User login
Host stress response may be a factor in early-stage HCV clearance
The cellular stress response in hepatocytes may play a major role in controlling hepatitis C virus (HCV). This response may be an important host factor for virus clearance during the early stages of HCV infection, according to a review of acute and chronic HCV infection by W. Alfredo Ríos-Ocampo, MD, and his colleagues (Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
The reviewers examined the mechanisms of induction and modulation of oxidative stress and endoplasmic-reticular stress with regard to viral persistence and cell survival. The accumulated research indicates that the activation of the eIF2-alpha/ATF4 pathway and selective autophagy induction are involved in the elimination of harmful viral proteins after oxidative stress induction. This all suggests a negative role of autophagy upon HCV infection or a negative regulation of viral replication.
“We conclude from published studies and our own research that viral protein synthesis activates adaptive responses, including autophagy pathways, that act to limit viral protein load and thereby reduce oxidative stress and cell death. Exploitation of these pathways to reduce viral replication will be the next goal and might be a valuable addition to antiviral therapy,” the reviewers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ríos-Ocampo, WA, et al. Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
The cellular stress response in hepatocytes may play a major role in controlling hepatitis C virus (HCV). This response may be an important host factor for virus clearance during the early stages of HCV infection, according to a review of acute and chronic HCV infection by W. Alfredo Ríos-Ocampo, MD, and his colleagues (Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).

The reviewers examined the mechanisms of induction and modulation of oxidative stress and endoplasmic-reticular stress with regard to viral persistence and cell survival. The accumulated research indicates that the activation of the eIF2-alpha/ATF4 pathway and selective autophagy induction are involved in the elimination of harmful viral proteins after oxidative stress induction. This all suggests a negative role of autophagy upon HCV infection or a negative regulation of viral replication.
“We conclude from published studies and our own research that viral protein synthesis activates adaptive responses, including autophagy pathways, that act to limit viral protein load and thereby reduce oxidative stress and cell death. Exploitation of these pathways to reduce viral replication will be the next goal and might be a valuable addition to antiviral therapy,” the reviewers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ríos-Ocampo, WA, et al. Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
The cellular stress response in hepatocytes may play a major role in controlling hepatitis C virus (HCV). This response may be an important host factor for virus clearance during the early stages of HCV infection, according to a review of acute and chronic HCV infection by W. Alfredo Ríos-Ocampo, MD, and his colleagues (Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).

