User login
Deuterium-altered ruxolitinib may be an effective treatment for alopecia areata
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
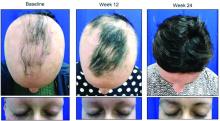
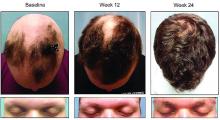
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
WASHINGTON – Yet another inhibitor of the Janus kinase enzyme has debuted with positive phase 2 results for patients with even longstanding alopecia areata.
About half of those who took 8 mg of CPT-543, a chemically altered form of ruxolitinib, twice a day for 24 weeks, regrew hair – including eyebrows and eyelashes – by at least 50%. The dose-ranging study also found that a 4 mg twice-daily dose promoted the same growth in 21%, James V. Cassella, PhD, said during a late breaking clinical trials session at the annual meeting of the American Academy of Dermatology.
Adverse events were mild, including headache, reported in 26% of the 8 mg group. However, investigators “are keeping an eye” on infections and blood chemistry, and in light of the confirmed increased risk of herpes zoster and suspected increased risk of thromboembolic events with ??ruxolitinib, said Dr. Cassella, chief development officer of Concert Pharmaceuticals, which is developing the molecule.
Ruxolitinib, an inhibitor of both JAK1 and JAK2, is available under the name Jakafi and is approved for the treatment of myelofibrosis and polycythemia vera. The addition of deuterium slows its metabolism, increasing half-life and bioavailability without affecting receptor selectivity or potency, according to the company.
The company’s (35). The primary endpoint was the proportion of patients with at least a 50% relative reduction in scalp hair loss as measured by the Severity of Alopecia Tool (SALT) at 24 weeks, from baseline. Secondarily, the trial examined response by alopecia subtype (patchy or complete) and individual SALT changes compared with baseline.
Patients were generally in their mid-30s, and about 75% were female. The mean current alopecia episode was about 5 years. The mean SALT score at baseline was about 89, with 100 being complete hair loss.
By week 24, 47% of those taking 8 mg twice daily and 21% of those taking 4 mg twice daily experienced the primary endpoint of at least 50% SALT reduction from baseline. Almost 9% of those taking placebo also hit the target. These patients all had patchy alopecia and may have been coming out of an alopecia episode during the trial, Dr. Cassella said.
Week 12 was the inflection point for response division, with both active groups significantly outperforming the placebo group. By week 16, 30% of the 8 mg group and about 15% of the 4 mg group had already hit the primary endpoint. Response in the 4 mg group climbed slowly until the end of the trial, while in the 8 mg group, response ascended more quickly. Response was still trending upward when the study stopped.
“We think we have not hit the ceiling effect with this drug,” Dr. Cassella said. “There is some evidence that response would continue to increase after 24 weeks.”
Patchy alopecia and alopecia universalis appeared to respond best to treatment in both dosage groups. There was no response in either group for patients with alopecia ophiasis or totalis.
Headache was the most common adverse event, and appeared to be dose-dependent, occurring in 11% of placebo patients, 17% of the 4 mg group, and 26% of the 8 mg group. Six patients developed increased blood creatinine phosphokinase levels (one in the placebo group, three in the 4 mg group, and two in the 8 mg group). There were no thromboembolic events. Three patients in the placebo group and two in the 8 mg group discontinued the medication due to unspecified adverse events.
In early March, the company announced an open-label dose-finding study, which will randomize 60 patients with moderate-to-severe alopecia areata to either 8 mg twice daily or 16 mg once daily over a 24-week treatment period. Concert intends to conduct a food-effect trial to assess the relative bioavailability of oral doses of CTP-543 under fasted and fed conditions in 14 healthy volunteers in the first half of 2019.
SOURCE: Casella J. AAD 2019; S034, Abstract 11291.
REPORTING FROM AAD 19
Analysis of Nail-Related Content in the Basic Dermatology Curriculum
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3
Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3


Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
Patients frequently present to dermatologists with nail disorders as their chief concern. Alternatively, nail conditions may be encountered by the examining physician as an incidental finding that may be a clue to underlying systemic disease. Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving,1 but many dermatologists find management of nail diseases challenging.2 Bridging this educational gap begins with dermatology resident and medical student education. In a collaboration with dermatology educators, the American Academy of Dermatology (AAD) prepared a free online core curriculum for medical students that covers the essential concepts of dermatology. We sought to determine the integration of nail education in the AAD Basic Dermatology Curriculum.
Methods
A cross-sectional study of the AAD Basic Dermatology Curriculum was conducted to determine nail disease content. The curriculum modules were downloaded in June 2018,
Results
Of 342 patients discussed in cases and quizzes, nails were mentioned for 19 patients (89 times total)(Table 1). Additionally, there were 2 mentions each of nail clippings and nail tumors, 0 mentions of nail biopsies, and 1 mention each of fungal cultures and microscopy on nail scrapings (Table 1). Of the 40 modules, nails were mentioned in 12 modules (Table 2) and 6 introductions to the modules (Table 1). There were no mentions of the terms nails, subungual, or onychomycosis in the learning objectives.3


Comment
Our study demonstrates a paucity of content relevant to nails in the AAD Basic Dermatology Curriculum. Medical students are missing an important opportunity to learn about diagnosis and management of nail conditions and may incorrectly conclude that nail expertise is not essential to becoming a competent board-certified dermatologist.
Particularly concerning is the exclusion of nail examinations in the skin exam module addressing full-body skin examinations (0 mentions in 31 slides). This curriculum may negatively influence medical students and may then follow at the resident level, with a study reporting that 50.3% (69/137) of residents examine nails only when the patient brings it to their attention.4
Most concerning was the inadequate coverage of nail unit melanoma in the melanoma module (1 mention in 53 slides). Furthermore, the ABCDE—asymmetry, border, color, diameter, and evolving—mnemonic for cutaneous melanoma was covered in 6 slides in this module, and the ABCDEF—family history added—mnemonic for nail unit melanoma was completely excluded. Not surprisingly, resident knowledge of melanonychia diagnosis is deficient, with a prior study demonstrating that 62% (88/142) of residents were not confident diagnosing and managing patients with melanonychia, and only 88% (125/142) of residents were aware of the nail melanoma mnemonic.4
Similarly, nail biopsy for melanonychia diagnosis was excluded from the curriculum, whereas skin biopsy was thoroughly discussed in the context of a cutaneous melanoma diagnosis. This deficient teaching may track to the dermatology resident curriculum, as a survey of third-year dermatology residents (N=240) showed that 58% performed 10 or fewer nail procedures, and one-third of residents felt incompetent in nail surgery.5
We acknowledge that the AAD Basic Dermatology Curriculum is simply an introduction to dermatology. However, given that dermatologists are among the major specialists who care for nail patients, we advocate for more content on nail diseases in this curriculum. Nails can easily be incorporated into existing modules, and a new module specifically dedicated to nail disease should be added. Moreover, we envision that our findings will positively reflect on competence in treating nail disease for dermatology residents.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
- Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713-714.
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- American Academy of Dermatology. Basic Dermatology Curriculum. https://www.aad.org/education/basic-derm-curriculum. Accessed March 25, 2019.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483, 483.e1-5.
Practice Points
- Competence in the diagnosis and treatment of nail diseases can drastically improve patient quality of life and can be lifesaving.
- Education on diagnosis and management of nail conditions is deficient in the American Academy of Dermatology (AAD) Basic Dermatology Curriculum.
- Increased efforts are needed to incorporate relevant nail education materials into the AAD Basic Dermatology Curriculum.
Clinical Pearl: Kinesiology Tape for Onychocryptosis
Practice Gap
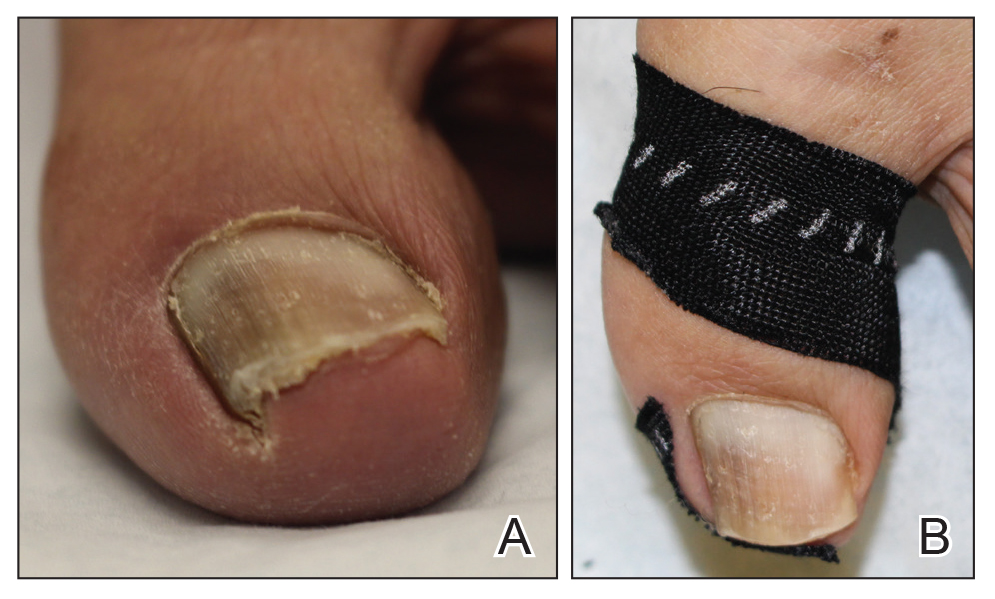
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.
Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
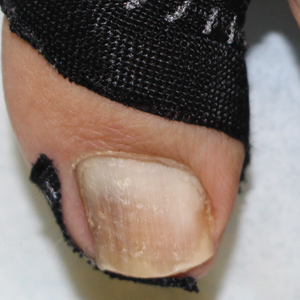
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.

Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
Practice Gap
Onychocryptosis, or ingrown toenail, is a highly prevalent nail condition characterized by penetration of the periungual skin by the nail plate (Figure, A). Patients may report pain either while at rest or walking, which may be debilitating in severe cases and may adversely affect daily living. Treatment may be approached using conservative or surgical therapies. Conservative methods are noninvasive and appropriate for mild cases but require excellent compliance. Although nail trimming is the simplest method, it may necessitate cutting soft tissue, particularly when the nail is anchored deep within the periungual skin. Another conservative method is taping, which aims to separate the nail fold from the offending nail edge by using an adhesive. In common practice, the adhesive often detaches within a few hours, which is further exacerbated by moisture from sweating or bathing.1 Therefore, for effective treatment of onychocryptosis, the tape typically must be reapplied multiple times per day, limiting compliance.

