hospital of Philadelphia; Matthew J. Elrick, MD, PhD, is from the department of neurology at Johns Hopkins University, Baltimore; and Kevin Messacar, MD, is from the department of pediatrics at the Children’s Hospital Colorado. These comments are taken from an accompanying viewpoint (JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4896). Dr. Messacar reported support from the National Institutes of Health/National Institute of Allergy and Infectious and Dr. Hopkins reported support from the Centers for Disease Control and Prevention.
Acute flaccid myelitis appears to present most commonly as asymmetric weakness after respiratory viral infection and has distinctive MRI features that could help with early diagnosis.
In a paper published in JAMA Pediatrics, researchers presented the results of a retrospective case series of 45 children who were diagnosed between 2012 and 2016 with acute flaccid myelitis, or “pseudo polio,” using the Centers for Disease Control’s case definition.
Matthew J. Elrick, MD, PhD, of Johns Hopkins University, Baltimore, and his coauthors came up with a set of reproducible and distinctive features of acute flaccid myelitis. These were the presence of a prodromal fever or viral syndrome; weakness in a lower motor neuron pattern involving one or more limbs, neck, face, and/or bulbar muscles; supportive evidence either from MRI, nerve conduction studies, or cerebrospinal fluid; and the absence of objective sensory deficits, supratentorial white matter, cortical lesions greater than 1 cm in size, encephalopathy, elevated cerebrospinal fluid without pleocytosis, or any other alternative diagnosis.
The researchers commented that, while the CDC case definition has helped with epidemiologic surveillance of acute flaccid myelitis, it may also pick up children with acute weakness caused by other conditions such as transverse myelitis, Guillain-Barré syndrome, ischemic myelopathy, and other myelopathies.
To identify clinical features that might help differentiate patients with acute flaccid myelitis, the researchers attempted to see how many alternative diagnoses were captured in the CDC case definition.
The patients in their study all presented with acute flaccid paralysis in at least one limb and with either an MRI showing a spinal cord lesion spanning one or more spinal segments but largely restricted to gray matter or pleocytosis of the cerebrospinal fluid. The researchers divided the cases into those who also met a well-defined alternative diagnosis – who they categorized as “acute flaccid myelitis with possible alternative diagnosis” (AFM-ad) – and those who were categorized as “restrictively defined AFM” (rAFM). Overall, 34 patients were classified as rAFM and 11 as AFM-ad.
Those in the rAFD group nearly all had asymmetric onset of symptoms, while those in the AFM-ad group were more likely to experience bilateral onset in their lower extremities, “reflecting the pattern of symptoms often seen in other causes of myelopathy such as transverse myelitis and ischemic injury,” the authors noted.
While both groups often presented with decreased muscle tone and reflexes, this was more likely to evolve to increased tone or hyperreflexia in the AFM-ad group. Patients with AFM-ad were also more likely to experience impaired bowel or bladder function.
On MRI, lesions were mostly or completely restricted to the spinal cord gray matter in patients with rAFM or to involve the dorsal pons. These patients did not have any supratentorial brain lesions.
Patients in the rAFM category also had lower cerebrospinal fluid protein values than those in the AFM-ad category, but this was the only cerebrospinal fluid difference between the two groups.
All patients categorized as having rAFM had an infectious prodrome – such as viral syndrome, fever, congestion, and cough – compared with 63.6% of the patients categorized as AFM-ad. The pathogen was identified in only 13 of the rAFM patients, and included 5 patients with enterovirus D68, 2 with unspecified enterovirus, 2 with rhinovirus, 2 with adenovirus, and 2 with mycoplasma. Of the three patients in the AFM-ad group whose pathogen was identified, one had an untyped rhinovirus/enterovirus and mycoplasma, one had a rhinovirus B, and one had enterovirus D68.
“These results highlight that the CDC case definition, while appropriately sensitive for epidemiologic ascertainment of possible AFM cases, also encompasses other neurologic diseases that can cause acute weakness,” the authors wrote. However, they acknowledged that acute flaccid myelitis was still poorly understood and their own definition of the disease may change as more children are diagnosed.
“We propose that the definition of rAFM presented here be used as a starting point for developing inclusion and exclusion criteria for future research studies of AFM,” they wrote.
The study was supported by Johns Hopkins University, the Bart McLean Fund for Neuroimmunology Research, and Project Restore. Two authors reported funding from private industry outside the submitted work and five reported support from or involvement with research and funding bodies.
SOURCE: Elrick MJ et al. JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4890.
Acute flaccid myelitis appears to present most commonly as asymmetric weakness after respiratory viral infection and has distinctive MRI features that could help with early diagnosis.
In a paper published in JAMA Pediatrics, researchers presented the results of a retrospective case series of 45 children who were diagnosed between 2012 and 2016 with acute flaccid myelitis, or “pseudo polio,” using the Centers for Disease Control’s case definition.
Matthew J. Elrick, MD, PhD, of Johns Hopkins University, Baltimore, and his coauthors came up with a set of reproducible and distinctive features of acute flaccid myelitis. These were the presence of a prodromal fever or viral syndrome; weakness in a lower motor neuron pattern involving one or more limbs, neck, face, and/or bulbar muscles; supportive evidence either from MRI, nerve conduction studies, or cerebrospinal fluid; and the absence of objective sensory deficits, supratentorial white matter, cortical lesions greater than 1 cm in size, encephalopathy, elevated cerebrospinal fluid without pleocytosis, or any other alternative diagnosis.
The researchers commented that, while the CDC case definition has helped with epidemiologic surveillance of acute flaccid myelitis, it may also pick up children with acute weakness caused by other conditions such as transverse myelitis, Guillain-Barré syndrome, ischemic myelopathy, and other myelopathies.
To identify clinical features that might help differentiate patients with acute flaccid myelitis, the researchers attempted to see how many alternative diagnoses were captured in the CDC case definition.
The patients in their study all presented with acute flaccid paralysis in at least one limb and with either an MRI showing a spinal cord lesion spanning one or more spinal segments but largely restricted to gray matter or pleocytosis of the cerebrospinal fluid. The researchers divided the cases into those who also met a well-defined alternative diagnosis – who they categorized as “acute flaccid myelitis with possible alternative diagnosis” (AFM-ad) – and those who were categorized as “restrictively defined AFM” (rAFM). Overall, 34 patients were classified as rAFM and 11 as AFM-ad.
Those in the rAFD group nearly all had asymmetric onset of symptoms, while those in the AFM-ad group were more likely to experience bilateral onset in their lower extremities, “reflecting the pattern of symptoms often seen in other causes of myelopathy such as transverse myelitis and ischemic injury,” the authors noted.
While both groups often presented with decreased muscle tone and reflexes, this was more likely to evolve to increased tone or hyperreflexia in the AFM-ad group. Patients with AFM-ad were also more likely to experience impaired bowel or bladder function.
On MRI, lesions were mostly or completely restricted to the spinal cord gray matter in patients with rAFM or to involve the dorsal pons. These patients did not have any supratentorial brain lesions.
Patients in the rAFM category also had lower cerebrospinal fluid protein values than those in the AFM-ad category, but this was the only cerebrospinal fluid difference between the two groups.
All patients categorized as having rAFM had an infectious prodrome – such as viral syndrome, fever, congestion, and cough – compared with 63.6% of the patients categorized as AFM-ad. The pathogen was identified in only 13 of the rAFM patients, and included 5 patients with enterovirus D68, 2 with unspecified enterovirus, 2 with rhinovirus, 2 with adenovirus, and 2 with mycoplasma. Of the three patients in the AFM-ad group whose pathogen was identified, one had an untyped rhinovirus/enterovirus and mycoplasma, one had a rhinovirus B, and one had enterovirus D68.
“These results highlight that the CDC case definition, while appropriately sensitive for epidemiologic ascertainment of possible AFM cases, also encompasses other neurologic diseases that can cause acute weakness,” the authors wrote. However, they acknowledged that acute flaccid myelitis was still poorly understood and their own definition of the disease may change as more children are diagnosed.
“We propose that the definition of rAFM presented here be used as a starting point for developing inclusion and exclusion criteria for future research studies of AFM,” they wrote.