The reviewers examined the mechanisms of induction and modulation of oxidative stress and endoplasmic-reticular stress with regard to viral persistence and cell survival. The accumulated research indicates that the activation of the eIF2-alpha/ATF4 pathway and selective autophagy induction are involved in the elimination of harmful viral proteins after oxidative stress induction. This all suggests a negative role of autophagy upon HCV infection or a negative regulation of viral replication.
“We conclude from published studies and our own research that viral protein synthesis activates adaptive responses, including autophagy pathways, that act to limit viral protein load and thereby reduce oxidative stress and cell death. Exploitation of these pathways to reduce viral replication will be the next goal and might be a valuable addition to antiviral therapy,” the reviewers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ríos-Ocampo, WA, et al. Virus Res. 2019. doi: 10.1016/j.virusres.2018.12.013).
FROM VIRUS RESEARCH
FDA approves Adacel for repeat Tdap vaccinations
The Food and Drug Administration has approved the expanded use of Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine Adsorbed) to include repeat vaccinations 8 years or more after the first vaccination in people aged 10-64 years.
The expanded indication was based on results of a randomized, controlled trial, published in the Journal of the Pediatric Infectious Diseases Society, in which more than 1,300 adults aged 18-64 years received either Adacel or a Td (tetanus-diphtheria) vaccine 8-12 years after receiving a previous dose of Adacel.
Over the course of the study, no significant difference in adverse event incidence was observed between groups. Injection-site reaction was the most common adverse event during the study, occurring in 87.7% of those who received Adacel and 88.0% of those who received the Td vaccine. Other common adverse events associated with Adacel include headache, body ache or muscle weakness, tiredness, muscle aches, and general discomfort.
“While strong vaccination programs are in place for young adolescents, a single Tdap immunization does not offer lifetime protection against pertussis due to waning immunity. The licensure of Adacel as the first Tdap vaccine in the U.S. for repeat vaccination is an important step for eligible patients and offers flexibility for health care providers to help manage their immunization schedules,” said David P. Greenberg, MD, regional medical head North America at Sanofi Pasteur, in the press release.
Find the full press release on the Sanofi website.
The Food and Drug Administration has approved the expanded use of Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine Adsorbed) to include repeat vaccinations 8 years or more after the first vaccination in people aged 10-64 years.
The expanded indication was based on results of a randomized, controlled trial, published in the Journal of the Pediatric Infectious Diseases Society, in which more than 1,300 adults aged 18-64 years received either Adacel or a Td (tetanus-diphtheria) vaccine 8-12 years after receiving a previous dose of Adacel.
Over the course of the study, no significant difference in adverse event incidence was observed between groups. Injection-site reaction was the most common adverse event during the study, occurring in 87.7% of those who received Adacel and 88.0% of those who received the Td vaccine. Other common adverse events associated with Adacel include headache, body ache or muscle weakness, tiredness, muscle aches, and general discomfort.
“While strong vaccination programs are in place for young adolescents, a single Tdap immunization does not offer lifetime protection against pertussis due to waning immunity. The licensure of Adacel as the first Tdap vaccine in the U.S. for repeat vaccination is an important step for eligible patients and offers flexibility for health care providers to help manage their immunization schedules,” said David P. Greenberg, MD, regional medical head North America at Sanofi Pasteur, in the press release.
Find the full press release on the Sanofi website.
The Food and Drug Administration has approved the expanded use of Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine Adsorbed) to include repeat vaccinations 8 years or more after the first vaccination in people aged 10-64 years.
The expanded indication was based on results of a randomized, controlled trial, published in the Journal of the Pediatric Infectious Diseases Society, in which more than 1,300 adults aged 18-64 years received either Adacel or a Td (tetanus-diphtheria) vaccine 8-12 years after receiving a previous dose of Adacel.
Over the course of the study, no significant difference in adverse event incidence was observed between groups. Injection-site reaction was the most common adverse event during the study, occurring in 87.7% of those who received Adacel and 88.0% of those who received the Td vaccine. Other common adverse events associated with Adacel include headache, body ache or muscle weakness, tiredness, muscle aches, and general discomfort.
“While strong vaccination programs are in place for young adolescents, a single Tdap immunization does not offer lifetime protection against pertussis due to waning immunity. The licensure of Adacel as the first Tdap vaccine in the U.S. for repeat vaccination is an important step for eligible patients and offers flexibility for health care providers to help manage their immunization schedules,” said David P. Greenberg, MD, regional medical head North America at Sanofi Pasteur, in the press release.
Find the full press release on the Sanofi website.
Incidence of late-onset GBS cases are higher than early-onset disease
according to a multistate study of invasive group B streptococcal disease published in JAMA Pediatrics.
Using data from the Active Bacterial Core surveillance (ABCs) program, Srinivas Acharya Nanduri, MD, MPH, at the Centers for Disease Control and Prevention, and colleagues performed an analysis of early-onset disease (EOD) and late-onset disease (LOD) cases of group B Streptococcus (GBS) in infants from 10 different states between 2006 and 2015, and whether mothers of infants with EOD received intrapartum antibiotic prophylaxis (IAP). EOD was defined as between 0 and 6 days old, while LOD occurred between 7 days and 89 days old.
They found 1,277 cases of EOD and 1,387 cases of LOD in total, with a decrease in incidence of EOD from 0.37 per 1,000 live births in 2006 to 0.23 per 1,000 live births in 2015 (P less than .001); LOD incidence remained stable at a mean 0.31 per 1,000 live births during the same time period.
In 2015, the national burden for EOD and LOD was estimated at 840 and 1,265 cases, respectively. Mothers of infants with EOD did not have indications for and did not receive IAP in 617 cases (48%) and did not receive IAP despite indications in 278 (22%) cases.
“While the current culture-based screening strategy has been highly successful in reducing EOD burden, our data show that almost half of remaining infants with EOD were born to mothers with no indication for receiving IAP,” Dr. Nanduri and colleagues wrote.
Because there currently is no effective prevention strategy against LOS GBS, the investigators wrote that a maternal vaccine against the most common serotypes “holds promise to prevent a substantial portion of this remaining burden,” and noted several GBS candidate vaccines were in advanced stages of development.
The researchers also looked at GBS serotype data in 1,743 patients from seven different centers. The most commonly found serotype isolates of 887 EOD cases were Ia (242 cases, 27%) and III (242 cases, 27%) overall. Serotype III was most common for LOD cases (481 cases, 56%) and increased in incidence from 0.12 per 1,000 live births to 0.20 per 1,000 live births during the study period (P less than .001), while serotype IV was responsible for 53 cases (6%) of both EOD and LOD.
Dr. Nanduri and associates wrote that over 99% of the serotyped EOD (881 cases) and serotyped LOD (853 cases) cases were caused by serotypes Ia, Ib, II, III, IV, and V. With regard to antimicrobial resistance, there were no cases of beta-lactam resistance, but there was constitutive clindamycin resistance in 359 isolate test results (21%).
The researchers noted that they were limited in the study by 1 year of whole-genome sequencing data, the ABCs capturing only 10% of live birth data in the United States, and conclusions on EOD prevention restricted to data from labor and delivery records.
This study was funded in part by the CDC. Paula S. Vagnone received grants from the CDC, while William S. Schaffner, MD, received grants from the CDC and personal fees from Pfizer, Merck, SutroVax, Shionogi, Dynavax, and Seqirus outside of the study. The other authors reported no relevant disclosures.
SOURCE: Nanduri SA et al. JAMA Pediatr. 2019 Jan 14. doi: 10.1001/jamapediatrics.2018.4826.
Perinatal group B Streptococcus (GBS) disease prevention guidelines are credited for the low rate of early-onset disease (EOD) cases of GBS in the United States, but the practice of intrapartum antibiotic prophylaxis (IAP) remains controversial in places like the United Kingdom where the National Health Service does not recommend screening-based IAP for GBS, Sagori Mukhopadhyay, MD, MMSc, and Karen M. Puopolo, MD, PhD, wrote in a related editorial.
One reason for concern about GBS IAP policies is that, despite the decreased number of EOD cases after implementation of IAP, the rate of late-onset disease (LOD) cases remain the same, the authors wrote. And implementation of IAP is not perfect: In some cases IAP was used for less than the recommended duration, used less effective drugs, or given too late so fetal infections were already established.
In addition, some may be uncomfortable with increased perinatal exposure to antibiotics – “a long-held concern about the extent to which widespread perinatal antibiotic use may contribute to the emergence and expansion of antibiotic-resistant GBS,” they added. However, despite the concern, the fatality ratio for EOD was 7% in the study by Nanduri et al., and one complication of GBS in survivors is neurodevelopmental impairment, according to a meta-analysis of 18 studies.
One solution that could address both EOD and LOD cases of GBS is the development of a GBS vaccine. Although there is reluctance to vaccinate pregnant women, recent studies have shown success in vaccinating women for influenza, tetanus, diphtheria, and pertussis; these recent efforts have “reinvigorated” academia’s interest in vaccine research for this population.
“Vaccination certainly could be a first step to eliminating neonatal GBS disease in the United States and may be the only available approach to addressing the substantial international burden of GBS-associated stillbirth, preterm birth, and neonatal disease morbidity and mortality,” the authors wrote. “But for now, while GBS IAP may be imperfect, it is the success we have.”
Dr. Mukhopadhyay and Dr. Puopolo are from the division of neonatology at the Children’s Hospital of Philadelphia. Dr. Mukhopadhyay and Dr. Puopolo commented on the study by Nanduri et al. in an accompanying editorial (Mukhopadhyay et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.4824). They reported no relevant conflicts of interest.
Perinatal group B Streptococcus (GBS) disease prevention guidelines are credited for the low rate of early-onset disease (EOD) cases of GBS in the United States, but the practice of intrapartum antibiotic prophylaxis (IAP) remains controversial in places like the United Kingdom where the National Health Service does not recommend screening-based IAP for GBS, Sagori Mukhopadhyay, MD, MMSc, and Karen M. Puopolo, MD, PhD, wrote in a related editorial.
