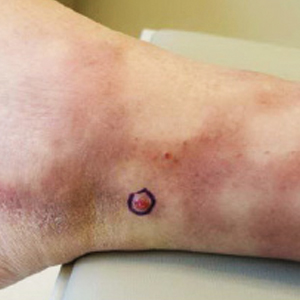
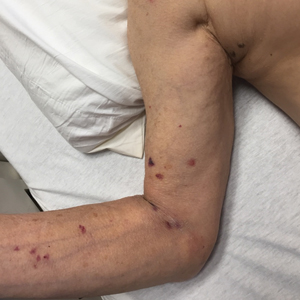
User login
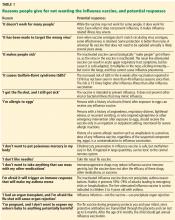
How to respond to flu vaccine doubters
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
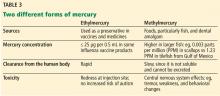
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
Click for Credit: PPI use & dementia; Weight loss after gastroplasty; more
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
ART treatment at birth found to benefit neonates with HIV
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Antiretroviral treatment initiation immediately after birth reduced HIV-1 viral reservoir size and alters innate immune responses in neonates.
Major finding: Very-early ART intervention in neonates infected with HIV limited the number of virally infected cells and improves immune response.
Study details: A cohort study of 10 infants infected with HIV who were born in Botswana, Africa.
Disclosures: The Early Infant Treatment study is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
Source: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
FDA approves Tula system for recurrent pediatric ear infections
The Food and Drug Administration has approved the Tubes Under Local Anesthesia (Tula) System for treatment of recurrent ear infections (otitis media) via tympanostomy in young children, according to a release from the agency.
Consisting of Tymbion anesthetic, tympanostomy tubes developed by Tusker Medical, and several devices that deliver them into the ear drum,
“This approval has the potential to expand patient access to a treatment that can be administered in a physician’s office with local anesthesia and minimal discomfort,” said Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health.
The approval was based on data from 222 children treated with the device, with a procedural success rate of 86% in children under 5 years and 89% in children aged 5-12 years. The most common adverse event was insufficient anesthetic.
The system should not be used in children with allergies to some local anesthetics or those younger than 6 months. It also is not intended for patients with preexisting issues with their eardrums, such as perforated ear drums, according to the press release.
The Tula system was granted a Breakthrough Device designation, which means the FDA provided intensive engagement and guidance during its development. The full release can be found on the FDA website.
The Food and Drug Administration has approved the Tubes Under Local Anesthesia (Tula) System for treatment of recurrent ear infections (otitis media) via tympanostomy in young children, according to a release from the agency.
Consisting of Tymbion anesthetic, tympanostomy tubes developed by Tusker Medical, and several devices that deliver them into the ear drum,
“This approval has the potential to expand patient access to a treatment that can be administered in a physician’s office with local anesthesia and minimal discomfort,” said Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health.
The approval was based on data from 222 children treated with the device, with a procedural success rate of 86% in children under 5 years and 89% in children aged 5-12 years. The most common adverse event was insufficient anesthetic.
The system should not be used in children with allergies to some local anesthetics or those younger than 6 months. It also is not intended for patients with preexisting issues with their eardrums, such as perforated ear drums, according to the press release.
The Tula system was granted a Breakthrough Device designation, which means the FDA provided intensive engagement and guidance during its development. The full release can be found on the FDA website.
The Food and Drug Administration has approved the Tubes Under Local Anesthesia (Tula) System for treatment of recurrent ear infections (otitis media) via tympanostomy in young children, according to a release from the agency.
Consisting of Tymbion anesthetic, tympanostomy tubes developed by Tusker Medical, and several devices that deliver them into the ear drum,
“This approval has the potential to expand patient access to a treatment that can be administered in a physician’s office with local anesthesia and minimal discomfort,” said Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health.
The approval was based on data from 222 children treated with the device, with a procedural success rate of 86% in children under 5 years and 89% in children aged 5-12 years. The most common adverse event was insufficient anesthetic.
The system should not be used in children with allergies to some local anesthetics or those younger than 6 months. It also is not intended for patients with preexisting issues with their eardrums, such as perforated ear drums, according to the press release.
The Tula system was granted a Breakthrough Device designation, which means the FDA provided intensive engagement and guidance during its development. The full release can be found on the FDA website.
Kaposi Sarcoma in a Patient With Postpolio Syndrome
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.
