User login
Racial Limitations of Fitzpatrick Skin Type
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
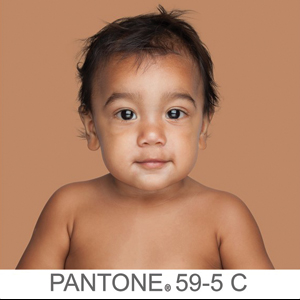
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
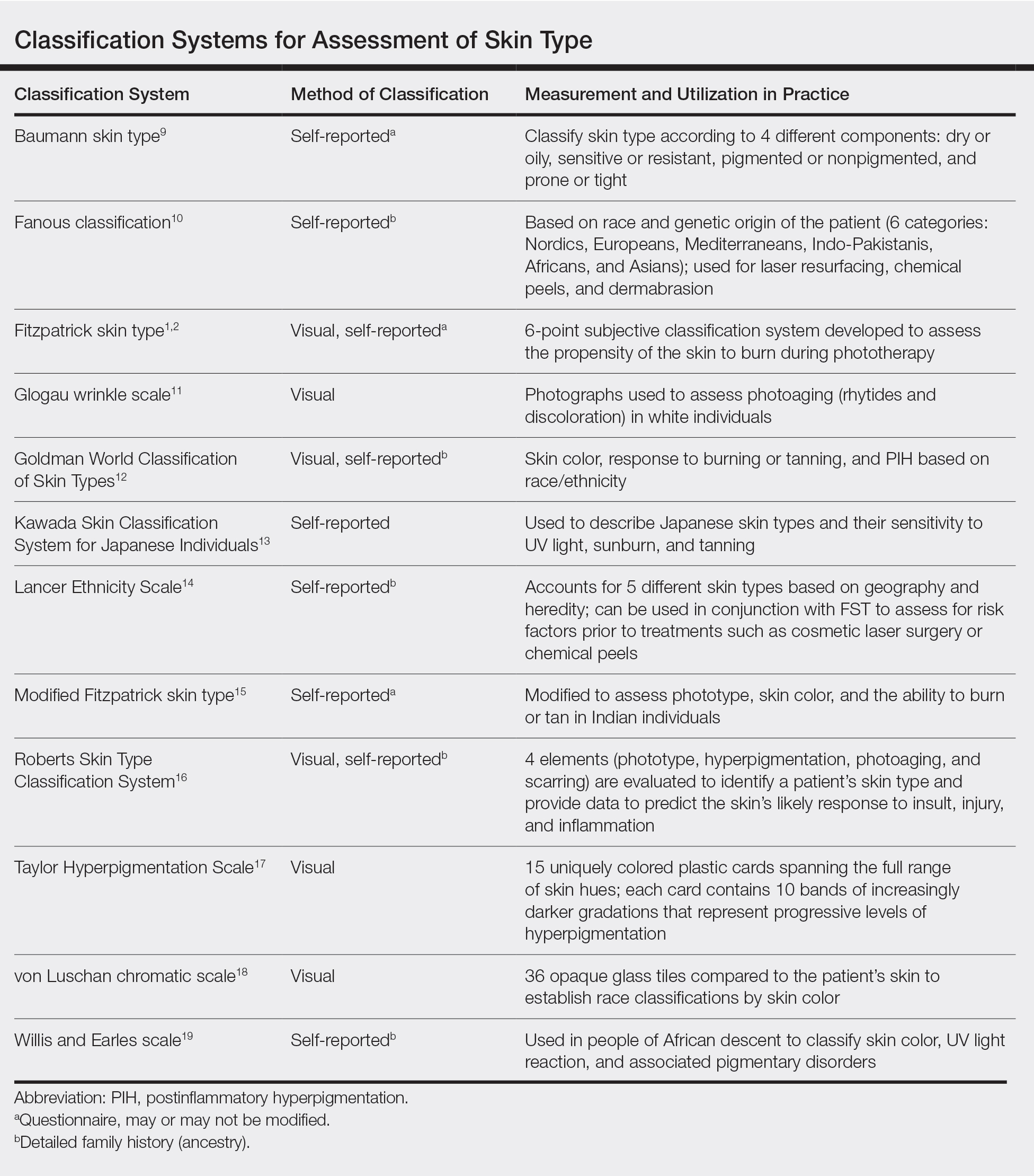
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Practice Points
- Medical providers should be cognizant of conflating race and ethnicity with Fitzpatrick skin type (FST).
