User login
Children aged 12-15 years continue to close COVID-19 vaccination gap
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.
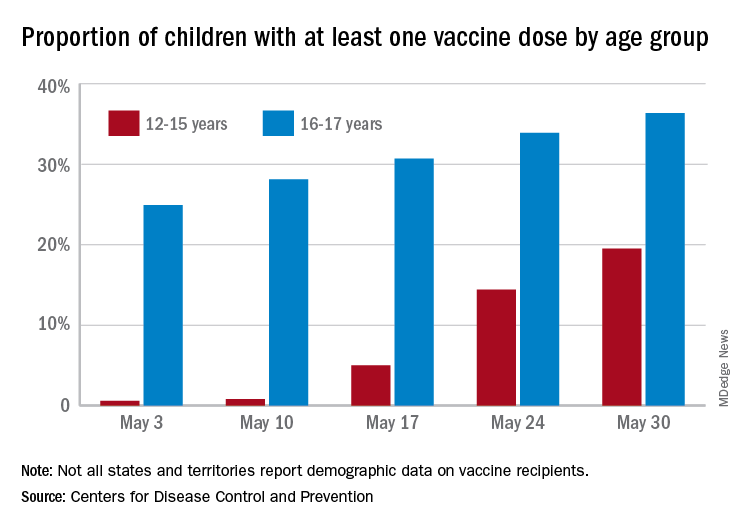
with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
Hospitalists innovate in ICU management
With intensive care units stretched to their limits – and beyond – during the COVID-19 pandemic, hospitalists became more central than ever in orchestrating the response.
At SHM Converge, the annual conference of the Society of Hospital Medicine, two hospitalists shared how their teams helped to develop new critical care units and strategies for best managing and allocating care to COVID patients in the ICU.
“The pandemic has been a selective pressure on us as a specialty,” said Jason Stein, MD, SFHM, a full-time clinical hospitalist at Roper Hospital, a 332-bed facility in Charleston, S.C.
Dr. Stein explained how hospitalists at Roper helped create the Progressive Care Unit – a negative-pressure unit with 12 high-flow oxygen beds overseen by a hospital medicine team, with the help of a respiratory therapist, pharmacist, and nurses. Patients in this unit had escalating acuity – quickly increasing oxygen needs – or deescalating acuity, such as ICU transfers, Dr. Stein said. Cardiac catheterization space was converted for the unit, which was intended to preserve beds in the hospital ICU for patients needing mechanical ventilation or vasoactive medication.
Interdisciplinary rounds – to assess oxygen and inflammatory marker trends, and run through a COVID care checklist – took place every day at 10 a.m.
“Consistency was the key,” Dr. Stein said.
At Weill Cornell Medical Center in New York, hospitalists helped build the COVID Recovery Unit, which was dedicated to the care of patients coming out of the ICU, said Vishwas Anand Singh, MD, MS, FHM, cochief of hospital medicine at New York Presbyterian–Lower Manhattan Hospital.
“The pandemic created an unprecedented need for critical care, and post-ICU care,” Dr. Singh said. “After extubation, patients remain very complicated and they have unique needs.”
The 30-bed COVID Recovery Unit – converted from a behavioral health unit – was designed to meet those needs. It was staffed by one lead hospitalist, 3 hospitalist physicians, 3 advanced practitioners, about 12 nurses and a neurologist, psychiatrist, and neuropsychologist.
The idea was to integrate medical care with careful attention to rehab and neuropsychological needs, Dr. Singh said. To be in the unit, patients had to be medically stable but with ongoing medical and rehabilitation needs and able to tolerate about half an hour of physical or occupational therapy each day.
The space was set up so that patients could interact with each other as well as staff, and this ability to share their experiences of trauma and recovery “led to an improved sense of psychological well-being and to healing,” according to Dr. Singh. Group therapy and meditation were also held several times a week.
“All this together, we thought we were really meeting the need for a lot of these patients from medical to psychosocial,” he said.
New York Presbyterian––Lower Manhattan Hospital also established a program called ICU Outreach to give hospitalists a “bird’s eye view” of the ICU in order to help move patients from unit to unit for optimized care. One hospitalist acted as a bridge between the ICU, the floors, and the emergency room.
The hospitalist on duty touched based with the ICU each day at 10 a.m., assessed the available beds, compiled a list of patients being discharged, met with all of the hospitalists and individual teams in inpatient and emergency services, and compiled a list of “watchers” – the sickest patients who needed help being managed.
The broad perspective was important, Dr. Singh said.
“We quickly found that each individual team or provider only knew the patients they were caring for, and the ICU Outreach person knew the whole big picture and could put the pieces together,” he said. “They could answer who was next in line for a bed, who benefited from a goals of care discussion, who could be managed on the floor with assistance. And this bridge, having this person fill this role, allowed the intensivists to focus on the patients they had in the unit.”
Palliative care and patient flow
Dr. Singh also described how hospitalists played an important role in palliative care for COVID patients. The hospital medicine team offered hospitalist palliative care services, which included COVIDtalk, a course on communicating about end of life, which helped to expand the pool of palliative care providers. Those trained were taught that these difficult conversations had to be honest and clear, with the goals of care addressed very early in the admission, should a patient decompensate soon after arrival.
A palliative “rapid response team” included a virtual hospitalist, a palliative care nurse practitioner, and a virtual psychiatrist – a team available 24 hours a day to have longer conversations so that clinicians could better tend to their patients when the in-person palliative care service was stretched thin, or at off hours like the middle of the night.
These innovations not only helped serve patients and families better, but also gave hospitalists training and experience in palliative care.
At Roper Hospital, Dr. Stein explained how hospitalists helped improve management of COVID patient flow. Depending on the time of day and the staffing on duty, there could be considerable confusion about where patients should go after the ED, or the COVID progressive unit, or the floor.
Hospitalists helped develop hospitalwide algorithms for escalating and deescalating acuity, Dr. Stein said, providing a “shared mental model for where a patient should go.”
“There are many ways hospitalists can and did rise to meet the unique demands of COVID,” Dr. Singh said, “whether it was innovating a new unit or service or work flow or leading a multidisciplinary team to extend or support other services that may have been strained.”
