User login
Hepatitis vaccination update
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.

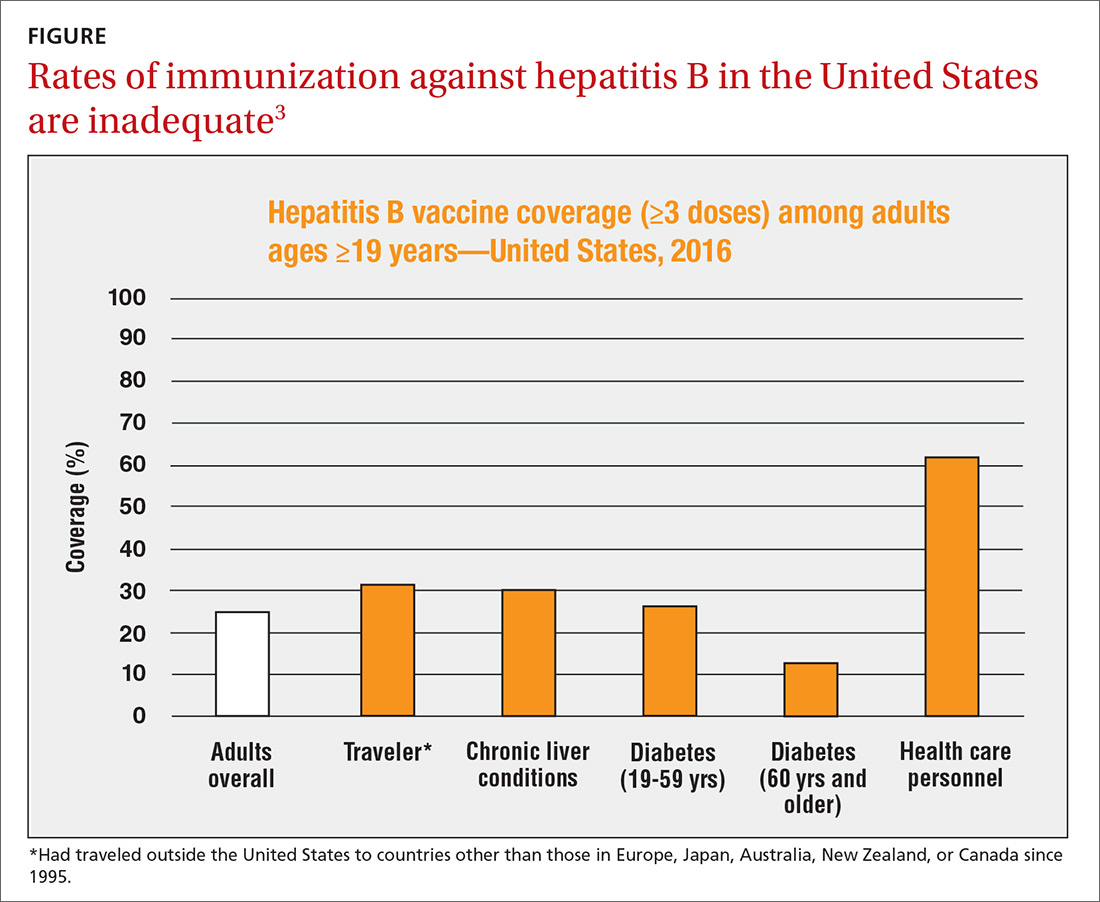
The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3
As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site

Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.

The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3

As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site

Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.

The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3

As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site

Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
Best practices lower postsepsis risk, but only if implemented
SAN DIEGO – North Carolina health care workers often failed to provide best-practice follow-up to patients who were released after hospitalization for sepsis, a small study has found. There may be a cost to this gap:
“It’s disappointing to see that we are not providing these seemingly common-sense care processes to our sepsis patients at discharge,” said study lead author Stephanie Parks Taylor, MD, of Atrium Health’s Carolinas Medical Center in Charlotte, in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “We need to develop and implement strategies to improve outcomes for sepsis patients, not just while they are in the hospital, but after discharge as well.”
A 2017 report estimated that 1.7 million adults were hospitalized for sepsis in the United States in 2014, and 270,000 died (JAMA. 2017;318[13]:1241-9). Age-adjusted sepsis death rates in the United States are highest in states in the Eastern and Southern regions, a 2017 report from the Centers for Disease Control and Prevention suggested; North Carolina has the 32nd-worst sepsis death rate in the country (12.4 deaths per 100,000 population).
Dr. Taylor said some recent news about sepsis is promising. “We’ve seen decreasing mortality rates from initiatives that improve the early detection of sepsis and rapid delivery of antibiotics, fluids, and other treatment. However, there is growing evidence that patients who survive an episode of sepsis face residual health deficits. Many sepsis survivors are left with new functional, cognitive, or mental health declines or worsening of their underlying comorbidities. Unfortunately, these patients have high rates of mortality and hospital readmission that persist for multiple years after hospitalization.”
Indeed, a 2013 report linked sepsis to significantly higher mortality risk over 5 years, after accounting for comorbidities. Postsepsis patients were 13 times more likely to die over the first year after hospitalization than counterparts who didn’t have sepsis (BMJ Open. 2014;4:e004283).
For the new study, Dr. Taylor said, “we aimed to evaluate current care practices with the hope to identify a postsepsis management strategy that could help nudge these patients towards a more meaningful recovery.”
The researchers retrospectively tracked a random sample of 100 patients (median age, 63 years), who were discharged following an admission for sepsis in 2017. They were treated at eight acute care hospitals in western and central North Carolina and hospitalized for a median of 5 days; 75 were discharged to home (17 received home health services there), 17 went to skilled nursing or long-term care facilities, and 8 went to hospice or another location.
The researchers analyzed whether the patients received four kinds of postsepsis care within 90 days, as recommended by a 2018 review: screening for common functional impairments (53/100 patients received this screening); adjustment of medications as needed following discharge (53/100 patients); monitoring for common and preventable causes for health deterioration, such as infection, chronic lung disease, or heart failure exacerbation (37/100); and assessment for palliative care (25/100 patients) (JAMA. 2018;319[1]:62-75).
Within 90 days of discharge, 34 patients were readmitted and 17 died. The 32 patients who received at least two recommended kinds of postsepsis care were less likely to be readmitted or die (9/32) than those who got zero or one recommended kind of care (34/68; odds ratio, 0.26; 95% confidence ratio, 0.09-0.82).
In an interview, study coauthor Marc Kowalkowski, PhD, associate professor with Atrium Health’s Center for Outcomes Research and Evaluation, said he was hesitant to only allocate blame to hospitals or outpatient providers. “Transition out of the hospital is an extremely complex event, involving often fragmented care settings, and sepsis patients tend to be more complicated than other patients. It probably makes sense to provide an added layer of support during the transition out of the hospital for patients who are at high risk for poor outcomes.”
Overall, the findings are “a call for clinicians to realize sepsis is more than just an acute illness. The combination of a growing number of sepsis survivors and the increased health problems following an episode of sepsis creates an urgent public health challenge,” Dr. Taylor said.
Is more home health an important part of a solution? It may be helpful, Dr. Taylor said, but “our data suggest that there really needs to be better coordination to bridge between the inpatient and outpatient transition. We are currently conducting a randomized study to investigate whether these types of care processes can be delivered effectively through a nurse navigator to improve patient outcomes.”
