User login
Nicotinamide-containing products gaining interest for aging, dermatologic disorders
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
NEW YORK – More patients are inquiring about the antiaging claims made for nicotinamide products, according to Christine DeWitt MD, of the department of dermatology, Georgetown University, Washington. She encouraged attendees at the American Academy of Dermatology summer meeting to gain familiarity with the underlying mechanisms and potential uses of nicotinamide for aging skin and prevention of skin cancer as well as for a variety of dermatologic disorders, including atopic dermatitis and bullous pemphigoid.
The ability of nicotinamide to increase oxidized nicotinamide adenine dinucleotide (NAD+) is credited for most of its dermatologic benefits, according to Dr. DeWitt. She explained that NAD+ has a central role in cell metabolism, including serving as a substrate for sirtuins, which help prevent deterioration of telomeres, now thought to be a critical event in aging.
Downstream effects include an improved barrier function to reduce transdermal water loss in patients with atopic dermatitis and anti-inflammatory effects that are relevant to acne and bullous pemphigoid.
The related but unique forms of vitamin B3, nicotinamide riboside and nicotinamide mononucleotide, appear to increase more directly and effectively NAD+ with the potential to provide more potent enzymatic antiaging effects, according to Dr. DeWitt. Not all of the more than 90 active and recruiting trials listed for these compounds on clinicaltrials.gov relate to aging, but many do list this or a related condition, such as frailty or sarcopenia, as the therapeutic target.
The trials are being conducted even as OTC nicotinamide riboside and nicotinamide mononucleotide products are being promoted with terms such as “antiaging DNA repair” and “sirtuins activator.” Dr. DeWitt said that favorable reviews of these products on Internet forums are leading many patients to ask her specifically about their clinical value.
“Patients are starting to look at aging and longevity as an entity to manage and to treat,” Dr. DeWitt explained. Increasingly, patients bring up terms like autophagy and ask about the science behind antiaging products.
The clinical role of nicotinamide-related products, whether to reduce events related to aging or provide other benefits, remains unproven.
Nevertheless, Dr. DeWitt often offers nicotinamide to her patients for such indications as acne and atopic dermatitis. In patients with bullous pemphigoid, nicotinamide is an adjunct to other therapies “in most of my patients.”
When recommending nicotinamide, Dr. DeWitt specifies a brand, not because there is evidence that one brand is better than another but because of a reputation of quality control with branded OTC products.
In general, nicotinamide, which is not generally associated with the flushing that accompanies niacin, is well tolerated. She recommends 500 mg twice daily for most indications.
Dr. DeWitt advised reviewing published studies on nicotinamide in order to respond appropriately to patient inquiries. She noted that many patients come to the clinician’s office already aware of the science behind the potential role of NAD+ to inhibit aging and will be seeking an objective point of view.
Dr. DeWitt reports no conflicts of interest.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Botulinum toxin injections: Err on the side of undercorrecting in first-time users
NEW YORK – In patients receiving first-time botulinum toxin injections for cosmetic enhancements, it is prudent to use a relatively low dose, Gary Goldenberg, MD, advised at the American Academy of Dermatology summer meeting.
Optimal dosing varies by individual, and undertreatment is easier to correct than is excess treatment. “This is a pearl. I always undercorrect, especially if I am injecting a patient for the first time,” said Dr. Goldenberg, an assistant clinical professor of dermatology and pathology at the Icahn School of Medicine at Mount Sinai, New York.
All patients are invited to return 2 weeks after their initial treatments, when the maximum effect is reached. Dr. Goldenberg does not charge for touch-ups administered at that time. “I want the patient to have the best possible experience,” he said.
The demand for botulinum toxin injections is skyrocketing, even among patients in their 20s. Also, men now represent a substantial proportion of those seeking cosmetic botulinum toxin injections.
Botulinum toxin injections are a source of high levels of patient satisfaction, according to Dr. Goldenberg. They are also a good way to get started in performing cosmetic procedures as skills in the injection of botulinum toxin are readily acquired, While some primary care physicians and gynecologists also are offering botulinum toxin injections for cosmetic purposes, dermatologists “are going to do a better job because we know the anatomy the best,” he said.
Dr. Goldenberg said botulinum toxin injections should be offered as a service in promotional efforts for one’s practice, but any mention to patients should be tactful. Patients should be informed that there are solutions for damaged or wrinkled skin, but the topic should be dropped if there is no apparent interest.
“I only suggest. I do not push,” he said. “I never talk about money. If they want to know how much (injections) will cost, they must speak to my office staff.”
With the recent approval of prabotulinumtoxinA (Jeuveau), there are four botulinum toxin injection products available in the United States. These include the original product, onabotulinumtoxinA (Botox), incobotulinumtoxinA (Xeomin), and abobotulinumtoxinA (Dysport). Dr. Goldenberg, who has administered them all, is not so far convinced there are important differences between them in regard to either efficacy or safety.
“There is another product now in clinical trials, so perhaps we will have a fifth product in a year or so,” said Dr. Goldenberg, who noted that the competition has resulted in claims and counterclaims regarding such issues as speed of onset and durability.
For dermatologists new to providing botulinum toxin injections, Dr. Goldenberg suggested restricting initial procedures to the face, particularly glabellar lines for which all of the available products are indicated. The companies that make these products also should offer a broad array of resources for improving skills, he said.
Dr. Goldenberg reports no potential conflicts of interest with companies that make botulinum toxins.