One reason for concern about GBS IAP policies is that, despite the decreased number of EOD cases after implementation of IAP, the rate of late-onset disease (LOD) cases remain the same, the authors wrote. And implementation of IAP is not perfect: In some cases IAP was used for less than the recommended duration, used less effective drugs, or given too late so fetal infections were already established.
In addition, some may be uncomfortable with increased perinatal exposure to antibiotics – “a long-held concern about the extent to which widespread perinatal antibiotic use may contribute to the emergence and expansion of antibiotic-resistant GBS,” they added. However, despite the concern, the fatality ratio for EOD was 7% in the study by Nanduri et al., and one complication of GBS in survivors is neurodevelopmental impairment, according to a meta-analysis of 18 studies.
One solution that could address both EOD and LOD cases of GBS is the development of a GBS vaccine. Although there is reluctance to vaccinate pregnant women, recent studies have shown success in vaccinating women for influenza, tetanus, diphtheria, and pertussis; these recent efforts have “reinvigorated” academia’s interest in vaccine research for this population.
“Vaccination certainly could be a first step to eliminating neonatal GBS disease in the United States and may be the only available approach to addressing the substantial international burden of GBS-associated stillbirth, preterm birth, and neonatal disease morbidity and mortality,” the authors wrote. “But for now, while GBS IAP may be imperfect, it is the success we have.”
Dr. Mukhopadhyay and Dr. Puopolo are from the division of neonatology at the Children’s Hospital of Philadelphia. Dr. Mukhopadhyay and Dr. Puopolo commented on the study by Nanduri et al. in an accompanying editorial (Mukhopadhyay et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.4824). They reported no relevant conflicts of interest.
Perinatal group B Streptococcus (GBS) disease prevention guidelines are credited for the low rate of early-onset disease (EOD) cases of GBS in the United States, but the practice of intrapartum antibiotic prophylaxis (IAP) remains controversial in places like the United Kingdom where the National Health Service does not recommend screening-based IAP for GBS, Sagori Mukhopadhyay, MD, MMSc, and Karen M. Puopolo, MD, PhD, wrote in a related editorial.
One reason for concern about GBS IAP policies is that, despite the decreased number of EOD cases after implementation of IAP, the rate of late-onset disease (LOD) cases remain the same, the authors wrote. And implementation of IAP is not perfect: In some cases IAP was used for less than the recommended duration, used less effective drugs, or given too late so fetal infections were already established.
In addition, some may be uncomfortable with increased perinatal exposure to antibiotics – “a long-held concern about the extent to which widespread perinatal antibiotic use may contribute to the emergence and expansion of antibiotic-resistant GBS,” they added. However, despite the concern, the fatality ratio for EOD was 7% in the study by Nanduri et al., and one complication of GBS in survivors is neurodevelopmental impairment, according to a meta-analysis of 18 studies.
One solution that could address both EOD and LOD cases of GBS is the development of a GBS vaccine. Although there is reluctance to vaccinate pregnant women, recent studies have shown success in vaccinating women for influenza, tetanus, diphtheria, and pertussis; these recent efforts have “reinvigorated” academia’s interest in vaccine research for this population.
“Vaccination certainly could be a first step to eliminating neonatal GBS disease in the United States and may be the only available approach to addressing the substantial international burden of GBS-associated stillbirth, preterm birth, and neonatal disease morbidity and mortality,” the authors wrote. “But for now, while GBS IAP may be imperfect, it is the success we have.”
Dr. Mukhopadhyay and Dr. Puopolo are from the division of neonatology at the Children’s Hospital of Philadelphia. Dr. Mukhopadhyay and Dr. Puopolo commented on the study by Nanduri et al. in an accompanying editorial (Mukhopadhyay et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.4824). They reported no relevant conflicts of interest.
according to a multistate study of invasive group B streptococcal disease published in JAMA Pediatrics.
Using data from the Active Bacterial Core surveillance (ABCs) program, Srinivas Acharya Nanduri, MD, MPH, at the Centers for Disease Control and Prevention, and colleagues performed an analysis of early-onset disease (EOD) and late-onset disease (LOD) cases of group B Streptococcus (GBS) in infants from 10 different states between 2006 and 2015, and whether mothers of infants with EOD received intrapartum antibiotic prophylaxis (IAP). EOD was defined as between 0 and 6 days old, while LOD occurred between 7 days and 89 days old.
They found 1,277 cases of EOD and 1,387 cases of LOD in total, with a decrease in incidence of EOD from 0.37 per 1,000 live births in 2006 to 0.23 per 1,000 live births in 2015 (P less than .001); LOD incidence remained stable at a mean 0.31 per 1,000 live births during the same time period.
In 2015, the national burden for EOD and LOD was estimated at 840 and 1,265 cases, respectively. Mothers of infants with EOD did not have indications for and did not receive IAP in 617 cases (48%) and did not receive IAP despite indications in 278 (22%) cases.
“While the current culture-based screening strategy has been highly successful in reducing EOD burden, our data show that almost half of remaining infants with EOD were born to mothers with no indication for receiving IAP,” Dr. Nanduri and colleagues wrote.
Because there currently is no effective prevention strategy against LOS GBS, the investigators wrote that a maternal vaccine against the most common serotypes “holds promise to prevent a substantial portion of this remaining burden,” and noted several GBS candidate vaccines were in advanced stages of development.
The researchers also looked at GBS serotype data in 1,743 patients from seven different centers. The most commonly found serotype isolates of 887 EOD cases were Ia (242 cases, 27%) and III (242 cases, 27%) overall. Serotype III was most common for LOD cases (481 cases, 56%) and increased in incidence from 0.12 per 1,000 live births to 0.20 per 1,000 live births during the study period (P less than .001), while serotype IV was responsible for 53 cases (6%) of both EOD and LOD.
Dr. Nanduri and associates wrote that over 99% of the serotyped EOD (881 cases) and serotyped LOD (853 cases) cases were caused by serotypes Ia, Ib, II, III, IV, and V. With regard to antimicrobial resistance, there were no cases of beta-lactam resistance, but there was constitutive clindamycin resistance in 359 isolate test results (21%).
The researchers noted that they were limited in the study by 1 year of whole-genome sequencing data, the ABCs capturing only 10% of live birth data in the United States, and conclusions on EOD prevention restricted to data from labor and delivery records.
This study was funded in part by the CDC. Paula S. Vagnone received grants from the CDC, while William S. Schaffner, MD, received grants from the CDC and personal fees from Pfizer, Merck, SutroVax, Shionogi, Dynavax, and Seqirus outside of the study. The other authors reported no relevant disclosures.
SOURCE: Nanduri SA et al. JAMA Pediatr. 2019 Jan 14. doi: 10.1001/jamapediatrics.2018.4826.
according to a multistate study of invasive group B streptococcal disease published in JAMA Pediatrics.
Using data from the Active Bacterial Core surveillance (ABCs) program, Srinivas Acharya Nanduri, MD, MPH, at the Centers for Disease Control and Prevention, and colleagues performed an analysis of early-onset disease (EOD) and late-onset disease (LOD) cases of group B Streptococcus (GBS) in infants from 10 different states between 2006 and 2015, and whether mothers of infants with EOD received intrapartum antibiotic prophylaxis (IAP). EOD was defined as between 0 and 6 days old, while LOD occurred between 7 days and 89 days old.
They found 1,277 cases of EOD and 1,387 cases of LOD in total, with a decrease in incidence of EOD from 0.37 per 1,000 live births in 2006 to 0.23 per 1,000 live births in 2015 (P less than .001); LOD incidence remained stable at a mean 0.31 per 1,000 live births during the same time period.
In 2015, the national burden for EOD and LOD was estimated at 840 and 1,265 cases, respectively. Mothers of infants with EOD did not have indications for and did not receive IAP in 617 cases (48%) and did not receive IAP despite indications in 278 (22%) cases.
“While the current culture-based screening strategy has been highly successful in reducing EOD burden, our data show that almost half of remaining infants with EOD were born to mothers with no indication for receiving IAP,” Dr. Nanduri and colleagues wrote.
Because there currently is no effective prevention strategy against LOS GBS, the investigators wrote that a maternal vaccine against the most common serotypes “holds promise to prevent a substantial portion of this remaining burden,” and noted several GBS candidate vaccines were in advanced stages of development.
The researchers also looked at GBS serotype data in 1,743 patients from seven different centers. The most commonly found serotype isolates of 887 EOD cases were Ia (242 cases, 27%) and III (242 cases, 27%) overall. Serotype III was most common for LOD cases (481 cases, 56%) and increased in incidence from 0.12 per 1,000 live births to 0.20 per 1,000 live births during the study period (P less than .001), while serotype IV was responsible for 53 cases (6%) of both EOD and LOD.
Dr. Nanduri and associates wrote that over 99% of the serotyped EOD (881 cases) and serotyped LOD (853 cases) cases were caused by serotypes Ia, Ib, II, III, IV, and V. With regard to antimicrobial resistance, there were no cases of beta-lactam resistance, but there was constitutive clindamycin resistance in 359 isolate test results (21%).
The researchers noted that they were limited in the study by 1 year of whole-genome sequencing data, the ABCs capturing only 10% of live birth data in the United States, and conclusions on EOD prevention restricted to data from labor and delivery records.
This study was funded in part by the CDC. Paula S. Vagnone received grants from the CDC, while William S. Schaffner, MD, received grants from the CDC and personal fees from Pfizer, Merck, SutroVax, Shionogi, Dynavax, and Seqirus outside of the study. The other authors reported no relevant disclosures.
SOURCE: Nanduri SA et al. JAMA Pediatr. 2019 Jan 14. doi: 10.1001/jamapediatrics.2018.4826.
FROM JAMA PEDIATRICS
Key clinical point: Between 2006 and 2015, early-onset disease cases of group B Streptococcus (GBS) declined, while the incidence of late-onset cases did not change.
Major finding: The rate of early-onset GBS declined from 0.37 to 0.23 per 1,000 live births and the rate of late-onset GBS cases remained at a mean 0.31 per 1,000 live births.
Study details: A population-based study of infants with early-onset disease and late-onset disease GBS from 10 different states in the Active Bacterial Core surveillance program between 2006 and 2015.
Disclosures: This study was funded in part by the Centers for Disease Control and Prevention. Paula S. Vagnone received grants from the CDC, while William S. Schaffner, MD, received grants from the CDC and personal fees from Pfizer, Merck, SutroVax, Shionogi, Dynavax, and Seqirus outside of the study. The other authors reported no relevant disclosures.
Source: Nanduri SA et al. JAMA Pediatr. 2019 Jan 14. doi: 10.1001/jamapediatrics.2018.4826.
Flu season showing signs of decline
The 2018-2019 flu season may have peaked as measures of influenza-like illness (ILI) activity dropped in the first week of the new year, according to the U.S. Centers for Disease Control and Prevention.

