User login
FDA approves hyaluronic acid filler for lip augmentation, perioral rhytids
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
The resurgence of Plaquenil (hydroxychloroquine)
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
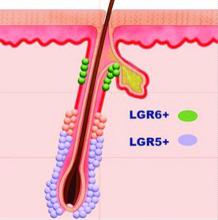
Mother of pearl: The power of pearl powder
Because of its dense protein and mineral composition, it has been used to treat several skin and bone disorders, as well as palpitations, insomnia, and epilepsy.3,4 The pearl-farming industry itself was established in Japan and has existed for more than a century; today, pearls are cultured globally and continue to receive attention for conferring health benefits.5
Calcium carbonate is the primary component of mollusk shells (roughly 95%), with the remainder an organic matrix including proteins, glycoproteins, and polysaccharides.6 Pearl powder is known to have exhibited antiaging, antioxidant, antiradiative, and tonic activities; in recent years, it has been incorporated into health foods for such properties and used in the clinical setting to treat ulcers (aphthous, gastric, and duodenal).4,7 Consisting of multiple active proteins, pearl powder is thought to be conducive to skin cell growth and effective for wound repair.4 This column focuses on recent research into the dermatologic potential of the powder derived from mother of pearl.
Wound healing
A decade ago, Jian-Ping et al. showed in mice that the water-soluble matrix of pearl powder (Hyriopsis cumingii) could significantly induce oral fibroblast proliferation and collagen accumulation, suppress matrix metalloproteinase-2 activity, and significantly foster TIMP-1 synthesis. The investigators concluded that the wound healing facilitated by pearl powder derives, in part, from its capacity to promote fibroblast mitosis, collagen deposition, and production of TIMP-1.8
Two years later, Lee et al. evaluated the effects of water-soluble nacre (mother of pearl) on second-degree burn wound healing in porcine skin as a proxy for human skin. They found that its application quickly led to burn-induced granulation areas filling with collagen, with normal skin appearance restored to wounded dermis and epidermis. Angiogenesis was also promoted by water-soluble nacre as was wound recovery in areas with apoptotic and necrotic cellular damage. Murine fibroblast NIH3T3 cells treated with water-soluble nacre also demonstrated augmented proliferation and collagen production. The researchers cited the restoration of angiogenesis and fibroblast activity as the primary benefits of water-soluble nacre, suggesting its potential as a wound therapy, preferable to powdered nacre due to better biocompatibility with less discomfort.9
The next year, Li et al. found that mother of pearl extract promoted cell migration of fibroblasts in cell culture, demonstrating its potential as a wound healing model.7In 2019, Chen et al. studied the effects of pearl powders of varying particle sizes to treat wounds in vitro and in vivo. They found that micro- and nanosized pearl powders augmented proliferation and migration of skin cells and shortened wound closure time. All powders also improved the biomechanical strength of healed skin, enhanced collagen formation and deposition, and expanded cutaneous angiogenesis, with nanoscale pearl powder displaying greatest efficiency.4
Skin tone and atopic dermatitis
In 2000, Lopez et al. implanted powdered nacre (mother of pearl derived from Pinctada maxima), which can promote and regulate bone-forming cells, into rat dermis to evaluate its effects on skin fibroblasts. They noted that the implant yielded well-vascularized tissue and improved extracellular matrix production, synthesis of substances involved in cellular adhesion and communication, and tissue regeneration (such as collagen types I and III). The investigators concluded that the powdered nacre contributed to the conditions necessary for improved skin tone and proper physiologic functioning of the skin.10
Rousseau et al. extracted lipids from the nacre of the oyster P. margaritifera to test on artificially dehydrated skin explants with the intention of developing new treatments for atopic dermatitis. The researchers determined that the lipids spurred a reconstitution of the intercellular material of the stratum corneum, concluding that new products to treat atopic dermatitis might be based on the signaling activity of nacre lipids.11
Antifibrotic and anti-inflammatory activity
A 2015 study by Yang et al. showed that a room-temperature superextraction system to yield the main active constituents of pearl was successful in enhancing their anti-inflammatory and antiapoptotic activity in human keratinocyte cells (HaCaT) exposed to low-dose UVB. The investigators combined pearl extract and poly (gamma-glutamic acid) hydrogels and observed reductions in inflammation and apoptosis of HaCaT cells. They concluded that a marketed pearl extract may be able to suppress radiation dermatitis present in keratinocytes.12
Two years later, Latire et al. used human dermal fibroblasts in primary culture to assess the potential biological activities of the matrix macromolecular components extracted from the shells of two edible mollusks (the blue mussel Mytilus edulis and the Pacific oyster Crassostrea gigas). The investigators found that both extracts influenced metabolic functions of the cells and reduced type I collagen levels, with an associated rise in matrix metalloproteinase-1 activity. Given their findings implying the effectiveness of the extracts in facilitating the catabolic pathway of human dermal fibroblasts, the authors suggest that these shell matrices present the potential for use in treating fibrosis, especially for scleroderma.6
Antioxidant and antiaging activity
Shao et al. demonstrated 10 years ago that pearl powder provides a moisturizing effect on the skin, with ultramicro pearl powder delivering a more robust moisturizing result than water-soluble pearl powder. These two types of pearl powder, along with another one tested (ultranano pearl powder), also significantly diminished the activation of tyrosinase and free radicals. Water-soluble pearl powder did not perform as well as the other two formulations in free radical scavenging. The investigators suggested that their results support the use of pearl powder to combat aging and enhance beauty, and could be used in the clinical setting.13
In 2017, Yang et al. reported on the in vitro antihemolytic and antioxidant activity of pearl powder in shielding human erythrocytes against 2,2’-azobis(2-amidinopropane) dihydrochloride–induced oxidative damage to membrane proteins/lipids. The researchers contend that the strong antioxidant qualities of pearl powder could be applied to prevent or protect against various diseases resulting from free radical damage.2
Human trials: Antioxidant, antiaging, skin appearance
Chiu et al. studied the antioxidant activity of various pearl powder extracts in a randomized, placebo-controlled trial in 2018. They also investigated the life span–prolonging effects of the powders using wild-type Caenorhabditis elegans. Twenty healthy middle-aged subjects were separated into two groups (experimental and placebo), with 3 g of pearl powder administered in capsules to the former and 3 g of placebo to the latter over 8 weeks. Blood samples taken at the beginning and every 2 weeks during the trial and in the 10th week revealed maximum antioxidant activity of the pearl powder and prolongation of C. elegans lifespan by 18.87%. Subjects using pearl powder demonstrated significant increases in total antioxidant capacity, thiols, glutathione, and enzymic antioxidant activity, along with notably inhibited lipid peroxidation products. The investigators concluded that pearl powder extract acted as a potent antioxidant and its use may be warranted to treat degenerative conditions related to aging.3
A recent study of the perception of blue light on Korean women’s faces using blue pearl pigment revealed that the pigment does indeed elicit the perception of the blue-light effect, notably transparency and gloss, which is particularly valued in Korea.14
Conclusion
The use of mother of pearl and pearl powder in traditional Chinese medicine and as a cosmetic and food additive has a rich and lengthy history. Contemporary research clearly suggests interesting avenues for further investigation and some promising results. Much more research is necessary, though, to delineate the potential roles of pearl powder in the skin care arsenal.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Zhang J et al. J Sep Sci. 2015 May;38(9):1552-60.
