User login
Snail mucus in skin care
Although it is not glamorous, .1 The modern consideration of using snail secretions in skin care arose serendipitously in the 1990s when Chilean farmers observed accelerated healing of their skin lesions without scarring after handling snails.1
Today, snail mucin is among the increasingly wide array of bioactive ingredients undergoing scientific validation and inclusion in the burgeoning Korean cosmeceutical market.2,3 In fact, a variety of Korean cosmeceuticals incorporate the mucus derived from Achatina fulica (African giant land snail) and Cryptomphalus (Helix) aspersa (common brown garden snail) based on their demonstrated antimicrobial and skin regenerative activity.1,3,4 The antioxidant properties also attributed to snail mucus are thought to originate in constituents such as glycosaminoglycans, as well as growth factors, and may justify the use of these ingredients in novel cosmeceuticals.5 The focus of this discussion is recent research into the novel use of this animal-derived product for dermatologic purposes.
Antioxidant activity, skin rejuvenation, and wound healing
In 2008, Brieva et al. reported on a screen for natural products yielding a molecular basis for the secretions of the mollusk Cryptomphalus aspersa, which displays skin-regenerative activity. Specifically, they found that the secretion exerts antioxidant superoxide dismutase and glutathione S-transferase, and spurred fibroblast proliferation and extracellular matrix assembly while regulating metalloproteinase function. The researchers concluded that such activities may support wound regeneration.5
Four years later, Cruz et al. found that secretions of C. aspersa promote in vitro cell proliferation and migration by localizing beta-catenin to the nuclei of human fibroblasts and keratinocytes, augment phosphorylated focal adhesion kinase, and thereby enhance cell survival. The investigators concluded that snail secretions may therefore impart regenerative and wound healing activity.3,6
Antimicrobial properties
In 2015, Pitt et al. investigated the antimicrobial properties of the mucus of the brown garden snail C. or H. aspersa, which had a reputation for exhibiting skin regeneration capabilities. Their results revealed that snail mucus displayed a strong antibacterial effect against multiple strains of Pseudomonas aeruginosa and a weak effect against Staphylococcus aureus.4
Indications for the use of snail mucin
Radiation-induced dermatitis and burns represented the first indication for the initial use of snail mucin as a cutaneous therapy.7 Experimental and clinical studies have since been performed to assess its applicability to treat acute radiation dermatitis, atopic dermatitis, partial-thickness burns, and photoaging.8-11
A 2017 in vitro investigation by Ellijimi et al. revealed that snail mucin displayed antimelanogenic and antitumoral activity against human melanoma cells, suggesting another possible application of this product.12
Human studies on photoaging
In a 2009 study by Tsoutsos et al. of an open, moist burn management protocol in deep partial-thickness facial burns, a cream containing H. aspersa secretions was identified to be an effective treatment option. For 14 days or until full epithelialization, 27 adult patients were treated with snail extract cream twice daily. Comparisons were made to 16 patients treated with moist exposure burn ointment. Visual analog scale pain scores were significantly lower in the group that received the H. aspersa cream, compared with the moist exposure burn group. The researchers concluded that the H. aspersa cream is a safe, effective, and natural option for treating partial-thickness burns in adults that acts by facilitating debris removal and accelerating reepithelialization.10
Also that year, Tribo-Boixareu et al. treated 15 patients with chronic photodamage with secretions of C. aspersa over a 3-month period, yielding significant amelioration in the clinical and histologic markers of photoaging.11
Four years later, a double-blind, split-face, randomized, controlled clinical study conducted by Fabi et al. over 12 weeks demonstrated that the topical application of an antiphotoaging formulation containing C. aspersa mucus diminished periocular and fine facial rhytides and enhanced skin texture within 8 weeks of treatment initiation.7
Snail eggs and photoaging
In 2015, Espada et al. determined in vitro that an extract derived from C. aspersa eggs could reorganize the cytoskeleton of keratinocytes and fibroblasts, as well as trigger the synthesis of the extracellular proteins collagen and fibronectin. They also found that gene expression declined in age-related genes including p53 and b-Gal. The researchers concluded that C. aspersa egg extract has the potential to reduce the signs of photoaging.3,13
Antiaging cosmeceuticals
In a 2017 assessment of the antiaging and skin-whitening activity of the nine most popular ingredients in the South Korean skin care product market, Quay et al. considered industry profit data from Euromonitor and conducted a comprehensive literature search. They identified licorice, niacinamide, green tea, soy, beta-glucan, snail mucus, ginkgo biloba, ginseng, and pomegranate as the nine most popular ingredients, with the first four associated with the most supportive data. They found a paucity of cogent evidence on the use of the other ingredients in antiaging and skin-whitening formulations.14
Conclusion
The use of snail mucin to treat skin dates back at least to the time of Hippocrates. Recent research suggests reasons for optimism, and further investigation, as this ingredient appears to have potential across various cutaneous conditions. As is often the case, though, much more research is necessary to ascertain what enduring benefits may be derived from the use of snail mucin. Nevertheless, this product has been available on the market for the last 20 years and is associated with anecdotal reports of efficacy.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]. She has no relevant disclosures.
References
1. Liu L et al. Snails and skin care – an uncovered combination. JAMA Dermatol. 2017 Jul 1;153(7):650.
2. Nguyen JK et al. J Cosmet Dermatol. 2020 Feb 26. doi: 10.1111/jocd.13344.
3. Juhász ML et al. J Cosmet Dermatol. 2018;17(3):305-12.
4. Pitt SJ et al. Br J Biomed Sci. 2015;72(4):174-81.
5. Brieva A et al. Skin Pharmacol Physiol. 2008;21(1):15-22.
6. Cruz MC et al. Int J Cosmet Sci. 2012 Apr;34(2):183-9.
7. Fabi SG et al. J Drugs Dermatol. 2013; Apr;12(4):453-7.
8. Ledo E et al. Radioproteccion. 1999;23(7):34-8.
9. Oh M-Jet al. J Korean Med Ophthalmol Otolaryngol Dermatol. 2010; Dec,23(3):138-53.
10. Tsoutsos D et al. J Dermatolog Treat. 2009;20(4):219-22.
11. Tribo-Boixareu MJ et al. Cosmet Dermatol. 2009;22(5):247-52.
12. Ellijimi C et al. Biomed Pharmacother. 2018 May;101:871-80.
13. Espada J et al. Int J Cosmet Sci. 2015 Feb;37(1):41-55.
14. Quay ER et al. J Drugs Dermatol. 2017 Apr 1;16(4):358-63.
Although it is not glamorous, .1 The modern consideration of using snail secretions in skin care arose serendipitously in the 1990s when Chilean farmers observed accelerated healing of their skin lesions without scarring after handling snails.1
Today, snail mucin is among the increasingly wide array of bioactive ingredients undergoing scientific validation and inclusion in the burgeoning Korean cosmeceutical market.2,3 In fact, a variety of Korean cosmeceuticals incorporate the mucus derived from Achatina fulica (African giant land snail) and Cryptomphalus (Helix) aspersa (common brown garden snail) based on their demonstrated antimicrobial and skin regenerative activity.1,3,4 The antioxidant properties also attributed to snail mucus are thought to originate in constituents such as glycosaminoglycans, as well as growth factors, and may justify the use of these ingredients in novel cosmeceuticals.5 The focus of this discussion is recent research into the novel use of this animal-derived product for dermatologic purposes.
Antioxidant activity, skin rejuvenation, and wound healing
In 2008, Brieva et al. reported on a screen for natural products yielding a molecular basis for the secretions of the mollusk Cryptomphalus aspersa, which displays skin-regenerative activity. Specifically, they found that the secretion exerts antioxidant superoxide dismutase and glutathione S-transferase, and spurred fibroblast proliferation and extracellular matrix assembly while regulating metalloproteinase function. The researchers concluded that such activities may support wound regeneration.5
Four years later, Cruz et al. found that secretions of C. aspersa promote in vitro cell proliferation and migration by localizing beta-catenin to the nuclei of human fibroblasts and keratinocytes, augment phosphorylated focal adhesion kinase, and thereby enhance cell survival. The investigators concluded that snail secretions may therefore impart regenerative and wound healing activity.3,6
Antimicrobial properties
In 2015, Pitt et al. investigated the antimicrobial properties of the mucus of the brown garden snail C. or H. aspersa, which had a reputation for exhibiting skin regeneration capabilities. Their results revealed that snail mucus displayed a strong antibacterial effect against multiple strains of Pseudomonas aeruginosa and a weak effect against Staphylococcus aureus.4
Indications for the use of snail mucin
Radiation-induced dermatitis and burns represented the first indication for the initial use of snail mucin as a cutaneous therapy.7 Experimental and clinical studies have since been performed to assess its applicability to treat acute radiation dermatitis, atopic dermatitis, partial-thickness burns, and photoaging.8-11
A 2017 in vitro investigation by Ellijimi et al. revealed that snail mucin displayed antimelanogenic and antitumoral activity against human melanoma cells, suggesting another possible application of this product.12
Human studies on photoaging
In a 2009 study by Tsoutsos et al. of an open, moist burn management protocol in deep partial-thickness facial burns, a cream containing H. aspersa secretions was identified to be an effective treatment option. For 14 days or until full epithelialization, 27 adult patients were treated with snail extract cream twice daily. Comparisons were made to 16 patients treated with moist exposure burn ointment. Visual analog scale pain scores were significantly lower in the group that received the H. aspersa cream, compared with the moist exposure burn group. The researchers concluded that the H. aspersa cream is a safe, effective, and natural option for treating partial-thickness burns in adults that acts by facilitating debris removal and accelerating reepithelialization.10
Also that year, Tribo-Boixareu et al. treated 15 patients with chronic photodamage with secretions of C. aspersa over a 3-month period, yielding significant amelioration in the clinical and histologic markers of photoaging.11
Four years later, a double-blind, split-face, randomized, controlled clinical study conducted by Fabi et al. over 12 weeks demonstrated that the topical application of an antiphotoaging formulation containing C. aspersa mucus diminished periocular and fine facial rhytides and enhanced skin texture within 8 weeks of treatment initiation.7
Snail eggs and photoaging
In 2015, Espada et al. determined in vitro that an extract derived from C. aspersa eggs could reorganize the cytoskeleton of keratinocytes and fibroblasts, as well as trigger the synthesis of the extracellular proteins collagen and fibronectin. They also found that gene expression declined in age-related genes including p53 and b-Gal. The researchers concluded that C. aspersa egg extract has the potential to reduce the signs of photoaging.3,13
Antiaging cosmeceuticals
In a 2017 assessment of the antiaging and skin-whitening activity of the nine most popular ingredients in the South Korean skin care product market, Quay et al. considered industry profit data from Euromonitor and conducted a comprehensive literature search. They identified licorice, niacinamide, green tea, soy, beta-glucan, snail mucus, ginkgo biloba, ginseng, and pomegranate as the nine most popular ingredients, with the first four associated with the most supportive data. They found a paucity of cogent evidence on the use of the other ingredients in antiaging and skin-whitening formulations.14
Conclusion
The use of snail mucin to treat skin dates back at least to the time of Hippocrates. Recent research suggests reasons for optimism, and further investigation, as this ingredient appears to have potential across various cutaneous conditions. As is often the case, though, much more research is necessary to ascertain what enduring benefits may be derived from the use of snail mucin. Nevertheless, this product has been available on the market for the last 20 years and is associated with anecdotal reports of efficacy.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]. She has no relevant disclosures.
