User login
FDA approves first live vaccine for smallpox, monkeypox prevention
The Food and Drug Administration has approved Jynneos, a live, nonreplicating vaccine based on the vaccinia virus, for smallpox and monkeypox, becoming the first FDA-approved vaccine for the prevention of monkeypox disease.
FDA approval for Jynneos for smallpox is based on results from a clinical trial that compared Jynneos with ACAM2000, a previously FDA-approved smallpox vaccine, in about 400 healthy adults aged 18-42 years. Adults who received Jynneos had a noninferior immune response to those who received ACAM2000. In addition, safety was assessed in 7,800 people who received at least one vaccine dose, with the most commonly reported side effects including pain, redness, swelling, itching, firmness at the injection site, muscle pain, headache, and fatigue.
The effectiveness of Jynneos to prevent monkeypox – a disease similar to but somewhat milder than smallpox caused by the non–U.S.-native monkeypox virus – was inferred from antibody responses of participants in the smallpox clinical trial and from studies on nonhuman primates that showed protection from the monkeypox virus after being vaccinated with Jynneos.
“Routine [smallpox] vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect.”
This vaccine is also part of the Strategic National Stockpile, the nation’s largest supply of potentially lifesaving pharmaceuticals and medical supplies for use in a public health emergency, according to the announcement.
Find the full press release on the FDA website.
The Food and Drug Administration has approved Jynneos, a live, nonreplicating vaccine based on the vaccinia virus, for smallpox and monkeypox, becoming the first FDA-approved vaccine for the prevention of monkeypox disease.
FDA approval for Jynneos for smallpox is based on results from a clinical trial that compared Jynneos with ACAM2000, a previously FDA-approved smallpox vaccine, in about 400 healthy adults aged 18-42 years. Adults who received Jynneos had a noninferior immune response to those who received ACAM2000. In addition, safety was assessed in 7,800 people who received at least one vaccine dose, with the most commonly reported side effects including pain, redness, swelling, itching, firmness at the injection site, muscle pain, headache, and fatigue.
The effectiveness of Jynneos to prevent monkeypox – a disease similar to but somewhat milder than smallpox caused by the non–U.S.-native monkeypox virus – was inferred from antibody responses of participants in the smallpox clinical trial and from studies on nonhuman primates that showed protection from the monkeypox virus after being vaccinated with Jynneos.
“Routine [smallpox] vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect.”
This vaccine is also part of the Strategic National Stockpile, the nation’s largest supply of potentially lifesaving pharmaceuticals and medical supplies for use in a public health emergency, according to the announcement.
Find the full press release on the FDA website.
The Food and Drug Administration has approved Jynneos, a live, nonreplicating vaccine based on the vaccinia virus, for smallpox and monkeypox, becoming the first FDA-approved vaccine for the prevention of monkeypox disease.
FDA approval for Jynneos for smallpox is based on results from a clinical trial that compared Jynneos with ACAM2000, a previously FDA-approved smallpox vaccine, in about 400 healthy adults aged 18-42 years. Adults who received Jynneos had a noninferior immune response to those who received ACAM2000. In addition, safety was assessed in 7,800 people who received at least one vaccine dose, with the most commonly reported side effects including pain, redness, swelling, itching, firmness at the injection site, muscle pain, headache, and fatigue.
The effectiveness of Jynneos to prevent monkeypox – a disease similar to but somewhat milder than smallpox caused by the non–U.S.-native monkeypox virus – was inferred from antibody responses of participants in the smallpox clinical trial and from studies on nonhuman primates that showed protection from the monkeypox virus after being vaccinated with Jynneos.
“Routine [smallpox] vaccination of the American public was stopped in 1972 after the disease was eradicated in the U.S. and, as a result, a large proportion of the U.S., as well as the global population has no immunity,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect.”
This vaccine is also part of the Strategic National Stockpile, the nation’s largest supply of potentially lifesaving pharmaceuticals and medical supplies for use in a public health emergency, according to the announcement.
Find the full press release on the FDA website.
USPSTF: Screening pregnant women for asymptomatic bacteriuria cuts pyelonephritis risk
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
FROM JAMA
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
Does this patient have bacterial conjunctivitis?
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
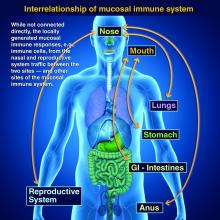
Taking vaccines to the next level via mucosal immunity
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Mycobacterium haemophilum: A Challenging Treatment Dilemma in an Immunocompromised Patient
To the Editor:
The increase in nontuberculous mycobacteria (NTM) infections over the last 3 decades likely is multifaceted, including increased clinical awareness, improved laboratory diagnostics, growing numbers of immunocompromised patients, and an aging population.1,2 Historically, the majority of mycobacteria-related diseases are due to Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium leprae.3
Mycobacterium haemophilum is a slow-growing acid-fast bacillus (AFB) that differs from other Mycobacterium species in that it requires iron-supplemented media and incubation temperatures of 30°C to 32°C for culture. As these requirements for growth are not standard for AFB cultures, M haemophilum infection may be underrecognized and underreported.3Mycobacterium haemophilum infections largely are cutaneous and generally are seen in AIDS patients and bone marrow transplant recipients who are iatrogenically immunosuppressed.4,5 No species-specific treatment guidelines exist2; however, triple-drug therapy combining a macrolide, rifamycin, and a quinolone for a minimum of 12 months often is recommended.
A 64-year-old man with a history of coronary artery disease, hypertension, hyperlipidemia, and acute myelogenous leukemia (AML) underwent allogenic stem cell transplantation. His posttransplant course was complicated by multiple deep vein thromboses, hypogammaglobulinemia, and graft-vs-host disease (GVHD) of the skin and gastrointestinal tract that manifested as chronic diarrhea, which was managed with chronic prednisone. Thirteen months after the transplant, the patient presented to his outpatient oncologist (M.K.) for evaluation of painless, nonpruritic, erythematous papules and nodules that had emerged on the right side of the chest, right arm, and left leg of approximately 2 weeks’ duration.
On review of systems by oncology, the patient denied any fevers, chills, or night sweats but noted chronic loose nonbloody stools without abdominal pain, likely related to the GVHD. The patient’s medications included prednisone 20 mg once daily, fluconazole, amitriptyline, atovaquone, budesonide, dabigatran, metoprolol, pantoprazole, rosuvastatin, senna glycoside, spironolactone, tramadol, and valacyclovir.
