User login
Risk of hypogammaglobulinemia, infections with rituximab increased in pediatric patients
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
A quarter of children receiving treatment with rituximab developed hypogammaglobulinemia within 18 months of starting the drug, according to preliminary research shared at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance. The findings lend support to previous research identifying a risk of hypogammaglobulinemia in children and adolescents taking rituximab and the need for monitoring immunoglobulin levels in those prescribed it.
“Our study highlights a role for heightened vigilance of rituximab-associated hypogammaglobulinemia and infections in pediatric patients with rheumatic conditions,” Mei-Sing Ong, PhD, of Harvard Medical School and the Harvard Pilgrim Health Care Institute, both in Boston, and colleagues concluded. “Increased risks appeared to be mediated, at least in part, by exposure to glucocorticoids (hypogammaglobulinemia and serious infections) or cyclophosphamide (hypogammaglobulinemia) administered prior to rituximab.”
The observational study involved a cohort of 93 patients, aged 2-25 years, treated at Boston Children’s Hospital during 2009-2019. The patients received rituximab for a wide range of rheumatic diseases, including systemic lupus erythematosus, vasculitis, juvenile idiopathic arthritis, and juvenile dermatomyositis or other polymyositis. The researchers excluded patients who had previously had hypogammaglobulinemia before using rituximab.
In this cohort, 26.9% of patients developed hypogammaglobulinemia, and 20.4% of patients developed an infectious complication within 18 months of beginning rituximab treatment. The infection was serious enough to require inpatient treatment in more than half of those who developed infections (57.9%).
Risk of new-onset hypogammaglobulinemia increased with decreasing age (P = .004), and males were more than four times more likely to develop the condition (odds ratio, 4.55; P = .012). Risk of an infection was also more likely among younger patients (OR, 0.87; P = .039).
Patients with vasculitis were fivefold more likely to develop the hypogammaglobulinemia than were those with other rheumatic diseases after the researchers accounted for age, sex, underlying disease, and medication use (OR, 5.04; P = .017). Risk was also greater in patients with exposure to cyclophosphamide in the year before starting rituximab (OR, 3.76; P = .032), although the finding narrowly reached statistical significance after adjustment for those covariates (OR, 4.41; P = .048).
Glucocorticoid treatment in the month before rituximab was associated with an elevated risk of hypogammaglobulinemia before adjustment (OR, 4.53; P = .007) but lost significance after adjustment. Those taking glucocorticoids had a greater than eightfold increase in infection risk (OR, 8.5; P = .006) before adjustment, which dropped to a fivefold risk after accounting for age, sex, underlying disease, and medication use (OR, 5.4; P = .040).
Monitoring needed for relatively common side effect
The findings are consistent with those seen in a cohort study conducted at Lurie Children’s Hospital of Chicago and published in 2019, said Amer M. Khojah, MD, an attending physician in allergy, immunology, and rheumatology at Lurie and an assistant professor of pediatrics at Northwestern University, also in Chicago. He was not involved in the current study.
“The main takeaway from this study is that we need to be careful about this side effect because it’s relatively common,” Dr. Khojah said in an interview.
At his institution, all patients undergo baseline labs to measure IgG levels prior to initiating rituximab and then have labs drawn again at 3 months and 1 year after starting the drug. Transient hypogammaglobulinemia may not require treatment, he said, but if it persists or the patient develops an infection, treatment with intravenous immunoglobulin is indicated. Yet the drug is so commonly used across a wide range of specialties that there’s a great deal of variability in clinical practice in terms of monitoring and follow-up, Dr. Khojah said.
“The problem is, if you don’t measure it, the patient might be get hypogammaglobulinemia and you don’t know it,” potentially leading to infections that the physician may or may not hear about, he said. “If you are the one who gives them the rituximab, you need to make sure they don’t get the side effects” or that they receive treatment if they do, he said.
Casey L. McAtee, MD, an instructor in the section of hematology and oncology in the department of pediatrics at Baylor College of Medicine, Houston, agreed that developing a consistent monitoring schedule is important.
“These data are supportive of the necessity to follow patients closely for infection after rituximab, especially considering that many infections may be severe and require hospitalization,” Dr. McAtee said in an interview. “The period of immunosuppression and subsequent infection risk following rituximab, even after single courses, may last well beyond a year following a single course. This is particularly true in patients receiving concurrent immunosuppressive therapy.”
Dr. McAtee similarly published data this year finding frequent infections among young patients receiving rituximab. Hypogammaglobulinemia is already more likely in patients who require rituximab because of other immunosuppressive medication they often take, but the risk “jumped substantially following rituximab,” he said. In addition to patients with low levels of IgG, 41% of patients showed low levels of IgM in that study.
“Nearly a third of patients with normal baseline IgM had persistently low levels more than a year after rituximab, consistent with prolonged B-cell recovery,” Dr. McAtee said. “It is necessary to highlight the importance of IgM in these patients, as common strategies to treat hypogammaglobulinemia, specifically intravenous immunoglobulin, do not replete IgM.”
Neither Dr. Khojah nor Dr. McAtee saw the risk of hypogammaglobulinemia as a reason to avoid rituximab when indicated.
“It is often the best choice for patients whose diseases have not responded to first-line therapies,” Dr. McAtee said. “This and similar studies inform the risk-benefit decision that the medical team must make, as well as the medical surveillance to be considered for patients following a course of rituximab. Going forward, strategies to mitigate infection risk after rituximab, particularly in the first 3 months when they are most common, should be pursued.”
The research was funded by CARRA, which receives funding from the Arthritis Foundation. The authors did not note whether they had any disclosures. Dr. Khojah and Dr. McAtee had no disclosures.
FROM CARRA 2021
CDC: STI rates rise for sixth year in a row
Annual cases of sexually transmitted infections in the United States jumped for the sixth year in a row in 2019, according to a new Centers for Disease Control and Prevention report that highlights an increase in congenital syphilis and rising rates of syphilis, chlamydia, and gonorrhea in men, especially men who have sex with men (MSM).
The report says nothing about STI rates during the COVID-19 pandemic, when both casual sex and disease screening and surveillance declined significantly, at least in the early months. But epidemiologist Patricia Kissinger, PhD, MPH, from Tulane University School, New Orleans, said in an interview that the findings reflect how “a confluence of factors” drove up rates before the age of COVID. Those factors include online dating, the opioid epidemic, the decline in condom use in the MSM community as HIV became more preventable, and indifference among policy makers and the community at large.
The CDC report, based on data from local health departments, says there were 129,813 cases of syphilis in 2019, up 74% since 2015. Almost 2,000 cases of congenital syphilis were reported, up 279% since 2015, and 128 infants died.
“There’s no reason for us to have congenital syphilis,” said Dr. Kissinger, who noted that the disease can cause birth defects and meningitis in addition to death. “Women should be screened, and it’s relatively easy to treat via penicillin injections.”
Indeed, medical guidelines suggest that pregnant women be routinely tested for syphilis. But that doesn’t always happen because “it falls through the cracks,” Dr. Kissinger said. Or, she added, women might not be tested enough times during their pregnancies: “You have to screen women in the third trimester. You can’t just do it in the first trimester because people do have sex when they’re pregnant.”
Rising congenital syphilis numbers have convinced at least one health system to take action. As of June 1, the University of California, San Diego, will routinely test pregnant women in the emergency department for syphilis in addition to HIV and hepatitis C, Martin Hoenigl, MD, a UCSF infectious disease specialist, said in an interview.
The CDC report also notes 1.8 million cases of chlamydia in 2019, a jump of 19% in 4 years, and a 56% increase in gonorrhea in that time period, to a total of 616,392 cases.
The report says increasing gonorrhea and chlamydia cases in men, especially MSM, could be caused by increased testing/screening, increased transmission, or both. Although women are generally diagnosed with chlamydia more often than men, the report says, numbers among men grew by 32% from 2015 to 2019. And since 2013, rates of gonorrhea among men have risen at a much faster clip than among women.
MSM accounted for most male cases of primary and secondary syphilis in 2019, although the report said the apparent long-term rise in these cases might be slowing.
Many MSM no longer use condoms because they’re using pre-exposure prophylaxis (PrEP) or have undetectable levels of HIV because of treatment, said Jeffrey Klausner, MD, MPH, an STI specialist at the University of Southern California in Los Angeles, said in an interview.
Many MSM might be getting screened much more often for STIs than in the past because frequent screening is required for those on PrEP. However, Dr. Kissinger said some clinics weren’t able to test at times during the pandemic because of a swab shortage. In addition, patients of all types avoided routine medical care during the pandemic, and some medical professionals in the infectious disease field were redirected to COVID care.
Clinical trials have been investigating a possible preventive STI strategy in MSM who don’t wear condoms – prophylaxis, either before or after exposure, with the antibiotic doxycycline. “That’s a very good solution,” Dr. Klausner said, but he believes bigger challenges remain. According to him, the existence of the report itself – which offers statistics from 2 years ago instead of more relevant recent numbers – is evidence of how the federal government isn’t doing enough to fight STIs. “If we’re taking the STD epidemic seriously, there should be timely and regular reporting.” Dr. Klausner said he likes the idea of monthly reports, as well as more funding for prevention.
Instead, he noted, the federal government cut STI prevention funding by 40% in inflation-adjusted dollars from 2002-2003 to 2018-2019, according to the National Coalition of STD Directors. “Burying your head in the sand and hoping the problem goes away is not an effective strategy,” he said.
It’s not clear whether STI rates are on the decline because of pandemic restrictions and stay-at-home orders. Surveys suggest that a dip in casual sex early in pandemic – when much of society shut down – was only temporary, Dr. Klausner said.
Dr. Kissinger disclosed no relevant financial relationships. Dr. Hoenigl reported receiving research funding via his university from Gilead. Dr. Klausner has recently provided consulting services to Danaher, Cepheid, Roche, GlaxoSmithKline, Talis Bio, SpeeDx, and Visby Medical, all manufacturers of diagnostic assays for STIs.
A version of this article first appeared on Medscape.com.
Annual cases of sexually transmitted infections in the United States jumped for the sixth year in a row in 2019, according to a new Centers for Disease Control and Prevention report that highlights an increase in congenital syphilis and rising rates of syphilis, chlamydia, and gonorrhea in men, especially men who have sex with men (MSM).
The report says nothing about STI rates during the COVID-19 pandemic, when both casual sex and disease screening and surveillance declined significantly, at least in the early months. But epidemiologist Patricia Kissinger, PhD, MPH, from Tulane University School, New Orleans, said in an interview that the findings reflect how “a confluence of factors” drove up rates before the age of COVID. Those factors include online dating, the opioid epidemic, the decline in condom use in the MSM community as HIV became more preventable, and indifference among policy makers and the community at large.
The CDC report, based on data from local health departments, says there were 129,813 cases of syphilis in 2019, up 74% since 2015. Almost 2,000 cases of congenital syphilis were reported, up 279% since 2015, and 128 infants died.
“There’s no reason for us to have congenital syphilis,” said Dr. Kissinger, who noted that the disease can cause birth defects and meningitis in addition to death. “Women should be screened, and it’s relatively easy to treat via penicillin injections.”
Indeed, medical guidelines suggest that pregnant women be routinely tested for syphilis. But that doesn’t always happen because “it falls through the cracks,” Dr. Kissinger said. Or, she added, women might not be tested enough times during their pregnancies: “You have to screen women in the third trimester. You can’t just do it in the first trimester because people do have sex when they’re pregnant.”
Rising congenital syphilis numbers have convinced at least one health system to take action. As of June 1, the University of California, San Diego, will routinely test pregnant women in the emergency department for syphilis in addition to HIV and hepatitis C, Martin Hoenigl, MD, a UCSF infectious disease specialist, said in an interview.
The CDC report also notes 1.8 million cases of chlamydia in 2019, a jump of 19% in 4 years, and a 56% increase in gonorrhea in that time period, to a total of 616,392 cases.
The report says increasing gonorrhea and chlamydia cases in men, especially MSM, could be caused by increased testing/screening, increased transmission, or both. Although women are generally diagnosed with chlamydia more often than men, the report says, numbers among men grew by 32% from 2015 to 2019. And since 2013, rates of gonorrhea among men have risen at a much faster clip than among women.
MSM accounted for most male cases of primary and secondary syphilis in 2019, although the report said the apparent long-term rise in these cases might be slowing.
Many MSM no longer use condoms because they’re using pre-exposure prophylaxis (PrEP) or have undetectable levels of HIV because of treatment, said Jeffrey Klausner, MD, MPH, an STI specialist at the University of Southern California in Los Angeles, said in an interview.
Many MSM might be getting screened much more often for STIs than in the past because frequent screening is required for those on PrEP. However, Dr. Kissinger said some clinics weren’t able to test at times during the pandemic because of a swab shortage. In addition, patients of all types avoided routine medical care during the pandemic, and some medical professionals in the infectious disease field were redirected to COVID care.
Clinical trials have been investigating a possible preventive STI strategy in MSM who don’t wear condoms – prophylaxis, either before or after exposure, with the antibiotic doxycycline. “That’s a very good solution,” Dr. Klausner said, but he believes bigger challenges remain. According to him, the existence of the report itself – which offers statistics from 2 years ago instead of more relevant recent numbers – is evidence of how the federal government isn’t doing enough to fight STIs. “If we’re taking the STD epidemic seriously, there should be timely and regular reporting.” Dr. Klausner said he likes the idea of monthly reports, as well as more funding for prevention.
Instead, he noted, the federal government cut STI prevention funding by 40% in inflation-adjusted dollars from 2002-2003 to 2018-2019, according to the National Coalition of STD Directors. “Burying your head in the sand and hoping the problem goes away is not an effective strategy,” he said.
It’s not clear whether STI rates are on the decline because of pandemic restrictions and stay-at-home orders. Surveys suggest that a dip in casual sex early in pandemic – when much of society shut down – was only temporary, Dr. Klausner said.
Dr. Kissinger disclosed no relevant financial relationships. Dr. Hoenigl reported receiving research funding via his university from Gilead. Dr. Klausner has recently provided consulting services to Danaher, Cepheid, Roche, GlaxoSmithKline, Talis Bio, SpeeDx, and Visby Medical, all manufacturers of diagnostic assays for STIs.
A version of this article first appeared on Medscape.com.
Annual cases of sexually transmitted infections in the United States jumped for the sixth year in a row in 2019, according to a new Centers for Disease Control and Prevention report that highlights an increase in congenital syphilis and rising rates of syphilis, chlamydia, and gonorrhea in men, especially men who have sex with men (MSM).
