User login
Ear tubes no better than antibiotics for otitis media in young kids
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-risk preterm infants may not need antibiotics
Selective use of antibiotics based on birth circumstances may reduce unnecessary antibiotic exposure for preterm infants at risk of early-onset sepsis, based on data from 340 preterm infants at a single center.
Preterm infants born because of preterm labor, premature rupture of membranes, and/or intraamniotic infection (IAI) are considered at increased risk for early-onset sepsis, and current management strategies include a blood culture and initiation of empirical antibiotics, said Kirtan Patel, MD, of Texas A&M University, Dallas, and colleagues in a poster (# 1720) presented at the Pediatric Academic Societies annual meeting.
However, this blanket approach “may increase the unnecessary early antibiotic exposure in preterm infants possibly leading to future adverse health outcomes,” and physicians are advised to review the risks and benefits, Dr. Patel said.
Data from previous studies suggest that preterm infants born as a result of preterm labor and/or premature rupture of membranes with adequate Group B Streptococcus (GBS) intrapartum antibiotic prophylaxis and no indication of IAI may be managed without empiric antibiotics because the early-onset sepsis risk in these infants is much lower than the ones born through IAI and inadequate GBS intrapartum antibiotic prophylaxis.
To better identify preterm birth circumstances in which antibiotics might be avoided, the researchers conducted a retrospective cohort study of preterm infants born at 28-34 weeks’ gestation during the period from Jan. 1, 2015, to Dec. 31, 2018. These infants were in the low-risk category of preterm birth because of preterm labor or premature rupture of membranes, with no IAI and adequate GBS intrapartum antibiotic prophylaxis, and no signs of cardiovascular or respiratory instability after birth. Of these, 157 (46.2%) received empiric antibiotics soon after birth and 183 infants (53.8%) did not receive empiric antibiotics.
The mean gestational age and birth weight were significantly lower in the empiric antibiotic group, but after correcting for these variables, the factors with the greatest influence on the initiation of antibiotics were maternal intrapartum antibiotic prophylaxis (odds ratio, 3.13); premature rupture of membranes (OR, 3.75); use of continuous positive airway pressure (CPAP) in the delivery room (OR, 1.84); CPAP on admission to the neonatal intensive care unit (OR, 1.94); drawing a blood culture (OR, 13.72); and a complete blood count with immature to total neutrophil ratio greater than 0.2 (OR, 3.84).
Three infants (2%) in the antibiotics group had culture-positive early-onset sepsis with Escherichia coli, compared with no infants in the no-antibiotics group. No differences in short-term hospital outcomes appeared between the two groups. The study was limited in part by the retrospective design and sample size, the researchers noted.
However, the results support a selective approach to antibiotics for preterm infants, taking various birth circumstances into account, they said.
Further risk factor identification could curb antibiotic use
In this study, empiric antibiotics were cast as a wide net to avoid missing serious infections in a few patients, said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“It is interesting in this retrospective review of 340 preterm infants that the three newborns that did have serious bacterial infection were correctly given empiric antibiotics from the start,” Dr. Joos noted. “The authors were very effective at elucidating the possible factors that go into starting or not starting empiric antibiotics, although there may be other factors in the clinician’s judgment that are being missed. … More studies are needed on this topic,” Dr. Joos said. “Further research examining how the septic newborns differ from the nonseptic ones could help to even further narrow the use of empiric antibiotics,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Selective use of antibiotics based on birth circumstances may reduce unnecessary antibiotic exposure for preterm infants at risk of early-onset sepsis, based on data from 340 preterm infants at a single center.
Preterm infants born because of preterm labor, premature rupture of membranes, and/or intraamniotic infection (IAI) are considered at increased risk for early-onset sepsis, and current management strategies include a blood culture and initiation of empirical antibiotics, said Kirtan Patel, MD, of Texas A&M University, Dallas, and colleagues in a poster (# 1720) presented at the Pediatric Academic Societies annual meeting.
However, this blanket approach “may increase the unnecessary early antibiotic exposure in preterm infants possibly leading to future adverse health outcomes,” and physicians are advised to review the risks and benefits, Dr. Patel said.
Data from previous studies suggest that preterm infants born as a result of preterm labor and/or premature rupture of membranes with adequate Group B Streptococcus (GBS) intrapartum antibiotic prophylaxis and no indication of IAI may be managed without empiric antibiotics because the early-onset sepsis risk in these infants is much lower than the ones born through IAI and inadequate GBS intrapartum antibiotic prophylaxis.
To better identify preterm birth circumstances in which antibiotics might be avoided, the researchers conducted a retrospective cohort study of preterm infants born at 28-34 weeks’ gestation during the period from Jan. 1, 2015, to Dec. 31, 2018. These infants were in the low-risk category of preterm birth because of preterm labor or premature rupture of membranes, with no IAI and adequate GBS intrapartum antibiotic prophylaxis, and no signs of cardiovascular or respiratory instability after birth. Of these, 157 (46.2%) received empiric antibiotics soon after birth and 183 infants (53.8%) did not receive empiric antibiotics.
The mean gestational age and birth weight were significantly lower in the empiric antibiotic group, but after correcting for these variables, the factors with the greatest influence on the initiation of antibiotics were maternal intrapartum antibiotic prophylaxis (odds ratio, 3.13); premature rupture of membranes (OR, 3.75); use of continuous positive airway pressure (CPAP) in the delivery room (OR, 1.84); CPAP on admission to the neonatal intensive care unit (OR, 1.94); drawing a blood culture (OR, 13.72); and a complete blood count with immature to total neutrophil ratio greater than 0.2 (OR, 3.84).
Three infants (2%) in the antibiotics group had culture-positive early-onset sepsis with Escherichia coli, compared with no infants in the no-antibiotics group. No differences in short-term hospital outcomes appeared between the two groups. The study was limited in part by the retrospective design and sample size, the researchers noted.
However, the results support a selective approach to antibiotics for preterm infants, taking various birth circumstances into account, they said.
Further risk factor identification could curb antibiotic use
In this study, empiric antibiotics were cast as a wide net to avoid missing serious infections in a few patients, said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“It is interesting in this retrospective review of 340 preterm infants that the three newborns that did have serious bacterial infection were correctly given empiric antibiotics from the start,” Dr. Joos noted. “The authors were very effective at elucidating the possible factors that go into starting or not starting empiric antibiotics, although there may be other factors in the clinician’s judgment that are being missed. … More studies are needed on this topic,” Dr. Joos said. “Further research examining how the septic newborns differ from the nonseptic ones could help to even further narrow the use of empiric antibiotics,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Selective use of antibiotics based on birth circumstances may reduce unnecessary antibiotic exposure for preterm infants at risk of early-onset sepsis, based on data from 340 preterm infants at a single center.
