User login
Journal retracts more articles for being ‘unethical, scientifically flawed, and based on racist ideas and agenda’
Eight months after a psychology journal retracted a pair of articles that were “unethical, scientifically flawed, and based on racist ideas and agenda,” the publication has pulled three more papers – all at least a quarter century old – for the same reason.
All five papers were written by J. Philippe Rushton, formerly of the University of Western Ontario, who died in 2012. As we wrote in December 2020, Rushton published dubious studies that promoted tropes of white supremacy, including that Blacks are less intelligent than Whites and that:
East Asians and their descendants average a larger brain size, greater intelligence, more sexual restraint, slower rates of maturation, and greater law abidingness and social organization than do Europeans and their descendants, who average higher scores on these dimensions than Africans and their descendants.
Here’s the new notice, whose language mirrors that of the earlier retraction statement:
The following articles have been retracted from Psychological Reports:
Rushton, J. P. (1987). An Evolutionary Theory of Health, Longevity, and Personality: Sociobiology and r/K Reproductive Strategies . Psychological Reports, 60, 539-49.
Rushton, J. P. (1992). Contributions to the History of Psychology: XC. Evolutionary Biology and Heritable Traits (with Reference to Oriental-White-Black Differences): The 1989 AAAS Paper . Psychological Reports, 71, 811-21.
Rushton, J. P. (1995). Race and Crime: International Data 1989-1990 . Psychological Reports, 76, 207-312.
This retraction is following a review that found that the research was unethical, scientifically flawed, and based on racist ideas and agenda. Specifically, these publications authored by Philippe Rushton on the subject of intelligence and race have been rejected based on the following findings:
A better understanding of the human genome (Yudell et al., 2016)
An inappropriately applied ecological theory that explains differences between species’ reproductive strategies to humans (Allen et al., 1992 ; Anderson, 1991)
A misuse of population genetic measures and misconceptions about heritability (Bailey, 1997)
Ignoring alternative explanations or evidence that did not support the racist theories being presented (Cain & Vanderwolf, 1990)
Rushton’s findings have not been replicated (Peregrine, Ember, & Ember, 2003)
Together, the papers have been cited 48 times, according to Clarivate Analytics’ Web of Science. At the time of this writing, none of the original abstracts include a link to the retraction notice.
When we wrote about the Rushton case last year, we received a copy of an email from the journal saying that it would be retracting Rushton’s 1992 paper, “Contributions to the History of Psychology: XC. Evolutionary Biology and Heritable Traits” along with the other two articles. That didn’t happen at the time.
We asked Cory Scherer, the editor of Psychological Reports, about the eight-month gap between retractions. He told us:
I got an email from a researcher who brought these articles to our attention and when I read them I moved fast on the first retraction and the rest were found when that retraction was already written and in press. I didn’t want to add them to the original retraction until I did my due diligence about the second set found.
Our search of the journal’s website turned up eight articles in total by Rushton in the journal, of which three remain unretracted. Mr. Scherer said he has created a committee to review the remnant papers to see if they require retraction.
Eight months after a psychology journal retracted a pair of articles that were “unethical, scientifically flawed, and based on racist ideas and agenda,” the publication has pulled three more papers – all at least a quarter century old – for the same reason.
All five papers were written by J. Philippe Rushton, formerly of the University of Western Ontario, who died in 2012. As we wrote in December 2020, Rushton published dubious studies that promoted tropes of white supremacy, including that Blacks are less intelligent than Whites and that:
East Asians and their descendants average a larger brain size, greater intelligence, more sexual restraint, slower rates of maturation, and greater law abidingness and social organization than do Europeans and their descendants, who average higher scores on these dimensions than Africans and their descendants.
Here’s the new notice, whose language mirrors that of the earlier retraction statement:
The following articles have been retracted from Psychological Reports:
Rushton, J. P. (1987). An Evolutionary Theory of Health, Longevity, and Personality: Sociobiology and r/K Reproductive Strategies . Psychological Reports, 60, 539-49.
Rushton, J. P. (1992). Contributions to the History of Psychology: XC. Evolutionary Biology and Heritable Traits (with Reference to Oriental-White-Black Differences): The 1989 AAAS Paper . Psychological Reports, 71, 811-21.
Rushton, J. P. (1995). Race and Crime: International Data 1989-1990 . Psychological Reports, 76, 207-312.
This retraction is following a review that found that the research was unethical, scientifically flawed, and based on racist ideas and agenda. Specifically, these publications authored by Philippe Rushton on the subject of intelligence and race have been rejected based on the following findings:
A better understanding of the human genome (Yudell et al., 2016)
An inappropriately applied ecological theory that explains differences between species’ reproductive strategies to humans (Allen et al., 1992 ; Anderson, 1991)
A misuse of population genetic measures and misconceptions about heritability (Bailey, 1997)
Ignoring alternative explanations or evidence that did not support the racist theories being presented (Cain & Vanderwolf, 1990)
Rushton’s findings have not been replicated (Peregrine, Ember, & Ember, 2003)
Together, the papers have been cited 48 times, according to Clarivate Analytics’ Web of Science. At the time of this writing, none of the original abstracts include a link to the retraction notice.
When we wrote about the Rushton case last year, we received a copy of an email from the journal saying that it would be retracting Rushton’s 1992 paper, “Contributions to the History of Psychology: XC. Evolutionary Biology and Heritable Traits” along with the other two articles. That didn’t happen at the time.
We asked Cory Scherer, the editor of Psychological Reports, about the eight-month gap between retractions. He told us:
I got an email from a researcher who brought these articles to our attention and when I read them I moved fast on the first retraction and the rest were found when that retraction was already written and in press. I didn’t want to add them to the original retraction until I did my due diligence about the second set found.
Our search of the journal’s website turned up eight articles in total by Rushton in the journal, of which three remain unretracted. Mr. Scherer said he has created a committee to review the remnant papers to see if they require retraction.
Eight months after a psychology journal retracted a pair of articles that were “unethical, scientifically flawed, and based on racist ideas and agenda,” the publication has pulled three more papers – all at least a quarter century old – for the same reason.
All five papers were written by J. Philippe Rushton, formerly of the University of Western Ontario, who died in 2012. As we wrote in December 2020, Rushton published dubious studies that promoted tropes of white supremacy, including that Blacks are less intelligent than Whites and that:
East Asians and their descendants average a larger brain size, greater intelligence, more sexual restraint, slower rates of maturation, and greater law abidingness and social organization than do Europeans and their descendants, who average higher scores on these dimensions than Africans and their descendants.
Here’s the new notice, whose language mirrors that of the earlier retraction statement:
The following articles have been retracted from Psychological Reports:
Rushton, J. P. (1987). An Evolutionary Theory of Health, Longevity, and Personality: Sociobiology and r/K Reproductive Strategies . Psychological Reports, 60, 539-49.
Rushton, J. P. (1992). Contributions to the History of Psychology: XC. Evolutionary Biology and Heritable Traits (with Reference to Oriental-White-Black Differences): The 1989 AAAS Paper . Psychological Reports, 71, 811-21.
Rushton, J. P. (1995). Race and Crime: International Data 1989-1990 . Psychological Reports, 76, 207-312.
This retraction is following a review that found that the research was unethical, scientifically flawed, and based on racist ideas and agenda. Specifically, these publications authored by Philippe Rushton on the subject of intelligence and race have been rejected based on the following findings:
A better understanding of the human genome (Yudell et al., 2016)
An inappropriately applied ecological theory that explains differences between species’ reproductive strategies to humans (Allen et al., 1992 ; Anderson, 1991)
A misuse of population genetic measures and misconceptions about heritability (Bailey, 1997)
Ignoring alternative explanations or evidence that did not support the racist theories being presented (Cain & Vanderwolf, 1990)
Rushton’s findings have not been replicated (Peregrine, Ember, & Ember, 2003)
Together, the papers have been cited 48 times, according to Clarivate Analytics’ Web of Science. At the time of this writing, none of the original abstracts include a link to the retraction notice.
When we wrote about the Rushton case last year, we received a copy of an email from the journal saying that it would be retracting Rushton’s 1992 paper, “Contributions to the History of Psychology: XC. Evolutionary Biology and Heritable Traits” along with the other two articles. That didn’t happen at the time.
We asked Cory Scherer, the editor of Psychological Reports, about the eight-month gap between retractions. He told us:
I got an email from a researcher who brought these articles to our attention and when I read them I moved fast on the first retraction and the rest were found when that retraction was already written and in press. I didn’t want to add them to the original retraction until I did my due diligence about the second set found.
Our search of the journal’s website turned up eight articles in total by Rushton in the journal, of which three remain unretracted. Mr. Scherer said he has created a committee to review the remnant papers to see if they require retraction.
Polygenic breast cancer risk scores strive to overcome racial bias
The potential of polygenic risk scores (PRSs) to become key components in the assessment of individual risk for disease in the clinical setting is inching closer to fruition; however, the technology is plagued by one glaring omission of most existing PRSs – the lack of applicability to those of non-European ancestry.
Polygenic risk scores predict an individual’s risk of disease based on common genetic variants identified in large genomewide association studies (GWASs). They have gained ground in research, as well as in the unregulated realm of the direct-to-consumer market where they are sold as add-ons to DNA ancestry kits such as 23andMe and MyHeritage.com.
While the risk scores show strong validation in estimating risk among people of European descent, their striking caveat is the lack of applicability to other ancestries, particularly African, and their use in practice outside of clinical trials is discouraged in National Comprehensive Cancer Network guidelines.
Study underscores need for ethnically diverse datasets
In a recent study published in JAMA Network Open, researchers evaluated the use of polygenic risk scores’ models in a clinical setting. Researchers tested 7 PRSs models for breast cancer risk against the medical records data of 39,591 women of European, African, and Latinx ancestry.
The PRSs models – all used only for research purposes – included three models involving European ancestry cohorts, two from Latinx cohorts, and two from women African descent.
After adjusting for factors including age, breast cancer family history, and ancestry, the PRSs from women with European ancestry highly corresponded to breast cancer risk, with a mean odds ratio of 1.46 per standard deviation increase in the score.
PRSs were also generalized relatively well among women of Latinx ancestry with a mean OR of 1.31. The authors noted that association is likely caused by Latinx individuals in the United States having a greater proportion of European ancestry than individuals with African ancestry. Importantly, however, the effect size was lower for women of African ancestry with a highest OR of 1.19 per standard deviation.
In the highest percentiles of breast cancer risk, women of European descent had odds ratio as high as 2.19-2.48, suggesting a statistically significant association with overall breast cancer risk. No statistically significant associations were found among women of Latinx and African-ancestry.
The PRSs models were smaller for women of non-European ancestry and included fewer genetic variants for women of non-European ancestry were notably smaller and hence reflected fewer genetic variants. Of the two risk scores involving African ancestry, the Women’s Health Initiative for Women with African ancestry risk score had just 75 variants, while the African diaspora study (ROOT) had 34 variants, compared with 3,820 and 5,218 in the two largest European ancestry PRSs, the Breast Cancer Association Consortium and the UK Biobank, respectively.
