User login
Polypharmacy in an Aging HIV Population
PORTLAND—“We are now working with an HIV population that is aging, and along with aging comes a lot of other diseases, such as heart and lung disease, that often result in the use of medications,” began Jennifer Cocohoba, PharmD, AAHIP, Professor of Clinical Pharmacy, UCSF School of Pharmacy. She explained that “the growing problem of having too many medications applies to all, but is particularly important among those living with HIV.”
Polypharmacy, as often defined in research studies, is the taking of at least 5 prescription medications. About 40% of US adults fall into this category, “and it’s not much different for people with HIV,” Cocohoba said. “Persons living with HIV experience polypharmacy at about the same rate.” But people living with HIV may reach that number faster, given that 2 to 3 of the 5 drugs may be for HIV. In addition, those living with HIV are potentially at greater risk for drug interactions.
Quality, not just quantity
Cocohoba explained that it’s important to pay attention to the number of medications a patient is taking because the greater the number, the greater the likelihood of interactions and adverse effects and the more difficult it is for patients to adhere to their medication regimens.
“But we should be looking also at appropriateness of using those medications in a particular person,” she continued, which moves into the realm of quality. She reviewed some criteria that can be used to evaluate the quality of prescribing.
The BEERS Criteria, published by the American Geriatric Society, present classes and specific medications that may be inappropriate in certain elderly persons. Benzodiazepines, for example, commonly used to treat anxiety, are not metabolized as efficiently in the elderly as they are in younger patients. As a result, older adults may experience sedation, falls, or other potentially harmful sequelae. “If a patient age 65 or older is taking a benzodiazepine, that’s a red flag for a clinician to look at that medication and see if it’s really the best choice or whether alternatives might be more appropriate,” explained Cocohoba.
START/STOPP Criteria. Other criteria include the Screening Tool To Alert To Right Treatment (START) and the Screening Tool Of Older People's Prescriptions (STOPP). START focuses on looking for medications that would be appropriate for a particular patient but that are missing from the patient’s medication list, while STOPP focuses on looking for medications that are on the list that might be inappropriate, much like the BEERS Criteria.
The Good Palliative Geriatric Practice Algorithm is a clinical decision-making tool that aids clinicians in thinking through whether a medication is appropriate or inappropriate and whether to maintain, alter, or discontinue the therapy. Cocohoba said it’s often used as a partner to BEERS.
Continue to: Reducing polypharmacy in HIV treatment
Reducing polypharmacy in HIV treatment
Cocohoba explained that there are essentially 2 frameworks for reducing polypharmacy that can be applied to HIV treatment regimens.
Consolidation. With consolidation, the focus is on reducing pill burden—but not by omitting medications. “It’s about looking into simpler dosage forms or combination medications to improve adherence and make life easier,” Cocohoba explained. “Same regimen, fewer pills.”
Simplification, on the other hand, is removing, and thus reducing the number of, agents a patient is taking. The question clinicians should be asking is, according to Cocohoba, “In what situations is it safe to strip down therapy to the bare essentials for the purposes of exposing people to fewer medications, reducing adverse effects, and keeping treatment as manageable as possible to optimize adherence?”
Simplification may be applied to either treatment-naïve or treatment-experienced patients. With the former, clinicians consider, for example, whether patients can be started on HIV treatment consisting of 2 rather than 3 medications. In the latter, “Clinicians may be dealing with patients who are fully virally suppressed on certain regimens and have been for a while; here we see if we can subtract medications, reducing say from triple to double therapy, while maintaining suppression,” explained Cocohoba.
“We want to offer people robust HIV treatment that is going to maintain viral suppression and prevent sequelae of HIV disease,” Cocohoba said, “but we need to balance that with safety, tolerability, and adherence.”
Continue to: An ongoing discussion and multidisciplinary effort
An ongoing discussion and multidisciplinary effort
“Medications can easily pile [up],” Cocohoba said, “so it’s important for clinicians to regularly review medication lists with patients to see if maybe they aren’t using certain agents anymore.” Another reason for clinicians to periodically review medication lists is to make certain they are aware of agents being prescribed by the patient’s other health care providers.
Polypharmacy is the responsibility of everyone on the health care team, both prescribers and nonprescribers, such as social workers and case managers, explained Cocohoba. “If ever there was a health care problem that could use an interdisciplinary approach, polypharmacy is it.”
PORTLAND—“We are now working with an HIV population that is aging, and along with aging comes a lot of other diseases, such as heart and lung disease, that often result in the use of medications,” began Jennifer Cocohoba, PharmD, AAHIP, Professor of Clinical Pharmacy, UCSF School of Pharmacy. She explained that “the growing problem of having too many medications applies to all, but is particularly important among those living with HIV.”
Polypharmacy, as often defined in research studies, is the taking of at least 5 prescription medications. About 40% of US adults fall into this category, “and it’s not much different for people with HIV,” Cocohoba said. “Persons living with HIV experience polypharmacy at about the same rate.” But people living with HIV may reach that number faster, given that 2 to 3 of the 5 drugs may be for HIV. In addition, those living with HIV are potentially at greater risk for drug interactions.
Quality, not just quantity
Cocohoba explained that it’s important to pay attention to the number of medications a patient is taking because the greater the number, the greater the likelihood of interactions and adverse effects and the more difficult it is for patients to adhere to their medication regimens.
“But we should be looking also at appropriateness of using those medications in a particular person,” she continued, which moves into the realm of quality. She reviewed some criteria that can be used to evaluate the quality of prescribing.
The BEERS Criteria, published by the American Geriatric Society, present classes and specific medications that may be inappropriate in certain elderly persons. Benzodiazepines, for example, commonly used to treat anxiety, are not metabolized as efficiently in the elderly as they are in younger patients. As a result, older adults may experience sedation, falls, or other potentially harmful sequelae. “If a patient age 65 or older is taking a benzodiazepine, that’s a red flag for a clinician to look at that medication and see if it’s really the best choice or whether alternatives might be more appropriate,” explained Cocohoba.
START/STOPP Criteria. Other criteria include the Screening Tool To Alert To Right Treatment (START) and the Screening Tool Of Older People's Prescriptions (STOPP). START focuses on looking for medications that would be appropriate for a particular patient but that are missing from the patient’s medication list, while STOPP focuses on looking for medications that are on the list that might be inappropriate, much like the BEERS Criteria.
The Good Palliative Geriatric Practice Algorithm is a clinical decision-making tool that aids clinicians in thinking through whether a medication is appropriate or inappropriate and whether to maintain, alter, or discontinue the therapy. Cocohoba said it’s often used as a partner to BEERS.
Continue to: Reducing polypharmacy in HIV treatment
Reducing polypharmacy in HIV treatment
Cocohoba explained that there are essentially 2 frameworks for reducing polypharmacy that can be applied to HIV treatment regimens.
Consolidation. With consolidation, the focus is on reducing pill burden—but not by omitting medications. “It’s about looking into simpler dosage forms or combination medications to improve adherence and make life easier,” Cocohoba explained. “Same regimen, fewer pills.”
Simplification, on the other hand, is removing, and thus reducing the number of, agents a patient is taking. The question clinicians should be asking is, according to Cocohoba, “In what situations is it safe to strip down therapy to the bare essentials for the purposes of exposing people to fewer medications, reducing adverse effects, and keeping treatment as manageable as possible to optimize adherence?”
Simplification may be applied to either treatment-naïve or treatment-experienced patients. With the former, clinicians consider, for example, whether patients can be started on HIV treatment consisting of 2 rather than 3 medications. In the latter, “Clinicians may be dealing with patients who are fully virally suppressed on certain regimens and have been for a while; here we see if we can subtract medications, reducing say from triple to double therapy, while maintaining suppression,” explained Cocohoba.
“We want to offer people robust HIV treatment that is going to maintain viral suppression and prevent sequelae of HIV disease,” Cocohoba said, “but we need to balance that with safety, tolerability, and adherence.”
Continue to: An ongoing discussion and multidisciplinary effort
An ongoing discussion and multidisciplinary effort
“Medications can easily pile [up],” Cocohoba said, “so it’s important for clinicians to regularly review medication lists with patients to see if maybe they aren’t using certain agents anymore.” Another reason for clinicians to periodically review medication lists is to make certain they are aware of agents being prescribed by the patient’s other health care providers.
Polypharmacy is the responsibility of everyone on the health care team, both prescribers and nonprescribers, such as social workers and case managers, explained Cocohoba. “If ever there was a health care problem that could use an interdisciplinary approach, polypharmacy is it.”
PORTLAND—“We are now working with an HIV population that is aging, and along with aging comes a lot of other diseases, such as heart and lung disease, that often result in the use of medications,” began Jennifer Cocohoba, PharmD, AAHIP, Professor of Clinical Pharmacy, UCSF School of Pharmacy. She explained that “the growing problem of having too many medications applies to all, but is particularly important among those living with HIV.”
Polypharmacy, as often defined in research studies, is the taking of at least 5 prescription medications. About 40% of US adults fall into this category, “and it’s not much different for people with HIV,” Cocohoba said. “Persons living with HIV experience polypharmacy at about the same rate.” But people living with HIV may reach that number faster, given that 2 to 3 of the 5 drugs may be for HIV. In addition, those living with HIV are potentially at greater risk for drug interactions.
Quality, not just quantity
Cocohoba explained that it’s important to pay attention to the number of medications a patient is taking because the greater the number, the greater the likelihood of interactions and adverse effects and the more difficult it is for patients to adhere to their medication regimens.
“But we should be looking also at appropriateness of using those medications in a particular person,” she continued, which moves into the realm of quality. She reviewed some criteria that can be used to evaluate the quality of prescribing.
