User login
Advances in digital otoscopy help improve AOM diagnoses
NEW ORLEANS – The incidence of acute otitis media has decreased by 25% to 35% in the past decade, thanks largely to the widespread and near universal use of the pneumococcal conjugate vaccine, according to Ellen R. Wald, MD.
“To a smaller degree, it is also attributable to the use of influenza vaccine, and to the use of more stringent diagnostic criteria,” Dr. Wald, who chairs the department of pediatrics at the University of Wisconsin, Madison, said at the annual meeting of the American Academy of Pediatrics. “The fact that we are decreasing the number of episodes of otitis media in children in the first year of life means that we’re going to have fewer otitis-prone children and therefore less of a need for tympanostomy tubes, either as a solution to the problem of recurrence of acute otitis media (AOM) or for the problem of persistent effusion.”
said Dr. Wald, pediatrician-in-chief at the American Family Children’s Hospital in Madison. She noted that OME is a nonbacterial inflammatory state that usually resolves spontaneously. It tends to occur before or after AOM, and often without ever progressing to AOM. “Its principal importance is as a cause of hearing loss and as a confounder in the diagnosis AOM,” she explained. “Because it is a nonbacterial process, antibiotics are not indicated in the management of OME. In contrast, children with AOM have a bacterial infection that will benefit from the use of antimicrobials.”*
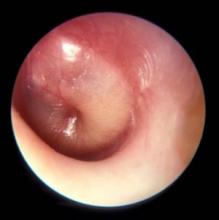
Middle ear effusion is common to both OME and AOM, she continued. To discriminate between the two conditions, clinicians must look for signs of acute inflammation of the tympanic membrane, “which we expect to see in AOM,” she said. “The most powerful sign of inflammation of the tympanic membrane is distinct fullness or bulging of the tympanic membrane on exam.”
Dr. Wald advises clinicians to be as systematic as possible when conducting the otoscopic exam, by looking at color and classifying it as pink, gray, white, yellow, red, amber, or blue, and by documenting the position as neutral, retracted, full, or bulging. “When we gauge how light passes through the tympanic membrane, we judge it as translucent, opaque, or partially opaque, and mobility as normal, decreased, or absent,” she added. “When we find decreased or absent mobility of the tympanic membrane, it tells us that we have fluid in the middle ear, but it does not discriminate between AOM and OME.”
Advances in digital otoscopy are helping pediatricians to improve their diagnostic skills. An early device, the iPhone otoscope by CellScope, uses an iOS smartphone to capture images and videos of the external ear canal and eardrum. “The image is pretty much the same as that seen through the eye of a hand-held otoscope,” Dr. Wald said. “The problem with this particular design is that the speculum is kind of large. It does still require the removal of cerumen, and the smartphone is kind of awkward to use as a handle during an otoscopic exam.”
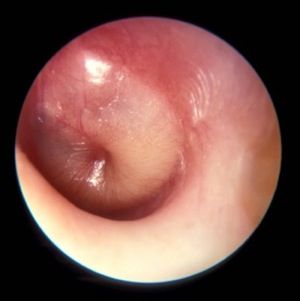
A new digital otoscope called Wispr was unveiled at the AAP meeting. First developed at the University of Wisconsin and now marketed by WiscMed, Wispr delivers high-resolution views of the eardrum in even small or partially obstructed ear canals with one-button image and video capture. WiscMed was founded by Jim Berbee, MD, MBA, an engineer turned emergency medicine physician.
“One of the advantages of this particular model is that it handles a lot more like a usual otoscope and can be attached to the rechargeable handles that are commercially available,” Dr. Wald said. “It has an extremely tiny speculum. Within the head, there is even a smaller camera that allows the photographs to be taken. Because the speculum is so tiny, it allows the device to sometimes avoid the presence of cerumen, or sometimes go through it and still obtain an image.”
Priced at $1,500, the Wispr also features a built-in USB port for computer download of captures images and video. “This way, multiple observers can look at the uploaded image and have an opportunity to view it at greater length,” she said. “Our hope is that the availability of digital otoscopy in the office setting may improve our diagnostic skills and therefore lead to more judicious use of antimicrobials. This remains to be seen. Prospective studies need to be done, but it’s an exciting development,” Dr. Wald said.
She reported having no financial disclosures.
*This article was updated 12/13/2019
NEW ORLEANS – The incidence of acute otitis media has decreased by 25% to 35% in the past decade, thanks largely to the widespread and near universal use of the pneumococcal conjugate vaccine, according to Ellen R. Wald, MD.
“To a smaller degree, it is also attributable to the use of influenza vaccine, and to the use of more stringent diagnostic criteria,” Dr. Wald, who chairs the department of pediatrics at the University of Wisconsin, Madison, said at the annual meeting of the American Academy of Pediatrics. “The fact that we are decreasing the number of episodes of otitis media in children in the first year of life means that we’re going to have fewer otitis-prone children and therefore less of a need for tympanostomy tubes, either as a solution to the problem of recurrence of acute otitis media (AOM) or for the problem of persistent effusion.”
said Dr. Wald, pediatrician-in-chief at the American Family Children’s Hospital in Madison. She noted that OME is a nonbacterial inflammatory state that usually resolves spontaneously. It tends to occur before or after AOM, and often without ever progressing to AOM. “Its principal importance is as a cause of hearing loss and as a confounder in the diagnosis AOM,” she explained. “Because it is a nonbacterial process, antibiotics are not indicated in the management of OME. In contrast, children with AOM have a bacterial infection that will benefit from the use of antimicrobials.”*
Middle ear effusion is common to both OME and AOM, she continued. To discriminate between the two conditions, clinicians must look for signs of acute inflammation of the tympanic membrane, “which we expect to see in AOM,” she said. “The most powerful sign of inflammation of the tympanic membrane is distinct fullness or bulging of the tympanic membrane on exam.”
Dr. Wald advises clinicians to be as systematic as possible when conducting the otoscopic exam, by looking at color and classifying it as pink, gray, white, yellow, red, amber, or blue, and by documenting the position as neutral, retracted, full, or bulging. “When we gauge how light passes through the tympanic membrane, we judge it as translucent, opaque, or partially opaque, and mobility as normal, decreased, or absent,” she added. “When we find decreased or absent mobility of the tympanic membrane, it tells us that we have fluid in the middle ear, but it does not discriminate between AOM and OME.”
Advances in digital otoscopy are helping pediatricians to improve their diagnostic skills. An early device, the iPhone otoscope by CellScope, uses an iOS smartphone to capture images and videos of the external ear canal and eardrum. “The image is pretty much the same as that seen through the eye of a hand-held otoscope,” Dr. Wald said. “The problem with this particular design is that the speculum is kind of large. It does still require the removal of cerumen, and the smartphone is kind of awkward to use as a handle during an otoscopic exam.”
A new digital otoscope called Wispr was unveiled at the AAP meeting. First developed at the University of Wisconsin and now marketed by WiscMed, Wispr delivers high-resolution views of the eardrum in even small or partially obstructed ear canals with one-button image and video capture. WiscMed was founded by Jim Berbee, MD, MBA, an engineer turned emergency medicine physician.
“One of the advantages of this particular model is that it handles a lot more like a usual otoscope and can be attached to the rechargeable handles that are commercially available,” Dr. Wald said. “It has an extremely tiny speculum. Within the head, there is even a smaller camera that allows the photographs to be taken. Because the speculum is so tiny, it allows the device to sometimes avoid the presence of cerumen, or sometimes go through it and still obtain an image.”
Priced at $1,500, the Wispr also features a built-in USB port for computer download of captures images and video. “This way, multiple observers can look at the uploaded image and have an opportunity to view it at greater length,” she said. “Our hope is that the availability of digital otoscopy in the office setting may improve our diagnostic skills and therefore lead to more judicious use of antimicrobials. This remains to be seen. Prospective studies need to be done, but it’s an exciting development,” Dr. Wald said.
She reported having no financial disclosures.
*This article was updated 12/13/2019
NEW ORLEANS – The incidence of acute otitis media has decreased by 25% to 35% in the past decade, thanks largely to the widespread and near universal use of the pneumococcal conjugate vaccine, according to Ellen R. Wald, MD.