Preterm infants born because of preterm labor, premature rupture of membranes, and/or intraamniotic infection (IAI) are considered at increased risk for early-onset sepsis, and current management strategies include a blood culture and initiation of empirical antibiotics, said Kirtan Patel, MD, of Texas A&M University, Dallas, and colleagues in a poster (# 1720) presented at the Pediatric Academic Societies annual meeting.
However, this blanket approach “may increase the unnecessary early antibiotic exposure in preterm infants possibly leading to future adverse health outcomes,” and physicians are advised to review the risks and benefits, Dr. Patel said.
Data from previous studies suggest that preterm infants born as a result of preterm labor and/or premature rupture of membranes with adequate Group B Streptococcus (GBS) intrapartum antibiotic prophylaxis and no indication of IAI may be managed without empiric antibiotics because the early-onset sepsis risk in these infants is much lower than the ones born through IAI and inadequate GBS intrapartum antibiotic prophylaxis.
To better identify preterm birth circumstances in which antibiotics might be avoided, the researchers conducted a retrospective cohort study of preterm infants born at 28-34 weeks’ gestation during the period from Jan. 1, 2015, to Dec. 31, 2018. These infants were in the low-risk category of preterm birth because of preterm labor or premature rupture of membranes, with no IAI and adequate GBS intrapartum antibiotic prophylaxis, and no signs of cardiovascular or respiratory instability after birth. Of these, 157 (46.2%) received empiric antibiotics soon after birth and 183 infants (53.8%) did not receive empiric antibiotics.
The mean gestational age and birth weight were significantly lower in the empiric antibiotic group, but after correcting for these variables, the factors with the greatest influence on the initiation of antibiotics were maternal intrapartum antibiotic prophylaxis (odds ratio, 3.13); premature rupture of membranes (OR, 3.75); use of continuous positive airway pressure (CPAP) in the delivery room (OR, 1.84); CPAP on admission to the neonatal intensive care unit (OR, 1.94); drawing a blood culture (OR, 13.72); and a complete blood count with immature to total neutrophil ratio greater than 0.2 (OR, 3.84).
Three infants (2%) in the antibiotics group had culture-positive early-onset sepsis with Escherichia coli, compared with no infants in the no-antibiotics group. No differences in short-term hospital outcomes appeared between the two groups. The study was limited in part by the retrospective design and sample size, the researchers noted.
However, the results support a selective approach to antibiotics for preterm infants, taking various birth circumstances into account, they said.
Further risk factor identification could curb antibiotic use
In this study, empiric antibiotics were cast as a wide net to avoid missing serious infections in a few patients, said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“It is interesting in this retrospective review of 340 preterm infants that the three newborns that did have serious bacterial infection were correctly given empiric antibiotics from the start,” Dr. Joos noted. “The authors were very effective at elucidating the possible factors that go into starting or not starting empiric antibiotics, although there may be other factors in the clinician’s judgment that are being missed. … More studies are needed on this topic,” Dr. Joos said. “Further research examining how the septic newborns differ from the nonseptic ones could help to even further narrow the use of empiric antibiotics,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Update in Hospital Medicine relays important findings
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
FROM SHM CONVERGE 2021
COVID-19 in children and adolescents: Disease burden and severity
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
Smart prescribing strategies improve antibiotic stewardship
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
FROM SHM CONVERGE 2021
CDC recommends use of Pfizer’s COVID vaccine in 12- to 15-year-olds
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
Keep antibiotics unchanged in breakthrough UTIs
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Palliative care in the pandemic: How one hospital met the challenge
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
FROM SGIM 2021
Small increase seen in new COVID-19 cases among children
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
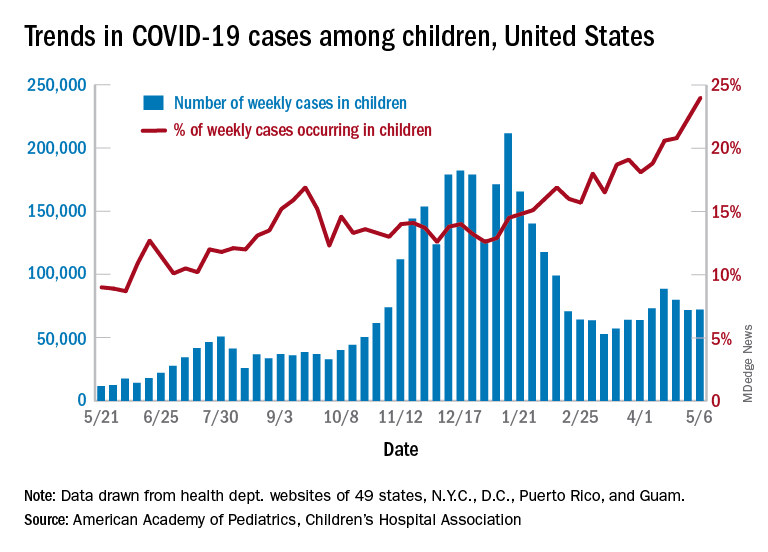
higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
What’s Eating You? Culex Mosquitoes and West Nile Virus
What is West Nile virus? How is it contracted, and who can become infected?
West Nile virus (WNV) is a single-stranded RNA virus of the Flaviviridae family and Flavivirus genus, a lineage that also includes the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses.1 Birds serve as the reservoir hosts of WNV, and mosquitoes acquire the virus during feeding.2 West Nile virus then is transmitted to humans primarily by bites from Culex mosquitoes, which are especially prevalent in wooded areas during peak mosquito season (summer through early fall in North America).1 Mosquitoes also can infect horses; however, humans and horses are dead-end hosts, meaning they do not pass the virus on to other biting mosquitoes.3 There also have been rare reports of transmission of WNV through blood and donation as well as mother-to-baby transmission.2
What is the epidemiology of WNV in the United States?