“These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts,” the authors wrote.
First author, Cong Liu, PhD, of Columbia University Irving Medical Center, New York, said that efforts are underway to improve the inclusivity in the Electronic Medical Records and Genomics network data set used in this study.
“Until well-developed and validated PRSs for women with non-European ancestry become available, the current PRSs based on cohorts with European ancestry could be adapted for Latinx women, but not women with African ancestry until additional data sets become available in this important and high-risk group,” Dr. Liu and colleagues wrote.
In a commentary published with the study, Payal D. Shah, MD, of the Basser Center for BRCA at the University of Pennsylvania, Philadelphia, said that PRSs are “disproportionately applicable to patients with European ancestry and are insufficiently vetted and developed in other populations. If an instrument exists that has clinical utility in informing effective cancer risk mitigation strategies, then we must strive to ensure that it is available and applicable to all.”
Higher morality among African American women
While American Cancer Society data shows women with African ancestry generally have incidence rates of breast cancer similar to White women, they have significantly higher mortality from the disease in part because of later-stage diagnosis and health care barriers.
Anne Marie McCarthy, PhD, of the University of Pennsylvania, and Katrina Armstrong, MD, of Harvard Medical School, Boston, wrote in the Journal of the National Cancer Institute that African American women “have 42% higher breast cancer mortality than white women, despite having lower disease incidence, and are more likely to be diagnosed with triple-negative breast cancer, which has poorer prognosis than other molecular subtypes.”
Dr. McCarthy and Dr. Armstrong wrote that African American women are chronically underrepresented in breast cancer studies. And as such, it is impossible to know the extent of the prevalence of mutations and risk.
Failing to address the lack of diversity in genomic studies may worsen health disparities for women with African ancestry, Dr. Liu and colleagues wrote. The higher mortality “underscores the urgent need to increase diversity in genomic studies so that future clinical applications of the PRS do not exacerbate existing health disparities. These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts.”
Potential PRS benefits underscore need to eliminate bias
The potentially important benefits of PRSs as risk prediction tools used in combination with family history, reproductive history and other factors, should provide strong incentive to push for improvement, Dr. Shah wrote.
For instance, if an individual is estrogen receptor positive and shows elevations in breast cancer risk on a reliable PRS, “this may inform antiestrogen chemoprevention strategies,” she wrote.
A risk score could furthermore influence the age at which breast cancer screening should begin or factor into whether a patient should also receive surveillance breast MRI.
Importantly, PRSs could also add to other risk factors to provide more precise risk estimates and inform management of women with a pathogenic variant in a breast cancer risk predisposition gene, Dr. Shah wrote.
Confluence project
Among the most promising developments in research is the National Cancer Institute’s Confluence Project, a large research resource aiming to include approximately 300,000 breast cancer cases and 300,000 controls of different races/ethnicities, utilizing the confluence of existing GWAS and new genomewide genotyping data.
Having started enrollment in 2018, the project is approaching implementation, said Montserrat García-Closas, MD, MPH, DrPH, deputy director of cancer epidemiology and genetics with the National Cancer Institute.
“We expect genotyping to be completed by the end of 2022 and for the data to be made available to the research community soon after that,” she said.
Among the project’s key objectives are the development of PRSs to be integrated with known risk factors to provide a personalized risk assessment for breast cancer, overall and by ancestral subtype.
“We plan to apply novel methods to derive multiancestry PRS that will account for differences and similarities in genetic architecture across ethnic/racial groups to develop breast cancer PRSs that can be applied in multiethnic/racial populations,” she said.
NCI is working with investigators in Africa, Central and South America, and Asia, and reaching out to non-European organizations such as AORTIC for studies of African populations.
Direct-to-consumer global PRS
In the commercial PRS market, efforts to address diversity shortcomings are also gaining momentum, with Myriad Genetics touting a first-of-its kind “global PRS.”
The PRS, a recalibrated version the company’s riskScore PRS, sold as part of its Myriad myRisk Hereditary Cancer test, will reportedly apply to all ethnicities in estimating an individual’s 5-year and lifetime risk of breast cancer.
A study presented in June at the American Society of Clinical Oncology meeting, describes the development of the model with the use of three large ancestry-specific PRSs based on African American, Asian, and European cohorts, with the system including a total of 149 single-nucleotide polymorphisms, including 93 well established for breast cancer and 56 that are ancestry specific.
In validation of the data in an independent cohort of 62,707 individuals, the global PRS was strongly associated with breast cancer in the full combined validation cohort as well as in all three of the ancestry subcohorts.
However, the effect size among women with African ancestry was still the lowest of all of the groups, with a mean OR of 1.24 per standard deviation, versus the highest rate of mixed ancestry (OR, 1.59).
According to senior author Holly Pederson, MD, director of medical breast services at the Cleveland Clinic, the applicability of the PRS to women with African ancestry is expected to further improve as additional data become available.
“The discriminatory power in women of African descent was significantly improved but still suboptimal,” she said. “The need for more data, particularly in Black women, is challenging not only because there is likely more diversity in the genomic landscape of women of African descent, but also because the barriers created by historical, cultural, institutional and interpersonal dynamics result in the paucity of this data.”
“We must be committed to ending bias resulting in health care disparities,” Dr. Pederson said. She noted that the global PRS is nevertheless “still clinically useful in Black women,” and recommended that clinicians be up front with patients on the status of the research challenges.
“As with any clinical shared decision-making conversation between a patient and her provider, it is important for Black women to know that data is limited in the African American population, particularly given the vast genomic diversity of the African continent,” she said. “This model, as models that have gone before it, will improve with additional data, particularly in this population.”
Commercial PRSs may benefit research
While the commercial marketing of PRSs in a direct-to-consumer fashion have raised some concerns, such as how individuals respond to their risk scores, there could be important benefits as well, commented Megan C. Roberts, PhD.
“There may be an opportunity to learn from these companies about how to engage diverse communities in genomic testing,” said Dr. Roberts, an assistant professor and director of implementation science in precision health and society at the University of North Carolina at Chapel Hill. “Moreover, the data they collect from their customers often can be used for research purposes as well.”
In a recent perspective, Dr. Roberts and colleagues addressed the role of health disparities in PRSs. She’ll be joining international precision public health researchers in October in hosting a free virtual conference at UNC on the topic.
“There is a huge need to improve racial and ethnic diversity in our genomic datasets,” Dr. Roberts said. “Without this, we will not be able to return on the promise of precision medicine and prevention for improving the health of our whole population.”
Dr. Pederson disclosed that she is a consultant for Myriad Genetics.
The potential of polygenic risk scores (PRSs) to become key components in the assessment of individual risk for disease in the clinical setting is inching closer to fruition; however, the technology is plagued by one glaring omission of most existing PRSs – the lack of applicability to those of non-European ancestry.
Polygenic risk scores predict an individual’s risk of disease based on common genetic variants identified in large genomewide association studies (GWASs). They have gained ground in research, as well as in the unregulated realm of the direct-to-consumer market where they are sold as add-ons to DNA ancestry kits such as 23andMe and MyHeritage.com.
While the risk scores show strong validation in estimating risk among people of European descent, their striking caveat is the lack of applicability to other ancestries, particularly African, and their use in practice outside of clinical trials is discouraged in National Comprehensive Cancer Network guidelines.
Study underscores need for ethnically diverse datasets
In a recent study published in JAMA Network Open, researchers evaluated the use of polygenic risk scores’ models in a clinical setting. Researchers tested 7 PRSs models for breast cancer risk against the medical records data of 39,591 women of European, African, and Latinx ancestry.
The PRSs models – all used only for research purposes – included three models involving European ancestry cohorts, two from Latinx cohorts, and two from women African descent.
After adjusting for factors including age, breast cancer family history, and ancestry, the PRSs from women with European ancestry highly corresponded to breast cancer risk, with a mean odds ratio of 1.46 per standard deviation increase in the score.
PRSs were also generalized relatively well among women of Latinx ancestry with a mean OR of 1.31. The authors noted that association is likely caused by Latinx individuals in the United States having a greater proportion of European ancestry than individuals with African ancestry. Importantly, however, the effect size was lower for women of African ancestry with a highest OR of 1.19 per standard deviation.
In the highest percentiles of breast cancer risk, women of European descent had odds ratio as high as 2.19-2.48, suggesting a statistically significant association with overall breast cancer risk. No statistically significant associations were found among women of Latinx and African-ancestry.
The PRSs models were smaller for women of non-European ancestry and included fewer genetic variants for women of non-European ancestry were notably smaller and hence reflected fewer genetic variants. Of the two risk scores involving African ancestry, the Women’s Health Initiative for Women with African ancestry risk score had just 75 variants, while the African diaspora study (ROOT) had 34 variants, compared with 3,820 and 5,218 in the two largest European ancestry PRSs, the Breast Cancer Association Consortium and the UK Biobank, respectively.
“These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts,” the authors wrote.
First author, Cong Liu, PhD, of Columbia University Irving Medical Center, New York, said that efforts are underway to improve the inclusivity in the Electronic Medical Records and Genomics network data set used in this study.
“Until well-developed and validated PRSs for women with non-European ancestry become available, the current PRSs based on cohorts with European ancestry could be adapted for Latinx women, but not women with African ancestry until additional data sets become available in this important and high-risk group,” Dr. Liu and colleagues wrote.
In a commentary published with the study, Payal D. Shah, MD, of the Basser Center for BRCA at the University of Pennsylvania, Philadelphia, said that PRSs are “disproportionately applicable to patients with European ancestry and are insufficiently vetted and developed in other populations. If an instrument exists that has clinical utility in informing effective cancer risk mitigation strategies, then we must strive to ensure that it is available and applicable to all.”
Higher morality among African American women
While American Cancer Society data shows women with African ancestry generally have incidence rates of breast cancer similar to White women, they have significantly higher mortality from the disease in part because of later-stage diagnosis and health care barriers.
Anne Marie McCarthy, PhD, of the University of Pennsylvania, and Katrina Armstrong, MD, of Harvard Medical School, Boston, wrote in the Journal of the National Cancer Institute that African American women “have 42% higher breast cancer mortality than white women, despite having lower disease incidence, and are more likely to be diagnosed with triple-negative breast cancer, which has poorer prognosis than other molecular subtypes.”
Dr. McCarthy and Dr. Armstrong wrote that African American women are chronically underrepresented in breast cancer studies. And as such, it is impossible to know the extent of the prevalence of mutations and risk.
Failing to address the lack of diversity in genomic studies may worsen health disparities for women with African ancestry, Dr. Liu and colleagues wrote. The higher mortality “underscores the urgent need to increase diversity in genomic studies so that future clinical applications of the PRS do not exacerbate existing health disparities. These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts.”
