User login
Drug-resistant TB trial stopped early after successful results
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
COVID-19 Monoclonal Antibody Infusions: A Multidisciplinary Initiative to Operationalize EUA Novel Treatment Options
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
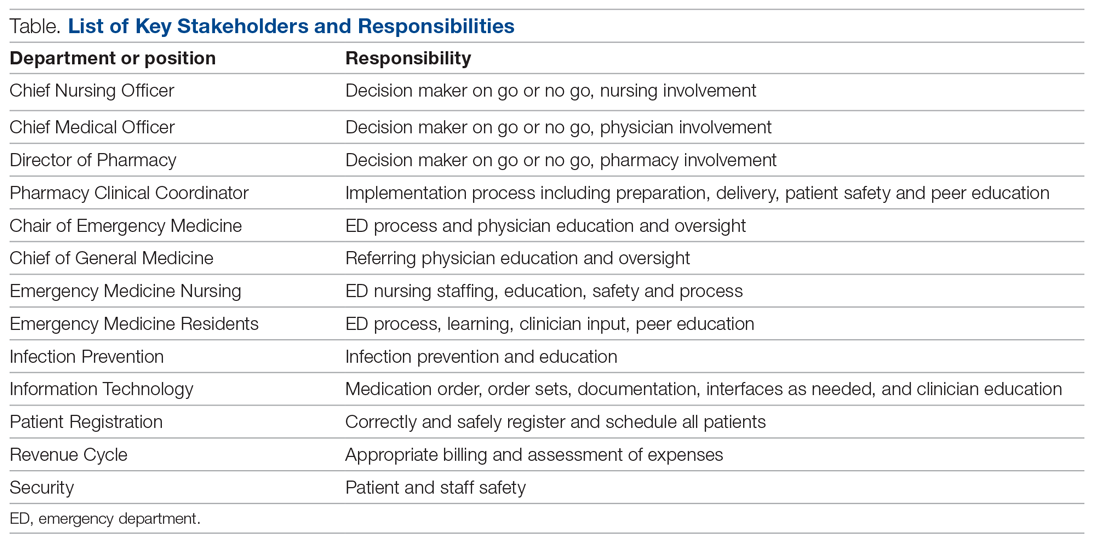
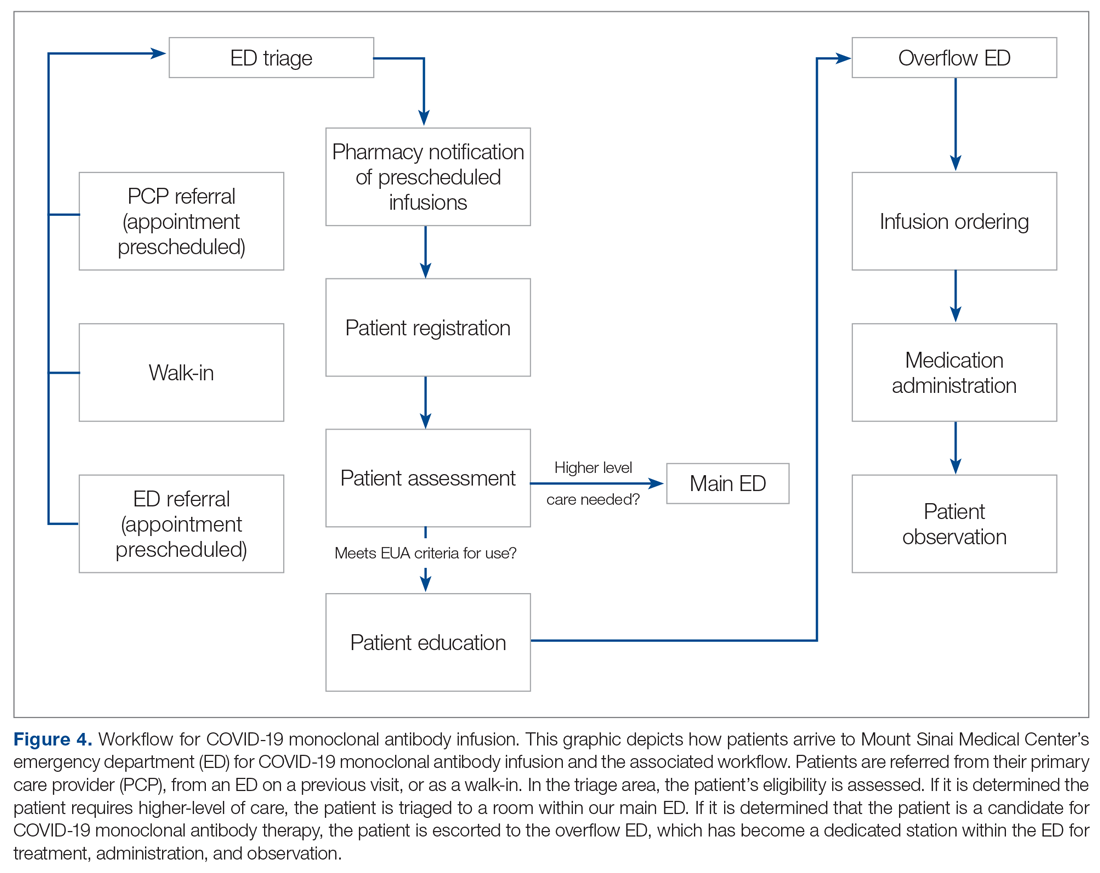
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).
The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.
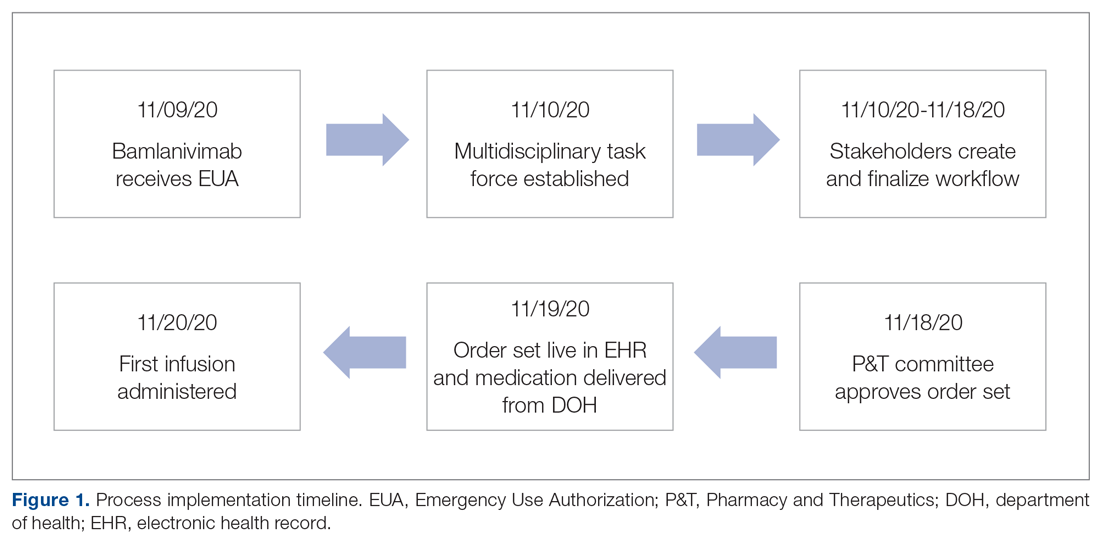
On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.

Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.
