User login
Conservatism spreads in prostate cancer
, the United States now has more than 100 measles cases for the year, e-cigarette use reverses progress in reducing teens’ tobacco use, and consider adopting the MESA 10-year coronary heart disease risk calculator.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
, the United States now has more than 100 measles cases for the year, e-cigarette use reverses progress in reducing teens’ tobacco use, and consider adopting the MESA 10-year coronary heart disease risk calculator.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
, the United States now has more than 100 measles cases for the year, e-cigarette use reverses progress in reducing teens’ tobacco use, and consider adopting the MESA 10-year coronary heart disease risk calculator.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Vaccination and antiviral treatment do not affect stroke risk following shingles
HONOLULU – according to findings from a retrospective study of Medicare beneficiaries with shingles and ischemic stroke.
The findings suggest that primary prevention of shingles through vaccination might be the most effective approach to prevent shingles-associated acute ischemic stroke, said the researchers, who presented the study at the International Stroke Conference sponsored by the American Heart Association.
Almost one in three people in the United States will develop shingles, also known as herpes zoster, in their lifetime, according to the Centers for Disease Control and Prevention. Previous research has not simultaneously examined the effect of shingles vaccination and antiviral treatment following shingles onset on the risk of acute ischemic stroke.
Quanhe Yang, PhD, a senior scientist at the CDC, and his colleagues examined data for 35,186 Medicare fee-for-service beneficiaries who were 66 years or older, diagnosed with shingles during 2008-2014, and diagnosed with acute ischemic stroke within a year of shingles diagnosis. Using a self-controlled case series design, the investigators analyzed the association between shingles and stroke. Dr. Yang and his colleagues estimated the incident rate ratio (IRR) by comparing the incidence of stroke during risk periods (i.e., periods following shingles), compared with control periods. To minimize confounding by age, they restricted their analyses to approximately 365 days from the shingles index date.
To investigate how vaccination against shingles with Zostavax and antiviral treatment following shingles affected stroke risk, the researchers classified beneficiaries into the following four groups: Group 1 had no vaccination and no antiviral treatment (49% of beneficiaries), Group 2 had vaccination only (9%), Group 3 had antiviral treatment only (34%), and Group 4 had vaccination and antiviral treatment (8%). The researchers tested for interaction to examine the changes in IRRs across the four groups.
IRRs for stroke progressively declined as time passed from the index shingles date, from 1.61 at 0-14 days following shingles to 1.35 at 15-30 days, 1.16 at 31-90 days, and 1.05 at 91-180 days. The researchers found no evidence that shingles vaccination and antiviral treatment modified the risk of acute ischemic stroke. The association between shingles and risk for acute ischemic stroke was consistent across age groups (i.e., 66-74 years, 75-84 years, and 85 years or older), sex, and race (i.e., non-Hispanic white, non-Hispanic black, and Hispanic, other).
One of the study’s strengths was that its sample was a large national cohort of Medicare fee-for-service beneficiaries, Dr. Yang said. In addition, the study design eliminated all fixed confounding effects. Potential weaknesses, however, included the fact that herpes zoster diagnosis was based on administrative data and that the vaccine’s efficacy declines over time.
The findings suggest that the importance of following the recommended shingles vaccination protocol in the prevention of shingles, Dr. Yang said. Shingrix, a vaccine that the Food and Drug Administration approved in 2017, prevents shingles with an efficacy greater than 90%, he added.
The investigators reported no funding source or disclosures for this study.
SOURCE: Yang Q et al. Circulation. 2019;50(Suppl_1): Abstract 39
HONOLULU – according to findings from a retrospective study of Medicare beneficiaries with shingles and ischemic stroke.
The findings suggest that primary prevention of shingles through vaccination might be the most effective approach to prevent shingles-associated acute ischemic stroke, said the researchers, who presented the study at the International Stroke Conference sponsored by the American Heart Association.
Almost one in three people in the United States will develop shingles, also known as herpes zoster, in their lifetime, according to the Centers for Disease Control and Prevention. Previous research has not simultaneously examined the effect of shingles vaccination and antiviral treatment following shingles onset on the risk of acute ischemic stroke.
Quanhe Yang, PhD, a senior scientist at the CDC, and his colleagues examined data for 35,186 Medicare fee-for-service beneficiaries who were 66 years or older, diagnosed with shingles during 2008-2014, and diagnosed with acute ischemic stroke within a year of shingles diagnosis. Using a self-controlled case series design, the investigators analyzed the association between shingles and stroke. Dr. Yang and his colleagues estimated the incident rate ratio (IRR) by comparing the incidence of stroke during risk periods (i.e., periods following shingles), compared with control periods. To minimize confounding by age, they restricted their analyses to approximately 365 days from the shingles index date.
To investigate how vaccination against shingles with Zostavax and antiviral treatment following shingles affected stroke risk, the researchers classified beneficiaries into the following four groups: Group 1 had no vaccination and no antiviral treatment (49% of beneficiaries), Group 2 had vaccination only (9%), Group 3 had antiviral treatment only (34%), and Group 4 had vaccination and antiviral treatment (8%). The researchers tested for interaction to examine the changes in IRRs across the four groups.
IRRs for stroke progressively declined as time passed from the index shingles date, from 1.61 at 0-14 days following shingles to 1.35 at 15-30 days, 1.16 at 31-90 days, and 1.05 at 91-180 days. The researchers found no evidence that shingles vaccination and antiviral treatment modified the risk of acute ischemic stroke. The association between shingles and risk for acute ischemic stroke was consistent across age groups (i.e., 66-74 years, 75-84 years, and 85 years or older), sex, and race (i.e., non-Hispanic white, non-Hispanic black, and Hispanic, other).
One of the study’s strengths was that its sample was a large national cohort of Medicare fee-for-service beneficiaries, Dr. Yang said. In addition, the study design eliminated all fixed confounding effects. Potential weaknesses, however, included the fact that herpes zoster diagnosis was based on administrative data and that the vaccine’s efficacy declines over time.
The findings suggest that the importance of following the recommended shingles vaccination protocol in the prevention of shingles, Dr. Yang said. Shingrix, a vaccine that the Food and Drug Administration approved in 2017, prevents shingles with an efficacy greater than 90%, he added.
The investigators reported no funding source or disclosures for this study.
SOURCE: Yang Q et al. Circulation. 2019;50(Suppl_1): Abstract 39
HONOLULU – according to findings from a retrospective study of Medicare beneficiaries with shingles and ischemic stroke.
The findings suggest that primary prevention of shingles through vaccination might be the most effective approach to prevent shingles-associated acute ischemic stroke, said the researchers, who presented the study at the International Stroke Conference sponsored by the American Heart Association.
Almost one in three people in the United States will develop shingles, also known as herpes zoster, in their lifetime, according to the Centers for Disease Control and Prevention. Previous research has not simultaneously examined the effect of shingles vaccination and antiviral treatment following shingles onset on the risk of acute ischemic stroke.
