User login
HHS effort aims to end new HIV cases within 10 years
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.
“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
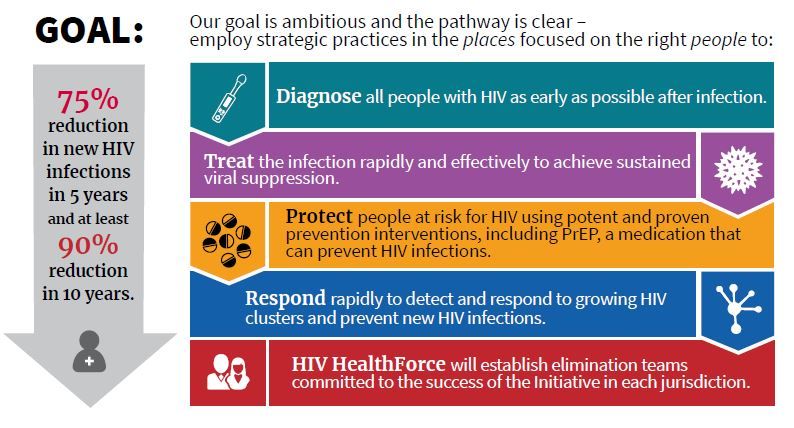
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.

“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.

“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
FROM A HEALTH AND HUMAN SERVICES BRIEFING
President Trump calls for end to HIV/AIDS, pediatric cancer
HIV/AIDS, pediatric cancer research, abortion, prescription drug prices, and preexisting conditions were among the health care highlights of President Donald Trump’s second State of the Union address Feb. 5.

Mr. Trump promised to push for funds to end HIV/AIDS and childhood cancer within in 10 years. “In recent years, we have made remarkable progress in the fight against HIV and AIDS. Scientific breakthroughs have brought a once-distant dream within reach,” he said to assembled members of Congress and leaders of the executive and judicial branches of government. “My budget will ask Democrats and Republicans to make the needed commitment to eliminate the HIV epidemic in the United States within 10 years.”
Following the speech, Alex Azar, secretary of the Department of Health and Human Services, offered more details in a blog post on the agency’s website.
Funding for the initiative, dubbed “Ending the HIV Epidemic: A Plan for America,” will have three components.
The first involves increasing investments in “geographic hotspots” though existing programs like the Ryan White HIV/AIDS Program and a new community health center–based program to provide antiretroviral therapy (ART) and preexposure prophylaxis (PrEP) to those at the highest risk of contracting the disease.
Second is the use of data to track where the disease is spreading most rapidly to help target prevention, care, and treatment at the local level. The third will provide funds for the creation of a local HIV HealthForce in these targeted areas to expand HIV prevention and treatment efforts.
A fact sheet on this initiative called for a 75% reduction in new cases of HIV infection in 5 years and at least a 90% reduction within 10 years.
President Trump called for similar efforts to address pediatric cancer.
“Tonight I am also asking you to join me in another fight that all American can get behind – the fight against childhood cancer,” he said, adding that his budget request will come with a line item of $500 million over 10 years to fund research. “Many childhood cancers have not seen new therapies in decades.”
President Trump also asked Congress to legislate a prohibition of late-term abortion.
“There could be no greater contrast to the beautiful image of a mother holding her infant child than the chilling displays our nation saw in recent days,” he said. “Lawmakers in New York cheered with delight upon the passage of legislation that would allow a baby to be ripped from the mother’s womb moments from birth. These are living, feeling beautiful babies who will never get the chance to share their love and their dreams with the world. ... Let us work together to build a culture that cherishes innocent life.”
He also touched on the recurring themes regarding lowering the cost of health care and prescription drugs, as well as protecting those with preexisting conditions, something he called a major priority.
“It’s unacceptable that Americans pay vastly more than people in other countries for the exact same drugs, often made in the exact same place. This is wrong. This is unfair and together we will stop it, and we will stop it fast,” he said.
He did not offer any specific policy recommendation on how to address prescription drug costs, other than a comment on the need for greater price transparency.
“I am asking Congress to pass legislation that finally takes on the problem of global freeloading and delivers fairness and price transparency for American patients,” he said.
“We should also require drug companies, insurance companies, and hospitals to disclose real prices to foster competition and bring costs way down.”
SOURCE: Trump D. State of the Union Address, Feb. 5, 2019.
HIV/AIDS, pediatric cancer research, abortion, prescription drug prices, and preexisting conditions were among the health care highlights of President Donald Trump’s second State of the Union address Feb. 5.

Mr. Trump promised to push for funds to end HIV/AIDS and childhood cancer within in 10 years. “In recent years, we have made remarkable progress in the fight against HIV and AIDS. Scientific breakthroughs have brought a once-distant dream within reach,” he said to assembled members of Congress and leaders of the executive and judicial branches of government. “My budget will ask Democrats and Republicans to make the needed commitment to eliminate the HIV epidemic in the United States within 10 years.”
Following the speech, Alex Azar, secretary of the Department of Health and Human Services, offered more details in a blog post on the agency’s website.
Funding for the initiative, dubbed “Ending the HIV Epidemic: A Plan for America,” will have three components.
The first involves increasing investments in “geographic hotspots” though existing programs like the Ryan White HIV/AIDS Program and a new community health center–based program to provide antiretroviral therapy (ART) and preexposure prophylaxis (PrEP) to those at the highest risk of contracting the disease.
Second is the use of data to track where the disease is spreading most rapidly to help target prevention, care, and treatment at the local level. The third will provide funds for the creation of a local HIV HealthForce in these targeted areas to expand HIV prevention and treatment efforts.
A fact sheet on this initiative called for a 75% reduction in new cases of HIV infection in 5 years and at least a 90% reduction within 10 years.
President Trump called for similar efforts to address pediatric cancer.
“Tonight I am also asking you to join me in another fight that all American can get behind – the fight against childhood cancer,” he said, adding that his budget request will come with a line item of $500 million over 10 years to fund research. “Many childhood cancers have not seen new therapies in decades.”
President Trump also asked Congress to legislate a prohibition of late-term abortion.
“There could be no greater contrast to the beautiful image of a mother holding her infant child than the chilling displays our nation saw in recent days,” he said. “Lawmakers in New York cheered with delight upon the passage of legislation that would allow a baby to be ripped from the mother’s womb moments from birth. These are living, feeling beautiful babies who will never get the chance to share their love and their dreams with the world. ... Let us work together to build a culture that cherishes innocent life.”
He also touched on the recurring themes regarding lowering the cost of health care and prescription drugs, as well as protecting those with preexisting conditions, something he called a major priority.
“It’s unacceptable that Americans pay vastly more than people in other countries for the exact same drugs, often made in the exact same place. This is wrong. This is unfair and together we will stop it, and we will stop it fast,” he said.
