User login
Antimicrobial, pH-modulating gel shows promise in preventing common STIs
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Cutaneous Manifestations of COVID-19: Characteristics, Pathogenesis, and the Role of Dermatology in the Pandemic
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
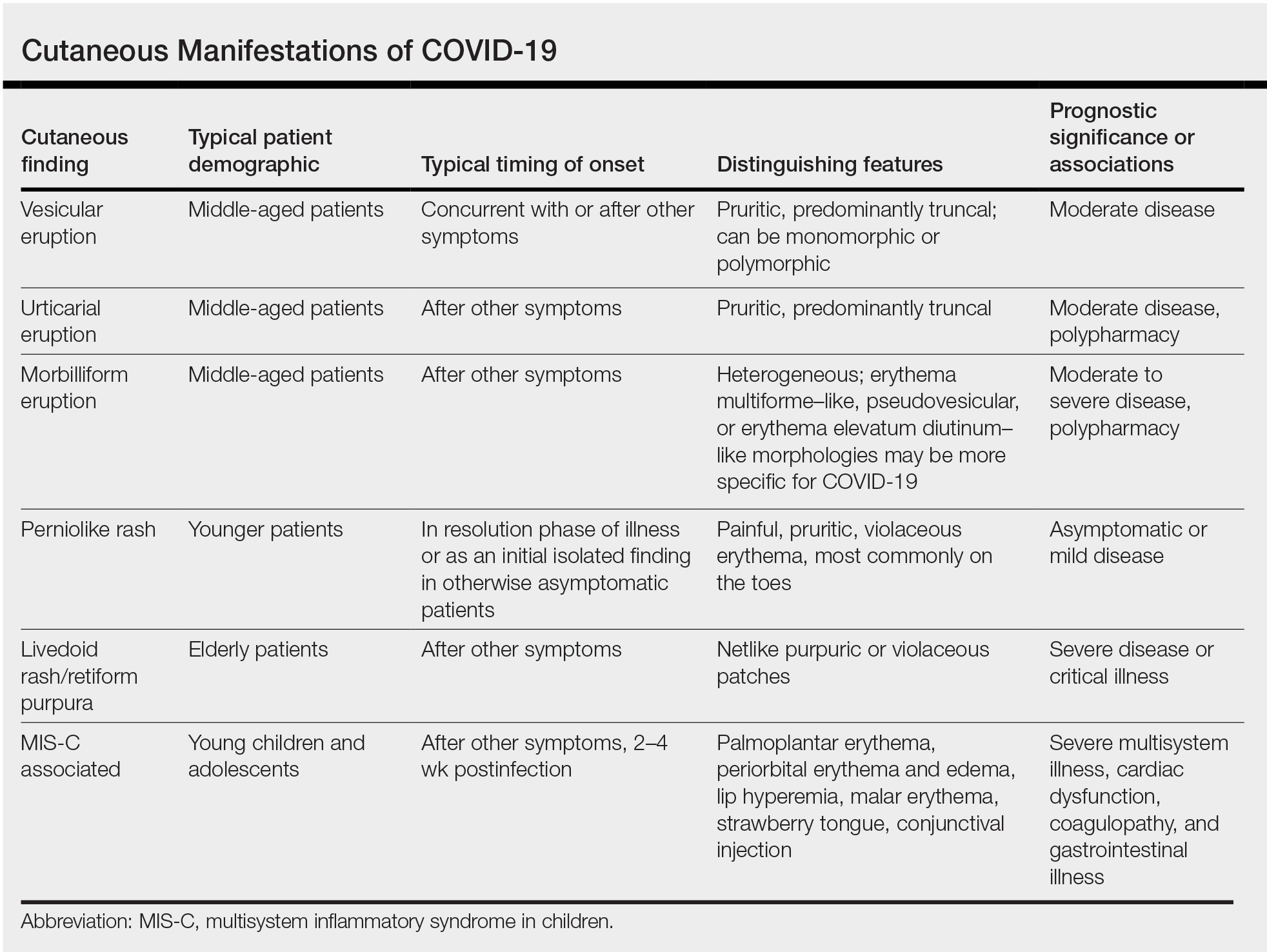
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
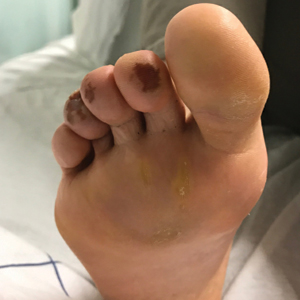
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
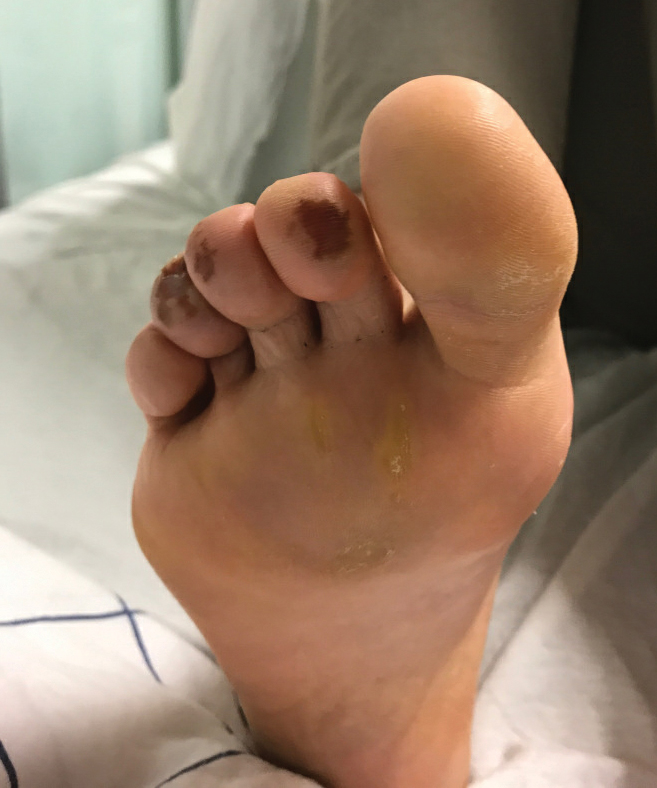
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
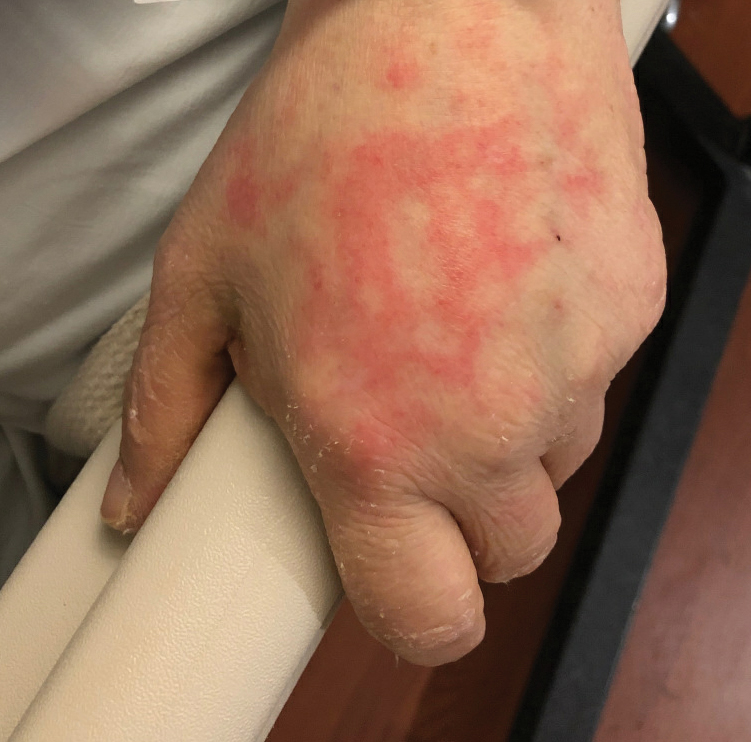
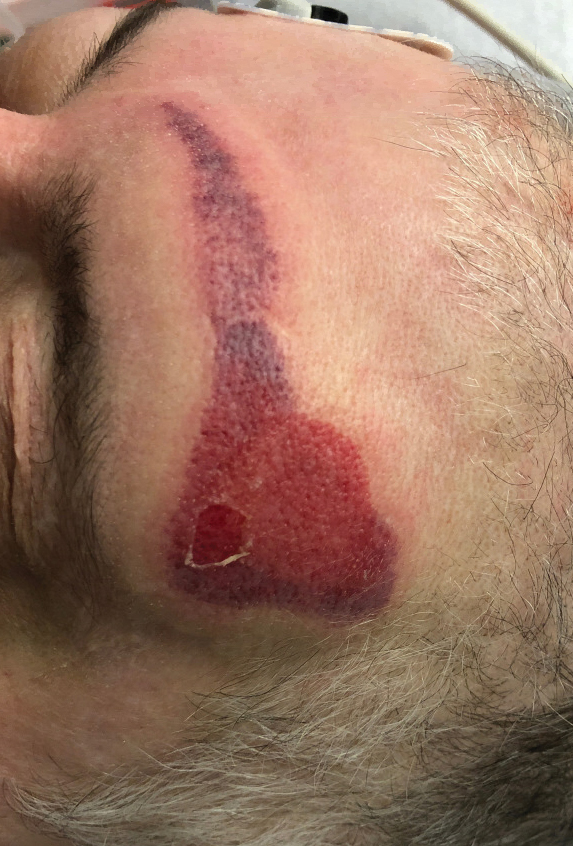
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18

Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25

Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14


Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18

Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25

Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14


Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
Practice Points
- Cutaneous manifestations of COVID-19 may reflect the range of host immunologic responses to SARS-CoV-2.
- Perniosis appears to be a late manifestation of COVID-19 associated with a comparatively benign disease course, whereas livedoid or other vasculopathic lesions portend poorer outcomes and may warrant further workup for occult thrombotic disease.