- Misuse of FST may occur more frequently among physicians who do not identify as having skin of color.
- Although alternative skin type classification systems have been proposed, more clinically relevant methods for describing skin of color need to be developed.
Social media may negatively influence acne treatment
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
A small survey suggests many patients consult social media for advice on acne treatment and follow recommendations that don’t align with clinical guidelines.
Of the 130 patients surveyed, 45% consulted social media for advice on acne treatment, and 52% of those patients followed recommendations that don’t correspond to American Academy of Dermatology (AAD) guidelines. Most patients reported no improvement (40%) or minimal improvement (53%) in their acne after following advice from social media.
“These results suggest that dermatologists should inquire about social media acne treatment advice and directly address misinformation,” wrote Ahmed Yousaf, of West Virginia University, Morgantown, W.Va., and colleagues. Their report is in Pediatric Dermatology.
They conducted the survey of 130 patients treated for acne at West Virginia University. Most patients were female (60%), and a majority were adolescents (54%) or adults (44%). About half of the patients (51%) said their acne was moderate, 38% said it was severe, and 11% said it was mild.
Most patients said they consulted a medical professional for their first acne treatment (58%). However, 16% of patients said they first went to social media for advice, 26% said they consulted family or friends, and 10% took “other” steps as their first approach to acne treatment.
In all, 45% of patients consulted social media for acne treatment advice at some point. This includes 54% of women, 31% of men, 41% of adolescents, and 51% of adults. Social media consultation was more common among patients with severe acne (54%) than among those with mild (36%) or moderate (39%) acne.
The most common social media platforms used were YouTube and Instagram (58% each), followed by Pinterest (31%), Facebook (19%), Twitter (9%), Snapchat (7%), and Tumblr (3%). (Patients could select more than one social media platform.)
Roughly half (52%) of patients who consulted social media followed advice that does not align with AAD guidelines, 31% made changes that are recommended by the AAD, and 17% did not provide information on recommendations they followed.
The social media advice patients followed included using over-the-counter products (81%), making dietary changes (40%), using self-made products (19%), taking supplements (16%), and making changes in exercise routines (7%). (Patients could select more than one treatment approach.)
Among the patients who followed social media advice, 40% said they saw no change in their acne, and 53% reported minimal improvement.
“Only 7% of social media users reported significant improvement in their acne,” Mr. Yousaf and colleagues wrote. “This may be due to less accurate content found on social media compared to other health care sources.”
The authors acknowledged that the patients surveyed were recruited from a dermatology clinic. Therefore, these results “likely underestimate the percentage of patients who improve from social media acne treatment advice and do not consult a medical professional.”
Mr. Yousaf and colleagues did not disclose any conflicts of interest.
SOURCE: Yousaf A et al. Pediatr Dermatol. 2020 Jan 15. doi: 10.1111/pde.14091.
FROM PEDIATRIC DERMATOLOGY
Lasers expunge mucosal tattoos
, researchers reported.
Mucocutaneous tattoos are relatively rare, and lasers have been used for their removal, but cases and results have not been well documented, wrote Hao Feng, MD, then of the Laser & Skin Surgery Center of New York, and the department of dermatology, New York University, and coauthors.
In a report published in Lasers in Surgery and Medicine, the clinicians noted significant improvement with no scarring or dyspigmentation at 1 month after the last treatment session in two patients, with mucosal tattoos that had not been previously treated.
In one case, a healthy 19-year-old woman with Fitzpatrick skin type II presented for removal of a 6‐month‐old, black tattoo on the mucosal surface of her lower lip. She received six treatment sessions at months 0, 1, 3, 5, 7, and 12 with a QS 694‐nm ruby laser at settings of 6-mm spot size, 20-nanosecond pulse duration, and 3.0-3.5 J/cm2.
