User login
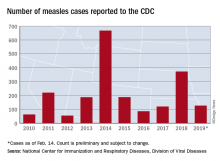
U.S. measles cases up to 159 for the year
Reported measles cases are now up to 159 for the year in the United States, according to the Centers for Disease Control and Prevention.

The most recent reporting week, which ended Feb. 21, brought another 32 cases of measles and one new outbreak of 4 cases in Illinois. The total number of outbreaks – an outbreak is defined as three or more cases – is now six, and cases have been reported in 10 states, the CDC said Feb. 25.
The majority (17) of those 32 new cases occurred in Brooklyn, one of New York state’s three outbreaks this year. The largest of the 2019 outbreaks is in Washington state, primarily in Clark County, and is up to 66 cases after 4 more were reported in the last week by the state’s department of health. The outbreaks are linked to travelers who brought the disease to the United States.
There are now two measures “advancing through the [Washington] state legislature that would bar parents from using personal or philosophical exemptions to avoid immunizing their school-age children. Both have bipartisan support despite strong antivaccination sentiment in parts of the state,” the Washington Post said on Feb. 25.
Reported measles cases are now up to 159 for the year in the United States, according to the Centers for Disease Control and Prevention.

The most recent reporting week, which ended Feb. 21, brought another 32 cases of measles and one new outbreak of 4 cases in Illinois. The total number of outbreaks – an outbreak is defined as three or more cases – is now six, and cases have been reported in 10 states, the CDC said Feb. 25.
The majority (17) of those 32 new cases occurred in Brooklyn, one of New York state’s three outbreaks this year. The largest of the 2019 outbreaks is in Washington state, primarily in Clark County, and is up to 66 cases after 4 more were reported in the last week by the state’s department of health. The outbreaks are linked to travelers who brought the disease to the United States.
There are now two measures “advancing through the [Washington] state legislature that would bar parents from using personal or philosophical exemptions to avoid immunizing their school-age children. Both have bipartisan support despite strong antivaccination sentiment in parts of the state,” the Washington Post said on Feb. 25.
Reported measles cases are now up to 159 for the year in the United States, according to the Centers for Disease Control and Prevention.

The most recent reporting week, which ended Feb. 21, brought another 32 cases of measles and one new outbreak of 4 cases in Illinois. The total number of outbreaks – an outbreak is defined as three or more cases – is now six, and cases have been reported in 10 states, the CDC said Feb. 25.
The majority (17) of those 32 new cases occurred in Brooklyn, one of New York state’s three outbreaks this year. The largest of the 2019 outbreaks is in Washington state, primarily in Clark County, and is up to 66 cases after 4 more were reported in the last week by the state’s department of health. The outbreaks are linked to travelers who brought the disease to the United States.
There are now two measures “advancing through the [Washington] state legislature that would bar parents from using personal or philosophical exemptions to avoid immunizing their school-age children. Both have bipartisan support despite strong antivaccination sentiment in parts of the state,” the Washington Post said on Feb. 25.
Peripheral perfusion fails septic shock test, but optimism remains
SAN DIEGO – During resuscitation of patients but missed statistical significance.

Although the paper, published online in JAMA, concludes that normalization of capillary refill time cannot be recommended over targeting serum lactate levels, Glenn Hernández, MD, PhD, sounded more optimistic after presenting the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “I think it’s good news to develop techniques that, even though they have this integrated variability, they can provide a signal that is also very close to the [underlying] physiology,” Dr. Hernández, who is a professor of intensive medicine at Pontifical Catholic University in Santiago, Chile. The Peripheral perfusion was also associated with lower mean Sequential Organ Failure Assessment (SOFA) Score at 72 hours.
The technique involves pressing a glass microscope slide to the ventral surface of the right index finger distal phalanx, increasing pressure and maintaining pressure for 10 seconds. After release, a chronometer assessed return of normal skin color, with refill times over 3 seconds considered abnormal. Clinicians applied the technique every 30 minutes during until normalization (every hour after that), compared with every 2 hours for the lactate arm of the study.
The ANDROMEDA-SHOCK randomized clinical trial was conducted at 28 hospitals in five countries (Argentina, Chile, Colombia, Ecuador, Uruguay). The trial did not demonstrate superiority of capillary refill, and it was not designed for noninferiority. It nevertheless seems unlikely that assessment of capillary refill is inferior to lactate levels, according an accompanying editorial by JAMA-associated editor Derek Angus, MD, who also is a professor of critical care medicine at the University of Pittsburgh. The simplicity of using a capillary refill could be particularly useful in resource-limited settings, since it can be accomplished visually.
It also a natural marker for resuscitation. The body slows fluid flow to peripheral tissues until vital organs are well perfused. Normal capillary refill time “is an indirect signal of reperfusion,” said Dr. Hernández.
The researchers are not calling for this technique to replace lactate measurements, noting that in many ways the techniques can be complementary, since lactate levels are a good indicator of the patient’s overall improvement. In any case, it would take more research to prove superiority of the capillary refill, and that’s not something Dr. Hernández is planning to undertake. The current study had no external funding and required about half of his time over a 2-year period. Getting the work done at all “was sort of a miracle. We would not repeat this,” he said.
