User login
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
CDC data strengthen link between obesity and severe COVID
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Missed visits during pandemic cause ‘detrimental ripple effects’
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Postoperative Neurologic Deficits in a Veteran With Recent COVID-19
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
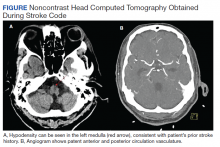
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
Physician Responsiveness to Positive Blood Culture Results at the Minneapolis Veterans Affairs Hospital—Is Anyone Paying Attention?
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
Decline in weekly child COVID-19 cases has almost stopped
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.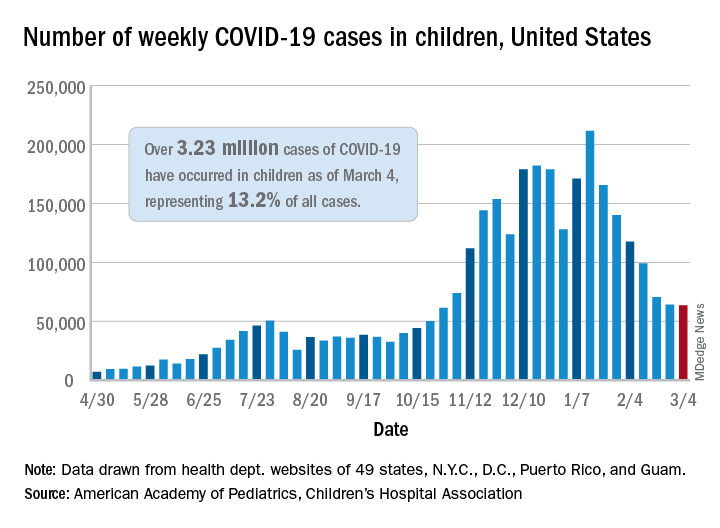
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
A third COVID-19 vaccine is now in circulation and states are starting to drop mask mandates, but the latest decline in weekly child cases barely registers as a decline, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
That’s only 702 cases – a drop of just 1.1% – the smallest by far since weekly cases peaked in mid-January, the AAP and CHA said in their weekly COVID-19 report. Since that peak, the last 7 weeks of declines have looked like this: 21.7%, 15.3%, 16.2%, 15.7%, 28.7%, 9.0%, and 1.1%.
Meanwhile, children’s share of the COVID-19 burden increased to its highest point ever: 18.0% of all new cases occurred in children during the week ending March 4, climbing from 15.7% the week before and eclipsing the previous high of 16.9%. Cumulatively, the 3.23 million cases in children represent 13.2% of all COVID-19 cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
At the state level, the new leader in cumulative share of cases is Vermont at 19.4%, which just edged past Wyoming’s 19.3% as of the week ending March 4. The other states above 18% are Alaska (19.2%) and South Carolina (18.2%). The lowest rates can be found in Florida (8.1%), New Jersey (10.2%), Iowa (10.4%), and Utah (10.5%), the AAP and CHA said.
The overall rate of COVID-19 cases nationwide was 4,294 cases per 100,000 children as of March 4, up from 4,209 per 100,000 the week before. That measure had doubled between Dec. 3 (1,941 per 100,000) and Feb. 4 (3,899) but has only risen about 10% in the last month, the AAP/CHA data show.
Perhaps the most surprising news of the week involves the number of COVID-19 deaths in children, which went from 256 the previous week to 253 after Ohio made a downward revision of its mortality data. So far, children represent just 0.06% of all coronavirus-related deaths, a figure that has held steady since last summer in the 43 states (along with New York City and Guam) that are reporting mortality data by age, the AAP and CHA said.
Pediatric TB – more work needed, especially with HIV-coinfection
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
Despite recent advances in the diagnosis, treatment, and prevention of pediatric tuberculosis in children living with HIV (CLHIV) and HIV-exposed uninfected children (HEU), several unmet needs remain, including studies evaluating the feasibility of shortened TB treatment regimens.
“Children living with HIV contribute disproportionately to pediatric TB mortality rates, accounting for 16% of child TB deaths, and many cases are underdiagnosed and underreported,” said Nicole Salazar-Austin, MD, of Johns Hopkins University in Baltimore. She provided an update on pediatric TB prevention and treatment during an educational symposium at this year’s virtual Conference on Retroviruses & Opportunistic Infections.
Dr. Salazar-Austin summarized current diagnostics for pediatric TB and reviewed options for the prevention and treatment of TB in CLHIV and HEU.
TB and CLHIV
Presently, TB is the most common opportunistic infection among CLHIV, and those with severe immune suppression have a fivefold greater risk of TB disease. While antiretroviral therapy (ART) is highly protective against TB disease in CLHIV, only about 50% of eligible children receive ART.
