User login
Influenza activity continues to be unusually high
The 2019-2020 flu season continues its unusually early rise in activity, with the Centers for Disease Control and Prevention estimating that 3.7 million cases have occurred through Dec. 14.
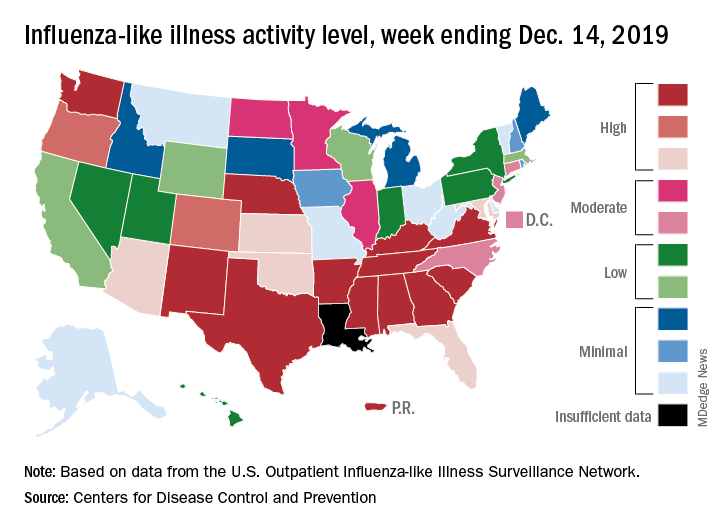
which is up from 3.2% the previous week and is the sixth consecutive week that the United States has been at or above the national baseline of 2.4%, the CDC reported Dec. 20. This year’s 3.9% is the highest mid-December rate recorded since 2003, when it reached almost 7.4%.
Most of the influenza activity so far this season is being driven by influenza B/Victoria viruses. Nationwide testing puts influenza B prevalence at 68.5% of all positive specimens, exactly the same as last week, but A(H1N1) viruses “are increasing in proportion relative to other influenza viruses in some regions,” the CDC’s influenza division said.
A look at this week’s activity map shows that 21 states, compared with 12 last week, were in the “high” range of activity – that’s levels 8-10 on the CDC’s 1-10 scale. Twelve of those states, along with Puerto Rico, were at level 10, which was up from nine a week earlier, the CDC said.
The overall hospitalization rate through the week of Dec. 8-14 (5.5 per 100,000 population) “is similar to what has been seen at this time during recent seasons,” the CDC noted. The highest rates are occurring among adults over age 65 years (12.7 per 100,000) and children aged 0-4 years (10.9 per 100,000).
Three ILI-related deaths among children that occurred last week were reported, which brings the total for the 2019-2020 season to 19, the CDC said.
The 2019-2020 flu season continues its unusually early rise in activity, with the Centers for Disease Control and Prevention estimating that 3.7 million cases have occurred through Dec. 14.

which is up from 3.2% the previous week and is the sixth consecutive week that the United States has been at or above the national baseline of 2.4%, the CDC reported Dec. 20. This year’s 3.9% is the highest mid-December rate recorded since 2003, when it reached almost 7.4%.
Most of the influenza activity so far this season is being driven by influenza B/Victoria viruses. Nationwide testing puts influenza B prevalence at 68.5% of all positive specimens, exactly the same as last week, but A(H1N1) viruses “are increasing in proportion relative to other influenza viruses in some regions,” the CDC’s influenza division said.
A look at this week’s activity map shows that 21 states, compared with 12 last week, were in the “high” range of activity – that’s levels 8-10 on the CDC’s 1-10 scale. Twelve of those states, along with Puerto Rico, were at level 10, which was up from nine a week earlier, the CDC said.
The overall hospitalization rate through the week of Dec. 8-14 (5.5 per 100,000 population) “is similar to what has been seen at this time during recent seasons,” the CDC noted. The highest rates are occurring among adults over age 65 years (12.7 per 100,000) and children aged 0-4 years (10.9 per 100,000).
Three ILI-related deaths among children that occurred last week were reported, which brings the total for the 2019-2020 season to 19, the CDC said.
The 2019-2020 flu season continues its unusually early rise in activity, with the Centers for Disease Control and Prevention estimating that 3.7 million cases have occurred through Dec. 14.

which is up from 3.2% the previous week and is the sixth consecutive week that the United States has been at or above the national baseline of 2.4%, the CDC reported Dec. 20. This year’s 3.9% is the highest mid-December rate recorded since 2003, when it reached almost 7.4%.
Most of the influenza activity so far this season is being driven by influenza B/Victoria viruses. Nationwide testing puts influenza B prevalence at 68.5% of all positive specimens, exactly the same as last week, but A(H1N1) viruses “are increasing in proportion relative to other influenza viruses in some regions,” the CDC’s influenza division said.
A look at this week’s activity map shows that 21 states, compared with 12 last week, were in the “high” range of activity – that’s levels 8-10 on the CDC’s 1-10 scale. Twelve of those states, along with Puerto Rico, were at level 10, which was up from nine a week earlier, the CDC said.
The overall hospitalization rate through the week of Dec. 8-14 (5.5 per 100,000 population) “is similar to what has been seen at this time during recent seasons,” the CDC noted. The highest rates are occurring among adults over age 65 years (12.7 per 100,000) and children aged 0-4 years (10.9 per 100,000).
Three ILI-related deaths among children that occurred last week were reported, which brings the total for the 2019-2020 season to 19, the CDC said.
Adolescents should know risks of tattoos and piercings
NEW ORLEANS – It wasn’t until her teenage daughter wanted to get her belly button pierced that Cora Breuner, MD, became interested in the safety of tattoos and piercings for adolescents.
“You’re a pediatrician,” her daughter said. “Where should I go? Should I get this done?” Although Dr. Breuner didn’t want her daughter to get the piercing, she knew saying “no” wasn’t likely to stop her teenager any more than it would another adolescent, so she looked to the medical literature … and didn’t find much.
“I couldn’t find an article summarizing complication rates or just about the legality of it or other issues around tattooing and piercing,” said Dr. Breuner a professor of pediatrics at Seattle Children’s Hospital and the University of Washington, also in Seattle. So she and the American Academy of Pediatrics’ Committee on Adolescent Health did the work themselves and wrote one.
“I want to make sure that you are talking to your teenagers about this,” she told attendees at the annual meeting of the American Academy of Pediatrics. In her presentation, she focused on knowing the legal age of consent for body modifications and what to watch for in terms of complications.
Tattoos growing in popularity
More than a third (38%) of people aged 18-29 years have at least one tattoo, according to a Pew Research Center report Dr. Breuner cited, and 23% had piercings somewhere on their body besides their ears. In fact, Americans spend about $1.65 billion on tattoos each year.
