User login
Novel body contouring device targets muscle, not fat
DENVER –
The device, known as CoolTone, is being developed by Allergan and uses high-powered coil electromagnetic stimulation applicators to induce eddy currents in the muscle tissue. CoolTone is pending Food and Drug Administration clearance and is not yet commercially available.
“Fat reduction is just one part of body contouring,” Mathew M. Avram, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “You have skin, fat, and muscle. More and more we’re targeting all three areas for patients’ best body contouring outcomes.”
According to Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston, CoolTone provides high-frequency electromagnetic muscle stimulation that triggers muscle contractions that cannot be achieved by normal exercise to increase muscle mass and strength. “You’re doing super physiological amounts of contractions with this stimulation – the equivalent of doing thousands of sit-ups, if you’re treating the abdomen,” he said. “It strengthens, tones, and firms muscles in abdomen, buttocks, arms, and legs. There is a history of this type of technology for athletes and other indications in physical therapy.”
The current FDA clearance for a predicate electromagnetic stimulation system for muscle conditioning is for the abdomen, buttocks, thighs, and arms. “This is for improvement of abdominal tone, strengthening of the abdominal muscles, and development of a firmer abdomen,” said Dr. Avram, who also is director of dermatologic surgery at Mass General. “It’s for strengthening, toning, and firming of buttocks and thighs, and for improvement of muscle tone and firmness, and for strengthening muscle in arms.”
The electrical current induced by the CoolTone device flows readily into muscle and not into fat, he continued. This brings the current to nearby motor nerve structures that stimulate contraction once the action potential is reached. “You’re getting maximal contractions that are extreme for a full range of muscle fibers,” explained Dr. Avram, who is the immediate past president of the ASLMS. “This requires an external electrical stimulus; it’s not something you do with normal exercise. With mild exercise, only the slow-twitch muscle fibers are activated, not the fast-twitch muscle fibers. Also, the pulsing sequences are designed to preferentially excite motor nerves rather than sensory nerves. So it’s really going after the ability for you to contract your muscles as much as possible.”
Dr. Avram has received consulting fees from Merz and Alastin and holds ownership interests with ZALEA, InMode, and Cytrellis. He has served on the advisory boards for ZELTIQ Aesthetics, Soliton, Sciton, and Sienna Biopharmaceuticals, and he has intellectual property rights with Cytrellis.
DENVER –
The device, known as CoolTone, is being developed by Allergan and uses high-powered coil electromagnetic stimulation applicators to induce eddy currents in the muscle tissue. CoolTone is pending Food and Drug Administration clearance and is not yet commercially available.
“Fat reduction is just one part of body contouring,” Mathew M. Avram, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “You have skin, fat, and muscle. More and more we’re targeting all three areas for patients’ best body contouring outcomes.”
According to Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston, CoolTone provides high-frequency electromagnetic muscle stimulation that triggers muscle contractions that cannot be achieved by normal exercise to increase muscle mass and strength. “You’re doing super physiological amounts of contractions with this stimulation – the equivalent of doing thousands of sit-ups, if you’re treating the abdomen,” he said. “It strengthens, tones, and firms muscles in abdomen, buttocks, arms, and legs. There is a history of this type of technology for athletes and other indications in physical therapy.”
The current FDA clearance for a predicate electromagnetic stimulation system for muscle conditioning is for the abdomen, buttocks, thighs, and arms. “This is for improvement of abdominal tone, strengthening of the abdominal muscles, and development of a firmer abdomen,” said Dr. Avram, who also is director of dermatologic surgery at Mass General. “It’s for strengthening, toning, and firming of buttocks and thighs, and for improvement of muscle tone and firmness, and for strengthening muscle in arms.”
The electrical current induced by the CoolTone device flows readily into muscle and not into fat, he continued. This brings the current to nearby motor nerve structures that stimulate contraction once the action potential is reached. “You’re getting maximal contractions that are extreme for a full range of muscle fibers,” explained Dr. Avram, who is the immediate past president of the ASLMS. “This requires an external electrical stimulus; it’s not something you do with normal exercise. With mild exercise, only the slow-twitch muscle fibers are activated, not the fast-twitch muscle fibers. Also, the pulsing sequences are designed to preferentially excite motor nerves rather than sensory nerves. So it’s really going after the ability for you to contract your muscles as much as possible.”
Dr. Avram has received consulting fees from Merz and Alastin and holds ownership interests with ZALEA, InMode, and Cytrellis. He has served on the advisory boards for ZELTIQ Aesthetics, Soliton, Sciton, and Sienna Biopharmaceuticals, and he has intellectual property rights with Cytrellis.
DENVER –
The device, known as CoolTone, is being developed by Allergan and uses high-powered coil electromagnetic stimulation applicators to induce eddy currents in the muscle tissue. CoolTone is pending Food and Drug Administration clearance and is not yet commercially available.
“Fat reduction is just one part of body contouring,” Mathew M. Avram, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “You have skin, fat, and muscle. More and more we’re targeting all three areas for patients’ best body contouring outcomes.”
According to Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston, CoolTone provides high-frequency electromagnetic muscle stimulation that triggers muscle contractions that cannot be achieved by normal exercise to increase muscle mass and strength. “You’re doing super physiological amounts of contractions with this stimulation – the equivalent of doing thousands of sit-ups, if you’re treating the abdomen,” he said. “It strengthens, tones, and firms muscles in abdomen, buttocks, arms, and legs. There is a history of this type of technology for athletes and other indications in physical therapy.”
The current FDA clearance for a predicate electromagnetic stimulation system for muscle conditioning is for the abdomen, buttocks, thighs, and arms. “This is for improvement of abdominal tone, strengthening of the abdominal muscles, and development of a firmer abdomen,” said Dr. Avram, who also is director of dermatologic surgery at Mass General. “It’s for strengthening, toning, and firming of buttocks and thighs, and for improvement of muscle tone and firmness, and for strengthening muscle in arms.”
The electrical current induced by the CoolTone device flows readily into muscle and not into fat, he continued. This brings the current to nearby motor nerve structures that stimulate contraction once the action potential is reached. “You’re getting maximal contractions that are extreme for a full range of muscle fibers,” explained Dr. Avram, who is the immediate past president of the ASLMS. “This requires an external electrical stimulus; it’s not something you do with normal exercise. With mild exercise, only the slow-twitch muscle fibers are activated, not the fast-twitch muscle fibers. Also, the pulsing sequences are designed to preferentially excite motor nerves rather than sensory nerves. So it’s really going after the ability for you to contract your muscles as much as possible.”
Dr. Avram has received consulting fees from Merz and Alastin and holds ownership interests with ZALEA, InMode, and Cytrellis. He has served on the advisory boards for ZELTIQ Aesthetics, Soliton, Sciton, and Sienna Biopharmaceuticals, and he has intellectual property rights with Cytrellis.
EXPERT ANALYSIS FROM ASLMS 2019
Energy-based devices for vaginal rejuvenation described in FDA adverse event reports
The use of was implicated in nearly four dozen adverse event reports found in the agency’s medical device adverse event reporting database, researchers report.
The 45 unique event reports, submitted to the FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning, said the researchers, led by Jusleen Ahluwalia, MD, of the department of dermatology at the University of California, San Diego, and her coauthors. They included 31 that were reported by the patients, 8 reported by the manufacturer; 4 reported by the distributor, and 2 not specified.
These findings emphasize the need for clinical trials to evaluate the safety and efficacy of the lasers and radiofrequency devices that have been marketed and used for so-called vaginal rejuvenation procedures, they wrote in Lasers in Surgery and Medicine. The coauthors are Arisa Ortiz, MD, also with the University of California, San Diego, and Mathew M. Avram, MD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston. “Randomized studies are necessary to compare these therapies with standard modalities and to establish the safety of these devices,” they wrote.
In July 2018, the FDA issued a safety communication alerting patients and health care providers that the safety and effectiveness of energy-based devices has not been established for procedures described as “vaginal rejuvenation.” Scott Gottlieb, MD, FDA commissioner at the time, issued a statement decrying “deceptive health claims and significant risks” related to devices marketed for those medical procedures. In a November 2018 update, the FDA said they contacted some device manufacturers to express concerns that the devices were being marketed inappropriately and that manufacturers they had contacted so far “responded with adequate corrections.”
In their report, Dr. Ahluwalia and her associates noted that “vaginal rejuvenation” is an ill-defined term that may encompass a variety of procedures related to tightening; dyspareunia; dysuria; urinary incontinence; vulvar issues including irritation, dryness, and atrophy; and orgasmic dysfunction.
They found a total of 58 records in their review of the Manufacturer and User Facility Device Experience database, of which 25 were reported prior to the FDA’s July 2018 statement. Of 45 unique event descriptions found in those records, 39 were categorized as patient-related injuries, while 2 were operator-related injuries, 2 were device malfunctions, and 2 were not specified.
Pain was the most commonly adverse event, accounting for 19 reports in their analysis, while 11 patients reported numbness or burning.
Among the laser- and energy-based devices specifically described in the 39 patient-report injuries, the MonaLisa Touch had the highest number of adverse event reports (16), the data show. “However, this may be reflective of length of time bias as it is one of the first devices utilized to promote vaginal rejuvenation,” the authors pointed out.
In light of these findings, the authors advised clinicians to ask patients about their reasons for seeking vaginal rejuvenation procedures. “Normal variety of female genital appearances should also be reviewed when patients express cosmetic concerns,” they added. Concerns about related to genitourinary syndrome of menopause “or optimizing sexual function may be alleviated by exploring nonprocedural, conservative approaches, such as hormonal creams, if not contraindicated, and/or counseling,” they noted.
The authors provided conflict of interest disclosures related to Zalea, Inmode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Adverse events related to devices and drugs can be reported to the FDA’s Medwatch program.
SOURCE: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
The use of was implicated in nearly four dozen adverse event reports found in the agency’s medical device adverse event reporting database, researchers report.
The 45 unique event reports, submitted to the FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning, said the researchers, led by Jusleen Ahluwalia, MD, of the department of dermatology at the University of California, San Diego, and her coauthors. They included 31 that were reported by the patients, 8 reported by the manufacturer; 4 reported by the distributor, and 2 not specified.
These findings emphasize the need for clinical trials to evaluate the safety and efficacy of the lasers and radiofrequency devices that have been marketed and used for so-called vaginal rejuvenation procedures, they wrote in Lasers in Surgery and Medicine. The coauthors are Arisa Ortiz, MD, also with the University of California, San Diego, and Mathew M. Avram, MD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston. “Randomized studies are necessary to compare these therapies with standard modalities and to establish the safety of these devices,” they wrote.