The BEERS Criteria, published by the American Geriatric Society, present classes and specific medications that may be inappropriate in certain elderly persons. Benzodiazepines, for example, commonly used to treat anxiety, are not metabolized as efficiently in the elderly as they are in younger patients. As a result, older adults may experience sedation, falls, or other potentially harmful sequelae. “If a patient age 65 or older is taking a benzodiazepine, that’s a red flag for a clinician to look at that medication and see if it’s really the best choice or whether alternatives might be more appropriate,” explained Cocohoba.
START/STOPP Criteria. Other criteria include the Screening Tool To Alert To Right Treatment (START) and the Screening Tool Of Older People's Prescriptions (STOPP). START focuses on looking for medications that would be appropriate for a particular patient but that are missing from the patient’s medication list, while STOPP focuses on looking for medications that are on the list that might be inappropriate, much like the BEERS Criteria.
The Good Palliative Geriatric Practice Algorithm is a clinical decision-making tool that aids clinicians in thinking through whether a medication is appropriate or inappropriate and whether to maintain, alter, or discontinue the therapy. Cocohoba said it’s often used as a partner to BEERS.
Continue to: Reducing polypharmacy in HIV treatment
Reducing polypharmacy in HIV treatment
Cocohoba explained that there are essentially 2 frameworks for reducing polypharmacy that can be applied to HIV treatment regimens.
Consolidation. With consolidation, the focus is on reducing pill burden—but not by omitting medications. “It’s about looking into simpler dosage forms or combination medications to improve adherence and make life easier,” Cocohoba explained. “Same regimen, fewer pills.”
Simplification, on the other hand, is removing, and thus reducing the number of, agents a patient is taking. The question clinicians should be asking is, according to Cocohoba, “In what situations is it safe to strip down therapy to the bare essentials for the purposes of exposing people to fewer medications, reducing adverse effects, and keeping treatment as manageable as possible to optimize adherence?”
Simplification may be applied to either treatment-naïve or treatment-experienced patients. With the former, clinicians consider, for example, whether patients can be started on HIV treatment consisting of 2 rather than 3 medications. In the latter, “Clinicians may be dealing with patients who are fully virally suppressed on certain regimens and have been for a while; here we see if we can subtract medications, reducing say from triple to double therapy, while maintaining suppression,” explained Cocohoba.
“We want to offer people robust HIV treatment that is going to maintain viral suppression and prevent sequelae of HIV disease,” Cocohoba said, “but we need to balance that with safety, tolerability, and adherence.”
Continue to: An ongoing discussion and multidisciplinary effort
An ongoing discussion and multidisciplinary effort
“Medications can easily pile [up],” Cocohoba said, “so it’s important for clinicians to regularly review medication lists with patients to see if maybe they aren’t using certain agents anymore.” Another reason for clinicians to periodically review medication lists is to make certain they are aware of agents being prescribed by the patient’s other health care providers.
Polypharmacy is the responsibility of everyone on the health care team, both prescribers and nonprescribers, such as social workers and case managers, explained Cocohoba. “If ever there was a health care problem that could use an interdisciplinary approach, polypharmacy is it.”
Association of Nurses in AIDS Care 2019
ARV Therapy: Current Issues and Controversies
PORTLAND—The investigational drug islatravir “could be a game changer in the field of HIV," said David H. Spach, MD, Professor of Medicine, Division of Infectious Diseases, University of Washington, Seattle, in a session called, "Antiretroviral therapy 2019 update: Mechanism of action, new medications, current guidelines, and controversies."
Dr. Spach reported that islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), is highly potent, is well tolerated, has a high genetic barrier to resistance, and has an extremely long half-life, according to the findings of preliminary studies. Its long half-life enables subdermal implantation and maintenance of therapeutic levels even after a year in place. Researchers are studying the compound in combination with other agents for treatment and independently for preexposure prophylaxis.
Another noteworthy investigational agent, according to Dr. Spach, is cabotegravir, an integrase strand transfer inhibitor (INSTI) with the potential for intramuscular administration every 4 to 8 weeks. Dr. Spach explained that researchers are studying the agent in 3 different clinical situations: oral cabotegravir in combination with rilpivirine for lead-in therapy; an extended-release injectable of cabotegravir and rilpivirine for maintenance antiretroviral (ARV) treatment every 4 weeks; and an extended-release cabotegravir injectable for preexposure prophylaxis every 8 weeks. The agent is highly potent, with a high genetic barrier to resistance.
ARV: The current state of affairs
"We are in an era now where everyone who is living with HIV ideally should be receiving fully suppressive antiretroviral therapy," remarked Dr. Spach. He explained that not only does this benefit the person living with HIV by reducing the onset and progression of chronic inflammatory disease states that occur along with HIV, such as cardiovascular disease, stroke, and cancer, but also "fully suppressive ARV therapy virtually eliminates sexual transmission of HIV to another person."
Spach explained that the most recent (2018) Health and Human Services ARV therapy guidelines have greatly simplified the choices for ARV therapy. The current recommendations for initial ARV therapy for most people are to use a 2-drug backbone regimen, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), combined with a single anchor drug, which should be an INSTI. The INSTI should have the highest potency and highest genetic barrier to resistance available, which effectively means using a regimen that contains either dolutegravir or bictegravir. The latter is available only in combination with emtricitabine and tenofovir alafenamide as a single-tablet regimen.
Spach also explained that "the guidelines have moved away from using boosted regimens for initial therapy, so elvitegravir boosted with cobicistat and protease inhibitors (PIs) boosted with ritonavir or cobicistat are no longer recommended as preferred initial therapy, although boosted PIs are still very useful as second- or third-line therapies."
Newer medications
Doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), was approved by the FDA in 2018. "It probably won't have a big impact on initial therapy, but it is likely to have a significant effect on second- and third-line therapy," Dr. Spach said. "Because of its high potency and high genetic barrier to resistance, those taking etravirine twice a day, for example, may be able to simplify to once-a-day doravirine." In addition, "Doravirine may have advantages over rilpivirine in that it has no restrictions when used with acid-suppressing agents such as H2 blockers or proton pump inhibitors."
Continue to: Ibalizumab
Ibalizumab. Another newer agent “worth mentioning,” according to Dr. Spach, is ibalizumab. This monoclonal antibody has a unique mechanism of action. It is 1 of only 2 drugs used for HIV treatment that targets human receptors (all of the others work by inhibiting either an HIV enzyme or binding to the HIV virus itself). Ibalizumab targets the D2 region of the CD4 receptor. It is an injectable (intravenous) compound administered with an initial loading dose and then every 2 weeks thereafter. Dr. Spach reported that the data surrounding ibalizumab show that it is effective as an add-on medication in more advanced resistant settings. Also, it provides an option for people who can't take oral drugs, such as those who have had major surgery or trauma.
Remaining questions
Dr. Spach explained that 1 of the questions that remains is whether to prescribe ARV therapy for patients who are viremic controllers (those who inherently control HIV through their own immunologic response to a level < 200 copies) or elite controllers (those whose own immunologic response controls the virus to a level < 50 copies, which is in the undetectable range). Both of these groups still have a higher risk for hospitalization and for HIV-related inflammatory conditions such as heart disease, according to Dr. Spach. Current thinking among most experts is to initiate and maintain therapy as long as it is tolerated well.
3 drugs to 2? Another question that remains is whether to switch patients who are doing well on 3-drug maintenance therapy to 2-drug maintenance therapy. According to Dr Spach, studies involving the combination dolutegravir/rilpivirine indicate that patients who have suppressed HIV RNA levels for at least 6 months on a 3-drug maintenance regimen do well after switching to the 2-drug combination dolutegravir/rilpivirine, as long as they do not have baseline resistance to either dolutegravir or rilpivirine. But he questioned the need for the change if the individual is tolerating a 3-drug regimen well, saying that given the safety of current regimens, the only broader motivating force may be cost savings. For now, he said, if patients are without complaints or tolerability issues on 3 drugs, leave them alone.
INSTIs and weight gain. The last issue is weight gain with the use of INSTIs. Preliminary data suggest disproportionate weight gain with these drugs (on the order of about 6 kg over a year and half, which may be 2-3 kg greater than that which occurs with PI-based and NNRTI-based regimens). At this point, experts do not recommend avoiding these agents, mainly because of the tremendous benefits that have been observed with INSTIs. Dr. Spach concluded, "Although we will continue to use INSTIs widely in clinical practice, there may be a subset of individuals taking an INSTI who have pronounced weight gain and may need to switch to another regimen that does not contain an INSTI.”
PORTLAND—The investigational drug islatravir “could be a game changer in the field of HIV," said David H. Spach, MD, Professor of Medicine, Division of Infectious Diseases, University of Washington, Seattle, in a session called, "Antiretroviral therapy 2019 update: Mechanism of action, new medications, current guidelines, and controversies."
Dr. Spach reported that islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), is highly potent, is well tolerated, has a high genetic barrier to resistance, and has an extremely long half-life, according to the findings of preliminary studies. Its long half-life enables subdermal implantation and maintenance of therapeutic levels even after a year in place. Researchers are studying the compound in combination with other agents for treatment and independently for preexposure prophylaxis.
Another noteworthy investigational agent, according to Dr. Spach, is cabotegravir, an integrase strand transfer inhibitor (INSTI) with the potential for intramuscular administration every 4 to 8 weeks. Dr. Spach explained that researchers are studying the agent in 3 different clinical situations: oral cabotegravir in combination with rilpivirine for lead-in therapy; an extended-release injectable of cabotegravir and rilpivirine for maintenance antiretroviral (ARV) treatment every 4 weeks; and an extended-release cabotegravir injectable for preexposure prophylaxis every 8 weeks. The agent is highly potent, with a high genetic barrier to resistance.
