User login
Itching and rash in a boy and his grandmother
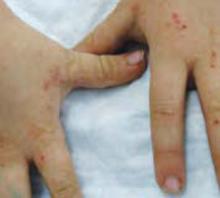
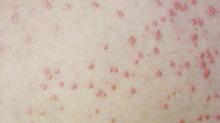
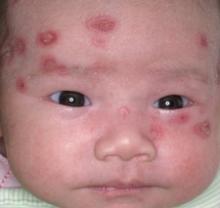
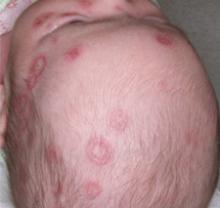
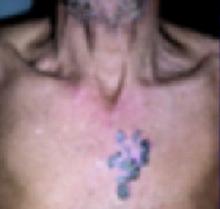
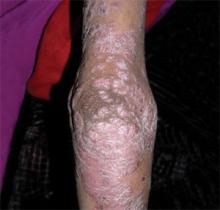
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
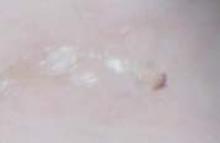
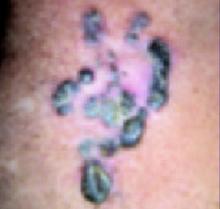
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
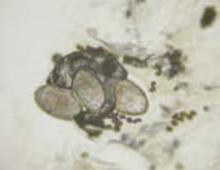
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: [email protected]
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: [email protected]
A boy came to the office with a rash and progressively severe itching for approximately 2 months (FIGURE 1). Examination showed an excoriated generalized papular eruption, including some urticarial-type papules and chronic eczematoid changes near the waist, axillae, hands, and wrists.
His grandmother, with whom he spends most weekends and a lot of time after school, also has had a rash and progressive itch for approximately 3 weeks. One feature of the dermopathy observed clinically, first located by hand lens examination and then confirmed by dermoscopy, is depicted in FIGURE 2.
FIGURE 1
Excoriated eruptions
FIGURE 2
Dermoscopic photograph of the dermatosis
What is your diagnosis?
Diagnosis: Scabies
The boy and his grandmother both have scabies, an infectious disease—in fact, the first human disease proven to be caused by a specific agent.1Sarcoptes scabiei var hominis, or scabies, is a mite in the arachnid class.2 In some states and localities, scabies cases or scabies outbreaks are reportable to the public health department.
The cardinal symptom of scabies is pruritus. The itch, especially with initial scabietic infestation, may be gradual in onset.3 Physical examination findings can vary from subtle and nonspecific to overwhelming and distinctive. Scabies can also mimic other dermopathies, complicating diagnosis. Undiagnosed and untreated, scabies can last a protracted period.
The dermopathy may be characterized by urticarial-type papules, vesicles, eczematoid change, excoriation, and bacterial superinfection, especially in children. Nodules may be present, particularly on the penis and scrotum. These may last for months after the infestation has cleared.3 The most commonly involved areas include fingers and finger webs, wrist folds, elbows, knees, the lower abdomen, armpits, thighs, male genitals, nipples, breasts, buttocks, and shoulder blades.3,4 In young children, scabies may be found anywhere, including palms, soles, face and scalp.
Affliction of multiple family members and finding dermatitis in these distinctive locations is helpful in diagnosis. Finding the mites’ burrows is considered pathognomonic because other burrowing diseases (eg, cutaneous larva migrans) are easily distinguished clinically.4 Extensive excoriation is a clinical clue to look for burrows.3
Transmission usually skin-to-skin
Scabies is generally transmitted by prolonged skin-to-skin contact, such as occurs in families or during sexual contact. It is possible to acquire scabies infestation via contaminated items of clothing or bed linens, but this is not regarded as a significant route of transmission.3 Transmission by casual contact, such as a handshake or hug, is unlikely.
Infestation with the S scabiei mite, referred to as scabies in man, is termed “mange” in other mammals known to host the mite (dogs, cats, rabbits, cattle, pigs, and horses). Mites from one host species generally do not establish themselves on another species, and thus are referred to as varieties, variants, or forms. Humans develop a transient dermopathy from infestation by animal scabies, but such infestations are mild and disappear spontaneously unless the person is in frequent contact with the infested animal.3,4
Differential diagnosis
The differential diagnosis of scabies—a great masquerader—is extensive, and includes atopic dermatitis, contact dermatitis, impetigo, insect bites, vasculitis, neurodermatitis, folliculitis, prurigo nodularis, psoriasis (crusted scabies), and a host of other dermopathies.3,4
Confirming the diagnosis
Finding the causative mite, its ova (eggs), or scybala (feces), confirms the diagnosis, although failure to find these does not rule out scabies. Papules or burrows that have not been excoriated are best for obtaining preparations for microscopic examination.3 Burrows may be found with nakedeye inspection, although use of a hand-held magnifier and good illumination make finding burrows easier.
Dermoscopy
Dermoscopy, performed with an otoscope-like, illuminated magnifier designed for skin assessment, provides reliable confirmation of S- or Z-shaped burrows. During dermoscopy, carefully examining the distal end of the burrows in the skin may reveal the “triangular black dot” of the scabies mite (FIGURE 2, top right)—the head of the mite.5 The body of the mite—light in color and oval—is not visible even with the most careful dermoscopic examination. The “black dot” of the mite may be visible with careful inspection with a hand lens. In the appropriate clinical setting, dermoscopic identification of an unequivocal burrow with the dark “triangle sign” at one end is diagnostic for scabies. When a digital photograph obtained through the dermatoscope is magnified, the distal end of the burrow (FIGURE 3) reveals the triangular head parts of the mite and the body within the burrow. This body is not evident with dermoscopy alone; the additional magnification via photography allows its visualization.
FIGURE 3
Magnification
Scabies mount
In instances where the physician is going to make an institution-wide recommendation with major ramifications, it is wise to positively identify the mite. A scabies mount performed at the location of the triangular dot will readily provide a mite for identification. Scabies mounts are prepared via a very superficial shave technique without anesthesia. The skin flakes are transferred to a slide and a drop of mineral oil is added. Alternatively, a drop of mineral oil can be placed on the skin and a superficial sample obtained.
Note that this technique differs from that of potassium hydroxide (KOH) preparation for fungal identification. The scabies mount technique is more like a superficial shave biopsy (with the knife blade parallel to the skin) than a KOH preparation (blade dragged along the skin surface more perpendicular to the skin).Microscopic examination of each slide reveals a mite (FIGURES 4 AND 5).
In our patient, 3 burrows were identified with a hand lens and confirmed by dermoscopy. In 2 burrows, the triangular dots were transferred to slides by doing a very superficial shave (without anesthesia) of the stratum corneum with a number 15 blade and handle. The material was placed on a slide, a drop of mineral oil added, and the slide examined microscopically (FIGURES 4 AND 5). These dermoscopically guided preparations each yielded a mite and little other debris.
FIGURE 4
Scabies mite
FIGURE 5
Mite, ova, and feces
Ink test
The “ink test” is another adjunct to help identify burrows. A nontoxic, watersoluble felt-tip marker is rubbed over an area suspected of having burrows. After waiting a few moments for the ink to sink into the disrupted stratum corneum overlying burrows, the ink is washed off, leaving an ink-demarcated burrow to examine.4 This can be performed as an adjunct to dermoscopy.5
The course of scabies
The mite’s life cycle
There are 4 stages in the mite’s life cycle: egg, larva, nymph, and adult. Female mites deposit 2 to 3 eggs per day as they create their burrows. The eggs are oval, 0.1 to 0.15 mm in length, and hatch in 3 to 8 days. The resultant larvae migrate to the skin surface and burrow into the intact stratum corneum to construct almost invisible, short burrows called molting pouches.
The larvae progress through 2 nymphal stages before a final molt to the adult stage. Larvae and nymphs live in molting pouches or in hair follicles. They appear similar to adults except for smaller size and, during the larval stage, 3 pair of legs. Adult female mites are 0.3 to 0.4 mm long and about 0.25 to 0.35 mm wide, about twice the size of males. Mating occurs when a male penetrates the molting pouch of the adult female. Impregnated females then extend their molting pouches to form the characteristic serpentine burrows, laying eggs in the process. The total period to progress from egg to the gravid female stage takes 10 to 14 days. The impregnated females spend the remaining 2 months of their lives in burrows.3,4
The mites live in and on the stratum corneum, burrowing into but never below the stratum corneum. The burrows appear as raised, serpentine lines varying from a few millimeters up to several centimeters long. Transmission occurs by the transfer of ova-bearing females.3,4
Cause of the rash and itch
The mites do not “bite.” Instead, the hallmark of scabies, when found, are the burrows created by the mites. However, it is common to see a papular urticarial type response as an allergic reaction to antigens associated with the mite itself, its scybala, and eggs. In fact, after acquiring scabies for the first time, itch does not appear for 2 to 6 weeks (average, 3 to 4 weeks) because the host needs to be sensitized to these antigens.4
It is not until the immunologic reactivity or sensitization develops that the host becomes symptomatic and aware of a problem. This requirement for sensitization explains the often gradual onset of itch. The incubation period is important in transmission to other individuals during the asymptomatic phase.3 However, a previously sensitized host may experience itch within hours to days after reinfestation.4
Epidemiology of scabies
Scabies infestations occur in all geographic areas and climates, and affects people of all ages and socioeconomic strata.7 For unexplained reasons, those with African ancestry rarely acquire scabies.7
It is most common in those who have close physical contact with others and, therefore, disproportionately affects children, mothers of young children, sexually active young adults, nursing home populations, and those in crowded living situations. Scabies is commonplace in developing countries. It is possible to acquire scabies after sleeping in unsanitary bedding. The scabies mite does not carry other diseases.7
Crusted scabies
Crusted scabies, a rare form of scabies also known as Norwegian scabies, is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons. Crusted scabies, because of the huge mite burden, is associated with greater transmissibility than scabies.7 Interestingly, because of impaired allergic response or indifference to itch, some of these patients may exhibit little pruritus.7
Treatment of scabies
Perhaps the most difficult job in treatment of scabies is treating asymptomatic contacts. Physicians may be reluctant to prescribe, and contacts themselves may be reluctant to take, appropriate treatment. These individuals often spread the infection for 4 to 6 weeks before they develop sensitization and clinical symptoms. Thus, it is essential that these asymptomatic contacts be treated or a cycle of reinfestation will be created.3 All sexual contacts, close personal contacts, and household contacts from within the preceding month should be examined and treated.8
Permethrin cream. The recommended treatment by the Centers for Disease Control and Prevention (CDC) is permethrin cream (5%) applied to all areas of the body from the neck down and thoroughly washed off after 8 to 14 hours. This recommendation includes careful application under fingernails, between toes, and on palms and soles. Infants may need the face and scalp treated in addition. Treatment of the face beyond infancy frequently results in a contact irritant dermatitis. Permethrin is effective and safe but costs more than lindane.8
Lindane. The CDC guidelines offer 2 alternatives to permethrin. One alternative, lindane 1% cream or lotion, can be applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours.
Lindane should not be used immediately after a bath or shower, and should not be used by persons who have extensive dermatitis, pregnant or lactating women, or children aged less than 2 years. Lindane resistance has been reported, including in the United States. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia following lindane use also has been reported. Infants, young children, and pregnant or lactating women should not be treated with lindane; they can be treated with permethrin.8
Applying topical treatments. Topical scabicides should be applied to all skin from neck down, including intertriginous areas and the gluteal fold. The medication needs to be reapplied to hands if the hands are washed after application. It is advisable to cut fingernails short before applying scabicides and to ensure that scabicide is applied under fingernails. A toothpick can be used if necessary to assist in application under nails. In infants and small children, medication should be applied to face and scalp, avoiding the periorbital area.6
Other treatments. Ivermectin, the third treatment recommended by the CDC, can be administered as a single dose of 200 mcg/kg orally, and repeated in 2 weeks. Ivermectin is not recommended for pregnant or lactating patients. The safety of ivermectin in children who weigh less than 15 kg has not been determined.8
Some specialists recommend retreatment after 1 to 2 weeks for patients who are still symptomatic. Patients who do not respond to the recommended treatment should be retreated with an alternative regimen.8
Patients who have uncomplicated scabies and also are infected with HIV should receive the same treatment regimens as those who are HIV-negative.8 For patients with crusted scabies, the optimal regimen is unknown because no controlled therapeutic trials have been conducted. Expert opinion suggests augmented and combined regimens should be used for this aggressive infestation. Lindane should be avoided because of risks of neurotoxicity with heavy applications.8 Control of scabies epidemics (eg, in nursing homes, hospitals, residential facilities) require treatment of the entire population at risk.
Ancillary measures
Scabies mites may survive for a few days after leaving human skin. Thus, frequent bed linen changes minimize transmission via bedding. Hot-water laundry in temperatures of 120°F (49°s mites in 10 minutes and is sufficient to disinfect all bedding, clothing, and washable items.3
Other methods of disinfection include placing items in a dryer on the hot cycle for 10 to 30 minutes, pressing them with a warm iron, dry-cleaning, or placing in a sealed plastic bag for 7 to 14 days. Carpets or upholstery should be vacuumed through the heavy traffic areas. Fumigation of living areas and furniture with insecticide is unnecessary.6-8 Pets do not need to be treated.6 Children may return to school and childcare immediately following initial treatment.6
Follow-up of scabies patient
The boy’s mother is allowed to view the mites through the microscope, fostering her accepting the diagnosis and enhancing the chance for compliance with treatment, which involves treating the entire family.
Patients should be informed that the rash and pruritus of scabies may persist for 4 weeks after treatment because scabietic antigenic material remains until natural epidermal sloughing and turnover occurs.3 When symptoms or signs persist, evaluation should ensue for faulty application of topical scabicides and for treatment failure.7
Acknowledgments
The author (GNF) wishes to acknowledge the assistance of Peggy Elston and Heather Martinez, without whose assistance photographs like these would never happen; and Lisa Nichols, without whose acquisitive skills it never would have occurred to me to mite-hunt with a dermatoscope.
CORRESPONDENCE
Gary N. Fox, MD, Defiance Clinic, 1400 East Second Street, Defiance, OH 43512. E-mail: [email protected]
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
1. Binder WD. Scabies. eMedicine [online database]. Available at: www.emedicine.com/emerg/topic517.htm. Accessed on July 6, 2006.
2. Arachnid. Wikipedia [online encyclopedia]. Available at: en.wikipedia.org/wiki/Arachnid. Accessed on July 6, 2006.
3. Scabies. DPDx—CDC Parasitology Diagnostic Website. Available at: www.dpd.cdc.gov/dpdx/HTML/Scabies.htm. Accessed on July 6, 2006.
4. Arya V, Molinaro MJ, Majewski SS, Schwartz RA. Pediatric scabies. Cutis 2003;71:193-196.
5. Vazquez-Lopez F, Kreusch JF, Marghoob AA. Other uses of dermoscopy. In: Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005:301,305-306.
6. FAQs—scabies. Texas Department of Health Services, Infectious Disease Control Unit website. Available at: www.dshs.state.tx.us/idcu/disease/scabies/faqs/. Accessed on July 6, 2006.
7. Infestations and bites [chapter 15]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004:497-503.
8. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR-6):68-69.
A 47-year-old man with eruptions on his trunk
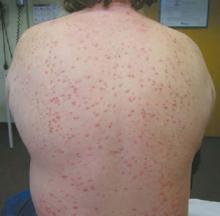
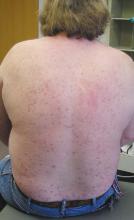
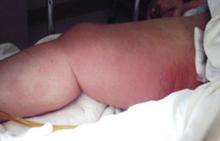
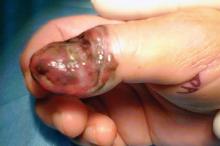
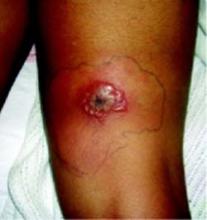
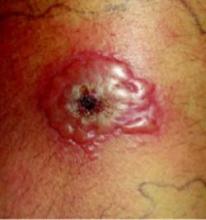
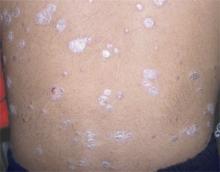
A 47-year-old white male came to the hospital emergency department complaining of chest pain. At admission, it was noted that the patient had numerous lesions on his buttocks, abdomen, back, and all extremities (FIGURES 1 AND 2). These lesions had been there for approximately 5 months—they developed after he discontinued his cholesterol medication due to lapsed insurance coverage. He had a similar eruption when he went off cholesterol medication on another occasion.
The patient’s medical history included type 2 diabetes mellitus, hypertension, coronary artery disease, and hyperlipidemia. He has had multiple heart catheterizations with stent placement, most recently 2 years ago. His mother also had diabetes mellitus, and she died at age 58 from a myocardial infarction.
On examination, his lesions were painless and nonpruritic. He had numerous yellow papules on his buttocks, abdomen, back, and upper and lower extremities. He had no lesions on his face. The rest of the physical exam showed no abnormal results.
FIGURE 1
Nodular lesions on back
The patient had yellow nodular lesions covering his entire trunk and all 4 extremities.
FIGURE 2
Close-up
What is your diagnosis?
What laboratory tests should be done to help make the diagnosis?
DIAGNOSIS: Eruptive xanthomas
This patient has eruptive xanthomas secondary to hypertriglyceridemia. His type IV hyperlipidemia has been worsened by long-standing, poorly controlled type 2 diabetes.
Xanthomas are lipid deposits in the skin and tendons that occur secondarily to a lipid abnormality. These lipid deposits are yellow and frequently firm.1 Although certain types of xanthomas are characteristic of particular lipid abnormalities, none is totally specific because the same form of xanthomas occurs in many different diseases.2
Xanthomas occur in various metabolic disorders and can also be associated with neoplasms. They may be associated with familial or acquired disorders resulting in hyperlipidemia or may be present with no underlying disorder.3
Types of xanthomas. Xanthomas are classified into 5 types based on clinical appearance. Tuberous and tendinous xanthomas both occur on the extensors of fingers and Achilles tendon. They appear as yellow nodules. Plane xanthomas are associated with biliary disease and appear as linear yellow lesions. Xanthelasmas appear as yellow plaques on the eyelids. Eruptive xanthomas are yellow papules that appear and disappear according to variations in lipid levels, especially triglycerides.3 As in this case, eruptive xanthomas usually appear over the buttocks, shoulders, back, and extensor surfaces of the extremities.4
Laboratory tests helpful in making the diagnosis
The patient’s tests on hospital admission showed normal cardiac enzymes and a normal electrocardiogram (ECG). His electrolytes were within normal limits except for a pseudohyponatremia of 133 mEq/dL due to an elevated glucose of 549 mg/dL.
A lipid profile the following morning revealed a triglyceride level of 1976 mg/dL, a total cholesterol level of 323 mg/dL, and a high-density lipoprotein (HDL) cholesterol level of 24 mg/dL. The low-density lipoprotein (LDL) cholesterol could not be calculated due to the high triglyceride level.
Differential diagnosis
Neurofibromas may have an appearance similar to eruptive xanthomas, but would usually be less numerous and less symptomatic. Prurigo nodularis would be another condition to be considered; however, this patient did not have any excoriations. Primary milia can also appear as keratinfilled cysts; however, these are much smaller and usually located on the face.
TREATMENT: Control the lipids and triglycerides
Patients may be prescribed HMG-CoA reductase inhibitors (statins) and fibric acid derivatives for control of lipid and triglyceride abnormalities. Further, counseling should involve diet modification, exercise, smoking cessation, and stringent control of diabetes.5 As a general rule, high doses of statins should not be given to patients who are taking fibrates.6 The combination of a statin and fibric acid derivative is not without risks: it may increase the risk of myopathy and rhabdomyolysis.
We started this patient on fenofibrate (Tricor) along with rosuvastatin (Crestor). When given in combination with any statin medication, fenofibrate resulted in fewer reports of rhabdomyolysis and myopathy than the older fibrate gemfibrozil (Lopid). It is believed that fenofibrate undergoes a different pathway of glucuronidation than gemfibrozil. Most statins undergo glucuronidation in the same family of enzymes as gemfibrozil, which could cause competition in converting the statin to a form that undergoes liver metabolism. Thus, metabolism of the statin is decreased and adverse effects such as rhabdomyolysis and myopathy occurs.7
Outcome
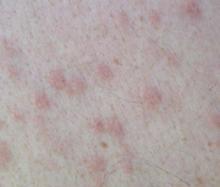
The patient started on rosuvastatin 10 mg once a day and fenofibrate 145 mg once a day. The patient’s xanthomas improved dramatically within a month (FIGURES 3 AND 4). His cholesterol, on the therapy described above, improved dramatically. His triglycerides decreased to 363 mg/dL. His total cholesterol was now 138 mg/dL, with an HDL of 34 mg/dL and an LDL of 31 mg/dL.
FIGURE 3
After treatment
FIGURE 4
Close-up
The patient reported no adverse effects from the medications. It was recommended that he continue on his present therapy indefinitely with the proper laboratory follow-up. We have not increased his dose of either medication, since his admission and no side effects have been encountered. We will continue to monitor his liver function tests and adjust the dose of his medications as needed.
CORRESPONDENCE
Jay Shubrook, DO, Parks Hall, Ohio University College of Medicine, Athens, OH 45701.
1. Damjanov I, Linder J. Diseases of the skin and connective tissues. In: Anderson’s Pathology. 10th ed. St Louis, Mo: Mosby, 1996:2454-2455.
2. Habif T. Xanthomas and dyslipoproteinemia. In: Clinical Dermatology. 4th ed. St Louis, Mo: Mosby, 2004:902-904.
3. Kumar V, Abbas A, Fausto N. Diseases of organ systems. In: Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, Pa: Elsevier Saunders, 2005:1248.
4. Freedberg I, Eisen A, Wolff K, et al. Xanthomatoses and lipoprotein disorders. In: Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill, 1999:1804-1809.
5. Braunwald E, Fauci A, Kasper D, Hauser S, Longo D, Jameson J. Disorders of lipoprotein metabolism. In: Harrison’s Principles of Internal Medicine. 15th ed. New York: McGraw-Hill, 2001:2245-2256.
6. Knopp RH. Drug treatment of lipid disorders. N Engl J Med 1999;341:498-511.
7. Corsini A, Bellosta S, Davidson MH. Pharmacokinetic interactions between statins and fibrates. Am J Cardiol 2005;96(9A):44K–49K; 34K-35K. Epub 2005 Oct 21.
A 47-year-old white male came to the hospital emergency department complaining of chest pain. At admission, it was noted that the patient had numerous lesions on his buttocks, abdomen, back, and all extremities (FIGURES 1 AND 2). These lesions had been there for approximately 5 months—they developed after he discontinued his cholesterol medication due to lapsed insurance coverage. He had a similar eruption when he went off cholesterol medication on another occasion.
The patient’s medical history included type 2 diabetes mellitus, hypertension, coronary artery disease, and hyperlipidemia. He has had multiple heart catheterizations with stent placement, most recently 2 years ago. His mother also had diabetes mellitus, and she died at age 58 from a myocardial infarction.
On examination, his lesions were painless and nonpruritic. He had numerous yellow papules on his buttocks, abdomen, back, and upper and lower extremities. He had no lesions on his face. The rest of the physical exam showed no abnormal results.
FIGURE 1
Nodular lesions on back
The patient had yellow nodular lesions covering his entire trunk and all 4 extremities.
FIGURE 2
Close-up
What is your diagnosis?
What laboratory tests should be done to help make the diagnosis?
DIAGNOSIS: Eruptive xanthomas
This patient has eruptive xanthomas secondary to hypertriglyceridemia. His type IV hyperlipidemia has been worsened by long-standing, poorly controlled type 2 diabetes.
Xanthomas are lipid deposits in the skin and tendons that occur secondarily to a lipid abnormality. These lipid deposits are yellow and frequently firm.1 Although certain types of xanthomas are characteristic of particular lipid abnormalities, none is totally specific because the same form of xanthomas occurs in many different diseases.2
Xanthomas occur in various metabolic disorders and can also be associated with neoplasms. They may be associated with familial or acquired disorders resulting in hyperlipidemia or may be present with no underlying disorder.3
Types of xanthomas. Xanthomas are classified into 5 types based on clinical appearance. Tuberous and tendinous xanthomas both occur on the extensors of fingers and Achilles tendon. They appear as yellow nodules. Plane xanthomas are associated with biliary disease and appear as linear yellow lesions. Xanthelasmas appear as yellow plaques on the eyelids. Eruptive xanthomas are yellow papules that appear and disappear according to variations in lipid levels, especially triglycerides.3 As in this case, eruptive xanthomas usually appear over the buttocks, shoulders, back, and extensor surfaces of the extremities.4
Laboratory tests helpful in making the diagnosis
The patient’s tests on hospital admission showed normal cardiac enzymes and a normal electrocardiogram (ECG). His electrolytes were within normal limits except for a pseudohyponatremia of 133 mEq/dL due to an elevated glucose of 549 mg/dL.
A lipid profile the following morning revealed a triglyceride level of 1976 mg/dL, a total cholesterol level of 323 mg/dL, and a high-density lipoprotein (HDL) cholesterol level of 24 mg/dL. The low-density lipoprotein (LDL) cholesterol could not be calculated due to the high triglyceride level.
Differential diagnosis
Neurofibromas may have an appearance similar to eruptive xanthomas, but would usually be less numerous and less symptomatic. Prurigo nodularis would be another condition to be considered; however, this patient did not have any excoriations. Primary milia can also appear as keratinfilled cysts; however, these are much smaller and usually located on the face.
TREATMENT: Control the lipids and triglycerides
Patients may be prescribed HMG-CoA reductase inhibitors (statins) and fibric acid derivatives for control of lipid and triglyceride abnormalities. Further, counseling should involve diet modification, exercise, smoking cessation, and stringent control of diabetes.5 As a general rule, high doses of statins should not be given to patients who are taking fibrates.6 The combination of a statin and fibric acid derivative is not without risks: it may increase the risk of myopathy and rhabdomyolysis.
We started this patient on fenofibrate (Tricor) along with rosuvastatin (Crestor). When given in combination with any statin medication, fenofibrate resulted in fewer reports of rhabdomyolysis and myopathy than the older fibrate gemfibrozil (Lopid). It is believed that fenofibrate undergoes a different pathway of glucuronidation than gemfibrozil. Most statins undergo glucuronidation in the same family of enzymes as gemfibrozil, which could cause competition in converting the statin to a form that undergoes liver metabolism. Thus, metabolism of the statin is decreased and adverse effects such as rhabdomyolysis and myopathy occurs.7
Outcome
The patient started on rosuvastatin 10 mg once a day and fenofibrate 145 mg once a day. The patient’s xanthomas improved dramatically within a month (FIGURES 3 AND 4). His cholesterol, on the therapy described above, improved dramatically. His triglycerides decreased to 363 mg/dL. His total cholesterol was now 138 mg/dL, with an HDL of 34 mg/dL and an LDL of 31 mg/dL.
FIGURE 3
After treatment
FIGURE 4
Close-up
The patient reported no adverse effects from the medications. It was recommended that he continue on his present therapy indefinitely with the proper laboratory follow-up. We have not increased his dose of either medication, since his admission and no side effects have been encountered. We will continue to monitor his liver function tests and adjust the dose of his medications as needed.
CORRESPONDENCE
Jay Shubrook, DO, Parks Hall, Ohio University College of Medicine, Athens, OH 45701.
A 47-year-old white male came to the hospital emergency department complaining of chest pain. At admission, it was noted that the patient had numerous lesions on his buttocks, abdomen, back, and all extremities (FIGURES 1 AND 2). These lesions had been there for approximately 5 months—they developed after he discontinued his cholesterol medication due to lapsed insurance coverage. He had a similar eruption when he went off cholesterol medication on another occasion.
The patient’s medical history included type 2 diabetes mellitus, hypertension, coronary artery disease, and hyperlipidemia. He has had multiple heart catheterizations with stent placement, most recently 2 years ago. His mother also had diabetes mellitus, and she died at age 58 from a myocardial infarction.
On examination, his lesions were painless and nonpruritic. He had numerous yellow papules on his buttocks, abdomen, back, and upper and lower extremities. He had no lesions on his face. The rest of the physical exam showed no abnormal results.
FIGURE 1
Nodular lesions on back
The patient had yellow nodular lesions covering his entire trunk and all 4 extremities.
FIGURE 2
Close-up
What is your diagnosis?
What laboratory tests should be done to help make the diagnosis?
DIAGNOSIS: Eruptive xanthomas
This patient has eruptive xanthomas secondary to hypertriglyceridemia. His type IV hyperlipidemia has been worsened by long-standing, poorly controlled type 2 diabetes.
Xanthomas are lipid deposits in the skin and tendons that occur secondarily to a lipid abnormality. These lipid deposits are yellow and frequently firm.1 Although certain types of xanthomas are characteristic of particular lipid abnormalities, none is totally specific because the same form of xanthomas occurs in many different diseases.2
Xanthomas occur in various metabolic disorders and can also be associated with neoplasms. They may be associated with familial or acquired disorders resulting in hyperlipidemia or may be present with no underlying disorder.3
Types of xanthomas. Xanthomas are classified into 5 types based on clinical appearance. Tuberous and tendinous xanthomas both occur on the extensors of fingers and Achilles tendon. They appear as yellow nodules. Plane xanthomas are associated with biliary disease and appear as linear yellow lesions. Xanthelasmas appear as yellow plaques on the eyelids. Eruptive xanthomas are yellow papules that appear and disappear according to variations in lipid levels, especially triglycerides.3 As in this case, eruptive xanthomas usually appear over the buttocks, shoulders, back, and extensor surfaces of the extremities.4
Laboratory tests helpful in making the diagnosis
The patient’s tests on hospital admission showed normal cardiac enzymes and a normal electrocardiogram (ECG). His electrolytes were within normal limits except for a pseudohyponatremia of 133 mEq/dL due to an elevated glucose of 549 mg/dL.
A lipid profile the following morning revealed a triglyceride level of 1976 mg/dL, a total cholesterol level of 323 mg/dL, and a high-density lipoprotein (HDL) cholesterol level of 24 mg/dL. The low-density lipoprotein (LDL) cholesterol could not be calculated due to the high triglyceride level.
Differential diagnosis
Neurofibromas may have an appearance similar to eruptive xanthomas, but would usually be less numerous and less symptomatic. Prurigo nodularis would be another condition to be considered; however, this patient did not have any excoriations. Primary milia can also appear as keratinfilled cysts; however, these are much smaller and usually located on the face.
TREATMENT: Control the lipids and triglycerides
Patients may be prescribed HMG-CoA reductase inhibitors (statins) and fibric acid derivatives for control of lipid and triglyceride abnormalities. Further, counseling should involve diet modification, exercise, smoking cessation, and stringent control of diabetes.5 As a general rule, high doses of statins should not be given to patients who are taking fibrates.6 The combination of a statin and fibric acid derivative is not without risks: it may increase the risk of myopathy and rhabdomyolysis.
