User login
Clinical Pearl: Advantages of the Scalp as a Split-Thickness Skin Graft Donor Site
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
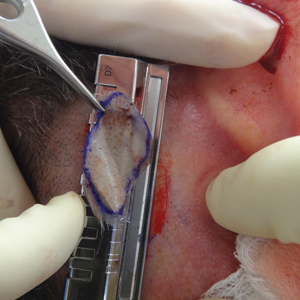
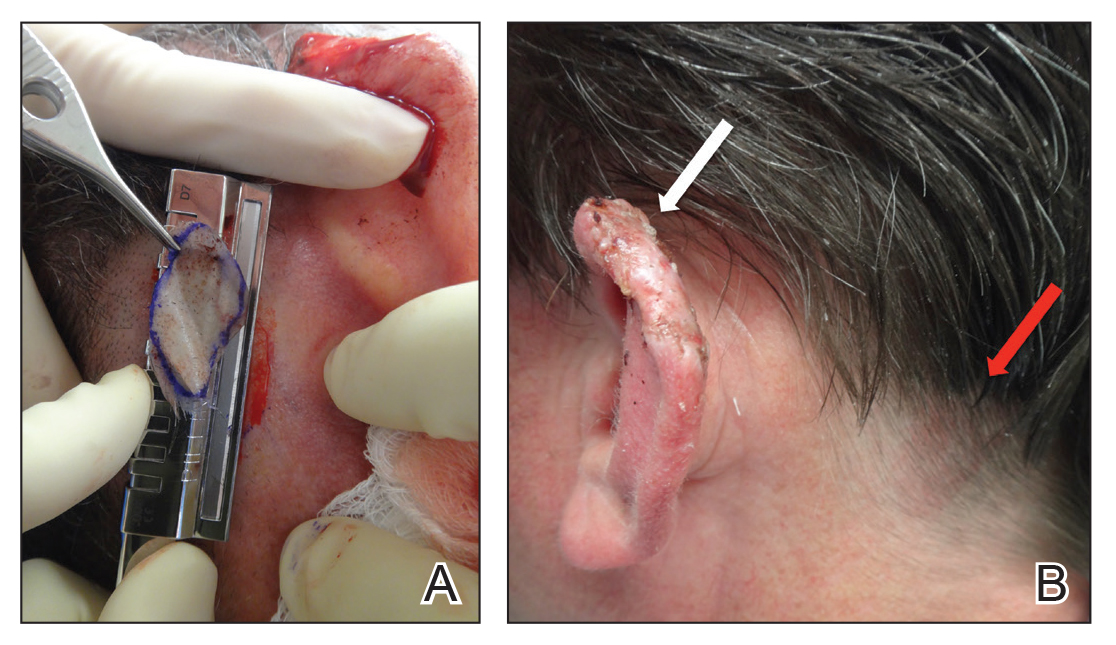
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).
Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).

Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
Practice Gap
Common donor sites for split-thickness skin grafts (STSGs) include the abdomen, buttocks, inner upper arms and forearms, and thighs. Challenges associated with donor site wounds in these areas include slow healing times and poor scar cosmesis. Although the scalp is not commonly considered when selecting a STSG donor site, harvesting from this area yields optimal results to improve these shortcomings.
Tools
A Weck knife facilitates STSG harvesting in an operationally timely, convenient fashion from larger donor sites up to 5.5 cm in width, such as the scalp, using adjustable thickness control guards.
The Technique
The donor site is lubricated with a sterile mineral oil. An assistant provides tension, leading the trajectory of the Weck knife with a guard. Small, gentle, back-and-forth strokes are made with the Weck knife to harvest the graft, which is then meshed with a No. 15 blade by placing the belly of the blade on the tissue and rolling it to-and-fro. The recipient site cartilage is fenestrated with a 2-mm punch biopsy.
A 48-year-old man underwent Mohs micrographic surgery for treatment of a primary basal cell carcinoma of the left helix, resulting in a 2.5×1.3-cm defect after 2 stages. A Weck knife with a 0.012-in guard was used to harvest an STSG from the postauricular scalp (Figure, A), and the graft was inset to the recipient wound bed. Hemostasis at the scalp donor site was achieved through application of pressure and sterile gauze that was saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine. Both recipient and donor sites were dressed with tie-over bolsters that were sutured into place. At 2-week follow-up, the donor site was fully reepithelialized and hair regrowth obscured the defect (Figure, B).

Practice Implications
Our case demonstrates the advantages of the scalp as an STSG donor site with prompt healing time and excellent cosmesis. Because grafts are harvested at a depth superficial to the hair follicle, the hair regrows to conceal the donor site scar. Additionally, the robust blood supply of the scalp and hair follicle density optimize healing time. The location of the donor site at the postauricular scalp facilitates accessibility for wound care by the patient. Electrocautery or chemical styptics used for hemostasis may traumatize the hair follicles and risk causing alopecia; therefore, as demonstrated in our case, the preferred method to achieve hemostasis is the use of pressure or application of sterile gauze that has been saturated with local 1% lidocaine anesthesia containing 1:400,000 epinephrine, followed by a pressure dressing provided by a sutured bolster.