ARV: The current state of affairs
"We are in an era now where everyone who is living with HIV ideally should be receiving fully suppressive antiretroviral therapy," remarked Dr. Spach. He explained that not only does this benefit the person living with HIV by reducing the onset and progression of chronic inflammatory disease states that occur along with HIV, such as cardiovascular disease, stroke, and cancer, but also "fully suppressive ARV therapy virtually eliminates sexual transmission of HIV to another person."
Spach explained that the most recent (2018) Health and Human Services ARV therapy guidelines have greatly simplified the choices for ARV therapy. The current recommendations for initial ARV therapy for most people are to use a 2-drug backbone regimen, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), combined with a single anchor drug, which should be an INSTI. The INSTI should have the highest potency and highest genetic barrier to resistance available, which effectively means using a regimen that contains either dolutegravir or bictegravir. The latter is available only in combination with emtricitabine and tenofovir alafenamide as a single-tablet regimen.
Spach also explained that "the guidelines have moved away from using boosted regimens for initial therapy, so elvitegravir boosted with cobicistat and protease inhibitors (PIs) boosted with ritonavir or cobicistat are no longer recommended as preferred initial therapy, although boosted PIs are still very useful as second- or third-line therapies."
Newer medications
Doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), was approved by the FDA in 2018. "It probably won't have a big impact on initial therapy, but it is likely to have a significant effect on second- and third-line therapy," Dr. Spach said. "Because of its high potency and high genetic barrier to resistance, those taking etravirine twice a day, for example, may be able to simplify to once-a-day doravirine." In addition, "Doravirine may have advantages over rilpivirine in that it has no restrictions when used with acid-suppressing agents such as H2 blockers or proton pump inhibitors."
Continue to: Ibalizumab
Ibalizumab. Another newer agent “worth mentioning,” according to Dr. Spach, is ibalizumab. This monoclonal antibody has a unique mechanism of action. It is 1 of only 2 drugs used for HIV treatment that targets human receptors (all of the others work by inhibiting either an HIV enzyme or binding to the HIV virus itself). Ibalizumab targets the D2 region of the CD4 receptor. It is an injectable (intravenous) compound administered with an initial loading dose and then every 2 weeks thereafter. Dr. Spach reported that the data surrounding ibalizumab show that it is effective as an add-on medication in more advanced resistant settings. Also, it provides an option for people who can't take oral drugs, such as those who have had major surgery or trauma.
Remaining questions
Dr. Spach explained that 1 of the questions that remains is whether to prescribe ARV therapy for patients who are viremic controllers (those who inherently control HIV through their own immunologic response to a level < 200 copies) or elite controllers (those whose own immunologic response controls the virus to a level < 50 copies, which is in the undetectable range). Both of these groups still have a higher risk for hospitalization and for HIV-related inflammatory conditions such as heart disease, according to Dr. Spach. Current thinking among most experts is to initiate and maintain therapy as long as it is tolerated well.
3 drugs to 2? Another question that remains is whether to switch patients who are doing well on 3-drug maintenance therapy to 2-drug maintenance therapy. According to Dr Spach, studies involving the combination dolutegravir/rilpivirine indicate that patients who have suppressed HIV RNA levels for at least 6 months on a 3-drug maintenance regimen do well after switching to the 2-drug combination dolutegravir/rilpivirine, as long as they do not have baseline resistance to either dolutegravir or rilpivirine. But he questioned the need for the change if the individual is tolerating a 3-drug regimen well, saying that given the safety of current regimens, the only broader motivating force may be cost savings. For now, he said, if patients are without complaints or tolerability issues on 3 drugs, leave them alone.
INSTIs and weight gain. The last issue is weight gain with the use of INSTIs. Preliminary data suggest disproportionate weight gain with these drugs (on the order of about 6 kg over a year and half, which may be 2-3 kg greater than that which occurs with PI-based and NNRTI-based regimens). At this point, experts do not recommend avoiding these agents, mainly because of the tremendous benefits that have been observed with INSTIs. Dr. Spach concluded, "Although we will continue to use INSTIs widely in clinical practice, there may be a subset of individuals taking an INSTI who have pronounced weight gain and may need to switch to another regimen that does not contain an INSTI.”
PORTLAND—The investigational drug islatravir “could be a game changer in the field of HIV," said David H. Spach, MD, Professor of Medicine, Division of Infectious Diseases, University of Washington, Seattle, in a session called, "Antiretroviral therapy 2019 update: Mechanism of action, new medications, current guidelines, and controversies."
Dr. Spach reported that islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), is highly potent, is well tolerated, has a high genetic barrier to resistance, and has an extremely long half-life, according to the findings of preliminary studies. Its long half-life enables subdermal implantation and maintenance of therapeutic levels even after a year in place. Researchers are studying the compound in combination with other agents for treatment and independently for preexposure prophylaxis.
Another noteworthy investigational agent, according to Dr. Spach, is cabotegravir, an integrase strand transfer inhibitor (INSTI) with the potential for intramuscular administration every 4 to 8 weeks. Dr. Spach explained that researchers are studying the agent in 3 different clinical situations: oral cabotegravir in combination with rilpivirine for lead-in therapy; an extended-release injectable of cabotegravir and rilpivirine for maintenance antiretroviral (ARV) treatment every 4 weeks; and an extended-release cabotegravir injectable for preexposure prophylaxis every 8 weeks. The agent is highly potent, with a high genetic barrier to resistance.
ARV: The current state of affairs
"We are in an era now where everyone who is living with HIV ideally should be receiving fully suppressive antiretroviral therapy," remarked Dr. Spach. He explained that not only does this benefit the person living with HIV by reducing the onset and progression of chronic inflammatory disease states that occur along with HIV, such as cardiovascular disease, stroke, and cancer, but also "fully suppressive ARV therapy virtually eliminates sexual transmission of HIV to another person."
Spach explained that the most recent (2018) Health and Human Services ARV therapy guidelines have greatly simplified the choices for ARV therapy. The current recommendations for initial ARV therapy for most people are to use a 2-drug backbone regimen, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), combined with a single anchor drug, which should be an INSTI. The INSTI should have the highest potency and highest genetic barrier to resistance available, which effectively means using a regimen that contains either dolutegravir or bictegravir. The latter is available only in combination with emtricitabine and tenofovir alafenamide as a single-tablet regimen.
Spach also explained that "the guidelines have moved away from using boosted regimens for initial therapy, so elvitegravir boosted with cobicistat and protease inhibitors (PIs) boosted with ritonavir or cobicistat are no longer recommended as preferred initial therapy, although boosted PIs are still very useful as second- or third-line therapies."
Newer medications
Doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), was approved by the FDA in 2018. "It probably won't have a big impact on initial therapy, but it is likely to have a significant effect on second- and third-line therapy," Dr. Spach said. "Because of its high potency and high genetic barrier to resistance, those taking etravirine twice a day, for example, may be able to simplify to once-a-day doravirine." In addition, "Doravirine may have advantages over rilpivirine in that it has no restrictions when used with acid-suppressing agents such as H2 blockers or proton pump inhibitors."
Continue to: Ibalizumab
Ibalizumab. Another newer agent “worth mentioning,” according to Dr. Spach, is ibalizumab. This monoclonal antibody has a unique mechanism of action. It is 1 of only 2 drugs used for HIV treatment that targets human receptors (all of the others work by inhibiting either an HIV enzyme or binding to the HIV virus itself). Ibalizumab targets the D2 region of the CD4 receptor. It is an injectable (intravenous) compound administered with an initial loading dose and then every 2 weeks thereafter. Dr. Spach reported that the data surrounding ibalizumab show that it is effective as an add-on medication in more advanced resistant settings. Also, it provides an option for people who can't take oral drugs, such as those who have had major surgery or trauma.
Remaining questions
Dr. Spach explained that 1 of the questions that remains is whether to prescribe ARV therapy for patients who are viremic controllers (those who inherently control HIV through their own immunologic response to a level < 200 copies) or elite controllers (those whose own immunologic response controls the virus to a level < 50 copies, which is in the undetectable range). Both of these groups still have a higher risk for hospitalization and for HIV-related inflammatory conditions such as heart disease, according to Dr. Spach. Current thinking among most experts is to initiate and maintain therapy as long as it is tolerated well.
3 drugs to 2? Another question that remains is whether to switch patients who are doing well on 3-drug maintenance therapy to 2-drug maintenance therapy. According to Dr Spach, studies involving the combination dolutegravir/rilpivirine indicate that patients who have suppressed HIV RNA levels for at least 6 months on a 3-drug maintenance regimen do well after switching to the 2-drug combination dolutegravir/rilpivirine, as long as they do not have baseline resistance to either dolutegravir or rilpivirine. But he questioned the need for the change if the individual is tolerating a 3-drug regimen well, saying that given the safety of current regimens, the only broader motivating force may be cost savings. For now, he said, if patients are without complaints or tolerability issues on 3 drugs, leave them alone.
INSTIs and weight gain. The last issue is weight gain with the use of INSTIs. Preliminary data suggest disproportionate weight gain with these drugs (on the order of about 6 kg over a year and half, which may be 2-3 kg greater than that which occurs with PI-based and NNRTI-based regimens). At this point, experts do not recommend avoiding these agents, mainly because of the tremendous benefits that have been observed with INSTIs. Dr. Spach concluded, "Although we will continue to use INSTIs widely in clinical practice, there may be a subset of individuals taking an INSTI who have pronounced weight gain and may need to switch to another regimen that does not contain an INSTI.”
