User login
Rupturing bullae not responding to antibiotics
A 33-year-old African American woman came to the office with a 2-week history of skin lesions and itching. The lesions started with a single blister on her left elbow; numerous other blisters subsequently appeared on her forearm and hands. One week before this visit, she had been given a presumptive diagnosis of bullous impetigo and was treated with cephalexin.
Despite the antibiotics, other lesions soon appeared in the nuchal and breast folds, axillae, and scalp areas. Several had ruptured, producing purulent, malodorous material. She had no known allergies, no medical problems aside from obesity, and no significant family history or recent travels. She denied any illicit drug use and had not been on any medications.
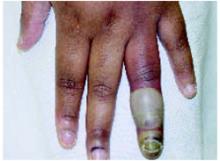
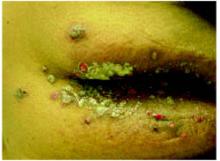
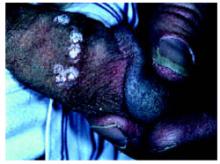
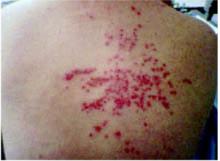
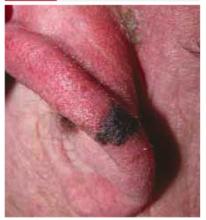
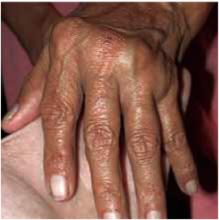
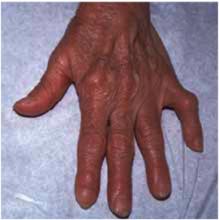
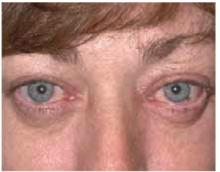
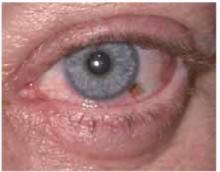
On physical exam, 1 large bulla was seen on the fourth digit of her left hand (Figure 1). The patient was obese, and inspection of the skin folds of her abdomen showed multiple suppurative lesions and erosions where previous bullae were found (Figure 2). No oral or gingival erosions were seen. Labs showed a white blood cell (WBC) count of 10.5 x109L], hemoglobin of 11.0 g/dL, and hemoglobin A1cof 5.5; liver function tests were normal. Gram stain showed no WBC and had rare Gram-positive bacilli. Potassium hydroxide prep of a skin lesion scraping showed no fungal elements. A herpes culture was performed along with a punch biopsy.
FIGURE 1
Bulla on the index finger
FIGURE 2
Multiple bullae on the trunk
What is the diagnosis?
Differential diagnosis
Many diseases manifest with bullae/vesicles. Workup should begin with a complete history and physical exam. A skin biopsy may be needed to make a definitive diagnosis.
Herpes zoster typically manifests with clustered pruritic vesicular lesions on a red base that follow a dermatomal distribution. Pemphigus vulgaris appears with flaccid blisters, erosions, and tend to have oral mucosal lesions. A positive Nikolsky’s sign is characteristic of pemphigus vulgaris. Bullous impetigo appears with scattered lesions of erythema and macules, progressing to thin roofed bullae and subsequently to “honey-crusted” lesions. In toxic epidermal necrolysis, the bullae are widespread and lead to sloughing of the skin. Pyoderma gangrenosum has ulcer formation preceded by pustules that typically expand rapidly to approximately 20 cm. These ulcers have necrotic bluish edges.
Diagnostic test results
The patient’s herpes culture was negative. Fortunately, the punch biopsy was sent for direct immunofluorescence. Direct immunofluorescence showed positive staining with immunoglobulin (Ig) G in the intercellular regions of the epidermis and no staining with IgA, IgM, C3, or fibrinogen. Hematoxylin and eosin-stained sections showed suprabasal blistering containing neutrophils and a few eosinophils. These results are consistent with pemphigus vulgaris.
Diagnosis: pemphigus vulgaris
Pemphigus vulgaris is blistering disease involving the skin and mucous membranes, with severe morbidity and occasional mortality. Prior to the development of effective treatment, the disease was 75% fatal within 5 years.1
Its prevalence is equal among men and women, with a rate of occurrence of 0.5/100,000 people per year. The average age of onset is in the fifth and sixth decades of life, but there is wide variation in age. It has multiple causes and risk factors (Table).
The clinical manifestation of pemphigus typically features mucocutaneous blisters followed by erosions. Often they appear first in mucous membranes and may not appear cutaneously until several months later.2 The skin lesions are painful flaccid blisters that may appear anywhere. A characteristic finding of pemphigus vulgaris is the Nikolsky sign, in which lateral stress applied to perilesional skin causes an expansion of the blistering.
There are 2 major subtypes of pemphigus. Pemphigus vulgaris has blisters extending to the deep epidermis, and pemphigus foliaceus has more superficial involvement of the epidermis. Pemphigus can also be seen in paraneoplastics syndromes.
In pemphigus, the epidermal cells lose normal cell contacts and form a blister. Electron microscopy shows desmosomal abnormalities at desmosomal junctions. It is these junctions that guarantee the integrity of the epithelium. Direct immunofluorescence shows IgG deposition in intercellular spaces.
TABLECauses and risk factors for pemphigus vulgaris
| Penicillamine |
| Captopril |
| Rifampin |
| Phenol-based drugs |
| Amide-based drugs |
| Foods: garlic, leek, onion |
| Pregnancy |
| Pesticide exposure |
| Herpes virus infection |
| Cytomegalovirus infection |
| Epstein-Barr virus infectyion |
| Adapted from: Benner et al 2003.4 |
Treatment: Steroids and adjuvant drugs
Inducing remission is the main goal of therapy for pemphigus vulgaris. Epidemiological studies have shown up to a 75% remission rate 10 years after initial diagnosis.3 Corticosteroids are the preferred therapy for the management of pemphigus vulgaris (based on expert opinion). Also, adjuvant drugs such as azathioprine and cyclophosphamide are commonly used in combination with corticosteroids, with the aim of increasing efficacy and of having a steroid-sparing action (level of evidence: 5, expert opinion).
A tailored dosing schedule of steroids has been advocated according to the severity of the disease. Mild disease can be treated with an initial prednisone dose of 40 to 60 mg/d; in severe cases, 60 to 100 mg/d. Other agents that have been used to treat pemphigus vulgaris are intramuscular gold, dapsone, and intravenous immunoglobulin.
Patient outcome
The patient was treated with oral prednisone starting at 60 mg/d and her skin began to clear. A full course of oral prednisone was continued and tapered over 1 month. Currently, she remains in remission off all medications.
Conclusion
Pemphigus vulgaris is a potentially life-threatening condition that must be recognized and treated promptly. With a lack of large-scale controlled studies, the diagnosis and management of pemphigus vulgaris has based on expert opinion.3 Complications such as superimposed infection of the lesions, cellulitis, and sepsis can occur. Its association with underlying neoplasm, thymomas, myasthenia gravis, and other autoimmune disorders warrants consideration for additional workup when indicated.
Correspondence
John Sauret, MD, Department of Family Medicine, State University of New York at Buffalo, 150 Family Medical Modular Complex, Buffalo, NY 14214-3013. E-mail: [email protected].
1. Sami N, Ahmed AR. Dual diagnosis of pemphigus and pemphigoid. Retrospective review of 30 cases in the literature. Dermatol 2001;202:293-301.
2. Ahmed AR, Graham J. Pemphigus: current concepts. Ann Intern Med 1980;92:396-405.
3. Harman KE, Albert S, Black MM. Guidelines for the management of pemphigus vulgaris. Br J Dermatol 2003;149:926-937.
4. Benner S, Mashiah J, Tamir E, Goldberg I, Wohl Y. PEMPHIGUS: an acronym for a disease with multiple etiologies. Skinmed 2003;2:163-167.
A 33-year-old African American woman came to the office with a 2-week history of skin lesions and itching. The lesions started with a single blister on her left elbow; numerous other blisters subsequently appeared on her forearm and hands. One week before this visit, she had been given a presumptive diagnosis of bullous impetigo and was treated with cephalexin.
Despite the antibiotics, other lesions soon appeared in the nuchal and breast folds, axillae, and scalp areas. Several had ruptured, producing purulent, malodorous material. She had no known allergies, no medical problems aside from obesity, and no significant family history or recent travels. She denied any illicit drug use and had not been on any medications.
On physical exam, 1 large bulla was seen on the fourth digit of her left hand (Figure 1). The patient was obese, and inspection of the skin folds of her abdomen showed multiple suppurative lesions and erosions where previous bullae were found (Figure 2). No oral or gingival erosions were seen. Labs showed a white blood cell (WBC) count of 10.5 x109L], hemoglobin of 11.0 g/dL, and hemoglobin A1cof 5.5; liver function tests were normal. Gram stain showed no WBC and had rare Gram-positive bacilli. Potassium hydroxide prep of a skin lesion scraping showed no fungal elements. A herpes culture was performed along with a punch biopsy.
FIGURE 1
Bulla on the index finger
FIGURE 2
Multiple bullae on the trunk
What is the diagnosis?
Differential diagnosis
Many diseases manifest with bullae/vesicles. Workup should begin with a complete history and physical exam. A skin biopsy may be needed to make a definitive diagnosis.
Herpes zoster typically manifests with clustered pruritic vesicular lesions on a red base that follow a dermatomal distribution. Pemphigus vulgaris appears with flaccid blisters, erosions, and tend to have oral mucosal lesions. A positive Nikolsky’s sign is characteristic of pemphigus vulgaris. Bullous impetigo appears with scattered lesions of erythema and macules, progressing to thin roofed bullae and subsequently to “honey-crusted” lesions. In toxic epidermal necrolysis, the bullae are widespread and lead to sloughing of the skin. Pyoderma gangrenosum has ulcer formation preceded by pustules that typically expand rapidly to approximately 20 cm. These ulcers have necrotic bluish edges.
Diagnostic test results
The patient’s herpes culture was negative. Fortunately, the punch biopsy was sent for direct immunofluorescence. Direct immunofluorescence showed positive staining with immunoglobulin (Ig) G in the intercellular regions of the epidermis and no staining with IgA, IgM, C3, or fibrinogen. Hematoxylin and eosin-stained sections showed suprabasal blistering containing neutrophils and a few eosinophils. These results are consistent with pemphigus vulgaris.
Diagnosis: pemphigus vulgaris
Pemphigus vulgaris is blistering disease involving the skin and mucous membranes, with severe morbidity and occasional mortality. Prior to the development of effective treatment, the disease was 75% fatal within 5 years.1
Its prevalence is equal among men and women, with a rate of occurrence of 0.5/100,000 people per year. The average age of onset is in the fifth and sixth decades of life, but there is wide variation in age. It has multiple causes and risk factors (Table).
The clinical manifestation of pemphigus typically features mucocutaneous blisters followed by erosions. Often they appear first in mucous membranes and may not appear cutaneously until several months later.2 The skin lesions are painful flaccid blisters that may appear anywhere. A characteristic finding of pemphigus vulgaris is the Nikolsky sign, in which lateral stress applied to perilesional skin causes an expansion of the blistering.
There are 2 major subtypes of pemphigus. Pemphigus vulgaris has blisters extending to the deep epidermis, and pemphigus foliaceus has more superficial involvement of the epidermis. Pemphigus can also be seen in paraneoplastics syndromes.
In pemphigus, the epidermal cells lose normal cell contacts and form a blister. Electron microscopy shows desmosomal abnormalities at desmosomal junctions. It is these junctions that guarantee the integrity of the epithelium. Direct immunofluorescence shows IgG deposition in intercellular spaces.
TABLECauses and risk factors for pemphigus vulgaris
| Penicillamine |
| Captopril |
| Rifampin |
| Phenol-based drugs |
| Amide-based drugs |
| Foods: garlic, leek, onion |
| Pregnancy |
| Pesticide exposure |
| Herpes virus infection |
| Cytomegalovirus infection |
| Epstein-Barr virus infectyion |
| Adapted from: Benner et al 2003.4 |
Treatment: Steroids and adjuvant drugs
Inducing remission is the main goal of therapy for pemphigus vulgaris. Epidemiological studies have shown up to a 75% remission rate 10 years after initial diagnosis.3 Corticosteroids are the preferred therapy for the management of pemphigus vulgaris (based on expert opinion). Also, adjuvant drugs such as azathioprine and cyclophosphamide are commonly used in combination with corticosteroids, with the aim of increasing efficacy and of having a steroid-sparing action (level of evidence: 5, expert opinion).
A tailored dosing schedule of steroids has been advocated according to the severity of the disease. Mild disease can be treated with an initial prednisone dose of 40 to 60 mg/d; in severe cases, 60 to 100 mg/d. Other agents that have been used to treat pemphigus vulgaris are intramuscular gold, dapsone, and intravenous immunoglobulin.
Patient outcome
The patient was treated with oral prednisone starting at 60 mg/d and her skin began to clear. A full course of oral prednisone was continued and tapered over 1 month. Currently, she remains in remission off all medications.
Conclusion
Pemphigus vulgaris is a potentially life-threatening condition that must be recognized and treated promptly. With a lack of large-scale controlled studies, the diagnosis and management of pemphigus vulgaris has based on expert opinion.3 Complications such as superimposed infection of the lesions, cellulitis, and sepsis can occur. Its association with underlying neoplasm, thymomas, myasthenia gravis, and other autoimmune disorders warrants consideration for additional workup when indicated.
Correspondence
John Sauret, MD, Department of Family Medicine, State University of New York at Buffalo, 150 Family Medical Modular Complex, Buffalo, NY 14214-3013. E-mail: [email protected].
A 33-year-old African American woman came to the office with a 2-week history of skin lesions and itching. The lesions started with a single blister on her left elbow; numerous other blisters subsequently appeared on her forearm and hands. One week before this visit, she had been given a presumptive diagnosis of bullous impetigo and was treated with cephalexin.
Despite the antibiotics, other lesions soon appeared in the nuchal and breast folds, axillae, and scalp areas. Several had ruptured, producing purulent, malodorous material. She had no known allergies, no medical problems aside from obesity, and no significant family history or recent travels. She denied any illicit drug use and had not been on any medications.
On physical exam, 1 large bulla was seen on the fourth digit of her left hand (Figure 1). The patient was obese, and inspection of the skin folds of her abdomen showed multiple suppurative lesions and erosions where previous bullae were found (Figure 2). No oral or gingival erosions were seen. Labs showed a white blood cell (WBC) count of 10.5 x109L], hemoglobin of 11.0 g/dL, and hemoglobin A1cof 5.5; liver function tests were normal. Gram stain showed no WBC and had rare Gram-positive bacilli. Potassium hydroxide prep of a skin lesion scraping showed no fungal elements. A herpes culture was performed along with a punch biopsy.
FIGURE 1
Bulla on the index finger
FIGURE 2
Multiple bullae on the trunk
What is the diagnosis?
Differential diagnosis
Many diseases manifest with bullae/vesicles. Workup should begin with a complete history and physical exam. A skin biopsy may be needed to make a definitive diagnosis.
Herpes zoster typically manifests with clustered pruritic vesicular lesions on a red base that follow a dermatomal distribution. Pemphigus vulgaris appears with flaccid blisters, erosions, and tend to have oral mucosal lesions. A positive Nikolsky’s sign is characteristic of pemphigus vulgaris. Bullous impetigo appears with scattered lesions of erythema and macules, progressing to thin roofed bullae and subsequently to “honey-crusted” lesions. In toxic epidermal necrolysis, the bullae are widespread and lead to sloughing of the skin. Pyoderma gangrenosum has ulcer formation preceded by pustules that typically expand rapidly to approximately 20 cm. These ulcers have necrotic bluish edges.
Diagnostic test results
The patient’s herpes culture was negative. Fortunately, the punch biopsy was sent for direct immunofluorescence. Direct immunofluorescence showed positive staining with immunoglobulin (Ig) G in the intercellular regions of the epidermis and no staining with IgA, IgM, C3, or fibrinogen. Hematoxylin and eosin-stained sections showed suprabasal blistering containing neutrophils and a few eosinophils. These results are consistent with pemphigus vulgaris.
Diagnosis: pemphigus vulgaris
Pemphigus vulgaris is blistering disease involving the skin and mucous membranes, with severe morbidity and occasional mortality. Prior to the development of effective treatment, the disease was 75% fatal within 5 years.1
Its prevalence is equal among men and women, with a rate of occurrence of 0.5/100,000 people per year. The average age of onset is in the fifth and sixth decades of life, but there is wide variation in age. It has multiple causes and risk factors (Table).
The clinical manifestation of pemphigus typically features mucocutaneous blisters followed by erosions. Often they appear first in mucous membranes and may not appear cutaneously until several months later.2 The skin lesions are painful flaccid blisters that may appear anywhere. A characteristic finding of pemphigus vulgaris is the Nikolsky sign, in which lateral stress applied to perilesional skin causes an expansion of the blistering.
There are 2 major subtypes of pemphigus. Pemphigus vulgaris has blisters extending to the deep epidermis, and pemphigus foliaceus has more superficial involvement of the epidermis. Pemphigus can also be seen in paraneoplastics syndromes.
In pemphigus, the epidermal cells lose normal cell contacts and form a blister. Electron microscopy shows desmosomal abnormalities at desmosomal junctions. It is these junctions that guarantee the integrity of the epithelium. Direct immunofluorescence shows IgG deposition in intercellular spaces.
TABLECauses and risk factors for pemphigus vulgaris
| Penicillamine |
| Captopril |
| Rifampin |
| Phenol-based drugs |
| Amide-based drugs |
| Foods: garlic, leek, onion |
| Pregnancy |
| Pesticide exposure |
| Herpes virus infection |
| Cytomegalovirus infection |
| Epstein-Barr virus infectyion |
| Adapted from: Benner et al 2003.4 |
Treatment: Steroids and adjuvant drugs
Inducing remission is the main goal of therapy for pemphigus vulgaris. Epidemiological studies have shown up to a 75% remission rate 10 years after initial diagnosis.3 Corticosteroids are the preferred therapy for the management of pemphigus vulgaris (based on expert opinion). Also, adjuvant drugs such as azathioprine and cyclophosphamide are commonly used in combination with corticosteroids, with the aim of increasing efficacy and of having a steroid-sparing action (level of evidence: 5, expert opinion).
A tailored dosing schedule of steroids has been advocated according to the severity of the disease. Mild disease can be treated with an initial prednisone dose of 40 to 60 mg/d; in severe cases, 60 to 100 mg/d. Other agents that have been used to treat pemphigus vulgaris are intramuscular gold, dapsone, and intravenous immunoglobulin.
Patient outcome
The patient was treated with oral prednisone starting at 60 mg/d and her skin began to clear. A full course of oral prednisone was continued and tapered over 1 month. Currently, she remains in remission off all medications.
Conclusion
Pemphigus vulgaris is a potentially life-threatening condition that must be recognized and treated promptly. With a lack of large-scale controlled studies, the diagnosis and management of pemphigus vulgaris has based on expert opinion.3 Complications such as superimposed infection of the lesions, cellulitis, and sepsis can occur. Its association with underlying neoplasm, thymomas, myasthenia gravis, and other autoimmune disorders warrants consideration for additional workup when indicated.
Correspondence
John Sauret, MD, Department of Family Medicine, State University of New York at Buffalo, 150 Family Medical Modular Complex, Buffalo, NY 14214-3013. E-mail: [email protected].
1. Sami N, Ahmed AR. Dual diagnosis of pemphigus and pemphigoid. Retrospective review of 30 cases in the literature. Dermatol 2001;202:293-301.
2. Ahmed AR, Graham J. Pemphigus: current concepts. Ann Intern Med 1980;92:396-405.
3. Harman KE, Albert S, Black MM. Guidelines for the management of pemphigus vulgaris. Br J Dermatol 2003;149:926-937.
4. Benner S, Mashiah J, Tamir E, Goldberg I, Wohl Y. PEMPHIGUS: an acronym for a disease with multiple etiologies. Skinmed 2003;2:163-167.
1. Sami N, Ahmed AR. Dual diagnosis of pemphigus and pemphigoid. Retrospective review of 30 cases in the literature. Dermatol 2001;202:293-301.
2. Ahmed AR, Graham J. Pemphigus: current concepts. Ann Intern Med 1980;92:396-405.
3. Harman KE, Albert S, Black MM. Guidelines for the management of pemphigus vulgaris. Br J Dermatol 2003;149:926-937.
4. Benner S, Mashiah J, Tamir E, Goldberg I, Wohl Y. PEMPHIGUS: an acronym for a disease with multiple etiologies. Skinmed 2003;2:163-167.
Pearly penile lesions
A 22-year-old man came into the office concerned he may have warts on his penis. He believed the warts appeared about 3 months ago. He was single and did not have a sexual partner. He had been dating a woman for 1 year until he graduated from college 4 months ago. His sexual history was serial monogamy with 5 lifetime female sexual partners.
After some hesitation, he noted he slept with a woman one night following a graduation party. He admitted that they were both drunk and that he did not use a condom. He asked if this was how the condition could have developed.
He denied any history of sexually transmitted diseases (STDs) and the result of an HIV test was negative when he donated blood last year. He did not have urethral discharge or burning on urination. The patient had no other symptoms and no chronic illnesses. He generally had a healthy lifestyle, without drug and tobacco use. He said he used to drink at college parties, but rarely had a drink since starting work full-time.
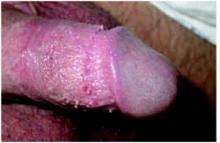
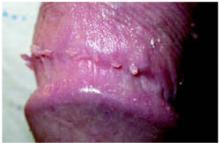
On physical exam, the patient had no fever or lymphadenopathy. A genital exam (Figure 1) with the foreskin retracted revealed skin-colored papules on the shaft of the penis that were somewhat verrucous. The papules seemed to make a ring around the shaft just proximal to the corona of the glans. Closer inspection showed smaller pearly papules surrounding the glans on the corona (Figure 2).
FIGURE 1
Large verrucuous papules
FIGURE 2
Detail of the smaller papules
Are The Larger Verrucous Papules Really Warts?
What Are The Smaller Papules And do they need treatment?
Diagnosis: Condyloma Acuminata
The larger verrucous papules are genital warts, also known as condyloma acuminata. These are caused by the human papillomavirus (HPV) and are sexually transmitted. The patient most likely acquired these from his last unprotected sexual encounter, but he may have been infected earlier and the warts just became visible in the last 3 months. The differential diagnosis includes condyloma lata, the flat warts of secondary syphilis, but these are much less common.
The small pearly papules on the corona are not warts but a variation of the normal male anatomy, called pearly penile papules. The reason to recognize these papules is to reassure worried men that they are normal and to avoid performing any invasive treatments to remove them.
Laboratory Examination
All patients with any sexually transmitted disease should be tested for syphilis and HIV regardless of other risk factors.1 In this case, testing for syphilis with either a rapid plasma reagin (RPR) or VDRL will also be helpful to rule out condyloma lata.
These genital warts do not need to be biopsied to make the diagnosis. No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts.1
Treatment: Removal With Medication Or Surgery
The primary goal of treating visible genital warts is the removal of symptomatic warts.1 Treatment can induce wart-free periods. Available therapies for genital warts may reduce, but probably do not eradicate, infectivity.1 No evidence suggests any available treatment is superior to another, and no single treatment is ideal for all patients or all warts. The natural history of genital warts is benign, and the types of HPV that usually cause external genital warts (HPV 6 and 11) are not associated with cancer.
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines1 for STDs recommend the following options. Cost data are given in the Table.
TABLE
Self-administered medications for HPV
| Medication | Method | Cost |
|---|---|---|
| Podofilox 0.5% solution or gel (podophyllotoxin) | Apply twice daily for 3 days, then off 4 days. May repeat cycle total of 4 times | Gel 0.5%, one 3.5-g tube:$164 Solution 0.5% one 3.5-g tube:$121* |
| Imiquimod 5% cream | Apply nightly 3 times per week. | 12 packets:$159* (enough for 4 weeks of therapy if 1 packet is used per application;using a packet for more than 1 day is possible) |
| Wash off after 6 to 10 hours. | ||
| May use up to 16 weeks. | ||
| * Prices from ePocrates,accessed on October 3,2004. | ||
Patient-applied treatments
- Podofilox 0.5% solution or gel. Patients should apply podofilox solution with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to 4 cycles. The health care provider may apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated.
- Imiquimod 5% cream. Patients should apply imiquimod cream once daily at bedtime, 3 times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application.
Provider-administered treatments
- Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1 to 2 weeks.
- Podophyllin resin 10% to 25% in a compound tincture of benzoin. A small amount should be applied to each wart and allowed to air-dry. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, some specialists recommend that application be limited to ≤0.5 mL of podophyllin or an area of <10 cm2 of warts per session. Some specialists suggest the preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established.
- Trichloroacetic acid (TCA): a small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops (Figure 3). This treatment can be repeated weekly, if necessary.
- Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
FIGURE 3
Treatment of genital warts
Keratinized vs nonkeratinized warts
In choosing the type of therapy for a patient, it is helpful to note that the soft, nonkeratinized warts respond well to the various forms of podophyllin and trichloroacetic acid. The more keratinized lesions, however, respond better to physical ablative methods such as cryotherapy, excision, or electrocautery. Imiquimod appears to work well for both types of lesions but is more effective for the nonkeratinized warts.
The softer, nonkeratinized warts are found more often on softer mucosa around the anus, under the foreskin, and around the female introitus. The firmer, more keratinized warts are found more often on more keratinized skin such as on the shaft of the penis.
Most options yield inadequate results
The reason for so many treatment options is in part due to the therapeutic inadequacy of any one of them. Cure rates are far less than optimal, and relapse rates are disappointing. While we have reasonable treatment guidelines from the CDC, studies on many of the treatment options are limited. There are few head-to-head studies of one method vs another.
One double-blind, randomized, multicenter, vehicle-controlled study2 demonstrated that 0.5% podofilox gel was significantly better than vehicle gel for successfully eliminating and reducing the number and size of anogenital warts. In the intention-to-treat population, 37% treated with podofilox gel had complete clearing of the treated areas, compared with 2% who had clearing of warts with the vehicle gel after 4 weeks (P<.001; number needed to treat=3) (level of evidence [LOE]: 1b).
The best systematic review of genital wart treatment was published in 2004 in the International Journal of STD & AIDS.3 Imiquimod has proven effective in many studies, including 3 randomized controlled trials in which clearance rates were 37% to 52% after 8 to 16 weeks of treatment (LOE=1a).3 One study using imiquimod found that clearance rates were twofold higher for women than for men (72% and 33%, respectively).4 This is probably because genital warts in females are less keratinized than warts on the usual site in males, the penile shaft. Similarly, clearance rates with imiquimod seem to be higher in uncircumcised men (62%) than circumcised men (33%), probably related to the degree of keratinization.3
Recurrence rates for sole therapy with imiquimod (9%–19%) are substantially lower than for most other genital wart treatments, including podophyllotoxin.3 Imiquimod may even be effective in reducing wart recurrence rates when used as an adjunct to surgical treatment.3
The Patient’s Treatment: Cryotherapy, Imiquimod Cream
The treatment options were discussed with the patient. The patient decided to have cryotherapy performed in the office and was given a prescription for imiquimod cream to be used on any remaining warts. The normal pearly penile papules on the corona were left alone and the patient tolerated the cryotherapy to the real warts with an acceptable level of temporary discomfort.