Potential PRS benefits underscore need to eliminate bias
The potentially important benefits of PRSs as risk prediction tools used in combination with family history, reproductive history and other factors, should provide strong incentive to push for improvement, Dr. Shah wrote.
For instance, if an individual is estrogen receptor positive and shows elevations in breast cancer risk on a reliable PRS, “this may inform antiestrogen chemoprevention strategies,” she wrote.
A risk score could furthermore influence the age at which breast cancer screening should begin or factor into whether a patient should also receive surveillance breast MRI.
Importantly, PRSs could also add to other risk factors to provide more precise risk estimates and inform management of women with a pathogenic variant in a breast cancer risk predisposition gene, Dr. Shah wrote.
Confluence project
Among the most promising developments in research is the National Cancer Institute’s Confluence Project, a large research resource aiming to include approximately 300,000 breast cancer cases and 300,000 controls of different races/ethnicities, utilizing the confluence of existing GWAS and new genomewide genotyping data.
Having started enrollment in 2018, the project is approaching implementation, said Montserrat García-Closas, MD, MPH, DrPH, deputy director of cancer epidemiology and genetics with the National Cancer Institute.
“We expect genotyping to be completed by the end of 2022 and for the data to be made available to the research community soon after that,” she said.
Among the project’s key objectives are the development of PRSs to be integrated with known risk factors to provide a personalized risk assessment for breast cancer, overall and by ancestral subtype.
“We plan to apply novel methods to derive multiancestry PRS that will account for differences and similarities in genetic architecture across ethnic/racial groups to develop breast cancer PRSs that can be applied in multiethnic/racial populations,” she said.
NCI is working with investigators in Africa, Central and South America, and Asia, and reaching out to non-European organizations such as AORTIC for studies of African populations.
Direct-to-consumer global PRS
In the commercial PRS market, efforts to address diversity shortcomings are also gaining momentum, with Myriad Genetics touting a first-of-its kind “global PRS.”
The PRS, a recalibrated version the company’s riskScore PRS, sold as part of its Myriad myRisk Hereditary Cancer test, will reportedly apply to all ethnicities in estimating an individual’s 5-year and lifetime risk of breast cancer.
A study presented in June at the American Society of Clinical Oncology meeting, describes the development of the model with the use of three large ancestry-specific PRSs based on African American, Asian, and European cohorts, with the system including a total of 149 single-nucleotide polymorphisms, including 93 well established for breast cancer and 56 that are ancestry specific.
In validation of the data in an independent cohort of 62,707 individuals, the global PRS was strongly associated with breast cancer in the full combined validation cohort as well as in all three of the ancestry subcohorts.
However, the effect size among women with African ancestry was still the lowest of all of the groups, with a mean OR of 1.24 per standard deviation, versus the highest rate of mixed ancestry (OR, 1.59).
According to senior author Holly Pederson, MD, director of medical breast services at the Cleveland Clinic, the applicability of the PRS to women with African ancestry is expected to further improve as additional data become available.
“The discriminatory power in women of African descent was significantly improved but still suboptimal,” she said. “The need for more data, particularly in Black women, is challenging not only because there is likely more diversity in the genomic landscape of women of African descent, but also because the barriers created by historical, cultural, institutional and interpersonal dynamics result in the paucity of this data.”
“We must be committed to ending bias resulting in health care disparities,” Dr. Pederson said. She noted that the global PRS is nevertheless “still clinically useful in Black women,” and recommended that clinicians be up front with patients on the status of the research challenges.
“As with any clinical shared decision-making conversation between a patient and her provider, it is important for Black women to know that data is limited in the African American population, particularly given the vast genomic diversity of the African continent,” she said. “This model, as models that have gone before it, will improve with additional data, particularly in this population.”
Commercial PRSs may benefit research
While the commercial marketing of PRSs in a direct-to-consumer fashion have raised some concerns, such as how individuals respond to their risk scores, there could be important benefits as well, commented Megan C. Roberts, PhD.
“There may be an opportunity to learn from these companies about how to engage diverse communities in genomic testing,” said Dr. Roberts, an assistant professor and director of implementation science in precision health and society at the University of North Carolina at Chapel Hill. “Moreover, the data they collect from their customers often can be used for research purposes as well.”
In a recent perspective, Dr. Roberts and colleagues addressed the role of health disparities in PRSs. She’ll be joining international precision public health researchers in October in hosting a free virtual conference at UNC on the topic.
“There is a huge need to improve racial and ethnic diversity in our genomic datasets,” Dr. Roberts said. “Without this, we will not be able to return on the promise of precision medicine and prevention for improving the health of our whole population.”
Dr. Pederson disclosed that she is a consultant for Myriad Genetics.
The potential of polygenic risk scores (PRSs) to become key components in the assessment of individual risk for disease in the clinical setting is inching closer to fruition; however, the technology is plagued by one glaring omission of most existing PRSs – the lack of applicability to those of non-European ancestry.
Polygenic risk scores predict an individual’s risk of disease based on common genetic variants identified in large genomewide association studies (GWASs). They have gained ground in research, as well as in the unregulated realm of the direct-to-consumer market where they are sold as add-ons to DNA ancestry kits such as 23andMe and MyHeritage.com.
While the risk scores show strong validation in estimating risk among people of European descent, their striking caveat is the lack of applicability to other ancestries, particularly African, and their use in practice outside of clinical trials is discouraged in National Comprehensive Cancer Network guidelines.
Study underscores need for ethnically diverse datasets
In a recent study published in JAMA Network Open, researchers evaluated the use of polygenic risk scores’ models in a clinical setting. Researchers tested 7 PRSs models for breast cancer risk against the medical records data of 39,591 women of European, African, and Latinx ancestry.
The PRSs models – all used only for research purposes – included three models involving European ancestry cohorts, two from Latinx cohorts, and two from women African descent.
After adjusting for factors including age, breast cancer family history, and ancestry, the PRSs from women with European ancestry highly corresponded to breast cancer risk, with a mean odds ratio of 1.46 per standard deviation increase in the score.
PRSs were also generalized relatively well among women of Latinx ancestry with a mean OR of 1.31. The authors noted that association is likely caused by Latinx individuals in the United States having a greater proportion of European ancestry than individuals with African ancestry. Importantly, however, the effect size was lower for women of African ancestry with a highest OR of 1.19 per standard deviation.
In the highest percentiles of breast cancer risk, women of European descent had odds ratio as high as 2.19-2.48, suggesting a statistically significant association with overall breast cancer risk. No statistically significant associations were found among women of Latinx and African-ancestry.
The PRSs models were smaller for women of non-European ancestry and included fewer genetic variants for women of non-European ancestry were notably smaller and hence reflected fewer genetic variants. Of the two risk scores involving African ancestry, the Women’s Health Initiative for Women with African ancestry risk score had just 75 variants, while the African diaspora study (ROOT) had 34 variants, compared with 3,820 and 5,218 in the two largest European ancestry PRSs, the Breast Cancer Association Consortium and the UK Biobank, respectively.
“These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts,” the authors wrote.
First author, Cong Liu, PhD, of Columbia University Irving Medical Center, New York, said that efforts are underway to improve the inclusivity in the Electronic Medical Records and Genomics network data set used in this study.
“Until well-developed and validated PRSs for women with non-European ancestry become available, the current PRSs based on cohorts with European ancestry could be adapted for Latinx women, but not women with African ancestry until additional data sets become available in this important and high-risk group,” Dr. Liu and colleagues wrote.
In a commentary published with the study, Payal D. Shah, MD, of the Basser Center for BRCA at the University of Pennsylvania, Philadelphia, said that PRSs are “disproportionately applicable to patients with European ancestry and are insufficiently vetted and developed in other populations. If an instrument exists that has clinical utility in informing effective cancer risk mitigation strategies, then we must strive to ensure that it is available and applicable to all.”
Higher morality among African American women
While American Cancer Society data shows women with African ancestry generally have incidence rates of breast cancer similar to White women, they have significantly higher mortality from the disease in part because of later-stage diagnosis and health care barriers.
Anne Marie McCarthy, PhD, of the University of Pennsylvania, and Katrina Armstrong, MD, of Harvard Medical School, Boston, wrote in the Journal of the National Cancer Institute that African American women “have 42% higher breast cancer mortality than white women, despite having lower disease incidence, and are more likely to be diagnosed with triple-negative breast cancer, which has poorer prognosis than other molecular subtypes.”
Dr. McCarthy and Dr. Armstrong wrote that African American women are chronically underrepresented in breast cancer studies. And as such, it is impossible to know the extent of the prevalence of mutations and risk.
Failing to address the lack of diversity in genomic studies may worsen health disparities for women with African ancestry, Dr. Liu and colleagues wrote. The higher mortality “underscores the urgent need to increase diversity in genomic studies so that future clinical applications of the PRS do not exacerbate existing health disparities. These results highlight the need to improve representation of diverse population groups, particularly women with African ancestry, in genomic research cohorts.”
Potential PRS benefits underscore need to eliminate bias
The potentially important benefits of PRSs as risk prediction tools used in combination with family history, reproductive history and other factors, should provide strong incentive to push for improvement, Dr. Shah wrote.
For instance, if an individual is estrogen receptor positive and shows elevations in breast cancer risk on a reliable PRS, “this may inform antiestrogen chemoprevention strategies,” she wrote.
A risk score could furthermore influence the age at which breast cancer screening should begin or factor into whether a patient should also receive surveillance breast MRI.
Importantly, PRSs could also add to other risk factors to provide more precise risk estimates and inform management of women with a pathogenic variant in a breast cancer risk predisposition gene, Dr. Shah wrote.
Confluence project
Among the most promising developments in research is the National Cancer Institute’s Confluence Project, a large research resource aiming to include approximately 300,000 breast cancer cases and 300,000 controls of different races/ethnicities, utilizing the confluence of existing GWAS and new genomewide genotyping data.
Having started enrollment in 2018, the project is approaching implementation, said Montserrat García-Closas, MD, MPH, DrPH, deputy director of cancer epidemiology and genetics with the National Cancer Institute.
“We expect genotyping to be completed by the end of 2022 and for the data to be made available to the research community soon after that,” she said.
Among the project’s key objectives are the development of PRSs to be integrated with known risk factors to provide a personalized risk assessment for breast cancer, overall and by ancestral subtype.
“We plan to apply novel methods to derive multiancestry PRS that will account for differences and similarities in genetic architecture across ethnic/racial groups to develop breast cancer PRSs that can be applied in multiethnic/racial populations,” she said.
NCI is working with investigators in Africa, Central and South America, and Asia, and reaching out to non-European organizations such as AORTIC for studies of African populations.
Direct-to-consumer global PRS
In the commercial PRS market, efforts to address diversity shortcomings are also gaining momentum, with Myriad Genetics touting a first-of-its kind “global PRS.”