Most of the people with tattoos (72%), however, had them in places that were covered and not visible, reinforcing the need to ask about them. The popularity of tattoos has been increasing in general, Dr. Breuner noted. In just the 4 years from 2012 to 2016, the prevalence of U.S. adults with at least one tattoo increased 20%.
And people don’t appear to be sorry to have them. According to a Harris Poll that Dr. Breuner cited, 86% of respondents in 2012 did not regret getting their tattoo, and respondents listed a number of feelings they associated with their tattoos: feeling sexy, rebellious, attractive, strong, spiritual, healthier, intelligent, and athletic.
Although the techniques for tattooing have changed over the years since the first documented ones in 4,000 B.C., the basic concept of injecting ink into the dermis hasn’t changed much. By injecting the ink below the epidermis, the ink remains visible for the rest of a person’s life.
The laws for tattoos vary by state, so you need to check the laws where they live. Not much data exist on infections and complaints, but data from the Michigan Department of Health & Human Services suggests the infection rate – at least those infections reported – is low while the rate of illegally operating facilities is a bigger risk. Local health districts in Michigan have received reports of only 18 infections since 2010, but they’ve received 85 reports of illegal operations and 69 reports of social media parties centered on all attendees getting a tattoo.
Risks of tattoos
The biggest concern for adolescents is ensuring they understand the risks of tattoos and piercings and what to look for. One risk for tattoos is hepatitis C. However, the studies on the risk of contracting hepatitis C from tattooing are confounded by the fact that many people getting tattoos also may be engaging in other risky behaviors, such as intravenous drug use or risky sexual behaviors. Still, some research suggests that “commercially acquired tattoos accounted for more than twice as many infections as injection-drug use,” Dr. Breuner said.
Another risk is tattoo-associated bacterial skin infections (Clin Infect Dis. 2019 Aug 30;69[6]:949-55; MMWR Morb Mortal Wkly Rep. 2012 Aug 24;61[33]:653-6).
Risks of body piercing
Although body piercing doesn’t date back quite as far as tattoos – about 700 A.D. – its history remains long. Research suggests the top reason people get body piercings is simply liking the way it looks, as 77% of respondents reported in one study (J Am Osteopath Assoc. 2007 Oct;107[10]:432-8). Other reasons including looking fashionable, catching attention, feeling different, making a personal statement, being daring, fitting in, pressuring from peers, and defying parents.
The most serious potential complication from piercings is gangrene, but the most common is infection. Other possible complications include an allergic reaction to the metal used, a bleeding complication (estimated in 1 of 10), a scar or site reaction (estimated in 1 of 15), or, much less commonly, toxic shock syndrome. In some areas, there’s a risk of nerve damage if the nerve is pierced, such as in the eyebrow or in the bridge of the nose.
Teens particularly should be aware of the average time it takes for a piercing to heal, depending on where they get it. A navel piercing, for example, can take up to 9 months to heal. Others with long healing times include the penis (3-9 months), labia majora (2-4 months), nipple (2-4 months), and scrotum (2-3 months). Other non-ear regions range from 2 to 8 weeks.
Bleeding definitely is a risk for piercings, Dr. Breuner said, especially now that so many teens are piercing body parts besides their ears. “The one I found most disturbing was that of the uvula,” she said. Bleeding risks tend to be low with ear and nose piercings, but the risk increases with the tongue, uvula, navel, nipples, and genitalia.
Another risk of mouth piercings, particularly tongue piercing, is damage to the teeth and gums, Dr. Breuner said. Barbells, the most popular type of mouth piercing, can lead to receding gums and chipped teeth with extended wear, especially because people wearing them have a tendency to frequently bite down on them.
One study found that half the participants who wore a long barbell piercing (1.59 cm or longer) for at least 2 years had lingual recession on their mandibular central incisors (J Periodontol. 2002 Mar;73[3]:289-97). Among those with a tongue piercing of at least 4 years, 47% had tooth chipping on their molars and premolars.
Another study found gingival recession was 11 times more likely among people with tongue piercings than without (J Clin Periodontol. 2010 Aug 1;37(8):712-8). Gingival recession also is a risk with lip piercings, but the risk is greater with tongue piercing, and only tongue piercings have been associated with tooth injuries (Aust Dent J. 2012 Mar;57[1]:71-8; Int J Dent Hyg. 2016 Feb;14[1]:62-73).
Hepatitis C also is a concern with body piercing. According to a systematic review of 12 studies, body piercing was a risk factor for hepatitis C infection in the majority of them (Am J Infect Control. 2001 Aug;29[4]:271-4).
Counseling adolescents on body modifications
You should ask teens about any tattoos or piercings they have at each visit and ask whether they have any plans to get any. Then you can answer questions about them and ensure the teens are aware of risks, particularly viral and bacterial infections and, with piercing, bleeding.
Beyond the medical risks, it’s important for teens to understand that tattoos have the potential to limit their employment in the future, depending on the job and how visible their tattoo is.
Social acceptance of tattoos and piercings have been increasing, but a survey of nearly 2,700 people conducted by Salary.com in 2013 found that 76% of respondents believed tattoos and piercings could reduce a job applicant’s chances of being hired.
If you want to learn more specifically about the safest places in your community for tattoos and piercings, Dr. Breuner recommended going out and visiting the shops. Tattoo artists generally are the most knowledgeable people in the community about the risks of their industry and often welcome local physicians who want to learn and see their equipment, she said.
NEW ORLEANS – It wasn’t until her teenage daughter wanted to get her belly button pierced that Cora Breuner, MD, became interested in the safety of tattoos and piercings for adolescents.
“You’re a pediatrician,” her daughter said. “Where should I go? Should I get this done?” Although Dr. Breuner didn’t want her daughter to get the piercing, she knew saying “no” wasn’t likely to stop her teenager any more than it would another adolescent, so she looked to the medical literature … and didn’t find much.
“I couldn’t find an article summarizing complication rates or just about the legality of it or other issues around tattooing and piercing,” said Dr. Breuner a professor of pediatrics at Seattle Children’s Hospital and the University of Washington, also in Seattle. So she and the American Academy of Pediatrics’ Committee on Adolescent Health did the work themselves and wrote one.
“I want to make sure that you are talking to your teenagers about this,” she told attendees at the annual meeting of the American Academy of Pediatrics. In her presentation, she focused on knowing the legal age of consent for body modifications and what to watch for in terms of complications.
Tattoos growing in popularity
More than a third (38%) of people aged 18-29 years have at least one tattoo, according to a Pew Research Center report Dr. Breuner cited, and 23% had piercings somewhere on their body besides their ears. In fact, Americans spend about $1.65 billion on tattoos each year.
Most of the people with tattoos (72%), however, had them in places that were covered and not visible, reinforcing the need to ask about them. The popularity of tattoos has been increasing in general, Dr. Breuner noted. In just the 4 years from 2012 to 2016, the prevalence of U.S. adults with at least one tattoo increased 20%.