We started this patient on fenofibrate (Tricor) along with rosuvastatin (Crestor). When given in combination with any statin medication, fenofibrate resulted in fewer reports of rhabdomyolysis and myopathy than the older fibrate gemfibrozil (Lopid). It is believed that fenofibrate undergoes a different pathway of glucuronidation than gemfibrozil. Most statins undergo glucuronidation in the same family of enzymes as gemfibrozil, which could cause competition in converting the statin to a form that undergoes liver metabolism. Thus, metabolism of the statin is decreased and adverse effects such as rhabdomyolysis and myopathy occurs.7
Outcome
The patient started on rosuvastatin 10 mg once a day and fenofibrate 145 mg once a day. The patient’s xanthomas improved dramatically within a month (FIGURES 3 AND 4). His cholesterol, on the therapy described above, improved dramatically. His triglycerides decreased to 363 mg/dL. His total cholesterol was now 138 mg/dL, with an HDL of 34 mg/dL and an LDL of 31 mg/dL.
FIGURE 3
After treatment
FIGURE 4
Close-up
The patient reported no adverse effects from the medications. It was recommended that he continue on his present therapy indefinitely with the proper laboratory follow-up. We have not increased his dose of either medication, since his admission and no side effects have been encountered. We will continue to monitor his liver function tests and adjust the dose of his medications as needed.
CORRESPONDENCE
Jay Shubrook, DO, Parks Hall, Ohio University College of Medicine, Athens, OH 45701.
1. Damjanov I, Linder J. Diseases of the skin and connective tissues. In: Anderson’s Pathology. 10th ed. St Louis, Mo: Mosby, 1996:2454-2455.
2. Habif T. Xanthomas and dyslipoproteinemia. In: Clinical Dermatology. 4th ed. St Louis, Mo: Mosby, 2004:902-904.
3. Kumar V, Abbas A, Fausto N. Diseases of organ systems. In: Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, Pa: Elsevier Saunders, 2005:1248.
4. Freedberg I, Eisen A, Wolff K, et al. Xanthomatoses and lipoprotein disorders. In: Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill, 1999:1804-1809.
5. Braunwald E, Fauci A, Kasper D, Hauser S, Longo D, Jameson J. Disorders of lipoprotein metabolism. In: Harrison’s Principles of Internal Medicine. 15th ed. New York: McGraw-Hill, 2001:2245-2256.
6. Knopp RH. Drug treatment of lipid disorders. N Engl J Med 1999;341:498-511.
7. Corsini A, Bellosta S, Davidson MH. Pharmacokinetic interactions between statins and fibrates. Am J Cardiol 2005;96(9A):44K–49K; 34K-35K. Epub 2005 Oct 21.
1. Damjanov I, Linder J. Diseases of the skin and connective tissues. In: Anderson’s Pathology. 10th ed. St Louis, Mo: Mosby, 1996:2454-2455.
2. Habif T. Xanthomas and dyslipoproteinemia. In: Clinical Dermatology. 4th ed. St Louis, Mo: Mosby, 2004:902-904.
3. Kumar V, Abbas A, Fausto N. Diseases of organ systems. In: Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, Pa: Elsevier Saunders, 2005:1248.
4. Freedberg I, Eisen A, Wolff K, et al. Xanthomatoses and lipoprotein disorders. In: Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill, 1999:1804-1809.
5. Braunwald E, Fauci A, Kasper D, Hauser S, Longo D, Jameson J. Disorders of lipoprotein metabolism. In: Harrison’s Principles of Internal Medicine. 15th ed. New York: McGraw-Hill, 2001:2245-2256.
6. Knopp RH. Drug treatment of lipid disorders. N Engl J Med 1999;341:498-511.
7. Corsini A, Bellosta S, Davidson MH. Pharmacokinetic interactions between statins and fibrates. Am J Cardiol 2005;96(9A):44K–49K; 34K-35K. Epub 2005 Oct 21.
Dark brown scaly plaques on face and ears
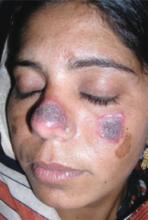
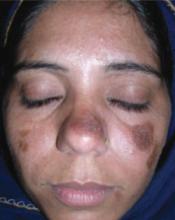
A 27-year-old woman, otherwise healthy, presented for evaluation of a mildly pruritic eruption of plaques on her face and ears. The eruption started as a few small, scaly red papules on her cheeks and nose about 3 months earlier. The papules slowly expanded to form well-demarcated, rounded, dark brown plaques covered with superficial scale. Similar lesions appeared on the concha of both ears. The older lesions started to regress after 2 months. Complete regression was followed by residual scarring and hyperpigmentation.
Physical examination revealed multiple well-defined, erythematous, hyperpigmented, round plaques covered with adherent scale affecting her nose, cheeks, and the concha of both ears (FIGURE 1). The mucosae and the rest of her skin and adnexa were unaffected. She had no personal or family history of similar skin findings or autoimmune disorders. She also had no history of any drug intake prior to the eruption. Her routine blood tests and urinalysis results were unremarkable, and the serological analysis for antinuclear antibodies had negative results.
A punch biopsy was taken from one of the lesions for histopathology. The epidermal histopathological findings were remarkable for hyperkeratosis, follicular plugging, pigment incontinence, and vacuolization of the basal cell layer. There was a predominantly lymphocytic infiltrate of the subepidermal and perivascular dermal areas.
FIGURE 1
Facial plaques
What is your diagnosis?
How would you treat this patient?
Diagnosis: Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is an autoimmune inflammatory disorder of the skin that often leads to scarring and alopecia. It may be localized to sun-exposed areas such as the face, ears, and scalp but occasionally is much more extensive, involving the trunk and extremities. Most patients are otherwise healthy, and DLE may be the only clinical finding.
Although its prevalence is not known, DLE is not uncommon. It affects females twice as often as males, and patients fall between the ages of 25 to 45 years, with no racial predilection. While about 15% to 20% of patients with systemic lupus erythematosus (SLE) manifest DLE lesions, only about 5% to 10% of patients with DLE go on to develop SLE.1
Like SLE, DLE is believed to be an autoimmune disorder. Unlike SLE, however, DLE patients do not have similar serologic abnormalities. Skin trauma and ultraviolet light exposure have been reported to induce or exacerbate the lesions of DLE. Sex hormones may also play a role: exacerbation may occur during pregnancy, during menstrual or premenstrual periods, or while taking oral contraceptives. Drugs such as procainamide, hydralazine, isoniazid, diphenylhydantoin, methyldopa, penicillamine, guanides, and lithium may also precipitate DLE lesions.2
DLE usually begins as dull red macules with adherent scales on sun-exposed areas. If the overlying scales are removed, an undersurface of horny plugs is revealed. These plugs fill the follicles and resemble carpet tacks or a cat’s tongue (langue au chat). The macules slowly expand to form large plaques.
Usually only a mild pruritus and tenderness is seen with DLE lesions. As the lesions progress, the scale may thicken and pigmentary changes become evident. The lesions heal with atrophy, scarring, telangiectasias, and changes in pigmentation. Morphological appearance of older lesions may vary from erythematous plaques to hyperkeratotic, dark gray plaques with centrally depressed scars.3 Scalp involvement in DLE results in more sclerotic and depressed scars with subsequent scarring alopecia.4
Differential diagnosis is wide. The differential diagnosis of DLE is extensive and includes actinic keratosis, dermatomyositis, granuloma annulare, granuloma faciale, keratoacanthoma, lichen planus, subacute cutaneous lupus erythematosus, psoriasis, rosacea, sarcoidosis, squamous cell carcinoma, syphilis, and nongenital warts.
Histopathology
Histopathological findings include hyperkeratosis, parakeratosis, follicular plugging, telangiectasias, and atrophy of the epidermis. Liquefaction or hydropic degeneration of the basal layer leads to pigmentary incontinence. A perivascular and perifollicular mononuclear inflammatory cell infiltrate is present in the superficial and deep dermis.5,6
Direct immunofluorescence demonstrates immunoglobulins and complement deposits at the dermoepidermal junction.5 This test was not recommended for this patient, as the diagnosis of DLE had already been confirmed on clinical and histopathologic grounds.
Workup: Exam, biopsy
In addition to a routine history and physical examination, the workup for DLE should include a complete blood count, antinuclear antibody levels, anti-Ro, anti-La, hepatic and renal function tests, and urinalysis. Consider a diagnosis of SLE by using the American College of Rheumatology criteria. A biopsy for histopathology of a fresh lesion or a biopsy for immunofluorescence of an old lesion can confirm the diagnosis.
Therapeutic options are varied
Effective early therapies for DLE are available, but patients who do not respond appropriately may end up with deep scars, alopecia, and pigmentary changes that are considerably disfiguring, especially for dark-skinned people. Therefore, the goal of treatment is not only to improve the appearance of the skin by minimizing the scarring and preventing further lesions, but also to prevent future complications.
Avoiding sunlight. The primary therapeutic approach is to educate patients regarding exposure to sunlight. Sun-protective measures include the use of high-SPF sunscreen lotions and protective clothing, such as baseball caps without vent holes and wide-brimmed hats.7
Medication options. Current treatment options for DLE include antimalarial agents such as chloroquine, topical and intralesional glucocorticoids, and thalidomide. The use of potent topical steroids may prevent significant scarring and deformity, especially of the face. Common side effects include steroid withdrawal syndrome, perioral dermatitis, steroid acne, and rosacea. All of these side effects can be treated and result in less long-term deformity than untreated DLE (FIGURE 2).
Systemic agents. Widespread disease may require you use systemic agents, such as antimalarials. Chloroquine has long been considered the gold standard in the treatment of DLE.8 Due to the frequency of ocular side effects with chloroquine, hydroxychloroquine is by far the most widely used agent. Hydroxychloroquine at a dose of 6.5 mg/kg/d for 3 months may lead to resolution of lesions for many patients.7 In resistant cases, higher dosages (eg, 400 mg/d) or combinations (eg, hydroxychloroquine 200 mg/d plus quinacrine 50 to 100 mg/d) may be required for months or even years. An ophthalmological evaluation is advisable before starting antimalarial treatment, and you should repeat it at 4- to 6-month intervals during treatment.9 Systemic corticosteroids may be needed to obtain timely initial control, especially for widespread and disfiguring lesions.
When this therapy is inadequate, other treatments for DLE include azathioprine, retinoids, and dapsone. Recent reports confirm the efficacy of thalidomide for cutaneous lupus erythematosus in dosages ranging from 50 to 200 mg/d.10 Twice-daily application of tacrolimus 0.01% has also been shown to be effective in a few clinical trials.11
Surgery. Surgical intervention is useful to remove scarred lesions, and laser therapy has also been effective, especially for lesions with prominent telangiectasias.12
Patient education. Patient education plays an important role in the treatment of DLE. Advising patients on how to avoid the sun and instructing them in the proper use of sunscreens is extremely important. Smoking cessation has also been shown to be beneficial.13
Patients with DLE generally have a favorable prognosis with regards to morbidity and mortality. Because DLE is usually self-limited, the course is most often benign; therefore, early recognition and adequate therapy may prevent clinical complications. Many patients with DLE go on to develop destructive or deforming scarring or pigmentary disturbances,14 especially in the spring and summer months when the sun is the strongest.
FIGURE 2
After treatment
This patient’s outcome
Our patient was treated with topical fluocinolone acetonide 0.025% ointment and oral hydroxychloroquine 200 mg/d for 1 month. The patient responded well to the treatment and the skin lesion regressed perceptibly (FIGURE 2). The treatment was nevertheless continued for another 5 months, and resulted in complete regression of the lesions, with minimal residual scarring.
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Department of Dermatology, 450 Clarkson Avenue Box 46, Brooklyn, NY 11203. E-mail: [email protected]
1. Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. Clinical and laboratory findings in seventeen patients. J Am Acad Dermatol 1985;12(2 Pt 1):278-288.
2. Hess E. Drug-related lupus. N Engl J Med 1988;318:1460-1462.
3. Callen JP, Fowler JF, Kulick KB. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol 1985;13(5 Pt 1):748-755.
4. Wilson CL, Burge SM, Dean D, Dawber RP. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol 1992;126:307-314.
5. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002;20:373-385, v.
6. Biesla I, et al. Histopathologic findings in cutaneous lupus erythematosus. Arch Dermatol 1994;130:54-58.
7. Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin 1994;12:201-206.
8. Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc 1953;152:1428-1429.
9. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983;75:35-39.
10. Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol 2000;39:218-222.
11. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1171.
12. Nunez M, Boixeda P, Miralles ES, et al. Pulsed dye laser treatment of telangiectatic chronic erythema of cutaneous lupus erythematosus. Arch Dermatol 1996;132:354-355.
13. Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology 2005;211:118-122.
14. de Berker D, Dissaneyeka M, Burge S. The sequelae of chronic cutaneous lupus erythematosus. Lupus 1992;1:181-186.
A 27-year-old woman, otherwise healthy, presented for evaluation of a mildly pruritic eruption of plaques on her face and ears. The eruption started as a few small, scaly red papules on her cheeks and nose about 3 months earlier. The papules slowly expanded to form well-demarcated, rounded, dark brown plaques covered with superficial scale. Similar lesions appeared on the concha of both ears. The older lesions started to regress after 2 months. Complete regression was followed by residual scarring and hyperpigmentation.
Physical examination revealed multiple well-defined, erythematous, hyperpigmented, round plaques covered with adherent scale affecting her nose, cheeks, and the concha of both ears (FIGURE 1). The mucosae and the rest of her skin and adnexa were unaffected. She had no personal or family history of similar skin findings or autoimmune disorders. She also had no history of any drug intake prior to the eruption. Her routine blood tests and urinalysis results were unremarkable, and the serological analysis for antinuclear antibodies had negative results.
A punch biopsy was taken from one of the lesions for histopathology. The epidermal histopathological findings were remarkable for hyperkeratosis, follicular plugging, pigment incontinence, and vacuolization of the basal cell layer. There was a predominantly lymphocytic infiltrate of the subepidermal and perivascular dermal areas.
FIGURE 1
Facial plaques
What is your diagnosis?
How would you treat this patient?
Diagnosis: Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is an autoimmune inflammatory disorder of the skin that often leads to scarring and alopecia. It may be localized to sun-exposed areas such as the face, ears, and scalp but occasionally is much more extensive, involving the trunk and extremities. Most patients are otherwise healthy, and DLE may be the only clinical finding.
Although its prevalence is not known, DLE is not uncommon. It affects females twice as often as males, and patients fall between the ages of 25 to 45 years, with no racial predilection. While about 15% to 20% of patients with systemic lupus erythematosus (SLE) manifest DLE lesions, only about 5% to 10% of patients with DLE go on to develop SLE.1
Like SLE, DLE is believed to be an autoimmune disorder. Unlike SLE, however, DLE patients do not have similar serologic abnormalities. Skin trauma and ultraviolet light exposure have been reported to induce or exacerbate the lesions of DLE. Sex hormones may also play a role: exacerbation may occur during pregnancy, during menstrual or premenstrual periods, or while taking oral contraceptives. Drugs such as procainamide, hydralazine, isoniazid, diphenylhydantoin, methyldopa, penicillamine, guanides, and lithium may also precipitate DLE lesions.2
DLE usually begins as dull red macules with adherent scales on sun-exposed areas. If the overlying scales are removed, an undersurface of horny plugs is revealed. These plugs fill the follicles and resemble carpet tacks or a cat’s tongue (langue au chat). The macules slowly expand to form large plaques.
Usually only a mild pruritus and tenderness is seen with DLE lesions. As the lesions progress, the scale may thicken and pigmentary changes become evident. The lesions heal with atrophy, scarring, telangiectasias, and changes in pigmentation. Morphological appearance of older lesions may vary from erythematous plaques to hyperkeratotic, dark gray plaques with centrally depressed scars.3 Scalp involvement in DLE results in more sclerotic and depressed scars with subsequent scarring alopecia.4
Differential diagnosis is wide. The differential diagnosis of DLE is extensive and includes actinic keratosis, dermatomyositis, granuloma annulare, granuloma faciale, keratoacanthoma, lichen planus, subacute cutaneous lupus erythematosus, psoriasis, rosacea, sarcoidosis, squamous cell carcinoma, syphilis, and nongenital warts.
Histopathology
Histopathological findings include hyperkeratosis, parakeratosis, follicular plugging, telangiectasias, and atrophy of the epidermis. Liquefaction or hydropic degeneration of the basal layer leads to pigmentary incontinence. A perivascular and perifollicular mononuclear inflammatory cell infiltrate is present in the superficial and deep dermis.5,6
Direct immunofluorescence demonstrates immunoglobulins and complement deposits at the dermoepidermal junction.5 This test was not recommended for this patient, as the diagnosis of DLE had already been confirmed on clinical and histopathologic grounds.
Workup: Exam, biopsy
In addition to a routine history and physical examination, the workup for DLE should include a complete blood count, antinuclear antibody levels, anti-Ro, anti-La, hepatic and renal function tests, and urinalysis. Consider a diagnosis of SLE by using the American College of Rheumatology criteria. A biopsy for histopathology of a fresh lesion or a biopsy for immunofluorescence of an old lesion can confirm the diagnosis.
Therapeutic options are varied
Effective early therapies for DLE are available, but patients who do not respond appropriately may end up with deep scars, alopecia, and pigmentary changes that are considerably disfiguring, especially for dark-skinned people. Therefore, the goal of treatment is not only to improve the appearance of the skin by minimizing the scarring and preventing further lesions, but also to prevent future complications.
Avoiding sunlight. The primary therapeutic approach is to educate patients regarding exposure to sunlight. Sun-protective measures include the use of high-SPF sunscreen lotions and protective clothing, such as baseball caps without vent holes and wide-brimmed hats.7
Medication options. Current treatment options for DLE include antimalarial agents such as chloroquine, topical and intralesional glucocorticoids, and thalidomide. The use of potent topical steroids may prevent significant scarring and deformity, especially of the face. Common side effects include steroid withdrawal syndrome, perioral dermatitis, steroid acne, and rosacea. All of these side effects can be treated and result in less long-term deformity than untreated DLE (FIGURE 2).
Systemic agents. Widespread disease may require you use systemic agents, such as antimalarials. Chloroquine has long been considered the gold standard in the treatment of DLE.8 Due to the frequency of ocular side effects with chloroquine, hydroxychloroquine is by far the most widely used agent. Hydroxychloroquine at a dose of 6.5 mg/kg/d for 3 months may lead to resolution of lesions for many patients.7 In resistant cases, higher dosages (eg, 400 mg/d) or combinations (eg, hydroxychloroquine 200 mg/d plus quinacrine 50 to 100 mg/d) may be required for months or even years. An ophthalmological evaluation is advisable before starting antimalarial treatment, and you should repeat it at 4- to 6-month intervals during treatment.9 Systemic corticosteroids may be needed to obtain timely initial control, especially for widespread and disfiguring lesions.
When this therapy is inadequate, other treatments for DLE include azathioprine, retinoids, and dapsone. Recent reports confirm the efficacy of thalidomide for cutaneous lupus erythematosus in dosages ranging from 50 to 200 mg/d.10 Twice-daily application of tacrolimus 0.01% has also been shown to be effective in a few clinical trials.11
Surgery. Surgical intervention is useful to remove scarred lesions, and laser therapy has also been effective, especially for lesions with prominent telangiectasias.12
Patient education. Patient education plays an important role in the treatment of DLE. Advising patients on how to avoid the sun and instructing them in the proper use of sunscreens is extremely important. Smoking cessation has also been shown to be beneficial.13
Patients with DLE generally have a favorable prognosis with regards to morbidity and mortality. Because DLE is usually self-limited, the course is most often benign; therefore, early recognition and adequate therapy may prevent clinical complications. Many patients with DLE go on to develop destructive or deforming scarring or pigmentary disturbances,14 especially in the spring and summer months when the sun is the strongest.
FIGURE 2
After treatment
This patient’s outcome
Our patient was treated with topical fluocinolone acetonide 0.025% ointment and oral hydroxychloroquine 200 mg/d for 1 month. The patient responded well to the treatment and the skin lesion regressed perceptibly (FIGURE 2). The treatment was nevertheless continued for another 5 months, and resulted in complete regression of the lesions, with minimal residual scarring.
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Department of Dermatology, 450 Clarkson Avenue Box 46, Brooklyn, NY 11203. E-mail: [email protected]
A 27-year-old woman, otherwise healthy, presented for evaluation of a mildly pruritic eruption of plaques on her face and ears. The eruption started as a few small, scaly red papules on her cheeks and nose about 3 months earlier. The papules slowly expanded to form well-demarcated, rounded, dark brown plaques covered with superficial scale. Similar lesions appeared on the concha of both ears. The older lesions started to regress after 2 months. Complete regression was followed by residual scarring and hyperpigmentation.
Physical examination revealed multiple well-defined, erythematous, hyperpigmented, round plaques covered with adherent scale affecting her nose, cheeks, and the concha of both ears (FIGURE 1). The mucosae and the rest of her skin and adnexa were unaffected. She had no personal or family history of similar skin findings or autoimmune disorders. She also had no history of any drug intake prior to the eruption. Her routine blood tests and urinalysis results were unremarkable, and the serological analysis for antinuclear antibodies had negative results.
A punch biopsy was taken from one of the lesions for histopathology. The epidermal histopathological findings were remarkable for hyperkeratosis, follicular plugging, pigment incontinence, and vacuolization of the basal cell layer. There was a predominantly lymphocytic infiltrate of the subepidermal and perivascular dermal areas.
FIGURE 1
Facial plaques
What is your diagnosis?
How would you treat this patient?
Diagnosis: Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is an autoimmune inflammatory disorder of the skin that often leads to scarring and alopecia. It may be localized to sun-exposed areas such as the face, ears, and scalp but occasionally is much more extensive, involving the trunk and extremities. Most patients are otherwise healthy, and DLE may be the only clinical finding.
Although its prevalence is not known, DLE is not uncommon. It affects females twice as often as males, and patients fall between the ages of 25 to 45 years, with no racial predilection. While about 15% to 20% of patients with systemic lupus erythematosus (SLE) manifest DLE lesions, only about 5% to 10% of patients with DLE go on to develop SLE.1
Like SLE, DLE is believed to be an autoimmune disorder. Unlike SLE, however, DLE patients do not have similar serologic abnormalities. Skin trauma and ultraviolet light exposure have been reported to induce or exacerbate the lesions of DLE. Sex hormones may also play a role: exacerbation may occur during pregnancy, during menstrual or premenstrual periods, or while taking oral contraceptives. Drugs such as procainamide, hydralazine, isoniazid, diphenylhydantoin, methyldopa, penicillamine, guanides, and lithium may also precipitate DLE lesions.2
DLE usually begins as dull red macules with adherent scales on sun-exposed areas. If the overlying scales are removed, an undersurface of horny plugs is revealed. These plugs fill the follicles and resemble carpet tacks or a cat’s tongue (langue au chat). The macules slowly expand to form large plaques.
Usually only a mild pruritus and tenderness is seen with DLE lesions. As the lesions progress, the scale may thicken and pigmentary changes become evident. The lesions heal with atrophy, scarring, telangiectasias, and changes in pigmentation. Morphological appearance of older lesions may vary from erythematous plaques to hyperkeratotic, dark gray plaques with centrally depressed scars.3 Scalp involvement in DLE results in more sclerotic and depressed scars with subsequent scarring alopecia.4
Differential diagnosis is wide. The differential diagnosis of DLE is extensive and includes actinic keratosis, dermatomyositis, granuloma annulare, granuloma faciale, keratoacanthoma, lichen planus, subacute cutaneous lupus erythematosus, psoriasis, rosacea, sarcoidosis, squamous cell carcinoma, syphilis, and nongenital warts.
Histopathology
Histopathological findings include hyperkeratosis, parakeratosis, follicular plugging, telangiectasias, and atrophy of the epidermis. Liquefaction or hydropic degeneration of the basal layer leads to pigmentary incontinence. A perivascular and perifollicular mononuclear inflammatory cell infiltrate is present in the superficial and deep dermis.5,6
Direct immunofluorescence demonstrates immunoglobulins and complement deposits at the dermoepidermal junction.5 This test was not recommended for this patient, as the diagnosis of DLE had already been confirmed on clinical and histopathologic grounds.
Workup: Exam, biopsy
In addition to a routine history and physical examination, the workup for DLE should include a complete blood count, antinuclear antibody levels, anti-Ro, anti-La, hepatic and renal function tests, and urinalysis. Consider a diagnosis of SLE by using the American College of Rheumatology criteria. A biopsy for histopathology of a fresh lesion or a biopsy for immunofluorescence of an old lesion can confirm the diagnosis.
Therapeutic options are varied
Effective early therapies for DLE are available, but patients who do not respond appropriately may end up with deep scars, alopecia, and pigmentary changes that are considerably disfiguring, especially for dark-skinned people. Therefore, the goal of treatment is not only to improve the appearance of the skin by minimizing the scarring and preventing further lesions, but also to prevent future complications.
Avoiding sunlight. The primary therapeutic approach is to educate patients regarding exposure to sunlight. Sun-protective measures include the use of high-SPF sunscreen lotions and protective clothing, such as baseball caps without vent holes and wide-brimmed hats.7
Medication options. Current treatment options for DLE include antimalarial agents such as chloroquine, topical and intralesional glucocorticoids, and thalidomide. The use of potent topical steroids may prevent significant scarring and deformity, especially of the face. Common side effects include steroid withdrawal syndrome, perioral dermatitis, steroid acne, and rosacea. All of these side effects can be treated and result in less long-term deformity than untreated DLE (FIGURE 2).
Systemic agents. Widespread disease may require you use systemic agents, such as antimalarials. Chloroquine has long been considered the gold standard in the treatment of DLE.8 Due to the frequency of ocular side effects with chloroquine, hydroxychloroquine is by far the most widely used agent. Hydroxychloroquine at a dose of 6.5 mg/kg/d for 3 months may lead to resolution of lesions for many patients.7 In resistant cases, higher dosages (eg, 400 mg/d) or combinations (eg, hydroxychloroquine 200 mg/d plus quinacrine 50 to 100 mg/d) may be required for months or even years. An ophthalmological evaluation is advisable before starting antimalarial treatment, and you should repeat it at 4- to 6-month intervals during treatment.9 Systemic corticosteroids may be needed to obtain timely initial control, especially for widespread and disfiguring lesions.
When this therapy is inadequate, other treatments for DLE include azathioprine, retinoids, and dapsone. Recent reports confirm the efficacy of thalidomide for cutaneous lupus erythematosus in dosages ranging from 50 to 200 mg/d.10 Twice-daily application of tacrolimus 0.01% has also been shown to be effective in a few clinical trials.11
Surgery. Surgical intervention is useful to remove scarred lesions, and laser therapy has also been effective, especially for lesions with prominent telangiectasias.12
Patient education. Patient education plays an important role in the treatment of DLE. Advising patients on how to avoid the sun and instructing them in the proper use of sunscreens is extremely important. Smoking cessation has also been shown to be beneficial.13
Patients with DLE generally have a favorable prognosis with regards to morbidity and mortality. Because DLE is usually self-limited, the course is most often benign; therefore, early recognition and adequate therapy may prevent clinical complications. Many patients with DLE go on to develop destructive or deforming scarring or pigmentary disturbances,14 especially in the spring and summer months when the sun is the strongest.
FIGURE 2
After treatment
This patient’s outcome
Our patient was treated with topical fluocinolone acetonide 0.025% ointment and oral hydroxychloroquine 200 mg/d for 1 month. The patient responded well to the treatment and the skin lesion regressed perceptibly (FIGURE 2). The treatment was nevertheless continued for another 5 months, and resulted in complete regression of the lesions, with minimal residual scarring.
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Department of Dermatology, 450 Clarkson Avenue Box 46, Brooklyn, NY 11203. E-mail: [email protected]
1. Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. Clinical and laboratory findings in seventeen patients. J Am Acad Dermatol 1985;12(2 Pt 1):278-288.
2. Hess E. Drug-related lupus. N Engl J Med 1988;318:1460-1462.
3. Callen JP, Fowler JF, Kulick KB. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol 1985;13(5 Pt 1):748-755.
4. Wilson CL, Burge SM, Dean D, Dawber RP. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol 1992;126:307-314.
5. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002;20:373-385, v.
6. Biesla I, et al. Histopathologic findings in cutaneous lupus erythematosus. Arch Dermatol 1994;130:54-58.
7. Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin 1994;12:201-206.
8. Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc 1953;152:1428-1429.
9. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983;75:35-39.
10. Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol 2000;39:218-222.
11. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1171.
12. Nunez M, Boixeda P, Miralles ES, et al. Pulsed dye laser treatment of telangiectatic chronic erythema of cutaneous lupus erythematosus. Arch Dermatol 1996;132:354-355.
13. Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology 2005;211:118-122.
14. de Berker D, Dissaneyeka M, Burge S. The sequelae of chronic cutaneous lupus erythematosus. Lupus 1992;1:181-186.
1. Callen JP. Systemic lupus erythematosus in patients with chronic cutaneous (discoid) lupus erythematosus. Clinical and laboratory findings in seventeen patients. J Am Acad Dermatol 1985;12(2 Pt 1):278-288.
2. Hess E. Drug-related lupus. N Engl J Med 1988;318:1460-1462.
3. Callen JP, Fowler JF, Kulick KB. Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol 1985;13(5 Pt 1):748-755.
4. Wilson CL, Burge SM, Dean D, Dawber RP. Scarring alopecia in discoid lupus erythematosus. Br J Dermatol 1992;126:307-314.
5. Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin 2002;20:373-385, v.
6. Biesla I, et al. Histopathologic findings in cutaneous lupus erythematosus. Arch Dermatol 1994;130:54-58.
7. Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin 1994;12:201-206.
8. Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc 1953;152:1428-1429.
9. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983;75:35-39.
10. Kyriakis KP, Kontochristopoulos GJ, Panteleos DN. Experience with low-dose thalidomide therapy in chronic discoid lupus erythematosus. Int J Dermatol 2000;39:218-222.
11. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1171.
12. Nunez M, Boixeda P, Miralles ES, et al. Pulsed dye laser treatment of telangiectatic chronic erythema of cutaneous lupus erythematosus. Arch Dermatol 1996;132:354-355.
13. Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology 2005;211:118-122.
14. de Berker D, Dissaneyeka M, Burge S. The sequelae of chronic cutaneous lupus erythematosus. Lupus 1992;1:181-186.
Simple cellulitis or a more serious infection?
A 54-year-old woman was admitted to the emergency department with a swollen right leg, fever, and altered mental status. Her family brought her in after finding her confused and lethargic. She was incontinent of stool and urine and complained of a rash with blisters on her right thigh. The patient had noted a pimple in her groin more than 5 days earlier; over the past few days she has been complaining of increasing leg pain. She related to her sisters that she had an appointment with her gynecologist in the next few days to have the lesion drained.
The patient had no fever, chest pain, shortness of breath, nausea, or vomiting. Her medical history included type 2 diabetes mellitus, hypertension, and cortical atrophy with mild mental retardation. She had been living independently in her own apartment, and was last seen by her sisters 6 days before with no apparent complaints. She had been wheelchair-bound for 6 months due to a fractured ankle from which she has not been able to completely rehabilitate.
