User login
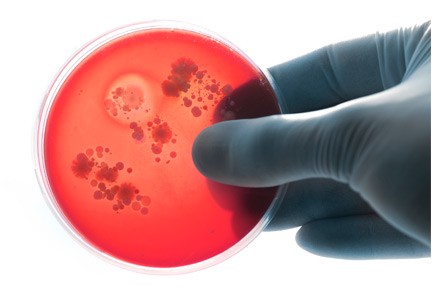
Follow-up blood cultures are often needed after bacteremia
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Procalcitonin, Will It Guide Us?
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Residential HCV program improves veterans’ diagnosis and care
Integrating comprehensive and collaborative hepatitis C virus (HCV) care within a Veterans Affairs residential treatment program can substantially increase diagnosis and treatment of HCV-infected veterans with substance use disorder (SUD), according to the results of an evaluation study for the period from December 2014 to April 2018.
A total of 97.5% (582/597) of patient admissions to the program were screened for HCV infection, and 12.7% (74/582) of the cases were confirmed to be HCV positive. All of the positive cases were sent to an infectious disease (ID) clinic for further evaluation and, if appropriate, to begin HCV pharmacotherapy, according to the report, published in the Journal of Substance Abuse Treatment.
Of the HCV-positive cases, 78.4% (58/74) received pharmacotherapy, with a sustained virologic response rate of 82.8% (48/58), wrote Mary Jane Burton, MD, of the G.V. (Sonny) Montgomery VA Medical Center, Jackson, Miss., and her colleagues.
As part of the program, all veterans admitted to the SUD residential program were offered screening for HCV. Veterans with negative screening results received education about how to remain HCV negative via handouts and veterans who screened positive received brief supportive counseling and were referred to the ID clinic via a consult. Veterans confirmed to have chronic HCV infection receive education and evaluation in the HCV clinic while they attend the residential SUD program. Treatment for HCV is instituted as early as feasible and prescribing is in accordance with VA guidelines (Department of Veterans Affairs, 2018), with the goal of initiating pharmacotherapy treatment for HCV while the veteran is still in the residential program, according to the researchers.
Following discharge from the program, veterans on HCV treatment are scheduled for follow-up every 2 weeks in the HCV treatment clinic for the remainder of their pharmacotherapy, the researchers added.
Patient-level barriers to HCV treatment among the SUD population include reduced health literacy, low health care utilization, comorbid mental health conditions, and poor social support, according to the literature. Because multidisciplinary approaches to HCV treatment that mitigate these barriers have been shown to increase treatment uptake among these patients, the VA program was initiated, the researchers stated. Dr. Burton and her colleagues reported that 18.9% (14/74) of the HCV-positive cases were newly diagnosed and would have likely gone undetected without this program (J Substance Abuse Treatment. 2019;98:9-14).
“We have demonstrated that integrating a comprehensive HCV screening, education, referral, and treatment program within residential SUD treatment is feasible and effective in diagnosing previously unrecognized HCV infections, transitioning veterans into HCV care, and promoting treatment initiation,” the researchers concluded.
The Department of Veterans Affairs and the VA Center for Innovation supported the study. Dr. Burton reported research support from Merck Sharpe & Dohme.
Integrating comprehensive and collaborative hepatitis C virus (HCV) care within a Veterans Affairs residential treatment program can substantially increase diagnosis and treatment of HCV-infected veterans with substance use disorder (SUD), according to the results of an evaluation study for the period from December 2014 to April 2018.
A total of 97.5% (582/597) of patient admissions to the program were screened for HCV infection, and 12.7% (74/582) of the cases were confirmed to be HCV positive. All of the positive cases were sent to an infectious disease (ID) clinic for further evaluation and, if appropriate, to begin HCV pharmacotherapy, according to the report, published in the Journal of Substance Abuse Treatment.
Of the HCV-positive cases, 78.4% (58/74) received pharmacotherapy, with a sustained virologic response rate of 82.8% (48/58), wrote Mary Jane Burton, MD, of the G.V. (Sonny) Montgomery VA Medical Center, Jackson, Miss., and her colleagues.
As part of the program, all veterans admitted to the SUD residential program were offered screening for HCV. Veterans with negative screening results received education about how to remain HCV negative via handouts and veterans who screened positive received brief supportive counseling and were referred to the ID clinic via a consult. Veterans confirmed to have chronic HCV infection receive education and evaluation in the HCV clinic while they attend the residential SUD program. Treatment for HCV is instituted as early as feasible and prescribing is in accordance with VA guidelines (Department of Veterans Affairs, 2018), with the goal of initiating pharmacotherapy treatment for HCV while the veteran is still in the residential program, according to the researchers.
Following discharge from the program, veterans on HCV treatment are scheduled for follow-up every 2 weeks in the HCV treatment clinic for the remainder of their pharmacotherapy, the researchers added.
Patient-level barriers to HCV treatment among the SUD population include reduced health literacy, low health care utilization, comorbid mental health conditions, and poor social support, according to the literature. Because multidisciplinary approaches to HCV treatment that mitigate these barriers have been shown to increase treatment uptake among these patients, the VA program was initiated, the researchers stated. Dr. Burton and her colleagues reported that 18.9% (14/74) of the HCV-positive cases were newly diagnosed and would have likely gone undetected without this program (J Substance Abuse Treatment. 2019;98:9-14).
“We have demonstrated that integrating a comprehensive HCV screening, education, referral, and treatment program within residential SUD treatment is feasible and effective in diagnosing previously unrecognized HCV infections, transitioning veterans into HCV care, and promoting treatment initiation,” the researchers concluded.
The Department of Veterans Affairs and the VA Center for Innovation supported the study. Dr. Burton reported research support from Merck Sharpe & Dohme.
Integrating comprehensive and collaborative hepatitis C virus (HCV) care within a Veterans Affairs residential treatment program can substantially increase diagnosis and treatment of HCV-infected veterans with substance use disorder (SUD), according to the results of an evaluation study for the period from December 2014 to April 2018.