References
1. Liu L et al. Snails and skin care – an uncovered combination. JAMA Dermatol. 2017 Jul 1;153(7):650.
2. Nguyen JK et al. J Cosmet Dermatol. 2020 Feb 26. doi: 10.1111/jocd.13344.
3. Juhász ML et al. J Cosmet Dermatol. 2018;17(3):305-12.
4. Pitt SJ et al. Br J Biomed Sci. 2015;72(4):174-81.
5. Brieva A et al. Skin Pharmacol Physiol. 2008;21(1):15-22.
6. Cruz MC et al. Int J Cosmet Sci. 2012 Apr;34(2):183-9.
7. Fabi SG et al. J Drugs Dermatol. 2013; Apr;12(4):453-7.
8. Ledo E et al. Radioproteccion. 1999;23(7):34-8.
9. Oh M-Jet al. J Korean Med Ophthalmol Otolaryngol Dermatol. 2010; Dec,23(3):138-53.
10. Tsoutsos D et al. J Dermatolog Treat. 2009;20(4):219-22.
11. Tribo-Boixareu MJ et al. Cosmet Dermatol. 2009;22(5):247-52.
12. Ellijimi C et al. Biomed Pharmacother. 2018 May;101:871-80.
13. Espada J et al. Int J Cosmet Sci. 2015 Feb;37(1):41-55.
14. Quay ER et al. J Drugs Dermatol. 2017 Apr 1;16(4):358-63.
Although it is not glamorous, .1 The modern consideration of using snail secretions in skin care arose serendipitously in the 1990s when Chilean farmers observed accelerated healing of their skin lesions without scarring after handling snails.1
Today, snail mucin is among the increasingly wide array of bioactive ingredients undergoing scientific validation and inclusion in the burgeoning Korean cosmeceutical market.2,3 In fact, a variety of Korean cosmeceuticals incorporate the mucus derived from Achatina fulica (African giant land snail) and Cryptomphalus (Helix) aspersa (common brown garden snail) based on their demonstrated antimicrobial and skin regenerative activity.1,3,4 The antioxidant properties also attributed to snail mucus are thought to originate in constituents such as glycosaminoglycans, as well as growth factors, and may justify the use of these ingredients in novel cosmeceuticals.5 The focus of this discussion is recent research into the novel use of this animal-derived product for dermatologic purposes.
Antioxidant activity, skin rejuvenation, and wound healing
In 2008, Brieva et al. reported on a screen for natural products yielding a molecular basis for the secretions of the mollusk Cryptomphalus aspersa, which displays skin-regenerative activity. Specifically, they found that the secretion exerts antioxidant superoxide dismutase and glutathione S-transferase, and spurred fibroblast proliferation and extracellular matrix assembly while regulating metalloproteinase function. The researchers concluded that such activities may support wound regeneration.5
Four years later, Cruz et al. found that secretions of C. aspersa promote in vitro cell proliferation and migration by localizing beta-catenin to the nuclei of human fibroblasts and keratinocytes, augment phosphorylated focal adhesion kinase, and thereby enhance cell survival. The investigators concluded that snail secretions may therefore impart regenerative and wound healing activity.3,6
Antimicrobial properties
In 2015, Pitt et al. investigated the antimicrobial properties of the mucus of the brown garden snail C. or H. aspersa, which had a reputation for exhibiting skin regeneration capabilities. Their results revealed that snail mucus displayed a strong antibacterial effect against multiple strains of Pseudomonas aeruginosa and a weak effect against Staphylococcus aureus.4
Indications for the use of snail mucin
Radiation-induced dermatitis and burns represented the first indication for the initial use of snail mucin as a cutaneous therapy.7 Experimental and clinical studies have since been performed to assess its applicability to treat acute radiation dermatitis, atopic dermatitis, partial-thickness burns, and photoaging.8-11
A 2017 in vitro investigation by Ellijimi et al. revealed that snail mucin displayed antimelanogenic and antitumoral activity against human melanoma cells, suggesting another possible application of this product.12
Human studies on photoaging
In a 2009 study by Tsoutsos et al. of an open, moist burn management protocol in deep partial-thickness facial burns, a cream containing H. aspersa secretions was identified to be an effective treatment option. For 14 days or until full epithelialization, 27 adult patients were treated with snail extract cream twice daily. Comparisons were made to 16 patients treated with moist exposure burn ointment. Visual analog scale pain scores were significantly lower in the group that received the H. aspersa cream, compared with the moist exposure burn group. The researchers concluded that the H. aspersa cream is a safe, effective, and natural option for treating partial-thickness burns in adults that acts by facilitating debris removal and accelerating reepithelialization.10
Also that year, Tribo-Boixareu et al. treated 15 patients with chronic photodamage with secretions of C. aspersa over a 3-month period, yielding significant amelioration in the clinical and histologic markers of photoaging.11
Four years later, a double-blind, split-face, randomized, controlled clinical study conducted by Fabi et al. over 12 weeks demonstrated that the topical application of an antiphotoaging formulation containing C. aspersa mucus diminished periocular and fine facial rhytides and enhanced skin texture within 8 weeks of treatment initiation.7
Snail eggs and photoaging
In 2015, Espada et al. determined in vitro that an extract derived from C. aspersa eggs could reorganize the cytoskeleton of keratinocytes and fibroblasts, as well as trigger the synthesis of the extracellular proteins collagen and fibronectin. They also found that gene expression declined in age-related genes including p53 and b-Gal. The researchers concluded that C. aspersa egg extract has the potential to reduce the signs of photoaging.3,13
Antiaging cosmeceuticals
In a 2017 assessment of the antiaging and skin-whitening activity of the nine most popular ingredients in the South Korean skin care product market, Quay et al. considered industry profit data from Euromonitor and conducted a comprehensive literature search. They identified licorice, niacinamide, green tea, soy, beta-glucan, snail mucus, ginkgo biloba, ginseng, and pomegranate as the nine most popular ingredients, with the first four associated with the most supportive data. They found a paucity of cogent evidence on the use of the other ingredients in antiaging and skin-whitening formulations.14
Conclusion
The use of snail mucin to treat skin dates back at least to the time of Hippocrates. Recent research suggests reasons for optimism, and further investigation, as this ingredient appears to have potential across various cutaneous conditions. As is often the case, though, much more research is necessary to ascertain what enduring benefits may be derived from the use of snail mucin. Nevertheless, this product has been available on the market for the last 20 years and is associated with anecdotal reports of efficacy.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]. She has no relevant disclosures.
References
1. Liu L et al. Snails and skin care – an uncovered combination. JAMA Dermatol. 2017 Jul 1;153(7):650.
2. Nguyen JK et al. J Cosmet Dermatol. 2020 Feb 26. doi: 10.1111/jocd.13344.
3. Juhász ML et al. J Cosmet Dermatol. 2018;17(3):305-12.
4. Pitt SJ et al. Br J Biomed Sci. 2015;72(4):174-81.
5. Brieva A et al. Skin Pharmacol Physiol. 2008;21(1):15-22.
6. Cruz MC et al. Int J Cosmet Sci. 2012 Apr;34(2):183-9.
7. Fabi SG et al. J Drugs Dermatol. 2013; Apr;12(4):453-7.
8. Ledo E et al. Radioproteccion. 1999;23(7):34-8.
9. Oh M-Jet al. J Korean Med Ophthalmol Otolaryngol Dermatol. 2010; Dec,23(3):138-53.
10. Tsoutsos D et al. J Dermatolog Treat. 2009;20(4):219-22.
11. Tribo-Boixareu MJ et al. Cosmet Dermatol. 2009;22(5):247-52.
12. Ellijimi C et al. Biomed Pharmacother. 2018 May;101:871-80.
13. Espada J et al. Int J Cosmet Sci. 2015 Feb;37(1):41-55.
14. Quay ER et al. J Drugs Dermatol. 2017 Apr 1;16(4):358-63.
Authors of picosecond laser review predict more widespread use of the technology
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
Ever since the first picosecond laser hit the market in 2012 as an option for treating unwanted tattoos and pigmented lesions, clinicians have used the technology to safely and effectively treat an expanding range of dermatologic conditions, from Nevus of Ota and melasma to rejuvenation.
. They called for further development of the technology and predicted that application of the devices will become more widespread.
“Future directions may include the development of even shorter pulse durations, improvements in fractionation method and delivery, and exploration of the utility of pulsing other laser wavelengths in the picosecond (or shorter) domain,” first author Douglas C. Wu, MD, PhD, of Cosmetic Laser Dermatology and colleagues wrote in the review. “The introduction of newer devices along with continued improvements in clinical technique and experience will drive the refinement and expansion of this technology.”
The authors evaluated medical literature on the topic published up to March 2020 and classified 78 studies into one of the following categories: discrete pigmented lesions, other nonmelasma pigmented conditions, rejuvenation, melasma, scar revision, and tattoo removal. They assessed the level of evidence for each indication according to modified criteria published by the Oxford Centre of Evidence-Based Medicine and proposed recommendations based on the medical literature in combination with the authors’ collective clinical experience with picosecond laser.
In the category of discrete pigmented lesions, the authors assigned level of evidence 1a to Nevus of Ota and Hori’s macules, level of evidence 2b to solar lentigines and freckles, level of evidence 3c to café au lait macules, and level of evidence 4 to all other benign pigmentary conditions. “Comparative studies utilizing clinical, histological, and microscopic endpoints further suggest that picosecond laser may be safer and more effective than nanosecond laser in some situations, with potentially reduced risk of inducing postinflammatory hyperpigmentation,” the authors wrote. “This increased safety level may be due to the reduction of non-specific photothermal damage of the melanocyte and dermal-epidermal junction,” they noted. They called for more robust clinical comparative data with a focus on shorter pulse durations and refined clinical endpoints “to further distinguish the differences between picosecond and nanosecond laser for the treatment of some benign pigmented lesions.”