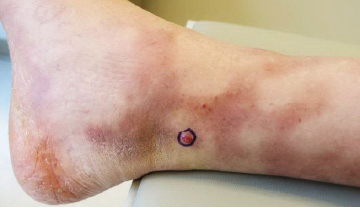
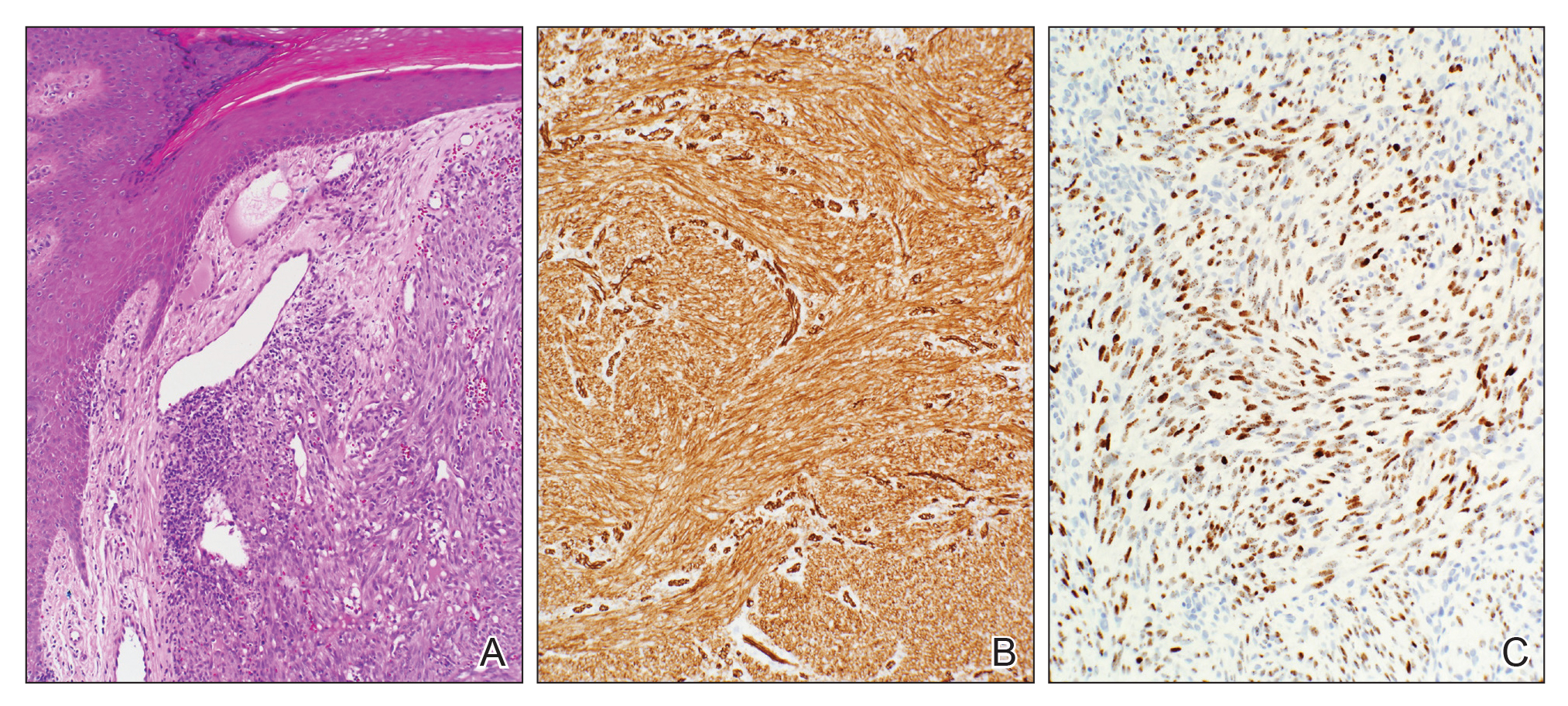
Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).
Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.


Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).


Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.


Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).


Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
Practice Points
- Cutaneous Kaposi sarcoma (KS) is a human herpesvirus 8–positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.
- In addition, patients with postpolio syndrome characterized by venous insufficiency may represent a population at increased risk for KS.
- Kaposi sarcoma is a radiosensitive vascular neoplasm, and radiation therapy can achieve local control.
What’s new in hepatitis C: Four themes that dominated at the Liver Meeting
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
REPORTING FROM THE LIVER MEETING 2019
Guideline: Diagnosis and treatment of adults with community-acquired pneumonia
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Two national analyses confirm safety of 9vHPV vaccine
most of which cannot be definitively tied to the vaccine, according to two large studies published simultaneously in Pediatrics.