The proportion of outpatients visits for ILI dropped to 3.5% for the week ending Jan. 5, 2019, after reaching 4.0% the previous week. Outpatient ILI visits first topped the national baseline of 2.2% during the week ending Dec. 8, 2018, and have remained above that value for 5 consecutive weeks, the CDC’s influenza division said on Jan. 11.
Flu activity reported by the states reflects the national drop: 10 states came in at level 10 on the CDC’s 1-10 scale of activity for the week ending Jan. 5 – down from 12 the week before – and a total of 15 were in the high range from 8 to 10, compared with 19 the previous week, the CDC said. Two states, Mississippi and Texas, dropped from level 10 to level 7, which the CDC categorizes as moderate activity.
A total of 73 ILI-related deaths were reported during the week ending Dec. 29 (the latest with data available; reporting less than 68% complete), which already exceeds the 71 deaths reported for the week ending Dec. 22 (reporting 85% complete). Flu deaths totaled 437 through the first 13 weeks of the 2018-2019 season, compared with the 1,659 that occurred during weeks 1-13 of the very severe 2017-2018 season, CDC data show.
For the week ending Jan. 5, the CDC received reports of three flu-related pediatric deaths, all of which occurred the previous week. For the season so far, there have been 16 pediatric deaths, compared with 20 at this point in the 2017-2018 season.
Estimates released during the flu season for the first time show that between 6 and 7 million Americans have been infected since Oct. 1, 2018, and that 69,000-84,000 people have been hospitalized with the flu through Jan. 5, 2019. These cumulative totals have previously been available only at the end of the season, the CDC noted.
The 2018-2019 flu season may have peaked as measures of influenza-like illness (ILI) activity dropped in the first week of the new year, according to the U.S. Centers for Disease Control and Prevention.