Tools
We propose using kinesiology tape to treat onychocryptosis. Kinesiology tape is a highly elastic adhesive that was originally employed by athletes to relieve pain while supporting muscles, tendons, and ligaments during strenuous activity. We hypothesized that its stronger adherent properties and greater elasticity would be advantageous for treatment of onychocryptosis compared to standard tape.
The Technique
A strip of tape is cut to approximately 10 to 15 mm×5 cm and is applied once daily to the lateral nail fold, pulling it away from the nail plate in oblique and proximal directions and then wrapping it around the plantar surface dorsally (Figure, B). Kinesiology tape properties allow for less frequent application and greater tension to be applied to the nail fold while reducing the risk for
Practice Implications
Kinesiology tape adheres more firmly than other tapes and requires less frequent applications. Use of kinesiology tape for onychocryptosis therapy often is effective and may negate the need for more invasive procedures and improve quality of life during and after treatment.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
1. Haneke E. Controversies in the treatment of ingrown nails [published online May 20, 2012]. Dermatol Res Pract. 2012;2012:783924.
Gene mutations may predispose women to alopecia subtype
in a study of women of African ancestry.
Central centrifugal cicatricial alopecia (CCCA) often runs in families, suggesting a possible genetic component, but specific genes have not been explored, wrote Liron Malki, of Tel Aviv Medical Center and his colleagues.
In a study published in the New England Journal of Medicine, the researchers used a genetic sequencing procedure to examine genes in 16 women with African ancestry with CCCA who served as a discovery set; they identified four heterozygous mutations in the PAD13 gene in 5 women, which included one splice site and three missense mutations. The PAD13 gene “is responsible for mediating the modification of proteins critical for normal hair shaft formation and shaping, such as trichohyalin, and may also play a role in interfollicular epidermal differentiation,” they wrote.
Mr. Malki and his associates then identified an additional 42 patients of African descent with CCCA and directly sequenced PADI3; they found 9 patients with genetic variations.
Overall, the researchers found six mutations in PAD13 that appeared in 14 of the 58 patients (24%) with CCCA.
In a post hoc analysis, the mutations were significantly more prevalent among CCCA patients, compared with 2,702 control women of African ancestry (P = .03 by the chi-square test and P = 0.04 by Fisher’s exact test after adjusting for relatedness of study participants).
The results were limited by several factors, including the small sample size and lack of data on individual hair grooming habits. However, the findings support data from previous studies indicating that PAD13 plays an important role in proper hair shaft formation, the researchers wrote.
“The different properties of hair among persons of African ancestry and those of European ancestry may explain, in part, the different clinical consequences of PADI3 mutations in CCCA and in the uncombable hair syndrome,” Mr. Malki and his associates wrote. “Alternatively, the distinct variants in PADI3 in each of the disorders may account for the difference in clinical outcomes.”
The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
SOURCE: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
Central centrifugal cicatricial alopecia (CCCA) is characterized by hair loss with rapid, progressive, and permanent hair follicle destruction, Jouni Uitto, MD, PhD, wrote in an accompanying editorial.
The current study showed six distinct genetic mutations in PAD13, a gene that allows normal hair follicle development, in 14 of 58 women with CCCA. The researchers also found that genes involved in hair shaft formation were expressed differently in women with CCCA than in unaffected women. Dr. Uitto wrote. Thus, variants in other genes likely contribute to CCCA pathogenesis.
“The observations by Malki et al. suggest that PADI3 mutations predispose persons to CCCA, which is then clinically manifested when hairstyling practices damage the hair. Thus, in the familial setting, such practices should be discouraged in both symptomatic and asymptomatic family members.” he wrote.
Dr. Uitto also noted that uncombable hair syndrome, a rare disorder not associated with alopecia or scarring, also has been associated with mutations in PAD13. However, uncombable hair syndrome is most common in children, who usually outgrow the condition, and it appears not to be inherited. “The mutations in PADI3 in these two conditions are distinct, which suggests different pathogenic consequences of specific PADI3 variants on hair development.”
The PAD13 mutations may predispose women to CCCA, but the data do not support screening asymptomatic women, Dr. Uitto wrote.
“The presence of variants in PADI3 in both CCCA and uncombable hair syndrome suggests that this gene has a pleiotropic effect on the determination of hair texture, and the finding holds implications for future development of therapy, such as the restoration of PADI3 activity,” he concluded.
Dr. Uitto is affiliated with the department of dermatology and cutaneous biology at Jefferson Medical College, Philadelphia. This is a summary of his editorial accompanying the article by Malki et al. (New Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMe1900042). He reported no relevant financial disclosures.
Central centrifugal cicatricial alopecia (CCCA) is characterized by hair loss with rapid, progressive, and permanent hair follicle destruction, Jouni Uitto, MD, PhD, wrote in an accompanying editorial.
The current study showed six distinct genetic mutations in PAD13, a gene that allows normal hair follicle development, in 14 of 58 women with CCCA. The researchers also found that genes involved in hair shaft formation were expressed differently in women with CCCA than in unaffected women. Dr. Uitto wrote. Thus, variants in other genes likely contribute to CCCA pathogenesis.
“The observations by Malki et al. suggest that PADI3 mutations predispose persons to CCCA, which is then clinically manifested when hairstyling practices damage the hair. Thus, in the familial setting, such practices should be discouraged in both symptomatic and asymptomatic family members.” he wrote.
Dr. Uitto also noted that uncombable hair syndrome, a rare disorder not associated with alopecia or scarring, also has been associated with mutations in PAD13. However, uncombable hair syndrome is most common in children, who usually outgrow the condition, and it appears not to be inherited. “The mutations in PADI3 in these two conditions are distinct, which suggests different pathogenic consequences of specific PADI3 variants on hair development.”
The PAD13 mutations may predispose women to CCCA, but the data do not support screening asymptomatic women, Dr. Uitto wrote.
“The presence of variants in PADI3 in both CCCA and uncombable hair syndrome suggests that this gene has a pleiotropic effect on the determination of hair texture, and the finding holds implications for future development of therapy, such as the restoration of PADI3 activity,” he concluded.
Dr. Uitto is affiliated with the department of dermatology and cutaneous biology at Jefferson Medical College, Philadelphia. This is a summary of his editorial accompanying the article by Malki et al. (New Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMe1900042). He reported no relevant financial disclosures.
Central centrifugal cicatricial alopecia (CCCA) is characterized by hair loss with rapid, progressive, and permanent hair follicle destruction, Jouni Uitto, MD, PhD, wrote in an accompanying editorial.
The current study showed six distinct genetic mutations in PAD13, a gene that allows normal hair follicle development, in 14 of 58 women with CCCA. The researchers also found that genes involved in hair shaft formation were expressed differently in women with CCCA than in unaffected women. Dr. Uitto wrote. Thus, variants in other genes likely contribute to CCCA pathogenesis.
“The observations by Malki et al. suggest that PADI3 mutations predispose persons to CCCA, which is then clinically manifested when hairstyling practices damage the hair. Thus, in the familial setting, such practices should be discouraged in both symptomatic and asymptomatic family members.” he wrote.
Dr. Uitto also noted that uncombable hair syndrome, a rare disorder not associated with alopecia or scarring, also has been associated with mutations in PAD13. However, uncombable hair syndrome is most common in children, who usually outgrow the condition, and it appears not to be inherited. “The mutations in PADI3 in these two conditions are distinct, which suggests different pathogenic consequences of specific PADI3 variants on hair development.”
The PAD13 mutations may predispose women to CCCA, but the data do not support screening asymptomatic women, Dr. Uitto wrote.
“The presence of variants in PADI3 in both CCCA and uncombable hair syndrome suggests that this gene has a pleiotropic effect on the determination of hair texture, and the finding holds implications for future development of therapy, such as the restoration of PADI3 activity,” he concluded.
Dr. Uitto is affiliated with the department of dermatology and cutaneous biology at Jefferson Medical College, Philadelphia. This is a summary of his editorial accompanying the article by Malki et al. (New Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMe1900042). He reported no relevant financial disclosures.
in a study of women of African ancestry.
Central centrifugal cicatricial alopecia (CCCA) often runs in families, suggesting a possible genetic component, but specific genes have not been explored, wrote Liron Malki, of Tel Aviv Medical Center and his colleagues.
In a study published in the New England Journal of Medicine, the researchers used a genetic sequencing procedure to examine genes in 16 women with African ancestry with CCCA who served as a discovery set; they identified four heterozygous mutations in the PAD13 gene in 5 women, which included one splice site and three missense mutations. The PAD13 gene “is responsible for mediating the modification of proteins critical for normal hair shaft formation and shaping, such as trichohyalin, and may also play a role in interfollicular epidermal differentiation,” they wrote.
Mr. Malki and his associates then identified an additional 42 patients of African descent with CCCA and directly sequenced PADI3; they found 9 patients with genetic variations.
Overall, the researchers found six mutations in PAD13 that appeared in 14 of the 58 patients (24%) with CCCA.
In a post hoc analysis, the mutations were significantly more prevalent among CCCA patients, compared with 2,702 control women of African ancestry (P = .03 by the chi-square test and P = 0.04 by Fisher’s exact test after adjusting for relatedness of study participants).
The results were limited by several factors, including the small sample size and lack of data on individual hair grooming habits. However, the findings support data from previous studies indicating that PAD13 plays an important role in proper hair shaft formation, the researchers wrote.
“The different properties of hair among persons of African ancestry and those of European ancestry may explain, in part, the different clinical consequences of PADI3 mutations in CCCA and in the uncombable hair syndrome,” Mr. Malki and his associates wrote. “Alternatively, the distinct variants in PADI3 in each of the disorders may account for the difference in clinical outcomes.”
The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
SOURCE: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
in a study of women of African ancestry.
Central centrifugal cicatricial alopecia (CCCA) often runs in families, suggesting a possible genetic component, but specific genes have not been explored, wrote Liron Malki, of Tel Aviv Medical Center and his colleagues.
In a study published in the New England Journal of Medicine, the researchers used a genetic sequencing procedure to examine genes in 16 women with African ancestry with CCCA who served as a discovery set; they identified four heterozygous mutations in the PAD13 gene in 5 women, which included one splice site and three missense mutations. The PAD13 gene “is responsible for mediating the modification of proteins critical for normal hair shaft formation and shaping, such as trichohyalin, and may also play a role in interfollicular epidermal differentiation,” they wrote.
Mr. Malki and his associates then identified an additional 42 patients of African descent with CCCA and directly sequenced PADI3; they found 9 patients with genetic variations.
Overall, the researchers found six mutations in PAD13 that appeared in 14 of the 58 patients (24%) with CCCA.
In a post hoc analysis, the mutations were significantly more prevalent among CCCA patients, compared with 2,702 control women of African ancestry (P = .03 by the chi-square test and P = 0.04 by Fisher’s exact test after adjusting for relatedness of study participants).
The results were limited by several factors, including the small sample size and lack of data on individual hair grooming habits. However, the findings support data from previous studies indicating that PAD13 plays an important role in proper hair shaft formation, the researchers wrote.
“The different properties of hair among persons of African ancestry and those of European ancestry may explain, in part, the different clinical consequences of PADI3 mutations in CCCA and in the uncombable hair syndrome,” Mr. Malki and his associates wrote. “Alternatively, the distinct variants in PADI3 in each of the disorders may account for the difference in clinical outcomes.”
The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
SOURCE: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Women with central centrifugal cicatricial alopecia (CCCA) were more likely to have a mutation of the PAD13 gene than unaffected women.
Major finding: Researchers identified six mutations in PAD13 that appeared in 24% of 58 women with CCCA.
Study details: The data come from 58 adult women with African ancestry who had a diagnosis of CCCA.
Disclosures: The study was supported in part by a grant to Eli Sprecher, MD, PhD, from the Ram Family Foundation and a grant to Dr. Sprecher and Regina C. Betz, MD, from the German-Israeli Foundation, a L’Oreal African Hair and Skin Research grant to Ncoza C. Dlova, a research grant from the Skin of Color Society to Amy McMichael, MD, and the Deutsche Forschungsgemeinschaft–funded Cluster of Excellence ImmunoSensation grant to Dr. Betz. Several other researchers – but not all – reported numerous financial disclosures from pharmaceutical and technology companies and universities and organizations.
Source: Malki L et al. N Engl J Med. 2019 Feb 13. doi: 10.1056/NEJMoa1816614.
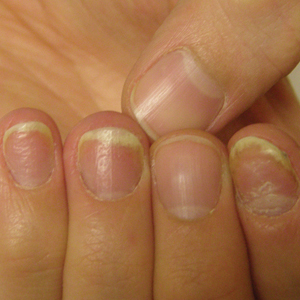
Radiographic Changes of Osteomyelitis in a Patient With Periungual Lichen Planus
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
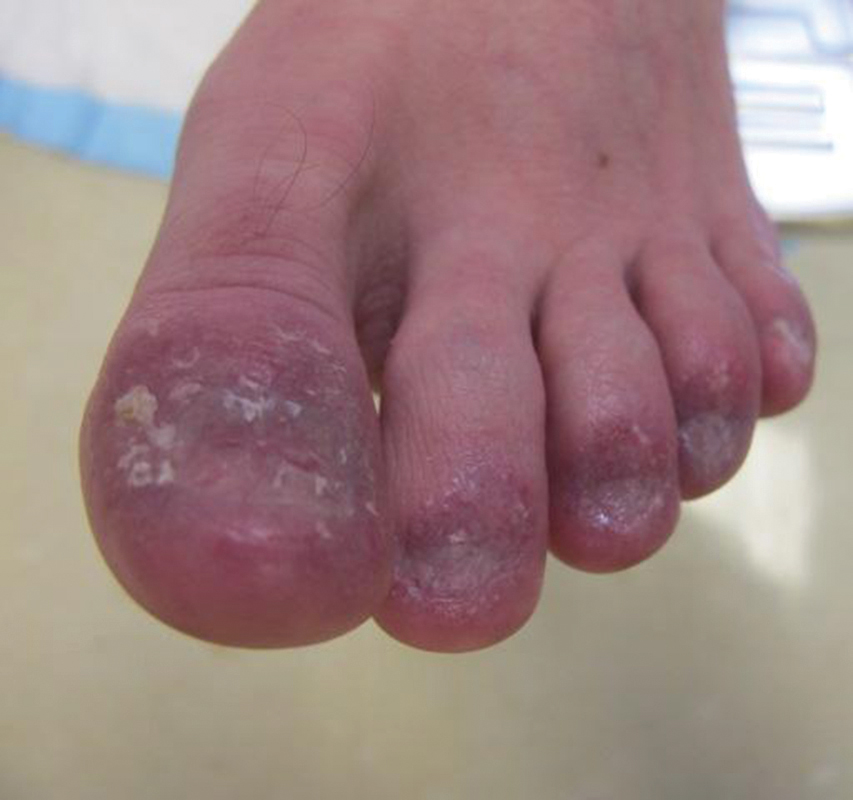
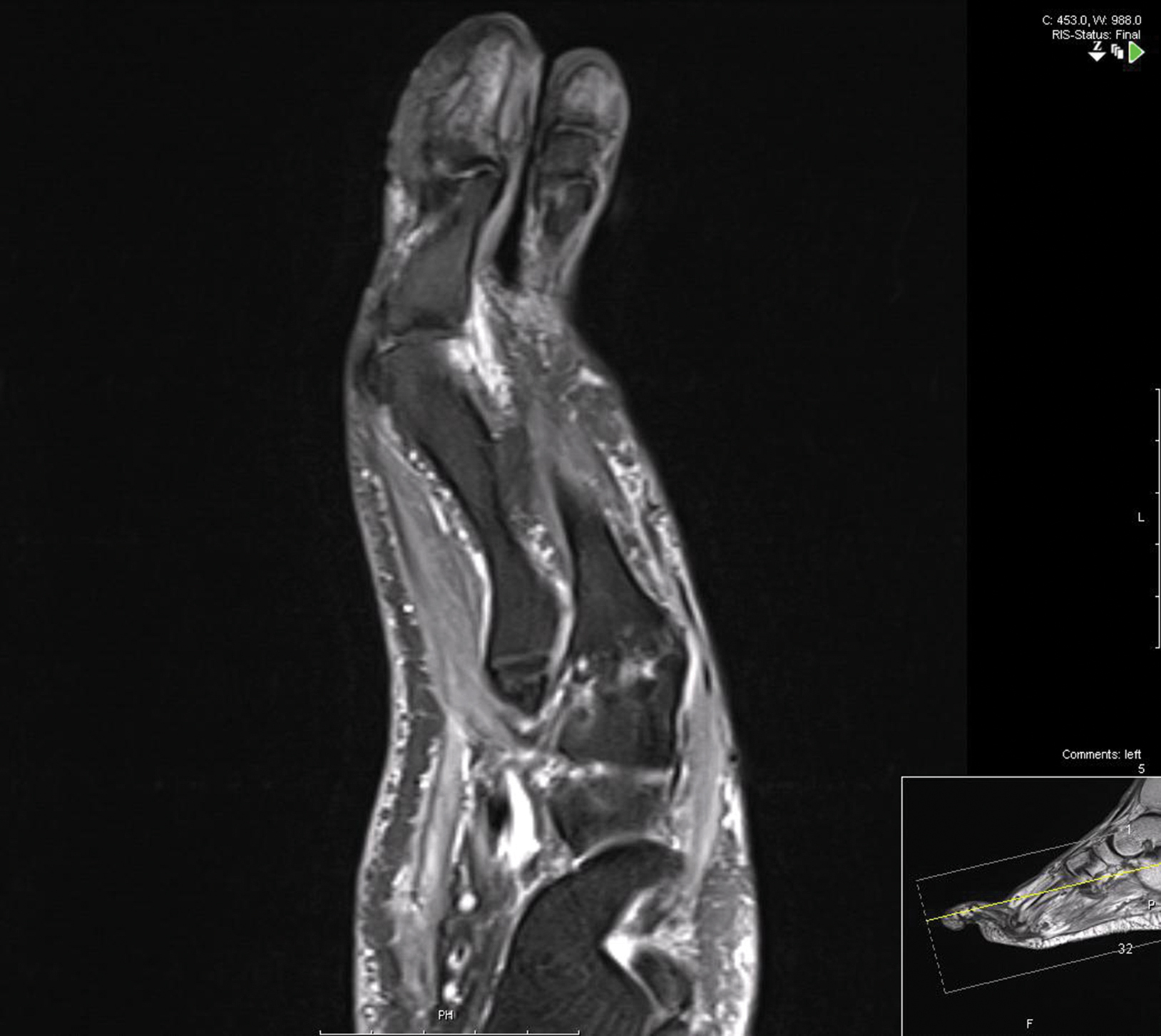
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.

Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.

On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.

Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.

On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder with variable presentations.
- With extensive nail involvement, nail LP may impart radiographic findings suggestive of osteomyelitis.
Frontal Fibrosing Alopecia Demographics: A Survey of 29 Patients
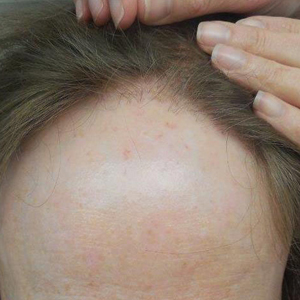
Frontal fibrosing alopecia (FFA) is a form of lymphocytic cicatricial alopecia that presents as frontotemporal hairline recession, typically in postmenopausal women.1 The condition is considered to be a variant of lichen planopilaris (LPP) due to its similar histologic appearance.2 Loss of eyebrow1-11 and body5-11 hair also is commonly present in FFA, and histologic findings are identical to those for hair loss on the scalp,8,9 suggesting that FFA may be a form of generalized alopecia.
The pathogenesis of FFA is unknown, but several etiologies have been postulated. Some suggest that as a variant of LPP, FFA is a hair-specific autoimmune disorder characterized by a T cell–mediated immune reaction against epithelial hair follicle stem cells, leading to fibrosis and depletion of hair regeneration potential.12 In support of this theory, FFA has been associated with other autoimmune diseases including hypothyroidism,6,8,13-16 mucocutaneous lichen planus,8,15,17 vitiligo,15,18 Sjögren syndrome,19 and lichen sclerosus et atrophicus.15,20 Another hypothesis suggests that the proandrogenic state in postmenopausal women may be related to the disease process.1 This hypothesis is supported by the reported success of antiandrogen therapy with 5α-reductase inhibitors (5α-RIs) in stabilizing FFA.3-5,7 Finally, genetic16,21 and environmental factors related to smoking and socioeconomic status5 also have been postulated to be risk factors for FFA. A variety of treatments have shown varying success, including topical and intralesional corticosteroids, hydroxychloroquine, immunomodulators, antibiotics, and 5α-RIs.1,3-6,8,15,17,22 However, FFA is considered to be relatively difficult to treat and commonly progresses regardless of treatment before spontaneously stabilizing.2-4,6,8,10
Since its discovery in 1994,1 FFA has become increasingly prevalent, comprising 17% of new referrals for hair loss in one study (N=57).6 Although growing recognition of the condition likely plays a role in its increasing presentation, other unidentified factors may contribute to its expanding incidence. In this report, we describe the demographics, clinical features, and disease progression of 29 cases of FFA treated within our division using a series of surveys and chart reviews.
Methods
Upon receiving approval for the project from the institutional review board, we identified 29 patients who met the criteria for diagnosis of FFA through a chart review of all patients being treated for hair loss by clinics within the Washington University Division of Dermatology (St. Louis, Missouri). Diagnostic criteria for FFA included scarring alopecia in the frontotemporal distribution with associated perifollicular erythema or papules and, if performed, a scalp biopsy of the involved area of alopecia showing lymphocytic cicatricial alopecia, compatible with LPP. The diagnosis was confirmed by biopsy in 18 patients (62%), while the remainder of the diagnoses were made clinically. Most biopsy specimens were diagnosed by board-certified dermatopathologists at Washington University, with the remainder diagnosed by outside pathologists if the patient was initially diagnosed at another institution.
Patients meeting criteria for FFA were mailed a study consent form, as well as a 2-page survey to assess demographics, clinical features of hair loss, medical histories, social and family histories, and treatments utilized. After receiving consent from patients, survey results were collected and summarized. If there was any need for clarification of answers, follow-up questions were conducted via email prior to any data analysis that was performed.
For analysis of treatment response, patients were asked what treatments they had utilized and about the progression of their hair loss. Patients reporting stabilization of hair loss or hair regrowth were classified as treatment responsive. Patients who underwent multiple treatments were included in the analyses for each of those treatments. Physician records for treatment response were not correlated with patient responses due to inconsistent documentation, care received outside of our medical system, and prolonged or loss to follow-up. Physician-reported data were only used to identify qualifying patients and their biopsy results, as described above.
Results
Patient Demographic
Between October 2013 and May 2014, 29 patients with FFA were recruited into the study. Patients were diagnosed between January 2006 and December 2013. There were 28 female patients (97%) and 1 male patient (3%). The average age of disease onset was 55.4 years (range, 29–75 years). Twenty-five patients (86%) self-identified as non-Hispanic white, 3 patients (10%) as Asian, and 1 patient (3%) as black. Patients also appeared to be a more affluent group than the general St. Louis County population, with a median household income between $75,000 and $100,000. In comparison, the median household income reported in St. Louis County from 2008 to 2012 was $58,485.23 The patient population was primarily composed of nonsmokers, with 22 (76%) patients who had never smoked, 6 (21%) who were present smokers, and 1 (3%) smoked in the past. These results were comparable to the reported number of female smokers in Missouri.24
Clinicopathologic Features
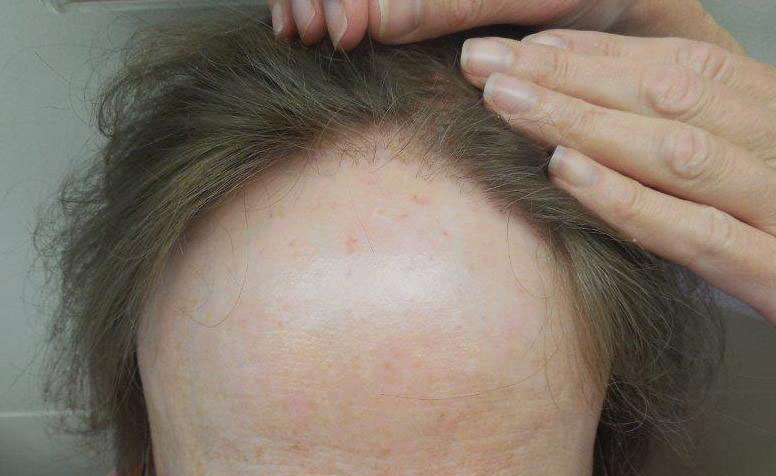
The clinical features of FFA are described in Table 1. All patients had frontotemporal recession of the hairline with some degree of scarring and perifollicular erythema (Figure 1). Most patients also reported hair loss at other sites, including 25 patients (86%) with eyebrow hair loss, 18 (62%) with limb hair loss, 11 (38%) with axillary hair loss, 11 (38%) with pubic hair loss, and 1 (3%) with eyelash hair loss. Patients also frequently reported inflammatory symptoms, including 19 patients (66%) with itching, 18 (62%) with redness, 3 (10%) with pain, 2 (7%) with papular lesions, and 1 (3%) with sores and erosions on the skin. Regarding progression of hair loss over time, 16 patients (55%) reported stabilization of hair loss, 11 (38%) reported progressive hair loss, and 2 (7%) reported some hair regrowth. Thirteen patients (45%) identified some inciting event that they believed to have triggered the disease. Ten patients (35%) identified stress as the inciting event, and 5 patients (17%) specifically referred to health-related stressors, including hip-replacement surgery, new diagnoses of systemic diseases, starting new medications, and stopping hormone replacement therapy. Furthermore, 2 (7%) patients reported exposure to chemicals and pesticides as suspected triggers.