“To a smaller degree, it is also attributable to the use of influenza vaccine, and to the use of more stringent diagnostic criteria,” Dr. Wald, who chairs the department of pediatrics at the University of Wisconsin, Madison, said at the annual meeting of the American Academy of Pediatrics. “The fact that we are decreasing the number of episodes of otitis media in children in the first year of life means that we’re going to have fewer otitis-prone children and therefore less of a need for tympanostomy tubes, either as a solution to the problem of recurrence of acute otitis media (AOM) or for the problem of persistent effusion.”
said Dr. Wald, pediatrician-in-chief at the American Family Children’s Hospital in Madison. She noted that OME is a nonbacterial inflammatory state that usually resolves spontaneously. It tends to occur before or after AOM, and often without ever progressing to AOM. “Its principal importance is as a cause of hearing loss and as a confounder in the diagnosis AOM,” she explained. “Because it is a nonbacterial process, antibiotics are not indicated in the management of OME. In contrast, children with AOM have a bacterial infection that will benefit from the use of antimicrobials.”*
Middle ear effusion is common to both OME and AOM, she continued. To discriminate between the two conditions, clinicians must look for signs of acute inflammation of the tympanic membrane, “which we expect to see in AOM,” she said. “The most powerful sign of inflammation of the tympanic membrane is distinct fullness or bulging of the tympanic membrane on exam.”
Dr. Wald advises clinicians to be as systematic as possible when conducting the otoscopic exam, by looking at color and classifying it as pink, gray, white, yellow, red, amber, or blue, and by documenting the position as neutral, retracted, full, or bulging. “When we gauge how light passes through the tympanic membrane, we judge it as translucent, opaque, or partially opaque, and mobility as normal, decreased, or absent,” she added. “When we find decreased or absent mobility of the tympanic membrane, it tells us that we have fluid in the middle ear, but it does not discriminate between AOM and OME.”
Advances in digital otoscopy are helping pediatricians to improve their diagnostic skills. An early device, the iPhone otoscope by CellScope, uses an iOS smartphone to capture images and videos of the external ear canal and eardrum. “The image is pretty much the same as that seen through the eye of a hand-held otoscope,” Dr. Wald said. “The problem with this particular design is that the speculum is kind of large. It does still require the removal of cerumen, and the smartphone is kind of awkward to use as a handle during an otoscopic exam.”
A new digital otoscope called Wispr was unveiled at the AAP meeting. First developed at the University of Wisconsin and now marketed by WiscMed, Wispr delivers high-resolution views of the eardrum in even small or partially obstructed ear canals with one-button image and video capture. WiscMed was founded by Jim Berbee, MD, MBA, an engineer turned emergency medicine physician.
“One of the advantages of this particular model is that it handles a lot more like a usual otoscope and can be attached to the rechargeable handles that are commercially available,” Dr. Wald said. “It has an extremely tiny speculum. Within the head, there is even a smaller camera that allows the photographs to be taken. Because the speculum is so tiny, it allows the device to sometimes avoid the presence of cerumen, or sometimes go through it and still obtain an image.”
Priced at $1,500, the Wispr also features a built-in USB port for computer download of captures images and video. “This way, multiple observers can look at the uploaded image and have an opportunity to view it at greater length,” she said. “Our hope is that the availability of digital otoscopy in the office setting may improve our diagnostic skills and therefore lead to more judicious use of antimicrobials. This remains to be seen. Prospective studies need to be done, but it’s an exciting development,” Dr. Wald said.
She reported having no financial disclosures.
*This article was updated 12/13/2019
EXPERT ANALYSIS AT AAP 19
Hepatitis B debrief: Key themes that emerged at AASLD
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
BOSTON – Some of the most notable abstracts presented here at the annual meeting of the American Association for the Study of Liver Diseases dealt with key topics including the natural history of hepatitis B virus, novel treatment approaches, and prevention, according to Marc Ghany, MD.
During a special hepatitis debriefing session held on the last day of the conference, Dr. Ghany reviewed his key selections in hepatitis B virus (HBV) research, including the following:
Natural history
Steatohepatitis may worsen HBV-related liver injury, according to results of an analysis of liver biopsies from adult patients enrolled in a North American cohort study (Abstract 162). Investigators found that steatohepatitis was associated with a 1.6-fold increased risk of advanced fibrosis.
“For all patients with hepatitis B, I think it’s important to screen and manage metabolic abnormalities to prevent liver disease progression,” said Dr. Ghany, who is with the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.
Risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B was evaluated in two notable studies, Dr. Ghany said, including one that found no difference in risk of HCC development among white patients who received long-term entecavir versus those who received long-term tenofovir (Abstract 454). This stands in contrast to a previous and controversial finding, according to investigators, that HCC incidence was lower in Asian patients treated with tenofovir versus those treated with entecavir.
In the other study, investigators found that dual-antiplatelet therapy (DAPT) was associated with a lower risk of HCC versus aspirin monotherapy in patients with chronic hepatitis B (Abstract 934). Antiplatelet therapy was associated with a near 20% reduction in HCC risk, while DAPT as compared with aspirin monotherapy was linked to a near 30% reduction. “These are very provocative findings,” Dr. Ghany said. “Of course they need to be confirmed, but if so, may open new avenues of HCC chemoprevention.”
Novel therapies
Several new and promising drugs are undergoing clinical trials, including JNJ-64530440 (JNJ-0440), a novel class N capsid assembly modulator. In phase 1a data presented here at The Liver Meeting, the treatment was safe, well tolerated, and resulted in potent inhibition of viral replication (Abstract 0089). “We await further studies on its effect on functional cure,” Dr. Ghany told attendees.
Another treatment to watch is GSK3389404 (GSK404), a liver-targeted antisense oligonucleotide; in a phase 2 placebo-controlled study in patients with chronic hepatitis B on stable nucleos(t)ide therapy, this treatment had acceptable safety and produced dose-dependent declines in hepatitis B surface antigen (HBsAg), according to investigators (Abstract 0695). Dr. Ghany said this constitutes “proof of principle” that antisense oligonucleotides can decrease HBsAg levels.
In a phase 2 randomized, placebo-controlled study in virally suppressed adults with chronic hepatitis B, the toll-like receptor 8 (TLR8) agonist GS-9688 was safe and well tolerated, and resulted in dose-dependent pharmacodynamic changes, with 5% of patients experiencing a 1 log10 IU/mL or greater decline in HBsAg levels or an HBsAg loss by week 24 (Abstract 0697). This is a “promising approach” that merits further study, according to Dr. Ghany.
Prevention: Vaccination and screening
A trivalent HBV vaccine is superior to monovalent vaccine, according to results from the double-blind, randomized, controlled, phase 3 PROTECT study presented here at the meeting (Abstract LP13). Known as Sci-B-Vac, the mammalian cell-derived trivalent vaccine had higher response rates versus the recombinant monovalent vaccine Engerix-B in difficult-to-vaccinate populations, according to Dr. Ghany.
A separate report based on a national health insurance cohort study in Korea demonstrated that regular follow-up, that is to say, every 3-6 months, significantly reduced liver cancer–related mortality (Abstract 0159). Patients compliant with screening in the study not only had a 44% reduction in risk of death from HCC, but also were more likely to receive curative treatments (23.1% versus 15.1%). “Notwithstanding the limitations or cohort studies, I think these data reinforce the need to screen patients with chronic hepatitis B,” Dr. Ghany said.
He provided no disclosures in his presentation.
REPORTING FROM THE LIVER MEETING 2019
HVPG predicts clinical benefit after sustained virologic response
BOSTON – For patients with hepatitis C virus infection who achieve sustained virologic response to interferon-free therapy, changes in hepatic venous pressure gradient (HVPG) predict clinical benefit, according to investigators.
This finding will allow investigators to use HVPG as a surrogate endpoint for etiologic therapies, which could accelerate future research, reported lead author Mattias Mandorfer, MD, PhD, of the Medical University of Vienna and colleagues.
“Sustained virologic response to interferon-free therapies ameliorates portal hypertension,” Dr. Mandorfer said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “[Previous research has shown that] nearly two-thirds of patients with pretreatment clinically significant portal hypertension had an HVPG decrease above or equal to 10%, which denotes a clinically meaningful change according to current recommendations. However, evidence is limited to studies evaluating the impact of HVPG response to nonselective beta-blockers, and nonselective beta-blockers have a completely different mode of action than etiological therapies. Accordingly, it is unclear whether a decrease in HVPG after the cure of hepatitis C translates into the same clinical benefit.”
To find out, the investigators enrolled 90 patients with hepatitis C virus who had an elevated HVPG of 6 mm Hg or higher prior to sustained virologic response. Before and after interferon-free therapy, patients underwent paired HVPG measurement. In addition, to evaluate noninvasive methods of HVPG assessment, transient elastography and von Willebrand factor to platelet count ratio testing were performed.