Based on seven prospective open-label trials and three split-face comparison trials involving the use of picosecond lasers for photorejuvenation, the authors assigned a level of evidence 2a to this category. “The studies show a high level of safety associated with a moderate level of efficacy,” they wrote. “Indeed, when compared with traditional non-ablative fractional laser, fractionated picosecond laser may have an improved side effect profile without sacrificing treatment efficacy. This could be due to the unique mechanism of action of fractionated picosecond laser, which results in greater confinement of tissue injury to focal and precise points within the epidermis and papillary dermis.”
Clinical data on using picosecond lasers to treat melasma remains “mixed and unclear,” but it may have a role as an adjunctive treatment combined with rigorous photoprotection, topical melanin inhibitors, “and potentially other laser or systemic therapies as dictated by clinical circumstance,” the authors said. They do not recommend the picosecond laser as a monotherapy for melasma, and they assigned a level of evidence 2a to this category.
Although the fractionated picosecond laser is cleared by the Food and Drug Administration for the treatment of acne scars, Dr. Wu and his colleagues noted that rigorous clinical data on using the technology for this indication is limited. “Encouragingly, reports thus far seem to suggest that the risk of post-inflammatory pigmentary alteration is low when using fractionated picosecond laser, which has added significance due to the high prevalence of acne scarring in skin of color,” they wrote. They assigned a level of evidence 2b to this category. Meanwhile, clinical data on the use of picosecond lasers for non-acne scars are limited to cases series and retrospective reviews, reaching evidence level 3c. “Although the level of evidence is weak, there is likely an effective role for fractionated picosecond laser for the improvement of hyperpigmented scars given its more robust track record for the treatment of hyperpigmentation due to other causes such as benign pigmentary conditions and photodamage,” the authors wrote.
The manuscript concludes with a discussion of the picosecond laser’s role in tattoo removal, which represents the oldest and most established dermatologic indication for the technology. “The accumulated scientific and clinical evidence to date concludes that the shorter pulse duration confers a distinct advantage when other laser parameters remain equal,” the authors wrote. “The evidence also suggests that the shorter the pulse gets (within currently commercially available and tested devices), the greater becomes the efficacy for tattoo removal. There is no evidence to suggest that larger tattoo particles are more optimally targeted by longer nanosecond pulses.” They assigned a level of evidence 1a to this category and described using the picosecond laser for tattoo removal of almost any color as “the gold standard.”
In an interview, Arisa Ortiz, MD, described the manuscript as a thorough review of the clinical indications for picosecond lasers. “Overall, the review shows evidence for slightly better improvement of efficacy with picosecond lasers compared to nanosecond lasers,” said Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, who was not involved with the review. “They also show a slightly improved side effect profile with picosecond lasers [and] notably, less risk of postinflammatory hyperpigmentation in darker skin types compared to nanosecond lasers. One issue that was not addressed was the cost of picosecond lasers. The cost of a picosecond lasers remains substantially higher than the cost of a nanosecond laser. I am not sure that this extra cost justifies a slightly improved efficacy or slightly improved side effect profile.”
According to Eric F. Bernstein, MD, director of the Main Line Center for Laser Surgery in Ardmore, Penn., the versatility of picosecond lasers offers an advantage to dermatologists. “Most of them have three wavelengths at least,” said Dr. Bernstein, who was not involved with the systematic review. “That means you can treat skin types I-VI. I was never able to offer much for my patients with skin types V and VI for fractionated rejuvenation and treatment of acne scarring. But now, with these lasers, I have an option for them. That’s a huge advantage.”
He credited laser engineers as “the real heroes” in the success of picosecond lasers in dermatology. “They’re passionate, they’re brilliant, and they’re creative,” Dr. Bernstein said. “They’re the ones that build and produce these devices for multiple manufacturers. In our space, the innovation really comes from industry.”
The review authors and Dr. Ortiz reported having no relevant disclosures. Dr. Bernstein disclosed that he is head of Candela’s medical advisory board.
SOURCE: Wu DC et al. Lasers Surg Med. 2020. doi: 10.1002/lsm.23244.
FROM LASERS IN SURGERY AND MEDICINE
Penile Paraffinoma: Dramatic Recurrence After Surgical Resection
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
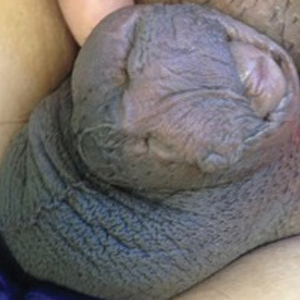
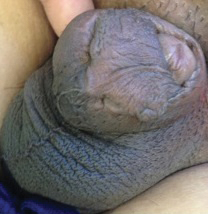
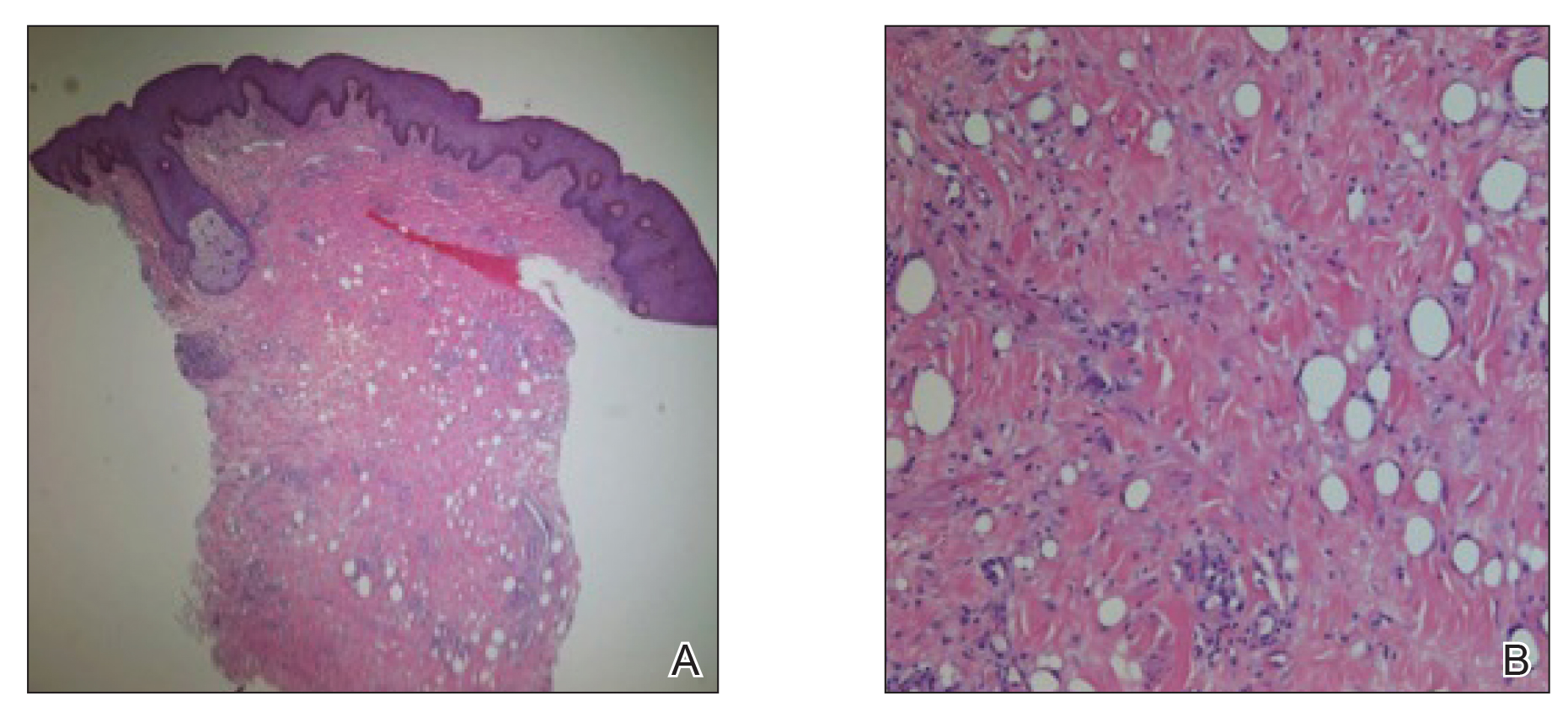
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.
The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.


The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.


The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
Practice Points
- Taking a thorough history in patients with possible paraffinomas is vital, including a history of injectables even in the genital region.
- Biopsies in cosmetically sensitive areas must be given careful consideration. Clinical history must support the decision to pursue a definitive diagnosis.
- Early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance.
Laser surgery precautions as clinics begin to reopen amid COVID-19
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Sericin, a versatile silk protein, has multiple potential roles in dermatology
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Experts recommend slow, steady approach to reopening laser and cosmetic surgery practices
“People talk about reinventing the wheel,” Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., said during an hour-long webinar on May 5 sponsored by the American Society for Laser Medicine and Surgery. “In this case, we’re inventing the wheel; no one’s ever done this before – not in our lifetimes. The last pandemic was over 100 years ago, when there wasn’t aesthetic medicine.”
Dr. Dover joined a panel of four other experts from around the country to discuss how to reopen practices safely and effectively. Paul M. Friedman, MD, director of the Houston Cosmetic Dermatology & Laser Center, moderated the event.
In Florida, which reopened certain businesses on May 4, 2020, Jill S. Waibel, MD, plans to start at 25% capacity at Miami Dermatology and Laser Institute, and build from there. “We’re trying to take care of skin cancer patients first,” said Dr. Waibel, a dermatologist who owns the practice. “Then we’re going to start doing less aggressive cosmetic procedures like injectables, nonablative procedures. We’ll move into the more aggressive procedures as we ease back into it. We really want to see what’s going to happen 2-3 weeks down the line now that things are starting to open up.”
In Maryland, where state officials announced on May 6 that guidelines would be issued to allow for nonmedical procedures, Elizabeth L. Tanzi, MD, founder and director of Capital Laser & Skin Care in Chevy Chase, expects things to “look very different” once her practice reopens. “We are taking it very slowly,” she said. “Teledermatology for acne and other follow-ups is not something we did before, but it is certainly something that we’ll continue.”
The way she sees it, having the proper personal protective equipment is a key part of any reopening discussion. “I am not going near anyone’s face without an N95 mask that fits well, and without a face shield,” she said. “If you’re delegating these procedures to people that you don’t trust to be wearing the PPE correctly, then you shouldn’t be delegating them, because a key is the PPE. You have to assume that everyone has the virus at every time.”
In Ardmore, Pa., the Main Line Center for Laser Surgery remains closed because of current state regulations. When practice director Eric F. Bernstein, MD, gets the green light to reopen, patients will undergo a consultation by phone or videoconference and pay their bill before they set foot in the office. “We’re on the second floor, so patients can take a stairwell and avoid the elevator,” Dr. Bernstein said. “They’ll come in, not check in at the desk; go right to the room. There will be one treater and one assistant. If the patient doesn’t come in with a mask, we’ll supply one. It’s going to be a very different process. People are setting their hours longer because they’re going to be seeing fewer people. There will be no sitting in the waiting room.”