NEW YORK – In patients receiving first-time botulinum toxin injections for cosmetic enhancements, it is prudent to use a relatively low dose, Gary Goldenberg, MD, advised at the American Academy of Dermatology summer meeting.
Optimal dosing varies by individual, and undertreatment is easier to correct than is excess treatment. “This is a pearl. I always undercorrect, especially if I am injecting a patient for the first time,” said Dr. Goldenberg, an assistant clinical professor of dermatology and pathology at the Icahn School of Medicine at Mount Sinai, New York.
All patients are invited to return 2 weeks after their initial treatments, when the maximum effect is reached. Dr. Goldenberg does not charge for touch-ups administered at that time. “I want the patient to have the best possible experience,” he said.
The demand for botulinum toxin injections is skyrocketing, even among patients in their 20s. Also, men now represent a substantial proportion of those seeking cosmetic botulinum toxin injections.
Botulinum toxin injections are a source of high levels of patient satisfaction, according to Dr. Goldenberg. They are also a good way to get started in performing cosmetic procedures as skills in the injection of botulinum toxin are readily acquired, While some primary care physicians and gynecologists also are offering botulinum toxin injections for cosmetic purposes, dermatologists “are going to do a better job because we know the anatomy the best,” he said.
Dr. Goldenberg said botulinum toxin injections should be offered as a service in promotional efforts for one’s practice, but any mention to patients should be tactful. Patients should be informed that there are solutions for damaged or wrinkled skin, but the topic should be dropped if there is no apparent interest.
“I only suggest. I do not push,” he said. “I never talk about money. If they want to know how much (injections) will cost, they must speak to my office staff.”
With the recent approval of prabotulinumtoxinA (Jeuveau), there are four botulinum toxin injection products available in the United States. These include the original product, onabotulinumtoxinA (Botox), incobotulinumtoxinA (Xeomin), and abobotulinumtoxinA (Dysport). Dr. Goldenberg, who has administered them all, is not so far convinced there are important differences between them in regard to either efficacy or safety.
“There is another product now in clinical trials, so perhaps we will have a fifth product in a year or so,” said Dr. Goldenberg, who noted that the competition has resulted in claims and counterclaims regarding such issues as speed of onset and durability.
For dermatologists new to providing botulinum toxin injections, Dr. Goldenberg suggested restricting initial procedures to the face, particularly glabellar lines for which all of the available products are indicated. The companies that make these products also should offer a broad array of resources for improving skills, he said.
Dr. Goldenberg reports no potential conflicts of interest with companies that make botulinum toxins.
NEW YORK – In patients receiving first-time botulinum toxin injections for cosmetic enhancements, it is prudent to use a relatively low dose, Gary Goldenberg, MD, advised at the American Academy of Dermatology summer meeting.
Optimal dosing varies by individual, and undertreatment is easier to correct than is excess treatment. “This is a pearl. I always undercorrect, especially if I am injecting a patient for the first time,” said Dr. Goldenberg, an assistant clinical professor of dermatology and pathology at the Icahn School of Medicine at Mount Sinai, New York.
All patients are invited to return 2 weeks after their initial treatments, when the maximum effect is reached. Dr. Goldenberg does not charge for touch-ups administered at that time. “I want the patient to have the best possible experience,” he said.
The demand for botulinum toxin injections is skyrocketing, even among patients in their 20s. Also, men now represent a substantial proportion of those seeking cosmetic botulinum toxin injections.
Botulinum toxin injections are a source of high levels of patient satisfaction, according to Dr. Goldenberg. They are also a good way to get started in performing cosmetic procedures as skills in the injection of botulinum toxin are readily acquired, While some primary care physicians and gynecologists also are offering botulinum toxin injections for cosmetic purposes, dermatologists “are going to do a better job because we know the anatomy the best,” he said.
Dr. Goldenberg said botulinum toxin injections should be offered as a service in promotional efforts for one’s practice, but any mention to patients should be tactful. Patients should be informed that there are solutions for damaged or wrinkled skin, but the topic should be dropped if there is no apparent interest.
“I only suggest. I do not push,” he said. “I never talk about money. If they want to know how much (injections) will cost, they must speak to my office staff.”
With the recent approval of prabotulinumtoxinA (Jeuveau), there are four botulinum toxin injection products available in the United States. These include the original product, onabotulinumtoxinA (Botox), incobotulinumtoxinA (Xeomin), and abobotulinumtoxinA (Dysport). Dr. Goldenberg, who has administered them all, is not so far convinced there are important differences between them in regard to either efficacy or safety.
“There is another product now in clinical trials, so perhaps we will have a fifth product in a year or so,” said Dr. Goldenberg, who noted that the competition has resulted in claims and counterclaims regarding such issues as speed of onset and durability.
For dermatologists new to providing botulinum toxin injections, Dr. Goldenberg suggested restricting initial procedures to the face, particularly glabellar lines for which all of the available products are indicated. The companies that make these products also should offer a broad array of resources for improving skills, he said.
Dr. Goldenberg reports no potential conflicts of interest with companies that make botulinum toxins.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Piercing art
Body art as a form of human expression is prevalent. The most common types are skin tattoos and piercings, but also include scarification, branding, subdermal implants, and body painting. Body painting has made headlines for its artistic creativity and artistic significance at annual week long temporary communities such as the annual Burning Man art festival.