The researchers randomized 416 patients with septic shock (mean age, 63 years; 53% of whom were women) to be managed by peripheral perfusion or lactate measurement. By day 28, 43.4% in the lactate group had died, compared with 34.9% in the peripheral perfusion group (hazard ratio, 0.75; P = .06). At 72 hours, the peripheral perfusion group had less organ dysfunction as measured by SOFA (mean, 5.6 vs. 6.6; P = .045). Six other secondary outcomes revealed no between-group differences.
The peripheral perfusion group received an average of 408 fewer mL of resuscitation fluids during the first 8 hours (P = .01).
That result fits with the greater responsiveness of peripheral perfusion measurements, and it’s relevant because some septic shock patients who are unresponsive to fluids often receive fluids anyway. “The general knowledge, though not correct, is that you treat lactate or blood pressure with fluids,” said coauthor Jan Bakker, MD, PhD, who is a professor at New York-Presbyterian Hospital Columbia University, and Erasmus University Rotterdam, the Netherlands.
After a series of observational studies suggested that warm, well-perfused patients were doing well, the idea was tested in a small interventional trial in which physicians were forbidden from giving fluids once patients were warm and well perfused. Patients did better than did those on standard of care. “We have said, if the patient is warm and well perfused, even if they are hypotensive, don’t give fluids, it won’t benefit them anymore. Give vasopressors or whatever, but don’t give fluids,” said Dr. Bakker.
The latest research also reinforced a signal from the earlier, smaller trial. “You get less organ failure if you use [fewer] fluids,” Dr. Bakker added.
The study received no external funding. Dr. Hernández and Dr. Bakker had no relevant financial disclosures. Dr. Angus received consulting fees from Ferring Pharmaceutical, Bristol-Myers Squibb, Bayer AG, and others outside the submitted work; he also has patents pending for compounds, compositions, and methods for treating sepsis and for proteomic biomarkers.
SOURCE: Hernández G et al. JAMA 2019 Feb 17. doi: 10.1001/jama.2019.0071.
SAN DIEGO – During resuscitation of patients but missed statistical significance.

Although the paper, published online in JAMA, concludes that normalization of capillary refill time cannot be recommended over targeting serum lactate levels, Glenn Hernández, MD, PhD, sounded more optimistic after presenting the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “I think it’s good news to develop techniques that, even though they have this integrated variability, they can provide a signal that is also very close to the [underlying] physiology,” Dr. Hernández, who is a professor of intensive medicine at Pontifical Catholic University in Santiago, Chile. The Peripheral perfusion was also associated with lower mean Sequential Organ Failure Assessment (SOFA) Score at 72 hours.
The technique involves pressing a glass microscope slide to the ventral surface of the right index finger distal phalanx, increasing pressure and maintaining pressure for 10 seconds. After release, a chronometer assessed return of normal skin color, with refill times over 3 seconds considered abnormal. Clinicians applied the technique every 30 minutes during until normalization (every hour after that), compared with every 2 hours for the lactate arm of the study.
The ANDROMEDA-SHOCK randomized clinical trial was conducted at 28 hospitals in five countries (Argentina, Chile, Colombia, Ecuador, Uruguay). The trial did not demonstrate superiority of capillary refill, and it was not designed for noninferiority. It nevertheless seems unlikely that assessment of capillary refill is inferior to lactate levels, according an accompanying editorial by JAMA-associated editor Derek Angus, MD, who also is a professor of critical care medicine at the University of Pittsburgh. The simplicity of using a capillary refill could be particularly useful in resource-limited settings, since it can be accomplished visually.
It also a natural marker for resuscitation. The body slows fluid flow to peripheral tissues until vital organs are well perfused. Normal capillary refill time “is an indirect signal of reperfusion,” said Dr. Hernández.
The researchers are not calling for this technique to replace lactate measurements, noting that in many ways the techniques can be complementary, since lactate levels are a good indicator of the patient’s overall improvement. In any case, it would take more research to prove superiority of the capillary refill, and that’s not something Dr. Hernández is planning to undertake. The current study had no external funding and required about half of his time over a 2-year period. Getting the work done at all “was sort of a miracle. We would not repeat this,” he said.
The researchers randomized 416 patients with septic shock (mean age, 63 years; 53% of whom were women) to be managed by peripheral perfusion or lactate measurement. By day 28, 43.4% in the lactate group had died, compared with 34.9% in the peripheral perfusion group (hazard ratio, 0.75; P = .06). At 72 hours, the peripheral perfusion group had less organ dysfunction as measured by SOFA (mean, 5.6 vs. 6.6; P = .045). Six other secondary outcomes revealed no between-group differences.
The peripheral perfusion group received an average of 408 fewer mL of resuscitation fluids during the first 8 hours (P = .01).
That result fits with the greater responsiveness of peripheral perfusion measurements, and it’s relevant because some septic shock patients who are unresponsive to fluids often receive fluids anyway. “The general knowledge, though not correct, is that you treat lactate or blood pressure with fluids,” said coauthor Jan Bakker, MD, PhD, who is a professor at New York-Presbyterian Hospital Columbia University, and Erasmus University Rotterdam, the Netherlands.