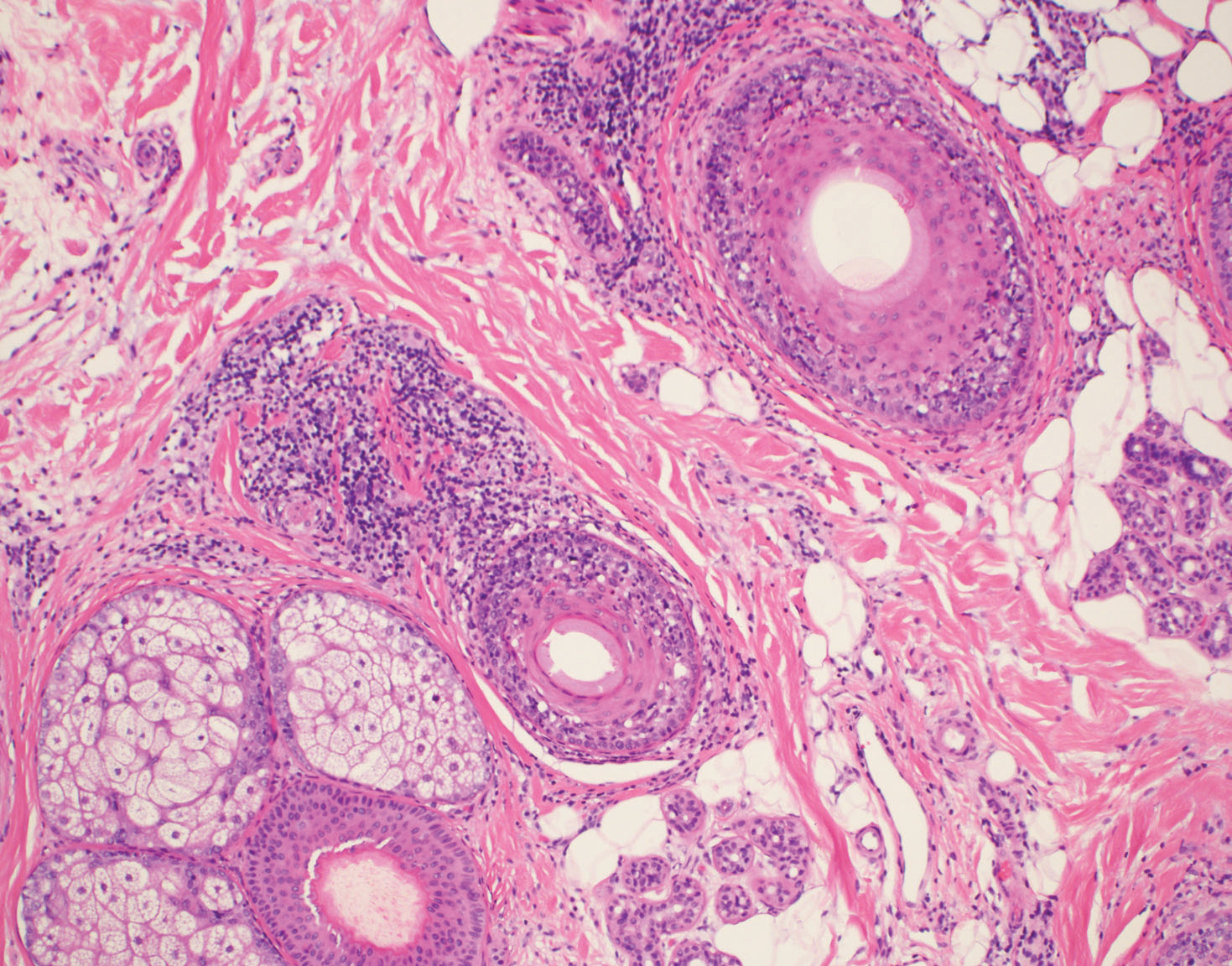
Typical biopsy results showed a perifollicular lymphocytic infiltrate and fibrosis surrounding the infundibulum and isthmus of hair follicles (Figure 2). There were associated vacuolar changes in the basal layer and scattered dyskeratosis throughout the follicular epithelium. As the disease progressed to end-stage scarring, there was marked reduction in the number of hair follicles, which were replaced by fibrous tracts, and a disappearance of the previous inflammatory infiltrate.
Medical History
Of the 26 female patients who provided data about menopause status at time of disease onset, 16 (62%) were postmenopausal, 5 (19%) were menopausal, and 5 (19%) were premenopausal. Of the 28 female patients in the study, 8 (29%) had a history of hysterectomy and 2 (7%) also had surgically induced menopause through bilateral surgical oophorectomy. Twenty-four patients (86%) had a childbearing history, with an average of 2.3 children. Twelve patients (43%) reported use of hormone replacement therapy after menopause. Twelve patients (43%) also reported a history of oral contraceptive use.
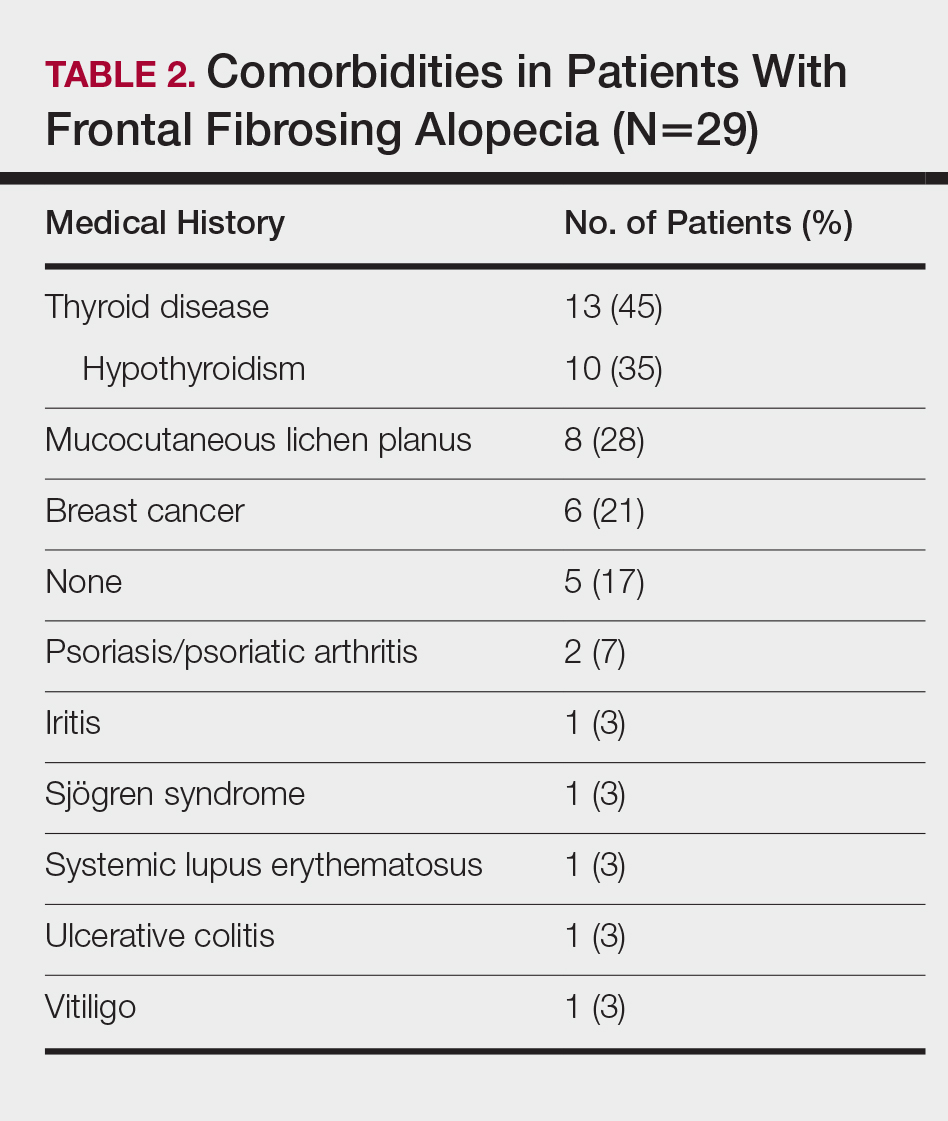
Table 2 describes the comorbidities of all 29 patients. A history of autoimmune disease was prominent, found in 16 patients (55%). Thirteen patients (45%) reported thyroid disease, including 10 patients (35%) with hypothyroidism. Additionally, 8 patients (28%) had a history of mucocutaneous lichen planus, 2 (7%) of psoriasis/psoriatic arthritis, 1 (3%) of vitiligo, 1 (3%) of systemic lupus erythematosus, 1 (3%) of iritis, 1 (3%) of Sjögren syndrome, and 1 (3%) of ulcerative colitis. Six patients (21%) also reported a history of breast cancer.
A dental history was obtained in 24 patients. All 24 patients reported having some dental implant or filling placed. Twenty-four patients (100%) had a history of metal amalgam implants, 8 (33%) had gold alloy implants, 4 (17%) had composite resin implants, and 3 (13%) had porcelain implants. Two patients had metal amalgam implants that had since been replaced by nonmetal implants. Both patients reported no change in their clinical conditions with removal of the metal implants. Six of 8 patients (75%) with mucocutaneous lichen planus reported having dental implants. Of them, all 6 patients (100%) reported having metal amalgam implants, and 3 patients (50%) additionally reported having gold alloy implants.
Treatments
On average, patients were treated with 3 different therapies for FFA (range, 0–14). The treatments utilized are listed in Table 3, and responses to treatments are summarized in Table 4. Topical steroids were the most popular treatment modality and were used by 21 patients (72%). Approximately half of those patients reported treatment response with stabilization of hair loss or regrowth (n=11; 52%). Hydroxychloroquine was the second most commonly used modality (16 patients [55%]), with 10 of those patients (63%) reporting treatment response. Intralesional steroids were used in 11 patients (38%), with a treatment response in 36% (4/11) of those patients. Topical pimecrolimus and tacrolimus were used by 6 patients (21%), with 5 of those patients (83%) reporting treatment response. UVB excimer laser therapy was used on 3 patients (10%) with 100% treatment response.
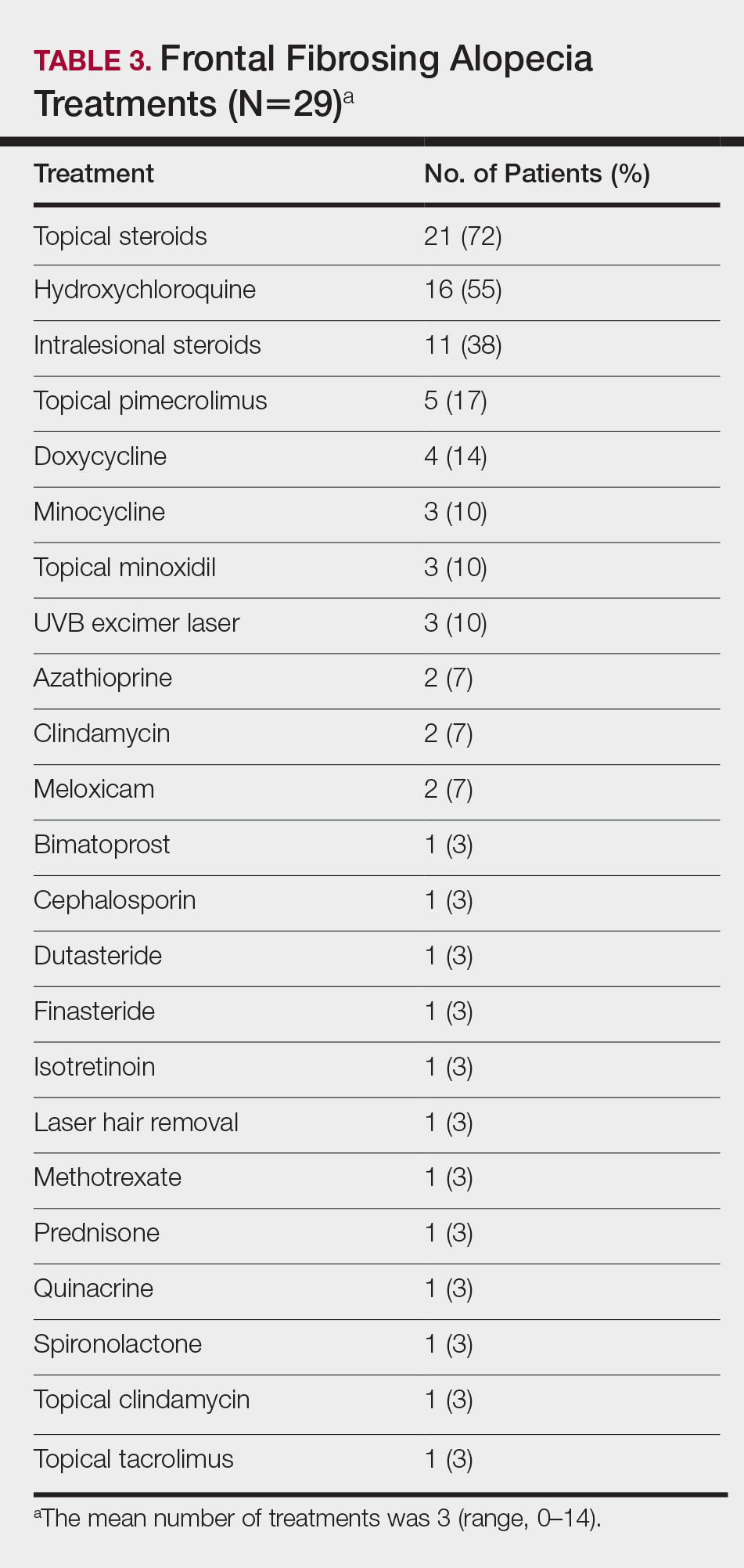
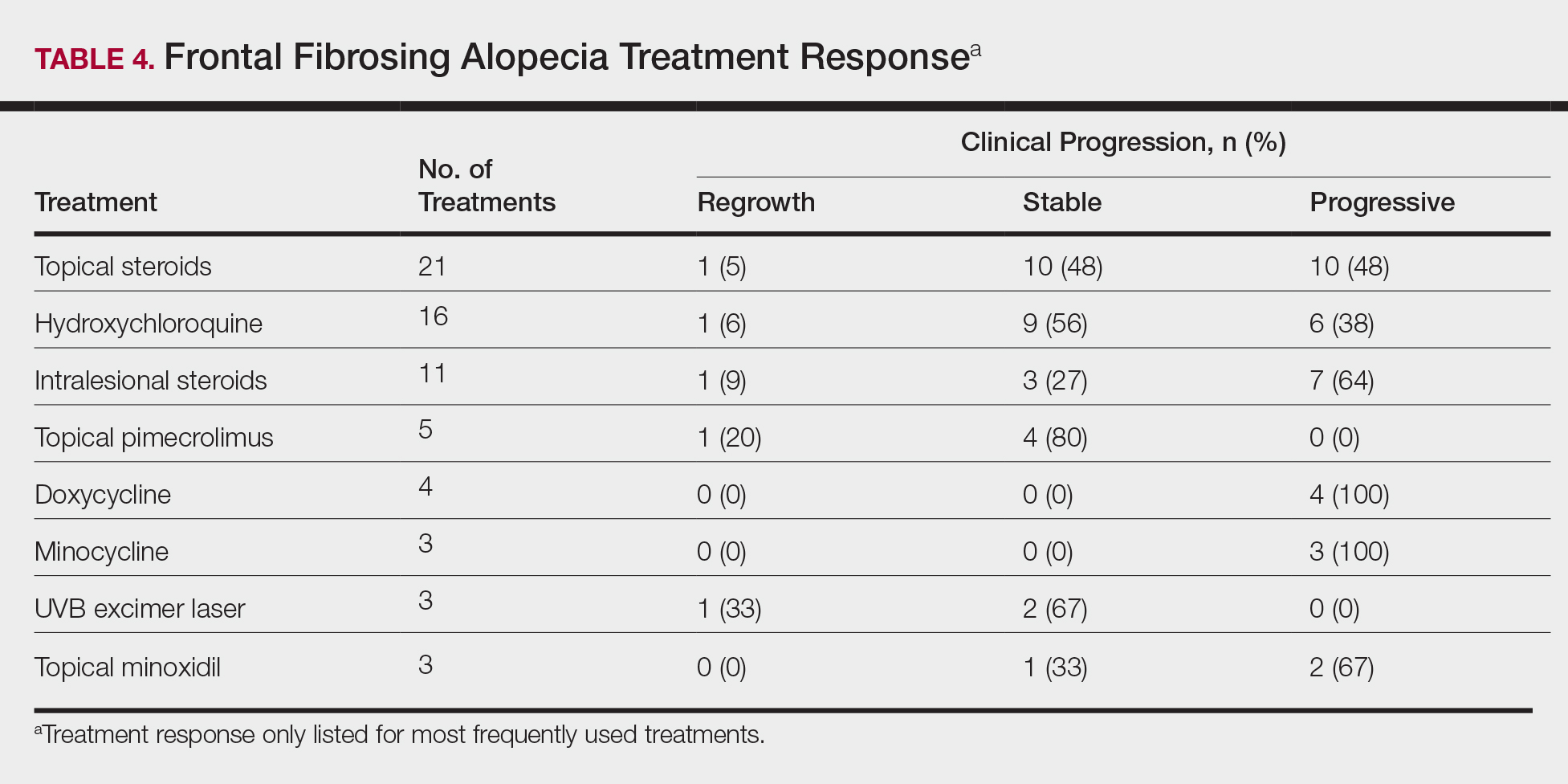
Treatments with little or no treatment response to hair loss include doxycycline, minocycline, and topical minoxidil. Seven patients (24%) were treated with doxycycline or minocycline, all of whom reported no clinical response. Topical minoxidil was used by 3 patients (10%), with only 1 patient (33%) reporting stabilization of hair loss but no regrowth of hair. 5α-reductase inhibitors such as finasteride and dutasteride were only used by 1 patient (3%), who reported no treatment response. Other treatments that were rarely used include meloxicam (n=2), azathioprine (n=2), oral clindamycin (n=2), bimatoprost (n=1), quinacrine (n=1), cephalosporin (n=1), prednisone (n=1), isotretinoin (n=1), methotrexate (n=1), spironolactone (n=1), topical clindamycin (n=1), and laser hair removal (n=1). Of these, only meloxicam and quinacrine were anecdotally associated with stabilization of hair loss, while the rest of the treatments were associated with progressive hair loss despite therapy.
Comment
Frontal fibrosing alopecia is a form of cicatricial alopecia considered to be a clinical subset of LPP. Although the pathogeneses of both diseases are poorly understood, LPP is the better-studied model and is generally considered to be an autoimmune disease specific to the hair follicle, involving a cell-mediated inflammatory response to epithelial hair follicle stem cells.12 In support of this hypothesis, FFA and LPP have been frequently associated with autoimmune diseases, particularly with hypothyroidism.6,13-15 We found that 55% of our patients had a history of autoimmune disease, including 35% with hypothyroidism, 28% with mucocutaneous lichen planus, 7% with psoriasis, 3% with vitiligo, 3% with systemic lupus erythematosus, 3% with iritis, 3% with Sjögren syndrome, and 3% with ulcerative colitis. The link between FFA and hypothyroidism has been the best studied, with a large study by Atanskova Mesinkovska et al14 finding that 34% of 166 patients with LPP and FFA have some kind of thyroid disease and 29% have hypothyroidism. Fron
Although FFA has been classically described to affect postmenopausal women, recent studies have consistently identified that premenopausal women4-6,8,16,17 and men14,16 also can be affected by the condition. In our patient cohort, there was 1 male patient (3%), and a substantial number of the female patients were premenopausal (19%) and menopausal (19%) at the time of disease onset. Most of the patients studied were white; Asian and black patients were a consistent minority across FFA studies,5,13-16,25 highlighting the importance of screening for FFA in all demographics.
In our study, FFA patients also appeared to be more affluent than the general population and were predominantly nonsmokers (76%). These statistics are consistent with the United Kingdom population studied by MacDonald et al,6 which demonstrated a higher socioeconomic status and higher incidence of nonsmoking in their cases of FFA. Another large retrospective study of FFA patients in Spain found that 87% of their FFA cases (N=355) were nonsmokers, though they did not note a difference from the general unaffected population.15 In our study, we replicated these trends, finding an above average affluence level and a high but not statistically significant incidence of nonsmokers. Although it is not clear how socioeconomic status or smoking factors into the pathology of FFA, these studies may show a general trend in the environmental demographics of the disease.
Clinically, patients with FFA typically present with hair loss of the scalp as well as other sites. The eyebrows are the most common site to be affected outside of the scalp, affecting 86% of our patients, whereas eyelashes are the least commonly affected, presenting in only 3% of our patients. Body hair loss also is common, with almost two-thirds of our cohort reporting hair loss on the limbs and more than one-third reporting loss of axillary and pubic hair. These findings are consistent with those of other studies.3-6,8,13,15 Eyelash loss, body hair loss, and facial papules have been found to be associated with more severe forms of FFA,15 though we did not investigate these forms in our study. Inflammatory symptoms are common, with pruritus affecting 66% of our patients and pain affecting 10% of patients, consistent with the published literature.3,13,15,17
Multiple studies have shown that female FFA patients have a higher incidence of hysterectomies in their medical history.5,8,15 This observation has been used to further support the hypothesis that a change in sex hormone balance may trigger the initial onset of disease.5,8,15 A considerable number of the female patients in our study had also undergone hysterectomies (29%). Only 2 patients (7%) underwent premature surgical menopause through bilateral removal of the ovaries, and neither of these patients had abnormally early onset of FFA (age at onset, 52 and 65 years). Many patients in our study also reported a history of pharmacologic manipulation of sex hormones with hormone replacement therapy (43%) and oral contraceptive use (43%). However, patients with FFA have not been identified to have abnormal hormone levels compared to unaffected postmenopausal women.1 Additionally, the disease does not exclusively affect androgen-dependent hair, as indicated by the high prevalence of eyebrow hair loss. We hypothesize that the link between increased prevalence of hysterectomy and FFA is not due to hormonal changes but rather from the stresses related to the hysterectomy or associated conditions that required the surgery. In our study, 35% of patients identified stress as the inciting event prior to their onset of hair loss, with 17% specifically referring to health-related stress such as surgery or new diagnoses as the cause. Although this pattern is purely observational, it is valuable to consider that stress could contribute to the initial onset of FFA as with alopecia areata.26
A dental history was obtained in 24 patients to explore the possibility of FFA as a manifestation of contact allergy secondary to exposure to metal dental implants. Contact allergies to metal amalgam and gold alloy dental implants/fillings frequently have been described as presenting as oral lichen planus in the literature.27-34 Given the histologic overlap between oral lichen planus and LPP/FFA, it is worth exploring the possibility that LPP and FFA are other manifestations of contact allergic response. In our study, 100% of the patients who provided a dental history had metal amalgam implants and 33% had gold alloy implants. It is an interesting observation, but it should be noted that none of the patients in our study had undergone patch testing for contact allergies to the metals in their dental implants, and further studies are required to explore this hypothesis.
Frontal fibrosing alopecia is a difficult condition to treat. In our study, patients tried an average of 3 different treatments, the most common being topical steroids (72%), hydroxychloroquine (55%), and intralesional steroids (38%).
A PubMed search of articles indexed for MEDLINE using the terms randomized control trial and frontal fibrosing alopecia yielded no randomized controlled trials that have been performed to demonstrate the most efficacious treatments of FFA. However, one systematic review of 114 patients found 5α-RIs, antimalarials, and intralesional corticosteroids to yield the best responses in treating FFA.22 Another large, multicenter, retrospective study of 355 patients also demonstrated that 5α-RIs and intralesional corticosteroids minimized hair loss most effectively across treatment modalities.15 One treatment that was not discussed in either study but was utilized in ours was the UVB excimer laser, which has been demonstrated to induce T-cell apoptosis and decrease inflammation in psoriasis but has been infrequently studied in the use of FFA or LPP. In one study of 13 patients with LPP, excimer laser treatment was successful in reducing inflammatory symptoms and improving hair loss.35 Our results reaffirm that laser therapy could be considered more frequently as a treatment of FFA.
