User login
Use hospital MRSA rates to guide pediatric osteomyelitis treatment
SEATTLE – If your hospital’s methicillin-resistant Staphylococcus aureus rate is less than 10%, cefazolin is a reasonable empiric choice for pediatric acute hematogenous osteomyelitis (AHO). It covers the usual suspects: methicillin-susceptible Staphylococcus aureus, group A Streptococcus, and Kingella.
Above the 10% mark, coverage should include considerations of MRSA; clindamycin is good option so long as 85% of isolates are susceptible. Above that, it’s time for vancomycin, according to Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University.
There are no practice guidelines in the United States for the diagnosis and management of AHO in children; Dr. Srinivas and colleagues sought to plug the gaps in a talk at Pediatric Hospitalist Medicine.
Pediatric AHO is more common in children under 5 years old and in boys. Lower extremities are the usual targets. Staphylococcus aureus, group B Streptococcus, and gram negatives are the most common causes in newborns; Staphylococcus aureus, group A Streptococcus, and Kingella in older infants and preschoolers; and Staphylococcus aureus and group A Streptococcus in older children.
About half the time, treatment remains empiric because nothing grows out on culture, and there are a few clinical pearls to keep in mind in those cases. A family history of boils or spider bites is suspicious for MRSA, and coverage should include Salmonella in children with abnormal hemoglobins and Streptococcus pneumoniae in children without a spleen or with functional asplenia. Pseudomonas has to be kept in mind with puncture wounds, and Brucella in children who drink unpasteurized milk, Dr. Srinivas said.
A switch from IV to oral therapy is appropriate when C-reactive protein (CRP) drops 50% from its peak or below 3 mg/dL, positive cultures – if any – turn negative, fever has been absent for 24 hours, there’s no sign of metastatic disease, and patients have markedly reduced pain and can bear weight on the infected limb, said copresenter Marie Wang, MD, also a pediatric infectious disease specialist at Stanford.
The oral switch, of course, must have similar coverage as the IV antibiotic: high-dose cephalexin for cefazolin, for instance. Children can be sent home on a PICC line to continue IV treatment, but they won’t do any better than children switched to an oral treatment, and the indwelling catheter can cause problems, she said.
Pleuritic or other sudden pain at a distant site suggests septic emboli. “[Staphylococcus aureus] is notorious for going places you don’t” expect it to go “and forming microabscesses, which become larger abscesses” and need to be drained, said the third presenter, Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
Four weeks of antibiotics are usually enough, so long as there aren’t complications such as septic thrombophlebitis, endocarditis, sickle cell disease, skull involvement, or immunodeficiencies. Source control and good, postdischarge care – including regular CRP and antibiotic toxicity labs – are critical. Monitoring is recommended for a year.
“X-rays are good at looking for longer-term complications, but bony abnormalities are not going to show up for the first 2 weeks,” Dr. McCulloh said.
The presenters didn’t have any relevant disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – If your hospital’s methicillin-resistant Staphylococcus aureus rate is less than 10%, cefazolin is a reasonable empiric choice for pediatric acute hematogenous osteomyelitis (AHO). It covers the usual suspects: methicillin-susceptible Staphylococcus aureus, group A Streptococcus, and Kingella.
Above the 10% mark, coverage should include considerations of MRSA; clindamycin is good option so long as 85% of isolates are susceptible. Above that, it’s time for vancomycin, according to Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University.
There are no practice guidelines in the United States for the diagnosis and management of AHO in children; Dr. Srinivas and colleagues sought to plug the gaps in a talk at Pediatric Hospitalist Medicine.
Pediatric AHO is more common in children under 5 years old and in boys. Lower extremities are the usual targets. Staphylococcus aureus, group B Streptococcus, and gram negatives are the most common causes in newborns; Staphylococcus aureus, group A Streptococcus, and Kingella in older infants and preschoolers; and Staphylococcus aureus and group A Streptococcus in older children.
About half the time, treatment remains empiric because nothing grows out on culture, and there are a few clinical pearls to keep in mind in those cases. A family history of boils or spider bites is suspicious for MRSA, and coverage should include Salmonella in children with abnormal hemoglobins and Streptococcus pneumoniae in children without a spleen or with functional asplenia. Pseudomonas has to be kept in mind with puncture wounds, and Brucella in children who drink unpasteurized milk, Dr. Srinivas said.
A switch from IV to oral therapy is appropriate when C-reactive protein (CRP) drops 50% from its peak or below 3 mg/dL, positive cultures – if any – turn negative, fever has been absent for 24 hours, there’s no sign of metastatic disease, and patients have markedly reduced pain and can bear weight on the infected limb, said copresenter Marie Wang, MD, also a pediatric infectious disease specialist at Stanford.
The oral switch, of course, must have similar coverage as the IV antibiotic: high-dose cephalexin for cefazolin, for instance. Children can be sent home on a PICC line to continue IV treatment, but they won’t do any better than children switched to an oral treatment, and the indwelling catheter can cause problems, she said.
Pleuritic or other sudden pain at a distant site suggests septic emboli. “[Staphylococcus aureus] is notorious for going places you don’t” expect it to go “and forming microabscesses, which become larger abscesses” and need to be drained, said the third presenter, Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
Four weeks of antibiotics are usually enough, so long as there aren’t complications such as septic thrombophlebitis, endocarditis, sickle cell disease, skull involvement, or immunodeficiencies. Source control and good, postdischarge care – including regular CRP and antibiotic toxicity labs – are critical. Monitoring is recommended for a year.
“X-rays are good at looking for longer-term complications, but bony abnormalities are not going to show up for the first 2 weeks,” Dr. McCulloh said.
The presenters didn’t have any relevant disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – If your hospital’s methicillin-resistant Staphylococcus aureus rate is less than 10%, cefazolin is a reasonable empiric choice for pediatric acute hematogenous osteomyelitis (AHO). It covers the usual suspects: methicillin-susceptible Staphylococcus aureus, group A Streptococcus, and Kingella.
Above the 10% mark, coverage should include considerations of MRSA; clindamycin is good option so long as 85% of isolates are susceptible. Above that, it’s time for vancomycin, according to Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University.
There are no practice guidelines in the United States for the diagnosis and management of AHO in children; Dr. Srinivas and colleagues sought to plug the gaps in a talk at Pediatric Hospitalist Medicine.
Pediatric AHO is more common in children under 5 years old and in boys. Lower extremities are the usual targets. Staphylococcus aureus, group B Streptococcus, and gram negatives are the most common causes in newborns; Staphylococcus aureus, group A Streptococcus, and Kingella in older infants and preschoolers; and Staphylococcus aureus and group A Streptococcus in older children.
About half the time, treatment remains empiric because nothing grows out on culture, and there are a few clinical pearls to keep in mind in those cases. A family history of boils or spider bites is suspicious for MRSA, and coverage should include Salmonella in children with abnormal hemoglobins and Streptococcus pneumoniae in children without a spleen or with functional asplenia. Pseudomonas has to be kept in mind with puncture wounds, and Brucella in children who drink unpasteurized milk, Dr. Srinivas said.
A switch from IV to oral therapy is appropriate when C-reactive protein (CRP) drops 50% from its peak or below 3 mg/dL, positive cultures – if any – turn negative, fever has been absent for 24 hours, there’s no sign of metastatic disease, and patients have markedly reduced pain and can bear weight on the infected limb, said copresenter Marie Wang, MD, also a pediatric infectious disease specialist at Stanford.
The oral switch, of course, must have similar coverage as the IV antibiotic: high-dose cephalexin for cefazolin, for instance. Children can be sent home on a PICC line to continue IV treatment, but they won’t do any better than children switched to an oral treatment, and the indwelling catheter can cause problems, she said.
Pleuritic or other sudden pain at a distant site suggests septic emboli. “[Staphylococcus aureus] is notorious for going places you don’t” expect it to go “and forming microabscesses, which become larger abscesses” and need to be drained, said the third presenter, Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
Four weeks of antibiotics are usually enough, so long as there aren’t complications such as septic thrombophlebitis, endocarditis, sickle cell disease, skull involvement, or immunodeficiencies. Source control and good, postdischarge care – including regular CRP and antibiotic toxicity labs – are critical. Monitoring is recommended for a year.
“X-rays are good at looking for longer-term complications, but bony abnormalities are not going to show up for the first 2 weeks,” Dr. McCulloh said.