In July 2018, the FDA issued a safety communication alerting patients and health care providers that the safety and effectiveness of energy-based devices has not been established for procedures described as “vaginal rejuvenation.” Scott Gottlieb, MD, FDA commissioner at the time, issued a statement decrying “deceptive health claims and significant risks” related to devices marketed for those medical procedures. In a November 2018 update, the FDA said they contacted some device manufacturers to express concerns that the devices were being marketed inappropriately and that manufacturers they had contacted so far “responded with adequate corrections.”
In their report, Dr. Ahluwalia and her associates noted that “vaginal rejuvenation” is an ill-defined term that may encompass a variety of procedures related to tightening; dyspareunia; dysuria; urinary incontinence; vulvar issues including irritation, dryness, and atrophy; and orgasmic dysfunction.
They found a total of 58 records in their review of the Manufacturer and User Facility Device Experience database, of which 25 were reported prior to the FDA’s July 2018 statement. Of 45 unique event descriptions found in those records, 39 were categorized as patient-related injuries, while 2 were operator-related injuries, 2 were device malfunctions, and 2 were not specified.
Pain was the most commonly adverse event, accounting for 19 reports in their analysis, while 11 patients reported numbness or burning.
Among the laser- and energy-based devices specifically described in the 39 patient-report injuries, the MonaLisa Touch had the highest number of adverse event reports (16), the data show. “However, this may be reflective of length of time bias as it is one of the first devices utilized to promote vaginal rejuvenation,” the authors pointed out.
In light of these findings, the authors advised clinicians to ask patients about their reasons for seeking vaginal rejuvenation procedures. “Normal variety of female genital appearances should also be reviewed when patients express cosmetic concerns,” they added. Concerns about related to genitourinary syndrome of menopause “or optimizing sexual function may be alleviated by exploring nonprocedural, conservative approaches, such as hormonal creams, if not contraindicated, and/or counseling,” they noted.
The authors provided conflict of interest disclosures related to Zalea, Inmode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Adverse events related to devices and drugs can be reported to the FDA’s Medwatch program.
SOURCE: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
The use of was implicated in nearly four dozen adverse event reports found in the agency’s medical device adverse event reporting database, researchers report.
The 45 unique event reports, submitted to the FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning, said the researchers, led by Jusleen Ahluwalia, MD, of the department of dermatology at the University of California, San Diego, and her coauthors. They included 31 that were reported by the patients, 8 reported by the manufacturer; 4 reported by the distributor, and 2 not specified.
These findings emphasize the need for clinical trials to evaluate the safety and efficacy of the lasers and radiofrequency devices that have been marketed and used for so-called vaginal rejuvenation procedures, they wrote in Lasers in Surgery and Medicine. The coauthors are Arisa Ortiz, MD, also with the University of California, San Diego, and Mathew M. Avram, MD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston. “Randomized studies are necessary to compare these therapies with standard modalities and to establish the safety of these devices,” they wrote.
In July 2018, the FDA issued a safety communication alerting patients and health care providers that the safety and effectiveness of energy-based devices has not been established for procedures described as “vaginal rejuvenation.” Scott Gottlieb, MD, FDA commissioner at the time, issued a statement decrying “deceptive health claims and significant risks” related to devices marketed for those medical procedures. In a November 2018 update, the FDA said they contacted some device manufacturers to express concerns that the devices were being marketed inappropriately and that manufacturers they had contacted so far “responded with adequate corrections.”
In their report, Dr. Ahluwalia and her associates noted that “vaginal rejuvenation” is an ill-defined term that may encompass a variety of procedures related to tightening; dyspareunia; dysuria; urinary incontinence; vulvar issues including irritation, dryness, and atrophy; and orgasmic dysfunction.
They found a total of 58 records in their review of the Manufacturer and User Facility Device Experience database, of which 25 were reported prior to the FDA’s July 2018 statement. Of 45 unique event descriptions found in those records, 39 were categorized as patient-related injuries, while 2 were operator-related injuries, 2 were device malfunctions, and 2 were not specified.
Pain was the most commonly adverse event, accounting for 19 reports in their analysis, while 11 patients reported numbness or burning.
Among the laser- and energy-based devices specifically described in the 39 patient-report injuries, the MonaLisa Touch had the highest number of adverse event reports (16), the data show. “However, this may be reflective of length of time bias as it is one of the first devices utilized to promote vaginal rejuvenation,” the authors pointed out.
In light of these findings, the authors advised clinicians to ask patients about their reasons for seeking vaginal rejuvenation procedures. “Normal variety of female genital appearances should also be reviewed when patients express cosmetic concerns,” they added. Concerns about related to genitourinary syndrome of menopause “or optimizing sexual function may be alleviated by exploring nonprocedural, conservative approaches, such as hormonal creams, if not contraindicated, and/or counseling,” they noted.
The authors provided conflict of interest disclosures related to Zalea, Inmode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Adverse events related to devices and drugs can be reported to the FDA’s Medwatch program.
SOURCE: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
FROM LASERS IN SURGERY AND MEDICINE
Key clinical point: Nearly four dozen distinct adverse event reports related to energy-based devices used for vaginal rejuvenation were found in an analysis of an FDA database.
Major finding: The 45 unique event reports, disclosed to FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning.
Study details: Cross-sectional analysis of records in the Manufacturer and User Facility Device Experience database entered during October 2015–January 2019.
Disclosures: Authors provided conflict of interest disclosures related to ZALEA, InMode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Source: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
Survey finds high rate of complications from laser tattoo removal in non-clinic settings
DENVER – A survey from a dermatology practice in Houston found that among patients seeking corrective treatment for laser tattoo removal, 79% had complications from previous removal attempts and 63% were treated in non-clinic facilities by a non-physician provider without physician supervision.
The findings come from a single-center study that sought to identify the type, burden, and frequency of complications from laser tattoo removal, a procedure offered by both physician and non-physician facilities. “Laser tattoo removal is increasing in popularity,” lead study author Amanda K. Suggs, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
Dr. Suggs and Paul M. Friedman, MD, of Houston-based Dermatology and Laser Surgery, have observed an increase in patients seeking corrective tattoo removal after complications from and lack of efficacy of prior treatments provided predominantly at non-clinic facilities, including medical spas and tattoo removal clinics –so they decided to interview 19 patients who presented to their practice seeking corrective laser tattoo removal. The majority (84%) were female, their mean age was 34 years old, and 53% had Fitzpatrick skin types IV or higher. Nearly three-quarters of tattoos (74%) consisted of multiple colors, which are known to be more difficult to treat. Of the patients seeking corrective treatment, 42% were seeking removal of more than one tattoo.
Prior to coming to their office, the patients had undergone an average of seven prior tattoo removal treatments and 72% of patients were treated by a non-physician provider at some point. Nearly two-thirds of patients (63%) were treated in non-clinic facilities. “All patients were unsatisfied with the degree of improvement, and 79% had at least one complication from their prior treatments,” said Dr. Suggs, who is a fellow at the practice.
Of the 15 patients with prior treatment complications, 64% were treated by a non-physician provider. The most common complication was scarring (53%), followed by dyspigmentation (47%), blistering (20%) and paradoxical darkening (20%). Six patients (40%) had more than one complication. Patients with Fitzpatrick skin types IV or higher had a higher proportion of scarring and dyspigmentation (63% and 71%, respectively) compared with those with other skin types. “This suggests that we should use caution when treating tattoos in patients with higher Fitzpatrick skin types, and use appropriate settings and endpoints when treating these patients,” Dr. Suggs said.
When she and Dr. Friedman interviewed the patients about their prior treatment experience elsewhere, all said they experienced excessive pain, only 33% received topical anesthesia, and none reported receiving an injectable anesthesia.
At the Dermatology and Laser Surgery Center, the protocol for corrective laser tattoo removal involves injectable anesthesia, Dr. Suggs said. They use a picosecond laser, a perfluorodecalin patch, and, if needed, nonablative fractional resurfacing at 1550 nm for scarring. The wavelength used for the picosecond laser (1064nm, 785nm or 532nm) is chosen based on patient characteristics and tattoo color or colors.
In a subset analysis, the investigators interviewed eight patients again after undergoing laser tattoo removal at their practice. All underwent treatment with a picosecond laser, perfluorodecalin patch, and injectable anesthesia. All reported minimal to no pain during the procedure and an optimal experience. No complications were noted.
Dr. Friedman and Dr. Suggs emphasized that consumers should be aware of the risks and potential for complications from laser tattoo removal. They recommend that all consumers – especially those at higher risk for complications such as higher Fitzpatrick skin type patients and those with multicolored tattoos – choose a provider with extensive training in the procedure, such as a board-certified dermatologist or plastic surgeon.
Dr. Suggs disclosed that she is an ambassador for Tri Sirena sun protective athletic apparel. Dr. Friedman disclosed that he is a member of the advisory board for Allergan, Solta Medical, Syneron-Candela, and Sienna Biopharmaceuticals. He is also a research investigator for Syneron-Candela and has received a research grant from Sienna.
DENVER – A survey from a dermatology practice in Houston found that among patients seeking corrective treatment for laser tattoo removal, 79% had complications from previous removal attempts and 63% were treated in non-clinic facilities by a non-physician provider without physician supervision.
The findings come from a single-center study that sought to identify the type, burden, and frequency of complications from laser tattoo removal, a procedure offered by both physician and non-physician facilities. “Laser tattoo removal is increasing in popularity,” lead study author Amanda K. Suggs, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
Dr. Suggs and Paul M. Friedman, MD, of Houston-based Dermatology and Laser Surgery, have observed an increase in patients seeking corrective tattoo removal after complications from and lack of efficacy of prior treatments provided predominantly at non-clinic facilities, including medical spas and tattoo removal clinics –so they decided to interview 19 patients who presented to their practice seeking corrective laser tattoo removal. The majority (84%) were female, their mean age was 34 years old, and 53% had Fitzpatrick skin types IV or higher. Nearly three-quarters of tattoos (74%) consisted of multiple colors, which are known to be more difficult to treat. Of the patients seeking corrective treatment, 42% were seeking removal of more than one tattoo.
Prior to coming to their office, the patients had undergone an average of seven prior tattoo removal treatments and 72% of patients were treated by a non-physician provider at some point. Nearly two-thirds of patients (63%) were treated in non-clinic facilities. “All patients were unsatisfied with the degree of improvement, and 79% had at least one complication from their prior treatments,” said Dr. Suggs, who is a fellow at the practice.
Of the 15 patients with prior treatment complications, 64% were treated by a non-physician provider. The most common complication was scarring (53%), followed by dyspigmentation (47%), blistering (20%) and paradoxical darkening (20%). Six patients (40%) had more than one complication. Patients with Fitzpatrick skin types IV or higher had a higher proportion of scarring and dyspigmentation (63% and 71%, respectively) compared with those with other skin types. “This suggests that we should use caution when treating tattoos in patients with higher Fitzpatrick skin types, and use appropriate settings and endpoints when treating these patients,” Dr. Suggs said.