Culture and history
Culturally, however, body painting has significant historical significance, with Henna painting described in the earliest Hindu Vedic ritual books dating back 5,000 years. Henna painting, most commonly of the hands and feet, known as Mehndi in the Indian subcontinent, signifies painting of symbolic representations of the outer and the inner sun, with the idea of “awakening the inner light.” It is also a common tradition of Hindu weddings and applied in Muslim tradition in India during Eid festivals. Body painting has also been used in other cultures for ceremonial, religious reasons, as well as forms of camouflage during hunting or war. Branding and scarification were used as methods of punishment during the Middle Ages in England and commonly during slavery in the Americas. Traditionally, though, branding and scarification have been seen in darker-skinned individuals as a form of self-expression where tattoos are not as effective visually. African tribes in Ethiopia and Sudan, as well as the Maasai people in Kenya, have used scarification and branding as an ancient art that can signify everything from beauty to transition to adulthood. Some black fraternities also use it as a mark of collegiality.
While tattoos are the most recognized form of body art, body and facial piercing are far more common in the general population among cultures throughout the world. While ear piercings are the most common, historically, nostril piercing has been documented in the Middle East as far back as 4,000 years ago, and both ear and nostril piercing and jewelry are mentioned historically in the Bible (Genesis 24:22, Isaiah 3:21). Ritual tongue piercing was reportedly performed by Aztec and Mayan Indians during ceremonies to honor their deities.
Current Practice
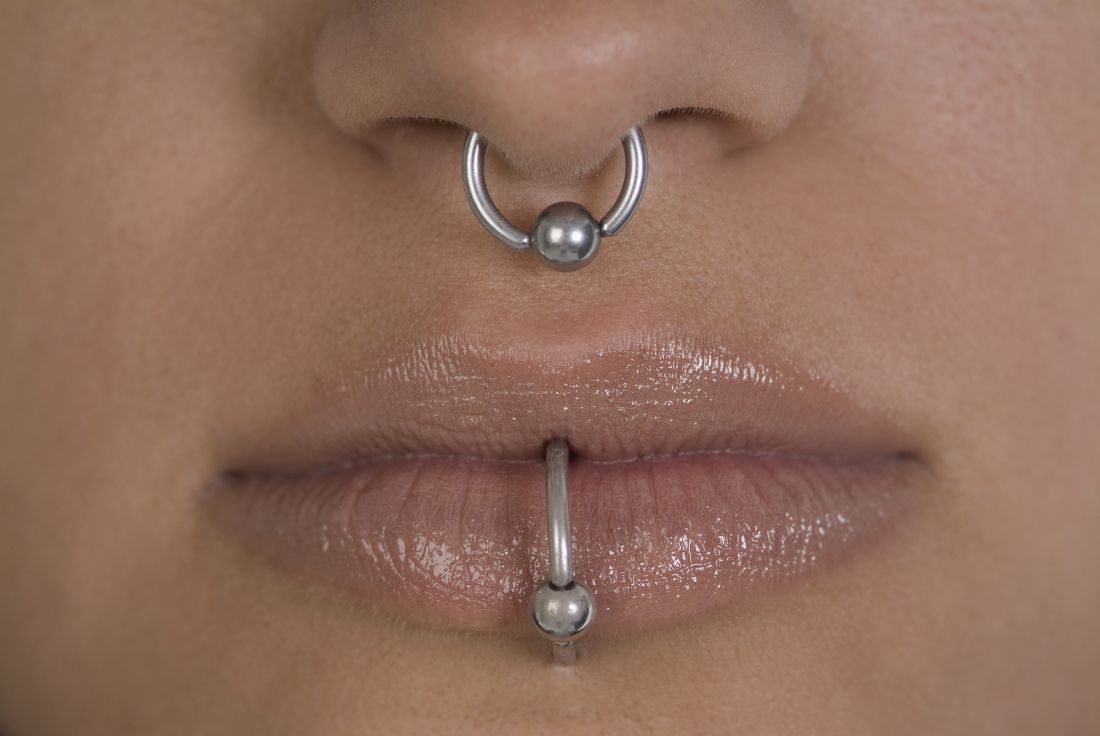
In practice, we see different types of piercings, including but not limited to ear, nose (alar, septum, bridge), eyebrow, lip, tongue, face, nipple, umbilical, and genital piercings. Ear piercings alone may come in many forms. Not only do location, cartilage versus no cartilage involvement, and age of piercing have different implications for care and potential risks/complications, so do the size, type, and shape of jewelry used for the piercing.
Having a better understanding of piercing art is important for dermatologists and dermatologic surgeons because we sometimes treat the sequelae, including infection, allergic reactions from the jewelry, and keloid scars. Patients may intentionally create large size piercings, known as gauge piercings, and decide later they no longer want them. Or earlobe piercings can unintentionally stretch and enlarge over time from prolonged wearing of heavy earrings or trauma, sometimes resulting in a partial or complete earlobe split, requiring surgical treatment for gauge or split earlobe repair. If repiercing earlobe repair is desired, most physicians wait at least 6-8 weeks. While different earlobe surgical repair techniques (most commonly Z-plasty) and even recommendations for subdermal implant removal are described in the literature, there are no real guidelines on when to repierce in the evidenced-based literature. Healing time in general for piercings also varies by site. For example, initial earlobe piercings typically take 1-2 months to heal, whereas ear cartilage and navel piercings may take 4-12 months.