Fortunately, she said, the findings suggest “we don’t have to reinvent the wheel. We just have to work on implementation of strategies for care processes that we are already familiar with.”
No funding was reported. None of the study authors reported relevant disclosures.
SOURCE: Taylor SP et al. CCC48, Abstract 1320.
SAN DIEGO – North Carolina health care workers often failed to provide best-practice follow-up to patients who were released after hospitalization for sepsis, a small study has found. There may be a cost to this gap:
“It’s disappointing to see that we are not providing these seemingly common-sense care processes to our sepsis patients at discharge,” said study lead author Stephanie Parks Taylor, MD, of Atrium Health’s Carolinas Medical Center in Charlotte, in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “We need to develop and implement strategies to improve outcomes for sepsis patients, not just while they are in the hospital, but after discharge as well.”
A 2017 report estimated that 1.7 million adults were hospitalized for sepsis in the United States in 2014, and 270,000 died (JAMA. 2017;318[13]:1241-9). Age-adjusted sepsis death rates in the United States are highest in states in the Eastern and Southern regions, a 2017 report from the Centers for Disease Control and Prevention suggested; North Carolina has the 32nd-worst sepsis death rate in the country (12.4 deaths per 100,000 population).
Dr. Taylor said some recent news about sepsis is promising. “We’ve seen decreasing mortality rates from initiatives that improve the early detection of sepsis and rapid delivery of antibiotics, fluids, and other treatment. However, there is growing evidence that patients who survive an episode of sepsis face residual health deficits. Many sepsis survivors are left with new functional, cognitive, or mental health declines or worsening of their underlying comorbidities. Unfortunately, these patients have high rates of mortality and hospital readmission that persist for multiple years after hospitalization.”
Indeed, a 2013 report linked sepsis to significantly higher mortality risk over 5 years, after accounting for comorbidities. Postsepsis patients were 13 times more likely to die over the first year after hospitalization than counterparts who didn’t have sepsis (BMJ Open. 2014;4:e004283).
For the new study, Dr. Taylor said, “we aimed to evaluate current care practices with the hope to identify a postsepsis management strategy that could help nudge these patients towards a more meaningful recovery.”
The researchers retrospectively tracked a random sample of 100 patients (median age, 63 years), who were discharged following an admission for sepsis in 2017. They were treated at eight acute care hospitals in western and central North Carolina and hospitalized for a median of 5 days; 75 were discharged to home (17 received home health services there), 17 went to skilled nursing or long-term care facilities, and 8 went to hospice or another location.
The researchers analyzed whether the patients received four kinds of postsepsis care within 90 days, as recommended by a 2018 review: screening for common functional impairments (53/100 patients received this screening); adjustment of medications as needed following discharge (53/100 patients); monitoring for common and preventable causes for health deterioration, such as infection, chronic lung disease, or heart failure exacerbation (37/100); and assessment for palliative care (25/100 patients) (JAMA. 2018;319[1]:62-75).
Within 90 days of discharge, 34 patients were readmitted and 17 died. The 32 patients who received at least two recommended kinds of postsepsis care were less likely to be readmitted or die (9/32) than those who got zero or one recommended kind of care (34/68; odds ratio, 0.26; 95% confidence ratio, 0.09-0.82).
In an interview, study coauthor Marc Kowalkowski, PhD, associate professor with Atrium Health’s Center for Outcomes Research and Evaluation, said he was hesitant to only allocate blame to hospitals or outpatient providers. “Transition out of the hospital is an extremely complex event, involving often fragmented care settings, and sepsis patients tend to be more complicated than other patients. It probably makes sense to provide an added layer of support during the transition out of the hospital for patients who are at high risk for poor outcomes.”
Overall, the findings are “a call for clinicians to realize sepsis is more than just an acute illness. The combination of a growing number of sepsis survivors and the increased health problems following an episode of sepsis creates an urgent public health challenge,” Dr. Taylor said.
Is more home health an important part of a solution? It may be helpful, Dr. Taylor said, but “our data suggest that there really needs to be better coordination to bridge between the inpatient and outpatient transition. We are currently conducting a randomized study to investigate whether these types of care processes can be delivered effectively through a nurse navigator to improve patient outcomes.”
Fortunately, she said, the findings suggest “we don’t have to reinvent the wheel. We just have to work on implementation of strategies for care processes that we are already familiar with.”
No funding was reported. None of the study authors reported relevant disclosures.
SOURCE: Taylor SP et al. CCC48, Abstract 1320.
SAN DIEGO – North Carolina health care workers often failed to provide best-practice follow-up to patients who were released after hospitalization for sepsis, a small study has found. There may be a cost to this gap:
“It’s disappointing to see that we are not providing these seemingly common-sense care processes to our sepsis patients at discharge,” said study lead author Stephanie Parks Taylor, MD, of Atrium Health’s Carolinas Medical Center in Charlotte, in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “We need to develop and implement strategies to improve outcomes for sepsis patients, not just while they are in the hospital, but after discharge as well.”
A 2017 report estimated that 1.7 million adults were hospitalized for sepsis in the United States in 2014, and 270,000 died (JAMA. 2017;318[13]:1241-9). Age-adjusted sepsis death rates in the United States are highest in states in the Eastern and Southern regions, a 2017 report from the Centers for Disease Control and Prevention suggested; North Carolina has the 32nd-worst sepsis death rate in the country (12.4 deaths per 100,000 population).
Dr. Taylor said some recent news about sepsis is promising. “We’ve seen decreasing mortality rates from initiatives that improve the early detection of sepsis and rapid delivery of antibiotics, fluids, and other treatment. However, there is growing evidence that patients who survive an episode of sepsis face residual health deficits. Many sepsis survivors are left with new functional, cognitive, or mental health declines or worsening of their underlying comorbidities. Unfortunately, these patients have high rates of mortality and hospital readmission that persist for multiple years after hospitalization.”
Indeed, a 2013 report linked sepsis to significantly higher mortality risk over 5 years, after accounting for comorbidities. Postsepsis patients were 13 times more likely to die over the first year after hospitalization than counterparts who didn’t have sepsis (BMJ Open. 2014;4:e004283).
For the new study, Dr. Taylor said, “we aimed to evaluate current care practices with the hope to identify a postsepsis management strategy that could help nudge these patients towards a more meaningful recovery.”
The researchers retrospectively tracked a random sample of 100 patients (median age, 63 years), who were discharged following an admission for sepsis in 2017. They were treated at eight acute care hospitals in western and central North Carolina and hospitalized for a median of 5 days; 75 were discharged to home (17 received home health services there), 17 went to skilled nursing or long-term care facilities, and 8 went to hospice or another location.
The researchers analyzed whether the patients received four kinds of postsepsis care within 90 days, as recommended by a 2018 review: screening for common functional impairments (53/100 patients received this screening); adjustment of medications as needed following discharge (53/100 patients); monitoring for common and preventable causes for health deterioration, such as infection, chronic lung disease, or heart failure exacerbation (37/100); and assessment for palliative care (25/100 patients) (JAMA. 2018;319[1]:62-75).