The proportion of outpatients visits for ILI dropped to 3.5% for the week ending Jan. 5, 2019, after reaching 4.0% the previous week. Outpatient ILI visits first topped the national baseline of 2.2% during the week ending Dec. 8, 2018, and have remained above that value for 5 consecutive weeks, the CDC’s influenza division said on Jan. 11.
Flu activity reported by the states reflects the national drop: 10 states came in at level 10 on the CDC’s 1-10 scale of activity for the week ending Jan. 5 – down from 12 the week before – and a total of 15 were in the high range from 8 to 10, compared with 19 the previous week, the CDC said. Two states, Mississippi and Texas, dropped from level 10 to level 7, which the CDC categorizes as moderate activity.
A total of 73 ILI-related deaths were reported during the week ending Dec. 29 (the latest with data available; reporting less than 68% complete), which already exceeds the 71 deaths reported for the week ending Dec. 22 (reporting 85% complete). Flu deaths totaled 437 through the first 13 weeks of the 2018-2019 season, compared with the 1,659 that occurred during weeks 1-13 of the very severe 2017-2018 season, CDC data show.
For the week ending Jan. 5, the CDC received reports of three flu-related pediatric deaths, all of which occurred the previous week. For the season so far, there have been 16 pediatric deaths, compared with 20 at this point in the 2017-2018 season.
Estimates released during the flu season for the first time show that between 6 and 7 million Americans have been infected since Oct. 1, 2018, and that 69,000-84,000 people have been hospitalized with the flu through Jan. 5, 2019. These cumulative totals have previously been available only at the end of the season, the CDC noted.
The 2018-2019 flu season may have peaked as measures of influenza-like illness (ILI) activity dropped in the first week of the new year, according to the U.S. Centers for Disease Control and Prevention.