When she and Dr. Friedman interviewed the patients about their prior treatment experience elsewhere, all said they experienced excessive pain, only 33% received topical anesthesia, and none reported receiving an injectable anesthesia.
At the Dermatology and Laser Surgery Center, the protocol for corrective laser tattoo removal involves injectable anesthesia, Dr. Suggs said. They use a picosecond laser, a perfluorodecalin patch, and, if needed, nonablative fractional resurfacing at 1550 nm for scarring. The wavelength used for the picosecond laser (1064nm, 785nm or 532nm) is chosen based on patient characteristics and tattoo color or colors.
In a subset analysis, the investigators interviewed eight patients again after undergoing laser tattoo removal at their practice. All underwent treatment with a picosecond laser, perfluorodecalin patch, and injectable anesthesia. All reported minimal to no pain during the procedure and an optimal experience. No complications were noted.
Dr. Friedman and Dr. Suggs emphasized that consumers should be aware of the risks and potential for complications from laser tattoo removal. They recommend that all consumers – especially those at higher risk for complications such as higher Fitzpatrick skin type patients and those with multicolored tattoos – choose a provider with extensive training in the procedure, such as a board-certified dermatologist or plastic surgeon.
Dr. Suggs disclosed that she is an ambassador for Tri Sirena sun protective athletic apparel. Dr. Friedman disclosed that he is a member of the advisory board for Allergan, Solta Medical, Syneron-Candela, and Sienna Biopharmaceuticals. He is also a research investigator for Syneron-Candela and has received a research grant from Sienna.
DENVER – A survey from a dermatology practice in Houston found that among patients seeking corrective treatment for laser tattoo removal, 79% had complications from previous removal attempts and 63% were treated in non-clinic facilities by a non-physician provider without physician supervision.
The findings come from a single-center study that sought to identify the type, burden, and frequency of complications from laser tattoo removal, a procedure offered by both physician and non-physician facilities. “Laser tattoo removal is increasing in popularity,” lead study author Amanda K. Suggs, MD, said at the annual conference of the American Society for Laser Medicine and Surgery.
Dr. Suggs and Paul M. Friedman, MD, of Houston-based Dermatology and Laser Surgery, have observed an increase in patients seeking corrective tattoo removal after complications from and lack of efficacy of prior treatments provided predominantly at non-clinic facilities, including medical spas and tattoo removal clinics –so they decided to interview 19 patients who presented to their practice seeking corrective laser tattoo removal. The majority (84%) were female, their mean age was 34 years old, and 53% had Fitzpatrick skin types IV or higher. Nearly three-quarters of tattoos (74%) consisted of multiple colors, which are known to be more difficult to treat. Of the patients seeking corrective treatment, 42% were seeking removal of more than one tattoo.
Prior to coming to their office, the patients had undergone an average of seven prior tattoo removal treatments and 72% of patients were treated by a non-physician provider at some point. Nearly two-thirds of patients (63%) were treated in non-clinic facilities. “All patients were unsatisfied with the degree of improvement, and 79% had at least one complication from their prior treatments,” said Dr. Suggs, who is a fellow at the practice.
Of the 15 patients with prior treatment complications, 64% were treated by a non-physician provider. The most common complication was scarring (53%), followed by dyspigmentation (47%), blistering (20%) and paradoxical darkening (20%). Six patients (40%) had more than one complication. Patients with Fitzpatrick skin types IV or higher had a higher proportion of scarring and dyspigmentation (63% and 71%, respectively) compared with those with other skin types. “This suggests that we should use caution when treating tattoos in patients with higher Fitzpatrick skin types, and use appropriate settings and endpoints when treating these patients,” Dr. Suggs said.
When she and Dr. Friedman interviewed the patients about their prior treatment experience elsewhere, all said they experienced excessive pain, only 33% received topical anesthesia, and none reported receiving an injectable anesthesia.
At the Dermatology and Laser Surgery Center, the protocol for corrective laser tattoo removal involves injectable anesthesia, Dr. Suggs said. They use a picosecond laser, a perfluorodecalin patch, and, if needed, nonablative fractional resurfacing at 1550 nm for scarring. The wavelength used for the picosecond laser (1064nm, 785nm or 532nm) is chosen based on patient characteristics and tattoo color or colors.
In a subset analysis, the investigators interviewed eight patients again after undergoing laser tattoo removal at their practice. All underwent treatment with a picosecond laser, perfluorodecalin patch, and injectable anesthesia. All reported minimal to no pain during the procedure and an optimal experience. No complications were noted.
Dr. Friedman and Dr. Suggs emphasized that consumers should be aware of the risks and potential for complications from laser tattoo removal. They recommend that all consumers – especially those at higher risk for complications such as higher Fitzpatrick skin type patients and those with multicolored tattoos – choose a provider with extensive training in the procedure, such as a board-certified dermatologist or plastic surgeon.
Dr. Suggs disclosed that she is an ambassador for Tri Sirena sun protective athletic apparel. Dr. Friedman disclosed that he is a member of the advisory board for Allergan, Solta Medical, Syneron-Candela, and Sienna Biopharmaceuticals. He is also a research investigator for Syneron-Candela and has received a research grant from Sienna.
REPORTING FROM ASLMS 2019
International registry of laser surgery outcomes in the works
DENVER – Researchers in the early stages of from existing studies in the medical literature. They’ve also observed insufficient reporting of outcome definitions and under-representation of life impact domains.
“Today, laser therapy is not only an important treatment modality for cosmetic indications, but also for medical skin disorders and diseases,” lead study author Frederike Fransen said at the annual conference of the American Society for Laser Medicine and Surgery. “These disorders include inflammatory lesions, vascular and pigmented lesions, tumors, and scars. Although there are a lot of options for laser therapy, the evidence for most of these skin conditions is quite low, consisting mostly of case reports and case series. However, if we want more evidence-based practice, we need more practice-based evidence.”
A new effort to gain insight into safety and effectiveness of laser treatments known as the international Laser Treatment Dermatology Registry (LEAD) is a platform to address this challenge. “We envision a registry that connects expertise and experience of a large international team of laser specialists, clinicians, and researchers,” said Ms. Fransen, a PhD candidate in the department of dermatology at Amsterdam University Medical Center. “Our goal is to gain insight into safety and effectiveness of laser treatments.” The collaboration includes researchers from the Netherlands, Denmark, France, Germany, Italy, and Switzerland, and the team will be complemented by experts from the United States, Asia, and North Africa.
For the first phase of the endeavor, Ms. Fransen and Albert Wolkerstorfer, MD, PhD, of the University of Amsterdam worked with colleagues from the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN) to develop a consensus of outcomes to be included in the registry. This involved a literature review of 350 articles to explore the outcomes used in laser research. From these, the researchers identified 100 articles for outcome mapping: 25 randomized, controlled trials, 44 trials that were not randomized or controlled, and 31 case reports. Their review yielded 98 outcomes and 53 outcome instruments.
Ultimately, outcomes were assigned to eight domains, Ms. Fransen said: appearance, long-term effects, physician-reported physical signs, patient-reported physical signs, satisfaction, health-related quality of life, psychological functioning, and adverse events. Of these domains, the most commonly used in existing medical literature were appearance such as clinical improvement and clearance (81%), followed by adverse effects such as erythema and scarring (55%), physician-reported signs such as morphology (30%), and long-term effects such as recurrence (27%). Ms. Fransen and Dr. Wolkerstorfer observed under-representation of patient-centered outcomes, including satisfaction of appearance or treatment (29%), patient-reported physical signs such as overall state and severity of disease (12%), health-related quality of life (4%), and psychological functioning such as anxiety and depression (1%).
The analysis also revealed that different outcomes measures were used in the various studies, and inconstant definitions within scaling systems. For example, for clearance of lesions/no clearance of lesions, some studies defined excellent clearance as 75% or greater, and others defined marked clearance as 70% or greater. In addition, some studies that used percentage quintile grading as an outcome defined grade 5 as a greater than 95% improvement, while others defined grade 5 as “clear,” or a greater than 90% improvement.
The next step in developing the LEAD registry involves performing an international e-Delphi survey, a method to obtain agreement on outcomes for the registry among health care professionals and patients with different opinions and backgrounds. “The process ends when sufficient agreement is obtained,” Ms. Fransen said. “Looking at future steps, the development of this collaborative initiative with a minimum set of outcomes is essential. When establishing this registry we can achieve sufficient sample size and confirmatory cases toward stronger evidence of laser treatments for orphan diseases.”
The project was supported by the European Academy of Dermatology and Venereology and by an educational grant from ASLMS.
[email protected]
DENVER – Researchers in the early stages of from existing studies in the medical literature. They’ve also observed insufficient reporting of outcome definitions and under-representation of life impact domains.
“Today, laser therapy is not only an important treatment modality for cosmetic indications, but also for medical skin disorders and diseases,” lead study author Frederike Fransen said at the annual conference of the American Society for Laser Medicine and Surgery. “These disorders include inflammatory lesions, vascular and pigmented lesions, tumors, and scars. Although there are a lot of options for laser therapy, the evidence for most of these skin conditions is quite low, consisting mostly of case reports and case series. However, if we want more evidence-based practice, we need more practice-based evidence.”
A new effort to gain insight into safety and effectiveness of laser treatments known as the international Laser Treatment Dermatology Registry (LEAD) is a platform to address this challenge. “We envision a registry that connects expertise and experience of a large international team of laser specialists, clinicians, and researchers,” said Ms. Fransen, a PhD candidate in the department of dermatology at Amsterdam University Medical Center. “Our goal is to gain insight into safety and effectiveness of laser treatments.” The collaboration includes researchers from the Netherlands, Denmark, France, Germany, Italy, and Switzerland, and the team will be complemented by experts from the United States, Asia, and North Africa.
For the first phase of the endeavor, Ms. Fransen and Albert Wolkerstorfer, MD, PhD, of the University of Amsterdam worked with colleagues from the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN) to develop a consensus of outcomes to be included in the registry. This involved a literature review of 350 articles to explore the outcomes used in laser research. From these, the researchers identified 100 articles for outcome mapping: 25 randomized, controlled trials, 44 trials that were not randomized or controlled, and 31 case reports. Their review yielded 98 outcomes and 53 outcome instruments.
Ultimately, outcomes were assigned to eight domains, Ms. Fransen said: appearance, long-term effects, physician-reported physical signs, patient-reported physical signs, satisfaction, health-related quality of life, psychological functioning, and adverse events. Of these domains, the most commonly used in existing medical literature were appearance such as clinical improvement and clearance (81%), followed by adverse effects such as erythema and scarring (55%), physician-reported signs such as morphology (30%), and long-term effects such as recurrence (27%). Ms. Fransen and Dr. Wolkerstorfer observed under-representation of patient-centered outcomes, including satisfaction of appearance or treatment (29%), patient-reported physical signs such as overall state and severity of disease (12%), health-related quality of life (4%), and psychological functioning such as anxiety and depression (1%).