“The body of evidence on the safety of 9vHPV now includes prelicensure clinical trial data on 15,000 study subjects, reassuring results from postlicensure near real-time sequential monitoring by the Centers for Disease Control and Prevention’s Vaccine Safety Datalink, on approximately 839 000 doses administered, and our review of VAERS [Vaccine Adverse Event Reporting System] reports over a 3-year period, during which time approximately 28 million doses were distributed in the United States,” Tom T. Shimabukuro, MD, and colleagues reported in Pediatrics.
James G. Donahue, PhD, and colleagues, authors of the Vaccine Safety Datalink study published in the same issue, concluded much the same thing.
The new numbers bolster extant safety data on the vaccine, which was approved in 2015, wrote Dr. Donahue, an epidemiologist at the Marshfield (Wis.) Clinic Research Institute, and coauthors. “With this large observational study, we contribute reassuring postlicensure data that will help bolster the safety profile of 9vHPV. Although we detected several unexpected potential safety signals, none were confirmed after further evaluation.”
The Vaccine Safety Datalink study of 838,991 doses looked for safety signals in a prespecified group of potential events, including anaphylaxis, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, pancreatitis, seizures, stroke, and venous thromboembolism.
Dr. Donahue and coauthors used real-time vaccination data and time-matched historical controls to evaluate any changes in expected disease rates, compared with those occurring in vaccine recipients.
Most doses in the study (76%) were given to children aged 9-17 years, with 48% going to girls. The remaining 24% of doses were given to persons aged 18-26 years, with 64% going to women.
The analysis found potential safety signals in allergic reactions (43 cases), appendicitis (30 cases), pancreatitis (8 cases), and syncope (67). None of these were confirmed after further investigation.
“The safety profile of 9vHPV is favorable and comparable to that of its predecessor, 4vHPV,” Dr. Donahue and associates concluded.
The VAERS analysis was similarly reassuring. It examined all reported adverse events, not predetermined events.
Among 28 million doses, there were 7,244 adverse event reports – a rate of about 1 event per 7 million doses. Of these, 97% were nonserious, wrote Dr. Shimabukuro, deputy director of the CDC’s Immunization Safety Office, and colleagues.
The vaccine manufacturer submitted 64% of these to VAERS; health care providers submitted 27%. Adverse events were reported from postvaccine day 0 to 2 years afterward. 9vHPV was the only vaccine given in 75% of reports. Coadministered vaccines included meningococcal conjugate (1,028); tetanus and diphtheria (Td) or Tdap (673); and hepatitis A (434).
There were nine reports of anaphylaxis (five males, four females); 9vHPV was the only vaccine administered in five cases. Three reports involved coadministration of meningococcal vaccine, two with hepatitis A, one with TDaP, and one with varicella.
There were eight reports of Guillain-Barré.
There were 17 reports of postural orthostatic tachycardia syndrome, most of which (71%) did not meet diagnostic criteria. Five cases, however, did.
One possible case of complex regional pain syndrome was reported in a 13-year-old girl with comorbid anxiety.
There were two reports of acute disseminated encephalomyelitis, both in boys. There were no reports of transverse myelitis or chronic inflammatory demyelinating polyneuropathy.
Seven vaccine recipients died after vaccination. Five of these reports did not contain medical information or any proof-of-death confirmation. The other two were verified by autopsy. A 14-year-old girl who received a flu vaccination with 9vHPV died of a thoracic aorta dissection 7 days postvaccination. The other death was a 16-year-old boy who received a concurrent hepatitis A vaccine. Four days later, he died of a cerebellar hemorrhage.
“We did not identify any unusual or unexpected safety concerns in our review of 9vHPV reports to the VAERS; most (97%) reports were nonserious, and adverse events were analogous to those observed in the prelicensure clinical trials,” Dr. Shimabukuro and associates concluded.
Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
SOURCES: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
most of which cannot be definitively tied to the vaccine, according to two large studies published simultaneously in Pediatrics.
“The body of evidence on the safety of 9vHPV now includes prelicensure clinical trial data on 15,000 study subjects, reassuring results from postlicensure near real-time sequential monitoring by the Centers for Disease Control and Prevention’s Vaccine Safety Datalink, on approximately 839 000 doses administered, and our review of VAERS [Vaccine Adverse Event Reporting System] reports over a 3-year period, during which time approximately 28 million doses were distributed in the United States,” Tom T. Shimabukuro, MD, and colleagues reported in Pediatrics.