Analysis showed that HVPG measurements after, but not before, interferon-free therapy predicted liver decompensation. Specifically, HVPG was associated with an 18% increased risk of hepatic decompensation per mm Hg. After 3 years, 40.1% of patients with posttherapy HVPG measurements of 16 mm Hg or more developed hepatic decompensation, an event that occurred in none of the patients with a posttherapy HVPG of 9 mm Hg or less. Among patients who had a baseline HVPG of 10 mm Hg or more, which is considered a clinically significant level of portal hypertension, a decrease in HVPG of least 10% after therapy was associated with a similar level of protection against decompensation, compared with those who had no such decrease (2.5% vs. 31.8%).
While the two noninvasive methods (transient elastography and von Willebrand factor to platelet count ratio) were able to detect clinically significant portal hypertension (at least 10 mm Hg), they were not accurate enough to detect the protective 10% drop in HVPG.
“These results support the concept of applying HVPG as a surrogate endpoint for interventions that primarily aim at decreasing intrahepatic resistance (e.g., etiological therapies),” the investigators concluded in their abstract.
Jaime Bosch, MD, PhD, of the University of Barcelona provided some expert insight into the findings.
“The significance of the work is very important,” Dr. Bosch said in a public comment. “This provides, for the first time, firm evidence that HVPG can be taken as a surrogate endpoint ... for studies involving portal hypertension and cirrhosis in general.”
In an interview, Dr. Bosch elaborated on this statement. “The problem is, it takes a long time to get rid of cirrhosis [after sustained virologic response], and meanwhile, as long as portal hypertension remains, there is a risk for decompensation, so the patients cannot be said to be cured. They are cured of the infection, of the consequences of the infection, but it may take 10 years or more [to resolve cirrhosis], so the patient needs clinical surveillance and treatment after curing the cause of the disease.
“An academic consequence of these findings is that they’ve proved that decreasing HVPG by means of achieving sustained virologic response is followed by an improvement in prognosis. ... And when you can influence prognosis, and the influence in prognosis is reflected by a measurement independent from the way that we achieve this effect on the measurement, it means that this measurement is robust and now has to be used as a surrogate marker of resolution of cirrhosis.”
The study was funded by the Medical Scientific Fund of the city of Vienna. The investigators disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead, and others.
SOURCE: Mandorfer M et al. The Liver Meeting 2019, Abstract 146.
BOSTON – For patients with hepatitis C virus infection who achieve sustained virologic response to interferon-free therapy, changes in hepatic venous pressure gradient (HVPG) predict clinical benefit, according to investigators.
This finding will allow investigators to use HVPG as a surrogate endpoint for etiologic therapies, which could accelerate future research, reported lead author Mattias Mandorfer, MD, PhD, of the Medical University of Vienna and colleagues.
“Sustained virologic response to interferon-free therapies ameliorates portal hypertension,” Dr. Mandorfer said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “[Previous research has shown that] nearly two-thirds of patients with pretreatment clinically significant portal hypertension had an HVPG decrease above or equal to 10%, which denotes a clinically meaningful change according to current recommendations. However, evidence is limited to studies evaluating the impact of HVPG response to nonselective beta-blockers, and nonselective beta-blockers have a completely different mode of action than etiological therapies. Accordingly, it is unclear whether a decrease in HVPG after the cure of hepatitis C translates into the same clinical benefit.”
To find out, the investigators enrolled 90 patients with hepatitis C virus who had an elevated HVPG of 6 mm Hg or higher prior to sustained virologic response. Before and after interferon-free therapy, patients underwent paired HVPG measurement. In addition, to evaluate noninvasive methods of HVPG assessment, transient elastography and von Willebrand factor to platelet count ratio testing were performed.
Analysis showed that HVPG measurements after, but not before, interferon-free therapy predicted liver decompensation. Specifically, HVPG was associated with an 18% increased risk of hepatic decompensation per mm Hg. After 3 years, 40.1% of patients with posttherapy HVPG measurements of 16 mm Hg or more developed hepatic decompensation, an event that occurred in none of the patients with a posttherapy HVPG of 9 mm Hg or less. Among patients who had a baseline HVPG of 10 mm Hg or more, which is considered a clinically significant level of portal hypertension, a decrease in HVPG of least 10% after therapy was associated with a similar level of protection against decompensation, compared with those who had no such decrease (2.5% vs. 31.8%).
While the two noninvasive methods (transient elastography and von Willebrand factor to platelet count ratio) were able to detect clinically significant portal hypertension (at least 10 mm Hg), they were not accurate enough to detect the protective 10% drop in HVPG.
“These results support the concept of applying HVPG as a surrogate endpoint for interventions that primarily aim at decreasing intrahepatic resistance (e.g., etiological therapies),” the investigators concluded in their abstract.
Jaime Bosch, MD, PhD, of the University of Barcelona provided some expert insight into the findings.
“The significance of the work is very important,” Dr. Bosch said in a public comment. “This provides, for the first time, firm evidence that HVPG can be taken as a surrogate endpoint ... for studies involving portal hypertension and cirrhosis in general.”
In an interview, Dr. Bosch elaborated on this statement. “The problem is, it takes a long time to get rid of cirrhosis [after sustained virologic response], and meanwhile, as long as portal hypertension remains, there is a risk for decompensation, so the patients cannot be said to be cured. They are cured of the infection, of the consequences of the infection, but it may take 10 years or more [to resolve cirrhosis], so the patient needs clinical surveillance and treatment after curing the cause of the disease.
“An academic consequence of these findings is that they’ve proved that decreasing HVPG by means of achieving sustained virologic response is followed by an improvement in prognosis. ... And when you can influence prognosis, and the influence in prognosis is reflected by a measurement independent from the way that we achieve this effect on the measurement, it means that this measurement is robust and now has to be used as a surrogate marker of resolution of cirrhosis.”
The study was funded by the Medical Scientific Fund of the city of Vienna. The investigators disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead, and others.
SOURCE: Mandorfer M et al. The Liver Meeting 2019, Abstract 146.
BOSTON – For patients with hepatitis C virus infection who achieve sustained virologic response to interferon-free therapy, changes in hepatic venous pressure gradient (HVPG) predict clinical benefit, according to investigators.
This finding will allow investigators to use HVPG as a surrogate endpoint for etiologic therapies, which could accelerate future research, reported lead author Mattias Mandorfer, MD, PhD, of the Medical University of Vienna and colleagues.
“Sustained virologic response to interferon-free therapies ameliorates portal hypertension,” Dr. Mandorfer said during a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “[Previous research has shown that] nearly two-thirds of patients with pretreatment clinically significant portal hypertension had an HVPG decrease above or equal to 10%, which denotes a clinically meaningful change according to current recommendations. However, evidence is limited to studies evaluating the impact of HVPG response to nonselective beta-blockers, and nonselective beta-blockers have a completely different mode of action than etiological therapies. Accordingly, it is unclear whether a decrease in HVPG after the cure of hepatitis C translates into the same clinical benefit.”
To find out, the investigators enrolled 90 patients with hepatitis C virus who had an elevated HVPG of 6 mm Hg or higher prior to sustained virologic response. Before and after interferon-free therapy, patients underwent paired HVPG measurement. In addition, to evaluate noninvasive methods of HVPG assessment, transient elastography and von Willebrand factor to platelet count ratio testing were performed.
Analysis showed that HVPG measurements after, but not before, interferon-free therapy predicted liver decompensation. Specifically, HVPG was associated with an 18% increased risk of hepatic decompensation per mm Hg. After 3 years, 40.1% of patients with posttherapy HVPG measurements of 16 mm Hg or more developed hepatic decompensation, an event that occurred in none of the patients with a posttherapy HVPG of 9 mm Hg or less. Among patients who had a baseline HVPG of 10 mm Hg or more, which is considered a clinically significant level of portal hypertension, a decrease in HVPG of least 10% after therapy was associated with a similar level of protection against decompensation, compared with those who had no such decrease (2.5% vs. 31.8%).
While the two noninvasive methods (transient elastography and von Willebrand factor to platelet count ratio) were able to detect clinically significant portal hypertension (at least 10 mm Hg), they were not accurate enough to detect the protective 10% drop in HVPG.
“These results support the concept of applying HVPG as a surrogate endpoint for interventions that primarily aim at decreasing intrahepatic resistance (e.g., etiological therapies),” the investigators concluded in their abstract.
