User login
A newborn with a skin lesion on the back
A 24-year-old woman without a family history of congenital anomalies delivered her first baby after a minimally complicated prenatal course. Poor weight gain led to an ultrasound at 33 weeks gestation; this test and others at 13 weeks to determine gestational age and at 20 weeks to evaluate for anatomic abnormalities all had normal results.
Prenatal screenings included a normal alpha feta-protein level. Review of the mother’s medical records suggested compliance with routine prenatal care and vitamins; however, she smoked cigarettes during the pregnancy and occasionally took a pill containing butalbital, acetaminophen, and caffeine for migraine headaches. She did not have gestational diabetes, but late in the pregnancy she developed hypertension, necessitating induction of labor.
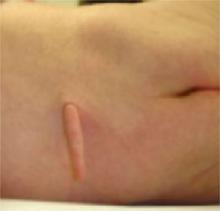
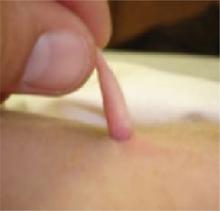
She delivered (via cesarean section) a full-term baby girl weighing 6 pounds, 9 ounces. The infant appeared healthy, with Apgar scores of 8 and 9 and a normal physical exam, except for a 2.5-cm appendage overlying the spine (Figures 1 and 2). The 2-day nursery stay was that of a normal neonate, without complications. The pediatric surgeon who was consulted arranged for outpatient follow-up after hospital dismissal.
FIGURE 1
Skin lesion
The small appendage was in the middle of the infant’s lower back.
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Pseudotail
Inspection of the lesion at the time of excision revealed fatty tissue within its core, consistent with the diagnosis of pseudotail. Ultrasound of the spine performed before hospital discharge was normal.
Tails and pseudotails
Tails and pseudotails appear similar on physical exam and are differentiated histologically. True tails—remnants of the embryologic tail—contain a core of muscle fibers, nerves, and blood vessels, whereas pseudotails contain primarily fatty tissue.1-3 From a practical and clinical point of view, you do not need to differentiate tails and pseudotails as they both can be associated with skin-covered congenital anomalies of the spine, or occult spinal dysraphism (OSD).
Although tail-like structures rarely occur, when they are present you should consider OSD.3 Two noteworthy literature reviews support this. The first identified 33 tails and pseudotails, of which 10 had OSD.1 An analysis of more recent cases (1960 through 1997) demonstrated OSD in 29 of 59 cases with lesions described as tails.2 A higher incidence of OSD occurred between 1980 and 1997 in this series. The authors attribute the higher incidence to the advent of computed tomography and magnetic resonance imaging (MRI), thereby stressing the importance of spine imaging with the presence of any caudal appendage resembling a tail.
Epidemiology of occult spinal dysraphism
The true incidence of OSD is not known. Congenital anomalies of the central nervous system, second only to birth defects of the cardiovascular system, are reported on birth certificates, but OSD may go unrecognized in the neonatal period. Frequently a benign-appearing midline cutaneous lesion is the only evidence of OSD at birth.4 Although 3% to 8% of newborns with a skin lesion over the spine have OSD,5 50% to 90% of patients with OSD have a skin marker.5-7 Of 2010 newborns in 1 study, 144 had a midline cutaneous lesion, and 5.5% of these had an abnormal ultrasound suggesting OSD.8 Additionally, the presence of more than 1 lesion increases the probability of finding OSD.5,9,10
You should look for underlying OSD in newborns with a suggestive skin lesion, since the embryologic origin of the spinal cord and the skin overlying the spine are the same: the ectoderm layer of cells within the trilaminar (endoderm, mesoderm, ectoderm) early embryo gives rise to both structures. Midline cutaneous lesions with high likelihood for OSD include hypertrichosis (hairy patch), atypical dimple (a dimple >5 mm diameter or >2.5 cm above the anus), hemangioma, lipoma, aplasia cutis, dermoid cyst or sinus, skin tags, tails, and pseudotails.3
Evaluation: The neonate with a midline cutaneous lesion
The incidence of OSD appears low in newborns with midline cutaneous lesions, but irreversible orthopedic, urologic, and neurologic problems from OSD may occur later in life. These complications can be prevented by early surgical intervention; therefore, it is critical that you do not miss a diagnosis of OSD and pursue spine imaging.
The definitive test, an MRI,3,6,9 usually requires sedation of infants,11 making high-resolution ultrasound the recommended choice for initial screening.3-10 Ultrasound is not invasive and may allow for testing in the hospital nursery. After age 3 months, when ossification of the posterior spine limits visualization of the spinal cord by ultrasound, consider an MRI first. If the ultrasound demonstrates OSD or is inconclusive, follow with an MRI for a more specific diagnosis.
