User login
FDA proposes new breast implant labeling with a boxed warning
Breast implants sold in the United States may soon require a boxed warning in their label, along with other label changes proposed by the Food and Drug Administration aimed at better informing prospective patients and clinicians of the potential risks from breast implants.
Other elements of the proposed labeling changes include creation of a patient-decision checklist, new recommendations for follow-up imaging to monitor for implant rupture, inclusion of detailed and understandable information about materials in the device, and provision of a device card to each patient with details on the specific implant they received.
These labeling changes all stemmed from a breast implant hearing held by the agency’s General and Plastic Surgery Devices Panel in March 2019, according to the draft guidance document officially released by the FDA on Oct. 24.
The proposed labeling changes were generally welcomed by patient advocates and by clinicians as a reasonable response to the concerns discussed at the March hearing. In an earlier move to address issues brought up at the hearing, the FDA in July arranged for a recall for certain Allergan models of textured breast implants because of their link with the development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).
The boxed warning proposed by the FDA would highlight four specific facts that patients, physicians, and surgeons should know about breast implants: They are not considered lifetime devices, the chance of developing complications from implants increases over time, some complications require additional surgery, and placement of breast implants has been associated with development of BIA-ALCL and may also be associated with certain systemic symptoms.
The FDA also proposed four other notable labeling changes:
- Creation of a patient-decision checklist to better systematize the informed consent process and make sure that certain aspects of breast implant placement are clearly brought to patients’ attention. The FDA proposed that patients sign their checklist attesting to having read and understood the information and that patients receive a take-home copy for their future reference. Proposed elements of the checklist include situations to not use breast implants; considerations for successful implant recipients; the risks of breast implant surgery; the importance of appropriate physician education, training, and experience; the risk for developing BIA-ALCL or systemic symptoms; and discussion of options other than breast implants.
- A new scheme for systematically and serially using imaging to screen for implant rupture that designates for the first time that ultrasound is an acceptable alternative to MRI and relies on a schedule by which either method initially screens the implant 5-6 years post operatively and then every 2 years thereafter.
- Detailed and understandable information about each material component of the implant with further information on possible adverse health effects of these compounds.
- A device card that patients should receive after their surgery with the implant’s name, serial number, and other identifiers; the boxed warning information; and a web link for accessing more up-to-date information.
The patient group Breast Implant Victim Advocacy praised the draft guidance. “The March Advisory Committee meeting seems to have prompted a shift by the FDA, surgeons, and industry,” said Jamee Cook, cofounder of the group. “We are definitely seeing a change in patient engagement. The FDA has been cooperating with patients and listening to our concerns. We still have a long way to go in raising public awareness of breast implant issues, but progress over the past 1-2 years has been amazing.”
Diana Zuckerman, PhD, president of the National Center for Health Research in Washington, gave the draft guidance a mixed review. “The FDA’s draft includes the types of information that we had proposed to the FDA in recent months in our work with patient advocates and plastic surgeons,” she said. “However, it is not as informative as it should be in describing well-designed studies indicating a risk of systemic illnesses. Patients deserve to make better-informed decisions in the future than most women considering breast implants have been able to make” in the past.
Patricia McGuire, MD, a St. Louis plastic surgeon who specializes in breast surgery and has studied breast implant illness, declared the guidance to be “reasonable.”
“I think the changes address the concerns expressed by patients during the [March] hearing; I agree with everything the FDA proposed in the guidance document,” Dr. McGuire said. “The boxed warning is reasonable and needs to be part of the informed consent process. I also agree with the changes in screening implants postoperatively. Most patients do not get MRI examinations. High-resolution ultrasound is more convenient and cost effective.”
The boxed warning was rated as “reasonably strong” and “the most serious step the FDA can take short of taking a device off the market,” but in the case of breast implants, a wider recall of textured implants than what the FDA arranged last July would be even more appropriate, commented Sidney M. Wolfe, MD, founder and senior adviser to Public Citizen. He also faulted the agency for not taking quicker action in mandating inclusion of the proposed boxed warning.
Issuing the labeling changes as draft guidance “is a ministep forward,” but also a process that “guarantees delay” and “creeps along at a dangerously slow pace,” Dr. Wolfe said. “The FDA is delaying what should be inevitable. The agency could put the boxed warning in place right now if they had the guts to do it.”
Dr. McGuire has been a consultant to Allergan, Establishment Labs, and Hans Biomed. Ms. Cook, Dr. Zuckerman, and Dr. Wolfe reported having no commercial disclosures.
Breast implants sold in the United States may soon require a boxed warning in their label, along with other label changes proposed by the Food and Drug Administration aimed at better informing prospective patients and clinicians of the potential risks from breast implants.
Other elements of the proposed labeling changes include creation of a patient-decision checklist, new recommendations for follow-up imaging to monitor for implant rupture, inclusion of detailed and understandable information about materials in the device, and provision of a device card to each patient with details on the specific implant they received.
These labeling changes all stemmed from a breast implant hearing held by the agency’s General and Plastic Surgery Devices Panel in March 2019, according to the draft guidance document officially released by the FDA on Oct. 24.
The proposed labeling changes were generally welcomed by patient advocates and by clinicians as a reasonable response to the concerns discussed at the March hearing. In an earlier move to address issues brought up at the hearing, the FDA in July arranged for a recall for certain Allergan models of textured breast implants because of their link with the development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).
The boxed warning proposed by the FDA would highlight four specific facts that patients, physicians, and surgeons should know about breast implants: They are not considered lifetime devices, the chance of developing complications from implants increases over time, some complications require additional surgery, and placement of breast implants has been associated with development of BIA-ALCL and may also be associated with certain systemic symptoms.
The FDA also proposed four other notable labeling changes:
- Creation of a patient-decision checklist to better systematize the informed consent process and make sure that certain aspects of breast implant placement are clearly brought to patients’ attention. The FDA proposed that patients sign their checklist attesting to having read and understood the information and that patients receive a take-home copy for their future reference. Proposed elements of the checklist include situations to not use breast implants; considerations for successful implant recipients; the risks of breast implant surgery; the importance of appropriate physician education, training, and experience; the risk for developing BIA-ALCL or systemic symptoms; and discussion of options other than breast implants.
- A new scheme for systematically and serially using imaging to screen for implant rupture that designates for the first time that ultrasound is an acceptable alternative to MRI and relies on a schedule by which either method initially screens the implant 5-6 years post operatively and then every 2 years thereafter.
- Detailed and understandable information about each material component of the implant with further information on possible adverse health effects of these compounds.
- A device card that patients should receive after their surgery with the implant’s name, serial number, and other identifiers; the boxed warning information; and a web link for accessing more up-to-date information.
The patient group Breast Implant Victim Advocacy praised the draft guidance. “The March Advisory Committee meeting seems to have prompted a shift by the FDA, surgeons, and industry,” said Jamee Cook, cofounder of the group. “We are definitely seeing a change in patient engagement. The FDA has been cooperating with patients and listening to our concerns. We still have a long way to go in raising public awareness of breast implant issues, but progress over the past 1-2 years has been amazing.”
Diana Zuckerman, PhD, president of the National Center for Health Research in Washington, gave the draft guidance a mixed review. “The FDA’s draft includes the types of information that we had proposed to the FDA in recent months in our work with patient advocates and plastic surgeons,” she said. “However, it is not as informative as it should be in describing well-designed studies indicating a risk of systemic illnesses. Patients deserve to make better-informed decisions in the future than most women considering breast implants have been able to make” in the past.
Patricia McGuire, MD, a St. Louis plastic surgeon who specializes in breast surgery and has studied breast implant illness, declared the guidance to be “reasonable.”
“I think the changes address the concerns expressed by patients during the [March] hearing; I agree with everything the FDA proposed in the guidance document,” Dr. McGuire said. “The boxed warning is reasonable and needs to be part of the informed consent process. I also agree with the changes in screening implants postoperatively. Most patients do not get MRI examinations. High-resolution ultrasound is more convenient and cost effective.”
The boxed warning was rated as “reasonably strong” and “the most serious step the FDA can take short of taking a device off the market,” but in the case of breast implants, a wider recall of textured implants than what the FDA arranged last July would be even more appropriate, commented Sidney M. Wolfe, MD, founder and senior adviser to Public Citizen. He also faulted the agency for not taking quicker action in mandating inclusion of the proposed boxed warning.
Issuing the labeling changes as draft guidance “is a ministep forward,” but also a process that “guarantees delay” and “creeps along at a dangerously slow pace,” Dr. Wolfe said. “The FDA is delaying what should be inevitable. The agency could put the boxed warning in place right now if they had the guts to do it.”