Within 90 days of discharge, 34 patients were readmitted and 17 died. The 32 patients who received at least two recommended kinds of postsepsis care were less likely to be readmitted or die (9/32) than those who got zero or one recommended kind of care (34/68; odds ratio, 0.26; 95% confidence ratio, 0.09-0.82).
In an interview, study coauthor Marc Kowalkowski, PhD, associate professor with Atrium Health’s Center for Outcomes Research and Evaluation, said he was hesitant to only allocate blame to hospitals or outpatient providers. “Transition out of the hospital is an extremely complex event, involving often fragmented care settings, and sepsis patients tend to be more complicated than other patients. It probably makes sense to provide an added layer of support during the transition out of the hospital for patients who are at high risk for poor outcomes.”
Overall, the findings are “a call for clinicians to realize sepsis is more than just an acute illness. The combination of a growing number of sepsis survivors and the increased health problems following an episode of sepsis creates an urgent public health challenge,” Dr. Taylor said.
Is more home health an important part of a solution? It may be helpful, Dr. Taylor said, but “our data suggest that there really needs to be better coordination to bridge between the inpatient and outpatient transition. We are currently conducting a randomized study to investigate whether these types of care processes can be delivered effectively through a nurse navigator to improve patient outcomes.”
Fortunately, she said, the findings suggest “we don’t have to reinvent the wheel. We just have to work on implementation of strategies for care processes that we are already familiar with.”
No funding was reported. None of the study authors reported relevant disclosures.
SOURCE: Taylor SP et al. CCC48, Abstract 1320.
REPORTING FROM CCC48
Rounding team boosts ICU liberation efforts
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
REPORTING FROM CCC48
HCV treatment with DAA regimens linked to reduced diabetes risk
SEATTLE – Treatment of hepatitis C virus (HCV) with new direct-acting antiviral (DAA) regimens is associated with improved glucose control and reduced incidence of type 2 diabetes when compared to treatment with pegylated interferon/ribavirin (PEG/RBV ) and untreated controls, according to a new analysis of the Electronically Retrieved Cohort of HCV Infected Veterans.
“Previously, people who had diabetes were considered slightly more difficult to treat because their virologic response was a little lower, but now this is not the case, and we have the added benefit of reducing the incidence of diabetes,” said Adeel Butt, MD, professor of medicine and health care policy and research at Weill Cornell Medicine, New York and Qatar, in an interview. Dr. Butt presented the study at the Conference on Retroviruses & Opportunistic Infections.
The incidence of diabetes dropped in the overall treated cohort, compared with untreated patients, but this benefit was driven by the effect of DAAs, as there was no significant difference between PEG/RBV–treated patients and controls. “It’s another reason to argue with people who make it difficult to treat. Our biggest barriers to treating everyone with hepatitis C has to do with reimbursement and the capacity of the health care system, and this is another reason that we need to overcome those barriers. It’s an important insight that provides one more reason to try to continue to eradicate hepatitis C in our population,” said Robert Schooley, MD, professor of medicine at the University of California, San Diego, in an interview.
Patients may also need some reassurance, given concerns that have arisen over the potential for older regimens to cause diabetes. Dr. Butt cited an example of a patient who has an acute myocardial infarction, has a high body mass, and wants to know if DAAs will help or hurt them. “We see [such patients] frequently. This is pretty reassuring not only that DAAs don’t increase risk, but they actually decrease the risk of diabetes as opposed to older treatments. There is a growing body of evidence that non–liver [related conditions] significantly improve with treatment,” he said.
The results could also help prioritize patients for treatment. “It may be important to the patients who are at elevated risk of developing diabetes. They may need to be monitored more closely and offered treatment earlier, perhaps, but that requires more study,” said Dr. Butt.
The researchers excluded patients with HIV or hepatitis B virus, and those who had prevalent diabetes. The cohort included 26,043 treated patients and 26,043 propensity score–matched untreated control patients. Treated patients underwent at least 8 weeks of DAA or 24 weeks of PEG/RBV. Demographically, 54% of patients were white, 29% were black, 3% were Hispanic, and 96% of the patients were male. About one-third had a body mass index of 30 or above.
The incidence of diabetes was 20.6 per 1,000 person-years of follow-up among untreated patients, compared with 15.5 among treated patients (P less than .0001). The incidence was 19.8 in patients treated with PEG/RBV (P =.39) and 9.9 in those treated with DAAs (P less than. 001; hazard ratio, 0.48; P less than .0001). The incidence of diabetes in those with a sustained viral response (SVR) was 13.3 per 1,000 person-years, compared with 19.2 in patients with no SVR (P less than .0001). The incidence of diabetes was lower in treated patients regardless of baseline FIB-4 (Fibrosis-4, a liver fibrosis score) levels.
The study was funded by Gilead. Dr. Butt has had research grants from Gilead and Dr. Schooley is on Gilead’s scientific advisory board.
SOURCE: A Butt et al. CROI 2019. Abstract 88.
SEATTLE – Treatment of hepatitis C virus (HCV) with new direct-acting antiviral (DAA) regimens is associated with improved glucose control and reduced incidence of type 2 diabetes when compared to treatment with pegylated interferon/ribavirin (PEG/RBV ) and untreated controls, according to a new analysis of the Electronically Retrieved Cohort of HCV Infected Veterans.
“Previously, people who had diabetes were considered slightly more difficult to treat because their virologic response was a little lower, but now this is not the case, and we have the added benefit of reducing the incidence of diabetes,” said Adeel Butt, MD, professor of medicine and health care policy and research at Weill Cornell Medicine, New York and Qatar, in an interview. Dr. Butt presented the study at the Conference on Retroviruses & Opportunistic Infections.
The incidence of diabetes dropped in the overall treated cohort, compared with untreated patients, but this benefit was driven by the effect of DAAs, as there was no significant difference between PEG/RBV–treated patients and controls. “It’s another reason to argue with people who make it difficult to treat. Our biggest barriers to treating everyone with hepatitis C has to do with reimbursement and the capacity of the health care system, and this is another reason that we need to overcome those barriers. It’s an important insight that provides one more reason to try to continue to eradicate hepatitis C in our population,” said Robert Schooley, MD, professor of medicine at the University of California, San Diego, in an interview.
Patients may also need some reassurance, given concerns that have arisen over the potential for older regimens to cause diabetes. Dr. Butt cited an example of a patient who has an acute myocardial infarction, has a high body mass, and wants to know if DAAs will help or hurt them. “We see [such patients] frequently. This is pretty reassuring not only that DAAs don’t increase risk, but they actually decrease the risk of diabetes as opposed to older treatments. There is a growing body of evidence that non–liver [related conditions] significantly improve with treatment,” he said.
The results could also help prioritize patients for treatment. “It may be important to the patients who are at elevated risk of developing diabetes. They may need to be monitored more closely and offered treatment earlier, perhaps, but that requires more study,” said Dr. Butt.
The researchers excluded patients with HIV or hepatitis B virus, and those who had prevalent diabetes. The cohort included 26,043 treated patients and 26,043 propensity score–matched untreated control patients. Treated patients underwent at least 8 weeks of DAA or 24 weeks of PEG/RBV. Demographically, 54% of patients were white, 29% were black, 3% were Hispanic, and 96% of the patients were male. About one-third had a body mass index of 30 or above.
