User login
Longer-lasting neuromodulators coming down the pike
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
EXPERT ANALYSIS FROM MOA 2019
Morinda citrifolia (Noni) tree: Many names, even more applications
, which has been in use on the islands for two millennia.1-4 The plant, found abundantly in Southeast Asia, Australia, the Pacific Basin, and the Caribbean, is called Great Morinda or cheese fruit in Australia, Nono in Tahiti, Indian Mulberry in India, and Ba ji tian in China.4-6 It is also deployed for a wide range of health purposes in Brazil.7
Noni has been credited with conferring various salutary benefits against arthritis, diabetes, fever, gingivitis, headaches, infections, inflammation, respiratory illnesses, and tuberculosis.3,8 In alternative medicine, the fruit juice, which has been found to be safe, is used for multiple indications, with a slew of studies presenting evidence for anti-inflammatory, antioxidant, and apoptosis-inducing benefits against cancer.5,6 All parts of M. citrifolia – leaves, fruits, roots, bark, flowers, and seeds – have been used in traditional medical practices.8 This column will focus on recent research into the broad array of biologic activities attributed to the plant and possible dermatologic uses.
Diverse biologic properties
In 2007, Nayak et al. showed that the juice of M. citrifolia fruit significantly lowered sugar levels in diabetic rats and facilitated their wound healing.1
Three years later, Thani et al. determined that the leaves of M. citrifolia exert antiproliferative and antioxidative activities, with chemopreventive benefits seen against epidermoid and cervical cancers.9
In 2011, Serafini et al. confirmed the antibacterial, anti-inflammatory, antioxidant, and antinociceptive qualities of the aqueous extract from M. citrifolia leaves, with the extract shown to significantly lower leukocyte migration in doses of 200 and 400 mg/kg. Mild antibacterial properties were seen as was an antinociceptive effect at the higher dose in the acetic-acid-induced writhing test.3
A comprehensive literature review in 2017 by Torres et al. identified a varied and extensive list of biological activities of M. citrifolia, including immunostimulatory, antitumor, antidiabetic, antiobesity, antibacterial and antiseptic, antifungal, antiviral, anti-inflammatory, antinociceptive and analgesic, antioxidant, neuroprotective, wound healing, antiallergic, photoprotective, and antiwrinkle among several others. Despite its use in disease prevention and treatment around the world, the researchers call for more in vitro and in vivo models in addition to clinical trials to further examine the health benefits of Noni.7
Early in 2019, De La Cruz-Sánchez et al. determined that the methanolic extract of M. citrifolia displayed marked activity against methicillin-resistant Staphylococcus aureus (MRSA), thus supporting its continuing applications in traditional medical practice.2
Photoprotection and antiaging potential
Based on their prior work demonstrating that M. citrifolia fruit upregulates the production of type I collagen and glycosaminoglycans in primary cultures of normal human fibroblasts, Kim et al. isolated anthraquinone from the fruit and showed that it dose-dependently decreased the expression of collagenase matrix metalloproteinase-1 in human dermal fibroblasts. The investigators also found that an anthraquinone-containing nano-emulsion raised type I procollagen in nude mouse skin. They concluded, in this 2005 study, that Noni extract warrants consideration as an antiwrinkle agent given its proclivity to induce the production of collagen.10
In 2009, West et al. assessed a carbomer gel base containing the ethanol extract and juice pressed from Noni leaves for possible allergenic activity in a repeat-insult patch test in 49 volunteers. They also used a UVB-induced erythema model in 25 subjects to test the topical photoprotective potential of the ethanol extract and leaf juice. The investigators reported no allergic potential evinced by the patch tests, and in a histamine H-1 receptor antagonism assay, the leaves hindered receptor binding by 57%, suggesting anti-inflammatory activity. In the UVB test, the dose necessary to engender erythema was nearly 3.5 times higher than in untreated skin. The team concluded that M. citrifolia leaves are safe for topical application and show promise in lessening UVB-induced skin damage.11
A 2014 study on mice by Serafini et al. showed that the dorsal skin of mice treated for 7 days with topical M. citrifolia was protected from damage by exposure to UVA-UVB radiation as measured by skin thickness, transepidermal water loss, erythema, and histological changes.12
Conclusion
Morinda citrifolia has been used in traditional medicine for at least 2,000 years. Its reported list of uses covers an impressive gamut of indications.
Modern medicine is beginning to catch up with new research conducted on this copious and beloved plant. That said, much more data, particularly from human clinical trials, are necessary to elucidate the most appropriate dermatologic roles for M. citrifolia. I just started growing a Noni tree in my yard because some patients have reported using it on their skin. I will report back and let you know how it goes. It is flowering now!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nayak BS et al. J Wound Care. 2007 Feb;16(2):83-6.