And people don’t appear to be sorry to have them. According to a Harris Poll that Dr. Breuner cited, 86% of respondents in 2012 did not regret getting their tattoo, and respondents listed a number of feelings they associated with their tattoos: feeling sexy, rebellious, attractive, strong, spiritual, healthier, intelligent, and athletic.
Although the techniques for tattooing have changed over the years since the first documented ones in 4,000 B.C., the basic concept of injecting ink into the dermis hasn’t changed much. By injecting the ink below the epidermis, the ink remains visible for the rest of a person’s life.
The laws for tattoos vary by state, so you need to check the laws where they live. Not much data exist on infections and complaints, but data from the Michigan Department of Health & Human Services suggests the infection rate – at least those infections reported – is low while the rate of illegally operating facilities is a bigger risk. Local health districts in Michigan have received reports of only 18 infections since 2010, but they’ve received 85 reports of illegal operations and 69 reports of social media parties centered on all attendees getting a tattoo.
Risks of tattoos
The biggest concern for adolescents is ensuring they understand the risks of tattoos and piercings and what to look for. One risk for tattoos is hepatitis C. However, the studies on the risk of contracting hepatitis C from tattooing are confounded by the fact that many people getting tattoos also may be engaging in other risky behaviors, such as intravenous drug use or risky sexual behaviors. Still, some research suggests that “commercially acquired tattoos accounted for more than twice as many infections as injection-drug use,” Dr. Breuner said.
Another risk is tattoo-associated bacterial skin infections (Clin Infect Dis. 2019 Aug 30;69[6]:949-55; MMWR Morb Mortal Wkly Rep. 2012 Aug 24;61[33]:653-6).
Risks of body piercing
Although body piercing doesn’t date back quite as far as tattoos – about 700 A.D. – its history remains long. Research suggests the top reason people get body piercings is simply liking the way it looks, as 77% of respondents reported in one study (J Am Osteopath Assoc. 2007 Oct;107[10]:432-8). Other reasons including looking fashionable, catching attention, feeling different, making a personal statement, being daring, fitting in, pressuring from peers, and defying parents.
The most serious potential complication from piercings is gangrene, but the most common is infection. Other possible complications include an allergic reaction to the metal used, a bleeding complication (estimated in 1 of 10), a scar or site reaction (estimated in 1 of 15), or, much less commonly, toxic shock syndrome. In some areas, there’s a risk of nerve damage if the nerve is pierced, such as in the eyebrow or in the bridge of the nose.
Teens particularly should be aware of the average time it takes for a piercing to heal, depending on where they get it. A navel piercing, for example, can take up to 9 months to heal. Others with long healing times include the penis (3-9 months), labia majora (2-4 months), nipple (2-4 months), and scrotum (2-3 months). Other non-ear regions range from 2 to 8 weeks.
Bleeding definitely is a risk for piercings, Dr. Breuner said, especially now that so many teens are piercing body parts besides their ears. “The one I found most disturbing was that of the uvula,” she said. Bleeding risks tend to be low with ear and nose piercings, but the risk increases with the tongue, uvula, navel, nipples, and genitalia.
Another risk of mouth piercings, particularly tongue piercing, is damage to the teeth and gums, Dr. Breuner said. Barbells, the most popular type of mouth piercing, can lead to receding gums and chipped teeth with extended wear, especially because people wearing them have a tendency to frequently bite down on them.
One study found that half the participants who wore a long barbell piercing (1.59 cm or longer) for at least 2 years had lingual recession on their mandibular central incisors (J Periodontol. 2002 Mar;73[3]:289-97). Among those with a tongue piercing of at least 4 years, 47% had tooth chipping on their molars and premolars.
Another study found gingival recession was 11 times more likely among people with tongue piercings than without (J Clin Periodontol. 2010 Aug 1;37(8):712-8). Gingival recession also is a risk with lip piercings, but the risk is greater with tongue piercing, and only tongue piercings have been associated with tooth injuries (Aust Dent J. 2012 Mar;57[1]:71-8; Int J Dent Hyg. 2016 Feb;14[1]:62-73).
Hepatitis C also is a concern with body piercing. According to a systematic review of 12 studies, body piercing was a risk factor for hepatitis C infection in the majority of them (Am J Infect Control. 2001 Aug;29[4]:271-4).
Counseling adolescents on body modifications
You should ask teens about any tattoos or piercings they have at each visit and ask whether they have any plans to get any. Then you can answer questions about them and ensure the teens are aware of risks, particularly viral and bacterial infections and, with piercing, bleeding.
Beyond the medical risks, it’s important for teens to understand that tattoos have the potential to limit their employment in the future, depending on the job and how visible their tattoo is.
Social acceptance of tattoos and piercings have been increasing, but a survey of nearly 2,700 people conducted by Salary.com in 2013 found that 76% of respondents believed tattoos and piercings could reduce a job applicant’s chances of being hired.
If you want to learn more specifically about the safest places in your community for tattoos and piercings, Dr. Breuner recommended going out and visiting the shops. Tattoo artists generally are the most knowledgeable people in the community about the risks of their industry and often welcome local physicians who want to learn and see their equipment, she said.
NEW ORLEANS – It wasn’t until her teenage daughter wanted to get her belly button pierced that Cora Breuner, MD, became interested in the safety of tattoos and piercings for adolescents.
“You’re a pediatrician,” her daughter said. “Where should I go? Should I get this done?” Although Dr. Breuner didn’t want her daughter to get the piercing, she knew saying “no” wasn’t likely to stop her teenager any more than it would another adolescent, so she looked to the medical literature … and didn’t find much.
“I couldn’t find an article summarizing complication rates or just about the legality of it or other issues around tattooing and piercing,” said Dr. Breuner a professor of pediatrics at Seattle Children’s Hospital and the University of Washington, also in Seattle. So she and the American Academy of Pediatrics’ Committee on Adolescent Health did the work themselves and wrote one.
“I want to make sure that you are talking to your teenagers about this,” she told attendees at the annual meeting of the American Academy of Pediatrics. In her presentation, she focused on knowing the legal age of consent for body modifications and what to watch for in terms of complications.
Tattoos growing in popularity
More than a third (38%) of people aged 18-29 years have at least one tattoo, according to a Pew Research Center report Dr. Breuner cited, and 23% had piercings somewhere on their body besides their ears. In fact, Americans spend about $1.65 billion on tattoos each year.
Most of the people with tattoos (72%), however, had them in places that were covered and not visible, reinforcing the need to ask about them. The popularity of tattoos has been increasing in general, Dr. Breuner noted. In just the 4 years from 2012 to 2016, the prevalence of U.S. adults with at least one tattoo increased 20%.
