User login
Cutaneous Mycobacterium haemophilum Infection Involving the Upper Extremities: Diagnosis and Management Guidelines
Infection with Mycobacterium haemophilum, a rare, slow-growing organism, most commonly presents as ulcerating cutaneous lesions and subcutaneous nodules in immunocompromised adults.1 The most common clinical presentation in adults includes cutaneous lesions, nodules, cysts, and papules, with signs and symptoms of erythema, pain, pruritus, and drainage.2 Disseminated disease states of septic arthritis, pulmonary infiltration, and osteomyelitis, though life-threatening, are less common manifestations reported in highly immunocompromised persons.3
Infection with M haemophilum presents a challenge to the dermatology community because it is infrequently suspected and misidentified, resulting in delayed diagnosis. Additionally, M haemophilum is an extremely fastidious organism that requires heme-supplemented culture media and a carefully regulated low temperature for many consecutive weeks to yield valid culture results.1 These features contribute to complications and delays in diagnosis of an already overlooked source of infection.
We discuss the clinical presentation, diagnosis, and treatment of 3 unusual cases of cutaneous M haemophilum infection involving the upper arms. The findings in these cases highlight the challenges inherent in diagnosis as well as the obstacles that arise in providing effective, long-term treatment of this infection.
Case Reports
Patient 1
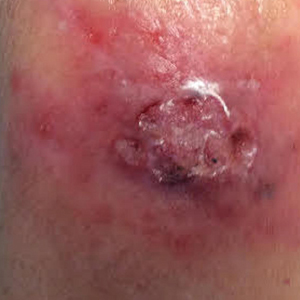
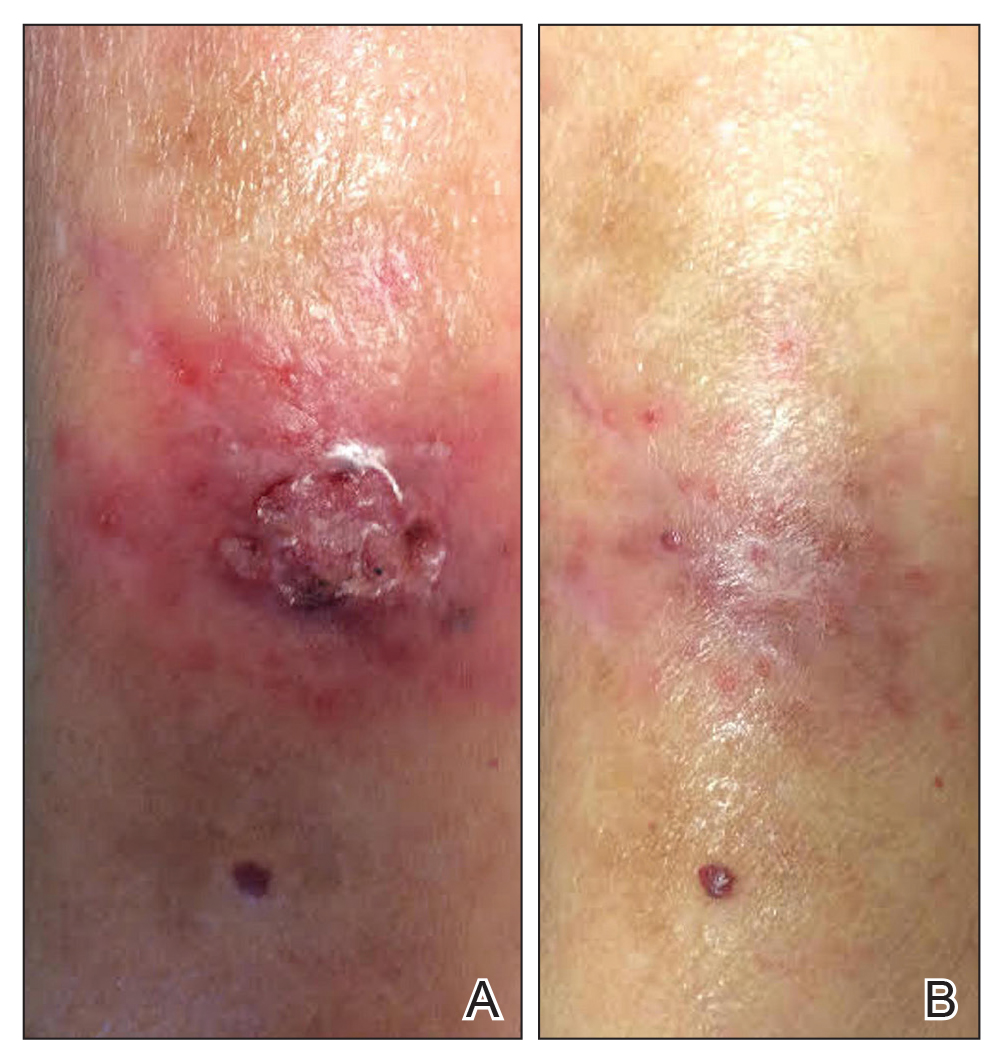
A 69-year-old woman with a medical history of a single functioning kidney and moderate psoriasis managed with low-dosage methotrexate presented with an erythematous nonhealing wound on the left forearm that developed after she was scratched by a dog. The pustules, appearing as bright red, tender, warm abscesses, had been present for 3 months and were distributed on the left proximal and distal dorsal forearm (Figure 1A). The patient reported no recent travel, sick contacts, allergies, or new medications.
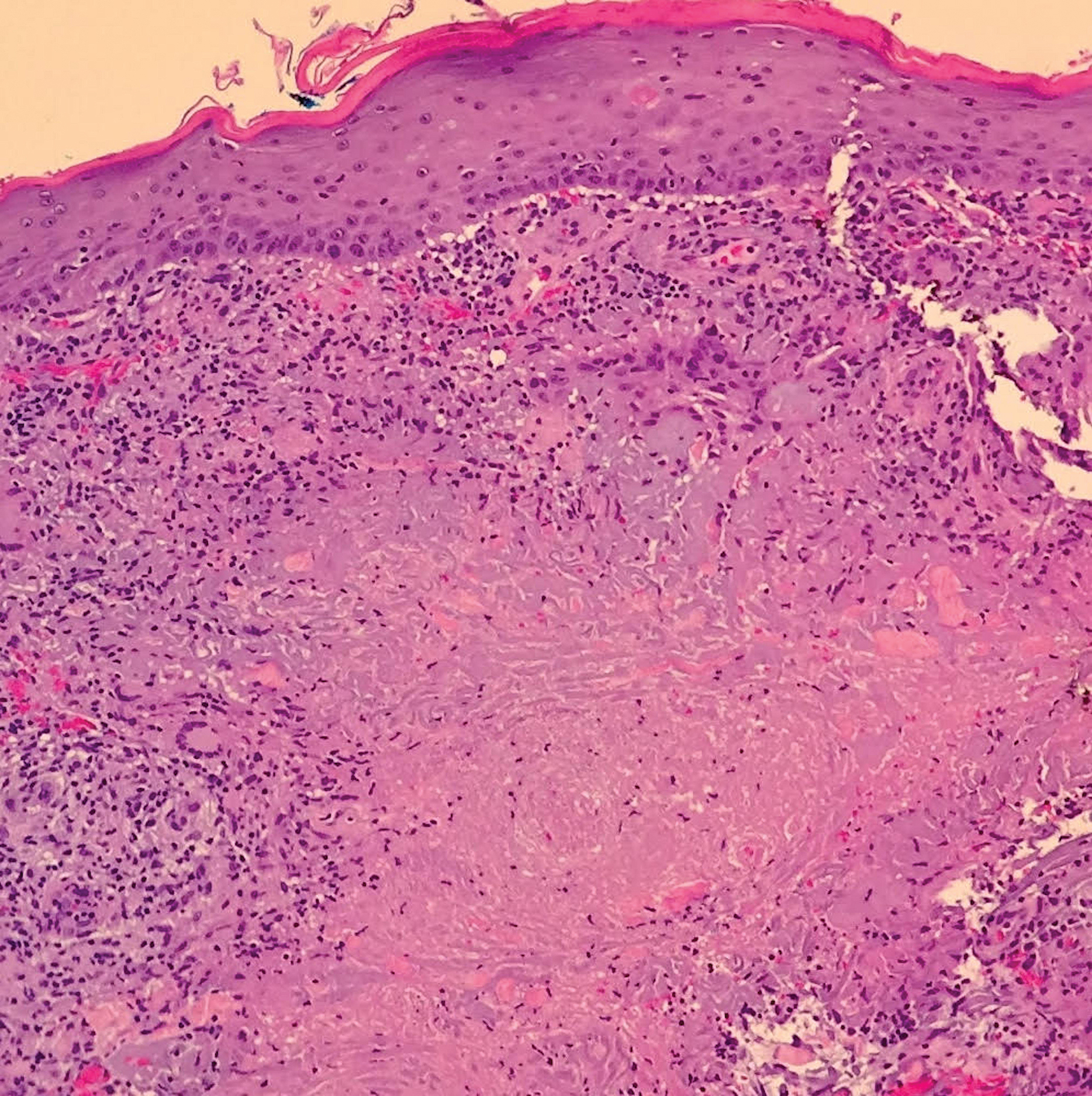
A shave biopsy was initially obtained. Swab specimens were sent for bacterial, fungal, and mycobacterial culture following discontinuation of methotrexate. Initial histopathologic analysis revealed aggregates of histiocytes and multinucleated giant cells within the dermis, surrounded by infiltrates of lymphocytes and neutrophils (Figure 2), consistent with a dermal noncaseating granulomatosis. Acid-fast bacilli (AFB), periodic acid–Schiff, Gram, and Grocott-Gomori methenamine-silver stains were negative for pathogenic microorganisms. There was no evidence of vasculitis.
Despite negative special stains, an infectious cause was still suspected. Oral doxycycline monohydrate 100 mg twice daily, oral fluconazole 200 mg daily, and econazole cream 1% were prescribed because of concern for mycobacterial infection and initial growth of Candida parapsilosis in the swab culture.
A punch biopsy also was performed at this time for both repeat histopathologic analysis and tissue culture. Follow-up appointments were scheduled every 2 weeks. Staining by AFB of the repeat histopathologic specimen was negative.
The patient demonstrated symptomatic and aesthetic improvement (Figure 1B) during consecutive regular follow-up appointments while culture results were pending. No lesions appeared above the left elbow and she had no lymphadenopathy. Results of blood chemistry analyses and complete blood cell count throughout follow-up were normal.
The final tissue culture report obtained 7 weeks after initial presentation showed growth of M haemophilum despite a negative smear. The swab culture that initially was taken did not grow pathogenic organisms.
The patient was referred to an infectious disease specialist who confirmed that the atypical mycobacterial infection likely was the main source of the cutaneous lesions. She was instructed to continue econazole cream 1% and was given prescriptions for clarithromycin 500 mg twice daily, ciprofloxacin 500 mg twice daily, and rifampin 300 mg twice daily for a total duration of 12 to 18 months. The patient has remained on this triple-drug regimen and demonstrated improvement in the lesions. She has been off methotrexate while on antibiotic therapy.
Patient 2
A 79-year-old man with a medical history of chronic lymphocytic leukemia, basal cell carcinoma, and squamous cell carcinoma presented with a nonhealing, painful, red lesion on the left forearm of 1 week’s duration. Physical examination revealed a violaceous nontender plaque with erosions and desquamation that was initially diagnosed as a carbuncle. The patient reported a similar eruption on the right foot that was successfully treated with silver sulfadiazine by another physician.
Biopsy was performed by the shave method for histologic analysis and tissue culture. Doxycycline 100 mg twice daily was prescribed because of high suspicion of infection. Histologic findings revealed granulomatous inflammation with pseudoepitheliomatous hyperplasia, reported as squamous cell carcinoma. A second opinion confirmed suspicion of an infectious process; the patient remained on doxycycline. During follow-up, the lesion progressed to a 5-cm plaque studded with pustules and satellite papules. Multiple additional tissue cultures were performed over 2 months until “light growth” of M haemophilum was reported.
The patient showed minimal improvement on tetracycline antibiotics. His condition was complicated by a photosensitivity reaction to doxycycline on the left and right forearms, hands, and nose. Consequently, triamcinolone was prescribed, doxycycline was discontinued, and minocycline 100 mg twice daily and ciprofloxacin 500 mg twice daily were prescribed.
Nine months after initial presentation, the lesions were still present but remarkably improved. The antibiotic regimen was discontinued after 11 months.
Patient 3
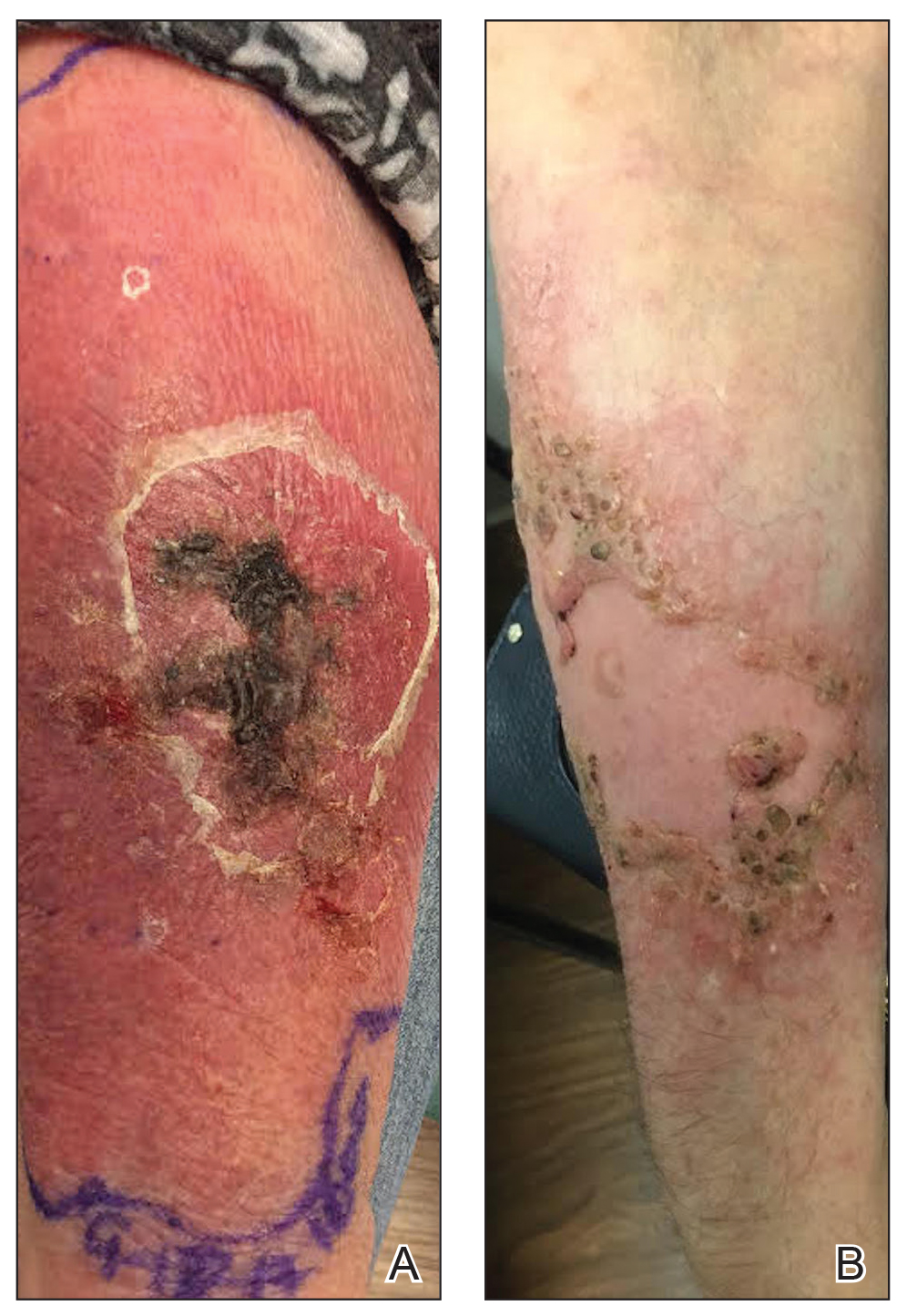
A 77-year-old woman with a history of rheumatoid arthritis treated with methotrexate and abatacept as well as cutaneous T-cell lymphoma treated with narrowband UVB radiation presented to the emergency department with fever and an inflamed right forearm (Figure 3A). Initial bacterial cultures of the wound and blood were negative.