Quanhe Yang, PhD, a senior scientist at the CDC, and his colleagues examined data for 35,186 Medicare fee-for-service beneficiaries who were 66 years or older, diagnosed with shingles during 2008-2014, and diagnosed with acute ischemic stroke within a year of shingles diagnosis. Using a self-controlled case series design, the investigators analyzed the association between shingles and stroke. Dr. Yang and his colleagues estimated the incident rate ratio (IRR) by comparing the incidence of stroke during risk periods (i.e., periods following shingles), compared with control periods. To minimize confounding by age, they restricted their analyses to approximately 365 days from the shingles index date.
To investigate how vaccination against shingles with Zostavax and antiviral treatment following shingles affected stroke risk, the researchers classified beneficiaries into the following four groups: Group 1 had no vaccination and no antiviral treatment (49% of beneficiaries), Group 2 had vaccination only (9%), Group 3 had antiviral treatment only (34%), and Group 4 had vaccination and antiviral treatment (8%). The researchers tested for interaction to examine the changes in IRRs across the four groups.
IRRs for stroke progressively declined as time passed from the index shingles date, from 1.61 at 0-14 days following shingles to 1.35 at 15-30 days, 1.16 at 31-90 days, and 1.05 at 91-180 days. The researchers found no evidence that shingles vaccination and antiviral treatment modified the risk of acute ischemic stroke. The association between shingles and risk for acute ischemic stroke was consistent across age groups (i.e., 66-74 years, 75-84 years, and 85 years or older), sex, and race (i.e., non-Hispanic white, non-Hispanic black, and Hispanic, other).
One of the study’s strengths was that its sample was a large national cohort of Medicare fee-for-service beneficiaries, Dr. Yang said. In addition, the study design eliminated all fixed confounding effects. Potential weaknesses, however, included the fact that herpes zoster diagnosis was based on administrative data and that the vaccine’s efficacy declines over time.
The findings suggest that the importance of following the recommended shingles vaccination protocol in the prevention of shingles, Dr. Yang said. Shingrix, a vaccine that the Food and Drug Administration approved in 2017, prevents shingles with an efficacy greater than 90%, he added.
The investigators reported no funding source or disclosures for this study.
SOURCE: Yang Q et al. Circulation. 2019;50(Suppl_1): Abstract 39
REPORTING FROM ISC 2019
Key clinical point: After a patient develops shingles, prior vaccination or treatment with antiviral medication does not change the risk of acute ischemic stroke.
Major finding: Stroke incidence increased by 61% within 14 days after shingles onset.
Study details: A self-controlled case series of 35,186 Medicare beneficiaries with shingles and acute ischemic stroke.
Disclosures: The authors reported no funding source or disclosures for this study.
Source: Yang Q et al. Circulation. 2019;50(Suppl_1), Abstract 39
Adult HIV patients should receive standard vaccinations, with caveats
Patients infected with HIV have an increased risk of mortality and morbidity from diseases that are preventable with vaccines. Undervaccination of these patients poses a major concern, according to a literature review of the vaccine response in the adult patient with HIV published in The American Journal of Medicine.
Despite the fact that data are limited, patients infected with HIV are advised to receive their age-specific and risk group−based vaccines, according to Firas El Chaer, MD, of the University of Maryland, Baltimore, and his colleague.
HIV patients are of particular concern regarding vaccination, because, despite the use of retroviral therapy, CD4+ T-lymphocytes in individuals infected with HIV remain lower than in those without HIV. In addition, HIV causes an inappropriate response to B-cell stimulation, which results in suboptimal primary and secondary response to vaccination, according to Dr. El Chaer and his colleague. Despite this and initial concerns about vaccine safety in this population, it is now recommended that adult patients infected with HIV receive their age-specific and risk group−based vaccines, they stated.
Inactivated or subunit vaccines
Haemophilus influenzae type b vaccine is not recommended under current guidelines for individuals older than age 18 with HIV infection, unless they have a clinical indication.
Vaccination against hepatitis A virus is recommended for HIV-infected patients who are hepatitis A virus seronegative and have chronic liver disease, men who have sex with men, intravenous drug users, and travelers to endemic regions. However, research has shown that the immunogenicity of the vaccine is lower in patients with HIV than in uninfected individuals. It was found that the CD4 count at the time of vaccination, not the CD4 low point, was the major predictor of the immune response.
Patients coinfected with HIV and hepatitis B virus have an 8-fold and 19-fold increase in mortality, respectively, compared with either virus monoinfection. Although vaccination is recommended, the optimal hepatitis B virus vaccination schedule in patients with HIV remains controversial, according to the authors. They indicated that new strategies to improve hepatitis B virus vaccine immunogenicity for those infected with HIV are needed.
Individuals infected with HIV have been found to have a higher risk of human papillomavirus (HPV) infection. The safety and immunogenicity results and prospect of benefits has led to a consensus on the benefit of vaccinating HIV-infected patients who meet the HPV vaccine age criteria, the authors indicated.
With regard to standard flu vaccinations: “An annual inactivated influenza vaccine is recommended during the influenza season for all adult individuals with HIV; however, a live attenuated influenza vaccine is contraindicated in this population,” according to the review.
Patients with HIV have a more than 10-fold increased risk of invasive meningococcal disease, compared with the general population, with the risk being particularly higher in those individuals with CD4 counts less than 200 cells/mm3 and in men who have sex with men in cities with meningococcal outbreaks. For these reasons, the “quadrivalent meningococcal vaccine is recommended for all patients with HIV regardless of their CD4 count, with 2-dose primary series at least 2 months apart and with a booster every 5 years.”
Pneumonia is known to be especially dangerous in the HIV-infected population. With regard to pneumonia vaccination, the 13-valent pneumococcal conjugate vaccine is recommended for all patients with HIV, regardless of their CD4 cell counts. According to Dr. El Chaer and his colleague, it should be followed by the 23-valent pneumococcal polysaccharide vaccine at least 8 weeks later as a prime-boost regimen, preferably when CD4 counts are greater than 200 cells/mm3 and in patients receiving ART.
“Tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccines are recommended once for all individuals infected with HIV, regardless of the CD4 count, with a tetanus toxoid and diphtheria toxoid booster every 10 years,” according to the review.
Live vaccines
Live vaccines are a concerning issue for HIV-infected adults and recommendations for use are generally tied to the CD4 T-cell count. The measles, mumps, and rubella vaccine seems to be safe in patients infected with HIV with a CD4 count greater than 200 cells/mm3, according to Dr. El Chaer and his colleague. Similarly, patients with HIV with CD4 counts greater than 200 cells/mm3 and no evidence of documented immunity to varicella should receive the varicella vaccine.
In contrast, the live, attenuated varicella zoster virus vaccine is not recommended for patients infected with HIV, and it is contraindicated if CD4 count is less than 200 cells/mm3. Recently, a herpes zoster subunit vaccine (HZ/su) was tested in a phase 1/2a randomized, placebo-controlled study and was found to be safe and immunogenic regardless of CD4 count, although it has not yet been given a specific recommendation for immunocompromised patients.
“With the widespread use of ART resulting in better HIV control, ,” the authors concluded.
The study was not sponsored. Dr. El Chaer and his colleague reported that they had no conflicts.
SOURCE: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
Patients infected with HIV have an increased risk of mortality and morbidity from diseases that are preventable with vaccines. Undervaccination of these patients poses a major concern, according to a literature review of the vaccine response in the adult patient with HIV published in The American Journal of Medicine.