James G. Donahue, PhD, and colleagues, authors of the Vaccine Safety Datalink study published in the same issue, concluded much the same thing.
The new numbers bolster extant safety data on the vaccine, which was approved in 2015, wrote Dr. Donahue, an epidemiologist at the Marshfield (Wis.) Clinic Research Institute, and coauthors. “With this large observational study, we contribute reassuring postlicensure data that will help bolster the safety profile of 9vHPV. Although we detected several unexpected potential safety signals, none were confirmed after further evaluation.”
The Vaccine Safety Datalink study of 838,991 doses looked for safety signals in a prespecified group of potential events, including anaphylaxis, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, pancreatitis, seizures, stroke, and venous thromboembolism.
Dr. Donahue and coauthors used real-time vaccination data and time-matched historical controls to evaluate any changes in expected disease rates, compared with those occurring in vaccine recipients.
Most doses in the study (76%) were given to children aged 9-17 years, with 48% going to girls. The remaining 24% of doses were given to persons aged 18-26 years, with 64% going to women.
The analysis found potential safety signals in allergic reactions (43 cases), appendicitis (30 cases), pancreatitis (8 cases), and syncope (67). None of these were confirmed after further investigation.
“The safety profile of 9vHPV is favorable and comparable to that of its predecessor, 4vHPV,” Dr. Donahue and associates concluded.
The VAERS analysis was similarly reassuring. It examined all reported adverse events, not predetermined events.
Among 28 million doses, there were 7,244 adverse event reports – a rate of about 1 event per 7 million doses. Of these, 97% were nonserious, wrote Dr. Shimabukuro, deputy director of the CDC’s Immunization Safety Office, and colleagues.
The vaccine manufacturer submitted 64% of these to VAERS; health care providers submitted 27%. Adverse events were reported from postvaccine day 0 to 2 years afterward. 9vHPV was the only vaccine given in 75% of reports. Coadministered vaccines included meningococcal conjugate (1,028); tetanus and diphtheria (Td) or Tdap (673); and hepatitis A (434).
There were nine reports of anaphylaxis (five males, four females); 9vHPV was the only vaccine administered in five cases. Three reports involved coadministration of meningococcal vaccine, two with hepatitis A, one with TDaP, and one with varicella.
There were eight reports of Guillain-Barré.
There were 17 reports of postural orthostatic tachycardia syndrome, most of which (71%) did not meet diagnostic criteria. Five cases, however, did.
One possible case of complex regional pain syndrome was reported in a 13-year-old girl with comorbid anxiety.
There were two reports of acute disseminated encephalomyelitis, both in boys. There were no reports of transverse myelitis or chronic inflammatory demyelinating polyneuropathy.
Seven vaccine recipients died after vaccination. Five of these reports did not contain medical information or any proof-of-death confirmation. The other two were verified by autopsy. A 14-year-old girl who received a flu vaccination with 9vHPV died of a thoracic aorta dissection 7 days postvaccination. The other death was a 16-year-old boy who received a concurrent hepatitis A vaccine. Four days later, he died of a cerebellar hemorrhage.
“We did not identify any unusual or unexpected safety concerns in our review of 9vHPV reports to the VAERS; most (97%) reports were nonserious, and adverse events were analogous to those observed in the prelicensure clinical trials,” Dr. Shimabukuro and associates concluded.
Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
SOURCES: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
most of which cannot be definitively tied to the vaccine, according to two large studies published simultaneously in Pediatrics.
“The body of evidence on the safety of 9vHPV now includes prelicensure clinical trial data on 15,000 study subjects, reassuring results from postlicensure near real-time sequential monitoring by the Centers for Disease Control and Prevention’s Vaccine Safety Datalink, on approximately 839 000 doses administered, and our review of VAERS [Vaccine Adverse Event Reporting System] reports over a 3-year period, during which time approximately 28 million doses were distributed in the United States,” Tom T. Shimabukuro, MD, and colleagues reported in Pediatrics.
James G. Donahue, PhD, and colleagues, authors of the Vaccine Safety Datalink study published in the same issue, concluded much the same thing.
The new numbers bolster extant safety data on the vaccine, which was approved in 2015, wrote Dr. Donahue, an epidemiologist at the Marshfield (Wis.) Clinic Research Institute, and coauthors. “With this large observational study, we contribute reassuring postlicensure data that will help bolster the safety profile of 9vHPV. Although we detected several unexpected potential safety signals, none were confirmed after further evaluation.”