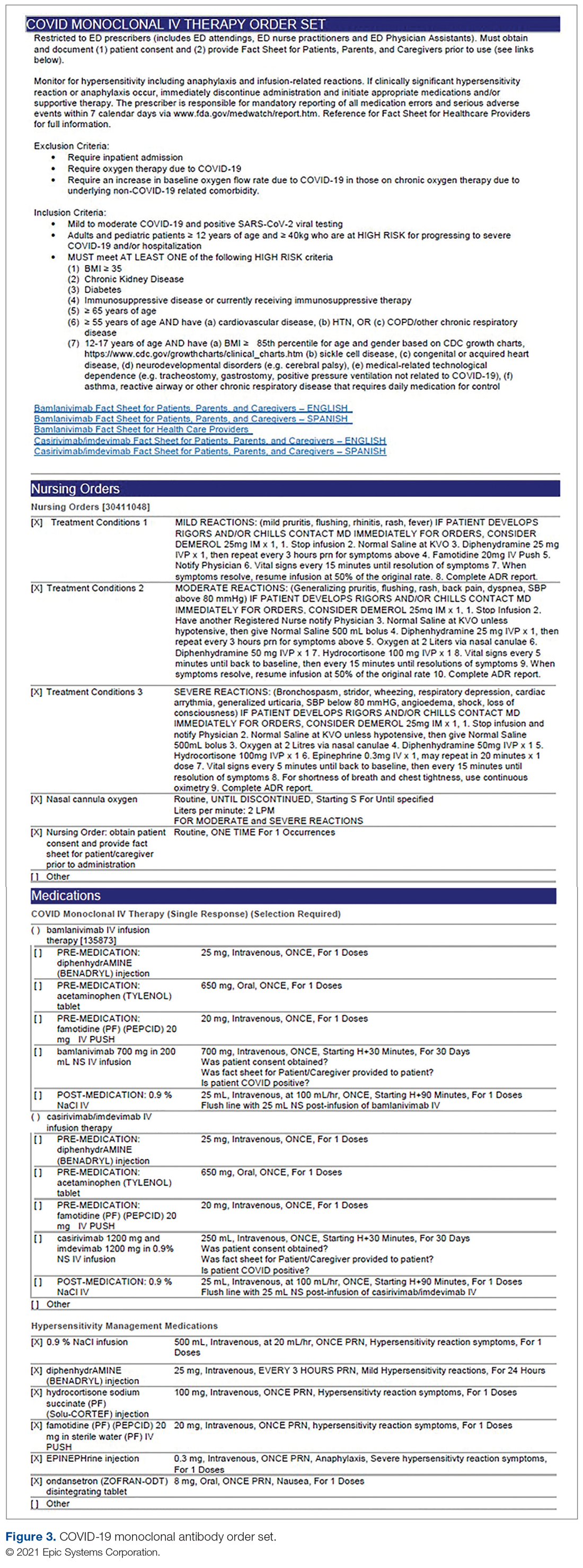
The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
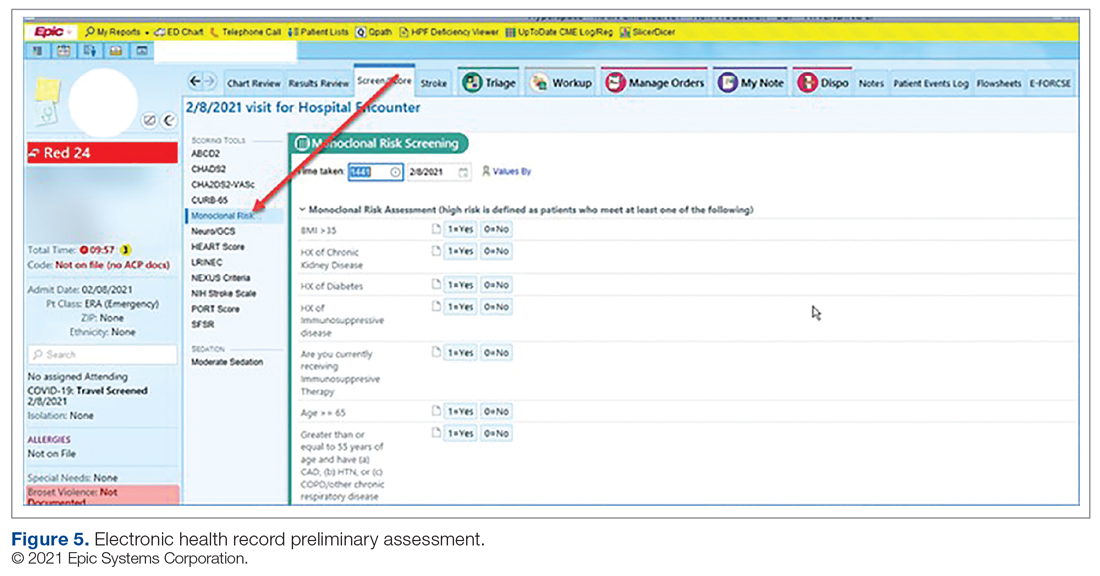
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.
Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.
Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).

The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.

On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.

Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.

The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.

Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.

Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
From Mount Sinai Medical Center, Miami Beach, FL.
Abstract
Objective: To develop and implement a process for administering COVID-19 monoclonal antibody infusions for outpatients with mild or moderate COVID-19 at high risk for hospitalization, using multidisciplinary collaboration, US Food and Drug Administration (FDA) guidance, and infection prevention standards.
Methods: When monoclonal antibody therapy became available for mild or moderate COVID-19 outpatients via Emergency Use Authorization (EUA), our institution sought to provide this therapy option to our patients. We describe the process for planning, implementing, and maintaining a successful program for administering novel therapies based on FDA guidance and infection prevention standards. Key components of our implementation process were multidisciplinary planning involving decision makers and stakeholders; setting realistic goals in the process; team communication; and measuring and reporting quality improvement on a regular basis.
Results: A total of 790 COVID-19 monoclonal antibody infusions were administered from November 20, 2020 to March 5, 2021. Steps to minimize the likelihood of adverse drug reactions were implemented and a low incidence (< 1%) has occurred. There has been no concern from staff regarding infection during the process. Rarely, patients have raised cost-related concerns, typically due to incomplete communication regarding billing prior to the infusion. Patients, families, nursing staff, physicians, pharmacy, and hospital administration have expressed satisfaction with the program.
Conclusion: This process can provide a template for other hospitals or health care delivery facilities to provide novel therapies to patients with mild or moderate COVID-19 in a safe and effective manner.
Keywords: COVID-19; monoclonal antibody; infusion; emergency use authorization.
SARS-CoV-2 and the disease it causes, COVID-19, have transformed from scientific vernacular to common household terms. It began with a cluster of pneumonia cases of unknown etiology in December 2019 in Wuhan, China, with physicians there reporting a novel coronavirus strain (2019-nCoV), now referred to as SARS-CoV-2. Rapid spread of this virus resulted in the World Health Organization (WHO) declaring an international public health emergency. Since this time, the virus has evolved into a worldwide pandemic. COVID-19 has dramatically impacted our society, resulting in more than 2.63 million global deaths as of this writing, of which more than 527,000 deaths have occurred in the United States.1 This novel virus has resulted in a flurry of literature, research, therapies, and collaboration across multiple disciplines in an effort to prevent, treat, and mitigate cases and complications of this disease.
On November 9, 2020, and November 21, 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for 2 novel COVID-19 monoclonal therapies, bamlanivimab2-3 and casirivimab/imdevimab,3-4 respectively. The EUAs granted permission for these therapies to be administered for the treatment of mild to moderate COVID-19 in adult and pediatric patients (≥ 12 years and weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19 and/or hospitalization. The therapies work by targeting the SARS-CoV-2 spike protein and subsequent attachment to human angiotensin-converting enzyme 2 receptors. Clinical trial data leading to the EUA demonstrated a reduction in viral load, safe outcome, and most importantly, fewer hospitalization and emergency room visits, as compared to the placebo group.5-7 The use of monoclonal antibodies is not new and gained recognition during the Ebola crisis, when the monoclonal antibody to the Ebola virus showed a significant survival benefit.8 Providing monoclonal antibody therapy soon after symptom onset aligns with a shift from the onset of the pandemic to the current focus on the administration of pharmaceutical therapy early in the disease course. This shift prevents progression to severe COVID-19, with the goal of reducing patient mortality, hospitalizations, and strain on health care systems.
The availability of novel neutralizing monoclonal antibodies for COVID-19 led to discussions of how to incorporate these therapies as new options for patients. Our institution networked with colleagues from multiple disciplines to discuss processes and policies for the safe administration of the monoclonal antibody infusion therapies. Federal health leaders urge more use of monoclonal antibodies, but many hospitals have been unable to successfully implement infusions due to staff and logistical challenges.9 This article presents a viable process that hospitals can use to provide these novel therapies to outpatients with mild to moderate COVID-19.
The Mount Sinai Medical Center, Florida Experience
Mount Sinai Medical Center in Miami Beach, Florida, is the largest private, independent, not-for-profit teaching hospital in South Florida, comprising 672 licensed beds and supporting 150,000 emergency department (ED) visits annually. Per the EUA criteria for use, COVID-19 monoclonal antibody therapies are not authorized for patients who are hospitalized or who require oxygen therapy due to COVID-19. Therefore, options for outpatient administration needed to be evaluated. Directly following the first EUA press release, a task force of key stakeholders was assembled to brainstorm and develop a process to offer this therapy to the community. A multidisciplinary task force with representation from the ED, nursing, primary care, hospital medicine, pharmacy, risk management, billing, information technology, infection prevention, and senior level leadership participated (Table).

The task force reviewed institutional outpatient locations to determine whether offering this service would be feasible (eg, ED, ambulatory care facilities, cancer center). The ED was selected because it would offer the largest array of appointment times to meet the community needs with around-the-clock availability. While Mount Sinai Medical Center offers care in 3 emergency center locations in Aventura, Hialeah, and Miami Beach, it was determined to initiate the infusions at the main campus center in Miami Beach only. The main campus affords an onsite pharmacy with suitable staffing to prepare the anticipated volume of infusions in a timely manner, as both therapies have short stabilities following preparation. Thus, it was decided that patients from freestanding emergency centers in Aventura and Hialeah would be moved to the Miami Beach ED location to receive therapy. Operating at a single site also allowed for more rapid implementation, monitoring, and ability to make modifications more easily. Discussions for the possible expansion of COVID-19 monoclonal antibody infusions at satellite locations are underway.