In a second case, a 30‐year‐old man with Fitzpatrick skin type IV presented for removal of a 10‐year‐old black tattoo on his left buccal mucosa. He received one treatment with 755-nm alexandrite picosecond lasers at settings of 2.5-mm spot size, 500-picosecond pulse duration, and 3.36 J/cm2.
Both patients experienced local mild discomfort, erythema, and edema after treatment.
“Older tattoos respond better and quicker on the skin to laser treatments, and it is likely the reason why the buccal mucosa tattoo (10 years) resolved with a single treatment whereas the lower lip tattoo (6 months) required six treatments,” the authors noted.
Mucosal tattoos, they added, “tend to respond better, faster, and with less unwanted side effects than tattoos on the skin. This may relate to the fact that mucosal skin is thinner, non-keratinized, well‐vascularized, and contains less melanin content.”
As to which laser is the best choice for removing mucosal tattoos, the authors noted that it is unclear, but while they said they have been using picosecond lasers for tattoo removals, QS lasers “remain excellent treatment modalities,” they wrote.
“Given the excellent clinical response combined with lack of scarring and dyspigmentation in our highly satisfied patients, it is the authors’ opinion that laser treatment should be considered as the first‐line treatment in removing unwanted cosmetic mucosal tattoos. This can be accomplished with various wavelengths in the picosecond and nanosecond domains,” they concluded.
Dr. Feng, who is now director of laser surgery and cosmetic dermatology at the University of Connecticut Health Center, Farmington, disclosed serving as a consultant and medical monitor for Cytrellis Biosystems. Another author disclosed serving on the advisory boards for Cytrellis, Syneron Candela, and Cynosure; owning stocks or having stock options with Cytrellis; and investing in Syneron Candela, Cynosure, and Cytrellis. The remaining two authors had no disclosures.
SOURCE: Feng H et al. Lasers Surg Med. 2019 Dec 30. doi: 10.1002/lsm.23207.
, researchers reported.
Mucocutaneous tattoos are relatively rare, and lasers have been used for their removal, but cases and results have not been well documented, wrote Hao Feng, MD, then of the Laser & Skin Surgery Center of New York, and the department of dermatology, New York University, and coauthors.
In a report published in Lasers in Surgery and Medicine, the clinicians noted significant improvement with no scarring or dyspigmentation at 1 month after the last treatment session in two patients, with mucosal tattoos that had not been previously treated.
In one case, a healthy 19-year-old woman with Fitzpatrick skin type II presented for removal of a 6‐month‐old, black tattoo on the mucosal surface of her lower lip. She received six treatment sessions at months 0, 1, 3, 5, 7, and 12 with a QS 694‐nm ruby laser at settings of 6-mm spot size, 20-nanosecond pulse duration, and 3.0-3.5 J/cm2.
In a second case, a 30‐year‐old man with Fitzpatrick skin type IV presented for removal of a 10‐year‐old black tattoo on his left buccal mucosa. He received one treatment with 755-nm alexandrite picosecond lasers at settings of 2.5-mm spot size, 500-picosecond pulse duration, and 3.36 J/cm2.
Both patients experienced local mild discomfort, erythema, and edema after treatment.
“Older tattoos respond better and quicker on the skin to laser treatments, and it is likely the reason why the buccal mucosa tattoo (10 years) resolved with a single treatment whereas the lower lip tattoo (6 months) required six treatments,” the authors noted.
Mucosal tattoos, they added, “tend to respond better, faster, and with less unwanted side effects than tattoos on the skin. This may relate to the fact that mucosal skin is thinner, non-keratinized, well‐vascularized, and contains less melanin content.”
As to which laser is the best choice for removing mucosal tattoos, the authors noted that it is unclear, but while they said they have been using picosecond lasers for tattoo removals, QS lasers “remain excellent treatment modalities,” they wrote.
“Given the excellent clinical response combined with lack of scarring and dyspigmentation in our highly satisfied patients, it is the authors’ opinion that laser treatment should be considered as the first‐line treatment in removing unwanted cosmetic mucosal tattoos. This can be accomplished with various wavelengths in the picosecond and nanosecond domains,” they concluded.