- Maculopapular, vesicular, and urticarial eruptions may be seen in association with COVID-19 but are nonspecific and necessitate a broad differential and workup.
- Challenges posed by the COVID-19 pandemic necessitate creative management strategies for immunosuppression and clinical assessment.
New guidelines on antibiotic prescribing focus on shorter courses
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antibiotic course of 5 days is usually just as effective as longer courses but with fewer side effects and decreased overall antibiotic exposure for a number of common bacterial conditions, according to new clinical guidelines published by the American College of Physicians.
The guidelines focus on treatment of uncomplicated cases involving pneumonia, urinary tract infections (UTIs), cellulitis, chronic obstructive pulmonary disease (COPD) exacerbations, and acute bronchitis. The goal of the guidelines is to continue improving antibiotic stewardship given the increasing threat of antibiotic resistance and the adverse effects of antibiotics.
“Any use of antibiotics (including necessary use) has downstream effects outside of treating infection,” Dawn Nolt, MD, MPH, a professor of pediatric infection disease at Oregon Health & Science University, Portland, said in an interview. Dr. Nolt was not involved in developing these guidelines. “Undesirable outcomes include allergic reactions, diarrhea, and antibiotic-resistant bacteria. When we reduce unnecessary antibiotic, we reduce undesirable outcomes,” she said.
