User login
People with HIV still at increased cardiovascular risk
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: U.S. insurance beneficiaries newly diagnosed with HIV had a significantly higher rate of CVD events than people without HIV.
Major finding: The adjusted rate of cardiovascular disease events was 21% higher in people infected with HIV, compared with matched, uninfected people.
Study details: A retrospective, case control study of 412,130 U.S. health insurance beneficiaries.
Disclosures: The study received partial funding from Amgen. Dr. Rosenson has received honoraria from Amgen, Akcaa, and Kowa; he has been an advisor to Amgen, Regeneron, and Sanofi; and he has received research funding from Amgen, Akcaa, AstraZeneca, and The Medicines Company.
Source: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CDC: Acute flaccid myelitis on the decline for 2018
, according to the Centers for Disease Control and Prevention.
Through Nov. 30, 134 cases of AFM in 33 states have been confirmed out of the 299 reported to the CDC. That represents “an increase of 18 confirmed cases from the previous week, but most of the latest confirmed AFM cases occurred in September and October,” the CDC reported Dec. 3.
There has been a pattern of increased AFM cases every other year for the previous 4 years: 120 cases in 2014, 22 cases in 2015, 149 cases in 2016, and 33 cases in 2017. “Most cases are reported between August and October, and a marked reduction in cases is seen in November. That pattern appears to be repeating in 2018 because states have reported fewer [persons under investigation] over the past couple of weeks. CDC expects this decline to continue,” the statement said.
The 16 confirmed cases in Texas are the most for any state this year, followed by Colorado with 15; Ohio with 10; and Illinois, New Jersey, and Washington with 9 each. California and Florida have not had any confirmed cases as of Nov. 30. Since 2014, over 90% of all confirmed AFM cases have occurred in children, the CDC noted.
More information on AFM is available at a CDC website for health care professionals.
, according to the Centers for Disease Control and Prevention.
Through Nov. 30, 134 cases of AFM in 33 states have been confirmed out of the 299 reported to the CDC. That represents “an increase of 18 confirmed cases from the previous week, but most of the latest confirmed AFM cases occurred in September and October,” the CDC reported Dec. 3.
There has been a pattern of increased AFM cases every other year for the previous 4 years: 120 cases in 2014, 22 cases in 2015, 149 cases in 2016, and 33 cases in 2017. “Most cases are reported between August and October, and a marked reduction in cases is seen in November. That pattern appears to be repeating in 2018 because states have reported fewer [persons under investigation] over the past couple of weeks. CDC expects this decline to continue,” the statement said.
The 16 confirmed cases in Texas are the most for any state this year, followed by Colorado with 15; Ohio with 10; and Illinois, New Jersey, and Washington with 9 each. California and Florida have not had any confirmed cases as of Nov. 30. Since 2014, over 90% of all confirmed AFM cases have occurred in children, the CDC noted.
More information on AFM is available at a CDC website for health care professionals.
, according to the Centers for Disease Control and Prevention.
Through Nov. 30, 134 cases of AFM in 33 states have been confirmed out of the 299 reported to the CDC. That represents “an increase of 18 confirmed cases from the previous week, but most of the latest confirmed AFM cases occurred in September and October,” the CDC reported Dec. 3.
There has been a pattern of increased AFM cases every other year for the previous 4 years: 120 cases in 2014, 22 cases in 2015, 149 cases in 2016, and 33 cases in 2017. “Most cases are reported between August and October, and a marked reduction in cases is seen in November. That pattern appears to be repeating in 2018 because states have reported fewer [persons under investigation] over the past couple of weeks. CDC expects this decline to continue,” the statement said.
The 16 confirmed cases in Texas are the most for any state this year, followed by Colorado with 15; Ohio with 10; and Illinois, New Jersey, and Washington with 9 each. California and Florida have not had any confirmed cases as of Nov. 30. Since 2014, over 90% of all confirmed AFM cases have occurred in children, the CDC noted.
More information on AFM is available at a CDC website for health care professionals.
Our missing microbes: Short-term antibiotic courses have long-term consequences
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
A new reason to reconsider that antibiotic prescription: The microbiome
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
ERRATUM
The September 2018 Practice Alert, “CDC recommendations for the 2018-2019 influenza season” contained an error (J Fam Pract. 2018. 67:550-553). On page 552, under “Available vaccine products,” the article listed “one standard dose IIV4 intradermal option.” This was incorrect. Sanofi Pasteur, the manufacturer of standard dose Intradermal IIV4, discontinued the production and supply of Fluzone Intradermal Quadrivalent vaccine at the conclusion of the 2017-2018 influenza season.
The September 2018 Practice Alert, “CDC recommendations for the 2018-2019 influenza season” contained an error (J Fam Pract. 2018. 67:550-553). On page 552, under “Available vaccine products,” the article listed “one standard dose IIV4 intradermal option.” This was incorrect. Sanofi Pasteur, the manufacturer of standard dose Intradermal IIV4, discontinued the production and supply of Fluzone Intradermal Quadrivalent vaccine at the conclusion of the 2017-2018 influenza season.
The September 2018 Practice Alert, “CDC recommendations for the 2018-2019 influenza season” contained an error (J Fam Pract. 2018. 67:550-553). On page 552, under “Available vaccine products,” the article listed “one standard dose IIV4 intradermal option.” This was incorrect. Sanofi Pasteur, the manufacturer of standard dose Intradermal IIV4, discontinued the production and supply of Fluzone Intradermal Quadrivalent vaccine at the conclusion of the 2017-2018 influenza season.
Diffuse facial rash in a former collegiate wrestler
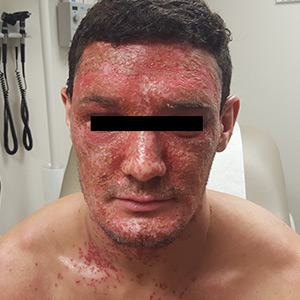
A 22-year-old Caucasian man with a history of atopic dermatitis (AD) was referred to our dermatology clinic for evaluation of a diffuse facial rash that had been present for the previous 7 days. The rash initially presented as erythema on the right malar cheek that rapidly spread to the entire face. Initially diagnosed as impetigo, empiric treatment with sulfamethoxazole/trimethoprim (800 mg/160 mg PO BID for 7 days), dicloxacillin (500 mg PO BID for 6 days), cephalexin (500 mg TID for 5 days), and mupirocin (2% topical cream applied TID for 6 days) failed to improve the patient’s symptoms. He reported mild pain associated with facial movements.