Since the introduction of WNV to the United States in 1999, it has become an important public health concern, with 48,183 cases and 2163 deaths reported since 1999.2,3 In 2018, Nebraska had the highest number of cases of WNV (n=251), followed by California (n=217), North Dakota (n=204), Illinois (n=176), and South Dakota (n=169).3 West Nile virus is endemic to all 48 contiguous states and Canada, though the Great Plains region is especially affected by WNV due to several factors, such as a greater percentage of rural land, forests, and irrigated areas.4 The Great Plains region also has been thought to be an ecological niche for a more virulent species (Culex tarsalis) compared to other regions in the United States.5
The annual incidence of WNV in the United States peaked in 2003 at 9862 cases (up from 62 cases in 1999), then declined gradually until 2008 to 2011, during which the incidence was stable at 700 to 1100 new cases per year. However, there was a resurgence of cases (n=5674) in 2012 that steadied at around 2200 cases annually in subsequent years.6 Although there likely are several factors affecting WNV incidence trends in the United States, interannual changes in temperature and precipitation have been described. An increased mean annual temperature (from September through October, the end of peak mosquito season) and an increased temperature in winter months (from January through March, prior to peak mosquito season) have both been associated with an increased incidence of WNV.7 An increased temperature is thought to increase population numbers of mosquitoes both by increasing reproductive rates and creating ideal breeding environments via pooled water areas.8 Depending on the region, both above average and below average precipitation levels in the United States can increase WNV incidence the following year.7,9
What are the signs and symptoms of WNV infection?
Up to 80% of those infected with WNV are asymptomatic.3 After an incubation period of roughly 2 to 14 days, the remaining 20% may develop symptoms of West Nile fever (WNF), typically a self-limited illness that consists of 3 to 10 days of nonspecific symptoms such as fever, headache, fatigue, muscle pain and/or weakness, eye pain, gastrointestinal tract upset, and a macular rash that usually presents on the trunk or extremities.1,3 Less than 1% of patients affected by WNV develop neuroinvasive disease, including meningitis, encephalitis, and/or acute flaccid paralysis.10 West Nile virus neuroinvasive disease can cause permanent neurologic sequelae such as muscle weakness, confusion, memory loss, and fatigue; it carries a mortality rate of 10% to 30%, which is mainly dependent on older age and immunosuppression status.1,10
What is the reported spectrum of cutaneous findings in WNV?
Of the roughly 20% of patients infected with WNV that develop WNF, approximately 25% to 50% will develop an associated rash.1 It most commonly is described as a morbilliform or maculopapular rash located on the chest, back, and arms, usually sparing the palms and soles, though 1 case report noted involvement with these areas (Figure).11,12 It typically appears 5 days after symptom onset, can be associated with defervescence, and lasts less than a week.1,13 Pruritus and dysesthesia are sometimes present.13 Other rare presentations that have been reported include an ill-defined pseudovesicular rash with erythematous papules on the palms and pink, scaly, psoriasiform papules on the feet and thighs, as well as neuroinvasive WNV leading to purpura fulminans.14,15 A diffuse, erythematous, petechial rash on the face, neck, trunk, and extremities was reported in a pediatric patient, but there have been no reports of a petechial rash associated with WNV in adult patients.16 These findings suggest some potential variability in the presentation of the WNV rash.
What role does the presence of rash play diagnostically and prognostically?
The rash of WNV has been implicated as a potential prognostic factor in predicting more favorable outcomes.17 Using 2002 data from the Illinois Department of Public Health and 2003 data from the Colorado Department of Public Health, Huhn and Dworkin17 found the age-adjusted risk of encephalitis and death to be decreased in WNV patients with a rash (relative risk, 0.44; 95% CI, 0.21-0.92). The reasons for this are not definitively known, but we hypothesize that the rash may prompt patients to seek earlier medical attention or indicate a more robust immune response. Additionally, a rash in WNV more commonly is seen in younger patients, whereas WNV neuroinvasive disease is more common in older patients, who also tend to have worse outcomes.10 One study found rash to be the only symptom that demonstrated a significant association with seropositivity (overall risk=6.35; P<.05; 95% CI, 3.75-10.80) by multivariate analysis.18
How is WNV diagnosed? What are the downsides to WNV testing?
Given that the presenting symptoms of WNV and WNF are nonspecific, it becomes challenging to arrive at the diagnosis based solely on physical examination. As such, the patient’s clinical and epidemiologic history, such as timing, pattern, and appearance of the rash or recent history of mosquito bites, is key to arriving at the correct diagnosis. With clinical suspicion, possible diagnostic tests include an IgM enzyme-linked immunosorbent assay (ELISA) for WNV, a plaque reduction neutralization test (PNRT), and blood polymerase chain reaction (PCR).
An ELISA is a confirmatory test to detect IgM antibodies to WNV in the serum. Because IgM seroconversion typically occurs between days 4 and 10 of symptom onset, there is a high probability of initial false-negative testing within the first 8 days after symptom onset.19,20 Clinical understanding of this fact is imperative, as an initial negative ELISA does not rule out WNV, and a retest is warranted if clinical suspicion is high. In addition to a high initial false-negative rate with ELISA, there are several other limitations to note. IgM antibodies remain elevated for 1 to 3 months or possibly up to a year in immunocompromised patients.1 Due to this, false positives may be present if there was a recent prior infection. Enzyme-linked immunosorbent assay may not distinguish from different flaviviruses, including the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses. Seropositivity has been estimated in some states, including 1999 data from New York (2.6%), 2003 data from Nebraska (9.5%), and 2012-2014 data from Connecticut (8.5%).21-23 Regional variance may be expected, as there also were significant differences in WNV seropositivity between different regions in Nebraska (P<.001).23
Because ELISA testing for WNV has readily apparent flaws, other tests have been utilized in its diagnosis. The PNRT is the most specific test, and it works by measuring neutralizing antibody titers for different flaviviruses. It has the ability to determine cross-reactivity with other flaviviruses; however, it does not discriminate between a current infection and a prior infection or prior flavivirus vaccine (ie, yellow fever vaccine). Despite this, a positive PNRT can lend credibility to a positive ELISA test and determine specificity for WNV for those with no prior flavivirus exposure.24 According to the Centers for Disease Control and Prevention (CDC), this test can be performed by the CDC or in reference laboratories designated by the CDC.3 Additionally, some state health laboratories may perform PRNTs.
Viral detection with PCR currently is used to screen blood donations and may be beneficial for immunocompromised patients that lack the ability to form a robust antibody response or if a patient presents early, as PCR works best within the first week of symptom onset.1 Tilley et al25 showed that a combination of PCR and ELISA were able to accurately predict 94.2% of patients (180/191) with documented WNV on a first blood sample compared to 45% and 58.1% for only viral detection or ELISA, respectively. Based on costs from a Midwest academic center, antibody detection tests are around $100 while PCR may range from $500 to $1000 and is only performed in reference laboratories. Although these tests remain in the repertoire for WNV diagnosis, financial stewardship is important.