According to background information in the paper, 1 in 10 patients receives an antibiotic prescription during visits, yet nearly a third of these (30%) are unnecessary and last too long, especially for sinusitis and bronchitis. Meanwhile, overuse of antibiotics, particularly broad-spectrum ones, leads to resistance and adverse effects in up to 20% of patients.
“Prescribing practices can vary based on the type of provider, the setting where the antibiotic is being prescribed, what geographic area you are looking at, the medical reason for which the antibiotic is being prescribed, the actual germ being targeted, and the type of patient,” Dr. Nolt said. “But this variability can be reduced when prescribing providers are aware and follow best practice standards as through this article.”
The new ACP guidelines are a distillation of recommendations from preexisting infectious disease organizations, Dr. Nolt said, but aimed specifically at those practicing internal medicine.
“We define appropriate antibiotic use as prescribing the right antibiotic at the right dose for the right duration for a specific condition,” Rachael A. Lee, MD, MSPH, of the University of Alabama at Birmingham, and colleagues wrote in the article detailing the new guidelines. “Despite evidence and guidelines supporting shorter durations of antibiotic use, many physicians do not prescribe short-course therapy, frequently defaulting to 10-day courses regardless of the condition.”
The reasons for this default response vary. Though some clinicians prescribe longer courses specifically to prevent antibiotic resistance, no evidence shows that continuing to take antibiotics after symptoms have resolved actually reduces likelihood of resistance, the authors noted.
“In fact, resistance is a documented side effect of prolonged antibiotic use due to natural selection pressure,” they wrote.
Another common reason is habit.
“This was the ‘conventional wisdom’ for so long, just trying to make sure all bacteria causing the infection were completely eradicated, with no stragglers that had been exposed to the antibiotic but were not gone and now could evolve into resistant organisms,” Jacqueline W. Fincher, MD, a primary care physician and president of the ACP, said in an interview. “While antibiotic stewardship has been very important for over a decade, we now have more recent head-to-head studies/data showing that, in these four conditions, shorter courses of treatment are just as efficacious with less side effects and adverse events.”
The researchers reviewed all existing clinical guidelines related to bronchitis with COPD exacerbations, community-acquired pneumonia, UTIs, and cellulitis, as well as any other relevant studies in the literature. Although they did not conduct a formal systematic review, they compiled the guidelines specifically for all internists, family physicians and other clinicians caring for patients with these conditions.
“Although most patients with these infections will be seen in the outpatient setting, these best-practice advice statements also apply to patients who present in the inpatient setting,” the authors wrote. They also note the importance of ensuring the patient has the correct diagnosis and appropriate corresponding antibiotic prescription. “If a patient is not improving with appropriate antibiotics, it is important for the clinician to reassess for other causes of symptoms rather than defaulting to a longer duration of antibiotic therapy,” they wrote, calling a longer course “the exception and not the rule.”
Acute bronchitis with COPD exacerbations
Antibiotic treatment for COPD exacerbations and acute uncomplicated bronchitis with signs of a bacterial infection should last no longer than 5 days. The authors define this condition as an acute respiratory infection with a normal chest x-ray, most often caused by a virus. Although patients with bronchitis do not automatically need antibiotics if there’s no evidence of pneumonia, the authors did advise antibiotics in cases involving COPD and a high likelihood of bacterial infection. Clinicians should base their choice of antibiotics on the most common bacterial etiology: Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Ideal candidates for therapy may include aminopenicillin with clavulanic acid, a macrolide, or a tetracycline.
Community-acquired pneumonia
The initial course of antibiotics should be at least 5 days for pneumonia and only extended after considering validated evidence of the patient’s clinical stability, such as resuming normal vital signs, mental activity, and the ability to eat. Multiple randomized, controlled trials have shown no improved benefit from longer courses, though longer courses are linked to increased adverse events and mortality.
Again, antibiotics used should “cover common pathogens, such as S. pneumoniae, H. influenzae, Mycoplasma pneumoniae, and Staphylococcus aureus, and atypical pathogens, such as Legionella species,” the authors wrote. Options include “amoxicillin, doxycycline, or a macrolide for healthy adults or a beta-lactam with a macrolide or a respiratory fluoroquinolone in patients with comorbidities.”
UTIs: Uncomplicated cystitis and pyelonephritis
For women’s bacterial cystitis – 75% of which is caused by Escherichia coli – the guidelines recommend nitrofurantoin for 5 days, trimethoprim-sulfamethoxazole for 3 days, or fosfomycin as a single dose. For uncomplicated pyelonephritis in both men and women, clinicians can consider fluoroquinolones for 5-7 days or trimethoprim-sulfamethoxazole for 14 days, depending on antibiotic susceptibility.
This recommendation does not include UTIs in women who are pregnant or UTIs with other functional abnormalities present, such as obstruction. The authors also intentionally left out acute bacterial prostatitis because of its complexity and how long it can take to treat.
Cellulitis
MRSA, which has been increasing in prevalence, is a leading cause of skin and soft-tissue infections, such as necrotizing infections, cellulitis, and erysipelas. Unless the patient has penetrating trauma, evidence of MRSA infection elsewhere, injection drug use, nasal colonization of MRSA, or systemic inflammatory response syndrome, the guidelines recommend a 5- to 6-day course of cephalosporin, penicillin, or clindamycin, extended only if the infection has not improved in 5 days. Further research can narrow down the most appropriate treatment course.
This guidance does not apply to purulent cellulitis, such as conditions with abscesses, furuncles, or carbuncles that typically require incision and drainage.
Continuing to get the message out
Dr. Fincher emphasized the importance of continuing to disseminate messaging for clinicians about reducing unnecessary antibiotic use.
