User login
Improving sepsis-related outcomes
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Early diagnosis a key goal
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Many Americans planning to avoid flu vaccination
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.
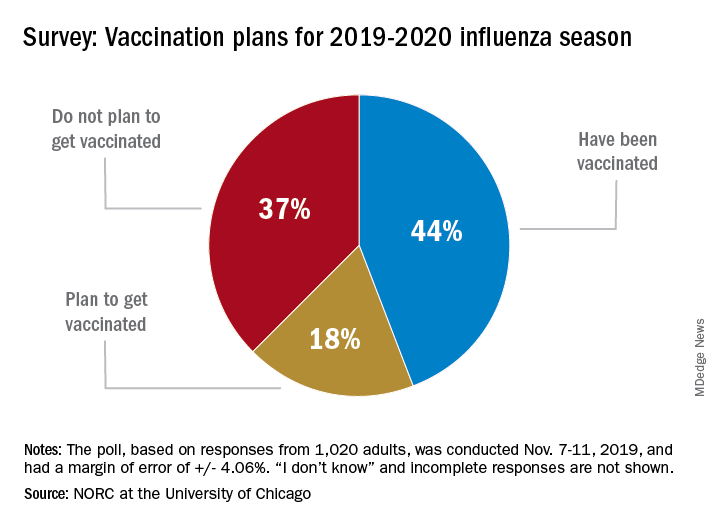
Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
Repeat LTBI testing best in patients taking biologics with new risk factors
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
REPORTING FROM ACR 2019
Perioperative antirheumatic drug use does not impact postsurgery infection rate in RA patients
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
REPORTING FROM ACR 2019
A triple-antibiotic cure for Crohn’s disease?
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
REPORTING FROM ACG 2019
Influenza already in midseason form
It’s been a decade since flu activity levels were this high this early in the season.
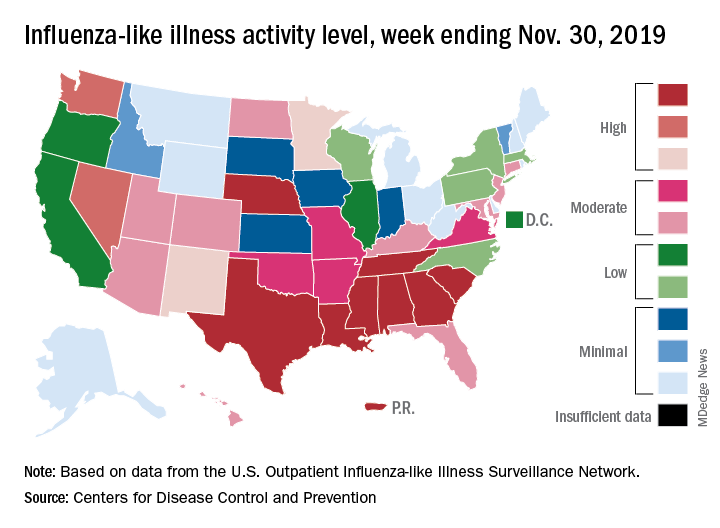
For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
Decreasing Overutilization of Echocardiograms and Abdominal Imaging in the Evaluation of Children with Fungemia
From the University of Miami, Department of Pediatrics and Department of Medicine, Miami, FL.
Abstract
- Objective: Pediatric fungemia is associated with a low risk of fungal endocarditis and renal infections. The majority of current guidelines do not recommend routine abdominal imaging/echocardiograms in the evaluation of fungemia, but such imaging has been routinely ordered for patients on the pediatric gastroenterology service at our institution. Our goals were to assess the financial impact of this deviation from current clinical guidelines and redefine the standard work to reduce overutilization of abdominal ultrasounds and echocardiograms. Specifically, our goal was to reduce imaging by 50% by 18 months.
- Methods: Root cause analysis showed a lack of familiarity with current evidence. Using this data, countermeasures were implemented, including practitioner education of guidelines and creation of a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Balancing measures were missed episodes of fungal endocarditis and renal infection.
- Results: During the period January 1, 2016 to November 19, 2017, 18 of 21 episodes of fungemia in our pediatric institution occurred in patients admitted to the pediatric gastroenterology service. Abdominal imaging and echocardiograms were done 100% of the time, with no positive findings and an estimated cost of approximately $58,000. Post-intervention from November 20, 2017 to April 3, 2019, 7 of 13 episodes of fungemia occurred on this service. Frequency of abdominal imaging and echocardiograms decreased to 43% and 57%, respectively. No episodes of fungal endocarditis or renal infection were identified.
- Conclusion: Overutilization of abdominal imaging and echocardiograms in pediatric fungemia evaluation can be safely decreased.
Keywords: guidelines; cost; candidemia; endocarditis.
Practitioners may remain under the impression that routine abdominal ultrasounds (US) and echocardiograms (echo) are indicated in fungemia to evaluate for fungal endocarditis and renal infection, although these conditions are rare and limited to a subset of the population.1-10 Risk factors include prematurity, immunosuppression, prior bacterial endocarditis, abnormal cardiac valves, and previous urogenital surgeries.11
The 2016 Infectious Diseases Society of America (IDSA) guidelines do not recommend routine US or echo but rather provide scenarios in which Candida endocarditis should be suspected, and these include: persistently positive blood cultures, persistent fevers despite appropriate therapy, and clinical signs that may suggest endocarditis, such as a new heart murmur, heart failure, or embolic phenomena.11 IDSA recommends abdominal imaging in neonates with persistently positive blood cultures to evaluate the urogenital system, in addition to the liver and spleen. They also recommend abdominal imaging in symptomatic ascending Candida pyelonephritis beyond the neonatal period and in chronic disseminated candidiasis; the latter is uncommon and seen almost exclusively in patients recovering from neutropenia with a hematologic malignancy.11
We also reviewed guidelines on fungemia originating outside the United States. The 2010 Canadian clinical guidelines on invasive candidiasis do not explicitly recommend routine imaging, but rather state that various imaging studies, including US and echo among others, may be helpful.12 The German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy published a joint recommendation against routine US and echo in uncomplicated candidemia in 2011.13
The European Society for Clinical Microbiology and Infectious Diseases is the only society that recommends routine echo. Their 2012 guidelines on candidiasis recommend transesophageal echo in adults14 and echocardiography in children,15 as well as abdominal imaging in the diagnosis of chronic disseminated candidiasis in adults with hematological malignancies/hematopoietic stem cell transplantation.16
The 2013 Brazilian guidelines explicitly recommend against routine abdominal imaging and echo because of the low frequency of visceral lesions in adults with candidemia and recommend reserving imaging for those with persistently positive blood cultures or with clinical signs/symptoms suggestive of endocarditis/abdominal infection or clinical deterioration.17 The 2014 Japanese guidelines recommend ruling out chronic disseminated candidiasis in these patients with symptoms during the neutrophil recovery phase, but do not mention routinely imaging other patients. They do not address the role of echocardiography.18
Although physicians in the United Sates typically follow IDSA guidelines, abdominal US and echo were ordered routinely for patients with fungemia on the pediatric gastroenterology service at our institution, leading to higher medical costs and waste of medical resources. Our goals were to assess the current standard work for fungemia evaluation on this service, assess the impact of its deviation from current clinical guidelines, and redefine the standard work by (1) presenting current evidence to practitioners taking care of patients on this service, (2) providing a clinical pathway that allowed for variations where appropriate, and (3) providing a plan for pediatric fungemia management. Our SMART (Specific, Measurable, Attainable, Relevant and Timely) goal was to reduce overutilization of abdominal US and echo in pediatric patients with fungemia on the pediatric gastroenterology service by 50%.
Methods
Study, Setting, and Participants
We executed this quality improvement project at a quaternary care pediatric hospital affiliated with a school of medicine. The project scope consisted of inpatient pediatric patients with fungemia on the pediatric gastroenterology service admitted to the wards or pediatric critical care unit at this institution, along with the practitioners caring for these patients. The project was part of an institutional quality improvement initiative program. The quality improvement team included quality improvement experts from the departments of medicine and pediatrics, a pediatric resident and student, and physicians from the divisions of pediatric infectious disease, pediatric critical care, and pediatric gastroenterology. This study qualified for Institutional Review Board (IRB) exemption based on the University’s IRB stipulations.
Current Condition
Root cause analysis was performed by creating a process map of the current standard work and a fishbone diagram (Figure 1). We incorporated feedback from voice of the customer in the root cause analysis. In this analysis, the voice of the customer came from the bedside floor nurses, ultrasound clerk and sonographer, echo technician, cardiology fellow, and microbiology medical technician. We got their feedback on our process map, its accuracy and ways to expand, their thoughts on the problem and why we have this problem, and any solutions they could offer to help improve the problem. Some of the key points obtained were: echos were not routinely done on the floors and were not considered urgent as they often did not change management; the sonographer and those from the cardiology department felt imaging was often overutilized because of misconceptions and lack of available hospital guidelines. Suggested solutions included provider education with reference to Duke’s criteria and establishing a clinical pathway approved by all concerned departments.
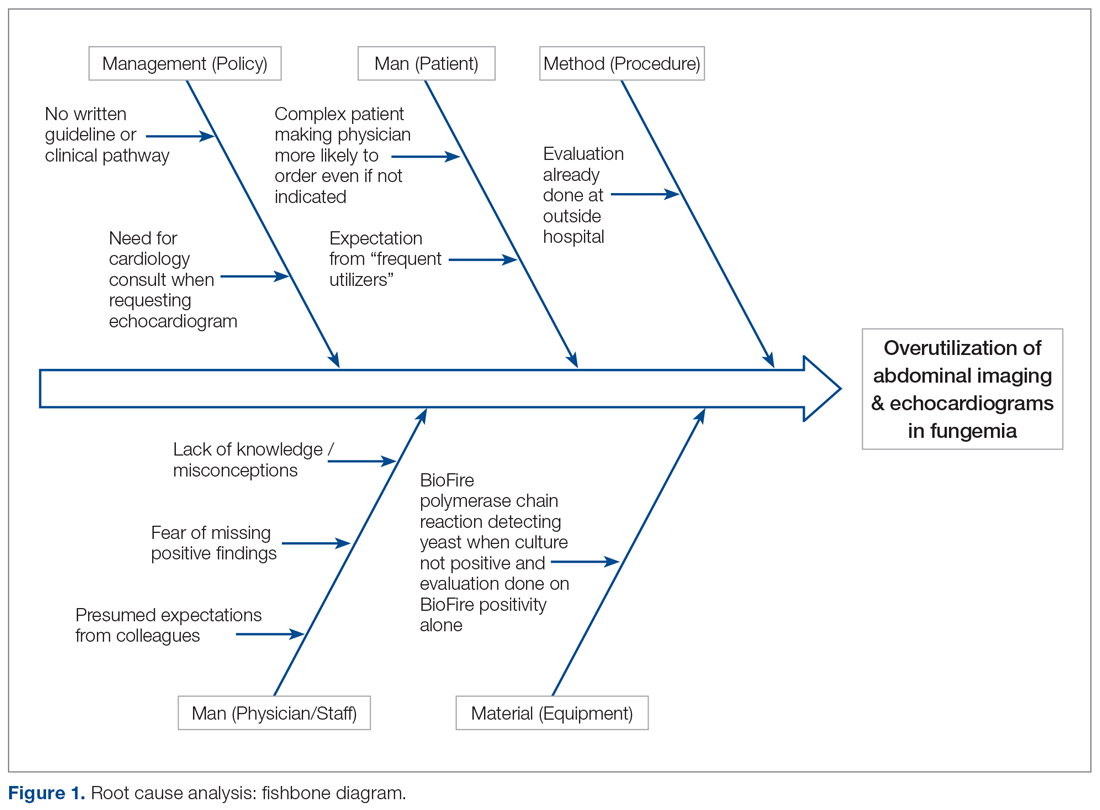
Prior to education, we surveyed current practices of practitioners on teams caring for these patients, which included physicians of all levels (attendings, fellows, residents) as well as nurse practitioners and medical students from the department of pediatrics and divisions of pediatric gastroenterology, pediatric infectious disease, and pediatric critical care medicine.