The report says nothing about STI rates during the COVID-19 pandemic, when both casual sex and disease screening and surveillance declined significantly, at least in the early months. But epidemiologist Patricia Kissinger, PhD, MPH, from Tulane University School, New Orleans, said in an interview that the findings reflect how “a confluence of factors” drove up rates before the age of COVID. Those factors include online dating, the opioid epidemic, the decline in condom use in the MSM community as HIV became more preventable, and indifference among policy makers and the community at large.
The CDC report, based on data from local health departments, says there were 129,813 cases of syphilis in 2019, up 74% since 2015. Almost 2,000 cases of congenital syphilis were reported, up 279% since 2015, and 128 infants died.
“There’s no reason for us to have congenital syphilis,” said Dr. Kissinger, who noted that the disease can cause birth defects and meningitis in addition to death. “Women should be screened, and it’s relatively easy to treat via penicillin injections.”
Indeed, medical guidelines suggest that pregnant women be routinely tested for syphilis. But that doesn’t always happen because “it falls through the cracks,” Dr. Kissinger said. Or, she added, women might not be tested enough times during their pregnancies: “You have to screen women in the third trimester. You can’t just do it in the first trimester because people do have sex when they’re pregnant.”
Rising congenital syphilis numbers have convinced at least one health system to take action. As of June 1, the University of California, San Diego, will routinely test pregnant women in the emergency department for syphilis in addition to HIV and hepatitis C, Martin Hoenigl, MD, a UCSF infectious disease specialist, said in an interview.
The CDC report also notes 1.8 million cases of chlamydia in 2019, a jump of 19% in 4 years, and a 56% increase in gonorrhea in that time period, to a total of 616,392 cases.
The report says increasing gonorrhea and chlamydia cases in men, especially MSM, could be caused by increased testing/screening, increased transmission, or both. Although women are generally diagnosed with chlamydia more often than men, the report says, numbers among men grew by 32% from 2015 to 2019. And since 2013, rates of gonorrhea among men have risen at a much faster clip than among women.
MSM accounted for most male cases of primary and secondary syphilis in 2019, although the report said the apparent long-term rise in these cases might be slowing.
Many MSM no longer use condoms because they’re using pre-exposure prophylaxis (PrEP) or have undetectable levels of HIV because of treatment, said Jeffrey Klausner, MD, MPH, an STI specialist at the University of Southern California in Los Angeles, said in an interview.
Many MSM might be getting screened much more often for STIs than in the past because frequent screening is required for those on PrEP. However, Dr. Kissinger said some clinics weren’t able to test at times during the pandemic because of a swab shortage. In addition, patients of all types avoided routine medical care during the pandemic, and some medical professionals in the infectious disease field were redirected to COVID care.
Clinical trials have been investigating a possible preventive STI strategy in MSM who don’t wear condoms – prophylaxis, either before or after exposure, with the antibiotic doxycycline. “That’s a very good solution,” Dr. Klausner said, but he believes bigger challenges remain. According to him, the existence of the report itself – which offers statistics from 2 years ago instead of more relevant recent numbers – is evidence of how the federal government isn’t doing enough to fight STIs. “If we’re taking the STD epidemic seriously, there should be timely and regular reporting.” Dr. Klausner said he likes the idea of monthly reports, as well as more funding for prevention.
Instead, he noted, the federal government cut STI prevention funding by 40% in inflation-adjusted dollars from 2002-2003 to 2018-2019, according to the National Coalition of STD Directors. “Burying your head in the sand and hoping the problem goes away is not an effective strategy,” he said.
It’s not clear whether STI rates are on the decline because of pandemic restrictions and stay-at-home orders. Surveys suggest that a dip in casual sex early in pandemic – when much of society shut down – was only temporary, Dr. Klausner said.
Dr. Kissinger disclosed no relevant financial relationships. Dr. Hoenigl reported receiving research funding via his university from Gilead. Dr. Klausner has recently provided consulting services to Danaher, Cepheid, Roche, GlaxoSmithKline, Talis Bio, SpeeDx, and Visby Medical, all manufacturers of diagnostic assays for STIs.
A version of this article first appeared on Medscape.com.
Phage-targeting PCR test picks up early Lyme disease
An investigational polymerase chain reaction (PCR) test that detects the presence of a viral gene in Lyme disease–causing bacteria can distinguish between early and late infection, according to the results of a study that the authors described as “systematic and comprehensive.”
“The current way of diagnosing Lyme disease is struggling to reflect the ‘true’ incidence of Lyme disease,” study investigator Jinyu Shan, PhD, said in an interview. Although there are tests for Lyme disease approved by the Food and Drug Administration, they are based on the development of antibodies in the blood, and the problem is that antibodies might not develop until several weeks after an infection.
Diagnosis therefore still relies heavily on the clinician’s experience. There are often telltale signs – such as a “bullseye” skin rash or having been to an area known to be infested with ticks that carry Lyme disease – but this might not always be the case.
For the new test, “we’re not targeting bacteria. We’re targeting bacteriophages,” said Dr. Shan, a research fellow in the department of genetics and genome biology at the University of Leicester (England).
Fortunately, there’s high correlation between the presence of the terL gene and the presence of Borrelia burgdorferi, the spirochete that causes Lyme disease. “If you find the bacteriophages, the bacteria are there,” said Dr. Shan.
“Importantly, there are 10 times more bacteriophages, compared with the bacteria, so you have a lot more targets,” he added.
In an evaluation of a total of 312 samples (156 whole blood and 156 serum samples), significantly fewer copies of the terL gene were found in samples from people with early Lyme disease than in those with late Lyme disease, whereas the fewest copies of terL were seen in healthy volunteers.
Most pathogenic bacteria carry viral DNA either as multiple complete or partial prophages, Dr. Shan explained. Knowing the prophage sequences means that quantitative PCR primers and probes can be designed and used to detect the presence of the associated bacteria.
Although the novel test still needs evaluation in a clinical trial, it could represent a “step-change” in the detection of Lyme disease, Dr. Shan and associates suggested in their report published in Frontiers in Microbiology.
Early treatment is key to the prevention of longer-term consequences of Lyme disease. Clinicians familiar with the treatment of Lyme disease might choose to initiate antibiotic treatment without a positive lab test. However, the lack of a test that can pick out people with Lyme disease in the first few weeks of infection means that many people are not diagnosed or treated early enough.
The new phage-based PCR test Dr. Shan and associates have developed could change all that. With only 0.3 mL of blood being needed, it can potentially be developed as a simple point-of-care test, but that’s a long way off.
At this stage, the research is very much a “proof of concept,” Dr. Shan said. One of the things he plans to try to work out next is whether the test can distinguish between active and dormant disease, which is a “big question” in the diagnosis of Lyme disease.
“Bacteriophages can only be sustained by actively growing bacteria,” explained Dr. Shan, so there is a chance that if they are present in a substantive amount the disease is active, and if they are not – or are in very low numbers – then the disease is dormant. The cutoff value, however, “is not trivial to establish, but we are working toward it,” added Dr. Shan.
Over the past 2 years, Dr. Shan and associates have been working with the Belgian-based diagnostics company, R.E.D Laboratories, to see how the test will fare in a real-world environment. This relationship is providing useful information to add to their bid to perform a clinical trial for which they are now seeking additional sponsorship.
“The lack of an early and effective diagnosis of Lyme disease remains a major cause of misdiagnosis and long-term patient suffering,” commented Rosie Milsom, charity manager for Caudwell LymeCo Charity in the United Kingdom.
It could be a game changer if the test passes the necessary clinical trial testing and validation stages, noted Ms. Milsom, who was not involved in the research.
“Not only would the test help to establish the level or length of infection,” she said, “but it could also act as a way to test after treatment to see if the infection levels are decreasing.” If levels are still high, “you would know more treatment is needed.
The research is being funded by the charity Phelix Research and Development with support from the University of Leicester and the Dutch-based Lyme Fund, Lymefonds. Dr. Shan is named as coinventor of the phage-targeting PCR test, alongside Martha R.J. Clokie, professor of microbiology at the University of Leicester and the senior author of the study. Dr. Shan is chief scientific officer for Phelix Research and Development. Ms. Clokie and other coauthors hold key positions within the medical research charity.
A version of this article first appeared on Medscape.com.
An investigational polymerase chain reaction (PCR) test that detects the presence of a viral gene in Lyme disease–causing bacteria can distinguish between early and late infection, according to the results of a study that the authors described as “systematic and comprehensive.”
“The current way of diagnosing Lyme disease is struggling to reflect the ‘true’ incidence of Lyme disease,” study investigator Jinyu Shan, PhD, said in an interview. Although there are tests for Lyme disease approved by the Food and Drug Administration, they are based on the development of antibodies in the blood, and the problem is that antibodies might not develop until several weeks after an infection.
Diagnosis therefore still relies heavily on the clinician’s experience. There are often telltale signs – such as a “bullseye” skin rash or having been to an area known to be infested with ticks that carry Lyme disease – but this might not always be the case.
For the new test, “we’re not targeting bacteria. We’re targeting bacteriophages,” said Dr. Shan, a research fellow in the department of genetics and genome biology at the University of Leicester (England).
Fortunately, there’s high correlation between the presence of the terL gene and the presence of Borrelia burgdorferi, the spirochete that causes Lyme disease. “If you find the bacteriophages, the bacteria are there,” said Dr. Shan.
“Importantly, there are 10 times more bacteriophages, compared with the bacteria, so you have a lot more targets,” he added.
In an evaluation of a total of 312 samples (156 whole blood and 156 serum samples), significantly fewer copies of the terL gene were found in samples from people with early Lyme disease than in those with late Lyme disease, whereas the fewest copies of terL were seen in healthy volunteers.
Most pathogenic bacteria carry viral DNA either as multiple complete or partial prophages, Dr. Shan explained. Knowing the prophage sequences means that quantitative PCR primers and probes can be designed and used to detect the presence of the associated bacteria.
Although the novel test still needs evaluation in a clinical trial, it could represent a “step-change” in the detection of Lyme disease, Dr. Shan and associates suggested in their report published in Frontiers in Microbiology.
Early treatment is key to the prevention of longer-term consequences of Lyme disease. Clinicians familiar with the treatment of Lyme disease might choose to initiate antibiotic treatment without a positive lab test. However, the lack of a test that can pick out people with Lyme disease in the first few weeks of infection means that many people are not diagnosed or treated early enough.
The new phage-based PCR test Dr. Shan and associates have developed could change all that. With only 0.3 mL of blood being needed, it can potentially be developed as a simple point-of-care test, but that’s a long way off.
At this stage, the research is very much a “proof of concept,” Dr. Shan said. One of the things he plans to try to work out next is whether the test can distinguish between active and dormant disease, which is a “big question” in the diagnosis of Lyme disease.
“Bacteriophages can only be sustained by actively growing bacteria,” explained Dr. Shan, so there is a chance that if they are present in a substantive amount the disease is active, and if they are not – or are in very low numbers – then the disease is dormant. The cutoff value, however, “is not trivial to establish, but we are working toward it,” added Dr. Shan.
Over the past 2 years, Dr. Shan and associates have been working with the Belgian-based diagnostics company, R.E.D Laboratories, to see how the test will fare in a real-world environment. This relationship is providing useful information to add to their bid to perform a clinical trial for which they are now seeking additional sponsorship.
“The lack of an early and effective diagnosis of Lyme disease remains a major cause of misdiagnosis and long-term patient suffering,” commented Rosie Milsom, charity manager for Caudwell LymeCo Charity in the United Kingdom.
It could be a game changer if the test passes the necessary clinical trial testing and validation stages, noted Ms. Milsom, who was not involved in the research.
“Not only would the test help to establish the level or length of infection,” she said, “but it could also act as a way to test after treatment to see if the infection levels are decreasing.” If levels are still high, “you would know more treatment is needed.
The research is being funded by the charity Phelix Research and Development with support from the University of Leicester and the Dutch-based Lyme Fund, Lymefonds. Dr. Shan is named as coinventor of the phage-targeting PCR test, alongside Martha R.J. Clokie, professor of microbiology at the University of Leicester and the senior author of the study. Dr. Shan is chief scientific officer for Phelix Research and Development. Ms. Clokie and other coauthors hold key positions within the medical research charity.
A version of this article first appeared on Medscape.com.
An investigational polymerase chain reaction (PCR) test that detects the presence of a viral gene in Lyme disease–causing bacteria can distinguish between early and late infection, according to the results of a study that the authors described as “systematic and comprehensive.”
“The current way of diagnosing Lyme disease is struggling to reflect the ‘true’ incidence of Lyme disease,” study investigator Jinyu Shan, PhD, said in an interview. Although there are tests for Lyme disease approved by the Food and Drug Administration, they are based on the development of antibodies in the blood, and the problem is that antibodies might not develop until several weeks after an infection.
Diagnosis therefore still relies heavily on the clinician’s experience. There are often telltale signs – such as a “bullseye” skin rash or having been to an area known to be infested with ticks that carry Lyme disease – but this might not always be the case.
For the new test, “we’re not targeting bacteria. We’re targeting bacteriophages,” said Dr. Shan, a research fellow in the department of genetics and genome biology at the University of Leicester (England).
Fortunately, there’s high correlation between the presence of the terL gene and the presence of Borrelia burgdorferi, the spirochete that causes Lyme disease. “If you find the bacteriophages, the bacteria are there,” said Dr. Shan.
“Importantly, there are 10 times more bacteriophages, compared with the bacteria, so you have a lot more targets,” he added.
In an evaluation of a total of 312 samples (156 whole blood and 156 serum samples), significantly fewer copies of the terL gene were found in samples from people with early Lyme disease than in those with late Lyme disease, whereas the fewest copies of terL were seen in healthy volunteers.
Most pathogenic bacteria carry viral DNA either as multiple complete or partial prophages, Dr. Shan explained. Knowing the prophage sequences means that quantitative PCR primers and probes can be designed and used to detect the presence of the associated bacteria.
Although the novel test still needs evaluation in a clinical trial, it could represent a “step-change” in the detection of Lyme disease, Dr. Shan and associates suggested in their report published in Frontiers in Microbiology.
Early treatment is key to the prevention of longer-term consequences of Lyme disease. Clinicians familiar with the treatment of Lyme disease might choose to initiate antibiotic treatment without a positive lab test. However, the lack of a test that can pick out people with Lyme disease in the first few weeks of infection means that many people are not diagnosed or treated early enough.
The new phage-based PCR test Dr. Shan and associates have developed could change all that. With only 0.3 mL of blood being needed, it can potentially be developed as a simple point-of-care test, but that’s a long way off.
At this stage, the research is very much a “proof of concept,” Dr. Shan said. One of the things he plans to try to work out next is whether the test can distinguish between active and dormant disease, which is a “big question” in the diagnosis of Lyme disease.