Association of Nurses in AIDS Care 2019
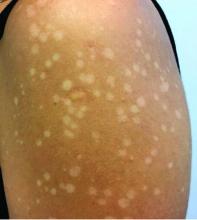
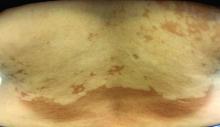
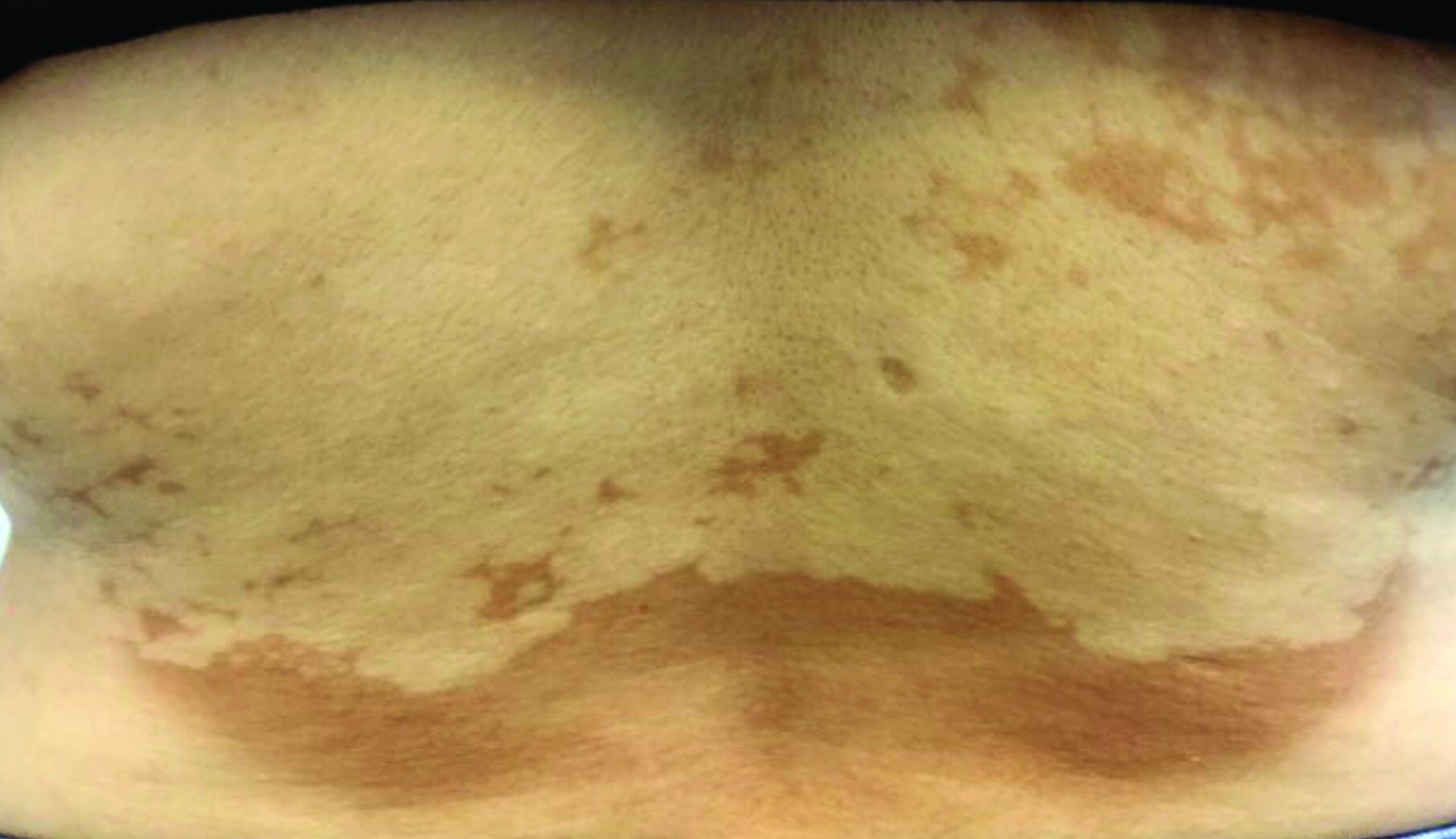
Asymptomatic hypopigmented macules and patches
, also known as Pityrosporum orbiculare or P. ovale. In its hyphal form, it produces skin lesions that appear as scaly, round or oval, hypopigmented, hyperpigmented, or pink macules or patches. Lesions are asymptomatic. The condition is more commonly seen in warm climates or during the summer months. Malassezia requires an oily environment for growth. Typically, TV appears in sebum-producing areas on the trunk. However, other sites may be affected such as the scalp, groin, and flexural areas. Infants may have facial lesions. Hypopigmentation may persist for months, even after lesions are treated, and takes time to resolve.
The differential diagnosis of hypopigmented lesions of tinea versicolor includes vitiligo, hypopigmented mycosis fungoides, progressive macular hypomelanosis (PMH), secondary syphilis, and pityriasis alba. Potassium hydroxide (KOH) preparations can be performed in the office for TV to reveal short, thick fungal hyphae with multiple spores, often referred to as “spaghetti and meatballs.” Use of a Wood’s light may aid in diagnosis. In TV, lesions may fluoresce yellow-green in adjacent follicles, unlike PMH, which characteristically show orange-red follicular fluorescence. A skin biopsy is necessary to rule out hypopgimented mycosis fungoides or syphilis. Histologically in TV, hyphae and spores will be present in the stratum corneum or in hair follicles. These are readily seen with PAS or GMS (Grocott methenamine silver) stains. There is usually no inflammation and skin appears “normal.” A biopsy was performed in this patient that revealed PAS positive hyphae.
Treatment for TV can be topical or systemic. Antifungal azole shampoo or creams, selenium sulfide shampoo, sulfur preparations, and allylamine creams have all been reported as useful treatments. Oral itraconazole or fluconazole are often given as systemic treatments. Monthly or weekly topical therapy may help prevent relapse.
This case and the photos were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
, also known as Pityrosporum orbiculare or P. ovale. In its hyphal form, it produces skin lesions that appear as scaly, round or oval, hypopigmented, hyperpigmented, or pink macules or patches. Lesions are asymptomatic. The condition is more commonly seen in warm climates or during the summer months. Malassezia requires an oily environment for growth. Typically, TV appears in sebum-producing areas on the trunk. However, other sites may be affected such as the scalp, groin, and flexural areas. Infants may have facial lesions. Hypopigmentation may persist for months, even after lesions are treated, and takes time to resolve.
The differential diagnosis of hypopigmented lesions of tinea versicolor includes vitiligo, hypopigmented mycosis fungoides, progressive macular hypomelanosis (PMH), secondary syphilis, and pityriasis alba. Potassium hydroxide (KOH) preparations can be performed in the office for TV to reveal short, thick fungal hyphae with multiple spores, often referred to as “spaghetti and meatballs.” Use of a Wood’s light may aid in diagnosis. In TV, lesions may fluoresce yellow-green in adjacent follicles, unlike PMH, which characteristically show orange-red follicular fluorescence. A skin biopsy is necessary to rule out hypopgimented mycosis fungoides or syphilis. Histologically in TV, hyphae and spores will be present in the stratum corneum or in hair follicles. These are readily seen with PAS or GMS (Grocott methenamine silver) stains. There is usually no inflammation and skin appears “normal.” A biopsy was performed in this patient that revealed PAS positive hyphae.
Treatment for TV can be topical or systemic. Antifungal azole shampoo or creams, selenium sulfide shampoo, sulfur preparations, and allylamine creams have all been reported as useful treatments. Oral itraconazole or fluconazole are often given as systemic treatments. Monthly or weekly topical therapy may help prevent relapse.
This case and the photos were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
, also known as Pityrosporum orbiculare or P. ovale. In its hyphal form, it produces skin lesions that appear as scaly, round or oval, hypopigmented, hyperpigmented, or pink macules or patches. Lesions are asymptomatic. The condition is more commonly seen in warm climates or during the summer months. Malassezia requires an oily environment for growth. Typically, TV appears in sebum-producing areas on the trunk. However, other sites may be affected such as the scalp, groin, and flexural areas. Infants may have facial lesions. Hypopigmentation may persist for months, even after lesions are treated, and takes time to resolve.
The differential diagnosis of hypopigmented lesions of tinea versicolor includes vitiligo, hypopigmented mycosis fungoides, progressive macular hypomelanosis (PMH), secondary syphilis, and pityriasis alba. Potassium hydroxide (KOH) preparations can be performed in the office for TV to reveal short, thick fungal hyphae with multiple spores, often referred to as “spaghetti and meatballs.” Use of a Wood’s light may aid in diagnosis. In TV, lesions may fluoresce yellow-green in adjacent follicles, unlike PMH, which characteristically show orange-red follicular fluorescence. A skin biopsy is necessary to rule out hypopgimented mycosis fungoides or syphilis. Histologically in TV, hyphae and spores will be present in the stratum corneum or in hair follicles. These are readily seen with PAS or GMS (Grocott methenamine silver) stains. There is usually no inflammation and skin appears “normal.” A biopsy was performed in this patient that revealed PAS positive hyphae.
Treatment for TV can be topical or systemic. Antifungal azole shampoo or creams, selenium sulfide shampoo, sulfur preparations, and allylamine creams have all been reported as useful treatments. Oral itraconazole or fluconazole are often given as systemic treatments. Monthly or weekly topical therapy may help prevent relapse.
This case and the photos were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Short-course DAA therapy may prevent hepatitis transmission in transplant patients
BOSTON – A short course of results of a recent study show.