This study is subject to several limitations. The study size was comprised of a relatively small number of patients with the condition. Additionally, only one-third of patients contacted agreed to participate in the study, and therefore the responses received may not be completely representative of all FFA patients. With a retrospective study, there is potential for recall bias in the data that are collected. Physician chart correlation to patient responses could not be reliably performed due to inconsistent documentation, care received outside our medical system, and prolonged or loss to follow-up. Another concern is that not all diagnoses of FFA in this study were biopsy confirmed. In one patient with systemic lupus erythematous who declined biopsy, it cannot be confirmed that her etiology of scarring alopecia was FFA rather than discoid lupus erythematous. Finally, because patients were treated with multiple medications, often concurrently, it was difficult to parse out which medications were efficacious and which were not. Despite these limitations, the findings in the study add to the growing literature about a rare but increasingly prevalent presentation.
Conclusion
Frontal fibrosing alopecia is a condition that predominantly affects white postmenopausal women but should not be overlooked in other demographics; higher socioeconomic status and nonsmoking are consistent with cases of FFA worldwide. Alopecia frequently involves other body hair, particularly the eyebrows, and is commonly associated with pruritus and pain. Many patients can identify an inciting event, usually stress, a health crisis, or new external exposures that they believe to have triggered the event. Consistent with data about LPP, FFA is frequently associated with autoimmune conditions, particularly hypothyroidism. A substantial portion of patients with FFA have had metal amalgam or gold alloy dental implants placed, though no patch testing was done to confirm that these patients have a contact allergy to these metals. Treatment for the condition is difficult, but topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy are efficacious in a large proportion of patients. Nevertheless, further research through prospective randomized trials is necessary to determine the best treatment modalities for FFA. Frontal fibrosing alopecia is a scarring form of hair loss that causes substantial emotional distress; therefore, it is critical to continue to investigate its etiology and treatments to improve patient care.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60.
- Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700-705.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Chew AL, Bashir SJ, Wain EM, et al. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660.
- Miteva M, Camacho I, Romanelli P, et al. Acute hair loss on the limbs in frontal fibrosing alopecia: a clinicopathological study of two cases. Br J Dermatol. 2010;163:426-428.
- Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J Am Acad Dermatol. 2007;57(2 suppl):S15-S18.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941.
- Atanaskova Mesinkovska N, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Miteva M, Aber C, Torres F, et al. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445-447.
- Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731.
- Feldmann R, Harms M, Saurat JH. Postmenopausal frontal fibrosing alopecia. Hautarzt. 1996;47:533-536.
- Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- QuickFacts: St. Louis County, Missouri. United States Census Bureau website. https://www.census.gov/quickfacts/fact/table/stlouiscountymissouri/PST045217. Accessed February 6, 2019.
- State tobacco activities tracking and evaluation (STATE) system. State highlights. Centers for Disease Control and Prevention website. https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_STATE.Highlights. Accessed February 6, 2019.
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210.
- Willemsen R, Vanderlinden J, Roseeuw D, et al. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388-393.
- Segura-Egea JJ, Bullón-Fernández P. Lichenoid reaction associated to amalgam restoration. Med Oral Patol Oral Cir Bucal. 2004;9:421-424.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol. 1994;30:205-209.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320-323.
- Nordlind K, Lidén S. Patch test reactions to metal salts in patients with oral mucosal lesions associated with amalgam restorations. Contact Dermatitis. 1992;27:157-160.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis. 1995;33:323-328.
- Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141-146.
- Yiannias JA, el-Azhary RA, Hand JH, et al. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J Am Acad Dermatol. 2000;42:177-182.
- Scalf LA, Fowler JF Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermat. 2001;12:146-150.
- Navarini AA, Kolios AG, Prinz-Vavricka BM, et al. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325-1326.
Frontal fibrosing alopecia (FFA) is a form of lymphocytic cicatricial alopecia that presents as frontotemporal hairline recession, typically in postmenopausal women.1 The condition is considered to be a variant of lichen planopilaris (LPP) due to its similar histologic appearance.2 Loss of eyebrow1-11 and body5-11 hair also is commonly present in FFA, and histologic findings are identical to those for hair loss on the scalp,8,9 suggesting that FFA may be a form of generalized alopecia.
The pathogenesis of FFA is unknown, but several etiologies have been postulated. Some suggest that as a variant of LPP, FFA is a hair-specific autoimmune disorder characterized by a T cell–mediated immune reaction against epithelial hair follicle stem cells, leading to fibrosis and depletion of hair regeneration potential.12 In support of this theory, FFA has been associated with other autoimmune diseases including hypothyroidism,6,8,13-16 mucocutaneous lichen planus,8,15,17 vitiligo,15,18 Sjögren syndrome,19 and lichen sclerosus et atrophicus.15,20 Another hypothesis suggests that the proandrogenic state in postmenopausal women may be related to the disease process.1 This hypothesis is supported by the reported success of antiandrogen therapy with 5α-reductase inhibitors (5α-RIs) in stabilizing FFA.3-5,7 Finally, genetic16,21 and environmental factors related to smoking and socioeconomic status5 also have been postulated to be risk factors for FFA. A variety of treatments have shown varying success, including topical and intralesional corticosteroids, hydroxychloroquine, immunomodulators, antibiotics, and 5α-RIs.1,3-6,8,15,17,22 However, FFA is considered to be relatively difficult to treat and commonly progresses regardless of treatment before spontaneously stabilizing.2-4,6,8,10
Since its discovery in 1994,1 FFA has become increasingly prevalent, comprising 17% of new referrals for hair loss in one study (N=57).6 Although growing recognition of the condition likely plays a role in its increasing presentation, other unidentified factors may contribute to its expanding incidence. In this report, we describe the demographics, clinical features, and disease progression of 29 cases of FFA treated within our division using a series of surveys and chart reviews.
Methods
Upon receiving approval for the project from the institutional review board, we identified 29 patients who met the criteria for diagnosis of FFA through a chart review of all patients being treated for hair loss by clinics within the Washington University Division of Dermatology (St. Louis, Missouri). Diagnostic criteria for FFA included scarring alopecia in the frontotemporal distribution with associated perifollicular erythema or papules and, if performed, a scalp biopsy of the involved area of alopecia showing lymphocytic cicatricial alopecia, compatible with LPP. The diagnosis was confirmed by biopsy in 18 patients (62%), while the remainder of the diagnoses were made clinically. Most biopsy specimens were diagnosed by board-certified dermatopathologists at Washington University, with the remainder diagnosed by outside pathologists if the patient was initially diagnosed at another institution.
Patients meeting criteria for FFA were mailed a study consent form, as well as a 2-page survey to assess demographics, clinical features of hair loss, medical histories, social and family histories, and treatments utilized. After receiving consent from patients, survey results were collected and summarized. If there was any need for clarification of answers, follow-up questions were conducted via email prior to any data analysis that was performed.
For analysis of treatment response, patients were asked what treatments they had utilized and about the progression of their hair loss. Patients reporting stabilization of hair loss or hair regrowth were classified as treatment responsive. Patients who underwent multiple treatments were included in the analyses for each of those treatments. Physician records for treatment response were not correlated with patient responses due to inconsistent documentation, care received outside of our medical system, and prolonged or loss to follow-up. Physician-reported data were only used to identify qualifying patients and their biopsy results, as described above.
Results
Patient Demographic
Between October 2013 and May 2014, 29 patients with FFA were recruited into the study. Patients were diagnosed between January 2006 and December 2013. There were 28 female patients (97%) and 1 male patient (3%). The average age of disease onset was 55.4 years (range, 29–75 years). Twenty-five patients (86%) self-identified as non-Hispanic white, 3 patients (10%) as Asian, and 1 patient (3%) as black. Patients also appeared to be a more affluent group than the general St. Louis County population, with a median household income between $75,000 and $100,000. In comparison, the median household income reported in St. Louis County from 2008 to 2012 was $58,485.23 The patient population was primarily composed of nonsmokers, with 22 (76%) patients who had never smoked, 6 (21%) who were present smokers, and 1 (3%) smoked in the past. These results were comparable to the reported number of female smokers in Missouri.24
Clinicopathologic Features
The clinical features of FFA are described in Table 1. All patients had frontotemporal recession of the hairline with some degree of scarring and perifollicular erythema (Figure 1). Most patients also reported hair loss at other sites, including 25 patients (86%) with eyebrow hair loss, 18 (62%) with limb hair loss, 11 (38%) with axillary hair loss, 11 (38%) with pubic hair loss, and 1 (3%) with eyelash hair loss. Patients also frequently reported inflammatory symptoms, including 19 patients (66%) with itching, 18 (62%) with redness, 3 (10%) with pain, 2 (7%) with papular lesions, and 1 (3%) with sores and erosions on the skin. Regarding progression of hair loss over time, 16 patients (55%) reported stabilization of hair loss, 11 (38%) reported progressive hair loss, and 2 (7%) reported some hair regrowth. Thirteen patients (45%) identified some inciting event that they believed to have triggered the disease. Ten patients (35%) identified stress as the inciting event, and 5 patients (17%) specifically referred to health-related stressors, including hip-replacement surgery, new diagnoses of systemic diseases, starting new medications, and stopping hormone replacement therapy. Furthermore, 2 (7%) patients reported exposure to chemicals and pesticides as suspected triggers.