“In medicine we are constantly bombarded with new information. It is those patients and disease states that we see and treat every day that are especially important for us as physicians and other clinicians to keep our skills and knowledge base up to date when it comes to use of antibiotics,” Dr. Fincher said in an interview. “We just need to continue to educate and push out the data, guidelines, and recommendations.”
Dr. Nolt added that it’s important to emphasize how to translate these national recommendations into local practices since local guidance can also raise awareness and encourage local compliance.
Other strategies for reducing overuse of antibiotics “include restriction on antibiotics available at health care systems (formulary restriction), not allowing use of antibiotics unless there is discussion about the patient’s case (preauthorization), and reviewing cases of patients on antibiotics and advising on next steps (prospective audit and feedback),” she said.
The research was funded by the ACP. Dr. Lee has received personal fees from this news organization and Prime Education. Dr. Fincher owns stock in Johnson & Johnson and Procter and Gamble. Dr. Nolt and the article’s coauthors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 in children: New cases back on the decline
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
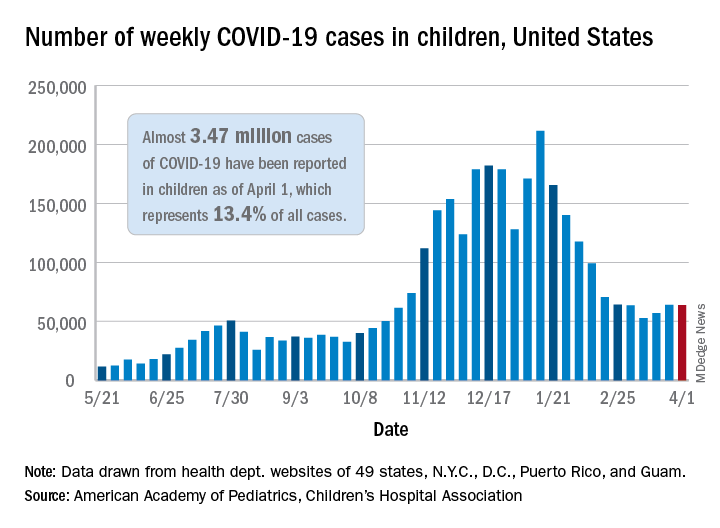
the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
Children likely the ‘leading edge’ in spread of COVID-19 variants
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
COVID-19 in 2020: Deaths and disparities
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.
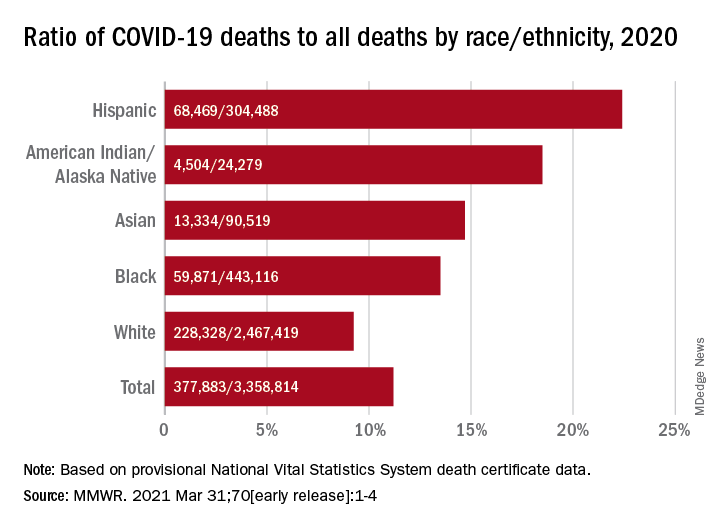
Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
FROM MMWR
Children could become eligible for a COVID-19 vaccine by fall, expert predicts
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
FROM THE SPD PRE-AAD MEETING
New guidelines on the diagnosis and treatment of adults with CAP
Background: More than a decade has passed since the last CAP guidelines. Since then there have been new trials and epidemiological studies. There have also been changes to the process for guideline development. This guideline has moved away from the narrative style of guidelines to the GRADE format and PICO framework with hopes of answering specific questions by looking at the quality of evidence.
Study design: Multidisciplinary panel conducted pragmatic systemic reviews of high-quality studies.
Setting: The panel revised and built upon the 2007 guidelines, addressing 16 clinical questions to be used in immunocompetent patients with radiographic evidence of CAP in the United States with no recent foreign travel.
Synopsis: Changes from the 2007 guidelines are as follows: Sputum and blood cultures, previously recommended only in patients with severe CAP, are now also recommended for inpatients being empirically treated for Pseudomonas or methicillin-resistant Staphylococcus aureus (MRSA) and for those who have received IV antibiotics in the previous 90 days; use of procalcitonin is not recommended to decide whether to withhold antibiotics; steroids are not recommended unless being used for shock; HCAP categorization should be abandoned and need for empiric coverage of MRSA and Pseudomonas should be based on local epidemiology and local validated risk factors; B-lactam/macrolide is favored over fluoroquinolone for severe CAP therapy; and routine follow-up chest x-ray is not recommended.
Other recommendations include not routinely testing for urine pneumococcal or legionella antigens in nonsevere CAP; using PSI over CURB-65, in addition to clinical judgment, to determine need for inpatient care; using severe CAP criteria and clinical judgment for determining ICU need; not adding anaerobic coverage for aspiration pneumonia; and treating most cases of CAP that are clinically stable and uncomplicated for 5-7 days.
Bottom line: Given new data, updated recommendations have been made to help optimize CAP therapy.
Citation: Metlay JP et al. Diagnosis and treatment of adults with community-acquired pneumonia: An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67.
Dr. Horton is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: More than a decade has passed since the last CAP guidelines. Since then there have been new trials and epidemiological studies. There have also been changes to the process for guideline development. This guideline has moved away from the narrative style of guidelines to the GRADE format and PICO framework with hopes of answering specific questions by looking at the quality of evidence.