The patient was treated with vancomycin and discharged on cephalexin once she became afebrile. She was seen at our office the next week for further evaluation. We recommended that she discontinue all immunosuppressant medications. A 4-mm tissue biopsy for hematoxylin and eosin staining and a separate 4-mm punch biopsy for culture were performed while she was taking cephalexin. Histopathologic analysis revealed numerous neutrophilic abscesses; however, Gram, AFB, and fungal stains were negative.
Arm edema and pustules slowly resolved, but the eschar and verrucous plaques continued to slowly progress while the patient was off immunosuppression. She was kept off antibiotics until mycobacterial culture was positive at 4 weeks, at which time she was placed on doxycycline and clarithromycin. Final identification of M haemophilum was made at 6 weeks; consequently, doxycycline was discontinued and she was referred to infectious disease for multidrug therapy. She remained afebrile during the entire 6 weeks until cultures were final.
While immunosuppressants were discontinued and clarithromycin was administered, the plaque changed from an edematous pustular dermatitis to a verrucous crusted plaque. Neither epitrochlear nor axillary lymphadenopathy was noted during the treatment period. The infectious disease specialist prescribed azithromycin, ethambutol, and rifampin, which produced marked improvement (Figure 3B). The patient has remained off immunosuppressive therapy while on antibiotics.
Comment
Clinical Presentation and Diagnosis
Mycobacterium haemophilum is a rare infectious organism that affects primarily immunocompromised adults but also has been identified in immunocompetent adults and pediatric patients.2 Commonly affected immunosuppressed groups include solid organ transplant recipients, bone marrow transplant recipients, human immunodeficiency virus–positive patients, and patients with rheumatoid arthritis.
The infection typically presents as small violaceous papules and pustules that become painful and erythematous, with progression and draining ulceration in later stages.2 In our cases, all lesions tended to evolve into a verrucous plaque that slowly resolved with antibiotic therapy.
Due to the rarity of this infection, the initial differential diagnosis can include infection with other mycobacteria, Sporothrix, Staphylococcus aureus, and other fungal pathogens. Misdiagnosis is a common obstacle in the treatment of M haemophilum due to its rarity, often negative AFB stains, and slow growth on culture media; therefore, tissue culture is essential to successful diagnosis and management. The natural reservoir of M haemophilum is unknown, but infection has been associated with contaminated water sources.1 In one case (patient 1), symptoms developed after a dog scratch; the other 2 patients were unaware of injury to the skin.Laboratory diagnosis of M haemophilum is inherently difficult and protracted. The species is a highly fastidious and slow-growing Mycobacterium that requires cooler (30°C) incubation for many weeks on agar medium enriched with hemin or ferric ammonium citrate to obtain valid growth.1 To secure timely diagnosis, the organism’s slow agar growth warrants immediate tissue culture and biopsy when an immunocompromised patient presents with clinical features of atypical infection of an extremity. Mycobacterium haemophilum infection likely is underreported because of these difficulties in diagnosis.
Management
Although there are no standard guidelines for antibiotic treatment of M haemophilum, the current literature recommends triple-drug therapy with clarithromycin, ciprofloxacin, and rifamycin for at least 12 to 24 months.2
Upon clinical suspicion of an atypical Mycobacterium, we recommend a macrolide antibiotic over doxycycline, however, because this class of agents maintains broad coverage while being more specific for atypical mycobacteria. Although an atypical Mycobacterium was suspected early in the presentation in our cases, we discourage immediate use of triple-agent antibiotic therapy until laboratory evidence is procured to minimize antibiotic overuse in patients who do not have a final diagnosis. Single-agent therapy for prolonged treatment is discouraged for atypical mycobacterial infections because of the high risk of antibiotic resistance. Therapy should be tailored to the needs of the individual based on the extent of dissemination of disease and the severity of immunosuppression.1,2
Additionally, underlying disease that results in immunosuppression might necessitate treatment reevaluation (as occurred in our cases) requiring cessation of immunosuppressive drugs, extended careful monitoring, and pharmacotherapeutic readjustment through the course of treatment. The degree to which antibiotics contribute to eradication of M haemophilum is unknown; therefore, it is recommended that long-term antibiotic use and treatment aimed at recovering the immunocompromised state (eg, highly active antiretroviral therapy in a patient with AIDS) be implemented.2
Conclusion
Our 3 cases of M haemophilum infection involved the upper extremities of immunosuppressed patients older than 65 years. This propensity to affect the upper extremities could possibly be due to the lower temperature required for growth of M haemophilum. Initial histopathologic study showed granulomatous and neutrophilic infiltrates, yet histopathologic specimens from all 3 patients failed to display positive AFB staining, which delayed the initial antibiotic choice. In all cases, diagnosis was made by tissue culture after swab culture failed to grow the pathogen. Furthermore, the 3 cases took approximately 6 weeks to achieve final identification of the organism. Neither clinical lymphadenopathy nor systemic spread was noted in our patients; immunosuppression was discontinued when possible.
Mycobacterium haemophilum is an uncommon but potentially life-threatening infection that should be suspected in immunocompromised adults who present with atypical cellulitis of the extremities. The ultimate diagnosis often is delayed because the organism grows slowly (as long as 8 weeks) in tissue culture. For that reason, empiric antibiotic treatment, including a macrolide, should be considered in patients with disseminated or severe infection or critical immunosuppression and in those who do not demonstrate improvement in symptoms once immunosuppressants are withheld. A prolonged course of multiple-drug antibiotic therapy has proved to be effective for treating cutaneous infection with M haemophilum.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24:701-717.
- Tangkosakul T, Hongmanee P, Malathum K. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a tertiary hospital in Bangkok, Thailand: under-reported/under-recognized infection. JMM Case Rep. 2014;1:E002618.
- Sabeti S, Pourabdollah Tootkaboni M, Abdolahi M, et al. Mycobacterium haemophilum: a report of cutaneous infection in a patient with end-stage renal disease. Int J Mycobacteriol. 2016;5(suppl 1):S236.
Infection with Mycobacterium haemophilum, a rare, slow-growing organism, most commonly presents as ulcerating cutaneous lesions and subcutaneous nodules in immunocompromised adults.1 The most common clinical presentation in adults includes cutaneous lesions, nodules, cysts, and papules, with signs and symptoms of erythema, pain, pruritus, and drainage.2 Disseminated disease states of septic arthritis, pulmonary infiltration, and osteomyelitis, though life-threatening, are less common manifestations reported in highly immunocompromised persons.3
Infection with M haemophilum presents a challenge to the dermatology community because it is infrequently suspected and misidentified, resulting in delayed diagnosis. Additionally, M haemophilum is an extremely fastidious organism that requires heme-supplemented culture media and a carefully regulated low temperature for many consecutive weeks to yield valid culture results.1 These features contribute to complications and delays in diagnosis of an already overlooked source of infection.
We discuss the clinical presentation, diagnosis, and treatment of 3 unusual cases of cutaneous M haemophilum infection involving the upper arms. The findings in these cases highlight the challenges inherent in diagnosis as well as the obstacles that arise in providing effective, long-term treatment of this infection.
Case Reports
Patient 1
A 69-year-old woman with a medical history of a single functioning kidney and moderate psoriasis managed with low-dosage methotrexate presented with an erythematous nonhealing wound on the left forearm that developed after she was scratched by a dog. The pustules, appearing as bright red, tender, warm abscesses, had been present for 3 months and were distributed on the left proximal and distal dorsal forearm (Figure 1A). The patient reported no recent travel, sick contacts, allergies, or new medications.

A shave biopsy was initially obtained. Swab specimens were sent for bacterial, fungal, and mycobacterial culture following discontinuation of methotrexate. Initial histopathologic analysis revealed aggregates of histiocytes and multinucleated giant cells within the dermis, surrounded by infiltrates of lymphocytes and neutrophils (Figure 2), consistent with a dermal noncaseating granulomatosis. Acid-fast bacilli (AFB), periodic acid–Schiff, Gram, and Grocott-Gomori methenamine-silver stains were negative for pathogenic microorganisms. There was no evidence of vasculitis.

Despite negative special stains, an infectious cause was still suspected. Oral doxycycline monohydrate 100 mg twice daily, oral fluconazole 200 mg daily, and econazole cream 1% were prescribed because of concern for mycobacterial infection and initial growth of Candida parapsilosis in the swab culture.
A punch biopsy also was performed at this time for both repeat histopathologic analysis and tissue culture. Follow-up appointments were scheduled every 2 weeks. Staining by AFB of the repeat histopathologic specimen was negative.
The patient demonstrated symptomatic and aesthetic improvement (Figure 1B) during consecutive regular follow-up appointments while culture results were pending. No lesions appeared above the left elbow and she had no lymphadenopathy. Results of blood chemistry analyses and complete blood cell count throughout follow-up were normal.
The final tissue culture report obtained 7 weeks after initial presentation showed growth of M haemophilum despite a negative smear. The swab culture that initially was taken did not grow pathogenic organisms.
The patient was referred to an infectious disease specialist who confirmed that the atypical mycobacterial infection likely was the main source of the cutaneous lesions. She was instructed to continue econazole cream 1% and was given prescriptions for clarithromycin 500 mg twice daily, ciprofloxacin 500 mg twice daily, and rifampin 300 mg twice daily for a total duration of 12 to 18 months. The patient has remained on this triple-drug regimen and demonstrated improvement in the lesions. She has been off methotrexate while on antibiotic therapy.
Patient 2
A 79-year-old man with a medical history of chronic lymphocytic leukemia, basal cell carcinoma, and squamous cell carcinoma presented with a nonhealing, painful, red lesion on the left forearm of 1 week’s duration. Physical examination revealed a violaceous nontender plaque with erosions and desquamation that was initially diagnosed as a carbuncle. The patient reported a similar eruption on the right foot that was successfully treated with silver sulfadiazine by another physician.
Biopsy was performed by the shave method for histologic analysis and tissue culture. Doxycycline 100 mg twice daily was prescribed because of high suspicion of infection. Histologic findings revealed granulomatous inflammation with pseudoepitheliomatous hyperplasia, reported as squamous cell carcinoma. A second opinion confirmed suspicion of an infectious process; the patient remained on doxycycline. During follow-up, the lesion progressed to a 5-cm plaque studded with pustules and satellite papules. Multiple additional tissue cultures were performed over 2 months until “light growth” of M haemophilum was reported.
The patient showed minimal improvement on tetracycline antibiotics. His condition was complicated by a photosensitivity reaction to doxycycline on the left and right forearms, hands, and nose. Consequently, triamcinolone was prescribed, doxycycline was discontinued, and minocycline 100 mg twice daily and ciprofloxacin 500 mg twice daily were prescribed.
Nine months after initial presentation, the lesions were still present but remarkably improved. The antibiotic regimen was discontinued after 11 months.
Patient 3
A 77-year-old woman with a history of rheumatoid arthritis treated with methotrexate and abatacept as well as cutaneous T-cell lymphoma treated with narrowband UVB radiation presented to the emergency department with fever and an inflamed right forearm (Figure 3A). Initial bacterial cultures of the wound and blood were negative.

The patient was treated with vancomycin and discharged on cephalexin once she became afebrile. She was seen at our office the next week for further evaluation. We recommended that she discontinue all immunosuppressant medications. A 4-mm tissue biopsy for hematoxylin and eosin staining and a separate 4-mm punch biopsy for culture were performed while she was taking cephalexin. Histopathologic analysis revealed numerous neutrophilic abscesses; however, Gram, AFB, and fungal stains were negative.
Arm edema and pustules slowly resolved, but the eschar and verrucous plaques continued to slowly progress while the patient was off immunosuppression. She was kept off antibiotics until mycobacterial culture was positive at 4 weeks, at which time she was placed on doxycycline and clarithromycin. Final identification of M haemophilum was made at 6 weeks; consequently, doxycycline was discontinued and she was referred to infectious disease for multidrug therapy. She remained afebrile during the entire 6 weeks until cultures were final.
While immunosuppressants were discontinued and clarithromycin was administered, the plaque changed from an edematous pustular dermatitis to a verrucous crusted plaque. Neither epitrochlear nor axillary lymphadenopathy was noted during the treatment period. The infectious disease specialist prescribed azithromycin, ethambutol, and rifampin, which produced marked improvement (Figure 3B). The patient has remained off immunosuppressive therapy while on antibiotics.
Comment
Clinical Presentation and Diagnosis
Mycobacterium haemophilum is a rare infectious organism that affects primarily immunocompromised adults but also has been identified in immunocompetent adults and pediatric patients.2 Commonly affected immunosuppressed groups include solid organ transplant recipients, bone marrow transplant recipients, human immunodeficiency virus–positive patients, and patients with rheumatoid arthritis.
