User login
Viral cause of acute flaccid myelitis eludes detection
A study of 305 cases of acute flaccid myelitis has found further evidence of a viral etiology but is yet to identify a single pathogen as the primary cause.
Writing in Pediatrics, researchers published an analysis of patients presenting with acute flaccid limb weakness from January 2015 to December 2017 across 43 states.
A total of 25 cases were judged as probable for acute flaccid myelitis (AFM) because they met clinical criteria and had a white blood cell count above 5 cells per mm3 in cerebrospinal fluid, while 193 were judged as confirmed cases based on the additional presence of spinal cord gray matter lesions on MRI.
Overall, 83% of patients had experienced fever, cough, runny nose, vomiting, and/or diarrhea for a median of 5 days before limb weakness began. Two-thirds of patients had experienced a respiratory illness, 62% had experienced a fever, and 29% had experienced gastrointestinal illness.
Overall, 47% of the 193 patients who had specimens tested at a Centers for Disease Control and Prevention or non-CDC laboratory had a pathogen found at any site, 10% had a pathogen detected from a sterile site such as cerebrospinal fluid or sera, and 42% had a pathogen detected from a nonsterile site.
Among 72 patients who had serum specimens tested at the CDC, 2 were positive for enteroviruses. Among the 90 patients who had upper respiratory specimens tested, 36% were positive for either enteroviruses or rhinoviruses.
A number of stool specimens were also tested; 15% were positive for enteroviruses or rhinoviruses and one was positive for parechovirus.
Cerebrospinal fluid was tested in 170 patients, of which 4 were positive for enteroviruses. The testing also found adenovirus, Epstein-Barr virus, human herpesvirus 6, and mycoplasma in six patients. Sera testing of 123 patients found 9 were positive for enteroviruses, West Nile virus, mycoplasma, and coxsackievirus B.
“In our summary of national AFM surveillance from 2015 to 2017, we demonstrate that cases were widely distributed across the United States, the majority of cases occurred in late summer or fall, children were predominantly affected, there is a spectrum of clinical severity, and no single pathogen was identified as the primary cause of AFM,” wrote Tracy Ayers, PhD, from the National Center for Immunization and Respiratory Diseases, and coauthors. “We conclude that symptoms of a viral syndrome within the week before limb weakness, detection of viral pathogens from sterile and nonsterile sites from almost half of patients, and seasonality of AFM incidence, particularly during the 2016 peak year, strongly suggest a viral etiology, including [enteroviruses].”
The authors of an accompanying editorial noted that the clinical syndrome of acute flaccid paralysis caused by myelitis in the gray matter of the spinal cord has previously been associated with a range of viruses, including poliovirus, enteroviruses, and flaviviruses, so a single etiology to explain all cases would not be expected.
“The central question remains: What is driving seasonal biennial nationwide outbreaks of AFM since 2014?” wrote Kevin Messaca, MD, and colleagues from the University of Colorado at Denver, Aurora.
Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared. One editorial author declared funding from the National Institute of Allergy and Infectious Diseases.
SOURCE: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
*Updated 10/14/2019.
A study of 305 cases of acute flaccid myelitis has found further evidence of a viral etiology but is yet to identify a single pathogen as the primary cause.
Writing in Pediatrics, researchers published an analysis of patients presenting with acute flaccid limb weakness from January 2015 to December 2017 across 43 states.
A total of 25 cases were judged as probable for acute flaccid myelitis (AFM) because they met clinical criteria and had a white blood cell count above 5 cells per mm3 in cerebrospinal fluid, while 193 were judged as confirmed cases based on the additional presence of spinal cord gray matter lesions on MRI.
Overall, 83% of patients had experienced fever, cough, runny nose, vomiting, and/or diarrhea for a median of 5 days before limb weakness began. Two-thirds of patients had experienced a respiratory illness, 62% had experienced a fever, and 29% had experienced gastrointestinal illness.
Overall, 47% of the 193 patients who had specimens tested at a Centers for Disease Control and Prevention or non-CDC laboratory had a pathogen found at any site, 10% had a pathogen detected from a sterile site such as cerebrospinal fluid or sera, and 42% had a pathogen detected from a nonsterile site.
Among 72 patients who had serum specimens tested at the CDC, 2 were positive for enteroviruses. Among the 90 patients who had upper respiratory specimens tested, 36% were positive for either enteroviruses or rhinoviruses.
A number of stool specimens were also tested; 15% were positive for enteroviruses or rhinoviruses and one was positive for parechovirus.
Cerebrospinal fluid was tested in 170 patients, of which 4 were positive for enteroviruses. The testing also found adenovirus, Epstein-Barr virus, human herpesvirus 6, and mycoplasma in six patients. Sera testing of 123 patients found 9 were positive for enteroviruses, West Nile virus, mycoplasma, and coxsackievirus B.
“In our summary of national AFM surveillance from 2015 to 2017, we demonstrate that cases were widely distributed across the United States, the majority of cases occurred in late summer or fall, children were predominantly affected, there is a spectrum of clinical severity, and no single pathogen was identified as the primary cause of AFM,” wrote Tracy Ayers, PhD, from the National Center for Immunization and Respiratory Diseases, and coauthors. “We conclude that symptoms of a viral syndrome within the week before limb weakness, detection of viral pathogens from sterile and nonsterile sites from almost half of patients, and seasonality of AFM incidence, particularly during the 2016 peak year, strongly suggest a viral etiology, including [enteroviruses].”
The authors of an accompanying editorial noted that the clinical syndrome of acute flaccid paralysis caused by myelitis in the gray matter of the spinal cord has previously been associated with a range of viruses, including poliovirus, enteroviruses, and flaviviruses, so a single etiology to explain all cases would not be expected.
“The central question remains: What is driving seasonal biennial nationwide outbreaks of AFM since 2014?” wrote Kevin Messaca, MD, and colleagues from the University of Colorado at Denver, Aurora.
Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared. One editorial author declared funding from the National Institute of Allergy and Infectious Diseases.
SOURCE: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
*Updated 10/14/2019.
A study of 305 cases of acute flaccid myelitis has found further evidence of a viral etiology but is yet to identify a single pathogen as the primary cause.
Writing in Pediatrics, researchers published an analysis of patients presenting with acute flaccid limb weakness from January 2015 to December 2017 across 43 states.
A total of 25 cases were judged as probable for acute flaccid myelitis (AFM) because they met clinical criteria and had a white blood cell count above 5 cells per mm3 in cerebrospinal fluid, while 193 were judged as confirmed cases based on the additional presence of spinal cord gray matter lesions on MRI.
Overall, 83% of patients had experienced fever, cough, runny nose, vomiting, and/or diarrhea for a median of 5 days before limb weakness began. Two-thirds of patients had experienced a respiratory illness, 62% had experienced a fever, and 29% had experienced gastrointestinal illness.
Overall, 47% of the 193 patients who had specimens tested at a Centers for Disease Control and Prevention or non-CDC laboratory had a pathogen found at any site, 10% had a pathogen detected from a sterile site such as cerebrospinal fluid or sera, and 42% had a pathogen detected from a nonsterile site.
Among 72 patients who had serum specimens tested at the CDC, 2 were positive for enteroviruses. Among the 90 patients who had upper respiratory specimens tested, 36% were positive for either enteroviruses or rhinoviruses.
A number of stool specimens were also tested; 15% were positive for enteroviruses or rhinoviruses and one was positive for parechovirus.
Cerebrospinal fluid was tested in 170 patients, of which 4 were positive for enteroviruses. The testing also found adenovirus, Epstein-Barr virus, human herpesvirus 6, and mycoplasma in six patients. Sera testing of 123 patients found 9 were positive for enteroviruses, West Nile virus, mycoplasma, and coxsackievirus B.
