User login
Children and COVID: Vaccination trends beginning to diverge
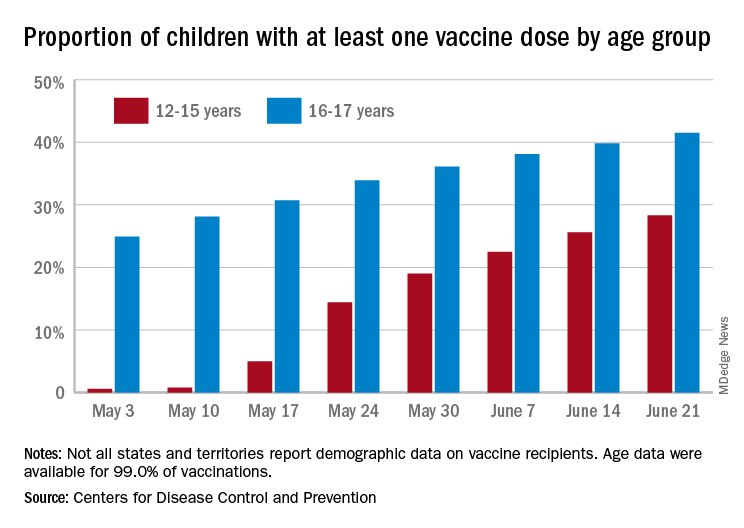
As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.
AAP updates guidance for return to sports and physical activities
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
New data on COVID-19’s cognitive fallout
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines are safe and effective for patients with migraine
according to a presentation at the American Headache Society’s 2021 annual meeting.
Amy Gelfand, MD, director of pediatric headache at University of California, San Francisco, reviewed common concerns migraine patients or their clinicians might have related any of the three vaccines, starting with a review of how the vaccines work – by targeting the spike protein of the SARS-CoV-2 virus.
“The vaccines induce response to that protein, but only that protein, so there’s no reason to think they’re going to cause the body to produce neutralizing antibodies against any of our migraine therapeutics,” Dr. Gelfand said. She added that the phase 3 clinical trials included participants from a wide range of ages and comorbidities, so there were likely many people in the trials who have migraine, though no subgroup analyses have been performed for this group or are likely to be performed.
Common questions
The two treatments people have the most questions about concerning the COVID-19 vaccine are onabotulinumtoxinA and CGRP pathway monoclonal antibodies (mAbs), likely because both of these are injections, as is the vaccine, Dr. Gelfand said. First, she reminded attendees that onabotulinumtoxinA is not a dermal filler, since some reports following administration of the Moderna vaccine suggested that some people with dermal fillers had swelling in those areas after vaccination.
In addition, “there’s no reason to think the onabotulinumtoxinA would influence our body’s immune response to any vaccine, so there’s no need to retime the onabotulinumtoxinA injections around COVID-19 vaccine administration,” Dr. Gelfand said.
Regarding mAbs, she acknowledged that some white blood cells have CGRP receptors, which may have a pro- or anti-inflammatory role, but clinical trials of mAbs did not show any evidence of being immunosuppressive or myelosuppressive.
“The monoclonal antibodies themselves have undergone engineering so that they are just going after their one target,” Dr. Gelfand said. “They’re not going to be expected to bind to anything else outside of their targets, so I don’t think there’s anything there to make us retime the monoclonal antibody administration relative to the COVID-19 vaccine.”
She did note that patients who choose to get mAbs injections in their arm instead of their thigh or abdomen may want to receive it in the opposite arm than they one they have gotten or will get the vaccine in since the vaccine can cause discomfort.
The other common question patients may have is whether taking any NSAIDs or acetaminophen before getting the COVID-19 vaccine will reduce their immune response to the vaccination. This concern arises because of past evidence showing that some infants tended to have lower immunologic responses when they received acetaminophen after their primary vaccines’ series, but the clinical significance of those reduced responses is not clear since they still had strong responses. Further, this effect was not seen with booster shots, suggesting it’s an age-dependent effect.
During the clinical trials of the AstraZeneca vaccine, several sites gave prophylactic paracetamol without any apparent detrimental effect on antibody response, Dr. Gelfand said. Further, the mRNA and adenovirus-vectored vaccines appear to induce antibodies far above what many believe is needed for protection.
