User login
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.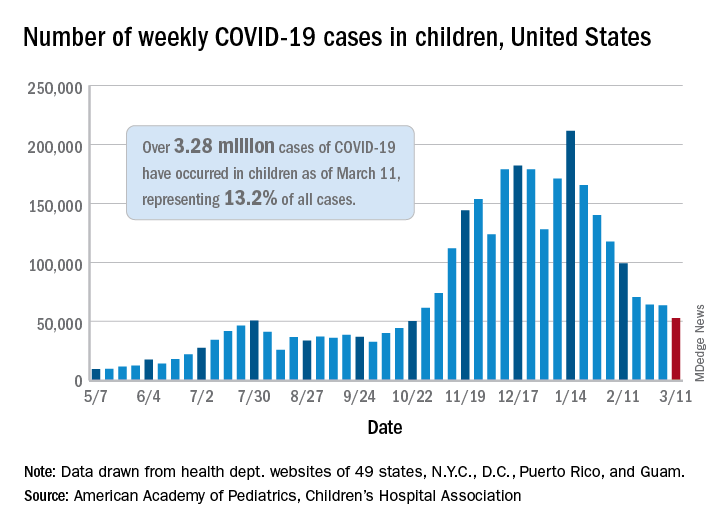
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
CDC’s new gonorrhea treatment recs: What’s changed, and when to retest
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
REFERENCES
- CDC. Expedited partner therapy. Accessed March 15, 2021. www.cdc.gov/std/ept/default.htm
- St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916. Accessed March 15, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm
- CDC. Gonococcal infections. Accessed March 15, 2021. www.cdc.gov/std/tg2015/gonorrhea.htm
Pregnant patients with severe COVID-19 disease at increased risk of complications
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
Pregnant patients with COVID-19 infections were more likely to experience severe disease if they had preexisting comorbidities, such as chronic hypertension, asthma, or pregestational diabetes, according to findings from a new study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The study included outcomes for the largest multistate cohort of pregnant patients with COVID-19 outside of what the Centers for Disease Control and Prevention is tracking. Its findings also mirrored those of a multicenter, retrospective study in Washington state, published in the American Journal of Obstetrics & Gynecology. That study also found that pregnant patients hospitalized for COVID-19 were more likely to have comorbidities, and both studies found an increased likelihood of preterm birth among pregnant patients with severe or critical disease.
Disease severity linked to risk of perinatal complications
In the abstract presented at the SMFM meeting, more severe disease was associated with older age and a higher median body mass index, as seen in the general population, but the researchers found no differences in disease severity occurred by race or ethnicity, Torri D. Metz, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, told attendees of the conference. The researchers also found that perinatal complications were more prevalent in those with severe or critical COVID-19 disease but not in those with mild or moderate disease. Vertical COVID-19 transmission from mother to child was rare.
The observational study included all patients who had a singleton pregnancy, had a positive SARS-CoV-2 test, and delivered between March 1 and July 31, 2020, at one of the 33 U.S. hospitals in the NICHD Maternal-Fetal Medicine Units Network, spread across 14 states. The researchers used electronic medical records to determine incidence of cesarean delivery, postpartum hemorrhage, hypertensive disorders of pregnancy, preterm birth (less than 37 weeks), maternal death, infant death, and positive infant COVID-19 test. They tracked mothers through 6 weeks post partum and newborns through delivery hospitalization.
Of 1,291 patients in the cohort, 1,219 received their first positive COVID-19 test during pregnancy. The others tested positive while in the hospital for delivery or within a month and a half after discharge. Limiting their analysis to those who developed COVID-19 while pregnant prior to delivery, nearly half (47%) were asymptomatic.
The disease was mild in 27%, moderate in 14%, severe in 8%, and critical in 4%. The researchers used the National Institutes of Health classifications for severity and included deaths in the critical group. The most common symptom was a cough, reported by a third of the patients (34%). Four of six maternal deaths that occurred were caused by COVID-19.