If there are symptoms of photophobia, phonophobia, nuchal rigidity, loss of consciousness, or marked personality changes, a lumbar puncture for WNV IgM in the cerebrospinal fluid can be performed. As with most viral infections, cerebrospinal fluid findings normally include an elevated protein and lymphocyte count, but neutrophils may be predominantly elevated if the infection is early in its course.26
What are the management options?
To date, there is no curative treatment for WNV, and management is largely supportive. For WNF, over-the-counter pain medications may be helpful to reduce fever and pain. If more severe disease develops, hospitalization for further supportive care may be needed.27 If meningitis or encephalitis is suspected, broad-spectrum antibiotics may need to be started until other common etiologies are ruled out.28
How can you prevent WNV infection?
Disease prevention largely consists of educating the public to avoid heavily wooded areas, especially in areas of high prevalence and during peak months, and to use protective clothing and insect repellant that has been approved by the Environmental Protection Agency.3 Insect repellants approved by the Environmental Protection Agency contain ingredients such as DEET (N, N-diethyl-meta-toluamide), picaridin, IR3535 (ethyl butylacetylaminopropionate), and oil of lemon eucalyptus, which have been proven safe and effective.29 Patients also can protect their homes by using window screens and promptly repairing screens with holes.3
What is the differential diagnosis for WNV?
The differential diagnosis for fever with generalized maculopapular rash broadly ranges from viral etiologies (eg, WNV, Zika, measles), to tick bites (eg, Rocky Mountain spotted fever, ehrlichiosis), to drug-induced rashes. A detailed patient history inquiring on recent sick contacts, travel (WNV in the Midwest, ehrlichiosis in the Southeast), environmental exposures (ticks, mosquitoes), and new medications (typically 7–10 days after starting) is imperative to narrow the differential.30 In addition, the distribution, timing, and clinical characteristics of the rash may aid in diagnosis, along with an appropriately correlated clinical picture. West Nile virus likely will present in the summer in mid central geographic locations and often develops on the trunk and extremities as a blanching, generalized, maculopapular rash around 5 days after symptom onset or with defervescence.1
- Petersen LR. Clinical manifestations and diagnosis of West Nile virus infection. UpToDate website. Updated August 7, 2020. Accessed April 16, 2021. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection?search=clinical-manifestations-and-diagnosis-of-west-nile-virusinfection.&source=search_result&selectedTitle=1~78&usage_type=default&display_rank=1
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137-1144.
- Centers for Disease Control and Prevention. West Nile virus. Updated June 3, 2020. Accessed April 16, 2021. https://www.cdc.gov/westnile/index.html
- Chuang TW, Hockett CW, Kightlinger L, et al. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724-731.
- Wimberly MC, Hildreth MB, Boyte SP, et al. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One. 2008;3:E3744. doi:10.1371/journal.pone.0003744
- Centers for Disease Control and Prevention. West Nile virus disease cases reported to CDC by state of residence, 1999-2019. Accessed April 26, 2021. https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2019-P.pdf
- Hahn MB, Monaghan AJ, Hayden MH, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92:1013-1022.
- Brown CM, DeMaria A Jr. The resurgence of West Nile virus. Ann Intern Med. 2012;157:823-824.
- Landesman WJ, Allan BF, Langerhans RB, et al. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7:337-343.
- Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
- Wu JJ, Huang DB, Tyring SK. West Nile virus rash on the palms and soles of the feet. J Eur Acad Dermatol Venereol. 2006;20:1393-1394.
- Sejvar J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-623.
- Ferguson DD, Gershman K, LeBailly A, et al. Characteristics of the rash associated with West Nile virus fever. Clin Infect Dis. 2005;41:1204-1207.
- Marszalek R, Chen A, Gjede J. Psoriasiform eruption in the setting of West Nile virus. J Am Acad Dermatol. 2014;70:AB4. doi:10.1016/j.jaad.2014.01.017
- Shah S, Fite LP, Lane N, et al. Purpura fulminans associated with acute West Nile virus encephalitis. J Clin Virol. 2016;75:1-4.
- Civen R, Villacorte F, Robles DT, et al. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
- Huhn GD, Dworkin MS. Rash as a prognostic factor in West Nile virus disease. Clin Infect Dis. 2006;43:388-389.
- Murphy TD, Grandpre J, Novick SL, et al. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246-251.
- Prince HE, Tobler LH, Lapé-Nixon M, et al. Development and persistence of West Nile virus–specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316-4320.
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile Virus infection. J Infect Dis. 2008;198:984-993.
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- Cahill ME, Yao Y, Nock D, et al. West Nile virus seroprevalence, Connecticut, USA, 2000–2014. Emerg Infect Dis. 2017;23:708-710.
- Schweitzer BK, Kramer WL, Sambol AR, et al. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin Vaccine Immunol. 2006;13:314-318.
- Maeda A, Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013;195:33-40.
- Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for west nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-1364.
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-315.
- Yu A, Ferenczi E, Moussa K, et al. Clinical spectrum of West Nile virus neuroinvasive disease. Neurohospitalist. 2020;10:43-47.
- Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981-986.
- United State Environmental Protection Agency. Skin-applied repellent ingredients. https://www.epa.gov/insect-repellents/skin-applied-repellent-ingredients. Accessed April 16, 2021.
- Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clin Dermatol. 2019;37:109-118.
What is West Nile virus? How is it contracted, and who can become infected?
West Nile virus (WNV) is a single-stranded RNA virus of the Flaviviridae family and Flavivirus genus, a lineage that also includes the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses.1 Birds serve as the reservoir hosts of WNV, and mosquitoes acquire the virus during feeding.2 West Nile virus then is transmitted to humans primarily by bites from Culex mosquitoes, which are especially prevalent in wooded areas during peak mosquito season (summer through early fall in North America).1 Mosquitoes also can infect horses; however, humans and horses are dead-end hosts, meaning they do not pass the virus on to other biting mosquitoes.3 There also have been rare reports of transmission of WNV through blood and donation as well as mother-to-baby transmission.2
What is the epidemiology of WNV in the United States?