Physical examination revealed multiple singular erythematous nodules on the right side of the chest (Figure 1A), right arm (Figure 1B), and left leg. There was no regional lymphadenopathy. The patient was afebrile and hemodynamically stable. A biopsy of the arm performed to rule out leukemia cutis revealed a granulomatous dermatitis with numerous AFB (Figures 2A and 2B), which were confirmed on Ziehl-Neelsen staining (Figures 2C and 2D). The presence of AFB raised concern for a disseminated mycobacterial infection. The patient was admitted to our institution approximately 1 week after the outpatient biopsy was performed. He was evaluated by infectious diseases (B.H.) and was recommended for repeat biopsy with AFB culture and for initiation of intravenous antibiotics.

The patient was evaluated by the dermatology consultation service on hospital day 1. At the time of consultation, the lesions were still painless but had enlarged. Two new satellite lesions were noted on his other extremities. Due to the widespread distribution of the lesions, there was concern for disseminated disease. The relatively rapid onset of new lesions increased concern for infection with rapid-growing mycobacteria, including Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium chelonae. A detailed history revealed that the patient’s wife had a fish tank, which supported the inclusion of Mycobacterium marinum in the differential; however, further questioning revealed that the patient never came in contact with the aquarium water. The initial outpatient biopsy had not been sent for culture. Following inpatient biopsy, the patient was initiated on empiric antimycobacterials, including imipenem, amikacin, clarithromycin, and levofloxacin. Computed tomography of the head was negative for cerebral involvement.
Acid-fast bacilli blood cultures were drawn per the recommendation from infectious diseases in an attempt to confirm disseminated disease; however, blood cultures remained negative. Tissue biopsy from the right arm was sent for AFB staining and culture. Many AFB were identified on microscopy, and growth was observed in the mycobacterial growth indicator tube after 6 days of incubation. The DNA probe was negative for M tuberculosis complex or Mycobacterium avium complex.
The patient was discharged on hospital day 6 on empiric therapy for rapid-growing mycobacteria while cultures were pending. The empiric regimen included intravenous imipenem 1 g every 6 hours, intravenous amikacin 1 g once daily, clarithromycin 500 mg every 12 hours, and levofloxacin 750 mg once daily. All solid media cultures were negative at the time of discharge.
The biopsy specimen proved difficult to culture on solid media using traditional methods. Three weeks after the inpatient biopsy, the microbiology laboratory reported that growth was observed on solid media that was incubated at 30°C and supplemented with iron. These findings were not characteristic of a rapidly growing mycobacteria (eg, M fortuitum, M chelonae, M abscessus) or M marinum but raised concern for infectionwith M haemophilum. Antimycobacterial treatment was adjusted to amikacin, clarithromycin, levofloxacin, and rifabutin.
Six weeks after the inpatient skin biopsy, final speciation confirmed infection with M haemophilum. The isolate proved susceptible to amikacin (minimal inhibitory concentration [MIC], 16), clarithromycin (MIC, 0.12), linezolid (MIC, <1), moxifloxacin (MIC, 0.5), rifabutin (MIC, <0.25), and trimethoprim-sulfamethoxazole (MIC, 0.5/9.5). The isolate was resistant to ciprofloxacin (MIC, 4), ethambutol (MIC, >16), and rifampin (MIC, 2). Based on these findings, an infectious disease specialist modified the treatment regimen to azithromycin 600 mg once daily, moxifloxacin 400 mg once daily, and rifabutin 300 mg once daily. Azithromycin was substituted for clarithromycin in an attempt to minimize the gastrointestinal side effects of the antibiotics. The infectious disease specialist was concerned that the clarithromycin could exacerbate the patient’s chronic GVHD-associated diarrhea, which posed a challenge to the oncologist, who was attempting to manage the patient’s GVHD and minimize the use of additional prednisone. At the time of this change, the patient was doing well clinically and denied any active skin lesions.
Four months later, he developed new left-sided neck swelling. Computed tomography revealed nonspecific enhancement involving the skin and superficial subcutaneous tissues in the left anterior neck. He was referred to otolaryngology given concern for recurrent infection vs leukemia cutis. He underwent excisional biopsy. Pathology was negative for malignancy but demonstrated subcutaneous necrotizing granulomatous inflammation with a positive AFB stain. Tissue AFB cultures revealed moderate AFB on direct stain, but there was no AFB growth at 12 weeks. Clarithromycin was restarted in place of azithromycin to increase the potency of the antimycobacterial regimen. Cultures from this neck biopsy were negative after 12 weeks of incubation.
In addition to this change in antibiotic coverage, the patient’s medical oncologist tapered the patient’s immunosuppression considerably. The patient subsequently completed 12 months of therapy with clarithromycin, moxifloxacin, and rifabutin starting from the time of the neck biopsy. He remained free of recurrence of mycobacterial infection for nearly 2 years until he died from an unrelated illness.
Nontuberculous mycobacteria are an ubiquitous environmental group.2 Sources include soil and natural water (M avium), fish tanks and swimming pools (M marinum), and tap water and occasionally domestic animals (Mycobacterium kansasii). Additionally, rapidly growing NTM such as M abscessus, M chelonae, and M fortuitum have been isolated from soil and natural water supplies.3
Mycobacterium haemophilum is a fastidious organism with a predilection for skin of the chest and extremities. Iatrogenically or inherently immunocompromised patients are most commonly affected6-11; however, there also have been reports in healthy patients.12,13 Infections typically present as painless erythematous papules or nodules that eventually suppurate, ulcerate, and become painful. Presentations involving Fitz-Hugh–Curtis syndrome,13 new B-cell lymphoma,10 and lymphadenitis12 also have been described. Beyond cutaneous involvement, M haemophilum has been cultured from bone, the synovium, the lungs, and the central nervous system.4,9 The majority of morbidities occur in patients with lung involvement.4 Therefore, even patients presenting with isolated cutaneous disease require close follow-up.
Mycobacterium haemophilum is a slowly proliferating organism that is unable to grow in standard egg-potato (Lowenstein-Jensen) medium or agar base (Middlebrook 7H10 or 7H11 agar) without iron supplementation (ferric ammonium citrate, hemin, or hemoglobin). It also requires temperatures of 30°C to 32°C for growth. Its iron requisite is unique, but species such as M marinum and Mycobacterium ulcerans also share reduced temperature requirements. Without a high index of suspicion, growth often is absent because standard Mycobacterium culture techniques will not foster organism growth. Our case demonstrated that special culture instructions must be relayed to the laboratory, even in the face of positive AFB smears. Failure to request hemin and modified incubation temperatures may have contributed to the negative AFB blood culture in our patient.
