User login
Skin rash and muscle weakness
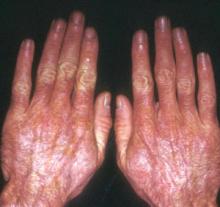
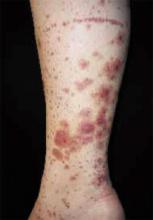
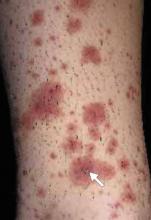
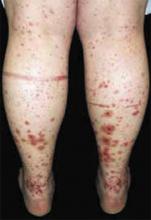
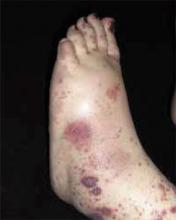
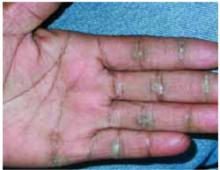
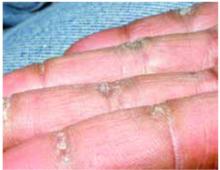
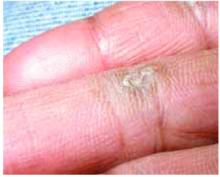
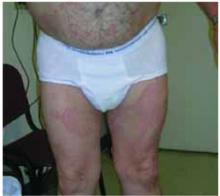
A 48-year-old Hispanic woman came to the clinic as a new patient—her chief complaint was a rash that appeared on her face 3 months before and had recently spread to her chest and hands (FIGURES 1-3). It itched occasionally and seemed to worsen after exposure to the sun.
She also said that for the last month she had been feeling very weak—she had difficulty rising from a seated position and walking up the stairs to her apartment. She also felt as if her arms were heavy, making it difficult for her to brush and dry her hair in the morning.
The patient was otherwise healthy with no known medical conditions, and she was not taking any medications. Her family history was noncontributory.
A musculoskeletal examination showed the following:
Upper extremities:
- 4/5 strength shoulder abduction, internal and external rotation
- 5/5 strength biceps, triceps, wrist extension/flexion, grip
- 2+ biceps and triceps deep tendon reflexes bilaterally
Lower extremities:
- 4/5 strength hip flexors, quadriceps, hamstrings
- 5/5 dorsiflexion/plantar flexion
- 2+ patellar and ankle deep tendon reflexes bilaterally.
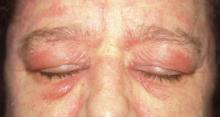
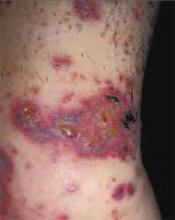
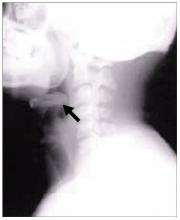
FIGURE 1
Facial rash and swelling
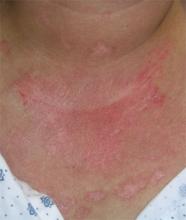
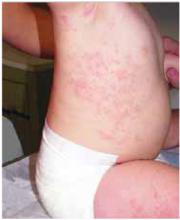
FIGURE 2
Rash spreading to chest
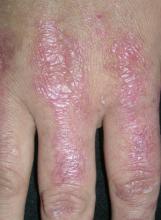
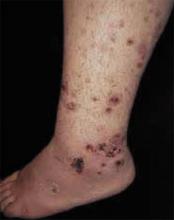
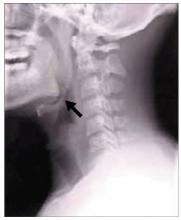
FIGURE 3
Plaques on knuckles
What is your diagnosis?
What diagnostic tests would you order for confirmation?
Diagnosis: Dermatomyositis
Dermatomyositis is a systemic disease classified as a type of idiopathic inflammatory myopathy. Dermatomyositis is a rare disease but one that may present initially to the family physician. It affects people at any age but is more commonly seen among children or adults aged >40 years.
Dermatomyositis involves the skin as well as skeletal muscle. Its cause is unknown; however, among those aged >50 years, malignancy may be an underlying cause. Cancers most commonly associated with dermatomyositis include those of the breast, ovary, lung, and gastrointestinal tract.
Skin manifestations may precede, follow, or present simultaneously with muscle involvement. Patients often complain of having difficulty ascending stairs, rising from a seated position, and performing overhead activities such as combing their hair. Patients may or may not have muscle tenderness and atrophy. Patients can have cutaneous involvement for more than a year before developing muscle weakness.1
The dermatologic signs of dermatomyositis to watch for:
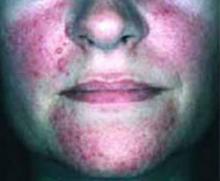
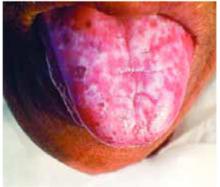
- Periorbital heliotrope erythema, usually associated with edema (FIGURE 1).
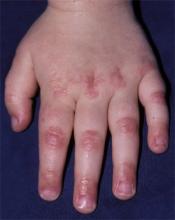
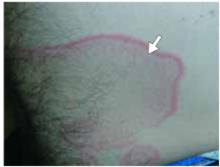
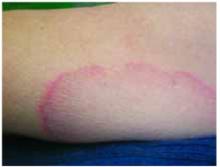
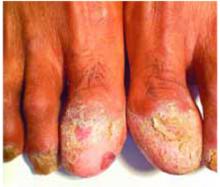
- Gottron’s papules—smooth, purple to red papules located over the knuckles, on the sides of the fingers, and sometimes on the elbows and knees. For adults, it is not uncommon to have plaques over the knuckles as opposed to the classic Gottron’s papules (FIGURE 3). In juvenile-onset dermatomyositis, distinct papules are much more evident upon presentation (FIGURE 4). Note that systemic lupus erythematosus (SLE) can present with a rash on the dorsum of the hands, but the rash spares the skin over the metacarpophalangeal and interphalangeal joints and affects the skin between the joints (FIGURE 5).
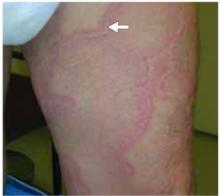
- Violaceous papular dermatitis with scale—may occur in localized areas, such as elbows and knees, or be diffusely distributed, starting off as a patchy erythema that coalesces and becomes slightly raised with scale. It tends to be confined to sun-exposed areas and worsens after sun exposure (FIGURE 2).
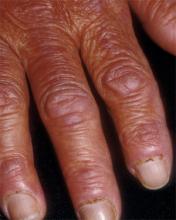
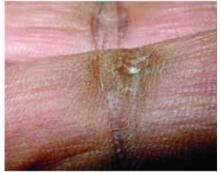
- Periungual erythema and telangiectasia—“moth-eaten” cuticles, a characteristic seen in other connective tissue diseases (FIGURE 6).1
FIGURE 4
Papules on knuckles
FIGURE 5
Sparing interphalangeal joints
FIGURE 6
Cuticles with erythema
Differential diagnosis
Seborrheic dermatitis—white or yellow, greasy scales on an erythematous base with distribution on scalp, nasolabial folds and chest.
Atopic dermatitis—chronic history of pruritic papules or plaques with scale localized to flexural areas or may be generalized; lichenification may be seen.
Contact dermatitis—papules and vesicles that correspond to contact with allergen.
Polymorphous light eruption—clusters of erythematous, pruritic papules or vesicles occurring most frequently on the neck, anterior chest, arms, and forearms following sun exposure; most common among women in their twenties.
Lichen planus—pruritic, purple, polygonal papules (4 Ps) that may involve hair, nails, and mucous membranes in addition to the skin; more common among women, with onset between 30 to 60 years of age; may last months to years.
Psoriasis—well-demarcated papules and plaques on an erythematous base with thick, silvery scale; characteristically found on elbows, knees, scalp, nails, and genitalia.
Steroid myopathy—A side effect of systemic steroids, usually seen 4 to 6 weeks after beginning of treatment.
Dermatomyositis-like reaction—onset of similar skin findings with initiation of the following medications and improvement with discontinuation: penicillamine, nonsteroidal anti-inflammatory drugs, and carbamazepine.
Overlap syndrome—The term “overlap” denotes that certain signs are seen in both dermatomyositis and other connective tissue diseases such as scleroderma, rheumatoid arthritis, and lupus erythematosus. Scleroderma and dermatomyositis are the most commonly associated conditions and have been termed sclerodermatomyositis or mixed connective disease. In mixed connective tissue disease, features of SLE, scleroderma, and polymyositis are evident such as malar rash, alopecia, Raynaud’s phenomenon, waxy-appearing skin, and proximal muscle weakness.1,2
Diagnostic tests: Muscle enzymes, EMG, biopsy
The diagnosis of dermatomyositis is confirmed by 3 laboratory tests: elevated muscle enzyme levels, electromyography, and muscle biopsy. A punch biopsy is helpful in differentiating dermatomyositis from other papulosquamous diseases such as lichen planus and psoriasis, but be careful as the histology of dermatomyositis is indistinguishable from cutaneous lupus erythematosus.
During the acute active phase, the following serum muscle enzymes may be elevated: creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), and aldolase. CK is elevated among 65% of patients and is most specific for muscle disease.3 Only one of the aforementioned enzymes may be elevated, so it is necessary to measure them all.
Measuring antibodies such as antinuclear antibody (ANA), Jo-1, SSA (Ro), SSB (La) supports the diagnosis if positive but dermatomyositis cannot be diagnosed solely on positive titers. It is not necessary to obtain an electromyograph or muscle biopsy for a patient with the characteristic skin findings and evidence of elevated muscle enzymes, as the diagnosis of dermatomyositis can be made with confidence. For a patient in whom the presentation is not as straightforward, it may be useful to obtain the electromyograph and muscle biopsy.
Management: Corticosteroids, watch for malignancy
Oral corticosteroids are the treatment of choice (strength of recommendation [SOR]: B).1,4 Prednisone 0.5 to 1.0 mg/kg body weight per day has been recommended until muscle enzyme levels trend toward normal limits, at which time you can taper the dose. Steroid myopathy is a potential side effect of this treatment regimen; it may occur 4 to 6 weeks after therapy starts.
Several steroid-sparing agents such as methotrexate and cyclosporine are being prescribed by clinicians for dermomyositis but with little published evidence to support effectiveness. Methotrexate is an option for those who do not respond to prednisone or are in need of a steroid-sparing agent secondary to side effects (SOR: C).2,3 A suggested regimen starts with 7.5 to 10 mg/wk, then increases the dose to 2.5 mg/wk until reaching a total dose of 25 mg/wk. The dose of prednisone should be decreased as the methotrexate dose increases. Azathioprine is another option to consider along with methotrexate, but choosing one agent over another or a combination of 2 agents remains empirical. With any of the immunosuppressants or immunomodulatory agents, it is important to look at their side-effect profiles and monitor the patient accordingly.
After initiating treatment, look for evidence of malignancy among those patients older than 50 years so as not to miss an underlying cancer as the cause for their dermatomyositis. For women without any risk factors, a complete annual physical exam—including pelvic, breast, and rectal exam—is sufficient.2 It is not necessary to order expensive radiological studies blindly searching for malignancy, especially more than 2 years after the diagnosis is made. The greatest risk of malignancy occurs during the first year after diagnosis with a six-fold increase.1 The risk drops during the second year and a patient’s risk for malignancy is comparable to the normal population in the years following. A mammogram and colonoscopy might be indicated after considering the patient’s age and family history.
The patient’s treatment and outcome
The patient was started on 60 mg of oral prednisone, taken in a single daily dose. She also began physical therapy twice a week in order to prevent muscle atrophy and maximize function. She took the prednisone for 1 month, at which time her creatine kinase level was trending towards normal. We then began slowly tapering the prednisone over the next 6 months.
She reported improvement in her strength 3 months after starting the systemic steroids. Little improvement was seen in the patient’s skin while on systemic steroids, but after prescribing 0.1% triamcinolone ointment, recommending a broad-spectrum sunscreen, and limiting sun exposure, the patient reported less erythema and edema.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia: Mosby; 2004.
2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982.
3. Woff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
4. Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2005;Issue 3:CD003643.
A 48-year-old Hispanic woman came to the clinic as a new patient—her chief complaint was a rash that appeared on her face 3 months before and had recently spread to her chest and hands (FIGURES 1-3). It itched occasionally and seemed to worsen after exposure to the sun.
She also said that for the last month she had been feeling very weak—she had difficulty rising from a seated position and walking up the stairs to her apartment. She also felt as if her arms were heavy, making it difficult for her to brush and dry her hair in the morning.
The patient was otherwise healthy with no known medical conditions, and she was not taking any medications. Her family history was noncontributory.
A musculoskeletal examination showed the following:
Upper extremities:
- 4/5 strength shoulder abduction, internal and external rotation
- 5/5 strength biceps, triceps, wrist extension/flexion, grip
- 2+ biceps and triceps deep tendon reflexes bilaterally
Lower extremities:
- 4/5 strength hip flexors, quadriceps, hamstrings
- 5/5 dorsiflexion/plantar flexion
- 2+ patellar and ankle deep tendon reflexes bilaterally.
FIGURE 1
Facial rash and swelling
FIGURE 2
Rash spreading to chest
FIGURE 3
Plaques on knuckles
What is your diagnosis?
What diagnostic tests would you order for confirmation?
Diagnosis: Dermatomyositis
Dermatomyositis is a systemic disease classified as a type of idiopathic inflammatory myopathy. Dermatomyositis is a rare disease but one that may present initially to the family physician. It affects people at any age but is more commonly seen among children or adults aged >40 years.
Dermatomyositis involves the skin as well as skeletal muscle. Its cause is unknown; however, among those aged >50 years, malignancy may be an underlying cause. Cancers most commonly associated with dermatomyositis include those of the breast, ovary, lung, and gastrointestinal tract.
Skin manifestations may precede, follow, or present simultaneously with muscle involvement. Patients often complain of having difficulty ascending stairs, rising from a seated position, and performing overhead activities such as combing their hair. Patients may or may not have muscle tenderness and atrophy. Patients can have cutaneous involvement for more than a year before developing muscle weakness.1
The dermatologic signs of dermatomyositis to watch for:
- Periorbital heliotrope erythema, usually associated with edema (FIGURE 1).
- Gottron’s papules—smooth, purple to red papules located over the knuckles, on the sides of the fingers, and sometimes on the elbows and knees. For adults, it is not uncommon to have plaques over the knuckles as opposed to the classic Gottron’s papules (FIGURE 3). In juvenile-onset dermatomyositis, distinct papules are much more evident upon presentation (FIGURE 4). Note that systemic lupus erythematosus (SLE) can present with a rash on the dorsum of the hands, but the rash spares the skin over the metacarpophalangeal and interphalangeal joints and affects the skin between the joints (FIGURE 5).
- Violaceous papular dermatitis with scale—may occur in localized areas, such as elbows and knees, or be diffusely distributed, starting off as a patchy erythema that coalesces and becomes slightly raised with scale. It tends to be confined to sun-exposed areas and worsens after sun exposure (FIGURE 2).
- Periungual erythema and telangiectasia—“moth-eaten” cuticles, a characteristic seen in other connective tissue diseases (FIGURE 6).1
FIGURE 4
Papules on knuckles
FIGURE 5
Sparing interphalangeal joints
FIGURE 6
Cuticles with erythema
Differential diagnosis
Seborrheic dermatitis—white or yellow, greasy scales on an erythematous base with distribution on scalp, nasolabial folds and chest.
Atopic dermatitis—chronic history of pruritic papules or plaques with scale localized to flexural areas or may be generalized; lichenification may be seen.
Contact dermatitis—papules and vesicles that correspond to contact with allergen.
Polymorphous light eruption—clusters of erythematous, pruritic papules or vesicles occurring most frequently on the neck, anterior chest, arms, and forearms following sun exposure; most common among women in their twenties.
Lichen planus—pruritic, purple, polygonal papules (4 Ps) that may involve hair, nails, and mucous membranes in addition to the skin; more common among women, with onset between 30 to 60 years of age; may last months to years.
Psoriasis—well-demarcated papules and plaques on an erythematous base with thick, silvery scale; characteristically found on elbows, knees, scalp, nails, and genitalia.
Steroid myopathy—A side effect of systemic steroids, usually seen 4 to 6 weeks after beginning of treatment.
Dermatomyositis-like reaction—onset of similar skin findings with initiation of the following medications and improvement with discontinuation: penicillamine, nonsteroidal anti-inflammatory drugs, and carbamazepine.
Overlap syndrome—The term “overlap” denotes that certain signs are seen in both dermatomyositis and other connective tissue diseases such as scleroderma, rheumatoid arthritis, and lupus erythematosus. Scleroderma and dermatomyositis are the most commonly associated conditions and have been termed sclerodermatomyositis or mixed connective disease. In mixed connective tissue disease, features of SLE, scleroderma, and polymyositis are evident such as malar rash, alopecia, Raynaud’s phenomenon, waxy-appearing skin, and proximal muscle weakness.1,2
Diagnostic tests: Muscle enzymes, EMG, biopsy
The diagnosis of dermatomyositis is confirmed by 3 laboratory tests: elevated muscle enzyme levels, electromyography, and muscle biopsy. A punch biopsy is helpful in differentiating dermatomyositis from other papulosquamous diseases such as lichen planus and psoriasis, but be careful as the histology of dermatomyositis is indistinguishable from cutaneous lupus erythematosus.
During the acute active phase, the following serum muscle enzymes may be elevated: creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), and aldolase. CK is elevated among 65% of patients and is most specific for muscle disease.3 Only one of the aforementioned enzymes may be elevated, so it is necessary to measure them all.
Measuring antibodies such as antinuclear antibody (ANA), Jo-1, SSA (Ro), SSB (La) supports the diagnosis if positive but dermatomyositis cannot be diagnosed solely on positive titers. It is not necessary to obtain an electromyograph or muscle biopsy for a patient with the characteristic skin findings and evidence of elevated muscle enzymes, as the diagnosis of dermatomyositis can be made with confidence. For a patient in whom the presentation is not as straightforward, it may be useful to obtain the electromyograph and muscle biopsy.
Management: Corticosteroids, watch for malignancy
Oral corticosteroids are the treatment of choice (strength of recommendation [SOR]: B).1,4 Prednisone 0.5 to 1.0 mg/kg body weight per day has been recommended until muscle enzyme levels trend toward normal limits, at which time you can taper the dose. Steroid myopathy is a potential side effect of this treatment regimen; it may occur 4 to 6 weeks after therapy starts.
Several steroid-sparing agents such as methotrexate and cyclosporine are being prescribed by clinicians for dermomyositis but with little published evidence to support effectiveness. Methotrexate is an option for those who do not respond to prednisone or are in need of a steroid-sparing agent secondary to side effects (SOR: C).2,3 A suggested regimen starts with 7.5 to 10 mg/wk, then increases the dose to 2.5 mg/wk until reaching a total dose of 25 mg/wk. The dose of prednisone should be decreased as the methotrexate dose increases. Azathioprine is another option to consider along with methotrexate, but choosing one agent over another or a combination of 2 agents remains empirical. With any of the immunosuppressants or immunomodulatory agents, it is important to look at their side-effect profiles and monitor the patient accordingly.
After initiating treatment, look for evidence of malignancy among those patients older than 50 years so as not to miss an underlying cancer as the cause for their dermatomyositis. For women without any risk factors, a complete annual physical exam—including pelvic, breast, and rectal exam—is sufficient.2 It is not necessary to order expensive radiological studies blindly searching for malignancy, especially more than 2 years after the diagnosis is made. The greatest risk of malignancy occurs during the first year after diagnosis with a six-fold increase.1 The risk drops during the second year and a patient’s risk for malignancy is comparable to the normal population in the years following. A mammogram and colonoscopy might be indicated after considering the patient’s age and family history.
The patient’s treatment and outcome
The patient was started on 60 mg of oral prednisone, taken in a single daily dose. She also began physical therapy twice a week in order to prevent muscle atrophy and maximize function. She took the prednisone for 1 month, at which time her creatine kinase level was trending towards normal. We then began slowly tapering the prednisone over the next 6 months.
She reported improvement in her strength 3 months after starting the systemic steroids. Little improvement was seen in the patient’s skin while on systemic steroids, but after prescribing 0.1% triamcinolone ointment, recommending a broad-spectrum sunscreen, and limiting sun exposure, the patient reported less erythema and edema.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 48-year-old Hispanic woman came to the clinic as a new patient—her chief complaint was a rash that appeared on her face 3 months before and had recently spread to her chest and hands (FIGURES 1-3). It itched occasionally and seemed to worsen after exposure to the sun.
She also said that for the last month she had been feeling very weak—she had difficulty rising from a seated position and walking up the stairs to her apartment. She also felt as if her arms were heavy, making it difficult for her to brush and dry her hair in the morning.
The patient was otherwise healthy with no known medical conditions, and she was not taking any medications. Her family history was noncontributory.
A musculoskeletal examination showed the following:
Upper extremities:
- 4/5 strength shoulder abduction, internal and external rotation
- 5/5 strength biceps, triceps, wrist extension/flexion, grip
- 2+ biceps and triceps deep tendon reflexes bilaterally
Lower extremities:
- 4/5 strength hip flexors, quadriceps, hamstrings
- 5/5 dorsiflexion/plantar flexion
- 2+ patellar and ankle deep tendon reflexes bilaterally.
FIGURE 1
Facial rash and swelling
FIGURE 2
Rash spreading to chest
FIGURE 3
Plaques on knuckles
What is your diagnosis?
What diagnostic tests would you order for confirmation?
Diagnosis: Dermatomyositis
Dermatomyositis is a systemic disease classified as a type of idiopathic inflammatory myopathy. Dermatomyositis is a rare disease but one that may present initially to the family physician. It affects people at any age but is more commonly seen among children or adults aged >40 years.
Dermatomyositis involves the skin as well as skeletal muscle. Its cause is unknown; however, among those aged >50 years, malignancy may be an underlying cause. Cancers most commonly associated with dermatomyositis include those of the breast, ovary, lung, and gastrointestinal tract.
Skin manifestations may precede, follow, or present simultaneously with muscle involvement. Patients often complain of having difficulty ascending stairs, rising from a seated position, and performing overhead activities such as combing their hair. Patients may or may not have muscle tenderness and atrophy. Patients can have cutaneous involvement for more than a year before developing muscle weakness.1
The dermatologic signs of dermatomyositis to watch for:
- Periorbital heliotrope erythema, usually associated with edema (FIGURE 1).
- Gottron’s papules—smooth, purple to red papules located over the knuckles, on the sides of the fingers, and sometimes on the elbows and knees. For adults, it is not uncommon to have plaques over the knuckles as opposed to the classic Gottron’s papules (FIGURE 3). In juvenile-onset dermatomyositis, distinct papules are much more evident upon presentation (FIGURE 4). Note that systemic lupus erythematosus (SLE) can present with a rash on the dorsum of the hands, but the rash spares the skin over the metacarpophalangeal and interphalangeal joints and affects the skin between the joints (FIGURE 5).
- Violaceous papular dermatitis with scale—may occur in localized areas, such as elbows and knees, or be diffusely distributed, starting off as a patchy erythema that coalesces and becomes slightly raised with scale. It tends to be confined to sun-exposed areas and worsens after sun exposure (FIGURE 2).
- Periungual erythema and telangiectasia—“moth-eaten” cuticles, a characteristic seen in other connective tissue diseases (FIGURE 6).1
FIGURE 4
Papules on knuckles
FIGURE 5
Sparing interphalangeal joints
FIGURE 6
Cuticles with erythema
Differential diagnosis
Seborrheic dermatitis—white or yellow, greasy scales on an erythematous base with distribution on scalp, nasolabial folds and chest.
Atopic dermatitis—chronic history of pruritic papules or plaques with scale localized to flexural areas or may be generalized; lichenification may be seen.
Contact dermatitis—papules and vesicles that correspond to contact with allergen.
Polymorphous light eruption—clusters of erythematous, pruritic papules or vesicles occurring most frequently on the neck, anterior chest, arms, and forearms following sun exposure; most common among women in their twenties.
Lichen planus—pruritic, purple, polygonal papules (4 Ps) that may involve hair, nails, and mucous membranes in addition to the skin; more common among women, with onset between 30 to 60 years of age; may last months to years.
Psoriasis—well-demarcated papules and plaques on an erythematous base with thick, silvery scale; characteristically found on elbows, knees, scalp, nails, and genitalia.
Steroid myopathy—A side effect of systemic steroids, usually seen 4 to 6 weeks after beginning of treatment.
Dermatomyositis-like reaction—onset of similar skin findings with initiation of the following medications and improvement with discontinuation: penicillamine, nonsteroidal anti-inflammatory drugs, and carbamazepine.
Overlap syndrome—The term “overlap” denotes that certain signs are seen in both dermatomyositis and other connective tissue diseases such as scleroderma, rheumatoid arthritis, and lupus erythematosus. Scleroderma and dermatomyositis are the most commonly associated conditions and have been termed sclerodermatomyositis or mixed connective disease. In mixed connective tissue disease, features of SLE, scleroderma, and polymyositis are evident such as malar rash, alopecia, Raynaud’s phenomenon, waxy-appearing skin, and proximal muscle weakness.1,2
Diagnostic tests: Muscle enzymes, EMG, biopsy
The diagnosis of dermatomyositis is confirmed by 3 laboratory tests: elevated muscle enzyme levels, electromyography, and muscle biopsy. A punch biopsy is helpful in differentiating dermatomyositis from other papulosquamous diseases such as lichen planus and psoriasis, but be careful as the histology of dermatomyositis is indistinguishable from cutaneous lupus erythematosus.
During the acute active phase, the following serum muscle enzymes may be elevated: creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), and aldolase. CK is elevated among 65% of patients and is most specific for muscle disease.3 Only one of the aforementioned enzymes may be elevated, so it is necessary to measure them all.
Measuring antibodies such as antinuclear antibody (ANA), Jo-1, SSA (Ro), SSB (La) supports the diagnosis if positive but dermatomyositis cannot be diagnosed solely on positive titers. It is not necessary to obtain an electromyograph or muscle biopsy for a patient with the characteristic skin findings and evidence of elevated muscle enzymes, as the diagnosis of dermatomyositis can be made with confidence. For a patient in whom the presentation is not as straightforward, it may be useful to obtain the electromyograph and muscle biopsy.
Management: Corticosteroids, watch for malignancy
Oral corticosteroids are the treatment of choice (strength of recommendation [SOR]: B).1,4 Prednisone 0.5 to 1.0 mg/kg body weight per day has been recommended until muscle enzyme levels trend toward normal limits, at which time you can taper the dose. Steroid myopathy is a potential side effect of this treatment regimen; it may occur 4 to 6 weeks after therapy starts.