Dr. Salazar-Austin explained that many individuals with TB/HIV coinfection are unaware of their coinfection and not receiving treatment. Despite recommendations, TB preventive therapy is poorly implemented in CLHIV, especially in high-burden settings.
Pediatric TB diagnosis
Smear microscopy, culture, and Xpert MTB/RIF Ultra are the main diagnostic modalities for pediatric TB. The Xpert MTB/RIF test is an automated PCR-based assay that simultaneously and rapidly detects Mycobacterium tuberculosis complex and resistance to rifampin. The test is currently recommended by the World Health Organization as the initial diagnostic method for presumptive TB cases in both adults and children.
However, under optimal conditions, only 40% of TB cases will be detected. This is in part due to limited implementation of sputum collection procedures, but recent evidence has shown that collection of multiple specimens improves sensitivity for both culture and Xpert MTB/RIF Ultra across all specimen types, Dr. Salazar-Austin explained.
In 2020, the WHO endorsed the use of stool samples for the diagnosis of pediatric pulmonary TB. Stool Xpert is an emerging alternative, noninvasive method for ruling in pediatric TB disease, and has shown sensitivity and specificity similar to that of Xpert MTB/RIF Ultra.
“TB diagnostics have limited sensitivity in children, and efforts are ongoing to maximize current diagnostics, but new diagnostics are needed,” said Dr. Salazar-Austin.
Pediatric TB treatment
Despite the high frequency of TB as an opportunistic infection in CLHIV, current data on co-treatment strategies are limited.
Dolutegravir-based regimens are the preferred first-line regimen for CLHIV. In June 2020, the Food and Drug Administration approved the dispersible dolutegravir tablet, and it is expected to become widely available in 2021.
In children with TB/HIV coinfection who receive dolutegravir and rifampicin, dolutegravir is typically dosed twice daily because of a known drug interaction, based on data from the ODYSSEY study. The WHO recommendations for treatment of pediatric TB/HIV coinfection were recently updated to reflect twice-daily dosing of dolutegravir.
Despite these new recommendations, data are currently limited, and observational pharmacokinetic studies evaluating twice daily dolutegravir with TB treatment in young children are needed.
“More work is needed to evaluate the drug-drug interactions and proper dosing of rifamycins with dolutegravir for the treatment and prevention of TB in CLHIV,” Dr. Salazar-Austin said.
Based on data from TBTC Study 31/ACTG A5349, high-dose rifapentine (a rifamycin) with moxifloxacin (a fluoroquinolone) was noninferior to rifapentine alone in newly diagnosed, culture positive, drug-susceptible TB in children 12 years and older.
Whether rifapentine and moxifloxacin (RPT-Mox) can be used in children under 12 years remains unknown, but future studies may help answer this question, Dr. Salazar-Austin noted. The FDA has restricted the use of fluoroquinolones in children because of a possible effect on cartilage development, she explained.
Furthermore, recent data from the SHINE trial suggested that shortened treatment regimens may hold promise for children with TB.
“While shortened TB treatment regimens hold promise, much work needs to be done in children to implement RPT-Mox, but the results from SHINE can be implemented rapidly,” Dr. Salazar-Austin said.
Dr. Salazar-Austin disclosed no conflicts of interest. The presentation was funded by NICHD, UNITAID, Fogarty Institute, and the IMPAACT network.
FROM CROI 2021
Are long-acting injectables the future of TB treatment?
Long-acting injectable (LAI) drug formulations represent a promising new strategy for the prevention and treatment of tuberculosis in women and children, according to an online presentation at the Conference on Retroviruses & Opportunistic Infections, held virtually.
“As a delivery strategy, LAIs hold the potential to unlock a vast chemical space of lipophilic compounds with very potent anti-TB activity that would otherwise not be developed due to poor predicted oral bioavailability,” explained presenter Eric Nuermberger, MD.
He summarized current preventive treatment options for TB and reviewed the potential impact of LAI formulations on TB therapy. In addition, he identified key challenges for future LAI development and proposed a new development path for clinical implementation.
Current TB preventive therapies
Despite widespread availability, the uptake of TB preventive therapy is poor and currently lags behind global targets. One key barrier to widespread uptake is the long duration of treatment, which may hinder patient adherence to therapy.
While shorter preventive regimens, such as 1 month of daily isoniazid plus rifapentine, show similar efficacy and higher completion rates, further shortening of therapy and reducing clinic visits are the most direct methods to increase adherence and treatment completion rates, Dr. Nuermberger said.
LAI drugs
LAI drug formulations allow for slow release of suitable drugs from a depot injected subcutaneously or intramuscularly.