The incidence of diabetes was 20.6 per 1,000 person-years of follow-up among untreated patients, compared with 15.5 among treated patients (P less than .0001). The incidence was 19.8 in patients treated with PEG/RBV (P =.39) and 9.9 in those treated with DAAs (P less than. 001; hazard ratio, 0.48; P less than .0001). The incidence of diabetes in those with a sustained viral response (SVR) was 13.3 per 1,000 person-years, compared with 19.2 in patients with no SVR (P less than .0001). The incidence of diabetes was lower in treated patients regardless of baseline FIB-4 (Fibrosis-4, a liver fibrosis score) levels.
The study was funded by Gilead. Dr. Butt has had research grants from Gilead and Dr. Schooley is on Gilead’s scientific advisory board.
SOURCE: A Butt et al. CROI 2019. Abstract 88.
SEATTLE – Treatment of hepatitis C virus (HCV) with new direct-acting antiviral (DAA) regimens is associated with improved glucose control and reduced incidence of type 2 diabetes when compared to treatment with pegylated interferon/ribavirin (PEG/RBV ) and untreated controls, according to a new analysis of the Electronically Retrieved Cohort of HCV Infected Veterans.
“Previously, people who had diabetes were considered slightly more difficult to treat because their virologic response was a little lower, but now this is not the case, and we have the added benefit of reducing the incidence of diabetes,” said Adeel Butt, MD, professor of medicine and health care policy and research at Weill Cornell Medicine, New York and Qatar, in an interview. Dr. Butt presented the study at the Conference on Retroviruses & Opportunistic Infections.
The incidence of diabetes dropped in the overall treated cohort, compared with untreated patients, but this benefit was driven by the effect of DAAs, as there was no significant difference between PEG/RBV–treated patients and controls. “It’s another reason to argue with people who make it difficult to treat. Our biggest barriers to treating everyone with hepatitis C has to do with reimbursement and the capacity of the health care system, and this is another reason that we need to overcome those barriers. It’s an important insight that provides one more reason to try to continue to eradicate hepatitis C in our population,” said Robert Schooley, MD, professor of medicine at the University of California, San Diego, in an interview.
Patients may also need some reassurance, given concerns that have arisen over the potential for older regimens to cause diabetes. Dr. Butt cited an example of a patient who has an acute myocardial infarction, has a high body mass, and wants to know if DAAs will help or hurt them. “We see [such patients] frequently. This is pretty reassuring not only that DAAs don’t increase risk, but they actually decrease the risk of diabetes as opposed to older treatments. There is a growing body of evidence that non–liver [related conditions] significantly improve with treatment,” he said.
The results could also help prioritize patients for treatment. “It may be important to the patients who are at elevated risk of developing diabetes. They may need to be monitored more closely and offered treatment earlier, perhaps, but that requires more study,” said Dr. Butt.
The researchers excluded patients with HIV or hepatitis B virus, and those who had prevalent diabetes. The cohort included 26,043 treated patients and 26,043 propensity score–matched untreated control patients. Treated patients underwent at least 8 weeks of DAA or 24 weeks of PEG/RBV. Demographically, 54% of patients were white, 29% were black, 3% were Hispanic, and 96% of the patients were male. About one-third had a body mass index of 30 or above.
The incidence of diabetes was 20.6 per 1,000 person-years of follow-up among untreated patients, compared with 15.5 among treated patients (P less than .0001). The incidence was 19.8 in patients treated with PEG/RBV (P =.39) and 9.9 in those treated with DAAs (P less than. 001; hazard ratio, 0.48; P less than .0001). The incidence of diabetes in those with a sustained viral response (SVR) was 13.3 per 1,000 person-years, compared with 19.2 in patients with no SVR (P less than .0001). The incidence of diabetes was lower in treated patients regardless of baseline FIB-4 (Fibrosis-4, a liver fibrosis score) levels.
The study was funded by Gilead. Dr. Butt has had research grants from Gilead and Dr. Schooley is on Gilead’s scientific advisory board.
SOURCE: A Butt et al. CROI 2019. Abstract 88.
REPORTING FROM CROI 2019
One-time, universal hepatitis C testing cost effective, researchers say
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Poor COPD management might increase MI risk in HIV
SEATTLE – Chronic obstructive pulmonary disease is independently associated with an increased risk of myocardial infarction in people with HIV, according to a report at the Conference on Retroviruses and Opportunistic Infections.
Chronic obstructive pulmonary disease (COPD) is known to increase the risk of myocardial infarction (MI) in the general population, but hadn’t been shown until now to do the same in HIV. The study raises the question of whether COPD is being managed adequately in patients with the virus, according to study lead Kristina Crothers, MD, associate professor in the division of pulmonary, critical care & sleep medicine at the University of Washington, Seattle.
The investigators reviewed 25,509 HIV patients in the Center for AIDS Research Network of Integrated Clinical Systems cohort, a large electronic database of HIV-infected people. They defined COPD by diagnostic codes and inhaler prescriptions. MIs were adjudicated by review.
The team identified 423 subjects with moderate to severe COPD, and 698 who had MIs, including 339 type 1 MIs (T1MI) from a ruptured plaque (54%), and 294 (46%) type 2 heart attacks (T2MI) from a supply-demand mismatch due to sepsis or some other problem. In general, T2MIs are far more common in people with HIV.
COPD was associated with a greater than twofold increased risk of MI after adjustment for age, sex, viral load, nadir CD4 count, hypertension, and other confounders. The risk dropped slightly when smoking – both current smoking and pack years – was added to the model (adjusted hazard ratio 1.88, 95% confidence interval, 1.34-2.63).
The association was particularly strong for T2MI, especially in the setting of bacteremia and sepsis, and unlike T1MI, it remained significant after adjustment for smoking.
The study establishes a link between COPD and MI in HIV, but it could not answer what’s going on. Chronic inflammation from the virus could be at play, but the team also found hints of inadequate COPD management.
“About 60% of patients were on inhalers ... but only about 25% of them were on long-acting inhalers. 75% were only on short-acting.” That’s a problem because long-acting inhalers are needed to control exacerbations, Dr. Crothers said.
The study didn’t capture exacerbation rates, but increased rates could help explain the MI risk. Increased rates of pneumonia could as well, since pneumonia is a common cause of sepsis.
“We need to better manage complications of COPD in this population. I think optimizing long-term COPD management could have many beneficial effects,” Dr. Crothers said.
The National Institutes of Health funded the work. Dr. Crothers had no disclosures.
SOURCE: Crothers K et al. CROI 2019, Abstract 31.
SEATTLE – Chronic obstructive pulmonary disease is independently associated with an increased risk of myocardial infarction in people with HIV, according to a report at the Conference on Retroviruses and Opportunistic Infections.
Chronic obstructive pulmonary disease (COPD) is known to increase the risk of myocardial infarction (MI) in the general population, but hadn’t been shown until now to do the same in HIV. The study raises the question of whether COPD is being managed adequately in patients with the virus, according to study lead Kristina Crothers, MD, associate professor in the division of pulmonary, critical care & sleep medicine at the University of Washington, Seattle.
The investigators reviewed 25,509 HIV patients in the Center for AIDS Research Network of Integrated Clinical Systems cohort, a large electronic database of HIV-infected people. They defined COPD by diagnostic codes and inhaler prescriptions. MIs were adjudicated by review.