The PRS, a recalibrated version the company’s riskScore PRS, sold as part of its Myriad myRisk Hereditary Cancer test, will reportedly apply to all ethnicities in estimating an individual’s 5-year and lifetime risk of breast cancer.
A study presented in June at the American Society of Clinical Oncology meeting, describes the development of the model with the use of three large ancestry-specific PRSs based on African American, Asian, and European cohorts, with the system including a total of 149 single-nucleotide polymorphisms, including 93 well established for breast cancer and 56 that are ancestry specific.
In validation of the data in an independent cohort of 62,707 individuals, the global PRS was strongly associated with breast cancer in the full combined validation cohort as well as in all three of the ancestry subcohorts.
However, the effect size among women with African ancestry was still the lowest of all of the groups, with a mean OR of 1.24 per standard deviation, versus the highest rate of mixed ancestry (OR, 1.59).
According to senior author Holly Pederson, MD, director of medical breast services at the Cleveland Clinic, the applicability of the PRS to women with African ancestry is expected to further improve as additional data become available.
“The discriminatory power in women of African descent was significantly improved but still suboptimal,” she said. “The need for more data, particularly in Black women, is challenging not only because there is likely more diversity in the genomic landscape of women of African descent, but also because the barriers created by historical, cultural, institutional and interpersonal dynamics result in the paucity of this data.”
“We must be committed to ending bias resulting in health care disparities,” Dr. Pederson said. She noted that the global PRS is nevertheless “still clinically useful in Black women,” and recommended that clinicians be up front with patients on the status of the research challenges.
“As with any clinical shared decision-making conversation between a patient and her provider, it is important for Black women to know that data is limited in the African American population, particularly given the vast genomic diversity of the African continent,” she said. “This model, as models that have gone before it, will improve with additional data, particularly in this population.”
Commercial PRSs may benefit research
While the commercial marketing of PRSs in a direct-to-consumer fashion have raised some concerns, such as how individuals respond to their risk scores, there could be important benefits as well, commented Megan C. Roberts, PhD.
“There may be an opportunity to learn from these companies about how to engage diverse communities in genomic testing,” said Dr. Roberts, an assistant professor and director of implementation science in precision health and society at the University of North Carolina at Chapel Hill. “Moreover, the data they collect from their customers often can be used for research purposes as well.”
In a recent perspective, Dr. Roberts and colleagues addressed the role of health disparities in PRSs. She’ll be joining international precision public health researchers in October in hosting a free virtual conference at UNC on the topic.
“There is a huge need to improve racial and ethnic diversity in our genomic datasets,” Dr. Roberts said. “Without this, we will not be able to return on the promise of precision medicine and prevention for improving the health of our whole population.”
Dr. Pederson disclosed that she is a consultant for Myriad Genetics.
FROM JAMA NETWORK OPEN
To be, or not to be? More counseling needed for gender dysphoria
Clinicians should not blindly accept a person’s self-diagnosis as transgender and desire to medically transition without closer inspection; rather, they should make a distinction between ‘acceptance’ and conducting an in-depth, respectful, and collaborative exploration of an individual’s claims about what they believe will best promote their well-being.
These are the conclusions of two experts in ethics and clinical psychology in an extended essay published in the Journal of Medical Ethics.
“It’s not about making life harder for people who wish to transition but about improving care for all people who identify as transgender,” lead author Alessandra Lemma, DClin Psych, visiting professor in the psychoanalysis unit at University College London, said in an interview.
She stressed that the argument is neither for nor against medical transitioning per se.
“It’s an invitation to think about how the medical and mental health care communities can best support anyone considering a transition, whether they eventually pursue that course of action or not. The provision of psychotherapy, irrespective of whether the individual medically transitions or not, makes for a better outcome either way,” said Dr. Lemma, who cares for adolescents as well as adults with gender dysphoria in her private practice in London.
Reflective space has been eroded in gender identity services for the young
There has been an exponential increase in the number of adolescents who identify as transgender in Western countries in recent years. This news organization has covered the debate in detail, which has intensified worldwide in the last 12 months, regarding how best to treat youth with gender dysphoria.
This has “raised concern about how the laudable aims of gender affirmative care may be ushering children and young people too quickly into medical transitioning,” generally defined as treatment with puberty blockers in minors followed by cross-sex hormones to transition to the opposite sex, “leading subsequently to a wish to detransition with all the attendant physical and psychological complications,” wrote Dr. Lemma and her coauthor, Julian Savulescu, MD, professor of practical ethics, University of Oxford (England).
While the United Kingdom and other countries such as Finland have tightened regulations regarding the treatment of minors, “these medical interventions continue to be provided in many other countries,” they noted.
Such affirmative care has recently been interpreted by “influential sections of the transgender community” as forbidding “’questioning’ of any kind of the person’s stated gender and what will help them,” the essayists stated.
But Dr. Lemma noted that, for teenagers, this is typically a time to “try on” different identities and ways of presenting oneself to the world.
“All this requires a reflective space during the decision-making process, and this has been eroded in many gender identity services for young people especially, with a massive pressure on services.”
Family issues, trauma, and comorbid conditions can all influence people too, she noted, adding that what may be happening unconsciously may be driving the decision to modify the body.
“I cannot see that it would be harmful to anyone to have the opportunity to really think about what they are doing before making decisions about medical interventions,” she asserted.
Decision to transition must be judged to be autonomous
Dr. Lemma noted that even in those instances where medical transitioning, on balance, is the best option, it’s important to acknowledge that the process has a psychological impact.
“What matters is that in facing a major life-changing decision, an individual has the opportunity to understand the developmental and social experiences that drive their experience of gender dysphoria such that the decisions they make about medical transitioning can be said to be taken more autonomously,” she and Dr. Savulescu wrote.
And for those people who opt for full gender reassignment surgery, they are the first to say, “I don’t think I could have got through this as well as I have without psychological support,” Dr. Lemma remarked.
Ultimately, what’s important is to ensure the protection of those individuals whose needs will most likely not be met by medical transitioning, while not making it impossible for those who are suffering to get the care they need in order to transition, she concluded.
Until 2016, Dr. Lemma was professor of psychological therapies at the Tavistock and Portman NHS Foundation Trust and Essex University. During that time she worked with adult transgender individuals at the Portman clinic but not at the Gender Identity Service at the Tavistock clinic. Currently, she works in private practice with transgender individuals at the Queen Anne Street Practice, London. Dr. Savulescu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should not blindly accept a person’s self-diagnosis as transgender and desire to medically transition without closer inspection; rather, they should make a distinction between ‘acceptance’ and conducting an in-depth, respectful, and collaborative exploration of an individual’s claims about what they believe will best promote their well-being.
These are the conclusions of two experts in ethics and clinical psychology in an extended essay published in the Journal of Medical Ethics.
“It’s not about making life harder for people who wish to transition but about improving care for all people who identify as transgender,” lead author Alessandra Lemma, DClin Psych, visiting professor in the psychoanalysis unit at University College London, said in an interview.
She stressed that the argument is neither for nor against medical transitioning per se.
“It’s an invitation to think about how the medical and mental health care communities can best support anyone considering a transition, whether they eventually pursue that course of action or not. The provision of psychotherapy, irrespective of whether the individual medically transitions or not, makes for a better outcome either way,” said Dr. Lemma, who cares for adolescents as well as adults with gender dysphoria in her private practice in London.
Reflective space has been eroded in gender identity services for the young
There has been an exponential increase in the number of adolescents who identify as transgender in Western countries in recent years. This news organization has covered the debate in detail, which has intensified worldwide in the last 12 months, regarding how best to treat youth with gender dysphoria.
This has “raised concern about how the laudable aims of gender affirmative care may be ushering children and young people too quickly into medical transitioning,” generally defined as treatment with puberty blockers in minors followed by cross-sex hormones to transition to the opposite sex, “leading subsequently to a wish to detransition with all the attendant physical and psychological complications,” wrote Dr. Lemma and her coauthor, Julian Savulescu, MD, professor of practical ethics, University of Oxford (England).
While the United Kingdom and other countries such as Finland have tightened regulations regarding the treatment of minors, “these medical interventions continue to be provided in many other countries,” they noted.
Such affirmative care has recently been interpreted by “influential sections of the transgender community” as forbidding “’questioning’ of any kind of the person’s stated gender and what will help them,” the essayists stated.
But Dr. Lemma noted that, for teenagers, this is typically a time to “try on” different identities and ways of presenting oneself to the world.
“All this requires a reflective space during the decision-making process, and this has been eroded in many gender identity services for young people especially, with a massive pressure on services.”
Family issues, trauma, and comorbid conditions can all influence people too, she noted, adding that what may be happening unconsciously may be driving the decision to modify the body.
“I cannot see that it would be harmful to anyone to have the opportunity to really think about what they are doing before making decisions about medical interventions,” she asserted.
Decision to transition must be judged to be autonomous
Dr. Lemma noted that even in those instances where medical transitioning, on balance, is the best option, it’s important to acknowledge that the process has a psychological impact.
“What matters is that in facing a major life-changing decision, an individual has the opportunity to understand the developmental and social experiences that drive their experience of gender dysphoria such that the decisions they make about medical transitioning can be said to be taken more autonomously,” she and Dr. Savulescu wrote.
And for those people who opt for full gender reassignment surgery, they are the first to say, “I don’t think I could have got through this as well as I have without psychological support,” Dr. Lemma remarked.
Ultimately, what’s important is to ensure the protection of those individuals whose needs will most likely not be met by medical transitioning, while not making it impossible for those who are suffering to get the care they need in order to transition, she concluded.
Until 2016, Dr. Lemma was professor of psychological therapies at the Tavistock and Portman NHS Foundation Trust and Essex University. During that time she worked with adult transgender individuals at the Portman clinic but not at the Gender Identity Service at the Tavistock clinic. Currently, she works in private practice with transgender individuals at the Queen Anne Street Practice, London. Dr. Savulescu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should not blindly accept a person’s self-diagnosis as transgender and desire to medically transition without closer inspection; rather, they should make a distinction between ‘acceptance’ and conducting an in-depth, respectful, and collaborative exploration of an individual’s claims about what they believe will best promote their well-being.
These are the conclusions of two experts in ethics and clinical psychology in an extended essay published in the Journal of Medical Ethics.
“It’s not about making life harder for people who wish to transition but about improving care for all people who identify as transgender,” lead author Alessandra Lemma, DClin Psych, visiting professor in the psychoanalysis unit at University College London, said in an interview.
She stressed that the argument is neither for nor against medical transitioning per se.
“It’s an invitation to think about how the medical and mental health care communities can best support anyone considering a transition, whether they eventually pursue that course of action or not. The provision of psychotherapy, irrespective of whether the individual medically transitions or not, makes for a better outcome either way,” said Dr. Lemma, who cares for adolescents as well as adults with gender dysphoria in her private practice in London.