After a series of observational studies suggested that warm, well-perfused patients were doing well, the idea was tested in a small interventional trial in which physicians were forbidden from giving fluids once patients were warm and well perfused. Patients did better than did those on standard of care. “We have said, if the patient is warm and well perfused, even if they are hypotensive, don’t give fluids, it won’t benefit them anymore. Give vasopressors or whatever, but don’t give fluids,” said Dr. Bakker.
The latest research also reinforced a signal from the earlier, smaller trial. “You get less organ failure if you use [fewer] fluids,” Dr. Bakker added.
The study received no external funding. Dr. Hernández and Dr. Bakker had no relevant financial disclosures. Dr. Angus received consulting fees from Ferring Pharmaceutical, Bristol-Myers Squibb, Bayer AG, and others outside the submitted work; he also has patents pending for compounds, compositions, and methods for treating sepsis and for proteomic biomarkers.
SOURCE: Hernández G et al. JAMA 2019 Feb 17. doi: 10.1001/jama.2019.0071.
SAN DIEGO – During resuscitation of patients but missed statistical significance.

Although the paper, published online in JAMA, concludes that normalization of capillary refill time cannot be recommended over targeting serum lactate levels, Glenn Hernández, MD, PhD, sounded more optimistic after presenting the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “I think it’s good news to develop techniques that, even though they have this integrated variability, they can provide a signal that is also very close to the [underlying] physiology,” Dr. Hernández, who is a professor of intensive medicine at Pontifical Catholic University in Santiago, Chile. The Peripheral perfusion was also associated with lower mean Sequential Organ Failure Assessment (SOFA) Score at 72 hours.
The technique involves pressing a glass microscope slide to the ventral surface of the right index finger distal phalanx, increasing pressure and maintaining pressure for 10 seconds. After release, a chronometer assessed return of normal skin color, with refill times over 3 seconds considered abnormal. Clinicians applied the technique every 30 minutes during until normalization (every hour after that), compared with every 2 hours for the lactate arm of the study.
The ANDROMEDA-SHOCK randomized clinical trial was conducted at 28 hospitals in five countries (Argentina, Chile, Colombia, Ecuador, Uruguay). The trial did not demonstrate superiority of capillary refill, and it was not designed for noninferiority. It nevertheless seems unlikely that assessment of capillary refill is inferior to lactate levels, according an accompanying editorial by JAMA-associated editor Derek Angus, MD, who also is a professor of critical care medicine at the University of Pittsburgh. The simplicity of using a capillary refill could be particularly useful in resource-limited settings, since it can be accomplished visually.
It also a natural marker for resuscitation. The body slows fluid flow to peripheral tissues until vital organs are well perfused. Normal capillary refill time “is an indirect signal of reperfusion,” said Dr. Hernández.
The researchers are not calling for this technique to replace lactate measurements, noting that in many ways the techniques can be complementary, since lactate levels are a good indicator of the patient’s overall improvement. In any case, it would take more research to prove superiority of the capillary refill, and that’s not something Dr. Hernández is planning to undertake. The current study had no external funding and required about half of his time over a 2-year period. Getting the work done at all “was sort of a miracle. We would not repeat this,” he said.
The researchers randomized 416 patients with septic shock (mean age, 63 years; 53% of whom were women) to be managed by peripheral perfusion or lactate measurement. By day 28, 43.4% in the lactate group had died, compared with 34.9% in the peripheral perfusion group (hazard ratio, 0.75; P = .06). At 72 hours, the peripheral perfusion group had less organ dysfunction as measured by SOFA (mean, 5.6 vs. 6.6; P = .045). Six other secondary outcomes revealed no between-group differences.
The peripheral perfusion group received an average of 408 fewer mL of resuscitation fluids during the first 8 hours (P = .01).
That result fits with the greater responsiveness of peripheral perfusion measurements, and it’s relevant because some septic shock patients who are unresponsive to fluids often receive fluids anyway. “The general knowledge, though not correct, is that you treat lactate or blood pressure with fluids,” said coauthor Jan Bakker, MD, PhD, who is a professor at New York-Presbyterian Hospital Columbia University, and Erasmus University Rotterdam, the Netherlands.
After a series of observational studies suggested that warm, well-perfused patients were doing well, the idea was tested in a small interventional trial in which physicians were forbidden from giving fluids once patients were warm and well perfused. Patients did better than did those on standard of care. “We have said, if the patient is warm and well perfused, even if they are hypotensive, don’t give fluids, it won’t benefit them anymore. Give vasopressors or whatever, but don’t give fluids,” said Dr. Bakker.
The latest research also reinforced a signal from the earlier, smaller trial. “You get less organ failure if you use [fewer] fluids,” Dr. Bakker added.
The study received no external funding. Dr. Hernández and Dr. Bakker had no relevant financial disclosures. Dr. Angus received consulting fees from Ferring Pharmaceutical, Bristol-Myers Squibb, Bayer AG, and others outside the submitted work; he also has patents pending for compounds, compositions, and methods for treating sepsis and for proteomic biomarkers.
SOURCE: Hernández G et al. JAMA 2019 Feb 17. doi: 10.1001/jama.2019.0071.
REPORTING FROM CCC48
Influenza activity continues to increase
The 2018-2019 flu season is showing no signs of decline as activity measures continued to increase into mid-February, according to the Centers for Disease Control and Prevention.