The team identified 423 subjects with moderate to severe COPD, and 698 who had MIs, including 339 type 1 MIs (T1MI) from a ruptured plaque (54%), and 294 (46%) type 2 heart attacks (T2MI) from a supply-demand mismatch due to sepsis or some other problem. In general, T2MIs are far more common in people with HIV.
COPD was associated with a greater than twofold increased risk of MI after adjustment for age, sex, viral load, nadir CD4 count, hypertension, and other confounders. The risk dropped slightly when smoking – both current smoking and pack years – was added to the model (adjusted hazard ratio 1.88, 95% confidence interval, 1.34-2.63).
The association was particularly strong for T2MI, especially in the setting of bacteremia and sepsis, and unlike T1MI, it remained significant after adjustment for smoking.
The study establishes a link between COPD and MI in HIV, but it could not answer what’s going on. Chronic inflammation from the virus could be at play, but the team also found hints of inadequate COPD management.
“About 60% of patients were on inhalers ... but only about 25% of them were on long-acting inhalers. 75% were only on short-acting.” That’s a problem because long-acting inhalers are needed to control exacerbations, Dr. Crothers said.
The study didn’t capture exacerbation rates, but increased rates could help explain the MI risk. Increased rates of pneumonia could as well, since pneumonia is a common cause of sepsis.
“We need to better manage complications of COPD in this population. I think optimizing long-term COPD management could have many beneficial effects,” Dr. Crothers said.
The National Institutes of Health funded the work. Dr. Crothers had no disclosures.
SOURCE: Crothers K et al. CROI 2019, Abstract 31.
SEATTLE – Chronic obstructive pulmonary disease is independently associated with an increased risk of myocardial infarction in people with HIV, according to a report at the Conference on Retroviruses and Opportunistic Infections.
Chronic obstructive pulmonary disease (COPD) is known to increase the risk of myocardial infarction (MI) in the general population, but hadn’t been shown until now to do the same in HIV. The study raises the question of whether COPD is being managed adequately in patients with the virus, according to study lead Kristina Crothers, MD, associate professor in the division of pulmonary, critical care & sleep medicine at the University of Washington, Seattle.
The investigators reviewed 25,509 HIV patients in the Center for AIDS Research Network of Integrated Clinical Systems cohort, a large electronic database of HIV-infected people. They defined COPD by diagnostic codes and inhaler prescriptions. MIs were adjudicated by review.