The medications she was taking included glyburide, raloxifene (Evista), and furosemide (Lasix). Surgical history was significant only for a cholecystectomy. She did not smoke or drink alcohol. Upon presentation to the ED she appeared ill, with a blood pressure of 124/50 mm Hg, pulse 110, respiratory rate 18, and temperature of 102°F. Her fingerstick blood sugar was 573. She was able to answer simple questions but was not oriented to time or place. Her skin was hot and dry. Chest exam revealed clear lungs with tachypnea and a 2/6 systolic murmur. Her abdomen was slightly obese, soft, and nontender with normal bowel sounds and a well-healed right upper quadrant incision. Her genitourinary exam revealed a purulent drainage in the groin near her vulva.
Her right leg was markedly swollen, erythematous, and had a brownish-red discoloration that extended from her groin circumferentially to her knee. The skin had a “woody” feel when palpated and large bullae were present (FIGURE 1).
The decision to obtain x-rays of her pelvis and femur was made to assess the extent of her infection (FIGURES 2 AND 3).
FIGURE 1
Cellulitis in the leg
FIGURE 2
Radiograph of thigh and hip area
FIGURE 3
Radiograph of knee
What is the differential diagnosis for this patient?
What tests might help delineate the extent of her infection?
Diagnosis: Acute necrotizing fasciitis
The patient was diagnosed with acute necrotizing fasciitis, a rare, often fatal, soft-tissue bacterial infection. According to the Centers for Disease Control and Prevention, only 500 to 1500 cases of necrotizing fasciitis are diagnosed each year in the US.1
Epidemiology
Peripheral vascular disease, diabetes, and a compromised immune system are significant risk factors for necrotizing fasciitis.2 Diabetes is present in 18% to 60% of cases;1,3 in addition, 19% to 77% of patients use intravenous drugs.1,3,4 Other significant predisposing factors include alcohol abuse (9%–31%),1,4 obesity,1,4 and malnutrition.3 Although risk factors are numerous, half of all cases of streptococcal necrotizing fasciitis occur in previously healthy individuals. Pathogenic agents can be introduced as a result of minor trauma, insect bites, or surgical incisions.
In this case the patient noted a “pimple” in the groin area and complained of pain for 5 days. By the time she reached the hospital she had mental status changes, fever, appeared toxic, and had signs of early septic shock. We can identify in this case the probable port of entry as the lesion in the groin that was visualized on physical exam to be draining pus.
Pathophysiology
Necrotizing fasciitis involves the superficial layer of skin, subcutaneous tissues, and fascia. The infection spreads rapidly along these layers, causing edema and compression of vasculature, which rapidly progresses to tissue necrosis and sepsis. Even with new broad-spectrum antibiotics, mortality can be as high as 75% in patients who become septic and develop renal failure.
Necrotizing fasciitis occurs when a mixed variety of organisms, both aerobic and anaerobic, invade the subcutaneous tissue and fascia.5 Most necrotizing soft-tissue infections are polymicrobial, with only a small percentage involving a single organism. In immune-compromised patients, Pseudomonas spp and gram-negative enteric organisms can be found. The organisms isolated most often in polymicrobial necrotizing soft-tissue infections are combinations of staphylococci (especially Staphylococcus epidermis with beta-hemolytic streptococcus), enterococci, Enterobacteriaceae spp (commonly Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, and Pseudomonas aeruginosa), streptococci, Bacterioides prevotella spp, anaerobic gram-positive cocci, and Clostridium spp.6
Patient presentation
The clinical history and a meticulous physical examination are essential in establishing an early diagnosis of necrotizing infections.5 Necrotizing fasciitis can be easily misdiagnosed as only cellulitis. Most often, a patient with necrotizing fasciitis appears ill, with constitutional symptoms of fever, chills, hypotension, dehydration, and rapid heart rate. You can also see erythema with bullae formation, serosanguineous fluids drainage, induration, and violaceous discoloration. Pain and crepitation may be noted.3,5,7 Rapid progression of edema and pain out of proportion to examination is seen in the early stages. The parts of the skin affected by the disease can become numb with progression of the infection; this is thought to be due to infarction of the cutaneous nerves located in necrotic subcutaneous fascia and soft tissue.5
Causative factors in this patient included diabetes and obesity. Diabetic neuropathy may have also delayed presentation and dulled her perception of pain. Diabetic microvascular disease may also have contributed to a faster progression of tissue hypoxia.
Diagnostic methods: Lab tests, biopsy, x-rays
Laboratory testing for necrotizing fasciitis is thought by most experts to be non-specific. Another investigative team found that 76% of patients with necrotizing soft-tissue infections had low platelet count or PT and PTT with higher than normal values; prolonged PT is associated with increase mortality.6 Hypocalcemia, hypoproteinemia, anemia, and acidosis have also been noted.
Diagnosis must be considered early when necrotizing fasciitis is suspected. Although the gold standard for diagnosis is biopsy or wound exploration and surgical debridement,6 diagnosis can be made early when necrotizing fasciitis is suspected.
The role of soft-tissue radiographs in the diagnosis of necrotizing fasciitis is unclear. Plain films can provide information such as soft-tissue thickening and internal gas formation. Unfortunately, plain radiographs typically show no specific abnormality until the necrotizing process is well advanced.
Treatment of necrotizing fasciitis
Resuscitation
Adequate fluid resuscitation and stabilization of any patient suspected of having necrotizing fasciitis is the first line of therapy. Large-bore IV lines or a central line may be necessary. Adequate monitoring should include a Foley catheter and pulse oximetry. Correction of any metabolic abnormalities needs to be addressed.
Antibiotics
Antibiotic treatment should be started as soon as possible, although no study has shown antibiotics to significantly alter mortality. A Gram stain of the infected material would be helpful to guide further antibiotic choices. However, initial therapy should be directed at both aerobic and anaerobic organisms.
Triple therapy is recommended: penicillin or ampicillin for Clostridia, Streptococci, and Peptostreptococcus; clindamycin or metronidazole for anaerobes, Bacteroides fragilis, Fusobacterium, and Peptostreptococcus; and gentamicin or another aminoglycoside for Enterobacteriaceae. Imipenem or meropenem can be used as the initial agent for high beta-lactamase resistance, wide-spectrum efficacy, and inhibition of endotoxin release from aerobic bacilli. Tetanus prophylaxis with absorbed tetanus toxoid and passive immune coverage with tetanus hyperimmune globulin is indicated for a patient whose history of immunization is unclear or unavailable.6
Surgery
Urgent surgical consultation is necessary. Early recognition and prompt aggressive debridement of all necrotic tissue is critical for survival—in fact, it is the only therapy demonstrated to improve the rate of survival.7 Necrotic tissue serves as a culture medium and creates an anaerobic environment, which hinders an adequate immune response. Sufficient debridement consists of exposure to all margins of viable tissue. Antibiotics are important but are secondary to urgent removal of the toxic tissue.
Hyperbaric oxygen therapy
All necrotizing infections are associated with ischemia, reduced tissue oxygen tension, and a decrease in host cellular immunity. The physciological rationale for increasing oxygen is that tension ischemia may be reversed and host defense mechanisms improved. Hyperbaric oxygen is generally considered an important adjunct in the treatment of clostridial myonecrosis or gas gangrene.
Studies have failed to show statistically significant outcome differences with respect to mortality and length of hospitalization.3 Some studies show improvement of survival rates or limb salvage; others show no difference in outcomes with hyperbaric oxygen. Note that these studies show no consistency in patient population or number of visits to the operating room. More evidence is needed, preferably by way of randomized controlled trials, before routine or wide-spread use of hyperbaric oxygen can be recommended.
The patient’s treatment and outcome
The emergency department physicians initiated intravenous antibiotics and obtained an urgent surgical consultation. In addition, they sent for blood cultures and other laboratory tests.
In the operating room, surgeons debrided her skin and removed all necrotic muscles and skin in her perineum and entire medial thigh during the first surgery. Eighteen hours later she returned to the operating room—the infection had spread to once-viable tissue from the symphysis pubis to the knee. The family was consulted concerning a more radical surgical approach, a hip disarticulation or hemipelvectomy. They declined. The patient was made comfortable; she died 12 hours later. Her wound culture later grew E coli, Proteus vulgaris, Coryne-bacterium, Enterococcus, Staphylococcus spp, and Peptostreptococcus.
CORRESPONDENCE
Susan Dufel, MD, Department Trauma and Emergency Medicine, University of Connecticut, 80 Seymour Street, Hartford CT 06102. E-mail: [email protected]
1. Faucher LD, Morris SE, Edelman LS, et al. Burn center management of necrotizing soft-tissue surgical infections in unburned patients. Am J Surg 2001;182:563-569.
2. Childers BJ, Potyondy LD, Nachreiner R, et al. Necrotizing fasciitis: a fourteen-year retrospective study of 163 patients. Am Surg 2002;68:109-116.
3. Kuncir EJ, Tillou A, St Hill CR. Necrotizing soft-tissue infections. Emerg Med Clin North Am 2003;21:1075-1087.
4. Bosshardt TL, Henderson VJ, Organ CH, Jr. Necrotizing soft-tissue infections. Arch Surg 1996;131:846-854.
5. Majeski J, John JF, Jr. Necrotizing soft tissue infections: a guide to early diagnosis and initial therapy. South Med J 2003;96:900-906.
6. Headley AJ. Necrotizing fasciitis: a primary care review. Am Fam Phys 2003;68:323-328.
7. Chin-Ho Wong, Haw-Chong Chang, Pasupathy S. Necrotizing fasciitis: clinical presentation, microbiology, determinants of mortality. J Bone Joint Surg 2003;85A(8):1454-1460.
A 54-year-old woman was admitted to the emergency department with a swollen right leg, fever, and altered mental status. Her family brought her in after finding her confused and lethargic. She was incontinent of stool and urine and complained of a rash with blisters on her right thigh. The patient had noted a pimple in her groin more than 5 days earlier; over the past few days she has been complaining of increasing leg pain. She related to her sisters that she had an appointment with her gynecologist in the next few days to have the lesion drained.
The patient had no fever, chest pain, shortness of breath, nausea, or vomiting. Her medical history included type 2 diabetes mellitus, hypertension, and cortical atrophy with mild mental retardation. She had been living independently in her own apartment, and was last seen by her sisters 6 days before with no apparent complaints. She had been wheelchair-bound for 6 months due to a fractured ankle from which she has not been able to completely rehabilitate.
The medications she was taking included glyburide, raloxifene (Evista), and furosemide (Lasix). Surgical history was significant only for a cholecystectomy. She did not smoke or drink alcohol. Upon presentation to the ED she appeared ill, with a blood pressure of 124/50 mm Hg, pulse 110, respiratory rate 18, and temperature of 102°F. Her fingerstick blood sugar was 573. She was able to answer simple questions but was not oriented to time or place. Her skin was hot and dry. Chest exam revealed clear lungs with tachypnea and a 2/6 systolic murmur. Her abdomen was slightly obese, soft, and nontender with normal bowel sounds and a well-healed right upper quadrant incision. Her genitourinary exam revealed a purulent drainage in the groin near her vulva.
Her right leg was markedly swollen, erythematous, and had a brownish-red discoloration that extended from her groin circumferentially to her knee. The skin had a “woody” feel when palpated and large bullae were present (FIGURE 1).
The decision to obtain x-rays of her pelvis and femur was made to assess the extent of her infection (FIGURES 2 AND 3).
FIGURE 1
Cellulitis in the leg
FIGURE 2
Radiograph of thigh and hip area
FIGURE 3
Radiograph of knee
What is the differential diagnosis for this patient?
What tests might help delineate the extent of her infection?
Diagnosis: Acute necrotizing fasciitis
The patient was diagnosed with acute necrotizing fasciitis, a rare, often fatal, soft-tissue bacterial infection. According to the Centers for Disease Control and Prevention, only 500 to 1500 cases of necrotizing fasciitis are diagnosed each year in the US.1
Epidemiology
Peripheral vascular disease, diabetes, and a compromised immune system are significant risk factors for necrotizing fasciitis.2 Diabetes is present in 18% to 60% of cases;1,3 in addition, 19% to 77% of patients use intravenous drugs.1,3,4 Other significant predisposing factors include alcohol abuse (9%–31%),1,4 obesity,1,4 and malnutrition.3 Although risk factors are numerous, half of all cases of streptococcal necrotizing fasciitis occur in previously healthy individuals. Pathogenic agents can be introduced as a result of minor trauma, insect bites, or surgical incisions.
In this case the patient noted a “pimple” in the groin area and complained of pain for 5 days. By the time she reached the hospital she had mental status changes, fever, appeared toxic, and had signs of early septic shock. We can identify in this case the probable port of entry as the lesion in the groin that was visualized on physical exam to be draining pus.
Pathophysiology
Necrotizing fasciitis involves the superficial layer of skin, subcutaneous tissues, and fascia. The infection spreads rapidly along these layers, causing edema and compression of vasculature, which rapidly progresses to tissue necrosis and sepsis. Even with new broad-spectrum antibiotics, mortality can be as high as 75% in patients who become septic and develop renal failure.
Necrotizing fasciitis occurs when a mixed variety of organisms, both aerobic and anaerobic, invade the subcutaneous tissue and fascia.5 Most necrotizing soft-tissue infections are polymicrobial, with only a small percentage involving a single organism. In immune-compromised patients, Pseudomonas spp and gram-negative enteric organisms can be found. The organisms isolated most often in polymicrobial necrotizing soft-tissue infections are combinations of staphylococci (especially Staphylococcus epidermis with beta-hemolytic streptococcus), enterococci, Enterobacteriaceae spp (commonly Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, and Pseudomonas aeruginosa), streptococci, Bacterioides prevotella spp, anaerobic gram-positive cocci, and Clostridium spp.6
Patient presentation
The clinical history and a meticulous physical examination are essential in establishing an early diagnosis of necrotizing infections.5 Necrotizing fasciitis can be easily misdiagnosed as only cellulitis. Most often, a patient with necrotizing fasciitis appears ill, with constitutional symptoms of fever, chills, hypotension, dehydration, and rapid heart rate. You can also see erythema with bullae formation, serosanguineous fluids drainage, induration, and violaceous discoloration. Pain and crepitation may be noted.3,5,7 Rapid progression of edema and pain out of proportion to examination is seen in the early stages. The parts of the skin affected by the disease can become numb with progression of the infection; this is thought to be due to infarction of the cutaneous nerves located in necrotic subcutaneous fascia and soft tissue.5
Causative factors in this patient included diabetes and obesity. Diabetic neuropathy may have also delayed presentation and dulled her perception of pain. Diabetic microvascular disease may also have contributed to a faster progression of tissue hypoxia.
Diagnostic methods: Lab tests, biopsy, x-rays
Laboratory testing for necrotizing fasciitis is thought by most experts to be non-specific. Another investigative team found that 76% of patients with necrotizing soft-tissue infections had low platelet count or PT and PTT with higher than normal values; prolonged PT is associated with increase mortality.6 Hypocalcemia, hypoproteinemia, anemia, and acidosis have also been noted.
Diagnosis must be considered early when necrotizing fasciitis is suspected. Although the gold standard for diagnosis is biopsy or wound exploration and surgical debridement,6 diagnosis can be made early when necrotizing fasciitis is suspected.
The role of soft-tissue radiographs in the diagnosis of necrotizing fasciitis is unclear. Plain films can provide information such as soft-tissue thickening and internal gas formation. Unfortunately, plain radiographs typically show no specific abnormality until the necrotizing process is well advanced.
Treatment of necrotizing fasciitis
Resuscitation
Adequate fluid resuscitation and stabilization of any patient suspected of having necrotizing fasciitis is the first line of therapy. Large-bore IV lines or a central line may be necessary. Adequate monitoring should include a Foley catheter and pulse oximetry. Correction of any metabolic abnormalities needs to be addressed.
Antibiotics
Antibiotic treatment should be started as soon as possible, although no study has shown antibiotics to significantly alter mortality. A Gram stain of the infected material would be helpful to guide further antibiotic choices. However, initial therapy should be directed at both aerobic and anaerobic organisms.
Triple therapy is recommended: penicillin or ampicillin for Clostridia, Streptococci, and Peptostreptococcus; clindamycin or metronidazole for anaerobes, Bacteroides fragilis, Fusobacterium, and Peptostreptococcus; and gentamicin or another aminoglycoside for Enterobacteriaceae. Imipenem or meropenem can be used as the initial agent for high beta-lactamase resistance, wide-spectrum efficacy, and inhibition of endotoxin release from aerobic bacilli. Tetanus prophylaxis with absorbed tetanus toxoid and passive immune coverage with tetanus hyperimmune globulin is indicated for a patient whose history of immunization is unclear or unavailable.6
Surgery
Urgent surgical consultation is necessary. Early recognition and prompt aggressive debridement of all necrotic tissue is critical for survival—in fact, it is the only therapy demonstrated to improve the rate of survival.7 Necrotic tissue serves as a culture medium and creates an anaerobic environment, which hinders an adequate immune response. Sufficient debridement consists of exposure to all margins of viable tissue. Antibiotics are important but are secondary to urgent removal of the toxic tissue.
Hyperbaric oxygen therapy
All necrotizing infections are associated with ischemia, reduced tissue oxygen tension, and a decrease in host cellular immunity. The physciological rationale for increasing oxygen is that tension ischemia may be reversed and host defense mechanisms improved. Hyperbaric oxygen is generally considered an important adjunct in the treatment of clostridial myonecrosis or gas gangrene.
Studies have failed to show statistically significant outcome differences with respect to mortality and length of hospitalization.3 Some studies show improvement of survival rates or limb salvage; others show no difference in outcomes with hyperbaric oxygen. Note that these studies show no consistency in patient population or number of visits to the operating room. More evidence is needed, preferably by way of randomized controlled trials, before routine or wide-spread use of hyperbaric oxygen can be recommended.
The patient’s treatment and outcome
The emergency department physicians initiated intravenous antibiotics and obtained an urgent surgical consultation. In addition, they sent for blood cultures and other laboratory tests.
In the operating room, surgeons debrided her skin and removed all necrotic muscles and skin in her perineum and entire medial thigh during the first surgery. Eighteen hours later she returned to the operating room—the infection had spread to once-viable tissue from the symphysis pubis to the knee. The family was consulted concerning a more radical surgical approach, a hip disarticulation or hemipelvectomy. They declined. The patient was made comfortable; she died 12 hours later. Her wound culture later grew E coli, Proteus vulgaris, Coryne-bacterium, Enterococcus, Staphylococcus spp, and Peptostreptococcus.
CORRESPONDENCE
Susan Dufel, MD, Department Trauma and Emergency Medicine, University of Connecticut, 80 Seymour Street, Hartford CT 06102. E-mail: [email protected]
A 54-year-old woman was admitted to the emergency department with a swollen right leg, fever, and altered mental status. Her family brought her in after finding her confused and lethargic. She was incontinent of stool and urine and complained of a rash with blisters on her right thigh. The patient had noted a pimple in her groin more than 5 days earlier; over the past few days she has been complaining of increasing leg pain. She related to her sisters that she had an appointment with her gynecologist in the next few days to have the lesion drained.
The patient had no fever, chest pain, shortness of breath, nausea, or vomiting. Her medical history included type 2 diabetes mellitus, hypertension, and cortical atrophy with mild mental retardation. She had been living independently in her own apartment, and was last seen by her sisters 6 days before with no apparent complaints. She had been wheelchair-bound for 6 months due to a fractured ankle from which she has not been able to completely rehabilitate.
The medications she was taking included glyburide, raloxifene (Evista), and furosemide (Lasix). Surgical history was significant only for a cholecystectomy. She did not smoke or drink alcohol. Upon presentation to the ED she appeared ill, with a blood pressure of 124/50 mm Hg, pulse 110, respiratory rate 18, and temperature of 102°F. Her fingerstick blood sugar was 573. She was able to answer simple questions but was not oriented to time or place. Her skin was hot and dry. Chest exam revealed clear lungs with tachypnea and a 2/6 systolic murmur. Her abdomen was slightly obese, soft, and nontender with normal bowel sounds and a well-healed right upper quadrant incision. Her genitourinary exam revealed a purulent drainage in the groin near her vulva.
Her right leg was markedly swollen, erythematous, and had a brownish-red discoloration that extended from her groin circumferentially to her knee. The skin had a “woody” feel when palpated and large bullae were present (FIGURE 1).
The decision to obtain x-rays of her pelvis and femur was made to assess the extent of her infection (FIGURES 2 AND 3).
FIGURE 1
Cellulitis in the leg
FIGURE 2
Radiograph of thigh and hip area
FIGURE 3
Radiograph of knee
What is the differential diagnosis for this patient?
What tests might help delineate the extent of her infection?
Diagnosis: Acute necrotizing fasciitis
The patient was diagnosed with acute necrotizing fasciitis, a rare, often fatal, soft-tissue bacterial infection. According to the Centers for Disease Control and Prevention, only 500 to 1500 cases of necrotizing fasciitis are diagnosed each year in the US.1
Epidemiology
Peripheral vascular disease, diabetes, and a compromised immune system are significant risk factors for necrotizing fasciitis.2 Diabetes is present in 18% to 60% of cases;1,3 in addition, 19% to 77% of patients use intravenous drugs.1,3,4 Other significant predisposing factors include alcohol abuse (9%–31%),1,4 obesity,1,4 and malnutrition.3 Although risk factors are numerous, half of all cases of streptococcal necrotizing fasciitis occur in previously healthy individuals. Pathogenic agents can be introduced as a result of minor trauma, insect bites, or surgical incisions.
In this case the patient noted a “pimple” in the groin area and complained of pain for 5 days. By the time she reached the hospital she had mental status changes, fever, appeared toxic, and had signs of early septic shock. We can identify in this case the probable port of entry as the lesion in the groin that was visualized on physical exam to be draining pus.
Pathophysiology
Necrotizing fasciitis involves the superficial layer of skin, subcutaneous tissues, and fascia. The infection spreads rapidly along these layers, causing edema and compression of vasculature, which rapidly progresses to tissue necrosis and sepsis. Even with new broad-spectrum antibiotics, mortality can be as high as 75% in patients who become septic and develop renal failure.
Necrotizing fasciitis occurs when a mixed variety of organisms, both aerobic and anaerobic, invade the subcutaneous tissue and fascia.5 Most necrotizing soft-tissue infections are polymicrobial, with only a small percentage involving a single organism. In immune-compromised patients, Pseudomonas spp and gram-negative enteric organisms can be found. The organisms isolated most often in polymicrobial necrotizing soft-tissue infections are combinations of staphylococci (especially Staphylococcus epidermis with beta-hemolytic streptococcus), enterococci, Enterobacteriaceae spp (commonly Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, and Pseudomonas aeruginosa), streptococci, Bacterioides prevotella spp, anaerobic gram-positive cocci, and Clostridium spp.6
Patient presentation
The clinical history and a meticulous physical examination are essential in establishing an early diagnosis of necrotizing infections.5 Necrotizing fasciitis can be easily misdiagnosed as only cellulitis. Most often, a patient with necrotizing fasciitis appears ill, with constitutional symptoms of fever, chills, hypotension, dehydration, and rapid heart rate. You can also see erythema with bullae formation, serosanguineous fluids drainage, induration, and violaceous discoloration. Pain and crepitation may be noted.3,5,7 Rapid progression of edema and pain out of proportion to examination is seen in the early stages. The parts of the skin affected by the disease can become numb with progression of the infection; this is thought to be due to infarction of the cutaneous nerves located in necrotic subcutaneous fascia and soft tissue.5
Causative factors in this patient included diabetes and obesity. Diabetic neuropathy may have also delayed presentation and dulled her perception of pain. Diabetic microvascular disease may also have contributed to a faster progression of tissue hypoxia.
Diagnostic methods: Lab tests, biopsy, x-rays
Laboratory testing for necrotizing fasciitis is thought by most experts to be non-specific. Another investigative team found that 76% of patients with necrotizing soft-tissue infections had low platelet count or PT and PTT with higher than normal values; prolonged PT is associated with increase mortality.6 Hypocalcemia, hypoproteinemia, anemia, and acidosis have also been noted.
Diagnosis must be considered early when necrotizing fasciitis is suspected. Although the gold standard for diagnosis is biopsy or wound exploration and surgical debridement,6 diagnosis can be made early when necrotizing fasciitis is suspected.
The role of soft-tissue radiographs in the diagnosis of necrotizing fasciitis is unclear. Plain films can provide information such as soft-tissue thickening and internal gas formation. Unfortunately, plain radiographs typically show no specific abnormality until the necrotizing process is well advanced.
Treatment of necrotizing fasciitis
Resuscitation
Adequate fluid resuscitation and stabilization of any patient suspected of having necrotizing fasciitis is the first line of therapy. Large-bore IV lines or a central line may be necessary. Adequate monitoring should include a Foley catheter and pulse oximetry. Correction of any metabolic abnormalities needs to be addressed.
Antibiotics
Antibiotic treatment should be started as soon as possible, although no study has shown antibiotics to significantly alter mortality. A Gram stain of the infected material would be helpful to guide further antibiotic choices. However, initial therapy should be directed at both aerobic and anaerobic organisms.
Triple therapy is recommended: penicillin or ampicillin for Clostridia, Streptococci, and Peptostreptococcus; clindamycin or metronidazole for anaerobes, Bacteroides fragilis, Fusobacterium, and Peptostreptococcus; and gentamicin or another aminoglycoside for Enterobacteriaceae. Imipenem or meropenem can be used as the initial agent for high beta-lactamase resistance, wide-spectrum efficacy, and inhibition of endotoxin release from aerobic bacilli. Tetanus prophylaxis with absorbed tetanus toxoid and passive immune coverage with tetanus hyperimmune globulin is indicated for a patient whose history of immunization is unclear or unavailable.6
Surgery
Urgent surgical consultation is necessary. Early recognition and prompt aggressive debridement of all necrotic tissue is critical for survival—in fact, it is the only therapy demonstrated to improve the rate of survival.7 Necrotic tissue serves as a culture medium and creates an anaerobic environment, which hinders an adequate immune response. Sufficient debridement consists of exposure to all margins of viable tissue. Antibiotics are important but are secondary to urgent removal of the toxic tissue.
Hyperbaric oxygen therapy
All necrotizing infections are associated with ischemia, reduced tissue oxygen tension, and a decrease in host cellular immunity. The physciological rationale for increasing oxygen is that tension ischemia may be reversed and host defense mechanisms improved. Hyperbaric oxygen is generally considered an important adjunct in the treatment of clostridial myonecrosis or gas gangrene.
Studies have failed to show statistically significant outcome differences with respect to mortality and length of hospitalization.3 Some studies show improvement of survival rates or limb salvage; others show no difference in outcomes with hyperbaric oxygen. Note that these studies show no consistency in patient population or number of visits to the operating room. More evidence is needed, preferably by way of randomized controlled trials, before routine or wide-spread use of hyperbaric oxygen can be recommended.
The patient’s treatment and outcome
The emergency department physicians initiated intravenous antibiotics and obtained an urgent surgical consultation. In addition, they sent for blood cultures and other laboratory tests.
In the operating room, surgeons debrided her skin and removed all necrotic muscles and skin in her perineum and entire medial thigh during the first surgery. Eighteen hours later she returned to the operating room—the infection had spread to once-viable tissue from the symphysis pubis to the knee. The family was consulted concerning a more radical surgical approach, a hip disarticulation or hemipelvectomy. They declined. The patient was made comfortable; she died 12 hours later. Her wound culture later grew E coli, Proteus vulgaris, Coryne-bacterium, Enterococcus, Staphylococcus spp, and Peptostreptococcus.
CORRESPONDENCE
Susan Dufel, MD, Department Trauma and Emergency Medicine, University of Connecticut, 80 Seymour Street, Hartford CT 06102. E-mail: [email protected]
1. Faucher LD, Morris SE, Edelman LS, et al. Burn center management of necrotizing soft-tissue surgical infections in unburned patients. Am J Surg 2001;182:563-569.
2. Childers BJ, Potyondy LD, Nachreiner R, et al. Necrotizing fasciitis: a fourteen-year retrospective study of 163 patients. Am Surg 2002;68:109-116.
3. Kuncir EJ, Tillou A, St Hill CR. Necrotizing soft-tissue infections. Emerg Med Clin North Am 2003;21:1075-1087.
4. Bosshardt TL, Henderson VJ, Organ CH, Jr. Necrotizing soft-tissue infections. Arch Surg 1996;131:846-854.
5. Majeski J, John JF, Jr. Necrotizing soft tissue infections: a guide to early diagnosis and initial therapy. South Med J 2003;96:900-906.
6. Headley AJ. Necrotizing fasciitis: a primary care review. Am Fam Phys 2003;68:323-328.
7. Chin-Ho Wong, Haw-Chong Chang, Pasupathy S. Necrotizing fasciitis: clinical presentation, microbiology, determinants of mortality. J Bone Joint Surg 2003;85A(8):1454-1460.
1. Faucher LD, Morris SE, Edelman LS, et al. Burn center management of necrotizing soft-tissue surgical infections in unburned patients. Am J Surg 2001;182:563-569.
2. Childers BJ, Potyondy LD, Nachreiner R, et al. Necrotizing fasciitis: a fourteen-year retrospective study of 163 patients. Am Surg 2002;68:109-116.
3. Kuncir EJ, Tillou A, St Hill CR. Necrotizing soft-tissue infections. Emerg Med Clin North Am 2003;21:1075-1087.
4. Bosshardt TL, Henderson VJ, Organ CH, Jr. Necrotizing soft-tissue infections. Arch Surg 1996;131:846-854.
5. Majeski J, John JF, Jr. Necrotizing soft tissue infections: a guide to early diagnosis and initial therapy. South Med J 2003;96:900-906.
6. Headley AJ. Necrotizing fasciitis: a primary care review. Am Fam Phys 2003;68:323-328.
7. Chin-Ho Wong, Haw-Chong Chang, Pasupathy S. Necrotizing fasciitis: clinical presentation, microbiology, determinants of mortality. J Bone Joint Surg 2003;85A(8):1454-1460.
Left-sided back pain that won’t go away
A 68-year-old man came to the emergency department complaining of left-side thoracic back pain, after 5 days of outpatient treatment with analgesics did not help him. His pain started after physical labor, but he did not recall any trauma. A review of his medical history revealed only coronary artery disease, with coronary stent placement several years before this event. He had not been hospitalized recently, undergone an invasive procedure, or taken antibiotics.
On examination, a left thoracic paraspinal muscle was tender without fluctuance, overlying skin redness, or a lesion. An elevated white blood cell count of 12,300/mcL was the only laboratory test with abnormal results. The patient did not have fever, and results of urinalysis, chest radiograph, and abdominal sonogram were normal. Computed tomography (CT) images of the abdomen and pelvis showed inflammation of a left thoracic paraspinal muscle.
Thirty-six hours after admission, the patient developed fever (38.8°C). His physicians obtained a blood culture. The fever recurred (at 38.9°C) the following day, and 2 more blood cultures were done. His back pain did not improve despite analgesics, intravenous antibiotics, and physical therapy; therefore, on the eighth day in the hospital, magnetic resonance imaging (MRI) was performed (FIGURE).