In the COVID-19 epicenter, Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, has been performing Mohs procedures and treating children with vascular malformations, but everything else is on hold. “Once the governor [Andrew Cuomo] lifts the stay-at-home restrictions, we’ll ease into things,” he said. “The issue of performing more invasive procedures – like ablative fractional resurfacing – is something that we are concerned about. I’m concerned about any laser that has environmental plume. For example, with our tattoo-removal procedures, I intend to treat every patient through a gel for the short term, and perhaps even for the long term. One can do that safely, and that eliminates the plume altogether.”
At the center, Dr. Geronemus added, “we do a fair amount of ablative fractional resurfacing and some fully ablative resurfacing. I intend to use large facial shields with these patients. We do use vacuum in each room as it stands right now, not only with electrosurgery, but we’ll be adding that to laser procedures as well. That will be helpful.”
In Chestnut Hill, Mass., Dr. Dover and his colleagues plan to practice what he termed “universal COVID precaution” by wearing a face mask, goggles, or a face shield, gloves, and protective clothing when necessary. “We are not going to do any ablative procedures, no procedures with plume, and we’re going to try and eliminate risk as much as we can,” he said. “We will have no waiting room; the patients will walk right to an exam room. They’ll be prescreened on the phone. The only thing they’ll have done when they first come in is to have their temperature taken, and they’ll be checked in and out with the doctor and the nurse in the room, and that’s it. There will be no other extraneous people to help to eliminate risk. We’re cutting our schedules down by 75% so that we can socially distance within our practice,” Dr. Dover said.
Dr. Dover served as lead author on “A path to resume aesthetic care: Executive summary of Project AesCert guidance supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” which was published online in Facial Plastic Surgery & Aesthetic Medicine (2002 May 5. doi: 10.1089/fpsam.2020.0239). His coauthors included a facial plastic surgeon and three infectious disease experts.
Dr. Dover said, “We took the advice of these experts in infectious diseases, who said, ‘we don’t know all the right answers [to resuming aesthetic care]. We can mitigate risk, but we cannot eliminate risk. You have to treat every patient in your office as if they’re COVID-19 positive. If you do that, you’ll have a safe office. It’ll be the safest place in your world, safer than a grocery store, where you have no idea who you’re standing beside.’ ”
“The problem with this virus, compared to, say, SARS-CoV-1, is that these patients are positive and shedding virus 2-3 days before they get a fever,” he added. “With SARS-CoV-1, they had a fever first and then they shed virus. What I learned was, treat everybody with universal precautions.” The document includes tips for communicating with patient about expectations for office visits, clinic schedule management, cleaning procedures, PPE, treatment room set-up, and employee health screening and training.
During the webinar, an ASLMS member posed a question to the panelists about their comfort level in performing mechanical microneedling and radiofrequency (RF) microneedling procedures as aesthetic practices begin to reopen. “Generally, there’s no plume with microneedling with or without RF,” Dr. Geronemus said. “Depending upon the procedure that you’re doing, some of the microneedling procedures are very bloody; that may carry a risk unto itself. Other procedures where you’re using a thermal component have less bleeding. I’m more inclined to proceed with an RF with microneedling procedure and less inclined to proceed with a bloody, more aggressive microneedling procedure.”
Dr. Waibel emphasized the importance of disinfecting the microneedling device between uses. “If you have disposable needle cartridges, I think it’s a lot safer than if you have to clean [them],” she said. “We know that COVID-19 can live up to 3 hours, at least in a lab scenario, so you don’t want to transmit it from patient to patient. If someone has COVID-19 on their nose, and you microneedle over it, and that’s not completely disinfected, you could spread it to the next patient. We have really amped up our cleaning in between rooms. We have a whole crew that cleans every surface with [disinfectant wipes] and 90% alcohol.”
With reported shortages of N95 in many health care settings, some panelists said that they plan to reuse masks until the supply chain improves. Dr. Dover said that one option is to “use a mask, label it, number it, drop it in into a paper bag or into a [sealed plastic food container] upside down without touching the front of it,” he said. “If it sits for a week and you see patients 5 days a week, that mask will be dried out and highly effective a week later. That’s what we’re going to do until there is a big supply of them.”
The pandemic has also thrown a monkey wrench into aesthetic and medical dermatology clinical research efforts. According to Dr. Dover, many aesthetic studies have been shut down, “and most companies are giving us little guidance,” he said. “As they figure things out, they ask us to do things over and over again. So, I hope that clinical research will improve because of COVID-19 in the long term, but in the short term, it’s been a bit of a nightmare.”
Dr. Geronemus added that, in order to fulfill criteria for most studies, clinicians are required to see patients in a certain number of days. “We’re out of protocol in many different studies, so we’re requesting that protocols be amended and that the FDA [Food and Drug Administration] and the sponsors will consider opportunities to make those changes,” he said. “We’ll do as much as we can virtually, but if you’re studying an acne scar, you really need to see the patient [in person].”
Strict social distancing measures are also disrupting agreements that dermatologists may have had with trainees and fellows before the pandemic hit. “We’ve had to send letters and e-mails to people who were planning visits and preceptorships,” Dr. Dover said. “Even with our fellows, we’re going to have to figure out a way to practice so as not to complicate the issue in the room. The more people in the room, the more risk there is for transmitting disease. It’s really an issue.”
Dr. Tanzi limits everyone in the room during procedures. “We’re screening patients beforehand and telling them no family members, unless there’s a disability; no kids, unless it’s a kid coming in for acne treatment and they have to bring their parent; no drivers – they can wait outside,” she said.
Another ASLMS member asked the panelists if they plan to incorporate an informed consent form for COVID-19 risk into their practices, similar to the one developed by the American Society of Plastic Surgeons. “That’s a tough one,” Dr. Waibel said. “Before patients enter our practice, we take their temperature and ask them several COVID-related symptoms and contact questions – which they validate as true.”
Dr. Geronemus said that he will consider the idea. “The downside is logistical,” he said. “Patients sign so many forms already; they’re complaining that it takes so long to get into see me, and my hand is tired from signing so many forms.’”
Dr. Dover said that he and his colleagues are planning to use a COVID-19 risk consent form. “I’d err on the side of yes rather than on the side of no, because you’re better off overdoing it than underdoing it,” he said. “This is not the time for shortcuts.”
[email protected]

“People talk about reinventing the wheel,” Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., said during an hour-long webinar on May 5 sponsored by the American Society for Laser Medicine and Surgery. “In this case, we’re inventing the wheel; no one’s ever done this before – not in our lifetimes. The last pandemic was over 100 years ago, when there wasn’t aesthetic medicine.”
Dr. Dover joined a panel of four other experts from around the country to discuss how to reopen practices safely and effectively. Paul M. Friedman, MD, director of the Houston Cosmetic Dermatology & Laser Center, moderated the event.
In Florida, which reopened certain businesses on May 4, 2020, Jill S. Waibel, MD, plans to start at 25% capacity at Miami Dermatology and Laser Institute, and build from there. “We’re trying to take care of skin cancer patients first,” said Dr. Waibel, a dermatologist who owns the practice. “Then we’re going to start doing less aggressive cosmetic procedures like injectables, nonablative procedures. We’ll move into the more aggressive procedures as we ease back into it. We really want to see what’s going to happen 2-3 weeks down the line now that things are starting to open up.”
In Maryland, where state officials announced on May 6 that guidelines would be issued to allow for nonmedical procedures, Elizabeth L. Tanzi, MD, founder and director of Capital Laser & Skin Care in Chevy Chase, expects things to “look very different” once her practice reopens. “We are taking it very slowly,” she said. “Teledermatology for acne and other follow-ups is not something we did before, but it is certainly something that we’ll continue.”
The way she sees it, having the proper personal protective equipment is a key part of any reopening discussion. “I am not going near anyone’s face without an N95 mask that fits well, and without a face shield,” she said. “If you’re delegating these procedures to people that you don’t trust to be wearing the PPE correctly, then you shouldn’t be delegating them, because a key is the PPE. You have to assume that everyone has the virus at every time.”
In Ardmore, Pa., the Main Line Center for Laser Surgery remains closed because of current state regulations. When practice director Eric F. Bernstein, MD, gets the green light to reopen, patients will undergo a consultation by phone or videoconference and pay their bill before they set foot in the office. “We’re on the second floor, so patients can take a stairwell and avoid the elevator,” Dr. Bernstein said. “They’ll come in, not check in at the desk; go right to the room. There will be one treater and one assistant. If the patient doesn’t come in with a mask, we’ll supply one. It’s going to be a very different process. People are setting their hours longer because they’re going to be seeing fewer people. There will be no sitting in the waiting room.”
In the COVID-19 epicenter, Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, has been performing Mohs procedures and treating children with vascular malformations, but everything else is on hold. “Once the governor [Andrew Cuomo] lifts the stay-at-home restrictions, we’ll ease into things,” he said. “The issue of performing more invasive procedures – like ablative fractional resurfacing – is something that we are concerned about. I’m concerned about any laser that has environmental plume. For example, with our tattoo-removal procedures, I intend to treat every patient through a gel for the short term, and perhaps even for the long term. One can do that safely, and that eliminates the plume altogether.”
At the center, Dr. Geronemus added, “we do a fair amount of ablative fractional resurfacing and some fully ablative resurfacing. I intend to use large facial shields with these patients. We do use vacuum in each room as it stands right now, not only with electrosurgery, but we’ll be adding that to laser procedures as well. That will be helpful.”
In Chestnut Hill, Mass., Dr. Dover and his colleagues plan to practice what he termed “universal COVID precaution” by wearing a face mask, goggles, or a face shield, gloves, and protective clothing when necessary. “We are not going to do any ablative procedures, no procedures with plume, and we’re going to try and eliminate risk as much as we can,” he said. “We will have no waiting room; the patients will walk right to an exam room. They’ll be prescreened on the phone. The only thing they’ll have done when they first come in is to have their temperature taken, and they’ll be checked in and out with the doctor and the nurse in the room, and that’s it. There will be no other extraneous people to help to eliminate risk. We’re cutting our schedules down by 75% so that we can socially distance within our practice,” Dr. Dover said.
Dr. Dover served as lead author on “A path to resume aesthetic care: Executive summary of Project AesCert guidance supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” which was published online in Facial Plastic Surgery & Aesthetic Medicine (2002 May 5. doi: 10.1089/fpsam.2020.0239). His coauthors included a facial plastic surgeon and three infectious disease experts.
