User login
Being overweight ups risk of severe COVID-19 in hospital
In a global meta-analysis of more than 7,000 patients who were hospitalized with COVID-19, individuals with overweight or obesity were more likely to need respiratory support but were not more likely to die in the hospital, compared to individuals of normal weight.
Compared to patients without diabetes, those with diabetes had higher odds of needing invasive respiratory support (with intubation) but not for needing noninvasive respiratory support or of dying in the hospital.
“Surprisingly,” among patients with diabetes, being overweight or having obesity did not further increase the odds of any of these outcomes, the researchers wrote. The finding needs to be confirmed in larger studies, they said, because the sample sizes in these subanalyses were small and the confidence intervals were large.
The study by Danielle K. Longmore, PhD, of Murdoch Children’s Research Institute (MCRI), Melbourne, and colleagues from the International BMI-COVID consortium, was published online April 15 in Diabetes Care.
This new research “adds to the known data on the associations between obesity and severe COVID-19 disease and extends these findings” to patients who are overweight and/or have diabetes, Dr. Longmore, a pediatric endocrinologist with a clinical and research interest in childhood and youth obesity, said in an interview.
Immunologist Siroon Bekkering, PhD, of Radboud University Medical Center, Nijmegen, the Netherlands, explained that never before have so much data of different types regarding obesity been combined in one large study. Dr. Bekkering is a coauthor of the article and was a principal investigator.
“Several national and international observations already showed the important role of overweight and obesity in a more severe COVID-19 course. This study adds to those observations by combining data from several countries with the possibility to look at the risk factors separately,” she said in a statement from her institution.
“Regardless of other risk factors (such as heart disease or diabetes), we now see that too high a BMI [body mass index] can actually lead to a more severe course in [coronavirus] infection,” she said.
Study implications: Data show that overweight, obesity add to risk
These latest findings highlight the urgent need to develop public health policies to address socioeconomic and psychological drivers of obesity, Dr. Longmore said.
“Although taking steps to address obesity in the short term is unlikely to have an immediate impact in the COVID-19 pandemic, it will likely reduce the disease burden in future viral pandemics and reduce risks of complications like heart disease and stroke,” she observed in a statement issued by MCRI.
Coauthor Kirsty R. Short, PhD, a research fellow at the University of Queensland, Brisbane, Australia, noted that “obesity is associated with numerous poor health outcomes, including increased risk of cardiometabolic and respiratory disease and more severe viral disease including influenza, dengue, and SARS-CoV-1.
“Given the large scale of this study,” she said, “we have conclusively shown that being overweight or obese are independent risk factors for worse outcomes in adults hospitalized with COVID-19.”
“At the moment, the World Health Organization has not had enough high-quality data to include being overweight or obese as a risk factor for severe COVID-19 disease,” added another author, David P. Burgner, PhD, a pediatric infectious diseases clinician scientist from MCRI.
“Our study should help inform decisions about which higher-risk groups should be vaccinated as a priority,” he observed.
Does being overweight up risk of worse COVID-19 outcomes?
About 13% of the world’s population are overweight, and 40% have obesity. There are wide between-country variations in these data, and about 90% of patients with type 2 diabetes are overweight or obese, the researchers noted.
The Organisation for Economic Co-operation and Development reported that the prevalence of obesity in 2016-2017 was 5.7% to 8.9% in Asia, 9.8% to 16.8% in Europe, 26.5% in South Africa, and 40.0% in the United States, they added.
Obesity is common and has emerged as an important risk factor for severe COVID-19. However, most previous studies of COVID-19 and elevated BMI were conducted in single centers and did not focus on patients with overweight.
To investigate, the researchers identified 7,244 patients (two-thirds were overweight or obese) who were hospitalized with COVID-19 in 69 hospitals (18 sites) in 11 countries from Jan. 17, 2020, to June 2, 2020.
Most patients were hospitalized with COVID-19 in the Netherlands (2,260), followed by New York City (1,682), Switzerland (920), St. Louis (805), Norway, Italy, China, South Africa, Indonesia, Denmark, Los Angeles, Austria, and Singapore.
Just over half (60%) of the individuals were male, and 52% were older than 65.
Overall, 34.8% were overweight, and 30.8% had obesity, but the average weight varied considerably between countries and sites.
Increased need for respiratory support, same mortality risk
Compared with patients with normal weight, patients who were overweight had a 44% increased risk of needing supplemental oxygen/noninvasive ventilation, and those with obesity had a 75% increased risk of this, after adjustment for age (< 65, ≥ 65), sex, hypertension, diabetes, or preexisting cardiovascular disease or respiratory conditions.
Patients who were overweight had a 22% increased risk of needing invasive (mechanical) ventilation, and those with obesity had a 73% increased risk of this, after multivariable adjustment.
Being overweight or having obesity was not associated with a significantly increased risk of dying in the hospital, however.
“In other viral respiratory infections, such as influenza, there is a similar pattern of increased requirement for ventilatory support but lower in-hospital mortality among individuals with obesity, when compared to those with normal range BMI,” Dr. Longmore noted. She said that larger studies are needed to further explore this finding regarding COVID-19.
Compared to patients without diabetes, those with diabetes had a 21% increased risk of requiring invasive ventilation, but they did not have an increased risk of needing noninvasive ventilation or of dying in the hospital.
As in previous studies, individuals who had cardiovascular and preexisting respiratory diseases were not at greater risk of needing oxygen or mechanical ventilation but were at increased risk for in-hospital death. Men had a greater risk of needing invasive mechanical ventilation, and individuals who were older than 65 had an increased risk of requiring oxygen or of dying in the hospital.
A living meta-analysis, call for more collaborators
“We consider this a ‘living meta-analysis’ and invite other centers to join us,” Dr. Longmore said. “We hope to update the analyses as more data are contributed.”
No specific project funded the study. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a global meta-analysis of more than 7,000 patients who were hospitalized with COVID-19, individuals with overweight or obesity were more likely to need respiratory support but were not more likely to die in the hospital, compared to individuals of normal weight.
Compared to patients without diabetes, those with diabetes had higher odds of needing invasive respiratory support (with intubation) but not for needing noninvasive respiratory support or of dying in the hospital.
“Surprisingly,” among patients with diabetes, being overweight or having obesity did not further increase the odds of any of these outcomes, the researchers wrote. The finding needs to be confirmed in larger studies, they said, because the sample sizes in these subanalyses were small and the confidence intervals were large.
The study by Danielle K. Longmore, PhD, of Murdoch Children’s Research Institute (MCRI), Melbourne, and colleagues from the International BMI-COVID consortium, was published online April 15 in Diabetes Care.
This new research “adds to the known data on the associations between obesity and severe COVID-19 disease and extends these findings” to patients who are overweight and/or have diabetes, Dr. Longmore, a pediatric endocrinologist with a clinical and research interest in childhood and youth obesity, said in an interview.
Immunologist Siroon Bekkering, PhD, of Radboud University Medical Center, Nijmegen, the Netherlands, explained that never before have so much data of different types regarding obesity been combined in one large study. Dr. Bekkering is a coauthor of the article and was a principal investigator.
“Several national and international observations already showed the important role of overweight and obesity in a more severe COVID-19 course. This study adds to those observations by combining data from several countries with the possibility to look at the risk factors separately,” she said in a statement from her institution.
“Regardless of other risk factors (such as heart disease or diabetes), we now see that too high a BMI [body mass index] can actually lead to a more severe course in [coronavirus] infection,” she said.
Study implications: Data show that overweight, obesity add to risk
These latest findings highlight the urgent need to develop public health policies to address socioeconomic and psychological drivers of obesity, Dr. Longmore said.
“Although taking steps to address obesity in the short term is unlikely to have an immediate impact in the COVID-19 pandemic, it will likely reduce the disease burden in future viral pandemics and reduce risks of complications like heart disease and stroke,” she observed in a statement issued by MCRI.
Coauthor Kirsty R. Short, PhD, a research fellow at the University of Queensland, Brisbane, Australia, noted that “obesity is associated with numerous poor health outcomes, including increased risk of cardiometabolic and respiratory disease and more severe viral disease including influenza, dengue, and SARS-CoV-1.