Compared with an average age of 28 in those without symptoms, the mean age was 29 in those with mild/moderate disease and 30 in those with severe/critical disease (P = .006). Similarly, the mean BMI was 28.3 in asymptomatic patients, 29 in those with mild/moderate disease, and 32.3 in those with severe/critical disease (P < .001). Despite a diverse cohort – 53% Hispanic, 23% Black, and 15% White – the researches found no racial/ethnic trends in disease severity.
Patients who had asthma, chronic obstructive pulmonary disorder, pregestational diabetes, chronic hypertension, chronic liver disease, or a seizure disorder were all significantly more likely to have critical/severe disease than mild/moderate disease, and more likely to have mild/moderate disease than asymptomatic (P values ranged from < .001 to .02).
The mothers with critical or severe illness were 1.6 times more likely to have cesarean births and to have hypertensive disorders of pregnancy, and they were twice as likely to have postpartum hemorrhage (P < .001; P = .007). Those with mild or moderate disease, however, had no increased risks for perinatal complications over asymptomatic patients.
Critical or severe illness was also associated with more than triple the risk of preterm birth (adjusted risk ratio, 3.6; P < .001). Newborns of mothers with critical or severe illness also had three times greater risk of neonatal ICU admission (ARR, 3.1; P <. 001) and weighed an average 385 g less than newborns of asymptomatic mothers. COVID-19 rate among infants was only 1% during delivery hospitalization.
Since the study cutoff was July 30 and COVID infections only became prevalent in March, the researchers were unable to evaluate women for outcomes resulting from COVID infections in early pregnancy, such as congenital anomalies or early miscarriage, Dr. Metz said. In addition, since many of the sites are urban centers, the data may not be generalizable to rural areas.
Peter S. Bernstein, MD, MPH, of Montefiore Medical Center, New York, asked whether the increased cesarean deliveries and preterm births in the group of women with severe disease were caused by usual obstetric causes or the treatment of COVID-19 infection. Dr. Metz said the vast majority of preterm deliveries were indicated, but only a small proportion were induced for COVID-19 alone. “A lot had hypertensive disorders of pregnancies or PPROM, so it’s partly driven by the infection itself but also partly driven by some of those perinatal complications,” she said.
Similar findings in Washington
In the Washington study, among 240 pregnant patients with confirmed COVID-19 infection between March 1 and July 30, 2020, 1 in 11 developed severe or critical disease, and 1 in 10 were hospitalized. The pregnant patients had more than triple the risk of hospitalization compared with adults of similar ages in the general population (10% vs. 2.8%; rate ratio, 3.5). Similar to the multistate NICHD study, women were more likely to be hospitalized if they had asthma, hypertension, type 2 diabetes, autoimmune disease, or class III obesity.
Three mothers died of COVID-19, resulting in a case fatality rate 13.6 times greater than nonpregnant patients with COVID-19 in the general population. The absolute difference in the rate was 1.2%. As seen in the NICHD study, preterm birth was more common in mothers with severe or critical COVID-19. Nearly half (45.4%) of mothers with severe or critical COVID-19 delivered preterm compared to 5.2% in those with mild COVID-19 (P < .001).
“Our finding that deaths in pregnant patients contributed disproportionately to deaths from COVID-19 among 20- to 39-year-olds in Washington state is similar to what was observed during the influenza A virus H1N1 2009 pandemic,” Erica M. Lokken, PhD, MS, of the departments of global health and ob.gyn. at the University of Washington, Seattle, and colleagues wrote in the Washington study. But they noted that it took 8 months into the pandemic before pregnant patients were identified as a high-risk group for COVID-19.
“Given the similarity in clinical course between COVID-19 and IAV H1N1 2009 with an increased risk for mortality during pregnancy and the postpartum period, we strongly recommend that pregnant patients should be considered a high-risk population to novel highly pathogenic respiratory viruses until proven otherwise by population-based studies with good ascertainment of pregnancy status,” they wrote.
Judette Louis, MD, MPH, associate professor of ob.gyn. and department chair at the University of South Florida, Tampa, said in an interview that the findings in these studies were fairly expected, but it’s important to have data from such a large cohort as the one presented at SMFM.