Typical biopsy results showed a perifollicular lymphocytic infiltrate and fibrosis surrounding the infundibulum and isthmus of hair follicles (Figure 2). There were associated vacuolar changes in the basal layer and scattered dyskeratosis throughout the follicular epithelium. As the disease progressed to end-stage scarring, there was marked reduction in the number of hair follicles, which were replaced by fibrous tracts, and a disappearance of the previous inflammatory infiltrate.

Medical History
Of the 26 female patients who provided data about menopause status at time of disease onset, 16 (62%) were postmenopausal, 5 (19%) were menopausal, and 5 (19%) were premenopausal. Of the 28 female patients in the study, 8 (29%) had a history of hysterectomy and 2 (7%) also had surgically induced menopause through bilateral surgical oophorectomy. Twenty-four patients (86%) had a childbearing history, with an average of 2.3 children. Twelve patients (43%) reported use of hormone replacement therapy after menopause. Twelve patients (43%) also reported a history of oral contraceptive use.
Table 2 describes the comorbidities of all 29 patients. A history of autoimmune disease was prominent, found in 16 patients (55%). Thirteen patients (45%) reported thyroid disease, including 10 patients (35%) with hypothyroidism. Additionally, 8 patients (28%) had a history of mucocutaneous lichen planus, 2 (7%) of psoriasis/psoriatic arthritis, 1 (3%) of vitiligo, 1 (3%) of systemic lupus erythematosus, 1 (3%) of iritis, 1 (3%) of Sjögren syndrome, and 1 (3%) of ulcerative colitis. Six patients (21%) also reported a history of breast cancer.

A dental history was obtained in 24 patients. All 24 patients reported having some dental implant or filling placed. Twenty-four patients (100%) had a history of metal amalgam implants, 8 (33%) had gold alloy implants, 4 (17%) had composite resin implants, and 3 (13%) had porcelain implants. Two patients had metal amalgam implants that had since been replaced by nonmetal implants. Both patients reported no change in their clinical conditions with removal of the metal implants. Six of 8 patients (75%) with mucocutaneous lichen planus reported having dental implants. Of them, all 6 patients (100%) reported having metal amalgam implants, and 3 patients (50%) additionally reported having gold alloy implants.
Treatments
On average, patients were treated with 3 different therapies for FFA (range, 0–14). The treatments utilized are listed in Table 3, and responses to treatments are summarized in Table 4. Topical steroids were the most popular treatment modality and were used by 21 patients (72%). Approximately half of those patients reported treatment response with stabilization of hair loss or regrowth (n=11; 52%). Hydroxychloroquine was the second most commonly used modality (16 patients [55%]), with 10 of those patients (63%) reporting treatment response. Intralesional steroids were used in 11 patients (38%), with a treatment response in 36% (4/11) of those patients. Topical pimecrolimus and tacrolimus were used by 6 patients (21%), with 5 of those patients (83%) reporting treatment response. UVB excimer laser therapy was used on 3 patients (10%) with 100% treatment response.


Treatments with little or no treatment response to hair loss include doxycycline, minocycline, and topical minoxidil. Seven patients (24%) were treated with doxycycline or minocycline, all of whom reported no clinical response. Topical minoxidil was used by 3 patients (10%), with only 1 patient (33%) reporting stabilization of hair loss but no regrowth of hair. 5α-reductase inhibitors such as finasteride and dutasteride were only used by 1 patient (3%), who reported no treatment response. Other treatments that were rarely used include meloxicam (n=2), azathioprine (n=2), oral clindamycin (n=2), bimatoprost (n=1), quinacrine (n=1), cephalosporin (n=1), prednisone (n=1), isotretinoin (n=1), methotrexate (n=1), spironolactone (n=1), topical clindamycin (n=1), and laser hair removal (n=1). Of these, only meloxicam and quinacrine were anecdotally associated with stabilization of hair loss, while the rest of the treatments were associated with progressive hair loss despite therapy.
Comment
Frontal fibrosing alopecia is a form of cicatricial alopecia considered to be a clinical subset of LPP. Although the pathogeneses of both diseases are poorly understood, LPP is the better-studied model and is generally considered to be an autoimmune disease specific to the hair follicle, involving a cell-mediated inflammatory response to epithelial hair follicle stem cells.12 In support of this hypothesis, FFA and LPP have been frequently associated with autoimmune diseases, particularly with hypothyroidism.6,13-15 We found that 55% of our patients had a history of autoimmune disease, including 35% with hypothyroidism, 28% with mucocutaneous lichen planus, 7% with psoriasis, 3% with vitiligo, 3% with systemic lupus erythematosus, 3% with iritis, 3% with Sjögren syndrome, and 3% with ulcerative colitis. The link between FFA and hypothyroidism has been the best studied, with a large study by Atanskova Mesinkovska et al14 finding that 34% of 166 patients with LPP and FFA have some kind of thyroid disease and 29% have hypothyroidism. Fron
Although FFA has been classically described to affect postmenopausal women, recent studies have consistently identified that premenopausal women4-6,8,16,17 and men14,16 also can be affected by the condition. In our patient cohort, there was 1 male patient (3%), and a substantial number of the female patients were premenopausal (19%) and menopausal (19%) at the time of disease onset. Most of the patients studied were white; Asian and black patients were a consistent minority across FFA studies,5,13-16,25 highlighting the importance of screening for FFA in all demographics.
In our study, FFA patients also appeared to be more affluent than the general population and were predominantly nonsmokers (76%). These statistics are consistent with the United Kingdom population studied by MacDonald et al,6 which demonstrated a higher socioeconomic status and higher incidence of nonsmoking in their cases of FFA. Another large retrospective study of FFA patients in Spain found that 87% of their FFA cases (N=355) were nonsmokers, though they did not note a difference from the general unaffected population.15 In our study, we replicated these trends, finding an above average affluence level and a high but not statistically significant incidence of nonsmokers. Although it is not clear how socioeconomic status or smoking factors into the pathology of FFA, these studies may show a general trend in the environmental demographics of the disease.
Clinically, patients with FFA typically present with hair loss of the scalp as well as other sites. The eyebrows are the most common site to be affected outside of the scalp, affecting 86% of our patients, whereas eyelashes are the least commonly affected, presenting in only 3% of our patients. Body hair loss also is common, with almost two-thirds of our cohort reporting hair loss on the limbs and more than one-third reporting loss of axillary and pubic hair. These findings are consistent with those of other studies.3-6,8,13,15 Eyelash loss, body hair loss, and facial papules have been found to be associated with more severe forms of FFA,15 though we did not investigate these forms in our study. Inflammatory symptoms are common, with pruritus affecting 66% of our patients and pain affecting 10% of patients, consistent with the published literature.3,13,15,17
Multiple studies have shown that female FFA patients have a higher incidence of hysterectomies in their medical history.5,8,15 This observation has been used to further support the hypothesis that a change in sex hormone balance may trigger the initial onset of disease.5,8,15 A considerable number of the female patients in our study had also undergone hysterectomies (29%). Only 2 patients (7%) underwent premature surgical menopause through bilateral removal of the ovaries, and neither of these patients had abnormally early onset of FFA (age at onset, 52 and 65 years). Many patients in our study also reported a history of pharmacologic manipulation of sex hormones with hormone replacement therapy (43%) and oral contraceptive use (43%). However, patients with FFA have not been identified to have abnormal hormone levels compared to unaffected postmenopausal women.1 Additionally, the disease does not exclusively affect androgen-dependent hair, as indicated by the high prevalence of eyebrow hair loss. We hypothesize that the link between increased prevalence of hysterectomy and FFA is not due to hormonal changes but rather from the stresses related to the hysterectomy or associated conditions that required the surgery. In our study, 35% of patients identified stress as the inciting event prior to their onset of hair loss, with 17% specifically referring to health-related stress such as surgery or new diagnoses as the cause. Although this pattern is purely observational, it is valuable to consider that stress could contribute to the initial onset of FFA as with alopecia areata.26
A dental history was obtained in 24 patients to explore the possibility of FFA as a manifestation of contact allergy secondary to exposure to metal dental implants. Contact allergies to metal amalgam and gold alloy dental implants/fillings frequently have been described as presenting as oral lichen planus in the literature.27-34 Given the histologic overlap between oral lichen planus and LPP/FFA, it is worth exploring the possibility that LPP and FFA are other manifestations of contact allergic response. In our study, 100% of the patients who provided a dental history had metal amalgam implants and 33% had gold alloy implants. It is an interesting observation, but it should be noted that none of the patients in our study had undergone patch testing for contact allergies to the metals in their dental implants, and further studies are required to explore this hypothesis.
Frontal fibrosing alopecia is a difficult condition to treat. In our study, patients tried an average of 3 different treatments, the most common being topical steroids (72%), hydroxychloroquine (55%), and intralesional steroids (38%).
A PubMed search of articles indexed for MEDLINE using the terms randomized control trial and frontal fibrosing alopecia yielded no randomized controlled trials that have been performed to demonstrate the most efficacious treatments of FFA. However, one systematic review of 114 patients found 5α-RIs, antimalarials, and intralesional corticosteroids to yield the best responses in treating FFA.22 Another large, multicenter, retrospective study of 355 patients also demonstrated that 5α-RIs and intralesional corticosteroids minimized hair loss most effectively across treatment modalities.15 One treatment that was not discussed in either study but was utilized in ours was the UVB excimer laser, which has been demonstrated to induce T-cell apoptosis and decrease inflammation in psoriasis but has been infrequently studied in the use of FFA or LPP. In one study of 13 patients with LPP, excimer laser treatment was successful in reducing inflammatory symptoms and improving hair loss.35 Our results reaffirm that laser therapy could be considered more frequently as a treatment of FFA.
This study is subject to several limitations. The study size was comprised of a relatively small number of patients with the condition. Additionally, only one-third of patients contacted agreed to participate in the study, and therefore the responses received may not be completely representative of all FFA patients. With a retrospective study, there is potential for recall bias in the data that are collected. Physician chart correlation to patient responses could not be reliably performed due to inconsistent documentation, care received outside our medical system, and prolonged or loss to follow-up. Another concern is that not all diagnoses of FFA in this study were biopsy confirmed. In one patient with systemic lupus erythematous who declined biopsy, it cannot be confirmed that her etiology of scarring alopecia was FFA rather than discoid lupus erythematous. Finally, because patients were treated with multiple medications, often concurrently, it was difficult to parse out which medications were efficacious and which were not. Despite these limitations, the findings in the study add to the growing literature about a rare but increasingly prevalent presentation.
Conclusion
Frontal fibrosing alopecia is a condition that predominantly affects white postmenopausal women but should not be overlooked in other demographics; higher socioeconomic status and nonsmoking are consistent with cases of FFA worldwide. Alopecia frequently involves other body hair, particularly the eyebrows, and is commonly associated with pruritus and pain. Many patients can identify an inciting event, usually stress, a health crisis, or new external exposures that they believe to have triggered the event. Consistent with data about LPP, FFA is frequently associated with autoimmune conditions, particularly hypothyroidism. A substantial portion of patients with FFA have had metal amalgam or gold alloy dental implants placed, though no patch testing was done to confirm that these patients have a contact allergy to these metals. Treatment for the condition is difficult, but topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy are efficacious in a large proportion of patients. Nevertheless, further research through prospective randomized trials is necessary to determine the best treatment modalities for FFA. Frontal fibrosing alopecia is a scarring form of hair loss that causes substantial emotional distress; therefore, it is critical to continue to investigate its etiology and treatments to improve patient care.
Frontal fibrosing alopecia (FFA) is a form of lymphocytic cicatricial alopecia that presents as frontotemporal hairline recession, typically in postmenopausal women.1 The condition is considered to be a variant of lichen planopilaris (LPP) due to its similar histologic appearance.2 Loss of eyebrow1-11 and body5-11 hair also is commonly present in FFA, and histologic findings are identical to those for hair loss on the scalp,8,9 suggesting that FFA may be a form of generalized alopecia.
The pathogenesis of FFA is unknown, but several etiologies have been postulated. Some suggest that as a variant of LPP, FFA is a hair-specific autoimmune disorder characterized by a T cell–mediated immune reaction against epithelial hair follicle stem cells, leading to fibrosis and depletion of hair regeneration potential.12 In support of this theory, FFA has been associated with other autoimmune diseases including hypothyroidism,6,8,13-16 mucocutaneous lichen planus,8,15,17 vitiligo,15,18 Sjögren syndrome,19 and lichen sclerosus et atrophicus.15,20 Another hypothesis suggests that the proandrogenic state in postmenopausal women may be related to the disease process.1 This hypothesis is supported by the reported success of antiandrogen therapy with 5α-reductase inhibitors (5α-RIs) in stabilizing FFA.3-5,7 Finally, genetic16,21 and environmental factors related to smoking and socioeconomic status5 also have been postulated to be risk factors for FFA. A variety of treatments have shown varying success, including topical and intralesional corticosteroids, hydroxychloroquine, immunomodulators, antibiotics, and 5α-RIs.1,3-6,8,15,17,22 However, FFA is considered to be relatively difficult to treat and commonly progresses regardless of treatment before spontaneously stabilizing.2-4,6,8,10
Since its discovery in 1994,1 FFA has become increasingly prevalent, comprising 17% of new referrals for hair loss in one study (N=57).6 Although growing recognition of the condition likely plays a role in its increasing presentation, other unidentified factors may contribute to its expanding incidence. In this report, we describe the demographics, clinical features, and disease progression of 29 cases of FFA treated within our division using a series of surveys and chart reviews.
Methods
Upon receiving approval for the project from the institutional review board, we identified 29 patients who met the criteria for diagnosis of FFA through a chart review of all patients being treated for hair loss by clinics within the Washington University Division of Dermatology (St. Louis, Missouri). Diagnostic criteria for FFA included scarring alopecia in the frontotemporal distribution with associated perifollicular erythema or papules and, if performed, a scalp biopsy of the involved area of alopecia showing lymphocytic cicatricial alopecia, compatible with LPP. The diagnosis was confirmed by biopsy in 18 patients (62%), while the remainder of the diagnoses were made clinically. Most biopsy specimens were diagnosed by board-certified dermatopathologists at Washington University, with the remainder diagnosed by outside pathologists if the patient was initially diagnosed at another institution.
Patients meeting criteria for FFA were mailed a study consent form, as well as a 2-page survey to assess demographics, clinical features of hair loss, medical histories, social and family histories, and treatments utilized. After receiving consent from patients, survey results were collected and summarized. If there was any need for clarification of answers, follow-up questions were conducted via email prior to any data analysis that was performed.
For analysis of treatment response, patients were asked what treatments they had utilized and about the progression of their hair loss. Patients reporting stabilization of hair loss or hair regrowth were classified as treatment responsive. Patients who underwent multiple treatments were included in the analyses for each of those treatments. Physician records for treatment response were not correlated with patient responses due to inconsistent documentation, care received outside of our medical system, and prolonged or loss to follow-up. Physician-reported data were only used to identify qualifying patients and their biopsy results, as described above.
Results
Patient Demographic
Between October 2013 and May 2014, 29 patients with FFA were recruited into the study. Patients were diagnosed between January 2006 and December 2013. There were 28 female patients (97%) and 1 male patient (3%). The average age of disease onset was 55.4 years (range, 29–75 years). Twenty-five patients (86%) self-identified as non-Hispanic white, 3 patients (10%) as Asian, and 1 patient (3%) as black. Patients also appeared to be a more affluent group than the general St. Louis County population, with a median household income between $75,000 and $100,000. In comparison, the median household income reported in St. Louis County from 2008 to 2012 was $58,485.23 The patient population was primarily composed of nonsmokers, with 22 (76%) patients who had never smoked, 6 (21%) who were present smokers, and 1 (3%) smoked in the past. These results were comparable to the reported number of female smokers in Missouri.24
Clinicopathologic Features
The clinical features of FFA are described in Table 1. All patients had frontotemporal recession of the hairline with some degree of scarring and perifollicular erythema (Figure 1). Most patients also reported hair loss at other sites, including 25 patients (86%) with eyebrow hair loss, 18 (62%) with limb hair loss, 11 (38%) with axillary hair loss, 11 (38%) with pubic hair loss, and 1 (3%) with eyelash hair loss. Patients also frequently reported inflammatory symptoms, including 19 patients (66%) with itching, 18 (62%) with redness, 3 (10%) with pain, 2 (7%) with papular lesions, and 1 (3%) with sores and erosions on the skin. Regarding progression of hair loss over time, 16 patients (55%) reported stabilization of hair loss, 11 (38%) reported progressive hair loss, and 2 (7%) reported some hair regrowth. Thirteen patients (45%) identified some inciting event that they believed to have triggered the disease. Ten patients (35%) identified stress as the inciting event, and 5 patients (17%) specifically referred to health-related stressors, including hip-replacement surgery, new diagnoses of systemic diseases, starting new medications, and stopping hormone replacement therapy. Furthermore, 2 (7%) patients reported exposure to chemicals and pesticides as suspected triggers.