“Given the large scale of this study,” she said, “we have conclusively shown that being overweight or obese are independent risk factors for worse outcomes in adults hospitalized with COVID-19.”
“At the moment, the World Health Organization has not had enough high-quality data to include being overweight or obese as a risk factor for severe COVID-19 disease,” added another author, David P. Burgner, PhD, a pediatric infectious diseases clinician scientist from MCRI.
“Our study should help inform decisions about which higher-risk groups should be vaccinated as a priority,” he observed.
Does being overweight up risk of worse COVID-19 outcomes?
About 13% of the world’s population are overweight, and 40% have obesity. There are wide between-country variations in these data, and about 90% of patients with type 2 diabetes are overweight or obese, the researchers noted.
The Organisation for Economic Co-operation and Development reported that the prevalence of obesity in 2016-2017 was 5.7% to 8.9% in Asia, 9.8% to 16.8% in Europe, 26.5% in South Africa, and 40.0% in the United States, they added.
Obesity is common and has emerged as an important risk factor for severe COVID-19. However, most previous studies of COVID-19 and elevated BMI were conducted in single centers and did not focus on patients with overweight.
To investigate, the researchers identified 7,244 patients (two-thirds were overweight or obese) who were hospitalized with COVID-19 in 69 hospitals (18 sites) in 11 countries from Jan. 17, 2020, to June 2, 2020.
Most patients were hospitalized with COVID-19 in the Netherlands (2,260), followed by New York City (1,682), Switzerland (920), St. Louis (805), Norway, Italy, China, South Africa, Indonesia, Denmark, Los Angeles, Austria, and Singapore.
Just over half (60%) of the individuals were male, and 52% were older than 65.
Overall, 34.8% were overweight, and 30.8% had obesity, but the average weight varied considerably between countries and sites.
Increased need for respiratory support, same mortality risk
Compared with patients with normal weight, patients who were overweight had a 44% increased risk of needing supplemental oxygen/noninvasive ventilation, and those with obesity had a 75% increased risk of this, after adjustment for age (< 65, ≥ 65), sex, hypertension, diabetes, or preexisting cardiovascular disease or respiratory conditions.
Patients who were overweight had a 22% increased risk of needing invasive (mechanical) ventilation, and those with obesity had a 73% increased risk of this, after multivariable adjustment.
Being overweight or having obesity was not associated with a significantly increased risk of dying in the hospital, however.
“In other viral respiratory infections, such as influenza, there is a similar pattern of increased requirement for ventilatory support but lower in-hospital mortality among individuals with obesity, when compared to those with normal range BMI,” Dr. Longmore noted. She said that larger studies are needed to further explore this finding regarding COVID-19.
Compared to patients without diabetes, those with diabetes had a 21% increased risk of requiring invasive ventilation, but they did not have an increased risk of needing noninvasive ventilation or of dying in the hospital.
As in previous studies, individuals who had cardiovascular and preexisting respiratory diseases were not at greater risk of needing oxygen or mechanical ventilation but were at increased risk for in-hospital death. Men had a greater risk of needing invasive mechanical ventilation, and individuals who were older than 65 had an increased risk of requiring oxygen or of dying in the hospital.
A living meta-analysis, call for more collaborators
“We consider this a ‘living meta-analysis’ and invite other centers to join us,” Dr. Longmore said. “We hope to update the analyses as more data are contributed.”
No specific project funded the study. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a global meta-analysis of more than 7,000 patients who were hospitalized with COVID-19, individuals with overweight or obesity were more likely to need respiratory support but were not more likely to die in the hospital, compared to individuals of normal weight.
Compared to patients without diabetes, those with diabetes had higher odds of needing invasive respiratory support (with intubation) but not for needing noninvasive respiratory support or of dying in the hospital.
“Surprisingly,” among patients with diabetes, being overweight or having obesity did not further increase the odds of any of these outcomes, the researchers wrote. The finding needs to be confirmed in larger studies, they said, because the sample sizes in these subanalyses were small and the confidence intervals were large.
The study by Danielle K. Longmore, PhD, of Murdoch Children’s Research Institute (MCRI), Melbourne, and colleagues from the International BMI-COVID consortium, was published online April 15 in Diabetes Care.
This new research “adds to the known data on the associations between obesity and severe COVID-19 disease and extends these findings” to patients who are overweight and/or have diabetes, Dr. Longmore, a pediatric endocrinologist with a clinical and research interest in childhood and youth obesity, said in an interview.
Immunologist Siroon Bekkering, PhD, of Radboud University Medical Center, Nijmegen, the Netherlands, explained that never before have so much data of different types regarding obesity been combined in one large study. Dr. Bekkering is a coauthor of the article and was a principal investigator.
“Several national and international observations already showed the important role of overweight and obesity in a more severe COVID-19 course. This study adds to those observations by combining data from several countries with the possibility to look at the risk factors separately,” she said in a statement from her institution.
“Regardless of other risk factors (such as heart disease or diabetes), we now see that too high a BMI [body mass index] can actually lead to a more severe course in [coronavirus] infection,” she said.
Study implications: Data show that overweight, obesity add to risk
These latest findings highlight the urgent need to develop public health policies to address socioeconomic and psychological drivers of obesity, Dr. Longmore said.
“Although taking steps to address obesity in the short term is unlikely to have an immediate impact in the COVID-19 pandemic, it will likely reduce the disease burden in future viral pandemics and reduce risks of complications like heart disease and stroke,” she observed in a statement issued by MCRI.
Coauthor Kirsty R. Short, PhD, a research fellow at the University of Queensland, Brisbane, Australia, noted that “obesity is associated with numerous poor health outcomes, including increased risk of cardiometabolic and respiratory disease and more severe viral disease including influenza, dengue, and SARS-CoV-1.
“Given the large scale of this study,” she said, “we have conclusively shown that being overweight or obese are independent risk factors for worse outcomes in adults hospitalized with COVID-19.”
“At the moment, the World Health Organization has not had enough high-quality data to include being overweight or obese as a risk factor for severe COVID-19 disease,” added another author, David P. Burgner, PhD, a pediatric infectious diseases clinician scientist from MCRI.
“Our study should help inform decisions about which higher-risk groups should be vaccinated as a priority,” he observed.
Does being overweight up risk of worse COVID-19 outcomes?
About 13% of the world’s population are overweight, and 40% have obesity. There are wide between-country variations in these data, and about 90% of patients with type 2 diabetes are overweight or obese, the researchers noted.
The Organisation for Economic Co-operation and Development reported that the prevalence of obesity in 2016-2017 was 5.7% to 8.9% in Asia, 9.8% to 16.8% in Europe, 26.5% in South Africa, and 40.0% in the United States, they added.
Obesity is common and has emerged as an important risk factor for severe COVID-19. However, most previous studies of COVID-19 and elevated BMI were conducted in single centers and did not focus on patients with overweight.
To investigate, the researchers identified 7,244 patients (two-thirds were overweight or obese) who were hospitalized with COVID-19 in 69 hospitals (18 sites) in 11 countries from Jan. 17, 2020, to June 2, 2020.
Most patients were hospitalized with COVID-19 in the Netherlands (2,260), followed by New York City (1,682), Switzerland (920), St. Louis (805), Norway, Italy, China, South Africa, Indonesia, Denmark, Los Angeles, Austria, and Singapore.
Just over half (60%) of the individuals were male, and 52% were older than 65.
Overall, 34.8% were overweight, and 30.8% had obesity, but the average weight varied considerably between countries and sites.
Increased need for respiratory support, same mortality risk
Compared with patients with normal weight, patients who were overweight had a 44% increased risk of needing supplemental oxygen/noninvasive ventilation, and those with obesity had a 75% increased risk of this, after adjustment for age (< 65, ≥ 65), sex, hypertension, diabetes, or preexisting cardiovascular disease or respiratory conditions.
Patients who were overweight had a 22% increased risk of needing invasive (mechanical) ventilation, and those with obesity had a 73% increased risk of this, after multivariable adjustment.
Being overweight or having obesity was not associated with a significantly increased risk of dying in the hospital, however.