“Bacteriophages can only be sustained by actively growing bacteria,” explained Dr. Shan, so there is a chance that if they are present in a substantive amount the disease is active, and if they are not – or are in very low numbers – then the disease is dormant. The cutoff value, however, “is not trivial to establish, but we are working toward it,” added Dr. Shan.
Over the past 2 years, Dr. Shan and associates have been working with the Belgian-based diagnostics company, R.E.D Laboratories, to see how the test will fare in a real-world environment. This relationship is providing useful information to add to their bid to perform a clinical trial for which they are now seeking additional sponsorship.
“The lack of an early and effective diagnosis of Lyme disease remains a major cause of misdiagnosis and long-term patient suffering,” commented Rosie Milsom, charity manager for Caudwell LymeCo Charity in the United Kingdom.
It could be a game changer if the test passes the necessary clinical trial testing and validation stages, noted Ms. Milsom, who was not involved in the research.
“Not only would the test help to establish the level or length of infection,” she said, “but it could also act as a way to test after treatment to see if the infection levels are decreasing.” If levels are still high, “you would know more treatment is needed.
The research is being funded by the charity Phelix Research and Development with support from the University of Leicester and the Dutch-based Lyme Fund, Lymefonds. Dr. Shan is named as coinventor of the phage-targeting PCR test, alongside Martha R.J. Clokie, professor of microbiology at the University of Leicester and the senior author of the study. Dr. Shan is chief scientific officer for Phelix Research and Development. Ms. Clokie and other coauthors hold key positions within the medical research charity.
A version of this article first appeared on Medscape.com.
Tick talk for families and pediatricians
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.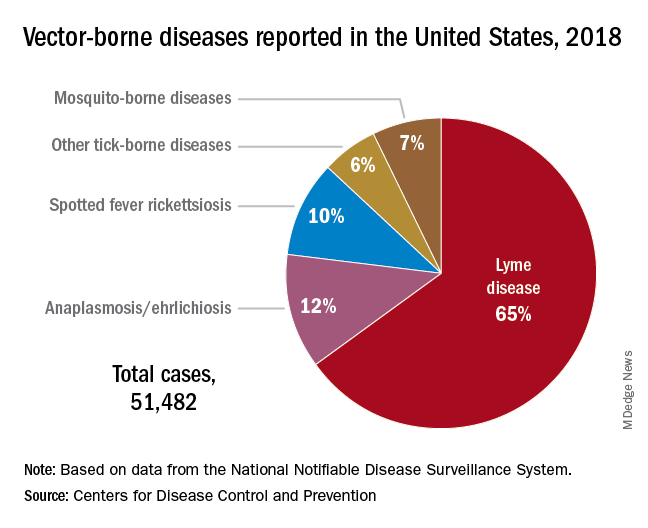
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The Natural History of a Patient With COVID-19 Pneumonia and Silent Hypoxemia
In less than a year, COVID-19 has infected nearly 100 million people worldwide and caused more than 2 million deaths and counting. Although the infection fatality rate is estimated to be 1% and the case fatality rate between 2% and 3%, COVID-19 has had a disproportionate effect on the older population and those with comorbidities. Some of these findings are mirrored in the US Department of Veterans Affairs (VA) population, which has seen a higher case fatality rate.1-4
As a respiratory tract infection, the most dreaded presentation is severe pneumonia with acute hypoxemia, which may rapidly deteriorate to acute respiratory distress syndrome (ARDS) and respiratory failure.5-7 This possibility has led to early intubation strategies aimed at preempting this rapid deterioration and minimizing viral exposure to health care workers. Intubation rates have varied widely with extremes of 6 to 88%.8,9
However, this early intubation strategy has waned as some of the rationale behind its endorsement has been called into question. Early intubation bypasses alternatives to intubation; high-flow nasal cannula oxygen, noninvasive ventilation, and awake proning are all effective maneuvers in the appropriate patient.10,11 The use of first-line high-flow nasal cannula oxygen and noninvasive ventilation has been widely reported. Reports of first-line use of high-flow nasal cannula oxygen has not demonstrated inferior outcomes, nor has the timing of intubation, suggesting a significant portion of patients could benefit from a trial of therapy and eventually avoid intubation.11-14 Other therapies, such as systemic corticosteroids, confer a mortality benefit in those patients with COVID-19 who require oxygen or mechanical ventilation, but their impact on the progression of respiratory failure and need for intubation are undetermined.
There also are reports of patients who report no signs of respiratory distress or dyspnea with their COVID-19 pneumonia despite profound hypoxemia or high oxygen requirements. Various terms, including silent hypoxemia or happy hypoxia, are descriptive of the demeanor of these patients, and treatment has invariably included oxygen.15,16 Nevertheless, low oxygen measurements have generally prompted higher levels of supplemental oxygen or more invasive therapies.
Treatment rendered may obscure the trajectory of response, which is important to understand to better position options for invasive therapies and other therapeutics. We recently encountered a patient with a course of illness that represented the natural history of COVID-19 pneumonia with low oxygen levels (referred to as hypoxemia for consistency) that highlighted several issues of management.
Case Presentation
A 62-year-old undomiciled woman with morbid obesity, prediabetes mellitus, long-standing schizophrenia, and bipolar disorder presented to our facility for evaluation of dry cough and need for tuberculosis clearance for admittance to a shelter. She appeared comfortable and was afebrile with blood pressure 111/74 mm Hg, heart rate 82 beats per minute. Her respiratory rate was 18 breaths per minute, but the pulse oximetry showed oxygen saturation of 70 to 75% on room air at rest. A chest X-ray showed bibasilar infiltrates (Figure 1), and a rapid COVID-19 nasopharyngeal polymerase chain reaction (PCR) test returned positive, confirmed by a second PCR test. Baseline inflammatory markers were elevated (Figure 2). In addition, the serum interleukin-6 also was elevated to 66.1 pg/mL (normal < 5.0), erythrocyte sedimentation rate elevated to 69 mm/h, but serum procalcitonin was essentially normal (0.22 ng/mL; normal < 20 ng/mL) as was the serum lactate (1.4 mmol/L).
The patient was admitted to the intensive care unit (ICU) for close monitoring in anticipation of the possibility of decompensation based on her age, hypoxia, and elevated inflammatory markers.17 Besides a subsequent low-grade fever (100.4 oF) and lymphopenia (manual count 550/uL), she remained clinically unchanged. Throughout her hospitalization, she maintained a persistent psychotic delusion that she did not have COVID-19, refusing all medical interventions, including a peripheral IV line and supplemental oxygen for the entire duration. Extensive efforts to identify family or a surrogate decision maker were unsuccessful. After consultation with Psychiatry, Bio-Ethics, and hospital leadership, the patient was deemed to lack decision-making capacity regarding treatment or disposition and was placed on a psychiatric hold. However, since any interventions against her will would require sedation, IV access, and potentially increase the risk of nosocomial COVID-19 transmission, she was allowed to remain untreated and was closely monitored for symptoms of worsening respiratory failure.
Over the next 2 weeks, her hypoxemia, inflammatory markers, and the infiltrates on imaging resolved (Figure 2). The lowest daily awake room air pulse oximetry readings are reported, initially with consistent readings in the low 80% range, but on day 12, readings were > 90% and remained > 90% for the remainder of her hospitalization. Therefore, shortly after hospital day 12, she was clinically stable for discharge from acute care to a subacute facility, but this required documentation of the clearance of her viral infection. She refused to undergo a subsequent nasopharyngeal swab but allowed an oropharyngeal COVID-19 PCR swab, which was negative. She remained stable and unchanged for the remainder of her hospitalization, awaiting identification of a receiving facility and was able to be discharged to transitional housing on day 38.
Discussion
The initial reports of COVID-19 pneumonia focused on ARDS and respiratory failure requiring mechanical ventilation with less emphasis on those with lower severity of illness. This was heightened by health care systems that were overwhelmed with large number of patients while faced with limited supplies and equipment. Given the risk to patients and providers of crash intubations, some recommended early intubation strategies.3 However, the natural history of COVID-19 pneumonia and the threshold for intubation of these patients remain poorly defined despite the creation of prognostic tools.17 This patient’s persistent hypoxemia and elevated inflammatory markers certainly met markers of disease associated with a high risk of progression.
The greatest concern would have been her level of hypoxemia. Acceptable thresholds of hypoxemia vary, but general consensus would classify pulse oximetry < 90% as hypoxemia and a threshold for administering supplemental oxygen. It is important to recognize how pulse oximetry readings translate to partial pressure of oxygen (PaO2) measurements (Table 1). Pulse oximetry readings of 90% corresponds to a PaO2 readings of 60 mm Hg in ideal conditions without the influence of acidosis, PaCO2, or temperature. While lower readings are of concern, these do not represent absolute indications for assisted ventilatory support as lower levels are well tolerated in a variety of conditions. A common example are patients with chronic obstructive pulmonary disease. Long-term mortality benefits of continuous supplemental oxygen are well established in specific populations, but the threshold for correction in the acute setting remains a case-by-case decision. This decision is complex and is based on more than an absolute number or the amount of oxygen required to achieve a threshold level of oxygenation.
The PaO2/FIO2 (fraction of inspired oxygen) is a common measure used to address severity of disease and oxygen requirements. It also has been used to define the severity of ARDS, but the ratio is based on intubated and mechanically ventilated patients and may not translate well to those not on assisted ventilation. Treatment with supplemental oxygen also involves entrained air with associated imprecision in oxygen delivery.18 For this discussion, the patient’s admission PaO2/FIO2 on room air would have been between 190 and 260. Coupled with the bilateral infiltrates on imaging, there was justified concern for progression to severe ARDS. Her presentation would have met most of the epidemiologic criteria used in initial case finding for severe COVID-19 cases, including a blood oxygen saturation ≤ 93%, PaO2/FIO2 < 300 with infiltrates involving close to if not exceeding 50% of the lung.
With COVID-19 pneumonia, the pathologic injury to the alveoli resembles that of any viral pneumonia with recruitment of predominantly lymphocytic inflammatory cells that fill the alveoli, derangements in ventilation/perfusion mismatch as the core mechanism of hypoxemia with interstitial edema and shuntlike physiology developing at the extremes of involvement. In later stages, the histologic appearance is similar to ARDS, including hyaline membrane formation and thickened alveolar septa with perivascular lymphocytic-plasmocytic infiltration. In addition, there also are findings of organizing pneumonia with fibroblastic proliferation, thrombosis, and diffuse alveolar damage, a constellation of findings similar to that seen in the latter stages of ARDS.2
Although these histologic findings resemble ARDS, many patients with respiratory failure due to COVID-19 have a different physiologic profile compared with those with typical ARDS, with the most striking finding of lungs with low elastance or high compliance. From the critical care standpoint, this meant that the lungs were relatively easy to ventilate with lower peak airway and plateau pressures and low driving pressures. This condition suggested that there was relatively less lung that could be recruited with positive end expiratory pressure; therefore, a somewhat different entity from that associated with ARDS.19 These findings were often noted early in the course of respiratory failure, and although there is debate about whether this represents a different phenotype or timepoint in the spectrum of disease, it clearly represents a subset that is distinct from that which had been previously encountered.
On the other hand, the clinical features seen in those patients with COVID-19 pneumonia who progressed to advanced respiratory failure were essentially indistinguishable from those patients with traditional ARDS. Other explanations for this respiratory failure have included a disrupted vasoregulatory response to hypoxemia with failed hypoxic vasoconstriction, intravascular microthrombi, and impaired diffusion, all contributing to impaired gas exchange and hypoxemia.19-21 This can lead to shuntlike conditions that neither respond well to supplemental oxygen nor manifest the type of physiologic response seen with other causes of hypoxemia.
The severity of hypoxemia manifested by this patient may have elicited additional findings of respiratory distress, such as dyspnea and tachypnea. However, in patients with severe COVID-19 pneumonia, dyspnea was not a universal finding, reported in the 20 to 60% range of cohorts, higher in those with ARDS and mechanical ventilation, although some report near universal dyspnea in their series.1,4,8,22,23 Tachypnea is another symptom of interest. Using a threshold of > 24 breaths/min, tachypnea was noted in 16 to 29% of patients with a much greater proportion (63%) in nonsurvivors.6,24 Several explanations have been proposed for the discordance between the presence and severity of hypoxemia and lack of symptoms of dyspnea and tachypnea. It is important to recognize that misclassification of the severity of hypoxemia can occur due to technical issues and potential errors involving pulse oximetry measurement and shifts in the oxyhemoglobin dissociation curve. However, this is more pertinent for those with mild disease as the severity of hypoxemia in severe pneumonia is beyond what can be attributed to technical issues.
More important, the ventilatory response curve to hypoxemia may not be normal for some patients, blunted by as much as 50% in older patients, especially in those with diabetes mellitus.7,25,26 In addition, the ventilatory response varies widely even among normal individuals. This would translate to lower levels of minute ventilation (less tachypnea or respiratory effort) with hypoxemia. Hypocapnic hypoxemia also blunts the ventilatory response to hypoxemia. Subjects do not increase their minute ventilation if the PaCO2 remains low despite oxygen desaturation to < 70%, especially if PaCO2 < 30 mm Hg or alternatively, increases in minute ventilation are not seen until the PaCO2 exceeds 39 mm Hg.27 Both scenarios occur in those with COVID-19 pneumonia and provide another explanation for the absence of respiratory symptoms or signs of respiratory distress in some patients.
The observation of more compliant lungs may help in the understanding of the variable presentation of these patients. Compliant lungs do not require the increased pressure needed to achieve a specific tidal volume that, in turn, may increase the work of breathing. This may add to the explanation of seemingly paradoxical silent hypoxemia in those patients where the combination of a blunted ventilatory response, hypocapnia, shunt physiology, and normal respiratory system compliance is represented by the absence of increased breathing effort despite severe hypoxemia.
If not for the patient’s refusal of medical services, this patient quite possibly would have been intubated due to hypoxemia and health care providers’ concern for her risk of deterioration. Reported intubation and mechanical ventilation rates have varied widely from extremes of from < 5 to 88% in severely ill patients.9,22 About 75% will need oxygen, but many can be treated and recover without the need for intubation and mechanical ventilation.
As previously mentioned, options for treatment include standard and high-flow oxygen delivery, noninvasive ventilation, and awake prone ventilation. Their role in patient management has been recently outlined, and instead of an early intubation strategy, represents gradual escalation of support that may be sufficient to treat hypoxemia and avoid the need for intubation and mechanical ventilation (Table 2).
In addition, the patient’s hospital course was notable for the decline in known markers of active inflammation that mirrored the resolution of her hypoxemia and pneumonia. This included elevated lactate dehydrogenase, D-dimer, ferritin, and C-reactive protein with all but the latter rising and decreasing over 2 weeks. These findings provide additional information of the time for recovery and supports the use of these markers to monitor the course of pneumonia.