A total of 97.5% (582/597) of patient admissions to the program were screened for HCV infection, and 12.7% (74/582) of the cases were confirmed to be HCV positive. All of the positive cases were sent to an infectious disease (ID) clinic for further evaluation and, if appropriate, to begin HCV pharmacotherapy, according to the report, published in the Journal of Substance Abuse Treatment.
Of the HCV-positive cases, 78.4% (58/74) received pharmacotherapy, with a sustained virologic response rate of 82.8% (48/58), wrote Mary Jane Burton, MD, of the G.V. (Sonny) Montgomery VA Medical Center, Jackson, Miss., and her colleagues.
As part of the program, all veterans admitted to the SUD residential program were offered screening for HCV. Veterans with negative screening results received education about how to remain HCV negative via handouts and veterans who screened positive received brief supportive counseling and were referred to the ID clinic via a consult. Veterans confirmed to have chronic HCV infection receive education and evaluation in the HCV clinic while they attend the residential SUD program. Treatment for HCV is instituted as early as feasible and prescribing is in accordance with VA guidelines (Department of Veterans Affairs, 2018), with the goal of initiating pharmacotherapy treatment for HCV while the veteran is still in the residential program, according to the researchers.
Following discharge from the program, veterans on HCV treatment are scheduled for follow-up every 2 weeks in the HCV treatment clinic for the remainder of their pharmacotherapy, the researchers added.
Patient-level barriers to HCV treatment among the SUD population include reduced health literacy, low health care utilization, comorbid mental health conditions, and poor social support, according to the literature. Because multidisciplinary approaches to HCV treatment that mitigate these barriers have been shown to increase treatment uptake among these patients, the VA program was initiated, the researchers stated. Dr. Burton and her colleagues reported that 18.9% (14/74) of the HCV-positive cases were newly diagnosed and would have likely gone undetected without this program (J Substance Abuse Treatment. 2019;98:9-14).
“We have demonstrated that integrating a comprehensive HCV screening, education, referral, and treatment program within residential SUD treatment is feasible and effective in diagnosing previously unrecognized HCV infections, transitioning veterans into HCV care, and promoting treatment initiation,” the researchers concluded.
The Department of Veterans Affairs and the VA Center for Innovation supported the study. Dr. Burton reported research support from Merck Sharpe & Dohme.
FROM THE JOURNAL OF SUBSTANCE ABUSE TREATMENT
Antibiotic use in dermatology declining, with one exception
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
FROM JAMA DERMATOLOGY
Key clinical point: Antibiotic prescriptions by dermatologists have decreased since 2008.
Major finding: Between 2008 and 2016, antibiotic prescriptions by dermatologists dropped by 36.6%.
Study details: Cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists from 2008 to 2016.
Disclosures: The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. The authors had no disclosures.
Source: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Vulvar disease treatment tips: From lice to lichen sclerosus
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS
2019 ID update for dermatologists: Ticks are the “ride of choice” for arthropods
ORLANDO – New tricks from ticks, near-zero Zika, and the perils of personal grooming: Dermatologists have a lot to think about along the infectious disease spectrum in 2019, according to Justin Finch, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Anaphylaxis from alpha-gal syndrome is on the rise, caused in part by the geographic spread of the Lone Star tick. Beginning in 2006, isolated cases of an anaphylactic reaction to cetuximab, the epidermal growth factor receptor antagonist used to treat certain cancers, began to be seen in a curious geographic distribution. “The anaphylaxis cases were restricted to the southeastern United States, the home of the Lone Star tick,” said Dr. Finch, of the department of dermatology at the University of Connecticut, Farmington.
With some detective work, physicians and epidemiologists eventually determined that patients were reacting to an oligosaccharide called galactose-alpha–1,3-galactose (alpha-gal) found in cetuximab. This protein is also found in the meat of nonprimate mammals; individuals in the southeastern United States, where the Lone Star tick is endemic, had been sensitized via exposure to alpha-gal from Lone Star tick bites.
“Alpha-gal syndrome is on the rise,” said Dr. Finch, driven by the increased spread of this tick. Individuals who are sensitized develop delayed anaphylaxis 2-7 hours after ingesting red meat such as beef, pork, or lamb. “Ask about it,” said Dr. Finch, in patients who develop urticaria, dyspnea, angioedema, or hypotension without a clear offender. Because of the delay between allergen ingestion and anaphylaxis, it can be hard to connect the dots.
A number of drugs other than cetuximab contain alpha-gal, so patients must also be told to avoid these agents, said Dr. Finch, who noted that alpha-gal syndrome isn’t the only emerging culprit for tick-borne diseases. “The tick is the ride of choice for arthropod-borne diseases in the U.S.,” he added. “Year after year, tick-borne diseases top mosquito-borne diseases in the U.S.” Zika’s explosion in 2016 made that year the exception to the rule.
Now, Zika virus may be on the wane – the number of case reports have plummeted both in the United States and in Central and South America this past year – but it hasn’t completely gone away. “It looks like it fell off all the maps,” but the virus is still present at low levels, he said.
When Zika virus is symptomatic, there’s often a nonspecific maculopapular rash. Critically, Dr. Finch said, “women with a rash are four times as likely to have adverse congenital outcomes. This is the important point for us to take home as dermatologists. ... It’s really important to have a high index of suspicion and to screen these women as they are coming into our clinic.”
Turning back to ticks, Lyme disease continues to be a problem in endemic areas in the Northeast, the mid-Atlantic region, and the Midwest, said Dr. Finch, so it’s a perennial on the differential diagnosis for dermatologists.