The study was supported by Johns Hopkins University, the Bart McLean Fund for Neuroimmunology Research, and Project Restore. Two authors reported funding from private industry outside the submitted work and five reported support from or involvement with research and funding bodies.
SOURCE: Elrick MJ et al. JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4890.
FROM JAMA PEDIATRICS
Key clinical point: Acute flaccid myelitis has distinct features that can distinguish it from other similar conditions.
Major finding: Asymmetric onset of symptoms and MRI signature can help distinguish acute flaccid myelitis from alternative diagnoses.
Study details: A retrospective case series in 45 children diagnosed with acute flaccid myelitis.
Disclosures: The study was supported by Johns Hopkins University, the Bart McLean Fund for Neuroimmunology Research, and Project Restore. Two authors reported funding from private industry outside the submitted work and five reported support from or involvement with research and funding bodies.
Source: Elrick MJ et al. JAMA Pediatr. 2018 Nov 30. doi: 10.1001/jamapediatrics.2018.4890.
DRESS Syndrome Induced by Telaprevir: A Potentially Fatal Adverse Event in Chronic Hepatitis C Therapy
To the Editor:
A 58-year-old woman with a history of hyperprolactinemia and gastrointestinal angiodysplasia presented to the dermatology department with a generalized skin rash of 3 weeks’ duration. She did not have a history of toxic habits. She had a history of chronic hepatitis C virus (HCV) genotype 1b (IL-28B locus) with severe hepatic fibrosis (stage 4) as assessed by ultrasound-based elastography. Due to lack of response, plasma HCV RNA was still detectable at week 12 of pegylated interferon and ribavirin (RIB) therapy, and triple therapy with pegylated interferon, RIB, and telaprevir was initiated.
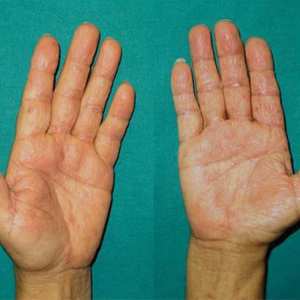
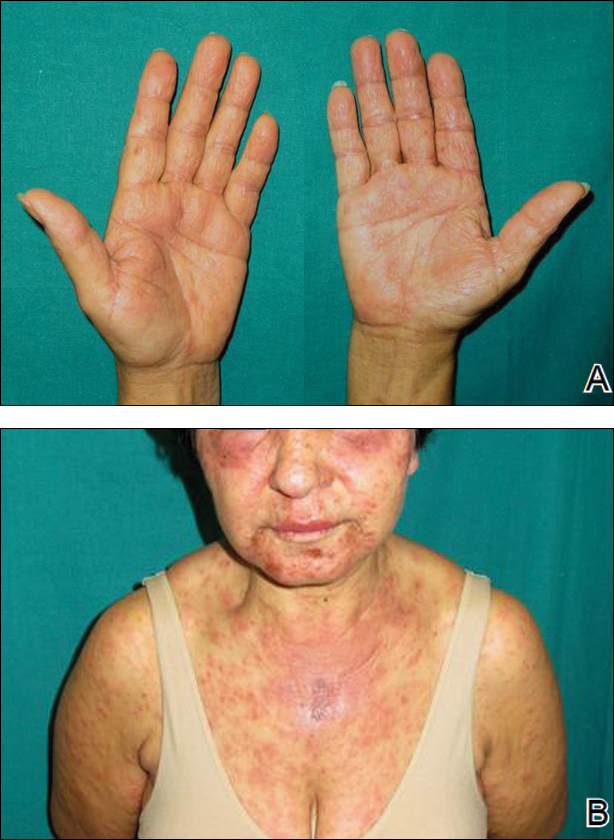
Two months later, she was admitted to the hospital after developing a generalized cutaneous rash that covered 90% of the body surface area (BSA) along with fever (temperature, 38.5°C). Laboratory blood tests showed an elevated absolute eosinophil count (2000 cells/µL [reference range, 0–500 cells/µL]), anemia (hemoglobin, 6.5 g/dL [reference range, 12–16 g/dL]), thrombocytopenia (26×103/µL [reference range, 150–400×103/µL]), and altered liver function tests (serum alanine aminotransferase, 60 U/L [reference range, 0–45 U/L]; aspartate aminotransferase, 80 U/L [reference range, 0–40 U/L]). Plasma HCV RNA was undetectable at this visit. On physical examination a generalized exanthema with coalescing plaques was observed, as well as crusted vesicles covering the arms, legs, chest, abdomen, and back. Palmoplantar papules (Figure, A) and facial swelling (Figure, B) also were present. A skin biopsy specimen taken from a papule on the left arm showed superficial perivascular lymphocytic infiltration with dermal edema. These findings were consistent with a diagnosis of DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Application of the Adverse Drug Reaction Probability Scale1 in our patient (total score of 5) suggested that DRESS syndrome was a moderate adverse event likely related to the use of telaprevir.
After diagnosis of DRESS syndrome, telaprevir was discontinued, and the doses of RIB and pegylated interferon were reduced to 200 mg and 180 µg weekly, respectively. Laboratory test values including liver function tests normalized within 3 weeks and remained normal on follow-up. Plasma HCV RNA continued to be undetectable.
Hepatitis C virus is relatively common with an incidence of 3% worldwide.2 It may present as an acute hepatitis or, more frequently, as asymptomatic chronic hepatitis. The acute process is self-limited and rarely causes hepatic failure. It usually leads to a chronic infection, which can result in cirrhosis, hepatocellular carcinoma, and the need for liver transplantation. The aim of treatment is eradication of HCV RNA, which is predicted by the attainment of a sustained virologic response. The latter is defined by the absence of HCV RNA by a polymerase chain reaction within 3 to 6 months after cessation of treatment.
Treatment of chronic HCV was based on the combination of pegylated interferon alfa-2a or -2b with RIB until 2015. Guidelines for the diagnosis and management of HCV infection have been published by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.2 These guidelines include new protease inhibitors, telaprevir and boceprevir, in the therapeutic approach of these patients. The main limitation of both drugs is the cutaneous toxicity.
Factors to be considered when treating HCV include viral genotype, if the patient is naïve or pretreated, the degree of fibrosis, established cirrhosis, and the treatment response. For patients with genotype 1,2 as in our case, combination therapy with 3 drugs is recommended: pegylated interferon 180 µg subcutaneous injection weekly, RIB 15 mg/kg daily, and telaprevir 2250 mg or boceprevir 2400 mg daily. Triple therapy has been shown to achieve a successful response in 75% of naïve patients and in 50% of patients refractory to standard therapy.3
Telaprevir is an NS3/4A protease inhibitor approved by the US Food and Drug Administration and the European Medicines Agency for treatment of chronic HCV infection in naïve patients and in those unresponsive to double therapy. In phase 2 clinical trials, 41% to 61% of patients treated with telaprevir developed cutaneous reactions, of which 5% to 8% required cessation of treatment.4 The predicting risk factors for developing a secondary rash to telaprevir include age older than 45 years, body mass index less than 30, Caucasian ethnicity, and receiving HCV therapy for the first time.4
This cutaneous side effect is managed depending on the extension of the lesions, the presence of systemic symptoms, and laboratory abnormalities.5 Therefore, the severity of the skin reaction can be divided into 4 stages4,5: (1) grade I or mild, defined as a localized rash with no systemic signs or mucosal involvement; (2) grade II or moderate, a maximum of 50% BSA involvement without epidermal detachment, and inflammation of the mucous membranes may be present without ulcers, as well as systemic symptoms such as fever, arthralgia, or eosinophilia; (3) grade III or severe, skin lesions affecting more than 50% BSA or less if any of the following lesions are present: vesicles or blisters, ulcers, epidermal detachment, palpable purpura, or erythema that does not blanch under pressure; (4) grade IV or life-threatening, when the clinical picture is consistent with acute generalized exanthematous pustulosis, DRESS syndrome, toxic epidermal necrolysis, or Stevens-Johnson syndrome.