With intensive care units stretched to their limits – and beyond – during the COVID-19 pandemic, hospitalists became more central than ever in orchestrating the response.
At SHM Converge, the annual conference of the Society of Hospital Medicine, two hospitalists shared how their teams helped to develop new critical care units and strategies for best managing and allocating care to COVID patients in the ICU.
“The pandemic has been a selective pressure on us as a specialty,” said Jason Stein, MD, SFHM, a full-time clinical hospitalist at Roper Hospital, a 332-bed facility in Charleston, S.C.
Dr. Stein explained how hospitalists at Roper helped create the Progressive Care Unit – a negative-pressure unit with 12 high-flow oxygen beds overseen by a hospital medicine team, with the help of a respiratory therapist, pharmacist, and nurses. Patients in this unit had escalating acuity – quickly increasing oxygen needs – or deescalating acuity, such as ICU transfers, Dr. Stein said. Cardiac catheterization space was converted for the unit, which was intended to preserve beds in the hospital ICU for patients needing mechanical ventilation or vasoactive medication.
Interdisciplinary rounds – to assess oxygen and inflammatory marker trends, and run through a COVID care checklist – took place every day at 10 a.m.
“Consistency was the key,” Dr. Stein said.
At Weill Cornell Medical Center in New York, hospitalists helped build the COVID Recovery Unit, which was dedicated to the care of patients coming out of the ICU, said Vishwas Anand Singh, MD, MS, FHM, cochief of hospital medicine at New York Presbyterian–Lower Manhattan Hospital.
“The pandemic created an unprecedented need for critical care, and post-ICU care,” Dr. Singh said. “After extubation, patients remain very complicated and they have unique needs.”
The 30-bed COVID Recovery Unit – converted from a behavioral health unit – was designed to meet those needs. It was staffed by one lead hospitalist, 3 hospitalist physicians, 3 advanced practitioners, about 12 nurses and a neurologist, psychiatrist, and neuropsychologist.
The idea was to integrate medical care with careful attention to rehab and neuropsychological needs, Dr. Singh said. To be in the unit, patients had to be medically stable but with ongoing medical and rehabilitation needs and able to tolerate about half an hour of physical or occupational therapy each day.
The space was set up so that patients could interact with each other as well as staff, and this ability to share their experiences of trauma and recovery “led to an improved sense of psychological well-being and to healing,” according to Dr. Singh. Group therapy and meditation were also held several times a week.
“All this together, we thought we were really meeting the need for a lot of these patients from medical to psychosocial,” he said.
New York Presbyterian––Lower Manhattan Hospital also established a program called ICU Outreach to give hospitalists a “bird’s eye view” of the ICU in order to help move patients from unit to unit for optimized care. One hospitalist acted as a bridge between the ICU, the floors, and the emergency room.
The hospitalist on duty touched based with the ICU each day at 10 a.m., assessed the available beds, compiled a list of patients being discharged, met with all of the hospitalists and individual teams in inpatient and emergency services, and compiled a list of “watchers” – the sickest patients who needed help being managed.
The broad perspective was important, Dr. Singh said.
“We quickly found that each individual team or provider only knew the patients they were caring for, and the ICU Outreach person knew the whole big picture and could put the pieces together,” he said. “They could answer who was next in line for a bed, who benefited from a goals of care discussion, who could be managed on the floor with assistance. And this bridge, having this person fill this role, allowed the intensivists to focus on the patients they had in the unit.”
Palliative care and patient flow
Dr. Singh also described how hospitalists played an important role in palliative care for COVID patients. The hospital medicine team offered hospitalist palliative care services, which included COVIDtalk, a course on communicating about end of life, which helped to expand the pool of palliative care providers. Those trained were taught that these difficult conversations had to be honest and clear, with the goals of care addressed very early in the admission, should a patient decompensate soon after arrival.
A palliative “rapid response team” included a virtual hospitalist, a palliative care nurse practitioner, and a virtual psychiatrist – a team available 24 hours a day to have longer conversations so that clinicians could better tend to their patients when the in-person palliative care service was stretched thin, or at off hours like the middle of the night.
These innovations not only helped serve patients and families better, but also gave hospitalists training and experience in palliative care.
At Roper Hospital, Dr. Stein explained how hospitalists helped improve management of COVID patient flow. Depending on the time of day and the staffing on duty, there could be considerable confusion about where patients should go after the ED, or the COVID progressive unit, or the floor.
Hospitalists helped develop hospitalwide algorithms for escalating and deescalating acuity, Dr. Stein said, providing a “shared mental model for where a patient should go.”
“There are many ways hospitalists can and did rise to meet the unique demands of COVID,” Dr. Singh said, “whether it was innovating a new unit or service or work flow or leading a multidisciplinary team to extend or support other services that may have been strained.”
With intensive care units stretched to their limits – and beyond – during the COVID-19 pandemic, hospitalists became more central than ever in orchestrating the response.
At SHM Converge, the annual conference of the Society of Hospital Medicine, two hospitalists shared how their teams helped to develop new critical care units and strategies for best managing and allocating care to COVID patients in the ICU.
“The pandemic has been a selective pressure on us as a specialty,” said Jason Stein, MD, SFHM, a full-time clinical hospitalist at Roper Hospital, a 332-bed facility in Charleston, S.C.
Dr. Stein explained how hospitalists at Roper helped create the Progressive Care Unit – a negative-pressure unit with 12 high-flow oxygen beds overseen by a hospital medicine team, with the help of a respiratory therapist, pharmacist, and nurses. Patients in this unit had escalating acuity – quickly increasing oxygen needs – or deescalating acuity, such as ICU transfers, Dr. Stein said. Cardiac catheterization space was converted for the unit, which was intended to preserve beds in the hospital ICU for patients needing mechanical ventilation or vasoactive medication.
Interdisciplinary rounds – to assess oxygen and inflammatory marker trends, and run through a COVID care checklist – took place every day at 10 a.m.
“Consistency was the key,” Dr. Stein said.
At Weill Cornell Medical Center in New York, hospitalists helped build the COVID Recovery Unit, which was dedicated to the care of patients coming out of the ICU, said Vishwas Anand Singh, MD, MS, FHM, cochief of hospital medicine at New York Presbyterian–Lower Manhattan Hospital.