Due to the relatively rare incidence of M haemophilum infection, there are no known randomized controlled trials guiding antibiotic regimens. Infectious disease specialists often treat empirically with triple-drug therapy derived from locally reported species susceptibilities. The largest case series to date did not identify resistance to amikacin, ciprofloxacin, or clarithromycin.4 Our case identified a novel finding of ciprofloxacin and rifampin resistance, which may highlight the emergence of a newly resistant strain of M haemophilum. Of note, one case of rifampin resistance has been reported, but the culture was drawn from a postmortem specimen in the setting of previously rifampin-sensitive isolates.4 Empiric therapies should be guided by hospital susceptibility reports and expert consultation.
Coinfection with 2 or more NTM—including M tuberculosis, M leprae, and M fortuitum—has been reported.8,14 Temporally distinct coinfections with M leprae and M haemophilum also have been described.15 Thus, practitioners should have a low threshold for repeat cultures in the context of new cutaneous nodules or granulomas, not only to detect concomitant infections but also to identify resistance patterns that might explain recurrent or recalcitrant disease. Immune reconstitution inflammatory syndrome also must be considered with new or worsening lesions, especially in the first months of therapy, as this is a common occurrence when immunosuppressive regimens are tapered to help manage infections.
In conclusion, M haemophilum is an underrecognized infection that presents as cutaneous nodules or lymphadenitis in immunocompromised or healthy individuals. Diagnosis requires a high index of suspicion because its unique growth requirements necessitate special laboratory techniques. Our case represents a classic presentation of this NTM infection in a patient with AML following allogenic stem cell transplantation. Repeat cultures, workup of potentially disseminated infections, and close follow-up are requisite to minimizing morbidity and mortality. A multidisciplinary approach involving infectious disease, medical oncology, radiology, and dermatology best manages this type of infection.
- Sheu LC, Tran TM, Jarlsberg LG, et al. Non-tuberculous mycobacterial infections at San Francisco General Hospital. Clin Respir J. 2015;9:436-442.
- Knoll BM. Update on nontuberculous mycobacterial infections in solid organ and hematopoietic stem cell transplant recipients. Curr Infect Dis Rep. 2014;16:421.
- Diagnosis and treatment of disease caused by nontuberculous mycobacteria. this official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156(2 pt 2):S1-S25.
- Shah MK, Sebti A, Kiehn TE, et al. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis. 2001;33:330-337.
- Griffiths DE, Aksamit T, Brown-Elliott BA. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Copeland NK, Arora NS, Ferguson TM. Mycobacterium haemophilum masquerading as leprosy in a renal transplant patient [published online November 28, 2013]. Case Rep Dermatol Med. 2013;2013:793127.
- Aslam A, Green RL, Motta L, et al. Cutaneous Mycobacterium haemophilum infection in a patient receiving infliximab for psoriasis. Br J Dermatol. 2013;168:446-447.
- Agrawal S, Sharma A. Dual mycobacterial infection in the setting of leflunomide treatment for rheumatoid arthritis. Ann Rheum Dis. 2007;66:277.
- Buppajarntham A, Apisarnthanarak A, Rutjanawech S, et al. Central nervous system infection due to Mycobacterium haemophilum in a patient with acquired immunodeficiency syndrome. Int J STD AIDS. 2015;26:288-290.
- Doherty T, Lynn M, Cavazza A, et al. Mycobacterium haemophilum as the initial presentation of a B-cell lymphoma in a liver transplant patient [published online January 12, 2014]. Case Rep Rheumatol. 2014;2014:742978.
- Ducharlet K, Murphy C, Tan SJ, et al. Recurrent Mycobacterium haemophilum in a renal transplant recipient. Nephrology (Carlton). 2014;(19 suppl 1):14-17.
- Dawson DJ, Blacklock ZM, Kane DW. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med J Aust. 1981;2:289-290.
- Jang HY, Burbelo PD, Chae YS, et al. Nontuberculous mycobacterial infection in a clinical presentation of Fitz-Hugh-Curtis syndrome: a case report with multigene diagnostic approach. BMC Womens Health. 2014;14:95.
- Scollard DM, Stryjewska BM, Prestigiacomo JF, et al. Hansen’s disease (leprosy) complicated by secondary mycobacterial infection. J Am Acad Dermatol. 2011;64:593-596.
- SoRelle JA, Beal SG, Scollard DM, et al. Mycobacterium leprae and Mycobacterium haemophilum co-infection in an iatrogenically immunosuppressed patient. Diagn Microbiol Infect Dis. 2014;78:494-496.
To the Editor:
The increase in nontuberculous mycobacteria (NTM) infections over the last 3 decades likely is multifaceted, including increased clinical awareness, improved laboratory diagnostics, growing numbers of immunocompromised patients, and an aging population.1,2 Historically, the majority of mycobacteria-related diseases are due to Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium leprae.3
Mycobacterium haemophilum is a slow-growing acid-fast bacillus (AFB) that differs from other Mycobacterium species in that it requires iron-supplemented media and incubation temperatures of 30°C to 32°C for culture. As these requirements for growth are not standard for AFB cultures, M haemophilum infection may be underrecognized and underreported.3Mycobacterium haemophilum infections largely are cutaneous and generally are seen in AIDS patients and bone marrow transplant recipients who are iatrogenically immunosuppressed.4,5 No species-specific treatment guidelines exist2; however, triple-drug therapy combining a macrolide, rifamycin, and a quinolone for a minimum of 12 months often is recommended.
A 64-year-old man with a history of coronary artery disease, hypertension, hyperlipidemia, and acute myelogenous leukemia (AML) underwent allogenic stem cell transplantation. His posttransplant course was complicated by multiple deep vein thromboses, hypogammaglobulinemia, and graft-vs-host disease (GVHD) of the skin and gastrointestinal tract that manifested as chronic diarrhea, which was managed with chronic prednisone. Thirteen months after the transplant, the patient presented to his outpatient oncologist (M.K.) for evaluation of painless, nonpruritic, erythematous papules and nodules that had emerged on the right side of the chest, right arm, and left leg of approximately 2 weeks’ duration.
On review of systems by oncology, the patient denied any fevers, chills, or night sweats but noted chronic loose nonbloody stools without abdominal pain, likely related to the GVHD. The patient’s medications included prednisone 20 mg once daily, fluconazole, amitriptyline, atovaquone, budesonide, dabigatran, metoprolol, pantoprazole, rosuvastatin, senna glycoside, spironolactone, tramadol, and valacyclovir.
Physical examination revealed multiple singular erythematous nodules on the right side of the chest (Figure 1A), right arm (Figure 1B), and left leg. There was no regional lymphadenopathy. The patient was afebrile and hemodynamically stable. A biopsy of the arm performed to rule out leukemia cutis revealed a granulomatous dermatitis with numerous AFB (Figures 2A and 2B), which were confirmed on Ziehl-Neelsen staining (Figures 2C and 2D). The presence of AFB raised concern for a disseminated mycobacterial infection. The patient was admitted to our institution approximately 1 week after the outpatient biopsy was performed. He was evaluated by infectious diseases (B.H.) and was recommended for repeat biopsy with AFB culture and for initiation of intravenous antibiotics.