The goal of LAI formulations is to free patients from the daily burden of oral administration. Other potential benefits include better adherence and efficacy, drug exposure, and the potential to overcome intrinsic poor oral bioavailability by bypassing the GI tract entirely.
Potential indications for LAIs include treatment of latent tuberculosis infection (LTBI), and as continuous therapy in people living with HIV in high-burden settings. There is also potential for treating younger children, such as household contacts, who have difficulty taking oral medications.
“We’ve already seen LAIs revolutionize other areas, such as psychiatry and contraception, and we appear to have another revolution in HIV prevention and treatment,” Dr. Nuermberger explained.
Not all existing TB drugs are suitable for LAI formulations, but drugs such as rifapentine, rifabutin, delamanid, and bedaquiline, show more promise than isoniazid or rifampin because of their physiochemical composition. Of all, bedaquiline may offer the best profile for LAI formulation, Dr. Nuermberger said.
Early proof-of-concept in vivo studies have shown potential use of LAI bedaquiline for TB prevention in both drug-sensitive and drug-resistant TB contacts. Translational PK modeling and simulation predicted that a 1-g intramuscular injection of LAI bedaquiline could maintain therapeutic plasma concentrations in humans for greater than 1 month.
Dr. Nuermberger noted that novel diarylquinoline-based therapies, currently in phase 1 studies, may be even better candidates for LAI-based TB preventive therapy. Early data suggests these compounds may be 10-20 times more potent and have a lower CV risk profile than that of bedaquiline.
Considerations for development and implementation
“Despite the promising potential of long-acting injectables for TB, we are still in the very early stages,” said Dr. Nuermberger.
Ensuring and optimizing acceptance of LAI formulations, especially in at-risk populations, will be very important, he explained. Early involvement of children and pregnant women in studies of who may benefit most from LAI drugs will also be essential.
Other important considerations include cost-effectiveness, particularly in at-risk and vulnerable populations. Furthermore, new dedicated research and development programs are needed to continue to develop more drug candidates suitable for LAI.
“Long-acting formulations hold enormous promise to be transformative for combating TB, through simplification of delivery and overcoming issues of adherence that can compromise success of current interventions,” said Andrew Owen, PhD, of the University of Liverpool (England).
“The ability to deliver an entire course of drug in a single visit promises to ensure missed doses don’t compromise outcomes or place unnecessary selective pressure in favor of drug resistance,” Dr. Owen said.
“Recent studies showing the value of one-month oral treatment regimens for LTBI make long-acting formulations seem more realistic and drugs such as long-acting bedaquiline put a one-shot regimen within reach,” Charles W. Flexner, MD, of Johns Hopkins University, Baltimore, said in an interview.
While no LAIs have been approved for TB, Dr. Nuermberger was optimistic that the recent success of LAI formulations for HIV treatment and prevention will catalyze further efforts in the TB landscape.
Dr. Nuermberger disclosed research support from Janssen Pharmaceuticals, TB Alliance, and the Gates Medical Research Institute. The presentation was sponsored by Janssen Pharmaceuticals, Johns Hopkins CFAR, NIH, Unitaid, and the TB Alliance.
Long-acting injectable (LAI) drug formulations represent a promising new strategy for the prevention and treatment of tuberculosis in women and children, according to an online presentation at the Conference on Retroviruses & Opportunistic Infections, held virtually.
“As a delivery strategy, LAIs hold the potential to unlock a vast chemical space of lipophilic compounds with very potent anti-TB activity that would otherwise not be developed due to poor predicted oral bioavailability,” explained presenter Eric Nuermberger, MD.
He summarized current preventive treatment options for TB and reviewed the potential impact of LAI formulations on TB therapy. In addition, he identified key challenges for future LAI development and proposed a new development path for clinical implementation.
Current TB preventive therapies
Despite widespread availability, the uptake of TB preventive therapy is poor and currently lags behind global targets. One key barrier to widespread uptake is the long duration of treatment, which may hinder patient adherence to therapy.
While shorter preventive regimens, such as 1 month of daily isoniazid plus rifapentine, show similar efficacy and higher completion rates, further shortening of therapy and reducing clinic visits are the most direct methods to increase adherence and treatment completion rates, Dr. Nuermberger said.
LAI drugs
LAI drug formulations allow for slow release of suitable drugs from a depot injected subcutaneously or intramuscularly.
The goal of LAI formulations is to free patients from the daily burden of oral administration. Other potential benefits include better adherence and efficacy, drug exposure, and the potential to overcome intrinsic poor oral bioavailability by bypassing the GI tract entirely.