Countermeasures
Practitioner Education. In October 2017 practitioners were given a 20-minute presentation on the latest international guidelines on fungemia. Fifty-nine practitioners completed pre- and post-test surveys. Eight respondents were excluded due to incomplete surveys. We compared self-reported frequencies of ordering abdominal imaging and echo before the presentation with intention to order post education. Intention to change clinical practice after the presentation was also surveyed.
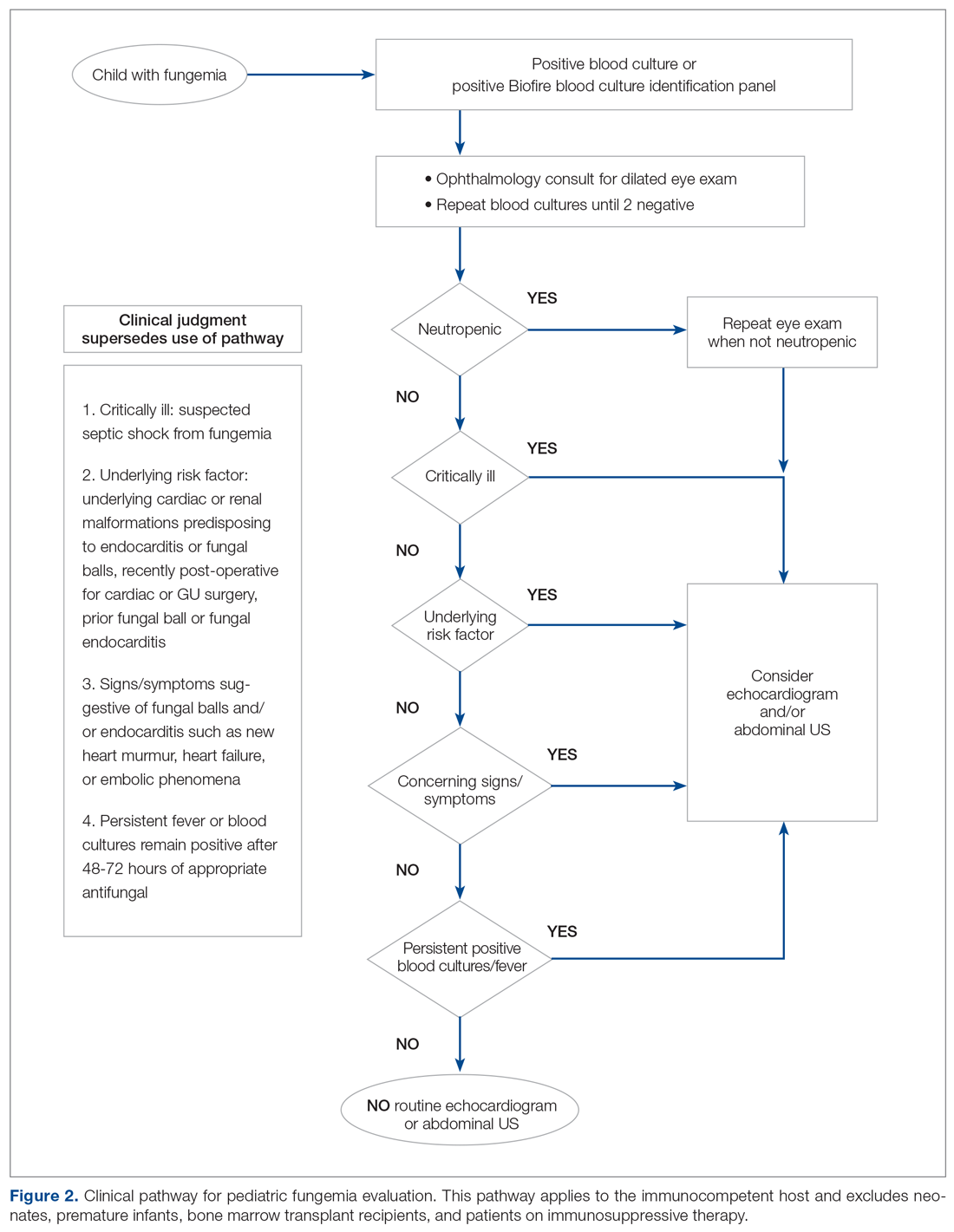
Clinical Pathway. Education alone may not result in sustainability, and thus we provided a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Inter-department buy-in was also necessary for success. It was important to get the input from the various teams (infectious disease, cardiology, gastroenterology, and critical care), which was done by incorporating members from those divisions in the project or getting their feedback through voice of the customer analysis.
We redefined standard work based on current evidence and created a clinical pathway during March 2018 that included variations when appropriate (Figure 2). We presented the clinical pathway to practitioners and distributed it via email. We also made it available to pediatric residents and fellows on their mobile institutional work resource application.
Electronic Order Set. We created an electronic order set for pediatric fungemia management and made it available in the electronic health record May 2018.
Measurement
Cases of fungemia were identified through the electronic health record pre-intervention (January 1, 2016 through November 19, 2017) and post-intervention (November 20, 2019 through April 3, 2019). An episode of fungemia was defined as an encounter with 1 or more positive blood culture(s) for Candida species or Cryptococcus species. We manually identified patients belonging to the pediatric gastroenterology service and reviewed these charts to determine the presenting complaint, organism isolated, transplant status, central lines status, risk factors, if abdominal imaging or echocardiography were done for the episode of fungemia, and their corresponding results. We calculated overall and per patient medical charges by using the average charges at our institution of US and echocardiography with a cardiology consult. These average charges were provided by patient financial services and the pediatric cardiology department, respectively. To address non-technical expenditures, we calculated the average time taken for transport to and from radiology and the echo suite for each identified patient. We identified missed fungal endocarditis and fungal balls as balancing measures.
Results
Survey
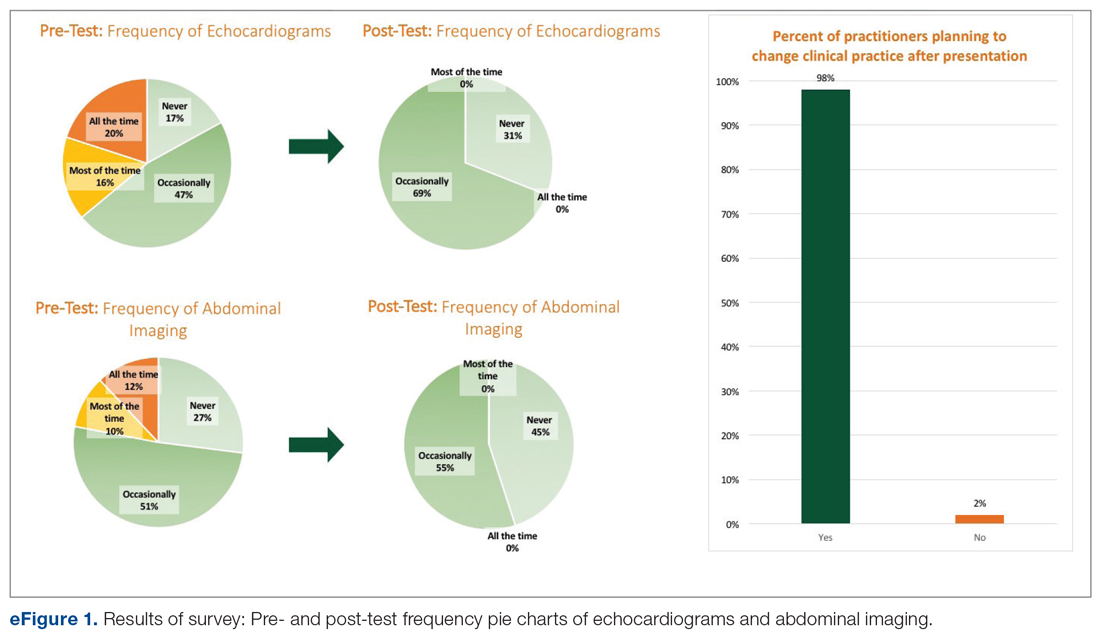
Among the 51 practitioners surveyed, 36% were performing routine echo and 22% self-reported performing routine abdominal imaging. After education, no respondents planned to routinely do echo or abdominal imaging. All but 1 respondent planned to change their practice for evaluation of fungemia patients based on the presentation (eFigure 1).
