User login
FDA approves 0.5-mL Fluzone Quadrivalent vaccine in young children
according to Sanofi Pasteur, the vaccine’s manufacturer.
FDA approval was based on results of a phase 4 safety and immunogenicity study of nearly 2,000 children. Children aged 6-35 months who received one or two doses of Fluzone at 0.50 mL had a safety profile similar to that of children who received one or two doses of Fluzone at 0.25 mL. Results from the study were presented at the Pediatric Academic Societies annual meeting in April 2018.
This flu vaccine should not be given to anyone with a severe allergic reaction (anaphylaxis) to egg or egg products, according to the press release.
In children, the most common adverse events are injection site reactions, muscle aches, fatigue, and headache; in young children, irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever are common.
“Offering pediatricians the convenience of the same 0.5-mL dose option for children may help streamline immunization efforts. The potentially life-threatening effects of influenza in children reported during the 2017-18 season, especially among those who were not vaccinated, is sobering,” David P. Greenberg, MD, regional medical head of Sanofi Pasteur of North America, said in the press release.
Find the full press release on the Sanofi website.
according to Sanofi Pasteur, the vaccine’s manufacturer.
FDA approval was based on results of a phase 4 safety and immunogenicity study of nearly 2,000 children. Children aged 6-35 months who received one or two doses of Fluzone at 0.50 mL had a safety profile similar to that of children who received one or two doses of Fluzone at 0.25 mL. Results from the study were presented at the Pediatric Academic Societies annual meeting in April 2018.
This flu vaccine should not be given to anyone with a severe allergic reaction (anaphylaxis) to egg or egg products, according to the press release.
In children, the most common adverse events are injection site reactions, muscle aches, fatigue, and headache; in young children, irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever are common.
“Offering pediatricians the convenience of the same 0.5-mL dose option for children may help streamline immunization efforts. The potentially life-threatening effects of influenza in children reported during the 2017-18 season, especially among those who were not vaccinated, is sobering,” David P. Greenberg, MD, regional medical head of Sanofi Pasteur of North America, said in the press release.
Find the full press release on the Sanofi website.
according to Sanofi Pasteur, the vaccine’s manufacturer.
FDA approval was based on results of a phase 4 safety and immunogenicity study of nearly 2,000 children. Children aged 6-35 months who received one or two doses of Fluzone at 0.50 mL had a safety profile similar to that of children who received one or two doses of Fluzone at 0.25 mL. Results from the study were presented at the Pediatric Academic Societies annual meeting in April 2018.
This flu vaccine should not be given to anyone with a severe allergic reaction (anaphylaxis) to egg or egg products, according to the press release.
In children, the most common adverse events are injection site reactions, muscle aches, fatigue, and headache; in young children, irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever are common.
“Offering pediatricians the convenience of the same 0.5-mL dose option for children may help streamline immunization efforts. The potentially life-threatening effects of influenza in children reported during the 2017-18 season, especially among those who were not vaccinated, is sobering,” David P. Greenberg, MD, regional medical head of Sanofi Pasteur of North America, said in the press release.
Find the full press release on the Sanofi website.
Bedbugs in the Workplace



Prescribed opioids increase pneumonia risk in patients with, without HIV
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Prescribed opioids were associated with an increase in community-acquired pneumonia in patients with and without HIV infection, according to results of a large database study.
People living with HIV (PLWH) appeared to have a greater community-acquired pneumonia (CAP) risk at lower opioid doses and particularly with immunosuppressive opioids compared with uninfected patients, although the difference was not significant, E. Jennifer Edelman, MD, of Yale University, New Haven, Conn., and her colleagues wrote in JAMA Internal Medicine.
The researchers performed a nested case-control study comprising 25,392 participants (98.9% men; mean age, 55 years) in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Dr. Edelman and her colleagues compared the characteristics of 4,246 CAP cases with those of 21,146 uninfected controls in the sample. They also compared cases and controls by HIV status. They ran bivariate and multivariate analysis to estimate odds ratios for CAP risk associated with opioid exposure. In addition, the researchers ran models stratified by HIV status and formally checked for an interaction between prescribed opioid characteristics and HIV status.
In unadjusted logistic regression, prescribed opioids were associated with increased odds of CAP, with the greatest risk observed with currently prescribed opioids, compared with past prescribed opioids or no opioids.