The Vaccine Safety Datalink study of 838,991 doses looked for safety signals in a prespecified group of potential events, including anaphylaxis, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, pancreatitis, seizures, stroke, and venous thromboembolism.
Dr. Donahue and coauthors used real-time vaccination data and time-matched historical controls to evaluate any changes in expected disease rates, compared with those occurring in vaccine recipients.
Most doses in the study (76%) were given to children aged 9-17 years, with 48% going to girls. The remaining 24% of doses were given to persons aged 18-26 years, with 64% going to women.
The analysis found potential safety signals in allergic reactions (43 cases), appendicitis (30 cases), pancreatitis (8 cases), and syncope (67). None of these were confirmed after further investigation.
“The safety profile of 9vHPV is favorable and comparable to that of its predecessor, 4vHPV,” Dr. Donahue and associates concluded.
The VAERS analysis was similarly reassuring. It examined all reported adverse events, not predetermined events.
Among 28 million doses, there were 7,244 adverse event reports – a rate of about 1 event per 7 million doses. Of these, 97% were nonserious, wrote Dr. Shimabukuro, deputy director of the CDC’s Immunization Safety Office, and colleagues.
The vaccine manufacturer submitted 64% of these to VAERS; health care providers submitted 27%. Adverse events were reported from postvaccine day 0 to 2 years afterward. 9vHPV was the only vaccine given in 75% of reports. Coadministered vaccines included meningococcal conjugate (1,028); tetanus and diphtheria (Td) or Tdap (673); and hepatitis A (434).
There were nine reports of anaphylaxis (five males, four females); 9vHPV was the only vaccine administered in five cases. Three reports involved coadministration of meningococcal vaccine, two with hepatitis A, one with TDaP, and one with varicella.
There were eight reports of Guillain-Barré.
There were 17 reports of postural orthostatic tachycardia syndrome, most of which (71%) did not meet diagnostic criteria. Five cases, however, did.
One possible case of complex regional pain syndrome was reported in a 13-year-old girl with comorbid anxiety.
There were two reports of acute disseminated encephalomyelitis, both in boys. There were no reports of transverse myelitis or chronic inflammatory demyelinating polyneuropathy.
Seven vaccine recipients died after vaccination. Five of these reports did not contain medical information or any proof-of-death confirmation. The other two were verified by autopsy. A 14-year-old girl who received a flu vaccination with 9vHPV died of a thoracic aorta dissection 7 days postvaccination. The other death was a 16-year-old boy who received a concurrent hepatitis A vaccine. Four days later, he died of a cerebellar hemorrhage.
“We did not identify any unusual or unexpected safety concerns in our review of 9vHPV reports to the VAERS; most (97%) reports were nonserious, and adverse events were analogous to those observed in the prelicensure clinical trials,” Dr. Shimabukuro and associates concluded.
Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
SOURCES: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
FROM PEDIATRICS
Key clinical point: Postlicensure studies confirm the safety of the 9vHPV vaccine.
Major finding: The adverse event rate is 1 in 7 million doses. Most of these events were not definitively tied to the vaccine.
Study details: The two studies covered all doses given in the United States since vaccine approval in 2015.
Disclosures: Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor on his study had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
Sources: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
Newborns’ maternal protection against measles wanes within 6 months
according to new research.
In fact, most of the 196 infants’ maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics.
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The findings are not surprising for a setting in which measles has been eliminated and align with results from past research, Huong Q. McLean, PhD, MPH, of the Marshfield (Wis.) Clinic Research Institute and Walter A. Orenstein, MD, of Emory University in Atlanta wrote in an accompanying editorial (Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-2541).
However, this susceptibility prior to receiving the MMR has taken on a new significance more recently, Dr. McLean and Dr. Orenstein suggested.
“In light of increasing measles outbreaks during the past year reaching levels not recorded in the United States since 1992 and increased measles elsewhere, coupled with the risk of severe illness in infants, there is increased concern regarding the protection of infants against measles,” the editorialists wrote.
Dr. Science and colleagues tested serum samples from 196 term infants, all under 12 months old, for antibodies against measles. The sera had been previously collected at a single tertiary care center in Ontario for clinical testing and then stored. Measles has been eliminated in Canada since 1998.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days); 2 months (61-89 days); 3 months (90-119 days); 4 months; 5 months; 6-9 months; and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test (PRNT) to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay (ELISA) because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL) , and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Dr. McLean and Dr. Orenstein noted in their editorial.
They discussed the rationale for various changes in the recommended schedule for measles immunization, based on changes in epidemiology of the disease and improved understanding of the immune response to vaccination since the vaccine became available in 1963. Then they posed the question of whether the recommendation should be revised again.