Our case also demonstrates the utility of the Weck knife, which was introduced in 1968 as a modification of existing instruments to improve the ease of harvesting STSGs by appending a fixed handle and interchangeable depth gauges to a straight razor.1,2 The Weck knife can obtain grafts up to 5.5 cm in width (length may be as long as anatomically available), often circumventing the need to overlap grafts of smaller widths for repair of larger defects. Furthermore, grafts are harvested at a depth superficial to the hair follicle, averting donor site alopecia. These characteristics make the technique an ideal option for harvesting grafts from the scalp and other large donor sites.
Limitations of the Weck knife technique include the inability to harvest grafts from small donor sites in difficult-to-access anatomic regions or from areas with notable 3-dimensional structure. For harvesting such grafts, we prefer the DermaBlade (AccuTec Blades). Furthermore, assistance for providing tension along the trajectory of the Weck blade with a guard is optimal when performing the procedure. For practices not already utilizing a Weck knife, the technique necessitates additional training and cost. Nonetheless, for STSGs in which large donor site surface area, adjustable thickness, and convenient and timely operational technique are desired, the Weck knife should be considered as part of the dermatologic surgeon’s armamentarium.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
- Aneer F, Singh AK, Kumar S. Evolution of instruments for harvest of the skin grafts. Indian J Plast Surg. 2013;46:28-35.
- Goulian D. A new economical dermatome. Plast Reconstr Surg. 1968;42:85-86.
Ocular Chemical Burns in the Dermatology Office: A Practical Approach to Managing Safety Precautions
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).
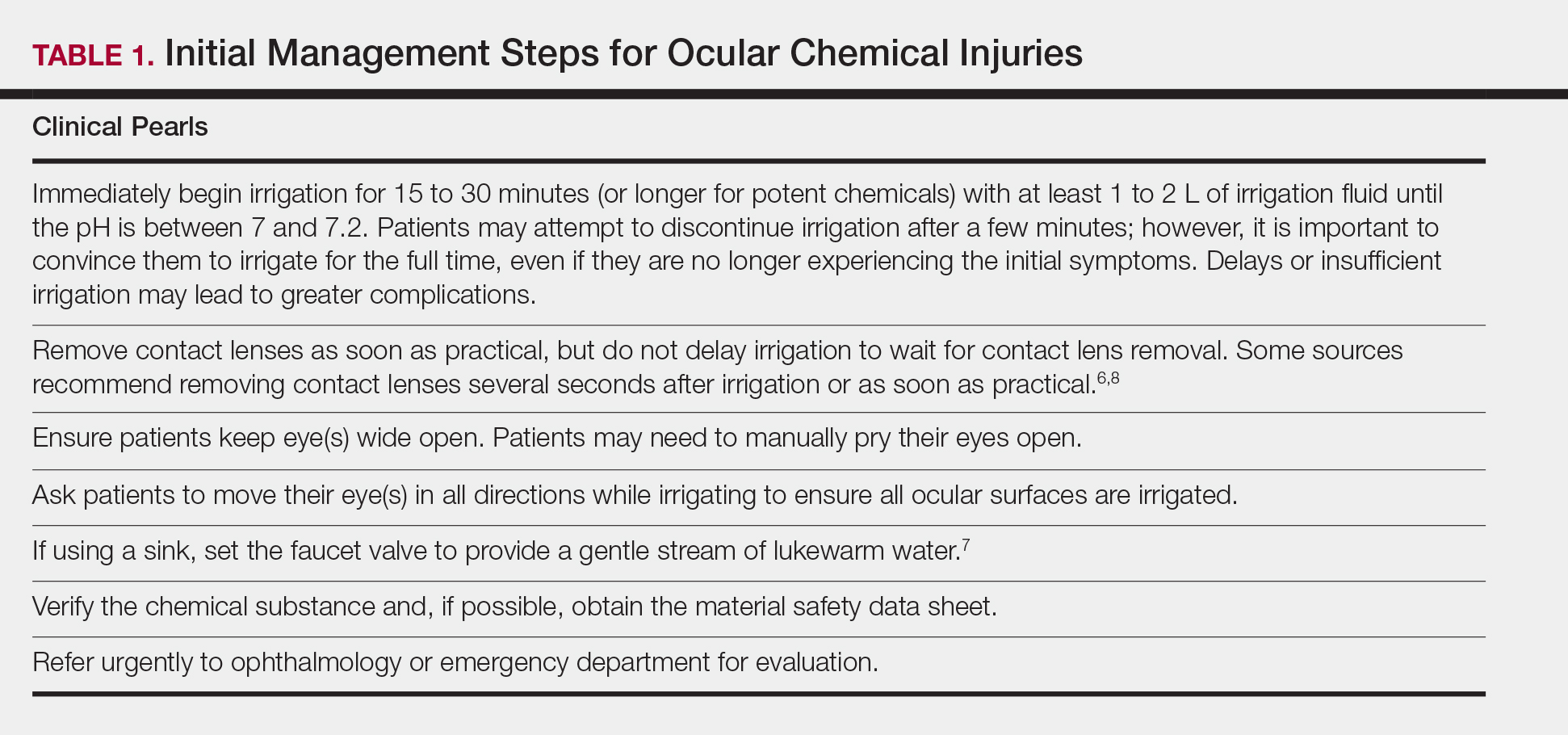
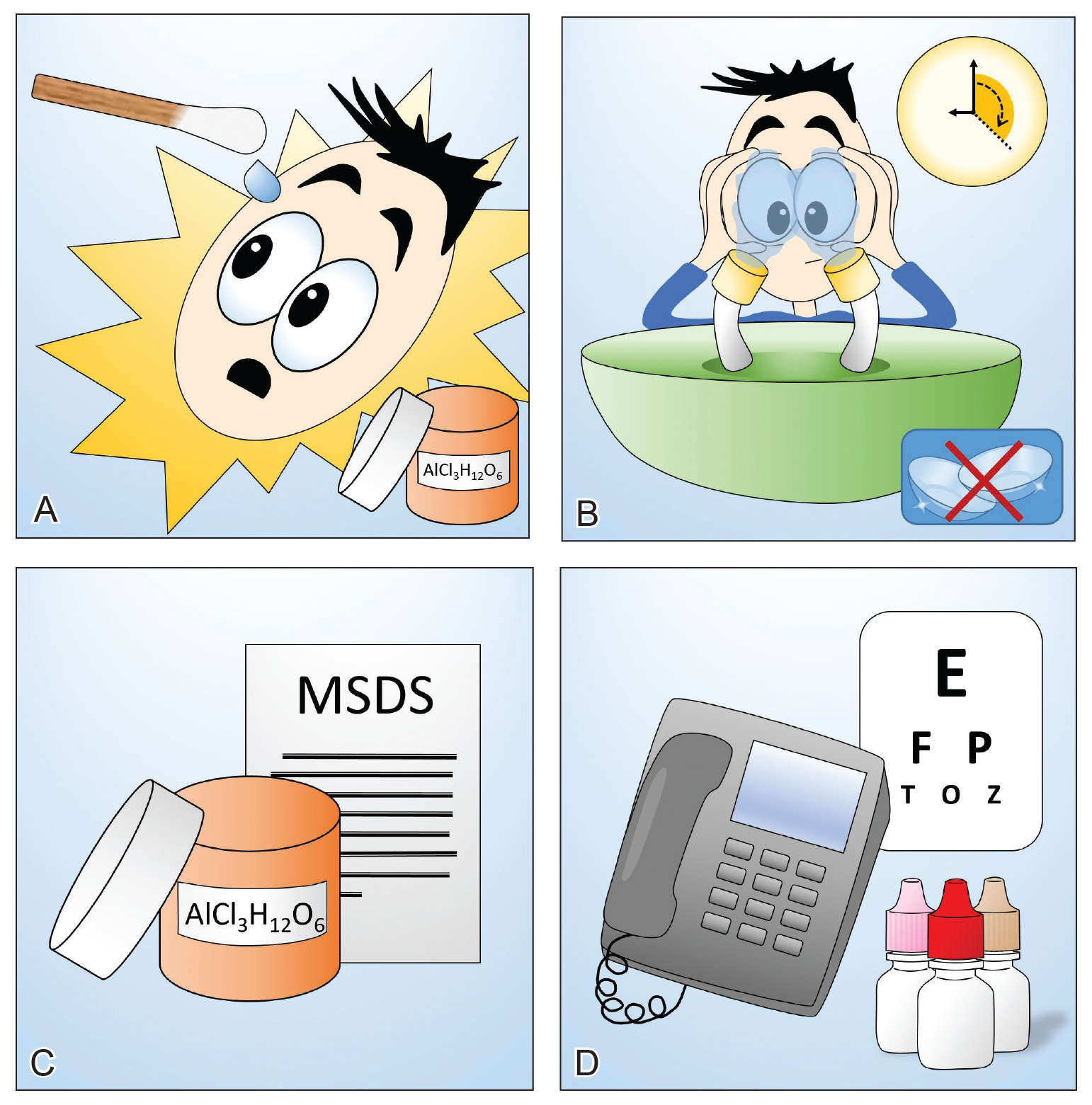
obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
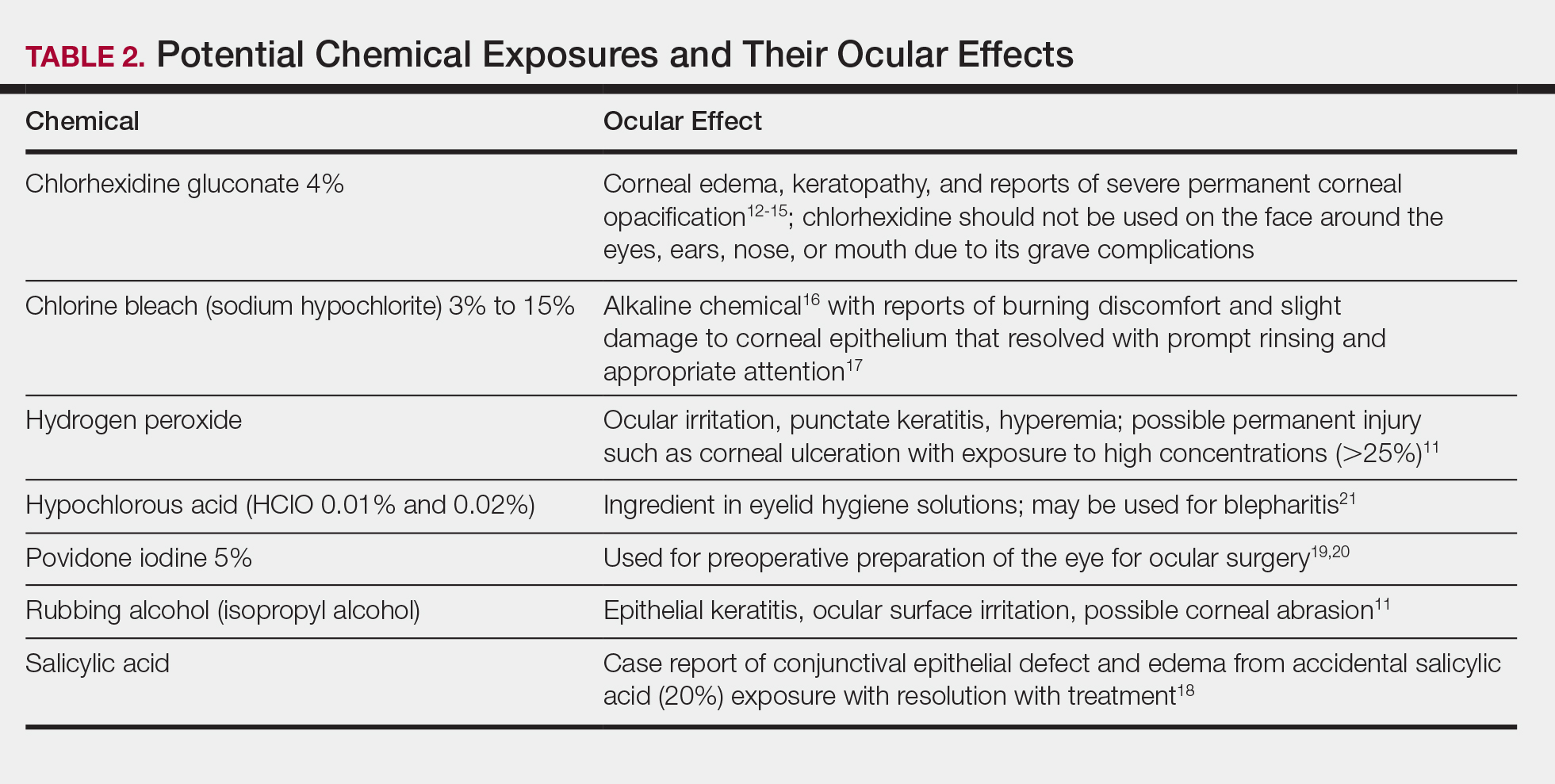
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27
Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
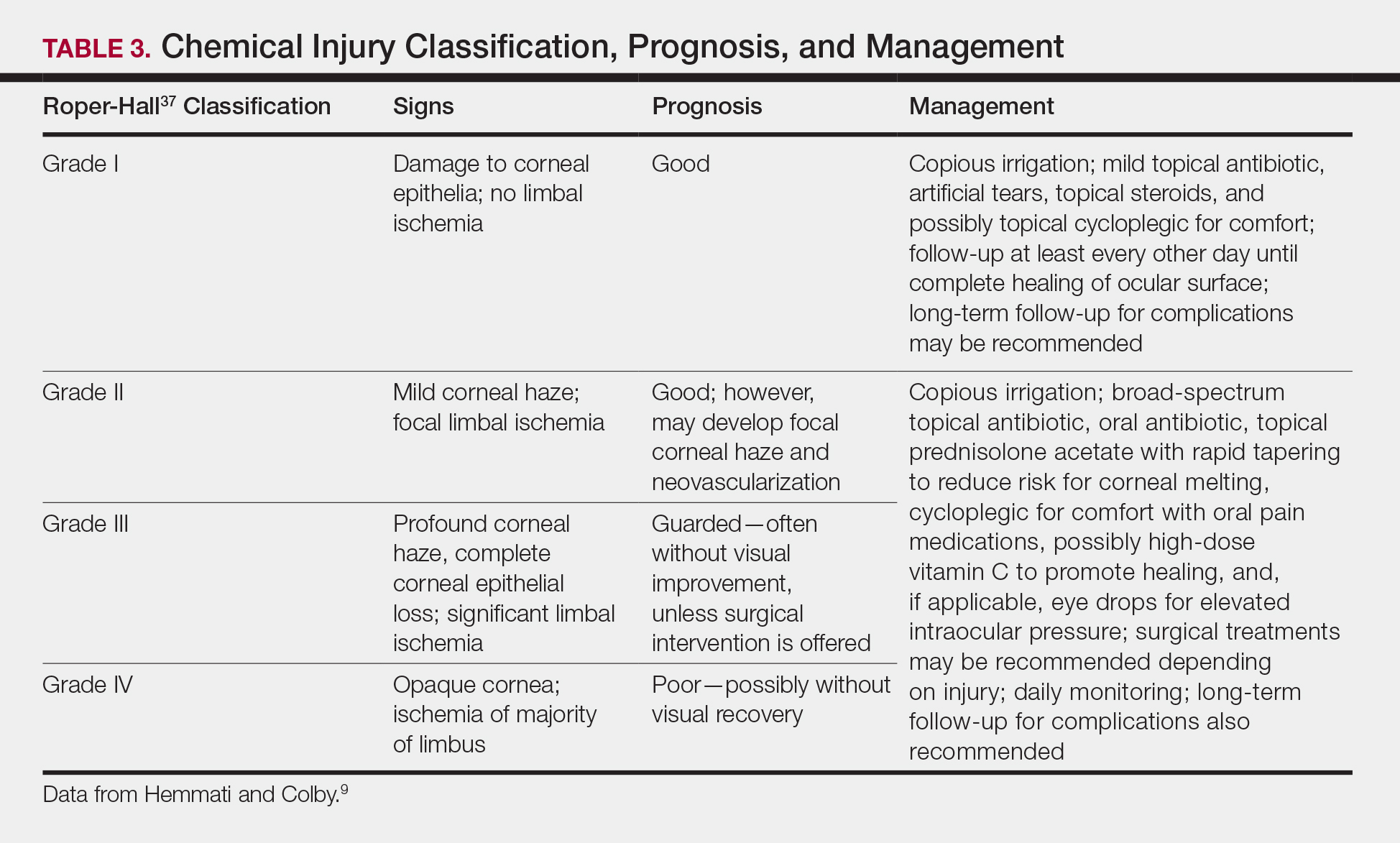
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9
Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).


obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27

Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9

Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
Many dermatologic procedures are performed on the face, such as skin biopsies, surgical excisions, and cosmetic procedures, which can increase the risk for accidental ocular injuries.1,2 Ocular chemical burns have been reported to account for approximately 3% to 20% of ocular injuries3,4 and are one of the few ocular emergencies dermatologists may encounter in practice. Given the potentially severe consequences of permanent vision changes or loss, it is important to take precautionary steps in preventing chemical exposures and know how to appropriately manage ophthalmic emergencies when they occur.1,5-8 In this article, we describe a patient with a transient ocular chemical injury from exposure to aluminum chloride hexahydrate that completely resolved with immediate care. We also offer practical guidance for the general dermatologist in the acute management of acidic chemical burns to the eye, highlighting immediate copious irrigation as the most important step in preventing severe permanent damage. Given that aluminum chloride hexahydrate is an acidic solution, we focus predominantly on the approach to acidic chemical exposures to the eye.