- Imiquimod • Aldara
- Podofilox • Condylox
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6):1-78.Available online at: http://www.cdc.gov/std/treatment/. (Note: The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at the following website: www.cdcnpin.org/scripts/std/pda.asp.)
2. Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-38.
3. Maw R. Critical appraisal of commonly used treatment for genital warts. Int J STD AIDS 2004;15:357-364.
4. Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003;Feb;30(2):124-8.
A 22-year-old man came into the office concerned he may have warts on his penis. He believed the warts appeared about 3 months ago. He was single and did not have a sexual partner. He had been dating a woman for 1 year until he graduated from college 4 months ago. His sexual history was serial monogamy with 5 lifetime female sexual partners.
After some hesitation, he noted he slept with a woman one night following a graduation party. He admitted that they were both drunk and that he did not use a condom. He asked if this was how the condition could have developed.
He denied any history of sexually transmitted diseases (STDs) and the result of an HIV test was negative when he donated blood last year. He did not have urethral discharge or burning on urination. The patient had no other symptoms and no chronic illnesses. He generally had a healthy lifestyle, without drug and tobacco use. He said he used to drink at college parties, but rarely had a drink since starting work full-time.
On physical exam, the patient had no fever or lymphadenopathy. A genital exam (Figure 1) with the foreskin retracted revealed skin-colored papules on the shaft of the penis that were somewhat verrucous. The papules seemed to make a ring around the shaft just proximal to the corona of the glans. Closer inspection showed smaller pearly papules surrounding the glans on the corona (Figure 2).
FIGURE 1
Large verrucuous papules
FIGURE 2
Detail of the smaller papules
Are The Larger Verrucous Papules Really Warts?
What Are The Smaller Papules And do they need treatment?
Diagnosis: Condyloma Acuminata
The larger verrucous papules are genital warts, also known as condyloma acuminata. These are caused by the human papillomavirus (HPV) and are sexually transmitted. The patient most likely acquired these from his last unprotected sexual encounter, but he may have been infected earlier and the warts just became visible in the last 3 months. The differential diagnosis includes condyloma lata, the flat warts of secondary syphilis, but these are much less common.
The small pearly papules on the corona are not warts but a variation of the normal male anatomy, called pearly penile papules. The reason to recognize these papules is to reassure worried men that they are normal and to avoid performing any invasive treatments to remove them.
Laboratory Examination
All patients with any sexually transmitted disease should be tested for syphilis and HIV regardless of other risk factors.1 In this case, testing for syphilis with either a rapid plasma reagin (RPR) or VDRL will also be helpful to rule out condyloma lata.
These genital warts do not need to be biopsied to make the diagnosis. No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts.1
Treatment: Removal With Medication Or Surgery
The primary goal of treating visible genital warts is the removal of symptomatic warts.1 Treatment can induce wart-free periods. Available therapies for genital warts may reduce, but probably do not eradicate, infectivity.1 No evidence suggests any available treatment is superior to another, and no single treatment is ideal for all patients or all warts. The natural history of genital warts is benign, and the types of HPV that usually cause external genital warts (HPV 6 and 11) are not associated with cancer.
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines1 for STDs recommend the following options. Cost data are given in the Table.
TABLE
Self-administered medications for HPV
| Medication | Method | Cost |
|---|---|---|
| Podofilox 0.5% solution or gel (podophyllotoxin) | Apply twice daily for 3 days, then off 4 days. May repeat cycle total of 4 times | Gel 0.5%, one 3.5-g tube:$164 Solution 0.5% one 3.5-g tube:$121* |
| Imiquimod 5% cream | Apply nightly 3 times per week. | 12 packets:$159* (enough for 4 weeks of therapy if 1 packet is used per application;using a packet for more than 1 day is possible) |
| Wash off after 6 to 10 hours. | ||
| May use up to 16 weeks. | ||
| * Prices from ePocrates,accessed on October 3,2004. | ||
Patient-applied treatments
- Podofilox 0.5% solution or gel. Patients should apply podofilox solution with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to 4 cycles. The health care provider may apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated.
- Imiquimod 5% cream. Patients should apply imiquimod cream once daily at bedtime, 3 times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application.
Provider-administered treatments
- Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1 to 2 weeks.
- Podophyllin resin 10% to 25% in a compound tincture of benzoin. A small amount should be applied to each wart and allowed to air-dry. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, some specialists recommend that application be limited to ≤0.5 mL of podophyllin or an area of <10 cm2 of warts per session. Some specialists suggest the preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established.
- Trichloroacetic acid (TCA): a small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops (Figure 3). This treatment can be repeated weekly, if necessary.
- Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
FIGURE 3
Treatment of genital warts
Keratinized vs nonkeratinized warts
In choosing the type of therapy for a patient, it is helpful to note that the soft, nonkeratinized warts respond well to the various forms of podophyllin and trichloroacetic acid. The more keratinized lesions, however, respond better to physical ablative methods such as cryotherapy, excision, or electrocautery. Imiquimod appears to work well for both types of lesions but is more effective for the nonkeratinized warts.
The softer, nonkeratinized warts are found more often on softer mucosa around the anus, under the foreskin, and around the female introitus. The firmer, more keratinized warts are found more often on more keratinized skin such as on the shaft of the penis.
Most options yield inadequate results
The reason for so many treatment options is in part due to the therapeutic inadequacy of any one of them. Cure rates are far less than optimal, and relapse rates are disappointing. While we have reasonable treatment guidelines from the CDC, studies on many of the treatment options are limited. There are few head-to-head studies of one method vs another.
One double-blind, randomized, multicenter, vehicle-controlled study2 demonstrated that 0.5% podofilox gel was significantly better than vehicle gel for successfully eliminating and reducing the number and size of anogenital warts. In the intention-to-treat population, 37% treated with podofilox gel had complete clearing of the treated areas, compared with 2% who had clearing of warts with the vehicle gel after 4 weeks (P<.001; number needed to treat=3) (level of evidence [LOE]: 1b).
The best systematic review of genital wart treatment was published in 2004 in the International Journal of STD & AIDS.3 Imiquimod has proven effective in many studies, including 3 randomized controlled trials in which clearance rates were 37% to 52% after 8 to 16 weeks of treatment (LOE=1a).3 One study using imiquimod found that clearance rates were twofold higher for women than for men (72% and 33%, respectively).4 This is probably because genital warts in females are less keratinized than warts on the usual site in males, the penile shaft. Similarly, clearance rates with imiquimod seem to be higher in uncircumcised men (62%) than circumcised men (33%), probably related to the degree of keratinization.3
Recurrence rates for sole therapy with imiquimod (9%–19%) are substantially lower than for most other genital wart treatments, including podophyllotoxin.3 Imiquimod may even be effective in reducing wart recurrence rates when used as an adjunct to surgical treatment.3
The Patient’s Treatment: Cryotherapy, Imiquimod Cream
The treatment options were discussed with the patient. The patient decided to have cryotherapy performed in the office and was given a prescription for imiquimod cream to be used on any remaining warts. The normal pearly penile papules on the corona were left alone and the patient tolerated the cryotherapy to the real warts with an acceptable level of temporary discomfort.
- Imiquimod • Aldara
- Podofilox • Condylox
A 22-year-old man came into the office concerned he may have warts on his penis. He believed the warts appeared about 3 months ago. He was single and did not have a sexual partner. He had been dating a woman for 1 year until he graduated from college 4 months ago. His sexual history was serial monogamy with 5 lifetime female sexual partners.
After some hesitation, he noted he slept with a woman one night following a graduation party. He admitted that they were both drunk and that he did not use a condom. He asked if this was how the condition could have developed.
He denied any history of sexually transmitted diseases (STDs) and the result of an HIV test was negative when he donated blood last year. He did not have urethral discharge or burning on urination. The patient had no other symptoms and no chronic illnesses. He generally had a healthy lifestyle, without drug and tobacco use. He said he used to drink at college parties, but rarely had a drink since starting work full-time.
On physical exam, the patient had no fever or lymphadenopathy. A genital exam (Figure 1) with the foreskin retracted revealed skin-colored papules on the shaft of the penis that were somewhat verrucous. The papules seemed to make a ring around the shaft just proximal to the corona of the glans. Closer inspection showed smaller pearly papules surrounding the glans on the corona (Figure 2).
FIGURE 1
Large verrucuous papules
FIGURE 2
Detail of the smaller papules
Are The Larger Verrucous Papules Really Warts?
What Are The Smaller Papules And do they need treatment?
Diagnosis: Condyloma Acuminata
The larger verrucous papules are genital warts, also known as condyloma acuminata. These are caused by the human papillomavirus (HPV) and are sexually transmitted. The patient most likely acquired these from his last unprotected sexual encounter, but he may have been infected earlier and the warts just became visible in the last 3 months. The differential diagnosis includes condyloma lata, the flat warts of secondary syphilis, but these are much less common.
The small pearly papules on the corona are not warts but a variation of the normal male anatomy, called pearly penile papules. The reason to recognize these papules is to reassure worried men that they are normal and to avoid performing any invasive treatments to remove them.
Laboratory Examination
All patients with any sexually transmitted disease should be tested for syphilis and HIV regardless of other risk factors.1 In this case, testing for syphilis with either a rapid plasma reagin (RPR) or VDRL will also be helpful to rule out condyloma lata.
These genital warts do not need to be biopsied to make the diagnosis. No data support the use of type-specific HPV nucleic acid tests in the routine diagnosis or management of visible genital warts.1
Treatment: Removal With Medication Or Surgery
The primary goal of treating visible genital warts is the removal of symptomatic warts.1 Treatment can induce wart-free periods. Available therapies for genital warts may reduce, but probably do not eradicate, infectivity.1 No evidence suggests any available treatment is superior to another, and no single treatment is ideal for all patients or all warts. The natural history of genital warts is benign, and the types of HPV that usually cause external genital warts (HPV 6 and 11) are not associated with cancer.
The Centers for Disease Control and Prevention (CDC) 2002 treatment guidelines1 for STDs recommend the following options. Cost data are given in the Table.
TABLE
Self-administered medications for HPV
| Medication | Method | Cost |
|---|---|---|
| Podofilox 0.5% solution or gel (podophyllotoxin) | Apply twice daily for 3 days, then off 4 days. May repeat cycle total of 4 times | Gel 0.5%, one 3.5-g tube:$164 Solution 0.5% one 3.5-g tube:$121* |
| Imiquimod 5% cream | Apply nightly 3 times per week. | 12 packets:$159* (enough for 4 weeks of therapy if 1 packet is used per application;using a packet for more than 1 day is possible) |
| Wash off after 6 to 10 hours. | ||
| May use up to 16 weeks. | ||
| * Prices from ePocrates,accessed on October 3,2004. | ||
Patient-applied treatments
- Podofilox 0.5% solution or gel. Patients should apply podofilox solution with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle may be repeated, as necessary, for up to 4 cycles. The health care provider may apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated.
- Imiquimod 5% cream. Patients should apply imiquimod cream once daily at bedtime, 3 times a week for up to 16 weeks. The treatment area should be washed with soap and water 6 to 10 hours after the application.
Provider-administered treatments
- Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1 to 2 weeks.
- Podophyllin resin 10% to 25% in a compound tincture of benzoin. A small amount should be applied to each wart and allowed to air-dry. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, some specialists recommend that application be limited to ≤0.5 mL of podophyllin or an area of <10 cm2 of warts per session. Some specialists suggest the preparation should be thoroughly washed off 1 to 4 hours after application to reduce local irritation. The safety of podophyllin during pregnancy has not been established.
- Trichloroacetic acid (TCA): a small amount should be applied only to warts and allowed to dry, at which time a white “frosting” develops (Figure 3). This treatment can be repeated weekly, if necessary.
- Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
FIGURE 3
Treatment of genital warts
Keratinized vs nonkeratinized warts
In choosing the type of therapy for a patient, it is helpful to note that the soft, nonkeratinized warts respond well to the various forms of podophyllin and trichloroacetic acid. The more keratinized lesions, however, respond better to physical ablative methods such as cryotherapy, excision, or electrocautery. Imiquimod appears to work well for both types of lesions but is more effective for the nonkeratinized warts.
The softer, nonkeratinized warts are found more often on softer mucosa around the anus, under the foreskin, and around the female introitus. The firmer, more keratinized warts are found more often on more keratinized skin such as on the shaft of the penis.
Most options yield inadequate results
The reason for so many treatment options is in part due to the therapeutic inadequacy of any one of them. Cure rates are far less than optimal, and relapse rates are disappointing. While we have reasonable treatment guidelines from the CDC, studies on many of the treatment options are limited. There are few head-to-head studies of one method vs another.
One double-blind, randomized, multicenter, vehicle-controlled study2 demonstrated that 0.5% podofilox gel was significantly better than vehicle gel for successfully eliminating and reducing the number and size of anogenital warts. In the intention-to-treat population, 37% treated with podofilox gel had complete clearing of the treated areas, compared with 2% who had clearing of warts with the vehicle gel after 4 weeks (P<.001; number needed to treat=3) (level of evidence [LOE]: 1b).
The best systematic review of genital wart treatment was published in 2004 in the International Journal of STD & AIDS.3 Imiquimod has proven effective in many studies, including 3 randomized controlled trials in which clearance rates were 37% to 52% after 8 to 16 weeks of treatment (LOE=1a).3 One study using imiquimod found that clearance rates were twofold higher for women than for men (72% and 33%, respectively).4 This is probably because genital warts in females are less keratinized than warts on the usual site in males, the penile shaft. Similarly, clearance rates with imiquimod seem to be higher in uncircumcised men (62%) than circumcised men (33%), probably related to the degree of keratinization.3
Recurrence rates for sole therapy with imiquimod (9%–19%) are substantially lower than for most other genital wart treatments, including podophyllotoxin.3 Imiquimod may even be effective in reducing wart recurrence rates when used as an adjunct to surgical treatment.3
The Patient’s Treatment: Cryotherapy, Imiquimod Cream
The treatment options were discussed with the patient. The patient decided to have cryotherapy performed in the office and was given a prescription for imiquimod cream to be used on any remaining warts. The normal pearly penile papules on the corona were left alone and the patient tolerated the cryotherapy to the real warts with an acceptable level of temporary discomfort.
- Imiquimod • Aldara
- Podofilox • Condylox
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6):1-78.Available online at: http://www.cdc.gov/std/treatment/. (Note: The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at the following website: www.cdcnpin.org/scripts/std/pda.asp.)
2. Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-38.
3. Maw R. Critical appraisal of commonly used treatment for genital warts. Int J STD AIDS 2004;15:357-364.
4. Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003;Feb;30(2):124-8.
1. Centers for Disease Control and Prevention. 2002 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2002;51(RR-6):1-78.Available online at: http://www.cdc.gov/std/treatment/. (Note: The CDC 2002 sexually transmitted diseases treatment guidelines are available for download and use on a Palm handheld computer at the following website: www.cdcnpin.org/scripts/std/pda.asp.)
2. Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Arch Dermatol 1998;134:33-38.
3. Maw R. Critical appraisal of commonly used treatment for genital warts. Int J STD AIDS 2004;15:357-364.
4. Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis 2003;Feb;30(2):124-8.
Facial lesion that came “out of nowhere”
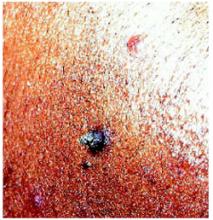
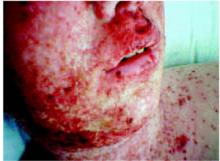
A 33-year-old woman had a facial lesion (Figures 1 and 2) that seemed to “come out of nowhere,” but it was months before she sought medical attention. She was certain that the duration was months, not years, but could not date the exact onset.
The lesion was asymptomatic except for its prominence and aesthetics. The patient had tried cutting the lesion off several times, but it regrew each time. She was married, mono-gamous by history, not pregnant, had no major underlying medical conditions, and had no personal or family history of skin malignancy. The remainder of the skin examination was normal.
FIGURE 1
Facial lesion with sudden onset
FIGURE 2
Detail of the lesion
What is your diagnosis?
What would be your management plan?
Diagnosis: Cutaneous horn
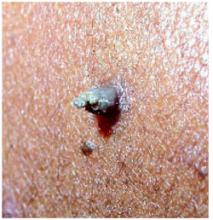
Cutaneous horn,also referred to as cornu cutaneum, is a clinical (morphologic) diagnosis, not a precise pathologic diagnosis. It describes an asymptomatic, projectile, conical, dense, hyperkeratotic lesion that resembles the horn of an animal.
Cutaneous horns can arise from a variety of primary underlying pathologic processes, including benign, premalignant, and malignant lesions. Thus, the important issue when confronted with a cutaneous horn is determining the causative pathologic process. Therefore, for treatment, most authors stress surgical excision with attention to removing the base of the specimen for histopathologic examination.1-4
Cutaneous horns may vary considerably in size and shape. Most are a few millimeters in length, but there are reports of some measuring up to 6 cm in length. They may be perpendicular or inclined in relation to the underlying skin. They usually occur singly and may grow slowly over decades.2,4
Cutaneous horns are more common in older and white individuals, although they have been reported in children and African Americans.5The higher prevalence in older and light-skinned individuals is secondary to the fact that many cutaneous horns are caused by cumulative sun damage over many years, leading to actinic keratoses and nonmelanoma skin cancer.
Differential diagnosis
The differential diagnosis of the underlying causes of cutaneous horns is extensive. Some causes are listed in the Table ; common ones include actinic keratoses (25%–35% of patients with cutaneous horns), verruca vulgaris (15%–25%), and cutaneous malignancies (15%–40%).1
Features that have been reported to increase the chance of an underlying malignancy include older age, male sex, lesion geometry (either alarge base or a large height-to-base ratio), and presence on a sun-exposed location (face, pinnae, dorsal hands and forearms, scalp). More than 70% of cutaneous horns with underlying premalignant or malignant lesions are found on these sun-exposed areas.3,6 Additionally, cutaneous horns on these locations are twice as likely to harbor underlying premalignant or malignant lesions.6 Of patients with malignancies underlying their cutaneous horns, up to one third have a history of skin malignancy.7
TABLE
Some causes of cutaneous horn
| Benign–noninfectious |
| Angiokeratoma |
| Angioma |
| Dermatofibroma |
| Epidermal inclusion cyst (“sebaceous cyst”) |
| Linear verrucous epidermal nevus |
| Fibroma |
| Lichen simplex chronicus (“neurodermatitis”) |
| Lichenoid keratosis |
| Prurigo nodularis |
| Pyogenic granuloma |
| Sebaceous adenoma |
| Seborrheic keratosis |
| Trichilemma |
| Benign–infectious |
| Condyloma acuminata (genital warts) |
| Molluscum contagiosum |
| Verruca vulgaris (common wart) |
| Premalignant/malignant |
| Actinic keratosis |
| Basal cell carcinoma |
| Bowen’s disease |
| Epidermoid carcinoma |
| Kaposi’s sarcoma |
| Keratoacanthoma |
| Malignant melanoma |
| Squamous cell carcinoma |
| Sources: Gould and Brodell 1999,1Akan et al 2001,6 Khaitan 1999.9 |
Treatment options
Cryosurgery
Some textbooks list cryosurgical therapy as an option.8 If there were a clearly benign pre-existing underlying dermopathy, such as verruca vulgaris or molluscum contagiosum, cryosurgery might be considered. However, cryosurgery is destructive; it does not preserve a specimen for pathologic examination. Because cutaneous horns have a 15% to 40% chance of underlying malignancy,1,4 it is difficult to recommend cryosurgical destruction without an initial biopsy-proven diagnosis.
Punch biopsy
In this patient, a 3-mm excisional punch biopsy was performed using a punch-to-ellipse technique. The skin is stretched parallel to the skin lines as the punch biopsy is performed. As the skin relaxes after removal of the punch instrument, an elliptical defect remains, enhancing cosmesis of the repair. Especially for a convex facial surface (which heals less well cosmetically than concave facial surfaces), this technique was believed to offer the potential for a better long-term cosmetic result.
In this case, a shave biopsy would have been a good option for both diagnosis and treatment. If the pathology from a punch biopsy or shave biopsy turned out to demonstrate an underlying skin cancer, then a fusiform excision would be needed to provide adequate surgical margins for the definitive treatment.
Results of histologic exam
With this patient, histologic examination revealed that the underlying condition was verruca vulgaris, or the common wart. Several months after removal of the cutaneous horn, the patient could not locate the surgical site, a cosmetically acceptable result to her and her physician ( Figure 3).
FIGURE 3
After successful treatment
Acknowledgments
The author would like to acknowledge the unfailing cooperation and expert assistance of the St. Vincent Mercy Medical Center library staff.
Correspondence
Gary N. Fox, MD, 2200 Jefferson Avenue, Toledo, OH 43624. E- mail: [email protected].
1. Gould JW, Brodell RT. Giant cutaneous horn associated with verruca vulgaris. Cutis. 1999;64:111-112.
2. Kastanioudakis I, Skevas A, Assimakopoulos D, Daneilidis B. Cutaneous horn of the auricle. Otolaryngol Head Neck Surg. 1998;118:735.-
3. Korkut T, Tan NB, Oztan Y. Giant cutaneous horn: a patient report. Ann Plast Surg. 1997;39:654-655.
4. Stavroulaki P, Mal RK. Squamous cell carcinoma presenting as a cutaneous horn. Auris Nasus Larynx. 2000;27:277-279.
5. Souza LN, Martins CR, de Paula AM. Cutaneous horn occurring on the lip of a child. Int J Paediatr Dent. 2003;13:365-367.
6. Akan M, Yildirim S, Avci G, Akoz T. Xeroderma pigmento sum with a giant cutaneous horn. Ann Plast Surg. 2001;46:665-666.
7. Spira J, Rabinovitz H. Cutaneous horn present for two months. Dermatol Online J. 2000;6:11.-
8. Benignkin tumors (Chapter 20)Cutaneous horn. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St. Louis, Mo: Mosby; 2004;706.:
9. Khaitan BK, Sood A, Singh MK. Lichen simplex chronicus with a cutaneous horn. Acta Derm Venereol. 1999;79:243.-
10. Agarwalla A, Agrawal CS, Thakur A, et al. Cutaneous horn on condyloma acuminatum. Acta Derm Venereol. 2000;80:159.
A 33-year-old woman had a facial lesion (Figures 1 and 2) that seemed to “come out of nowhere,” but it was months before she sought medical attention. She was certain that the duration was months, not years, but could not date the exact onset.
The lesion was asymptomatic except for its prominence and aesthetics. The patient had tried cutting the lesion off several times, but it regrew each time. She was married, mono-gamous by history, not pregnant, had no major underlying medical conditions, and had no personal or family history of skin malignancy. The remainder of the skin examination was normal.
FIGURE 1
Facial lesion with sudden onset
FIGURE 2
Detail of the lesion
What is your diagnosis?
What would be your management plan?
Diagnosis: Cutaneous horn
Cutaneous horn,also referred to as cornu cutaneum, is a clinical (morphologic) diagnosis, not a precise pathologic diagnosis. It describes an asymptomatic, projectile, conical, dense, hyperkeratotic lesion that resembles the horn of an animal.
Cutaneous horns can arise from a variety of primary underlying pathologic processes, including benign, premalignant, and malignant lesions. Thus, the important issue when confronted with a cutaneous horn is determining the causative pathologic process. Therefore, for treatment, most authors stress surgical excision with attention to removing the base of the specimen for histopathologic examination.1-4
Cutaneous horns may vary considerably in size and shape. Most are a few millimeters in length, but there are reports of some measuring up to 6 cm in length. They may be perpendicular or inclined in relation to the underlying skin. They usually occur singly and may grow slowly over decades.2,4
Cutaneous horns are more common in older and white individuals, although they have been reported in children and African Americans.5The higher prevalence in older and light-skinned individuals is secondary to the fact that many cutaneous horns are caused by cumulative sun damage over many years, leading to actinic keratoses and nonmelanoma skin cancer.
Differential diagnosis
The differential diagnosis of the underlying causes of cutaneous horns is extensive. Some causes are listed in the Table ; common ones include actinic keratoses (25%–35% of patients with cutaneous horns), verruca vulgaris (15%–25%), and cutaneous malignancies (15%–40%).1
Features that have been reported to increase the chance of an underlying malignancy include older age, male sex, lesion geometry (either alarge base or a large height-to-base ratio), and presence on a sun-exposed location (face, pinnae, dorsal hands and forearms, scalp). More than 70% of cutaneous horns with underlying premalignant or malignant lesions are found on these sun-exposed areas.3,6 Additionally, cutaneous horns on these locations are twice as likely to harbor underlying premalignant or malignant lesions.6 Of patients with malignancies underlying their cutaneous horns, up to one third have a history of skin malignancy.7
TABLE
Some causes of cutaneous horn
| Benign–noninfectious |
| Angiokeratoma |
| Angioma |
| Dermatofibroma |
| Epidermal inclusion cyst (“sebaceous cyst”) |
| Linear verrucous epidermal nevus |
| Fibroma |
| Lichen simplex chronicus (“neurodermatitis”) |
| Lichenoid keratosis |
| Prurigo nodularis |
| Pyogenic granuloma |
| Sebaceous adenoma |
| Seborrheic keratosis |
| Trichilemma |
| Benign–infectious |
| Condyloma acuminata (genital warts) |
| Molluscum contagiosum |
| Verruca vulgaris (common wart) |
| Premalignant/malignant |
| Actinic keratosis |
| Basal cell carcinoma |
| Bowen’s disease |
| Epidermoid carcinoma |
| Kaposi’s sarcoma |
| Keratoacanthoma |
| Malignant melanoma |
| Squamous cell carcinoma |
| Sources: Gould and Brodell 1999,1Akan et al 2001,6 Khaitan 1999.9 |
Treatment options
Cryosurgery
Some textbooks list cryosurgical therapy as an option.8 If there were a clearly benign pre-existing underlying dermopathy, such as verruca vulgaris or molluscum contagiosum, cryosurgery might be considered. However, cryosurgery is destructive; it does not preserve a specimen for pathologic examination. Because cutaneous horns have a 15% to 40% chance of underlying malignancy,1,4 it is difficult to recommend cryosurgical destruction without an initial biopsy-proven diagnosis.
Punch biopsy
In this patient, a 3-mm excisional punch biopsy was performed using a punch-to-ellipse technique. The skin is stretched parallel to the skin lines as the punch biopsy is performed. As the skin relaxes after removal of the punch instrument, an elliptical defect remains, enhancing cosmesis of the repair. Especially for a convex facial surface (which heals less well cosmetically than concave facial surfaces), this technique was believed to offer the potential for a better long-term cosmetic result.