He did not offer any specific policy recommendation on how to address prescription drug costs, other than a comment on the need for greater price transparency.
“I am asking Congress to pass legislation that finally takes on the problem of global freeloading and delivers fairness and price transparency for American patients,” he said.
“We should also require drug companies, insurance companies, and hospitals to disclose real prices to foster competition and bring costs way down.”
SOURCE: Trump D. State of the Union Address, Feb. 5, 2019.
HIV/AIDS, pediatric cancer research, abortion, prescription drug prices, and preexisting conditions were among the health care highlights of President Donald Trump’s second State of the Union address Feb. 5.

Mr. Trump promised to push for funds to end HIV/AIDS and childhood cancer within in 10 years. “In recent years, we have made remarkable progress in the fight against HIV and AIDS. Scientific breakthroughs have brought a once-distant dream within reach,” he said to assembled members of Congress and leaders of the executive and judicial branches of government. “My budget will ask Democrats and Republicans to make the needed commitment to eliminate the HIV epidemic in the United States within 10 years.”
Following the speech, Alex Azar, secretary of the Department of Health and Human Services, offered more details in a blog post on the agency’s website.
Funding for the initiative, dubbed “Ending the HIV Epidemic: A Plan for America,” will have three components.
The first involves increasing investments in “geographic hotspots” though existing programs like the Ryan White HIV/AIDS Program and a new community health center–based program to provide antiretroviral therapy (ART) and preexposure prophylaxis (PrEP) to those at the highest risk of contracting the disease.
Second is the use of data to track where the disease is spreading most rapidly to help target prevention, care, and treatment at the local level. The third will provide funds for the creation of a local HIV HealthForce in these targeted areas to expand HIV prevention and treatment efforts.
A fact sheet on this initiative called for a 75% reduction in new cases of HIV infection in 5 years and at least a 90% reduction within 10 years.
President Trump called for similar efforts to address pediatric cancer.
“Tonight I am also asking you to join me in another fight that all American can get behind – the fight against childhood cancer,” he said, adding that his budget request will come with a line item of $500 million over 10 years to fund research. “Many childhood cancers have not seen new therapies in decades.”
President Trump also asked Congress to legislate a prohibition of late-term abortion.
“There could be no greater contrast to the beautiful image of a mother holding her infant child than the chilling displays our nation saw in recent days,” he said. “Lawmakers in New York cheered with delight upon the passage of legislation that would allow a baby to be ripped from the mother’s womb moments from birth. These are living, feeling beautiful babies who will never get the chance to share their love and their dreams with the world. ... Let us work together to build a culture that cherishes innocent life.”
He also touched on the recurring themes regarding lowering the cost of health care and prescription drugs, as well as protecting those with preexisting conditions, something he called a major priority.
“It’s unacceptable that Americans pay vastly more than people in other countries for the exact same drugs, often made in the exact same place. This is wrong. This is unfair and together we will stop it, and we will stop it fast,” he said.
He did not offer any specific policy recommendation on how to address prescription drug costs, other than a comment on the need for greater price transparency.
“I am asking Congress to pass legislation that finally takes on the problem of global freeloading and delivers fairness and price transparency for American patients,” he said.
“We should also require drug companies, insurance companies, and hospitals to disclose real prices to foster competition and bring costs way down.”
SOURCE: Trump D. State of the Union Address, Feb. 5, 2019.
Key clinical point: President Trump calls for an end to HIV/AIDS and pediatric cancer in 10 years.
Major finding: His budget will request $500 million for cancer research and as yet undisclosed amount for HIV/AIDS research.
Study details: More specific details on the proposals will likely come when the president makes his budget submission to Congress in the coming weeks.
Disclosures: There are no disclosures.
Source: Trump D. State of the Union Address, Feb. 5, 2019.
Rise in HCV infection rates linked to OxyContin reformulation
Public health experts have attributed the alarming rise in hepatitis C virus (HCV) infection rates in recent years to the opioid epidemic, and a new Rand study suggests that an effort to deter opioid abuse – namely the 2010 abuse-deterrent reformulation of OxyContin – is partly to blame.
Between 2004 and 2015, HCV infection rates in the United States nearly tripled, but a closer look showed that states with above-median rates of OxyContin misuse prior to the reformulation had a 222% increase in HCV rates, compared with a 75% increase in states with below-median OxyContin misuse, said David Powell, PhD, a senior economist at Rand in Arlington, Va., and his colleagues, Abby Alpert, PhD, and Rosalie L. Pacula, PhD. The report was published in Health Affairs.
The coauthors found that hepatitis C infection rates were not significantly different between the two groups of states before the reformulation (0.350 vs. 0.260). But after 2010, there were large and statistically significant differences in the rates (1.128 vs. 0.455; P less than 0.01), they wrote, noting that the above-median states experienced an additional 0.58 HCV infections per 100,000 population through 2015 relative to the below-median states).
HCV infection rates declined during the 1990s followed by a plateau beginning around 2003, then rose sharply beginning in 2010, coinciding with the introduction of the release of the abuse-deterrent formulation of OxyContin, which is one of the most commonly misused opioid analgesics, the investigators said, explaining that the reformulated version was harder to crush or dissolve, making it more difficult to inhale or inject.
“Prior studies have shown that, after OxyContin became more difficult to abuse, some nonmedical users of OxyContin switched to heroin (a pharmacologically similar opiate),” they noted.
This led to a decline of more than 40% in OxyContin misuse but also to a sharp increase in heroin overdoses after 2010.
The investigators assessed whether the related increase in heroin use might explain the increase in HCV infections, which can be transmitted through shared needle use.
Using a quasi-experimental difference-in-differences approach, they examined whether states with higher exposure to the reformulated OxyContin had faster growth of HCV infection rates after the reformulations, and as a falsification exercise, they also looked at whether the nonmedical use of pain relievers other than OxyContin predicted post-reformulation HCV infection rate increases.
HCV infection rates for each calendar year from 2004 to 2015 were assessed using confirmed case reports collected by the Centers for Disease Control and Prevention, and nonmedical OxyContin use was measured using self-reported data from the National Survey on Drug Use and Health, which is the largest U.S. survey on substance use disorder.
The two groups of states had similar demographic and economic conditions, except that the above-median misuse states had smaller populations and a larger proportion of white residents.
Of note, the patterns of HCV infection mirrored those of heroin overdoses. There was small relative increase in HCV infection rates in 2010 in the above-median OxyContin misuse states, and the gap between above- and below-median misuse states widened more rapidly from 2011 to 2013. “This striking inflection point in the trend of hepatitis C infections for high-misuse states after 2010 mimics the inflection in heroin overdoses that occurred as a result of the reformulation,” they said, noting that heroin morality per 100,000 population was nearly identical in the two groups of states in the pre-reformulation period (0.859 and 0.847).