“It confirmed that those who had severe disease were more likely to have chronic medical conditions, mirroring what we saw in the general population who isn’t pregnant,” Dr. Louis said. “I thought this was very crucial because as pregnant women are trying to decide whether they should get the COVID vaccine, this provides support to say that if you’re pregnant, you’re more likely to have severe disease [if you have] other chronic medical conditions.”
The findings also confirm the importance of pregnant people taking precautions to avoid infection.
“Even though these individuals are, as a group, in an age cohort that mostly has asymptomatic disease, for some of them, it results in severe disease and even maternal death,” she said. “They should still take it seriously if they’re pregnant.”
The SMFM abstract study was funded by the NICHD. The Washington study was funded by the University of Washington Population Health Initiative, the National Institutes of Health, and philanthropic gift funds. One coauthor of the Washington study is on a Pfizer and GlaxoSmithKline advisory board for immunizations. No other authors or individuals interviewed reported any disclosures.
FROM THE PREGNANCY MEETING
Let’s apply the lessons from the AIDS crisis to the COVID-19 pandemic
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
Doxorubicin-pomalidomide combo shows promise for Kaposi sarcoma
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
FROM CROI 2021
Vaginal pH may predict CIN 2 progression in HIV-positive women
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
Elevated vaginal pH at the time of cervical intraepithelial neoplasia 2 diagnosis may be a useful marker of CIN 2 persistence/progression, as well as the rate of persistence/progression in HIV-positive women, new research suggests.
“We analyzed data from the Women’s Interagency HIV Study [WIHS], an observational, longitudinal cohort of women with and without HIV to determine factors that may influence CIN 2 natural history,” said Kate Michel, PhD, MPH, of Georgetown University, Washington. She presented the results at the Conference on Retroviruses and Opportunistic Infections.
As previous data have shown a high incidence of CIN 2 progression among women with HIV, the researchers evaluated the role of human papillomavirus (HPV) type, local immune response, and markers of the cervicovaginal microbiome on the risk of CIN 2 persistence/progression.
Within the cohort, follow-up visits occur every 6 months, and clinical data is collected via questionnaires, physical and gynecologic exams, and biological samples. As no specific treatment is offered in the WIHS, treatment for cervical abnormalities is abstracted from medical records.
In the present study, Dr. Michel and colleagues selected up to four banked cervicovaginal lavage (CVL) samples per woman, with the first sample selected 6-12 months prior to CIN 2 diagnosis, the second at CIN 2 diagnosis, the third between CIN 2 diagnosis and outcome, and the fourth at the outcome visit.
The investigators performed HPV typing and muiltiplex immune mediator testing on each CVL sample. Lab results from WIHS core testing were also extracted, including plasma CD4+ T-cell count and HIV viral load, as well as vaginal pH and Nugent’s score.
Study outcomes included persistence/progression and regression, defined as a subsequent CIN 2 or CIN 3 diagnosis and subsequent CIN 1 or normal diagnosis, respectively. Logistic regression models were used to determine CIN 2 regression versus persistence/progression.
Results
A total of 337 samples were obtained and 94 women were included in the analysis. Key demographic and behavioral factor were similar at CIN 2 diagnosis.
The majority of participants were African American (53.2%) and on antiretroviral therapy (66.0%). The most prevalent high-risk types were HPV-58 (18.4%) and HPV-16 (17.5%).
After a median 12.5 years of follow-up, 33 participants (35.1%) with incident CIN 2 had a subsequent CIN 2/CIN 3 diagnosis and those who regressed had a higher CD4 T-cell count at CIN 2 diagnosis (P = .02).
Each subsequent high-risk HPV type identified at the pre–CIN 2 visit was associated with higher odds of CIN2 persistence/progression (odds ratio, 2.27; 95% confidence interval, 1.15-4.50).
Bacterial vaginosis (adjusted OR, 5.08; 95% CI, 1.30-19.94) and vaginal pH (aOR, 2.27; 95% CI, 1.15-4.50) at the CIN 2 diagnosis visit were each associated with increased odds of CIN 2 persistence/progression.