Baseline Data
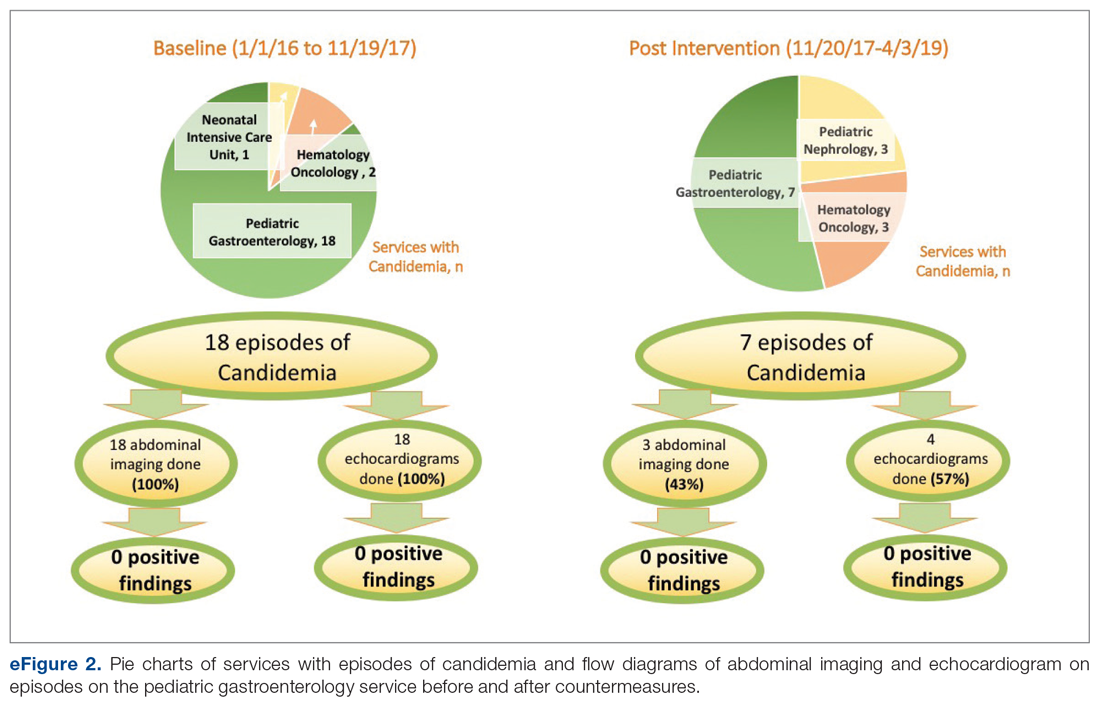
Over the 23-month period from January 1, 2016 to November 19, 2017, there were 21 episodes of fungemia, 18 of which occurred in patients on the pediatric gastroenterology service (2 of the 18 were transplant recipients). For the 18 episodes on this service, abdominal imaging and echo were done 100% of the time, with 0 positive findings (eFigure 2).
Of those 18 episodes, the average age was 4.6 years, with two-thirds of the population being male. There were 3 patients with multiple episodes that accounted for 8 of the episodes (3, 3, and 2 episodes each). Fever was the most common presenting complaint. The most common organism was Candida parapsilosis (6 of the 18 episodes). All episodes but one involved a central line, and all central lines were removed when present except for one case. Of the risk factors, 3 episodes occurred in neutropenic patients, and for 1 episode the patient had a questionable history of fungal endocarditis (and was on fungal prophylaxis). There were no patients with recent cardiac/urogenital surgery or prior fungal balls. No episodes had clinical symptoms suggestive of fungal endocarditis or fungal balls.
Post-Intervention Data
Over the subsequent 17-month period (November 11, 2017 to April 3, 2019), there were 13 episodes of candidemia. There were no episodes of Cryptococcus fungemia. Seven episodes occurred in patients on the pediatric gastroenterology service (2 of the 7 occurred in transplant recipients). Abdominal imaging was done in 3 of these episodes (43%), and in 2 of these 3 episodes, imaging was done at an outside institution prior to arrival, with no positive results (eFigure 2).
Echocardiography was done 57% of the time (n = 4), with echo being done at an outside institution prior to arrival half of the time (n = 2), with no endocarditis identified. The cases of abdominal imaging and echo done at outside institutions prior to arrival were not impacted by the countermeasures. Excluding those 2 patients who had both abdominal imaging and echocardiography done prior to arrival, the overall rate of imaging (both abdominal imaging and echo) done after countermeasures were instituted was 30% (Figure 3).
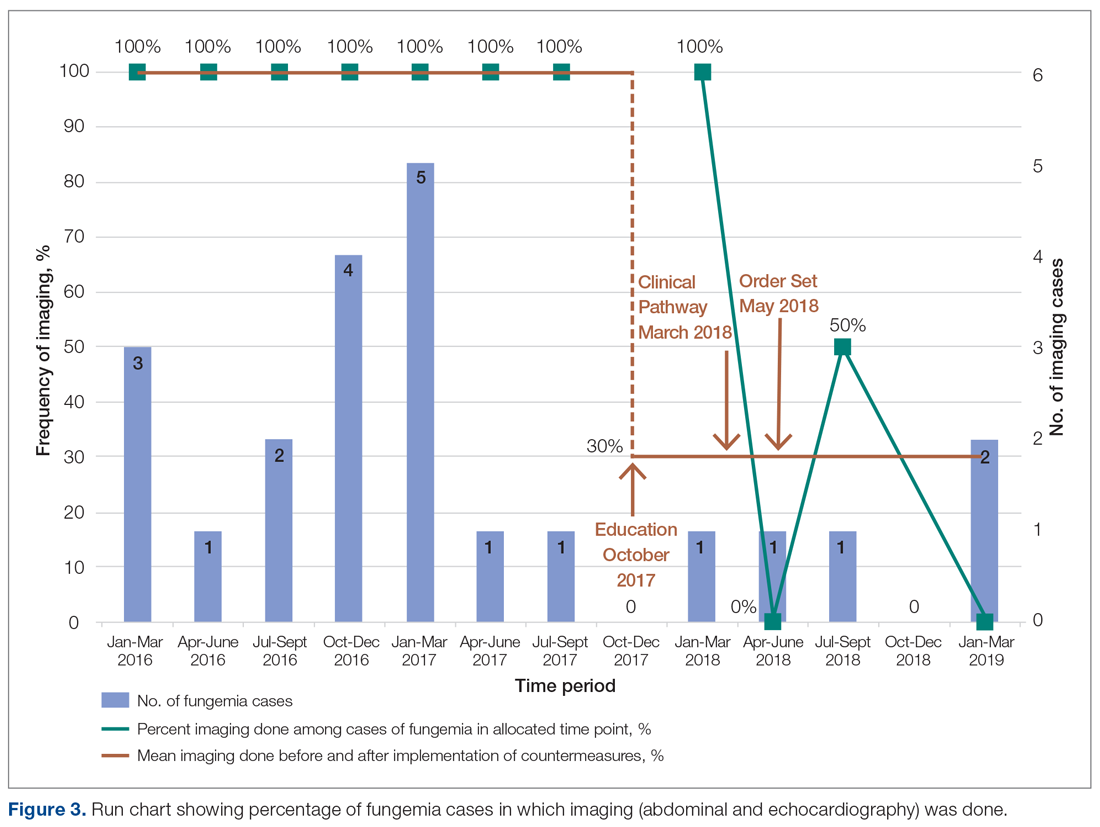
Of those 7 episodes, the average age was 6.8 years (57% female). There were no patients with multiple episodes. The most common presenting complaint was fever. The most common organism was Candida albicans (3 of the 7 episodes). All episodes involved a central line, which was removed in all cases except for one. Of the risk factors, 2 episodes were in neutropenic patients, and 1 episode had a history of bacterial endocarditis (not related to fungemia). No episodes occurred in patients with prior fungal renal infection, urogenital malformations, or recent cardiac/urogenital surgery. No episodes had clinical symptoms suggestive of fungal endocarditis or renal infection. No episodes of fungal endocarditis or renal infection were identified.
On average, a patient at our institution undergoing abdominal US and echo with a cardiology consult results in medical waste of approximately $3200 per patient. This cost does not take into account other miscellaneous charges possibly incurred, such as the radiologist interpreting the findings and transportation. Baseline data calculations show that patients waste on average 55 minutes in physical transport, and this does not take into account wait times.
Discussion
Candidemia contributes to 10% of central-line associated blood stream infections (CLABSI).19 Increased usage of indwelling central catheters for administration of parenteral nutrition will inevitably result in practitioners encountering cases of candidemia when caring for this population. As seen from our results, the majority of episodes of candidemia at our institution occurred on the pediatric gastroenterology service, and thus redefining standard work on this service will be impactful.
Candida parapsilosis and Candida albicans were the most common causative agents before and after intervention, respectively, but overall the most common organism was Candida albicans, which is in keeping with that of CLABSI in the literature.19 Growth of Candida parapsilosis has been particularly linked to CLABSI.19 The third most common organism in our study was Candida glabrata, which is the second most common cause of candidemia in CLABSI.19
The cases of positive abdominal imaging in fungemia in the literature are limited to the neonatal population1-4 and chronic disseminated candidiasis in patients with hematologic malignancies/neutropenia/immunosuppression.5,6 In fungal endocarditis, the reported cases were generally in neonates,1,3,7 critically ill patients,8 patients with hematologic malignancies/neutropenia/immunosuppression,6,9 or those with a cardiac history.9,10 This population differs from the patient population on the pediatric gastroenterology service. Patients on this service may not need US or echo. Performing abdominal US and echo in fungemia patients in whom such imaging is not indicated may result in medical waste of approximately $3200 per patient. There is also a waste of medical resources and time.
We found almost all practitioners are willing to change clinical practice once provided with current guidelines. Face-to-face oral presentations allowed for questions and interaction, making this form of information dissemination better than e-mails or handouts.
Though the numbers were small over the short study period, we were able to decrease overutilization of abdominal imaging and echo after implementing countermeasures. Frequency decreased from 100% to 43% and 57% for abdominal imaging and echo, respectively. Imaging that was done after the countermeasures were implemented was mainly attributed to imaging patients underwent prior to presenting to our institution. This reinforces the need for education at other institutions as well. Of the balancing measures assessed, there were no missed cases of fungal balls or fungal endocarditis. Additionally,
The findings from this quality improvement project underscore current recommendations that, despite common misconceptions, routine abdominal US and echo are not indicated in all cases of fungemia. Case-by-case assessment based on the clinical scenario remains key to management of fungemia to avoid unnecessary medical interventions.
Corresponding author: Donna Cheung, MBBS, 200 Hawkins Drive, BT 1120-G, Iowa City, IA 52242; [email protected].
Financial support: None.
1. Benjamin DK Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634-640.
2. Wynn JL, Tan S, Gantz MG, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. 2012;54:331-339.
3. Noyola DE, Fernandez M, Moylett EH, et al. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018-1023.
4. Phillips JR, Karlowicz MG Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190-194.
5. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87:535-541.
6. Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641.
7. Levy I, Shalit I, Birk E, et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses. 2006;49:43-48.
8. Aspesberro F, Beghetti M, Oberhansli I, et al. Fungal endocarditis in critically ill children. Eur J Pediatr. 1999;158:275-280.
9. Fernandez-Cruz A, Cruz Menarguez M, Munoz P, et al. The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549.
10. Hernandez-Torres A, Garcia-Vazquez E, Laso-Ortiz A, et al. [Candida sp endocarditis. Experience in a third-level hospital and review of the literature]. Rev Esp Quimioter. 2013;26:51-55.
11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
12. Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122-50.
13. Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54:279-310.
14. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
15. Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52.
16. Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53-67.
17. Colombo AL, Guimaraes T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis. 2013;17:283-312.
18. Kohno S, Tamura K, Niki Y, et al. Executive Summary of Japanese Domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117-E163.
19. Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiology Rev. 2004;17:255-267.