The falsification exercise looking at nonmedical use of pain relievers other than OxyContin in the two groups of states showed that after 2010 groups’ rates of hepatitis C infections grew at virtually identical rates.
“Thus, the differential risk in hepatitis C infections was uniquely associated with OxyContin misuse, rather than prescription pain reliever misuse more generally,” they said. “This suggests that it was the OxyContin reformulation, not other policies broadly affecting opioids, that drove much of the differential growth.”
The investigators controlled for numerous other factors, including opioid policies that might have an impact on OxyContin and heroin use, prescription drug monitoring programs and pain clinic regulations, as well as the role of major pill-mill crackdowns in 2010 and 2011.
The findings represent a “substantial public health concern,” they said, explaining that, while “considerable policy attention is being given to managing the opioid epidemic ... a ‘silent epidemic’ of hepatitis C has emerged as a result of a transition in the mode of administration toward injection drug use.”
In 2017, the CDC reported on this link between the opioid epidemic and rising HCV infection rates, as well.
Dr. Powell and his colleagues wrote.
Their findings regarding the unintended consequences of the OxyContin reformulation suggest that caution is warranted with respect to future interventions that limit the supply of abusable prescription opioids, they said, adding that “such interventions must be paired with polices that alleviate the harms associated with switching to illicit drugs, such as improved access to substance use disorder treatment and increased efforts aimed at identifying and treating diseases associated with injection drug use.”
However, policy makers and medical professionals also must recognize that reducing opioid-related mortality and increasing access to drug treatment might not be sufficient to fully address all of the public health consequences associated with the opioid crisis. As additional reformulations of opioids are promoted and more policies seek to limit access to prescription opioids, “both the medical and the law enforcement communities must recognize the critical transition from prescription opioids to other drugs, particularly those that are injected, and be prepared to consider complementary strategies that can effectively reduce the additional harms from the particular mode of drug use,” they concluded.
The coauthors cited several limitations, including the possibility that true hepatitis C infection rates might have been underestimated in the study.
He and Dr. Pacula received funding from the National Institute on Drug Abuse. Dr. Powell also cited funding from the Rand Alumni Impact Award.
SOURCE: Powell D et al. Health Aff. 2019;38(2):287-94.
Increases have been seen not only in infectious diseases but also in cardiovascular diseases as intravenous opioid use has risen, Mark S. Gold, MD, said in an interview. “These emerging co-occurring diseases tend to lag behind drug deaths and other data,” he said.
The study by Powell et al. shows that drugs of abuse are dangerous, and that, with addictive use, we find consequences. “Each change appears to bring with it intended consequences we study, but over time, unintended consequences emerge,” he said. “It is important to remain vigilant.”
Dr. Gold is 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
Increases have been seen not only in infectious diseases but also in cardiovascular diseases as intravenous opioid use has risen, Mark S. Gold, MD, said in an interview. “These emerging co-occurring diseases tend to lag behind drug deaths and other data,” he said.
The study by Powell et al. shows that drugs of abuse are dangerous, and that, with addictive use, we find consequences. “Each change appears to bring with it intended consequences we study, but over time, unintended consequences emerge,” he said. “It is important to remain vigilant.”
Dr. Gold is 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
Increases have been seen not only in infectious diseases but also in cardiovascular diseases as intravenous opioid use has risen, Mark S. Gold, MD, said in an interview. “These emerging co-occurring diseases tend to lag behind drug deaths and other data,” he said.
The study by Powell et al. shows that drugs of abuse are dangerous, and that, with addictive use, we find consequences. “Each change appears to bring with it intended consequences we study, but over time, unintended consequences emerge,” he said. “It is important to remain vigilant.”
Dr. Gold is 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis.
Public health experts have attributed the alarming rise in hepatitis C virus (HCV) infection rates in recent years to the opioid epidemic, and a new Rand study suggests that an effort to deter opioid abuse – namely the 2010 abuse-deterrent reformulation of OxyContin – is partly to blame.
Between 2004 and 2015, HCV infection rates in the United States nearly tripled, but a closer look showed that states with above-median rates of OxyContin misuse prior to the reformulation had a 222% increase in HCV rates, compared with a 75% increase in states with below-median OxyContin misuse, said David Powell, PhD, a senior economist at Rand in Arlington, Va., and his colleagues, Abby Alpert, PhD, and Rosalie L. Pacula, PhD. The report was published in Health Affairs.
The coauthors found that hepatitis C infection rates were not significantly different between the two groups of states before the reformulation (0.350 vs. 0.260). But after 2010, there were large and statistically significant differences in the rates (1.128 vs. 0.455; P less than 0.01), they wrote, noting that the above-median states experienced an additional 0.58 HCV infections per 100,000 population through 2015 relative to the below-median states).
HCV infection rates declined during the 1990s followed by a plateau beginning around 2003, then rose sharply beginning in 2010, coinciding with the introduction of the release of the abuse-deterrent formulation of OxyContin, which is one of the most commonly misused opioid analgesics, the investigators said, explaining that the reformulated version was harder to crush or dissolve, making it more difficult to inhale or inject.
“Prior studies have shown that, after OxyContin became more difficult to abuse, some nonmedical users of OxyContin switched to heroin (a pharmacologically similar opiate),” they noted.
This led to a decline of more than 40% in OxyContin misuse but also to a sharp increase in heroin overdoses after 2010.
The investigators assessed whether the related increase in heroin use might explain the increase in HCV infections, which can be transmitted through shared needle use.
Using a quasi-experimental difference-in-differences approach, they examined whether states with higher exposure to the reformulated OxyContin had faster growth of HCV infection rates after the reformulations, and as a falsification exercise, they also looked at whether the nonmedical use of pain relievers other than OxyContin predicted post-reformulation HCV infection rate increases.
HCV infection rates for each calendar year from 2004 to 2015 were assessed using confirmed case reports collected by the Centers for Disease Control and Prevention, and nonmedical OxyContin use was measured using self-reported data from the National Survey on Drug Use and Health, which is the largest U.S. survey on substance use disorder.
The two groups of states had similar demographic and economic conditions, except that the above-median misuse states had smaller populations and a larger proportion of white residents.
Of note, the patterns of HCV infection mirrored those of heroin overdoses. There was small relative increase in HCV infection rates in 2010 in the above-median OxyContin misuse states, and the gap between above- and below-median misuse states widened more rapidly from 2011 to 2013. “This striking inflection point in the trend of hepatitis C infections for high-misuse states after 2010 mimics the inflection in heroin overdoses that occurred as a result of the reformulation,” they said, noting that heroin morality per 100,000 population was nearly identical in the two groups of states in the pre-reformulation period (0.859 and 0.847).
The falsification exercise looking at nonmedical use of pain relievers other than OxyContin in the two groups of states showed that after 2010 groups’ rates of hepatitis C infections grew at virtually identical rates.