“In our summary of national AFM surveillance from 2015 to 2017, we demonstrate that cases were widely distributed across the United States, the majority of cases occurred in late summer or fall, children were predominantly affected, there is a spectrum of clinical severity, and no single pathogen was identified as the primary cause of AFM,” wrote Tracy Ayers, PhD, from the National Center for Immunization and Respiratory Diseases, and coauthors. “We conclude that symptoms of a viral syndrome within the week before limb weakness, detection of viral pathogens from sterile and nonsterile sites from almost half of patients, and seasonality of AFM incidence, particularly during the 2016 peak year, strongly suggest a viral etiology, including [enteroviruses].”
The authors of an accompanying editorial noted that the clinical syndrome of acute flaccid paralysis caused by myelitis in the gray matter of the spinal cord has previously been associated with a range of viruses, including poliovirus, enteroviruses, and flaviviruses, so a single etiology to explain all cases would not be expected.
“The central question remains: What is driving seasonal biennial nationwide outbreaks of AFM since 2014?” wrote Kevin Messaca, MD, and colleagues from the University of Colorado at Denver, Aurora.
Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared. One editorial author declared funding from the National Institute of Allergy and Infectious Diseases.
SOURCE: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
*Updated 10/14/2019.
FROM PEDIATRICS
Key clinical point: Acute flaccid myelitis shows a strong suggestion of viral etiology but a single causal virus is not identified.
Major finding: Patients with acute flaccid myelitis show infection with a range of viruses including enteroviruses.
Study details: A study of 305 cases of acute flaccid myelitis in the United States.
Disclosures: Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
Source: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
IDWeek examined hot topics in the clinical treatment of infectious diseases
WASHINGTON – The top existential threats to health today are climate change and overpopulation, but third in this list is antimicrobial resistance, according to Helen Boucher, MD, of Tufts Medical Center, Boston. In her talk at an annual scientific meeting on infectious diseases, however, she focused on the last, presenting the hottest developments in the clinical science of treating and identifying disease-causing agents.
In particular, she discussed two of the most important developments in the area of rapid diagnostics: cell-free microbial DNA in plasma and the use of next-generation gene sequencing for determining disease etiology.
Using a meta-genomics test, cell-free microbial DNA can be identified in plasma from more than 1,000 relevant bacteria, DNA viruses, fungi, and parasites. Though importantly, RNA viruses are not detectable using this technology, she added. Although current sampling is of plasma, this might expand to the ability to use urine in the future. She discussed its particular use in sepsis, as outlined in a paper in Nature Microbiology (2019;4[4]:663-74). The researchers examined 350 suspected sepsis patients and they found a 93% sensitivity, compared with reference standards, using this new test. The main issue with the test was a high incidence of false positives.
Another test Dr. Boucher discussed was the use of meta-genomic next-generation sequencing. She referred to a 2019 paper in the New England Journal of Medicine, which discussed the use of clinical meta-genomic next-generation sequencing of cerebrospinal fluid for the diagnosis of meningitis and encephalitis (2019;380[27]:2327-40). Next-generation sequencing identified 13% of patients positive who were missed using standard screening. However, a number of patients were not diagnosed using the new test, showing that this technique was an improvement over current methods, but not 100% successful.
Dr. Boucher stressed the need for “diagnostic stewardship” to identify the correct microbial agent causing disease, allowing for the use of appropriate treatment rather than shotgun approaches to prevent the development of antibiotic resistance. This practice requires collaboration between the clinical laboratory, pharmacists, and infectious disease specialists.
Dr. Boucher then switched to the area of therapeutics, focusing on the introduction of new antibiotics and other innovations in disease treatment methodologies, especially in the field of transplant ID.
“We have new drugs. That is the good news,” with the goals of the 10 x ’20 initiative to develop 10 new systemic antibiotics by 2020, having “been met and then some,” said Dr. Boucher.
“We now have 13 new drugs, systemically available antibiotics, available by August 2019,” she added, discussing several of the new drugs.
In addition, she pointed out several studies that have indicated that shorter courses of antibiotics are better than longer, and that, in many cases, oral therapy is better than intravenous.
In the burgeoning area of transplant ID studies, Dr. Boucher discussed new research showing that vaccinations in transplanted patients can be advised in several instances, though may require higher dosing, and how the use of hepatitis C virus–positive organs for transplant is showing good results and increasing the availability of organs for transplant.
Dr. Boucher has served on data review committees for Actelion and Medtronix and has served as a consultant/advisor for Cerexa, Durata Therapeutics, Merck (adjudication committee), Rib-X, and Wyeth/Pfizer (data safety monitoring committee).
WASHINGTON – The top existential threats to health today are climate change and overpopulation, but third in this list is antimicrobial resistance, according to Helen Boucher, MD, of Tufts Medical Center, Boston. In her talk at an annual scientific meeting on infectious diseases, however, she focused on the last, presenting the hottest developments in the clinical science of treating and identifying disease-causing agents.
In particular, she discussed two of the most important developments in the area of rapid diagnostics: cell-free microbial DNA in plasma and the use of next-generation gene sequencing for determining disease etiology.
Using a meta-genomics test, cell-free microbial DNA can be identified in plasma from more than 1,000 relevant bacteria, DNA viruses, fungi, and parasites. Though importantly, RNA viruses are not detectable using this technology, she added. Although current sampling is of plasma, this might expand to the ability to use urine in the future. She discussed its particular use in sepsis, as outlined in a paper in Nature Microbiology (2019;4[4]:663-74). The researchers examined 350 suspected sepsis patients and they found a 93% sensitivity, compared with reference standards, using this new test. The main issue with the test was a high incidence of false positives.
Another test Dr. Boucher discussed was the use of meta-genomic next-generation sequencing. She referred to a 2019 paper in the New England Journal of Medicine, which discussed the use of clinical meta-genomic next-generation sequencing of cerebrospinal fluid for the diagnosis of meningitis and encephalitis (2019;380[27]:2327-40). Next-generation sequencing identified 13% of patients positive who were missed using standard screening. However, a number of patients were not diagnosed using the new test, showing that this technique was an improvement over current methods, but not 100% successful.
Dr. Boucher stressed the need for “diagnostic stewardship” to identify the correct microbial agent causing disease, allowing for the use of appropriate treatment rather than shotgun approaches to prevent the development of antibiotic resistance. This practice requires collaboration between the clinical laboratory, pharmacists, and infectious disease specialists.
Dr. Boucher then switched to the area of therapeutics, focusing on the introduction of new antibiotics and other innovations in disease treatment methodologies, especially in the field of transplant ID.
“We have new drugs. That is the good news,” with the goals of the 10 x ’20 initiative to develop 10 new systemic antibiotics by 2020, having “been met and then some,” said Dr. Boucher.
“We now have 13 new drugs, systemically available antibiotics, available by August 2019,” she added, discussing several of the new drugs.
In addition, she pointed out several studies that have indicated that shorter courses of antibiotics are better than longer, and that, in many cases, oral therapy is better than intravenous.
In the burgeoning area of transplant ID studies, Dr. Boucher discussed new research showing that vaccinations in transplanted patients can be advised in several instances, though may require higher dosing, and how the use of hepatitis C virus–positive organs for transplant is showing good results and increasing the availability of organs for transplant.
Dr. Boucher has served on data review committees for Actelion and Medtronix and has served as a consultant/advisor for Cerexa, Durata Therapeutics, Merck (adjudication committee), Rib-X, and Wyeth/Pfizer (data safety monitoring committee).
WASHINGTON – The top existential threats to health today are climate change and overpopulation, but third in this list is antimicrobial resistance, according to Helen Boucher, MD, of Tufts Medical Center, Boston. In her talk at an annual scientific meeting on infectious diseases, however, she focused on the last, presenting the hottest developments in the clinical science of treating and identifying disease-causing agents.
In particular, she discussed two of the most important developments in the area of rapid diagnostics: cell-free microbial DNA in plasma and the use of next-generation gene sequencing for determining disease etiology.