The infection typically presents as small violaceous papules and pustules that become painful and erythematous, with progression and draining ulceration in later stages.2 In our cases, all lesions tended to evolve into a verrucous plaque that slowly resolved with antibiotic therapy.
Due to the rarity of this infection, the initial differential diagnosis can include infection with other mycobacteria, Sporothrix, Staphylococcus aureus, and other fungal pathogens. Misdiagnosis is a common obstacle in the treatment of M haemophilum due to its rarity, often negative AFB stains, and slow growth on culture media; therefore, tissue culture is essential to successful diagnosis and management. The natural reservoir of M haemophilum is unknown, but infection has been associated with contaminated water sources.1 In one case (patient 1), symptoms developed after a dog scratch; the other 2 patients were unaware of injury to the skin.Laboratory diagnosis of M haemophilum is inherently difficult and protracted. The species is a highly fastidious and slow-growing Mycobacterium that requires cooler (30°C) incubation for many weeks on agar medium enriched with hemin or ferric ammonium citrate to obtain valid growth.1 To secure timely diagnosis, the organism’s slow agar growth warrants immediate tissue culture and biopsy when an immunocompromised patient presents with clinical features of atypical infection of an extremity. Mycobacterium haemophilum infection likely is underreported because of these difficulties in diagnosis.
Management
Although there are no standard guidelines for antibiotic treatment of M haemophilum, the current literature recommends triple-drug therapy with clarithromycin, ciprofloxacin, and rifamycin for at least 12 to 24 months.2
Upon clinical suspicion of an atypical Mycobacterium, we recommend a macrolide antibiotic over doxycycline, however, because this class of agents maintains broad coverage while being more specific for atypical mycobacteria. Although an atypical Mycobacterium was suspected early in the presentation in our cases, we discourage immediate use of triple-agent antibiotic therapy until laboratory evidence is procured to minimize antibiotic overuse in patients who do not have a final diagnosis. Single-agent therapy for prolonged treatment is discouraged for atypical mycobacterial infections because of the high risk of antibiotic resistance. Therapy should be tailored to the needs of the individual based on the extent of dissemination of disease and the severity of immunosuppression.1,2
Additionally, underlying disease that results in immunosuppression might necessitate treatment reevaluation (as occurred in our cases) requiring cessation of immunosuppressive drugs, extended careful monitoring, and pharmacotherapeutic readjustment through the course of treatment. The degree to which antibiotics contribute to eradication of M haemophilum is unknown; therefore, it is recommended that long-term antibiotic use and treatment aimed at recovering the immunocompromised state (eg, highly active antiretroviral therapy in a patient with AIDS) be implemented.2
Conclusion
Our 3 cases of M haemophilum infection involved the upper extremities of immunosuppressed patients older than 65 years. This propensity to affect the upper extremities could possibly be due to the lower temperature required for growth of M haemophilum. Initial histopathologic study showed granulomatous and neutrophilic infiltrates, yet histopathologic specimens from all 3 patients failed to display positive AFB staining, which delayed the initial antibiotic choice. In all cases, diagnosis was made by tissue culture after swab culture failed to grow the pathogen. Furthermore, the 3 cases took approximately 6 weeks to achieve final identification of the organism. Neither clinical lymphadenopathy nor systemic spread was noted in our patients; immunosuppression was discontinued when possible.
Mycobacterium haemophilum is an uncommon but potentially life-threatening infection that should be suspected in immunocompromised adults who present with atypical cellulitis of the extremities. The ultimate diagnosis often is delayed because the organism grows slowly (as long as 8 weeks) in tissue culture. For that reason, empiric antibiotic treatment, including a macrolide, should be considered in patients with disseminated or severe infection or critical immunosuppression and in those who do not demonstrate improvement in symptoms once immunosuppressants are withheld. A prolonged course of multiple-drug antibiotic therapy has proved to be effective for treating cutaneous infection with M haemophilum.
Infection with Mycobacterium haemophilum, a rare, slow-growing organism, most commonly presents as ulcerating cutaneous lesions and subcutaneous nodules in immunocompromised adults.1 The most common clinical presentation in adults includes cutaneous lesions, nodules, cysts, and papules, with signs and symptoms of erythema, pain, pruritus, and drainage.2 Disseminated disease states of septic arthritis, pulmonary infiltration, and osteomyelitis, though life-threatening, are less common manifestations reported in highly immunocompromised persons.3
Infection with M haemophilum presents a challenge to the dermatology community because it is infrequently suspected and misidentified, resulting in delayed diagnosis. Additionally, M haemophilum is an extremely fastidious organism that requires heme-supplemented culture media and a carefully regulated low temperature for many consecutive weeks to yield valid culture results.1 These features contribute to complications and delays in diagnosis of an already overlooked source of infection.
We discuss the clinical presentation, diagnosis, and treatment of 3 unusual cases of cutaneous M haemophilum infection involving the upper arms. The findings in these cases highlight the challenges inherent in diagnosis as well as the obstacles that arise in providing effective, long-term treatment of this infection.
Case Reports
Patient 1
A 69-year-old woman with a medical history of a single functioning kidney and moderate psoriasis managed with low-dosage methotrexate presented with an erythematous nonhealing wound on the left forearm that developed after she was scratched by a dog. The pustules, appearing as bright red, tender, warm abscesses, had been present for 3 months and were distributed on the left proximal and distal dorsal forearm (Figure 1A). The patient reported no recent travel, sick contacts, allergies, or new medications.

A shave biopsy was initially obtained. Swab specimens were sent for bacterial, fungal, and mycobacterial culture following discontinuation of methotrexate. Initial histopathologic analysis revealed aggregates of histiocytes and multinucleated giant cells within the dermis, surrounded by infiltrates of lymphocytes and neutrophils (Figure 2), consistent with a dermal noncaseating granulomatosis. Acid-fast bacilli (AFB), periodic acid–Schiff, Gram, and Grocott-Gomori methenamine-silver stains were negative for pathogenic microorganisms. There was no evidence of vasculitis.

Despite negative special stains, an infectious cause was still suspected. Oral doxycycline monohydrate 100 mg twice daily, oral fluconazole 200 mg daily, and econazole cream 1% were prescribed because of concern for mycobacterial infection and initial growth of Candida parapsilosis in the swab culture.
A punch biopsy also was performed at this time for both repeat histopathologic analysis and tissue culture. Follow-up appointments were scheduled every 2 weeks. Staining by AFB of the repeat histopathologic specimen was negative.
The patient demonstrated symptomatic and aesthetic improvement (Figure 1B) during consecutive regular follow-up appointments while culture results were pending. No lesions appeared above the left elbow and she had no lymphadenopathy. Results of blood chemistry analyses and complete blood cell count throughout follow-up were normal.
The final tissue culture report obtained 7 weeks after initial presentation showed growth of M haemophilum despite a negative smear. The swab culture that initially was taken did not grow pathogenic organisms.
The patient was referred to an infectious disease specialist who confirmed that the atypical mycobacterial infection likely was the main source of the cutaneous lesions. She was instructed to continue econazole cream 1% and was given prescriptions for clarithromycin 500 mg twice daily, ciprofloxacin 500 mg twice daily, and rifampin 300 mg twice daily for a total duration of 12 to 18 months. The patient has remained on this triple-drug regimen and demonstrated improvement in the lesions. She has been off methotrexate while on antibiotic therapy.
Patient 2
A 79-year-old man with a medical history of chronic lymphocytic leukemia, basal cell carcinoma, and squamous cell carcinoma presented with a nonhealing, painful, red lesion on the left forearm of 1 week’s duration. Physical examination revealed a violaceous nontender plaque with erosions and desquamation that was initially diagnosed as a carbuncle. The patient reported a similar eruption on the right foot that was successfully treated with silver sulfadiazine by another physician.
Biopsy was performed by the shave method for histologic analysis and tissue culture. Doxycycline 100 mg twice daily was prescribed because of high suspicion of infection. Histologic findings revealed granulomatous inflammation with pseudoepitheliomatous hyperplasia, reported as squamous cell carcinoma. A second opinion confirmed suspicion of an infectious process; the patient remained on doxycycline. During follow-up, the lesion progressed to a 5-cm plaque studded with pustules and satellite papules. Multiple additional tissue cultures were performed over 2 months until “light growth” of M haemophilum was reported.
The patient showed minimal improvement on tetracycline antibiotics. His condition was complicated by a photosensitivity reaction to doxycycline on the left and right forearms, hands, and nose. Consequently, triamcinolone was prescribed, doxycycline was discontinued, and minocycline 100 mg twice daily and ciprofloxacin 500 mg twice daily were prescribed.
Nine months after initial presentation, the lesions were still present but remarkably improved. The antibiotic regimen was discontinued after 11 months.
Patient 3
A 77-year-old woman with a history of rheumatoid arthritis treated with methotrexate and abatacept as well as cutaneous T-cell lymphoma treated with narrowband UVB radiation presented to the emergency department with fever and an inflamed right forearm (Figure 3A). Initial bacterial cultures of the wound and blood were negative.

The patient was treated with vancomycin and discharged on cephalexin once she became afebrile. She was seen at our office the next week for further evaluation. We recommended that she discontinue all immunosuppressant medications. A 4-mm tissue biopsy for hematoxylin and eosin staining and a separate 4-mm punch biopsy for culture were performed while she was taking cephalexin. Histopathologic analysis revealed numerous neutrophilic abscesses; however, Gram, AFB, and fungal stains were negative.
Arm edema and pustules slowly resolved, but the eschar and verrucous plaques continued to slowly progress while the patient was off immunosuppression. She was kept off antibiotics until mycobacterial culture was positive at 4 weeks, at which time she was placed on doxycycline and clarithromycin. Final identification of M haemophilum was made at 6 weeks; consequently, doxycycline was discontinued and she was referred to infectious disease for multidrug therapy. She remained afebrile during the entire 6 weeks until cultures were final.
While immunosuppressants were discontinued and clarithromycin was administered, the plaque changed from an edematous pustular dermatitis to a verrucous crusted plaque. Neither epitrochlear nor axillary lymphadenopathy was noted during the treatment period. The infectious disease specialist prescribed azithromycin, ethambutol, and rifampin, which produced marked improvement (Figure 3B). The patient has remained off immunosuppressive therapy while on antibiotics.
Comment
Clinical Presentation and Diagnosis
Mycobacterium haemophilum is a rare infectious organism that affects primarily immunocompromised adults but also has been identified in immunocompetent adults and pediatric patients.2 Commonly affected immunosuppressed groups include solid organ transplant recipients, bone marrow transplant recipients, human immunodeficiency virus–positive patients, and patients with rheumatoid arthritis.
The infection typically presents as small violaceous papules and pustules that become painful and erythematous, with progression and draining ulceration in later stages.2 In our cases, all lesions tended to evolve into a verrucous plaque that slowly resolved with antibiotic therapy.
Due to the rarity of this infection, the initial differential diagnosis can include infection with other mycobacteria, Sporothrix, Staphylococcus aureus, and other fungal pathogens. Misdiagnosis is a common obstacle in the treatment of M haemophilum due to its rarity, often negative AFB stains, and slow growth on culture media; therefore, tissue culture is essential to successful diagnosis and management. The natural reservoir of M haemophilum is unknown, but infection has been associated with contaminated water sources.1 In one case (patient 1), symptoms developed after a dog scratch; the other 2 patients were unaware of injury to the skin.Laboratory diagnosis of M haemophilum is inherently difficult and protracted. The species is a highly fastidious and slow-growing Mycobacterium that requires cooler (30°C) incubation for many weeks on agar medium enriched with hemin or ferric ammonium citrate to obtain valid growth.1 To secure timely diagnosis, the organism’s slow agar growth warrants immediate tissue culture and biopsy when an immunocompromised patient presents with clinical features of atypical infection of an extremity. Mycobacterium haemophilum infection likely is underreported because of these difficulties in diagnosis.
Management
Although there are no standard guidelines for antibiotic treatment of M haemophilum, the current literature recommends triple-drug therapy with clarithromycin, ciprofloxacin, and rifamycin for at least 12 to 24 months.2
Upon clinical suspicion of an atypical Mycobacterium, we recommend a macrolide antibiotic over doxycycline, however, because this class of agents maintains broad coverage while being more specific for atypical mycobacteria. Although an atypical Mycobacterium was suspected early in the presentation in our cases, we discourage immediate use of triple-agent antibiotic therapy until laboratory evidence is procured to minimize antibiotic overuse in patients who do not have a final diagnosis. Single-agent therapy for prolonged treatment is discouraged for atypical mycobacterial infections because of the high risk of antibiotic resistance. Therapy should be tailored to the needs of the individual based on the extent of dissemination of disease and the severity of immunosuppression.1,2
Additionally, underlying disease that results in immunosuppression might necessitate treatment reevaluation (as occurred in our cases) requiring cessation of immunosuppressive drugs, extended careful monitoring, and pharmacotherapeutic readjustment through the course of treatment. The degree to which antibiotics contribute to eradication of M haemophilum is unknown; therefore, it is recommended that long-term antibiotic use and treatment aimed at recovering the immunocompromised state (eg, highly active antiretroviral therapy in a patient with AIDS) be implemented.2
Conclusion
Our 3 cases of M haemophilum infection involved the upper extremities of immunosuppressed patients older than 65 years. This propensity to affect the upper extremities could possibly be due to the lower temperature required for growth of M haemophilum. Initial histopathologic study showed granulomatous and neutrophilic infiltrates, yet histopathologic specimens from all 3 patients failed to display positive AFB staining, which delayed the initial antibiotic choice. In all cases, diagnosis was made by tissue culture after swab culture failed to grow the pathogen. Furthermore, the 3 cases took approximately 6 weeks to achieve final identification of the organism. Neither clinical lymphadenopathy nor systemic spread was noted in our patients; immunosuppression was discontinued when possible.
Mycobacterium haemophilum is an uncommon but potentially life-threatening infection that should be suspected in immunocompromised adults who present with atypical cellulitis of the extremities. The ultimate diagnosis often is delayed because the organism grows slowly (as long as 8 weeks) in tissue culture. For that reason, empiric antibiotic treatment, including a macrolide, should be considered in patients with disseminated or severe infection or critical immunosuppression and in those who do not demonstrate improvement in symptoms once immunosuppressants are withheld. A prolonged course of multiple-drug antibiotic therapy has proved to be effective for treating cutaneous infection with M haemophilum.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24:701-717.
- Tangkosakul T, Hongmanee P, Malathum K. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a tertiary hospital in Bangkok, Thailand: under-reported/under-recognized infection. JMM Case Rep. 2014;1:E002618.