FIGURE
MRI of the back
What is the diagnosis?
Diagnosis: Methicillin-resistant Staphylococcus aureus pyomyositis
Reports of the 3 blood cultures, acquired on the seventh day in the hospital, showed growth of methicillin-resistant Staphylococcus aureus (MRSA) with antibiotic susceptibility patterns common to community-acquired MRSA.1 A purulent aspirate was obtained from the lesion in the FIGURE with CT-guided drainage. Community-acquired MRSA grew in the aspirate culture. The final diagnosis was a primary skeletal muscle abscess without contiguous spread from an adjacent site, or pyomyositis.
Epidemiology of pyomyositis
Pyomyositis is uncommon in immunocompetent individuals living outside of the tropics. It is usually caused by S aureus. In the United States between 1981 and 2002, 330 cases were identified in a literature review,2 which noted an increasing incidence. Of these cases, 70% were caused by S aureus and 61.5% of patients were immunocompromised; 1 involved MRSA.
In 2003, 2 of 3 MRSA cases were reported in patients with hematologic disorders.3 In 2005, 4 community-acquired MRSA cases were reported;1 2 had other illnesses leading to increased risk. MRSA pyomyositis is now being reported in immunocompetent individuals, but most cases arise in patients with cancer, diabetes mellitus, rheumatologic disorders, hematologic disorders, renal failure, liver cirrhosis, intravenous drug use, or HIV infection.2,4,5 With staphylococcal infections increasing,6 including MRSA and community-acquired MRSA, the incidence of pyomyositis may increase correspondingly.
Consider the possibility of pyomyositis in patients with localized muscle pain and tenderness and who have risk factors for acquiring MRSA—living in a community with prevalent MRSA, recent hospitalization, antibiotic use, invasive procedures, or chronic venous catheters.
Most commonly, pyomyositis occurs in a leg or arm.2 In the 330 cases mentioned above, the sites of infection, in descending order of frequency, were lower extremity, upper extremity, buttocks, chest wall, paraspinal, and psoas.
Diagnostic evaluation: Know the stages
Making the correct diagnosis at the initial presentation is difficult because nonspecific symptoms resemble a muscle strain. But this infection progresses to bacteremia, making early identification important. To recognize pyomyositis before bacteremia develops, rely on clinical suspicion and familiarity with the 3 stages of the disease.
Stage 1 (invasive) is characterized by muscle pain and tenderness without systemic evidence of illness. Stage 2 (suppurative) is the formation of an abscess. At this time systemic symptoms such as fever may occur; otherwise physical exam findings remain vague with tenderness and induration without overlying skin redness or palpable fluctuance common to skin abscesses. Stage 3 is bacteremia or sepsis. Awareness of the risks and the stages of pyomyositis will aid in quicker diagnosis.
Diagnostic delay occurs because the first stage presents with nonspecific symptoms and examination findings, and also because initial laboratory and radiographic testing are inconclusive. Elevation of the white blood cell count and the erythrocyte sedimentation rate occur early, while serum muscle enzymes remain normal.2,4,5
Imaging studies performed during the first stage may not be productive; however, in the second stage imaging identifies the abscess. Although limited data exist regarding the best imaging modality, MRI and CT appear most useful.1,3,4,7 In 1 retrospective analysis, ultrasound identified 5 of 8 cases correctly, while MRI and CT were diagnostic in 5 of 6 and 9 of 9, respectively.7
Treatment: Antibiotics, drainage
Choose an antibiotic empirically that covers S aureus and is also appropriate for MRSA. Pyomyositis discovered in the first stage may resolve with antibiotics alone.4 In addition, early intervention may be especially important with MRSA, since MRSA bacteremia is associated with increased mortality compared with methicillin-sensitive S aureus bacteremia.6
If the condition progresses to the second stage with abscess development, surgical or percutaneous drainage will be necessary. Identification and removal of the site of infection in patients with community-acquired staphylococcal bacteremia results in improved outcomes,8 and pyomyositis can be an overlooked source.
Patient outcome
The day following drainage of the abscess, the patient was discharged from the hospital. He was treated for 6 weeks with intravenous antibiotics because of staphylococcal bacteremia, and he has fully recovered.
Acknowledgments
The authors would like to thank Robert Martin, Lead Operator, Information Technology, Texas Tech University Health Sciences Center at Amarillo for assistance in computer image enhancement and development.
CORRESPONDENCE
Timothy J. Benton, MD, Texas Tech University Health Sciences Center at Amarillo, Department of Family and Community Medicine, 1600 Wallace Boulevard, Amarillo, TX 79106. E-mail: [email protected]
1. Ruiz M, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2005;352:1488-1489.
2. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420-428.
3. Hayashi T. Pyomyositis as a focus of infection in hematological disorders: a report of 3 cases. Int J Hematol 2003;77:171-174.
4. Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 2002;16:697-712.
5. Hossain A, Reis ED, Soundararajan K, Kerstain MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg 2000;66:1064-1066.
6. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59.
7. Boniotti V. Contribution of imaging to the evaluation of pyomyositis. Radiol Med 2005;109:404-13.
8. Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J Hosp Infect 2002;52:29-36.
A 68-year-old man came to the emergency department complaining of left-side thoracic back pain, after 5 days of outpatient treatment with analgesics did not help him. His pain started after physical labor, but he did not recall any trauma. A review of his medical history revealed only coronary artery disease, with coronary stent placement several years before this event. He had not been hospitalized recently, undergone an invasive procedure, or taken antibiotics.
On examination, a left thoracic paraspinal muscle was tender without fluctuance, overlying skin redness, or a lesion. An elevated white blood cell count of 12,300/mcL was the only laboratory test with abnormal results. The patient did not have fever, and results of urinalysis, chest radiograph, and abdominal sonogram were normal. Computed tomography (CT) images of the abdomen and pelvis showed inflammation of a left thoracic paraspinal muscle.
Thirty-six hours after admission, the patient developed fever (38.8°C). His physicians obtained a blood culture. The fever recurred (at 38.9°C) the following day, and 2 more blood cultures were done. His back pain did not improve despite analgesics, intravenous antibiotics, and physical therapy; therefore, on the eighth day in the hospital, magnetic resonance imaging (MRI) was performed (FIGURE).
FIGURE
MRI of the back
What is the diagnosis?
Diagnosis: Methicillin-resistant Staphylococcus aureus pyomyositis
Reports of the 3 blood cultures, acquired on the seventh day in the hospital, showed growth of methicillin-resistant Staphylococcus aureus (MRSA) with antibiotic susceptibility patterns common to community-acquired MRSA.1 A purulent aspirate was obtained from the lesion in the FIGURE with CT-guided drainage. Community-acquired MRSA grew in the aspirate culture. The final diagnosis was a primary skeletal muscle abscess without contiguous spread from an adjacent site, or pyomyositis.
Epidemiology of pyomyositis
Pyomyositis is uncommon in immunocompetent individuals living outside of the tropics. It is usually caused by S aureus. In the United States between 1981 and 2002, 330 cases were identified in a literature review,2 which noted an increasing incidence. Of these cases, 70% were caused by S aureus and 61.5% of patients were immunocompromised; 1 involved MRSA.
In 2003, 2 of 3 MRSA cases were reported in patients with hematologic disorders.3 In 2005, 4 community-acquired MRSA cases were reported;1 2 had other illnesses leading to increased risk. MRSA pyomyositis is now being reported in immunocompetent individuals, but most cases arise in patients with cancer, diabetes mellitus, rheumatologic disorders, hematologic disorders, renal failure, liver cirrhosis, intravenous drug use, or HIV infection.2,4,5 With staphylococcal infections increasing,6 including MRSA and community-acquired MRSA, the incidence of pyomyositis may increase correspondingly.
Consider the possibility of pyomyositis in patients with localized muscle pain and tenderness and who have risk factors for acquiring MRSA—living in a community with prevalent MRSA, recent hospitalization, antibiotic use, invasive procedures, or chronic venous catheters.
Most commonly, pyomyositis occurs in a leg or arm.2 In the 330 cases mentioned above, the sites of infection, in descending order of frequency, were lower extremity, upper extremity, buttocks, chest wall, paraspinal, and psoas.
Diagnostic evaluation: Know the stages
Making the correct diagnosis at the initial presentation is difficult because nonspecific symptoms resemble a muscle strain. But this infection progresses to bacteremia, making early identification important. To recognize pyomyositis before bacteremia develops, rely on clinical suspicion and familiarity with the 3 stages of the disease.
Stage 1 (invasive) is characterized by muscle pain and tenderness without systemic evidence of illness. Stage 2 (suppurative) is the formation of an abscess. At this time systemic symptoms such as fever may occur; otherwise physical exam findings remain vague with tenderness and induration without overlying skin redness or palpable fluctuance common to skin abscesses. Stage 3 is bacteremia or sepsis. Awareness of the risks and the stages of pyomyositis will aid in quicker diagnosis.
Diagnostic delay occurs because the first stage presents with nonspecific symptoms and examination findings, and also because initial laboratory and radiographic testing are inconclusive. Elevation of the white blood cell count and the erythrocyte sedimentation rate occur early, while serum muscle enzymes remain normal.2,4,5
Imaging studies performed during the first stage may not be productive; however, in the second stage imaging identifies the abscess. Although limited data exist regarding the best imaging modality, MRI and CT appear most useful.1,3,4,7 In 1 retrospective analysis, ultrasound identified 5 of 8 cases correctly, while MRI and CT were diagnostic in 5 of 6 and 9 of 9, respectively.7
Treatment: Antibiotics, drainage
Choose an antibiotic empirically that covers S aureus and is also appropriate for MRSA. Pyomyositis discovered in the first stage may resolve with antibiotics alone.4 In addition, early intervention may be especially important with MRSA, since MRSA bacteremia is associated with increased mortality compared with methicillin-sensitive S aureus bacteremia.6
If the condition progresses to the second stage with abscess development, surgical or percutaneous drainage will be necessary. Identification and removal of the site of infection in patients with community-acquired staphylococcal bacteremia results in improved outcomes,8 and pyomyositis can be an overlooked source.
Patient outcome
The day following drainage of the abscess, the patient was discharged from the hospital. He was treated for 6 weeks with intravenous antibiotics because of staphylococcal bacteremia, and he has fully recovered.
Acknowledgments
The authors would like to thank Robert Martin, Lead Operator, Information Technology, Texas Tech University Health Sciences Center at Amarillo for assistance in computer image enhancement and development.
CORRESPONDENCE
Timothy J. Benton, MD, Texas Tech University Health Sciences Center at Amarillo, Department of Family and Community Medicine, 1600 Wallace Boulevard, Amarillo, TX 79106. E-mail: [email protected]
A 68-year-old man came to the emergency department complaining of left-side thoracic back pain, after 5 days of outpatient treatment with analgesics did not help him. His pain started after physical labor, but he did not recall any trauma. A review of his medical history revealed only coronary artery disease, with coronary stent placement several years before this event. He had not been hospitalized recently, undergone an invasive procedure, or taken antibiotics.
On examination, a left thoracic paraspinal muscle was tender without fluctuance, overlying skin redness, or a lesion. An elevated white blood cell count of 12,300/mcL was the only laboratory test with abnormal results. The patient did not have fever, and results of urinalysis, chest radiograph, and abdominal sonogram were normal. Computed tomography (CT) images of the abdomen and pelvis showed inflammation of a left thoracic paraspinal muscle.
Thirty-six hours after admission, the patient developed fever (38.8°C). His physicians obtained a blood culture. The fever recurred (at 38.9°C) the following day, and 2 more blood cultures were done. His back pain did not improve despite analgesics, intravenous antibiotics, and physical therapy; therefore, on the eighth day in the hospital, magnetic resonance imaging (MRI) was performed (FIGURE).
FIGURE
MRI of the back
What is the diagnosis?
Diagnosis: Methicillin-resistant Staphylococcus aureus pyomyositis
Reports of the 3 blood cultures, acquired on the seventh day in the hospital, showed growth of methicillin-resistant Staphylococcus aureus (MRSA) with antibiotic susceptibility patterns common to community-acquired MRSA.1 A purulent aspirate was obtained from the lesion in the FIGURE with CT-guided drainage. Community-acquired MRSA grew in the aspirate culture. The final diagnosis was a primary skeletal muscle abscess without contiguous spread from an adjacent site, or pyomyositis.
Epidemiology of pyomyositis
Pyomyositis is uncommon in immunocompetent individuals living outside of the tropics. It is usually caused by S aureus. In the United States between 1981 and 2002, 330 cases were identified in a literature review,2 which noted an increasing incidence. Of these cases, 70% were caused by S aureus and 61.5% of patients were immunocompromised; 1 involved MRSA.
In 2003, 2 of 3 MRSA cases were reported in patients with hematologic disorders.3 In 2005, 4 community-acquired MRSA cases were reported;1 2 had other illnesses leading to increased risk. MRSA pyomyositis is now being reported in immunocompetent individuals, but most cases arise in patients with cancer, diabetes mellitus, rheumatologic disorders, hematologic disorders, renal failure, liver cirrhosis, intravenous drug use, or HIV infection.2,4,5 With staphylococcal infections increasing,6 including MRSA and community-acquired MRSA, the incidence of pyomyositis may increase correspondingly.
Consider the possibility of pyomyositis in patients with localized muscle pain and tenderness and who have risk factors for acquiring MRSA—living in a community with prevalent MRSA, recent hospitalization, antibiotic use, invasive procedures, or chronic venous catheters.
Most commonly, pyomyositis occurs in a leg or arm.2 In the 330 cases mentioned above, the sites of infection, in descending order of frequency, were lower extremity, upper extremity, buttocks, chest wall, paraspinal, and psoas.
Diagnostic evaluation: Know the stages
Making the correct diagnosis at the initial presentation is difficult because nonspecific symptoms resemble a muscle strain. But this infection progresses to bacteremia, making early identification important. To recognize pyomyositis before bacteremia develops, rely on clinical suspicion and familiarity with the 3 stages of the disease.
Stage 1 (invasive) is characterized by muscle pain and tenderness without systemic evidence of illness. Stage 2 (suppurative) is the formation of an abscess. At this time systemic symptoms such as fever may occur; otherwise physical exam findings remain vague with tenderness and induration without overlying skin redness or palpable fluctuance common to skin abscesses. Stage 3 is bacteremia or sepsis. Awareness of the risks and the stages of pyomyositis will aid in quicker diagnosis.
Diagnostic delay occurs because the first stage presents with nonspecific symptoms and examination findings, and also because initial laboratory and radiographic testing are inconclusive. Elevation of the white blood cell count and the erythrocyte sedimentation rate occur early, while serum muscle enzymes remain normal.2,4,5
Imaging studies performed during the first stage may not be productive; however, in the second stage imaging identifies the abscess. Although limited data exist regarding the best imaging modality, MRI and CT appear most useful.1,3,4,7 In 1 retrospective analysis, ultrasound identified 5 of 8 cases correctly, while MRI and CT were diagnostic in 5 of 6 and 9 of 9, respectively.7
Treatment: Antibiotics, drainage
Choose an antibiotic empirically that covers S aureus and is also appropriate for MRSA. Pyomyositis discovered in the first stage may resolve with antibiotics alone.4 In addition, early intervention may be especially important with MRSA, since MRSA bacteremia is associated with increased mortality compared with methicillin-sensitive S aureus bacteremia.6
If the condition progresses to the second stage with abscess development, surgical or percutaneous drainage will be necessary. Identification and removal of the site of infection in patients with community-acquired staphylococcal bacteremia results in improved outcomes,8 and pyomyositis can be an overlooked source.
Patient outcome
The day following drainage of the abscess, the patient was discharged from the hospital. He was treated for 6 weeks with intravenous antibiotics because of staphylococcal bacteremia, and he has fully recovered.
Acknowledgments
The authors would like to thank Robert Martin, Lead Operator, Information Technology, Texas Tech University Health Sciences Center at Amarillo for assistance in computer image enhancement and development.
CORRESPONDENCE
Timothy J. Benton, MD, Texas Tech University Health Sciences Center at Amarillo, Department of Family and Community Medicine, 1600 Wallace Boulevard, Amarillo, TX 79106. E-mail: [email protected]
1. Ruiz M, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2005;352:1488-1489.
2. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420-428.
3. Hayashi T. Pyomyositis as a focus of infection in hematological disorders: a report of 3 cases. Int J Hematol 2003;77:171-174.
4. Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 2002;16:697-712.
5. Hossain A, Reis ED, Soundararajan K, Kerstain MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg 2000;66:1064-1066.
6. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59.
7. Boniotti V. Contribution of imaging to the evaluation of pyomyositis. Radiol Med 2005;109:404-13.
8. Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J Hosp Infect 2002;52:29-36.
1. Ruiz M, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2005;352:1488-1489.
2. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420-428.
3. Hayashi T. Pyomyositis as a focus of infection in hematological disorders: a report of 3 cases. Int J Hematol 2003;77:171-174.
4. Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 2002;16:697-712.
5. Hossain A, Reis ED, Soundararajan K, Kerstain MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg 2000;66:1064-1066.
6. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59.
7. Boniotti V. Contribution of imaging to the evaluation of pyomyositis. Radiol Med 2005;109:404-13.
8. Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J Hosp Infect 2002;52:29-36.
A non-healing ulcerated fingertip following injury
A man went to his primary care physician 3 months after slamming his right thumb in a car door. The nail had turned black and sloughed off several weeks later, leaving a red, draining wound on the tip of his thumb. The wound drained continuously for the next 2 months and showed little progress in healing.
His physician started him on antibiotics, but the wound still showed no progress in healing over the next 6 weeks. Cultures were obtained that grew out Staphylococcus and Streptococcus spp. Another course of antibiotics was given, but the patient’s condition failed to improve.
At this point the patient was referred to a surgeon. He missed several appointments before finally presenting to the surgery clinic nearly 6 months after his original office visit. He was diagnosed clinically as having a giant pyogenic granuloma and was given antibiotics as well as silver nitrate sticks to cauterize the wound daily. After missing several more follow-up appointments, the patient returned with a spongy, weeping soft-tissue wound over the dorsum of his right thumb [that] doubled in size over the past 3 months (FIGURE). Radiographs obtained at that time were normal, but a bone scan revealed late uptake, cause for concern that this was osteomyelitis.
FIGURE
Ulcerated tip of the thumb
What is the differential diagnosis, and what tests are necessary?
Diagnosis: Subungual melanoma
Due to the aggressive nature of the wound and the large soft-tissue defect, a plastic surgeon was consulted. Biopsy showed malignant melanoma, and the thumb was amputated at the base of the proximal phalanx. Pathology revealed a 3×3.5 cm ulceration at the distal portion of the specimen with malignant melanoma involving the skin, subcutaneous tissue, and bone marrow. Two of 3 axillary lymph nodes were also positive for metastatic malignant melanoma.
History and epidemiology
While public awareness of cutaneous melanoma has been increasing, subungual melanoma remains obscure. First described in 1834 by Alexis Boyer, surgeon to Napoleon, it was later characterized in 1886 by Sir Jonathan Hutchinson, who reported 6 cases of “melanotic whitlow.”1 Hutchinson reported that the lesion was usually first attributed to an injury, and because of this the diagnosis was nearly always missed in the early stages.
Today, subungual melanoma is often neglected by patients and frequently misdiagnosed by physicians. The estimated mean delay in diagnosis ranges from 3 to 24 months—nearly double the diagnostic delay observed with cutaneous melanoma.1,2
One study found 52% of subungual melanomas were mistaken for benign or traumatic lesions of the nail bed such as pyogenic granuloma, paronychia, onychomycosis, chronic infection, subungual hematoma, or pigmented nevus. This mistake is not surprising as these lesions are all in the differential diagnosis of a single pigmented nail streak, and all are far more common than subungual melanoma.
Another study found that two thirds of patients underwent some inappropriate surgical procedure before the correct diagnosis was considered.1 Because of its poor prognosis, often related to delay in diagnosis, maintain a high index of suspicion for subungual melanoma in the proper setting and a sound understanding of which lesions need to be biopsied.
Subungual melanoma disproportionately affects nonwhites. While the total number of cases of subungual melanoma accounts for only 0.3% to 3% of all new cases of malignant melanoma, one study found subungual melanoma accounted for up to 23% of all malignant melanomas in Japanese persons, 17% of malignant melanomas in Hong Kong Chinese persons, and 25 % of malignant melanomas in African Americans.1
The thumb and big toe are the most frequently involved regions, perhaps due to the larger proportion of nail matrix on these digits, with 55% of lesions arising on the hands, and more than half of those involving the thumb.2
Cause is unclear
The cause of subungual melanoma is thought to be different from that of cutaneous melanoma, but it remains unclear. Because the nail filters out UVB light and subungual melanoma most frequently arises from a portion of the nail matrix that is not sun-exposed, UV exposure is not thought to play the same role in pathogenesis of subungual melanoma as it clearly does in cutaneous melanoma.1
Since the time of Hutchinson’s original report there have been numerous case reports and several series that describe antecedent trauma in subungual melanoma; however, there has never been conclusive evidence that trauma is a causative factor.3,4
Clinical presentation
Nail pigmentation is the first clinical sign of subungual melanoma in more than 75% of cases, but few patients present at this stage. Instead most patients delay presentation until changes in the nail contour are evident, secondary infection supervenes, or ulceration of the nail bed with granuloma formation manifests.1
Levit et al5 surveyed the world literature on “subungual melanoma” in 2000 and, based on their findings, described the ABCDEF mnemonic (TABLE 1) to describe the salient features. When considering a nail bed lesion, the presence of any one of these features should raise the clinician’s index of suspicion for subungual melanoma, while the presence of multiple features should raise a significantly higher concern.
TABLE 1
Salient features of subungual melanoma
| A=Age (5th to 7th decades), African-American, Asian, American Indian |
| B=Brown/Black pigment, Breadth (>3mm), Border variegation |
| C=Change in nail band or lack of change in nail morphology despite, presumably, adequate treatment |
| D=Digits most commonly involved (thumb, hallux) |
| E=Extension of pigment onto the proximal/lateral nail fold (Hutchinson’s sign) |
| F=Family or personal history of melanoma |
Nail pigmentation
The 2 most important signs of subungual melanoma are melanonychia striata (longitudinal brown to black pigmented streaks in the nail) and Hutchinson’s sign, which is the spread of brown or black pigment from the nail bed, nail matrix, or nail plate onto the adjacent cuticle or onto the proximal or the lateral nail fold.
Many patients with subungual melanoma have a history of a thin pigmented streak that had remained unchanged for years and then suddenly began to enlarge—eventually involving the entire nail bed with subsequent penetration to the eponychium or paronychium, ulceration, or granuloma formation.
Melanonychia striata. The differential diagnosis of melanonychia striata is quite long, and most of these streaks are benign (TABLE 2). Dark brown or black lines in the nails are common in Asians, African Americans, and in dark-skinned individuals, and may simply represent ethnic variation in pigment. Multiple streaks and streaks that do not extend distally from the proximal nail fold are nearly always benign.
Single streaks greater than 6 mm wide, those appearing in the sixth and seventh decade of life, streaks with a variegated color, or those that exhibit a broader proximal base or undergo any morphological change (indicating an active process) are suspicious for subungual melanoma.
Hutchinson’s sign. Hutchinson’s sign is pathognomonic for subungual melanoma only when accompanied by ulceration of the nail bed or obliteration of the nail plate by granuloma. When present, a tissue diagnosis must always be sought.
TABLE 2
Common differential diagnoses of multiple pigmented nail streaks or periungual pigmentation
| DRUGS | ||
| Antimalarials | Hydroxyurea | Sulfonamide |
| Bleomycin | Ketoconazole | Tetracycline |
| Cyclophosphamide | Methotrexate | Zidovudine |
| Doxorubicin | Minocycline | |
| 5-FU | Phenytoin | |
| SYSTEMIC CONDITIONS | ||
| Addison’s disease | Peutz-Jeghers syndrome | |
| Hemosiderosis | Vitamin B12 deficiency | |
| Porphyria | Pregnancy | |
| Radiation therapy | Laugler-Hunziker syndrome | |
| Subungual melanoma | ||
| MICROBIAL INFECTIONS | ||
| AIDS | Proteus mirabilis | |
| Blastomycetes | Secondary syphilis | |
| Candida | Trichophyton | |
Amelanotic subungual melanoma
Fewer than 7% of cutaneous melanomas lack pigment. In contrast, 20% to 33% of subungual melanomas are amelanotic. This makes the diagnosis of amelanotic subungual melanoma at best difficult, and often times impossible without a biopsy.1
Management of subungual melanoma
Nail biopsy
Untreated melanoma is potentially fatal, while nail biopsy is technically difficult, potentially disfiguring, and can be complicated by scarring or pterygium formation. The physician must thus carefully determine which lesions require biopsy. Except in the special case when the clinician deems the probability of subungual hematoma to be very high (discussed below), it may be best to refer these patients to a specialist when available.
As a general rule any nail lesion, whether pigmented or not, that does not heal with 6 to 12 weeks of conservative treatment should be biopsied. Lesions causing nail dystrophy, ulcerating lesions, and those presenting with Hutchinson’s sign should all be biopsied.
Caucasians. Managed conservatively, all single pigmented nail streaks in adult Caucasians unresolved within 6 to 12 weeks need to be biopsied. The clinician should thoroughly investigate non-melanotic causes of single pigmented streaks with a careful history and a thorough physical examination. Seek out clues, especially from the patient’s drug and treatment history. Unchanged, lightly pigmented streaks already present for many years on adult Caucasians at the time of initial presentation warrant close follow-up, and those with such lesions that remain unchanged after 6 to 12 months of follow-up may be safely discharged with instructions to seek medical evaluation if the lesion shows any subsequent morphological change.
Asians and dark-skinned persons. In contrast to pigmented nail streaks in Caucasians, dark-complected persons commonly exhibit melanonychia striata, and these streaks should not be routinely biopsied unless there has been some morphological change. Multiple streaks, streaks present on multiple nails, and those streaks that do not originate from the proximal nail fold are also unlikely to be melanoma in adults or children of any ethnicity, and these warrant clinical observation and a diligent search for another cause.1 The single exception to this is metastatic melanoma to the nail bed, which may present as multiple pigmented lesions on the same or different nails.
Children. Subungual melanoma is quite rare in children of any race, though the incidence is not zero. As such, any new pigmented streak warrants observation by the physician, while pre-existing streaks unchanged for several years may by monitored by the parent and child. Any morphological change in a pre-existing streak or change in a new streak requires biopsy in all adults and children.
Conducting a nail biopsy
When the clinician believes a diagnosis of subungual hematoma is likely, a simple office procedure and test will often confirm the diagnosis.6 Any family physician can perform this procedure and test, saving the patient both time and the expense of obtaining a consultation, and sparing both patient and physician the anxiety of waiting days or even weeks to rule out a potentially life-threatening diagnosis.
Procedure. First, soak the hand (or foot) in warm water for 10 to 15 minutes to soften the nail plate. Next, drive a 3- or 4-mm punch biopsy through the nail plate, being careful not to injure the underlying nail bed, as any bleeding will invalidate the subsequent test. To minimize the risk of traumatizing the nail bed, avoid local anesthesia. Once you remove the specimen, grasp it with a pair of forceps and inspect it closely.
If the pigment does not adhere to the undersurface of the nail plate, but instead the biopsy specimen is clean, with the pigmented part being the nail bed itself, the diagnosis of subungual hematoma is virtually excluded, mandating a biopsy of the nail bed. If the underside of the nail specimen is pigmented, use a #15 blade to carefully scrape the pigment onto the sample surface of a standard hemoccult card. Add a drop of water to the pigment scrapings; then the card is developed in the usual fashion. A positive test indicates the presence of old blood and confirms the diagnosis of subungual hematoma. You should make sure to follow-up periodically to assure that the pigment recedes as the nail grows, as well as to confirm the absence of any further lesion is still required—keeping in mind that nail trauma precedes a large percentage of subungual hematoma.
Staging
Once the diagnosis of subungual melanoma is confirmed the depth of invasion must be determined. Clark’s level of invasion may be poorly defined in subungual melanoma, due to unique histopathological characteristics of the nail bed. Breslow’s absolute depth correlates less closely with prognosis of subungual melanoma than with cutaneous melanoma, but tumor thickness has been found to be a good prognostic indicator of subungual melanoma.1
Treatment: Excision and amputation
Amputation or wide excision are the only accepted treatments for subungual melanoma. However, there is no clear consensus regarding the most appropriate type of excision, biopsy, or level of amputation.5 Limb perfusion chemotherapy is thought to increase survival in selected cases.
Outcomes
Subungual melanoma typically has a worse outcome than cutaneous melanoma, but the causes remain unclear. Anatomic location has often been cited as a prognostic marker, and many studies attribute the unusual location of subungual melanomas to their poorer prognosis.7
Some recent studies have found that worse outcomes are tied to the fact that subungual melanoma often presents at a much more advanced stage than cutaneous melanoma, while other studies have shown that even when controlled for diseased and stage, subungual melanoma typically has a worse prognosis than cutaneous melanoma.1 Racial differences in outcomes may also exist, as one study found that African Americans with subungual melanoma have a 3.5 times greater rate of death than Caucasians (95% confidence interval, 1.4–8.6). When rates were controlled for Clark and Wihm’s level, they continued to have a 2.6 times greater rate of death.2 This indicates that the greater mortality rate is not due entirely to either later stage of disease at presentation or to later diagnosis.
CORRESPONDENCE
Adam Leight, MD. E-mail: [email protected]
1. Thai K, Young R, Sinclair R. Nail apparatus melanoma. Australian J Dermatol 2001;42:71-83.
2. O’Leary J, Berend K, Johnson J, Levin S, Seigler H. Subungual melanoma: A review of 93 cases with identification of prognostic variables. Clinical Orthopedica and Related Research 2000;378:206-212.
3. Blessing K, Kernohan N, Park K. Subungual malignant melanoma: clinicopathological features of 100 cases. Histopathology 1991;19:425-429.
4. O’Toole E, Stephens R, Young M, Tanner A, Barnes L. Subungual melanoma: a relation to direct injury? J Am Acad Dermatol 1995;33:525-528.
5. Levit E, Kagen M, Scher R, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol 2000;42:269-274.
6. Elpern D. Subungual melanoma vs hematoma. Virtual Grand Rounds in Dermatology. Presented in Williamstown, MA, 16 June 2001. Available at: www.vgrd.org.
7. Harrington P, Kelly A, Trail I, Freemont A. Amelanotic subungual melanoma mimicking pyogenic granuloma of the hand. J R Coll Surg Edin 2002;47:638-640.
A man went to his primary care physician 3 months after slamming his right thumb in a car door. The nail had turned black and sloughed off several weeks later, leaving a red, draining wound on the tip of his thumb. The wound drained continuously for the next 2 months and showed little progress in healing.
His physician started him on antibiotics, but the wound still showed no progress in healing over the next 6 weeks. Cultures were obtained that grew out Staphylococcus and Streptococcus spp. Another course of antibiotics was given, but the patient’s condition failed to improve.