“The pandemic created an unprecedented need for critical care, and post-ICU care,” Dr. Singh said. “After extubation, patients remain very complicated and they have unique needs.”
The 30-bed COVID Recovery Unit – converted from a behavioral health unit – was designed to meet those needs. It was staffed by one lead hospitalist, 3 hospitalist physicians, 3 advanced practitioners, about 12 nurses and a neurologist, psychiatrist, and neuropsychologist.
The idea was to integrate medical care with careful attention to rehab and neuropsychological needs, Dr. Singh said. To be in the unit, patients had to be medically stable but with ongoing medical and rehabilitation needs and able to tolerate about half an hour of physical or occupational therapy each day.
The space was set up so that patients could interact with each other as well as staff, and this ability to share their experiences of trauma and recovery “led to an improved sense of psychological well-being and to healing,” according to Dr. Singh. Group therapy and meditation were also held several times a week.
“All this together, we thought we were really meeting the need for a lot of these patients from medical to psychosocial,” he said.
New York Presbyterian––Lower Manhattan Hospital also established a program called ICU Outreach to give hospitalists a “bird’s eye view” of the ICU in order to help move patients from unit to unit for optimized care. One hospitalist acted as a bridge between the ICU, the floors, and the emergency room.
The hospitalist on duty touched based with the ICU each day at 10 a.m., assessed the available beds, compiled a list of patients being discharged, met with all of the hospitalists and individual teams in inpatient and emergency services, and compiled a list of “watchers” – the sickest patients who needed help being managed.
The broad perspective was important, Dr. Singh said.
“We quickly found that each individual team or provider only knew the patients they were caring for, and the ICU Outreach person knew the whole big picture and could put the pieces together,” he said. “They could answer who was next in line for a bed, who benefited from a goals of care discussion, who could be managed on the floor with assistance. And this bridge, having this person fill this role, allowed the intensivists to focus on the patients they had in the unit.”
Palliative care and patient flow
Dr. Singh also described how hospitalists played an important role in palliative care for COVID patients. The hospital medicine team offered hospitalist palliative care services, which included COVIDtalk, a course on communicating about end of life, which helped to expand the pool of palliative care providers. Those trained were taught that these difficult conversations had to be honest and clear, with the goals of care addressed very early in the admission, should a patient decompensate soon after arrival.
A palliative “rapid response team” included a virtual hospitalist, a palliative care nurse practitioner, and a virtual psychiatrist – a team available 24 hours a day to have longer conversations so that clinicians could better tend to their patients when the in-person palliative care service was stretched thin, or at off hours like the middle of the night.
These innovations not only helped serve patients and families better, but also gave hospitalists training and experience in palliative care.
At Roper Hospital, Dr. Stein explained how hospitalists helped improve management of COVID patient flow. Depending on the time of day and the staffing on duty, there could be considerable confusion about where patients should go after the ED, or the COVID progressive unit, or the floor.
Hospitalists helped develop hospitalwide algorithms for escalating and deescalating acuity, Dr. Stein said, providing a “shared mental model for where a patient should go.”
“There are many ways hospitalists can and did rise to meet the unique demands of COVID,” Dr. Singh said, “whether it was innovating a new unit or service or work flow or leading a multidisciplinary team to extend or support other services that may have been strained.”
FROM SHM CONVERGE 2021
Psychiatric fallout from long-COVID: How to prepare
As mounting evidence points to a significant psychiatric component of COVID-19, experts are concerned about an influx of survivors presenting with persistent mental health problems and how best to prepare.
Clinicians should be aware that patients who have had COVID frequently develop psychiatric symptoms, Silvia S. Martins, MD, PhD, associate professor of epidemiology, Columbia University, New York, said in an interview.
“There should be more screening of all patients recovering from a COVID infection for anxiety, posttraumatic stress disorder, and depression, as well as referral to services, including psychotherapy, and medication as needed,” said Dr. Martins, who, along with colleagues, uncovered a high rate of these symptoms in patients who had the disease.
The COVID-19 pandemic has taken an enormous social, emotional, and public health toll. It has disrupted lives and caused stress, fear, and uncertainty about loss of health and income, not to mention forced isolation.
In addition, a significant number of patients who contract COVID-19 continue to have symptoms after the acute phase of the illness. This post-COVID, or “long-haul,” syndrome isn’t well defined; experts cite a range of symptoms that persist for weeks or months.
These ongoing symptoms can include cough, fatigue, and chronic pain, as well as psychiatric complaints. As reported by this news organization, an observational study of more than 230,000 U.S. patient health records revealed that one in three COVID-19 survivors received a psychiatric or neurologic diagnosis within 6 months of contracting the virus.
The most common psychiatric diagnoses were anxiety disorders, mood disorders, substance misuse disorders, and insomnia.
Significant symptoms even in mild cases
Another study showed that even those with mild COVID-19 may experience psychiatric symptoms independently of previous psychiatric diagnoses. Results revealed that 26% of the sample of almost 900 patients reported depression, 22% reported anxiety, and 17% reported symptoms of posttraumatic stress 2 months after testing positive for the virus. This finding is important because the majority of individuals who contract COVID-19 have a mild case.
“We saw very high levels of clinically significant depression, anxiety, and posttraumatic stress symptoms in people who had mild disease,” study investigator João Mauricio Castaldelli-Maia, MD, PhD, postdoctoral fellow, department of epidemiology, Columbia University, said in an interview.
He attributed these symptoms in part to long periods of isolation, even from relatives in the same household, in cramped spaces typical of large cities such as São Paulo.
Social isolation can have a huge impact on persons who depend on social connections and relationships, Vivian Pender, MD, president of the American Psychiatric Association and clinical professor of psychiatry, Weill Cornell Medical Center, New York, said in an interview.
“The fact that we have not been able to see our colleagues, our friends, our family, and in the case of psychiatrists, even our patients has taken a toll on everyone, and that leads to more stress, more anxiety,” she said.
National surveys show that psychiatric symptoms occur after acute COVID. One survey revealed that over 50% of 3,900 respondents who had COVID reported having at least moderate symptoms of major depression.