“In other viral respiratory infections, such as influenza, there is a similar pattern of increased requirement for ventilatory support but lower in-hospital mortality among individuals with obesity, when compared to those with normal range BMI,” Dr. Longmore noted. She said that larger studies are needed to further explore this finding regarding COVID-19.
Compared to patients without diabetes, those with diabetes had a 21% increased risk of requiring invasive ventilation, but they did not have an increased risk of needing noninvasive ventilation or of dying in the hospital.
As in previous studies, individuals who had cardiovascular and preexisting respiratory diseases were not at greater risk of needing oxygen or mechanical ventilation but were at increased risk for in-hospital death. Men had a greater risk of needing invasive mechanical ventilation, and individuals who were older than 65 had an increased risk of requiring oxygen or of dying in the hospital.
A living meta-analysis, call for more collaborators
“We consider this a ‘living meta-analysis’ and invite other centers to join us,” Dr. Longmore said. “We hope to update the analyses as more data are contributed.”
No specific project funded the study. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IV drug users: The new face of candidemia
Background: Intravenous drug use is an increasingly common risk factor for candidemia as the opioid crisis worsens. This study quantifies this change and characterizes the changing epidemiology of candidemia.
Study design: A cross-sectional study.
Setting: Health departments in nine states.
Synopsis: IV drug users typically have a very distinctive phenotype among all patients with candidemia: They are younger (35 vs. 63 years), are more likely to be homeless, are not black, are smokers; they have hepatitis C, have no malignancies, have polymicrobial bacteremia, and have acquired the infection outside of the hospital. They are much less likely to die of the infection (8.6% vs 27.5%), compared with the non-IV drug users. In four states, the proportion of candidemia associated with IV drug use more than doubled, from 7% to 15% during 2014-2017, representing a possible shift in the epidemiology of candidemia.
The study did not quantify or address complications that many hospitalists see, such as endocarditis, endophthalmitis, and osteomyelitis. The study looked at only nine states, so results may not be generalizable. Nevertheless, the robust analysis suggests an alarming, increasing trend.
Bottom line: As the opioid crisis worsens, hospitalists should consider candidemia in hospitalized IV drug users and should evaluate patients with candidemia for IV drug use.
Citation: Zhang AY et al. The changing epidemiology of candidemia in the United States: Injection drug use as an increasingly common risk factor – Active surveillance in selected sites, United States, 2014-2017. Clin Infect Dis. 2019 Nov 2. doi: 10.1093/cid/ciz1061.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Intravenous drug use is an increasingly common risk factor for candidemia as the opioid crisis worsens. This study quantifies this change and characterizes the changing epidemiology of candidemia.
Study design: A cross-sectional study.
Setting: Health departments in nine states.
Synopsis: IV drug users typically have a very distinctive phenotype among all patients with candidemia: They are younger (35 vs. 63 years), are more likely to be homeless, are not black, are smokers; they have hepatitis C, have no malignancies, have polymicrobial bacteremia, and have acquired the infection outside of the hospital. They are much less likely to die of the infection (8.6% vs 27.5%), compared with the non-IV drug users. In four states, the proportion of candidemia associated with IV drug use more than doubled, from 7% to 15% during 2014-2017, representing a possible shift in the epidemiology of candidemia.
The study did not quantify or address complications that many hospitalists see, such as endocarditis, endophthalmitis, and osteomyelitis. The study looked at only nine states, so results may not be generalizable. Nevertheless, the robust analysis suggests an alarming, increasing trend.
Bottom line: As the opioid crisis worsens, hospitalists should consider candidemia in hospitalized IV drug users and should evaluate patients with candidemia for IV drug use.
Citation: Zhang AY et al. The changing epidemiology of candidemia in the United States: Injection drug use as an increasingly common risk factor – Active surveillance in selected sites, United States, 2014-2017. Clin Infect Dis. 2019 Nov 2. doi: 10.1093/cid/ciz1061.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Intravenous drug use is an increasingly common risk factor for candidemia as the opioid crisis worsens. This study quantifies this change and characterizes the changing epidemiology of candidemia.
Study design: A cross-sectional study.
Setting: Health departments in nine states.
Synopsis: IV drug users typically have a very distinctive phenotype among all patients with candidemia: They are younger (35 vs. 63 years), are more likely to be homeless, are not black, are smokers; they have hepatitis C, have no malignancies, have polymicrobial bacteremia, and have acquired the infection outside of the hospital. They are much less likely to die of the infection (8.6% vs 27.5%), compared with the non-IV drug users. In four states, the proportion of candidemia associated with IV drug use more than doubled, from 7% to 15% during 2014-2017, representing a possible shift in the epidemiology of candidemia.
The study did not quantify or address complications that many hospitalists see, such as endocarditis, endophthalmitis, and osteomyelitis. The study looked at only nine states, so results may not be generalizable. Nevertheless, the robust analysis suggests an alarming, increasing trend.
Bottom line: As the opioid crisis worsens, hospitalists should consider candidemia in hospitalized IV drug users and should evaluate patients with candidemia for IV drug use.
Citation: Zhang AY et al. The changing epidemiology of candidemia in the United States: Injection drug use as an increasingly common risk factor – Active surveillance in selected sites, United States, 2014-2017. Clin Infect Dis. 2019 Nov 2. doi: 10.1093/cid/ciz1061.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Head to Toe: Recommendations for Physician Head and Shoe Coverings to Limit COVID-19 Transmission
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).
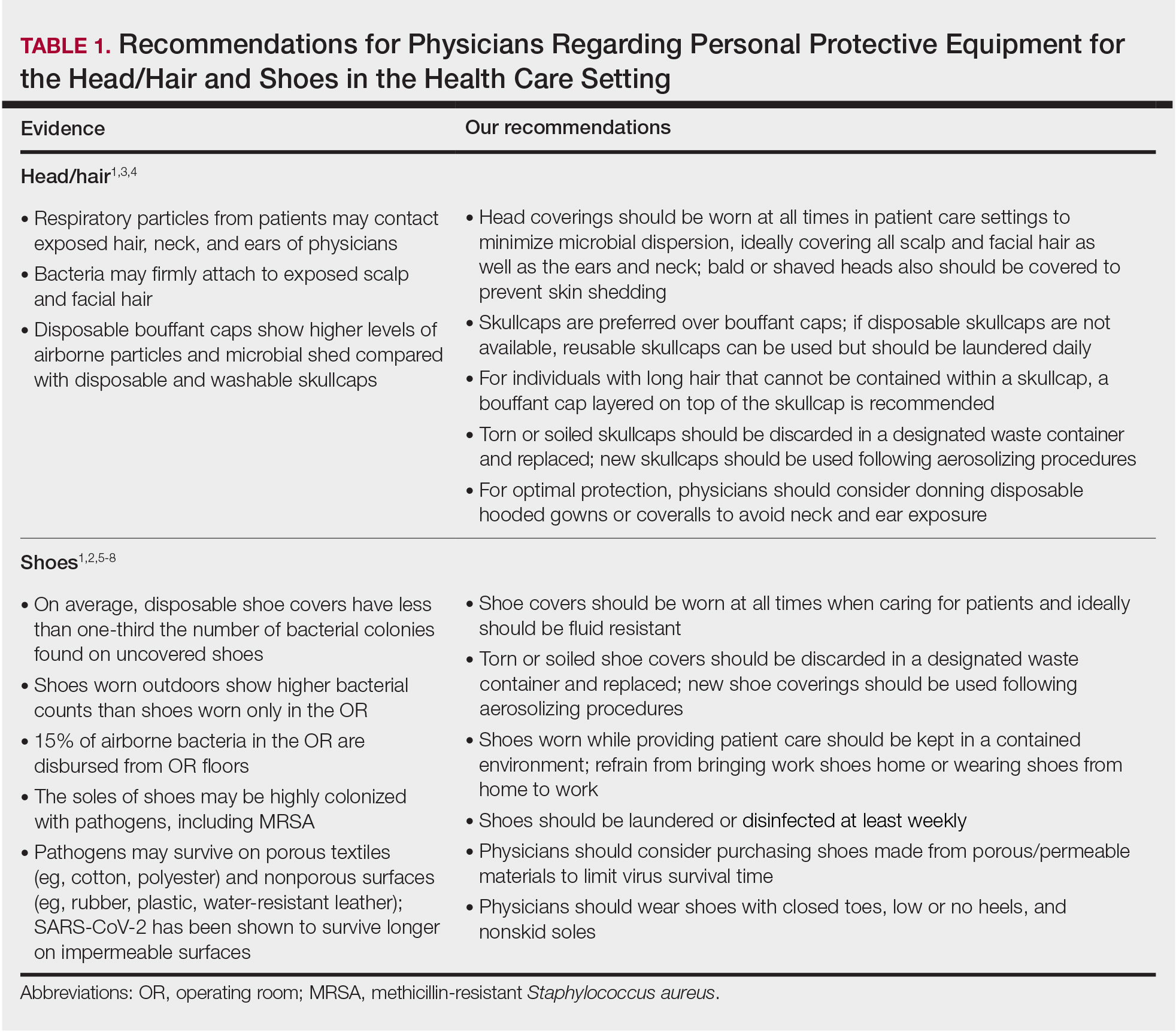
Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).

Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.

Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).

Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.

- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
Practice Points
- Consistent use of personal protective equipment, including masks, face shields, goggles, and gloves, may limit transmission of SARS-CoV-2.
- Hair and shoes also may transmit SARS-CoV-2, but recommendations for hair and shoe coverings to prevent SARS-CoV-2 are lacking.
Line of therapy matters for assessing biologic’s serious infection risk in RA
The order in which tocilizumab (Actemra) is used in the sequence of treatments for rheumatoid arthritis could be muddying the waters when it comes to evaluating patients’ risk for serious infection.
According to new data emerging from the British Society for Rheumatology Biologics Register – Rheumatoid Arthritis (BSRBR-RA), the line of therapy is a confounding factor when examining the risk for serious infection with not only tocilizumab but also other biologic agents.
The good news for patients, however, is that there doesn’t appear to be any overall greater risk for serious infection with one biologic over another when the line of therapy is taken into account.
“We don’t have any strong signal that there is an increased risk of serious infections with tocilizumab, compared to TNF inhibitors,” rheumatologist Kim Lauper, MD, of Geneva University Hospitals, said in an interview after presenting the data at the annual conference of the British Society for Rheumatology.
This is in contrast to studies where an increased risk of infections with tocilizumab has been seen when compared to TNF inhibitors. However, those studies did not account for the line of therapy, explained Dr. Lauper, who is also a clinical research fellow in the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), where the BSRBR-RA is managed.
“Tocilizumab is a treatment that we often give to patients after several other treatments, so they’re really different patients,” Dr. Lauper observed. Indeed, in the “real-world” setting, patients taking tocilizumab tend to be older, have longer disease duration, and have worse functional status than do those who might receive other biologics.
To look at the effect of line of therapy on the serious infection risk associated with commonly used biologic drugs, Dr. Lauper and associates examined data on more than 33,000 treatment courses, representing more than 62,500 patient-years.
Using etanercept as the comparator – because it represents the largest group of patients in the BSRBR-RA – the serious infection risk for tocilizumab, rituximab, adalimumab, infliximab, certolizumab pegol, and abatacept were calculated as an overall rate, and for their use as first-, second-, third-, fourth-, or fifth-line therapy.
The researchers adjusted their analysis for some clear baseline differences between the treatment groups, including age, prior treatment, disease duration, and comorbidities. Seropositivity, smoking status, general health status, and disease activity scores were also adjusted for in the analysis.
Crude hazard ratios (HRs), compared with etanercept, before and after adjusting for these already-known confounding factors were 1.0 and 1.2 for tocilizumab, 1.1 and 1.1 for adalimumab, 1.4 and 1.3 for infliximab, 0.6 and 0.8 for certolizumab pegol, 0.9 and 1.0 for rituximab, and 0.9 and 1.2 for abatacept.
Stratifying by line of therapy, however, changed the results: HRs were no longer significantly different, compared with etanercept, for tocilizumab, adalimumab, and infliximab for most lines of therapy.
Indeed, while the risk for serious infection occurring with tocilizumab was 20% higher overall, compared with etanercept, that risk was actually lower if tocilizumab had been used as first- or fifth-line therapy (HRs for both, 0.9) but higher if it had been used as a third- or fourth-line therapy (HR of 1.4 for both).
“We often use tocilizumab as a second-line, third-line, or even fourth-line therapy, and if we don’t adjust for anything, we can have the impression that there are more infections with tocilizumab. But then, when we adjust for confounding factors and the line of therapy, we don’t have this anymore,” Dr. Lauper said.
“Line of therapy in itself is not a risk for serious infections,” she said in qualifying the conclusions that could be drawn from the study. “It may be a marker of the disease or some patient characteristic that is associated with a higher risk of infections.” Nevertheless, it should be taken into account when evaluating serious outcomes and possibly other safety and effectiveness outcomes.
“I understand concentrating on the hospitalized infections because the data are so much more robust,” observed consultant rheumatologist Jon Packham, BM, DM, of Haywood Hospital in Stoke-on-Trent, England, who chaired the session. He queried if there were any data on milder or just antibiotic-treated infections. At present, there aren’t those data to look at, Dr. Lauper responded, as this is something that’s difficult for registers to capture because doctors often do not log them in the databases.
There are also too few data on Janus kinase (JAK) inhibitors currently in the BSRBR-RA at present to be able to look at their rate of serious infection by line of therapy, Dr. Lauper noted. Because JAK inhibitors act on cytokines different from those affected by biologics for RA, there may be a difference there, but more data are needed on the JAK inhibitors before that question can be analyzed.
Dr. Lauper did not state having any disclosures. The BSRBR-RA is funded by the BSR via restricted income grants from several U.K. pharmaceutical companies, which has included or currently includes AbbVie, Celltrion, Hospira, Pfizer, UCB, Roche, Swedish Orphan Biovitrum, and Merck.
The order in which tocilizumab (Actemra) is used in the sequence of treatments for rheumatoid arthritis could be muddying the waters when it comes to evaluating patients’ risk for serious infection.
According to new data emerging from the British Society for Rheumatology Biologics Register – Rheumatoid Arthritis (BSRBR-RA), the line of therapy is a confounding factor when examining the risk for serious infection with not only tocilizumab but also other biologic agents.
The good news for patients, however, is that there doesn’t appear to be any overall greater risk for serious infection with one biologic over another when the line of therapy is taken into account.
“We don’t have any strong signal that there is an increased risk of serious infections with tocilizumab, compared to TNF inhibitors,” rheumatologist Kim Lauper, MD, of Geneva University Hospitals, said in an interview after presenting the data at the annual conference of the British Society for Rheumatology.
This is in contrast to studies where an increased risk of infections with tocilizumab has been seen when compared to TNF inhibitors. However, those studies did not account for the line of therapy, explained Dr. Lauper, who is also a clinical research fellow in the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), where the BSRBR-RA is managed.
“Tocilizumab is a treatment that we often give to patients after several other treatments, so they’re really different patients,” Dr. Lauper observed. Indeed, in the “real-world” setting, patients taking tocilizumab tend to be older, have longer disease duration, and have worse functional status than do those who might receive other biologics.
To look at the effect of line of therapy on the serious infection risk associated with commonly used biologic drugs, Dr. Lauper and associates examined data on more than 33,000 treatment courses, representing more than 62,500 patient-years.
Using etanercept as the comparator – because it represents the largest group of patients in the BSRBR-RA – the serious infection risk for tocilizumab, rituximab, adalimumab, infliximab, certolizumab pegol, and abatacept were calculated as an overall rate, and for their use as first-, second-, third-, fourth-, or fifth-line therapy.
The researchers adjusted their analysis for some clear baseline differences between the treatment groups, including age, prior treatment, disease duration, and comorbidities. Seropositivity, smoking status, general health status, and disease activity scores were also adjusted for in the analysis.
Crude hazard ratios (HRs), compared with etanercept, before and after adjusting for these already-known confounding factors were 1.0 and 1.2 for tocilizumab, 1.1 and 1.1 for adalimumab, 1.4 and 1.3 for infliximab, 0.6 and 0.8 for certolizumab pegol, 0.9 and 1.0 for rituximab, and 0.9 and 1.2 for abatacept.