Some medical practitioners may not be aware of tips known to top piercing professionals that can help guide patients on piercing care. Cartilage piercings can sometimes present with inflammation and nodule formation, even prior to true keloid formation. In my experience, a simple solution of washing daily with a highly alkaline but gentle natural soap, such as Dr. Bronner’s mild baby soap, or compresses or soaks with warm salt water, can sometimes reduce the inflammation and resolve nodule formation before topical, intralesional corticosteroids, or surgery is needed (a situation in which surgery may lead to further cartilage inflammation and hypertrophic scar formation). Additionally, certain pressure earrings may be used to help prevent keloid formation, in addition to wearing jewelry of a metal that is nonallergenic to the user, to prevent further inflammation.
Piercing is a common form of body art and self-expression. but also develop a better understanding of and relationship with our patients by virtue of their means of artistic self-expression.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Body art as a form of human expression is prevalent. The most common types are skin tattoos and piercings, but also include scarification, branding, subdermal implants, and body painting. Body painting has made headlines for its artistic creativity and artistic significance at annual week long temporary communities such as the annual Burning Man art festival.
Culture and history
Culturally, however, body painting has significant historical significance, with Henna painting described in the earliest Hindu Vedic ritual books dating back 5,000 years. Henna painting, most commonly of the hands and feet, known as Mehndi in the Indian subcontinent, signifies painting of symbolic representations of the outer and the inner sun, with the idea of “awakening the inner light.” It is also a common tradition of Hindu weddings and applied in Muslim tradition in India during Eid festivals. Body painting has also been used in other cultures for ceremonial, religious reasons, as well as forms of camouflage during hunting or war. Branding and scarification were used as methods of punishment during the Middle Ages in England and commonly during slavery in the Americas. Traditionally, though, branding and scarification have been seen in darker-skinned individuals as a form of self-expression where tattoos are not as effective visually. African tribes in Ethiopia and Sudan, as well as the Maasai people in Kenya, have used scarification and branding as an ancient art that can signify everything from beauty to transition to adulthood. Some black fraternities also use it as a mark of collegiality.
While tattoos are the most recognized form of body art, body and facial piercing are far more common in the general population among cultures throughout the world. While ear piercings are the most common, historically, nostril piercing has been documented in the Middle East as far back as 4,000 years ago, and both ear and nostril piercing and jewelry are mentioned historically in the Bible (Genesis 24:22, Isaiah 3:21). Ritual tongue piercing was reportedly performed by Aztec and Mayan Indians during ceremonies to honor their deities.
Current Practice
In practice, we see different types of piercings, including but not limited to ear, nose (alar, septum, bridge), eyebrow, lip, tongue, face, nipple, umbilical, and genital piercings. Ear piercings alone may come in many forms. Not only do location, cartilage versus no cartilage involvement, and age of piercing have different implications for care and potential risks/complications, so do the size, type, and shape of jewelry used for the piercing.
Having a better understanding of piercing art is important for dermatologists and dermatologic surgeons because we sometimes treat the sequelae, including infection, allergic reactions from the jewelry, and keloid scars. Patients may intentionally create large size piercings, known as gauge piercings, and decide later they no longer want them. Or earlobe piercings can unintentionally stretch and enlarge over time from prolonged wearing of heavy earrings or trauma, sometimes resulting in a partial or complete earlobe split, requiring surgical treatment for gauge or split earlobe repair. If repiercing earlobe repair is desired, most physicians wait at least 6-8 weeks. While different earlobe surgical repair techniques (most commonly Z-plasty) and even recommendations for subdermal implant removal are described in the literature, there are no real guidelines on when to repierce in the evidenced-based literature. Healing time in general for piercings also varies by site. For example, initial earlobe piercings typically take 1-2 months to heal, whereas ear cartilage and navel piercings may take 4-12 months.
Some medical practitioners may not be aware of tips known to top piercing professionals that can help guide patients on piercing care. Cartilage piercings can sometimes present with inflammation and nodule formation, even prior to true keloid formation. In my experience, a simple solution of washing daily with a highly alkaline but gentle natural soap, such as Dr. Bronner’s mild baby soap, or compresses or soaks with warm salt water, can sometimes reduce the inflammation and resolve nodule formation before topical, intralesional corticosteroids, or surgery is needed (a situation in which surgery may lead to further cartilage inflammation and hypertrophic scar formation). Additionally, certain pressure earrings may be used to help prevent keloid formation, in addition to wearing jewelry of a metal that is nonallergenic to the user, to prevent further inflammation.
Piercing is a common form of body art and self-expression. but also develop a better understanding of and relationship with our patients by virtue of their means of artistic self-expression.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Body art as a form of human expression is prevalent. The most common types are skin tattoos and piercings, but also include scarification, branding, subdermal implants, and body painting. Body painting has made headlines for its artistic creativity and artistic significance at annual week long temporary communities such as the annual Burning Man art festival.
Culture and history
Culturally, however, body painting has significant historical significance, with Henna painting described in the earliest Hindu Vedic ritual books dating back 5,000 years. Henna painting, most commonly of the hands and feet, known as Mehndi in the Indian subcontinent, signifies painting of symbolic representations of the outer and the inner sun, with the idea of “awakening the inner light.” It is also a common tradition of Hindu weddings and applied in Muslim tradition in India during Eid festivals. Body painting has also been used in other cultures for ceremonial, religious reasons, as well as forms of camouflage during hunting or war. Branding and scarification were used as methods of punishment during the Middle Ages in England and commonly during slavery in the Americas. Traditionally, though, branding and scarification have been seen in darker-skinned individuals as a form of self-expression where tattoos are not as effective visually. African tribes in Ethiopia and Sudan, as well as the Maasai people in Kenya, have used scarification and branding as an ancient art that can signify everything from beauty to transition to adulthood. Some black fraternities also use it as a mark of collegiality.