The patient had a history of similar (but more limited) rashes, which he described as “recurrent impetigo,” that began during his career as a high school and collegiate wrestler. These rashes were different from the rashes he described as his history of AD, which consisted of pruritic and erythematous skin in his antecubital and popliteal fossae. He denied any history of herpes simplex virus (HSV) infection.
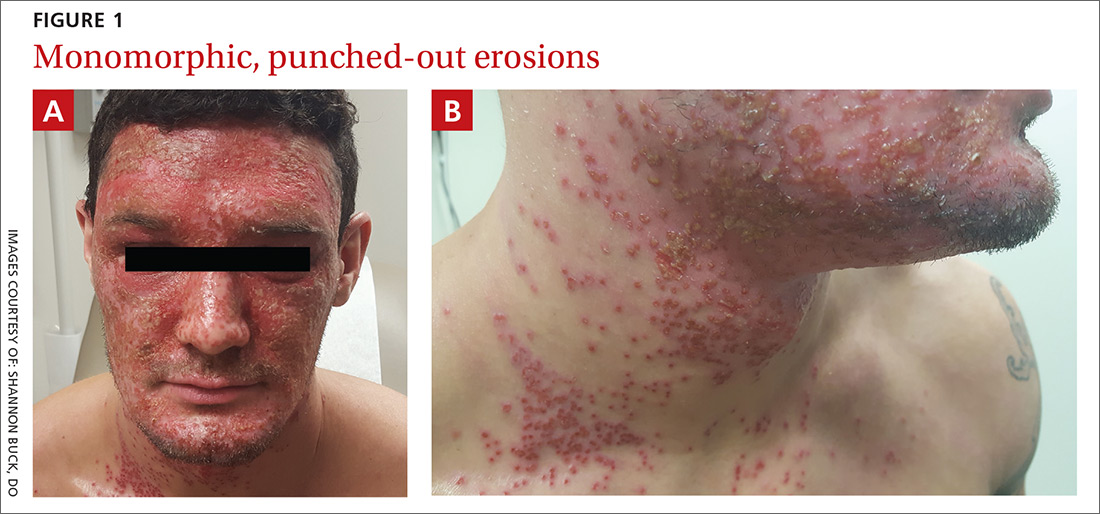
A physical examination revealed numerous monomorphic, 1- to 3-mm, punched-out erosions and ulcers with overlying yellow-brown crust encompassing the patient’s entire face and portions of his anterior neck. Several clustered vesicles on erythematous bases also were noted (FIGUREs 1A and 1B). We used a Dermablade to unroof some of the vesicles and sent the scrapings to the lab for Tzanck, direct fluorescent antibody assay (DFA), and HSV polymerase chain reaction (PCR) testing.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum secondary to herpes gladiatorum
The patient’s laboratory results came back and the Tzanck preparation was positive for multinucleated giant cells, and both the DFA and HSV PCR were positive for HSV infection. This, paired with the widely disseminated rash observed on examination and the patient’s history of AD, was consistent with a diagnosis of eczema herpeticum (EH).
Rather than primary impetigo, the patient’s self-described history of recurrent rashes was felt to represent a history of HSV outbreaks. Given his denial of prior oral or genital HSV infection, as well as the coincident onset of these outbreaks during his career as a competitive wrestler, the most likely primary infection source was direct contact with another HSV-infected wrestler.
Herpes gladiatorum refers to a primary cutaneous HSV infection contracted by an athlete through direct skin-to-skin contact with another athlete.1 It is common in contact sports, such as rugby and wrestling, and particularly common at organized wrestling camps, where mass outbreaks are a frequent occurrence.2 Herpes gladiatorum is so common at these camps that many recommend prophylactic valacyclovir treatment for all participants to mitigate the risk of contracting HSV. In a 2016 review, Anderson et al concluded that prophylactic valacyclovir treatment at a 28-day high school wrestling camp effectively reduced outbreak incidence by 89.5%.2
The lesions of herpes gladiatorum are classically limited in distribution and reflective of the areas of direct contact with infected skin, most commonly the face, neck, and arms. Our patient’s history of more limited outbreaks on his face was consistent with this typical presentation. His current outbreak, however, had become much more widely disseminated, which led to the diagnosis of EH secondary to herpes gladiatorum.
Eczema herpeticum: Pathogenesis and diagnosis
Also known as Kaposi’s varicelliform eruption, EH is a rapid, widespread cutaneous dissemination of HSV infection in areas of dermatitis or skin barrier disruption, most commonly caused by HSV-1 infection.3 It is classically associated with AD, but also can occur in patients with impaired epidermal barrier function due to other conditions, such as burns, pemphigus vulgaris, mycosis fungoides, and Darier disease.4 It occurs in <3% of patients with AD and is more commonly observed in infants and children with AD than adults.5
Continue to: Clinically, the most common manifestations are discrete..
Clinically, the most common manifestations are discrete, monomorphic, 2- to 3-mm, punched-out erosions with hemorrhagic crusts; intact vesicles are less commonly observed.4 Involved skin is typically painful and may be pruritic. Clinical diagnosis should be confirmed by laboratory evaluation, typically Tzanck preparation, DFA, and/or HSV PCR.
Complications and the importance of rapid treatment
The most common complication of EH is bacterial superinfection (impetigo), usually by Staphylococcus aureus or group A streptococci. Signs of bacterial superinfection include weeping lesions, pustules, honey-colored/golden crusting, worsening of existing dermatitis, and failure to respond to antiviral treatment. Topical mupirocin 2% cream is generally effective for controlling limited infection. However, systemic antibiotics (cephalosporins or penicillinase-resistant penicillins) may be necessary to control widespread disease.4 Clinical improvement should be observed within a single course of an appropriate antibiotic.
In contrast to impetigo, less common but more serious complications of EH can be life threatening. Systemic dissemination of disease is of particular importance in vulnerable populations such as pediatric and immunocompromised patients. Meningoencephalitis, secondary bacteremia, and herpes keratitis can all develop secondary to EH and incur significant morbidity and mortality.1
Fever, malaise, lymphadenopathy, or eye pain should prompt immediate consideration of inpatient evaluation and treatment for these potentially deadly or debilitating complications. All patients with EH distributed near the eyes should be referred to ophthalmology to rule out ocular involvement.
Immediately treat with antivirals
Due to the potential complications discussed above, a diagnosis of EH necessitates immediate treatment with oral or intravenous antiviral medication. Acyclovir, valacyclovir, or famciclovir may be used, with typical treatment courses ranging from 10 to 14 days or until all mucocutaneous lesions are healed.4 Although typically reserved for patients with recurrent genital herpes resulting in 6 or more outbreaks annually, chronic suppressive therapy also may be considered for patients with EH who suffer from frequent or severe recurrent outbreaks.
Continue to: Our patient
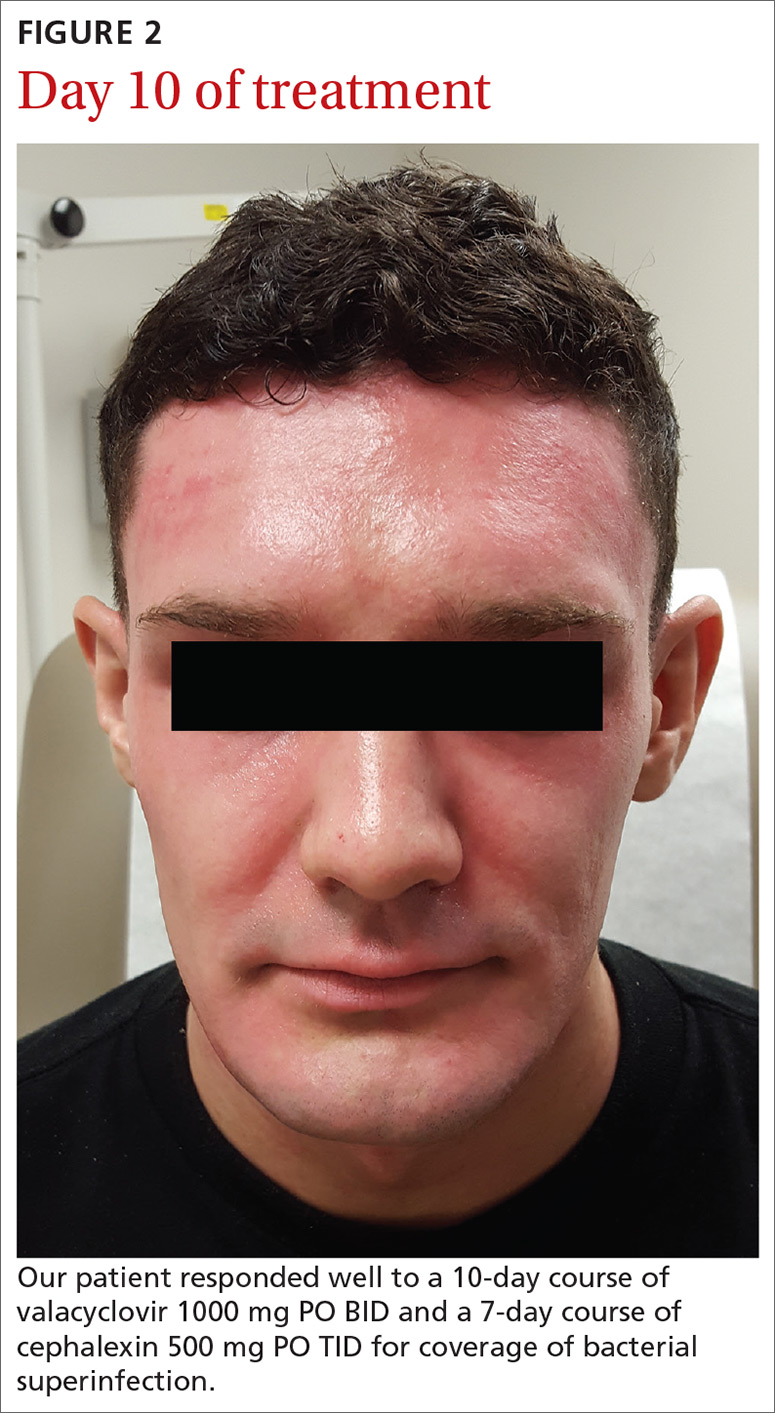
Our patient. Given his otherwise excellent health and the absence of symptoms of potentially serious complications, our patient was treated as an outpatient with a 10-day course of valacyclovir 1000 mg PO BID. He was additionally prescribed a 7-day course of cephalexin 500 mg PO TID for coverage of bacterial superinfection. He responded well to treatment.
Ten days after his initial presentation to our clinic, his erosions and vesicles had completely cleared, and the associated erythema had significantly improved (FIGURE 2). Given the severity of his presentation and his history of 2 to 3 outbreaks annually, he opted to continue prophylactic valacyclovir (500 mg/d) for long-term suppression.
CORRESPONDENCE
Jonathan Madden, MD, 221 3rd Street West, JBSA-Randolph, TX 78150, [email protected]
1. Shenoy R, Mostow E, Cain G. Eczema herpeticum in a wrestler. Clin J Sport Med. 2015;25:e18-e19.
2. Anderson BJ, McGuire DP, Reed M, et al. Prophylactic valacyclovir to prevent outbreaks of primary herpes gladiatorum at a 28-day wrestling camp: a 10-year review. Clin J Sport Med. 2016;26:272-278.
3. Olson J, Robles DT, Kirby P, et al. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14:18.
4. Downing C, Mendoza N, Tyring S. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1400-1424.
5. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
