User login
Quinolones and tendon health: Third-generation drugs may be safer
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
Mohs Micrographic Surgery During the COVID-19 Pandemic: Considering the Patient Perspective
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
Practice Points
- There is little evidence that supports a high risk for SARS-CoV-2 transmission during Mohs micrographic surgery when proper personal protective equipment and strict infection control protocols are in place.
- The effects of treatment delays due to COVID-19 on the patient experience have not been well studied, but the limited literature suggests a negative association.
- Shared decision-making and evaluation of individual patient risk factors and concerns should be considered when deciding whether to postpone skin cancer treatment.
Progress stalling on malaria elimination
In its final report on the E-2020 initiative, the World Health Organization touted its progress on its goal of eliminating malaria throughout the world. But critics are charging that progress has stalled.
The E-2020 initiative supported the efforts of 21 countries in eliminating malaria. In a remarkable achievement, especially during the COVID-19 pandemic, eight E-2020 member countries reported zero cases of malaria in 2020. The WHO’s next target is the elimination of malaria in 20 of those countries by 2025.
While applauding these successes, in an interview with this news organization, Sir Nicholas J. White, FRS, professor of tropical medicine, Mahidol University, Salaya, Thailand, and Oxford (England) University, also put those successes in perspective. For one thing, the original 2020 goal was the elimination of malaria in 10 countries. Prof. White acknowledged that there had been very “substantial reductions in global morbidity and mortality” from 2000 to 2015, but he pointed out that those advances have not been sustained.
Prof. White added, “There has never been a really good, detailed inquiry as to why progress has stalled” in the high-burden countries.
Prof. White also provided important historical context, explaining that “100 years ago, malaria was pretty much a global disease. There were few places in the world which did not have malaria. You had malaria up to the Arctic Circle. You had malaria in the United States, particularly in the Tennessee Valley in the southeastern part of the United States. The Centers for Disease Control was formed specifically to counter malaria and malaria interfering with the building of the Erie and Ottawa canals.”
Kim Lindblade, PhD, malaria elimination team lead of the WHO’s Global Malaria Program, addressed those concerns with this news organization. “It’s not completely clear why [progress] has stalled,” she said. “There are lots of potential reasons for it, including stagnating funding.”
Dr. Lindblade added that high-burden countries are “facing big challenges. [Since 2015] there’s this stagnation. We’re fighting against population growth, and countries need to get back on track to continue to decrease their malaria burden. So that’s the big focus right now, to reorganize efforts to help countries achieve the goals of the World Health Assembly.”
Asked how these countries might approach the problem differently, Dr. Lindblade said that in the recent past, there was “almost a one-size-fits-all strategy. Now we’re looking much more carefully at conditions at the district level or provincial level and saying, What is it that this particular district or province needs? … It’s becoming much more tailored to the environment and to the specific epidemiological situation. … and I think that’s gotten a lot of people very excited.”
Because of travel restrictions and lockdowns because of COVID-19, the number of imported cases of malaria has declined. That’s the good news. But the pandemic has made elimination more difficult in other ways. For example, the delivery of insecticide-treated bed nets has been delayed in some areas, as has targeted indoor spraying. People in many areas have put off seeking medical care. Diagnostic capabilities have been reduced because of health care personnel having been diverted to address the COVID-19 crisis.
Still, some of the successes in eliminating malaria have been striking. Iran, for example, reduced its cases from about 98,000 in 1991 to 12,000 just 10 years later. Since then, Iran has established rapid response teams equipped with insecticide-impregnated nets, rapid diagnostic tests, and antimalarials. A network of more than 3,700 community health volunteers has been trained and deployed throughout the country.
A key element of Iran’s success – and that of some of the other countries – is the political will to tackle malaria. This translates to funding. Notably, the most successful countries provide free primary health care to everyone, regardless of their legal or residency status. Volunteer migrant workers are trained to diagnose malaria and to educate fellow migrants about the disease and prevention strategies.
Malaysia and China are examples of two countries at risk of importing malaria through their many people who work abroad in malaria-endemic regions. They have had to increase their surveillance.
Although Malaysia has eliminated most malaria species – those transmitted through people – they still have problems with the malaria parasite hosted by monkeys.
The WHO report stresses the lessons learned through their E-2020 program. Two key criteria are political commitment and associated funding. Next are surveillance and efforts to reach everyone, even in geographically remote or marginalized communities. Close surveillance also enables strategies to be modified to local needs.
Countries need to cooperate, especially along border areas and in regard to communications. The WHO stressed the need for countries to have an integrated response in their approach to malaria, including accurate surveillance, diagnostic testing, treatment, and robust education in preventive measures.
Although these successes were not as evident in some high-burden countries, Prof. White applauded their perseverance, noting, “It’s quite difficult to sustain the political momentum. … That endgame to keep the motivation, keep the support, to getting rid of something is hard.”
Prof. White and Dr. Lindberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In its final report on the E-2020 initiative, the World Health Organization touted its progress on its goal of eliminating malaria throughout the world. But critics are charging that progress has stalled.
The E-2020 initiative supported the efforts of 21 countries in eliminating malaria. In a remarkable achievement, especially during the COVID-19 pandemic, eight E-2020 member countries reported zero cases of malaria in 2020. The WHO’s next target is the elimination of malaria in 20 of those countries by 2025.
While applauding these successes, in an interview with this news organization, Sir Nicholas J. White, FRS, professor of tropical medicine, Mahidol University, Salaya, Thailand, and Oxford (England) University, also put those successes in perspective. For one thing, the original 2020 goal was the elimination of malaria in 10 countries. Prof. White acknowledged that there had been very “substantial reductions in global morbidity and mortality” from 2000 to 2015, but he pointed out that those advances have not been sustained.
Prof. White added, “There has never been a really good, detailed inquiry as to why progress has stalled” in the high-burden countries.
Prof. White also provided important historical context, explaining that “100 years ago, malaria was pretty much a global disease. There were few places in the world which did not have malaria. You had malaria up to the Arctic Circle. You had malaria in the United States, particularly in the Tennessee Valley in the southeastern part of the United States. The Centers for Disease Control was formed specifically to counter malaria and malaria interfering with the building of the Erie and Ottawa canals.”
Kim Lindblade, PhD, malaria elimination team lead of the WHO’s Global Malaria Program, addressed those concerns with this news organization. “It’s not completely clear why [progress] has stalled,” she said. “There are lots of potential reasons for it, including stagnating funding.”