On November 20, 2020, 11 days after the formation of the multidisciplinary task force, the first COVID-19 monoclonal infusion was successfully administered. Figure 1 depicts the timeline from assessment to program implementation. Critical to implementation was the involvement of decision makers from all necessary departments early in the planning process to ensure that standard operating procedures were followed and that the patients, community, and organization had a positive experience. This allowed for simultaneous planning of electronic health record (Epic; EHR) builds, departmental workflows, and staff education, as described in the following section. Figure 2 shows the patient safety activities included in the implementation process.

Key Stakeholder Involvement and Workflow
On the day of bamlanivimab EUA release, email communication was shared among hospital leadership with details of the press release. Departments were quickly involved to initiate a task force to assess if and how this therapy could be offered at Mount Sinai Medical Center. The following sections explain the role of each stakeholder and their essential role to operationalize these novel EUA treatment options. The task force was organized and led by our chief medical officer and chief nursing officer.
Information Technology
Medication Ordering and Documentation EHR and Smart Pumps. Early in the pandemic, the antimicrobial stewardship (ASP) clinical coordinator became the designated point person for pharmacy assessment of novel COVID-19 therapies. As such, this pharmacist began reviewing the bamlanivimab and, later, the casirivimab/imdevimab EUA Fact Sheet for Health Care Providers. All necessary elements for the complete and safe ordering and dispensing of the medication were developed and reviewed by pharmacy administration and ED nursing leadership for input, prior to submitting to the information technology team for implementation. Building the COVID-19 monoclonal medication records into the EHR allowed for detailed direction (ie, administration and preparation instructions) to be consistently applied. The medication records were also built into hospital smart pumps so that nurses could access prepopulated, accurate volumes and infusion rates to minimize errors.
Order Set Development. The pharmacy medication build was added to a comprehensive order set (Figure 3), which was then developed to guide prescribers and standardize the process around ordering of COVID-19 monoclonal therapies. While these therapies are new, oncology monoclonal therapies are regularly administered to outpatients at Mount Sinai Cancer Center. The cancer center was therefore consulted on their process surrounding best practices in administration of monoclonal antibody therapies. This included protocols for medications used in pretreatment and management of hypersensitivity reactions and potential adverse drug reactions of both COVID-19 monoclonal therapies. These medication orders were selected by default in the order set to ensure that all patients received premedications aimed at minimizing the risk of hypersensitivity reaction, and had as-needed medication orders, in the event a hypersensitivity reaction occurred. Reducing hypersensitivity reaction risk is important as well to increase the likelihood that the patient would receive full therapy, as management of this adverse drug reactions involves possible cessation of therapy depending on the level of severity. The pharmacy department also ensured these medications were stocked in ED automated dispensing cabinets to promote quick access. In addition to the aforementioned nursing orders, we added EUA criteria for use and hyperlinks to the Fact Sheets for Patients and Caregivers and Health Care Providers for each monoclonal therapy, and restricted ordering to ED physicians, nurse practitioners, and physician assistants.

The order set underwent multidisciplinary review by pharmacy administration, the chair of emergency medicine, physicians, and ED nursing leadership prior to presentation and approval by the Pharmacy and Therapeutics Committee. Lastly, at time of implementation, the order set was added to the ED preference list, preventing inpatient access. Additionally, as a patient safety action, free- standing orders of COVID-19 monoclonal therapies were disabled, so providers could only order therapies via the approved, comprehensive order set.
Preliminary Assessment Tool. A provider assessment tool was developed to document patient-specific EUA criteria for use during initial assessment (Figure 4). This tool serves as a checklist and is visible to the full multidisciplinary team in the patient’s EHR. It is used as a resource at the time of pharmacist verification and ED physician assessment to ensure criteria for use are met.

Outpatient Offices
Patient Referral. Patients with symptoms or concerns of COVID-19 exposure can make physician appointments via telemedicine or in person at Mount Sinai Medical Center’s primary care and specialty offices. At the time of patient encounter, physicians suspecting a COVID-19 diagnosis will refer patients for outpatient COVID-19 polymerase chain reaction (PCR) laboratory testing, which has an approximate 24-hour turnaround to results. Physicians also assess whether the patient meets EUA criteria for use, pending results of testing. In the event a patient meets EUA criteria for use, the physician provides patient counseling and requests verbal consent. Following this, the physician enters a note in the EHR describing the patient’s condition, criteria for use evaluation, and the patient’s verbal agreement to therapy. This preliminary screening is beneficial to begin planning with both the patient and ED to minimize delays. Patients are notified of the results of their test once available. If the COVID-19 PCR test returns positive, the physician will call the ED at the main campus and schedule the patient for COVID-19 monoclonal therapy. As the desired timeframe for administering COVID-19 monoclonal therapies is within less than 10 days of symptom onset, timely scheduling of appointments is crucial. Infusion appointments are typically provided the same or next day. The patients are informed that they must bring documentation of their positive COVID-19 PCR test to their ED visit. Lastly, because patients are pretreated with medication that may potentially impair driving, they are instructed that they cannot drive themselves home; ride shares also are not allowed in order to limit the spread of infection.
Emergency Department
Patient Arrival and Screening. A COVID-19 patient can be evaluated in the ED 1 of 2 ways. The first option is via outpatient office referral, as described previously. Upon arrival to the ED, a second screening is performed to ensure the patient still meets EUA criteria for use and the positive COVID-19 PCR test result is confirmed. If the patient no longer meets criteria, the patient is triaged accordingly, including evaluation for higher-level care (eg, supplemental oxygen, hospital admission). The second optoion is via new patient walk-ins without outpatient physician referral (Figure 4). In these cases, an initial screening is performed, documenting EUA criteria for use in the preliminary assessment (Figure 5). Physicians will consider an outside COVID-19 test as valid, so long as documentation is readily available confirming a positive PCR result. Otherwise, an in-house COVID-19 PCR test will be performed, which has a 2-hour turnaround time.

Infusion Schedule. The ED offers a total of 16 COVID-19 monoclonal infusions slots daily. These are broken up into 4 infusion time blocks (eg, 8
Patient Education. Prior to administration of the monoclonal therapy, physician and nursing staff obtain a formal, written patient consent for therapy and provide patients with the option of participating in the institutional review board (IRB) approved study. Details of this are discussed in the risk management and IRB sections of the article. Nursing staff also provides the medication-specific Fact Sheet for Patients and Caregivers in either Spanish or English, which is also included as a hyperlink on the COVID-19 Monoclonal Antibody Order Set for ease of access. Interpreter services are available for patients who speak other languages. An ED decentralized pharmacist is also available onsite Monday through Friday from 12
Infusion Ordering. Once the patient is ready to begin therapy, the he/she is brought to a dedicated overflow area of the ED. There are few, if any, patients in this location, and it is adjacent to the main emergency center for easy access by the patients, nurses, pharmacists, and physicians. The physician then enters orders in the EHR using the COVID-19 Monoclonal Antibody Order Set (Figure 3). Three discrete questions were built into the medication order: (1) Was patient consent obtained? (2) Was the Fact Sheet for Patient/Caregiver provided to the patient? (3) Is the patient COVID-19 PCR-positive? These questions were built as hard stops so that the medication orders cannot be placed without a response. This serves as another double-check to ensure processes are followed and helps facilitate timely verification by the pharmacist.
Medication Administration. One nurse is dedicated to administering the monoclonal therapies scheduled at 8
Pharmacy Department
Medication Receipt Process. Inventory is currently allocated biweekly from the state department of health and will soon be transitioning to a direct order system. The pharmacy technician in charge of deliveries notifies the pharmacy Antimicrobial Stewardship Program (ASP) clinical coordinator upon receipt of the monoclonal therapies. Bamlanivimab is supplied as 1 vial per dose, whereas casirivimab/imdevimab is supplied as 4 vials or 8 vials per dose, depending how it is shipped. To reduce the likelihood of medication errors, the ASP clinical coordinator assembles each of the casirivimab/imdevimab vials into kits, where 1 kit equals 1 dose. Labels are then affixed to each kit indicating the medication name, number of vials which equal a full dose, and pharmacist signature. The kits are stored in a dedicated refrigerator, and inventory logs are affixed to the outside of the refrigerator and updated daily. This inventory is also communicated daily to ED physician, nursing, and pharmacy leadership, as well as the director of patient safety, who reports weekly usage to the state Department of Health and Human Services. These weekly reports are used to determine allocation amounts.