The analysis also revealed that different outcomes measures were used in the various studies, and inconstant definitions within scaling systems. For example, for clearance of lesions/no clearance of lesions, some studies defined excellent clearance as 75% or greater, and others defined marked clearance as 70% or greater. In addition, some studies that used percentage quintile grading as an outcome defined grade 5 as a greater than 95% improvement, while others defined grade 5 as “clear,” or a greater than 90% improvement.
The next step in developing the LEAD registry involves performing an international e-Delphi survey, a method to obtain agreement on outcomes for the registry among health care professionals and patients with different opinions and backgrounds. “The process ends when sufficient agreement is obtained,” Ms. Fransen said. “Looking at future steps, the development of this collaborative initiative with a minimum set of outcomes is essential. When establishing this registry we can achieve sufficient sample size and confirmatory cases toward stronger evidence of laser treatments for orphan diseases.”
The project was supported by the European Academy of Dermatology and Venereology and by an educational grant from ASLMS.
[email protected]
DENVER – Researchers in the early stages of from existing studies in the medical literature. They’ve also observed insufficient reporting of outcome definitions and under-representation of life impact domains.
“Today, laser therapy is not only an important treatment modality for cosmetic indications, but also for medical skin disorders and diseases,” lead study author Frederike Fransen said at the annual conference of the American Society for Laser Medicine and Surgery. “These disorders include inflammatory lesions, vascular and pigmented lesions, tumors, and scars. Although there are a lot of options for laser therapy, the evidence for most of these skin conditions is quite low, consisting mostly of case reports and case series. However, if we want more evidence-based practice, we need more practice-based evidence.”
A new effort to gain insight into safety and effectiveness of laser treatments known as the international Laser Treatment Dermatology Registry (LEAD) is a platform to address this challenge. “We envision a registry that connects expertise and experience of a large international team of laser specialists, clinicians, and researchers,” said Ms. Fransen, a PhD candidate in the department of dermatology at Amsterdam University Medical Center. “Our goal is to gain insight into safety and effectiveness of laser treatments.” The collaboration includes researchers from the Netherlands, Denmark, France, Germany, Italy, and Switzerland, and the team will be complemented by experts from the United States, Asia, and North Africa.
For the first phase of the endeavor, Ms. Fransen and Albert Wolkerstorfer, MD, PhD, of the University of Amsterdam worked with colleagues from the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN) to develop a consensus of outcomes to be included in the registry. This involved a literature review of 350 articles to explore the outcomes used in laser research. From these, the researchers identified 100 articles for outcome mapping: 25 randomized, controlled trials, 44 trials that were not randomized or controlled, and 31 case reports. Their review yielded 98 outcomes and 53 outcome instruments.
Ultimately, outcomes were assigned to eight domains, Ms. Fransen said: appearance, long-term effects, physician-reported physical signs, patient-reported physical signs, satisfaction, health-related quality of life, psychological functioning, and adverse events. Of these domains, the most commonly used in existing medical literature were appearance such as clinical improvement and clearance (81%), followed by adverse effects such as erythema and scarring (55%), physician-reported signs such as morphology (30%), and long-term effects such as recurrence (27%). Ms. Fransen and Dr. Wolkerstorfer observed under-representation of patient-centered outcomes, including satisfaction of appearance or treatment (29%), patient-reported physical signs such as overall state and severity of disease (12%), health-related quality of life (4%), and psychological functioning such as anxiety and depression (1%).
The analysis also revealed that different outcomes measures were used in the various studies, and inconstant definitions within scaling systems. For example, for clearance of lesions/no clearance of lesions, some studies defined excellent clearance as 75% or greater, and others defined marked clearance as 70% or greater. In addition, some studies that used percentage quintile grading as an outcome defined grade 5 as a greater than 95% improvement, while others defined grade 5 as “clear,” or a greater than 90% improvement.
The next step in developing the LEAD registry involves performing an international e-Delphi survey, a method to obtain agreement on outcomes for the registry among health care professionals and patients with different opinions and backgrounds. “The process ends when sufficient agreement is obtained,” Ms. Fransen said. “Looking at future steps, the development of this collaborative initiative with a minimum set of outcomes is essential. When establishing this registry we can achieve sufficient sample size and confirmatory cases toward stronger evidence of laser treatments for orphan diseases.”
The project was supported by the European Academy of Dermatology and Venereology and by an educational grant from ASLMS.
[email protected]
REPORTING FROM ASLMS 2019
Powerful breast-implant testimony constrained by limited evidence
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
What’s the role of anecdotal medical histories in the era of evidence-based medicine?
But the anecdotal histories fell short of producing a clear committee consensus on dramatic, immediate changes in FDA policy, such as joining a renewed ban on certain types of breast implants linked with a rare lymphoma, a step recently taken by 38 other countries, including 33 European countries acting in concert through the European Union.
The disconnect between gripping testimony and limited panel recommendations was most stark for a complication that’s been named Breast Implant Illness (BII) by patients on the Internet. Many breast implant recipients have reported life-changing symptoms that appeared after implant placement, most often fatigue, joint and muscle pain, brain fog, neurologic symptoms, immune dysfunction, skin manifestations, and autoimmune disease or symptoms. By my count, 22 people spoke about their harrowing experiences with BII symptoms out of the 77 who stepped to the panel’s public-comment mic during 4 hours of public testimony over 2-days of hearings, often saying that they had experienced dramatic improvements after their implants came out. The meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee also heard presentations from two experts who ran some of the first reported studies on BII, or a BII-like syndrome called Autoimmune Syndrome Induced by Adjuvants (ASIA) described by Jan W.C. Tervaert, MD, professor of medicine and director of rheumatology at the University of Alberta in Edmonton. Dr. Tervaert and his associates published their findings about ASIA in the rheumatology literature last year (Clin Rheumatol. 2018 Feb;37[2]:483-93), and during his talk before the FDA panel, he said that silicone breast implants and the surgical mesh often used with them could be ASIA triggers.
Panel members seemed to mostly believe that the evidence they heard about BII did no more than hint at a possible association between breast implants and BII symptoms that required additional study. Many agreed on the need to include mention of the most common BII-linked patient complaints in informed consent material, but some were reluctant about even taking that step.
“I do not mention BII to patients. It’s not a disease; it’s a constellation of symptoms,” said panel member and plastic surgeon Pierre M. Chevray, MD, from Houston Methodist Hospital. The evidence for BII “is extremely anecdotal,” he said in an interview at the end of the 2-day session. Descriptions of BII “have been mainly published on social media. One reason why I don’t tell patients [about BII as part of informed consent] is because right now the evidence of a link is weak. We don’t yet even have a definition of this as an illness. A first step is to define it,” said Dr. Chevray, who has a very active implant practice. Other plastic surgeons were more accepting of BII as a real complication, although they agreed it needs much more study. During the testimony period, St. Louis plastic surgeon Patricia A. McGuire, MD, highlighted the challenge of teasing apart whether real symptoms are truly related to implants or are simply common ailments that accumulate during middle-age in many women. Dr. McGuire and some of her associates published an assessment of the challenges and possible solutions to studying BII that appeared shortly before the hearing (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S),
Consensus recommendations from the panel to the FDA to address BII included having a single registry that would include all U.S. patients who receive breast implants (recently launched as the National Breast Implant Registry), inclusion of a control group, and collection of data at baseline and after regular follow-up intervals that includes a variety of measures relevant to autoimmune and rheumatologic disorders. Several panel members cited inadequate postmarketing safety surveillance by manufacturers in the years since breast implants returned to the U.S. market, and earlier in March, the FDA issued warning letters to two of the four companies that market U.S. breast implants over their inadequate long-term safety follow-up.
The panel’s decisions about the other major implant-associated health risk it considered, breast implant associated anaplastic large cell lymphoma (BIA-ALCL), faced a different sort of challenge. First described as linked to breast implants in 2011, today there is little doubt that BIA-ALCL is a consequence of breast implants, what several patients derisively called a “man-made cancer.” The key issue the committee grappled with was whether the calculated incidence of BIA-ALCL was at a frequency that warranted a ban on at least selected breast implant types. Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, told the panel that he calculated the Allergan Biocell group of implants, which have textured surfaces that allows for easier and more stable placement in patients, linked with an incidence of BIA-ALCL that was sevenfold to eightfold higher than that with smooth implants. That’s against a background of an overall incidence of about one case for every 20,000 U.S. implant recipients, Dr. Clemens said.
Many testifying patients, including several of the eight who described a personal history of BIA-ALCL, called for a ban on the sale of at least some breast implants because of their role in causing lymphoma. That sentiment was shared by Dr. Chevray, who endorsed a ban on “salt-loss” implants (the method that makes Biocell implants) during his closing comments to his fellow panel members. But earlier during panel discussions, others on the committee pushed back against implant bans, leaving the FDA’s eventual decision on this issue unclear. Evidence presented during the hearings suggests that implants cause ALCL by triggering a local “inflammatory milieu” and that different types of implants can have varying levels of potency for producing this milieu.
Perhaps the closest congruence between what patients called for and what the committee recommended was on informed consent. “No doubt, patients feel that informed consent failed them,” concluded panel member Karen E. Burke, MD, a New York dermatologist who was one of two panel discussants for the topic.
In addition to many suggestions on how to improve informed consent and public awareness lobbed at FDA staffers during the session by panel members, the final public comment of the 2 days came from Laurie A. Casas, MD, a Chicago plastic surgeon affiliated with the University of Chicago and a member of the board of directors of the American Society of Aesthetic Plastic Surgery (also know as the Aesthetic Society). During her testimony, Dr. Casas said “Over the past 2 days, we heard that patients need a structured educational checklist for informed consent. The Aesthetic Society hears you,” and promised that the website of the Society’s publication, the Aesthetic Surgery Journal, will soon feature a safety checklist for people receiving breast implants that will get updated as new information becomes available. She also highlighted the need for a comprehensive registry and long-term follow-up of implant recipients by the plastic surgeons who treated them.
In addition to better informed consent, patients who came to the hearing clearly also hoped to raise awareness in the general American public about the potential dangers from breast implants and the need to follow patients who receive implants. The 2 days of hearing accomplished that in part just by taking place. The New York Times and The Washington Post ran at least a couple of articles apiece on implant safety just before or during the hearings, while a more regional paper, the Philadelphia Inquirer, ran one article, as presumably did many other newspapers, broadcast outlets, and websites across America. Much of the coverage focused on compelling and moving personal stories from patients.