Dr. Feng, who is now director of laser surgery and cosmetic dermatology at the University of Connecticut Health Center, Farmington, disclosed serving as a consultant and medical monitor for Cytrellis Biosystems. Another author disclosed serving on the advisory boards for Cytrellis, Syneron Candela, and Cynosure; owning stocks or having stock options with Cytrellis; and investing in Syneron Candela, Cynosure, and Cytrellis. The remaining two authors had no disclosures.
SOURCE: Feng H et al. Lasers Surg Med. 2019 Dec 30. doi: 10.1002/lsm.23207.
, researchers reported.
Mucocutaneous tattoos are relatively rare, and lasers have been used for their removal, but cases and results have not been well documented, wrote Hao Feng, MD, then of the Laser & Skin Surgery Center of New York, and the department of dermatology, New York University, and coauthors.
In a report published in Lasers in Surgery and Medicine, the clinicians noted significant improvement with no scarring or dyspigmentation at 1 month after the last treatment session in two patients, with mucosal tattoos that had not been previously treated.
In one case, a healthy 19-year-old woman with Fitzpatrick skin type II presented for removal of a 6‐month‐old, black tattoo on the mucosal surface of her lower lip. She received six treatment sessions at months 0, 1, 3, 5, 7, and 12 with a QS 694‐nm ruby laser at settings of 6-mm spot size, 20-nanosecond pulse duration, and 3.0-3.5 J/cm2.
In a second case, a 30‐year‐old man with Fitzpatrick skin type IV presented for removal of a 10‐year‐old black tattoo on his left buccal mucosa. He received one treatment with 755-nm alexandrite picosecond lasers at settings of 2.5-mm spot size, 500-picosecond pulse duration, and 3.36 J/cm2.
Both patients experienced local mild discomfort, erythema, and edema after treatment.
“Older tattoos respond better and quicker on the skin to laser treatments, and it is likely the reason why the buccal mucosa tattoo (10 years) resolved with a single treatment whereas the lower lip tattoo (6 months) required six treatments,” the authors noted.
Mucosal tattoos, they added, “tend to respond better, faster, and with less unwanted side effects than tattoos on the skin. This may relate to the fact that mucosal skin is thinner, non-keratinized, well‐vascularized, and contains less melanin content.”
As to which laser is the best choice for removing mucosal tattoos, the authors noted that it is unclear, but while they said they have been using picosecond lasers for tattoo removals, QS lasers “remain excellent treatment modalities,” they wrote.
“Given the excellent clinical response combined with lack of scarring and dyspigmentation in our highly satisfied patients, it is the authors’ opinion that laser treatment should be considered as the first‐line treatment in removing unwanted cosmetic mucosal tattoos. This can be accomplished with various wavelengths in the picosecond and nanosecond domains,” they concluded.
Dr. Feng, who is now director of laser surgery and cosmetic dermatology at the University of Connecticut Health Center, Farmington, disclosed serving as a consultant and medical monitor for Cytrellis Biosystems. Another author disclosed serving on the advisory boards for Cytrellis, Syneron Candela, and Cynosure; owning stocks or having stock options with Cytrellis; and investing in Syneron Candela, Cynosure, and Cytrellis. The remaining two authors had no disclosures.
SOURCE: Feng H et al. Lasers Surg Med. 2019 Dec 30. doi: 10.1002/lsm.23207.
FROM LASERS IN SURGERY AND MEDICINE
CD1a and cosmetic-related contact dermatitis
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
Pyrrolidone carboxylic acid may be a key cutaneous biomarker
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Makeup is contaminated with pathogenic bacteria
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Nonablative laser improved PIH in patients with darker skin
Yoon‐Soo Cindy Bae, MD, and colleagues reported.
Among patients treated with the nonablative fractional 1,927 nm laser, there was a mean improvement of about 43% in hyperpigmented areas, and no side effects were reported, wrote Dr. Bae, of the department of dermatology at New York University and the Laser & Skin Surgery Center of New York, and coauthors in Lasers in Surgery and Medicine.
Lasers have not been the first choice for hyperpigmentation in Fitzpatrick skin types IV, V, and VI, they pointed out. More commonly used treatments are hydroquinone and chemical peels that use glycolic acid or salicylic acid. But these are not always ideal options, Dr. Bae said in an interview.