While tattoos are the most recognized form of body art, body and facial piercing are far more common in the general population among cultures throughout the world. While ear piercings are the most common, historically, nostril piercing has been documented in the Middle East as far back as 4,000 years ago, and both ear and nostril piercing and jewelry are mentioned historically in the Bible (Genesis 24:22, Isaiah 3:21). Ritual tongue piercing was reportedly performed by Aztec and Mayan Indians during ceremonies to honor their deities.
Current Practice
In practice, we see different types of piercings, including but not limited to ear, nose (alar, septum, bridge), eyebrow, lip, tongue, face, nipple, umbilical, and genital piercings. Ear piercings alone may come in many forms. Not only do location, cartilage versus no cartilage involvement, and age of piercing have different implications for care and potential risks/complications, so do the size, type, and shape of jewelry used for the piercing.
Having a better understanding of piercing art is important for dermatologists and dermatologic surgeons because we sometimes treat the sequelae, including infection, allergic reactions from the jewelry, and keloid scars. Patients may intentionally create large size piercings, known as gauge piercings, and decide later they no longer want them. Or earlobe piercings can unintentionally stretch and enlarge over time from prolonged wearing of heavy earrings or trauma, sometimes resulting in a partial or complete earlobe split, requiring surgical treatment for gauge or split earlobe repair. If repiercing earlobe repair is desired, most physicians wait at least 6-8 weeks. While different earlobe surgical repair techniques (most commonly Z-plasty) and even recommendations for subdermal implant removal are described in the literature, there are no real guidelines on when to repierce in the evidenced-based literature. Healing time in general for piercings also varies by site. For example, initial earlobe piercings typically take 1-2 months to heal, whereas ear cartilage and navel piercings may take 4-12 months.
Some medical practitioners may not be aware of tips known to top piercing professionals that can help guide patients on piercing care. Cartilage piercings can sometimes present with inflammation and nodule formation, even prior to true keloid formation. In my experience, a simple solution of washing daily with a highly alkaline but gentle natural soap, such as Dr. Bronner’s mild baby soap, or compresses or soaks with warm salt water, can sometimes reduce the inflammation and resolve nodule formation before topical, intralesional corticosteroids, or surgery is needed (a situation in which surgery may lead to further cartilage inflammation and hypertrophic scar formation). Additionally, certain pressure earrings may be used to help prevent keloid formation, in addition to wearing jewelry of a metal that is nonallergenic to the user, to prevent further inflammation.
Piercing is a common form of body art and self-expression. but also develop a better understanding of and relationship with our patients by virtue of their means of artistic self-expression.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Parabens – friend or foe?
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
<i>Mycobacterium abscessus</i> Infection Following Home Dermabrasion
Case Report
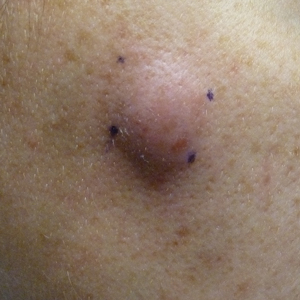
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).
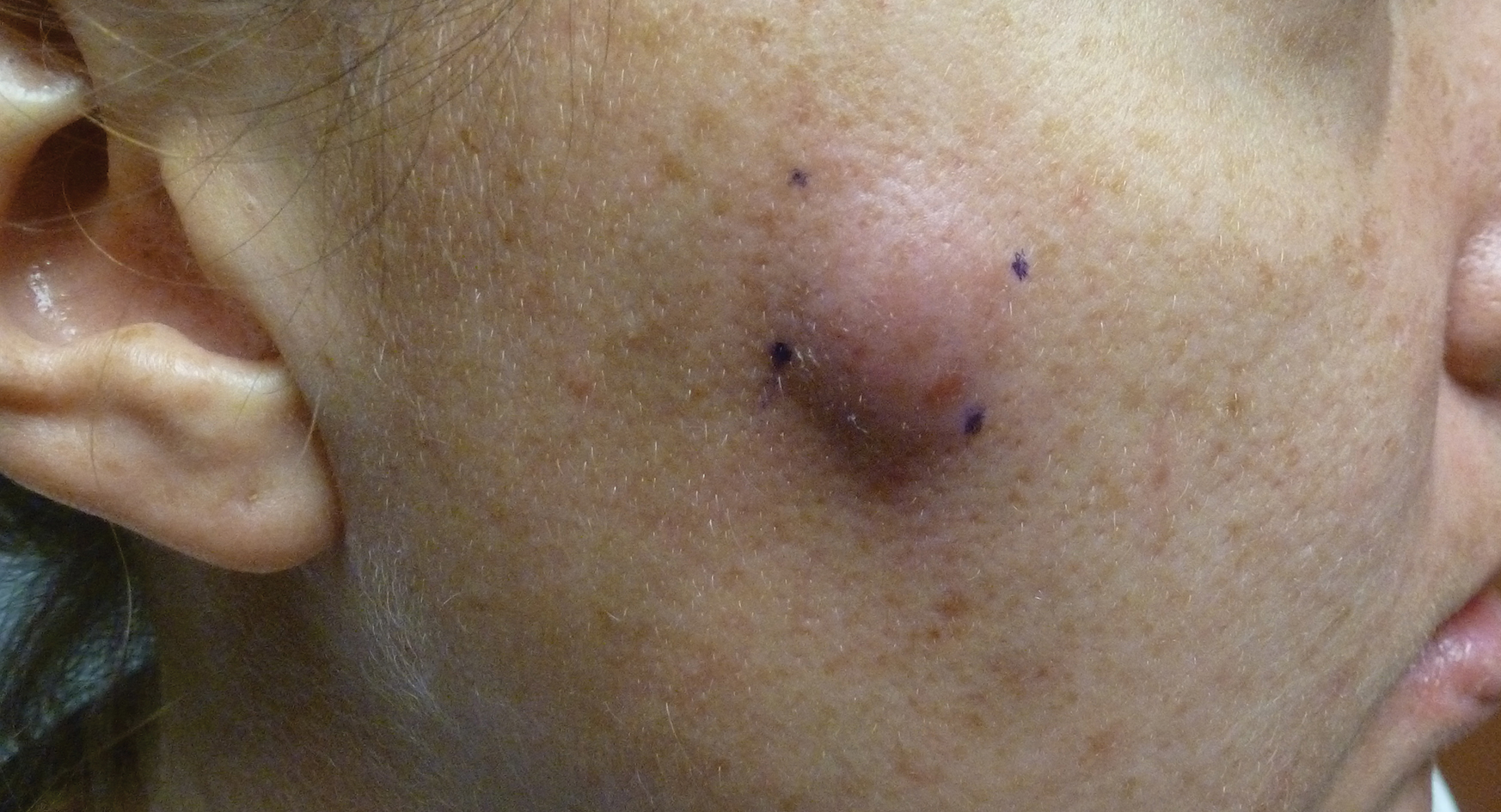
A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
Case Report
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).