Jaime Bosch, MD, PhD, of the University of Barcelona provided some expert insight into the findings.
“The significance of the work is very important,” Dr. Bosch said in a public comment. “This provides, for the first time, firm evidence that HVPG can be taken as a surrogate endpoint ... for studies involving portal hypertension and cirrhosis in general.”
In an interview, Dr. Bosch elaborated on this statement. “The problem is, it takes a long time to get rid of cirrhosis [after sustained virologic response], and meanwhile, as long as portal hypertension remains, there is a risk for decompensation, so the patients cannot be said to be cured. They are cured of the infection, of the consequences of the infection, but it may take 10 years or more [to resolve cirrhosis], so the patient needs clinical surveillance and treatment after curing the cause of the disease.
“An academic consequence of these findings is that they’ve proved that decreasing HVPG by means of achieving sustained virologic response is followed by an improvement in prognosis. ... And when you can influence prognosis, and the influence in prognosis is reflected by a measurement independent from the way that we achieve this effect on the measurement, it means that this measurement is robust and now has to be used as a surrogate marker of resolution of cirrhosis.”
The study was funded by the Medical Scientific Fund of the city of Vienna. The investigators disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead, and others.
SOURCE: Mandorfer M et al. The Liver Meeting 2019, Abstract 146.
REPORTING FROM THE LIVER MEETING 2019
Treatment of recurrent C. difficile infection
FMT is an option for some patients
The case
A 67-year-old woman with a past medical history significant for diabetes mellitus type 2 and chronic kidney disease stage 3 was recently hospitalized for a community acquired pneumonia and treated for 5 days with moxifloxacin. In the week following this hospitalization, she began to have watery diarrhea and was found to have Clostridioides difficile diarrhea. She was treated with 10 days of oral vancomycin for her C. difficile infection (CDI). Approximately 3 weeks later, she again developed watery diarrhea with some abdominal cramping and has a leukocyte count of 22.4.
Key clinical questions
When is C. difficile considered recurrent?
C. difficile is considered recurrent when a patient experiences symptom onset and has a positive test in the 2-8 week period following the resolution of symptoms from the previous episode that had been confirmed with a positive test.1
What is the recurrence rate for C. difficile?
Of patients who are initially diagnosed with C. difficile, about 20%-35% develop recurrence of their infection, and of those who experience recurrence, roughly 40%-60% will experience a second recurrence.2
What are the risk factors for recurrent C. difficile?
Risk factors for recurrence of C. difficile include older age (older than 65 years), female sex, Caucasian ethnicity, ongoing antibiotic use, concurrent proton pump inhibitor use, and more severe initial disease.
Also, receiving antineoplastic chemotherapy, being an organ transplant recipient, chronic kidney disease, inflammatory bowel disease, hypogammaglobulinemia, or other immunodeficiency, as well as having exposure to infected adult or infant carrier of C. difficile have all been risk factors for recurrent disease. There is still some degree of ongoing controversy over the role of proton pump inhibitors as a risk factor.2
What are the treatment options for initial C. difficile infection?
The recent Infectious Diseases Society of America (IDSA) guidelines recommend treating for an initial CDI with a 10-day course of oral vancomycin or fidaxomicin instead of metronidazole. This change is based on a combined analysis of two large randomized controlled trials that demonstrated better clinical response rates with vancomycin, compared with metronidazole (81.1% vs. 72.7%; P = .002).1,3
What are the treatment options for first recurrence?
The data is overall limited in treatment of first recurrence of CDI. The IDSA guidelines recommend that a first recurrence of CDI may be treated with oral vancomycin followed by a tapered and pulsed regimen or with a 10-day course of fidaxomicin. If metronidazole was used for the first episode, a 10-day course of vancomycin can be used.1
What are the treatment options for second and subsequent recurrences?
Second or subsequent CDI recurrences may be treated with oral vancomycin as a tapered and pulsed-dose regimen or with fidaxomicin as described above, but this is based on low quality of evidence.
The IDSA guidelines strongly recommend fecal microbiota transplantation (FMT) for patients who have two or more C. difficile recurrences and in whom standard antibiotic treatment has not been successful. FMT has demonstrated high efficacy rates of 80%-90% for clinical remission of recurrent CDI.
FMT can be administered through various routes. The choice of delivery depends in part on local expertise, patient preference, cost, and risk of the procedure.1,4,5,6
What new therapies exist for reducing recurrence?
Bezlotoxumab is a humanized monoclonal antibody directed against C. difficile toxin B that was approved by the Food and Drug Administration in 2016 for prevention of recurrent CDI. Randomized placebo-controlled trials demonstrated that a single infusion of bezlotoxumab, given in combination with usual antibiotics for CDI in adults, was effective in reducing CDI recurrence within 12 weeks (rate of recurrent infection in both trials was 16.5% in the bezlotoxumab groups and 26.6% in the placebo groups).
In a post hoc analysis, the highest benefit was in patients with three or more risk factors: older than 65 years, history of CDI, immunocompromised status, or severe CDI. Although the best strategy for prevention of CDI recurrence remains to be determined, bezlotoxumab remains an option.7,8
Back to the case
The patient had a C. difficile polymerase chain reaction test sent that came back positive for C. difficile. Because she had previously been treated with a 10-day course of oral vancomycin, she was started on a tapered and pulsed-dose regimen of oral vancomycin. Five days later her diarrhea resolved, and her leukocyte count returned to normal.
Dr. Bell is associate clinical professor in the division of hospital medicine at the University of California, San Diego, Medical Center. Dr. Farkhondehpour is a hospitalist and assistant clinical professor at UC San Diego Health.
References
1. McDonald L et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66(7):987-994.
2. Hopkins R and Wilson R. Treatment of recurrent Clostridium difficile colitis: A narrative review. Gastroenterol Rep (Oxf). 2018 Feb;6(1):21-8.
3. Johnson S et al. (2014). Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014 Aug 1;59(3):345-54.
4. van Nood E et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013 Jan 31;368(5):407-15.
5. Cammarota G et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015 May;41(9):835-43.
6. Kelly C et al. Effect of fecal microbiota transplantation on recurrence in multiple recurrent Clostridium difficile infection. Ann Intern Med. 2016 Nov 1;165(9):609-16.
7. Gerding DN et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018 Aug 16;67(5):649-56.
8. Wilcox M et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017 Jan 26;376(4):305-17.
FMT is an option for some patients
FMT is an option for some patients
The case
A 67-year-old woman with a past medical history significant for diabetes mellitus type 2 and chronic kidney disease stage 3 was recently hospitalized for a community acquired pneumonia and treated for 5 days with moxifloxacin. In the week following this hospitalization, she began to have watery diarrhea and was found to have Clostridioides difficile diarrhea. She was treated with 10 days of oral vancomycin for her C. difficile infection (CDI). Approximately 3 weeks later, she again developed watery diarrhea with some abdominal cramping and has a leukocyte count of 22.4.
Key clinical questions
When is C. difficile considered recurrent?
C. difficile is considered recurrent when a patient experiences symptom onset and has a positive test in the 2-8 week period following the resolution of symptoms from the previous episode that had been confirmed with a positive test.1
What is the recurrence rate for C. difficile?
Of patients who are initially diagnosed with C. difficile, about 20%-35% develop recurrence of their infection, and of those who experience recurrence, roughly 40%-60% will experience a second recurrence.2
What are the risk factors for recurrent C. difficile?
Risk factors for recurrence of C. difficile include older age (older than 65 years), female sex, Caucasian ethnicity, ongoing antibiotic use, concurrent proton pump inhibitor use, and more severe initial disease.
Also, receiving antineoplastic chemotherapy, being an organ transplant recipient, chronic kidney disease, inflammatory bowel disease, hypogammaglobulinemia, or other immunodeficiency, as well as having exposure to infected adult or infant carrier of C. difficile have all been risk factors for recurrent disease. There is still some degree of ongoing controversy over the role of proton pump inhibitors as a risk factor.2
What are the treatment options for initial C. difficile infection?
The recent Infectious Diseases Society of America (IDSA) guidelines recommend treating for an initial CDI with a 10-day course of oral vancomycin or fidaxomicin instead of metronidazole. This change is based on a combined analysis of two large randomized controlled trials that demonstrated better clinical response rates with vancomycin, compared with metronidazole (81.1% vs. 72.7%; P = .002).1,3
What are the treatment options for first recurrence?