In this case, a shave biopsy would have been a good option for both diagnosis and treatment. If the pathology from a punch biopsy or shave biopsy turned out to demonstrate an underlying skin cancer, then a fusiform excision would be needed to provide adequate surgical margins for the definitive treatment.
Results of histologic exam
With this patient, histologic examination revealed that the underlying condition was verruca vulgaris, or the common wart. Several months after removal of the cutaneous horn, the patient could not locate the surgical site, a cosmetically acceptable result to her and her physician ( Figure 3).
FIGURE 3
After successful treatment
Acknowledgments
The author would like to acknowledge the unfailing cooperation and expert assistance of the St. Vincent Mercy Medical Center library staff.
Correspondence
Gary N. Fox, MD, 2200 Jefferson Avenue, Toledo, OH 43624. E- mail: [email protected].
A 33-year-old woman had a facial lesion (Figures 1 and 2) that seemed to “come out of nowhere,” but it was months before she sought medical attention. She was certain that the duration was months, not years, but could not date the exact onset.
The lesion was asymptomatic except for its prominence and aesthetics. The patient had tried cutting the lesion off several times, but it regrew each time. She was married, mono-gamous by history, not pregnant, had no major underlying medical conditions, and had no personal or family history of skin malignancy. The remainder of the skin examination was normal.
FIGURE 1
Facial lesion with sudden onset
FIGURE 2
Detail of the lesion
What is your diagnosis?
What would be your management plan?
Diagnosis: Cutaneous horn
Cutaneous horn,also referred to as cornu cutaneum, is a clinical (morphologic) diagnosis, not a precise pathologic diagnosis. It describes an asymptomatic, projectile, conical, dense, hyperkeratotic lesion that resembles the horn of an animal.
Cutaneous horns can arise from a variety of primary underlying pathologic processes, including benign, premalignant, and malignant lesions. Thus, the important issue when confronted with a cutaneous horn is determining the causative pathologic process. Therefore, for treatment, most authors stress surgical excision with attention to removing the base of the specimen for histopathologic examination.1-4
Cutaneous horns may vary considerably in size and shape. Most are a few millimeters in length, but there are reports of some measuring up to 6 cm in length. They may be perpendicular or inclined in relation to the underlying skin. They usually occur singly and may grow slowly over decades.2,4
Cutaneous horns are more common in older and white individuals, although they have been reported in children and African Americans.5The higher prevalence in older and light-skinned individuals is secondary to the fact that many cutaneous horns are caused by cumulative sun damage over many years, leading to actinic keratoses and nonmelanoma skin cancer.
Differential diagnosis
The differential diagnosis of the underlying causes of cutaneous horns is extensive. Some causes are listed in the Table ; common ones include actinic keratoses (25%–35% of patients with cutaneous horns), verruca vulgaris (15%–25%), and cutaneous malignancies (15%–40%).1
Features that have been reported to increase the chance of an underlying malignancy include older age, male sex, lesion geometry (either alarge base or a large height-to-base ratio), and presence on a sun-exposed location (face, pinnae, dorsal hands and forearms, scalp). More than 70% of cutaneous horns with underlying premalignant or malignant lesions are found on these sun-exposed areas.3,6 Additionally, cutaneous horns on these locations are twice as likely to harbor underlying premalignant or malignant lesions.6 Of patients with malignancies underlying their cutaneous horns, up to one third have a history of skin malignancy.7
TABLE
Some causes of cutaneous horn
| Benign–noninfectious |
| Angiokeratoma |
| Angioma |
| Dermatofibroma |
| Epidermal inclusion cyst (“sebaceous cyst”) |
| Linear verrucous epidermal nevus |
| Fibroma |
| Lichen simplex chronicus (“neurodermatitis”) |
| Lichenoid keratosis |
| Prurigo nodularis |
| Pyogenic granuloma |
| Sebaceous adenoma |
| Seborrheic keratosis |
| Trichilemma |
| Benign–infectious |
| Condyloma acuminata (genital warts) |
| Molluscum contagiosum |
| Verruca vulgaris (common wart) |
| Premalignant/malignant |
| Actinic keratosis |
| Basal cell carcinoma |
| Bowen’s disease |
| Epidermoid carcinoma |
| Kaposi’s sarcoma |
| Keratoacanthoma |
| Malignant melanoma |
| Squamous cell carcinoma |
| Sources: Gould and Brodell 1999,1Akan et al 2001,6 Khaitan 1999.9 |
Treatment options
Cryosurgery
Some textbooks list cryosurgical therapy as an option.8 If there were a clearly benign pre-existing underlying dermopathy, such as verruca vulgaris or molluscum contagiosum, cryosurgery might be considered. However, cryosurgery is destructive; it does not preserve a specimen for pathologic examination. Because cutaneous horns have a 15% to 40% chance of underlying malignancy,1,4 it is difficult to recommend cryosurgical destruction without an initial biopsy-proven diagnosis.
Punch biopsy
In this patient, a 3-mm excisional punch biopsy was performed using a punch-to-ellipse technique. The skin is stretched parallel to the skin lines as the punch biopsy is performed. As the skin relaxes after removal of the punch instrument, an elliptical defect remains, enhancing cosmesis of the repair. Especially for a convex facial surface (which heals less well cosmetically than concave facial surfaces), this technique was believed to offer the potential for a better long-term cosmetic result.
In this case, a shave biopsy would have been a good option for both diagnosis and treatment. If the pathology from a punch biopsy or shave biopsy turned out to demonstrate an underlying skin cancer, then a fusiform excision would be needed to provide adequate surgical margins for the definitive treatment.
Results of histologic exam
With this patient, histologic examination revealed that the underlying condition was verruca vulgaris, or the common wart. Several months after removal of the cutaneous horn, the patient could not locate the surgical site, a cosmetically acceptable result to her and her physician ( Figure 3).
FIGURE 3
After successful treatment
Acknowledgments
The author would like to acknowledge the unfailing cooperation and expert assistance of the St. Vincent Mercy Medical Center library staff.
Correspondence
Gary N. Fox, MD, 2200 Jefferson Avenue, Toledo, OH 43624. E- mail: [email protected].
1. Gould JW, Brodell RT. Giant cutaneous horn associated with verruca vulgaris. Cutis. 1999;64:111-112.
2. Kastanioudakis I, Skevas A, Assimakopoulos D, Daneilidis B. Cutaneous horn of the auricle. Otolaryngol Head Neck Surg. 1998;118:735.-
3. Korkut T, Tan NB, Oztan Y. Giant cutaneous horn: a patient report. Ann Plast Surg. 1997;39:654-655.
4. Stavroulaki P, Mal RK. Squamous cell carcinoma presenting as a cutaneous horn. Auris Nasus Larynx. 2000;27:277-279.
5. Souza LN, Martins CR, de Paula AM. Cutaneous horn occurring on the lip of a child. Int J Paediatr Dent. 2003;13:365-367.
6. Akan M, Yildirim S, Avci G, Akoz T. Xeroderma pigmento sum with a giant cutaneous horn. Ann Plast Surg. 2001;46:665-666.
7. Spira J, Rabinovitz H. Cutaneous horn present for two months. Dermatol Online J. 2000;6:11.-
8. Benignkin tumors (Chapter 20)Cutaneous horn. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St. Louis, Mo: Mosby; 2004;706.:
9. Khaitan BK, Sood A, Singh MK. Lichen simplex chronicus with a cutaneous horn. Acta Derm Venereol. 1999;79:243.-
10. Agarwalla A, Agrawal CS, Thakur A, et al. Cutaneous horn on condyloma acuminatum. Acta Derm Venereol. 2000;80:159.
1. Gould JW, Brodell RT. Giant cutaneous horn associated with verruca vulgaris. Cutis. 1999;64:111-112.
2. Kastanioudakis I, Skevas A, Assimakopoulos D, Daneilidis B. Cutaneous horn of the auricle. Otolaryngol Head Neck Surg. 1998;118:735.-
3. Korkut T, Tan NB, Oztan Y. Giant cutaneous horn: a patient report. Ann Plast Surg. 1997;39:654-655.
4. Stavroulaki P, Mal RK. Squamous cell carcinoma presenting as a cutaneous horn. Auris Nasus Larynx. 2000;27:277-279.
5. Souza LN, Martins CR, de Paula AM. Cutaneous horn occurring on the lip of a child. Int J Paediatr Dent. 2003;13:365-367.
6. Akan M, Yildirim S, Avci G, Akoz T. Xeroderma pigmento sum with a giant cutaneous horn. Ann Plast Surg. 2001;46:665-666.
7. Spira J, Rabinovitz H. Cutaneous horn present for two months. Dermatol Online J. 2000;6:11.-
8. Benignkin tumors (Chapter 20)Cutaneous horn. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St. Louis, Mo: Mosby; 2004;706.:
9. Khaitan BK, Sood A, Singh MK. Lichen simplex chronicus with a cutaneous horn. Acta Derm Venereol. 1999;79:243.-
10. Agarwalla A, Agrawal CS, Thakur A, et al. Cutaneous horn on condyloma acuminatum. Acta Derm Venereol. 2000;80:159.
Excoriations and ulcers on the arms and legs
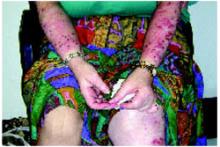
A 55-year-old woman came to the clinic complaining of severe itching on her arms and legs. Although she itched throughout the day, it became intolerable at night, disrupting her sleep. She would sometimes scratch her arms and legs until exhaustion but could find no relief. Being outside in warm and sunny weather aggravated the problem. She had used moisturizers, emollients, and topical corticosteroids, but they only alleviated the itching temporarily. The itching began 10 months earlier, just after she finalized the divorce from her husband of 20 years.
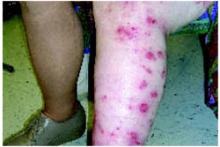
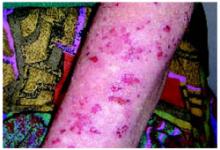
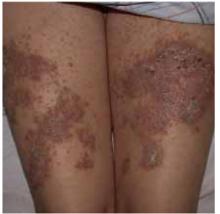
Examination of the skin revealed numerous excoriations with ulcerations and xerosis on the arms and left leg (Figure 1 and 2). The excoriations were located extensively from the dorsum of her left foot to above the knee and bilaterally from the wrist to above the elbow. They also showed signs of infection. The patient admitted they were self-inflicted. The patient’s right leg had been amputated 5 years before after a car accident, and she wore a prosthetic leg. Examination of other areas showed nothing remarkable.
The patient readily admitted to a great deal of psychological distress. She described feeling depressed since her divorce. She has had difficulty securing a full-time job and has high anxiety about being able to pay her rent and bills.
FIGURE 1
Excoriations on the arms…
FIGURE 2
…and the left leg
WHAT IS THE DIAGNOSIS?
WHAT IS THE TREATMENT STRATEGY?
DIFFERENTIAL DIAGNOSIS: PSYCHODERMATOLOGIC DISORDER
The patient’s history and physical examination points to a psychodermatologic disorder. Psycho-dermatologic disorders are conditions involving an interaction between the mind and skin and are classified as:3
- Psychophysiologic disorders:skin disorders worsened by emotional stress
- Primary psychiatric disorders:usually caused by psychological conditions with self-induced skin damage
- Secondary psychiatric disorders: psychological problems developed as a consequence of a disfiguring skin disorder, which negatively effects self-esteem and body image.3
This patient’s excoriations and ulcers are due to self-mutilation. The differential diagnosis includes psychogenic parasitosis, factitial dermatitis, and neurotic excoriations. These 3 skin conditions are primary psychiatric symptoms, and proper diagnosis revolves around being able to assess the dermatologic features and associated psychological disorder.2
Psychogenic parasitosis
Also known as delusional parasitosis, psychogenic parasitosis is a psychodermatologic disorder in which patients believe they are infested with parasites. Patients with this disorder report seeing or feeling parasites on their skin, and they damage their skin in an attempt to remove them. Patients create excoriations and ulcers on easily reached areas, usually the ears, eyes, nose, and extremities. They often present with what is termed the “matchbox sign,” in which they bring containers filled with “small bits of excoriated skin, dried blood, debris, or insect parts as proof of infestation.”2
Women over the age of 50 years are more often affected with psychogenic parasitosis, and the disorder is associated with anxiety, depression, and hypochondriasis.
Factitial dermatitis
Factitial dermatitis, also known as dermatitis artefacta, is a psychodermatologic disorder in which patients damage their skin but deny their self-involvement. This disorder encompasses a wide range of lesions, including blisters, cuts, ulcers, and burns. Patients often are unable to describe how the lesions evolved. Lesions exhibit bizarre patterns not characteristic of any disease.
Young adults and adolescents are more commonly affected, and it is 4 times more common among women than men. Psychological disorders involved with factitial dermatitis include personality disorders and posttraumatic stress disorder.2
Neurotic excoriations
Neurotic excoriations, sometimes referred to as neurodermatitis, are a result of a psychodermatologic disorder in which patients inflict excoriations and ulcers on their skin and admit to their involvement.3 The condition is characterized by excoriations similar in size and shape that are localized on areas easily reached by the patient, such as the arms, legs, and upper back.
The lesions may present in various stages, varying from dugout ulcers to ulcers covered with crusts and surrounded by erythema to areas receding into depressed scars. These lesions are a result of repetitive scratching and digging by the patient, usually without an underlying physical pathology but sometimes initiated by pruritus.1,2
Studies show the condition primarily affects women, with a mean onset between the ages of 30 to 45 years.1 Common psychiatric problems associated with neurotic excoriations include significant social stress, depression, anxiety, and obsessive-compulsive disorder.
Diagnosis: Neurotic excoriations due to depression and stress
The patient’s history and physical examination suggests a diagnosis of neurotic excoriations due to the characteristics of the excoriations and ulcers on her arms and leg, her admission of their self-inflicted nature, and the associated depression and psychosocial stress.
Laboratory tests
Although there are no available laboratory tests to confirm a positive finding of neurotic excoriations, tests could be performed to disprove any systemic causes of pruritus and the resulting dermatological damage.5 These tests include complete blood count with differential, chemistry profile, determination of thyroid-stimulating hormone levels, fasting plasma glucose level, and skin biopsies.1,
Laboratory tests for systemic causes of pruritus should be based on the patient’s physical and history, avoiding a broad approach.
Treatment: address the skin and the psyche
The treatment of neurotic excoriations requires a dual approach, addressing both the dermatological problems and the underlying psychological disorder.3 Supportive dermatologic care is necessary to avoid secondary complications and to ensure that the patient feels supported.3
Dermatologic care
Topical steroids can be helpful to decrease the pruritus and inflammation. The steroid strength should be chosen based upon the severity of the lesions and the thickness of the skin. Steroid ointments are preferred to creams when there are skin ulcers and deep excoriations.
If there is significant crusting or exudate, there is probably a secondary bacterial infection and oral antibiotics are indicated ( Figure 3). A first-generation cephalosporin or dicloxacillin should provide adequate coverage against the most likely organisms, Staphylococcus aureus and Streptococcus pyogenes.
FIGURE 3
Detail of lesions on the arm
Psychological care
The selective serotonin reuptake inhibitors produce a strong antipruritic response in patients with neurotic excoriations. Doxepin is a tricyclic antidepressant with one of the most powerful antihistamines for pruritus. When prescribing doxepin, limit the amount of medication dispensed at one time to minimize the risk of suicide.3 The patient should trim the fingernails to reduce the amount of damage caused by scratching and digging.
Treating the underlying psychological disorder requires supportive and empathic counseling. It may be necessary to collaborate with other mental health specialists. Management options include psychotropic medication, stress management courses, and referral to a psychiatrist. Patients with psychodermatologic disorders frequently resist referral to mental health professionals.3 If this is the case, family physicians are well positioned to help patients with psychological problems.
Alternative courses of treatment include hypnosis to disrupt the itch-scratch cycle, and acupuncture and supportive therapy to reduce underlying stressors.1
Patient’s outcome
By showing support and concern for the patient’s health during the visit, the physician strengthened the relationship with the patient, who felt comfortable disclosing many of her concerns and troubles. She received prescriptions for cephalexin 500 mg orally 3 times daily, 0.1% triamcinolone ointment to be applied twice daily, and doxepin 25 mg once nightly. She also received a referral for counseling.
At first she refused to trim her fingernails, but as she began to see that her ulcers were self-inflicted, she reconsidered and agreed. In fact, she asked to borrow our nail clippers before leaving the office.
- Cephalexin • Biocef, Keflex, Keftab
- Dicloxacillin • Dycill, Dynapen, Pathocil
- Doxepin • Adapin, Sinequan
Corresponding author
Richard P. Usatine, MD, University of Texas Health Sceicnes Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900.
Note: A handout developed by the American Academy of Family Physicians for patients with neurotic excoriations is available to print or photocopy at the following website: www.aafp.org/afp/20011215/neurph.html.
1. Cyr PR, Dreher GK. Neurotic excoriations. Am Fam Physician 2001;64:1981-1984.
2. Habif TP. Clinical Dermatology.4th ed. New York: Mosby; 2004.
3. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician 2001;64:1873-1878.
4. Moses S. Pruritic condition. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER258.htm. Accessed July 23, 2004.
5. Moses S. Pruritus causes. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER259.htm. Accessed July 23, 2004.
A 55-year-old woman came to the clinic complaining of severe itching on her arms and legs. Although she itched throughout the day, it became intolerable at night, disrupting her sleep. She would sometimes scratch her arms and legs until exhaustion but could find no relief. Being outside in warm and sunny weather aggravated the problem. She had used moisturizers, emollients, and topical corticosteroids, but they only alleviated the itching temporarily. The itching began 10 months earlier, just after she finalized the divorce from her husband of 20 years.
Examination of the skin revealed numerous excoriations with ulcerations and xerosis on the arms and left leg (Figure 1 and 2). The excoriations were located extensively from the dorsum of her left foot to above the knee and bilaterally from the wrist to above the elbow. They also showed signs of infection. The patient admitted they were self-inflicted. The patient’s right leg had been amputated 5 years before after a car accident, and she wore a prosthetic leg. Examination of other areas showed nothing remarkable.
The patient readily admitted to a great deal of psychological distress. She described feeling depressed since her divorce. She has had difficulty securing a full-time job and has high anxiety about being able to pay her rent and bills.
FIGURE 1
Excoriations on the arms…
FIGURE 2
…and the left leg
WHAT IS THE DIAGNOSIS?
WHAT IS THE TREATMENT STRATEGY?
DIFFERENTIAL DIAGNOSIS: PSYCHODERMATOLOGIC DISORDER
The patient’s history and physical examination points to a psychodermatologic disorder. Psycho-dermatologic disorders are conditions involving an interaction between the mind and skin and are classified as:3
- Psychophysiologic disorders:skin disorders worsened by emotional stress
- Primary psychiatric disorders:usually caused by psychological conditions with self-induced skin damage
- Secondary psychiatric disorders: psychological problems developed as a consequence of a disfiguring skin disorder, which negatively effects self-esteem and body image.3
This patient’s excoriations and ulcers are due to self-mutilation. The differential diagnosis includes psychogenic parasitosis, factitial dermatitis, and neurotic excoriations. These 3 skin conditions are primary psychiatric symptoms, and proper diagnosis revolves around being able to assess the dermatologic features and associated psychological disorder.2
Psychogenic parasitosis
Also known as delusional parasitosis, psychogenic parasitosis is a psychodermatologic disorder in which patients believe they are infested with parasites. Patients with this disorder report seeing or feeling parasites on their skin, and they damage their skin in an attempt to remove them. Patients create excoriations and ulcers on easily reached areas, usually the ears, eyes, nose, and extremities. They often present with what is termed the “matchbox sign,” in which they bring containers filled with “small bits of excoriated skin, dried blood, debris, or insect parts as proof of infestation.”2
Women over the age of 50 years are more often affected with psychogenic parasitosis, and the disorder is associated with anxiety, depression, and hypochondriasis.
Factitial dermatitis
Factitial dermatitis, also known as dermatitis artefacta, is a psychodermatologic disorder in which patients damage their skin but deny their self-involvement. This disorder encompasses a wide range of lesions, including blisters, cuts, ulcers, and burns. Patients often are unable to describe how the lesions evolved. Lesions exhibit bizarre patterns not characteristic of any disease.
Young adults and adolescents are more commonly affected, and it is 4 times more common among women than men. Psychological disorders involved with factitial dermatitis include personality disorders and posttraumatic stress disorder.2
Neurotic excoriations
Neurotic excoriations, sometimes referred to as neurodermatitis, are a result of a psychodermatologic disorder in which patients inflict excoriations and ulcers on their skin and admit to their involvement.3 The condition is characterized by excoriations similar in size and shape that are localized on areas easily reached by the patient, such as the arms, legs, and upper back.
The lesions may present in various stages, varying from dugout ulcers to ulcers covered with crusts and surrounded by erythema to areas receding into depressed scars. These lesions are a result of repetitive scratching and digging by the patient, usually without an underlying physical pathology but sometimes initiated by pruritus.1,2
Studies show the condition primarily affects women, with a mean onset between the ages of 30 to 45 years.1 Common psychiatric problems associated with neurotic excoriations include significant social stress, depression, anxiety, and obsessive-compulsive disorder.
Diagnosis: Neurotic excoriations due to depression and stress
The patient’s history and physical examination suggests a diagnosis of neurotic excoriations due to the characteristics of the excoriations and ulcers on her arms and leg, her admission of their self-inflicted nature, and the associated depression and psychosocial stress.
Laboratory tests
Although there are no available laboratory tests to confirm a positive finding of neurotic excoriations, tests could be performed to disprove any systemic causes of pruritus and the resulting dermatological damage.5 These tests include complete blood count with differential, chemistry profile, determination of thyroid-stimulating hormone levels, fasting plasma glucose level, and skin biopsies.1,
Laboratory tests for systemic causes of pruritus should be based on the patient’s physical and history, avoiding a broad approach.
Treatment: address the skin and the psyche
The treatment of neurotic excoriations requires a dual approach, addressing both the dermatological problems and the underlying psychological disorder.3 Supportive dermatologic care is necessary to avoid secondary complications and to ensure that the patient feels supported.3
Dermatologic care
Topical steroids can be helpful to decrease the pruritus and inflammation. The steroid strength should be chosen based upon the severity of the lesions and the thickness of the skin. Steroid ointments are preferred to creams when there are skin ulcers and deep excoriations.
If there is significant crusting or exudate, there is probably a secondary bacterial infection and oral antibiotics are indicated ( Figure 3). A first-generation cephalosporin or dicloxacillin should provide adequate coverage against the most likely organisms, Staphylococcus aureus and Streptococcus pyogenes.
FIGURE 3
Detail of lesions on the arm
Psychological care
The selective serotonin reuptake inhibitors produce a strong antipruritic response in patients with neurotic excoriations. Doxepin is a tricyclic antidepressant with one of the most powerful antihistamines for pruritus. When prescribing doxepin, limit the amount of medication dispensed at one time to minimize the risk of suicide.3 The patient should trim the fingernails to reduce the amount of damage caused by scratching and digging.
Treating the underlying psychological disorder requires supportive and empathic counseling. It may be necessary to collaborate with other mental health specialists. Management options include psychotropic medication, stress management courses, and referral to a psychiatrist. Patients with psychodermatologic disorders frequently resist referral to mental health professionals.3 If this is the case, family physicians are well positioned to help patients with psychological problems.
Alternative courses of treatment include hypnosis to disrupt the itch-scratch cycle, and acupuncture and supportive therapy to reduce underlying stressors.1
Patient’s outcome
By showing support and concern for the patient’s health during the visit, the physician strengthened the relationship with the patient, who felt comfortable disclosing many of her concerns and troubles. She received prescriptions for cephalexin 500 mg orally 3 times daily, 0.1% triamcinolone ointment to be applied twice daily, and doxepin 25 mg once nightly. She also received a referral for counseling.
At first she refused to trim her fingernails, but as she began to see that her ulcers were self-inflicted, she reconsidered and agreed. In fact, she asked to borrow our nail clippers before leaving the office.
- Cephalexin • Biocef, Keflex, Keftab
- Dicloxacillin • Dycill, Dynapen, Pathocil
- Doxepin • Adapin, Sinequan
Corresponding author
Richard P. Usatine, MD, University of Texas Health Sceicnes Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900.
Note: A handout developed by the American Academy of Family Physicians for patients with neurotic excoriations is available to print or photocopy at the following website: www.aafp.org/afp/20011215/neurph.html.
A 55-year-old woman came to the clinic complaining of severe itching on her arms and legs. Although she itched throughout the day, it became intolerable at night, disrupting her sleep. She would sometimes scratch her arms and legs until exhaustion but could find no relief. Being outside in warm and sunny weather aggravated the problem. She had used moisturizers, emollients, and topical corticosteroids, but they only alleviated the itching temporarily. The itching began 10 months earlier, just after she finalized the divorce from her husband of 20 years.
Examination of the skin revealed numerous excoriations with ulcerations and xerosis on the arms and left leg (Figure 1 and 2). The excoriations were located extensively from the dorsum of her left foot to above the knee and bilaterally from the wrist to above the elbow. They also showed signs of infection. The patient admitted they were self-inflicted. The patient’s right leg had been amputated 5 years before after a car accident, and she wore a prosthetic leg. Examination of other areas showed nothing remarkable.
The patient readily admitted to a great deal of psychological distress. She described feeling depressed since her divorce. She has had difficulty securing a full-time job and has high anxiety about being able to pay her rent and bills.
FIGURE 1
Excoriations on the arms…
FIGURE 2
…and the left leg
WHAT IS THE DIAGNOSIS?
WHAT IS THE TREATMENT STRATEGY?
DIFFERENTIAL DIAGNOSIS: PSYCHODERMATOLOGIC DISORDER
The patient’s history and physical examination points to a psychodermatologic disorder. Psycho-dermatologic disorders are conditions involving an interaction between the mind and skin and are classified as:3
- Psychophysiologic disorders:skin disorders worsened by emotional stress
- Primary psychiatric disorders:usually caused by psychological conditions with self-induced skin damage
- Secondary psychiatric disorders: psychological problems developed as a consequence of a disfiguring skin disorder, which negatively effects self-esteem and body image.3
This patient’s excoriations and ulcers are due to self-mutilation. The differential diagnosis includes psychogenic parasitosis, factitial dermatitis, and neurotic excoriations. These 3 skin conditions are primary psychiatric symptoms, and proper diagnosis revolves around being able to assess the dermatologic features and associated psychological disorder.2
Psychogenic parasitosis
Also known as delusional parasitosis, psychogenic parasitosis is a psychodermatologic disorder in which patients believe they are infested with parasites. Patients with this disorder report seeing or feeling parasites on their skin, and they damage their skin in an attempt to remove them. Patients create excoriations and ulcers on easily reached areas, usually the ears, eyes, nose, and extremities. They often present with what is termed the “matchbox sign,” in which they bring containers filled with “small bits of excoriated skin, dried blood, debris, or insect parts as proof of infestation.”2
Women over the age of 50 years are more often affected with psychogenic parasitosis, and the disorder is associated with anxiety, depression, and hypochondriasis.