Indications for an ultrasound of the newborn’s spine include any one of the following: an abnormal antenatal ultrasound; a midline cutaneous lesion other than a simple dimple; presence of other OSD related anomalies; or an abnormal neurologic exam.5 The authors of this protocol retrospectively applied it to 223 infants who had an ultrasound, and it reduced the number of tests by 50% without missing a single case of OSD.
If you are caring for a newborn infant you should resist the temptation to simply remove a midline cutaneous lesion, including a pseudotail, in the nursery without first searching for OSD. If OSD is present, neurosurgical consultation should be obtained as soon as possible. The optimal time for surgical intervention is within the first 4 months of life.4
Patient outcome
The infant’s pseudotail was removed without consequence and postoperatively the patient has thrived.
CORRESPONDENCE
Timothy J. Benton, MD Assistant Professor, Associate Residency Program Director, Texas Tech University Health Sciences Center at Amarillo, School of Medicine, Department of Family and Community Medicine, 1400 Wallace Boulevard, Amarillo, Texas 79106. E-mail: [email protected]
1. Dao AH, Netsky MG. Human tails and pseudotails. Hum Pathol 1984;56:339-340.
2. Lu FL, Wang PJ, Teng RJ, Yau KI. The human tail. Pediatr Neurol 1998;19:230-233.
3. Drolet B. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am 2000;47:813-823.
4. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr 1995;34:650-654.
5. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72-77.
6. Mcatee-smith J, Hebert AA, Rapini RP, Goldberg NS. Skin lesions of the spinal axis and spinal dysraphism. Fifteen cases and a review of the literature. Arch Pediatr Adolesc Med 1994;148:740-748.
7. Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol 2002;75:384-392.
8. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmao S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol 2005;63(suppl 1):s8-s12.
9. Guggisberg D, Hadj-rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 2004;140:1109-1115.
10. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687-1692.
11. Keengwe IN, Hegde S, Dearlove O, Wilson B, Yates RW, Sharples A. Structured sedation programme for magnetic resonance imaging examination in children. Anaesthesia 1999;54:1069-1072.
A 24-year-old woman without a family history of congenital anomalies delivered her first baby after a minimally complicated prenatal course. Poor weight gain led to an ultrasound at 33 weeks gestation; this test and others at 13 weeks to determine gestational age and at 20 weeks to evaluate for anatomic abnormalities all had normal results.
Prenatal screenings included a normal alpha feta-protein level. Review of the mother’s medical records suggested compliance with routine prenatal care and vitamins; however, she smoked cigarettes during the pregnancy and occasionally took a pill containing butalbital, acetaminophen, and caffeine for migraine headaches. She did not have gestational diabetes, but late in the pregnancy she developed hypertension, necessitating induction of labor.
She delivered (via cesarean section) a full-term baby girl weighing 6 pounds, 9 ounces. The infant appeared healthy, with Apgar scores of 8 and 9 and a normal physical exam, except for a 2.5-cm appendage overlying the spine (Figures 1 and 2). The 2-day nursery stay was that of a normal neonate, without complications. The pediatric surgeon who was consulted arranged for outpatient follow-up after hospital dismissal.
FIGURE 1
Skin lesion
The small appendage was in the middle of the infant’s lower back.
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Pseudotail
Inspection of the lesion at the time of excision revealed fatty tissue within its core, consistent with the diagnosis of pseudotail. Ultrasound of the spine performed before hospital discharge was normal.
Tails and pseudotails
Tails and pseudotails appear similar on physical exam and are differentiated histologically. True tails—remnants of the embryologic tail—contain a core of muscle fibers, nerves, and blood vessels, whereas pseudotails contain primarily fatty tissue.1-3 From a practical and clinical point of view, you do not need to differentiate tails and pseudotails as they both can be associated with skin-covered congenital anomalies of the spine, or occult spinal dysraphism (OSD).
Although tail-like structures rarely occur, when they are present you should consider OSD.3 Two noteworthy literature reviews support this. The first identified 33 tails and pseudotails, of which 10 had OSD.1 An analysis of more recent cases (1960 through 1997) demonstrated OSD in 29 of 59 cases with lesions described as tails.2 A higher incidence of OSD occurred between 1980 and 1997 in this series. The authors attribute the higher incidence to the advent of computed tomography and magnetic resonance imaging (MRI), thereby stressing the importance of spine imaging with the presence of any caudal appendage resembling a tail.