Potential indications for LAIs include treatment of latent tuberculosis infection (LTBI), and as continuous therapy in people living with HIV in high-burden settings. There is also potential for treating younger children, such as household contacts, who have difficulty taking oral medications.
“We’ve already seen LAIs revolutionize other areas, such as psychiatry and contraception, and we appear to have another revolution in HIV prevention and treatment,” Dr. Nuermberger explained.
Not all existing TB drugs are suitable for LAI formulations, but drugs such as rifapentine, rifabutin, delamanid, and bedaquiline, show more promise than isoniazid or rifampin because of their physiochemical composition. Of all, bedaquiline may offer the best profile for LAI formulation, Dr. Nuermberger said.
Early proof-of-concept in vivo studies have shown potential use of LAI bedaquiline for TB prevention in both drug-sensitive and drug-resistant TB contacts. Translational PK modeling and simulation predicted that a 1-g intramuscular injection of LAI bedaquiline could maintain therapeutic plasma concentrations in humans for greater than 1 month.
Dr. Nuermberger noted that novel diarylquinoline-based therapies, currently in phase 1 studies, may be even better candidates for LAI-based TB preventive therapy. Early data suggests these compounds may be 10-20 times more potent and have a lower CV risk profile than that of bedaquiline.
Considerations for development and implementation
“Despite the promising potential of long-acting injectables for TB, we are still in the very early stages,” said Dr. Nuermberger.
Ensuring and optimizing acceptance of LAI formulations, especially in at-risk populations, will be very important, he explained. Early involvement of children and pregnant women in studies of who may benefit most from LAI drugs will also be essential.
Other important considerations include cost-effectiveness, particularly in at-risk and vulnerable populations. Furthermore, new dedicated research and development programs are needed to continue to develop more drug candidates suitable for LAI.
“Long-acting formulations hold enormous promise to be transformative for combating TB, through simplification of delivery and overcoming issues of adherence that can compromise success of current interventions,” said Andrew Owen, PhD, of the University of Liverpool (England).
“The ability to deliver an entire course of drug in a single visit promises to ensure missed doses don’t compromise outcomes or place unnecessary selective pressure in favor of drug resistance,” Dr. Owen said.
“Recent studies showing the value of one-month oral treatment regimens for LTBI make long-acting formulations seem more realistic and drugs such as long-acting bedaquiline put a one-shot regimen within reach,” Charles W. Flexner, MD, of Johns Hopkins University, Baltimore, said in an interview.
While no LAIs have been approved for TB, Dr. Nuermberger was optimistic that the recent success of LAI formulations for HIV treatment and prevention will catalyze further efforts in the TB landscape.
Dr. Nuermberger disclosed research support from Janssen Pharmaceuticals, TB Alliance, and the Gates Medical Research Institute. The presentation was sponsored by Janssen Pharmaceuticals, Johns Hopkins CFAR, NIH, Unitaid, and the TB Alliance.
Long-acting injectable (LAI) drug formulations represent a promising new strategy for the prevention and treatment of tuberculosis in women and children, according to an online presentation at the Conference on Retroviruses & Opportunistic Infections, held virtually.
“As a delivery strategy, LAIs hold the potential to unlock a vast chemical space of lipophilic compounds with very potent anti-TB activity that would otherwise not be developed due to poor predicted oral bioavailability,” explained presenter Eric Nuermberger, MD.
He summarized current preventive treatment options for TB and reviewed the potential impact of LAI formulations on TB therapy. In addition, he identified key challenges for future LAI development and proposed a new development path for clinical implementation.
Current TB preventive therapies
Despite widespread availability, the uptake of TB preventive therapy is poor and currently lags behind global targets. One key barrier to widespread uptake is the long duration of treatment, which may hinder patient adherence to therapy.
While shorter preventive regimens, such as 1 month of daily isoniazid plus rifapentine, show similar efficacy and higher completion rates, further shortening of therapy and reducing clinic visits are the most direct methods to increase adherence and treatment completion rates, Dr. Nuermberger said.
LAI drugs
LAI drug formulations allow for slow release of suitable drugs from a depot injected subcutaneously or intramuscularly.
The goal of LAI formulations is to free patients from the daily burden of oral administration. Other potential benefits include better adherence and efficacy, drug exposure, and the potential to overcome intrinsic poor oral bioavailability by bypassing the GI tract entirely.
Potential indications for LAIs include treatment of latent tuberculosis infection (LTBI), and as continuous therapy in people living with HIV in high-burden settings. There is also potential for treating younger children, such as household contacts, who have difficulty taking oral medications.
“We’ve already seen LAIs revolutionize other areas, such as psychiatry and contraception, and we appear to have another revolution in HIV prevention and treatment,” Dr. Nuermberger explained.