At this point the patient was referred to a surgeon. He missed several appointments before finally presenting to the surgery clinic nearly 6 months after his original office visit. He was diagnosed clinically as having a giant pyogenic granuloma and was given antibiotics as well as silver nitrate sticks to cauterize the wound daily. After missing several more follow-up appointments, the patient returned with a spongy, weeping soft-tissue wound over the dorsum of his right thumb [that] doubled in size over the past 3 months (FIGURE). Radiographs obtained at that time were normal, but a bone scan revealed late uptake, cause for concern that this was osteomyelitis.
FIGURE
Ulcerated tip of the thumb
What is the differential diagnosis, and what tests are necessary?
Diagnosis: Subungual melanoma
Due to the aggressive nature of the wound and the large soft-tissue defect, a plastic surgeon was consulted. Biopsy showed malignant melanoma, and the thumb was amputated at the base of the proximal phalanx. Pathology revealed a 3×3.5 cm ulceration at the distal portion of the specimen with malignant melanoma involving the skin, subcutaneous tissue, and bone marrow. Two of 3 axillary lymph nodes were also positive for metastatic malignant melanoma.
History and epidemiology
While public awareness of cutaneous melanoma has been increasing, subungual melanoma remains obscure. First described in 1834 by Alexis Boyer, surgeon to Napoleon, it was later characterized in 1886 by Sir Jonathan Hutchinson, who reported 6 cases of “melanotic whitlow.”1 Hutchinson reported that the lesion was usually first attributed to an injury, and because of this the diagnosis was nearly always missed in the early stages.
Today, subungual melanoma is often neglected by patients and frequently misdiagnosed by physicians. The estimated mean delay in diagnosis ranges from 3 to 24 months—nearly double the diagnostic delay observed with cutaneous melanoma.1,2
One study found 52% of subungual melanomas were mistaken for benign or traumatic lesions of the nail bed such as pyogenic granuloma, paronychia, onychomycosis, chronic infection, subungual hematoma, or pigmented nevus. This mistake is not surprising as these lesions are all in the differential diagnosis of a single pigmented nail streak, and all are far more common than subungual melanoma.
Another study found that two thirds of patients underwent some inappropriate surgical procedure before the correct diagnosis was considered.1 Because of its poor prognosis, often related to delay in diagnosis, maintain a high index of suspicion for subungual melanoma in the proper setting and a sound understanding of which lesions need to be biopsied.
Subungual melanoma disproportionately affects nonwhites. While the total number of cases of subungual melanoma accounts for only 0.3% to 3% of all new cases of malignant melanoma, one study found subungual melanoma accounted for up to 23% of all malignant melanomas in Japanese persons, 17% of malignant melanomas in Hong Kong Chinese persons, and 25 % of malignant melanomas in African Americans.1
The thumb and big toe are the most frequently involved regions, perhaps due to the larger proportion of nail matrix on these digits, with 55% of lesions arising on the hands, and more than half of those involving the thumb.2
Cause is unclear
The cause of subungual melanoma is thought to be different from that of cutaneous melanoma, but it remains unclear. Because the nail filters out UVB light and subungual melanoma most frequently arises from a portion of the nail matrix that is not sun-exposed, UV exposure is not thought to play the same role in pathogenesis of subungual melanoma as it clearly does in cutaneous melanoma.1
Since the time of Hutchinson’s original report there have been numerous case reports and several series that describe antecedent trauma in subungual melanoma; however, there has never been conclusive evidence that trauma is a causative factor.3,4
Clinical presentation
Nail pigmentation is the first clinical sign of subungual melanoma in more than 75% of cases, but few patients present at this stage. Instead most patients delay presentation until changes in the nail contour are evident, secondary infection supervenes, or ulceration of the nail bed with granuloma formation manifests.1
Levit et al5 surveyed the world literature on “subungual melanoma” in 2000 and, based on their findings, described the ABCDEF mnemonic (TABLE 1) to describe the salient features. When considering a nail bed lesion, the presence of any one of these features should raise the clinician’s index of suspicion for subungual melanoma, while the presence of multiple features should raise a significantly higher concern.
TABLE 1
Salient features of subungual melanoma
| A=Age (5th to 7th decades), African-American, Asian, American Indian |
| B=Brown/Black pigment, Breadth (>3mm), Border variegation |
| C=Change in nail band or lack of change in nail morphology despite, presumably, adequate treatment |
| D=Digits most commonly involved (thumb, hallux) |
| E=Extension of pigment onto the proximal/lateral nail fold (Hutchinson’s sign) |
| F=Family or personal history of melanoma |
Nail pigmentation
The 2 most important signs of subungual melanoma are melanonychia striata (longitudinal brown to black pigmented streaks in the nail) and Hutchinson’s sign, which is the spread of brown or black pigment from the nail bed, nail matrix, or nail plate onto the adjacent cuticle or onto the proximal or the lateral nail fold.
Many patients with subungual melanoma have a history of a thin pigmented streak that had remained unchanged for years and then suddenly began to enlarge—eventually involving the entire nail bed with subsequent penetration to the eponychium or paronychium, ulceration, or granuloma formation.
Melanonychia striata. The differential diagnosis of melanonychia striata is quite long, and most of these streaks are benign (TABLE 2). Dark brown or black lines in the nails are common in Asians, African Americans, and in dark-skinned individuals, and may simply represent ethnic variation in pigment. Multiple streaks and streaks that do not extend distally from the proximal nail fold are nearly always benign.
Single streaks greater than 6 mm wide, those appearing in the sixth and seventh decade of life, streaks with a variegated color, or those that exhibit a broader proximal base or undergo any morphological change (indicating an active process) are suspicious for subungual melanoma.
Hutchinson’s sign. Hutchinson’s sign is pathognomonic for subungual melanoma only when accompanied by ulceration of the nail bed or obliteration of the nail plate by granuloma. When present, a tissue diagnosis must always be sought.
TABLE 2
Common differential diagnoses of multiple pigmented nail streaks or periungual pigmentation
| DRUGS | ||
| Antimalarials | Hydroxyurea | Sulfonamide |
| Bleomycin | Ketoconazole | Tetracycline |
| Cyclophosphamide | Methotrexate | Zidovudine |
| Doxorubicin | Minocycline | |
| 5-FU | Phenytoin | |
| SYSTEMIC CONDITIONS | ||
| Addison’s disease | Peutz-Jeghers syndrome | |
| Hemosiderosis | Vitamin B12 deficiency | |
| Porphyria | Pregnancy | |
| Radiation therapy | Laugler-Hunziker syndrome | |
| Subungual melanoma | ||
| MICROBIAL INFECTIONS | ||
| AIDS | Proteus mirabilis | |
| Blastomycetes | Secondary syphilis | |
| Candida | Trichophyton | |
Amelanotic subungual melanoma
Fewer than 7% of cutaneous melanomas lack pigment. In contrast, 20% to 33% of subungual melanomas are amelanotic. This makes the diagnosis of amelanotic subungual melanoma at best difficult, and often times impossible without a biopsy.1
Management of subungual melanoma
Nail biopsy
Untreated melanoma is potentially fatal, while nail biopsy is technically difficult, potentially disfiguring, and can be complicated by scarring or pterygium formation. The physician must thus carefully determine which lesions require biopsy. Except in the special case when the clinician deems the probability of subungual hematoma to be very high (discussed below), it may be best to refer these patients to a specialist when available.
As a general rule any nail lesion, whether pigmented or not, that does not heal with 6 to 12 weeks of conservative treatment should be biopsied. Lesions causing nail dystrophy, ulcerating lesions, and those presenting with Hutchinson’s sign should all be biopsied.
Caucasians. Managed conservatively, all single pigmented nail streaks in adult Caucasians unresolved within 6 to 12 weeks need to be biopsied. The clinician should thoroughly investigate non-melanotic causes of single pigmented streaks with a careful history and a thorough physical examination. Seek out clues, especially from the patient’s drug and treatment history. Unchanged, lightly pigmented streaks already present for many years on adult Caucasians at the time of initial presentation warrant close follow-up, and those with such lesions that remain unchanged after 6 to 12 months of follow-up may be safely discharged with instructions to seek medical evaluation if the lesion shows any subsequent morphological change.
Asians and dark-skinned persons. In contrast to pigmented nail streaks in Caucasians, dark-complected persons commonly exhibit melanonychia striata, and these streaks should not be routinely biopsied unless there has been some morphological change. Multiple streaks, streaks present on multiple nails, and those streaks that do not originate from the proximal nail fold are also unlikely to be melanoma in adults or children of any ethnicity, and these warrant clinical observation and a diligent search for another cause.1 The single exception to this is metastatic melanoma to the nail bed, which may present as multiple pigmented lesions on the same or different nails.
Children. Subungual melanoma is quite rare in children of any race, though the incidence is not zero. As such, any new pigmented streak warrants observation by the physician, while pre-existing streaks unchanged for several years may by monitored by the parent and child. Any morphological change in a pre-existing streak or change in a new streak requires biopsy in all adults and children.
Conducting a nail biopsy
When the clinician believes a diagnosis of subungual hematoma is likely, a simple office procedure and test will often confirm the diagnosis.6 Any family physician can perform this procedure and test, saving the patient both time and the expense of obtaining a consultation, and sparing both patient and physician the anxiety of waiting days or even weeks to rule out a potentially life-threatening diagnosis.
Procedure. First, soak the hand (or foot) in warm water for 10 to 15 minutes to soften the nail plate. Next, drive a 3- or 4-mm punch biopsy through the nail plate, being careful not to injure the underlying nail bed, as any bleeding will invalidate the subsequent test. To minimize the risk of traumatizing the nail bed, avoid local anesthesia. Once you remove the specimen, grasp it with a pair of forceps and inspect it closely.
If the pigment does not adhere to the undersurface of the nail plate, but instead the biopsy specimen is clean, with the pigmented part being the nail bed itself, the diagnosis of subungual hematoma is virtually excluded, mandating a biopsy of the nail bed. If the underside of the nail specimen is pigmented, use a #15 blade to carefully scrape the pigment onto the sample surface of a standard hemoccult card. Add a drop of water to the pigment scrapings; then the card is developed in the usual fashion. A positive test indicates the presence of old blood and confirms the diagnosis of subungual hematoma. You should make sure to follow-up periodically to assure that the pigment recedes as the nail grows, as well as to confirm the absence of any further lesion is still required—keeping in mind that nail trauma precedes a large percentage of subungual hematoma.
Staging
Once the diagnosis of subungual melanoma is confirmed the depth of invasion must be determined. Clark’s level of invasion may be poorly defined in subungual melanoma, due to unique histopathological characteristics of the nail bed. Breslow’s absolute depth correlates less closely with prognosis of subungual melanoma than with cutaneous melanoma, but tumor thickness has been found to be a good prognostic indicator of subungual melanoma.1
Treatment: Excision and amputation
Amputation or wide excision are the only accepted treatments for subungual melanoma. However, there is no clear consensus regarding the most appropriate type of excision, biopsy, or level of amputation.5 Limb perfusion chemotherapy is thought to increase survival in selected cases.
Outcomes
Subungual melanoma typically has a worse outcome than cutaneous melanoma, but the causes remain unclear. Anatomic location has often been cited as a prognostic marker, and many studies attribute the unusual location of subungual melanomas to their poorer prognosis.7
Some recent studies have found that worse outcomes are tied to the fact that subungual melanoma often presents at a much more advanced stage than cutaneous melanoma, while other studies have shown that even when controlled for diseased and stage, subungual melanoma typically has a worse prognosis than cutaneous melanoma.1 Racial differences in outcomes may also exist, as one study found that African Americans with subungual melanoma have a 3.5 times greater rate of death than Caucasians (95% confidence interval, 1.4–8.6). When rates were controlled for Clark and Wihm’s level, they continued to have a 2.6 times greater rate of death.2 This indicates that the greater mortality rate is not due entirely to either later stage of disease at presentation or to later diagnosis.
CORRESPONDENCE
Adam Leight, MD. E-mail: [email protected]
A man went to his primary care physician 3 months after slamming his right thumb in a car door. The nail had turned black and sloughed off several weeks later, leaving a red, draining wound on the tip of his thumb. The wound drained continuously for the next 2 months and showed little progress in healing.
His physician started him on antibiotics, but the wound still showed no progress in healing over the next 6 weeks. Cultures were obtained that grew out Staphylococcus and Streptococcus spp. Another course of antibiotics was given, but the patient’s condition failed to improve.
At this point the patient was referred to a surgeon. He missed several appointments before finally presenting to the surgery clinic nearly 6 months after his original office visit. He was diagnosed clinically as having a giant pyogenic granuloma and was given antibiotics as well as silver nitrate sticks to cauterize the wound daily. After missing several more follow-up appointments, the patient returned with a spongy, weeping soft-tissue wound over the dorsum of his right thumb [that] doubled in size over the past 3 months (FIGURE). Radiographs obtained at that time were normal, but a bone scan revealed late uptake, cause for concern that this was osteomyelitis.
FIGURE
Ulcerated tip of the thumb
What is the differential diagnosis, and what tests are necessary?
Diagnosis: Subungual melanoma
Due to the aggressive nature of the wound and the large soft-tissue defect, a plastic surgeon was consulted. Biopsy showed malignant melanoma, and the thumb was amputated at the base of the proximal phalanx. Pathology revealed a 3×3.5 cm ulceration at the distal portion of the specimen with malignant melanoma involving the skin, subcutaneous tissue, and bone marrow. Two of 3 axillary lymph nodes were also positive for metastatic malignant melanoma.
History and epidemiology
While public awareness of cutaneous melanoma has been increasing, subungual melanoma remains obscure. First described in 1834 by Alexis Boyer, surgeon to Napoleon, it was later characterized in 1886 by Sir Jonathan Hutchinson, who reported 6 cases of “melanotic whitlow.”1 Hutchinson reported that the lesion was usually first attributed to an injury, and because of this the diagnosis was nearly always missed in the early stages.
Today, subungual melanoma is often neglected by patients and frequently misdiagnosed by physicians. The estimated mean delay in diagnosis ranges from 3 to 24 months—nearly double the diagnostic delay observed with cutaneous melanoma.1,2
One study found 52% of subungual melanomas were mistaken for benign or traumatic lesions of the nail bed such as pyogenic granuloma, paronychia, onychomycosis, chronic infection, subungual hematoma, or pigmented nevus. This mistake is not surprising as these lesions are all in the differential diagnosis of a single pigmented nail streak, and all are far more common than subungual melanoma.
Another study found that two thirds of patients underwent some inappropriate surgical procedure before the correct diagnosis was considered.1 Because of its poor prognosis, often related to delay in diagnosis, maintain a high index of suspicion for subungual melanoma in the proper setting and a sound understanding of which lesions need to be biopsied.
Subungual melanoma disproportionately affects nonwhites. While the total number of cases of subungual melanoma accounts for only 0.3% to 3% of all new cases of malignant melanoma, one study found subungual melanoma accounted for up to 23% of all malignant melanomas in Japanese persons, 17% of malignant melanomas in Hong Kong Chinese persons, and 25 % of malignant melanomas in African Americans.1
The thumb and big toe are the most frequently involved regions, perhaps due to the larger proportion of nail matrix on these digits, with 55% of lesions arising on the hands, and more than half of those involving the thumb.2
Cause is unclear
The cause of subungual melanoma is thought to be different from that of cutaneous melanoma, but it remains unclear. Because the nail filters out UVB light and subungual melanoma most frequently arises from a portion of the nail matrix that is not sun-exposed, UV exposure is not thought to play the same role in pathogenesis of subungual melanoma as it clearly does in cutaneous melanoma.1
Since the time of Hutchinson’s original report there have been numerous case reports and several series that describe antecedent trauma in subungual melanoma; however, there has never been conclusive evidence that trauma is a causative factor.3,4
Clinical presentation
Nail pigmentation is the first clinical sign of subungual melanoma in more than 75% of cases, but few patients present at this stage. Instead most patients delay presentation until changes in the nail contour are evident, secondary infection supervenes, or ulceration of the nail bed with granuloma formation manifests.1
Levit et al5 surveyed the world literature on “subungual melanoma” in 2000 and, based on their findings, described the ABCDEF mnemonic (TABLE 1) to describe the salient features. When considering a nail bed lesion, the presence of any one of these features should raise the clinician’s index of suspicion for subungual melanoma, while the presence of multiple features should raise a significantly higher concern.
TABLE 1
Salient features of subungual melanoma
| A=Age (5th to 7th decades), African-American, Asian, American Indian |
| B=Brown/Black pigment, Breadth (>3mm), Border variegation |
| C=Change in nail band or lack of change in nail morphology despite, presumably, adequate treatment |
| D=Digits most commonly involved (thumb, hallux) |
| E=Extension of pigment onto the proximal/lateral nail fold (Hutchinson’s sign) |
| F=Family or personal history of melanoma |
Nail pigmentation
The 2 most important signs of subungual melanoma are melanonychia striata (longitudinal brown to black pigmented streaks in the nail) and Hutchinson’s sign, which is the spread of brown or black pigment from the nail bed, nail matrix, or nail plate onto the adjacent cuticle or onto the proximal or the lateral nail fold.
Many patients with subungual melanoma have a history of a thin pigmented streak that had remained unchanged for years and then suddenly began to enlarge—eventually involving the entire nail bed with subsequent penetration to the eponychium or paronychium, ulceration, or granuloma formation.
Melanonychia striata. The differential diagnosis of melanonychia striata is quite long, and most of these streaks are benign (TABLE 2). Dark brown or black lines in the nails are common in Asians, African Americans, and in dark-skinned individuals, and may simply represent ethnic variation in pigment. Multiple streaks and streaks that do not extend distally from the proximal nail fold are nearly always benign.
Single streaks greater than 6 mm wide, those appearing in the sixth and seventh decade of life, streaks with a variegated color, or those that exhibit a broader proximal base or undergo any morphological change (indicating an active process) are suspicious for subungual melanoma.
Hutchinson’s sign. Hutchinson’s sign is pathognomonic for subungual melanoma only when accompanied by ulceration of the nail bed or obliteration of the nail plate by granuloma. When present, a tissue diagnosis must always be sought.
TABLE 2
Common differential diagnoses of multiple pigmented nail streaks or periungual pigmentation
| DRUGS | ||
| Antimalarials | Hydroxyurea | Sulfonamide |
| Bleomycin | Ketoconazole | Tetracycline |
| Cyclophosphamide | Methotrexate | Zidovudine |
| Doxorubicin | Minocycline | |
| 5-FU | Phenytoin | |
| SYSTEMIC CONDITIONS | ||
| Addison’s disease | Peutz-Jeghers syndrome | |
| Hemosiderosis | Vitamin B12 deficiency | |
| Porphyria | Pregnancy | |
| Radiation therapy | Laugler-Hunziker syndrome | |
| Subungual melanoma | ||
| MICROBIAL INFECTIONS | ||
| AIDS | Proteus mirabilis | |
| Blastomycetes | Secondary syphilis | |
| Candida | Trichophyton | |
Amelanotic subungual melanoma
Fewer than 7% of cutaneous melanomas lack pigment. In contrast, 20% to 33% of subungual melanomas are amelanotic. This makes the diagnosis of amelanotic subungual melanoma at best difficult, and often times impossible without a biopsy.1
Management of subungual melanoma
Nail biopsy
Untreated melanoma is potentially fatal, while nail biopsy is technically difficult, potentially disfiguring, and can be complicated by scarring or pterygium formation. The physician must thus carefully determine which lesions require biopsy. Except in the special case when the clinician deems the probability of subungual hematoma to be very high (discussed below), it may be best to refer these patients to a specialist when available.
As a general rule any nail lesion, whether pigmented or not, that does not heal with 6 to 12 weeks of conservative treatment should be biopsied. Lesions causing nail dystrophy, ulcerating lesions, and those presenting with Hutchinson’s sign should all be biopsied.
Caucasians. Managed conservatively, all single pigmented nail streaks in adult Caucasians unresolved within 6 to 12 weeks need to be biopsied. The clinician should thoroughly investigate non-melanotic causes of single pigmented streaks with a careful history and a thorough physical examination. Seek out clues, especially from the patient’s drug and treatment history. Unchanged, lightly pigmented streaks already present for many years on adult Caucasians at the time of initial presentation warrant close follow-up, and those with such lesions that remain unchanged after 6 to 12 months of follow-up may be safely discharged with instructions to seek medical evaluation if the lesion shows any subsequent morphological change.
Asians and dark-skinned persons. In contrast to pigmented nail streaks in Caucasians, dark-complected persons commonly exhibit melanonychia striata, and these streaks should not be routinely biopsied unless there has been some morphological change. Multiple streaks, streaks present on multiple nails, and those streaks that do not originate from the proximal nail fold are also unlikely to be melanoma in adults or children of any ethnicity, and these warrant clinical observation and a diligent search for another cause.1 The single exception to this is metastatic melanoma to the nail bed, which may present as multiple pigmented lesions on the same or different nails.
Children. Subungual melanoma is quite rare in children of any race, though the incidence is not zero. As such, any new pigmented streak warrants observation by the physician, while pre-existing streaks unchanged for several years may by monitored by the parent and child. Any morphological change in a pre-existing streak or change in a new streak requires biopsy in all adults and children.
Conducting a nail biopsy
When the clinician believes a diagnosis of subungual hematoma is likely, a simple office procedure and test will often confirm the diagnosis.6 Any family physician can perform this procedure and test, saving the patient both time and the expense of obtaining a consultation, and sparing both patient and physician the anxiety of waiting days or even weeks to rule out a potentially life-threatening diagnosis.
Procedure. First, soak the hand (or foot) in warm water for 10 to 15 minutes to soften the nail plate. Next, drive a 3- or 4-mm punch biopsy through the nail plate, being careful not to injure the underlying nail bed, as any bleeding will invalidate the subsequent test. To minimize the risk of traumatizing the nail bed, avoid local anesthesia. Once you remove the specimen, grasp it with a pair of forceps and inspect it closely.
If the pigment does not adhere to the undersurface of the nail plate, but instead the biopsy specimen is clean, with the pigmented part being the nail bed itself, the diagnosis of subungual hematoma is virtually excluded, mandating a biopsy of the nail bed. If the underside of the nail specimen is pigmented, use a #15 blade to carefully scrape the pigment onto the sample surface of a standard hemoccult card. Add a drop of water to the pigment scrapings; then the card is developed in the usual fashion. A positive test indicates the presence of old blood and confirms the diagnosis of subungual hematoma. You should make sure to follow-up periodically to assure that the pigment recedes as the nail grows, as well as to confirm the absence of any further lesion is still required—keeping in mind that nail trauma precedes a large percentage of subungual hematoma.
Staging
Once the diagnosis of subungual melanoma is confirmed the depth of invasion must be determined. Clark’s level of invasion may be poorly defined in subungual melanoma, due to unique histopathological characteristics of the nail bed. Breslow’s absolute depth correlates less closely with prognosis of subungual melanoma than with cutaneous melanoma, but tumor thickness has been found to be a good prognostic indicator of subungual melanoma.1
Treatment: Excision and amputation
Amputation or wide excision are the only accepted treatments for subungual melanoma. However, there is no clear consensus regarding the most appropriate type of excision, biopsy, or level of amputation.5 Limb perfusion chemotherapy is thought to increase survival in selected cases.
Outcomes
Subungual melanoma typically has a worse outcome than cutaneous melanoma, but the causes remain unclear. Anatomic location has often been cited as a prognostic marker, and many studies attribute the unusual location of subungual melanomas to their poorer prognosis.7
Some recent studies have found that worse outcomes are tied to the fact that subungual melanoma often presents at a much more advanced stage than cutaneous melanoma, while other studies have shown that even when controlled for diseased and stage, subungual melanoma typically has a worse prognosis than cutaneous melanoma.1 Racial differences in outcomes may also exist, as one study found that African Americans with subungual melanoma have a 3.5 times greater rate of death than Caucasians (95% confidence interval, 1.4–8.6). When rates were controlled for Clark and Wihm’s level, they continued to have a 2.6 times greater rate of death.2 This indicates that the greater mortality rate is not due entirely to either later stage of disease at presentation or to later diagnosis.
CORRESPONDENCE
Adam Leight, MD. E-mail: [email protected]
1. Thai K, Young R, Sinclair R. Nail apparatus melanoma. Australian J Dermatol 2001;42:71-83.
2. O’Leary J, Berend K, Johnson J, Levin S, Seigler H. Subungual melanoma: A review of 93 cases with identification of prognostic variables. Clinical Orthopedica and Related Research 2000;378:206-212.
3. Blessing K, Kernohan N, Park K. Subungual malignant melanoma: clinicopathological features of 100 cases. Histopathology 1991;19:425-429.
4. O’Toole E, Stephens R, Young M, Tanner A, Barnes L. Subungual melanoma: a relation to direct injury? J Am Acad Dermatol 1995;33:525-528.
5. Levit E, Kagen M, Scher R, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol 2000;42:269-274.
6. Elpern D. Subungual melanoma vs hematoma. Virtual Grand Rounds in Dermatology. Presented in Williamstown, MA, 16 June 2001. Available at: www.vgrd.org.
7. Harrington P, Kelly A, Trail I, Freemont A. Amelanotic subungual melanoma mimicking pyogenic granuloma of the hand. J R Coll Surg Edin 2002;47:638-640.
1. Thai K, Young R, Sinclair R. Nail apparatus melanoma. Australian J Dermatol 2001;42:71-83.
2. O’Leary J, Berend K, Johnson J, Levin S, Seigler H. Subungual melanoma: A review of 93 cases with identification of prognostic variables. Clinical Orthopedica and Related Research 2000;378:206-212.
3. Blessing K, Kernohan N, Park K. Subungual malignant melanoma: clinicopathological features of 100 cases. Histopathology 1991;19:425-429.
4. O’Toole E, Stephens R, Young M, Tanner A, Barnes L. Subungual melanoma: a relation to direct injury? J Am Acad Dermatol 1995;33:525-528.
5. Levit E, Kagen M, Scher R, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol 2000;42:269-274.
6. Elpern D. Subungual melanoma vs hematoma. Virtual Grand Rounds in Dermatology. Presented in Williamstown, MA, 16 June 2001. Available at: www.vgrd.org.
7. Harrington P, Kelly A, Trail I, Freemont A. Amelanotic subungual melanoma mimicking pyogenic granuloma of the hand. J R Coll Surg Edin 2002;47:638-640.
Annular rash on a newborn
A full-term, healthy female newborn was delivered via cesarean section because the labor did not adequately progress. The mother, age 33 years and of Asian ancestry, had a significant medical and obstetrical history: chronic hepatitis B carrier without cirrhosis, cutaneous lupus erythematosus (positive anti-Ro and anti-La antibodies), and a positive group B streptococcal recto-vaginal culture at 35 weeks’ gestation. The mother received 4 doses of intravenous ampicillin during labor.
The infant’s initial hospital course was complicated by a transient and otherwise asymptomatic bradycardia. An electrocardiogram (ECG) confirmed a heart rate of 96 with normal interval parameters, but there were changes suggestive of left ventricular hypertrophy. An echocardiogram was normal.
Follow-up office visits for common newborn feeding problems demonstrated consistent weight gain and normal vital signs, including heart rate and facial milia. However, by age 4 weeks an erythematous eruption extending from the frontal scalp and forehead to the cheek area had developed (FIGURES 1 AND 2).
FIGURE 1
Rash on a newborn’s face …
FIGURE 2
…and on the scalp
What is the differential diagnosis?
What tests should be done to make the diagnosis?
Diagnosis: Neonatal lupus
This infant has neonatal lupus erythematosus, a rare syndrome in which maternal autoantibodies are passively transferred to the baby and cause cutaneous lesions or isolated congenital heart block. The skin rash generally appears a few days to weeks after birth, typically after sun exposure, and shows well-demarcated erythematous, scaling patches that are often annular and predominately on the scalp, neck, or face. It is self-limited and generally resolves without scarring by 6 to 7 months of age.
Differential diagnosis
The differential diagnosis of neonatal lupus syndrome includes annular urticaria, tinea corporis, seborrheic dermatitis, erythema annulare centrifugum, familial annular erythema, erythema multiforme, systemic lupus erythematosus, pityrosporum infection, and photosensitive genodermatoses.1 These can be differentiated from neonatal lupus by several defining characteristics:
- Annular urticaria is transient and pruritic
- In tinea corporis, a KOH prep reveals numerous branching hyphae
- Seborrheic dermatitis is characterized by greasy scales over red, inflamed skin and distributed in facial and body folds
- Erythema annulare centrifugum usually affects the trunks and legs; the characteristic rings enlarge daily
- Familial annular erythema is generally found on the back and shoulders and accompanied by a family history of similar lesions in infancy
- Erythema multiforme is more often on extensor surfaces and is uncommon in infancy
- Systemic lupus erythematosus should be suspected if the infant has the clinical manifestations or positive serological tests while autoantibodies are absent in the mother
- Pityrosporum infection can be diagnosed by hyphae and spores (“spaghetti and meatballs”) on KOH preparation
- Photosensitive genodermatoses are a rare group of disorders that are characterized by chronicity without autoantibodies.
Causes and pathophysiology of neonatal lupus
Neonatal lupus is caused by the IgG autoantibodies anti-Ro/SSA and anti-La/SSB, which pass from the mother to the fetus via the placenta, although anti-U1-RNP antibodies can sometimes cause the cutaneous manifestations of neonatal lupus.2 The mother may or may not demonstrate features of connective tissue disease at the time of birth. Lupus has a higher incidence in Asian women than in the overall US population.
Possible symptoms in the neonate include a characteristic skin eruption or congenital heart block. Neonatal lupus accounts for 85% of cases of congenital complete heart block diagnosed in utero or in the neonatal period.3 The incidence of complete heart block in offspring of women with anti-Ro/SSA or anti-La/SSB antibodies is about 2%. The incidence of complete heart block in infants with cutaneous neonatal lupus is 15% to 30%. Incomplete heart block, which may progress to complete heart block, may also occur. A less common and self-limited sinus bradycardia has also been described in fetuses.4
Other manifestations of neonatal lupus include thrombocytopenia, aplastic anemia, hepatobiliary dysfunction, and central nervous system vasculopathy.
Diagnosis and treatment
Diagnosis is based on physical findings in an infant aged <6 months and detection of anti-Ro/SSA and anti-La/SSB antibodies in the child or mother. It should be suspected in children with congenital heart block as it is responsible for over 85% of cases.
All infants diagnosed with neonatal lupus erythematosus or born to women with anti-Ro/SSA or anti-La/SSB antibodies should have an ECG to detect heart block. If this is abnormal, referral to a pediatric cardiologist is warranted. All pregnant women with anti-Ro/SSA or anti-La/SSB antibodies should undergo regular fetal echocardiography to detect heart block. The initiation of dexamethasone or plasmapheresis as a preventative measure against congenital heart block in high-risk pregnant women is currently under consideration but is not yet justified.3
Treatment includes photoprotection (avoiding sunlight using clothing and other protective measures) and time, as nearly all cases resolve within 6 months without scarring. Mild topical steroids may be helpful. It is important to note that children with neonatal lupus may be at higher risk of developing autoimmune disorders or rheumatic disease later in life.2
Outcome
For this patient, the skin lesions were scraped with the side of a microscope slide and KOH with DMSO was used to dissolve the epithelial cells. The preparation was negative for fungal elements.This infant had anti-Ro, anti-La, and anti-RNP levels of 128.3, 150.3, and 128, respectively. Normal range is 0 to 19 for each autoantibody. Her mother’s autoantibody levels were similarly abnormal at 138.1, 158.8, and 169.1.