Women who have been having adverse effects from breast implants “have felt dismissed,” noted panel member Natalie C. Portis, PhD, a clinical psychologist from Oakland, Calif., and the patient representative on the advisory committee. “We need to listen to women that something real is happening.”
Dr. Tervaert, Dr. Chevray, Dr. McGuire, Dr. Clemens, Dr. Burke, Dr. Casas, and Dr. Portis had no relevant commercial disclosures.
Topical Natural Products in Managing Dermatologic Conditions: Observations and Recommendations
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
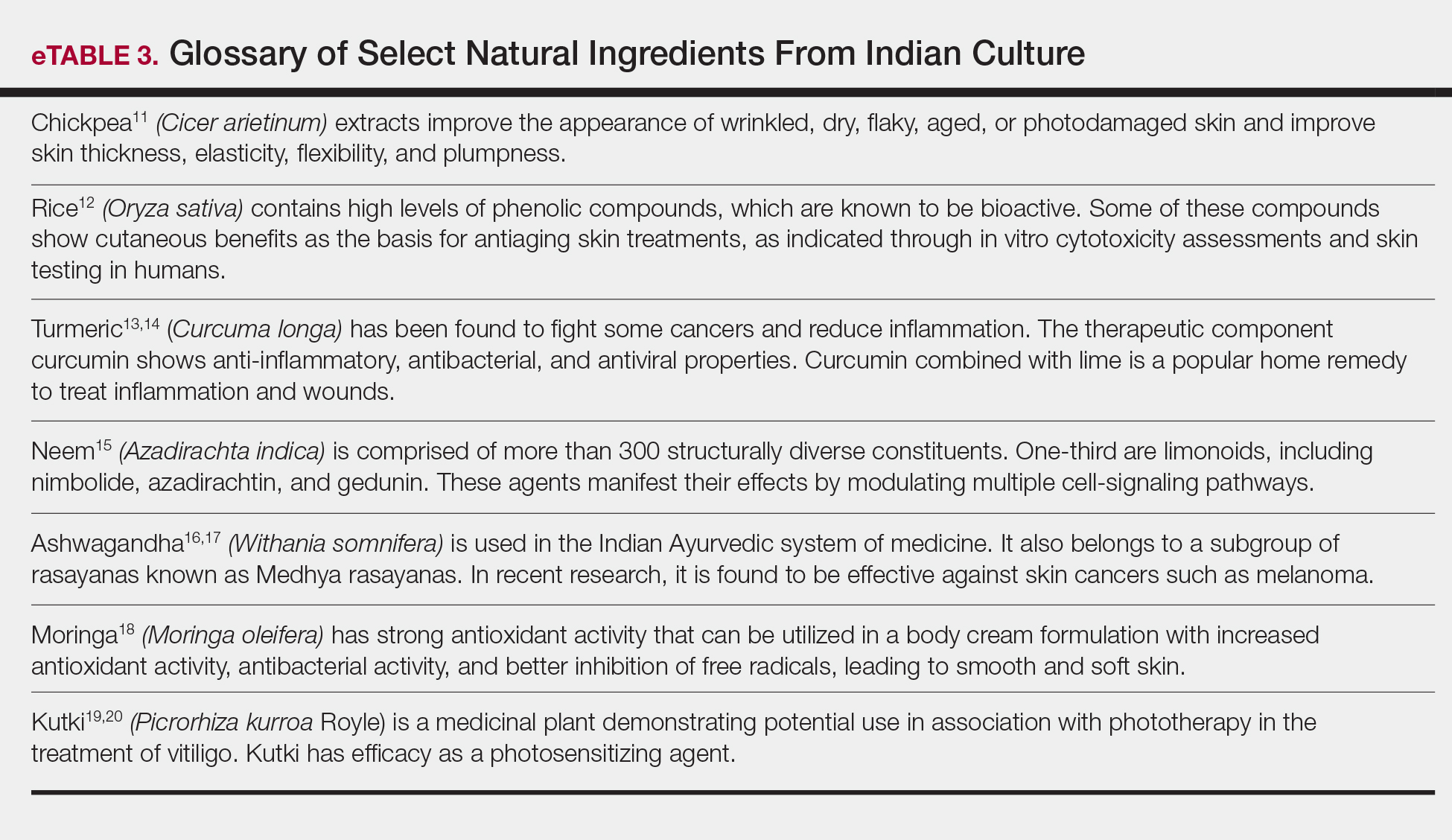
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.


For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.



For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
Patients seek healthy skin that conveys overall health and well-being. Cosmeceuticals claim to therapeutically affect the structure and function of the skin, and it is rational to hold them to scientific standards that substantiate efficacy claims.1 Notably, it is increasingly important to consider nature-based products in helping patients and consumers to achieve healthier skin. Despite the availability of sophisticated efficacy testing, explanations of the underlying physiologic and pharmacologic principles of nature-based products lag behind those of conventional formulations. In many instances, simple form and function information cannot adequately support their desired use and expected benefits. In addition, cosmetic regulations do not even permit structure-function claims that are allowed for dietary supplements.
Physicians whose patients want recommendations for nature-based products often do not know where to turn for definitive product and use information. Unlike prescription medications or even beauty-from-within dietary supplement products, natural cosmetics and cosmeceuticals are barred from communicating scientific evidence and experience of use to form proper opinions for recommendations. Without the benefit of full product labeling, physicians are left to mine sparse, confusing, and often contradictory literature in an effort to self-educate. Here, we share our experiences with patients, our operating knowledge base, and our recommendations for investigation to improve the available information and ensure practicing physicians have the information they need to appropriately recommend nature-based products.
General Observations Pertaining to Patients and Nature-Based Products
Ethnic and cultural customs and traditions have accepted and employed nature-based products for skin health for millennia (eTables 1–3).2-20 African and the derived Caribbean cultures frequently use shea butter, black soap, or coconut oil. East Asian ethnobotanical practices include the use of ginseng, green tea, almond, and angelica root in skin care. Indian culture employs Ayurvedic medicine principles that include herbal remedies comprised of ground chickpeas, rice, turmeric, neem, ashwagandha, moringa, and kutki. These cultural traditions continue into modern times, and patients regularly use these products. Modern social trends that focus on a healthy lifestyle also create demand for nature-based products for skin health. In our opinion, the current growing interest in nature-based products implies continued growth in their use as patients become more familiar and comfortable with them.



For beauty and skin health, a new trend has evolved in which the first source of advice is rarely a dermatologist. Social media, nonphysician influencers, and pseudoscience have created an authority previously reserved for dermatologists among patients and consumers. Bloggers and social media influencers, posting their individual real-world experiences, shape the perceptions of consumers and patients.21,22 Nonphysician influencers leverage their celebrity to provide guidance and advice on beauty and cosmetic tips.23 Much of the evidence supporting cosmetic and especially nature-based products for skin care and health often is believed to be less rigorous and of lower quality than that typically supporting physician recommendations.24-26
Nature-Based Products in Skin Health and Dermatologic Conditions
Patients turn to nature-based products for skin care and health for many reasons. The simplest reason is that they grew up with such products and continue their use. Many patients find nature-based products themselves, have favorable experiences, and seek advice on their efficacy and safety for continued use. Patients also use these products as part of a holistic approach to health in which diet and exercise coincide with the idea of ministering to the whole self instead of preventing or treating an illness. These nature-based treatment options fit their natural lifestyles. Patients sometimes express concerns about synthetic products that lead them to seek out nature-based products. Chemicals and preservatives (eg, parabens, sunscreens, nanoparticles) may evoke concerns about negative health consequences, which can be a cause of great anxiety to patients.
Nature-based products, when recommended by physicians, can fulfill important roles. As healthier alternatives, they can address health concerns in the belief that plant-based ingredients may be more compatible with overall health than synthetic ingredients. This compatibility may have resulted from the human species coevolving with plant species containing therapeutic utility, leading to the development of specific receptors for many natural products, such as digoxin from foxglove (Digitalis purpurea), opioids from poppies (Papaver somniferum), and cannabinoids (Cannabis sativa and hybrids). Natural products can become alternatives to synthetic products or adjuncts to prescription medications. Often, inclusion of nature-based products into a treatment plan enables patients to feel that they are a more integral part of the care team treating their conditions. By virtue of physician recommendations, patients may have expectations on product efficacy being as robust as prescription products with the safety profile of plant-based products. Patients should be advised to accept a realistic view of the efficacy and tolerability profiles. In the end, patients consider physician recommendations based on the assumption that they are credible and derived from experience and knowledge.
Physician Perceptions of Nature-Based Products
Physicians recommend nature-based products based on several factors. Central to the recommendation is an understanding, through appropriate documentation, that the product will be reasonably efficacious. Critical to this point, physicians must understand what ingredients are in nature-based products, their concentrations or amounts, and why they are present. However, our experience with nature-based products suggests that many of these factors are not met. Limited or unclear information on the efficacy of nature-based products fails to satisfy a physician’s need for adequate information to support recommendations. Although natural ingredients are listed on product labels, their intended benefit and efficacy characteristics often are unclear or poorly stated, in some cases resulting from improper labeling and in other cases due to claim restrictions imposed on cosmetics. In addition, insufficient details on formulation, such as type and percentages of oils, antioxidants, and vitamins, hinder the physician’s ability to identify and explain mechanisms that bring benefit to the patient. Universal benchmarks do not exist for amounts or concentrations of ingredients that are required for a stated benefit.27 Currently, no standards exist for assurances that product quality, control, and efficacy are consistently reproducible. For example, angel dusting is a practice that discloses that an active ingredient is present, yet these ingredients may be present in quantities that are insufficient to provide measurable benefit. Sourcing of ingredients also can be concerning, as they may not always meet manufacturer, physician, or patient expectations for characterization or efficacy.28,29 Dry testing, which is when a manufacturer contracts a laboratory to certify their ingredients without performing assays, has been increasingly reported in lay and botanical literature over the last few years.30
It is unknown if many nature-based products clinically exhibit their stated efficacy. Empirical evidence or well-conducted clinical studies on which to base recommendations of these products are limited. Individual natural ingredients, however, do have some supporting evidence of efficacy: shea butter moisturizes31; coconut oil exhibits anti-inflammatory properties32,33; and vinegar, yogurt, and diluted tea tree oil exhibit antibacterial properties in postprocedure care and fungal infections, and as adjuvants to prescription antibiotics in atopic dermatitis, acne, and rosacea.34-41 Honey also has been shown to improve wound healing and is even available as a medical device for wounds.42,43 Although nature-based products are an interesting alternative to synthetic products, they require a fulsome understanding of characteristics and efficacy properties to support physician recommendations.
Physician Recommendations
Physicians must be educated to understand when and how to recommend nature-based products. Although we recommend increased product information to guide physicians, current laws, including the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act, are satisfactory from a regulatory standpoint.44 Here, we discuss the information physicians could use to support an informed recommendation of nature-based products.