Stratifying by line of therapy, however, changed the results: HRs were no longer significantly different, compared with etanercept, for tocilizumab, adalimumab, and infliximab for most lines of therapy.
Indeed, while the risk for serious infection occurring with tocilizumab was 20% higher overall, compared with etanercept, that risk was actually lower if tocilizumab had been used as first- or fifth-line therapy (HRs for both, 0.9) but higher if it had been used as a third- or fourth-line therapy (HR of 1.4 for both).
“We often use tocilizumab as a second-line, third-line, or even fourth-line therapy, and if we don’t adjust for anything, we can have the impression that there are more infections with tocilizumab. But then, when we adjust for confounding factors and the line of therapy, we don’t have this anymore,” Dr. Lauper said.
“Line of therapy in itself is not a risk for serious infections,” she said in qualifying the conclusions that could be drawn from the study. “It may be a marker of the disease or some patient characteristic that is associated with a higher risk of infections.” Nevertheless, it should be taken into account when evaluating serious outcomes and possibly other safety and effectiveness outcomes.
“I understand concentrating on the hospitalized infections because the data are so much more robust,” observed consultant rheumatologist Jon Packham, BM, DM, of Haywood Hospital in Stoke-on-Trent, England, who chaired the session. He queried if there were any data on milder or just antibiotic-treated infections. At present, there aren’t those data to look at, Dr. Lauper responded, as this is something that’s difficult for registers to capture because doctors often do not log them in the databases.
There are also too few data on Janus kinase (JAK) inhibitors currently in the BSRBR-RA at present to be able to look at their rate of serious infection by line of therapy, Dr. Lauper noted. Because JAK inhibitors act on cytokines different from those affected by biologics for RA, there may be a difference there, but more data are needed on the JAK inhibitors before that question can be analyzed.
Dr. Lauper did not state having any disclosures. The BSRBR-RA is funded by the BSR via restricted income grants from several U.K. pharmaceutical companies, which has included or currently includes AbbVie, Celltrion, Hospira, Pfizer, UCB, Roche, Swedish Orphan Biovitrum, and Merck.
The order in which tocilizumab (Actemra) is used in the sequence of treatments for rheumatoid arthritis could be muddying the waters when it comes to evaluating patients’ risk for serious infection.
According to new data emerging from the British Society for Rheumatology Biologics Register – Rheumatoid Arthritis (BSRBR-RA), the line of therapy is a confounding factor when examining the risk for serious infection with not only tocilizumab but also other biologic agents.
The good news for patients, however, is that there doesn’t appear to be any overall greater risk for serious infection with one biologic over another when the line of therapy is taken into account.
“We don’t have any strong signal that there is an increased risk of serious infections with tocilizumab, compared to TNF inhibitors,” rheumatologist Kim Lauper, MD, of Geneva University Hospitals, said in an interview after presenting the data at the annual conference of the British Society for Rheumatology.
This is in contrast to studies where an increased risk of infections with tocilizumab has been seen when compared to TNF inhibitors. However, those studies did not account for the line of therapy, explained Dr. Lauper, who is also a clinical research fellow in the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), where the BSRBR-RA is managed.
“Tocilizumab is a treatment that we often give to patients after several other treatments, so they’re really different patients,” Dr. Lauper observed. Indeed, in the “real-world” setting, patients taking tocilizumab tend to be older, have longer disease duration, and have worse functional status than do those who might receive other biologics.
To look at the effect of line of therapy on the serious infection risk associated with commonly used biologic drugs, Dr. Lauper and associates examined data on more than 33,000 treatment courses, representing more than 62,500 patient-years.
Using etanercept as the comparator – because it represents the largest group of patients in the BSRBR-RA – the serious infection risk for tocilizumab, rituximab, adalimumab, infliximab, certolizumab pegol, and abatacept were calculated as an overall rate, and for their use as first-, second-, third-, fourth-, or fifth-line therapy.
The researchers adjusted their analysis for some clear baseline differences between the treatment groups, including age, prior treatment, disease duration, and comorbidities. Seropositivity, smoking status, general health status, and disease activity scores were also adjusted for in the analysis.
Crude hazard ratios (HRs), compared with etanercept, before and after adjusting for these already-known confounding factors were 1.0 and 1.2 for tocilizumab, 1.1 and 1.1 for adalimumab, 1.4 and 1.3 for infliximab, 0.6 and 0.8 for certolizumab pegol, 0.9 and 1.0 for rituximab, and 0.9 and 1.2 for abatacept.
Stratifying by line of therapy, however, changed the results: HRs were no longer significantly different, compared with etanercept, for tocilizumab, adalimumab, and infliximab for most lines of therapy.
Indeed, while the risk for serious infection occurring with tocilizumab was 20% higher overall, compared with etanercept, that risk was actually lower if tocilizumab had been used as first- or fifth-line therapy (HRs for both, 0.9) but higher if it had been used as a third- or fourth-line therapy (HR of 1.4 for both).
“We often use tocilizumab as a second-line, third-line, or even fourth-line therapy, and if we don’t adjust for anything, we can have the impression that there are more infections with tocilizumab. But then, when we adjust for confounding factors and the line of therapy, we don’t have this anymore,” Dr. Lauper said.
“Line of therapy in itself is not a risk for serious infections,” she said in qualifying the conclusions that could be drawn from the study. “It may be a marker of the disease or some patient characteristic that is associated with a higher risk of infections.” Nevertheless, it should be taken into account when evaluating serious outcomes and possibly other safety and effectiveness outcomes.
“I understand concentrating on the hospitalized infections because the data are so much more robust,” observed consultant rheumatologist Jon Packham, BM, DM, of Haywood Hospital in Stoke-on-Trent, England, who chaired the session. He queried if there were any data on milder or just antibiotic-treated infections. At present, there aren’t those data to look at, Dr. Lauper responded, as this is something that’s difficult for registers to capture because doctors often do not log them in the databases.
There are also too few data on Janus kinase (JAK) inhibitors currently in the BSRBR-RA at present to be able to look at their rate of serious infection by line of therapy, Dr. Lauper noted. Because JAK inhibitors act on cytokines different from those affected by biologics for RA, there may be a difference there, but more data are needed on the JAK inhibitors before that question can be analyzed.
Dr. Lauper did not state having any disclosures. The BSRBR-RA is funded by the BSR via restricted income grants from several U.K. pharmaceutical companies, which has included or currently includes AbbVie, Celltrion, Hospira, Pfizer, UCB, Roche, Swedish Orphan Biovitrum, and Merck.
FROM BSR 2021
Pediatric bronchiolitis: Less is more
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
Trend reversed: New cases of COVID-19 decline in children
New cases of COVID-19 dropped among children for just the second time in the past 6 weeks, but that was not enough to reverse the trend in children’s share of the weekly total, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

of all COVID-19 cases reported for the week, surpassing the pandemic-high 20.6% seen just a week earlier, the AAP/CHA report shows.
The total number of cases in children is now over 3.7 million – that’s 13.7% of cases in all ages – since the start of the pandemic, and the cumulative rate of infection has reached 4,931 per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Cases of more severe illness in children continue to trend lower. The cumulative number of hospitalizations in children (15,187) is only 2.0% of the total of almost 760,000 in the 25 jurisdictions (24 states and New York City) that report such data, and deaths in children now number 296, which is just 0.06% of all COVID-19–related mortality in 43 states, New York City, Puerto Rico, and Guam, the AAP and CHA said in their report.
Among those 46 jurisdictions, Texas has reported the most deaths (51) in children, followed by Arizona (29) and New York City (23), while 9 states and the District of Columbia have reported no deaths so far. Children represent the highest proportion of deaths (0.19%) in Colorado, but Guam, with 2 child deaths among its total of 136, has by far the highest rate at 1.47%, the AAP/CHA data show.
Data from the 25 reporting jurisdictions show that children make up the largest share of hospitalizations (3.1%) in Colorado and Minnesota, while New York City (1.9%), Georgia (1.3%), and Rhode Island (1.3%) have the highest hospitalization rates among children diagnosed with SARS-CoV-2 infection, the two groups reported.