Despite the fact that data are limited, patients infected with HIV are advised to receive their age-specific and risk group−based vaccines, according to Firas El Chaer, MD, of the University of Maryland, Baltimore, and his colleague.
HIV patients are of particular concern regarding vaccination, because, despite the use of retroviral therapy, CD4+ T-lymphocytes in individuals infected with HIV remain lower than in those without HIV. In addition, HIV causes an inappropriate response to B-cell stimulation, which results in suboptimal primary and secondary response to vaccination, according to Dr. El Chaer and his colleague. Despite this and initial concerns about vaccine safety in this population, it is now recommended that adult patients infected with HIV receive their age-specific and risk group−based vaccines, they stated.
Inactivated or subunit vaccines
Haemophilus influenzae type b vaccine is not recommended under current guidelines for individuals older than age 18 with HIV infection, unless they have a clinical indication.
Vaccination against hepatitis A virus is recommended for HIV-infected patients who are hepatitis A virus seronegative and have chronic liver disease, men who have sex with men, intravenous drug users, and travelers to endemic regions. However, research has shown that the immunogenicity of the vaccine is lower in patients with HIV than in uninfected individuals. It was found that the CD4 count at the time of vaccination, not the CD4 low point, was the major predictor of the immune response.
Patients coinfected with HIV and hepatitis B virus have an 8-fold and 19-fold increase in mortality, respectively, compared with either virus monoinfection. Although vaccination is recommended, the optimal hepatitis B virus vaccination schedule in patients with HIV remains controversial, according to the authors. They indicated that new strategies to improve hepatitis B virus vaccine immunogenicity for those infected with HIV are needed.
Individuals infected with HIV have been found to have a higher risk of human papillomavirus (HPV) infection. The safety and immunogenicity results and prospect of benefits has led to a consensus on the benefit of vaccinating HIV-infected patients who meet the HPV vaccine age criteria, the authors indicated.
With regard to standard flu vaccinations: “An annual inactivated influenza vaccine is recommended during the influenza season for all adult individuals with HIV; however, a live attenuated influenza vaccine is contraindicated in this population,” according to the review.
Patients with HIV have a more than 10-fold increased risk of invasive meningococcal disease, compared with the general population, with the risk being particularly higher in those individuals with CD4 counts less than 200 cells/mm3 and in men who have sex with men in cities with meningococcal outbreaks. For these reasons, the “quadrivalent meningococcal vaccine is recommended for all patients with HIV regardless of their CD4 count, with 2-dose primary series at least 2 months apart and with a booster every 5 years.”
Pneumonia is known to be especially dangerous in the HIV-infected population. With regard to pneumonia vaccination, the 13-valent pneumococcal conjugate vaccine is recommended for all patients with HIV, regardless of their CD4 cell counts. According to Dr. El Chaer and his colleague, it should be followed by the 23-valent pneumococcal polysaccharide vaccine at least 8 weeks later as a prime-boost regimen, preferably when CD4 counts are greater than 200 cells/mm3 and in patients receiving ART.
“Tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccines are recommended once for all individuals infected with HIV, regardless of the CD4 count, with a tetanus toxoid and diphtheria toxoid booster every 10 years,” according to the review.
Live vaccines
Live vaccines are a concerning issue for HIV-infected adults and recommendations for use are generally tied to the CD4 T-cell count. The measles, mumps, and rubella vaccine seems to be safe in patients infected with HIV with a CD4 count greater than 200 cells/mm3, according to Dr. El Chaer and his colleague. Similarly, patients with HIV with CD4 counts greater than 200 cells/mm3 and no evidence of documented immunity to varicella should receive the varicella vaccine.
In contrast, the live, attenuated varicella zoster virus vaccine is not recommended for patients infected with HIV, and it is contraindicated if CD4 count is less than 200 cells/mm3. Recently, a herpes zoster subunit vaccine (HZ/su) was tested in a phase 1/2a randomized, placebo-controlled study and was found to be safe and immunogenic regardless of CD4 count, although it has not yet been given a specific recommendation for immunocompromised patients.
“With the widespread use of ART resulting in better HIV control, ,” the authors concluded.
The study was not sponsored. Dr. El Chaer and his colleague reported that they had no conflicts.
SOURCE: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
Patients infected with HIV have an increased risk of mortality and morbidity from diseases that are preventable with vaccines. Undervaccination of these patients poses a major concern, according to a literature review of the vaccine response in the adult patient with HIV published in The American Journal of Medicine.
Despite the fact that data are limited, patients infected with HIV are advised to receive their age-specific and risk group−based vaccines, according to Firas El Chaer, MD, of the University of Maryland, Baltimore, and his colleague.
HIV patients are of particular concern regarding vaccination, because, despite the use of retroviral therapy, CD4+ T-lymphocytes in individuals infected with HIV remain lower than in those without HIV. In addition, HIV causes an inappropriate response to B-cell stimulation, which results in suboptimal primary and secondary response to vaccination, according to Dr. El Chaer and his colleague. Despite this and initial concerns about vaccine safety in this population, it is now recommended that adult patients infected with HIV receive their age-specific and risk group−based vaccines, they stated.
Inactivated or subunit vaccines
Haemophilus influenzae type b vaccine is not recommended under current guidelines for individuals older than age 18 with HIV infection, unless they have a clinical indication.
Vaccination against hepatitis A virus is recommended for HIV-infected patients who are hepatitis A virus seronegative and have chronic liver disease, men who have sex with men, intravenous drug users, and travelers to endemic regions. However, research has shown that the immunogenicity of the vaccine is lower in patients with HIV than in uninfected individuals. It was found that the CD4 count at the time of vaccination, not the CD4 low point, was the major predictor of the immune response.
Patients coinfected with HIV and hepatitis B virus have an 8-fold and 19-fold increase in mortality, respectively, compared with either virus monoinfection. Although vaccination is recommended, the optimal hepatitis B virus vaccination schedule in patients with HIV remains controversial, according to the authors. They indicated that new strategies to improve hepatitis B virus vaccine immunogenicity for those infected with HIV are needed.
Individuals infected with HIV have been found to have a higher risk of human papillomavirus (HPV) infection. The safety and immunogenicity results and prospect of benefits has led to a consensus on the benefit of vaccinating HIV-infected patients who meet the HPV vaccine age criteria, the authors indicated.
With regard to standard flu vaccinations: “An annual inactivated influenza vaccine is recommended during the influenza season for all adult individuals with HIV; however, a live attenuated influenza vaccine is contraindicated in this population,” according to the review.
Patients with HIV have a more than 10-fold increased risk of invasive meningococcal disease, compared with the general population, with the risk being particularly higher in those individuals with CD4 counts less than 200 cells/mm3 and in men who have sex with men in cities with meningococcal outbreaks. For these reasons, the “quadrivalent meningococcal vaccine is recommended for all patients with HIV regardless of their CD4 count, with 2-dose primary series at least 2 months apart and with a booster every 5 years.”