Epidemiology of occult spinal dysraphism
The true incidence of OSD is not known. Congenital anomalies of the central nervous system, second only to birth defects of the cardiovascular system, are reported on birth certificates, but OSD may go unrecognized in the neonatal period. Frequently a benign-appearing midline cutaneous lesion is the only evidence of OSD at birth.4 Although 3% to 8% of newborns with a skin lesion over the spine have OSD,5 50% to 90% of patients with OSD have a skin marker.5-7 Of 2010 newborns in 1 study, 144 had a midline cutaneous lesion, and 5.5% of these had an abnormal ultrasound suggesting OSD.8 Additionally, the presence of more than 1 lesion increases the probability of finding OSD.5,9,10
You should look for underlying OSD in newborns with a suggestive skin lesion, since the embryologic origin of the spinal cord and the skin overlying the spine are the same: the ectoderm layer of cells within the trilaminar (endoderm, mesoderm, ectoderm) early embryo gives rise to both structures. Midline cutaneous lesions with high likelihood for OSD include hypertrichosis (hairy patch), atypical dimple (a dimple >5 mm diameter or >2.5 cm above the anus), hemangioma, lipoma, aplasia cutis, dermoid cyst or sinus, skin tags, tails, and pseudotails.3
Evaluation: The neonate with a midline cutaneous lesion
The incidence of OSD appears low in newborns with midline cutaneous lesions, but irreversible orthopedic, urologic, and neurologic problems from OSD may occur later in life. These complications can be prevented by early surgical intervention; therefore, it is critical that you do not miss a diagnosis of OSD and pursue spine imaging.
The definitive test, an MRI,3,6,9 usually requires sedation of infants,11 making high-resolution ultrasound the recommended choice for initial screening.3-10 Ultrasound is not invasive and may allow for testing in the hospital nursery. After age 3 months, when ossification of the posterior spine limits visualization of the spinal cord by ultrasound, consider an MRI first. If the ultrasound demonstrates OSD or is inconclusive, follow with an MRI for a more specific diagnosis.
Indications for an ultrasound of the newborn’s spine include any one of the following: an abnormal antenatal ultrasound; a midline cutaneous lesion other than a simple dimple; presence of other OSD related anomalies; or an abnormal neurologic exam.5 The authors of this protocol retrospectively applied it to 223 infants who had an ultrasound, and it reduced the number of tests by 50% without missing a single case of OSD.
If you are caring for a newborn infant you should resist the temptation to simply remove a midline cutaneous lesion, including a pseudotail, in the nursery without first searching for OSD. If OSD is present, neurosurgical consultation should be obtained as soon as possible. The optimal time for surgical intervention is within the first 4 months of life.4
Patient outcome
The infant’s pseudotail was removed without consequence and postoperatively the patient has thrived.
CORRESPONDENCE
Timothy J. Benton, MD Assistant Professor, Associate Residency Program Director, Texas Tech University Health Sciences Center at Amarillo, School of Medicine, Department of Family and Community Medicine, 1400 Wallace Boulevard, Amarillo, Texas 79106. E-mail: [email protected]
A 24-year-old woman without a family history of congenital anomalies delivered her first baby after a minimally complicated prenatal course. Poor weight gain led to an ultrasound at 33 weeks gestation; this test and others at 13 weeks to determine gestational age and at 20 weeks to evaluate for anatomic abnormalities all had normal results.
Prenatal screenings included a normal alpha feta-protein level. Review of the mother’s medical records suggested compliance with routine prenatal care and vitamins; however, she smoked cigarettes during the pregnancy and occasionally took a pill containing butalbital, acetaminophen, and caffeine for migraine headaches. She did not have gestational diabetes, but late in the pregnancy she developed hypertension, necessitating induction of labor.
She delivered (via cesarean section) a full-term baby girl weighing 6 pounds, 9 ounces. The infant appeared healthy, with Apgar scores of 8 and 9 and a normal physical exam, except for a 2.5-cm appendage overlying the spine (Figures 1 and 2). The 2-day nursery stay was that of a normal neonate, without complications. The pediatric surgeon who was consulted arranged for outpatient follow-up after hospital dismissal.
FIGURE 1
Skin lesion
The small appendage was in the middle of the infant’s lower back.
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Pseudotail
Inspection of the lesion at the time of excision revealed fatty tissue within its core, consistent with the diagnosis of pseudotail. Ultrasound of the spine performed before hospital discharge was normal.
Tails and pseudotails
Tails and pseudotails appear similar on physical exam and are differentiated histologically. True tails—remnants of the embryologic tail—contain a core of muscle fibers, nerves, and blood vessels, whereas pseudotails contain primarily fatty tissue.1-3 From a practical and clinical point of view, you do not need to differentiate tails and pseudotails as they both can be associated with skin-covered congenital anomalies of the spine, or occult spinal dysraphism (OSD).
Although tail-like structures rarely occur, when they are present you should consider OSD.3 Two noteworthy literature reviews support this. The first identified 33 tails and pseudotails, of which 10 had OSD.1 An analysis of more recent cases (1960 through 1997) demonstrated OSD in 29 of 59 cases with lesions described as tails.2 A higher incidence of OSD occurred between 1980 and 1997 in this series. The authors attribute the higher incidence to the advent of computed tomography and magnetic resonance imaging (MRI), thereby stressing the importance of spine imaging with the presence of any caudal appendage resembling a tail.