2. De La Cruz-Sánchez NG et al. Microb Pathog. 2019 Mar;128:347-53.
3. Serafini MR et al. J Med Food. 2011 Oct;14(10):1159-66.
4. Wang MY, Su C. Ann N Y Acad Sci. 2001 Dec;952:161-8.
5. Gupta RK, Patel AK. Asian Pac J Cancer Prev. 2013;14(8):4495-9.
6. Brown AC. Phytother Res. 2012 Oct;26(10):1427-40.
7. Torres MAO et al. Phytother Res. 2017 Jul;31(7):971-9.
8. Potterat O, Hamburger M. Planta Med. 2007 Mar;73(3):191-9.
9. Thani W et al. Southeast Asian J Trop Med Public Health. 2010 Mar;41(2):482-9.
10. Kim SW et al. J Med Food. 2005 Winter;8(4):552-5.
11. West BJ et al. J Nat Med. 2009 Jul;63(3):351-4.
12. Serafini MR et al. Biomed Res Int. 2014;2014:587819. doi: 10.1155/2014/587819.
, which has been in use on the islands for two millennia.1-4 The plant, found abundantly in Southeast Asia, Australia, the Pacific Basin, and the Caribbean, is called Great Morinda or cheese fruit in Australia, Nono in Tahiti, Indian Mulberry in India, and Ba ji tian in China.4-6 It is also deployed for a wide range of health purposes in Brazil.7
Noni has been credited with conferring various salutary benefits against arthritis, diabetes, fever, gingivitis, headaches, infections, inflammation, respiratory illnesses, and tuberculosis.3,8 In alternative medicine, the fruit juice, which has been found to be safe, is used for multiple indications, with a slew of studies presenting evidence for anti-inflammatory, antioxidant, and apoptosis-inducing benefits against cancer.5,6 All parts of M. citrifolia – leaves, fruits, roots, bark, flowers, and seeds – have been used in traditional medical practices.8 This column will focus on recent research into the broad array of biologic activities attributed to the plant and possible dermatologic uses.
Diverse biologic properties
In 2007, Nayak et al. showed that the juice of M. citrifolia fruit significantly lowered sugar levels in diabetic rats and facilitated their wound healing.1
Three years later, Thani et al. determined that the leaves of M. citrifolia exert antiproliferative and antioxidative activities, with chemopreventive benefits seen against epidermoid and cervical cancers.9
In 2011, Serafini et al. confirmed the antibacterial, anti-inflammatory, antioxidant, and antinociceptive qualities of the aqueous extract from M. citrifolia leaves, with the extract shown to significantly lower leukocyte migration in doses of 200 and 400 mg/kg. Mild antibacterial properties were seen as was an antinociceptive effect at the higher dose in the acetic-acid-induced writhing test.3
A comprehensive literature review in 2017 by Torres et al. identified a varied and extensive list of biological activities of M. citrifolia, including immunostimulatory, antitumor, antidiabetic, antiobesity, antibacterial and antiseptic, antifungal, antiviral, anti-inflammatory, antinociceptive and analgesic, antioxidant, neuroprotective, wound healing, antiallergic, photoprotective, and antiwrinkle among several others. Despite its use in disease prevention and treatment around the world, the researchers call for more in vitro and in vivo models in addition to clinical trials to further examine the health benefits of Noni.7
Early in 2019, De La Cruz-Sánchez et al. determined that the methanolic extract of M. citrifolia displayed marked activity against methicillin-resistant Staphylococcus aureus (MRSA), thus supporting its continuing applications in traditional medical practice.2
Photoprotection and antiaging potential
Based on their prior work demonstrating that M. citrifolia fruit upregulates the production of type I collagen and glycosaminoglycans in primary cultures of normal human fibroblasts, Kim et al. isolated anthraquinone from the fruit and showed that it dose-dependently decreased the expression of collagenase matrix metalloproteinase-1 in human dermal fibroblasts. The investigators also found that an anthraquinone-containing nano-emulsion raised type I procollagen in nude mouse skin. They concluded, in this 2005 study, that Noni extract warrants consideration as an antiwrinkle agent given its proclivity to induce the production of collagen.10
In 2009, West et al. assessed a carbomer gel base containing the ethanol extract and juice pressed from Noni leaves for possible allergenic activity in a repeat-insult patch test in 49 volunteers. They also used a UVB-induced erythema model in 25 subjects to test the topical photoprotective potential of the ethanol extract and leaf juice. The investigators reported no allergic potential evinced by the patch tests, and in a histamine H-1 receptor antagonism assay, the leaves hindered receptor binding by 57%, suggesting anti-inflammatory activity. In the UVB test, the dose necessary to engender erythema was nearly 3.5 times higher than in untreated skin. The team concluded that M. citrifolia leaves are safe for topical application and show promise in lessening UVB-induced skin damage.11
A 2014 study on mice by Serafini et al. showed that the dorsal skin of mice treated for 7 days with topical M. citrifolia was protected from damage by exposure to UVA-UVB radiation as measured by skin thickness, transepidermal water loss, erythema, and histological changes.12
Conclusion
Morinda citrifolia has been used in traditional medicine for at least 2,000 years. Its reported list of uses covers an impressive gamut of indications.
Modern medicine is beginning to catch up with new research conducted on this copious and beloved plant. That said, much more data, particularly from human clinical trials, are necessary to elucidate the most appropriate dermatologic roles for M. citrifolia. I just started growing a Noni tree in my yard because some patients have reported using it on their skin. I will report back and let you know how it goes. It is flowering now!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nayak BS et al. J Wound Care. 2007 Feb;16(2):83-6.