“Thus, the differential risk in hepatitis C infections was uniquely associated with OxyContin misuse, rather than prescription pain reliever misuse more generally,” they said. “This suggests that it was the OxyContin reformulation, not other policies broadly affecting opioids, that drove much of the differential growth.”
The investigators controlled for numerous other factors, including opioid policies that might have an impact on OxyContin and heroin use, prescription drug monitoring programs and pain clinic regulations, as well as the role of major pill-mill crackdowns in 2010 and 2011.
The findings represent a “substantial public health concern,” they said, explaining that, while “considerable policy attention is being given to managing the opioid epidemic ... a ‘silent epidemic’ of hepatitis C has emerged as a result of a transition in the mode of administration toward injection drug use.”
In 2017, the CDC reported on this link between the opioid epidemic and rising HCV infection rates, as well.
Dr. Powell and his colleagues wrote.
Their findings regarding the unintended consequences of the OxyContin reformulation suggest that caution is warranted with respect to future interventions that limit the supply of abusable prescription opioids, they said, adding that “such interventions must be paired with polices that alleviate the harms associated with switching to illicit drugs, such as improved access to substance use disorder treatment and increased efforts aimed at identifying and treating diseases associated with injection drug use.”
However, policy makers and medical professionals also must recognize that reducing opioid-related mortality and increasing access to drug treatment might not be sufficient to fully address all of the public health consequences associated with the opioid crisis. As additional reformulations of opioids are promoted and more policies seek to limit access to prescription opioids, “both the medical and the law enforcement communities must recognize the critical transition from prescription opioids to other drugs, particularly those that are injected, and be prepared to consider complementary strategies that can effectively reduce the additional harms from the particular mode of drug use,” they concluded.
The coauthors cited several limitations, including the possibility that true hepatitis C infection rates might have been underestimated in the study.
He and Dr. Pacula received funding from the National Institute on Drug Abuse. Dr. Powell also cited funding from the Rand Alumni Impact Award.
SOURCE: Powell D et al. Health Aff. 2019;38(2):287-94.
Public health experts have attributed the alarming rise in hepatitis C virus (HCV) infection rates in recent years to the opioid epidemic, and a new Rand study suggests that an effort to deter opioid abuse – namely the 2010 abuse-deterrent reformulation of OxyContin – is partly to blame.
Between 2004 and 2015, HCV infection rates in the United States nearly tripled, but a closer look showed that states with above-median rates of OxyContin misuse prior to the reformulation had a 222% increase in HCV rates, compared with a 75% increase in states with below-median OxyContin misuse, said David Powell, PhD, a senior economist at Rand in Arlington, Va., and his colleagues, Abby Alpert, PhD, and Rosalie L. Pacula, PhD. The report was published in Health Affairs.
The coauthors found that hepatitis C infection rates were not significantly different between the two groups of states before the reformulation (0.350 vs. 0.260). But after 2010, there were large and statistically significant differences in the rates (1.128 vs. 0.455; P less than 0.01), they wrote, noting that the above-median states experienced an additional 0.58 HCV infections per 100,000 population through 2015 relative to the below-median states).
HCV infection rates declined during the 1990s followed by a plateau beginning around 2003, then rose sharply beginning in 2010, coinciding with the introduction of the release of the abuse-deterrent formulation of OxyContin, which is one of the most commonly misused opioid analgesics, the investigators said, explaining that the reformulated version was harder to crush or dissolve, making it more difficult to inhale or inject.
“Prior studies have shown that, after OxyContin became more difficult to abuse, some nonmedical users of OxyContin switched to heroin (a pharmacologically similar opiate),” they noted.
This led to a decline of more than 40% in OxyContin misuse but also to a sharp increase in heroin overdoses after 2010.
The investigators assessed whether the related increase in heroin use might explain the increase in HCV infections, which can be transmitted through shared needle use.
Using a quasi-experimental difference-in-differences approach, they examined whether states with higher exposure to the reformulated OxyContin had faster growth of HCV infection rates after the reformulations, and as a falsification exercise, they also looked at whether the nonmedical use of pain relievers other than OxyContin predicted post-reformulation HCV infection rate increases.
HCV infection rates for each calendar year from 2004 to 2015 were assessed using confirmed case reports collected by the Centers for Disease Control and Prevention, and nonmedical OxyContin use was measured using self-reported data from the National Survey on Drug Use and Health, which is the largest U.S. survey on substance use disorder.
The two groups of states had similar demographic and economic conditions, except that the above-median misuse states had smaller populations and a larger proportion of white residents.
Of note, the patterns of HCV infection mirrored those of heroin overdoses. There was small relative increase in HCV infection rates in 2010 in the above-median OxyContin misuse states, and the gap between above- and below-median misuse states widened more rapidly from 2011 to 2013. “This striking inflection point in the trend of hepatitis C infections for high-misuse states after 2010 mimics the inflection in heroin overdoses that occurred as a result of the reformulation,” they said, noting that heroin morality per 100,000 population was nearly identical in the two groups of states in the pre-reformulation period (0.859 and 0.847).
The falsification exercise looking at nonmedical use of pain relievers other than OxyContin in the two groups of states showed that after 2010 groups’ rates of hepatitis C infections grew at virtually identical rates.
“Thus, the differential risk in hepatitis C infections was uniquely associated with OxyContin misuse, rather than prescription pain reliever misuse more generally,” they said. “This suggests that it was the OxyContin reformulation, not other policies broadly affecting opioids, that drove much of the differential growth.”
The investigators controlled for numerous other factors, including opioid policies that might have an impact on OxyContin and heroin use, prescription drug monitoring programs and pain clinic regulations, as well as the role of major pill-mill crackdowns in 2010 and 2011.
The findings represent a “substantial public health concern,” they said, explaining that, while “considerable policy attention is being given to managing the opioid epidemic ... a ‘silent epidemic’ of hepatitis C has emerged as a result of a transition in the mode of administration toward injection drug use.”
In 2017, the CDC reported on this link between the opioid epidemic and rising HCV infection rates, as well.
Dr. Powell and his colleagues wrote.
Their findings regarding the unintended consequences of the OxyContin reformulation suggest that caution is warranted with respect to future interventions that limit the supply of abusable prescription opioids, they said, adding that “such interventions must be paired with polices that alleviate the harms associated with switching to illicit drugs, such as improved access to substance use disorder treatment and increased efforts aimed at identifying and treating diseases associated with injection drug use.”
However, policy makers and medical professionals also must recognize that reducing opioid-related mortality and increasing access to drug treatment might not be sufficient to fully address all of the public health consequences associated with the opioid crisis. As additional reformulations of opioids are promoted and more policies seek to limit access to prescription opioids, “both the medical and the law enforcement communities must recognize the critical transition from prescription opioids to other drugs, particularly those that are injected, and be prepared to consider complementary strategies that can effectively reduce the additional harms from the particular mode of drug use,” they concluded.