Using a meta-genomics test, cell-free microbial DNA can be identified in plasma from more than 1,000 relevant bacteria, DNA viruses, fungi, and parasites. Though importantly, RNA viruses are not detectable using this technology, she added. Although current sampling is of plasma, this might expand to the ability to use urine in the future. She discussed its particular use in sepsis, as outlined in a paper in Nature Microbiology (2019;4[4]:663-74). The researchers examined 350 suspected sepsis patients and they found a 93% sensitivity, compared with reference standards, using this new test. The main issue with the test was a high incidence of false positives.
Another test Dr. Boucher discussed was the use of meta-genomic next-generation sequencing. She referred to a 2019 paper in the New England Journal of Medicine, which discussed the use of clinical meta-genomic next-generation sequencing of cerebrospinal fluid for the diagnosis of meningitis and encephalitis (2019;380[27]:2327-40). Next-generation sequencing identified 13% of patients positive who were missed using standard screening. However, a number of patients were not diagnosed using the new test, showing that this technique was an improvement over current methods, but not 100% successful.
Dr. Boucher stressed the need for “diagnostic stewardship” to identify the correct microbial agent causing disease, allowing for the use of appropriate treatment rather than shotgun approaches to prevent the development of antibiotic resistance. This practice requires collaboration between the clinical laboratory, pharmacists, and infectious disease specialists.
Dr. Boucher then switched to the area of therapeutics, focusing on the introduction of new antibiotics and other innovations in disease treatment methodologies, especially in the field of transplant ID.
“We have new drugs. That is the good news,” with the goals of the 10 x ’20 initiative to develop 10 new systemic antibiotics by 2020, having “been met and then some,” said Dr. Boucher.
“We now have 13 new drugs, systemically available antibiotics, available by August 2019,” she added, discussing several of the new drugs.
In addition, she pointed out several studies that have indicated that shorter courses of antibiotics are better than longer, and that, in many cases, oral therapy is better than intravenous.
In the burgeoning area of transplant ID studies, Dr. Boucher discussed new research showing that vaccinations in transplanted patients can be advised in several instances, though may require higher dosing, and how the use of hepatitis C virus–positive organs for transplant is showing good results and increasing the availability of organs for transplant.
Dr. Boucher has served on data review committees for Actelion and Medtronix and has served as a consultant/advisor for Cerexa, Durata Therapeutics, Merck (adjudication committee), Rib-X, and Wyeth/Pfizer (data safety monitoring committee).
EXPERT ANALYSIS FROM IDWEEK 2019
Oral beta-lactams provide noninferior postdischarge pyelonephritis treatment
WASHINGTON – Patients hospitalized for pyelonephritis and discharged after receiving intravenous antibiotic treatment who then received step-down treatment with an oral beta-lactam had 30-day outcomes that were noninferior to patients who received an oral fluoroquinolone or trimethoprim-sulfamethoxazole as their discharge regimen, in a retrospective study of 211 patients managed at either of two U.S. hospitals.
This was the largest comparison reported on oral beta-lactam drugs for postdischarge treatment of pyelonephritis relative to the standard oral agents, fluoroquinolones and trimethoprim-sulfamethoxazole (Bactrim), Athena Hobbs, PharmD, said at an annual scientific meeting on infectious diseases. The superiority of an oral fluoroquinolone or trimethoprim-sulfamethoxazole and inferiority of oral beta-lactam drugs were cited in 2010 guidelines for managing pyelonephritis from the Infectious Diseases Society of America (Clin Infect Dis. 2011 March 1;52 [5]: e103-20).
Although limited as a nonrandomized, retrospective comparison, the finding of at least similar efficacy by beta-lactam agents “opens new treatment options” that avoid issues with drug resistance and adverse effects from treatment with fluoroquinolones or trimethoprim-sulfamethoxazole, Dr. Hobbs said in a video interview. Beta-lactams have already been embraced for this indication by some hospitalists, demonstrated by their use of beta-lactam antibiotics for 122 (58%) of the 211 patients included in the study. Among the 89 patients discharged on a non–beta-lactam, 69 (78%) had fluoroquinolone treatment and the remaining 20 patients went home taking trimethoprim-sulfamethoxazole. The new finding “confirms that we are not doing harm to patients,” with this existing practice of mostly prescribing an oral beta-lactam drug, noted Dr. Hobbs, an infectious diseases pharmacy specialist at Baptist Memorial Hospital in Memphis.
The study included patients aged 18-89 years hospitalized during 2014-2017 for a primary diagnosis of pyelonephritis at Baptist or at a second Hospital in Austin, Tex. The study excluded patients in intensive care, with a urologic abnormality, pregnant women, and patients treated with an intravenous antibiotic other than a beta-lactam for more than 24 hours. The most commonly used intravenous drugs were cefazolin and ceftriaxone. The enrolled patients averaged just over 40 years old, and more than 90% were women.
The study’s primary outcome was the 30-day rate of either hospital readmission or an ED visit for pyelonephritis or a urinary tract infection. This occurred in 4.9% of the patients discharged on an oral course of a beta-lactam drug, and in 5.6% of those discharged on either a fluoroquinolone or trimethoprim-sulfamethoxazole, a difference that was not statistically significant and that met the prespecified criteria for noninferiority, Dr. Hobbs reported. The most commonly prescribed oral beta-lactam was cefuroxime in about half the patients, followed by cephalexin or cefadroxil in about a quarter of patients, and amoxicillin with clavulanate in 19%. The two arms of the study also showed no significant difference in infection recurrences during 90-day follow-up.
The study received no commercial funding. Dr. Hobbs had no relevant disclosures.
WASHINGTON – Patients hospitalized for pyelonephritis and discharged after receiving intravenous antibiotic treatment who then received step-down treatment with an oral beta-lactam had 30-day outcomes that were noninferior to patients who received an oral fluoroquinolone or trimethoprim-sulfamethoxazole as their discharge regimen, in a retrospective study of 211 patients managed at either of two U.S. hospitals.
This was the largest comparison reported on oral beta-lactam drugs for postdischarge treatment of pyelonephritis relative to the standard oral agents, fluoroquinolones and trimethoprim-sulfamethoxazole (Bactrim), Athena Hobbs, PharmD, said at an annual scientific meeting on infectious diseases. The superiority of an oral fluoroquinolone or trimethoprim-sulfamethoxazole and inferiority of oral beta-lactam drugs were cited in 2010 guidelines for managing pyelonephritis from the Infectious Diseases Society of America (Clin Infect Dis. 2011 March 1;52 [5]: e103-20).
Although limited as a nonrandomized, retrospective comparison, the finding of at least similar efficacy by beta-lactam agents “opens new treatment options” that avoid issues with drug resistance and adverse effects from treatment with fluoroquinolones or trimethoprim-sulfamethoxazole, Dr. Hobbs said in a video interview. Beta-lactams have already been embraced for this indication by some hospitalists, demonstrated by their use of beta-lactam antibiotics for 122 (58%) of the 211 patients included in the study. Among the 89 patients discharged on a non–beta-lactam, 69 (78%) had fluoroquinolone treatment and the remaining 20 patients went home taking trimethoprim-sulfamethoxazole. The new finding “confirms that we are not doing harm to patients,” with this existing practice of mostly prescribing an oral beta-lactam drug, noted Dr. Hobbs, an infectious diseases pharmacy specialist at Baptist Memorial Hospital in Memphis.
The study included patients aged 18-89 years hospitalized during 2014-2017 for a primary diagnosis of pyelonephritis at Baptist or at a second Hospital in Austin, Tex. The study excluded patients in intensive care, with a urologic abnormality, pregnant women, and patients treated with an intravenous antibiotic other than a beta-lactam for more than 24 hours. The most commonly used intravenous drugs were cefazolin and ceftriaxone. The enrolled patients averaged just over 40 years old, and more than 90% were women.
The study’s primary outcome was the 30-day rate of either hospital readmission or an ED visit for pyelonephritis or a urinary tract infection. This occurred in 4.9% of the patients discharged on an oral course of a beta-lactam drug, and in 5.6% of those discharged on either a fluoroquinolone or trimethoprim-sulfamethoxazole, a difference that was not statistically significant and that met the prespecified criteria for noninferiority, Dr. Hobbs reported. The most commonly prescribed oral beta-lactam was cefuroxime in about half the patients, followed by cephalexin or cefadroxil in about a quarter of patients, and amoxicillin with clavulanate in 19%. The two arms of the study also showed no significant difference in infection recurrences during 90-day follow-up.