Prescribed opioids remained associated with CAP in the adjusted models for past unknown or nonimmunosuppressive (adjusted OR, 1.24; 95% confidence interval, 1.09-1.40) and past immunosuppressive opioid use (aOR, 1.42; 95% CI, 1.21-1.67).
For currently prescribed opioids, nonimmunosuppressive or unknown, the aOR was 1.23 (95% CI, 1.03-1.48). For currently prescribed immunosuppressive opioids, the aOR was 3.18 (95% CI, 2.44-4.14).
The researchers also found evidence of a dose-response effect such that currently prescribed high-dose opioids were associated with the greatest CAP risk, followed by medium- and then by low-dose opioids, whether immunosuppressive or not.
With regard to the effect of HIV status in stratified, adjusted analyses, CAP risk tended to be greater among PLWH with current prescribed opioids, especially immunosuppressive opioids, compared with uninfected patients. However, the overall interaction term for opioid × HIV status was not significant (P = .36).
Although the researchers stated that a limitation of their study was an inability to prove causality or rule out respiratory depression (vs. immunosuppression) as the cause of the increased CAP risk, “the observed effects of opioid immunosuppressive properties and CAP risk lend support to our hypothesis that opioids have clinically relevant immunosuppressive properties.”
Dr. Edelman and her colleagues cited several limitations. For example, they were not able to determine whether patients took their prescribed medications appropriately and assess whether the patients took nonmedically prescribed opioids. Also, because men made up such a large portion of the study population, it is unclear whether the results are generalizable to women.
Nevertheless, the study “adds to growing evidence of potential medical harms associated with prescribed opioids,” they wrote.
“Health care professionals should be aware of this additional CAP risk when they prescribe opioids, and future studies should investigate the effects of opioids prescribed for longer durations and on other immune-related outcomes,” wrote Dr. Edelman and her colleagues. “Understanding whether mitigating the risk of prescribed opioids for CAP is possible by using a lower dose and nonimmunosuppressive opioids awaits further study.”
However, without such data, when prescribed opioids are warranted, physicians should attempt to modify other factors known to affect CAP risk, including smoking and lack of vaccination, Dr. Edelman and her colleagues concluded.
Several U.S. government agencies and Yale University provided funding for the study. The authors reported that they had no conflicts.
SOURCE: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Prescribed opioids, especially those with immunosuppressive properties, are associated with increased community-acquired pneumonia risk.
Major finding: For currently prescribed immunosuppressive opioids, the adjusted odds ratio for community-acquired pneumonia was 3.18 (95% confidence interval, 2.44-4.14).
Study details: A nested case-control study of 25,392 patients in the Veterans Aging Cohort Study from Jan. 1, 2000, through Dec. 31, 2012.
Disclosures: Funding was provided by a variety of government organizations and Yale University, New Haven, Conn. The authors reported that they had no conflicts.
Source: Edelman EJ et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6101.
Study shows evidence of herd immunity with HPV vaccine
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
FROM PEDIATRICS
Key clinical point:
Major finding: Infection rates for quadrivalent vaccine-covered HPV strains declined by 81% among vaccinated women.
Study details: Surveillance studies in 1,580 vaccinated and unvaccinated young women.
Disclosures: The study was funded by the National Institutes of Health. One author declared shares of Merck, but no other conflicts of interest were declared.
Source: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Emphasize disease prevention in communications about HPV vaccine
Parents were much more confident about vaccinating their children against the human papillomavirus (HPV) when they were told about the diseases that the vaccine prevents rather than about safety, new research found.
In Pediatrics, researchers reported the outcomes of an online video-messaging study that attempted to address the most common parental questions and concerns about the HPV vaccine. They surveyed a national sample of 1,196 parents of children (aged 9-17 years) who watched four brief videos of a pediatrician talking about one of seven common concerns regarding HPV vaccination. The parents then were asked how each video affected them.
Parents who were exposed to messages about the diseases that the HPV vaccine prevented had the highest confidence in the HPV vaccine (46%). These messages included “HPV is a common virus that millions of people get every year. The HPV vaccine will protect your child from some cancers and genital warts” and “HPV infection can cause cancer in both men and women. The HPV vaccine will protect your child from many of these cancers.”
Similarly, parents exposed to messages about the need for HPV vaccination for both boys and girls also had the highest levels of confidence about HPV vaccination (44%).