Not all existing TB drugs are suitable for LAI formulations, but drugs such as rifapentine, rifabutin, delamanid, and bedaquiline, show more promise than isoniazid or rifampin because of their physiochemical composition. Of all, bedaquiline may offer the best profile for LAI formulation, Dr. Nuermberger said.
Early proof-of-concept in vivo studies have shown potential use of LAI bedaquiline for TB prevention in both drug-sensitive and drug-resistant TB contacts. Translational PK modeling and simulation predicted that a 1-g intramuscular injection of LAI bedaquiline could maintain therapeutic plasma concentrations in humans for greater than 1 month.
Dr. Nuermberger noted that novel diarylquinoline-based therapies, currently in phase 1 studies, may be even better candidates for LAI-based TB preventive therapy. Early data suggests these compounds may be 10-20 times more potent and have a lower CV risk profile than that of bedaquiline.
Considerations for development and implementation
“Despite the promising potential of long-acting injectables for TB, we are still in the very early stages,” said Dr. Nuermberger.
Ensuring and optimizing acceptance of LAI formulations, especially in at-risk populations, will be very important, he explained. Early involvement of children and pregnant women in studies of who may benefit most from LAI drugs will also be essential.
Other important considerations include cost-effectiveness, particularly in at-risk and vulnerable populations. Furthermore, new dedicated research and development programs are needed to continue to develop more drug candidates suitable for LAI.
“Long-acting formulations hold enormous promise to be transformative for combating TB, through simplification of delivery and overcoming issues of adherence that can compromise success of current interventions,” said Andrew Owen, PhD, of the University of Liverpool (England).
“The ability to deliver an entire course of drug in a single visit promises to ensure missed doses don’t compromise outcomes or place unnecessary selective pressure in favor of drug resistance,” Dr. Owen said.
“Recent studies showing the value of one-month oral treatment regimens for LTBI make long-acting formulations seem more realistic and drugs such as long-acting bedaquiline put a one-shot regimen within reach,” Charles W. Flexner, MD, of Johns Hopkins University, Baltimore, said in an interview.
While no LAIs have been approved for TB, Dr. Nuermberger was optimistic that the recent success of LAI formulations for HIV treatment and prevention will catalyze further efforts in the TB landscape.
Dr. Nuermberger disclosed research support from Janssen Pharmaceuticals, TB Alliance, and the Gates Medical Research Institute. The presentation was sponsored by Janssen Pharmaceuticals, Johns Hopkins CFAR, NIH, Unitaid, and the TB Alliance.
FROM CROI 2021
Five-day course of oral antiviral appears to stop SARS-CoV-2 in its tracks
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
Management of a Child vs an Adult Presenting With Acral Lesions During the COVID-19 Pandemic: A Practical Review
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
There has been a rise in the prevalence of perniolike lesions—erythematous to violaceous, edematous papules or nodules on the fingers or toes—during the coronavirus disease 2019 (COVID-19) pandemic. These lesions are referred to as “COVID toes.” Although several studies have suggested an association with these lesions and COVID-19, and coronavirus particles have been identified in endothelial cells of biopsies of pernio lesions, questions remain on the management, pathophysiology, and implications of these lesions.1 We provide a practical review for primary care clinicians and dermatologists on the current management, recommendations, and remaining questions, with particular attention to the distinctions for children vs adults presenting with pernio lesions.
Hypothetical Case of a Child Presenting With Acral Lesions
A 7-year-old boy presents with acute-onset, violaceous, mildly painful and pruritic macules on the distal toes that began 3 days earlier and have progressed to involve more toes and appear more purpuric. A review of symptoms reveals no fever, cough, fatigue, or viral symptoms. He has been staying at home for the last few weeks with his brother, mother, and father. His father is working in delivery services and is social distancing at work but not at home. His mother is concerned about the lesions, if they could be COVID toes, and if testing is needed for the patient or family. In your assessment and management of this patient, you consider the following questions.
What Is the Relationship Between These Clinical Findings and COVID-19?