The patient was evaluated by the dermatology consultation service on hospital day 1. At the time of consultation, the lesions were still painless but had enlarged. Two new satellite lesions were noted on his other extremities. Due to the widespread distribution of the lesions, there was concern for disseminated disease. The relatively rapid onset of new lesions increased concern for infection with rapid-growing mycobacteria, including Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium chelonae. A detailed history revealed that the patient’s wife had a fish tank, which supported the inclusion of Mycobacterium marinum in the differential; however, further questioning revealed that the patient never came in contact with the aquarium water. The initial outpatient biopsy had not been sent for culture. Following inpatient biopsy, the patient was initiated on empiric antimycobacterials, including imipenem, amikacin, clarithromycin, and levofloxacin. Computed tomography of the head was negative for cerebral involvement.
Acid-fast bacilli blood cultures were drawn per the recommendation from infectious diseases in an attempt to confirm disseminated disease; however, blood cultures remained negative. Tissue biopsy from the right arm was sent for AFB staining and culture. Many AFB were identified on microscopy, and growth was observed in the mycobacterial growth indicator tube after 6 days of incubation. The DNA probe was negative for M tuberculosis complex or Mycobacterium avium complex.
The patient was discharged on hospital day 6 on empiric therapy for rapid-growing mycobacteria while cultures were pending. The empiric regimen included intravenous imipenem 1 g every 6 hours, intravenous amikacin 1 g once daily, clarithromycin 500 mg every 12 hours, and levofloxacin 750 mg once daily. All solid media cultures were negative at the time of discharge.
The biopsy specimen proved difficult to culture on solid media using traditional methods. Three weeks after the inpatient biopsy, the microbiology laboratory reported that growth was observed on solid media that was incubated at 30°C and supplemented with iron. These findings were not characteristic of a rapidly growing mycobacteria (eg, M fortuitum, M chelonae, M abscessus) or M marinum but raised concern for infectionwith M haemophilum. Antimycobacterial treatment was adjusted to amikacin, clarithromycin, levofloxacin, and rifabutin.
Six weeks after the inpatient skin biopsy, final speciation confirmed infection with M haemophilum. The isolate proved susceptible to amikacin (minimal inhibitory concentration [MIC], 16), clarithromycin (MIC, 0.12), linezolid (MIC, <1), moxifloxacin (MIC, 0.5), rifabutin (MIC, <0.25), and trimethoprim-sulfamethoxazole (MIC, 0.5/9.5). The isolate was resistant to ciprofloxacin (MIC, 4), ethambutol (MIC, >16), and rifampin (MIC, 2). Based on these findings, an infectious disease specialist modified the treatment regimen to azithromycin 600 mg once daily, moxifloxacin 400 mg once daily, and rifabutin 300 mg once daily. Azithromycin was substituted for clarithromycin in an attempt to minimize the gastrointestinal side effects of the antibiotics. The infectious disease specialist was concerned that the clarithromycin could exacerbate the patient’s chronic GVHD-associated diarrhea, which posed a challenge to the oncologist, who was attempting to manage the patient’s GVHD and minimize the use of additional prednisone. At the time of this change, the patient was doing well clinically and denied any active skin lesions.
Four months later, he developed new left-sided neck swelling. Computed tomography revealed nonspecific enhancement involving the skin and superficial subcutaneous tissues in the left anterior neck. He was referred to otolaryngology given concern for recurrent infection vs leukemia cutis. He underwent excisional biopsy. Pathology was negative for malignancy but demonstrated subcutaneous necrotizing granulomatous inflammation with a positive AFB stain. Tissue AFB cultures revealed moderate AFB on direct stain, but there was no AFB growth at 12 weeks. Clarithromycin was restarted in place of azithromycin to increase the potency of the antimycobacterial regimen. Cultures from this neck biopsy were negative after 12 weeks of incubation.
In addition to this change in antibiotic coverage, the patient’s medical oncologist tapered the patient’s immunosuppression considerably. The patient subsequently completed 12 months of therapy with clarithromycin, moxifloxacin, and rifabutin starting from the time of the neck biopsy. He remained free of recurrence of mycobacterial infection for nearly 2 years until he died from an unrelated illness.
Nontuberculous mycobacteria are an ubiquitous environmental group.2 Sources include soil and natural water (M avium), fish tanks and swimming pools (M marinum), and tap water and occasionally domestic animals (Mycobacterium kansasii). Additionally, rapidly growing NTM such as M abscessus, M chelonae, and M fortuitum have been isolated from soil and natural water supplies.3
Mycobacterium haemophilum is a fastidious organism with a predilection for skin of the chest and extremities. Iatrogenically or inherently immunocompromised patients are most commonly affected6-11; however, there also have been reports in healthy patients.12,13 Infections typically present as painless erythematous papules or nodules that eventually suppurate, ulcerate, and become painful. Presentations involving Fitz-Hugh–Curtis syndrome,13 new B-cell lymphoma,10 and lymphadenitis12 also have been described. Beyond cutaneous involvement, M haemophilum has been cultured from bone, the synovium, the lungs, and the central nervous system.4,9 The majority of morbidities occur in patients with lung involvement.4 Therefore, even patients presenting with isolated cutaneous disease require close follow-up.
Mycobacterium haemophilum is a slowly proliferating organism that is unable to grow in standard egg-potato (Lowenstein-Jensen) medium or agar base (Middlebrook 7H10 or 7H11 agar) without iron supplementation (ferric ammonium citrate, hemin, or hemoglobin). It also requires temperatures of 30°C to 32°C for growth. Its iron requisite is unique, but species such as M marinum and Mycobacterium ulcerans also share reduced temperature requirements. Without a high index of suspicion, growth often is absent because standard Mycobacterium culture techniques will not foster organism growth. Our case demonstrated that special culture instructions must be relayed to the laboratory, even in the face of positive AFB smears. Failure to request hemin and modified incubation temperatures may have contributed to the negative AFB blood culture in our patient.
Due to the relatively rare incidence of M haemophilum infection, there are no known randomized controlled trials guiding antibiotic regimens. Infectious disease specialists often treat empirically with triple-drug therapy derived from locally reported species susceptibilities. The largest case series to date did not identify resistance to amikacin, ciprofloxacin, or clarithromycin.4 Our case identified a novel finding of ciprofloxacin and rifampin resistance, which may highlight the emergence of a newly resistant strain of M haemophilum. Of note, one case of rifampin resistance has been reported, but the culture was drawn from a postmortem specimen in the setting of previously rifampin-sensitive isolates.4 Empiric therapies should be guided by hospital susceptibility reports and expert consultation.