- Sabeti S, Pourabdollah Tootkaboni M, Abdolahi M, et al. Mycobacterium haemophilum: a report of cutaneous infection in a patient with end-stage renal disease. Int J Mycobacteriol. 2016;5(suppl 1):S236.
- Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, et al. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev. 2011;24:701-717.
- Tangkosakul T, Hongmanee P, Malathum K. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a tertiary hospital in Bangkok, Thailand: under-reported/under-recognized infection. JMM Case Rep. 2014;1:E002618.
- Sabeti S, Pourabdollah Tootkaboni M, Abdolahi M, et al. Mycobacterium haemophilum: a report of cutaneous infection in a patient with end-stage renal disease. Int J Mycobacteriol. 2016;5(suppl 1):S236.
Practice Points
- Mycobacterium haemophilum infections typically occur on the extremities of immunosuppressed patients.
- Acid-fast bacilli staining may be negative.
- Mycobacterial cultures may take up to 6 weeks for growth.
- Prolonged triple-antibiotic therapy and lowering of immunosuppression is ideal treatment.
Cutaneous Nocardiosis in an Immunocompromised Patient
Case Report
A 79-year-old man with chronic lymphocytic leukemia (CLL) who was being treated with ibrutinib presented to the emergency department with a dry cough, ataxia and falls, and vision loss. Physical examination was remarkable for diffuse crackles heard throughout the right lung and bilateral lower extremity weakness. Additionally, he had 4 pink mobile nodules on the left side of the forehead, right side of the chin, left submental area, and left postauricular scalp, which arose approximately 2 weeks prior to presentation. The left postauricular lesion had been tender at times and had developed a crust. The cutaneous lesions were all smaller than 2 cm.
The patient had a history of squamous cell carcinoma of the skin and was under the care of a dermatologist as an outpatient. His dermatologist had described him as an active gardener; he was noted to have healing abrasions on the forearms due to gardening raspberry bushes.
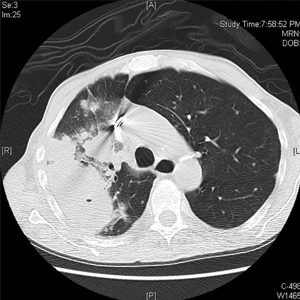
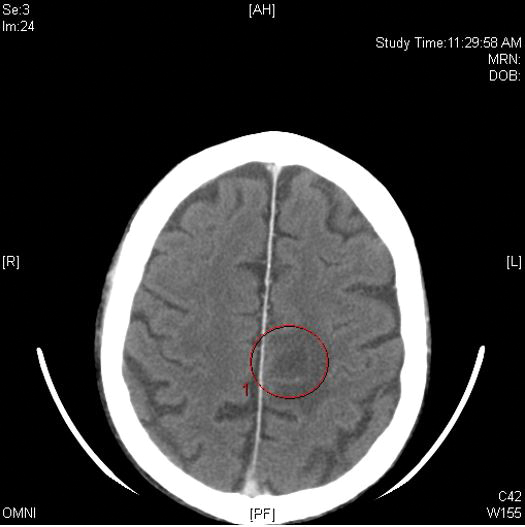
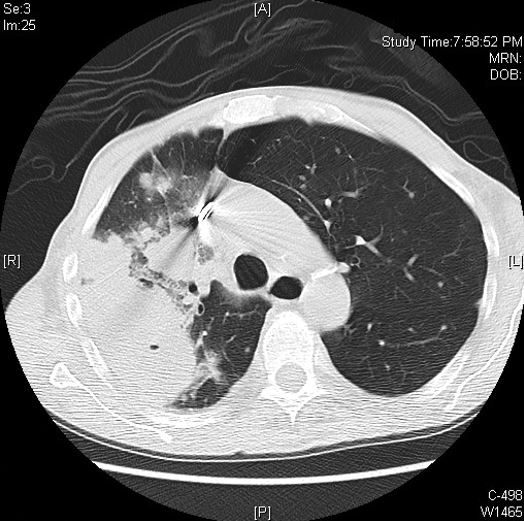
Computed tomography of the head revealed a 14-mm, ring-enhancing lesion in the left paramedian posterior frontal lobe with surrounding white matter vasogenic edema (Figure 1). Computed tomography of the chest revealed a peripheral mass on the right upper lobe measuring 6.3 cm at its greatest dimension (Figure 2).
Empiric antibiotic therapy with vancomycin and piperacillin-tazobactam was initiated. A dermatology consultation was placed by the hospitalist service; the consulting dermatologist noted that the patient had subepidermal nodules on the anterior thigh and abdomen, of which the patient had not been aware.
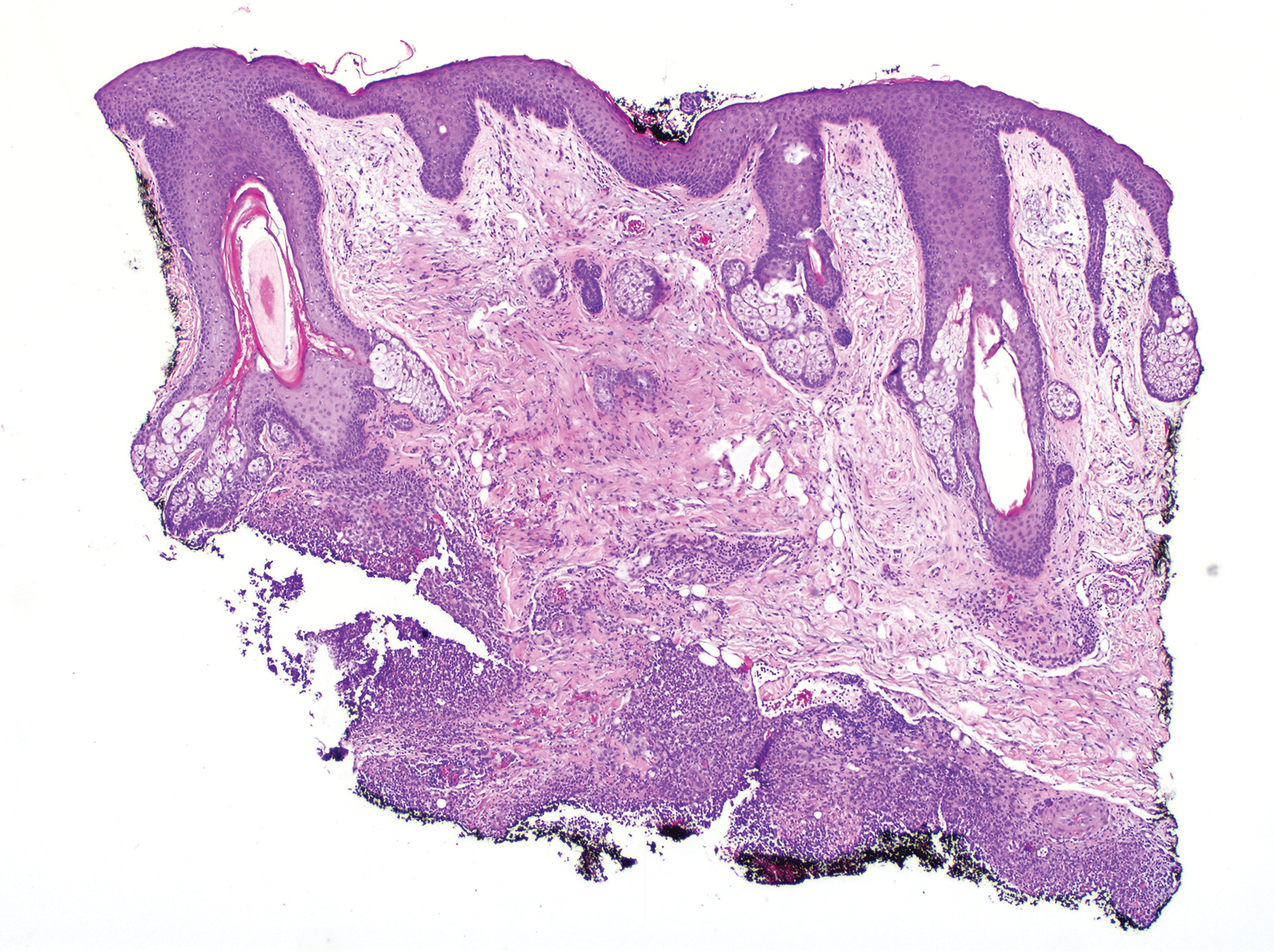
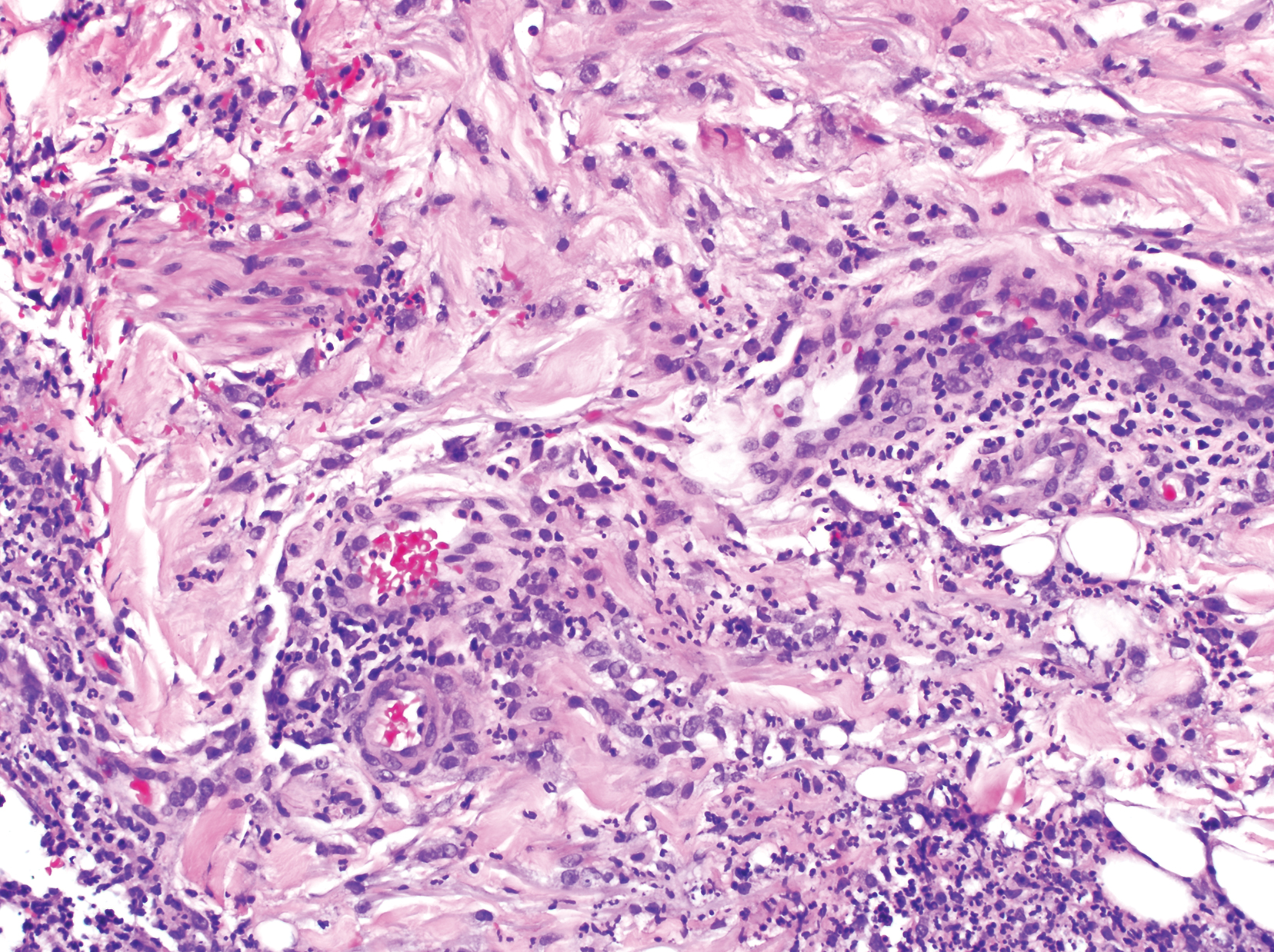
Clinically, the constellation of symptoms was thought to represent an infectious process or less likely metastatic malignancy. Biopsies of the nodule on the right side of the chin were performed and sent for culture and histologic examination. Sections from the anterior right chin showed compact orthokeratosis overlying a slightly spongiotic epidermis (Figure 3). Within the deep dermis, there was a dense mixed inflammatory infiltrate comprising predominantly neutrophils, with occasional eosinophils, lymphocytes, and histiocytes (Figure 4).
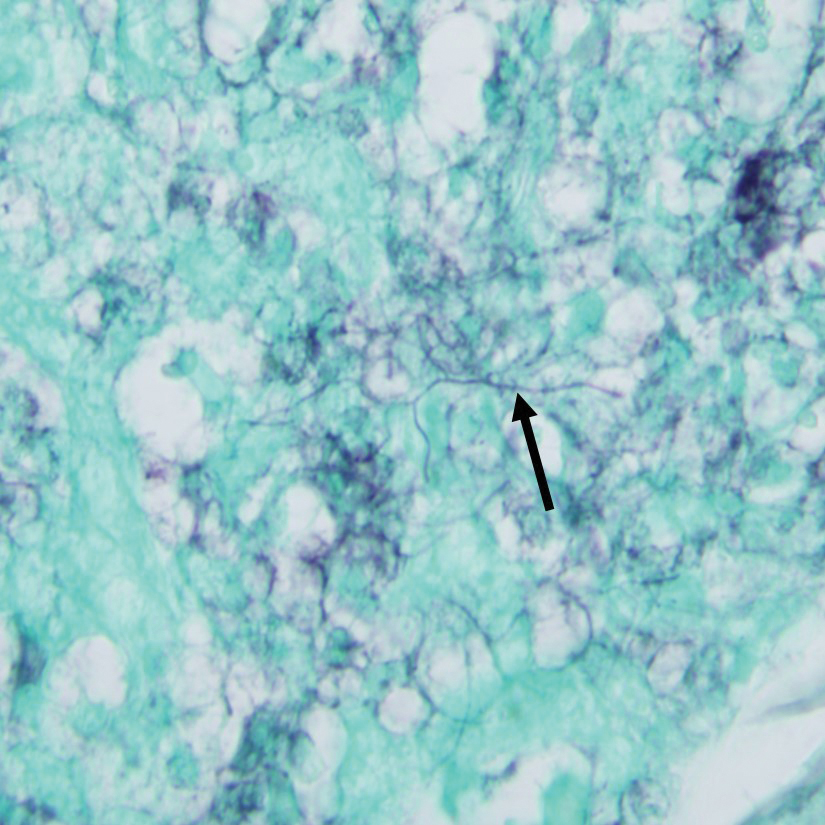
Gram stain revealed gram-variable, branching, bacterial organisms morphologically consistent with Nocardia. Grocott-Gomori methenamine-silver and periodic acid–Schiff stains also highlighted the bacterial organisms (Figure 5). An auramine-O stain was negative for acid-fast microorganisms. After 3 days on a blood agar plate, cultures of a specimen of the chin nodule grew branching filamentous bacterial organisms consistent with Nocardia.
