User login
Giving flu and COVID-19 shots at same time appears safe, effective: Study
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
New AMA president discusses pandemic during inaugural address
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
He has encountered “all manner of unexpected situations” and feels “more than prepared” to serve as president of the American Medical Association, he said.
At the same time, “I still find myself a little nervous about it,” Dr. Harmon said in an interview the day after he was sworn in as president. “I would be less than candid if I didn’t tell you that. I don’t mean intimidated. ... It’s almost like before an athletic event.”
Dr. Harmon was sworn in June 15 as the 176th president of the AMA during the virtual Special Meeting of the AMA House of Delegates. He follows Susan R. Bailey, MD, an allergist from Fort Worth, Tex., in leading the organization, which has more than 270,000 members.
Advancing health equity
During his inaugural address, Dr. Harmon discussed the pandemic and the AMA’s plan to advance health equity.
COVID-19 “has revealed enormous gaps in how we care for people and communities in America, demonstrated in the disproportionate impact of this pandemic on communities of color and in the weaknesses of our underfunded and underresourced public health infrastructure,” Dr. Harmon said.
He described medical professionals as being “at war against seemingly formidable adversaries,” including the pandemic, the effects of prolonged isolation on emotional and behavioral health, and political and racial tension. There is an “immense battle to rid our health system – and society – of health disparities and racism,” he said. “As we face these battles, we must remember that our actions as physicians and as leaders will have far-reaching consequences.”
Other challenges before the AMA include vaccinating patients, recovering from the ongoing pandemic, removing unnecessary obstacles to care, ending an epidemic of drug overdoses, improving outcomes for patients with chronic disease, incorporating technology in ways that benefit doctors and patients, and preparing future physicians, Dr. Harmon noted.
“We are going to embed the principles of equity and racial justice within the AMA and throughout our health system,” added Dr. Harmon, who has been an AMA board member since 2013 and served as board chair from 2017 to 2018. He highlighted the AMA’s strategic plan, released in May 2021, to advance health equity and justice and improve the quality of care for people who have been marginalized.
“Meaningful progress won’t happen until we, as doctors, recognize how profoundly systemic racism influences the health of our patients, and until we commit to taking action within our own spheres of influence,” Dr. Harmon said. “As a family doctor in a very diverse state, I have treated people from all backgrounds, and have seen inequities up close, inequities that understandably lead to distrust.”
Commenting in an interview on JAMA’s controversial tweet and podcast related to structural racism from earlier this year that have been deleted and removed from JAMA’s website, Dr. Harmon said, JAMA maintains editorial independence from the AMA, but that direction from a journal oversight committee could lead to changes at the journal that could help prevent similar incidents.
“We’ll support whatever the journal oversight committee suggests,” Dr. Harmon said.
“We had public statements about [the podcast]. I do think that we’ll be able to move very quickly in a stronger direction to address the issue of systemic racism,” Dr. Harmon said. “The AMA has acknowledged that it is a public health threat. We have acknowledged that it is ... a political description versus a biologic construct. So, I would anticipate that you’ll find changes.”
The AMA began developing its strategic plan to advance equity several years ago, Dr. Harmon noted. “I think we are very well poised to move forward and attack this enemy of health disparity.”
AAFP president supporting Dr. Harmon’s inauguration
Among those congratulating Dr. Harmon on his inauguration was Ada Stewart, MD, a fellow family physician and South Carolina resident who is the president of the American Academy of Family Physicians.
“We are very excited that family physician Dr. Gerald Harmon will serve as president of the AMA this coming year,” Dr. Stewart said. “Family medicine encompasses the very essence of medicine – treating the whole person, in the context of family, community, and each individual’s unique circumstances. As a family physician, Dr. Harmon brings important perspectives from the front lines of primary care. His commitment to health equity and evidence-based care, as well as his concern for practice sustainability and physician well-being, will serve him well as he leads the house of medicine into the future.”
Dr. Harmon has practiced as a family medicine specialist in Georgetown, S.C., for more than 30 years. He is a member of the clinical faculty for the Tidelands Health Medical University of South Carolina family medicine residency program, advises a community health system, and is vice president of a multispecialty physician practice. In addition, Dr. Harmon is the medical director of a nonprofit hospice and volunteers as medical supervisor for his local school district.
Dr. Harmon received his undergraduate degree in physics and mathematics from the University of South Carolina, Columbia, and received his medical degree from the Medical University of South Carolina, Charleston. He completed a residency training program in family medicine with the U.S. Air Force at Eglin (Fla.) AFB, Florida.
During a 35-year military career, Dr. Harmon served as chief surgeon for the National Guard Bureau and assistant surgeon general for the U.S. Air Force. He retired from the military as a major general.
Dr. Harmon and his wife, Linda, have three married children and eight grandchildren.
Every now and then, a bucket of tomatoes or even a half bushel of corn shows up in the back of Dr. Harmon’s pickup truck, with a note on the window thanking him. “That really touches you deeply,” Dr. Harmon said. “I practice that type of medicine and I’m honored to be able to do that every day.”
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
‘COVID toes’ chilblain-like lesions not related to COVID-19
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
As new cases fall, U.S. passes 4 million children with COVID-19
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.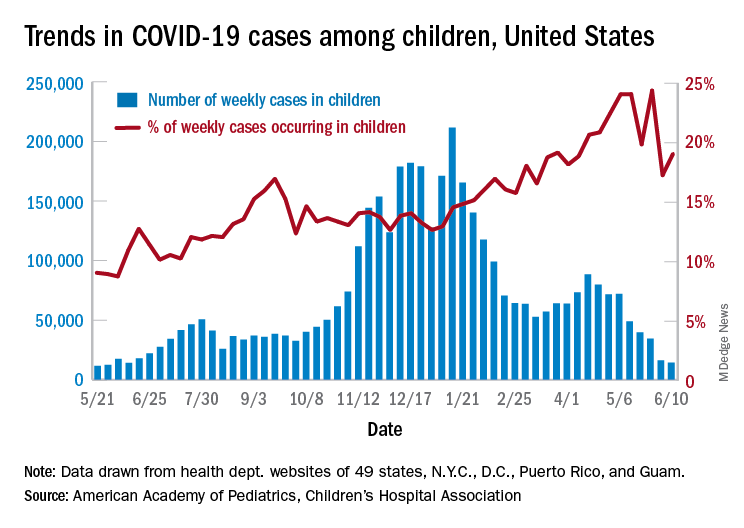
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Third COVID-19 vaccine dose helped some transplant recipients
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
Judge tosses hospital staff suit over vaccine mandate
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
Is your patient having an existential crisis?
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
OSHA issues new rules on COVID-19 safety for health care workers
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
COVID-19 death toll higher for international medical graduates
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.