Case Report
A 61-year-old woman was seen in the dermatology outpatient clinic for a shave biopsy on the left cheek followed by aluminum chloride application for hemostasis. Following the biopsy, the patient stated she felt the sensation that something had dripped into the left eye and she felt a burning pain. There was a 30- to 60-second delay in irrigation of the eye, as it was at first unclear what had occurred. The patient reported an increased burning sensation, and at that point she was instructed to begin flushing the eye with tap water from the examination room sink for 15 to 20 minutes; she wanted to stop irrigation after a few minutes, and convincing her to continue thorough irrigation was somewhat challenging. It was determined that aluminum chloride hexahydrate had dripped from an oversaturated cotton swab in transit from the tray to the biopsy site.
The patient was urgently directed to the ophthalmology clinic and evaluated by an ophthalmologist within 1 to 2 hours of chemical exposure. Visual acuity of the affected left eye was noted to be 20/30 -2 with correctional glasses, and slit lamp examination revealed moderate injection of the conjunctiva and sclera, and at least 3 punctate epithelial erosions and punctate staining of the inferior aspects of the cornea, consistent with a chemical injury. The remaining ocular examination was normal for both eyes. She was diagnosed with keratitis of the left eye from chemical exposure to aluminum chloride and was prescribed loteprednol etabonate ophthalmic suspension 0.5% and tobramycin ophthalmic solution 0.3% to be applied to the left eye 4 times daily, with follow-up 4 days later.
At follow-up, the patient denied any pain, though she was not using the prescribed eye drops consistently. On examination, the patient showed improvement in visual acuity to 20/20 -2 and complete resolution of the keratitis, with slit lamp examination showing clear conjunctiva, sclera, and cornea. Given complete resolution, the eye drops were discontinued.
Comment
Factors Contributing to Ocular Chemical Injuries
Chemical burns to the eyes during cosmetic or surgical procedures are one of the few acute ocular emergencies dermatologists may encounter in practice. If not managed properly, the eye may be permanently damaged. Therefore, dermatologists must be confident in the initial management of ocular chemical burns (Table 1; Figure).


obtain the material safety data sheet. D, Refer the patient urgently to ophthalmology for a visual acuity test and treatment. Images courtesy of Deborah J. Moon, MD (Los Angeles, California).
Mechanism of Ocular Chemical Burns
The extent of injury is predominantly determined by 2 factors: (1) the chemical properties of the substance, and (2) the length of exposure.5,9,10 Potential chemical exposures and their reported ocular effects are listed in Table 2.11-21 Alkaline chemical burns often have the gravest outcome, as they can rapidly penetrate into the internal ocular structures, potentially leading to cataracts and glaucoma.9 Hydroxyl ions, often found in alkaline chemicals, are capable of rapidly denaturing the corneal matrix and triggering release of proteolytic enzymes through a series of inflammatory responses. Conversely, ocular damage from most acidic chemicals often is limited to the more superficial structures, such as the cornea and conjunctiva, given that acids may cause corneal proteins to coagulate, thus forming a barrier that slows further penetration into deeper structures.9 Nonetheless, corneal damage can still have a devastating impact on visual acuity, as the cornea provides 65% to 75% of the eye’s total focusing power.22 For both alkaline and acidic chemicals, immediate profuse irrigation is most critical in determining the clinical course.23-26 To provide perspective, potent alkaline chemicals may penetrate into the anterior chamber of the eye within 15 seconds,9 and delayed initiation of irrigation by even 5 to 15 minutes may lead to irreversible intraocular damage.27

Symptoms of Ocular Chemical Exposure
Signs and symptoms associated with ocular chemical exposures include erythema, pain, tearing, photosensitivity, eyelid swelling, foreign body sensation, changes in vision, and corneal clouding.3,5,9,28 Specifically, aluminum chloride hexahydrate, a hemostatic agent commonly used by dermatologists, has potentially caused eye irritation and conjunctivitis, according to its material safety data sheet,29 as well as blepharospasms, transient disturbances in corneal epithelium, and a persistent faint nebula in the corneal stroma.30 Similar antiperspirants also showed damaging effects to bovine lenses, ocular irritation, and subjective reports of burning and watery eyes.31-33
Immediate Management
If potential chemical exposure to the eye is suspected either by the health care provider or patient, immediately irrigate the affected eye(s) for at least 15 to 30 minutes (longer for alkaline burns) with at least 1 to 2 L of irrigation fluid until the pH is between 7 and 7.2.3-5,9,27,34,35 Irrigation fluids reported to be used include normal saline, Ringer lactate solution, normal saline with sodium bicarbonate, and balanced salt solution.5 If no solutions are readily available, immediate irrigation with tap water is sufficient for diluting and washing away the chemical and has been reported to have better clinical outcomes than delaying irrigation.5,24-26 Studies have shown that prolonged irrigation corresponded with reduced severity, shortened healing time, shorter in-hospital treatment duration, and quicker return to work.5,26
If an eye wash station is not available, the patient can gently flush the eye under a sink faucet set to a gentle stream of lukewarm water.6,7 The health care provider also may manually irrigate the eye. Necessary equipment includes a large syringe or clean eyecup, irrigating fluid, local anesthetic drops for comfort, a towel to soak up excessive fluid, and a bowl or kidney dish to collect the irrigated fluid.34 Providers should first wash their hands. If necessary, anesthetic eye drops may be added for comfort. Lay a towel over the patient’s neck and shoulders and position the patient at a comfortable angle. Place a bowl adjacent to the patient’s cheek to collect the irrigating fluid and have the patient tilt his/her head such that the irrigated fluid would flow into the bowl. Pour a steady stream of the irrigating fluid over the eye from a height of no more than 5 cm.6,7,34
During irrigation, ensure that the patient’s eye(s) is wide open and that all ocular surfaces, including the area underneath the eyelids, are thoroughly washed; everting the eyelids may be beneficial. Ask the patient to move his/her eye(s) in all directions while irrigating. If available, place a litmus strip in the conjunctival fornix to ensure that the goal pH of 7 to 7.2 is reached.9 The pH should be rechecked every 15 to 30 minutes to ensure there has been no change, as hidden crystalized chemical particles may continue to elute chemicals, causing further injury.3 Contact lenses, if present, should be removed as soon as practical, as lenses can trap chemicals; however, immediate initiation of irrigation should not be delayed8 (Table 1).
Identify and verify the chemical suspected to have been exposed to the patient’s eye. The material safety data sheet, which may often be found online if a hard copy is not available, may provide valuable information for the ophthalmologist.36 After thorough irrigation, refer the patient urgently to ophthalmology or the emergency department for prompt evaluation. The emergency department is frequently equipped with polymethylmethacrylate scleral lenses, also called Morgan Lens, which consist of a plastic lens connected via tubing to a bag of irrigation fluid (eg, Ringer lactate solution), allowing for prolonged continuous irrigation of the conjunctiva and cornea. The ophthalmologist will conduct a visual acuity test and complete a thorough eye examination to assess the extent of ischemic injury to the conjunctiva or sclera and damage to the corneal epithelium and internal ocular structures.9
Generally, topical antibiotics, artificial tears, and topical steroids may be provided to patients with mild injury with close follow-up.9,37 For higher-grade injuries, broad-spectrum topical antibiotics, oral antibiotics, topical corticosteroids, vitamin C, and surgical treatments may be additionally recommended (Table 3). Long-term follow-up may be recommended by the ophthalmologist to monitor for potential late complications, such as glaucoma from damage to the trabecular meshwork, corneal abnormalities and limbal stem cell deficiency, symblepharon formation, or eyelid abnormalities.9

Conclusion
We report a case of a transient chemical burn to the eye secondary to exposure to aluminum chloride hexahydrate. Complete resolution of the injury was achieved with prompt irrigation and urgent medical management by ophthalmology. This case emphasizes the potential for ocular emergencies in the dermatology setting and highlights the steps for appropriate management should a chemical burn to the eye occur. We emphasize the importance of immediate profuse irrigation for 15 to 30 minutes and urgent evaluation by an ophthalmologist. Dermatologists should be cognizant of potential hazards to the eye during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
- Ricci LH, Navajas SV, Carneiro PR, et al. Ocular adverse effects after facial cosmetic procedures: a review of case reports. J Cosmet Dermatol. 2015;14:145-151.
- Boonsiri M, Marks KC, Ditre CM. Benzocaine/lidocaine/tetracainecream: report of corneal damage and review. J Clin Aesthet Dermatol. 2016;9:48-50.
- Gelston CD. Common eye emergencies. Am Fam Physician. 2013;88:515-519.
- Sharma N, Kaur M, Agarwal T, et al. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235.
- Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- Sears W, Sears M, Sears R, et al. The Portable Pediatrician: Everything You Need to Know About Your Child’s Health. New York, NY: Little, Brown and Company; 2011.
- Kuckelkorn R, Schrage N, Keller G, et al. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80:4-10.
- Schulte PA, Ahlers HW, Jackson LL, et al. Contact Lens Use in a Chemical Environment. Cincinnati, OH: National Institute for Occupational Safety and Health, US Department of Health and Human Services; 2005. NIOSH publication 2005-139.
- Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet. October 2012. https://www.aao.org/eyenet/article/treating-acute-chemical-injuries-of-cornea. Accessed May 28, 2019.
- Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26:689-699.
- Gattey D. Chemical-induced ocular side effects. In: Fraunfelder FT, Fraunfelder FW, Chambers WA, eds. Clinical Ocular Toxicology. Edinburgh, Scotland: W.B. Saunders; 2008:289-306.
- Apt L, Isenberg SJ. Hibiclens keratitis. Am J Ophthalmol. 1987;104:670-671.
- Tabor E, Bostwick DC, Evans C. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA. 1989;261:557-558.
- Galor A, Jeng BH, Lowder CY. A curious case of corneal edema. Eyenet. January 2007. https://www.aao.org/eyenet/article/curious-case-of-corneal-edema. Accessed May 28, 2019.
- Hamed LM, Ellis FD, Boudreault G, et al. Hibiclens keratitis. Am J Ophthalmol. 1987;104:50-56.
- Haring R, Sheffield ID, Channa R, et al. Epidemiologic trends of chemical ocular burns in the United States. JAMA Ophthalmol. 2016;134:1119-1124.
- Racioppi F, Daskaleros PA, Besbelli N, et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem Toxicol. 1994;32:845-861.
- Shazly TA. Ocular acid burn due to 20% concentrated salicylic acid. Cutan Ocul Toxicol. 2011;30:84-86.
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769-1775.
- Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery: III. effect of povidone-iodine on the conjunctiva. Arch Ophthalmol. 1984;102:728-729.
- Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707-714.
- Paul M, Sieving A. Facts about the cornea and corneal disease. National Eye Institute, National Institutes of Health website. https://nei.nih.gov/health/cornealdisease. Accessed May 20, 2019.
- Khaw P, Shah P, Elkington A. Injury to the eye. BMJ. 2004;328:36-38.
- Duffy B. Managing chemical eye injuries: Bernice Duffy says initial management of potentially devastating chemical eye injuries by emergency nurses can affect patients’ future prognosis as much as subsequent ophthalmic treatment. Emerg Nurse. 2008;16:25-30.
- Burns F, Paterson C. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58:33-36.
- Ikeda N, Hayasaka S, Hayasaka Y, et al. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220:225-228.
- Eslani M, Baradaran-Rafii A, Movahedan A, et al. The ocular surface chemical burns. J Ophthalmol. 2014;2014:196827.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician. 2007;76:829-836.