Dr. Lindblade added that high-burden countries are “facing big challenges. [Since 2015] there’s this stagnation. We’re fighting against population growth, and countries need to get back on track to continue to decrease their malaria burden. So that’s the big focus right now, to reorganize efforts to help countries achieve the goals of the World Health Assembly.”
Asked how these countries might approach the problem differently, Dr. Lindblade said that in the recent past, there was “almost a one-size-fits-all strategy. Now we’re looking much more carefully at conditions at the district level or provincial level and saying, What is it that this particular district or province needs? … It’s becoming much more tailored to the environment and to the specific epidemiological situation. … and I think that’s gotten a lot of people very excited.”
Because of travel restrictions and lockdowns because of COVID-19, the number of imported cases of malaria has declined. That’s the good news. But the pandemic has made elimination more difficult in other ways. For example, the delivery of insecticide-treated bed nets has been delayed in some areas, as has targeted indoor spraying. People in many areas have put off seeking medical care. Diagnostic capabilities have been reduced because of health care personnel having been diverted to address the COVID-19 crisis.
Still, some of the successes in eliminating malaria have been striking. Iran, for example, reduced its cases from about 98,000 in 1991 to 12,000 just 10 years later. Since then, Iran has established rapid response teams equipped with insecticide-impregnated nets, rapid diagnostic tests, and antimalarials. A network of more than 3,700 community health volunteers has been trained and deployed throughout the country.
A key element of Iran’s success – and that of some of the other countries – is the political will to tackle malaria. This translates to funding. Notably, the most successful countries provide free primary health care to everyone, regardless of their legal or residency status. Volunteer migrant workers are trained to diagnose malaria and to educate fellow migrants about the disease and prevention strategies.
Malaysia and China are examples of two countries at risk of importing malaria through their many people who work abroad in malaria-endemic regions. They have had to increase their surveillance.
Although Malaysia has eliminated most malaria species – those transmitted through people – they still have problems with the malaria parasite hosted by monkeys.
The WHO report stresses the lessons learned through their E-2020 program. Two key criteria are political commitment and associated funding. Next are surveillance and efforts to reach everyone, even in geographically remote or marginalized communities. Close surveillance also enables strategies to be modified to local needs.
Countries need to cooperate, especially along border areas and in regard to communications. The WHO stressed the need for countries to have an integrated response in their approach to malaria, including accurate surveillance, diagnostic testing, treatment, and robust education in preventive measures.
Although these successes were not as evident in some high-burden countries, Prof. White applauded their perseverance, noting, “It’s quite difficult to sustain the political momentum. … That endgame to keep the motivation, keep the support, to getting rid of something is hard.”
Prof. White and Dr. Lindberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In its final report on the E-2020 initiative, the World Health Organization touted its progress on its goal of eliminating malaria throughout the world. But critics are charging that progress has stalled.
The E-2020 initiative supported the efforts of 21 countries in eliminating malaria. In a remarkable achievement, especially during the COVID-19 pandemic, eight E-2020 member countries reported zero cases of malaria in 2020. The WHO’s next target is the elimination of malaria in 20 of those countries by 2025.
While applauding these successes, in an interview with this news organization, Sir Nicholas J. White, FRS, professor of tropical medicine, Mahidol University, Salaya, Thailand, and Oxford (England) University, also put those successes in perspective. For one thing, the original 2020 goal was the elimination of malaria in 10 countries. Prof. White acknowledged that there had been very “substantial reductions in global morbidity and mortality” from 2000 to 2015, but he pointed out that those advances have not been sustained.
Prof. White added, “There has never been a really good, detailed inquiry as to why progress has stalled” in the high-burden countries.
Prof. White also provided important historical context, explaining that “100 years ago, malaria was pretty much a global disease. There were few places in the world which did not have malaria. You had malaria up to the Arctic Circle. You had malaria in the United States, particularly in the Tennessee Valley in the southeastern part of the United States. The Centers for Disease Control was formed specifically to counter malaria and malaria interfering with the building of the Erie and Ottawa canals.”
Kim Lindblade, PhD, malaria elimination team lead of the WHO’s Global Malaria Program, addressed those concerns with this news organization. “It’s not completely clear why [progress] has stalled,” she said. “There are lots of potential reasons for it, including stagnating funding.”
Dr. Lindblade added that high-burden countries are “facing big challenges. [Since 2015] there’s this stagnation. We’re fighting against population growth, and countries need to get back on track to continue to decrease their malaria burden. So that’s the big focus right now, to reorganize efforts to help countries achieve the goals of the World Health Assembly.”
Asked how these countries might approach the problem differently, Dr. Lindblade said that in the recent past, there was “almost a one-size-fits-all strategy. Now we’re looking much more carefully at conditions at the district level or provincial level and saying, What is it that this particular district or province needs? … It’s becoming much more tailored to the environment and to the specific epidemiological situation. … and I think that’s gotten a lot of people very excited.”
Because of travel restrictions and lockdowns because of COVID-19, the number of imported cases of malaria has declined. That’s the good news. But the pandemic has made elimination more difficult in other ways. For example, the delivery of insecticide-treated bed nets has been delayed in some areas, as has targeted indoor spraying. People in many areas have put off seeking medical care. Diagnostic capabilities have been reduced because of health care personnel having been diverted to address the COVID-19 crisis.
Still, some of the successes in eliminating malaria have been striking. Iran, for example, reduced its cases from about 98,000 in 1991 to 12,000 just 10 years later. Since then, Iran has established rapid response teams equipped with insecticide-impregnated nets, rapid diagnostic tests, and antimalarials. A network of more than 3,700 community health volunteers has been trained and deployed throughout the country.
A key element of Iran’s success – and that of some of the other countries – is the political will to tackle malaria. This translates to funding. Notably, the most successful countries provide free primary health care to everyone, regardless of their legal or residency status. Volunteer migrant workers are trained to diagnose malaria and to educate fellow migrants about the disease and prevention strategies.
Malaysia and China are examples of two countries at risk of importing malaria through their many people who work abroad in malaria-endemic regions. They have had to increase their surveillance.
Although Malaysia has eliminated most malaria species – those transmitted through people – they still have problems with the malaria parasite hosted by monkeys.
The WHO report stresses the lessons learned through their E-2020 program. Two key criteria are political commitment and associated funding. Next are surveillance and efforts to reach everyone, even in geographically remote or marginalized communities. Close surveillance also enables strategies to be modified to local needs.
Countries need to cooperate, especially along border areas and in regard to communications. The WHO stressed the need for countries to have an integrated response in their approach to malaria, including accurate surveillance, diagnostic testing, treatment, and robust education in preventive measures.