Reflective space has been eroded in gender identity services for the young
There has been an exponential increase in the number of adolescents who identify as transgender in Western countries in recent years. This news organization has covered the debate in detail, which has intensified worldwide in the last 12 months, regarding how best to treat youth with gender dysphoria.
This has “raised concern about how the laudable aims of gender affirmative care may be ushering children and young people too quickly into medical transitioning,” generally defined as treatment with puberty blockers in minors followed by cross-sex hormones to transition to the opposite sex, “leading subsequently to a wish to detransition with all the attendant physical and psychological complications,” wrote Dr. Lemma and her coauthor, Julian Savulescu, MD, professor of practical ethics, University of Oxford (England).
While the United Kingdom and other countries such as Finland have tightened regulations regarding the treatment of minors, “these medical interventions continue to be provided in many other countries,” they noted.
Such affirmative care has recently been interpreted by “influential sections of the transgender community” as forbidding “’questioning’ of any kind of the person’s stated gender and what will help them,” the essayists stated.
But Dr. Lemma noted that, for teenagers, this is typically a time to “try on” different identities and ways of presenting oneself to the world.
“All this requires a reflective space during the decision-making process, and this has been eroded in many gender identity services for young people especially, with a massive pressure on services.”
Family issues, trauma, and comorbid conditions can all influence people too, she noted, adding that what may be happening unconsciously may be driving the decision to modify the body.
“I cannot see that it would be harmful to anyone to have the opportunity to really think about what they are doing before making decisions about medical interventions,” she asserted.
Decision to transition must be judged to be autonomous
Dr. Lemma noted that even in those instances where medical transitioning, on balance, is the best option, it’s important to acknowledge that the process has a psychological impact.
“What matters is that in facing a major life-changing decision, an individual has the opportunity to understand the developmental and social experiences that drive their experience of gender dysphoria such that the decisions they make about medical transitioning can be said to be taken more autonomously,” she and Dr. Savulescu wrote.
And for those people who opt for full gender reassignment surgery, they are the first to say, “I don’t think I could have got through this as well as I have without psychological support,” Dr. Lemma remarked.
Ultimately, what’s important is to ensure the protection of those individuals whose needs will most likely not be met by medical transitioning, while not making it impossible for those who are suffering to get the care they need in order to transition, she concluded.
Until 2016, Dr. Lemma was professor of psychological therapies at the Tavistock and Portman NHS Foundation Trust and Essex University. During that time she worked with adult transgender individuals at the Portman clinic but not at the Gender Identity Service at the Tavistock clinic. Currently, she works in private practice with transgender individuals at the Queen Anne Street Practice, London. Dr. Savulescu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Connecticut chapter of ACC at center of Twitter dustup
Tweets from a black female medical student about the perils of being on call after lengthy hospital shifts was met with a stinging rebuke from the Twitter account of the Connecticut chapter of the American College of Cardiology – prompting an apology and some high-octane exchanges on medical Twitter.
In a series of Tweets, “queen of anonymous medicine” @QueenMD202X describes one friend “working 87 hours this week and 13 days straight” and a second, a third-year medical student working a 15-hour surgical shift. “That is cruel,” she writes, “15-hour shift? For what?????”
In response to a Tweet suggesting that being on call can be a valuable experience for students to know what they’re facing once they get to residency, @QueenMD202X pointed out the 15-hour shifts aren’t just a one-off.
In a now-deleted Tweet that nevertheless appears in several additional tweets as a screenshot, @ConnecticutACC replied: “You might be in the wrong field. You sound very angry probably unsuitable for patient care when your mental state is as you describe it. Emotions are contagious.”
The response from the medical and broader Twitter community was swift, with several tweets calling the chapter’s reply insensitive and racist.
In another Tweet, @BrittGratreak responded by stating: “I think institutions need to be more transparent how they basically weigh the costs & benefits of writing a memorial statement for students who die by suicide rather than investing in changing the toxic culture of medical education to prevent deaths & producing harmed physicians.”
Within hours, Connecticut-ACC issued an apology from their now-deleted account and questioned the origins of the Tweet. “We sincerely apologize for the earlier post as the views do not represent the values or beliefs of the Chapter or broader ACC. We are working to ID its origins. Burnout & well-being are critical issues [that] ACC/CCACC is working to address on behalf of members at all career stages.”
Speaking to this news organization, Connecticut-ACC president and governor Craig McPherson, MD, Yale University, New Haven, Conn., said the chapter believes its account was hacked.
“We provide limited password access to our Twitter account, and we assume, since we’ve contacted most of the individuals who had access to the current password and all of the them deny any knowledge, the account got hacked … it’s just one of those unfortunate aspects of social media,” he said.
The password was quickly changed after the chapter learned of the Tweet on Wednesday and the account has since been closed, at Dr. McPherson’s request.
“We don’t condone that kind of language, that kind of remark. It’s highly inappropriate, and I certainly agree with anyone that voiced that opinion in the Twitterstorm that followed,” he said. “But as I said at the outset, I have no control over what people say on social media once it’s out there. All we can do is apologize for the fact our Twitter feed was used as a vehicle for those comments, which we consider inappropriate.”
Asked whether he considered the remarks racist, Dr. McPherson replied: “That’s not for me to judge.”
ACC president Dipti Itchhaporia, MD, however, weighed in this afternoon with a Tweet citing the need to address clinician well-being and an inclusive workplace.
Some on Twitter recalled their own long hours as a medical student or defended the need to inculcate students in the long hours they’ll face as physicians. Others observed that neither ACC nor its Connecticut chapter addressed the issue of medical student hours in their response. Although fellow and resident hours are regulated, Dr. McPherson pointed out that it’s up to each individual medical school to set the hours for their students.
A version of this article first appeared on Medscape.com.
Tweets from a black female medical student about the perils of being on call after lengthy hospital shifts was met with a stinging rebuke from the Twitter account of the Connecticut chapter of the American College of Cardiology – prompting an apology and some high-octane exchanges on medical Twitter.
In a series of Tweets, “queen of anonymous medicine” @QueenMD202X describes one friend “working 87 hours this week and 13 days straight” and a second, a third-year medical student working a 15-hour surgical shift. “That is cruel,” she writes, “15-hour shift? For what?????”
In response to a Tweet suggesting that being on call can be a valuable experience for students to know what they’re facing once they get to residency, @QueenMD202X pointed out the 15-hour shifts aren’t just a one-off.
In a now-deleted Tweet that nevertheless appears in several additional tweets as a screenshot, @ConnecticutACC replied: “You might be in the wrong field. You sound very angry probably unsuitable for patient care when your mental state is as you describe it. Emotions are contagious.”
The response from the medical and broader Twitter community was swift, with several tweets calling the chapter’s reply insensitive and racist.
In another Tweet, @BrittGratreak responded by stating: “I think institutions need to be more transparent how they basically weigh the costs & benefits of writing a memorial statement for students who die by suicide rather than investing in changing the toxic culture of medical education to prevent deaths & producing harmed physicians.”
Within hours, Connecticut-ACC issued an apology from their now-deleted account and questioned the origins of the Tweet. “We sincerely apologize for the earlier post as the views do not represent the values or beliefs of the Chapter or broader ACC. We are working to ID its origins. Burnout & well-being are critical issues [that] ACC/CCACC is working to address on behalf of members at all career stages.”
Speaking to this news organization, Connecticut-ACC president and governor Craig McPherson, MD, Yale University, New Haven, Conn., said the chapter believes its account was hacked.
“We provide limited password access to our Twitter account, and we assume, since we’ve contacted most of the individuals who had access to the current password and all of the them deny any knowledge, the account got hacked … it’s just one of those unfortunate aspects of social media,” he said.
The password was quickly changed after the chapter learned of the Tweet on Wednesday and the account has since been closed, at Dr. McPherson’s request.
“We don’t condone that kind of language, that kind of remark. It’s highly inappropriate, and I certainly agree with anyone that voiced that opinion in the Twitterstorm that followed,” he said. “But as I said at the outset, I have no control over what people say on social media once it’s out there. All we can do is apologize for the fact our Twitter feed was used as a vehicle for those comments, which we consider inappropriate.”
Asked whether he considered the remarks racist, Dr. McPherson replied: “That’s not for me to judge.”
ACC president Dipti Itchhaporia, MD, however, weighed in this afternoon with a Tweet citing the need to address clinician well-being and an inclusive workplace.
Some on Twitter recalled their own long hours as a medical student or defended the need to inculcate students in the long hours they’ll face as physicians. Others observed that neither ACC nor its Connecticut chapter addressed the issue of medical student hours in their response. Although fellow and resident hours are regulated, Dr. McPherson pointed out that it’s up to each individual medical school to set the hours for their students.
A version of this article first appeared on Medscape.com.
Tweets from a black female medical student about the perils of being on call after lengthy hospital shifts was met with a stinging rebuke from the Twitter account of the Connecticut chapter of the American College of Cardiology – prompting an apology and some high-octane exchanges on medical Twitter.
In a series of Tweets, “queen of anonymous medicine” @QueenMD202X describes one friend “working 87 hours this week and 13 days straight” and a second, a third-year medical student working a 15-hour surgical shift. “That is cruel,” she writes, “15-hour shift? For what?????”
In response to a Tweet suggesting that being on call can be a valuable experience for students to know what they’re facing once they get to residency, @QueenMD202X pointed out the 15-hour shifts aren’t just a one-off.
In a now-deleted Tweet that nevertheless appears in several additional tweets as a screenshot, @ConnecticutACC replied: “You might be in the wrong field. You sound very angry probably unsuitable for patient care when your mental state is as you describe it. Emotions are contagious.”
The response from the medical and broader Twitter community was swift, with several tweets calling the chapter’s reply insensitive and racist.
In another Tweet, @BrittGratreak responded by stating: “I think institutions need to be more transparent how they basically weigh the costs & benefits of writing a memorial statement for students who die by suicide rather than investing in changing the toxic culture of medical education to prevent deaths & producing harmed physicians.”
Within hours, Connecticut-ACC issued an apology from their now-deleted account and questioned the origins of the Tweet. “We sincerely apologize for the earlier post as the views do not represent the values or beliefs of the Chapter or broader ACC. We are working to ID its origins. Burnout & well-being are critical issues [that] ACC/CCACC is working to address on behalf of members at all career stages.”
Speaking to this news organization, Connecticut-ACC president and governor Craig McPherson, MD, Yale University, New Haven, Conn., said the chapter believes its account was hacked.
“We provide limited password access to our Twitter account, and we assume, since we’ve contacted most of the individuals who had access to the current password and all of the them deny any knowledge, the account got hacked … it’s just one of those unfortunate aspects of social media,” he said.
The password was quickly changed after the chapter learned of the Tweet on Wednesday and the account has since been closed, at Dr. McPherson’s request.