DRESS syndrome is a condition clinically characterized by a generalized skin rash, facial angioedema, high fever, lymph node enlargement, and leukocytosis with eosinophilia or atypical lymphocytosis, along with abnormal renal and hepatic function tests. Cutaneous histopathologic examination may be unspecific, though atypical lymphocytes with a marked epidermotropism mimicking fungoid mycosis also have been described.6 In addition, human herpesvirus 6 serology may be negative, despite infection with this herpesvirus subtype having been associated with the development of DRESS syndrome. The pathophysiologic mechanism of DRESS syndrome is not completely understood; however, one theory ascribes an immunologic activation due to drug metabolite formation as the main mechanism.1
Eleven patients7 with possible DRESS syndrome have been reported in clinical trials (less than 5% of the total of patients), with an addition of 1 more by Montaudié et al.8 No notable differences were found between telaprevir levels in these patients with respect to those of the control group.
For the management of DRESS syndrome, the occurrence of early signs of a severe acute skin reaction requires the immediate cessation of the drug, telaprevir in this case. The withdrawal of the dual therapy will depend on the short-term clinical course, according to the general condition of the patient, as well as the analytical abnormalities observed.9
In conclusion, telaprevir is a promising novel therapy for the treatment of HCV infection, but its cutaneous side effects still need to be properly established.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther. 1981;30:239-245.
- HCV guidance: recommendations for testing, managing, and treating hepatitis C. HCV Guidelines website. http://www.hcvguidelines.org. Accessed August 11, 2018.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Roujeau JC, Mockenhaupt M, Tahan SR, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- De Vriese AS, Philippe J, Van Renterghem DM, et al. Carbamazepine hypersensitivity syndrome: report of 4 cases and review of the literature. Medicine (Baltimore). 1995;74:144-151.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review [published online May 17, 2011]. Am J Med. 2011;124:588-597.
- Montaudié H, Passeron T, Cardot-Leccia N, et al. Drug rash with eosinophilia and systemic symptoms due to telaprevir. Dermatology. 2010;221:303-305.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
To the Editor:
A 58-year-old woman with a history of hyperprolactinemia and gastrointestinal angiodysplasia presented to the dermatology department with a generalized skin rash of 3 weeks’ duration. She did not have a history of toxic habits. She had a history of chronic hepatitis C virus (HCV) genotype 1b (IL-28B locus) with severe hepatic fibrosis (stage 4) as assessed by ultrasound-based elastography. Due to lack of response, plasma HCV RNA was still detectable at week 12 of pegylated interferon and ribavirin (RIB) therapy, and triple therapy with pegylated interferon, RIB, and telaprevir was initiated.
Two months later, she was admitted to the hospital after developing a generalized cutaneous rash that covered 90% of the body surface area (BSA) along with fever (temperature, 38.5°C). Laboratory blood tests showed an elevated absolute eosinophil count (2000 cells/µL [reference range, 0–500 cells/µL]), anemia (hemoglobin, 6.5 g/dL [reference range, 12–16 g/dL]), thrombocytopenia (26×103/µL [reference range, 150–400×103/µL]), and altered liver function tests (serum alanine aminotransferase, 60 U/L [reference range, 0–45 U/L]; aspartate aminotransferase, 80 U/L [reference range, 0–40 U/L]). Plasma HCV RNA was undetectable at this visit. On physical examination a generalized exanthema with coalescing plaques was observed, as well as crusted vesicles covering the arms, legs, chest, abdomen, and back. Palmoplantar papules (Figure, A) and facial swelling (Figure, B) also were present. A skin biopsy specimen taken from a papule on the left arm showed superficial perivascular lymphocytic infiltration with dermal edema. These findings were consistent with a diagnosis of DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Application of the Adverse Drug Reaction Probability Scale1 in our patient (total score of 5) suggested that DRESS syndrome was a moderate adverse event likely related to the use of telaprevir.

After diagnosis of DRESS syndrome, telaprevir was discontinued, and the doses of RIB and pegylated interferon were reduced to 200 mg and 180 µg weekly, respectively. Laboratory test values including liver function tests normalized within 3 weeks and remained normal on follow-up. Plasma HCV RNA continued to be undetectable.
Hepatitis C virus is relatively common with an incidence of 3% worldwide.2 It may present as an acute hepatitis or, more frequently, as asymptomatic chronic hepatitis. The acute process is self-limited and rarely causes hepatic failure. It usually leads to a chronic infection, which can result in cirrhosis, hepatocellular carcinoma, and the need for liver transplantation. The aim of treatment is eradication of HCV RNA, which is predicted by the attainment of a sustained virologic response. The latter is defined by the absence of HCV RNA by a polymerase chain reaction within 3 to 6 months after cessation of treatment.
Treatment of chronic HCV was based on the combination of pegylated interferon alfa-2a or -2b with RIB until 2015. Guidelines for the diagnosis and management of HCV infection have been published by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.2 These guidelines include new protease inhibitors, telaprevir and boceprevir, in the therapeutic approach of these patients. The main limitation of both drugs is the cutaneous toxicity.
Factors to be considered when treating HCV include viral genotype, if the patient is naïve or pretreated, the degree of fibrosis, established cirrhosis, and the treatment response. For patients with genotype 1,2 as in our case, combination therapy with 3 drugs is recommended: pegylated interferon 180 µg subcutaneous injection weekly, RIB 15 mg/kg daily, and telaprevir 2250 mg or boceprevir 2400 mg daily. Triple therapy has been shown to achieve a successful response in 75% of naïve patients and in 50% of patients refractory to standard therapy.3
Telaprevir is an NS3/4A protease inhibitor approved by the US Food and Drug Administration and the European Medicines Agency for treatment of chronic HCV infection in naïve patients and in those unresponsive to double therapy. In phase 2 clinical trials, 41% to 61% of patients treated with telaprevir developed cutaneous reactions, of which 5% to 8% required cessation of treatment.4 The predicting risk factors for developing a secondary rash to telaprevir include age older than 45 years, body mass index less than 30, Caucasian ethnicity, and receiving HCV therapy for the first time.4
This cutaneous side effect is managed depending on the extension of the lesions, the presence of systemic symptoms, and laboratory abnormalities.5 Therefore, the severity of the skin reaction can be divided into 4 stages4,5: (1) grade I or mild, defined as a localized rash with no systemic signs or mucosal involvement; (2) grade II or moderate, a maximum of 50% BSA involvement without epidermal detachment, and inflammation of the mucous membranes may be present without ulcers, as well as systemic symptoms such as fever, arthralgia, or eosinophilia; (3) grade III or severe, skin lesions affecting more than 50% BSA or less if any of the following lesions are present: vesicles or blisters, ulcers, epidermal detachment, palpable purpura, or erythema that does not blanch under pressure; (4) grade IV or life-threatening, when the clinical picture is consistent with acute generalized exanthematous pustulosis, DRESS syndrome, toxic epidermal necrolysis, or Stevens-Johnson syndrome.
DRESS syndrome is a condition clinically characterized by a generalized skin rash, facial angioedema, high fever, lymph node enlargement, and leukocytosis with eosinophilia or atypical lymphocytosis, along with abnormal renal and hepatic function tests. Cutaneous histopathologic examination may be unspecific, though atypical lymphocytes with a marked epidermotropism mimicking fungoid mycosis also have been described.6 In addition, human herpesvirus 6 serology may be negative, despite infection with this herpesvirus subtype having been associated with the development of DRESS syndrome. The pathophysiologic mechanism of DRESS syndrome is not completely understood; however, one theory ascribes an immunologic activation due to drug metabolite formation as the main mechanism.1
Eleven patients7 with possible DRESS syndrome have been reported in clinical trials (less than 5% of the total of patients), with an addition of 1 more by Montaudié et al.8 No notable differences were found between telaprevir levels in these patients with respect to those of the control group.
For the management of DRESS syndrome, the occurrence of early signs of a severe acute skin reaction requires the immediate cessation of the drug, telaprevir in this case. The withdrawal of the dual therapy will depend on the short-term clinical course, according to the general condition of the patient, as well as the analytical abnormalities observed.9
In conclusion, telaprevir is a promising novel therapy for the treatment of HCV infection, but its cutaneous side effects still need to be properly established.