Pneumonia is known to be especially dangerous in the HIV-infected population. With regard to pneumonia vaccination, the 13-valent pneumococcal conjugate vaccine is recommended for all patients with HIV, regardless of their CD4 cell counts. According to Dr. El Chaer and his colleague, it should be followed by the 23-valent pneumococcal polysaccharide vaccine at least 8 weeks later as a prime-boost regimen, preferably when CD4 counts are greater than 200 cells/mm3 and in patients receiving ART.
“Tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccines are recommended once for all individuals infected with HIV, regardless of the CD4 count, with a tetanus toxoid and diphtheria toxoid booster every 10 years,” according to the review.
Live vaccines
Live vaccines are a concerning issue for HIV-infected adults and recommendations for use are generally tied to the CD4 T-cell count. The measles, mumps, and rubella vaccine seems to be safe in patients infected with HIV with a CD4 count greater than 200 cells/mm3, according to Dr. El Chaer and his colleague. Similarly, patients with HIV with CD4 counts greater than 200 cells/mm3 and no evidence of documented immunity to varicella should receive the varicella vaccine.
In contrast, the live, attenuated varicella zoster virus vaccine is not recommended for patients infected with HIV, and it is contraindicated if CD4 count is less than 200 cells/mm3. Recently, a herpes zoster subunit vaccine (HZ/su) was tested in a phase 1/2a randomized, placebo-controlled study and was found to be safe and immunogenic regardless of CD4 count, although it has not yet been given a specific recommendation for immunocompromised patients.
“With the widespread use of ART resulting in better HIV control, ,” the authors concluded.
The study was not sponsored. Dr. El Chaer and his colleague reported that they had no conflicts.
SOURCE: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
FROM THE AMERICAN JOURNAL OF MEDICINE
Key clinical point: Undervaccination is too common among HIV-infected patients.
Major finding: Data on vaccine effectiveness in HIV patients are limited, but do not contraindicate the need for vaccination.
Study details: Literature review of immunogenicity and vaccine efficacy in HIV-infected adults.
Disclosures: The study was unsponsored and the authors reported they had no conflicts.
Source: El Chaer F et al. Am J Med. 2019. doi: 10.1016/j.amjmed.2018.12.011.
Adenovirus: More than just another viral illness
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Dengue antibodies may reduce Zika infection risk
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
FROM SCIENCE
Key clinical point: Higher dengue antibody titers are associated with a lower risk of Zika virus infection.
Major finding: The highest tertile of dengue antibody titers was associated with a 44% reduction in the risk of Zika seropositivity.
Study details: A prospective cohort study of 1,453 residents in Salvador, Brazil.
Disclosures: The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
Source: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
DAAs reduce mortality, cancer risk in HCV study
Direct-acting antivirals significantly decrease risk of hepatocellular carcinoma and mortality in persons with hepatitis C, according to results of the first prospective, longitudinal study to evaluate the effect of the drugs on complications related to the infection.
Compared with no treatment, DAA therapy cut risk of hepatocellular carcinoma by about one-third and all-cause mortality by about half in the study, which included about 10,000 adult patients with chronic hepatitis C virus (HCV) infection treated at 1 of 32 hepatology centers in France (NCT01953458).
There were no signs of increased risk of hepatocellular carcinoma during treatment with DAAs, providing more evidence refuting earlier, single-center reports that had suggested an increased incidence early after treatment. These findings also counterbalance a recent Cochrane review that could not confirm or reject a potential benefit of drugs on long-term morbidity and mortality.
Results of the study, published in the Lancet, are based on analysis of 9,895 patients, including 7,344 who started DAA treatment and 2,551 who remained untreated at a median follow-up of more than 31 months. The median patient age was 56 years, and 53% were men.
Treatment with DAAs reduced risk of hepatocellular carcinoma when compared with no DAA treatment, with a hazard ratio of 0.66 (95% confidence interval, 0.46-0.93), and reduced risk of all-cause mortality, with an HR of 0.48 (95% CI, 0.33-0.70), investigators reported in a multivariable analysis that adjusted for variables including age, sex, fibrosis score, HCV genotype, alcohol use, and more.
“These inverse associations persisted in the subgroup of patients who achieved a sustained virological response, whereas those who did not achieve a sustained virological response were a higher risk for hepatocellular carcinoma,” said the investigators, led by Fabrice Carrat, PhD, of Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris.
Sustained virologic response was observed in 94% of patients who had known response status and sufficient follow-up, investigators said.
In patients with cirrhosis at baseline, DAA treatment had a similarly strong association with reduced hepatocellular carcinoma and mortality, with a sustained virologic response rate of 92% in those for whom sufficient data was available, they said.
There was no evidence for an increased risk of hepatocellular carcinoma on treatment, with an adjusted HR of 0.74 (95% CI, 0.49-1.13; P = 0.17), they added.
“Our results support urgent treatment of patients with advanced liver disease and extension of the follow-up of treated patients with less severe disease to assess the long-term clinical effect of direct-acting antiviral treatment,” Dr. Carrat and colleagues said in a commentary on their results.
However, the long-term effect of DAAs on liver decompensation has yet to be clarified, they added, noting that their study excluded patients with decompensated cirrhosis or a history of hepatocellular carcinoma.
Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to Gilead, AbbVie, Bristol-Myers Squibb, MSD, and Janssen, among others.
SOURCE: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/S0140-6736(18)32111-1
This study provides “substantive evidence” that curing hepatitis C virus with all-oral direct-acting antiviral regimens provides clinical benefits, according to Raymond T. Chung, MD, and his coauthors of a related editorial.
Investigators in this study provide the best evidence so far in support of guidelines that advise direct-acting antiviral (DAA) treatment for all patients with chronic hepatitis C virus (HCV) infection, the editorial’s authors stated.
Results of the French study provide a strong counterpoint to the findings of a recent Cochrane review of DAA trials that could not confirm or reject whether DAAs had effects on long-term morbidity and mortality related to HCV, added Dr. Chung and his coauthors. “Finally, they provide credence to the achievability of the goals set out by the World Health Organization (WHO), not only to eliminate HCV but also to substantially reduce its complications.”
The WHO targets were established in light of earlier evidence that sustained virologic responses are linked to reductions in hepatocellular carcinoma, liver transplantation, and mortality, they said.
“In view of the high sustained virological response and excellent tolerability achieved with DAAs, it seemed highly plausible to envision reductions in chronic HCV infection–related complications with these drugs,” they said in reference to the study by Carrat and colleagues.
This editorial appearing in the Lancet was authored by Jacinta A. Holmes, Stephanie M. Rutledge, and Raymond T. Chung of the Liver Center, Gastrointestinal Division, Massachusetts General Hospital, Boston. Dr. Chung provided disclosures related to AbbVie, Gilead, Merck, Bristol-Myers Squibb, Roche, Janssen, and Boehringer Ingelheim.
This study provides “substantive evidence” that curing hepatitis C virus with all-oral direct-acting antiviral regimens provides clinical benefits, according to Raymond T. Chung, MD, and his coauthors of a related editorial.
Investigators in this study provide the best evidence so far in support of guidelines that advise direct-acting antiviral (DAA) treatment for all patients with chronic hepatitis C virus (HCV) infection, the editorial’s authors stated.