The presenters didn’t have any relevant disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
EXPERT ANALYSIS FROM PHM 2019
USPSTF issues draft recommendation statement for HCV screening in adults
and now suggests that all adults aged 18-79 years receive screening.
This proposal represents an update and expansion of its current recommendation for screening for HCV infection. The draft recommendation incorporates new evidence and would replace the recommendation made in 2013, which calls for screening in persons at high risk for infection and one-time screening in adults born between 1945 and 1965 (Grade B).
“Today, more people are infected with hepatitis C than there were a decade ago, but there are now better treatments available. The evidence now shows more people can benefit from screening; therefore, we are recommending to screen all adults ages 18-79 for hepatitis C,” task force chair Douglas K. Owens, MD, MS, said in a bulletin issued by the USPSTF.
To update the previous recommendation, the USPSTF conducted a systematic review that included a total of 97 studies. No direct evidence was found regarding the benefits of HCV screening versus no screening or repeat versus one-time screening, and no new studies analyzed the psychological and social consequences of HCV screening.
Evidence concerning direct-acting antiviral (DAA) treatment was more compelling given that 49 trials found DAA therapy to be associated with pooled sustained virologic response (SVR) rates between 95.5% and 98.9% across genotypes. The rate of serious adverse events caused by DAA treatment was 1.9%, and the discontinuation of treatment because of adverse events was 0.4%. In seven trials involving adolescents, SVR rates after antiviral treatment were similar to those in adults.
Achieving an SVR after DAA treatment was associated with a decreased risk in those treated of all-cause mortality (hazard ratio, 0.40; 95% confidence interval, 0.28-0.56), liver mortality (HR, 0.11; 95% CI, 0.04-0.27), cirrhosis (HR, 0.36; 95% CI, 0.33-0.40), and hepatocellular carcinoma (HR, 0.29; 95% CI, 0.23-0.38), compared with those who did not respond.
Because of the evidence collected, the USPSTF issued a B recommendation for HCV screening in adults and recommended screening for all people aged 18-79 years in the draft recommendation statement. “Clinicians may want to consider screening in adolescents younger than age 18 years and in adults older than age 79 years who are at high risk [for HCV],” the proposal says.
The draft recommendation statement and evidence review is available at www.uspreventiveservicestaskforce.org. The public comment period will last until Sept. 23, 2019.
and now suggests that all adults aged 18-79 years receive screening.
This proposal represents an update and expansion of its current recommendation for screening for HCV infection. The draft recommendation incorporates new evidence and would replace the recommendation made in 2013, which calls for screening in persons at high risk for infection and one-time screening in adults born between 1945 and 1965 (Grade B).
“Today, more people are infected with hepatitis C than there were a decade ago, but there are now better treatments available. The evidence now shows more people can benefit from screening; therefore, we are recommending to screen all adults ages 18-79 for hepatitis C,” task force chair Douglas K. Owens, MD, MS, said in a bulletin issued by the USPSTF.
To update the previous recommendation, the USPSTF conducted a systematic review that included a total of 97 studies. No direct evidence was found regarding the benefits of HCV screening versus no screening or repeat versus one-time screening, and no new studies analyzed the psychological and social consequences of HCV screening.
Evidence concerning direct-acting antiviral (DAA) treatment was more compelling given that 49 trials found DAA therapy to be associated with pooled sustained virologic response (SVR) rates between 95.5% and 98.9% across genotypes. The rate of serious adverse events caused by DAA treatment was 1.9%, and the discontinuation of treatment because of adverse events was 0.4%. In seven trials involving adolescents, SVR rates after antiviral treatment were similar to those in adults.
Achieving an SVR after DAA treatment was associated with a decreased risk in those treated of all-cause mortality (hazard ratio, 0.40; 95% confidence interval, 0.28-0.56), liver mortality (HR, 0.11; 95% CI, 0.04-0.27), cirrhosis (HR, 0.36; 95% CI, 0.33-0.40), and hepatocellular carcinoma (HR, 0.29; 95% CI, 0.23-0.38), compared with those who did not respond.
Because of the evidence collected, the USPSTF issued a B recommendation for HCV screening in adults and recommended screening for all people aged 18-79 years in the draft recommendation statement. “Clinicians may want to consider screening in adolescents younger than age 18 years and in adults older than age 79 years who are at high risk [for HCV],” the proposal says.
The draft recommendation statement and evidence review is available at www.uspreventiveservicestaskforce.org. The public comment period will last until Sept. 23, 2019.
and now suggests that all adults aged 18-79 years receive screening.
This proposal represents an update and expansion of its current recommendation for screening for HCV infection. The draft recommendation incorporates new evidence and would replace the recommendation made in 2013, which calls for screening in persons at high risk for infection and one-time screening in adults born between 1945 and 1965 (Grade B).
“Today, more people are infected with hepatitis C than there were a decade ago, but there are now better treatments available. The evidence now shows more people can benefit from screening; therefore, we are recommending to screen all adults ages 18-79 for hepatitis C,” task force chair Douglas K. Owens, MD, MS, said in a bulletin issued by the USPSTF.
To update the previous recommendation, the USPSTF conducted a systematic review that included a total of 97 studies. No direct evidence was found regarding the benefits of HCV screening versus no screening or repeat versus one-time screening, and no new studies analyzed the psychological and social consequences of HCV screening.
Evidence concerning direct-acting antiviral (DAA) treatment was more compelling given that 49 trials found DAA therapy to be associated with pooled sustained virologic response (SVR) rates between 95.5% and 98.9% across genotypes. The rate of serious adverse events caused by DAA treatment was 1.9%, and the discontinuation of treatment because of adverse events was 0.4%. In seven trials involving adolescents, SVR rates after antiviral treatment were similar to those in adults.
Achieving an SVR after DAA treatment was associated with a decreased risk in those treated of all-cause mortality (hazard ratio, 0.40; 95% confidence interval, 0.28-0.56), liver mortality (HR, 0.11; 95% CI, 0.04-0.27), cirrhosis (HR, 0.36; 95% CI, 0.33-0.40), and hepatocellular carcinoma (HR, 0.29; 95% CI, 0.23-0.38), compared with those who did not respond.
Because of the evidence collected, the USPSTF issued a B recommendation for HCV screening in adults and recommended screening for all people aged 18-79 years in the draft recommendation statement. “Clinicians may want to consider screening in adolescents younger than age 18 years and in adults older than age 79 years who are at high risk [for HCV],” the proposal says.
The draft recommendation statement and evidence review is available at www.uspreventiveservicestaskforce.org. The public comment period will last until Sept. 23, 2019.
CPAP safety for infants with bronchiolitis on the general pediatrics floor
SEATTLE – Rady Children’s Hospital in San Diego has been doing continuous positive airway pressure for infants with bronchiolitis on the general pediatrics floors safely and with no problems for nearly 20 years, according to a presentation at Pediatric Hospital Medicine.
It’s newsworthy because “very, very few” hospitals do bronchiolitis continuous positive airway pressure (CPAP) outside of the ICU. “The perception is that there are complications, and you might miss kids that are really sick if you keep them on the floor.” However, “we have been doing it safely for so long that no one thinks twice about it,” said Christiane Lenzen, MD, a pediatric hospitalist at Rady and an assistant clinical professor of pediatrics at the University of California, San Diego.
It doesn’t matter if children have congenital heart disease, chronic lung disease, or other problems, she said, “if they are stable enough for the floor, we will see if it’s okay.”
Rady’s hand was forced on the issue because it has a large catchment area but limited ICU beds, so for practical reasons and within certain limits, CPAP moved to the floors. One of Dr. Lenzen’s colleagues noted that, as long as there’s nurse and respiratory leadership buy in, “it’s actually quite easy to pull off in a very safe manner.”
Rady has a significant advantage over community hospitals and other places considering the approach, because it has onsite pediatric ICU services for when things head south. Over the past 3 or so years, 52% of the children the pediatric hospital medicine service started on CPAP (168/324) had to be transferred to the ICU; 17% were ultimately intubated.
Many of those transfers were caused by comorbidities, not CPAP failure, but other times children needed greater respiratory support; in general, the floor CPAP limit is 6 cm H2O and a fraction of inspired oxygen of 50%. Also, sometimes children needed to be sedated for CPAP, which isn’t done on the floor.