The patient was referred to a pediatric cardiologist for follow-up; results of a second echocardiogram and ECG were normal. The infant was seen again at 7 weeks; cutaneous lesions were present but somewhat improved, and she was otherwise doing well. Given the high probability of lupus occurring in future pregnancies, the mother received appropriate education.
CORRESPONDENCE
Keith A. Frey, MD, Department of Family Medicine, Mayo Clinic Arizona, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: [email protected]
1. Silverman ED. Neonatal lupus. In Cassidy JT, Pett RE (eds): Textbook of Pediatric Rheumatology. Philadelphia: WB Saunders; 2001:456.
2. Habif TP. Clinical Dermatology. Edinburgh: Mosby; 2004.
3. Buyon JP, Clancy RM. Neonatal lupus: Basic research and clinical perspective. Rheum Dis Clin North Am 2005;31:299-313.
4. Askanase AD, Friedman DM, Copel J, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La anti-bodies. Rheum Dis Clin North Am 2002;11:145.
A full-term, healthy female newborn was delivered via cesarean section because the labor did not adequately progress. The mother, age 33 years and of Asian ancestry, had a significant medical and obstetrical history: chronic hepatitis B carrier without cirrhosis, cutaneous lupus erythematosus (positive anti-Ro and anti-La antibodies), and a positive group B streptococcal recto-vaginal culture at 35 weeks’ gestation. The mother received 4 doses of intravenous ampicillin during labor.
The infant’s initial hospital course was complicated by a transient and otherwise asymptomatic bradycardia. An electrocardiogram (ECG) confirmed a heart rate of 96 with normal interval parameters, but there were changes suggestive of left ventricular hypertrophy. An echocardiogram was normal.
Follow-up office visits for common newborn feeding problems demonstrated consistent weight gain and normal vital signs, including heart rate and facial milia. However, by age 4 weeks an erythematous eruption extending from the frontal scalp and forehead to the cheek area had developed (FIGURES 1 AND 2).
FIGURE 1
Rash on a newborn’s face …
FIGURE 2
…and on the scalp
What is the differential diagnosis?
What tests should be done to make the diagnosis?
Diagnosis: Neonatal lupus
This infant has neonatal lupus erythematosus, a rare syndrome in which maternal autoantibodies are passively transferred to the baby and cause cutaneous lesions or isolated congenital heart block. The skin rash generally appears a few days to weeks after birth, typically after sun exposure, and shows well-demarcated erythematous, scaling patches that are often annular and predominately on the scalp, neck, or face. It is self-limited and generally resolves without scarring by 6 to 7 months of age.
Differential diagnosis
The differential diagnosis of neonatal lupus syndrome includes annular urticaria, tinea corporis, seborrheic dermatitis, erythema annulare centrifugum, familial annular erythema, erythema multiforme, systemic lupus erythematosus, pityrosporum infection, and photosensitive genodermatoses.1 These can be differentiated from neonatal lupus by several defining characteristics:
- Annular urticaria is transient and pruritic
- In tinea corporis, a KOH prep reveals numerous branching hyphae
- Seborrheic dermatitis is characterized by greasy scales over red, inflamed skin and distributed in facial and body folds
- Erythema annulare centrifugum usually affects the trunks and legs; the characteristic rings enlarge daily
- Familial annular erythema is generally found on the back and shoulders and accompanied by a family history of similar lesions in infancy
- Erythema multiforme is more often on extensor surfaces and is uncommon in infancy
- Systemic lupus erythematosus should be suspected if the infant has the clinical manifestations or positive serological tests while autoantibodies are absent in the mother
- Pityrosporum infection can be diagnosed by hyphae and spores (“spaghetti and meatballs”) on KOH preparation
- Photosensitive genodermatoses are a rare group of disorders that are characterized by chronicity without autoantibodies.
Causes and pathophysiology of neonatal lupus
Neonatal lupus is caused by the IgG autoantibodies anti-Ro/SSA and anti-La/SSB, which pass from the mother to the fetus via the placenta, although anti-U1-RNP antibodies can sometimes cause the cutaneous manifestations of neonatal lupus.2 The mother may or may not demonstrate features of connective tissue disease at the time of birth. Lupus has a higher incidence in Asian women than in the overall US population.
Possible symptoms in the neonate include a characteristic skin eruption or congenital heart block. Neonatal lupus accounts for 85% of cases of congenital complete heart block diagnosed in utero or in the neonatal period.3 The incidence of complete heart block in offspring of women with anti-Ro/SSA or anti-La/SSB antibodies is about 2%. The incidence of complete heart block in infants with cutaneous neonatal lupus is 15% to 30%. Incomplete heart block, which may progress to complete heart block, may also occur. A less common and self-limited sinus bradycardia has also been described in fetuses.4
Other manifestations of neonatal lupus include thrombocytopenia, aplastic anemia, hepatobiliary dysfunction, and central nervous system vasculopathy.
Diagnosis and treatment
Diagnosis is based on physical findings in an infant aged <6 months and detection of anti-Ro/SSA and anti-La/SSB antibodies in the child or mother. It should be suspected in children with congenital heart block as it is responsible for over 85% of cases.
All infants diagnosed with neonatal lupus erythematosus or born to women with anti-Ro/SSA or anti-La/SSB antibodies should have an ECG to detect heart block. If this is abnormal, referral to a pediatric cardiologist is warranted. All pregnant women with anti-Ro/SSA or anti-La/SSB antibodies should undergo regular fetal echocardiography to detect heart block. The initiation of dexamethasone or plasmapheresis as a preventative measure against congenital heart block in high-risk pregnant women is currently under consideration but is not yet justified.3
Treatment includes photoprotection (avoiding sunlight using clothing and other protective measures) and time, as nearly all cases resolve within 6 months without scarring. Mild topical steroids may be helpful. It is important to note that children with neonatal lupus may be at higher risk of developing autoimmune disorders or rheumatic disease later in life.2
Outcome
For this patient, the skin lesions were scraped with the side of a microscope slide and KOH with DMSO was used to dissolve the epithelial cells. The preparation was negative for fungal elements.This infant had anti-Ro, anti-La, and anti-RNP levels of 128.3, 150.3, and 128, respectively. Normal range is 0 to 19 for each autoantibody. Her mother’s autoantibody levels were similarly abnormal at 138.1, 158.8, and 169.1.
The patient was referred to a pediatric cardiologist for follow-up; results of a second echocardiogram and ECG were normal. The infant was seen again at 7 weeks; cutaneous lesions were present but somewhat improved, and she was otherwise doing well. Given the high probability of lupus occurring in future pregnancies, the mother received appropriate education.
CORRESPONDENCE
Keith A. Frey, MD, Department of Family Medicine, Mayo Clinic Arizona, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: [email protected]
A full-term, healthy female newborn was delivered via cesarean section because the labor did not adequately progress. The mother, age 33 years and of Asian ancestry, had a significant medical and obstetrical history: chronic hepatitis B carrier without cirrhosis, cutaneous lupus erythematosus (positive anti-Ro and anti-La antibodies), and a positive group B streptococcal recto-vaginal culture at 35 weeks’ gestation. The mother received 4 doses of intravenous ampicillin during labor.
The infant’s initial hospital course was complicated by a transient and otherwise asymptomatic bradycardia. An electrocardiogram (ECG) confirmed a heart rate of 96 with normal interval parameters, but there were changes suggestive of left ventricular hypertrophy. An echocardiogram was normal.
Follow-up office visits for common newborn feeding problems demonstrated consistent weight gain and normal vital signs, including heart rate and facial milia. However, by age 4 weeks an erythematous eruption extending from the frontal scalp and forehead to the cheek area had developed (FIGURES 1 AND 2).
FIGURE 1
Rash on a newborn’s face …
FIGURE 2
…and on the scalp
What is the differential diagnosis?
What tests should be done to make the diagnosis?
Diagnosis: Neonatal lupus
This infant has neonatal lupus erythematosus, a rare syndrome in which maternal autoantibodies are passively transferred to the baby and cause cutaneous lesions or isolated congenital heart block. The skin rash generally appears a few days to weeks after birth, typically after sun exposure, and shows well-demarcated erythematous, scaling patches that are often annular and predominately on the scalp, neck, or face. It is self-limited and generally resolves without scarring by 6 to 7 months of age.
Differential diagnosis
The differential diagnosis of neonatal lupus syndrome includes annular urticaria, tinea corporis, seborrheic dermatitis, erythema annulare centrifugum, familial annular erythema, erythema multiforme, systemic lupus erythematosus, pityrosporum infection, and photosensitive genodermatoses.1 These can be differentiated from neonatal lupus by several defining characteristics:
- Annular urticaria is transient and pruritic
- In tinea corporis, a KOH prep reveals numerous branching hyphae
- Seborrheic dermatitis is characterized by greasy scales over red, inflamed skin and distributed in facial and body folds
- Erythema annulare centrifugum usually affects the trunks and legs; the characteristic rings enlarge daily
- Familial annular erythema is generally found on the back and shoulders and accompanied by a family history of similar lesions in infancy
- Erythema multiforme is more often on extensor surfaces and is uncommon in infancy
- Systemic lupus erythematosus should be suspected if the infant has the clinical manifestations or positive serological tests while autoantibodies are absent in the mother
- Pityrosporum infection can be diagnosed by hyphae and spores (“spaghetti and meatballs”) on KOH preparation
- Photosensitive genodermatoses are a rare group of disorders that are characterized by chronicity without autoantibodies.
Causes and pathophysiology of neonatal lupus
Neonatal lupus is caused by the IgG autoantibodies anti-Ro/SSA and anti-La/SSB, which pass from the mother to the fetus via the placenta, although anti-U1-RNP antibodies can sometimes cause the cutaneous manifestations of neonatal lupus.2 The mother may or may not demonstrate features of connective tissue disease at the time of birth. Lupus has a higher incidence in Asian women than in the overall US population.
Possible symptoms in the neonate include a characteristic skin eruption or congenital heart block. Neonatal lupus accounts for 85% of cases of congenital complete heart block diagnosed in utero or in the neonatal period.3 The incidence of complete heart block in offspring of women with anti-Ro/SSA or anti-La/SSB antibodies is about 2%. The incidence of complete heart block in infants with cutaneous neonatal lupus is 15% to 30%. Incomplete heart block, which may progress to complete heart block, may also occur. A less common and self-limited sinus bradycardia has also been described in fetuses.4
Other manifestations of neonatal lupus include thrombocytopenia, aplastic anemia, hepatobiliary dysfunction, and central nervous system vasculopathy.
Diagnosis and treatment
Diagnosis is based on physical findings in an infant aged <6 months and detection of anti-Ro/SSA and anti-La/SSB antibodies in the child or mother. It should be suspected in children with congenital heart block as it is responsible for over 85% of cases.
All infants diagnosed with neonatal lupus erythematosus or born to women with anti-Ro/SSA or anti-La/SSB antibodies should have an ECG to detect heart block. If this is abnormal, referral to a pediatric cardiologist is warranted. All pregnant women with anti-Ro/SSA or anti-La/SSB antibodies should undergo regular fetal echocardiography to detect heart block. The initiation of dexamethasone or plasmapheresis as a preventative measure against congenital heart block in high-risk pregnant women is currently under consideration but is not yet justified.3
Treatment includes photoprotection (avoiding sunlight using clothing and other protective measures) and time, as nearly all cases resolve within 6 months without scarring. Mild topical steroids may be helpful. It is important to note that children with neonatal lupus may be at higher risk of developing autoimmune disorders or rheumatic disease later in life.2
Outcome
For this patient, the skin lesions were scraped with the side of a microscope slide and KOH with DMSO was used to dissolve the epithelial cells. The preparation was negative for fungal elements.This infant had anti-Ro, anti-La, and anti-RNP levels of 128.3, 150.3, and 128, respectively. Normal range is 0 to 19 for each autoantibody. Her mother’s autoantibody levels were similarly abnormal at 138.1, 158.8, and 169.1.
The patient was referred to a pediatric cardiologist for follow-up; results of a second echocardiogram and ECG were normal. The infant was seen again at 7 weeks; cutaneous lesions were present but somewhat improved, and she was otherwise doing well. Given the high probability of lupus occurring in future pregnancies, the mother received appropriate education.
CORRESPONDENCE
Keith A. Frey, MD, Department of Family Medicine, Mayo Clinic Arizona, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: [email protected]
1. Silverman ED. Neonatal lupus. In Cassidy JT, Pett RE (eds): Textbook of Pediatric Rheumatology. Philadelphia: WB Saunders; 2001:456.
2. Habif TP. Clinical Dermatology. Edinburgh: Mosby; 2004.
3. Buyon JP, Clancy RM. Neonatal lupus: Basic research and clinical perspective. Rheum Dis Clin North Am 2005;31:299-313.
4. Askanase AD, Friedman DM, Copel J, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La anti-bodies. Rheum Dis Clin North Am 2002;11:145.
1. Silverman ED. Neonatal lupus. In Cassidy JT, Pett RE (eds): Textbook of Pediatric Rheumatology. Philadelphia: WB Saunders; 2001:456.
2. Habif TP. Clinical Dermatology. Edinburgh: Mosby; 2004.
3. Buyon JP, Clancy RM. Neonatal lupus: Basic research and clinical perspective. Rheum Dis Clin North Am 2005;31:299-313.
4. Askanase AD, Friedman DM, Copel J, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La anti-bodies. Rheum Dis Clin North Am 2002;11:145.
A man with a pigmented growth on his chest
A 50-year-old man came to the office with a 7-year history of a slowly growing lesion on his chest. The patient, an outdoor worker with excessive sun exposure for 20 years, had skin phototype IV (burns minimally, always tans well to moderately brown). He reported that this lesion has never healed, has bled on occasion, and that regular wound care over several months was ineffective. He had suffered no trauma, nor had he applied any caustic products to this area. The patient was otherwise healthy and was not taking any medications. His family history was not contributory.
Physical examination revealed a large plaque about 4.5 by 3 cm with irregular borders (FIGURE 1). It seemed to have been expanding centrifugally for a long time, with areas of skipping, individual nodules, and papules. Close exam showed central ulcerations and crusting on scattered papules and nodules (FIGURE 2). The borders were rolled up and had a somewhat pearly appearance with erythema and telangiectasia. The area immediately surrounding the plaque was also remarkable for faint erythema. The central part of the lesion was atrophic with pinkish discoloration; on finger pinching of the central, the skin seemed to be sclerotic. The rest of the skin, mucosal, adnexal examination and review of systems was unremarkable. No lymphadenopathy was present. A skin biopsy was done to confirm the clinical impression.
FIGURE 1
Pigmented plaque on chest
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Basal cell carcinoma, pigmented subtype
Basal cell carcinoma (BCC) is the most common cancer among Caucasians1 and accounts for approximately 75% of all skin cancers. Resultant mortality is very low, but it may cause destruction to local and surrounding structures. Metastasis to lymph nodes and other organs and subsequent death have been reported with BCC.2,3 Other names for BCC are basalioma, basal cell epithelioma, rodent ulcer, and Jacobs’ ulcer.
Clinical presentations: Location, subtypes, risk of recurrence
The face (particularly the nose) is the most common site for BCC. A small percentage of BCCs occur on the trunk; it is rare on areas not exposed to the sun, such as the penis, vulva, perianal areas and axilla. Many subtypes of BCCs exist, including nodular, superficial, cystic, micronodular, morpheaform, and pigmented. In addition to features seen in lesions of nodular BCC, the pigmented subtype contains increased brown or black pigment, and it is seen more commonly in persons with dark skin.
Histology and new investigations
BCC arises from the basal layer of the epidermis. There are many histologic subtypes. The basaloid cells form tumor aggregates or nests of varying sizes. Cells tend to align more densely in a palisade pattern at the periphery of these nests. In the morpheaform subtype, the cells are embedded in fibrous stroma.
In a recent article, Goldberg et al4 used histopathology and special staining to show that BCC lesions had melanin pigment (positive for Fontana-Masson stain and negative for Perl’s stain) within nests of tumor cells. The authors concluded that speckled pigmentation of a basal cell carcinoma is a distinguishing feature, which may be useful in differentiating this tumor from other discrete skin tumors.
More recently, endothelins (ETs) have been implicated as participating in the pigmentation process of BCC. Enhanced ET-1 expression in pigmented BCC plays an important role in the hyperpigmentation of this tumor.5
Risk factors for BCC: Environment, genetics
Solar radiation is the chief environmental cause of BCC. Therapeutic radiation, such as PUVA for psoriasis or radiation for the treatment of acne or tinea capitis may result in BCC many years after exposure. Genetic characteristics such as fair skin, light-colored eyes, and red hair are also important risk factors. Another reported risk factor is chronic arsenic exposure, which may result from ingestion of contaminated water or seafood.
Basal cell nevus syndrome is an autosomal dominant genetic disorder with multiple characteristic clinical features. These features include the development of multiple BCCs at a relatively young age, macrocephaly, frontal bossing, hypertelorism, bifid ribs, palmar and plantar pitting, and bone cysts in the mandible. These patients have a mutation in the tumor suppressor gene patched (PTCH) on chromosome 9.6 Xeroderma pigmentosum and other rare genetic diseases also increase the likelihood of BCC tumors. Risk of BCC recurrence is related to tumor location and size, histologic type, and treatment modality and history of UV exposure.
Differential diagnosis for pigmented BCC
- Malignant melanoma
- Melanocytic nevus
- Spitz nevus
- Pigmented seborrheic keratosis
- Pigmented dermatofibroma
- Squamous cell carcinoma
- Pigmented Bowen’s disease (squamous cell carcinoma in situ)
- Keratoacanthoma
- Trichoepithelioma
- Sebaceous hyperplasia
- Fibrous papule of the nose
Treatment: Local destruction, photodynamic therapy, immune modulators
Treatment options available for BCC depend on characteristics of the tumor type, location, individual patient, and economic resources.
Cryosurgery, electrodessication and curettage, CO2 laser destruction, surgical excision, Mohs micrographic surgery, radiation therapy, topical therapy such as 5-fluorouracil (5-FU) and imiquimod (Aldara), intralesional interferon, and photodynamic therapy are used to treat BCC.
Older treatments for low-risk BCCs in cosmetically less significant areas
Cryosurgery induces cytotoxicity by production of extracellular and intracellular ice crystals. It is rapid and cost-effective, and demonstrates high cure rates.
Electrosurgery can be used for superficial and nodular BCC on the trunk and extremities.
Surgical excision is an effective method for BCCs located on low-risk areas without aggressive histologic features.
Radiation therapy is an option for the treatment of both primary and recurrent BCCs.
Topical chemotherapeutic agent 5-fluorouracil (Efudex, Carac, Fluoroplex) is also effective against BCC.
Interferon (interferon alpha 2b) has also been used as intralesional injections to treat superficial and nodular BCCs.
Treatment for high-risk tumors
Mohs micrographic surgery is currently the standard of treatment for high-risk BCCs and BCCs located in cosmetically sensitive locations such as the nasolabial fold and periorbital areas. It has a higher cure rate than other modalities.
Relatively new methods
Imiquimod is used for primary superficial BCC (not on head or neck) in adults with normal immune systems. It is used for tumors 2 cm or smaller in diameter on certain areas of the body. Imiquimod treatment is indicated only when surgical methods are not appropriate.7
Photodynamic therapy includes the use of various intravenous and topical photosensitizers—ie, intravenous verteporfin, topical 5-aminolevulinic acid (ALA), topical methyl aminolevulinate (mALA)—combined with different sources of visible light. Photodynamic therapy is being used for some BCCs but is not recommended for pigmented BCC.8
Given the ill-defined nature of this lesion, and its size, we recommended surgical excision using either regular excision with staining or marking of margins, or Mohs micrographic surgery. Unfortunately, the patient was lost to follow-up.
Acknowledgments
The author wants to thank Dr. Shahbaz A. Janjua with his assistance in the preparation of this manuscript.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, CWS, SUNY Downstate Medical Center, Department of Dermatology Box 46, 450 Clarkson Ave, Brooklyn, NY 11203. E-mail: [email protected]
1. Marks R. An overview of skin cancers: incidence and causation. Cancer 1995;75:607-612.
2. Robinson JK, Dahiya M. Basal cell carcinoma with pulmonary and lymph node metastasis causing death. Arch Dermatol 2003;139:643-648.
3. Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol 2005;152:1108-1124.
4. Goldberg LH, Friedman RH, Silapunt S. Pigmented speckling as a sign of basal cell carcinoma. Dermatol Surg 2004;30:1553-1555.
5. Lan CC, Wu CS, Cheng CM, Yu CL, Chen GS, Yu HS. Pigmentation in basal cell carcinoma involves enhanced endothelin-1 expression. Exp Dermatol 2005;14:528-534.
6. Blyumin ML, Khachemoune A. Self-assessment examination. Man with multiple papulonodules on the back. Identification no. 804-203. J Am Acad Dermatol 2004;50:498-494.
7. Oldfield V, Keating GM, Perry CM. Imiquimod: in superficial basal cell carcinoma. Am J Clin Dermatol 2005;6:195-200.
8. Kaviani A, Ataie-Fashtami L, Fateh M, Sheikhbahaee N, Ghodsi M, Zand N, Djavid GE. Photodynamic therapy of head and neck basal cell carcinoma according to different clinicopathologic features. Lasers Surg Med 2005;36:377-382.
A 50-year-old man came to the office with a 7-year history of a slowly growing lesion on his chest. The patient, an outdoor worker with excessive sun exposure for 20 years, had skin phototype IV (burns minimally, always tans well to moderately brown). He reported that this lesion has never healed, has bled on occasion, and that regular wound care over several months was ineffective. He had suffered no trauma, nor had he applied any caustic products to this area. The patient was otherwise healthy and was not taking any medications. His family history was not contributory.
Physical examination revealed a large plaque about 4.5 by 3 cm with irregular borders (FIGURE 1). It seemed to have been expanding centrifugally for a long time, with areas of skipping, individual nodules, and papules. Close exam showed central ulcerations and crusting on scattered papules and nodules (FIGURE 2). The borders were rolled up and had a somewhat pearly appearance with erythema and telangiectasia. The area immediately surrounding the plaque was also remarkable for faint erythema. The central part of the lesion was atrophic with pinkish discoloration; on finger pinching of the central, the skin seemed to be sclerotic. The rest of the skin, mucosal, adnexal examination and review of systems was unremarkable. No lymphadenopathy was present. A skin biopsy was done to confirm the clinical impression.
FIGURE 1
Pigmented plaque on chest
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Basal cell carcinoma, pigmented subtype
Basal cell carcinoma (BCC) is the most common cancer among Caucasians1 and accounts for approximately 75% of all skin cancers. Resultant mortality is very low, but it may cause destruction to local and surrounding structures. Metastasis to lymph nodes and other organs and subsequent death have been reported with BCC.2,3 Other names for BCC are basalioma, basal cell epithelioma, rodent ulcer, and Jacobs’ ulcer.
Clinical presentations: Location, subtypes, risk of recurrence
The face (particularly the nose) is the most common site for BCC. A small percentage of BCCs occur on the trunk; it is rare on areas not exposed to the sun, such as the penis, vulva, perianal areas and axilla. Many subtypes of BCCs exist, including nodular, superficial, cystic, micronodular, morpheaform, and pigmented. In addition to features seen in lesions of nodular BCC, the pigmented subtype contains increased brown or black pigment, and it is seen more commonly in persons with dark skin.
Histology and new investigations
BCC arises from the basal layer of the epidermis. There are many histologic subtypes. The basaloid cells form tumor aggregates or nests of varying sizes. Cells tend to align more densely in a palisade pattern at the periphery of these nests. In the morpheaform subtype, the cells are embedded in fibrous stroma.
In a recent article, Goldberg et al4 used histopathology and special staining to show that BCC lesions had melanin pigment (positive for Fontana-Masson stain and negative for Perl’s stain) within nests of tumor cells. The authors concluded that speckled pigmentation of a basal cell carcinoma is a distinguishing feature, which may be useful in differentiating this tumor from other discrete skin tumors.
More recently, endothelins (ETs) have been implicated as participating in the pigmentation process of BCC. Enhanced ET-1 expression in pigmented BCC plays an important role in the hyperpigmentation of this tumor.5
Risk factors for BCC: Environment, genetics
Solar radiation is the chief environmental cause of BCC. Therapeutic radiation, such as PUVA for psoriasis or radiation for the treatment of acne or tinea capitis may result in BCC many years after exposure. Genetic characteristics such as fair skin, light-colored eyes, and red hair are also important risk factors. Another reported risk factor is chronic arsenic exposure, which may result from ingestion of contaminated water or seafood.
Basal cell nevus syndrome is an autosomal dominant genetic disorder with multiple characteristic clinical features. These features include the development of multiple BCCs at a relatively young age, macrocephaly, frontal bossing, hypertelorism, bifid ribs, palmar and plantar pitting, and bone cysts in the mandible. These patients have a mutation in the tumor suppressor gene patched (PTCH) on chromosome 9.6 Xeroderma pigmentosum and other rare genetic diseases also increase the likelihood of BCC tumors. Risk of BCC recurrence is related to tumor location and size, histologic type, and treatment modality and history of UV exposure.
Differential diagnosis for pigmented BCC
- Malignant melanoma
- Melanocytic nevus
- Spitz nevus
- Pigmented seborrheic keratosis
- Pigmented dermatofibroma
- Squamous cell carcinoma
- Pigmented Bowen’s disease (squamous cell carcinoma in situ)
- Keratoacanthoma
- Trichoepithelioma
- Sebaceous hyperplasia
- Fibrous papule of the nose
Treatment: Local destruction, photodynamic therapy, immune modulators
Treatment options available for BCC depend on characteristics of the tumor type, location, individual patient, and economic resources.
Cryosurgery, electrodessication and curettage, CO2 laser destruction, surgical excision, Mohs micrographic surgery, radiation therapy, topical therapy such as 5-fluorouracil (5-FU) and imiquimod (Aldara), intralesional interferon, and photodynamic therapy are used to treat BCC.
Older treatments for low-risk BCCs in cosmetically less significant areas
Cryosurgery induces cytotoxicity by production of extracellular and intracellular ice crystals. It is rapid and cost-effective, and demonstrates high cure rates.
Electrosurgery can be used for superficial and nodular BCC on the trunk and extremities.
Surgical excision is an effective method for BCCs located on low-risk areas without aggressive histologic features.
Radiation therapy is an option for the treatment of both primary and recurrent BCCs.
Topical chemotherapeutic agent 5-fluorouracil (Efudex, Carac, Fluoroplex) is also effective against BCC.
Interferon (interferon alpha 2b) has also been used as intralesional injections to treat superficial and nodular BCCs.
Treatment for high-risk tumors
Mohs micrographic surgery is currently the standard of treatment for high-risk BCCs and BCCs located in cosmetically sensitive locations such as the nasolabial fold and periorbital areas. It has a higher cure rate than other modalities.
Relatively new methods
Imiquimod is used for primary superficial BCC (not on head or neck) in adults with normal immune systems. It is used for tumors 2 cm or smaller in diameter on certain areas of the body. Imiquimod treatment is indicated only when surgical methods are not appropriate.7
Photodynamic therapy includes the use of various intravenous and topical photosensitizers—ie, intravenous verteporfin, topical 5-aminolevulinic acid (ALA), topical methyl aminolevulinate (mALA)—combined with different sources of visible light. Photodynamic therapy is being used for some BCCs but is not recommended for pigmented BCC.8
Given the ill-defined nature of this lesion, and its size, we recommended surgical excision using either regular excision with staining or marking of margins, or Mohs micrographic surgery. Unfortunately, the patient was lost to follow-up.
Acknowledgments
The author wants to thank Dr. Shahbaz A. Janjua with his assistance in the preparation of this manuscript.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, CWS, SUNY Downstate Medical Center, Department of Dermatology Box 46, 450 Clarkson Ave, Brooklyn, NY 11203. E-mail: [email protected]
A 50-year-old man came to the office with a 7-year history of a slowly growing lesion on his chest. The patient, an outdoor worker with excessive sun exposure for 20 years, had skin phototype IV (burns minimally, always tans well to moderately brown). He reported that this lesion has never healed, has bled on occasion, and that regular wound care over several months was ineffective. He had suffered no trauma, nor had he applied any caustic products to this area. The patient was otherwise healthy and was not taking any medications. His family history was not contributory.
Physical examination revealed a large plaque about 4.5 by 3 cm with irregular borders (FIGURE 1). It seemed to have been expanding centrifugally for a long time, with areas of skipping, individual nodules, and papules. Close exam showed central ulcerations and crusting on scattered papules and nodules (FIGURE 2). The borders were rolled up and had a somewhat pearly appearance with erythema and telangiectasia. The area immediately surrounding the plaque was also remarkable for faint erythema. The central part of the lesion was atrophic with pinkish discoloration; on finger pinching of the central, the skin seemed to be sclerotic. The rest of the skin, mucosal, adnexal examination and review of systems was unremarkable. No lymphadenopathy was present. A skin biopsy was done to confirm the clinical impression.
FIGURE 1
Pigmented plaque on chest
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Basal cell carcinoma, pigmented subtype
Basal cell carcinoma (BCC) is the most common cancer among Caucasians1 and accounts for approximately 75% of all skin cancers. Resultant mortality is very low, but it may cause destruction to local and surrounding structures. Metastasis to lymph nodes and other organs and subsequent death have been reported with BCC.2,3 Other names for BCC are basalioma, basal cell epithelioma, rodent ulcer, and Jacobs’ ulcer.
Clinical presentations: Location, subtypes, risk of recurrence
The face (particularly the nose) is the most common site for BCC. A small percentage of BCCs occur on the trunk; it is rare on areas not exposed to the sun, such as the penis, vulva, perianal areas and axilla. Many subtypes of BCCs exist, including nodular, superficial, cystic, micronodular, morpheaform, and pigmented. In addition to features seen in lesions of nodular BCC, the pigmented subtype contains increased brown or black pigment, and it is seen more commonly in persons with dark skin.
Histology and new investigations
BCC arises from the basal layer of the epidermis. There are many histologic subtypes. The basaloid cells form tumor aggregates or nests of varying sizes. Cells tend to align more densely in a palisade pattern at the periphery of these nests. In the morpheaform subtype, the cells are embedded in fibrous stroma.
In a recent article, Goldberg et al4 used histopathology and special staining to show that BCC lesions had melanin pigment (positive for Fontana-Masson stain and negative for Perl’s stain) within nests of tumor cells. The authors concluded that speckled pigmentation of a basal cell carcinoma is a distinguishing feature, which may be useful in differentiating this tumor from other discrete skin tumors.