A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
Case Report
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).

A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
Practice Points
- Atypical mycobacteria are included in the differential for cutaneous abscesses.
- At-home cosmetic treatments often carry unrecognized risks for adverse events.
- Obtain culture prior to initiation of empiric antibiotics.
Nonsurgical Hair Restoration Treatment
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Practice Points
- Hair loss is a common phenomenon in both men and women and can seriously impact psychosocial functioning.
- There are numerous US Food and Drug Administration–approved and off-label nonsurgical treatment options for alopecia. Dermatologists should be well versed in these treatment modalities and the associated sideeffect profiles to select the appropriate therapy for each patient.
Bakuchiol
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Minimally invasive cosmetic surgery: Steady growth in 2018
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.
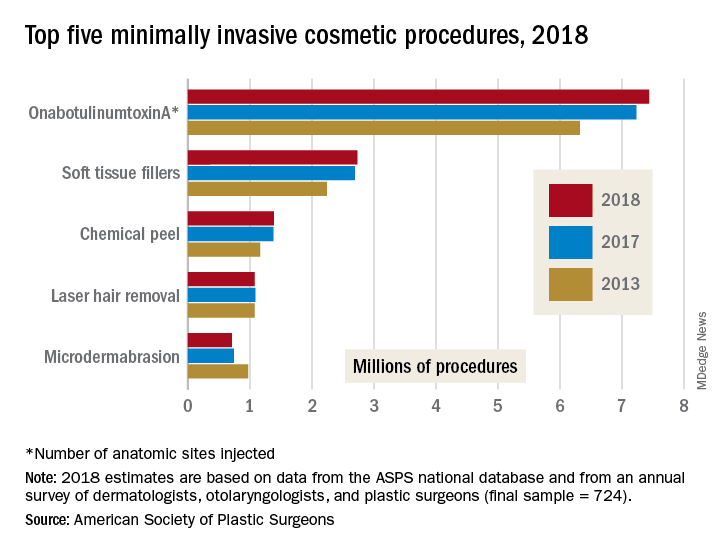
The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.

The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.

The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
Social media use linked to acceptance of cosmetic surgery
Use of social media platforms such as Tinder, Snapchat, and Instagram, particularly in conjunction with photo-editing applications, may increase an individual’s acceptance of cosmetic surgery, a new study suggests.
In JAMA Facial Plastic Surgery, researchers report the outcomes of a web-based survey study involving 252 participants, 73.0% of whom were female. The survey asked participants about their use of social media, photo-editing tools such as Photoshop, VSCO, and Snapchat filters, and answered questionnaires to assess their self-esteem, self-worth, and attitudes toward cosmetic surgery.
All participants used at least one social media platform, with a mean of seven, and used a mean of two photo-editing applications; the analysis found that those who used more social media platforms were more likely to consider cosmetic surgery.
People who used Tinder and Snapchat – with or without photo filters – showed greater acceptance of cosmetic surgery, while those who used the photography mobile app VSCO and Instagram photo filters showed greater consideration but not acceptance of cosmetic surgery, compared with nonusers.
Participants whose self-worth was more closely tied to their appearance showed greater acceptance of cosmetic surgery. When it came to self-esteem, participants who used YouTube, WhatsApp, VSCO, and Photoshop had lower self-esteem scores, compared with nonusers.
Overall, nearly two-thirds of survey participants said they used photo-editing applications to change the lighting of images, but only 5.16% said they used these applications to make changes to face or body shape. This distinction was also seen in their acceptance of cosmetic surgery scores: Those who said they made changes to face and body shape showed higher acceptance scores than nonusers, but this was not seen in those who only used it for lighting adjustments.
“The rising trend of pursuing cosmetic surgery based on social media inspiration highlights the need to better understand patients’ motivations to seek cosmetic surgery,” wrote Jonlin Chen, a medical student at Johns Hopkins University, Baltimore, and coauthors.