“Even if there were a slight decrease, it’s not clear that that would have any kind of clinical significance for that person in terms of their level of protection against COVID-19,” she said. “Bottom line, it’s fine for patients to use either of these after administration of the COVID-19 vaccine.” The Centers for Disease Control and Prevention doesn’t recommend it prophylactically beforehand, but it’s fine to take it for a fever, aches or headache after getting the vaccine.
Migraine or vaccine reaction?
Dr. Gelfand then addressed whether it should affect physicians’ headache differential if seeing a patient who recently received an adenovirus-vectored vaccine, such as the Johnson & Johnson or AstraZeneca vaccines. The question relates to the discovery of a very rare potential adverse event from these vaccines: cerebral venous sinus thrombosis (CVST) with thrombocytopenia and thromboses in other major vessels, together called thrombosis thrombocytopenia syndrome (TTS). No TTS cases have been reported following mRNA vaccines.
TTS’s mechanism appears similar to autoimmune heparin-induced thrombocytopenia, where the body produces platelet-activating antibodies. TTS currently has three diagnostic criteria: new-onset thrombocytopenia (<150,000/microliter) without evidence of platelet clumping, venous or arterial thrombosis, and absence of prior exposure to heparin.
So far, TTS has been limited only to the vaccines that use an adenovirus vector. One male clinical trial participant experienced CVST with thrombocytopenia in Johnson & Johnson phase 3 trials, and 12 cases out of approximately 8 million Johnson & Johnson doses were reported to the Vaccine Adverse Event Reporting System between March 2 and April 21, 2021. Three TTS more cases followed these, resulting in 15 TTS events per 8 million doses.
In terms of clinical features, all 15 cases were females under age 60, mostly white, and all 11 who were tested were positive for the heparin-platelet factor 4 antibody test. TTS occurred 6-15 days after vaccination for these cases, and all but one had a headache. Their platelet count was 9,000-127,000. None were pregnant or postpartum.
“For us, as headache clinicians, the epidemiology of TTS overlaps with the epidemiology of migraine – they’re happening to the same group of patients,” Dr. Gelfand said. Most of the cases occurred in women aged 30-39 years, while the estimated incidence in women aged 50 or older is 0.9 cases per million doses.
The CDC has proceeded with the Johnson & Johnson vaccine because a risk-benefit analysis revealed that use of the vaccine will result in fewer hospitalization and deaths from COVID-19, compared with adverse events from the vaccine, Dr. Gelfand explained. However, the CDC notes that “women younger than 50 years old should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.”
For clinicians, the existence of TTS raises a question when patients with a history of migraine call after having received the Johnson & Johnson vaccine, Dr. Gelfand said: “How do we know if this is a spontaneous attack, if it’s a headache provoked by receiving the vaccine, or they have one of these rare cases of [TTS]?”
Three things help with this differential, she said: timing, epidemiology, and headache phenotype. Headache after a vaccine is very common, but it usually happens within the first couple of hours or days after the vaccine. By day 4 after vaccination, few people had headaches in the clinical trials. Since TTS requires production of antibodies, a headache within a few hours of vaccination should not raise concerns about TTS. It should be considered, however, for patients who experience a headache within a week or 2 after vaccination.
Then consider the epidemiology: If it’s a woman between ages 18 and49 calling, the risk is higher than if it’s a male over age 50. Then consider whether there are any unusual headache features, positionality, encephalopathy, or clinical features that could suggest clots in other parts of the body, such as abdominal pain, shortness of breath, or pain in the legs.
“At the end of the day, if it’s a person who’s in this epidemiological window and they’re calling a week or 2 out from the Johnson & Johnson vaccine, we may just need to work it up and see,” Dr. Gelfand said. Work-up involves a CBC, a platelet count to see if they’re thrombocytopenic, and perhaps imaging, preferentially using MRI/MRV over CT since it’s a younger population. Treatment for CVST with thrombocytopenia is a nonheparin anticoagulant, and platelet transfusion should not occur before consulting with hematology.
Continue to vaccinate
“The big take home is that we should continue to vaccinate patients with migraine and that your current therapies do not interfere with the vaccine working and that the vaccine does not interact with our therapies,” Brian D. Loftus, MD, BSChE, immediate past president of the Southern Headache Society and a neurologist at Bellaire (Pa.) Neurology, said of the presentation. He also felt it was helpful to know that NSAIDs likely have no impact on the vaccines’ effectiveness as well.