Several steroid-sparing agents such as methotrexate and cyclosporine are being prescribed by clinicians for dermomyositis but with little published evidence to support effectiveness. Methotrexate is an option for those who do not respond to prednisone or are in need of a steroid-sparing agent secondary to side effects (SOR: C).2,3 A suggested regimen starts with 7.5 to 10 mg/wk, then increases the dose to 2.5 mg/wk until reaching a total dose of 25 mg/wk. The dose of prednisone should be decreased as the methotrexate dose increases. Azathioprine is another option to consider along with methotrexate, but choosing one agent over another or a combination of 2 agents remains empirical. With any of the immunosuppressants or immunomodulatory agents, it is important to look at their side-effect profiles and monitor the patient accordingly.
After initiating treatment, look for evidence of malignancy among those patients older than 50 years so as not to miss an underlying cancer as the cause for their dermatomyositis. For women without any risk factors, a complete annual physical exam—including pelvic, breast, and rectal exam—is sufficient.2 It is not necessary to order expensive radiological studies blindly searching for malignancy, especially more than 2 years after the diagnosis is made. The greatest risk of malignancy occurs during the first year after diagnosis with a six-fold increase.1 The risk drops during the second year and a patient’s risk for malignancy is comparable to the normal population in the years following. A mammogram and colonoscopy might be indicated after considering the patient’s age and family history.
The patient’s treatment and outcome
The patient was started on 60 mg of oral prednisone, taken in a single daily dose. She also began physical therapy twice a week in order to prevent muscle atrophy and maximize function. She took the prednisone for 1 month, at which time her creatine kinase level was trending towards normal. We then began slowly tapering the prednisone over the next 6 months.
She reported improvement in her strength 3 months after starting the systemic steroids. Little improvement was seen in the patient’s skin while on systemic steroids, but after prescribing 0.1% triamcinolone ointment, recommending a broad-spectrum sunscreen, and limiting sun exposure, the patient reported less erythema and edema.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia: Mosby; 2004.
2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982.
3. Woff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
4. Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2005;Issue 3:CD003643.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia: Mosby; 2004.
2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982.
3. Woff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
4. Choy EHS, Hoogendijk JE, Lecky B, Winer JB. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2005;Issue 3:CD003643.
Red facial rash with “granitos”
A 56-year-old Hispanic woman came to the office distraught about the red rash on her face. The rash has been on her cheeks and nose for 5 years, but over the past 2 weeks she has developed red, mildly pruritic, and very tender papules that she calls granitos (the Spanish term for pimples). Foods do not make the rash better or worse, but it gets redder with sun exposure. She had not tried any lotions or sought medical attention until now, when she noticed that the papules were increasing in number but not size.
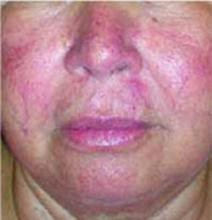
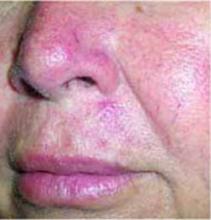
A few years ago she first noticed small blood vessels becoming more prominent, especially on her cheeks. The lesions have not bled or ulcerated. Her health has been generally good within the past year, with no recent infections. Review of systems indicated no changes in vision, upper respiratory illness, or systemic conditions. She is being treated for type 2 diabetes and hypertension. She does not have a family history of the same rash. FIGURES 1 AND 2 show erythema and telangiectasias distributed symmetrically on both cheeks and nose. A cluster of smooth papules were seen under the nose. No scales were noted.
FIGURE 1
Facial rash
FIGURE 2
Close-up
What is the diagnosis?
How would you treat this condition?
Diagnosis: Rosacea
The patient has rosacea. Rosacea is a common inflammatory skin condition characterized by erythema, edema, papules, pustules, or telangiectasias, most notably found on the concave portions of the cheeks, forehead, chin, and nose.1 Its peak incidence is between the ages of 30 and 50 years.
Because of the marked flushing due to inflammation and capillary hyperreactivity and dilation, the rash has brought a certain degree of social embarrassment for some patients. Moreover, some equate rosacea with excessive alcohol consumption, but this is simply not true.
Although rosacea is a chronic disorder, it does alternate between periods of flare-up and remission. Flares may be triggered by stress, sun exposure, heat, hot drinks and spicy foods, alcohol, exercise, wind, and hot baths. In 50% of all rosacea patients, there are some ocular symptoms, ranging from mild dryness and grittiness to blepharitis, conjunctivitis, and even keratitis.2
Differential diagnosis
Although the differential diagnosis of rosacea is wide—acne, folliculitis, sarcoid, seborrheic dermatitis, and systemic lupus erythematosus [SLE]—there are unique characteristics that can help distinguish these from rosacea.
For example, the age of onset for rosacea tends to be 30 to 50 years, much later than the onset for acne vulgaris. Similarly, in acne vulgaris comedones are often present, but they are absent in rosacea.
Seborrheic dermatitis tends to produce scales while rosacea does not. Moreover, while both seborrheic dermatitis and rosacea can affect the central facial area, papules and telangiectasias are absent in the former while present with the latter.
SLE can be scarring, does not produce papules or pustules, and it spares the nasolabial folds and nose. Whereas rosacea has a more central face distribution, folliculitis typically presents with pustules visibly surrounding hair follicles and has associated tenderness to palpation and sometimes severe pruritus.
Causes and pathophysiology of rosacea
Although the cause of rosacea is unknown, its mechanism is understood to be nonspecific inflammation followed by dilation around follicles and hyperreactive capillaries. Oftentimes these dilated capillaries present as telangiectasias, which collectively exacerbate the red flushing. As the disorder progresses, diffuse hypertrophy of the connective tissue and sebaceous glands ensues.
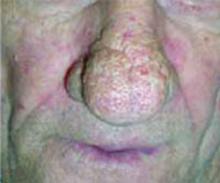
Rosacea is more common in women than men (FIGURE 3). Men are typically more prone to the extreme forms of hyperplasia, which causes rhinophymatous rosacea (FIGURE 4)—eg,W C Fields’s nose.
Alcohol may accentuate erythema, but does not cause the disease. Sun exposure may precipitate an acute rosacea flare, but flare-ups can happen without sun exposure.
A significant increase in the hair follicle mite Demadex folliculorum is sometimes found in rosacea. It is theorized that these mites play a role because they incite an inflammatory or allergic reaction by mechanical blockage of follicles.
FIGURE 3
Papulopustular rosacea
FIGURE 4
Rhinophymatous rosacea
Stages of rosacea
There are 4 stages or subtypes of rosacea.
- Subtype 1: Erythematotelangiectatic rosacea. This stage is characterized by frequent mild to severe blushing with trace persistent central facial erythema in an individual.
- Subtype 2: Papulopustular rosacea (seen in FIGURES 1 , 2, AND 3). This is a highly vascular stage that involves longer periods of flushing than the first stage—often lasting from days to weeks. Minute telangiectasias and papules start to form by this stage, and some patients begin having very mild ocular complaints such as ocular grittiness or conjunctivitis.
Subtype 3: Phymatous rosacea. The third stage is also called the rhinophymatous stage. It is characterized by deepening shades of erythema and more papules and pustules. In the chronic forms of rosacea, hyperplasia of the sebaceous glands occurs, which forms a thickened confluent plaque of erythema at the tip of the nose known as rhinophyma. This hyperplasia can cause significant disfigurement to the forehead, eyelids, chin, and nose. The nasal disfiguration is seen more commonly in men than women (FIGURE 4).
Subtype 4: Ocular rosacea.The final or fourth stage is an advanced variation of rosacea that is characterized by impressive, severe flushing with persistent telangiectasias, papules, and pustules. At this point, more severe forms of conjunctivitis and blepharitis are more fullblown. The patient may complain of watery eyes, a foreign body sensation, burning, dryness, vision changes, and lid or periocular erythema.3
Diagnosis and treatment
Diagnostic tests
The diagnosis of rosacea is a clinical one. There is no confirmatory laboratory test. Biopsy is warranted only to rule out alternative diagnoses, since histopathological findings are not diagnostic. Scrapings may reveal Demadex folliculorum infection.1
Treatment: Target the inflammation It is important to reassure patients about the benign nature of the disorder as well as explain that its cause is unknown. It may be useful to direct patients to information, such as web sites like those of the National Rosacea Society (www.rosacea.org). Advise patients to keep a daily diary to identify precipitating factors. These can include hot and humid weather, alcohol, hot beverages, spicy foods, and large hot meals. Suggest daily application of sunscreen, which protects against UVA and UVB rays.
Depending on the severity of the skin rosacea, the first-line treatment is an oral antibiotic (tetracycline or erythromycin) and/or topical metronidazole (0.75%–1.0%) twice daily. These antibiotics target the inflammation because rosacea is not a true infection. There is concern in the medical literature about how long-term use of antibiotics can promote drug resistance. Wolf et al4 proposed that once a patient’s lesions have improved after treatment with full-dose antibiotics, the clinician can consider switching to a lower dose and adding a topical agent such as metronidazole for maintenance.4 Studies have shown that 1.0% and 0.75% cream are equally effective.
TABLERosacea treatments
| TREATMENT | DELIVERY | ODDS RATIO* (MEDICATION VS PLACEBO) |
|---|---|---|
| Azelaic acid | Topical | 2.45 |
| Metronidazole | Topical | 5.96 |
| Tetracycline | Oral | 6.06 |
| * Larger number indicates a more effective response to treatment. | ||
Another useful therapy is topical azelaic acid. While the evidence for scabicides in rosacea is limited, some clinicians use these medications for rosacea that is refractory to antibiotics. This is based on the idea that the Demadex mite is a causative agent in rosacea. If a patient has severe papulopustular disease refractory to antibiotics and topical treatments, the physician can start an oral isotretinoin regimen at a low dose of 0.1 to 0.5 mg/kg. Pulse-dye laser and electrosurgery can be used to treat the telangiectasias associated with rosacea.
A systematic review by Van Zuuren et al5 examined the efficacy of metronidazole, tetracycline, and azelaic acid in treating rosacea. Twenty-nine randomized controlled trials were found. Pooled data from 2 of the trials involving 174 participants indicated that, according to the participants, topical metronidazole was more effective than placebo (odds ratio [OR]=5.96; 95% confidence interval [CI], 2.95–12.06).
There was a definite improvement in the azelaic acid group; the rates of treatment success were approximately 70 to 80% versus 50% to 55% (OR=2.45; 95% CI, 1.82–3.28). Data pooled from 3 studies of oral tetracycline vs placebo involving 152 participants showed that, according to physicians, tetracycline was more effective than placebo (OR=6.06; 95% CI, 2.96–12.42).
Maintenance therapy
Because relapse occurs within weeks in about 25% of patients after the cessation of systemic therapy, topical therapy is usually used in an effort to maintain remission.6 The required duration of maintenance therapy is unknown, but a period of 6 months is generally advised. After this time, some patients report that they can keep their skin free of papulopustular lesions with topical therapy applied on alternate days or twice weekly, whereas others require repeated courses of systemic medication. After a few years, the disease may disappear spontaneously.
Patient management
Our patient was counseled about her rosacea and given the web site address for the National Rosacea Organization. She was advised to avoid sun exposure as much as possible and to use sunscreen and a hat to protect her face. She was prescribed tetracycline 500 mg and topical metronidazole to be used twice daily. Follow-up was set for 1 month. At that time, patient will be offered the option of electrocoagulation of her most prominent telangiectasias.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Wolf K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
2. Randleman J, Loft E. Ocular rosacea. eMedicine website. Available at: www.emedicine.com/oph/topic115.htm. Accessed on August 2, 2005.
3. National Rosacea Organization Website. Available at rosacea.org/grading/gradingsystem.pdf. Accessed on August 2, 2005.
4. Wolf J, Parkerson R. Preventing antibiotic resistance in the treatment of rosacea. Fam Pract Recertification 2005;27:50-55.
5. Van Zuuren E, Graber M, Hollis S, Chaudhry M, Gupta A, Gover M. Interventions for rosacea. Cochrane Database Syst Rev 2005;(3):CD003262.-
6. Powell F. Rosacea. N Engl J Med 2005;352:793-803.
A 56-year-old Hispanic woman came to the office distraught about the red rash on her face. The rash has been on her cheeks and nose for 5 years, but over the past 2 weeks she has developed red, mildly pruritic, and very tender papules that she calls granitos (the Spanish term for pimples). Foods do not make the rash better or worse, but it gets redder with sun exposure. She had not tried any lotions or sought medical attention until now, when she noticed that the papules were increasing in number but not size.
A few years ago she first noticed small blood vessels becoming more prominent, especially on her cheeks. The lesions have not bled or ulcerated. Her health has been generally good within the past year, with no recent infections. Review of systems indicated no changes in vision, upper respiratory illness, or systemic conditions. She is being treated for type 2 diabetes and hypertension. She does not have a family history of the same rash. FIGURES 1 AND 2 show erythema and telangiectasias distributed symmetrically on both cheeks and nose. A cluster of smooth papules were seen under the nose. No scales were noted.
FIGURE 1
Facial rash
FIGURE 2
Close-up
What is the diagnosis?
How would you treat this condition?
Diagnosis: Rosacea
The patient has rosacea. Rosacea is a common inflammatory skin condition characterized by erythema, edema, papules, pustules, or telangiectasias, most notably found on the concave portions of the cheeks, forehead, chin, and nose.1 Its peak incidence is between the ages of 30 and 50 years.
Because of the marked flushing due to inflammation and capillary hyperreactivity and dilation, the rash has brought a certain degree of social embarrassment for some patients. Moreover, some equate rosacea with excessive alcohol consumption, but this is simply not true.
Although rosacea is a chronic disorder, it does alternate between periods of flare-up and remission. Flares may be triggered by stress, sun exposure, heat, hot drinks and spicy foods, alcohol, exercise, wind, and hot baths. In 50% of all rosacea patients, there are some ocular symptoms, ranging from mild dryness and grittiness to blepharitis, conjunctivitis, and even keratitis.2
Differential diagnosis
Although the differential diagnosis of rosacea is wide—acne, folliculitis, sarcoid, seborrheic dermatitis, and systemic lupus erythematosus [SLE]—there are unique characteristics that can help distinguish these from rosacea.
For example, the age of onset for rosacea tends to be 30 to 50 years, much later than the onset for acne vulgaris. Similarly, in acne vulgaris comedones are often present, but they are absent in rosacea.
Seborrheic dermatitis tends to produce scales while rosacea does not. Moreover, while both seborrheic dermatitis and rosacea can affect the central facial area, papules and telangiectasias are absent in the former while present with the latter.
SLE can be scarring, does not produce papules or pustules, and it spares the nasolabial folds and nose. Whereas rosacea has a more central face distribution, folliculitis typically presents with pustules visibly surrounding hair follicles and has associated tenderness to palpation and sometimes severe pruritus.
Causes and pathophysiology of rosacea
Although the cause of rosacea is unknown, its mechanism is understood to be nonspecific inflammation followed by dilation around follicles and hyperreactive capillaries. Oftentimes these dilated capillaries present as telangiectasias, which collectively exacerbate the red flushing. As the disorder progresses, diffuse hypertrophy of the connective tissue and sebaceous glands ensues.
Rosacea is more common in women than men (FIGURE 3). Men are typically more prone to the extreme forms of hyperplasia, which causes rhinophymatous rosacea (FIGURE 4)—eg,W C Fields’s nose.
Alcohol may accentuate erythema, but does not cause the disease. Sun exposure may precipitate an acute rosacea flare, but flare-ups can happen without sun exposure.
A significant increase in the hair follicle mite Demadex folliculorum is sometimes found in rosacea. It is theorized that these mites play a role because they incite an inflammatory or allergic reaction by mechanical blockage of follicles.
FIGURE 3
Papulopustular rosacea
FIGURE 4
Rhinophymatous rosacea
Stages of rosacea
There are 4 stages or subtypes of rosacea.
- Subtype 1: Erythematotelangiectatic rosacea. This stage is characterized by frequent mild to severe blushing with trace persistent central facial erythema in an individual.
- Subtype 2: Papulopustular rosacea (seen in FIGURES 1 , 2, AND 3). This is a highly vascular stage that involves longer periods of flushing than the first stage—often lasting from days to weeks. Minute telangiectasias and papules start to form by this stage, and some patients begin having very mild ocular complaints such as ocular grittiness or conjunctivitis.
Subtype 3: Phymatous rosacea. The third stage is also called the rhinophymatous stage. It is characterized by deepening shades of erythema and more papules and pustules. In the chronic forms of rosacea, hyperplasia of the sebaceous glands occurs, which forms a thickened confluent plaque of erythema at the tip of the nose known as rhinophyma. This hyperplasia can cause significant disfigurement to the forehead, eyelids, chin, and nose. The nasal disfiguration is seen more commonly in men than women (FIGURE 4).
Subtype 4: Ocular rosacea.The final or fourth stage is an advanced variation of rosacea that is characterized by impressive, severe flushing with persistent telangiectasias, papules, and pustules. At this point, more severe forms of conjunctivitis and blepharitis are more fullblown. The patient may complain of watery eyes, a foreign body sensation, burning, dryness, vision changes, and lid or periocular erythema.3
Diagnosis and treatment
Diagnostic tests
The diagnosis of rosacea is a clinical one. There is no confirmatory laboratory test. Biopsy is warranted only to rule out alternative diagnoses, since histopathological findings are not diagnostic. Scrapings may reveal Demadex folliculorum infection.1
Treatment: Target the inflammation It is important to reassure patients about the benign nature of the disorder as well as explain that its cause is unknown. It may be useful to direct patients to information, such as web sites like those of the National Rosacea Society (www.rosacea.org). Advise patients to keep a daily diary to identify precipitating factors. These can include hot and humid weather, alcohol, hot beverages, spicy foods, and large hot meals. Suggest daily application of sunscreen, which protects against UVA and UVB rays.
Depending on the severity of the skin rosacea, the first-line treatment is an oral antibiotic (tetracycline or erythromycin) and/or topical metronidazole (0.75%–1.0%) twice daily. These antibiotics target the inflammation because rosacea is not a true infection. There is concern in the medical literature about how long-term use of antibiotics can promote drug resistance. Wolf et al4 proposed that once a patient’s lesions have improved after treatment with full-dose antibiotics, the clinician can consider switching to a lower dose and adding a topical agent such as metronidazole for maintenance.4 Studies have shown that 1.0% and 0.75% cream are equally effective.
TABLERosacea treatments
| TREATMENT | DELIVERY | ODDS RATIO* (MEDICATION VS PLACEBO) |
|---|---|---|
| Azelaic acid | Topical | 2.45 |
| Metronidazole | Topical | 5.96 |
| Tetracycline | Oral | 6.06 |
| * Larger number indicates a more effective response to treatment. | ||
Another useful therapy is topical azelaic acid. While the evidence for scabicides in rosacea is limited, some clinicians use these medications for rosacea that is refractory to antibiotics. This is based on the idea that the Demadex mite is a causative agent in rosacea. If a patient has severe papulopustular disease refractory to antibiotics and topical treatments, the physician can start an oral isotretinoin regimen at a low dose of 0.1 to 0.5 mg/kg. Pulse-dye laser and electrosurgery can be used to treat the telangiectasias associated with rosacea.
A systematic review by Van Zuuren et al5 examined the efficacy of metronidazole, tetracycline, and azelaic acid in treating rosacea. Twenty-nine randomized controlled trials were found. Pooled data from 2 of the trials involving 174 participants indicated that, according to the participants, topical metronidazole was more effective than placebo (odds ratio [OR]=5.96; 95% confidence interval [CI], 2.95–12.06).
There was a definite improvement in the azelaic acid group; the rates of treatment success were approximately 70 to 80% versus 50% to 55% (OR=2.45; 95% CI, 1.82–3.28). Data pooled from 3 studies of oral tetracycline vs placebo involving 152 participants showed that, according to physicians, tetracycline was more effective than placebo (OR=6.06; 95% CI, 2.96–12.42).
Maintenance therapy
Because relapse occurs within weeks in about 25% of patients after the cessation of systemic therapy, topical therapy is usually used in an effort to maintain remission.6 The required duration of maintenance therapy is unknown, but a period of 6 months is generally advised. After this time, some patients report that they can keep their skin free of papulopustular lesions with topical therapy applied on alternate days or twice weekly, whereas others require repeated courses of systemic medication. After a few years, the disease may disappear spontaneously.
Patient management
Our patient was counseled about her rosacea and given the web site address for the National Rosacea Organization. She was advised to avoid sun exposure as much as possible and to use sunscreen and a hat to protect her face. She was prescribed tetracycline 500 mg and topical metronidazole to be used twice daily. Follow-up was set for 1 month. At that time, patient will be offered the option of electrocoagulation of her most prominent telangiectasias.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 56-year-old Hispanic woman came to the office distraught about the red rash on her face. The rash has been on her cheeks and nose for 5 years, but over the past 2 weeks she has developed red, mildly pruritic, and very tender papules that she calls granitos (the Spanish term for pimples). Foods do not make the rash better or worse, but it gets redder with sun exposure. She had not tried any lotions or sought medical attention until now, when she noticed that the papules were increasing in number but not size.
A few years ago she first noticed small blood vessels becoming more prominent, especially on her cheeks. The lesions have not bled or ulcerated. Her health has been generally good within the past year, with no recent infections. Review of systems indicated no changes in vision, upper respiratory illness, or systemic conditions. She is being treated for type 2 diabetes and hypertension. She does not have a family history of the same rash. FIGURES 1 AND 2 show erythema and telangiectasias distributed symmetrically on both cheeks and nose. A cluster of smooth papules were seen under the nose. No scales were noted.
FIGURE 1
Facial rash
FIGURE 2
Close-up
What is the diagnosis?
How would you treat this condition?
Diagnosis: Rosacea
The patient has rosacea. Rosacea is a common inflammatory skin condition characterized by erythema, edema, papules, pustules, or telangiectasias, most notably found on the concave portions of the cheeks, forehead, chin, and nose.1 Its peak incidence is between the ages of 30 and 50 years.
Because of the marked flushing due to inflammation and capillary hyperreactivity and dilation, the rash has brought a certain degree of social embarrassment for some patients. Moreover, some equate rosacea with excessive alcohol consumption, but this is simply not true.
Although rosacea is a chronic disorder, it does alternate between periods of flare-up and remission. Flares may be triggered by stress, sun exposure, heat, hot drinks and spicy foods, alcohol, exercise, wind, and hot baths. In 50% of all rosacea patients, there are some ocular symptoms, ranging from mild dryness and grittiness to blepharitis, conjunctivitis, and even keratitis.2
Differential diagnosis
Although the differential diagnosis of rosacea is wide—acne, folliculitis, sarcoid, seborrheic dermatitis, and systemic lupus erythematosus [SLE]—there are unique characteristics that can help distinguish these from rosacea.
For example, the age of onset for rosacea tends to be 30 to 50 years, much later than the onset for acne vulgaris. Similarly, in acne vulgaris comedones are often present, but they are absent in rosacea.
Seborrheic dermatitis tends to produce scales while rosacea does not. Moreover, while both seborrheic dermatitis and rosacea can affect the central facial area, papules and telangiectasias are absent in the former while present with the latter.
SLE can be scarring, does not produce papules or pustules, and it spares the nasolabial folds and nose. Whereas rosacea has a more central face distribution, folliculitis typically presents with pustules visibly surrounding hair follicles and has associated tenderness to palpation and sometimes severe pruritus.
Causes and pathophysiology of rosacea
Although the cause of rosacea is unknown, its mechanism is understood to be nonspecific inflammation followed by dilation around follicles and hyperreactive capillaries. Oftentimes these dilated capillaries present as telangiectasias, which collectively exacerbate the red flushing. As the disorder progresses, diffuse hypertrophy of the connective tissue and sebaceous glands ensues.
Rosacea is more common in women than men (FIGURE 3). Men are typically more prone to the extreme forms of hyperplasia, which causes rhinophymatous rosacea (FIGURE 4)—eg,W C Fields’s nose.
Alcohol may accentuate erythema, but does not cause the disease. Sun exposure may precipitate an acute rosacea flare, but flare-ups can happen without sun exposure.
A significant increase in the hair follicle mite Demadex folliculorum is sometimes found in rosacea. It is theorized that these mites play a role because they incite an inflammatory or allergic reaction by mechanical blockage of follicles.
FIGURE 3
Papulopustular rosacea
FIGURE 4
Rhinophymatous rosacea
Stages of rosacea
There are 4 stages or subtypes of rosacea.
- Subtype 1: Erythematotelangiectatic rosacea. This stage is characterized by frequent mild to severe blushing with trace persistent central facial erythema in an individual.
- Subtype 2: Papulopustular rosacea (seen in FIGURES 1 , 2, AND 3). This is a highly vascular stage that involves longer periods of flushing than the first stage—often lasting from days to weeks. Minute telangiectasias and papules start to form by this stage, and some patients begin having very mild ocular complaints such as ocular grittiness or conjunctivitis.
Subtype 3: Phymatous rosacea. The third stage is also called the rhinophymatous stage. It is characterized by deepening shades of erythema and more papules and pustules. In the chronic forms of rosacea, hyperplasia of the sebaceous glands occurs, which forms a thickened confluent plaque of erythema at the tip of the nose known as rhinophyma. This hyperplasia can cause significant disfigurement to the forehead, eyelids, chin, and nose. The nasal disfiguration is seen more commonly in men than women (FIGURE 4).
Subtype 4: Ocular rosacea.The final or fourth stage is an advanced variation of rosacea that is characterized by impressive, severe flushing with persistent telangiectasias, papules, and pustules. At this point, more severe forms of conjunctivitis and blepharitis are more fullblown. The patient may complain of watery eyes, a foreign body sensation, burning, dryness, vision changes, and lid or periocular erythema.3
Diagnosis and treatment
Diagnostic tests
The diagnosis of rosacea is a clinical one. There is no confirmatory laboratory test. Biopsy is warranted only to rule out alternative diagnoses, since histopathological findings are not diagnostic. Scrapings may reveal Demadex folliculorum infection.1
Treatment: Target the inflammation It is important to reassure patients about the benign nature of the disorder as well as explain that its cause is unknown. It may be useful to direct patients to information, such as web sites like those of the National Rosacea Society (www.rosacea.org). Advise patients to keep a daily diary to identify precipitating factors. These can include hot and humid weather, alcohol, hot beverages, spicy foods, and large hot meals. Suggest daily application of sunscreen, which protects against UVA and UVB rays.
Depending on the severity of the skin rosacea, the first-line treatment is an oral antibiotic (tetracycline or erythromycin) and/or topical metronidazole (0.75%–1.0%) twice daily. These antibiotics target the inflammation because rosacea is not a true infection. There is concern in the medical literature about how long-term use of antibiotics can promote drug resistance. Wolf et al4 proposed that once a patient’s lesions have improved after treatment with full-dose antibiotics, the clinician can consider switching to a lower dose and adding a topical agent such as metronidazole for maintenance.4 Studies have shown that 1.0% and 0.75% cream are equally effective.