2. Yang HL et al. J Food Drug Anal. 2017 Oct;25(4):898-907.
3. Chiu HF et al. J Food Drug Anal. 2018 Jan;26(1):309-17.
4. Chen X et al. Drug Dev Ind Pharm. 2019 Jun;45(6):1009-16.
5. Nagai K. Zoolog Sci. 2013 Oct;30(10):783-93.
6. Latire T et al. Cytotechnology. 2017 Oct;69(5):815-29.
7. Li YC et al. Pharm Biol. 2013 Mar;51(3):289-97.
8. Jian-Ping D et al. Pharm Biol. 2010 Feb;48(2):122-7.
9. Lee K et al. Mol Biol Rep. 2012 Mar;39(3):3211-8.
10. Lopez E et al. Tissue Cell. 2000 Feb;32(1):95-101.
11. Rousseau M et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):1-9.
12. Yang YL et al. Biomed Mater Eng. 2015;26 Suppl 1:S139-45.
13. Shao DZ et al. J Cosmet Sci. 2010 Mar-Apr;61(2):133-45.
14. Lee M et al. Skin Res Technol. 2020 Jan;26(1):76-80.
Because of its dense protein and mineral composition, it has been used to treat several skin and bone disorders, as well as palpitations, insomnia, and epilepsy.3,4 The pearl-farming industry itself was established in Japan and has existed for more than a century; today, pearls are cultured globally and continue to receive attention for conferring health benefits.5
Calcium carbonate is the primary component of mollusk shells (roughly 95%), with the remainder an organic matrix including proteins, glycoproteins, and polysaccharides.6 Pearl powder is known to have exhibited antiaging, antioxidant, antiradiative, and tonic activities; in recent years, it has been incorporated into health foods for such properties and used in the clinical setting to treat ulcers (aphthous, gastric, and duodenal).4,7 Consisting of multiple active proteins, pearl powder is thought to be conducive to skin cell growth and effective for wound repair.4 This column focuses on recent research into the dermatologic potential of the powder derived from mother of pearl.
Wound healing
A decade ago, Jian-Ping et al. showed in mice that the water-soluble matrix of pearl powder (Hyriopsis cumingii) could significantly induce oral fibroblast proliferation and collagen accumulation, suppress matrix metalloproteinase-2 activity, and significantly foster TIMP-1 synthesis. The investigators concluded that the wound healing facilitated by pearl powder derives, in part, from its capacity to promote fibroblast mitosis, collagen deposition, and production of TIMP-1.8
Two years later, Lee et al. evaluated the effects of water-soluble nacre (mother of pearl) on second-degree burn wound healing in porcine skin as a proxy for human skin. They found that its application quickly led to burn-induced granulation areas filling with collagen, with normal skin appearance restored to wounded dermis and epidermis. Angiogenesis was also promoted by water-soluble nacre as was wound recovery in areas with apoptotic and necrotic cellular damage. Murine fibroblast NIH3T3 cells treated with water-soluble nacre also demonstrated augmented proliferation and collagen production. The researchers cited the restoration of angiogenesis and fibroblast activity as the primary benefits of water-soluble nacre, suggesting its potential as a wound therapy, preferable to powdered nacre due to better biocompatibility with less discomfort.9
The next year, Li et al. found that mother of pearl extract promoted cell migration of fibroblasts in cell culture, demonstrating its potential as a wound healing model.7In 2019, Chen et al. studied the effects of pearl powders of varying particle sizes to treat wounds in vitro and in vivo. They found that micro- and nanosized pearl powders augmented proliferation and migration of skin cells and shortened wound closure time. All powders also improved the biomechanical strength of healed skin, enhanced collagen formation and deposition, and expanded cutaneous angiogenesis, with nanoscale pearl powder displaying greatest efficiency.4
Skin tone and atopic dermatitis
In 2000, Lopez et al. implanted powdered nacre (mother of pearl derived from Pinctada maxima), which can promote and regulate bone-forming cells, into rat dermis to evaluate its effects on skin fibroblasts. They noted that the implant yielded well-vascularized tissue and improved extracellular matrix production, synthesis of substances involved in cellular adhesion and communication, and tissue regeneration (such as collagen types I and III). The investigators concluded that the powdered nacre contributed to the conditions necessary for improved skin tone and proper physiologic functioning of the skin.10
Rousseau et al. extracted lipids from the nacre of the oyster P. margaritifera to test on artificially dehydrated skin explants with the intention of developing new treatments for atopic dermatitis. The researchers determined that the lipids spurred a reconstitution of the intercellular material of the stratum corneum, concluding that new products to treat atopic dermatitis might be based on the signaling activity of nacre lipids.11
Antifibrotic and anti-inflammatory activity
A 2015 study by Yang et al. showed that a room-temperature superextraction system to yield the main active constituents of pearl was successful in enhancing their anti-inflammatory and antiapoptotic activity in human keratinocyte cells (HaCaT) exposed to low-dose UVB. The investigators combined pearl extract and poly (gamma-glutamic acid) hydrogels and observed reductions in inflammation and apoptosis of HaCaT cells. They concluded that a marketed pearl extract may be able to suppress radiation dermatitis present in keratinocytes.12
Two years later, Latire et al. used human dermal fibroblasts in primary culture to assess the potential biological activities of the matrix macromolecular components extracted from the shells of two edible mollusks (the blue mussel Mytilus edulis and the Pacific oyster Crassostrea gigas). The investigators found that both extracts influenced metabolic functions of the cells and reduced type I collagen levels, with an associated rise in matrix metalloproteinase-1 activity. Given their findings implying the effectiveness of the extracts in facilitating the catabolic pathway of human dermal fibroblasts, the authors suggest that these shell matrices present the potential for use in treating fibrosis, especially for scleroderma.6
Antioxidant and antiaging activity
Shao et al. demonstrated 10 years ago that pearl powder provides a moisturizing effect on the skin, with ultramicro pearl powder delivering a more robust moisturizing result than water-soluble pearl powder. These two types of pearl powder, along with another one tested (ultranano pearl powder), also significantly diminished the activation of tyrosinase and free radicals. Water-soluble pearl powder did not perform as well as the other two formulations in free radical scavenging. The investigators suggested that their results support the use of pearl powder to combat aging and enhance beauty, and could be used in the clinical setting.13
In 2017, Yang et al. reported on the in vitro antihemolytic and antioxidant activity of pearl powder in shielding human erythrocytes against 2,2’-azobis(2-amidinopropane) dihydrochloride–induced oxidative damage to membrane proteins/lipids. The researchers contend that the strong antioxidant qualities of pearl powder could be applied to prevent or protect against various diseases resulting from free radical damage.2
Human trials: Antioxidant, antiaging, skin appearance
Chiu et al. studied the antioxidant activity of various pearl powder extracts in a randomized, placebo-controlled trial in 2018. They also investigated the life span–prolonging effects of the powders using wild-type Caenorhabditis elegans. Twenty healthy middle-aged subjects were separated into two groups (experimental and placebo), with 3 g of pearl powder administered in capsules to the former and 3 g of placebo to the latter over 8 weeks. Blood samples taken at the beginning and every 2 weeks during the trial and in the 10th week revealed maximum antioxidant activity of the pearl powder and prolongation of C. elegans lifespan by 18.87%. Subjects using pearl powder demonstrated significant increases in total antioxidant capacity, thiols, glutathione, and enzymic antioxidant activity, along with notably inhibited lipid peroxidation products. The investigators concluded that pearl powder extract acted as a potent antioxidant and its use may be warranted to treat degenerative conditions related to aging.3
A recent study of the perception of blue light on Korean women’s faces using blue pearl pigment revealed that the pigment does indeed elicit the perception of the blue-light effect, notably transparency and gloss, which is particularly valued in Korea.14
Conclusion
The use of mother of pearl and pearl powder in traditional Chinese medicine and as a cosmetic and food additive has a rich and lengthy history. Contemporary research clearly suggests interesting avenues for further investigation and some promising results. Much more research is necessary, though, to delineate the potential roles of pearl powder in the skin care arsenal.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Zhang J et al. J Sep Sci. 2015 May;38(9):1552-60.