The coauthors cited several limitations, including the possibility that true hepatitis C infection rates might have been underestimated in the study.
He and Dr. Pacula received funding from the National Institute on Drug Abuse. Dr. Powell also cited funding from the Rand Alumni Impact Award.
SOURCE: Powell D et al. Health Aff. 2019;38(2):287-94.
FROM HEALTH AFFAIRS
Key clinical point: Physicians and others must be “prepared to consider complementary strategies that can effectively reduce the additional harms from the particular mode of drug use.”
Major finding: HCV rates increased 222% in states that had above-median OxyContin misuse rates, compared with an increase of 75% in states with below-median misuse.
Study details: A review of data from 2004 to 2015.
Disclosures: Dr. Powell and Dr. Pacula received funding from the National Institute on Drug Abuse. Dr. Powell also cited funding from the Rand Alumni Impact Award.
Source: Powell D et al. Health Aff. 2019;38(2):287-94.
No increase in severe community-acquired pneumonia after PCV13
Despite concern about the rise of nonvaccine serotypes following widespread PCV13 immunization, cases of community-acquired pneumonia (CAP) remain nearly as low as after initial implementation of the vaccine and severe cases have not risen at all.
This was the finding of a prospective time-series analysis study from eight French pediatric emergency departments between June 2009 and May 2017.
The 12,587 children with CAP enrolled in the study between June 2009 and May 2017 were all aged 15 years or younger and came from one of eight French pediatric EDs.
Pediatric pneumonia cases per 1,000 ED visits dropped 44% after PCV13 was implemented, a decrease from 6.3 to 3.5 cases of CAP per 1,000 pediatric visits from June 2011 to May 2014, with a slight but statistically significant increase to 3.8 cases of CAP per 1,000 pediatric visits from June 2014 to May 2017. However, there was no statistically significant increase in cases with pleural effusion, hospitalization, or high inflammatory biomarkers.
“These results contrast with the recent increase in frequency of invasive pneumococcal disease observed in several countries during the same period linked to serotype replacement beyond 5 years after PCV13 implementation,” reported Naïm Ouldali, MD, of the Association Clinique et Thérapeutique Infantile du Val-de-Marne in France, and associates. The report is in JAMA Pediatrics.
“This difference in the trends suggests different consequences of serotype replacement on pneumococcal CAP vs invasive pneumococcal disease,” they wrote. “The recent slight increase in the number of all CAP cases and virus involvement may reflect changes in the epidemiology of other pathogens and/or serotype replacement with less pathogenic serotypes.”
This latter point arose from discovering no dominant serotype during the study period. Of the 11 serotypes not covered by PCV13, none appeared in more than four cases.
“The implementation of PCV13 has led to the quasi-disappearance of the more invasive serotypes and increase in others in nasopharyngeal flora, which greatly reduces the frequency of the more severe forms of CAP, but could also play a role in the slight increase in frequency of the more benign forms,” the authors reported.
Among the study’s limitations was lack of a control group, precluding the ability to attribute findings to any changes in case reporting. And “participating physicians were encouraged to not change their practice, including test use, and no other potential interfering intervention.”
Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research and a Pfizer Investigator Initiated Research grant.
Dr Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received non-financial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer and/or Sanofi Pasteur.
SOURCE: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
Despite concern about the rise of nonvaccine serotypes following widespread PCV13 immunization, cases of community-acquired pneumonia (CAP) remain nearly as low as after initial implementation of the vaccine and severe cases have not risen at all.
This was the finding of a prospective time-series analysis study from eight French pediatric emergency departments between June 2009 and May 2017.
The 12,587 children with CAP enrolled in the study between June 2009 and May 2017 were all aged 15 years or younger and came from one of eight French pediatric EDs.
Pediatric pneumonia cases per 1,000 ED visits dropped 44% after PCV13 was implemented, a decrease from 6.3 to 3.5 cases of CAP per 1,000 pediatric visits from June 2011 to May 2014, with a slight but statistically significant increase to 3.8 cases of CAP per 1,000 pediatric visits from June 2014 to May 2017. However, there was no statistically significant increase in cases with pleural effusion, hospitalization, or high inflammatory biomarkers.
“These results contrast with the recent increase in frequency of invasive pneumococcal disease observed in several countries during the same period linked to serotype replacement beyond 5 years after PCV13 implementation,” reported Naïm Ouldali, MD, of the Association Clinique et Thérapeutique Infantile du Val-de-Marne in France, and associates. The report is in JAMA Pediatrics.
“This difference in the trends suggests different consequences of serotype replacement on pneumococcal CAP vs invasive pneumococcal disease,” they wrote. “The recent slight increase in the number of all CAP cases and virus involvement may reflect changes in the epidemiology of other pathogens and/or serotype replacement with less pathogenic serotypes.”
This latter point arose from discovering no dominant serotype during the study period. Of the 11 serotypes not covered by PCV13, none appeared in more than four cases.
“The implementation of PCV13 has led to the quasi-disappearance of the more invasive serotypes and increase in others in nasopharyngeal flora, which greatly reduces the frequency of the more severe forms of CAP, but could also play a role in the slight increase in frequency of the more benign forms,” the authors reported.
Among the study’s limitations was lack of a control group, precluding the ability to attribute findings to any changes in case reporting. And “participating physicians were encouraged to not change their practice, including test use, and no other potential interfering intervention.”
Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research and a Pfizer Investigator Initiated Research grant.
Dr Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received non-financial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer and/or Sanofi Pasteur.
SOURCE: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
Despite concern about the rise of nonvaccine serotypes following widespread PCV13 immunization, cases of community-acquired pneumonia (CAP) remain nearly as low as after initial implementation of the vaccine and severe cases have not risen at all.
This was the finding of a prospective time-series analysis study from eight French pediatric emergency departments between June 2009 and May 2017.
The 12,587 children with CAP enrolled in the study between June 2009 and May 2017 were all aged 15 years or younger and came from one of eight French pediatric EDs.
Pediatric pneumonia cases per 1,000 ED visits dropped 44% after PCV13 was implemented, a decrease from 6.3 to 3.5 cases of CAP per 1,000 pediatric visits from June 2011 to May 2014, with a slight but statistically significant increase to 3.8 cases of CAP per 1,000 pediatric visits from June 2014 to May 2017. However, there was no statistically significant increase in cases with pleural effusion, hospitalization, or high inflammatory biomarkers.
“These results contrast with the recent increase in frequency of invasive pneumococcal disease observed in several countries during the same period linked to serotype replacement beyond 5 years after PCV13 implementation,” reported Naïm Ouldali, MD, of the Association Clinique et Thérapeutique Infantile du Val-de-Marne in France, and associates. The report is in JAMA Pediatrics.