A 22-year-old Caucasian man with a history of atopic dermatitis (AD) was referred to our dermatology clinic for evaluation of a diffuse facial rash that had been present for the previous 7 days. The rash initially presented as erythema on the right malar cheek that rapidly spread to the entire face. Initially diagnosed as impetigo, empiric treatment with sulfamethoxazole/trimethoprim (800 mg/160 mg PO BID for 7 days), dicloxacillin (500 mg PO BID for 6 days), cephalexin (500 mg TID for 5 days), and mupirocin (2% topical cream applied TID for 6 days) failed to improve the patient’s symptoms. He reported mild pain associated with facial movements.
The patient had a history of similar (but more limited) rashes, which he described as “recurrent impetigo,” that began during his career as a high school and collegiate wrestler. These rashes were different from the rashes he described as his history of AD, which consisted of pruritic and erythematous skin in his antecubital and popliteal fossae. He denied any history of herpes simplex virus (HSV) infection.
A physical examination revealed numerous monomorphic, 1- to 3-mm, punched-out erosions and ulcers with overlying yellow-brown crust encompassing the patient’s entire face and portions of his anterior neck. Several clustered vesicles on erythematous bases also were noted (FIGUREs 1A and 1B). We used a Dermablade to unroof some of the vesicles and sent the scrapings to the lab for Tzanck, direct fluorescent antibody assay (DFA), and HSV polymerase chain reaction (PCR) testing.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum secondary to herpes gladiatorum
The patient’s laboratory results came back and the Tzanck preparation was positive for multinucleated giant cells, and both the DFA and HSV PCR were positive for HSV infection. This, paired with the widely disseminated rash observed on examination and the patient’s history of AD, was consistent with a diagnosis of eczema herpeticum (EH).
Rather than primary impetigo, the patient’s self-described history of recurrent rashes was felt to represent a history of HSV outbreaks. Given his denial of prior oral or genital HSV infection, as well as the coincident onset of these outbreaks during his career as a competitive wrestler, the most likely primary infection source was direct contact with another HSV-infected wrestler.
Herpes gladiatorum refers to a primary cutaneous HSV infection contracted by an athlete through direct skin-to-skin contact with another athlete.1 It is common in contact sports, such as rugby and wrestling, and particularly common at organized wrestling camps, where mass outbreaks are a frequent occurrence.2 Herpes gladiatorum is so common at these camps that many recommend prophylactic valacyclovir treatment for all participants to mitigate the risk of contracting HSV. In a 2016 review, Anderson et al concluded that prophylactic valacyclovir treatment at a 28-day high school wrestling camp effectively reduced outbreak incidence by 89.5%.2
The lesions of herpes gladiatorum are classically limited in distribution and reflective of the areas of direct contact with infected skin, most commonly the face, neck, and arms. Our patient’s history of more limited outbreaks on his face was consistent with this typical presentation. His current outbreak, however, had become much more widely disseminated, which led to the diagnosis of EH secondary to herpes gladiatorum.
Eczema herpeticum: Pathogenesis and diagnosis
Also known as Kaposi’s varicelliform eruption, EH is a rapid, widespread cutaneous dissemination of HSV infection in areas of dermatitis or skin barrier disruption, most commonly caused by HSV-1 infection.3 It is classically associated with AD, but also can occur in patients with impaired epidermal barrier function due to other conditions, such as burns, pemphigus vulgaris, mycosis fungoides, and Darier disease.4 It occurs in <3% of patients with AD and is more commonly observed in infants and children with AD than adults.5
Continue to: Clinically, the most common manifestations are discrete..
Clinically, the most common manifestations are discrete, monomorphic, 2- to 3-mm, punched-out erosions with hemorrhagic crusts; intact vesicles are less commonly observed.4 Involved skin is typically painful and may be pruritic. Clinical diagnosis should be confirmed by laboratory evaluation, typically Tzanck preparation, DFA, and/or HSV PCR.
Complications and the importance of rapid treatment
The most common complication of EH is bacterial superinfection (impetigo), usually by Staphylococcus aureus or group A streptococci. Signs of bacterial superinfection include weeping lesions, pustules, honey-colored/golden crusting, worsening of existing dermatitis, and failure to respond to antiviral treatment. Topical mupirocin 2% cream is generally effective for controlling limited infection. However, systemic antibiotics (cephalosporins or penicillinase-resistant penicillins) may be necessary to control widespread disease.4 Clinical improvement should be observed within a single course of an appropriate antibiotic.
In contrast to impetigo, less common but more serious complications of EH can be life threatening. Systemic dissemination of disease is of particular importance in vulnerable populations such as pediatric and immunocompromised patients. Meningoencephalitis, secondary bacteremia, and herpes keratitis can all develop secondary to EH and incur significant morbidity and mortality.1
Fever, malaise, lymphadenopathy, or eye pain should prompt immediate consideration of inpatient evaluation and treatment for these potentially deadly or debilitating complications. All patients with EH distributed near the eyes should be referred to ophthalmology to rule out ocular involvement.
Immediately treat with antivirals
Due to the potential complications discussed above, a diagnosis of EH necessitates immediate treatment with oral or intravenous antiviral medication. Acyclovir, valacyclovir, or famciclovir may be used, with typical treatment courses ranging from 10 to 14 days or until all mucocutaneous lesions are healed.4 Although typically reserved for patients with recurrent genital herpes resulting in 6 or more outbreaks annually, chronic suppressive therapy also may be considered for patients with EH who suffer from frequent or severe recurrent outbreaks.
Continue to: Our patient
Our patient. Given his otherwise excellent health and the absence of symptoms of potentially serious complications, our patient was treated as an outpatient with a 10-day course of valacyclovir 1000 mg PO BID. He was additionally prescribed a 7-day course of cephalexin 500 mg PO TID for coverage of bacterial superinfection. He responded well to treatment.
Ten days after his initial presentation to our clinic, his erosions and vesicles had completely cleared, and the associated erythema had significantly improved (FIGURE 2). Given the severity of his presentation and his history of 2 to 3 outbreaks annually, he opted to continue prophylactic valacyclovir (500 mg/d) for long-term suppression.