Although these successes were not as evident in some high-burden countries, Prof. White applauded their perseverance, noting, “It’s quite difficult to sustain the political momentum. … That endgame to keep the motivation, keep the support, to getting rid of something is hard.”
Prof. White and Dr. Lindberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Genital Primary Herpetic Infection With Concurrent Hepatitis in an Infant
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
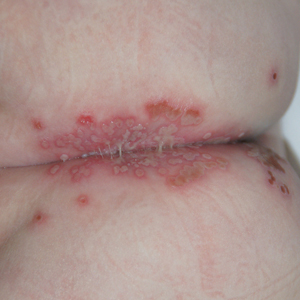
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.
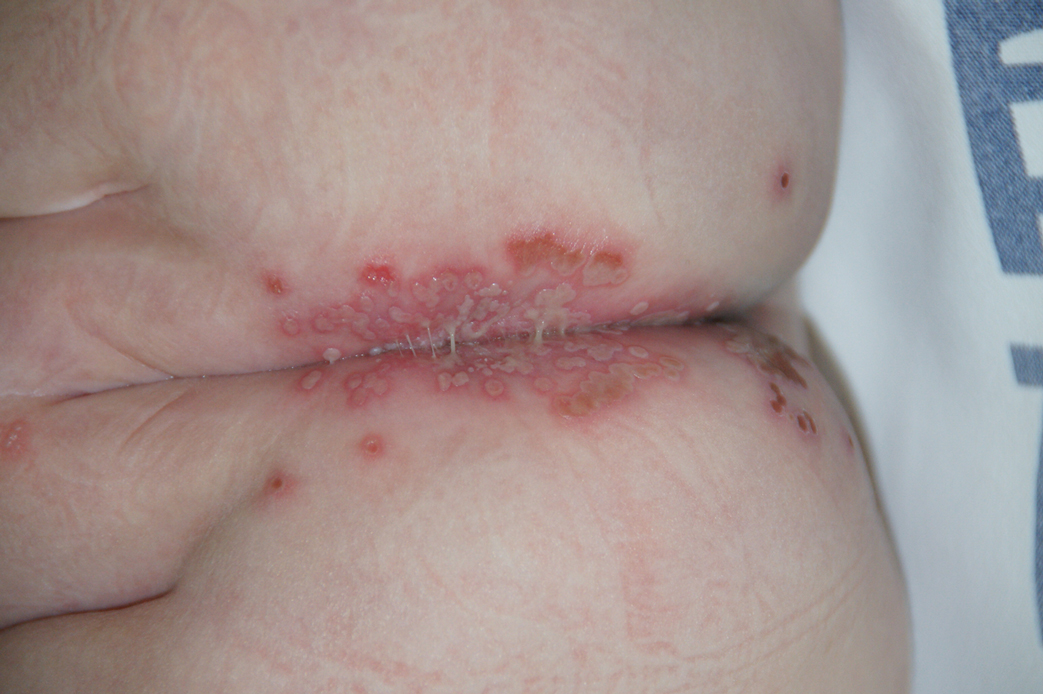
A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.

A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.

A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
Practice Points
- Parents with a history of herpes simplex virus (HSV) need to be educated before the baby is born to be careful about direct skin contact with the child to prevent the spread of HSV infection.
- Although systemic involvement is not typical, additional tests to rule out internal organ involvement may be required, especially in children.
Jack Remington, MD, noted toxoplasmosis researcher, dies at 90
Jack. S. Remington, MD, the Stanford (Calif.) University clinical scientist who developed a test to identify babies at risk for dangerous toxoplasmosis, died on April 8 at the age of 90.
Dr. Remington was professor emeritus of infectious diseases at Stanford Medicine. A legendary researcher, Dr. Remington was described by colleagues and trainees as a dogged clinician. Known as “Stat Jack” for his sense of urgency, he retired in 2005.
He died after a fall; it was the last of many. When he wasn’t treating patients or conducting research, Dr. Remington was often rock climbing. Friends said he had broken many bones but was always a passionate climber.
Dr. Remington was retired when Upinder Singh, MD, arrived at Stanford. Now she is chief of infectious diseases and geographic medicine at Stanford Medicine. Dr. Singh said in an interview that Dr. Remington was a bright, forward-thinking scientist.
Dr. Remington conducted research at the Palo Alto Medical Foundation (PAMF), part of the Sutter Health network. He ran a toxoplasmosis serology lab, and it was his baby, Dr. Singh said. In 2019, it was renamed for him: The Dr Jack S. Remington Laboratory for Specialty Diagnostics.
While he conducted research at PAMF, he treated patients at Stanford, where he could see his research benefit them.
“What he held closest to his heart was that scientific endeavors should help patients,” Dr. Singh said.
Born in Chicago in 1931, Dr. Remington did his undergraduate work at Loyola University in Chicago and the University of Illinois, where he graduated from medical school in 1956, according to a statement from Stanford. He spent 2 years as a senior assistant surgeon for the United States Public Health Service and as a researcher at the National Institute of Allergy and Infectious Diseases.
There, he conducted key research on Toxoplasma gondii, a usually dormant parasite that poses a serious risk to anyone with a compromised immune system – a group that includes babies, transplant recipients, and people with HIV. T gondii is the reason pregnant women are told not to clean out litter boxes, because it can be spread through cat feces. Humans also contract toxoplasmosis by eating contaminated meat. The Centers for Disease Control and Prevention estimates that 300 to 4,000 babies are exposed each year and develop toxoplasmosis. Often symptom-free for a period, the children can go on to develop vision problems or developmental delays.
Dr. Remington developed a blood test that measures a baby’s exposure and, therefore, risk for toxoplasmosis. According to the Stanford announcement, “The test distinguished between antibodies that a newborn has passively acquired from its mother through the placental barrier and antibodies that indicate a newborn has actually been infected in the womb by pathogens, notably T. gondii, that had been residing in the mother’s tissues. The latter case meant a baby needed immediate treatment to stave off active toxoplasmosis.”
Dr. Remington also led clinical trials and developed drugs to treat the condition. Stanford reports that he authored or coauthored more than 600 articles and held 11 patents.
He also coauthored the most authoritative textbook in the field. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant is now in its eighth edition.
Dr. Remington was elected a fellow of the American College of Physicians in 1966, the London-based Royal College of Physicians in 1999, the American Association for the Advancement of Science in 2000, and the American Academy of Microbiology in 2000. He was a past president of the Western Society for Clinical Research, the Infectious Diseases Society of America, and the International Immunocompromised Host Society.