With the 52% transfer rate, “I would worry about patients who are sick enough to need CPAP staying” in a hospital without quick access to ICU services, Dr. Lenzen said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Even so, among 324 children who at least initially were treated with CPAP on the floor – out of 2,424 admitted to the pediatric hospital medicine service with bronchiolitis – there hasn’t been a single pneumothorax, aspiration event, or CPAP equipment–related injury, she said.
CPAP on the floor has several benefits. ICU resources are conserved, patient handoffs and the work of transfers into and out of the ICU are avoided, families don’t have to get used to a new treatment team, and infants aren’t subjected to the jarring ICU environment.
For it to work, though, staff “really need to be on top of this,” and “it needs to be very tightly controlled” with order sets and other measures, the presenters said. There’s regular training at Rady for nurses, respiratory therapists, and hospitalists on CPAP equipment, airway management, monitoring, troubleshooting, and other essentials.
Almost all children on the pediatric floors have a trial of high-flow nasal cannula with an upper limit of 8 L/min. If the Respiratory Assessment Score hasn’t improved in an hour, CPAP is considered. If a child is admitted with a score above 10 and they seem to be worsening, they go straight to CPAP.
Children alternate between nasal prongs and nasal masks to prevent pressure necrosis, and are kept nil per os while on CPAP. They are on continual pulse oximetry and cardiorespiratory monitoring. Vital signs and respiratory scores are checked frequently, more so for children who are struggling.
The patient-to-nurse ratio drops from the usual 4:1 to 3:1 when a child goes on CPAP, and to 2:1 if necessary. Traveling nurses aren’t allowed to take CPAP cases.
The presenters didn’t report any disclosures.
This article was updated 8/27/19.
SEATTLE – Rady Children’s Hospital in San Diego has been doing continuous positive airway pressure for infants with bronchiolitis on the general pediatrics floors safely and with no problems for nearly 20 years, according to a presentation at Pediatric Hospital Medicine.
It’s newsworthy because “very, very few” hospitals do bronchiolitis continuous positive airway pressure (CPAP) outside of the ICU. “The perception is that there are complications, and you might miss kids that are really sick if you keep them on the floor.” However, “we have been doing it safely for so long that no one thinks twice about it,” said Christiane Lenzen, MD, a pediatric hospitalist at Rady and an assistant clinical professor of pediatrics at the University of California, San Diego.
It doesn’t matter if children have congenital heart disease, chronic lung disease, or other problems, she said, “if they are stable enough for the floor, we will see if it’s okay.”
Rady’s hand was forced on the issue because it has a large catchment area but limited ICU beds, so for practical reasons and within certain limits, CPAP moved to the floors. One of Dr. Lenzen’s colleagues noted that, as long as there’s nurse and respiratory leadership buy in, “it’s actually quite easy to pull off in a very safe manner.”
Rady has a significant advantage over community hospitals and other places considering the approach, because it has onsite pediatric ICU services for when things head south. Over the past 3 or so years, 52% of the children the pediatric hospital medicine service started on CPAP (168/324) had to be transferred to the ICU; 17% were ultimately intubated.
Many of those transfers were caused by comorbidities, not CPAP failure, but other times children needed greater respiratory support; in general, the floor CPAP limit is 6 cm H2O and a fraction of inspired oxygen of 50%. Also, sometimes children needed to be sedated for CPAP, which isn’t done on the floor.
With the 52% transfer rate, “I would worry about patients who are sick enough to need CPAP staying” in a hospital without quick access to ICU services, Dr. Lenzen said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Even so, among 324 children who at least initially were treated with CPAP on the floor – out of 2,424 admitted to the pediatric hospital medicine service with bronchiolitis – there hasn’t been a single pneumothorax, aspiration event, or CPAP equipment–related injury, she said.
CPAP on the floor has several benefits. ICU resources are conserved, patient handoffs and the work of transfers into and out of the ICU are avoided, families don’t have to get used to a new treatment team, and infants aren’t subjected to the jarring ICU environment.
For it to work, though, staff “really need to be on top of this,” and “it needs to be very tightly controlled” with order sets and other measures, the presenters said. There’s regular training at Rady for nurses, respiratory therapists, and hospitalists on CPAP equipment, airway management, monitoring, troubleshooting, and other essentials.
Almost all children on the pediatric floors have a trial of high-flow nasal cannula with an upper limit of 8 L/min. If the Respiratory Assessment Score hasn’t improved in an hour, CPAP is considered. If a child is admitted with a score above 10 and they seem to be worsening, they go straight to CPAP.
Children alternate between nasal prongs and nasal masks to prevent pressure necrosis, and are kept nil per os while on CPAP. They are on continual pulse oximetry and cardiorespiratory monitoring. Vital signs and respiratory scores are checked frequently, more so for children who are struggling.
The patient-to-nurse ratio drops from the usual 4:1 to 3:1 when a child goes on CPAP, and to 2:1 if necessary. Traveling nurses aren’t allowed to take CPAP cases.
The presenters didn’t report any disclosures.
This article was updated 8/27/19.
SEATTLE – Rady Children’s Hospital in San Diego has been doing continuous positive airway pressure for infants with bronchiolitis on the general pediatrics floors safely and with no problems for nearly 20 years, according to a presentation at Pediatric Hospital Medicine.
It’s newsworthy because “very, very few” hospitals do bronchiolitis continuous positive airway pressure (CPAP) outside of the ICU. “The perception is that there are complications, and you might miss kids that are really sick if you keep them on the floor.” However, “we have been doing it safely for so long that no one thinks twice about it,” said Christiane Lenzen, MD, a pediatric hospitalist at Rady and an assistant clinical professor of pediatrics at the University of California, San Diego.
It doesn’t matter if children have congenital heart disease, chronic lung disease, or other problems, she said, “if they are stable enough for the floor, we will see if it’s okay.”
Rady’s hand was forced on the issue because it has a large catchment area but limited ICU beds, so for practical reasons and within certain limits, CPAP moved to the floors. One of Dr. Lenzen’s colleagues noted that, as long as there’s nurse and respiratory leadership buy in, “it’s actually quite easy to pull off in a very safe manner.”
Rady has a significant advantage over community hospitals and other places considering the approach, because it has onsite pediatric ICU services for when things head south. Over the past 3 or so years, 52% of the children the pediatric hospital medicine service started on CPAP (168/324) had to be transferred to the ICU; 17% were ultimately intubated.
Many of those transfers were caused by comorbidities, not CPAP failure, but other times children needed greater respiratory support; in general, the floor CPAP limit is 6 cm H2O and a fraction of inspired oxygen of 50%. Also, sometimes children needed to be sedated for CPAP, which isn’t done on the floor.
With the 52% transfer rate, “I would worry about patients who are sick enough to need CPAP staying” in a hospital without quick access to ICU services, Dr. Lenzen said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Even so, among 324 children who at least initially were treated with CPAP on the floor – out of 2,424 admitted to the pediatric hospital medicine service with bronchiolitis – there hasn’t been a single pneumothorax, aspiration event, or CPAP equipment–related injury, she said.
CPAP on the floor has several benefits. ICU resources are conserved, patient handoffs and the work of transfers into and out of the ICU are avoided, families don’t have to get used to a new treatment team, and infants aren’t subjected to the jarring ICU environment.
For it to work, though, staff “really need to be on top of this,” and “it needs to be very tightly controlled” with order sets and other measures, the presenters said. There’s regular training at Rady for nurses, respiratory therapists, and hospitalists on CPAP equipment, airway management, monitoring, troubleshooting, and other essentials.
Almost all children on the pediatric floors have a trial of high-flow nasal cannula with an upper limit of 8 L/min. If the Respiratory Assessment Score hasn’t improved in an hour, CPAP is considered. If a child is admitted with a score above 10 and they seem to be worsening, they go straight to CPAP.
Children alternate between nasal prongs and nasal masks to prevent pressure necrosis, and are kept nil per os while on CPAP. They are on continual pulse oximetry and cardiorespiratory monitoring. Vital signs and respiratory scores are checked frequently, more so for children who are struggling.
The patient-to-nurse ratio drops from the usual 4:1 to 3:1 when a child goes on CPAP, and to 2:1 if necessary. Traveling nurses aren’t allowed to take CPAP cases.
The presenters didn’t report any disclosures.
This article was updated 8/27/19.
EXPERT ANALYSIS FROM PHM 2019
Fifty-one percent of U.S. adolescents fully vaccinated against HPV
according to a report published in Morbidity and Mortality Weekly Report.