Despite negative polymerase chain reaction (PCR) tests reported in cases of chilblains during the COVID-19 pandemic as well as the possibility that these lesions are an indirect result of environmental factors or behavioral changes during quarantine, the majority of studies favor an association between these chilblains lesions and COVID-19 infection.2,3 Most compellingly, COVID-19 viral particles have been identified by immunohistochemistry and electron microscopy in the endothelial cells of biopsies of these lesions.1 Additionally, there is evidence for possible associations of other viruses, including Epstein-Barr virus and parvovirus B19, with chilblains lesions.4,5 In sum, with the lack of any large prospective study, the weight of current evidence suggests that these perniolike skin lesions are not specific markers of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6
Published studies differ in reporting the coincidence of perniolike lesions with typical COVID-19 symptoms, including fever, dyspnea, cough, fatigue, myalgia, headache, and anosmia, among others. Some studies have reported that up to 63% of patients with reported perniolike lesions developed typical COVID-19 symptoms, but other studies found that no patients with these lesions developed symptoms.6-11 Studies with younger cohorts tended to report lower prevalence of COVID-19 symptoms, and within cohorts, younger patients tended to have less severe symptoms. For example, 78.8% of patients in a cohort (n=58) with an average age of 14 years did not experience COVID-19–related symptoms.6 Based on these data, it has been hypothesized that patients with chilblainslike lesions may represent a subpopulation who will have a robust interferon response that is protective from more symptomatic and severe COVID-19.12-14
Current evidence suggests that these lesions are most likely to occur between 9 days and 2 months after the onset of COVID-19 symptoms.4,9,10 Most cases have been only mildly symptomatic, with an overall favorable prognosis of both lesions and any viral symptoms.8,10 The lesions typically resolve without treatment within a few days of initial onset.15,16
What Should Be the Workup and Management of These Lesions?
Given the currently available information and favorable prognosis, usually no further workup specific to the perniolike lesions is required in the case of an asymptomatic child presenting with acral lesions, and the majority of management will center around patient and parent/guardian education and reassurance. When asked by the patient’s parent, “What does it mean that my child has these lesions?”, clinicians can provide information on the possible association with COVID-19 and the excellent, self-resolving prognosis. An example of honest and reasonable phrasing with current understanding might be, “We are currently not certain if COVID-19 causes these lesions, although there are data to suggest that they are associated. There are a lot of data showing that children with these lesions either do not have any symptoms or have very mild symptoms that resolve without treatment.”
For management, important considerations include how painful the lesions are to the individual patient and how they affect quality of life. If less severe, clinicians can reassure patients and parents/guardians that the lesions will likely self-resolve without treatment. If worsening or symptomatic, clinicians can try typical treatments for chilblains, such as topical steroids, whole-body warming, and nifedipine.17-19 Obtaining a review of symptoms, including COVID-19 symptoms and general viral symptoms, is important given the rare cases of children with severe COVID-19.20,21
The question of COVID-19 testing as related to these lesions remains controversial, and currently there are still differing perspectives on the need for biopsy, PCR for COVID-19, or serologies for COVID-19 in patients presenting with these lesions. Some experts report that additional testing is not needed in the pediatric population because of the high frequency of negative testing reported to date.22,23 However, these children may be silent carriers, and until more is known about their potential to transmit the virus, testing may be considered if resources allow, particularly if the patient has a known exposure.10,12,16,24 The ultimate decision to pursue biopsy or serologic workup for COVID-19 remains up to clinical discretion with consideration of symptoms, severity, and immunocompromised household contacts. If lesions developed after infection, PCR likely will result negative, whereas serologic testing may reveal antibodies.
Hypothetical Case of an Adult Presenting With Acral Lesions and COVID-19 Symptoms
A 50-year-old man presents with acute-onset, violaceous, painful, edematous plaques on the distal toes that began 3 days earlier and have progressed to include the soles. A review of symptoms reveals fever (temperature, 38.4 °C [101 °F]), cough, dyspnea, diarrhea, and severe asthenia. He has had interactions with a coworker who recently tested positive for COVID-19.
How Should You Consider These Lesions in the Context of the Other Symptoms Concerning for COVID-19?
In contrast to the asymptomatic child above, this adult has chilblainslike lesions and viral symptoms. In adults, chilblainslike lesions have been associated with relatively mild COVID-19, and patients with these lesions who are otherwise asymptomatic have largely tested negative for COVID-19 by PCR and serologic antibody testing.11,25,26
True acral ischemia, which is more severe and should be differentiated from chilblains, has been reported in critically ill patients.9 Additionally, studies have found that retiform purpura is the most common cutaneous finding in patients with severe COVID-19.27 For this patient, who has an examination consistent with progressive and severe chilblainslike lesions and suspicion for COVID-19 infection, it is important to observe and monitor these lesions, as clinical progression suggestive of acral ischemia or retiform purpura should be taken seriously and may indicate worsening of the underlying disease. Early intervention with anticoagulation might be considered, though there currently is no evidence of successful treatment.28
What Causes These Lesions in a Patient With COVID-19?