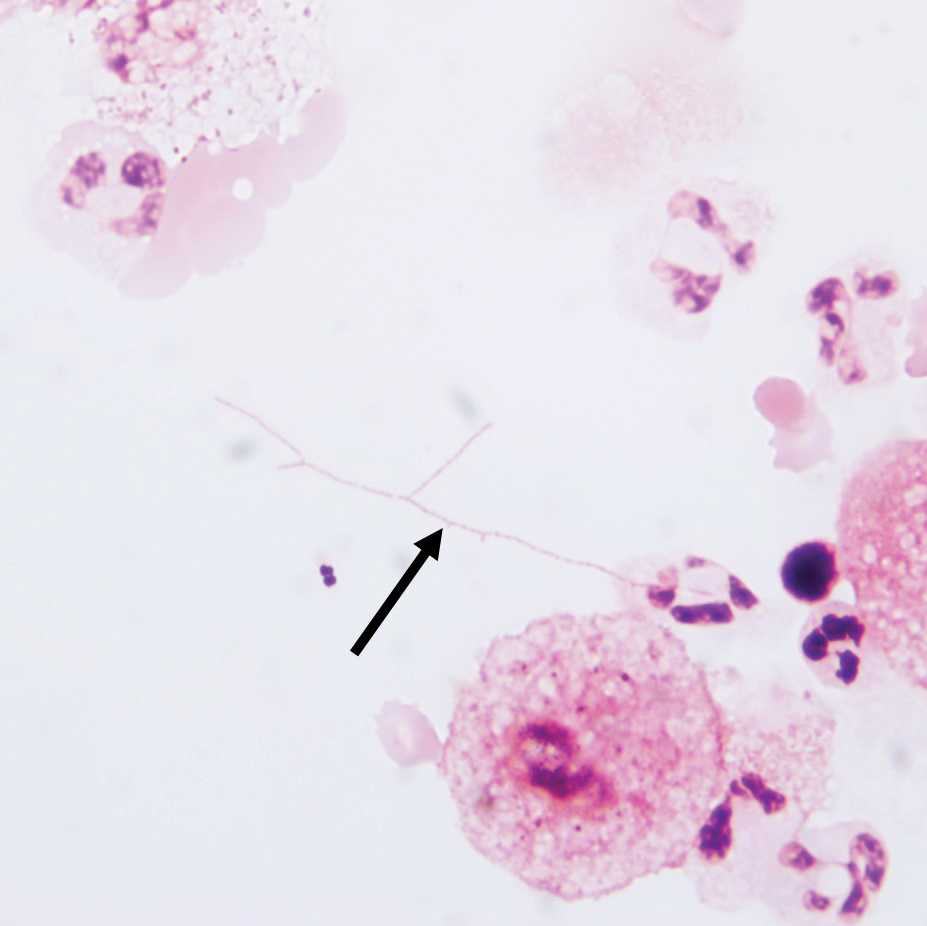
Additionally, morphologically similar microorganisms were identified on a specimen of bronchoalveolar lavage (Figure 6). Blood cultures also returned positive for Nocardia. The specimen was sent to the South Dakota Public Health Laboratory (Pierre, South Dakota), which identified the organism as Nocardia asteroides. Given the findings in skin and the lungs, it was thought that the ring-enhancing lesion in the brain was most likely the result of Nocardia infection.
Antibiotic therapy was switched to trimethoprim-sulfamethoxazole. The patient’s mental status deteriorated; vital signs became unstable. He was transferred to the intensive care unit and was found to be hyponatremic, most likely a result of the brain lesion causing the syndrome of inappropriate antidiuretic hormone secretion. Mental status and clinical condition continued to deteriorate; the patient and his family decided to stop all aggressive care and move to a comfort-only approach. He was transferred to a hospice facility and died shortly thereafter.
Comment
Presentation and Diagnosis
Nocardiosis is an infrequently encountered opportunistic infection that typically targets skin, lungs, and the central nervous system (CNS). Nocardia species characteristically are gram-positive, thin rods that form beaded, right-angle, branching filaments.1 More than 50 Nocardia species have been clinically isolated.2
Definitive diagnosis requires culture. Nocardia grows well on nonselective media, such as blood or Löwenstein-Jensen agar; growth can be enhanced with 10% CO2. Growth can be slow, however, and takes from 48 hours to several weeks. Nocardia typically grows as buff or pigmented, waxy, cerebriform colonies at 3 to 5 days’ incubation.1
Cause of Infection
Nocardia species are commonly found in the environment—soil, plant matter, water, and decomposing organic material—as well as in the gastrointestinal tract and skin of animals. Infection has been reported in cattle, dogs, horses, swine, birds, cats, foxes, and a few other animals.2 A history of exposure, such as gardening or handling animals, should increase suspicion of Nocardia.3 Although infection is classically thought to affect immunocompromised patients, there are case reports of immunocompetent individuals developing disseminated infection.4-7 However, infected immunocompetent individuals typically have localized cutaneous infection, which often includes cellulitis, abscesses, or sporotrichoid patterns.2 Cutaneous infections typically are the result of direct inoculation of the skin through a penetrating injury.8
Disseminated nocardiosis can be caused by numerous species and generally is the result of primary pulmonary infection.9 In these cases, skin disease is present in approximately 10% of patients. Disseminated infection from cutaneous nocardiosis is uncommon; when it does occur, the most common site of dissemination is the CNS, resulting in abscess or cerebritis.10 Therefore, CNS involvement should always be ruled out on diagnosis in immunocompromised patients, even if neurologic symptoms are absent.9 Nearly 80% of patients with disseminated disease are, in fact, immunocompromised.8
Association With CLL
Chronic lymphocytic leukemia is associated with profound immunodeficiency caused by quantitative and qualitative aberrations in both innate and adaptive immunity. This perturbation of the immune system predisposes the patient to infection.11,12 Early in the course of CLL, a patient develops neutropenia, which predisposes to bacterial infection; later, the patient develops a sustained B- and T-cell immunodeficiency that predisposes to opportunistic infection.13 Treatment-naïve patients with CLL are commonly diagnosed with respiratory and urinary tract infections.12 Chronic lymphocytic leukemia patients treated with alemtuzumab or purine analogs have been reported to have the highest risk for major infection.14
Ibrutinib is a commonly used treatment of CLL because it induces apoptosis in B cells, which are abnormal in CLL. Ibrutinib functions by inhibiting the Bruton tyrosine kinase pathway, which is essential in B-cell production and maintenance.15 Studies have reported a high rate of infection in ibrutinib-treated CLL patients14,16; salvage ibrutinib therapy has been associated with higher infection risk than primary ibrutinib therapy.16,17 Long-term follow-up studies have shown a decreased rate of infection in ibrutinib-treated CLL after 2 years or longer of treatment, suggesting a reconstitution of normal B cells and humoral immunity with longer ibrutinib therapy.16,17
Many infections have been identified in association with ibrutinib therapy, including invasive aspergillosis, disseminated fusariosis, cerebral mucormycosis, disseminated cryptococcosis, and Pneumocystis jirovecii pneumonia.18-22 Disseminated nocardiosis has been reported in a few patients with CLL, though the treatment they received for CLL varied from case to case.23-25
Identification and Treatment
Clinical and microscopic identification of Nocardia organisms can be exceedingly difficult. Primary cutaneous nocardiosis clinically presents as tumors or nodules that often have a sporotrichoid pattern along the lymphatics. In disease that disseminates to skin, nocardiosis presents as vesiculopustules or abscesses. The biopsy specimen most often shows a dense dermal and subcutaneous infiltrate of neutrophils with abscess formation. Long-standing lesions might show chronic inflammation and nonspecific granulomas.
The appearance of Nocardia organisms is quite subtle on hematoxylin and eosin staining and can be easily missed. Special stains, such as Gram and Grocott-Gomori methenamine-silver stains as well as stains for acid-fast organisms, can be invaluable in diagnosing this disease. Biopsy in immunocompromised patients when nocardiosis is part of the differential diagnosis requires extra attention because the organisms can be gram variable and only partially acid fast, as was the case in our patient. Organisms typically will be positive with silver stains.
Trimethoprim-sulfamethoxazole typically is the first-line treatment of nocardiosis. Although prognosis is excellent when disease is confined to skin, disseminated infection has 25% mortality.8 Diagnosticians should maintain a high index of suspicion for the disease, especially in immunocompromised patients, because clinical and imaging findings can be nonspecific.
Conclusion
Our patient’s primary risk factor for nocardiosis was his immunocompromised state. In addition, he was an avid gardener, which increased his risk for exposure to the microorganism. Given the timing of disease progression, our case most likely represents primary cutaneous nocardiosis with dissemination to brain, lungs, and other organs, leading to death, and serves as a reminder to dermatologists and pathologists to establish a broad differential diagnosis when dealing with an infectious process in immunocompromised patients.
- Ferri F. Ferri’s Clinical Advisor 2016: 5 Books in 1. Philadelphia, PA: Elsevier; 2016.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Grau Pérez M, Casabella Pernas A, de la Rosa Del Rey MDP, et al. Primary cutaneous nocardiosis: a pitfall in the diagnosis of skin infection. Infection. 2017;45:927-928.
- Oda R, Sekikawa Y, Hongo I. Primary cutaneous nocardiosis in an immunocompetent patient. Intern Med. 2017;56:469-470.
- Jiang Y, Huang A, Fang Q. Disseminated nocardiosis caused by Nocardia otitidiscaviarum in an immunocompetent host: a case report and literature review. Exp Ther Med. 2016;12:3339-3346.
- Cooper CJ, Said S, Popp M, et al. A complicated case of an immunocompetent patient with disseminated nocardiosis. Infect Dis Rep. 2014;6:5327.
- Kim MS, Choi H, Choi KC, et al. Primary cutaneous nocardiosis due to Nocardia vinacea: first case in an immunocompetent patient. Clin Exp Dermatol. 2011;36:812-814.
- Hall BJ, Hall JC, Cockerell CJ. Diagnostic Pathology. Nonneoplastic Dermatopathology. Salt Lake City, UT: Amirsys; 2012.
- Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
- Bosamiya SS, Vaishnani JB, Momin AM. Sporotrichoid nocardiosis with cutaneous dissemination. Indian J Dermatol Venereol Leprol. 2011;77:535.
- Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207-235.
- Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573-581.
- Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2018;11:57-70.
- Williams AM, Baran AM, Meacham PJ, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma. 2018;59:625-632.
- Dias AL, Jain D. Ibrutinib: a new frontier in the treatment of chronic lymphocytic leukemia by Bruton’s tyrosine kinase inhibition. Cardiovasc Hematol Agents Med Chem. 2013;11:265-271.
- Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213-2219.
- Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497-2506.
- Arthurs B, Wunderle K, Hsu M, et al. Invasive aspergillosis related to ibrutinib therapy for chronic lymphocytic leukemia. Respir Med Case Rep. 2017;21:27-29.
- Chan TS, Au-Yeung R, Chim CS, et al. Disseminated fusarium infection after ibrutinib therapy in chronic lymphocytic leukaemia. Ann Hematol. 2017;96:871-872.
- Farid S, AbuSaleh O, Liesman R, et al. Isolated cerebral mucormycosis caused by Rhizomucor pusillus [published online October 4, 2017]. BMJ Case Rep. pii:bcr-2017-221473.
- Okamoto K, Proia LA, Demarais PL. Disseminated cryptococcal disease in a patient with chronic lymphocytic leukemia on ibrutinib. Case Rep Infect Dis. 2016;2016:4642831.
- Ahn IE, Jerussi T, Farooqui M, et al. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128:1940-1943.
- Roberts AL, Davidson RM, Freifeld AG, et al. Nocardia arthritidis as a cause of disseminated nocardiosis in a patient with chronic lymphocytic leukemia. IDCases. 2016;6:68-71.
- Rámila E, Martino R, Santamaría A, et al. Inappropriate secretion of antidiuretic hormone as the initial sign of central nervous system progression of nocardiosis in a patient with chronic lymphocytic leukemia. Haematologica. 1999;84:1155-1156.
- Phillips WB, Shields CL, Shields JA, et al. Nocardia choroidal abscess. Br J Ophthalmol. 1992;76:694-696.
Case Report
A 79-year-old man with chronic lymphocytic leukemia (CLL) who was being treated with ibrutinib presented to the emergency department with a dry cough, ataxia and falls, and vision loss. Physical examination was remarkable for diffuse crackles heard throughout the right lung and bilateral lower extremity weakness. Additionally, he had 4 pink mobile nodules on the left side of the forehead, right side of the chin, left submental area, and left postauricular scalp, which arose approximately 2 weeks prior to presentation. The left postauricular lesion had been tender at times and had developed a crust. The cutaneous lesions were all smaller than 2 cm.
The patient had a history of squamous cell carcinoma of the skin and was under the care of a dermatologist as an outpatient. His dermatologist had described him as an active gardener; he was noted to have healing abrasions on the forearms due to gardening raspberry bushes.
Computed tomography of the head revealed a 14-mm, ring-enhancing lesion in the left paramedian posterior frontal lobe with surrounding white matter vasogenic edema (Figure 1). Computed tomography of the chest revealed a peripheral mass on the right upper lobe measuring 6.3 cm at its greatest dimension (Figure 2).


Empiric antibiotic therapy with vancomycin and piperacillin-tazobactam was initiated. A dermatology consultation was placed by the hospitalist service; the consulting dermatologist noted that the patient had subepidermal nodules on the anterior thigh and abdomen, of which the patient had not been aware.
Clinically, the constellation of symptoms was thought to represent an infectious process or less likely metastatic malignancy. Biopsies of the nodule on the right side of the chin were performed and sent for culture and histologic examination. Sections from the anterior right chin showed compact orthokeratosis overlying a slightly spongiotic epidermis (Figure 3). Within the deep dermis, there was a dense mixed inflammatory infiltrate comprising predominantly neutrophils, with occasional eosinophils, lymphocytes, and histiocytes (Figure 4).