To the Editor:
A 58-year-old woman with a history of hyperprolactinemia and gastrointestinal angiodysplasia presented to the dermatology department with a generalized skin rash of 3 weeks’ duration. She did not have a history of toxic habits. She had a history of chronic hepatitis C virus (HCV) genotype 1b (IL-28B locus) with severe hepatic fibrosis (stage 4) as assessed by ultrasound-based elastography. Due to lack of response, plasma HCV RNA was still detectable at week 12 of pegylated interferon and ribavirin (RIB) therapy, and triple therapy with pegylated interferon, RIB, and telaprevir was initiated.
Two months later, she was admitted to the hospital after developing a generalized cutaneous rash that covered 90% of the body surface area (BSA) along with fever (temperature, 38.5°C). Laboratory blood tests showed an elevated absolute eosinophil count (2000 cells/µL [reference range, 0–500 cells/µL]), anemia (hemoglobin, 6.5 g/dL [reference range, 12–16 g/dL]), thrombocytopenia (26×103/µL [reference range, 150–400×103/µL]), and altered liver function tests (serum alanine aminotransferase, 60 U/L [reference range, 0–45 U/L]; aspartate aminotransferase, 80 U/L [reference range, 0–40 U/L]). Plasma HCV RNA was undetectable at this visit. On physical examination a generalized exanthema with coalescing plaques was observed, as well as crusted vesicles covering the arms, legs, chest, abdomen, and back. Palmoplantar papules (Figure, A) and facial swelling (Figure, B) also were present. A skin biopsy specimen taken from a papule on the left arm showed superficial perivascular lymphocytic infiltration with dermal edema. These findings were consistent with a diagnosis of DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Application of the Adverse Drug Reaction Probability Scale1 in our patient (total score of 5) suggested that DRESS syndrome was a moderate adverse event likely related to the use of telaprevir.

After diagnosis of DRESS syndrome, telaprevir was discontinued, and the doses of RIB and pegylated interferon were reduced to 200 mg and 180 µg weekly, respectively. Laboratory test values including liver function tests normalized within 3 weeks and remained normal on follow-up. Plasma HCV RNA continued to be undetectable.
Hepatitis C virus is relatively common with an incidence of 3% worldwide.2 It may present as an acute hepatitis or, more frequently, as asymptomatic chronic hepatitis. The acute process is self-limited and rarely causes hepatic failure. It usually leads to a chronic infection, which can result in cirrhosis, hepatocellular carcinoma, and the need for liver transplantation. The aim of treatment is eradication of HCV RNA, which is predicted by the attainment of a sustained virologic response. The latter is defined by the absence of HCV RNA by a polymerase chain reaction within 3 to 6 months after cessation of treatment.
Treatment of chronic HCV was based on the combination of pegylated interferon alfa-2a or -2b with RIB until 2015. Guidelines for the diagnosis and management of HCV infection have been published by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.2 These guidelines include new protease inhibitors, telaprevir and boceprevir, in the therapeutic approach of these patients. The main limitation of both drugs is the cutaneous toxicity.
Factors to be considered when treating HCV include viral genotype, if the patient is naïve or pretreated, the degree of fibrosis, established cirrhosis, and the treatment response. For patients with genotype 1,2 as in our case, combination therapy with 3 drugs is recommended: pegylated interferon 180 µg subcutaneous injection weekly, RIB 15 mg/kg daily, and telaprevir 2250 mg or boceprevir 2400 mg daily. Triple therapy has been shown to achieve a successful response in 75% of naïve patients and in 50% of patients refractory to standard therapy.3
Telaprevir is an NS3/4A protease inhibitor approved by the US Food and Drug Administration and the European Medicines Agency for treatment of chronic HCV infection in naïve patients and in those unresponsive to double therapy. In phase 2 clinical trials, 41% to 61% of patients treated with telaprevir developed cutaneous reactions, of which 5% to 8% required cessation of treatment.4 The predicting risk factors for developing a secondary rash to telaprevir include age older than 45 years, body mass index less than 30, Caucasian ethnicity, and receiving HCV therapy for the first time.4
This cutaneous side effect is managed depending on the extension of the lesions, the presence of systemic symptoms, and laboratory abnormalities.5 Therefore, the severity of the skin reaction can be divided into 4 stages4,5: (1) grade I or mild, defined as a localized rash with no systemic signs or mucosal involvement; (2) grade II or moderate, a maximum of 50% BSA involvement without epidermal detachment, and inflammation of the mucous membranes may be present without ulcers, as well as systemic symptoms such as fever, arthralgia, or eosinophilia; (3) grade III or severe, skin lesions affecting more than 50% BSA or less if any of the following lesions are present: vesicles or blisters, ulcers, epidermal detachment, palpable purpura, or erythema that does not blanch under pressure; (4) grade IV or life-threatening, when the clinical picture is consistent with acute generalized exanthematous pustulosis, DRESS syndrome, toxic epidermal necrolysis, or Stevens-Johnson syndrome.
DRESS syndrome is a condition clinically characterized by a generalized skin rash, facial angioedema, high fever, lymph node enlargement, and leukocytosis with eosinophilia or atypical lymphocytosis, along with abnormal renal and hepatic function tests. Cutaneous histopathologic examination may be unspecific, though atypical lymphocytes with a marked epidermotropism mimicking fungoid mycosis also have been described.6 In addition, human herpesvirus 6 serology may be negative, despite infection with this herpesvirus subtype having been associated with the development of DRESS syndrome. The pathophysiologic mechanism of DRESS syndrome is not completely understood; however, one theory ascribes an immunologic activation due to drug metabolite formation as the main mechanism.1
Eleven patients7 with possible DRESS syndrome have been reported in clinical trials (less than 5% of the total of patients), with an addition of 1 more by Montaudié et al.8 No notable differences were found between telaprevir levels in these patients with respect to those of the control group.
For the management of DRESS syndrome, the occurrence of early signs of a severe acute skin reaction requires the immediate cessation of the drug, telaprevir in this case. The withdrawal of the dual therapy will depend on the short-term clinical course, according to the general condition of the patient, as well as the analytical abnormalities observed.9
In conclusion, telaprevir is a promising novel therapy for the treatment of HCV infection, but its cutaneous side effects still need to be properly established.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther. 1981;30:239-245.
- HCV guidance: recommendations for testing, managing, and treating hepatitis C. HCV Guidelines website. http://www.hcvguidelines.org. Accessed August 11, 2018.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Roujeau JC, Mockenhaupt M, Tahan SR, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- De Vriese AS, Philippe J, Van Renterghem DM, et al. Carbamazepine hypersensitivity syndrome: report of 4 cases and review of the literature. Medicine (Baltimore). 1995;74:144-151.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review [published online May 17, 2011]. Am J Med. 2011;124:588-597.
- Montaudié H, Passeron T, Cardot-Leccia N, et al. Drug rash with eosinophilia and systemic symptoms due to telaprevir. Dermatology. 2010;221:303-305.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther. 1981;30:239-245.
- HCV guidance: recommendations for testing, managing, and treating hepatitis C. HCV Guidelines website. http://www.hcvguidelines.org. Accessed August 11, 2018.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Roujeau JC, Mockenhaupt M, Tahan SR, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- De Vriese AS, Philippe J, Van Renterghem DM, et al. Carbamazepine hypersensitivity syndrome: report of 4 cases and review of the literature. Medicine (Baltimore). 1995;74:144-151.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review [published online May 17, 2011]. Am J Med. 2011;124:588-597.
- Montaudié H, Passeron T, Cardot-Leccia N, et al. Drug rash with eosinophilia and systemic symptoms due to telaprevir. Dermatology. 2010;221:303-305.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
Practice Points
- DRESS syndrome is characterized by a generalized skin rash, facial angioedema, high fever, lymph node enlargement, and leukocytosis with eosinophilia or atypical lymphocytosis, along with abnormal renal and hepatic function tests.
- Severity of the skin reaction can be divided into 4 stages; in the third and fourth stages, adequate patient monitoring is necessary.