Dr. McGuire has been a consultant to Allergan, Establishment Labs, and Hans Biomed. Ms. Cook, Dr. Zuckerman, and Dr. Wolfe reported having no commercial disclosures.
Breast implants sold in the United States may soon require a boxed warning in their label, along with other label changes proposed by the Food and Drug Administration aimed at better informing prospective patients and clinicians of the potential risks from breast implants.
Other elements of the proposed labeling changes include creation of a patient-decision checklist, new recommendations for follow-up imaging to monitor for implant rupture, inclusion of detailed and understandable information about materials in the device, and provision of a device card to each patient with details on the specific implant they received.
These labeling changes all stemmed from a breast implant hearing held by the agency’s General and Plastic Surgery Devices Panel in March 2019, according to the draft guidance document officially released by the FDA on Oct. 24.
The proposed labeling changes were generally welcomed by patient advocates and by clinicians as a reasonable response to the concerns discussed at the March hearing. In an earlier move to address issues brought up at the hearing, the FDA in July arranged for a recall for certain Allergan models of textured breast implants because of their link with the development of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL).
The boxed warning proposed by the FDA would highlight four specific facts that patients, physicians, and surgeons should know about breast implants: They are not considered lifetime devices, the chance of developing complications from implants increases over time, some complications require additional surgery, and placement of breast implants has been associated with development of BIA-ALCL and may also be associated with certain systemic symptoms.
The FDA also proposed four other notable labeling changes:
- Creation of a patient-decision checklist to better systematize the informed consent process and make sure that certain aspects of breast implant placement are clearly brought to patients’ attention. The FDA proposed that patients sign their checklist attesting to having read and understood the information and that patients receive a take-home copy for their future reference. Proposed elements of the checklist include situations to not use breast implants; considerations for successful implant recipients; the risks of breast implant surgery; the importance of appropriate physician education, training, and experience; the risk for developing BIA-ALCL or systemic symptoms; and discussion of options other than breast implants.
- A new scheme for systematically and serially using imaging to screen for implant rupture that designates for the first time that ultrasound is an acceptable alternative to MRI and relies on a schedule by which either method initially screens the implant 5-6 years post operatively and then every 2 years thereafter.
- Detailed and understandable information about each material component of the implant with further information on possible adverse health effects of these compounds.
- A device card that patients should receive after their surgery with the implant’s name, serial number, and other identifiers; the boxed warning information; and a web link for accessing more up-to-date information.
The patient group Breast Implant Victim Advocacy praised the draft guidance. “The March Advisory Committee meeting seems to have prompted a shift by the FDA, surgeons, and industry,” said Jamee Cook, cofounder of the group. “We are definitely seeing a change in patient engagement. The FDA has been cooperating with patients and listening to our concerns. We still have a long way to go in raising public awareness of breast implant issues, but progress over the past 1-2 years has been amazing.”
Diana Zuckerman, PhD, president of the National Center for Health Research in Washington, gave the draft guidance a mixed review. “The FDA’s draft includes the types of information that we had proposed to the FDA in recent months in our work with patient advocates and plastic surgeons,” she said. “However, it is not as informative as it should be in describing well-designed studies indicating a risk of systemic illnesses. Patients deserve to make better-informed decisions in the future than most women considering breast implants have been able to make” in the past.
Patricia McGuire, MD, a St. Louis plastic surgeon who specializes in breast surgery and has studied breast implant illness, declared the guidance to be “reasonable.”
“I think the changes address the concerns expressed by patients during the [March] hearing; I agree with everything the FDA proposed in the guidance document,” Dr. McGuire said. “The boxed warning is reasonable and needs to be part of the informed consent process. I also agree with the changes in screening implants postoperatively. Most patients do not get MRI examinations. High-resolution ultrasound is more convenient and cost effective.”
The boxed warning was rated as “reasonably strong” and “the most serious step the FDA can take short of taking a device off the market,” but in the case of breast implants, a wider recall of textured implants than what the FDA arranged last July would be even more appropriate, commented Sidney M. Wolfe, MD, founder and senior adviser to Public Citizen. He also faulted the agency for not taking quicker action in mandating inclusion of the proposed boxed warning.
Issuing the labeling changes as draft guidance “is a ministep forward,” but also a process that “guarantees delay” and “creeps along at a dangerously slow pace,” Dr. Wolfe said. “The FDA is delaying what should be inevitable. The agency could put the boxed warning in place right now if they had the guts to do it.”
Dr. McGuire has been a consultant to Allergan, Establishment Labs, and Hans Biomed. Ms. Cook, Dr. Zuckerman, and Dr. Wolfe reported having no commercial disclosures.
Acoustic pulse boosts laser tattoo removal
In tattoo removal, the impact of laser treatments in single office visits is limited because of the laser’s effects on the skin. Now, a new study suggests that a
“As a result,” the authors of the study wrote, “a lower total number of office visits will likely be required for complete tattoo removal leading to improved convenience and efficiency as well as increased satisfaction for both patients and clinicians.” The study, led by cosmetic surgeon Michael S. Kaminer, MD, of SkinCare Physicians in Chestnut Hill, Mass., appeared in Lasers in Surgery and Medicine.
In the study, he and his coauthors pointed out that tattoos are most frequently removed with short-pulse high-fluence lasers, such as a 1064-nm Nd:YAG Q‐switched (QS) laser. However, “the QS laser has a limited ability to affect the tattoo ink pigment particles in each treatment session due to shielding of the pigment particles caused by both the agglomeration of the pigment particles and laser‐induced epidermal and dermal vacuoles known as ‘whitening.’ ” Therefore, “use of the QS laser often requires 10 or more single‐pass office sessions to achieve acceptable fading results.”
The study evaluated whether the use of a rapid acoustic pulse (RAP) device could reduce the whitening effect by clearing vacuoles and make it possible to increase the number of potential laser passes. In the single-center, prospective trial, they treated 32 black-ink tattoos in 21 patients, dividing the tattoos into zones and treating them differently: One zone received at least three consecutive laser passes alternating with a minute of treatment with the RAP device, one received single-pass laser treatment, and one received no treatment.
Reviewers assessed the tattoos for fading at 12 weeks. Average percent fading was higher in the laser/RAP group, compared with the laser-only group (44% and 25%, respectively, P less than .01). The percentages of tattoos with more than 50% fading (38% vs. 9%, P less than .01) and more than 75% fading (22% vs. 3%, P less than .05) were also higher in the laser/RAP group, compared with the laser-only group.
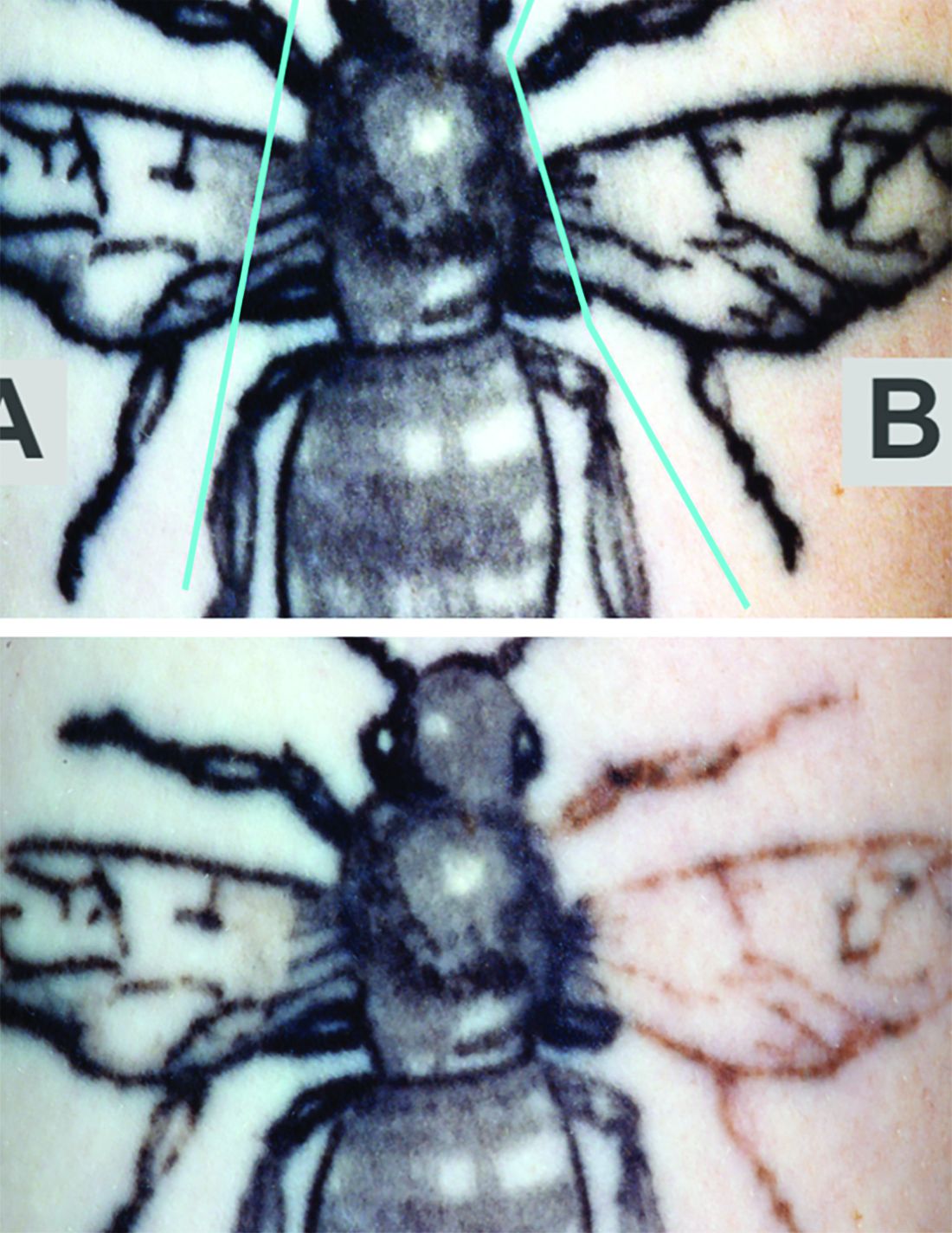
“Further clinical studies will be performed to investigate the broader applicability of the RAP device as an accessory device to reduce the number of laser tattoo removal sessions for other tattoo ink colors in a broader range of skin types,” the researchers commented, noting that the study included patients with Fitzpatrick skin types I-III.