Factitial dermatitis
Factitial dermatitis, also known as dermatitis artefacta, is a psychodermatologic disorder in which patients damage their skin but deny their self-involvement. This disorder encompasses a wide range of lesions, including blisters, cuts, ulcers, and burns. Patients often are unable to describe how the lesions evolved. Lesions exhibit bizarre patterns not characteristic of any disease.
Young adults and adolescents are more commonly affected, and it is 4 times more common among women than men. Psychological disorders involved with factitial dermatitis include personality disorders and posttraumatic stress disorder.2
Neurotic excoriations
Neurotic excoriations, sometimes referred to as neurodermatitis, are a result of a psychodermatologic disorder in which patients inflict excoriations and ulcers on their skin and admit to their involvement.3 The condition is characterized by excoriations similar in size and shape that are localized on areas easily reached by the patient, such as the arms, legs, and upper back.
The lesions may present in various stages, varying from dugout ulcers to ulcers covered with crusts and surrounded by erythema to areas receding into depressed scars. These lesions are a result of repetitive scratching and digging by the patient, usually without an underlying physical pathology but sometimes initiated by pruritus.1,2
Studies show the condition primarily affects women, with a mean onset between the ages of 30 to 45 years.1 Common psychiatric problems associated with neurotic excoriations include significant social stress, depression, anxiety, and obsessive-compulsive disorder.
Diagnosis: Neurotic excoriations due to depression and stress
The patient’s history and physical examination suggests a diagnosis of neurotic excoriations due to the characteristics of the excoriations and ulcers on her arms and leg, her admission of their self-inflicted nature, and the associated depression and psychosocial stress.
Laboratory tests
Although there are no available laboratory tests to confirm a positive finding of neurotic excoriations, tests could be performed to disprove any systemic causes of pruritus and the resulting dermatological damage.5 These tests include complete blood count with differential, chemistry profile, determination of thyroid-stimulating hormone levels, fasting plasma glucose level, and skin biopsies.1,
Laboratory tests for systemic causes of pruritus should be based on the patient’s physical and history, avoiding a broad approach.
Treatment: address the skin and the psyche
The treatment of neurotic excoriations requires a dual approach, addressing both the dermatological problems and the underlying psychological disorder.3 Supportive dermatologic care is necessary to avoid secondary complications and to ensure that the patient feels supported.3
Dermatologic care
Topical steroids can be helpful to decrease the pruritus and inflammation. The steroid strength should be chosen based upon the severity of the lesions and the thickness of the skin. Steroid ointments are preferred to creams when there are skin ulcers and deep excoriations.
If there is significant crusting or exudate, there is probably a secondary bacterial infection and oral antibiotics are indicated ( Figure 3). A first-generation cephalosporin or dicloxacillin should provide adequate coverage against the most likely organisms, Staphylococcus aureus and Streptococcus pyogenes.
FIGURE 3
Detail of lesions on the arm
Psychological care
The selective serotonin reuptake inhibitors produce a strong antipruritic response in patients with neurotic excoriations. Doxepin is a tricyclic antidepressant with one of the most powerful antihistamines for pruritus. When prescribing doxepin, limit the amount of medication dispensed at one time to minimize the risk of suicide.3 The patient should trim the fingernails to reduce the amount of damage caused by scratching and digging.
Treating the underlying psychological disorder requires supportive and empathic counseling. It may be necessary to collaborate with other mental health specialists. Management options include psychotropic medication, stress management courses, and referral to a psychiatrist. Patients with psychodermatologic disorders frequently resist referral to mental health professionals.3 If this is the case, family physicians are well positioned to help patients with psychological problems.
Alternative courses of treatment include hypnosis to disrupt the itch-scratch cycle, and acupuncture and supportive therapy to reduce underlying stressors.1
Patient’s outcome
By showing support and concern for the patient’s health during the visit, the physician strengthened the relationship with the patient, who felt comfortable disclosing many of her concerns and troubles. She received prescriptions for cephalexin 500 mg orally 3 times daily, 0.1% triamcinolone ointment to be applied twice daily, and doxepin 25 mg once nightly. She also received a referral for counseling.
At first she refused to trim her fingernails, but as she began to see that her ulcers were self-inflicted, she reconsidered and agreed. In fact, she asked to borrow our nail clippers before leaving the office.
- Cephalexin • Biocef, Keflex, Keftab
- Dicloxacillin • Dycill, Dynapen, Pathocil
- Doxepin • Adapin, Sinequan
Corresponding author
Richard P. Usatine, MD, University of Texas Health Sceicnes Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900.
Note: A handout developed by the American Academy of Family Physicians for patients with neurotic excoriations is available to print or photocopy at the following website: www.aafp.org/afp/20011215/neurph.html.
1. Cyr PR, Dreher GK. Neurotic excoriations. Am Fam Physician 2001;64:1981-1984.
2. Habif TP. Clinical Dermatology.4th ed. New York: Mosby; 2004.
3. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician 2001;64:1873-1878.
4. Moses S. Pruritic condition. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER258.htm. Accessed July 23, 2004.
5. Moses S. Pruritus causes. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER259.htm. Accessed July 23, 2004.
1. Cyr PR, Dreher GK. Neurotic excoriations. Am Fam Physician 2001;64:1981-1984.
2. Habif TP. Clinical Dermatology.4th ed. New York: Mosby; 2004.
3. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician 2001;64:1873-1878.
4. Moses S. Pruritic condition. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER258.htm. Accessed July 23, 2004.
5. Moses S. Pruritus causes. In Family Practice Notebook [database online]. 2000; updated June 6, 2004. Available at:www.fpnotebook.com/DER259.htm. Accessed July 23, 2004.
Severe rash after dermatitis
An 18-year-old Caucasian woman came to the emergency department with a pruritic rash and localized swelling, most marked in the periorbital area. The rash had started 5 days earlier on her upper lip and subsequently spread to her face and upper chest.
Two days before, the patient was treated with amoxicillin/clavulanate (Augmentin), prednisone, and hydroxyzine (Atarax), but her symptoms worsened. She said she felt feverish but did not have any visual disturbances. She had no contacts with others ill with herpes or Varicella, although she did admit to having an unprotected sexual encounter 2 weeks before the rash’s onset. Her medical history was significant for untreated atopic dermatitis.
On exam, the patient was afebrile and had a diffuse maculopapular rash with areas of confluence over her face and hands (Figure 1). The face and hands also showed crusting and scaling. Discrete lesions were found on her upper and lower back (Figure 2), chest, volar wrist, and popliteal fossa. No vesicular lesions were present, although some isolated scabbed areas suggested previous vesicular lesions. The facial lesions were tender to palpation, and there was periorbital edema. No oral lesions were seen. Wound cultures grew methicillin-resistant Staphylococcus aureus.
FIGURE 1
Rash covering the face
FIGURE 2
Lesions on the upper back
What is the diagnosis?
How can the diagnosis be confirmed?
How should this disease be treated?
Diagnosis: Eczema herpeticum
Eczema herpeticum is an overwhelming herpesvirus infection on skin already affected by atopic dermatitis. It is a dermatologic emergency—untreated infections may lead to complications, including herpes keratitis and disseminated herpes simplex virus (HSV) infections with visceral involvement. Mortality is 1%–9%,1 although before antiviral therapy it was as high as 75%.2
The rash begins as dome-shaped vesicles, which subsequently disappear and become punched-out excoriations, crusts, and erythematous plaques. The head, neck, and trunk are the most commonly affected areas. Systemic symptoms such as fever and malaise usually accompany the rash.
Causes of eczema herpeticum
The cause of eczema herpeticum is always HSV type I.3 The exact pathophysiology is unknown, but it is thought to involve HSV entering the skin when skin barrier function is compromised due to dermatitis. Defective cytokine secretion in the affected skin also plays an important role.3
The severity of preexisting eczema does not seem to dictate the severity of eczema herpeticum.4 Secondary bacterial skin infections are very common. A mixture of aerobic and anaerobic bacteria are commonly isolated, the most common being S aureus, Group A β-hemolytic Streptococcus, Pseudomonas, and Peptostreptococcus.6,7
Risks
It is not clear which patients with atopic dermatitis are more at risk for developing eczema herpeticum. High total serum immunoglobulin E (IgE) and early age of onset are 2 risk factors that have been identified.7,8
Some researchers have suggested that use of topical corticosteroids predisposes those with atopic dermatitis to develop eczema herpeticum, but larger studies do not support this.8 However, topical calcineurin inhibitors do seem to pose a higher risk and are thus contraindicated during an eczema herpeticum infection.9
Differential diagnosis
Kaposi’s varicelliform eruption is a disseminated eruption of HSV on skin already affected with another dermatitis; eczema herpeticum refers specifically to the occurrence of an eruption on skin affected by atopic dermatitis. Thus, other types of Kaposi’s varicelliform eruption should be considered in the differential diagnosis. These include HSV infections on skin affected by Darier-White disease, pemphigus foliaceus, and mycosis fungoides. A good history taken from the patient regarding coexisting skin disorders makes the difference clear.
Other generalized vesicular eruptions such as Varicella should also be considered before making the diagnosis. Since distinct vesicles are not often present by the time the patient presents for care, the rash may also be confused with impetigo or other bacterial infections.
Laboratory tests
Several tests are available to detect the presence of HSV in the skin lesions of eczema herpeticum. These include polymerase chain reaction (PCR), immunofluorescence, and electron microscopy. Electron microscopy is not widely available and PCR can take several days, so often it is helpful to do direct fluorescent antibody testing while PCR results are pending. Light microscopy can be used to do a Tzanck test, which looks for multinucleated giant cells in blister fluid.
Serologies are often ordered, but results are nonspecific. Viral cultures are not very sensitive and take a while to get results. Aerobic and anaerobic bacterial cultures should be done because superinfection is common.
Treatment: systemic antivirals, antibiotics
Systemic antiviral medications are the mainstay of treatment for eczema herpeticum. Before the advent of acyclovir, the mortality rate of eczema herpeticum was 75%.2
The nucleoside analogs, which work by inhibiting viral DNA replication, are the most commonly used antiviral agents. Most of the studies on the treatment of eczema herpeticum have been on acyclovir; a 7-day course of IV therapy is typical. Alternatives such as valacyclovir have also been shown to be effective and have better oral bioavailability. Topical antivirals are often used for prophylaxis of keratoconjunctivitis if lesions are found around eyelids.
Antibiotics are often necessary to treat bacterial superinfection. Topical or systemic corticosteroids are not generally recommended and may worsen disease by attenuating the immune response.
Patient follow-up
This patient was initially treated with levofloxacin (Levaquin) for a presumed diagnosis of dermatitis with bacterial superinfection. Vancomycin was added on hospital day 2 when the rash was not improving. Due to a high suspicion for eczema herpeticum, a direct fluorescent antibody test sent for herpes, When the test results came back positive, the patient was started on intravenous acyclovir. A HSV PCR also had positive results. An ophthalmology specialist found no evidence for herpes keratoconjunctivitis and made no further recommendations.
The patient responded to the treatment and was discharged on hospital day 5. She was given a 10-day course of valacyclovir and a 21-day course of levofloxacin to complete at home. The patient fully recovered from the acute infections with some residual scarring.
1. Atherton DJ, Marshall WC. Eczema herpeticum. Practitioner 1982;226:971-973.
2. Wollenberg A, Wetzel S, Burgdorf W, Haas J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J Allergy Clin Immunol 2003;112:667-674.
3. Goodyear HM, et al. Growth of herpes simplex type 1 on skin explants of atopic eczema. Clin Exp Dermatol 1996;21:185-189.
4. Harindra V, Paffett MC. Recurrent eczema herpeticum: an underrecognised condition. Sex Transm Infect 2001;77:76.-
5. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol 1998;38:627-629.
6. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002;51:808-812.
7. Bork K, Brauninger W. Increasing evidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol 1988;19:1024-1029.
8. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum—a retrospective analysis of 100 cases. J Amer Acad Dermatol 2003;49:198-205.
9. Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110:e2.
An 18-year-old Caucasian woman came to the emergency department with a pruritic rash and localized swelling, most marked in the periorbital area. The rash had started 5 days earlier on her upper lip and subsequently spread to her face and upper chest.
Two days before, the patient was treated with amoxicillin/clavulanate (Augmentin), prednisone, and hydroxyzine (Atarax), but her symptoms worsened. She said she felt feverish but did not have any visual disturbances. She had no contacts with others ill with herpes or Varicella, although she did admit to having an unprotected sexual encounter 2 weeks before the rash’s onset. Her medical history was significant for untreated atopic dermatitis.
On exam, the patient was afebrile and had a diffuse maculopapular rash with areas of confluence over her face and hands (Figure 1). The face and hands also showed crusting and scaling. Discrete lesions were found on her upper and lower back (Figure 2), chest, volar wrist, and popliteal fossa. No vesicular lesions were present, although some isolated scabbed areas suggested previous vesicular lesions. The facial lesions were tender to palpation, and there was periorbital edema. No oral lesions were seen. Wound cultures grew methicillin-resistant Staphylococcus aureus.
FIGURE 1
Rash covering the face
FIGURE 2
Lesions on the upper back
What is the diagnosis?
How can the diagnosis be confirmed?
How should this disease be treated?
Diagnosis: Eczema herpeticum
Eczema herpeticum is an overwhelming herpesvirus infection on skin already affected by atopic dermatitis. It is a dermatologic emergency—untreated infections may lead to complications, including herpes keratitis and disseminated herpes simplex virus (HSV) infections with visceral involvement. Mortality is 1%–9%,1 although before antiviral therapy it was as high as 75%.2
The rash begins as dome-shaped vesicles, which subsequently disappear and become punched-out excoriations, crusts, and erythematous plaques. The head, neck, and trunk are the most commonly affected areas. Systemic symptoms such as fever and malaise usually accompany the rash.
Causes of eczema herpeticum
The cause of eczema herpeticum is always HSV type I.3 The exact pathophysiology is unknown, but it is thought to involve HSV entering the skin when skin barrier function is compromised due to dermatitis. Defective cytokine secretion in the affected skin also plays an important role.3
The severity of preexisting eczema does not seem to dictate the severity of eczema herpeticum.4 Secondary bacterial skin infections are very common. A mixture of aerobic and anaerobic bacteria are commonly isolated, the most common being S aureus, Group A β-hemolytic Streptococcus, Pseudomonas, and Peptostreptococcus.6,7
Risks
It is not clear which patients with atopic dermatitis are more at risk for developing eczema herpeticum. High total serum immunoglobulin E (IgE) and early age of onset are 2 risk factors that have been identified.7,8
Some researchers have suggested that use of topical corticosteroids predisposes those with atopic dermatitis to develop eczema herpeticum, but larger studies do not support this.8 However, topical calcineurin inhibitors do seem to pose a higher risk and are thus contraindicated during an eczema herpeticum infection.9
Differential diagnosis
Kaposi’s varicelliform eruption is a disseminated eruption of HSV on skin already affected with another dermatitis; eczema herpeticum refers specifically to the occurrence of an eruption on skin affected by atopic dermatitis. Thus, other types of Kaposi’s varicelliform eruption should be considered in the differential diagnosis. These include HSV infections on skin affected by Darier-White disease, pemphigus foliaceus, and mycosis fungoides. A good history taken from the patient regarding coexisting skin disorders makes the difference clear.
Other generalized vesicular eruptions such as Varicella should also be considered before making the diagnosis. Since distinct vesicles are not often present by the time the patient presents for care, the rash may also be confused with impetigo or other bacterial infections.
Laboratory tests
Several tests are available to detect the presence of HSV in the skin lesions of eczema herpeticum. These include polymerase chain reaction (PCR), immunofluorescence, and electron microscopy. Electron microscopy is not widely available and PCR can take several days, so often it is helpful to do direct fluorescent antibody testing while PCR results are pending. Light microscopy can be used to do a Tzanck test, which looks for multinucleated giant cells in blister fluid.
Serologies are often ordered, but results are nonspecific. Viral cultures are not very sensitive and take a while to get results. Aerobic and anaerobic bacterial cultures should be done because superinfection is common.
Treatment: systemic antivirals, antibiotics
Systemic antiviral medications are the mainstay of treatment for eczema herpeticum. Before the advent of acyclovir, the mortality rate of eczema herpeticum was 75%.2
The nucleoside analogs, which work by inhibiting viral DNA replication, are the most commonly used antiviral agents. Most of the studies on the treatment of eczema herpeticum have been on acyclovir; a 7-day course of IV therapy is typical. Alternatives such as valacyclovir have also been shown to be effective and have better oral bioavailability. Topical antivirals are often used for prophylaxis of keratoconjunctivitis if lesions are found around eyelids.
Antibiotics are often necessary to treat bacterial superinfection. Topical or systemic corticosteroids are not generally recommended and may worsen disease by attenuating the immune response.
Patient follow-up
This patient was initially treated with levofloxacin (Levaquin) for a presumed diagnosis of dermatitis with bacterial superinfection. Vancomycin was added on hospital day 2 when the rash was not improving. Due to a high suspicion for eczema herpeticum, a direct fluorescent antibody test sent for herpes, When the test results came back positive, the patient was started on intravenous acyclovir. A HSV PCR also had positive results. An ophthalmology specialist found no evidence for herpes keratoconjunctivitis and made no further recommendations.
The patient responded to the treatment and was discharged on hospital day 5. She was given a 10-day course of valacyclovir and a 21-day course of levofloxacin to complete at home. The patient fully recovered from the acute infections with some residual scarring.
An 18-year-old Caucasian woman came to the emergency department with a pruritic rash and localized swelling, most marked in the periorbital area. The rash had started 5 days earlier on her upper lip and subsequently spread to her face and upper chest.
Two days before, the patient was treated with amoxicillin/clavulanate (Augmentin), prednisone, and hydroxyzine (Atarax), but her symptoms worsened. She said she felt feverish but did not have any visual disturbances. She had no contacts with others ill with herpes or Varicella, although she did admit to having an unprotected sexual encounter 2 weeks before the rash’s onset. Her medical history was significant for untreated atopic dermatitis.
On exam, the patient was afebrile and had a diffuse maculopapular rash with areas of confluence over her face and hands (Figure 1). The face and hands also showed crusting and scaling. Discrete lesions were found on her upper and lower back (Figure 2), chest, volar wrist, and popliteal fossa. No vesicular lesions were present, although some isolated scabbed areas suggested previous vesicular lesions. The facial lesions were tender to palpation, and there was periorbital edema. No oral lesions were seen. Wound cultures grew methicillin-resistant Staphylococcus aureus.
FIGURE 1
Rash covering the face
FIGURE 2
Lesions on the upper back
What is the diagnosis?
How can the diagnosis be confirmed?
How should this disease be treated?
Diagnosis: Eczema herpeticum
Eczema herpeticum is an overwhelming herpesvirus infection on skin already affected by atopic dermatitis. It is a dermatologic emergency—untreated infections may lead to complications, including herpes keratitis and disseminated herpes simplex virus (HSV) infections with visceral involvement. Mortality is 1%–9%,1 although before antiviral therapy it was as high as 75%.2
The rash begins as dome-shaped vesicles, which subsequently disappear and become punched-out excoriations, crusts, and erythematous plaques. The head, neck, and trunk are the most commonly affected areas. Systemic symptoms such as fever and malaise usually accompany the rash.
Causes of eczema herpeticum
The cause of eczema herpeticum is always HSV type I.3 The exact pathophysiology is unknown, but it is thought to involve HSV entering the skin when skin barrier function is compromised due to dermatitis. Defective cytokine secretion in the affected skin also plays an important role.3
The severity of preexisting eczema does not seem to dictate the severity of eczema herpeticum.4 Secondary bacterial skin infections are very common. A mixture of aerobic and anaerobic bacteria are commonly isolated, the most common being S aureus, Group A β-hemolytic Streptococcus, Pseudomonas, and Peptostreptococcus.6,7
Risks
It is not clear which patients with atopic dermatitis are more at risk for developing eczema herpeticum. High total serum immunoglobulin E (IgE) and early age of onset are 2 risk factors that have been identified.7,8
Some researchers have suggested that use of topical corticosteroids predisposes those with atopic dermatitis to develop eczema herpeticum, but larger studies do not support this.8 However, topical calcineurin inhibitors do seem to pose a higher risk and are thus contraindicated during an eczema herpeticum infection.9
Differential diagnosis
Kaposi’s varicelliform eruption is a disseminated eruption of HSV on skin already affected with another dermatitis; eczema herpeticum refers specifically to the occurrence of an eruption on skin affected by atopic dermatitis. Thus, other types of Kaposi’s varicelliform eruption should be considered in the differential diagnosis. These include HSV infections on skin affected by Darier-White disease, pemphigus foliaceus, and mycosis fungoides. A good history taken from the patient regarding coexisting skin disorders makes the difference clear.
Other generalized vesicular eruptions such as Varicella should also be considered before making the diagnosis. Since distinct vesicles are not often present by the time the patient presents for care, the rash may also be confused with impetigo or other bacterial infections.
Laboratory tests
Several tests are available to detect the presence of HSV in the skin lesions of eczema herpeticum. These include polymerase chain reaction (PCR), immunofluorescence, and electron microscopy. Electron microscopy is not widely available and PCR can take several days, so often it is helpful to do direct fluorescent antibody testing while PCR results are pending. Light microscopy can be used to do a Tzanck test, which looks for multinucleated giant cells in blister fluid.
Serologies are often ordered, but results are nonspecific. Viral cultures are not very sensitive and take a while to get results. Aerobic and anaerobic bacterial cultures should be done because superinfection is common.
Treatment: systemic antivirals, antibiotics
Systemic antiviral medications are the mainstay of treatment for eczema herpeticum. Before the advent of acyclovir, the mortality rate of eczema herpeticum was 75%.2
The nucleoside analogs, which work by inhibiting viral DNA replication, are the most commonly used antiviral agents. Most of the studies on the treatment of eczema herpeticum have been on acyclovir; a 7-day course of IV therapy is typical. Alternatives such as valacyclovir have also been shown to be effective and have better oral bioavailability. Topical antivirals are often used for prophylaxis of keratoconjunctivitis if lesions are found around eyelids.
Antibiotics are often necessary to treat bacterial superinfection. Topical or systemic corticosteroids are not generally recommended and may worsen disease by attenuating the immune response.
Patient follow-up
This patient was initially treated with levofloxacin (Levaquin) for a presumed diagnosis of dermatitis with bacterial superinfection. Vancomycin was added on hospital day 2 when the rash was not improving. Due to a high suspicion for eczema herpeticum, a direct fluorescent antibody test sent for herpes, When the test results came back positive, the patient was started on intravenous acyclovir. A HSV PCR also had positive results. An ophthalmology specialist found no evidence for herpes keratoconjunctivitis and made no further recommendations.
The patient responded to the treatment and was discharged on hospital day 5. She was given a 10-day course of valacyclovir and a 21-day course of levofloxacin to complete at home. The patient fully recovered from the acute infections with some residual scarring.
1. Atherton DJ, Marshall WC. Eczema herpeticum. Practitioner 1982;226:971-973.
2. Wollenberg A, Wetzel S, Burgdorf W, Haas J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J Allergy Clin Immunol 2003;112:667-674.
3. Goodyear HM, et al. Growth of herpes simplex type 1 on skin explants of atopic eczema. Clin Exp Dermatol 1996;21:185-189.
4. Harindra V, Paffett MC. Recurrent eczema herpeticum: an underrecognised condition. Sex Transm Infect 2001;77:76.-
5. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol 1998;38:627-629.
6. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002;51:808-812.
7. Bork K, Brauninger W. Increasing evidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol 1988;19:1024-1029.
8. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum—a retrospective analysis of 100 cases. J Amer Acad Dermatol 2003;49:198-205.
9. Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110:e2.
1. Atherton DJ, Marshall WC. Eczema herpeticum. Practitioner 1982;226:971-973.
2. Wollenberg A, Wetzel S, Burgdorf W, Haas J. Viral infections in atopic dermatitis: Pathogenic aspects and clinical management. J Allergy Clin Immunol 2003;112:667-674.
3. Goodyear HM, et al. Growth of herpes simplex type 1 on skin explants of atopic eczema. Clin Exp Dermatol 1996;21:185-189.
4. Harindra V, Paffett MC. Recurrent eczema herpeticum: an underrecognised condition. Sex Transm Infect 2001;77:76.-
5. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol 1998;38:627-629.
6. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002;51:808-812.
7. Bork K, Brauninger W. Increasing evidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol 1988;19:1024-1029.
8. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum—a retrospective analysis of 100 cases. J Amer Acad Dermatol 2003;49:198-205.
9. Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110:e2.
Pigmented lesion on the ear
A 65-year-old farmer came to the office with a pigmented lesion on his left ear; the lesion had been there for about 2 years. He noticed different shades of black developing in the lesion during the previous 3 months.
On physical examination, we observed a 13 mm x 7 mm asymmetrical dark-brown-to-black papule, with pigment fading at its borders, on the lower helix of the patient’s left ear. No ulceration was noted.
As an incidental finding, we noted accumulation of a yellowish waxy material in the left retroauricular area. The right ear was normal on examination, and no mucosal lesions were found. Lymph nodes of the retroauricular, submandibular, occipital, and supraclavicular areas were normal. An excisional biopsy was performed.
What is the diagnosis?
How would you manage this case?
FIGURE 1
Pigmented papule on the ear
Diagnosis: Malignant melanoma
Our patient’s excisional biopsy, which included 3-mm lateral margins, demonstrated clear architectural and cytological abnormalities consistent with superficial spreading malignant melanoma. Pronounced anisocytosis with prominent nucleoli and unevenly distributed melanin was noted, with atypical melanocytes extending into the papillary dermis. The Breslow thickness was 0.55 mm (Clark level II), and the TNM stage was T1a.
Incidence of malignant melanoma
The incidence of malignant melanoma has more than tripled among Caucasians in the US over the last 40 years; it is the fastest-growing1 and seventh most frequent cancer in the country.2 The risk of developing malignant melanoma is expected to reach 1 in 50 by 2010,3 increasing from 1 in 250 less than a quarter-century ago.1 Elderly men are particularly at risk.
Roughly 20% of melanomas develop in the head and neck regions, and of these approximately 7% to 14% are located on the external ear.4 Melanoma of the external ear most frequently develops on the left side (possibly due to increased sun exposure while driving), usually on the helix.1,4 In a small series by Benmeir et al,4 most patients reported having had an ear nevus whose features (size, color) began changing before diagnosis. Of note, only about one quarter of cutaneous melanomas are discovered directly by physicians.3 Moreover, neoplasms in certain regions of the ear may easily go unnoticed, causing a delay in diagnosis and treatment.1
Lesions are generally found in peripheral areas of the ear and are usually the superficial spreading type; however, nodular melanoma predominated in 1 relatively recent series.7 The lack of subcutaneous tissue on the external ear may contribute to the ease of invasion and poor prognosis identified in several reports.4,7 Hudson et al8 noted more deeply penetrating and thicker lesions at presentation on the external ear in comparison with malignant melanoma of other head and neck areas.