TABLERosacea treatments
| TREATMENT | DELIVERY | ODDS RATIO* (MEDICATION VS PLACEBO) |
|---|---|---|
| Azelaic acid | Topical | 2.45 |
| Metronidazole | Topical | 5.96 |
| Tetracycline | Oral | 6.06 |
| * Larger number indicates a more effective response to treatment. | ||
Another useful therapy is topical azelaic acid. While the evidence for scabicides in rosacea is limited, some clinicians use these medications for rosacea that is refractory to antibiotics. This is based on the idea that the Demadex mite is a causative agent in rosacea. If a patient has severe papulopustular disease refractory to antibiotics and topical treatments, the physician can start an oral isotretinoin regimen at a low dose of 0.1 to 0.5 mg/kg. Pulse-dye laser and electrosurgery can be used to treat the telangiectasias associated with rosacea.
A systematic review by Van Zuuren et al5 examined the efficacy of metronidazole, tetracycline, and azelaic acid in treating rosacea. Twenty-nine randomized controlled trials were found. Pooled data from 2 of the trials involving 174 participants indicated that, according to the participants, topical metronidazole was more effective than placebo (odds ratio [OR]=5.96; 95% confidence interval [CI], 2.95–12.06).
There was a definite improvement in the azelaic acid group; the rates of treatment success were approximately 70 to 80% versus 50% to 55% (OR=2.45; 95% CI, 1.82–3.28). Data pooled from 3 studies of oral tetracycline vs placebo involving 152 participants showed that, according to physicians, tetracycline was more effective than placebo (OR=6.06; 95% CI, 2.96–12.42).
Maintenance therapy
Because relapse occurs within weeks in about 25% of patients after the cessation of systemic therapy, topical therapy is usually used in an effort to maintain remission.6 The required duration of maintenance therapy is unknown, but a period of 6 months is generally advised. After this time, some patients report that they can keep their skin free of papulopustular lesions with topical therapy applied on alternate days or twice weekly, whereas others require repeated courses of systemic medication. After a few years, the disease may disappear spontaneously.
Patient management
Our patient was counseled about her rosacea and given the web site address for the National Rosacea Organization. She was advised to avoid sun exposure as much as possible and to use sunscreen and a hat to protect her face. She was prescribed tetracycline 500 mg and topical metronidazole to be used twice daily. Follow-up was set for 1 month. At that time, patient will be offered the option of electrocoagulation of her most prominent telangiectasias.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Wolf K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
2. Randleman J, Loft E. Ocular rosacea. eMedicine website. Available at: www.emedicine.com/oph/topic115.htm. Accessed on August 2, 2005.
3. National Rosacea Organization Website. Available at rosacea.org/grading/gradingsystem.pdf. Accessed on August 2, 2005.
4. Wolf J, Parkerson R. Preventing antibiotic resistance in the treatment of rosacea. Fam Pract Recertification 2005;27:50-55.
5. Van Zuuren E, Graber M, Hollis S, Chaudhry M, Gupta A, Gover M. Interventions for rosacea. Cochrane Database Syst Rev 2005;(3):CD003262.-
6. Powell F. Rosacea. N Engl J Med 2005;352:793-803.
1. Wolf K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York: McGraw-Hill; 2005.
2. Randleman J, Loft E. Ocular rosacea. eMedicine website. Available at: www.emedicine.com/oph/topic115.htm. Accessed on August 2, 2005.
3. National Rosacea Organization Website. Available at rosacea.org/grading/gradingsystem.pdf. Accessed on August 2, 2005.
4. Wolf J, Parkerson R. Preventing antibiotic resistance in the treatment of rosacea. Fam Pract Recertification 2005;27:50-55.
5. Van Zuuren E, Graber M, Hollis S, Chaudhry M, Gupta A, Gover M. Interventions for rosacea. Cochrane Database Syst Rev 2005;(3):CD003262.-
6. Powell F. Rosacea. N Engl J Med 2005;352:793-803.
Abdominal pain in a pregnant woman
A 24-year-old woman, pregnant with a fetus at 22 weeks gestational age, came to the OB triage area with abdominal pain, nausea, and vomiting. She described a sharp pain that began the night before, starting at the umbilicus and radiating toward her right side; she rated it 7 out of 10.
The patient said there had been no contractions, vaginal bleeding or fluid leaking, or dysuria. She reported having GERD at times. She experienced chills the day before, but no fever. She had similar pain 1 month before that resolved spontaneously, and for which a cause was never determined. She had nothing significant in her medical history; family history was noncontributory.
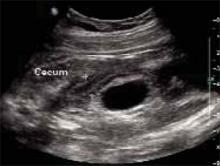
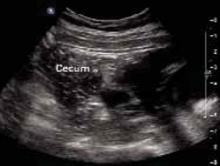
On examination, she was afebrile, normotensive, and in no apparent distress. Her heart and lungs were normal. Her abdomen was soft and gravid with a fundal height of 22 cm. Bowel sounds were present in all 4 quadrants. Fetal heart tones were normal, and there was no indication of contractions. Her abdomen was diffusely tender, with significant tenderness to deep palpation in the right upper quadrant at first. There was no rebound or guarding. The psoas sign was negative. The obturator sign was positive, with increased pain 4 out of 10 in the right lower quadrant. There were no abdominal masses. Digital rectal examination revealed no rectal masses, and a guaiac stool test result was negative. A few hours later, the tenderness seemed to move toward the right lower quadrant (FIGURES 1 AND 2).
What is the most likely diagnosis?
How do the ultrasound images help you make the diagnosis?
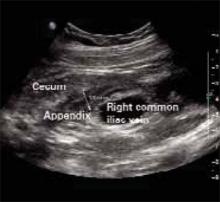
FIGURE 1
Ultrasound of RLQ
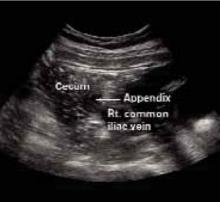
FIGURE 2
A second ultrasound of RLQ
Differential diagnosis
The differential diagnosis of abdominal pain in a gravid patient includes placental abruption, cholecystitis, pancreatitis, appendicitis, intussusception, pyelonephritis, round ligament syndrome, hydronephrosis, ovarian torsion, uterine fibroid degeneration, ovarian cysts or tumors, intra-abdominal and rectus muscle abscesses, and Crohn’s disease with diffuse peritoneal inflammation. Given the location of the pain and the lack of vaginal bleeding, the most likely diagnoses are cholecystitis and appendicitis.
Making the diagnosis
We performed several laboratory analyses, including a complete blood count, chemistry panel (including electrolytes and liver function studies), amylase, lipase, and a urinalysis. The test results were all normal. She had a white blood cell count of 15,000/μL, which can be normal in pregnancy. The initial evaluating physician had obtained a right upper quadrant ultrasound, which showed no gallstones or bilateral hydronephrosis; unfortunately, no attempt was made to visualize the right lower quadrant or appendix at that time.
In light of the physical exam findings and the absence of gallstones, the patient was admitted to rule out appendicitis. The surgery team at the university hospital was consulted. They requested a computed tomography (CT) scan of the abdomen with and without contrast. To avoid the risk of radiation to the fetus, the family medicine team spoke with Radiology to obtain another ultrasound.
The ultrasound showed an enlarged and inflamed appendix with a transverse diameter of 13 mm (normal is <6 mm) (FIGURES 3 AND 4).1 A graded compression technique was used to assess the appendix. This involves using pressure of the ultrasound probe starting above the area of tenderness and working toward the tender area while scanning for the appendix. This showed obvious peristalsis in the cecum and no movement within the appendix, indicating obstruction or inflammation.
FIGURE 3
Appendix: Longitudinal view
FIGURE 4
Appendix: Transverse view
Patient management and outcome
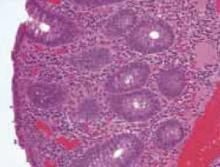
An open appendectomy was performed. The appendix was inflamed and enlarged as suspected. The histology showed neutrophilic infiltration of mucosa, muscle, and serosa (FIGURE 5). Postoperatively, the patient recovered in Labor and Delivery to monitor for possible preterm labor. She did not develop any signs or symptoms of preterm labor, and was transferred to a regular antepartum floor after being observed for 6 hours.
She did well during her hospitalization, and was sent home on post-op day 2. Her abdominal pain had resolved, and she had very little post-op tenderness.
FIGURE 5
Histology
Discussion: Appendicitis in pregnancy
Acute appendicitis is the most common condition requiring surgery during pregnancy.2 Suspected appendicitis accounts for nearly two thirds of all nonobstetric exploratory laparotomies performed during pregnancy; most cases occur in the second and third trimesters.
The incidence of appendicitis is 0.4 to 1.4 per 1000 pregnancies.2 Although the incidence of appendicitis in not increased during pregnancy, rupture of the appendix occurs 2 to 3 times more frequently in pregnancy secondary to delays in diagnosis and operation. Maternal and perinatal mortality and morbidity rates are greatly increased when appendicitis is complicated by peritonitis.
A difficult diagnosis
Diagnosis is difficult because many symptoms are considered to be normal during pregnancy. Many times, pain in the right lower quadrant of the abdomen may be attributed to round ligament pain or urinary tract infection. After the first trimester, the appendix is gradually displaced above McBurney’s point, with horizontal rotation of its base. This upward displacement occurs until the eighth month of gestation, when more than 90% of appendices lie above the iliac crest, and 80% rotate upward and toward the right subcostal area.2,3
The most consistent clinical symptom encountered in pregnant women with appendicitis is vague right-sided abdominal pain.2 Depending on the gestation, muscle guarding and rebound tenderness may or may not be present. Nausea, vomiting, and anorexia are usually present as in the nonpregnant patient. Twenty five percent of pregnant patients with appendicitis are afebrile, as our patient was.2,4
The leukocytosis of pregnancy makes it difficult to determine if there is an infection. Not all pregnant patients with appendicitis will have a white blood cell count greater than 16,000/μL, but approximately 75% of them will have a left shift in the differential.2 A urinalysis may reveal pyuria and hematuria and can mislead the physician to explain the symptoms as pyelonephritis.2
Treatment: Appendectomy, antibiotics if needed
Treatment of nonperforated acute appendicitis in pregnancy is appendectomy. In the first trimester, a laparoscopic appendectomy may be performed.2 Intravenous antibiotics are indicated with perforation, peritonitis or abscess formation.2,5
Tocolysis is unnecessary in uncomplicated appendicitis, but may be indicated if the patient goes into labor after surgery. In the late third trimester, with perforation or peritonitis, a cesarean section is indicated.
Evaluation is imperative
Fetal loss may occur in association with preterm labor and delivery or with generalized peritonitis and sepsis, and occurs only rarely in uncomplicated appendicitis. Fetal loss appears to be more closely associated with severity of appendicitis than with surgical intervention.2,5,6
Imaging test characteristics: Is sonography enough?
Thus, it is imperative that any pregnant patient that comes in to the hospital or clinic with abdominal pain be evaluated for appendicitis. Ultrasound was a valuable diagnostic tool in this case and saved both the patient and developing fetus the radiation exposure of a CT scan. Ultrasound has a high specificity for diagnosing appendicitis if the appendix is visualized with abnormal findings. However, the sensitivity is not as high as CT, and failure to visualize the appendix adequately would have required a decision between appendectomy on clinical grounds only or going through with the CT scan.
The sensitivity, specificity, and positive and negative predictive values for ultrasonography and CT scans in the diagnosis of appendicitis are given in the TABLE (level of evidence [LOE]=1a).7
In a prospective study of patients with clinical signs and symptoms of acute appendicitis using a graded compression technique of ultrasonography, sonographic testing was as accurate as the focused unenhanced single-detector helical CT. The primary sonographic criterion for diagnosing acute appendicitis was an incompressible appendix with a transverse outer diameter of 6 mm or larger, as seen in this patient. The sensitivity of CT and sonography was 76% and 79%, respectively; the specificity was 83% and 78%; the accuracy was 78% and 78%; the positive predictive value was 90% and 87%; and the negative predictive value was 64% and 65% (LOE=2a).8
In conclusion, it is reasonable to use graded compression ultrasonography in a pregnant woman with suspected appendicitis. If the suspicion for appendicitis is high, a negative result may still need further evaluation with a CT or ultimately lead to abdominal surgery despite negative imaging studies.
TABLEUltrasound and CT in the diagnosis of appendicitis
| TEST | SN | SP | LR+ | LR– |
|---|---|---|---|---|
| Ultrasound | 0.86 (0.83–0.88) | 0.81 (0.78–0.84) | 5.8 (3.5–9.5) | .019 (0.13–0.27) |
| CT | 0.94 (0.91–0.95) | 0.95 (0.93–0.96) | 13.3 (9.9–17.9) | 0.09 (0.07–0.12) |
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; CT, computed tomography. | ||||
| Source: Teresawa et al, Ann Intern Med 2004.7 | ||||
Corresponding Author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Hansen GC, Toot PJ, Lynch CO. Subtle ultrasound signs of appendicitis in pregnancy. A case report. J Reprod Med 1993;3:223-224.
2. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571-576.
3. Hodjati H, Kazerooni T. Location of the appendix in the gravid patient: a re-evaluation of the established concept. Int J Gynecol Obstet 2003;81:245-247.
4. Morad JDO, Elliott JP, Lisboa L. Appendicitis in pregnancy:new information that contradicts long held clinical beliefs. Am J Obstet Gynecol 2002;182:1027-1029.
5. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999;78:758-762.
6. Thurnau GR, Hales KA. Appendicitis in pregnancy. Female Patient 1992;17:81.
7. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537-546.
8. Poortman P, Lohle PN, Schoemaker CM, et al. Comparison of CT and sonography in the diagnosis of acute appendicitis: a blinded prospective study. AJR Am J Roentgenol 2003;181:1355-1359.
A 24-year-old woman, pregnant with a fetus at 22 weeks gestational age, came to the OB triage area with abdominal pain, nausea, and vomiting. She described a sharp pain that began the night before, starting at the umbilicus and radiating toward her right side; she rated it 7 out of 10.
The patient said there had been no contractions, vaginal bleeding or fluid leaking, or dysuria. She reported having GERD at times. She experienced chills the day before, but no fever. She had similar pain 1 month before that resolved spontaneously, and for which a cause was never determined. She had nothing significant in her medical history; family history was noncontributory.
On examination, she was afebrile, normotensive, and in no apparent distress. Her heart and lungs were normal. Her abdomen was soft and gravid with a fundal height of 22 cm. Bowel sounds were present in all 4 quadrants. Fetal heart tones were normal, and there was no indication of contractions. Her abdomen was diffusely tender, with significant tenderness to deep palpation in the right upper quadrant at first. There was no rebound or guarding. The psoas sign was negative. The obturator sign was positive, with increased pain 4 out of 10 in the right lower quadrant. There were no abdominal masses. Digital rectal examination revealed no rectal masses, and a guaiac stool test result was negative. A few hours later, the tenderness seemed to move toward the right lower quadrant (FIGURES 1 AND 2).
What is the most likely diagnosis?
How do the ultrasound images help you make the diagnosis?
FIGURE 1
Ultrasound of RLQ
FIGURE 2
A second ultrasound of RLQ
Differential diagnosis
The differential diagnosis of abdominal pain in a gravid patient includes placental abruption, cholecystitis, pancreatitis, appendicitis, intussusception, pyelonephritis, round ligament syndrome, hydronephrosis, ovarian torsion, uterine fibroid degeneration, ovarian cysts or tumors, intra-abdominal and rectus muscle abscesses, and Crohn’s disease with diffuse peritoneal inflammation. Given the location of the pain and the lack of vaginal bleeding, the most likely diagnoses are cholecystitis and appendicitis.
Making the diagnosis
We performed several laboratory analyses, including a complete blood count, chemistry panel (including electrolytes and liver function studies), amylase, lipase, and a urinalysis. The test results were all normal. She had a white blood cell count of 15,000/μL, which can be normal in pregnancy. The initial evaluating physician had obtained a right upper quadrant ultrasound, which showed no gallstones or bilateral hydronephrosis; unfortunately, no attempt was made to visualize the right lower quadrant or appendix at that time.
In light of the physical exam findings and the absence of gallstones, the patient was admitted to rule out appendicitis. The surgery team at the university hospital was consulted. They requested a computed tomography (CT) scan of the abdomen with and without contrast. To avoid the risk of radiation to the fetus, the family medicine team spoke with Radiology to obtain another ultrasound.
The ultrasound showed an enlarged and inflamed appendix with a transverse diameter of 13 mm (normal is <6 mm) (FIGURES 3 AND 4).1 A graded compression technique was used to assess the appendix. This involves using pressure of the ultrasound probe starting above the area of tenderness and working toward the tender area while scanning for the appendix. This showed obvious peristalsis in the cecum and no movement within the appendix, indicating obstruction or inflammation.
FIGURE 3
Appendix: Longitudinal view
FIGURE 4
Appendix: Transverse view
Patient management and outcome
An open appendectomy was performed. The appendix was inflamed and enlarged as suspected. The histology showed neutrophilic infiltration of mucosa, muscle, and serosa (FIGURE 5). Postoperatively, the patient recovered in Labor and Delivery to monitor for possible preterm labor. She did not develop any signs or symptoms of preterm labor, and was transferred to a regular antepartum floor after being observed for 6 hours.
She did well during her hospitalization, and was sent home on post-op day 2. Her abdominal pain had resolved, and she had very little post-op tenderness.
FIGURE 5
Histology
Discussion: Appendicitis in pregnancy
Acute appendicitis is the most common condition requiring surgery during pregnancy.2 Suspected appendicitis accounts for nearly two thirds of all nonobstetric exploratory laparotomies performed during pregnancy; most cases occur in the second and third trimesters.
The incidence of appendicitis is 0.4 to 1.4 per 1000 pregnancies.2 Although the incidence of appendicitis in not increased during pregnancy, rupture of the appendix occurs 2 to 3 times more frequently in pregnancy secondary to delays in diagnosis and operation. Maternal and perinatal mortality and morbidity rates are greatly increased when appendicitis is complicated by peritonitis.
A difficult diagnosis
Diagnosis is difficult because many symptoms are considered to be normal during pregnancy. Many times, pain in the right lower quadrant of the abdomen may be attributed to round ligament pain or urinary tract infection. After the first trimester, the appendix is gradually displaced above McBurney’s point, with horizontal rotation of its base. This upward displacement occurs until the eighth month of gestation, when more than 90% of appendices lie above the iliac crest, and 80% rotate upward and toward the right subcostal area.2,3
The most consistent clinical symptom encountered in pregnant women with appendicitis is vague right-sided abdominal pain.2 Depending on the gestation, muscle guarding and rebound tenderness may or may not be present. Nausea, vomiting, and anorexia are usually present as in the nonpregnant patient. Twenty five percent of pregnant patients with appendicitis are afebrile, as our patient was.2,4
The leukocytosis of pregnancy makes it difficult to determine if there is an infection. Not all pregnant patients with appendicitis will have a white blood cell count greater than 16,000/μL, but approximately 75% of them will have a left shift in the differential.2 A urinalysis may reveal pyuria and hematuria and can mislead the physician to explain the symptoms as pyelonephritis.2
Treatment: Appendectomy, antibiotics if needed
Treatment of nonperforated acute appendicitis in pregnancy is appendectomy. In the first trimester, a laparoscopic appendectomy may be performed.2 Intravenous antibiotics are indicated with perforation, peritonitis or abscess formation.2,5
Tocolysis is unnecessary in uncomplicated appendicitis, but may be indicated if the patient goes into labor after surgery. In the late third trimester, with perforation or peritonitis, a cesarean section is indicated.
Evaluation is imperative
Fetal loss may occur in association with preterm labor and delivery or with generalized peritonitis and sepsis, and occurs only rarely in uncomplicated appendicitis. Fetal loss appears to be more closely associated with severity of appendicitis than with surgical intervention.2,5,6
Imaging test characteristics: Is sonography enough?
Thus, it is imperative that any pregnant patient that comes in to the hospital or clinic with abdominal pain be evaluated for appendicitis. Ultrasound was a valuable diagnostic tool in this case and saved both the patient and developing fetus the radiation exposure of a CT scan. Ultrasound has a high specificity for diagnosing appendicitis if the appendix is visualized with abnormal findings. However, the sensitivity is not as high as CT, and failure to visualize the appendix adequately would have required a decision between appendectomy on clinical grounds only or going through with the CT scan.
The sensitivity, specificity, and positive and negative predictive values for ultrasonography and CT scans in the diagnosis of appendicitis are given in the TABLE (level of evidence [LOE]=1a).7
In a prospective study of patients with clinical signs and symptoms of acute appendicitis using a graded compression technique of ultrasonography, sonographic testing was as accurate as the focused unenhanced single-detector helical CT. The primary sonographic criterion for diagnosing acute appendicitis was an incompressible appendix with a transverse outer diameter of 6 mm or larger, as seen in this patient. The sensitivity of CT and sonography was 76% and 79%, respectively; the specificity was 83% and 78%; the accuracy was 78% and 78%; the positive predictive value was 90% and 87%; and the negative predictive value was 64% and 65% (LOE=2a).8
In conclusion, it is reasonable to use graded compression ultrasonography in a pregnant woman with suspected appendicitis. If the suspicion for appendicitis is high, a negative result may still need further evaluation with a CT or ultimately lead to abdominal surgery despite negative imaging studies.
TABLEUltrasound and CT in the diagnosis of appendicitis
| TEST | SN | SP | LR+ | LR– |
|---|---|---|---|---|
| Ultrasound | 0.86 (0.83–0.88) | 0.81 (0.78–0.84) | 5.8 (3.5–9.5) | .019 (0.13–0.27) |
| CT | 0.94 (0.91–0.95) | 0.95 (0.93–0.96) | 13.3 (9.9–17.9) | 0.09 (0.07–0.12) |
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; CT, computed tomography. | ||||
| Source: Teresawa et al, Ann Intern Med 2004.7 | ||||
Corresponding Author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 24-year-old woman, pregnant with a fetus at 22 weeks gestational age, came to the OB triage area with abdominal pain, nausea, and vomiting. She described a sharp pain that began the night before, starting at the umbilicus and radiating toward her right side; she rated it 7 out of 10.
The patient said there had been no contractions, vaginal bleeding or fluid leaking, or dysuria. She reported having GERD at times. She experienced chills the day before, but no fever. She had similar pain 1 month before that resolved spontaneously, and for which a cause was never determined. She had nothing significant in her medical history; family history was noncontributory.
On examination, she was afebrile, normotensive, and in no apparent distress. Her heart and lungs were normal. Her abdomen was soft and gravid with a fundal height of 22 cm. Bowel sounds were present in all 4 quadrants. Fetal heart tones were normal, and there was no indication of contractions. Her abdomen was diffusely tender, with significant tenderness to deep palpation in the right upper quadrant at first. There was no rebound or guarding. The psoas sign was negative. The obturator sign was positive, with increased pain 4 out of 10 in the right lower quadrant. There were no abdominal masses. Digital rectal examination revealed no rectal masses, and a guaiac stool test result was negative. A few hours later, the tenderness seemed to move toward the right lower quadrant (FIGURES 1 AND 2).
What is the most likely diagnosis?
How do the ultrasound images help you make the diagnosis?
FIGURE 1
Ultrasound of RLQ
FIGURE 2
A second ultrasound of RLQ
Differential diagnosis
The differential diagnosis of abdominal pain in a gravid patient includes placental abruption, cholecystitis, pancreatitis, appendicitis, intussusception, pyelonephritis, round ligament syndrome, hydronephrosis, ovarian torsion, uterine fibroid degeneration, ovarian cysts or tumors, intra-abdominal and rectus muscle abscesses, and Crohn’s disease with diffuse peritoneal inflammation. Given the location of the pain and the lack of vaginal bleeding, the most likely diagnoses are cholecystitis and appendicitis.
Making the diagnosis
We performed several laboratory analyses, including a complete blood count, chemistry panel (including electrolytes and liver function studies), amylase, lipase, and a urinalysis. The test results were all normal. She had a white blood cell count of 15,000/μL, which can be normal in pregnancy. The initial evaluating physician had obtained a right upper quadrant ultrasound, which showed no gallstones or bilateral hydronephrosis; unfortunately, no attempt was made to visualize the right lower quadrant or appendix at that time.
In light of the physical exam findings and the absence of gallstones, the patient was admitted to rule out appendicitis. The surgery team at the university hospital was consulted. They requested a computed tomography (CT) scan of the abdomen with and without contrast. To avoid the risk of radiation to the fetus, the family medicine team spoke with Radiology to obtain another ultrasound.
The ultrasound showed an enlarged and inflamed appendix with a transverse diameter of 13 mm (normal is <6 mm) (FIGURES 3 AND 4).1 A graded compression technique was used to assess the appendix. This involves using pressure of the ultrasound probe starting above the area of tenderness and working toward the tender area while scanning for the appendix. This showed obvious peristalsis in the cecum and no movement within the appendix, indicating obstruction or inflammation.
FIGURE 3
Appendix: Longitudinal view
FIGURE 4
Appendix: Transverse view
Patient management and outcome
An open appendectomy was performed. The appendix was inflamed and enlarged as suspected. The histology showed neutrophilic infiltration of mucosa, muscle, and serosa (FIGURE 5). Postoperatively, the patient recovered in Labor and Delivery to monitor for possible preterm labor. She did not develop any signs or symptoms of preterm labor, and was transferred to a regular antepartum floor after being observed for 6 hours.
She did well during her hospitalization, and was sent home on post-op day 2. Her abdominal pain had resolved, and she had very little post-op tenderness.
FIGURE 5
Histology
Discussion: Appendicitis in pregnancy
Acute appendicitis is the most common condition requiring surgery during pregnancy.2 Suspected appendicitis accounts for nearly two thirds of all nonobstetric exploratory laparotomies performed during pregnancy; most cases occur in the second and third trimesters.
The incidence of appendicitis is 0.4 to 1.4 per 1000 pregnancies.2 Although the incidence of appendicitis in not increased during pregnancy, rupture of the appendix occurs 2 to 3 times more frequently in pregnancy secondary to delays in diagnosis and operation. Maternal and perinatal mortality and morbidity rates are greatly increased when appendicitis is complicated by peritonitis.