- Telaprevir is an NS3/4A protease inhibitor approved for treatment of chronic hepatitis C virus infection in naïve patients and in those unresponsive to double therapy. Its cutaneous side effects still need to be properly established.
Eumycetoma Pedis in an Albanian Farmer
To the Editor:
Mycetoma is a noncontagious chronic infection of the skin and subcutaneous tissue caused by exogenous fungi or bacteria that can involve deeper structures such as the fasciae, muscles, and bones. Clinically it is characterized by increased swelling of the affected area, fibrosis, nodules, tumefaction, formation of draining sinuses, and abscesses that drain pus-containing grains through fistulae.1 The initiation of the infection is related to local trauma and can involve muscle, underlying bone, and adjacent organs. The feet are the most commonly affected region, and the incubation period is variable. Patients rarely report prior trauma to the affected area and only seek medical consultation when the nodules and draining sinuses become evident. The etiopathogenesis of mycetoma is associated with aerobic actinomycetes (ie, Nocardia, Actinomadura, Streptomyces), known as actinomycetoma, and fungal infections, known as eumycetomas.1
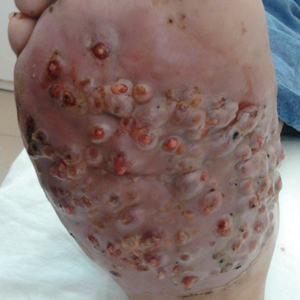
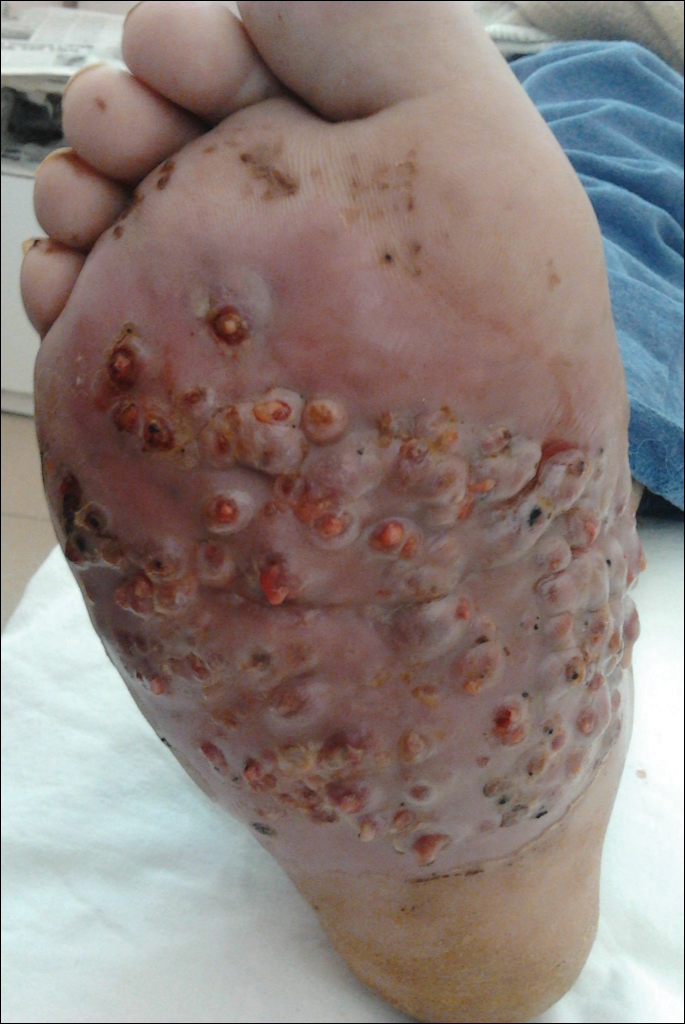
We report the case of a 57-year-old Albanian man who was referred to the outpatient clinic of our dermatology department for diagnosis and treatment of a chronic, suppurative, subcutaneous infection on the right foot presenting as abscesses and draining sinuses. The patient was a farmer and reported that the condition appeared 4 years prior following a laceration he sustained while at work. Dermatologic examination revealed local tumefaction, fistulated nodules, and abscesses discharging a serohemorrhagic fluid on the right foot (Figure 1). Perilesional erythema and subcutaneous swelling were evident. There was no regional lymphadenopathy. Standard laboratory examination was normal. Radiography of the right foot showed no osteolytic lesions or evidence of osteomyelitis.
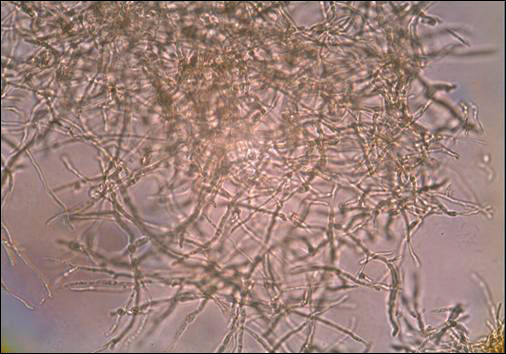
A skin biopsy from a lesion on the right foot was performed, and identification of the possible etiologic agent was based on direct microscopic examination of the granules, culture isolation of the agent, and fungal microscopic morphology.2 Granules were studied under direct examination with potassium hydroxide solution 20% and showed septate branching hyphae (Figure 2). The culture produced colonies that were white, yellow, and brown. Colonies were comprised of dense mycelium with melanin pigment and were grown at 37°C. A lactose tolerance test was positive.2 Therefore, the strain was identified as Madurella mycetomatis, and a diagnosis of eumycetoma pedis was made.
The patient was hospitalized for 2 weeks and treated with intravenous fluconazole, then treatment with oral itraconazole 200 mg once daily was initiated. At 4-month follow-up, he had self-discontinued treatment but demonstrated partial improvement of the tumefaction, healing of sinus tracts, and functional recovery of the right foot.
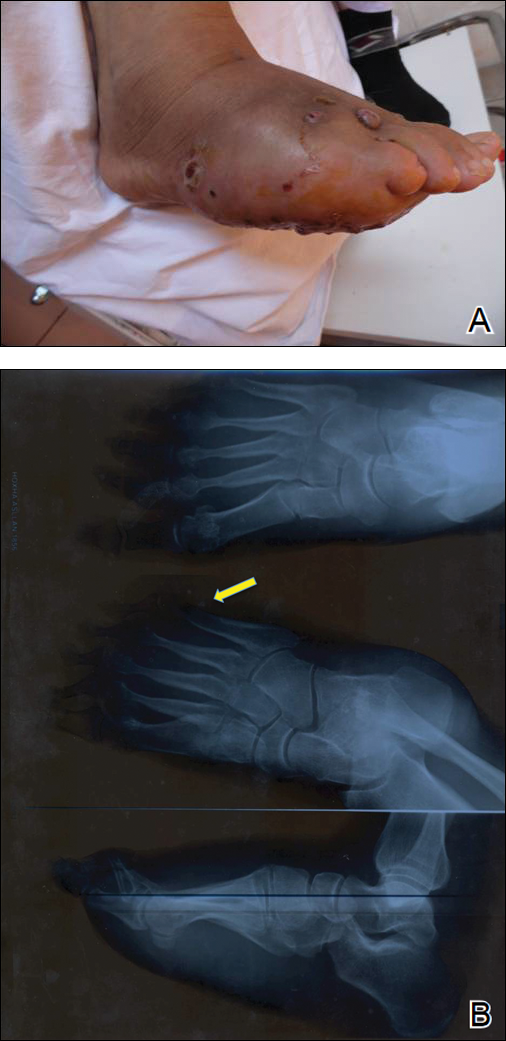
One year following the initial presentation, the patient’s clinical condition worsened (Figure 3A). Radiography of the right foot showed osteolytic lesions on bones in the right foot (Figure 3B), and a repeat culture showed the presence of Staphylococcus aureus; thus, treatment with itraconazole 200 mg once daily along with antibiotics (cefuroxime and gentamicin) was started immediately. Surgical treatment was recommended, but the patient refused treatment.