“There are side effects to medical therapy. The drawbacks of medical therapy include compliance issues, risk of skin irritation from the product ... and a risk of hyperpigmentation specifically for hydroquinone. There are also risks to laser therapy, including dyspigmentation and scarring,” she added. “However, the laser we used is a low energy, nonablative type of laser, so the risk of scarring is extremely rare and the dyspigmentation is actually what we are aiming to treat.”
The retrospective study comprised 61 patients with PIH who had received more than one treatment with the low energy fractionated 1,927 nm diode laser between 2013 and 2016. Most were Fitzpatrick type IV (73.8%). The remainder were Type V (16.4%) and Type VI (9.8%). The most common treatment site was the face or cheeks (68.9%), followed by legs (13%), the rest of the cases were unspecified.
Patients had received treatment with the laser with fixed fluence at 5 mJ, fixed spot size of 140 micrometers, depth of 170 micrometers, and 5% coverage. They required several treatments: 15 had two, 14 had three, 16 had four, and the remainder had five or more. Topical treatment data were not collected. Photographs taken before treatment and before the last treatment were evaluated by dermatologists who had not treated the patients. Based on those evaluations, the mean improvement was a statistically significant 43.2%.
There did not, however, appear to be much difference between the treatment groups. The mean improvement among patients with two treatments was 44.5%; three treatments, 44.29%; four treatments, 40.63%; five or more treatments, 43.75%.
Although those with darker skin types tended to have better results, there were no statistically significant differences between the skin-type groups. Among those with Fitzpatrick skin type IV, the mean improvement was 40.39%; skin type V, 47.25%; and skin type VI, 57.92%.
“The fact that there was no correlation between Fitzpatrick skin type … and average percent improvement demonstrates that this laser is a viable treatment option for patients with very dark skin,” the authors wrote. “There were also no significant differences between the average percent improvements for people receiving different numbers of treatments. A trend was observed that favored treating patients with darker skin type; however, this lacked statistical significance. This may have been due to an underpowered study.”
Limitations of the study included the retrospective design and nonstandardization of photographs; “further studies with prospective controlled designs are needed to confirm our findings,” they added.
No funding or disclosure information was provided.
[email protected]
SOURCE: Bae YS et al. Lasers Surg Med. 2019 Oct 29. doi: 10.1002/lsm.23173.
Yoon‐Soo Cindy Bae, MD, and colleagues reported.
Among patients treated with the nonablative fractional 1,927 nm laser, there was a mean improvement of about 43% in hyperpigmented areas, and no side effects were reported, wrote Dr. Bae, of the department of dermatology at New York University and the Laser & Skin Surgery Center of New York, and coauthors in Lasers in Surgery and Medicine.
Lasers have not been the first choice for hyperpigmentation in Fitzpatrick skin types IV, V, and VI, they pointed out. More commonly used treatments are hydroquinone and chemical peels that use glycolic acid or salicylic acid. But these are not always ideal options, Dr. Bae said in an interview.
“There are side effects to medical therapy. The drawbacks of medical therapy include compliance issues, risk of skin irritation from the product ... and a risk of hyperpigmentation specifically for hydroquinone. There are also risks to laser therapy, including dyspigmentation and scarring,” she added. “However, the laser we used is a low energy, nonablative type of laser, so the risk of scarring is extremely rare and the dyspigmentation is actually what we are aiming to treat.”
The retrospective study comprised 61 patients with PIH who had received more than one treatment with the low energy fractionated 1,927 nm diode laser between 2013 and 2016. Most were Fitzpatrick type IV (73.8%). The remainder were Type V (16.4%) and Type VI (9.8%). The most common treatment site was the face or cheeks (68.9%), followed by legs (13%), the rest of the cases were unspecified.
Patients had received treatment with the laser with fixed fluence at 5 mJ, fixed spot size of 140 micrometers, depth of 170 micrometers, and 5% coverage. They required several treatments: 15 had two, 14 had three, 16 had four, and the remainder had five or more. Topical treatment data were not collected. Photographs taken before treatment and before the last treatment were evaluated by dermatologists who had not treated the patients. Based on those evaluations, the mean improvement was a statistically significant 43.2%.