- Drysol. MSDS No. BLVCL; Glendale, CA: Person & Covey Inc; March 9, 1991. http://msdsreport.com/msds/blvcl. Accessed May 20, 2019.
- Grant WM, Schuman JS. Toxicology of the Eye: Effects on the Eyes and Visual System From Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: Also Systemic Side Effects From Eye Medications. Vol 1. Springfield, IL: Charles C. Thomas Publisher; 1993.
- Wong W, Sivak JG, Moran KL. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicol In Vitro. 2003;17:785-790.
- Donahue DA, Kaufman LE, Avalos J, et al. Survey of ocular irritation predictive capacity using chorioallantoic membrane vascular assay (CAMVA) and bovine corneal opacity and permeability (BCOP) test historical data for 319 personal care products over fourteen years. Toxicol In Vitro. 2011;25:563-572.
- Groot AC, Nater JP, Lender R, et al. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9:255-259.
- Stevens S. Ophthalmic practice. Community Eye Health. 2005;18:109-110.
- Hoyt KS, Haley RJ. Innovations in advanced practice: assessment and management of eye emergencies. Adv Emerg Nurs J. 2005;27:101-117.
- LaDou J, Harrison RJ, eds. CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine. 5th ed. New York, NY: McGraw-Hill Education; 2013.
- Roper-Hall M. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631-653.
Practice Points
- Dermatologists should be cognizant of potential hazards to the eyes during facial procedures and always take proper precautions to decrease the risk for ocular injuries.
- If a patient’s eye(s) becomes exposed to a chemical during a dermatologic procedure, immediate copious irrigation for at least 15 to 30 minutes (longer for alkaline burns) is crucial, followed by prompt evaluation by an ophthalmologist.
- The patient should be instructed to manually hold open the eye and move the eyeball in all directions to achieve the most effective irrigation of the chemical.
- If the patient is wearing contact lenses, they should be removed promptly, but do not delay the irrigation to do so. Lenses should be removed once irrigation is underway.
The great sunscreen ingredient debate
In a commentary issued on May 6, the Food and Drug Administration stated that “with sunscreens now being used with greater frequency, in larger amounts, and by broader populations, it is more important than ever to ensure that sunscreens are safe and effective for daily, lifelong use.” The statement coincided with the publication of the randomized study, “Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients,” by Matta et al. of the FDA and others in JAMA (2019 May 6. doi: 10.1001/jama.2019.5586). A maximal usage trial examines the systemic absorption of a topical drug when used according to the guidelines given for the product’s maximum usage. In this study, adult participants were randomized to one of four commercially available sunscreen products: spray 1 (n = 6), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two mg of sunscreen per 1 cm2 was applied to 75% of body surface area four times per day for 4 days, and blood samples were collected from each individual over 7 days.
The FDA’s guidance for industry and proposed rule on OTC sunscreens state that active ingredients with systemic absorption at 0.5 ng/mL or higher or with possible safety concerns need to undergo further nonclinical toxicology assessment to evaluate risk of systemic carcinogenicity, developmental/reproductive abnormalities, or other adverse effects.
Absorption of some sunscreen ingredients has been detected in other studies. Despite systemic absorption, two active ingredients – zinc oxide and titanium dioxide – have been found by the FDA to be generally recognized as safe and effective. But for 12 other active ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there are insufficient data to make a “generally recognized as safe and effective” determination; thus, more data have been requested from the manufacturers. While physical blocking sunscreens have improved in their UV-blocking ability without compromising cosmesis over the past several years, some sunscreens containing chemical blockers are able to achieve higher SPFs with good cosmesis when applied to the skin.
Our skin acts as the ultimate barrier between ourselves and the environment, and it is not uncommon for substances to be blocked, absorbed, or excreted from the skin. Absorption of an ingredient through the skin and into the body does not indicate that the ingredient is unsafe. Rather, findings such as these call for further testing and research to determine the safety of that ingredient with repeated use. Per the FDA, such testing is part of the standard premarket safety evaluation of most chronically administered drugs with appreciable systemic absorption.
In February 2019, the FDA’s proposed rule was issued to “update regulatory requirements for most sunscreen products in the United States,” with the goal of bringing OTC sunscreens “up to date with the latest scientific standards,” according to the FDA May 6 commentary. “As part of this rule, the FDA is asking industry and other interested parties for additional safety data on the 12 active sunscreen ingredients currently available in marketed products” mentioned previously. These rules are being put into place to address the “key data gap” for these 12 ingredients, which is “understanding whether, and to what extent the ingredient is absorbed into the body after topical application.”
In other previously published studies, oxybenzone, along with some other sunscreen active ingredients including octocrylene, have been found in human breast milk. In addition, oxybenzone has been detected in amniotic fluid, urine, and blood. Whether these findings have any clinical implications needs to be further assessed. Some studies in the literature have raised questions about the potential for oxybenzone to affect endocrine activity.
Another issue that has been raised is the potential impact of sunscreen on the environment, specifically, coral reefs. In July 2018, Hawaii Governor David Ige (D) signed a bill (SB 2571) that bans the sale of sunscreens containing oxybenzone and octinoxate beginning in 2021, making Hawaii the first state to ban the sale of sunscreens containing these two chemicals. Shortly afterward, the Republic of Palau and city of Key West, Fla., also took action to ban sunscreens containing chemicals potentially harmful to marine life. In Hawaii, what’s know as “reef safe” sunscreen is sold.
More research in this area is needed, but studies have linked these ingredients to harming coral by bleaching, disease, and damage to DNA, and also to decreasing fertility in fish, impairing algae growth, inducing defects in mussel and sea urchin young, and accumulating in the tissues of dolphins. According to NASA, as much as 27% of monitored reef formation have already been lost and over the following 32 years, 32% more are at risk. Reefs cover a mere 0.2% of the ocean’s floor, but it is estimated that reefs are home to and protect nearly 1 million species of fish, invertebrates, and algae.
In early May, Rep. Tulsi Gabbard (D-Hawaii) and Sen. Tim Ryan (D-Ohio) introduced legislation known as the Oxybenzone and Octinoxate Impact Study Act of 2019 (H.R. 2588) to require the Environmental Protection Agency to study the impact of those two chemicals on human health and the environment and to provide findings to Congress and the public within 18 months.
The importance of sun protection and prevention of sunburns is paramount. We know that multiple sunburn events during childhood double a child’s risk of developing skin cancer later in life, and skin cancer is the most common cancer diagnosed in the United States, with 5 million cases treated every year. One in five Americans will develop skin cancer by age 70 years.
As a Mohs and a cosmetic dermatologic surgeon, I appreciate the unquestionable protective effects of sunscreen products with regards to skin cancer, dyspigmentation, solar elastosis, and rhytids associated with photoaging. We can applaud the FDA for improving testing and regulation of OTC ingredients, including those in sunscreen. These types of studies are important and monumental in ensuring that we are utilizing the right type of ingredients to protect our patients, our oceans, and our reefs.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
- Matta MK et al. JAMA. 2019 May 6. doi: 10.1001/jama.2019.5586.
- Shedding new light on sunscreen absorption, by Janet Woodcock, MD, director, Center for Drug Evaluation and Research, and Theresa M. Michele, MD, director, CDER’s Division of Nonprescription Drug Products, Office of New Drugs
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed Regist. 2019;84(38):6204-75.
- Schlumpf M et al. Chemosphere. 2010 Nov;81(10):1171-83.
- Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
In a commentary issued on May 6, the Food and Drug Administration stated that “with sunscreens now being used with greater frequency, in larger amounts, and by broader populations, it is more important than ever to ensure that sunscreens are safe and effective for daily, lifelong use.” The statement coincided with the publication of the randomized study, “Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients,” by Matta et al. of the FDA and others in JAMA (2019 May 6. doi: 10.1001/jama.2019.5586). A maximal usage trial examines the systemic absorption of a topical drug when used according to the guidelines given for the product’s maximum usage. In this study, adult participants were randomized to one of four commercially available sunscreen products: spray 1 (n = 6), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two mg of sunscreen per 1 cm2 was applied to 75% of body surface area four times per day for 4 days, and blood samples were collected from each individual over 7 days.
The FDA’s guidance for industry and proposed rule on OTC sunscreens state that active ingredients with systemic absorption at 0.5 ng/mL or higher or with possible safety concerns need to undergo further nonclinical toxicology assessment to evaluate risk of systemic carcinogenicity, developmental/reproductive abnormalities, or other adverse effects.
Absorption of some sunscreen ingredients has been detected in other studies. Despite systemic absorption, two active ingredients – zinc oxide and titanium dioxide – have been found by the FDA to be generally recognized as safe and effective. But for 12 other active ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there are insufficient data to make a “generally recognized as safe and effective” determination; thus, more data have been requested from the manufacturers. While physical blocking sunscreens have improved in their UV-blocking ability without compromising cosmesis over the past several years, some sunscreens containing chemical blockers are able to achieve higher SPFs with good cosmesis when applied to the skin.
Our skin acts as the ultimate barrier between ourselves and the environment, and it is not uncommon for substances to be blocked, absorbed, or excreted from the skin. Absorption of an ingredient through the skin and into the body does not indicate that the ingredient is unsafe. Rather, findings such as these call for further testing and research to determine the safety of that ingredient with repeated use. Per the FDA, such testing is part of the standard premarket safety evaluation of most chronically administered drugs with appreciable systemic absorption.
In February 2019, the FDA’s proposed rule was issued to “update regulatory requirements for most sunscreen products in the United States,” with the goal of bringing OTC sunscreens “up to date with the latest scientific standards,” according to the FDA May 6 commentary. “As part of this rule, the FDA is asking industry and other interested parties for additional safety data on the 12 active sunscreen ingredients currently available in marketed products” mentioned previously. These rules are being put into place to address the “key data gap” for these 12 ingredients, which is “understanding whether, and to what extent the ingredient is absorbed into the body after topical application.”
In other previously published studies, oxybenzone, along with some other sunscreen active ingredients including octocrylene, have been found in human breast milk. In addition, oxybenzone has been detected in amniotic fluid, urine, and blood. Whether these findings have any clinical implications needs to be further assessed. Some studies in the literature have raised questions about the potential for oxybenzone to affect endocrine activity.
Another issue that has been raised is the potential impact of sunscreen on the environment, specifically, coral reefs. In July 2018, Hawaii Governor David Ige (D) signed a bill (SB 2571) that bans the sale of sunscreens containing oxybenzone and octinoxate beginning in 2021, making Hawaii the first state to ban the sale of sunscreens containing these two chemicals. Shortly afterward, the Republic of Palau and city of Key West, Fla., also took action to ban sunscreens containing chemicals potentially harmful to marine life. In Hawaii, what’s know as “reef safe” sunscreen is sold.
More research in this area is needed, but studies have linked these ingredients to harming coral by bleaching, disease, and damage to DNA, and also to decreasing fertility in fish, impairing algae growth, inducing defects in mussel and sea urchin young, and accumulating in the tissues of dolphins. According to NASA, as much as 27% of monitored reef formation have already been lost and over the following 32 years, 32% more are at risk. Reefs cover a mere 0.2% of the ocean’s floor, but it is estimated that reefs are home to and protect nearly 1 million species of fish, invertebrates, and algae.
In early May, Rep. Tulsi Gabbard (D-Hawaii) and Sen. Tim Ryan (D-Ohio) introduced legislation known as the Oxybenzone and Octinoxate Impact Study Act of 2019 (H.R. 2588) to require the Environmental Protection Agency to study the impact of those two chemicals on human health and the environment and to provide findings to Congress and the public within 18 months.