The study received no commercial funding. Dr. Hobbs had no relevant disclosures.
WASHINGTON – Patients hospitalized for pyelonephritis and discharged after receiving intravenous antibiotic treatment who then received step-down treatment with an oral beta-lactam had 30-day outcomes that were noninferior to patients who received an oral fluoroquinolone or trimethoprim-sulfamethoxazole as their discharge regimen, in a retrospective study of 211 patients managed at either of two U.S. hospitals.
This was the largest comparison reported on oral beta-lactam drugs for postdischarge treatment of pyelonephritis relative to the standard oral agents, fluoroquinolones and trimethoprim-sulfamethoxazole (Bactrim), Athena Hobbs, PharmD, said at an annual scientific meeting on infectious diseases. The superiority of an oral fluoroquinolone or trimethoprim-sulfamethoxazole and inferiority of oral beta-lactam drugs were cited in 2010 guidelines for managing pyelonephritis from the Infectious Diseases Society of America (Clin Infect Dis. 2011 March 1;52 [5]: e103-20).
Although limited as a nonrandomized, retrospective comparison, the finding of at least similar efficacy by beta-lactam agents “opens new treatment options” that avoid issues with drug resistance and adverse effects from treatment with fluoroquinolones or trimethoprim-sulfamethoxazole, Dr. Hobbs said in a video interview. Beta-lactams have already been embraced for this indication by some hospitalists, demonstrated by their use of beta-lactam antibiotics for 122 (58%) of the 211 patients included in the study. Among the 89 patients discharged on a non–beta-lactam, 69 (78%) had fluoroquinolone treatment and the remaining 20 patients went home taking trimethoprim-sulfamethoxazole. The new finding “confirms that we are not doing harm to patients,” with this existing practice of mostly prescribing an oral beta-lactam drug, noted Dr. Hobbs, an infectious diseases pharmacy specialist at Baptist Memorial Hospital in Memphis.
The study included patients aged 18-89 years hospitalized during 2014-2017 for a primary diagnosis of pyelonephritis at Baptist or at a second Hospital in Austin, Tex. The study excluded patients in intensive care, with a urologic abnormality, pregnant women, and patients treated with an intravenous antibiotic other than a beta-lactam for more than 24 hours. The most commonly used intravenous drugs were cefazolin and ceftriaxone. The enrolled patients averaged just over 40 years old, and more than 90% were women.
The study’s primary outcome was the 30-day rate of either hospital readmission or an ED visit for pyelonephritis or a urinary tract infection. This occurred in 4.9% of the patients discharged on an oral course of a beta-lactam drug, and in 5.6% of those discharged on either a fluoroquinolone or trimethoprim-sulfamethoxazole, a difference that was not statistically significant and that met the prespecified criteria for noninferiority, Dr. Hobbs reported. The most commonly prescribed oral beta-lactam was cefuroxime in about half the patients, followed by cephalexin or cefadroxil in about a quarter of patients, and amoxicillin with clavulanate in 19%. The two arms of the study also showed no significant difference in infection recurrences during 90-day follow-up.
The study received no commercial funding. Dr. Hobbs had no relevant disclosures.
REPORTING FROM IDWEEK 2019
Measles 2019: Most cases occurred in close-knit, undervaccinated communities
While 22 outbreaks were reported in 17 states during 2019, the majority of measles cases occurred in a pair of outbreaks that started in late 2018, one in New York City and the other in New York state. Theses two outbreaks, which occurred in underimmunized, close-knit communities, accounted for 934 (75%) of the 2019 total. An additional six outbreaks in similar communities accounted for nearly half of the remaining reported cases.
The overall median patient age was 6 years, with 31% being children aged 1-4 years, 27% being school-age children aged 5-17 years, and 29% were adults aged at least 18 years. However, when excluding the New York City (NYC) and New York state outbreaks, the median patient age was 19 years. Outbreak length also differed significantly between the NYC and New York state outbreaks, compared with all other outbreaks; the NYC outbreak lasted for 9.5 months, involving 702 patients from start to finish, the New York state outbreak lasted for 10.5 months and involved 412 cases.
The rate of patients who were either unvaccinated or had unknown vaccination status was similar in the New York outbreaks and in the other U.S. outbreaks, ranging from 87% to 91%. A total of 119 patients were hospitalized, 20% of whom were younger than 1 year; no deaths were reported. A total of 81 cases were internationally imported; the rate of patients who were unvaccinated or had unknown status in this group was 90%.
While most outbreaks in 2019 were similar to those previously seen, the outbreaks in NYC and New York state were more sustained for three reasons, the CDC investigators said: pockets of low vaccination coverage and variable vaccine acceptance, relatively high population density and closed social nature of the community, and repeated importations of measles cases among unvaccinated persons traveling internationally and returning to or visiting the affected communities.
“Public health authorities need to identify pockets of undervaccinated persons to prevent these outbreaks, which require substantial resources to control. A preventive strategy to build vaccine confidence is important, especially one that uses culturally appropriate communication strategies to offset misinformation and disseminate accurate information about the safety and importance of vaccination in advance of outbreaks,” the CDC investigators concluded.
The CDC investigators reported that they had no conflicts of interest.
[email protected]
SOURCE: Patel M et al. MMWR Morb Mortal Wkly Rep. 2019 Oct 4. doi: 10.15585/mmwr.mm6840e2.
While 22 outbreaks were reported in 17 states during 2019, the majority of measles cases occurred in a pair of outbreaks that started in late 2018, one in New York City and the other in New York state. Theses two outbreaks, which occurred in underimmunized, close-knit communities, accounted for 934 (75%) of the 2019 total. An additional six outbreaks in similar communities accounted for nearly half of the remaining reported cases.
The overall median patient age was 6 years, with 31% being children aged 1-4 years, 27% being school-age children aged 5-17 years, and 29% were adults aged at least 18 years. However, when excluding the New York City (NYC) and New York state outbreaks, the median patient age was 19 years. Outbreak length also differed significantly between the NYC and New York state outbreaks, compared with all other outbreaks; the NYC outbreak lasted for 9.5 months, involving 702 patients from start to finish, the New York state outbreak lasted for 10.5 months and involved 412 cases.
The rate of patients who were either unvaccinated or had unknown vaccination status was similar in the New York outbreaks and in the other U.S. outbreaks, ranging from 87% to 91%. A total of 119 patients were hospitalized, 20% of whom were younger than 1 year; no deaths were reported. A total of 81 cases were internationally imported; the rate of patients who were unvaccinated or had unknown status in this group was 90%.
While most outbreaks in 2019 were similar to those previously seen, the outbreaks in NYC and New York state were more sustained for three reasons, the CDC investigators said: pockets of low vaccination coverage and variable vaccine acceptance, relatively high population density and closed social nature of the community, and repeated importations of measles cases among unvaccinated persons traveling internationally and returning to or visiting the affected communities.
“Public health authorities need to identify pockets of undervaccinated persons to prevent these outbreaks, which require substantial resources to control. A preventive strategy to build vaccine confidence is important, especially one that uses culturally appropriate communication strategies to offset misinformation and disseminate accurate information about the safety and importance of vaccination in advance of outbreaks,” the CDC investigators concluded.
The CDC investigators reported that they had no conflicts of interest.
[email protected]
SOURCE: Patel M et al. MMWR Morb Mortal Wkly Rep. 2019 Oct 4. doi: 10.15585/mmwr.mm6840e2.
While 22 outbreaks were reported in 17 states during 2019, the majority of measles cases occurred in a pair of outbreaks that started in late 2018, one in New York City and the other in New York state. Theses two outbreaks, which occurred in underimmunized, close-knit communities, accounted for 934 (75%) of the 2019 total. An additional six outbreaks in similar communities accounted for nearly half of the remaining reported cases.