Confidence was lower in parents exposed to messages about safety and side effects (30%)
“As such, reiterating vaccination benefits (including cancer prevention) when addressing concerns may also improve the impact of messages,” wrote Parth D. Shah, PhD, from the Fred Hutchinson Cancer Research Center, Seattle, and his coauthors.
Parents who received messages that expressed urgency about vaccination had lower confidence in the HPV vaccine.
“One reason may be that parents who are hesitant feel inappropriately rushed or that their concerns are not being treated with appropriate care,” the authors wrote.
However, messages that required a higher reading grade level and messages that were longer also seemed to inspire more confidence among parents. Parents who were exposed to messages about cancer prevention additionally were even more confident in HPV vaccine, Dr. Shah and his associates reported.
The study also found that 84% of parents wanted to talk to their children’s doctor about the diseases that the HPV vaccine prevented, while 68% wanted to talk about safety and side effects.
The study was funded by the Centers for Disease Control and Prevention and the National Cancer Institute. Dr. Shah was partially supported by an Agency for Healthcare Research and Quality grant. Another author declared being on paid advisory boards of research grants from Merck, Pfizer, and GlaxoSmithKline. No other conflicts of interest were declared.
SOURCE: Shah PD et al. Pediatrics. 2019 Feb. doi: 10.1542/peds.2018-1872.
Parents were much more confident about vaccinating their children against the human papillomavirus (HPV) when they were told about the diseases that the vaccine prevents rather than about safety, new research found.
In Pediatrics, researchers reported the outcomes of an online video-messaging study that attempted to address the most common parental questions and concerns about the HPV vaccine. They surveyed a national sample of 1,196 parents of children (aged 9-17 years) who watched four brief videos of a pediatrician talking about one of seven common concerns regarding HPV vaccination. The parents then were asked how each video affected them.
Parents who were exposed to messages about the diseases that the HPV vaccine prevented had the highest confidence in the HPV vaccine (46%). These messages included “HPV is a common virus that millions of people get every year. The HPV vaccine will protect your child from some cancers and genital warts” and “HPV infection can cause cancer in both men and women. The HPV vaccine will protect your child from many of these cancers.”
Similarly, parents exposed to messages about the need for HPV vaccination for both boys and girls also had the highest levels of confidence about HPV vaccination (44%).
Confidence was lower in parents exposed to messages about safety and side effects (30%)
“As such, reiterating vaccination benefits (including cancer prevention) when addressing concerns may also improve the impact of messages,” wrote Parth D. Shah, PhD, from the Fred Hutchinson Cancer Research Center, Seattle, and his coauthors.
Parents who received messages that expressed urgency about vaccination had lower confidence in the HPV vaccine.
“One reason may be that parents who are hesitant feel inappropriately rushed or that their concerns are not being treated with appropriate care,” the authors wrote.
However, messages that required a higher reading grade level and messages that were longer also seemed to inspire more confidence among parents. Parents who were exposed to messages about cancer prevention additionally were even more confident in HPV vaccine, Dr. Shah and his associates reported.
The study also found that 84% of parents wanted to talk to their children’s doctor about the diseases that the HPV vaccine prevented, while 68% wanted to talk about safety and side effects.
The study was funded by the Centers for Disease Control and Prevention and the National Cancer Institute. Dr. Shah was partially supported by an Agency for Healthcare Research and Quality grant. Another author declared being on paid advisory boards of research grants from Merck, Pfizer, and GlaxoSmithKline. No other conflicts of interest were declared.
SOURCE: Shah PD et al. Pediatrics. 2019 Feb. doi: 10.1542/peds.2018-1872.
Parents were much more confident about vaccinating their children against the human papillomavirus (HPV) when they were told about the diseases that the vaccine prevents rather than about safety, new research found.
In Pediatrics, researchers reported the outcomes of an online video-messaging study that attempted to address the most common parental questions and concerns about the HPV vaccine. They surveyed a national sample of 1,196 parents of children (aged 9-17 years) who watched four brief videos of a pediatrician talking about one of seven common concerns regarding HPV vaccination. The parents then were asked how each video affected them.
Parents who were exposed to messages about the diseases that the HPV vaccine prevented had the highest confidence in the HPV vaccine (46%). These messages included “HPV is a common virus that millions of people get every year. The HPV vaccine will protect your child from some cancers and genital warts” and “HPV infection can cause cancer in both men and women. The HPV vaccine will protect your child from many of these cancers.”