An Asian tick new to North America was seen for the first time in New Jersey in the summer of 2017. The Asian longhorned tick carries a phlebovirus that causes severe fever with thrombocytopenia syndrome, a disease with a 15% fatality rate. The reservoir host of this virus in Asia isn’t known, said Dr. Finch, adding that no cases of the virus have yet been seen in the United States. As of November 2018, according to the Centers for Disease Control and Prevention, the tick had been found in nine states (Arkansas, Connecticut, Maryland, North Carolina, New Jersey, New York, Pennsylvania, Virginia, and West Virginia).
“What’s not on the rise? Pubic lice. We are destroying their natural habitat!” said Dr. Finch, citing surveys about personal grooming that show that more than 90% of women remove at least some of their pubic hair. Most college campuses are currently reporting essentially no cases of pubic lice, he noted.
However, the same personal grooming practices may be contributing to increases in molluscum contagiosum, herpes simplex virus, some strains of human papillomavirus, and cutaneous Streptococcus pyogenes infections, he said.
Another STI has had a resurgence in geographic pockets around the nation and among specific populations, said Dr. Finch. Syphilis is on the rise among gay and bisexual men and African Americans. Known as the “great imitator,” syphilis should be on the differential for dermatologists when the clinical picture isn’t quite adding up. “Think of this, and screen with an RPR [rapid plasma reagin],” he said.
Finally, an old enemy is back: A total of 11 measles outbreaks were reported in 2018. “We need to know about measles because of the complications,” said Dr. Finch. Even years later, such dire sequelae as subacute sclerosing panencephalitis can crop up, he added.
After a 2-week incubation period, measles begins with a fever and cough, congestion, and conjunctivitis. The rash begins on the head and spreads inferiorly by day 3. As the rash blooms, the classic morbilliform eruption becomes apparent. A biopsy of affected skin will be nonspecific; measles is diagnosed with a nasopharyngeal culture and serologic assay. Dr. Finch pointed out that dermatologists are unlikely to see measles in its earliest stages because their expertise will be called on only after it becomes clear that the patient is not experiencing just a mild illness with a viral exanthem.
When there’s suspicion for measles, a full-body skin exam is needed. “Koplik’s spots – the gray white papules on the buccal mucosa – are not pathognomonic in themselves, but in the clinical scenario of a person with measles” they can help the dermatologist make a definitive call, he said.
Vitamin A can be given to a patient with active measles, but prevention via immunization at age 12 months and 5 years is the only way to stop the disease, Dr. Finch noted.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – New tricks from ticks, near-zero Zika, and the perils of personal grooming: Dermatologists have a lot to think about along the infectious disease spectrum in 2019, according to Justin Finch, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Anaphylaxis from alpha-gal syndrome is on the rise, caused in part by the geographic spread of the Lone Star tick. Beginning in 2006, isolated cases of an anaphylactic reaction to cetuximab, the epidermal growth factor receptor antagonist used to treat certain cancers, began to be seen in a curious geographic distribution. “The anaphylaxis cases were restricted to the southeastern United States, the home of the Lone Star tick,” said Dr. Finch, of the department of dermatology at the University of Connecticut, Farmington.
With some detective work, physicians and epidemiologists eventually determined that patients were reacting to an oligosaccharide called galactose-alpha–1,3-galactose (alpha-gal) found in cetuximab. This protein is also found in the meat of nonprimate mammals; individuals in the southeastern United States, where the Lone Star tick is endemic, had been sensitized via exposure to alpha-gal from Lone Star tick bites.
“Alpha-gal syndrome is on the rise,” said Dr. Finch, driven by the increased spread of this tick. Individuals who are sensitized develop delayed anaphylaxis 2-7 hours after ingesting red meat such as beef, pork, or lamb. “Ask about it,” said Dr. Finch, in patients who develop urticaria, dyspnea, angioedema, or hypotension without a clear offender. Because of the delay between allergen ingestion and anaphylaxis, it can be hard to connect the dots.
A number of drugs other than cetuximab contain alpha-gal, so patients must also be told to avoid these agents, said Dr. Finch, who noted that alpha-gal syndrome isn’t the only emerging culprit for tick-borne diseases. “The tick is the ride of choice for arthropod-borne diseases in the U.S.,” he added. “Year after year, tick-borne diseases top mosquito-borne diseases in the U.S.” Zika’s explosion in 2016 made that year the exception to the rule.
Now, Zika virus may be on the wane – the number of case reports have plummeted both in the United States and in Central and South America this past year – but it hasn’t completely gone away. “It looks like it fell off all the maps,” but the virus is still present at low levels, he said.
When Zika virus is symptomatic, there’s often a nonspecific maculopapular rash. Critically, Dr. Finch said, “women with a rash are four times as likely to have adverse congenital outcomes. This is the important point for us to take home as dermatologists. ... It’s really important to have a high index of suspicion and to screen these women as they are coming into our clinic.”
Turning back to ticks, Lyme disease continues to be a problem in endemic areas in the Northeast, the mid-Atlantic region, and the Midwest, said Dr. Finch, so it’s a perennial on the differential diagnosis for dermatologists.
An Asian tick new to North America was seen for the first time in New Jersey in the summer of 2017. The Asian longhorned tick carries a phlebovirus that causes severe fever with thrombocytopenia syndrome, a disease with a 15% fatality rate. The reservoir host of this virus in Asia isn’t known, said Dr. Finch, adding that no cases of the virus have yet been seen in the United States. As of November 2018, according to the Centers for Disease Control and Prevention, the tick had been found in nine states (Arkansas, Connecticut, Maryland, North Carolina, New Jersey, New York, Pennsylvania, Virginia, and West Virginia).
“What’s not on the rise? Pubic lice. We are destroying their natural habitat!” said Dr. Finch, citing surveys about personal grooming that show that more than 90% of women remove at least some of their pubic hair. Most college campuses are currently reporting essentially no cases of pubic lice, he noted.
However, the same personal grooming practices may be contributing to increases in molluscum contagiosum, herpes simplex virus, some strains of human papillomavirus, and cutaneous Streptococcus pyogenes infections, he said.