Dr. Dover said, “We took the advice of these experts in infectious diseases, who said, ‘we don’t know all the right answers [to resuming aesthetic care]. We can mitigate risk, but we cannot eliminate risk. You have to treat every patient in your office as if they’re COVID-19 positive. If you do that, you’ll have a safe office. It’ll be the safest place in your world, safer than a grocery store, where you have no idea who you’re standing beside.’ ”
“The problem with this virus, compared to, say, SARS-CoV-1, is that these patients are positive and shedding virus 2-3 days before they get a fever,” he added. “With SARS-CoV-1, they had a fever first and then they shed virus. What I learned was, treat everybody with universal precautions.” The document includes tips for communicating with patient about expectations for office visits, clinic schedule management, cleaning procedures, PPE, treatment room set-up, and employee health screening and training.
During the webinar, an ASLMS member posed a question to the panelists about their comfort level in performing mechanical microneedling and radiofrequency (RF) microneedling procedures as aesthetic practices begin to reopen. “Generally, there’s no plume with microneedling with or without RF,” Dr. Geronemus said. “Depending upon the procedure that you’re doing, some of the microneedling procedures are very bloody; that may carry a risk unto itself. Other procedures where you’re using a thermal component have less bleeding. I’m more inclined to proceed with an RF with microneedling procedure and less inclined to proceed with a bloody, more aggressive microneedling procedure.”
Dr. Waibel emphasized the importance of disinfecting the microneedling device between uses. “If you have disposable needle cartridges, I think it’s a lot safer than if you have to clean [them],” she said. “We know that COVID-19 can live up to 3 hours, at least in a lab scenario, so you don’t want to transmit it from patient to patient. If someone has COVID-19 on their nose, and you microneedle over it, and that’s not completely disinfected, you could spread it to the next patient. We have really amped up our cleaning in between rooms. We have a whole crew that cleans every surface with [disinfectant wipes] and 90% alcohol.”
With reported shortages of N95 in many health care settings, some panelists said that they plan to reuse masks until the supply chain improves. Dr. Dover said that one option is to “use a mask, label it, number it, drop it in into a paper bag or into a [sealed plastic food container] upside down without touching the front of it,” he said. “If it sits for a week and you see patients 5 days a week, that mask will be dried out and highly effective a week later. That’s what we’re going to do until there is a big supply of them.”
The pandemic has also thrown a monkey wrench into aesthetic and medical dermatology clinical research efforts. According to Dr. Dover, many aesthetic studies have been shut down, “and most companies are giving us little guidance,” he said. “As they figure things out, they ask us to do things over and over again. So, I hope that clinical research will improve because of COVID-19 in the long term, but in the short term, it’s been a bit of a nightmare.”
Dr. Geronemus added that, in order to fulfill criteria for most studies, clinicians are required to see patients in a certain number of days. “We’re out of protocol in many different studies, so we’re requesting that protocols be amended and that the FDA [Food and Drug Administration] and the sponsors will consider opportunities to make those changes,” he said. “We’ll do as much as we can virtually, but if you’re studying an acne scar, you really need to see the patient [in person].”
Strict social distancing measures are also disrupting agreements that dermatologists may have had with trainees and fellows before the pandemic hit. “We’ve had to send letters and e-mails to people who were planning visits and preceptorships,” Dr. Dover said. “Even with our fellows, we’re going to have to figure out a way to practice so as not to complicate the issue in the room. The more people in the room, the more risk there is for transmitting disease. It’s really an issue.”
Dr. Tanzi limits everyone in the room during procedures. “We’re screening patients beforehand and telling them no family members, unless there’s a disability; no kids, unless it’s a kid coming in for acne treatment and they have to bring their parent; no drivers – they can wait outside,” she said.
Another ASLMS member asked the panelists if they plan to incorporate an informed consent form for COVID-19 risk into their practices, similar to the one developed by the American Society of Plastic Surgeons. “That’s a tough one,” Dr. Waibel said. “Before patients enter our practice, we take their temperature and ask them several COVID-related symptoms and contact questions – which they validate as true.”
Dr. Geronemus said that he will consider the idea. “The downside is logistical,” he said. “Patients sign so many forms already; they’re complaining that it takes so long to get into see me, and my hand is tired from signing so many forms.’”
Dr. Dover said that he and his colleagues are planning to use a COVID-19 risk consent form. “I’d err on the side of yes rather than on the side of no, because you’re better off overdoing it than underdoing it,” he said. “This is not the time for shortcuts.”
[email protected]

“People talk about reinventing the wheel,” Jeffrey S. Dover, MD, codirector of SkinCare Physicians in Chestnut Hill, Mass., said during an hour-long webinar on May 5 sponsored by the American Society for Laser Medicine and Surgery. “In this case, we’re inventing the wheel; no one’s ever done this before – not in our lifetimes. The last pandemic was over 100 years ago, when there wasn’t aesthetic medicine.”
Dr. Dover joined a panel of four other experts from around the country to discuss how to reopen practices safely and effectively. Paul M. Friedman, MD, director of the Houston Cosmetic Dermatology & Laser Center, moderated the event.
In Florida, which reopened certain businesses on May 4, 2020, Jill S. Waibel, MD, plans to start at 25% capacity at Miami Dermatology and Laser Institute, and build from there. “We’re trying to take care of skin cancer patients first,” said Dr. Waibel, a dermatologist who owns the practice. “Then we’re going to start doing less aggressive cosmetic procedures like injectables, nonablative procedures. We’ll move into the more aggressive procedures as we ease back into it. We really want to see what’s going to happen 2-3 weeks down the line now that things are starting to open up.”
In Maryland, where state officials announced on May 6 that guidelines would be issued to allow for nonmedical procedures, Elizabeth L. Tanzi, MD, founder and director of Capital Laser & Skin Care in Chevy Chase, expects things to “look very different” once her practice reopens. “We are taking it very slowly,” she said. “Teledermatology for acne and other follow-ups is not something we did before, but it is certainly something that we’ll continue.”
The way she sees it, having the proper personal protective equipment is a key part of any reopening discussion. “I am not going near anyone’s face without an N95 mask that fits well, and without a face shield,” she said. “If you’re delegating these procedures to people that you don’t trust to be wearing the PPE correctly, then you shouldn’t be delegating them, because a key is the PPE. You have to assume that everyone has the virus at every time.”
In Ardmore, Pa., the Main Line Center for Laser Surgery remains closed because of current state regulations. When practice director Eric F. Bernstein, MD, gets the green light to reopen, patients will undergo a consultation by phone or videoconference and pay their bill before they set foot in the office. “We’re on the second floor, so patients can take a stairwell and avoid the elevator,” Dr. Bernstein said. “They’ll come in, not check in at the desk; go right to the room. There will be one treater and one assistant. If the patient doesn’t come in with a mask, we’ll supply one. It’s going to be a very different process. People are setting their hours longer because they’re going to be seeing fewer people. There will be no sitting in the waiting room.”
In the COVID-19 epicenter, Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, has been performing Mohs procedures and treating children with vascular malformations, but everything else is on hold. “Once the governor [Andrew Cuomo] lifts the stay-at-home restrictions, we’ll ease into things,” he said. “The issue of performing more invasive procedures – like ablative fractional resurfacing – is something that we are concerned about. I’m concerned about any laser that has environmental plume. For example, with our tattoo-removal procedures, I intend to treat every patient through a gel for the short term, and perhaps even for the long term. One can do that safely, and that eliminates the plume altogether.”
At the center, Dr. Geronemus added, “we do a fair amount of ablative fractional resurfacing and some fully ablative resurfacing. I intend to use large facial shields with these patients. We do use vacuum in each room as it stands right now, not only with electrosurgery, but we’ll be adding that to laser procedures as well. That will be helpful.”
In Chestnut Hill, Mass., Dr. Dover and his colleagues plan to practice what he termed “universal COVID precaution” by wearing a face mask, goggles, or a face shield, gloves, and protective clothing when necessary. “We are not going to do any ablative procedures, no procedures with plume, and we’re going to try and eliminate risk as much as we can,” he said. “We will have no waiting room; the patients will walk right to an exam room. They’ll be prescreened on the phone. The only thing they’ll have done when they first come in is to have their temperature taken, and they’ll be checked in and out with the doctor and the nurse in the room, and that’s it. There will be no other extraneous people to help to eliminate risk. We’re cutting our schedules down by 75% so that we can socially distance within our practice,” Dr. Dover said.
Dr. Dover served as lead author on “A path to resume aesthetic care: Executive summary of Project AesCert guidance supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” which was published online in Facial Plastic Surgery & Aesthetic Medicine (2002 May 5. doi: 10.1089/fpsam.2020.0239). His coauthors included a facial plastic surgeon and three infectious disease experts.
Dr. Dover said, “We took the advice of these experts in infectious diseases, who said, ‘we don’t know all the right answers [to resuming aesthetic care]. We can mitigate risk, but we cannot eliminate risk. You have to treat every patient in your office as if they’re COVID-19 positive. If you do that, you’ll have a safe office. It’ll be the safest place in your world, safer than a grocery store, where you have no idea who you’re standing beside.’ ”
“The problem with this virus, compared to, say, SARS-CoV-1, is that these patients are positive and shedding virus 2-3 days before they get a fever,” he added. “With SARS-CoV-1, they had a fever first and then they shed virus. What I learned was, treat everybody with universal precautions.” The document includes tips for communicating with patient about expectations for office visits, clinic schedule management, cleaning procedures, PPE, treatment room set-up, and employee health screening and training.
During the webinar, an ASLMS member posed a question to the panelists about their comfort level in performing mechanical microneedling and radiofrequency (RF) microneedling procedures as aesthetic practices begin to reopen. “Generally, there’s no plume with microneedling with or without RF,” Dr. Geronemus said. “Depending upon the procedure that you’re doing, some of the microneedling procedures are very bloody; that may carry a risk unto itself. Other procedures where you’re using a thermal component have less bleeding. I’m more inclined to proceed with an RF with microneedling procedure and less inclined to proceed with a bloody, more aggressive microneedling procedure.”
Dr. Waibel emphasized the importance of disinfecting the microneedling device between uses. “If you have disposable needle cartridges, I think it’s a lot safer than if you have to clean [them],” she said. “We know that COVID-19 can live up to 3 hours, at least in a lab scenario, so you don’t want to transmit it from patient to patient. If someone has COVID-19 on their nose, and you microneedle over it, and that’s not completely disinfected, you could spread it to the next patient. We have really amped up our cleaning in between rooms. We have a whole crew that cleans every surface with [disinfectant wipes] and 90% alcohol.”
With reported shortages of N95 in many health care settings, some panelists said that they plan to reuse masks until the supply chain improves. Dr. Dover said that one option is to “use a mask, label it, number it, drop it in into a paper bag or into a [sealed plastic food container] upside down without touching the front of it,” he said. “If it sits for a week and you see patients 5 days a week, that mask will be dried out and highly effective a week later. That’s what we’re going to do until there is a big supply of them.”