Medication Verification and Delivery. The Mount Sinai Medical Center pharmacist staffing model consists of centralized order entry and specialized, decentralized positions. All orders are verified by the ED pharmacist when scheduled (not a 24/7 service) and by the designated pharmacist for all other times. At the time of medication verification, the pharmacist documents patient-specific EUA criteria for use and confirms that consent was obtained and the Fact Sheet for Patients/Caregivers was provided. A pharmacist intervention was developed to assist with this documentation. Pharmacists input smart text “.COVIDmonoclonal” and a drop-down menu of EUA criteria for use appears. The pharmacist reviews the patient care notes and medication order question responses to ascertain this information, contacting the ED prescriber if further clarification is required. This verification serves as another check to ensure processes put in place are followed. Lastly, intravenous preparation and delivery are electronically recorded in the EHR, and the medications require nursing signature at the time of delivery to ensure a formal chain of custody.
Risk Management
At Mount Sinai Medical Center, all EUA and investigational therapies require patient consent. Consistent with this requirement, a COVID-19 monoclonal specific consent was developed by risk management. This is provided to every patient receiving a COVID-19 monoclonal infusion, in addition to the FDA EUA Fact Sheet for Patients and Caregivers, and documented as part of their EHR. The questions providers must answer are built into the order set to ensure this process is followed and these patient safety checks are incorporated into the workflow.
Billing and Finance Department
In alignment with Mount Sinai Medical Center’s mission to provide high-quality health care to its diverse community through teaching, research, charity care, and financial responsibility, it was determined that this therapy would be provided to all patients regardless of insurance type, including those who are uninsured. The billing and finance department was consulted prior to this service being offered, to provide patients with accurate and pertinent information. The billing and finance department provided guidance on how to document patient encounters at time of registration to facilitate appropriate billing. At this time, the medication is free of charge, but nonmedication-related ED fees apply. This is explained to patients so there is a clear understanding prior to booking their appointment.
Infection Prevention
As patients receiving COVID-19 monoclonal therapies can transmit the virus to others, measures to ensure protection for other patients and staff are vital. To minimize exposure, specific nursing and physician staff from the ED are assigned to the treatment of these patients, and patients receive infusions and postobservation monitoring in a designated wing of the ED. Additionally, all staff who interact with these patients are required to don full personal protective equipment. This includes not only physicians and nurses but all specialties such as physician assistants, nurse practitioners, pharmacists, and laboratory technicians. Moreover, patients are not permitted to go home in a ride share and are counseled on Centers for Disease Control and Prevention quarantining following infusion.
Measurement of Process and Outcomes and Reporting
IRB approval was sought and obtained early during initiation of this service, allowing study consent to be offered to patients at the time general consent was obtained, which maximized patient recruitment and streamlined workflow. The study is a prospective observational research study to determine the impact of administration of COVID-19 monoclonal antibody therapy on length of symptoms, chronic illness, and rate of hospitalization. Most patients were eager to participate and offer their assistance to the scientific community during this pandemic.
Staff Education
In order to successfully implement this multidisciplinary EUA treatment option, comprehensive staff education was paramount after the workflow was developed. Prior to the first day of infusions, nurses and pharmacists were provided education during multiple huddle announcements. The pharmacy team also provided screen captures via email to the pharmacists so they could become familiar with the order set, intervention documentation, and location of the preliminary assessment of EUA criteria for use at the time of order verification. The emergency medicine department chair and chief medical officer also provided education via several virtual meetings and email to referring physicians (specialists and primary care) and residents in the emergency centers involved in COVID-19 monoclonal therapy-related patient care.
Factors Contributing to Success
We believe the reasons for continued success of this process are multifactorial and include the following key elements. Multidisciplinary planning, which included decision makers and all stakeholders, began at the time the idea was conceived. This allowed quick implementation of this service by efficiently navigating barriers to engaging impacted staff early on. Throughout this process, the authors set realistic step-wise goals. While navigating through the many details to implementation described, we also kept in mind the big picture, which was to provide this potentially lifesaving therapy to as many qualifying members of our community as possible. This included being flexible with the process and adapting when needed to achieve this ultimate goal. A focus on safety remained a priority to minimize possible errors and enhance patient and staff satisfaction. The optimization of the EHR streamlined workflow, provided point-of-care resources, and enhanced patient safety. Additionally, the target date set for implementation allowed staff and department leads adequate time to plan for and anticipate the changes. Serving only 1 patient on the first day allowed time for staff to experience this new process hands-on and provided opportunity for focused education. This team communication was essential to implementing this project, including staff training of processes and procedures prior to go-live. Early incorporation of IRB approval allowed the experience to be assessed and considered for contribution to the scientific literature to tackle this novel virus that has impacted our communities locally, nationally, and abroad. Moreover, continued measurement and reporting on a regular basis leads to performance improvement. The process outlined here can be adapted to incorporate other new therapies in the future, such as the recent February 9, 2021, EUA of the COVID-19 monoclonal antibody combination bamlanivimab and etesevimab.10
Conclusion
We administered 790 COVID-19 monoclonal antibody infusions between November 20, 2020 and March 5, 2021. Steps to minimize the likelihood of hypersensitivity reactions were implemented, and a low incidence (< 1%) has been observed. There has been no incidence of infection, concern from staff about infection prevention, or risk of infection during the processes. There have been very infrequent cost-related concerns raised by patients, typically due to incomplete communication regarding billing prior to the infusion. To address these issues, staff education has been provided to enhance patient instruction on this topic. The program has provided patient and family satisfaction, as well nursing, physician, pharmacist, clinical staff, and hospital administration pride and gratification. Setting up a new program to provide a 4-hour patient encounter to infuse therapy to high-risk patients with COVID-19 requires commitment and effort. This article describes the experience, ideas, and formula others may consider using to set up such a program. Through networking and formal phone calls and meetings about monoclonal antibody therapy, we have heard about other institutions who have not been able to institute this program due to various barriers to implementation. We hope our experience serves as a resource for others to provide this therapy to their patients and expand access in an effort to mitigate COVID-19 consequences and cases affecting our communities.
Corresponding author: Kathleen Jodoin, PharmD, BCPS, Mount Sinai Medical Center, 4300 Alton Rd, Miami Beach, FL 33140; [email protected].
Financial disclosures: None.
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
1. COVID Data Tracker. Center for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 12, 2021.
2. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/143603/download
3. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed February 14, 2021.
4. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. US Food and Drug Administration. Updated December 2020. Accessed March 9, 2021. https://www.fda.gov/media/143892/download
5. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
6. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. 10.1JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
7. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. 10.1N Engl J Med. 2021;384:238-251. doi:10.1056/nejmoa2035002
8. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. 10.1N Engl J Med. 2019;381:2293-2303. doi:10.1056/NEJMoa1910993
9. Boyle, P. Can an experimental treatment keep COVID-19 patients out of hospitals? Association of American Medical Colleges. January 29, 2021. Accessed March 9, 2021. https://www.aamc.org/news-insights/can-experimental-treatment-keep-covid-19-patients-out-hospitals
10. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. US Food and Drug Administration. Updated February 2021. Accessed March 9, 2021. https://www.fda.gov/media/145802/download
COVID-19 maternal antibodies transferred to fetus, newborn from pregnant and lactating vaccine recipients
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Here we go again? Rate of COVID-19 in children takes a turn for the worse
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
Emergent ERCP in acute cholangitis linked with better outcomes
Background: Acute cholangitis (AC) in its most severe form is associated with a high mortality rate. Most patients respond to medical management involving intravenous hydration and antibiotics, though a sizable portion require biliary drainage. Current guidelines advocate for urgent drainage depending on the severity of AC, though do not specify optimal timing. Existing literature is conflicting on when ERCP should ideally be done for AC.
Study design: Systematic review and meta-analysis.
Setting: Literature search involving PubMed, Medline, and Embase databases.
Synopsis: Nine studies with 7,534 patients were included in the final meta-analysis. Emergent ERCP was associated with a lower in-hospital mortality (IHM; odds ratio, 0.52; 95% confidence interval, 0.28-0.98) and shorter length of stay (LOS; mean difference, –2.87 days; 95% CI, –1.55 to –4.18), compared to urgent ERCP. The IHM mortality difference was true for both patients with severe AC (as defined by evidence of end-organ dysfunction) and mild-moderate AC. There was a trend toward lower 30-day mortality in patients who underwent emergent ERCP, though it did not reach statistical significance.
The studies included in the analysis were observational studies, so no causal relationship can be established. Only two of the nine studies reported outcome differences stratified by severity of presentation. Etiology of the AC was inconsistently reported amongst studies.
Bottom line: Emergent ERCP appears to be associated with reduced mortality and LOS in patients presenting with AC, though larger randomized controlled trials are needed to better delineate the optimal timing for biliary drainage in these patients.
Citation: Iqbal U et al. Emergent versus urgent ERCP in acute cholangitis: A systematic review and meta-analysis. Gastrointes Endosc. 2019 Oct 16. doi: 10.1016/j.gie.2019.09.040.
Dr. Babbel is a hospitalist and assistant professor of medicine at the University of Utah, Salt Lake City.