Risk factors
Risk factors for developing malignant melanoma include intense intermittent sunlight exposure (primarily UVB) and blistering sunburns at an early age; skin types and certain ethnicities with limited tanning capability; personal or family history of melanoma; multiple nevi; and immunosup-pression.3 The vast majority of malignant melanomas arise de novo, although very rarely a nevus (usually a giant congenital melanocytic nevus) may undergo malignant transformation.3
Differential diagnosis
The differential diagnosis of malignant melanoma includes atypical nevi, dermatofibromas, lentigos, basal and squamous cell carcinomas, keloids and hypertrophic scars, and seborrheic keratoses.2
Making an accurate diagnosis
The ABCD approach
The ABCD approach to recognizing potentially malignant melanotic lesions is evaluation for Asymmetry, Border irregularity, Color variegation, and Diameter >6 mm (roughly pencil eraser size). Patients who report recent changes in the characteristics of existing nevi should be examined carefully.
Biopsy, histology, dermatoscopy
Excisional biopsy and histologic examination are required for diagnosis, which is facilitated by histochemistry and immunohistochemistry techniques. Architectural criteria are of greater diagnostic significance than cytologic features, rendering fine- needle aspiration or curettage less helpful and unnecessary for diagnosis.3 Before excision, corresponding areas of lymphatic drainage should be examined, and subsequently a full-thickness biopsy with 2- to 5-mm lateral margins should be performed.9
Dermatoscopy is an excellent noninvasive method for in vivo examination of suspected melanomas, being a potentially powerful resource for general practitioners and dermatologists alike. In this procedure, the suspected melanoma is covered with mineral oil, alcohol, or water and viewed with a hand-held dermatoscope, which magnifies from 10 to 100 times, allowing visualization of structures at and below the skin surface. In comparison with clinical analysis, the sensitivity of dermatoscopic diagnosis is increased by 10% to 30%.3
Management: Surgical excision and special considerations
Difficulties in managing ear melanomas arise due to the ear’s importance in daily functioning and to patient’s cosmetic concerns. Initial reports of malignant melanoma of the external ear indicated a poorer prognosis compared with lesions in other areas,5,6 but subsequent studies did not corroborate these findings.
Surgical excision
Surgical excision is the standard of care for malignant melanoma. The World Health Organization recommends excision margins of 5 mm for in situ lesions, and 20 mm for melanomas >2.1 mm thick,10 although treatment of external ear lesions must be individualized given the thin skin and various anatomic subdivisions of the ear. Pockaj et al1 found margins of at least 10 mm to be associated with the lowest recurrence risk.
Several techniques have been employed in lesion excision and postexcisional defect repair, including wedge resection, partial and total auriculectomy, wide excision and skin grafting, and Mohs micrographic surgery.4 Wedge resection was associated in 1 study8 with significantly increased melanoma recurrence when compared with wide local excision using 10-mm margins or total auriculectomy.
Skin and subcutaneous tissue should always be excised, but perichondrium is generally spared unless involved with the tumor.1 However, Narayan et al11 suggested cartilaginous excision in melanomas >1 mm thick, regardless of the presence of tumor infiltration. Various types of flaps are used in reconstructing surgical defects.11
Lymph node dissection
Elective lymph node dissection, as well as superficial parotidectomy in cases with suspected metastasis, have been performed; however, sentinel lymph node mapping can drastically reduce the morbidity associated with unnecessary lymph node dissection.12 This technique has been shown to be of benefit in managing malignant melanoma of the ear due to its highly ambiguous and variable lymphatic drainage patterns.12
Sentinel node biopsy of the parotid gland can be performed as well with low morbidity and a high success rate.1 Byers et al6 suggested that neck dissections be reserved for patients with Clark level IV or V melanomas.
Immunotherapies
Immunotherapeutic agents, including interleukin-2 and interferon alpha 2b, have recently become significant adjuvant therapies for malignant melanoma, and investigation into a potential melanoma vaccine is currently underway.3 Consultations with surgical, medical, and possibly radiation oncology, nuclear medicine, and pathology may be needed in treating patients with malignant melanoma depending on tumor invasiveness and metastasis.
Evidence of metastatic spread should routinely be sought when examining patients. For patients with lesions <1 mm thick, close follow-up with biannual full-body skin examination is recommended for 2 years following excision, and subsequently each year for the next 8 years.3 Melanomas thicker than 1 mm may require up to 4 annual visits during the first 2 years, followed by biannual and annual exams. Chest x-ray is recommended annually during the first 5 years for lesions <3 mm thick, and biannually for those >3 mm thick.3
The patient’s follow-up
Our patient’s lesion was very superficial. Following excisional biopsy, a wedge excision with appropriate 10-mm margins was performed by a plastic surgeon. The result of the chest x-ray was normal. The patient is scheduled for follow-up examination every 6 months for 2 years and yearly thereafter.
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: [email protected].
1. Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for malignant melanoma of the external ear. Ann Surg Oncol 2003;10:689-696.
2. Swetter SM. Malignant melanoma. EMedicine 2003. Available at www.emedicine.com/derm/topic257.htm. Accessed on May 10, 2004.
3. Zalaudek I, Ferrara G, Argenziano G, et al. Diagnosis and treatment of cutaneous melanoma: a practical guide. SKINmed 2003;2:20-31.
4. Benmeir P, Baruchin A, Weinberg A. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg 1995;23:50-53.
5. Batsakis JG. Melanomas (cutaneous and mucosal) of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore, Md, and London: Williams & Wilkins; 1980;431-447.
6. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg 1980;140:518-521.
7. Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol 1993;107:798-802.
8. Hudson DA, Krige JEJ, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg 1990;43:608-611.
9. Eedy DJ. Surgical treatment of melanoma. Br J Dermatol 2003;149:2-12.
10. Lens MB, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma. A systemic review of randomized controlled trials comparing narrow vs. wide excision. Arch Surg 2002;137:1101-1105.
11. Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg 2001;107:20-24.
12. Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg 1998;40:506-509.
A 65-year-old farmer came to the office with a pigmented lesion on his left ear; the lesion had been there for about 2 years. He noticed different shades of black developing in the lesion during the previous 3 months.
On physical examination, we observed a 13 mm x 7 mm asymmetrical dark-brown-to-black papule, with pigment fading at its borders, on the lower helix of the patient’s left ear. No ulceration was noted.
As an incidental finding, we noted accumulation of a yellowish waxy material in the left retroauricular area. The right ear was normal on examination, and no mucosal lesions were found. Lymph nodes of the retroauricular, submandibular, occipital, and supraclavicular areas were normal. An excisional biopsy was performed.
What is the diagnosis?
How would you manage this case?
FIGURE 1
Pigmented papule on the ear
Diagnosis: Malignant melanoma
Our patient’s excisional biopsy, which included 3-mm lateral margins, demonstrated clear architectural and cytological abnormalities consistent with superficial spreading malignant melanoma. Pronounced anisocytosis with prominent nucleoli and unevenly distributed melanin was noted, with atypical melanocytes extending into the papillary dermis. The Breslow thickness was 0.55 mm (Clark level II), and the TNM stage was T1a.
Incidence of malignant melanoma
The incidence of malignant melanoma has more than tripled among Caucasians in the US over the last 40 years; it is the fastest-growing1 and seventh most frequent cancer in the country.2 The risk of developing malignant melanoma is expected to reach 1 in 50 by 2010,3 increasing from 1 in 250 less than a quarter-century ago.1 Elderly men are particularly at risk.
Roughly 20% of melanomas develop in the head and neck regions, and of these approximately 7% to 14% are located on the external ear.4 Melanoma of the external ear most frequently develops on the left side (possibly due to increased sun exposure while driving), usually on the helix.1,4 In a small series by Benmeir et al,4 most patients reported having had an ear nevus whose features (size, color) began changing before diagnosis. Of note, only about one quarter of cutaneous melanomas are discovered directly by physicians.3 Moreover, neoplasms in certain regions of the ear may easily go unnoticed, causing a delay in diagnosis and treatment.1
Lesions are generally found in peripheral areas of the ear and are usually the superficial spreading type; however, nodular melanoma predominated in 1 relatively recent series.7 The lack of subcutaneous tissue on the external ear may contribute to the ease of invasion and poor prognosis identified in several reports.4,7 Hudson et al8 noted more deeply penetrating and thicker lesions at presentation on the external ear in comparison with malignant melanoma of other head and neck areas.
Risk factors
Risk factors for developing malignant melanoma include intense intermittent sunlight exposure (primarily UVB) and blistering sunburns at an early age; skin types and certain ethnicities with limited tanning capability; personal or family history of melanoma; multiple nevi; and immunosup-pression.3 The vast majority of malignant melanomas arise de novo, although very rarely a nevus (usually a giant congenital melanocytic nevus) may undergo malignant transformation.3
Differential diagnosis
The differential diagnosis of malignant melanoma includes atypical nevi, dermatofibromas, lentigos, basal and squamous cell carcinomas, keloids and hypertrophic scars, and seborrheic keratoses.2
Making an accurate diagnosis
The ABCD approach
The ABCD approach to recognizing potentially malignant melanotic lesions is evaluation for Asymmetry, Border irregularity, Color variegation, and Diameter >6 mm (roughly pencil eraser size). Patients who report recent changes in the characteristics of existing nevi should be examined carefully.
Biopsy, histology, dermatoscopy
Excisional biopsy and histologic examination are required for diagnosis, which is facilitated by histochemistry and immunohistochemistry techniques. Architectural criteria are of greater diagnostic significance than cytologic features, rendering fine- needle aspiration or curettage less helpful and unnecessary for diagnosis.3 Before excision, corresponding areas of lymphatic drainage should be examined, and subsequently a full-thickness biopsy with 2- to 5-mm lateral margins should be performed.9
Dermatoscopy is an excellent noninvasive method for in vivo examination of suspected melanomas, being a potentially powerful resource for general practitioners and dermatologists alike. In this procedure, the suspected melanoma is covered with mineral oil, alcohol, or water and viewed with a hand-held dermatoscope, which magnifies from 10 to 100 times, allowing visualization of structures at and below the skin surface. In comparison with clinical analysis, the sensitivity of dermatoscopic diagnosis is increased by 10% to 30%.3
Management: Surgical excision and special considerations
Difficulties in managing ear melanomas arise due to the ear’s importance in daily functioning and to patient’s cosmetic concerns. Initial reports of malignant melanoma of the external ear indicated a poorer prognosis compared with lesions in other areas,5,6 but subsequent studies did not corroborate these findings.
Surgical excision
Surgical excision is the standard of care for malignant melanoma. The World Health Organization recommends excision margins of 5 mm for in situ lesions, and 20 mm for melanomas >2.1 mm thick,10 although treatment of external ear lesions must be individualized given the thin skin and various anatomic subdivisions of the ear. Pockaj et al1 found margins of at least 10 mm to be associated with the lowest recurrence risk.
Several techniques have been employed in lesion excision and postexcisional defect repair, including wedge resection, partial and total auriculectomy, wide excision and skin grafting, and Mohs micrographic surgery.4 Wedge resection was associated in 1 study8 with significantly increased melanoma recurrence when compared with wide local excision using 10-mm margins or total auriculectomy.
Skin and subcutaneous tissue should always be excised, but perichondrium is generally spared unless involved with the tumor.1 However, Narayan et al11 suggested cartilaginous excision in melanomas >1 mm thick, regardless of the presence of tumor infiltration. Various types of flaps are used in reconstructing surgical defects.11
Lymph node dissection
Elective lymph node dissection, as well as superficial parotidectomy in cases with suspected metastasis, have been performed; however, sentinel lymph node mapping can drastically reduce the morbidity associated with unnecessary lymph node dissection.12 This technique has been shown to be of benefit in managing malignant melanoma of the ear due to its highly ambiguous and variable lymphatic drainage patterns.12
Sentinel node biopsy of the parotid gland can be performed as well with low morbidity and a high success rate.1 Byers et al6 suggested that neck dissections be reserved for patients with Clark level IV or V melanomas.
Immunotherapies
Immunotherapeutic agents, including interleukin-2 and interferon alpha 2b, have recently become significant adjuvant therapies for malignant melanoma, and investigation into a potential melanoma vaccine is currently underway.3 Consultations with surgical, medical, and possibly radiation oncology, nuclear medicine, and pathology may be needed in treating patients with malignant melanoma depending on tumor invasiveness and metastasis.
Evidence of metastatic spread should routinely be sought when examining patients. For patients with lesions <1 mm thick, close follow-up with biannual full-body skin examination is recommended for 2 years following excision, and subsequently each year for the next 8 years.3 Melanomas thicker than 1 mm may require up to 4 annual visits during the first 2 years, followed by biannual and annual exams. Chest x-ray is recommended annually during the first 5 years for lesions <3 mm thick, and biannually for those >3 mm thick.3
The patient’s follow-up
Our patient’s lesion was very superficial. Following excisional biopsy, a wedge excision with appropriate 10-mm margins was performed by a plastic surgeon. The result of the chest x-ray was normal. The patient is scheduled for follow-up examination every 6 months for 2 years and yearly thereafter.
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: [email protected].
A 65-year-old farmer came to the office with a pigmented lesion on his left ear; the lesion had been there for about 2 years. He noticed different shades of black developing in the lesion during the previous 3 months.
On physical examination, we observed a 13 mm x 7 mm asymmetrical dark-brown-to-black papule, with pigment fading at its borders, on the lower helix of the patient’s left ear. No ulceration was noted.
As an incidental finding, we noted accumulation of a yellowish waxy material in the left retroauricular area. The right ear was normal on examination, and no mucosal lesions were found. Lymph nodes of the retroauricular, submandibular, occipital, and supraclavicular areas were normal. An excisional biopsy was performed.
What is the diagnosis?
How would you manage this case?
FIGURE 1
Pigmented papule on the ear
Diagnosis: Malignant melanoma
Our patient’s excisional biopsy, which included 3-mm lateral margins, demonstrated clear architectural and cytological abnormalities consistent with superficial spreading malignant melanoma. Pronounced anisocytosis with prominent nucleoli and unevenly distributed melanin was noted, with atypical melanocytes extending into the papillary dermis. The Breslow thickness was 0.55 mm (Clark level II), and the TNM stage was T1a.
Incidence of malignant melanoma
The incidence of malignant melanoma has more than tripled among Caucasians in the US over the last 40 years; it is the fastest-growing1 and seventh most frequent cancer in the country.2 The risk of developing malignant melanoma is expected to reach 1 in 50 by 2010,3 increasing from 1 in 250 less than a quarter-century ago.1 Elderly men are particularly at risk.
Roughly 20% of melanomas develop in the head and neck regions, and of these approximately 7% to 14% are located on the external ear.4 Melanoma of the external ear most frequently develops on the left side (possibly due to increased sun exposure while driving), usually on the helix.1,4 In a small series by Benmeir et al,4 most patients reported having had an ear nevus whose features (size, color) began changing before diagnosis. Of note, only about one quarter of cutaneous melanomas are discovered directly by physicians.3 Moreover, neoplasms in certain regions of the ear may easily go unnoticed, causing a delay in diagnosis and treatment.1
Lesions are generally found in peripheral areas of the ear and are usually the superficial spreading type; however, nodular melanoma predominated in 1 relatively recent series.7 The lack of subcutaneous tissue on the external ear may contribute to the ease of invasion and poor prognosis identified in several reports.4,7 Hudson et al8 noted more deeply penetrating and thicker lesions at presentation on the external ear in comparison with malignant melanoma of other head and neck areas.
Risk factors
Risk factors for developing malignant melanoma include intense intermittent sunlight exposure (primarily UVB) and blistering sunburns at an early age; skin types and certain ethnicities with limited tanning capability; personal or family history of melanoma; multiple nevi; and immunosup-pression.3 The vast majority of malignant melanomas arise de novo, although very rarely a nevus (usually a giant congenital melanocytic nevus) may undergo malignant transformation.3
Differential diagnosis
The differential diagnosis of malignant melanoma includes atypical nevi, dermatofibromas, lentigos, basal and squamous cell carcinomas, keloids and hypertrophic scars, and seborrheic keratoses.2
Making an accurate diagnosis
The ABCD approach
The ABCD approach to recognizing potentially malignant melanotic lesions is evaluation for Asymmetry, Border irregularity, Color variegation, and Diameter >6 mm (roughly pencil eraser size). Patients who report recent changes in the characteristics of existing nevi should be examined carefully.
Biopsy, histology, dermatoscopy
Excisional biopsy and histologic examination are required for diagnosis, which is facilitated by histochemistry and immunohistochemistry techniques. Architectural criteria are of greater diagnostic significance than cytologic features, rendering fine- needle aspiration or curettage less helpful and unnecessary for diagnosis.3 Before excision, corresponding areas of lymphatic drainage should be examined, and subsequently a full-thickness biopsy with 2- to 5-mm lateral margins should be performed.9
Dermatoscopy is an excellent noninvasive method for in vivo examination of suspected melanomas, being a potentially powerful resource for general practitioners and dermatologists alike. In this procedure, the suspected melanoma is covered with mineral oil, alcohol, or water and viewed with a hand-held dermatoscope, which magnifies from 10 to 100 times, allowing visualization of structures at and below the skin surface. In comparison with clinical analysis, the sensitivity of dermatoscopic diagnosis is increased by 10% to 30%.3
Management: Surgical excision and special considerations
Difficulties in managing ear melanomas arise due to the ear’s importance in daily functioning and to patient’s cosmetic concerns. Initial reports of malignant melanoma of the external ear indicated a poorer prognosis compared with lesions in other areas,5,6 but subsequent studies did not corroborate these findings.
Surgical excision
Surgical excision is the standard of care for malignant melanoma. The World Health Organization recommends excision margins of 5 mm for in situ lesions, and 20 mm for melanomas >2.1 mm thick,10 although treatment of external ear lesions must be individualized given the thin skin and various anatomic subdivisions of the ear. Pockaj et al1 found margins of at least 10 mm to be associated with the lowest recurrence risk.
Several techniques have been employed in lesion excision and postexcisional defect repair, including wedge resection, partial and total auriculectomy, wide excision and skin grafting, and Mohs micrographic surgery.4 Wedge resection was associated in 1 study8 with significantly increased melanoma recurrence when compared with wide local excision using 10-mm margins or total auriculectomy.
Skin and subcutaneous tissue should always be excised, but perichondrium is generally spared unless involved with the tumor.1 However, Narayan et al11 suggested cartilaginous excision in melanomas >1 mm thick, regardless of the presence of tumor infiltration. Various types of flaps are used in reconstructing surgical defects.11
Lymph node dissection
Elective lymph node dissection, as well as superficial parotidectomy in cases with suspected metastasis, have been performed; however, sentinel lymph node mapping can drastically reduce the morbidity associated with unnecessary lymph node dissection.12 This technique has been shown to be of benefit in managing malignant melanoma of the ear due to its highly ambiguous and variable lymphatic drainage patterns.12
Sentinel node biopsy of the parotid gland can be performed as well with low morbidity and a high success rate.1 Byers et al6 suggested that neck dissections be reserved for patients with Clark level IV or V melanomas.
Immunotherapies
Immunotherapeutic agents, including interleukin-2 and interferon alpha 2b, have recently become significant adjuvant therapies for malignant melanoma, and investigation into a potential melanoma vaccine is currently underway.3 Consultations with surgical, medical, and possibly radiation oncology, nuclear medicine, and pathology may be needed in treating patients with malignant melanoma depending on tumor invasiveness and metastasis.
Evidence of metastatic spread should routinely be sought when examining patients. For patients with lesions <1 mm thick, close follow-up with biannual full-body skin examination is recommended for 2 years following excision, and subsequently each year for the next 8 years.3 Melanomas thicker than 1 mm may require up to 4 annual visits during the first 2 years, followed by biannual and annual exams. Chest x-ray is recommended annually during the first 5 years for lesions <3 mm thick, and biannually for those >3 mm thick.3
The patient’s follow-up
Our patient’s lesion was very superficial. Following excisional biopsy, a wedge excision with appropriate 10-mm margins was performed by a plastic surgeon. The result of the chest x-ray was normal. The patient is scheduled for follow-up examination every 6 months for 2 years and yearly thereafter.
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: [email protected].
1. Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for malignant melanoma of the external ear. Ann Surg Oncol 2003;10:689-696.
2. Swetter SM. Malignant melanoma. EMedicine 2003. Available at www.emedicine.com/derm/topic257.htm. Accessed on May 10, 2004.
3. Zalaudek I, Ferrara G, Argenziano G, et al. Diagnosis and treatment of cutaneous melanoma: a practical guide. SKINmed 2003;2:20-31.
4. Benmeir P, Baruchin A, Weinberg A. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg 1995;23:50-53.
5. Batsakis JG. Melanomas (cutaneous and mucosal) of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore, Md, and London: Williams & Wilkins; 1980;431-447.
6. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg 1980;140:518-521.
7. Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol 1993;107:798-802.
8. Hudson DA, Krige JEJ, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg 1990;43:608-611.
9. Eedy DJ. Surgical treatment of melanoma. Br J Dermatol 2003;149:2-12.
10. Lens MB, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma. A systemic review of randomized controlled trials comparing narrow vs. wide excision. Arch Surg 2002;137:1101-1105.
11. Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg 2001;107:20-24.
12. Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg 1998;40:506-509.
1. Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for malignant melanoma of the external ear. Ann Surg Oncol 2003;10:689-696.
2. Swetter SM. Malignant melanoma. EMedicine 2003. Available at www.emedicine.com/derm/topic257.htm. Accessed on May 10, 2004.
3. Zalaudek I, Ferrara G, Argenziano G, et al. Diagnosis and treatment of cutaneous melanoma: a practical guide. SKINmed 2003;2:20-31.
4. Benmeir P, Baruchin A, Weinberg A. Rare sites of melanoma: melanoma of the external ear. J Craniomaxillofac Surg 1995;23:50-53.
5. Batsakis JG. Melanomas (cutaneous and mucosal) of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore, Md, and London: Williams & Wilkins; 1980;431-447.
6. Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear. Review of 102 cases. Am J Surg 1980;140:518-521.
7. Davidsson A, Hellquist HB, Villman K, et al. Malignant melanoma of the ear. J Laryngol Otol 1993;107:798-802.
8. Hudson DA, Krige JEJ, Strover RM, et al. Malignant melanoma of the external ear. Br J Plast Surg 1990;43:608-611.
9. Eedy DJ. Surgical treatment of melanoma. Br J Dermatol 2003;149:2-12.
10. Lens MB, Dawes M, Goodacre T, et al. Excision margins in the treatment of primary cutaneous melanoma. A systemic review of randomized controlled trials comparing narrow vs. wide excision. Arch Surg 2002;137:1101-1105.
11. Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg 2001;107:20-24.
12. Wey PD, De La Cruz C, Goydos JS, et al. Sentinel lymph node mapping in melanoma of the ear. Ann Plast Surg 1998;40:506-509.
Incidental splenic lesions
A 43-year-old Caucasian male presented to the emergency department complaining of left lower quadrant pain that had lasted 1 day. He described the pain as “a wrench twisting my insides,” and he rated it as 8 out of 10. He also complained of nausea and non-bloody, nonbilious emesis. He did not have fever, chills, or weight loss. His medical history included diverticulitis, sigmoid colectomy, and nephrolithiasis.
The physical exam revealed a heart rate of 102 beats per minute, blood pressure of 153/89 mm Hg, respiratory rate of 20, and a temperature of 99.2°F. Abdominal exam demonstrated mild left lower quadrant tenderness to palpation without rebound or guarding. The remainder of the physical exam was unremarkable. Laboratory studies demonstrated a normal blood count and differential, and a normal comprehensive metabolic profile. The urinalysis showed 60–80 red blood cells per high-power field (RBC/HPF). A computed tomography (CT) scan of his abdomen revealed a 3-mm calculus in the mid-left ureter without hydronephrosis, and a few small descending colon diverticuli. The patient was treated conservatively with hydration, analgesics, and antiemetic medications.
Radiographic findings
The following day, the radiologist called the patient’s primary care physician to report that upon rereading the abdominal CT scan there appeared to be “patchy enhancement of the spleen” (Figure 1). Follow-up abdominal ultrasound revealed multiple irregular areas of low density within the spleen. A CT-guided spleen biopsy discovered multiple noncaseating, non-necrotizing granulomas. Subsequent chest CT revealed multiple bilateral micronodules and nodules (Figure 2).
FIGURE 1
Abdominal CT scan
Chest CT scan
What are the management options?
What is the prognosis?
Diagnosis: Sarcoidosis
Sarcoidosis is an idiopathic systemic disorder identified by noncaseating granulomas in affected organs. Clinical presentation is variable and can involve virtually any organ system. Because of this, sarcoidosis is often misdiagnosed. The diagnosis of sarcoidosis requires clinical and radiographic features known to be associated with sarcoidosis, noncaseating granulomas found on tissue biopsy, and exclusion of known causes of granulomatous diseases such as tuberculosis or berylliosis.1
Sarcoidosis most commonly affects adults in their third decade of life.2 It is unusual to see the initial presentation of sarcoidosis after the age of 40 years—except in Scandinavia and Japan, where a second peak occurs among women after age 50 years.3,4,5 There seems to be a slightly higher rate of sarcoidosis in women.1 The incidence of sarcoidosis is higher in Swedes, Danes, and African Americans. The lifetime risk of sarcoidosis is 0.85% for US whites and 2.4% for US blacks.6
Possible causes of sarcoidosis
Although the cause of sarcoidosis is unknown, there is evidence of spatial, seasonal (winter and early spring), and familial clustering. Multiple theories have been proposed suggesting possible transmissible agents as the cause of sarcoidosis, including infectious agents such as Mycoplasma and Borrelia burgdorferi.
Other proposed agents include metals and inorganic substances such as clay and pine tree pollen.10 Despite extensive research, these hypotheses have not been validated. None-theless, there seems to be an emerging pattern of immunologic abnormalities in patients with sarcoidosis, suggesting an abnormal host response.
Clinical presentation of sarcoidosis
The local Th1-type T lymphocyte is now believed to be the central cell responsible for granuloma formation.10 It is hypothesized that sarcoid granulomas are formed in response to chronic antigenic stimulation of genetically susceptible Th-1 T cells and subsequent macrocyte-mediated inflammation.10,11 Certain HLA haplotypes have been implicated in the pathogenesis of sarcoidosis, yet there is no consistent data linking any one haplotype to the disease.11
The clinical presentation of sarcoidosis is related to the organ(s) involved. Specifically, symptoms may be a result of granuloma mass-effect, immune complex vasculitis, metabolically active granulomas, or fibrotic organ destruction. The lungs are the most commonly affected organ and lung involvement is seen in 88% of patients diagnosed with sarcoidosis.1
The most common presentation of intrathoracic sarcoidosis is bilateral hilar lymphadenopathy with or without diffuse pulmonary infiltration. This may present clinically as cough, wheeze, shortness of breath, or dyspnea on exertion.