Researchers analyzed data from 18,700 adolescents aged 13-17 years – 48% of whom were female – in the 2018 National Immunization Survey–Teen to discover that 51% of adolescents were up to date with the human papillomavirus (HPV) vaccine, and 68% had received at least one dose of the vaccine.
There was an increase in HPV vaccination coverage from 2017 to 2018, but this was attributable to a 4.4 percentage point increase in males who were up to date, compared with a 0.6 percentage point increase in females.
“Although HPV vaccination coverage improved, increases among all adolescents were modest compared with increases in previous years and were observed only among males,” wrote Tanja Y. Walker of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, and coauthors.
The number of adolescents who had at least one dose of the quadrivalent meningococcal conjugate (4MenB) vaccine increased by 1.5 percentage points to 86.6%, while among individuals aged 17 years, coverage with two or more doses of 4MenB vaccine increased by 6.5 percentage points to 50.8%. Tdap coverage remained the same at 89% (MMWR 2019;68(33):718-23).
However, the study saw no significant increases in coverage with three or more hepatitis B vaccine doses, two or more MMR vaccine doses, or with one or more varicella vaccine doses in adolescents without a history of varicella disease.
Adolescents with Medicaid had higher HPV vaccination coverage than did adolescents with private health insurance. Uninsured adolescents had lower coverage overall, ranging from 4 percentage points lower for one or more varicella vaccine doses to 19 percentage points lower for two or more 4MenB vaccines, compared with adolescents with private health insurance.
Vaccination rates were lower among adolescents outside metropolitan areas, particularly when it came to being up to date with HPV vaccination, where there was a 15 percentage point difference, and with two or more doses of the quadrivalent meningococcal conjugate vaccine, where there was a 20 percentage point difference.
Provider recommendations to parents were associated with a higher rate of coverage with one or more doses of the HPV vaccine, but the prevalence of provider recommendations varied significantly from state to state. Overall, 78% of parents said they received a provider recommendation for the adolescent HPV vaccine, but that figure was as low as 60% in Mississippi and as high as 91% in Massachusetts.
Parents living in nonmetropolitan areas were less likely to report receiving a provider recommendation than were those in metropolitan principal cities.
“Equipping providers with the tools they need to give strong recommendations that emphasize the importance of HPV vaccination in preventing cancer and effectively address parental concerns is a priority, especially in states where provider recommendations were less commonly reported,” Ms. Walker and associates said.
No conflicts of interest were declared.
according to a report published in Morbidity and Mortality Weekly Report.
Researchers analyzed data from 18,700 adolescents aged 13-17 years – 48% of whom were female – in the 2018 National Immunization Survey–Teen to discover that 51% of adolescents were up to date with the human papillomavirus (HPV) vaccine, and 68% had received at least one dose of the vaccine.
There was an increase in HPV vaccination coverage from 2017 to 2018, but this was attributable to a 4.4 percentage point increase in males who were up to date, compared with a 0.6 percentage point increase in females.
“Although HPV vaccination coverage improved, increases among all adolescents were modest compared with increases in previous years and were observed only among males,” wrote Tanja Y. Walker of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, and coauthors.
The number of adolescents who had at least one dose of the quadrivalent meningococcal conjugate (4MenB) vaccine increased by 1.5 percentage points to 86.6%, while among individuals aged 17 years, coverage with two or more doses of 4MenB vaccine increased by 6.5 percentage points to 50.8%. Tdap coverage remained the same at 89% (MMWR 2019;68(33):718-23).
However, the study saw no significant increases in coverage with three or more hepatitis B vaccine doses, two or more MMR vaccine doses, or with one or more varicella vaccine doses in adolescents without a history of varicella disease.
Adolescents with Medicaid had higher HPV vaccination coverage than did adolescents with private health insurance. Uninsured adolescents had lower coverage overall, ranging from 4 percentage points lower for one or more varicella vaccine doses to 19 percentage points lower for two or more 4MenB vaccines, compared with adolescents with private health insurance.
Vaccination rates were lower among adolescents outside metropolitan areas, particularly when it came to being up to date with HPV vaccination, where there was a 15 percentage point difference, and with two or more doses of the quadrivalent meningococcal conjugate vaccine, where there was a 20 percentage point difference.
Provider recommendations to parents were associated with a higher rate of coverage with one or more doses of the HPV vaccine, but the prevalence of provider recommendations varied significantly from state to state. Overall, 78% of parents said they received a provider recommendation for the adolescent HPV vaccine, but that figure was as low as 60% in Mississippi and as high as 91% in Massachusetts.
Parents living in nonmetropolitan areas were less likely to report receiving a provider recommendation than were those in metropolitan principal cities.
“Equipping providers with the tools they need to give strong recommendations that emphasize the importance of HPV vaccination in preventing cancer and effectively address parental concerns is a priority, especially in states where provider recommendations were less commonly reported,” Ms. Walker and associates said.
No conflicts of interest were declared.
according to a report published in Morbidity and Mortality Weekly Report.
Researchers analyzed data from 18,700 adolescents aged 13-17 years – 48% of whom were female – in the 2018 National Immunization Survey–Teen to discover that 51% of adolescents were up to date with the human papillomavirus (HPV) vaccine, and 68% had received at least one dose of the vaccine.
There was an increase in HPV vaccination coverage from 2017 to 2018, but this was attributable to a 4.4 percentage point increase in males who were up to date, compared with a 0.6 percentage point increase in females.
“Although HPV vaccination coverage improved, increases among all adolescents were modest compared with increases in previous years and were observed only among males,” wrote Tanja Y. Walker of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, and coauthors.
The number of adolescents who had at least one dose of the quadrivalent meningococcal conjugate (4MenB) vaccine increased by 1.5 percentage points to 86.6%, while among individuals aged 17 years, coverage with two or more doses of 4MenB vaccine increased by 6.5 percentage points to 50.8%. Tdap coverage remained the same at 89% (MMWR 2019;68(33):718-23).
However, the study saw no significant increases in coverage with three or more hepatitis B vaccine doses, two or more MMR vaccine doses, or with one or more varicella vaccine doses in adolescents without a history of varicella disease.
Adolescents with Medicaid had higher HPV vaccination coverage than did adolescents with private health insurance. Uninsured adolescents had lower coverage overall, ranging from 4 percentage points lower for one or more varicella vaccine doses to 19 percentage points lower for two or more 4MenB vaccines, compared with adolescents with private health insurance.
Vaccination rates were lower among adolescents outside metropolitan areas, particularly when it came to being up to date with HPV vaccination, where there was a 15 percentage point difference, and with two or more doses of the quadrivalent meningococcal conjugate vaccine, where there was a 20 percentage point difference.
Provider recommendations to parents were associated with a higher rate of coverage with one or more doses of the HPV vaccine, but the prevalence of provider recommendations varied significantly from state to state. Overall, 78% of parents said they received a provider recommendation for the adolescent HPV vaccine, but that figure was as low as 60% in Mississippi and as high as 91% in Massachusetts.
Parents living in nonmetropolitan areas were less likely to report receiving a provider recommendation than were those in metropolitan principal cities.
“Equipping providers with the tools they need to give strong recommendations that emphasize the importance of HPV vaccination in preventing cancer and effectively address parental concerns is a priority, especially in states where provider recommendations were less commonly reported,” Ms. Walker and associates said.
No conflicts of interest were declared.
FROM MMWR
Key clinical point: Slightly more than half of adolescents in the United States are fully vaccinated with the HPV vaccine.
Major finding: Rates of full HPV vaccination are 51% among adolescents aged 13-17 years.
Study details: Analysis of data from 18,700 adolescents aged 13-17 years in the 2018 National Immunization Survey–Teen.
Disclosures: No conflicts of interest were declared.
Source: Walker T et al. MMWR 2019 Aug 23;68(33):718-23.