Since the introduction of WNV to the United States in 1999, it has become an important public health concern, with 48,183 cases and 2163 deaths reported since 1999.2,3 In 2018, Nebraska had the highest number of cases of WNV (n=251), followed by California (n=217), North Dakota (n=204), Illinois (n=176), and South Dakota (n=169).3 West Nile virus is endemic to all 48 contiguous states and Canada, though the Great Plains region is especially affected by WNV due to several factors, such as a greater percentage of rural land, forests, and irrigated areas.4 The Great Plains region also has been thought to be an ecological niche for a more virulent species (Culex tarsalis) compared to other regions in the United States.5
The annual incidence of WNV in the United States peaked in 2003 at 9862 cases (up from 62 cases in 1999), then declined gradually until 2008 to 2011, during which the incidence was stable at 700 to 1100 new cases per year. However, there was a resurgence of cases (n=5674) in 2012 that steadied at around 2200 cases annually in subsequent years.6 Although there likely are several factors affecting WNV incidence trends in the United States, interannual changes in temperature and precipitation have been described. An increased mean annual temperature (from September through October, the end of peak mosquito season) and an increased temperature in winter months (from January through March, prior to peak mosquito season) have both been associated with an increased incidence of WNV.7 An increased temperature is thought to increase population numbers of mosquitoes both by increasing reproductive rates and creating ideal breeding environments via pooled water areas.8 Depending on the region, both above average and below average precipitation levels in the United States can increase WNV incidence the following year.7,9
What are the signs and symptoms of WNV infection?
Up to 80% of those infected with WNV are asymptomatic.3 After an incubation period of roughly 2 to 14 days, the remaining 20% may develop symptoms of West Nile fever (WNF), typically a self-limited illness that consists of 3 to 10 days of nonspecific symptoms such as fever, headache, fatigue, muscle pain and/or weakness, eye pain, gastrointestinal tract upset, and a macular rash that usually presents on the trunk or extremities.1,3 Less than 1% of patients affected by WNV develop neuroinvasive disease, including meningitis, encephalitis, and/or acute flaccid paralysis.10 West Nile virus neuroinvasive disease can cause permanent neurologic sequelae such as muscle weakness, confusion, memory loss, and fatigue; it carries a mortality rate of 10% to 30%, which is mainly dependent on older age and immunosuppression status.1,10
What is the reported spectrum of cutaneous findings in WNV?
Of the roughly 20% of patients infected with WNV that develop WNF, approximately 25% to 50% will develop an associated rash.1 It most commonly is described as a morbilliform or maculopapular rash located on the chest, back, and arms, usually sparing the palms and soles, though 1 case report noted involvement with these areas (Figure).11,12 It typically appears 5 days after symptom onset, can be associated with defervescence, and lasts less than a week.1,13 Pruritus and dysesthesia are sometimes present.13 Other rare presentations that have been reported include an ill-defined pseudovesicular rash with erythematous papules on the palms and pink, scaly, psoriasiform papules on the feet and thighs, as well as neuroinvasive WNV leading to purpura fulminans.14,15 A diffuse, erythematous, petechial rash on the face, neck, trunk, and extremities was reported in a pediatric patient, but there have been no reports of a petechial rash associated with WNV in adult patients.16 These findings suggest some potential variability in the presentation of the WNV rash.

What role does the presence of rash play diagnostically and prognostically?
The rash of WNV has been implicated as a potential prognostic factor in predicting more favorable outcomes.17 Using 2002 data from the Illinois Department of Public Health and 2003 data from the Colorado Department of Public Health, Huhn and Dworkin17 found the age-adjusted risk of encephalitis and death to be decreased in WNV patients with a rash (relative risk, 0.44; 95% CI, 0.21-0.92). The reasons for this are not definitively known, but we hypothesize that the rash may prompt patients to seek earlier medical attention or indicate a more robust immune response. Additionally, a rash in WNV more commonly is seen in younger patients, whereas WNV neuroinvasive disease is more common in older patients, who also tend to have worse outcomes.10 One study found rash to be the only symptom that demonstrated a significant association with seropositivity (overall risk=6.35; P<.05; 95% CI, 3.75-10.80) by multivariate analysis.18
How is WNV diagnosed? What are the downsides to WNV testing?
Given that the presenting symptoms of WNV and WNF are nonspecific, it becomes challenging to arrive at the diagnosis based solely on physical examination. As such, the patient’s clinical and epidemiologic history, such as timing, pattern, and appearance of the rash or recent history of mosquito bites, is key to arriving at the correct diagnosis. With clinical suspicion, possible diagnostic tests include an IgM enzyme-linked immunosorbent assay (ELISA) for WNV, a plaque reduction neutralization test (PNRT), and blood polymerase chain reaction (PCR).
An ELISA is a confirmatory test to detect IgM antibodies to WNV in the serum. Because IgM seroconversion typically occurs between days 4 and 10 of symptom onset, there is a high probability of initial false-negative testing within the first 8 days after symptom onset.19,20 Clinical understanding of this fact is imperative, as an initial negative ELISA does not rule out WNV, and a retest is warranted if clinical suspicion is high. In addition to a high initial false-negative rate with ELISA, there are several other limitations to note. IgM antibodies remain elevated for 1 to 3 months or possibly up to a year in immunocompromised patients.1 Due to this, false positives may be present if there was a recent prior infection. Enzyme-linked immunosorbent assay may not distinguish from different flaviviruses, including the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses. Seropositivity has been estimated in some states, including 1999 data from New York (2.6%), 2003 data from Nebraska (9.5%), and 2012-2014 data from Connecticut (8.5%).21-23 Regional variance may be expected, as there also were significant differences in WNV seropositivity between different regions in Nebraska (P<.001).23
Because ELISA testing for WNV has readily apparent flaws, other tests have been utilized in its diagnosis. The PNRT is the most specific test, and it works by measuring neutralizing antibody titers for different flaviviruses. It has the ability to determine cross-reactivity with other flaviviruses; however, it does not discriminate between a current infection and a prior infection or prior flavivirus vaccine (ie, yellow fever vaccine). Despite this, a positive PNRT can lend credibility to a positive ELISA test and determine specificity for WNV for those with no prior flavivirus exposure.24 According to the Centers for Disease Control and Prevention (CDC), this test can be performed by the CDC or in reference laboratories designated by the CDC.3 Additionally, some state health laboratories may perform PRNTs.
Viral detection with PCR currently is used to screen blood donations and may be beneficial for immunocompromised patients that lack the ability to form a robust antibody response or if a patient presents early, as PCR works best within the first week of symptom onset.1 Tilley et al25 showed that a combination of PCR and ELISA were able to accurately predict 94.2% of patients (180/191) with documented WNV on a first blood sample compared to 45% and 58.1% for only viral detection or ELISA, respectively. Based on costs from a Midwest academic center, antibody detection tests are around $100 while PCR may range from $500 to $1000 and is only performed in reference laboratories. Although these tests remain in the repertoire for WNV diagnosis, financial stewardship is important.