Friends and colleagues remember him as a dedicated mentor, evidenced by the many trainees who traveled to his 70th birthday party, said Philip Pizzo, MD, professor of pediatrics and immunology at Stanford Medicine. Dr. Pizzo, the former dean of the School of Medicine, met Dr. Remington in 1977 after presenting a research paper on the subject of the immunocompromised host at a New York meeting of the Infectious Diseases Society of America. They became lifelong colleagues and friends.
Dr. Remington had his own kind of confidence and self-assurance, Dr. Pizzo said: “He climbed the most challenging rock faces in the world. It takes a certain kind of personality to do that.”
A version of this article first appeared on Medscape.com.
Jack. S. Remington, MD, the Stanford (Calif.) University clinical scientist who developed a test to identify babies at risk for dangerous toxoplasmosis, died on April 8 at the age of 90.
Dr. Remington was professor emeritus of infectious diseases at Stanford Medicine. A legendary researcher, Dr. Remington was described by colleagues and trainees as a dogged clinician. Known as “Stat Jack” for his sense of urgency, he retired in 2005.
He died after a fall; it was the last of many. When he wasn’t treating patients or conducting research, Dr. Remington was often rock climbing. Friends said he had broken many bones but was always a passionate climber.
Dr. Remington was retired when Upinder Singh, MD, arrived at Stanford. Now she is chief of infectious diseases and geographic medicine at Stanford Medicine. Dr. Singh said in an interview that Dr. Remington was a bright, forward-thinking scientist.
Dr. Remington conducted research at the Palo Alto Medical Foundation (PAMF), part of the Sutter Health network. He ran a toxoplasmosis serology lab, and it was his baby, Dr. Singh said. In 2019, it was renamed for him: The Dr Jack S. Remington Laboratory for Specialty Diagnostics.
While he conducted research at PAMF, he treated patients at Stanford, where he could see his research benefit them.
“What he held closest to his heart was that scientific endeavors should help patients,” Dr. Singh said.
Born in Chicago in 1931, Dr. Remington did his undergraduate work at Loyola University in Chicago and the University of Illinois, where he graduated from medical school in 1956, according to a statement from Stanford. He spent 2 years as a senior assistant surgeon for the United States Public Health Service and as a researcher at the National Institute of Allergy and Infectious Diseases.
There, he conducted key research on Toxoplasma gondii, a usually dormant parasite that poses a serious risk to anyone with a compromised immune system – a group that includes babies, transplant recipients, and people with HIV. T gondii is the reason pregnant women are told not to clean out litter boxes, because it can be spread through cat feces. Humans also contract toxoplasmosis by eating contaminated meat. The Centers for Disease Control and Prevention estimates that 300 to 4,000 babies are exposed each year and develop toxoplasmosis. Often symptom-free for a period, the children can go on to develop vision problems or developmental delays.
Dr. Remington developed a blood test that measures a baby’s exposure and, therefore, risk for toxoplasmosis. According to the Stanford announcement, “The test distinguished between antibodies that a newborn has passively acquired from its mother through the placental barrier and antibodies that indicate a newborn has actually been infected in the womb by pathogens, notably T. gondii, that had been residing in the mother’s tissues. The latter case meant a baby needed immediate treatment to stave off active toxoplasmosis.”
Dr. Remington also led clinical trials and developed drugs to treat the condition. Stanford reports that he authored or coauthored more than 600 articles and held 11 patents.
He also coauthored the most authoritative textbook in the field. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant is now in its eighth edition.
Dr. Remington was elected a fellow of the American College of Physicians in 1966, the London-based Royal College of Physicians in 1999, the American Association for the Advancement of Science in 2000, and the American Academy of Microbiology in 2000. He was a past president of the Western Society for Clinical Research, the Infectious Diseases Society of America, and the International Immunocompromised Host Society.
Friends and colleagues remember him as a dedicated mentor, evidenced by the many trainees who traveled to his 70th birthday party, said Philip Pizzo, MD, professor of pediatrics and immunology at Stanford Medicine. Dr. Pizzo, the former dean of the School of Medicine, met Dr. Remington in 1977 after presenting a research paper on the subject of the immunocompromised host at a New York meeting of the Infectious Diseases Society of America. They became lifelong colleagues and friends.
Dr. Remington had his own kind of confidence and self-assurance, Dr. Pizzo said: “He climbed the most challenging rock faces in the world. It takes a certain kind of personality to do that.”
A version of this article first appeared on Medscape.com.
Jack. S. Remington, MD, the Stanford (Calif.) University clinical scientist who developed a test to identify babies at risk for dangerous toxoplasmosis, died on April 8 at the age of 90.
Dr. Remington was professor emeritus of infectious diseases at Stanford Medicine. A legendary researcher, Dr. Remington was described by colleagues and trainees as a dogged clinician. Known as “Stat Jack” for his sense of urgency, he retired in 2005.
He died after a fall; it was the last of many. When he wasn’t treating patients or conducting research, Dr. Remington was often rock climbing. Friends said he had broken many bones but was always a passionate climber.
Dr. Remington was retired when Upinder Singh, MD, arrived at Stanford. Now she is chief of infectious diseases and geographic medicine at Stanford Medicine. Dr. Singh said in an interview that Dr. Remington was a bright, forward-thinking scientist.
Dr. Remington conducted research at the Palo Alto Medical Foundation (PAMF), part of the Sutter Health network. He ran a toxoplasmosis serology lab, and it was his baby, Dr. Singh said. In 2019, it was renamed for him: The Dr Jack S. Remington Laboratory for Specialty Diagnostics.
While he conducted research at PAMF, he treated patients at Stanford, where he could see his research benefit them.
“What he held closest to his heart was that scientific endeavors should help patients,” Dr. Singh said.
Born in Chicago in 1931, Dr. Remington did his undergraduate work at Loyola University in Chicago and the University of Illinois, where he graduated from medical school in 1956, according to a statement from Stanford. He spent 2 years as a senior assistant surgeon for the United States Public Health Service and as a researcher at the National Institute of Allergy and Infectious Diseases.
There, he conducted key research on Toxoplasma gondii, a usually dormant parasite that poses a serious risk to anyone with a compromised immune system – a group that includes babies, transplant recipients, and people with HIV. T gondii is the reason pregnant women are told not to clean out litter boxes, because it can be spread through cat feces. Humans also contract toxoplasmosis by eating contaminated meat. The Centers for Disease Control and Prevention estimates that 300 to 4,000 babies are exposed each year and develop toxoplasmosis. Often symptom-free for a period, the children can go on to develop vision problems or developmental delays.