“This difference in the trends suggests different consequences of serotype replacement on pneumococcal CAP vs invasive pneumococcal disease,” they wrote. “The recent slight increase in the number of all CAP cases and virus involvement may reflect changes in the epidemiology of other pathogens and/or serotype replacement with less pathogenic serotypes.”
This latter point arose from discovering no dominant serotype during the study period. Of the 11 serotypes not covered by PCV13, none appeared in more than four cases.
“The implementation of PCV13 has led to the quasi-disappearance of the more invasive serotypes and increase in others in nasopharyngeal flora, which greatly reduces the frequency of the more severe forms of CAP, but could also play a role in the slight increase in frequency of the more benign forms,” the authors reported.
Among the study’s limitations was lack of a control group, precluding the ability to attribute findings to any changes in case reporting. And “participating physicians were encouraged to not change their practice, including test use, and no other potential interfering intervention.”
Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research and a Pfizer Investigator Initiated Research grant.
Dr Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received non-financial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer and/or Sanofi Pasteur.
SOURCE: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Pediatric community-acquired pneumonia cases dropped from 6.3 to 3.5 cases per 1,000 visits from 2010 to 2014 and increased to 3.8 cases per 1,000 visits in May 2017.
Study details: The findings are based on a prospective time series analysis of 12,587 pediatric pneumonia cases (under 15 years old) in eight French emergency departments from June 2009 to May 2017.
Disclosures: Funding sources for this study included the Pediatric Infectious Diseases Group of the French Pediatrics Society, Association Clinique et Thérapeutique Infantile du Val-de-Marne, the Foundation for Medical Research, and a Pfizer Investigator Initiated Research grant. Dr. Ouldali has received grants from GlaxoSmithKline, and many of the authors have financial ties and/or have received nonfinancial support from AstraZeneca, Biocodex, GlaxoSmithKline, Merck, Novartis, Pfizer, and/or Sanofi Pasteur.
Source: Ouldali N et al. JAMA Pediatrics. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5273.
Flu activity & measles outbreaks: Where we stand, steps we can take
Resources
Measles (Robeola). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/measles/index.html. Updated January 28, 2019. Accessed January 31, 2019.
Influenza (Flu). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/flu/index.htm. Updated January 25, 2019. Accessed January 31, 2019.
Resources
Measles (Robeola). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/measles/index.html. Updated January 28, 2019. Accessed January 31, 2019.
Influenza (Flu). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/flu/index.htm. Updated January 25, 2019. Accessed January 31, 2019.
Resources
Measles (Robeola). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/measles/index.html. Updated January 28, 2019. Accessed January 31, 2019.
Influenza (Flu). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/flu/index.htm. Updated January 25, 2019. Accessed January 31, 2019.
Click for Credit: Missed HIV screening opps; aspirin & preeclampsia; more
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Flu activity ticks up for second week in a row
Influenza activity increased for a second straight week after a 2-week drop and by one measure has topped the high reached in late December, according to the Centers for Disease Control and Prevention.
For the week ending Jan. 26, 2019, there were 16 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, compared with 12 states during the week ending Dec. 29. With another seven states at levels 8 and 9, that makes 23 in the high range for the week ending Jan. 26, again putting it above the 19 reported for Dec. 29, the CDC’s influenza division reported Feb. 1.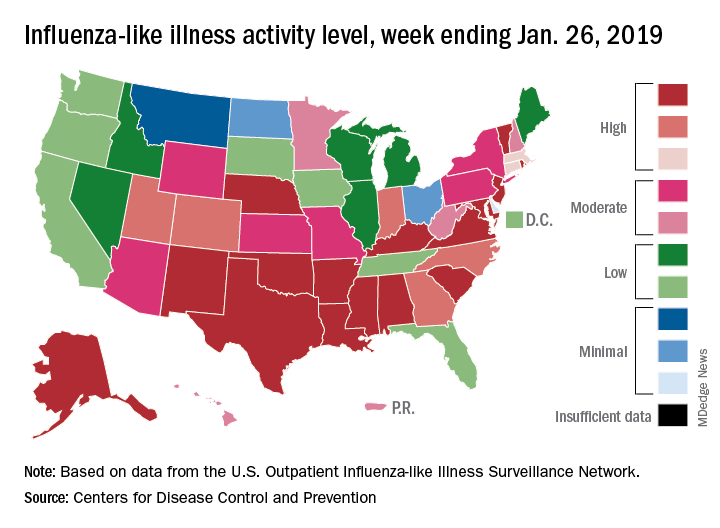
By another measure, however, that December peak in activity remains the seasonal high. The proportion of outpatient visits for ILI that week was 4.0%, compared with the 3.8% reported for Jan. 26. That’s up from 3.3% the week before and 3.1% the week before that, which in turn was the second week of a 2-week decline in activity in early January, CDC data show.
Two flu-related pediatric deaths were reported during the week ending Jan. 26, but both occurred the previous week. For the 2018-2019 flu season so far, a total of 24 pediatric flu deaths have been reported, the CDC said. At the same point in the 2017-2018 flu season, there had been 84 such deaths, according to the CDC’s Influenza-Associated Pediatric Mortality Surveillance System.
There were 143 overall flu-related deaths during the week of Jan. 19, which is the most recent week available. That is down from 189 the week before, but the Jan. 19 reporting is only 75% complete, data from the National Center for Health Statistics show.
Influenza activity increased for a second straight week after a 2-week drop and by one measure has topped the high reached in late December, according to the Centers for Disease Control and Prevention.
For the week ending Jan. 26, 2019, there were 16 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, compared with 12 states during the week ending Dec. 29. With another seven states at levels 8 and 9, that makes 23 in the high range for the week ending Jan. 26, again putting it above the 19 reported for Dec. 29, the CDC’s influenza division reported Feb. 1.
By another measure, however, that December peak in activity remains the seasonal high. The proportion of outpatient visits for ILI that week was 4.0%, compared with the 3.8% reported for Jan. 26. That’s up from 3.3% the week before and 3.1% the week before that, which in turn was the second week of a 2-week decline in activity in early January, CDC data show.
Two flu-related pediatric deaths were reported during the week ending Jan. 26, but both occurred the previous week. For the 2018-2019 flu season so far, a total of 24 pediatric flu deaths have been reported, the CDC said. At the same point in the 2017-2018 flu season, there had been 84 such deaths, according to the CDC’s Influenza-Associated Pediatric Mortality Surveillance System.
There were 143 overall flu-related deaths during the week of Jan. 19, which is the most recent week available. That is down from 189 the week before, but the Jan. 19 reporting is only 75% complete, data from the National Center for Health Statistics show.
Influenza activity increased for a second straight week after a 2-week drop and by one measure has topped the high reached in late December, according to the Centers for Disease Control and Prevention.