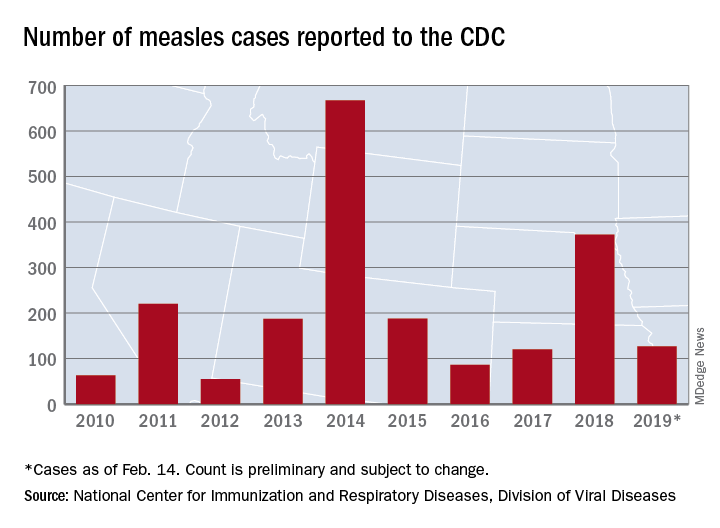
Eight of the last 10 flu seasons had already reached their peak before mid-February, but another rise brought the proportion of outpatient visits for influenza-like illness (ILI) to 5.1% for the week ending Feb. 16, compared with 4.8% the week before, the CDC’s influenza division reported Feb. 22. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
The week also brought more ILI to more states, as the number reporting an activity level of 10 on the CDC’s 1-10 scale rose from 21 to 24 and the number in the high range of 8-10 increased from 26 to 30. Another seven states – including California, which was at level 5 the previous week – and the District of Columbia were at level 7 for the current reporting week, the CDC said.
Two flu-related pediatric deaths occurred during the week ending Feb. 16 and another five were reported from previous weeks, which brings the total to 41 for the 2018-2019 season. Data for influenza deaths at all ages, which are reported a week later, show that 205 occurred in the week ending Feb. 9, with reporting 75% complete. There were 236 total deaths for the week ending Feb. 2 (94% reporting) and 218 deaths during the week ending Jan. 26 (99% reporting), the CDC said.
The 2018-2019 flu season is showing no signs of decline as activity measures continued to increase into mid-February, according to the Centers for Disease Control and Prevention.

Eight of the last 10 flu seasons had already reached their peak before mid-February, but another rise brought the proportion of outpatient visits for influenza-like illness (ILI) to 5.1% for the week ending Feb. 16, compared with 4.8% the week before, the CDC’s influenza division reported Feb. 22. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
The week also brought more ILI to more states, as the number reporting an activity level of 10 on the CDC’s 1-10 scale rose from 21 to 24 and the number in the high range of 8-10 increased from 26 to 30. Another seven states – including California, which was at level 5 the previous week – and the District of Columbia were at level 7 for the current reporting week, the CDC said.
Two flu-related pediatric deaths occurred during the week ending Feb. 16 and another five were reported from previous weeks, which brings the total to 41 for the 2018-2019 season. Data for influenza deaths at all ages, which are reported a week later, show that 205 occurred in the week ending Feb. 9, with reporting 75% complete. There were 236 total deaths for the week ending Feb. 2 (94% reporting) and 218 deaths during the week ending Jan. 26 (99% reporting), the CDC said.
The 2018-2019 flu season is showing no signs of decline as activity measures continued to increase into mid-February, according to the Centers for Disease Control and Prevention.

Eight of the last 10 flu seasons had already reached their peak before mid-February, but another rise brought the proportion of outpatient visits for influenza-like illness (ILI) to 5.1% for the week ending Feb. 16, compared with 4.8% the week before, the CDC’s influenza division reported Feb. 22. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
The week also brought more ILI to more states, as the number reporting an activity level of 10 on the CDC’s 1-10 scale rose from 21 to 24 and the number in the high range of 8-10 increased from 26 to 30. Another seven states – including California, which was at level 5 the previous week – and the District of Columbia were at level 7 for the current reporting week, the CDC said.
Two flu-related pediatric deaths occurred during the week ending Feb. 16 and another five were reported from previous weeks, which brings the total to 41 for the 2018-2019 season. Data for influenza deaths at all ages, which are reported a week later, show that 205 occurred in the week ending Feb. 9, with reporting 75% complete. There were 236 total deaths for the week ending Feb. 2 (94% reporting) and 218 deaths during the week ending Jan. 26 (99% reporting), the CDC said.
Baby boomers account for more than 74% of chronic HCV cases
The overall prevalence of chronic hepatitis C virus (HCV) in the United States was 1.19%, giving an estimate of 2,347,852 infected adults. Baby boomers had the highest prevalence at 2.23%, accounting for more than 74% of all chronic HCV cases, according to the results of a database analysis done by Kevin J. Moore, MD, of the University of Miami and his colleagues.
In the analysis, published in the Journal of Infection and Public Health, the researchers assessed data from National Health and Nutrition Examination Survey for the years 1999-2012. They separated three age categories: baby boomers (BB), younger than BB (YG), and older than BB (OG). BBs showed an HCV prevalence over four times higher than YG or OG. BBs also had more significant predictors of positive HCV status than YGs or OGs.