Mycetomas are rare in Albania but are common in countries of tropical and subtropical regions. K
Clinical features of eumycetoma include lesions with clear margins, few sinuses, black grains, slow progression, and long-term involvement of bone. The grains represent an aggregate of hyphae produced by fungi; thus, the characteristic feature of eumycetoma is the formation of large granules that can involve bone.1 A critical diagnostic step is to distinguish between eumycetoma and actinomycetoma. If possible, it is important to culture the organism because treatment varies depending on the cause of the infection.
Fungal identification is crucial in the diagnosis of mycetoma. In our case, diagnosis of eumycetoma pedis was based on clinical examination and detection of fungal species by microscopic examination and culture. The color of small granules (black grains) is a parameter used to identify different pathogens on histology but is not sufficient for diagnosis.5 The examination by potassium hydroxide preparation is helpful to identify the hyphae; however, culture is necessary.2
Therapeutic management of eumycetoma needs a combined strategy that includes systemic treatment and surgical therapy. Eumycetomas generally are more difficult to treat then actinomycetomas. Some authors recommend a high dose of amphotericin B as the treatment of choice for eumycetoma,6,7 but there are some that emphasize that amphotericin B is partially effective.8,9 There also is evidence in the literature of resistance of eumycetoma to ketoconazole treatment10,11 and successful treatment with fluconazole and itraconazole.10-13 For this reason, we treated our patient with the latter agents. In cases of osteolysis, amputation often is required.
In conclusion, eumycetoma pedis is a rare deep fungal infection that can cause considerable morbidity. P
- Rook A, Burns T. Rook’s Textbook of Dermatology. 8th ed. West Sussex, UK; Hoboken, NJ: Wiley-Blackwell; 2010.
- Balows A, Hausler WJ, eds. Manual of Clinical Microbiology. 5th ed. Washington, DC: American Society for Microbiology; 1991.
- Carter HV. On a new striking form of fungus disease principally affecting the foot and prevailing endemically in many parts of India. Trans Med Phys Soc Bombay. 1860;6:104-142.
- Kwon-Chung KJ, Bennet JE. Medical Mycology. Philadelphia, PA: Lea & Febiger; 1992.
- Venugopal PV, Venugopal TV. Pale grain eumycetomas in Madras. Australas J Dermatol. 1995;36:149-151.
- Guarro J, Gams W, Pujol I, et al. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin Infec Dis. 1997;25:1222-1229.
- Lau YL, Yuen KY, Lee CW, et al. Invasive Acremonium falciforme infection in a patient with severe combined immunodeficiency. Clin Infect Dis. 1995;20:197-198.
- Fincher RM, Fisher JF, Lovell RD, et al. Infection due to the fungus Acremonium (cephalosporium). Medicine (Baltimore). 1991;70:398-409.
- Milburn PB, Papayanopulos DM, Pomerantz BM. Mycetoma due to Acremonium falciforme. Int J Dermatol. 1988;27:408-410.
- Welsh O, Salinas MC, Rodriguez MA. Treatment of eumycetoma and actinomycetoma. Cur Top Med Mycol. 1995;6:47-71.
- Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31:S91-S102.
- Gugnani HC, Ezeanolue BC, Khalil M, et al. Fluconazole in the therapy of tropical deep mycoses. Mycoses. 1995;38:485-488.
- Welsh O. Mycetoma. current concepts in treatment. Int J Dermatol. 1991;30:387-398.
To the Editor:
Mycetoma is a noncontagious chronic infection of the skin and subcutaneous tissue caused by exogenous fungi or bacteria that can involve deeper structures such as the fasciae, muscles, and bones. Clinically it is characterized by increased swelling of the affected area, fibrosis, nodules, tumefaction, formation of draining sinuses, and abscesses that drain pus-containing grains through fistulae.1 The initiation of the infection is related to local trauma and can involve muscle, underlying bone, and adjacent organs. The feet are the most commonly affected region, and the incubation period is variable. Patients rarely report prior trauma to the affected area and only seek medical consultation when the nodules and draining sinuses become evident. The etiopathogenesis of mycetoma is associated with aerobic actinomycetes (ie, Nocardia, Actinomadura, Streptomyces), known as actinomycetoma, and fungal infections, known as eumycetomas.1
We report the case of a 57-year-old Albanian man who was referred to the outpatient clinic of our dermatology department for diagnosis and treatment of a chronic, suppurative, subcutaneous infection on the right foot presenting as abscesses and draining sinuses. The patient was a farmer and reported that the condition appeared 4 years prior following a laceration he sustained while at work. Dermatologic examination revealed local tumefaction, fistulated nodules, and abscesses discharging a serohemorrhagic fluid on the right foot (Figure 1). Perilesional erythema and subcutaneous swelling were evident. There was no regional lymphadenopathy. Standard laboratory examination was normal. Radiography of the right foot showed no osteolytic lesions or evidence of osteomyelitis.

A skin biopsy from a lesion on the right foot was performed, and identification of the possible etiologic agent was based on direct microscopic examination of the granules, culture isolation of the agent, and fungal microscopic morphology.2 Granules were studied under direct examination with potassium hydroxide solution 20% and showed septate branching hyphae (Figure 2). The culture produced colonies that were white, yellow, and brown. Colonies were comprised of dense mycelium with melanin pigment and were grown at 37°C. A lactose tolerance test was positive.2 Therefore, the strain was identified as Madurella mycetomatis, and a diagnosis of eumycetoma pedis was made.

The patient was hospitalized for 2 weeks and treated with intravenous fluconazole, then treatment with oral itraconazole 200 mg once daily was initiated. At 4-month follow-up, he had self-discontinued treatment but demonstrated partial improvement of the tumefaction, healing of sinus tracts, and functional recovery of the right foot.
One year following the initial presentation, the patient’s clinical condition worsened (Figure 3A). Radiography of the right foot showed osteolytic lesions on bones in the right foot (Figure 3B), and a repeat culture showed the presence of Staphylococcus aureus; thus, treatment with itraconazole 200 mg once daily along with antibiotics (cefuroxime and gentamicin) was started immediately. Surgical treatment was recommended, but the patient refused treatment.
Mycetomas are rare in Albania but are common in countries of tropical and subtropical regions. K

Clinical features of eumycetoma include lesions with clear margins, few sinuses, black grains, slow progression, and long-term involvement of bone. The grains represent an aggregate of hyphae produced by fungi; thus, the characteristic feature of eumycetoma is the formation of large granules that can involve bone.1 A critical diagnostic step is to distinguish between eumycetoma and actinomycetoma. If possible, it is important to culture the organism because treatment varies depending on the cause of the infection.
Fungal identification is crucial in the diagnosis of mycetoma. In our case, diagnosis of eumycetoma pedis was based on clinical examination and detection of fungal species by microscopic examination and culture. The color of small granules (black grains) is a parameter used to identify different pathogens on histology but is not sufficient for diagnosis.5 The examination by potassium hydroxide preparation is helpful to identify the hyphae; however, culture is necessary.2
Therapeutic management of eumycetoma needs a combined strategy that includes systemic treatment and surgical therapy. Eumycetomas generally are more difficult to treat then actinomycetomas. Some authors recommend a high dose of amphotericin B as the treatment of choice for eumycetoma,6,7 but there are some that emphasize that amphotericin B is partially effective.8,9 There also is evidence in the literature of resistance of eumycetoma to ketoconazole treatment10,11 and successful treatment with fluconazole and itraconazole.10-13 For this reason, we treated our patient with the latter agents. In cases of osteolysis, amputation often is required.