2. De La Cruz-Sánchez NG et al. Microb Pathog. 2019 Mar;128:347-53.
3. Serafini MR et al. J Med Food. 2011 Oct;14(10):1159-66.
4. Wang MY, Su C. Ann N Y Acad Sci. 2001 Dec;952:161-8.
5. Gupta RK, Patel AK. Asian Pac J Cancer Prev. 2013;14(8):4495-9.
6. Brown AC. Phytother Res. 2012 Oct;26(10):1427-40.
7. Torres MAO et al. Phytother Res. 2017 Jul;31(7):971-9.
8. Potterat O, Hamburger M. Planta Med. 2007 Mar;73(3):191-9.
9. Thani W et al. Southeast Asian J Trop Med Public Health. 2010 Mar;41(2):482-9.
10. Kim SW et al. J Med Food. 2005 Winter;8(4):552-5.
11. West BJ et al. J Nat Med. 2009 Jul;63(3):351-4.
12. Serafini MR et al. Biomed Res Int. 2014;2014:587819. doi: 10.1155/2014/587819.
, which has been in use on the islands for two millennia.1-4 The plant, found abundantly in Southeast Asia, Australia, the Pacific Basin, and the Caribbean, is called Great Morinda or cheese fruit in Australia, Nono in Tahiti, Indian Mulberry in India, and Ba ji tian in China.4-6 It is also deployed for a wide range of health purposes in Brazil.7
Noni has been credited with conferring various salutary benefits against arthritis, diabetes, fever, gingivitis, headaches, infections, inflammation, respiratory illnesses, and tuberculosis.3,8 In alternative medicine, the fruit juice, which has been found to be safe, is used for multiple indications, with a slew of studies presenting evidence for anti-inflammatory, antioxidant, and apoptosis-inducing benefits against cancer.5,6 All parts of M. citrifolia – leaves, fruits, roots, bark, flowers, and seeds – have been used in traditional medical practices.8 This column will focus on recent research into the broad array of biologic activities attributed to the plant and possible dermatologic uses.
Diverse biologic properties
In 2007, Nayak et al. showed that the juice of M. citrifolia fruit significantly lowered sugar levels in diabetic rats and facilitated their wound healing.1
Three years later, Thani et al. determined that the leaves of M. citrifolia exert antiproliferative and antioxidative activities, with chemopreventive benefits seen against epidermoid and cervical cancers.9
In 2011, Serafini et al. confirmed the antibacterial, anti-inflammatory, antioxidant, and antinociceptive qualities of the aqueous extract from M. citrifolia leaves, with the extract shown to significantly lower leukocyte migration in doses of 200 and 400 mg/kg. Mild antibacterial properties were seen as was an antinociceptive effect at the higher dose in the acetic-acid-induced writhing test.3
A comprehensive literature review in 2017 by Torres et al. identified a varied and extensive list of biological activities of M. citrifolia, including immunostimulatory, antitumor, antidiabetic, antiobesity, antibacterial and antiseptic, antifungal, antiviral, anti-inflammatory, antinociceptive and analgesic, antioxidant, neuroprotective, wound healing, antiallergic, photoprotective, and antiwrinkle among several others. Despite its use in disease prevention and treatment around the world, the researchers call for more in vitro and in vivo models in addition to clinical trials to further examine the health benefits of Noni.7
Early in 2019, De La Cruz-Sánchez et al. determined that the methanolic extract of M. citrifolia displayed marked activity against methicillin-resistant Staphylococcus aureus (MRSA), thus supporting its continuing applications in traditional medical practice.2
Photoprotection and antiaging potential
Based on their prior work demonstrating that M. citrifolia fruit upregulates the production of type I collagen and glycosaminoglycans in primary cultures of normal human fibroblasts, Kim et al. isolated anthraquinone from the fruit and showed that it dose-dependently decreased the expression of collagenase matrix metalloproteinase-1 in human dermal fibroblasts. The investigators also found that an anthraquinone-containing nano-emulsion raised type I procollagen in nude mouse skin. They concluded, in this 2005 study, that Noni extract warrants consideration as an antiwrinkle agent given its proclivity to induce the production of collagen.10
In 2009, West et al. assessed a carbomer gel base containing the ethanol extract and juice pressed from Noni leaves for possible allergenic activity in a repeat-insult patch test in 49 volunteers. They also used a UVB-induced erythema model in 25 subjects to test the topical photoprotective potential of the ethanol extract and leaf juice. The investigators reported no allergic potential evinced by the patch tests, and in a histamine H-1 receptor antagonism assay, the leaves hindered receptor binding by 57%, suggesting anti-inflammatory activity. In the UVB test, the dose necessary to engender erythema was nearly 3.5 times higher than in untreated skin. The team concluded that M. citrifolia leaves are safe for topical application and show promise in lessening UVB-induced skin damage.11
A 2014 study on mice by Serafini et al. showed that the dorsal skin of mice treated for 7 days with topical M. citrifolia was protected from damage by exposure to UVA-UVB radiation as measured by skin thickness, transepidermal water loss, erythema, and histological changes.12
Conclusion
Morinda citrifolia has been used in traditional medicine for at least 2,000 years. Its reported list of uses covers an impressive gamut of indications.
Modern medicine is beginning to catch up with new research conducted on this copious and beloved plant. That said, much more data, particularly from human clinical trials, are necessary to elucidate the most appropriate dermatologic roles for M. citrifolia. I just started growing a Noni tree in my yard because some patients have reported using it on their skin. I will report back and let you know how it goes. It is flowering now!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nayak BS et al. J Wound Care. 2007 Feb;16(2):83-6.