Gram stain revealed gram-variable, branching, bacterial organisms morphologically consistent with Nocardia. Grocott-Gomori methenamine-silver and periodic acid–Schiff stains also highlighted the bacterial organisms (Figure 5). An auramine-O stain was negative for acid-fast microorganisms. After 3 days on a blood agar plate, cultures of a specimen of the chin nodule grew branching filamentous bacterial organisms consistent with Nocardia.

Additionally, morphologically similar microorganisms were identified on a specimen of bronchoalveolar lavage (Figure 6). Blood cultures also returned positive for Nocardia. The specimen was sent to the South Dakota Public Health Laboratory (Pierre, South Dakota), which identified the organism as Nocardia asteroides. Given the findings in skin and the lungs, it was thought that the ring-enhancing lesion in the brain was most likely the result of Nocardia infection.

Antibiotic therapy was switched to trimethoprim-sulfamethoxazole. The patient’s mental status deteriorated; vital signs became unstable. He was transferred to the intensive care unit and was found to be hyponatremic, most likely a result of the brain lesion causing the syndrome of inappropriate antidiuretic hormone secretion. Mental status and clinical condition continued to deteriorate; the patient and his family decided to stop all aggressive care and move to a comfort-only approach. He was transferred to a hospice facility and died shortly thereafter.
Comment
Presentation and Diagnosis
Nocardiosis is an infrequently encountered opportunistic infection that typically targets skin, lungs, and the central nervous system (CNS). Nocardia species characteristically are gram-positive, thin rods that form beaded, right-angle, branching filaments.1 More than 50 Nocardia species have been clinically isolated.2
Definitive diagnosis requires culture. Nocardia grows well on nonselective media, such as blood or Löwenstein-Jensen agar; growth can be enhanced with 10% CO2. Growth can be slow, however, and takes from 48 hours to several weeks. Nocardia typically grows as buff or pigmented, waxy, cerebriform colonies at 3 to 5 days’ incubation.1
Cause of Infection
Nocardia species are commonly found in the environment—soil, plant matter, water, and decomposing organic material—as well as in the gastrointestinal tract and skin of animals. Infection has been reported in cattle, dogs, horses, swine, birds, cats, foxes, and a few other animals.2 A history of exposure, such as gardening or handling animals, should increase suspicion of Nocardia.3 Although infection is classically thought to affect immunocompromised patients, there are case reports of immunocompetent individuals developing disseminated infection.4-7 However, infected immunocompetent individuals typically have localized cutaneous infection, which often includes cellulitis, abscesses, or sporotrichoid patterns.2 Cutaneous infections typically are the result of direct inoculation of the skin through a penetrating injury.8
Disseminated nocardiosis can be caused by numerous species and generally is the result of primary pulmonary infection.9 In these cases, skin disease is present in approximately 10% of patients. Disseminated infection from cutaneous nocardiosis is uncommon; when it does occur, the most common site of dissemination is the CNS, resulting in abscess or cerebritis.10 Therefore, CNS involvement should always be ruled out on diagnosis in immunocompromised patients, even if neurologic symptoms are absent.9 Nearly 80% of patients with disseminated disease are, in fact, immunocompromised.8
Association With CLL
Chronic lymphocytic leukemia is associated with profound immunodeficiency caused by quantitative and qualitative aberrations in both innate and adaptive immunity. This perturbation of the immune system predisposes the patient to infection.11,12 Early in the course of CLL, a patient develops neutropenia, which predisposes to bacterial infection; later, the patient develops a sustained B- and T-cell immunodeficiency that predisposes to opportunistic infection.13 Treatment-naïve patients with CLL are commonly diagnosed with respiratory and urinary tract infections.12 Chronic lymphocytic leukemia patients treated with alemtuzumab or purine analogs have been reported to have the highest risk for major infection.14
Ibrutinib is a commonly used treatment of CLL because it induces apoptosis in B cells, which are abnormal in CLL. Ibrutinib functions by inhibiting the Bruton tyrosine kinase pathway, which is essential in B-cell production and maintenance.15 Studies have reported a high rate of infection in ibrutinib-treated CLL patients14,16; salvage ibrutinib therapy has been associated with higher infection risk than primary ibrutinib therapy.16,17 Long-term follow-up studies have shown a decreased rate of infection in ibrutinib-treated CLL after 2 years or longer of treatment, suggesting a reconstitution of normal B cells and humoral immunity with longer ibrutinib therapy.16,17
Many infections have been identified in association with ibrutinib therapy, including invasive aspergillosis, disseminated fusariosis, cerebral mucormycosis, disseminated cryptococcosis, and Pneumocystis jirovecii pneumonia.18-22 Disseminated nocardiosis has been reported in a few patients with CLL, though the treatment they received for CLL varied from case to case.23-25
Identification and Treatment
Clinical and microscopic identification of Nocardia organisms can be exceedingly difficult. Primary cutaneous nocardiosis clinically presents as tumors or nodules that often have a sporotrichoid pattern along the lymphatics. In disease that disseminates to skin, nocardiosis presents as vesiculopustules or abscesses. The biopsy specimen most often shows a dense dermal and subcutaneous infiltrate of neutrophils with abscess formation. Long-standing lesions might show chronic inflammation and nonspecific granulomas.
The appearance of Nocardia organisms is quite subtle on hematoxylin and eosin staining and can be easily missed. Special stains, such as Gram and Grocott-Gomori methenamine-silver stains as well as stains for acid-fast organisms, can be invaluable in diagnosing this disease. Biopsy in immunocompromised patients when nocardiosis is part of the differential diagnosis requires extra attention because the organisms can be gram variable and only partially acid fast, as was the case in our patient. Organisms typically will be positive with silver stains.
Trimethoprim-sulfamethoxazole typically is the first-line treatment of nocardiosis. Although prognosis is excellent when disease is confined to skin, disseminated infection has 25% mortality.8 Diagnosticians should maintain a high index of suspicion for the disease, especially in immunocompromised patients, because clinical and imaging findings can be nonspecific.
Conclusion
Our patient’s primary risk factor for nocardiosis was his immunocompromised state. In addition, he was an avid gardener, which increased his risk for exposure to the microorganism. Given the timing of disease progression, our case most likely represents primary cutaneous nocardiosis with dissemination to brain, lungs, and other organs, leading to death, and serves as a reminder to dermatologists and pathologists to establish a broad differential diagnosis when dealing with an infectious process in immunocompromised patients.
Case Report
A 79-year-old man with chronic lymphocytic leukemia (CLL) who was being treated with ibrutinib presented to the emergency department with a dry cough, ataxia and falls, and vision loss. Physical examination was remarkable for diffuse crackles heard throughout the right lung and bilateral lower extremity weakness. Additionally, he had 4 pink mobile nodules on the left side of the forehead, right side of the chin, left submental area, and left postauricular scalp, which arose approximately 2 weeks prior to presentation. The left postauricular lesion had been tender at times and had developed a crust. The cutaneous lesions were all smaller than 2 cm.
The patient had a history of squamous cell carcinoma of the skin and was under the care of a dermatologist as an outpatient. His dermatologist had described him as an active gardener; he was noted to have healing abrasions on the forearms due to gardening raspberry bushes.
Computed tomography of the head revealed a 14-mm, ring-enhancing lesion in the left paramedian posterior frontal lobe with surrounding white matter vasogenic edema (Figure 1). Computed tomography of the chest revealed a peripheral mass on the right upper lobe measuring 6.3 cm at its greatest dimension (Figure 2).


Empiric antibiotic therapy with vancomycin and piperacillin-tazobactam was initiated. A dermatology consultation was placed by the hospitalist service; the consulting dermatologist noted that the patient had subepidermal nodules on the anterior thigh and abdomen, of which the patient had not been aware.
Clinically, the constellation of symptoms was thought to represent an infectious process or less likely metastatic malignancy. Biopsies of the nodule on the right side of the chin were performed and sent for culture and histologic examination. Sections from the anterior right chin showed compact orthokeratosis overlying a slightly spongiotic epidermis (Figure 3). Within the deep dermis, there was a dense mixed inflammatory infiltrate comprising predominantly neutrophils, with occasional eosinophils, lymphocytes, and histiocytes (Figure 4).


Gram stain revealed gram-variable, branching, bacterial organisms morphologically consistent with Nocardia. Grocott-Gomori methenamine-silver and periodic acid–Schiff stains also highlighted the bacterial organisms (Figure 5). An auramine-O stain was negative for acid-fast microorganisms. After 3 days on a blood agar plate, cultures of a specimen of the chin nodule grew branching filamentous bacterial organisms consistent with Nocardia.

Additionally, morphologically similar microorganisms were identified on a specimen of bronchoalveolar lavage (Figure 6). Blood cultures also returned positive for Nocardia. The specimen was sent to the South Dakota Public Health Laboratory (Pierre, South Dakota), which identified the organism as Nocardia asteroides. Given the findings in skin and the lungs, it was thought that the ring-enhancing lesion in the brain was most likely the result of Nocardia infection.

Antibiotic therapy was switched to trimethoprim-sulfamethoxazole. The patient’s mental status deteriorated; vital signs became unstable. He was transferred to the intensive care unit and was found to be hyponatremic, most likely a result of the brain lesion causing the syndrome of inappropriate antidiuretic hormone secretion. Mental status and clinical condition continued to deteriorate; the patient and his family decided to stop all aggressive care and move to a comfort-only approach. He was transferred to a hospice facility and died shortly thereafter.
Comment
Presentation and Diagnosis
Nocardiosis is an infrequently encountered opportunistic infection that typically targets skin, lungs, and the central nervous system (CNS). Nocardia species characteristically are gram-positive, thin rods that form beaded, right-angle, branching filaments.1 More than 50 Nocardia species have been clinically isolated.2
Definitive diagnosis requires culture. Nocardia grows well on nonselective media, such as blood or Löwenstein-Jensen agar; growth can be enhanced with 10% CO2. Growth can be slow, however, and takes from 48 hours to several weeks. Nocardia typically grows as buff or pigmented, waxy, cerebriform colonies at 3 to 5 days’ incubation.1
Cause of Infection
Nocardia species are commonly found in the environment—soil, plant matter, water, and decomposing organic material—as well as in the gastrointestinal tract and skin of animals. Infection has been reported in cattle, dogs, horses, swine, birds, cats, foxes, and a few other animals.2 A history of exposure, such as gardening or handling animals, should increase suspicion of Nocardia.3 Although infection is classically thought to affect immunocompromised patients, there are case reports of immunocompetent individuals developing disseminated infection.4-7 However, infected immunocompetent individuals typically have localized cutaneous infection, which often includes cellulitis, abscesses, or sporotrichoid patterns.2 Cutaneous infections typically are the result of direct inoculation of the skin through a penetrating injury.8
Disseminated nocardiosis can be caused by numerous species and generally is the result of primary pulmonary infection.9 In these cases, skin disease is present in approximately 10% of patients. Disseminated infection from cutaneous nocardiosis is uncommon; when it does occur, the most common site of dissemination is the CNS, resulting in abscess or cerebritis.10 Therefore, CNS involvement should always be ruled out on diagnosis in immunocompromised patients, even if neurologic symptoms are absent.9 Nearly 80% of patients with disseminated disease are, in fact, immunocompromised.8
Association With CLL
Chronic lymphocytic leukemia is associated with profound immunodeficiency caused by quantitative and qualitative aberrations in both innate and adaptive immunity. This perturbation of the immune system predisposes the patient to infection.11,12 Early in the course of CLL, a patient develops neutropenia, which predisposes to bacterial infection; later, the patient develops a sustained B- and T-cell immunodeficiency that predisposes to opportunistic infection.13 Treatment-naïve patients with CLL are commonly diagnosed with respiratory and urinary tract infections.12 Chronic lymphocytic leukemia patients treated with alemtuzumab or purine analogs have been reported to have the highest risk for major infection.14
Ibrutinib is a commonly used treatment of CLL because it induces apoptosis in B cells, which are abnormal in CLL. Ibrutinib functions by inhibiting the Bruton tyrosine kinase pathway, which is essential in B-cell production and maintenance.15 Studies have reported a high rate of infection in ibrutinib-treated CLL patients14,16; salvage ibrutinib therapy has been associated with higher infection risk than primary ibrutinib therapy.16,17 Long-term follow-up studies have shown a decreased rate of infection in ibrutinib-treated CLL after 2 years or longer of treatment, suggesting a reconstitution of normal B cells and humoral immunity with longer ibrutinib therapy.16,17
Many infections have been identified in association with ibrutinib therapy, including invasive aspergillosis, disseminated fusariosis, cerebral mucormycosis, disseminated cryptococcosis, and Pneumocystis jirovecii pneumonia.18-22 Disseminated nocardiosis has been reported in a few patients with CLL, though the treatment they received for CLL varied from case to case.23-25
Identification and Treatment
Clinical and microscopic identification of Nocardia organisms can be exceedingly difficult. Primary cutaneous nocardiosis clinically presents as tumors or nodules that often have a sporotrichoid pattern along the lymphatics. In disease that disseminates to skin, nocardiosis presents as vesiculopustules or abscesses. The biopsy specimen most often shows a dense dermal and subcutaneous infiltrate of neutrophils with abscess formation. Long-standing lesions might show chronic inflammation and nonspecific granulomas.
The appearance of Nocardia organisms is quite subtle on hematoxylin and eosin staining and can be easily missed. Special stains, such as Gram and Grocott-Gomori methenamine-silver stains as well as stains for acid-fast organisms, can be invaluable in diagnosing this disease. Biopsy in immunocompromised patients when nocardiosis is part of the differential diagnosis requires extra attention because the organisms can be gram variable and only partially acid fast, as was the case in our patient. Organisms typically will be positive with silver stains.
Trimethoprim-sulfamethoxazole typically is the first-line treatment of nocardiosis. Although prognosis is excellent when disease is confined to skin, disseminated infection has 25% mortality.8 Diagnosticians should maintain a high index of suspicion for the disease, especially in immunocompromised patients, because clinical and imaging findings can be nonspecific.
Conclusion
Our patient’s primary risk factor for nocardiosis was his immunocompromised state. In addition, he was an avid gardener, which increased his risk for exposure to the microorganism. Given the timing of disease progression, our case most likely represents primary cutaneous nocardiosis with dissemination to brain, lungs, and other organs, leading to death, and serves as a reminder to dermatologists and pathologists to establish a broad differential diagnosis when dealing with an infectious process in immunocompromised patients.
- Ferri F. Ferri’s Clinical Advisor 2016: 5 Books in 1. Philadelphia, PA: Elsevier; 2016.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Grau Pérez M, Casabella Pernas A, de la Rosa Del Rey MDP, et al. Primary cutaneous nocardiosis: a pitfall in the diagnosis of skin infection. Infection. 2017;45:927-928.