Coinfection with 2 or more NTM—including M tuberculosis, M leprae, and M fortuitum—has been reported.8,14 Temporally distinct coinfections with M leprae and M haemophilum also have been described.15 Thus, practitioners should have a low threshold for repeat cultures in the context of new cutaneous nodules or granulomas, not only to detect concomitant infections but also to identify resistance patterns that might explain recurrent or recalcitrant disease. Immune reconstitution inflammatory syndrome also must be considered with new or worsening lesions, especially in the first months of therapy, as this is a common occurrence when immunosuppressive regimens are tapered to help manage infections.
In conclusion, M haemophilum is an underrecognized infection that presents as cutaneous nodules or lymphadenitis in immunocompromised or healthy individuals. Diagnosis requires a high index of suspicion because its unique growth requirements necessitate special laboratory techniques. Our case represents a classic presentation of this NTM infection in a patient with AML following allogenic stem cell transplantation. Repeat cultures, workup of potentially disseminated infections, and close follow-up are requisite to minimizing morbidity and mortality. A multidisciplinary approach involving infectious disease, medical oncology, radiology, and dermatology best manages this type of infection.
To the Editor:
The increase in nontuberculous mycobacteria (NTM) infections over the last 3 decades likely is multifaceted, including increased clinical awareness, improved laboratory diagnostics, growing numbers of immunocompromised patients, and an aging population.1,2 Historically, the majority of mycobacteria-related diseases are due to Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium leprae.3
Mycobacterium haemophilum is a slow-growing acid-fast bacillus (AFB) that differs from other Mycobacterium species in that it requires iron-supplemented media and incubation temperatures of 30°C to 32°C for culture. As these requirements for growth are not standard for AFB cultures, M haemophilum infection may be underrecognized and underreported.3Mycobacterium haemophilum infections largely are cutaneous and generally are seen in AIDS patients and bone marrow transplant recipients who are iatrogenically immunosuppressed.4,5 No species-specific treatment guidelines exist2; however, triple-drug therapy combining a macrolide, rifamycin, and a quinolone for a minimum of 12 months often is recommended.
A 64-year-old man with a history of coronary artery disease, hypertension, hyperlipidemia, and acute myelogenous leukemia (AML) underwent allogenic stem cell transplantation. His posttransplant course was complicated by multiple deep vein thromboses, hypogammaglobulinemia, and graft-vs-host disease (GVHD) of the skin and gastrointestinal tract that manifested as chronic diarrhea, which was managed with chronic prednisone. Thirteen months after the transplant, the patient presented to his outpatient oncologist (M.K.) for evaluation of painless, nonpruritic, erythematous papules and nodules that had emerged on the right side of the chest, right arm, and left leg of approximately 2 weeks’ duration.
On review of systems by oncology, the patient denied any fevers, chills, or night sweats but noted chronic loose nonbloody stools without abdominal pain, likely related to the GVHD. The patient’s medications included prednisone 20 mg once daily, fluconazole, amitriptyline, atovaquone, budesonide, dabigatran, metoprolol, pantoprazole, rosuvastatin, senna glycoside, spironolactone, tramadol, and valacyclovir.
Physical examination revealed multiple singular erythematous nodules on the right side of the chest (Figure 1A), right arm (Figure 1B), and left leg. There was no regional lymphadenopathy. The patient was afebrile and hemodynamically stable. A biopsy of the arm performed to rule out leukemia cutis revealed a granulomatous dermatitis with numerous AFB (Figures 2A and 2B), which were confirmed on Ziehl-Neelsen staining (Figures 2C and 2D). The presence of AFB raised concern for a disseminated mycobacterial infection. The patient was admitted to our institution approximately 1 week after the outpatient biopsy was performed. He was evaluated by infectious diseases (B.H.) and was recommended for repeat biopsy with AFB culture and for initiation of intravenous antibiotics.


The patient was evaluated by the dermatology consultation service on hospital day 1. At the time of consultation, the lesions were still painless but had enlarged. Two new satellite lesions were noted on his other extremities. Due to the widespread distribution of the lesions, there was concern for disseminated disease. The relatively rapid onset of new lesions increased concern for infection with rapid-growing mycobacteria, including Mycobacterium abscessus, Mycobacterium fortuitum, and Mycobacterium chelonae. A detailed history revealed that the patient’s wife had a fish tank, which supported the inclusion of Mycobacterium marinum in the differential; however, further questioning revealed that the patient never came in contact with the aquarium water. The initial outpatient biopsy had not been sent for culture. Following inpatient biopsy, the patient was initiated on empiric antimycobacterials, including imipenem, amikacin, clarithromycin, and levofloxacin. Computed tomography of the head was negative for cerebral involvement.
Acid-fast bacilli blood cultures were drawn per the recommendation from infectious diseases in an attempt to confirm disseminated disease; however, blood cultures remained negative. Tissue biopsy from the right arm was sent for AFB staining and culture. Many AFB were identified on microscopy, and growth was observed in the mycobacterial growth indicator tube after 6 days of incubation. The DNA probe was negative for M tuberculosis complex or Mycobacterium avium complex.
The patient was discharged on hospital day 6 on empiric therapy for rapid-growing mycobacteria while cultures were pending. The empiric regimen included intravenous imipenem 1 g every 6 hours, intravenous amikacin 1 g once daily, clarithromycin 500 mg every 12 hours, and levofloxacin 750 mg once daily. All solid media cultures were negative at the time of discharge.
The biopsy specimen proved difficult to culture on solid media using traditional methods. Three weeks after the inpatient biopsy, the microbiology laboratory reported that growth was observed on solid media that was incubated at 30°C and supplemented with iron. These findings were not characteristic of a rapidly growing mycobacteria (eg, M fortuitum, M chelonae, M abscessus) or M marinum but raised concern for infectionwith M haemophilum. Antimycobacterial treatment was adjusted to amikacin, clarithromycin, levofloxacin, and rifabutin.
Six weeks after the inpatient skin biopsy, final speciation confirmed infection with M haemophilum. The isolate proved susceptible to amikacin (minimal inhibitory concentration [MIC], 16), clarithromycin (MIC, 0.12), linezolid (MIC, <1), moxifloxacin (MIC, 0.5), rifabutin (MIC, <0.25), and trimethoprim-sulfamethoxazole (MIC, 0.5/9.5). The isolate was resistant to ciprofloxacin (MIC, 4), ethambutol (MIC, >16), and rifampin (MIC, 2). Based on these findings, an infectious disease specialist modified the treatment regimen to azithromycin 600 mg once daily, moxifloxacin 400 mg once daily, and rifabutin 300 mg once daily. Azithromycin was substituted for clarithromycin in an attempt to minimize the gastrointestinal side effects of the antibiotics. The infectious disease specialist was concerned that the clarithromycin could exacerbate the patient’s chronic GVHD-associated diarrhea, which posed a challenge to the oncologist, who was attempting to manage the patient’s GVHD and minimize the use of additional prednisone. At the time of this change, the patient was doing well clinically and denied any active skin lesions.