Results of the French study provide a strong counterpoint to the findings of a recent Cochrane review of DAA trials that could not confirm or reject whether DAAs had effects on long-term morbidity and mortality related to HCV, added Dr. Chung and his coauthors. “Finally, they provide credence to the achievability of the goals set out by the World Health Organization (WHO), not only to eliminate HCV but also to substantially reduce its complications.”
The WHO targets were established in light of earlier evidence that sustained virologic responses are linked to reductions in hepatocellular carcinoma, liver transplantation, and mortality, they said.
“In view of the high sustained virological response and excellent tolerability achieved with DAAs, it seemed highly plausible to envision reductions in chronic HCV infection–related complications with these drugs,” they said in reference to the study by Carrat and colleagues.
This editorial appearing in the Lancet was authored by Jacinta A. Holmes, Stephanie M. Rutledge, and Raymond T. Chung of the Liver Center, Gastrointestinal Division, Massachusetts General Hospital, Boston. Dr. Chung provided disclosures related to AbbVie, Gilead, Merck, Bristol-Myers Squibb, Roche, Janssen, and Boehringer Ingelheim.
This study provides “substantive evidence” that curing hepatitis C virus with all-oral direct-acting antiviral regimens provides clinical benefits, according to Raymond T. Chung, MD, and his coauthors of a related editorial.
Investigators in this study provide the best evidence so far in support of guidelines that advise direct-acting antiviral (DAA) treatment for all patients with chronic hepatitis C virus (HCV) infection, the editorial’s authors stated.
Results of the French study provide a strong counterpoint to the findings of a recent Cochrane review of DAA trials that could not confirm or reject whether DAAs had effects on long-term morbidity and mortality related to HCV, added Dr. Chung and his coauthors. “Finally, they provide credence to the achievability of the goals set out by the World Health Organization (WHO), not only to eliminate HCV but also to substantially reduce its complications.”
The WHO targets were established in light of earlier evidence that sustained virologic responses are linked to reductions in hepatocellular carcinoma, liver transplantation, and mortality, they said.
“In view of the high sustained virological response and excellent tolerability achieved with DAAs, it seemed highly plausible to envision reductions in chronic HCV infection–related complications with these drugs,” they said in reference to the study by Carrat and colleagues.
This editorial appearing in the Lancet was authored by Jacinta A. Holmes, Stephanie M. Rutledge, and Raymond T. Chung of the Liver Center, Gastrointestinal Division, Massachusetts General Hospital, Boston. Dr. Chung provided disclosures related to AbbVie, Gilead, Merck, Bristol-Myers Squibb, Roche, Janssen, and Boehringer Ingelheim.
Direct-acting antivirals significantly decrease risk of hepatocellular carcinoma and mortality in persons with hepatitis C, according to results of the first prospective, longitudinal study to evaluate the effect of the drugs on complications related to the infection.
Compared with no treatment, DAA therapy cut risk of hepatocellular carcinoma by about one-third and all-cause mortality by about half in the study, which included about 10,000 adult patients with chronic hepatitis C virus (HCV) infection treated at 1 of 32 hepatology centers in France (NCT01953458).
There were no signs of increased risk of hepatocellular carcinoma during treatment with DAAs, providing more evidence refuting earlier, single-center reports that had suggested an increased incidence early after treatment. These findings also counterbalance a recent Cochrane review that could not confirm or reject a potential benefit of drugs on long-term morbidity and mortality.
Results of the study, published in the Lancet, are based on analysis of 9,895 patients, including 7,344 who started DAA treatment and 2,551 who remained untreated at a median follow-up of more than 31 months. The median patient age was 56 years, and 53% were men.
Treatment with DAAs reduced risk of hepatocellular carcinoma when compared with no DAA treatment, with a hazard ratio of 0.66 (95% confidence interval, 0.46-0.93), and reduced risk of all-cause mortality, with an HR of 0.48 (95% CI, 0.33-0.70), investigators reported in a multivariable analysis that adjusted for variables including age, sex, fibrosis score, HCV genotype, alcohol use, and more.
“These inverse associations persisted in the subgroup of patients who achieved a sustained virological response, whereas those who did not achieve a sustained virological response were a higher risk for hepatocellular carcinoma,” said the investigators, led by Fabrice Carrat, PhD, of Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris.
Sustained virologic response was observed in 94% of patients who had known response status and sufficient follow-up, investigators said.
In patients with cirrhosis at baseline, DAA treatment had a similarly strong association with reduced hepatocellular carcinoma and mortality, with a sustained virologic response rate of 92% in those for whom sufficient data was available, they said.
There was no evidence for an increased risk of hepatocellular carcinoma on treatment, with an adjusted HR of 0.74 (95% CI, 0.49-1.13; P = 0.17), they added.
“Our results support urgent treatment of patients with advanced liver disease and extension of the follow-up of treated patients with less severe disease to assess the long-term clinical effect of direct-acting antiviral treatment,” Dr. Carrat and colleagues said in a commentary on their results.
However, the long-term effect of DAAs on liver decompensation has yet to be clarified, they added, noting that their study excluded patients with decompensated cirrhosis or a history of hepatocellular carcinoma.
Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to Gilead, AbbVie, Bristol-Myers Squibb, MSD, and Janssen, among others.
SOURCE: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/S0140-6736(18)32111-1
Direct-acting antivirals significantly decrease risk of hepatocellular carcinoma and mortality in persons with hepatitis C, according to results of the first prospective, longitudinal study to evaluate the effect of the drugs on complications related to the infection.
Compared with no treatment, DAA therapy cut risk of hepatocellular carcinoma by about one-third and all-cause mortality by about half in the study, which included about 10,000 adult patients with chronic hepatitis C virus (HCV) infection treated at 1 of 32 hepatology centers in France (NCT01953458).
There were no signs of increased risk of hepatocellular carcinoma during treatment with DAAs, providing more evidence refuting earlier, single-center reports that had suggested an increased incidence early after treatment. These findings also counterbalance a recent Cochrane review that could not confirm or reject a potential benefit of drugs on long-term morbidity and mortality.
Results of the study, published in the Lancet, are based on analysis of 9,895 patients, including 7,344 who started DAA treatment and 2,551 who remained untreated at a median follow-up of more than 31 months. The median patient age was 56 years, and 53% were men.
Treatment with DAAs reduced risk of hepatocellular carcinoma when compared with no DAA treatment, with a hazard ratio of 0.66 (95% confidence interval, 0.46-0.93), and reduced risk of all-cause mortality, with an HR of 0.48 (95% CI, 0.33-0.70), investigators reported in a multivariable analysis that adjusted for variables including age, sex, fibrosis score, HCV genotype, alcohol use, and more.
“These inverse associations persisted in the subgroup of patients who achieved a sustained virological response, whereas those who did not achieve a sustained virological response were a higher risk for hepatocellular carcinoma,” said the investigators, led by Fabrice Carrat, PhD, of Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris.
Sustained virologic response was observed in 94% of patients who had known response status and sufficient follow-up, investigators said.
In patients with cirrhosis at baseline, DAA treatment had a similarly strong association with reduced hepatocellular carcinoma and mortality, with a sustained virologic response rate of 92% in those for whom sufficient data was available, they said.