Similarly, parents exposed to messages about the need for HPV vaccination for both boys and girls also had the highest levels of confidence about HPV vaccination (44%).
Confidence was lower in parents exposed to messages about safety and side effects (30%)
“As such, reiterating vaccination benefits (including cancer prevention) when addressing concerns may also improve the impact of messages,” wrote Parth D. Shah, PhD, from the Fred Hutchinson Cancer Research Center, Seattle, and his coauthors.
Parents who received messages that expressed urgency about vaccination had lower confidence in the HPV vaccine.
“One reason may be that parents who are hesitant feel inappropriately rushed or that their concerns are not being treated with appropriate care,” the authors wrote.
However, messages that required a higher reading grade level and messages that were longer also seemed to inspire more confidence among parents. Parents who were exposed to messages about cancer prevention additionally were even more confident in HPV vaccine, Dr. Shah and his associates reported.
The study also found that 84% of parents wanted to talk to their children’s doctor about the diseases that the HPV vaccine prevented, while 68% wanted to talk about safety and side effects.
The study was funded by the Centers for Disease Control and Prevention and the National Cancer Institute. Dr. Shah was partially supported by an Agency for Healthcare Research and Quality grant. Another author declared being on paid advisory boards of research grants from Merck, Pfizer, and GlaxoSmithKline. No other conflicts of interest were declared.
SOURCE: Shah PD et al. Pediatrics. 2019 Feb. doi: 10.1542/peds.2018-1872.
FROM PEDIATRICS
Key clinical point: Information on the benefits of HPV vaccination can improve parent confidence.
Major finding: Messages about the disease and cancer prevention benefits of HPV vaccination inspired greater parent confidence.
Study details: Study in 1,196 parents of children aged 9-17 years.
Disclosures: The study was funded by the Centers for Disease Control and Prevention and the National Cancer Institute. Dr. Shah was partially supported by an Agency for Healthcare Research and Quality grant. Another author declared being on paid advisory boards of research grants from Merck, Pfizer, and GlaxoSmithKline. No other conflicts of interest were declared.
Source: Shah P et al. Pediatrics. 2019 Feb. doi. 10.1542/peds.2018-1872.
Doctors: Shutdown affects patient health
Also today, a mental disorder diagnosis increases the risk for all mental disorders, frailty may affect the expression of dementia, and nearly one-quarter of antibiotic fills are deemed unnecessary.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, a mental disorder diagnosis increases the risk for all mental disorders, frailty may affect the expression of dementia, and nearly one-quarter of antibiotic fills are deemed unnecessary.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, a mental disorder diagnosis increases the risk for all mental disorders, frailty may affect the expression of dementia, and nearly one-quarter of antibiotic fills are deemed unnecessary.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Flu activity down for second consecutive week
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
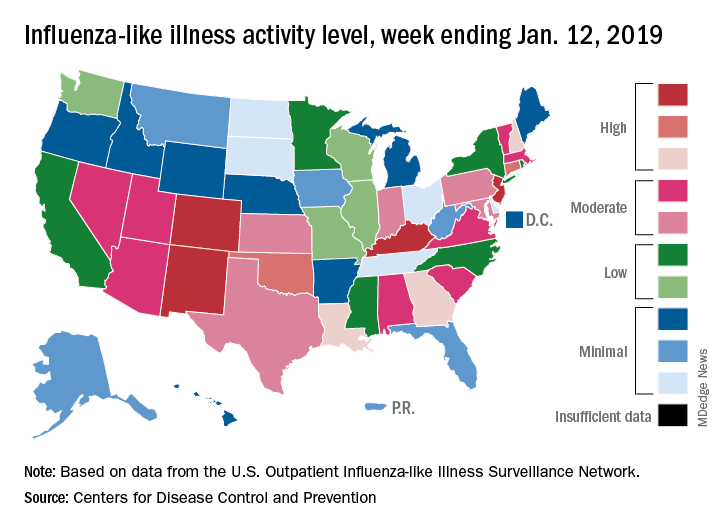
The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
Intrapartum molecular GBS screening reduced newborn early-onset disease, antibiotic use
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The rate of early-onset disease group B Streptococcus decreased from 1.01/1,000 cases to 0.21/1,000 cases across the antenatal and intrapartum periods.