2. Yang HL et al. J Food Drug Anal. 2017 Oct;25(4):898-907.
3. Chiu HF et al. J Food Drug Anal. 2018 Jan;26(1):309-17.
4. Chen X et al. Drug Dev Ind Pharm. 2019 Jun;45(6):1009-16.
5. Nagai K. Zoolog Sci. 2013 Oct;30(10):783-93.
6. Latire T et al. Cytotechnology. 2017 Oct;69(5):815-29.
7. Li YC et al. Pharm Biol. 2013 Mar;51(3):289-97.
8. Jian-Ping D et al. Pharm Biol. 2010 Feb;48(2):122-7.
9. Lee K et al. Mol Biol Rep. 2012 Mar;39(3):3211-8.
10. Lopez E et al. Tissue Cell. 2000 Feb;32(1):95-101.
11. Rousseau M et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):1-9.
12. Yang YL et al. Biomed Mater Eng. 2015;26 Suppl 1:S139-45.
13. Shao DZ et al. J Cosmet Sci. 2010 Mar-Apr;61(2):133-45.
14. Lee M et al. Skin Res Technol. 2020 Jan;26(1):76-80.
Because of its dense protein and mineral composition, it has been used to treat several skin and bone disorders, as well as palpitations, insomnia, and epilepsy.3,4 The pearl-farming industry itself was established in Japan and has existed for more than a century; today, pearls are cultured globally and continue to receive attention for conferring health benefits.5
Calcium carbonate is the primary component of mollusk shells (roughly 95%), with the remainder an organic matrix including proteins, glycoproteins, and polysaccharides.6 Pearl powder is known to have exhibited antiaging, antioxidant, antiradiative, and tonic activities; in recent years, it has been incorporated into health foods for such properties and used in the clinical setting to treat ulcers (aphthous, gastric, and duodenal).4,7 Consisting of multiple active proteins, pearl powder is thought to be conducive to skin cell growth and effective for wound repair.4 This column focuses on recent research into the dermatologic potential of the powder derived from mother of pearl.
Wound healing
A decade ago, Jian-Ping et al. showed in mice that the water-soluble matrix of pearl powder (Hyriopsis cumingii) could significantly induce oral fibroblast proliferation and collagen accumulation, suppress matrix metalloproteinase-2 activity, and significantly foster TIMP-1 synthesis. The investigators concluded that the wound healing facilitated by pearl powder derives, in part, from its capacity to promote fibroblast mitosis, collagen deposition, and production of TIMP-1.8
Two years later, Lee et al. evaluated the effects of water-soluble nacre (mother of pearl) on second-degree burn wound healing in porcine skin as a proxy for human skin. They found that its application quickly led to burn-induced granulation areas filling with collagen, with normal skin appearance restored to wounded dermis and epidermis. Angiogenesis was also promoted by water-soluble nacre as was wound recovery in areas with apoptotic and necrotic cellular damage. Murine fibroblast NIH3T3 cells treated with water-soluble nacre also demonstrated augmented proliferation and collagen production. The researchers cited the restoration of angiogenesis and fibroblast activity as the primary benefits of water-soluble nacre, suggesting its potential as a wound therapy, preferable to powdered nacre due to better biocompatibility with less discomfort.9
The next year, Li et al. found that mother of pearl extract promoted cell migration of fibroblasts in cell culture, demonstrating its potential as a wound healing model.7In 2019, Chen et al. studied the effects of pearl powders of varying particle sizes to treat wounds in vitro and in vivo. They found that micro- and nanosized pearl powders augmented proliferation and migration of skin cells and shortened wound closure time. All powders also improved the biomechanical strength of healed skin, enhanced collagen formation and deposition, and expanded cutaneous angiogenesis, with nanoscale pearl powder displaying greatest efficiency.4
Skin tone and atopic dermatitis
In 2000, Lopez et al. implanted powdered nacre (mother of pearl derived from Pinctada maxima), which can promote and regulate bone-forming cells, into rat dermis to evaluate its effects on skin fibroblasts. They noted that the implant yielded well-vascularized tissue and improved extracellular matrix production, synthesis of substances involved in cellular adhesion and communication, and tissue regeneration (such as collagen types I and III). The investigators concluded that the powdered nacre contributed to the conditions necessary for improved skin tone and proper physiologic functioning of the skin.10