Unique depression subtype?
Another survey, slated for publication later this year, shows that lead investigator Roy Perlis, MD, professor of psychiatry, Harvard Medical School, Boston, said in an interview.
This might suggest a neurobiological element. Researchers are speculating as to whether lingering psychiatric problems that occur after having COVID are linked to the psychosocial impact of the disease or to pathological processes, such as inflammation, that affect the brain.
Although rates of post-COVID psychiatric symptoms vary from study to study, “they seem to be pretty enduring,” noted Faith Gunning, PhD, vice chair of research, department of psychology, Weill Cornell Medicine, who specializes in clinical neuropsychology.
“So they’re not just a brief response” to getting sick, a fact that points to the possible need for treatment, she told this news organization. “In some of the work that’s starting to emerge, it does appear that the symptoms persist, at least for a relatively large subset of individuals.”
Although depression typically affects twice as many women as men, these new surveys show that, after COVID, “that difference is not so distinct,” said Dr. Gunning.
It’s unclear why this is, but it could be cause by financial stresses that may affect men to a greater extent, she added. “There is so much we’re still learning.”
Increased suicide risk?
Other researchers, including Leo Sher, MD, professor of psychiatry, Icahn School of Medicine at Mount Sinai, and director of inpatient psychiatry, James J. Peters Veterans Affairs Medical Center, both in New York, are concerned that higher rates of psychiatric symptoms among patients with long-haul COVID raise the risk for suicidal ideation and behavior.
Studies of suicidality in COVID-19 survivors “are urgently needed,” said Dr. Sher in an article published in the Monthly Journal of the Association of Physicians.
“We need to study what factors may increase suicide risk among the COVID-19 survivors during and after the recovery. We also need to investigate whether there is a long-term increased suicide risk among COVID-19 survivors,” Dr. Sher said.
COVID-19 is not unique among viral respiratory diseases in being associated with long-term mental health problems. Research shows that survivors of the 2003 outbreak of severe acute respiratory syndrome experienced increased psychological distress that persisted for at least a year, as did patients who in 2015 had Middle East respiratory syndrome coronavirus (MERS-CoV).
Some experts believe clinicians should screen patients for mental health symptoms after the acute phase of COVID and offer early and prolonged care.
“Early mental health intervention such as psychotherapy and supportive groups could play an important role in preventing incident mental health problems for post-COVID sufferers,” said Dr. Castaldelli-Maia.
A version of this article first appeared on Medscape.com.
As mounting evidence points to a significant psychiatric component of COVID-19, experts are concerned about an influx of survivors presenting with persistent mental health problems and how best to prepare.
Clinicians should be aware that patients who have had COVID frequently develop psychiatric symptoms, Silvia S. Martins, MD, PhD, associate professor of epidemiology, Columbia University, New York, said in an interview.
“There should be more screening of all patients recovering from a COVID infection for anxiety, posttraumatic stress disorder, and depression, as well as referral to services, including psychotherapy, and medication as needed,” said Dr. Martins, who, along with colleagues, uncovered a high rate of these symptoms in patients who had the disease.
The COVID-19 pandemic has taken an enormous social, emotional, and public health toll. It has disrupted lives and caused stress, fear, and uncertainty about loss of health and income, not to mention forced isolation.
In addition, a significant number of patients who contract COVID-19 continue to have symptoms after the acute phase of the illness. This post-COVID, or “long-haul,” syndrome isn’t well defined; experts cite a range of symptoms that persist for weeks or months.
These ongoing symptoms can include cough, fatigue, and chronic pain, as well as psychiatric complaints. As reported by this news organization, an observational study of more than 230,000 U.S. patient health records revealed that one in three COVID-19 survivors received a psychiatric or neurologic diagnosis within 6 months of contracting the virus.
The most common psychiatric diagnoses were anxiety disorders, mood disorders, substance misuse disorders, and insomnia.
Significant symptoms even in mild cases
Another study showed that even those with mild COVID-19 may experience psychiatric symptoms independently of previous psychiatric diagnoses. Results revealed that 26% of the sample of almost 900 patients reported depression, 22% reported anxiety, and 17% reported symptoms of posttraumatic stress 2 months after testing positive for the virus. This finding is important because the majority of individuals who contract COVID-19 have a mild case.
“We saw very high levels of clinically significant depression, anxiety, and posttraumatic stress symptoms in people who had mild disease,” study investigator João Mauricio Castaldelli-Maia, MD, PhD, postdoctoral fellow, department of epidemiology, Columbia University, said in an interview.
He attributed these symptoms in part to long periods of isolation, even from relatives in the same household, in cramped spaces typical of large cities such as São Paulo.
Social isolation can have a huge impact on persons who depend on social connections and relationships, Vivian Pender, MD, president of the American Psychiatric Association and clinical professor of psychiatry, Weill Cornell Medical Center, New York, said in an interview.
“The fact that we have not been able to see our colleagues, our friends, our family, and in the case of psychiatrists, even our patients has taken a toll on everyone, and that leads to more stress, more anxiety,” she said.
National surveys show that psychiatric symptoms occur after acute COVID. One survey revealed that over 50% of 3,900 respondents who had COVID reported having at least moderate symptoms of major depression.
Unique depression subtype?
Another survey, slated for publication later this year, shows that lead investigator Roy Perlis, MD, professor of psychiatry, Harvard Medical School, Boston, said in an interview.
This might suggest a neurobiological element. Researchers are speculating as to whether lingering psychiatric problems that occur after having COVID are linked to the psychosocial impact of the disease or to pathological processes, such as inflammation, that affect the brain.
Although rates of post-COVID psychiatric symptoms vary from study to study, “they seem to be pretty enduring,” noted Faith Gunning, PhD, vice chair of research, department of psychology, Weill Cornell Medicine, who specializes in clinical neuropsychology.
“So they’re not just a brief response” to getting sick, a fact that points to the possible need for treatment, she told this news organization. “In some of the work that’s starting to emerge, it does appear that the symptoms persist, at least for a relatively large subset of individuals.”
Although depression typically affects twice as many women as men, these new surveys show that, after COVID, “that difference is not so distinct,” said Dr. Gunning.