Study details: A single-center study of antenatal culture screening for 11,226 deliveries during 2006-2009 and intrapartum PCR screening for 18,835 deliveries during 2010-2015.
Disclosures: The authors reported no relevant conflicts of interest.
Source: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Digoxin-furosemide reduces viral load, diameter of cutaneous warts
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The combination of digoxin and furosemide in a topical gel reduced the diameter of cutaneous warts caused by HPV.
Major finding: Wart diameter was reduced by a mean of 3 mm among those treated with the combination.
Study details: The randomized, placebo-controlled phase 2a study compared the furosemide-digoxin combination with the two components separately, and placebo separately, in 80 adults.
Disclosures: One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
Source: Rijsbergen M et al. J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
More than 23% of antibiotic fills deemed unnecessary
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.
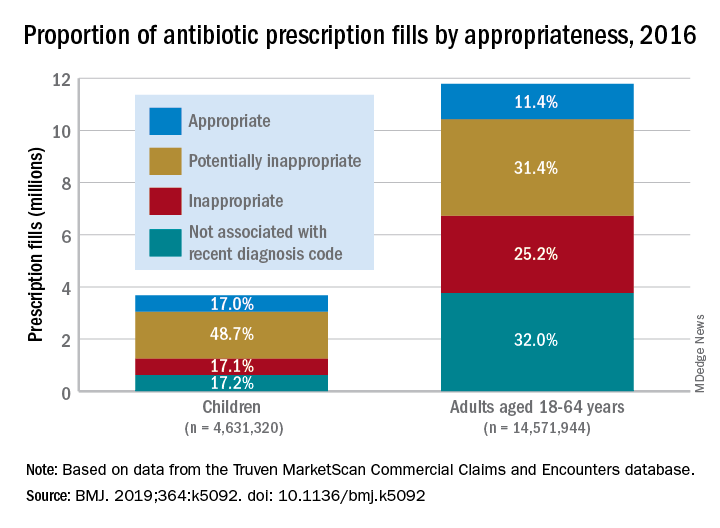
Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.

Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
More than 23% of all antibiotic prescriptions filled in 2016 were medically unnecessary, and another 36% were questionable, according to an analysis of prescribing data for 19.2 million children and nonelderly adults.

Based on the diagnosis codes for 15.5 million prescriptions filled that year, at least 3.6 million (23.2%) were “inappropriate” – prescribed for conditions for which an antibiotic is almost never recommended, such as acute upper respiratory conditions – and 5.5 million (35.5%) were “potentially inappropriate” – conditions such as acute sinusitis or otitis media, for which an antibiotic is only sometimes recommended, Kao-Ping Chua, MD, PhD, of the University of Michigan, Ann Arbor, and his associates reported in the BMJ.
Only 12.8% of filled prescriptions for the 39 oral antibiotics assessed were classified as “appropriate” under the investigators’ scheme, which assigned an antibiotic appropriateness level to all 91,738 diagnostic codes in the 2016 ICD-10-CM. Finally, 28.5% of antibiotic fills were not associated with a recent diagnosis code, suggesting that they involved phone consultations that did not result in claims or visits that were paid out of pocket and did not make it into the Truven MarketScan Commercial Claims and Encounters database used in the study, the investigators said.
The three highest levels of inappropriate fills were 70.7% in office-based settings, 6.2% in urgent care centers, and 4.7% in emergency departments.
“The unacceptable scale of inappropriate antibiotic prescribing in the United States ... underscores the need to learn more about prescriptions that aren’t justified by a diagnosis – or are written after no diagnosis at all,” coinvestigator Jeffrey Linder, MD, of Northwestern University, Chicago, said in a written statement.
Prescriptions for children, who represented almost a quarter of all antibiotic fills, were less likely to be inappropriate than those for adults aged 18-64 years. Proportions for children were 17.1% inappropriate, 48.7% potentially inappropriate, and 17.0% appropriate, compared with 25.2%, 31.4%, and 11.4%, respectively, for adults, Dr. Chua and his associates said.
“This study shows how data and analytics can help us identify and understand important challenges facing the American health care system,” said Gopal Khanna, director of the Agency for Healthcare Research and Quality, which funded the study. “We now need to use these data to spur change in the prescribing of these very common medications.”
SOURCE: Chua K-P et al. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092.
FROM THE BMJ