There was no evidence for an increased risk of hepatocellular carcinoma on treatment, with an adjusted HR of 0.74 (95% CI, 0.49-1.13; P = 0.17), they added.
“Our results support urgent treatment of patients with advanced liver disease and extension of the follow-up of treated patients with less severe disease to assess the long-term clinical effect of direct-acting antiviral treatment,” Dr. Carrat and colleagues said in a commentary on their results.
However, the long-term effect of DAAs on liver decompensation has yet to be clarified, they added, noting that their study excluded patients with decompensated cirrhosis or a history of hepatocellular carcinoma.
Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to Gilead, AbbVie, Bristol-Myers Squibb, MSD, and Janssen, among others.
SOURCE: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/S0140-6736(18)32111-1
FROM THE LANCET
Key clinical point:
Major finding: DAAs reduced risk of hepatocellular carcinoma (HR, 0.66; 95% confidence interval, 0.46-0.93) and all-cause mortality (HR, 0.48; 95% CI, 0.33-0.70).
Study details: A prospective study including about 10,000 adults with chronic HCV infection enrolled at 1 of 32 centers in France.
Disclosures: Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to the study pharma sponsors among others.
Source: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/20S0140-6736(18)32111-1.
United States now over 100 measles cases for the year
according to the Centers for Disease Control and Prevention.
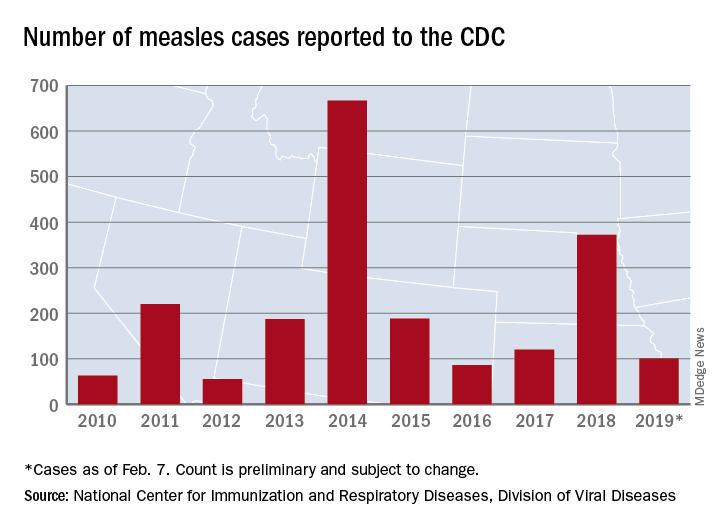
Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
Flu activity hits seasonal high
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
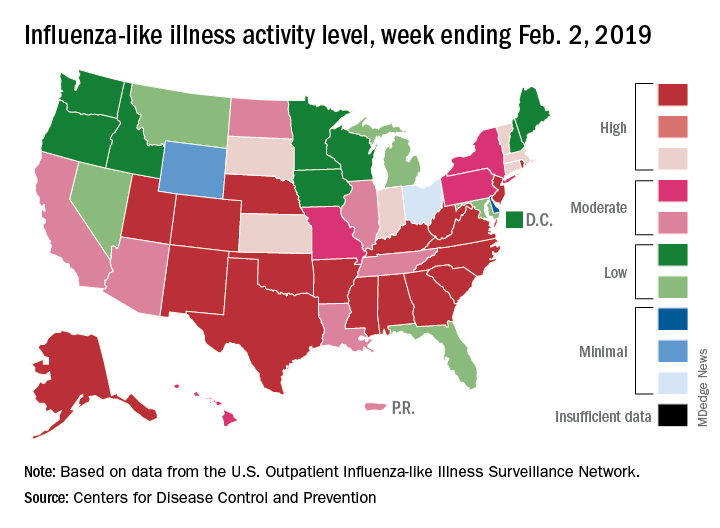
The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Marijuana smoking is an independent risk factor for lung disease in HIV+
Long-term marijuana smoking was associated with lung disease in HIV-infected (HIV+) but not HIV uninfected (HIV–) men who have sex with men (MSM), according to the results of a large, prospective cohort study.
“There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors,” wrote David R. Lorenz, PhD, of the Dana-Farber Cancer Institute, Boston, and his colleagues.
These findings are especially important given that the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population in the United States, and has increased in recent years, according to the report, published online in EClinicalMedicine.
The study examined 2,704 MSM who met eligibility criteria (1,352 HIV+ and 1,352 HIV− individuals), with a median age of 44 years at baseline and a median follow-up of 10.5 years. A total of 27% of HIV+ participants reported daily or weekly marijuana smoking for 1 year or more during follow-up, compared with 18% of the HIV− participants.
HIV+ participants who smoked marijuana were more likely to report one or more pulmonary diagnoses, versus nonsmoking HIV+ individuals during follow-up (41.0% vs. 30.0% infectious, and 24.8% vs. 19.0% noninfectious), according to the authors. In contrast, there was no association between marijuana smoking and either an infectious or noninfectious pulmonary diagnosis among HIV− participants (24.2% vs. 20.9%, and 14.8% vs. 17.7%, respectively).
For HIV+ individuals, each 10 days/month increase in marijuana smoking in the prior 2-year period was found to be associated with a 6% increased risk of infectious pulmonary diagnosis (hazard risk 1.06 [95% confidence interval 1.00-1.11]; P = .041). Overall, they found that from the 53,000 person-visits in the study, marijuana smoking was associated with increased risk of both infectious and noninfectious pulmonary diagnoses among the 1,352 HIV-infected participants independent of CD4 count, antiretroviral therapy (ART) adherence, and demographic factors as well.
In particular, viral suppression did not seem to interfere with this association between marijuana smoking and infectious pulmonary diagnoses, as it remained significant in models restricted to those person-visits with suppressed HIV viral load (HR 1.41 [1.03-1.91], P = .029).
The authors suggested that HIV-specific factors such as lung immune cell depletion and dysfunction, persistent immune cell activation, systemic inflammation, respiratory microbiome alterations, and oxidative stress, or a combination of these effects, may interact with the alveolar macrophage dysfunction seen in both humans and mouse models exposed to marijuana smoke. Thus, “a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses,” Dr. Lorenz and his colleagues stated.
“These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease,” they concluded.
The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
SOURCE: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
Long-term marijuana smoking was associated with lung disease in HIV-infected (HIV+) but not HIV uninfected (HIV–) men who have sex with men (MSM), according to the results of a large, prospective cohort study.
“There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors,” wrote David R. Lorenz, PhD, of the Dana-Farber Cancer Institute, Boston, and his colleagues.
These findings are especially important given that the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population in the United States, and has increased in recent years, according to the report, published online in EClinicalMedicine.
The study examined 2,704 MSM who met eligibility criteria (1,352 HIV+ and 1,352 HIV− individuals), with a median age of 44 years at baseline and a median follow-up of 10.5 years. A total of 27% of HIV+ participants reported daily or weekly marijuana smoking for 1 year or more during follow-up, compared with 18% of the HIV− participants.