The patient declined all intervention, including oxygen, and recovered to her presumed prehospitalization condition. This experiment of nature due to unique circumstances may shed light on the natural time course of untreated hypoxemic COVID-19 pneumonia that has not previously been well appreciated. It is important to recognize that recovery occurred over 2 weeks. This is close to the observed and expected time for recovery that has been reported for those with severe COVID-19 pneumonia.
Conclusions
Since the emergence of the COVID-19, evidence has accumulated for the benefit of several adjunctive therapies in the treatment of this type of pneumonia, with corticosteroids providing a mortality benefit. Although unknown whether this patient’s experience can be generalized to others or whether it represents her unique response, this case provides another perspective for comparison of treatments and reinforces the need for prospective, randomized clinical trials to establish treatment efficacy. The exact nature of silent hypoxemia of COVID-19 remains incompletely understood; however, this case highlights the importance of treating the individual instead of clinical markers and provides a time course for recovery from pneumonia and severe hypoxemia that occurs without oxygen or any other treatment over about 2 weeks.
1. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi:10.1001/jamanetworkopen.2020.22310
2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. doi:10.1001/jama.2020.12839
3. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
4. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. doi:10.1164/rccm.202004-1163LE
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S01406736(20)30566-3
7. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. doi:10.1164/rccm.202006-2157CP
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi:10.1056/NEJMoa2002032
9. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi:10.1001/jama.2020.5394
10. Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992-2002. doi:10.1016/j.chest.2020.07.013
11. Ackermann M, Mentzer SJ, Jonigk D. Pulmonary vascular pathology in COVID-19. Reply. N Engl J Med. 2020;383(9):888-889. doi:10.1056/NEJMc2022068
12. McDonough G, Khaing P, Treacy T, McGrath C, Yoo EJ. The use of high-flow nasal oxygen in the ICU as a first-line therapy for acute hypoxemic respiratory failure secondary to coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0257. doi:10.1097/CCE.0000000000000257
13. Hernandez-Romieu AC, Adelman MW, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045-e1053. doi:10.1097/CCM.0000000000004600
14. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
15. Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5
16. Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243.e5-2243.e6. doi:10.1016/j.ajem.2020.05.044
17. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833-840. doi:10.1093/cid/ciaa443
18. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
19. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329-2330. doi:10.1001/jama.2020.6825
20. Schaller T, Hirschbuhl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518-2520. doi:10.1001/jama.2020.8907
21. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. doi:10.1056/NEJMoa2015432
22. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943. doi:10.1001/jamainternmed.2020.0994. Published correction appeared May 11, 2020. Errors in data and units of measure. doi:10.1001/jamainternmed.2020.1429
23. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. doi:10.1016/j.ijid.2020.03.017
24. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
25. Tobin MJ, Jubran A, Laghi F. Misconceptions of pathophysiology of happy hypoxemia and implications for management of COVID-19. Respir Res. 2020;21(1):249. doi:10.1186/s12931-020-01520-y
26. Bickler PE, Feiner JR, Lipnick MS, McKleroy W. “Silent” presentation of hypoxemia and cardiorespiratory compensation in COVID-19. Anesthesiology. 2020;134(2):262-269. doi:10.1097/ALN.0000000000003578
27. Jounieaux V, Parreira VF, Aubert G, Dury M, Delguste P, Rodenstein DO. Effects of hypocapnic hyperventilation on the response to hypoxia in normal subjects receiving intermittent positive-pressure ventilation. Chest. 2002;121(4):1141-1148. doi:10.1378/chest.121.4.1141
In less than a year, COVID-19 has infected nearly 100 million people worldwide and caused more than 2 million deaths and counting. Although the infection fatality rate is estimated to be 1% and the case fatality rate between 2% and 3%, COVID-19 has had a disproportionate effect on the older population and those with comorbidities. Some of these findings are mirrored in the US Department of Veterans Affairs (VA) population, which has seen a higher case fatality rate.1-4
As a respiratory tract infection, the most dreaded presentation is severe pneumonia with acute hypoxemia, which may rapidly deteriorate to acute respiratory distress syndrome (ARDS) and respiratory failure.5-7 This possibility has led to early intubation strategies aimed at preempting this rapid deterioration and minimizing viral exposure to health care workers. Intubation rates have varied widely with extremes of 6 to 88%.8,9
However, this early intubation strategy has waned as some of the rationale behind its endorsement has been called into question. Early intubation bypasses alternatives to intubation; high-flow nasal cannula oxygen, noninvasive ventilation, and awake proning are all effective maneuvers in the appropriate patient.10,11 The use of first-line high-flow nasal cannula oxygen and noninvasive ventilation has been widely reported. Reports of first-line use of high-flow nasal cannula oxygen has not demonstrated inferior outcomes, nor has the timing of intubation, suggesting a significant portion of patients could benefit from a trial of therapy and eventually avoid intubation.11-14 Other therapies, such as systemic corticosteroids, confer a mortality benefit in those patients with COVID-19 who require oxygen or mechanical ventilation, but their impact on the progression of respiratory failure and need for intubation are undetermined.
There also are reports of patients who report no signs of respiratory distress or dyspnea with their COVID-19 pneumonia despite profound hypoxemia or high oxygen requirements. Various terms, including silent hypoxemia or happy hypoxia, are descriptive of the demeanor of these patients, and treatment has invariably included oxygen.15,16 Nevertheless, low oxygen measurements have generally prompted higher levels of supplemental oxygen or more invasive therapies.
Treatment rendered may obscure the trajectory of response, which is important to understand to better position options for invasive therapies and other therapeutics. We recently encountered a patient with a course of illness that represented the natural history of COVID-19 pneumonia with low oxygen levels (referred to as hypoxemia for consistency) that highlighted several issues of management.
Case Presentation
A 62-year-old undomiciled woman with morbid obesity, prediabetes mellitus, long-standing schizophrenia, and bipolar disorder presented to our facility for evaluation of dry cough and need for tuberculosis clearance for admittance to a shelter. She appeared comfortable and was afebrile with blood pressure 111/74 mm Hg, heart rate 82 beats per minute. Her respiratory rate was 18 breaths per minute, but the pulse oximetry showed oxygen saturation of 70 to 75% on room air at rest. A chest X-ray showed bibasilar infiltrates (Figure 1), and a rapid COVID-19 nasopharyngeal polymerase chain reaction (PCR) test returned positive, confirmed by a second PCR test. Baseline inflammatory markers were elevated (Figure 2). In addition, the serum interleukin-6 also was elevated to 66.1 pg/mL (normal < 5.0), erythrocyte sedimentation rate elevated to 69 mm/h, but serum procalcitonin was essentially normal (0.22 ng/mL; normal < 20 ng/mL) as was the serum lactate (1.4 mmol/L).
The patient was admitted to the intensive care unit (ICU) for close monitoring in anticipation of the possibility of decompensation based on her age, hypoxia, and elevated inflammatory markers.17 Besides a subsequent low-grade fever (100.4 oF) and lymphopenia (manual count 550/uL), she remained clinically unchanged. Throughout her hospitalization, she maintained a persistent psychotic delusion that she did not have COVID-19, refusing all medical interventions, including a peripheral IV line and supplemental oxygen for the entire duration. Extensive efforts to identify family or a surrogate decision maker were unsuccessful. After consultation with Psychiatry, Bio-Ethics, and hospital leadership, the patient was deemed to lack decision-making capacity regarding treatment or disposition and was placed on a psychiatric hold. However, since any interventions against her will would require sedation, IV access, and potentially increase the risk of nosocomial COVID-19 transmission, she was allowed to remain untreated and was closely monitored for symptoms of worsening respiratory failure.
Over the next 2 weeks, her hypoxemia, inflammatory markers, and the infiltrates on imaging resolved (Figure 2). The lowest daily awake room air pulse oximetry readings are reported, initially with consistent readings in the low 80% range, but on day 12, readings were > 90% and remained > 90% for the remainder of her hospitalization. Therefore, shortly after hospital day 12, she was clinically stable for discharge from acute care to a subacute facility, but this required documentation of the clearance of her viral infection. She refused to undergo a subsequent nasopharyngeal swab but allowed an oropharyngeal COVID-19 PCR swab, which was negative. She remained stable and unchanged for the remainder of her hospitalization, awaiting identification of a receiving facility and was able to be discharged to transitional housing on day 38.
Discussion
The initial reports of COVID-19 pneumonia focused on ARDS and respiratory failure requiring mechanical ventilation with less emphasis on those with lower severity of illness. This was heightened by health care systems that were overwhelmed with large number of patients while faced with limited supplies and equipment. Given the risk to patients and providers of crash intubations, some recommended early intubation strategies.3 However, the natural history of COVID-19 pneumonia and the threshold for intubation of these patients remain poorly defined despite the creation of prognostic tools.17 This patient’s persistent hypoxemia and elevated inflammatory markers certainly met markers of disease associated with a high risk of progression.
The greatest concern would have been her level of hypoxemia. Acceptable thresholds of hypoxemia vary, but general consensus would classify pulse oximetry < 90% as hypoxemia and a threshold for administering supplemental oxygen. It is important to recognize how pulse oximetry readings translate to partial pressure of oxygen (PaO2) measurements (Table 1). Pulse oximetry readings of 90% corresponds to a PaO2 readings of 60 mm Hg in ideal conditions without the influence of acidosis, PaCO2, or temperature. While lower readings are of concern, these do not represent absolute indications for assisted ventilatory support as lower levels are well tolerated in a variety of conditions. A common example are patients with chronic obstructive pulmonary disease. Long-term mortality benefits of continuous supplemental oxygen are well established in specific populations, but the threshold for correction in the acute setting remains a case-by-case decision. This decision is complex and is based on more than an absolute number or the amount of oxygen required to achieve a threshold level of oxygenation.
The PaO2/FIO2 (fraction of inspired oxygen) is a common measure used to address severity of disease and oxygen requirements. It also has been used to define the severity of ARDS, but the ratio is based on intubated and mechanically ventilated patients and may not translate well to those not on assisted ventilation. Treatment with supplemental oxygen also involves entrained air with associated imprecision in oxygen delivery.18 For this discussion, the patient’s admission PaO2/FIO2 on room air would have been between 190 and 260. Coupled with the bilateral infiltrates on imaging, there was justified concern for progression to severe ARDS. Her presentation would have met most of the epidemiologic criteria used in initial case finding for severe COVID-19 cases, including a blood oxygen saturation ≤ 93%, PaO2/FIO2 < 300 with infiltrates involving close to if not exceeding 50% of the lung.
With COVID-19 pneumonia, the pathologic injury to the alveoli resembles that of any viral pneumonia with recruitment of predominantly lymphocytic inflammatory cells that fill the alveoli, derangements in ventilation/perfusion mismatch as the core mechanism of hypoxemia with interstitial edema and shuntlike physiology developing at the extremes of involvement. In later stages, the histologic appearance is similar to ARDS, including hyaline membrane formation and thickened alveolar septa with perivascular lymphocytic-plasmocytic infiltration. In addition, there also are findings of organizing pneumonia with fibroblastic proliferation, thrombosis, and diffuse alveolar damage, a constellation of findings similar to that seen in the latter stages of ARDS.2
Although these histologic findings resemble ARDS, many patients with respiratory failure due to COVID-19 have a different physiologic profile compared with those with typical ARDS, with the most striking finding of lungs with low elastance or high compliance. From the critical care standpoint, this meant that the lungs were relatively easy to ventilate with lower peak airway and plateau pressures and low driving pressures. This condition suggested that there was relatively less lung that could be recruited with positive end expiratory pressure; therefore, a somewhat different entity from that associated with ARDS.19 These findings were often noted early in the course of respiratory failure, and although there is debate about whether this represents a different phenotype or timepoint in the spectrum of disease, it clearly represents a subset that is distinct from that which had been previously encountered.
On the other hand, the clinical features seen in those patients with COVID-19 pneumonia who progressed to advanced respiratory failure were essentially indistinguishable from those patients with traditional ARDS. Other explanations for this respiratory failure have included a disrupted vasoregulatory response to hypoxemia with failed hypoxic vasoconstriction, intravascular microthrombi, and impaired diffusion, all contributing to impaired gas exchange and hypoxemia.19-21 This can lead to shuntlike conditions that neither respond well to supplemental oxygen nor manifest the type of physiologic response seen with other causes of hypoxemia.
The severity of hypoxemia manifested by this patient may have elicited additional findings of respiratory distress, such as dyspnea and tachypnea. However, in patients with severe COVID-19 pneumonia, dyspnea was not a universal finding, reported in the 20 to 60% range of cohorts, higher in those with ARDS and mechanical ventilation, although some report near universal dyspnea in their series.1,4,8,22,23 Tachypnea is another symptom of interest. Using a threshold of > 24 breaths/min, tachypnea was noted in 16 to 29% of patients with a much greater proportion (63%) in nonsurvivors.6,24 Several explanations have been proposed for the discordance between the presence and severity of hypoxemia and lack of symptoms of dyspnea and tachypnea. It is important to recognize that misclassification of the severity of hypoxemia can occur due to technical issues and potential errors involving pulse oximetry measurement and shifts in the oxyhemoglobin dissociation curve. However, this is more pertinent for those with mild disease as the severity of hypoxemia in severe pneumonia is beyond what can be attributed to technical issues.
More important, the ventilatory response curve to hypoxemia may not be normal for some patients, blunted by as much as 50% in older patients, especially in those with diabetes mellitus.7,25,26 In addition, the ventilatory response varies widely even among normal individuals. This would translate to lower levels of minute ventilation (less tachypnea or respiratory effort) with hypoxemia. Hypocapnic hypoxemia also blunts the ventilatory response to hypoxemia. Subjects do not increase their minute ventilation if the PaCO2 remains low despite oxygen desaturation to < 70%, especially if PaCO2 < 30 mm Hg or alternatively, increases in minute ventilation are not seen until the PaCO2 exceeds 39 mm Hg.27 Both scenarios occur in those with COVID-19 pneumonia and provide another explanation for the absence of respiratory symptoms or signs of respiratory distress in some patients.
The observation of more compliant lungs may help in the understanding of the variable presentation of these patients. Compliant lungs do not require the increased pressure needed to achieve a specific tidal volume that, in turn, may increase the work of breathing. This may add to the explanation of seemingly paradoxical silent hypoxemia in those patients where the combination of a blunted ventilatory response, hypocapnia, shunt physiology, and normal respiratory system compliance is represented by the absence of increased breathing effort despite severe hypoxemia.
If not for the patient’s refusal of medical services, this patient quite possibly would have been intubated due to hypoxemia and health care providers’ concern for her risk of deterioration. Reported intubation and mechanical ventilation rates have varied widely from extremes of from < 5 to 88% in severely ill patients.9,22 About 75% will need oxygen, but many can be treated and recover without the need for intubation and mechanical ventilation.