It’s unclear why this is, but it could be cause by financial stresses that may affect men to a greater extent, she added. “There is so much we’re still learning.”
Increased suicide risk?
Other researchers, including Leo Sher, MD, professor of psychiatry, Icahn School of Medicine at Mount Sinai, and director of inpatient psychiatry, James J. Peters Veterans Affairs Medical Center, both in New York, are concerned that higher rates of psychiatric symptoms among patients with long-haul COVID raise the risk for suicidal ideation and behavior.
Studies of suicidality in COVID-19 survivors “are urgently needed,” said Dr. Sher in an article published in the Monthly Journal of the Association of Physicians.
“We need to study what factors may increase suicide risk among the COVID-19 survivors during and after the recovery. We also need to investigate whether there is a long-term increased suicide risk among COVID-19 survivors,” Dr. Sher said.
COVID-19 is not unique among viral respiratory diseases in being associated with long-term mental health problems. Research shows that survivors of the 2003 outbreak of severe acute respiratory syndrome experienced increased psychological distress that persisted for at least a year, as did patients who in 2015 had Middle East respiratory syndrome coronavirus (MERS-CoV).
Some experts believe clinicians should screen patients for mental health symptoms after the acute phase of COVID and offer early and prolonged care.
“Early mental health intervention such as psychotherapy and supportive groups could play an important role in preventing incident mental health problems for post-COVID sufferers,” said Dr. Castaldelli-Maia.
A version of this article first appeared on Medscape.com.
As mounting evidence points to a significant psychiatric component of COVID-19, experts are concerned about an influx of survivors presenting with persistent mental health problems and how best to prepare.
Clinicians should be aware that patients who have had COVID frequently develop psychiatric symptoms, Silvia S. Martins, MD, PhD, associate professor of epidemiology, Columbia University, New York, said in an interview.
“There should be more screening of all patients recovering from a COVID infection for anxiety, posttraumatic stress disorder, and depression, as well as referral to services, including psychotherapy, and medication as needed,” said Dr. Martins, who, along with colleagues, uncovered a high rate of these symptoms in patients who had the disease.
The COVID-19 pandemic has taken an enormous social, emotional, and public health toll. It has disrupted lives and caused stress, fear, and uncertainty about loss of health and income, not to mention forced isolation.
In addition, a significant number of patients who contract COVID-19 continue to have symptoms after the acute phase of the illness. This post-COVID, or “long-haul,” syndrome isn’t well defined; experts cite a range of symptoms that persist for weeks or months.
These ongoing symptoms can include cough, fatigue, and chronic pain, as well as psychiatric complaints. As reported by this news organization, an observational study of more than 230,000 U.S. patient health records revealed that one in three COVID-19 survivors received a psychiatric or neurologic diagnosis within 6 months of contracting the virus.
The most common psychiatric diagnoses were anxiety disorders, mood disorders, substance misuse disorders, and insomnia.
Significant symptoms even in mild cases
Another study showed that even those with mild COVID-19 may experience psychiatric symptoms independently of previous psychiatric diagnoses. Results revealed that 26% of the sample of almost 900 patients reported depression, 22% reported anxiety, and 17% reported symptoms of posttraumatic stress 2 months after testing positive for the virus. This finding is important because the majority of individuals who contract COVID-19 have a mild case.
“We saw very high levels of clinically significant depression, anxiety, and posttraumatic stress symptoms in people who had mild disease,” study investigator João Mauricio Castaldelli-Maia, MD, PhD, postdoctoral fellow, department of epidemiology, Columbia University, said in an interview.
He attributed these symptoms in part to long periods of isolation, even from relatives in the same household, in cramped spaces typical of large cities such as São Paulo.
Social isolation can have a huge impact on persons who depend on social connections and relationships, Vivian Pender, MD, president of the American Psychiatric Association and clinical professor of psychiatry, Weill Cornell Medical Center, New York, said in an interview.
“The fact that we have not been able to see our colleagues, our friends, our family, and in the case of psychiatrists, even our patients has taken a toll on everyone, and that leads to more stress, more anxiety,” she said.
National surveys show that psychiatric symptoms occur after acute COVID. One survey revealed that over 50% of 3,900 respondents who had COVID reported having at least moderate symptoms of major depression.
Unique depression subtype?
Another survey, slated for publication later this year, shows that lead investigator Roy Perlis, MD, professor of psychiatry, Harvard Medical School, Boston, said in an interview.
This might suggest a neurobiological element. Researchers are speculating as to whether lingering psychiatric problems that occur after having COVID are linked to the psychosocial impact of the disease or to pathological processes, such as inflammation, that affect the brain.
Although rates of post-COVID psychiatric symptoms vary from study to study, “they seem to be pretty enduring,” noted Faith Gunning, PhD, vice chair of research, department of psychology, Weill Cornell Medicine, who specializes in clinical neuropsychology.
“So they’re not just a brief response” to getting sick, a fact that points to the possible need for treatment, she told this news organization. “In some of the work that’s starting to emerge, it does appear that the symptoms persist, at least for a relatively large subset of individuals.”
Although depression typically affects twice as many women as men, these new surveys show that, after COVID, “that difference is not so distinct,” said Dr. Gunning.
It’s unclear why this is, but it could be cause by financial stresses that may affect men to a greater extent, she added. “There is so much we’re still learning.”
Increased suicide risk?
Other researchers, including Leo Sher, MD, professor of psychiatry, Icahn School of Medicine at Mount Sinai, and director of inpatient psychiatry, James J. Peters Veterans Affairs Medical Center, both in New York, are concerned that higher rates of psychiatric symptoms among patients with long-haul COVID raise the risk for suicidal ideation and behavior.
Studies of suicidality in COVID-19 survivors “are urgently needed,” said Dr. Sher in an article published in the Monthly Journal of the Association of Physicians.
“We need to study what factors may increase suicide risk among the COVID-19 survivors during and after the recovery. We also need to investigate whether there is a long-term increased suicide risk among COVID-19 survivors,” Dr. Sher said.
COVID-19 is not unique among viral respiratory diseases in being associated with long-term mental health problems. Research shows that survivors of the 2003 outbreak of severe acute respiratory syndrome experienced increased psychological distress that persisted for at least a year, as did patients who in 2015 had Middle East respiratory syndrome coronavirus (MERS-CoV).