Background: Acute cholangitis (AC) in its most severe form is associated with a high mortality rate. Most patients respond to medical management involving intravenous hydration and antibiotics, though a sizable portion require biliary drainage. Current guidelines advocate for urgent drainage depending on the severity of AC, though do not specify optimal timing. Existing literature is conflicting on when ERCP should ideally be done for AC.
Study design: Systematic review and meta-analysis.
Setting: Literature search involving PubMed, Medline, and Embase databases.
Synopsis: Nine studies with 7,534 patients were included in the final meta-analysis. Emergent ERCP was associated with a lower in-hospital mortality (IHM; odds ratio, 0.52; 95% confidence interval, 0.28-0.98) and shorter length of stay (LOS; mean difference, –2.87 days; 95% CI, –1.55 to –4.18), compared to urgent ERCP. The IHM mortality difference was true for both patients with severe AC (as defined by evidence of end-organ dysfunction) and mild-moderate AC. There was a trend toward lower 30-day mortality in patients who underwent emergent ERCP, though it did not reach statistical significance.
The studies included in the analysis were observational studies, so no causal relationship can be established. Only two of the nine studies reported outcome differences stratified by severity of presentation. Etiology of the AC was inconsistently reported amongst studies.
Bottom line: Emergent ERCP appears to be associated with reduced mortality and LOS in patients presenting with AC, though larger randomized controlled trials are needed to better delineate the optimal timing for biliary drainage in these patients.
Citation: Iqbal U et al. Emergent versus urgent ERCP in acute cholangitis: A systematic review and meta-analysis. Gastrointes Endosc. 2019 Oct 16. doi: 10.1016/j.gie.2019.09.040.
Dr. Babbel is a hospitalist and assistant professor of medicine at the University of Utah, Salt Lake City.
Background: Acute cholangitis (AC) in its most severe form is associated with a high mortality rate. Most patients respond to medical management involving intravenous hydration and antibiotics, though a sizable portion require biliary drainage. Current guidelines advocate for urgent drainage depending on the severity of AC, though do not specify optimal timing. Existing literature is conflicting on when ERCP should ideally be done for AC.
Study design: Systematic review and meta-analysis.
Setting: Literature search involving PubMed, Medline, and Embase databases.
Synopsis: Nine studies with 7,534 patients were included in the final meta-analysis. Emergent ERCP was associated with a lower in-hospital mortality (IHM; odds ratio, 0.52; 95% confidence interval, 0.28-0.98) and shorter length of stay (LOS; mean difference, –2.87 days; 95% CI, –1.55 to –4.18), compared to urgent ERCP. The IHM mortality difference was true for both patients with severe AC (as defined by evidence of end-organ dysfunction) and mild-moderate AC. There was a trend toward lower 30-day mortality in patients who underwent emergent ERCP, though it did not reach statistical significance.
The studies included in the analysis were observational studies, so no causal relationship can be established. Only two of the nine studies reported outcome differences stratified by severity of presentation. Etiology of the AC was inconsistently reported amongst studies.
Bottom line: Emergent ERCP appears to be associated with reduced mortality and LOS in patients presenting with AC, though larger randomized controlled trials are needed to better delineate the optimal timing for biliary drainage in these patients.
Citation: Iqbal U et al. Emergent versus urgent ERCP in acute cholangitis: A systematic review and meta-analysis. Gastrointes Endosc. 2019 Oct 16. doi: 10.1016/j.gie.2019.09.040.
Dr. Babbel is a hospitalist and assistant professor of medicine at the University of Utah, Salt Lake City.
How to talk to patients reluctant to get a COVID-19 vaccine
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Candida Esophagitis Associated With Adalimumab for Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
Practice Points
- Adalimumab is an effective treatment for patients with hidradenitis suppurativa.
- There is risk for opportunistic infections with adalimumab, and patients should be monitored closely.
‘Reassuring’ data on COVID-19 vaccines in pregnancy
Pregnant women can safely get vaccinated with the Pfizer-BioNTech and Moderna vaccines for COVID-19, surveillance data from the Centers for Disease Control and Prevention suggest.
More than 30,000 women who received these vaccines have reported pregnancies through the CDC’s V-Safe voluntary reporting system, and their rates of complications are not significantly different from those of unvaccinated pregnant women, said Tom Shimabukuro, MD, MPH, MBA, deputy director of the CDC Immunization Safety Office.
“Overall, the data are reassuring with respect to vaccine safety in pregnant women,” he told this news organization.
Dr. Shimabukuro presented the data during a March 1 meeting of the Advisory Committee on Immunization Practices, a group of health experts selected by the Secretary of the U.S. Department of Health & Human Services.
The CDC has included pregnancy along with other underlying conditions that qualify people to be offered vaccines in the third priority tier (Phase 1c).
“There is evidence that pregnant women who get COVID-19 are at increased risk of severe illness and complications from severe illness,” Dr. Shimabukuro explained. “And there is also evidence that pregnant persons who get COVID-19 may be at increased risk for adverse pregnancy outcomes.”
The American College of Obstetrics and Gynecology recommends that “COVID-19 vaccines should not be withheld from pregnant individuals.”
By contrast, the World Health Organization recommends the vaccines only for those pregnant women who are “at high risk of exposure to SARS-CoV-2 (for example, health workers) or who have comorbidities which add to their risk of severe disease.”
Not enough information was available from the pivotal trials of the Moderna and Pfizer vaccines to assess risk in pregnant women, according to these manufacturers. Pfizer has announced a follow-up trial of its vaccine in healthy pregnant women.
Analyzing surveillance data
To better assess whether the Pfizer or Moderna vaccines cause problems in pregnancy or childbirth, Dr. Shimabukuro and colleagues analyzed data from V-Safe and the Vaccine Adverse Event Reporting System (VAERS).
The CDC encourages providers to inform people they vaccinate about the V-Safe program. Participants can voluntarily enter their data through a website, and may receive follow-up text messages and phone calls from the CDC asking for additional information at various times after vaccination. It is not a systematic survey, and the sample is not necessarily representative of everyone who gets the vaccine, Dr. Shimabukuro noted.
At the time of the study, V-Safe recorded 55,220,364 reports from people who received at least one dose of the Pfizer or Moderna vaccine through Feb. 16. These included 30,494 pregnancies, of which 16,039 were in women who received the Pfizer vaccine and 14,455 in women who received the Moderna vaccine.
Analyzing data collected through Jan. 13, 2021, the researchers found that both local and systemic reactions were similar between pregnant and nonpregnant women aged 16-54 years.
Most women reported pain, and some reported swelling, redness, and itching at the injection site. Of systemic reactions, fatigue was the most common, followed by headache, myalgia, chills, nausea, and fever. The systemic reactions were more common with the second Pfizer dose; fatigue affected a majority of both pregnant and nonpregnant women. Data on the second Moderna dose were not available.
The CDC enrolled 1,815 pregnant women for additional follow-up, among whom there were 275 completed pregnancies and 232 live births.
Rates of outcomes “of interest” were no higher among these women than in the general population. 
In contrast to V-Safe, data from VAERS, comanaged by the CDC and U.S. Food and Drug Administration, are from spontaneous reports of adverse events. The sources for those reports are varied. “That could be the health care provider,” Dr. Shimabukuro said. “That could be the patient themselves. It could be a caregiver for children.”
Just 154 VAERS reports through Feb. 16 concerned pregnant women, and of these, only 42 (27%) were for pregnancy-specific conditions, with the other 73% representing the types of adverse events reported for the general population of vaccinated people, such as headache and fatigue.
Of the 42 pregnancy-related events, there were 29 spontaneous abortions or miscarriages, with the remainder divided among 10 other pregnancy and neonatal conditions.
“When we looked at those outcomes and we compared the reporting rates, based on known background rates of these conditions, we did not see anything unexpected or concerning with respect to pregnancy or neonatal-specific conditions,” Dr. Shimabukuro said about the VAERS data.
The CDC did not collect data on fertility. “We’ve done a lot of work with other vaccines,” said Dr. Shimabukuro. “And just from a biological basis, we don’t have any evidence that vaccination, just in general, causes fertility problems.”
Also, Dr. Shimabukuro noted that the COVID-19 vaccine made by Janssen/Johnson & Johnson did not receive emergency authorization from the FDA in time to be included in the current report, but is being tracked for future reports.
Vaccination could benefit infants
In addition to the new safety data, experts continue to remind clinicians and the public that vaccination during pregnancy could benefit offspring. The unborn babies of pregnant women who receive the COVID-19 vaccine could be protected from the virus for the first several months of their lives, said White House COVID-19 czar Anthony Fauci, MD, at a briefing on March 10.
“We’ve seen this with many other vaccines,” Dr. Fauci said. “That’s a very good way you can get protection for the mother during pregnancy and also a transfer of protection for the infant, which will last a few months following the birth.”
Dr. Fauci also noted that the same vaccine platform used in Johnson & Johnson’s COVID-19 vaccine was successfully used for Ebola in pregnant women in Africa.