A difficult diagnosis
Diagnosis is difficult because many symptoms are considered to be normal during pregnancy. Many times, pain in the right lower quadrant of the abdomen may be attributed to round ligament pain or urinary tract infection. After the first trimester, the appendix is gradually displaced above McBurney’s point, with horizontal rotation of its base. This upward displacement occurs until the eighth month of gestation, when more than 90% of appendices lie above the iliac crest, and 80% rotate upward and toward the right subcostal area.2,3
The most consistent clinical symptom encountered in pregnant women with appendicitis is vague right-sided abdominal pain.2 Depending on the gestation, muscle guarding and rebound tenderness may or may not be present. Nausea, vomiting, and anorexia are usually present as in the nonpregnant patient. Twenty five percent of pregnant patients with appendicitis are afebrile, as our patient was.2,4
The leukocytosis of pregnancy makes it difficult to determine if there is an infection. Not all pregnant patients with appendicitis will have a white blood cell count greater than 16,000/μL, but approximately 75% of them will have a left shift in the differential.2 A urinalysis may reveal pyuria and hematuria and can mislead the physician to explain the symptoms as pyelonephritis.2
Treatment: Appendectomy, antibiotics if needed
Treatment of nonperforated acute appendicitis in pregnancy is appendectomy. In the first trimester, a laparoscopic appendectomy may be performed.2 Intravenous antibiotics are indicated with perforation, peritonitis or abscess formation.2,5
Tocolysis is unnecessary in uncomplicated appendicitis, but may be indicated if the patient goes into labor after surgery. In the late third trimester, with perforation or peritonitis, a cesarean section is indicated.
Evaluation is imperative
Fetal loss may occur in association with preterm labor and delivery or with generalized peritonitis and sepsis, and occurs only rarely in uncomplicated appendicitis. Fetal loss appears to be more closely associated with severity of appendicitis than with surgical intervention.2,5,6
Imaging test characteristics: Is sonography enough?
Thus, it is imperative that any pregnant patient that comes in to the hospital or clinic with abdominal pain be evaluated for appendicitis. Ultrasound was a valuable diagnostic tool in this case and saved both the patient and developing fetus the radiation exposure of a CT scan. Ultrasound has a high specificity for diagnosing appendicitis if the appendix is visualized with abnormal findings. However, the sensitivity is not as high as CT, and failure to visualize the appendix adequately would have required a decision between appendectomy on clinical grounds only or going through with the CT scan.
The sensitivity, specificity, and positive and negative predictive values for ultrasonography and CT scans in the diagnosis of appendicitis are given in the TABLE (level of evidence [LOE]=1a).7
In a prospective study of patients with clinical signs and symptoms of acute appendicitis using a graded compression technique of ultrasonography, sonographic testing was as accurate as the focused unenhanced single-detector helical CT. The primary sonographic criterion for diagnosing acute appendicitis was an incompressible appendix with a transverse outer diameter of 6 mm or larger, as seen in this patient. The sensitivity of CT and sonography was 76% and 79%, respectively; the specificity was 83% and 78%; the accuracy was 78% and 78%; the positive predictive value was 90% and 87%; and the negative predictive value was 64% and 65% (LOE=2a).8
In conclusion, it is reasonable to use graded compression ultrasonography in a pregnant woman with suspected appendicitis. If the suspicion for appendicitis is high, a negative result may still need further evaluation with a CT or ultimately lead to abdominal surgery despite negative imaging studies.
TABLEUltrasound and CT in the diagnosis of appendicitis
| TEST | SN | SP | LR+ | LR– |
|---|---|---|---|---|
| Ultrasound | 0.86 (0.83–0.88) | 0.81 (0.78–0.84) | 5.8 (3.5–9.5) | .019 (0.13–0.27) |
| CT | 0.94 (0.91–0.95) | 0.95 (0.93–0.96) | 13.3 (9.9–17.9) | 0.09 (0.07–0.12) |
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; CT, computed tomography. | ||||
| Source: Teresawa et al, Ann Intern Med 2004.7 | ||||
Corresponding Author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Hansen GC, Toot PJ, Lynch CO. Subtle ultrasound signs of appendicitis in pregnancy. A case report. J Reprod Med 1993;3:223-224.
2. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571-576.
3. Hodjati H, Kazerooni T. Location of the appendix in the gravid patient: a re-evaluation of the established concept. Int J Gynecol Obstet 2003;81:245-247.
4. Morad JDO, Elliott JP, Lisboa L. Appendicitis in pregnancy:new information that contradicts long held clinical beliefs. Am J Obstet Gynecol 2002;182:1027-1029.
5. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999;78:758-762.
6. Thurnau GR, Hales KA. Appendicitis in pregnancy. Female Patient 1992;17:81.
7. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537-546.
8. Poortman P, Lohle PN, Schoemaker CM, et al. Comparison of CT and sonography in the diagnosis of acute appendicitis: a blinded prospective study. AJR Am J Roentgenol 2003;181:1355-1359.
1. Hansen GC, Toot PJ, Lynch CO. Subtle ultrasound signs of appendicitis in pregnancy. A case report. J Reprod Med 1993;3:223-224.
2. Tamir IL, Bongard FS, Klein SR. Acute appendicitis in the pregnant patient. Am J Surg 1990;160:571-576.
3. Hodjati H, Kazerooni T. Location of the appendix in the gravid patient: a re-evaluation of the established concept. Int J Gynecol Obstet 2003;81:245-247.
4. Morad JDO, Elliott JP, Lisboa L. Appendicitis in pregnancy:new information that contradicts long held clinical beliefs. Am J Obstet Gynecol 2002;182:1027-1029.
5. Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999;78:758-762.
6. Thurnau GR, Hales KA. Appendicitis in pregnancy. Female Patient 1992;17:81.
7. Terasawa T, Blackmore CC, Bent S, Kohlwes RJ. Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537-546.
8. Poortman P, Lohle PN, Schoemaker CM, et al. Comparison of CT and sonography in the diagnosis of acute appendicitis: a blinded prospective study. AJR Am J Roentgenol 2003;181:1355-1359.
Pits on the soles of the feet
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected]
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected]
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected]
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
Palpable purpura and a visible sock line
A 21-year-old woman came to the clinic, frightened by a painful purpuric rash on her lower extremities (FIGURES 1 AND 2). The lesions appeared suddenly 3 days before, with no prior similar episodes.
The pain, and some swelling that happened when she stood, had finally driven her to take some time off from her job and seek medical advice. She was diagnosed with a case of pharyngitis earlier that week; due to multiple drug allergies, she was prescribed a course of clindamycin. She had not experienced any nausea or vomiting, fever, abdominal cramping, or gross hematuria.
On examination, the patient was friendly and good-humored, although she was concerned about her rash and visibly uncomfortable. She was walking with the aid of a borrowed cane, but her lesions were no longer tender to palpation.
The rash consisted mainly of purpuric papules almost entirely limited to her legs, although some isolated lesions were on her back as well. The papules were concentrated around her distal lower extremities, with a clear line of lesions encircling her calves bilaterally where her knee-high socks had applied pressure for the last 2 days (FIGURE 3) Mild edema was noted, but the rest of her physical exam was normal. By dip-stick, the patient had blood in her urine but no protein.
FIGURE 1
Purpura on the lower leg
FIGURE 2
Close-up
FIGURE 3
Visible sock lines
What is the diagnosis?
What is the treatment for this condition?
Diagnosis: Henoch-Schönlein purpura
This patient has Henoch-Schönlein purpura (HSP), a systemic vasculitis, secondary to hypersensitivity, occurring most commonly in children and young adults. Its classic triad includes palpable purpura, abdominal pain or renal involvement, and arthritis.1
Purpura is typically nonblanching, as it represents extravasation of blood into skin, 2 but lesions may also include urticarial wheals or occasional target lesions. As seen in this patient, the lower extremities and buttocks are typically affected. This rash is pressure- and gravity-dependent and pressure lines may develop, causing “sock lines” such as those seen on this young woman’s calves.1,2 Arthritis also tends to affect large joints in lower extremities more severely than upper extremities, and ankles and knees may be swollen and tender, as observed in our patient.
Abdominal pain, not present in this case, develops in up to 65% of cases, and may be accompanied by vomiting, hematemesis, or blood in stools.
Renal involvement is by far the most long-reaching and potentially serious complication of HSP, particularly in adults. Although only 1% of all HSP patients may develop end-stage renal failure, it is estimated that in adults, this number may be as high as 11%.3,4 The young woman in question presented with microscopic hematuria initially, which had resolved by the time of her next visit, 4 days later. A worse prognosis is associated with those patients who exhibit both nephritic and nephrotic features, with hematuria and proteinuria, where clinical remission has been shown to present only in 20% of these patients. Finally, as renal involvement may develop up to years after initial diagnosis, follow-up is crucial to monitor renal function.4
Cause is unknown
The cause of HSP is unknown, although it is thought to be secondary to deposition of immune complexes, and has been associated with vaccinations, viral infections, allergens in foods, drug reactions, exposure to cold, and bacterial respiratory infections, particularly involving group A streptococci.1,2 This patient had been diagnosed with streptococcal pharyngitis and had been given a course of clindamycin due to her numerous drug allergies. Although the precise cause of her HSP is not definite, drugs have been shown to cause approximately 10% of acute cutaneous vasculitis cases, and she had never before been given clindamycin, possibly pointing to the origin of her disease.5
Differential diagnoses
Differential diagnoses for HSP depend on the level of specificity with which the triad presents. When symptoms are not typical, abdominal cramping alone may be confused with acute abdomen. Rash may be predominantly in the form of target lesions, and can be mistaken for erythema multiforme, particularly after a suspected drug reaction. Rheumatoid arthritis or systemic lupus erythematosus may be suspected with arthralgias. Idiopathic thrombocytopenic purpura can be distinguished from HSP by platelet count. Trauma or meningococcal septicemia may also be mistaken for the correct diagnosis in the presence of an atypical rash with few accompanying symptoms.
Treatment: Steroids, NSAIDs, and careful monitoring of renal function
Corticosteroids may be administered for severe abdominal symptoms, but they have not been shown to be useful in the absence of such symptoms. Conservative management with NSAIDs is the treatment of choice, and careful monitoring of renal function, even in the absence of renal involvement.1,3,5
Outcome and discussion
Due to the presence of the classic HSP triad in our patient, we deemed a biopsy necessary, and managed the patient with ibupro-fen. One week later, her rash had receded and her microscopic hematuria was gone, but the vasculitis had led to lower-extremity edema. The patient’s feet were painfully swollen, and where the patient had scratched a lesion, her foot exhibited honey crusting and warmth, indicating a probable infection (FIGURES 4 AND 5). Her feet and ankles were so swollen and painful that she had difficulty walking. She was given cephalexin 500 mg 4 times daily for her infection, and was also restarted on oral prednisone at 60 mg daily until she could see the rheumatologist 2 days later. She was also issued crutches.
The rheumatologist agreed that this was HSP and told her that she would need to continue the prednisone for 2 months due to the severity of her cutaneous vasculitis. Within 5 days, the patient’s rash had diminished significantly. She was able to walk with less pain, as her ankle swelling decreased (FIGURE 6).
FIGURE 4
Swollen ankle
FIGURE 5
Probable infection
FIGURE 6
The same patient after 5 days
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician 1998;58:405-8,411.-
2. Leung AKC, Chan KW. Evaluating the child with purpura. Am Fam Physician 2001;64:419-428.
3. Usatine RP. A 4-year-old girl with a rash and joint pain. West J Med 1999;171:116-117.
4. Pillebout E, Thervet E, Hill G, et al. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002;13:1271-1278.
5. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-1285.
A 21-year-old woman came to the clinic, frightened by a painful purpuric rash on her lower extremities (FIGURES 1 AND 2). The lesions appeared suddenly 3 days before, with no prior similar episodes.
The pain, and some swelling that happened when she stood, had finally driven her to take some time off from her job and seek medical advice. She was diagnosed with a case of pharyngitis earlier that week; due to multiple drug allergies, she was prescribed a course of clindamycin. She had not experienced any nausea or vomiting, fever, abdominal cramping, or gross hematuria.
On examination, the patient was friendly and good-humored, although she was concerned about her rash and visibly uncomfortable. She was walking with the aid of a borrowed cane, but her lesions were no longer tender to palpation.
The rash consisted mainly of purpuric papules almost entirely limited to her legs, although some isolated lesions were on her back as well. The papules were concentrated around her distal lower extremities, with a clear line of lesions encircling her calves bilaterally where her knee-high socks had applied pressure for the last 2 days (FIGURE 3) Mild edema was noted, but the rest of her physical exam was normal. By dip-stick, the patient had blood in her urine but no protein.
FIGURE 1
Purpura on the lower leg
FIGURE 2
Close-up
FIGURE 3
Visible sock lines
What is the diagnosis?
What is the treatment for this condition?
Diagnosis: Henoch-Schönlein purpura
This patient has Henoch-Schönlein purpura (HSP), a systemic vasculitis, secondary to hypersensitivity, occurring most commonly in children and young adults. Its classic triad includes palpable purpura, abdominal pain or renal involvement, and arthritis.1
Purpura is typically nonblanching, as it represents extravasation of blood into skin, 2 but lesions may also include urticarial wheals or occasional target lesions. As seen in this patient, the lower extremities and buttocks are typically affected. This rash is pressure- and gravity-dependent and pressure lines may develop, causing “sock lines” such as those seen on this young woman’s calves.1,2 Arthritis also tends to affect large joints in lower extremities more severely than upper extremities, and ankles and knees may be swollen and tender, as observed in our patient.
Abdominal pain, not present in this case, develops in up to 65% of cases, and may be accompanied by vomiting, hematemesis, or blood in stools.
Renal involvement is by far the most long-reaching and potentially serious complication of HSP, particularly in adults. Although only 1% of all HSP patients may develop end-stage renal failure, it is estimated that in adults, this number may be as high as 11%.3,4 The young woman in question presented with microscopic hematuria initially, which had resolved by the time of her next visit, 4 days later. A worse prognosis is associated with those patients who exhibit both nephritic and nephrotic features, with hematuria and proteinuria, where clinical remission has been shown to present only in 20% of these patients. Finally, as renal involvement may develop up to years after initial diagnosis, follow-up is crucial to monitor renal function.4
Cause is unknown
The cause of HSP is unknown, although it is thought to be secondary to deposition of immune complexes, and has been associated with vaccinations, viral infections, allergens in foods, drug reactions, exposure to cold, and bacterial respiratory infections, particularly involving group A streptococci.1,2 This patient had been diagnosed with streptococcal pharyngitis and had been given a course of clindamycin due to her numerous drug allergies. Although the precise cause of her HSP is not definite, drugs have been shown to cause approximately 10% of acute cutaneous vasculitis cases, and she had never before been given clindamycin, possibly pointing to the origin of her disease.5
Differential diagnoses
Differential diagnoses for HSP depend on the level of specificity with which the triad presents. When symptoms are not typical, abdominal cramping alone may be confused with acute abdomen. Rash may be predominantly in the form of target lesions, and can be mistaken for erythema multiforme, particularly after a suspected drug reaction. Rheumatoid arthritis or systemic lupus erythematosus may be suspected with arthralgias. Idiopathic thrombocytopenic purpura can be distinguished from HSP by platelet count. Trauma or meningococcal septicemia may also be mistaken for the correct diagnosis in the presence of an atypical rash with few accompanying symptoms.
Treatment: Steroids, NSAIDs, and careful monitoring of renal function
Corticosteroids may be administered for severe abdominal symptoms, but they have not been shown to be useful in the absence of such symptoms. Conservative management with NSAIDs is the treatment of choice, and careful monitoring of renal function, even in the absence of renal involvement.1,3,5
Outcome and discussion
Due to the presence of the classic HSP triad in our patient, we deemed a biopsy necessary, and managed the patient with ibupro-fen. One week later, her rash had receded and her microscopic hematuria was gone, but the vasculitis had led to lower-extremity edema. The patient’s feet were painfully swollen, and where the patient had scratched a lesion, her foot exhibited honey crusting and warmth, indicating a probable infection (FIGURES 4 AND 5). Her feet and ankles were so swollen and painful that she had difficulty walking. She was given cephalexin 500 mg 4 times daily for her infection, and was also restarted on oral prednisone at 60 mg daily until she could see the rheumatologist 2 days later. She was also issued crutches.
The rheumatologist agreed that this was HSP and told her that she would need to continue the prednisone for 2 months due to the severity of her cutaneous vasculitis. Within 5 days, the patient’s rash had diminished significantly. She was able to walk with less pain, as her ankle swelling decreased (FIGURE 6).
FIGURE 4
Swollen ankle
FIGURE 5
Probable infection
FIGURE 6
The same patient after 5 days
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 21-year-old woman came to the clinic, frightened by a painful purpuric rash on her lower extremities (FIGURES 1 AND 2). The lesions appeared suddenly 3 days before, with no prior similar episodes.
The pain, and some swelling that happened when she stood, had finally driven her to take some time off from her job and seek medical advice. She was diagnosed with a case of pharyngitis earlier that week; due to multiple drug allergies, she was prescribed a course of clindamycin. She had not experienced any nausea or vomiting, fever, abdominal cramping, or gross hematuria.
On examination, the patient was friendly and good-humored, although she was concerned about her rash and visibly uncomfortable. She was walking with the aid of a borrowed cane, but her lesions were no longer tender to palpation.
The rash consisted mainly of purpuric papules almost entirely limited to her legs, although some isolated lesions were on her back as well. The papules were concentrated around her distal lower extremities, with a clear line of lesions encircling her calves bilaterally where her knee-high socks had applied pressure for the last 2 days (FIGURE 3) Mild edema was noted, but the rest of her physical exam was normal. By dip-stick, the patient had blood in her urine but no protein.
FIGURE 1
Purpura on the lower leg
FIGURE 2
Close-up
FIGURE 3
Visible sock lines
What is the diagnosis?
What is the treatment for this condition?
Diagnosis: Henoch-Schönlein purpura
This patient has Henoch-Schönlein purpura (HSP), a systemic vasculitis, secondary to hypersensitivity, occurring most commonly in children and young adults. Its classic triad includes palpable purpura, abdominal pain or renal involvement, and arthritis.1
Purpura is typically nonblanching, as it represents extravasation of blood into skin, 2 but lesions may also include urticarial wheals or occasional target lesions. As seen in this patient, the lower extremities and buttocks are typically affected. This rash is pressure- and gravity-dependent and pressure lines may develop, causing “sock lines” such as those seen on this young woman’s calves.1,2 Arthritis also tends to affect large joints in lower extremities more severely than upper extremities, and ankles and knees may be swollen and tender, as observed in our patient.
Abdominal pain, not present in this case, develops in up to 65% of cases, and may be accompanied by vomiting, hematemesis, or blood in stools.
Renal involvement is by far the most long-reaching and potentially serious complication of HSP, particularly in adults. Although only 1% of all HSP patients may develop end-stage renal failure, it is estimated that in adults, this number may be as high as 11%.3,4 The young woman in question presented with microscopic hematuria initially, which had resolved by the time of her next visit, 4 days later. A worse prognosis is associated with those patients who exhibit both nephritic and nephrotic features, with hematuria and proteinuria, where clinical remission has been shown to present only in 20% of these patients. Finally, as renal involvement may develop up to years after initial diagnosis, follow-up is crucial to monitor renal function.4
Cause is unknown
The cause of HSP is unknown, although it is thought to be secondary to deposition of immune complexes, and has been associated with vaccinations, viral infections, allergens in foods, drug reactions, exposure to cold, and bacterial respiratory infections, particularly involving group A streptococci.1,2 This patient had been diagnosed with streptococcal pharyngitis and had been given a course of clindamycin due to her numerous drug allergies. Although the precise cause of her HSP is not definite, drugs have been shown to cause approximately 10% of acute cutaneous vasculitis cases, and she had never before been given clindamycin, possibly pointing to the origin of her disease.5
Differential diagnoses
Differential diagnoses for HSP depend on the level of specificity with which the triad presents. When symptoms are not typical, abdominal cramping alone may be confused with acute abdomen. Rash may be predominantly in the form of target lesions, and can be mistaken for erythema multiforme, particularly after a suspected drug reaction. Rheumatoid arthritis or systemic lupus erythematosus may be suspected with arthralgias. Idiopathic thrombocytopenic purpura can be distinguished from HSP by platelet count. Trauma or meningococcal septicemia may also be mistaken for the correct diagnosis in the presence of an atypical rash with few accompanying symptoms.
Treatment: Steroids, NSAIDs, and careful monitoring of renal function
Corticosteroids may be administered for severe abdominal symptoms, but they have not been shown to be useful in the absence of such symptoms. Conservative management with NSAIDs is the treatment of choice, and careful monitoring of renal function, even in the absence of renal involvement.1,3,5
Outcome and discussion
Due to the presence of the classic HSP triad in our patient, we deemed a biopsy necessary, and managed the patient with ibupro-fen. One week later, her rash had receded and her microscopic hematuria was gone, but the vasculitis had led to lower-extremity edema. The patient’s feet were painfully swollen, and where the patient had scratched a lesion, her foot exhibited honey crusting and warmth, indicating a probable infection (FIGURES 4 AND 5). Her feet and ankles were so swollen and painful that she had difficulty walking. She was given cephalexin 500 mg 4 times daily for her infection, and was also restarted on oral prednisone at 60 mg daily until she could see the rheumatologist 2 days later. She was also issued crutches.
The rheumatologist agreed that this was HSP and told her that she would need to continue the prednisone for 2 months due to the severity of her cutaneous vasculitis. Within 5 days, the patient’s rash had diminished significantly. She was able to walk with less pain, as her ankle swelling decreased (FIGURE 6).
FIGURE 4
Swollen ankle
FIGURE 5
Probable infection
FIGURE 6
The same patient after 5 days
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician 1998;58:405-8,411.-
2. Leung AKC, Chan KW. Evaluating the child with purpura. Am Fam Physician 2001;64:419-428.
3. Usatine RP. A 4-year-old girl with a rash and joint pain. West J Med 1999;171:116-117.
4. Pillebout E, Thervet E, Hill G, et al. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002;13:1271-1278.
5. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-1285.
1. Kraft DM, McKee D, Scott C. Henoch-Schonlein purpura: a review. Am Fam Physician 1998;58:405-8,411.-
2. Leung AKC, Chan KW. Evaluating the child with purpura. Am Fam Physician 2001;64:419-428.
3. Usatine RP. A 4-year-old girl with a rash and joint pain. West J Med 1999;171:116-117.
4. Pillebout E, Thervet E, Hill G, et al. Henoch-Schonlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002;13:1271-1278.
5. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-1285.
"My airway is closing"
A 29-year-old man came to the emergency department with a 3-day history of progressively worsening sore throat, dysphagia, odynophagia (painon swallowing), and shortness of breath after unsuccessful treatment for Streptococcus pharyngitis. He reported having fevers and chills at home, and he had not slept the past 2 nights for fear that his “airway was closing.” He had tachycardia and tachypnea at presentation.
Physical examination was significant for an erythematous posterior oropharynx without tonsillar enlargement or exudates. The white blood cell count was 29,300 cells/mm3, with 92.6% neutrophils and 89% bands. A lateral soft-tissue x-ray of the neck was obtained (FIGURE 1), and compared with a cervical spine x-ray view taken the year before (FIGURE 2).
FIGURE 1
Soft-tissue neck radiograph
FIGURE 2
The same patient a year before
What is your diagnosis?
How should the diagnosis be confirmed?
How should the patient be treated?
Diagnosis: Acute adult epiglottitis
The lateral soft-tissue view of the neck demonstrates marked enlargement of the epiglottis (FIGURE 3, arrow) in comparison with the patient’s normal epiglottis from films taken the year before for neck pain (FIGURE 4, arrow); this is indicative of acute adult epiglottitis. The epiglottis occupies most of the supraglottic space and displays the classic “thumbprint sign,” which is pathognomonic for epiglottitis.
FIGURE 3
Enlargement of the epiglottis
FIGURE 4
The same patient a year before
Differential diagnoses
Given the patient’s fever, sore throat, odynophagia, and shortness of breath, other diagnoses to consider are pharyngitis, tonsillitis, peritonsillar abscess, retropharyngeal abscess, and angioedema.
Pharyngitis and tonsillitis can easily be evaluated by visualizing the oropharynx. If epiglottitis is suspected, however, a tongue depressor should not be used as it may precipitate loss of the airway.
A retropharyngeal abscess will result in enlargement of the prevertebral soft tissues on the lateral soft tissue view of the neck. Diagnostic confirmation of a retropharyngeal abscess is made with a contrast enhanced computed tomography scan of the neck, demonstrating rim-enhancing fluid collections within the retropharyngeal space.
Angioedema may be associated with cutaneous manifestations, such as urticaria, and sometimes an inciting agent can be identified.
Epidemiology and pathophysiology of acute epiglottitis
Acute epiglottitis is a rapidly progressive supraglottic infection that can lead to life-threatening airway obstruction. Although it is becoming less common in pediatric populations secondary to the Haemophilus influenzae type b vaccine, the adult incidence of approximately 1.8 per 100,000 persons remains stable, if not increasing.1
As in childhood epiglottitis, H influenzae is the most common causative agent in acute adult epiglottitis. Other causative agents include Streptococcus pneumoniae, Group A Streptococci, Staphylococcus aureus, viruses, and caustic agents. Patients most often present with nonspecific symptoms of odynophagia, dysphagia, sore throat, and a muffled voice. In more serious cases, adults will present with respiratory complaints, indicating that the supraglottic infection is jeopardizing the patient’s airway. The mortality rate for acute adult epiglottitis is approximately 7%.2
Diagnostic work-up: laryngoscopy and x-rays
The diagnosis of epiglottitis is usually made clinically but can be confirmed by direct visualization with a laryngoscope under monitored conditions.3 This should be done by personnel trained in management of a difficult airway—ie, anesthesiol-ogists and otolaryngologists—and a cricothyrotomy tray should be readily available in the event that the patient’s airway becomes compromised.
Lateral soft-tissue x-rays of the neck can be obtained if the patient is stable. However, the interpretation of these films can be difficult in equivocal cases. For this reason, a negative lateral soft-tissue view should not exclude the diagnosis of epiglottitis if clinical suspicion is high.
Once the diagnosis of epiglottitis is confirmed and the patient is stabilized, blood cultures and throat cultures may be obtained; however, their utility is questionable. More often than not, a causative agent will not be identified. Regardless of the laboratory results, current recommendations call for broad-spectrum antibiotic coverage, since many of these infections can be polymicrobial.
Management: Monitor the airway, administer antibiotics
Airway management is crucial in patients with epiglottitis. There is a debate in the literature as to whether or not these patients require immediate intubation due the danger of quickly losing an airway with little warning. Though most authors believe children with epiglottitis require a definitive airway at the time of presentation, adults can be closely monitored and treated conservatively4 —ie, hospital admission to a medical intensive care unit in a facility that has immediate access to anesthesiology and otolaryngology support.