There did not, however, appear to be much difference between the treatment groups. The mean improvement among patients with two treatments was 44.5%; three treatments, 44.29%; four treatments, 40.63%; five or more treatments, 43.75%.
Although those with darker skin types tended to have better results, there were no statistically significant differences between the skin-type groups. Among those with Fitzpatrick skin type IV, the mean improvement was 40.39%; skin type V, 47.25%; and skin type VI, 57.92%.
“The fact that there was no correlation between Fitzpatrick skin type … and average percent improvement demonstrates that this laser is a viable treatment option for patients with very dark skin,” the authors wrote. “There were also no significant differences between the average percent improvements for people receiving different numbers of treatments. A trend was observed that favored treating patients with darker skin type; however, this lacked statistical significance. This may have been due to an underpowered study.”
Limitations of the study included the retrospective design and nonstandardization of photographs; “further studies with prospective controlled designs are needed to confirm our findings,” they added.
No funding or disclosure information was provided.
[email protected]
SOURCE: Bae YS et al. Lasers Surg Med. 2019 Oct 29. doi: 10.1002/lsm.23173.
Yoon‐Soo Cindy Bae, MD, and colleagues reported.
Among patients treated with the nonablative fractional 1,927 nm laser, there was a mean improvement of about 43% in hyperpigmented areas, and no side effects were reported, wrote Dr. Bae, of the department of dermatology at New York University and the Laser & Skin Surgery Center of New York, and coauthors in Lasers in Surgery and Medicine.
Lasers have not been the first choice for hyperpigmentation in Fitzpatrick skin types IV, V, and VI, they pointed out. More commonly used treatments are hydroquinone and chemical peels that use glycolic acid or salicylic acid. But these are not always ideal options, Dr. Bae said in an interview.
“There are side effects to medical therapy. The drawbacks of medical therapy include compliance issues, risk of skin irritation from the product ... and a risk of hyperpigmentation specifically for hydroquinone. There are also risks to laser therapy, including dyspigmentation and scarring,” she added. “However, the laser we used is a low energy, nonablative type of laser, so the risk of scarring is extremely rare and the dyspigmentation is actually what we are aiming to treat.”
The retrospective study comprised 61 patients with PIH who had received more than one treatment with the low energy fractionated 1,927 nm diode laser between 2013 and 2016. Most were Fitzpatrick type IV (73.8%). The remainder were Type V (16.4%) and Type VI (9.8%). The most common treatment site was the face or cheeks (68.9%), followed by legs (13%), the rest of the cases were unspecified.
Patients had received treatment with the laser with fixed fluence at 5 mJ, fixed spot size of 140 micrometers, depth of 170 micrometers, and 5% coverage. They required several treatments: 15 had two, 14 had three, 16 had four, and the remainder had five or more. Topical treatment data were not collected. Photographs taken before treatment and before the last treatment were evaluated by dermatologists who had not treated the patients. Based on those evaluations, the mean improvement was a statistically significant 43.2%.
There did not, however, appear to be much difference between the treatment groups. The mean improvement among patients with two treatments was 44.5%; three treatments, 44.29%; four treatments, 40.63%; five or more treatments, 43.75%.
Although those with darker skin types tended to have better results, there were no statistically significant differences between the skin-type groups. Among those with Fitzpatrick skin type IV, the mean improvement was 40.39%; skin type V, 47.25%; and skin type VI, 57.92%.
“The fact that there was no correlation between Fitzpatrick skin type … and average percent improvement demonstrates that this laser is a viable treatment option for patients with very dark skin,” the authors wrote. “There were also no significant differences between the average percent improvements for people receiving different numbers of treatments. A trend was observed that favored treating patients with darker skin type; however, this lacked statistical significance. This may have been due to an underpowered study.”
Limitations of the study included the retrospective design and nonstandardization of photographs; “further studies with prospective controlled designs are needed to confirm our findings,” they added.
No funding or disclosure information was provided.
[email protected]
SOURCE: Bae YS et al. Lasers Surg Med. 2019 Oct 29. doi: 10.1002/lsm.23173.