2. De La Cruz-Sánchez NG et al. Microb Pathog. 2019 Mar;128:347-53.
3. Serafini MR et al. J Med Food. 2011 Oct;14(10):1159-66.
4. Wang MY, Su C. Ann N Y Acad Sci. 2001 Dec;952:161-8.
5. Gupta RK, Patel AK. Asian Pac J Cancer Prev. 2013;14(8):4495-9.
6. Brown AC. Phytother Res. 2012 Oct;26(10):1427-40.
7. Torres MAO et al. Phytother Res. 2017 Jul;31(7):971-9.
8. Potterat O, Hamburger M. Planta Med. 2007 Mar;73(3):191-9.
9. Thani W et al. Southeast Asian J Trop Med Public Health. 2010 Mar;41(2):482-9.
10. Kim SW et al. J Med Food. 2005 Winter;8(4):552-5.
11. West BJ et al. J Nat Med. 2009 Jul;63(3):351-4.
12. Serafini MR et al. Biomed Res Int. 2014;2014:587819. doi: 10.1155/2014/587819.
Expert shares tips for laser hair removal prior to gender reassignment surgery
SAN DIEGO – prior to undergoing the procedures.
“In the last year, in terms of hair removal, this has been the biggest change in my practice,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium.
R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Melanie Grossman, MD, who practices in New York City, developed laser hair removal in the 1990s, and today laser hair removal stands as the most common laser treatment in medicine, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Laser hair removal works by the extended theory of selective photothermolysis. “You’re targeting by proxy,” Dr. Avram explained. “The laser targets eumelanin in darkly pigmented hairs, with the secondary target being the follicular stem cells. Pigment is a prerequisite for effective treatment. So if there is no pigment in the hair, with current technology, it’s not going to work.”
He advises clinicians to avoid a cookbook approach to fluences when performing laser hair removal. Even though higher fluences have been correlated with greater permanent hair removal, they are also more likely to cause unexpected side effects. “The recommended treatment fluences are often provided with each individual laser device for nonexperienced operators, but I would not recommend doing that,” he said. “You want to evaluate for the desired clinical endpoint of perifollicular erythema and edema. The highest possible tolerated fluence, which yields this endpoint, without any adverse effects, is often the best fluence for treatment.” In 2016, Dr. Avram and his colleagues published a paper that focuses on desirable and therapeutic endpoints when performing laser and light treatments (J Am Acad Dermatol 2016;74[5]:821-33).
The best candidates for laser hair removal are those with light skin color and dark hair. “The more pigment that’s in the hair, the more it’s going to absorb the energy,” he said. Coarse, thick hair responds better than thin vellus hairs, and blond, gray hairs do not respond. A new silver nanoparticle technology is being developed that may improve efficacy for people with blond or gray hair in the future. “Modest initial data showed that it works, but it requires several treatments,” Dr. Avram said.
A past president of the American Society for Laser Medicine and Surgery, Dr. Avram went on to note that laser hair removal is often delegated to nonphysicians and is the most common cause of lawsuits for laser injury. “The rates of lawsuits rise dramatically when delegated to nonphysicians,” he said. “They even rise higher when performed by nonphysicians without supervision such as in medi-spas. Some of the side effects when performed by nonexperienced users can include temporary hyperpigmentation and longterm hypopigmentation.”
One of his clinical pearls is to never perform laser hair removal on suntanned individuals (“you will get obvious, bizarre-appearing hypopigmentation,” he said) and to exercise caution in patients with darker skin types. “If you do a test spot, give it a couple of weeks to see if hyperpigmentation develops,” he advised. “However, their sun exposure may change, and the area you treat with a test spot may be different than the entire area you intend to treat, so don’t think that a test spot is going to guarantee a particular result. You also have to be aware of paradoxical hypertrichosis, where you get more hair growth rather than less.”
Laser hair removal is mandatory prior to neovaginoplasty surgery. Surgeons use skin from the penile shaft and the midscrotum to create the new vagina, Dr. Avram said, so all hair must be removed prior to surgery so that the inside of the new vagina will be free of hair.
“You can use laser or electrolysis for this,” he said. “Electrolysis takes a lot more treatments and is going to be much more tedious than laser hair removal.” Areas to be targeted include all hair on the scrotum and all hair on the penile shaft, plus one inch around the base. “In the perineum, you want to remove hair from the bottom of the scrotum to one inch above the anus in order to clear a 2.5-inch-wide strip,” he said.
For a phalloplasty, surgeons use skin from the underside of arm to create a urethra. This means that all hair should be removed from the crease of the wrist to 15-18 cm up the arm. “You treat the underside of the arm at 4 cm distally and 5.5 cm proximally,” Dr. Avram said. “It should be 15-18 cm in length, and you cannot have any hair that remains within the new urethra.”
To create a penis, surgeons use skin from the prone arm and around. This requires removing hair at 10 cm distally, 13 cm proximally, and 14 cm in length.
Dr. Avram emphasized the importance of patient and staff education and use of preferred pronouns when performing laser hair removal on patients prior to their gender reassignment surgery. “It requires an explanation that this requires multiple treatments and will not remove all hair,” he said. “You can work with an experienced electrologist for nonresponsive hair.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
SAN DIEGO – prior to undergoing the procedures.
“In the last year, in terms of hair removal, this has been the biggest change in my practice,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium.