“The most important new information for me was that the median onset of the CSVT was 8 days post vaccine,” Dr. Loftus said. “Typically, postvaccine headache is seen much sooner, within 1-2 days, so this is a useful clinical feature to separate out who needs to closer follow-up and possible neuroimaging.”
Given the epidemiology of those most likely to have TTS, Dr. Loftus said he would advise his female patients younger than 60 to simply get the Pfizer or Moderna vaccine since they appear safer for this demographic.
Dr. Gelfand is editor of the journal Headache but has no industry disclosures. Her spouse has received clinical trial grant support from Genentech and honoraria for editorial work from Dynamed Plus. Dr. Loftus has received grants or fees from Teva, Amgen, Abbvie, and Biohaven.
according to a presentation at the American Headache Society’s 2021 annual meeting.
Amy Gelfand, MD, director of pediatric headache at University of California, San Francisco, reviewed common concerns migraine patients or their clinicians might have related any of the three vaccines, starting with a review of how the vaccines work – by targeting the spike protein of the SARS-CoV-2 virus.
“The vaccines induce response to that protein, but only that protein, so there’s no reason to think they’re going to cause the body to produce neutralizing antibodies against any of our migraine therapeutics,” Dr. Gelfand said. She added that the phase 3 clinical trials included participants from a wide range of ages and comorbidities, so there were likely many people in the trials who have migraine, though no subgroup analyses have been performed for this group or are likely to be performed.
Common questions
The two treatments people have the most questions about concerning the COVID-19 vaccine are onabotulinumtoxinA and CGRP pathway monoclonal antibodies (mAbs), likely because both of these are injections, as is the vaccine, Dr. Gelfand said. First, she reminded attendees that onabotulinumtoxinA is not a dermal filler, since some reports following administration of the Moderna vaccine suggested that some people with dermal fillers had swelling in those areas after vaccination.
In addition, “there’s no reason to think the onabotulinumtoxinA would influence our body’s immune response to any vaccine, so there’s no need to retime the onabotulinumtoxinA injections around COVID-19 vaccine administration,” Dr. Gelfand said.
Regarding mAbs, she acknowledged that some white blood cells have CGRP receptors, which may have a pro- or anti-inflammatory role, but clinical trials of mAbs did not show any evidence of being immunosuppressive or myelosuppressive.
“The monoclonal antibodies themselves have undergone engineering so that they are just going after their one target,” Dr. Gelfand said. “They’re not going to be expected to bind to anything else outside of their targets, so I don’t think there’s anything there to make us retime the monoclonal antibody administration relative to the COVID-19 vaccine.”
She did note that patients who choose to get mAbs injections in their arm instead of their thigh or abdomen may want to receive it in the opposite arm than they one they have gotten or will get the vaccine in since the vaccine can cause discomfort.
The other common question patients may have is whether taking any NSAIDs or acetaminophen before getting the COVID-19 vaccine will reduce their immune response to the vaccination. This concern arises because of past evidence showing that some infants tended to have lower immunologic responses when they received acetaminophen after their primary vaccines’ series, but the clinical significance of those reduced responses is not clear since they still had strong responses. Further, this effect was not seen with booster shots, suggesting it’s an age-dependent effect.
During the clinical trials of the AstraZeneca vaccine, several sites gave prophylactic paracetamol without any apparent detrimental effect on antibody response, Dr. Gelfand said. Further, the mRNA and adenovirus-vectored vaccines appear to induce antibodies far above what many believe is needed for protection.
“Even if there were a slight decrease, it’s not clear that that would have any kind of clinical significance for that person in terms of their level of protection against COVID-19,” she said. “Bottom line, it’s fine for patients to use either of these after administration of the COVID-19 vaccine.” The Centers for Disease Control and Prevention doesn’t recommend it prophylactically beforehand, but it’s fine to take it for a fever, aches or headache after getting the vaccine.
Migraine or vaccine reaction?
Dr. Gelfand then addressed whether it should affect physicians’ headache differential if seeing a patient who recently received an adenovirus-vectored vaccine, such as the Johnson & Johnson or AstraZeneca vaccines. The question relates to the discovery of a very rare potential adverse event from these vaccines: cerebral venous sinus thrombosis (CVST) with thrombocytopenia and thromboses in other major vessels, together called thrombosis thrombocytopenia syndrome (TTS). No TTS cases have been reported following mRNA vaccines.