As previously mentioned, options for treatment include standard and high-flow oxygen delivery, noninvasive ventilation, and awake prone ventilation. Their role in patient management has been recently outlined, and instead of an early intubation strategy, represents gradual escalation of support that may be sufficient to treat hypoxemia and avoid the need for intubation and mechanical ventilation (Table 2).
In addition, the patient’s hospital course was notable for the decline in known markers of active inflammation that mirrored the resolution of her hypoxemia and pneumonia. This included elevated lactate dehydrogenase, D-dimer, ferritin, and C-reactive protein with all but the latter rising and decreasing over 2 weeks. These findings provide additional information of the time for recovery and supports the use of these markers to monitor the course of pneumonia.
The patient declined all intervention, including oxygen, and recovered to her presumed prehospitalization condition. This experiment of nature due to unique circumstances may shed light on the natural time course of untreated hypoxemic COVID-19 pneumonia that has not previously been well appreciated. It is important to recognize that recovery occurred over 2 weeks. This is close to the observed and expected time for recovery that has been reported for those with severe COVID-19 pneumonia.
Conclusions
Since the emergence of the COVID-19, evidence has accumulated for the benefit of several adjunctive therapies in the treatment of this type of pneumonia, with corticosteroids providing a mortality benefit. Although unknown whether this patient’s experience can be generalized to others or whether it represents her unique response, this case provides another perspective for comparison of treatments and reinforces the need for prospective, randomized clinical trials to establish treatment efficacy. The exact nature of silent hypoxemia of COVID-19 remains incompletely understood; however, this case highlights the importance of treating the individual instead of clinical markers and provides a time course for recovery from pneumonia and severe hypoxemia that occurs without oxygen or any other treatment over about 2 weeks.
In less than a year, COVID-19 has infected nearly 100 million people worldwide and caused more than 2 million deaths and counting. Although the infection fatality rate is estimated to be 1% and the case fatality rate between 2% and 3%, COVID-19 has had a disproportionate effect on the older population and those with comorbidities. Some of these findings are mirrored in the US Department of Veterans Affairs (VA) population, which has seen a higher case fatality rate.1-4
As a respiratory tract infection, the most dreaded presentation is severe pneumonia with acute hypoxemia, which may rapidly deteriorate to acute respiratory distress syndrome (ARDS) and respiratory failure.5-7 This possibility has led to early intubation strategies aimed at preempting this rapid deterioration and minimizing viral exposure to health care workers. Intubation rates have varied widely with extremes of 6 to 88%.8,9
However, this early intubation strategy has waned as some of the rationale behind its endorsement has been called into question. Early intubation bypasses alternatives to intubation; high-flow nasal cannula oxygen, noninvasive ventilation, and awake proning are all effective maneuvers in the appropriate patient.10,11 The use of first-line high-flow nasal cannula oxygen and noninvasive ventilation has been widely reported. Reports of first-line use of high-flow nasal cannula oxygen has not demonstrated inferior outcomes, nor has the timing of intubation, suggesting a significant portion of patients could benefit from a trial of therapy and eventually avoid intubation.11-14 Other therapies, such as systemic corticosteroids, confer a mortality benefit in those patients with COVID-19 who require oxygen or mechanical ventilation, but their impact on the progression of respiratory failure and need for intubation are undetermined.
There also are reports of patients who report no signs of respiratory distress or dyspnea with their COVID-19 pneumonia despite profound hypoxemia or high oxygen requirements. Various terms, including silent hypoxemia or happy hypoxia, are descriptive of the demeanor of these patients, and treatment has invariably included oxygen.15,16 Nevertheless, low oxygen measurements have generally prompted higher levels of supplemental oxygen or more invasive therapies.
Treatment rendered may obscure the trajectory of response, which is important to understand to better position options for invasive therapies and other therapeutics. We recently encountered a patient with a course of illness that represented the natural history of COVID-19 pneumonia with low oxygen levels (referred to as hypoxemia for consistency) that highlighted several issues of management.
Case Presentation
A 62-year-old undomiciled woman with morbid obesity, prediabetes mellitus, long-standing schizophrenia, and bipolar disorder presented to our facility for evaluation of dry cough and need for tuberculosis clearance for admittance to a shelter. She appeared comfortable and was afebrile with blood pressure 111/74 mm Hg, heart rate 82 beats per minute. Her respiratory rate was 18 breaths per minute, but the pulse oximetry showed oxygen saturation of 70 to 75% on room air at rest. A chest X-ray showed bibasilar infiltrates (Figure 1), and a rapid COVID-19 nasopharyngeal polymerase chain reaction (PCR) test returned positive, confirmed by a second PCR test. Baseline inflammatory markers were elevated (Figure 2). In addition, the serum interleukin-6 also was elevated to 66.1 pg/mL (normal < 5.0), erythrocyte sedimentation rate elevated to 69 mm/h, but serum procalcitonin was essentially normal (0.22 ng/mL; normal < 20 ng/mL) as was the serum lactate (1.4 mmol/L).
The patient was admitted to the intensive care unit (ICU) for close monitoring in anticipation of the possibility of decompensation based on her age, hypoxia, and elevated inflammatory markers.17 Besides a subsequent low-grade fever (100.4 oF) and lymphopenia (manual count 550/uL), she remained clinically unchanged. Throughout her hospitalization, she maintained a persistent psychotic delusion that she did not have COVID-19, refusing all medical interventions, including a peripheral IV line and supplemental oxygen for the entire duration. Extensive efforts to identify family or a surrogate decision maker were unsuccessful. After consultation with Psychiatry, Bio-Ethics, and hospital leadership, the patient was deemed to lack decision-making capacity regarding treatment or disposition and was placed on a psychiatric hold. However, since any interventions against her will would require sedation, IV access, and potentially increase the risk of nosocomial COVID-19 transmission, she was allowed to remain untreated and was closely monitored for symptoms of worsening respiratory failure.
Over the next 2 weeks, her hypoxemia, inflammatory markers, and the infiltrates on imaging resolved (Figure 2). The lowest daily awake room air pulse oximetry readings are reported, initially with consistent readings in the low 80% range, but on day 12, readings were > 90% and remained > 90% for the remainder of her hospitalization. Therefore, shortly after hospital day 12, she was clinically stable for discharge from acute care to a subacute facility, but this required documentation of the clearance of her viral infection. She refused to undergo a subsequent nasopharyngeal swab but allowed an oropharyngeal COVID-19 PCR swab, which was negative. She remained stable and unchanged for the remainder of her hospitalization, awaiting identification of a receiving facility and was able to be discharged to transitional housing on day 38.
Discussion
The initial reports of COVID-19 pneumonia focused on ARDS and respiratory failure requiring mechanical ventilation with less emphasis on those with lower severity of illness. This was heightened by health care systems that were overwhelmed with large number of patients while faced with limited supplies and equipment. Given the risk to patients and providers of crash intubations, some recommended early intubation strategies.3 However, the natural history of COVID-19 pneumonia and the threshold for intubation of these patients remain poorly defined despite the creation of prognostic tools.17 This patient’s persistent hypoxemia and elevated inflammatory markers certainly met markers of disease associated with a high risk of progression.
The greatest concern would have been her level of hypoxemia. Acceptable thresholds of hypoxemia vary, but general consensus would classify pulse oximetry < 90% as hypoxemia and a threshold for administering supplemental oxygen. It is important to recognize how pulse oximetry readings translate to partial pressure of oxygen (PaO2) measurements (Table 1). Pulse oximetry readings of 90% corresponds to a PaO2 readings of 60 mm Hg in ideal conditions without the influence of acidosis, PaCO2, or temperature. While lower readings are of concern, these do not represent absolute indications for assisted ventilatory support as lower levels are well tolerated in a variety of conditions. A common example are patients with chronic obstructive pulmonary disease. Long-term mortality benefits of continuous supplemental oxygen are well established in specific populations, but the threshold for correction in the acute setting remains a case-by-case decision. This decision is complex and is based on more than an absolute number or the amount of oxygen required to achieve a threshold level of oxygenation.
The PaO2/FIO2 (fraction of inspired oxygen) is a common measure used to address severity of disease and oxygen requirements. It also has been used to define the severity of ARDS, but the ratio is based on intubated and mechanically ventilated patients and may not translate well to those not on assisted ventilation. Treatment with supplemental oxygen also involves entrained air with associated imprecision in oxygen delivery.18 For this discussion, the patient’s admission PaO2/FIO2 on room air would have been between 190 and 260. Coupled with the bilateral infiltrates on imaging, there was justified concern for progression to severe ARDS. Her presentation would have met most of the epidemiologic criteria used in initial case finding for severe COVID-19 cases, including a blood oxygen saturation ≤ 93%, PaO2/FIO2 < 300 with infiltrates involving close to if not exceeding 50% of the lung.
With COVID-19 pneumonia, the pathologic injury to the alveoli resembles that of any viral pneumonia with recruitment of predominantly lymphocytic inflammatory cells that fill the alveoli, derangements in ventilation/perfusion mismatch as the core mechanism of hypoxemia with interstitial edema and shuntlike physiology developing at the extremes of involvement. In later stages, the histologic appearance is similar to ARDS, including hyaline membrane formation and thickened alveolar septa with perivascular lymphocytic-plasmocytic infiltration. In addition, there also are findings of organizing pneumonia with fibroblastic proliferation, thrombosis, and diffuse alveolar damage, a constellation of findings similar to that seen in the latter stages of ARDS.2
Although these histologic findings resemble ARDS, many patients with respiratory failure due to COVID-19 have a different physiologic profile compared with those with typical ARDS, with the most striking finding of lungs with low elastance or high compliance. From the critical care standpoint, this meant that the lungs were relatively easy to ventilate with lower peak airway and plateau pressures and low driving pressures. This condition suggested that there was relatively less lung that could be recruited with positive end expiratory pressure; therefore, a somewhat different entity from that associated with ARDS.19 These findings were often noted early in the course of respiratory failure, and although there is debate about whether this represents a different phenotype or timepoint in the spectrum of disease, it clearly represents a subset that is distinct from that which had been previously encountered.
On the other hand, the clinical features seen in those patients with COVID-19 pneumonia who progressed to advanced respiratory failure were essentially indistinguishable from those patients with traditional ARDS. Other explanations for this respiratory failure have included a disrupted vasoregulatory response to hypoxemia with failed hypoxic vasoconstriction, intravascular microthrombi, and impaired diffusion, all contributing to impaired gas exchange and hypoxemia.19-21 This can lead to shuntlike conditions that neither respond well to supplemental oxygen nor manifest the type of physiologic response seen with other causes of hypoxemia.
The severity of hypoxemia manifested by this patient may have elicited additional findings of respiratory distress, such as dyspnea and tachypnea. However, in patients with severe COVID-19 pneumonia, dyspnea was not a universal finding, reported in the 20 to 60% range of cohorts, higher in those with ARDS and mechanical ventilation, although some report near universal dyspnea in their series.1,4,8,22,23 Tachypnea is another symptom of interest. Using a threshold of > 24 breaths/min, tachypnea was noted in 16 to 29% of patients with a much greater proportion (63%) in nonsurvivors.6,24 Several explanations have been proposed for the discordance between the presence and severity of hypoxemia and lack of symptoms of dyspnea and tachypnea. It is important to recognize that misclassification of the severity of hypoxemia can occur due to technical issues and potential errors involving pulse oximetry measurement and shifts in the oxyhemoglobin dissociation curve. However, this is more pertinent for those with mild disease as the severity of hypoxemia in severe pneumonia is beyond what can be attributed to technical issues.
More important, the ventilatory response curve to hypoxemia may not be normal for some patients, blunted by as much as 50% in older patients, especially in those with diabetes mellitus.7,25,26 In addition, the ventilatory response varies widely even among normal individuals. This would translate to lower levels of minute ventilation (less tachypnea or respiratory effort) with hypoxemia. Hypocapnic hypoxemia also blunts the ventilatory response to hypoxemia. Subjects do not increase their minute ventilation if the PaCO2 remains low despite oxygen desaturation to < 70%, especially if PaCO2 < 30 mm Hg or alternatively, increases in minute ventilation are not seen until the PaCO2 exceeds 39 mm Hg.27 Both scenarios occur in those with COVID-19 pneumonia and provide another explanation for the absence of respiratory symptoms or signs of respiratory distress in some patients.
The observation of more compliant lungs may help in the understanding of the variable presentation of these patients. Compliant lungs do not require the increased pressure needed to achieve a specific tidal volume that, in turn, may increase the work of breathing. This may add to the explanation of seemingly paradoxical silent hypoxemia in those patients where the combination of a blunted ventilatory response, hypocapnia, shunt physiology, and normal respiratory system compliance is represented by the absence of increased breathing effort despite severe hypoxemia.
If not for the patient’s refusal of medical services, this patient quite possibly would have been intubated due to hypoxemia and health care providers’ concern for her risk of deterioration. Reported intubation and mechanical ventilation rates have varied widely from extremes of from < 5 to 88% in severely ill patients.9,22 About 75% will need oxygen, but many can be treated and recover without the need for intubation and mechanical ventilation.
As previously mentioned, options for treatment include standard and high-flow oxygen delivery, noninvasive ventilation, and awake prone ventilation. Their role in patient management has been recently outlined, and instead of an early intubation strategy, represents gradual escalation of support that may be sufficient to treat hypoxemia and avoid the need for intubation and mechanical ventilation (Table 2).
In addition, the patient’s hospital course was notable for the decline in known markers of active inflammation that mirrored the resolution of her hypoxemia and pneumonia. This included elevated lactate dehydrogenase, D-dimer, ferritin, and C-reactive protein with all but the latter rising and decreasing over 2 weeks. These findings provide additional information of the time for recovery and supports the use of these markers to monitor the course of pneumonia.
The patient declined all intervention, including oxygen, and recovered to her presumed prehospitalization condition. This experiment of nature due to unique circumstances may shed light on the natural time course of untreated hypoxemic COVID-19 pneumonia that has not previously been well appreciated. It is important to recognize that recovery occurred over 2 weeks. This is close to the observed and expected time for recovery that has been reported for those with severe COVID-19 pneumonia.
Conclusions
Since the emergence of the COVID-19, evidence has accumulated for the benefit of several adjunctive therapies in the treatment of this type of pneumonia, with corticosteroids providing a mortality benefit. Although unknown whether this patient’s experience can be generalized to others or whether it represents her unique response, this case provides another perspective for comparison of treatments and reinforces the need for prospective, randomized clinical trials to establish treatment efficacy. The exact nature of silent hypoxemia of COVID-19 remains incompletely understood; however, this case highlights the importance of treating the individual instead of clinical markers and provides a time course for recovery from pneumonia and severe hypoxemia that occurs without oxygen or any other treatment over about 2 weeks.
1. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi:10.1001/jamanetworkopen.2020.22310
2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. doi:10.1001/jama.2020.12839
3. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
4. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. doi:10.1164/rccm.202004-1163LE
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S01406736(20)30566-3
7. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. doi:10.1164/rccm.202006-2157CP
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi:10.1056/NEJMoa2002032
9. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi:10.1001/jama.2020.5394
10. Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992-2002. doi:10.1016/j.chest.2020.07.013
11. Ackermann M, Mentzer SJ, Jonigk D. Pulmonary vascular pathology in COVID-19. Reply. N Engl J Med. 2020;383(9):888-889. doi:10.1056/NEJMc2022068
12. McDonough G, Khaing P, Treacy T, McGrath C, Yoo EJ. The use of high-flow nasal oxygen in the ICU as a first-line therapy for acute hypoxemic respiratory failure secondary to coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0257. doi:10.1097/CCE.0000000000000257
13. Hernandez-Romieu AC, Adelman MW, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045-e1053. doi:10.1097/CCM.0000000000004600
14. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
15. Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5
16. Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243.e5-2243.e6. doi:10.1016/j.ajem.2020.05.044
17. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833-840. doi:10.1093/cid/ciaa443
18. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
19. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329-2330. doi:10.1001/jama.2020.6825
20. Schaller T, Hirschbuhl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518-2520. doi:10.1001/jama.2020.8907
21. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. doi:10.1056/NEJMoa2015432
22. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943. doi:10.1001/jamainternmed.2020.0994. Published correction appeared May 11, 2020. Errors in data and units of measure. doi:10.1001/jamainternmed.2020.1429
23. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. doi:10.1016/j.ijid.2020.03.017
24. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
25. Tobin MJ, Jubran A, Laghi F. Misconceptions of pathophysiology of happy hypoxemia and implications for management of COVID-19. Respir Res. 2020;21(1):249. doi:10.1186/s12931-020-01520-y
26. Bickler PE, Feiner JR, Lipnick MS, McKleroy W. “Silent” presentation of hypoxemia and cardiorespiratory compensation in COVID-19. Anesthesiology. 2020;134(2):262-269. doi:10.1097/ALN.0000000000003578
27. Jounieaux V, Parreira VF, Aubert G, Dury M, Delguste P, Rodenstein DO. Effects of hypocapnic hyperventilation on the response to hypoxia in normal subjects receiving intermittent positive-pressure ventilation. Chest. 2002;121(4):1141-1148. doi:10.1378/chest.121.4.1141
1. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi:10.1001/jamanetworkopen.2020.22310
2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. doi:10.1001/jama.2020.12839
3. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
4. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. doi:10.1164/rccm.202004-1163LE
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S01406736(20)30566-3
7. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. doi:10.1164/rccm.202006-2157CP
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi:10.1056/NEJMoa2002032
9. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi:10.1001/jama.2020.5394
10. Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992-2002. doi:10.1016/j.chest.2020.07.013
11. Ackermann M, Mentzer SJ, Jonigk D. Pulmonary vascular pathology in COVID-19. Reply. N Engl J Med. 2020;383(9):888-889. doi:10.1056/NEJMc2022068
12. McDonough G, Khaing P, Treacy T, McGrath C, Yoo EJ. The use of high-flow nasal oxygen in the ICU as a first-line therapy for acute hypoxemic respiratory failure secondary to coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0257. doi:10.1097/CCE.0000000000000257
13. Hernandez-Romieu AC, Adelman MW, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045-e1053. doi:10.1097/CCM.0000000000004600
14. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
15. Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi:10.1186/s12931-020-01462-5
16. Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243.e5-2243.e6. doi:10.1016/j.ajem.2020.05.044
17. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833-840. doi:10.1093/cid/ciaa443
18. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
19. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329-2330. doi:10.1001/jama.2020.6825
20. Schaller T, Hirschbuhl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518-2520. doi:10.1001/jama.2020.8907
21. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. doi:10.1056/NEJMoa2015432
22. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943. doi:10.1001/jamainternmed.2020.0994. Published correction appeared May 11, 2020. Errors in data and units of measure. doi:10.1001/jamainternmed.2020.1429
23. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. doi:10.1016/j.ijid.2020.03.017
24. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
25. Tobin MJ, Jubran A, Laghi F. Misconceptions of pathophysiology of happy hypoxemia and implications for management of COVID-19. Respir Res. 2020;21(1):249. doi:10.1186/s12931-020-01520-y
26. Bickler PE, Feiner JR, Lipnick MS, McKleroy W. “Silent” presentation of hypoxemia and cardiorespiratory compensation in COVID-19. Anesthesiology. 2020;134(2):262-269. doi:10.1097/ALN.0000000000003578
27. Jounieaux V, Parreira VF, Aubert G, Dury M, Delguste P, Rodenstein DO. Effects of hypocapnic hyperventilation on the response to hypoxia in normal subjects receiving intermittent positive-pressure ventilation. Chest. 2002;121(4):1141-1148. doi:10.1378/chest.121.4.1141
The Plague Year Revisited
In April 2020, I pledged to focus my editorials on the pandemic. In subsequent editorials I renewed that intention. And it is a promise I have kept during the long plague year for all my editorials. When I announced my plan to write solely on COVID-19, my astute editor asked me, “How are you going to know when to stop?” I reminded myself of his question as I sat down to write each month and never arrived at a satisfactory answer. Nor do I have an answer now for why I am asking readers to release me from my vow—except for the somewhat trivial reason that a year seems enough. Is there more to say about the pandemic? Yes, there is so much more that needs to be discovered and unraveled, contemplated and analyzed; no doubt oceans of print and electronic pages will wash over us in the coming decade from thousands of scientists and journalists commenting on the topic of this public health crisis.2
Nevertheless, I have run the gauntlet of salient subjects within my wheelhouse: The plague year of editorials opened with a primer on public health ethics; the May column studied the duty to care for health care professionals in the midst of the first surge of virus; June examined the controversy around remdesivir and hydroxcholoroquine as medicine frantically sought some way to treat the sick; in July, I took a lighter look at the “Dog Days” of COVID-19 staring my Labrador Retriever mix, Reed, snoozing on his couch on the patio; August celebrated the amazing outreach of the US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA) in service to the community; September discussed the adverse effects of the prolonged pandemic on the human psyche and some positive ways of handling the stress; October lamented the exponential rise in substance misuse as human beings struggled to manage the emotional toll of the pandemic; in December, COVID-19 was the sole subject of my annual Best and Worst ethics column; the new year saw the emergency use authorizations of the first and second vaccines and the editorial laid out the critical challenges for vaccination; in February my esteemed colleague Anita Tarzian joined me in an article explaining the ethical approach to vaccine allocation developed by the VA.3-12
A reader might aptly ask whether I am laying down the COVID-19 gauntlet because I believe the pandemic is over and done with us. The news is full of pundits opining when things will return to normal (if that ever existed or will again) and soothsayers divining the signs of the plague’s end.13 What I think is that we are more than done with the pandemic and unfortunately that may be the central cause of its perpetuation; which brings me to Daniel Defoe’s A Journal of the Plague Year.1
Defoe is better known to most of us if at all from modern films of his best-seller Robinson Crusoe. Yet A Journal of the Plague Year and other books about epidemics have become popular reading as we seek clues to the mystery of how to affirm life amid a death-dealing infectious disease.14 There is even an emerging lockdown literature genre. (Before anyone asks, I am in no way so pretentious as to suggest my columns should be included in that scholarly body of work).
Defoe’s book chronicles the last episode of the bubonic plague that afflicted London in 1665 and claimed 100,000 lives. Defoe was only 5 years old when the epidemic devastated one of the greatest cities in Europe. In 1772 he published what one recent reviewer called “a fascinating record of trying to cope with the capital’s last plague.”15 Defoe presciently documented the central reason I think the pandemic may not end anytime soon despite the increasing success of vaccination, at least in the United States. “But the Case was this...that the infection was propagated insensibly, and by such Persons, as were not visibly infected, who neither knew who they infected, or who they were infected by.”1
Ignorance and apathy are not confined to the streets of 17th century England: We see state after state lift restrictions prematurely, guaranteeing the scientists prediction that the wave now hitting Europe could again breach our shores. Defoe wrote long before germ theory and the ascendancy of public health, yet he knew that the inability or unwillingness to stick close to home kept the plague circulating. “And here I must observe again, that this Necessity of going out of our Houses to buy Provisions, was in a Great Measure the Ruin of the whole City, for the people catch’d the Distemper, on those Occasions, one of another...”1 While provisions may equate to food for many, for others necessities include going to bars, dining inside restaurants, and working out at gyms—all are natural laboratories for the spread and mutation of COVID-19 into variants against which physicians warn that the vaccine may not offer protection.
Defoe’s insights were at least in part due to his distance from the horror of the plague, which enabled him to study it with both empathy and objectivity, critical thinking, and creative observation. Similarly, it is time to take a brief breathing space from the pandemic as the central preoccupation of our existence: not just for me but for all of us to the extent possible given that unlike Defoe’s epoch it is still very much our reality. Even a few moments imagining a world without COVID-19 or more accurately one where it is under some reasonable control can help us reconceive how we want to live in it.
Can we use that luminal period to reenvision society along the lines Defoe idealistically drew even while we contribute to the collective search for the Holy Grail of herd immunity? During this second plague year, in coming editorials and in my own small circle of concern I will try to take a different less frustrated, embittered view of our lives scarred as they may be. It is only such a reorientation of perspectives in the shadow of so much death and suffering that can give us the energy and empathy to wear masks, go only where we must, follow public health measures and direction, and persuade the hesitant to be vaccinated so this truly is the last plague year at least for a long, quiet while.
1. Defoe D. A Journal of the Plague Year . Revised edition. Oxford World Classics; 2010.
2. Balch BT. One year into COVID, scientists are still learning about how the virus spreads, why disease symptoms and severity vary, and more. Published March 11, 2021. Accessed March 22, 2021. https://www.aamc.org/news-insights/one-year-covid-scientists-are-still-learning-about-how-virus-spreads-why-disease-symptoms-and
3. Geppert CMA. The return of the plague: a primer on pandemic ethics. Fed Pract. 2020;37(4):158-159.
4. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
5. Geppert CMA. A tale of 2 medications: a desperate race for hope. Fed Pract. 2020;37(6):256-257.
6. Geppert CMA. The dog days of COVID-19. Fed Pract. 2020;37(7):300-301.
7. Geppert CMA. All hands on deck: the federal health care response to the COVID-19 national emergency. Fed Pract. 2020;37(8):346-347. doi:10.12788/fp.0036
8. Geppert CMA. The brain in COVID-19: no one is okay. Fed Pract. 2020;37(9):396-397. doi:10.12788/fp.0046
9. Geppert CMA. The other pandemic: addiction. Fed Pract. 2020;37(10):440-441. doi:10.12788/fp.0059
10. Geppert CMA. Recalled to life: the best and worst of 2020 is the year 2020. Fed Pract . 2020;37(12):550-551. doi:10.12788/fp.0077
11. Geppert CMA. Trust in a vial. Fed Pract. 2021;38(1):4-5. doi:10.12788/fp.0084
12. Tarzian AJ, Geppert CMA. The Veterans Health Administration approach to COVID-19 vaccine allocation-balancing utility and equity. Fed Pract. 2021;38(2):52-54. doi:10.12788/fp.0093
13. Madrigal AG. A simple rule of thumb for knowing when the pandemic is over. Published February 23, 2021. Accessed March 22, 2021. https://www.theatlantic.com/health/archive/2021/02/how-know-when-pandemic-over/618122
14. Ford-Smith A. A Journal of the Plague Year book review. Med History. 2012;56(1):98-99. doi:10.1017/S0025727300000338
15. Jordison S. A Journal of the Plague Year by Daniel Defoe is our reading group book for May. The Guardian . Published April 28, 2020. Accessed March 22, 2021. https://www.theguardian.com/books/booksblog/2020/apr/28/a-journal-of-the-plague-year-by-daniel-defoe-is-our-reading-group-book-for-may
In April 2020, I pledged to focus my editorials on the pandemic. In subsequent editorials I renewed that intention. And it is a promise I have kept during the long plague year for all my editorials. When I announced my plan to write solely on COVID-19, my astute editor asked me, “How are you going to know when to stop?” I reminded myself of his question as I sat down to write each month and never arrived at a satisfactory answer. Nor do I have an answer now for why I am asking readers to release me from my vow—except for the somewhat trivial reason that a year seems enough. Is there more to say about the pandemic? Yes, there is so much more that needs to be discovered and unraveled, contemplated and analyzed; no doubt oceans of print and electronic pages will wash over us in the coming decade from thousands of scientists and journalists commenting on the topic of this public health crisis.2
Nevertheless, I have run the gauntlet of salient subjects within my wheelhouse: The plague year of editorials opened with a primer on public health ethics; the May column studied the duty to care for health care professionals in the midst of the first surge of virus; June examined the controversy around remdesivir and hydroxcholoroquine as medicine frantically sought some way to treat the sick; in July, I took a lighter look at the “Dog Days” of COVID-19 staring my Labrador Retriever mix, Reed, snoozing on his couch on the patio; August celebrated the amazing outreach of the US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA) in service to the community; September discussed the adverse effects of the prolonged pandemic on the human psyche and some positive ways of handling the stress; October lamented the exponential rise in substance misuse as human beings struggled to manage the emotional toll of the pandemic; in December, COVID-19 was the sole subject of my annual Best and Worst ethics column; the new year saw the emergency use authorizations of the first and second vaccines and the editorial laid out the critical challenges for vaccination; in February my esteemed colleague Anita Tarzian joined me in an article explaining the ethical approach to vaccine allocation developed by the VA.3-12
A reader might aptly ask whether I am laying down the COVID-19 gauntlet because I believe the pandemic is over and done with us. The news is full of pundits opining when things will return to normal (if that ever existed or will again) and soothsayers divining the signs of the plague’s end.13 What I think is that we are more than done with the pandemic and unfortunately that may be the central cause of its perpetuation; which brings me to Daniel Defoe’s A Journal of the Plague Year.1
Defoe is better known to most of us if at all from modern films of his best-seller Robinson Crusoe. Yet A Journal of the Plague Year and other books about epidemics have become popular reading as we seek clues to the mystery of how to affirm life amid a death-dealing infectious disease.14 There is even an emerging lockdown literature genre. (Before anyone asks, I am in no way so pretentious as to suggest my columns should be included in that scholarly body of work).