Study design: Multidisciplinary panel conducted pragmatic systemic reviews of high-quality studies.
Setting: The panel revised and built upon the 2007 guidelines, addressing 16 clinical questions to be used in immunocompetent patients with radiographic evidence of CAP in the United States with no recent foreign travel.
Synopsis: Changes from the 2007 guidelines are as follows: Sputum and blood cultures, previously recommended only in patients with severe CAP, are now also recommended for inpatients being empirically treated for Pseudomonas or methicillin-resistant Staphylococcus aureus (MRSA) and for those who have received IV antibiotics in the previous 90 days; use of procalcitonin is not recommended to decide whether to withhold antibiotics; steroids are not recommended unless being used for shock; HCAP categorization should be abandoned and need for empiric coverage of MRSA and Pseudomonas should be based on local epidemiology and local validated risk factors; B-lactam/macrolide is favored over fluoroquinolone for severe CAP therapy; and routine follow-up chest x-ray is not recommended.
Other recommendations include not routinely testing for urine pneumococcal or legionella antigens in nonsevere CAP; using PSI over CURB-65, in addition to clinical judgment, to determine need for inpatient care; using severe CAP criteria and clinical judgment for determining ICU need; not adding anaerobic coverage for aspiration pneumonia; and treating most cases of CAP that are clinically stable and uncomplicated for 5-7 days.
Bottom line: Given new data, updated recommendations have been made to help optimize CAP therapy.
Citation: Metlay JP et al. Diagnosis and treatment of adults with community-acquired pneumonia: An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67.
Dr. Horton is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: More than a decade has passed since the last CAP guidelines. Since then there have been new trials and epidemiological studies. There have also been changes to the process for guideline development. This guideline has moved away from the narrative style of guidelines to the GRADE format and PICO framework with hopes of answering specific questions by looking at the quality of evidence.
Study design: Multidisciplinary panel conducted pragmatic systemic reviews of high-quality studies.
Setting: The panel revised and built upon the 2007 guidelines, addressing 16 clinical questions to be used in immunocompetent patients with radiographic evidence of CAP in the United States with no recent foreign travel.
Synopsis: Changes from the 2007 guidelines are as follows: Sputum and blood cultures, previously recommended only in patients with severe CAP, are now also recommended for inpatients being empirically treated for Pseudomonas or methicillin-resistant Staphylococcus aureus (MRSA) and for those who have received IV antibiotics in the previous 90 days; use of procalcitonin is not recommended to decide whether to withhold antibiotics; steroids are not recommended unless being used for shock; HCAP categorization should be abandoned and need for empiric coverage of MRSA and Pseudomonas should be based on local epidemiology and local validated risk factors; B-lactam/macrolide is favored over fluoroquinolone for severe CAP therapy; and routine follow-up chest x-ray is not recommended.
Other recommendations include not routinely testing for urine pneumococcal or legionella antigens in nonsevere CAP; using PSI over CURB-65, in addition to clinical judgment, to determine need for inpatient care; using severe CAP criteria and clinical judgment for determining ICU need; not adding anaerobic coverage for aspiration pneumonia; and treating most cases of CAP that are clinically stable and uncomplicated for 5-7 days.
Bottom line: Given new data, updated recommendations have been made to help optimize CAP therapy.
Citation: Metlay JP et al. Diagnosis and treatment of adults with community-acquired pneumonia: An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67.
Dr. Horton is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
New COVID-19 cases rise again in children
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
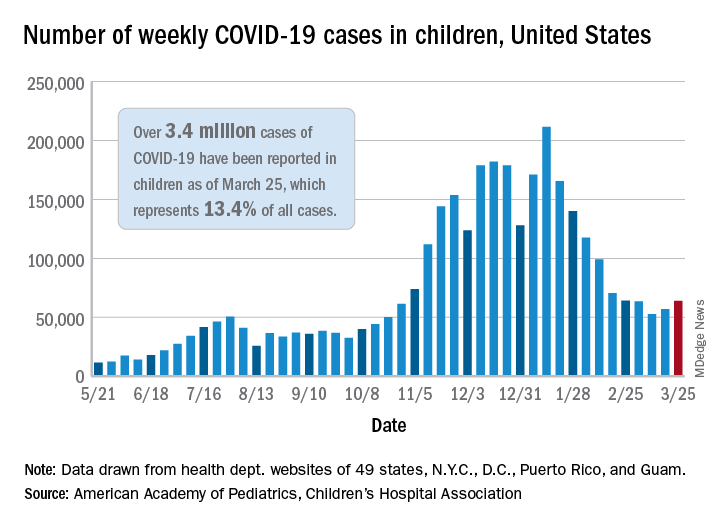
That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
The number of new COVID-19 cases in children increased for the second consecutive week in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That brings the number of children infected with the coronavirus to over 3.4 million since the beginning of the pandemic, or 13.4% of all reported cases, the AAP and CHA said in their weekly COVID-19 report.
For just the week of March 19-25, however, the proportion of all cases occurring in children was quite a bit higher, 19.1%. That’s higher than at any other point during the pandemic, passing the previous high of 18.7% set just a week earlier, based on the data collected by AAP/CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The national infection rate was 4,525 cases per 100,000 children for the week of March 19-25, compared with 4,440 per 100,000 the previous week. States falling the farthest from that national mark were Hawaii at 1,101 per 100,000 and North Dakota at 8,848, the AAP and CHA said.
There was double-digit increase, 11, in the number of child deaths, as the total went from 268 to 279 despite Virginia’s revising its mortality data downward. The mortality rate for children remains 0.01%, and children represent only 0.06% of all COVID-19–related deaths in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting deaths by age, the report shows.