The pandemic has also thrown a monkey wrench into aesthetic and medical dermatology clinical research efforts. According to Dr. Dover, many aesthetic studies have been shut down, “and most companies are giving us little guidance,” he said. “As they figure things out, they ask us to do things over and over again. So, I hope that clinical research will improve because of COVID-19 in the long term, but in the short term, it’s been a bit of a nightmare.”
Dr. Geronemus added that, in order to fulfill criteria for most studies, clinicians are required to see patients in a certain number of days. “We’re out of protocol in many different studies, so we’re requesting that protocols be amended and that the FDA [Food and Drug Administration] and the sponsors will consider opportunities to make those changes,” he said. “We’ll do as much as we can virtually, but if you’re studying an acne scar, you really need to see the patient [in person].”
Strict social distancing measures are also disrupting agreements that dermatologists may have had with trainees and fellows before the pandemic hit. “We’ve had to send letters and e-mails to people who were planning visits and preceptorships,” Dr. Dover said. “Even with our fellows, we’re going to have to figure out a way to practice so as not to complicate the issue in the room. The more people in the room, the more risk there is for transmitting disease. It’s really an issue.”
Dr. Tanzi limits everyone in the room during procedures. “We’re screening patients beforehand and telling them no family members, unless there’s a disability; no kids, unless it’s a kid coming in for acne treatment and they have to bring their parent; no drivers – they can wait outside,” she said.
Another ASLMS member asked the panelists if they plan to incorporate an informed consent form for COVID-19 risk into their practices, similar to the one developed by the American Society of Plastic Surgeons. “That’s a tough one,” Dr. Waibel said. “Before patients enter our practice, we take their temperature and ask them several COVID-related symptoms and contact questions – which they validate as true.”
Dr. Geronemus said that he will consider the idea. “The downside is logistical,” he said. “Patients sign so many forms already; they’re complaining that it takes so long to get into see me, and my hand is tired from signing so many forms.’”
Dr. Dover said that he and his colleagues are planning to use a COVID-19 risk consent form. “I’d err on the side of yes rather than on the side of no, because you’re better off overdoing it than underdoing it,” he said. “This is not the time for shortcuts.”
[email protected]
Over-the-counter Topical Products in Dermatology
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
Resident Pearls
- Several branches of the US Food and Drug Administration are responsible for regulation of overthe-counter (OTC) topical products with both direct and indirect oversight.
- There are several excellent resources available to dermatologists in training who are interested in learning about pharmacology and tolerability of OTC products.
- Firsthand experience in personally sampling a variety of products also helps clinicians provide practical recommendations to patients.
FDA approves hyaluronic acid filler for lip augmentation, perioral rhytids
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
The resurgence of Plaquenil (hydroxychloroquine)
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Two of the most unusual dermatologic drugs have resurged as possible first-line therapy for rescue treatment of hospitalized patients with SARS-CoV-2, despite extremely limited clinical data supporting their efficacy, optimal dose, treatment duration, and potential adverse effects.
Chloroquine and hydroxychloroquine were introduced as treatment and prophylaxis of malaria and approved by the Food and Drug Administration in 1949 and 1955, respectively. They belong to a class of drugs called 4-aminoquinolones and have a flat aromatic core and a basic side chain. The basic property of these drugs contribute to their ability to accumulate in lysosomes. They have a large volume of distribution in the blood and a half-life of 40-60 days. Important interactions include use with tamoxifen, proton pump inhibitors, and with smoking. Although both drugs cross the placenta, they don’t have any notable effects on the fetus.
Chloroquine and hydroxychloroquine enter the cell and accumulate in the lysosomes along a pH gradient. Within the lysosome, they increase the pH, thereby stabilizing lysosomes and inhibiting eosinophil and neutrophil chemotaxis and phagocytic activity. They also inhibit complement-mediated hemolysis, reduce acute phase reactants, and prevent MHC class II–mediated auto antigen presentation. Additionally, they decrease cell-mediated immunity by decreasing the production of interleukin-1 and plasma cell synthesis. Hydroxychloroquine can also accumulate in endosomes and inhibit toll-like receptor signaling, thereby reducing the production of proinflammatory cytokines.
One of the ways SARS-CoV-2 enters cells is by up-regulating and binding to ACE2. Chloroquine/hydroxychloroquine reduce glycosylation of ACE2 and thus inhibit viral entry. Additionally, by increasing the endosomal pH, they potentially inactivate enzymes that viruses require for replication. Their lifesaving benefits, however, are thought to involve blocking the proinflammatory cytokine IL-6 and suppressing the cytokine storm thought to induce acute respiratory distress syndrome. Interestingly, chloroquine has also been shown to allow zinc ions into the cell, and zinc is a potent inhibitor of coronavirus RNA polymerase.
Side effects of chloroquine and hydroxychloroquine include GI upset, retinal toxicity with long-term use, hypoglycemia, cardiomyopathy, QT prolongation, ventricular arrhythmias, and renal and liver toxicity. Adverse effects have been observed with long-term daily doses of more than 3.5 mg/kg of chloroquine or more than 6.5 mg/kg of hydroxychloroquine. Cutaneous effects include pruritus, morbilliform rashes (in an estimated 10% of those treated) and psoriasis flares, and blue-black hyperpigmentation (in about 25%) of the shins, face, oral palate, and nails.
Initial In February 2020, the first clinical results of 100 patients treated with chloroquine were reported in a news briefing by the Chinese government. On March 20, the first clinical trial was published offering guidelines for the treatment of COVID-19 using hydroxychloroquine and azithromycin combination therapy – albeit with many limitations and reported biases in the study. Despite the poorly designed studies and inconclusive evidence, on March 28, the FDA issued an Emergency Use Authorization that allows providers to request a supply of hydroxychloroquine or chloroquine for hospitalized patients with COVID-19 who are unable to join a clinical trial.
On April 2, the first clinical trial to evaluate the safety and efficacy of hydroxychloroquine in adults hospitalized with COVID-19 began at Vanderbilt University Medical Center, Nashville, Tenn. The ORCHID trial (Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease), funded by the National Heart, Lung, and Blood Institute. This blinded, placebo-controlled study is evaluating hydroxychloroquine treatment of hospitalized patients with COVID-19 in hopes of treating the severe complications of acute respiratory distress syndrome. Participants are randomly assigned to receive 400 mg hydroxychloroquine twice daily as a loading dose and then 200 mg twice daily thereafter on days 2-5. As of this writing, this study is currently underway and outcomes are expected in the upcoming weeks.
There is now a shortage of chloroquine and hydroxychloroquine in patients who have severe dermatologic and rheumatologic diseases, which include some who been in remission for years because of these medications and are in grave danger of recurrence. During this crisis, we desperately need well-controlled, randomized studies to test the efficacy and prolonged safety profile of these drugs in COVID-19 patients, as well as appropriate funding to source these medications for hospitalized and nonhospitalized patients in need.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Sources
Liu J et al. Cell Discov. 2020 Mar 18. doi: 10.1038/s41421-020-0156-0.
Vincent MJ et al. Virol J. 2005 Aug 22;2:69.
Gautret P et al. Int J Antimicrob Agents. 2020 Mar 20. doi: 10.1016/j.ijantimicag.2020.105949.
Devaux CA et al. Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Aronson J et al. COVID-19 trials registered up to 8 March 2020 – an analysis of 382 studies. 2020. Centre for Evidence-Based Medicine. https://www.cebm.net/oxford-covid-19/covid-19-registered-trials-and-analysis/
Savarino A et al. Lancet Infect Dis. 2003 Nov;3(11):722-7.
Yazdany J, Kim AHJ. Ann Intern Med. 2020 Mar 31. doi: 10.7326/M20-1334.
Xue J et al. PLoS One. 2014 Oct 1;9(10):e109180.
te Velthuis AJ et al. PLoS Pathog. 2010 Nov 4;6(11):e1001176.
Mother of pearl: The power of pearl powder
Because of its dense protein and mineral composition, it has been used to treat several skin and bone disorders, as well as palpitations, insomnia, and epilepsy.3,4 The pearl-farming industry itself was established in Japan and has existed for more than a century; today, pearls are cultured globally and continue to receive attention for conferring health benefits.5
Calcium carbonate is the primary component of mollusk shells (roughly 95%), with the remainder an organic matrix including proteins, glycoproteins, and polysaccharides.6 Pearl powder is known to have exhibited antiaging, antioxidant, antiradiative, and tonic activities; in recent years, it has been incorporated into health foods for such properties and used in the clinical setting to treat ulcers (aphthous, gastric, and duodenal).4,7 Consisting of multiple active proteins, pearl powder is thought to be conducive to skin cell growth and effective for wound repair.4 This column focuses on recent research into the dermatologic potential of the powder derived from mother of pearl.