From the University of Miami, Department of Pediatrics and Department of Medicine, Miami, FL.
Abstract
- Objective: Pediatric fungemia is associated with a low risk of fungal endocarditis and renal infections. The majority of current guidelines do not recommend routine abdominal imaging/echocardiograms in the evaluation of fungemia, but such imaging has been routinely ordered for patients on the pediatric gastroenterology service at our institution. Our goals were to assess the financial impact of this deviation from current clinical guidelines and redefine the standard work to reduce overutilization of abdominal ultrasounds and echocardiograms. Specifically, our goal was to reduce imaging by 50% by 18 months.
- Methods: Root cause analysis showed a lack of familiarity with current evidence. Using this data, countermeasures were implemented, including practitioner education of guidelines and creation of a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Balancing measures were missed episodes of fungal endocarditis and renal infection.
- Results: During the period January 1, 2016 to November 19, 2017, 18 of 21 episodes of fungemia in our pediatric institution occurred in patients admitted to the pediatric gastroenterology service. Abdominal imaging and echocardiograms were done 100% of the time, with no positive findings and an estimated cost of approximately $58,000. Post-intervention from November 20, 2017 to April 3, 2019, 7 of 13 episodes of fungemia occurred on this service. Frequency of abdominal imaging and echocardiograms decreased to 43% and 57%, respectively. No episodes of fungal endocarditis or renal infection were identified.
- Conclusion: Overutilization of abdominal imaging and echocardiograms in pediatric fungemia evaluation can be safely decreased.
Keywords: guidelines; cost; candidemia; endocarditis.
Practitioners may remain under the impression that routine abdominal ultrasounds (US) and echocardiograms (echo) are indicated in fungemia to evaluate for fungal endocarditis and renal infection, although these conditions are rare and limited to a subset of the population.1-10 Risk factors include prematurity, immunosuppression, prior bacterial endocarditis, abnormal cardiac valves, and previous urogenital surgeries.11
The 2016 Infectious Diseases Society of America (IDSA) guidelines do not recommend routine US or echo but rather provide scenarios in which Candida endocarditis should be suspected, and these include: persistently positive blood cultures, persistent fevers despite appropriate therapy, and clinical signs that may suggest endocarditis, such as a new heart murmur, heart failure, or embolic phenomena.11 IDSA recommends abdominal imaging in neonates with persistently positive blood cultures to evaluate the urogenital system, in addition to the liver and spleen. They also recommend abdominal imaging in symptomatic ascending Candida pyelonephritis beyond the neonatal period and in chronic disseminated candidiasis; the latter is uncommon and seen almost exclusively in patients recovering from neutropenia with a hematologic malignancy.11
We also reviewed guidelines on fungemia originating outside the United States. The 2010 Canadian clinical guidelines on invasive candidiasis do not explicitly recommend routine imaging, but rather state that various imaging studies, including US and echo among others, may be helpful.12 The German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy published a joint recommendation against routine US and echo in uncomplicated candidemia in 2011.13
The European Society for Clinical Microbiology and Infectious Diseases is the only society that recommends routine echo. Their 2012 guidelines on candidiasis recommend transesophageal echo in adults14 and echocardiography in children,15 as well as abdominal imaging in the diagnosis of chronic disseminated candidiasis in adults with hematological malignancies/hematopoietic stem cell transplantation.16
The 2013 Brazilian guidelines explicitly recommend against routine abdominal imaging and echo because of the low frequency of visceral lesions in adults with candidemia and recommend reserving imaging for those with persistently positive blood cultures or with clinical signs/symptoms suggestive of endocarditis/abdominal infection or clinical deterioration.17 The 2014 Japanese guidelines recommend ruling out chronic disseminated candidiasis in these patients with symptoms during the neutrophil recovery phase, but do not mention routinely imaging other patients. They do not address the role of echocardiography.18
Although physicians in the United Sates typically follow IDSA guidelines, abdominal US and echo were ordered routinely for patients with fungemia on the pediatric gastroenterology service at our institution, leading to higher medical costs and waste of medical resources. Our goals were to assess the current standard work for fungemia evaluation on this service, assess the impact of its deviation from current clinical guidelines, and redefine the standard work by (1) presenting current evidence to practitioners taking care of patients on this service, (2) providing a clinical pathway that allowed for variations where appropriate, and (3) providing a plan for pediatric fungemia management. Our SMART (Specific, Measurable, Attainable, Relevant and Timely) goal was to reduce overutilization of abdominal US and echo in pediatric patients with fungemia on the pediatric gastroenterology service by 50%.
Methods
Study, Setting, and Participants
We executed this quality improvement project at a quaternary care pediatric hospital affiliated with a school of medicine. The project scope consisted of inpatient pediatric patients with fungemia on the pediatric gastroenterology service admitted to the wards or pediatric critical care unit at this institution, along with the practitioners caring for these patients. The project was part of an institutional quality improvement initiative program. The quality improvement team included quality improvement experts from the departments of medicine and pediatrics, a pediatric resident and student, and physicians from the divisions of pediatric infectious disease, pediatric critical care, and pediatric gastroenterology. This study qualified for Institutional Review Board (IRB) exemption based on the University’s IRB stipulations.
Current Condition
Root cause analysis was performed by creating a process map of the current standard work and a fishbone diagram (Figure 1). We incorporated feedback from voice of the customer in the root cause analysis. In this analysis, the voice of the customer came from the bedside floor nurses, ultrasound clerk and sonographer, echo technician, cardiology fellow, and microbiology medical technician. We got their feedback on our process map, its accuracy and ways to expand, their thoughts on the problem and why we have this problem, and any solutions they could offer to help improve the problem. Some of the key points obtained were: echos were not routinely done on the floors and were not considered urgent as they often did not change management; the sonographer and those from the cardiology department felt imaging was often overutilized because of misconceptions and lack of available hospital guidelines. Suggested solutions included provider education with reference to Duke’s criteria and establishing a clinical pathway approved by all concerned departments.

Prior to education, we surveyed current practices of practitioners on teams caring for these patients, which included physicians of all levels (attendings, fellows, residents) as well as nurse practitioners and medical students from the department of pediatrics and divisions of pediatric gastroenterology, pediatric infectious disease, and pediatric critical care medicine.
Countermeasures
Practitioner Education. In October 2017 practitioners were given a 20-minute presentation on the latest international guidelines on fungemia. Fifty-nine practitioners completed pre- and post-test surveys. Eight respondents were excluded due to incomplete surveys. We compared self-reported frequencies of ordering abdominal imaging and echo before the presentation with intention to order post education. Intention to change clinical practice after the presentation was also surveyed.
Clinical Pathway. Education alone may not result in sustainability, and thus we provided a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Inter-department buy-in was also necessary for success. It was important to get the input from the various teams (infectious disease, cardiology, gastroenterology, and critical care), which was done by incorporating members from those divisions in the project or getting their feedback through voice of the customer analysis.
We redefined standard work based on current evidence and created a clinical pathway during March 2018 that included variations when appropriate (Figure 2). We presented the clinical pathway to practitioners and distributed it via email. We also made it available to pediatric residents and fellows on their mobile institutional work resource application.

Electronic Order Set. We created an electronic order set for pediatric fungemia management and made it available in the electronic health record May 2018.
Measurement
Cases of fungemia were identified through the electronic health record pre-intervention (January 1, 2016 through November 19, 2017) and post-intervention (November 20, 2019 through April 3, 2019). An episode of fungemia was defined as an encounter with 1 or more positive blood culture(s) for Candida species or Cryptococcus species. We manually identified patients belonging to the pediatric gastroenterology service and reviewed these charts to determine the presenting complaint, organism isolated, transplant status, central lines status, risk factors, if abdominal imaging or echocardiography were done for the episode of fungemia, and their corresponding results. We calculated overall and per patient medical charges by using the average charges at our institution of US and echocardiography with a cardiology consult. These average charges were provided by patient financial services and the pediatric cardiology department, respectively. To address non-technical expenditures, we calculated the average time taken for transport to and from radiology and the echo suite for each identified patient. We identified missed fungal endocarditis and fungal balls as balancing measures.
Results
Survey
Among the 51 practitioners surveyed, 36% were performing routine echo and 22% self-reported performing routine abdominal imaging. After education, no respondents planned to routinely do echo or abdominal imaging. All but 1 respondent planned to change their practice for evaluation of fungemia patients based on the presentation (eFigure 1).

Baseline Data
Over the 23-month period from January 1, 2016 to November 19, 2017, there were 21 episodes of fungemia, 18 of which occurred in patients on the pediatric gastroenterology service (2 of the 18 were transplant recipients). For the 18 episodes on this service, abdominal imaging and echo were done 100% of the time, with 0 positive findings (eFigure 2).

Of those 18 episodes, the average age was 4.6 years, with two-thirds of the population being male. There were 3 patients with multiple episodes that accounted for 8 of the episodes (3, 3, and 2 episodes each). Fever was the most common presenting complaint. The most common organism was Candida parapsilosis (6 of the 18 episodes). All episodes but one involved a central line, and all central lines were removed when present except for one case. Of the risk factors, 3 episodes occurred in neutropenic patients, and for 1 episode the patient had a questionable history of fungal endocarditis (and was on fungal prophylaxis). There were no patients with recent cardiac/urogenital surgery or prior fungal balls. No episodes had clinical symptoms suggestive of fungal endocarditis or fungal balls.
Post-Intervention Data
Over the subsequent 17-month period (November 11, 2017 to April 3, 2019), there were 13 episodes of candidemia. There were no episodes of Cryptococcus fungemia. Seven episodes occurred in patients on the pediatric gastroenterology service (2 of the 7 occurred in transplant recipients). Abdominal imaging was done in 3 of these episodes (43%), and in 2 of these 3 episodes, imaging was done at an outside institution prior to arrival, with no positive results (eFigure 2).
Echocardiography was done 57% of the time (n = 4), with echo being done at an outside institution prior to arrival half of the time (n = 2), with no endocarditis identified. The cases of abdominal imaging and echo done at outside institutions prior to arrival were not impacted by the countermeasures. Excluding those 2 patients who had both abdominal imaging and echocardiography done prior to arrival, the overall rate of imaging (both abdominal imaging and echo) done after countermeasures were instituted was 30% (Figure 3).

Of those 7 episodes, the average age was 6.8 years (57% female). There were no patients with multiple episodes. The most common presenting complaint was fever. The most common organism was Candida albicans (3 of the 7 episodes). All episodes involved a central line, which was removed in all cases except for one. Of the risk factors, 2 episodes were in neutropenic patients, and 1 episode had a history of bacterial endocarditis (not related to fungemia). No episodes occurred in patients with prior fungal renal infection, urogenital malformations, or recent cardiac/urogenital surgery. No episodes had clinical symptoms suggestive of fungal endocarditis or renal infection. No episodes of fungal endocarditis or renal infection were identified.