“We don’t condone that kind of language, that kind of remark. It’s highly inappropriate, and I certainly agree with anyone that voiced that opinion in the Twitterstorm that followed,” he said. “But as I said at the outset, I have no control over what people say on social media once it’s out there. All we can do is apologize for the fact our Twitter feed was used as a vehicle for those comments, which we consider inappropriate.”
Asked whether he considered the remarks racist, Dr. McPherson replied: “That’s not for me to judge.”
ACC president Dipti Itchhaporia, MD, however, weighed in this afternoon with a Tweet citing the need to address clinician well-being and an inclusive workplace.
Some on Twitter recalled their own long hours as a medical student or defended the need to inculcate students in the long hours they’ll face as physicians. Others observed that neither ACC nor its Connecticut chapter addressed the issue of medical student hours in their response. Although fellow and resident hours are regulated, Dr. McPherson pointed out that it’s up to each individual medical school to set the hours for their students.
A version of this article first appeared on Medscape.com.
Medical students lead event addressing disparity in skin cancer morbidity and mortality
WASHINGTON – Those who self-identify as Hispanic or Black have a lower self-perceived risk of melanoma. In fact, people of color receive little to no information concerning skin cancer risks and prevention strategies and experience a longer time from diagnosis to definitive surgery, resulting in far worse outcomes, compared with non-Hispanic Whites.

This disparity is reflected in statistics showing that the average 5-year survival rate for melanoma is 92% in White patients but drops down to 67% in Black patients. Low income is also a contributing factor: Patients with lower incomes experience greater difficulty accessing health care and have greater time to diagnosis and a worse prognosis and survival time with melanoma. Despite economic advancements, Black Americans are still economically deprived when compared with White Americans.
This reality is what led Sarah Millan, a 4th-year medical student at George Washington University, Washington, to focus on the Ward 8 community in Washington – one of the poorest regions in our nation’s capital – well known for limited access to medical care and referred to as a health care desert. “Ward 8 has a population that is 92% Black and does not have a single dermatology clinic in the vicinity – my vision was to bring together the community through an enjoyable attraction conducive to the delivery of quality dermatologic care and education to a community that has none,” said Ms. Millan.
This low-resource population that is socioeconomically and geographically isolated is likely unaware of skin cancer risks, prevention strategies, and signs or symptoms that would warrant a visit to the dermatologist.
, while also exploring the attitudes and behaviors around skin cancer and sunscreen use in the community through data collected from optional surveys.
On Saturday, July 10, 2021, dermatologists from George Washington University, department of dermatology and medical students from George Washington School of Medicine and Health Sciences and Howard University College of Medicine in Washington, transformed Martha’s Outfitters in Ward 8 into a decorated, music-filled venue. Part of the Ward 8 council member’s 40 Days of Peace initiative, the Learn2Derm fair provided free skin cancer screenings by dermatologists, while students staffed various stations, delivering fun and interactive educational lessons organized by Ms. Millan under the mentorship of Adam Friedman, MD, chair of dermatology at George Washington University.

“It is our responsibility to support our communities through care, but even more importantly, combating misinformation and misperceptions that could interfere with healthy living,” said Dr. Friedman.
Activities included arts and crafts sponsored by the American Academy of Dermatology Good Skin Knowledge lessons, games with giveaways sponsored by the Polka Dot Mama Melanoma Foundation and IMPACT Melanoma, Skin Analyzers (to see where sunscreen was applied, and where it was missed) supplied by the Melanoma Research Foundation (MRF) and Children’s Melanoma Prevention Foundation (CMPF), and even Viva Vita virtual reality headsets that are catered towards the senior population – but enjoyable to anyone. Prizes and giveaways ranged from ultraviolet-induced color-changing bracelets and Frisbees, SPF lip balms, sunglasses – and of course – an abundant supply of free sunscreen. Many community members expressed their gratitude for this event and were impressed by the education that was enlivened through interactive games, activities, and giveaways. One participant shared the news of the event with a friend who immediately stopped what she was doing to come by for some education, a skin cancer screening, and free skincare products. While parents went in for a free skin cancer screening, their children were supervised by medical student volunteers as they colored or participated in other stations.

Ms. Millan’s involvement with the National Council on Skin Cancer Prevention’s Skin Smart Campus Initiative facilitated the support and partnership with multiple national organizations central to the event’s success, including the AAD, the National Council on Skin Cancer Prevention, the Skin Cancer Foundation, IMPACT Melanoma, Polka Dot Mama Melanoma Foundation, MRF, and CMPF. The donations of these organizations and businesses in the sun protection industry, along with faculty and medical students who share a passion for delivering dermatologic care and resources brought this exciting plan into fruition. The aim of Learn2Derm is not for this to be a single event, but rather the first of many that will continue to deliver this type of care to a community that is in need of greater dermatologic attention – an ongoing occurrence that can have a lasting impact on the Ward 8 community.
Major sunscreen manufacturers that donated sunscreen for this event included Avène, Black Girl Sunscreen, CeraVe, Cetaphil, EltaMD, and Neutrogena. Coolibar, which specializes in sun-protective clothing, also made a donation of multistyle hats, gaiters, and clothes for attendees.
References
1: Harvey VM et al. Cancer Control. 2014 Oct;21(4):343-9.
2: Tripathi R et al. J Am Acad Dermatol. 2020 Sep;83(3):854-9.
3. Beyer Don. “The Economic State of Black America in 2020” U.S. Congress: Joint Economic Committee.
4. Culp MaryBeth B and Lunsford Natasha Buchanan. “Melanoma Among Non-Hispanic Black Americans” Prev Chronic Dis;16. 2019 Jun 20. doi: 10.5888/pcd16.180640.
5. “Ask the Expert: Is There a Skin Cancer Crisis in People of Color?” The Skin Cancer Foundation. 2020 Jul 5.
6. Salvaggio C et al. Oncology. 2016;90(2):79-87.
WASHINGTON – Those who self-identify as Hispanic or Black have a lower self-perceived risk of melanoma. In fact, people of color receive little to no information concerning skin cancer risks and prevention strategies and experience a longer time from diagnosis to definitive surgery, resulting in far worse outcomes, compared with non-Hispanic Whites.

This disparity is reflected in statistics showing that the average 5-year survival rate for melanoma is 92% in White patients but drops down to 67% in Black patients. Low income is also a contributing factor: Patients with lower incomes experience greater difficulty accessing health care and have greater time to diagnosis and a worse prognosis and survival time with melanoma. Despite economic advancements, Black Americans are still economically deprived when compared with White Americans.
This reality is what led Sarah Millan, a 4th-year medical student at George Washington University, Washington, to focus on the Ward 8 community in Washington – one of the poorest regions in our nation’s capital – well known for limited access to medical care and referred to as a health care desert. “Ward 8 has a population that is 92% Black and does not have a single dermatology clinic in the vicinity – my vision was to bring together the community through an enjoyable attraction conducive to the delivery of quality dermatologic care and education to a community that has none,” said Ms. Millan.
This low-resource population that is socioeconomically and geographically isolated is likely unaware of skin cancer risks, prevention strategies, and signs or symptoms that would warrant a visit to the dermatologist.
, while also exploring the attitudes and behaviors around skin cancer and sunscreen use in the community through data collected from optional surveys.
On Saturday, July 10, 2021, dermatologists from George Washington University, department of dermatology and medical students from George Washington School of Medicine and Health Sciences and Howard University College of Medicine in Washington, transformed Martha’s Outfitters in Ward 8 into a decorated, music-filled venue. Part of the Ward 8 council member’s 40 Days of Peace initiative, the Learn2Derm fair provided free skin cancer screenings by dermatologists, while students staffed various stations, delivering fun and interactive educational lessons organized by Ms. Millan under the mentorship of Adam Friedman, MD, chair of dermatology at George Washington University.

“It is our responsibility to support our communities through care, but even more importantly, combating misinformation and misperceptions that could interfere with healthy living,” said Dr. Friedman.
Activities included arts and crafts sponsored by the American Academy of Dermatology Good Skin Knowledge lessons, games with giveaways sponsored by the Polka Dot Mama Melanoma Foundation and IMPACT Melanoma, Skin Analyzers (to see where sunscreen was applied, and where it was missed) supplied by the Melanoma Research Foundation (MRF) and Children’s Melanoma Prevention Foundation (CMPF), and even Viva Vita virtual reality headsets that are catered towards the senior population – but enjoyable to anyone. Prizes and giveaways ranged from ultraviolet-induced color-changing bracelets and Frisbees, SPF lip balms, sunglasses – and of course – an abundant supply of free sunscreen. Many community members expressed their gratitude for this event and were impressed by the education that was enlivened through interactive games, activities, and giveaways. One participant shared the news of the event with a friend who immediately stopped what she was doing to come by for some education, a skin cancer screening, and free skincare products. While parents went in for a free skin cancer screening, their children were supervised by medical student volunteers as they colored or participated in other stations.

Ms. Millan’s involvement with the National Council on Skin Cancer Prevention’s Skin Smart Campus Initiative facilitated the support and partnership with multiple national organizations central to the event’s success, including the AAD, the National Council on Skin Cancer Prevention, the Skin Cancer Foundation, IMPACT Melanoma, Polka Dot Mama Melanoma Foundation, MRF, and CMPF. The donations of these organizations and businesses in the sun protection industry, along with faculty and medical students who share a passion for delivering dermatologic care and resources brought this exciting plan into fruition. The aim of Learn2Derm is not for this to be a single event, but rather the first of many that will continue to deliver this type of care to a community that is in need of greater dermatologic attention – an ongoing occurrence that can have a lasting impact on the Ward 8 community.
Major sunscreen manufacturers that donated sunscreen for this event included Avène, Black Girl Sunscreen, CeraVe, Cetaphil, EltaMD, and Neutrogena. Coolibar, which specializes in sun-protective clothing, also made a donation of multistyle hats, gaiters, and clothes for attendees.
References
1: Harvey VM et al. Cancer Control. 2014 Oct;21(4):343-9.
2: Tripathi R et al. J Am Acad Dermatol. 2020 Sep;83(3):854-9.
3. Beyer Don. “The Economic State of Black America in 2020” U.S. Congress: Joint Economic Committee.
4. Culp MaryBeth B and Lunsford Natasha Buchanan. “Melanoma Among Non-Hispanic Black Americans” Prev Chronic Dis;16. 2019 Jun 20. doi: 10.5888/pcd16.180640.
5. “Ask the Expert: Is There a Skin Cancer Crisis in People of Color?” The Skin Cancer Foundation. 2020 Jul 5.
6. Salvaggio C et al. Oncology. 2016;90(2):79-87.
WASHINGTON – Those who self-identify as Hispanic or Black have a lower self-perceived risk of melanoma. In fact, people of color receive little to no information concerning skin cancer risks and prevention strategies and experience a longer time from diagnosis to definitive surgery, resulting in far worse outcomes, compared with non-Hispanic Whites.