If there are symptoms of photophobia, phonophobia, nuchal rigidity, loss of consciousness, or marked personality changes, a lumbar puncture for WNV IgM in the cerebrospinal fluid can be performed. As with most viral infections, cerebrospinal fluid findings normally include an elevated protein and lymphocyte count, but neutrophils may be predominantly elevated if the infection is early in its course.26
What are the management options?
To date, there is no curative treatment for WNV, and management is largely supportive. For WNF, over-the-counter pain medications may be helpful to reduce fever and pain. If more severe disease develops, hospitalization for further supportive care may be needed.27 If meningitis or encephalitis is suspected, broad-spectrum antibiotics may need to be started until other common etiologies are ruled out.28
How can you prevent WNV infection?
Disease prevention largely consists of educating the public to avoid heavily wooded areas, especially in areas of high prevalence and during peak months, and to use protective clothing and insect repellant that has been approved by the Environmental Protection Agency.3 Insect repellants approved by the Environmental Protection Agency contain ingredients such as DEET (N, N-diethyl-meta-toluamide), picaridin, IR3535 (ethyl butylacetylaminopropionate), and oil of lemon eucalyptus, which have been proven safe and effective.29 Patients also can protect their homes by using window screens and promptly repairing screens with holes.3
What is the differential diagnosis for WNV?
The differential diagnosis for fever with generalized maculopapular rash broadly ranges from viral etiologies (eg, WNV, Zika, measles), to tick bites (eg, Rocky Mountain spotted fever, ehrlichiosis), to drug-induced rashes. A detailed patient history inquiring on recent sick contacts, travel (WNV in the Midwest, ehrlichiosis in the Southeast), environmental exposures (ticks, mosquitoes), and new medications (typically 7–10 days after starting) is imperative to narrow the differential.30 In addition, the distribution, timing, and clinical characteristics of the rash may aid in diagnosis, along with an appropriately correlated clinical picture. West Nile virus likely will present in the summer in mid central geographic locations and often develops on the trunk and extremities as a blanching, generalized, maculopapular rash around 5 days after symptom onset or with defervescence.1
What is West Nile virus? How is it contracted, and who can become infected?
West Nile virus (WNV) is a single-stranded RNA virus of the Flaviviridae family and Flavivirus genus, a lineage that also includes the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses.1 Birds serve as the reservoir hosts of WNV, and mosquitoes acquire the virus during feeding.2 West Nile virus then is transmitted to humans primarily by bites from Culex mosquitoes, which are especially prevalent in wooded areas during peak mosquito season (summer through early fall in North America).1 Mosquitoes also can infect horses; however, humans and horses are dead-end hosts, meaning they do not pass the virus on to other biting mosquitoes.3 There also have been rare reports of transmission of WNV through blood and donation as well as mother-to-baby transmission.2
What is the epidemiology of WNV in the United States?
Since the introduction of WNV to the United States in 1999, it has become an important public health concern, with 48,183 cases and 2163 deaths reported since 1999.2,3 In 2018, Nebraska had the highest number of cases of WNV (n=251), followed by California (n=217), North Dakota (n=204), Illinois (n=176), and South Dakota (n=169).3 West Nile virus is endemic to all 48 contiguous states and Canada, though the Great Plains region is especially affected by WNV due to several factors, such as a greater percentage of rural land, forests, and irrigated areas.4 The Great Plains region also has been thought to be an ecological niche for a more virulent species (Culex tarsalis) compared to other regions in the United States.5
The annual incidence of WNV in the United States peaked in 2003 at 9862 cases (up from 62 cases in 1999), then declined gradually until 2008 to 2011, during which the incidence was stable at 700 to 1100 new cases per year. However, there was a resurgence of cases (n=5674) in 2012 that steadied at around 2200 cases annually in subsequent years.6 Although there likely are several factors affecting WNV incidence trends in the United States, interannual changes in temperature and precipitation have been described. An increased mean annual temperature (from September through October, the end of peak mosquito season) and an increased temperature in winter months (from January through March, prior to peak mosquito season) have both been associated with an increased incidence of WNV.7 An increased temperature is thought to increase population numbers of mosquitoes both by increasing reproductive rates and creating ideal breeding environments via pooled water areas.8 Depending on the region, both above average and below average precipitation levels in the United States can increase WNV incidence the following year.7,9
What are the signs and symptoms of WNV infection?
Up to 80% of those infected with WNV are asymptomatic.3 After an incubation period of roughly 2 to 14 days, the remaining 20% may develop symptoms of West Nile fever (WNF), typically a self-limited illness that consists of 3 to 10 days of nonspecific symptoms such as fever, headache, fatigue, muscle pain and/or weakness, eye pain, gastrointestinal tract upset, and a macular rash that usually presents on the trunk or extremities.1,3 Less than 1% of patients affected by WNV develop neuroinvasive disease, including meningitis, encephalitis, and/or acute flaccid paralysis.10 West Nile virus neuroinvasive disease can cause permanent neurologic sequelae such as muscle weakness, confusion, memory loss, and fatigue; it carries a mortality rate of 10% to 30%, which is mainly dependent on older age and immunosuppression status.1,10
What is the reported spectrum of cutaneous findings in WNV?
Of the roughly 20% of patients infected with WNV that develop WNF, approximately 25% to 50% will develop an associated rash.1 It most commonly is described as a morbilliform or maculopapular rash located on the chest, back, and arms, usually sparing the palms and soles, though 1 case report noted involvement with these areas (Figure).11,12 It typically appears 5 days after symptom onset, can be associated with defervescence, and lasts less than a week.1,13 Pruritus and dysesthesia are sometimes present.13 Other rare presentations that have been reported include an ill-defined pseudovesicular rash with erythematous papules on the palms and pink, scaly, psoriasiform papules on the feet and thighs, as well as neuroinvasive WNV leading to purpura fulminans.14,15 A diffuse, erythematous, petechial rash on the face, neck, trunk, and extremities was reported in a pediatric patient, but there have been no reports of a petechial rash associated with WNV in adult patients.16 These findings suggest some potential variability in the presentation of the WNV rash.

What role does the presence of rash play diagnostically and prognostically?
The rash of WNV has been implicated as a potential prognostic factor in predicting more favorable outcomes.17 Using 2002 data from the Illinois Department of Public Health and 2003 data from the Colorado Department of Public Health, Huhn and Dworkin17 found the age-adjusted risk of encephalitis and death to be decreased in WNV patients with a rash (relative risk, 0.44; 95% CI, 0.21-0.92). The reasons for this are not definitively known, but we hypothesize that the rash may prompt patients to seek earlier medical attention or indicate a more robust immune response. Additionally, a rash in WNV more commonly is seen in younger patients, whereas WNV neuroinvasive disease is more common in older patients, who also tend to have worse outcomes.10 One study found rash to be the only symptom that demonstrated a significant association with seropositivity (overall risk=6.35; P<.05; 95% CI, 3.75-10.80) by multivariate analysis.18
How is WNV diagnosed? What are the downsides to WNV testing?