On average, a patient at our institution undergoing abdominal US and echo with a cardiology consult results in medical waste of approximately $3200 per patient. This cost does not take into account other miscellaneous charges possibly incurred, such as the radiologist interpreting the findings and transportation. Baseline data calculations show that patients waste on average 55 minutes in physical transport, and this does not take into account wait times.
Discussion
Candidemia contributes to 10% of central-line associated blood stream infections (CLABSI).19 Increased usage of indwelling central catheters for administration of parenteral nutrition will inevitably result in practitioners encountering cases of candidemia when caring for this population. As seen from our results, the majority of episodes of candidemia at our institution occurred on the pediatric gastroenterology service, and thus redefining standard work on this service will be impactful.
Candida parapsilosis and Candida albicans were the most common causative agents before and after intervention, respectively, but overall the most common organism was Candida albicans, which is in keeping with that of CLABSI in the literature.19 Growth of Candida parapsilosis has been particularly linked to CLABSI.19 The third most common organism in our study was Candida glabrata, which is the second most common cause of candidemia in CLABSI.19
The cases of positive abdominal imaging in fungemia in the literature are limited to the neonatal population1-4 and chronic disseminated candidiasis in patients with hematologic malignancies/neutropenia/immunosuppression.5,6 In fungal endocarditis, the reported cases were generally in neonates,1,3,7 critically ill patients,8 patients with hematologic malignancies/neutropenia/immunosuppression,6,9 or those with a cardiac history.9,10 This population differs from the patient population on the pediatric gastroenterology service. Patients on this service may not need US or echo. Performing abdominal US and echo in fungemia patients in whom such imaging is not indicated may result in medical waste of approximately $3200 per patient. There is also a waste of medical resources and time.
We found almost all practitioners are willing to change clinical practice once provided with current guidelines. Face-to-face oral presentations allowed for questions and interaction, making this form of information dissemination better than e-mails or handouts.
Though the numbers were small over the short study period, we were able to decrease overutilization of abdominal imaging and echo after implementing countermeasures. Frequency decreased from 100% to 43% and 57% for abdominal imaging and echo, respectively. Imaging that was done after the countermeasures were implemented was mainly attributed to imaging patients underwent prior to presenting to our institution. This reinforces the need for education at other institutions as well. Of the balancing measures assessed, there were no missed cases of fungal balls or fungal endocarditis. Additionally,
The findings from this quality improvement project underscore current recommendations that, despite common misconceptions, routine abdominal US and echo are not indicated in all cases of fungemia. Case-by-case assessment based on the clinical scenario remains key to management of fungemia to avoid unnecessary medical interventions.
Corresponding author: Donna Cheung, MBBS, 200 Hawkins Drive, BT 1120-G, Iowa City, IA 52242; [email protected].
Financial support: None.
From the University of Miami, Department of Pediatrics and Department of Medicine, Miami, FL.
Abstract
- Objective: Pediatric fungemia is associated with a low risk of fungal endocarditis and renal infections. The majority of current guidelines do not recommend routine abdominal imaging/echocardiograms in the evaluation of fungemia, but such imaging has been routinely ordered for patients on the pediatric gastroenterology service at our institution. Our goals were to assess the financial impact of this deviation from current clinical guidelines and redefine the standard work to reduce overutilization of abdominal ultrasounds and echocardiograms. Specifically, our goal was to reduce imaging by 50% by 18 months.
- Methods: Root cause analysis showed a lack of familiarity with current evidence. Using this data, countermeasures were implemented, including practitioner education of guidelines and creation of a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Balancing measures were missed episodes of fungal endocarditis and renal infection.
- Results: During the period January 1, 2016 to November 19, 2017, 18 of 21 episodes of fungemia in our pediatric institution occurred in patients admitted to the pediatric gastroenterology service. Abdominal imaging and echocardiograms were done 100% of the time, with no positive findings and an estimated cost of approximately $58,000. Post-intervention from November 20, 2017 to April 3, 2019, 7 of 13 episodes of fungemia occurred on this service. Frequency of abdominal imaging and echocardiograms decreased to 43% and 57%, respectively. No episodes of fungal endocarditis or renal infection were identified.
- Conclusion: Overutilization of abdominal imaging and echocardiograms in pediatric fungemia evaluation can be safely decreased.
Keywords: guidelines; cost; candidemia; endocarditis.
Practitioners may remain under the impression that routine abdominal ultrasounds (US) and echocardiograms (echo) are indicated in fungemia to evaluate for fungal endocarditis and renal infection, although these conditions are rare and limited to a subset of the population.1-10 Risk factors include prematurity, immunosuppression, prior bacterial endocarditis, abnormal cardiac valves, and previous urogenital surgeries.11
The 2016 Infectious Diseases Society of America (IDSA) guidelines do not recommend routine US or echo but rather provide scenarios in which Candida endocarditis should be suspected, and these include: persistently positive blood cultures, persistent fevers despite appropriate therapy, and clinical signs that may suggest endocarditis, such as a new heart murmur, heart failure, or embolic phenomena.11 IDSA recommends abdominal imaging in neonates with persistently positive blood cultures to evaluate the urogenital system, in addition to the liver and spleen. They also recommend abdominal imaging in symptomatic ascending Candida pyelonephritis beyond the neonatal period and in chronic disseminated candidiasis; the latter is uncommon and seen almost exclusively in patients recovering from neutropenia with a hematologic malignancy.11
We also reviewed guidelines on fungemia originating outside the United States. The 2010 Canadian clinical guidelines on invasive candidiasis do not explicitly recommend routine imaging, but rather state that various imaging studies, including US and echo among others, may be helpful.12 The German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy published a joint recommendation against routine US and echo in uncomplicated candidemia in 2011.13
The European Society for Clinical Microbiology and Infectious Diseases is the only society that recommends routine echo. Their 2012 guidelines on candidiasis recommend transesophageal echo in adults14 and echocardiography in children,15 as well as abdominal imaging in the diagnosis of chronic disseminated candidiasis in adults with hematological malignancies/hematopoietic stem cell transplantation.16
The 2013 Brazilian guidelines explicitly recommend against routine abdominal imaging and echo because of the low frequency of visceral lesions in adults with candidemia and recommend reserving imaging for those with persistently positive blood cultures or with clinical signs/symptoms suggestive of endocarditis/abdominal infection or clinical deterioration.17 The 2014 Japanese guidelines recommend ruling out chronic disseminated candidiasis in these patients with symptoms during the neutrophil recovery phase, but do not mention routinely imaging other patients. They do not address the role of echocardiography.18
Although physicians in the United Sates typically follow IDSA guidelines, abdominal US and echo were ordered routinely for patients with fungemia on the pediatric gastroenterology service at our institution, leading to higher medical costs and waste of medical resources. Our goals were to assess the current standard work for fungemia evaluation on this service, assess the impact of its deviation from current clinical guidelines, and redefine the standard work by (1) presenting current evidence to practitioners taking care of patients on this service, (2) providing a clinical pathway that allowed for variations where appropriate, and (3) providing a plan for pediatric fungemia management. Our SMART (Specific, Measurable, Attainable, Relevant and Timely) goal was to reduce overutilization of abdominal US and echo in pediatric patients with fungemia on the pediatric gastroenterology service by 50%.
Methods
Study, Setting, and Participants
We executed this quality improvement project at a quaternary care pediatric hospital affiliated with a school of medicine. The project scope consisted of inpatient pediatric patients with fungemia on the pediatric gastroenterology service admitted to the wards or pediatric critical care unit at this institution, along with the practitioners caring for these patients. The project was part of an institutional quality improvement initiative program. The quality improvement team included quality improvement experts from the departments of medicine and pediatrics, a pediatric resident and student, and physicians from the divisions of pediatric infectious disease, pediatric critical care, and pediatric gastroenterology. This study qualified for Institutional Review Board (IRB) exemption based on the University’s IRB stipulations.
Current Condition
Root cause analysis was performed by creating a process map of the current standard work and a fishbone diagram (Figure 1). We incorporated feedback from voice of the customer in the root cause analysis. In this analysis, the voice of the customer came from the bedside floor nurses, ultrasound clerk and sonographer, echo technician, cardiology fellow, and microbiology medical technician. We got their feedback on our process map, its accuracy and ways to expand, their thoughts on the problem and why we have this problem, and any solutions they could offer to help improve the problem. Some of the key points obtained were: echos were not routinely done on the floors and were not considered urgent as they often did not change management; the sonographer and those from the cardiology department felt imaging was often overutilized because of misconceptions and lack of available hospital guidelines. Suggested solutions included provider education with reference to Duke’s criteria and establishing a clinical pathway approved by all concerned departments.

Prior to education, we surveyed current practices of practitioners on teams caring for these patients, which included physicians of all levels (attendings, fellows, residents) as well as nurse practitioners and medical students from the department of pediatrics and divisions of pediatric gastroenterology, pediatric infectious disease, and pediatric critical care medicine.
Countermeasures
Practitioner Education. In October 2017 practitioners were given a 20-minute presentation on the latest international guidelines on fungemia. Fifty-nine practitioners completed pre- and post-test surveys. Eight respondents were excluded due to incomplete surveys. We compared self-reported frequencies of ordering abdominal imaging and echo before the presentation with intention to order post education. Intention to change clinical practice after the presentation was also surveyed.
Clinical Pathway. Education alone may not result in sustainability, and thus we provided a readily accessible clinical pathway and an electronic order set for pediatric fungemia management. Inter-department buy-in was also necessary for success. It was important to get the input from the various teams (infectious disease, cardiology, gastroenterology, and critical care), which was done by incorporating members from those divisions in the project or getting their feedback through voice of the customer analysis.
We redefined standard work based on current evidence and created a clinical pathway during March 2018 that included variations when appropriate (Figure 2). We presented the clinical pathway to practitioners and distributed it via email. We also made it available to pediatric residents and fellows on their mobile institutional work resource application.

Electronic Order Set. We created an electronic order set for pediatric fungemia management and made it available in the electronic health record May 2018.
Measurement
Cases of fungemia were identified through the electronic health record pre-intervention (January 1, 2016 through November 19, 2017) and post-intervention (November 20, 2019 through April 3, 2019). An episode of fungemia was defined as an encounter with 1 or more positive blood culture(s) for Candida species or Cryptococcus species. We manually identified patients belonging to the pediatric gastroenterology service and reviewed these charts to determine the presenting complaint, organism isolated, transplant status, central lines status, risk factors, if abdominal imaging or echocardiography were done for the episode of fungemia, and their corresponding results. We calculated overall and per patient medical charges by using the average charges at our institution of US and echocardiography with a cardiology consult. These average charges were provided by patient financial services and the pediatric cardiology department, respectively. To address non-technical expenditures, we calculated the average time taken for transport to and from radiology and the echo suite for each identified patient. We identified missed fungal endocarditis and fungal balls as balancing measures.