The study was funded by Soliton, which provided the equipment. One of the six authors is an employee of the company The other authors reported no relevant disclosures.
SOURCE: Kaminer MS et al. Lasers Surg Med. 2019 Sep 19. doi: 10.1002/lsm.23163.
In tattoo removal, the impact of laser treatments in single office visits is limited because of the laser’s effects on the skin. Now, a new study suggests that a
“As a result,” the authors of the study wrote, “a lower total number of office visits will likely be required for complete tattoo removal leading to improved convenience and efficiency as well as increased satisfaction for both patients and clinicians.” The study, led by cosmetic surgeon Michael S. Kaminer, MD, of SkinCare Physicians in Chestnut Hill, Mass., appeared in Lasers in Surgery and Medicine.
In the study, he and his coauthors pointed out that tattoos are most frequently removed with short-pulse high-fluence lasers, such as a 1064-nm Nd:YAG Q‐switched (QS) laser. However, “the QS laser has a limited ability to affect the tattoo ink pigment particles in each treatment session due to shielding of the pigment particles caused by both the agglomeration of the pigment particles and laser‐induced epidermal and dermal vacuoles known as ‘whitening.’ ” Therefore, “use of the QS laser often requires 10 or more single‐pass office sessions to achieve acceptable fading results.”
The study evaluated whether the use of a rapid acoustic pulse (RAP) device could reduce the whitening effect by clearing vacuoles and make it possible to increase the number of potential laser passes. In the single-center, prospective trial, they treated 32 black-ink tattoos in 21 patients, dividing the tattoos into zones and treating them differently: One zone received at least three consecutive laser passes alternating with a minute of treatment with the RAP device, one received single-pass laser treatment, and one received no treatment.
Reviewers assessed the tattoos for fading at 12 weeks. Average percent fading was higher in the laser/RAP group, compared with the laser-only group (44% and 25%, respectively, P less than .01). The percentages of tattoos with more than 50% fading (38% vs. 9%, P less than .01) and more than 75% fading (22% vs. 3%, P less than .05) were also higher in the laser/RAP group, compared with the laser-only group.

“Further clinical studies will be performed to investigate the broader applicability of the RAP device as an accessory device to reduce the number of laser tattoo removal sessions for other tattoo ink colors in a broader range of skin types,” the researchers commented, noting that the study included patients with Fitzpatrick skin types I-III.
The study was funded by Soliton, which provided the equipment. One of the six authors is an employee of the company The other authors reported no relevant disclosures.
SOURCE: Kaminer MS et al. Lasers Surg Med. 2019 Sep 19. doi: 10.1002/lsm.23163.
In tattoo removal, the impact of laser treatments in single office visits is limited because of the laser’s effects on the skin. Now, a new study suggests that a
“As a result,” the authors of the study wrote, “a lower total number of office visits will likely be required for complete tattoo removal leading to improved convenience and efficiency as well as increased satisfaction for both patients and clinicians.” The study, led by cosmetic surgeon Michael S. Kaminer, MD, of SkinCare Physicians in Chestnut Hill, Mass., appeared in Lasers in Surgery and Medicine.
In the study, he and his coauthors pointed out that tattoos are most frequently removed with short-pulse high-fluence lasers, such as a 1064-nm Nd:YAG Q‐switched (QS) laser. However, “the QS laser has a limited ability to affect the tattoo ink pigment particles in each treatment session due to shielding of the pigment particles caused by both the agglomeration of the pigment particles and laser‐induced epidermal and dermal vacuoles known as ‘whitening.’ ” Therefore, “use of the QS laser often requires 10 or more single‐pass office sessions to achieve acceptable fading results.”
The study evaluated whether the use of a rapid acoustic pulse (RAP) device could reduce the whitening effect by clearing vacuoles and make it possible to increase the number of potential laser passes. In the single-center, prospective trial, they treated 32 black-ink tattoos in 21 patients, dividing the tattoos into zones and treating them differently: One zone received at least three consecutive laser passes alternating with a minute of treatment with the RAP device, one received single-pass laser treatment, and one received no treatment.
Reviewers assessed the tattoos for fading at 12 weeks. Average percent fading was higher in the laser/RAP group, compared with the laser-only group (44% and 25%, respectively, P less than .01). The percentages of tattoos with more than 50% fading (38% vs. 9%, P less than .01) and more than 75% fading (22% vs. 3%, P less than .05) were also higher in the laser/RAP group, compared with the laser-only group.

“Further clinical studies will be performed to investigate the broader applicability of the RAP device as an accessory device to reduce the number of laser tattoo removal sessions for other tattoo ink colors in a broader range of skin types,” the researchers commented, noting that the study included patients with Fitzpatrick skin types I-III.
The study was funded by Soliton, which provided the equipment. One of the six authors is an employee of the company The other authors reported no relevant disclosures.
SOURCE: Kaminer MS et al. Lasers Surg Med. 2019 Sep 19. doi: 10.1002/lsm.23163.
FROM LASERS IN SURGERY AND MEDICINE
Online resources influencing cosmetic treatment choices
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.
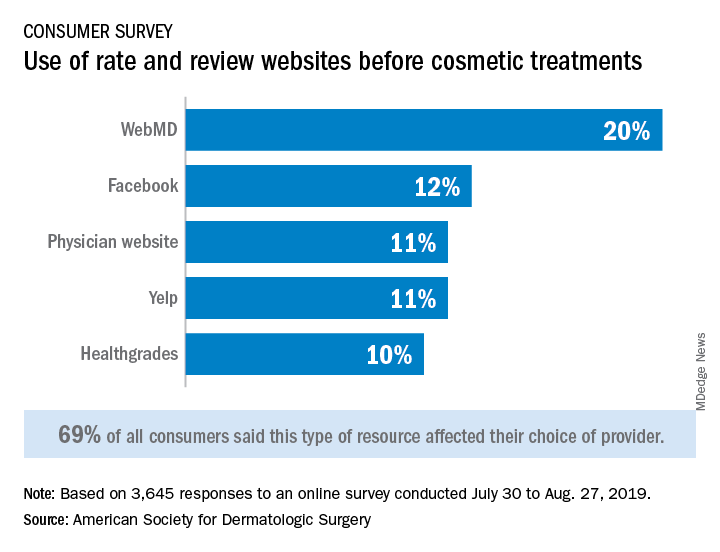
Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.

Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.

Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
‘Clean’ and ‘natural’ beauty products
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Holy basil: A member of the Ocimum family
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
Combined treatments provide control of pseudofolliculitis barbae in women
NEW YORK – For has been found highly effective, Wendy Roberts, MD, reported at the Skin of Color Update 2019.