Dr. Shimabukuro has reported no relevant financial relationships.
Lindsay Kalter contributed to the reporting for this story.
A version of this article first appeared on Medscape.com.
Pregnant women can safely get vaccinated with the Pfizer-BioNTech and Moderna vaccines for COVID-19, surveillance data from the Centers for Disease Control and Prevention suggest.
More than 30,000 women who received these vaccines have reported pregnancies through the CDC’s V-Safe voluntary reporting system, and their rates of complications are not significantly different from those of unvaccinated pregnant women, said Tom Shimabukuro, MD, MPH, MBA, deputy director of the CDC Immunization Safety Office.
“Overall, the data are reassuring with respect to vaccine safety in pregnant women,” he told this news organization.
Dr. Shimabukuro presented the data during a March 1 meeting of the Advisory Committee on Immunization Practices, a group of health experts selected by the Secretary of the U.S. Department of Health & Human Services.
The CDC has included pregnancy along with other underlying conditions that qualify people to be offered vaccines in the third priority tier (Phase 1c).
“There is evidence that pregnant women who get COVID-19 are at increased risk of severe illness and complications from severe illness,” Dr. Shimabukuro explained. “And there is also evidence that pregnant persons who get COVID-19 may be at increased risk for adverse pregnancy outcomes.”
The American College of Obstetrics and Gynecology recommends that “COVID-19 vaccines should not be withheld from pregnant individuals.”
By contrast, the World Health Organization recommends the vaccines only for those pregnant women who are “at high risk of exposure to SARS-CoV-2 (for example, health workers) or who have comorbidities which add to their risk of severe disease.”
Not enough information was available from the pivotal trials of the Moderna and Pfizer vaccines to assess risk in pregnant women, according to these manufacturers. Pfizer has announced a follow-up trial of its vaccine in healthy pregnant women.
Analyzing surveillance data
To better assess whether the Pfizer or Moderna vaccines cause problems in pregnancy or childbirth, Dr. Shimabukuro and colleagues analyzed data from V-Safe and the Vaccine Adverse Event Reporting System (VAERS).
The CDC encourages providers to inform people they vaccinate about the V-Safe program. Participants can voluntarily enter their data through a website, and may receive follow-up text messages and phone calls from the CDC asking for additional information at various times after vaccination. It is not a systematic survey, and the sample is not necessarily representative of everyone who gets the vaccine, Dr. Shimabukuro noted.
At the time of the study, V-Safe recorded 55,220,364 reports from people who received at least one dose of the Pfizer or Moderna vaccine through Feb. 16. These included 30,494 pregnancies, of which 16,039 were in women who received the Pfizer vaccine and 14,455 in women who received the Moderna vaccine.
Analyzing data collected through Jan. 13, 2021, the researchers found that both local and systemic reactions were similar between pregnant and nonpregnant women aged 16-54 years.
Most women reported pain, and some reported swelling, redness, and itching at the injection site. Of systemic reactions, fatigue was the most common, followed by headache, myalgia, chills, nausea, and fever. The systemic reactions were more common with the second Pfizer dose; fatigue affected a majority of both pregnant and nonpregnant women. Data on the second Moderna dose were not available.
The CDC enrolled 1,815 pregnant women for additional follow-up, among whom there were 275 completed pregnancies and 232 live births.
Rates of outcomes “of interest” were no higher among these women than in the general population. 
In contrast to V-Safe, data from VAERS, comanaged by the CDC and U.S. Food and Drug Administration, are from spontaneous reports of adverse events. The sources for those reports are varied. “That could be the health care provider,” Dr. Shimabukuro said. “That could be the patient themselves. It could be a caregiver for children.”
Just 154 VAERS reports through Feb. 16 concerned pregnant women, and of these, only 42 (27%) were for pregnancy-specific conditions, with the other 73% representing the types of adverse events reported for the general population of vaccinated people, such as headache and fatigue.
Of the 42 pregnancy-related events, there were 29 spontaneous abortions or miscarriages, with the remainder divided among 10 other pregnancy and neonatal conditions.
“When we looked at those outcomes and we compared the reporting rates, based on known background rates of these conditions, we did not see anything unexpected or concerning with respect to pregnancy or neonatal-specific conditions,” Dr. Shimabukuro said about the VAERS data.
The CDC did not collect data on fertility. “We’ve done a lot of work with other vaccines,” said Dr. Shimabukuro. “And just from a biological basis, we don’t have any evidence that vaccination, just in general, causes fertility problems.”
Also, Dr. Shimabukuro noted that the COVID-19 vaccine made by Janssen/Johnson & Johnson did not receive emergency authorization from the FDA in time to be included in the current report, but is being tracked for future reports.
Vaccination could benefit infants
In addition to the new safety data, experts continue to remind clinicians and the public that vaccination during pregnancy could benefit offspring. The unborn babies of pregnant women who receive the COVID-19 vaccine could be protected from the virus for the first several months of their lives, said White House COVID-19 czar Anthony Fauci, MD, at a briefing on March 10.
“We’ve seen this with many other vaccines,” Dr. Fauci said. “That’s a very good way you can get protection for the mother during pregnancy and also a transfer of protection for the infant, which will last a few months following the birth.”
Dr. Fauci also noted that the same vaccine platform used in Johnson & Johnson’s COVID-19 vaccine was successfully used for Ebola in pregnant women in Africa.
Dr. Shimabukuro has reported no relevant financial relationships.
Lindsay Kalter contributed to the reporting for this story.
A version of this article first appeared on Medscape.com.
Pregnant women can safely get vaccinated with the Pfizer-BioNTech and Moderna vaccines for COVID-19, surveillance data from the Centers for Disease Control and Prevention suggest.
More than 30,000 women who received these vaccines have reported pregnancies through the CDC’s V-Safe voluntary reporting system, and their rates of complications are not significantly different from those of unvaccinated pregnant women, said Tom Shimabukuro, MD, MPH, MBA, deputy director of the CDC Immunization Safety Office.
“Overall, the data are reassuring with respect to vaccine safety in pregnant women,” he told this news organization.
Dr. Shimabukuro presented the data during a March 1 meeting of the Advisory Committee on Immunization Practices, a group of health experts selected by the Secretary of the U.S. Department of Health & Human Services.
The CDC has included pregnancy along with other underlying conditions that qualify people to be offered vaccines in the third priority tier (Phase 1c).
“There is evidence that pregnant women who get COVID-19 are at increased risk of severe illness and complications from severe illness,” Dr. Shimabukuro explained. “And there is also evidence that pregnant persons who get COVID-19 may be at increased risk for adverse pregnancy outcomes.”
The American College of Obstetrics and Gynecology recommends that “COVID-19 vaccines should not be withheld from pregnant individuals.”
By contrast, the World Health Organization recommends the vaccines only for those pregnant women who are “at high risk of exposure to SARS-CoV-2 (for example, health workers) or who have comorbidities which add to their risk of severe disease.”
Not enough information was available from the pivotal trials of the Moderna and Pfizer vaccines to assess risk in pregnant women, according to these manufacturers. Pfizer has announced a follow-up trial of its vaccine in healthy pregnant women.
Analyzing surveillance data
To better assess whether the Pfizer or Moderna vaccines cause problems in pregnancy or childbirth, Dr. Shimabukuro and colleagues analyzed data from V-Safe and the Vaccine Adverse Event Reporting System (VAERS).
The CDC encourages providers to inform people they vaccinate about the V-Safe program. Participants can voluntarily enter their data through a website, and may receive follow-up text messages and phone calls from the CDC asking for additional information at various times after vaccination. It is not a systematic survey, and the sample is not necessarily representative of everyone who gets the vaccine, Dr. Shimabukuro noted.
At the time of the study, V-Safe recorded 55,220,364 reports from people who received at least one dose of the Pfizer or Moderna vaccine through Feb. 16. These included 30,494 pregnancies, of which 16,039 were in women who received the Pfizer vaccine and 14,455 in women who received the Moderna vaccine.
Analyzing data collected through Jan. 13, 2021, the researchers found that both local and systemic reactions were similar between pregnant and nonpregnant women aged 16-54 years.
Most women reported pain, and some reported swelling, redness, and itching at the injection site. Of systemic reactions, fatigue was the most common, followed by headache, myalgia, chills, nausea, and fever. The systemic reactions were more common with the second Pfizer dose; fatigue affected a majority of both pregnant and nonpregnant women. Data on the second Moderna dose were not available.
The CDC enrolled 1,815 pregnant women for additional follow-up, among whom there were 275 completed pregnancies and 232 live births.
Rates of outcomes “of interest” were no higher among these women than in the general population. 
In contrast to V-Safe, data from VAERS, comanaged by the CDC and U.S. Food and Drug Administration, are from spontaneous reports of adverse events. The sources for those reports are varied. “That could be the health care provider,” Dr. Shimabukuro said. “That could be the patient themselves. It could be a caregiver for children.”