The data is overall limited in treatment of first recurrence of CDI. The IDSA guidelines recommend that a first recurrence of CDI may be treated with oral vancomycin followed by a tapered and pulsed regimen or with a 10-day course of fidaxomicin. If metronidazole was used for the first episode, a 10-day course of vancomycin can be used.1
What are the treatment options for second and subsequent recurrences?
Second or subsequent CDI recurrences may be treated with oral vancomycin as a tapered and pulsed-dose regimen or with fidaxomicin as described above, but this is based on low quality of evidence.
The IDSA guidelines strongly recommend fecal microbiota transplantation (FMT) for patients who have two or more C. difficile recurrences and in whom standard antibiotic treatment has not been successful. FMT has demonstrated high efficacy rates of 80%-90% for clinical remission of recurrent CDI.
FMT can be administered through various routes. The choice of delivery depends in part on local expertise, patient preference, cost, and risk of the procedure.1,4,5,6
What new therapies exist for reducing recurrence?
Bezlotoxumab is a humanized monoclonal antibody directed against C. difficile toxin B that was approved by the Food and Drug Administration in 2016 for prevention of recurrent CDI. Randomized placebo-controlled trials demonstrated that a single infusion of bezlotoxumab, given in combination with usual antibiotics for CDI in adults, was effective in reducing CDI recurrence within 12 weeks (rate of recurrent infection in both trials was 16.5% in the bezlotoxumab groups and 26.6% in the placebo groups).
In a post hoc analysis, the highest benefit was in patients with three or more risk factors: older than 65 years, history of CDI, immunocompromised status, or severe CDI. Although the best strategy for prevention of CDI recurrence remains to be determined, bezlotoxumab remains an option.7,8
Back to the case
The patient had a C. difficile polymerase chain reaction test sent that came back positive for C. difficile. Because she had previously been treated with a 10-day course of oral vancomycin, she was started on a tapered and pulsed-dose regimen of oral vancomycin. Five days later her diarrhea resolved, and her leukocyte count returned to normal.
Dr. Bell is associate clinical professor in the division of hospital medicine at the University of California, San Diego, Medical Center. Dr. Farkhondehpour is a hospitalist and assistant clinical professor at UC San Diego Health.
References
1. McDonald L et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66(7):987-994.
2. Hopkins R and Wilson R. Treatment of recurrent Clostridium difficile colitis: A narrative review. Gastroenterol Rep (Oxf). 2018 Feb;6(1):21-8.
3. Johnson S et al. (2014). Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014 Aug 1;59(3):345-54.
4. van Nood E et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013 Jan 31;368(5):407-15.
5. Cammarota G et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015 May;41(9):835-43.
6. Kelly C et al. Effect of fecal microbiota transplantation on recurrence in multiple recurrent Clostridium difficile infection. Ann Intern Med. 2016 Nov 1;165(9):609-16.
7. Gerding DN et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018 Aug 16;67(5):649-56.
8. Wilcox M et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017 Jan 26;376(4):305-17.
The case
A 67-year-old woman with a past medical history significant for diabetes mellitus type 2 and chronic kidney disease stage 3 was recently hospitalized for a community acquired pneumonia and treated for 5 days with moxifloxacin. In the week following this hospitalization, she began to have watery diarrhea and was found to have Clostridioides difficile diarrhea. She was treated with 10 days of oral vancomycin for her C. difficile infection (CDI). Approximately 3 weeks later, she again developed watery diarrhea with some abdominal cramping and has a leukocyte count of 22.4.
Key clinical questions
When is C. difficile considered recurrent?
C. difficile is considered recurrent when a patient experiences symptom onset and has a positive test in the 2-8 week period following the resolution of symptoms from the previous episode that had been confirmed with a positive test.1
What is the recurrence rate for C. difficile?
Of patients who are initially diagnosed with C. difficile, about 20%-35% develop recurrence of their infection, and of those who experience recurrence, roughly 40%-60% will experience a second recurrence.2
What are the risk factors for recurrent C. difficile?
Risk factors for recurrence of C. difficile include older age (older than 65 years), female sex, Caucasian ethnicity, ongoing antibiotic use, concurrent proton pump inhibitor use, and more severe initial disease.
Also, receiving antineoplastic chemotherapy, being an organ transplant recipient, chronic kidney disease, inflammatory bowel disease, hypogammaglobulinemia, or other immunodeficiency, as well as having exposure to infected adult or infant carrier of C. difficile have all been risk factors for recurrent disease. There is still some degree of ongoing controversy over the role of proton pump inhibitors as a risk factor.2
What are the treatment options for initial C. difficile infection?
The recent Infectious Diseases Society of America (IDSA) guidelines recommend treating for an initial CDI with a 10-day course of oral vancomycin or fidaxomicin instead of metronidazole. This change is based on a combined analysis of two large randomized controlled trials that demonstrated better clinical response rates with vancomycin, compared with metronidazole (81.1% vs. 72.7%; P = .002).1,3
What are the treatment options for first recurrence?
The data is overall limited in treatment of first recurrence of CDI. The IDSA guidelines recommend that a first recurrence of CDI may be treated with oral vancomycin followed by a tapered and pulsed regimen or with a 10-day course of fidaxomicin. If metronidazole was used for the first episode, a 10-day course of vancomycin can be used.1
What are the treatment options for second and subsequent recurrences?
Second or subsequent CDI recurrences may be treated with oral vancomycin as a tapered and pulsed-dose regimen or with fidaxomicin as described above, but this is based on low quality of evidence.
The IDSA guidelines strongly recommend fecal microbiota transplantation (FMT) for patients who have two or more C. difficile recurrences and in whom standard antibiotic treatment has not been successful. FMT has demonstrated high efficacy rates of 80%-90% for clinical remission of recurrent CDI.
FMT can be administered through various routes. The choice of delivery depends in part on local expertise, patient preference, cost, and risk of the procedure.1,4,5,6
What new therapies exist for reducing recurrence?
Bezlotoxumab is a humanized monoclonal antibody directed against C. difficile toxin B that was approved by the Food and Drug Administration in 2016 for prevention of recurrent CDI. Randomized placebo-controlled trials demonstrated that a single infusion of bezlotoxumab, given in combination with usual antibiotics for CDI in adults, was effective in reducing CDI recurrence within 12 weeks (rate of recurrent infection in both trials was 16.5% in the bezlotoxumab groups and 26.6% in the placebo groups).
In a post hoc analysis, the highest benefit was in patients with three or more risk factors: older than 65 years, history of CDI, immunocompromised status, or severe CDI. Although the best strategy for prevention of CDI recurrence remains to be determined, bezlotoxumab remains an option.7,8
Back to the case
The patient had a C. difficile polymerase chain reaction test sent that came back positive for C. difficile. Because she had previously been treated with a 10-day course of oral vancomycin, she was started on a tapered and pulsed-dose regimen of oral vancomycin. Five days later her diarrhea resolved, and her leukocyte count returned to normal.
Dr. Bell is associate clinical professor in the division of hospital medicine at the University of California, San Diego, Medical Center. Dr. Farkhondehpour is a hospitalist and assistant clinical professor at UC San Diego Health.
References
1. McDonald L et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66(7):987-994.
2. Hopkins R and Wilson R. Treatment of recurrent Clostridium difficile colitis: A narrative review. Gastroenterol Rep (Oxf). 2018 Feb;6(1):21-8.
3. Johnson S et al. (2014). Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014 Aug 1;59(3):345-54.
4. van Nood E et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013 Jan 31;368(5):407-15.
5. Cammarota G et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015 May;41(9):835-43.
6. Kelly C et al. Effect of fecal microbiota transplantation on recurrence in multiple recurrent Clostridium difficile infection. Ann Intern Med. 2016 Nov 1;165(9):609-16.
7. Gerding DN et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018 Aug 16;67(5):649-56.
8. Wilcox M et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017 Jan 26;376(4):305-17.
Free HIV self-tests for at-risk groups can increase awareness, testing frequency
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Providing free HIV self-tests can lead to increased testing and more newly identified infections.
Major finding: About 77% of participants in the self-testing group tested three times or more in 12 months, compared with 22% of controls.
Study details: A 12-month longitudinal, two-group, randomized clinical trial of 2,665 men who have sex with men.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network.