Dr. Remington developed a blood test that measures a baby’s exposure and, therefore, risk for toxoplasmosis. According to the Stanford announcement, “The test distinguished between antibodies that a newborn has passively acquired from its mother through the placental barrier and antibodies that indicate a newborn has actually been infected in the womb by pathogens, notably T. gondii, that had been residing in the mother’s tissues. The latter case meant a baby needed immediate treatment to stave off active toxoplasmosis.”
Dr. Remington also led clinical trials and developed drugs to treat the condition. Stanford reports that he authored or coauthored more than 600 articles and held 11 patents.
He also coauthored the most authoritative textbook in the field. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant is now in its eighth edition.
Dr. Remington was elected a fellow of the American College of Physicians in 1966, the London-based Royal College of Physicians in 1999, the American Association for the Advancement of Science in 2000, and the American Academy of Microbiology in 2000. He was a past president of the Western Society for Clinical Research, the Infectious Diseases Society of America, and the International Immunocompromised Host Society.
Friends and colleagues remember him as a dedicated mentor, evidenced by the many trainees who traveled to his 70th birthday party, said Philip Pizzo, MD, professor of pediatrics and immunology at Stanford Medicine. Dr. Pizzo, the former dean of the School of Medicine, met Dr. Remington in 1977 after presenting a research paper on the subject of the immunocompromised host at a New York meeting of the Infectious Diseases Society of America. They became lifelong colleagues and friends.
Dr. Remington had his own kind of confidence and self-assurance, Dr. Pizzo said: “He climbed the most challenging rock faces in the world. It takes a certain kind of personality to do that.”
A version of this article first appeared on Medscape.com.
Evidence or anecdote: Clinical judgment in COVID care
As the COVID-19 pandemic continues and evidence evolves, clinical judgment is the bottom line for clinical care, according to Adarsh Bhimraj, MD, of the Cleveland Clinic, and James Walter, MD, of Northwestern Medicine, Chicago.
In a debate/discussion presented at SHM Converge, the annual conference of the Society of Hospital Medicine, Dr. Bhimraj and Dr. Walter took sides in a friendly debate on the value of remdesivir and tocilizumab for hospitalized COVID-19 patients.
Dr. Bhimraj argued for the use of remdesivir or tocilizumab in patients hospitalized with COVID-19 pneumonia, and Dr. Walter presented the case against their use.
Referendum on remdesivir
The main sources referenced by the presenters regarding remdesivir were the WHO Solidarity Trial (N Engl J Med. 2021 Feb 11. doi: 10.1056/NEJMoa2023184) and the Adaptive Covid-19 Treatment Trial (ACCT) final report (N Engl J Med. 2020 Nov 5. doi: 10.1056/NEJMoa2007764).
“The ‘debate’ is partly artificial,” and meant to illustrate how clinicians can use their own clinical faculties and reasoning to make an informed decision when treating COVID-19 patients, Dr. Bhimraj said.
The ACCT trial compared remdesivir with placebo in patients with severe enough COVID-19 to require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation. The primary outcome in the study was time to recovery, and “the devil is in the details,” Dr. Bhimraj said. The outcomes clinicians should look for in studies are those that matter to patients, such as death, disability, and discomfort, he noted. Disease-oriented endpoints are easier to measure, but not always meaningful for patients, he said. The study showed an average 5-day decrease in illness, “but the fact is that it did not show a mortality benefit,” he noted.
Another large, open-label study of remdesivir across 30 countries showed no survival benefit associated with the drug, compared with standard of care, said Dr. Bhimraj. Patients treated with remdesivir remained in the hospital longer, but Dr. Bhimraj said he believed that was a bias. “I think the physicians kept the patients in the hospital longer to give the treatment rather than the treatments themselves prolonging the treatment duration,” he said.
In conclusion for remdesivir, “the solid data show that there is an early recovery,” he said. “At least for severe disease, even if there is no mortality benefit, there is a role. I argue that, if someone asks if you want to use remdesivir in severe COVID-19 patients, say yes, especially if you value people getting out of the hospital sooner. In a crisis situation, there is a role for remdesivir.”
Dr. Walter discussed the “con” side of using remdesivir. “We can start with a predata hypothesis, but integrate new data about the efficacy into a postdata hypothesis,” he said.
Dr. Walter made several points against the use of remdesivir in hospitalized COVID-19 patients. First, it has not shown any improvement in mortality and may increase the length of hospital stay, he noted.
Data from the ACCT-1 trial and the WHO solidarity trial, showed “no signal of mortality benefit at all,” he said. In addition, the World Health Organization, American College of Physicians, and National Institutes of Health all recommend against remdesivir for patients who require mechanical ventilation or extracorporeal membrane oxygenation, he said. The efficacy when used with steroids remains unclear, and long-term safety data are lacking, he added.
Taking on tocilizumab
Tocilizumab, an anti-inflammatory agent, has demonstrated an impact on several surrogate markers, notably C-reactive protein, temperature, and oxygenation. Dr. Bhimraj said. He reviewed data from eight published studies on the use of tocilizumab in COVID-19 patients.
Arguably, some trials may not have been powered adequately, and in combination, some trials show an effect on clinical deterioration, if not a mortality benefit, he said.
Consequently, in the context of COVID-19, tocilizumab “should be used early in the disease process, especially if steroids are not working,” said Dr. Bhimraj. Despite the limited evidence, “there is a niche population where this might be beneficial,” he said.
By contrast, Dr. Walter took the position of skepticism about the value of tocilizumab for COVID-19 patients.
Notably, decades of research show that tocilizumab has shown no benefit in patients with sepsis or septic shock, or those with acute respiratory distress syndrome, which have similarities to COVID-19 (JAMA. 2020 Sep 3. doi: 10.1001/jama.2020.17052).
He cited a research letter published in JAMA in September 2020, which showed that cytokine levels were in fact lower in critically ill patients with COVID-19, compared with those who had conditions including sepsis with and without ARDS.
Dr. Walter also cited data on the questionable benefit of tocilizumab when used with steroids and the negligible impact on mortality in hospitalized COVID-19 patients seen in the RECOVERY trial.
Limited data mean that therapeutic decisions related to COVID-19 are more nuanced, but they can be made, the presenters agreed.
Ultimately, when trying to decide whether a drug is efficacious, futile, or harmful, “What we have to do is consider the grand totality of the evidence,” Dr. Bhimraj emphasized.
Dr. Bhimraj and Dr. Walter had no relevant financial conflicts to disclose.