“Ideally, the schedule should minimize the risk of measles and its complications and optimize vaccine-induced protection,” Dr. McLean and Dr. Orenstein wrote.
They argued that the evidence cannot currently support changing the first MMR dose to a younger age because measles incidence in the United States remains extremely low outside of the extraordinary outbreaks in 2014 and 2019. Further, infants under 12 months of age make up less than 15% of measles cases during outbreaks, and unvaccinated people make up more than 70% of cases.
Rather, they stated, this new study emphasizes the importance of following the current schedule, with consideration of an earlier schedule only warranted during outbreaks.
“Health care providers must work to maintain high levels of coverage with 2 doses of MMR among vaccine-eligible populations and minimize pockets of susceptibility to prevent transmission to infants and prevent reestablishment of endemic transmission,” they concluded.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures. The editorialists had no external funding and no relevant financial disclosures.
SOURCE: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
according to new research.
In fact, most of the 196 infants’ maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics.
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The findings are not surprising for a setting in which measles has been eliminated and align with results from past research, Huong Q. McLean, PhD, MPH, of the Marshfield (Wis.) Clinic Research Institute and Walter A. Orenstein, MD, of Emory University in Atlanta wrote in an accompanying editorial (Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-2541).
However, this susceptibility prior to receiving the MMR has taken on a new significance more recently, Dr. McLean and Dr. Orenstein suggested.
“In light of increasing measles outbreaks during the past year reaching levels not recorded in the United States since 1992 and increased measles elsewhere, coupled with the risk of severe illness in infants, there is increased concern regarding the protection of infants against measles,” the editorialists wrote.
Dr. Science and colleagues tested serum samples from 196 term infants, all under 12 months old, for antibodies against measles. The sera had been previously collected at a single tertiary care center in Ontario for clinical testing and then stored. Measles has been eliminated in Canada since 1998.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days); 2 months (61-89 days); 3 months (90-119 days); 4 months; 5 months; 6-9 months; and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test (PRNT) to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay (ELISA) because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL) , and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Dr. McLean and Dr. Orenstein noted in their editorial.
They discussed the rationale for various changes in the recommended schedule for measles immunization, based on changes in epidemiology of the disease and improved understanding of the immune response to vaccination since the vaccine became available in 1963. Then they posed the question of whether the recommendation should be revised again.
“Ideally, the schedule should minimize the risk of measles and its complications and optimize vaccine-induced protection,” Dr. McLean and Dr. Orenstein wrote.
They argued that the evidence cannot currently support changing the first MMR dose to a younger age because measles incidence in the United States remains extremely low outside of the extraordinary outbreaks in 2014 and 2019. Further, infants under 12 months of age make up less than 15% of measles cases during outbreaks, and unvaccinated people make up more than 70% of cases.
Rather, they stated, this new study emphasizes the importance of following the current schedule, with consideration of an earlier schedule only warranted during outbreaks.
“Health care providers must work to maintain high levels of coverage with 2 doses of MMR among vaccine-eligible populations and minimize pockets of susceptibility to prevent transmission to infants and prevent reestablishment of endemic transmission,” they concluded.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures. The editorialists had no external funding and no relevant financial disclosures.
SOURCE: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
according to new research.
In fact, most of the 196 infants’ maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics.
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The findings are not surprising for a setting in which measles has been eliminated and align with results from past research, Huong Q. McLean, PhD, MPH, of the Marshfield (Wis.) Clinic Research Institute and Walter A. Orenstein, MD, of Emory University in Atlanta wrote in an accompanying editorial (Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-2541).
However, this susceptibility prior to receiving the MMR has taken on a new significance more recently, Dr. McLean and Dr. Orenstein suggested.
“In light of increasing measles outbreaks during the past year reaching levels not recorded in the United States since 1992 and increased measles elsewhere, coupled with the risk of severe illness in infants, there is increased concern regarding the protection of infants against measles,” the editorialists wrote.
Dr. Science and colleagues tested serum samples from 196 term infants, all under 12 months old, for antibodies against measles. The sera had been previously collected at a single tertiary care center in Ontario for clinical testing and then stored. Measles has been eliminated in Canada since 1998.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days); 2 months (61-89 days); 3 months (90-119 days); 4 months; 5 months; 6-9 months; and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test (PRNT) to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay (ELISA) because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL) , and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Dr. McLean and Dr. Orenstein noted in their editorial.