Four months later, he developed new left-sided neck swelling. Computed tomography revealed nonspecific enhancement involving the skin and superficial subcutaneous tissues in the left anterior neck. He was referred to otolaryngology given concern for recurrent infection vs leukemia cutis. He underwent excisional biopsy. Pathology was negative for malignancy but demonstrated subcutaneous necrotizing granulomatous inflammation with a positive AFB stain. Tissue AFB cultures revealed moderate AFB on direct stain, but there was no AFB growth at 12 weeks. Clarithromycin was restarted in place of azithromycin to increase the potency of the antimycobacterial regimen. Cultures from this neck biopsy were negative after 12 weeks of incubation.
In addition to this change in antibiotic coverage, the patient’s medical oncologist tapered the patient’s immunosuppression considerably. The patient subsequently completed 12 months of therapy with clarithromycin, moxifloxacin, and rifabutin starting from the time of the neck biopsy. He remained free of recurrence of mycobacterial infection for nearly 2 years until he died from an unrelated illness.
Nontuberculous mycobacteria are an ubiquitous environmental group.2 Sources include soil and natural water (M avium), fish tanks and swimming pools (M marinum), and tap water and occasionally domestic animals (Mycobacterium kansasii). Additionally, rapidly growing NTM such as M abscessus, M chelonae, and M fortuitum have been isolated from soil and natural water supplies.3
Mycobacterium haemophilum is a fastidious organism with a predilection for skin of the chest and extremities. Iatrogenically or inherently immunocompromised patients are most commonly affected6-11; however, there also have been reports in healthy patients.12,13 Infections typically present as painless erythematous papules or nodules that eventually suppurate, ulcerate, and become painful. Presentations involving Fitz-Hugh–Curtis syndrome,13 new B-cell lymphoma,10 and lymphadenitis12 also have been described. Beyond cutaneous involvement, M haemophilum has been cultured from bone, the synovium, the lungs, and the central nervous system.4,9 The majority of morbidities occur in patients with lung involvement.4 Therefore, even patients presenting with isolated cutaneous disease require close follow-up.
Mycobacterium haemophilum is a slowly proliferating organism that is unable to grow in standard egg-potato (Lowenstein-Jensen) medium or agar base (Middlebrook 7H10 or 7H11 agar) without iron supplementation (ferric ammonium citrate, hemin, or hemoglobin). It also requires temperatures of 30°C to 32°C for growth. Its iron requisite is unique, but species such as M marinum and Mycobacterium ulcerans also share reduced temperature requirements. Without a high index of suspicion, growth often is absent because standard Mycobacterium culture techniques will not foster organism growth. Our case demonstrated that special culture instructions must be relayed to the laboratory, even in the face of positive AFB smears. Failure to request hemin and modified incubation temperatures may have contributed to the negative AFB blood culture in our patient.
Due to the relatively rare incidence of M haemophilum infection, there are no known randomized controlled trials guiding antibiotic regimens. Infectious disease specialists often treat empirically with triple-drug therapy derived from locally reported species susceptibilities. The largest case series to date did not identify resistance to amikacin, ciprofloxacin, or clarithromycin.4 Our case identified a novel finding of ciprofloxacin and rifampin resistance, which may highlight the emergence of a newly resistant strain of M haemophilum. Of note, one case of rifampin resistance has been reported, but the culture was drawn from a postmortem specimen in the setting of previously rifampin-sensitive isolates.4 Empiric therapies should be guided by hospital susceptibility reports and expert consultation.
Coinfection with 2 or more NTM—including M tuberculosis, M leprae, and M fortuitum—has been reported.8,14 Temporally distinct coinfections with M leprae and M haemophilum also have been described.15 Thus, practitioners should have a low threshold for repeat cultures in the context of new cutaneous nodules or granulomas, not only to detect concomitant infections but also to identify resistance patterns that might explain recurrent or recalcitrant disease. Immune reconstitution inflammatory syndrome also must be considered with new or worsening lesions, especially in the first months of therapy, as this is a common occurrence when immunosuppressive regimens are tapered to help manage infections.
In conclusion, M haemophilum is an underrecognized infection that presents as cutaneous nodules or lymphadenitis in immunocompromised or healthy individuals. Diagnosis requires a high index of suspicion because its unique growth requirements necessitate special laboratory techniques. Our case represents a classic presentation of this NTM infection in a patient with AML following allogenic stem cell transplantation. Repeat cultures, workup of potentially disseminated infections, and close follow-up are requisite to minimizing morbidity and mortality. A multidisciplinary approach involving infectious disease, medical oncology, radiology, and dermatology best manages this type of infection.
- Sheu LC, Tran TM, Jarlsberg LG, et al. Non-tuberculous mycobacterial infections at San Francisco General Hospital. Clin Respir J. 2015;9:436-442.
- Knoll BM. Update on nontuberculous mycobacterial infections in solid organ and hematopoietic stem cell transplant recipients. Curr Infect Dis Rep. 2014;16:421.
- Diagnosis and treatment of disease caused by nontuberculous mycobacteria. this official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156(2 pt 2):S1-S25.
- Shah MK, Sebti A, Kiehn TE, et al. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis. 2001;33:330-337.
- Griffiths DE, Aksamit T, Brown-Elliott BA. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Copeland NK, Arora NS, Ferguson TM. Mycobacterium haemophilum masquerading as leprosy in a renal transplant patient [published online November 28, 2013]. Case Rep Dermatol Med. 2013;2013:793127.
- Aslam A, Green RL, Motta L, et al. Cutaneous Mycobacterium haemophilum infection in a patient receiving infliximab for psoriasis. Br J Dermatol. 2013;168:446-447.
- Agrawal S, Sharma A. Dual mycobacterial infection in the setting of leflunomide treatment for rheumatoid arthritis. Ann Rheum Dis. 2007;66:277.
- Buppajarntham A, Apisarnthanarak A, Rutjanawech S, et al. Central nervous system infection due to Mycobacterium haemophilum in a patient with acquired immunodeficiency syndrome. Int J STD AIDS. 2015;26:288-290.
- Doherty T, Lynn M, Cavazza A, et al. Mycobacterium haemophilum as the initial presentation of a B-cell lymphoma in a liver transplant patient [published online January 12, 2014]. Case Rep Rheumatol. 2014;2014:742978.