Epidemiology of occult spinal dysraphism
The true incidence of OSD is not known. Congenital anomalies of the central nervous system, second only to birth defects of the cardiovascular system, are reported on birth certificates, but OSD may go unrecognized in the neonatal period. Frequently a benign-appearing midline cutaneous lesion is the only evidence of OSD at birth.4 Although 3% to 8% of newborns with a skin lesion over the spine have OSD,5 50% to 90% of patients with OSD have a skin marker.5-7 Of 2010 newborns in 1 study, 144 had a midline cutaneous lesion, and 5.5% of these had an abnormal ultrasound suggesting OSD.8 Additionally, the presence of more than 1 lesion increases the probability of finding OSD.5,9,10
You should look for underlying OSD in newborns with a suggestive skin lesion, since the embryologic origin of the spinal cord and the skin overlying the spine are the same: the ectoderm layer of cells within the trilaminar (endoderm, mesoderm, ectoderm) early embryo gives rise to both structures. Midline cutaneous lesions with high likelihood for OSD include hypertrichosis (hairy patch), atypical dimple (a dimple >5 mm diameter or >2.5 cm above the anus), hemangioma, lipoma, aplasia cutis, dermoid cyst or sinus, skin tags, tails, and pseudotails.3
Evaluation: The neonate with a midline cutaneous lesion
The incidence of OSD appears low in newborns with midline cutaneous lesions, but irreversible orthopedic, urologic, and neurologic problems from OSD may occur later in life. These complications can be prevented by early surgical intervention; therefore, it is critical that you do not miss a diagnosis of OSD and pursue spine imaging.
The definitive test, an MRI,3,6,9 usually requires sedation of infants,11 making high-resolution ultrasound the recommended choice for initial screening.3-10 Ultrasound is not invasive and may allow for testing in the hospital nursery. After age 3 months, when ossification of the posterior spine limits visualization of the spinal cord by ultrasound, consider an MRI first. If the ultrasound demonstrates OSD or is inconclusive, follow with an MRI for a more specific diagnosis.
Indications for an ultrasound of the newborn’s spine include any one of the following: an abnormal antenatal ultrasound; a midline cutaneous lesion other than a simple dimple; presence of other OSD related anomalies; or an abnormal neurologic exam.5 The authors of this protocol retrospectively applied it to 223 infants who had an ultrasound, and it reduced the number of tests by 50% without missing a single case of OSD.
If you are caring for a newborn infant you should resist the temptation to simply remove a midline cutaneous lesion, including a pseudotail, in the nursery without first searching for OSD. If OSD is present, neurosurgical consultation should be obtained as soon as possible. The optimal time for surgical intervention is within the first 4 months of life.4
Patient outcome
The infant’s pseudotail was removed without consequence and postoperatively the patient has thrived.
CORRESPONDENCE
Timothy J. Benton, MD Assistant Professor, Associate Residency Program Director, Texas Tech University Health Sciences Center at Amarillo, School of Medicine, Department of Family and Community Medicine, 1400 Wallace Boulevard, Amarillo, Texas 79106. E-mail: [email protected]
1. Dao AH, Netsky MG. Human tails and pseudotails. Hum Pathol 1984;56:339-340.
2. Lu FL, Wang PJ, Teng RJ, Yau KI. The human tail. Pediatr Neurol 1998;19:230-233.
3. Drolet B. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am 2000;47:813-823.
4. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr 1995;34:650-654.
5. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72-77.
6. Mcatee-smith J, Hebert AA, Rapini RP, Goldberg NS. Skin lesions of the spinal axis and spinal dysraphism. Fifteen cases and a review of the literature. Arch Pediatr Adolesc Med 1994;148:740-748.
7. Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol 2002;75:384-392.
8. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmao S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol 2005;63(suppl 1):s8-s12.
9. Guggisberg D, Hadj-rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 2004;140:1109-1115.
10. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687-1692.
11. Keengwe IN, Hegde S, Dearlove O, Wilson B, Yates RW, Sharples A. Structured sedation programme for magnetic resonance imaging examination in children. Anaesthesia 1999;54:1069-1072.
1. Dao AH, Netsky MG. Human tails and pseudotails. Hum Pathol 1984;56:339-340.
2. Lu FL, Wang PJ, Teng RJ, Yau KI. The human tail. Pediatr Neurol 1998;19:230-233.
3. Drolet B. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am 2000;47:813-823.
4. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr 1995;34:650-654.
5. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72-77.
6. Mcatee-smith J, Hebert AA, Rapini RP, Goldberg NS. Skin lesions of the spinal axis and spinal dysraphism. Fifteen cases and a review of the literature. Arch Pediatr Adolesc Med 1994;148:740-748.
7. Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol 2002;75:384-392.
8. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmao S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol 2005;63(suppl 1):s8-s12.
9. Guggisberg D, Hadj-rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 2004;140:1109-1115.
10. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687-1692.