Vaginal pH greater than 4.5 at CIN 2 diagnosis was also associated with unadjusted time to CIN 2 persistence/progression (log rank P = .002) and an increased rate of CIN 2 persistence/progression (adjusted hazard ratio, 3.37; 95% CI, 1.26-8.99).
Furthermore, among participants who did not receive CIN 2 treatment, vaginal pH remained associated with greater odds of CIN 2 persistence/progression (OR, 2.46; 95% CI, 1.19-5.13). Cervicovaginal immune mediator levels were not associated with CIN 2 persistence/progression.
“The most striking finding from this work was that vaginal pH was associated with higher odds of, quicker time to, and increased hazard of CIN 2 persistence/progression,” Dr. Michel said. “We postulate this effect is mediated by the cervical microbiome, but more work is needed to establish the exact mechanism.”
“It would be interesting to test whether this association might be explained by different vaginal cleaning techniques, such as douching,” said moderator Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles.
“We’re currently working on an analysis of cervicovaginal bacterial species to explore the microbiome in more detail,” Dr. Michel concluded.
Dr. Michel disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the Georgetown-Howard Universities Center for Clinical and Translational Science.
FROM CROI 2021
The vanguard of HIV care: Don’t forget this screening
In response, clinical care is continually adapting to the dramatically altered natural history of disease.
Today, the cutting edge of clinical care overlaps with primary care. The clinical vanguard addresses the medical vulnerabilities of patients with HIV, seeking to eliminate preventable morbidity and premature death. Among this clinical vanguard is the screening for and prevention of anal cancer. With the increased longevity of people living with HIV and the nearly universal exposure to human papillomavirus (HPV), there is now potential for progression to mucosal cellular dysplasia and eventual malignancy.
We know that prevention is possible because of the example of cervical cancer, the etiology of which is exposure to oncogenic serotypes of HPV (16 and 18 are most common). Screenings for cervical cancer (regular clinical examinations and Pap smears) and treatments to eliminate high-grade dysplasia have decreased the incidence rate by over 50% since the 1970s. Vaccination against HPV has been available since 2006 and offers the prospect of preventing HPV-associated malignancies, including head and neck cancer, in future decades.
However, rates of anal cancer are increasing. The CDC estimates that about 4,700 new cases of HPV-associated anal cancers are diagnosed in women and about 2,300 are diagnosed in men each year in the United States. Anal cancer rates in individuals with HIV have increased in the era of effective antiretrovirals and greater longevity. The highest rates, at 95 per 100,000, are in HIV-positive men who have sex with men. Very similar rates were noted in a more recent study that found increased risk with advancing age and in those with an AIDS diagnosis.
All patients with HIV should be screened
The New York State AIDS Institute Clinical Guidelines Program recommends screening for anal dysplasia in all patients with HIV. A proactive approach similar to cervical cancer screening is appropriate and includes measures easily implemented by all clinicians.
- History: Assess for rectal symptoms, anal pain, discharge, and lumps.
- Physical exam: Assess for presence of perianal lesions; perform a thorough digital rectal exam.
- Anal Pap test for anal cytology: Insert a Dacron swab moistened with tap water about 3 inches into the anal canal, applying pressure to lateral anal walls and rotating the swab. Then remove and place the swab into liquid cytology solution, shake vigorously for a full 30 seconds, and assess for any dysplasia (high-grade squamous intraepithelial lesion, low-grade intraepithelial lesion, atypical squamous cells of undetermined significance), which would warrant further evaluation by high-resolution anoscopy (HRA).
High-resolution anoscopy
HRA for anal dysplasia corresponds to colposcopy for cervical dysplasia. The ability to treat and eliminate high-risk precursor lesions interrupts the progression to malignancy. The efficacy of this strategy is being evaluated in a National Institutes of Health prospective trial called the Anchor Study. The epidemiology of HPV; the clinical horror of witnessing the painful, preventable deaths of young patients with well-controlled HIV caused by anal cancer; and the example of controlling cervical cancer have motivated my practice to assure comprehensive care for our patients.