The importance of sun protection and prevention of sunburns is paramount. We know that multiple sunburn events during childhood double a child’s risk of developing skin cancer later in life, and skin cancer is the most common cancer diagnosed in the United States, with 5 million cases treated every year. One in five Americans will develop skin cancer by age 70 years.
As a Mohs and a cosmetic dermatologic surgeon, I appreciate the unquestionable protective effects of sunscreen products with regards to skin cancer, dyspigmentation, solar elastosis, and rhytids associated with photoaging. We can applaud the FDA for improving testing and regulation of OTC ingredients, including those in sunscreen. These types of studies are important and monumental in ensuring that we are utilizing the right type of ingredients to protect our patients, our oceans, and our reefs.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
- Matta MK et al. JAMA. 2019 May 6. doi: 10.1001/jama.2019.5586.
- Shedding new light on sunscreen absorption, by Janet Woodcock, MD, director, Center for Drug Evaluation and Research, and Theresa M. Michele, MD, director, CDER’s Division of Nonprescription Drug Products, Office of New Drugs
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed Regist. 2019;84(38):6204-75.
- Schlumpf M et al. Chemosphere. 2010 Nov;81(10):1171-83.
- Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
In a commentary issued on May 6, the Food and Drug Administration stated that “with sunscreens now being used with greater frequency, in larger amounts, and by broader populations, it is more important than ever to ensure that sunscreens are safe and effective for daily, lifelong use.” The statement coincided with the publication of the randomized study, “Effect of sunscreen application under maximal use conditions on plasma concentrations of sunscreen active ingredients,” by Matta et al. of the FDA and others in JAMA (2019 May 6. doi: 10.1001/jama.2019.5586). A maximal usage trial examines the systemic absorption of a topical drug when used according to the guidelines given for the product’s maximum usage. In this study, adult participants were randomized to one of four commercially available sunscreen products: spray 1 (n = 6), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two mg of sunscreen per 1 cm2 was applied to 75% of body surface area four times per day for 4 days, and blood samples were collected from each individual over 7 days.
The FDA’s guidance for industry and proposed rule on OTC sunscreens state that active ingredients with systemic absorption at 0.5 ng/mL or higher or with possible safety concerns need to undergo further nonclinical toxicology assessment to evaluate risk of systemic carcinogenicity, developmental/reproductive abnormalities, or other adverse effects.
Absorption of some sunscreen ingredients has been detected in other studies. Despite systemic absorption, two active ingredients – zinc oxide and titanium dioxide – have been found by the FDA to be generally recognized as safe and effective. But for 12 other active ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there are insufficient data to make a “generally recognized as safe and effective” determination; thus, more data have been requested from the manufacturers. While physical blocking sunscreens have improved in their UV-blocking ability without compromising cosmesis over the past several years, some sunscreens containing chemical blockers are able to achieve higher SPFs with good cosmesis when applied to the skin.
Our skin acts as the ultimate barrier between ourselves and the environment, and it is not uncommon for substances to be blocked, absorbed, or excreted from the skin. Absorption of an ingredient through the skin and into the body does not indicate that the ingredient is unsafe. Rather, findings such as these call for further testing and research to determine the safety of that ingredient with repeated use. Per the FDA, such testing is part of the standard premarket safety evaluation of most chronically administered drugs with appreciable systemic absorption.
In February 2019, the FDA’s proposed rule was issued to “update regulatory requirements for most sunscreen products in the United States,” with the goal of bringing OTC sunscreens “up to date with the latest scientific standards,” according to the FDA May 6 commentary. “As part of this rule, the FDA is asking industry and other interested parties for additional safety data on the 12 active sunscreen ingredients currently available in marketed products” mentioned previously. These rules are being put into place to address the “key data gap” for these 12 ingredients, which is “understanding whether, and to what extent the ingredient is absorbed into the body after topical application.”
In other previously published studies, oxybenzone, along with some other sunscreen active ingredients including octocrylene, have been found in human breast milk. In addition, oxybenzone has been detected in amniotic fluid, urine, and blood. Whether these findings have any clinical implications needs to be further assessed. Some studies in the literature have raised questions about the potential for oxybenzone to affect endocrine activity.
Another issue that has been raised is the potential impact of sunscreen on the environment, specifically, coral reefs. In July 2018, Hawaii Governor David Ige (D) signed a bill (SB 2571) that bans the sale of sunscreens containing oxybenzone and octinoxate beginning in 2021, making Hawaii the first state to ban the sale of sunscreens containing these two chemicals. Shortly afterward, the Republic of Palau and city of Key West, Fla., also took action to ban sunscreens containing chemicals potentially harmful to marine life. In Hawaii, what’s know as “reef safe” sunscreen is sold.
More research in this area is needed, but studies have linked these ingredients to harming coral by bleaching, disease, and damage to DNA, and also to decreasing fertility in fish, impairing algae growth, inducing defects in mussel and sea urchin young, and accumulating in the tissues of dolphins. According to NASA, as much as 27% of monitored reef formation have already been lost and over the following 32 years, 32% more are at risk. Reefs cover a mere 0.2% of the ocean’s floor, but it is estimated that reefs are home to and protect nearly 1 million species of fish, invertebrates, and algae.
In early May, Rep. Tulsi Gabbard (D-Hawaii) and Sen. Tim Ryan (D-Ohio) introduced legislation known as the Oxybenzone and Octinoxate Impact Study Act of 2019 (H.R. 2588) to require the Environmental Protection Agency to study the impact of those two chemicals on human health and the environment and to provide findings to Congress and the public within 18 months.
The importance of sun protection and prevention of sunburns is paramount. We know that multiple sunburn events during childhood double a child’s risk of developing skin cancer later in life, and skin cancer is the most common cancer diagnosed in the United States, with 5 million cases treated every year. One in five Americans will develop skin cancer by age 70 years.
As a Mohs and a cosmetic dermatologic surgeon, I appreciate the unquestionable protective effects of sunscreen products with regards to skin cancer, dyspigmentation, solar elastosis, and rhytids associated with photoaging. We can applaud the FDA for improving testing and regulation of OTC ingredients, including those in sunscreen. These types of studies are important and monumental in ensuring that we are utilizing the right type of ingredients to protect our patients, our oceans, and our reefs.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
- Matta MK et al. JAMA. 2019 May 6. doi: 10.1001/jama.2019.5586.
- Shedding new light on sunscreen absorption, by Janet Woodcock, MD, director, Center for Drug Evaluation and Research, and Theresa M. Michele, MD, director, CDER’s Division of Nonprescription Drug Products, Office of New Drugs
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed Regist. 2019;84(38):6204-75.
- Schlumpf M et al. Chemosphere. 2010 Nov;81(10):1171-83.
- Krause M et al. Int J Androl. 2012 Jun;35(3):424-36.
A primer on cannabis for cosmeceuticals: Research and treatments for particular skin conditions
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at [email protected].
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at [email protected].
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at [email protected].
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
Infographic: Hyperhidrosis Survey Results
Hyperhidrosis: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on hyperhidrosis. Here’s what we found.
In which areas do patients report hyperhidrosis most frequently?
Nearly 70% of dermatologists see patients with hyperhidrosis of the axillae, followed by the palms and soles (27%). Only 4% of dermatologists indicated that they see hyperhidrosis all over the body.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Hyperhidrosis affects up to 5% of the US population and may remarkably affect quality of life. Primary hyperhidrosis accounts for 93% of cases. Before puberty, hyperhidrosis affects the palms and soles in up to 90% of patients. In adults, the axillae are most commonly affected (51%), followed by plantar (30%), palmar (24%), and facial (10%) areas (Strutton et al).
Next page: Topical treatment
Approximately what percentage of patients are satisfied with topical treatments for hyperhidrosis?
The majority of dermatologists (88%) reported that less than half of their patients are satisfied with topical treatments for hyperhidrosis. Only 12% indicated that 51% to 70% of their patients were satisfied, and none of the respondents indicated that >70% were satisfied.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
There is clearly a need for safe and effective treatments for hyperhidrosis. Treatment of hyperhidrosis should include lifestyle and behavioral modifications. It is helpful to try to avoid hot crowded rooms when feasible, as well as stress, tight clothing, occlusive shoes, alcohol, and spicy foods. Patients should be instructed on proper use of medications, as well as the need to continue therapy for maintenance. Patients should be encouraged to follow up for alternative treatment options in cases of therapy failure.
Next page: Botulinum toxin
On average, how long do the effects of botulinum toxin last in your axillary hyperhidrosis patients?
The effects of botulinum toxin last at least 4 months and up to 6 months in most patients, according to 58% of dermatologists surveyed. Thirty percent reported 2 to 4 months, and 13% reported more than 6 months.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
OnabotulinumtoxinA is approved by the US Food and Drug Administration for severe primary axillary hyperhidrosis. Injections are ideally placed at the dermal-subcutaneous junction, with 1 unit placed every 1 to 2 cm. Dosing is 50 to 100 U per axilla with higher dosing required for the palms and soles (off label). Reported efficacy for axillary hyperhidrosis is 82% to 87%; however, 50% of patients with plantar hyperhidrosis are dissatisfied with the treatment. Sweat reduction is most apparent after 2 weeks and typically persists 6 to 8 months in clinical trials (Botox package insert).
Next page: Systemic anticholinergics
When prescribing systemic anticholinergics for hyperhidrosis, what side effect is most common among your patients?
More than three-quarters of dermatologists (81%) reported that dry mouth is the most common side effect of systemic anticholinergics. Dry eyes is the second most common side effect (15%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Systemic anticholinergics are commonly used off label for the treatment of hyperhidrosis. Adverse effects include dry mouth, blurred vision, dry eyes, orthostatic hypotension, gastrointestinal, urinary retention, tachycardia, and drowsiness. Unfortunately, these side effects cause one-third of patients to discontinue treatment (Bajaj and Langtry). A slow escalation of the dose may increase tolerability and reduce these side effects. These anticholinergics should not be taken with other medications with anticholinergic activity to avoid exacerbating these side effects.
Next page: Surgical treatment
What percentage of patients require surgery for treatment of hyperhidrosis after topical, injectable, systemic options and devices have failed?
According to 62% of dermatologists, 10% or less of patients require surgery for treatment of hyperhidrosis after other therapies have failed. Almost one-third indicated that none of their patients require surgical treatment. None of the dermatologists surveyed reported that more than 60% of patients need surgery.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Surgery is an option to treat hyperhidrosis when conservative methods have failed. Surgical therapies include curettage, liposuction, and excision. A last resort is considered sympathectomy. Endoscopic thoracic sympathectomy is employed for palmar, facial, and axillary hyperhidrosis, while endoscopic lumbar sympathectomy is indicated for plantar hyperhidrosis.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Patients with focal idiopathic hyperhidrosis of the axillae as well as palms/soles report that this condition interferes with the quality of life in major ways, from social interactions to professional interactions. They often don't even know they have a problem and internalize that they must be overly anxious about things. I have patients that buy 3 of the same shirts and change a few times a day, costing a great deal of money (plus cleaning bills for 3 shirts as well) and costing a great deal of wasted time when they could be doing something more productive. It's great that not only botulinum toxins can be helpful for the underarms but also even less-invasive topical anticholinergics (easy to use, no discomfort, predictable, and helping make treatment for axillary hyperhidrosis much more on the radar).—Joel L. Cohen, MD (Denver, Colorado)
More and more patients are presenting to request relief from hyperhidrosis, and increasingly in nontraditional areas (ie, areas other than the axilla and forehead). These include the palms and scalp most commonly, and then the breast, chest, and back. Patients with hyperhidrosis of the feet often present requesting help for their malodorous or smelly feet and shoes.—Fran E. Cook-Bolden, MD (New York, New York)
I have found that systemic hyperhidrosis has usually been responsive to oral glycopyrrolate. But localized hyperhidrosis is more difficult to treat. Glycopyrronium has made life so much easier for my axillary hyperhidrosis patients. Now I am waiting for some game changer for palms and soles.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from March 11, 2019, to April 8, 2019. A total of 26 usable responses were received.
Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118-121.
Botox [package insert]. Madison, NJ: Allergan, Inc; 2018.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241-248.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on hyperhidrosis. Here’s what we found.
In which areas do patients report hyperhidrosis most frequently?
Nearly 70% of dermatologists see patients with hyperhidrosis of the axillae, followed by the palms and soles (27%). Only 4% of dermatologists indicated that they see hyperhidrosis all over the body.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Hyperhidrosis affects up to 5% of the US population and may remarkably affect quality of life. Primary hyperhidrosis accounts for 93% of cases. Before puberty, hyperhidrosis affects the palms and soles in up to 90% of patients. In adults, the axillae are most commonly affected (51%), followed by plantar (30%), palmar (24%), and facial (10%) areas (Strutton et al).
Next page: Topical treatment
Approximately what percentage of patients are satisfied with topical treatments for hyperhidrosis?
The majority of dermatologists (88%) reported that less than half of their patients are satisfied with topical treatments for hyperhidrosis. Only 12% indicated that 51% to 70% of their patients were satisfied, and none of the respondents indicated that >70% were satisfied.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
There is clearly a need for safe and effective treatments for hyperhidrosis. Treatment of hyperhidrosis should include lifestyle and behavioral modifications. It is helpful to try to avoid hot crowded rooms when feasible, as well as stress, tight clothing, occlusive shoes, alcohol, and spicy foods. Patients should be instructed on proper use of medications, as well as the need to continue therapy for maintenance. Patients should be encouraged to follow up for alternative treatment options in cases of therapy failure.
Next page: Botulinum toxin
On average, how long do the effects of botulinum toxin last in your axillary hyperhidrosis patients?
The effects of botulinum toxin last at least 4 months and up to 6 months in most patients, according to 58% of dermatologists surveyed. Thirty percent reported 2 to 4 months, and 13% reported more than 6 months.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
OnabotulinumtoxinA is approved by the US Food and Drug Administration for severe primary axillary hyperhidrosis. Injections are ideally placed at the dermal-subcutaneous junction, with 1 unit placed every 1 to 2 cm. Dosing is 50 to 100 U per axilla with higher dosing required for the palms and soles (off label). Reported efficacy for axillary hyperhidrosis is 82% to 87%; however, 50% of patients with plantar hyperhidrosis are dissatisfied with the treatment. Sweat reduction is most apparent after 2 weeks and typically persists 6 to 8 months in clinical trials (Botox package insert).
Next page: Systemic anticholinergics
When prescribing systemic anticholinergics for hyperhidrosis, what side effect is most common among your patients?
More than three-quarters of dermatologists (81%) reported that dry mouth is the most common side effect of systemic anticholinergics. Dry eyes is the second most common side effect (15%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Systemic anticholinergics are commonly used off label for the treatment of hyperhidrosis. Adverse effects include dry mouth, blurred vision, dry eyes, orthostatic hypotension, gastrointestinal, urinary retention, tachycardia, and drowsiness. Unfortunately, these side effects cause one-third of patients to discontinue treatment (Bajaj and Langtry). A slow escalation of the dose may increase tolerability and reduce these side effects. These anticholinergics should not be taken with other medications with anticholinergic activity to avoid exacerbating these side effects.
Next page: Surgical treatment
What percentage of patients require surgery for treatment of hyperhidrosis after topical, injectable, systemic options and devices have failed?
According to 62% of dermatologists, 10% or less of patients require surgery for treatment of hyperhidrosis after other therapies have failed. Almost one-third indicated that none of their patients require surgical treatment. None of the dermatologists surveyed reported that more than 60% of patients need surgery.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Surgery is an option to treat hyperhidrosis when conservative methods have failed. Surgical therapies include curettage, liposuction, and excision. A last resort is considered sympathectomy. Endoscopic thoracic sympathectomy is employed for palmar, facial, and axillary hyperhidrosis, while endoscopic lumbar sympathectomy is indicated for plantar hyperhidrosis.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Patients with focal idiopathic hyperhidrosis of the axillae as well as palms/soles report that this condition interferes with the quality of life in major ways, from social interactions to professional interactions. They often don't even know they have a problem and internalize that they must be overly anxious about things. I have patients that buy 3 of the same shirts and change a few times a day, costing a great deal of money (plus cleaning bills for 3 shirts as well) and costing a great deal of wasted time when they could be doing something more productive. It's great that not only botulinum toxins can be helpful for the underarms but also even less-invasive topical anticholinergics (easy to use, no discomfort, predictable, and helping make treatment for axillary hyperhidrosis much more on the radar).—Joel L. Cohen, MD (Denver, Colorado)
More and more patients are presenting to request relief from hyperhidrosis, and increasingly in nontraditional areas (ie, areas other than the axilla and forehead). These include the palms and scalp most commonly, and then the breast, chest, and back. Patients with hyperhidrosis of the feet often present requesting help for their malodorous or smelly feet and shoes.—Fran E. Cook-Bolden, MD (New York, New York)
I have found that systemic hyperhidrosis has usually been responsive to oral glycopyrrolate. But localized hyperhidrosis is more difficult to treat. Glycopyrronium has made life so much easier for my axillary hyperhidrosis patients. Now I am waiting for some game changer for palms and soles.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from March 11, 2019, to April 8, 2019. A total of 26 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on hyperhidrosis. Here’s what we found.
In which areas do patients report hyperhidrosis most frequently?
Nearly 70% of dermatologists see patients with hyperhidrosis of the axillae, followed by the palms and soles (27%). Only 4% of dermatologists indicated that they see hyperhidrosis all over the body.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Hyperhidrosis affects up to 5% of the US population and may remarkably affect quality of life. Primary hyperhidrosis accounts for 93% of cases. Before puberty, hyperhidrosis affects the palms and soles in up to 90% of patients. In adults, the axillae are most commonly affected (51%), followed by plantar (30%), palmar (24%), and facial (10%) areas (Strutton et al).
Next page: Topical treatment
Approximately what percentage of patients are satisfied with topical treatments for hyperhidrosis?
The majority of dermatologists (88%) reported that less than half of their patients are satisfied with topical treatments for hyperhidrosis. Only 12% indicated that 51% to 70% of their patients were satisfied, and none of the respondents indicated that >70% were satisfied.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
There is clearly a need for safe and effective treatments for hyperhidrosis. Treatment of hyperhidrosis should include lifestyle and behavioral modifications. It is helpful to try to avoid hot crowded rooms when feasible, as well as stress, tight clothing, occlusive shoes, alcohol, and spicy foods. Patients should be instructed on proper use of medications, as well as the need to continue therapy for maintenance. Patients should be encouraged to follow up for alternative treatment options in cases of therapy failure.
Next page: Botulinum toxin
On average, how long do the effects of botulinum toxin last in your axillary hyperhidrosis patients?
The effects of botulinum toxin last at least 4 months and up to 6 months in most patients, according to 58% of dermatologists surveyed. Thirty percent reported 2 to 4 months, and 13% reported more than 6 months.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
OnabotulinumtoxinA is approved by the US Food and Drug Administration for severe primary axillary hyperhidrosis. Injections are ideally placed at the dermal-subcutaneous junction, with 1 unit placed every 1 to 2 cm. Dosing is 50 to 100 U per axilla with higher dosing required for the palms and soles (off label). Reported efficacy for axillary hyperhidrosis is 82% to 87%; however, 50% of patients with plantar hyperhidrosis are dissatisfied with the treatment. Sweat reduction is most apparent after 2 weeks and typically persists 6 to 8 months in clinical trials (Botox package insert).
Next page: Systemic anticholinergics
When prescribing systemic anticholinergics for hyperhidrosis, what side effect is most common among your patients?
More than three-quarters of dermatologists (81%) reported that dry mouth is the most common side effect of systemic anticholinergics. Dry eyes is the second most common side effect (15%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Systemic anticholinergics are commonly used off label for the treatment of hyperhidrosis. Adverse effects include dry mouth, blurred vision, dry eyes, orthostatic hypotension, gastrointestinal, urinary retention, tachycardia, and drowsiness. Unfortunately, these side effects cause one-third of patients to discontinue treatment (Bajaj and Langtry). A slow escalation of the dose may increase tolerability and reduce these side effects. These anticholinergics should not be taken with other medications with anticholinergic activity to avoid exacerbating these side effects.
Next page: Surgical treatment
What percentage of patients require surgery for treatment of hyperhidrosis after topical, injectable, systemic options and devices have failed?
According to 62% of dermatologists, 10% or less of patients require surgery for treatment of hyperhidrosis after other therapies have failed. Almost one-third indicated that none of their patients require surgical treatment. None of the dermatologists surveyed reported that more than 60% of patients need surgery.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Surgery is an option to treat hyperhidrosis when conservative methods have failed. Surgical therapies include curettage, liposuction, and excision. A last resort is considered sympathectomy. Endoscopic thoracic sympathectomy is employed for palmar, facial, and axillary hyperhidrosis, while endoscopic lumbar sympathectomy is indicated for plantar hyperhidrosis.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Patients with focal idiopathic hyperhidrosis of the axillae as well as palms/soles report that this condition interferes with the quality of life in major ways, from social interactions to professional interactions. They often don't even know they have a problem and internalize that they must be overly anxious about things. I have patients that buy 3 of the same shirts and change a few times a day, costing a great deal of money (plus cleaning bills for 3 shirts as well) and costing a great deal of wasted time when they could be doing something more productive. It's great that not only botulinum toxins can be helpful for the underarms but also even less-invasive topical anticholinergics (easy to use, no discomfort, predictable, and helping make treatment for axillary hyperhidrosis much more on the radar).—Joel L. Cohen, MD (Denver, Colorado)
More and more patients are presenting to request relief from hyperhidrosis, and increasingly in nontraditional areas (ie, areas other than the axilla and forehead). These include the palms and scalp most commonly, and then the breast, chest, and back. Patients with hyperhidrosis of the feet often present requesting help for their malodorous or smelly feet and shoes.—Fran E. Cook-Bolden, MD (New York, New York)
I have found that systemic hyperhidrosis has usually been responsive to oral glycopyrrolate. But localized hyperhidrosis is more difficult to treat. Glycopyrronium has made life so much easier for my axillary hyperhidrosis patients. Now I am waiting for some game changer for palms and soles.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from March 11, 2019, to April 8, 2019. A total of 26 usable responses were received.
Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118-121.
Botox [package insert]. Madison, NJ: Allergan, Inc; 2018.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241-248.
Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118-121.
Botox [package insert]. Madison, NJ: Allergan, Inc; 2018.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241-248.
Tips for preventing complications in resurfacing skin of color
DENVER – In the opinion of
“You have to have the right patients and the right indication,” Dr. Brauer said at the annual conference of the American Society for Laser Medicine and Surgery. “What are they coming in for? Are they asking for what they really need?”