New cases of COVID-19 dropped among children for just the second time in the past 6 weeks, but that was not enough to reverse the trend in children’s share of the weekly total, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

of all COVID-19 cases reported for the week, surpassing the pandemic-high 20.6% seen just a week earlier, the AAP/CHA report shows.
The total number of cases in children is now over 3.7 million – that’s 13.7% of cases in all ages – since the start of the pandemic, and the cumulative rate of infection has reached 4,931 per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Cases of more severe illness in children continue to trend lower. The cumulative number of hospitalizations in children (15,187) is only 2.0% of the total of almost 760,000 in the 25 jurisdictions (24 states and New York City) that report such data, and deaths in children now number 296, which is just 0.06% of all COVID-19–related mortality in 43 states, New York City, Puerto Rico, and Guam, the AAP and CHA said in their report.
Among those 46 jurisdictions, Texas has reported the most deaths (51) in children, followed by Arizona (29) and New York City (23), while 9 states and the District of Columbia have reported no deaths so far. Children represent the highest proportion of deaths (0.19%) in Colorado, but Guam, with 2 child deaths among its total of 136, has by far the highest rate at 1.47%, the AAP/CHA data show.
Data from the 25 reporting jurisdictions show that children make up the largest share of hospitalizations (3.1%) in Colorado and Minnesota, while New York City (1.9%), Georgia (1.3%), and Rhode Island (1.3%) have the highest hospitalization rates among children diagnosed with SARS-CoV-2 infection, the two groups reported.
New cases of COVID-19 dropped among children for just the second time in the past 6 weeks, but that was not enough to reverse the trend in children’s share of the weekly total, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

of all COVID-19 cases reported for the week, surpassing the pandemic-high 20.6% seen just a week earlier, the AAP/CHA report shows.
The total number of cases in children is now over 3.7 million – that’s 13.7% of cases in all ages – since the start of the pandemic, and the cumulative rate of infection has reached 4,931 per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Cases of more severe illness in children continue to trend lower. The cumulative number of hospitalizations in children (15,187) is only 2.0% of the total of almost 760,000 in the 25 jurisdictions (24 states and New York City) that report such data, and deaths in children now number 296, which is just 0.06% of all COVID-19–related mortality in 43 states, New York City, Puerto Rico, and Guam, the AAP and CHA said in their report.
Among those 46 jurisdictions, Texas has reported the most deaths (51) in children, followed by Arizona (29) and New York City (23), while 9 states and the District of Columbia have reported no deaths so far. Children represent the highest proportion of deaths (0.19%) in Colorado, but Guam, with 2 child deaths among its total of 136, has by far the highest rate at 1.47%, the AAP/CHA data show.
Data from the 25 reporting jurisdictions show that children make up the largest share of hospitalizations (3.1%) in Colorado and Minnesota, while New York City (1.9%), Georgia (1.3%), and Rhode Island (1.3%) have the highest hospitalization rates among children diagnosed with SARS-CoV-2 infection, the two groups reported.
Tinea Incognito Mimicking Pustular Psoriasis in a Patient With Psoriasis and Cushing Syndrome
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.
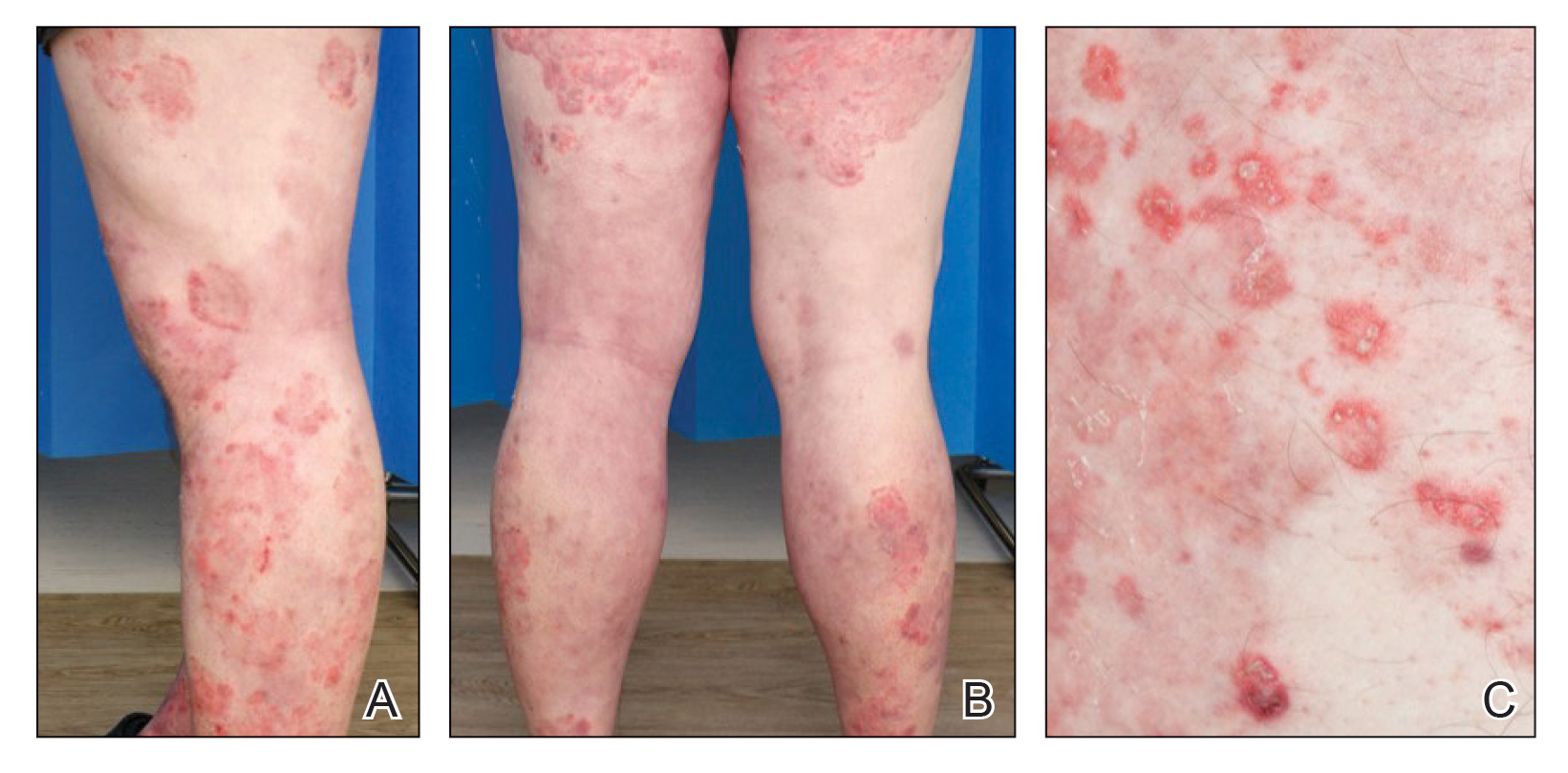
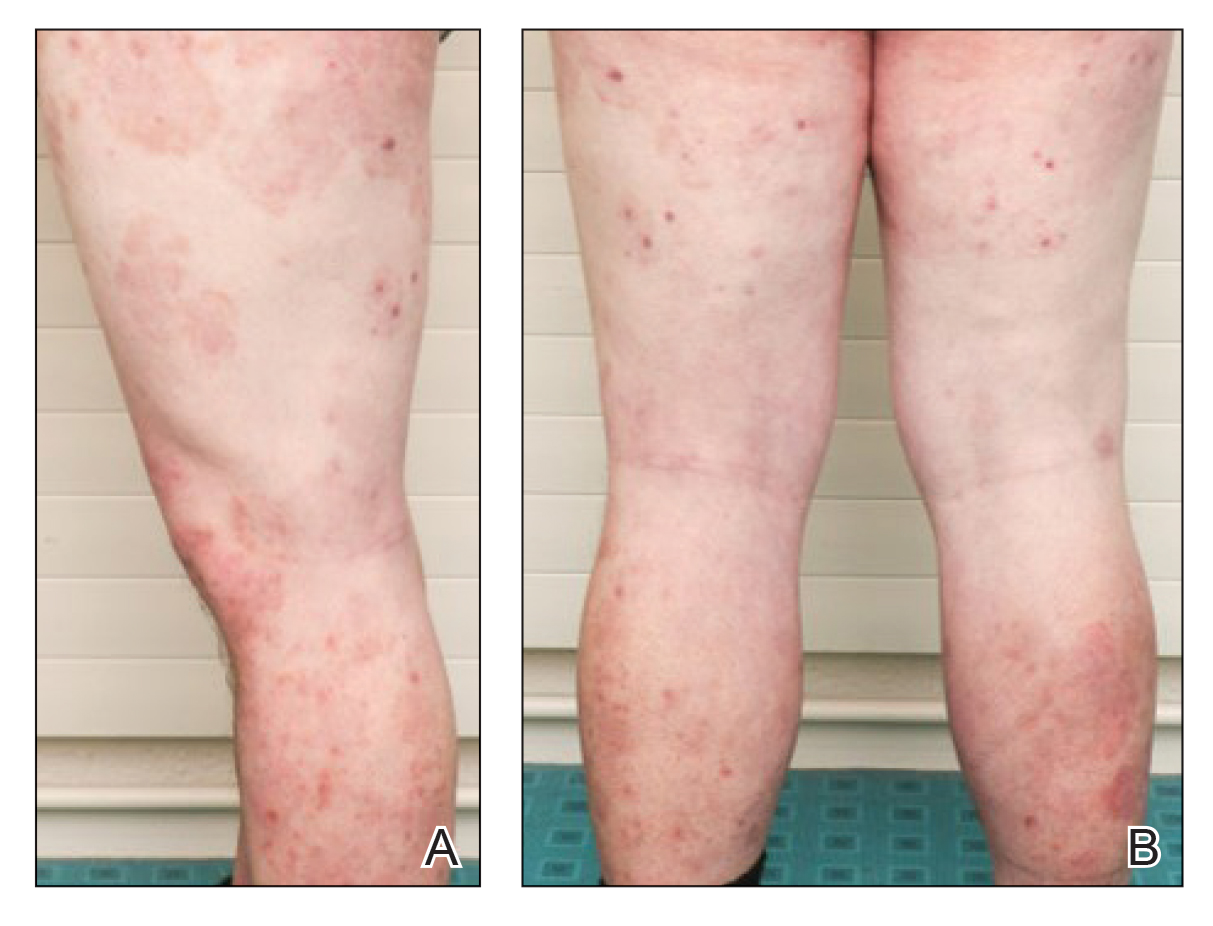
This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.


This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.


This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
Practice Points
- Tinea incognito and its altered clinical presentation can provide clinical challenges and often is diagnosed with delay.
- Immunosuppression, such as iatrogenic Cushing syndrome, is a risk factor for tinea incognito.
- Pustular tinea incognito is a differential diagnosis of pustular psoriasis that can mimic tumor necrosis factor inhibitor treatment failure in patients with psoriasis.
Vaccinating homebound patients is an uphill battle

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.
“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.
Malaria resistant to artemisinin emerging in Africa
A new study shows disturbing evidence that malaria is becoming resistant to artemisinin, a drug critical for treatment in Africa. Although artemisinin resistance has long plagued the Mekong Delta, it is relatively new to Africa.
In a study published online April 14 in The Lancet Infectious Diseases, researchers found that the typical 3-day course of treatment did not totally eradicate Plasmodium falciparum, the parasite that causes malaria. A delayed clearance of the parasite was shown and found to be associated with a genetic mutation called Pfkelch13 R561H.
P. falciparum isolates with this mutation were found in 7.5% of infected children in one area of Rwanda. Further genomic studies showed that this mutation was locally acquired and did not emerge from Southeast Asia. This is well illustrated in a genomic tree published in Nature Medicine in August, 2020. That study reported data collected from adults from 2013 to 2015.