Unfortunately, establishing HRA in one’s practice is challenging. Barriers to practice include the expense of required equipment and the absence of consensus on specific products. In addition, hands-on precepting to ease newcomers to competence is not generally available. Considerable skill is required for complete visualization of the anal transformative zone in the folds of the anal canal, and recognizing high-risk lesions requires study and accumulated experience. The International Anal Neoplasia Society is a useful resource that also offers a training course. We are invited to train ourselves, and to rely on the eventual feedback of biopsy results and the forbearance of our early patients.
The expanding scope of our medical practices must shift to meet the evolving needs of the growing population of virologically suppressed patients who are living longer. HIV care involves curing life-threatening opportunistic infections, encouraging antiretroviral adherence, and providing comprehensive care – which now includes preventing anal cancer.
A version of this article first appeared on Medscape.com.
In response, clinical care is continually adapting to the dramatically altered natural history of disease.
Today, the cutting edge of clinical care overlaps with primary care. The clinical vanguard addresses the medical vulnerabilities of patients with HIV, seeking to eliminate preventable morbidity and premature death. Among this clinical vanguard is the screening for and prevention of anal cancer. With the increased longevity of people living with HIV and the nearly universal exposure to human papillomavirus (HPV), there is now potential for progression to mucosal cellular dysplasia and eventual malignancy.
We know that prevention is possible because of the example of cervical cancer, the etiology of which is exposure to oncogenic serotypes of HPV (16 and 18 are most common). Screenings for cervical cancer (regular clinical examinations and Pap smears) and treatments to eliminate high-grade dysplasia have decreased the incidence rate by over 50% since the 1970s. Vaccination against HPV has been available since 2006 and offers the prospect of preventing HPV-associated malignancies, including head and neck cancer, in future decades.
However, rates of anal cancer are increasing. The CDC estimates that about 4,700 new cases of HPV-associated anal cancers are diagnosed in women and about 2,300 are diagnosed in men each year in the United States. Anal cancer rates in individuals with HIV have increased in the era of effective antiretrovirals and greater longevity. The highest rates, at 95 per 100,000, are in HIV-positive men who have sex with men. Very similar rates were noted in a more recent study that found increased risk with advancing age and in those with an AIDS diagnosis.
All patients with HIV should be screened
The New York State AIDS Institute Clinical Guidelines Program recommends screening for anal dysplasia in all patients with HIV. A proactive approach similar to cervical cancer screening is appropriate and includes measures easily implemented by all clinicians.
- History: Assess for rectal symptoms, anal pain, discharge, and lumps.
- Physical exam: Assess for presence of perianal lesions; perform a thorough digital rectal exam.
- Anal Pap test for anal cytology: Insert a Dacron swab moistened with tap water about 3 inches into the anal canal, applying pressure to lateral anal walls and rotating the swab. Then remove and place the swab into liquid cytology solution, shake vigorously for a full 30 seconds, and assess for any dysplasia (high-grade squamous intraepithelial lesion, low-grade intraepithelial lesion, atypical squamous cells of undetermined significance), which would warrant further evaluation by high-resolution anoscopy (HRA).
High-resolution anoscopy
HRA for anal dysplasia corresponds to colposcopy for cervical dysplasia. The ability to treat and eliminate high-risk precursor lesions interrupts the progression to malignancy. The efficacy of this strategy is being evaluated in a National Institutes of Health prospective trial called the Anchor Study. The epidemiology of HPV; the clinical horror of witnessing the painful, preventable deaths of young patients with well-controlled HIV caused by anal cancer; and the example of controlling cervical cancer have motivated my practice to assure comprehensive care for our patients.
Unfortunately, establishing HRA in one’s practice is challenging. Barriers to practice include the expense of required equipment and the absence of consensus on specific products. In addition, hands-on precepting to ease newcomers to competence is not generally available. Considerable skill is required for complete visualization of the anal transformative zone in the folds of the anal canal, and recognizing high-risk lesions requires study and accumulated experience. The International Anal Neoplasia Society is a useful resource that also offers a training course. We are invited to train ourselves, and to rely on the eventual feedback of biopsy results and the forbearance of our early patients.
The expanding scope of our medical practices must shift to meet the evolving needs of the growing population of virologically suppressed patients who are living longer. HIV care involves curing life-threatening opportunistic infections, encouraging antiretroviral adherence, and providing comprehensive care – which now includes preventing anal cancer.