The regimen, given right before transplantation and for 7 days afterward, reduced the cost of direct-acting antiviral (DAA) therapy and allowed patients to complete hepatitis C virus (HCV) therapy before hospital discharge, according to authors of the study, which was presented at the annual meeting of the American Association for the Study of Liver Diseases.
If confirmed in subsequent studies, this regimen could become the standard of care for donor-positive, recipient-negative transplantation, said lead study author Jordan J. Feld, MD, R. Phelan Chair in translational liver disease research at the University of Toronto and research director at the Toronto Centre for Liver Disease.
“Transplant recipients are understandably nervous about accepting organs from people with HCV infection,” said Dr. Feld in a press release. “This very short therapy allows them to leave hospital free of HCV, which is a huge benefit. Not only is it cheaper and likely safer, but the patients really prefer not having to worry about HCV with all of the other challenges after a transplant.”
Results of this study come at a time when the proportion of overdose death organ donors is on the rise, from just 1% in 2000 to 15% in 2016, according to Dr. Feld. Overdose deaths account for the largest percentage of HCV-infected donors, most of whom are young and often otherwise healthy, he added.
Recipients of HCV-infected organs can be cured after transplant as a number of studies have previously shown. However, preventing transmission would be better than cure, Dr. Feld said, in part because of issues with drug-drug interactions, potential for relapse, and issues with procuring the drugs after transplant.
Accordingly, Dr. Feld and colleagues sought to evaluate “preemptive” treatment with DAA therapy combined with ezetimibe, which they said has been shown to inhibit HCV entry blockers. The recipients, who were listed for heart, lung, kidney, or kidney-pancreas transplant, were given glecaprevir/pibrentasvir plus ezetimibe starting 6-12 hours prior to transplantation, and then daily for 7 days.
The median age was 36 years for the 16 donors reported, and 61 years for the 25 recipients. Most recipients (12 patients) had a lung transplant, while 8 had a heart transplant, 4 had a kidney transplant, and 1 had a kidney-pancreas transplant.
There were no virologic failures, according to the investigators, with sustained virologic response (SVR) after 6 weeks in 7 patients, and SVR after 12 weeks in the remaining 18. Three recipients did have detectable HCV RNA, though all cleared and had SVR at 6 weeks in one case, and SVR at 12 weeks in the other two, according to the investigators’ report.
Of 22 serious adverse events noted in the study, 1 was considered treatment related, according to the report, and there were 2 deaths among lung transplant patients, caused by sepsis in 1 case to sepsis and subarachnoid hemorrhage in another.
It’s not clear whether ezetimibe is needed in this short-duration regimen, but in any case, it is well tolerated and inexpensive, and so there is “minimal downside” to include it, Dr. Feld and coinvestigators wrote in their report.
Dr. Feld reported disclosures related to Abbvie, Abbott, Enanta Pharmaceuticals, Gilead, Janssen, Merck, and Roche.
SOURCE: Feld JJ et al. The Liver Meeting 2019, Abstract 38.
BOSTON – A short course of results of a recent study show.
The regimen, given right before transplantation and for 7 days afterward, reduced the cost of direct-acting antiviral (DAA) therapy and allowed patients to complete hepatitis C virus (HCV) therapy before hospital discharge, according to authors of the study, which was presented at the annual meeting of the American Association for the Study of Liver Diseases.
If confirmed in subsequent studies, this regimen could become the standard of care for donor-positive, recipient-negative transplantation, said lead study author Jordan J. Feld, MD, R. Phelan Chair in translational liver disease research at the University of Toronto and research director at the Toronto Centre for Liver Disease.
“Transplant recipients are understandably nervous about accepting organs from people with HCV infection,” said Dr. Feld in a press release. “This very short therapy allows them to leave hospital free of HCV, which is a huge benefit. Not only is it cheaper and likely safer, but the patients really prefer not having to worry about HCV with all of the other challenges after a transplant.”
Results of this study come at a time when the proportion of overdose death organ donors is on the rise, from just 1% in 2000 to 15% in 2016, according to Dr. Feld. Overdose deaths account for the largest percentage of HCV-infected donors, most of whom are young and often otherwise healthy, he added.
Recipients of HCV-infected organs can be cured after transplant as a number of studies have previously shown. However, preventing transmission would be better than cure, Dr. Feld said, in part because of issues with drug-drug interactions, potential for relapse, and issues with procuring the drugs after transplant.
Accordingly, Dr. Feld and colleagues sought to evaluate “preemptive” treatment with DAA therapy combined with ezetimibe, which they said has been shown to inhibit HCV entry blockers. The recipients, who were listed for heart, lung, kidney, or kidney-pancreas transplant, were given glecaprevir/pibrentasvir plus ezetimibe starting 6-12 hours prior to transplantation, and then daily for 7 days.
The median age was 36 years for the 16 donors reported, and 61 years for the 25 recipients. Most recipients (12 patients) had a lung transplant, while 8 had a heart transplant, 4 had a kidney transplant, and 1 had a kidney-pancreas transplant.
There were no virologic failures, according to the investigators, with sustained virologic response (SVR) after 6 weeks in 7 patients, and SVR after 12 weeks in the remaining 18. Three recipients did have detectable HCV RNA, though all cleared and had SVR at 6 weeks in one case, and SVR at 12 weeks in the other two, according to the investigators’ report.
Of 22 serious adverse events noted in the study, 1 was considered treatment related, according to the report, and there were 2 deaths among lung transplant patients, caused by sepsis in 1 case to sepsis and subarachnoid hemorrhage in another.
It’s not clear whether ezetimibe is needed in this short-duration regimen, but in any case, it is well tolerated and inexpensive, and so there is “minimal downside” to include it, Dr. Feld and coinvestigators wrote in their report.
Dr. Feld reported disclosures related to Abbvie, Abbott, Enanta Pharmaceuticals, Gilead, Janssen, Merck, and Roche.
SOURCE: Feld JJ et al. The Liver Meeting 2019, Abstract 38.
BOSTON – A short course of results of a recent study show.
The regimen, given right before transplantation and for 7 days afterward, reduced the cost of direct-acting antiviral (DAA) therapy and allowed patients to complete hepatitis C virus (HCV) therapy before hospital discharge, according to authors of the study, which was presented at the annual meeting of the American Association for the Study of Liver Diseases.
If confirmed in subsequent studies, this regimen could become the standard of care for donor-positive, recipient-negative transplantation, said lead study author Jordan J. Feld, MD, R. Phelan Chair in translational liver disease research at the University of Toronto and research director at the Toronto Centre for Liver Disease.
“Transplant recipients are understandably nervous about accepting organs from people with HCV infection,” said Dr. Feld in a press release. “This very short therapy allows them to leave hospital free of HCV, which is a huge benefit. Not only is it cheaper and likely safer, but the patients really prefer not having to worry about HCV with all of the other challenges after a transplant.”
Results of this study come at a time when the proportion of overdose death organ donors is on the rise, from just 1% in 2000 to 15% in 2016, according to Dr. Feld. Overdose deaths account for the largest percentage of HCV-infected donors, most of whom are young and often otherwise healthy, he added.
Recipients of HCV-infected organs can be cured after transplant as a number of studies have previously shown. However, preventing transmission would be better than cure, Dr. Feld said, in part because of issues with drug-drug interactions, potential for relapse, and issues with procuring the drugs after transplant.
Accordingly, Dr. Feld and colleagues sought to evaluate “preemptive” treatment with DAA therapy combined with ezetimibe, which they said has been shown to inhibit HCV entry blockers. The recipients, who were listed for heart, lung, kidney, or kidney-pancreas transplant, were given glecaprevir/pibrentasvir plus ezetimibe starting 6-12 hours prior to transplantation, and then daily for 7 days.
The median age was 36 years for the 16 donors reported, and 61 years for the 25 recipients. Most recipients (12 patients) had a lung transplant, while 8 had a heart transplant, 4 had a kidney transplant, and 1 had a kidney-pancreas transplant.
There were no virologic failures, according to the investigators, with sustained virologic response (SVR) after 6 weeks in 7 patients, and SVR after 12 weeks in the remaining 18. Three recipients did have detectable HCV RNA, though all cleared and had SVR at 6 weeks in one case, and SVR at 12 weeks in the other two, according to the investigators’ report.
Of 22 serious adverse events noted in the study, 1 was considered treatment related, according to the report, and there were 2 deaths among lung transplant patients, caused by sepsis in 1 case to sepsis and subarachnoid hemorrhage in another.
It’s not clear whether ezetimibe is needed in this short-duration regimen, but in any case, it is well tolerated and inexpensive, and so there is “minimal downside” to include it, Dr. Feld and coinvestigators wrote in their report.
Dr. Feld reported disclosures related to Abbvie, Abbott, Enanta Pharmaceuticals, Gilead, Janssen, Merck, and Roche.
SOURCE: Feld JJ et al. The Liver Meeting 2019, Abstract 38.
REPORTING FROM THE LIVER MEETING 2019
Even low-dose steroids increase DMARD infection risk
ATLANTA – Concomitant use of even low-dose steroids increases the risk of serious infections with antirheumatic drugs, according to a review of 170,357 Medicare patients by investigators at the University of Pennsylvania, Philadelphia.
Infections are a well-known side effect of high-dose glucocorticoids, but there’s been debate about prednisone doses in the 5-10 mg/day range. Guidelines generally advise tapering RA patients off steroids after they start a biologic or methotrexate, but that doesn’t always happen because there’s a common perception that low-dose steroids are safe, said lead investigator Michael George, MD, assistant professor of medicine and epidemiology at the university.
“Many people continue low-dose steroids over the long term, but even low dose seems to be associated with infection. It’s a small risk, but it should be something you are aware of; for some patients, it might be quite important,” he said in an interview at the annual meeting of the American College of Rheumatology.