The state/local-level data show that Texas has the highest number of child deaths (48), followed by Arizona (26), New York City (22), California (16), and Illinois (16), while nine states and the District of Columbia have not yet reported a death, the AAP and CHA said.
New NAS report seeks to modernize STI paradigm
Approximately 68 million cases of sexually transmitted infections are reported in the United States each year, yet antiquated approaches to STI prevention, in addition to health care inequities and lack of funding, have substantially prevented providers and officials from curbing the spread. In response to rising case numbers, the National Academies of Sciences, Engineering, and Medicine released a report this week with recommendations to modernize the nation’s STI surveillance and monitoring systems, increase the capabilities of the STI workforce, and address structural barriers to STI prevention and access to care.
Given the rising rates of STIs and the urgent, unmet need for prevention and treatment, the Centers for Disease Control and Prevention’s National Association of County and City Health Officials commissioned the National Academies to develop actionable recommendations to control STIs. The new report marks a long road toward the public’s willingness to discuss STDs, or what a 1997 Institute of Medicine report described as a “hidden epidemic” that had been largely neglected in public discourse.
Jeffrey Crowley, MPH, committee member and an author of the new National Academies report, said in an interview that, despite the increased openness to discuss STIs in today’s society, STD rates since the late 1990s have gotten much worse. Lack of appropriate governmental funding for research and drug development, structural inequities, and persisting stigmatization are key drivers for rising rates, explained Mr. Crowley.
Addressing structural barriers to STI prevention
Playing a prominent role in the National Academies report are issues of structural and institutional barriers to STI prevention and care. In the report, the authors argued that a policy-based approach should seek to promote sexual health and eliminate structural racism and inequities to drive improvements in STI management.
“We think it’s these structural factors that are central to all the inequities that play out,” said Mr. Crowley, “and they either don’t get any attention or, if they do get attention, people don’t really speak concretely enough about how we address them.”
The concrete steps, as outlined in the report, begin with addressing factors that involve the health care industry at large. Automatic STI screening as part of routine visits, alerts in electronic health records that remind clinicians to screen patients, and reminders to test patients can be initial low-cost actions health care systems can take to improve STI testing, particularly in marginalized communities. Mr. Crowley noted that greater evidence is needed to support further steps to address structural factors that contribute to barriers in STI screening and treatment access.
Given the complexities inherent in structural barriers to STI care, the report calls on a whole-government response, in partnership with affected communities, to normalize discussions involving sexual well-being. “We have to ask ourselves how we can build healthier communities and how can we integrate sexual health into that dialogue in a way that improves our response to STI prevention and control,” Mr. Crowley explained.
Harnessing AI and dating apps
The report also addresses the power of artificial intelligence to predict STI rates and to discover trends in risk factors, both of which may improve STI surveillance and assist in the development of tailored interventions. The report calls for policy that will enable companies and the government to capitalize on AI to evaluate large collections of data in EHRs, insurance claims databases, social media, search engines, and even dating apps.
In particular, dating apps could be an avenue through which the public and private sectors could improve STI prevention, diagnosis, and treatment. “People want to focus on this idea of whether these apps increase transmission risk,” said Mr. Crowley. “But we would say that this is asking the wrong question, because these technologies are not going away.” He noted that private and public enterprises could work together to leverage these technologies to increase awareness of prevention and testing.
Unifying the STI/HIV and COVID-19 workforce
The report also recommends that the nation unify the STI/HIV workforce with the COVID-19 workforce. Given the high levels of expertise in these professional working groups, the report suggests unification could potentially address both the current crisis and possible future disease outbreaks. Combining COVID-19 response teams with underresourced STI/HIV programs may also improve the delivery of STI testing, considering that STI testing programs have had to compete for resources during the pandemic.
Addressing stigma
The National Academies report also addresses the ongoing issue of stigma, which results from “blaming” individuals and the choices they make so as to create shame, embarrassment, and discrimination. Because of stigma, sexually active people may be unwilling to seek recommended screening, which can lead to delays in diagnosis and treatment and can increase the risk for negative health outcomes.
“As a nation, we’ve almost focused too intently on individual-level factors in a way that’s driven stigma and really hasn’t been helpful for combating the problem,” said Mr. Crowley. He added that, instead of focusing solely on individual-level choices, the nation should instead work to reframe sexual health as a key aspect of overall physical, mental, and emotional well-being. Doing so could create more opportunities to address structural barriers to STI prevention and ensure that more prevention and screening services are available in stigma-free environments.
“I know what we’re recommending is ambitious, but it’s not too big to be achieved, and we’re not saying tomorrow we’re going to transform the world,” Mr. Crowley concluded. “It’s a puzzle with many pieces, but the long-term impact is really all of these pieces fitting together so that, over time, we can reduce the burden STIs have on the population.”
Implications for real-world change
H. Hunter Handsfield, MD, professor emeritus of medicine for the Center for AIDS and STD at the University of Washington, Seattle, said in an interview that this report essentially is a response to evolving societal changes, new and emerging means of social engagement, and increased focus on racial/ethnic disparities. “These features have all come to the forefront of health care and general policy discussions in recent years,” said Dr. Handsfield, who was not part of the committee that developed the NAS report.
Greater scrutiny on public health infrastructure and its relationship with health disparities in the United States makes the publication of these new recommendations especially appropriate during this era of enhanced focus on social justice. Although the report features the tone and quality needed to bolster bipartisan support, said Dr. Handsfield, it’s hard to predict whether such support will come to fruition in today’s political environment.