Defoe’s book chronicles the last episode of the bubonic plague that afflicted London in 1665 and claimed 100,000 lives. Defoe was only 5 years old when the epidemic devastated one of the greatest cities in Europe. In 1772 he published what one recent reviewer called “a fascinating record of trying to cope with the capital’s last plague.”15 Defoe presciently documented the central reason I think the pandemic may not end anytime soon despite the increasing success of vaccination, at least in the United States. “But the Case was this...that the infection was propagated insensibly, and by such Persons, as were not visibly infected, who neither knew who they infected, or who they were infected by.”1
Ignorance and apathy are not confined to the streets of 17th century England: We see state after state lift restrictions prematurely, guaranteeing the scientists prediction that the wave now hitting Europe could again breach our shores. Defoe wrote long before germ theory and the ascendancy of public health, yet he knew that the inability or unwillingness to stick close to home kept the plague circulating. “And here I must observe again, that this Necessity of going out of our Houses to buy Provisions, was in a Great Measure the Ruin of the whole City, for the people catch’d the Distemper, on those Occasions, one of another...”1 While provisions may equate to food for many, for others necessities include going to bars, dining inside restaurants, and working out at gyms—all are natural laboratories for the spread and mutation of COVID-19 into variants against which physicians warn that the vaccine may not offer protection.
Defoe’s insights were at least in part due to his distance from the horror of the plague, which enabled him to study it with both empathy and objectivity, critical thinking, and creative observation. Similarly, it is time to take a brief breathing space from the pandemic as the central preoccupation of our existence: not just for me but for all of us to the extent possible given that unlike Defoe’s epoch it is still very much our reality. Even a few moments imagining a world without COVID-19 or more accurately one where it is under some reasonable control can help us reconceive how we want to live in it.
Can we use that luminal period to reenvision society along the lines Defoe idealistically drew even while we contribute to the collective search for the Holy Grail of herd immunity? During this second plague year, in coming editorials and in my own small circle of concern I will try to take a different less frustrated, embittered view of our lives scarred as they may be. It is only such a reorientation of perspectives in the shadow of so much death and suffering that can give us the energy and empathy to wear masks, go only where we must, follow public health measures and direction, and persuade the hesitant to be vaccinated so this truly is the last plague year at least for a long, quiet while.
In April 2020, I pledged to focus my editorials on the pandemic. In subsequent editorials I renewed that intention. And it is a promise I have kept during the long plague year for all my editorials. When I announced my plan to write solely on COVID-19, my astute editor asked me, “How are you going to know when to stop?” I reminded myself of his question as I sat down to write each month and never arrived at a satisfactory answer. Nor do I have an answer now for why I am asking readers to release me from my vow—except for the somewhat trivial reason that a year seems enough. Is there more to say about the pandemic? Yes, there is so much more that needs to be discovered and unraveled, contemplated and analyzed; no doubt oceans of print and electronic pages will wash over us in the coming decade from thousands of scientists and journalists commenting on the topic of this public health crisis.2
Nevertheless, I have run the gauntlet of salient subjects within my wheelhouse: The plague year of editorials opened with a primer on public health ethics; the May column studied the duty to care for health care professionals in the midst of the first surge of virus; June examined the controversy around remdesivir and hydroxcholoroquine as medicine frantically sought some way to treat the sick; in July, I took a lighter look at the “Dog Days” of COVID-19 staring my Labrador Retriever mix, Reed, snoozing on his couch on the patio; August celebrated the amazing outreach of the US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA) in service to the community; September discussed the adverse effects of the prolonged pandemic on the human psyche and some positive ways of handling the stress; October lamented the exponential rise in substance misuse as human beings struggled to manage the emotional toll of the pandemic; in December, COVID-19 was the sole subject of my annual Best and Worst ethics column; the new year saw the emergency use authorizations of the first and second vaccines and the editorial laid out the critical challenges for vaccination; in February my esteemed colleague Anita Tarzian joined me in an article explaining the ethical approach to vaccine allocation developed by the VA.3-12
A reader might aptly ask whether I am laying down the COVID-19 gauntlet because I believe the pandemic is over and done with us. The news is full of pundits opining when things will return to normal (if that ever existed or will again) and soothsayers divining the signs of the plague’s end.13 What I think is that we are more than done with the pandemic and unfortunately that may be the central cause of its perpetuation; which brings me to Daniel Defoe’s A Journal of the Plague Year.1
Defoe is better known to most of us if at all from modern films of his best-seller Robinson Crusoe. Yet A Journal of the Plague Year and other books about epidemics have become popular reading as we seek clues to the mystery of how to affirm life amid a death-dealing infectious disease.14 There is even an emerging lockdown literature genre. (Before anyone asks, I am in no way so pretentious as to suggest my columns should be included in that scholarly body of work).
Defoe’s book chronicles the last episode of the bubonic plague that afflicted London in 1665 and claimed 100,000 lives. Defoe was only 5 years old when the epidemic devastated one of the greatest cities in Europe. In 1772 he published what one recent reviewer called “a fascinating record of trying to cope with the capital’s last plague.”15 Defoe presciently documented the central reason I think the pandemic may not end anytime soon despite the increasing success of vaccination, at least in the United States. “But the Case was this...that the infection was propagated insensibly, and by such Persons, as were not visibly infected, who neither knew who they infected, or who they were infected by.”1
Ignorance and apathy are not confined to the streets of 17th century England: We see state after state lift restrictions prematurely, guaranteeing the scientists prediction that the wave now hitting Europe could again breach our shores. Defoe wrote long before germ theory and the ascendancy of public health, yet he knew that the inability or unwillingness to stick close to home kept the plague circulating. “And here I must observe again, that this Necessity of going out of our Houses to buy Provisions, was in a Great Measure the Ruin of the whole City, for the people catch’d the Distemper, on those Occasions, one of another...”1 While provisions may equate to food for many, for others necessities include going to bars, dining inside restaurants, and working out at gyms—all are natural laboratories for the spread and mutation of COVID-19 into variants against which physicians warn that the vaccine may not offer protection.
Defoe’s insights were at least in part due to his distance from the horror of the plague, which enabled him to study it with both empathy and objectivity, critical thinking, and creative observation. Similarly, it is time to take a brief breathing space from the pandemic as the central preoccupation of our existence: not just for me but for all of us to the extent possible given that unlike Defoe’s epoch it is still very much our reality. Even a few moments imagining a world without COVID-19 or more accurately one where it is under some reasonable control can help us reconceive how we want to live in it.
Can we use that luminal period to reenvision society along the lines Defoe idealistically drew even while we contribute to the collective search for the Holy Grail of herd immunity? During this second plague year, in coming editorials and in my own small circle of concern I will try to take a different less frustrated, embittered view of our lives scarred as they may be. It is only such a reorientation of perspectives in the shadow of so much death and suffering that can give us the energy and empathy to wear masks, go only where we must, follow public health measures and direction, and persuade the hesitant to be vaccinated so this truly is the last plague year at least for a long, quiet while.
1. Defoe D. A Journal of the Plague Year . Revised edition. Oxford World Classics; 2010.
2. Balch BT. One year into COVID, scientists are still learning about how the virus spreads, why disease symptoms and severity vary, and more. Published March 11, 2021. Accessed March 22, 2021. https://www.aamc.org/news-insights/one-year-covid-scientists-are-still-learning-about-how-virus-spreads-why-disease-symptoms-and
3. Geppert CMA. The return of the plague: a primer on pandemic ethics. Fed Pract. 2020;37(4):158-159.
4. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
5. Geppert CMA. A tale of 2 medications: a desperate race for hope. Fed Pract. 2020;37(6):256-257.
6. Geppert CMA. The dog days of COVID-19. Fed Pract. 2020;37(7):300-301.
7. Geppert CMA. All hands on deck: the federal health care response to the COVID-19 national emergency. Fed Pract. 2020;37(8):346-347. doi:10.12788/fp.0036
8. Geppert CMA. The brain in COVID-19: no one is okay. Fed Pract. 2020;37(9):396-397. doi:10.12788/fp.0046
9. Geppert CMA. The other pandemic: addiction. Fed Pract. 2020;37(10):440-441. doi:10.12788/fp.0059
10. Geppert CMA. Recalled to life: the best and worst of 2020 is the year 2020. Fed Pract . 2020;37(12):550-551. doi:10.12788/fp.0077
11. Geppert CMA. Trust in a vial. Fed Pract. 2021;38(1):4-5. doi:10.12788/fp.0084
12. Tarzian AJ, Geppert CMA. The Veterans Health Administration approach to COVID-19 vaccine allocation-balancing utility and equity. Fed Pract. 2021;38(2):52-54. doi:10.12788/fp.0093
13. Madrigal AG. A simple rule of thumb for knowing when the pandemic is over. Published February 23, 2021. Accessed March 22, 2021. https://www.theatlantic.com/health/archive/2021/02/how-know-when-pandemic-over/618122
14. Ford-Smith A. A Journal of the Plague Year book review. Med History. 2012;56(1):98-99. doi:10.1017/S0025727300000338
15. Jordison S. A Journal of the Plague Year by Daniel Defoe is our reading group book for May. The Guardian . Published April 28, 2020. Accessed March 22, 2021. https://www.theguardian.com/books/booksblog/2020/apr/28/a-journal-of-the-plague-year-by-daniel-defoe-is-our-reading-group-book-for-may
1. Defoe D. A Journal of the Plague Year . Revised edition. Oxford World Classics; 2010.
2. Balch BT. One year into COVID, scientists are still learning about how the virus spreads, why disease symptoms and severity vary, and more. Published March 11, 2021. Accessed March 22, 2021. https://www.aamc.org/news-insights/one-year-covid-scientists-are-still-learning-about-how-virus-spreads-why-disease-symptoms-and
3. Geppert CMA. The return of the plague: a primer on pandemic ethics. Fed Pract. 2020;37(4):158-159.
4. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
5. Geppert CMA. A tale of 2 medications: a desperate race for hope. Fed Pract. 2020;37(6):256-257.
6. Geppert CMA. The dog days of COVID-19. Fed Pract. 2020;37(7):300-301.
7. Geppert CMA. All hands on deck: the federal health care response to the COVID-19 national emergency. Fed Pract. 2020;37(8):346-347. doi:10.12788/fp.0036
8. Geppert CMA. The brain in COVID-19: no one is okay. Fed Pract. 2020;37(9):396-397. doi:10.12788/fp.0046
9. Geppert CMA. The other pandemic: addiction. Fed Pract. 2020;37(10):440-441. doi:10.12788/fp.0059
10. Geppert CMA. Recalled to life: the best and worst of 2020 is the year 2020. Fed Pract . 2020;37(12):550-551. doi:10.12788/fp.0077
11. Geppert CMA. Trust in a vial. Fed Pract. 2021;38(1):4-5. doi:10.12788/fp.0084
12. Tarzian AJ, Geppert CMA. The Veterans Health Administration approach to COVID-19 vaccine allocation-balancing utility and equity. Fed Pract. 2021;38(2):52-54. doi:10.12788/fp.0093
13. Madrigal AG. A simple rule of thumb for knowing when the pandemic is over. Published February 23, 2021. Accessed March 22, 2021. https://www.theatlantic.com/health/archive/2021/02/how-know-when-pandemic-over/618122
14. Ford-Smith A. A Journal of the Plague Year book review. Med History. 2012;56(1):98-99. doi:10.1017/S0025727300000338
15. Jordison S. A Journal of the Plague Year by Daniel Defoe is our reading group book for May. The Guardian . Published April 28, 2020. Accessed March 22, 2021. https://www.theguardian.com/books/booksblog/2020/apr/28/a-journal-of-the-plague-year-by-daniel-defoe-is-our-reading-group-book-for-may
Confidently rule out CAP in the outpatient setting
ILLUSTRATIVE CASE
An otherwise healthy 56-year-old woman presents to the emergency department (ED) with a productive cough of 4 days’ duration. A review of her history is negative for recurrent upper respiratory infections, smoking, or environmental exposures. Her physical exam is unremarkable and, more specifically, her pulmonary exam and vital signs (temperature, respiratory rate, and heart rate) are within normal limits. The patient states that last year her friend had similar symptoms and was given a diagnosis of pneumonia. Is it necessary to order a chest x-ray in this patient to rule out community-acquired pneumonia (CAP)?
CAP is a common pulmonary condition seen in the outpatient setting in the United States, representing more than 4.5 million outpatient visits in the years 2009 to 2010.2 Historically, a diagnosis of CAP has been based on clinical findings in conjunction with infiltrates seen on chest x-ray.
In 2017, more than 5 million visits to the ED were due to a cough.3 The use of radiographic imaging in EDs has been increasing. There were 49 million x-rays and 2.7 million noncardiac chest computed tomography (CT) scans performed in 2016, many of which were for patients with cough.3,4 Although imaging is an extremely useful tool and indicated in many instances, the ability to rule out CAP in an adult who presents with a cough by using a set of simple, clinically based heuristics without requiring imaging would help to increase efficiency, limit cost, and decrease exposure of patients to unnecessary and potentially harmful diagnostic studies.
Clinical decision rules (CDRs) are simple heuristics that can stratify patients as either high risk or low risk for specific diseases. Two older large, prospective cross-sectional studies developed CDRs to determine the probability of CAP based on symptoms (eg, night sweats, myalgias, and sputum production) and clinical findings (eg, temperature > 37.8 °C [100 °F], tachypnea, tachycardia, rales, and decreased breath sounds).5,6 This meta-analysis includes these studies and more recent studies7-9 used to develop a CDR that focuses solely on a few specific signs and symptoms that can reliably rule out CAP without imaging, and so prove highly useful for busy primary care clinicians.
STUDY SUMMARY
This simple approach rules out CAP in outpatients 99.6% of the time
This systematic review and meta-analysis included studies that used 2 or more signs, symptoms, or point-of-care tests to determine the patient’s risk for CAP.1 Twelve studies (N = 10,254) met inclusion criteria by applying a CDR to adults or adolescents presenting with respiratory signs or symptoms potentially suggestive of CAP to either an outpatient setting or an ED. Prospective cohort, cross-sectional, and case-control studies were included when a chest x-ray or CT was utilized as the primary reference standard. Exclusion criteria included studies of military or nursing home populations and studies in which the majority of patients had hospital- or ventilator-associated pneumonia or were immunocompromised.
A simple, highly useful CDR emerged from 3 of the studies (N = 1865).7-9 Two of these studies were described as case-control studies with prospective enrollment of patients older than 17 years in both outpatient and ED settings.7,8 One study was conducted in the United States (mean age, 65 years) and the other in Iran (mean age, 60 years). The third was a Chilean prospective cohort study of ED patients older than 15 years (mean age, 53 years).9 In each of these studies, the outpatient or ED physicians collected all clinical data and documented their physical exam prior to receiving the chest radiograph results. The radiologists were masked to the clinical findings at the time of their interpretation.