TTS’s mechanism appears similar to autoimmune heparin-induced thrombocytopenia, where the body produces platelet-activating antibodies. TTS currently has three diagnostic criteria: new-onset thrombocytopenia (<150,000/microliter) without evidence of platelet clumping, venous or arterial thrombosis, and absence of prior exposure to heparin.
So far, TTS has been limited only to the vaccines that use an adenovirus vector. One male clinical trial participant experienced CVST with thrombocytopenia in Johnson & Johnson phase 3 trials, and 12 cases out of approximately 8 million Johnson & Johnson doses were reported to the Vaccine Adverse Event Reporting System between March 2 and April 21, 2021. Three TTS more cases followed these, resulting in 15 TTS events per 8 million doses.
In terms of clinical features, all 15 cases were females under age 60, mostly white, and all 11 who were tested were positive for the heparin-platelet factor 4 antibody test. TTS occurred 6-15 days after vaccination for these cases, and all but one had a headache. Their platelet count was 9,000-127,000. None were pregnant or postpartum.
“For us, as headache clinicians, the epidemiology of TTS overlaps with the epidemiology of migraine – they’re happening to the same group of patients,” Dr. Gelfand said. Most of the cases occurred in women aged 30-39 years, while the estimated incidence in women aged 50 or older is 0.9 cases per million doses.
The CDC has proceeded with the Johnson & Johnson vaccine because a risk-benefit analysis revealed that use of the vaccine will result in fewer hospitalization and deaths from COVID-19, compared with adverse events from the vaccine, Dr. Gelfand explained. However, the CDC notes that “women younger than 50 years old should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.”
For clinicians, the existence of TTS raises a question when patients with a history of migraine call after having received the Johnson & Johnson vaccine, Dr. Gelfand said: “How do we know if this is a spontaneous attack, if it’s a headache provoked by receiving the vaccine, or they have one of these rare cases of [TTS]?”
Three things help with this differential, she said: timing, epidemiology, and headache phenotype. Headache after a vaccine is very common, but it usually happens within the first couple of hours or days after the vaccine. By day 4 after vaccination, few people had headaches in the clinical trials. Since TTS requires production of antibodies, a headache within a few hours of vaccination should not raise concerns about TTS. It should be considered, however, for patients who experience a headache within a week or 2 after vaccination.
Then consider the epidemiology: If it’s a woman between ages 18 and49 calling, the risk is higher than if it’s a male over age 50. Then consider whether there are any unusual headache features, positionality, encephalopathy, or clinical features that could suggest clots in other parts of the body, such as abdominal pain, shortness of breath, or pain in the legs.
“At the end of the day, if it’s a person who’s in this epidemiological window and they’re calling a week or 2 out from the Johnson & Johnson vaccine, we may just need to work it up and see,” Dr. Gelfand said. Work-up involves a CBC, a platelet count to see if they’re thrombocytopenic, and perhaps imaging, preferentially using MRI/MRV over CT since it’s a younger population. Treatment for CVST with thrombocytopenia is a nonheparin anticoagulant, and platelet transfusion should not occur before consulting with hematology.
Continue to vaccinate
“The big take home is that we should continue to vaccinate patients with migraine and that your current therapies do not interfere with the vaccine working and that the vaccine does not interact with our therapies,” Brian D. Loftus, MD, BSChE, immediate past president of the Southern Headache Society and a neurologist at Bellaire (Pa.) Neurology, said of the presentation. He also felt it was helpful to know that NSAIDs likely have no impact on the vaccines’ effectiveness as well.
“The most important new information for me was that the median onset of the CSVT was 8 days post vaccine,” Dr. Loftus said. “Typically, postvaccine headache is seen much sooner, within 1-2 days, so this is a useful clinical feature to separate out who needs to closer follow-up and possible neuroimaging.”
Given the epidemiology of those most likely to have TTS, Dr. Loftus said he would advise his female patients younger than 60 to simply get the Pfizer or Moderna vaccine since they appear safer for this demographic.