Some experts believe clinicians should screen patients for mental health symptoms after the acute phase of COVID and offer early and prolonged care.
“Early mental health intervention such as psychotherapy and supportive groups could play an important role in preventing incident mental health problems for post-COVID sufferers,” said Dr. Castaldelli-Maia.
A version of this article first appeared on Medscape.com.
How COVID-19 affects peripartum women’s mental health
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
GI symptoms and chronic fatigue may persist months after COVID-19
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
Gastrointestinal symptoms and chronic fatigue may persist months after the COVID-19 virus infection resolves, results of a recent cohort-controlled study suggest.
About 5 months after SARS-CoV-2 infection, relative risks of loose stools, somatization, and chronic fatigue were increased by approximately two- to three fold, compared to individuals who had not been infected, according to study results presented at the annual Digestive Disease Week® (DDW).
These longer-term consequences of SARS-CoV-2 appeared to be more severe in patients who had experienced diarrhea during the acute infection, according to investigator Daniele Noviello, MD, a second-year resident in gastroenterology and hepatology at the University of Milan.
This is the first cohort-controlled study that specifically investigates gastrointestinal symptoms and somatoform disorders, Dr. Noviello said in a virtual presentation of the results.
“Based on our data, chronic fatigue, gastrointestinal, and somatoform symptoms may have a common postinfectious origin, and they should be investigated in the follow-up of SARS-CoV-2 patients,” he said.
Links between SARS-CoV-2 and gastrointestinal symptoms
Gastrointestinal symptoms are known to be relatively common during acute infection. According to Dr. Noviello, the most frequent gastrointestinal symptom associated with SARS-CoV-2 is diarrhea, occurring in 4% to nearly 40% of patients in case series to date.
However, data on the longer-term gastrointestinal impacts of SARS-CoV-2 remain scarce.
In one noncontrolled cohort study in China, loss of appetite, nausea, acid reflux, and diarrhea were seen in 15%-24% of patients 3 months after the infection, Dr. Noviello said. In another cohort study in China, diarrhea and vomiting were reported in 5% of patients 6 months after infection.
In any case, it is known that viral, bacterial, and protozoal infections of the gastrointestinal tract are a risk factor for development of functional disorders including irritable bowel syndrome (IBS), functional dyspepsia, and chronic fatigue, according to Dr. Noviello.
Accordingly, the results of the present study suggest that SARS-CoV-2 also “may affect the brain-gut axis in the long term,” Dr. Noviello and coauthors wrote in an abstract of the study.
It is plausible that SARS-CoV-2 infection could be a trigger for longer-term gastrointestinal symptoms, especially given the previous evidence linking infections and IBS symptoms, or postinfectious IBS, said Juan Pablo Stefanolo, MD, a physician with the neurogastroenterology and motility section, Hospital de Clínicas José de San Martín, Buenos Aires University.
“If it is demonstrated [that SARS-CoV-2 infection is a trigger], the microbiota-gut-brain axis concept in IBS pathophysiology is reinforced,” Dr. Stefanolo said in an interview.
In the meantime, practitioners may want to take into account COVID-19 infection history in the evaluation of a patient with IBS-like symptoms and, in case of a known positive COVID-19 result in an IBS patient, be aware of the possibility of symptom exacerbation, Dr. Stefanolo said.
Pandemic in Italy: Unique study opportunity
The severe outbreak in the Milan region early in the COVID-19 pandemic provided a “unique opportunity” to assess the long-term impact of infection on gastrointestinal and extraintestinal somatoform symptoms, said Dr. Noviello.
The investigators sent an online questionnaire to patients who had a molecular diagnosis of SARS-CoV-2 infection by nasal swab between February and April of 2020. To form a control group, they also sent questionnaires to hospital employees and health care providers who had tested negative over that same time period.
In all, 378 questionnaires were completed by 177 SARS-CoV-2–positive individuals and 201 controls. The SARS-CoV-2–positive patients were somewhat older (about 44 years vs. 40 years for controls), were less often female (40% vs. 61%), had a lower education level, and smoked less than did controls, according to the investigators.
A mean of 4.8 months had elapsed between the time of SARS-CoV-2 infection and when the questionnaires were compiled, said Dr. Noviello.
In the acute phase, diarrhea was the most common gastrointestinal symptom among virus-positive individuals, occurring in about 50% compared to 20% of controls (P < .001), data show. Other symptoms reported by 40% of SARS-CoV-2–infected individuals included fever, dyspnea, loss of smell or taste, weight loss, myalgia, arthralgia, and asthenia in the acute phase controls in the acute phase, Dr. Noviello said.
Persistent gastrointestinal symptoms after SARS-CoV-2
Persistent symptoms included loose stools, as measured by the Bristol Stool scale, occurring in 17.8% of SARS-CoV-2–positive individuals, but only 9.3% of the SARS-CoV-2–negative controls, according to Dr. Noviello, with an adjusted risk ratio of 1.88 (95% confidence interval, 0.99-3.54).
Chronic fatigue symptoms, as measured by the Structured Assessment of Gastrointestinal Symptoms questionnaire, were reported by about 30% of SARS-CoV-2–positive patients and about 15% of controls, for an adjusted risk ratio of 2.24 (95% CI, 1.48-3.37), according to Dr. Noviello’s presentation.
The mean t-score on the Symptom Checklist–12 for somatoform disorders was higher for the virus-positive patients compared to controls, according to Dr. Noviello. The scores were 54.6 and 50.5, respectively, with an adjusted score difference of 3.6 (95% CI, 1.0-6.2).
The longer-term sequelae of SARS-CoV-2 infection might be more severe in individuals who experienced diarrhea during acute infection, according to Dr. Noviello. In a post hoc analysis, reports of irritable bowel syndrome and loose stools were significantly higher in SARS-CoV-2–infected individuals who had diarrhea in the acute phase compared to those who did not experience diarrhea, he said.
Somatoform disorder scores were significantly higher, and reports of headache, back pain, and chronic fatigue were significantly more common, in individuals who had diarrhea at the time of SARS-CoV-2 infection, he added.