Medical management consists of broad-spectrum antibiotic coverage with a third-generation cephalosporin.5 The use of steroids to reduce airway inflammation and potentially avoid the need for intubation is controversial, since the literature fails to show a direct benefit regarding the need for intubation, the length of intubation, or duration of hospital stay.6 Despite the lack of supporting evidence, steroids are often used as adjuvant treatment for epiglottitis.
Patient follow-up
The patient in this case was admitted to the hospital for close airway monitoring. He was treated conservatively with intravenous ceftriaxone, clindamycin, and Decadron, and his symptoms were significantly reduced by the second day in the hospital. He was discharged to home in stable condition on day 3. The patient fully recovered after a 10-day outpatient course of clindamycin and cefpodoxime.
DISCLAIMER
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the US government, the Department of Defense, or the Department of the Air Force.
CORRESPONDING AUTHOR
William T. O’Brien, Sr, DO, Department of Radiology, David Grant US Air Force Medical Center, Travis Air Force Base, CA. E-mail: [email protected]
1. Berg S, Trollfors B, Nylen O, Hugosson S, Prellner K, Carenfelt C. Incidence, aetiology, and prognosis of acute epiglottitis in children and adults in Sweden. Scand J Infect Dis 1996;28:261-264.
2. Mayo-Smith MF, Hirsch PJ, Wodzinski SF, Schiffmann FJ. Acute epiglottitis in adults, an eight-year experience in the State of Rhode Island. N Engl J Med 1986;314:133-139.
3. Ames WA, Ward VM, Tranter RM, Street M. Adult epiglottitis: an under-recognized, life-threatening condition. Br J Anesth 2000;85:795-797.
4. Wolf M, Strauss B, Kronenberg J, Leventon G. Conservative management of adult epiglottitis. Laryngoscope 1990;110:183-185.
5. Carey MJ. Epiglottitis in adults. Am J Emerg Med 1996;14:421-424.
6. Dort JC, Frohlich AM, Tate RB. Acute epiglottitis in adults: diagnosis and treatment in 43 patients. J Otolaryngol 1994;23:281-285.
A 29-year-old man came to the emergency department with a 3-day history of progressively worsening sore throat, dysphagia, odynophagia (painon swallowing), and shortness of breath after unsuccessful treatment for Streptococcus pharyngitis. He reported having fevers and chills at home, and he had not slept the past 2 nights for fear that his “airway was closing.” He had tachycardia and tachypnea at presentation.
Physical examination was significant for an erythematous posterior oropharynx without tonsillar enlargement or exudates. The white blood cell count was 29,300 cells/mm3, with 92.6% neutrophils and 89% bands. A lateral soft-tissue x-ray of the neck was obtained (FIGURE 1), and compared with a cervical spine x-ray view taken the year before (FIGURE 2).
FIGURE 1
Soft-tissue neck radiograph
FIGURE 2
The same patient a year before
What is your diagnosis?
How should the diagnosis be confirmed?
How should the patient be treated?
Diagnosis: Acute adult epiglottitis
The lateral soft-tissue view of the neck demonstrates marked enlargement of the epiglottis (FIGURE 3, arrow) in comparison with the patient’s normal epiglottis from films taken the year before for neck pain (FIGURE 4, arrow); this is indicative of acute adult epiglottitis. The epiglottis occupies most of the supraglottic space and displays the classic “thumbprint sign,” which is pathognomonic for epiglottitis.
FIGURE 3
Enlargement of the epiglottis
FIGURE 4
The same patient a year before
Differential diagnoses
Given the patient’s fever, sore throat, odynophagia, and shortness of breath, other diagnoses to consider are pharyngitis, tonsillitis, peritonsillar abscess, retropharyngeal abscess, and angioedema.
Pharyngitis and tonsillitis can easily be evaluated by visualizing the oropharynx. If epiglottitis is suspected, however, a tongue depressor should not be used as it may precipitate loss of the airway.
A retropharyngeal abscess will result in enlargement of the prevertebral soft tissues on the lateral soft tissue view of the neck. Diagnostic confirmation of a retropharyngeal abscess is made with a contrast enhanced computed tomography scan of the neck, demonstrating rim-enhancing fluid collections within the retropharyngeal space.
Angioedema may be associated with cutaneous manifestations, such as urticaria, and sometimes an inciting agent can be identified.
Epidemiology and pathophysiology of acute epiglottitis
Acute epiglottitis is a rapidly progressive supraglottic infection that can lead to life-threatening airway obstruction. Although it is becoming less common in pediatric populations secondary to the Haemophilus influenzae type b vaccine, the adult incidence of approximately 1.8 per 100,000 persons remains stable, if not increasing.1
As in childhood epiglottitis, H influenzae is the most common causative agent in acute adult epiglottitis. Other causative agents include Streptococcus pneumoniae, Group A Streptococci, Staphylococcus aureus, viruses, and caustic agents. Patients most often present with nonspecific symptoms of odynophagia, dysphagia, sore throat, and a muffled voice. In more serious cases, adults will present with respiratory complaints, indicating that the supraglottic infection is jeopardizing the patient’s airway. The mortality rate for acute adult epiglottitis is approximately 7%.2
Diagnostic work-up: laryngoscopy and x-rays
The diagnosis of epiglottitis is usually made clinically but can be confirmed by direct visualization with a laryngoscope under monitored conditions.3 This should be done by personnel trained in management of a difficult airway—ie, anesthesiol-ogists and otolaryngologists—and a cricothyrotomy tray should be readily available in the event that the patient’s airway becomes compromised.
Lateral soft-tissue x-rays of the neck can be obtained if the patient is stable. However, the interpretation of these films can be difficult in equivocal cases. For this reason, a negative lateral soft-tissue view should not exclude the diagnosis of epiglottitis if clinical suspicion is high.
Once the diagnosis of epiglottitis is confirmed and the patient is stabilized, blood cultures and throat cultures may be obtained; however, their utility is questionable. More often than not, a causative agent will not be identified. Regardless of the laboratory results, current recommendations call for broad-spectrum antibiotic coverage, since many of these infections can be polymicrobial.
Management: Monitor the airway, administer antibiotics
Airway management is crucial in patients with epiglottitis. There is a debate in the literature as to whether or not these patients require immediate intubation due the danger of quickly losing an airway with little warning. Though most authors believe children with epiglottitis require a definitive airway at the time of presentation, adults can be closely monitored and treated conservatively4 —ie, hospital admission to a medical intensive care unit in a facility that has immediate access to anesthesiology and otolaryngology support.
Medical management consists of broad-spectrum antibiotic coverage with a third-generation cephalosporin.5 The use of steroids to reduce airway inflammation and potentially avoid the need for intubation is controversial, since the literature fails to show a direct benefit regarding the need for intubation, the length of intubation, or duration of hospital stay.6 Despite the lack of supporting evidence, steroids are often used as adjuvant treatment for epiglottitis.
Patient follow-up
The patient in this case was admitted to the hospital for close airway monitoring. He was treated conservatively with intravenous ceftriaxone, clindamycin, and Decadron, and his symptoms were significantly reduced by the second day in the hospital. He was discharged to home in stable condition on day 3. The patient fully recovered after a 10-day outpatient course of clindamycin and cefpodoxime.
DISCLAIMER
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the US government, the Department of Defense, or the Department of the Air Force.
CORRESPONDING AUTHOR
William T. O’Brien, Sr, DO, Department of Radiology, David Grant US Air Force Medical Center, Travis Air Force Base, CA. E-mail: [email protected]
A 29-year-old man came to the emergency department with a 3-day history of progressively worsening sore throat, dysphagia, odynophagia (painon swallowing), and shortness of breath after unsuccessful treatment for Streptococcus pharyngitis. He reported having fevers and chills at home, and he had not slept the past 2 nights for fear that his “airway was closing.” He had tachycardia and tachypnea at presentation.
Physical examination was significant for an erythematous posterior oropharynx without tonsillar enlargement or exudates. The white blood cell count was 29,300 cells/mm3, with 92.6% neutrophils and 89% bands. A lateral soft-tissue x-ray of the neck was obtained (FIGURE 1), and compared with a cervical spine x-ray view taken the year before (FIGURE 2).
FIGURE 1
Soft-tissue neck radiograph
FIGURE 2
The same patient a year before
What is your diagnosis?
How should the diagnosis be confirmed?
How should the patient be treated?
Diagnosis: Acute adult epiglottitis
The lateral soft-tissue view of the neck demonstrates marked enlargement of the epiglottis (FIGURE 3, arrow) in comparison with the patient’s normal epiglottis from films taken the year before for neck pain (FIGURE 4, arrow); this is indicative of acute adult epiglottitis. The epiglottis occupies most of the supraglottic space and displays the classic “thumbprint sign,” which is pathognomonic for epiglottitis.
FIGURE 3
Enlargement of the epiglottis
FIGURE 4
The same patient a year before
Differential diagnoses
Given the patient’s fever, sore throat, odynophagia, and shortness of breath, other diagnoses to consider are pharyngitis, tonsillitis, peritonsillar abscess, retropharyngeal abscess, and angioedema.
Pharyngitis and tonsillitis can easily be evaluated by visualizing the oropharynx. If epiglottitis is suspected, however, a tongue depressor should not be used as it may precipitate loss of the airway.
A retropharyngeal abscess will result in enlargement of the prevertebral soft tissues on the lateral soft tissue view of the neck. Diagnostic confirmation of a retropharyngeal abscess is made with a contrast enhanced computed tomography scan of the neck, demonstrating rim-enhancing fluid collections within the retropharyngeal space.
Angioedema may be associated with cutaneous manifestations, such as urticaria, and sometimes an inciting agent can be identified.
Epidemiology and pathophysiology of acute epiglottitis
Acute epiglottitis is a rapidly progressive supraglottic infection that can lead to life-threatening airway obstruction. Although it is becoming less common in pediatric populations secondary to the Haemophilus influenzae type b vaccine, the adult incidence of approximately 1.8 per 100,000 persons remains stable, if not increasing.1
As in childhood epiglottitis, H influenzae is the most common causative agent in acute adult epiglottitis. Other causative agents include Streptococcus pneumoniae, Group A Streptococci, Staphylococcus aureus, viruses, and caustic agents. Patients most often present with nonspecific symptoms of odynophagia, dysphagia, sore throat, and a muffled voice. In more serious cases, adults will present with respiratory complaints, indicating that the supraglottic infection is jeopardizing the patient’s airway. The mortality rate for acute adult epiglottitis is approximately 7%.2
Diagnostic work-up: laryngoscopy and x-rays
The diagnosis of epiglottitis is usually made clinically but can be confirmed by direct visualization with a laryngoscope under monitored conditions.3 This should be done by personnel trained in management of a difficult airway—ie, anesthesiol-ogists and otolaryngologists—and a cricothyrotomy tray should be readily available in the event that the patient’s airway becomes compromised.
Lateral soft-tissue x-rays of the neck can be obtained if the patient is stable. However, the interpretation of these films can be difficult in equivocal cases. For this reason, a negative lateral soft-tissue view should not exclude the diagnosis of epiglottitis if clinical suspicion is high.
Once the diagnosis of epiglottitis is confirmed and the patient is stabilized, blood cultures and throat cultures may be obtained; however, their utility is questionable. More often than not, a causative agent will not be identified. Regardless of the laboratory results, current recommendations call for broad-spectrum antibiotic coverage, since many of these infections can be polymicrobial.
Management: Monitor the airway, administer antibiotics
Airway management is crucial in patients with epiglottitis. There is a debate in the literature as to whether or not these patients require immediate intubation due the danger of quickly losing an airway with little warning. Though most authors believe children with epiglottitis require a definitive airway at the time of presentation, adults can be closely monitored and treated conservatively4 —ie, hospital admission to a medical intensive care unit in a facility that has immediate access to anesthesiology and otolaryngology support.
Medical management consists of broad-spectrum antibiotic coverage with a third-generation cephalosporin.5 The use of steroids to reduce airway inflammation and potentially avoid the need for intubation is controversial, since the literature fails to show a direct benefit regarding the need for intubation, the length of intubation, or duration of hospital stay.6 Despite the lack of supporting evidence, steroids are often used as adjuvant treatment for epiglottitis.
Patient follow-up
The patient in this case was admitted to the hospital for close airway monitoring. He was treated conservatively with intravenous ceftriaxone, clindamycin, and Decadron, and his symptoms were significantly reduced by the second day in the hospital. He was discharged to home in stable condition on day 3. The patient fully recovered after a 10-day outpatient course of clindamycin and cefpodoxime.
DISCLAIMER
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the US government, the Department of Defense, or the Department of the Air Force.
CORRESPONDING AUTHOR
William T. O’Brien, Sr, DO, Department of Radiology, David Grant US Air Force Medical Center, Travis Air Force Base, CA. E-mail: [email protected]
1. Berg S, Trollfors B, Nylen O, Hugosson S, Prellner K, Carenfelt C. Incidence, aetiology, and prognosis of acute epiglottitis in children and adults in Sweden. Scand J Infect Dis 1996;28:261-264.
2. Mayo-Smith MF, Hirsch PJ, Wodzinski SF, Schiffmann FJ. Acute epiglottitis in adults, an eight-year experience in the State of Rhode Island. N Engl J Med 1986;314:133-139.
3. Ames WA, Ward VM, Tranter RM, Street M. Adult epiglottitis: an under-recognized, life-threatening condition. Br J Anesth 2000;85:795-797.
4. Wolf M, Strauss B, Kronenberg J, Leventon G. Conservative management of adult epiglottitis. Laryngoscope 1990;110:183-185.
5. Carey MJ. Epiglottitis in adults. Am J Emerg Med 1996;14:421-424.
6. Dort JC, Frohlich AM, Tate RB. Acute epiglottitis in adults: diagnosis and treatment in 43 patients. J Otolaryngol 1994;23:281-285.
1. Berg S, Trollfors B, Nylen O, Hugosson S, Prellner K, Carenfelt C. Incidence, aetiology, and prognosis of acute epiglottitis in children and adults in Sweden. Scand J Infect Dis 1996;28:261-264.
2. Mayo-Smith MF, Hirsch PJ, Wodzinski SF, Schiffmann FJ. Acute epiglottitis in adults, an eight-year experience in the State of Rhode Island. N Engl J Med 1986;314:133-139.
3. Ames WA, Ward VM, Tranter RM, Street M. Adult epiglottitis: an under-recognized, life-threatening condition. Br J Anesth 2000;85:795-797.
4. Wolf M, Strauss B, Kronenberg J, Leventon G. Conservative management of adult epiglottitis. Laryngoscope 1990;110:183-185.
5. Carey MJ. Epiglottitis in adults. Am J Emerg Med 1996;14:421-424.
6. Dort JC, Frohlich AM, Tate RB. Acute epiglottitis in adults: diagnosis and treatment in 43 patients. J Otolaryngol 1994;23:281-285.
Unilateral rash on a baby girl
An 11-month-old baby girl came to the clinic with a pruritic rash. The rash initially appeared in her popliteal fossa 2 weeks before the visit. The eruption extended to the right leg, arm, and flank the week before the visit, subsequently spreading to the contralateral flank. Three weeks before to the eruption’s appearance, the patient had an upper respiratory infection with a dry nonproductive cough, which resolved spontaneously without antibiotics.
The physical examination revealed a healthy-appearing infant girl with excoriated erythematous papules coalescing into plaques on her right flexural arm that continued to the axilla and down the right flank to the flexural aspect of her leg (FIGURE 1). Her left side was essentially free of any rash (FIGURE 2). No cervical or axillary lymphadenopathy was noted, and the remainder of her exam was normal.
FIGURE 1
The right side has a rash…
FIGURE 2
…and the left side is clear
What is your diagnosis?
How Would You Manage This Condition?
Diagnosis: Asymmetric periflexural exanthem of childhood
Asymmetric periflexural exanthem of childhood (APEC) is a diagnosis defined by its unique clinical presentation. Since its original description in 1962 by Brunner 1 as a new papular erythema of childhood, a number of names have been used to describe the same clinical process: unilateral laterothoracic exanthema,2 asymmetric periflexural exanthem of childhood,3 and lichen miliaris.4
Clinical picture of APEC
The initial clinical finding is a unilateral erythematous macular and papular eruption, often beginning in or around the axilla. Over the following 1 to 3 weeks, centrifugal spread involves the upper and lower extremities. Approximately 70% of APEC cases have involvement of the contralateral trunk. Despite the progression to the contralateral side, the eruption remains asymmetric throughout its course.
Additional findings include lymphadenopathy and pruritus in 70% and 65% of cases, respectively. 3-5 In contrast to other exanthems, APEC rarely involves the face.5 A study by Coustou reported that 60% of cases had a preceding prodrome including rhinitis, pharyngitis, otitis, and fever.4,5
Cause is unknown
Although the precise cause of APEC is not known, it has features consistent with a viral exanthem. A viral source is supported by a springtime and pediatric predominance with spontaneous resolution. In addition, 1 adult case of APEC has been attributed to an acute Parvo B19 infection.6
However, consistent serologic evidence supporting a viral cause is lacking,2, 7 and no human transmissions have been documented except for reports of 2 familial cases.8 Some have proposed that this could be a childhood form of pityriasis rosea possibly caused by human herpes virus 7.4
Differential diagnosis
The differential diagnosis for APEC includes viral exanthems, eczema, scabies, pityriasis rosea, contact dermatitis, and miliaria (heat rash). APEC mainly affects children aged 2 to 3 years but can occur at a younger age. There are no laboratory tests that help establish the diagnosis of APEC. The diagnosis is based on the clinical picture of an asymmetric macular and papular exanthem in a young child with a viral-like prodrome.
Treatment and outcome
There is no specific treatment for APEC other than to treat the symptoms. No treatment has been shown to shorten the course of this disease. A low-potency topical steroid along with an antihistamine provides adequate symptomatic treatment.
This child had no significant symptoms and therefore no medications were prescribed. The parents were told they may get 1% hydrocortisone cream over-the-counter if their daughter developed troublesome itching. Reassurance was provided about the limited nature of this exanthem. The parents were advised to bring the child for follow-up if the rash did not completely resolve in 2 months. By the time for the child’s 1-year check-up, the rash was gone.
Corresponding Author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine (BAR 314), Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street, Boston, MA 02114. E-mail: [email protected].
1. Brunner MJ, Rubin L, Dunlap F. A new papular erythema of childhood. Arch Dermatol 1962;85:539-540.
2. Bodemer C, de Prost Y. Unilateral laterothoracic exanthem in children: a new disease? J Am Acad Dermato 1992;27:693-696.
3. Taïeb A, Megraud F, Le Roy JM, Magne F, Reguilhem MO, Maleville J. Érythème localisé avec adénopathie régionale de l’enfant: Une maladie d’inoculation? Ann Dermatol Venereol 1986;113:1023-1024.
4. Laur WE. Unilateral laterothoracic exanthem in children. J Am Acad Dermatol 1993;29:799-800.
5. Coustou D, Leaute-Labreze C, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood: a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol 1999;135:799-803.
6. Pauluzzi P, Festini G, Gelmetti C. Asymmetric periflexural exanthem of childhood in an adult patient with parvovirus B19. J Eur Acad Dermatol Venereol 2001;15:372-374.
7. Jhin MH, Eidelman M, Cohen SR, Husain S. Unilateral eruption in a child. Arch Dermatol 2002;138:1371-1376.
8. McCuaig CC, Russo P, Powell J, et al. Unilateral laterothoracic exanthem. A clinicopathologic study of forty-eight patients. J Am Acad Dermatol 1996;34:979-984.
An 11-month-old baby girl came to the clinic with a pruritic rash. The rash initially appeared in her popliteal fossa 2 weeks before the visit. The eruption extended to the right leg, arm, and flank the week before the visit, subsequently spreading to the contralateral flank. Three weeks before to the eruption’s appearance, the patient had an upper respiratory infection with a dry nonproductive cough, which resolved spontaneously without antibiotics.
The physical examination revealed a healthy-appearing infant girl with excoriated erythematous papules coalescing into plaques on her right flexural arm that continued to the axilla and down the right flank to the flexural aspect of her leg (FIGURE 1). Her left side was essentially free of any rash (FIGURE 2). No cervical or axillary lymphadenopathy was noted, and the remainder of her exam was normal.
FIGURE 1
The right side has a rash…
FIGURE 2
…and the left side is clear
What is your diagnosis?
How Would You Manage This Condition?
Diagnosis: Asymmetric periflexural exanthem of childhood
Asymmetric periflexural exanthem of childhood (APEC) is a diagnosis defined by its unique clinical presentation. Since its original description in 1962 by Brunner 1 as a new papular erythema of childhood, a number of names have been used to describe the same clinical process: unilateral laterothoracic exanthema,2 asymmetric periflexural exanthem of childhood,3 and lichen miliaris.4
Clinical picture of APEC
The initial clinical finding is a unilateral erythematous macular and papular eruption, often beginning in or around the axilla. Over the following 1 to 3 weeks, centrifugal spread involves the upper and lower extremities. Approximately 70% of APEC cases have involvement of the contralateral trunk. Despite the progression to the contralateral side, the eruption remains asymmetric throughout its course.
Additional findings include lymphadenopathy and pruritus in 70% and 65% of cases, respectively. 3-5 In contrast to other exanthems, APEC rarely involves the face.5 A study by Coustou reported that 60% of cases had a preceding prodrome including rhinitis, pharyngitis, otitis, and fever.4,5
Cause is unknown
Although the precise cause of APEC is not known, it has features consistent with a viral exanthem. A viral source is supported by a springtime and pediatric predominance with spontaneous resolution. In addition, 1 adult case of APEC has been attributed to an acute Parvo B19 infection.6
However, consistent serologic evidence supporting a viral cause is lacking,2, 7 and no human transmissions have been documented except for reports of 2 familial cases.8 Some have proposed that this could be a childhood form of pityriasis rosea possibly caused by human herpes virus 7.4
Differential diagnosis
The differential diagnosis for APEC includes viral exanthems, eczema, scabies, pityriasis rosea, contact dermatitis, and miliaria (heat rash). APEC mainly affects children aged 2 to 3 years but can occur at a younger age. There are no laboratory tests that help establish the diagnosis of APEC. The diagnosis is based on the clinical picture of an asymmetric macular and papular exanthem in a young child with a viral-like prodrome.
Treatment and outcome
There is no specific treatment for APEC other than to treat the symptoms. No treatment has been shown to shorten the course of this disease. A low-potency topical steroid along with an antihistamine provides adequate symptomatic treatment.
This child had no significant symptoms and therefore no medications were prescribed. The parents were told they may get 1% hydrocortisone cream over-the-counter if their daughter developed troublesome itching. Reassurance was provided about the limited nature of this exanthem. The parents were advised to bring the child for follow-up if the rash did not completely resolve in 2 months. By the time for the child’s 1-year check-up, the rash was gone.
Corresponding Author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine (BAR 314), Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street, Boston, MA 02114. E-mail: [email protected].
An 11-month-old baby girl came to the clinic with a pruritic rash. The rash initially appeared in her popliteal fossa 2 weeks before the visit. The eruption extended to the right leg, arm, and flank the week before the visit, subsequently spreading to the contralateral flank. Three weeks before to the eruption’s appearance, the patient had an upper respiratory infection with a dry nonproductive cough, which resolved spontaneously without antibiotics.
The physical examination revealed a healthy-appearing infant girl with excoriated erythematous papules coalescing into plaques on her right flexural arm that continued to the axilla and down the right flank to the flexural aspect of her leg (FIGURE 1). Her left side was essentially free of any rash (FIGURE 2). No cervical or axillary lymphadenopathy was noted, and the remainder of her exam was normal.
FIGURE 1
The right side has a rash…
FIGURE 2
…and the left side is clear
What is your diagnosis?
How Would You Manage This Condition?
Diagnosis: Asymmetric periflexural exanthem of childhood
Asymmetric periflexural exanthem of childhood (APEC) is a diagnosis defined by its unique clinical presentation. Since its original description in 1962 by Brunner 1 as a new papular erythema of childhood, a number of names have been used to describe the same clinical process: unilateral laterothoracic exanthema,2 asymmetric periflexural exanthem of childhood,3 and lichen miliaris.4
Clinical picture of APEC
The initial clinical finding is a unilateral erythematous macular and papular eruption, often beginning in or around the axilla. Over the following 1 to 3 weeks, centrifugal spread involves the upper and lower extremities. Approximately 70% of APEC cases have involvement of the contralateral trunk. Despite the progression to the contralateral side, the eruption remains asymmetric throughout its course.
Additional findings include lymphadenopathy and pruritus in 70% and 65% of cases, respectively. 3-5 In contrast to other exanthems, APEC rarely involves the face.5 A study by Coustou reported that 60% of cases had a preceding prodrome including rhinitis, pharyngitis, otitis, and fever.4,5
Cause is unknown
Although the precise cause of APEC is not known, it has features consistent with a viral exanthem. A viral source is supported by a springtime and pediatric predominance with spontaneous resolution. In addition, 1 adult case of APEC has been attributed to an acute Parvo B19 infection.6
However, consistent serologic evidence supporting a viral cause is lacking,2, 7 and no human transmissions have been documented except for reports of 2 familial cases.8 Some have proposed that this could be a childhood form of pityriasis rosea possibly caused by human herpes virus 7.4
Differential diagnosis
The differential diagnosis for APEC includes viral exanthems, eczema, scabies, pityriasis rosea, contact dermatitis, and miliaria (heat rash). APEC mainly affects children aged 2 to 3 years but can occur at a younger age. There are no laboratory tests that help establish the diagnosis of APEC. The diagnosis is based on the clinical picture of an asymmetric macular and papular exanthem in a young child with a viral-like prodrome.
Treatment and outcome
There is no specific treatment for APEC other than to treat the symptoms. No treatment has been shown to shorten the course of this disease. A low-potency topical steroid along with an antihistamine provides adequate symptomatic treatment.
This child had no significant symptoms and therefore no medications were prescribed. The parents were told they may get 1% hydrocortisone cream over-the-counter if their daughter developed troublesome itching. Reassurance was provided about the limited nature of this exanthem. The parents were advised to bring the child for follow-up if the rash did not completely resolve in 2 months. By the time for the child’s 1-year check-up, the rash was gone.
Corresponding Author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine (BAR 314), Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street, Boston, MA 02114. E-mail: [email protected].
1. Brunner MJ, Rubin L, Dunlap F. A new papular erythema of childhood. Arch Dermatol 1962;85:539-540.
2. Bodemer C, de Prost Y. Unilateral laterothoracic exanthem in children: a new disease? J Am Acad Dermato 1992;27:693-696.
3. Taïeb A, Megraud F, Le Roy JM, Magne F, Reguilhem MO, Maleville J. Érythème localisé avec adénopathie régionale de l’enfant: Une maladie d’inoculation? Ann Dermatol Venereol 1986;113:1023-1024.