Results
Survey
Among the 51 practitioners surveyed, 36% were performing routine echo and 22% self-reported performing routine abdominal imaging. After education, no respondents planned to routinely do echo or abdominal imaging. All but 1 respondent planned to change their practice for evaluation of fungemia patients based on the presentation (eFigure 1).

Baseline Data
Over the 23-month period from January 1, 2016 to November 19, 2017, there were 21 episodes of fungemia, 18 of which occurred in patients on the pediatric gastroenterology service (2 of the 18 were transplant recipients). For the 18 episodes on this service, abdominal imaging and echo were done 100% of the time, with 0 positive findings (eFigure 2).

Of those 18 episodes, the average age was 4.6 years, with two-thirds of the population being male. There were 3 patients with multiple episodes that accounted for 8 of the episodes (3, 3, and 2 episodes each). Fever was the most common presenting complaint. The most common organism was Candida parapsilosis (6 of the 18 episodes). All episodes but one involved a central line, and all central lines were removed when present except for one case. Of the risk factors, 3 episodes occurred in neutropenic patients, and for 1 episode the patient had a questionable history of fungal endocarditis (and was on fungal prophylaxis). There were no patients with recent cardiac/urogenital surgery or prior fungal balls. No episodes had clinical symptoms suggestive of fungal endocarditis or fungal balls.
Post-Intervention Data
Over the subsequent 17-month period (November 11, 2017 to April 3, 2019), there were 13 episodes of candidemia. There were no episodes of Cryptococcus fungemia. Seven episodes occurred in patients on the pediatric gastroenterology service (2 of the 7 occurred in transplant recipients). Abdominal imaging was done in 3 of these episodes (43%), and in 2 of these 3 episodes, imaging was done at an outside institution prior to arrival, with no positive results (eFigure 2).
Echocardiography was done 57% of the time (n = 4), with echo being done at an outside institution prior to arrival half of the time (n = 2), with no endocarditis identified. The cases of abdominal imaging and echo done at outside institutions prior to arrival were not impacted by the countermeasures. Excluding those 2 patients who had both abdominal imaging and echocardiography done prior to arrival, the overall rate of imaging (both abdominal imaging and echo) done after countermeasures were instituted was 30% (Figure 3).

Of those 7 episodes, the average age was 6.8 years (57% female). There were no patients with multiple episodes. The most common presenting complaint was fever. The most common organism was Candida albicans (3 of the 7 episodes). All episodes involved a central line, which was removed in all cases except for one. Of the risk factors, 2 episodes were in neutropenic patients, and 1 episode had a history of bacterial endocarditis (not related to fungemia). No episodes occurred in patients with prior fungal renal infection, urogenital malformations, or recent cardiac/urogenital surgery. No episodes had clinical symptoms suggestive of fungal endocarditis or renal infection. No episodes of fungal endocarditis or renal infection were identified.
On average, a patient at our institution undergoing abdominal US and echo with a cardiology consult results in medical waste of approximately $3200 per patient. This cost does not take into account other miscellaneous charges possibly incurred, such as the radiologist interpreting the findings and transportation. Baseline data calculations show that patients waste on average 55 minutes in physical transport, and this does not take into account wait times.
Discussion
Candidemia contributes to 10% of central-line associated blood stream infections (CLABSI).19 Increased usage of indwelling central catheters for administration of parenteral nutrition will inevitably result in practitioners encountering cases of candidemia when caring for this population. As seen from our results, the majority of episodes of candidemia at our institution occurred on the pediatric gastroenterology service, and thus redefining standard work on this service will be impactful.
Candida parapsilosis and Candida albicans were the most common causative agents before and after intervention, respectively, but overall the most common organism was Candida albicans, which is in keeping with that of CLABSI in the literature.19 Growth of Candida parapsilosis has been particularly linked to CLABSI.19 The third most common organism in our study was Candida glabrata, which is the second most common cause of candidemia in CLABSI.19
The cases of positive abdominal imaging in fungemia in the literature are limited to the neonatal population1-4 and chronic disseminated candidiasis in patients with hematologic malignancies/neutropenia/immunosuppression.5,6 In fungal endocarditis, the reported cases were generally in neonates,1,3,7 critically ill patients,8 patients with hematologic malignancies/neutropenia/immunosuppression,6,9 or those with a cardiac history.9,10 This population differs from the patient population on the pediatric gastroenterology service. Patients on this service may not need US or echo. Performing abdominal US and echo in fungemia patients in whom such imaging is not indicated may result in medical waste of approximately $3200 per patient. There is also a waste of medical resources and time.
We found almost all practitioners are willing to change clinical practice once provided with current guidelines. Face-to-face oral presentations allowed for questions and interaction, making this form of information dissemination better than e-mails or handouts.
Though the numbers were small over the short study period, we were able to decrease overutilization of abdominal imaging and echo after implementing countermeasures. Frequency decreased from 100% to 43% and 57% for abdominal imaging and echo, respectively. Imaging that was done after the countermeasures were implemented was mainly attributed to imaging patients underwent prior to presenting to our institution. This reinforces the need for education at other institutions as well. Of the balancing measures assessed, there were no missed cases of fungal balls or fungal endocarditis. Additionally,
The findings from this quality improvement project underscore current recommendations that, despite common misconceptions, routine abdominal US and echo are not indicated in all cases of fungemia. Case-by-case assessment based on the clinical scenario remains key to management of fungemia to avoid unnecessary medical interventions.
Corresponding author: Donna Cheung, MBBS, 200 Hawkins Drive, BT 1120-G, Iowa City, IA 52242; [email protected].
Financial support: None.
1. Benjamin DK Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634-640.
2. Wynn JL, Tan S, Gantz MG, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. 2012;54:331-339.
3. Noyola DE, Fernandez M, Moylett EH, et al. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018-1023.
4. Phillips JR, Karlowicz MG Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190-194.
5. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87:535-541.
6. Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641.
7. Levy I, Shalit I, Birk E, et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses. 2006;49:43-48.
8. Aspesberro F, Beghetti M, Oberhansli I, et al. Fungal endocarditis in critically ill children. Eur J Pediatr. 1999;158:275-280.
9. Fernandez-Cruz A, Cruz Menarguez M, Munoz P, et al. The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549.
10. Hernandez-Torres A, Garcia-Vazquez E, Laso-Ortiz A, et al. [Candida sp endocarditis. Experience in a third-level hospital and review of the literature]. Rev Esp Quimioter. 2013;26:51-55.
11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
12. Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122-50.
13. Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54:279-310.
14. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
15. Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52.
16. Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53-67.
17. Colombo AL, Guimaraes T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis. 2013;17:283-312.
18. Kohno S, Tamura K, Niki Y, et al. Executive Summary of Japanese Domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117-E163.
19. Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiology Rev. 2004;17:255-267.
1. Benjamin DK Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634-640.
2. Wynn JL, Tan S, Gantz MG, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. 2012;54:331-339.
3. Noyola DE, Fernandez M, Moylett EH, et al. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018-1023.
4. Phillips JR, Karlowicz MG Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190-194.
5. Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87:535-541.
6. Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641.
7. Levy I, Shalit I, Birk E, et al. Candida endocarditis in neonates: report of five cases and review of the literature. Mycoses. 2006;49:43-48.
8. Aspesberro F, Beghetti M, Oberhansli I, et al. Fungal endocarditis in critically ill children. Eur J Pediatr. 1999;158:275-280.
9. Fernandez-Cruz A, Cruz Menarguez M, Munoz P, et al. The search for endocarditis in patients with candidemia: a systematic recommendation for echocardiography? A prospective cohort. Eur J Clin Microbiol Infect Dis. 2015;34:1543-1549.
10. Hernandez-Torres A, Garcia-Vazquez E, Laso-Ortiz A, et al. [Candida sp endocarditis. Experience in a third-level hospital and review of the literature]. Rev Esp Quimioter. 2013;26:51-55.
11. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50.
12. Bow EJ, Evans G, Fuller J, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122-50.
13. Ruhnke M, Rickerts V, Cornely OA, et al. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses. 2011;54:279-310.
14. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
15. Hope WW, Castagnola E, Groll AH, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18 Suppl 7:38-52.
16. Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18 Suppl 7:53-67.
17. Colombo AL, Guimaraes T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis. 2013;17:283-312.
18. Kohno S, Tamura K, Niki Y, et al. Executive Summary of Japanese Domestic guidelines for management of deep-seated mycosis 2014. Med Mycol J. 2016;57:E117-E163.
19. Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiology Rev. 2004;17:255-267.
CDC finds that efforts to reduce new HIV infections have stalled
, according to a Vital Signs report published by the Centers for Disease Control and Prevention based upon a simultaneous MMWR Early Release. The report indicates that many Americans with HIV are not aware of their status or are not receiving effective treatment. Furthermore, the data suggest that few Americans who could benefit from preexposure prophylaxis (PrEP), a daily pill that prevents HIV, are receiving it.
The report “shows that HIV testing, treatment, and prevention have not reached enough Americans, and it emphasizes the continued urgent need to increase these interventions,” said Jay C. Butler, MD, deputy director for infectious diseases at the CDC, at a press conference. “We made a lot of progress in the late ’90s and into the early part of the 21st century in reducing the number of new cases of HIV. But HIV prevention progress has stalled in America since 2013. This stalling underscores the need to increase resources, deploy new technologies, and build expertise, particularly in areas where they’re needed most.”
To achieve these objectives, the CDC has proposed a federal initiative called Ending the HIV Epidemic: A Plan for America. The goal of the initiative is to reduce new HIV infections by 90% by 2030, in part by expanding access to PrEP medications.
Data suggest shortcomings in diagnosis, treatment, and prevention
In its review of data on HIV testing and treatment in 2017, the Vital Signs report found that approximately 154,000 people with HIV (that is, 14% of the total population with HIV) were unaware that they had the virus. These patients consequently could not take advantage of HIV treatment to maintain health, control the virus, and prevent HIV transmission. Young people aged 13-24 years were less likely to know their HIV status than did those aged 25 years and older, according to the report.
Furthermore, approximately two-thirds (63%) of patients who knew that they had HIV had the virus under control through effective treatment. Young people and African Americans were least likely to have the virus under control, according to the report.
The report also examines data about treatment with PrEP in 2018. About 1.2 million Americans could benefit from PrEP, but only 219,700 (18%) of them had received a prescription for the drug. The eligible groups with the lowest rates of coverage were young people, African Americans, and Latinos.
The report presents a conservative estimate of PrEP coverage, however. Researchers examined data from 92% of prescriptions from retail pharmacies in the United States but did not include prescriptions from closed health care systems such as managed care organizations and military health plans. PrEP coverage in 2018 likely was higher than these estimates indicate, according to the CDC.