A clear specific explanation of natural ingredient sources, their intended efficacy, and rigorous scientific clinical evidence supporting their use should be given. Manufacturers are needed to document and report the structure and function of natural ingredients, leading to a common understanding by practicing dermatologists.45 For this reason, manufacturers must provide nonambiguous and standardized methods and measures to demonstrate the mechanism of ingredient efficacy and the limits of safety and tolerability.
We recommend that manufacturers provide standardized transparency into the composition of nature-based formulations, including amounts and concentrations of ingredients; geographic sources; parts of plants used; and if extracted, what agent(s) this standard is based on (eg, hypericin in Saint-John’s-wort or kavalactones in kava kava). Most natural products contain an aqueous phase and therefore will likely require preservatives such as synthetic parabens or alcohols to avoid degradation. Unnecessary ingredients, including fragrances, fillers, and support chemicals, should be absent since inert agents may exhibit biologic effects, obscuring the boundary between active and inert. A clear explanation of the origins of these nature-based ingredients and the concentration, purity, and activity assessment should be provided. In the context of an authoritative review with standardized measures, labels that provide the common name, plant name, part used, how it was obtained, concentrations and/or amounts, and standardized activity measures can be helpful to the recommending physician, who will then know the efficacy patients should expect from the ingredients. They also can assess the expected tolerability based on the concentrations and their own experience managing a particular disorder, tempered by the patient’s experiences with prior therapies. Transparent and standardized labeling describing the formulation, quantities of ingredients, and intended activity will help inform expectations of efficacy.
We recommend clear preclinical and clinical demonstrations of the efficacy and benefits that are claimed by nature-based formulations. Properly designed placebo- or active-controlled, blinded, randomized studies with standardized measures and end points are recommended to determine efficacy and safety. These demonstrations of efficacy can provide physicians with credible evidence on which to base their recommendations and guide the use of products for the patient’s best experience. Given sufficient involvement from manufacturers and publication of the information in peer-reviewed journals, the relative benefits for each nature-based product can be cataloged as a resource for physicians.
Conclusion
Patients turn to nature-based products for many reasons. They have high expectations but also harbor concerns as to the efficacy of these products for skin and health care. Physicians seek to recommend nature-based products for these patients but often find themselves disadvantaged by limited published evidence and insufficient labeling information on composition and efficacy, which should support recommendations for use. To remedy this situation, we suggest research to allow a clear explanation of the activity of natural ingredients, clear demonstrations of the efficacy of nature-based formulas using clinical standardized measures and end points, and clear education and disclosure of ingredients contained within nature-based products.
Acknowledgments—Burt’s Bees (Durham, North Carolina) provided funding for editorial support by Medical Dynamics, Inc (New York, New York).
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
- Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
- Ajala EO, Aberuagba F, Olaniyan AM, et al. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53:730-738.
- Lin A, Nabatian A, Halverstam CP. Discovering black soap: a survey on the attitudes and practices of black soap users. J Clin Aesthet Dermatol. 2017;10:18-22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19. pii:E70. doi:10.3390/ijms19010070.
- Dua K, Sheshala R, Ling TY, et al. Anti-inflammatory, antibacterial and analgesic potential of cocos nucifera linn.: a review. Antiinflamm Antiallergy Agents Med Chem. 2013;12:158-164.
- Hyun TK, Jang KI. Are berries useless by-products of ginseng? recent research on the potential health benefits of ginseng berry. EXCLI J. 2017;16:780-784.
- Truong VL, Bak MJ, Lee C, et al. Hair regenerative mechanisms of red ginseng oil and its major components in the testosterone-induced delay of anagen entry in C57BL/6 mice. Molecules. 2017;22. pii:E1505. doi:10.3390/molecules22091505.
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. 2017;33:931-936.
- Yi M, Fu J, Zhou L, et al. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J Int Soc Sports Nutr. 2014;11:18.
- Sowndhararajan K, Deepa P, Kim M, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm. 2017;85. pii:E33. doi:10.3390/scipharm85030033.
- Mahjour M, Khoushabi A, Noras M, et al. Effectiveness of Cicer arietinum in cutaneous problems: viewpoint of Avicenna and Razi. Curr Drug Discov Technol. 2018;15:243-250.
- Kanlayavattanakul M, Laurits N, Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607-616.
- Aggarwal BB, Yuan W, Li S, et al. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
- Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582-1592.
- Gupta SC, Prasad S, Tyagi AK, et al. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14-20.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599-612.
- Halder B, Singh S, Thakur SS. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS One. 2015;10:E0137498.
- Nadeem M, Imran M. Promising features of Moringa oleifera oil: recent updates and perspectives. Lipids Health Dis. 2016;15:212.
- Sultan P, Jan A, Pervaiz Q. Phytochemical studies for quantitative estimation of iridoid glycosides in Picrorhiza kurroa Royle. Bot Stud. 2016;57:7.
- Gianfaldoni S, Wollina U, Tirant M, et al. Herbal compounds for the treatment of vitiligo: a review. Open Access Maced J Med Sci. 2018;6:203-207.
- Diamantoglou M, Platz J, Vienken J. Cellulose carbamates and derivatives as hemocompatible membrane materials for hemodialysis. Artif Organs. 1999;23:15-22.
- Respiratory syncytial virus (RSV). Centers for Disease Control and Prevention website. http://www.cdc.gov/rsv/research/us-surveillance.html. Updated June 26, 2018. Accessed February 1, 2019.
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409-415.
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys Rev. 2012;4:107-116.
- Liu Z. How cosmeceuticals companies get away with pseudoscience. Pacific Standard website. https://psmag.com/environment/cosmetic-companies-get-away-pseudoscience-placebo-week-92455. Published October 15, 2014. Accessed February 1, 2019.
- Beyerstein BL. Alternative medicine and common errors of reasoning. Acad Med. 2001;76:230-237.
- Topical antimicrobial drug products for over-the-counter human use. US Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=333.310. Accessed February 1, 2019.
- Natural personal care. Natural Products Association website. https://www.npanational.org/certifications/natural-seal/natural-seal-personal-care/. Accessed March 27, 2019.
- Natural Cosmetics Standard. GFaW Web site. https://gfaw.eu/en/ncs-for-all-who-love-nature-and-cosmetics/ncs-information-for-consumer/. Accessed February 1, 2019.
- Brown PN, Betz JM, Jasch F. How to qualify an analytical laboratory for analysis of herbal dietary ingredients and avoid using a “dry lab”: a review of issues related to using a contract analytical laboratory by industry, academia, and regulatory agencies. HerbalGram. 2013:52-59.
- Oh MJ, Cho YH, Cha SY, et al. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin Cosmet Investig Dermatol. 2017;10:363-371.
- Famurewa AC, Aja PM, Maduagwuna EK, et al. Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomed Pharmacother. 2017;96:905-911.
- Intahphuak S, Khonsung P, Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. 2010;48:151-157.
- McKenna PJ, Lehr GS, Leist P, et al. Antiseptic effectiveness with fibroblast preservation. Ann Plast Surg. 1991;27:265-268.
- Brockow K, Grabenhorst P, Abeck D, et al. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus aureus-colonized atopic eczema. Dermatology. 1999;199:231-236.
- Larson D, Jacob SE. Tea tree oil. Dermatitis. 2012;23:48-49.
- Misner BD. A novel aromatic oil compound inhibits microbial overgrowth on feet: a case study. J Int Soc Sports Nutr. 2007;4:3.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Fuchs-Tarlovsky V, Marquez-Barba MF, Sriram K. Probiotics in dermatologic practice. Nutrition. 2016;32:289-295.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185-199.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18:237-251.
- Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50-57.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug AdministrationWeb site. https://www.fda.gov/cosmetics/guidanceregulation/lawsregulations/ucm074162.htm. Updated July 24, 2018. Accessed February 1, 2019.
- Wohlrab J. Topical preparations and their use in dermatology. J Dtsch Dermatol Ges. 2016;4:1061-1070
Practice Points
- Patients are increasingly interested in and asking for nature-based products and formulations to manage dermatologic conditions.
- Physicians can satisfy patient interests with nature-based formulations that are as beneficial or more so than synthetic formulations because of the physiologic activity of the ingredients within these formulations.
- Physicians should have resources available to them that adequately educate on nature-based ingredients and how to recommend them.
Lactobionic acid
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
What does a Google search for cosmetic and laser procedures reveal?
DENVER – of professional societies only 8% of the time, and even less frequently to websites of academic centers and peer-reviewed medical journals, results from a novel study showed.
“An increasing number of patients are seeking information about cosmetic and laser dermatology from online sources,” Jennifer L. Sawaya, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “There are several studies that have discussed the role of the Internet and social media in dermatology. To our knowledge, this is the first study to specifically look at the results of Google search terms within our field to investigate which sources are providing this information.”
Dr. Sawaya, a fellow at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, and her colleagues cross-measured keyword analytics provided by Zalea, an online resource on cosmetic treatments for consumers, with the most used Instagram hashtags to obtain 10 online keywords: body contouring, Botox, fillers, CoolSculpting, laser hair removal, tattoo removal, skin tightening, skin rejuvenation, cosmetic surgery, and liposuction. Next, they used an advanced Google search to obtain the top 25 search results for each of those 10 keywords and categorized information sources as professional societies, peer-reviewed journals, non–peer-reviewed online health information, news/media, device/cosmeceutical companies, clinical practices, academic centers, or medical spas.
Overall, the top search results came from clinical practices 23% of the time, followed by online health information sites (19%), medical spas (16%), and news/media (15%). A much smaller percentage of the search results came from professional societies (8%), academic centers (6%), and peer-reviewed medical journals (5%). Within the clinical practices and medical spas, nearly half of these sources were plastic surgeons, while board-certified dermatologists comprised only 21% of the clinical information sources.
When Dr. Sawaya and her associates evaluated the source of search results for each keyword, results varied. For example, search results for “body contouring” came most frequently from professional societies and clinical practices (20% each), “Botox” from news/media (36%), “fillers” from online health information (28%), “CoolSculpting” from clinical practices (40%), “laser hair removal” from news/media (32%), “tattoo removal” from medical spas (28%), “skin tightening” from news/media (24%), “skin rejuvenation” from medical spas (28%), “cosmetic surgery” from clinical practices (52%), and “liposuction” from online health information (36%).
“Our clinical take-home message is essentially a call for an increasing amount of evidence-based, academic content to be made available for online consumption,” Dr. Sawaya said. “In an era when patients seek a lot of medical information online and make important decisions through this manner, we have an obligation to understand what is out there and do our best to improve the quality of available information.”