Wound healing
A decade ago, Jian-Ping et al. showed in mice that the water-soluble matrix of pearl powder (Hyriopsis cumingii) could significantly induce oral fibroblast proliferation and collagen accumulation, suppress matrix metalloproteinase-2 activity, and significantly foster TIMP-1 synthesis. The investigators concluded that the wound healing facilitated by pearl powder derives, in part, from its capacity to promote fibroblast mitosis, collagen deposition, and production of TIMP-1.8
Two years later, Lee et al. evaluated the effects of water-soluble nacre (mother of pearl) on second-degree burn wound healing in porcine skin as a proxy for human skin. They found that its application quickly led to burn-induced granulation areas filling with collagen, with normal skin appearance restored to wounded dermis and epidermis. Angiogenesis was also promoted by water-soluble nacre as was wound recovery in areas with apoptotic and necrotic cellular damage. Murine fibroblast NIH3T3 cells treated with water-soluble nacre also demonstrated augmented proliferation and collagen production. The researchers cited the restoration of angiogenesis and fibroblast activity as the primary benefits of water-soluble nacre, suggesting its potential as a wound therapy, preferable to powdered nacre due to better biocompatibility with less discomfort.9
The next year, Li et al. found that mother of pearl extract promoted cell migration of fibroblasts in cell culture, demonstrating its potential as a wound healing model.7In 2019, Chen et al. studied the effects of pearl powders of varying particle sizes to treat wounds in vitro and in vivo. They found that micro- and nanosized pearl powders augmented proliferation and migration of skin cells and shortened wound closure time. All powders also improved the biomechanical strength of healed skin, enhanced collagen formation and deposition, and expanded cutaneous angiogenesis, with nanoscale pearl powder displaying greatest efficiency.4
Skin tone and atopic dermatitis
In 2000, Lopez et al. implanted powdered nacre (mother of pearl derived from Pinctada maxima), which can promote and regulate bone-forming cells, into rat dermis to evaluate its effects on skin fibroblasts. They noted that the implant yielded well-vascularized tissue and improved extracellular matrix production, synthesis of substances involved in cellular adhesion and communication, and tissue regeneration (such as collagen types I and III). The investigators concluded that the powdered nacre contributed to the conditions necessary for improved skin tone and proper physiologic functioning of the skin.10
Rousseau et al. extracted lipids from the nacre of the oyster P. margaritifera to test on artificially dehydrated skin explants with the intention of developing new treatments for atopic dermatitis. The researchers determined that the lipids spurred a reconstitution of the intercellular material of the stratum corneum, concluding that new products to treat atopic dermatitis might be based on the signaling activity of nacre lipids.11
Antifibrotic and anti-inflammatory activity
A 2015 study by Yang et al. showed that a room-temperature superextraction system to yield the main active constituents of pearl was successful in enhancing their anti-inflammatory and antiapoptotic activity in human keratinocyte cells (HaCaT) exposed to low-dose UVB. The investigators combined pearl extract and poly (gamma-glutamic acid) hydrogels and observed reductions in inflammation and apoptosis of HaCaT cells. They concluded that a marketed pearl extract may be able to suppress radiation dermatitis present in keratinocytes.12
Two years later, Latire et al. used human dermal fibroblasts in primary culture to assess the potential biological activities of the matrix macromolecular components extracted from the shells of two edible mollusks (the blue mussel Mytilus edulis and the Pacific oyster Crassostrea gigas). The investigators found that both extracts influenced metabolic functions of the cells and reduced type I collagen levels, with an associated rise in matrix metalloproteinase-1 activity. Given their findings implying the effectiveness of the extracts in facilitating the catabolic pathway of human dermal fibroblasts, the authors suggest that these shell matrices present the potential for use in treating fibrosis, especially for scleroderma.6
Antioxidant and antiaging activity
Shao et al. demonstrated 10 years ago that pearl powder provides a moisturizing effect on the skin, with ultramicro pearl powder delivering a more robust moisturizing result than water-soluble pearl powder. These two types of pearl powder, along with another one tested (ultranano pearl powder), also significantly diminished the activation of tyrosinase and free radicals. Water-soluble pearl powder did not perform as well as the other two formulations in free radical scavenging. The investigators suggested that their results support the use of pearl powder to combat aging and enhance beauty, and could be used in the clinical setting.13
In 2017, Yang et al. reported on the in vitro antihemolytic and antioxidant activity of pearl powder in shielding human erythrocytes against 2,2’-azobis(2-amidinopropane) dihydrochloride–induced oxidative damage to membrane proteins/lipids. The researchers contend that the strong antioxidant qualities of pearl powder could be applied to prevent or protect against various diseases resulting from free radical damage.2
Human trials: Antioxidant, antiaging, skin appearance
Chiu et al. studied the antioxidant activity of various pearl powder extracts in a randomized, placebo-controlled trial in 2018. They also investigated the life span–prolonging effects of the powders using wild-type Caenorhabditis elegans. Twenty healthy middle-aged subjects were separated into two groups (experimental and placebo), with 3 g of pearl powder administered in capsules to the former and 3 g of placebo to the latter over 8 weeks. Blood samples taken at the beginning and every 2 weeks during the trial and in the 10th week revealed maximum antioxidant activity of the pearl powder and prolongation of C. elegans lifespan by 18.87%. Subjects using pearl powder demonstrated significant increases in total antioxidant capacity, thiols, glutathione, and enzymic antioxidant activity, along with notably inhibited lipid peroxidation products. The investigators concluded that pearl powder extract acted as a potent antioxidant and its use may be warranted to treat degenerative conditions related to aging.3
A recent study of the perception of blue light on Korean women’s faces using blue pearl pigment revealed that the pigment does indeed elicit the perception of the blue-light effect, notably transparency and gloss, which is particularly valued in Korea.14
Conclusion
The use of mother of pearl and pearl powder in traditional Chinese medicine and as a cosmetic and food additive has a rich and lengthy history. Contemporary research clearly suggests interesting avenues for further investigation and some promising results. Much more research is necessary, though, to delineate the potential roles of pearl powder in the skin care arsenal.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Zhang J et al. J Sep Sci. 2015 May;38(9):1552-60.
2. Yang HL et al. J Food Drug Anal. 2017 Oct;25(4):898-907.
3. Chiu HF et al. J Food Drug Anal. 2018 Jan;26(1):309-17.
4. Chen X et al. Drug Dev Ind Pharm. 2019 Jun;45(6):1009-16.
5. Nagai K. Zoolog Sci. 2013 Oct;30(10):783-93.
6. Latire T et al. Cytotechnology. 2017 Oct;69(5):815-29.
7. Li YC et al. Pharm Biol. 2013 Mar;51(3):289-97.
8. Jian-Ping D et al. Pharm Biol. 2010 Feb;48(2):122-7.
9. Lee K et al. Mol Biol Rep. 2012 Mar;39(3):3211-8.
10. Lopez E et al. Tissue Cell. 2000 Feb;32(1):95-101.
11. Rousseau M et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):1-9.
12. Yang YL et al. Biomed Mater Eng. 2015;26 Suppl 1:S139-45.
13. Shao DZ et al. J Cosmet Sci. 2010 Mar-Apr;61(2):133-45.
14. Lee M et al. Skin Res Technol. 2020 Jan;26(1):76-80.
Because of its dense protein and mineral composition, it has been used to treat several skin and bone disorders, as well as palpitations, insomnia, and epilepsy.3,4 The pearl-farming industry itself was established in Japan and has existed for more than a century; today, pearls are cultured globally and continue to receive attention for conferring health benefits.5
Calcium carbonate is the primary component of mollusk shells (roughly 95%), with the remainder an organic matrix including proteins, glycoproteins, and polysaccharides.6 Pearl powder is known to have exhibited antiaging, antioxidant, antiradiative, and tonic activities; in recent years, it has been incorporated into health foods for such properties and used in the clinical setting to treat ulcers (aphthous, gastric, and duodenal).4,7 Consisting of multiple active proteins, pearl powder is thought to be conducive to skin cell growth and effective for wound repair.4 This column focuses on recent research into the dermatologic potential of the powder derived from mother of pearl.
Wound healing
A decade ago, Jian-Ping et al. showed in mice that the water-soluble matrix of pearl powder (Hyriopsis cumingii) could significantly induce oral fibroblast proliferation and collagen accumulation, suppress matrix metalloproteinase-2 activity, and significantly foster TIMP-1 synthesis. The investigators concluded that the wound healing facilitated by pearl powder derives, in part, from its capacity to promote fibroblast mitosis, collagen deposition, and production of TIMP-1.8
Two years later, Lee et al. evaluated the effects of water-soluble nacre (mother of pearl) on second-degree burn wound healing in porcine skin as a proxy for human skin. They found that its application quickly led to burn-induced granulation areas filling with collagen, with normal skin appearance restored to wounded dermis and epidermis. Angiogenesis was also promoted by water-soluble nacre as was wound recovery in areas with apoptotic and necrotic cellular damage. Murine fibroblast NIH3T3 cells treated with water-soluble nacre also demonstrated augmented proliferation and collagen production. The researchers cited the restoration of angiogenesis and fibroblast activity as the primary benefits of water-soluble nacre, suggesting its potential as a wound therapy, preferable to powdered nacre due to better biocompatibility with less discomfort.9
The next year, Li et al. found that mother of pearl extract promoted cell migration of fibroblasts in cell culture, demonstrating its potential as a wound healing model.7In 2019, Chen et al. studied the effects of pearl powders of varying particle sizes to treat wounds in vitro and in vivo. They found that micro- and nanosized pearl powders augmented proliferation and migration of skin cells and shortened wound closure time. All powders also improved the biomechanical strength of healed skin, enhanced collagen formation and deposition, and expanded cutaneous angiogenesis, with nanoscale pearl powder displaying greatest efficiency.4
Skin tone and atopic dermatitis
In 2000, Lopez et al. implanted powdered nacre (mother of pearl derived from Pinctada maxima), which can promote and regulate bone-forming cells, into rat dermis to evaluate its effects on skin fibroblasts. They noted that the implant yielded well-vascularized tissue and improved extracellular matrix production, synthesis of substances involved in cellular adhesion and communication, and tissue regeneration (such as collagen types I and III). The investigators concluded that the powdered nacre contributed to the conditions necessary for improved skin tone and proper physiologic functioning of the skin.10
Rousseau et al. extracted lipids from the nacre of the oyster P. margaritifera to test on artificially dehydrated skin explants with the intention of developing new treatments for atopic dermatitis. The researchers determined that the lipids spurred a reconstitution of the intercellular material of the stratum corneum, concluding that new products to treat atopic dermatitis might be based on the signaling activity of nacre lipids.11
Antifibrotic and anti-inflammatory activity
A 2015 study by Yang et al. showed that a room-temperature superextraction system to yield the main active constituents of pearl was successful in enhancing their anti-inflammatory and antiapoptotic activity in human keratinocyte cells (HaCaT) exposed to low-dose UVB. The investigators combined pearl extract and poly (gamma-glutamic acid) hydrogels and observed reductions in inflammation and apoptosis of HaCaT cells. They concluded that a marketed pearl extract may be able to suppress radiation dermatitis present in keratinocytes.12
Two years later, Latire et al. used human dermal fibroblasts in primary culture to assess the potential biological activities of the matrix macromolecular components extracted from the shells of two edible mollusks (the blue mussel Mytilus edulis and the Pacific oyster Crassostrea gigas). The investigators found that both extracts influenced metabolic functions of the cells and reduced type I collagen levels, with an associated rise in matrix metalloproteinase-1 activity. Given their findings implying the effectiveness of the extracts in facilitating the catabolic pathway of human dermal fibroblasts, the authors suggest that these shell matrices present the potential for use in treating fibrosis, especially for scleroderma.6
Antioxidant and antiaging activity
Shao et al. demonstrated 10 years ago that pearl powder provides a moisturizing effect on the skin, with ultramicro pearl powder delivering a more robust moisturizing result than water-soluble pearl powder. These two types of pearl powder, along with another one tested (ultranano pearl powder), also significantly diminished the activation of tyrosinase and free radicals. Water-soluble pearl powder did not perform as well as the other two formulations in free radical scavenging. The investigators suggested that their results support the use of pearl powder to combat aging and enhance beauty, and could be used in the clinical setting.13
In 2017, Yang et al. reported on the in vitro antihemolytic and antioxidant activity of pearl powder in shielding human erythrocytes against 2,2’-azobis(2-amidinopropane) dihydrochloride–induced oxidative damage to membrane proteins/lipids. The researchers contend that the strong antioxidant qualities of pearl powder could be applied to prevent or protect against various diseases resulting from free radical damage.2
Human trials: Antioxidant, antiaging, skin appearance
Chiu et al. studied the antioxidant activity of various pearl powder extracts in a randomized, placebo-controlled trial in 2018. They also investigated the life span–prolonging effects of the powders using wild-type Caenorhabditis elegans. Twenty healthy middle-aged subjects were separated into two groups (experimental and placebo), with 3 g of pearl powder administered in capsules to the former and 3 g of placebo to the latter over 8 weeks. Blood samples taken at the beginning and every 2 weeks during the trial and in the 10th week revealed maximum antioxidant activity of the pearl powder and prolongation of C. elegans lifespan by 18.87%. Subjects using pearl powder demonstrated significant increases in total antioxidant capacity, thiols, glutathione, and enzymic antioxidant activity, along with notably inhibited lipid peroxidation products. The investigators concluded that pearl powder extract acted as a potent antioxidant and its use may be warranted to treat degenerative conditions related to aging.3
A recent study of the perception of blue light on Korean women’s faces using blue pearl pigment revealed that the pigment does indeed elicit the perception of the blue-light effect, notably transparency and gloss, which is particularly valued in Korea.14
Conclusion
The use of mother of pearl and pearl powder in traditional Chinese medicine and as a cosmetic and food additive has a rich and lengthy history. Contemporary research clearly suggests interesting avenues for further investigation and some promising results. Much more research is necessary, though, to delineate the potential roles of pearl powder in the skin care arsenal.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Zhang J et al. J Sep Sci. 2015 May;38(9):1552-60.