For the week ending Jan. 26, 2019, there were 16 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, compared with 12 states during the week ending Dec. 29. With another seven states at levels 8 and 9, that makes 23 in the high range for the week ending Jan. 26, again putting it above the 19 reported for Dec. 29, the CDC’s influenza division reported Feb. 1.
By another measure, however, that December peak in activity remains the seasonal high. The proportion of outpatient visits for ILI that week was 4.0%, compared with the 3.8% reported for Jan. 26. That’s up from 3.3% the week before and 3.1% the week before that, which in turn was the second week of a 2-week decline in activity in early January, CDC data show.
Two flu-related pediatric deaths were reported during the week ending Jan. 26, but both occurred the previous week. For the 2018-2019 flu season so far, a total of 24 pediatric flu deaths have been reported, the CDC said. At the same point in the 2017-2018 flu season, there had been 84 such deaths, according to the CDC’s Influenza-Associated Pediatric Mortality Surveillance System.
There were 143 overall flu-related deaths during the week of Jan. 19, which is the most recent week available. That is down from 189 the week before, but the Jan. 19 reporting is only 75% complete, data from the National Center for Health Statistics show.
Penicillin allergy
A 75-year-old man presents with fever, chills, and facial pain. He had an upper respiratory infection 3 weeks ago and has had persistent sinus drainage since. He has tried nasal irrigation and nasal steroids without improvement.
Over the past 5 days, he has had thicker postnasal drip, the development of facial pain, and today fevers as high as 102 degrees. He has a history of giant cell arteritis, for which he takes 30 mg of prednisone daily; coronary artery disease; and hypertension. He has a penicillin allergy (rash on chest, back, and arms 25 years ago). Exam reveals temperature of 101.5 and tenderness over left maxillary sinus.
What treatment do you recommend?
A. Amoxicillin/clavulanate.
B. Cefpodoxime.
C. Levofloxacin.
D. Trimethoprim/sulfamethoxazole.
I think cefpodoxime is probably the best of these choices to treat sinusitis in this patient. Choosing amoxicillin /clavulanate is an option only if you could give the patient a test dose in a controlled setting. I think giving this patient levofloxacin poses greater risk than a penicillin rechallenge. This patient is elderly and on prednisone, both of which increase his risk of tendon rupture if given a quinolone. Also, the Food and Drug Administration released a warning recently regarding increased risk of aortic disease in patients with cardiovascular risk factors who receive fluoroquinolones.1
Merin Kuruvilla, MD, and colleagues described oral amoxicillin challenge for patients with a history of low-risk penicillin allergy (described as benign rash, benign somatic symptoms, or unknown history with penicillin exposure more than 12 months prior).2 The study was done in a single allergy practice where 38 of 50 patients with penicillin allergy histories qualified for the study. Of the 38 eligible patients, 20 consented to oral rechallenge in clinic, and none of them developed immediate or delayed hypersensitivity reactions.
Melissa Iammatteo, MD, et al. studied 155 patients with a history of non–life-threatening penicillin reactions.3 Study participants received placebo followed by a two-step graded challenge to amoxicillin. No reaction occurred in 77% of patients, while 20% of patients had nonallergic reactions, which were equal between placebo and amoxicillin. Only 2.6 % had allergic reactions, all of which were classified as mild.
Reported penicillin allergy occurs in about 10% of community patients, but 90% of these patients can tolerate penicillins.4 Patients reporting a penicillin allergy have increased risk for drug resistance and prolonged hospital stays.5
The American Academy of Allergy, Asthma & Immunology recommended more widespread and routine performance of penicillin allergy testing in patients with a history of allergy to penicillin or other beta-lactam antibiotics.6 Patients who have penicillin allergy histories are more likely to receive drugs, such as clindamycin or a fluoroquinolone, that may carry much greater risks than a beta-lactam antibiotic. It also leads to more vancomycin use, which increases risk of vancomycin resistance.
Allergic reactions to cephalosporins are very infrequent in patients with a penicillin allergy. Eric Macy, MD, and colleagues studied all members of Kaiser Permanente Southern California health plan who had received cephalosporins over a 2-year period.7 More than 275,000 courses were given to patients with penicillin allergy, with only about 1% having an allergic reaction and only three cases of anaphylaxis.
Pearl: Most patients with a history of penicillin allergy will tolerate penicillins and cephalosporins. Penicillin allergy testing should be done to assess if they have a penicillin allergy, and in low-risk patients (patients who do not recall the allergy or had a maculopapular rash), consideration for oral rechallenge in a controlled setting may be an option. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Food and Drug Administration. “FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients,” 2018 Dec 20.
2. Ann Allergy Asthma Immunol. 2018 Nov;121(5):627-8.
3. J Allergy Clin Immunol Pract. 2019 Jan;7(1):236-43.
4. Immunol Allergy Clin North Am. 2017 Nov;37(4):643-62.
5. J Allergy Clin Immunol. 2014 Mar;133(3):790-6.
6. J Allergy Clin Immunol Pract. 2017 Mar - Apr;5(2):333-4.
7. J Allergy Clin Immunol. 2015 Mar;135(3):745-52.e5.
A 75-year-old man presents with fever, chills, and facial pain. He had an upper respiratory infection 3 weeks ago and has had persistent sinus drainage since. He has tried nasal irrigation and nasal steroids without improvement.
Over the past 5 days, he has had thicker postnasal drip, the development of facial pain, and today fevers as high as 102 degrees. He has a history of giant cell arteritis, for which he takes 30 mg of prednisone daily; coronary artery disease; and hypertension. He has a penicillin allergy (rash on chest, back, and arms 25 years ago). Exam reveals temperature of 101.5 and tenderness over left maxillary sinus.
What treatment do you recommend?
A. Amoxicillin/clavulanate.
B. Cefpodoxime.
C. Levofloxacin.
D. Trimethoprim/sulfamethoxazole.
I think cefpodoxime is probably the best of these choices to treat sinusitis in this patient. Choosing amoxicillin /clavulanate is an option only if you could give the patient a test dose in a controlled setting. I think giving this patient levofloxacin poses greater risk than a penicillin rechallenge. This patient is elderly and on prednisone, both of which increase his risk of tendon rupture if given a quinolone. Also, the Food and Drug Administration released a warning recently regarding increased risk of aortic disease in patients with cardiovascular risk factors who receive fluoroquinolones.1
Merin Kuruvilla, MD, and colleagues described oral amoxicillin challenge for patients with a history of low-risk penicillin allergy (described as benign rash, benign somatic symptoms, or unknown history with penicillin exposure more than 12 months prior).2 The study was done in a single allergy practice where 38 of 50 patients with penicillin allergy histories qualified for the study. Of the 38 eligible patients, 20 consented to oral rechallenge in clinic, and none of them developed immediate or delayed hypersensitivity reactions.