Given that the presenting symptoms of WNV and WNF are nonspecific, it becomes challenging to arrive at the diagnosis based solely on physical examination. As such, the patient’s clinical and epidemiologic history, such as timing, pattern, and appearance of the rash or recent history of mosquito bites, is key to arriving at the correct diagnosis. With clinical suspicion, possible diagnostic tests include an IgM enzyme-linked immunosorbent assay (ELISA) for WNV, a plaque reduction neutralization test (PNRT), and blood polymerase chain reaction (PCR).
An ELISA is a confirmatory test to detect IgM antibodies to WNV in the serum. Because IgM seroconversion typically occurs between days 4 and 10 of symptom onset, there is a high probability of initial false-negative testing within the first 8 days after symptom onset.19,20 Clinical understanding of this fact is imperative, as an initial negative ELISA does not rule out WNV, and a retest is warranted if clinical suspicion is high. In addition to a high initial false-negative rate with ELISA, there are several other limitations to note. IgM antibodies remain elevated for 1 to 3 months or possibly up to a year in immunocompromised patients.1 Due to this, false positives may be present if there was a recent prior infection. Enzyme-linked immunosorbent assay may not distinguish from different flaviviruses, including the yellow fever, dengue, Zika, Japanese encephalitis, and Saint Louis encephalitis viruses. Seropositivity has been estimated in some states, including 1999 data from New York (2.6%), 2003 data from Nebraska (9.5%), and 2012-2014 data from Connecticut (8.5%).21-23 Regional variance may be expected, as there also were significant differences in WNV seropositivity between different regions in Nebraska (P<.001).23
Because ELISA testing for WNV has readily apparent flaws, other tests have been utilized in its diagnosis. The PNRT is the most specific test, and it works by measuring neutralizing antibody titers for different flaviviruses. It has the ability to determine cross-reactivity with other flaviviruses; however, it does not discriminate between a current infection and a prior infection or prior flavivirus vaccine (ie, yellow fever vaccine). Despite this, a positive PNRT can lend credibility to a positive ELISA test and determine specificity for WNV for those with no prior flavivirus exposure.24 According to the Centers for Disease Control and Prevention (CDC), this test can be performed by the CDC or in reference laboratories designated by the CDC.3 Additionally, some state health laboratories may perform PRNTs.
Viral detection with PCR currently is used to screen blood donations and may be beneficial for immunocompromised patients that lack the ability to form a robust antibody response or if a patient presents early, as PCR works best within the first week of symptom onset.1 Tilley et al25 showed that a combination of PCR and ELISA were able to accurately predict 94.2% of patients (180/191) with documented WNV on a first blood sample compared to 45% and 58.1% for only viral detection or ELISA, respectively. Based on costs from a Midwest academic center, antibody detection tests are around $100 while PCR may range from $500 to $1000 and is only performed in reference laboratories. Although these tests remain in the repertoire for WNV diagnosis, financial stewardship is important.
If there are symptoms of photophobia, phonophobia, nuchal rigidity, loss of consciousness, or marked personality changes, a lumbar puncture for WNV IgM in the cerebrospinal fluid can be performed. As with most viral infections, cerebrospinal fluid findings normally include an elevated protein and lymphocyte count, but neutrophils may be predominantly elevated if the infection is early in its course.26
What are the management options?
To date, there is no curative treatment for WNV, and management is largely supportive. For WNF, over-the-counter pain medications may be helpful to reduce fever and pain. If more severe disease develops, hospitalization for further supportive care may be needed.27 If meningitis or encephalitis is suspected, broad-spectrum antibiotics may need to be started until other common etiologies are ruled out.28
How can you prevent WNV infection?
Disease prevention largely consists of educating the public to avoid heavily wooded areas, especially in areas of high prevalence and during peak months, and to use protective clothing and insect repellant that has been approved by the Environmental Protection Agency.3 Insect repellants approved by the Environmental Protection Agency contain ingredients such as DEET (N, N-diethyl-meta-toluamide), picaridin, IR3535 (ethyl butylacetylaminopropionate), and oil of lemon eucalyptus, which have been proven safe and effective.29 Patients also can protect their homes by using window screens and promptly repairing screens with holes.3
What is the differential diagnosis for WNV?
The differential diagnosis for fever with generalized maculopapular rash broadly ranges from viral etiologies (eg, WNV, Zika, measles), to tick bites (eg, Rocky Mountain spotted fever, ehrlichiosis), to drug-induced rashes. A detailed patient history inquiring on recent sick contacts, travel (WNV in the Midwest, ehrlichiosis in the Southeast), environmental exposures (ticks, mosquitoes), and new medications (typically 7–10 days after starting) is imperative to narrow the differential.30 In addition, the distribution, timing, and clinical characteristics of the rash may aid in diagnosis, along with an appropriately correlated clinical picture. West Nile virus likely will present in the summer in mid central geographic locations and often develops on the trunk and extremities as a blanching, generalized, maculopapular rash around 5 days after symptom onset or with defervescence.1
- Petersen LR. Clinical manifestations and diagnosis of West Nile virus infection. UpToDate website. Updated August 7, 2020. Accessed April 16, 2021. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection?search=clinical-manifestations-and-diagnosis-of-west-nile-virusinfection.&source=search_result&selectedTitle=1~78&usage_type=default&display_rank=1
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137-1144.
- Centers for Disease Control and Prevention. West Nile virus. Updated June 3, 2020. Accessed April 16, 2021. https://www.cdc.gov/westnile/index.html
- Chuang TW, Hockett CW, Kightlinger L, et al. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724-731.
- Wimberly MC, Hildreth MB, Boyte SP, et al. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One. 2008;3:E3744. doi:10.1371/journal.pone.0003744
- Centers for Disease Control and Prevention. West Nile virus disease cases reported to CDC by state of residence, 1999-2019. Accessed April 26, 2021. https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2019-P.pdf
- Hahn MB, Monaghan AJ, Hayden MH, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92:1013-1022.
- Brown CM, DeMaria A Jr. The resurgence of West Nile virus. Ann Intern Med. 2012;157:823-824.