Source: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
FDA approves cefiderocol for multidrug-resistant, complicated urinary tract infections
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
FROM THE FDA
Without action, every child will be affected by climate change
As wildfires increase the likelihood of respiratory illnesses for residents in California and Queensland, Australia, a new report from the Lancet warns that such health risks will become increasingly common without action to address climate change. But, the authors stressed, it’s still possible to prevent some health effects and mitigate others.
Given the magnitude of the issue, lead author Nick Watts, MBBS, MA, framed the issue in terms of what an individual child born today will face in his or her future. If the world continues on its current trajectory, such a child will eventually live in a world at least 4º C above average preindustrial temperatures.
“We roughly know what that looks like from a climate perspective,” said Dr. Watts, executive director of The Lancet Countdown: Tracking Progress on Health and Climate Change, during a telebriefing on the report.
“We have no idea of what that looks like from a public health perspective, but we know it is catastrophic,” he continued. “We know that it has the potential to undermine the last 50 years of gains in public health and overwhelm the health systems that we rely on.”
Health sector a significant, growing contributor
The report described the changes to which climate change has already contributed and addresses both the health threats and the way institutions and states are currently responding to those threats. It also included policy briefs specific to individual countries and an extensive appendix with projections data.
The authors noted that progress in mitigating fossil fuel combustion – the biggest driver of rising temperatures – is “intermittent at best,” with carbon dioxide emissions continuing to rise in 2018. The past decade has included 8 of the 10 hottest years on record. “Many of the indicators contained in this report suggest the world is following this ‘business as usual’ pathway,” the authors wrote.
In fact, the trend of coal-produced energy that had been declining actually increased 1.7% between 2016 and 2018. Perhaps ironically, given the focus of the report, “the healthcare sector is responsible for about 4.6% of global emissions, a value which is steadily rising across most major economies,” Dr. Watts and colleagues reported.
The potential health risks from climate change range from increased chronic illness, such as asthma and cardiovascular disease, to the increased spread of infectious diseases, especially vector-borne diseases, including dengue fever, malaria, and chikungunya. Increases in the frequency and intensity of severe weather events can lead to increased acute and longer-term morbidity and mortality.
Though children will suffer the brunt of negative health impact from climate change, the effects will touch people at every stage of life, from in utero development through old age, the authors emphasized.
“Downward trends in global yield potential for all major crops tracked since 1960 threaten food production and food security, with infants often the worst affected by the potentially permanent effects of undernutrition,” the authors reported. Children are also most susceptible to diarrheal disease and infectious diseases, particularly dengue.
Mitigating actions available
But the report focused as much on solutions and mitigation strategies as it did on the worst-case scenario without action. Speakers during the telebriefing emphasized the responsibility of all people, including physicians and other health care providers, to play a role in countering the public health disaster that could result from inaction on climate.
“Thankfully, here we have the treatment for climate change, solutions to shift away from the carbon pollution and towards clean energy and working to find the best way to protect ourselves and each other from climate change,” Renee N. Salas, MD, MPH, lead author of the 2019 Lancet Countdown U.S. Policy Brief and a Harvard C-CHANGE Fellow, said during the press briefing. “All we need is political will.”
Salas compared the present moment to that period when a physician still has the ability to save a critically ill patient’s life with fast action.
“If I don’t act quickly, the patient may still die even though that treatment would have saved their life earlier,” she said. “We are in that narrow window.”
Physicians have a responsibility to speak to patients and families frankly about not only specific conditions, such as asthma, but also the climate-related causes of those conditions, such as increasing air pollution, said Gina McCarthy, director of the Harvard Center for Climate, Health and the Global Environment and the 13th administrator U.S. Environmental Policy Administration. Physicians are trusted advisers and therefore need to speak up because climate change is “about the health and well-being and the future of children,” she said.
Political polarization is one of the biggest challenges to addressing climate change and stymies efforts to take action, according to Richard Carmona, MD, who served as the 17th U.S. Surgeon General.
“The thing that frustrated me as a surgeon general and continues to frustrate me today is that these very scientifically vetted issues are reduced to political currency that creates divisiveness, and things don’t get done,” he said during the briefing.
“We have to move beyond that and elevate this discussion to one of the survival of our civilization and the health and safety and security of all nations in the world,” continued Dr. Carmona, who is also a professor of public health at the University of Arizona in Tucson.
The report notes that the warming is already “occurring faster than governments are able, or willing, to respond,” likely contributing to the increased outcry across the world from youth about the need to act.
And anyone can take some kind of action, Ms. McCarthy said. Her aim is to make the reality of climate change effects personal so that people understand its impact on them as well as what they can do.
“The report provides a list of actions that policy makers can take today to reduce the threat of climate change” as well as information on “how we can adapt and be more resilient as communities” while facing climate change’s challenges, she said.
Ms. McCarthy encouraged people to pay particular attention to the report’s mitigation and adaptation recommendations, “because I want them to know that climate change isn’t a lost cause,” she said. The actions people can demand of policymakers will not only avoid the worst-case health scenario but can also improve health today, she added.
“We can do better than to dwell on the problem,” Ms. McCarthy said. “We need people now to be hopeful about climate change, to do as others have suggested and demand action and take action in their own lives. We can use that to really drive solutions.”
Annual report assesses numerous indicators
The Lancet Countdown is an annual report supported by the Wellcome Trust that pulls together research from 35 academic institutions and United Nations agencies across the world to provide an update on what the authors described as “41 health indicators across five key domains: climate change impacts, exposures and vulnerability; adaptation, planning, and resilience for health; mitigation action and health cobenefits; economics and finance; [and] public and political engagement.”
Given the complexity of the issue of climate change and the wide range of possible effects and preventive measures, contributing researchers included not just climate scientists but also ecologists, mathematicians, engineers, hydrologists, social and political scientists, physicians and other public health professionals, and experts in energy, food, and transportation.
The research was supported by the Wellcome Trust. Multiple authors also received support from a range of government institutions and public and private foundations and fellowships. No relevant financial relationships were noted.
SOURCE: Watts N et al. Lancet. 2019 Nov 13. doi: 10.1016/S0140-6736(19)32596-6.
This story first appeared in Medscape.com.
As wildfires increase the likelihood of respiratory illnesses for residents in California and Queensland, Australia, a new report from the Lancet warns that such health risks will become increasingly common without action to address climate change. But, the authors stressed, it’s still possible to prevent some health effects and mitigate others.
Given the magnitude of the issue, lead author Nick Watts, MBBS, MA, framed the issue in terms of what an individual child born today will face in his or her future. If the world continues on its current trajectory, such a child will eventually live in a world at least 4º C above average preindustrial temperatures.
“We roughly know what that looks like from a climate perspective,” said Dr. Watts, executive director of The Lancet Countdown: Tracking Progress on Health and Climate Change, during a telebriefing on the report.
“We have no idea of what that looks like from a public health perspective, but we know it is catastrophic,” he continued. “We know that it has the potential to undermine the last 50 years of gains in public health and overwhelm the health systems that we rely on.”
Health sector a significant, growing contributor
The report described the changes to which climate change has already contributed and addresses both the health threats and the way institutions and states are currently responding to those threats. It also included policy briefs specific to individual countries and an extensive appendix with projections data.
The authors noted that progress in mitigating fossil fuel combustion – the biggest driver of rising temperatures – is “intermittent at best,” with carbon dioxide emissions continuing to rise in 2018. The past decade has included 8 of the 10 hottest years on record. “Many of the indicators contained in this report suggest the world is following this ‘business as usual’ pathway,” the authors wrote.
In fact, the trend of coal-produced energy that had been declining actually increased 1.7% between 2016 and 2018. Perhaps ironically, given the focus of the report, “the healthcare sector is responsible for about 4.6% of global emissions, a value which is steadily rising across most major economies,” Dr. Watts and colleagues reported.
The potential health risks from climate change range from increased chronic illness, such as asthma and cardiovascular disease, to the increased spread of infectious diseases, especially vector-borne diseases, including dengue fever, malaria, and chikungunya. Increases in the frequency and intensity of severe weather events can lead to increased acute and longer-term morbidity and mortality.
Though children will suffer the brunt of negative health impact from climate change, the effects will touch people at every stage of life, from in utero development through old age, the authors emphasized.
“Downward trends in global yield potential for all major crops tracked since 1960 threaten food production and food security, with infants often the worst affected by the potentially permanent effects of undernutrition,” the authors reported. Children are also most susceptible to diarrheal disease and infectious diseases, particularly dengue.
Mitigating actions available
But the report focused as much on solutions and mitigation strategies as it did on the worst-case scenario without action. Speakers during the telebriefing emphasized the responsibility of all people, including physicians and other health care providers, to play a role in countering the public health disaster that could result from inaction on climate.