The overall median patient age was 6 years, with 31% being children aged 1-4 years, 27% being school-age children aged 5-17 years, and 29% were adults aged at least 18 years. However, when excluding the New York City (NYC) and New York state outbreaks, the median patient age was 19 years. Outbreak length also differed significantly between the NYC and New York state outbreaks, compared with all other outbreaks; the NYC outbreak lasted for 9.5 months, involving 702 patients from start to finish, the New York state outbreak lasted for 10.5 months and involved 412 cases.
The rate of patients who were either unvaccinated or had unknown vaccination status was similar in the New York outbreaks and in the other U.S. outbreaks, ranging from 87% to 91%. A total of 119 patients were hospitalized, 20% of whom were younger than 1 year; no deaths were reported. A total of 81 cases were internationally imported; the rate of patients who were unvaccinated or had unknown status in this group was 90%.
While most outbreaks in 2019 were similar to those previously seen, the outbreaks in NYC and New York state were more sustained for three reasons, the CDC investigators said: pockets of low vaccination coverage and variable vaccine acceptance, relatively high population density and closed social nature of the community, and repeated importations of measles cases among unvaccinated persons traveling internationally and returning to or visiting the affected communities.
“Public health authorities need to identify pockets of undervaccinated persons to prevent these outbreaks, which require substantial resources to control. A preventive strategy to build vaccine confidence is important, especially one that uses culturally appropriate communication strategies to offset misinformation and disseminate accurate information about the safety and importance of vaccination in advance of outbreaks,” the CDC investigators concluded.
The CDC investigators reported that they had no conflicts of interest.
[email protected]
SOURCE: Patel M et al. MMWR Morb Mortal Wkly Rep. 2019 Oct 4. doi: 10.15585/mmwr.mm6840e2.
FROM THE MMWR
Oral drug cut viral respiratory tract infections in elderly
WASHINGTON – An investigational, oral, small molecule designed to boost innate antiviral immunity safely cut the incidence of various viral respiratory infections in elderly people during a winter season by nearly a third when administered once daily in a placebo-controlled, multicenter, phase 2 study of 952 patients. Based on these and other findings the drug, RTB101, is now undergoing testing in a phase 3 study, Joan Mannick, MD, said at an annual scientific meeting on infectious diseases.
At a dosage of 10 mg once daily, RTB101 was “well tolerated, upregulated innate antiviral gene expression, and reduced the incidence” of laboratory-confirmed respiratory tract infections caused by several different viruses, said Dr. Mannick, who disclosed that she is a cofounder and chief medical officer of resTORbio, a Boston-based company that’s developing the drug.
During 16 weeks of treatment during the winter virus season, once-daily dosing led to cuts in the rates of respiratory infections compared with placebo by rhinovirus and enterovirus, respiratory syncytial virus, coronavirus, influenza virus, metapneuomovirus, and parainfluenza virus, especially in patients whom the results identified as having the best drug responses: those who were at least 85 years old, and those who were at least 65 years old and also had asthma. Enrolled patients who were at least 65 years old and had other risk factors – current smoking, chronic obstructive pulmonary disease, or diabetes – had notably less robust responses to treatment, and the phase 3 study is not enrolling elderly people who currently smoke or have chronic obstructive pulmonary disease, Dr. Mannick said in an interview.
RTB101 inhibits the active site of the “mechanistic target of rapamycin” (mTOR) protein, the key player of the TORC1 protein complex that appears to downregulate innate antiviral immunity when active. Hence inhibiting mTOR and TORC1 activity should boost innate antiviral immunity. Once-daily dosing with 10 mg of RTB101 appears to mimic the normal daily cycle of high and low levels of TORC1 activity seen in younger adults but which is missing the elderly who generally have persistently elevated levels of TORC1 activity, Dr. Mannick explained.
The study she reported enrolled a total of 952 people at any of 10 sites in the Southern Hemisphere or 17 Northern Hemisphere study sites. The researchers randomized patients to receive either RTB101 or placebo at either of two once-daily dosages or either of two twice-daily regimens. The best drug performance was among the 356 patients treated with 10 mg once daily or placebo. Those who received the active drug at this level had a 19% incidence of any laboratory-confirmed respiratory tract infection, while those who received placebo had a 28% incidence, a 30.6% relative risk reduction with RTB101 treatment that was statistically significant.
The actively-treated patients showed upregulation for 19 of 20 “antiviral” genes assessed in the study compared with upregulation of just five of these genes in the those who received placebo. Two post hoc analyses showed that the people who received 10 mg once daily had about half the rate of all-cause hospitalizations compared with those on placebo, and among those who had respiratory infections treated patients had alleviation of their moderate or severe symptoms in about half the time compared with patients on placebo.
The 10-mg daily dosage of RTB101 is less than 1% of the maximum-tolerated dose in people, and the safety data collected in the current study showed adverse events occurring at similar rates in the patients who received the active drug and those who got placebo. Discontinuations because of adverse events occurred in 5% of people who received RTB101 and in 6% of those on placebo.
The researchers are planning to run a cost-effectiveness study to see whether the observed prevention of respiratory tract infections and their consequences can offset the cost of taking RTB101 daily for 16 weeks, Dr. Mannick said.
WASHINGTON – An investigational, oral, small molecule designed to boost innate antiviral immunity safely cut the incidence of various viral respiratory infections in elderly people during a winter season by nearly a third when administered once daily in a placebo-controlled, multicenter, phase 2 study of 952 patients. Based on these and other findings the drug, RTB101, is now undergoing testing in a phase 3 study, Joan Mannick, MD, said at an annual scientific meeting on infectious diseases.
At a dosage of 10 mg once daily, RTB101 was “well tolerated, upregulated innate antiviral gene expression, and reduced the incidence” of laboratory-confirmed respiratory tract infections caused by several different viruses, said Dr. Mannick, who disclosed that she is a cofounder and chief medical officer of resTORbio, a Boston-based company that’s developing the drug.
During 16 weeks of treatment during the winter virus season, once-daily dosing led to cuts in the rates of respiratory infections compared with placebo by rhinovirus and enterovirus, respiratory syncytial virus, coronavirus, influenza virus, metapneuomovirus, and parainfluenza virus, especially in patients whom the results identified as having the best drug responses: those who were at least 85 years old, and those who were at least 65 years old and also had asthma. Enrolled patients who were at least 65 years old and had other risk factors – current smoking, chronic obstructive pulmonary disease, or diabetes – had notably less robust responses to treatment, and the phase 3 study is not enrolling elderly people who currently smoke or have chronic obstructive pulmonary disease, Dr. Mannick said in an interview.
RTB101 inhibits the active site of the “mechanistic target of rapamycin” (mTOR) protein, the key player of the TORC1 protein complex that appears to downregulate innate antiviral immunity when active. Hence inhibiting mTOR and TORC1 activity should boost innate antiviral immunity. Once-daily dosing with 10 mg of RTB101 appears to mimic the normal daily cycle of high and low levels of TORC1 activity seen in younger adults but which is missing the elderly who generally have persistently elevated levels of TORC1 activity, Dr. Mannick explained.
The study she reported enrolled a total of 952 people at any of 10 sites in the Southern Hemisphere or 17 Northern Hemisphere study sites. The researchers randomized patients to receive either RTB101 or placebo at either of two once-daily dosages or either of two twice-daily regimens. The best drug performance was among the 356 patients treated with 10 mg once daily or placebo. Those who received the active drug at this level had a 19% incidence of any laboratory-confirmed respiratory tract infection, while those who received placebo had a 28% incidence, a 30.6% relative risk reduction with RTB101 treatment that was statistically significant.
The actively-treated patients showed upregulation for 19 of 20 “antiviral” genes assessed in the study compared with upregulation of just five of these genes in the those who received placebo. Two post hoc analyses showed that the people who received 10 mg once daily had about half the rate of all-cause hospitalizations compared with those on placebo, and among those who had respiratory infections treated patients had alleviation of their moderate or severe symptoms in about half the time compared with patients on placebo.