- Landesman WJ, Allan BF, Langerhans RB, et al. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7:337-343.
- Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
- Wu JJ, Huang DB, Tyring SK. West Nile virus rash on the palms and soles of the feet. J Eur Acad Dermatol Venereol. 2006;20:1393-1394.
- Sejvar J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-623.
- Ferguson DD, Gershman K, LeBailly A, et al. Characteristics of the rash associated with West Nile virus fever. Clin Infect Dis. 2005;41:1204-1207.
- Marszalek R, Chen A, Gjede J. Psoriasiform eruption in the setting of West Nile virus. J Am Acad Dermatol. 2014;70:AB4. doi:10.1016/j.jaad.2014.01.017
- Shah S, Fite LP, Lane N, et al. Purpura fulminans associated with acute West Nile virus encephalitis. J Clin Virol. 2016;75:1-4.
- Civen R, Villacorte F, Robles DT, et al. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
- Huhn GD, Dworkin MS. Rash as a prognostic factor in West Nile virus disease. Clin Infect Dis. 2006;43:388-389.
- Murphy TD, Grandpre J, Novick SL, et al. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246-251.
- Prince HE, Tobler LH, Lapé-Nixon M, et al. Development and persistence of West Nile virus–specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316-4320.
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile Virus infection. J Infect Dis. 2008;198:984-993.
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- Cahill ME, Yao Y, Nock D, et al. West Nile virus seroprevalence, Connecticut, USA, 2000–2014. Emerg Infect Dis. 2017;23:708-710.
- Schweitzer BK, Kramer WL, Sambol AR, et al. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin Vaccine Immunol. 2006;13:314-318.
- Maeda A, Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013;195:33-40.
- Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for west nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-1364.
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-315.
- Yu A, Ferenczi E, Moussa K, et al. Clinical spectrum of West Nile virus neuroinvasive disease. Neurohospitalist. 2020;10:43-47.
- Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981-986.
- United State Environmental Protection Agency. Skin-applied repellent ingredients. https://www.epa.gov/insect-repellents/skin-applied-repellent-ingredients. Accessed April 16, 2021.
- Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clin Dermatol. 2019;37:109-118.
- Petersen LR. Clinical manifestations and diagnosis of West Nile virus infection. UpToDate website. Updated August 7, 2020. Accessed April 16, 2021. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection?search=clinical-manifestations-and-diagnosis-of-west-nile-virusinfection.&source=search_result&selectedTitle=1~78&usage_type=default&display_rank=1
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo Clin Proc. 2003;78:1137-1144.
- Centers for Disease Control and Prevention. West Nile virus. Updated June 3, 2020. Accessed April 16, 2021. https://www.cdc.gov/westnile/index.html
- Chuang TW, Hockett CW, Kightlinger L, et al. Landscape-level spatial patterns of West Nile virus risk in the northern Great Plains. Am J Trop Med Hyg. 2012;86:724-731.
- Wimberly MC, Hildreth MB, Boyte SP, et al. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One. 2008;3:E3744. doi:10.1371/journal.pone.0003744
- Centers for Disease Control and Prevention. West Nile virus disease cases reported to CDC by state of residence, 1999-2019. Accessed April 26, 2021. https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2019-P.pdf
- Hahn MB, Monaghan AJ, Hayden MH, et al. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am J Trop Med Hyg. 2015;92:1013-1022.
- Brown CM, DeMaria A Jr. The resurgence of West Nile virus. Ann Intern Med. 2012;157:823-824.
- Landesman WJ, Allan BF, Langerhans RB, et al. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne Zoonotic Dis. 2007;7:337-343.
- Hart J Jr, Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
- Wu JJ, Huang DB, Tyring SK. West Nile virus rash on the palms and soles of the feet. J Eur Acad Dermatol Venereol. 2006;20:1393-1394.
- Sejvar J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-623.
- Ferguson DD, Gershman K, LeBailly A, et al. Characteristics of the rash associated with West Nile virus fever. Clin Infect Dis. 2005;41:1204-1207.
- Marszalek R, Chen A, Gjede J. Psoriasiform eruption in the setting of West Nile virus. J Am Acad Dermatol. 2014;70:AB4. doi:10.1016/j.jaad.2014.01.017
- Shah S, Fite LP, Lane N, et al. Purpura fulminans associated with acute West Nile virus encephalitis. J Clin Virol. 2016;75:1-4.
- Civen R, Villacorte F, Robles DT, et al. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
- Huhn GD, Dworkin MS. Rash as a prognostic factor in West Nile virus disease. Clin Infect Dis. 2006;43:388-389.
- Murphy TD, Grandpre J, Novick SL, et al. West Nile virus infection among health-fair participants, Wyoming 2003: assessment of symptoms and risk factors. Vector Borne Zoonotic Dis. 2005;5:246-251.
- Prince HE, Tobler LH, Lapé-Nixon M, et al. Development and persistence of West Nile virus–specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316-4320.
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile Virus infection. J Infect Dis. 2008;198:984-993.
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- Cahill ME, Yao Y, Nock D, et al. West Nile virus seroprevalence, Connecticut, USA, 2000–2014. Emerg Infect Dis. 2017;23:708-710.
- Schweitzer BK, Kramer WL, Sambol AR, et al. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin Vaccine Immunol. 2006;13:314-318.
- Maeda A, Maeda J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet J. 2013;195:33-40.
- Tilley PA, Fox JD, Jayaraman GC, et al. Nucleic acid testing for west nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-1364.
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-315.
- Yu A, Ferenczi E, Moussa K, et al. Clinical spectrum of West Nile virus neuroinvasive disease. Neurohospitalist. 2020;10:43-47.
- Michaelis M, Kleinschmidt MC, Doerr HW, et al. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981-986.
- United State Environmental Protection Agency. Skin-applied repellent ingredients. https://www.epa.gov/insect-repellents/skin-applied-repellent-ingredients. Accessed April 16, 2021.
- Muzumdar S, Rothe MJ, Grant-Kels JM. The rash with maculopapules and fever in adults. Clin Dermatol. 2019;37:109-118.
Practice Points
- Dermatologists should be aware of the most common rash associated with West Nile virus (WNV), which is a nonspecific maculopapular rash appearing on the trunk and extremities around 5 days after the onset of fever, fatigue, and other nonspecific symptoms.
- Rash may serve as a prognostic indicator for improved outcomes in WNV due to its association with decreased risk of encephalitis and death.
- An IgM enzyme-linked immunosorbent assay for WNV initially may yield false-negative results, as the development of detectable antibodies against the virus may take up to 8 days after symptom onset.