Another STI has had a resurgence in geographic pockets around the nation and among specific populations, said Dr. Finch. Syphilis is on the rise among gay and bisexual men and African Americans. Known as the “great imitator,” syphilis should be on the differential for dermatologists when the clinical picture isn’t quite adding up. “Think of this, and screen with an RPR [rapid plasma reagin],” he said.
Finally, an old enemy is back: A total of 11 measles outbreaks were reported in 2018. “We need to know about measles because of the complications,” said Dr. Finch. Even years later, such dire sequelae as subacute sclerosing panencephalitis can crop up, he added.
After a 2-week incubation period, measles begins with a fever and cough, congestion, and conjunctivitis. The rash begins on the head and spreads inferiorly by day 3. As the rash blooms, the classic morbilliform eruption becomes apparent. A biopsy of affected skin will be nonspecific; measles is diagnosed with a nasopharyngeal culture and serologic assay. Dr. Finch pointed out that dermatologists are unlikely to see measles in its earliest stages because their expertise will be called on only after it becomes clear that the patient is not experiencing just a mild illness with a viral exanthem.
When there’s suspicion for measles, a full-body skin exam is needed. “Koplik’s spots – the gray white papules on the buccal mucosa – are not pathognomonic in themselves, but in the clinical scenario of a person with measles” they can help the dermatologist make a definitive call, he said.
Vitamin A can be given to a patient with active measles, but prevention via immunization at age 12 months and 5 years is the only way to stop the disease, Dr. Finch noted.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – New tricks from ticks, near-zero Zika, and the perils of personal grooming: Dermatologists have a lot to think about along the infectious disease spectrum in 2019, according to Justin Finch, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Anaphylaxis from alpha-gal syndrome is on the rise, caused in part by the geographic spread of the Lone Star tick. Beginning in 2006, isolated cases of an anaphylactic reaction to cetuximab, the epidermal growth factor receptor antagonist used to treat certain cancers, began to be seen in a curious geographic distribution. “The anaphylaxis cases were restricted to the southeastern United States, the home of the Lone Star tick,” said Dr. Finch, of the department of dermatology at the University of Connecticut, Farmington.
With some detective work, physicians and epidemiologists eventually determined that patients were reacting to an oligosaccharide called galactose-alpha–1,3-galactose (alpha-gal) found in cetuximab. This protein is also found in the meat of nonprimate mammals; individuals in the southeastern United States, where the Lone Star tick is endemic, had been sensitized via exposure to alpha-gal from Lone Star tick bites.
“Alpha-gal syndrome is on the rise,” said Dr. Finch, driven by the increased spread of this tick. Individuals who are sensitized develop delayed anaphylaxis 2-7 hours after ingesting red meat such as beef, pork, or lamb. “Ask about it,” said Dr. Finch, in patients who develop urticaria, dyspnea, angioedema, or hypotension without a clear offender. Because of the delay between allergen ingestion and anaphylaxis, it can be hard to connect the dots.
A number of drugs other than cetuximab contain alpha-gal, so patients must also be told to avoid these agents, said Dr. Finch, who noted that alpha-gal syndrome isn’t the only emerging culprit for tick-borne diseases. “The tick is the ride of choice for arthropod-borne diseases in the U.S.,” he added. “Year after year, tick-borne diseases top mosquito-borne diseases in the U.S.” Zika’s explosion in 2016 made that year the exception to the rule.
Now, Zika virus may be on the wane – the number of case reports have plummeted both in the United States and in Central and South America this past year – but it hasn’t completely gone away. “It looks like it fell off all the maps,” but the virus is still present at low levels, he said.
When Zika virus is symptomatic, there’s often a nonspecific maculopapular rash. Critically, Dr. Finch said, “women with a rash are four times as likely to have adverse congenital outcomes. This is the important point for us to take home as dermatologists. ... It’s really important to have a high index of suspicion and to screen these women as they are coming into our clinic.”
Turning back to ticks, Lyme disease continues to be a problem in endemic areas in the Northeast, the mid-Atlantic region, and the Midwest, said Dr. Finch, so it’s a perennial on the differential diagnosis for dermatologists.
An Asian tick new to North America was seen for the first time in New Jersey in the summer of 2017. The Asian longhorned tick carries a phlebovirus that causes severe fever with thrombocytopenia syndrome, a disease with a 15% fatality rate. The reservoir host of this virus in Asia isn’t known, said Dr. Finch, adding that no cases of the virus have yet been seen in the United States. As of November 2018, according to the Centers for Disease Control and Prevention, the tick had been found in nine states (Arkansas, Connecticut, Maryland, North Carolina, New Jersey, New York, Pennsylvania, Virginia, and West Virginia).
“What’s not on the rise? Pubic lice. We are destroying their natural habitat!” said Dr. Finch, citing surveys about personal grooming that show that more than 90% of women remove at least some of their pubic hair. Most college campuses are currently reporting essentially no cases of pubic lice, he noted.
However, the same personal grooming practices may be contributing to increases in molluscum contagiosum, herpes simplex virus, some strains of human papillomavirus, and cutaneous Streptococcus pyogenes infections, he said.
Another STI has had a resurgence in geographic pockets around the nation and among specific populations, said Dr. Finch. Syphilis is on the rise among gay and bisexual men and African Americans. Known as the “great imitator,” syphilis should be on the differential for dermatologists when the clinical picture isn’t quite adding up. “Think of this, and screen with an RPR [rapid plasma reagin],” he said.
Finally, an old enemy is back: A total of 11 measles outbreaks were reported in 2018. “We need to know about measles because of the complications,” said Dr. Finch. Even years later, such dire sequelae as subacute sclerosing panencephalitis can crop up, he added.