They discussed the rationale for various changes in the recommended schedule for measles immunization, based on changes in epidemiology of the disease and improved understanding of the immune response to vaccination since the vaccine became available in 1963. Then they posed the question of whether the recommendation should be revised again.
“Ideally, the schedule should minimize the risk of measles and its complications and optimize vaccine-induced protection,” Dr. McLean and Dr. Orenstein wrote.
They argued that the evidence cannot currently support changing the first MMR dose to a younger age because measles incidence in the United States remains extremely low outside of the extraordinary outbreaks in 2014 and 2019. Further, infants under 12 months of age make up less than 15% of measles cases during outbreaks, and unvaccinated people make up more than 70% of cases.
Rather, they stated, this new study emphasizes the importance of following the current schedule, with consideration of an earlier schedule only warranted during outbreaks.
“Health care providers must work to maintain high levels of coverage with 2 doses of MMR among vaccine-eligible populations and minimize pockets of susceptibility to prevent transmission to infants and prevent reestablishment of endemic transmission,” they concluded.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures. The editorialists had no external funding and no relevant financial disclosures.
SOURCE: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
FROM PEDIATRICS
Key clinical point: Infants’ maternal measles antibodies fell below protective levels by 6 months old.
Major finding: Infants were twice as likely not to have protective immunity against measles for each month of age after birth (odds ratio, 2.13).
Study details: The findings are based on measles antibody testing of 196 serum samples from infants born in a tertiary care center in Ontario.
Disclosures: The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Source: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
Papulonecrotic Tuberculid Secondary to Mycobacterium avium Complex
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.
During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.
Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.

During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.

Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
To the Editor:
Papulonecrotic tuberculid (PNT) is a cutaneous hypersensitivity reaction to antigenic components of Mycobacterium species, most commonly Mycobacterium tuberculosis. According to a PubMed search of articles indexed for MEDLINE using the terms papulonecrotic tuberculid, Mycobacterium avium complex, and Mycobacterium, only 1 case of PNT secondary to infection with Mycobacterium avium complex (MAC) has been reported.1,2 Papulonecrotic tuberculid classically presents with symmetrical, dusky red papules with necrosis on the extremities.3 Patients may or may not have associated symptoms of fever and weight loss. It is diagnosed through skin biopsy as well as identification of a distant source of mycobacterial infection. Papulonecrotic tuberculid is considered a reactive process to a distant site of mycobacterial infection, and skin lesions contain few, if any, mycobacteria.4
A 65-year-old man was admitted to the hospital for expedited workup of chronic fevers, 20-lb weight loss, and night sweats of 8 months’ duration. He had a medical history of myelodysplastic syndrome and autoimmune hemolytic anemia. During hospitalization, positron emission tomography revealed multilevel vertebral lytic and sclerotic lesions. Subsequent T10 vertebral biopsy showed necrotizing granulomatous inflammation with extensive necrosis and acid-fast bacilli–positive organisms. The patient was empirically started on rifampicin, isoniazid, pyrazinamide, ethambutol, and pyridoxine for presumed M tuberculosis and placed on respiratory isolation.
Dermatology was consulted for a recurrent tender rash on the bilateral upper and lower extremities of 5 years’ duration. Physical examination revealed numerous erythematous papulonecrotic lesions in various states of healing on the bilateral upper and lower extremities (Figure 1). Three years prior to the current presentation, 2 lesions were biopsied and demonstrated leukocytoclastic vasculitis with neutrophilic panniculitis and vasculopathy. A presumptive diagnosis of Sweet syndrome was made given the history of myelodysplastic syndrome, though an infectious etiology could not be ruled out at that time. Concurrently, the patient was diagnosed with autoimmune hemolytic anemia and was started on prednisone. Initially, the skin lesions improved with prednisone but never fully resolved; however, as the dosage of oral steroids decreased, the skin lesions worsened and presented in larger numbers with more frequency. The patient was titrated down to prednisone 5 mg daily with no additional treatment of the skin lesions at that time.

During the current hospitalization, 2 additional biopsies were taken from the arm for routine histopathology and tissue culture. Dermatopathology revealed robust neutrophilic and granulomatous inflammation as well as remarkable necrosis with a few mycobacteria identified on acid-fast and Fite stains (Figure 2). Tissue culture was negative. Additionally, the patient’s spinal biopsy was sent for polymerase chain reaction analysis for Mycobacterium typing, which confirmed MAC. The patient was diagnosed with Pott disease, a mycobacterial infection of the spine, as well as cutaneous papulonecrotic tuberculid secondary to MAC.