Dr. Gelfand is editor of the journal Headache but has no industry disclosures. Her spouse has received clinical trial grant support from Genentech and honoraria for editorial work from Dynamed Plus. Dr. Loftus has received grants or fees from Teva, Amgen, Abbvie, and Biohaven.
according to a presentation at the American Headache Society’s 2021 annual meeting.
Amy Gelfand, MD, director of pediatric headache at University of California, San Francisco, reviewed common concerns migraine patients or their clinicians might have related any of the three vaccines, starting with a review of how the vaccines work – by targeting the spike protein of the SARS-CoV-2 virus.
“The vaccines induce response to that protein, but only that protein, so there’s no reason to think they’re going to cause the body to produce neutralizing antibodies against any of our migraine therapeutics,” Dr. Gelfand said. She added that the phase 3 clinical trials included participants from a wide range of ages and comorbidities, so there were likely many people in the trials who have migraine, though no subgroup analyses have been performed for this group or are likely to be performed.
Common questions
The two treatments people have the most questions about concerning the COVID-19 vaccine are onabotulinumtoxinA and CGRP pathway monoclonal antibodies (mAbs), likely because both of these are injections, as is the vaccine, Dr. Gelfand said. First, she reminded attendees that onabotulinumtoxinA is not a dermal filler, since some reports following administration of the Moderna vaccine suggested that some people with dermal fillers had swelling in those areas after vaccination.
In addition, “there’s no reason to think the onabotulinumtoxinA would influence our body’s immune response to any vaccine, so there’s no need to retime the onabotulinumtoxinA injections around COVID-19 vaccine administration,” Dr. Gelfand said.
Regarding mAbs, she acknowledged that some white blood cells have CGRP receptors, which may have a pro- or anti-inflammatory role, but clinical trials of mAbs did not show any evidence of being immunosuppressive or myelosuppressive.
“The monoclonal antibodies themselves have undergone engineering so that they are just going after their one target,” Dr. Gelfand said. “They’re not going to be expected to bind to anything else outside of their targets, so I don’t think there’s anything there to make us retime the monoclonal antibody administration relative to the COVID-19 vaccine.”
She did note that patients who choose to get mAbs injections in their arm instead of their thigh or abdomen may want to receive it in the opposite arm than they one they have gotten or will get the vaccine in since the vaccine can cause discomfort.
The other common question patients may have is whether taking any NSAIDs or acetaminophen before getting the COVID-19 vaccine will reduce their immune response to the vaccination. This concern arises because of past evidence showing that some infants tended to have lower immunologic responses when they received acetaminophen after their primary vaccines’ series, but the clinical significance of those reduced responses is not clear since they still had strong responses. Further, this effect was not seen with booster shots, suggesting it’s an age-dependent effect.
During the clinical trials of the AstraZeneca vaccine, several sites gave prophylactic paracetamol without any apparent detrimental effect on antibody response, Dr. Gelfand said. Further, the mRNA and adenovirus-vectored vaccines appear to induce antibodies far above what many believe is needed for protection.
“Even if there were a slight decrease, it’s not clear that that would have any kind of clinical significance for that person in terms of their level of protection against COVID-19,” she said. “Bottom line, it’s fine for patients to use either of these after administration of the COVID-19 vaccine.” The Centers for Disease Control and Prevention doesn’t recommend it prophylactically beforehand, but it’s fine to take it for a fever, aches or headache after getting the vaccine.
Migraine or vaccine reaction?
Dr. Gelfand then addressed whether it should affect physicians’ headache differential if seeing a patient who recently received an adenovirus-vectored vaccine, such as the Johnson & Johnson or AstraZeneca vaccines. The question relates to the discovery of a very rare potential adverse event from these vaccines: cerebral venous sinus thrombosis (CVST) with thrombocytopenia and thromboses in other major vessels, together called thrombosis thrombocytopenia syndrome (TTS). No TTS cases have been reported following mRNA vaccines.
TTS’s mechanism appears similar to autoimmune heparin-induced thrombocytopenia, where the body produces platelet-activating antibodies. TTS currently has three diagnostic criteria: new-onset thrombocytopenia (<150,000/microliter) without evidence of platelet clumping, venous or arterial thrombosis, and absence of prior exposure to heparin.