“There has been a rapid increase in the number of people taking PrEP over the past 3 years, but there is no doubt that PrEP uptake is too low,” said Eugene McCray, MD, director of CDC’s division of HIV/AIDS prevention. “We are working hard to increase access to PrEP, especially among gay and bisexual men, women, transgender people, young people, African Americans, and Latinos.”
The rate of new HIV infections has not decreased, but remained stable, according to the report. The CDC estimates that there were about 38,000 new infections per year from 2013 to 2017.
Proposed initiative focuses on areas of greatest need
The proposed Ending the HIV Epidemic initiative, if it is funded, will target the locations of greatest need throughout the country. Its initial focus will be on 50 areas that account for more than half of new HIV diagnoses, including 48 counties; San Juan, Puerto Rico; and Washington, D.C. It also will direct resources to seven states with high rates of infection in rural areas. In a second phase, the initiative will expand nationwide, provided that additional resources are made available.
The proposed initiative relies on four science-based strategies. First, it will aim to diagnose all Americans with HIV (at least 95% of HIV infections) as early as possible. Second, the initiative will enable people with HIV to receive treatment rapidly and effectively. The CDC’s target is to achieve viral suppression in at least 95% of people with diagnosed HIV. Third, the initiative will use proven interventions such as PrEP and syringe services programs to prevent new HIV transmissions. One related goal is for at least 50% of people who could benefit from PrEP to receive a prescription. Finally, the initiative is intended to respond quickly to potential HIV outbreaks and provide prevention and treatment to those who need them.
The U.S. Department of Health & Human Services already has taken steps to enable the initiative to be implemented quickly if it is funded in 2020. The department has provided funding to Baltimore City, Md.; DeKalb County, Ga.; and East Baton Rouge Parish, La. to begin pursuing parts of the initiative. These communities are encouraged to share the lessons of their experiences with other communities. HHS also has supported local efforts to develop plans under the initiative in all priority geographic areas. These plans draw upon recommendations from the community, HIV-planning bodies, and health care providers.
“Ending the HIV epidemic would be one of the greatest public health triumphs in our nation’s history,” said Dr. McCray.
SOURCES: Centers for Disease Control and Prevention. CDC Vital Signs. 2019 Dec 3. and Harris NS et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 3.
, according to a Vital Signs report published by the Centers for Disease Control and Prevention based upon a simultaneous MMWR Early Release. The report indicates that many Americans with HIV are not aware of their status or are not receiving effective treatment. Furthermore, the data suggest that few Americans who could benefit from preexposure prophylaxis (PrEP), a daily pill that prevents HIV, are receiving it.

The report “shows that HIV testing, treatment, and prevention have not reached enough Americans, and it emphasizes the continued urgent need to increase these interventions,” said Jay C. Butler, MD, deputy director for infectious diseases at the CDC, at a press conference. “We made a lot of progress in the late ’90s and into the early part of the 21st century in reducing the number of new cases of HIV. But HIV prevention progress has stalled in America since 2013. This stalling underscores the need to increase resources, deploy new technologies, and build expertise, particularly in areas where they’re needed most.”
To achieve these objectives, the CDC has proposed a federal initiative called Ending the HIV Epidemic: A Plan for America. The goal of the initiative is to reduce new HIV infections by 90% by 2030, in part by expanding access to PrEP medications.
Data suggest shortcomings in diagnosis, treatment, and prevention
In its review of data on HIV testing and treatment in 2017, the Vital Signs report found that approximately 154,000 people with HIV (that is, 14% of the total population with HIV) were unaware that they had the virus. These patients consequently could not take advantage of HIV treatment to maintain health, control the virus, and prevent HIV transmission. Young people aged 13-24 years were less likely to know their HIV status than did those aged 25 years and older, according to the report.
Furthermore, approximately two-thirds (63%) of patients who knew that they had HIV had the virus under control through effective treatment. Young people and African Americans were least likely to have the virus under control, according to the report.
The report also examines data about treatment with PrEP in 2018. About 1.2 million Americans could benefit from PrEP, but only 219,700 (18%) of them had received a prescription for the drug. The eligible groups with the lowest rates of coverage were young people, African Americans, and Latinos.
The report presents a conservative estimate of PrEP coverage, however. Researchers examined data from 92% of prescriptions from retail pharmacies in the United States but did not include prescriptions from closed health care systems such as managed care organizations and military health plans. PrEP coverage in 2018 likely was higher than these estimates indicate, according to the CDC.
“There has been a rapid increase in the number of people taking PrEP over the past 3 years, but there is no doubt that PrEP uptake is too low,” said Eugene McCray, MD, director of CDC’s division of HIV/AIDS prevention. “We are working hard to increase access to PrEP, especially among gay and bisexual men, women, transgender people, young people, African Americans, and Latinos.”
The rate of new HIV infections has not decreased, but remained stable, according to the report. The CDC estimates that there were about 38,000 new infections per year from 2013 to 2017.
Proposed initiative focuses on areas of greatest need
The proposed Ending the HIV Epidemic initiative, if it is funded, will target the locations of greatest need throughout the country. Its initial focus will be on 50 areas that account for more than half of new HIV diagnoses, including 48 counties; San Juan, Puerto Rico; and Washington, D.C. It also will direct resources to seven states with high rates of infection in rural areas. In a second phase, the initiative will expand nationwide, provided that additional resources are made available.
The proposed initiative relies on four science-based strategies. First, it will aim to diagnose all Americans with HIV (at least 95% of HIV infections) as early as possible. Second, the initiative will enable people with HIV to receive treatment rapidly and effectively. The CDC’s target is to achieve viral suppression in at least 95% of people with diagnosed HIV. Third, the initiative will use proven interventions such as PrEP and syringe services programs to prevent new HIV transmissions. One related goal is for at least 50% of people who could benefit from PrEP to receive a prescription. Finally, the initiative is intended to respond quickly to potential HIV outbreaks and provide prevention and treatment to those who need them.
The U.S. Department of Health & Human Services already has taken steps to enable the initiative to be implemented quickly if it is funded in 2020. The department has provided funding to Baltimore City, Md.; DeKalb County, Ga.; and East Baton Rouge Parish, La. to begin pursuing parts of the initiative. These communities are encouraged to share the lessons of their experiences with other communities. HHS also has supported local efforts to develop plans under the initiative in all priority geographic areas. These plans draw upon recommendations from the community, HIV-planning bodies, and health care providers.
“Ending the HIV epidemic would be one of the greatest public health triumphs in our nation’s history,” said Dr. McCray.
SOURCES: Centers for Disease Control and Prevention. CDC Vital Signs. 2019 Dec 3. and Harris NS et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 3.
, according to a Vital Signs report published by the Centers for Disease Control and Prevention based upon a simultaneous MMWR Early Release. The report indicates that many Americans with HIV are not aware of their status or are not receiving effective treatment. Furthermore, the data suggest that few Americans who could benefit from preexposure prophylaxis (PrEP), a daily pill that prevents HIV, are receiving it.

The report “shows that HIV testing, treatment, and prevention have not reached enough Americans, and it emphasizes the continued urgent need to increase these interventions,” said Jay C. Butler, MD, deputy director for infectious diseases at the CDC, at a press conference. “We made a lot of progress in the late ’90s and into the early part of the 21st century in reducing the number of new cases of HIV. But HIV prevention progress has stalled in America since 2013. This stalling underscores the need to increase resources, deploy new technologies, and build expertise, particularly in areas where they’re needed most.”
To achieve these objectives, the CDC has proposed a federal initiative called Ending the HIV Epidemic: A Plan for America. The goal of the initiative is to reduce new HIV infections by 90% by 2030, in part by expanding access to PrEP medications.
Data suggest shortcomings in diagnosis, treatment, and prevention
In its review of data on HIV testing and treatment in 2017, the Vital Signs report found that approximately 154,000 people with HIV (that is, 14% of the total population with HIV) were unaware that they had the virus. These patients consequently could not take advantage of HIV treatment to maintain health, control the virus, and prevent HIV transmission. Young people aged 13-24 years were less likely to know their HIV status than did those aged 25 years and older, according to the report.
Furthermore, approximately two-thirds (63%) of patients who knew that they had HIV had the virus under control through effective treatment. Young people and African Americans were least likely to have the virus under control, according to the report.
The report also examines data about treatment with PrEP in 2018. About 1.2 million Americans could benefit from PrEP, but only 219,700 (18%) of them had received a prescription for the drug. The eligible groups with the lowest rates of coverage were young people, African Americans, and Latinos.
The report presents a conservative estimate of PrEP coverage, however. Researchers examined data from 92% of prescriptions from retail pharmacies in the United States but did not include prescriptions from closed health care systems such as managed care organizations and military health plans. PrEP coverage in 2018 likely was higher than these estimates indicate, according to the CDC.
“There has been a rapid increase in the number of people taking PrEP over the past 3 years, but there is no doubt that PrEP uptake is too low,” said Eugene McCray, MD, director of CDC’s division of HIV/AIDS prevention. “We are working hard to increase access to PrEP, especially among gay and bisexual men, women, transgender people, young people, African Americans, and Latinos.”
The rate of new HIV infections has not decreased, but remained stable, according to the report. The CDC estimates that there were about 38,000 new infections per year from 2013 to 2017.
Proposed initiative focuses on areas of greatest need
The proposed Ending the HIV Epidemic initiative, if it is funded, will target the locations of greatest need throughout the country. Its initial focus will be on 50 areas that account for more than half of new HIV diagnoses, including 48 counties; San Juan, Puerto Rico; and Washington, D.C. It also will direct resources to seven states with high rates of infection in rural areas. In a second phase, the initiative will expand nationwide, provided that additional resources are made available.
The proposed initiative relies on four science-based strategies. First, it will aim to diagnose all Americans with HIV (at least 95% of HIV infections) as early as possible. Second, the initiative will enable people with HIV to receive treatment rapidly and effectively. The CDC’s target is to achieve viral suppression in at least 95% of people with diagnosed HIV. Third, the initiative will use proven interventions such as PrEP and syringe services programs to prevent new HIV transmissions. One related goal is for at least 50% of people who could benefit from PrEP to receive a prescription. Finally, the initiative is intended to respond quickly to potential HIV outbreaks and provide prevention and treatment to those who need them.
The U.S. Department of Health & Human Services already has taken steps to enable the initiative to be implemented quickly if it is funded in 2020. The department has provided funding to Baltimore City, Md.; DeKalb County, Ga.; and East Baton Rouge Parish, La. to begin pursuing parts of the initiative. These communities are encouraged to share the lessons of their experiences with other communities. HHS also has supported local efforts to develop plans under the initiative in all priority geographic areas. These plans draw upon recommendations from the community, HIV-planning bodies, and health care providers.