Taking a thorough medical history during consultations and follow-up visits is also key. “What medical or surgical problems do they have?” he asked. “Do they have a history of keloid formation? Are they on isotretinoin? What allergies do they have? What are their expectations, and are they realistic? For example, do they believe that you are going to erase all of their acne scars? On physical exam, be sure that what you’re looking at is what they’re concerned about, so that you agree upon what can and can’t be effectively treated.”
Above all else, stay true to your gut. “If you perceive that someone is not a suitable candidate for resurfacing or has unrealistic expectations, and they are insistent, it is important to stand your ground, and even find a way to politely walk away,” said Dr. Brauer of the department of dermatology at New York University.
Most complications from laser resurfacing are not unique to skin of color, he continued. A review of the topic revealed that mild complications may include prolonged erythema, acne and milia, delayed purpura, superficial erosions, contact dermatitis, and recall phenomenon (Dermatol Surg. 2010;36[3]:299-306). Moderate complications may include infection, pigmentary alteration, anesthesia toxicity, and eruptive keratoacanthomas, while severe complications may include hypertrophic scarring, ectropion formation, and disseminated infection.
An earlier analysis of fractional laser treatment found that patients with darker skin types had a significantly higher proportion of certain side effects, namely postinflammatory hyperpigmentation (Dermatol Surg. 2008;34[3]:301-7). “Additionally, the researchers found that this presented both later and lasted longer than in individuals with lighter skin types,” said Dr. Brauer, who was not involved with the study.
He listed pigmentary alterations and hypertrophic scarring/keloid formation as the potential complications from resurfacing to be most concerned about in skin of color patients. “In addition to appropriate device selection, the correct device parameters are key,” he said. “You have to make sure you use appropriate energy, but you can use higher energies with lower densities to minimize the risk of postinflammatory pigmentation. You also want to protect the epidermis by use of epidermal cooling, avoid bulk heating, and perform sessions at prolonged treatment intervals, to safely achieve optimal results.”
Dr. Brauer reported having received honoraria or being a member of the medical advisory board for Cutera, Cynosure/Hologic, and Merz.
DENVER – In the opinion of
“You have to have the right patients and the right indication,” Dr. Brauer said at the annual conference of the American Society for Laser Medicine and Surgery. “What are they coming in for? Are they asking for what they really need?”
Taking a thorough medical history during consultations and follow-up visits is also key. “What medical or surgical problems do they have?” he asked. “Do they have a history of keloid formation? Are they on isotretinoin? What allergies do they have? What are their expectations, and are they realistic? For example, do they believe that you are going to erase all of their acne scars? On physical exam, be sure that what you’re looking at is what they’re concerned about, so that you agree upon what can and can’t be effectively treated.”
Above all else, stay true to your gut. “If you perceive that someone is not a suitable candidate for resurfacing or has unrealistic expectations, and they are insistent, it is important to stand your ground, and even find a way to politely walk away,” said Dr. Brauer of the department of dermatology at New York University.
Most complications from laser resurfacing are not unique to skin of color, he continued. A review of the topic revealed that mild complications may include prolonged erythema, acne and milia, delayed purpura, superficial erosions, contact dermatitis, and recall phenomenon (Dermatol Surg. 2010;36[3]:299-306). Moderate complications may include infection, pigmentary alteration, anesthesia toxicity, and eruptive keratoacanthomas, while severe complications may include hypertrophic scarring, ectropion formation, and disseminated infection.
An earlier analysis of fractional laser treatment found that patients with darker skin types had a significantly higher proportion of certain side effects, namely postinflammatory hyperpigmentation (Dermatol Surg. 2008;34[3]:301-7). “Additionally, the researchers found that this presented both later and lasted longer than in individuals with lighter skin types,” said Dr. Brauer, who was not involved with the study.
He listed pigmentary alterations and hypertrophic scarring/keloid formation as the potential complications from resurfacing to be most concerned about in skin of color patients. “In addition to appropriate device selection, the correct device parameters are key,” he said. “You have to make sure you use appropriate energy, but you can use higher energies with lower densities to minimize the risk of postinflammatory pigmentation. You also want to protect the epidermis by use of epidermal cooling, avoid bulk heating, and perform sessions at prolonged treatment intervals, to safely achieve optimal results.”
Dr. Brauer reported having received honoraria or being a member of the medical advisory board for Cutera, Cynosure/Hologic, and Merz.
DENVER – In the opinion of
“You have to have the right patients and the right indication,” Dr. Brauer said at the annual conference of the American Society for Laser Medicine and Surgery. “What are they coming in for? Are they asking for what they really need?”
Taking a thorough medical history during consultations and follow-up visits is also key. “What medical or surgical problems do they have?” he asked. “Do they have a history of keloid formation? Are they on isotretinoin? What allergies do they have? What are their expectations, and are they realistic? For example, do they believe that you are going to erase all of their acne scars? On physical exam, be sure that what you’re looking at is what they’re concerned about, so that you agree upon what can and can’t be effectively treated.”
Above all else, stay true to your gut. “If you perceive that someone is not a suitable candidate for resurfacing or has unrealistic expectations, and they are insistent, it is important to stand your ground, and even find a way to politely walk away,” said Dr. Brauer of the department of dermatology at New York University.
Most complications from laser resurfacing are not unique to skin of color, he continued. A review of the topic revealed that mild complications may include prolonged erythema, acne and milia, delayed purpura, superficial erosions, contact dermatitis, and recall phenomenon (Dermatol Surg. 2010;36[3]:299-306). Moderate complications may include infection, pigmentary alteration, anesthesia toxicity, and eruptive keratoacanthomas, while severe complications may include hypertrophic scarring, ectropion formation, and disseminated infection.
An earlier analysis of fractional laser treatment found that patients with darker skin types had a significantly higher proportion of certain side effects, namely postinflammatory hyperpigmentation (Dermatol Surg. 2008;34[3]:301-7). “Additionally, the researchers found that this presented both later and lasted longer than in individuals with lighter skin types,” said Dr. Brauer, who was not involved with the study.
He listed pigmentary alterations and hypertrophic scarring/keloid formation as the potential complications from resurfacing to be most concerned about in skin of color patients. “In addition to appropriate device selection, the correct device parameters are key,” he said. “You have to make sure you use appropriate energy, but you can use higher energies with lower densities to minimize the risk of postinflammatory pigmentation. You also want to protect the epidermis by use of epidermal cooling, avoid bulk heating, and perform sessions at prolonged treatment intervals, to safely achieve optimal results.”
Dr. Brauer reported having received honoraria or being a member of the medical advisory board for Cutera, Cynosure/Hologic, and Merz.
EXPERT ANALYSIS FROM ASLMS 2019
Split-face trial compares outcomes of two different lasers on photoaging
DENVER – The fractionated picosecond Nd:YAG laser and fractionated thulium fiber laser can be equally effective for facial rejuvenation, results from a small split-face trial showed. However, the fractionated picosecond Nd:YAG laser may result in significantly less postoperative downtime, compared with the fractionated thulium fiber laser.
The findings from the prospective, evaluator-blinded trial were presented by Douglas C. Wu, MD, PhD, at the annual conference of the American Society for Laser Medicine and Surgery. Dr. Wu, of San Diego–based Cosmetic Laser Dermatology, and his colleague, Mitchel P. Goldman, MD, enrolled 20 subjects with at least moderate photoaging who randomly received three treatments with either the 1064/532-nm fractionated picosecond Nd:YAG laser or with the 1927-nm fractionated thulium fiber laser on each side of the face, 4 weeks apart. The primary endpoint was the degree of rhytids, laxity, dyschromia, erythema-telangiectasia, keratoses, and texture rated on a four-point scale and performed by a blinded evaluator at baseline, and 12, 20, and 30 weeks from baseline. Secondary endpoints were the global aesthetic improvement score, investigator satisfaction questionnaire, and a subject satisfaction questionnaire administered at weeks 12, 20, and 30. Recovery time and adverse events were assessed through a 14-day subject diary administered after each treatment.
All but 1 of the 20 patients were female and their mean age was 57 years. Six had Fitzpatrick skin type II, seven had type III, six had type IV, and one had type V. The device settings were on medium for both devices. The researchers observed significant improvements in elastosis, erythema, dyschromia, and texture at all treatment follow-up time points (P less than .01 for all endpoints).
There were no differences between the two lasers in terms of efficacy. “Clinically, the efficacy was rated to be the same,” Dr. Wu said. “However, when we analyzed the patient diaries, we found some very interesting results. In terms of redness, at days 3 and 4, there was a consistently increased amount of redness on the side treated with the fractionated thulium fiber laser, with swelling also being significantly increased at day 5.” Similarly, he said, the side treated with the fractionated picosecond laser experienced significantly less crusting on posttreatment days 1 through 9, less peeling on days 3 through 5, and less itching on day 4. Posttreatment pain was minimal on both sides and did not differ significantly.
Dr. Wu disclosed having numerous financial ties to pharmaceutical and device companies.
DENVER – The fractionated picosecond Nd:YAG laser and fractionated thulium fiber laser can be equally effective for facial rejuvenation, results from a small split-face trial showed. However, the fractionated picosecond Nd:YAG laser may result in significantly less postoperative downtime, compared with the fractionated thulium fiber laser.
The findings from the prospective, evaluator-blinded trial were presented by Douglas C. Wu, MD, PhD, at the annual conference of the American Society for Laser Medicine and Surgery. Dr. Wu, of San Diego–based Cosmetic Laser Dermatology, and his colleague, Mitchel P. Goldman, MD, enrolled 20 subjects with at least moderate photoaging who randomly received three treatments with either the 1064/532-nm fractionated picosecond Nd:YAG laser or with the 1927-nm fractionated thulium fiber laser on each side of the face, 4 weeks apart. The primary endpoint was the degree of rhytids, laxity, dyschromia, erythema-telangiectasia, keratoses, and texture rated on a four-point scale and performed by a blinded evaluator at baseline, and 12, 20, and 30 weeks from baseline. Secondary endpoints were the global aesthetic improvement score, investigator satisfaction questionnaire, and a subject satisfaction questionnaire administered at weeks 12, 20, and 30. Recovery time and adverse events were assessed through a 14-day subject diary administered after each treatment.
All but 1 of the 20 patients were female and their mean age was 57 years. Six had Fitzpatrick skin type II, seven had type III, six had type IV, and one had type V. The device settings were on medium for both devices. The researchers observed significant improvements in elastosis, erythema, dyschromia, and texture at all treatment follow-up time points (P less than .01 for all endpoints).
There were no differences between the two lasers in terms of efficacy. “Clinically, the efficacy was rated to be the same,” Dr. Wu said. “However, when we analyzed the patient diaries, we found some very interesting results. In terms of redness, at days 3 and 4, there was a consistently increased amount of redness on the side treated with the fractionated thulium fiber laser, with swelling also being significantly increased at day 5.” Similarly, he said, the side treated with the fractionated picosecond laser experienced significantly less crusting on posttreatment days 1 through 9, less peeling on days 3 through 5, and less itching on day 4. Posttreatment pain was minimal on both sides and did not differ significantly.
Dr. Wu disclosed having numerous financial ties to pharmaceutical and device companies.
DENVER – The fractionated picosecond Nd:YAG laser and fractionated thulium fiber laser can be equally effective for facial rejuvenation, results from a small split-face trial showed. However, the fractionated picosecond Nd:YAG laser may result in significantly less postoperative downtime, compared with the fractionated thulium fiber laser.