Appearance on the skin
Skin manifestations of sarcoidosis are also relatively common, affecting up to one third of patients.1,7 Cutaneous sarcoidosis is divided into nonspecific and specific lesions. Nonspecific skin lesions do not contain granulomas on biopsy.
Erythema nodosum, the most common nonspecific skin lesion of sarcoidosis, is commonly seen in European, Puerto Rican, and Mexican patients.8 It presents as tender, subcutaneous erythematous nodules on the lower legs.
Lofgren’s syndrome is the combination of erythema nodosum, bilateral hilar lympha-denopathy, fever, and polyarthritis.
Specific sarcoid lesions may present as cutaneous papules, plaques, or enlarging scars.
Lupus pernio is another specific skin lesion characteristic of sarcoidosis. It presents as chronic, indurated, violaceous plaques on the nose, cheeks, lips, ears, or nasal mucosa. Lupus pernio is more common in African American women.8
Other manifestations
Ocular manifestations of sarcoidosis include uveitis or chorioretinitis and are present in 27% of patients with sarcoidosis.1
Sarcoidosis in peripheral lymph nodes, spleen, and liver is seen in 10% to 30% of patients with sarcoidosis; however, these tend to be incidental findings.1 Occasionally, hepatomegaly and splenomegaly are present.
Liver enzymes are typically normal, however a mildly elevated alkaline phosphatase is sometimes seen.1 Neurological, cardiac, and renal involvement are uncommon, affecting less than 5% of patients with sarcoidosis.1,13 Hypercalciuria with or without hypercalcemia is commonly seen secondary to overproduction of 1,25-vitamin D.8
Differential diagnosis
The differential diagnoses of solid splenic lesions can be divided into 3 categories: granulomatous disease, metastasis, and primary masses. Granulomatous diseases include tuberculosis, histoplasmosis, and sarcoid.
Metastasis is most commonly from melanoma, lymphoma, breast, or lung cancer, although prostate, colon, stomach, ovarian, and pancreatic metastasis have occurred. Primary masses include hemangioma, hemangiosarcoma, lymphangioma, and splenic infarction. Occasionally, regenerating nodules of hematopoeisis are seen in the spleen.
Testing: A Diagnosis Of Exclusion
The diagnosis of sarcoidosis is ultimately a diagnosis of exclusion. No laboratory tests can confirm the diagnosis of sarcoid. However, serum levels of angiotensin-converting enzyme are elevated in 50% to 80% of patients with sarcoidosis. Also, cutaneous anergy to common antigens (mumps, candida) is seen in 50% of patients with sarcoidosis.1,9
Once the diagnosis of sarcoidosis is made, additional clinical information is needed. In addition to a detailed history and physical, a chest x-ray, pulmonary function tests (PFT), electrocardiogram, comprehensive metabolic profile, and slit lamp evaluation should be obtained.
Some clinicians follow angiotensin-converting enzyme levels to monitor disease activity; however, treatment is ultimately based on the patient’s symptoms.
Treatment: Corticosteroids Treat The Symptoms
Corticosteroids are the cornerstone of treatment for sarcoidosis. Asymptomatic, early disease can be observed with routine follow-up. Oral corticosteroids should be prescribed for symptomatic cardiac, neurologic, and ocular sarcoidosis. Oral corticosteroids should also be prescribed for any symptomatic pulmonary disease, especially in the setting of worsening PFTs, severe hypercalcemia, and painful or disfiguring skin lesions (LOE: 2a).7,8,10
For symptomatic or worsening pulmonary sarcoidosis, patients are prescribed prednisone 20 to 40 mg daily for 3 months. If the patient responses to prednisone, it should slowly be tapered to 5 to 10 mg and continued for a period of 12 months.7,11,12
Topical steroids can be used for uveitis and skin lesions;1,8 the latter also respond well to intralesional steroids.7 Inhaled corticosteroids are now being used to treat pulmonary disease, but their efficacy is uncertain.1,8,10
Of patients being treated with corticosteroids, 25% to 40% will relapse and require further treatment.1,8 The addition of methotrexate, azathioprine, or hydroychloroquine is sometimes required in recalcitrant cases of sarcoidosis (LOE: 2b).1,8,11
Prognosis: Most Experience Spontaneous Remission
Overall, 70% of patients with sarcoidosis will experience a spontaneous remission.1,8,11 Serious extrapulmonary involvement is seen in 4% to 7% of patients at presentation and 1% to 5% of patients with sarcoidosis will die from respiratory, cardiac, or neurological sequela of sarcoidosis.1,8
Higher rates of chronic and more serious sarcoidosis are found in patients of African heritage or who are older than 40 years at the onset of disease, and in those who have lupus pernio, chronic uveitis, chronic hypercalcemia, progressive pulmonary sarcoidosis, nasal mucosal involvement, cystic bone lesions, neu-rosarcoidosis, cardiac involvement, and chronic respiratory insufficiency.8
Patient follow-up
All of the recommended tests were done for this patient. Pulmonary function testing was normal, and he never developed any respiratory symptoms. Therefore, treatment with cortico-steroids was deferred. He is now being seen every 4 to 6 months for follow-up PFTs and laboratory testing.
- Azathioprine • Imuran
- Hydroxychloroquine • Plaquenil
- Methotrexate • Folex
Corresponding author
Rade Nicholas Pejic, MD, Santa Monica/UCLA Medical Center Family Medicine Residency, 1920 Colorado Avenue, Santa Monica, CA 90404. E-mail: [email protected]. Submissions: Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58:2041-2058.
2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003;289:3300-3303.
3. Alsbirk PH. Epidemiologic studies of sarcoidosis in Denmark based on a nation wide central register. A preliminary report. Acta Med Scand Suppl 1964;176:106-109.
4. Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994;11:26-31.
5. Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
6. Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 997;145:234-241.
7. Katta R. Cutaneous sarcoidosis: A dermatologic masquer-ader. Am Fam Physician 2002;65:1581-1584.
8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-755.
9. Chesnutt AN. Enigmas in sarcoidosis. West J Med 1995;162:519-526.
10. Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002;287:1301-1307.
11. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224-1234.
12. Baughman RP, Lower EE, Lynch JP. Treatment modalities for sarcoidosis. Clin Pulmonol Med 1994;1:223-231.
A 43-year-old Caucasian male presented to the emergency department complaining of left lower quadrant pain that had lasted 1 day. He described the pain as “a wrench twisting my insides,” and he rated it as 8 out of 10. He also complained of nausea and non-bloody, nonbilious emesis. He did not have fever, chills, or weight loss. His medical history included diverticulitis, sigmoid colectomy, and nephrolithiasis.
The physical exam revealed a heart rate of 102 beats per minute, blood pressure of 153/89 mm Hg, respiratory rate of 20, and a temperature of 99.2°F. Abdominal exam demonstrated mild left lower quadrant tenderness to palpation without rebound or guarding. The remainder of the physical exam was unremarkable. Laboratory studies demonstrated a normal blood count and differential, and a normal comprehensive metabolic profile. The urinalysis showed 60–80 red blood cells per high-power field (RBC/HPF). A computed tomography (CT) scan of his abdomen revealed a 3-mm calculus in the mid-left ureter without hydronephrosis, and a few small descending colon diverticuli. The patient was treated conservatively with hydration, analgesics, and antiemetic medications.
Radiographic findings
The following day, the radiologist called the patient’s primary care physician to report that upon rereading the abdominal CT scan there appeared to be “patchy enhancement of the spleen” (Figure 1). Follow-up abdominal ultrasound revealed multiple irregular areas of low density within the spleen. A CT-guided spleen biopsy discovered multiple noncaseating, non-necrotizing granulomas. Subsequent chest CT revealed multiple bilateral micronodules and nodules (Figure 2).
FIGURE 1
Abdominal CT scan
Chest CT scan
What are the management options?
What is the prognosis?
Diagnosis: Sarcoidosis
Sarcoidosis is an idiopathic systemic disorder identified by noncaseating granulomas in affected organs. Clinical presentation is variable and can involve virtually any organ system. Because of this, sarcoidosis is often misdiagnosed. The diagnosis of sarcoidosis requires clinical and radiographic features known to be associated with sarcoidosis, noncaseating granulomas found on tissue biopsy, and exclusion of known causes of granulomatous diseases such as tuberculosis or berylliosis.1
Sarcoidosis most commonly affects adults in their third decade of life.2 It is unusual to see the initial presentation of sarcoidosis after the age of 40 years—except in Scandinavia and Japan, where a second peak occurs among women after age 50 years.3,4,5 There seems to be a slightly higher rate of sarcoidosis in women.1 The incidence of sarcoidosis is higher in Swedes, Danes, and African Americans. The lifetime risk of sarcoidosis is 0.85% for US whites and 2.4% for US blacks.6
Possible causes of sarcoidosis
Although the cause of sarcoidosis is unknown, there is evidence of spatial, seasonal (winter and early spring), and familial clustering. Multiple theories have been proposed suggesting possible transmissible agents as the cause of sarcoidosis, including infectious agents such as Mycoplasma and Borrelia burgdorferi.
Other proposed agents include metals and inorganic substances such as clay and pine tree pollen.10 Despite extensive research, these hypotheses have not been validated. None-theless, there seems to be an emerging pattern of immunologic abnormalities in patients with sarcoidosis, suggesting an abnormal host response.
Clinical presentation of sarcoidosis
The local Th1-type T lymphocyte is now believed to be the central cell responsible for granuloma formation.10 It is hypothesized that sarcoid granulomas are formed in response to chronic antigenic stimulation of genetically susceptible Th-1 T cells and subsequent macrocyte-mediated inflammation.10,11 Certain HLA haplotypes have been implicated in the pathogenesis of sarcoidosis, yet there is no consistent data linking any one haplotype to the disease.11
The clinical presentation of sarcoidosis is related to the organ(s) involved. Specifically, symptoms may be a result of granuloma mass-effect, immune complex vasculitis, metabolically active granulomas, or fibrotic organ destruction. The lungs are the most commonly affected organ and lung involvement is seen in 88% of patients diagnosed with sarcoidosis.1
The most common presentation of intrathoracic sarcoidosis is bilateral hilar lymphadenopathy with or without diffuse pulmonary infiltration. This may present clinically as cough, wheeze, shortness of breath, or dyspnea on exertion.
Appearance on the skin
Skin manifestations of sarcoidosis are also relatively common, affecting up to one third of patients.1,7 Cutaneous sarcoidosis is divided into nonspecific and specific lesions. Nonspecific skin lesions do not contain granulomas on biopsy.
Erythema nodosum, the most common nonspecific skin lesion of sarcoidosis, is commonly seen in European, Puerto Rican, and Mexican patients.8 It presents as tender, subcutaneous erythematous nodules on the lower legs.
Lofgren’s syndrome is the combination of erythema nodosum, bilateral hilar lympha-denopathy, fever, and polyarthritis.
Specific sarcoid lesions may present as cutaneous papules, plaques, or enlarging scars.
Lupus pernio is another specific skin lesion characteristic of sarcoidosis. It presents as chronic, indurated, violaceous plaques on the nose, cheeks, lips, ears, or nasal mucosa. Lupus pernio is more common in African American women.8
Other manifestations
Ocular manifestations of sarcoidosis include uveitis or chorioretinitis and are present in 27% of patients with sarcoidosis.1
Sarcoidosis in peripheral lymph nodes, spleen, and liver is seen in 10% to 30% of patients with sarcoidosis; however, these tend to be incidental findings.1 Occasionally, hepatomegaly and splenomegaly are present.
Liver enzymes are typically normal, however a mildly elevated alkaline phosphatase is sometimes seen.1 Neurological, cardiac, and renal involvement are uncommon, affecting less than 5% of patients with sarcoidosis.1,13 Hypercalciuria with or without hypercalcemia is commonly seen secondary to overproduction of 1,25-vitamin D.8
Differential diagnosis
The differential diagnoses of solid splenic lesions can be divided into 3 categories: granulomatous disease, metastasis, and primary masses. Granulomatous diseases include tuberculosis, histoplasmosis, and sarcoid.
Metastasis is most commonly from melanoma, lymphoma, breast, or lung cancer, although prostate, colon, stomach, ovarian, and pancreatic metastasis have occurred. Primary masses include hemangioma, hemangiosarcoma, lymphangioma, and splenic infarction. Occasionally, regenerating nodules of hematopoeisis are seen in the spleen.
Testing: A Diagnosis Of Exclusion
The diagnosis of sarcoidosis is ultimately a diagnosis of exclusion. No laboratory tests can confirm the diagnosis of sarcoid. However, serum levels of angiotensin-converting enzyme are elevated in 50% to 80% of patients with sarcoidosis. Also, cutaneous anergy to common antigens (mumps, candida) is seen in 50% of patients with sarcoidosis.1,9
Once the diagnosis of sarcoidosis is made, additional clinical information is needed. In addition to a detailed history and physical, a chest x-ray, pulmonary function tests (PFT), electrocardiogram, comprehensive metabolic profile, and slit lamp evaluation should be obtained.
Some clinicians follow angiotensin-converting enzyme levels to monitor disease activity; however, treatment is ultimately based on the patient’s symptoms.
Treatment: Corticosteroids Treat The Symptoms
Corticosteroids are the cornerstone of treatment for sarcoidosis. Asymptomatic, early disease can be observed with routine follow-up. Oral corticosteroids should be prescribed for symptomatic cardiac, neurologic, and ocular sarcoidosis. Oral corticosteroids should also be prescribed for any symptomatic pulmonary disease, especially in the setting of worsening PFTs, severe hypercalcemia, and painful or disfiguring skin lesions (LOE: 2a).7,8,10
For symptomatic or worsening pulmonary sarcoidosis, patients are prescribed prednisone 20 to 40 mg daily for 3 months. If the patient responses to prednisone, it should slowly be tapered to 5 to 10 mg and continued for a period of 12 months.7,11,12
Topical steroids can be used for uveitis and skin lesions;1,8 the latter also respond well to intralesional steroids.7 Inhaled corticosteroids are now being used to treat pulmonary disease, but their efficacy is uncertain.1,8,10
Of patients being treated with corticosteroids, 25% to 40% will relapse and require further treatment.1,8 The addition of methotrexate, azathioprine, or hydroychloroquine is sometimes required in recalcitrant cases of sarcoidosis (LOE: 2b).1,8,11
Prognosis: Most Experience Spontaneous Remission
Overall, 70% of patients with sarcoidosis will experience a spontaneous remission.1,8,11 Serious extrapulmonary involvement is seen in 4% to 7% of patients at presentation and 1% to 5% of patients with sarcoidosis will die from respiratory, cardiac, or neurological sequela of sarcoidosis.1,8
Higher rates of chronic and more serious sarcoidosis are found in patients of African heritage or who are older than 40 years at the onset of disease, and in those who have lupus pernio, chronic uveitis, chronic hypercalcemia, progressive pulmonary sarcoidosis, nasal mucosal involvement, cystic bone lesions, neu-rosarcoidosis, cardiac involvement, and chronic respiratory insufficiency.8
Patient follow-up
All of the recommended tests were done for this patient. Pulmonary function testing was normal, and he never developed any respiratory symptoms. Therefore, treatment with cortico-steroids was deferred. He is now being seen every 4 to 6 months for follow-up PFTs and laboratory testing.
- Azathioprine • Imuran
- Hydroxychloroquine • Plaquenil
- Methotrexate • Folex
Corresponding author
Rade Nicholas Pejic, MD, Santa Monica/UCLA Medical Center Family Medicine Residency, 1920 Colorado Avenue, Santa Monica, CA 90404. E-mail: [email protected]. Submissions: Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
A 43-year-old Caucasian male presented to the emergency department complaining of left lower quadrant pain that had lasted 1 day. He described the pain as “a wrench twisting my insides,” and he rated it as 8 out of 10. He also complained of nausea and non-bloody, nonbilious emesis. He did not have fever, chills, or weight loss. His medical history included diverticulitis, sigmoid colectomy, and nephrolithiasis.
The physical exam revealed a heart rate of 102 beats per minute, blood pressure of 153/89 mm Hg, respiratory rate of 20, and a temperature of 99.2°F. Abdominal exam demonstrated mild left lower quadrant tenderness to palpation without rebound or guarding. The remainder of the physical exam was unremarkable. Laboratory studies demonstrated a normal blood count and differential, and a normal comprehensive metabolic profile. The urinalysis showed 60–80 red blood cells per high-power field (RBC/HPF). A computed tomography (CT) scan of his abdomen revealed a 3-mm calculus in the mid-left ureter without hydronephrosis, and a few small descending colon diverticuli. The patient was treated conservatively with hydration, analgesics, and antiemetic medications.
Radiographic findings
The following day, the radiologist called the patient’s primary care physician to report that upon rereading the abdominal CT scan there appeared to be “patchy enhancement of the spleen” (Figure 1). Follow-up abdominal ultrasound revealed multiple irregular areas of low density within the spleen. A CT-guided spleen biopsy discovered multiple noncaseating, non-necrotizing granulomas. Subsequent chest CT revealed multiple bilateral micronodules and nodules (Figure 2).
FIGURE 1
Abdominal CT scan
Chest CT scan
What are the management options?
What is the prognosis?
Diagnosis: Sarcoidosis
Sarcoidosis is an idiopathic systemic disorder identified by noncaseating granulomas in affected organs. Clinical presentation is variable and can involve virtually any organ system. Because of this, sarcoidosis is often misdiagnosed. The diagnosis of sarcoidosis requires clinical and radiographic features known to be associated with sarcoidosis, noncaseating granulomas found on tissue biopsy, and exclusion of known causes of granulomatous diseases such as tuberculosis or berylliosis.1
Sarcoidosis most commonly affects adults in their third decade of life.2 It is unusual to see the initial presentation of sarcoidosis after the age of 40 years—except in Scandinavia and Japan, where a second peak occurs among women after age 50 years.3,4,5 There seems to be a slightly higher rate of sarcoidosis in women.1 The incidence of sarcoidosis is higher in Swedes, Danes, and African Americans. The lifetime risk of sarcoidosis is 0.85% for US whites and 2.4% for US blacks.6
Possible causes of sarcoidosis
Although the cause of sarcoidosis is unknown, there is evidence of spatial, seasonal (winter and early spring), and familial clustering. Multiple theories have been proposed suggesting possible transmissible agents as the cause of sarcoidosis, including infectious agents such as Mycoplasma and Borrelia burgdorferi.
Other proposed agents include metals and inorganic substances such as clay and pine tree pollen.10 Despite extensive research, these hypotheses have not been validated. None-theless, there seems to be an emerging pattern of immunologic abnormalities in patients with sarcoidosis, suggesting an abnormal host response.
Clinical presentation of sarcoidosis
The local Th1-type T lymphocyte is now believed to be the central cell responsible for granuloma formation.10 It is hypothesized that sarcoid granulomas are formed in response to chronic antigenic stimulation of genetically susceptible Th-1 T cells and subsequent macrocyte-mediated inflammation.10,11 Certain HLA haplotypes have been implicated in the pathogenesis of sarcoidosis, yet there is no consistent data linking any one haplotype to the disease.11
The clinical presentation of sarcoidosis is related to the organ(s) involved. Specifically, symptoms may be a result of granuloma mass-effect, immune complex vasculitis, metabolically active granulomas, or fibrotic organ destruction. The lungs are the most commonly affected organ and lung involvement is seen in 88% of patients diagnosed with sarcoidosis.1
The most common presentation of intrathoracic sarcoidosis is bilateral hilar lymphadenopathy with or without diffuse pulmonary infiltration. This may present clinically as cough, wheeze, shortness of breath, or dyspnea on exertion.
Appearance on the skin
Skin manifestations of sarcoidosis are also relatively common, affecting up to one third of patients.1,7 Cutaneous sarcoidosis is divided into nonspecific and specific lesions. Nonspecific skin lesions do not contain granulomas on biopsy.
Erythema nodosum, the most common nonspecific skin lesion of sarcoidosis, is commonly seen in European, Puerto Rican, and Mexican patients.8 It presents as tender, subcutaneous erythematous nodules on the lower legs.
Lofgren’s syndrome is the combination of erythema nodosum, bilateral hilar lympha-denopathy, fever, and polyarthritis.
Specific sarcoid lesions may present as cutaneous papules, plaques, or enlarging scars.
Lupus pernio is another specific skin lesion characteristic of sarcoidosis. It presents as chronic, indurated, violaceous plaques on the nose, cheeks, lips, ears, or nasal mucosa. Lupus pernio is more common in African American women.8
Other manifestations
Ocular manifestations of sarcoidosis include uveitis or chorioretinitis and are present in 27% of patients with sarcoidosis.1
Sarcoidosis in peripheral lymph nodes, spleen, and liver is seen in 10% to 30% of patients with sarcoidosis; however, these tend to be incidental findings.1 Occasionally, hepatomegaly and splenomegaly are present.
Liver enzymes are typically normal, however a mildly elevated alkaline phosphatase is sometimes seen.1 Neurological, cardiac, and renal involvement are uncommon, affecting less than 5% of patients with sarcoidosis.1,13 Hypercalciuria with or without hypercalcemia is commonly seen secondary to overproduction of 1,25-vitamin D.8
Differential diagnosis
The differential diagnoses of solid splenic lesions can be divided into 3 categories: granulomatous disease, metastasis, and primary masses. Granulomatous diseases include tuberculosis, histoplasmosis, and sarcoid.
Metastasis is most commonly from melanoma, lymphoma, breast, or lung cancer, although prostate, colon, stomach, ovarian, and pancreatic metastasis have occurred. Primary masses include hemangioma, hemangiosarcoma, lymphangioma, and splenic infarction. Occasionally, regenerating nodules of hematopoeisis are seen in the spleen.
Testing: A Diagnosis Of Exclusion
The diagnosis of sarcoidosis is ultimately a diagnosis of exclusion. No laboratory tests can confirm the diagnosis of sarcoid. However, serum levels of angiotensin-converting enzyme are elevated in 50% to 80% of patients with sarcoidosis. Also, cutaneous anergy to common antigens (mumps, candida) is seen in 50% of patients with sarcoidosis.1,9
Once the diagnosis of sarcoidosis is made, additional clinical information is needed. In addition to a detailed history and physical, a chest x-ray, pulmonary function tests (PFT), electrocardiogram, comprehensive metabolic profile, and slit lamp evaluation should be obtained.
Some clinicians follow angiotensin-converting enzyme levels to monitor disease activity; however, treatment is ultimately based on the patient’s symptoms.
Treatment: Corticosteroids Treat The Symptoms
Corticosteroids are the cornerstone of treatment for sarcoidosis. Asymptomatic, early disease can be observed with routine follow-up. Oral corticosteroids should be prescribed for symptomatic cardiac, neurologic, and ocular sarcoidosis. Oral corticosteroids should also be prescribed for any symptomatic pulmonary disease, especially in the setting of worsening PFTs, severe hypercalcemia, and painful or disfiguring skin lesions (LOE: 2a).7,8,10
For symptomatic or worsening pulmonary sarcoidosis, patients are prescribed prednisone 20 to 40 mg daily for 3 months. If the patient responses to prednisone, it should slowly be tapered to 5 to 10 mg and continued for a period of 12 months.7,11,12
Topical steroids can be used for uveitis and skin lesions;1,8 the latter also respond well to intralesional steroids.7 Inhaled corticosteroids are now being used to treat pulmonary disease, but their efficacy is uncertain.1,8,10
Of patients being treated with corticosteroids, 25% to 40% will relapse and require further treatment.1,8 The addition of methotrexate, azathioprine, or hydroychloroquine is sometimes required in recalcitrant cases of sarcoidosis (LOE: 2b).1,8,11
Prognosis: Most Experience Spontaneous Remission
Overall, 70% of patients with sarcoidosis will experience a spontaneous remission.1,8,11 Serious extrapulmonary involvement is seen in 4% to 7% of patients at presentation and 1% to 5% of patients with sarcoidosis will die from respiratory, cardiac, or neurological sequela of sarcoidosis.1,8
Higher rates of chronic and more serious sarcoidosis are found in patients of African heritage or who are older than 40 years at the onset of disease, and in those who have lupus pernio, chronic uveitis, chronic hypercalcemia, progressive pulmonary sarcoidosis, nasal mucosal involvement, cystic bone lesions, neu-rosarcoidosis, cardiac involvement, and chronic respiratory insufficiency.8
Patient follow-up
All of the recommended tests were done for this patient. Pulmonary function testing was normal, and he never developed any respiratory symptoms. Therefore, treatment with cortico-steroids was deferred. He is now being seen every 4 to 6 months for follow-up PFTs and laboratory testing.
- Azathioprine • Imuran
- Hydroxychloroquine • Plaquenil
- Methotrexate • Folex
Corresponding author
Rade Nicholas Pejic, MD, Santa Monica/UCLA Medical Center Family Medicine Residency, 1920 Colorado Avenue, Santa Monica, CA 90404. E-mail: [email protected]. Submissions: Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58:2041-2058.
2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003;289:3300-3303.
3. Alsbirk PH. Epidemiologic studies of sarcoidosis in Denmark based on a nation wide central register. A preliminary report. Acta Med Scand Suppl 1964;176:106-109.
4. Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994;11:26-31.
5. Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
6. Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 997;145:234-241.
7. Katta R. Cutaneous sarcoidosis: A dermatologic masquer-ader. Am Fam Physician 2002;65:1581-1584.
8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-755.
9. Chesnutt AN. Enigmas in sarcoidosis. West J Med 1995;162:519-526.
10. Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002;287:1301-1307.
11. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224-1234.
12. Baughman RP, Lower EE, Lynch JP. Treatment modalities for sarcoidosis. Clin Pulmonol Med 1994;1:223-231.
1. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58:2041-2058.
2. Thomas KW, Hunninghake GW. Sarcoidosis. JAMA 2003;289:3300-3303.
3. Alsbirk PH. Epidemiologic studies of sarcoidosis in Denmark based on a nation wide central register. A preliminary report. Acta Med Scand Suppl 1964;176:106-109.
4. Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994;11:26-31.
5. Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982. Epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
6. Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 997;145:234-241.
7. Katta R. Cutaneous sarcoidosis: A dermatologic masquer-ader. Am Fam Physician 2002;65:1581-1584.
8. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-755.
9. Chesnutt AN. Enigmas in sarcoidosis. West J Med 1995;162:519-526.
10. Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002;287:1301-1307.
11. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224-1234.
12. Baughman RP, Lower EE, Lynch JP. Treatment modalities for sarcoidosis. Clin Pulmonol Med 1994;1:223-231.
Chronic papules on the back and extremities
A 45-year-old Caucasian woman presented with pruritic erythematous scaling eruptions on her back and lower extremities. The patient said these skin lesions, which she has had since childhood, become worse in the summer. Additionally, her nails were fragile and often cracked. Her father and son had similar skin lesions.
On skin examination, we saw erythematous hyperkeratotic confluent papules and plaques on her back, chest, thighs, and lower legs. Multiple 3- to 5-mm keratotic papules were noted on the dorsa of her hands bilaterally. Fingernail examination revealed longitudinal lines with notching at the distal edges (Figures 1 and 2). Examination of other systems was unremarkable.
FIGURE 1
Papules on the back...