After a 2-week incubation period, measles begins with a fever and cough, congestion, and conjunctivitis. The rash begins on the head and spreads inferiorly by day 3. As the rash blooms, the classic morbilliform eruption becomes apparent. A biopsy of affected skin will be nonspecific; measles is diagnosed with a nasopharyngeal culture and serologic assay. Dr. Finch pointed out that dermatologists are unlikely to see measles in its earliest stages because their expertise will be called on only after it becomes clear that the patient is not experiencing just a mild illness with a viral exanthem.
When there’s suspicion for measles, a full-body skin exam is needed. “Koplik’s spots – the gray white papules on the buccal mucosa – are not pathognomonic in themselves, but in the clinical scenario of a person with measles” they can help the dermatologist make a definitive call, he said.
Vitamin A can be given to a patient with active measles, but prevention via immunization at age 12 months and 5 years is the only way to stop the disease, Dr. Finch noted.
Dr. Finch reported that he has no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2019
FDA permits marketing of first M. genitalium diagnostic test
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
Flu activity increases after 2 weeks of declines
according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
Single-dose tafenoquine appears to prevent malaria relapse
Single-dose tafenoquine therapy safely reduces the risk of Plasmodium vivax relapse in patients with normal glucose-6-phosphate dehydrogenase (G6PD) activity, according to the results of two phase 3, double-blind, randomized controlled trials.
Findings from both studies were published in two separate reports in the New England Journal of Medicine.
In the first study, the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine in Vivax Elimination (DETECTIVE), the risk of P. vivax recurrence was approximately 70% lower with tafenoquine versus placebo, wrote Marcus V.G. Lacerda, MD, of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Brazil, and his colleagues.
The study included 522 patients with confirmed P. vivax infection from Peru, Brazil, Ethiopia, Cambodia, Thailand, and the Philippines. Patients received 3 days of chloroquine therapy (600 mg on days 1 and 2, and 300 mg on day 3) and were randomly assigned on a 2:1:1 basis to receive a single 300-mg dose of tafenoquine on day 1 or 2, primaquine once daily for 14 days, or placebo.
Since primaquine and tafenoquine can cause clinically significant hemolysis in individuals with G6PD deficiency, the study included only patients with normal G6PD activity.
In the intention-to-treat analysis, 62% of tafenoquine recipients were free from P. vivax recurrence (95% confidence interval [CI], 55%-69%) at 6 months, as were 70% of primaquine recipients (95% CI, 60%-77%) and 28% of placebo recipients (95% CI, 20%-37%). Compared with placebo, the reduction in risk of recurrence was 70% with tafenoquine (hazard ratio [HR], 0.30; 95% CI, 0.22-0.40; P less than .001) and 74% with primaquine.
Declines in hemoglobin levels were greatest in the tafenoquine group but were not associated with symptomatic anemia and resolved without intervention, the investigators wrote.
In addition to the quantitative G6PD test, the investigators also evaluated a qualitative test, which “failed to identify 16 patients most at risk for hemolysis,” they reported. “If tafenoquine use is expanded, adoption of reliable quantitative point-of-care G6PD tests will be needed; such tests are not currently available but are in development.”
In the second study, Global Assessment of Tafenoquine Hemolytic Risk (GATHER), Alejandro Llanos-Cuentas, MD, of Universidad Peruana Cayetano Heredia, Lima, Peru, and his colleagues enrolled 251 patients with confirmed P. vivax infection from Peru, Brazil, Columbia, Vietnam, and Thailand. They attempted to recruit women with moderate G6PD levels, but only one participant met this criterion – all others had normal G6PD activity.
Patients received 3-day course of chloroquine and were randomly assigned on a 2:1 basis to either tafenoquine or primaquine at the same doses as in the DETECTIVE trial.
At 6 months, 2% (95% CI, 1%-6%) of tafenoquine recipients and 1% (95% CI, 0.2%-6%) of primaquine recipients had decreased hemoglobin levels, but none consequently needed treatment. The medications also caused a similar degree and time course of hemoglobin decrease, the investigators noted.
A meta-analysis of GATHER and DETECTIVE confirmed that tafenoquine more often led to a decreased hemoglobin level (4% versus 1.5% with primaquine). Tafenoquine also did not meet prespecified criteria for noninferiority compared with primaquine, with respective 6-month recurrence rates of 67% versus 73%.
However, GATHER deployed “extensive” support to help patients adhere to the 15-day primaquine course, Dr. Llanos-Cuentas and his colleagues wrote. “Without such interventions, adherence to primaquine has been reported to be as low as 24% in Southeast Asia, with a corresponding attenuation of efficacy.”
GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having no conflicts of interest.
SOURCES: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
The studies show that tafenoquine reduces the risk of Plasmodium vivax recurrence in patients with quantitatively confirmed normal glucose-6-phosphate dehydrogenase (G6PD) activity, Nicholas J. White, FRS, wrote in an accompanying editorial.
But the need for this test and current prescribing restrictions will “limit the potential deployment of tafenoquine, at least in the immediate future,” he said. He praised the developers of tafenoquine “for persevering with this potentially valuable antimalarial drug, despite the difficulties,” but cautioned that it’s too soon to conclude that tafenoquine can be used safely and routinely on a large scale “and thus fulfill its promise as a radical improvement in the treatment of malaria.”
Currently, tafenoquine may not be used during pregnancy, lactation, or in patients younger than 16 years, Dr. White noted. Tafenoquine, like primaquine, causes dose-dependent hemolysis in patients with G6PD deficiency, but unlike primaquine, it is given as a single large dose. Hence, pretreatment quantitative G6PD testing is necessary. Point-of-care quantitative G6PD tests have been developed but await extensive field testing, Dr. White said.
Dr. White is with Mahidol University, Bangkok, Thailand, and University of Oxford, England. He reported having no financial disclosures. These comments are from his accompanying editorial ( N Engl J Med. 2019;380:285-6 ).
The studies show that tafenoquine reduces the risk of Plasmodium vivax recurrence in patients with quantitatively confirmed normal glucose-6-phosphate dehydrogenase (G6PD) activity, Nicholas J. White, FRS, wrote in an accompanying editorial.