11. Keengwe IN, Hegde S, Dearlove O, Wilson B, Yates RW, Sharples A. Structured sedation programme for magnetic resonance imaging examination in children. Anaesthesia 1999;54:1069-1072.
Left-sided back pain that won’t go away
A 68-year-old man came to the emergency department complaining of left-side thoracic back pain, after 5 days of outpatient treatment with analgesics did not help him. His pain started after physical labor, but he did not recall any trauma. A review of his medical history revealed only coronary artery disease, with coronary stent placement several years before this event. He had not been hospitalized recently, undergone an invasive procedure, or taken antibiotics.
On examination, a left thoracic paraspinal muscle was tender without fluctuance, overlying skin redness, or a lesion. An elevated white blood cell count of 12,300/mcL was the only laboratory test with abnormal results. The patient did not have fever, and results of urinalysis, chest radiograph, and abdominal sonogram were normal. Computed tomography (CT) images of the abdomen and pelvis showed inflammation of a left thoracic paraspinal muscle.
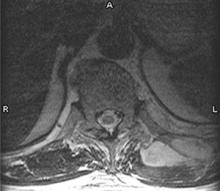
Thirty-six hours after admission, the patient developed fever (38.8°C). His physicians obtained a blood culture. The fever recurred (at 38.9°C) the following day, and 2 more blood cultures were done. His back pain did not improve despite analgesics, intravenous antibiotics, and physical therapy; therefore, on the eighth day in the hospital, magnetic resonance imaging (MRI) was performed (FIGURE).
FIGURE
MRI of the back
What is the diagnosis?
Diagnosis: Methicillin-resistant Staphylococcus aureus pyomyositis
Reports of the 3 blood cultures, acquired on the seventh day in the hospital, showed growth of methicillin-resistant Staphylococcus aureus (MRSA) with antibiotic susceptibility patterns common to community-acquired MRSA.1 A purulent aspirate was obtained from the lesion in the FIGURE with CT-guided drainage. Community-acquired MRSA grew in the aspirate culture. The final diagnosis was a primary skeletal muscle abscess without contiguous spread from an adjacent site, or pyomyositis.
Epidemiology of pyomyositis
Pyomyositis is uncommon in immunocompetent individuals living outside of the tropics. It is usually caused by S aureus. In the United States between 1981 and 2002, 330 cases were identified in a literature review,2 which noted an increasing incidence. Of these cases, 70% were caused by S aureus and 61.5% of patients were immunocompromised; 1 involved MRSA.
In 2003, 2 of 3 MRSA cases were reported in patients with hematologic disorders.3 In 2005, 4 community-acquired MRSA cases were reported;1 2 had other illnesses leading to increased risk. MRSA pyomyositis is now being reported in immunocompetent individuals, but most cases arise in patients with cancer, diabetes mellitus, rheumatologic disorders, hematologic disorders, renal failure, liver cirrhosis, intravenous drug use, or HIV infection.2,4,5 With staphylococcal infections increasing,6 including MRSA and community-acquired MRSA, the incidence of pyomyositis may increase correspondingly.
Consider the possibility of pyomyositis in patients with localized muscle pain and tenderness and who have risk factors for acquiring MRSA—living in a community with prevalent MRSA, recent hospitalization, antibiotic use, invasive procedures, or chronic venous catheters.
Most commonly, pyomyositis occurs in a leg or arm.2 In the 330 cases mentioned above, the sites of infection, in descending order of frequency, were lower extremity, upper extremity, buttocks, chest wall, paraspinal, and psoas.
Diagnostic evaluation: Know the stages
Making the correct diagnosis at the initial presentation is difficult because nonspecific symptoms resemble a muscle strain. But this infection progresses to bacteremia, making early identification important. To recognize pyomyositis before bacteremia develops, rely on clinical suspicion and familiarity with the 3 stages of the disease.
Stage 1 (invasive) is characterized by muscle pain and tenderness without systemic evidence of illness. Stage 2 (suppurative) is the formation of an abscess. At this time systemic symptoms such as fever may occur; otherwise physical exam findings remain vague with tenderness and induration without overlying skin redness or palpable fluctuance common to skin abscesses. Stage 3 is bacteremia or sepsis. Awareness of the risks and the stages of pyomyositis will aid in quicker diagnosis.