In terms of the effects the recommendations may have on STI rates, Dr. Handsfield noted that cherry-picking elements from the report to direct policy may result in its having only a trivial impact. “The report is really an appropriate and necessary response, and almost all the recommendations made can be helpful,” he said, “but for true effectiveness, all the elements need to be implemented to drive policy and funding.”
A version of this article first appeared on Medscape.com.
Approximately 68 million cases of sexually transmitted infections are reported in the United States each year, yet antiquated approaches to STI prevention, in addition to health care inequities and lack of funding, have substantially prevented providers and officials from curbing the spread. In response to rising case numbers, the National Academies of Sciences, Engineering, and Medicine released a report this week with recommendations to modernize the nation’s STI surveillance and monitoring systems, increase the capabilities of the STI workforce, and address structural barriers to STI prevention and access to care.
Given the rising rates of STIs and the urgent, unmet need for prevention and treatment, the Centers for Disease Control and Prevention’s National Association of County and City Health Officials commissioned the National Academies to develop actionable recommendations to control STIs. The new report marks a long road toward the public’s willingness to discuss STDs, or what a 1997 Institute of Medicine report described as a “hidden epidemic” that had been largely neglected in public discourse.
Jeffrey Crowley, MPH, committee member and an author of the new National Academies report, said in an interview that, despite the increased openness to discuss STIs in today’s society, STD rates since the late 1990s have gotten much worse. Lack of appropriate governmental funding for research and drug development, structural inequities, and persisting stigmatization are key drivers for rising rates, explained Mr. Crowley.
Addressing structural barriers to STI prevention
Playing a prominent role in the National Academies report are issues of structural and institutional barriers to STI prevention and care. In the report, the authors argued that a policy-based approach should seek to promote sexual health and eliminate structural racism and inequities to drive improvements in STI management.
“We think it’s these structural factors that are central to all the inequities that play out,” said Mr. Crowley, “and they either don’t get any attention or, if they do get attention, people don’t really speak concretely enough about how we address them.”
The concrete steps, as outlined in the report, begin with addressing factors that involve the health care industry at large. Automatic STI screening as part of routine visits, alerts in electronic health records that remind clinicians to screen patients, and reminders to test patients can be initial low-cost actions health care systems can take to improve STI testing, particularly in marginalized communities. Mr. Crowley noted that greater evidence is needed to support further steps to address structural factors that contribute to barriers in STI screening and treatment access.
Given the complexities inherent in structural barriers to STI care, the report calls on a whole-government response, in partnership with affected communities, to normalize discussions involving sexual well-being. “We have to ask ourselves how we can build healthier communities and how can we integrate sexual health into that dialogue in a way that improves our response to STI prevention and control,” Mr. Crowley explained.
Harnessing AI and dating apps
The report also addresses the power of artificial intelligence to predict STI rates and to discover trends in risk factors, both of which may improve STI surveillance and assist in the development of tailored interventions. The report calls for policy that will enable companies and the government to capitalize on AI to evaluate large collections of data in EHRs, insurance claims databases, social media, search engines, and even dating apps.
In particular, dating apps could be an avenue through which the public and private sectors could improve STI prevention, diagnosis, and treatment. “People want to focus on this idea of whether these apps increase transmission risk,” said Mr. Crowley. “But we would say that this is asking the wrong question, because these technologies are not going away.” He noted that private and public enterprises could work together to leverage these technologies to increase awareness of prevention and testing.
Unifying the STI/HIV and COVID-19 workforce
The report also recommends that the nation unify the STI/HIV workforce with the COVID-19 workforce. Given the high levels of expertise in these professional working groups, the report suggests unification could potentially address both the current crisis and possible future disease outbreaks. Combining COVID-19 response teams with underresourced STI/HIV programs may also improve the delivery of STI testing, considering that STI testing programs have had to compete for resources during the pandemic.
Addressing stigma
The National Academies report also addresses the ongoing issue of stigma, which results from “blaming” individuals and the choices they make so as to create shame, embarrassment, and discrimination. Because of stigma, sexually active people may be unwilling to seek recommended screening, which can lead to delays in diagnosis and treatment and can increase the risk for negative health outcomes.
“As a nation, we’ve almost focused too intently on individual-level factors in a way that’s driven stigma and really hasn’t been helpful for combating the problem,” said Mr. Crowley. He added that, instead of focusing solely on individual-level choices, the nation should instead work to reframe sexual health as a key aspect of overall physical, mental, and emotional well-being. Doing so could create more opportunities to address structural barriers to STI prevention and ensure that more prevention and screening services are available in stigma-free environments.
“I know what we’re recommending is ambitious, but it’s not too big to be achieved, and we’re not saying tomorrow we’re going to transform the world,” Mr. Crowley concluded. “It’s a puzzle with many pieces, but the long-term impact is really all of these pieces fitting together so that, over time, we can reduce the burden STIs have on the population.”
Implications for real-world change
H. Hunter Handsfield, MD, professor emeritus of medicine for the Center for AIDS and STD at the University of Washington, Seattle, said in an interview that this report essentially is a response to evolving societal changes, new and emerging means of social engagement, and increased focus on racial/ethnic disparities. “These features have all come to the forefront of health care and general policy discussions in recent years,” said Dr. Handsfield, who was not part of the committee that developed the NAS report.
Greater scrutiny on public health infrastructure and its relationship with health disparities in the United States makes the publication of these new recommendations especially appropriate during this era of enhanced focus on social justice. Although the report features the tone and quality needed to bolster bipartisan support, said Dr. Handsfield, it’s hard to predict whether such support will come to fruition in today’s political environment.
In terms of the effects the recommendations may have on STI rates, Dr. Handsfield noted that cherry-picking elements from the report to direct policy may result in its having only a trivial impact. “The report is really an appropriate and necessary response, and almost all the recommendations made can be helpful,” he said, “but for true effectiveness, all the elements need to be implemented to drive policy and funding.”