R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Melanie Grossman, MD, who practices in New York City, developed laser hair removal in the 1990s, and today laser hair removal stands as the most common laser treatment in medicine, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Laser hair removal works by the extended theory of selective photothermolysis. “You’re targeting by proxy,” Dr. Avram explained. “The laser targets eumelanin in darkly pigmented hairs, with the secondary target being the follicular stem cells. Pigment is a prerequisite for effective treatment. So if there is no pigment in the hair, with current technology, it’s not going to work.”
He advises clinicians to avoid a cookbook approach to fluences when performing laser hair removal. Even though higher fluences have been correlated with greater permanent hair removal, they are also more likely to cause unexpected side effects. “The recommended treatment fluences are often provided with each individual laser device for nonexperienced operators, but I would not recommend doing that,” he said. “You want to evaluate for the desired clinical endpoint of perifollicular erythema and edema. The highest possible tolerated fluence, which yields this endpoint, without any adverse effects, is often the best fluence for treatment.” In 2016, Dr. Avram and his colleagues published a paper that focuses on desirable and therapeutic endpoints when performing laser and light treatments (J Am Acad Dermatol 2016;74[5]:821-33).
The best candidates for laser hair removal are those with light skin color and dark hair. “The more pigment that’s in the hair, the more it’s going to absorb the energy,” he said. Coarse, thick hair responds better than thin vellus hairs, and blond, gray hairs do not respond. A new silver nanoparticle technology is being developed that may improve efficacy for people with blond or gray hair in the future. “Modest initial data showed that it works, but it requires several treatments,” Dr. Avram said.
A past president of the American Society for Laser Medicine and Surgery, Dr. Avram went on to note that laser hair removal is often delegated to nonphysicians and is the most common cause of lawsuits for laser injury. “The rates of lawsuits rise dramatically when delegated to nonphysicians,” he said. “They even rise higher when performed by nonphysicians without supervision such as in medi-spas. Some of the side effects when performed by nonexperienced users can include temporary hyperpigmentation and longterm hypopigmentation.”
One of his clinical pearls is to never perform laser hair removal on suntanned individuals (“you will get obvious, bizarre-appearing hypopigmentation,” he said) and to exercise caution in patients with darker skin types. “If you do a test spot, give it a couple of weeks to see if hyperpigmentation develops,” he advised. “However, their sun exposure may change, and the area you treat with a test spot may be different than the entire area you intend to treat, so don’t think that a test spot is going to guarantee a particular result. You also have to be aware of paradoxical hypertrichosis, where you get more hair growth rather than less.”
Laser hair removal is mandatory prior to neovaginoplasty surgery. Surgeons use skin from the penile shaft and the midscrotum to create the new vagina, Dr. Avram said, so all hair must be removed prior to surgery so that the inside of the new vagina will be free of hair.
“You can use laser or electrolysis for this,” he said. “Electrolysis takes a lot more treatments and is going to be much more tedious than laser hair removal.” Areas to be targeted include all hair on the scrotum and all hair on the penile shaft, plus one inch around the base. “In the perineum, you want to remove hair from the bottom of the scrotum to one inch above the anus in order to clear a 2.5-inch-wide strip,” he said.
For a phalloplasty, surgeons use skin from the underside of arm to create a urethra. This means that all hair should be removed from the crease of the wrist to 15-18 cm up the arm. “You treat the underside of the arm at 4 cm distally and 5.5 cm proximally,” Dr. Avram said. “It should be 15-18 cm in length, and you cannot have any hair that remains within the new urethra.”
To create a penis, surgeons use skin from the prone arm and around. This requires removing hair at 10 cm distally, 13 cm proximally, and 14 cm in length.
Dr. Avram emphasized the importance of patient and staff education and use of preferred pronouns when performing laser hair removal on patients prior to their gender reassignment surgery. “It requires an explanation that this requires multiple treatments and will not remove all hair,” he said. “You can work with an experienced electrologist for nonresponsive hair.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
SAN DIEGO – prior to undergoing the procedures.
“In the last year, in terms of hair removal, this has been the biggest change in my practice,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium.
R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Melanie Grossman, MD, who practices in New York City, developed laser hair removal in the 1990s, and today laser hair removal stands as the most common laser treatment in medicine, said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Laser hair removal works by the extended theory of selective photothermolysis. “You’re targeting by proxy,” Dr. Avram explained. “The laser targets eumelanin in darkly pigmented hairs, with the secondary target being the follicular stem cells. Pigment is a prerequisite for effective treatment. So if there is no pigment in the hair, with current technology, it’s not going to work.”
He advises clinicians to avoid a cookbook approach to fluences when performing laser hair removal. Even though higher fluences have been correlated with greater permanent hair removal, they are also more likely to cause unexpected side effects. “The recommended treatment fluences are often provided with each individual laser device for nonexperienced operators, but I would not recommend doing that,” he said. “You want to evaluate for the desired clinical endpoint of perifollicular erythema and edema. The highest possible tolerated fluence, which yields this endpoint, without any adverse effects, is often the best fluence for treatment.” In 2016, Dr. Avram and his colleagues published a paper that focuses on desirable and therapeutic endpoints when performing laser and light treatments (J Am Acad Dermatol 2016;74[5]:821-33).
The best candidates for laser hair removal are those with light skin color and dark hair. “The more pigment that’s in the hair, the more it’s going to absorb the energy,” he said. Coarse, thick hair responds better than thin vellus hairs, and blond, gray hairs do not respond. A new silver nanoparticle technology is being developed that may improve efficacy for people with blond or gray hair in the future. “Modest initial data showed that it works, but it requires several treatments,” Dr. Avram said.