The proportion of outpatients visits for ILI dropped to 3.5% for the week ending Jan. 5, 2019, after reaching 4.0% the previous week. Outpatient ILI visits first topped the national baseline of 2.2% during the week ending Dec. 8, 2018, and have remained above that value for 5 consecutive weeks, the CDC’s influenza division said on Jan. 11.
Flu activity reported by the states reflects the national drop: 10 states came in at level 10 on the CDC’s 1-10 scale of activity for the week ending Jan. 5 – down from 12 the week before – and a total of 15 were in the high range from 8 to 10, compared with 19 the previous week, the CDC said. Two states, Mississippi and Texas, dropped from level 10 to level 7, which the CDC categorizes as moderate activity.
A total of 73 ILI-related deaths were reported during the week ending Dec. 29 (the latest with data available; reporting less than 68% complete), which already exceeds the 71 deaths reported for the week ending Dec. 22 (reporting 85% complete). Flu deaths totaled 437 through the first 13 weeks of the 2018-2019 season, compared with the 1,659 that occurred during weeks 1-13 of the very severe 2017-2018 season, CDC data show.
For the week ending Jan. 5, the CDC received reports of three flu-related pediatric deaths, all of which occurred the previous week. For the season so far, there have been 16 pediatric deaths, compared with 20 at this point in the 2017-2018 season.
Estimates released during the flu season for the first time show that between 6 and 7 million Americans have been infected since Oct. 1, 2018, and that 69,000-84,000 people have been hospitalized with the flu through Jan. 5, 2019. These cumulative totals have previously been available only at the end of the season, the CDC noted.
Children who are coughing: Is it flu or bacterial pneumonia?
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
Chronic infections such as HCV, HIV, and TB cause unique problems for psoriasis patients
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Opioid clinic physicians report lack of competency in managing patients with HCV
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
FROM THE INTERNATIONAL JOURNAL OF DRUG POLICY
Necrobiosis Lipoidica With Superimposed Pyoderma Vegetans
Case Report
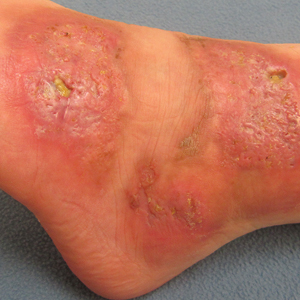
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
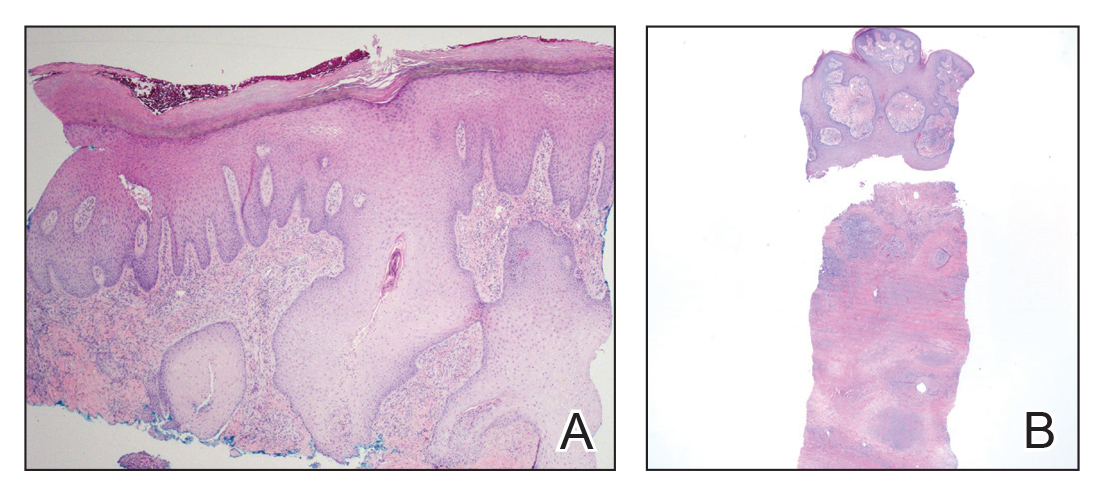
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.

The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.


The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.