The team identified 423 subjects with moderate to severe COPD, and 698 who had MIs, including 339 type 1 MIs (T1MI) from a ruptured plaque (54%), and 294 (46%) type 2 heart attacks (T2MI) from a supply-demand mismatch due to sepsis or some other problem. In general, T2MIs are far more common in people with HIV.
COPD was associated with a greater than twofold increased risk of MI after adjustment for age, sex, viral load, nadir CD4 count, hypertension, and other confounders. The risk dropped slightly when smoking – both current smoking and pack years – was added to the model (adjusted hazard ratio 1.88, 95% confidence interval, 1.34-2.63).
The association was particularly strong for T2MI, especially in the setting of bacteremia and sepsis, and unlike T1MI, it remained significant after adjustment for smoking.
The study establishes a link between COPD and MI in HIV, but it could not answer what’s going on. Chronic inflammation from the virus could be at play, but the team also found hints of inadequate COPD management.
“About 60% of patients were on inhalers ... but only about 25% of them were on long-acting inhalers. 75% were only on short-acting.” That’s a problem because long-acting inhalers are needed to control exacerbations, Dr. Crothers said.
The study didn’t capture exacerbation rates, but increased rates could help explain the MI risk. Increased rates of pneumonia could as well, since pneumonia is a common cause of sepsis.
“We need to better manage complications of COPD in this population. I think optimizing long-term COPD management could have many beneficial effects,” Dr. Crothers said.
The National Institutes of Health funded the work. Dr. Crothers had no disclosures.
SOURCE: Crothers K et al. CROI 2019, Abstract 31.
REPORTING FROM CROI 2019
Vitamin C for sepsis? Experts take sides in sharp debate
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
EXPERT ANALYSIS FROM CCC48
Infective endocarditis isn’t what it used to be
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
REPORTING FROM ACC SNOWMASS 2019
Cutaneous Gummatous Tuberculosis in a Kidney Transplant Patient
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.
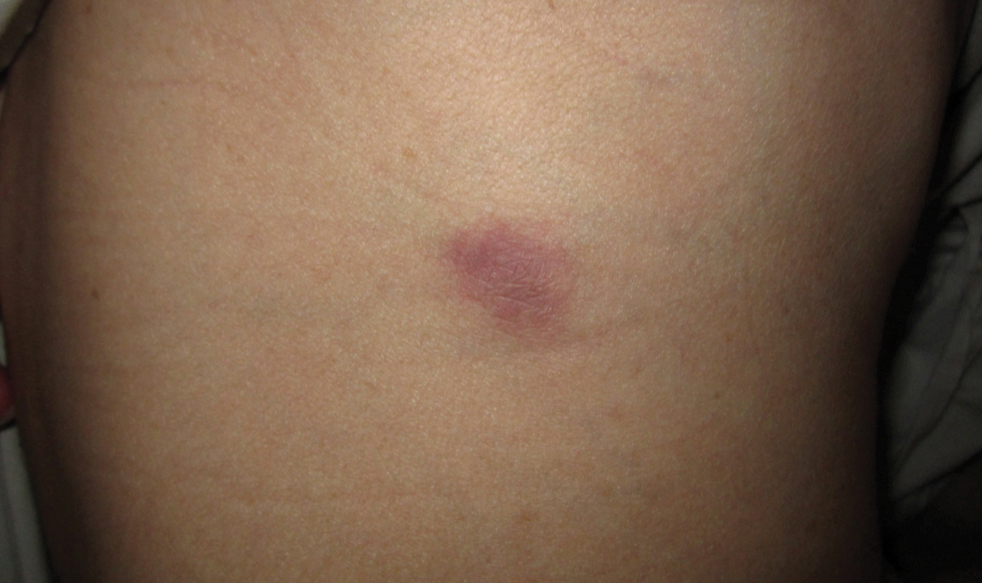
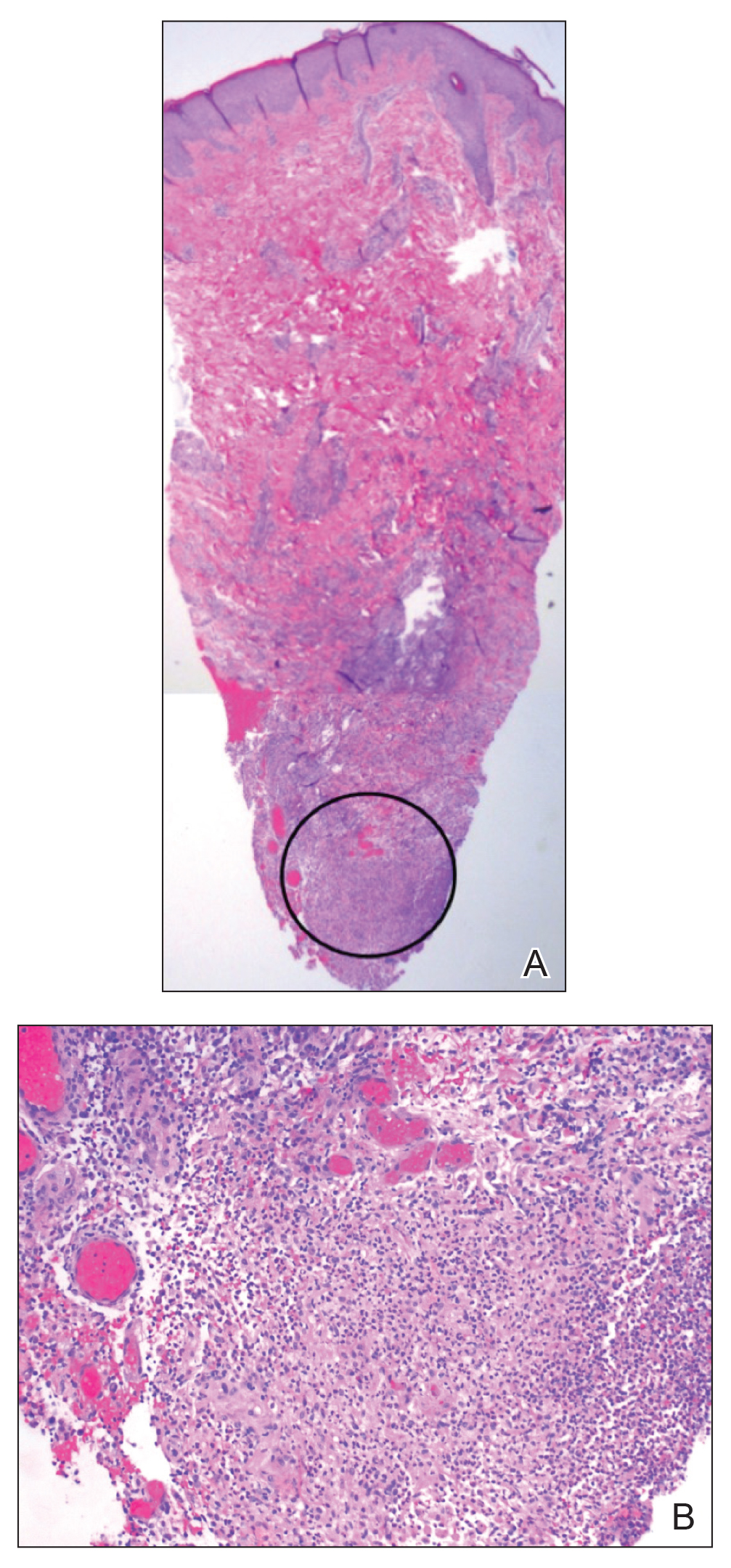
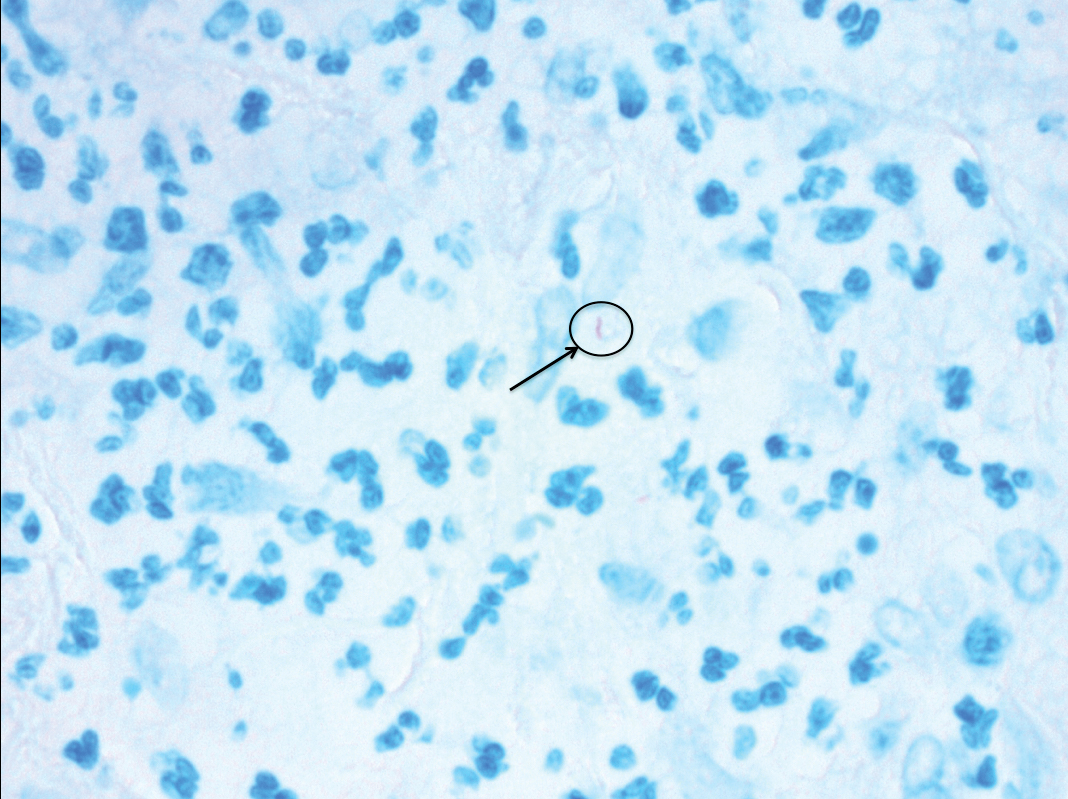
Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12
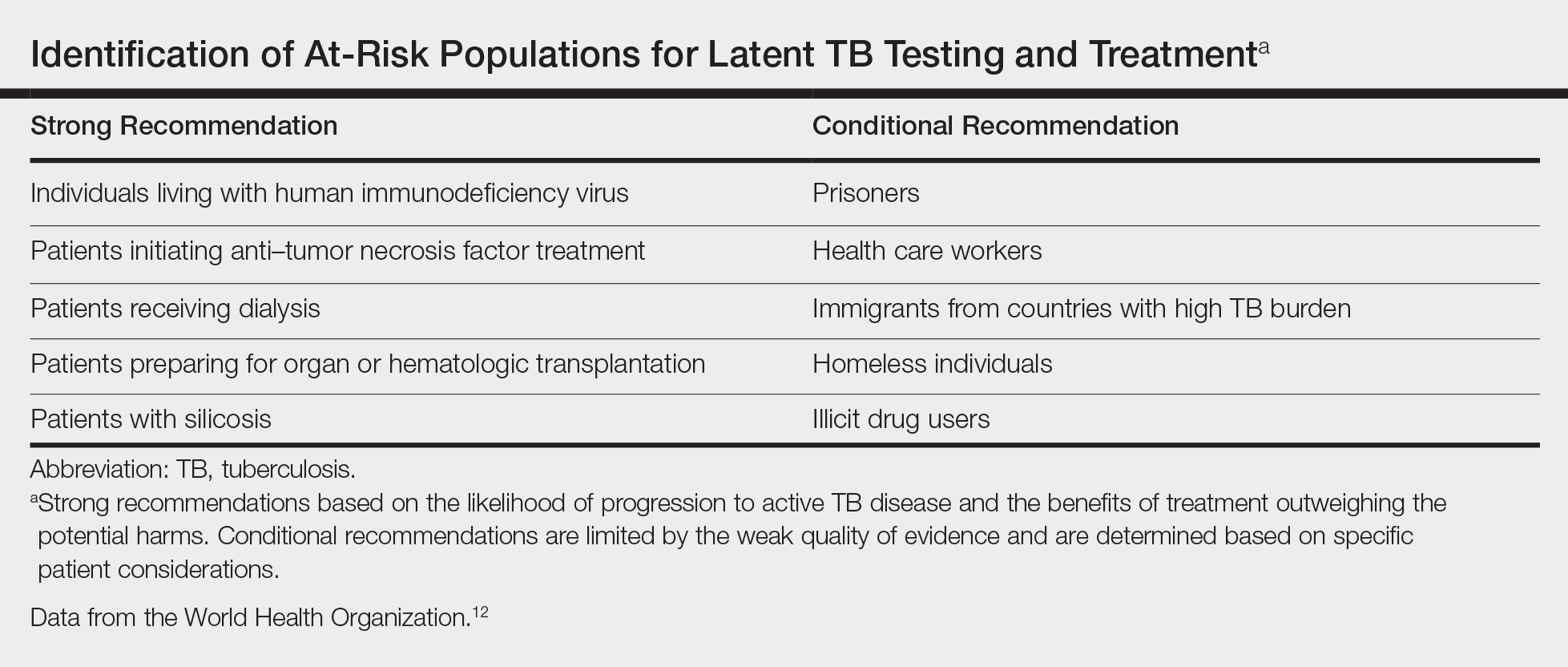
Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.



Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12

Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.



Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12

Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
Practice Points
- Transplant patients are at increased risk for infection given their immunosuppressed state.
- Although rare, cutaneous tuberculosis should be considered in the differential for cutaneous lesions in an immunosuppressed patient.
Annular Atrophic Plaques on the Forearm
Sarcoidosis is a systemic noncaseating granulomatous disease of unknown etiology. The skin is the second most common location for disease manifestation following the lungs.1 Cutaneous sarcoidosis is present in 35% of patients with sarcoidosis and may be further subtyped by its morphologic characteristics (eg, hyperpigmented, papular, nodular, atrophic, ulcerative, psoriasiform). Cutaneous sarcoidosis has an increased tendency to occur at areas of prior injury such as surgeries or tattoos.2 Although sarcoidosis affects all races and sexes, it is more prevalent in women and in the black population.3
The clinical presentation of sarcoidosis is difficult due to its morphologic variation, allowing for a wide differential diagnosis. With our patient’s presentation of atrophic plaques, the differential diagnosis included granuloma annulare, necrobiosis lipoidica, tumid lupus erythematosus, leprosy, and sarcoidosis; however, biopsy is required for definitive diagnosis. The characteristic histopathology for cutaneous sarcoidosis includes noncaseating granulomas (Figure, A) composed of epithelioid histiocytes with giant cells surrounded by a lymphocytic infiltrate. Noncaseating granulomas are considered specific to sarcoidosis and are present in 71% to 89% of biopsied lesions.4 Interestingly, our patient presented with a rare subtype of atrophic ulcerative cutaneous sarcoidosis, necrobiosis lipoidica–like sarcoidosis, which is more common in females and in the black population. It is characterized by pink to violaceous plaques with depressed centers and prominent necrotizing granuloma (Figure, B) on histopathology. In a small case series, all 3 patients with necrobiosis lipoidica–like sarcoidosis were female and had systemic involvement at the time of diagnosis.5
Sarcoidosis typically is a systemic disease with only a limited number of cases presenting with isolated cutaneous findings. Therefore, patients require a systemic evaluation, which may include a chest radiograph, complete blood cell count, ophthalmologic examinations, thyroid testing, and vitamin D monitoring, as well as an echocardiogram and electrocardiogram.2
Treatment is guided by the severity of disease. For isolated cutaneous lesions, topical or intralesional high-potency steroids have been shown to be effective.6,7 Several studies also have shown phototherapy and laser therapy as well as surgical excision to be beneficial.8-10 Once cutaneous lesions become disfiguring or systemic involvement is found, systemic corticosteroids or other immunomodulatory medications may be warranted.11 Our patient was started on intralesional and topical high-potency steroids, which failed, and she was transitioned to methotrexate and adalimumab. Unfortunately, even with advanced therapies, our patient did not have notableresolution of the lesions.
- Mañá J, Marcoval J. Skin manifestations of sarcoidosis. Presse Med. 2012;41 (6, pt 2): E355-E374.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med.2015; 36:685-702.
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics ofpatients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10, pt 1):1885-1889.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004; 31:160-168.
- Mendoza V, Vahid B, Kozic H, et al. Clinical and pathologic manifestations of necrobiosis lipoidica-like skin involvement in sarcoidosis. Joint Bone Spine. 2007; 74:647-649.
- Khatri KA, Chotzen VA, Burrall BA. Lupus pernio: successful treatment with a potent topical corticosteroid. Arch Dermatol. 1995; 131:617-618.
- Singh SK, Singh S, Pandey SS. Cutaneous sarcoidosis without systemic involvement: response to intralesional corticosteroid. Indian J Dermatol Venereol Leprol. 1996; 62:273-274.
- Karrer S, Abels C, Wimmershoff MB, et al. Successful treatment of cutaneous sarcoidosis using topical photodynamic therapy. Arch Dermatol. 2002; 138:581-584.
- Mahnke N, Medve-koenigs K, Berneburg M, et al. Cutaneous sarcoidosis treated with medium-dose UVA1. J Am Acad Dermatol. 2004; 50:978-979.
- Frederiksen LG, Jørgensen K. Sarcoidosis of the nose treated with laser surgery. Rhinology. 1996; 34:245-246.
- Baughman RP, Lower EE. Evidence-based therapy for cutaneous sarcoidosis. Clin Dermatol. 2007; 25:334-340.