A version of this article first appeared on Medscape.com.
In response, clinical care is continually adapting to the dramatically altered natural history of disease.
Today, the cutting edge of clinical care overlaps with primary care. The clinical vanguard addresses the medical vulnerabilities of patients with HIV, seeking to eliminate preventable morbidity and premature death. Among this clinical vanguard is the screening for and prevention of anal cancer. With the increased longevity of people living with HIV and the nearly universal exposure to human papillomavirus (HPV), there is now potential for progression to mucosal cellular dysplasia and eventual malignancy.
We know that prevention is possible because of the example of cervical cancer, the etiology of which is exposure to oncogenic serotypes of HPV (16 and 18 are most common). Screenings for cervical cancer (regular clinical examinations and Pap smears) and treatments to eliminate high-grade dysplasia have decreased the incidence rate by over 50% since the 1970s. Vaccination against HPV has been available since 2006 and offers the prospect of preventing HPV-associated malignancies, including head and neck cancer, in future decades.
However, rates of anal cancer are increasing. The CDC estimates that about 4,700 new cases of HPV-associated anal cancers are diagnosed in women and about 2,300 are diagnosed in men each year in the United States. Anal cancer rates in individuals with HIV have increased in the era of effective antiretrovirals and greater longevity. The highest rates, at 95 per 100,000, are in HIV-positive men who have sex with men. Very similar rates were noted in a more recent study that found increased risk with advancing age and in those with an AIDS diagnosis.
All patients with HIV should be screened
The New York State AIDS Institute Clinical Guidelines Program recommends screening for anal dysplasia in all patients with HIV. A proactive approach similar to cervical cancer screening is appropriate and includes measures easily implemented by all clinicians.
- History: Assess for rectal symptoms, anal pain, discharge, and lumps.
- Physical exam: Assess for presence of perianal lesions; perform a thorough digital rectal exam.
- Anal Pap test for anal cytology: Insert a Dacron swab moistened with tap water about 3 inches into the anal canal, applying pressure to lateral anal walls and rotating the swab. Then remove and place the swab into liquid cytology solution, shake vigorously for a full 30 seconds, and assess for any dysplasia (high-grade squamous intraepithelial lesion, low-grade intraepithelial lesion, atypical squamous cells of undetermined significance), which would warrant further evaluation by high-resolution anoscopy (HRA).
High-resolution anoscopy
HRA for anal dysplasia corresponds to colposcopy for cervical dysplasia. The ability to treat and eliminate high-risk precursor lesions interrupts the progression to malignancy. The efficacy of this strategy is being evaluated in a National Institutes of Health prospective trial called the Anchor Study. The epidemiology of HPV; the clinical horror of witnessing the painful, preventable deaths of young patients with well-controlled HIV caused by anal cancer; and the example of controlling cervical cancer have motivated my practice to assure comprehensive care for our patients.
Unfortunately, establishing HRA in one’s practice is challenging. Barriers to practice include the expense of required equipment and the absence of consensus on specific products. In addition, hands-on precepting to ease newcomers to competence is not generally available. Considerable skill is required for complete visualization of the anal transformative zone in the folds of the anal canal, and recognizing high-risk lesions requires study and accumulated experience. The International Anal Neoplasia Society is a useful resource that also offers a training course. We are invited to train ourselves, and to rely on the eventual feedback of biopsy results and the forbearance of our early patients.
The expanding scope of our medical practices must shift to meet the evolving needs of the growing population of virologically suppressed patients who are living longer. HIV care involves curing life-threatening opportunistic infections, encouraging antiretroviral adherence, and providing comprehensive care – which now includes preventing anal cancer.
A version of this article first appeared on Medscape.com.
New ‘minimal monitoring’ approach to HCV treatment may simplify care
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
FROM CROI 2021
Nearly 20% of lupus patients have severe infection in first decade after diagnosis
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
FROM RHEUMATOLOGY
HBV viremia linked to HCC risk in HIV/HBV coinfection
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
FROM CROI 2021