CORRESPONDENCE
Jonathan Madden, MD, 221 3rd Street West, JBSA-Randolph, TX 78150, [email protected]
A 22-year-old Caucasian man with a history of atopic dermatitis (AD) was referred to our dermatology clinic for evaluation of a diffuse facial rash that had been present for the previous 7 days. The rash initially presented as erythema on the right malar cheek that rapidly spread to the entire face. Initially diagnosed as impetigo, empiric treatment with sulfamethoxazole/trimethoprim (800 mg/160 mg PO BID for 7 days), dicloxacillin (500 mg PO BID for 6 days), cephalexin (500 mg TID for 5 days), and mupirocin (2% topical cream applied TID for 6 days) failed to improve the patient’s symptoms. He reported mild pain associated with facial movements.
The patient had a history of similar (but more limited) rashes, which he described as “recurrent impetigo,” that began during his career as a high school and collegiate wrestler. These rashes were different from the rashes he described as his history of AD, which consisted of pruritic and erythematous skin in his antecubital and popliteal fossae. He denied any history of herpes simplex virus (HSV) infection.
A physical examination revealed numerous monomorphic, 1- to 3-mm, punched-out erosions and ulcers with overlying yellow-brown crust encompassing the patient’s entire face and portions of his anterior neck. Several clustered vesicles on erythematous bases also were noted (FIGUREs 1A and 1B). We used a Dermablade to unroof some of the vesicles and sent the scrapings to the lab for Tzanck, direct fluorescent antibody assay (DFA), and HSV polymerase chain reaction (PCR) testing.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum secondary to herpes gladiatorum
The patient’s laboratory results came back and the Tzanck preparation was positive for multinucleated giant cells, and both the DFA and HSV PCR were positive for HSV infection. This, paired with the widely disseminated rash observed on examination and the patient’s history of AD, was consistent with a diagnosis of eczema herpeticum (EH).
Rather than primary impetigo, the patient’s self-described history of recurrent rashes was felt to represent a history of HSV outbreaks. Given his denial of prior oral or genital HSV infection, as well as the coincident onset of these outbreaks during his career as a competitive wrestler, the most likely primary infection source was direct contact with another HSV-infected wrestler.
Herpes gladiatorum refers to a primary cutaneous HSV infection contracted by an athlete through direct skin-to-skin contact with another athlete.1 It is common in contact sports, such as rugby and wrestling, and particularly common at organized wrestling camps, where mass outbreaks are a frequent occurrence.2 Herpes gladiatorum is so common at these camps that many recommend prophylactic valacyclovir treatment for all participants to mitigate the risk of contracting HSV. In a 2016 review, Anderson et al concluded that prophylactic valacyclovir treatment at a 28-day high school wrestling camp effectively reduced outbreak incidence by 89.5%.2
The lesions of herpes gladiatorum are classically limited in distribution and reflective of the areas of direct contact with infected skin, most commonly the face, neck, and arms. Our patient’s history of more limited outbreaks on his face was consistent with this typical presentation. His current outbreak, however, had become much more widely disseminated, which led to the diagnosis of EH secondary to herpes gladiatorum.
Eczema herpeticum: Pathogenesis and diagnosis
Also known as Kaposi’s varicelliform eruption, EH is a rapid, widespread cutaneous dissemination of HSV infection in areas of dermatitis or skin barrier disruption, most commonly caused by HSV-1 infection.3 It is classically associated with AD, but also can occur in patients with impaired epidermal barrier function due to other conditions, such as burns, pemphigus vulgaris, mycosis fungoides, and Darier disease.4 It occurs in <3% of patients with AD and is more commonly observed in infants and children with AD than adults.5
Continue to: Clinically, the most common manifestations are discrete..
Clinically, the most common manifestations are discrete, monomorphic, 2- to 3-mm, punched-out erosions with hemorrhagic crusts; intact vesicles are less commonly observed.4 Involved skin is typically painful and may be pruritic. Clinical diagnosis should be confirmed by laboratory evaluation, typically Tzanck preparation, DFA, and/or HSV PCR.
Complications and the importance of rapid treatment
The most common complication of EH is bacterial superinfection (impetigo), usually by Staphylococcus aureus or group A streptococci. Signs of bacterial superinfection include weeping lesions, pustules, honey-colored/golden crusting, worsening of existing dermatitis, and failure to respond to antiviral treatment. Topical mupirocin 2% cream is generally effective for controlling limited infection. However, systemic antibiotics (cephalosporins or penicillinase-resistant penicillins) may be necessary to control widespread disease.4 Clinical improvement should be observed within a single course of an appropriate antibiotic.
In contrast to impetigo, less common but more serious complications of EH can be life threatening. Systemic dissemination of disease is of particular importance in vulnerable populations such as pediatric and immunocompromised patients. Meningoencephalitis, secondary bacteremia, and herpes keratitis can all develop secondary to EH and incur significant morbidity and mortality.1
Fever, malaise, lymphadenopathy, or eye pain should prompt immediate consideration of inpatient evaluation and treatment for these potentially deadly or debilitating complications. All patients with EH distributed near the eyes should be referred to ophthalmology to rule out ocular involvement.
Immediately treat with antivirals
Due to the potential complications discussed above, a diagnosis of EH necessitates immediate treatment with oral or intravenous antiviral medication. Acyclovir, valacyclovir, or famciclovir may be used, with typical treatment courses ranging from 10 to 14 days or until all mucocutaneous lesions are healed.4 Although typically reserved for patients with recurrent genital herpes resulting in 6 or more outbreaks annually, chronic suppressive therapy also may be considered for patients with EH who suffer from frequent or severe recurrent outbreaks.
Continue to: Our patient
Our patient. Given his otherwise excellent health and the absence of symptoms of potentially serious complications, our patient was treated as an outpatient with a 10-day course of valacyclovir 1000 mg PO BID. He was additionally prescribed a 7-day course of cephalexin 500 mg PO TID for coverage of bacterial superinfection. He responded well to treatment.
Ten days after his initial presentation to our clinic, his erosions and vesicles had completely cleared, and the associated erythema had significantly improved (FIGURE 2). Given the severity of his presentation and his history of 2 to 3 outbreaks annually, he opted to continue prophylactic valacyclovir (500 mg/d) for long-term suppression.