In addition to the overall difference in incidence of HCV infection in boomers and nonboomers, they found that significant predictors of chronic HCV positivity among BBs were being male or non-Hispanic black, having a positive blood transfusion history, being a current or former smoker, and living below the poverty line.
The most significant risk factor for HCV positivity differed across age groups – being a current smoker in YG and BB and being non-Hispanic black in OG, the researchers noted.
“These results show that HCV infection continues to be a significant public health issue and warrants further attention given the aging BB population. Future studies should seek to further identify age-specific risk factors for HCV infection to optimize HCV screening and prevention programs through public health interventions,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Moore KJ et al. J Infect Public Health. 2019 Jan-Feb;12(1):32-6.
The overall prevalence of chronic hepatitis C virus (HCV) in the United States was 1.19%, giving an estimate of 2,347,852 infected adults. Baby boomers had the highest prevalence at 2.23%, accounting for more than 74% of all chronic HCV cases, according to the results of a database analysis done by Kevin J. Moore, MD, of the University of Miami and his colleagues.
In the analysis, published in the Journal of Infection and Public Health, the researchers assessed data from National Health and Nutrition Examination Survey for the years 1999-2012. They separated three age categories: baby boomers (BB), younger than BB (YG), and older than BB (OG). BBs showed an HCV prevalence over four times higher than YG or OG. BBs also had more significant predictors of positive HCV status than YGs or OGs.
In addition to the overall difference in incidence of HCV infection in boomers and nonboomers, they found that significant predictors of chronic HCV positivity among BBs were being male or non-Hispanic black, having a positive blood transfusion history, being a current or former smoker, and living below the poverty line.
The most significant risk factor for HCV positivity differed across age groups – being a current smoker in YG and BB and being non-Hispanic black in OG, the researchers noted.
“These results show that HCV infection continues to be a significant public health issue and warrants further attention given the aging BB population. Future studies should seek to further identify age-specific risk factors for HCV infection to optimize HCV screening and prevention programs through public health interventions,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Moore KJ et al. J Infect Public Health. 2019 Jan-Feb;12(1):32-6.
The overall prevalence of chronic hepatitis C virus (HCV) in the United States was 1.19%, giving an estimate of 2,347,852 infected adults. Baby boomers had the highest prevalence at 2.23%, accounting for more than 74% of all chronic HCV cases, according to the results of a database analysis done by Kevin J. Moore, MD, of the University of Miami and his colleagues.
In the analysis, published in the Journal of Infection and Public Health, the researchers assessed data from National Health and Nutrition Examination Survey for the years 1999-2012. They separated three age categories: baby boomers (BB), younger than BB (YG), and older than BB (OG). BBs showed an HCV prevalence over four times higher than YG or OG. BBs also had more significant predictors of positive HCV status than YGs or OGs.
In addition to the overall difference in incidence of HCV infection in boomers and nonboomers, they found that significant predictors of chronic HCV positivity among BBs were being male or non-Hispanic black, having a positive blood transfusion history, being a current or former smoker, and living below the poverty line.
The most significant risk factor for HCV positivity differed across age groups – being a current smoker in YG and BB and being non-Hispanic black in OG, the researchers noted.
“These results show that HCV infection continues to be a significant public health issue and warrants further attention given the aging BB population. Future studies should seek to further identify age-specific risk factors for HCV infection to optimize HCV screening and prevention programs through public health interventions,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Moore KJ et al. J Infect Public Health. 2019 Jan-Feb;12(1):32-6.
FROM THE JOURNAL OF INFECTION AND PUBLIC HEALTH
Onychomycosis that fails terbinafine probably isn’t T. rubrum
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Tropical travelers’ top dermatologic infestations
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
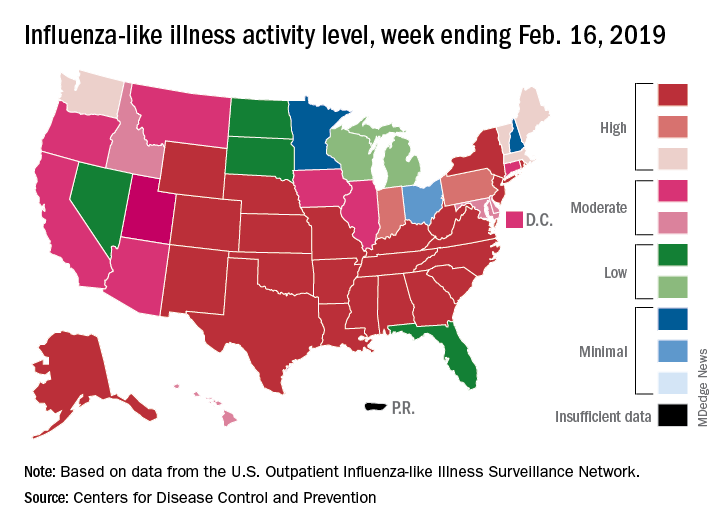
Cutaneous larva migrans
This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
HCV-infected patients in the ED should be tested for advanced liver fibrosis
More than one-third of hepatitis C virus-infected patients in the emergency department (ED) were found to have advanced liver fibrosis and higher mortality, according to the results of a retrospective study of 113 known patients with HCV at a single institution.