“Ending the HIV epidemic would be one of the greatest public health triumphs in our nation’s history,” said Dr. McCray.
SOURCES: Centers for Disease Control and Prevention. CDC Vital Signs. 2019 Dec 3. and Harris NS et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 3.
FROM THE CDC
Antibiotic use may increase the risk of Parkinson’s disease
according to a report published in Movement Disorders. Associations were found for broad-spectrum antibiotics and those that act against anaerobic bacteria and fungi. The timing of antibiotic exposure also seemed to matter.
In a nationwide case-control study, Finnish researchers compared data on antibiotic use in 13,976 individuals diagnosed with Parkinson’s disease between 1998 and 2014 with antibiotic-use data from 40,697 controls. The strongest connection with Parkinson’s disease risk was found for oral exposure to macrolides and lincosamides (adjusted odds ratio up to 1.416). After correction for multiple comparisons, exposure to antianaerobics and tetracyclines 10-15 years before the index date, and antifungal medications 1-5 years before the index date were positively associated with Parkinson’s disease risk. In post hoc analyses, further positive associations were found for broad-spectrum antibiotics.
Tuomas H. Mertsalmi, MD, from the Helsinki University Hospital and coauthors reported that this was the first study to explore a possible connection between antimicrobial use and Parkinson’s disease.
“In Parkinson’s disease, several studies have described alterations of gut microbiota composition, and changes in fecal microbiota abundance have been found to be associated with gastrointestinal and motor symptoms,” they wrote.
Commenting on the delay between the exposure and diagnosis for the most strongly associated antimicrobials, the authors noted that this 10-15 year lag was comparable with what has been found between the peripheral initiation of Parkinson’s disease and its motor manifestation.
“This would also explain the lack of association between antibiotic exposure 1-5 years before index date – if antibiotic exposure could induce or contribute to the pathogenesis of Parkinson’s disease in the gastrointestinal tract, it would probably take several years before the clinical manifestation of Parkinson’s disease,” they wrote.
With regards to the association seen for sulfonamides and trimethoprim – which was 1-5 years before the index date – they speculated this could reflect treatment for urinary tract infections, which individuals with Parkinson’s disease might be more susceptible to in the prodromal phase of the disease.
The authors noted that infectious disease has also been associated with Parkinson’s disease, and that their analysis did not include information about why the antimicrobial agents were prescribed. However, they pointed out that the associations were only for certain antibiotic classes, which makes it unlikely that the association was related to greater burden of infectious disease among individuals with Parkinson’s disease.
The pattern of associations supports the hypothesis that effects on gut microbiota could link antibiotics to Parkinson’s disease. “The link between antibiotic exposure and Parkinson’s disease fits the current view that in a significant proportion of patients the pathology of Parkinson’s disease may originate in the gut, possibly related to microbial changes, years before the onset of typical Parkinson’s disease motor symptoms such as slowness, muscle stiffness, and shaking of the extremities. It was known that bacterial composition of the intestine in patients with Parkinson’s disease is abnormal, but the cause is unclear. Our results suggest that some commonly used antibiotics, which are known to strongly influence the gut microbiota, could be a predisposing factor,” said lead investigator Filip Scheperjans, MD, PhD, from the department of neurology at Helsinki University Hospital.
The findings may have implications for antibiotic prescribing practices in the future, said Dr. Scheperjans. “In addition to the problem of antibiotic resistance, antimicrobial prescribing should also take into account their potentially long-lasting effects on the gut microbiome and the development of certain diseases.”
The study was funded by the Finnish Parkinson Foundation, the Finnish Medical Foundation, the Maire Taponen Foundation, and the Academy of Finland. One author declared relevant patents and his position as founder and chief executive of a private company. No other conflicts of interest were declared.
SOURCE: Mertsalmi TH et al. Mov Disord. 2019 Nov 18. doi: 10.1002/mds.27924.
according to a report published in Movement Disorders. Associations were found for broad-spectrum antibiotics and those that act against anaerobic bacteria and fungi. The timing of antibiotic exposure also seemed to matter.
In a nationwide case-control study, Finnish researchers compared data on antibiotic use in 13,976 individuals diagnosed with Parkinson’s disease between 1998 and 2014 with antibiotic-use data from 40,697 controls. The strongest connection with Parkinson’s disease risk was found for oral exposure to macrolides and lincosamides (adjusted odds ratio up to 1.416). After correction for multiple comparisons, exposure to antianaerobics and tetracyclines 10-15 years before the index date, and antifungal medications 1-5 years before the index date were positively associated with Parkinson’s disease risk. In post hoc analyses, further positive associations were found for broad-spectrum antibiotics.
Tuomas H. Mertsalmi, MD, from the Helsinki University Hospital and coauthors reported that this was the first study to explore a possible connection between antimicrobial use and Parkinson’s disease.
“In Parkinson’s disease, several studies have described alterations of gut microbiota composition, and changes in fecal microbiota abundance have been found to be associated with gastrointestinal and motor symptoms,” they wrote.
Commenting on the delay between the exposure and diagnosis for the most strongly associated antimicrobials, the authors noted that this 10-15 year lag was comparable with what has been found between the peripheral initiation of Parkinson’s disease and its motor manifestation.
“This would also explain the lack of association between antibiotic exposure 1-5 years before index date – if antibiotic exposure could induce or contribute to the pathogenesis of Parkinson’s disease in the gastrointestinal tract, it would probably take several years before the clinical manifestation of Parkinson’s disease,” they wrote.
With regards to the association seen for sulfonamides and trimethoprim – which was 1-5 years before the index date – they speculated this could reflect treatment for urinary tract infections, which individuals with Parkinson’s disease might be more susceptible to in the prodromal phase of the disease.
The authors noted that infectious disease has also been associated with Parkinson’s disease, and that their analysis did not include information about why the antimicrobial agents were prescribed. However, they pointed out that the associations were only for certain antibiotic classes, which makes it unlikely that the association was related to greater burden of infectious disease among individuals with Parkinson’s disease.
The pattern of associations supports the hypothesis that effects on gut microbiota could link antibiotics to Parkinson’s disease. “The link between antibiotic exposure and Parkinson’s disease fits the current view that in a significant proportion of patients the pathology of Parkinson’s disease may originate in the gut, possibly related to microbial changes, years before the onset of typical Parkinson’s disease motor symptoms such as slowness, muscle stiffness, and shaking of the extremities. It was known that bacterial composition of the intestine in patients with Parkinson’s disease is abnormal, but the cause is unclear. Our results suggest that some commonly used antibiotics, which are known to strongly influence the gut microbiota, could be a predisposing factor,” said lead investigator Filip Scheperjans, MD, PhD, from the department of neurology at Helsinki University Hospital.
The findings may have implications for antibiotic prescribing practices in the future, said Dr. Scheperjans. “In addition to the problem of antibiotic resistance, antimicrobial prescribing should also take into account their potentially long-lasting effects on the gut microbiome and the development of certain diseases.”
The study was funded by the Finnish Parkinson Foundation, the Finnish Medical Foundation, the Maire Taponen Foundation, and the Academy of Finland. One author declared relevant patents and his position as founder and chief executive of a private company. No other conflicts of interest were declared.
SOURCE: Mertsalmi TH et al. Mov Disord. 2019 Nov 18. doi: 10.1002/mds.27924.
according to a report published in Movement Disorders. Associations were found for broad-spectrum antibiotics and those that act against anaerobic bacteria and fungi. The timing of antibiotic exposure also seemed to matter.
In a nationwide case-control study, Finnish researchers compared data on antibiotic use in 13,976 individuals diagnosed with Parkinson’s disease between 1998 and 2014 with antibiotic-use data from 40,697 controls. The strongest connection with Parkinson’s disease risk was found for oral exposure to macrolides and lincosamides (adjusted odds ratio up to 1.416). After correction for multiple comparisons, exposure to antianaerobics and tetracyclines 10-15 years before the index date, and antifungal medications 1-5 years before the index date were positively associated with Parkinson’s disease risk. In post hoc analyses, further positive associations were found for broad-spectrum antibiotics.
Tuomas H. Mertsalmi, MD, from the Helsinki University Hospital and coauthors reported that this was the first study to explore a possible connection between antimicrobial use and Parkinson’s disease.
“In Parkinson’s disease, several studies have described alterations of gut microbiota composition, and changes in fecal microbiota abundance have been found to be associated with gastrointestinal and motor symptoms,” they wrote.
Commenting on the delay between the exposure and diagnosis for the most strongly associated antimicrobials, the authors noted that this 10-15 year lag was comparable with what has been found between the peripheral initiation of Parkinson’s disease and its motor manifestation.
“This would also explain the lack of association between antibiotic exposure 1-5 years before index date – if antibiotic exposure could induce or contribute to the pathogenesis of Parkinson’s disease in the gastrointestinal tract, it would probably take several years before the clinical manifestation of Parkinson’s disease,” they wrote.
With regards to the association seen for sulfonamides and trimethoprim – which was 1-5 years before the index date – they speculated this could reflect treatment for urinary tract infections, which individuals with Parkinson’s disease might be more susceptible to in the prodromal phase of the disease.
The authors noted that infectious disease has also been associated with Parkinson’s disease, and that their analysis did not include information about why the antimicrobial agents were prescribed. However, they pointed out that the associations were only for certain antibiotic classes, which makes it unlikely that the association was related to greater burden of infectious disease among individuals with Parkinson’s disease.
The pattern of associations supports the hypothesis that effects on gut microbiota could link antibiotics to Parkinson’s disease. “The link between antibiotic exposure and Parkinson’s disease fits the current view that in a significant proportion of patients the pathology of Parkinson’s disease may originate in the gut, possibly related to microbial changes, years before the onset of typical Parkinson’s disease motor symptoms such as slowness, muscle stiffness, and shaking of the extremities. It was known that bacterial composition of the intestine in patients with Parkinson’s disease is abnormal, but the cause is unclear. Our results suggest that some commonly used antibiotics, which are known to strongly influence the gut microbiota, could be a predisposing factor,” said lead investigator Filip Scheperjans, MD, PhD, from the department of neurology at Helsinki University Hospital.
The findings may have implications for antibiotic prescribing practices in the future, said Dr. Scheperjans. “In addition to the problem of antibiotic resistance, antimicrobial prescribing should also take into account their potentially long-lasting effects on the gut microbiome and the development of certain diseases.”
The study was funded by the Finnish Parkinson Foundation, the Finnish Medical Foundation, the Maire Taponen Foundation, and the Academy of Finland. One author declared relevant patents and his position as founder and chief executive of a private company. No other conflicts of interest were declared.
SOURCE: Mertsalmi TH et al. Mov Disord. 2019 Nov 18. doi: 10.1002/mds.27924.
FROM MOVEMENT DISORDERS
2019-2020 flu season starts off full throttle
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.