Typical biopsy results showed a perifollicular lymphocytic infiltrate and fibrosis surrounding the infundibulum and isthmus of hair follicles (Figure 2). There were associated vacuolar changes in the basal layer and scattered dyskeratosis throughout the follicular epithelium. As the disease progressed to end-stage scarring, there was marked reduction in the number of hair follicles, which were replaced by fibrous tracts, and a disappearance of the previous inflammatory infiltrate.

Medical History
Of the 26 female patients who provided data about menopause status at time of disease onset, 16 (62%) were postmenopausal, 5 (19%) were menopausal, and 5 (19%) were premenopausal. Of the 28 female patients in the study, 8 (29%) had a history of hysterectomy and 2 (7%) also had surgically induced menopause through bilateral surgical oophorectomy. Twenty-four patients (86%) had a childbearing history, with an average of 2.3 children. Twelve patients (43%) reported use of hormone replacement therapy after menopause. Twelve patients (43%) also reported a history of oral contraceptive use.
Table 2 describes the comorbidities of all 29 patients. A history of autoimmune disease was prominent, found in 16 patients (55%). Thirteen patients (45%) reported thyroid disease, including 10 patients (35%) with hypothyroidism. Additionally, 8 patients (28%) had a history of mucocutaneous lichen planus, 2 (7%) of psoriasis/psoriatic arthritis, 1 (3%) of vitiligo, 1 (3%) of systemic lupus erythematosus, 1 (3%) of iritis, 1 (3%) of Sjögren syndrome, and 1 (3%) of ulcerative colitis. Six patients (21%) also reported a history of breast cancer.

A dental history was obtained in 24 patients. All 24 patients reported having some dental implant or filling placed. Twenty-four patients (100%) had a history of metal amalgam implants, 8 (33%) had gold alloy implants, 4 (17%) had composite resin implants, and 3 (13%) had porcelain implants. Two patients had metal amalgam implants that had since been replaced by nonmetal implants. Both patients reported no change in their clinical conditions with removal of the metal implants. Six of 8 patients (75%) with mucocutaneous lichen planus reported having dental implants. Of them, all 6 patients (100%) reported having metal amalgam implants, and 3 patients (50%) additionally reported having gold alloy implants.
Treatments
On average, patients were treated with 3 different therapies for FFA (range, 0–14). The treatments utilized are listed in Table 3, and responses to treatments are summarized in Table 4. Topical steroids were the most popular treatment modality and were used by 21 patients (72%). Approximately half of those patients reported treatment response with stabilization of hair loss or regrowth (n=11; 52%). Hydroxychloroquine was the second most commonly used modality (16 patients [55%]), with 10 of those patients (63%) reporting treatment response. Intralesional steroids were used in 11 patients (38%), with a treatment response in 36% (4/11) of those patients. Topical pimecrolimus and tacrolimus were used by 6 patients (21%), with 5 of those patients (83%) reporting treatment response. UVB excimer laser therapy was used on 3 patients (10%) with 100% treatment response.