Dr. Noviello and coauthors reported no competing interests related to the study. Dr. Stefanolo had no disclosures to report.
FROM DDW 2021
COVID-19: One Patient at a Time
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
Obstructive sleep apnea linked to COVID-19 risk
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
FROM ATS 2021
Pandemic experience taught lessons about clinician wellness
As a member of the Society of Hospital Medicine Wellbeing Task Force, Mark Rudolph, MD, SFHM, thought he understood a thing or two about resilience, but nothing could prepare him for the vulnerability he felt when his parents became infected with COVID-19 following a visit to New York City in March 2020 – which soon became an epicenter of disease outbreak.
“They were both quite ill but fortunately they recovered,” Dr. Rudolph, chief experience officer for Sound Physicians said during SHM Converge, the annual conference of the Society of Hospital Medicine. He had completed his residency training in New York, where he cared for patients following the 9/11 terrorist attacks, “so I had a lot of PTSD related to all that stuff,” he recalled. Then he started to worry about the clinicians who work for Sound Physicians, a multispecialty group with roots in hospital medicine. “I found it difficult knowing there was someone in the hospital somewhere taking care of our patients all day long, all night long,” he said. “I felt fearful for them.”
Other members of the SHM Wellbeing Task Force shared challenges they faced during the pandemic’s early stages, as well as lessons learned. Task force chair Sarah Richards, MD, said the COVID-19 pandemic brought on feelings of guilt after hearing from fellow hospitalists about the surge of cases they were caring for, or that their best friend or colleague died by suicide. “I felt a sense of guilt because I didn’t have a loved one get COVID or die from COVID,” said Dr. Richards, a hospitalist at the University of Nebraska Medical Center in Omaha. “I felt like the world was crumbling around me and I was still okay. That guilt was almost like a helplessness. I didn’t know how make it better. I didn’t know how to help people because the problem was so big, especially during the height of the pandemic. That was tough for me because I’m a helper. I think we go into this field wanting to help and I feel like we didn’t know how to help make things better.”
Sonia George, MD, recalled first hearing about COVID-19 as she was preparing to attend the 2020 SHM annual conference in San Diego, which was planned for April but was canceled amid the escalating health concerns. “That was difficult for me, because I wanted to travel more in 2020,” said Dr. George, a hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y. “Traveling is something that I’ve been wanting to do ever since I finished residency, after all that training. I wanted to reward myself. What I have learned about myself is that I’ve learned to be more patient, to take every day as it is, to find some small moments of joy within each day and try to take that forward with me, and try to remember what I do have, and celebrate that a bit more every day.”
Over the past 14 months or so, Dr. Rudolph said that he grew to appreciate the importance of connecting with colleagues, “however short [the time] may be, where we can talk with one another, commiserate, discuss situations and experiences – whether virtually or in person. Those have been critical. If you add those all up, that’s what’s keeping us all going. At least it’s keeping me going.”
Dr. Richards echoed that sentiment. “The lesson I learned is that people really do want to share and to talk,” she said. “I can’t tell you how many times I told people about my [sense of] guilt and they would say things like, ‘Me, too!’ Knowing ‘it’s not just me’ made me feel so much better.”
During the course of the pandemic, the SHM Wellbeing Task Force created a one-page resource for clinicians known as the “Hospital Medicine COVID-19 Check-in Guide for Self & Peers,” which can be accessed here:. The three main recommended steps are to identify (“self-assess” to see if you are experiencing physical, emotional, cognitive, or behavioral stress); initiate (“reach out to your colleagues one-one-one or in small informal groups”); and intervene (“take action to make change or get help.”)
“Wellness and thriving are a team sport,” observed task force member Patrick Kneeland, MD, vice president of medical affairs at DispatchHealth, which provides hospital to home services. “It’s not an individual task to achieve. The team sport thing is complicated by gowns and masks and the lack of in-person meetings. You can’t even grab a cup of coffee with colleagues. That part has impacted most of us.” However, he said, he learned that clinicians can “double down on those small practices that form human connection” by using virtual communication platforms like Zoom. “For me, it’s been a great reminder [of] why presence with others matters, even if it’s in an unusual format, and how sharing our humanity across [communication] channels or through several layers of PPE is so critical.” Dr. Kneeland said.
None of the presenters reported having financial disclosures.
As a member of the Society of Hospital Medicine Wellbeing Task Force, Mark Rudolph, MD, SFHM, thought he understood a thing or two about resilience, but nothing could prepare him for the vulnerability he felt when his parents became infected with COVID-19 following a visit to New York City in March 2020 – which soon became an epicenter of disease outbreak.
“They were both quite ill but fortunately they recovered,” Dr. Rudolph, chief experience officer for Sound Physicians said during SHM Converge, the annual conference of the Society of Hospital Medicine. He had completed his residency training in New York, where he cared for patients following the 9/11 terrorist attacks, “so I had a lot of PTSD related to all that stuff,” he recalled. Then he started to worry about the clinicians who work for Sound Physicians, a multispecialty group with roots in hospital medicine. “I found it difficult knowing there was someone in the hospital somewhere taking care of our patients all day long, all night long,” he said. “I felt fearful for them.”
Other members of the SHM Wellbeing Task Force shared challenges they faced during the pandemic’s early stages, as well as lessons learned. Task force chair Sarah Richards, MD, said the COVID-19 pandemic brought on feelings of guilt after hearing from fellow hospitalists about the surge of cases they were caring for, or that their best friend or colleague died by suicide. “I felt a sense of guilt because I didn’t have a loved one get COVID or die from COVID,” said Dr. Richards, a hospitalist at the University of Nebraska Medical Center in Omaha. “I felt like the world was crumbling around me and I was still okay. That guilt was almost like a helplessness. I didn’t know how make it better. I didn’t know how to help people because the problem was so big, especially during the height of the pandemic. That was tough for me because I’m a helper. I think we go into this field wanting to help and I feel like we didn’t know how to help make things better.”