HIV+ participants who smoked marijuana were more likely to report one or more pulmonary diagnoses, versus nonsmoking HIV+ individuals during follow-up (41.0% vs. 30.0% infectious, and 24.8% vs. 19.0% noninfectious), according to the authors. In contrast, there was no association between marijuana smoking and either an infectious or noninfectious pulmonary diagnosis among HIV− participants (24.2% vs. 20.9%, and 14.8% vs. 17.7%, respectively).
For HIV+ individuals, each 10 days/month increase in marijuana smoking in the prior 2-year period was found to be associated with a 6% increased risk of infectious pulmonary diagnosis (hazard risk 1.06 [95% confidence interval 1.00-1.11]; P = .041). Overall, they found that from the 53,000 person-visits in the study, marijuana smoking was associated with increased risk of both infectious and noninfectious pulmonary diagnoses among the 1,352 HIV-infected participants independent of CD4 count, antiretroviral therapy (ART) adherence, and demographic factors as well.
In particular, viral suppression did not seem to interfere with this association between marijuana smoking and infectious pulmonary diagnoses, as it remained significant in models restricted to those person-visits with suppressed HIV viral load (HR 1.41 [1.03-1.91], P = .029).
The authors suggested that HIV-specific factors such as lung immune cell depletion and dysfunction, persistent immune cell activation, systemic inflammation, respiratory microbiome alterations, and oxidative stress, or a combination of these effects, may interact with the alveolar macrophage dysfunction seen in both humans and mouse models exposed to marijuana smoke. Thus, “a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses,” Dr. Lorenz and his colleagues stated.
“These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease,” they concluded.
The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
SOURCE: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
Long-term marijuana smoking was associated with lung disease in HIV-infected (HIV+) but not HIV uninfected (HIV–) men who have sex with men (MSM), according to the results of a large, prospective cohort study.
“There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors,” wrote David R. Lorenz, PhD, of the Dana-Farber Cancer Institute, Boston, and his colleagues.
These findings are especially important given that the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population in the United States, and has increased in recent years, according to the report, published online in EClinicalMedicine.
The study examined 2,704 MSM who met eligibility criteria (1,352 HIV+ and 1,352 HIV− individuals), with a median age of 44 years at baseline and a median follow-up of 10.5 years. A total of 27% of HIV+ participants reported daily or weekly marijuana smoking for 1 year or more during follow-up, compared with 18% of the HIV− participants.
HIV+ participants who smoked marijuana were more likely to report one or more pulmonary diagnoses, versus nonsmoking HIV+ individuals during follow-up (41.0% vs. 30.0% infectious, and 24.8% vs. 19.0% noninfectious), according to the authors. In contrast, there was no association between marijuana smoking and either an infectious or noninfectious pulmonary diagnosis among HIV− participants (24.2% vs. 20.9%, and 14.8% vs. 17.7%, respectively).
For HIV+ individuals, each 10 days/month increase in marijuana smoking in the prior 2-year period was found to be associated with a 6% increased risk of infectious pulmonary diagnosis (hazard risk 1.06 [95% confidence interval 1.00-1.11]; P = .041). Overall, they found that from the 53,000 person-visits in the study, marijuana smoking was associated with increased risk of both infectious and noninfectious pulmonary diagnoses among the 1,352 HIV-infected participants independent of CD4 count, antiretroviral therapy (ART) adherence, and demographic factors as well.
In particular, viral suppression did not seem to interfere with this association between marijuana smoking and infectious pulmonary diagnoses, as it remained significant in models restricted to those person-visits with suppressed HIV viral load (HR 1.41 [1.03-1.91], P = .029).
The authors suggested that HIV-specific factors such as lung immune cell depletion and dysfunction, persistent immune cell activation, systemic inflammation, respiratory microbiome alterations, and oxidative stress, or a combination of these effects, may interact with the alveolar macrophage dysfunction seen in both humans and mouse models exposed to marijuana smoke. Thus, “a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses,” Dr. Lorenz and his colleagues stated.
“These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease,” they concluded.
The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
SOURCE: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
FROM ECLINICALMEDICINE
Key clinical point: HIV+ but not HIV– marijuana smokers had an increased rate of pulmonary diagnoses.
Major finding: HIV+ marijuana smokers were more likely to report one or more infectious or noninfectious pulmonary diagnoses, compared with nonsmoking HIV+ individuals (41.0% vs. 30.0%, and 24.8% vs. 19.0%, respectively).
Study details: A prospective cohort study of 1,352 HIV+ vs. 1,352 HIV– men who have sex with men.
Disclosures: The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
Source: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
Measles outbreak sends vaccine demand soaring, even among the hesitant
Demand for measles vaccine has surged in the Washington county in which the highly contagious virus is linked to more than 50 confirmed illnesses this year – including among people who had previously shunned the shots.
Orders for two types of measles vaccines in Clark County were up nearly 500% in January, compared with the same month last year, jumping from 530 doses to 3,150, according to state health department figures.
Area health clinics are scrambling to keep up with sudden demand, mostly among parents of children who had not been inoculated.
“During an outbreak is when you see an influx of patients who would otherwise be vaccine hesitant,” said Virginia Ramos, infection control nurse with Sea Mar Community Health Center, which runs six sites that offer vaccines in Clark County.
“We’re just happy that we’re prepared and that there is vaccine available.”
The Vancouver Clinic, which operates medical offices and urgent care centers in the area, reported that shots administered jumped from 263 in January 2018 to 1,444 last month, a nearly 450% increase.
That’s a huge rise in a county in which vaccination rates lag – only 76.5% of kindergartners had all the required immunizations for the 2017-2018 school year. Health officials have long worried about the potential for an outbreak in the region.
Statewide in Washington, orders for measles vaccine jumped about 30% in January, compared with the same month last year, climbing from 12,140 doses to 15,780 doses, figures showed. The vaccines include MMR, which protects against measles, mumps and rubella, and MMR-V, which also protects against the varicella-zoster virus, which causes chickenpox. The vaccine takes effect within 72 hours, health officials said.
The orders represent only state-supplied vaccines requested through the federal Vaccines for Children program, which provides free immunizations to children who otherwise couldn’t afford them.
But it’s a snapshot of the scare an outbreak can cause, said Alan Melnick, MD, the health officer and public health director for Clark County overseeing the response.
“I would rather it not take an outbreak for this to happen,” he said.
Since Jan. 1, 2019, 50 cases of measles have been confirmed in Clark County, with 11 more cases suspected, officials said. The Pacific Northwest outbreak includes one confirmed case in King County, where Seattle is located, and four in Multnomah County, which includes Portland, Ore.
On Feb. 6, officials sent letters to families of 5,000 children in Multnomah County telling them they’ll be excluded from school if they don’t have up-to-date immunizations or valid exemptions by Feb. 20.
Most of the infections have occurred in children, under age 18 years, who were unvaccinated. The outbreak includes 43 cases among those who were not immunized, 6 cases in which immunization has not been verified, and 1 case in which the person had received only a single dose of vaccine.
The Centers for Disease Control and Prevention recommends two doses of measles vaccine, one given at between 12 and 15 months of age and one between ages 4 and 6. Health officials say the shots are safe and effective, providing about 93% protection with one dose and 97% with two doses.