Diagnostic delay occurs because the first stage presents with nonspecific symptoms and examination findings, and also because initial laboratory and radiographic testing are inconclusive. Elevation of the white blood cell count and the erythrocyte sedimentation rate occur early, while serum muscle enzymes remain normal.2,4,5
Imaging studies performed during the first stage may not be productive; however, in the second stage imaging identifies the abscess. Although limited data exist regarding the best imaging modality, MRI and CT appear most useful.1,3,4,7 In 1 retrospective analysis, ultrasound identified 5 of 8 cases correctly, while MRI and CT were diagnostic in 5 of 6 and 9 of 9, respectively.7
Treatment: Antibiotics, drainage
Choose an antibiotic empirically that covers S aureus and is also appropriate for MRSA. Pyomyositis discovered in the first stage may resolve with antibiotics alone.4 In addition, early intervention may be especially important with MRSA, since MRSA bacteremia is associated with increased mortality compared with methicillin-sensitive S aureus bacteremia.6
If the condition progresses to the second stage with abscess development, surgical or percutaneous drainage will be necessary. Identification and removal of the site of infection in patients with community-acquired staphylococcal bacteremia results in improved outcomes,8 and pyomyositis can be an overlooked source.
Patient outcome
The day following drainage of the abscess, the patient was discharged from the hospital. He was treated for 6 weeks with intravenous antibiotics because of staphylococcal bacteremia, and he has fully recovered.
Acknowledgments
The authors would like to thank Robert Martin, Lead Operator, Information Technology, Texas Tech University Health Sciences Center at Amarillo for assistance in computer image enhancement and development.
CORRESPONDENCE
Timothy J. Benton, MD, Texas Tech University Health Sciences Center at Amarillo, Department of Family and Community Medicine, 1600 Wallace Boulevard, Amarillo, TX 79106. E-mail: [email protected]
1. Ruiz M, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2005;352:1488-1489.
2. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420-428.
3. Hayashi T. Pyomyositis as a focus of infection in hematological disorders: a report of 3 cases. Int J Hematol 2003;77:171-174.
4. Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 2002;16:697-712.
5. Hossain A, Reis ED, Soundararajan K, Kerstain MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg 2000;66:1064-1066.
6. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59.
7. Boniotti V. Contribution of imaging to the evaluation of pyomyositis. Radiol Med 2005;109:404-13.
8. Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J Hosp Infect 2002;52:29-36.
A 68-year-old man came to the emergency department complaining of left-side thoracic back pain, after 5 days of outpatient treatment with analgesics did not help him. His pain started after physical labor, but he did not recall any trauma. A review of his medical history revealed only coronary artery disease, with coronary stent placement several years before this event. He had not been hospitalized recently, undergone an invasive procedure, or taken antibiotics.
On examination, a left thoracic paraspinal muscle was tender without fluctuance, overlying skin redness, or a lesion. An elevated white blood cell count of 12,300/mcL was the only laboratory test with abnormal results. The patient did not have fever, and results of urinalysis, chest radiograph, and abdominal sonogram were normal. Computed tomography (CT) images of the abdomen and pelvis showed inflammation of a left thoracic paraspinal muscle.
Thirty-six hours after admission, the patient developed fever (38.8°C). His physicians obtained a blood culture. The fever recurred (at 38.9°C) the following day, and 2 more blood cultures were done. His back pain did not improve despite analgesics, intravenous antibiotics, and physical therapy; therefore, on the eighth day in the hospital, magnetic resonance imaging (MRI) was performed (FIGURE).
FIGURE
MRI of the back
What is the diagnosis?
Diagnosis: Methicillin-resistant Staphylococcus aureus pyomyositis
Reports of the 3 blood cultures, acquired on the seventh day in the hospital, showed growth of methicillin-resistant Staphylococcus aureus (MRSA) with antibiotic susceptibility patterns common to community-acquired MRSA.1 A purulent aspirate was obtained from the lesion in the FIGURE with CT-guided drainage. Community-acquired MRSA grew in the aspirate culture. The final diagnosis was a primary skeletal muscle abscess without contiguous spread from an adjacent site, or pyomyositis.
Epidemiology of pyomyositis
Pyomyositis is uncommon in immunocompetent individuals living outside of the tropics. It is usually caused by S aureus. In the United States between 1981 and 2002, 330 cases were identified in a literature review,2 which noted an increasing incidence. Of these cases, 70% were caused by S aureus and 61.5% of patients were immunocompromised; 1 involved MRSA.
In 2003, 2 of 3 MRSA cases were reported in patients with hematologic disorders.3 In 2005, 4 community-acquired MRSA cases were reported;1 2 had other illnesses leading to increased risk. MRSA pyomyositis is now being reported in immunocompetent individuals, but most cases arise in patients with cancer, diabetes mellitus, rheumatologic disorders, hematologic disorders, renal failure, liver cirrhosis, intravenous drug use, or HIV infection.2,4,5 With staphylococcal infections increasing,6 including MRSA and community-acquired MRSA, the incidence of pyomyositis may increase correspondingly.
Consider the possibility of pyomyositis in patients with localized muscle pain and tenderness and who have risk factors for acquiring MRSA—living in a community with prevalent MRSA, recent hospitalization, antibiotic use, invasive procedures, or chronic venous catheters.
Most commonly, pyomyositis occurs in a leg or arm.2 In the 330 cases mentioned above, the sites of infection, in descending order of frequency, were lower extremity, upper extremity, buttocks, chest wall, paraspinal, and psoas.