4. Laur WE. Unilateral laterothoracic exanthem in children. J Am Acad Dermatol 1993;29:799-800.
5. Coustou D, Leaute-Labreze C, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood: a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol 1999;135:799-803.
6. Pauluzzi P, Festini G, Gelmetti C. Asymmetric periflexural exanthem of childhood in an adult patient with parvovirus B19. J Eur Acad Dermatol Venereol 2001;15:372-374.
7. Jhin MH, Eidelman M, Cohen SR, Husain S. Unilateral eruption in a child. Arch Dermatol 2002;138:1371-1376.
8. McCuaig CC, Russo P, Powell J, et al. Unilateral laterothoracic exanthem. A clinicopathologic study of forty-eight patients. J Am Acad Dermatol 1996;34:979-984.
1. Brunner MJ, Rubin L, Dunlap F. A new papular erythema of childhood. Arch Dermatol 1962;85:539-540.
2. Bodemer C, de Prost Y. Unilateral laterothoracic exanthem in children: a new disease? J Am Acad Dermato 1992;27:693-696.
3. Taïeb A, Megraud F, Le Roy JM, Magne F, Reguilhem MO, Maleville J. Érythème localisé avec adénopathie régionale de l’enfant: Une maladie d’inoculation? Ann Dermatol Venereol 1986;113:1023-1024.
4. Laur WE. Unilateral laterothoracic exanthem in children. J Am Acad Dermatol 1993;29:799-800.
5. Coustou D, Leaute-Labreze C, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood: a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol 1999;135:799-803.
6. Pauluzzi P, Festini G, Gelmetti C. Asymmetric periflexural exanthem of childhood in an adult patient with parvovirus B19. J Eur Acad Dermatol Venereol 2001;15:372-374.
7. Jhin MH, Eidelman M, Cohen SR, Husain S. Unilateral eruption in a child. Arch Dermatol 2002;138:1371-1376.
8. McCuaig CC, Russo P, Powell J, et al. Unilateral laterothoracic exanthem. A clinicopathologic study of forty-eight patients. J Am Acad Dermatol 1996;34:979-984.
Puzzling palmar papules and pits
A 54-year-old African American woman came to the office with a problem on her hands that began about 10 years before: small, hard plugs that formed on her palms (Figures 1 and 2). These areas remain tender for 1 to 2 days after the plugs first form and while they “stick up.” After a few days, the plugs fall out, leaving small pits. The patient experienced no other symptoms once the plugs fall out; just the appearance of her palms.
Many years ago, a physician tried freezing the lesions, believing them to be warts. That therapy provided no benefit. The patient found that filing down the plugs and lubricating them with white petrolatum helped during the symptomatic phase.
The patient was married, with no history of sexually transmitted diseases or significant occupational exposures. She did take medication to control her hypertension, hyperlipidemia, and hypothyroidism. However, the problem with her hands predated taking these medications. There was no personal or family history of skin malignancy. The remainder of the skin examination was unremarkable.
FIGURE 1
Lesions on left palm
FIGURE 2
Lateral view
What is your diagnosis?
Diagnosis: keratosis punctata of the palmar creases
Keratosis punctata of the palmar creases (KPPC) is a benign, largely asymptomatic condition of the hands, seen almost exclusively those with African ancestry. KPPC presents as small keratotic papules (Figure 3) that evolve into discreet conical pits (Figure 4).1,2 Although KPPC is not a novel or rare condition among African Americans, it is not found in standard dermatology texts used by primary care physicians. However, reference to KPPC may be found in ethnic dermatology texts, including reference to it as “a common normal finding in the black palm.”1
The lesions of KPPC characteristically are 1 to 5 mm in diameter, sharply defined hyperkeratotic pits that occur in the flexural creases of the hands, both on the palms and volar surfaces of the fingers. KPPC has also referred to as keratotic pits of the palmar creases, punctate keratoses of the palmar creases, keratoderma punctata, hyperkeratosis penetrans, lenticular atrophia of the palmar creases, and hyperkeratosis punctata of the palmar creases.
FIGURE 3
Close-up of a “plug”
FIGURE 4
Close-up of a “pit”
Distinguishing KPPC from KPPP
KPPC has also been regarded as a variant of keratosis punctata palmaris et plantaris (KPPP).3,4 KPPP and KPPC share some similarities with respect to the size and number of lesions per palm, probable exacerbation by trauma, and predilection for occurring in those of Afro-Caribbean descent.1,3,4
Historically, there has been some confusion in distinguishing KPPC from KPPP—in fact, it is possible for the 2 conditions to occur simultaneously. The papular lesions of KPPP tend to occur over the entire palm, volar wrist, and medial aspects of the feet. These entities differ in age at onset, prevalence, symptoms, and prognosis.
Sources differ regarding the average age of onset for KPPP; some report onset from infancy to 70 years.5-7 For KPPC, the age of onset generally is between 15 and 40 years.3 Among African Americans, the prevalence of KPPC is between 1.9% and 3.1%,3,6,8 whereas the prevalence of KPPP may be up to 11%.3,4 While KPPP is largely asymptomatic, the lesions of KPPC tend to be noticed more often. Once present, KPPP lesions usually remain stable over time, whereas, KPPC lesions usually increase in number and size.3
Demographics and causes
KPPC is rarely seen in Caucasians. Of 1001 white patients examined for palmar lesions, none fulfilled the diagnostic criteria for KPPC.9 In a study of 534 patients, Weiss et al discovered 7 cases— all in African American patients and representing 3.1% of this racial group.8
The cause of KPPC is unknown. No medications have been implicated, and it has been difficult to link it to a virus.3 Although some authors have suggested that KPPC represents flexural calluses related to manual labor, lesions also occur in patients without this history.1,5 There is no association between KPPC and arsenical agents or syphilis.8
It is generally believed that KPPC does not have a recognizable heritable pattern, though there may be exceptions.8 There may be a familial association with ichthyosis vulgaris and other disorders of keratinization. One report included 5 patients in 1 family with keratotic plugs of the palmar creases consistent with an autosomal dominant pattern of inheritance. The syndrome was associated with ichthyosis vulgaris in several family members.10 KPPP and KPPC might be the result of abnormal callus formation in predisposed individuals, as both conditions seem to be due to an abnormal hyperproliferative response to local trauma.3
Differential diagnosis
Punctate keratoses of the palms are fairly common frequently overlooked lesions. The differential diagnosis is extensive (Table), but there are several clinical features of KPPC that distinguish it from other hyperkeratotic conditions. The lesions of KPPC can be painful, have a predilection for jointcreases, and evolve into pits.3 KPPP is similar except not localized to the creases.4
Aquagenic keratoderma is a transitory condition afflicting young women and defined clinically by the appearance of palmar lesions accentuated after immersion in water. These lesions have a characteristic histological appearance (hyperkeratosis, dilated eccrine ducts).13
Palmoplantar pustulosis is characterized by chronically recurring sterile pustules on the palms and soles, usually found on an erythematous base, and a strong association with tobacco use.14Palmoplantar lichen planus may exhibit a variety of morphologic patterns including papules or plaques with pruritus, erythema, and compact hyperkeratosis.15
Cole disease is an uncommon disorder typified by distinctive cutaneous hyperpigmentation and punctate keratoses on the palms and soles. It is a congenital disease with an autosomal dominant inheritance pattern and phenotypic variability.16
Palmoplantar psoriasis is associated with manual labor in 50% of cases. Lesions are restricted to areas exposed to pressure. All patients with unilateral palmar lesions had them on their dominant hand. Biopsy may be necessary to differentiate hyperkeratotic eczema from psoriasis when just localized to the palms and soles.17
TABLE
Differential diagnosis of punctate keratoses of the hands and feet
| Acquired keratoses | Classic clinical description | Associations |
|---|---|---|
| Arsenical | Round, verrucous, or acuminate keratotic papules most common on palms and soles. Typically occur decades after chronic arsenic ingestion | Angiosarcoma of the liver, nonmelanoma skin cancer, bronchial adenocarcinoma |
| Idiopathic filiform porokeratoses | Multiple thin spiny keratotic projections on palms and soles | Breast, renal, colon, and lung cancer |
| Keratosis punctata of the palmar creases | Discrete, sharply marginated, hyperkeratotic, conical, 1–5 mm depressions confined to flexural creases | Dupuytren’s contracture, striate keratoderma, knuckle pads |
| Hereditary keratoses | Classic clinical description | Associations |
| Keratosis punctata palmoplantaris (type I), Buschke-Fischer-Brauer disease | Multiple 1–2 mm punctate keratoses of the palms and soles | Longitudinal nail dystrophy, lichen nitidus, ichthyosis, atopy, recalcitrant warts. Increased risk of malignancy |
| Spiny keratoderma (type II) | Small keratotic spines over entire palmoplantar surfaces. Resembles the spines of an old-fashioned music box | No predisposition to malignancy |
| Acrokeratoelastoidosis lichenoids (type III) | 2–4 mm round to oval papules on the borders of hands, feet and wrists. May be umbilicated and become confluent | Darier’s disease, Cowden’s disease |
| Adapted from Rustad et al 1990,5 Kong et al 2004,7 Asadi 2003,9 Habif 2004,11 and Osman et al 1992.12 | ||
Treatment options: keratolytic agents may help temporarily
The mainstay of therapy is informing the patient of the benign nature of the diagnosis and avoiding unnecessary and unhelpful therapies and diagnostic modalities. Therapy with keratolytic agents or systemic retinoids may temporarily improve symptoms of KPPC.
However, lesions tend to recur when the medications are stopped or decreased.5,7 Temporizing treatment of symptomatic keratoses, such as applying emollients and paring them, is all that is usually necessary. Systemic retinoids have far too many side effects to consider using in this completely benign condition.
Patient outcome
The patient was reassured by the explanation of the condition and chose to try a keratolytic/emollient agent, Lac-Hydrin, for symptomatic recurrences. At her last visit for another health issue, she has reported this to be helpful.
Acknowledgments
The authors would like to acknowledge the unfailingly cheerful cooperation and expert assistance of the St. Vincent Mercy Medical Center library staff.
Corresponding author
Gary N. Fox, MD, 2200 Jefferson Avenue, Toledo, OH 43624. E-mail: [email protected].
1. Rosen T, Martin S. Variants of normal skin in blacks. In: Atlas of Black Dermatology. 1st ed. Boston, Mass: Little, Brown; 1981;12-13.
2. Dilaimy MS, Owen WR, Sina B. Keratosis punctata of the palmar creases. Cutis 1984;33:394-396.
3. Rustad OJ, Vance JC. Punctate keratoses of the palms and soles and keratotic pits of the palmar creases. J Am Acad Dermatol 1990;22:468-476.
4. Kinsley-Scott TR, Young RJ, 3rd, Meffert JJ. Keratosis punctata of the instep. Cutis 2003;72:451-452.
5. Kong MS, Harford R, O’Neill JT. Keratosis punctata palmoplantaris controlled with topical retinoids: a case report and review of the literature. Cutis 2004;74:173-179.
6. Anderson WA, Elam MD, Lambert WC. Keratosis punctata and atopy. Report of 31 cases with a prospective study of prevalence. Arch Dermatol 1984;120:884-890.
7. Asadi AK. Type I hereditary punctate keratoderma. Dermatol Online J 2003;9:38.-
8. Weiss RM, Rasmussen JE. Keratosis punctata of the palmar creases. Arch Dermatol 1980;116:669-671.
9. Penas PF, Rios-Buceta L, Sanchez-Perez J, Dorado-Bris JM, Aragues M. Keratosis punctata of the palmar creases: case report and prevalence study in Caucasians. Dermatology 1994;188:200-202.
10. Del-Rio E, Vazquez-Veiga H, Aguilar A, Velez A, Sanchez Yus E. Keratosis punctata of the palmar creases. A report on three generations, demonstrating an association with ichthyosis vulgaris and evidence of involvement of the acrosyringium. Clin Exp Dermatol 1994;19:165-167.
11. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St. Louis, Mo: Mosby; 2004.
12. Osman Y, Daly TJ, Don PC. Spiny keratoderma of the palms and soles. J Am Acad Dermatol 1992;26:879-881.
13. Betlloch I, Vergara G, Albares MP, Pascual JC, Silvestre JF, Botella R. Aquagenic keratoderma. J Eur Acad Dermatol Venereol 2003;17:306-307.
14. Gimenez-Garcia R, Sanchez-Ramon S, Cuellar-Olmedo LA. Palmoplantar pustulosis: a clinicoepidemiological study. The relationship between tobacco use and thyroid function. J Eur Acad Dermatol Venereol 2003;17:276-279.
15. Gunduz K, Inanir I, Turkdogan P, Sacar H. Palmoplantar lichen planus presenting with vesicle-like papules. J Dermatol 2003;30:337-340. 0
16. Vignale R, Yusin A, Panuncio A, Abulafia J, Reyno Z, Vaglio A. Cole disease: hypopigmentation with punctate keratosis of the palms and soles. Pediatr Dermatol 2002;19:302-306.
17. Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis. Acta Derm Venereol 2002;82:192-195.
A 54-year-old African American woman came to the office with a problem on her hands that began about 10 years before: small, hard plugs that formed on her palms (Figures 1 and 2). These areas remain tender for 1 to 2 days after the plugs first form and while they “stick up.” After a few days, the plugs fall out, leaving small pits. The patient experienced no other symptoms once the plugs fall out; just the appearance of her palms.
Many years ago, a physician tried freezing the lesions, believing them to be warts. That therapy provided no benefit. The patient found that filing down the plugs and lubricating them with white petrolatum helped during the symptomatic phase.
The patient was married, with no history of sexually transmitted diseases or significant occupational exposures. She did take medication to control her hypertension, hyperlipidemia, and hypothyroidism. However, the problem with her hands predated taking these medications. There was no personal or family history of skin malignancy. The remainder of the skin examination was unremarkable.
FIGURE 1
Lesions on left palm
FIGURE 2
Lateral view
What is your diagnosis?
Diagnosis: keratosis punctata of the palmar creases
Keratosis punctata of the palmar creases (KPPC) is a benign, largely asymptomatic condition of the hands, seen almost exclusively those with African ancestry. KPPC presents as small keratotic papules (Figure 3) that evolve into discreet conical pits (Figure 4).1,2 Although KPPC is not a novel or rare condition among African Americans, it is not found in standard dermatology texts used by primary care physicians. However, reference to KPPC may be found in ethnic dermatology texts, including reference to it as “a common normal finding in the black palm.”1
The lesions of KPPC characteristically are 1 to 5 mm in diameter, sharply defined hyperkeratotic pits that occur in the flexural creases of the hands, both on the palms and volar surfaces of the fingers. KPPC has also referred to as keratotic pits of the palmar creases, punctate keratoses of the palmar creases, keratoderma punctata, hyperkeratosis penetrans, lenticular atrophia of the palmar creases, and hyperkeratosis punctata of the palmar creases.
FIGURE 3
Close-up of a “plug”
FIGURE 4
Close-up of a “pit”
Distinguishing KPPC from KPPP
KPPC has also been regarded as a variant of keratosis punctata palmaris et plantaris (KPPP).3,4 KPPP and KPPC share some similarities with respect to the size and number of lesions per palm, probable exacerbation by trauma, and predilection for occurring in those of Afro-Caribbean descent.1,3,4
Historically, there has been some confusion in distinguishing KPPC from KPPP—in fact, it is possible for the 2 conditions to occur simultaneously. The papular lesions of KPPP tend to occur over the entire palm, volar wrist, and medial aspects of the feet. These entities differ in age at onset, prevalence, symptoms, and prognosis.
Sources differ regarding the average age of onset for KPPP; some report onset from infancy to 70 years.5-7 For KPPC, the age of onset generally is between 15 and 40 years.3 Among African Americans, the prevalence of KPPC is between 1.9% and 3.1%,3,6,8 whereas the prevalence of KPPP may be up to 11%.3,4 While KPPP is largely asymptomatic, the lesions of KPPC tend to be noticed more often. Once present, KPPP lesions usually remain stable over time, whereas, KPPC lesions usually increase in number and size.3
Demographics and causes
KPPC is rarely seen in Caucasians. Of 1001 white patients examined for palmar lesions, none fulfilled the diagnostic criteria for KPPC.9 In a study of 534 patients, Weiss et al discovered 7 cases— all in African American patients and representing 3.1% of this racial group.8
The cause of KPPC is unknown. No medications have been implicated, and it has been difficult to link it to a virus.3 Although some authors have suggested that KPPC represents flexural calluses related to manual labor, lesions also occur in patients without this history.1,5 There is no association between KPPC and arsenical agents or syphilis.8
It is generally believed that KPPC does not have a recognizable heritable pattern, though there may be exceptions.8 There may be a familial association with ichthyosis vulgaris and other disorders of keratinization. One report included 5 patients in 1 family with keratotic plugs of the palmar creases consistent with an autosomal dominant pattern of inheritance. The syndrome was associated with ichthyosis vulgaris in several family members.10 KPPP and KPPC might be the result of abnormal callus formation in predisposed individuals, as both conditions seem to be due to an abnormal hyperproliferative response to local trauma.3
Differential diagnosis
Punctate keratoses of the palms are fairly common frequently overlooked lesions. The differential diagnosis is extensive (Table), but there are several clinical features of KPPC that distinguish it from other hyperkeratotic conditions. The lesions of KPPC can be painful, have a predilection for jointcreases, and evolve into pits.3 KPPP is similar except not localized to the creases.4
Aquagenic keratoderma is a transitory condition afflicting young women and defined clinically by the appearance of palmar lesions accentuated after immersion in water. These lesions have a characteristic histological appearance (hyperkeratosis, dilated eccrine ducts).13
Palmoplantar pustulosis is characterized by chronically recurring sterile pustules on the palms and soles, usually found on an erythematous base, and a strong association with tobacco use.14Palmoplantar lichen planus may exhibit a variety of morphologic patterns including papules or plaques with pruritus, erythema, and compact hyperkeratosis.15
Cole disease is an uncommon disorder typified by distinctive cutaneous hyperpigmentation and punctate keratoses on the palms and soles. It is a congenital disease with an autosomal dominant inheritance pattern and phenotypic variability.16
Palmoplantar psoriasis is associated with manual labor in 50% of cases. Lesions are restricted to areas exposed to pressure. All patients with unilateral palmar lesions had them on their dominant hand. Biopsy may be necessary to differentiate hyperkeratotic eczema from psoriasis when just localized to the palms and soles.17
TABLE
Differential diagnosis of punctate keratoses of the hands and feet
| Acquired keratoses | Classic clinical description | Associations |
|---|---|---|
| Arsenical | Round, verrucous, or acuminate keratotic papules most common on palms and soles. Typically occur decades after chronic arsenic ingestion | Angiosarcoma of the liver, nonmelanoma skin cancer, bronchial adenocarcinoma |
| Idiopathic filiform porokeratoses | Multiple thin spiny keratotic projections on palms and soles | Breast, renal, colon, and lung cancer |
| Keratosis punctata of the palmar creases | Discrete, sharply marginated, hyperkeratotic, conical, 1–5 mm depressions confined to flexural creases | Dupuytren’s contracture, striate keratoderma, knuckle pads |
| Hereditary keratoses | Classic clinical description | Associations |
| Keratosis punctata palmoplantaris (type I), Buschke-Fischer-Brauer disease | Multiple 1–2 mm punctate keratoses of the palms and soles | Longitudinal nail dystrophy, lichen nitidus, ichthyosis, atopy, recalcitrant warts. Increased risk of malignancy |
| Spiny keratoderma (type II) | Small keratotic spines over entire palmoplantar surfaces. Resembles the spines of an old-fashioned music box | No predisposition to malignancy |
| Acrokeratoelastoidosis lichenoids (type III) | 2–4 mm round to oval papules on the borders of hands, feet and wrists. May be umbilicated and become confluent | Darier’s disease, Cowden’s disease |
| Adapted from Rustad et al 1990,5 Kong et al 2004,7 Asadi 2003,9 Habif 2004,11 and Osman et al 1992.12 | ||
Treatment options: keratolytic agents may help temporarily
The mainstay of therapy is informing the patient of the benign nature of the diagnosis and avoiding unnecessary and unhelpful therapies and diagnostic modalities. Therapy with keratolytic agents or systemic retinoids may temporarily improve symptoms of KPPC.
However, lesions tend to recur when the medications are stopped or decreased.5,7 Temporizing treatment of symptomatic keratoses, such as applying emollients and paring them, is all that is usually necessary. Systemic retinoids have far too many side effects to consider using in this completely benign condition.
Patient outcome
The patient was reassured by the explanation of the condition and chose to try a keratolytic/emollient agent, Lac-Hydrin, for symptomatic recurrences. At her last visit for another health issue, she has reported this to be helpful.
Acknowledgments
The authors would like to acknowledge the unfailingly cheerful cooperation and expert assistance of the St. Vincent Mercy Medical Center library staff.
Corresponding author
Gary N. Fox, MD, 2200 Jefferson Avenue, Toledo, OH 43624. E-mail: [email protected].
A 54-year-old African American woman came to the office with a problem on her hands that began about 10 years before: small, hard plugs that formed on her palms (Figures 1 and 2). These areas remain tender for 1 to 2 days after the plugs first form and while they “stick up.” After a few days, the plugs fall out, leaving small pits. The patient experienced no other symptoms once the plugs fall out; just the appearance of her palms.
Many years ago, a physician tried freezing the lesions, believing them to be warts. That therapy provided no benefit. The patient found that filing down the plugs and lubricating them with white petrolatum helped during the symptomatic phase.
The patient was married, with no history of sexually transmitted diseases or significant occupational exposures. She did take medication to control her hypertension, hyperlipidemia, and hypothyroidism. However, the problem with her hands predated taking these medications. There was no personal or family history of skin malignancy. The remainder of the skin examination was unremarkable.
FIGURE 1
Lesions on left palm
FIGURE 2
Lateral view
What is your diagnosis?
Diagnosis: keratosis punctata of the palmar creases
Keratosis punctata of the palmar creases (KPPC) is a benign, largely asymptomatic condition of the hands, seen almost exclusively those with African ancestry. KPPC presents as small keratotic papules (Figure 3) that evolve into discreet conical pits (Figure 4).1,2 Although KPPC is not a novel or rare condition among African Americans, it is not found in standard dermatology texts used by primary care physicians. However, reference to KPPC may be found in ethnic dermatology texts, including reference to it as “a common normal finding in the black palm.”1
The lesions of KPPC characteristically are 1 to 5 mm in diameter, sharply defined hyperkeratotic pits that occur in the flexural creases of the hands, both on the palms and volar surfaces of the fingers. KPPC has also referred to as keratotic pits of the palmar creases, punctate keratoses of the palmar creases, keratoderma punctata, hyperkeratosis penetrans, lenticular atrophia of the palmar creases, and hyperkeratosis punctata of the palmar creases.
FIGURE 3
Close-up of a “plug”
FIGURE 4
Close-up of a “pit”
Distinguishing KPPC from KPPP
KPPC has also been regarded as a variant of keratosis punctata palmaris et plantaris (KPPP).3,4 KPPP and KPPC share some similarities with respect to the size and number of lesions per palm, probable exacerbation by trauma, and predilection for occurring in those of Afro-Caribbean descent.1,3,4
Historically, there has been some confusion in distinguishing KPPC from KPPP—in fact, it is possible for the 2 conditions to occur simultaneously. The papular lesions of KPPP tend to occur over the entire palm, volar wrist, and medial aspects of the feet. These entities differ in age at onset, prevalence, symptoms, and prognosis.
Sources differ regarding the average age of onset for KPPP; some report onset from infancy to 70 years.5-7 For KPPC, the age of onset generally is between 15 and 40 years.3 Among African Americans, the prevalence of KPPC is between 1.9% and 3.1%,3,6,8 whereas the prevalence of KPPP may be up to 11%.3,4 While KPPP is largely asymptomatic, the lesions of KPPC tend to be noticed more often. Once present, KPPP lesions usually remain stable over time, whereas, KPPC lesions usually increase in number and size.3
Demographics and causes
KPPC is rarely seen in Caucasians. Of 1001 white patients examined for palmar lesions, none fulfilled the diagnostic criteria for KPPC.9 In a study of 534 patients, Weiss et al discovered 7 cases— all in African American patients and representing 3.1% of this racial group.8
The cause of KPPC is unknown. No medications have been implicated, and it has been difficult to link it to a virus.3 Although some authors have suggested that KPPC represents flexural calluses related to manual labor, lesions also occur in patients without this history.1,5 There is no association between KPPC and arsenical agents or syphilis.8
It is generally believed that KPPC does not have a recognizable heritable pattern, though there may be exceptions.8 There may be a familial association with ichthyosis vulgaris and other disorders of keratinization. One report included 5 patients in 1 family with keratotic plugs of the palmar creases consistent with an autosomal dominant pattern of inheritance. The syndrome was associated with ichthyosis vulgaris in several family members.10 KPPP and KPPC might be the result of abnormal callus formation in predisposed individuals, as both conditions seem to be due to an abnormal hyperproliferative response to local trauma.3
Differential diagnosis
Punctate keratoses of the palms are fairly common frequently overlooked lesions. The differential diagnosis is extensive (Table), but there are several clinical features of KPPC that distinguish it from other hyperkeratotic conditions. The lesions of KPPC can be painful, have a predilection for jointcreases, and evolve into pits.3 KPPP is similar except not localized to the creases.4
Aquagenic keratoderma is a transitory condition afflicting young women and defined clinically by the appearance of palmar lesions accentuated after immersion in water. These lesions have a characteristic histological appearance (hyperkeratosis, dilated eccrine ducts).13
Palmoplantar pustulosis is characterized by chronically recurring sterile pustules on the palms and soles, usually found on an erythematous base, and a strong association with tobacco use.14Palmoplantar lichen planus may exhibit a variety of morphologic patterns including papules or plaques with pruritus, erythema, and compact hyperkeratosis.15
Cole disease is an uncommon disorder typified by distinctive cutaneous hyperpigmentation and punctate keratoses on the palms and soles. It is a congenital disease with an autosomal dominant inheritance pattern and phenotypic variability.16
Palmoplantar psoriasis is associated with manual labor in 50% of cases. Lesions are restricted to areas exposed to pressure. All patients with unilateral palmar lesions had them on their dominant hand. Biopsy may be necessary to differentiate hyperkeratotic eczema from psoriasis when just localized to the palms and soles.17
TABLE
Differential diagnosis of punctate keratoses of the hands and feet
| Acquired keratoses | Classic clinical description | Associations |
|---|---|---|
| Arsenical | Round, verrucous, or acuminate keratotic papules most common on palms and soles. Typically occur decades after chronic arsenic ingestion | Angiosarcoma of the liver, nonmelanoma skin cancer, bronchial adenocarcinoma |
| Idiopathic filiform porokeratoses | Multiple thin spiny keratotic projections on palms and soles | Breast, renal, colon, and lung cancer |
| Keratosis punctata of the palmar creases | Discrete, sharply marginated, hyperkeratotic, conical, 1–5 mm depressions confined to flexural creases | Dupuytren’s contracture, striate keratoderma, knuckle pads |
| Hereditary keratoses | Classic clinical description | Associations |
| Keratosis punctata palmoplantaris (type I), Buschke-Fischer-Brauer disease | Multiple 1–2 mm punctate keratoses of the palms and soles | Longitudinal nail dystrophy, lichen nitidus, ichthyosis, atopy, recalcitrant warts. Increased risk of malignancy |
| Spiny keratoderma (type II) | Small keratotic spines over entire palmoplantar surfaces. Resembles the spines of an old-fashioned music box | No predisposition to malignancy |
| Acrokeratoelastoidosis lichenoids (type III) | 2–4 mm round to oval papules on the borders of hands, feet and wrists. May be umbilicated and become confluent | Darier’s disease, Cowden’s disease |
| Adapted from Rustad et al 1990,5 Kong et al 2004,7 Asadi 2003,9 Habif 2004,11 and Osman et al 1992.12 | ||
Treatment options: keratolytic agents may help temporarily
The mainstay of therapy is informing the patient of the benign nature of the diagnosis and avoiding unnecessary and unhelpful therapies and diagnostic modalities. Therapy with keratolytic agents or systemic retinoids may temporarily improve symptoms of KPPC.