“Thankfully, here we have the treatment for climate change, solutions to shift away from the carbon pollution and towards clean energy and working to find the best way to protect ourselves and each other from climate change,” Renee N. Salas, MD, MPH, lead author of the 2019 Lancet Countdown U.S. Policy Brief and a Harvard C-CHANGE Fellow, said during the press briefing. “All we need is political will.”
Salas compared the present moment to that period when a physician still has the ability to save a critically ill patient’s life with fast action.
“If I don’t act quickly, the patient may still die even though that treatment would have saved their life earlier,” she said. “We are in that narrow window.”
Physicians have a responsibility to speak to patients and families frankly about not only specific conditions, such as asthma, but also the climate-related causes of those conditions, such as increasing air pollution, said Gina McCarthy, director of the Harvard Center for Climate, Health and the Global Environment and the 13th administrator U.S. Environmental Policy Administration. Physicians are trusted advisers and therefore need to speak up because climate change is “about the health and well-being and the future of children,” she said.
Political polarization is one of the biggest challenges to addressing climate change and stymies efforts to take action, according to Richard Carmona, MD, who served as the 17th U.S. Surgeon General.
“The thing that frustrated me as a surgeon general and continues to frustrate me today is that these very scientifically vetted issues are reduced to political currency that creates divisiveness, and things don’t get done,” he said during the briefing.
“We have to move beyond that and elevate this discussion to one of the survival of our civilization and the health and safety and security of all nations in the world,” continued Dr. Carmona, who is also a professor of public health at the University of Arizona in Tucson.
The report notes that the warming is already “occurring faster than governments are able, or willing, to respond,” likely contributing to the increased outcry across the world from youth about the need to act.
And anyone can take some kind of action, Ms. McCarthy said. Her aim is to make the reality of climate change effects personal so that people understand its impact on them as well as what they can do.
“The report provides a list of actions that policy makers can take today to reduce the threat of climate change” as well as information on “how we can adapt and be more resilient as communities” while facing climate change’s challenges, she said.
Ms. McCarthy encouraged people to pay particular attention to the report’s mitigation and adaptation recommendations, “because I want them to know that climate change isn’t a lost cause,” she said. The actions people can demand of policymakers will not only avoid the worst-case health scenario but can also improve health today, she added.
“We can do better than to dwell on the problem,” Ms. McCarthy said. “We need people now to be hopeful about climate change, to do as others have suggested and demand action and take action in their own lives. We can use that to really drive solutions.”
Annual report assesses numerous indicators
The Lancet Countdown is an annual report supported by the Wellcome Trust that pulls together research from 35 academic institutions and United Nations agencies across the world to provide an update on what the authors described as “41 health indicators across five key domains: climate change impacts, exposures and vulnerability; adaptation, planning, and resilience for health; mitigation action and health cobenefits; economics and finance; [and] public and political engagement.”
Given the complexity of the issue of climate change and the wide range of possible effects and preventive measures, contributing researchers included not just climate scientists but also ecologists, mathematicians, engineers, hydrologists, social and political scientists, physicians and other public health professionals, and experts in energy, food, and transportation.
The research was supported by the Wellcome Trust. Multiple authors also received support from a range of government institutions and public and private foundations and fellowships. No relevant financial relationships were noted.
SOURCE: Watts N et al. Lancet. 2019 Nov 13. doi: 10.1016/S0140-6736(19)32596-6.
This story first appeared in Medscape.com.
As wildfires increase the likelihood of respiratory illnesses for residents in California and Queensland, Australia, a new report from the Lancet warns that such health risks will become increasingly common without action to address climate change. But, the authors stressed, it’s still possible to prevent some health effects and mitigate others.
Given the magnitude of the issue, lead author Nick Watts, MBBS, MA, framed the issue in terms of what an individual child born today will face in his or her future. If the world continues on its current trajectory, such a child will eventually live in a world at least 4º C above average preindustrial temperatures.
“We roughly know what that looks like from a climate perspective,” said Dr. Watts, executive director of The Lancet Countdown: Tracking Progress on Health and Climate Change, during a telebriefing on the report.
“We have no idea of what that looks like from a public health perspective, but we know it is catastrophic,” he continued. “We know that it has the potential to undermine the last 50 years of gains in public health and overwhelm the health systems that we rely on.”
Health sector a significant, growing contributor
The report described the changes to which climate change has already contributed and addresses both the health threats and the way institutions and states are currently responding to those threats. It also included policy briefs specific to individual countries and an extensive appendix with projections data.
The authors noted that progress in mitigating fossil fuel combustion – the biggest driver of rising temperatures – is “intermittent at best,” with carbon dioxide emissions continuing to rise in 2018. The past decade has included 8 of the 10 hottest years on record. “Many of the indicators contained in this report suggest the world is following this ‘business as usual’ pathway,” the authors wrote.
In fact, the trend of coal-produced energy that had been declining actually increased 1.7% between 2016 and 2018. Perhaps ironically, given the focus of the report, “the healthcare sector is responsible for about 4.6% of global emissions, a value which is steadily rising across most major economies,” Dr. Watts and colleagues reported.
The potential health risks from climate change range from increased chronic illness, such as asthma and cardiovascular disease, to the increased spread of infectious diseases, especially vector-borne diseases, including dengue fever, malaria, and chikungunya. Increases in the frequency and intensity of severe weather events can lead to increased acute and longer-term morbidity and mortality.
Though children will suffer the brunt of negative health impact from climate change, the effects will touch people at every stage of life, from in utero development through old age, the authors emphasized.
“Downward trends in global yield potential for all major crops tracked since 1960 threaten food production and food security, with infants often the worst affected by the potentially permanent effects of undernutrition,” the authors reported. Children are also most susceptible to diarrheal disease and infectious diseases, particularly dengue.
Mitigating actions available
But the report focused as much on solutions and mitigation strategies as it did on the worst-case scenario without action. Speakers during the telebriefing emphasized the responsibility of all people, including physicians and other health care providers, to play a role in countering the public health disaster that could result from inaction on climate.
“Thankfully, here we have the treatment for climate change, solutions to shift away from the carbon pollution and towards clean energy and working to find the best way to protect ourselves and each other from climate change,” Renee N. Salas, MD, MPH, lead author of the 2019 Lancet Countdown U.S. Policy Brief and a Harvard C-CHANGE Fellow, said during the press briefing. “All we need is political will.”
Salas compared the present moment to that period when a physician still has the ability to save a critically ill patient’s life with fast action.
“If I don’t act quickly, the patient may still die even though that treatment would have saved their life earlier,” she said. “We are in that narrow window.”
Physicians have a responsibility to speak to patients and families frankly about not only specific conditions, such as asthma, but also the climate-related causes of those conditions, such as increasing air pollution, said Gina McCarthy, director of the Harvard Center for Climate, Health and the Global Environment and the 13th administrator U.S. Environmental Policy Administration. Physicians are trusted advisers and therefore need to speak up because climate change is “about the health and well-being and the future of children,” she said.
Political polarization is one of the biggest challenges to addressing climate change and stymies efforts to take action, according to Richard Carmona, MD, who served as the 17th U.S. Surgeon General.
“The thing that frustrated me as a surgeon general and continues to frustrate me today is that these very scientifically vetted issues are reduced to political currency that creates divisiveness, and things don’t get done,” he said during the briefing.
“We have to move beyond that and elevate this discussion to one of the survival of our civilization and the health and safety and security of all nations in the world,” continued Dr. Carmona, who is also a professor of public health at the University of Arizona in Tucson.
The report notes that the warming is already “occurring faster than governments are able, or willing, to respond,” likely contributing to the increased outcry across the world from youth about the need to act.
And anyone can take some kind of action, Ms. McCarthy said. Her aim is to make the reality of climate change effects personal so that people understand its impact on them as well as what they can do.
“The report provides a list of actions that policy makers can take today to reduce the threat of climate change” as well as information on “how we can adapt and be more resilient as communities” while facing climate change’s challenges, she said.
Ms. McCarthy encouraged people to pay particular attention to the report’s mitigation and adaptation recommendations, “because I want them to know that climate change isn’t a lost cause,” she said. The actions people can demand of policymakers will not only avoid the worst-case health scenario but can also improve health today, she added.
“We can do better than to dwell on the problem,” Ms. McCarthy said. “We need people now to be hopeful about climate change, to do as others have suggested and demand action and take action in their own lives. We can use that to really drive solutions.”