Just 154 VAERS reports through Feb. 16 concerned pregnant women, and of these, only 42 (27%) were for pregnancy-specific conditions, with the other 73% representing the types of adverse events reported for the general population of vaccinated people, such as headache and fatigue.
Of the 42 pregnancy-related events, there were 29 spontaneous abortions or miscarriages, with the remainder divided among 10 other pregnancy and neonatal conditions.
“When we looked at those outcomes and we compared the reporting rates, based on known background rates of these conditions, we did not see anything unexpected or concerning with respect to pregnancy or neonatal-specific conditions,” Dr. Shimabukuro said about the VAERS data.
The CDC did not collect data on fertility. “We’ve done a lot of work with other vaccines,” said Dr. Shimabukuro. “And just from a biological basis, we don’t have any evidence that vaccination, just in general, causes fertility problems.”
Also, Dr. Shimabukuro noted that the COVID-19 vaccine made by Janssen/Johnson & Johnson did not receive emergency authorization from the FDA in time to be included in the current report, but is being tracked for future reports.
Vaccination could benefit infants
In addition to the new safety data, experts continue to remind clinicians and the public that vaccination during pregnancy could benefit offspring. The unborn babies of pregnant women who receive the COVID-19 vaccine could be protected from the virus for the first several months of their lives, said White House COVID-19 czar Anthony Fauci, MD, at a briefing on March 10.
“We’ve seen this with many other vaccines,” Dr. Fauci said. “That’s a very good way you can get protection for the mother during pregnancy and also a transfer of protection for the infant, which will last a few months following the birth.”
Dr. Fauci also noted that the same vaccine platform used in Johnson & Johnson’s COVID-19 vaccine was successfully used for Ebola in pregnant women in Africa.
Dr. Shimabukuro has reported no relevant financial relationships.
Lindsay Kalter contributed to the reporting for this story.
A version of this article first appeared on Medscape.com.
Baby born to partially vaccinated mom has COVID-19 antibodies
A baby girl who was born 3 weeks after her mom got the first dose of the Moderna COVID-19 vaccine has antibodies against the coronavirus, according to a preprint paper published on the medRxiv server Feb. 5. The paper hasn’t yet been peer reviewed.
The mom, a health care worker in Florida, developed COVID-19 antibodies after she received the shot. Testing showed that the antibodies passed through the placenta to the baby.
“Maternal vaccination for influenza and TDaP have been well studied in terms of safety and efficacy for protection of the newborn by placental passage of antibodies,” Paul Gilbert, MD, and Chad Rudnick, MD, pediatricians and researchers at Florida Atlantic University, wrote in the paper.
Previous research has indicated that moms who have recovered from COVID-19 can deliver babies with antibodies, according to Insider, but this may be the first report that shows how vaccination during pregnancy can provide antibodies as well.
Dr. Gilbert and Dr. Rudnick said they were fortunate to connect with the mom in Boca Raton. She hadn’t contracted COVID-19 and was able to get the vaccine at the end of her pregnancy in January. When the baby was born, they were able to test the cord blood to look for antibodies specifically from the vaccine.
“We were very excited to see, once the test result came back, that the antibodies from the mom’s vaccine did in fact pass through the placenta to the newborn,” Dr. Rudnick told WPTV, an NBC affiliate in West Palm Beach.
“We knew that we were going to be potentially one of the first in the world to report it, and that opportunity probably only comes once in a career,” Dr. Gilbert told WPTV.
In the preprint, Dr. Gilbert and Dr. Rudnick said a “vigorous, healthy, full-term” baby was born, and the mom received the second dose of the Moderna vaccine during the postpartum period. The newborn received a normal “well-infant” evaluation and was breastfeeding.
The two doctors called for a “significant and urgent need” to research the safety and efficacy of COVID-19 vaccines during pregnancy. They also encouraged other researchers to create pregnancy and breastfeeding registries to study COVID-19 vaccines in pregnant and breastfeeding moms and newborns.
Dr. Gilbert and Dr. Rudnick are now preparing their research for publication and hope future studies will investigate the amount and length of antibody response in newborns.
“Total antibody measurements may be used to determine how long protection is expected, which may help to determine when the best time would be to begin vaccination,” they wrote.
A version of this article first appeared on Medscape.com.
A baby girl who was born 3 weeks after her mom got the first dose of the Moderna COVID-19 vaccine has antibodies against the coronavirus, according to a preprint paper published on the medRxiv server Feb. 5. The paper hasn’t yet been peer reviewed.
The mom, a health care worker in Florida, developed COVID-19 antibodies after she received the shot. Testing showed that the antibodies passed through the placenta to the baby.
“Maternal vaccination for influenza and TDaP have been well studied in terms of safety and efficacy for protection of the newborn by placental passage of antibodies,” Paul Gilbert, MD, and Chad Rudnick, MD, pediatricians and researchers at Florida Atlantic University, wrote in the paper.
Previous research has indicated that moms who have recovered from COVID-19 can deliver babies with antibodies, according to Insider, but this may be the first report that shows how vaccination during pregnancy can provide antibodies as well.
Dr. Gilbert and Dr. Rudnick said they were fortunate to connect with the mom in Boca Raton. She hadn’t contracted COVID-19 and was able to get the vaccine at the end of her pregnancy in January. When the baby was born, they were able to test the cord blood to look for antibodies specifically from the vaccine.
“We were very excited to see, once the test result came back, that the antibodies from the mom’s vaccine did in fact pass through the placenta to the newborn,” Dr. Rudnick told WPTV, an NBC affiliate in West Palm Beach.
“We knew that we were going to be potentially one of the first in the world to report it, and that opportunity probably only comes once in a career,” Dr. Gilbert told WPTV.
In the preprint, Dr. Gilbert and Dr. Rudnick said a “vigorous, healthy, full-term” baby was born, and the mom received the second dose of the Moderna vaccine during the postpartum period. The newborn received a normal “well-infant” evaluation and was breastfeeding.
The two doctors called for a “significant and urgent need” to research the safety and efficacy of COVID-19 vaccines during pregnancy. They also encouraged other researchers to create pregnancy and breastfeeding registries to study COVID-19 vaccines in pregnant and breastfeeding moms and newborns.
Dr. Gilbert and Dr. Rudnick are now preparing their research for publication and hope future studies will investigate the amount and length of antibody response in newborns.
“Total antibody measurements may be used to determine how long protection is expected, which may help to determine when the best time would be to begin vaccination,” they wrote.
A version of this article first appeared on Medscape.com.
A baby girl who was born 3 weeks after her mom got the first dose of the Moderna COVID-19 vaccine has antibodies against the coronavirus, according to a preprint paper published on the medRxiv server Feb. 5. The paper hasn’t yet been peer reviewed.
The mom, a health care worker in Florida, developed COVID-19 antibodies after she received the shot. Testing showed that the antibodies passed through the placenta to the baby.
“Maternal vaccination for influenza and TDaP have been well studied in terms of safety and efficacy for protection of the newborn by placental passage of antibodies,” Paul Gilbert, MD, and Chad Rudnick, MD, pediatricians and researchers at Florida Atlantic University, wrote in the paper.
Previous research has indicated that moms who have recovered from COVID-19 can deliver babies with antibodies, according to Insider, but this may be the first report that shows how vaccination during pregnancy can provide antibodies as well.
Dr. Gilbert and Dr. Rudnick said they were fortunate to connect with the mom in Boca Raton. She hadn’t contracted COVID-19 and was able to get the vaccine at the end of her pregnancy in January. When the baby was born, they were able to test the cord blood to look for antibodies specifically from the vaccine.
“We were very excited to see, once the test result came back, that the antibodies from the mom’s vaccine did in fact pass through the placenta to the newborn,” Dr. Rudnick told WPTV, an NBC affiliate in West Palm Beach.
“We knew that we were going to be potentially one of the first in the world to report it, and that opportunity probably only comes once in a career,” Dr. Gilbert told WPTV.
In the preprint, Dr. Gilbert and Dr. Rudnick said a “vigorous, healthy, full-term” baby was born, and the mom received the second dose of the Moderna vaccine during the postpartum period. The newborn received a normal “well-infant” evaluation and was breastfeeding.
The two doctors called for a “significant and urgent need” to research the safety and efficacy of COVID-19 vaccines during pregnancy. They also encouraged other researchers to create pregnancy and breastfeeding registries to study COVID-19 vaccines in pregnant and breastfeeding moms and newborns.
Dr. Gilbert and Dr. Rudnick are now preparing their research for publication and hope future studies will investigate the amount and length of antibody response in newborns.
“Total antibody measurements may be used to determine how long protection is expected, which may help to determine when the best time would be to begin vaccination,” they wrote.
A version of this article first appeared on Medscape.com.
New guidelines dispel myths about COVID-19 treatment
Recommendations, as well as conspiracy theories about COVID-19, have changed at distressing rates over the past year. No disease has ever been more politicized, or more polarizing.