Sonia George, MD, recalled first hearing about COVID-19 as she was preparing to attend the 2020 SHM annual conference in San Diego, which was planned for April but was canceled amid the escalating health concerns. “That was difficult for me, because I wanted to travel more in 2020,” said Dr. George, a hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y. “Traveling is something that I’ve been wanting to do ever since I finished residency, after all that training. I wanted to reward myself. What I have learned about myself is that I’ve learned to be more patient, to take every day as it is, to find some small moments of joy within each day and try to take that forward with me, and try to remember what I do have, and celebrate that a bit more every day.”
Over the past 14 months or so, Dr. Rudolph said that he grew to appreciate the importance of connecting with colleagues, “however short [the time] may be, where we can talk with one another, commiserate, discuss situations and experiences – whether virtually or in person. Those have been critical. If you add those all up, that’s what’s keeping us all going. At least it’s keeping me going.”
Dr. Richards echoed that sentiment. “The lesson I learned is that people really do want to share and to talk,” she said. “I can’t tell you how many times I told people about my [sense of] guilt and they would say things like, ‘Me, too!’ Knowing ‘it’s not just me’ made me feel so much better.”
During the course of the pandemic, the SHM Wellbeing Task Force created a one-page resource for clinicians known as the “Hospital Medicine COVID-19 Check-in Guide for Self & Peers,” which can be accessed here:. The three main recommended steps are to identify (“self-assess” to see if you are experiencing physical, emotional, cognitive, or behavioral stress); initiate (“reach out to your colleagues one-one-one or in small informal groups”); and intervene (“take action to make change or get help.”)
“Wellness and thriving are a team sport,” observed task force member Patrick Kneeland, MD, vice president of medical affairs at DispatchHealth, which provides hospital to home services. “It’s not an individual task to achieve. The team sport thing is complicated by gowns and masks and the lack of in-person meetings. You can’t even grab a cup of coffee with colleagues. That part has impacted most of us.” However, he said, he learned that clinicians can “double down on those small practices that form human connection” by using virtual communication platforms like Zoom. “For me, it’s been a great reminder [of] why presence with others matters, even if it’s in an unusual format, and how sharing our humanity across [communication] channels or through several layers of PPE is so critical.” Dr. Kneeland said.
None of the presenters reported having financial disclosures.
As a member of the Society of Hospital Medicine Wellbeing Task Force, Mark Rudolph, MD, SFHM, thought he understood a thing or two about resilience, but nothing could prepare him for the vulnerability he felt when his parents became infected with COVID-19 following a visit to New York City in March 2020 – which soon became an epicenter of disease outbreak.
“They were both quite ill but fortunately they recovered,” Dr. Rudolph, chief experience officer for Sound Physicians said during SHM Converge, the annual conference of the Society of Hospital Medicine. He had completed his residency training in New York, where he cared for patients following the 9/11 terrorist attacks, “so I had a lot of PTSD related to all that stuff,” he recalled. Then he started to worry about the clinicians who work for Sound Physicians, a multispecialty group with roots in hospital medicine. “I found it difficult knowing there was someone in the hospital somewhere taking care of our patients all day long, all night long,” he said. “I felt fearful for them.”
Other members of the SHM Wellbeing Task Force shared challenges they faced during the pandemic’s early stages, as well as lessons learned. Task force chair Sarah Richards, MD, said the COVID-19 pandemic brought on feelings of guilt after hearing from fellow hospitalists about the surge of cases they were caring for, or that their best friend or colleague died by suicide. “I felt a sense of guilt because I didn’t have a loved one get COVID or die from COVID,” said Dr. Richards, a hospitalist at the University of Nebraska Medical Center in Omaha. “I felt like the world was crumbling around me and I was still okay. That guilt was almost like a helplessness. I didn’t know how make it better. I didn’t know how to help people because the problem was so big, especially during the height of the pandemic. That was tough for me because I’m a helper. I think we go into this field wanting to help and I feel like we didn’t know how to help make things better.”
Sonia George, MD, recalled first hearing about COVID-19 as she was preparing to attend the 2020 SHM annual conference in San Diego, which was planned for April but was canceled amid the escalating health concerns. “That was difficult for me, because I wanted to travel more in 2020,” said Dr. George, a hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y. “Traveling is something that I’ve been wanting to do ever since I finished residency, after all that training. I wanted to reward myself. What I have learned about myself is that I’ve learned to be more patient, to take every day as it is, to find some small moments of joy within each day and try to take that forward with me, and try to remember what I do have, and celebrate that a bit more every day.”
Over the past 14 months or so, Dr. Rudolph said that he grew to appreciate the importance of connecting with colleagues, “however short [the time] may be, where we can talk with one another, commiserate, discuss situations and experiences – whether virtually or in person. Those have been critical. If you add those all up, that’s what’s keeping us all going. At least it’s keeping me going.”
Dr. Richards echoed that sentiment. “The lesson I learned is that people really do want to share and to talk,” she said. “I can’t tell you how many times I told people about my [sense of] guilt and they would say things like, ‘Me, too!’ Knowing ‘it’s not just me’ made me feel so much better.”
During the course of the pandemic, the SHM Wellbeing Task Force created a one-page resource for clinicians known as the “Hospital Medicine COVID-19 Check-in Guide for Self & Peers,” which can be accessed here:. The three main recommended steps are to identify (“self-assess” to see if you are experiencing physical, emotional, cognitive, or behavioral stress); initiate (“reach out to your colleagues one-one-one or in small informal groups”); and intervene (“take action to make change or get help.”)
“Wellness and thriving are a team sport,” observed task force member Patrick Kneeland, MD, vice president of medical affairs at DispatchHealth, which provides hospital to home services. “It’s not an individual task to achieve. The team sport thing is complicated by gowns and masks and the lack of in-person meetings. You can’t even grab a cup of coffee with colleagues. That part has impacted most of us.” However, he said, he learned that clinicians can “double down on those small practices that form human connection” by using virtual communication platforms like Zoom. “For me, it’s been a great reminder [of] why presence with others matters, even if it’s in an unusual format, and how sharing our humanity across [communication] channels or through several layers of PPE is so critical.” Dr. Kneeland said.
None of the presenters reported having financial disclosures.
FROM SHM CONVERGE 2021
No-cancel culture: How telehealth is making it easier to keep that therapy session
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.