And people don’t appear to be sorry to have them. According to a Harris Poll that Dr. Breuner cited, 86% of respondents in 2012 did not regret getting their tattoo, and respondents listed a number of feelings they associated with their tattoos: feeling sexy, rebellious, attractive, strong, spiritual, healthier, intelligent, and athletic.
Although the techniques for tattooing have changed over the years since the first documented ones in 4,000 B.C., the basic concept of injecting ink into the dermis hasn’t changed much. By injecting the ink below the epidermis, the ink remains visible for the rest of a person’s life.
The laws for tattoos vary by state, so you need to check the laws where they live. Not much data exist on infections and complaints, but data from the Michigan Department of Health & Human Services suggests the infection rate – at least those infections reported – is low while the rate of illegally operating facilities is a bigger risk. Local health districts in Michigan have received reports of only 18 infections since 2010, but they’ve received 85 reports of illegal operations and 69 reports of social media parties centered on all attendees getting a tattoo.
Risks of tattoos
The biggest concern for adolescents is ensuring they understand the risks of tattoos and piercings and what to look for. One risk for tattoos is hepatitis C. However, the studies on the risk of contracting hepatitis C from tattooing are confounded by the fact that many people getting tattoos also may be engaging in other risky behaviors, such as intravenous drug use or risky sexual behaviors. Still, some research suggests that “commercially acquired tattoos accounted for more than twice as many infections as injection-drug use,” Dr. Breuner said.
Another risk is tattoo-associated bacterial skin infections (Clin Infect Dis. 2019 Aug 30;69[6]:949-55; MMWR Morb Mortal Wkly Rep. 2012 Aug 24;61[33]:653-6).
Risks of body piercing
Although body piercing doesn’t date back quite as far as tattoos – about 700 A.D. – its history remains long. Research suggests the top reason people get body piercings is simply liking the way it looks, as 77% of respondents reported in one study (J Am Osteopath Assoc. 2007 Oct;107[10]:432-8). Other reasons including looking fashionable, catching attention, feeling different, making a personal statement, being daring, fitting in, pressuring from peers, and defying parents.
The most serious potential complication from piercings is gangrene, but the most common is infection. Other possible complications include an allergic reaction to the metal used, a bleeding complication (estimated in 1 of 10), a scar or site reaction (estimated in 1 of 15), or, much less commonly, toxic shock syndrome. In some areas, there’s a risk of nerve damage if the nerve is pierced, such as in the eyebrow or in the bridge of the nose.
Teens particularly should be aware of the average time it takes for a piercing to heal, depending on where they get it. A navel piercing, for example, can take up to 9 months to heal. Others with long healing times include the penis (3-9 months), labia majora (2-4 months), nipple (2-4 months), and scrotum (2-3 months). Other non-ear regions range from 2 to 8 weeks.
Bleeding definitely is a risk for piercings, Dr. Breuner said, especially now that so many teens are piercing body parts besides their ears. “The one I found most disturbing was that of the uvula,” she said. Bleeding risks tend to be low with ear and nose piercings, but the risk increases with the tongue, uvula, navel, nipples, and genitalia.
Another risk of mouth piercings, particularly tongue piercing, is damage to the teeth and gums, Dr. Breuner said. Barbells, the most popular type of mouth piercing, can lead to receding gums and chipped teeth with extended wear, especially because people wearing them have a tendency to frequently bite down on them.
One study found that half the participants who wore a long barbell piercing (1.59 cm or longer) for at least 2 years had lingual recession on their mandibular central incisors (J Periodontol. 2002 Mar;73[3]:289-97). Among those with a tongue piercing of at least 4 years, 47% had tooth chipping on their molars and premolars.
Another study found gingival recession was 11 times more likely among people with tongue piercings than without (J Clin Periodontol. 2010 Aug 1;37(8):712-8). Gingival recession also is a risk with lip piercings, but the risk is greater with tongue piercing, and only tongue piercings have been associated with tooth injuries (Aust Dent J. 2012 Mar;57[1]:71-8; Int J Dent Hyg. 2016 Feb;14[1]:62-73).
Hepatitis C also is a concern with body piercing. According to a systematic review of 12 studies, body piercing was a risk factor for hepatitis C infection in the majority of them (Am J Infect Control. 2001 Aug;29[4]:271-4).
Counseling adolescents on body modifications
You should ask teens about any tattoos or piercings they have at each visit and ask whether they have any plans to get any. Then you can answer questions about them and ensure the teens are aware of risks, particularly viral and bacterial infections and, with piercing, bleeding.
Beyond the medical risks, it’s important for teens to understand that tattoos have the potential to limit their employment in the future, depending on the job and how visible their tattoo is.
Social acceptance of tattoos and piercings have been increasing, but a survey of nearly 2,700 people conducted by Salary.com in 2013 found that 76% of respondents believed tattoos and piercings could reduce a job applicant’s chances of being hired.
If you want to learn more specifically about the safest places in your community for tattoos and piercings, Dr. Breuner recommended going out and visiting the shops. Tattoo artists generally are the most knowledgeable people in the community about the risks of their industry and often welcome local physicians who want to learn and see their equipment, she said.