Experts, as well as the least educated, take a stand on what they believe is the most important way to prevent and treat this virus.
Just recently, a study was published revealing that ivermectin is not effective as a COVID-19 treatment while people continue to claim it works. It has never been more important for doctors, and especially family physicians, to have accurate and updated guidelines.
The NIH and CDC have been publishing recommendations and guidelines for the prevention and treatment of COVID-19 since the start of the pandemic. Like any new disease, these have been changing to keep up as new knowledge related to the disease becomes available.
NIH updates treatment guidelines
A recent update to the NIH COVID-19 treatment guidelines was published on March 5, 2021. While the complete guidelines are quite extensive, spanning over 200 pages, it’s most important to understand the most recent updates in them.
Since preventative medicine is an integral part of primary care, it is important to note that no medications have been advised to prevent infection with COVID-19. In fact, taking drugs for pre-exposure prophylaxis (PrEp) is not recommended even in the highest-risk patients, such as health care workers.
In the updated guidelines, tocilizumab in a single IV dose of 8 mg/kg up to a maximum of 800 mg can be given only in combination with dexamethasone (or equivalent corticosteroid) in certain hospitalized patients exhibiting rapid respiratory decompensation. These patients include recently hospitalized patients who have been admitted to the ICU within the previous 24 hours and now require mechanical ventilation or high-flow oxygen via nasal cannula. Those not in the ICU who require rapidly increasing oxygen levels and have significantly increased levels of inflammatory markers should also receive this therapy. In the new guidance, the NIH recommends treating other hospitalized patients who require oxygen with remdesivir, remdesivir + dexamethasone, or dexamethasone alone.
In outpatients, those who have mild to moderate infection and are at increased risk of developing severe disease and/or hospitalization can be treated with bamlanivimab 700 mg + etesevimab 1,400 mg. This should be started as soon as possible after a confirmed diagnosis and within 10 days of symptom onset, according to the NIH recommendations. There is no evidence to support its use in patients hospitalized because of infection. However, it can be used in patients hospitalized for other reasons who have mild to moderate infection, but should be reserved – because of limited supply – for those with the highest risk of complications.
Hydroxychloroquine and casirivimab + imdevimab
One medication that has been touted in the media as a tool to treat COVID-19 has been hydroxychloroquine. Past guidelines recommended against this medication as a treatment because it lacked efficacy and posed risks for no therapeutic benefit. The most recent guidelines also recommend against the use of hydroxychloroquine for pre- and postexposure prophylaxis.
Casirivimab + imdevimab has been another talked about therapy. However, current guidelines recommend against its use in hospitalized patients. In addition, it is advised that hospitalized patients be enrolled in a clinical trial to receive it.
Since the pandemic began, the world has seen more than 120 million infections and more than 2 million deaths. Family physicians have a vital role to play as we are often the first ones patients turn to for treatment and advice. It is imperative we stay current with the guidelines and follow the most recent updates as research data are published.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Recommendations, as well as conspiracy theories about COVID-19, have changed at distressing rates over the past year. No disease has ever been more politicized, or more polarizing.
Experts, as well as the least educated, take a stand on what they believe is the most important way to prevent and treat this virus.
Just recently, a study was published revealing that ivermectin is not effective as a COVID-19 treatment while people continue to claim it works. It has never been more important for doctors, and especially family physicians, to have accurate and updated guidelines.
The NIH and CDC have been publishing recommendations and guidelines for the prevention and treatment of COVID-19 since the start of the pandemic. Like any new disease, these have been changing to keep up as new knowledge related to the disease becomes available.
NIH updates treatment guidelines
A recent update to the NIH COVID-19 treatment guidelines was published on March 5, 2021. While the complete guidelines are quite extensive, spanning over 200 pages, it’s most important to understand the most recent updates in them.
Since preventative medicine is an integral part of primary care, it is important to note that no medications have been advised to prevent infection with COVID-19. In fact, taking drugs for pre-exposure prophylaxis (PrEp) is not recommended even in the highest-risk patients, such as health care workers.
In the updated guidelines, tocilizumab in a single IV dose of 8 mg/kg up to a maximum of 800 mg can be given only in combination with dexamethasone (or equivalent corticosteroid) in certain hospitalized patients exhibiting rapid respiratory decompensation. These patients include recently hospitalized patients who have been admitted to the ICU within the previous 24 hours and now require mechanical ventilation or high-flow oxygen via nasal cannula. Those not in the ICU who require rapidly increasing oxygen levels and have significantly increased levels of inflammatory markers should also receive this therapy. In the new guidance, the NIH recommends treating other hospitalized patients who require oxygen with remdesivir, remdesivir + dexamethasone, or dexamethasone alone.
In outpatients, those who have mild to moderate infection and are at increased risk of developing severe disease and/or hospitalization can be treated with bamlanivimab 700 mg + etesevimab 1,400 mg. This should be started as soon as possible after a confirmed diagnosis and within 10 days of symptom onset, according to the NIH recommendations. There is no evidence to support its use in patients hospitalized because of infection. However, it can be used in patients hospitalized for other reasons who have mild to moderate infection, but should be reserved – because of limited supply – for those with the highest risk of complications.
Hydroxychloroquine and casirivimab + imdevimab
One medication that has been touted in the media as a tool to treat COVID-19 has been hydroxychloroquine. Past guidelines recommended against this medication as a treatment because it lacked efficacy and posed risks for no therapeutic benefit. The most recent guidelines also recommend against the use of hydroxychloroquine for pre- and postexposure prophylaxis.
Casirivimab + imdevimab has been another talked about therapy. However, current guidelines recommend against its use in hospitalized patients. In addition, it is advised that hospitalized patients be enrolled in a clinical trial to receive it.
Since the pandemic began, the world has seen more than 120 million infections and more than 2 million deaths. Family physicians have a vital role to play as we are often the first ones patients turn to for treatment and advice. It is imperative we stay current with the guidelines and follow the most recent updates as research data are published.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Recommendations, as well as conspiracy theories about COVID-19, have changed at distressing rates over the past year. No disease has ever been more politicized, or more polarizing.
Experts, as well as the least educated, take a stand on what they believe is the most important way to prevent and treat this virus.
Just recently, a study was published revealing that ivermectin is not effective as a COVID-19 treatment while people continue to claim it works. It has never been more important for doctors, and especially family physicians, to have accurate and updated guidelines.
The NIH and CDC have been publishing recommendations and guidelines for the prevention and treatment of COVID-19 since the start of the pandemic. Like any new disease, these have been changing to keep up as new knowledge related to the disease becomes available.
NIH updates treatment guidelines
A recent update to the NIH COVID-19 treatment guidelines was published on March 5, 2021. While the complete guidelines are quite extensive, spanning over 200 pages, it’s most important to understand the most recent updates in them.
Since preventative medicine is an integral part of primary care, it is important to note that no medications have been advised to prevent infection with COVID-19. In fact, taking drugs for pre-exposure prophylaxis (PrEp) is not recommended even in the highest-risk patients, such as health care workers.
In the updated guidelines, tocilizumab in a single IV dose of 8 mg/kg up to a maximum of 800 mg can be given only in combination with dexamethasone (or equivalent corticosteroid) in certain hospitalized patients exhibiting rapid respiratory decompensation. These patients include recently hospitalized patients who have been admitted to the ICU within the previous 24 hours and now require mechanical ventilation or high-flow oxygen via nasal cannula. Those not in the ICU who require rapidly increasing oxygen levels and have significantly increased levels of inflammatory markers should also receive this therapy. In the new guidance, the NIH recommends treating other hospitalized patients who require oxygen with remdesivir, remdesivir + dexamethasone, or dexamethasone alone.
In outpatients, those who have mild to moderate infection and are at increased risk of developing severe disease and/or hospitalization can be treated with bamlanivimab 700 mg + etesevimab 1,400 mg. This should be started as soon as possible after a confirmed diagnosis and within 10 days of symptom onset, according to the NIH recommendations. There is no evidence to support its use in patients hospitalized because of infection. However, it can be used in patients hospitalized for other reasons who have mild to moderate infection, but should be reserved – because of limited supply – for those with the highest risk of complications.
Hydroxychloroquine and casirivimab + imdevimab
One medication that has been touted in the media as a tool to treat COVID-19 has been hydroxychloroquine. Past guidelines recommended against this medication as a treatment because it lacked efficacy and posed risks for no therapeutic benefit. The most recent guidelines also recommend against the use of hydroxychloroquine for pre- and postexposure prophylaxis.
Casirivimab + imdevimab has been another talked about therapy. However, current guidelines recommend against its use in hospitalized patients. In addition, it is advised that hospitalized patients be enrolled in a clinical trial to receive it.
Since the pandemic began, the world has seen more than 120 million infections and more than 2 million deaths. Family physicians have a vital role to play as we are often the first ones patients turn to for treatment and advice. It is imperative we stay current with the guidelines and follow the most recent updates as research data are published.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].