So far, TTS has been limited only to the vaccines that use an adenovirus vector. One male clinical trial participant experienced CVST with thrombocytopenia in Johnson & Johnson phase 3 trials, and 12 cases out of approximately 8 million Johnson & Johnson doses were reported to the Vaccine Adverse Event Reporting System between March 2 and April 21, 2021. Three TTS more cases followed these, resulting in 15 TTS events per 8 million doses.
In terms of clinical features, all 15 cases were females under age 60, mostly white, and all 11 who were tested were positive for the heparin-platelet factor 4 antibody test. TTS occurred 6-15 days after vaccination for these cases, and all but one had a headache. Their platelet count was 9,000-127,000. None were pregnant or postpartum.
“For us, as headache clinicians, the epidemiology of TTS overlaps with the epidemiology of migraine – they’re happening to the same group of patients,” Dr. Gelfand said. Most of the cases occurred in women aged 30-39 years, while the estimated incidence in women aged 50 or older is 0.9 cases per million doses.
The CDC has proceeded with the Johnson & Johnson vaccine because a risk-benefit analysis revealed that use of the vaccine will result in fewer hospitalization and deaths from COVID-19, compared with adverse events from the vaccine, Dr. Gelfand explained. However, the CDC notes that “women younger than 50 years old should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.”
For clinicians, the existence of TTS raises a question when patients with a history of migraine call after having received the Johnson & Johnson vaccine, Dr. Gelfand said: “How do we know if this is a spontaneous attack, if it’s a headache provoked by receiving the vaccine, or they have one of these rare cases of [TTS]?”
Three things help with this differential, she said: timing, epidemiology, and headache phenotype. Headache after a vaccine is very common, but it usually happens within the first couple of hours or days after the vaccine. By day 4 after vaccination, few people had headaches in the clinical trials. Since TTS requires production of antibodies, a headache within a few hours of vaccination should not raise concerns about TTS. It should be considered, however, for patients who experience a headache within a week or 2 after vaccination.
Then consider the epidemiology: If it’s a woman between ages 18 and49 calling, the risk is higher than if it’s a male over age 50. Then consider whether there are any unusual headache features, positionality, encephalopathy, or clinical features that could suggest clots in other parts of the body, such as abdominal pain, shortness of breath, or pain in the legs.
“At the end of the day, if it’s a person who’s in this epidemiological window and they’re calling a week or 2 out from the Johnson & Johnson vaccine, we may just need to work it up and see,” Dr. Gelfand said. Work-up involves a CBC, a platelet count to see if they’re thrombocytopenic, and perhaps imaging, preferentially using MRI/MRV over CT since it’s a younger population. Treatment for CVST with thrombocytopenia is a nonheparin anticoagulant, and platelet transfusion should not occur before consulting with hematology.
Continue to vaccinate
“The big take home is that we should continue to vaccinate patients with migraine and that your current therapies do not interfere with the vaccine working and that the vaccine does not interact with our therapies,” Brian D. Loftus, MD, BSChE, immediate past president of the Southern Headache Society and a neurologist at Bellaire (Pa.) Neurology, said of the presentation. He also felt it was helpful to know that NSAIDs likely have no impact on the vaccines’ effectiveness as well.
“The most important new information for me was that the median onset of the CSVT was 8 days post vaccine,” Dr. Loftus said. “Typically, postvaccine headache is seen much sooner, within 1-2 days, so this is a useful clinical feature to separate out who needs to closer follow-up and possible neuroimaging.”
Given the epidemiology of those most likely to have TTS, Dr. Loftus said he would advise his female patients younger than 60 to simply get the Pfizer or Moderna vaccine since they appear safer for this demographic.
Dr. Gelfand is editor of the journal Headache but has no industry disclosures. Her spouse has received clinical trial grant support from Genentech and honoraria for editorial work from Dynamed Plus. Dr. Loftus has received grants or fees from Teva, Amgen, Abbvie, and Biohaven.
FROM AHS 2021
Why getting a COVID-19 vaccine to children could take time
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation tied to lower death rate in COVID
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S., international MIS-C studies yield disparate results
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Trial offers first look at how tralokinumab-treated patients weather COVID-19
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
FROM REVOLUTIONIZING AD 2021
Fewer dangerous COPD flare-ups during COVID-19
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Understanding the grieving process
Loss is inevitable – and understanding essential
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
Loss is inevitable – and understanding essential
Loss is inevitable – and understanding essential
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.