A version of this article first appeared on Medscape.com.
Approximately 68 million cases of sexually transmitted infections are reported in the United States each year, yet antiquated approaches to STI prevention, in addition to health care inequities and lack of funding, have substantially prevented providers and officials from curbing the spread. In response to rising case numbers, the National Academies of Sciences, Engineering, and Medicine released a report this week with recommendations to modernize the nation’s STI surveillance and monitoring systems, increase the capabilities of the STI workforce, and address structural barriers to STI prevention and access to care.
Given the rising rates of STIs and the urgent, unmet need for prevention and treatment, the Centers for Disease Control and Prevention’s National Association of County and City Health Officials commissioned the National Academies to develop actionable recommendations to control STIs. The new report marks a long road toward the public’s willingness to discuss STDs, or what a 1997 Institute of Medicine report described as a “hidden epidemic” that had been largely neglected in public discourse.
Jeffrey Crowley, MPH, committee member and an author of the new National Academies report, said in an interview that, despite the increased openness to discuss STIs in today’s society, STD rates since the late 1990s have gotten much worse. Lack of appropriate governmental funding for research and drug development, structural inequities, and persisting stigmatization are key drivers for rising rates, explained Mr. Crowley.
Addressing structural barriers to STI prevention
Playing a prominent role in the National Academies report are issues of structural and institutional barriers to STI prevention and care. In the report, the authors argued that a policy-based approach should seek to promote sexual health and eliminate structural racism and inequities to drive improvements in STI management.
“We think it’s these structural factors that are central to all the inequities that play out,” said Mr. Crowley, “and they either don’t get any attention or, if they do get attention, people don’t really speak concretely enough about how we address them.”
The concrete steps, as outlined in the report, begin with addressing factors that involve the health care industry at large. Automatic STI screening as part of routine visits, alerts in electronic health records that remind clinicians to screen patients, and reminders to test patients can be initial low-cost actions health care systems can take to improve STI testing, particularly in marginalized communities. Mr. Crowley noted that greater evidence is needed to support further steps to address structural factors that contribute to barriers in STI screening and treatment access.
Given the complexities inherent in structural barriers to STI care, the report calls on a whole-government response, in partnership with affected communities, to normalize discussions involving sexual well-being. “We have to ask ourselves how we can build healthier communities and how can we integrate sexual health into that dialogue in a way that improves our response to STI prevention and control,” Mr. Crowley explained.
Harnessing AI and dating apps
The report also addresses the power of artificial intelligence to predict STI rates and to discover trends in risk factors, both of which may improve STI surveillance and assist in the development of tailored interventions. The report calls for policy that will enable companies and the government to capitalize on AI to evaluate large collections of data in EHRs, insurance claims databases, social media, search engines, and even dating apps.
In particular, dating apps could be an avenue through which the public and private sectors could improve STI prevention, diagnosis, and treatment. “People want to focus on this idea of whether these apps increase transmission risk,” said Mr. Crowley. “But we would say that this is asking the wrong question, because these technologies are not going away.” He noted that private and public enterprises could work together to leverage these technologies to increase awareness of prevention and testing.
Unifying the STI/HIV and COVID-19 workforce
The report also recommends that the nation unify the STI/HIV workforce with the COVID-19 workforce. Given the high levels of expertise in these professional working groups, the report suggests unification could potentially address both the current crisis and possible future disease outbreaks. Combining COVID-19 response teams with underresourced STI/HIV programs may also improve the delivery of STI testing, considering that STI testing programs have had to compete for resources during the pandemic.
Addressing stigma
The National Academies report also addresses the ongoing issue of stigma, which results from “blaming” individuals and the choices they make so as to create shame, embarrassment, and discrimination. Because of stigma, sexually active people may be unwilling to seek recommended screening, which can lead to delays in diagnosis and treatment and can increase the risk for negative health outcomes.
“As a nation, we’ve almost focused too intently on individual-level factors in a way that’s driven stigma and really hasn’t been helpful for combating the problem,” said Mr. Crowley. He added that, instead of focusing solely on individual-level choices, the nation should instead work to reframe sexual health as a key aspect of overall physical, mental, and emotional well-being. Doing so could create more opportunities to address structural barriers to STI prevention and ensure that more prevention and screening services are available in stigma-free environments.
“I know what we’re recommending is ambitious, but it’s not too big to be achieved, and we’re not saying tomorrow we’re going to transform the world,” Mr. Crowley concluded. “It’s a puzzle with many pieces, but the long-term impact is really all of these pieces fitting together so that, over time, we can reduce the burden STIs have on the population.”
Implications for real-world change
H. Hunter Handsfield, MD, professor emeritus of medicine for the Center for AIDS and STD at the University of Washington, Seattle, said in an interview that this report essentially is a response to evolving societal changes, new and emerging means of social engagement, and increased focus on racial/ethnic disparities. “These features have all come to the forefront of health care and general policy discussions in recent years,” said Dr. Handsfield, who was not part of the committee that developed the NAS report.
Greater scrutiny on public health infrastructure and its relationship with health disparities in the United States makes the publication of these new recommendations especially appropriate during this era of enhanced focus on social justice. Although the report features the tone and quality needed to bolster bipartisan support, said Dr. Handsfield, it’s hard to predict whether such support will come to fruition in today’s political environment.
In terms of the effects the recommendations may have on STI rates, Dr. Handsfield noted that cherry-picking elements from the report to direct policy may result in its having only a trivial impact. “The report is really an appropriate and necessary response, and almost all the recommendations made can be helpful,” he said, “but for true effectiveness, all the elements need to be implemented to drive policy and funding.”
A version of this article first appeared on Medscape.com.