A past president of the American Society for Laser Medicine and Surgery, Dr. Avram went on to note that laser hair removal is often delegated to nonphysicians and is the most common cause of lawsuits for laser injury. “The rates of lawsuits rise dramatically when delegated to nonphysicians,” he said. “They even rise higher when performed by nonphysicians without supervision such as in medi-spas. Some of the side effects when performed by nonexperienced users can include temporary hyperpigmentation and longterm hypopigmentation.”
One of his clinical pearls is to never perform laser hair removal on suntanned individuals (“you will get obvious, bizarre-appearing hypopigmentation,” he said) and to exercise caution in patients with darker skin types. “If you do a test spot, give it a couple of weeks to see if hyperpigmentation develops,” he advised. “However, their sun exposure may change, and the area you treat with a test spot may be different than the entire area you intend to treat, so don’t think that a test spot is going to guarantee a particular result. You also have to be aware of paradoxical hypertrichosis, where you get more hair growth rather than less.”
Laser hair removal is mandatory prior to neovaginoplasty surgery. Surgeons use skin from the penile shaft and the midscrotum to create the new vagina, Dr. Avram said, so all hair must be removed prior to surgery so that the inside of the new vagina will be free of hair.
“You can use laser or electrolysis for this,” he said. “Electrolysis takes a lot more treatments and is going to be much more tedious than laser hair removal.” Areas to be targeted include all hair on the scrotum and all hair on the penile shaft, plus one inch around the base. “In the perineum, you want to remove hair from the bottom of the scrotum to one inch above the anus in order to clear a 2.5-inch-wide strip,” he said.
For a phalloplasty, surgeons use skin from the underside of arm to create a urethra. This means that all hair should be removed from the crease of the wrist to 15-18 cm up the arm. “You treat the underside of the arm at 4 cm distally and 5.5 cm proximally,” Dr. Avram said. “It should be 15-18 cm in length, and you cannot have any hair that remains within the new urethra.”
To create a penis, surgeons use skin from the prone arm and around. This requires removing hair at 10 cm distally, 13 cm proximally, and 14 cm in length.
Dr. Avram emphasized the importance of patient and staff education and use of preferred pronouns when performing laser hair removal on patients prior to their gender reassignment surgery. “It requires an explanation that this requires multiple treatments and will not remove all hair,” he said. “You can work with an experienced electrologist for nonresponsive hair.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
EXPERT ANALYSIS FROM MOA 2019
Novel dermal microcoring device holds promise for moderate to severe wrinkles
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
EXPERT ANALYSIS FROM MOA 2019
Thread lifts making a comeback, but long-term effects remain unclear
SAN DIEGO – The use of threads to improve skin laxity is making a comeback, thanks largely to advances in absorbable sutures.
“Thread lifts were popularized in the 1990s, but I think they were misrepresented as an alternative to a surgical face-lift, which remains the gold standard,” Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium. “A thread lift is certainly not like a traditional face-lift; it’s much more subtle.”
In the 1990s, clinicians used nonabsorbable sutures for thread lifts, including polypropylene-barbed threads, which caused adverse events ranging from extrusion and migration to thread expulsion, dimpling, granuloma formation, and prolonged pain. As a result, the Food and Drug Administration withdrew its approval of contour thread aesthetic procedures in 2009. Since then, the development of absorbable threads made from polydioxanone (PDO) and poly-
“There are some nice benefits to thread lifts,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “You get immediate results, which is always nice for patients, but with tissue tightening using energy-based devices, results are unpredictable and it can take 6 months to see the results. With resorbable sutures, we’re seeing fewer complications, and the amount of lifting is more predictable because you’re physically lifting the tissue. In some cases, threads are able to lift tissue more than energy-based devices. There is minimal recovery, it requires local anesthesia, and it’s less expensive than a surgical face-lift, which can run $10,000-$15,000 or more.”
For skin lifting, clinicians implant threads subcutaneously. When tugged in the opposite direction, the barbs anchor in adipose tissue, increasing tensile strength while suspended in the dermis and overlying tissue. This produces a fibrous adhesion capsule that helps to solidify anchorage of the suture long term. Fibrosis has been shown to increase local collagen production. PDO and PLLA are known collagen stimulants and are postulated to stimulate a long-term benefit in rejuvenation, Dr. Ortiz said, but overall evidence regarding their use in thread lifts is weak.
“Existing studies have a very short follow-up period and there is really no standardized protocol, so we don’t know really know a lot about them yet,” she said. Lana Tong, MD, and Evan A. Rieder, MD, of New York University recently published a systematic review of the literature on the topic (Dermatol Surg. 2019 45[7]:931-40).
PDO is biodegradable by hydrolysis over 4-8 months and is used as absorbable suture material for prolonged tension–bearing areas. “It causes neocollagenesis with a foreign-body reaction,” Dr. Ortiz said. Meanwhile, PLLA is a collagen stimulator used for prolonged volume restoration. “It’s used an aesthetic filler, but a known complication with PLLA injections is the formation of subcutaneous nodules and late onset granulomas,” she said.
Early in 2019, Korean researchers published results of a study that set out to evaluate the collagen-producing effects of powdered PDO injection, compared with PLLA injection, in a murine model (J Cosmet Dermatol. 2019 Feb 27. doi: 10.1111/jocd.12894). “It showed both PDO and PLLA induced granulomatous reactions and collagen formation, but this decreased at 12 weeks,” said Dr. Ortiz, who was not involved with the work. “PDO had slightly more collagen formation than PLLA.”