Rousseau et al. extracted lipids from the nacre of the oyster P. margaritifera to test on artificially dehydrated skin explants with the intention of developing new treatments for atopic dermatitis. The researchers determined that the lipids spurred a reconstitution of the intercellular material of the stratum corneum, concluding that new products to treat atopic dermatitis might be based on the signaling activity of nacre lipids.11
Antifibrotic and anti-inflammatory activity
A 2015 study by Yang et al. showed that a room-temperature superextraction system to yield the main active constituents of pearl was successful in enhancing their anti-inflammatory and antiapoptotic activity in human keratinocyte cells (HaCaT) exposed to low-dose UVB. The investigators combined pearl extract and poly (gamma-glutamic acid) hydrogels and observed reductions in inflammation and apoptosis of HaCaT cells. They concluded that a marketed pearl extract may be able to suppress radiation dermatitis present in keratinocytes.12
Two years later, Latire et al. used human dermal fibroblasts in primary culture to assess the potential biological activities of the matrix macromolecular components extracted from the shells of two edible mollusks (the blue mussel Mytilus edulis and the Pacific oyster Crassostrea gigas). The investigators found that both extracts influenced metabolic functions of the cells and reduced type I collagen levels, with an associated rise in matrix metalloproteinase-1 activity. Given their findings implying the effectiveness of the extracts in facilitating the catabolic pathway of human dermal fibroblasts, the authors suggest that these shell matrices present the potential for use in treating fibrosis, especially for scleroderma.6
Antioxidant and antiaging activity
Shao et al. demonstrated 10 years ago that pearl powder provides a moisturizing effect on the skin, with ultramicro pearl powder delivering a more robust moisturizing result than water-soluble pearl powder. These two types of pearl powder, along with another one tested (ultranano pearl powder), also significantly diminished the activation of tyrosinase and free radicals. Water-soluble pearl powder did not perform as well as the other two formulations in free radical scavenging. The investigators suggested that their results support the use of pearl powder to combat aging and enhance beauty, and could be used in the clinical setting.13
In 2017, Yang et al. reported on the in vitro antihemolytic and antioxidant activity of pearl powder in shielding human erythrocytes against 2,2’-azobis(2-amidinopropane) dihydrochloride–induced oxidative damage to membrane proteins/lipids. The researchers contend that the strong antioxidant qualities of pearl powder could be applied to prevent or protect against various diseases resulting from free radical damage.2
Human trials: Antioxidant, antiaging, skin appearance
Chiu et al. studied the antioxidant activity of various pearl powder extracts in a randomized, placebo-controlled trial in 2018. They also investigated the life span–prolonging effects of the powders using wild-type Caenorhabditis elegans. Twenty healthy middle-aged subjects were separated into two groups (experimental and placebo), with 3 g of pearl powder administered in capsules to the former and 3 g of placebo to the latter over 8 weeks. Blood samples taken at the beginning and every 2 weeks during the trial and in the 10th week revealed maximum antioxidant activity of the pearl powder and prolongation of C. elegans lifespan by 18.87%. Subjects using pearl powder demonstrated significant increases in total antioxidant capacity, thiols, glutathione, and enzymic antioxidant activity, along with notably inhibited lipid peroxidation products. The investigators concluded that pearl powder extract acted as a potent antioxidant and its use may be warranted to treat degenerative conditions related to aging.3
A recent study of the perception of blue light on Korean women’s faces using blue pearl pigment revealed that the pigment does indeed elicit the perception of the blue-light effect, notably transparency and gloss, which is particularly valued in Korea.14
Conclusion
The use of mother of pearl and pearl powder in traditional Chinese medicine and as a cosmetic and food additive has a rich and lengthy history. Contemporary research clearly suggests interesting avenues for further investigation and some promising results. Much more research is necessary, though, to delineate the potential roles of pearl powder in the skin care arsenal.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Zhang J et al. J Sep Sci. 2015 May;38(9):1552-60.
2. Yang HL et al. J Food Drug Anal. 2017 Oct;25(4):898-907.
3. Chiu HF et al. J Food Drug Anal. 2018 Jan;26(1):309-17.