In conclusion, eumycetoma pedis is a rare deep fungal infection that can cause considerable morbidity. P
To the Editor:
Mycetoma is a noncontagious chronic infection of the skin and subcutaneous tissue caused by exogenous fungi or bacteria that can involve deeper structures such as the fasciae, muscles, and bones. Clinically it is characterized by increased swelling of the affected area, fibrosis, nodules, tumefaction, formation of draining sinuses, and abscesses that drain pus-containing grains through fistulae.1 The initiation of the infection is related to local trauma and can involve muscle, underlying bone, and adjacent organs. The feet are the most commonly affected region, and the incubation period is variable. Patients rarely report prior trauma to the affected area and only seek medical consultation when the nodules and draining sinuses become evident. The etiopathogenesis of mycetoma is associated with aerobic actinomycetes (ie, Nocardia, Actinomadura, Streptomyces), known as actinomycetoma, and fungal infections, known as eumycetomas.1
We report the case of a 57-year-old Albanian man who was referred to the outpatient clinic of our dermatology department for diagnosis and treatment of a chronic, suppurative, subcutaneous infection on the right foot presenting as abscesses and draining sinuses. The patient was a farmer and reported that the condition appeared 4 years prior following a laceration he sustained while at work. Dermatologic examination revealed local tumefaction, fistulated nodules, and abscesses discharging a serohemorrhagic fluid on the right foot (Figure 1). Perilesional erythema and subcutaneous swelling were evident. There was no regional lymphadenopathy. Standard laboratory examination was normal. Radiography of the right foot showed no osteolytic lesions or evidence of osteomyelitis.

A skin biopsy from a lesion on the right foot was performed, and identification of the possible etiologic agent was based on direct microscopic examination of the granules, culture isolation of the agent, and fungal microscopic morphology.2 Granules were studied under direct examination with potassium hydroxide solution 20% and showed septate branching hyphae (Figure 2). The culture produced colonies that were white, yellow, and brown. Colonies were comprised of dense mycelium with melanin pigment and were grown at 37°C. A lactose tolerance test was positive.2 Therefore, the strain was identified as Madurella mycetomatis, and a diagnosis of eumycetoma pedis was made.

The patient was hospitalized for 2 weeks and treated with intravenous fluconazole, then treatment with oral itraconazole 200 mg once daily was initiated. At 4-month follow-up, he had self-discontinued treatment but demonstrated partial improvement of the tumefaction, healing of sinus tracts, and functional recovery of the right foot.
One year following the initial presentation, the patient’s clinical condition worsened (Figure 3A). Radiography of the right foot showed osteolytic lesions on bones in the right foot (Figure 3B), and a repeat culture showed the presence of Staphylococcus aureus; thus, treatment with itraconazole 200 mg once daily along with antibiotics (cefuroxime and gentamicin) was started immediately. Surgical treatment was recommended, but the patient refused treatment.
Mycetomas are rare in Albania but are common in countries of tropical and subtropical regions. K

Clinical features of eumycetoma include lesions with clear margins, few sinuses, black grains, slow progression, and long-term involvement of bone. The grains represent an aggregate of hyphae produced by fungi; thus, the characteristic feature of eumycetoma is the formation of large granules that can involve bone.1 A critical diagnostic step is to distinguish between eumycetoma and actinomycetoma. If possible, it is important to culture the organism because treatment varies depending on the cause of the infection.
Fungal identification is crucial in the diagnosis of mycetoma. In our case, diagnosis of eumycetoma pedis was based on clinical examination and detection of fungal species by microscopic examination and culture. The color of small granules (black grains) is a parameter used to identify different pathogens on histology but is not sufficient for diagnosis.5 The examination by potassium hydroxide preparation is helpful to identify the hyphae; however, culture is necessary.2
Therapeutic management of eumycetoma needs a combined strategy that includes systemic treatment and surgical therapy. Eumycetomas generally are more difficult to treat then actinomycetomas. Some authors recommend a high dose of amphotericin B as the treatment of choice for eumycetoma,6,7 but there are some that emphasize that amphotericin B is partially effective.8,9 There also is evidence in the literature of resistance of eumycetoma to ketoconazole treatment10,11 and successful treatment with fluconazole and itraconazole.10-13 For this reason, we treated our patient with the latter agents. In cases of osteolysis, amputation often is required.
In conclusion, eumycetoma pedis is a rare deep fungal infection that can cause considerable morbidity. P
- Rook A, Burns T. Rook’s Textbook of Dermatology. 8th ed. West Sussex, UK; Hoboken, NJ: Wiley-Blackwell; 2010.
- Balows A, Hausler WJ, eds. Manual of Clinical Microbiology. 5th ed. Washington, DC: American Society for Microbiology; 1991.
- Carter HV. On a new striking form of fungus disease principally affecting the foot and prevailing endemically in many parts of India. Trans Med Phys Soc Bombay. 1860;6:104-142.
- Kwon-Chung KJ, Bennet JE. Medical Mycology. Philadelphia, PA: Lea & Febiger; 1992.
- Venugopal PV, Venugopal TV. Pale grain eumycetomas in Madras. Australas J Dermatol. 1995;36:149-151.
- Guarro J, Gams W, Pujol I, et al. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin Infec Dis. 1997;25:1222-1229.
- Lau YL, Yuen KY, Lee CW, et al. Invasive Acremonium falciforme infection in a patient with severe combined immunodeficiency. Clin Infect Dis. 1995;20:197-198.
- Fincher RM, Fisher JF, Lovell RD, et al. Infection due to the fungus Acremonium (cephalosporium). Medicine (Baltimore). 1991;70:398-409.
- Milburn PB, Papayanopulos DM, Pomerantz BM. Mycetoma due to Acremonium falciforme. Int J Dermatol. 1988;27:408-410.
- Welsh O, Salinas MC, Rodriguez MA. Treatment of eumycetoma and actinomycetoma. Cur Top Med Mycol. 1995;6:47-71.
- Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31:S91-S102.
- Gugnani HC, Ezeanolue BC, Khalil M, et al. Fluconazole in the therapy of tropical deep mycoses. Mycoses. 1995;38:485-488.
- Welsh O. Mycetoma. current concepts in treatment. Int J Dermatol. 1991;30:387-398.
- Rook A, Burns T. Rook’s Textbook of Dermatology. 8th ed. West Sussex, UK; Hoboken, NJ: Wiley-Blackwell; 2010.
- Balows A, Hausler WJ, eds. Manual of Clinical Microbiology. 5th ed. Washington, DC: American Society for Microbiology; 1991.
- Carter HV. On a new striking form of fungus disease principally affecting the foot and prevailing endemically in many parts of India. Trans Med Phys Soc Bombay. 1860;6:104-142.
- Kwon-Chung KJ, Bennet JE. Medical Mycology. Philadelphia, PA: Lea & Febiger; 1992.
- Venugopal PV, Venugopal TV. Pale grain eumycetomas in Madras. Australas J Dermatol. 1995;36:149-151.
- Guarro J, Gams W, Pujol I, et al. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin Infec Dis. 1997;25:1222-1229.
- Lau YL, Yuen KY, Lee CW, et al. Invasive Acremonium falciforme infection in a patient with severe combined immunodeficiency. Clin Infect Dis. 1995;20:197-198.
- Fincher RM, Fisher JF, Lovell RD, et al. Infection due to the fungus Acremonium (cephalosporium). Medicine (Baltimore). 1991;70:398-409.
- Milburn PB, Papayanopulos DM, Pomerantz BM. Mycetoma due to Acremonium falciforme. Int J Dermatol. 1988;27:408-410.
- Welsh O, Salinas MC, Rodriguez MA. Treatment of eumycetoma and actinomycetoma. Cur Top Med Mycol. 1995;6:47-71.
- Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31:S91-S102.
- Gugnani HC, Ezeanolue BC, Khalil M, et al. Fluconazole in the therapy of tropical deep mycoses. Mycoses. 1995;38:485-488.
- Welsh O. Mycetoma. current concepts in treatment. Int J Dermatol. 1991;30:387-398.
Practice Points
- A critical step in the diagnosis of mycetomas is to distinguish between eumycetoma and actinomycetoma.
- Potassium hydroxide preparation is helpful to identify fungal infection.
- Eumycetomas generally are more difficult to treat and require a combined strategy including systemic treatment and surgical therapy.
HIV prevention: Mandating insurance coverage of PrEP
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.