As the COVID-19 pandemic continues and evidence evolves, clinical judgment is the bottom line for clinical care, according to Adarsh Bhimraj, MD, of the Cleveland Clinic, and James Walter, MD, of Northwestern Medicine, Chicago.
In a debate/discussion presented at SHM Converge, the annual conference of the Society of Hospital Medicine, Dr. Bhimraj and Dr. Walter took sides in a friendly debate on the value of remdesivir and tocilizumab for hospitalized COVID-19 patients.
Dr. Bhimraj argued for the use of remdesivir or tocilizumab in patients hospitalized with COVID-19 pneumonia, and Dr. Walter presented the case against their use.
Referendum on remdesivir
The main sources referenced by the presenters regarding remdesivir were the WHO Solidarity Trial (N Engl J Med. 2021 Feb 11. doi: 10.1056/NEJMoa2023184) and the Adaptive Covid-19 Treatment Trial (ACCT) final report (N Engl J Med. 2020 Nov 5. doi: 10.1056/NEJMoa2007764).
“The ‘debate’ is partly artificial,” and meant to illustrate how clinicians can use their own clinical faculties and reasoning to make an informed decision when treating COVID-19 patients, Dr. Bhimraj said.
The ACCT trial compared remdesivir with placebo in patients with severe enough COVID-19 to require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation. The primary outcome in the study was time to recovery, and “the devil is in the details,” Dr. Bhimraj said. The outcomes clinicians should look for in studies are those that matter to patients, such as death, disability, and discomfort, he noted. Disease-oriented endpoints are easier to measure, but not always meaningful for patients, he said. The study showed an average 5-day decrease in illness, “but the fact is that it did not show a mortality benefit,” he noted.
Another large, open-label study of remdesivir across 30 countries showed no survival benefit associated with the drug, compared with standard of care, said Dr. Bhimraj. Patients treated with remdesivir remained in the hospital longer, but Dr. Bhimraj said he believed that was a bias. “I think the physicians kept the patients in the hospital longer to give the treatment rather than the treatments themselves prolonging the treatment duration,” he said.
In conclusion for remdesivir, “the solid data show that there is an early recovery,” he said. “At least for severe disease, even if there is no mortality benefit, there is a role. I argue that, if someone asks if you want to use remdesivir in severe COVID-19 patients, say yes, especially if you value people getting out of the hospital sooner. In a crisis situation, there is a role for remdesivir.”
Dr. Walter discussed the “con” side of using remdesivir. “We can start with a predata hypothesis, but integrate new data about the efficacy into a postdata hypothesis,” he said.
Dr. Walter made several points against the use of remdesivir in hospitalized COVID-19 patients. First, it has not shown any improvement in mortality and may increase the length of hospital stay, he noted.
Data from the ACCT-1 trial and the WHO solidarity trial, showed “no signal of mortality benefit at all,” he said. In addition, the World Health Organization, American College of Physicians, and National Institutes of Health all recommend against remdesivir for patients who require mechanical ventilation or extracorporeal membrane oxygenation, he said. The efficacy when used with steroids remains unclear, and long-term safety data are lacking, he added.
Taking on tocilizumab
Tocilizumab, an anti-inflammatory agent, has demonstrated an impact on several surrogate markers, notably C-reactive protein, temperature, and oxygenation. Dr. Bhimraj said. He reviewed data from eight published studies on the use of tocilizumab in COVID-19 patients.
Arguably, some trials may not have been powered adequately, and in combination, some trials show an effect on clinical deterioration, if not a mortality benefit, he said.
Consequently, in the context of COVID-19, tocilizumab “should be used early in the disease process, especially if steroids are not working,” said Dr. Bhimraj. Despite the limited evidence, “there is a niche population where this might be beneficial,” he said.
By contrast, Dr. Walter took the position of skepticism about the value of tocilizumab for COVID-19 patients.
Notably, decades of research show that tocilizumab has shown no benefit in patients with sepsis or septic shock, or those with acute respiratory distress syndrome, which have similarities to COVID-19 (JAMA. 2020 Sep 3. doi: 10.1001/jama.2020.17052).
He cited a research letter published in JAMA in September 2020, which showed that cytokine levels were in fact lower in critically ill patients with COVID-19, compared with those who had conditions including sepsis with and without ARDS.
Dr. Walter also cited data on the questionable benefit of tocilizumab when used with steroids and the negligible impact on mortality in hospitalized COVID-19 patients seen in the RECOVERY trial.
Limited data mean that therapeutic decisions related to COVID-19 are more nuanced, but they can be made, the presenters agreed.
Ultimately, when trying to decide whether a drug is efficacious, futile, or harmful, “What we have to do is consider the grand totality of the evidence,” Dr. Bhimraj emphasized.
Dr. Bhimraj and Dr. Walter had no relevant financial conflicts to disclose.
As the COVID-19 pandemic continues and evidence evolves, clinical judgment is the bottom line for clinical care, according to Adarsh Bhimraj, MD, of the Cleveland Clinic, and James Walter, MD, of Northwestern Medicine, Chicago.
In a debate/discussion presented at SHM Converge, the annual conference of the Society of Hospital Medicine, Dr. Bhimraj and Dr. Walter took sides in a friendly debate on the value of remdesivir and tocilizumab for hospitalized COVID-19 patients.
Dr. Bhimraj argued for the use of remdesivir or tocilizumab in patients hospitalized with COVID-19 pneumonia, and Dr. Walter presented the case against their use.
Referendum on remdesivir
The main sources referenced by the presenters regarding remdesivir were the WHO Solidarity Trial (N Engl J Med. 2021 Feb 11. doi: 10.1056/NEJMoa2023184) and the Adaptive Covid-19 Treatment Trial (ACCT) final report (N Engl J Med. 2020 Nov 5. doi: 10.1056/NEJMoa2007764).
“The ‘debate’ is partly artificial,” and meant to illustrate how clinicians can use their own clinical faculties and reasoning to make an informed decision when treating COVID-19 patients, Dr. Bhimraj said.
The ACCT trial compared remdesivir with placebo in patients with severe enough COVID-19 to require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation. The primary outcome in the study was time to recovery, and “the devil is in the details,” Dr. Bhimraj said. The outcomes clinicians should look for in studies are those that matter to patients, such as death, disability, and discomfort, he noted. Disease-oriented endpoints are easier to measure, but not always meaningful for patients, he said. The study showed an average 5-day decrease in illness, “but the fact is that it did not show a mortality benefit,” he noted.