Melissa Iammatteo, MD, et al. studied 155 patients with a history of non–life-threatening penicillin reactions.3 Study participants received placebo followed by a two-step graded challenge to amoxicillin. No reaction occurred in 77% of patients, while 20% of patients had nonallergic reactions, which were equal between placebo and amoxicillin. Only 2.6 % had allergic reactions, all of which were classified as mild.
Reported penicillin allergy occurs in about 10% of community patients, but 90% of these patients can tolerate penicillins.4 Patients reporting a penicillin allergy have increased risk for drug resistance and prolonged hospital stays.5
The American Academy of Allergy, Asthma & Immunology recommended more widespread and routine performance of penicillin allergy testing in patients with a history of allergy to penicillin or other beta-lactam antibiotics.6 Patients who have penicillin allergy histories are more likely to receive drugs, such as clindamycin or a fluoroquinolone, that may carry much greater risks than a beta-lactam antibiotic. It also leads to more vancomycin use, which increases risk of vancomycin resistance.
Allergic reactions to cephalosporins are very infrequent in patients with a penicillin allergy. Eric Macy, MD, and colleagues studied all members of Kaiser Permanente Southern California health plan who had received cephalosporins over a 2-year period.7 More than 275,000 courses were given to patients with penicillin allergy, with only about 1% having an allergic reaction and only three cases of anaphylaxis.
Pearl: Most patients with a history of penicillin allergy will tolerate penicillins and cephalosporins. Penicillin allergy testing should be done to assess if they have a penicillin allergy, and in low-risk patients (patients who do not recall the allergy or had a maculopapular rash), consideration for oral rechallenge in a controlled setting may be an option. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Food and Drug Administration. “FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients,” 2018 Dec 20.
2. Ann Allergy Asthma Immunol. 2018 Nov;121(5):627-8.
3. J Allergy Clin Immunol Pract. 2019 Jan;7(1):236-43.
4. Immunol Allergy Clin North Am. 2017 Nov;37(4):643-62.
5. J Allergy Clin Immunol. 2014 Mar;133(3):790-6.
6. J Allergy Clin Immunol Pract. 2017 Mar - Apr;5(2):333-4.
7. J Allergy Clin Immunol. 2015 Mar;135(3):745-52.e5.
A 75-year-old man presents with fever, chills, and facial pain. He had an upper respiratory infection 3 weeks ago and has had persistent sinus drainage since. He has tried nasal irrigation and nasal steroids without improvement.
Over the past 5 days, he has had thicker postnasal drip, the development of facial pain, and today fevers as high as 102 degrees. He has a history of giant cell arteritis, for which he takes 30 mg of prednisone daily; coronary artery disease; and hypertension. He has a penicillin allergy (rash on chest, back, and arms 25 years ago). Exam reveals temperature of 101.5 and tenderness over left maxillary sinus.
What treatment do you recommend?
A. Amoxicillin/clavulanate.
B. Cefpodoxime.
C. Levofloxacin.
D. Trimethoprim/sulfamethoxazole.
I think cefpodoxime is probably the best of these choices to treat sinusitis in this patient. Choosing amoxicillin /clavulanate is an option only if you could give the patient a test dose in a controlled setting. I think giving this patient levofloxacin poses greater risk than a penicillin rechallenge. This patient is elderly and on prednisone, both of which increase his risk of tendon rupture if given a quinolone. Also, the Food and Drug Administration released a warning recently regarding increased risk of aortic disease in patients with cardiovascular risk factors who receive fluoroquinolones.1
Merin Kuruvilla, MD, and colleagues described oral amoxicillin challenge for patients with a history of low-risk penicillin allergy (described as benign rash, benign somatic symptoms, or unknown history with penicillin exposure more than 12 months prior).2 The study was done in a single allergy practice where 38 of 50 patients with penicillin allergy histories qualified for the study. Of the 38 eligible patients, 20 consented to oral rechallenge in clinic, and none of them developed immediate or delayed hypersensitivity reactions.
Melissa Iammatteo, MD, et al. studied 155 patients with a history of non–life-threatening penicillin reactions.3 Study participants received placebo followed by a two-step graded challenge to amoxicillin. No reaction occurred in 77% of patients, while 20% of patients had nonallergic reactions, which were equal between placebo and amoxicillin. Only 2.6 % had allergic reactions, all of which were classified as mild.
Reported penicillin allergy occurs in about 10% of community patients, but 90% of these patients can tolerate penicillins.4 Patients reporting a penicillin allergy have increased risk for drug resistance and prolonged hospital stays.5
The American Academy of Allergy, Asthma & Immunology recommended more widespread and routine performance of penicillin allergy testing in patients with a history of allergy to penicillin or other beta-lactam antibiotics.6 Patients who have penicillin allergy histories are more likely to receive drugs, such as clindamycin or a fluoroquinolone, that may carry much greater risks than a beta-lactam antibiotic. It also leads to more vancomycin use, which increases risk of vancomycin resistance.
Allergic reactions to cephalosporins are very infrequent in patients with a penicillin allergy. Eric Macy, MD, and colleagues studied all members of Kaiser Permanente Southern California health plan who had received cephalosporins over a 2-year period.7 More than 275,000 courses were given to patients with penicillin allergy, with only about 1% having an allergic reaction and only three cases of anaphylaxis.
Pearl: Most patients with a history of penicillin allergy will tolerate penicillins and cephalosporins. Penicillin allergy testing should be done to assess if they have a penicillin allergy, and in low-risk patients (patients who do not recall the allergy or had a maculopapular rash), consideration for oral rechallenge in a controlled setting may be an option. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Food and Drug Administration. “FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients,” 2018 Dec 20.
2. Ann Allergy Asthma Immunol. 2018 Nov;121(5):627-8.
3. J Allergy Clin Immunol Pract. 2019 Jan;7(1):236-43.
4. Immunol Allergy Clin North Am. 2017 Nov;37(4):643-62.
5. J Allergy Clin Immunol. 2014 Mar;133(3):790-6.
6. J Allergy Clin Immunol Pract. 2017 Mar - Apr;5(2):333-4.
7. J Allergy Clin Immunol. 2015 Mar;135(3):745-52.e5.
Breast augmentation surgery: Clinical considerations
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12 Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12 Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12 Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
KEY POINTS
- Nearly 300,000 breast augmentation surgeries are performed annually, making this the second most common aesthetic procedure in US women (after liposuction).
- Today, silicone gel implants dominate the world market, and in the United States, approximately 60% of implants contain silicone gel filler.
- Capsular contracture is the most common complication of breast augmentation, typically presenting within the first postoperative year and with increasing risk over time. It occurs with both silicone and saline breast implants.
- Numerous studies have demonstrated the safety of silicone breast implants with regard to autoimmune disease incidence. However, the risk of associated anaplastic large-cell lymphoma must be discussed at every consultation, and confirmed cases should be reported to a national registry.
Repeating blood cultures after initial bacteremia: When and how often?
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527