CORRESPONDENCE
Jonathan Madden, MD, 221 3rd Street West, JBSA-Randolph, TX 78150, [email protected]
1. Shenoy R, Mostow E, Cain G. Eczema herpeticum in a wrestler. Clin J Sport Med. 2015;25:e18-e19.
2. Anderson BJ, McGuire DP, Reed M, et al. Prophylactic valacyclovir to prevent outbreaks of primary herpes gladiatorum at a 28-day wrestling camp: a 10-year review. Clin J Sport Med. 2016;26:272-278.
3. Olson J, Robles DT, Kirby P, et al. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14:18.
4. Downing C, Mendoza N, Tyring S. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1400-1424.
5. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
1. Shenoy R, Mostow E, Cain G. Eczema herpeticum in a wrestler. Clin J Sport Med. 2015;25:e18-e19.
2. Anderson BJ, McGuire DP, Reed M, et al. Prophylactic valacyclovir to prevent outbreaks of primary herpes gladiatorum at a 28-day wrestling camp: a 10-year review. Clin J Sport Med. 2016;26:272-278.
3. Olson J, Robles DT, Kirby P, et al. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14:18.
4. Downing C, Mendoza N, Tyring S. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1400-1424.
5. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
6-day history of fever • groin pain and swelling • recent hiking trip in Colorado • Dx?
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
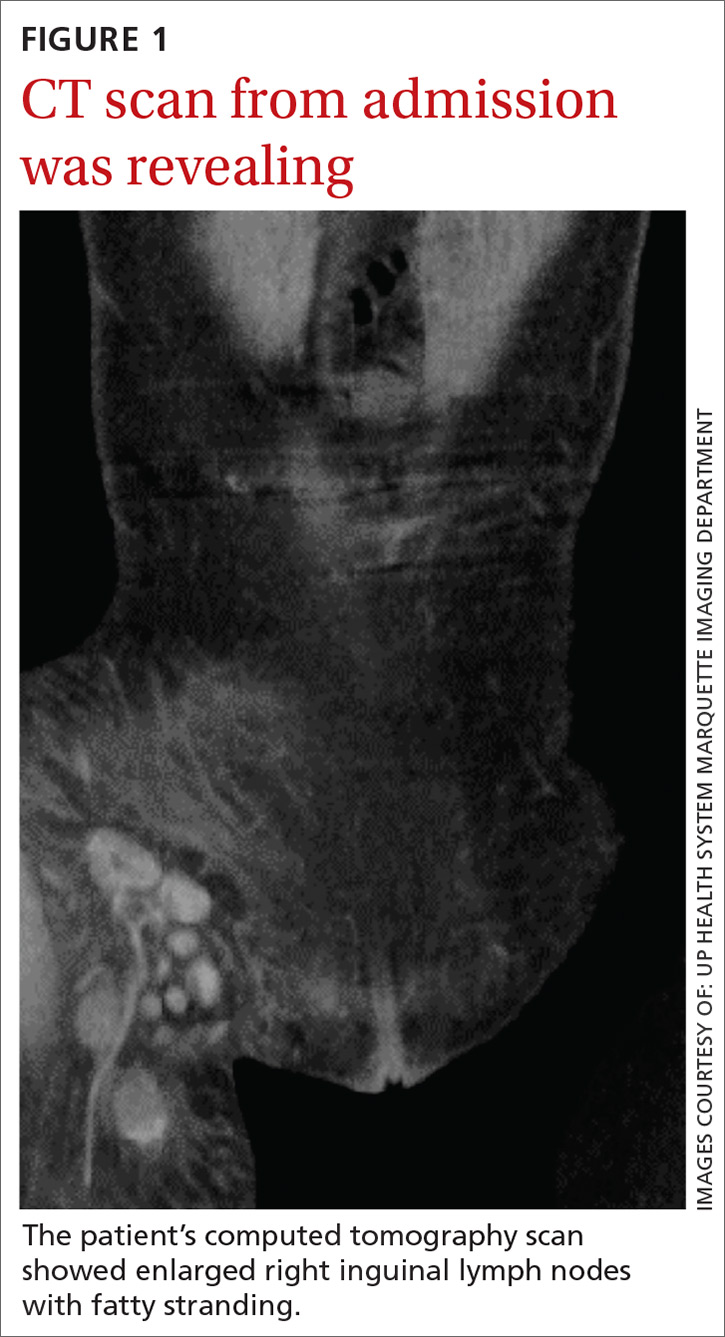
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
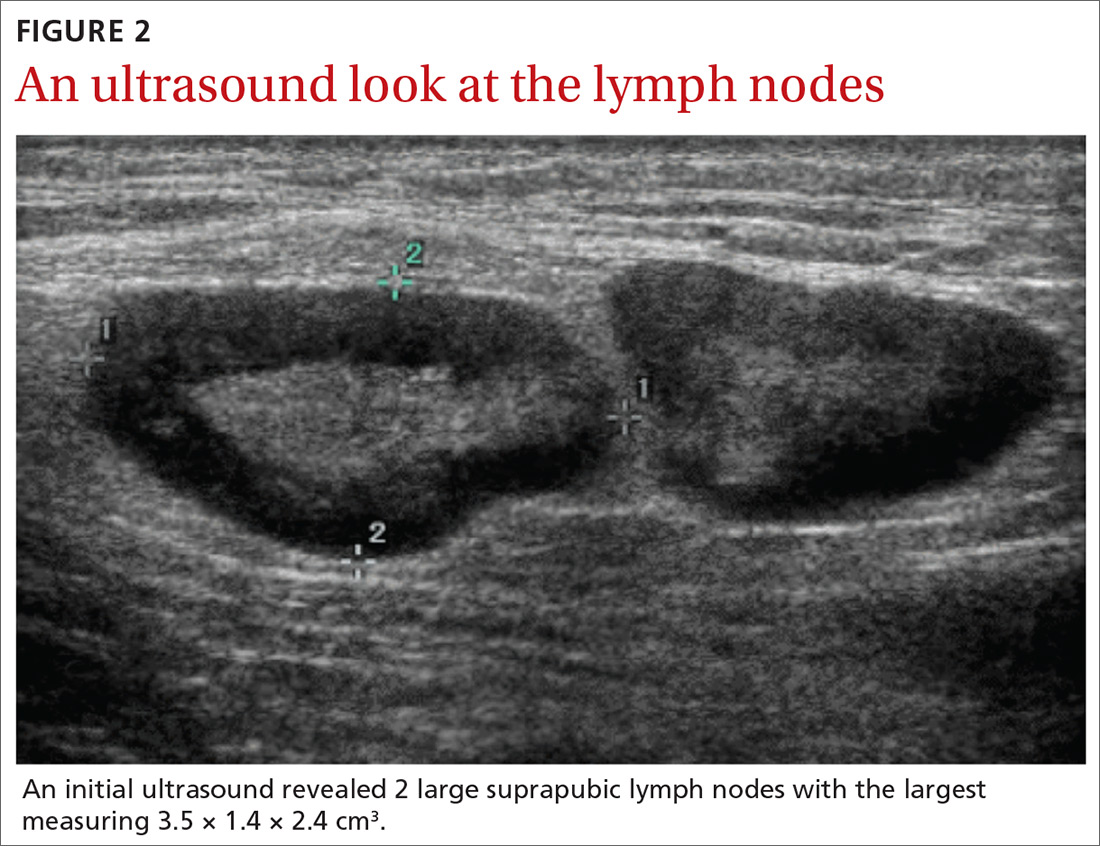
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
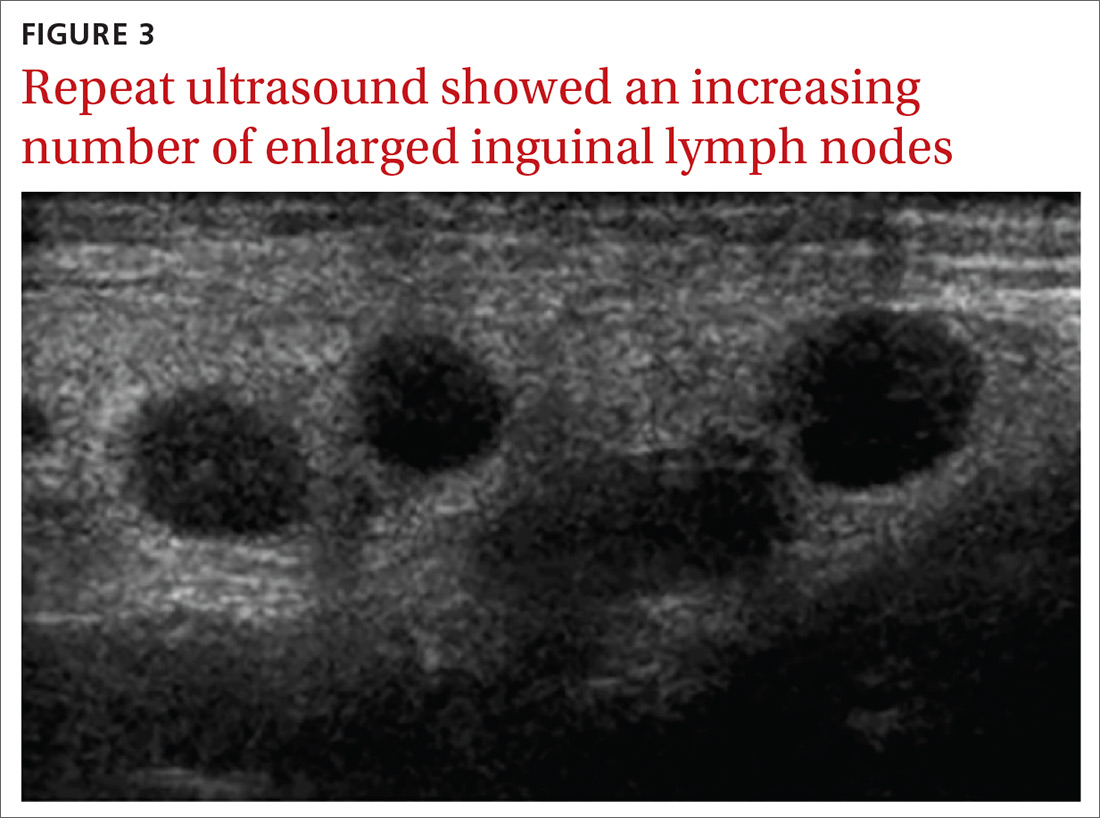
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; [email protected]
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.

Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.

The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).

Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; [email protected]
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.

Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.

The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).

Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; [email protected]
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
A look at new guidelines for HIV treatment and prevention
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
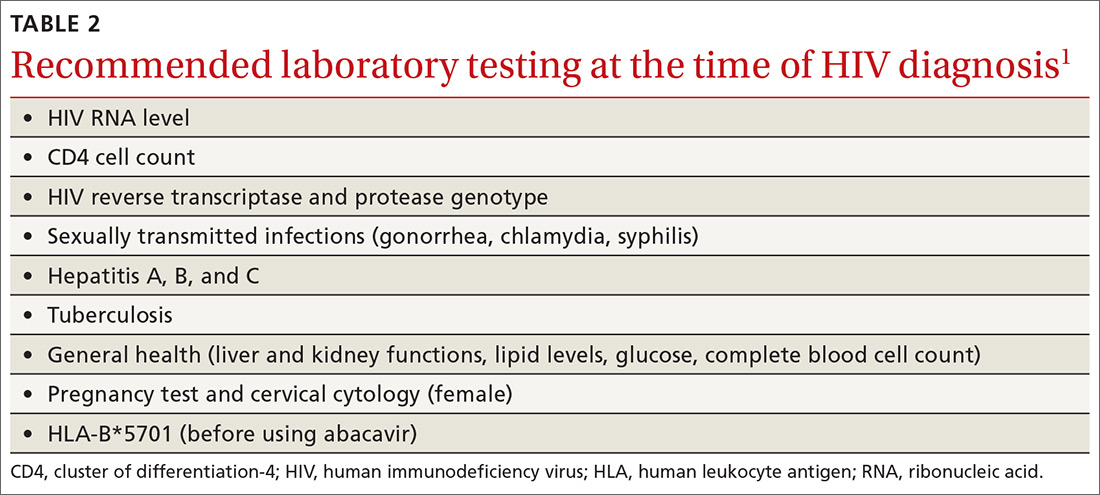
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
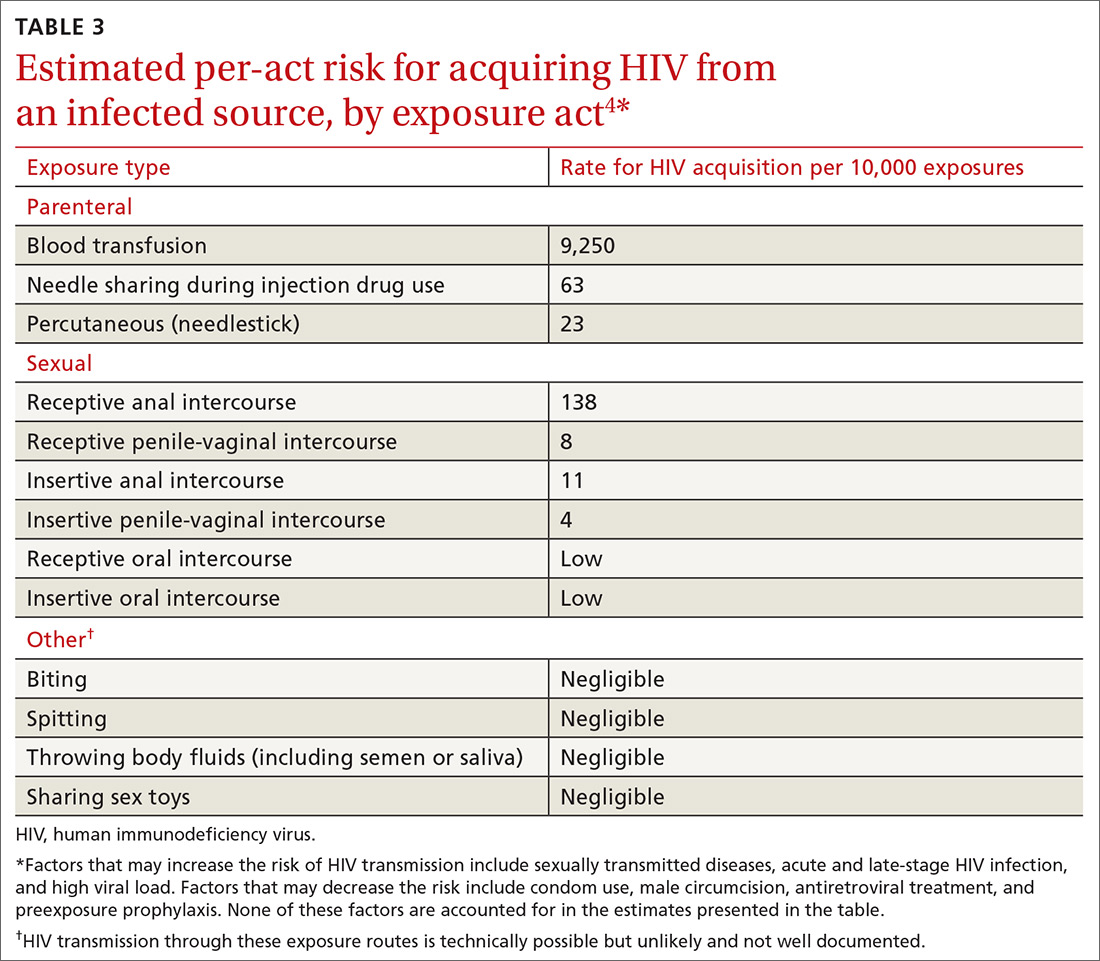