The team wanted to mimic real-world practice, so they compared infection incidence between the 53% of patients who were not on low-dose steroids with the 47% who were after at least 6 months of disease-modifying antirheumatic drug (DMARD) therapy. About 56% of patients were on methotrexate, with the rest on biologics or a targeted synthetic DMARD (tsDMARD). Average follow up was an additional 6 months, but some people were followed for several years; prednisone 5 mg/day or less was the most common dose.
There were 20,630 serious infections requiring hospitalization, most often urinary tract infection, pneumonia, bacteremia/septicemia, and skin or soft-tissue infections. The crude incidence was 11 per 100 person-years.
After propensity-score weighting to balance out about 50 potential confounders, the predicted 1-year incidence of infection was 9.3% among patients not on steroids. Among those on up to 5 mg/day of prednisone, it was 12.5%; among those on 5-10 mg/day, 17.2%; and among those on more than 10 mg/day, 23.9%.
Glucocorticoids were associated with a 37% increased rate of serious infections, even with doses at or below 5mg/day. The effect “was really similar” whether people were on a biologic, tsDMARD, or methotrexate, which was “surprising,” Dr. George said.
“When I see a patient now who is on long-term, low-dose prednisone, I don’t just say ‘okay, that’s probably safe.’ I think really hard about how much benefit they’re getting. For some people, that means I try to get them off it,” he said. For those who flare otherwise, “I might continue them on it, but recognize there is likely some risk.”
The magnitude of the infection risk was similar to that reported with tumor necrosis factors inhibitors, which might reassure patients who are reluctant to switch to a tumor necrosis factor inhibitor.
“Now I can say you’ve been taking 10 mg prednisone a day, and that’s probably at least as risky,” Dr. George said.
Frequency of office visits, hospitalizations, and ED visits, as well as prior infections, comorbidities, nursing-home admissions, and use of durable medical equipment were among the potential confounders controlled for in the analysis. They stood in for direct markers of RA severity, which weren’t available in the data. “We spent a lot of time trying to make sure our groups were as similar as possible in every way except prednisone use,” he said.
Patients were in their late 60s on average, 71% white, and 81% were women. People with other autoimmune rheumatic diseases, cancer, or HIV were excluded. Dr. George said the next step is to run the same analysis in a younger cohort.
The work was funded by the National Institutes of Health. Dr. George disclosed relationships with AbbVie and Bristol-Myers Squibb.
SOURCE: George M et al. ACR 2019, Abstract 848
ATLANTA – Concomitant use of even low-dose steroids increases the risk of serious infections with antirheumatic drugs, according to a review of 170,357 Medicare patients by investigators at the University of Pennsylvania, Philadelphia.
Infections are a well-known side effect of high-dose glucocorticoids, but there’s been debate about prednisone doses in the 5-10 mg/day range. Guidelines generally advise tapering RA patients off steroids after they start a biologic or methotrexate, but that doesn’t always happen because there’s a common perception that low-dose steroids are safe, said lead investigator Michael George, MD, assistant professor of medicine and epidemiology at the university.
“Many people continue low-dose steroids over the long term, but even low dose seems to be associated with infection. It’s a small risk, but it should be something you are aware of; for some patients, it might be quite important,” he said in an interview at the annual meeting of the American College of Rheumatology.
The team wanted to mimic real-world practice, so they compared infection incidence between the 53% of patients who were not on low-dose steroids with the 47% who were after at least 6 months of disease-modifying antirheumatic drug (DMARD) therapy. About 56% of patients were on methotrexate, with the rest on biologics or a targeted synthetic DMARD (tsDMARD). Average follow up was an additional 6 months, but some people were followed for several years; prednisone 5 mg/day or less was the most common dose.
There were 20,630 serious infections requiring hospitalization, most often urinary tract infection, pneumonia, bacteremia/septicemia, and skin or soft-tissue infections. The crude incidence was 11 per 100 person-years.
After propensity-score weighting to balance out about 50 potential confounders, the predicted 1-year incidence of infection was 9.3% among patients not on steroids. Among those on up to 5 mg/day of prednisone, it was 12.5%; among those on 5-10 mg/day, 17.2%; and among those on more than 10 mg/day, 23.9%.
Glucocorticoids were associated with a 37% increased rate of serious infections, even with doses at or below 5mg/day. The effect “was really similar” whether people were on a biologic, tsDMARD, or methotrexate, which was “surprising,” Dr. George said.
“When I see a patient now who is on long-term, low-dose prednisone, I don’t just say ‘okay, that’s probably safe.’ I think really hard about how much benefit they’re getting. For some people, that means I try to get them off it,” he said. For those who flare otherwise, “I might continue them on it, but recognize there is likely some risk.”
The magnitude of the infection risk was similar to that reported with tumor necrosis factors inhibitors, which might reassure patients who are reluctant to switch to a tumor necrosis factor inhibitor.
“Now I can say you’ve been taking 10 mg prednisone a day, and that’s probably at least as risky,” Dr. George said.
Frequency of office visits, hospitalizations, and ED visits, as well as prior infections, comorbidities, nursing-home admissions, and use of durable medical equipment were among the potential confounders controlled for in the analysis. They stood in for direct markers of RA severity, which weren’t available in the data. “We spent a lot of time trying to make sure our groups were as similar as possible in every way except prednisone use,” he said.
Patients were in their late 60s on average, 71% white, and 81% were women. People with other autoimmune rheumatic diseases, cancer, or HIV were excluded. Dr. George said the next step is to run the same analysis in a younger cohort.
The work was funded by the National Institutes of Health. Dr. George disclosed relationships with AbbVie and Bristol-Myers Squibb.
SOURCE: George M et al. ACR 2019, Abstract 848
ATLANTA – Concomitant use of even low-dose steroids increases the risk of serious infections with antirheumatic drugs, according to a review of 170,357 Medicare patients by investigators at the University of Pennsylvania, Philadelphia.
Infections are a well-known side effect of high-dose glucocorticoids, but there’s been debate about prednisone doses in the 5-10 mg/day range. Guidelines generally advise tapering RA patients off steroids after they start a biologic or methotrexate, but that doesn’t always happen because there’s a common perception that low-dose steroids are safe, said lead investigator Michael George, MD, assistant professor of medicine and epidemiology at the university.
“Many people continue low-dose steroids over the long term, but even low dose seems to be associated with infection. It’s a small risk, but it should be something you are aware of; for some patients, it might be quite important,” he said in an interview at the annual meeting of the American College of Rheumatology.
The team wanted to mimic real-world practice, so they compared infection incidence between the 53% of patients who were not on low-dose steroids with the 47% who were after at least 6 months of disease-modifying antirheumatic drug (DMARD) therapy. About 56% of patients were on methotrexate, with the rest on biologics or a targeted synthetic DMARD (tsDMARD). Average follow up was an additional 6 months, but some people were followed for several years; prednisone 5 mg/day or less was the most common dose.
There were 20,630 serious infections requiring hospitalization, most often urinary tract infection, pneumonia, bacteremia/septicemia, and skin or soft-tissue infections. The crude incidence was 11 per 100 person-years.
After propensity-score weighting to balance out about 50 potential confounders, the predicted 1-year incidence of infection was 9.3% among patients not on steroids. Among those on up to 5 mg/day of prednisone, it was 12.5%; among those on 5-10 mg/day, 17.2%; and among those on more than 10 mg/day, 23.9%.
Glucocorticoids were associated with a 37% increased rate of serious infections, even with doses at or below 5mg/day. The effect “was really similar” whether people were on a biologic, tsDMARD, or methotrexate, which was “surprising,” Dr. George said.
“When I see a patient now who is on long-term, low-dose prednisone, I don’t just say ‘okay, that’s probably safe.’ I think really hard about how much benefit they’re getting. For some people, that means I try to get them off it,” he said. For those who flare otherwise, “I might continue them on it, but recognize there is likely some risk.”
The magnitude of the infection risk was similar to that reported with tumor necrosis factors inhibitors, which might reassure patients who are reluctant to switch to a tumor necrosis factor inhibitor.
“Now I can say you’ve been taking 10 mg prednisone a day, and that’s probably at least as risky,” Dr. George said.
Frequency of office visits, hospitalizations, and ED visits, as well as prior infections, comorbidities, nursing-home admissions, and use of durable medical equipment were among the potential confounders controlled for in the analysis. They stood in for direct markers of RA severity, which weren’t available in the data. “We spent a lot of time trying to make sure our groups were as similar as possible in every way except prednisone use,” he said.
Patients were in their late 60s on average, 71% white, and 81% were women. People with other autoimmune rheumatic diseases, cancer, or HIV were excluded. Dr. George said the next step is to run the same analysis in a younger cohort.
The work was funded by the National Institutes of Health. Dr. George disclosed relationships with AbbVie and Bristol-Myers Squibb.
SOURCE: George M et al. ACR 2019, Abstract 848
REPORTING FROM ACR 2019
Don’t let a foodborne illness dampen the holiday season
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.