The 10-mg daily dosage of RTB101 is less than 1% of the maximum-tolerated dose in people, and the safety data collected in the current study showed adverse events occurring at similar rates in the patients who received the active drug and those who got placebo. Discontinuations because of adverse events occurred in 5% of people who received RTB101 and in 6% of those on placebo.
The researchers are planning to run a cost-effectiveness study to see whether the observed prevention of respiratory tract infections and their consequences can offset the cost of taking RTB101 daily for 16 weeks, Dr. Mannick said.
WASHINGTON – An investigational, oral, small molecule designed to boost innate antiviral immunity safely cut the incidence of various viral respiratory infections in elderly people during a winter season by nearly a third when administered once daily in a placebo-controlled, multicenter, phase 2 study of 952 patients. Based on these and other findings the drug, RTB101, is now undergoing testing in a phase 3 study, Joan Mannick, MD, said at an annual scientific meeting on infectious diseases.
At a dosage of 10 mg once daily, RTB101 was “well tolerated, upregulated innate antiviral gene expression, and reduced the incidence” of laboratory-confirmed respiratory tract infections caused by several different viruses, said Dr. Mannick, who disclosed that she is a cofounder and chief medical officer of resTORbio, a Boston-based company that’s developing the drug.
During 16 weeks of treatment during the winter virus season, once-daily dosing led to cuts in the rates of respiratory infections compared with placebo by rhinovirus and enterovirus, respiratory syncytial virus, coronavirus, influenza virus, metapneuomovirus, and parainfluenza virus, especially in patients whom the results identified as having the best drug responses: those who were at least 85 years old, and those who were at least 65 years old and also had asthma. Enrolled patients who were at least 65 years old and had other risk factors – current smoking, chronic obstructive pulmonary disease, or diabetes – had notably less robust responses to treatment, and the phase 3 study is not enrolling elderly people who currently smoke or have chronic obstructive pulmonary disease, Dr. Mannick said in an interview.
RTB101 inhibits the active site of the “mechanistic target of rapamycin” (mTOR) protein, the key player of the TORC1 protein complex that appears to downregulate innate antiviral immunity when active. Hence inhibiting mTOR and TORC1 activity should boost innate antiviral immunity. Once-daily dosing with 10 mg of RTB101 appears to mimic the normal daily cycle of high and low levels of TORC1 activity seen in younger adults but which is missing the elderly who generally have persistently elevated levels of TORC1 activity, Dr. Mannick explained.
The study she reported enrolled a total of 952 people at any of 10 sites in the Southern Hemisphere or 17 Northern Hemisphere study sites. The researchers randomized patients to receive either RTB101 or placebo at either of two once-daily dosages or either of two twice-daily regimens. The best drug performance was among the 356 patients treated with 10 mg once daily or placebo. Those who received the active drug at this level had a 19% incidence of any laboratory-confirmed respiratory tract infection, while those who received placebo had a 28% incidence, a 30.6% relative risk reduction with RTB101 treatment that was statistically significant.
The actively-treated patients showed upregulation for 19 of 20 “antiviral” genes assessed in the study compared with upregulation of just five of these genes in the those who received placebo. Two post hoc analyses showed that the people who received 10 mg once daily had about half the rate of all-cause hospitalizations compared with those on placebo, and among those who had respiratory infections treated patients had alleviation of their moderate or severe symptoms in about half the time compared with patients on placebo.
The 10-mg daily dosage of RTB101 is less than 1% of the maximum-tolerated dose in people, and the safety data collected in the current study showed adverse events occurring at similar rates in the patients who received the active drug and those who got placebo. Discontinuations because of adverse events occurred in 5% of people who received RTB101 and in 6% of those on placebo.
The researchers are planning to run a cost-effectiveness study to see whether the observed prevention of respiratory tract infections and their consequences can offset the cost of taking RTB101 daily for 16 weeks, Dr. Mannick said.
REPORTING FROM IDWEEK 2019
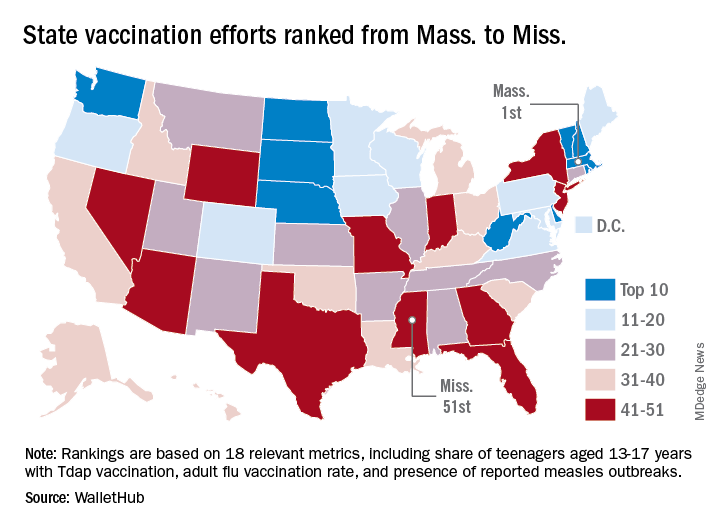
Massachusetts tops state vaccination rankings
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
FDA approves Descovy as HIV PrEP for men and transgender women who have sex with men
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
IPD in children may be a signal of immunodeficiency
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
FROM JAMA PEDIATRICS
Guttate Psoriasis Following Presumed Coxsackievirus A
There are 4 variants of psoriasis: plaque, guttate, pustular, and erythroderma (in order of prevalence).2 Guttate psoriasis is characterized by small, 2- to 10-mm, raindroplike lesions on the skin.1 It accounts for approximately 2% of total psoriasis cases and is commonly triggered by group A streptococcal pharyngitis or tonsillitis.3,4
Hand-foot-and-mouth disease (HFMD) is an illness most commonly caused by a coxsackievirus A infection but also can be caused by other enteroviruses.5,6 Coxsackievirus is a serotype of the Enterovirus species within the Picornaviridae family.7 Hand-foot-and-mouth disease is characterized by a brief fever and vesicular rashes on the palms, soles, or buttocks, as well as oropharyngeal ulcers.8 Typically, the rash is benign and short-lived.9 In rare cases, neurologic complications develop. There have been no reported cases of guttate psoriasis following a coxsackievirus A infection.
The involvement of coxsackievirus B in the etiopathogenesis of psoriasis has been previously reported.10 We report the case of guttate psoriasis following presumed coxsackievirus A HFMD.
Case Report
A 56-year-old woman presented with a vesicular rash on the hands, feet, and lips. The patient reported having a sore throat that started around the same time that the rash developed. The severity of the sore throat was rated as moderate. No fever was reported. One day prior, the patient’s primary care physician prescribed a tapered course of prednisone for the rash. The patient reported a medical history of herpes zoster virus, sunburn, and genital herpes. She was taking clonazepam and had a known allergy to penicillin.
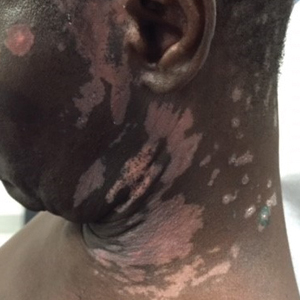
Physical examination revealed erythematous vesicular and papular lesions on the extensor surfaces of the hands and feet. Vesicles also were noted on the vermilion border of the lip. Examination of the patient’s mouth showed blisters and shallow ulcerations in the oral cavity. A clinical diagnosis of coxsackievirus A HFMD was made, and the treatment plan included triamcinolone acetonide ointment 0.025% applied twice daily for 2 weeks and oral valacyclovir hydrochloride 1 g taken 3 times daily for 7 days. A topical emollient also was recommended for the lips when necessary. The lesions all resolved within a 2-week period with no sequela.