But the need for this test and current prescribing restrictions will “limit the potential deployment of tafenoquine, at least in the immediate future,” he said. He praised the developers of tafenoquine “for persevering with this potentially valuable antimalarial drug, despite the difficulties,” but cautioned that it’s too soon to conclude that tafenoquine can be used safely and routinely on a large scale “and thus fulfill its promise as a radical improvement in the treatment of malaria.”
Currently, tafenoquine may not be used during pregnancy, lactation, or in patients younger than 16 years, Dr. White noted. Tafenoquine, like primaquine, causes dose-dependent hemolysis in patients with G6PD deficiency, but unlike primaquine, it is given as a single large dose. Hence, pretreatment quantitative G6PD testing is necessary. Point-of-care quantitative G6PD tests have been developed but await extensive field testing, Dr. White said.
Dr. White is with Mahidol University, Bangkok, Thailand, and University of Oxford, England. He reported having no financial disclosures. These comments are from his accompanying editorial ( N Engl J Med. 2019;380:285-6 ).
The studies show that tafenoquine reduces the risk of Plasmodium vivax recurrence in patients with quantitatively confirmed normal glucose-6-phosphate dehydrogenase (G6PD) activity, Nicholas J. White, FRS, wrote in an accompanying editorial.
But the need for this test and current prescribing restrictions will “limit the potential deployment of tafenoquine, at least in the immediate future,” he said. He praised the developers of tafenoquine “for persevering with this potentially valuable antimalarial drug, despite the difficulties,” but cautioned that it’s too soon to conclude that tafenoquine can be used safely and routinely on a large scale “and thus fulfill its promise as a radical improvement in the treatment of malaria.”
Currently, tafenoquine may not be used during pregnancy, lactation, or in patients younger than 16 years, Dr. White noted. Tafenoquine, like primaquine, causes dose-dependent hemolysis in patients with G6PD deficiency, but unlike primaquine, it is given as a single large dose. Hence, pretreatment quantitative G6PD testing is necessary. Point-of-care quantitative G6PD tests have been developed but await extensive field testing, Dr. White said.
Dr. White is with Mahidol University, Bangkok, Thailand, and University of Oxford, England. He reported having no financial disclosures. These comments are from his accompanying editorial ( N Engl J Med. 2019;380:285-6 ).
Single-dose tafenoquine therapy safely reduces the risk of Plasmodium vivax relapse in patients with normal glucose-6-phosphate dehydrogenase (G6PD) activity, according to the results of two phase 3, double-blind, randomized controlled trials.
Findings from both studies were published in two separate reports in the New England Journal of Medicine.
In the first study, the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine in Vivax Elimination (DETECTIVE), the risk of P. vivax recurrence was approximately 70% lower with tafenoquine versus placebo, wrote Marcus V.G. Lacerda, MD, of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Brazil, and his colleagues.
The study included 522 patients with confirmed P. vivax infection from Peru, Brazil, Ethiopia, Cambodia, Thailand, and the Philippines. Patients received 3 days of chloroquine therapy (600 mg on days 1 and 2, and 300 mg on day 3) and were randomly assigned on a 2:1:1 basis to receive a single 300-mg dose of tafenoquine on day 1 or 2, primaquine once daily for 14 days, or placebo.
Since primaquine and tafenoquine can cause clinically significant hemolysis in individuals with G6PD deficiency, the study included only patients with normal G6PD activity.
In the intention-to-treat analysis, 62% of tafenoquine recipients were free from P. vivax recurrence (95% confidence interval [CI], 55%-69%) at 6 months, as were 70% of primaquine recipients (95% CI, 60%-77%) and 28% of placebo recipients (95% CI, 20%-37%). Compared with placebo, the reduction in risk of recurrence was 70% with tafenoquine (hazard ratio [HR], 0.30; 95% CI, 0.22-0.40; P less than .001) and 74% with primaquine.
Declines in hemoglobin levels were greatest in the tafenoquine group but were not associated with symptomatic anemia and resolved without intervention, the investigators wrote.
In addition to the quantitative G6PD test, the investigators also evaluated a qualitative test, which “failed to identify 16 patients most at risk for hemolysis,” they reported. “If tafenoquine use is expanded, adoption of reliable quantitative point-of-care G6PD tests will be needed; such tests are not currently available but are in development.”
In the second study, Global Assessment of Tafenoquine Hemolytic Risk (GATHER), Alejandro Llanos-Cuentas, MD, of Universidad Peruana Cayetano Heredia, Lima, Peru, and his colleagues enrolled 251 patients with confirmed P. vivax infection from Peru, Brazil, Columbia, Vietnam, and Thailand. They attempted to recruit women with moderate G6PD levels, but only one participant met this criterion – all others had normal G6PD activity.
Patients received 3-day course of chloroquine and were randomly assigned on a 2:1 basis to either tafenoquine or primaquine at the same doses as in the DETECTIVE trial.
At 6 months, 2% (95% CI, 1%-6%) of tafenoquine recipients and 1% (95% CI, 0.2%-6%) of primaquine recipients had decreased hemoglobin levels, but none consequently needed treatment. The medications also caused a similar degree and time course of hemoglobin decrease, the investigators noted.
A meta-analysis of GATHER and DETECTIVE confirmed that tafenoquine more often led to a decreased hemoglobin level (4% versus 1.5% with primaquine). Tafenoquine also did not meet prespecified criteria for noninferiority compared with primaquine, with respective 6-month recurrence rates of 67% versus 73%.
However, GATHER deployed “extensive” support to help patients adhere to the 15-day primaquine course, Dr. Llanos-Cuentas and his colleagues wrote. “Without such interventions, adherence to primaquine has been reported to be as low as 24% in Southeast Asia, with a corresponding attenuation of efficacy.”
GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having no conflicts of interest.
SOURCES: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
Single-dose tafenoquine therapy safely reduces the risk of Plasmodium vivax relapse in patients with normal glucose-6-phosphate dehydrogenase (G6PD) activity, according to the results of two phase 3, double-blind, randomized controlled trials.
Findings from both studies were published in two separate reports in the New England Journal of Medicine.
In the first study, the Dose and Efficacy Trial Evaluating Chloroquine and Tafenoquine in Vivax Elimination (DETECTIVE), the risk of P. vivax recurrence was approximately 70% lower with tafenoquine versus placebo, wrote Marcus V.G. Lacerda, MD, of Fundação de Medicina Tropical Doutor Heitor Vieira Dourado in Manaus, Brazil, and his colleagues.
The study included 522 patients with confirmed P. vivax infection from Peru, Brazil, Ethiopia, Cambodia, Thailand, and the Philippines. Patients received 3 days of chloroquine therapy (600 mg on days 1 and 2, and 300 mg on day 3) and were randomly assigned on a 2:1:1 basis to receive a single 300-mg dose of tafenoquine on day 1 or 2, primaquine once daily for 14 days, or placebo.
Since primaquine and tafenoquine can cause clinically significant hemolysis in individuals with G6PD deficiency, the study included only patients with normal G6PD activity.
In the intention-to-treat analysis, 62% of tafenoquine recipients were free from P. vivax recurrence (95% confidence interval [CI], 55%-69%) at 6 months, as were 70% of primaquine recipients (95% CI, 60%-77%) and 28% of placebo recipients (95% CI, 20%-37%). Compared with placebo, the reduction in risk of recurrence was 70% with tafenoquine (hazard ratio [HR], 0.30; 95% CI, 0.22-0.40; P less than .001) and 74% with primaquine.
Declines in hemoglobin levels were greatest in the tafenoquine group but were not associated with symptomatic anemia and resolved without intervention, the investigators wrote.
In addition to the quantitative G6PD test, the investigators also evaluated a qualitative test, which “failed to identify 16 patients most at risk for hemolysis,” they reported. “If tafenoquine use is expanded, adoption of reliable quantitative point-of-care G6PD tests will be needed; such tests are not currently available but are in development.”
In the second study, Global Assessment of Tafenoquine Hemolytic Risk (GATHER), Alejandro Llanos-Cuentas, MD, of Universidad Peruana Cayetano Heredia, Lima, Peru, and his colleagues enrolled 251 patients with confirmed P. vivax infection from Peru, Brazil, Columbia, Vietnam, and Thailand. They attempted to recruit women with moderate G6PD levels, but only one participant met this criterion – all others had normal G6PD activity.
Patients received 3-day course of chloroquine and were randomly assigned on a 2:1 basis to either tafenoquine or primaquine at the same doses as in the DETECTIVE trial.
At 6 months, 2% (95% CI, 1%-6%) of tafenoquine recipients and 1% (95% CI, 0.2%-6%) of primaquine recipients had decreased hemoglobin levels, but none consequently needed treatment. The medications also caused a similar degree and time course of hemoglobin decrease, the investigators noted.
A meta-analysis of GATHER and DETECTIVE confirmed that tafenoquine more often led to a decreased hemoglobin level (4% versus 1.5% with primaquine). Tafenoquine also did not meet prespecified criteria for noninferiority compared with primaquine, with respective 6-month recurrence rates of 67% versus 73%.
However, GATHER deployed “extensive” support to help patients adhere to the 15-day primaquine course, Dr. Llanos-Cuentas and his colleagues wrote. “Without such interventions, adherence to primaquine has been reported to be as low as 24% in Southeast Asia, with a corresponding attenuation of efficacy.”
GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having no conflicts of interest.
SOURCES: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: In DETECTIVE, 6-month rates of freedom from recurrence from Plasmodium vivax infection were 62% with tafenoquine, 70% with primaquine, and 28% with placebo.
Study details: Two randomized, phase 3, double-blind controlled trials of patients with confirmed P. vivax infection (DETECTIVE and GATHER) and without deficient G6PD activity.
Disclosures: GlaxoSmithKline and Medicines for Malaria Venture funded both studies, and GSK funded and conducted the meta-analysis. Dr. Llanos-Cuentas and several coinvestigators reported ties to GSK, Medicines for Malaria Venture, the Gambia, and LSTMH. Dr. Lacerda reported having financial disclosures.
Source: Lacerda MVG et al. N Engl J Med 2019;380:215-28, and Llanos-Cuentas A et al. N Engl J Med 2019;380:229-41.
New study determines factors that can send flu patients to the ICU
Numerous independent factors – including a history of obstructive/central sleep apnea syndrome (OSAS/CSAS) or myocardial infarction, along with a body mass index greater than 30 g/m2 – could be related to ICU admission and subsequent high mortality rates in influenza patients, according to an analysis of patients in the Netherlands who were treated during the influenza epidemic of 2015-2016.
Along with determining these factors, lead author M.C. Beumer, of Radboud University Medical Center, the Netherlands, and his coauthors found that “coinfections with bacterial, fungal, and viral pathogens developed more often in patients who were admitted to the ICU.” The study was published in the Journal of Critical Care.
The coauthors reviewed 199 influenza patients who were admitted to two medical centers in the Netherlands during October 2015–April 2016. Of those patients, 45 (23%) were admitted to the ICU, primarily because of respiratory failure, and their mortality rate was 17/45 (38%) versus an overall mortality rate of 18/199 (9%).
Compared with patients in the normal ward, patients admitted to the ICU more frequently had a history of OSAS/CSAS (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), along with a BMI higher than 30 g/m2 (30% vs. 15%; P = .04) and dyspnea as a symptom (77% vs. 48%,; P = .001). In addition, more ICU-admitted patients had influenza A rather than influenza B, compared with those not admitted (87% vs. 66%; P = .009).