She acknowledged certain limitations of the study, including the fact that results of a Google search may vary depending on the type of device used (mobile, desktop) as well as the location of the device. “An additional limitation is how the search history on the device may impact results,” she said. “To control for this, the device history, cache, and cookies were cleared prior to the search. Despite these controls, it is unclear how and to what extent prior searches affect the Google ranking algorithm. We acknowledge that the findings in this study reflect a single point in time and that the results of a Google search will change dynamically based on many factors. Finally, we acknowledge that our study is based on a single search engine site and that the trends we observe with Google may not be extrapolated to other online channels.”
Dr. Sawaya reported having no financial disclosures.
DENVER – of professional societies only 8% of the time, and even less frequently to websites of academic centers and peer-reviewed medical journals, results from a novel study showed.
“An increasing number of patients are seeking information about cosmetic and laser dermatology from online sources,” Jennifer L. Sawaya, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “There are several studies that have discussed the role of the Internet and social media in dermatology. To our knowledge, this is the first study to specifically look at the results of Google search terms within our field to investigate which sources are providing this information.”
Dr. Sawaya, a fellow at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, and her colleagues cross-measured keyword analytics provided by Zalea, an online resource on cosmetic treatments for consumers, with the most used Instagram hashtags to obtain 10 online keywords: body contouring, Botox, fillers, CoolSculpting, laser hair removal, tattoo removal, skin tightening, skin rejuvenation, cosmetic surgery, and liposuction. Next, they used an advanced Google search to obtain the top 25 search results for each of those 10 keywords and categorized information sources as professional societies, peer-reviewed journals, non–peer-reviewed online health information, news/media, device/cosmeceutical companies, clinical practices, academic centers, or medical spas.
Overall, the top search results came from clinical practices 23% of the time, followed by online health information sites (19%), medical spas (16%), and news/media (15%). A much smaller percentage of the search results came from professional societies (8%), academic centers (6%), and peer-reviewed medical journals (5%). Within the clinical practices and medical spas, nearly half of these sources were plastic surgeons, while board-certified dermatologists comprised only 21% of the clinical information sources.
When Dr. Sawaya and her associates evaluated the source of search results for each keyword, results varied. For example, search results for “body contouring” came most frequently from professional societies and clinical practices (20% each), “Botox” from news/media (36%), “fillers” from online health information (28%), “CoolSculpting” from clinical practices (40%), “laser hair removal” from news/media (32%), “tattoo removal” from medical spas (28%), “skin tightening” from news/media (24%), “skin rejuvenation” from medical spas (28%), “cosmetic surgery” from clinical practices (52%), and “liposuction” from online health information (36%).
“Our clinical take-home message is essentially a call for an increasing amount of evidence-based, academic content to be made available for online consumption,” Dr. Sawaya said. “In an era when patients seek a lot of medical information online and make important decisions through this manner, we have an obligation to understand what is out there and do our best to improve the quality of available information.”
She acknowledged certain limitations of the study, including the fact that results of a Google search may vary depending on the type of device used (mobile, desktop) as well as the location of the device. “An additional limitation is how the search history on the device may impact results,” she said. “To control for this, the device history, cache, and cookies were cleared prior to the search. Despite these controls, it is unclear how and to what extent prior searches affect the Google ranking algorithm. We acknowledge that the findings in this study reflect a single point in time and that the results of a Google search will change dynamically based on many factors. Finally, we acknowledge that our study is based on a single search engine site and that the trends we observe with Google may not be extrapolated to other online channels.”
Dr. Sawaya reported having no financial disclosures.
DENVER – of professional societies only 8% of the time, and even less frequently to websites of academic centers and peer-reviewed medical journals, results from a novel study showed.
“An increasing number of patients are seeking information about cosmetic and laser dermatology from online sources,” Jennifer L. Sawaya, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “There are several studies that have discussed the role of the Internet and social media in dermatology. To our knowledge, this is the first study to specifically look at the results of Google search terms within our field to investigate which sources are providing this information.”
Dr. Sawaya, a fellow at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, and her colleagues cross-measured keyword analytics provided by Zalea, an online resource on cosmetic treatments for consumers, with the most used Instagram hashtags to obtain 10 online keywords: body contouring, Botox, fillers, CoolSculpting, laser hair removal, tattoo removal, skin tightening, skin rejuvenation, cosmetic surgery, and liposuction. Next, they used an advanced Google search to obtain the top 25 search results for each of those 10 keywords and categorized information sources as professional societies, peer-reviewed journals, non–peer-reviewed online health information, news/media, device/cosmeceutical companies, clinical practices, academic centers, or medical spas.
Overall, the top search results came from clinical practices 23% of the time, followed by online health information sites (19%), medical spas (16%), and news/media (15%). A much smaller percentage of the search results came from professional societies (8%), academic centers (6%), and peer-reviewed medical journals (5%). Within the clinical practices and medical spas, nearly half of these sources were plastic surgeons, while board-certified dermatologists comprised only 21% of the clinical information sources.
When Dr. Sawaya and her associates evaluated the source of search results for each keyword, results varied. For example, search results for “body contouring” came most frequently from professional societies and clinical practices (20% each), “Botox” from news/media (36%), “fillers” from online health information (28%), “CoolSculpting” from clinical practices (40%), “laser hair removal” from news/media (32%), “tattoo removal” from medical spas (28%), “skin tightening” from news/media (24%), “skin rejuvenation” from medical spas (28%), “cosmetic surgery” from clinical practices (52%), and “liposuction” from online health information (36%).
“Our clinical take-home message is essentially a call for an increasing amount of evidence-based, academic content to be made available for online consumption,” Dr. Sawaya said. “In an era when patients seek a lot of medical information online and make important decisions through this manner, we have an obligation to understand what is out there and do our best to improve the quality of available information.”
She acknowledged certain limitations of the study, including the fact that results of a Google search may vary depending on the type of device used (mobile, desktop) as well as the location of the device. “An additional limitation is how the search history on the device may impact results,” she said. “To control for this, the device history, cache, and cookies were cleared prior to the search. Despite these controls, it is unclear how and to what extent prior searches affect the Google ranking algorithm. We acknowledge that the findings in this study reflect a single point in time and that the results of a Google search will change dynamically based on many factors. Finally, we acknowledge that our study is based on a single search engine site and that the trends we observe with Google may not be extrapolated to other online channels.”
Dr. Sawaya reported having no financial disclosures.
REPORTING FROM ASLMS 2019
Key clinical point: There is a paucity of online information regarding cosmetic and laser dermatology from professional societies and academic, peer-reviewed sources.
Major finding: Top Google search results came from clinical practices 23% of the time and from professional societies only 8% of the time.
Study details: An online review of 25 Google search results for 10 keywords associated with cosmetic and laser dermatology.
Disclosures: Dr. Sawaya reported having no financial disclosures.
Study finds pain perception disconnect during vascular laser procedures
DENVER – There is an apparent disconnect between the level of periprocedural pain experienced by patients during vascular laser procedures and what device manufacturers say that level of pain should be, results from a retrospective study showed.
“Although there is an abundance of research on how pain signals are transmitted in the nervous system and how pain is perceived among certain patient demographics, there is not much known about how pain perception differs from that put forth by industry,” Lauren Bonati, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “This study is unique because we are questioning not whether pain perception is reproducible between patients, but rather if it reflects what industry and device manufacturers are telling us.”
Dr. Bonati, a dermatologist at Edwards, Colo.–based Mountain Dermatology Specialists, and her colleagues collected median and mode pain scores from a past clinical trial that investigated a dual wavelength laser used for different types of treatments. “The treatment type (laser wavelength and treatment area) was largely based on the severity of facial redness for each individual patient,” she explained. “The options were spot treatment, nose and cheeks, or a global facial treatment with either wavelength.” The researchers reviewed industry-provided materials to determine language regarding procedural pain, and they interviewed the clinical trial’s principal investigator about how pain expectations were set during the trial. Next, they transferred subject-reported pain scores and verbal pain descriptors to the validated Numerical Rating Scale and the Verbal Rating Scale, for comparison.
In all, 85 procedural pain scores were collected from 22 subject charts. The researchers found that the average procedural pain scores for treatment types reported by subjects were translated to entirely different verbal and numerical categories of pain from those described by industry materials. “It was surprising to see how vague pain descriptions can be in device manuals and industry materials, if even addressed at all,” Dr. Bonati said.
She advised clinicians to be wary of whom they rely on for information related to pain expectations. “Also, remember that wrongly set pain expectations can have physiologic and emotional effects that may positively or negatively impact patient experience,” Dr. Bonati said.
She acknowledged certain limitations of the study, including the fact that it was a review of a previously conducted clinical trial, “which is not a perfect representation of real-life clinic.”
She reported having no conflicts of interest.
DENVER – There is an apparent disconnect between the level of periprocedural pain experienced by patients during vascular laser procedures and what device manufacturers say that level of pain should be, results from a retrospective study showed.
“Although there is an abundance of research on how pain signals are transmitted in the nervous system and how pain is perceived among certain patient demographics, there is not much known about how pain perception differs from that put forth by industry,” Lauren Bonati, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “This study is unique because we are questioning not whether pain perception is reproducible between patients, but rather if it reflects what industry and device manufacturers are telling us.”
Dr. Bonati, a dermatologist at Edwards, Colo.–based Mountain Dermatology Specialists, and her colleagues collected median and mode pain scores from a past clinical trial that investigated a dual wavelength laser used for different types of treatments. “The treatment type (laser wavelength and treatment area) was largely based on the severity of facial redness for each individual patient,” she explained. “The options were spot treatment, nose and cheeks, or a global facial treatment with either wavelength.” The researchers reviewed industry-provided materials to determine language regarding procedural pain, and they interviewed the clinical trial’s principal investigator about how pain expectations were set during the trial. Next, they transferred subject-reported pain scores and verbal pain descriptors to the validated Numerical Rating Scale and the Verbal Rating Scale, for comparison.
In all, 85 procedural pain scores were collected from 22 subject charts. The researchers found that the average procedural pain scores for treatment types reported by subjects were translated to entirely different verbal and numerical categories of pain from those described by industry materials. “It was surprising to see how vague pain descriptions can be in device manuals and industry materials, if even addressed at all,” Dr. Bonati said.
She advised clinicians to be wary of whom they rely on for information related to pain expectations. “Also, remember that wrongly set pain expectations can have physiologic and emotional effects that may positively or negatively impact patient experience,” Dr. Bonati said.
She acknowledged certain limitations of the study, including the fact that it was a review of a previously conducted clinical trial, “which is not a perfect representation of real-life clinic.”
She reported having no conflicts of interest.
DENVER – There is an apparent disconnect between the level of periprocedural pain experienced by patients during vascular laser procedures and what device manufacturers say that level of pain should be, results from a retrospective study showed.