Another large, open-label study of remdesivir across 30 countries showed no survival benefit associated with the drug, compared with standard of care, said Dr. Bhimraj. Patients treated with remdesivir remained in the hospital longer, but Dr. Bhimraj said he believed that was a bias. “I think the physicians kept the patients in the hospital longer to give the treatment rather than the treatments themselves prolonging the treatment duration,” he said.
In conclusion for remdesivir, “the solid data show that there is an early recovery,” he said. “At least for severe disease, even if there is no mortality benefit, there is a role. I argue that, if someone asks if you want to use remdesivir in severe COVID-19 patients, say yes, especially if you value people getting out of the hospital sooner. In a crisis situation, there is a role for remdesivir.”
Dr. Walter discussed the “con” side of using remdesivir. “We can start with a predata hypothesis, but integrate new data about the efficacy into a postdata hypothesis,” he said.
Dr. Walter made several points against the use of remdesivir in hospitalized COVID-19 patients. First, it has not shown any improvement in mortality and may increase the length of hospital stay, he noted.
Data from the ACCT-1 trial and the WHO solidarity trial, showed “no signal of mortality benefit at all,” he said. In addition, the World Health Organization, American College of Physicians, and National Institutes of Health all recommend against remdesivir for patients who require mechanical ventilation or extracorporeal membrane oxygenation, he said. The efficacy when used with steroids remains unclear, and long-term safety data are lacking, he added.
Taking on tocilizumab
Tocilizumab, an anti-inflammatory agent, has demonstrated an impact on several surrogate markers, notably C-reactive protein, temperature, and oxygenation. Dr. Bhimraj said. He reviewed data from eight published studies on the use of tocilizumab in COVID-19 patients.
Arguably, some trials may not have been powered adequately, and in combination, some trials show an effect on clinical deterioration, if not a mortality benefit, he said.
Consequently, in the context of COVID-19, tocilizumab “should be used early in the disease process, especially if steroids are not working,” said Dr. Bhimraj. Despite the limited evidence, “there is a niche population where this might be beneficial,” he said.
By contrast, Dr. Walter took the position of skepticism about the value of tocilizumab for COVID-19 patients.
Notably, decades of research show that tocilizumab has shown no benefit in patients with sepsis or septic shock, or those with acute respiratory distress syndrome, which have similarities to COVID-19 (JAMA. 2020 Sep 3. doi: 10.1001/jama.2020.17052).
He cited a research letter published in JAMA in September 2020, which showed that cytokine levels were in fact lower in critically ill patients with COVID-19, compared with those who had conditions including sepsis with and without ARDS.
Dr. Walter also cited data on the questionable benefit of tocilizumab when used with steroids and the negligible impact on mortality in hospitalized COVID-19 patients seen in the RECOVERY trial.
Limited data mean that therapeutic decisions related to COVID-19 are more nuanced, but they can be made, the presenters agreed.
Ultimately, when trying to decide whether a drug is efficacious, futile, or harmful, “What we have to do is consider the grand totality of the evidence,” Dr. Bhimraj emphasized.
Dr. Bhimraj and Dr. Walter had no relevant financial conflicts to disclose.
FROM SHM CONVERGE 2021
New child COVID-19 cases drop for second consecutive week
New cases of COVID-19 in children are trending downward again after dropping for a second consecutive week, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite that drop, however, based on data in the weekly AAP/CHA report.
New cases totaled 71,649 for the week of April 23-29, down by 10.3% from the week before and by 19.0% over this most recent 2-week decline, but still a ways to go before reaching the low point of the year (52,695) recorded during the second week of March, the report shows.
Since the beginning of the pandemic, just over 3.78 million children have been infected by SARS-CoV-2, which is 13.8% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The overall rate of COVID-19 has reached 5,026 cases per 100,000 children, or 5% of the total pediatric population, although there is considerable variation among the states regarding age ranges used to define child cases. Most states use a range of 0-17 or 0-19 years, but Florida and Utah use a range of 0-14 years and South Carolina and Tennessee go with 0-20, the AAP and CHA noted.
There is also much variation between the states when it comes to cumulative child COVID-19 rates, with the lowest rate reported in Hawaii (1,264 per 100,000) and the highest in North Dakota (9,416 per 100,000). The lowest proportion of child cases to all cases is found in Florida (8.7%) and the highest in Vermont (22.2%), the AAP and CHA said.
The number of COVID-19–related deaths was 303 as of April 29, up by 7 from the previous week in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting mortality data by age. The proportion of child deaths to child cases remains at 0.01%, and children represent just 0.06% of all COVID-19 deaths, according to the AAP/CHA report.
New cases of COVID-19 in children are trending downward again after dropping for a second consecutive week, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite that drop, however, based on data in the weekly AAP/CHA report.
New cases totaled 71,649 for the week of April 23-29, down by 10.3% from the week before and by 19.0% over this most recent 2-week decline, but still a ways to go before reaching the low point of the year (52,695) recorded during the second week of March, the report shows.
Since the beginning of the pandemic, just over 3.78 million children have been infected by SARS-CoV-2, which is 13.8% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The overall rate of COVID-19 has reached 5,026 cases per 100,000 children, or 5% of the total pediatric population, although there is considerable variation among the states regarding age ranges used to define child cases. Most states use a range of 0-17 or 0-19 years, but Florida and Utah use a range of 0-14 years and South Carolina and Tennessee go with 0-20, the AAP and CHA noted.
There is also much variation between the states when it comes to cumulative child COVID-19 rates, with the lowest rate reported in Hawaii (1,264 per 100,000) and the highest in North Dakota (9,416 per 100,000). The lowest proportion of child cases to all cases is found in Florida (8.7%) and the highest in Vermont (22.2%), the AAP and CHA said.
The number of COVID-19–related deaths was 303 as of April 29, up by 7 from the previous week in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting mortality data by age. The proportion of child deaths to child cases remains at 0.01%, and children represent just 0.06% of all COVID-19 deaths, according to the AAP/CHA report.
New cases of COVID-19 in children are trending downward again after dropping for a second consecutive week, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite that drop, however, based on data in the weekly AAP/CHA report.
New cases totaled 71,649 for the week of April 23-29, down by 10.3% from the week before and by 19.0% over this most recent 2-week decline, but still a ways to go before reaching the low point of the year (52,695) recorded during the second week of March, the report shows.