As part of an ongoing HCV linkage-to-care (LTC) program, HCV-infected ED patients were retrospectively identified. Components of FIB-4 (a noninvasive serum fibrosis index, which includes age, alanine aminotransferase, aspartate aminotransferase, and platelet count), were abstracted. Patients with an FIB-4 greater than 3.25 were classified with advanced fibrosis and characterized with regard to downstream outcomes at 1 year after enrollment.
The 1-year outcomes after the ED encounter for the 113 patients showed 38 with and 75 patients without advanced fibrosis. Among these, 72 (96%) and 34 (89.5%), respectively, agreed to be linked to HCV care. Ten patients of the total number of patients died within the 1-year follow-up. For those HCV-infected patients with advanced liver fibrosis compared to those without, all-cause mortality was more than fourfold higher, (18.4% [7 patients] vs. 4.0% [3 patients], P = .030), according to Yu-Hsiang Hsieh, PhD, associate professor of emergency medicine at Johns Hopkins University, Baltimore, and his colleagues (Am J Emerg Med. 2019;37[2]:286-90).
“Given the substantial burden of HCV-related illness in urban ED patients nationally, and the recognized fact that EDs are often the only point of contact with the health care system for many of these patients, we propose incorporating FIB-4 based rapid assessment into ED-based HCV screening and LTC programs in order to prioritize LTC for patients with advanced liver fibrosis, as well as routine ED clinical practice,” the researchers concluded.
They reported having no conflicts.
SOURCE: Yu-Hsiang Hsieh Y-H, Am J Emerg Med. 2019;37[2]:286-90.
More than one-third of hepatitis C virus-infected patients in the emergency department (ED) were found to have advanced liver fibrosis and higher mortality, according to the results of a retrospective study of 113 known patients with HCV at a single institution.
As part of an ongoing HCV linkage-to-care (LTC) program, HCV-infected ED patients were retrospectively identified. Components of FIB-4 (a noninvasive serum fibrosis index, which includes age, alanine aminotransferase, aspartate aminotransferase, and platelet count), were abstracted. Patients with an FIB-4 greater than 3.25 were classified with advanced fibrosis and characterized with regard to downstream outcomes at 1 year after enrollment.
The 1-year outcomes after the ED encounter for the 113 patients showed 38 with and 75 patients without advanced fibrosis. Among these, 72 (96%) and 34 (89.5%), respectively, agreed to be linked to HCV care. Ten patients of the total number of patients died within the 1-year follow-up. For those HCV-infected patients with advanced liver fibrosis compared to those without, all-cause mortality was more than fourfold higher, (18.4% [7 patients] vs. 4.0% [3 patients], P = .030), according to Yu-Hsiang Hsieh, PhD, associate professor of emergency medicine at Johns Hopkins University, Baltimore, and his colleagues (Am J Emerg Med. 2019;37[2]:286-90).
“Given the substantial burden of HCV-related illness in urban ED patients nationally, and the recognized fact that EDs are often the only point of contact with the health care system for many of these patients, we propose incorporating FIB-4 based rapid assessment into ED-based HCV screening and LTC programs in order to prioritize LTC for patients with advanced liver fibrosis, as well as routine ED clinical practice,” the researchers concluded.
They reported having no conflicts.
SOURCE: Yu-Hsiang Hsieh Y-H, Am J Emerg Med. 2019;37[2]:286-90.
More than one-third of hepatitis C virus-infected patients in the emergency department (ED) were found to have advanced liver fibrosis and higher mortality, according to the results of a retrospective study of 113 known patients with HCV at a single institution.
As part of an ongoing HCV linkage-to-care (LTC) program, HCV-infected ED patients were retrospectively identified. Components of FIB-4 (a noninvasive serum fibrosis index, which includes age, alanine aminotransferase, aspartate aminotransferase, and platelet count), were abstracted. Patients with an FIB-4 greater than 3.25 were classified with advanced fibrosis and characterized with regard to downstream outcomes at 1 year after enrollment.
The 1-year outcomes after the ED encounter for the 113 patients showed 38 with and 75 patients without advanced fibrosis. Among these, 72 (96%) and 34 (89.5%), respectively, agreed to be linked to HCV care. Ten patients of the total number of patients died within the 1-year follow-up. For those HCV-infected patients with advanced liver fibrosis compared to those without, all-cause mortality was more than fourfold higher, (18.4% [7 patients] vs. 4.0% [3 patients], P = .030), according to Yu-Hsiang Hsieh, PhD, associate professor of emergency medicine at Johns Hopkins University, Baltimore, and his colleagues (Am J Emerg Med. 2019;37[2]:286-90).
“Given the substantial burden of HCV-related illness in urban ED patients nationally, and the recognized fact that EDs are often the only point of contact with the health care system for many of these patients, we propose incorporating FIB-4 based rapid assessment into ED-based HCV screening and LTC programs in order to prioritize LTC for patients with advanced liver fibrosis, as well as routine ED clinical practice,” the researchers concluded.
They reported having no conflicts.
SOURCE: Yu-Hsiang Hsieh Y-H, Am J Emerg Med. 2019;37[2]:286-90.