“Although there is an abundance of research on how pain signals are transmitted in the nervous system and how pain is perceived among certain patient demographics, there is not much known about how pain perception differs from that put forth by industry,” Lauren Bonati, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “This study is unique because we are questioning not whether pain perception is reproducible between patients, but rather if it reflects what industry and device manufacturers are telling us.”
Dr. Bonati, a dermatologist at Edwards, Colo.–based Mountain Dermatology Specialists, and her colleagues collected median and mode pain scores from a past clinical trial that investigated a dual wavelength laser used for different types of treatments. “The treatment type (laser wavelength and treatment area) was largely based on the severity of facial redness for each individual patient,” she explained. “The options were spot treatment, nose and cheeks, or a global facial treatment with either wavelength.” The researchers reviewed industry-provided materials to determine language regarding procedural pain, and they interviewed the clinical trial’s principal investigator about how pain expectations were set during the trial. Next, they transferred subject-reported pain scores and verbal pain descriptors to the validated Numerical Rating Scale and the Verbal Rating Scale, for comparison.
In all, 85 procedural pain scores were collected from 22 subject charts. The researchers found that the average procedural pain scores for treatment types reported by subjects were translated to entirely different verbal and numerical categories of pain from those described by industry materials. “It was surprising to see how vague pain descriptions can be in device manuals and industry materials, if even addressed at all,” Dr. Bonati said.
She advised clinicians to be wary of whom they rely on for information related to pain expectations. “Also, remember that wrongly set pain expectations can have physiologic and emotional effects that may positively or negatively impact patient experience,” Dr. Bonati said.
She acknowledged certain limitations of the study, including the fact that it was a review of a previously conducted clinical trial, “which is not a perfect representation of real-life clinic.”
She reported having no conflicts of interest.
REPORTING FROM ASLMS 2019
Key clinical point: Industry-provided materials failed to capture the range of procedural pain scores reported by patients undergoing a variety of vascular laser procedures.
Major finding: The average procedural pain scores for treatment types reported by subjects were translated to entirely different verbal and numerical categories of pain from those described by industry materials.
Study details: A retrospective evaluation of 85 procedural pain scores collected from 22 subject charts.
Disclosures: Dr. Bonati reported having no financial disclosures.
Investigative magnetic device found effective for skin tightening in a small study
DENVER – Patients treated with results from a small trial showed.
“There are many different modalities for tissue tightening, including lights, radiofrequency, ultrasound and thermal energy,” Jerome M. Garden, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “The idea behind all of these technologies is to heat up the skin’s collagen and to stimulate further collagen production, which can then result in improved skin tightening and textural improvement.”
In a trial conducted at the Chicago-based Physicians Laser and Dermatology Institute, Dr. Garden and his colleagues evaluated a new technology for tissue tightening that involves magnetic energy. Developed by Rocky Mountain Biosystems and BioFusionary Corp., the investigative device produces a magnetic field in the targeted tissue, which then results in the heating and eventual tightening of the tissue. “By using magnetic energy, which relies on the polarity of the molecules, it allows for a safe way to target specifically the polar dermis, without heating the relatively dry epidermis or nonpolar adipose layer, resulting in a more tolerable and potentially safer alternative to tissue tightening,” said Dr. Garden, a dermatologist who is the director of the Physicians Laser and Dermatology Institute.
For the trial, 20 patients with facial and upper skin laxity underwent a mean of 4.3 treatment sessions with the 27MHz magnetic device that used a 3-cm spot size, with a minimum of 4 weeks between each session. No anesthetics or analgesics were used. “No gels or skin prep was performed before the treatment, other than a gentle soap beforehand,” Dr. Garden said. “A bland moisturizer was applied to the treated skin after treatments.” The majority of patients (85%) had paid for their procedures (a price comparable to other skin-tightening procedures), and two board-certified dermatologists evaluated both before and after photographs for overall improvement of skin laxity and texture. Follow-ups were done 2-4 months after the last treatment. The observers were not informed which photographs were before or after.
Dr. Garden reported that the observers correctly chose 19 out of 20 patients’ before and after photographs, and they rated the mean grade level of improvement as 43%. Nearly half of the patients (48%) were graded at 50% or greater improvement. At the same time, patients rated their own improvement as a mean 6.5 out of 10. Nearly half of patients graded their outcome at 7 or better, which was designated as “very satisfied.” The procedures were well tolerated, Dr. Garden said, and the most common side effects were minor transient erythema and edema. The erythema generally faded after 2-4 hours, and the mild edema lasted up to 24 hours.
“Magnetic energy is a new technology that can be used to treat lower face and neck laxity,” said Dr. Garden, who is also a professor of clinical dermatology at Northwestern University, Chicago. “We only treated patients with skin types I-IV, but we feel that this technology is likely safe for higher skin types as well.”
Rocky Mountain Biosystems and BioFusionary Corp. provided the device used for the study. Dr. Garden and his colleagues are currently extending the ongoing trial. He reported having no financial disclosures.
DENVER – Patients treated with results from a small trial showed.
“There are many different modalities for tissue tightening, including lights, radiofrequency, ultrasound and thermal energy,” Jerome M. Garden, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “The idea behind all of these technologies is to heat up the skin’s collagen and to stimulate further collagen production, which can then result in improved skin tightening and textural improvement.”
In a trial conducted at the Chicago-based Physicians Laser and Dermatology Institute, Dr. Garden and his colleagues evaluated a new technology for tissue tightening that involves magnetic energy. Developed by Rocky Mountain Biosystems and BioFusionary Corp., the investigative device produces a magnetic field in the targeted tissue, which then results in the heating and eventual tightening of the tissue. “By using magnetic energy, which relies on the polarity of the molecules, it allows for a safe way to target specifically the polar dermis, without heating the relatively dry epidermis or nonpolar adipose layer, resulting in a more tolerable and potentially safer alternative to tissue tightening,” said Dr. Garden, a dermatologist who is the director of the Physicians Laser and Dermatology Institute.
For the trial, 20 patients with facial and upper skin laxity underwent a mean of 4.3 treatment sessions with the 27MHz magnetic device that used a 3-cm spot size, with a minimum of 4 weeks between each session. No anesthetics or analgesics were used. “No gels or skin prep was performed before the treatment, other than a gentle soap beforehand,” Dr. Garden said. “A bland moisturizer was applied to the treated skin after treatments.” The majority of patients (85%) had paid for their procedures (a price comparable to other skin-tightening procedures), and two board-certified dermatologists evaluated both before and after photographs for overall improvement of skin laxity and texture. Follow-ups were done 2-4 months after the last treatment. The observers were not informed which photographs were before or after.
Dr. Garden reported that the observers correctly chose 19 out of 20 patients’ before and after photographs, and they rated the mean grade level of improvement as 43%. Nearly half of the patients (48%) were graded at 50% or greater improvement. At the same time, patients rated their own improvement as a mean 6.5 out of 10. Nearly half of patients graded their outcome at 7 or better, which was designated as “very satisfied.” The procedures were well tolerated, Dr. Garden said, and the most common side effects were minor transient erythema and edema. The erythema generally faded after 2-4 hours, and the mild edema lasted up to 24 hours.
“Magnetic energy is a new technology that can be used to treat lower face and neck laxity,” said Dr. Garden, who is also a professor of clinical dermatology at Northwestern University, Chicago. “We only treated patients with skin types I-IV, but we feel that this technology is likely safe for higher skin types as well.”
Rocky Mountain Biosystems and BioFusionary Corp. provided the device used for the study. Dr. Garden and his colleagues are currently extending the ongoing trial. He reported having no financial disclosures.
DENVER – Patients treated with results from a small trial showed.
“There are many different modalities for tissue tightening, including lights, radiofrequency, ultrasound and thermal energy,” Jerome M. Garden, MD, said in an interview in advance of the annual conference of the American Society for Laser Medicine and Surgery. “The idea behind all of these technologies is to heat up the skin’s collagen and to stimulate further collagen production, which can then result in improved skin tightening and textural improvement.”
In a trial conducted at the Chicago-based Physicians Laser and Dermatology Institute, Dr. Garden and his colleagues evaluated a new technology for tissue tightening that involves magnetic energy. Developed by Rocky Mountain Biosystems and BioFusionary Corp., the investigative device produces a magnetic field in the targeted tissue, which then results in the heating and eventual tightening of the tissue. “By using magnetic energy, which relies on the polarity of the molecules, it allows for a safe way to target specifically the polar dermis, without heating the relatively dry epidermis or nonpolar adipose layer, resulting in a more tolerable and potentially safer alternative to tissue tightening,” said Dr. Garden, a dermatologist who is the director of the Physicians Laser and Dermatology Institute.
For the trial, 20 patients with facial and upper skin laxity underwent a mean of 4.3 treatment sessions with the 27MHz magnetic device that used a 3-cm spot size, with a minimum of 4 weeks between each session. No anesthetics or analgesics were used. “No gels or skin prep was performed before the treatment, other than a gentle soap beforehand,” Dr. Garden said. “A bland moisturizer was applied to the treated skin after treatments.” The majority of patients (85%) had paid for their procedures (a price comparable to other skin-tightening procedures), and two board-certified dermatologists evaluated both before and after photographs for overall improvement of skin laxity and texture. Follow-ups were done 2-4 months after the last treatment. The observers were not informed which photographs were before or after.
Dr. Garden reported that the observers correctly chose 19 out of 20 patients’ before and after photographs, and they rated the mean grade level of improvement as 43%. Nearly half of the patients (48%) were graded at 50% or greater improvement. At the same time, patients rated their own improvement as a mean 6.5 out of 10. Nearly half of patients graded their outcome at 7 or better, which was designated as “very satisfied.” The procedures were well tolerated, Dr. Garden said, and the most common side effects were minor transient erythema and edema. The erythema generally faded after 2-4 hours, and the mild edema lasted up to 24 hours.
“Magnetic energy is a new technology that can be used to treat lower face and neck laxity,” said Dr. Garden, who is also a professor of clinical dermatology at Northwestern University, Chicago. “We only treated patients with skin types I-IV, but we feel that this technology is likely safe for higher skin types as well.”
Rocky Mountain Biosystems and BioFusionary Corp. provided the device used for the study. Dr. Garden and his colleagues are currently extending the ongoing trial. He reported having no financial disclosures.
REPORTING FROM ASLMS 2019
Key clinical point: A device that delivers high magnetic energy was found safe and effective for treatment of skin laxity.
Major finding: Following treatment, dermatologists graded nearly half of the patients (48%) at 50% or greater improvement.
Study details: A single-center trial of 20 patients with facial and upper skin laxity who underwent a mean of 4.3 treatment sessions.
Disclosures: Rocky Mountain Biosystems and BioFusionary Corp. provided the device used for the study. Dr. Garden reported having no financial disclosures.


