FROM LASERS IN SURGERY AND MEDICINE
Halal nail polish
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ever heard of halal nail polish? As an expert on all things hair, skin, and nails, I was dismayed when I walked into my local nail salon and saw this new category of nail polish I’d never heard of before. About 10 halal nail polishes were available in an array of beautiful colors. This nail salon was already branded as “nontoxic,” carrying only “8-free” nail polishes, vegan, and cruelty-free body and cleaning products – as well as no acrylics or UV light devices used for drying manicured nails or processing gel nails. With the salon already providing 8-free nail polishes, what was the difference between those and halal nail polishes?
As I did my Google search while sitting in the salon chair, I got the answer both from salon employees and the Internet, and also found several other brands of halal nail polishes sold on Amazon.
The main ingredient in traditional nail lacquer is nitrocellulose, a mixture of an indigestible plant fiber. Once used for gunpowder and blast mining in the 19th century, today, nitrocellulose is used for many purposes for holding materials together, such as photography film, and diagnostic tests that involve antigen-antibody binding, such as pregnancy tests. In a bottle of traditional nail polish, nitrocellulose is dissolved in a chemical solvent (typically ethyl acetate), along with pigment colors and plasticizers. The solvent quickly evaporates and is what gives nail polish its chemical smell. Once painted on the nail, the solvent gradually evaporates away entirely and the nitrocellulose is left behind, drying into a solid film on the nail. The same solvent molecule is in nonacetone nail polish remover, which simply redissolves the nitrocellulose back into a liquid so it can be wiped off.
Some nail polish may also include “pearl essence” to give a shiny look, like the silvery iridescence of fish scales. In fact, these polishes have contained ground up iridescent fish scales, but because of overfishing and cost, cheaper mineral alternatives are now more commonly used to give this shiny appearance.
Traditional nail polish contains tight molecular bonds that are impermeable to air and water. The tight bonds create fewer interstitial spaces for water to pass through. Nail polishes with polymer blends that help them withstand or make them more impermeable to water often chip less quickly and stay shinier longer.
While nail polishes are generally deemed safe, newer categories of 3-, 5-, or 8-free nail polishes containing fewer or different ingredients to preserve the product or give it it’s finish have been developed because of health concerns over some ingredients, for both users and cosmetologists. The 8-free nail polish does not contain dibutyl phthalate (DBP), toluene, formaldehyde, formaldehyde resin, camphor, ethyl tosylamide, parabens, or xylene. Three-, 5-, or 8- free doesn’t always mean that the lacquer has fewer chemicals; it may have alternative ingredients that also warrant study comparison to traditional ingredients.
Halal nail polish is in another category of “breathable nail polish,” which is not purely a function of the ingredient or lack of ingredients, but has to do with the way it is formulated. Compared with the tight molecular bonds of traditional nail polish, “breathable” polishes have a more staggered structure, which allows air and water molecules to pass through the polish. Halal nail polish is often free of the same ingredients as 8-free polishes, and some brands are even 13-free, animal product free, and do not require a base or top coat, but may also contain ingredients like bis (glycidoxyphenyl) propane/bisaminomethylnorbornae. Those ingredients are not typically used in traditional nail polish and may play a role in the unique staggered structure allowing air and water to pass through the polish. Halal nail polish may not last as long on nails as does traditional nail polish, usually a few days to a week.
In the past, some Islamic women would not wear nail polish because it is not porous, and so would interfere with wudu or ablution, the Islamic tradition of washing parts of the body before prayer. Halal translates to what is permissible and is most often associated with diet and procuring of meat. While the custom is not the same, the purpose is analogous to kosher preparation of foods in Judaism and dietary traditions in Hinduism. Having the opportunity to learn about this nail polish has been an interesting way to learn more about how different traditions, cultures, and faith affect skin and nail care.