“We didn’t have great treatments for this problem in the past, but the technology has evolved, and you can now get most women clear,” Dr. Roberts, a dermatologist who practices in Rancho Mirage, Calif., said at the meeting.
This approach is appropriate in all women, but Dr. Roberts focused on her experience with black patients, for whom an antioxidant cream is added to address the inflammatory-associated hyperpigmentation that often accompanies pseudofolliculitis barbae, a chronic inflammatory skin condition typically characterized by small, painful papules and pustules.
Start with microdermabrasion to treat the hypertrophic hair follicles and address keratin plugs, Dr. Roberts said. The microdermabrasion smooths the skin and increases penetration of subsequent creams and topics, she said.
“In the same session, I treat with Nd-YAG 1064 nm laser using short pulses,” she noted. For black women, she makes four passes with the laser at a level of moderate intensity. For those with lighter skin, she might perform as many as six passes with the laser set higher.
The microdermabrasion is repeated monthly for three or four treatments, but can be extended for those with persistent symptoms, Dr. Roberts pointed out. She presented a case of a patient who required seven treatments to achieve a satisfactory response.
Patients are instructed to avoid hair plucking and over the course of treatment nightly topical tretinoin is recommended for maintenance. Regular use of emollients is also recommended. For black women who have developed hyperpigmentation as a complication of pseudofolliculitis barbae, Dr. Roberts prescribes a lightening cream.
“I have pretty much moved away from hydroquinone,” said Dr. Roberts, explaining that she has achieved better results with topical cysteamine, a product that she has been using for about 3 years.
In outlining her treatment strategy, she employed case studies of two black women, both of whom achieved resolution of the problem and were satisfied with the results. She said that the same approach is suitable for women of other racial and ethnic groups.
Most commonly seen in black men, pseudofolliculitis barbae – also known as razor bumps – can occur as a complication of shaving in men or women from any racial and ethnic group. However, because of their embarrassment, women often fail to volunteer that they are struggling with this problem. Some women have been afflicted for years and have developed a regular routine of shaving or plucking hairs and then applying makeup for camouflage, Dr. Roberts said.
“This is a patient who rarely presents the problem to the dermatologist. Yet, she is in every one of our practices,” she added. Due to the frequency with which she has identified pseudofolliculitis barbae in patients who are being seen for a different complaint, she now routinely asks patients about this issue when taking a history. Early detection is useful because pseudofolliculitis barbae is more easily resolved in younger women than in older women.
When the problem is resolved, patient satisfaction is very high. For this reason, Dr. Roberts called diagnosis and treatment of pseudofolliculitis barbae “a practice builder.” Based on her approach, “you can really get these ladies clear.”
Dr. Roberts reports financial relationships with an extensive list of companies that market dermatologic and cosmetic products.
NEW YORK – For has been found highly effective, Wendy Roberts, MD, reported at the Skin of Color Update 2019.
“We didn’t have great treatments for this problem in the past, but the technology has evolved, and you can now get most women clear,” Dr. Roberts, a dermatologist who practices in Rancho Mirage, Calif., said at the meeting.
This approach is appropriate in all women, but Dr. Roberts focused on her experience with black patients, for whom an antioxidant cream is added to address the inflammatory-associated hyperpigmentation that often accompanies pseudofolliculitis barbae, a chronic inflammatory skin condition typically characterized by small, painful papules and pustules.
Start with microdermabrasion to treat the hypertrophic hair follicles and address keratin plugs, Dr. Roberts said. The microdermabrasion smooths the skin and increases penetration of subsequent creams and topics, she said.
“In the same session, I treat with Nd-YAG 1064 nm laser using short pulses,” she noted. For black women, she makes four passes with the laser at a level of moderate intensity. For those with lighter skin, she might perform as many as six passes with the laser set higher.
The microdermabrasion is repeated monthly for three or four treatments, but can be extended for those with persistent symptoms, Dr. Roberts pointed out. She presented a case of a patient who required seven treatments to achieve a satisfactory response.
Patients are instructed to avoid hair plucking and over the course of treatment nightly topical tretinoin is recommended for maintenance. Regular use of emollients is also recommended. For black women who have developed hyperpigmentation as a complication of pseudofolliculitis barbae, Dr. Roberts prescribes a lightening cream.
“I have pretty much moved away from hydroquinone,” said Dr. Roberts, explaining that she has achieved better results with topical cysteamine, a product that she has been using for about 3 years.
In outlining her treatment strategy, she employed case studies of two black women, both of whom achieved resolution of the problem and were satisfied with the results. She said that the same approach is suitable for women of other racial and ethnic groups.
Most commonly seen in black men, pseudofolliculitis barbae – also known as razor bumps – can occur as a complication of shaving in men or women from any racial and ethnic group. However, because of their embarrassment, women often fail to volunteer that they are struggling with this problem. Some women have been afflicted for years and have developed a regular routine of shaving or plucking hairs and then applying makeup for camouflage, Dr. Roberts said.
“This is a patient who rarely presents the problem to the dermatologist. Yet, she is in every one of our practices,” she added. Due to the frequency with which she has identified pseudofolliculitis barbae in patients who are being seen for a different complaint, she now routinely asks patients about this issue when taking a history. Early detection is useful because pseudofolliculitis barbae is more easily resolved in younger women than in older women.
When the problem is resolved, patient satisfaction is very high. For this reason, Dr. Roberts called diagnosis and treatment of pseudofolliculitis barbae “a practice builder.” Based on her approach, “you can really get these ladies clear.”
Dr. Roberts reports financial relationships with an extensive list of companies that market dermatologic and cosmetic products.
NEW YORK – For has been found highly effective, Wendy Roberts, MD, reported at the Skin of Color Update 2019.
“We didn’t have great treatments for this problem in the past, but the technology has evolved, and you can now get most women clear,” Dr. Roberts, a dermatologist who practices in Rancho Mirage, Calif., said at the meeting.
This approach is appropriate in all women, but Dr. Roberts focused on her experience with black patients, for whom an antioxidant cream is added to address the inflammatory-associated hyperpigmentation that often accompanies pseudofolliculitis barbae, a chronic inflammatory skin condition typically characterized by small, painful papules and pustules.
Start with microdermabrasion to treat the hypertrophic hair follicles and address keratin plugs, Dr. Roberts said. The microdermabrasion smooths the skin and increases penetration of subsequent creams and topics, she said.
“In the same session, I treat with Nd-YAG 1064 nm laser using short pulses,” she noted. For black women, she makes four passes with the laser at a level of moderate intensity. For those with lighter skin, she might perform as many as six passes with the laser set higher.
The microdermabrasion is repeated monthly for three or four treatments, but can be extended for those with persistent symptoms, Dr. Roberts pointed out. She presented a case of a patient who required seven treatments to achieve a satisfactory response.
Patients are instructed to avoid hair plucking and over the course of treatment nightly topical tretinoin is recommended for maintenance. Regular use of emollients is also recommended. For black women who have developed hyperpigmentation as a complication of pseudofolliculitis barbae, Dr. Roberts prescribes a lightening cream.
“I have pretty much moved away from hydroquinone,” said Dr. Roberts, explaining that she has achieved better results with topical cysteamine, a product that she has been using for about 3 years.
In outlining her treatment strategy, she employed case studies of two black women, both of whom achieved resolution of the problem and were satisfied with the results. She said that the same approach is suitable for women of other racial and ethnic groups.
Most commonly seen in black men, pseudofolliculitis barbae – also known as razor bumps – can occur as a complication of shaving in men or women from any racial and ethnic group. However, because of their embarrassment, women often fail to volunteer that they are struggling with this problem. Some women have been afflicted for years and have developed a regular routine of shaving or plucking hairs and then applying makeup for camouflage, Dr. Roberts said.
“This is a patient who rarely presents the problem to the dermatologist. Yet, she is in every one of our practices,” she added. Due to the frequency with which she has identified pseudofolliculitis barbae in patients who are being seen for a different complaint, she now routinely asks patients about this issue when taking a history. Early detection is useful because pseudofolliculitis barbae is more easily resolved in younger women than in older women.
When the problem is resolved, patient satisfaction is very high. For this reason, Dr. Roberts called diagnosis and treatment of pseudofolliculitis barbae “a practice builder.” Based on her approach, “you can really get these ladies clear.”
Dr. Roberts reports financial relationships with an extensive list of companies that market dermatologic and cosmetic products.
REPORTING FROM SOC 2019
Whitening of skin remains charged topic at Skin of Color meeting
NEW YORK – , judging from an informal survey of those attending the Skin of Color Update 2019, where this topic was introduced.
When the Skin of Color conference chair, Eliot Battle, MD, founder of Cultura Dermatology and Laser Center, Washington, asked who in the audience considered total body whitening to be “wrong,” the show of hands was substantial. He then offered some perspective.