More recently, endothelins (ETs) have been implicated as participating in the pigmentation process of BCC. Enhanced ET-1 expression in pigmented BCC plays an important role in the hyperpigmentation of this tumor.5
Risk factors for BCC: Environment, genetics
Solar radiation is the chief environmental cause of BCC. Therapeutic radiation, such as PUVA for psoriasis or radiation for the treatment of acne or tinea capitis may result in BCC many years after exposure. Genetic characteristics such as fair skin, light-colored eyes, and red hair are also important risk factors. Another reported risk factor is chronic arsenic exposure, which may result from ingestion of contaminated water or seafood.
Basal cell nevus syndrome is an autosomal dominant genetic disorder with multiple characteristic clinical features. These features include the development of multiple BCCs at a relatively young age, macrocephaly, frontal bossing, hypertelorism, bifid ribs, palmar and plantar pitting, and bone cysts in the mandible. These patients have a mutation in the tumor suppressor gene patched (PTCH) on chromosome 9.6 Xeroderma pigmentosum and other rare genetic diseases also increase the likelihood of BCC tumors. Risk of BCC recurrence is related to tumor location and size, histologic type, and treatment modality and history of UV exposure.
Differential diagnosis for pigmented BCC
- Malignant melanoma
- Melanocytic nevus
- Spitz nevus
- Pigmented seborrheic keratosis
- Pigmented dermatofibroma
- Squamous cell carcinoma
- Pigmented Bowen’s disease (squamous cell carcinoma in situ)
- Keratoacanthoma
- Trichoepithelioma
- Sebaceous hyperplasia
- Fibrous papule of the nose
Treatment: Local destruction, photodynamic therapy, immune modulators
Treatment options available for BCC depend on characteristics of the tumor type, location, individual patient, and economic resources.
Cryosurgery, electrodessication and curettage, CO2 laser destruction, surgical excision, Mohs micrographic surgery, radiation therapy, topical therapy such as 5-fluorouracil (5-FU) and imiquimod (Aldara), intralesional interferon, and photodynamic therapy are used to treat BCC.
Older treatments for low-risk BCCs in cosmetically less significant areas
Cryosurgery induces cytotoxicity by production of extracellular and intracellular ice crystals. It is rapid and cost-effective, and demonstrates high cure rates.
Electrosurgery can be used for superficial and nodular BCC on the trunk and extremities.
Surgical excision is an effective method for BCCs located on low-risk areas without aggressive histologic features.
Radiation therapy is an option for the treatment of both primary and recurrent BCCs.
Topical chemotherapeutic agent 5-fluorouracil (Efudex, Carac, Fluoroplex) is also effective against BCC.
Interferon (interferon alpha 2b) has also been used as intralesional injections to treat superficial and nodular BCCs.
Treatment for high-risk tumors
Mohs micrographic surgery is currently the standard of treatment for high-risk BCCs and BCCs located in cosmetically sensitive locations such as the nasolabial fold and periorbital areas. It has a higher cure rate than other modalities.
Relatively new methods
Imiquimod is used for primary superficial BCC (not on head or neck) in adults with normal immune systems. It is used for tumors 2 cm or smaller in diameter on certain areas of the body. Imiquimod treatment is indicated only when surgical methods are not appropriate.7
Photodynamic therapy includes the use of various intravenous and topical photosensitizers—ie, intravenous verteporfin, topical 5-aminolevulinic acid (ALA), topical methyl aminolevulinate (mALA)—combined with different sources of visible light. Photodynamic therapy is being used for some BCCs but is not recommended for pigmented BCC.8
Given the ill-defined nature of this lesion, and its size, we recommended surgical excision using either regular excision with staining or marking of margins, or Mohs micrographic surgery. Unfortunately, the patient was lost to follow-up.
Acknowledgments
The author wants to thank Dr. Shahbaz A. Janjua with his assistance in the preparation of this manuscript.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, CWS, SUNY Downstate Medical Center, Department of Dermatology Box 46, 450 Clarkson Ave, Brooklyn, NY 11203. E-mail: [email protected]
1. Marks R. An overview of skin cancers: incidence and causation. Cancer 1995;75:607-612.
2. Robinson JK, Dahiya M. Basal cell carcinoma with pulmonary and lymph node metastasis causing death. Arch Dermatol 2003;139:643-648.
3. Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol 2005;152:1108-1124.
4. Goldberg LH, Friedman RH, Silapunt S. Pigmented speckling as a sign of basal cell carcinoma. Dermatol Surg 2004;30:1553-1555.
5. Lan CC, Wu CS, Cheng CM, Yu CL, Chen GS, Yu HS. Pigmentation in basal cell carcinoma involves enhanced endothelin-1 expression. Exp Dermatol 2005;14:528-534.
6. Blyumin ML, Khachemoune A. Self-assessment examination. Man with multiple papulonodules on the back. Identification no. 804-203. J Am Acad Dermatol 2004;50:498-494.
7. Oldfield V, Keating GM, Perry CM. Imiquimod: in superficial basal cell carcinoma. Am J Clin Dermatol 2005;6:195-200.
8. Kaviani A, Ataie-Fashtami L, Fateh M, Sheikhbahaee N, Ghodsi M, Zand N, Djavid GE. Photodynamic therapy of head and neck basal cell carcinoma according to different clinicopathologic features. Lasers Surg Med 2005;36:377-382.
1. Marks R. An overview of skin cancers: incidence and causation. Cancer 1995;75:607-612.
2. Robinson JK, Dahiya M. Basal cell carcinoma with pulmonary and lymph node metastasis causing death. Arch Dermatol 2003;139:643-648.
3. Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol 2005;152:1108-1124.
4. Goldberg LH, Friedman RH, Silapunt S. Pigmented speckling as a sign of basal cell carcinoma. Dermatol Surg 2004;30:1553-1555.
5. Lan CC, Wu CS, Cheng CM, Yu CL, Chen GS, Yu HS. Pigmentation in basal cell carcinoma involves enhanced endothelin-1 expression. Exp Dermatol 2005;14:528-534.
6. Blyumin ML, Khachemoune A. Self-assessment examination. Man with multiple papulonodules on the back. Identification no. 804-203. J Am Acad Dermatol 2004;50:498-494.
7. Oldfield V, Keating GM, Perry CM. Imiquimod: in superficial basal cell carcinoma. Am J Clin Dermatol 2005;6:195-200.
8. Kaviani A, Ataie-Fashtami L, Fateh M, Sheikhbahaee N, Ghodsi M, Zand N, Djavid GE. Photodynamic therapy of head and neck basal cell carcinoma according to different clinicopathologic features. Lasers Surg Med 2005;36:377-382.
Bullous eruption on the posterior thigh
A healthy 11-year-old girl visited her family physician with a lesion on her right posterior thigh. The lesion was a 1-cm plaque that was tender, firm, erythematous, and indurated, with a central pustule. It had been present for 3 days; it was noticed by the patient after returning from a camping trip in southeastern Pennsylvania. The pustular area was incised, drained, and cultured, and the patient was started on cephalexin.
Two days later, the lesion did not improve, showing increased induration, erythema, and blistering. The patient went to the emergency department with an 8 cm by 6 cm coalescence of thin-walled vesicles and bullae with surrounding erythema (FIGURES 1 AND 2). A thick, honey-yellow adherent crust covered the eroded center of the lesion. The girl’s temperature was 37.1°C, and she reported no burning, pain, or pruritus. She had full range of motion of her right hip and knee, and no lymphadenopathy was detected. Her white blood cell count was normal; blood and wound cultures were taken.
FIGURE 1
Bullous eruption on the thigh
FIGURE 2
Close-up
What is the most likely diagnosis?
How would you empirically treat this condition?
Diagnosis: Bullous impetigo, caused by methicillin resistant S aureus
Impetigo is a highly contagious superficial skin infection, with peak incidence among children aged 2 to 6 years.1,2 Nonbullous impetigo (70% of cases) is caused by Staphylococcus aureus or betahemolytic Streptococcus.3 Bullous impetigo is almost always caused by S aureus. Epidermolytic toxins produced by phage group II strains cause loss of cell adhesion in the stratum granulosum due to proteolytic attack of desmoglein 1, resulting in bullae.4
Bullous impetigo may occur after minor skin injury, such as an insect bite, abrasion, or dermatitis. Lesions generally start as small vesicles on the face, buttocks, extremities, or perineum, and may progress to a coalescence of thin-roofed bullae. The flaccid bullae rupture easily, draining serous or purulent fluid.
Lesions are usually painless, and systemic findings are rare. Lymphadenopathy is rare in bullous impetigo but common in nonbullous impetigo. The disease is generally self-limited and complications are uncommon. However, ecthyma (ulcerative impetigo) may result from an untreated impetigo infection.5
Differential diagnosis
The differential diagnosis for bullous impetigo is broad, and may include allergic contact dermatitis, herpes simplex, herpes zoster, pemphigus foliaceus, bullous pemphigoid, pemphigus vulgaris, and (in this case specifically) erythema migrans.
Allergic contact dermatitis is a delayed hypersensitivity reaction, usually caused by skin contact with an allergen. Lesions can be vesicular, edematous, erythematous, and pruritic. In this case, the patient did not have allergen exposure or a pruritic lesion.
Herpes zoster is a reactivation of the varicella zoster virus, characterized by stabbing, neuritic pain in a dermatomal distribution. Clear vesicles on an erythematous, edematous base distributed along a dermatome constitutes the classic appearance. This was not the case with this patient.
Pemphigus foliaceous is an autoimmune intraepidermal blistering disease with lesions occurring on the face, scalp, chest, and upper back.5 Intact blisters are not commonly seen. The vesicle roof is very thin and ruptures easily, forming broad areas of crust. Skin biopsy reveals intraepidermal bulla or acantholysis in the upper epidermis.
Pemphigus vulgaris is also an autoimmune blistering disease that affects the skin and mucous membranes. It is generally seen among patients aged >40 years.
Bullous pemphigoid is an autoimmune disorder presenting with chronic eruption of erythematous, papular, urticaria lesions often evolving into bullae. Childhood cases are rare. Biopsy of the lesions demonstrates subepidermal bulla with an infiltration of eosinophils within the dermis.5
Erythema migrans with central vesiculation must be considered given the patient’s camping trip. Recent evidence shows that erythema migrans with central redness accounts for most cases in areas endemic for Lyme disease. Only 10% of the patients with early Lyme disease show the classic bulls-eye lesion with concentric erythematous rings and central clearing. Vesiculation can occur in up to 30% of lesions.6
Staphylococcus aureus and antibiotic resistance
As many as 61% of community-acquired methicillin-resistant S aureus (MRSA) infections are initially treated only with beta-lactam antibiotics, to which they are resistant.7 Risk factors for community-acquired MRSA infection include day-care attendance, recent hospitalization, recent antibiotic use, chronic illness, and frequent health care visits.8 A growing number of cases are reported among patients without risk factors.
Community-acquired MRSA isolates are usually genetically different from nosocomial isolates, and have been relatively susceptible to non–beta-lactam antibiotics. These strains vary substantially, however, and it is important to check the susceptibility of the isolate.
Virulent new strains of S aureus are infecting children—these strains have a novel transpeptidase, which offers them a mechanism of resistance to beta-lactams different from hospital-and community-acquired types.
Awareness of the local antimicrobial susceptibility patterns of community S aureus isolates is also helpful. Oral antibiotics that have been successful include clindamycin, minocycline, doxycycline, and trimethoprim-sulfamethoxazole. Cephalexin has no therapeutic value in treating community-acquired MRSA.
Preventing disease spread in the patient and contacts
Preventive efforts should be directed at patients with recurrent episodes of MRSA skin abscesses. Metabolic and immunologic screening should be performed to rule out underlying disease processes causing increased risk for infection. In most cases these test results are normal, and patients with recurrent MRSA skin abscesses should also be empirically treated for presumed nasal carriage of MRSA.
Mupirocin ointment (Bactroban) should be applied to the nares twice daily for 5 days in an effort to prevent recurrent self-inoculation and lateral transmission of MRSA.
Patients and families should also be instructed in hygienic measures such as daily changing of underwear and personal use only of towels, washcloths, and sleepwear. Fingernails should be kept short and clean. Open insect bites or superficial skin abrasions should be kept clean and covered. Benefit from the daily use of antimicrobial soaps is controversial.
Empiric treatment of impetigo: Consider a culture for MRSA
For localized impetigo, topical therapy with mupirocin 2% ointment 3 times a day for 10 days is usually adequate. A 10-day course of oral antibiotic therapy with dicloxacillin or cephalexin is indicated in more widespread impetigo presumed to be methicillin-sensitive S aureus. Azithromycin (Zithromax) or clarithromycin (Biaxin) may be given to patients allergic to penicillin.
However, it is becoming increasingly important to consider community-acquired methicillin-resistant S aureus species in cases such as this that do not respond to traditional therapy. Hence, culture and sensitivity of all suspicious lesions is highly suggested.
Patient’s treatment and recovery
In this case, the patient was diagnosed with bullous impetigo and admitted to the hospital. She was started on intravenous clindamycin at 380 mg (30 mg/kg) every 8 hours. Clindamycin was chosen because most cases of community-acquired MRSA in this geographic area are resistant to trimethoprim-sulfamethoxazole and susceptible to clindamycin.
Although doxycycline would have covered both community-acquired MRSA and Lyme disease, we were less suspicious of Lyme given the physical exam of the patient, and we were reluctant to start this patient on doxycycline due to the fact she did not have complete maturation of her dentition.
Within 24 hours of intravenous clindamycin, the lesion was markedly improved and the culture confirmed that the MRSA was sensitive to clindamycin. She was discharged on oral clindamycin at 375 mg 3 times daily, to complete a 14-day course of therapy. The lesion was completely resolved without recurrence within 2 weeks.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Dagan R. Impetigo in children: changing epidemiology and new treatments. Pediatric Annals 1993;22:235-240.
2. Bruijnzeels MA, van Suijlekom-Smit LW, van der Velden J, van der Wouden JC. The child in general practice. Dutch national survey of morbidity and interventions in general practice. Rotterdam: Erasmus University Rotterdam, 1993.
3. Allen CH, Patel M, Endom E. Primary bacterial infections of the skin and soft tissues changes in epidemiology and management. Clin Ped Emerg Med 2004;5:246-255.
Amagai M, Matsuyoshi N, Wang ZH, et al. Toxin in bullous impetigo and staphylococcal scalded skin syndrome targets desmoglein 1. Nat Med 2000;6:1275.-
5. Habif TP. Skin Disease Diagnosis and Treatment. 2nd ed. Philadelphia, Pa: Elsevier-Mosby 2005;136-141.
6. Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002;136:477-479.
7. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976.-
8. Cohen P. Community-acquired methicillin-resistant staphylococcus aureus: skin infection presenting as an axillary abscess with cellulites in a college athlete. Skin Med 2005;4:115-117.
A healthy 11-year-old girl visited her family physician with a lesion on her right posterior thigh. The lesion was a 1-cm plaque that was tender, firm, erythematous, and indurated, with a central pustule. It had been present for 3 days; it was noticed by the patient after returning from a camping trip in southeastern Pennsylvania. The pustular area was incised, drained, and cultured, and the patient was started on cephalexin.
Two days later, the lesion did not improve, showing increased induration, erythema, and blistering. The patient went to the emergency department with an 8 cm by 6 cm coalescence of thin-walled vesicles and bullae with surrounding erythema (FIGURES 1 AND 2). A thick, honey-yellow adherent crust covered the eroded center of the lesion. The girl’s temperature was 37.1°C, and she reported no burning, pain, or pruritus. She had full range of motion of her right hip and knee, and no lymphadenopathy was detected. Her white blood cell count was normal; blood and wound cultures were taken.
FIGURE 1
Bullous eruption on the thigh
FIGURE 2
Close-up
What is the most likely diagnosis?
How would you empirically treat this condition?
Diagnosis: Bullous impetigo, caused by methicillin resistant S aureus
Impetigo is a highly contagious superficial skin infection, with peak incidence among children aged 2 to 6 years.1,2 Nonbullous impetigo (70% of cases) is caused by Staphylococcus aureus or betahemolytic Streptococcus.3 Bullous impetigo is almost always caused by S aureus. Epidermolytic toxins produced by phage group II strains cause loss of cell adhesion in the stratum granulosum due to proteolytic attack of desmoglein 1, resulting in bullae.4
Bullous impetigo may occur after minor skin injury, such as an insect bite, abrasion, or dermatitis. Lesions generally start as small vesicles on the face, buttocks, extremities, or perineum, and may progress to a coalescence of thin-roofed bullae. The flaccid bullae rupture easily, draining serous or purulent fluid.
Lesions are usually painless, and systemic findings are rare. Lymphadenopathy is rare in bullous impetigo but common in nonbullous impetigo. The disease is generally self-limited and complications are uncommon. However, ecthyma (ulcerative impetigo) may result from an untreated impetigo infection.5
Differential diagnosis
The differential diagnosis for bullous impetigo is broad, and may include allergic contact dermatitis, herpes simplex, herpes zoster, pemphigus foliaceus, bullous pemphigoid, pemphigus vulgaris, and (in this case specifically) erythema migrans.
Allergic contact dermatitis is a delayed hypersensitivity reaction, usually caused by skin contact with an allergen. Lesions can be vesicular, edematous, erythematous, and pruritic. In this case, the patient did not have allergen exposure or a pruritic lesion.
Herpes zoster is a reactivation of the varicella zoster virus, characterized by stabbing, neuritic pain in a dermatomal distribution. Clear vesicles on an erythematous, edematous base distributed along a dermatome constitutes the classic appearance. This was not the case with this patient.
Pemphigus foliaceous is an autoimmune intraepidermal blistering disease with lesions occurring on the face, scalp, chest, and upper back.5 Intact blisters are not commonly seen. The vesicle roof is very thin and ruptures easily, forming broad areas of crust. Skin biopsy reveals intraepidermal bulla or acantholysis in the upper epidermis.
Pemphigus vulgaris is also an autoimmune blistering disease that affects the skin and mucous membranes. It is generally seen among patients aged >40 years.
Bullous pemphigoid is an autoimmune disorder presenting with chronic eruption of erythematous, papular, urticaria lesions often evolving into bullae. Childhood cases are rare. Biopsy of the lesions demonstrates subepidermal bulla with an infiltration of eosinophils within the dermis.5
Erythema migrans with central vesiculation must be considered given the patient’s camping trip. Recent evidence shows that erythema migrans with central redness accounts for most cases in areas endemic for Lyme disease. Only 10% of the patients with early Lyme disease show the classic bulls-eye lesion with concentric erythematous rings and central clearing. Vesiculation can occur in up to 30% of lesions.6
Staphylococcus aureus and antibiotic resistance
As many as 61% of community-acquired methicillin-resistant S aureus (MRSA) infections are initially treated only with beta-lactam antibiotics, to which they are resistant.7 Risk factors for community-acquired MRSA infection include day-care attendance, recent hospitalization, recent antibiotic use, chronic illness, and frequent health care visits.8 A growing number of cases are reported among patients without risk factors.
Community-acquired MRSA isolates are usually genetically different from nosocomial isolates, and have been relatively susceptible to non–beta-lactam antibiotics. These strains vary substantially, however, and it is important to check the susceptibility of the isolate.
Virulent new strains of S aureus are infecting children—these strains have a novel transpeptidase, which offers them a mechanism of resistance to beta-lactams different from hospital-and community-acquired types.
Awareness of the local antimicrobial susceptibility patterns of community S aureus isolates is also helpful. Oral antibiotics that have been successful include clindamycin, minocycline, doxycycline, and trimethoprim-sulfamethoxazole. Cephalexin has no therapeutic value in treating community-acquired MRSA.
Preventing disease spread in the patient and contacts
Preventive efforts should be directed at patients with recurrent episodes of MRSA skin abscesses. Metabolic and immunologic screening should be performed to rule out underlying disease processes causing increased risk for infection. In most cases these test results are normal, and patients with recurrent MRSA skin abscesses should also be empirically treated for presumed nasal carriage of MRSA.
Mupirocin ointment (Bactroban) should be applied to the nares twice daily for 5 days in an effort to prevent recurrent self-inoculation and lateral transmission of MRSA.
Patients and families should also be instructed in hygienic measures such as daily changing of underwear and personal use only of towels, washcloths, and sleepwear. Fingernails should be kept short and clean. Open insect bites or superficial skin abrasions should be kept clean and covered. Benefit from the daily use of antimicrobial soaps is controversial.
Empiric treatment of impetigo: Consider a culture for MRSA
For localized impetigo, topical therapy with mupirocin 2% ointment 3 times a day for 10 days is usually adequate. A 10-day course of oral antibiotic therapy with dicloxacillin or cephalexin is indicated in more widespread impetigo presumed to be methicillin-sensitive S aureus. Azithromycin (Zithromax) or clarithromycin (Biaxin) may be given to patients allergic to penicillin.
However, it is becoming increasingly important to consider community-acquired methicillin-resistant S aureus species in cases such as this that do not respond to traditional therapy. Hence, culture and sensitivity of all suspicious lesions is highly suggested.
Patient’s treatment and recovery
In this case, the patient was diagnosed with bullous impetigo and admitted to the hospital. She was started on intravenous clindamycin at 380 mg (30 mg/kg) every 8 hours. Clindamycin was chosen because most cases of community-acquired MRSA in this geographic area are resistant to trimethoprim-sulfamethoxazole and susceptible to clindamycin.
Although doxycycline would have covered both community-acquired MRSA and Lyme disease, we were less suspicious of Lyme given the physical exam of the patient, and we were reluctant to start this patient on doxycycline due to the fact she did not have complete maturation of her dentition.
Within 24 hours of intravenous clindamycin, the lesion was markedly improved and the culture confirmed that the MRSA was sensitive to clindamycin. She was discharged on oral clindamycin at 375 mg 3 times daily, to complete a 14-day course of therapy. The lesion was completely resolved without recurrence within 2 weeks.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A healthy 11-year-old girl visited her family physician with a lesion on her right posterior thigh. The lesion was a 1-cm plaque that was tender, firm, erythematous, and indurated, with a central pustule. It had been present for 3 days; it was noticed by the patient after returning from a camping trip in southeastern Pennsylvania. The pustular area was incised, drained, and cultured, and the patient was started on cephalexin.
Two days later, the lesion did not improve, showing increased induration, erythema, and blistering. The patient went to the emergency department with an 8 cm by 6 cm coalescence of thin-walled vesicles and bullae with surrounding erythema (FIGURES 1 AND 2). A thick, honey-yellow adherent crust covered the eroded center of the lesion. The girl’s temperature was 37.1°C, and she reported no burning, pain, or pruritus. She had full range of motion of her right hip and knee, and no lymphadenopathy was detected. Her white blood cell count was normal; blood and wound cultures were taken.
FIGURE 1
Bullous eruption on the thigh
FIGURE 2
Close-up
What is the most likely diagnosis?
How would you empirically treat this condition?
Diagnosis: Bullous impetigo, caused by methicillin resistant S aureus
Impetigo is a highly contagious superficial skin infection, with peak incidence among children aged 2 to 6 years.1,2 Nonbullous impetigo (70% of cases) is caused by Staphylococcus aureus or betahemolytic Streptococcus.3 Bullous impetigo is almost always caused by S aureus. Epidermolytic toxins produced by phage group II strains cause loss of cell adhesion in the stratum granulosum due to proteolytic attack of desmoglein 1, resulting in bullae.4
Bullous impetigo may occur after minor skin injury, such as an insect bite, abrasion, or dermatitis. Lesions generally start as small vesicles on the face, buttocks, extremities, or perineum, and may progress to a coalescence of thin-roofed bullae. The flaccid bullae rupture easily, draining serous or purulent fluid.
Lesions are usually painless, and systemic findings are rare. Lymphadenopathy is rare in bullous impetigo but common in nonbullous impetigo. The disease is generally self-limited and complications are uncommon. However, ecthyma (ulcerative impetigo) may result from an untreated impetigo infection.5
Differential diagnosis
The differential diagnosis for bullous impetigo is broad, and may include allergic contact dermatitis, herpes simplex, herpes zoster, pemphigus foliaceus, bullous pemphigoid, pemphigus vulgaris, and (in this case specifically) erythema migrans.
Allergic contact dermatitis is a delayed hypersensitivity reaction, usually caused by skin contact with an allergen. Lesions can be vesicular, edematous, erythematous, and pruritic. In this case, the patient did not have allergen exposure or a pruritic lesion.
Herpes zoster is a reactivation of the varicella zoster virus, characterized by stabbing, neuritic pain in a dermatomal distribution. Clear vesicles on an erythematous, edematous base distributed along a dermatome constitutes the classic appearance. This was not the case with this patient.
Pemphigus foliaceous is an autoimmune intraepidermal blistering disease with lesions occurring on the face, scalp, chest, and upper back.5 Intact blisters are not commonly seen. The vesicle roof is very thin and ruptures easily, forming broad areas of crust. Skin biopsy reveals intraepidermal bulla or acantholysis in the upper epidermis.
Pemphigus vulgaris is also an autoimmune blistering disease that affects the skin and mucous membranes. It is generally seen among patients aged >40 years.
Bullous pemphigoid is an autoimmune disorder presenting with chronic eruption of erythematous, papular, urticaria lesions often evolving into bullae. Childhood cases are rare. Biopsy of the lesions demonstrates subepidermal bulla with an infiltration of eosinophils within the dermis.5
Erythema migrans with central vesiculation must be considered given the patient’s camping trip. Recent evidence shows that erythema migrans with central redness accounts for most cases in areas endemic for Lyme disease. Only 10% of the patients with early Lyme disease show the classic bulls-eye lesion with concentric erythematous rings and central clearing. Vesiculation can occur in up to 30% of lesions.6
Staphylococcus aureus and antibiotic resistance
As many as 61% of community-acquired methicillin-resistant S aureus (MRSA) infections are initially treated only with beta-lactam antibiotics, to which they are resistant.7 Risk factors for community-acquired MRSA infection include day-care attendance, recent hospitalization, recent antibiotic use, chronic illness, and frequent health care visits.8 A growing number of cases are reported among patients without risk factors.
Community-acquired MRSA isolates are usually genetically different from nosocomial isolates, and have been relatively susceptible to non–beta-lactam antibiotics. These strains vary substantially, however, and it is important to check the susceptibility of the isolate.
Virulent new strains of S aureus are infecting children—these strains have a novel transpeptidase, which offers them a mechanism of resistance to beta-lactams different from hospital-and community-acquired types.
Awareness of the local antimicrobial susceptibility patterns of community S aureus isolates is also helpful. Oral antibiotics that have been successful include clindamycin, minocycline, doxycycline, and trimethoprim-sulfamethoxazole. Cephalexin has no therapeutic value in treating community-acquired MRSA.
Preventing disease spread in the patient and contacts
Preventive efforts should be directed at patients with recurrent episodes of MRSA skin abscesses. Metabolic and immunologic screening should be performed to rule out underlying disease processes causing increased risk for infection. In most cases these test results are normal, and patients with recurrent MRSA skin abscesses should also be empirically treated for presumed nasal carriage of MRSA.
Mupirocin ointment (Bactroban) should be applied to the nares twice daily for 5 days in an effort to prevent recurrent self-inoculation and lateral transmission of MRSA.
Patients and families should also be instructed in hygienic measures such as daily changing of underwear and personal use only of towels, washcloths, and sleepwear. Fingernails should be kept short and clean. Open insect bites or superficial skin abrasions should be kept clean and covered. Benefit from the daily use of antimicrobial soaps is controversial.
Empiric treatment of impetigo: Consider a culture for MRSA
For localized impetigo, topical therapy with mupirocin 2% ointment 3 times a day for 10 days is usually adequate. A 10-day course of oral antibiotic therapy with dicloxacillin or cephalexin is indicated in more widespread impetigo presumed to be methicillin-sensitive S aureus. Azithromycin (Zithromax) or clarithromycin (Biaxin) may be given to patients allergic to penicillin.
However, it is becoming increasingly important to consider community-acquired methicillin-resistant S aureus species in cases such as this that do not respond to traditional therapy. Hence, culture and sensitivity of all suspicious lesions is highly suggested.
Patient’s treatment and recovery
In this case, the patient was diagnosed with bullous impetigo and admitted to the hospital. She was started on intravenous clindamycin at 380 mg (30 mg/kg) every 8 hours. Clindamycin was chosen because most cases of community-acquired MRSA in this geographic area are resistant to trimethoprim-sulfamethoxazole and susceptible to clindamycin.
Although doxycycline would have covered both community-acquired MRSA and Lyme disease, we were less suspicious of Lyme given the physical exam of the patient, and we were reluctant to start this patient on doxycycline due to the fact she did not have complete maturation of her dentition.
Within 24 hours of intravenous clindamycin, the lesion was markedly improved and the culture confirmed that the MRSA was sensitive to clindamycin. She was discharged on oral clindamycin at 375 mg 3 times daily, to complete a 14-day course of therapy. The lesion was completely resolved without recurrence within 2 weeks.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Dagan R. Impetigo in children: changing epidemiology and new treatments. Pediatric Annals 1993;22:235-240.
2. Bruijnzeels MA, van Suijlekom-Smit LW, van der Velden J, van der Wouden JC. The child in general practice. Dutch national survey of morbidity and interventions in general practice. Rotterdam: Erasmus University Rotterdam, 1993.
3. Allen CH, Patel M, Endom E. Primary bacterial infections of the skin and soft tissues changes in epidemiology and management. Clin Ped Emerg Med 2004;5:246-255.
Amagai M, Matsuyoshi N, Wang ZH, et al. Toxin in bullous impetigo and staphylococcal scalded skin syndrome targets desmoglein 1. Nat Med 2000;6:1275.-
5. Habif TP. Skin Disease Diagnosis and Treatment. 2nd ed. Philadelphia, Pa: Elsevier-Mosby 2005;136-141.
6. Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002;136:477-479.
7. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976.-
8. Cohen P. Community-acquired methicillin-resistant staphylococcus aureus: skin infection presenting as an axillary abscess with cellulites in a college athlete. Skin Med 2005;4:115-117.
1. Dagan R. Impetigo in children: changing epidemiology and new treatments. Pediatric Annals 1993;22:235-240.
2. Bruijnzeels MA, van Suijlekom-Smit LW, van der Velden J, van der Wouden JC. The child in general practice. Dutch national survey of morbidity and interventions in general practice. Rotterdam: Erasmus University Rotterdam, 1993.
3. Allen CH, Patel M, Endom E. Primary bacterial infections of the skin and soft tissues changes in epidemiology and management. Clin Ped Emerg Med 2004;5:246-255.
Amagai M, Matsuyoshi N, Wang ZH, et al. Toxin in bullous impetigo and staphylococcal scalded skin syndrome targets desmoglein 1. Nat Med 2000;6:1275.-
5. Habif TP. Skin Disease Diagnosis and Treatment. 2nd ed. Philadelphia, Pa: Elsevier-Mosby 2005;136-141.
6. Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002;136:477-479.
7. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976.-
8. Cohen P. Community-acquired methicillin-resistant staphylococcus aureus: skin infection presenting as an axillary abscess with cellulites in a college athlete. Skin Med 2005;4:115-117.