Our nails are circulating breathing structures, with our nail plates being appendages over our nail beds with a rich pulse and blood supply. The main oxygen supply to the ends of our digits comes from our blood supply, not via oxygen through the nail plate, but wearing nail polish continuously can affect our nails. Oxygen saturation is detected through the end of our digits and nails when vital signs are being checked (less so when nail polish is present). As a continual wearer of nail polish for over 30 years, I can personally attest to certain types of onychodystrophy (white spots and discoloration on toe nails) from overuse of dark nail polish colors. Taking a break from polish and using these more “breathable” polishes could also potentially be a solution to this common complaint of nonfungal onychodystrophy.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Piceatannol: The other potent antioxidant in grapes and wine
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Comment on “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences”
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
To the Editor:
We read with great interest the recent Cutis article by Golda et al,1 “Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences.” We applaud the growing interest in the topic of dermatologist safety, as there are currently no established guidelines for precautions while performing surgical procedures. In 2018 we conducted a comprehensive review2 to characterize the specific risks, hazard reduction strategies available, and current use of surgical smoke safety techniques during surgery among dermatologists, and ultimately recommend guidance based on the current available evidence. To conduct this review, we collected data from 45 manuscripts in the dermatology, surgery, infectious disease, obstetrics, and cancer biology literature. Herein, we summarize key findings.2
Dermatologic surgeons, residents, staff, and patients are exposed to many infectious, inhalational, chemical, and mutagenic hazards when performing procedures that liberate smoke and plume. These risks are commonplace; however, they are particularly notable during ablative laser and laser hair removal procedures, which produce a heavy plume (averaging >100,000 particles/cm3). Brief periods of heavy plume exposure also are commonplace during electrosurgery.
Infectious particles in surgical plume have been extensively studied, and viral transmission has been demonstrated in animal studies. Human papillomavirus transmission appears to be the most prevalent risk. Surgical smoke has been shown to cause acute and chronic inhalational injury in rat and sheep studies.3-6
Additionally, chemicals with carcinogenic potential are present in surgical smoke and have been described.7,8 Chemicals in the greatest quantity include hydrocarbons, nitriles, fatty acids, and phenols. Although there have been no human studies on smoke carcinogenesis to date, surgical smoke has been shown to have carcinogenic properties in vitro.
Given these risks—both evidence based and theoretical—we believe that diligent hazard reduction strategies should be employed whenever possible. Surgical masks and high-efficiency particulate air respirators, such as N95 respirator masks, have been well studied and do provide smoke protection. High-efficiency particulate air masks can be worn when possible, especially during procedures that produce heavy plume, though surgical masks are capable of filtering most of the noxious chemicals in surgical smoke. It should be noted that proper fit with minimal air leak is the most important aspect of overall performance.
Smoke evacuators provide another level of protection. The physician should consider the evacuator’s filtration efficiency, capture velocity, and suction strength when evaluating overall performance. Furthermore, the smoke collection tip should be within 2 in of the surgical field to maximize efficacy. Maintenance for smoke evacuation systems should include regular (as defined by manufacturer instructions) flushing of the smoke evacuator lines.
Despite the risks of surgical smoke and the available options of minimizing these risks, the hazards of surgical smoke and the importance of protection are likely underemphasized. Many dermatologic surgeons do not use surgical masks or smoke evacuators in routine practice, according to several survey studies.9-11
It is important for the dermatologic community to consider effective ways of spreading awareness. We propose that surgical smoke safety be taught early in residency training. Additionally, smoke safety can be implemented into certification examinations. Access to masks and smoke evacuation devices are an important part of dermatology training. Accreditation Council for Graduate Medical Education funds should be appropriated to provide for such resources.
Finally, and perhaps most importantly, continued awareness should be established in the dermatology community via standardized guidelines and periodic updates in the dermatology literature and lectures at local and national conferences. Not until these strategies are implemented will surgical smoke protection be viewed as a necessary and important component of routine practice when performing dermatologic surgical procedures.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Golda N, Merrill B, Neill B. Intraoperative electrosurgical smoke during outpatient surgery: a survey of dermatologic surgeon and staff preferences. Cutis. 2019;104:120-124.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Wenig BL, Stenson KM, Wenig BM, et al. Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med. 1993;13:242-245.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc. 2003;17:979-987.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. Surg Endosc. 1998;12:1017-1019.
- Edwards BE, Reiman RE. Results of a survey on current surgical smoke control practices. AORN J. 2008;87:739-749.
- Oganesyan G, Eimpunth S, Kim SS, et al. Surgical smoke in dermatologic surgery. Dermatol Surg. 2014;40:1373-1377.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.