“How many think breast augmentation is wrong?” he asked. “How many think changing your hair color is wrong? Before we cast judgment, let’s think a little about how our patients feel.”
Although he acknowledged the difficulty of separating a racial context from the cultural perception of lighter skin as desirable, Dr. Battle contended that choices regarding appearance are complex. He cautioned against moral judgments blind to this complexity.
“As physicians we need to keep ourselves in check, to keep ourselves from making judgments [regarding lightening agents],” he said.
The two other panelists participating in the same session made compatible observations. Although the other two panelists limited most of their presentations to skin lightening for clinical indications, such as melasma and other disorders of hyperpigmentation, they acknowledged and addressed the sense of discomfort the topic raises.
“To many patients, depigmentation is a passport to society,” said Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute, Los Angeles. Although she considers this a global issue, not an issue unique to the black population, she counseled dermatologists to “respect the vicissitudes and issues of pigmentation” that she said include the patient’s concerns about beauty, class, and privilege.
Sensitive to the desire of some patients for lighter skin, Cheryl Burgess, MD, founder of the Center for Dermatology and Dermatologic Surgery, Washington, opened her talk by displaying the Time Magazine cover of O.J. Simpson at the time he was accused of murder. The photo appeared to have been intentionally darkened in an effort that was thought by many to make him appear more sinister.
This might be an appropriate example of what skin pigment represents to some segments of American society, but Dr. Battle said that the quest for lighter skin is a global phenomenon. He claims that Asia, India, and Africa are now among the fastest growing and largest markets for skin lightening strategies. The options in those areas of the world, like the United States, are proliferating quickly.
Many of the rapidly expanding options have not yet proved to be effective or safe. The antioxidant glutathione, which is being used for a long list of proven and unproven indications, is among these, according to Dr. Battle. In many clinics where this drug is administered intravenously to avoid degradation in the gastrointestinal tract, he suggested there is reason to believe the staff has little training in safety monitoring.
There are no long-term studies evaluating the safety and efficacy of glutathione for skin lightening, according to Dr. Battle, but there are many case reports of serious toxicities, including death. He listed thyroid dysfunction, renal impairment, and liver dysfunction among adverse events potentially related to glutathione.
“When I gave this talk a year ago, there were no clinics in Washington [offering glutathione]. Now there are seven,” he said.
Even for those dermatologists uncomfortable offering skin lightening for cosmetic purposes, ignoring the demand is ill advised, he said. Evaluating and advising patients on the safety of these agents is one reason to become involved, said Dr. Battle, who noted that specialists in dermatology are uniquely trained to monitor drugs for this application.
“You can tell a patient to stop, but they won’t stop,” said Dr. Battle. He maintained that organized medicine, including the American Academy of Dermatology, should take a role in evaluating the safety and efficacy of lightening agents even when used only for cosmetic indications.
Currently, there are no Food and Drug Administration–approved therapies for whitening of the skin.
“This is such an important question, and I think we need to figure it out,” Dr. Battle said. “Not a day goes by in our practice when we are not asked about skin lightening.”
Dr. Battle reported no relevant disclosures; Dr. Grimes and Dr. Burgess reported multiple financial relationships with industry that are not necessarily relevant to this topic.
NEW YORK – , judging from an informal survey of those attending the Skin of Color Update 2019, where this topic was introduced.
When the Skin of Color conference chair, Eliot Battle, MD, founder of Cultura Dermatology and Laser Center, Washington, asked who in the audience considered total body whitening to be “wrong,” the show of hands was substantial. He then offered some perspective.
“How many think breast augmentation is wrong?” he asked. “How many think changing your hair color is wrong? Before we cast judgment, let’s think a little about how our patients feel.”
Although he acknowledged the difficulty of separating a racial context from the cultural perception of lighter skin as desirable, Dr. Battle contended that choices regarding appearance are complex. He cautioned against moral judgments blind to this complexity.
“As physicians we need to keep ourselves in check, to keep ourselves from making judgments [regarding lightening agents],” he said.
The two other panelists participating in the same session made compatible observations. Although the other two panelists limited most of their presentations to skin lightening for clinical indications, such as melasma and other disorders of hyperpigmentation, they acknowledged and addressed the sense of discomfort the topic raises.
“To many patients, depigmentation is a passport to society,” said Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute, Los Angeles. Although she considers this a global issue, not an issue unique to the black population, she counseled dermatologists to “respect the vicissitudes and issues of pigmentation” that she said include the patient’s concerns about beauty, class, and privilege.
Sensitive to the desire of some patients for lighter skin, Cheryl Burgess, MD, founder of the Center for Dermatology and Dermatologic Surgery, Washington, opened her talk by displaying the Time Magazine cover of O.J. Simpson at the time he was accused of murder. The photo appeared to have been intentionally darkened in an effort that was thought by many to make him appear more sinister.
This might be an appropriate example of what skin pigment represents to some segments of American society, but Dr. Battle said that the quest for lighter skin is a global phenomenon. He claims that Asia, India, and Africa are now among the fastest growing and largest markets for skin lightening strategies. The options in those areas of the world, like the United States, are proliferating quickly.
Many of the rapidly expanding options have not yet proved to be effective or safe. The antioxidant glutathione, which is being used for a long list of proven and unproven indications, is among these, according to Dr. Battle. In many clinics where this drug is administered intravenously to avoid degradation in the gastrointestinal tract, he suggested there is reason to believe the staff has little training in safety monitoring.
There are no long-term studies evaluating the safety and efficacy of glutathione for skin lightening, according to Dr. Battle, but there are many case reports of serious toxicities, including death. He listed thyroid dysfunction, renal impairment, and liver dysfunction among adverse events potentially related to glutathione.
“When I gave this talk a year ago, there were no clinics in Washington [offering glutathione]. Now there are seven,” he said.
Even for those dermatologists uncomfortable offering skin lightening for cosmetic purposes, ignoring the demand is ill advised, he said. Evaluating and advising patients on the safety of these agents is one reason to become involved, said Dr. Battle, who noted that specialists in dermatology are uniquely trained to monitor drugs for this application.
“You can tell a patient to stop, but they won’t stop,” said Dr. Battle. He maintained that organized medicine, including the American Academy of Dermatology, should take a role in evaluating the safety and efficacy of lightening agents even when used only for cosmetic indications.
Currently, there are no Food and Drug Administration–approved therapies for whitening of the skin.
“This is such an important question, and I think we need to figure it out,” Dr. Battle said. “Not a day goes by in our practice when we are not asked about skin lightening.”
Dr. Battle reported no relevant disclosures; Dr. Grimes and Dr. Burgess reported multiple financial relationships with industry that are not necessarily relevant to this topic.
NEW YORK – , judging from an informal survey of those attending the Skin of Color Update 2019, where this topic was introduced.
When the Skin of Color conference chair, Eliot Battle, MD, founder of Cultura Dermatology and Laser Center, Washington, asked who in the audience considered total body whitening to be “wrong,” the show of hands was substantial. He then offered some perspective.
“How many think breast augmentation is wrong?” he asked. “How many think changing your hair color is wrong? Before we cast judgment, let’s think a little about how our patients feel.”
Although he acknowledged the difficulty of separating a racial context from the cultural perception of lighter skin as desirable, Dr. Battle contended that choices regarding appearance are complex. He cautioned against moral judgments blind to this complexity.
“As physicians we need to keep ourselves in check, to keep ourselves from making judgments [regarding lightening agents],” he said.
The two other panelists participating in the same session made compatible observations. Although the other two panelists limited most of their presentations to skin lightening for clinical indications, such as melasma and other disorders of hyperpigmentation, they acknowledged and addressed the sense of discomfort the topic raises.
“To many patients, depigmentation is a passport to society,” said Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute, Los Angeles. Although she considers this a global issue, not an issue unique to the black population, she counseled dermatologists to “respect the vicissitudes and issues of pigmentation” that she said include the patient’s concerns about beauty, class, and privilege.
Sensitive to the desire of some patients for lighter skin, Cheryl Burgess, MD, founder of the Center for Dermatology and Dermatologic Surgery, Washington, opened her talk by displaying the Time Magazine cover of O.J. Simpson at the time he was accused of murder. The photo appeared to have been intentionally darkened in an effort that was thought by many to make him appear more sinister.
This might be an appropriate example of what skin pigment represents to some segments of American society, but Dr. Battle said that the quest for lighter skin is a global phenomenon. He claims that Asia, India, and Africa are now among the fastest growing and largest markets for skin lightening strategies. The options in those areas of the world, like the United States, are proliferating quickly.