A young girl with scaly skin plaques
An otherwise healthy 12-year-old girl came to the office with a 1-year history of a symmetric, generalized scaly eruption. These skin plaques did not itch; she had no recent history of sore throat. She also had no personal or family history of atopy or any similar eruption. She mentioned that the appearance of her skin and the “flaking” was making her very self-conscious and she wanted to have some intervention to make the skin clear.
The patient had multiple red, scaly, thick plaques on her back and the extensor surfaces of her elbows, knees, legs, and forearms (FIGURES 1 AND 2). There were no scalp, mucosal, or nail involvements. Rheumatologic examination and review of systems were unremarkable.
FIGURE 1
Plaques on the arm…
FIGURE 2
…and on the back
What is your diagnosis?
How would you manage this case?
Diagnosis: Chronic plaque psoriasis
Psoriasis is a noninfectious inflammatory skin disorder characterized by well-defined erythematous plaques that bear large, adherent silvery scales. It can appear at any age, but 75% of patients have an onset before the age of 40 years.1
Psoriasis was once considered a hyperproliferative disorder, but is now recognized as an autoimmune phenomenon involving activation of T-cells. As a result, new immunosuppressive agents have been added to the list of traditional therapies, opening a new chapter of immunomodulatory therapy.
Clinical presentations of psoriasis
In its classic presentation, psoriasis does not pose a diagnosis challenge to most clinicians—it presents as a sharply demarcated erythematous plaques with silvery white scales. Although many classification systems exist, a concise classification is included in TABLE 1. Skin conditions to be considered for differential diagnosis are summarized in TABLE 2. The US Food and Drug Administration defines “severe” psoriasis as involving more than 20% of body surface area.2
TABLE 1
Classification of psoriasis
| Chronic plaque psoriasis | Symmetrical plaques up to 20 cm in diameter, with a predilection for the elbows, knees, presacrum, scalp, hands, and feet |
| 5%–30% of patients develop a seronegative arthropathy | |
| Guttate psoriasis | Numerous small papules and plaques over the upper trunk and proximal extremities |
| Most common form of psoriasis in children, and may be triggered by any streptococcal infection including streptococcal perianal dermatitis | |
| Spontaneous remission is the rule | |
| Generalized pustular and erythrodermic psoriasis | Uncommon variants associated with high morbidity that may be fatal |
TABLE 2
Differential diagnosis
| Fungal infections |
| Squamous cell carcinoma in situ |
| Cutaneous T-cell lymphoma |
| Discoid eczema |
| Seborrhoeic eczema |
| Pityriasis rosea |
| Secondary syphilis |
| Hypertrophic lichen planus |
| Nummular dermatitis |
Pathogenesis of psoriasis
Psoriasis is a polygenic disorder. Susceptibility is determined by a large number of genes, each with a low penetrance. Important genetic associations are with HLA-Cw6, HLA-B27, and the genes PSOR-S1 and PSOR-S2 on chromosome 6 and 17, respectively.
Several triggering factors are identified, of which streptococcal antigens and certain drugs seem important, but the specific antigens are still unknown. The end result is activation of T-cells, overexpression of cytokines, and an inflammatory response.
Review of the classic treatments for psoriasis
Topical therapy
Topical therapy is indicated when psoriasis is limited to less than 20% of the body surface. Potent class I and II topical corticosteroids are the most widely used treatment for mild disease. After plaque clearance they can be given intermittently for maintenance.
The vitamin D3 derivative calcipotriene (Dovonex) is another first-line agent. Tazarotene (Tazorac), a topical retinoid prodrug, is a second-line agent used as monotherapy or in combination. Many combined regimens use topical corticosteroids, calcipotriene, and tazarotene. Coal tar—different concentrations in liquid form—is useful in treating extensive areas of the body and scalp psoriasis.
Phototherapy
Failure of topical therapy or extensive disease are indications for phototherapy or systemic medications. The trend is to use phototherapy in the form of narrowband UVB, which has proven more effective than broadband UVB and to have fewer adverse effects than psoralen UVA therapy (PUVA).3 Other light sources for home use are being developed.
Systemic agents
Methotrexate, given as a single weekly dose or in divided doses, has been used for more than 30 years; it inhibits the enzyme dihydrofolate reductase. An alternative immunosuppressive agent is cyclosporine. These 2 agents have high efficacy, but due to potential adverse effects they require careful patient selection and close follow-up.
Acitretin (Soriatane), an oral retinoid, is the treatment of choice for generalized pustular and erythrodermic psoriasis. It is also used in chronic plaque psoriasis, often in combination with phototherapy, which has a synergistic effect.New treatments
Our understanding of the immunopathogenesis of psoriasis has led to the development of therapies designed specifically to interfere with T-cell activation and effector functions. Three new immunomodulatory biologics are FDA-approved for the treatment of moderate to severe psoriasis. Typical cost of this therapy is more than $1000/month.
Anti-TNF-α strategies
Etanercept (Enbrel) is an antibody against the cytokine tumor necrosis factor alpha (TNF-α). It is self-administered at 25 mg to 50 mg subcutaneously twice weekly. Studies with 50-mg injections have shown a 75% clinical improvement in 49% of patients at 12 weeks and 59% of patients at 24 weeks.4
The most common side effect is reaction at the injection site. It was reported to produce dramatic remission of psoriatic arthritis and prevent radiographic progression of the disease.4-5 Due to raised concern about the risk of opportunistic infections, a purified protein derivative (PPD) test is advised before initiation of therapy to detect potential latent tuberculosis. Other risks include sepsis, pancytopenia, and worsening of multiple sclerosis.
Antibodies against T-lymphocyte surface molecules
Alefacept (Amevive) is a fusion protein that blocks T-cell activation and triggers apoptosis of activated T-cells. It is given as 15mg weekly intramuscular injections. Thirty-three percent of patients reported a 75% clinical improvement in their psoriasis within 12 weeks.6 Alefacept also decreases synovial tissue T-cell count, and may have a future role in psoriatic arthritis.
It has few side effects, but patients need weekly monitoring of their CD4+ count. Ongoing studies on combining it with ultraviolet light or extending the dose to 16 weeks are showing promise.6
Antibodies against adhesion molecules
Efalizumab (Raptiva) is a monoclonal antibody that blocks T-cell activation and migration. It is self-administered by the patient as a 1 mg/kg weekly subcutaneous injection. Forty-four percent of patients achieve a 75% clinical improvement in their psoriasis by 24 weeks.
The most common side effects are headache, fever, nausea, vomiting, and myalgia. A “rebound” phenomenon after discontinuation is observed in 14%, and it may worsen psoriasis in those unresponsive to treatment.7
Other anti-TNF medications such as infliximab (Remicade), adalimumab (Humira), and onercept are still in clinical trials.
Combination therapy: Achieving goals while reducing adverse effects
Some patients require therapy with several agents to maintain adequate clearing of their psoriasis. The ideal combination therapy should lead to enhanced clinical response with reduction of adverse effects.
It is important to choose agents with synergistic effects without additive toxicities. Examples are combination of a systemic agent with topical calcipotriene, topical steroids, or with phototherapy. PUVA should be used with care for patients taking immunosuppressive agents due to risk of squamous cell carcinoma.8 A combination of 2 immunosuppressive agents is generally avoided due to risk of opportunistic infections, but has proven beneficial in a few therapy-resistant patients.9 Further clinical experience is needed for the inclusion of the new biologics in combination therapy.
New directions in treating psoriasis
A summary of future directions and current investigations in the management of psoriasis is given in TABLE 3.
Our patient’s treatment consisted of topical emollients, mid-potency topical corticosteroids, and tar shampoos/tar baths. She was responding well to the treatment. Introducing calcipotriene and reducing topical steroids is our next step. Regular follow-up visits are scheduled every 4 to 6 weeks.
TABLE 3
Future investigations
| Photodynamic therapy | The use of a photosensitizing drug in combination with a light source is showing promise, and clinical studies are under way for the treatment of psoriasis |
| Excimer lasers | Deliver high-dose narrowband UVB to a localized area sparing uninvolved skin. Clear psoriasis faster than conventional phototherapy, and may become predominant in the future |
| CNTO-1275 | Anti-interleukin-12 antibody that switches the immune response from a T-helper cell 1 cytokine reaction most commonly seen in psoriasis to a T-helper cell 2 cytokine response |
| T-cell receptor vaccines | Have been developed and are undergoing clinical trials in patients with psoriasis |
| Pimecrolimus | Used orally. It is a cytokine-release inhibitor with lesser immunosuppressive effects and side effects than tacrolimus and cyclosporin |
| Angiogenesis | Cutaneous blood vessels in psoriatic plaques are dilated, tortuous, and leaky |
| Vascular endothelial growth factor (VEGF) is overexpressed, and VEGF or its receptors are potential therapeutic targets for psoriasis | |
| Gene therapy | Chromosomes involved in psoriasis are being mapped |
| Gene therapy promises to be one of the most important areas of treatment of psoriasis for the new millennium |
Conclusion
Patients with mild localized psoriasis can easily and effectively be managed by family physicians using topical treatments or combinations modalities as outlined above. Patients with extensive disease or resistant to treatment should be referred to a dermatologist in conjunction with a rheumatologist if psoriatic arthritis is suspected.
With improved understanding of the immunopathogenesis and genetics of psoriasis, advent of the biologic agents, and future strategies under investigation, our approach to treating psoriasis may be very different in the years to come.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine (BAR 314), Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street, Boston, MA 02114. E-mail: [email protected]
1. Khachemoune A, Phillips TJ. Current treatment options in psoriasis. Hosp Pract (Off Ed) 2000;35:93-96,101-104,107.-
2. Winterfield LS, Menter A, Gordon A, Gottlieb A. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis 2005;64:ii87-ii90.
3. Zanolli M. Phototherapy arsenal in the treatment of psoriasis. Dermatol Clin 2004;22:397-406.
4. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept Psoriasis Study Group. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2005;349:2014-22.
5. Lebwohl M. Innovations in the treatment of psoriasis. J Am Acad Dermatol 2004;51:40-41.
6. Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE. Alefacept Clinical Study Group. An international, randomized, double blind placebo-controlled phase 3 trial of intramuscular Alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003;139:719-727.
7. Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab Study Group. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 2003;290:3073-3080.
8. Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and cyclosporin: nested cohort crossover study. Lancet 2001;358:1942-1945.
9. Clark CM, Kirby B, Morris AO, et al. Combination treatment with methotrexate and cyclosporin for severe and recalcitrant psoriasis. Br J Dermatol 1999;141:279-282.
An otherwise healthy 12-year-old girl came to the office with a 1-year history of a symmetric, generalized scaly eruption. These skin plaques did not itch; she had no recent history of sore throat. She also had no personal or family history of atopy or any similar eruption. She mentioned that the appearance of her skin and the “flaking” was making her very self-conscious and she wanted to have some intervention to make the skin clear.
The patient had multiple red, scaly, thick plaques on her back and the extensor surfaces of her elbows, knees, legs, and forearms (FIGURES 1 AND 2). There were no scalp, mucosal, or nail involvements. Rheumatologic examination and review of systems were unremarkable.
FIGURE 1
Plaques on the arm…
FIGURE 2
…and on the back
What is your diagnosis?
How would you manage this case?
Diagnosis: Chronic plaque psoriasis
Psoriasis is a noninfectious inflammatory skin disorder characterized by well-defined erythematous plaques that bear large, adherent silvery scales. It can appear at any age, but 75% of patients have an onset before the age of 40 years.1
Psoriasis was once considered a hyperproliferative disorder, but is now recognized as an autoimmune phenomenon involving activation of T-cells. As a result, new immunosuppressive agents have been added to the list of traditional therapies, opening a new chapter of immunomodulatory therapy.
Clinical presentations of psoriasis
In its classic presentation, psoriasis does not pose a diagnosis challenge to most clinicians—it presents as a sharply demarcated erythematous plaques with silvery white scales. Although many classification systems exist, a concise classification is included in TABLE 1. Skin conditions to be considered for differential diagnosis are summarized in TABLE 2. The US Food and Drug Administration defines “severe” psoriasis as involving more than 20% of body surface area.2
TABLE 1
Classification of psoriasis
| Chronic plaque psoriasis | Symmetrical plaques up to 20 cm in diameter, with a predilection for the elbows, knees, presacrum, scalp, hands, and feet |
| 5%–30% of patients develop a seronegative arthropathy | |
| Guttate psoriasis | Numerous small papules and plaques over the upper trunk and proximal extremities |
| Most common form of psoriasis in children, and may be triggered by any streptococcal infection including streptococcal perianal dermatitis | |
| Spontaneous remission is the rule | |
| Generalized pustular and erythrodermic psoriasis | Uncommon variants associated with high morbidity that may be fatal |
TABLE 2
Differential diagnosis
| Fungal infections |
| Squamous cell carcinoma in situ |
| Cutaneous T-cell lymphoma |
| Discoid eczema |
| Seborrhoeic eczema |
| Pityriasis rosea |
| Secondary syphilis |
| Hypertrophic lichen planus |
| Nummular dermatitis |
Pathogenesis of psoriasis
Psoriasis is a polygenic disorder. Susceptibility is determined by a large number of genes, each with a low penetrance. Important genetic associations are with HLA-Cw6, HLA-B27, and the genes PSOR-S1 and PSOR-S2 on chromosome 6 and 17, respectively.
Several triggering factors are identified, of which streptococcal antigens and certain drugs seem important, but the specific antigens are still unknown. The end result is activation of T-cells, overexpression of cytokines, and an inflammatory response.
Review of the classic treatments for psoriasis
Topical therapy
Topical therapy is indicated when psoriasis is limited to less than 20% of the body surface. Potent class I and II topical corticosteroids are the most widely used treatment for mild disease. After plaque clearance they can be given intermittently for maintenance.
The vitamin D3 derivative calcipotriene (Dovonex) is another first-line agent. Tazarotene (Tazorac), a topical retinoid prodrug, is a second-line agent used as monotherapy or in combination. Many combined regimens use topical corticosteroids, calcipotriene, and tazarotene. Coal tar—different concentrations in liquid form—is useful in treating extensive areas of the body and scalp psoriasis.
Phototherapy
Failure of topical therapy or extensive disease are indications for phototherapy or systemic medications. The trend is to use phototherapy in the form of narrowband UVB, which has proven more effective than broadband UVB and to have fewer adverse effects than psoralen UVA therapy (PUVA).3 Other light sources for home use are being developed.
Systemic agents
Methotrexate, given as a single weekly dose or in divided doses, has been used for more than 30 years; it inhibits the enzyme dihydrofolate reductase. An alternative immunosuppressive agent is cyclosporine. These 2 agents have high efficacy, but due to potential adverse effects they require careful patient selection and close follow-up.
Acitretin (Soriatane), an oral retinoid, is the treatment of choice for generalized pustular and erythrodermic psoriasis. It is also used in chronic plaque psoriasis, often in combination with phototherapy, which has a synergistic effect.New treatments
Our understanding of the immunopathogenesis of psoriasis has led to the development of therapies designed specifically to interfere with T-cell activation and effector functions. Three new immunomodulatory biologics are FDA-approved for the treatment of moderate to severe psoriasis. Typical cost of this therapy is more than $1000/month.
Anti-TNF-α strategies
Etanercept (Enbrel) is an antibody against the cytokine tumor necrosis factor alpha (TNF-α). It is self-administered at 25 mg to 50 mg subcutaneously twice weekly. Studies with 50-mg injections have shown a 75% clinical improvement in 49% of patients at 12 weeks and 59% of patients at 24 weeks.4
The most common side effect is reaction at the injection site. It was reported to produce dramatic remission of psoriatic arthritis and prevent radiographic progression of the disease.4-5 Due to raised concern about the risk of opportunistic infections, a purified protein derivative (PPD) test is advised before initiation of therapy to detect potential latent tuberculosis. Other risks include sepsis, pancytopenia, and worsening of multiple sclerosis.
Antibodies against T-lymphocyte surface molecules
Alefacept (Amevive) is a fusion protein that blocks T-cell activation and triggers apoptosis of activated T-cells. It is given as 15mg weekly intramuscular injections. Thirty-three percent of patients reported a 75% clinical improvement in their psoriasis within 12 weeks.6 Alefacept also decreases synovial tissue T-cell count, and may have a future role in psoriatic arthritis.
It has few side effects, but patients need weekly monitoring of their CD4+ count. Ongoing studies on combining it with ultraviolet light or extending the dose to 16 weeks are showing promise.6
Antibodies against adhesion molecules
Efalizumab (Raptiva) is a monoclonal antibody that blocks T-cell activation and migration. It is self-administered by the patient as a 1 mg/kg weekly subcutaneous injection. Forty-four percent of patients achieve a 75% clinical improvement in their psoriasis by 24 weeks.
The most common side effects are headache, fever, nausea, vomiting, and myalgia. A “rebound” phenomenon after discontinuation is observed in 14%, and it may worsen psoriasis in those unresponsive to treatment.7
Other anti-TNF medications such as infliximab (Remicade), adalimumab (Humira), and onercept are still in clinical trials.
Combination therapy: Achieving goals while reducing adverse effects
Some patients require therapy with several agents to maintain adequate clearing of their psoriasis. The ideal combination therapy should lead to enhanced clinical response with reduction of adverse effects.
It is important to choose agents with synergistic effects without additive toxicities. Examples are combination of a systemic agent with topical calcipotriene, topical steroids, or with phototherapy. PUVA should be used with care for patients taking immunosuppressive agents due to risk of squamous cell carcinoma.8 A combination of 2 immunosuppressive agents is generally avoided due to risk of opportunistic infections, but has proven beneficial in a few therapy-resistant patients.9 Further clinical experience is needed for the inclusion of the new biologics in combination therapy.
New directions in treating psoriasis
A summary of future directions and current investigations in the management of psoriasis is given in TABLE 3.
Our patient’s treatment consisted of topical emollients, mid-potency topical corticosteroids, and tar shampoos/tar baths. She was responding well to the treatment. Introducing calcipotriene and reducing topical steroids is our next step. Regular follow-up visits are scheduled every 4 to 6 weeks.
TABLE 3
Future investigations
| Photodynamic therapy | The use of a photosensitizing drug in combination with a light source is showing promise, and clinical studies are under way for the treatment of psoriasis |
| Excimer lasers | Deliver high-dose narrowband UVB to a localized area sparing uninvolved skin. Clear psoriasis faster than conventional phototherapy, and may become predominant in the future |
| CNTO-1275 | Anti-interleukin-12 antibody that switches the immune response from a T-helper cell 1 cytokine reaction most commonly seen in psoriasis to a T-helper cell 2 cytokine response |
| T-cell receptor vaccines | Have been developed and are undergoing clinical trials in patients with psoriasis |
| Pimecrolimus | Used orally. It is a cytokine-release inhibitor with lesser immunosuppressive effects and side effects than tacrolimus and cyclosporin |
| Angiogenesis | Cutaneous blood vessels in psoriatic plaques are dilated, tortuous, and leaky |
| Vascular endothelial growth factor (VEGF) is overexpressed, and VEGF or its receptors are potential therapeutic targets for psoriasis | |
| Gene therapy | Chromosomes involved in psoriasis are being mapped |
| Gene therapy promises to be one of the most important areas of treatment of psoriasis for the new millennium |
Conclusion
Patients with mild localized psoriasis can easily and effectively be managed by family physicians using topical treatments or combinations modalities as outlined above. Patients with extensive disease or resistant to treatment should be referred to a dermatologist in conjunction with a rheumatologist if psoriatic arthritis is suspected.
With improved understanding of the immunopathogenesis and genetics of psoriasis, advent of the biologic agents, and future strategies under investigation, our approach to treating psoriasis may be very different in the years to come.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine (BAR 314), Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street, Boston, MA 02114. E-mail: [email protected]
An otherwise healthy 12-year-old girl came to the office with a 1-year history of a symmetric, generalized scaly eruption. These skin plaques did not itch; she had no recent history of sore throat. She also had no personal or family history of atopy or any similar eruption. She mentioned that the appearance of her skin and the “flaking” was making her very self-conscious and she wanted to have some intervention to make the skin clear.
The patient had multiple red, scaly, thick plaques on her back and the extensor surfaces of her elbows, knees, legs, and forearms (FIGURES 1 AND 2). There were no scalp, mucosal, or nail involvements. Rheumatologic examination and review of systems were unremarkable.
FIGURE 1
Plaques on the arm…
FIGURE 2
…and on the back
What is your diagnosis?
How would you manage this case?
Diagnosis: Chronic plaque psoriasis
Psoriasis is a noninfectious inflammatory skin disorder characterized by well-defined erythematous plaques that bear large, adherent silvery scales. It can appear at any age, but 75% of patients have an onset before the age of 40 years.1
Psoriasis was once considered a hyperproliferative disorder, but is now recognized as an autoimmune phenomenon involving activation of T-cells. As a result, new immunosuppressive agents have been added to the list of traditional therapies, opening a new chapter of immunomodulatory therapy.
Clinical presentations of psoriasis
In its classic presentation, psoriasis does not pose a diagnosis challenge to most clinicians—it presents as a sharply demarcated erythematous plaques with silvery white scales. Although many classification systems exist, a concise classification is included in TABLE 1. Skin conditions to be considered for differential diagnosis are summarized in TABLE 2. The US Food and Drug Administration defines “severe” psoriasis as involving more than 20% of body surface area.2
TABLE 1
Classification of psoriasis
| Chronic plaque psoriasis | Symmetrical plaques up to 20 cm in diameter, with a predilection for the elbows, knees, presacrum, scalp, hands, and feet |
| 5%–30% of patients develop a seronegative arthropathy | |
| Guttate psoriasis | Numerous small papules and plaques over the upper trunk and proximal extremities |
| Most common form of psoriasis in children, and may be triggered by any streptococcal infection including streptococcal perianal dermatitis | |
| Spontaneous remission is the rule | |
| Generalized pustular and erythrodermic psoriasis | Uncommon variants associated with high morbidity that may be fatal |
TABLE 2
Differential diagnosis
| Fungal infections |
| Squamous cell carcinoma in situ |
| Cutaneous T-cell lymphoma |
| Discoid eczema |
| Seborrhoeic eczema |
| Pityriasis rosea |
| Secondary syphilis |
| Hypertrophic lichen planus |
| Nummular dermatitis |
Pathogenesis of psoriasis
Psoriasis is a polygenic disorder. Susceptibility is determined by a large number of genes, each with a low penetrance. Important genetic associations are with HLA-Cw6, HLA-B27, and the genes PSOR-S1 and PSOR-S2 on chromosome 6 and 17, respectively.
Several triggering factors are identified, of which streptococcal antigens and certain drugs seem important, but the specific antigens are still unknown. The end result is activation of T-cells, overexpression of cytokines, and an inflammatory response.
Review of the classic treatments for psoriasis
Topical therapy
Topical therapy is indicated when psoriasis is limited to less than 20% of the body surface. Potent class I and II topical corticosteroids are the most widely used treatment for mild disease. After plaque clearance they can be given intermittently for maintenance.
The vitamin D3 derivative calcipotriene (Dovonex) is another first-line agent. Tazarotene (Tazorac), a topical retinoid prodrug, is a second-line agent used as monotherapy or in combination. Many combined regimens use topical corticosteroids, calcipotriene, and tazarotene. Coal tar—different concentrations in liquid form—is useful in treating extensive areas of the body and scalp psoriasis.
Phototherapy
Failure of topical therapy or extensive disease are indications for phototherapy or systemic medications. The trend is to use phototherapy in the form of narrowband UVB, which has proven more effective than broadband UVB and to have fewer adverse effects than psoralen UVA therapy (PUVA).3 Other light sources for home use are being developed.
Systemic agents
Methotrexate, given as a single weekly dose or in divided doses, has been used for more than 30 years; it inhibits the enzyme dihydrofolate reductase. An alternative immunosuppressive agent is cyclosporine. These 2 agents have high efficacy, but due to potential adverse effects they require careful patient selection and close follow-up.
Acitretin (Soriatane), an oral retinoid, is the treatment of choice for generalized pustular and erythrodermic psoriasis. It is also used in chronic plaque psoriasis, often in combination with phototherapy, which has a synergistic effect.New treatments
Our understanding of the immunopathogenesis of psoriasis has led to the development of therapies designed specifically to interfere with T-cell activation and effector functions. Three new immunomodulatory biologics are FDA-approved for the treatment of moderate to severe psoriasis. Typical cost of this therapy is more than $1000/month.
Anti-TNF-α strategies
Etanercept (Enbrel) is an antibody against the cytokine tumor necrosis factor alpha (TNF-α). It is self-administered at 25 mg to 50 mg subcutaneously twice weekly. Studies with 50-mg injections have shown a 75% clinical improvement in 49% of patients at 12 weeks and 59% of patients at 24 weeks.4
The most common side effect is reaction at the injection site. It was reported to produce dramatic remission of psoriatic arthritis and prevent radiographic progression of the disease.4-5 Due to raised concern about the risk of opportunistic infections, a purified protein derivative (PPD) test is advised before initiation of therapy to detect potential latent tuberculosis. Other risks include sepsis, pancytopenia, and worsening of multiple sclerosis.
Antibodies against T-lymphocyte surface molecules
Alefacept (Amevive) is a fusion protein that blocks T-cell activation and triggers apoptosis of activated T-cells. It is given as 15mg weekly intramuscular injections. Thirty-three percent of patients reported a 75% clinical improvement in their psoriasis within 12 weeks.6 Alefacept also decreases synovial tissue T-cell count, and may have a future role in psoriatic arthritis.
It has few side effects, but patients need weekly monitoring of their CD4+ count. Ongoing studies on combining it with ultraviolet light or extending the dose to 16 weeks are showing promise.6
Antibodies against adhesion molecules
Efalizumab (Raptiva) is a monoclonal antibody that blocks T-cell activation and migration. It is self-administered by the patient as a 1 mg/kg weekly subcutaneous injection. Forty-four percent of patients achieve a 75% clinical improvement in their psoriasis by 24 weeks.
The most common side effects are headache, fever, nausea, vomiting, and myalgia. A “rebound” phenomenon after discontinuation is observed in 14%, and it may worsen psoriasis in those unresponsive to treatment.7
Other anti-TNF medications such as infliximab (Remicade), adalimumab (Humira), and onercept are still in clinical trials.
Combination therapy: Achieving goals while reducing adverse effects
Some patients require therapy with several agents to maintain adequate clearing of their psoriasis. The ideal combination therapy should lead to enhanced clinical response with reduction of adverse effects.
It is important to choose agents with synergistic effects without additive toxicities. Examples are combination of a systemic agent with topical calcipotriene, topical steroids, or with phototherapy. PUVA should be used with care for patients taking immunosuppressive agents due to risk of squamous cell carcinoma.8 A combination of 2 immunosuppressive agents is generally avoided due to risk of opportunistic infections, but has proven beneficial in a few therapy-resistant patients.9 Further clinical experience is needed for the inclusion of the new biologics in combination therapy.
New directions in treating psoriasis
A summary of future directions and current investigations in the management of psoriasis is given in TABLE 3.
Our patient’s treatment consisted of topical emollients, mid-potency topical corticosteroids, and tar shampoos/tar baths. She was responding well to the treatment. Introducing calcipotriene and reducing topical steroids is our next step. Regular follow-up visits are scheduled every 4 to 6 weeks.
TABLE 3
Future investigations
| Photodynamic therapy | The use of a photosensitizing drug in combination with a light source is showing promise, and clinical studies are under way for the treatment of psoriasis |
| Excimer lasers | Deliver high-dose narrowband UVB to a localized area sparing uninvolved skin. Clear psoriasis faster than conventional phototherapy, and may become predominant in the future |
| CNTO-1275 | Anti-interleukin-12 antibody that switches the immune response from a T-helper cell 1 cytokine reaction most commonly seen in psoriasis to a T-helper cell 2 cytokine response |
| T-cell receptor vaccines | Have been developed and are undergoing clinical trials in patients with psoriasis |
| Pimecrolimus | Used orally. It is a cytokine-release inhibitor with lesser immunosuppressive effects and side effects than tacrolimus and cyclosporin |
| Angiogenesis | Cutaneous blood vessels in psoriatic plaques are dilated, tortuous, and leaky |
| Vascular endothelial growth factor (VEGF) is overexpressed, and VEGF or its receptors are potential therapeutic targets for psoriasis | |
| Gene therapy | Chromosomes involved in psoriasis are being mapped |
| Gene therapy promises to be one of the most important areas of treatment of psoriasis for the new millennium |
Conclusion
Patients with mild localized psoriasis can easily and effectively be managed by family physicians using topical treatments or combinations modalities as outlined above. Patients with extensive disease or resistant to treatment should be referred to a dermatologist in conjunction with a rheumatologist if psoriatic arthritis is suspected.
With improved understanding of the immunopathogenesis and genetics of psoriasis, advent of the biologic agents, and future strategies under investigation, our approach to treating psoriasis may be very different in the years to come.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, CWS, Wellman Center for Photomedicine (BAR 314), Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street, Boston, MA 02114. E-mail: [email protected]
1. Khachemoune A, Phillips TJ. Current treatment options in psoriasis. Hosp Pract (Off Ed) 2000;35:93-96,101-104,107.-
2. Winterfield LS, Menter A, Gordon A, Gottlieb A. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis 2005;64:ii87-ii90.
3. Zanolli M. Phototherapy arsenal in the treatment of psoriasis. Dermatol Clin 2004;22:397-406.
4. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept Psoriasis Study Group. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2005;349:2014-22.
5. Lebwohl M. Innovations in the treatment of psoriasis. J Am Acad Dermatol 2004;51:40-41.
6. Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE. Alefacept Clinical Study Group. An international, randomized, double blind placebo-controlled phase 3 trial of intramuscular Alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003;139:719-727.
7. Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab Study Group. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 2003;290:3073-3080.
8. Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and cyclosporin: nested cohort crossover study. Lancet 2001;358:1942-1945.
9. Clark CM, Kirby B, Morris AO, et al. Combination treatment with methotrexate and cyclosporin for severe and recalcitrant psoriasis. Br J Dermatol 1999;141:279-282.
1. Khachemoune A, Phillips TJ. Current treatment options in psoriasis. Hosp Pract (Off Ed) 2000;35:93-96,101-104,107.-
2. Winterfield LS, Menter A, Gordon A, Gottlieb A. Psoriasis treatment: current and emerging directed therapies. Ann Rheum Dis 2005;64:ii87-ii90.
3. Zanolli M. Phototherapy arsenal in the treatment of psoriasis. Dermatol Clin 2004;22:397-406.
4. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept Psoriasis Study Group. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2005;349:2014-22.
5. Lebwohl M. Innovations in the treatment of psoriasis. J Am Acad Dermatol 2004;51:40-41.
6. Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE. Alefacept Clinical Study Group. An international, randomized, double blind placebo-controlled phase 3 trial of intramuscular Alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003;139:719-727.
7. Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab Study Group. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 2003;290:3073-3080.
8. Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and cyclosporin: nested cohort crossover study. Lancet 2001;358:1942-1945.
9. Clark CM, Kirby B, Morris AO, et al. Combination treatment with methotrexate and cyclosporin for severe and recalcitrant psoriasis. Br J Dermatol 1999;141:279-282.