EXPERT ANALYSIS FROM AAP 19
Verruciform Plaques Within a Tattoo of an HIV-Positive Patient
The Diagnosis: Lichenoid Reaction With Pseudoepitheliomatous Hyperplasia
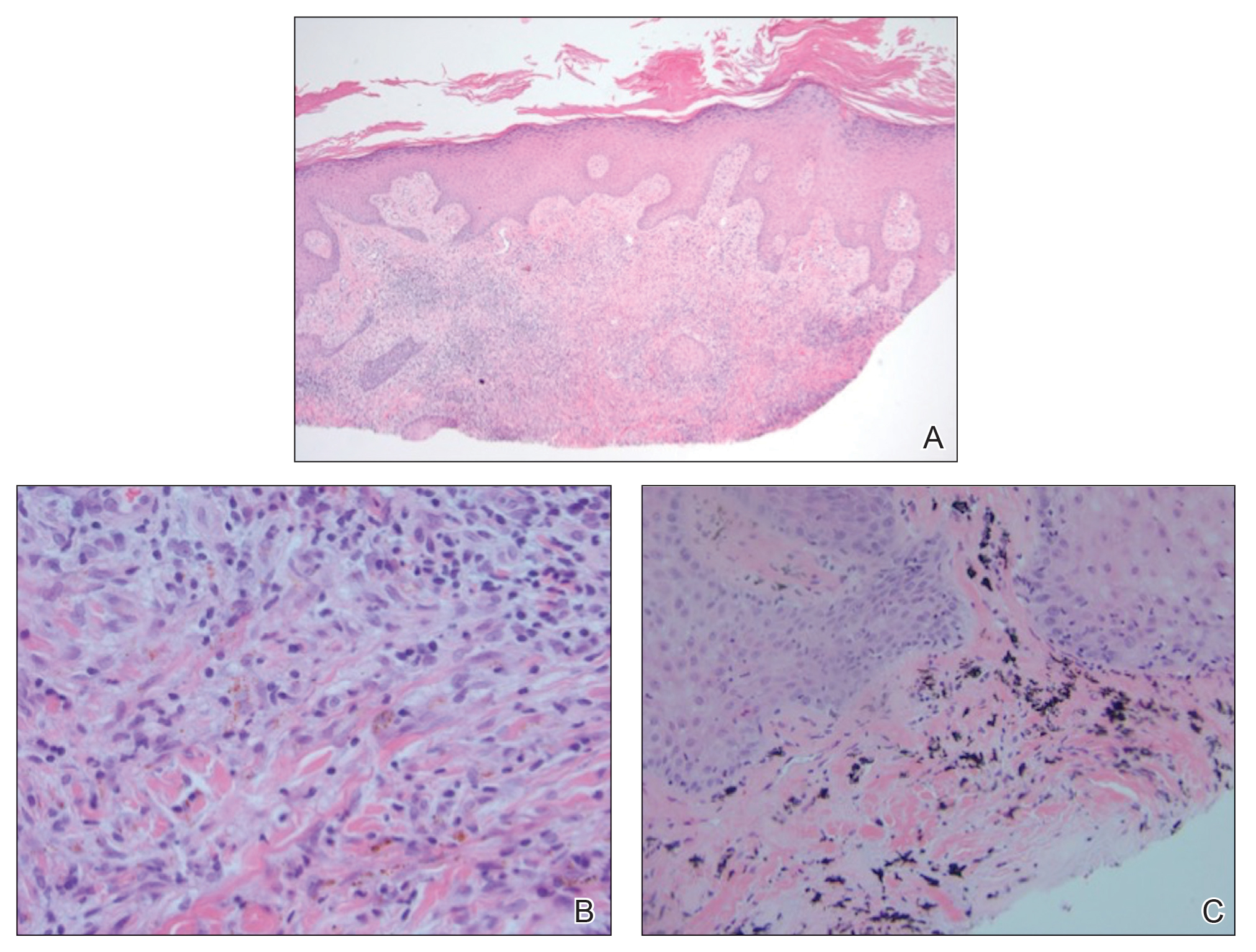
A shave biopsy of the left ankle and a punch biopsy of the left medial calf were performed and sent for histologic examination and acid-fast stain. Bacterial, fungal, and mycobacterial tissue cultures also were sent for testing. The findings from direct examination were negative, and tissue cultures exhibited no growth. The shave and punch biopsies and histology revealed pseudoepitheliomatous hyperplasia (PEH) with keratinocyte necrosis, satellitosis, and areas of acute folliculitis (Figure 1). A lichenoid hypersensitivity mixed infiltrate that included histiocytes admixed with anthracotic and red-orange pigment, lymphocytes, plasma cells, neutrophils, and rare eosinophils was noted in the dermis. Given these clinical and histopathologic findings, the patient was diagnosed with red-pigment tattoo lichenoid reaction with PEH.
Tattoo-related inflammatory reactions can manifest clinically as allergic contact dermatitis, photodermatitis, infection, malignancy, foreign body granulomas, and delayed hypersensitivity reactions with myriad associated histopathologic patterns including spongiotic, psoriasiform, granulomatous, and lichenoid (as seen in our patient). Lichenoid tattoo reactions are the most common histopathologic variants of delayed hypersensitivity seen, mostly with cinnabar or red dye.1 However, there is a paucity of cases in the literature of PEH following tattooing with red dye. Interestingly, lichenoid tissue reaction accompanies PEH in all reported cases.2
Pseudoepitheliomatous hyperplasia can mimic squamous cell carcinoma and keratoacanthoma (KA) both clinically and histologically. All 3 conditions may exhibit epithelial hyperplasia with prominent dilated hyperplastic infundibula. In a case series of 11 presumed KAs within tattoos, Fraga and Prossick2 reported 82% (9/11) of the lesions were located strictly in areas with red pigment, and many were associated with a lichenoid tissue reaction. Kazlouskaya and Junkins-Hopkins3 previously described cases of KAs in tattoos that may represent PEH.
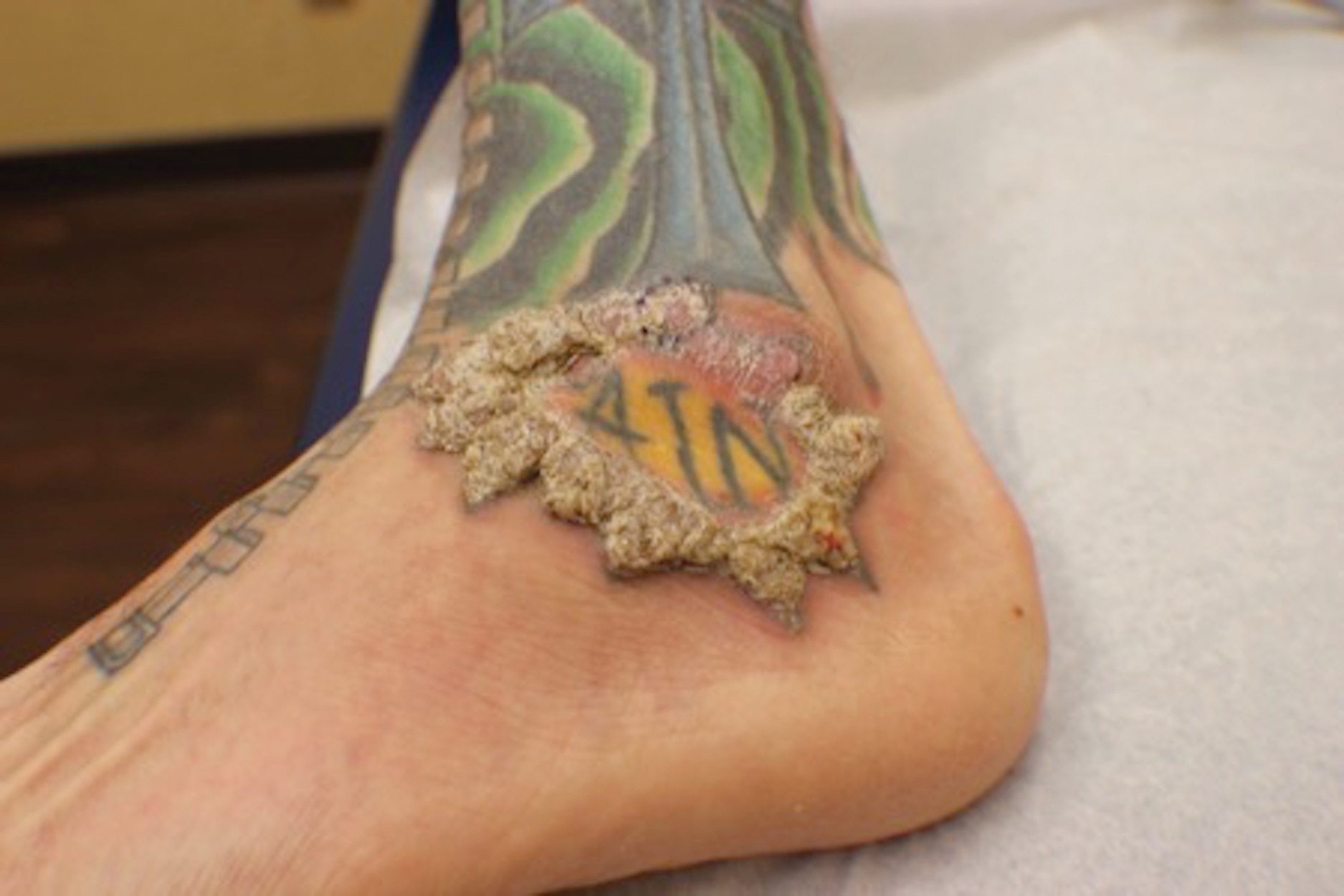
When treating lesions with this histologic appearance, consider the clinical and histologic overlap between KAs and PEH. Our patient was managed with clobetasol ointment 0.05% under occlusion followed by intralesional triamcinolone acetonide with notable improvement of the verrucous plaques on the left lateral malleolus (Figure 2). He also noted near resolution of the papules on the pretibial shin and complete resolution of all associated pruritus and burning. Calcineurin inhibitors, photochemotherapy, CO2 laser, excimer laser, and surgical removal with interval grafting also were considered.