The delay in reporting the mutation was due, in part, to the burdensome process of whole-genome sequencing and transfection studies, Pascal Ringwald, MD, PhD, coordinator of the Global Malaria Programme at WHO, and coauthor of the Nature Medicine study, said in an interview. In transfection studies, the mutation is inserted into parasites and the resultant effect is observed.
Aline Uwimana, MD, of Rwanda Biomedical Centre. is the lead author on both studies.
Meera Venkatesan, PhD, chief of the Case Management, Monitoring and Evaluation Branch, President’s Malaria Initiative, USAID, noted that the Lancet Infectious Diseases study was a therapeutic efficacy study (TES) on samples from children from 2018. In an interview, Dr. Venkatesan explained that the study is noteworthy because it demonstrated the clinical significance of this mutation with delayed parasite clearance. She did note that although there was a lag in publication of the initial reports of artemisinin resistance mutations, that information – and its implications – was promptly shared with the global malaria research community, as are other findings of public health importance.
Although most of the children got better, this “partial resistance” emerged while patients were taking artemether–lumefantrine. This is a type of artemisinin-based combination therapy (ACT) with two drugs intended to stall the emergence of resistance.
The delayed clearance will be a problem because it can contribute to the selection and spread of the partially resistant malaria parasite.
To slow the spread of artemisinin resistance, Dr. Ringwald emphasized the need to add a gametocidal drug to block the transmission to humans. “You give a single dose of primaquine, which will help stop the spread,” he said in an interview. “Continuing surveillance and mapping. These are priorities.”
So are following national guidelines and banning the use of artemisinin monotherapy. Dr. Ringwald stressed two additional priorities: the need for accurate diagnosis of malaria, and the need to use “good-quality drugs and to avoid substandard or fake medicines” by not purchasing drugs on the street.
Unscrupulous individuals are also selling artemisia preparations to treat or prevent COVID-19, when it has no such activity. Similarly, artemisia teas are sold as herbal remedies and nutraceuticals.
Philippe Guérin, MD, director of the Worldwide Antimalarial Resistance Network (WWARN), listed the same recommendations, focusing a bit more on accurate detection of malaria and treatment with a multidrug regimen plus primaquine. You need “to have different first-line treatment (different ACTs) to avoid drug pressure” and resistance to the partner drug emerging, he said in an interview.
Such multiple first-line treatments rely on artemisinin in combination with various drugs, but this can cause some logistical challenges. Resistance is so problematic that the MORU (Mahidol Oxford Tropical Medicine Research Unit) Tropical Health Network in Bangkok is studying triple drug combinations, adding amodiaquine or mefloquine to an artemisinin-based combination.
Dr. Guérin emphasized two other problems regarding the monitoring of malaria resistance in Africa (although not specifically Rwanda). One is the inability to do adequate surveillance in active conflict zones and areas of instability. The other is that COVID-19 is causing resources to be taken away from malaria and redirected to the more immediate crisis. By having to focus on the immediate viral pandemic, public-health authorities are missing the chance to address other critically important infectious diseases with large burdens – specifically malaria, TB, and HIV – which might have greater impacts on future generations.
Dr. Guérin noted that although we now have solid evidence of artemisinin resistance in Rwanda, and isolated cases in other African countries, we have little idea of the magnitude of the problem because testing is not widespread throughout parts of the continent.
What would widespread P. falciparum malaria resistance in Africa mean? Children are the most vulnerable to malaria, and account for two-thirds of the deaths. One study suggests there could be 78 million more cases over a 5-year period, along with far more deaths. Hence, there is a heightened urgency to implement the outlined strategies to prevent a looming catastrophe.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study shows disturbing evidence that malaria is becoming resistant to artemisinin, a drug critical for treatment in Africa. Although artemisinin resistance has long plagued the Mekong Delta, it is relatively new to Africa.
In a study published online April 14 in The Lancet Infectious Diseases, researchers found that the typical 3-day course of treatment did not totally eradicate Plasmodium falciparum, the parasite that causes malaria. A delayed clearance of the parasite was shown and found to be associated with a genetic mutation called Pfkelch13 R561H.
P. falciparum isolates with this mutation were found in 7.5% of infected children in one area of Rwanda. Further genomic studies showed that this mutation was locally acquired and did not emerge from Southeast Asia. This is well illustrated in a genomic tree published in Nature Medicine in August, 2020. That study reported data collected from adults from 2013 to 2015.