Treatments with little or no treatment response to hair loss include doxycycline, minocycline, and topical minoxidil. Seven patients (24%) were treated with doxycycline or minocycline, all of whom reported no clinical response. Topical minoxidil was used by 3 patients (10%), with only 1 patient (33%) reporting stabilization of hair loss but no regrowth of hair. 5α-reductase inhibitors such as finasteride and dutasteride were only used by 1 patient (3%), who reported no treatment response. Other treatments that were rarely used include meloxicam (n=2), azathioprine (n=2), oral clindamycin (n=2), bimatoprost (n=1), quinacrine (n=1), cephalosporin (n=1), prednisone (n=1), isotretinoin (n=1), methotrexate (n=1), spironolactone (n=1), topical clindamycin (n=1), and laser hair removal (n=1). Of these, only meloxicam and quinacrine were anecdotally associated with stabilization of hair loss, while the rest of the treatments were associated with progressive hair loss despite therapy.
Comment
Frontal fibrosing alopecia is a form of cicatricial alopecia considered to be a clinical subset of LPP. Although the pathogeneses of both diseases are poorly understood, LPP is the better-studied model and is generally considered to be an autoimmune disease specific to the hair follicle, involving a cell-mediated inflammatory response to epithelial hair follicle stem cells.12 In support of this hypothesis, FFA and LPP have been frequently associated with autoimmune diseases, particularly with hypothyroidism.6,13-15 We found that 55% of our patients had a history of autoimmune disease, including 35% with hypothyroidism, 28% with mucocutaneous lichen planus, 7% with psoriasis, 3% with vitiligo, 3% with systemic lupus erythematosus, 3% with iritis, 3% with Sjögren syndrome, and 3% with ulcerative colitis. The link between FFA and hypothyroidism has been the best studied, with a large study by Atanskova Mesinkovska et al14 finding that 34% of 166 patients with LPP and FFA have some kind of thyroid disease and 29% have hypothyroidism. Fron
Although FFA has been classically described to affect postmenopausal women, recent studies have consistently identified that premenopausal women4-6,8,16,17 and men14,16 also can be affected by the condition. In our patient cohort, there was 1 male patient (3%), and a substantial number of the female patients were premenopausal (19%) and menopausal (19%) at the time of disease onset. Most of the patients studied were white; Asian and black patients were a consistent minority across FFA studies,5,13-16,25 highlighting the importance of screening for FFA in all demographics.
In our study, FFA patients also appeared to be more affluent than the general population and were predominantly nonsmokers (76%). These statistics are consistent with the United Kingdom population studied by MacDonald et al,6 which demonstrated a higher socioeconomic status and higher incidence of nonsmoking in their cases of FFA. Another large retrospective study of FFA patients in Spain found that 87% of their FFA cases (N=355) were nonsmokers, though they did not note a difference from the general unaffected population.15 In our study, we replicated these trends, finding an above average affluence level and a high but not statistically significant incidence of nonsmokers. Although it is not clear how socioeconomic status or smoking factors into the pathology of FFA, these studies may show a general trend in the environmental demographics of the disease.
Clinically, patients with FFA typically present with hair loss of the scalp as well as other sites. The eyebrows are the most common site to be affected outside of the scalp, affecting 86% of our patients, whereas eyelashes are the least commonly affected, presenting in only 3% of our patients. Body hair loss also is common, with almost two-thirds of our cohort reporting hair loss on the limbs and more than one-third reporting loss of axillary and pubic hair. These findings are consistent with those of other studies.3-6,8,13,15 Eyelash loss, body hair loss, and facial papules have been found to be associated with more severe forms of FFA,15 though we did not investigate these forms in our study. Inflammatory symptoms are common, with pruritus affecting 66% of our patients and pain affecting 10% of patients, consistent with the published literature.3,13,15,17
Multiple studies have shown that female FFA patients have a higher incidence of hysterectomies in their medical history.5,8,15 This observation has been used to further support the hypothesis that a change in sex hormone balance may trigger the initial onset of disease.5,8,15 A considerable number of the female patients in our study had also undergone hysterectomies (29%). Only 2 patients (7%) underwent premature surgical menopause through bilateral removal of the ovaries, and neither of these patients had abnormally early onset of FFA (age at onset, 52 and 65 years). Many patients in our study also reported a history of pharmacologic manipulation of sex hormones with hormone replacement therapy (43%) and oral contraceptive use (43%). However, patients with FFA have not been identified to have abnormal hormone levels compared to unaffected postmenopausal women.1 Additionally, the disease does not exclusively affect androgen-dependent hair, as indicated by the high prevalence of eyebrow hair loss. We hypothesize that the link between increased prevalence of hysterectomy and FFA is not due to hormonal changes but rather from the stresses related to the hysterectomy or associated conditions that required the surgery. In our study, 35% of patients identified stress as the inciting event prior to their onset of hair loss, with 17% specifically referring to health-related stress such as surgery or new diagnoses as the cause. Although this pattern is purely observational, it is valuable to consider that stress could contribute to the initial onset of FFA as with alopecia areata.26
A dental history was obtained in 24 patients to explore the possibility of FFA as a manifestation of contact allergy secondary to exposure to metal dental implants. Contact allergies to metal amalgam and gold alloy dental implants/fillings frequently have been described as presenting as oral lichen planus in the literature.27-34 Given the histologic overlap between oral lichen planus and LPP/FFA, it is worth exploring the possibility that LPP and FFA are other manifestations of contact allergic response. In our study, 100% of the patients who provided a dental history had metal amalgam implants and 33% had gold alloy implants. It is an interesting observation, but it should be noted that none of the patients in our study had undergone patch testing for contact allergies to the metals in their dental implants, and further studies are required to explore this hypothesis.
Frontal fibrosing alopecia is a difficult condition to treat. In our study, patients tried an average of 3 different treatments, the most common being topical steroids (72%), hydroxychloroquine (55%), and intralesional steroids (38%).
A PubMed search of articles indexed for MEDLINE using the terms randomized control trial and frontal fibrosing alopecia yielded no randomized controlled trials that have been performed to demonstrate the most efficacious treatments of FFA. However, one systematic review of 114 patients found 5α-RIs, antimalarials, and intralesional corticosteroids to yield the best responses in treating FFA.22 Another large, multicenter, retrospective study of 355 patients also demonstrated that 5α-RIs and intralesional corticosteroids minimized hair loss most effectively across treatment modalities.15 One treatment that was not discussed in either study but was utilized in ours was the UVB excimer laser, which has been demonstrated to induce T-cell apoptosis and decrease inflammation in psoriasis but has been infrequently studied in the use of FFA or LPP. In one study of 13 patients with LPP, excimer laser treatment was successful in reducing inflammatory symptoms and improving hair loss.35 Our results reaffirm that laser therapy could be considered more frequently as a treatment of FFA.
This study is subject to several limitations. The study size was comprised of a relatively small number of patients with the condition. Additionally, only one-third of patients contacted agreed to participate in the study, and therefore the responses received may not be completely representative of all FFA patients. With a retrospective study, there is potential for recall bias in the data that are collected. Physician chart correlation to patient responses could not be reliably performed due to inconsistent documentation, care received outside our medical system, and prolonged or loss to follow-up. Another concern is that not all diagnoses of FFA in this study were biopsy confirmed. In one patient with systemic lupus erythematous who declined biopsy, it cannot be confirmed that her etiology of scarring alopecia was FFA rather than discoid lupus erythematous. Finally, because patients were treated with multiple medications, often concurrently, it was difficult to parse out which medications were efficacious and which were not. Despite these limitations, the findings in the study add to the growing literature about a rare but increasingly prevalent presentation.
Conclusion
Frontal fibrosing alopecia is a condition that predominantly affects white postmenopausal women but should not be overlooked in other demographics; higher socioeconomic status and nonsmoking are consistent with cases of FFA worldwide. Alopecia frequently involves other body hair, particularly the eyebrows, and is commonly associated with pruritus and pain. Many patients can identify an inciting event, usually stress, a health crisis, or new external exposures that they believe to have triggered the event. Consistent with data about LPP, FFA is frequently associated with autoimmune conditions, particularly hypothyroidism. A substantial portion of patients with FFA have had metal amalgam or gold alloy dental implants placed, though no patch testing was done to confirm that these patients have a contact allergy to these metals. Treatment for the condition is difficult, but topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy are efficacious in a large proportion of patients. Nevertheless, further research through prospective randomized trials is necessary to determine the best treatment modalities for FFA. Frontal fibrosing alopecia is a scarring form of hair loss that causes substantial emotional distress; therefore, it is critical to continue to investigate its etiology and treatments to improve patient care.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60.
- Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700-705.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Chew AL, Bashir SJ, Wain EM, et al. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660.
- Miteva M, Camacho I, Romanelli P, et al. Acute hair loss on the limbs in frontal fibrosing alopecia: a clinicopathological study of two cases. Br J Dermatol. 2010;163:426-428.
- Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J Am Acad Dermatol. 2007;57(2 suppl):S15-S18.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941.
- Atanaskova Mesinkovska N, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Miteva M, Aber C, Torres F, et al. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445-447.
- Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731.
- Feldmann R, Harms M, Saurat JH. Postmenopausal frontal fibrosing alopecia. Hautarzt. 1996;47:533-536.
- Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- QuickFacts: St. Louis County, Missouri. United States Census Bureau website. https://www.census.gov/quickfacts/fact/table/stlouiscountymissouri/PST045217. Accessed February 6, 2019.
- State tobacco activities tracking and evaluation (STATE) system. State highlights. Centers for Disease Control and Prevention website. https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_STATE.Highlights. Accessed February 6, 2019.
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210.
- Willemsen R, Vanderlinden J, Roseeuw D, et al. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388-393.
- Segura-Egea JJ, Bullón-Fernández P. Lichenoid reaction associated to amalgam restoration. Med Oral Patol Oral Cir Bucal. 2004;9:421-424.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol. 1994;30:205-209.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320-323.
- Nordlind K, Lidén S. Patch test reactions to metal salts in patients with oral mucosal lesions associated with amalgam restorations. Contact Dermatitis. 1992;27:157-160.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis. 1995;33:323-328.
- Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141-146.
- Yiannias JA, el-Azhary RA, Hand JH, et al. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J Am Acad Dermatol. 2000;42:177-182.
- Scalf LA, Fowler JF Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermat. 2001;12:146-150.
- Navarini AA, Kolios AG, Prinz-Vavricka BM, et al. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325-1326.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Tosti A, Piraccini BM, Iorizzo M, et al. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55-60.
- Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700-705.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157-158.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Chew AL, Bashir SJ, Wain EM, et al. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63:653-660.
- Miteva M, Camacho I, Romanelli P, et al. Acute hair loss on the limbs in frontal fibrosing alopecia: a clinicopathological study of two cases. Br J Dermatol. 2010;163:426-428.
- Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome. J Am Acad Dermatol. 2007;57(2 suppl):S15-S18.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Dlova NC, Jordaan HF, Skenjane A, et al. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939-941.
- Atanaskova Mesinkovska N, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168:220-222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Miteva M, Aber C, Torres F, et al. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445-447.
- Sato M, Saga K, Takahashi H. Postmenopausal frontal fibrosing alopecia in a Japanese woman with Sjögren’s syndrome. J Dermatol. 2008;35:729-731.
- Feldmann R, Harms M, Saurat JH. Postmenopausal frontal fibrosing alopecia. Hautarzt. 1996;47:533-536.
- Junqueira Ribeiro Pereira AF, Vincenzi C, Tosti A. Frontal fibrosing alopecia in two sisters. Br J Dermatol. 2010;162:1154-1155.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- QuickFacts: St. Louis County, Missouri. United States Census Bureau website. https://www.census.gov/quickfacts/fact/table/stlouiscountymissouri/PST045217. Accessed February 6, 2019.
- State tobacco activities tracking and evaluation (STATE) system. State highlights. Centers for Disease Control and Prevention website. https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_STATE.Highlights. Accessed February 6, 2019.
- Miteva M, Whiting D, Harries M, et al. Frontal fibrosing alopecia in black patients. Br J Dermatol. 2012;167:208-210.
- Willemsen R, Vanderlinden J, Roseeuw D, et al. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J Am Acad Dermatol. 2009;60:388-393.
- Segura-Egea JJ, Bullón-Fernández P. Lichenoid reaction associated to amalgam restoration. Med Oral Patol Oral Cir Bucal. 2004;9:421-424.
- Laeijendecker R, van Joost T. Oral manifestations of gold allergy. J Am Acad Dermatol. 1994;30:205-209.
- Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320-323.
- Nordlind K, Lidén S. Patch test reactions to metal salts in patients with oral mucosal lesions associated with amalgam restorations. Contact Dermatitis. 1992;27:157-160.
- Koch P, Bahmer FA. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermatitis. 1995;33:323-328.
- Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141-146.
- Yiannias JA, el-Azhary RA, Hand JH, et al. Relevant contact sensitivities in patients with the diagnosis of oral lichen planus. J Am Acad Dermatol. 2000;42:177-182.
- Scalf LA, Fowler JF Jr, Morgan KW, et al. Dental metal allergy in patients with oral, cutaneous, and genital lichenoid reactions. Am J Contact Dermat. 2001;12:146-150.
- Navarini AA, Kolios AG, Prinz-Vavricka BM, et al. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147:1325-1326.
Practice Points
- Frontal fibrosing alopecia (FFA) may be associated with other autoimmune conditions, and patients should be screened accordingly.
- The most efficacious treatments for FFA include topical and intralesional steroids, hydroxychloroquine, calcineurin inhibitors, and excimer laser therapy.
- A stressful precipitating event or metal dental implants/fillings are 2 possible environmental triggers for this condition.
Onychomycosis that fails terbinafine probably isn’t T. rubrum
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Laser Hair Removal: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?
Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?
The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?

Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?

The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?

Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?

The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
What is your diagnosis?
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at [email protected].
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at [email protected].
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at [email protected].
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
A 5-year-old female is brought to clinic for hair loss. The mother reports that when styling her daughter's hair, she has noticed areas of hair thinning, especially at the temples and at the occiput. The mother denies scale, pruritus, and erythema. The patient reports that her scalp does not hurt. She has never had a haircut because her hair hasn't grown long enough to cut. There is no history of specific bald spots. The patient has no personal history of psoriasis, seborrheic dermatitis, or autoimmune disease. No picking has been noted, and there is no history of compulsive behaviors or anxiety. The patient's mother has a history of Graves disease. The mother reports that the patient's older sister may have had hair thinning when she was younger as well, but no longer has thin hair.
The patient was previously seen by another provider who prescribed hydrocortisone 2.5% ointment, which the mother has been applying nightly without improvement.
The child is otherwise medically well and thriving, with no recent change in activity level and with growth parameters consistently around the 75th percentile for height and weight. On physical exam, the patient has blondish, fine hair with areas of poorly demarcated hair thinning at the left temple and at the occiput. The hair remaining at the occiput is normal in texture. There are no areas of complete hair loss. There is no scale, erythema, or abnormal pigmentation, and no cervical or occipital adenopathy. The patient has intact eyebrows and eyelashes.
Nail Psoriasis Tips
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
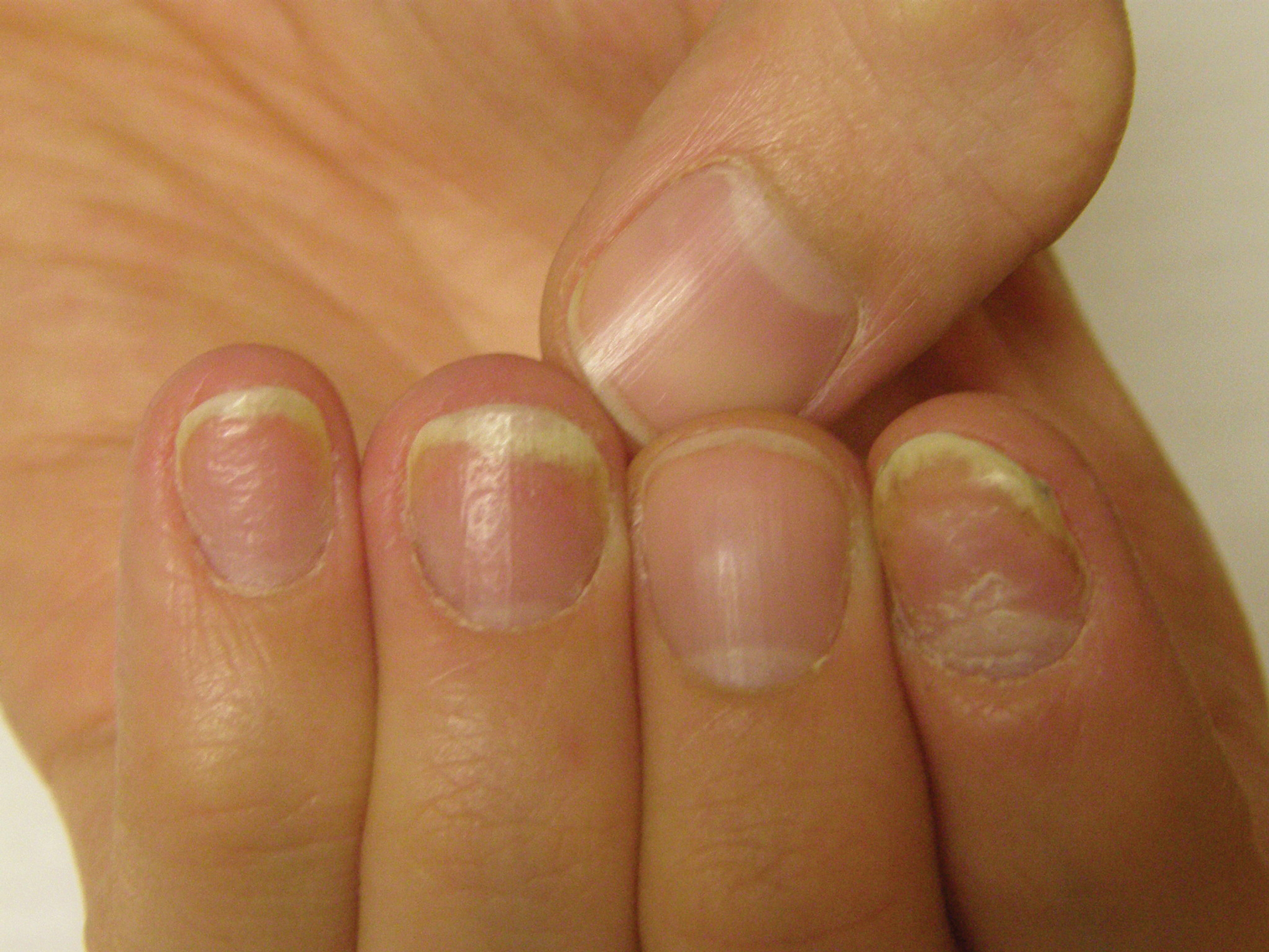
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.