This disparity is reflected in statistics showing that the average 5-year survival rate for melanoma is 92% in White patients but drops down to 67% in Black patients. Low income is also a contributing factor: Patients with lower incomes experience greater difficulty accessing health care and have greater time to diagnosis and a worse prognosis and survival time with melanoma. Despite economic advancements, Black Americans are still economically deprived when compared with White Americans.
This reality is what led Sarah Millan, a 4th-year medical student at George Washington University, Washington, to focus on the Ward 8 community in Washington – one of the poorest regions in our nation’s capital – well known for limited access to medical care and referred to as a health care desert. “Ward 8 has a population that is 92% Black and does not have a single dermatology clinic in the vicinity – my vision was to bring together the community through an enjoyable attraction conducive to the delivery of quality dermatologic care and education to a community that has none,” said Ms. Millan.
This low-resource population that is socioeconomically and geographically isolated is likely unaware of skin cancer risks, prevention strategies, and signs or symptoms that would warrant a visit to the dermatologist.
, while also exploring the attitudes and behaviors around skin cancer and sunscreen use in the community through data collected from optional surveys.
On Saturday, July 10, 2021, dermatologists from George Washington University, department of dermatology and medical students from George Washington School of Medicine and Health Sciences and Howard University College of Medicine in Washington, transformed Martha’s Outfitters in Ward 8 into a decorated, music-filled venue. Part of the Ward 8 council member’s 40 Days of Peace initiative, the Learn2Derm fair provided free skin cancer screenings by dermatologists, while students staffed various stations, delivering fun and interactive educational lessons organized by Ms. Millan under the mentorship of Adam Friedman, MD, chair of dermatology at George Washington University.

“It is our responsibility to support our communities through care, but even more importantly, combating misinformation and misperceptions that could interfere with healthy living,” said Dr. Friedman.
Activities included arts and crafts sponsored by the American Academy of Dermatology Good Skin Knowledge lessons, games with giveaways sponsored by the Polka Dot Mama Melanoma Foundation and IMPACT Melanoma, Skin Analyzers (to see where sunscreen was applied, and where it was missed) supplied by the Melanoma Research Foundation (MRF) and Children’s Melanoma Prevention Foundation (CMPF), and even Viva Vita virtual reality headsets that are catered towards the senior population – but enjoyable to anyone. Prizes and giveaways ranged from ultraviolet-induced color-changing bracelets and Frisbees, SPF lip balms, sunglasses – and of course – an abundant supply of free sunscreen. Many community members expressed their gratitude for this event and were impressed by the education that was enlivened through interactive games, activities, and giveaways. One participant shared the news of the event with a friend who immediately stopped what she was doing to come by for some education, a skin cancer screening, and free skincare products. While parents went in for a free skin cancer screening, their children were supervised by medical student volunteers as they colored or participated in other stations.

Ms. Millan’s involvement with the National Council on Skin Cancer Prevention’s Skin Smart Campus Initiative facilitated the support and partnership with multiple national organizations central to the event’s success, including the AAD, the National Council on Skin Cancer Prevention, the Skin Cancer Foundation, IMPACT Melanoma, Polka Dot Mama Melanoma Foundation, MRF, and CMPF. The donations of these organizations and businesses in the sun protection industry, along with faculty and medical students who share a passion for delivering dermatologic care and resources brought this exciting plan into fruition. The aim of Learn2Derm is not for this to be a single event, but rather the first of many that will continue to deliver this type of care to a community that is in need of greater dermatologic attention – an ongoing occurrence that can have a lasting impact on the Ward 8 community.
Major sunscreen manufacturers that donated sunscreen for this event included Avène, Black Girl Sunscreen, CeraVe, Cetaphil, EltaMD, and Neutrogena. Coolibar, which specializes in sun-protective clothing, also made a donation of multistyle hats, gaiters, and clothes for attendees.
References
1: Harvey VM et al. Cancer Control. 2014 Oct;21(4):343-9.
2: Tripathi R et al. J Am Acad Dermatol. 2020 Sep;83(3):854-9.
3. Beyer Don. “The Economic State of Black America in 2020” U.S. Congress: Joint Economic Committee.
4. Culp MaryBeth B and Lunsford Natasha Buchanan. “Melanoma Among Non-Hispanic Black Americans” Prev Chronic Dis;16. 2019 Jun 20. doi: 10.5888/pcd16.180640.
5. “Ask the Expert: Is There a Skin Cancer Crisis in People of Color?” The Skin Cancer Foundation. 2020 Jul 5.
6. Salvaggio C et al. Oncology. 2016;90(2):79-87.
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.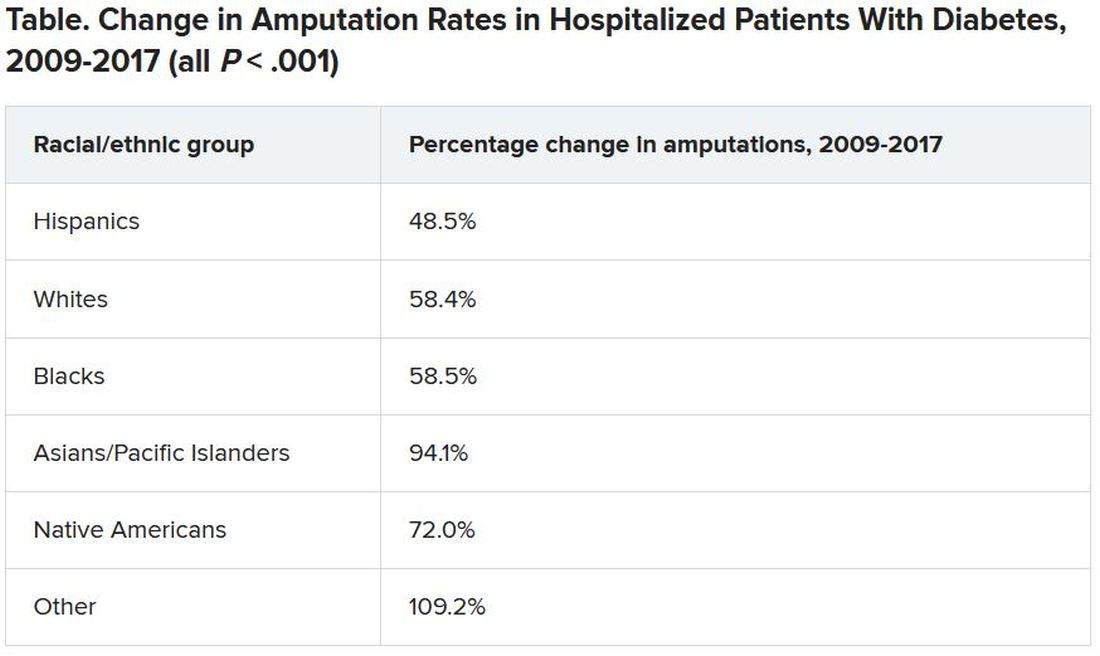
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ulcerated and Verrucous Plaque on the Chest
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
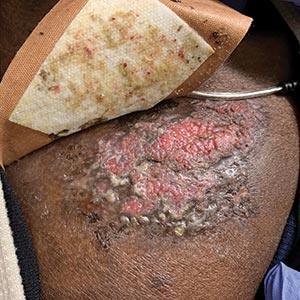
A 36-year-old man presented to an emergency department in the southwestern United States with a cough, fatigue, and worsening back pain associated with night sweats of 1 month’s duration. He experienced a 9.07-kg weight loss, as well as development of a rough, nontender, nonpruritic rash along the left upper chest over the prior month. The patient was born in West Africa and reported that he had moved to the southwestern United States from the eastern United States approximately 6 years prior to presentation. Physical examination on admission revealed a 5×3-cm, purple-brown, verrucous plaque with a central pink cobblestone appearance and ulceration. Chest radiography was notable for perihilar adenopathy with no focal infiltrates or cavitary lesions. Computed tomography and magnetic resonance imaging of the chest were notable for miliary nodules throughout the lungs; extensive lytic spine lesions of cervical, thoracic, and lumbar vertebral bodies and left twelfth rib; and a left paraspinal thoracic epidural soft tissue phlegmon. Initial laboratory investigations revealed peripheral eosinophilia without absolute leukocytosis and a microcytic anemia.
Shedding the super-doctor myth requires an honest look at systemic racism
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Move from awareness to action to combat racism in medicine
Structural racism and implicit bias are connected, and both must be addressed to move from awareness of racism to action, said Nathan Chomilo, MD, of HealthPartners/Park Nicollet, Brooklyn Center, Minn., in a presentation at the virtual Pediatric Hospital Medicine annual conference.
“We need pediatricians with the courage to address racism head on,” he said.
One step in moving from awareness to action against structural and institutional racism in medicine is examining policies, Dr. Chomilo said. He cited the creation of Medicare and Medicaid in 1965 as examples of how policy changes can make a difference, illustrated by data from 1955-1975 that showed a significant decrease in infant deaths among Black infants in Mississippi after 1965.
Medicaid expansion has helped to narrow, but not eliminate, racial disparities in health care, Dr. Chomilo said. The impact of Medicare and Medicaid is evident in the current COVID-19 pandemic, as county level data show that areas where more than 25% of the population are uninsured have higher rates of COVID-19 infections, said Dr. Chomilo. Policies that impact access to care also impact their incidence of chronic diseases and risk for severe disease, he noted.
“If you don’t have ready access to a health care provider, you don’t have access to the vaccine, and you don’t have information that would inform your getting the vaccine,” he added.
Prioritizing the power of voting
“Voting is one of many ways we can impact structural racism in health care policy,” Dr. Chomilo emphasized.
However, voting inequity remains a challenge, Dr. Chomilo noted. Community level disparities lead to inequity in voting access and subsequent disparities in voter participation, he said. “Leaders are less responsive to nonvoting constituents,” which can result in policies that impact health inequitably, and loop back to community level health disparities, he explained.
Historically, physicians have had an 8%-9% lower voter turnout than the general public, although this may have changed in recent elections, Dr. Chomilo said. He encouraged all clinicians to set an example and vote, and to empower their patients to vote. Evidence shows that enfranchisement of Black voters is associated with reductions in education gaps for Blacks and Whites, and that enfranchisement of women is associated with increased spending on children and lower child mortality, he said. Dr. Chomilo encouraged pediatricians and all clinicians to take advantage of the resources on voting available from the American Academy of Pediatrics (aap.org/votekids).
“When we see more people in a community vote, leaders are more responsive to their needs,” he said.
Informing racial identity
“Racial identity is informed by racial socialization,” Dr. Chomilo said. “All of us are socialized along the lines of race; it happens in conversations with parents, family, peers, community.” Another point in moving from awareness to action in eliminating structural racism is recognizing that children are not too young to talk about race, Dr. Chomilo emphasized.