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
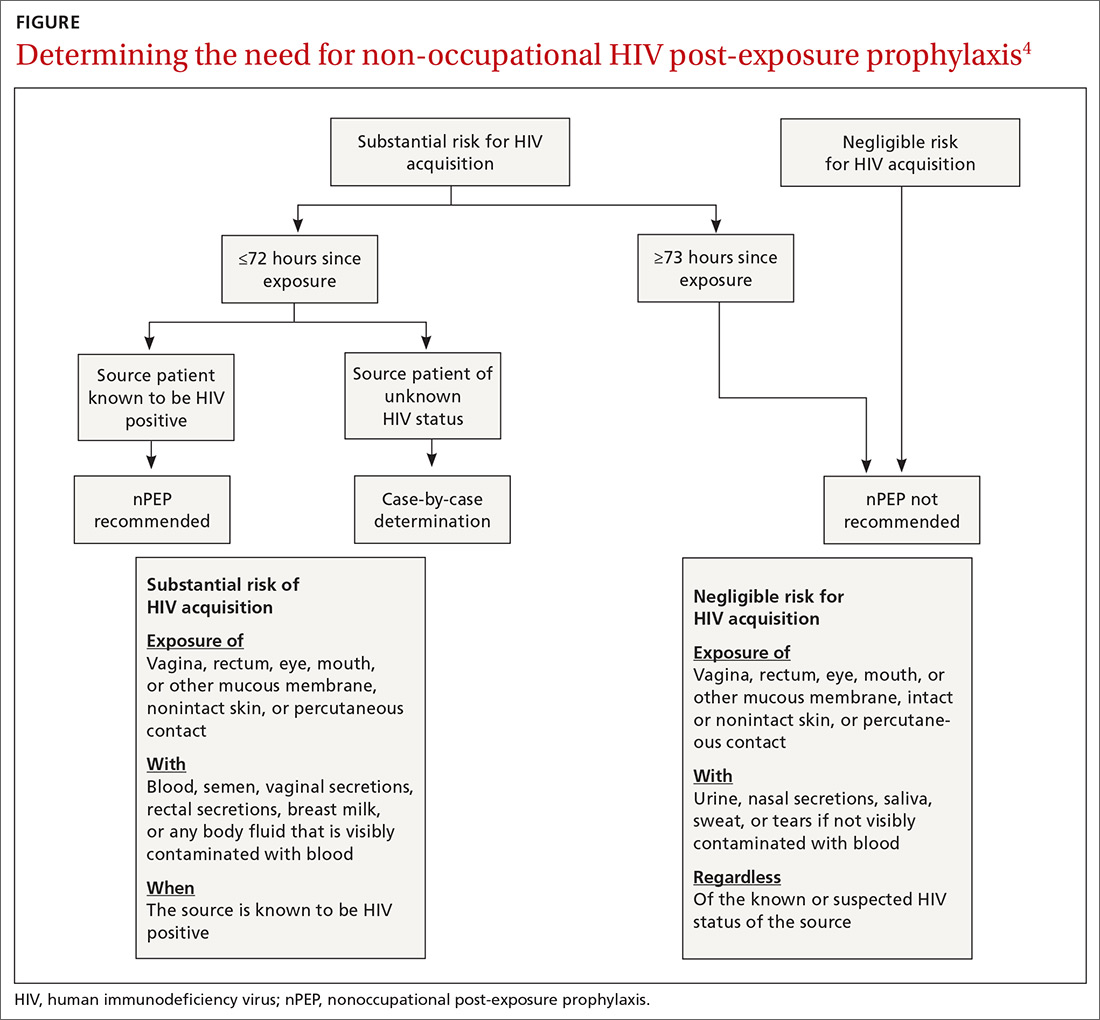
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
Sexually Transmitted Infections Caused by Mycoplasma genitalium and Neisseria gonorrhoeae: Diagnosis and Treatment
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
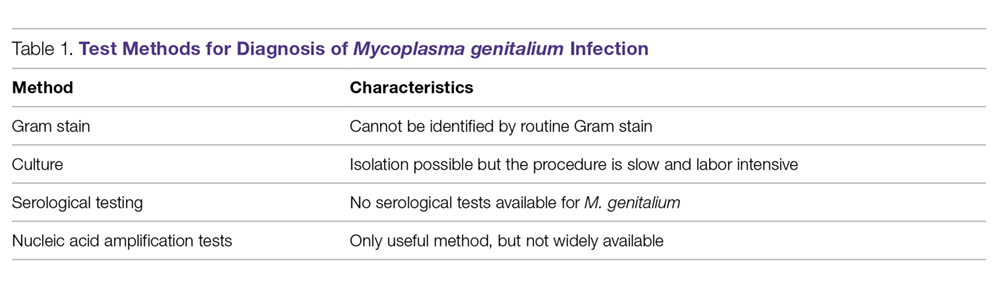
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
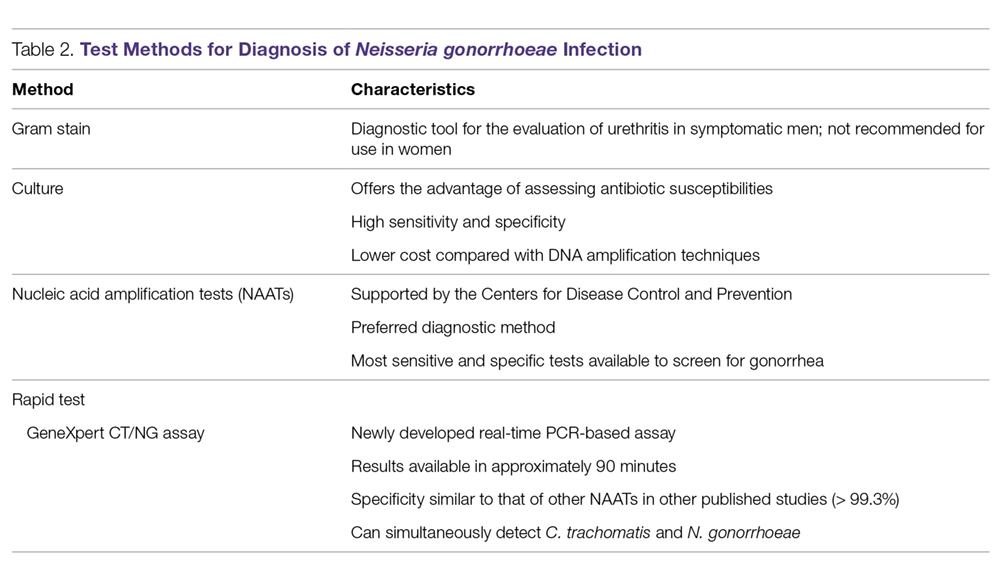
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
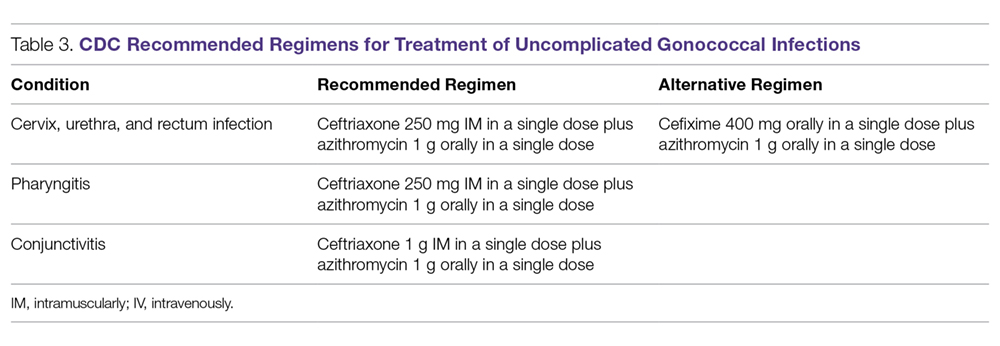
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
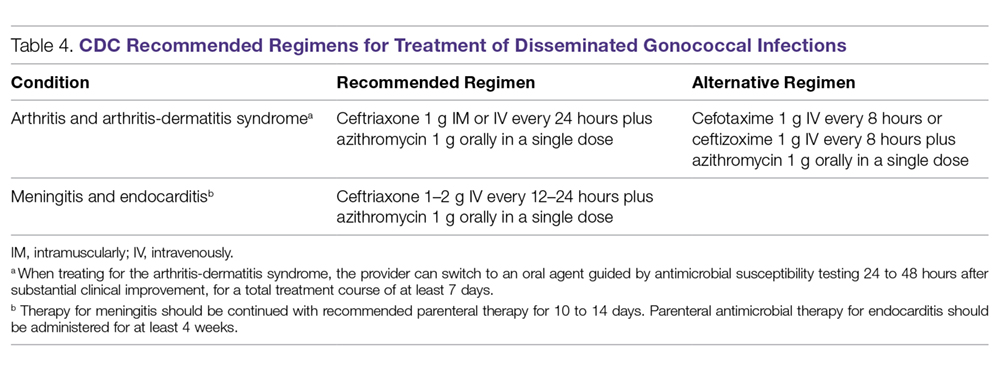
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.