4. Chen X et al. Drug Dev Ind Pharm. 2019 Jun;45(6):1009-16.
5. Nagai K. Zoolog Sci. 2013 Oct;30(10):783-93.
6. Latire T et al. Cytotechnology. 2017 Oct;69(5):815-29.
7. Li YC et al. Pharm Biol. 2013 Mar;51(3):289-97.
8. Jian-Ping D et al. Pharm Biol. 2010 Feb;48(2):122-7.
9. Lee K et al. Mol Biol Rep. 2012 Mar;39(3):3211-8.
10. Lopez E et al. Tissue Cell. 2000 Feb;32(1):95-101.
11. Rousseau M et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):1-9.
12. Yang YL et al. Biomed Mater Eng. 2015;26 Suppl 1:S139-45.
13. Shao DZ et al. J Cosmet Sci. 2010 Mar-Apr;61(2):133-45.
14. Lee M et al. Skin Res Technol. 2020 Jan;26(1):76-80.
Hand washing and hand sanitizer on the skin and COVID-19 infection risk
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at [email protected].
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
Rapid Development of Perifolliculitis Following Mesotherapy
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.

A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.
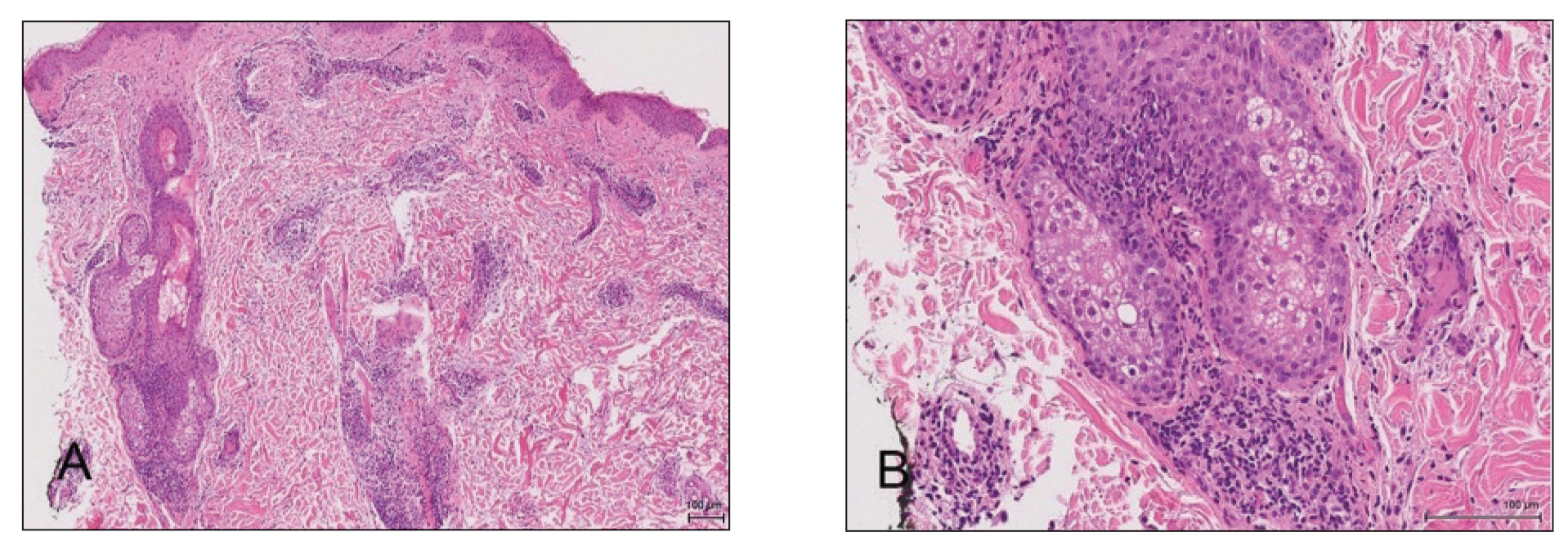
A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18
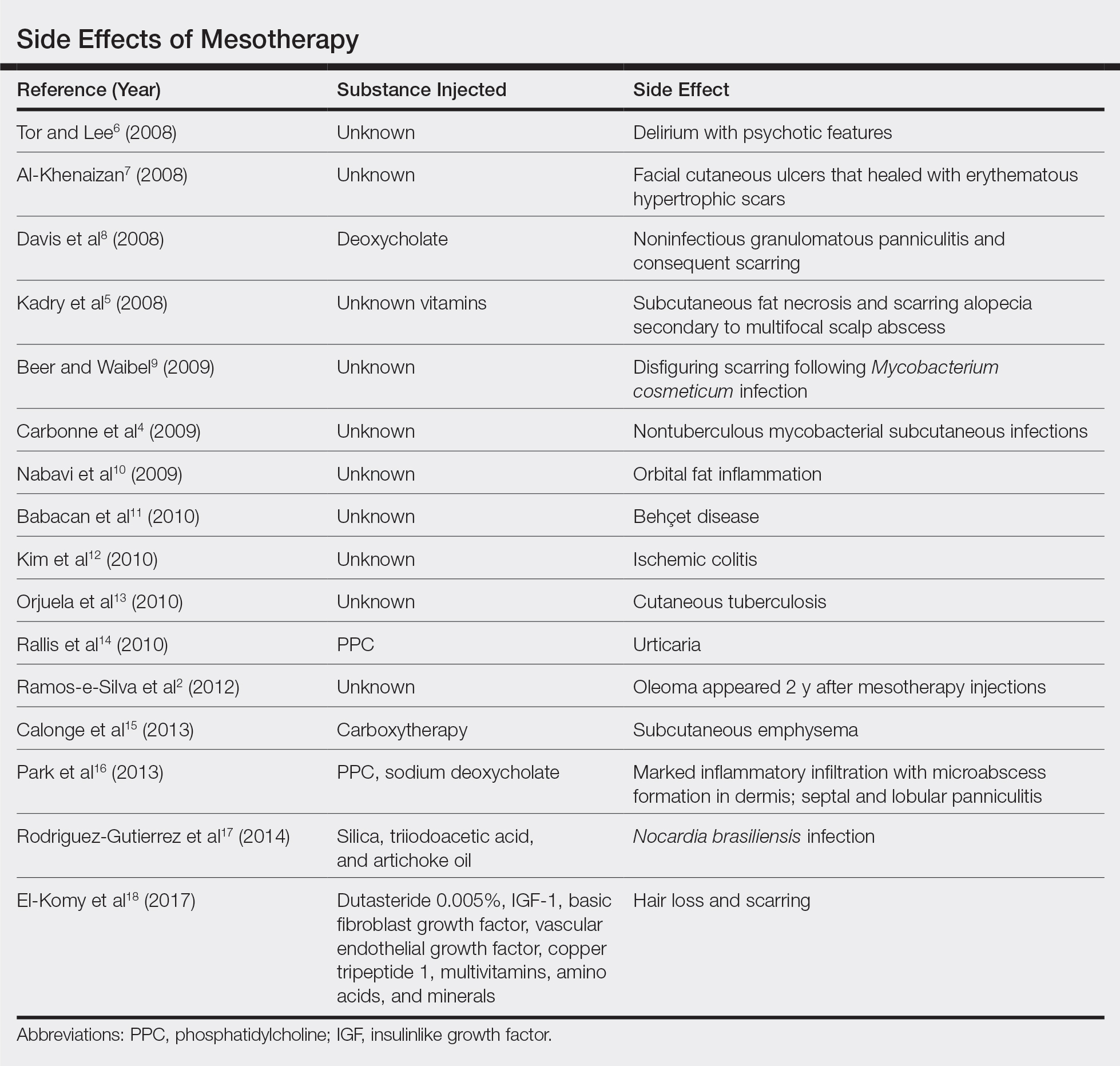
Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.

A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.

A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18

Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
To the Editor:
Mesotherapy, also known as intradermotherapy, is a cosmetic procedure in which multiple intradermal or subcutaneous injections of homeopathic substances, vitamins, chemicals, and plant extracts are administered.1 First conceived in Europe, mesotherapy is not approved by the US Food and Drug Administration but is gaining popularity in the United States as an alternative cosmetic procedure for various purposes, including lipolysis, body contouring, stretch marks, acne scars, actinic damage, and skin rejuvenation.1,2 We report a case of a healthy woman who developed perifolliculitis, transaminitis, and neutropenia 2 weeks after mesotherapy administration to the face, neck, and chest. We also review other potential side effects of this procedure.
A 36-year-old woman with no notable medical history presented to the emergency department with a worsening pruritic and painful rash on the face, chest, and neck of 2 weeks’ duration. The rash had developed 3 days after the patient received mesotherapy with an unknown substance for cosmetic rejuvenation; the rash was localized only to the injection sites. She did not note any fever, chills, nausea, vomiting, diarrhea, headache, arthralgia, or upper respiratory tract symptoms. She further denied starting any new medications, herbal products, or topical therapies apart from the procedure she had received 2 weeks prior.
The patient was found to be in no acute distress and vital signs were stable. Laboratory testing was remarkable for elevations in alanine aminotransferase (62 U/L [reference range, 10–40 U/L]) and aspartate aminotransferase (72 U/L [reference range 10–30 U/L]). Moreover, she had an absolute neutrophil count of 0.5×103 cells/µL (reference range 1.8–8.0×103 cells/µL). An electrolyte panel, creatinine level, and urinalysis were normal. Physical examination revealed numerous 4- to 5-mm erythematous papules in a gridlike distribution across the face, neck, and chest (Figure 1). No pustules or nodules were present. There was no discharge, crust, excoriations, or secondary lesions. Additionally, there was no lymphadenopathy and no mucous membrane or ocular involvement.

A 4-mm punch biopsy from a representative papule on the right lateral aspect of the neck demonstrated a perifollicular and perivascular lymphohistiocytic infiltrate with some focal granulomatous changes. No polarizable foreign body material was found (Figure 2). Bacterial, fungal, mycobacterial, and skin cultures were obtained, and results were all negative after several weeks.

A diagnosis of perifolliculitis from the mesotherapy procedure was on the top of the differential vs a fast-growing mycobacterial or granulomatous reaction. The patient was started on a prednisone taper at 40 mg once daily tapered down completely over 3 weeks in addition to triamcinolone cream 0.1% applied 2 to 4 times daily as needed. Although she did not return to our outpatient clinic for follow-up, she informed us that her rash had improved 1 month after starting the prednisone taper. She was later lost to follow-up. It is unclear if the transaminitis and neutropenia were related to the materials injected during the mesotherapy procedure or from long-standing health issues.