EULAR updates vaccination recommendations for autoimmune inflammatory rheumatic disease patients
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
FROM ANNALS OF THE RHEUMATIC DISEASES
FUO, pneumonia often distinguishes influenza from RSV in hospitalized young children
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
REPORTING FROM ESPID 2019
Green thumbnail
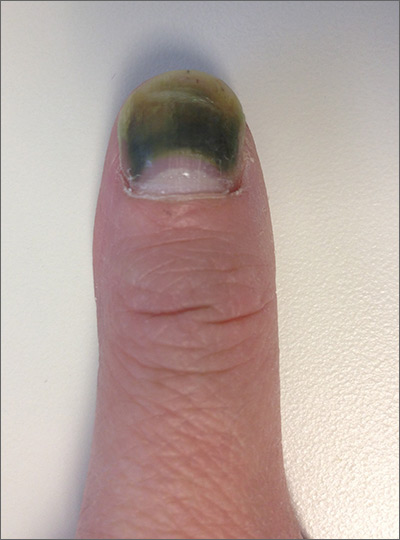
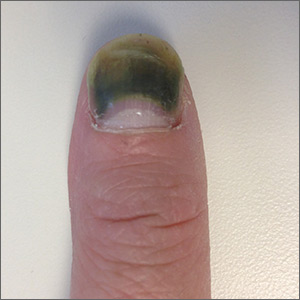
The patient was given a diagnosis of green nail syndrome (GNS), an infection of the nail bed caused by Pseudomonas aeruginosa. These bacteria produce pyocyanin, a blue-green pigment that discolors the nail. GNS often occurs in patients with prior nail problems, such as onychomycosis, onycholysis, trauma, chronic paronychia, or psoriasis.
Nail disease disrupts the integumentary barrier and allows a portal of entry for bacteria. Scanning electron microscopy of patients with GNS has shown that fungal infections create tunnel-like structures in the nail keratin, and P aeruginosa can grow in these spaces. Nails with prior nail disease that are chronically exposed to moisture are at greatest risk of developing GNS, and it is typical for only one nail to be involved.
It’s likely that this patient’s earlier nail problem had been a case of onycholysis, based on her description of a “spongy” nail bed and loose nail. This created a favorable environment for an infection by allowing moisture and bacteria to infiltrate the space. The patient also acknowledged that she washed dishes by hand and bathed her young children. This frequent soaking of her hands likely helped to provide a moist environment in which P aeruginosa could thrive. In addition, onycholysis is associated with hypothyroidism, which the patient also had.
GNS can be diagnosed by clinical observation and characteristic pigmentation along with an appropriate patient history. Nail discoloration, or chromonychia, can present in a variety of colors. Nail findings may represent an isolated disease or provide an important clinical clue to other systemic diseases. The specific shade of discoloration helps to differentiate the underlying pathology. Culture of the nail bed may be helpful if bacterial resistance or co-infection with fungal organisms is suspected. GNS is often painless, but may be accompanied by mild tenderness of the nail.
The patient was prescribed ciprofloxacin 500 mg twice a day for 10 days, plus bleach soaks (1 part bleach to 4 parts water) twice a day. She also was advised to wear gloves for household tasks that involved immersing her hands in water, and to dry her finger with a hair dryer after bathing.
This case was adapted from: Gish D, Romero BJ. Green fingernail. J Fam Pract. 2017;66:E7-E9.

The patient was given a diagnosis of green nail syndrome (GNS), an infection of the nail bed caused by Pseudomonas aeruginosa. These bacteria produce pyocyanin, a blue-green pigment that discolors the nail. GNS often occurs in patients with prior nail problems, such as onychomycosis, onycholysis, trauma, chronic paronychia, or psoriasis.
Nail disease disrupts the integumentary barrier and allows a portal of entry for bacteria. Scanning electron microscopy of patients with GNS has shown that fungal infections create tunnel-like structures in the nail keratin, and P aeruginosa can grow in these spaces. Nails with prior nail disease that are chronically exposed to moisture are at greatest risk of developing GNS, and it is typical for only one nail to be involved.
It’s likely that this patient’s earlier nail problem had been a case of onycholysis, based on her description of a “spongy” nail bed and loose nail. This created a favorable environment for an infection by allowing moisture and bacteria to infiltrate the space. The patient also acknowledged that she washed dishes by hand and bathed her young children. This frequent soaking of her hands likely helped to provide a moist environment in which P aeruginosa could thrive. In addition, onycholysis is associated with hypothyroidism, which the patient also had.
GNS can be diagnosed by clinical observation and characteristic pigmentation along with an appropriate patient history. Nail discoloration, or chromonychia, can present in a variety of colors. Nail findings may represent an isolated disease or provide an important clinical clue to other systemic diseases. The specific shade of discoloration helps to differentiate the underlying pathology. Culture of the nail bed may be helpful if bacterial resistance or co-infection with fungal organisms is suspected. GNS is often painless, but may be accompanied by mild tenderness of the nail.
The patient was prescribed ciprofloxacin 500 mg twice a day for 10 days, plus bleach soaks (1 part bleach to 4 parts water) twice a day. She also was advised to wear gloves for household tasks that involved immersing her hands in water, and to dry her finger with a hair dryer after bathing.
This case was adapted from: Gish D, Romero BJ. Green fingernail. J Fam Pract. 2017;66:E7-E9.

The patient was given a diagnosis of green nail syndrome (GNS), an infection of the nail bed caused by Pseudomonas aeruginosa. These bacteria produce pyocyanin, a blue-green pigment that discolors the nail. GNS often occurs in patients with prior nail problems, such as onychomycosis, onycholysis, trauma, chronic paronychia, or psoriasis.
Nail disease disrupts the integumentary barrier and allows a portal of entry for bacteria. Scanning electron microscopy of patients with GNS has shown that fungal infections create tunnel-like structures in the nail keratin, and P aeruginosa can grow in these spaces. Nails with prior nail disease that are chronically exposed to moisture are at greatest risk of developing GNS, and it is typical for only one nail to be involved.
It’s likely that this patient’s earlier nail problem had been a case of onycholysis, based on her description of a “spongy” nail bed and loose nail. This created a favorable environment for an infection by allowing moisture and bacteria to infiltrate the space. The patient also acknowledged that she washed dishes by hand and bathed her young children. This frequent soaking of her hands likely helped to provide a moist environment in which P aeruginosa could thrive. In addition, onycholysis is associated with hypothyroidism, which the patient also had.
GNS can be diagnosed by clinical observation and characteristic pigmentation along with an appropriate patient history. Nail discoloration, or chromonychia, can present in a variety of colors. Nail findings may represent an isolated disease or provide an important clinical clue to other systemic diseases. The specific shade of discoloration helps to differentiate the underlying pathology. Culture of the nail bed may be helpful if bacterial resistance or co-infection with fungal organisms is suspected. GNS is often painless, but may be accompanied by mild tenderness of the nail.
The patient was prescribed ciprofloxacin 500 mg twice a day for 10 days, plus bleach soaks (1 part bleach to 4 parts water) twice a day. She also was advised to wear gloves for household tasks that involved immersing her hands in water, and to dry her finger with a hair dryer after bathing.
This case was adapted from: Gish D, Romero BJ. Green fingernail. J Fam Pract. 2017;66:E7-E9.
Impact of climate change on mortality underlined by global study
Regardless of where people live in the world, air pollution is linked to increased rates of cardiovascular disease, respiratory problems, and all-cause mortality, according to one of the largest studies ever to assess the effects of inhalable particulate matter (PM), published Aug. 21 in the New England Journal of Medicine.
“These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies,” reported Cong Liu of the Huazhong University of Science and Technology in Wuhan, China, and an international team of researchers.
“Many people are experiencing worse allergy and asthma symptoms in the setting of increased heat and worse air quality,” Caren G. Solomon, MD, of Harvard Medical School, Boston, said in an interview. “It is often not appreciated that these are complications of climate change.”
Other such complications include heat-related illnesses and severe weather events, as well as the less visible manifestations, such as shifts in the epidemiology of vector-borne infectious disease, Dr. Solomon and colleagues wrote in an editorial accompanying Mr. Liu’s study.
“The stark reality is that high levels of greenhouse gases caused by the combustion of fossil fuels – and the resulting rise in temperature and sea levels and intensification of extreme weather – are having profound consequences for human health and health systems,” Dr. Solomon and colleagues wrote (N Engl J Med. 2019;381:773-4.).
In the new air pollution study, Mr. Liu and colleagues analyzed 59.6 million deaths from 652 cities across 24 countries, “thereby greatly increasing the generalizability of the association and decreasing the likelihood that the reported associations are subject to confounding bias,” wrote John R. Balmes, MD, of the University of California, San Francisco, and the University of California, Berkeley, in an editorial about the study (N Engl J Med. 2019;381:774-6).