Commenting on the association between YouTube use, lower self-esteem, and higher acceptance of cosmetic surgery, the authors suggested that the platform may generate appearance comparisons between users by allowing them to access beauty-related videos and connect with other users interested in cosmetics.
Michael J. Reilly, MD, department of otolaryngology–head and neck surgery and Keon M. Parsa, MD, from the department of psychiatry at MedStar Georgetown University Hospital in Washington, commented in an accompanying editorial that the findings of this study illustrate an increased trend seen by facial plastic surgeons (JAMA Facial Plast Surg. 2019 June 27. doi: 10.1001/jamafacial.2019.0419). The study “shows the importance of understanding the underlying motives and characteristics of individuals seeking cosmetic surgery.” They noted that facial plastic surgeons can play a role in helping patients to improve their self-esteem, but it is also important to be aware of the clinical signs of depression, anxiety, and social isolation and refer for appropriate nonsurgical support when there are mental health concerns that go beyond the knife and needle.
The authors of the study did note that their choice of a web-based survey meant the demographic was likely to be skewed toward a younger, more social media–savvy demographic, and may not necessarily represent the broader population of individuals seeking cosmetic surgery.
No funding or conflicts of interest were declared.
SOURCE: Chen J et al. JAMA Facial Plast Surg. 2019 Jun 27. doi: 10.1001/jamafacial.2019.0328.
Use of social media platforms such as Tinder, Snapchat, and Instagram, particularly in conjunction with photo-editing applications, may increase an individual’s acceptance of cosmetic surgery, a new study suggests.
In JAMA Facial Plastic Surgery, researchers report the outcomes of a web-based survey study involving 252 participants, 73.0% of whom were female. The survey asked participants about their use of social media, photo-editing tools such as Photoshop, VSCO, and Snapchat filters, and answered questionnaires to assess their self-esteem, self-worth, and attitudes toward cosmetic surgery.
All participants used at least one social media platform, with a mean of seven, and used a mean of two photo-editing applications; the analysis found that those who used more social media platforms were more likely to consider cosmetic surgery.
People who used Tinder and Snapchat – with or without photo filters – showed greater acceptance of cosmetic surgery, while those who used the photography mobile app VSCO and Instagram photo filters showed greater consideration but not acceptance of cosmetic surgery, compared with nonusers.
Participants whose self-worth was more closely tied to their appearance showed greater acceptance of cosmetic surgery. When it came to self-esteem, participants who used YouTube, WhatsApp, VSCO, and Photoshop had lower self-esteem scores, compared with nonusers.
Overall, nearly two-thirds of survey participants said they used photo-editing applications to change the lighting of images, but only 5.16% said they used these applications to make changes to face or body shape. This distinction was also seen in their acceptance of cosmetic surgery scores: Those who said they made changes to face and body shape showed higher acceptance scores than nonusers, but this was not seen in those who only used it for lighting adjustments.
“The rising trend of pursuing cosmetic surgery based on social media inspiration highlights the need to better understand patients’ motivations to seek cosmetic surgery,” wrote Jonlin Chen, a medical student at Johns Hopkins University, Baltimore, and coauthors.
Commenting on the association between YouTube use, lower self-esteem, and higher acceptance of cosmetic surgery, the authors suggested that the platform may generate appearance comparisons between users by allowing them to access beauty-related videos and connect with other users interested in cosmetics.
Michael J. Reilly, MD, department of otolaryngology–head and neck surgery and Keon M. Parsa, MD, from the department of psychiatry at MedStar Georgetown University Hospital in Washington, commented in an accompanying editorial that the findings of this study illustrate an increased trend seen by facial plastic surgeons (JAMA Facial Plast Surg. 2019 June 27. doi: 10.1001/jamafacial.2019.0419). The study “shows the importance of understanding the underlying motives and characteristics of individuals seeking cosmetic surgery.” They noted that facial plastic surgeons can play a role in helping patients to improve their self-esteem, but it is also important to be aware of the clinical signs of depression, anxiety, and social isolation and refer for appropriate nonsurgical support when there are mental health concerns that go beyond the knife and needle.
The authors of the study did note that their choice of a web-based survey meant the demographic was likely to be skewed toward a younger, more social media–savvy demographic, and may not necessarily represent the broader population of individuals seeking cosmetic surgery.
No funding or conflicts of interest were declared.
SOURCE: Chen J et al. JAMA Facial Plast Surg. 2019 Jun 27. doi: 10.1001/jamafacial.2019.0328.
Use of social media platforms such as Tinder, Snapchat, and Instagram, particularly in conjunction with photo-editing applications, may increase an individual’s acceptance of cosmetic surgery, a new study suggests.
In JAMA Facial Plastic Surgery, researchers report the outcomes of a web-based survey study involving 252 participants, 73.0% of whom were female. The survey asked participants about their use of social media, photo-editing tools such as Photoshop, VSCO, and Snapchat filters, and answered questionnaires to assess their self-esteem, self-worth, and attitudes toward cosmetic surgery.
All participants used at least one social media platform, with a mean of seven, and used a mean of two photo-editing applications; the analysis found that those who used more social media platforms were more likely to consider cosmetic surgery.
People who used Tinder and Snapchat – with or without photo filters – showed greater acceptance of cosmetic surgery, while those who used the photography mobile app VSCO and Instagram photo filters showed greater consideration but not acceptance of cosmetic surgery, compared with nonusers.