Papulonecrotic tuberculid is the rarest form of cutaneous tuberculosis infection and rarely has been reported in connection to MAC.1 This condition is considered a hypersensitivity reaction that occurs in response to antigenic components of mycobacteria.4 Patients with PNT typically present with recurrent crops of painful papulonecrotic lesions distributed on the extremities. Histopathology in PNT classically reveals necrosis, notable inflammatory infiltrate, and lack of observed organisms.5 Diagnosis often is made through skin biopsy, though histopathology varies based on lesion maturity.4 Early lesions often reveal leukocytoclastic vasculitis, whereas late lesions usually demonstrate granulomatous inflammation.4 Mycobacterium avium complex is difficult to culture, as it is a slow-growing, fastidious bacterium and therefore polymerase chain reaction genotyping is useful for bacterial classification.6
Disseminated MAC infection also was on the differential for our patient; however, we felt it was less likely than PNT for several reasons. First, disseminated infection rarely presents with cutaneous involvement and is associated with pulmonary involvement in 90% of cases.7-9 Second, the granuloma formation noted on our patient’s skin biopsy was not typical for disseminated MAC but is well described in cases of PNT.4,8,9 Finally, in the rare cases in which cutaneous involvement has occurred with disseminated mycobacterial infections, skin biopsies typically revealed numerous Mycobacterium organisms.8,10 In contrast, skin lesions associated with PNT usually reveal few, if any, organisms, as was seen with our patient.2
The patient’s initial biopsies also supported a diagnosis of PNT, as early lesions of PNT typically show leukocytoclastic vasculitis. His response to low and high doses of prednisone also fit well with a PNT diagnosis. In fact, a case of PNT secondary to Mycobacterium bovis similarly showed an improvement in the rash with high-dose steroids but progression with lower doses.11 It is possible that our patient’s response to steroids complicated the diagnosis of his rash.
The treatment of PNT is clearance of the underlying infection. Macrolide antibiotics, such as clarithromycin and azithromycin, have the best efficacy against MAC, in combination with ethambutol and/or rifabutin.6,12 Treatment duration should be 1 year. Amikacin or streptomycin may be added to this regimen during early treatment.6 Mycobacterium avium complex is resistant to many antibiotics, including typical antituberculosis drugs, and sensitivities should be identified at the onset of treatment.11,12
Albeit rare, clinicians should be aware of PNT secondary to MAC or other mycobacterial infections. Because this condition is difficult to diagnose with varying histologic findings and often negative tissue cultures, a high index of suspicion is necessary when a patient presents with recurrent papulonecrotic lesions, especially in immunocompromised hosts and patients with exposure to mycobacteria.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
- Williams JT, Pulitzer DR, DeVillez RL. Papulonecrotic tuberculid secondary to disseminated Mycobacterium avium complex. Int J Dermatol. 1994;33:109-112.
- Jordaan HF, Schneider JW. Papulonecrotic tuberculid. Int J Dermatol. 1995;34:217-219.
- Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and leprosy: classical granulomatous diseases in the twenty-first century. Dermatol Clin. 2015;33:541-562.
- Kim GW, Park HJ, Kim HS, et al. Simultaneous occurrence of papulonecrotic tuberculid and erythema induratum in a patient with pulmonary tuberculosis. Pediatr Dermatol. 2013;30:256-259.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Dyer J, Weiss J, Steiner WS, et al. Primary cutaneous Mycobacterium avium complex infection following squamous cell carcinoma excision. Cutis. 2016;98:E8-E11.
- Kollipara R, Richards K, Tschen J, et al. Disseminated Mycobacterium avium complex with cutaneous lesions. J Cutan Med Surg. 2016;20:272-274.
- Endly DC, Ackerman LS. Disseminated cutaneous Mycobacterium avium complex in a person with AIDS. Dermatol Online J. 2014;20:22616.
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443.
- Iden DL, Rogers RS 3rd, Schroeter AL. Papulonecrotic tuberculid secondary to Mycobacterium bovis. Arch Dermatol. 1978;114:564-566.
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong). 2008;16:359-363.
Practice Points
- Papulonecrotic tuberculid (PNT) is a hypersensitivity reaction that presents with reddish papules with central necrosis on the extremities.
- Early PNT histopathology shows leukocytoclastic vasculitis. Later lesions demonstrate granulomatous inflammation on histopathology.
- Mycobacterium avium is difficult to culture; therefore, if you suspect it, we recommend polymerase chain reaction genotyping for bacterial classification.