Mesotherapy promises aesthetic benefits through a minimally invasive procedure and therefore is rapidly gaining popularity in aesthetic spas and treatment centers. Due to the lack of regulation in treatment protocols and substances used, there have been numerous reported cases of adverse side effects following mesotherapy, such as pain, allergic reactions, urticaria, panniculitis, ulceration, hair loss, necrosis, paraffinoma, cutaneous tuberculosis, and rapidly growing nontuberculous mycobacterial infections.1-5 More serious side effects also have been reported, such as permanent scarring, deformities, delirium, and massive subcutaneous emphysema (Table).2,4-18

Given the potential complications of mesotherapy documented in the literature, we believe clinical investigations and trials must be performed to appropriately assess the safety and efficacy of this potentially hazardous procedure. Because there currently is insufficient research showing why certain patients are developing these adverse side effects, aesthetic spas and treatment centers should inform patients of all potential side effects associated with mesotherapy for the patient to make an informed decision about the procedure. Mesotherapy should be a point of focus for both the US Food and Drug Administration and researchers to determine its efficacy, safety, and standardization of the procedure.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
- Bishara AS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842-849.
- Ramos-e-Silva M, Pereira AL, Ramos-e-Silva S, et al. Oleoma: a rare complication of mesotherapy for cellulite. Int J Dermatol. 2012;51:162-167.
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465-480.
- Carbonne A, Brossier F, Arnaud I, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. 2009;47:1961-1964.
- Kadry R, Hamadah I, Al-Issa A, et al. Multifocal scalp abscess with subcutaneous fat necrosis and scarring alopecia as a complication of scalp mesotherapy. J Drugs Dermatol. 2008;7:72-73.
- Tor PC, Lee TS. Delirium with psychotic features possibly associated with mesotherapy. Psychosomatics. 2008;49:273-274.
- Al-Khenaizan S. Facial cutaneous ulcers following mesotherapy. Dermatol Surg. 2008;34:832-834.
- Davis MD, Wright TI, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-809.
- Beer K, Waibel J. Disfiguring scarring following mesotherapy-associated Mycobacterium cosmeticum infection. J Drugs Dermatol. 2009;8:391-393.
- Nabavi CB, Minckler DS, Tao JP. Histologic features of mesotherapy-induced orbital fat inflammation. Opthalmic Plast Reconstr Surg. 2009;25:69-70.
- Babacan T, Onat AM, Pehlivan Y, et al. A case of Behçet’s disease diagnosed by the panniculitis after mesotherapy. Rheumatol Int. 2010;30:1657-1659.
- Kim JB, Moon W, Park SJ, et al. Ischemic colitis after mesotherapy combined with anti-obesity medications. World J Gastroenterol. 2010;16:1537-1540.
- Orjuela D, Puerto G, Mejia G, et al. Cutaneous tuberculosis after mesotherapy: report of six cases. Biomedica. 2010;30:321-326.
- Rallis E, Kintzoglou S, Moussatou V, et al. Mesotherapy-induced urticaria. Dermatol Surg. 2010;36:1355-1356.
- Calonge WM, Lesbros-Pantoflickova D, Hodina M, et al. Massive subcutaneous emphysema after carbon dioxide mesotherapy. Aesthetic Plast Surg. 2013;37:194-197.
- Park EJ, Kim HS, Kim M, et al. Histological changes after treatment for localized fat deposits with phosphatidylcholine and sodium deoxycholate. J Cosmet Dermatol. 2013;3:240-243.
- Rodriguez-Gutierrez G, Toussaint S, Hernandez-Castro R, et al. Norcardia brasiliensis infection: an emergent suppurative granuloma after mesotherapy. Int J Dermatol. 2014;53:888-890.
- El-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia [published online February 3, 2017]. J Cosmet Dermatol. 2017;16:E28-E30.
Practice Points
- Mesotherapy—the delivery of vitamins, chemicals, and plant extracts directly into the dermis via injections—is a common procedure performed in both medical and nonmedical settings for cosmetic rejuvenation.
- Complications can occur from mesotherapy treatment.
- Patients should be advised to seek medical care with US Food and Drug Administration–approved cosmetic techniques and substances only
Morning and evening skin care: What to tell patients
LAHAINA, HAWAII – That’s the simple message about daily skin care that clinicians can offer patients, according to Brooke Sikora, MD.
“At a very basic level, you want to tell your patients [to] use an antioxidant and use your sunscreen” early in the day, said Dr. Sikora, who is in private practice in Chestnut Hill, Mass. “In the evening, it’s all about repairing their damage, so make sure they’re getting on a retinol,” and if they can’t tolerate prescription strength, try a nonprescription product, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Aging factors create oxidative stress on the skin that leads to the development of these reactive oxygen species on the skin,” decreasing collagen production and increasing collagen breakdown, she explained during a presentation on cosmeceuticals at the meeting. Applying an antioxidant to the skin, however, can help neutralize “a lot of these reactive oxygen species and help to slow the breakdown of collagen.”
There is good evidence that peptides and growth factors – although expensive – work well and are worth recommending for patients “who really want to take their skin care to the next level,” Dr. Sikora said. “Then you can add corrective products like hyperpigmentation or acne products to treat ... specific concerns” as needed.
In an interview at the meeting, Dr. Sikora discussed these recommendations, as well as vitamin C use in the daily skin care routine. (To listen to the interview, click on the play button below.)
Vitamin C is the best-studied antioxidant, she noted during her presentation, and in vivo studies have shown it can stimulate collagen synthesis, reduce erythema of rosacea (which is why she has all her rosacea patients on vitamin C), reduce post-UVB erythema, decrease facial wrinkles, and increase dermal papillae.
Dr. Sikora disclosed that she is a consultant to and on the advisory board of SkinCeuticals, La Roche–Posay, Silk Therapeutics, Galderma, Evolus, and Allergen. She is on the speakers bureau for SkinCeuticals, La Roche–Posay, Galderma, and Aclaris.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click on the play button below.
LAHAINA, HAWAII – That’s the simple message about daily skin care that clinicians can offer patients, according to Brooke Sikora, MD.
“At a very basic level, you want to tell your patients [to] use an antioxidant and use your sunscreen” early in the day, said Dr. Sikora, who is in private practice in Chestnut Hill, Mass. “In the evening, it’s all about repairing their damage, so make sure they’re getting on a retinol,” and if they can’t tolerate prescription strength, try a nonprescription product, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Aging factors create oxidative stress on the skin that leads to the development of these reactive oxygen species on the skin,” decreasing collagen production and increasing collagen breakdown, she explained during a presentation on cosmeceuticals at the meeting. Applying an antioxidant to the skin, however, can help neutralize “a lot of these reactive oxygen species and help to slow the breakdown of collagen.”
There is good evidence that peptides and growth factors – although expensive – work well and are worth recommending for patients “who really want to take their skin care to the next level,” Dr. Sikora said. “Then you can add corrective products like hyperpigmentation or acne products to treat ... specific concerns” as needed.
In an interview at the meeting, Dr. Sikora discussed these recommendations, as well as vitamin C use in the daily skin care routine. (To listen to the interview, click on the play button below.)
Vitamin C is the best-studied antioxidant, she noted during her presentation, and in vivo studies have shown it can stimulate collagen synthesis, reduce erythema of rosacea (which is why she has all her rosacea patients on vitamin C), reduce post-UVB erythema, decrease facial wrinkles, and increase dermal papillae.