Diagnostic evaluation: Know the stages
Making the correct diagnosis at the initial presentation is difficult because nonspecific symptoms resemble a muscle strain. But this infection progresses to bacteremia, making early identification important. To recognize pyomyositis before bacteremia develops, rely on clinical suspicion and familiarity with the 3 stages of the disease.
Stage 1 (invasive) is characterized by muscle pain and tenderness without systemic evidence of illness. Stage 2 (suppurative) is the formation of an abscess. At this time systemic symptoms such as fever may occur; otherwise physical exam findings remain vague with tenderness and induration without overlying skin redness or palpable fluctuance common to skin abscesses. Stage 3 is bacteremia or sepsis. Awareness of the risks and the stages of pyomyositis will aid in quicker diagnosis.
Diagnostic delay occurs because the first stage presents with nonspecific symptoms and examination findings, and also because initial laboratory and radiographic testing are inconclusive. Elevation of the white blood cell count and the erythrocyte sedimentation rate occur early, while serum muscle enzymes remain normal.2,4,5
Imaging studies performed during the first stage may not be productive; however, in the second stage imaging identifies the abscess. Although limited data exist regarding the best imaging modality, MRI and CT appear most useful.1,3,4,7 In 1 retrospective analysis, ultrasound identified 5 of 8 cases correctly, while MRI and CT were diagnostic in 5 of 6 and 9 of 9, respectively.7
Treatment: Antibiotics, drainage
Choose an antibiotic empirically that covers S aureus and is also appropriate for MRSA. Pyomyositis discovered in the first stage may resolve with antibiotics alone.4 In addition, early intervention may be especially important with MRSA, since MRSA bacteremia is associated with increased mortality compared with methicillin-sensitive S aureus bacteremia.6
If the condition progresses to the second stage with abscess development, surgical or percutaneous drainage will be necessary. Identification and removal of the site of infection in patients with community-acquired staphylococcal bacteremia results in improved outcomes,8 and pyomyositis can be an overlooked source.
Patient outcome
The day following drainage of the abscess, the patient was discharged from the hospital. He was treated for 6 weeks with intravenous antibiotics because of staphylococcal bacteremia, and he has fully recovered.
Acknowledgments
The authors would like to thank Robert Martin, Lead Operator, Information Technology, Texas Tech University Health Sciences Center at Amarillo for assistance in computer image enhancement and development.
CORRESPONDENCE
Timothy J. Benton, MD, Texas Tech University Health Sciences Center at Amarillo, Department of Family and Community Medicine, 1600 Wallace Boulevard, Amarillo, TX 79106. E-mail: [email protected]
A 68-year-old man came to the emergency department complaining of left-side thoracic back pain, after 5 days of outpatient treatment with analgesics did not help him. His pain started after physical labor, but he did not recall any trauma. A review of his medical history revealed only coronary artery disease, with coronary stent placement several years before this event. He had not been hospitalized recently, undergone an invasive procedure, or taken antibiotics.
On examination, a left thoracic paraspinal muscle was tender without fluctuance, overlying skin redness, or a lesion. An elevated white blood cell count of 12,300/mcL was the only laboratory test with abnormal results. The patient did not have fever, and results of urinalysis, chest radiograph, and abdominal sonogram were normal. Computed tomography (CT) images of the abdomen and pelvis showed inflammation of a left thoracic paraspinal muscle.
Thirty-six hours after admission, the patient developed fever (38.8°C). His physicians obtained a blood culture. The fever recurred (at 38.9°C) the following day, and 2 more blood cultures were done. His back pain did not improve despite analgesics, intravenous antibiotics, and physical therapy; therefore, on the eighth day in the hospital, magnetic resonance imaging (MRI) was performed (FIGURE).
FIGURE
MRI of the back
What is the diagnosis?
Diagnosis: Methicillin-resistant Staphylococcus aureus pyomyositis
Reports of the 3 blood cultures, acquired on the seventh day in the hospital, showed growth of methicillin-resistant Staphylococcus aureus (MRSA) with antibiotic susceptibility patterns common to community-acquired MRSA.1 A purulent aspirate was obtained from the lesion in the FIGURE with CT-guided drainage. Community-acquired MRSA grew in the aspirate culture. The final diagnosis was a primary skeletal muscle abscess without contiguous spread from an adjacent site, or pyomyositis.
Epidemiology of pyomyositis
Pyomyositis is uncommon in immunocompetent individuals living outside of the tropics. It is usually caused by S aureus. In the United States between 1981 and 2002, 330 cases were identified in a literature review,2 which noted an increasing incidence. Of these cases, 70% were caused by S aureus and 61.5% of patients were immunocompromised; 1 involved MRSA.