Many of the rapidly expanding options have not yet proved to be effective or safe. The antioxidant glutathione, which is being used for a long list of proven and unproven indications, is among these, according to Dr. Battle. In many clinics where this drug is administered intravenously to avoid degradation in the gastrointestinal tract, he suggested there is reason to believe the staff has little training in safety monitoring.
There are no long-term studies evaluating the safety and efficacy of glutathione for skin lightening, according to Dr. Battle, but there are many case reports of serious toxicities, including death. He listed thyroid dysfunction, renal impairment, and liver dysfunction among adverse events potentially related to glutathione.
“When I gave this talk a year ago, there were no clinics in Washington [offering glutathione]. Now there are seven,” he said.
Even for those dermatologists uncomfortable offering skin lightening for cosmetic purposes, ignoring the demand is ill advised, he said. Evaluating and advising patients on the safety of these agents is one reason to become involved, said Dr. Battle, who noted that specialists in dermatology are uniquely trained to monitor drugs for this application.
“You can tell a patient to stop, but they won’t stop,” said Dr. Battle. He maintained that organized medicine, including the American Academy of Dermatology, should take a role in evaluating the safety and efficacy of lightening agents even when used only for cosmetic indications.
Currently, there are no Food and Drug Administration–approved therapies for whitening of the skin.
“This is such an important question, and I think we need to figure it out,” Dr. Battle said. “Not a day goes by in our practice when we are not asked about skin lightening.”
Dr. Battle reported no relevant disclosures; Dr. Grimes and Dr. Burgess reported multiple financial relationships with industry that are not necessarily relevant to this topic.
REPORTING FROM SOC 2019
Cosmetic surgery and the secret world of Instagram dolls
They use names and hashtags that connect the work to their provider. So, for example, KathySmithDoll would be a woman who underwent surgery with a Dr. Kathy Smith.
In an era of patient empowerment, these pages – they’re called “Sx pages,” with Sx mimicking the prescriptive “Rx” – form a just-out-of-sight Instagram community. They serve as a cosmetic surgery shopping guide, a best-practices education system, and can also sound the alarm about bad experiences with practitioners. Some presurgery doll pages are more like inspiration pages or mood boards, collecting images of desired shapes.
That way, “other girls doing research can find someone with a similar build to theirs and follow their journey for a glimpse at what they might look like if they got similar procedures,” said Tai Hall, a massage therapist in Maryland. On her Instagram page, she showcases before-and-after body-contouring results; in her Facebook group, she teaches postoperative self-massage and how people can best take care of themselves while healing.
These Instagram pages, she said, “are really big deals.”
The Sx Instagram pages are private and anonymous, to some extent, and follow strict rules to stay that way, particularly since many feature nudity. (As a social media practice, Sx pages are fairly similar to teenager’s private “finsta” friends-only accounts. They are similarly unverified and what they report is unverifiable.) Many of the bios on these pages indicate they won’t allow access to men.
Each Instagram page bio often unveils elaborate details, often including height and weight. The patient – the doll – will list surgery dates and tag her surgeon, recovery house, any postoperative care specialists or private nurses, and her postoperative massage therapist.
Recovery houses, surgery providers, and massage therapists also use the hashtags to promote their services. Some of these are flooded with ads or spam. Some are used by practitioners for education about surgery.
The surgery age
According to the American Society of Plastic Surgeons, more than 1.8 million cosmetic surgeries were performed in the United States in 2018. Breast augmentation and liposuction accounted for about a third of those.
And the number of “cosmetic minimally invasive procedures” – Botox, laser hair removal, soft-tissue fillers, and more – has grown rapidly in the United States. There were fewer than 5 million procedures in 2000. In 2018, there were nearly 16 million. (Almost half of those procedures are Botox treatments.)
Cosmetic procedures are also becoming more popular among people of color. The American Society for Aesthetic Plastic Surgery reports that cosmetic augmentation, like liposuctions and buttocks lifts, increased 56% among African Americans from 2005 to 2013, and is still rising.
As the number of savvy customers grows, doll pages provide a useful glimpse into the less-glamorous side of before and after – the details that people like to overlook, like bruising, drainage, and the often painfully long process of healing after significant surgeries.
Patients become online advertisements for their surgeons. Surgeons develop a reputation on social media for being the best at certain procedures, for delivering a desired look, or for working with certain ethnic groups and body types.
“They’ll cry and upload videos of pain and success and their struggles, or whatever they’re going through, and their surgery sisters help uplift them,” Ms. Hall said.
And there is a lot to talk about, from surgeons to procedures to recovery houses to advice on how to travel with the least hassle from airport security or airline staff when patients are clad in fajas – a kind of postoperative girdle – or other foam paddings.
How we shop for surgeons now
Sx pages can be an effective patient empowerment tool if done honestly and fairly, said Alan Matarasso, MD, a plastic surgeon in New York and the president of the American Society of Plastic Surgeons.
“It makes sense because this is a small group of people,” he said. “Not a lot of doctors do Brazilian butt lifts, but patients need to realize that they are not rating a restaurant.”
Dr. Matarasso encourages prospective patients who rely on Sx pages to research and prepare in other ways as well.
“The standards have to be even greater than if you had a sick gallbladder, because you don’t have to do this,” he said. “This is not like vetting a hotel room. You have to be careful.”
Dr. Matarasso recommends that prospective patients ask to see the surgeon’s best and worst results, or a random case – say, the 37th case they did that year. He suggests that prospective clients visit the American Board of Plastic Surgery websites to do research and that patients query the licensing state and find out what, if any, violations a surgeon may have had. Patients can ask board-certified surgeons their specialty and whether they are certified in it.
Ms. Hall, the massage therapist, warned that patients may see women who heal faster or achieve different results than they might. As is often the case on Instagram, people tend to post fewer of their struggles and more of their highlight reels.
Patients taking care of patients
Sx pages might be even more valuable for patients who plan to travel internationally for their surgery. Many people in the United States do this to save money. Doll pages serve to warn prospective patients about problems that surgeons and hospitals don’t disclose.
After surgery, especially if extensive travel is needed, patients may recuperate at recovery houses for a few days. Procedures like fat transfer to the buttocks leave patients unable to move around or sit; doctors may install drains to help remove fluid after surgery.
In a recovery house, a caretaker can tend to their incisions; help with bathing, food, and pain medications; and even perform regular postoperative massages.
In May, the mother of an Instagram model named Yatnaa Rivera died during a procedure in the Dominican Republic. Ms. Rivera took to Instagram to ask for help and to warn others. The doctor who performed the operation, Hector Cabral, MD, had been fined for operating in the United States without a license. He is linked to several deaths and is still practicing. (Dr. Cabral did not respond to inquiries via social media; his office answered calls but said he was on vacation.)
Instagram accounts tagged into his doll hashtag (#CabralDoll) to spread the message.
Every day women are bombarded with images of beauty. With filters and editing apps, and the army of social media influencers who receive money or free cosmetic services in exchange for their Instagram posts, it’s often hard to know what’s real. Authentic depictions of what cosmetic surgery entails can be a reality check on what is attainable with cosmetic surgery.
In May 2019, the American College of Surgeons released voluntary ethical guidelines for social media by surgeons. Many of them address patient privacy, but they also advise practitioners to provide trustworthy medical advice and to be cautious around these “powerful educational tools.” Even so, now a real-time, crowdsourced system allows patients to cut through the surgeons’ marketing and advertising efforts.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
They use names and hashtags that connect the work to their provider. So, for example, KathySmithDoll would be a woman who underwent surgery with a Dr. Kathy Smith.
In an era of patient empowerment, these pages – they’re called “Sx pages,” with Sx mimicking the prescriptive “Rx” – form a just-out-of-sight Instagram community. They serve as a cosmetic surgery shopping guide, a best-practices education system, and can also sound the alarm about bad experiences with practitioners. Some presurgery doll pages are more like inspiration pages or mood boards, collecting images of desired shapes.
That way, “other girls doing research can find someone with a similar build to theirs and follow their journey for a glimpse at what they might look like if they got similar procedures,” said Tai Hall, a massage therapist in Maryland. On her Instagram page, she showcases before-and-after body-contouring results; in her Facebook group, she teaches postoperative self-massage and how people can best take care of themselves while healing.
These Instagram pages, she said, “are really big deals.”
The Sx Instagram pages are private and anonymous, to some extent, and follow strict rules to stay that way, particularly since many feature nudity. (As a social media practice, Sx pages are fairly similar to teenager’s private “finsta” friends-only accounts. They are similarly unverified and what they report is unverifiable.) Many of the bios on these pages indicate they won’t allow access to men.