Since the beginning of the pandemic, just over 3.78 million children have been infected by SARS-CoV-2, which is 13.8% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The overall rate of COVID-19 has reached 5,026 cases per 100,000 children, or 5% of the total pediatric population, although there is considerable variation among the states regarding age ranges used to define child cases. Most states use a range of 0-17 or 0-19 years, but Florida and Utah use a range of 0-14 years and South Carolina and Tennessee go with 0-20, the AAP and CHA noted.
There is also much variation between the states when it comes to cumulative child COVID-19 rates, with the lowest rate reported in Hawaii (1,264 per 100,000) and the highest in North Dakota (9,416 per 100,000). The lowest proportion of child cases to all cases is found in Florida (8.7%) and the highest in Vermont (22.2%), the AAP and CHA said.
The number of COVID-19–related deaths was 303 as of April 29, up by 7 from the previous week in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting mortality data by age. The proportion of child deaths to child cases remains at 0.01%, and children represent just 0.06% of all COVID-19 deaths, according to the AAP/CHA report.
Success in LGBTQ+ medicine requires awareness of risk
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
HPV vaccination rates continue to climb among young adults in U.S.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
FMT cuts risk of bloodstream infections in patients with recurrent CDI
Background: After a first episode of CDI, almost 20% of patients will have a recurrence. Recurrent CDI (rCDI) is more likely to be associated with life-threatening complications including toxic megacolon, perforation, bloodstream infections, and death. Most BSIs are caused by intestinal microbes. Some evidence suggests that vancomycin therapy creates conditions that favor intestinal colonization by health care–associated pathogens. FMT aims to restore the normal composition of gut microbiota, is superior to vancomycin, and might decrease the incidence of BSI and related complications including death.
Study design: Prospective cohort study.
Setting: Fondazione Policlinico Universitario Agostino Gemelli in Rome.
Synopsis: In this study, 290 patients with rCDIs were randomized to FMT (109 patients) or antibiotic therapy (181 patients). Only patients with their first rCDIs were included. The primary outcome was the development of primary BSI after treatment of rCDI and within a 90-day follow-up period. Secondary outcomes were length of hospitalization and overall survival at 90 days.
Five patients in the FMT group and 40 in the antibiotic group (16% of total patients) developed BSIs during the 90-day follow-up. Because of baseline characteristic differences in the patients treated with FMT versus antibiotics, comparative analyses were limited to the matched cohort. Risk for BSIs was 23% lower in the FMT group (95% confidence interval, 10%-35%); the FMT group also had 14 fewer days of hospitalization (95% CI, 9-20 fewer days) and a 32% increase in overall survival (95% CI, 16%-47%), compared with the antibiotic group. Limitations of the study include its observational nature, single-center design, and differences in several baseline characteristics between the groups remaining after the match.
Bottom line: Patients with rCDI who received FMT were less likely to develop primary BSIs and related complications, including hospital length of stay and death when compared with patients who received antibiotics.
Citation: Ianiro G et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: A prospective cohort study. Ann Intern Med. 2019, Nov 5;171:695-702.
Dr. Santa is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: After a first episode of CDI, almost 20% of patients will have a recurrence. Recurrent CDI (rCDI) is more likely to be associated with life-threatening complications including toxic megacolon, perforation, bloodstream infections, and death. Most BSIs are caused by intestinal microbes. Some evidence suggests that vancomycin therapy creates conditions that favor intestinal colonization by health care–associated pathogens. FMT aims to restore the normal composition of gut microbiota, is superior to vancomycin, and might decrease the incidence of BSI and related complications including death.
Study design: Prospective cohort study.
Setting: Fondazione Policlinico Universitario Agostino Gemelli in Rome.
Synopsis: In this study, 290 patients with rCDIs were randomized to FMT (109 patients) or antibiotic therapy (181 patients). Only patients with their first rCDIs were included. The primary outcome was the development of primary BSI after treatment of rCDI and within a 90-day follow-up period. Secondary outcomes were length of hospitalization and overall survival at 90 days.
Five patients in the FMT group and 40 in the antibiotic group (16% of total patients) developed BSIs during the 90-day follow-up. Because of baseline characteristic differences in the patients treated with FMT versus antibiotics, comparative analyses were limited to the matched cohort. Risk for BSIs was 23% lower in the FMT group (95% confidence interval, 10%-35%); the FMT group also had 14 fewer days of hospitalization (95% CI, 9-20 fewer days) and a 32% increase in overall survival (95% CI, 16%-47%), compared with the antibiotic group. Limitations of the study include its observational nature, single-center design, and differences in several baseline characteristics between the groups remaining after the match.
Bottom line: Patients with rCDI who received FMT were less likely to develop primary BSIs and related complications, including hospital length of stay and death when compared with patients who received antibiotics.
Citation: Ianiro G et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: A prospective cohort study. Ann Intern Med. 2019, Nov 5;171:695-702.
Dr. Santa is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: After a first episode of CDI, almost 20% of patients will have a recurrence. Recurrent CDI (rCDI) is more likely to be associated with life-threatening complications including toxic megacolon, perforation, bloodstream infections, and death. Most BSIs are caused by intestinal microbes. Some evidence suggests that vancomycin therapy creates conditions that favor intestinal colonization by health care–associated pathogens. FMT aims to restore the normal composition of gut microbiota, is superior to vancomycin, and might decrease the incidence of BSI and related complications including death.
Study design: Prospective cohort study.
Setting: Fondazione Policlinico Universitario Agostino Gemelli in Rome.
Synopsis: In this study, 290 patients with rCDIs were randomized to FMT (109 patients) or antibiotic therapy (181 patients). Only patients with their first rCDIs were included. The primary outcome was the development of primary BSI after treatment of rCDI and within a 90-day follow-up period. Secondary outcomes were length of hospitalization and overall survival at 90 days.
Five patients in the FMT group and 40 in the antibiotic group (16% of total patients) developed BSIs during the 90-day follow-up. Because of baseline characteristic differences in the patients treated with FMT versus antibiotics, comparative analyses were limited to the matched cohort. Risk for BSIs was 23% lower in the FMT group (95% confidence interval, 10%-35%); the FMT group also had 14 fewer days of hospitalization (95% CI, 9-20 fewer days) and a 32% increase in overall survival (95% CI, 16%-47%), compared with the antibiotic group. Limitations of the study include its observational nature, single-center design, and differences in several baseline characteristics between the groups remaining after the match.
Bottom line: Patients with rCDI who received FMT were less likely to develop primary BSIs and related complications, including hospital length of stay and death when compared with patients who received antibiotics.
Citation: Ianiro G et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: A prospective cohort study. Ann Intern Med. 2019, Nov 5;171:695-702.
Dr. Santa is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.