It is important to recognize PEH in the differential of eruptions occurring within tattoos to avoid unnecessary invasive surgical procedures such as complete surgical excision of a KA to avoid malignant transformation.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
The Diagnosis: Lichenoid Reaction With Pseudoepitheliomatous Hyperplasia
A shave biopsy of the left ankle and a punch biopsy of the left medial calf were performed and sent for histologic examination and acid-fast stain. Bacterial, fungal, and mycobacterial tissue cultures also were sent for testing. The findings from direct examination were negative, and tissue cultures exhibited no growth. The shave and punch biopsies and histology revealed pseudoepitheliomatous hyperplasia (PEH) with keratinocyte necrosis, satellitosis, and areas of acute folliculitis (Figure 1). A lichenoid hypersensitivity mixed infiltrate that included histiocytes admixed with anthracotic and red-orange pigment, lymphocytes, plasma cells, neutrophils, and rare eosinophils was noted in the dermis. Given these clinical and histopathologic findings, the patient was diagnosed with red-pigment tattoo lichenoid reaction with PEH.
Tattoo-related inflammatory reactions can manifest clinically as allergic contact dermatitis, photodermatitis, infection, malignancy, foreign body granulomas, and delayed hypersensitivity reactions with myriad associated histopathologic patterns including spongiotic, psoriasiform, granulomatous, and lichenoid (as seen in our patient). Lichenoid tattoo reactions are the most common histopathologic variants of delayed hypersensitivity seen, mostly with cinnabar or red dye.1 However, there is a paucity of cases in the literature of PEH following tattooing with red dye. Interestingly, lichenoid tissue reaction accompanies PEH in all reported cases.2
Pseudoepitheliomatous hyperplasia can mimic squamous cell carcinoma and keratoacanthoma (KA) both clinically and histologically. All 3 conditions may exhibit epithelial hyperplasia with prominent dilated hyperplastic infundibula. In a case series of 11 presumed KAs within tattoos, Fraga and Prossick2 reported 82% (9/11) of the lesions were located strictly in areas with red pigment, and many were associated with a lichenoid tissue reaction. Kazlouskaya and Junkins-Hopkins3 previously described cases of KAs in tattoos that may represent PEH.
When treating lesions with this histologic appearance, consider the clinical and histologic overlap between KAs and PEH. Our patient was managed with clobetasol ointment 0.05% under occlusion followed by intralesional triamcinolone acetonide with notable improvement of the verrucous plaques on the left lateral malleolus (Figure 2). He also noted near resolution of the papules on the pretibial shin and complete resolution of all associated pruritus and burning. Calcineurin inhibitors, photochemotherapy, CO2 laser, excimer laser, and surgical removal with interval grafting also were considered.
It is important to recognize PEH in the differential of eruptions occurring within tattoos to avoid unnecessary invasive surgical procedures such as complete surgical excision of a KA to avoid malignant transformation.
The Diagnosis: Lichenoid Reaction With Pseudoepitheliomatous Hyperplasia
A shave biopsy of the left ankle and a punch biopsy of the left medial calf were performed and sent for histologic examination and acid-fast stain. Bacterial, fungal, and mycobacterial tissue cultures also were sent for testing. The findings from direct examination were negative, and tissue cultures exhibited no growth. The shave and punch biopsies and histology revealed pseudoepitheliomatous hyperplasia (PEH) with keratinocyte necrosis, satellitosis, and areas of acute folliculitis (Figure 1). A lichenoid hypersensitivity mixed infiltrate that included histiocytes admixed with anthracotic and red-orange pigment, lymphocytes, plasma cells, neutrophils, and rare eosinophils was noted in the dermis. Given these clinical and histopathologic findings, the patient was diagnosed with red-pigment tattoo lichenoid reaction with PEH.
Tattoo-related inflammatory reactions can manifest clinically as allergic contact dermatitis, photodermatitis, infection, malignancy, foreign body granulomas, and delayed hypersensitivity reactions with myriad associated histopathologic patterns including spongiotic, psoriasiform, granulomatous, and lichenoid (as seen in our patient). Lichenoid tattoo reactions are the most common histopathologic variants of delayed hypersensitivity seen, mostly with cinnabar or red dye.1 However, there is a paucity of cases in the literature of PEH following tattooing with red dye. Interestingly, lichenoid tissue reaction accompanies PEH in all reported cases.2
Pseudoepitheliomatous hyperplasia can mimic squamous cell carcinoma and keratoacanthoma (KA) both clinically and histologically. All 3 conditions may exhibit epithelial hyperplasia with prominent dilated hyperplastic infundibula. In a case series of 11 presumed KAs within tattoos, Fraga and Prossick2 reported 82% (9/11) of the lesions were located strictly in areas with red pigment, and many were associated with a lichenoid tissue reaction. Kazlouskaya and Junkins-Hopkins3 previously described cases of KAs in tattoos that may represent PEH.