- Oda R, Sekikawa Y, Hongo I. Primary cutaneous nocardiosis in an immunocompetent patient. Intern Med. 2017;56:469-470.
- Jiang Y, Huang A, Fang Q. Disseminated nocardiosis caused by Nocardia otitidiscaviarum in an immunocompetent host: a case report and literature review. Exp Ther Med. 2016;12:3339-3346.
- Cooper CJ, Said S, Popp M, et al. A complicated case of an immunocompetent patient with disseminated nocardiosis. Infect Dis Rep. 2014;6:5327.
- Kim MS, Choi H, Choi KC, et al. Primary cutaneous nocardiosis due to Nocardia vinacea: first case in an immunocompetent patient. Clin Exp Dermatol. 2011;36:812-814.
- Hall BJ, Hall JC, Cockerell CJ. Diagnostic Pathology. Nonneoplastic Dermatopathology. Salt Lake City, UT: Amirsys; 2012.
- Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
- Bosamiya SS, Vaishnani JB, Momin AM. Sporotrichoid nocardiosis with cutaneous dissemination. Indian J Dermatol Venereol Leprol. 2011;77:535.
- Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207-235.
- Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573-581.
- Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2018;11:57-70.
- Williams AM, Baran AM, Meacham PJ, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma. 2018;59:625-632.
- Dias AL, Jain D. Ibrutinib: a new frontier in the treatment of chronic lymphocytic leukemia by Bruton’s tyrosine kinase inhibition. Cardiovasc Hematol Agents Med Chem. 2013;11:265-271.
- Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213-2219.
- Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497-2506.
- Arthurs B, Wunderle K, Hsu M, et al. Invasive aspergillosis related to ibrutinib therapy for chronic lymphocytic leukemia. Respir Med Case Rep. 2017;21:27-29.
- Chan TS, Au-Yeung R, Chim CS, et al. Disseminated fusarium infection after ibrutinib therapy in chronic lymphocytic leukaemia. Ann Hematol. 2017;96:871-872.
- Farid S, AbuSaleh O, Liesman R, et al. Isolated cerebral mucormycosis caused by Rhizomucor pusillus [published online October 4, 2017]. BMJ Case Rep. pii:bcr-2017-221473.
- Okamoto K, Proia LA, Demarais PL. Disseminated cryptococcal disease in a patient with chronic lymphocytic leukemia on ibrutinib. Case Rep Infect Dis. 2016;2016:4642831.
- Ahn IE, Jerussi T, Farooqui M, et al. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128:1940-1943.
- Roberts AL, Davidson RM, Freifeld AG, et al. Nocardia arthritidis as a cause of disseminated nocardiosis in a patient with chronic lymphocytic leukemia. IDCases. 2016;6:68-71.
- Rámila E, Martino R, Santamaría A, et al. Inappropriate secretion of antidiuretic hormone as the initial sign of central nervous system progression of nocardiosis in a patient with chronic lymphocytic leukemia. Haematologica. 1999;84:1155-1156.
- Phillips WB, Shields CL, Shields JA, et al. Nocardia choroidal abscess. Br J Ophthalmol. 1992;76:694-696.
- Ferri F. Ferri’s Clinical Advisor 2016: 5 Books in 1. Philadelphia, PA: Elsevier; 2016.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Grau Pérez M, Casabella Pernas A, de la Rosa Del Rey MDP, et al. Primary cutaneous nocardiosis: a pitfall in the diagnosis of skin infection. Infection. 2017;45:927-928.
- Oda R, Sekikawa Y, Hongo I. Primary cutaneous nocardiosis in an immunocompetent patient. Intern Med. 2017;56:469-470.
- Jiang Y, Huang A, Fang Q. Disseminated nocardiosis caused by Nocardia otitidiscaviarum in an immunocompetent host: a case report and literature review. Exp Ther Med. 2016;12:3339-3346.
- Cooper CJ, Said S, Popp M, et al. A complicated case of an immunocompetent patient with disseminated nocardiosis. Infect Dis Rep. 2014;6:5327.
- Kim MS, Choi H, Choi KC, et al. Primary cutaneous nocardiosis due to Nocardia vinacea: first case in an immunocompetent patient. Clin Exp Dermatol. 2011;36:812-814.
- Hall BJ, Hall JC, Cockerell CJ. Diagnostic Pathology. Nonneoplastic Dermatopathology. Salt Lake City, UT: Amirsys; 2012.
- Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
- Bosamiya SS, Vaishnani JB, Momin AM. Sporotrichoid nocardiosis with cutaneous dissemination. Indian J Dermatol Venereol Leprol. 2011;77:535.
- Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207-235.
- Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573-581.
- Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2018;11:57-70.
- Williams AM, Baran AM, Meacham PJ, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma. 2018;59:625-632.
- Dias AL, Jain D. Ibrutinib: a new frontier in the treatment of chronic lymphocytic leukemia by Bruton’s tyrosine kinase inhibition. Cardiovasc Hematol Agents Med Chem. 2013;11:265-271.
- Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213-2219.
- Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497-2506.
- Arthurs B, Wunderle K, Hsu M, et al. Invasive aspergillosis related to ibrutinib therapy for chronic lymphocytic leukemia. Respir Med Case Rep. 2017;21:27-29.
- Chan TS, Au-Yeung R, Chim CS, et al. Disseminated fusarium infection after ibrutinib therapy in chronic lymphocytic leukaemia. Ann Hematol. 2017;96:871-872.
- Farid S, AbuSaleh O, Liesman R, et al. Isolated cerebral mucormycosis caused by Rhizomucor pusillus [published online October 4, 2017]. BMJ Case Rep. pii:bcr-2017-221473.
- Okamoto K, Proia LA, Demarais PL. Disseminated cryptococcal disease in a patient with chronic lymphocytic leukemia on ibrutinib. Case Rep Infect Dis. 2016;2016:4642831.
- Ahn IE, Jerussi T, Farooqui M, et al. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128:1940-1943.
- Roberts AL, Davidson RM, Freifeld AG, et al. Nocardia arthritidis as a cause of disseminated nocardiosis in a patient with chronic lymphocytic leukemia. IDCases. 2016;6:68-71.
- Rámila E, Martino R, Santamaría A, et al. Inappropriate secretion of antidiuretic hormone as the initial sign of central nervous system progression of nocardiosis in a patient with chronic lymphocytic leukemia. Haematologica. 1999;84:1155-1156.
- Phillips WB, Shields CL, Shields JA, et al. Nocardia choroidal abscess. Br J Ophthalmol. 1992;76:694-696.
Practice Points
- Clinicians should consider a broad differential when dealing with infectious diseases in immunocompromised patients.
- Primary cutaneous nocardiosis classically presents as tumors or nodules with a sporotrichoid pattern along the lymphatics. Vesiculopustules and abscesses are seen in disseminated disease, which usually involves the skin, lungs, and/or central nervous system.
- Nocardia species are characteristically gram-positive, thin rods that form beaded, right-angle branching filaments.
- When nocardiosis is in the differential, special care should be taken, as organisms can be gram variable or only partially acid fast. Gram, Grocott-Gomori methenamine-silver, and acid-fast staining may be essential to making the diagnosis.
What’s Eating You? The South African Fattail Scorpion Revisited
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
Practice Points
- Exotic and dangerous pets are becoming more popular. Scorpion stings cause potentially life-threatening neurotoxicity, with children particularly susceptible.
- Fattail scorpions are particularly dangerous and physicians should be aware that their stings may be encountered worldwide.
- Symptoms present 1 to 8 hours after envenomation, with severe cases showing hyperreflexia, clonus, difficulty swallowing, and respiratory distress. The sting site may be unimpressive.
What Neglected Tropical Diseases Teach Us About Stigma
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
Epidemiology and costs of sepsis in the United States
Background: Sepsis is responsible for an increasingly disproportionate fraction of health care burden. Delays in diagnosis of sepsis are associated with worse outcomes.
Study design: Retrospective observational study.
Setting: Premier Healthcare database, including 20% of U.S. private/academic hospitals.
Synopsis: With use of the Premier Healthcare database, researchers identified 2,566,689 cases of sepsis using ICD-9 and MS-DRG codes between Jan. 1, 2010, and Sept. 30, 2016. Increasing severity of sepsis was associated with increasing mortality and cost, but there was a large discrepancy in cost in patients with sepsis present at admission versus those without it at admission ($18,023 vs. $51,022) and was associated with increases in both mean hospital length of stay and mortality rate across all levels of sepsis severity.
Bottom line: Early identification of sepsis (at admission vs. later in the stay) may be important as a factor to reduce its overall burden on the health care system.
Citation: Paoli CJ et al. Epidemiology and costs of sepsis in the United States – An analysis based on timing of diagnosis and severity level. Crit Care Med. 2018 Dec;46(12):1889-97.
Dr. Ho is an assistant professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Sepsis is responsible for an increasingly disproportionate fraction of health care burden. Delays in diagnosis of sepsis are associated with worse outcomes.
Study design: Retrospective observational study.
Setting: Premier Healthcare database, including 20% of U.S. private/academic hospitals.
Synopsis: With use of the Premier Healthcare database, researchers identified 2,566,689 cases of sepsis using ICD-9 and MS-DRG codes between Jan. 1, 2010, and Sept. 30, 2016. Increasing severity of sepsis was associated with increasing mortality and cost, but there was a large discrepancy in cost in patients with sepsis present at admission versus those without it at admission ($18,023 vs. $51,022) and was associated with increases in both mean hospital length of stay and mortality rate across all levels of sepsis severity.
Bottom line: Early identification of sepsis (at admission vs. later in the stay) may be important as a factor to reduce its overall burden on the health care system.
Citation: Paoli CJ et al. Epidemiology and costs of sepsis in the United States – An analysis based on timing of diagnosis and severity level. Crit Care Med. 2018 Dec;46(12):1889-97.
Dr. Ho is an assistant professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Sepsis is responsible for an increasingly disproportionate fraction of health care burden. Delays in diagnosis of sepsis are associated with worse outcomes.
Study design: Retrospective observational study.
Setting: Premier Healthcare database, including 20% of U.S. private/academic hospitals.
Synopsis: With use of the Premier Healthcare database, researchers identified 2,566,689 cases of sepsis using ICD-9 and MS-DRG codes between Jan. 1, 2010, and Sept. 30, 2016. Increasing severity of sepsis was associated with increasing mortality and cost, but there was a large discrepancy in cost in patients with sepsis present at admission versus those without it at admission ($18,023 vs. $51,022) and was associated with increases in both mean hospital length of stay and mortality rate across all levels of sepsis severity.
Bottom line: Early identification of sepsis (at admission vs. later in the stay) may be important as a factor to reduce its overall burden on the health care system.
Citation: Paoli CJ et al. Epidemiology and costs of sepsis in the United States – An analysis based on timing of diagnosis and severity level. Crit Care Med. 2018 Dec;46(12):1889-97.
Dr. Ho is an assistant professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Lefamulin found noninferior to moxifloxacin for bacterial pneumonia
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
FROM JAMA
Vitamin C infusion falls short for sepsis and ARDS patients
Vitamin C infusion did not improve outcomes related to organ failure, inflammation, or vascular injury for patients with sepsis and acute respiratory distress syndrome, based on data from 167 adults.
“Previous research found that vitamin C attenuates systemic inflammation, corrects sepsis-induced coagulopathy, and attenuates vascular injury,” wrote Alpha A. Fowler III, MD, of Virginia Commonwealth University, Richmond, and colleagues.
To examine the impact of vitamin C infusion on patients with sepsis and acute respiratory distress syndrome (ARDS), the researchers designed the CITRIS-ALI trial, a randomized, double-blind, placebo-controlled study conducted at 7 medical intensive care units in the United States.
In the study, published in JAMA, the researchers randomized 167 adults with sepsis and ARDS to receive high-dose intravenous vitamin C (50 mg/kg in 5% dextrose in water) or placebo (5% dextrose in water only) every 6 hours for 96 hours. The primary outcomes were measures of organ failure based on changes in the modified Sequential Organ Failure Assessment score (mSOFA), inflammation (based on changes in C-reactive protein), and vascular injury based on thrombomodulin.
Overall, no significant differences appeared between the vitamin C and placebo groups, respectively in the three primary outcome measures: change in average SOFA score (3-point change vs. a 3.5-point change) at 96 hours; change in C-reactive protein levels (change of 54.1 mcg/mL vs. 46.1 mcg/mL) at 168 hours; and change in thrombomodulin levels (14.5 ng/mL vs. 13.8 ng/mL) at 168 hours.
The average age of the patients was 55 years, and 54% were men.
The researchers also assessed 46 secondary outcomes. Most of these showed no significant differences between the groups, but 28-day all-cause mortality was significantly lower in the vitamin C group, compared with the placebo group (46.3% vs. 29.8%), the researchers said. Vitamin C also was significantly associated with increased ICU-free days to day 28 and hospital-free days to day 60, compared with placebo.
No significant differences were seen between the groups on 43 other secondary outcomes including ventilator-free days and vasopressor use. However, “these findings were based on analyses that did not account for multiple comparisons and therefore must be considered exploratory,” they said.
“The inability of vitamin C to affect C-reactive protein and thrombomodulin levels in this trial possibly resulted from the advanced stages of sepsis that were present before the development of ARDS,” the researchers noted.
The findings were limited by several factors including the variability in the timing of vitamin C administration and the use of a single high dose of vitamin C, they emphasized. However, the results suggest that further research may be needed to determine the potential of vitamin C for improving outcomes in patients with sepsis and ARDS, they said.
The study was supported by the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Polytechnic Institute and State University, Richmond; the NHLBI; and study materials from McGuff Pharmaceuticals.
SOURCE: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi:10.1001/jama.2019.11825.
Although none of the primary outcomes was significant, “the difference in mortality is tantalizing and likely to spur much debate,” wrote Emily B. Brant, MD, and Derek C. Angus, MD, in an accompanying editorial.
“However, this outcome was one of many secondary outcomes, and although reported as statistically significant, that finding was without adjustment for multiple comparisons,” they said.
The study was well-designed, and resulted in the collection of considerable patient data, they said. Previous studies have suggested that approximately 40% of sepsis patients are vitamin C deficient, and vitamin C is considered safe and inexpensive, which may be reason to pursue research in this area, they added.
Study design for addition research should keep in mind the timing and dosage that were limitations in the current study; the lack of effect on organ dysfunction may have occurred because vitamin C was given too late, they said.
Researchers planning further evaluation might “reconsider optimal dosing and timing, as well as the likelihood that any potential benefits may only accrue to subsets of patients, given the underlying heterogeneity of sepsis,” they concluded (JAMA. 2019 Oct 1; 322:1257-8).