FROM THE AMERICAN JOURNAL OF EMERGENCY MEDICINE
Measles: 26 new cases reported last week
according to the Centers for Disease Control and Prevention.
On Jan. 31, total measles cases stood at 79, which means that the number of individuals with measles has risen by 61% in just the last 2 weeks. Of the five outbreaks (defined as three or more cases) so far in 2019, three have occurred in New York (57 cases in three counties), one in Texas (8 cases in five counties), and one in Washington (62 cases in two counties), the CDC reported Feb. 18.
The majority of the Washington cases (61 of the 62) have occurred in Clark County, which is located just across the Columbia River from Portland, Ore. Oregon, in turn, has a higher percentage of kindergartners with nonmedical exemptions from vaccination (7.5%) than any other state, the CDC reported in October 2018. Washington’s rate of 3.9% was nearly double the national median of 2.0% for the 2017-2018 school year, while Texas (1.8%) and New York (1.0%) were below it, the CDC said.
In the Pacific Northwest, however, some parents may be changing their minds about vaccinations, according to the New York Times, which reported that “about triple the number of children have been vaccinated this year, compared with the same period in 2018,” in Oregon and southwest Washington.
Individual cases of measles have been reported to the CDC by seven other states: California, Colorado, Connecticut, Georgia, Illinois, Kentucky, and Oregon.
according to the Centers for Disease Control and Prevention.
On Jan. 31, total measles cases stood at 79, which means that the number of individuals with measles has risen by 61% in just the last 2 weeks. Of the five outbreaks (defined as three or more cases) so far in 2019, three have occurred in New York (57 cases in three counties), one in Texas (8 cases in five counties), and one in Washington (62 cases in two counties), the CDC reported Feb. 18.
The majority of the Washington cases (61 of the 62) have occurred in Clark County, which is located just across the Columbia River from Portland, Ore. Oregon, in turn, has a higher percentage of kindergartners with nonmedical exemptions from vaccination (7.5%) than any other state, the CDC reported in October 2018. Washington’s rate of 3.9% was nearly double the national median of 2.0% for the 2017-2018 school year, while Texas (1.8%) and New York (1.0%) were below it, the CDC said.
In the Pacific Northwest, however, some parents may be changing their minds about vaccinations, according to the New York Times, which reported that “about triple the number of children have been vaccinated this year, compared with the same period in 2018,” in Oregon and southwest Washington.
Individual cases of measles have been reported to the CDC by seven other states: California, Colorado, Connecticut, Georgia, Illinois, Kentucky, and Oregon.
according to the Centers for Disease Control and Prevention.
On Jan. 31, total measles cases stood at 79, which means that the number of individuals with measles has risen by 61% in just the last 2 weeks. Of the five outbreaks (defined as three or more cases) so far in 2019, three have occurred in New York (57 cases in three counties), one in Texas (8 cases in five counties), and one in Washington (62 cases in two counties), the CDC reported Feb. 18.
The majority of the Washington cases (61 of the 62) have occurred in Clark County, which is located just across the Columbia River from Portland, Ore. Oregon, in turn, has a higher percentage of kindergartners with nonmedical exemptions from vaccination (7.5%) than any other state, the CDC reported in October 2018. Washington’s rate of 3.9% was nearly double the national median of 2.0% for the 2017-2018 school year, while Texas (1.8%) and New York (1.0%) were below it, the CDC said.
In the Pacific Northwest, however, some parents may be changing their minds about vaccinations, according to the New York Times, which reported that “about triple the number of children have been vaccinated this year, compared with the same period in 2018,” in Oregon and southwest Washington.
Individual cases of measles have been reported to the CDC by seven other states: California, Colorado, Connecticut, Georgia, Illinois, Kentucky, and Oregon.
Enterovirus in at-risk children associated with later celiac disease
“We found a significant association between exposure to enterovirus and subsequent risk of celiac disease,” wrote lead author Christian R. Kahrs of the University of Oslo and his coauthors, adding that “adenovirus was not associated with celiac disease.” The study was published in the BMJ.
From 2001 to 2007, 46,939 newborns in Norway were screened for the HLA-DQ2/DQ8 genotype, which is associated with an increased risk of celiac disease. The genotype was identified in 912 children, and blood and stool sample collection began at 3 months. Children who were still contributing blood samples by 2014-2016 were invited to be screened for celiac disease.
Of the 220 children screened, 25 were diagnosed with celiac disease. Enterovirus was detected in 370 (17%) of the 2,135 stool samples and was more frequent in children who developed celiac disease antibodies than in matched controls (adjusted odds ratio, 1.49; 95% confidence interval, 1.07-2.06; P = .02). There was a significant association between later development of celiac disease and the commonly identified enterovirus A (aOR, 1.62; 95% CI, 1.04-2.53; P = .03) and enterovirus B (aOR, 2.27; 95% CI, 1.33-3.88; P = .003). No adenovirus types were associated with development of celiac disease.
The authors acknowledged their study’s limitations, including the possibility that children might be diagnosed with celiac disease later than the study’s roughly 10-year follow-up and the limited number of children with the disease despite a large number of analyzed samples. They noted that, “given the limited number of cases, we call for corroboration in similar studies and preferably interventional studies to reach conclusions about causality.”