The delay in reporting the mutation was due, in part, to the burdensome process of whole-genome sequencing and transfection studies, Pascal Ringwald, MD, PhD, coordinator of the Global Malaria Programme at WHO, and coauthor of the Nature Medicine study, said in an interview. In transfection studies, the mutation is inserted into parasites and the resultant effect is observed.
Aline Uwimana, MD, of Rwanda Biomedical Centre. is the lead author on both studies.
Meera Venkatesan, PhD, chief of the Case Management, Monitoring and Evaluation Branch, President’s Malaria Initiative, USAID, noted that the Lancet Infectious Diseases study was a therapeutic efficacy study (TES) on samples from children from 2018. In an interview, Dr. Venkatesan explained that the study is noteworthy because it demonstrated the clinical significance of this mutation with delayed parasite clearance. She did note that although there was a lag in publication of the initial reports of artemisinin resistance mutations, that information – and its implications – was promptly shared with the global malaria research community, as are other findings of public health importance.
Although most of the children got better, this “partial resistance” emerged while patients were taking artemether–lumefantrine. This is a type of artemisinin-based combination therapy (ACT) with two drugs intended to stall the emergence of resistance.
The delayed clearance will be a problem because it can contribute to the selection and spread of the partially resistant malaria parasite.
To slow the spread of artemisinin resistance, Dr. Ringwald emphasized the need to add a gametocidal drug to block the transmission to humans. “You give a single dose of primaquine, which will help stop the spread,” he said in an interview. “Continuing surveillance and mapping. These are priorities.”
So are following national guidelines and banning the use of artemisinin monotherapy. Dr. Ringwald stressed two additional priorities: the need for accurate diagnosis of malaria, and the need to use “good-quality drugs and to avoid substandard or fake medicines” by not purchasing drugs on the street.
Unscrupulous individuals are also selling artemisia preparations to treat or prevent COVID-19, when it has no such activity. Similarly, artemisia teas are sold as herbal remedies and nutraceuticals.
Philippe Guérin, MD, director of the Worldwide Antimalarial Resistance Network (WWARN), listed the same recommendations, focusing a bit more on accurate detection of malaria and treatment with a multidrug regimen plus primaquine. You need “to have different first-line treatment (different ACTs) to avoid drug pressure” and resistance to the partner drug emerging, he said in an interview.
Such multiple first-line treatments rely on artemisinin in combination with various drugs, but this can cause some logistical challenges. Resistance is so problematic that the MORU (Mahidol Oxford Tropical Medicine Research Unit) Tropical Health Network in Bangkok is studying triple drug combinations, adding amodiaquine or mefloquine to an artemisinin-based combination.
Dr. Guérin emphasized two other problems regarding the monitoring of malaria resistance in Africa (although not specifically Rwanda). One is the inability to do adequate surveillance in active conflict zones and areas of instability. The other is that COVID-19 is causing resources to be taken away from malaria and redirected to the more immediate crisis. By having to focus on the immediate viral pandemic, public-health authorities are missing the chance to address other critically important infectious diseases with large burdens – specifically malaria, TB, and HIV – which might have greater impacts on future generations.
Dr. Guérin noted that although we now have solid evidence of artemisinin resistance in Rwanda, and isolated cases in other African countries, we have little idea of the magnitude of the problem because testing is not widespread throughout parts of the continent.
What would widespread P. falciparum malaria resistance in Africa mean? Children are the most vulnerable to malaria, and account for two-thirds of the deaths. One study suggests there could be 78 million more cases over a 5-year period, along with far more deaths. Hence, there is a heightened urgency to implement the outlined strategies to prevent a looming catastrophe.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study shows disturbing evidence that malaria is becoming resistant to artemisinin, a drug critical for treatment in Africa. Although artemisinin resistance has long plagued the Mekong Delta, it is relatively new to Africa.
In a study published online April 14 in The Lancet Infectious Diseases, researchers found that the typical 3-day course of treatment did not totally eradicate Plasmodium falciparum, the parasite that causes malaria. A delayed clearance of the parasite was shown and found to be associated with a genetic mutation called Pfkelch13 R561H.
P. falciparum isolates with this mutation were found in 7.5% of infected children in one area of Rwanda. Further genomic studies showed that this mutation was locally acquired and did not emerge from Southeast Asia. This is well illustrated in a genomic tree published in Nature Medicine in August, 2020. That study reported data collected from adults from 2013 to 2015.
The delay in reporting the mutation was due, in part, to the burdensome process of whole-genome sequencing and transfection studies, Pascal Ringwald, MD, PhD, coordinator of the Global Malaria Programme at WHO, and coauthor of the Nature Medicine study, said in an interview. In transfection studies, the mutation is inserted into parasites and the resultant effect is observed.
Aline Uwimana, MD, of Rwanda Biomedical Centre. is the lead author on both studies.
Meera Venkatesan, PhD, chief of the Case Management, Monitoring and Evaluation Branch, President’s Malaria Initiative, USAID, noted that the Lancet Infectious Diseases study was a therapeutic efficacy study (TES) on samples from children from 2018. In an interview, Dr. Venkatesan explained that the study is noteworthy because it demonstrated the clinical significance of this mutation with delayed parasite clearance. She did note that although there was a lag in publication of the initial reports of artemisinin resistance mutations, that information – and its implications – was promptly shared with the global malaria research community, as are other findings of public health importance.
Although most of the children got better, this “partial resistance” emerged while patients were taking artemether–lumefantrine. This is a type of artemisinin-based combination therapy (ACT) with two drugs intended to stall the emergence of resistance.
The delayed clearance will be a problem because it can contribute to the selection and spread of the partially resistant malaria parasite.
To slow the spread of artemisinin resistance, Dr. Ringwald emphasized the need to add a gametocidal drug to block the transmission to humans. “You give a single dose of primaquine, which will help stop the spread,” he said in an interview. “Continuing surveillance and mapping. These are priorities.”
So are following national guidelines and banning the use of artemisinin monotherapy. Dr. Ringwald stressed two additional priorities: the need for accurate diagnosis of malaria, and the need to use “good-quality drugs and to avoid substandard or fake medicines” by not purchasing drugs on the street.
Unscrupulous individuals are also selling artemisia preparations to treat or prevent COVID-19, when it has no such activity. Similarly, artemisia teas are sold as herbal remedies and nutraceuticals.
Philippe Guérin, MD, director of the Worldwide Antimalarial Resistance Network (WWARN), listed the same recommendations, focusing a bit more on accurate detection of malaria and treatment with a multidrug regimen plus primaquine. You need “to have different first-line treatment (different ACTs) to avoid drug pressure” and resistance to the partner drug emerging, he said in an interview.
Such multiple first-line treatments rely on artemisinin in combination with various drugs, but this can cause some logistical challenges. Resistance is so problematic that the MORU (Mahidol Oxford Tropical Medicine Research Unit) Tropical Health Network in Bangkok is studying triple drug combinations, adding amodiaquine or mefloquine to an artemisinin-based combination.
Dr. Guérin emphasized two other problems regarding the monitoring of malaria resistance in Africa (although not specifically Rwanda). One is the inability to do adequate surveillance in active conflict zones and areas of instability. The other is that COVID-19 is causing resources to be taken away from malaria and redirected to the more immediate crisis. By having to focus on the immediate viral pandemic, public-health authorities are missing the chance to address other critically important infectious diseases with large burdens – specifically malaria, TB, and HIV – which might have greater impacts on future generations.
Dr. Guérin noted that although we now have solid evidence of artemisinin resistance in Rwanda, and isolated cases in other African countries, we have little idea of the magnitude of the problem because testing is not widespread throughout parts of the continent.
What would widespread P. falciparum malaria resistance in Africa mean? Children are the most vulnerable to malaria, and account for two-thirds of the deaths. One study suggests there could be 78 million more cases over a 5-year period, along with far more deaths. Hence, there is a heightened urgency to implement the outlined strategies to prevent a looming catastrophe.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ten reasons airborne transmission of SARS-CoV-2 appears airtight
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.