Dr. Brant and Dr. Angus are affiliated with the department of critical care medicine, University of Pittsburgh. Dr. Angus serves as a associate editor for JAMA and disclosed receiving consulting fees from Ferring, Bristol-Myers Squibb, and Beckman Coulter; holding stock in Alung Technologies; and holding pending patents for selepressin and for proteomic biomarkers of sepsis in elderly patients. Dr. Brant had no financial conflicts to disclose.
Although none of the primary outcomes was significant, “the difference in mortality is tantalizing and likely to spur much debate,” wrote Emily B. Brant, MD, and Derek C. Angus, MD, in an accompanying editorial.
“However, this outcome was one of many secondary outcomes, and although reported as statistically significant, that finding was without adjustment for multiple comparisons,” they said.
The study was well-designed, and resulted in the collection of considerable patient data, they said. Previous studies have suggested that approximately 40% of sepsis patients are vitamin C deficient, and vitamin C is considered safe and inexpensive, which may be reason to pursue research in this area, they added.
Study design for addition research should keep in mind the timing and dosage that were limitations in the current study; the lack of effect on organ dysfunction may have occurred because vitamin C was given too late, they said.
Researchers planning further evaluation might “reconsider optimal dosing and timing, as well as the likelihood that any potential benefits may only accrue to subsets of patients, given the underlying heterogeneity of sepsis,” they concluded (JAMA. 2019 Oct 1; 322:1257-8).
Dr. Brant and Dr. Angus are affiliated with the department of critical care medicine, University of Pittsburgh. Dr. Angus serves as a associate editor for JAMA and disclosed receiving consulting fees from Ferring, Bristol-Myers Squibb, and Beckman Coulter; holding stock in Alung Technologies; and holding pending patents for selepressin and for proteomic biomarkers of sepsis in elderly patients. Dr. Brant had no financial conflicts to disclose.
Although none of the primary outcomes was significant, “the difference in mortality is tantalizing and likely to spur much debate,” wrote Emily B. Brant, MD, and Derek C. Angus, MD, in an accompanying editorial.
“However, this outcome was one of many secondary outcomes, and although reported as statistically significant, that finding was without adjustment for multiple comparisons,” they said.
The study was well-designed, and resulted in the collection of considerable patient data, they said. Previous studies have suggested that approximately 40% of sepsis patients are vitamin C deficient, and vitamin C is considered safe and inexpensive, which may be reason to pursue research in this area, they added.
Study design for addition research should keep in mind the timing and dosage that were limitations in the current study; the lack of effect on organ dysfunction may have occurred because vitamin C was given too late, they said.
Researchers planning further evaluation might “reconsider optimal dosing and timing, as well as the likelihood that any potential benefits may only accrue to subsets of patients, given the underlying heterogeneity of sepsis,” they concluded (JAMA. 2019 Oct 1; 322:1257-8).
Dr. Brant and Dr. Angus are affiliated with the department of critical care medicine, University of Pittsburgh. Dr. Angus serves as a associate editor for JAMA and disclosed receiving consulting fees from Ferring, Bristol-Myers Squibb, and Beckman Coulter; holding stock in Alung Technologies; and holding pending patents for selepressin and for proteomic biomarkers of sepsis in elderly patients. Dr. Brant had no financial conflicts to disclose.
Vitamin C infusion did not improve outcomes related to organ failure, inflammation, or vascular injury for patients with sepsis and acute respiratory distress syndrome, based on data from 167 adults.
“Previous research found that vitamin C attenuates systemic inflammation, corrects sepsis-induced coagulopathy, and attenuates vascular injury,” wrote Alpha A. Fowler III, MD, of Virginia Commonwealth University, Richmond, and colleagues.
To examine the impact of vitamin C infusion on patients with sepsis and acute respiratory distress syndrome (ARDS), the researchers designed the CITRIS-ALI trial, a randomized, double-blind, placebo-controlled study conducted at 7 medical intensive care units in the United States.
In the study, published in JAMA, the researchers randomized 167 adults with sepsis and ARDS to receive high-dose intravenous vitamin C (50 mg/kg in 5% dextrose in water) or placebo (5% dextrose in water only) every 6 hours for 96 hours. The primary outcomes were measures of organ failure based on changes in the modified Sequential Organ Failure Assessment score (mSOFA), inflammation (based on changes in C-reactive protein), and vascular injury based on thrombomodulin.
Overall, no significant differences appeared between the vitamin C and placebo groups, respectively in the three primary outcome measures: change in average SOFA score (3-point change vs. a 3.5-point change) at 96 hours; change in C-reactive protein levels (change of 54.1 mcg/mL vs. 46.1 mcg/mL) at 168 hours; and change in thrombomodulin levels (14.5 ng/mL vs. 13.8 ng/mL) at 168 hours.
The average age of the patients was 55 years, and 54% were men.
The researchers also assessed 46 secondary outcomes. Most of these showed no significant differences between the groups, but 28-day all-cause mortality was significantly lower in the vitamin C group, compared with the placebo group (46.3% vs. 29.8%), the researchers said. Vitamin C also was significantly associated with increased ICU-free days to day 28 and hospital-free days to day 60, compared with placebo.
No significant differences were seen between the groups on 43 other secondary outcomes including ventilator-free days and vasopressor use. However, “these findings were based on analyses that did not account for multiple comparisons and therefore must be considered exploratory,” they said.
“The inability of vitamin C to affect C-reactive protein and thrombomodulin levels in this trial possibly resulted from the advanced stages of sepsis that were present before the development of ARDS,” the researchers noted.
The findings were limited by several factors including the variability in the timing of vitamin C administration and the use of a single high dose of vitamin C, they emphasized. However, the results suggest that further research may be needed to determine the potential of vitamin C for improving outcomes in patients with sepsis and ARDS, they said.
The study was supported by the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Polytechnic Institute and State University, Richmond; the NHLBI; and study materials from McGuff Pharmaceuticals.
SOURCE: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi:10.1001/jama.2019.11825.
Vitamin C infusion did not improve outcomes related to organ failure, inflammation, or vascular injury for patients with sepsis and acute respiratory distress syndrome, based on data from 167 adults.
“Previous research found that vitamin C attenuates systemic inflammation, corrects sepsis-induced coagulopathy, and attenuates vascular injury,” wrote Alpha A. Fowler III, MD, of Virginia Commonwealth University, Richmond, and colleagues.
To examine the impact of vitamin C infusion on patients with sepsis and acute respiratory distress syndrome (ARDS), the researchers designed the CITRIS-ALI trial, a randomized, double-blind, placebo-controlled study conducted at 7 medical intensive care units in the United States.
In the study, published in JAMA, the researchers randomized 167 adults with sepsis and ARDS to receive high-dose intravenous vitamin C (50 mg/kg in 5% dextrose in water) or placebo (5% dextrose in water only) every 6 hours for 96 hours. The primary outcomes were measures of organ failure based on changes in the modified Sequential Organ Failure Assessment score (mSOFA), inflammation (based on changes in C-reactive protein), and vascular injury based on thrombomodulin.
Overall, no significant differences appeared between the vitamin C and placebo groups, respectively in the three primary outcome measures: change in average SOFA score (3-point change vs. a 3.5-point change) at 96 hours; change in C-reactive protein levels (change of 54.1 mcg/mL vs. 46.1 mcg/mL) at 168 hours; and change in thrombomodulin levels (14.5 ng/mL vs. 13.8 ng/mL) at 168 hours.
The average age of the patients was 55 years, and 54% were men.
The researchers also assessed 46 secondary outcomes. Most of these showed no significant differences between the groups, but 28-day all-cause mortality was significantly lower in the vitamin C group, compared with the placebo group (46.3% vs. 29.8%), the researchers said. Vitamin C also was significantly associated with increased ICU-free days to day 28 and hospital-free days to day 60, compared with placebo.
No significant differences were seen between the groups on 43 other secondary outcomes including ventilator-free days and vasopressor use. However, “these findings were based on analyses that did not account for multiple comparisons and therefore must be considered exploratory,” they said.
“The inability of vitamin C to affect C-reactive protein and thrombomodulin levels in this trial possibly resulted from the advanced stages of sepsis that were present before the development of ARDS,” the researchers noted.
The findings were limited by several factors including the variability in the timing of vitamin C administration and the use of a single high dose of vitamin C, they emphasized. However, the results suggest that further research may be needed to determine the potential of vitamin C for improving outcomes in patients with sepsis and ARDS, they said.
The study was supported by the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Polytechnic Institute and State University, Richmond; the NHLBI; and study materials from McGuff Pharmaceuticals.
SOURCE: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi:10.1001/jama.2019.11825.
FROM JAMA
Key clinical point: Vitamin C infusion failed to improve outcomes for patients with ARDS and sepsis.
Major finding: The average SOFA score to measure organ failure changed by 3 points in the vitamin C group vs. 3.5 points in the placebo group.
Study details: The data come from a randomized trial of 167 adults with ARDS and sepsis.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute, the National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Tech School of Medicine, the NHLBI, and study materials from McGuff Pharmaceuticals.
Source: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi: 10.1001/jama.2019.11825.
What are the risks to inpatients during hospital construction or renovation?
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3 A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3 A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3 A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Direct-acting antiviral therapy boosts survival for infected HCC patients
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
FROM GASTROENTEROLOGY
Delaying revision knee replacement increases the odds of infection
According to a study on patients undergoing revision knee replacement, a delay of more than 24 hours between hospital admission and total knee arthroplasty (TKA) for periprosthetic fracture (PPF) led to increased odds of complications such as surgical site and urinary tract infections.
“Although this association is an important finding, the confounding factors that cause delay to surgery must be elucidated in non-database studies,” wrote Venkat Boddapati, MD, of Columbia University Medical Center, New York, and coauthors. The study was published in Arthroplasty Today.
To assess the best time for revision TKA after PPF of the knee, the researchers analyzed data from 484 patients who underwent another TKA from 2005 to 2016. Of those patients, 377 (78%) had expedited surgery – defined as less than or equal to 24 hours from hospital admission – and 107 (22%) had non-expedited surgery. Non-expedited patients averaged 3.2 days from admission to surgery.
After multivariate analysis, non-expedited patients had more complications overall, compared with expedited patients (odds ratio 2.35, P = .037). They also had comparative increases in surgical site infections (OR 12.87, P = .029), urinary tract infections (OR 10.46, P = .048), non-home discharge (OR 4.27, P less than .001), and blood transfusions (OR 4.53, P less than .001). The two groups saw no statistical difference in mortality.
The authors noted their study’s limitations, including an inability to assess complications beyond 30 days after surgery, which may affect tracking longer-term outcomes such as mortality. In addition, they were only able to classify surgery as expedited or non-expedited based on when the patient was admitted to the hospital, not the time since their injury. Finally, they lacked “relevant variables that may have contributed to this analysis,” including the type of fracture and the revision implants used.
Three authors reported being paid consultants for, and receiving research support from, several medical companies. The others reported no conflicts of interest.
SOURCE: Boddapati V et al. Arthroplast Today. 2019 Sep 1. doi: 10.1016/j.artd.2019.05.002.
According to a study on patients undergoing revision knee replacement, a delay of more than 24 hours between hospital admission and total knee arthroplasty (TKA) for periprosthetic fracture (PPF) led to increased odds of complications such as surgical site and urinary tract infections.
“Although this association is an important finding, the confounding factors that cause delay to surgery must be elucidated in non-database studies,” wrote Venkat Boddapati, MD, of Columbia University Medical Center, New York, and coauthors. The study was published in Arthroplasty Today.
To assess the best time for revision TKA after PPF of the knee, the researchers analyzed data from 484 patients who underwent another TKA from 2005 to 2016. Of those patients, 377 (78%) had expedited surgery – defined as less than or equal to 24 hours from hospital admission – and 107 (22%) had non-expedited surgery. Non-expedited patients averaged 3.2 days from admission to surgery.
After multivariate analysis, non-expedited patients had more complications overall, compared with expedited patients (odds ratio 2.35, P = .037). They also had comparative increases in surgical site infections (OR 12.87, P = .029), urinary tract infections (OR 10.46, P = .048), non-home discharge (OR 4.27, P less than .001), and blood transfusions (OR 4.53, P less than .001). The two groups saw no statistical difference in mortality.
The authors noted their study’s limitations, including an inability to assess complications beyond 30 days after surgery, which may affect tracking longer-term outcomes such as mortality. In addition, they were only able to classify surgery as expedited or non-expedited based on when the patient was admitted to the hospital, not the time since their injury. Finally, they lacked “relevant variables that may have contributed to this analysis,” including the type of fracture and the revision implants used.
Three authors reported being paid consultants for, and receiving research support from, several medical companies. The others reported no conflicts of interest.
SOURCE: Boddapati V et al. Arthroplast Today. 2019 Sep 1. doi: 10.1016/j.artd.2019.05.002.
According to a study on patients undergoing revision knee replacement, a delay of more than 24 hours between hospital admission and total knee arthroplasty (TKA) for periprosthetic fracture (PPF) led to increased odds of complications such as surgical site and urinary tract infections.
“Although this association is an important finding, the confounding factors that cause delay to surgery must be elucidated in non-database studies,” wrote Venkat Boddapati, MD, of Columbia University Medical Center, New York, and coauthors. The study was published in Arthroplasty Today.
To assess the best time for revision TKA after PPF of the knee, the researchers analyzed data from 484 patients who underwent another TKA from 2005 to 2016. Of those patients, 377 (78%) had expedited surgery – defined as less than or equal to 24 hours from hospital admission – and 107 (22%) had non-expedited surgery. Non-expedited patients averaged 3.2 days from admission to surgery.
After multivariate analysis, non-expedited patients had more complications overall, compared with expedited patients (odds ratio 2.35, P = .037). They also had comparative increases in surgical site infections (OR 12.87, P = .029), urinary tract infections (OR 10.46, P = .048), non-home discharge (OR 4.27, P less than .001), and blood transfusions (OR 4.53, P less than .001). The two groups saw no statistical difference in mortality.
The authors noted their study’s limitations, including an inability to assess complications beyond 30 days after surgery, which may affect tracking longer-term outcomes such as mortality. In addition, they were only able to classify surgery as expedited or non-expedited based on when the patient was admitted to the hospital, not the time since their injury. Finally, they lacked “relevant variables that may have contributed to this analysis,” including the type of fracture and the revision implants used.
Three authors reported being paid consultants for, and receiving research support from, several medical companies. The others reported no conflicts of interest.
SOURCE: Boddapati V et al. Arthroplast Today. 2019 Sep 1. doi: 10.1016/j.artd.2019.05.002.
FROM ARTHROPLASTY TODAY