The underlying pathophysiology has been proposed to be a monocytic-macrophage–induced hyperinflammatory systemic state that damages the lungs, as well as the gastrointestinal, renal, and endothelial systems. The activation of the innate immune system triggers a cytokine storm that creates a hypercoagulable state that ultimately can manifest as superficial thromboses, leading to gangrene of the extremities. Additionally, interferon response and resulting hypercytokinemia may cause direct cytopathic damage to the endothelium of arterioles and capillaries, causing the development of papulovesicular lesions that resemble the chilblainslike lesions observed in children.29 In contrast to children, who typically have no or mild COVID-19 symptoms, adults may have a delayed interferon response, which has been proposed to allow for more severe manifestations of infection.12,30
How Should an Adult With Perniolike Lesions Be Managed?
Adults with chilblainslike lesions and no other signs or symptoms of COVID-19 infection do not necessarily need be tested for COVID-19, given the reports demonstrating most patients in this clinical situation will have negative PCRs and serologies for antibodies. However, there have been several reports of adults with acro-ischemic skin findings who also had severe COVID-19, with an observed incidence of 23% in intensive care unit patients with COVID-19.27,28,31,32 If there is suspicion of infection with COVID-19, it is advisable to first obtain workup for COVID-19 and other viruses that can cause acral lesions, including Epstein-Barr virus and parvovirus. Other pertinent laboratory tests may include D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, antithrombin activity, platelet count, neutrophil count, procalcitonin, triglycerides, ferritin, C-reactive protein, and hemoglobin. For patients with evidence of worsening acro-ischemia, regular monitoring of these values up to several times per week can allow for initiation of vascular intervention, including angiontensin-converting enzyme inhibitors, statins, or antiplatelet drugs.32 The presence of antiphospholipid antibodies also has been associated with critically ill patients who develop digit ischemia as part of the sequelae of COVID-19 infection and therefore may act as an important marker for the potential to develop disseminated intravascular coagulation in this patient.33 Even if COVID-19 infection is not suspected, a thorough review of systems is important to look for an underlying connective tissue disease, such as systemic lupus erythematosus, which is associated with pernio. Associated symptoms may warrant workup with antinuclear antibodies and other appropriate autoimmune serologies.
If there is any doubt of the diagnosis, the patient is experiencing symptoms from the lesion, or the patient is experiencing other viral symptoms, it is appropriate to biopsy immediately to confirm the diagnosis. Prior studies have identified fibrin clots, angiocentric and eccrinotropic lymphocytic infiltrates, lymphocytic vasculopathy, and papillary dermal edema as the most common features in chilblainslike lesions during the COVID-19 pandemic.9
For COVID-19 testing, many studies have revealed adult patients with an acute hypercoagulable state testing positive by SARS-CoV-2 PCR. These same patients also experienced thromboembolic events shortly after testing positive for COVID-19, which suggests that patients with elevated D-dimer and fibrinogen likely will have a viral load that is sufficient to test positive for COVID-19.32,34-36 It is appropriate to test all patients with suspected COVID-19, especially adults who are more likely to experience adverse complications secondary to infection.
This patient experiencing COVID-19 symptoms with signs of acral ischemia is likely to test positive by PCR, and additional testing for serologic antibodies is unlikely to be clinically meaningful in this patient’s state. Furthermore, there is little evidence that serology is reliable because of the markedly high levels of both false-negative and false-positive results when using the available antibody testing kits.37 The latter evidence makes serology testing of little value for the general population, but particularly for patients with acute COVID-19.
Conclusion and Outstanding Questions
There is evidence suggesting an association between chilblainslike lesions and COVID-19.11,22,38,39 Children presenting with these lesions have an excellent prognosis and only need a workup or treatment if there are other symptoms, as the lesions self-resolve in the majority of reported cases.7-9 Adults presenting with these lesions and without symptoms likewise are unlikely to test positive for COVID-19, and the lesions typically resolve spontaneously or with first-line treatment. However, adults presenting with these lesions and COVID-19 symptoms should raise clinical concern for evolving skin manifestations of acro-ischemia. If the diagnosis is uncertain or systemic symptoms are concerning, biopsy, COVID-19 PCR, and other appropriate laboratory workup should be obtained.