Dr. Sikora disclosed that she is a consultant to and on the advisory board of SkinCeuticals, La Roche–Posay, Silk Therapeutics, Galderma, Evolus, and Allergen. She is on the speakers bureau for SkinCeuticals, La Roche–Posay, Galderma, and Aclaris.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click on the play button below.
LAHAINA, HAWAII – That’s the simple message about daily skin care that clinicians can offer patients, according to Brooke Sikora, MD.
“At a very basic level, you want to tell your patients [to] use an antioxidant and use your sunscreen” early in the day, said Dr. Sikora, who is in private practice in Chestnut Hill, Mass. “In the evening, it’s all about repairing their damage, so make sure they’re getting on a retinol,” and if they can’t tolerate prescription strength, try a nonprescription product, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Aging factors create oxidative stress on the skin that leads to the development of these reactive oxygen species on the skin,” decreasing collagen production and increasing collagen breakdown, she explained during a presentation on cosmeceuticals at the meeting. Applying an antioxidant to the skin, however, can help neutralize “a lot of these reactive oxygen species and help to slow the breakdown of collagen.”
There is good evidence that peptides and growth factors – although expensive – work well and are worth recommending for patients “who really want to take their skin care to the next level,” Dr. Sikora said. “Then you can add corrective products like hyperpigmentation or acne products to treat ... specific concerns” as needed.
In an interview at the meeting, Dr. Sikora discussed these recommendations, as well as vitamin C use in the daily skin care routine. (To listen to the interview, click on the play button below.)
Vitamin C is the best-studied antioxidant, she noted during her presentation, and in vivo studies have shown it can stimulate collagen synthesis, reduce erythema of rosacea (which is why she has all her rosacea patients on vitamin C), reduce post-UVB erythema, decrease facial wrinkles, and increase dermal papillae.
Dr. Sikora disclosed that she is a consultant to and on the advisory board of SkinCeuticals, La Roche–Posay, Silk Therapeutics, Galderma, Evolus, and Allergen. She is on the speakers bureau for SkinCeuticals, La Roche–Posay, Galderma, and Aclaris.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click on the play button below.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Patient counseling about what to expect with noninvasive skin tightening is key
LAHAINA, HAWAII – It’s important to counsel patients about the degree of improvement to expect with noninvasive skin tightening procedures, Nazanin Saedi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Many and we really need to educate our patients about what we can do so that they have realistic expectations,” said Dr. Saedi, director of laser surgery and cosmetic dermatology at Sidney Kimmel Medical College, Philadelphia.
Treatment with these devices improve skin laxity, and some improve skin texture as well, she said. These devices are not an option for patients who want to have several inches of excess skin removed.
“You have to tell patients that this isn’t a replacement for a face-lift or a mini face-lift,” but patients can expect to see mild and modest improvement, and they’ll continue to see improvement for 3-6 months.
Patient selection is also important. Patients with mild to moderate laxity who do not want to undergo surgery and anesthesia are good candidates, as opposed to those who are older and have thin, sagging skin, Dr. Saedi said, noting that there still is no standard method of defining laxity.
She referred to a recent study illustrating the importance of counseling patients about what to expect. Of the 83 patients in a practice who had undergone microfocused ultrasound treatments and responded to an anonymous survey about the results of treatment, almost 80% reported at least mild improvement (14.5% said the improvement was significant, almost 28% said it was moderate, 37.3% said it was mild, and 20.5% said there was no improvement).
However, although about half (53.1%) reported being satisfied with their results, almost 45% said that the results did not meet their expectations (Lasers Surg Med. 2019;51[6]:495-9).
In an interview at the meeting, Dr. Saedi commented on these results and the importance of counseling.
Listen to the interview by clicking the play button at the end of this story.
During the presentation, Dr. Saedi, who is also codirector of cutaneous surgery in the department of dermatology and cutaneous biology at Sidney Kimmel Medical College, reviewed different technologies used for noninvasive skin tightening, including ablative and fractional laser resurfacing, radiofrequency, and microfocused ultrasound with visualization.
She disclosed serving on the advisory board and/or as a consultant for Aerolase, Alastin, Alma, Cartessa Aesthetics, Cynosure, and Vivo Capital, and that she has equipment from these companies, except for Vivo Capital and Alastin.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
LAHAINA, HAWAII – It’s important to counsel patients about the degree of improvement to expect with noninvasive skin tightening procedures, Nazanin Saedi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Many and we really need to educate our patients about what we can do so that they have realistic expectations,” said Dr. Saedi, director of laser surgery and cosmetic dermatology at Sidney Kimmel Medical College, Philadelphia.
Treatment with these devices improve skin laxity, and some improve skin texture as well, she said. These devices are not an option for patients who want to have several inches of excess skin removed.
“You have to tell patients that this isn’t a replacement for a face-lift or a mini face-lift,” but patients can expect to see mild and modest improvement, and they’ll continue to see improvement for 3-6 months.
Patient selection is also important. Patients with mild to moderate laxity who do not want to undergo surgery and anesthesia are good candidates, as opposed to those who are older and have thin, sagging skin, Dr. Saedi said, noting that there still is no standard method of defining laxity.
She referred to a recent study illustrating the importance of counseling patients about what to expect. Of the 83 patients in a practice who had undergone microfocused ultrasound treatments and responded to an anonymous survey about the results of treatment, almost 80% reported at least mild improvement (14.5% said the improvement was significant, almost 28% said it was moderate, 37.3% said it was mild, and 20.5% said there was no improvement).
However, although about half (53.1%) reported being satisfied with their results, almost 45% said that the results did not meet their expectations (Lasers Surg Med. 2019;51[6]:495-9).
In an interview at the meeting, Dr. Saedi commented on these results and the importance of counseling.
Listen to the interview by clicking the play button at the end of this story.
During the presentation, Dr. Saedi, who is also codirector of cutaneous surgery in the department of dermatology and cutaneous biology at Sidney Kimmel Medical College, reviewed different technologies used for noninvasive skin tightening, including ablative and fractional laser resurfacing, radiofrequency, and microfocused ultrasound with visualization.
She disclosed serving on the advisory board and/or as a consultant for Aerolase, Alastin, Alma, Cartessa Aesthetics, Cynosure, and Vivo Capital, and that she has equipment from these companies, except for Vivo Capital and Alastin.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
LAHAINA, HAWAII – It’s important to counsel patients about the degree of improvement to expect with noninvasive skin tightening procedures, Nazanin Saedi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Many and we really need to educate our patients about what we can do so that they have realistic expectations,” said Dr. Saedi, director of laser surgery and cosmetic dermatology at Sidney Kimmel Medical College, Philadelphia.
Treatment with these devices improve skin laxity, and some improve skin texture as well, she said. These devices are not an option for patients who want to have several inches of excess skin removed.
“You have to tell patients that this isn’t a replacement for a face-lift or a mini face-lift,” but patients can expect to see mild and modest improvement, and they’ll continue to see improvement for 3-6 months.