FIGURE 2
...and the thighs
What is your diagnosis?
Are any diagnostic tests Necessary to confirm it?
Diagnosis: darier’s disease
Darier’s disease, also called Darier-White disease or keratosis follicularis, is a genodermatosis that affects 1 in 55,000 to 100,000 persons.1 It is inherited in an autosomal dominant fashion, resulting from a mutation in chromosome 12q23-q24.1, which encodes the gene ATP 2A2, which in turn encodes a SERCA2 (sarco/endoplasmic reticulum calcium)-ATPase pump. This defect results in instability of desmosomes.2-4
With Darier’s disease, skin lesions are often present in the second decade of life but rarely appear in adulthood. The clinical findings are described as yellowish, greasy hyperkeratotic papules coalescing to warty plaques in a seborrheic distribution on the face, scalp, flexures, and groin. The nails can have red and white alternating longitudinal bands, as well as a V-shaped nicking at the distal nail plate, with resultant splitting and subungual hyperkeratosis. Oral and anogenital mucosa can have a cobblestone appearance. Some families have had associated cases of schizophrenia and mental retardation.1,5
The histologic findings on skin biopsies of Darier’s disease show acantholysis (loss of epidermal adhesion) and dyskeratosis (abnormal keratinization) as the 2 main features.
Laboratory tests: biopsy confirms diagnosis
Biopsy of a characteristic papule from the patient’s back revealed diffuse acantholytic dyskeratosis, which confirmed the clinical diagnosis of Darier’s disease.
Two types of dyskeratotic cells are present: corps ronds and grains. Corps ronds, found in the stratum spinosum, are characterized by an irregular eccentric pyknotic nucleus, a clear perinuclear halo, and a brightly eosinophilic cytoplasm. Grains are mostly located in the stratum corneum; they consist of oval cells with elongated cigar-shaped nuclei. The patient declined genetic testing.
Differential diagnosis
For hyperkeratotic plaques on the back and extremities, several diagnoses should be considered.
Psoriasis is a papulosquamous hyperproliferative disorder, with underlying autoimmune mechanisms.
Seborrheic dermatitis is another papulosquamous disorder involving the sebum-rich areas of the scalp, face, and trunk. In addition to sebum, is is linked to Pityrosporum ovale, immunologic abnormalities, and activation of complement.
Transient acantholytic dermatosis, or Grover’s disease, is a benign, self-limited disorder; however, it may be persistent and difficult to manage. The process usually begins as an eruption on the anterior part of the chest, the upper part of the back, and the lower part of the chest.
Familial benign pemphigus is a chronic autosomal dominant disorder with incomplete penetrance, which manifests clinically as vesicles and erythematous plaques with overlying crusts, which typically occur in the genital area, as well as the chest, neck, and axillary areas.
Management: retinoids, laser surgery
Therapeutic options are palliative. A multidisciplinary approach is often necessary; family physicians as well as dermatologists, and occasionally plastic surgeons, have important roles in managing this chronic condition.
Treatment options for Darier’s disease range from topical to systemic medications and laser therapies. Successful topical therapies include corticosteroids, retinoids, urea, salicylic acid, 5-fluorouracil, and topical antibiotics.5,6
The most successful treatment option is systemic use of retinoids, most often acitretin (Soriatane).1,5 This medication, like isotretinoin (Accutane), requires meticulous monitoring and counseling. Women of childbearing age should be instructed to use 2 birth-control methods and, according to current recommendations, instructed not to conceive for 3 years following cessation of treatment (2 years in Europe). Cyclosporine may be used to control acute, severe flares.
Laser excision, excision and grafting, and dermabrasion have been used to treat hypertrophic lesions.1,5 More recently, photodynamic therapy (using photosensitizers in conjunction with a source of visible light) was also used in the treatment of Darier’s disease.7
Keep in mind that patients with Darier’s disease are more susceptible to cutaneous infections with bacteria, herpes simplex virus, and poxvirus. Awareness of this susceptibility can facilitate early diagnosis and treatment.8
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: [email protected].
Submissions
Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In Freedberg IM, Eisen AZ, Wolff K, Austen KF, et al, eds: Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999;614-618.
2. Jacobsen NJ, Lyons I, Hoogendoorn B, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet 1999;8:1631-1636.
3. Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca+2 pump, cause Darier disease. Nat Genet 1999;21:271-277.
4. Ahn W, Lee MG, Kim KH, Muallem S. Multiple effects of SERCA2b mutations associated with Darier’s disease. J Biol Chem 2003;278:20795-20801.
5. Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol 2003;4:97-105.
6. Knulst AC, De La Faille HB, Van Vloten WA. Topical 5-fluorouracil in the treatment of Darier’s disease. Br J Dermatol 1995;133:463-466.
7. Exadaktylou D, Kurwa HA, Calonje E, Barlow RJ. Treatment of Darier’s disease with photodynamic therapy. Br J Dermatol 2003;149:606-610.
8. Parslew R, Verbov JL. Kaposi’s varicelliform eruption due to herpes simplex in Darier’s disease. Clin Exp Dermatol 1994;19:428-429.
A 45-year-old Caucasian woman presented with pruritic erythematous scaling eruptions on her back and lower extremities. The patient said these skin lesions, which she has had since childhood, become worse in the summer. Additionally, her nails were fragile and often cracked. Her father and son had similar skin lesions.
On skin examination, we saw erythematous hyperkeratotic confluent papules and plaques on her back, chest, thighs, and lower legs. Multiple 3- to 5-mm keratotic papules were noted on the dorsa of her hands bilaterally. Fingernail examination revealed longitudinal lines with notching at the distal edges (Figures 1 and 2). Examination of other systems was unremarkable.
FIGURE 1
Papules on the back...
FIGURE 2
...and the thighs
What is your diagnosis?
Are any diagnostic tests Necessary to confirm it?
Diagnosis: darier’s disease
Darier’s disease, also called Darier-White disease or keratosis follicularis, is a genodermatosis that affects 1 in 55,000 to 100,000 persons.1 It is inherited in an autosomal dominant fashion, resulting from a mutation in chromosome 12q23-q24.1, which encodes the gene ATP 2A2, which in turn encodes a SERCA2 (sarco/endoplasmic reticulum calcium)-ATPase pump. This defect results in instability of desmosomes.2-4
With Darier’s disease, skin lesions are often present in the second decade of life but rarely appear in adulthood. The clinical findings are described as yellowish, greasy hyperkeratotic papules coalescing to warty plaques in a seborrheic distribution on the face, scalp, flexures, and groin. The nails can have red and white alternating longitudinal bands, as well as a V-shaped nicking at the distal nail plate, with resultant splitting and subungual hyperkeratosis. Oral and anogenital mucosa can have a cobblestone appearance. Some families have had associated cases of schizophrenia and mental retardation.1,5
The histologic findings on skin biopsies of Darier’s disease show acantholysis (loss of epidermal adhesion) and dyskeratosis (abnormal keratinization) as the 2 main features.
Laboratory tests: biopsy confirms diagnosis
Biopsy of a characteristic papule from the patient’s back revealed diffuse acantholytic dyskeratosis, which confirmed the clinical diagnosis of Darier’s disease.
Two types of dyskeratotic cells are present: corps ronds and grains. Corps ronds, found in the stratum spinosum, are characterized by an irregular eccentric pyknotic nucleus, a clear perinuclear halo, and a brightly eosinophilic cytoplasm. Grains are mostly located in the stratum corneum; they consist of oval cells with elongated cigar-shaped nuclei. The patient declined genetic testing.
Differential diagnosis
For hyperkeratotic plaques on the back and extremities, several diagnoses should be considered.
Psoriasis is a papulosquamous hyperproliferative disorder, with underlying autoimmune mechanisms.
Seborrheic dermatitis is another papulosquamous disorder involving the sebum-rich areas of the scalp, face, and trunk. In addition to sebum, is is linked to Pityrosporum ovale, immunologic abnormalities, and activation of complement.
Transient acantholytic dermatosis, or Grover’s disease, is a benign, self-limited disorder; however, it may be persistent and difficult to manage. The process usually begins as an eruption on the anterior part of the chest, the upper part of the back, and the lower part of the chest.
Familial benign pemphigus is a chronic autosomal dominant disorder with incomplete penetrance, which manifests clinically as vesicles and erythematous plaques with overlying crusts, which typically occur in the genital area, as well as the chest, neck, and axillary areas.
Management: retinoids, laser surgery
Therapeutic options are palliative. A multidisciplinary approach is often necessary; family physicians as well as dermatologists, and occasionally plastic surgeons, have important roles in managing this chronic condition.
Treatment options for Darier’s disease range from topical to systemic medications and laser therapies. Successful topical therapies include corticosteroids, retinoids, urea, salicylic acid, 5-fluorouracil, and topical antibiotics.5,6
The most successful treatment option is systemic use of retinoids, most often acitretin (Soriatane).1,5 This medication, like isotretinoin (Accutane), requires meticulous monitoring and counseling. Women of childbearing age should be instructed to use 2 birth-control methods and, according to current recommendations, instructed not to conceive for 3 years following cessation of treatment (2 years in Europe). Cyclosporine may be used to control acute, severe flares.
Laser excision, excision and grafting, and dermabrasion have been used to treat hypertrophic lesions.1,5 More recently, photodynamic therapy (using photosensitizers in conjunction with a source of visible light) was also used in the treatment of Darier’s disease.7
Keep in mind that patients with Darier’s disease are more susceptible to cutaneous infections with bacteria, herpes simplex virus, and poxvirus. Awareness of this susceptibility can facilitate early diagnosis and treatment.8
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: [email protected].
Submissions
Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
A 45-year-old Caucasian woman presented with pruritic erythematous scaling eruptions on her back and lower extremities. The patient said these skin lesions, which she has had since childhood, become worse in the summer. Additionally, her nails were fragile and often cracked. Her father and son had similar skin lesions.
On skin examination, we saw erythematous hyperkeratotic confluent papules and plaques on her back, chest, thighs, and lower legs. Multiple 3- to 5-mm keratotic papules were noted on the dorsa of her hands bilaterally. Fingernail examination revealed longitudinal lines with notching at the distal edges (Figures 1 and 2). Examination of other systems was unremarkable.
FIGURE 1
Papules on the back...
FIGURE 2
...and the thighs
What is your diagnosis?
Are any diagnostic tests Necessary to confirm it?
Diagnosis: darier’s disease
Darier’s disease, also called Darier-White disease or keratosis follicularis, is a genodermatosis that affects 1 in 55,000 to 100,000 persons.1 It is inherited in an autosomal dominant fashion, resulting from a mutation in chromosome 12q23-q24.1, which encodes the gene ATP 2A2, which in turn encodes a SERCA2 (sarco/endoplasmic reticulum calcium)-ATPase pump. This defect results in instability of desmosomes.2-4
With Darier’s disease, skin lesions are often present in the second decade of life but rarely appear in adulthood. The clinical findings are described as yellowish, greasy hyperkeratotic papules coalescing to warty plaques in a seborrheic distribution on the face, scalp, flexures, and groin. The nails can have red and white alternating longitudinal bands, as well as a V-shaped nicking at the distal nail plate, with resultant splitting and subungual hyperkeratosis. Oral and anogenital mucosa can have a cobblestone appearance. Some families have had associated cases of schizophrenia and mental retardation.1,5
The histologic findings on skin biopsies of Darier’s disease show acantholysis (loss of epidermal adhesion) and dyskeratosis (abnormal keratinization) as the 2 main features.
Laboratory tests: biopsy confirms diagnosis
Biopsy of a characteristic papule from the patient’s back revealed diffuse acantholytic dyskeratosis, which confirmed the clinical diagnosis of Darier’s disease.
Two types of dyskeratotic cells are present: corps ronds and grains. Corps ronds, found in the stratum spinosum, are characterized by an irregular eccentric pyknotic nucleus, a clear perinuclear halo, and a brightly eosinophilic cytoplasm. Grains are mostly located in the stratum corneum; they consist of oval cells with elongated cigar-shaped nuclei. The patient declined genetic testing.
Differential diagnosis
For hyperkeratotic plaques on the back and extremities, several diagnoses should be considered.
Psoriasis is a papulosquamous hyperproliferative disorder, with underlying autoimmune mechanisms.
Seborrheic dermatitis is another papulosquamous disorder involving the sebum-rich areas of the scalp, face, and trunk. In addition to sebum, is is linked to Pityrosporum ovale, immunologic abnormalities, and activation of complement.
Transient acantholytic dermatosis, or Grover’s disease, is a benign, self-limited disorder; however, it may be persistent and difficult to manage. The process usually begins as an eruption on the anterior part of the chest, the upper part of the back, and the lower part of the chest.
Familial benign pemphigus is a chronic autosomal dominant disorder with incomplete penetrance, which manifests clinically as vesicles and erythematous plaques with overlying crusts, which typically occur in the genital area, as well as the chest, neck, and axillary areas.
Management: retinoids, laser surgery
Therapeutic options are palliative. A multidisciplinary approach is often necessary; family physicians as well as dermatologists, and occasionally plastic surgeons, have important roles in managing this chronic condition.
Treatment options for Darier’s disease range from topical to systemic medications and laser therapies. Successful topical therapies include corticosteroids, retinoids, urea, salicylic acid, 5-fluorouracil, and topical antibiotics.5,6
The most successful treatment option is systemic use of retinoids, most often acitretin (Soriatane).1,5 This medication, like isotretinoin (Accutane), requires meticulous monitoring and counseling. Women of childbearing age should be instructed to use 2 birth-control methods and, according to current recommendations, instructed not to conceive for 3 years following cessation of treatment (2 years in Europe). Cyclosporine may be used to control acute, severe flares.
Laser excision, excision and grafting, and dermabrasion have been used to treat hypertrophic lesions.1,5 More recently, photodynamic therapy (using photosensitizers in conjunction with a source of visible light) was also used in the treatment of Darier’s disease.7
Keep in mind that patients with Darier’s disease are more susceptible to cutaneous infections with bacteria, herpes simplex virus, and poxvirus. Awareness of this susceptibility can facilitate early diagnosis and treatment.8
Corresponding author
Amor Khachemoune, MD, CWS, Georgetown University Medical Center, Division of Dermatology, 3800 Reservoir Road, NW, 5PHC, Washington, DC 20007. E-mail: [email protected].
Submissions
Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In Freedberg IM, Eisen AZ, Wolff K, Austen KF, et al, eds: Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999;614-618.
2. Jacobsen NJ, Lyons I, Hoogendoorn B, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet 1999;8:1631-1636.
3. Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca+2 pump, cause Darier disease. Nat Genet 1999;21:271-277.
4. Ahn W, Lee MG, Kim KH, Muallem S. Multiple effects of SERCA2b mutations associated with Darier’s disease. J Biol Chem 2003;278:20795-20801.
5. Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol 2003;4:97-105.
6. Knulst AC, De La Faille HB, Van Vloten WA. Topical 5-fluorouracil in the treatment of Darier’s disease. Br J Dermatol 1995;133:463-466.
7. Exadaktylou D, Kurwa HA, Calonje E, Barlow RJ. Treatment of Darier’s disease with photodynamic therapy. Br J Dermatol 2003;149:606-610.
8. Parslew R, Verbov JL. Kaposi’s varicelliform eruption due to herpes simplex in Darier’s disease. Clin Exp Dermatol 1994;19:428-429.
1. Goldsmith LA, Baden HP. Darier-White disease (keratosis follicularis) and acrokeratosis verruciformis. In Freedberg IM, Eisen AZ, Wolff K, Austen KF, et al, eds: Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999;614-618.
2. Jacobsen NJ, Lyons I, Hoogendoorn B, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet 1999;8:1631-1636.
3. Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca+2 pump, cause Darier disease. Nat Genet 1999;21:271-277.
4. Ahn W, Lee MG, Kim KH, Muallem S. Multiple effects of SERCA2b mutations associated with Darier’s disease. J Biol Chem 2003;278:20795-20801.
5. Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol 2003;4:97-105.
6. Knulst AC, De La Faille HB, Van Vloten WA. Topical 5-fluorouracil in the treatment of Darier’s disease. Br J Dermatol 1995;133:463-466.
7. Exadaktylou D, Kurwa HA, Calonje E, Barlow RJ. Treatment of Darier’s disease with photodynamic therapy. Br J Dermatol 2003;149:606-610.
8. Parslew R, Verbov JL. Kaposi’s varicelliform eruption due to herpes simplex in Darier’s disease. Clin Exp Dermatol 1994;19:428-429.
Painful and swollen hands
A 67-year-old woman came to the office with pain in her hands. She had just arrived from Panama to live with her son. She has had this pain for decades: she refers to it as artritisin Spanish but does not know what type of arthritis. Aspirin had helped in the past, but lately she had not been getting enough relief from it. Her hands feel stiff in the morning for at least 1 hour, which interferes with cooking and sewing.
Her hands showed signs of joint swelling and deformities (Figure 1). Her swollen joints felt warm. She also had knee pain.
FIGURE 1
Painful,swollen hands
What type of arthritis does she have?
Are any diagnostic tests necessary?
What are the best treatments available?
That same day, another patient was seen with painful hands (Figure 2). What type of arthritis does she have, and how does it differ from the condition of the patient in Figure 1 ?
FIGURE 2
Another patient with hand pain
The obvious ulnar deviation of her fingers and the swelling of the metacarpophalangeal (MCP) joints (Figure 1) are strongly indicative of rheumatoid arthritis. The patient in Figure 2 has swelling and deformities in her proximal inter-phalangeal (PIP) and distal interphalangeal (DIP) joints, indicating that she most likely has osteoarthritis. The swelling of the PIP joints is called Bouchard’s nodes; the swelling of the DIP joints is called Heberden’s nodes.
Differential diagnosis: types of arthritis
The first decision point in diagnosing chronic (>6 weeks) polyarticular joint pain is distinguishing between inflammatory and noninflammatory arthritis. Key features of inflammatory arthritis are stiffness in the morning or after inactivity, and visible joint swelling. The differential diagnosis of inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus (SLE).
After the age of 50, maturity-onset seronegative synovitis syndrome and crystal-induced synovitis should also be considered.1 Although osteoarthritis is considered noninflammatory, inflammation of the joint tissue occurs occasionally due to the joint’s degenerative loss of cartilage and bony overgrowth.
It is critical to identify rheumatoid arthritis early, as prompt intervention can delay disease progression, and reduce the substantial morbidity and mortality of rheumatoid arthritis.2 A diagnosis of rheumatoid arthritis requires 4 of the following: morning stiffness; arthritis in 3 or more joints; arthritis in the wrist, MCP joints, or PIP joints; symmetric arthritis; rheumatoid nodules; positive rheumatoid factor; radiographic changes.1 This patient has the morning stiffness, arthritis in more than 3 joints, symmetric arthritis, and rheumatoid nodules on her feet (Figure 3).
The most common noninflammatory arthritis is osteoarthritis, which affects 21 million Americans.2 The weight-bearing joints are usually affected, and damage may occur because of trauma or repetitive impact. When the hands are involved, the DIP and PIP joints are more likely to be involved than the MCP joints.
FIGURE 3
Rheumatoid nodules on feet
Diagnostic tests can differentiate between causes
Laboratory tests can help differentiate between conditions causing inflammatory arthritis. Rheumatoid factor is positive in 70% of patients with rheumatoid arthritis, and the antinuclear antibody is invariably positive for patients with SLE. Maturity-onset seronegative synovitis has negative rheumatoid factor and antinuclear antibody tests with marked elevation in erythrocyte sedimentation rate. Polyarticular gout may have increased serum uric acid, and is best diagnosed by demonstrating crystals in the joint fluid.
Laboratory tests are not helpful in diagnosing psoriatic arthritis, seronegative spondylo-arthropathies, and osteoarthritis with inflammation.1 Radiographic studies may show joint erosions in patients with active rheumatoid arthritis for more than a year, and typical osteophytes and joint-space narrowing in osteoarthritis.1
Radiographs are not necessary for the diagnosis of this patient but may help management, especially if hand surgery is going to be considered. In this case, the radiographs showed joint erosions and unequivocal juxta-auricular osteopenia.
Management: decrease pain, optimize mobility
The management of osteoarthritis and rheumatoid arthritis is different. However, in all types of arthritis the goals of therapy are to decrease pain, optimize mobility, and maximize quality of life. In rheumatoid arthritis, another goal is to slow the progression of the disease with disease-modifying antirheumatic drugs.
Osteoarthritis. First-line therapy for osteo arthritis includes exercise, weight loss (if indicated), and acetaminophen in scheduled doses up to 1000 mg 4 times a day. A recent Cochrane Review concluded that acetaminophen is clearly superior to placebo, but slightly less efficacious than nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief in osteoarthritis (level of evidence [LOE]: 1a). Acetaminophen and NSAIDs were equivalent in improving function. This evidence supports the use of acetaminophen first, reserving NSAIDs for those who do not respond.3 Adding NSAIDs may improve pain relief, but carries an increased risk of gastrointestinal ulcerations or bleeding.
A cyclo-oxygenase-2 (COX-2) inhibitor may be preferred to NSAIDs for patients at high risk for gastrointestinal complications. Other treatments include topical analgesics, glucosamine, and chondroitin. Large clinical trials for glucosamine and chondroitin are ongoing.
Rheumatoid arthritis. In rheumatoid arthritis, the recommendation from the American College of Rheumatology is for early, aggressive intervention with disease-modifying antirheumatic drugs, often within a few months of the onset of the disease.4 Unfortunately, the patient in Figure 1 has had rheumatoid arthritis for decades. Patients with rheumatoid arthritis also benefit from exercise and physical therapy.
Other treatments include low-dose corticosteroids and NSAIDs or COX-2 inhibitors. COX-2 inhibitors have similar efficacy to NSAIDs, with a lower risk of gastrointestinal complications (LOE: 1a).5,6 COX-2 inhibitors should be considered in place of NSAIDs for patients with rheumatoid arthritis, as these patients are almost twice as likely to suffer from serious gastrointestinal complications as the general population.2 COX-2 inhibitors, however, are much more expensive than NSAIDs, which limits their use.
Patient’s outcome
The patient was started on an anti-inflammatory medication and referred to a rheumatologist for consideration of a disease-modifying antirheumatic drug.
Acknowledgments
The authors would like to acknowledge Michael Fischbach, MD, in the Rheumatology Department of the University of Texas Health Science Center for his contribution to this article.
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas HealthSciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd CurlDrive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162:1833-1838
2. Kuritzky L, Weaver A. Advances in rheumatology: coxibs and beyond. J Pain Symptom Manage 2003;25(2 Suppl):S6-S20.
3. Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;(2):CD004257-
4. Guidelines for the Management of Rheumatoid Arthritis from the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Arthritis Rheum 2002;46:328-346.
5. Garner S, Fidan D, Frankish R, et al. Rofecoxib for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;(3):CD003685.-
6. Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev 2002;(4):CD003831.
A 67-year-old woman came to the office with pain in her hands. She had just arrived from Panama to live with her son. She has had this pain for decades: she refers to it as artritisin Spanish but does not know what type of arthritis. Aspirin had helped in the past, but lately she had not been getting enough relief from it. Her hands feel stiff in the morning for at least 1 hour, which interferes with cooking and sewing.
Her hands showed signs of joint swelling and deformities (Figure 1). Her swollen joints felt warm. She also had knee pain.
FIGURE 1
Painful,swollen hands
What type of arthritis does she have?
Are any diagnostic tests necessary?
What are the best treatments available?
That same day, another patient was seen with painful hands (Figure 2). What type of arthritis does she have, and how does it differ from the condition of the patient in Figure 1 ?
FIGURE 2
Another patient with hand pain
The obvious ulnar deviation of her fingers and the swelling of the metacarpophalangeal (MCP) joints (Figure 1) are strongly indicative of rheumatoid arthritis. The patient in Figure 2 has swelling and deformities in her proximal inter-phalangeal (PIP) and distal interphalangeal (DIP) joints, indicating that she most likely has osteoarthritis. The swelling of the PIP joints is called Bouchard’s nodes; the swelling of the DIP joints is called Heberden’s nodes.
Differential diagnosis: types of arthritis
The first decision point in diagnosing chronic (>6 weeks) polyarticular joint pain is distinguishing between inflammatory and noninflammatory arthritis. Key features of inflammatory arthritis are stiffness in the morning or after inactivity, and visible joint swelling. The differential diagnosis of inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus (SLE).
After the age of 50, maturity-onset seronegative synovitis syndrome and crystal-induced synovitis should also be considered.1 Although osteoarthritis is considered noninflammatory, inflammation of the joint tissue occurs occasionally due to the joint’s degenerative loss of cartilage and bony overgrowth.
It is critical to identify rheumatoid arthritis early, as prompt intervention can delay disease progression, and reduce the substantial morbidity and mortality of rheumatoid arthritis.2 A diagnosis of rheumatoid arthritis requires 4 of the following: morning stiffness; arthritis in 3 or more joints; arthritis in the wrist, MCP joints, or PIP joints; symmetric arthritis; rheumatoid nodules; positive rheumatoid factor; radiographic changes.1 This patient has the morning stiffness, arthritis in more than 3 joints, symmetric arthritis, and rheumatoid nodules on her feet (Figure 3).
The most common noninflammatory arthritis is osteoarthritis, which affects 21 million Americans.2 The weight-bearing joints are usually affected, and damage may occur because of trauma or repetitive impact. When the hands are involved, the DIP and PIP joints are more likely to be involved than the MCP joints.
FIGURE 3
Rheumatoid nodules on feet
Diagnostic tests can differentiate between causes
Laboratory tests can help differentiate between conditions causing inflammatory arthritis. Rheumatoid factor is positive in 70% of patients with rheumatoid arthritis, and the antinuclear antibody is invariably positive for patients with SLE. Maturity-onset seronegative synovitis has negative rheumatoid factor and antinuclear antibody tests with marked elevation in erythrocyte sedimentation rate. Polyarticular gout may have increased serum uric acid, and is best diagnosed by demonstrating crystals in the joint fluid.
Laboratory tests are not helpful in diagnosing psoriatic arthritis, seronegative spondylo-arthropathies, and osteoarthritis with inflammation.1 Radiographic studies may show joint erosions in patients with active rheumatoid arthritis for more than a year, and typical osteophytes and joint-space narrowing in osteoarthritis.1
Radiographs are not necessary for the diagnosis of this patient but may help management, especially if hand surgery is going to be considered. In this case, the radiographs showed joint erosions and unequivocal juxta-auricular osteopenia.
Management: decrease pain, optimize mobility
The management of osteoarthritis and rheumatoid arthritis is different. However, in all types of arthritis the goals of therapy are to decrease pain, optimize mobility, and maximize quality of life. In rheumatoid arthritis, another goal is to slow the progression of the disease with disease-modifying antirheumatic drugs.