The findings from the prospective, evaluator-blinded trial were presented by Douglas C. Wu, MD, PhD, at the annual conference of the American Society for Laser Medicine and Surgery. Dr. Wu, of San Diego–based Cosmetic Laser Dermatology, and his colleague, Mitchel P. Goldman, MD, enrolled 20 subjects with at least moderate photoaging who randomly received three treatments with either the 1064/532-nm fractionated picosecond Nd:YAG laser or with the 1927-nm fractionated thulium fiber laser on each side of the face, 4 weeks apart. The primary endpoint was the degree of rhytids, laxity, dyschromia, erythema-telangiectasia, keratoses, and texture rated on a four-point scale and performed by a blinded evaluator at baseline, and 12, 20, and 30 weeks from baseline. Secondary endpoints were the global aesthetic improvement score, investigator satisfaction questionnaire, and a subject satisfaction questionnaire administered at weeks 12, 20, and 30. Recovery time and adverse events were assessed through a 14-day subject diary administered after each treatment.
All but 1 of the 20 patients were female and their mean age was 57 years. Six had Fitzpatrick skin type II, seven had type III, six had type IV, and one had type V. The device settings were on medium for both devices. The researchers observed significant improvements in elastosis, erythema, dyschromia, and texture at all treatment follow-up time points (P less than .01 for all endpoints).
There were no differences between the two lasers in terms of efficacy. “Clinically, the efficacy was rated to be the same,” Dr. Wu said. “However, when we analyzed the patient diaries, we found some very interesting results. In terms of redness, at days 3 and 4, there was a consistently increased amount of redness on the side treated with the fractionated thulium fiber laser, with swelling also being significantly increased at day 5.” Similarly, he said, the side treated with the fractionated picosecond laser experienced significantly less crusting on posttreatment days 1 through 9, less peeling on days 3 through 5, and less itching on day 4. Posttreatment pain was minimal on both sides and did not differ significantly.
Dr. Wu disclosed having numerous financial ties to pharmaceutical and device companies.
REPORTING FROM ASLMS 2019
A primer on cannabis for cosmeceuticals: The endocannabinoid system
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
Nitrous oxide in dermatology
. When used properly, with meticulous patient monitoring, it is safe and effective. In my practice, I have used it for procedures as simple as a skin biopsy. While we have excellent topical numbing options for pain control, nitrous oxide works well as an anxiolytic and can help calm the patient who is nervous or has a fear of needles.
Nitrous oxide is a tasteless gas synthesized and released by cells. Inhalational nitrous oxide is absorbed from the lungs and diffuses into plasma, where it acts on the central nervous system as an anxiolytic and analgesic by blocking the NMDA receptor. It has a quick onset of action and short duration, is easily titrated, and has a low side effect profile.
Initially used to provide pain relief during labor in the late 1800s, nitrous oxide is now rarely used in the United States as inhalational analgesia during surgery or labor; however, use in dentistry and pediatrics is common. In a recent review of PubMed and Cochrane databases by Brotzman et al., eight studies on the use of nitrous oxide in dermatology were identified. Studies reported favorable safety and efficacy of nitrous oxide in providing analgesia during dermatologic procedures, which included facial rejuvenation, hair transplantation, and pediatric procedures. Several other studies also discussed the use of nitrous oxide in combination with tumescent anesthesia for venous ablation and liposuction. All adverse effects were limited to the time of inhalation and included euphoria, laughter, nausea, dizziness, and vertigo. There are no studies reviewing the risk of nitrous oxide used during CO2 resurfacing procedures.
In five of the eight studies, vital signs and oxygen saturation were recorded during the period of inhalation. Almost all patients maintained adequate oxygen saturation and vitals also remained stable in these five studies, except for a slight increase in systolic and diastolic arterial pressure after ulcer debridement. In four of the eight studies, a 50% nitrous oxide/50% oxygen mixture delivered through an on-demand valve activated by a patient’s inspired breaths was used to minimize the risk of oversedation and to prevent hypoxia.
Contraindications for using nitrous oxide are pregnancy (in patients, health care providers, and assistants). Relative contraindications include nasal obstruction, chronic obstructive pulmonary disease, active cystic fibrosis, recent tympanic membrane surgery, and claustrophobia. According to the National Institute for Occupational Safety and Health, occupational exposure to nitrous oxide can lead to adverse effects that include reduced fertility and spontaneous abortion, as well as neurologic, renal, and hepatic diseases. The consensus of the majority of the studies in the PubMed/Cochrane review is that nitrous oxide provided a significant reduction in pain during dermatologic procedures, with mild and transient adverse effects. The effects dissipated quickly and thus patients could drive themselves home. But studies remain limited, and more well designed, randomized clinical trials are needed to provide clinical guidelines, safety monitoring protocols, and evidence for the use of nitrous oxide in dermatology. In my opinion, when more data are available, it will become one of the mainstays of analgesia in dermatologic procedures, particularly for pediatric, Mohs, and facial rejuvenation procedures.
Dr. Talakoub Dr. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Brotzman EA et al. Dermatol Surg. 2018 May;44(5):661-9.
“Controlling Exposures to Nitrous Oxide During Anesthetic Administration,” National Institute for Occupational Safety and Health (https://www.cdc.gov/niosh/docs/94-100/default.html).
. When used properly, with meticulous patient monitoring, it is safe and effective. In my practice, I have used it for procedures as simple as a skin biopsy. While we have excellent topical numbing options for pain control, nitrous oxide works well as an anxiolytic and can help calm the patient who is nervous or has a fear of needles.
Nitrous oxide is a tasteless gas synthesized and released by cells. Inhalational nitrous oxide is absorbed from the lungs and diffuses into plasma, where it acts on the central nervous system as an anxiolytic and analgesic by blocking the NMDA receptor. It has a quick onset of action and short duration, is easily titrated, and has a low side effect profile.
Initially used to provide pain relief during labor in the late 1800s, nitrous oxide is now rarely used in the United States as inhalational analgesia during surgery or labor; however, use in dentistry and pediatrics is common. In a recent review of PubMed and Cochrane databases by Brotzman et al., eight studies on the use of nitrous oxide in dermatology were identified. Studies reported favorable safety and efficacy of nitrous oxide in providing analgesia during dermatologic procedures, which included facial rejuvenation, hair transplantation, and pediatric procedures. Several other studies also discussed the use of nitrous oxide in combination with tumescent anesthesia for venous ablation and liposuction. All adverse effects were limited to the time of inhalation and included euphoria, laughter, nausea, dizziness, and vertigo. There are no studies reviewing the risk of nitrous oxide used during CO2 resurfacing procedures.
In five of the eight studies, vital signs and oxygen saturation were recorded during the period of inhalation. Almost all patients maintained adequate oxygen saturation and vitals also remained stable in these five studies, except for a slight increase in systolic and diastolic arterial pressure after ulcer debridement. In four of the eight studies, a 50% nitrous oxide/50% oxygen mixture delivered through an on-demand valve activated by a patient’s inspired breaths was used to minimize the risk of oversedation and to prevent hypoxia.
Contraindications for using nitrous oxide are pregnancy (in patients, health care providers, and assistants). Relative contraindications include nasal obstruction, chronic obstructive pulmonary disease, active cystic fibrosis, recent tympanic membrane surgery, and claustrophobia. According to the National Institute for Occupational Safety and Health, occupational exposure to nitrous oxide can lead to adverse effects that include reduced fertility and spontaneous abortion, as well as neurologic, renal, and hepatic diseases. The consensus of the majority of the studies in the PubMed/Cochrane review is that nitrous oxide provided a significant reduction in pain during dermatologic procedures, with mild and transient adverse effects. The effects dissipated quickly and thus patients could drive themselves home. But studies remain limited, and more well designed, randomized clinical trials are needed to provide clinical guidelines, safety monitoring protocols, and evidence for the use of nitrous oxide in dermatology. In my opinion, when more data are available, it will become one of the mainstays of analgesia in dermatologic procedures, particularly for pediatric, Mohs, and facial rejuvenation procedures.
Dr. Talakoub Dr. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Brotzman EA et al. Dermatol Surg. 2018 May;44(5):661-9.
“Controlling Exposures to Nitrous Oxide During Anesthetic Administration,” National Institute for Occupational Safety and Health (https://www.cdc.gov/niosh/docs/94-100/default.html).
. When used properly, with meticulous patient monitoring, it is safe and effective. In my practice, I have used it for procedures as simple as a skin biopsy. While we have excellent topical numbing options for pain control, nitrous oxide works well as an anxiolytic and can help calm the patient who is nervous or has a fear of needles.
Nitrous oxide is a tasteless gas synthesized and released by cells. Inhalational nitrous oxide is absorbed from the lungs and diffuses into plasma, where it acts on the central nervous system as an anxiolytic and analgesic by blocking the NMDA receptor. It has a quick onset of action and short duration, is easily titrated, and has a low side effect profile.
Initially used to provide pain relief during labor in the late 1800s, nitrous oxide is now rarely used in the United States as inhalational analgesia during surgery or labor; however, use in dentistry and pediatrics is common. In a recent review of PubMed and Cochrane databases by Brotzman et al., eight studies on the use of nitrous oxide in dermatology were identified. Studies reported favorable safety and efficacy of nitrous oxide in providing analgesia during dermatologic procedures, which included facial rejuvenation, hair transplantation, and pediatric procedures. Several other studies also discussed the use of nitrous oxide in combination with tumescent anesthesia for venous ablation and liposuction. All adverse effects were limited to the time of inhalation and included euphoria, laughter, nausea, dizziness, and vertigo. There are no studies reviewing the risk of nitrous oxide used during CO2 resurfacing procedures.
In five of the eight studies, vital signs and oxygen saturation were recorded during the period of inhalation. Almost all patients maintained adequate oxygen saturation and vitals also remained stable in these five studies, except for a slight increase in systolic and diastolic arterial pressure after ulcer debridement. In four of the eight studies, a 50% nitrous oxide/50% oxygen mixture delivered through an on-demand valve activated by a patient’s inspired breaths was used to minimize the risk of oversedation and to prevent hypoxia.
Contraindications for using nitrous oxide are pregnancy (in patients, health care providers, and assistants). Relative contraindications include nasal obstruction, chronic obstructive pulmonary disease, active cystic fibrosis, recent tympanic membrane surgery, and claustrophobia. According to the National Institute for Occupational Safety and Health, occupational exposure to nitrous oxide can lead to adverse effects that include reduced fertility and spontaneous abortion, as well as neurologic, renal, and hepatic diseases. The consensus of the majority of the studies in the PubMed/Cochrane review is that nitrous oxide provided a significant reduction in pain during dermatologic procedures, with mild and transient adverse effects. The effects dissipated quickly and thus patients could drive themselves home. But studies remain limited, and more well designed, randomized clinical trials are needed to provide clinical guidelines, safety monitoring protocols, and evidence for the use of nitrous oxide in dermatology. In my opinion, when more data are available, it will become one of the mainstays of analgesia in dermatologic procedures, particularly for pediatric, Mohs, and facial rejuvenation procedures.
Dr. Talakoub Dr. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Sources
Brotzman EA et al. Dermatol Surg. 2018 May;44(5):661-9.
“Controlling Exposures to Nitrous Oxide During Anesthetic Administration,” National Institute for Occupational Safety and Health (https://www.cdc.gov/niosh/docs/94-100/default.html).