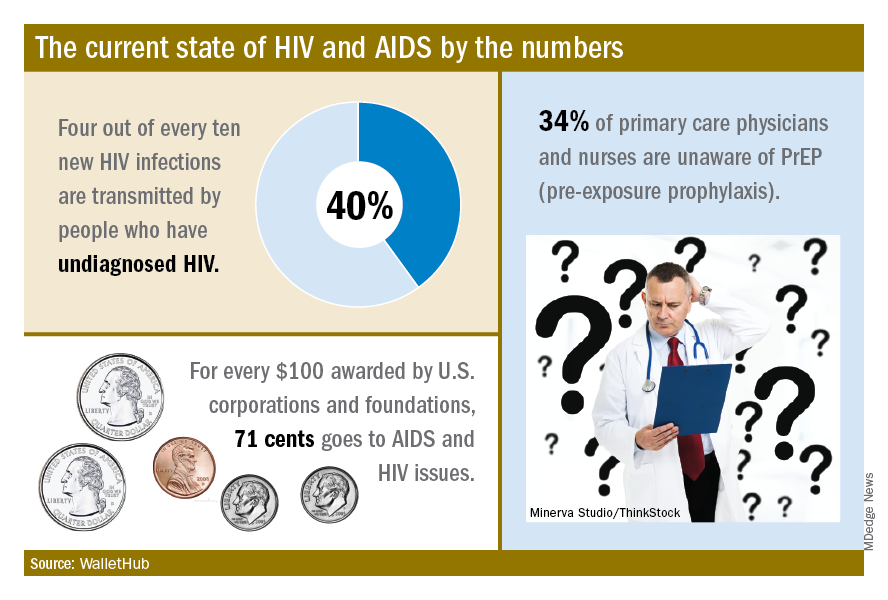
The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.

The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.

The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Hamstring tendinopathy implicated in persistent Lyme arthritis
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
CHICAGO – The big news regarding Lyme disease at the annual meeting of the American College of Rheumatology was a report that hamstring tendon calcification is extremely common among patients who have persistent Lyme arthritis despite having undergone appropriate antibiotic therapy.
“This is a fascinating study,” Robert A. Kalish, MD, a Lyme disease expert not involved in the research, said regarding the report by Sheila L. Arvikar, MD, and her coworkers at Massachusetts General Hospital, Boston.
One implication of this finding by a renowned group of Lyme disease researchers is that persistent posttreatment Lyme arthritis may in many cases be due to ongoing immunostimulation by spirochete remains located in hamstring tendons, a privileged, relatively avascular site where the foreign material may be able to evade immune clearance.
Also, as Dr. Arvikar pointed out in her presentation, calcific tendinopathy implies prior inflammation or degenerative changes. Thus, these calcific hamstring abnormalities implicate the hamstring tendons as a potential initial site of infection by hematogenously-spread Borrelia burgdorferi during the prearthritis phase of Lyme disease.
A further implication of the study is the possibility that hamstring tendon calcification could serve as a useful diagnostic aid in distinguishing Lyme arthritis from arthritis due to other causes. In the study, hamstring calcific tendinopathy was found in 28 of 31 adults and children with Lyme arthritis, 3 of 22 with knee osteoarthritis, and 1 of 14 patients with inflammatory arthritis, Dr. Arvikar noted.
She and her coinvestigators evaluated tendon pathology in their retrospective study of patients at the Massachusetts General Hospital Rheumatology Musculoskeletal Ultrasound Clinic. They used ultrasound because they have found it offers far better spatial resolution of calcification than does MRI or x-rays. The semimembranosus tendon was the hamstring tendon that most commonly exhibited calcification, although 11 patients with Lyme arthritis also had involvement of the semitendinosus tendon, compared with none of the controls with osteoarthritis or inflammatory arthritis.
In the eight patients with serial ultrasound evaluations over a period of up to 12 months, the calcification persisted but the symptoms of tendinitis and synovitis improved.
Dr. Arvikar and her colleagues are expanding the scope of their ongoing study by examining patients whose Lyme arthritis is milder than that of the initial population, including patients who haven’t yet received antibiotics. They are also evaluating more controls with inflammatory arthritis.
In a separate presentation, Dr. Kalish noted that Lyme arthritis, the manifestation of Lyme disease of greatest interest to rheumatologists, occurs in about 60% of untreated patients, with onset a mean of 6 months after the tick bite. It typically entails recurrent mono- or oligoarthritis of large joints. The knee is involved in roughly 95% of cases.
The natural history of untreated Lyme arthritis is a spontaneous resolution rate of 10%-20% per year. Since the 1980s, however, 4 weeks of oral doxycycline or amoxicillin has been the treatment of choice. About 10% of patients with Lyme arthritis continue to have active synovitis 3 months after their course of antibiotics.
“There are some patients you give the treatment to and their arthritis just melts away in a month, but some, no matter what you do with antibiotics, continue to have synovitis, often developing a highly proliferative palpable synovitis that is really gunked up and features obliterative microvascular lesions,” observed Dr. Kalish, a rheumatologist at Tufts University in Boston.
Dr. Kalish said that persistent posttreatment Lyme arthritis is most often due to a self-perpetuating immune response after the spirochete has been killed by antibiotics. He noted that patients with certain HLA-DRB1 haplotypes are more likely to experience persistent Lyme arthritis after standard recommended courses of antibiotics, and these DRB1 alleles correlate closely with the shared epitope associated with increased susceptibility to rheumatoid arthritis. Several candidate autoantigens have already been identified.
He noted that the Massachusetts General group, in an earlier study, demonstrated that the presence of B. burgdorferi DNA by PCR in synovial fluid from patients with persistent Lyme arthritis after antibiotic therapy was not a reliable indicator of active joint infection (Arthritis Rheum. 2011 Aug;63[8]:2238-47).
“This was a paradigm change for me in seeing this study, because prior to that I had used PCR somewhat to guide treatment and make management decisions,” Dr. Kalish said.
What’s a reasonable treatment strategy in patients with persistent Lyme arthritis despite 30 days of oral antibiotics? Dr. Kalish favors an algorithm similar to one published by Dr. Arvikar and Allen C. Steere, MD (Infect Dis Clin North Am. 2015 Jun;29[2]:269-80). In the case of mild persistent arthritis, he opts for another 30 days of oral doxycycline. If the arthritis is moderate or severe, he goes with either another 30 days of doxycycline or 30 days of intravenous ceftriaxone.
If the arthritis still hasn’t resolved despite two 30-day rounds of antibiotic therapy, he prescribes an NSAID or hydroxychloroquine if the persistent arthritis is mild, or methotrexate if it’s moderate to severe. And if the arthritis still persists after 3-6 months of disease-modifying antirheumatic drug therapy, he’ll consider synovectomy, which has a good success rate.
Neither Dr. Arvikar nor Dr. Kalish reported having any financial conflicts regarding their presentations.
SOURCE: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Ultrasound evidence of hamstring calcific tendinopathy was found in 28 of 31 patients with persistent posttreatment Lyme arthritis, compared with 3 of 22 patients with knee osteoarthritis.
Study details: This was a retrospective imaging study of hamstring tendon status in 31 patients with persistent posttreatment Lyme arthritis, 22 patients with osteoarthritis, and 14 with inflammatory arthritis.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Source: Arvikar SL et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 950.
Missed HIV screening opportunities found among subsequently infected youth
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
FROM THE JOURNAL OF ADOLESCENT HEALTH
Key clinical point: Only 51% of youth with symptoms suggesting acute retroviral syndrome were tested.
Major finding: HIV testing was performed in only 48% of the subjects seen in the year prior to their diagnosis.
Study details: Retrospective review of subjects with HIV aged 14-26 years, comparing those with and without HIV screening within the year prior to diagnosis.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
Recurrent Infections: Viral or Something More Sinister?
Early treatment with direct-acting antivirals linked to reduced medical costs in noncirrhotic HCV
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Noncirrhotic chronic hepatitis C patients incur high medical costs after their first diagnosis if left untreated.
Major finding: In the first 3 years post index, the average medical costs incurred in the direct-acting antiviral–treated and untreated groups were $28,392 and $42,914, respectively.
Study details: A database sample of 3,069 adults first diagnosed with chronic hepatitis C in or after 2013.
Disclosures: AbbVie provided funding for the study. The company employs Dr. Bao and two of the study coauthors.
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.