Osteoarthritis. First-line therapy for osteo arthritis includes exercise, weight loss (if indicated), and acetaminophen in scheduled doses up to 1000 mg 4 times a day. A recent Cochrane Review concluded that acetaminophen is clearly superior to placebo, but slightly less efficacious than nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief in osteoarthritis (level of evidence [LOE]: 1a). Acetaminophen and NSAIDs were equivalent in improving function. This evidence supports the use of acetaminophen first, reserving NSAIDs for those who do not respond.3 Adding NSAIDs may improve pain relief, but carries an increased risk of gastrointestinal ulcerations or bleeding.
A cyclo-oxygenase-2 (COX-2) inhibitor may be preferred to NSAIDs for patients at high risk for gastrointestinal complications. Other treatments include topical analgesics, glucosamine, and chondroitin. Large clinical trials for glucosamine and chondroitin are ongoing.
Rheumatoid arthritis. In rheumatoid arthritis, the recommendation from the American College of Rheumatology is for early, aggressive intervention with disease-modifying antirheumatic drugs, often within a few months of the onset of the disease.4 Unfortunately, the patient in Figure 1 has had rheumatoid arthritis for decades. Patients with rheumatoid arthritis also benefit from exercise and physical therapy.
Other treatments include low-dose corticosteroids and NSAIDs or COX-2 inhibitors. COX-2 inhibitors have similar efficacy to NSAIDs, with a lower risk of gastrointestinal complications (LOE: 1a).5,6 COX-2 inhibitors should be considered in place of NSAIDs for patients with rheumatoid arthritis, as these patients are almost twice as likely to suffer from serious gastrointestinal complications as the general population.2 COX-2 inhibitors, however, are much more expensive than NSAIDs, which limits their use.
Patient’s outcome
The patient was started on an anti-inflammatory medication and referred to a rheumatologist for consideration of a disease-modifying antirheumatic drug.
Acknowledgments
The authors would like to acknowledge Michael Fischbach, MD, in the Rheumatology Department of the University of Texas Health Science Center for his contribution to this article.
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas HealthSciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd CurlDrive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 67-year-old woman came to the office with pain in her hands. She had just arrived from Panama to live with her son. She has had this pain for decades: she refers to it as artritisin Spanish but does not know what type of arthritis. Aspirin had helped in the past, but lately she had not been getting enough relief from it. Her hands feel stiff in the morning for at least 1 hour, which interferes with cooking and sewing.
Her hands showed signs of joint swelling and deformities (Figure 1). Her swollen joints felt warm. She also had knee pain.
FIGURE 1
Painful,swollen hands
What type of arthritis does she have?
Are any diagnostic tests necessary?
What are the best treatments available?
That same day, another patient was seen with painful hands (Figure 2). What type of arthritis does she have, and how does it differ from the condition of the patient in Figure 1 ?
FIGURE 2
Another patient with hand pain
The obvious ulnar deviation of her fingers and the swelling of the metacarpophalangeal (MCP) joints (Figure 1) are strongly indicative of rheumatoid arthritis. The patient in Figure 2 has swelling and deformities in her proximal inter-phalangeal (PIP) and distal interphalangeal (DIP) joints, indicating that she most likely has osteoarthritis. The swelling of the PIP joints is called Bouchard’s nodes; the swelling of the DIP joints is called Heberden’s nodes.
Differential diagnosis: types of arthritis
The first decision point in diagnosing chronic (>6 weeks) polyarticular joint pain is distinguishing between inflammatory and noninflammatory arthritis. Key features of inflammatory arthritis are stiffness in the morning or after inactivity, and visible joint swelling. The differential diagnosis of inflammatory arthritis includes rheumatoid arthritis, psoriatic arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus (SLE).
After the age of 50, maturity-onset seronegative synovitis syndrome and crystal-induced synovitis should also be considered.1 Although osteoarthritis is considered noninflammatory, inflammation of the joint tissue occurs occasionally due to the joint’s degenerative loss of cartilage and bony overgrowth.
It is critical to identify rheumatoid arthritis early, as prompt intervention can delay disease progression, and reduce the substantial morbidity and mortality of rheumatoid arthritis.2 A diagnosis of rheumatoid arthritis requires 4 of the following: morning stiffness; arthritis in 3 or more joints; arthritis in the wrist, MCP joints, or PIP joints; symmetric arthritis; rheumatoid nodules; positive rheumatoid factor; radiographic changes.1 This patient has the morning stiffness, arthritis in more than 3 joints, symmetric arthritis, and rheumatoid nodules on her feet (Figure 3).
The most common noninflammatory arthritis is osteoarthritis, which affects 21 million Americans.2 The weight-bearing joints are usually affected, and damage may occur because of trauma or repetitive impact. When the hands are involved, the DIP and PIP joints are more likely to be involved than the MCP joints.
FIGURE 3
Rheumatoid nodules on feet
Diagnostic tests can differentiate between causes
Laboratory tests can help differentiate between conditions causing inflammatory arthritis. Rheumatoid factor is positive in 70% of patients with rheumatoid arthritis, and the antinuclear antibody is invariably positive for patients with SLE. Maturity-onset seronegative synovitis has negative rheumatoid factor and antinuclear antibody tests with marked elevation in erythrocyte sedimentation rate. Polyarticular gout may have increased serum uric acid, and is best diagnosed by demonstrating crystals in the joint fluid.
Laboratory tests are not helpful in diagnosing psoriatic arthritis, seronegative spondylo-arthropathies, and osteoarthritis with inflammation.1 Radiographic studies may show joint erosions in patients with active rheumatoid arthritis for more than a year, and typical osteophytes and joint-space narrowing in osteoarthritis.1
Radiographs are not necessary for the diagnosis of this patient but may help management, especially if hand surgery is going to be considered. In this case, the radiographs showed joint erosions and unequivocal juxta-auricular osteopenia.
Management: decrease pain, optimize mobility
The management of osteoarthritis and rheumatoid arthritis is different. However, in all types of arthritis the goals of therapy are to decrease pain, optimize mobility, and maximize quality of life. In rheumatoid arthritis, another goal is to slow the progression of the disease with disease-modifying antirheumatic drugs.
Osteoarthritis. First-line therapy for osteo arthritis includes exercise, weight loss (if indicated), and acetaminophen in scheduled doses up to 1000 mg 4 times a day. A recent Cochrane Review concluded that acetaminophen is clearly superior to placebo, but slightly less efficacious than nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief in osteoarthritis (level of evidence [LOE]: 1a). Acetaminophen and NSAIDs were equivalent in improving function. This evidence supports the use of acetaminophen first, reserving NSAIDs for those who do not respond.3 Adding NSAIDs may improve pain relief, but carries an increased risk of gastrointestinal ulcerations or bleeding.
A cyclo-oxygenase-2 (COX-2) inhibitor may be preferred to NSAIDs for patients at high risk for gastrointestinal complications. Other treatments include topical analgesics, glucosamine, and chondroitin. Large clinical trials for glucosamine and chondroitin are ongoing.
Rheumatoid arthritis. In rheumatoid arthritis, the recommendation from the American College of Rheumatology is for early, aggressive intervention with disease-modifying antirheumatic drugs, often within a few months of the onset of the disease.4 Unfortunately, the patient in Figure 1 has had rheumatoid arthritis for decades. Patients with rheumatoid arthritis also benefit from exercise and physical therapy.
Other treatments include low-dose corticosteroids and NSAIDs or COX-2 inhibitors. COX-2 inhibitors have similar efficacy to NSAIDs, with a lower risk of gastrointestinal complications (LOE: 1a).5,6 COX-2 inhibitors should be considered in place of NSAIDs for patients with rheumatoid arthritis, as these patients are almost twice as likely to suffer from serious gastrointestinal complications as the general population.2 COX-2 inhibitors, however, are much more expensive than NSAIDs, which limits their use.
Patient’s outcome
The patient was started on an anti-inflammatory medication and referred to a rheumatologist for consideration of a disease-modifying antirheumatic drug.
Acknowledgments
The authors would like to acknowledge Michael Fischbach, MD, in the Rheumatology Department of the University of Texas Health Science Center for his contribution to this article.
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas HealthSciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd CurlDrive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162:1833-1838
2. Kuritzky L, Weaver A. Advances in rheumatology: coxibs and beyond. J Pain Symptom Manage 2003;25(2 Suppl):S6-S20.
3. Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;(2):CD004257-
4. Guidelines for the Management of Rheumatoid Arthritis from the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Arthritis Rheum 2002;46:328-346.
5. Garner S, Fidan D, Frankish R, et al. Rofecoxib for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;(3):CD003685.-
6. Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev 2002;(4):CD003831.
1. Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162:1833-1838
2. Kuritzky L, Weaver A. Advances in rheumatology: coxibs and beyond. J Pain Symptom Manage 2003;25(2 Suppl):S6-S20.
3. Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2003;(2):CD004257-
4. Guidelines for the Management of Rheumatoid Arthritis from the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Arthritis Rheum 2002;46:328-346.
5. Garner S, Fidan D, Frankish R, et al. Rofecoxib for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;(3):CD003685.-
6. Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database Syst Rev 2002;(4):CD003831.
Red eyes with a brown spot
A 32-year-old woman came to the rescue mission clinic with her 2 sons because she had red eyes and a runny nose. Her sons both had symptoms highly suggestive of viral upper respiratory infection. They lived in a homeless shelter.
The patient stated she did not use contact lenses or have any eye trauma, itching, photophobia, loss or change of vision, eye pain, eye discharge, or previous episodes of pinkeye. She had no other medical problems or history of allergies.
On physical exam, her vital signs were normal. She had conjunctival injection, without purulent discharge or limbal blush (Figures 1 and 2). Eyelids were mildly erythematous with no cobble-stoning. Pupils were equal, round, and reactive to light. The anterior chamber by flashlight exam from the side did not show a narrow angle. Visual acuity was normal by Snellen exam. She had clear nasal discharge and bilateral preauricular lymphadenopathy.
In addition, she had a brown macule under the left iris on the conjunctiva. The patient said this had been present since childhood and it had not changed.
FIGURE 1
Red eyes
FIGURE 2
Brown macule
What is the differential iagnosis?
What about the brown macule?
Are any diagnostic tests Necessary for either condition?
Differential diagnosis
The differential diagnosis of a red eye includes:
- Conjunctivitis
- Uveitis
- Acute glaucoma
- Corneal disease or foreign body trauma
- Scleritis and episcleritis.1
For this patient, conjunctivitis is the most likely diagnosis. The absence of eye pain or loss of vision makes uveitis, acute glaucoma, or corneal disease (including foreign-body trauma) less likely. The round shape of the pupil and the absence of the limbal blush also make uveitis less likely. The pattern of injection does not match the wedge-shaped inflammation of episcleritis or the depth of scleritis.
Diagnosis: viral conjunctivitis
This patient has viral conjunctivitis. Conjunctivitis can be infectious, allergic, chemical/irritative, or autoimmune in origin. The most common infectious agents are viral—specifically, the adenoviruses. Other infectious agents include bacteria (Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae), chlamydia, and herpes simplex virus.
Both infectious and allergic conjunctivitis are common. In this patient, the presence of nasal discharge, preauricular lymphadenopathy, and the lack of pruritus make viral infection more likely than allergic. Conjunctivitis that is bilateral without purulent discharge is more likely to be viral than bacterial.
What about the brown macule?
This patient also had a brown macule on her conjunctiva. The differential diagnosis of pigmented areas on the conjunctiva includes nevus, racial melanosis, primary acquired melanosis, secondary pigmentary deposition, and ocular melanoma.2 These conditions (besides ocular melanoma) are benign.
Although ocular melanoma is the most common primary intraocular malignancy in adults, and the second most likely location for primary melanoma after the skin, it is still exceedingly rare. In the Causasian population, it has an average annual incidence of 6 cases per million, with approximately 1200 cases diagnosed each year. Ocular melanoma occurs in the uvea much more commonly than in the conjunctiva, at a ratio of 35:1. Conjunctival melanomas have a propensity for regional spread to the lymph nodes analogous to cutaneous disease, with 10-year survival rates of more than 80%.3
In this case, the patient had this dark spot since childhood and had noted no growth or change. It was consistent with a conjunctival nevus and did not need biopsy.
Diagnostic tests: only for cases that do not respond
When viral conjunctivitis is suspected, no laboratory tests are routinely recommended. Bacterial and viral cultures may be helpful to establish the diagnosis in cases that do not resolve or when patients have recurrent episodes. Infections that do not respond to empiric treatment should be cultured for the suspected organisms (bacteria, chlamydia, or herpes simplex). When chlamydial conjunctivitis is suspected, the diagnosis should be confirmed by means of an immunodiagnostic test (direct fluorescent antibody [DFA]) or culture (level of evidence [LOE]=1a).4
A fluorescein exam is helpful in cases with a question of corneal involvement from foreign-body trauma, herpes simplex, or epidemic kerato-conjunctivitis. Herpes simplex infections have a dendritic pattern of ulceration, and epidemic keratoconjunctivitis infections cause multiple small areas of increased fluorescein uptake.
Management: conjunctivitis usually self-limiting
Typical viral conjunctivitis caused by the adenoviruses or other common viruses (not herpes) does not require medication. Warm compresses may be recommended to reduce discomfort. Infectious conjunctivitis caused by bacteria are also usually self-limiting; however, a recent meta-analysis indicates that treatment with antibiotics can shorten the clinical duration (LOE=1a).5
Appropriate medications for bacterial conjunctivitis include 0.3% tobramycin or gentamycin, 10% sodium sulfacetamide, or erythromycin ophthalmic ointment. If herpetic keratoconjuntivitis is suspected, prompt ophthalmologic referral is indicated.1
Patient’s treatment and outcome
This patient was managed with reassurance and symptomatic treatment of her viral respiratory illness. Her red eyes and upper respiratory infection both resolved spontaneously within 1 week.
As her nevus had not changed in many years, she was instructed to continue to watch the nevus and report any changes to a physician for evaluation. If the lesion changed in the future, she should be referred to an ophthalmologist.
- Erythromycin (ophthalmic) • Ilotycin
- Gentamycin (ophthalmic) • Garamycin, Genoptic Liquifilm, Genoptic SOP, Gentacidin, Gentafair, Gentak, Ocu-Mycin, Spectro-Genta
- Sulfacetamide (ophthalmic) • AK-Sulf, Bleph-10, Cetamide, Isopto Cetamide, Ocusulf-10, Sodium Sulamyd, Sulf-10
- Tobramycin (ophthalmic) • AKTob, Tobrex
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Ricketson CW. Conjunctivitis in 5-minute clinical consult. Dambro, MR (ed). InfoRetriever [electronic database]. Charlottesville, Va: InfoPOEMs, Inc; 2004.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol 2004;49:3-24.
3. Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Arch Dermatol 2003;139:1067-1073.
4. American Academy of Ophthalmology. Preferred Practice Patterns. Conjunctivitis. Available at: www.aao.org/aao/education/library/ppp/index.cfm. Accessed on February 3, 2004.
5. Sheikh A, Hurwitz B, Cave J. Antibiotics versus placebo for acute bacterial conjunctivitis (Cochrane Review). In: The Cochrane Library, Issue 4, 2003.
A 32-year-old woman came to the rescue mission clinic with her 2 sons because she had red eyes and a runny nose. Her sons both had symptoms highly suggestive of viral upper respiratory infection. They lived in a homeless shelter.
The patient stated she did not use contact lenses or have any eye trauma, itching, photophobia, loss or change of vision, eye pain, eye discharge, or previous episodes of pinkeye. She had no other medical problems or history of allergies.
On physical exam, her vital signs were normal. She had conjunctival injection, without purulent discharge or limbal blush (Figures 1 and 2). Eyelids were mildly erythematous with no cobble-stoning. Pupils were equal, round, and reactive to light. The anterior chamber by flashlight exam from the side did not show a narrow angle. Visual acuity was normal by Snellen exam. She had clear nasal discharge and bilateral preauricular lymphadenopathy.
In addition, she had a brown macule under the left iris on the conjunctiva. The patient said this had been present since childhood and it had not changed.
FIGURE 1
Red eyes
FIGURE 2
Brown macule
What is the differential iagnosis?
What about the brown macule?
Are any diagnostic tests Necessary for either condition?
Differential diagnosis
The differential diagnosis of a red eye includes:
- Conjunctivitis
- Uveitis
- Acute glaucoma
- Corneal disease or foreign body trauma
- Scleritis and episcleritis.1
For this patient, conjunctivitis is the most likely diagnosis. The absence of eye pain or loss of vision makes uveitis, acute glaucoma, or corneal disease (including foreign-body trauma) less likely. The round shape of the pupil and the absence of the limbal blush also make uveitis less likely. The pattern of injection does not match the wedge-shaped inflammation of episcleritis or the depth of scleritis.
Diagnosis: viral conjunctivitis
This patient has viral conjunctivitis. Conjunctivitis can be infectious, allergic, chemical/irritative, or autoimmune in origin. The most common infectious agents are viral—specifically, the adenoviruses. Other infectious agents include bacteria (Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae), chlamydia, and herpes simplex virus.
Both infectious and allergic conjunctivitis are common. In this patient, the presence of nasal discharge, preauricular lymphadenopathy, and the lack of pruritus make viral infection more likely than allergic. Conjunctivitis that is bilateral without purulent discharge is more likely to be viral than bacterial.
What about the brown macule?
This patient also had a brown macule on her conjunctiva. The differential diagnosis of pigmented areas on the conjunctiva includes nevus, racial melanosis, primary acquired melanosis, secondary pigmentary deposition, and ocular melanoma.2 These conditions (besides ocular melanoma) are benign.
Although ocular melanoma is the most common primary intraocular malignancy in adults, and the second most likely location for primary melanoma after the skin, it is still exceedingly rare. In the Causasian population, it has an average annual incidence of 6 cases per million, with approximately 1200 cases diagnosed each year. Ocular melanoma occurs in the uvea much more commonly than in the conjunctiva, at a ratio of 35:1. Conjunctival melanomas have a propensity for regional spread to the lymph nodes analogous to cutaneous disease, with 10-year survival rates of more than 80%.3
In this case, the patient had this dark spot since childhood and had noted no growth or change. It was consistent with a conjunctival nevus and did not need biopsy.
Diagnostic tests: only for cases that do not respond
When viral conjunctivitis is suspected, no laboratory tests are routinely recommended. Bacterial and viral cultures may be helpful to establish the diagnosis in cases that do not resolve or when patients have recurrent episodes. Infections that do not respond to empiric treatment should be cultured for the suspected organisms (bacteria, chlamydia, or herpes simplex). When chlamydial conjunctivitis is suspected, the diagnosis should be confirmed by means of an immunodiagnostic test (direct fluorescent antibody [DFA]) or culture (level of evidence [LOE]=1a).4
A fluorescein exam is helpful in cases with a question of corneal involvement from foreign-body trauma, herpes simplex, or epidemic kerato-conjunctivitis. Herpes simplex infections have a dendritic pattern of ulceration, and epidemic keratoconjunctivitis infections cause multiple small areas of increased fluorescein uptake.
Management: conjunctivitis usually self-limiting
Typical viral conjunctivitis caused by the adenoviruses or other common viruses (not herpes) does not require medication. Warm compresses may be recommended to reduce discomfort. Infectious conjunctivitis caused by bacteria are also usually self-limiting; however, a recent meta-analysis indicates that treatment with antibiotics can shorten the clinical duration (LOE=1a).5
Appropriate medications for bacterial conjunctivitis include 0.3% tobramycin or gentamycin, 10% sodium sulfacetamide, or erythromycin ophthalmic ointment. If herpetic keratoconjuntivitis is suspected, prompt ophthalmologic referral is indicated.1
Patient’s treatment and outcome
This patient was managed with reassurance and symptomatic treatment of her viral respiratory illness. Her red eyes and upper respiratory infection both resolved spontaneously within 1 week.
As her nevus had not changed in many years, she was instructed to continue to watch the nevus and report any changes to a physician for evaluation. If the lesion changed in the future, she should be referred to an ophthalmologist.
- Erythromycin (ophthalmic) • Ilotycin
- Gentamycin (ophthalmic) • Garamycin, Genoptic Liquifilm, Genoptic SOP, Gentacidin, Gentafair, Gentak, Ocu-Mycin, Spectro-Genta
- Sulfacetamide (ophthalmic) • AK-Sulf, Bleph-10, Cetamide, Isopto Cetamide, Ocusulf-10, Sodium Sulamyd, Sulf-10
- Tobramycin (ophthalmic) • AKTob, Tobrex
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
A 32-year-old woman came to the rescue mission clinic with her 2 sons because she had red eyes and a runny nose. Her sons both had symptoms highly suggestive of viral upper respiratory infection. They lived in a homeless shelter.
The patient stated she did not use contact lenses or have any eye trauma, itching, photophobia, loss or change of vision, eye pain, eye discharge, or previous episodes of pinkeye. She had no other medical problems or history of allergies.
On physical exam, her vital signs were normal. She had conjunctival injection, without purulent discharge or limbal blush (Figures 1 and 2). Eyelids were mildly erythematous with no cobble-stoning. Pupils were equal, round, and reactive to light. The anterior chamber by flashlight exam from the side did not show a narrow angle. Visual acuity was normal by Snellen exam. She had clear nasal discharge and bilateral preauricular lymphadenopathy.
In addition, she had a brown macule under the left iris on the conjunctiva. The patient said this had been present since childhood and it had not changed.
FIGURE 1
Red eyes
FIGURE 2
Brown macule
What is the differential iagnosis?
What about the brown macule?
Are any diagnostic tests Necessary for either condition?
Differential diagnosis
The differential diagnosis of a red eye includes:
- Conjunctivitis
- Uveitis
- Acute glaucoma
- Corneal disease or foreign body trauma
- Scleritis and episcleritis.1
For this patient, conjunctivitis is the most likely diagnosis. The absence of eye pain or loss of vision makes uveitis, acute glaucoma, or corneal disease (including foreign-body trauma) less likely. The round shape of the pupil and the absence of the limbal blush also make uveitis less likely. The pattern of injection does not match the wedge-shaped inflammation of episcleritis or the depth of scleritis.
Diagnosis: viral conjunctivitis
This patient has viral conjunctivitis. Conjunctivitis can be infectious, allergic, chemical/irritative, or autoimmune in origin. The most common infectious agents are viral—specifically, the adenoviruses. Other infectious agents include bacteria (Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae), chlamydia, and herpes simplex virus.
Both infectious and allergic conjunctivitis are common. In this patient, the presence of nasal discharge, preauricular lymphadenopathy, and the lack of pruritus make viral infection more likely than allergic. Conjunctivitis that is bilateral without purulent discharge is more likely to be viral than bacterial.
What about the brown macule?
This patient also had a brown macule on her conjunctiva. The differential diagnosis of pigmented areas on the conjunctiva includes nevus, racial melanosis, primary acquired melanosis, secondary pigmentary deposition, and ocular melanoma.2 These conditions (besides ocular melanoma) are benign.
Although ocular melanoma is the most common primary intraocular malignancy in adults, and the second most likely location for primary melanoma after the skin, it is still exceedingly rare. In the Causasian population, it has an average annual incidence of 6 cases per million, with approximately 1200 cases diagnosed each year. Ocular melanoma occurs in the uvea much more commonly than in the conjunctiva, at a ratio of 35:1. Conjunctival melanomas have a propensity for regional spread to the lymph nodes analogous to cutaneous disease, with 10-year survival rates of more than 80%.3
In this case, the patient had this dark spot since childhood and had noted no growth or change. It was consistent with a conjunctival nevus and did not need biopsy.
Diagnostic tests: only for cases that do not respond
When viral conjunctivitis is suspected, no laboratory tests are routinely recommended. Bacterial and viral cultures may be helpful to establish the diagnosis in cases that do not resolve or when patients have recurrent episodes. Infections that do not respond to empiric treatment should be cultured for the suspected organisms (bacteria, chlamydia, or herpes simplex). When chlamydial conjunctivitis is suspected, the diagnosis should be confirmed by means of an immunodiagnostic test (direct fluorescent antibody [DFA]) or culture (level of evidence [LOE]=1a).4
A fluorescein exam is helpful in cases with a question of corneal involvement from foreign-body trauma, herpes simplex, or epidemic kerato-conjunctivitis. Herpes simplex infections have a dendritic pattern of ulceration, and epidemic keratoconjunctivitis infections cause multiple small areas of increased fluorescein uptake.
Management: conjunctivitis usually self-limiting
Typical viral conjunctivitis caused by the adenoviruses or other common viruses (not herpes) does not require medication. Warm compresses may be recommended to reduce discomfort. Infectious conjunctivitis caused by bacteria are also usually self-limiting; however, a recent meta-analysis indicates that treatment with antibiotics can shorten the clinical duration (LOE=1a).5
Appropriate medications for bacterial conjunctivitis include 0.3% tobramycin or gentamycin, 10% sodium sulfacetamide, or erythromycin ophthalmic ointment. If herpetic keratoconjuntivitis is suspected, prompt ophthalmologic referral is indicated.1
Patient’s treatment and outcome
This patient was managed with reassurance and symptomatic treatment of her viral respiratory illness. Her red eyes and upper respiratory infection both resolved spontaneously within 1 week.
As her nevus had not changed in many years, she was instructed to continue to watch the nevus and report any changes to a physician for evaluation. If the lesion changed in the future, she should be referred to an ophthalmologist.
- Erythromycin (ophthalmic) • Ilotycin
- Gentamycin (ophthalmic) • Garamycin, Genoptic Liquifilm, Genoptic SOP, Gentacidin, Gentafair, Gentak, Ocu-Mycin, Spectro-Genta
- Sulfacetamide (ophthalmic) • AK-Sulf, Bleph-10, Cetamide, Isopto Cetamide, Ocusulf-10, Sodium Sulamyd, Sulf-10
- Tobramycin (ophthalmic) • AKTob, Tobrex
Correspondence
Richard P. Usatine, Editor, Photo Rounds, University of Texas Health Sciences Center at San Antonio, Dept of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Ricketson CW. Conjunctivitis in 5-minute clinical consult. Dambro, MR (ed). InfoRetriever [electronic database]. Charlottesville, Va: InfoPOEMs, Inc; 2004.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol 2004;49:3-24.
3. Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Arch Dermatol 2003;139:1067-1073.
4. American Academy of Ophthalmology. Preferred Practice Patterns. Conjunctivitis. Available at: www.aao.org/aao/education/library/ppp/index.cfm. Accessed on February 3, 2004.
5. Sheikh A, Hurwitz B, Cave J. Antibiotics versus placebo for acute bacterial conjunctivitis (Cochrane Review). In: The Cochrane Library, Issue 4, 2003.
1. Ricketson CW. Conjunctivitis in 5-minute clinical consult. Dambro, MR (ed). InfoRetriever [electronic database]. Charlottesville, Va: InfoPOEMs, Inc; 2004.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol 2004;49:3-24.
3. Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Arch Dermatol 2003;139:1067-1073.
4. American Academy of Ophthalmology. Preferred Practice Patterns. Conjunctivitis. Available at: www.aao.org/aao/education/library/ppp/index.cfm. Accessed on February 3, 2004.
5. Sheikh A, Hurwitz B, Cave J. Antibiotics versus placebo for acute bacterial conjunctivitis (Cochrane Review). In: The Cochrane Library, Issue 4, 2003.