There remains controversy and uncertainty over the relationship between these skin findings and SARS-CoV-2 infection, with clinical evidence to support both a direct relationship representing convalescent-phase cutaneous reaction as well as an indirect epiphenomenon. If there was a direct relationship, we would have expected to see a rise in the incidence of acral lesions proportionate to the rising caseload of COVID-19 after the reopening of many states in the summer of 2020. Similarly, because young adults represent the largest demographic of increasing cases and as some schools have remained open for in-person instruction during the current academic year, we also would have expected the incidence of chilblains-like lesions presenting to dermatologists and pediatricians to increase alongside these cases. Continued evaluation of emerging literature and ongoing efforts to understand the cause of this observed phenomenon will hopefully help us arrive at a future understanding of the pathophysiology of this puzzling skin manifestation.40
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737. doi:10.1111/bjd.19327
- Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric “true” primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635. doi:10.1111/jdv.16751
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions [letter]. Lancet Infect Dis. June 18, 2020. doi:10.1016/S1473-3099(20)30518-1
- Pistorius MA, Blaise S, Le Hello C, et al. Chilblains and COVID19 infection: causality or coincidence? How to proceed? J Med Vasc. 2020;45:221-223. doi:10.1016/j.jdmv.2020.05.002
- Massey PR, Jones KM. Going viral: a brief history of Chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330-334. doi:10.1053/j.seminoncol.2020.05.012
- Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G, et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review [letter]. J Eur Acad Dermatol Venereol. 2020;34:e445-e446. doi:10.1111/jdv.16665
- El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620-2629. doi:10.1111/jdv.16682
- Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346-e347. doi:10.1111/jdv.16533
- Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406-411. doi:10.1111/pde.14215
- Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID-19) infection–induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489-492. doi:10.1016/j.jdcr.2020.04.011
- Damsky W, Peterson D, King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:E269-E270. doi:10.1016/j.jaad.2020.06.052
- Lipsker D. A chilblain epidemic during the COVID-19 pandemic. A sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi:10.1016/j.mehy.2020.109959
- Kaya G, Kaya A, Saurat J-H. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. 2020;7:3-16. doi:10.3390/dermatopathology7010002
- Pavone P, Marino S, Marino L, et al. Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 2021;34:E14502. doi:https://doi.org/10.1111/dth.14502
- Dowd PM, Rustin MH, Lanigan S. Nifedipine in the treatment of chilblains. Br Med J (Clin Res Ed). 1986;293:923-924. doi:10.1136/bmj.293.6552.923-a
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267-275. doi:10.1111/j.1365-2133.1989.tb07792.x
- Almahameed A, Pinto DS. Pernio (chilblains). Curr Treat Options Cardiovasc Med. 2008;10:128-135. doi:10.1007/s11936-008-0014-0
- Chen F, Liu ZS, Zhang FR, et al. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:179-182. doi:10.3760/cma.j.issn.0578-1310.2020.03.003
- Choi S-H, Kim HW, Kang J-M, et al. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125-132. doi:10.3345/cep.2020.00535
- Piccolo V, Neri I, Manunza F, et al. Chilblain-like lesions during the COVID-19 pandemic: should we really worry? Int J Dermatol. 2020;59:1026-1027. doi:10.1111/ijd.1499
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992-997. doi:10.1001/jamadermatol.2020.2340
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998-1003. doi:10.1001/jamadermatol.2020.2368
- Daneshjou R, Rana J, Dickman M, et al. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892-897. doi:10.1016/j.jdcr.2020.07.009
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi:10.3760/cma.j.issn.0253-2727.2020.0006
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756. doi:10.1007/s00011-020-01370-w
- Park A, Iwasaki A. Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870-878. doi:10.1016/j.chom.2020.05.008
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:E13549. doi:10.1111/dth.13549
- Alonso MN, Mata-Forte T, García-León N, et al. Incidence, characteristics, laboratory findings and outcomes in acro-ischemia in COVID-19 patients. Vasc Health Risk Manag. 2020;16:467-478. doi:10.2147/VHRM.S276530
- Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324-1329. doi:10.1111/jth.14859
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. doi:10.1007/s00134-020-06062-x
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725-733. doi:10.1093/ajcp/aqaa062
- Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268-277. doi:10.7326/M20-2003
- Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of
COVID -19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77. doi:10.1111/bjd.19163 - Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Deutsch A, Blasiak R, Keyes A, et al. COVID toes: phenomenon or epiphenomenon? J Am Acad Dermatol. 2020;83:E347-E348. doi:10.1016/j.jaad.2020.07.037
Practice Points
- Children with chilblainslike lesions generally have a favorable prognosis. As lesions self-resolve, treatment should focus on symptom management and education.
- In children with chilblainslike lesions and no systemic symptoms, further workup for coronavirus disease 2019 (COVID-19) is not necessary for the care of the individual patient.
- In adults with acral lesions, it is important to distinguish between chilblainslike lesions, true acral ischemia, and retiform purpura. Chilblainslike lesions have been associated with mild COVID-19 disease, whereas acral ischemia and retiform purpura have been associated with severe and fatal disease.
- Biopsy and COVID-19 testing should be obtained in adults if there is diagnostic uncertainty or if there are worsening symptoms.