The patient returned 1 month later, citing newer red abdominal skin lesions. Fever was denied. She reported that both prescribed treatments had not been helping for the newer lesions. She noticed similar lesions on the groin and brought them to the attention of her gynecologist. Physical examination revealed salmon pink papules and plaques with silvery scaling involving the abdomen, bilateral upper extremities and ears, and scalp. The patient was then clinically diagnosed with guttate psoriasis. A shave biopsy of a representative lesion on the abdomen was performed. The treatment plan included betamethasone dipropionate cream 0.05% applied twice daily for 2 weeks, clobetasol propionate solution 0.05% applied twice daily for 14 days (for the scalp), and hydrocortisone valerate cream 0.2% applied twice daily for 14 days (for the groin).
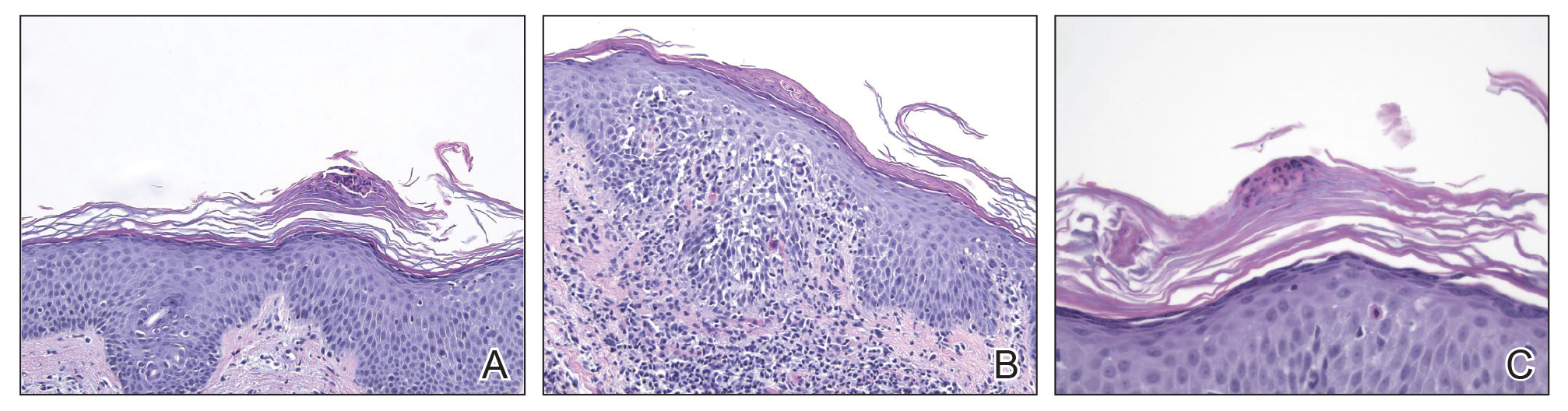
The skin biopsy shown in the Figure was received in 10% buffered formalin, measuring 5×4×1 mm of skin. Sections showed an acanthotic epidermis with foci of spongiosis and hypergranulosis covered by mounds of parakeratosis infiltrated by neutrophils. Superficial perivascular and interstitial lymphocytic inflammation was present. Tortuous blood vessels within the papillary dermis also were present. Results showed psoriasiform dermatitis with mild spongiosis. Periodic acid–Schiff stain did not reveal any fungal organisms. These findings were consistent with a diagnosis of guttate psoriasis.
The patient then returned 1 month later mentioning continued flare-ups of the scalp as well as newer patches on the arms and hands that were less eruptive and faded more quickly. The plaques in the groin area had resolved. Physical examination showed fewer pink papules and plaques with silvery scaling on the abdomen, bilateral upper extremities and ears, and scalp. Topical medications were continued, and possible apremilast therapy for the psoriasis was discussed.
Comment
Enterovirus-derived HFMD likely is caused by coxsackie-virus A. Current evidence supports the theory that guttate psoriasis can be environmentally triggered in genetically susceptible individuals, often but not exclusively by a streptococcal infection. The causative agent elicits a T-cell–mediated reaction leading to increased type 1 helper T cells, IFN-γ, and IL-2 cytokine levels. HLA-Cw∗0602–positive patients are considered genetically susceptible and more likely to develop guttate psoriasis following an environmental trigger. Based on the coincidence in timing of both diagnoses, this reported case of guttate psoriasis may have been triggered by a coxsackievirus A infection.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;1:79-82.
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? an immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326-332.
- Telfer N, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Seitsonen J, Shakeel S, Susi P, et al. Structural analysis of coxsackievirus A7 reveals conformational changes associated with uncoating. J Virol. 2012;86:7207-7215.
- Wu Y, Yeo A, Phoon M, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Tesini BL. Hand-foot-and-mouth-disease (HFMD). May 2018. https://www.msdmanuals.com/professional/infectious-diseases/enteroviruses/hand-foot-and-mouth-disease-hfmd. Accessed September 25, 2019.
- Korzhova TP, Shyrobokov VP, Koliadenko VH, et al. Coxsackie B viral infection in the etiology and clinical pathogenesis of psoriasis [in Ukrainian]. Lik Sprava. 2001:54-58.
There are 4 variants of psoriasis: plaque, guttate, pustular, and erythroderma (in order of prevalence).2 Guttate psoriasis is characterized by small, 2- to 10-mm, raindroplike lesions on the skin.1 It accounts for approximately 2% of total psoriasis cases and is commonly triggered by group A streptococcal pharyngitis or tonsillitis.3,4
Hand-foot-and-mouth disease (HFMD) is an illness most commonly caused by a coxsackievirus A infection but also can be caused by other enteroviruses.5,6 Coxsackievirus is a serotype of the Enterovirus species within the Picornaviridae family.7 Hand-foot-and-mouth disease is characterized by a brief fever and vesicular rashes on the palms, soles, or buttocks, as well as oropharyngeal ulcers.8 Typically, the rash is benign and short-lived.9 In rare cases, neurologic complications develop. There have been no reported cases of guttate psoriasis following a coxsackievirus A infection.
The involvement of coxsackievirus B in the etiopathogenesis of psoriasis has been previously reported.10 We report the case of guttate psoriasis following presumed coxsackievirus A HFMD.
Case Report
A 56-year-old woman presented with a vesicular rash on the hands, feet, and lips. The patient reported having a sore throat that started around the same time that the rash developed. The severity of the sore throat was rated as moderate. No fever was reported. One day prior, the patient’s primary care physician prescribed a tapered course of prednisone for the rash. The patient reported a medical history of herpes zoster virus, sunburn, and genital herpes. She was taking clonazepam and had a known allergy to penicillin.
Physical examination revealed erythematous vesicular and papular lesions on the extensor surfaces of the hands and feet. Vesicles also were noted on the vermilion border of the lip. Examination of the patient’s mouth showed blisters and shallow ulcerations in the oral cavity. A clinical diagnosis of coxsackievirus A HFMD was made, and the treatment plan included triamcinolone acetonide ointment 0.025% applied twice daily for 2 weeks and oral valacyclovir hydrochloride 1 g taken 3 times daily for 7 days. A topical emollient also was recommended for the lips when necessary. The lesions all resolved within a 2-week period with no sequela.
The patient returned 1 month later, citing newer red abdominal skin lesions. Fever was denied. She reported that both prescribed treatments had not been helping for the newer lesions. She noticed similar lesions on the groin and brought them to the attention of her gynecologist. Physical examination revealed salmon pink papules and plaques with silvery scaling involving the abdomen, bilateral upper extremities and ears, and scalp. The patient was then clinically diagnosed with guttate psoriasis. A shave biopsy of a representative lesion on the abdomen was performed. The treatment plan included betamethasone dipropionate cream 0.05% applied twice daily for 2 weeks, clobetasol propionate solution 0.05% applied twice daily for 14 days (for the scalp), and hydrocortisone valerate cream 0.2% applied twice daily for 14 days (for the groin).