However, lesions tend to recur when the medications are stopped or decreased.5,7 Temporizing treatment of symptomatic keratoses, such as applying emollients and paring them, is all that is usually necessary. Systemic retinoids have far too many side effects to consider using in this completely benign condition.
Patient outcome
The patient was reassured by the explanation of the condition and chose to try a keratolytic/emollient agent, Lac-Hydrin, for symptomatic recurrences. At her last visit for another health issue, she has reported this to be helpful.
Acknowledgments
The authors would like to acknowledge the unfailingly cheerful cooperation and expert assistance of the St. Vincent Mercy Medical Center library staff.
Corresponding author
Gary N. Fox, MD, 2200 Jefferson Avenue, Toledo, OH 43624. E-mail: [email protected].
1. Rosen T, Martin S. Variants of normal skin in blacks. In: Atlas of Black Dermatology. 1st ed. Boston, Mass: Little, Brown; 1981;12-13.
2. Dilaimy MS, Owen WR, Sina B. Keratosis punctata of the palmar creases. Cutis 1984;33:394-396.
3. Rustad OJ, Vance JC. Punctate keratoses of the palms and soles and keratotic pits of the palmar creases. J Am Acad Dermatol 1990;22:468-476.
4. Kinsley-Scott TR, Young RJ, 3rd, Meffert JJ. Keratosis punctata of the instep. Cutis 2003;72:451-452.
5. Kong MS, Harford R, O’Neill JT. Keratosis punctata palmoplantaris controlled with topical retinoids: a case report and review of the literature. Cutis 2004;74:173-179.
6. Anderson WA, Elam MD, Lambert WC. Keratosis punctata and atopy. Report of 31 cases with a prospective study of prevalence. Arch Dermatol 1984;120:884-890.
7. Asadi AK. Type I hereditary punctate keratoderma. Dermatol Online J 2003;9:38.-
8. Weiss RM, Rasmussen JE. Keratosis punctata of the palmar creases. Arch Dermatol 1980;116:669-671.
9. Penas PF, Rios-Buceta L, Sanchez-Perez J, Dorado-Bris JM, Aragues M. Keratosis punctata of the palmar creases: case report and prevalence study in Caucasians. Dermatology 1994;188:200-202.
10. Del-Rio E, Vazquez-Veiga H, Aguilar A, Velez A, Sanchez Yus E. Keratosis punctata of the palmar creases. A report on three generations, demonstrating an association with ichthyosis vulgaris and evidence of involvement of the acrosyringium. Clin Exp Dermatol 1994;19:165-167.
11. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St. Louis, Mo: Mosby; 2004.
12. Osman Y, Daly TJ, Don PC. Spiny keratoderma of the palms and soles. J Am Acad Dermatol 1992;26:879-881.
13. Betlloch I, Vergara G, Albares MP, Pascual JC, Silvestre JF, Botella R. Aquagenic keratoderma. J Eur Acad Dermatol Venereol 2003;17:306-307.
14. Gimenez-Garcia R, Sanchez-Ramon S, Cuellar-Olmedo LA. Palmoplantar pustulosis: a clinicoepidemiological study. The relationship between tobacco use and thyroid function. J Eur Acad Dermatol Venereol 2003;17:276-279.
15. Gunduz K, Inanir I, Turkdogan P, Sacar H. Palmoplantar lichen planus presenting with vesicle-like papules. J Dermatol 2003;30:337-340. 0
16. Vignale R, Yusin A, Panuncio A, Abulafia J, Reyno Z, Vaglio A. Cole disease: hypopigmentation with punctate keratosis of the palms and soles. Pediatr Dermatol 2002;19:302-306.
17. Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis. Acta Derm Venereol 2002;82:192-195.
1. Rosen T, Martin S. Variants of normal skin in blacks. In: Atlas of Black Dermatology. 1st ed. Boston, Mass: Little, Brown; 1981;12-13.
2. Dilaimy MS, Owen WR, Sina B. Keratosis punctata of the palmar creases. Cutis 1984;33:394-396.
3. Rustad OJ, Vance JC. Punctate keratoses of the palms and soles and keratotic pits of the palmar creases. J Am Acad Dermatol 1990;22:468-476.
4. Kinsley-Scott TR, Young RJ, 3rd, Meffert JJ. Keratosis punctata of the instep. Cutis 2003;72:451-452.
5. Kong MS, Harford R, O’Neill JT. Keratosis punctata palmoplantaris controlled with topical retinoids: a case report and review of the literature. Cutis 2004;74:173-179.
6. Anderson WA, Elam MD, Lambert WC. Keratosis punctata and atopy. Report of 31 cases with a prospective study of prevalence. Arch Dermatol 1984;120:884-890.
7. Asadi AK. Type I hereditary punctate keratoderma. Dermatol Online J 2003;9:38.-
8. Weiss RM, Rasmussen JE. Keratosis punctata of the palmar creases. Arch Dermatol 1980;116:669-671.
9. Penas PF, Rios-Buceta L, Sanchez-Perez J, Dorado-Bris JM, Aragues M. Keratosis punctata of the palmar creases: case report and prevalence study in Caucasians. Dermatology 1994;188:200-202.
10. Del-Rio E, Vazquez-Veiga H, Aguilar A, Velez A, Sanchez Yus E. Keratosis punctata of the palmar creases. A report on three generations, demonstrating an association with ichthyosis vulgaris and evidence of involvement of the acrosyringium. Clin Exp Dermatol 1994;19:165-167.
11. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St. Louis, Mo: Mosby; 2004.
12. Osman Y, Daly TJ, Don PC. Spiny keratoderma of the palms and soles. J Am Acad Dermatol 1992;26:879-881.
13. Betlloch I, Vergara G, Albares MP, Pascual JC, Silvestre JF, Botella R. Aquagenic keratoderma. J Eur Acad Dermatol Venereol 2003;17:306-307.
14. Gimenez-Garcia R, Sanchez-Ramon S, Cuellar-Olmedo LA. Palmoplantar pustulosis: a clinicoepidemiological study. The relationship between tobacco use and thyroid function. J Eur Acad Dermatol Venereol 2003;17:276-279.
15. Gunduz K, Inanir I, Turkdogan P, Sacar H. Palmoplantar lichen planus presenting with vesicle-like papules. J Dermatol 2003;30:337-340. 0
16. Vignale R, Yusin A, Panuncio A, Abulafia J, Reyno Z, Vaglio A. Cole disease: hypopigmentation with punctate keratosis of the palms and soles. Pediatr Dermatol 2002;19:302-306.
17. Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis. Acta Derm Venereol 2002;82:192-195.
Persistent itchy pink rings
A 57-year-old retired farm worker came into the clinic with itchy pink rings all over his abdomen, legs, and arms. He said that the rash began 13 years ago, soon after he had retired from working in the fields. He said he had been exposed to pesticide chemicals but no other toxins. He’d never received a diagnosis of atopy or “sensitive skin.” He had sun damage on his lower arms, but none on the areas where the rash appeared.
Over the years, he had tried over-the-counter topical steroids, antifungals, and oral antihistamines, all with minimal relief. Furthermore, the lesions did not respond to 2 courses of oral antifungal medications. He said, “some days it itches more than others, but I just deal with it.” The patient was otherwise healthy and on no medications. He had been treated for active tuberculosis in the past but had no symptoms at this time. Out of his frustration, he recently began using paint thinner on the lesions. He thought the paint thinner “dried the rash out” and decreased the itching.
The distribution of the lesions can be seen in Figure 1. Erythematous rings can be seen on the abdomen and legs. These vary in size and shape. Figure 2 is a close-up of the left thigh showing the scale. There was no blanching erythema, no induration, no warmth, and no tenderness to palpation.
FIGURE 1
Rings on the trunk and legs
FIGURE 2
Close-up of thigh
What is the Diagnosis?
Diagnosis: Erythema Annulare Centrifugum
This patient has a rare skin condition called erythema annulare centrifugum (EAC). The primary lesion begins as a group of pink/erythematous papules that spread peripherally as they clear centrally, forming a “trailing scale” (Figure 3) They can enlarge at a rate of 2 to 5 mm/d, producing a plaque or patch of annular, arcuate, or polycyclic shape.1 The margin is indurated and can vary in width from 4 to 6 mm. Vesicles may also be present. Distribution is usually on the thighs and legs but can also appear on the upper extremities (Figure 4), trunk, or face.
Differential diagnosis
Other causes of similar erythematous itchy rings to consider include pityriasis rosea, tinea corporis, psoriasis, nummular eczema, atopic dermatitis, drug reaction, and erythema migrans. The physical exam is the most effective method to differentiate between the above diagnoses—specifically, the distribution and morphology of the lesions. The natural history of the lesions can further narrow the diagnosis.
Pityriasis rosea has erythematous patches distributed on the trunk and extremities. These patches have a distinctive collarette border. The “herald patch,” the first patch to appear, is often oval and about 1 to 3 inches in diameter. Lesions classically follow skin lines to create a “Christmas tree” pattern on the back. Unlike EAC, pityriasis only lasts 6 to 8 weeks.
Tinea corporis (ringworm) is a fungal infection that is often a single scaly annular patch. Like EAC, it can be erythematous and is distributed anywhere on the body. When it presents as multiple lesions, it more closely resembles EAC. Unlike EAC, however, it should show branched hyphae with septae on a KOH prep and fungal culture would show growth of dermatophytes. Furthermore, it responds well to 4 to 6 weeks of antifungal treatment.
Psoriasis may present as plaques on the extensor surfaces of extremities. “Guttate” psoriasis (meaning “like water drops”) appears like small drops on the back and abdomen. Psoriatic plaques do not have central clearing or the trailing scale characteristic of EAC. Psoriatic plaques are often more responsive to steroids than EAC.
FIGURE 3
“Trailing scale”on abdomen
FIGURE 4
Ring on inner arm
Epidemiology and Pathophysiology
EAC is an uncommon inflammatory skin condition of unknown cause. Histologically, it resembles pityriasis rosea, having a superficial perivascular lymphocytic infiltrate with moderate epidermal acanthosis.1,2 The primary lesions spread peripherally and clear centrally at a maximum rate of 2 to 5 mm/d. Pruritus is common but not always present.1
Although the cause and exact pathogenesis are unknown, EAC may in some cases be triggered by drugs, bacterial infections (like tuberculosis), fungi, viruses, parasites, autoimmune diseases, neoplasms, liver diseases, and dysproteinemias.1,3 Other hypotheses suggest that it may be a type IV hypersensitivity reaction.1 The mean duration is 11 months, but it can vary from as little as 4 weeks to 34 years.1
Diagnostic Tests
No specific tests are needed to make the diagnosis of EAC. However, it is a good idea to do a KOH prep to rule out tinea corporis or candidiasis. This technique has a sensitivity of 88% and a specificity of 95% for tinea.4 Thus, a positive result is sufficient for diagnosis. Fungal culture may be useful if you believe that the diagnosis is truly fungal but the KOH result is negative.
If physical exam leads to suspicion of underlying disease, it is important to order the appropriate diagnostic lab tests. A few case reports have described EAC as the presenting sign in patients diagnosed with cancer approximately 2 years later.1
Treatment
It is helpful if you can find an underlying disease process because most cases of EAC clear as the underlying disease is treated. Review the current drugs and consider replacing any drug reported to have been associated with EAC: chloroquine, hydroxychloroquine, estrogen, cimetidine, penicillin, salicylates, piroxicam, hydrochlorothiazide, and amitriptyline.1
If you have minimal suspicion for an underlying disease process and no causative drug agent is found, most cases of EAC require no treatment and resolve spontaneously.1 However, if pruritus is significant, treat symptomatically. One option is to start with a topical high-potency steroid ointment twice daily. Use this regimen for 2 weeks, then assess response. If the response is good, you may continue a mid-potency steroid as needed or pulse-dosing of a high-potency steroid on the weekends. The goal is to minimize the itching while also minimizing the side effects associated with long-term steroid treatment. Treat as needed and taper when possible. Steroids may cause resolution of lesions but do not prevent recurrence.
A second option is topical calcipotriol, a topical vitamin D derivative. A recent case report has shown this to be beneficial,2 but this is an off-label indication. To date, no randomized clinical trials have been done to demonstrate the efficacy of any other treatment.
Patient Outcome
A KOH prep and a fungal culture were obtained to confidently rule out tinea. Both results were negative. We decided to order a chest x-ray because of his past history of active tuberculosis; the test result was negative. To investigate for any underlying diseases, we ordered a complete blood count, chemistry panel, and erythrocyte sedimentation rate, which all showed normal results.
We explained the diagnosis of EAC as a chronic condition of unknown cause. Since steroids did not provide any relief for him in the past, we offered the option of using calcipotriol ointment, even though the evidence is only based on 1 case report.2 He chose to try the calcipotriol. We warned the patient to not use paint thinner because inhalation and skin exposure are harmful. The patient agreed to stop using it.
Corresponding author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Willard RJ, Montemarano AD. Erythema annulare centrifugum. eMedicine website. Available at: http://www.emedicine.com/derm/topic131.htm.
2. Gniadecki R. Case report: Calcipotriol for erythema annulare centrifugum. 2002 British Association of Dermatologists.British Journal of Dermatology 2002;146:317-319.
3. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-462.
4. Bergus, GR, Johnson JS. Superficial tinea infections. Am Fam Physician 1993;48:259-268.
A 57-year-old retired farm worker came into the clinic with itchy pink rings all over his abdomen, legs, and arms. He said that the rash began 13 years ago, soon after he had retired from working in the fields. He said he had been exposed to pesticide chemicals but no other toxins. He’d never received a diagnosis of atopy or “sensitive skin.” He had sun damage on his lower arms, but none on the areas where the rash appeared.
Over the years, he had tried over-the-counter topical steroids, antifungals, and oral antihistamines, all with minimal relief. Furthermore, the lesions did not respond to 2 courses of oral antifungal medications. He said, “some days it itches more than others, but I just deal with it.” The patient was otherwise healthy and on no medications. He had been treated for active tuberculosis in the past but had no symptoms at this time. Out of his frustration, he recently began using paint thinner on the lesions. He thought the paint thinner “dried the rash out” and decreased the itching.
The distribution of the lesions can be seen in Figure 1. Erythematous rings can be seen on the abdomen and legs. These vary in size and shape. Figure 2 is a close-up of the left thigh showing the scale. There was no blanching erythema, no induration, no warmth, and no tenderness to palpation.
FIGURE 1
Rings on the trunk and legs
FIGURE 2
Close-up of thigh
What is the Diagnosis?
Diagnosis: Erythema Annulare Centrifugum
This patient has a rare skin condition called erythema annulare centrifugum (EAC). The primary lesion begins as a group of pink/erythematous papules that spread peripherally as they clear centrally, forming a “trailing scale” (Figure 3) They can enlarge at a rate of 2 to 5 mm/d, producing a plaque or patch of annular, arcuate, or polycyclic shape.1 The margin is indurated and can vary in width from 4 to 6 mm. Vesicles may also be present. Distribution is usually on the thighs and legs but can also appear on the upper extremities (Figure 4), trunk, or face.
Differential diagnosis
Other causes of similar erythematous itchy rings to consider include pityriasis rosea, tinea corporis, psoriasis, nummular eczema, atopic dermatitis, drug reaction, and erythema migrans. The physical exam is the most effective method to differentiate between the above diagnoses—specifically, the distribution and morphology of the lesions. The natural history of the lesions can further narrow the diagnosis.
Pityriasis rosea has erythematous patches distributed on the trunk and extremities. These patches have a distinctive collarette border. The “herald patch,” the first patch to appear, is often oval and about 1 to 3 inches in diameter. Lesions classically follow skin lines to create a “Christmas tree” pattern on the back. Unlike EAC, pityriasis only lasts 6 to 8 weeks.
Tinea corporis (ringworm) is a fungal infection that is often a single scaly annular patch. Like EAC, it can be erythematous and is distributed anywhere on the body. When it presents as multiple lesions, it more closely resembles EAC. Unlike EAC, however, it should show branched hyphae with septae on a KOH prep and fungal culture would show growth of dermatophytes. Furthermore, it responds well to 4 to 6 weeks of antifungal treatment.
Psoriasis may present as plaques on the extensor surfaces of extremities. “Guttate” psoriasis (meaning “like water drops”) appears like small drops on the back and abdomen. Psoriatic plaques do not have central clearing or the trailing scale characteristic of EAC. Psoriatic plaques are often more responsive to steroids than EAC.
FIGURE 3
“Trailing scale”on abdomen
FIGURE 4
Ring on inner arm
Epidemiology and Pathophysiology
EAC is an uncommon inflammatory skin condition of unknown cause. Histologically, it resembles pityriasis rosea, having a superficial perivascular lymphocytic infiltrate with moderate epidermal acanthosis.1,2 The primary lesions spread peripherally and clear centrally at a maximum rate of 2 to 5 mm/d. Pruritus is common but not always present.1
Although the cause and exact pathogenesis are unknown, EAC may in some cases be triggered by drugs, bacterial infections (like tuberculosis), fungi, viruses, parasites, autoimmune diseases, neoplasms, liver diseases, and dysproteinemias.1,3 Other hypotheses suggest that it may be a type IV hypersensitivity reaction.1 The mean duration is 11 months, but it can vary from as little as 4 weeks to 34 years.1
Diagnostic Tests
No specific tests are needed to make the diagnosis of EAC. However, it is a good idea to do a KOH prep to rule out tinea corporis or candidiasis. This technique has a sensitivity of 88% and a specificity of 95% for tinea.4 Thus, a positive result is sufficient for diagnosis. Fungal culture may be useful if you believe that the diagnosis is truly fungal but the KOH result is negative.
If physical exam leads to suspicion of underlying disease, it is important to order the appropriate diagnostic lab tests. A few case reports have described EAC as the presenting sign in patients diagnosed with cancer approximately 2 years later.1
Treatment
It is helpful if you can find an underlying disease process because most cases of EAC clear as the underlying disease is treated. Review the current drugs and consider replacing any drug reported to have been associated with EAC: chloroquine, hydroxychloroquine, estrogen, cimetidine, penicillin, salicylates, piroxicam, hydrochlorothiazide, and amitriptyline.1
If you have minimal suspicion for an underlying disease process and no causative drug agent is found, most cases of EAC require no treatment and resolve spontaneously.1 However, if pruritus is significant, treat symptomatically. One option is to start with a topical high-potency steroid ointment twice daily. Use this regimen for 2 weeks, then assess response. If the response is good, you may continue a mid-potency steroid as needed or pulse-dosing of a high-potency steroid on the weekends. The goal is to minimize the itching while also minimizing the side effects associated with long-term steroid treatment. Treat as needed and taper when possible. Steroids may cause resolution of lesions but do not prevent recurrence.
A second option is topical calcipotriol, a topical vitamin D derivative. A recent case report has shown this to be beneficial,2 but this is an off-label indication. To date, no randomized clinical trials have been done to demonstrate the efficacy of any other treatment.
Patient Outcome
A KOH prep and a fungal culture were obtained to confidently rule out tinea. Both results were negative. We decided to order a chest x-ray because of his past history of active tuberculosis; the test result was negative. To investigate for any underlying diseases, we ordered a complete blood count, chemistry panel, and erythrocyte sedimentation rate, which all showed normal results.
We explained the diagnosis of EAC as a chronic condition of unknown cause. Since steroids did not provide any relief for him in the past, we offered the option of using calcipotriol ointment, even though the evidence is only based on 1 case report.2 He chose to try the calcipotriol. We warned the patient to not use paint thinner because inhalation and skin exposure are harmful. The patient agreed to stop using it.
Corresponding author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
A 57-year-old retired farm worker came into the clinic with itchy pink rings all over his abdomen, legs, and arms. He said that the rash began 13 years ago, soon after he had retired from working in the fields. He said he had been exposed to pesticide chemicals but no other toxins. He’d never received a diagnosis of atopy or “sensitive skin.” He had sun damage on his lower arms, but none on the areas where the rash appeared.
Over the years, he had tried over-the-counter topical steroids, antifungals, and oral antihistamines, all with minimal relief. Furthermore, the lesions did not respond to 2 courses of oral antifungal medications. He said, “some days it itches more than others, but I just deal with it.” The patient was otherwise healthy and on no medications. He had been treated for active tuberculosis in the past but had no symptoms at this time. Out of his frustration, he recently began using paint thinner on the lesions. He thought the paint thinner “dried the rash out” and decreased the itching.
The distribution of the lesions can be seen in Figure 1. Erythematous rings can be seen on the abdomen and legs. These vary in size and shape. Figure 2 is a close-up of the left thigh showing the scale. There was no blanching erythema, no induration, no warmth, and no tenderness to palpation.
FIGURE 1
Rings on the trunk and legs
FIGURE 2
Close-up of thigh
What is the Diagnosis?
Diagnosis: Erythema Annulare Centrifugum
This patient has a rare skin condition called erythema annulare centrifugum (EAC). The primary lesion begins as a group of pink/erythematous papules that spread peripherally as they clear centrally, forming a “trailing scale” (Figure 3) They can enlarge at a rate of 2 to 5 mm/d, producing a plaque or patch of annular, arcuate, or polycyclic shape.1 The margin is indurated and can vary in width from 4 to 6 mm. Vesicles may also be present. Distribution is usually on the thighs and legs but can also appear on the upper extremities (Figure 4), trunk, or face.
Differential diagnosis
Other causes of similar erythematous itchy rings to consider include pityriasis rosea, tinea corporis, psoriasis, nummular eczema, atopic dermatitis, drug reaction, and erythema migrans. The physical exam is the most effective method to differentiate between the above diagnoses—specifically, the distribution and morphology of the lesions. The natural history of the lesions can further narrow the diagnosis.
Pityriasis rosea has erythematous patches distributed on the trunk and extremities. These patches have a distinctive collarette border. The “herald patch,” the first patch to appear, is often oval and about 1 to 3 inches in diameter. Lesions classically follow skin lines to create a “Christmas tree” pattern on the back. Unlike EAC, pityriasis only lasts 6 to 8 weeks.
Tinea corporis (ringworm) is a fungal infection that is often a single scaly annular patch. Like EAC, it can be erythematous and is distributed anywhere on the body. When it presents as multiple lesions, it more closely resembles EAC. Unlike EAC, however, it should show branched hyphae with septae on a KOH prep and fungal culture would show growth of dermatophytes. Furthermore, it responds well to 4 to 6 weeks of antifungal treatment.
Psoriasis may present as plaques on the extensor surfaces of extremities. “Guttate” psoriasis (meaning “like water drops”) appears like small drops on the back and abdomen. Psoriatic plaques do not have central clearing or the trailing scale characteristic of EAC. Psoriatic plaques are often more responsive to steroids than EAC.
FIGURE 3
“Trailing scale”on abdomen
FIGURE 4
Ring on inner arm
Epidemiology and Pathophysiology
EAC is an uncommon inflammatory skin condition of unknown cause. Histologically, it resembles pityriasis rosea, having a superficial perivascular lymphocytic infiltrate with moderate epidermal acanthosis.1,2 The primary lesions spread peripherally and clear centrally at a maximum rate of 2 to 5 mm/d. Pruritus is common but not always present.1
Although the cause and exact pathogenesis are unknown, EAC may in some cases be triggered by drugs, bacterial infections (like tuberculosis), fungi, viruses, parasites, autoimmune diseases, neoplasms, liver diseases, and dysproteinemias.1,3 Other hypotheses suggest that it may be a type IV hypersensitivity reaction.1 The mean duration is 11 months, but it can vary from as little as 4 weeks to 34 years.1
Diagnostic Tests
No specific tests are needed to make the diagnosis of EAC. However, it is a good idea to do a KOH prep to rule out tinea corporis or candidiasis. This technique has a sensitivity of 88% and a specificity of 95% for tinea.4 Thus, a positive result is sufficient for diagnosis. Fungal culture may be useful if you believe that the diagnosis is truly fungal but the KOH result is negative.
If physical exam leads to suspicion of underlying disease, it is important to order the appropriate diagnostic lab tests. A few case reports have described EAC as the presenting sign in patients diagnosed with cancer approximately 2 years later.1
Treatment
It is helpful if you can find an underlying disease process because most cases of EAC clear as the underlying disease is treated. Review the current drugs and consider replacing any drug reported to have been associated with EAC: chloroquine, hydroxychloroquine, estrogen, cimetidine, penicillin, salicylates, piroxicam, hydrochlorothiazide, and amitriptyline.1
If you have minimal suspicion for an underlying disease process and no causative drug agent is found, most cases of EAC require no treatment and resolve spontaneously.1 However, if pruritus is significant, treat symptomatically. One option is to start with a topical high-potency steroid ointment twice daily. Use this regimen for 2 weeks, then assess response. If the response is good, you may continue a mid-potency steroid as needed or pulse-dosing of a high-potency steroid on the weekends. The goal is to minimize the itching while also minimizing the side effects associated with long-term steroid treatment. Treat as needed and taper when possible. Steroids may cause resolution of lesions but do not prevent recurrence.
A second option is topical calcipotriol, a topical vitamin D derivative. A recent case report has shown this to be beneficial,2 but this is an off-label indication. To date, no randomized clinical trials have been done to demonstrate the efficacy of any other treatment.
Patient Outcome
A KOH prep and a fungal culture were obtained to confidently rule out tinea. Both results were negative. We decided to order a chest x-ray because of his past history of active tuberculosis; the test result was negative. To investigate for any underlying diseases, we ordered a complete blood count, chemistry panel, and erythrocyte sedimentation rate, which all showed normal results.