- Ducharlet K, Murphy C, Tan SJ, et al. Recurrent Mycobacterium haemophilum in a renal transplant recipient. Nephrology (Carlton). 2014;(19 suppl 1):14-17.
- Dawson DJ, Blacklock ZM, Kane DW. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med J Aust. 1981;2:289-290.
- Jang HY, Burbelo PD, Chae YS, et al. Nontuberculous mycobacterial infection in a clinical presentation of Fitz-Hugh-Curtis syndrome: a case report with multigene diagnostic approach. BMC Womens Health. 2014;14:95.
- Scollard DM, Stryjewska BM, Prestigiacomo JF, et al. Hansen’s disease (leprosy) complicated by secondary mycobacterial infection. J Am Acad Dermatol. 2011;64:593-596.
- SoRelle JA, Beal SG, Scollard DM, et al. Mycobacterium leprae and Mycobacterium haemophilum co-infection in an iatrogenically immunosuppressed patient. Diagn Microbiol Infect Dis. 2014;78:494-496.
- Sheu LC, Tran TM, Jarlsberg LG, et al. Non-tuberculous mycobacterial infections at San Francisco General Hospital. Clin Respir J. 2015;9:436-442.
- Knoll BM. Update on nontuberculous mycobacterial infections in solid organ and hematopoietic stem cell transplant recipients. Curr Infect Dis Rep. 2014;16:421.
- Diagnosis and treatment of disease caused by nontuberculous mycobacteria. this official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156(2 pt 2):S1-S25.
- Shah MK, Sebti A, Kiehn TE, et al. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis. 2001;33:330-337.
- Griffiths DE, Aksamit T, Brown-Elliott BA. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Copeland NK, Arora NS, Ferguson TM. Mycobacterium haemophilum masquerading as leprosy in a renal transplant patient [published online November 28, 2013]. Case Rep Dermatol Med. 2013;2013:793127.
- Aslam A, Green RL, Motta L, et al. Cutaneous Mycobacterium haemophilum infection in a patient receiving infliximab for psoriasis. Br J Dermatol. 2013;168:446-447.
- Agrawal S, Sharma A. Dual mycobacterial infection in the setting of leflunomide treatment for rheumatoid arthritis. Ann Rheum Dis. 2007;66:277.
- Buppajarntham A, Apisarnthanarak A, Rutjanawech S, et al. Central nervous system infection due to Mycobacterium haemophilum in a patient with acquired immunodeficiency syndrome. Int J STD AIDS. 2015;26:288-290.
- Doherty T, Lynn M, Cavazza A, et al. Mycobacterium haemophilum as the initial presentation of a B-cell lymphoma in a liver transplant patient [published online January 12, 2014]. Case Rep Rheumatol. 2014;2014:742978.
- Ducharlet K, Murphy C, Tan SJ, et al. Recurrent Mycobacterium haemophilum in a renal transplant recipient. Nephrology (Carlton). 2014;(19 suppl 1):14-17.
- Dawson DJ, Blacklock ZM, Kane DW. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med J Aust. 1981;2:289-290.
- Jang HY, Burbelo PD, Chae YS, et al. Nontuberculous mycobacterial infection in a clinical presentation of Fitz-Hugh-Curtis syndrome: a case report with multigene diagnostic approach. BMC Womens Health. 2014;14:95.
- Scollard DM, Stryjewska BM, Prestigiacomo JF, et al. Hansen’s disease (leprosy) complicated by secondary mycobacterial infection. J Am Acad Dermatol. 2011;64:593-596.
- SoRelle JA, Beal SG, Scollard DM, et al. Mycobacterium leprae and Mycobacterium haemophilum co-infection in an iatrogenically immunosuppressed patient. Diagn Microbiol Infect Dis. 2014;78:494-496.
Practice Points
- Mycobacterium haemophilum is a slow-growing acid-fast bacillus that requires iron-supplemented media and incubation temperatures of 30°C to 32°C for culture. Because these requirements for growth are not standard for acid-fast bacteria cultures, M haemophilum infection may be underrecognized and underreported.
- There are no species-specific treatment guidelines, but extended course of treatment with multiple active antibacterials typically is recommended.
New engineered HIV-1 vaccine candidate shows improved immunogenicity in early trial
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
ALVAC-HIV vaccine showed immunogenicity across several HIV clades in an early trial involving 100 healthy patients at low risk of HIV infection, according to a study by Glenda E. Gray, MBBCH, FCPaed, of the University of the Witwatersrand, Johannesburg, South Africa, and colleagues that was published online in the Sep. 18 issue of Science Translational Medicine.
ALVAC-HIV (vCP1521) is a live attenuated recombinant canarypox-derived virus that expresses gene products from the HIV-1 gp120 (92TH023/clade E), Gag (clade B), and Pro (clade B) that is cultured in chicken embryo fibroblast cells.
Four injections of ALVAC-HIV were given at months 0, 1, 3, and 6. At months 3 and 6, two booster injections were given of AIDSVAX/BE, a bivalent HIV glycoprotein 120 (gp120) that was previously studied in the RV144 trial. The HVTN 097 trial examined primary immunogenicity endpoints including the frequency and magnitude of IgG and IgG3 antibody binding, measured in serum specimens obtained at baseline, at a peak time point (2 weeks after second ALVAC/AIDSVAX vaccination), a durability time point (6 months after second ALVAC/AIDSVAX vaccination), and the response rates and magnitudes of CD4+ and CD8+ T-cell responses at the baseline, peak, and durability time points. One hundred healthy adults at low risk for HIV infection were randomized in 3:1:1 ratio to group T1 (HIV vaccines, tetanus vaccine, and hepatitis B vaccine), group T2 (HIV vaccine only), and the placebo group T3 (tetanus vaccine and hepatitis B vaccine). There were no meaningful differences in HIV immune responses between the HIV vaccine recipients with or without the tetanus and hepatitis B vaccines, so the researchers pooled the data from groups T1 and T2 in their analysis.
At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env; this was measured by expression of interleukin-2 and/or interferon-gamma. The Env-specific CD4+ T-cell response rate was significantly higher in HVTN 097 vaccine recipients than it was in those in the RV144 trial (51.9% vs. 36.4%; P = .043). The HVTN 097 trial also showed significantly higher response rates for CD40L(59.3% for HVTN 097 vs. 33.7% for RV144; P less than .001) and for interferon-gamma (42.6% in HVTN 097 vs. 19.5% in RV144; P = .001).
However, durability at 6 months after the second vaccine injection remained an issue, with the frequency of circulating Env-specific CD4+ T-cell responses among vaccine recipients declining significantly; the response rate dropped from 70.8% to 36.1%.