Sarcoidosis is a systemic noncaseating granulomatous disease of unknown etiology. The skin is the second most common location for disease manifestation following the lungs.1 Cutaneous sarcoidosis is present in 35% of patients with sarcoidosis and may be further subtyped by its morphologic characteristics (eg, hyperpigmented, papular, nodular, atrophic, ulcerative, psoriasiform). Cutaneous sarcoidosis has an increased tendency to occur at areas of prior injury such as surgeries or tattoos.2 Although sarcoidosis affects all races and sexes, it is more prevalent in women and in the black population.3
The clinical presentation of sarcoidosis is difficult due to its morphologic variation, allowing for a wide differential diagnosis. With our patient’s presentation of atrophic plaques, the differential diagnosis included granuloma annulare, necrobiosis lipoidica, tumid lupus erythematosus, leprosy, and sarcoidosis; however, biopsy is required for definitive diagnosis. The characteristic histopathology for cutaneous sarcoidosis includes noncaseating granulomas (Figure, A) composed of epithelioid histiocytes with giant cells surrounded by a lymphocytic infiltrate. Noncaseating granulomas are considered specific to sarcoidosis and are present in 71% to 89% of biopsied lesions.4 Interestingly, our patient presented with a rare subtype of atrophic ulcerative cutaneous sarcoidosis, necrobiosis lipoidica–like sarcoidosis, which is more common in females and in the black population. It is characterized by pink to violaceous plaques with depressed centers and prominent necrotizing granuloma (Figure, B) on histopathology. In a small case series, all 3 patients with necrobiosis lipoidica–like sarcoidosis were female and had systemic involvement at the time of diagnosis.5

Sarcoidosis typically is a systemic disease with only a limited number of cases presenting with isolated cutaneous findings. Therefore, patients require a systemic evaluation, which may include a chest radiograph, complete blood cell count, ophthalmologic examinations, thyroid testing, and vitamin D monitoring, as well as an echocardiogram and electrocardiogram.2
Treatment is guided by the severity of disease. For isolated cutaneous lesions, topical or intralesional high-potency steroids have been shown to be effective.6,7 Several studies also have shown phototherapy and laser therapy as well as surgical excision to be beneficial.8-10 Once cutaneous lesions become disfiguring or systemic involvement is found, systemic corticosteroids or other immunomodulatory medications may be warranted.11 Our patient was started on intralesional and topical high-potency steroids, which failed, and she was transitioned to methotrexate and adalimumab. Unfortunately, even with advanced therapies, our patient did not have notableresolution of the lesions.
Sarcoidosis is a systemic noncaseating granulomatous disease of unknown etiology. The skin is the second most common location for disease manifestation following the lungs.1 Cutaneous sarcoidosis is present in 35% of patients with sarcoidosis and may be further subtyped by its morphologic characteristics (eg, hyperpigmented, papular, nodular, atrophic, ulcerative, psoriasiform). Cutaneous sarcoidosis has an increased tendency to occur at areas of prior injury such as surgeries or tattoos.2 Although sarcoidosis affects all races and sexes, it is more prevalent in women and in the black population.3
The clinical presentation of sarcoidosis is difficult due to its morphologic variation, allowing for a wide differential diagnosis. With our patient’s presentation of atrophic plaques, the differential diagnosis included granuloma annulare, necrobiosis lipoidica, tumid lupus erythematosus, leprosy, and sarcoidosis; however, biopsy is required for definitive diagnosis. The characteristic histopathology for cutaneous sarcoidosis includes noncaseating granulomas (Figure, A) composed of epithelioid histiocytes with giant cells surrounded by a lymphocytic infiltrate. Noncaseating granulomas are considered specific to sarcoidosis and are present in 71% to 89% of biopsied lesions.4 Interestingly, our patient presented with a rare subtype of atrophic ulcerative cutaneous sarcoidosis, necrobiosis lipoidica–like sarcoidosis, which is more common in females and in the black population. It is characterized by pink to violaceous plaques with depressed centers and prominent necrotizing granuloma (Figure, B) on histopathology. In a small case series, all 3 patients with necrobiosis lipoidica–like sarcoidosis were female and had systemic involvement at the time of diagnosis.5

Sarcoidosis typically is a systemic disease with only a limited number of cases presenting with isolated cutaneous findings. Therefore, patients require a systemic evaluation, which may include a chest radiograph, complete blood cell count, ophthalmologic examinations, thyroid testing, and vitamin D monitoring, as well as an echocardiogram and electrocardiogram.2
Treatment is guided by the severity of disease. For isolated cutaneous lesions, topical or intralesional high-potency steroids have been shown to be effective.6,7 Several studies also have shown phototherapy and laser therapy as well as surgical excision to be beneficial.8-10 Once cutaneous lesions become disfiguring or systemic involvement is found, systemic corticosteroids or other immunomodulatory medications may be warranted.11 Our patient was started on intralesional and topical high-potency steroids, which failed, and she was transitioned to methotrexate and adalimumab. Unfortunately, even with advanced therapies, our patient did not have notableresolution of the lesions.
- Mañá J, Marcoval J. Skin manifestations of sarcoidosis. Presse Med. 2012;41 (6, pt 2): E355-E374.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med.2015; 36:685-702.
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics ofpatients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10, pt 1):1885-1889.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004; 31:160-168.
- Mendoza V, Vahid B, Kozic H, et al. Clinical and pathologic manifestations of necrobiosis lipoidica-like skin involvement in sarcoidosis. Joint Bone Spine. 2007; 74:647-649.
- Khatri KA, Chotzen VA, Burrall BA. Lupus pernio: successful treatment with a potent topical corticosteroid. Arch Dermatol. 1995; 131:617-618.
- Singh SK, Singh S, Pandey SS. Cutaneous sarcoidosis without systemic involvement: response to intralesional corticosteroid. Indian J Dermatol Venereol Leprol. 1996; 62:273-274.
- Karrer S, Abels C, Wimmershoff MB, et al. Successful treatment of cutaneous sarcoidosis using topical photodynamic therapy. Arch Dermatol. 2002; 138:581-584.
- Mahnke N, Medve-koenigs K, Berneburg M, et al. Cutaneous sarcoidosis treated with medium-dose UVA1. J Am Acad Dermatol. 2004; 50:978-979.
- Frederiksen LG, Jørgensen K. Sarcoidosis of the nose treated with laser surgery. Rhinology. 1996; 34:245-246.
- Baughman RP, Lower EE. Evidence-based therapy for cutaneous sarcoidosis. Clin Dermatol. 2007; 25:334-340.
- Mañá J, Marcoval J. Skin manifestations of sarcoidosis. Presse Med. 2012;41 (6, pt 2): E355-E374.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med.2015; 36:685-702.
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics ofpatients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10, pt 1):1885-1889.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004; 31:160-168.
- Mendoza V, Vahid B, Kozic H, et al. Clinical and pathologic manifestations of necrobiosis lipoidica-like skin involvement in sarcoidosis. Joint Bone Spine. 2007; 74:647-649.
- Khatri KA, Chotzen VA, Burrall BA. Lupus pernio: successful treatment with a potent topical corticosteroid. Arch Dermatol. 1995; 131:617-618.
- Singh SK, Singh S, Pandey SS. Cutaneous sarcoidosis without systemic involvement: response to intralesional corticosteroid. Indian J Dermatol Venereol Leprol. 1996; 62:273-274.
- Karrer S, Abels C, Wimmershoff MB, et al. Successful treatment of cutaneous sarcoidosis using topical photodynamic therapy. Arch Dermatol. 2002; 138:581-584.
- Mahnke N, Medve-koenigs K, Berneburg M, et al. Cutaneous sarcoidosis treated with medium-dose UVA1. J Am Acad Dermatol. 2004; 50:978-979.
- Frederiksen LG, Jørgensen K. Sarcoidosis of the nose treated with laser surgery. Rhinology. 1996; 34:245-246.
- Baughman RP, Lower EE. Evidence-based therapy for cutaneous sarcoidosis. Clin Dermatol. 2007; 25:334-340.
A 57-year-old woman presented with several lesions on the left extensor forearm of 10 years’ duration. A single annular indurated lesion with central atrophy initially developed near a prior surgical site. The lesions were pruritic with no associated pain or bleeding. Over 5 years, similar lesions had developed extending up the arm. No benefit was seen with low-potency topical steroid application. Biopsy for histopathologic examination was performed to confirm the diagnosis.