The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
Practice Points
- Necrobiosis lipoidica (NL), a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial-wall thickening, is most often seen in association with insulin-dependent diabetes mellitus (DM).
- Asymptomatic, well-circumscribed, violaceous papules and nodules coalesce into plaques on the lower extremities, face, or trunk in NL.
- Treatment mainstay is topical and intralesional corticosteroids at active borders of lesions. Other treatments used with some success include tumor necrosis factor 11α inhibitors, topical tretinoin, topical tacrolimus, and skin grafting. Control and management of DM can be helpful.
What’s Eating You? Bedbugs
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.
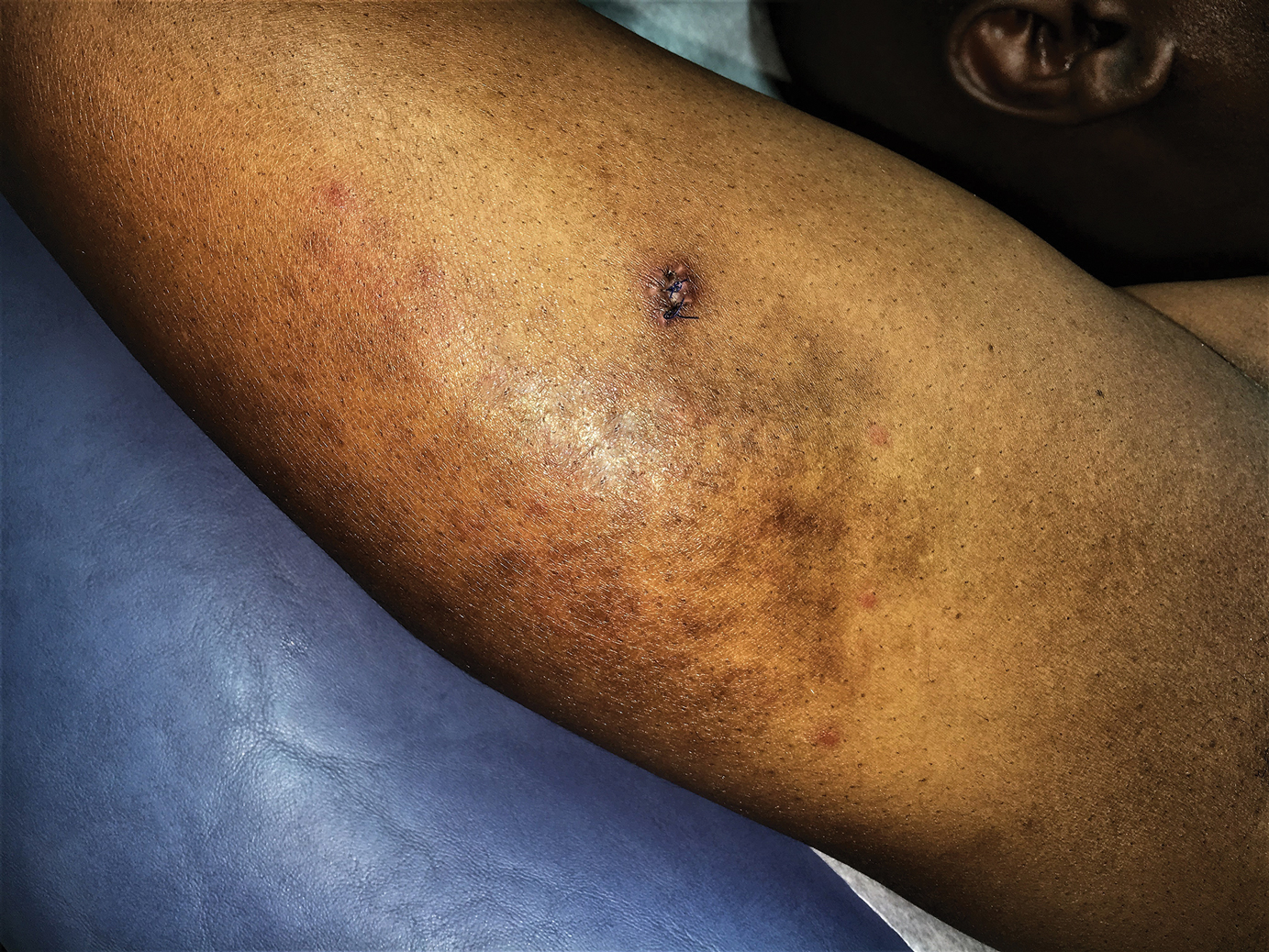
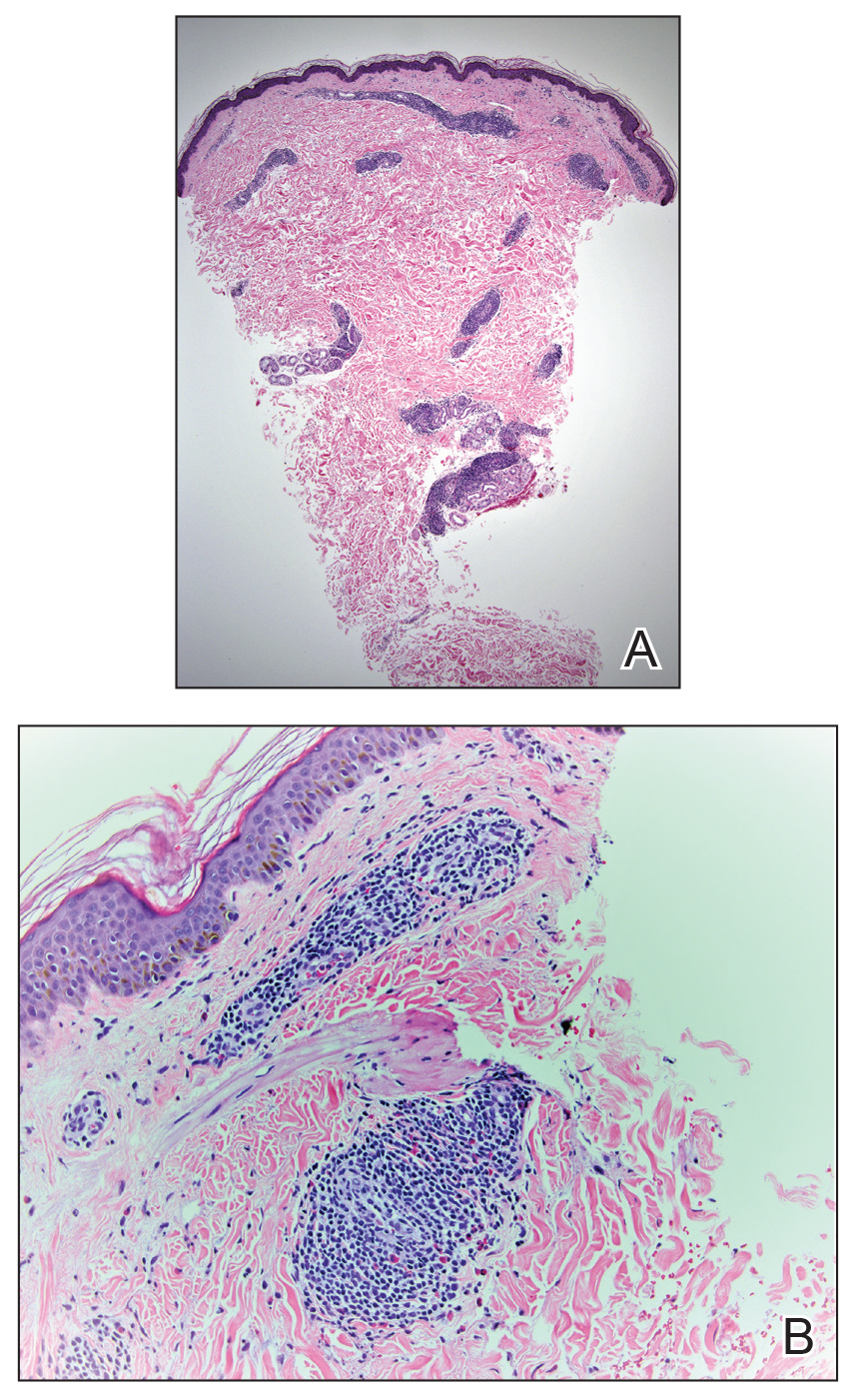
At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.