Participants whose self-worth was more closely tied to their appearance showed greater acceptance of cosmetic surgery. When it came to self-esteem, participants who used YouTube, WhatsApp, VSCO, and Photoshop had lower self-esteem scores, compared with nonusers.
Overall, nearly two-thirds of survey participants said they used photo-editing applications to change the lighting of images, but only 5.16% said they used these applications to make changes to face or body shape. This distinction was also seen in their acceptance of cosmetic surgery scores: Those who said they made changes to face and body shape showed higher acceptance scores than nonusers, but this was not seen in those who only used it for lighting adjustments.
“The rising trend of pursuing cosmetic surgery based on social media inspiration highlights the need to better understand patients’ motivations to seek cosmetic surgery,” wrote Jonlin Chen, a medical student at Johns Hopkins University, Baltimore, and coauthors.
Commenting on the association between YouTube use, lower self-esteem, and higher acceptance of cosmetic surgery, the authors suggested that the platform may generate appearance comparisons between users by allowing them to access beauty-related videos and connect with other users interested in cosmetics.
Michael J. Reilly, MD, department of otolaryngology–head and neck surgery and Keon M. Parsa, MD, from the department of psychiatry at MedStar Georgetown University Hospital in Washington, commented in an accompanying editorial that the findings of this study illustrate an increased trend seen by facial plastic surgeons (JAMA Facial Plast Surg. 2019 June 27. doi: 10.1001/jamafacial.2019.0419). The study “shows the importance of understanding the underlying motives and characteristics of individuals seeking cosmetic surgery.” They noted that facial plastic surgeons can play a role in helping patients to improve their self-esteem, but it is also important to be aware of the clinical signs of depression, anxiety, and social isolation and refer for appropriate nonsurgical support when there are mental health concerns that go beyond the knife and needle.
The authors of the study did note that their choice of a web-based survey meant the demographic was likely to be skewed toward a younger, more social media–savvy demographic, and may not necessarily represent the broader population of individuals seeking cosmetic surgery.
No funding or conflicts of interest were declared.
SOURCE: Chen J et al. JAMA Facial Plast Surg. 2019 Jun 27. doi: 10.1001/jamafacial.2019.0328.
FROM JAMA FACIAL PLASTIC SURGERY
Are nutritional supplements important in the treatment of female pattern hair loss?
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.
Although genetics, hormones, age, environment, stress, and nutrition all play a role in the etiology of FPHL, the underlying pathophysiology is poorly understood. The only Food and Drug Administration–approved medication to treat FPHL is topical minoxidil. The armamentarium is limited so alternative treatments such as platelet-rich plasma, topical hair loss preparations, and nutritional supplements are now being used in an effort to slow down progression of this disease.
Hair follicles are metabolically active and thus nutrient deficiency as well as calorie and protein restriction impact the hair growth cycle. Patients often inquire if dietary changes or supplementation can help prevent the loss or increase the growth of the hair. Unfortunately, the quality of evidence on nutritional supplements for this use is poor. Furthermore, it is unclear whether patients with FPHL should be routinely tested for nutritional deficiencies, and which type and concentration of supplementation will be of benefit to patients.
Iron deficiency is one of the most well-known factors for hair loss. Risk factors include heavy bleeding during menses, gastrointestinal blood loss, and malabsorption. Studies have shown that iron supplementation does help increase hair growth in iron-deficient mice. Zinc is also a key mineral in hair follicle development, and zinc deficiency is seen in genetic diseases or malabsorption syndromes and has been linked to hair loss.
Deficiencies in selenium, essential fatty acids, vitamin D, vitamin A, vitamin E, folic acid, and biotin have been documented in relation to hair loss. However, no studies have effectively shown that supplementation of these nutrients helps hair growth in patients without a documented deficiency. Currently, it is difficult to ascertain which nutrients and in what concentrations are both safe and effective to correct hair loss.
In the vast hair supplement market, some of the more popular supplements for FPHL are DeeplyRooted (Hush & Hush), Viviscal, Nutrafol, and Nature’s Bounty and Sugarbearhair products. These supplements contain a combination of micronutrients (such as vitamin D, niacin, zinc, biotin, and selenium) and adaptogens (a natural substance that helps the body heal with stress and increased cortisol production during stress) that may stimulate the growth and health of the hair follicle and minimize the production of stress hormones and dihydrotestosterone.
In my practice, we see over 100 hair loss patients a week; 30%-40% are patients with FPHL who are often suffering from depression, anxiety, and emotional distress. Our combination treatments always include nutritional supplementation and we have had success not only halting subclinical shedding, but also increasing hair growth. Until the complex pathophysiology of FPHL is identified and new therapeutics are developed, practitioners should consider adding nutritional supplements for the treatment of women with FPHL. Monitoring of supplement use is essential given the risk of toxicity from some vitamins and supplements when taken without proper supervision. More research is also needed to help delineate both the guidelines of micronutrient testing and parameters for supplementation.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Guo EL et al. Dermatol Pract Concept. 2017 Jan 31;7(1):1-10.
Goldberg LJ et al. Clin Dermatol. 2010 Jul-Aug;28(4):412-9.
Finner AM. Dermatol Clin. 2013 Jan;31(1):167-72.
St Pierre SA et al. J Am Acad Dermatol. 2010 Dec;63(6):1070-6.
Rasheed H et al. Skin Pharmacol Physiol. 2013;26(2):101-7.
Rogers NE et al. J Am Acad Dermatol. 2008 Oct;59(4):547-66.
Ablon G et al. J Drugs Dermatol. 2018 May 1;17(5):558-65.