Children start to navigate racial identity and to take note of other differences at an early age. For example, a 3-year-old might ask, “why does that person talk funny, why is that person being pushed in a chair?” Dr. Chomilo said, and it is important for parents and as pediatricians to be prepared for these questions, which are part of normal development. As children get older, they start to reflect on what differences mean for them, which is not rooted in anything negative, he noted.
Children first develop racial identity at home, but children solidify their identities in child care and school settings, Dr. Chomilo said. The American Academy of Pediatrics has acknowledged the potential for racial bias in education and child care, and said in a statement that, “it is critical for pediatricians to recognize the institutional personally mediated, and internalized levels of racism that occur in the educational setting, because education is a critical social determinant of health for children.” In fact, data from children in preschool show that they use racial categories to identify themselves and others, to include or exclude children from activities, and to negotiate power in their social and play networks.
Early intervention matters in educating children about racism, Dr. Chomilo said. “If we were not taught to talk about race, it is on us to learn about it ourselves as well,” he said.
Ultimately, the goal is to create active antiracism among adults and children, said Dr. Chomilo. He encouraged pediatricians and parents not to shut down or discourage children when they raise questions of race, but to take the opportunity to teach. “There may be hurt feelings around what a child said, even if they didn’t mean to offend someone,” he noted. Take the topic seriously, and make racism conversations ongoing; teach children to safely oppose negative messages and behaviors in others, and replace them with something positive, he emphasized.
Addressing bias in clinical settings
Dr. Chomilo also encouraged hospitalists to consider internalized racism in clinical settings and take action to build confidence and cultural pride in all patients by ensuring that a pediatric hospital unit is welcoming and representative of the diversity in a given community, with appropriate options for books, movies, and toys. He also encouraged pediatric hospitalists to assess children for experiences of racism as part of a social assessment. Be aware of signs of posttraumatic stress, anxiety, depression, or grief that might have a racial component, he said.
Dr. Chomilo had no financial conflicts to disclose.
Structural racism and implicit bias are connected, and both must be addressed to move from awareness of racism to action, said Nathan Chomilo, MD, of HealthPartners/Park Nicollet, Brooklyn Center, Minn., in a presentation at the virtual Pediatric Hospital Medicine annual conference.
“We need pediatricians with the courage to address racism head on,” he said.
One step in moving from awareness to action against structural and institutional racism in medicine is examining policies, Dr. Chomilo said. He cited the creation of Medicare and Medicaid in 1965 as examples of how policy changes can make a difference, illustrated by data from 1955-1975 that showed a significant decrease in infant deaths among Black infants in Mississippi after 1965.
Medicaid expansion has helped to narrow, but not eliminate, racial disparities in health care, Dr. Chomilo said. The impact of Medicare and Medicaid is evident in the current COVID-19 pandemic, as county level data show that areas where more than 25% of the population are uninsured have higher rates of COVID-19 infections, said Dr. Chomilo. Policies that impact access to care also impact their incidence of chronic diseases and risk for severe disease, he noted.
“If you don’t have ready access to a health care provider, you don’t have access to the vaccine, and you don’t have information that would inform your getting the vaccine,” he added.
Prioritizing the power of voting
“Voting is one of many ways we can impact structural racism in health care policy,” Dr. Chomilo emphasized.
However, voting inequity remains a challenge, Dr. Chomilo noted. Community level disparities lead to inequity in voting access and subsequent disparities in voter participation, he said. “Leaders are less responsive to nonvoting constituents,” which can result in policies that impact health inequitably, and loop back to community level health disparities, he explained.
Historically, physicians have had an 8%-9% lower voter turnout than the general public, although this may have changed in recent elections, Dr. Chomilo said. He encouraged all clinicians to set an example and vote, and to empower their patients to vote. Evidence shows that enfranchisement of Black voters is associated with reductions in education gaps for Blacks and Whites, and that enfranchisement of women is associated with increased spending on children and lower child mortality, he said. Dr. Chomilo encouraged pediatricians and all clinicians to take advantage of the resources on voting available from the American Academy of Pediatrics (aap.org/votekids).
“When we see more people in a community vote, leaders are more responsive to their needs,” he said.
Informing racial identity
“Racial identity is informed by racial socialization,” Dr. Chomilo said. “All of us are socialized along the lines of race; it happens in conversations with parents, family, peers, community.” Another point in moving from awareness to action in eliminating structural racism is recognizing that children are not too young to talk about race, Dr. Chomilo emphasized.
Children start to navigate racial identity and to take note of other differences at an early age. For example, a 3-year-old might ask, “why does that person talk funny, why is that person being pushed in a chair?” Dr. Chomilo said, and it is important for parents and as pediatricians to be prepared for these questions, which are part of normal development. As children get older, they start to reflect on what differences mean for them, which is not rooted in anything negative, he noted.
Children first develop racial identity at home, but children solidify their identities in child care and school settings, Dr. Chomilo said. The American Academy of Pediatrics has acknowledged the potential for racial bias in education and child care, and said in a statement that, “it is critical for pediatricians to recognize the institutional personally mediated, and internalized levels of racism that occur in the educational setting, because education is a critical social determinant of health for children.” In fact, data from children in preschool show that they use racial categories to identify themselves and others, to include or exclude children from activities, and to negotiate power in their social and play networks.
Early intervention matters in educating children about racism, Dr. Chomilo said. “If we were not taught to talk about race, it is on us to learn about it ourselves as well,” he said.
Ultimately, the goal is to create active antiracism among adults and children, said Dr. Chomilo. He encouraged pediatricians and parents not to shut down or discourage children when they raise questions of race, but to take the opportunity to teach. “There may be hurt feelings around what a child said, even if they didn’t mean to offend someone,” he noted. Take the topic seriously, and make racism conversations ongoing; teach children to safely oppose negative messages and behaviors in others, and replace them with something positive, he emphasized.
Addressing bias in clinical settings
Dr. Chomilo also encouraged hospitalists to consider internalized racism in clinical settings and take action to build confidence and cultural pride in all patients by ensuring that a pediatric hospital unit is welcoming and representative of the diversity in a given community, with appropriate options for books, movies, and toys. He also encouraged pediatric hospitalists to assess children for experiences of racism as part of a social assessment. Be aware of signs of posttraumatic stress, anxiety, depression, or grief that might have a racial component, he said.
Dr. Chomilo had no financial conflicts to disclose.
Structural racism and implicit bias are connected, and both must be addressed to move from awareness of racism to action, said Nathan Chomilo, MD, of HealthPartners/Park Nicollet, Brooklyn Center, Minn., in a presentation at the virtual Pediatric Hospital Medicine annual conference.
“We need pediatricians with the courage to address racism head on,” he said.
One step in moving from awareness to action against structural and institutional racism in medicine is examining policies, Dr. Chomilo said. He cited the creation of Medicare and Medicaid in 1965 as examples of how policy changes can make a difference, illustrated by data from 1955-1975 that showed a significant decrease in infant deaths among Black infants in Mississippi after 1965.
Medicaid expansion has helped to narrow, but not eliminate, racial disparities in health care, Dr. Chomilo said. The impact of Medicare and Medicaid is evident in the current COVID-19 pandemic, as county level data show that areas where more than 25% of the population are uninsured have higher rates of COVID-19 infections, said Dr. Chomilo. Policies that impact access to care also impact their incidence of chronic diseases and risk for severe disease, he noted.
“If you don’t have ready access to a health care provider, you don’t have access to the vaccine, and you don’t have information that would inform your getting the vaccine,” he added.
Prioritizing the power of voting
“Voting is one of many ways we can impact structural racism in health care policy,” Dr. Chomilo emphasized.
However, voting inequity remains a challenge, Dr. Chomilo noted. Community level disparities lead to inequity in voting access and subsequent disparities in voter participation, he said. “Leaders are less responsive to nonvoting constituents,” which can result in policies that impact health inequitably, and loop back to community level health disparities, he explained.
Historically, physicians have had an 8%-9% lower voter turnout than the general public, although this may have changed in recent elections, Dr. Chomilo said. He encouraged all clinicians to set an example and vote, and to empower their patients to vote. Evidence shows that enfranchisement of Black voters is associated with reductions in education gaps for Blacks and Whites, and that enfranchisement of women is associated with increased spending on children and lower child mortality, he said. Dr. Chomilo encouraged pediatricians and all clinicians to take advantage of the resources on voting available from the American Academy of Pediatrics (aap.org/votekids).
“When we see more people in a community vote, leaders are more responsive to their needs,” he said.
Informing racial identity
“Racial identity is informed by racial socialization,” Dr. Chomilo said. “All of us are socialized along the lines of race; it happens in conversations with parents, family, peers, community.” Another point in moving from awareness to action in eliminating structural racism is recognizing that children are not too young to talk about race, Dr. Chomilo emphasized.
Children start to navigate racial identity and to take note of other differences at an early age. For example, a 3-year-old might ask, “why does that person talk funny, why is that person being pushed in a chair?” Dr. Chomilo said, and it is important for parents and as pediatricians to be prepared for these questions, which are part of normal development. As children get older, they start to reflect on what differences mean for them, which is not rooted in anything negative, he noted.
Children first develop racial identity at home, but children solidify their identities in child care and school settings, Dr. Chomilo said. The American Academy of Pediatrics has acknowledged the potential for racial bias in education and child care, and said in a statement that, “it is critical for pediatricians to recognize the institutional personally mediated, and internalized levels of racism that occur in the educational setting, because education is a critical social determinant of health for children.” In fact, data from children in preschool show that they use racial categories to identify themselves and others, to include or exclude children from activities, and to negotiate power in their social and play networks.
Early intervention matters in educating children about racism, Dr. Chomilo said. “If we were not taught to talk about race, it is on us to learn about it ourselves as well,” he said.
Ultimately, the goal is to create active antiracism among adults and children, said Dr. Chomilo. He encouraged pediatricians and parents not to shut down or discourage children when they raise questions of race, but to take the opportunity to teach. “There may be hurt feelings around what a child said, even if they didn’t mean to offend someone,” he noted. Take the topic seriously, and make racism conversations ongoing; teach children to safely oppose negative messages and behaviors in others, and replace them with something positive, he emphasized.
Addressing bias in clinical settings
Dr. Chomilo also encouraged hospitalists to consider internalized racism in clinical settings and take action to build confidence and cultural pride in all patients by ensuring that a pediatric hospital unit is welcoming and representative of the diversity in a given community, with appropriate options for books, movies, and toys. He also encouraged pediatric hospitalists to assess children for experiences of racism as part of a social assessment. Be aware of signs of posttraumatic stress, anxiety, depression, or grief that might have a racial component, he said.
Dr. Chomilo had no financial conflicts to disclose.
FROM PHM 2021
Insurance coverage for vitiligo varies widely in the U.S., analysis finds
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
FROM PEDIATRIC DERMATOLOGY