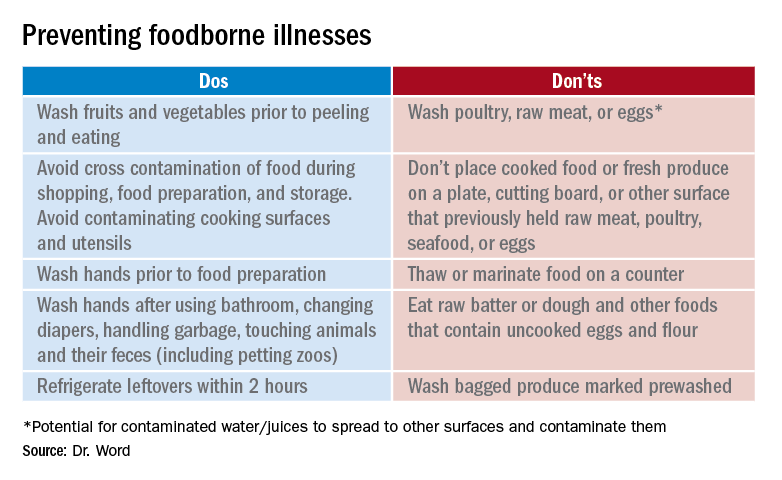
Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
Oral vs. IV antibiotic therapy in early treatment of complex bone and joint infections
Background: The standard of care for complex bone and joint infections includes the use of IV antibiotics. A prior meta-analysis suggested that the outcomes for bone and joint infections treated with oral and IV antibiotics are similar.
Study design: Randomized, controlled trial.
Setting: Twenty-six U.K. sites during June 2010–October 2015.
Synopsis: The study enrolled 1,054 adults with bone or joint infections who would have been treated with 6 weeks of IV antibiotics; they were then randomized to receive either IV or oral antibiotics. Treatment regimens were selected by infectious disease specialists. The rate of the primary endpoint, definite treatment failure at 1 year after randomization, was 14.6% in the intravenous group and 13.2% in the oral group. The difference in the risk of definite treatment failure between the two groups was –1.4% (95% confidence interval, –5.6 to 2.9), which met the predefined noninferiority criteria. The use of oral antibiotics also was associated with a shorter hospital stay and fewer complications. The conclusions of the trial are limited by the open-label design. An associated editorial advocated for additional research before widespread change to current treatment recommendations.
Bottom line: Bone and joint infections treated with oral versus IV antibiotics may have similar treatment failure rates.
Citation: Li HK et al. Oral versus intravenous antibiotics for bone and joint infection. N Eng J Med. 2019 Jan 31;380(5):425-36.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine at Harvard Medical School.
Background: The standard of care for complex bone and joint infections includes the use of IV antibiotics. A prior meta-analysis suggested that the outcomes for bone and joint infections treated with oral and IV antibiotics are similar.
Study design: Randomized, controlled trial.
Setting: Twenty-six U.K. sites during June 2010–October 2015.
Synopsis: The study enrolled 1,054 adults with bone or joint infections who would have been treated with 6 weeks of IV antibiotics; they were then randomized to receive either IV or oral antibiotics. Treatment regimens were selected by infectious disease specialists. The rate of the primary endpoint, definite treatment failure at 1 year after randomization, was 14.6% in the intravenous group and 13.2% in the oral group. The difference in the risk of definite treatment failure between the two groups was –1.4% (95% confidence interval, –5.6 to 2.9), which met the predefined noninferiority criteria. The use of oral antibiotics also was associated with a shorter hospital stay and fewer complications. The conclusions of the trial are limited by the open-label design. An associated editorial advocated for additional research before widespread change to current treatment recommendations.
Bottom line: Bone and joint infections treated with oral versus IV antibiotics may have similar treatment failure rates.
Citation: Li HK et al. Oral versus intravenous antibiotics for bone and joint infection. N Eng J Med. 2019 Jan 31;380(5):425-36.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine at Harvard Medical School.
Background: The standard of care for complex bone and joint infections includes the use of IV antibiotics. A prior meta-analysis suggested that the outcomes for bone and joint infections treated with oral and IV antibiotics are similar.
Study design: Randomized, controlled trial.
Setting: Twenty-six U.K. sites during June 2010–October 2015.
Synopsis: The study enrolled 1,054 adults with bone or joint infections who would have been treated with 6 weeks of IV antibiotics; they were then randomized to receive either IV or oral antibiotics. Treatment regimens were selected by infectious disease specialists. The rate of the primary endpoint, definite treatment failure at 1 year after randomization, was 14.6% in the intravenous group and 13.2% in the oral group. The difference in the risk of definite treatment failure between the two groups was –1.4% (95% confidence interval, –5.6 to 2.9), which met the predefined noninferiority criteria. The use of oral antibiotics also was associated with a shorter hospital stay and fewer complications. The conclusions of the trial are limited by the open-label design. An associated editorial advocated for additional research before widespread change to current treatment recommendations.
Bottom line: Bone and joint infections treated with oral versus IV antibiotics may have similar treatment failure rates.
Citation: Li HK et al. Oral versus intravenous antibiotics for bone and joint infection. N Eng J Med. 2019 Jan 31;380(5):425-36.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine at Harvard Medical School.
Tide beginning to turn on vaccine hesitancy
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
HDV combo therapy reduces viral loads
BOSTON – For most patients with chronic hepatitis D virus (HDV) infection, combination therapy with lonafarnib, ritonavir, and peginterferon may significantly decrease viral loads, based on interim results from the phase IIa LIFT trial.
After 6 months of therapy, more than one-third of evaluable patients (37%) achieved undetectable levels of HDV RNA in serum, according to lead author Christopher Koh, MD, of the National Institute of Diabetes, Digestive and Kidney Diseases at the National Institutes of Health and colleagues.
The open-label LIFT trial, which is ongoing, initially recruited 26 patients with HDV RNA who had serum levels of at least 40 IU/mL (lower limit of quantification). After starting tenofovir or entecavir, patients began a combination regimen of twice-daily oral lonafarnib (50 mg) and ritonavir (100 mg) plus weekly subcutaneous injections of Peginterferon Lambda-1a (180 mcg).
The median patient age was 40 years, with a slightly higher proportion of male participants (60%). Approximately half of the patients were of Asian descent (52%), followed by patients who were white (32%), or African (16%). The investigators reported median baseline measurements of modified histology activity index (9) and Ishak fibrosis stage (3), as well as serum levels of alanine aminotransferase (64 IU/mL), aspartate aminotransferase (47 IU/mL), hepatitis B virus DNA (less than 21 IU/mL), and log HDV RNA (4.74 IU/mL), with this latter measurement serving as a key determinant of efficacy.
After 12 weeks of therapy, the median decrease in HDV RNA among 21 evaluable patients was 3.6 log IU/mL with an interquartile range from 2.6 to 4.2 (P less than .0001). Of these patients, 5 (24%) achieved undetectable levels of HDV RNA, while another 5 tested below the lower limit of quantification.
Following an additional 12 weeks of therapy, 19 patients remained evaluable, among whom the median decrease in HDV RNA was 3.4 log IU/mL with an interquartile range from 2.9 to 4.5 (P less than .0001). Seven of these patients (37%) achieved undetectable HDV RNA, whereas 3 others fell below the lower limit of quantification. Furthermore, 18 out of 19 of these patients (95%) experienced a decline in HDV RNA of more than 2 log IU/mL.
According to the investigators, the trial regimen was safe and well tolerated. Adverse events were mild to moderate; most common were anemia, hyperbilirubinemia, weight loss, and gastrointestinal issues. Doses were reduced in three patients while four others discontinued therapy prematurely.
“These interim results support continued exploration of this therapeutic combination in HDV,” the investigators concluded.
The above findings will be presented in an oral abstract session at the annual meeting of the American Association for the Study of Liver Diseases.
The investigators disclosed relationships with I-Cubed Therapeutics, Eiger BioPharmaceuticals, Riboscience, and others.
SOURCE: Koh C et al. The Liver Meeting 2019. Abstract LO8.
BOSTON – For most patients with chronic hepatitis D virus (HDV) infection, combination therapy with lonafarnib, ritonavir, and peginterferon may significantly decrease viral loads, based on interim results from the phase IIa LIFT trial.
After 6 months of therapy, more than one-third of evaluable patients (37%) achieved undetectable levels of HDV RNA in serum, according to lead author Christopher Koh, MD, of the National Institute of Diabetes, Digestive and Kidney Diseases at the National Institutes of Health and colleagues.
The open-label LIFT trial, which is ongoing, initially recruited 26 patients with HDV RNA who had serum levels of at least 40 IU/mL (lower limit of quantification). After starting tenofovir or entecavir, patients began a combination regimen of twice-daily oral lonafarnib (50 mg) and ritonavir (100 mg) plus weekly subcutaneous injections of Peginterferon Lambda-1a (180 mcg).
The median patient age was 40 years, with a slightly higher proportion of male participants (60%). Approximately half of the patients were of Asian descent (52%), followed by patients who were white (32%), or African (16%). The investigators reported median baseline measurements of modified histology activity index (9) and Ishak fibrosis stage (3), as well as serum levels of alanine aminotransferase (64 IU/mL), aspartate aminotransferase (47 IU/mL), hepatitis B virus DNA (less than 21 IU/mL), and log HDV RNA (4.74 IU/mL), with this latter measurement serving as a key determinant of efficacy.
After 12 weeks of therapy, the median decrease in HDV RNA among 21 evaluable patients was 3.6 log IU/mL with an interquartile range from 2.6 to 4.2 (P less than .0001). Of these patients, 5 (24%) achieved undetectable levels of HDV RNA, while another 5 tested below the lower limit of quantification.
Following an additional 12 weeks of therapy, 19 patients remained evaluable, among whom the median decrease in HDV RNA was 3.4 log IU/mL with an interquartile range from 2.9 to 4.5 (P less than .0001). Seven of these patients (37%) achieved undetectable HDV RNA, whereas 3 others fell below the lower limit of quantification. Furthermore, 18 out of 19 of these patients (95%) experienced a decline in HDV RNA of more than 2 log IU/mL.
According to the investigators, the trial regimen was safe and well tolerated. Adverse events were mild to moderate; most common were anemia, hyperbilirubinemia, weight loss, and gastrointestinal issues. Doses were reduced in three patients while four others discontinued therapy prematurely.