Patient selection is also important. Patients with mild to moderate laxity who do not want to undergo surgery and anesthesia are good candidates, as opposed to those who are older and have thin, sagging skin, Dr. Saedi said, noting that there still is no standard method of defining laxity.
She referred to a recent study illustrating the importance of counseling patients about what to expect. Of the 83 patients in a practice who had undergone microfocused ultrasound treatments and responded to an anonymous survey about the results of treatment, almost 80% reported at least mild improvement (14.5% said the improvement was significant, almost 28% said it was moderate, 37.3% said it was mild, and 20.5% said there was no improvement).
However, although about half (53.1%) reported being satisfied with their results, almost 45% said that the results did not meet their expectations (Lasers Surg Med. 2019;51[6]:495-9).
In an interview at the meeting, Dr. Saedi commented on these results and the importance of counseling.
Listen to the interview by clicking the play button at the end of this story.
During the presentation, Dr. Saedi, who is also codirector of cutaneous surgery in the department of dermatology and cutaneous biology at Sidney Kimmel Medical College, reviewed different technologies used for noninvasive skin tightening, including ablative and fractional laser resurfacing, radiofrequency, and microfocused ultrasound with visualization.
She disclosed serving on the advisory board and/or as a consultant for Aerolase, Alastin, Alma, Cartessa Aesthetics, Cynosure, and Vivo Capital, and that she has equipment from these companies, except for Vivo Capital and Alastin.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Data back botulinum toxin for facial flushing, androgenetic alopecia
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Vascular occlusion management
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.
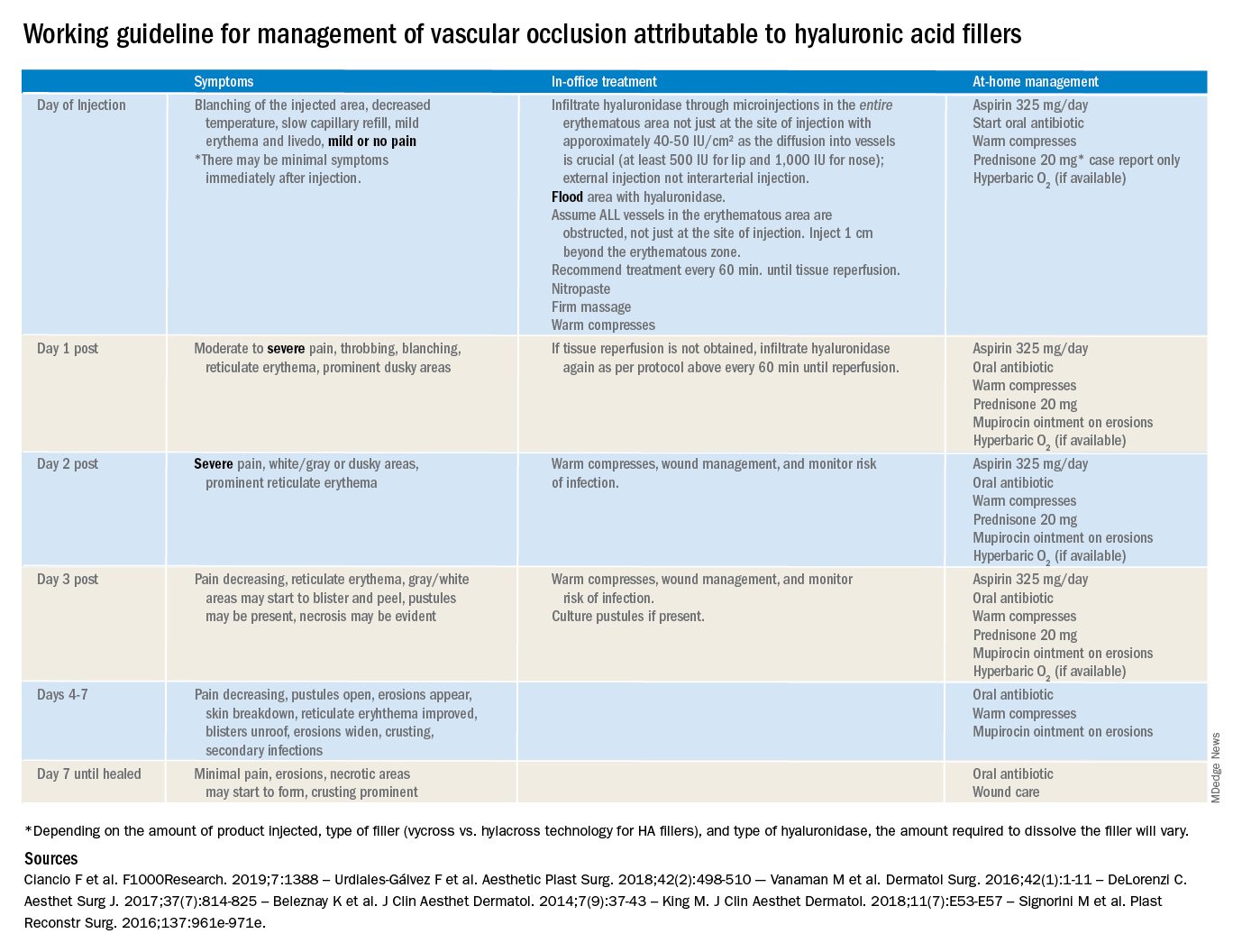
There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
The time course and proper management of vascular occlusion attributable to interarterial hyaluronic acid fillers is critical. Albeit a rare complication, off-label uses of HA fillers, lack of proper training of injectors, and lack of clear appropriate guidelines in the management of these complications are some of the causes of delayed treatment and necrotic complications.

There are currently no definitive guidelines for the management of filler-associated cutaneous necrosis as experience with its treatment continues to evolve and be reported. In an attempt to consolidate the published data, as well as to give somewhat of a clear guideline of expectations, a time course and treatment guide has been outlined. The following is a working guideline for management of vascular occlusion attributable to HA fillers based on reports in the literature. This is not a consensus statement, rather it is a consolidation of the anecdotal reports and case studies outlined to help practitioners. It is also not inclusive of all the presentations of vascular occlusion. There are delayed cases of vascular occlusion beginning several days after injection, as well as alternative treatment options that may be considered.
These guidelines also are not for the devastating complication of blindness because of vascular occlusion secondary to fillers. Blindness is beyond the scope of the current article; however, we believe all experienced injectors should have emergency preparations in place and a relationship with an ophthalmologist or other trained surgeons experienced in performing retrobulbar hyaluronidase injections who can be reached in the event of a suspected occlusion. Any symptoms of eye pain, headache, or visual changes need to be immediately treated. Vascular occlusion is an emergency and timing is critical to prevent permanent blindness and facial deformities.
As with all filler injections, risks and complications can happen, and we cannot stress enough the appropriate level of training, as well as expert understanding of anatomy and injection technique, in minimizing potential risks. We encourage regulations and a required level of training to perform these procedures.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Cosmeceutical ingredients to use before and after antiaging procedures
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.