The researchers compared air pollution data from 1986-2015 from the Multi-City Multi-Country (MCC) Collaborative Research Network to mortality data reported from individual countries. They assessed PM with an aerodynamic diameter of 10 mcg or less (PM10; n = 598 cities) and PM with an aerodynamic diameter of 2.5 mcg or less (PM2.5; n=499 cities).
Mr. Liu’s team used a time-series analysis – a standard upon which the majority of air pollution research relies. These studies “include daily measures of health events (e.g., daily mortality), regressed against concentrations of PM (e.g., 24-hour average PM2.5) and weather variables (e.g., daily average temperature) for a given geographic area,” Dr. Balmes wrote. “The population serves as its own control, and confounding by population characteristics is negligible because these are stable over short time frames.”
The researchers found a 0.44% increase in daily all-cause mortality for each 10-mcg/m3 increase in the 2-day moving average (current and previous day) of PM10. The same increase was linked to a 0.36% increase in daily cardiovascular mortality and a 0.47% increase in daily respiratory mortality. Similarly, a 10-mcg/m3 increase in the PM2.5 average was linked to 0.68% increase in all-cause mortality, a 0.55% increase in cardiovascular mortality, and 0.74% increase in respiratory mortality.
Locations with higher annual mean temperatures showed stronger associations, and all these associations remained statistically significant after the researchers adjusted for gaseous pollutants.
Although the majority of countries and cities included in the study came from the northern hemisphere, the researchers noted that the magnitude of effect they found, particularly for PM10 concentrations, matched up with that seen in previous studies of multiple cities or countries.
Still, they found “significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions.” Among the factors that could contribute to those variations are “different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods,” they speculated.
What makes this study remarkable – despite decades of previous similar studies – is its size and the implications of a curvilinear shape in its concentration-response relation, according to Dr. Balmes.
“The current study of PM data from many regions around the world provides the strongest evidence to date that higher levels of exposure may be associated with a lower per-unit risk,” Dr. Balmes wrote. “Regions that have lower exposures had a higher per-unit risk. This finding has profound policy implications, especially given that no threshold of effect was found. Even high-income countries, such as the United States, with relatively good air quality could still see public health benefits from further reduction of ambient PM concentrations.”
The policy implications, however, extend well beyond clean air regulations because the findings represent just one aspect of climate change’s negative effects on health, which are “frighteningly broad,” Dr. Solomon and colleagues wrote.
“As climate change continues to alter disease patterns and disrupt health systems, its effects on human health will become harder to ignore,” they wrote. “We, as a medical community, have the responsibility and the opportunity to mobilize the urgent, large-scale climate action required to protect health – as well as the ingenuity to develop novel and bold interventions to avert the most catastrophic outcomes.”
The new research and associated commentary marked the introduction of a new NEJM topic on climate change effects on health and health systems.
SOURCE: Liu C et al. N Engl J Med. 2019;381:705-15.
This article was updated 8/22/19.
The negative effects of climate change on global public health are already playing out around us, but scientific research shows that they will only get worse – unless we begin addressing the issue in earnest now.
At the macro level nationally, effective policy is actually being stripped away right now. “[While] scientists tell us we have little time to wait if we hope to avoid the most devastating effects of climate change, leaders in Washington, D.C., are attacking science and rolling back Obama-era rules from the Environmental Protection Agency,” such as working to weaken vehicle fuel-efficiency standards, relaxing methane emissions rules, ending mercury emissions regulation and taking other actions that will only increase air pollution.
“If these EPA rollbacks are successful, they will diminish our ability to mitigate health effects and diseases related to the burning of fossil fuels and the immense toll they take on our families. ... If we stop supporting and listening to the best available science, if we allow more pollution to be emitted, and if we start limiting the EPA’s ability to monitor and enforce pollution standards, then we put at risk everyone’s health – and especially the health and future of our children.”
Engaging in advocacy and communicating to our representatives that we want stronger regulations is one way people can personally take action, but we can take immediate actions in our everyday lives too. Rather than dwelling on the despair of helplessness and hopelessness that grips many people when it comes to climate change, this moment can be reframed as an opportunity for people to make decisions that immediately begin improving their health — and also happen to be good for the planet.
“To me, the most urgent challenge when it comes to health and climate change is the reality that, when climate change comes up, in the U.S. audience, the first thing that should come into people’s minds is that we need to do this now because we need to protect our children’s health. ... Too many people either don’t get that it matters to health at all, or they don’t get that the actions we need to take are exactly what we need to do to address the health problems that have been nearly impossible to deal with.”
For example, problems like rising child obesity and type 2 diabetes rates have plagued public health, yet people can make changes that reduce obesity and diabetes risk that also decrease their carbon footprints, he said. “One of the best ways to deal with obesity is to eat more plants, and it turns out that’s really good for the climate” Additionally, getting people out of cars and walking and cycling can reduce individuals’ risk of diabetes – while simultaneously decreasing air pollution. “We need to be doing these things regardless of climate change, and if parents and children understood that the pathway to a healthier future was through tackling climate change, we would see a transformation.”
The value of local policy actions should be emphasized, such as ones that call for a reduction in a city’s use of concrete – which increases localized heat – and constructing more efficient buildings. Healthcare providers have an opportunity – and responsibility – not only to recognize this reality but to help their patients recognize it too.
“We can also use our roles as trusted advisers to inform and motivate actions that are increasingly necessary to protect the health of the communities we serve.” They also need to be vigilant about conditions that will worsen as the planet heats up: For example, medications such as diuretics carry more risks in higher temperatures, and patients taking them need to know that.
The need to address climate change matters because we face the challenge of protecting the world’s most vulnerable people.
“One of the great things about climate change is if it causes us to rethink about what we need to do to protect the future, it’s going to help our health today. ... If we can use that as the motivator, then maybe we can stop arguing and start thinking about climate as a positive issue, as a more personal issue we can all participate in and be willing to invest in.”
Gina McCarthy, MS, was administrator of the Environmental Protection Agency during 2013-2017, and Aaron Bernstein, MD, MPH, is a pediatrician at Boston Children’s Hospital. Both are from the Center for Climate, Health, and the Global Environment (Harvard C-CHANGE) at the Harvard T.H. Chan School of Public Health in Boston. Their comments came from their perspective (N Engl J Med. 2019 Aug 22. doi: 10.1056/NEJMp1909643) published in NEJM along with this article and editorial and a phone interview. They reported not having any disclosures.
The negative effects of climate change on global public health are already playing out around us, but scientific research shows that they will only get worse – unless we begin addressing the issue in earnest now.
At the macro level nationally, effective policy is actually being stripped away right now. “[While] scientists tell us we have little time to wait if we hope to avoid the most devastating effects of climate change, leaders in Washington, D.C., are attacking science and rolling back Obama-era rules from the Environmental Protection Agency,” such as working to weaken vehicle fuel-efficiency standards, relaxing methane emissions rules, ending mercury emissions regulation and taking other actions that will only increase air pollution.
“If these EPA rollbacks are successful, they will diminish our ability to mitigate health effects and diseases related to the burning of fossil fuels and the immense toll they take on our families. ... If we stop supporting and listening to the best available science, if we allow more pollution to be emitted, and if we start limiting the EPA’s ability to monitor and enforce pollution standards, then we put at risk everyone’s health – and especially the health and future of our children.”
Engaging in advocacy and communicating to our representatives that we want stronger regulations is one way people can personally take action, but we can take immediate actions in our everyday lives too. Rather than dwelling on the despair of helplessness and hopelessness that grips many people when it comes to climate change, this moment can be reframed as an opportunity for people to make decisions that immediately begin improving their health — and also happen to be good for the planet.
“To me, the most urgent challenge when it comes to health and climate change is the reality that, when climate change comes up, in the U.S. audience, the first thing that should come into people’s minds is that we need to do this now because we need to protect our children’s health. ... Too many people either don’t get that it matters to health at all, or they don’t get that the actions we need to take are exactly what we need to do to address the health problems that have been nearly impossible to deal with.”
For example, problems like rising child obesity and type 2 diabetes rates have plagued public health, yet people can make changes that reduce obesity and diabetes risk that also decrease their carbon footprints, he said. “One of the best ways to deal with obesity is to eat more plants, and it turns out that’s really good for the climate” Additionally, getting people out of cars and walking and cycling can reduce individuals’ risk of diabetes – while simultaneously decreasing air pollution. “We need to be doing these things regardless of climate change, and if parents and children understood that the pathway to a healthier future was through tackling climate change, we would see a transformation.”