We explained the diagnosis of EAC as a chronic condition of unknown cause. Since steroids did not provide any relief for him in the past, we offered the option of using calcipotriol ointment, even though the evidence is only based on 1 case report.2 He chose to try the calcipotriol. We warned the patient to not use paint thinner because inhalation and skin exposure are harmful. The patient agreed to stop using it.
Corresponding author
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected].
1. Willard RJ, Montemarano AD. Erythema annulare centrifugum. eMedicine website. Available at: http://www.emedicine.com/derm/topic131.htm.
2. Gniadecki R. Case report: Calcipotriol for erythema annulare centrifugum. 2002 British Association of Dermatologists.British Journal of Dermatology 2002;146:317-319.
3. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-462.
4. Bergus, GR, Johnson JS. Superficial tinea infections. Am Fam Physician 1993;48:259-268.
1. Willard RJ, Montemarano AD. Erythema annulare centrifugum. eMedicine website. Available at: http://www.emedicine.com/derm/topic131.htm.
2. Gniadecki R. Case report: Calcipotriol for erythema annulare centrifugum. 2002 British Association of Dermatologists.British Journal of Dermatology 2002;146:317-319.
3. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-462.
4. Bergus, GR, Johnson JS. Superficial tinea infections. Am Fam Physician 1993;48:259-268.
A sore and sensitive tongue
A 40-year-old woman came into the office with a 1-year history of painful red lesions with white striations on her tongue (Figure 1). The lesions caused burning after she ate spicy food and an increased sensitivity to mouthwash. She reported a slow, progressive onset and worsening of her condition, with intensifying symptoms during periods of emotional stress.
On physical examination, we observed an erythematous and cream-colored reticulated patch with a few small focal areas of erosion covering the dorsal tongue. The remainder of the oral mucosa appeared normal. She also had thinning and ridging of the toenails (Figure 2).
What is the most likely diagnosis?
How would you manage this case?
FIGURE 1
Tongue with painful lesions
FIGURE 2
Thinning toenails
Diagnosis: oral lichen planus
Compared with the more self-limited nature of its cutaneous counterpart, oral lichen planus (OLP) causes more chronic inflammatory lesions, resulting in increased morbidity and a greater therapeutic challenge for physicians. Of the 3 basic clinical morphologies of the disease (reticular, erythematous, and erosive), the last form tends to be more symptomatic and prompts patients to seek treatment.1,2 The more asymptomatic reticular form is the most easily recognizable variant and may cause increased symptoms when involving the tongue, typically manifesting on the dorsal surface.1
In contrast to reticular lesions in other oral sites, tongue lesions do not usually exhibit the characteristic interlacing pattern of white, raised striae; instead they manifest as well-demarcated areas with a patch-like pattern of erythematous, atrophic, and keratotic regions. 3 Erosive OLP more commonly involves the lateral tongue and displays erythematous or ulcerated areas with peripheral keratosis, forming fine centrifugal striae.3
Oral lichen planus affects approximately 1% to 4% of the population 4 and is seen most commonly in older women.1,5,6 It has a predilection for bilateral involvement of the buccal mucosa but may also affect (in descending order of frequency) the tongue, gingiva, lips, floor of the mouth, and palate.1,2 Isolated involvement of only 1 oral site is infrequent, with the exception of gingival lesions.1 Extraoral cutaneous lichen planus has been reported in 16% to 44% of patients with oral disease; it most frequently involves the genital mucosa, as well as the scalp, nails, and esophageal and ocular mucosae.4 A multidisciplinary approach including generalists, dermatologists, otolaryngologists, ophthalmologists, and gynecologists may be necessary for patient evaluation and management.
Pathogenesis of oral lichen planus
Although the precise cause of OLP is unknown, its pathogenesis has been linked to an autoimmune mechanism involving autocytotoxic CD8+ T cells, which trigger apoptosis (programmed cell death) of basal keratinocytes.4,7 An imbalance between T cell helper and suppressor activity has also been observed.1,7
Complications: Carcinoma, chronic liver disease
Malignant transformation to squamous cell carcinoma (SCC) is seen in 0.4% to 5% of patients with OLP, particularly those with erosive and erythematous disease.1,2 Increased risk factors for SCC have not been identified in these patients, but a greater prevalence of Candida albicansmay be associated with carcinogenesis, as may herpes simplex and human papilloma viruses, immunosuppressive therapy, and an inflammatory cytokine-rich microenvironment.1
In Japan and parts of Southern Europe, OLP has been associated with hepatitis C infection and chronic liver disease, but these findings have not been reproduced in patients in the US.
Making the diagnosis: exam, biopsy, immunofluorescence
A detailed history and physical examination are usually sufficient to diagnose OLP, although laboratory studies and biopsy may be required to exclude malignancy or distinguish OLP from conditions such as pemphigus vulgaris and mucous membrane pemphigoid. A biopsy is rarely needed, and should only be performed by physicians that have training in a tongue biopsy.
If necessary, biopsy samples should be obtained from the most representative sample area of the tongue. After administration of local anesthesia (lidocaine 1:100,000 with epinephrine) near the periphery of the site, biopsy is performed using a #15 blade scalpel or biopsy punch. An assistant may be needed to grasp the tongue by wrapping a gauze sponge over the tip and securing it firmly. When tongue biopsy sites are closed, deep sutures should be placed if necessary, and mucosal sutures should be placed relatively close together, as inadequately closed wounds on the tongue tend to reopen, resulting in bleeding and prolonged healing time.
Specimens should be placed in a 10% formalin solution for transportation to a pathologist. Patients should return to the clinic after 7 to 14 days for examination of the biopsy site and suture removal.
Microscopically, OLP demonstrates vacuolar degeneration, basal cell lysis, and liquefactive degeneration1 along with focal hyperkeratosis, a characteristic amorphous eosinophilic band at the basement membrane level, and a dense, bandlike subepithelial lymphocytic infiltrate.5,6 Direct immunofluorescence, displaying granular fibrino-gen and variable immunoglobulin deposited linearly near the basement membrane, and possibly cytoid bodies, is useful in cases of suspected OLP with nondiagnostic histological findings, as well as in those with gingival involvement.1
Differential diagnosis
The clinical differential diagnosis of OLP includes the autoimmune bullous diseases pemphigus vulgaris, mucous membrane pemphigoid, dermatitis herpetiformis, and linear immuno-globulin A disease, which can all be excluded with direct and indirect immunofluorescence studies. When clinically appropriate, biopsy specimens submitted for direct immunofluorescence must be preserved in Michel solution. Reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, anemic states, and several gastrointestinal diseases including oral Crohn disease and chronic hepatic disease, must also be ruled out.
Oral lichenoid reactions may develop as a hypersensitivity to dental materials and drugs, and must also be considered.1 Patch testing may help identify a contact allergy to dental prosthesis components, including gold, mercury, and palladium salts.
Management: steroids, immunosuppressants, surgery
Because fewer than 20% of patients experience total remission,5 treatment of OLP is chronic and palliative. Most symptomatic patients are taught to avoid exacerbating factors and given topical steroid therapy (dexamethasone rinse, fluocinolone gel, triamcinolone cream). Observation, intralesional and systemic corticosteroids, immunosuppressants, antifungals, retinoids, antimalarials, dapsone, oral psoralen-ultraviolet-light (PUVA) treatment, and surgical techniques (CO2 and neodymium: yttrium-aluminum-garnet [Nd:YAG] laser therapy, cryotherapy, and excision) are also employed, alone and in combination.5,8
Tacrolimus (Protopic), an immunomodulator, administered topically in 0.03% to 0.3% concentrations using Aquaphor or paraffin ointment base, has been shown effective and well tolerated in controlling symptoms,8,9 and is a less costly alternative to topical cyclosporine.9 It has been demonstrated effective prospective-ly,10 and is safe in long-term therapy11 of erosive OLP, but it has been reported in one case to cause hyperpigmentation of oral mucosa.12
A recent study13 demonstrated the utility of low-dose 308-nm excimer laser radiation for symptomatic OLP.
Patient management
Our patient was instructed to avoid foods and substances that caused irritation of her tongue and oral mucosa. In addition, she was prescribed topical fluocinolone gel 0.025% 3 times daily, and was given information about alternative treatment options, including tacrolimus and surgical therapy. She was instructed to perform gentle yet thorough daily oral hygiene and to follow-up in 6 months for re-examination.
Corresponding author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected].
December 2004’s Photo Rounds, “Rupturing bullae not respondign to antiobiotics,” left out the names of two of the article’s authors. The correct authors of the piece are John Sauret, MD, FAAFP, Sandra Yale, DO, and Ahunna Ahiarah, MD. We regret the error.
1. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2002;46:207-214.
2. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:644-649.
3. Allen CM, Blozis GG. Oral mucosal lesions. In Cummings CW, Schuller DE (eds): Otolaryngology: Head and Neck Surgery, vol 2, 2nd ed. St Louis, Mo: Mosby-Yearbook; 1993;1374-1375.
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:431-436.
5. Brown RS, Bottomley WK, Puente E, Lavigne GL. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med 1993;22:69-72.
6. Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A retrospective follow-up study of 611 patients. J Oral Pathol 1998;17:213-218.
7. Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:358-366.
8. Rozycki TW, Rogers RS, Pittelkow MR, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: A series of 13 patients. J Am Acad Dermatol 2002;46:27-34.
9. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcer-ative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 2002;46:35-41.
10. Olivier V, Lacour JP, Mousnier A, Garraffo R, Monteil RA, Ortonne JP. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus. An open prospective study. Arch Dermatol 2002;138:1335-1338.
11. Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol 2003;13:466-470.
12. Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol 2004;50-326.
13. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol 2004;140:415-420.
A 40-year-old woman came into the office with a 1-year history of painful red lesions with white striations on her tongue (Figure 1). The lesions caused burning after she ate spicy food and an increased sensitivity to mouthwash. She reported a slow, progressive onset and worsening of her condition, with intensifying symptoms during periods of emotional stress.
On physical examination, we observed an erythematous and cream-colored reticulated patch with a few small focal areas of erosion covering the dorsal tongue. The remainder of the oral mucosa appeared normal. She also had thinning and ridging of the toenails (Figure 2).
What is the most likely diagnosis?
How would you manage this case?
FIGURE 1
Tongue with painful lesions
FIGURE 2
Thinning toenails
Diagnosis: oral lichen planus
Compared with the more self-limited nature of its cutaneous counterpart, oral lichen planus (OLP) causes more chronic inflammatory lesions, resulting in increased morbidity and a greater therapeutic challenge for physicians. Of the 3 basic clinical morphologies of the disease (reticular, erythematous, and erosive), the last form tends to be more symptomatic and prompts patients to seek treatment.1,2 The more asymptomatic reticular form is the most easily recognizable variant and may cause increased symptoms when involving the tongue, typically manifesting on the dorsal surface.1
In contrast to reticular lesions in other oral sites, tongue lesions do not usually exhibit the characteristic interlacing pattern of white, raised striae; instead they manifest as well-demarcated areas with a patch-like pattern of erythematous, atrophic, and keratotic regions. 3 Erosive OLP more commonly involves the lateral tongue and displays erythematous or ulcerated areas with peripheral keratosis, forming fine centrifugal striae.3
Oral lichen planus affects approximately 1% to 4% of the population 4 and is seen most commonly in older women.1,5,6 It has a predilection for bilateral involvement of the buccal mucosa but may also affect (in descending order of frequency) the tongue, gingiva, lips, floor of the mouth, and palate.1,2 Isolated involvement of only 1 oral site is infrequent, with the exception of gingival lesions.1 Extraoral cutaneous lichen planus has been reported in 16% to 44% of patients with oral disease; it most frequently involves the genital mucosa, as well as the scalp, nails, and esophageal and ocular mucosae.4 A multidisciplinary approach including generalists, dermatologists, otolaryngologists, ophthalmologists, and gynecologists may be necessary for patient evaluation and management.
Pathogenesis of oral lichen planus
Although the precise cause of OLP is unknown, its pathogenesis has been linked to an autoimmune mechanism involving autocytotoxic CD8+ T cells, which trigger apoptosis (programmed cell death) of basal keratinocytes.4,7 An imbalance between T cell helper and suppressor activity has also been observed.1,7
Complications: Carcinoma, chronic liver disease
Malignant transformation to squamous cell carcinoma (SCC) is seen in 0.4% to 5% of patients with OLP, particularly those with erosive and erythematous disease.1,2 Increased risk factors for SCC have not been identified in these patients, but a greater prevalence of Candida albicansmay be associated with carcinogenesis, as may herpes simplex and human papilloma viruses, immunosuppressive therapy, and an inflammatory cytokine-rich microenvironment.1
In Japan and parts of Southern Europe, OLP has been associated with hepatitis C infection and chronic liver disease, but these findings have not been reproduced in patients in the US.
Making the diagnosis: exam, biopsy, immunofluorescence
A detailed history and physical examination are usually sufficient to diagnose OLP, although laboratory studies and biopsy may be required to exclude malignancy or distinguish OLP from conditions such as pemphigus vulgaris and mucous membrane pemphigoid. A biopsy is rarely needed, and should only be performed by physicians that have training in a tongue biopsy.
If necessary, biopsy samples should be obtained from the most representative sample area of the tongue. After administration of local anesthesia (lidocaine 1:100,000 with epinephrine) near the periphery of the site, biopsy is performed using a #15 blade scalpel or biopsy punch. An assistant may be needed to grasp the tongue by wrapping a gauze sponge over the tip and securing it firmly. When tongue biopsy sites are closed, deep sutures should be placed if necessary, and mucosal sutures should be placed relatively close together, as inadequately closed wounds on the tongue tend to reopen, resulting in bleeding and prolonged healing time.
Specimens should be placed in a 10% formalin solution for transportation to a pathologist. Patients should return to the clinic after 7 to 14 days for examination of the biopsy site and suture removal.
Microscopically, OLP demonstrates vacuolar degeneration, basal cell lysis, and liquefactive degeneration1 along with focal hyperkeratosis, a characteristic amorphous eosinophilic band at the basement membrane level, and a dense, bandlike subepithelial lymphocytic infiltrate.5,6 Direct immunofluorescence, displaying granular fibrino-gen and variable immunoglobulin deposited linearly near the basement membrane, and possibly cytoid bodies, is useful in cases of suspected OLP with nondiagnostic histological findings, as well as in those with gingival involvement.1
Differential diagnosis
The clinical differential diagnosis of OLP includes the autoimmune bullous diseases pemphigus vulgaris, mucous membrane pemphigoid, dermatitis herpetiformis, and linear immuno-globulin A disease, which can all be excluded with direct and indirect immunofluorescence studies. When clinically appropriate, biopsy specimens submitted for direct immunofluorescence must be preserved in Michel solution. Reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, anemic states, and several gastrointestinal diseases including oral Crohn disease and chronic hepatic disease, must also be ruled out.
Oral lichenoid reactions may develop as a hypersensitivity to dental materials and drugs, and must also be considered.1 Patch testing may help identify a contact allergy to dental prosthesis components, including gold, mercury, and palladium salts.
Management: steroids, immunosuppressants, surgery
Because fewer than 20% of patients experience total remission,5 treatment of OLP is chronic and palliative. Most symptomatic patients are taught to avoid exacerbating factors and given topical steroid therapy (dexamethasone rinse, fluocinolone gel, triamcinolone cream). Observation, intralesional and systemic corticosteroids, immunosuppressants, antifungals, retinoids, antimalarials, dapsone, oral psoralen-ultraviolet-light (PUVA) treatment, and surgical techniques (CO2 and neodymium: yttrium-aluminum-garnet [Nd:YAG] laser therapy, cryotherapy, and excision) are also employed, alone and in combination.5,8
Tacrolimus (Protopic), an immunomodulator, administered topically in 0.03% to 0.3% concentrations using Aquaphor or paraffin ointment base, has been shown effective and well tolerated in controlling symptoms,8,9 and is a less costly alternative to topical cyclosporine.9 It has been demonstrated effective prospective-ly,10 and is safe in long-term therapy11 of erosive OLP, but it has been reported in one case to cause hyperpigmentation of oral mucosa.12
A recent study13 demonstrated the utility of low-dose 308-nm excimer laser radiation for symptomatic OLP.
Patient management
Our patient was instructed to avoid foods and substances that caused irritation of her tongue and oral mucosa. In addition, she was prescribed topical fluocinolone gel 0.025% 3 times daily, and was given information about alternative treatment options, including tacrolimus and surgical therapy. She was instructed to perform gentle yet thorough daily oral hygiene and to follow-up in 6 months for re-examination.
Corresponding author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected].
December 2004’s Photo Rounds, “Rupturing bullae not respondign to antiobiotics,” left out the names of two of the article’s authors. The correct authors of the piece are John Sauret, MD, FAAFP, Sandra Yale, DO, and Ahunna Ahiarah, MD. We regret the error.
A 40-year-old woman came into the office with a 1-year history of painful red lesions with white striations on her tongue (Figure 1). The lesions caused burning after she ate spicy food and an increased sensitivity to mouthwash. She reported a slow, progressive onset and worsening of her condition, with intensifying symptoms during periods of emotional stress.
On physical examination, we observed an erythematous and cream-colored reticulated patch with a few small focal areas of erosion covering the dorsal tongue. The remainder of the oral mucosa appeared normal. She also had thinning and ridging of the toenails (Figure 2).
What is the most likely diagnosis?
How would you manage this case?
FIGURE 1
Tongue with painful lesions
FIGURE 2
Thinning toenails
Diagnosis: oral lichen planus
Compared with the more self-limited nature of its cutaneous counterpart, oral lichen planus (OLP) causes more chronic inflammatory lesions, resulting in increased morbidity and a greater therapeutic challenge for physicians. Of the 3 basic clinical morphologies of the disease (reticular, erythematous, and erosive), the last form tends to be more symptomatic and prompts patients to seek treatment.1,2 The more asymptomatic reticular form is the most easily recognizable variant and may cause increased symptoms when involving the tongue, typically manifesting on the dorsal surface.1
In contrast to reticular lesions in other oral sites, tongue lesions do not usually exhibit the characteristic interlacing pattern of white, raised striae; instead they manifest as well-demarcated areas with a patch-like pattern of erythematous, atrophic, and keratotic regions. 3 Erosive OLP more commonly involves the lateral tongue and displays erythematous or ulcerated areas with peripheral keratosis, forming fine centrifugal striae.3
Oral lichen planus affects approximately 1% to 4% of the population 4 and is seen most commonly in older women.1,5,6 It has a predilection for bilateral involvement of the buccal mucosa but may also affect (in descending order of frequency) the tongue, gingiva, lips, floor of the mouth, and palate.1,2 Isolated involvement of only 1 oral site is infrequent, with the exception of gingival lesions.1 Extraoral cutaneous lichen planus has been reported in 16% to 44% of patients with oral disease; it most frequently involves the genital mucosa, as well as the scalp, nails, and esophageal and ocular mucosae.4 A multidisciplinary approach including generalists, dermatologists, otolaryngologists, ophthalmologists, and gynecologists may be necessary for patient evaluation and management.
Pathogenesis of oral lichen planus
Although the precise cause of OLP is unknown, its pathogenesis has been linked to an autoimmune mechanism involving autocytotoxic CD8+ T cells, which trigger apoptosis (programmed cell death) of basal keratinocytes.4,7 An imbalance between T cell helper and suppressor activity has also been observed.1,7
Complications: Carcinoma, chronic liver disease
Malignant transformation to squamous cell carcinoma (SCC) is seen in 0.4% to 5% of patients with OLP, particularly those with erosive and erythematous disease.1,2 Increased risk factors for SCC have not been identified in these patients, but a greater prevalence of Candida albicansmay be associated with carcinogenesis, as may herpes simplex and human papilloma viruses, immunosuppressive therapy, and an inflammatory cytokine-rich microenvironment.1
In Japan and parts of Southern Europe, OLP has been associated with hepatitis C infection and chronic liver disease, but these findings have not been reproduced in patients in the US.
Making the diagnosis: exam, biopsy, immunofluorescence
A detailed history and physical examination are usually sufficient to diagnose OLP, although laboratory studies and biopsy may be required to exclude malignancy or distinguish OLP from conditions such as pemphigus vulgaris and mucous membrane pemphigoid. A biopsy is rarely needed, and should only be performed by physicians that have training in a tongue biopsy.
If necessary, biopsy samples should be obtained from the most representative sample area of the tongue. After administration of local anesthesia (lidocaine 1:100,000 with epinephrine) near the periphery of the site, biopsy is performed using a #15 blade scalpel or biopsy punch. An assistant may be needed to grasp the tongue by wrapping a gauze sponge over the tip and securing it firmly. When tongue biopsy sites are closed, deep sutures should be placed if necessary, and mucosal sutures should be placed relatively close together, as inadequately closed wounds on the tongue tend to reopen, resulting in bleeding and prolonged healing time.
Specimens should be placed in a 10% formalin solution for transportation to a pathologist. Patients should return to the clinic after 7 to 14 days for examination of the biopsy site and suture removal.
Microscopically, OLP demonstrates vacuolar degeneration, basal cell lysis, and liquefactive degeneration1 along with focal hyperkeratosis, a characteristic amorphous eosinophilic band at the basement membrane level, and a dense, bandlike subepithelial lymphocytic infiltrate.5,6 Direct immunofluorescence, displaying granular fibrino-gen and variable immunoglobulin deposited linearly near the basement membrane, and possibly cytoid bodies, is useful in cases of suspected OLP with nondiagnostic histological findings, as well as in those with gingival involvement.1
Differential diagnosis
The clinical differential diagnosis of OLP includes the autoimmune bullous diseases pemphigus vulgaris, mucous membrane pemphigoid, dermatitis herpetiformis, and linear immuno-globulin A disease, which can all be excluded with direct and indirect immunofluorescence studies. When clinically appropriate, biopsy specimens submitted for direct immunofluorescence must be preserved in Michel solution. Reactive keratoses, chronic hyperplastic candidosis, epithelial dysplasia, discoid lupus erythematosus, anemic states, and several gastrointestinal diseases including oral Crohn disease and chronic hepatic disease, must also be ruled out.
Oral lichenoid reactions may develop as a hypersensitivity to dental materials and drugs, and must also be considered.1 Patch testing may help identify a contact allergy to dental prosthesis components, including gold, mercury, and palladium salts.
Management: steroids, immunosuppressants, surgery
Because fewer than 20% of patients experience total remission,5 treatment of OLP is chronic and palliative. Most symptomatic patients are taught to avoid exacerbating factors and given topical steroid therapy (dexamethasone rinse, fluocinolone gel, triamcinolone cream). Observation, intralesional and systemic corticosteroids, immunosuppressants, antifungals, retinoids, antimalarials, dapsone, oral psoralen-ultraviolet-light (PUVA) treatment, and surgical techniques (CO2 and neodymium: yttrium-aluminum-garnet [Nd:YAG] laser therapy, cryotherapy, and excision) are also employed, alone and in combination.5,8
Tacrolimus (Protopic), an immunomodulator, administered topically in 0.03% to 0.3% concentrations using Aquaphor or paraffin ointment base, has been shown effective and well tolerated in controlling symptoms,8,9 and is a less costly alternative to topical cyclosporine.9 It has been demonstrated effective prospective-ly,10 and is safe in long-term therapy11 of erosive OLP, but it has been reported in one case to cause hyperpigmentation of oral mucosa.12
A recent study13 demonstrated the utility of low-dose 308-nm excimer laser radiation for symptomatic OLP.
Patient management
Our patient was instructed to avoid foods and substances that caused irritation of her tongue and oral mucosa. In addition, she was prescribed topical fluocinolone gel 0.025% 3 times daily, and was given information about alternative treatment options, including tacrolimus and surgical therapy. She was instructed to perform gentle yet thorough daily oral hygiene and to follow-up in 6 months for re-examination.
Corresponding author
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected].
December 2004’s Photo Rounds, “Rupturing bullae not respondign to antiobiotics,” left out the names of two of the article’s authors. The correct authors of the piece are John Sauret, MD, FAAFP, Sandra Yale, DO, and Ahunna Ahiarah, MD. We regret the error.
1. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2002;46:207-214.
2. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:644-649.
3. Allen CM, Blozis GG. Oral mucosal lesions. In Cummings CW, Schuller DE (eds): Otolaryngology: Head and Neck Surgery, vol 2, 2nd ed. St Louis, Mo: Mosby-Yearbook; 1993;1374-1375.
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:431-436.
5. Brown RS, Bottomley WK, Puente E, Lavigne GL. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med 1993;22:69-72.
6. Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A retrospective follow-up study of 611 patients. J Oral Pathol 1998;17:213-218.
7. Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:358-366.
8. Rozycki TW, Rogers RS, Pittelkow MR, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: A series of 13 patients. J Am Acad Dermatol 2002;46:27-34.
9. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcer-ative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 2002;46:35-41.
10. Olivier V, Lacour JP, Mousnier A, Garraffo R, Monteil RA, Ortonne JP. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus. An open prospective study. Arch Dermatol 2002;138:1335-1338.
11. Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol 2003;13:466-470.
12. Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol 2004;50-326.
13. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol 2004;140:415-420.
1. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2002;46:207-214.
2. Gorsky M, Raviv M, Moskona D, Laufer M, Bodner L. Clinical characteristics and treatment of patients with oral lichen planus in Israel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:644-649.
3. Allen CM, Blozis GG. Oral mucosal lesions. In Cummings CW, Schuller DE (eds): Otolaryngology: Head and Neck Surgery, vol 2, 2nd ed. St Louis, Mo: Mosby-Yearbook; 1993;1374-1375.
4. Eisen D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:431-436.
5. Brown RS, Bottomley WK, Puente E, Lavigne GL. A retrospective evaluation of 193 patients with oral lichen planus. J Oral Pathol Med 1993;22:69-72.
6. Thorn JJ, Holmstrup P, Rindum J, Pindborg JJ. Course of various clinical forms of oral lichen planus. A retrospective follow-up study of 611 patients. J Oral Pathol 1998;17:213-218.
7. Porter SR, Kirby A, Olsen I, Barrett W. Immunologic aspects of dermal and oral lichen planus. A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;83:358-366.
8. Rozycki TW, Rogers RS, Pittelkow MR, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: A series of 13 patients. J Am Acad Dermatol 2002;46:27-34.
9. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcer-ative oral lichen planus with topical tacrolimus. J Am Acad Dermatol 2002;46:35-41.
10. Olivier V, Lacour JP, Mousnier A, Garraffo R, Monteil RA, Ortonne JP. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus. An open prospective study. Arch Dermatol 2002;138:1335-1338.
11. Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol 2003;13:466-470.
12. Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol 2004;50-326.
13. Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol 2004;140:415-420.