Annual report assesses numerous indicators
The Lancet Countdown is an annual report supported by the Wellcome Trust that pulls together research from 35 academic institutions and United Nations agencies across the world to provide an update on what the authors described as “41 health indicators across five key domains: climate change impacts, exposures and vulnerability; adaptation, planning, and resilience for health; mitigation action and health cobenefits; economics and finance; [and] public and political engagement.”
Given the complexity of the issue of climate change and the wide range of possible effects and preventive measures, contributing researchers included not just climate scientists but also ecologists, mathematicians, engineers, hydrologists, social and political scientists, physicians and other public health professionals, and experts in energy, food, and transportation.
The research was supported by the Wellcome Trust. Multiple authors also received support from a range of government institutions and public and private foundations and fellowships. No relevant financial relationships were noted.
SOURCE: Watts N et al. Lancet. 2019 Nov 13. doi: 10.1016/S0140-6736(19)32596-6.
This story first appeared in Medscape.com.
CDC releases update of 2013 Antibiotic Resistance Threats Report
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
Rifabutin-based triple therapy for H. pylori gets high marks
SAN ANTONIO – David Y. Graham, MD, asserted at the annual meeting of the American College of Gastroenterology.
The drug, recently approved as Talicia, is a rifabutin-based triple therapy. Each capsule contains 50 mg of rifabutin, 1,000 mg of amoxicillin, and 40 mg of omeprazole. As in the pivotal phase 3 trial led by Dr. Graham, the approved treatment regimen calls for adults to take four capsules every 8 hours for 14 days.
The impetus for developing the new therapy centers on the growing problem of resistance to long-standard agents for H. pylori eradication, including metronidazole and clarithromycin. The World Health Organization has declared H. pylori eradication to be a high priority for therapeutic development. Rifabutin resistance is rare: In one study, 413 of 414 strains of H. pylori were sensitive to the antibiotic, noted Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
He presented the results of the pivotal phase 3, double-blind, multicenter, active comparator trial, known as ERADICATE Hp2, in which 455 participants with confirmed H. pylori infection were randomized to a course of the all-in-one-capsule triple drug combo or to dual therapy with four capsules, each containing 1,000 mg of amoxicillin and 40 mg of omeprazole, every 8 hours for 14 days.
The primary endpoint was H. pylori eradication as documented by a negative urea breath test obtained 4-6 weeks after completing 14 days of treatment. The rate was 84% with the rifabutin-based combo, compared with 58% seen with the high-dose dual therapy. Moreover, in a prespecified secondary analysis restricted to the 391 participants who were confirmed to be actually taking their medication as evidenced by a positive blood level measured on day 13, the eradication rates rose to 90% and 65%, respectively.
The antimicrobial resistance rates documented in this study were eye opening: 17% of patients’ strains were resistant to clarithromycin, 44% to metronidazole, and 10.5% to both. Of concern, 6.4% of participants’ strains were amoxicillin resistant.
“For the first time we saw a low level – but a definite level – of amoxicillin resistance. That’s something we had not seen previously,” Dr. Graham said.
No rifabutin resistance was detected before or after treatment.
The side effect profiles of the two treatment regimens were similar. Diarrhea was reported by 9% of participants, headache by 7%, and nausea by 5%. No serious adverse events occurred in the 14-day study.
The efficacy of the rifabutin-based therapy wasn’t affected by metronidazole or clarithromycin resistance.
The ERADICATE Hp2 trial was sponsored by RedHill Biopharma of Tel Aviv. Dr. Graham reported having no financial conflicts.
SAN ANTONIO – David Y. Graham, MD, asserted at the annual meeting of the American College of Gastroenterology.
The drug, recently approved as Talicia, is a rifabutin-based triple therapy. Each capsule contains 50 mg of rifabutin, 1,000 mg of amoxicillin, and 40 mg of omeprazole. As in the pivotal phase 3 trial led by Dr. Graham, the approved treatment regimen calls for adults to take four capsules every 8 hours for 14 days.
The impetus for developing the new therapy centers on the growing problem of resistance to long-standard agents for H. pylori eradication, including metronidazole and clarithromycin. The World Health Organization has declared H. pylori eradication to be a high priority for therapeutic development. Rifabutin resistance is rare: In one study, 413 of 414 strains of H. pylori were sensitive to the antibiotic, noted Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
He presented the results of the pivotal phase 3, double-blind, multicenter, active comparator trial, known as ERADICATE Hp2, in which 455 participants with confirmed H. pylori infection were randomized to a course of the all-in-one-capsule triple drug combo or to dual therapy with four capsules, each containing 1,000 mg of amoxicillin and 40 mg of omeprazole, every 8 hours for 14 days.
The primary endpoint was H. pylori eradication as documented by a negative urea breath test obtained 4-6 weeks after completing 14 days of treatment. The rate was 84% with the rifabutin-based combo, compared with 58% seen with the high-dose dual therapy. Moreover, in a prespecified secondary analysis restricted to the 391 participants who were confirmed to be actually taking their medication as evidenced by a positive blood level measured on day 13, the eradication rates rose to 90% and 65%, respectively.
The antimicrobial resistance rates documented in this study were eye opening: 17% of patients’ strains were resistant to clarithromycin, 44% to metronidazole, and 10.5% to both. Of concern, 6.4% of participants’ strains were amoxicillin resistant.
“For the first time we saw a low level – but a definite level – of amoxicillin resistance. That’s something we had not seen previously,” Dr. Graham said.
No rifabutin resistance was detected before or after treatment.
The side effect profiles of the two treatment regimens were similar. Diarrhea was reported by 9% of participants, headache by 7%, and nausea by 5%. No serious adverse events occurred in the 14-day study.
The efficacy of the rifabutin-based therapy wasn’t affected by metronidazole or clarithromycin resistance.
The ERADICATE Hp2 trial was sponsored by RedHill Biopharma of Tel Aviv. Dr. Graham reported having no financial conflicts.
SAN ANTONIO – David Y. Graham, MD, asserted at the annual meeting of the American College of Gastroenterology.
The drug, recently approved as Talicia, is a rifabutin-based triple therapy. Each capsule contains 50 mg of rifabutin, 1,000 mg of amoxicillin, and 40 mg of omeprazole. As in the pivotal phase 3 trial led by Dr. Graham, the approved treatment regimen calls for adults to take four capsules every 8 hours for 14 days.
The impetus for developing the new therapy centers on the growing problem of resistance to long-standard agents for H. pylori eradication, including metronidazole and clarithromycin. The World Health Organization has declared H. pylori eradication to be a high priority for therapeutic development. Rifabutin resistance is rare: In one study, 413 of 414 strains of H. pylori were sensitive to the antibiotic, noted Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
He presented the results of the pivotal phase 3, double-blind, multicenter, active comparator trial, known as ERADICATE Hp2, in which 455 participants with confirmed H. pylori infection were randomized to a course of the all-in-one-capsule triple drug combo or to dual therapy with four capsules, each containing 1,000 mg of amoxicillin and 40 mg of omeprazole, every 8 hours for 14 days.
The primary endpoint was H. pylori eradication as documented by a negative urea breath test obtained 4-6 weeks after completing 14 days of treatment. The rate was 84% with the rifabutin-based combo, compared with 58% seen with the high-dose dual therapy. Moreover, in a prespecified secondary analysis restricted to the 391 participants who were confirmed to be actually taking their medication as evidenced by a positive blood level measured on day 13, the eradication rates rose to 90% and 65%, respectively.
The antimicrobial resistance rates documented in this study were eye opening: 17% of patients’ strains were resistant to clarithromycin, 44% to metronidazole, and 10.5% to both. Of concern, 6.4% of participants’ strains were amoxicillin resistant.
“For the first time we saw a low level – but a definite level – of amoxicillin resistance. That’s something we had not seen previously,” Dr. Graham said.
No rifabutin resistance was detected before or after treatment.
The side effect profiles of the two treatment regimens were similar. Diarrhea was reported by 9% of participants, headache by 7%, and nausea by 5%. No serious adverse events occurred in the 14-day study.
The efficacy of the rifabutin-based therapy wasn’t affected by metronidazole or clarithromycin resistance.
The ERADICATE Hp2 trial was sponsored by RedHill Biopharma of Tel Aviv. Dr. Graham reported having no financial conflicts.
REPORTING FROM ACG 2019
Monoclonal Antibodies in MS
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.