The value of local policy actions should be emphasized, such as ones that call for a reduction in a city’s use of concrete – which increases localized heat – and constructing more efficient buildings. Healthcare providers have an opportunity – and responsibility – not only to recognize this reality but to help their patients recognize it too.
“We can also use our roles as trusted advisers to inform and motivate actions that are increasingly necessary to protect the health of the communities we serve.” They also need to be vigilant about conditions that will worsen as the planet heats up: For example, medications such as diuretics carry more risks in higher temperatures, and patients taking them need to know that.
The need to address climate change matters because we face the challenge of protecting the world’s most vulnerable people.
“One of the great things about climate change is if it causes us to rethink about what we need to do to protect the future, it’s going to help our health today. ... If we can use that as the motivator, then maybe we can stop arguing and start thinking about climate as a positive issue, as a more personal issue we can all participate in and be willing to invest in.”
Gina McCarthy, MS, was administrator of the Environmental Protection Agency during 2013-2017, and Aaron Bernstein, MD, MPH, is a pediatrician at Boston Children’s Hospital. Both are from the Center for Climate, Health, and the Global Environment (Harvard C-CHANGE) at the Harvard T.H. Chan School of Public Health in Boston. Their comments came from their perspective (N Engl J Med. 2019 Aug 22. doi: 10.1056/NEJMp1909643) published in NEJM along with this article and editorial and a phone interview. They reported not having any disclosures.
The negative effects of climate change on global public health are already playing out around us, but scientific research shows that they will only get worse – unless we begin addressing the issue in earnest now.
At the macro level nationally, effective policy is actually being stripped away right now. “[While] scientists tell us we have little time to wait if we hope to avoid the most devastating effects of climate change, leaders in Washington, D.C., are attacking science and rolling back Obama-era rules from the Environmental Protection Agency,” such as working to weaken vehicle fuel-efficiency standards, relaxing methane emissions rules, ending mercury emissions regulation and taking other actions that will only increase air pollution.
“If these EPA rollbacks are successful, they will diminish our ability to mitigate health effects and diseases related to the burning of fossil fuels and the immense toll they take on our families. ... If we stop supporting and listening to the best available science, if we allow more pollution to be emitted, and if we start limiting the EPA’s ability to monitor and enforce pollution standards, then we put at risk everyone’s health – and especially the health and future of our children.”
Engaging in advocacy and communicating to our representatives that we want stronger regulations is one way people can personally take action, but we can take immediate actions in our everyday lives too. Rather than dwelling on the despair of helplessness and hopelessness that grips many people when it comes to climate change, this moment can be reframed as an opportunity for people to make decisions that immediately begin improving their health — and also happen to be good for the planet.
“To me, the most urgent challenge when it comes to health and climate change is the reality that, when climate change comes up, in the U.S. audience, the first thing that should come into people’s minds is that we need to do this now because we need to protect our children’s health. ... Too many people either don’t get that it matters to health at all, or they don’t get that the actions we need to take are exactly what we need to do to address the health problems that have been nearly impossible to deal with.”
For example, problems like rising child obesity and type 2 diabetes rates have plagued public health, yet people can make changes that reduce obesity and diabetes risk that also decrease their carbon footprints, he said. “One of the best ways to deal with obesity is to eat more plants, and it turns out that’s really good for the climate” Additionally, getting people out of cars and walking and cycling can reduce individuals’ risk of diabetes – while simultaneously decreasing air pollution. “We need to be doing these things regardless of climate change, and if parents and children understood that the pathway to a healthier future was through tackling climate change, we would see a transformation.”
The value of local policy actions should be emphasized, such as ones that call for a reduction in a city’s use of concrete – which increases localized heat – and constructing more efficient buildings. Healthcare providers have an opportunity – and responsibility – not only to recognize this reality but to help their patients recognize it too.
“We can also use our roles as trusted advisers to inform and motivate actions that are increasingly necessary to protect the health of the communities we serve.” They also need to be vigilant about conditions that will worsen as the planet heats up: For example, medications such as diuretics carry more risks in higher temperatures, and patients taking them need to know that.
The need to address climate change matters because we face the challenge of protecting the world’s most vulnerable people.
“One of the great things about climate change is if it causes us to rethink about what we need to do to protect the future, it’s going to help our health today. ... If we can use that as the motivator, then maybe we can stop arguing and start thinking about climate as a positive issue, as a more personal issue we can all participate in and be willing to invest in.”
Gina McCarthy, MS, was administrator of the Environmental Protection Agency during 2013-2017, and Aaron Bernstein, MD, MPH, is a pediatrician at Boston Children’s Hospital. Both are from the Center for Climate, Health, and the Global Environment (Harvard C-CHANGE) at the Harvard T.H. Chan School of Public Health in Boston. Their comments came from their perspective (N Engl J Med. 2019 Aug 22. doi: 10.1056/NEJMp1909643) published in NEJM along with this article and editorial and a phone interview. They reported not having any disclosures.
Regardless of where people live in the world, air pollution is linked to increased rates of cardiovascular disease, respiratory problems, and all-cause mortality, according to one of the largest studies ever to assess the effects of inhalable particulate matter (PM), published Aug. 21 in the New England Journal of Medicine.
“These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies,” reported Cong Liu of the Huazhong University of Science and Technology in Wuhan, China, and an international team of researchers.
“Many people are experiencing worse allergy and asthma symptoms in the setting of increased heat and worse air quality,” Caren G. Solomon, MD, of Harvard Medical School, Boston, said in an interview. “It is often not appreciated that these are complications of climate change.”
Other such complications include heat-related illnesses and severe weather events, as well as the less visible manifestations, such as shifts in the epidemiology of vector-borne infectious disease, Dr. Solomon and colleagues wrote in an editorial accompanying Mr. Liu’s study.
“The stark reality is that high levels of greenhouse gases caused by the combustion of fossil fuels – and the resulting rise in temperature and sea levels and intensification of extreme weather – are having profound consequences for human health and health systems,” Dr. Solomon and colleagues wrote (N Engl J Med. 2019;381:773-4.).
In the new air pollution study, Mr. Liu and colleagues analyzed 59.6 million deaths from 652 cities across 24 countries, “thereby greatly increasing the generalizability of the association and decreasing the likelihood that the reported associations are subject to confounding bias,” wrote John R. Balmes, MD, of the University of California, San Francisco, and the University of California, Berkeley, in an editorial about the study (N Engl J Med. 2019;381:774-6).
The researchers compared air pollution data from 1986-2015 from the Multi-City Multi-Country (MCC) Collaborative Research Network to mortality data reported from individual countries. They assessed PM with an aerodynamic diameter of 10 mcg or less (PM10; n = 598 cities) and PM with an aerodynamic diameter of 2.5 mcg or less (PM2.5; n=499 cities).
Mr. Liu’s team used a time-series analysis – a standard upon which the majority of air pollution research relies. These studies “include daily measures of health events (e.g., daily mortality), regressed against concentrations of PM (e.g., 24-hour average PM2.5) and weather variables (e.g., daily average temperature) for a given geographic area,” Dr. Balmes wrote. “The population serves as its own control, and confounding by population characteristics is negligible because these are stable over short time frames.”
The researchers found a 0.44% increase in daily all-cause mortality for each 10-mcg/m3 increase in the 2-day moving average (current and previous day) of PM10. The same increase was linked to a 0.36% increase in daily cardiovascular mortality and a 0.47% increase in daily respiratory mortality. Similarly, a 10-mcg/m3 increase in the PM2.5 average was linked to 0.68% increase in all-cause mortality, a 0.55% increase in cardiovascular mortality, and 0.74% increase in respiratory mortality.
Locations with higher annual mean temperatures showed stronger associations, and all these associations remained statistically significant after the researchers adjusted for gaseous pollutants.
Although the majority of countries and cities included in the study came from the northern hemisphere, the researchers noted that the magnitude of effect they found, particularly for PM10 concentrations, matched up with that seen in previous studies of multiple cities or countries.
Still, they found “significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions.” Among the factors that could contribute to those variations are “different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods,” they speculated.
What makes this study remarkable – despite decades of previous similar studies – is its size and the implications of a curvilinear shape in its concentration-response relation, according to Dr. Balmes.