When treating lesions with this histologic appearance, consider the clinical and histologic overlap between KAs and PEH. Our patient was managed with clobetasol ointment 0.05% under occlusion followed by intralesional triamcinolone acetonide with notable improvement of the verrucous plaques on the left lateral malleolus (Figure 2). He also noted near resolution of the papules on the pretibial shin and complete resolution of all associated pruritus and burning. Calcineurin inhibitors, photochemotherapy, CO2 laser, excimer laser, and surgical removal with interval grafting also were considered.
It is important to recognize PEH in the differential of eruptions occurring within tattoos to avoid unnecessary invasive surgical procedures such as complete surgical excision of a KA to avoid malignant transformation.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Kazlouskaya V, Junkins-Hopkins JM. Pseudoepitheliomatous hyperplasia in a red pigment tattoo: a separate entity or hypertrophic lichen planus-like reaction? J Clin Aesthet Dermatol. 2015;8:48-52.
A 40-year-old man with a medical history of human immunodeficiency virus infection managed with highly active antiretroviral therapy (CD4 count, 888 cells/mm3 and an undetectable viral load), psoriasis, and recurrent condyloma acuminatum presented with exophytic, annular, hyperkeratotic, verrucous plaques on the left lateral malleolus with multiple erythematous hyperkeratotic papules on the pretibial shin of the left leg of 6 months' duration. These plaques and papules were localized to areas where red dye was used in a tattoo the patient had received 2 years prior to presentation. There was no associated fluctuance or drainage. The patient reported paroxysmal pruritus and burning pain.
Flu activity dropped in early December
according to the Centers for Disease Control and Prevention.
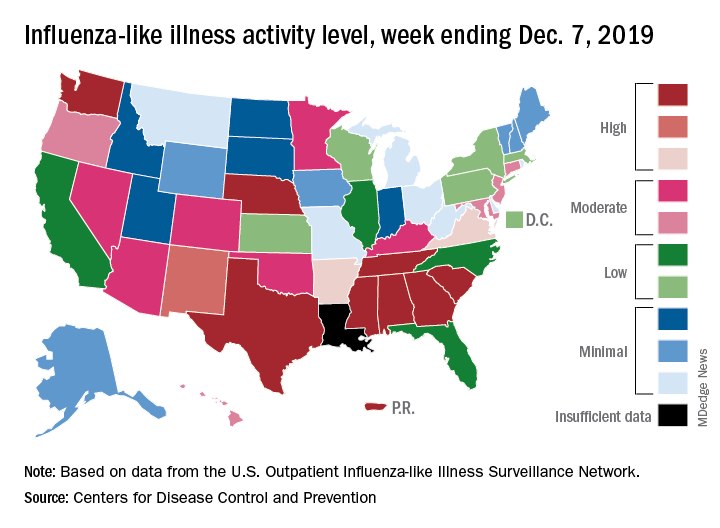
Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
ID Consult: It’s not necessarily over when measles infection clears
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at [email protected].
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at [email protected].
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at [email protected].
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
Improving sepsis-related outcomes
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Early diagnosis a key goal
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Many Americans planning to avoid flu vaccination
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.
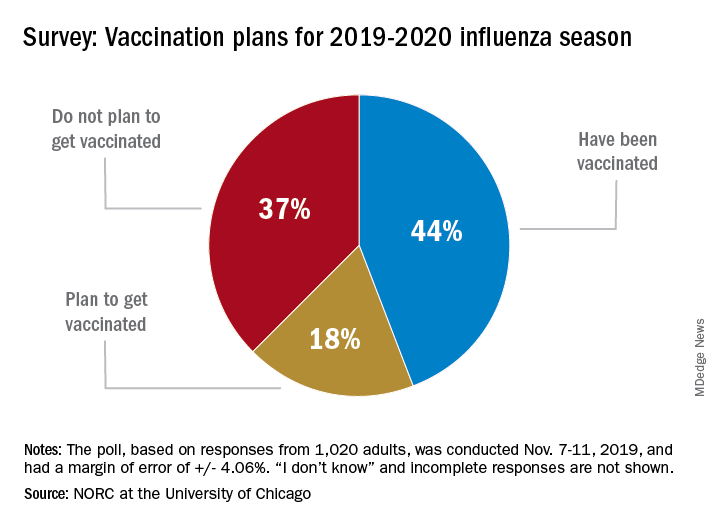
Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
Repeat LTBI testing best in patients taking biologics with new risk factors
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
ATLANTA – Patients taking biologics who received latent tuberculosis testing on an annual basis were unlikely to convert from a negative QuantiFERON test to a positive result, which suggests that the test may be unnecessary for patients without new tuberculosis risk factors, according to research presented at the annual meeting of the American College of Rheumatology.
In addition, nearly all of the cost of repeat testing for latent tuberculosis infection (LTBI) went to patients who were not diagnosed with or treated for LTBI, noted Urmi Khanna, MD, a dermatologist with the Cleveland Clinic.
“All in all, about $1.4 million U.S. dollars was spent just on additional QuantiFERON testing, and only 1% of this additional cost was actually spent on testing patients who were diagnosed with and treated for latent tuberculosis,” Dr. Khanna said in her presentation at the meeting.
“Based on this study, we would like to propose that, in low incidence TB regions such as the United States, repeat LTBI testing in patients on biologic therapies should be focused on patients who have new risk factors for TB infection since their last screening,” she said.
The National Psoriasis Foundation has recommended patients be screened annually for LTBI, and the Centers for Disease Control and Prevention and the ACR have recommended patients taking biologics be screened annually for LTBI if they have new risk factors for TB, such as coming into contact with immigrants, a person infected with TB, immunosuppressed individuals, or persons working in areas where TB might be present. Annual screening was also recently added to the Medicare Merit-Based Incentive Payment System (MIPS), which will affect physician reimbursement. “Based on [the addition of this quality outcome measure], we expect that more and more physicians will adopt this practice of annual LTBI screening in all patients on biologics,” Dr. Khanna said.
She and her colleagues examined QuantiFERON tuberculosis test (QFT) results of 10,914 patients from the Cleveland Clinic Foundation between August 2007 and March 2019 where patients were receiving systemic biologic therapy for inflammatory or autoimmune conditions, including nearly 32% with inflammatory bowel disease, 29% with rheumatoid arthritis, and 25% with psoriatic disease. Overall, 5,212 patients were included in the final analysis, and patients had a median of three QFT results. Patients had a median age of 41 years, had taken an average of 1.80 biologics during follow-up, and had a median biologic therapy duration of about 49 months. The most common biologics used were adalimumab (33%), etanercept (17%), and infliximab (17%).
Of these patients, 4,561 patients had negative QFTs (88%), 172 patients had one or more positive QFTs (3%), and 479 patients had one or more indeterminate QFTs (9%). For patients who converted from a negative QFT to a positive QFT, the most common risk factors were exposure to someone with TB (26%), immigrating or traveling to an endemic area (26%), and occupational exposure (16%).