“These data may indicate that cross-clade immune responses, especially to non-neutralizing epitopes correlated with decreased HIV-1 risk, can be achieved for a globally effective vaccine by using unique HIV Env strains,” Dr. Gray and associates concluded.
The authors declared that they had no competing interests.
SOURCE: Gray GE et al. Sci. Transl. Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880..
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: “These data may indicate that cross-clade immune responses ... can be achieved for a globally effective vaccine by using unique HIV Env strains.”
Major finding: At the peak immunogenicity time point, the vaccine schedule predominantly induced CD4+ T cells directed to HIV-1 Env .
Study details: A phase 1b randomized, double-blind, placebo-controlled trial to assess the safety and immunogenicity of the ALVAC-HIV vaccine in 100 healthy patients at low risk of HIV infection.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases and other global health agencies. The authors declared that they had no competing interests.
Source: Gray GE et al. Sci Transl Med. 2019 Sep 18. doi: 10.1126/scitranslmed.aax1880.
Australia’s rotavirus outbreak wasn’t caused by vaccine effectiveness decline
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
FROM PEDIATRICS
Pneumonia with tender, dry, crusted lips
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Hospital-acquired C. diff. tied to four ‘high-risk’ antibiotic classes
The use of four antibiotic classes designated “high risk” was found to be an independent predictor of hospital-acquired Clostridioides difficile (CDI), based upon an analysis of microbiologic and pharmacy data from 171 hospitals in the United States.
The high-risk antibiotic classes were second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides, according to a report by Ying P. Tabak, PhD, of Becton Dickinson in Franklin Lakes, N.J., and colleagues published in Infection Control & Hospital Epidemiology.
Of the 171 study sites studied, 66 (39%) were teaching hospitals and 105 (61%) were nonteaching hospitals. The high-risk antibiotics most frequently used were cephalosporins (47.9%), fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). The sites were distributed across various regions of the United States. The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP).
The study was not able to determine specific links to individual antibiotic classes but to the use of high-risk antibiotics as a whole, except for cephalosporins, which were significantly correlated with hospital-acquired CDI (r = 0.23; P less than .01).
The overall correlation of high-risk antibiotic use and hospital-acquired CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (r = 0.38; P = .002) versus nonteaching hospitals (r = 0.19; P = .055), according to the researchers. The authors attributed this to the possibility of teaching hospitals dealing with more elderly and sicker patients.
After adjusting for significant confounders, the use of high-risk antibiotics was still independently associated with significant risk for hospital-acquired CDI. “For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in [hospital-acquired] CDI (RR, 1.12; 95% [confidence interval], 1.04-1.21; P = .002),” according to the authors. This translated to four additional hospital-acquired CDI cases with every 100 DOT increase per 1,000 DP.
“Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on [hospital-acquired] CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status,” the researchers concluded.
Funding was provided by Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including CDI, and one author was an employee of Nabriva Therapeutics.
SOURCE: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.
The use of four antibiotic classes designated “high risk” was found to be an independent predictor of hospital-acquired Clostridioides difficile (CDI), based upon an analysis of microbiologic and pharmacy data from 171 hospitals in the United States.
The high-risk antibiotic classes were second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides, according to a report by Ying P. Tabak, PhD, of Becton Dickinson in Franklin Lakes, N.J., and colleagues published in Infection Control & Hospital Epidemiology.
Of the 171 study sites studied, 66 (39%) were teaching hospitals and 105 (61%) were nonteaching hospitals. The high-risk antibiotics most frequently used were cephalosporins (47.9%), fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). The sites were distributed across various regions of the United States. The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP).
The study was not able to determine specific links to individual antibiotic classes but to the use of high-risk antibiotics as a whole, except for cephalosporins, which were significantly correlated with hospital-acquired CDI (r = 0.23; P less than .01).
The overall correlation of high-risk antibiotic use and hospital-acquired CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (r = 0.38; P = .002) versus nonteaching hospitals (r = 0.19; P = .055), according to the researchers. The authors attributed this to the possibility of teaching hospitals dealing with more elderly and sicker patients.
After adjusting for significant confounders, the use of high-risk antibiotics was still independently associated with significant risk for hospital-acquired CDI. “For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in [hospital-acquired] CDI (RR, 1.12; 95% [confidence interval], 1.04-1.21; P = .002),” according to the authors. This translated to four additional hospital-acquired CDI cases with every 100 DOT increase per 1,000 DP.
“Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on [hospital-acquired] CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status,” the researchers concluded.
Funding was provided by Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including CDI, and one author was an employee of Nabriva Therapeutics.
SOURCE: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.
The use of four antibiotic classes designated “high risk” was found to be an independent predictor of hospital-acquired Clostridioides difficile (CDI), based upon an analysis of microbiologic and pharmacy data from 171 hospitals in the United States.
The high-risk antibiotic classes were second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides, according to a report by Ying P. Tabak, PhD, of Becton Dickinson in Franklin Lakes, N.J., and colleagues published in Infection Control & Hospital Epidemiology.
Of the 171 study sites studied, 66 (39%) were teaching hospitals and 105 (61%) were nonteaching hospitals. The high-risk antibiotics most frequently used were cephalosporins (47.9%), fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). The sites were distributed across various regions of the United States. The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP).
The study was not able to determine specific links to individual antibiotic classes but to the use of high-risk antibiotics as a whole, except for cephalosporins, which were significantly correlated with hospital-acquired CDI (r = 0.23; P less than .01).
The overall correlation of high-risk antibiotic use and hospital-acquired CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (r = 0.38; P = .002) versus nonteaching hospitals (r = 0.19; P = .055), according to the researchers. The authors attributed this to the possibility of teaching hospitals dealing with more elderly and sicker patients.
After adjusting for significant confounders, the use of high-risk antibiotics was still independently associated with significant risk for hospital-acquired CDI. “For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in [hospital-acquired] CDI (RR, 1.12; 95% [confidence interval], 1.04-1.21; P = .002),” according to the authors. This translated to four additional hospital-acquired CDI cases with every 100 DOT increase per 1,000 DP.
“Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on [hospital-acquired] CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status,” the researchers concluded.
Funding was provided by Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including CDI, and one author was an employee of Nabriva Therapeutics.
SOURCE: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Key clinical point:
Major finding: For every 100-day increase in high-risk antibiotic therapy, there was a 12% increase in hospital-acquired C. difficile.
Study details: Microbiological and pharmacy data from 171 hospitals comparing hospitalwide use of four antibiotics classes on hospital-acquired C. difficile.
Disclosures: Funding was provided Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including C. difficile, and one author was an employee of Nabriva Therapeutics.
Source: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.