At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.


At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
Practice Points
- Bedbug exposures in the workplace are on the rise.
- High clinical suspicion is required when atypical dermatoses are not responding to therapy and histology suggests arthropod exposure.
- Once detected, partnership with occupational health and pest management experts is critical to eradicate bedbugs.
Pediatric Warts: Update on Interventions
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10
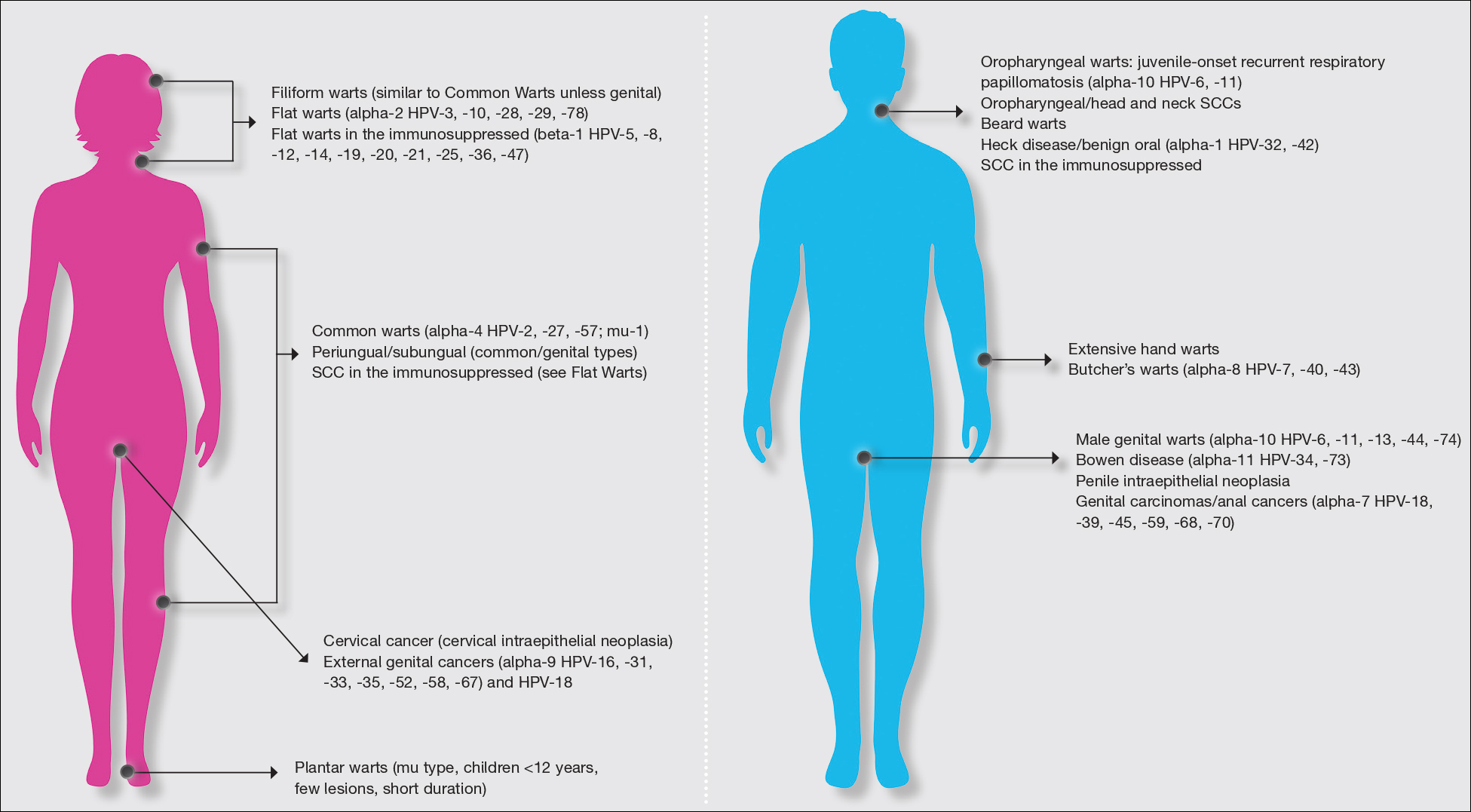
Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).
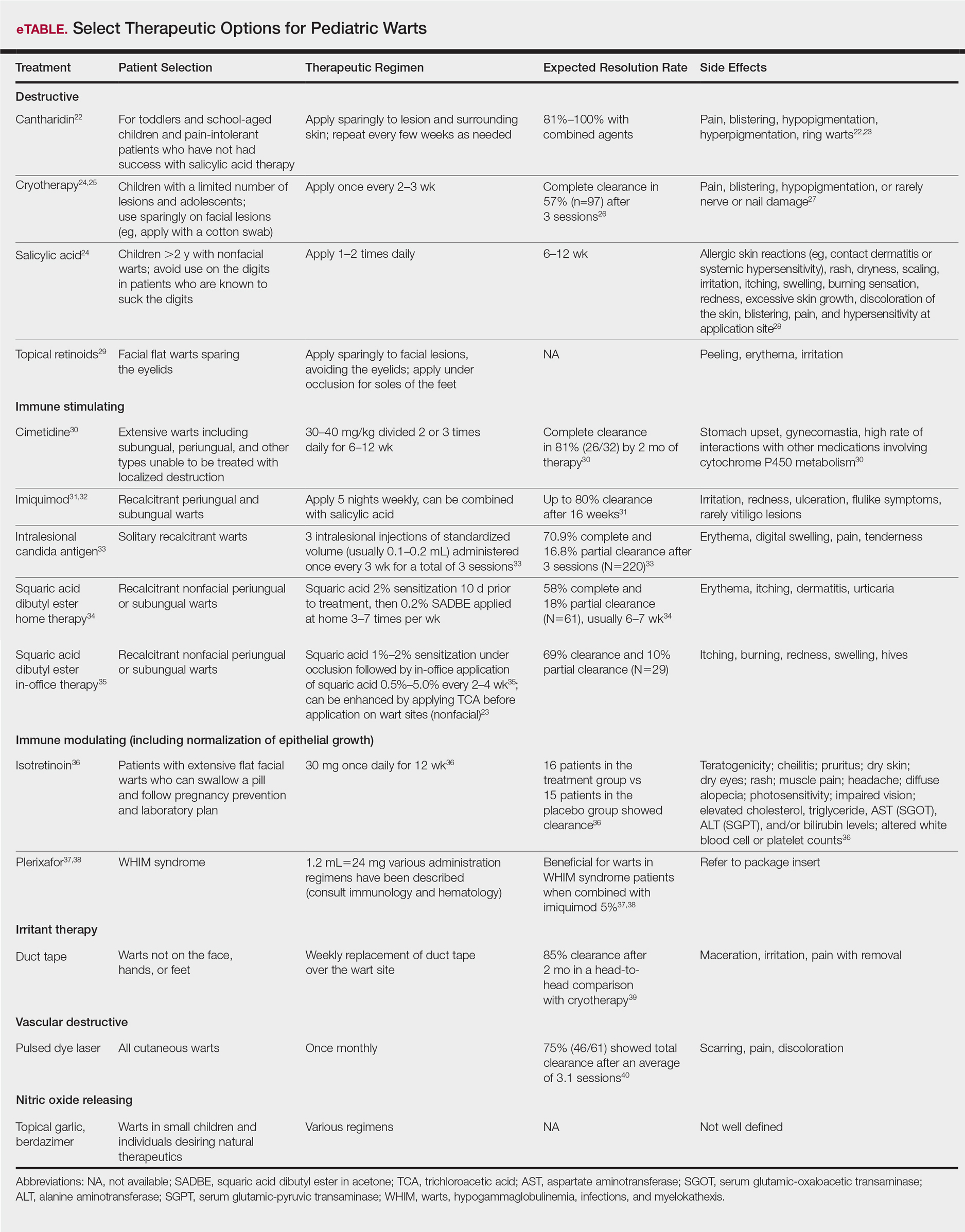
Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.
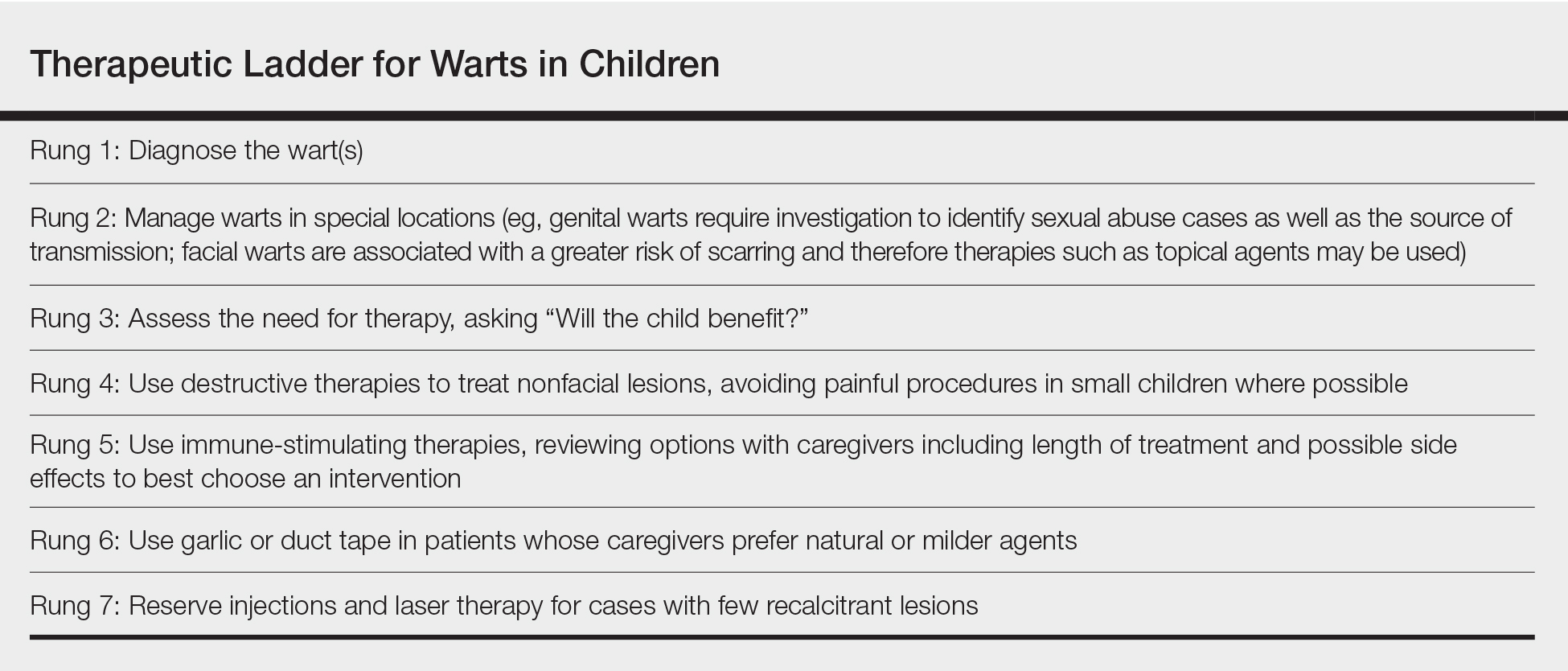
Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10

Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).

Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.

Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10

Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).

Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.

Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
Practice Points
- Warts are caused by infection with the human papillomavirus.
- Warts are extremely common in all age groups, but risk factors and types of lesions vary by age and location of lesions.
- Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; vascular destructive; irritant; and nitric oxide releasing.