Each Instagram page bio often unveils elaborate details, often including height and weight. The patient – the doll – will list surgery dates and tag her surgeon, recovery house, any postoperative care specialists or private nurses, and her postoperative massage therapist.
Recovery houses, surgery providers, and massage therapists also use the hashtags to promote their services. Some of these are flooded with ads or spam. Some are used by practitioners for education about surgery.
The surgery age
According to the American Society of Plastic Surgeons, more than 1.8 million cosmetic surgeries were performed in the United States in 2018. Breast augmentation and liposuction accounted for about a third of those.
And the number of “cosmetic minimally invasive procedures” – Botox, laser hair removal, soft-tissue fillers, and more – has grown rapidly in the United States. There were fewer than 5 million procedures in 2000. In 2018, there were nearly 16 million. (Almost half of those procedures are Botox treatments.)
Cosmetic procedures are also becoming more popular among people of color. The American Society for Aesthetic Plastic Surgery reports that cosmetic augmentation, like liposuctions and buttocks lifts, increased 56% among African Americans from 2005 to 2013, and is still rising.
As the number of savvy customers grows, doll pages provide a useful glimpse into the less-glamorous side of before and after – the details that people like to overlook, like bruising, drainage, and the often painfully long process of healing after significant surgeries.
Patients become online advertisements for their surgeons. Surgeons develop a reputation on social media for being the best at certain procedures, for delivering a desired look, or for working with certain ethnic groups and body types.
“They’ll cry and upload videos of pain and success and their struggles, or whatever they’re going through, and their surgery sisters help uplift them,” Ms. Hall said.
And there is a lot to talk about, from surgeons to procedures to recovery houses to advice on how to travel with the least hassle from airport security or airline staff when patients are clad in fajas – a kind of postoperative girdle – or other foam paddings.
How we shop for surgeons now
Sx pages can be an effective patient empowerment tool if done honestly and fairly, said Alan Matarasso, MD, a plastic surgeon in New York and the president of the American Society of Plastic Surgeons.
“It makes sense because this is a small group of people,” he said. “Not a lot of doctors do Brazilian butt lifts, but patients need to realize that they are not rating a restaurant.”
Dr. Matarasso encourages prospective patients who rely on Sx pages to research and prepare in other ways as well.
“The standards have to be even greater than if you had a sick gallbladder, because you don’t have to do this,” he said. “This is not like vetting a hotel room. You have to be careful.”
Dr. Matarasso recommends that prospective patients ask to see the surgeon’s best and worst results, or a random case – say, the 37th case they did that year. He suggests that prospective clients visit the American Board of Plastic Surgery websites to do research and that patients query the licensing state and find out what, if any, violations a surgeon may have had. Patients can ask board-certified surgeons their specialty and whether they are certified in it.
Ms. Hall, the massage therapist, warned that patients may see women who heal faster or achieve different results than they might. As is often the case on Instagram, people tend to post fewer of their struggles and more of their highlight reels.
Patients taking care of patients
Sx pages might be even more valuable for patients who plan to travel internationally for their surgery. Many people in the United States do this to save money. Doll pages serve to warn prospective patients about problems that surgeons and hospitals don’t disclose.
After surgery, especially if extensive travel is needed, patients may recuperate at recovery houses for a few days. Procedures like fat transfer to the buttocks leave patients unable to move around or sit; doctors may install drains to help remove fluid after surgery.
In a recovery house, a caretaker can tend to their incisions; help with bathing, food, and pain medications; and even perform regular postoperative massages.
In May, the mother of an Instagram model named Yatnaa Rivera died during a procedure in the Dominican Republic. Ms. Rivera took to Instagram to ask for help and to warn others. The doctor who performed the operation, Hector Cabral, MD, had been fined for operating in the United States without a license. He is linked to several deaths and is still practicing. (Dr. Cabral did not respond to inquiries via social media; his office answered calls but said he was on vacation.)
Instagram accounts tagged into his doll hashtag (#CabralDoll) to spread the message.
Every day women are bombarded with images of beauty. With filters and editing apps, and the army of social media influencers who receive money or free cosmetic services in exchange for their Instagram posts, it’s often hard to know what’s real. Authentic depictions of what cosmetic surgery entails can be a reality check on what is attainable with cosmetic surgery.
In May 2019, the American College of Surgeons released voluntary ethical guidelines for social media by surgeons. Many of them address patient privacy, but they also advise practitioners to provide trustworthy medical advice and to be cautious around these “powerful educational tools.” Even so, now a real-time, crowdsourced system allows patients to cut through the surgeons’ marketing and advertising efforts.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
They use names and hashtags that connect the work to their provider. So, for example, KathySmithDoll would be a woman who underwent surgery with a Dr. Kathy Smith.
In an era of patient empowerment, these pages – they’re called “Sx pages,” with Sx mimicking the prescriptive “Rx” – form a just-out-of-sight Instagram community. They serve as a cosmetic surgery shopping guide, a best-practices education system, and can also sound the alarm about bad experiences with practitioners. Some presurgery doll pages are more like inspiration pages or mood boards, collecting images of desired shapes.
That way, “other girls doing research can find someone with a similar build to theirs and follow their journey for a glimpse at what they might look like if they got similar procedures,” said Tai Hall, a massage therapist in Maryland. On her Instagram page, she showcases before-and-after body-contouring results; in her Facebook group, she teaches postoperative self-massage and how people can best take care of themselves while healing.
These Instagram pages, she said, “are really big deals.”
The Sx Instagram pages are private and anonymous, to some extent, and follow strict rules to stay that way, particularly since many feature nudity. (As a social media practice, Sx pages are fairly similar to teenager’s private “finsta” friends-only accounts. They are similarly unverified and what they report is unverifiable.) Many of the bios on these pages indicate they won’t allow access to men.
Each Instagram page bio often unveils elaborate details, often including height and weight. The patient – the doll – will list surgery dates and tag her surgeon, recovery house, any postoperative care specialists or private nurses, and her postoperative massage therapist.
Recovery houses, surgery providers, and massage therapists also use the hashtags to promote their services. Some of these are flooded with ads or spam. Some are used by practitioners for education about surgery.
The surgery age
According to the American Society of Plastic Surgeons, more than 1.8 million cosmetic surgeries were performed in the United States in 2018. Breast augmentation and liposuction accounted for about a third of those.
And the number of “cosmetic minimally invasive procedures” – Botox, laser hair removal, soft-tissue fillers, and more – has grown rapidly in the United States. There were fewer than 5 million procedures in 2000. In 2018, there were nearly 16 million. (Almost half of those procedures are Botox treatments.)
Cosmetic procedures are also becoming more popular among people of color. The American Society for Aesthetic Plastic Surgery reports that cosmetic augmentation, like liposuctions and buttocks lifts, increased 56% among African Americans from 2005 to 2013, and is still rising.
As the number of savvy customers grows, doll pages provide a useful glimpse into the less-glamorous side of before and after – the details that people like to overlook, like bruising, drainage, and the often painfully long process of healing after significant surgeries.
Patients become online advertisements for their surgeons. Surgeons develop a reputation on social media for being the best at certain procedures, for delivering a desired look, or for working with certain ethnic groups and body types.
“They’ll cry and upload videos of pain and success and their struggles, or whatever they’re going through, and their surgery sisters help uplift them,” Ms. Hall said.
And there is a lot to talk about, from surgeons to procedures to recovery houses to advice on how to travel with the least hassle from airport security or airline staff when patients are clad in fajas – a kind of postoperative girdle – or other foam paddings.
How we shop for surgeons now
Sx pages can be an effective patient empowerment tool if done honestly and fairly, said Alan Matarasso, MD, a plastic surgeon in New York and the president of the American Society of Plastic Surgeons.
“It makes sense because this is a small group of people,” he said. “Not a lot of doctors do Brazilian butt lifts, but patients need to realize that they are not rating a restaurant.”
Dr. Matarasso encourages prospective patients who rely on Sx pages to research and prepare in other ways as well.
“The standards have to be even greater than if you had a sick gallbladder, because you don’t have to do this,” he said. “This is not like vetting a hotel room. You have to be careful.”
Dr. Matarasso recommends that prospective patients ask to see the surgeon’s best and worst results, or a random case – say, the 37th case they did that year. He suggests that prospective clients visit the American Board of Plastic Surgery websites to do research and that patients query the licensing state and find out what, if any, violations a surgeon may have had. Patients can ask board-certified surgeons their specialty and whether they are certified in it.