The Northwest cases are among three ongoing measles outbreaks in the United States that sickened 79 people in January, according to the CDC. Last year, 372 measles cases were confirmed nationwide, the most since an outbreak in 2014 sickened 667 people.
Washington and Oregon are among 17 states that allow nonmedical exemptions from vaccination requirements for school entry, according to the National Conference of State Legislatures.
Washington state Rep. Paul Harris (R-Vancouver) has introduced a measure that would remove personal belief exemptions for the MMR vaccine.
Research has confirmed that vaccines don’t cause autism, a common reason cited by parents who reject vaccinations. Others object to the timing and combinations of the vaccines and to being forced to inoculate their children.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Demand for measles vaccine has surged in the Washington county in which the highly contagious virus is linked to more than 50 confirmed illnesses this year – including among people who had previously shunned the shots.
Orders for two types of measles vaccines in Clark County were up nearly 500% in January, compared with the same month last year, jumping from 530 doses to 3,150, according to state health department figures.
Area health clinics are scrambling to keep up with sudden demand, mostly among parents of children who had not been inoculated.
“During an outbreak is when you see an influx of patients who would otherwise be vaccine hesitant,” said Virginia Ramos, infection control nurse with Sea Mar Community Health Center, which runs six sites that offer vaccines in Clark County.
“We’re just happy that we’re prepared and that there is vaccine available.”
The Vancouver Clinic, which operates medical offices and urgent care centers in the area, reported that shots administered jumped from 263 in January 2018 to 1,444 last month, a nearly 450% increase.
That’s a huge rise in a county in which vaccination rates lag – only 76.5% of kindergartners had all the required immunizations for the 2017-2018 school year. Health officials have long worried about the potential for an outbreak in the region.
Statewide in Washington, orders for measles vaccine jumped about 30% in January, compared with the same month last year, climbing from 12,140 doses to 15,780 doses, figures showed. The vaccines include MMR, which protects against measles, mumps and rubella, and MMR-V, which also protects against the varicella-zoster virus, which causes chickenpox. The vaccine takes effect within 72 hours, health officials said.
The orders represent only state-supplied vaccines requested through the federal Vaccines for Children program, which provides free immunizations to children who otherwise couldn’t afford them.
But it’s a snapshot of the scare an outbreak can cause, said Alan Melnick, MD, the health officer and public health director for Clark County overseeing the response.
“I would rather it not take an outbreak for this to happen,” he said.
Since Jan. 1, 2019, 50 cases of measles have been confirmed in Clark County, with 11 more cases suspected, officials said. The Pacific Northwest outbreak includes one confirmed case in King County, where Seattle is located, and four in Multnomah County, which includes Portland, Ore.
On Feb. 6, officials sent letters to families of 5,000 children in Multnomah County telling them they’ll be excluded from school if they don’t have up-to-date immunizations or valid exemptions by Feb. 20.
Most of the infections have occurred in children, under age 18 years, who were unvaccinated. The outbreak includes 43 cases among those who were not immunized, 6 cases in which immunization has not been verified, and 1 case in which the person had received only a single dose of vaccine.
The Centers for Disease Control and Prevention recommends two doses of measles vaccine, one given at between 12 and 15 months of age and one between ages 4 and 6. Health officials say the shots are safe and effective, providing about 93% protection with one dose and 97% with two doses.
The Northwest cases are among three ongoing measles outbreaks in the United States that sickened 79 people in January, according to the CDC. Last year, 372 measles cases were confirmed nationwide, the most since an outbreak in 2014 sickened 667 people.
Washington and Oregon are among 17 states that allow nonmedical exemptions from vaccination requirements for school entry, according to the National Conference of State Legislatures.
Washington state Rep. Paul Harris (R-Vancouver) has introduced a measure that would remove personal belief exemptions for the MMR vaccine.
Research has confirmed that vaccines don’t cause autism, a common reason cited by parents who reject vaccinations. Others object to the timing and combinations of the vaccines and to being forced to inoculate their children.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Demand for measles vaccine has surged in the Washington county in which the highly contagious virus is linked to more than 50 confirmed illnesses this year – including among people who had previously shunned the shots.
Orders for two types of measles vaccines in Clark County were up nearly 500% in January, compared with the same month last year, jumping from 530 doses to 3,150, according to state health department figures.
Area health clinics are scrambling to keep up with sudden demand, mostly among parents of children who had not been inoculated.
“During an outbreak is when you see an influx of patients who would otherwise be vaccine hesitant,” said Virginia Ramos, infection control nurse with Sea Mar Community Health Center, which runs six sites that offer vaccines in Clark County.
“We’re just happy that we’re prepared and that there is vaccine available.”
The Vancouver Clinic, which operates medical offices and urgent care centers in the area, reported that shots administered jumped from 263 in January 2018 to 1,444 last month, a nearly 450% increase.
That’s a huge rise in a county in which vaccination rates lag – only 76.5% of kindergartners had all the required immunizations for the 2017-2018 school year. Health officials have long worried about the potential for an outbreak in the region.
Statewide in Washington, orders for measles vaccine jumped about 30% in January, compared with the same month last year, climbing from 12,140 doses to 15,780 doses, figures showed. The vaccines include MMR, which protects against measles, mumps and rubella, and MMR-V, which also protects against the varicella-zoster virus, which causes chickenpox. The vaccine takes effect within 72 hours, health officials said.
The orders represent only state-supplied vaccines requested through the federal Vaccines for Children program, which provides free immunizations to children who otherwise couldn’t afford them.
But it’s a snapshot of the scare an outbreak can cause, said Alan Melnick, MD, the health officer and public health director for Clark County overseeing the response.
“I would rather it not take an outbreak for this to happen,” he said.
Since Jan. 1, 2019, 50 cases of measles have been confirmed in Clark County, with 11 more cases suspected, officials said. The Pacific Northwest outbreak includes one confirmed case in King County, where Seattle is located, and four in Multnomah County, which includes Portland, Ore.
On Feb. 6, officials sent letters to families of 5,000 children in Multnomah County telling them they’ll be excluded from school if they don’t have up-to-date immunizations or valid exemptions by Feb. 20.
Most of the infections have occurred in children, under age 18 years, who were unvaccinated. The outbreak includes 43 cases among those who were not immunized, 6 cases in which immunization has not been verified, and 1 case in which the person had received only a single dose of vaccine.
The Centers for Disease Control and Prevention recommends two doses of measles vaccine, one given at between 12 and 15 months of age and one between ages 4 and 6. Health officials say the shots are safe and effective, providing about 93% protection with one dose and 97% with two doses.
The Northwest cases are among three ongoing measles outbreaks in the United States that sickened 79 people in January, according to the CDC. Last year, 372 measles cases were confirmed nationwide, the most since an outbreak in 2014 sickened 667 people.
Washington and Oregon are among 17 states that allow nonmedical exemptions from vaccination requirements for school entry, according to the National Conference of State Legislatures.
Washington state Rep. Paul Harris (R-Vancouver) has introduced a measure that would remove personal belief exemptions for the MMR vaccine.
Research has confirmed that vaccines don’t cause autism, a common reason cited by parents who reject vaccinations. Others object to the timing and combinations of the vaccines and to being forced to inoculate their children.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.