2. Yang HL et al. J Food Drug Anal. 2017 Oct;25(4):898-907.
3. Chiu HF et al. J Food Drug Anal. 2018 Jan;26(1):309-17.
4. Chen X et al. Drug Dev Ind Pharm. 2019 Jun;45(6):1009-16.
5. Nagai K. Zoolog Sci. 2013 Oct;30(10):783-93.
6. Latire T et al. Cytotechnology. 2017 Oct;69(5):815-29.
7. Li YC et al. Pharm Biol. 2013 Mar;51(3):289-97.
8. Jian-Ping D et al. Pharm Biol. 2010 Feb;48(2):122-7.
9. Lee K et al. Mol Biol Rep. 2012 Mar;39(3):3211-8.
10. Lopez E et al. Tissue Cell. 2000 Feb;32(1):95-101.
11. Rousseau M et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):1-9.
12. Yang YL et al. Biomed Mater Eng. 2015;26 Suppl 1:S139-45.
13. Shao DZ et al. J Cosmet Sci. 2010 Mar-Apr;61(2):133-45.
14. Lee M et al. Skin Res Technol. 2020 Jan;26(1):76-80.
Because of its dense protein and mineral composition, it has been used to treat several skin and bone disorders, as well as palpitations, insomnia, and epilepsy.3,4 The pearl-farming industry itself was established in Japan and has existed for more than a century; today, pearls are cultured globally and continue to receive attention for conferring health benefits.5
Calcium carbonate is the primary component of mollusk shells (roughly 95%), with the remainder an organic matrix including proteins, glycoproteins, and polysaccharides.6 Pearl powder is known to have exhibited antiaging, antioxidant, antiradiative, and tonic activities; in recent years, it has been incorporated into health foods for such properties and used in the clinical setting to treat ulcers (aphthous, gastric, and duodenal).4,7 Consisting of multiple active proteins, pearl powder is thought to be conducive to skin cell growth and effective for wound repair.4 This column focuses on recent research into the dermatologic potential of the powder derived from mother of pearl.
Wound healing
A decade ago, Jian-Ping et al. showed in mice that the water-soluble matrix of pearl powder (Hyriopsis cumingii) could significantly induce oral fibroblast proliferation and collagen accumulation, suppress matrix metalloproteinase-2 activity, and significantly foster TIMP-1 synthesis. The investigators concluded that the wound healing facilitated by pearl powder derives, in part, from its capacity to promote fibroblast mitosis, collagen deposition, and production of TIMP-1.8
Two years later, Lee et al. evaluated the effects of water-soluble nacre (mother of pearl) on second-degree burn wound healing in porcine skin as a proxy for human skin. They found that its application quickly led to burn-induced granulation areas filling with collagen, with normal skin appearance restored to wounded dermis and epidermis. Angiogenesis was also promoted by water-soluble nacre as was wound recovery in areas with apoptotic and necrotic cellular damage. Murine fibroblast NIH3T3 cells treated with water-soluble nacre also demonstrated augmented proliferation and collagen production. The researchers cited the restoration of angiogenesis and fibroblast activity as the primary benefits of water-soluble nacre, suggesting its potential as a wound therapy, preferable to powdered nacre due to better biocompatibility with less discomfort.9
The next year, Li et al. found that mother of pearl extract promoted cell migration of fibroblasts in cell culture, demonstrating its potential as a wound healing model.7In 2019, Chen et al. studied the effects of pearl powders of varying particle sizes to treat wounds in vitro and in vivo. They found that micro- and nanosized pearl powders augmented proliferation and migration of skin cells and shortened wound closure time. All powders also improved the biomechanical strength of healed skin, enhanced collagen formation and deposition, and expanded cutaneous angiogenesis, with nanoscale pearl powder displaying greatest efficiency.4
Skin tone and atopic dermatitis
In 2000, Lopez et al. implanted powdered nacre (mother of pearl derived from Pinctada maxima), which can promote and regulate bone-forming cells, into rat dermis to evaluate its effects on skin fibroblasts. They noted that the implant yielded well-vascularized tissue and improved extracellular matrix production, synthesis of substances involved in cellular adhesion and communication, and tissue regeneration (such as collagen types I and III). The investigators concluded that the powdered nacre contributed to the conditions necessary for improved skin tone and proper physiologic functioning of the skin.10
Rousseau et al. extracted lipids from the nacre of the oyster P. margaritifera to test on artificially dehydrated skin explants with the intention of developing new treatments for atopic dermatitis. The researchers determined that the lipids spurred a reconstitution of the intercellular material of the stratum corneum, concluding that new products to treat atopic dermatitis might be based on the signaling activity of nacre lipids.11
Antifibrotic and anti-inflammatory activity
A 2015 study by Yang et al. showed that a room-temperature superextraction system to yield the main active constituents of pearl was successful in enhancing their anti-inflammatory and antiapoptotic activity in human keratinocyte cells (HaCaT) exposed to low-dose UVB. The investigators combined pearl extract and poly (gamma-glutamic acid) hydrogels and observed reductions in inflammation and apoptosis of HaCaT cells. They concluded that a marketed pearl extract may be able to suppress radiation dermatitis present in keratinocytes.12
Two years later, Latire et al. used human dermal fibroblasts in primary culture to assess the potential biological activities of the matrix macromolecular components extracted from the shells of two edible mollusks (the blue mussel Mytilus edulis and the Pacific oyster Crassostrea gigas). The investigators found that both extracts influenced metabolic functions of the cells and reduced type I collagen levels, with an associated rise in matrix metalloproteinase-1 activity. Given their findings implying the effectiveness of the extracts in facilitating the catabolic pathway of human dermal fibroblasts, the authors suggest that these shell matrices present the potential for use in treating fibrosis, especially for scleroderma.6
Antioxidant and antiaging activity
Shao et al. demonstrated 10 years ago that pearl powder provides a moisturizing effect on the skin, with ultramicro pearl powder delivering a more robust moisturizing result than water-soluble pearl powder. These two types of pearl powder, along with another one tested (ultranano pearl powder), also significantly diminished the activation of tyrosinase and free radicals. Water-soluble pearl powder did not perform as well as the other two formulations in free radical scavenging. The investigators suggested that their results support the use of pearl powder to combat aging and enhance beauty, and could be used in the clinical setting.13
In 2017, Yang et al. reported on the in vitro antihemolytic and antioxidant activity of pearl powder in shielding human erythrocytes against 2,2’-azobis(2-amidinopropane) dihydrochloride–induced oxidative damage to membrane proteins/lipids. The researchers contend that the strong antioxidant qualities of pearl powder could be applied to prevent or protect against various diseases resulting from free radical damage.2
Human trials: Antioxidant, antiaging, skin appearance
Chiu et al. studied the antioxidant activity of various pearl powder extracts in a randomized, placebo-controlled trial in 2018. They also investigated the life span–prolonging effects of the powders using wild-type Caenorhabditis elegans. Twenty healthy middle-aged subjects were separated into two groups (experimental and placebo), with 3 g of pearl powder administered in capsules to the former and 3 g of placebo to the latter over 8 weeks. Blood samples taken at the beginning and every 2 weeks during the trial and in the 10th week revealed maximum antioxidant activity of the pearl powder and prolongation of C. elegans lifespan by 18.87%. Subjects using pearl powder demonstrated significant increases in total antioxidant capacity, thiols, glutathione, and enzymic antioxidant activity, along with notably inhibited lipid peroxidation products. The investigators concluded that pearl powder extract acted as a potent antioxidant and its use may be warranted to treat degenerative conditions related to aging.3
A recent study of the perception of blue light on Korean women’s faces using blue pearl pigment revealed that the pigment does indeed elicit the perception of the blue-light effect, notably transparency and gloss, which is particularly valued in Korea.14
Conclusion
The use of mother of pearl and pearl powder in traditional Chinese medicine and as a cosmetic and food additive has a rich and lengthy history. Contemporary research clearly suggests interesting avenues for further investigation and some promising results. Much more research is necessary, though, to delineate the potential roles of pearl powder in the skin care arsenal.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Zhang J et al. J Sep Sci. 2015 May;38(9):1552-60.
2. Yang HL et al. J Food Drug Anal. 2017 Oct;25(4):898-907.
3. Chiu HF et al. J Food Drug Anal. 2018 Jan;26(1):309-17.
4. Chen X et al. Drug Dev Ind Pharm. 2019 Jun;45(6):1009-16.
5. Nagai K. Zoolog Sci. 2013 Oct;30(10):783-93.
6. Latire T et al. Cytotechnology. 2017 Oct;69(5):815-29.
7. Li YC et al. Pharm Biol. 2013 Mar;51(3):289-97.
8. Jian-Ping D et al. Pharm Biol. 2010 Feb;48(2):122-7.
9. Lee K et al. Mol Biol Rep. 2012 Mar;39(3):3211-8.
10. Lopez E et al. Tissue Cell. 2000 Feb;32(1):95-101.
11. Rousseau M et al. Comp Biochem Physiol B Biochem Mol Biol. 2006 Sep;145(1):1-9.
12. Yang YL et al. Biomed Mater Eng. 2015;26 Suppl 1:S139-45.
13. Shao DZ et al. J Cosmet Sci. 2010 Mar-Apr;61(2):133-45.
14. Lee M et al. Skin Res Technol. 2020 Jan;26(1):76-80.