N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.

Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45

In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.

Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.

N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.

Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45

In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.

Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
Bundled Hospital-at-Home and Transitional Care Program Is Associated with Reduced Rate of Hospital Readmission
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of a hospital-at-home (HaH) and transitional care program on clinical outcomes and patient experiences when compared with inpatient hospitalization.
Design. Cohort study with matched controls.
Setting and participants. The study was conducted in a single center and aimed to evaluate a HaH program bundled with a 30-day postacute period of home-based transitional care. The program is funded by the Center for Medicare and Medicaid Innovation of the Centers for Medicare and Medicaid Services (CMS) with the goal of establishing a new HaH program that provides acute hospital-level care in a patient’s home as a substitute for transitional inpatient care.
Patients were eligible for the program if they were aged 18 years or older, lived in Manhattan, New York, had fee-for-service Medicare or private insurer that had contracted for HaH services, and required inpatient hospital admission for eligible conditions. Eligible conditions included acute exacerbations of asthma or chronic obstructive pulmonary disease, congestive heart failure (CHF), urinary tract infections (UTI), community-acquired pneumonia (CAP), cellulitis of lower extremities, deep venous thrombosis, pulmonary embolism, hypertensive urgency, hyperglycemia, and dehydration; this list was later expanded to 19 conditions representing 65 diagnosis-related groups. Patients were excluded if they were clinically unstable, required cardiac monitoring or intensive care, or lived in an unsafe home environment. Patients were identified in the emergency department (ED) and approached for enrollment in the program. Patients who were eligible for admission but refused HaH admission, or those who were identified as eligible for admission but for whom HaH clinicians were not available were enrolled as control patients.
Intervention. The HaH intervention included physician or nurse practitioner visits at home to provide acute care services including physical examination, illness and vital signs monitoring, intravenous infusions, wound care, and education regarding the illness. Nurses visited patients once or more a day to provide most of the care, and a physician or nurse practitioner saw patients at least daily in person or via video call facilitated by the nurse. A social worker also visited each patient at least once. Medical equipment, phlebotomy, and home radiography were also provided at home as needed. Patients were discharged from acute care when their acute illness resolved; subsequently, nurses and social workers provided self-
Main outcome measures. Main study outcome measures include duration of the acute care period (length of stay [LOS]) and 30-day all-cause hospital readmissions or ED visits, transfer to a skilled nursing facility, and referral to a certified home health care agency. LOS was defined as being from the date the patient was listed for admission by an ED physician to the date that post-acute care was initiated (for HaH) or hospital discharge (for control patients). Other measures include patient’s rating of care measured using items in 6 of the 9 domains of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey that were most salient to care at home, including communication with nurses, communication with physicians, pain management, communication about medicines, discharge information, and overall hospital rating.
Main results. The HaH clinical team approached 460 patients and enrolled 295 to the program. A total of 212 patients who were admitted to the hospital were enrolled as control patients. HaH patients were older than control patients, with an average age of 76.9 years (SD, 16.6) and 71.5 years (SD 13.8), respectively, and more likely to have at least 1 functional limitation (71.5% vs. 55.5%). The most frequent admission diagnoses to HaH were UTIs, CAP, cellulitis, and CHF. HaH patients had a shorter hospitalization LOS (3.2 days) compared with the control group (5.5 days; 95% confidence interval [CI], –1.8 to –2.7 days). HaH patients were less likely to have 30-day all-cause hospital readmissions (8.6% vs. 15.6%; 95% CI, –12.9% to –1.1%) and 30-day ED revisits (5.8% vs. 11.7%) compared to controls. Analysis adjusted for age, sex, race, ethnicity, education, insurance type, physical function, general health, and admitting diagnosis found that HaH patients had lower odds of hospital readmission (odds ratio [OR], 0.43; 95% CI, 0.36-0.52) and lower odds of ED revisits (OR, 0.39; 95% CI, 0.31-0.49). HaH patients reported higher ratings for communication with nurses and physicians and communication about medicines when compared with controls; they were also more likely to report the highest rating for overall hospital care (68.8% vs. 45.3%). Scores for pain management were lower for HaH patients when compared with controls.
Conclusions. Patients receiving care through the HaH program were less likely to be readmitted at 30 days after hospital discharge, had lower hospital LOS and reported higher ratings of care when compared to patients receiving care in the hospital. The study demonstrated the potential benefits of the HaH model of care for adults who need inpatient hospitalization.
Commentary
This study adds to the literature on outcomes associated with HaH programs. The first study of the HaH model in the United States was published in 2005,1 and despite the early demonstration of its feasibility and outcomes in this and subsequent studies,2,3 HaH models have not been widely adopted, unlike in other countries with integrated health care systems.4 One of the primary reasons this model has not been adopted is the lack of a specific payment mechanism in Medicare fee for service for HaH. Implementation of the HaH program described in the current study was an effort funded by a CMS innovation award to test the effect of models of care with the potential of developing payment mechanisms that would support further dissemination of these models. The results from the current study were encouraging and have led to the Physician-Focused Payment Model Technical Advisory Committee’s unanimous recommendation to the U.S. Department of Health and Human Services for full implementation in 2017.
The current study does have certain limitations. It is not a randomized trial, and thus control group selection could be affected by selection bias. Also, the study was conducted in a single health system and thus may have limited generalizability. Nevertheless, this study was designed based on prior studies of HaH, including randomized and non-randomized studies, that have demonstrated benefits similar to the current study. The finding that HaH patients reported worse pain control than did patients hospitalized in the inpatient setting, where staff is available 24 hours a day, may suggest differences in care that is feasible at home versus in the inpatient setting. Finally, because it is a bundled program that includes both HaH and a post-discharge care transition program, it is unclear if the effects found in this evaluation can be attributed to specific components within the bundled program.
Applications for Clinical Practice
Patients, particularly older adults, may prefer to have hospital-level care delivered at home; clinicians may consider how HaH may allow patients to avoid potential hazards of hospitalization,5 such as inpatient falls, delirium, and other iatrogenic events. The HaH program is feasible and safe, and is associated with improved outcomes of care for patients.
—William W. Hung, MD, MPH
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.
1. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
2. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital at home”. Med J Aust. 2012;197:512-519.
3. Mader SL, Medcraft MC, Joseph C, et al. Program at home: a Veteran Affairs healthcare program to deliver hospital care in the home. J Am Geriatr Soc. 2008;56: 2317-2322.
4. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health Review of the hospital in the home program. Med J Aust. 2010;193:598-601.
5. Creditor MC. Hazards of hospitalization. Ann Intern Med. 1993;118:219-223.