Indications for thread lifts, she continued, are for jawline lift, cheek enhancement, brow lift, wrinkle reduction, body contouring, acne scarring, and texturing. “Choose patients with good skin quality: not too thick/heavy, and not too thin. Patients with moderate skin sagging are going to better candidates than those with severe skin sagging.”
One type of absorbable suspension suture, the Silhouette InstaLift, is made of polyglycolide/
In terms of adverse events following thread lift procedures, patients usually feel tender for about a week or 2. “They can have some bruising, mostly from the anesthesia,” she said.
To prevent temporary dimpling, Dr. Ortiz undermines with an 18-gauge needle and inserts perpendicular to the skin surface. “Extrusions can still occur,” she said. To prevent this, she pulls on the end and makes sure it’s buried subcutaneously.
Dr. Ortiz reported having financial relationships with numerous pharmaceutical and device companies, though none related to the content of her presentation. She is also cochair of the Masters of Aesthetics symposium.
SAN DIEGO – The use of threads to improve skin laxity is making a comeback, thanks largely to advances in absorbable sutures.
“Thread lifts were popularized in the 1990s, but I think they were misrepresented as an alternative to a surgical face-lift, which remains the gold standard,” Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium. “A thread lift is certainly not like a traditional face-lift; it’s much more subtle.”
In the 1990s, clinicians used nonabsorbable sutures for thread lifts, including polypropylene-barbed threads, which caused adverse events ranging from extrusion and migration to thread expulsion, dimpling, granuloma formation, and prolonged pain. As a result, the Food and Drug Administration withdrew its approval of contour thread aesthetic procedures in 2009. Since then, the development of absorbable threads made from polydioxanone (PDO) and poly-
“There are some nice benefits to thread lifts,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “You get immediate results, which is always nice for patients, but with tissue tightening using energy-based devices, results are unpredictable and it can take 6 months to see the results. With resorbable sutures, we’re seeing fewer complications, and the amount of lifting is more predictable because you’re physically lifting the tissue. In some cases, threads are able to lift tissue more than energy-based devices. There is minimal recovery, it requires local anesthesia, and it’s less expensive than a surgical face-lift, which can run $10,000-$15,000 or more.”
For skin lifting, clinicians implant threads subcutaneously. When tugged in the opposite direction, the barbs anchor in adipose tissue, increasing tensile strength while suspended in the dermis and overlying tissue. This produces a fibrous adhesion capsule that helps to solidify anchorage of the suture long term. Fibrosis has been shown to increase local collagen production. PDO and PLLA are known collagen stimulants and are postulated to stimulate a long-term benefit in rejuvenation, Dr. Ortiz said, but overall evidence regarding their use in thread lifts is weak.
“Existing studies have a very short follow-up period and there is really no standardized protocol, so we don’t know really know a lot about them yet,” she said. Lana Tong, MD, and Evan A. Rieder, MD, of New York University recently published a systematic review of the literature on the topic (Dermatol Surg. 2019 45[7]:931-40).
PDO is biodegradable by hydrolysis over 4-8 months and is used as absorbable suture material for prolonged tension–bearing areas. “It causes neocollagenesis with a foreign-body reaction,” Dr. Ortiz said. Meanwhile, PLLA is a collagen stimulator used for prolonged volume restoration. “It’s used an aesthetic filler, but a known complication with PLLA injections is the formation of subcutaneous nodules and late onset granulomas,” she said.
Early in 2019, Korean researchers published results of a study that set out to evaluate the collagen-producing effects of powdered PDO injection, compared with PLLA injection, in a murine model (J Cosmet Dermatol. 2019 Feb 27. doi: 10.1111/jocd.12894). “It showed both PDO and PLLA induced granulomatous reactions and collagen formation, but this decreased at 12 weeks,” said Dr. Ortiz, who was not involved with the work. “PDO had slightly more collagen formation than PLLA.”
Indications for thread lifts, she continued, are for jawline lift, cheek enhancement, brow lift, wrinkle reduction, body contouring, acne scarring, and texturing. “Choose patients with good skin quality: not too thick/heavy, and not too thin. Patients with moderate skin sagging are going to better candidates than those with severe skin sagging.”
One type of absorbable suspension suture, the Silhouette InstaLift, is made of polyglycolide/
In terms of adverse events following thread lift procedures, patients usually feel tender for about a week or 2. “They can have some bruising, mostly from the anesthesia,” she said.
To prevent temporary dimpling, Dr. Ortiz undermines with an 18-gauge needle and inserts perpendicular to the skin surface. “Extrusions can still occur,” she said. To prevent this, she pulls on the end and makes sure it’s buried subcutaneously.
Dr. Ortiz reported having financial relationships with numerous pharmaceutical and device companies, though none related to the content of her presentation. She is also cochair of the Masters of Aesthetics symposium.
SAN DIEGO – The use of threads to improve skin laxity is making a comeback, thanks largely to advances in absorbable sutures.
“Thread lifts were popularized in the 1990s, but I think they were misrepresented as an alternative to a surgical face-lift, which remains the gold standard,” Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium. “A thread lift is certainly not like a traditional face-lift; it’s much more subtle.”