Pulmonary coinfections – including bacterial, fungal, and viral pathogens – were also proportionally higher among the 45 ICU patients (56% vs. 20%; P less than .0001). The most common bacterial pathogens were Staphylococcus aureus (11%) and Streptococcus pneumoniae (7%) while Aspergillus fumigatus (18%) and Pneumocystis jirovecii (7%) topped the fungal pathogens.
Mr. Beumer and his colleagues noted potential limitations of their work, including the selection of patients from among the “most severely ill” contributing to an ICU admission rate that surpassed the 5%-10% described elsewhere. They also admitted that their study relied on a “relatively small sample size,” focusing on one seasonal influenza outbreak. However, “despite the limited validity,” they reiterated that “the identified factors may contribute to a complicated disease course and could represent a tool for early recognition of the influenza patients at risk for a complicated disease course.”
The authors reported no conflicts of interest.
SOURCE: Beumer MC et al. J Crit Care. 2019;50:59-65.
.
Numerous independent factors – including a history of obstructive/central sleep apnea syndrome (OSAS/CSAS) or myocardial infarction, along with a body mass index greater than 30 g/m2 – could be related to ICU admission and subsequent high mortality rates in influenza patients, according to an analysis of patients in the Netherlands who were treated during the influenza epidemic of 2015-2016.
Along with determining these factors, lead author M.C. Beumer, of Radboud University Medical Center, the Netherlands, and his coauthors found that “coinfections with bacterial, fungal, and viral pathogens developed more often in patients who were admitted to the ICU.” The study was published in the Journal of Critical Care.
The coauthors reviewed 199 influenza patients who were admitted to two medical centers in the Netherlands during October 2015–April 2016. Of those patients, 45 (23%) were admitted to the ICU, primarily because of respiratory failure, and their mortality rate was 17/45 (38%) versus an overall mortality rate of 18/199 (9%).
Compared with patients in the normal ward, patients admitted to the ICU more frequently had a history of OSAS/CSAS (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), along with a BMI higher than 30 g/m2 (30% vs. 15%; P = .04) and dyspnea as a symptom (77% vs. 48%,; P = .001). In addition, more ICU-admitted patients had influenza A rather than influenza B, compared with those not admitted (87% vs. 66%; P = .009).
Pulmonary coinfections – including bacterial, fungal, and viral pathogens – were also proportionally higher among the 45 ICU patients (56% vs. 20%; P less than .0001). The most common bacterial pathogens were Staphylococcus aureus (11%) and Streptococcus pneumoniae (7%) while Aspergillus fumigatus (18%) and Pneumocystis jirovecii (7%) topped the fungal pathogens.
Mr. Beumer and his colleagues noted potential limitations of their work, including the selection of patients from among the “most severely ill” contributing to an ICU admission rate that surpassed the 5%-10% described elsewhere. They also admitted that their study relied on a “relatively small sample size,” focusing on one seasonal influenza outbreak. However, “despite the limited validity,” they reiterated that “the identified factors may contribute to a complicated disease course and could represent a tool for early recognition of the influenza patients at risk for a complicated disease course.”
The authors reported no conflicts of interest.
SOURCE: Beumer MC et al. J Crit Care. 2019;50:59-65.
.
Numerous independent factors – including a history of obstructive/central sleep apnea syndrome (OSAS/CSAS) or myocardial infarction, along with a body mass index greater than 30 g/m2 – could be related to ICU admission and subsequent high mortality rates in influenza patients, according to an analysis of patients in the Netherlands who were treated during the influenza epidemic of 2015-2016.
Along with determining these factors, lead author M.C. Beumer, of Radboud University Medical Center, the Netherlands, and his coauthors found that “coinfections with bacterial, fungal, and viral pathogens developed more often in patients who were admitted to the ICU.” The study was published in the Journal of Critical Care.
The coauthors reviewed 199 influenza patients who were admitted to two medical centers in the Netherlands during October 2015–April 2016. Of those patients, 45 (23%) were admitted to the ICU, primarily because of respiratory failure, and their mortality rate was 17/45 (38%) versus an overall mortality rate of 18/199 (9%).
Compared with patients in the normal ward, patients admitted to the ICU more frequently had a history of OSAS/CSAS (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), along with a BMI higher than 30 g/m2 (30% vs. 15%; P = .04) and dyspnea as a symptom (77% vs. 48%,; P = .001). In addition, more ICU-admitted patients had influenza A rather than influenza B, compared with those not admitted (87% vs. 66%; P = .009).
Pulmonary coinfections – including bacterial, fungal, and viral pathogens – were also proportionally higher among the 45 ICU patients (56% vs. 20%; P less than .0001). The most common bacterial pathogens were Staphylococcus aureus (11%) and Streptococcus pneumoniae (7%) while Aspergillus fumigatus (18%) and Pneumocystis jirovecii (7%) topped the fungal pathogens.
Mr. Beumer and his colleagues noted potential limitations of their work, including the selection of patients from among the “most severely ill” contributing to an ICU admission rate that surpassed the 5%-10% described elsewhere. They also admitted that their study relied on a “relatively small sample size,” focusing on one seasonal influenza outbreak. However, “despite the limited validity,” they reiterated that “the identified factors may contribute to a complicated disease course and could represent a tool for early recognition of the influenza patients at risk for a complicated disease course.”
The authors reported no conflicts of interest.
SOURCE: Beumer MC et al. J Crit Care. 2019;50:59-65.
.
FROM THE JOURNAL OF CRITICAL CARE
Key clinical point:
Major finding: Flu patients in the ICU more frequently had a history of obstructive/central sleep apnea syndrome (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), compared with non-ICU flu patients.
Study details: A retrospective cohort study of 199 flu patients who were admitted to two academic hospitals in the Netherlands.
Disclosures: The authors reported no conflicts of interest.
Source: Beumer MC et al. J Crit Care. 2019; 50:59-65.