Results. From the meta-analysis, a simple CDR emerged for patients with normal vital signs (temperature, respiratory rate, and heart rate) and a normal pulmonary exam that virtually ruled out CAP (sensitivity = 96%; 95% CI, 92%–98%; and negative likelihood ratio = 0.10; 95% CI, 0.07–0.13). In patients presenting to an outpatient clinic with acute cough with a 4% baseline prevalence rate of pneumonia, this CDR ruled out CAP 99.6% of the time.
Continue to: WHAT'S NEW
WHAT’S NEW
A clinical decision rule validated for accuracy
This is the first validated CDR that accurately rules out CAP in the outpatient or ED setting using parameters easily obtainable during a clinical exam.
CAVEATS
Proceed with caution in the young and the very old
Two of the 3 studies in this CDR had an overall moderate risk of bias, whereas the third study was determined to be at low risk of bias, based on appraisal with the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) framework.10
The mean age range in these 3 studies was 53 to 66 years (without further data such as standard deviation), suggesting that application of the CDR to adults who fall at extremes of age should be done with a modicum of caution.
Additionally, although the symptom complex of COVID-19 pneumonia would suggest that this CDR would likely remain accurate today, it has not been validated in patients with COVID-19 infection.
CHALLENGES TO IMPLEMENTATION
Potential reluctance to forgo imaging
Beyond the caveats regarding COVID-19, the use of a simple CDR to reliably exclude pneumonia should have no barrier to implementation in an outpatient primary care setting or ED, although there could be reluctance on the part of both providers and patients to fully embrace this simple tool without a confirmatory chest x-ray.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
2. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56-67.
3. CDC. National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2017. Emergency Department Summary Tables. Accessed March 24, 2021. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
4. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427.
5. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664-670.
6. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis. 1984;37:215-225.
7. O’Brien WT Sr, Rohweder DA, Lattin GE Jr, et al. Clinical indicators of radiographic findings in patients with suspected community-acquired pneumonia: who needs a chest x-ray? J Am Coll Radiol. 2006;3:703-706.
8. Ebrahimzadeh A, Mohammadifard M, Naseh G, et al. Clinical and laboratory findings in patients with acute respiratory symptoms that suggest the necessity of chest x-ray for community-acquired pneumonia. Iran J Radiol. 2015;12:e13547.
9. Saldías PF, Cabrera TD, de Solminihac LI, et al. Valor predictivo de la historia clínica y examen físico en el diagnóstico de neumonía del adulto adquirida en la comunidad [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Abstract in English. Rev Med Chil. 2007;135:143-152.
10. Whiting PF, Rutjes AWS, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
ILLUSTRATIVE CASE
An otherwise healthy 56-year-old woman presents to the emergency department (ED) with a productive cough of 4 days’ duration. A review of her history is negative for recurrent upper respiratory infections, smoking, or environmental exposures. Her physical exam is unremarkable and, more specifically, her pulmonary exam and vital signs (temperature, respiratory rate, and heart rate) are within normal limits. The patient states that last year her friend had similar symptoms and was given a diagnosis of pneumonia. Is it necessary to order a chest x-ray in this patient to rule out community-acquired pneumonia (CAP)?
CAP is a common pulmonary condition seen in the outpatient setting in the United States, representing more than 4.5 million outpatient visits in the years 2009 to 2010.2 Historically, a diagnosis of CAP has been based on clinical findings in conjunction with infiltrates seen on chest x-ray.
In 2017, more than 5 million visits to the ED were due to a cough.3 The use of radiographic imaging in EDs has been increasing. There were 49 million x-rays and 2.7 million noncardiac chest computed tomography (CT) scans performed in 2016, many of which were for patients with cough.3,4 Although imaging is an extremely useful tool and indicated in many instances, the ability to rule out CAP in an adult who presents with a cough by using a set of simple, clinically based heuristics without requiring imaging would help to increase efficiency, limit cost, and decrease exposure of patients to unnecessary and potentially harmful diagnostic studies.
Clinical decision rules (CDRs) are simple heuristics that can stratify patients as either high risk or low risk for specific diseases. Two older large, prospective cross-sectional studies developed CDRs to determine the probability of CAP based on symptoms (eg, night sweats, myalgias, and sputum production) and clinical findings (eg, temperature > 37.8 °C [100 °F], tachypnea, tachycardia, rales, and decreased breath sounds).5,6 This meta-analysis includes these studies and more recent studies7-9 used to develop a CDR that focuses solely on a few specific signs and symptoms that can reliably rule out CAP without imaging, and so prove highly useful for busy primary care clinicians.
STUDY SUMMARY
This simple approach rules out CAP in outpatients 99.6% of the time
This systematic review and meta-analysis included studies that used 2 or more signs, symptoms, or point-of-care tests to determine the patient’s risk for CAP.1 Twelve studies (N = 10,254) met inclusion criteria by applying a CDR to adults or adolescents presenting with respiratory signs or symptoms potentially suggestive of CAP to either an outpatient setting or an ED. Prospective cohort, cross-sectional, and case-control studies were included when a chest x-ray or CT was utilized as the primary reference standard. Exclusion criteria included studies of military or nursing home populations and studies in which the majority of patients had hospital- or ventilator-associated pneumonia or were immunocompromised.
A simple, highly useful CDR emerged from 3 of the studies (N = 1865).7-9 Two of these studies were described as case-control studies with prospective enrollment of patients older than 17 years in both outpatient and ED settings.7,8 One study was conducted in the United States (mean age, 65 years) and the other in Iran (mean age, 60 years). The third was a Chilean prospective cohort study of ED patients older than 15 years (mean age, 53 years).9 In each of these studies, the outpatient or ED physicians collected all clinical data and documented their physical exam prior to receiving the chest radiograph results. The radiologists were masked to the clinical findings at the time of their interpretation.
Results. From the meta-analysis, a simple CDR emerged for patients with normal vital signs (temperature, respiratory rate, and heart rate) and a normal pulmonary exam that virtually ruled out CAP (sensitivity = 96%; 95% CI, 92%–98%; and negative likelihood ratio = 0.10; 95% CI, 0.07–0.13). In patients presenting to an outpatient clinic with acute cough with a 4% baseline prevalence rate of pneumonia, this CDR ruled out CAP 99.6% of the time.
Continue to: WHAT'S NEW
WHAT’S NEW
A clinical decision rule validated for accuracy
This is the first validated CDR that accurately rules out CAP in the outpatient or ED setting using parameters easily obtainable during a clinical exam.
CAVEATS
Proceed with caution in the young and the very old
Two of the 3 studies in this CDR had an overall moderate risk of bias, whereas the third study was determined to be at low risk of bias, based on appraisal with the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) framework.10
The mean age range in these 3 studies was 53 to 66 years (without further data such as standard deviation), suggesting that application of the CDR to adults who fall at extremes of age should be done with a modicum of caution.
Additionally, although the symptom complex of COVID-19 pneumonia would suggest that this CDR would likely remain accurate today, it has not been validated in patients with COVID-19 infection.
CHALLENGES TO IMPLEMENTATION
Potential reluctance to forgo imaging
Beyond the caveats regarding COVID-19, the use of a simple CDR to reliably exclude pneumonia should have no barrier to implementation in an outpatient primary care setting or ED, although there could be reluctance on the part of both providers and patients to fully embrace this simple tool without a confirmatory chest x-ray.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
An otherwise healthy 56-year-old woman presents to the emergency department (ED) with a productive cough of 4 days’ duration. A review of her history is negative for recurrent upper respiratory infections, smoking, or environmental exposures. Her physical exam is unremarkable and, more specifically, her pulmonary exam and vital signs (temperature, respiratory rate, and heart rate) are within normal limits. The patient states that last year her friend had similar symptoms and was given a diagnosis of pneumonia. Is it necessary to order a chest x-ray in this patient to rule out community-acquired pneumonia (CAP)?
CAP is a common pulmonary condition seen in the outpatient setting in the United States, representing more than 4.5 million outpatient visits in the years 2009 to 2010.2 Historically, a diagnosis of CAP has been based on clinical findings in conjunction with infiltrates seen on chest x-ray.
In 2017, more than 5 million visits to the ED were due to a cough.3 The use of radiographic imaging in EDs has been increasing. There were 49 million x-rays and 2.7 million noncardiac chest computed tomography (CT) scans performed in 2016, many of which were for patients with cough.3,4 Although imaging is an extremely useful tool and indicated in many instances, the ability to rule out CAP in an adult who presents with a cough by using a set of simple, clinically based heuristics without requiring imaging would help to increase efficiency, limit cost, and decrease exposure of patients to unnecessary and potentially harmful diagnostic studies.
Clinical decision rules (CDRs) are simple heuristics that can stratify patients as either high risk or low risk for specific diseases. Two older large, prospective cross-sectional studies developed CDRs to determine the probability of CAP based on symptoms (eg, night sweats, myalgias, and sputum production) and clinical findings (eg, temperature > 37.8 °C [100 °F], tachypnea, tachycardia, rales, and decreased breath sounds).5,6 This meta-analysis includes these studies and more recent studies7-9 used to develop a CDR that focuses solely on a few specific signs and symptoms that can reliably rule out CAP without imaging, and so prove highly useful for busy primary care clinicians.
STUDY SUMMARY
This simple approach rules out CAP in outpatients 99.6% of the time
This systematic review and meta-analysis included studies that used 2 or more signs, symptoms, or point-of-care tests to determine the patient’s risk for CAP.1 Twelve studies (N = 10,254) met inclusion criteria by applying a CDR to adults or adolescents presenting with respiratory signs or symptoms potentially suggestive of CAP to either an outpatient setting or an ED. Prospective cohort, cross-sectional, and case-control studies were included when a chest x-ray or CT was utilized as the primary reference standard. Exclusion criteria included studies of military or nursing home populations and studies in which the majority of patients had hospital- or ventilator-associated pneumonia or were immunocompromised.
A simple, highly useful CDR emerged from 3 of the studies (N = 1865).7-9 Two of these studies were described as case-control studies with prospective enrollment of patients older than 17 years in both outpatient and ED settings.7,8 One study was conducted in the United States (mean age, 65 years) and the other in Iran (mean age, 60 years). The third was a Chilean prospective cohort study of ED patients older than 15 years (mean age, 53 years).9 In each of these studies, the outpatient or ED physicians collected all clinical data and documented their physical exam prior to receiving the chest radiograph results. The radiologists were masked to the clinical findings at the time of their interpretation.
Results. From the meta-analysis, a simple CDR emerged for patients with normal vital signs (temperature, respiratory rate, and heart rate) and a normal pulmonary exam that virtually ruled out CAP (sensitivity = 96%; 95% CI, 92%–98%; and negative likelihood ratio = 0.10; 95% CI, 0.07–0.13). In patients presenting to an outpatient clinic with acute cough with a 4% baseline prevalence rate of pneumonia, this CDR ruled out CAP 99.6% of the time.
Continue to: WHAT'S NEW
WHAT’S NEW
A clinical decision rule validated for accuracy
This is the first validated CDR that accurately rules out CAP in the outpatient or ED setting using parameters easily obtainable during a clinical exam.
CAVEATS
Proceed with caution in the young and the very old
Two of the 3 studies in this CDR had an overall moderate risk of bias, whereas the third study was determined to be at low risk of bias, based on appraisal with the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS-2) framework.10
The mean age range in these 3 studies was 53 to 66 years (without further data such as standard deviation), suggesting that application of the CDR to adults who fall at extremes of age should be done with a modicum of caution.
Additionally, although the symptom complex of COVID-19 pneumonia would suggest that this CDR would likely remain accurate today, it has not been validated in patients with COVID-19 infection.
CHALLENGES TO IMPLEMENTATION
Potential reluctance to forgo imaging
Beyond the caveats regarding COVID-19, the use of a simple CDR to reliably exclude pneumonia should have no barrier to implementation in an outpatient primary care setting or ED, although there could be reluctance on the part of both providers and patients to fully embrace this simple tool without a confirmatory chest x-ray.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
2. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56-67.
3. CDC. National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2017. Emergency Department Summary Tables. Accessed March 24, 2021. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
4. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427.
5. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664-670.
6. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis. 1984;37:215-225.
7. O’Brien WT Sr, Rohweder DA, Lattin GE Jr, et al. Clinical indicators of radiographic findings in patients with suspected community-acquired pneumonia: who needs a chest x-ray? J Am Coll Radiol. 2006;3:703-706.
8. Ebrahimzadeh A, Mohammadifard M, Naseh G, et al. Clinical and laboratory findings in patients with acute respiratory symptoms that suggest the necessity of chest x-ray for community-acquired pneumonia. Iran J Radiol. 2015;12:e13547.
9. Saldías PF, Cabrera TD, de Solminihac LI, et al. Valor predictivo de la historia clínica y examen físico en el diagnóstico de neumonía del adulto adquirida en la comunidad [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Abstract in English. Rev Med Chil. 2007;135:143-152.
10. Whiting PF, Rutjes AWS, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
1. Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
2. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56-67.
3. CDC. National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2017. Emergency Department Summary Tables. Accessed March 24, 2021. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
4. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415-427.
5. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664-670.
6. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis. 1984;37:215-225.
7. O’Brien WT Sr, Rohweder DA, Lattin GE Jr, et al. Clinical indicators of radiographic findings in patients with suspected community-acquired pneumonia: who needs a chest x-ray? J Am Coll Radiol. 2006;3:703-706.
8. Ebrahimzadeh A, Mohammadifard M, Naseh G, et al. Clinical and laboratory findings in patients with acute respiratory symptoms that suggest the necessity of chest x-ray for community-acquired pneumonia. Iran J Radiol. 2015;12:e13547.
9. Saldías PF, Cabrera TD, de Solminihac LI, et al. Valor predictivo de la historia clínica y examen físico en el diagnóstico de neumonía del adulto adquirida en la comunidad [Predictive value of history and physical examination for the diagnosis of community-acquired pneumonia in adults]. Abstract in English. Rev Med Chil. 2007;135:143-152.
10. Whiting PF, Rutjes AWS, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536.
PRACTICE CHANGER
You can safely rule out community-acquired pneumonia (CAP)—without requiring a chest x-ray—in an otherwise healthy adult outpatient who has an acute cough, a normal pulmonary exam, and normal vital signs using this simple clinical decision rule (CDR).1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review of prospective case-control studies and randomized controlled trials in the outpatient setting.1
Marchello CS, Ebell MH, Dale AP, et al. Signs and symptoms that rule out community-acquired pneumonia in outpatient adults: a systematic review and meta-analysis. J Am Board Fam Med. 2019;32:234-247.
University taking aim at racial disparities in COVID vaccine trials
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.