The study was funded by the Research Council of Norway, the Project for the Conceptual Development of Research Organization, and the Norwegian Coeliac Society. Two authors reported grant support from trusts and foundations in Norway and Switzerland; no conflicts of interest were reported.
SOURCE: Kahrs CR et al. BMJ. 2019 Feb 13. doi: 10.1136/bmj.l231.
“We found a significant association between exposure to enterovirus and subsequent risk of celiac disease,” wrote lead author Christian R. Kahrs of the University of Oslo and his coauthors, adding that “adenovirus was not associated with celiac disease.” The study was published in the BMJ.
From 2001 to 2007, 46,939 newborns in Norway were screened for the HLA-DQ2/DQ8 genotype, which is associated with an increased risk of celiac disease. The genotype was identified in 912 children, and blood and stool sample collection began at 3 months. Children who were still contributing blood samples by 2014-2016 were invited to be screened for celiac disease.
Of the 220 children screened, 25 were diagnosed with celiac disease. Enterovirus was detected in 370 (17%) of the 2,135 stool samples and was more frequent in children who developed celiac disease antibodies than in matched controls (adjusted odds ratio, 1.49; 95% confidence interval, 1.07-2.06; P = .02). There was a significant association between later development of celiac disease and the commonly identified enterovirus A (aOR, 1.62; 95% CI, 1.04-2.53; P = .03) and enterovirus B (aOR, 2.27; 95% CI, 1.33-3.88; P = .003). No adenovirus types were associated with development of celiac disease.
The authors acknowledged their study’s limitations, including the possibility that children might be diagnosed with celiac disease later than the study’s roughly 10-year follow-up and the limited number of children with the disease despite a large number of analyzed samples. They noted that, “given the limited number of cases, we call for corroboration in similar studies and preferably interventional studies to reach conclusions about causality.”
The study was funded by the Research Council of Norway, the Project for the Conceptual Development of Research Organization, and the Norwegian Coeliac Society. Two authors reported grant support from trusts and foundations in Norway and Switzerland; no conflicts of interest were reported.
SOURCE: Kahrs CR et al. BMJ. 2019 Feb 13. doi: 10.1136/bmj.l231.
“We found a significant association between exposure to enterovirus and subsequent risk of celiac disease,” wrote lead author Christian R. Kahrs of the University of Oslo and his coauthors, adding that “adenovirus was not associated with celiac disease.” The study was published in the BMJ.
From 2001 to 2007, 46,939 newborns in Norway were screened for the HLA-DQ2/DQ8 genotype, which is associated with an increased risk of celiac disease. The genotype was identified in 912 children, and blood and stool sample collection began at 3 months. Children who were still contributing blood samples by 2014-2016 were invited to be screened for celiac disease.
Of the 220 children screened, 25 were diagnosed with celiac disease. Enterovirus was detected in 370 (17%) of the 2,135 stool samples and was more frequent in children who developed celiac disease antibodies than in matched controls (adjusted odds ratio, 1.49; 95% confidence interval, 1.07-2.06; P = .02). There was a significant association between later development of celiac disease and the commonly identified enterovirus A (aOR, 1.62; 95% CI, 1.04-2.53; P = .03) and enterovirus B (aOR, 2.27; 95% CI, 1.33-3.88; P = .003). No adenovirus types were associated with development of celiac disease.
The authors acknowledged their study’s limitations, including the possibility that children might be diagnosed with celiac disease later than the study’s roughly 10-year follow-up and the limited number of children with the disease despite a large number of analyzed samples. They noted that, “given the limited number of cases, we call for corroboration in similar studies and preferably interventional studies to reach conclusions about causality.”
The study was funded by the Research Council of Norway, the Project for the Conceptual Development of Research Organization, and the Norwegian Coeliac Society. Two authors reported grant support from trusts and foundations in Norway and Switzerland; no conflicts of interest were reported.
SOURCE: Kahrs CR et al. BMJ. 2019 Feb 13. doi: 10.1136/bmj.l231.
FROM THE BMJ
Flu season showing its staying power
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.
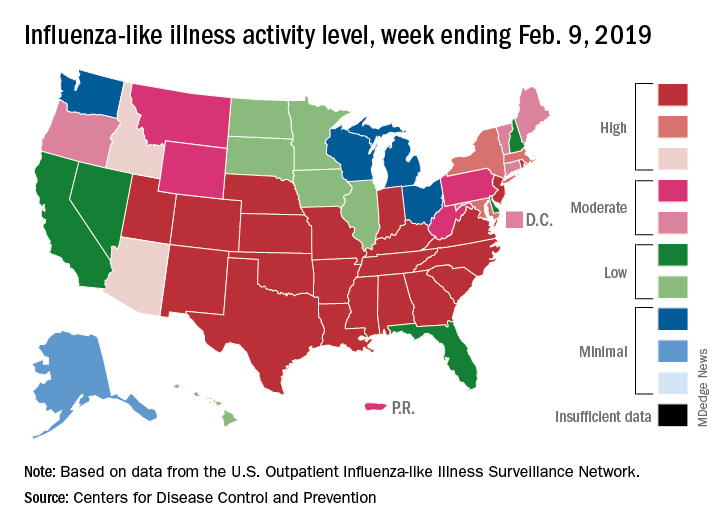
There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.