Ms. Hall, the massage therapist, warned that patients may see women who heal faster or achieve different results than they might. As is often the case on Instagram, people tend to post fewer of their struggles and more of their highlight reels.
Patients taking care of patients
Sx pages might be even more valuable for patients who plan to travel internationally for their surgery. Many people in the United States do this to save money. Doll pages serve to warn prospective patients about problems that surgeons and hospitals don’t disclose.
After surgery, especially if extensive travel is needed, patients may recuperate at recovery houses for a few days. Procedures like fat transfer to the buttocks leave patients unable to move around or sit; doctors may install drains to help remove fluid after surgery.
In a recovery house, a caretaker can tend to their incisions; help with bathing, food, and pain medications; and even perform regular postoperative massages.
In May, the mother of an Instagram model named Yatnaa Rivera died during a procedure in the Dominican Republic. Ms. Rivera took to Instagram to ask for help and to warn others. The doctor who performed the operation, Hector Cabral, MD, had been fined for operating in the United States without a license. He is linked to several deaths and is still practicing. (Dr. Cabral did not respond to inquiries via social media; his office answered calls but said he was on vacation.)
Instagram accounts tagged into his doll hashtag (#CabralDoll) to spread the message.
Every day women are bombarded with images of beauty. With filters and editing apps, and the army of social media influencers who receive money or free cosmetic services in exchange for their Instagram posts, it’s often hard to know what’s real. Authentic depictions of what cosmetic surgery entails can be a reality check on what is attainable with cosmetic surgery.
In May 2019, the American College of Surgeons released voluntary ethical guidelines for social media by surgeons. Many of them address patient privacy, but they also advise practitioners to provide trustworthy medical advice and to be cautious around these “powerful educational tools.” Even so, now a real-time, crowdsourced system allows patients to cut through the surgeons’ marketing and advertising efforts.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Three distinct scenarios for treating facial redness with lasers and light
SAN DIEGO – In the clinical experience of J. Stuart Nelson, MD, PhD,
“There’s the patient with telangiectasia without diffuse redness, the patient who has telangiectasia with diffuse redness, and the patient who has diffuse redness,” Dr. Nelson said at the annual Masters of Aesthetics Symposium. “Because the vessel sizes are different, your approach to the clinical management of each one of these patients is going to be very different.”
For patients with telangiectasia without the redness, using pulsed dye lasers with a wavelength of 585-600 nm can be effective. “If someone has a single isolated telangiectasia, it’s the simplest thing you’ll do that day in your office,” said Dr. Nelson, professor of surgery and biomedical engineering at the Beckman Laser Institute and Medical Clinic at the University of California, Irvine. “It’s like Tiger Woods putting for a 2-foot birdie. Similarly, with the millisecond green devices, you can focus the laser beam onto the spot and you will see the blood vessels go away in real time.”
Treating patients who have telangiectasia and diffuse redness requires two steps. First, treat the larger telangiectasia with pulse durations of 20 ms, he said, and then treat the global background redness with shorter pulse durations (of 3 ms and 6 ms). “You can do this with pulse dye lasers and with green millisecond devices,” he noted.
For patients who present with diffuse global redness, “you don’t have to worry about the larger blood vessels, so you’re not going to be using the long pulse durations of the laser exposure,” said Dr. Nelson, past president of the American Society for Laser Medicine and Surgery. “You’re going to be using much shorter pulse durations, because you’re targeting blood vessels that are much smaller. You’re trying to tease out that background redness.”
If you’re concerned about how a particular patient will fare, consider performing a test spot. “This allows you to check for any unusual tissue reaction and to gauge the potential success of the laser treatment you’re doing,” he said. “It allows the patient to sort of experience the swelling and healing process they’re going to be going through.”
Dr. Nelson advised against applying a “cookbook” approach to using lasers and light sources in dermatology. “Don’t memorize treatment parameters,” he said. “What you really need to do is look for the clinical endpoints. What is the tissue response you want to see? You also want to exercise caution in patients who are tanned. The epidermal melanin absorption by tanned patients can be significant, even with some of the cooling technologies we have.”
Dr. Nelson reported having intellectual property rights with Syneron/Candela.
SAN DIEGO – In the clinical experience of J. Stuart Nelson, MD, PhD,
“There’s the patient with telangiectasia without diffuse redness, the patient who has telangiectasia with diffuse redness, and the patient who has diffuse redness,” Dr. Nelson said at the annual Masters of Aesthetics Symposium. “Because the vessel sizes are different, your approach to the clinical management of each one of these patients is going to be very different.”
For patients with telangiectasia without the redness, using pulsed dye lasers with a wavelength of 585-600 nm can be effective. “If someone has a single isolated telangiectasia, it’s the simplest thing you’ll do that day in your office,” said Dr. Nelson, professor of surgery and biomedical engineering at the Beckman Laser Institute and Medical Clinic at the University of California, Irvine. “It’s like Tiger Woods putting for a 2-foot birdie. Similarly, with the millisecond green devices, you can focus the laser beam onto the spot and you will see the blood vessels go away in real time.”
Treating patients who have telangiectasia and diffuse redness requires two steps. First, treat the larger telangiectasia with pulse durations of 20 ms, he said, and then treat the global background redness with shorter pulse durations (of 3 ms and 6 ms). “You can do this with pulse dye lasers and with green millisecond devices,” he noted.
For patients who present with diffuse global redness, “you don’t have to worry about the larger blood vessels, so you’re not going to be using the long pulse durations of the laser exposure,” said Dr. Nelson, past president of the American Society for Laser Medicine and Surgery. “You’re going to be using much shorter pulse durations, because you’re targeting blood vessels that are much smaller. You’re trying to tease out that background redness.”
If you’re concerned about how a particular patient will fare, consider performing a test spot. “This allows you to check for any unusual tissue reaction and to gauge the potential success of the laser treatment you’re doing,” he said. “It allows the patient to sort of experience the swelling and healing process they’re going to be going through.”
Dr. Nelson advised against applying a “cookbook” approach to using lasers and light sources in dermatology. “Don’t memorize treatment parameters,” he said. “What you really need to do is look for the clinical endpoints. What is the tissue response you want to see? You also want to exercise caution in patients who are tanned. The epidermal melanin absorption by tanned patients can be significant, even with some of the cooling technologies we have.”
Dr. Nelson reported having intellectual property rights with Syneron/Candela.
SAN DIEGO – In the clinical experience of J. Stuart Nelson, MD, PhD,
“There’s the patient with telangiectasia without diffuse redness, the patient who has telangiectasia with diffuse redness, and the patient who has diffuse redness,” Dr. Nelson said at the annual Masters of Aesthetics Symposium. “Because the vessel sizes are different, your approach to the clinical management of each one of these patients is going to be very different.”
For patients with telangiectasia without the redness, using pulsed dye lasers with a wavelength of 585-600 nm can be effective. “If someone has a single isolated telangiectasia, it’s the simplest thing you’ll do that day in your office,” said Dr. Nelson, professor of surgery and biomedical engineering at the Beckman Laser Institute and Medical Clinic at the University of California, Irvine. “It’s like Tiger Woods putting for a 2-foot birdie. Similarly, with the millisecond green devices, you can focus the laser beam onto the spot and you will see the blood vessels go away in real time.”
Treating patients who have telangiectasia and diffuse redness requires two steps. First, treat the larger telangiectasia with pulse durations of 20 ms, he said, and then treat the global background redness with shorter pulse durations (of 3 ms and 6 ms). “You can do this with pulse dye lasers and with green millisecond devices,” he noted.
For patients who present with diffuse global redness, “you don’t have to worry about the larger blood vessels, so you’re not going to be using the long pulse durations of the laser exposure,” said Dr. Nelson, past president of the American Society for Laser Medicine and Surgery. “You’re going to be using much shorter pulse durations, because you’re targeting blood vessels that are much smaller. You’re trying to tease out that background redness.”
If you’re concerned about how a particular patient will fare, consider performing a test spot. “This allows you to check for any unusual tissue reaction and to gauge the potential success of the laser treatment you’re doing,” he said. “It allows the patient to sort of experience the swelling and healing process they’re going to be going through.”
Dr. Nelson advised against applying a “cookbook” approach to using lasers and light sources in dermatology. “Don’t memorize treatment parameters,” he said. “What you really need to do is look for the clinical endpoints. What is the tissue response you want to see? You also want to exercise caution in patients who are tanned. The epidermal melanin absorption by tanned patients can be significant, even with some of the cooling technologies we have.”
Dr. Nelson reported having intellectual property rights with Syneron/Candela.
EXPERT ANALYSIS FROM MOA 2019
CBD in beauty products
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).




