In 2003, 2 of 3 MRSA cases were reported in patients with hematologic disorders.3 In 2005, 4 community-acquired MRSA cases were reported;1 2 had other illnesses leading to increased risk. MRSA pyomyositis is now being reported in immunocompetent individuals, but most cases arise in patients with cancer, diabetes mellitus, rheumatologic disorders, hematologic disorders, renal failure, liver cirrhosis, intravenous drug use, or HIV infection.2,4,5 With staphylococcal infections increasing,6 including MRSA and community-acquired MRSA, the incidence of pyomyositis may increase correspondingly.
Consider the possibility of pyomyositis in patients with localized muscle pain and tenderness and who have risk factors for acquiring MRSA—living in a community with prevalent MRSA, recent hospitalization, antibiotic use, invasive procedures, or chronic venous catheters.
Most commonly, pyomyositis occurs in a leg or arm.2 In the 330 cases mentioned above, the sites of infection, in descending order of frequency, were lower extremity, upper extremity, buttocks, chest wall, paraspinal, and psoas.
Diagnostic evaluation: Know the stages
Making the correct diagnosis at the initial presentation is difficult because nonspecific symptoms resemble a muscle strain. But this infection progresses to bacteremia, making early identification important. To recognize pyomyositis before bacteremia develops, rely on clinical suspicion and familiarity with the 3 stages of the disease.
Stage 1 (invasive) is characterized by muscle pain and tenderness without systemic evidence of illness. Stage 2 (suppurative) is the formation of an abscess. At this time systemic symptoms such as fever may occur; otherwise physical exam findings remain vague with tenderness and induration without overlying skin redness or palpable fluctuance common to skin abscesses. Stage 3 is bacteremia or sepsis. Awareness of the risks and the stages of pyomyositis will aid in quicker diagnosis.
Diagnostic delay occurs because the first stage presents with nonspecific symptoms and examination findings, and also because initial laboratory and radiographic testing are inconclusive. Elevation of the white blood cell count and the erythrocyte sedimentation rate occur early, while serum muscle enzymes remain normal.2,4,5
Imaging studies performed during the first stage may not be productive; however, in the second stage imaging identifies the abscess. Although limited data exist regarding the best imaging modality, MRI and CT appear most useful.1,3,4,7 In 1 retrospective analysis, ultrasound identified 5 of 8 cases correctly, while MRI and CT were diagnostic in 5 of 6 and 9 of 9, respectively.7
Treatment: Antibiotics, drainage
Choose an antibiotic empirically that covers S aureus and is also appropriate for MRSA. Pyomyositis discovered in the first stage may resolve with antibiotics alone.4 In addition, early intervention may be especially important with MRSA, since MRSA bacteremia is associated with increased mortality compared with methicillin-sensitive S aureus bacteremia.6
If the condition progresses to the second stage with abscess development, surgical or percutaneous drainage will be necessary. Identification and removal of the site of infection in patients with community-acquired staphylococcal bacteremia results in improved outcomes,8 and pyomyositis can be an overlooked source.
Patient outcome
The day following drainage of the abscess, the patient was discharged from the hospital. He was treated for 6 weeks with intravenous antibiotics because of staphylococcal bacteremia, and he has fully recovered.
Acknowledgments
The authors would like to thank Robert Martin, Lead Operator, Information Technology, Texas Tech University Health Sciences Center at Amarillo for assistance in computer image enhancement and development.
CORRESPONDENCE
Timothy J. Benton, MD, Texas Tech University Health Sciences Center at Amarillo, Department of Family and Community Medicine, 1600 Wallace Boulevard, Amarillo, TX 79106. E-mail: [email protected]
1. Ruiz M, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2005;352:1488-1489.
2. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420-428.
3. Hayashi T. Pyomyositis as a focus of infection in hematological disorders: a report of 3 cases. Int J Hematol 2003;77:171-174.
4. Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 2002;16:697-712.
5. Hossain A, Reis ED, Soundararajan K, Kerstain MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg 2000;66:1064-1066.
6. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59.
7. Boniotti V. Contribution of imaging to the evaluation of pyomyositis. Radiol Med 2005;109:404-13.
8. Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J Hosp Infect 2002;52:29-36.
1. Ruiz M, Yohannes S, Wladyka CG. Pyomyositis caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2005;352:1488-1489.
2. Crum NF. Bacterial pyomyositis in the United States. Am J Med 2004;117:420-428.
3. Hayashi T. Pyomyositis as a focus of infection in hematological disorders: a report of 3 cases. Int J Hematol 2003;77:171-174.
4. Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 2002;16:697-712.
5. Hossain A, Reis ED, Soundararajan K, Kerstain MD, Hollier LH. Nontropical pyomyositis: analysis of eight patients in an urban center. Am Surg 2000;66:1064-1066.
6. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-59.
7. Boniotti V. Contribution of imaging to the evaluation of pyomyositis. Radiol Med 2005;109:404-13.
8. Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J Hosp Infect 2002;52:29-36.