“These interim results support continued exploration of this therapeutic combination in HDV,” the investigators concluded.
The above findings will be presented in an oral abstract session at the annual meeting of the American Association for the Study of Liver Diseases.
The investigators disclosed relationships with I-Cubed Therapeutics, Eiger BioPharmaceuticals, Riboscience, and others.
SOURCE: Koh C et al. The Liver Meeting 2019. Abstract LO8.
BOSTON – For most patients with chronic hepatitis D virus (HDV) infection, combination therapy with lonafarnib, ritonavir, and peginterferon may significantly decrease viral loads, based on interim results from the phase IIa LIFT trial.
After 6 months of therapy, more than one-third of evaluable patients (37%) achieved undetectable levels of HDV RNA in serum, according to lead author Christopher Koh, MD, of the National Institute of Diabetes, Digestive and Kidney Diseases at the National Institutes of Health and colleagues.
The open-label LIFT trial, which is ongoing, initially recruited 26 patients with HDV RNA who had serum levels of at least 40 IU/mL (lower limit of quantification). After starting tenofovir or entecavir, patients began a combination regimen of twice-daily oral lonafarnib (50 mg) and ritonavir (100 mg) plus weekly subcutaneous injections of Peginterferon Lambda-1a (180 mcg).
The median patient age was 40 years, with a slightly higher proportion of male participants (60%). Approximately half of the patients were of Asian descent (52%), followed by patients who were white (32%), or African (16%). The investigators reported median baseline measurements of modified histology activity index (9) and Ishak fibrosis stage (3), as well as serum levels of alanine aminotransferase (64 IU/mL), aspartate aminotransferase (47 IU/mL), hepatitis B virus DNA (less than 21 IU/mL), and log HDV RNA (4.74 IU/mL), with this latter measurement serving as a key determinant of efficacy.
After 12 weeks of therapy, the median decrease in HDV RNA among 21 evaluable patients was 3.6 log IU/mL with an interquartile range from 2.6 to 4.2 (P less than .0001). Of these patients, 5 (24%) achieved undetectable levels of HDV RNA, while another 5 tested below the lower limit of quantification.
Following an additional 12 weeks of therapy, 19 patients remained evaluable, among whom the median decrease in HDV RNA was 3.4 log IU/mL with an interquartile range from 2.9 to 4.5 (P less than .0001). Seven of these patients (37%) achieved undetectable HDV RNA, whereas 3 others fell below the lower limit of quantification. Furthermore, 18 out of 19 of these patients (95%) experienced a decline in HDV RNA of more than 2 log IU/mL.
According to the investigators, the trial regimen was safe and well tolerated. Adverse events were mild to moderate; most common were anemia, hyperbilirubinemia, weight loss, and gastrointestinal issues. Doses were reduced in three patients while four others discontinued therapy prematurely.
“These interim results support continued exploration of this therapeutic combination in HDV,” the investigators concluded.
The above findings will be presented in an oral abstract session at the annual meeting of the American Association for the Study of Liver Diseases.
The investigators disclosed relationships with I-Cubed Therapeutics, Eiger BioPharmaceuticals, Riboscience, and others.
SOURCE: Koh C et al. The Liver Meeting 2019. Abstract LO8.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: For most patients with chronic hepatitis D virus infection, combination therapy with lonafarnib, ritonavir, and peginterferon may significantly decrease viral loads.
Major finding: After 6 months of therapy, 37% of evaluable patients achieved undetectable levels of hepatitis D virus RNA.
Study details: The phase IIa open-label LIFT trial involving 26 patients with chronic hepatitis delta virus (HDV).
Disclosures: The investigators disclosed relationships with I-Cubed Therapeutics, Eiger BioPharmaceuticals, Riboscience, and others.
Source: Koh C et al. The Liver Meeting 2019. Abstract LO8.
Hepatitis C vaccine alters viral trajectory, but fails in chronic infection protection
BOSTON – A prime-boost hepatitis C virus (HCV) vaccine regimen did not protect against chronic infection, but it did evoke immune responses and differences in viral trajectory, according to investigators in what is believed to be the first randomized, placebo-controlled efficacy trial in this setting.
There were no apparent safety concerns with the vaccine according to investigators, led by Kimberly Page, PhD, MPH, of the University of New Mexico, Albuquerque.
“A safe and effective vaccine to prevent chronic hepatitis C virus infection is essential to reduce transmission,” Dr. Page and coauthors said in a late-breaking abstract of the study results, which will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase 1/2 trial described by Dr. Page and colleagues included 455 adults at risk of HCV infection because of injection drug use. They were randomized to vaccine, which consisted of a recombinant chimpanzee adenovirus-3 vectored vaccine prime plus a recombinant Modified Vaccinia virus Ankara boost, or to two doses of placebo at days 0 and 56 of the study.
There was no difference in chronic HCV infection at 6 months, the primary endpoint of the study. There were 14 chronically infected participants in the vaccine group, as well as 14 in the placebo group, for an overall incidence of infection of 13.0/100 person-years, Dr. Page and coauthors reported in the abstract.
However, there were significant differences in HCV RNA geometric mean peak at 1 month, which was 193,795 IU/L in the vaccine group and 1,078,092 IU/L in the placebo group, according to investigators. Similarly, geometric mean fold rise after infection was 0.2 in the vaccine group and 13.5 in the placebo group.
A total of 78% of vaccinated individuals had T-cell responses to at least one vaccine antigen pool, investigators said, adding that the vaccine was safe, well tolerated, and not associated with any serious adverse events.
Dr. Page had no disclosures related to the abstract.
SOURCE: Page K et al. The Liver Meeting 2019. Abstract LP17.
BOSTON – A prime-boost hepatitis C virus (HCV) vaccine regimen did not protect against chronic infection, but it did evoke immune responses and differences in viral trajectory, according to investigators in what is believed to be the first randomized, placebo-controlled efficacy trial in this setting.
There were no apparent safety concerns with the vaccine according to investigators, led by Kimberly Page, PhD, MPH, of the University of New Mexico, Albuquerque.
“A safe and effective vaccine to prevent chronic hepatitis C virus infection is essential to reduce transmission,” Dr. Page and coauthors said in a late-breaking abstract of the study results, which will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase 1/2 trial described by Dr. Page and colleagues included 455 adults at risk of HCV infection because of injection drug use. They were randomized to vaccine, which consisted of a recombinant chimpanzee adenovirus-3 vectored vaccine prime plus a recombinant Modified Vaccinia virus Ankara boost, or to two doses of placebo at days 0 and 56 of the study.
There was no difference in chronic HCV infection at 6 months, the primary endpoint of the study. There were 14 chronically infected participants in the vaccine group, as well as 14 in the placebo group, for an overall incidence of infection of 13.0/100 person-years, Dr. Page and coauthors reported in the abstract.
However, there were significant differences in HCV RNA geometric mean peak at 1 month, which was 193,795 IU/L in the vaccine group and 1,078,092 IU/L in the placebo group, according to investigators. Similarly, geometric mean fold rise after infection was 0.2 in the vaccine group and 13.5 in the placebo group.
A total of 78% of vaccinated individuals had T-cell responses to at least one vaccine antigen pool, investigators said, adding that the vaccine was safe, well tolerated, and not associated with any serious adverse events.
Dr. Page had no disclosures related to the abstract.
SOURCE: Page K et al. The Liver Meeting 2019. Abstract LP17.
BOSTON – A prime-boost hepatitis C virus (HCV) vaccine regimen did not protect against chronic infection, but it did evoke immune responses and differences in viral trajectory, according to investigators in what is believed to be the first randomized, placebo-controlled efficacy trial in this setting.
There were no apparent safety concerns with the vaccine according to investigators, led by Kimberly Page, PhD, MPH, of the University of New Mexico, Albuquerque.
“A safe and effective vaccine to prevent chronic hepatitis C virus infection is essential to reduce transmission,” Dr. Page and coauthors said in a late-breaking abstract of the study results, which will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase 1/2 trial described by Dr. Page and colleagues included 455 adults at risk of HCV infection because of injection drug use. They were randomized to vaccine, which consisted of a recombinant chimpanzee adenovirus-3 vectored vaccine prime plus a recombinant Modified Vaccinia virus Ankara boost, or to two doses of placebo at days 0 and 56 of the study.
There was no difference in chronic HCV infection at 6 months, the primary endpoint of the study. There were 14 chronically infected participants in the vaccine group, as well as 14 in the placebo group, for an overall incidence of infection of 13.0/100 person-years, Dr. Page and coauthors reported in the abstract.
However, there were significant differences in HCV RNA geometric mean peak at 1 month, which was 193,795 IU/L in the vaccine group and 1,078,092 IU/L in the placebo group, according to investigators. Similarly, geometric mean fold rise after infection was 0.2 in the vaccine group and 13.5 in the placebo group.
A total of 78% of vaccinated individuals had T-cell responses to at least one vaccine antigen pool, investigators said, adding that the vaccine was safe, well tolerated, and not associated with any serious adverse events.
Dr. Page had no disclosures related to the abstract.
SOURCE: Page K et al. The Liver Meeting 2019. Abstract LP17.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: A prime-boost HCV vaccine altered viral trajectory but did not protect against chronic infection.
Major finding: At 6 months after vaccination, there were 14 chronically infected participants in the vaccine group, and 14 in the placebo group.
Study details: A randomized, placebo controlled phase 1/2 trial including 455 adults at risk of HCV infection.
Disclosures: The first author reported no disclosures.
Source: Page K et al. The Liver Meeting 2019. Abstract LP17.