“The current study of PM data from many regions around the world provides the strongest evidence to date that higher levels of exposure may be associated with a lower per-unit risk,” Dr. Balmes wrote. “Regions that have lower exposures had a higher per-unit risk. This finding has profound policy implications, especially given that no threshold of effect was found. Even high-income countries, such as the United States, with relatively good air quality could still see public health benefits from further reduction of ambient PM concentrations.”
The policy implications, however, extend well beyond clean air regulations because the findings represent just one aspect of climate change’s negative effects on health, which are “frighteningly broad,” Dr. Solomon and colleagues wrote.
“As climate change continues to alter disease patterns and disrupt health systems, its effects on human health will become harder to ignore,” they wrote. “We, as a medical community, have the responsibility and the opportunity to mobilize the urgent, large-scale climate action required to protect health – as well as the ingenuity to develop novel and bold interventions to avert the most catastrophic outcomes.”
The new research and associated commentary marked the introduction of a new NEJM topic on climate change effects on health and health systems.
SOURCE: Liu C et al. N Engl J Med. 2019;381:705-15.
This article was updated 8/22/19.
Regardless of where people live in the world, air pollution is linked to increased rates of cardiovascular disease, respiratory problems, and all-cause mortality, according to one of the largest studies ever to assess the effects of inhalable particulate matter (PM), published Aug. 21 in the New England Journal of Medicine.
“These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies,” reported Cong Liu of the Huazhong University of Science and Technology in Wuhan, China, and an international team of researchers.
“Many people are experiencing worse allergy and asthma symptoms in the setting of increased heat and worse air quality,” Caren G. Solomon, MD, of Harvard Medical School, Boston, said in an interview. “It is often not appreciated that these are complications of climate change.”
Other such complications include heat-related illnesses and severe weather events, as well as the less visible manifestations, such as shifts in the epidemiology of vector-borne infectious disease, Dr. Solomon and colleagues wrote in an editorial accompanying Mr. Liu’s study.
“The stark reality is that high levels of greenhouse gases caused by the combustion of fossil fuels – and the resulting rise in temperature and sea levels and intensification of extreme weather – are having profound consequences for human health and health systems,” Dr. Solomon and colleagues wrote (N Engl J Med. 2019;381:773-4.).
In the new air pollution study, Mr. Liu and colleagues analyzed 59.6 million deaths from 652 cities across 24 countries, “thereby greatly increasing the generalizability of the association and decreasing the likelihood that the reported associations are subject to confounding bias,” wrote John R. Balmes, MD, of the University of California, San Francisco, and the University of California, Berkeley, in an editorial about the study (N Engl J Med. 2019;381:774-6).
The researchers compared air pollution data from 1986-2015 from the Multi-City Multi-Country (MCC) Collaborative Research Network to mortality data reported from individual countries. They assessed PM with an aerodynamic diameter of 10 mcg or less (PM10; n = 598 cities) and PM with an aerodynamic diameter of 2.5 mcg or less (PM2.5; n=499 cities).
Mr. Liu’s team used a time-series analysis – a standard upon which the majority of air pollution research relies. These studies “include daily measures of health events (e.g., daily mortality), regressed against concentrations of PM (e.g., 24-hour average PM2.5) and weather variables (e.g., daily average temperature) for a given geographic area,” Dr. Balmes wrote. “The population serves as its own control, and confounding by population characteristics is negligible because these are stable over short time frames.”
The researchers found a 0.44% increase in daily all-cause mortality for each 10-mcg/m3 increase in the 2-day moving average (current and previous day) of PM10. The same increase was linked to a 0.36% increase in daily cardiovascular mortality and a 0.47% increase in daily respiratory mortality. Similarly, a 10-mcg/m3 increase in the PM2.5 average was linked to 0.68% increase in all-cause mortality, a 0.55% increase in cardiovascular mortality, and 0.74% increase in respiratory mortality.
Locations with higher annual mean temperatures showed stronger associations, and all these associations remained statistically significant after the researchers adjusted for gaseous pollutants.
Although the majority of countries and cities included in the study came from the northern hemisphere, the researchers noted that the magnitude of effect they found, particularly for PM10 concentrations, matched up with that seen in previous studies of multiple cities or countries.
Still, they found “significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions.” Among the factors that could contribute to those variations are “different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods,” they speculated.
What makes this study remarkable – despite decades of previous similar studies – is its size and the implications of a curvilinear shape in its concentration-response relation, according to Dr. Balmes.
“The current study of PM data from many regions around the world provides the strongest evidence to date that higher levels of exposure may be associated with a lower per-unit risk,” Dr. Balmes wrote. “Regions that have lower exposures had a higher per-unit risk. This finding has profound policy implications, especially given that no threshold of effect was found. Even high-income countries, such as the United States, with relatively good air quality could still see public health benefits from further reduction of ambient PM concentrations.”
The policy implications, however, extend well beyond clean air regulations because the findings represent just one aspect of climate change’s negative effects on health, which are “frighteningly broad,” Dr. Solomon and colleagues wrote.
“As climate change continues to alter disease patterns and disrupt health systems, its effects on human health will become harder to ignore,” they wrote. “We, as a medical community, have the responsibility and the opportunity to mobilize the urgent, large-scale climate action required to protect health – as well as the ingenuity to develop novel and bold interventions to avert the most catastrophic outcomes.”
The new research and associated commentary marked the introduction of a new NEJM topic on climate change effects on health and health systems.
SOURCE: Liu C et al. N Engl J Med. 2019;381:705-15.
This article was updated 8/22/19.
FROM NEJM
HCV coinfection adds to cardiovascular risk in HIV-infected patients
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
Hepatitis C virus (HCV) coinfection, as well as an accumulation of viral and bacterial infections, was independently associated with the risk of developing a cardiovascular event in HIV-infected patients, according to the results of a large retrospective analysis.
The study comprised 823 patients at a single institution during 1982-2018. The researchers assessed those patients who had at least two visits to the HIV clinic, data concerning herpes varicella zoster virus (VZV) reactivation, and bacterial infections. Data on HCV coinfection status (as determined by HCV antibodies and qualitative HCV-PCR) were also available, according to Miguel Genebat, MD, of Virgen del Rocío University Hospital, Seville, Spain, and colleagues.
During the observational period, 58 patients (7%) experienced a cardiovascular event at a median age of 47 years. Most of these patients (50, 86%) had effective HIV treatment, with their viral load being persistently undetectable.
In terms of standard cardiovascular disease (CVD) risk factors, hypercholesterolemia was present in 31 patients (53%) and only 11 subjects (19%) had diabetes. This left 24 “low-risk” subjects, 5 of whom (21%) developed recurrent CVD and 8 of whom (33%) died after the development of cardiovascular disease.
The most frequent cardiovascular event was acute coronary syndrome (ACS), developed by 38 patients, with 14 (24%) of these individuals having recurrent CVD events. Among the 58 patients who experienced a cardiovascular event, 21 (36%) died, 17 from cardiovascular disease, 2 from cancer, and 2 each from acute bacterial infection and end-stage liver disease.
The researchers examined other variables potentially associated with the development of cardiovascular disease. They performed a multivariate analysis considering the added burden of infections and found that advanced age at HIV-1 diagnosis (OR, 1.07), a T-CD4 nadir of less than 200 cells/mcL (OR, 2.01), a diagnosis of HIV prior to combined antiretroviral therapy availability in 1996 (OR, 2.35), and cumulative infections greater than 2 (OR, 3.63), were all significantly and independently associated with the risk of developing a cardiovascular event.
They also found that HCV coinfection (OR, 2.84) on its own in simple multivariate analysis increased the risk of developing a CVD event in HIV-infected subjects. There was insufficient power to tease out the individual risk of other infections, such as herpes zoster virus and bacterial infections, hence the use of cumulative infections reported above.
The researchers concluded that potential strategies to minimize cardiovascular risk in these subjects could be treating HCV coinfection in all subjects independently of liver fibrosis stage, starting cART as soon as possible, and immunizing for those infections for which effective vaccine are available.
The authors reported that they had no conflicts of interest.
SOURCE: Genebat M. et al. Antiviral Res. 2019 Sep;169:104527.
FROM ANTIVIRAL RESEARCH
FDA approves Xenleta for community-acquired bacterial pneumonia treatment
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.