In the 1990s, clinicians used nonabsorbable sutures for thread lifts, including polypropylene-barbed threads, which caused adverse events ranging from extrusion and migration to thread expulsion, dimpling, granuloma formation, and prolonged pain. As a result, the Food and Drug Administration withdrew its approval of contour thread aesthetic procedures in 2009. Since then, the development of absorbable threads made from polydioxanone (PDO) and poly-
“There are some nice benefits to thread lifts,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego. “You get immediate results, which is always nice for patients, but with tissue tightening using energy-based devices, results are unpredictable and it can take 6 months to see the results. With resorbable sutures, we’re seeing fewer complications, and the amount of lifting is more predictable because you’re physically lifting the tissue. In some cases, threads are able to lift tissue more than energy-based devices. There is minimal recovery, it requires local anesthesia, and it’s less expensive than a surgical face-lift, which can run $10,000-$15,000 or more.”
For skin lifting, clinicians implant threads subcutaneously. When tugged in the opposite direction, the barbs anchor in adipose tissue, increasing tensile strength while suspended in the dermis and overlying tissue. This produces a fibrous adhesion capsule that helps to solidify anchorage of the suture long term. Fibrosis has been shown to increase local collagen production. PDO and PLLA are known collagen stimulants and are postulated to stimulate a long-term benefit in rejuvenation, Dr. Ortiz said, but overall evidence regarding their use in thread lifts is weak.
“Existing studies have a very short follow-up period and there is really no standardized protocol, so we don’t know really know a lot about them yet,” she said. Lana Tong, MD, and Evan A. Rieder, MD, of New York University recently published a systematic review of the literature on the topic (Dermatol Surg. 2019 45[7]:931-40).
PDO is biodegradable by hydrolysis over 4-8 months and is used as absorbable suture material for prolonged tension–bearing areas. “It causes neocollagenesis with a foreign-body reaction,” Dr. Ortiz said. Meanwhile, PLLA is a collagen stimulator used for prolonged volume restoration. “It’s used an aesthetic filler, but a known complication with PLLA injections is the formation of subcutaneous nodules and late onset granulomas,” she said.
Early in 2019, Korean researchers published results of a study that set out to evaluate the collagen-producing effects of powdered PDO injection, compared with PLLA injection, in a murine model (J Cosmet Dermatol. 2019 Feb 27. doi: 10.1111/jocd.12894). “It showed both PDO and PLLA induced granulomatous reactions and collagen formation, but this decreased at 12 weeks,” said Dr. Ortiz, who was not involved with the work. “PDO had slightly more collagen formation than PLLA.”
Indications for thread lifts, she continued, are for jawline lift, cheek enhancement, brow lift, wrinkle reduction, body contouring, acne scarring, and texturing. “Choose patients with good skin quality: not too thick/heavy, and not too thin. Patients with moderate skin sagging are going to better candidates than those with severe skin sagging.”
One type of absorbable suspension suture, the Silhouette InstaLift, is made of polyglycolide/
In terms of adverse events following thread lift procedures, patients usually feel tender for about a week or 2. “They can have some bruising, mostly from the anesthesia,” she said.
To prevent temporary dimpling, Dr. Ortiz undermines with an 18-gauge needle and inserts perpendicular to the skin surface. “Extrusions can still occur,” she said. To prevent this, she pulls on the end and makes sure it’s buried subcutaneously.
Dr. Ortiz reported having financial relationships with numerous pharmaceutical and device companies, though none related to the content of her presentation. She is also cochair of the Masters of Aesthetics symposium.
EXPERT ANALYSIS FROM MOA 2019
Body sculpting, microneedling show strong growth
, according to a survey by the American Society for Dermatologic Surgery.
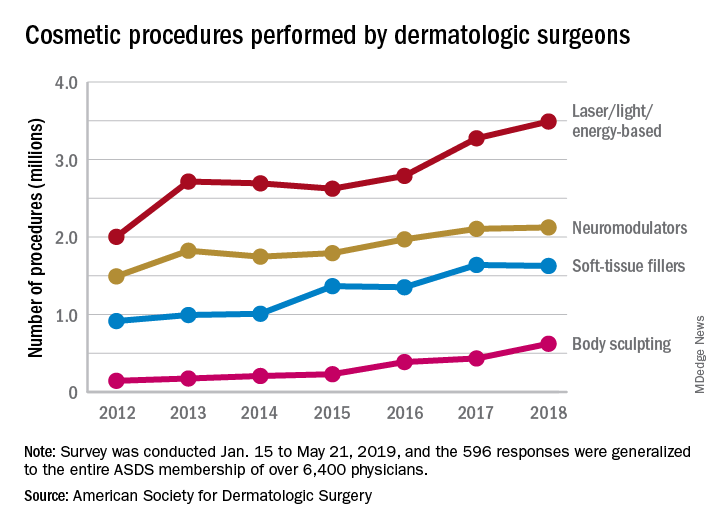
The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
, according to a survey by the American Society for Dermatologic Surgery.

The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
, according to a survey by the American Society for Dermatologic Surgery.

The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
Tips for adding cosmeceuticals to your aesthetic practice
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
EXPERT ANALYSIS FROM MOAS 2019
Should you market your aesthetic services to the ‘Me Me Me Generation’?
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
EXPERT ANALYSIS FROM MOAS 2019
Windshield and UV exposure
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Beware of natural fruit and nut ingredients in latex-allergic patients
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.