The skin biopsy shown in the Figure was received in 10% buffered formalin, measuring 5×4×1 mm of skin. Sections showed an acanthotic epidermis with foci of spongiosis and hypergranulosis covered by mounds of parakeratosis infiltrated by neutrophils. Superficial perivascular and interstitial lymphocytic inflammation was present. Tortuous blood vessels within the papillary dermis also were present. Results showed psoriasiform dermatitis with mild spongiosis. Periodic acid–Schiff stain did not reveal any fungal organisms. These findings were consistent with a diagnosis of guttate psoriasis.

The patient then returned 1 month later mentioning continued flare-ups of the scalp as well as newer patches on the arms and hands that were less eruptive and faded more quickly. The plaques in the groin area had resolved. Physical examination showed fewer pink papules and plaques with silvery scaling on the abdomen, bilateral upper extremities and ears, and scalp. Topical medications were continued, and possible apremilast therapy for the psoriasis was discussed.
Comment
Enterovirus-derived HFMD likely is caused by coxsackie-virus A. Current evidence supports the theory that guttate psoriasis can be environmentally triggered in genetically susceptible individuals, often but not exclusively by a streptococcal infection. The causative agent elicits a T-cell–mediated reaction leading to increased type 1 helper T cells, IFN-γ, and IL-2 cytokine levels. HLA-Cw∗0602–positive patients are considered genetically susceptible and more likely to develop guttate psoriasis following an environmental trigger. Based on the coincidence in timing of both diagnoses, this reported case of guttate psoriasis may have been triggered by a coxsackievirus A infection.
There are 4 variants of psoriasis: plaque, guttate, pustular, and erythroderma (in order of prevalence).2 Guttate psoriasis is characterized by small, 2- to 10-mm, raindroplike lesions on the skin.1 It accounts for approximately 2% of total psoriasis cases and is commonly triggered by group A streptococcal pharyngitis or tonsillitis.3,4
Hand-foot-and-mouth disease (HFMD) is an illness most commonly caused by a coxsackievirus A infection but also can be caused by other enteroviruses.5,6 Coxsackievirus is a serotype of the Enterovirus species within the Picornaviridae family.7 Hand-foot-and-mouth disease is characterized by a brief fever and vesicular rashes on the palms, soles, or buttocks, as well as oropharyngeal ulcers.8 Typically, the rash is benign and short-lived.9 In rare cases, neurologic complications develop. There have been no reported cases of guttate psoriasis following a coxsackievirus A infection.
The involvement of coxsackievirus B in the etiopathogenesis of psoriasis has been previously reported.10 We report the case of guttate psoriasis following presumed coxsackievirus A HFMD.
Case Report
A 56-year-old woman presented with a vesicular rash on the hands, feet, and lips. The patient reported having a sore throat that started around the same time that the rash developed. The severity of the sore throat was rated as moderate. No fever was reported. One day prior, the patient’s primary care physician prescribed a tapered course of prednisone for the rash. The patient reported a medical history of herpes zoster virus, sunburn, and genital herpes. She was taking clonazepam and had a known allergy to penicillin.
Physical examination revealed erythematous vesicular and papular lesions on the extensor surfaces of the hands and feet. Vesicles also were noted on the vermilion border of the lip. Examination of the patient’s mouth showed blisters and shallow ulcerations in the oral cavity. A clinical diagnosis of coxsackievirus A HFMD was made, and the treatment plan included triamcinolone acetonide ointment 0.025% applied twice daily for 2 weeks and oral valacyclovir hydrochloride 1 g taken 3 times daily for 7 days. A topical emollient also was recommended for the lips when necessary. The lesions all resolved within a 2-week period with no sequela.
The patient returned 1 month later, citing newer red abdominal skin lesions. Fever was denied. She reported that both prescribed treatments had not been helping for the newer lesions. She noticed similar lesions on the groin and brought them to the attention of her gynecologist. Physical examination revealed salmon pink papules and plaques with silvery scaling involving the abdomen, bilateral upper extremities and ears, and scalp. The patient was then clinically diagnosed with guttate psoriasis. A shave biopsy of a representative lesion on the abdomen was performed. The treatment plan included betamethasone dipropionate cream 0.05% applied twice daily for 2 weeks, clobetasol propionate solution 0.05% applied twice daily for 14 days (for the scalp), and hydrocortisone valerate cream 0.2% applied twice daily for 14 days (for the groin).
The skin biopsy shown in the Figure was received in 10% buffered formalin, measuring 5×4×1 mm of skin. Sections showed an acanthotic epidermis with foci of spongiosis and hypergranulosis covered by mounds of parakeratosis infiltrated by neutrophils. Superficial perivascular and interstitial lymphocytic inflammation was present. Tortuous blood vessels within the papillary dermis also were present. Results showed psoriasiform dermatitis with mild spongiosis. Periodic acid–Schiff stain did not reveal any fungal organisms. These findings were consistent with a diagnosis of guttate psoriasis.

The patient then returned 1 month later mentioning continued flare-ups of the scalp as well as newer patches on the arms and hands that were less eruptive and faded more quickly. The plaques in the groin area had resolved. Physical examination showed fewer pink papules and plaques with silvery scaling on the abdomen, bilateral upper extremities and ears, and scalp. Topical medications were continued, and possible apremilast therapy for the psoriasis was discussed.
Comment
Enterovirus-derived HFMD likely is caused by coxsackie-virus A. Current evidence supports the theory that guttate psoriasis can be environmentally triggered in genetically susceptible individuals, often but not exclusively by a streptococcal infection. The causative agent elicits a T-cell–mediated reaction leading to increased type 1 helper T cells, IFN-γ, and IL-2 cytokine levels. HLA-Cw∗0602–positive patients are considered genetically susceptible and more likely to develop guttate psoriasis following an environmental trigger. Based on the coincidence in timing of both diagnoses, this reported case of guttate psoriasis may have been triggered by a coxsackievirus A infection.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;1:79-82.
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? an immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326-332.
- Telfer N, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Seitsonen J, Shakeel S, Susi P, et al. Structural analysis of coxsackievirus A7 reveals conformational changes associated with uncoating. J Virol. 2012;86:7207-7215.
- Wu Y, Yeo A, Phoon M, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Tesini BL. Hand-foot-and-mouth-disease (HFMD). May 2018. https://www.msdmanuals.com/professional/infectious-diseases/enteroviruses/hand-foot-and-mouth-disease-hfmd. Accessed September 25, 2019.
- Korzhova TP, Shyrobokov VP, Koliadenko VH, et al. Coxsackie B viral infection in the etiology and clinical pathogenesis of psoriasis [in Ukrainian]. Lik Sprava. 2001:54-58.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23.
- Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin Istanb. 2016;1:79-82.
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? an immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326-332.
- Telfer N, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:O150-O156.
- Li Y, Chang Z, Wu P, et al. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010-2016. Emerg Infect Dis. 2018;24:1902-1906.
- Seitsonen J, Shakeel S, Susi P, et al. Structural analysis of coxsackievirus A7 reveals conformational changes associated with uncoating. J Virol. 2012;86:7207-7215.
- Wu Y, Yeo A, Phoon M, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:E1076-E1081.
- Tesini BL. Hand-foot-and-mouth-disease (HFMD). May 2018. https://www.msdmanuals.com/professional/infectious-diseases/enteroviruses/hand-foot-and-mouth-disease-hfmd. Accessed September 25, 2019.
- Korzhova TP, Shyrobokov VP, Koliadenko VH, et al. Coxsackie B viral infection in the etiology and clinical pathogenesis of psoriasis [in Ukrainian]. Lik Sprava. 2001:54-58.
Practice Points
- Inquire about any illnesses preceding derma-tologic diseases.
- There may be additional microbial triggers for dermatologic diseases.
Photolichenoid Dermatitis: A Presenting Sign of Human Immunodeficiency Virus
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.
A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.
Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.


A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.

Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.


A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.

Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
Practice Points
- There are few reports in the literature of human immunodeficiency virus (HIV) presenting as a photolichenoid eruption.
- We report the case of a 62-year-old African man who presented with a new-onset photodistributed eruption and was subsequently diagnosed with HIV.
- This case supports testing for HIV in patients with a similar clinical presentation.