Within the group with one or more positive QFTs, there were 108 patients with baseline positive QFTs prior to starting biologic therapy (2.1%), 61 patients who converted from a baseline negative QFT to a positive QFT (1.2%), and 3 patients where a positive result overlapped with a negative result (0.1%). The majority of patients who converted to a positive QFT result had borderline positive results (70.5%), defined as 0.35 to 1 IU/mL, compared with 29.5% of converters who had a positive QFT result of more than 1.0 IU/mL.
Among the 61 patients who converted to a positive QFT result, 28 patients with LTBI (46%) and 1 patient with an active case of TB (2%) were diagnosed and treated. The active TB case was a 29-year-old patient with inflammatory bowel disease and ankylosing spondylitis receiving adalimumab who had recently traveled to India.
The researchers also examined the cost of additional QFTs in each group. Among negative QFTs, the cost of an additional 9,611 tests was $1,201,375. The cost of additional tests for indeterminate QFTs was $136,200, but Dr. Khanna noted that 99.99% of additional tests in this group were for patients never diagnosed with or treated for LTBI. Additional tests for positive QFTs cost another $47,700, and 26.1% of patients in this group were diagnosed and received treatment for LTBI, compared with 73.9% who did not receive an LTBI diagnosis or treatment.
In the discussion session following the presentation, Dr. Khanna emphasized that discontinuing annual screening in low-risk patients was not standard of care at the Cleveland Clinic, and this study was conducted to raise awareness of focusing testing on patients with new TB risk factors.
Dr. Khanna reported no relevant financial disclosures. A few of her coauthors reported financial relationships with pharmaceutical companies.
SOURCE: Khanna U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1802.
REPORTING FROM ACR 2019
Perioperative antirheumatic drug use does not impact postsurgery infection rate in RA patients
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
REPORTING FROM ACR 2019
A triple-antibiotic cure for Crohn’s disease?
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
SAN ANTONIO – A proprietary oral fixed-dose, triple-antibiotic combination pill offers a promising new approach to the treatment of Crohn’s disease, David Y. Graham, MD, declared at the annual meeting of the American College of Gastroenterology.
In the phase 3 MAP US trial, patients with Crohn’s disease who were randomized to the fixed-dose combination of 45 mg rifabutin, 95 mg clarithromycin, and 10 mg clofazimine, known for now as RHB-104, experienced significantly higher rates of clinical remission and improvement in inflammation as assessed endoscopically and via biomarkers, compared with placebo-treated controls, reported Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
RHB-104 is effective against Mycobacterium avium paratuberculosis (MAP) – and therein hangs a tale.
“MAP has been considered as a possible cause of Crohn’s disease since the disease was described by Crohn in 1932,” the gastroenterologist noted. “These randomized trial data provide further evidence suggesting an important role for MAP or similar microorganisms in the pathogenesis of Crohn’s disease.”
For Dr. Graham, this is a case of deja vu all over again. More than a quarter century ago he was lead author of a highly influential randomized, controlled trial which established that treatment with antibiotics directed against Helicobacter pylori cured peptic ulcer disease. As such, he became internationally recognized as a key figure in the resultant revolution in peptic ulcer treatment. He hears an echo of that earlier transformative change in the MAP US results.
“This is either an additional therapy or it’s the beginning of a paradigm shift. I mean, I see this as we’re standing at the same place now as we were standing with Helicobacter pylori 30 years ago, when the question was: Have we found something that we can eradicate and change the natural history of the disease and cure it? You can say this [MAP-directed therapy] is going in that direction, but it certainly hasn’t gotten to the point of proof yet. The results have to be reproduced,” he said.
The MAP US trial included 331 patients with moderate to severely active Crohn’s disease at 92 sites who had failed to achieve an adequate response with conventional therapies. Participants were randomized double blind to twice-daily RHB-104 or placebo for 52 weeks. Those not in remission at 26 weeks could opt for open-label RHB-104. Background concomitant treatment with corticosteroids, tumor necrosis factor inhibitors, and immunosuppressives was permitted.
The primary outcome was clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 at week 26. This was achieved in 36.7% of the active treatment group and 23% of controls, a highly significant difference. The clinical remission rates at week 16 were 42.2% and 29.1%, respectively. At week 26, 44% of RHB-104-treated patients had achieved at least a 100-point reduction in CDAI score, compared with baseline, as did 30.9% of controls. The key symptom score provided by the sum of the abdominal pain and bowel movement components of the CDAI was significantly lower in the RHB-104 group than in controls from week 16 on.
The remission rate at week 26 in the group on RHB-104 was similarly favorable regardless of whether or not they were on anti–tumor necrosis factor therapy.
“This suggests that RHB-104 can be used effectively and safely as an adjunct treatment to other medications to enhance the response to medical therapy,” according to Dr. Graham, who was principal investigator for MAP US.
The composite endpoint of clinical remission plus at least a 50% reduction from baseline in fecal calprotectin or C-reactive protein was achieved in 21.1% of the RHB-104 group and 9.1% of controls at week 26, and by 16.9% on RHB-104 and 7.9% on placebo at week 52.
In the 35 patients who underwent endoscopy at week 26, a 50% or greater reduction in the Simple Endoscopic Score in Crohn’s Disease was documented in 28.6% of patients on RHB-104 versus 4.8% of controls.
Durable remission, defined as a CDAI score below 150 at all study visits from week 16 to week 52, was achieved in 18.7% of the RHB-104 group, compared with 8.5% of controls.
The side effect profiles of RHB-104 and placebo were similar, with no serious adverse events recorded in the 52-week study. An increase in the QT interval on ECG was noted in the RHB-104 group from week 4 on, but it wasn’t associated with any clinical findings. Further study of this ECG finding is underway.
Several audience members rose to urge caution in interpreting the MAP US data.
“We must adhere to Koch’s postulates before we make conclusions about causative agents of an infectious disease, and I didn’t see those data here. So I look forward to a future presentation that shares that,” one gastroenterologist commented.
“I haven’t seen any data here that shows Mycobacterium was present in these patients,” noted another.
Dr. Graham replied that MAP US was a hypothesis-driven clinical trial: Crohn’s disease has much in common with an inflammatory bowel disease occurring in ruminant animals, where RHB-104 has shown treatment efficacy.
“This is a Mycobacterium avium organism, so it’s not something you’re going to cure in 2 weeks or 2 months. But the question is, do you have an effect on the disease, and the answer in MAP US was unquestionably yes. It’s very positive data to further pursue the hypothesis, but the study doesn’t provide a definitive answer,” he said.
Dr. Graham reported serving as a consultant to RedHill Biopharma, the study sponsor.
REPORTING FROM ACG 2019